Abstract
Mitochondrial biogenesis and destruction in skeletal muscle are coordinated by distinct signaling pathways that are influenced by internal and exogenous variables including, but not limited to, muscle phenotype, physical activity, dietary composition, or drug administration. Previously we found that long-term resveratrol administration (up to 480 mg/day) ameliorates the slow-to-fast phenotypic shift in soleus muscles and promotes the expression in slow myosin heavy chain in the mixed plantaris muscle of non-human primates consuming a high fat/sugar (HFS) diet. Here, we expand on these earlier findings by examining whether mitochondrial content and the markers that dictate their biogenesis and mitophagy/autophagy are similarly affected by HFS and/or influenced by resveratrol while consuming this diet (HFSR). Compared to controls (n=9), there was a ~20–25% decrease in mitochondrial content in HFS (n=8) muscles as reflected in the COX2- and CYTB-to-GAPDH ratios using PCR analysis, which was blunted by resveratrol in HFSR (n=7) soleus and, to a lesser degree, in plantaris muscles. A ~1.5 and 3-fold increase in Rev-erb-α protein was detected in HFSR soleus and plantaris muscles compared to controls, respectively. Unlike in HFSR animals, HFS soleus and plantaris muscles exhibited a ~2-fold elevation in phosphor-AMPKα (Thr172). HFS soleus muscles had elevated phosphorylated-to-total TANK binding protein-1 (TBK1) ratio suggesting an enhancement in mito/autophagic events. Taken together, resveratrol appears to blunt mitochondrial losses with a high fat / sugar diet by tempering mito/autophagy rather than promoting mitochondrial biogenesis, suggesting that the quantity of daily resveratrol supplement ingested and / or its long-term consumption are important considerations.
Keywords: biogenesis, mitophagy, Rev-erb-α, TBK1
Introduction
Skeletal muscle mitochondrial size, number, and location (i.e., subsarcolemmal, intra-fibrillar, paravascular) define, in part, the characteristics of slow (aerobic) or fast (glycolytic) phenotypes through the oxidation of glucose and/or fatty acids (Chen et al., 2011; Glancy et al., 2015; Memme et al., 2019). Changes to muscle/fiber phenotype resulting from fluctuations in physical activity or nutritional balance are largely paralleled by changes in mitochondrial content (Baldwin et al., 1972), which ultimately influences total oxygen consumption (VO2) and the relative time to the onset of fatigue. Adaptation of the mitochondria within a muscle fiber, therefore, centers on the relative rate of mitochondrial biogenesis, which is coordinated through nuclear-mitochondrial crosstalk, versus their destruction (e.g., mitophagy) (Devarshi et al., 2017; Gureev et al., 2019; Memme et al., 2019; Sparks et al., 2005).
Alterations in mitochondrial volume and function are dependent on dietary and temporal variables (Rasool et al., 2018). Formulated diets exclusively high in fat have been shown to augment mitochondrial content within weeks (Garcia-Roves et al., 2007; Hancock et al., 2008; Li et al., 2018) and sustained for months (Gómez-Pérez et al., 2012; Malik et al., 2019) following dietary shifts; yet, despite this increase, skeletal muscle mitochondria exhibit signs of dysfunction (Chanseaume et al., 2006; Jana et al, 2019; Johannsen and Ravussin, 2009; Kim et al., 2000; Mitotto et al., 2018; Ritov et al., 2005; Ritov et al., 2010). Diets high in fat and sugar (e.g., sucrose), however, decrease (Bonnard et al., 2008; Wang et al., 2014) or have no impact (Malik et al., 2019) on mitochondrial content in various tissues; interestingly, this reduction may not manifest for months, or up to a year, following diet interventions. Relative to lean individuals, obese or type 2 diabetic patients also show impaired mitochondrial content in sub-sarcolemmal regions of skeletal muscle (Ritov et al., 2005). Positive or negative changes in mitochondrial content are generally paralleled by concomitant shifts in markers associated with mitochondrial biogenesis (Bonnard et al., 2008; Garcia-Roves et al., 2007; Gómez-Pérez et al., 2012; Hancock et al., 2008; Wang et al., 2014).
Resveratrol (RESV), a polyphenolic plant compound, has been employed as a long-term counter-strategy to high fat/sucrose consumption (Fiori et al., 2013; Hou et al., 2019; Hyatt et al., 2016; Jimenez-Gomez et al., 2013; Mattison et al., 2014). Although acute or single-dose RESV consumption appears to have to no impact on mitochondrial respiration (Williams et al., 2014), long-term supplementation is thought to enhance mitochondrial biogenesis through the deacetylation (e.g., activation) of peroxisome proliferator-activated receptor γ coactivator (PGC-1α) by phosphorylated Sirtuin-1 (SIRT1) (Gurd, 2011); an increase mitochondria enhances expression of oxidative phosphorylation (OXPHOS) proteins and, consequently, increases aerobic respiration (Lagouge et al, 2006). In the phenotypically fast tibialis anterior muscle, RESV restored expression of nuclear-encoded mitochondrial genes that were blunted by an 8-week high-fat diet and improved β-oxidation in subsarcolemmal mitochondria (Chen et al., 2011); however, recent work noted that RESV does not impact the signals associated with mitophagy in the livers of mice fed a diet high in fat for 8 weeks (Meza-Torres et al., 2020). Given the strong positive relationship between phenotype and mitochondrial density and quality (Adhihetty et al., 2003; Memme et al., 2019) and that a high fat/sugar diet and RESV can influence shifts in myosin heavy chain isoform expression, we hypothesized that a loss in mitochondria would parallel the slow-to-fast shift in muscle phenotype that was observed in our earlier work (Hyatt et al., 2016). Specifically, we expected a decrease in mitochondrial content in HFS muscles attributed to blunted expression of markers of biogenesis, elevated markers of mito/autophagy, or both. We hypothesized that these responses would be normalized with RESV supplementation.
Methods
The experimental procedures, including the animals, diet, and resveratrol supplementation used for this project have been detailed previously (Bernier et al., 2016; Fiori et al., 2013; Hyatt et al., 2016; Jimenez-Gomez et al., 2013; Mattison et al., 2014). This and our earlier work (Hyatt et al., 2016) were a part of a project entitled “Effects of resveratrol with a high fat and sugar diet in monkeys” which aimed to elucidate the long-term impacts of resveratrol supplementation on overall health. The hypotheses tested in the present study were generated post hoc through collaboration.
Animals, diet, and resveratrol supplementation
Twenty-four adult (7–13 years old) male rhesus monkeys (Macaca mulatta) were housed continuously at the National Institutes of Health (NIH) Animal Center (Poolesville, MD). It should be noted that at the time of project was conceptualized, a cohort of male rhesus macaque monkeys was available and an age-matched cohort of females was not. All experimental and animal care procedures were approved by the Animal Care and Use Committee of the National Institute on Aging Intramural Research Program and are detailed elsewhere (Bernier et al., 2016; Fiori et al., 2013; Jimenez-Gomez et al., 2013; Mattison et al., 2014). Monkeys were randomized into one of three groups: a high fat/sucrose diet in combination with either placebo (HFS; n = 8) or resveratrol (HFSR; n = 7) and age-matched controls (CON) on a healthy standard diet (n = 4). Additional control samples (n=5) were obtained from historic controls that were not part of this longitudinal study.
The CON animals received a standard purified monkey chow consisting of 18.2, 13.1, and 68.7% of kcal from protein, fat, carbohydrate, respectively; approximately 2.2% of this diet consisted of sucrose by weight (Diet #5038; Purina Mills, St. Louis, MO; Supplemental Table 1). During baseline periods, the HFS and HFSR animals received the control diet and were gradually moved to the HFS diet over a 3-week period; it consisted of 15.8, 42.3, 41.9% of kcals from protein, fat, and carbohydrate, respectively, and 27% sucrose by weight (Teklad #TD.07802; Harlan, Indianapolis, IN; Supplemental Table 2). Monkeys received two meals per day at estimated ad libitum levels throughout the study. This controlled feeding regimen allowed for a closer control of food consumption and prevented obesity due to overeating, thus ensuring that experimental effects were due to the treatment and not differences in food consumption. Food allotments were determined by calculating food consumption while monkeys had access to excess food. Water was available ad libitum. The average food consumption for weekly periods was the same for all three groups (Supplemental Table 3). Monkeys were monitored at least three times daily by trained animal care staff and, during baseline assessment, all monkeys were maintained on a commercially available closed formula monkey chow.
Resveratrol was supplied by DSM Nutritional Products North America (Parsippany, NJ). Dosages were determined based on earlier work denoting the protective dose (22 mg/kg) from a rodent model (Baur et al., 2006). Equivalent dosages for monkeys in the present study were calculated using an average monkey body weight of 12.1 kg. Dosages were derived by allometric scaling from rodents to monkeys and are specified in previous reports from this longitudinal study (Fiori et al., 2013; Jimenez-Gomez et al., 2013). For the first year, HFSR monkeys received a total dose of 80 mg/day (40 mg/meal); during the second year, 480 mg/day (240 mg/meal) was provided. The resveratrol was incorporated into a cherry-flavored primate treat (Bio-Serv, Frenchtown, NJ) that was given to the monkeys prior to each meal. Non-resveratrol CON and HFS animals received a cherry-flavored placebo treat (PRIMA-Treats® Bio-Serv).
Experiment termination and tissue collection
At 24 months from the start of the project, the monkeys were deeply anesthetized with a lethal dose of sodium pentobarbital (50 mg/kg, intraperitoneal). Once sedated, each monkey was perfused with cold lactated Ringer’s solution until death. The soleus (SOL) and plantaris (PLT) muscles were removed from the hindlimbs, trimmed and cleaned of excess connective tissue, weighed, and flash frozen in liquid nitrogen. Whole muscles were stored at −80° C until further analysis.
Mitochondrial DNA (mtDNA) copy number
Total DNA was extracted using a modified protocol detailed by Strauss (1998). Approximately 45–60 mg of 15 μm-thick cross-sectioned muscle samples were collected and digested at 40°C for 18 hours in buffer containing 1M NaCl, 0.1M Tris-HCl, 0.1M EDTA, 10% SDS, and fresh 0.1 mg/mL proteinase K. DNA was then extracted using standard phenol:chloroform:isoamyl alcohol methods (Strauss, 1998) and resuspended in 1X Tris-EDTA buffer (pH 8.0). Semi-quantitative end-point PCR was performed using primers generated against the rhesus macaque nuclear and mitochondrial targets including glyceraldehyde-3-phosphate dehydrogenase (GAPDH), cytochrome c oxidase subunit II (COX2) and cytochrome b (CYTB) are shown in Table 2. Samples were prepared using 2X master mix containing optimized concentrations of Taq polymerase, dNTPs, MgCl2 (Promega, Madison, WI); 100 ng of DNA per reaction per sample was used. The cycling conditions consisted of one cycle at 94°C for 5 min, 27 cycles at 94°C for 30 sec, 27 cycles at 58°C for 45 sec, 27 cycles at 72°C for 45 sec, and a final extension at 72°C for 5 min (Benchmark, Edison, NJ). Amplicons were separated in a 0.8% agarose gel containing 1X GelGreen (Biotium, Freemont, CA) at 45V for 60 min, photographed and quantified using densitometry. The mitochondrial-to-nuclear gene ratio was then determined and statistically compared.
Table 2.
Primer sequences used for PCR analysis
| Target |
Primer sequences (5’→ 3’) |
Size (bp) |
|
|---|---|---|---|
| COX 2 | CCTACAAGACGCCACATCCC ATTTAGACGCCCAGGTACGG |
forward reverse |
517 |
| Cytochrome B | TTTCCTACACATCGGTCGGG AGTAGGTGCACGGTTGTGAG |
forward reverse |
312 |
| GAPDH | TCAATGGAAGCCCCATCACC CATTCCCCAGCTCTCATACCA |
forward reverse |
638 |
Total protein isolation
To ensure homogenous total protein isolation, SOL and PLT muscle sample chunks from the approximate muscle belly were mounted on cork and cut cross-sectionally using a cryostat (CM1950; Leica, Buffalo Grove, IL). Approximately 30–80 15 μm-thick cross sections were acquired, which equated to ~50–100 mg sampled from each muscle. Samples were then bead homogenized for 2.5 min at 3,000 rpm in 10-volume ice-cold buffer (pH 7.8) containing 50 mM Tris-HCl, 2 mM EDTA, 2 mM EGTA, 10% glycerol, 1% Triton-X, and a 1% v/v final concentration of a protease and phosphatase inhibitor cocktail (PPC1010; Sigma, St. Louis, MO) containing 4-(2-Aminoethyl)benzenesulfonyl fluoride hydrochloride, aprotinin, bestatin hydrochloride, N-(trans-Epoxysuccinyl)-L-leucine 4-guanidinobutylamide, leupeptin, pepstatin A, cantharidin, (−)-p-Bromolevamisole oxalate, and calyculin A. After homogenization, the samples were centrifuged at 12,000g for 10 min at 4°C, and the supernatant was transferred to clean tubes in a biological safety cabinet, and then frozen at −80°C.
Western analysis
Loading of total protein for immunoblotting ranged between 10–50 μg depending on the target (Table 1). Total protein was separated using either 10, 12, or 15% acrylamide tris-glycine gels. Samples were mixed in 2X sample buffer (0.2% SDS, 20% glycerol, 25% 4X buffer, 5% β-mercaptoethanol, and 0.025% bromophenol blue) and heated to either 42.5°C when probing membranes with OXPHOS antibodies or 100°C for 3 min and electrophoresed at 80 V for 20 min and then 140 V for 60–70 min. The proteins were transferred to PVDF membranes for 3 h at 60V and the membranes were placed in a solution of Ponceau S (Sigma) to verify that the transfer was uniform and artifact-free. Ponceau-stained membranes were partially de-stained in ddH2O and then digitally scanned for sample loading control used in subsequent analyses and quantification. The membranes were then placed in 5% nonfat dry milk (NFM) dissolved in tris buffered saline with 0.05% Tween-20 (T-TBS) for a minimum of 0.5 hr. The membranes were incubated in primary antibody diluted in NFM for 1 hr at room temperature or overnight at 4°C (Table 1). A custom anti-rat rabbit polyclonal antibody was developed (Abcam Inc., Cambridge, MA) against the small peptide, mitochondrial ORF of the 12S rRNA type-c (MOTS-c) (Lee et al., 2015). To confirm specificity, ~1 μg of antibody was spiked with 3 μg of peptide (GenScript, Piscataway NJ) for 1 hr at room temperature prior to incubation with a membrane containing samples from each group. Skeletal muscle MOTS-c was determined to be at ~14kDa, which is in agreement with others (T. Merry, University of Auckland, personal communication). This antibody also has demonstrated cross-reactivity against rat and mouse skeletal and cardiac muscle (data not shown). In addition, the antibody used against mitochondrial transcription factor A (Tfam) detected both pre-Tfam and the mature cleaved Tfam isoforms, which was confirmed based on their presence in cytosolic and mitochondrial locations, respectively (data not shown; Tryon et al., 2015). After primary antibody incubation, the membranes were washed 6 × 10 min in T-TBS and incubated for 1 h in a secondary antibody cocktail (Table 1) for 1 hr at room temperature. The membranes were developed using an ECL detection kit (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) per the manufacturer’s instructions. Densitometry and quantification were performed using ImageJ software (Rasband, 2020).
Table 1.
Antibodies and conditions.
| Targets | SDS-PAGE (μg / sample) | Vendor Details | Conditions |
|
|---|---|---|---|---|
| 1° Antibody | 2° Antibody* | |||
|
|
|
|
|
|
| Rev-Erb-la | 25 | Abeam; Cat#: ab 174309; Species: rabbit | 1:1,000; overnight @4°C+ 1 hratRT | 1:10,000; lhr@ RT |
| phospho AMPKa (Thrl72) | 30 | Cell Signaling; Cat#: 2535; Species: rabbit | 1:1,000; overnight @ 4°C |
1:2,000; lhr@RT |
| total AMPKa | 30 | Cell Signaling; Cat#: 2532; Species: rabbit | 1:1,000; overnight @4°C | 1:2,000; lhr@RT |
| PGC-la | 25 | Abeam; Cat#: ab54481; Species: rabbit | 1:2,000; 1 hr@RT | 1:2,000; lhr@RT |
| phospho SIRT1 (Ser47) | 50 | Cell Signaling; Cat#: 2314; Species: rabbit | 1:1,000; overnight @4°C | 1:2,000; lhr@RT |
| S1RT1 | 30 | Abeam; Cat#: abl 10304; Species: mouse | 1:1,000; overnight @4°C | 1:4,000; lhr@RT |
| NRF1 | 25 | Abeam; Cat#: abl75932; Species: rabbit | 1:1,750; 1 hr@RT | 1:4,000; lhr@RT |
| NRF2 | 25 | Abeam; Cat#: ab31163; Species: rabbit | 1:1,000; 1 hr@RT | 1:5,000; lhr@RT |
| Tfam & pre-Tfam | 25 | Abeam; Cat#: abl31607; Species: rabbit | 1:1,000; 1 hr@RT | 1:4,000; lhr@RT |
| OXPHOS | ||||
| Complex V - ATP5A Complex IV - MTC01 Complex III - U0CRC2 Complex II - SDHB Complex I - NDUFB8 |
10 | Abeam; Cat#: abl 10413; Species: mouse | 1:1,000; 1 hr@RT | 1:10,000; lhr@ RT |
| MOTS-c | 10 | Abeam; Custom**; Species: rabbit | 1:10,000; 1 hr@ RT | 1:5,000; lhr@RT |
| Humanin | 50 | Sigma; Cat#: H2414; Species: rabbit | 1:1,200; 1.5 hr @ RT | 1:1,500; lhr@RT |
| Mfn2 | 10 | Abeam; Cat#: abl24773; Species: rabbit | 1:1,000; overnight @4°C | 1:2,000; lhr@RT |
| FIS-1 | 15 | Abeam; Cat#: ab96764; Species: rabbit | 1:1,000; overnight @4°C | 1:2,000; lhr@RT |
| DRP-1 | 7.5 | Abeam; Cat#: ab56788; Species: mouse | 1:2,500; overnight @4°C | 1:4,000; lhr@RT |
| p62 | 10 | Abeam; Cat#: ab56416; Species: rabbit | 1:1,500; 1 hr@RT | 1:4,000; lhr@RT |
| PINKl | 50 | Abeam; Cat#: ab23707; Species: rabbit | 1:750; overnight @ 4°C | 1:2,000; lhr@RT |
| Parkin | 10 | Abeam; Cat#: ab77924; Species: mouse | 1:1,000; overnight @4°C | 1:3,000; lhr@RT |
| phospho TBK1 (Scrl72) | 30 | Cell Signaling; Cat#: 5483; Species: rabbit | 1:1,000; 1 hr@RT | 1:2,000; lhr@RT |
| total TBK1 | 20 | Cell Signaling; Cat#: 3504; Species: rabbit | 1:1,000; 1 hr @ RT | 1:2,000; lhr@RT |
| LC3 I/II | 50 | Abeam; Cat#: abl28025; Species: rabbit | 1:2,000; 1 hr@RT | 1:5,000; lhr@RT |
RT: room temperature
Seconday antibodies: goat anti-mouse IgG-HRP; goat anti-rabbit IgG-HRP
Polyclonal anti-rat MOTS-c antibody raised against peptide sequence: MKRKEMGYIFF (Lee et al., 2015)
Statistical Analyses
Values are presented as means ± SE. Animal age, body mass, and muscle masses were reported in our earlier work (Hyatt et al., 2016), which detected a significant difference in age for the control animals, termed CON-Y (n=5) and CON-O (n=4) for “young” and “old,” respectively. For all variables in the present study, we performed age comparisons between the CON-Y and CON-O animals using an unpaired, two-tailed t-test with equal variance and Mann Whitney tests to determine the presence of age effects. No differences were detected in expression levels for all analyses between CON-Y and CON-O animals; as such, the values between these age cohorts were combined into a single CON group. For group comparisons between CON, HFS, and HFSR, a one-way analysis of variance (ANOVA) determined overall differences between groups for all variables and a Tukey’s two-tailed test was used for post hoc comparisons. For all statistical analyses, SPSS (v24) was used and significance was set at p < 0.05.
Results
Mitochondrial content.
To evaluate the impact of long-term HFS and HFSR treatment on mitochondrial content, the mtDNA-to-nuclear DNA copy number was evaluated using PCR. Mitochondrial content was significantly lower in HFS soleus and plantaris muscles compared to CON (Fig. 1). In the soleus muscle, a significant difference in the CYTB:GAPDH ratio was detected between HFS and HFSR groups and a non-significant trend was observed for the COX2:GAPDH ratio between these groups (p = 0.1; Fig. 1). Compared to CON, COX2:GAPDH ratio was statistically lower in HFSR plantaris muscles, whereas both mtDNA-to-nuclear ratios trended higher in HFSR than in HFS (Fig. 1).
Figure 1.

Mitochondrial DNA (mtDNA) copy number in rhesus macaque soleus (SOL) and plantaris (PLT) muscles. Values are shown as means (± S.E.M) relative to control (CON) conditions. HFS: high fat/sucrose diet; HFSR: high fat/sucrose diet with resveratrol supplementation; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; COX2: cytochrome c oxidase subunit II; CYTB: cytochrome b. *: significantly different from CON (p < 0.05); †: significantly different from HFS (p < 0.05).
Upstream Signals.
Rev-Erb-α and AMPK are divergent signals that modulate numerous cellular actions, including mitochondrial biogenesis and autophagy. A general expression pattern emerged in HFS and HFSR soleus and plantaris muscles (Fig. 2). Rev-Erb-α protein was elevated ~3-fold in HFSR plantaris muscles compared to CON and trended higher when compared HFS expression (p = 0.08). Rev-Erb-α protein expression trended higher in HFS than in CON plantaris muscles (p = 0.09). In the soleus muscle, Rev-Erb-α protein expression trended greater in HFSR compared to control animals (p = 0.08). Compared to CON, phospho-AMPK (Thr172) was elevated >2-fold in both HFS soleus and plantaris muscles; conversely, expression in HFSR muscles was near CON levels and significantly different when compared to the HFS group. The phosphor-AMPK-to-total AMPK ratio was significantly elevated from CON and HFSR groups in both soleus and plantaris muscles.
Figure 2.
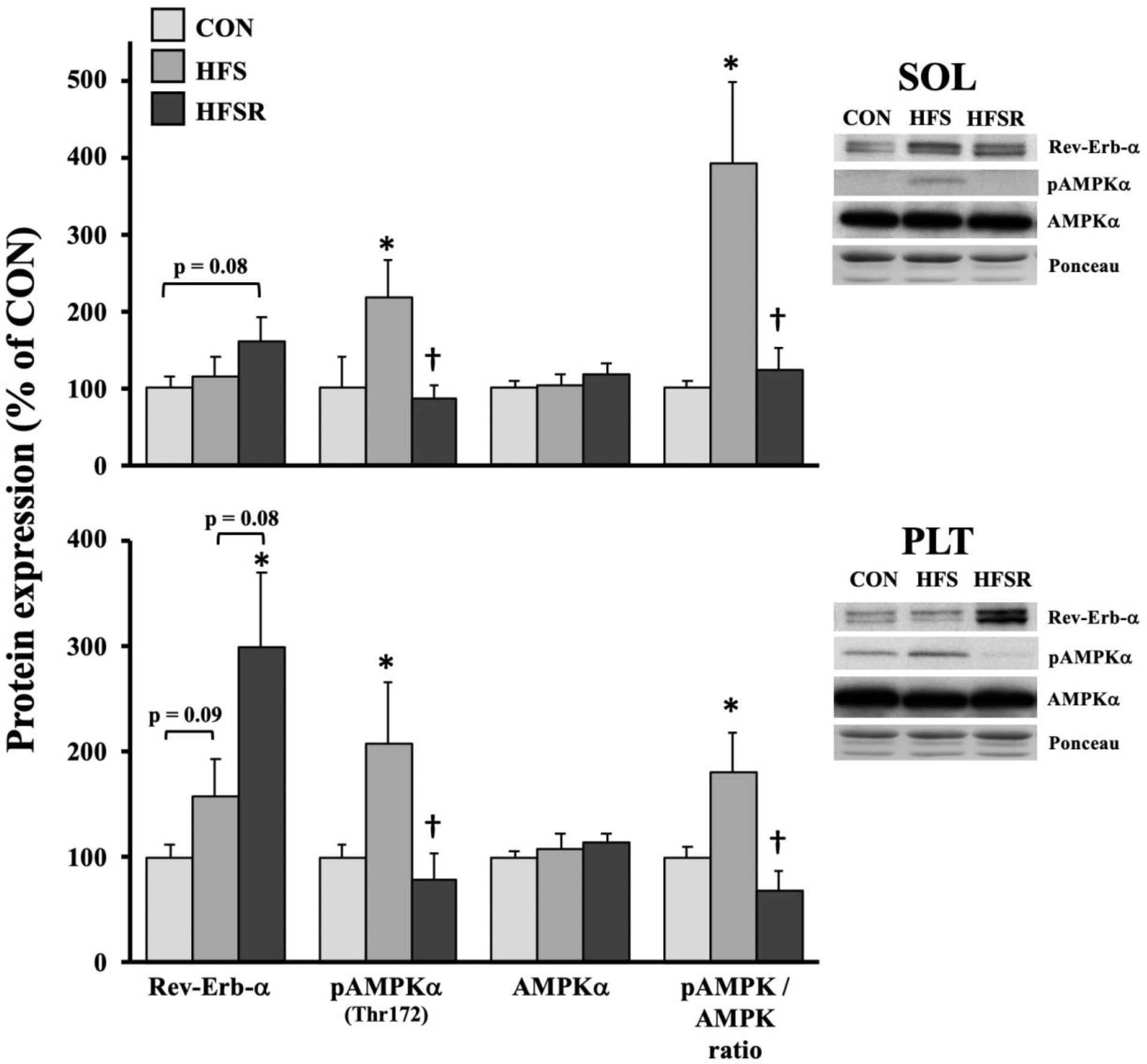
Expression of upstream protein markers, Rev-erb-α and AMP kinase-α (AMPKα) in rhesus macaque soleus (SOL) and plantaris (PLT) muscles. Expression of total and phosphorylated (pAMPKα) AMPKα are shown and presented as a ratio. Representative blots for each marker are shown (right). Values are shown as means (± S.E.M) relative to control (CON) conditions. HFS: high fat/sucrose diet; HFSR: high fat/sucrose diet with resveratrol supplementation. *: significantly different from CON (p < 0.05); †: significantly different from HFS (p < 0.05)
Regulators of mitochondrial biogenesis.
There was a differential response for proteins associated with mitochondrial biogenesis in the soleus and plantaris muscles (Fig. 3). In the soleus muscle, no group differences were detected for any markers. Nuclear respiratory factors (NRF)-1 NRF-1 protein expression was ~25% lower in HFS and HFSR plantaris when compared to CON; NRF-2 protein was lower in HFS vs. HFSR plantaris muscles (Fig. 3). There was lower expression of the cytosolic protein pre-Tfam in HFS animals compared to CON and HFSR groups (Fig. 3); the mature/cleaved Tfam protein, located in the mitochondria, trended lower in HFS versus CON.
Figure 3.
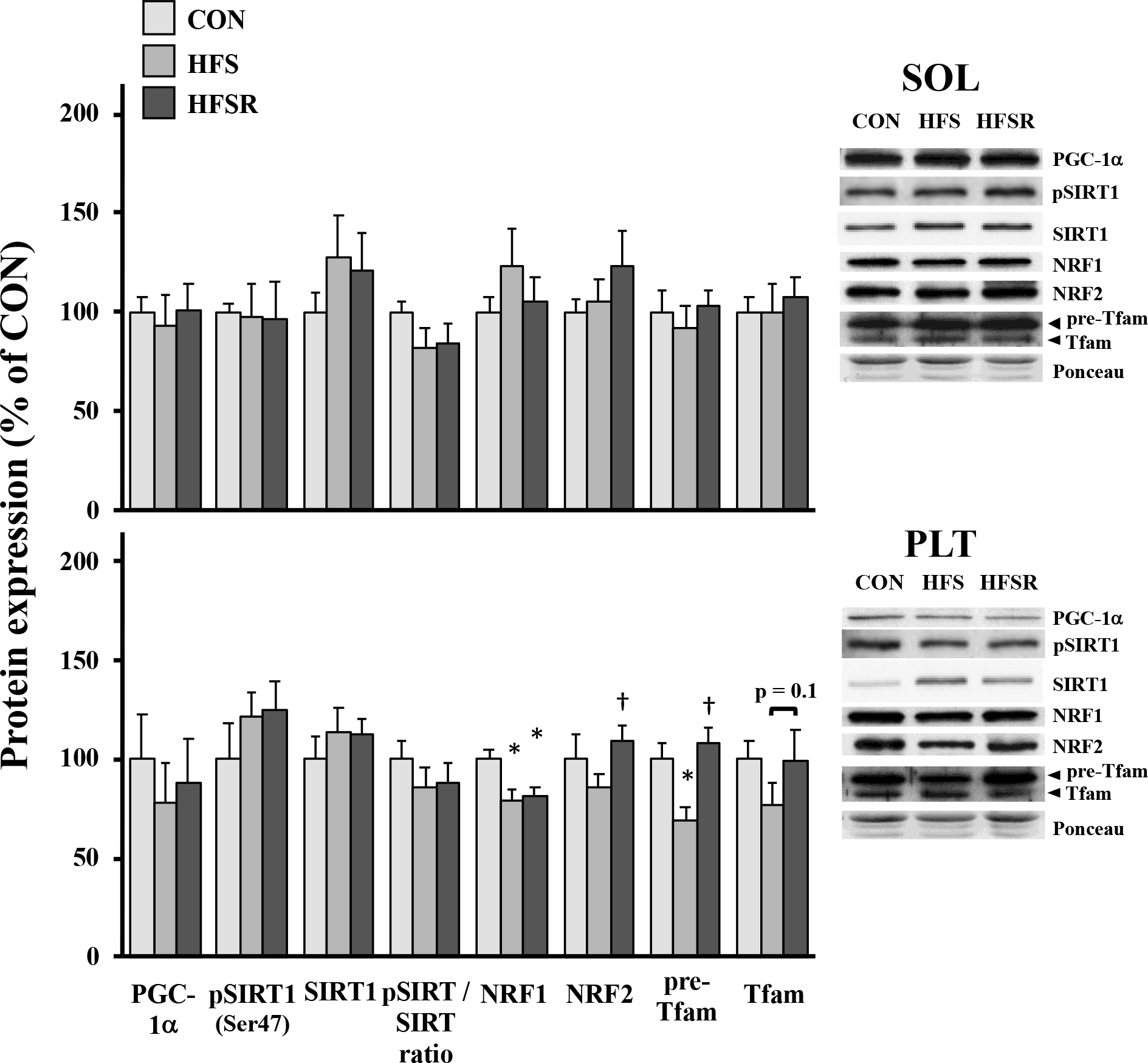
Expression of protein markers of mitochondrial biogenesis in rhesus macaque soleus (SOL) and plantaris (PLT) muscles. Expression of total and phosphorylated Sirtuin-I (pSIRT) are shown and presented as a ratio. Representative blots for each marker are shown (right). Values are shown as means (± S.E.M) relative to control (CON) conditions. HFS: high fat/sucrose diet; HFSR: high fat/sucrose diet with resveratrol supplementation;*: significantly different from CON (p < 0.05); †: significantly different from HFS (p < 0.05)
Mitochondrial protein markers.
Mitochondrial OXPHOS complexes are aggregated protein assemblies expressed primarily from the nuclear genome and secondarily from the mitochondrial DNA. Given that proteins associated with mitochondrial biogenesis can promote the expression nuclear and mitochondrial genes and that changes in mtDNA expression may signal dysfunction (Malik and Czajka, 2013), we evaluated the expression of OXPHOS-related proteins from nuclear- and mitochondrial-encoded genes as well as two mitochondria-derived peptides, MOTS-c and humanin, that purportedly have local-acting and systemic impacts on glucose and metabolism (Gidlund et al., 2016; Gong et al., 2015; Kim et al., 2017; Lee et al., 2015; Lee et al., 2016; Muzumdar et al., 2009; Reynolds et al., 2021). The generation of a novel rabbit anti-rat polyclonal antibody showed specificity to MOTS-c in protein isolate from monkey skeletal muscle (Fig. 4). In HFSR soleus muscles, nuclear-encoded OXPHOS proteins UQCRC2 (Complex III), SDHB (Complex II), and NDUFB8 (Complex I) were generally higher than in CON and HFS animals (Fig. 4). In the plantaris muscle, no group differences were detected in OXPHOS proteins; humanin expression was ~25% lower in HFS than in CON and trended lower compared to HFSR plantaris muscles (Fig. 4).
Figure 4.

Expression of nuclear-encoded (nDNA) and mitochondrial-encoded (mtDNA) proteins associated with complexes of oxidative phosphorylation (OXPHOS) and metabolism in rhesus macaque soleus (SOL) and plantaris (PLT) muscles. Top: Specificity of a custom rabbit anti-rat MOTS-c polyclonal antibody was pre-tested in rhesus macaque skeletal muscle following 1 hr pre-incubation with a MOTS-c peptide; detection of MOTS-c occurred at 14 kDa. Representative blots for each marker are shown (right). Values are shown as means (± S.E.M) relative to control (CON) conditions. HFS: high fat/sucrose diet; HFSR: high fat/sucrose diet with resveratrol supplementation. *: significantly different from CON (p < 0.05); †: significantly different from HFSR (p < 0.05).
Regulators of mitochondrial fission and fusion.
Mitochondrial size and health are, in part, coordinated by proteins associated with the expansion or reduction of this organelle. Mitofusion-2 (Mfn2), which modulates fusion events, was elevated ~50% above CON values in HFS and HFSR soleus muscles (Fig. 5). Conversely, mitochondrial fission 1 protein (FIS-1) trended lower in HFS than CON soleus muscles. In the plantaris, FIS-1 expression was significantly lower in HFS and HFSR than in CON samples. No differences in expression were detected for dynamin-1-like protein (DRP-1).
Figure 5.
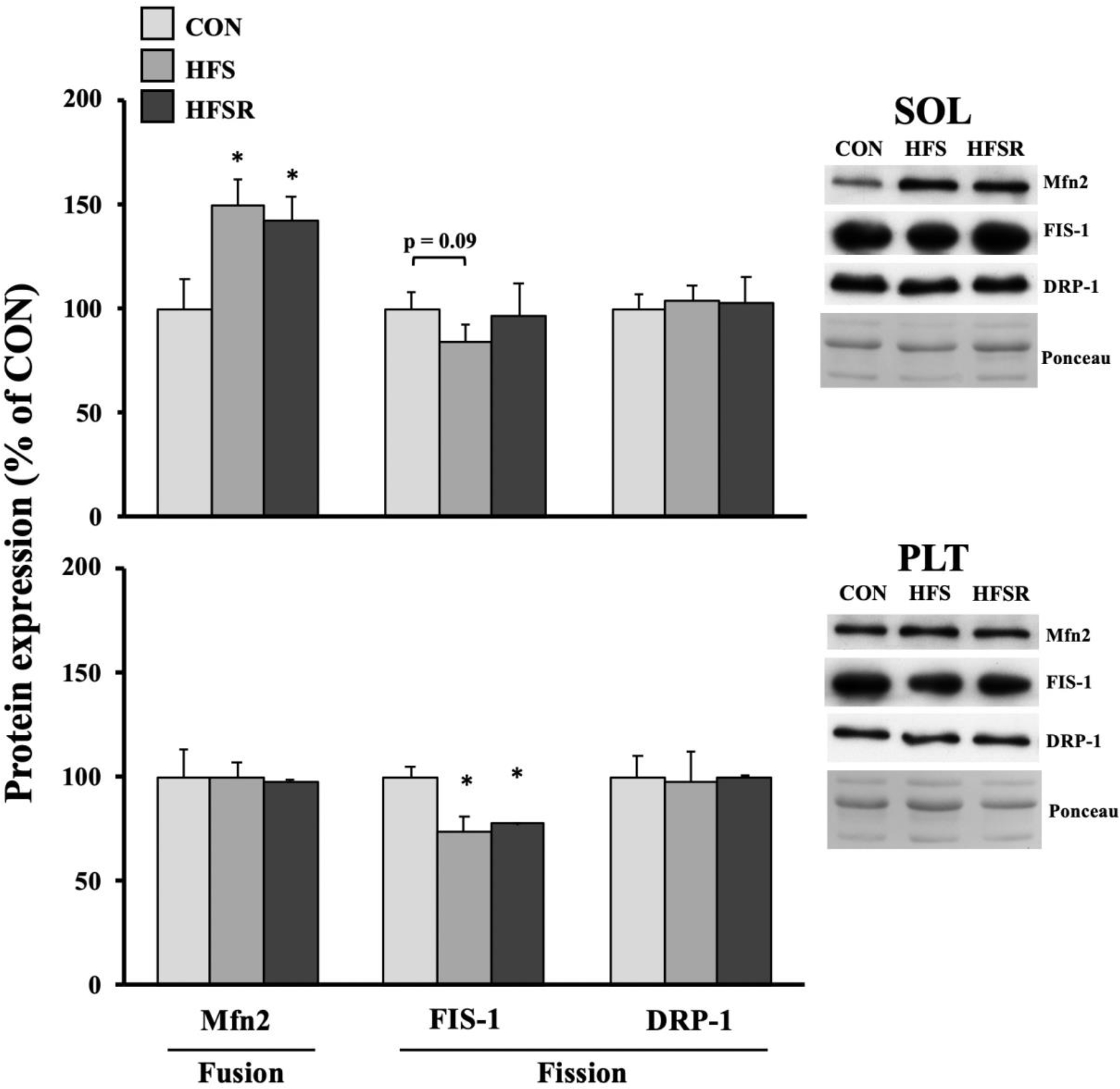
Expression of mitochondrial fusion and fission protein markers in rhesus macaque soleus (SOL) and plantaris (PLT) muscles. Representative blots for each marker are shown (right). Values are shown as means (± S.E.M) relative to control (CON) conditions. HFS: high fat/sucrose diet; HFSR: high fat/sucrose diet with resveratrol supplementation. *: significantly different from CON (p < 0.05)
Regulators of mito/autophagy.
The events encompassing mitophagy / autophagy are orchestrated by a host of proteins and protein complexes that ultimately sequester and destroy damaged mitochondria (Fig. 6). Given the relationship between a high fat/sugar diet and changes in mitochondrial morphology and function (Chanseaume et al., 2006; Chen et al., 2018; Mitotto et al., 2018; Ritov et al., 2005; Ritov et al., 2010), we assembled a general profile of the markers associated with this process in HFS muscles and ascertained whether resveratrol treatment impacted expression of these mito/autophagy-associated proteins. p62 expression in both HFS and HFSR soleus muscles was significantly elevated ~25% above CON and no group differences were observed in plantaris muscles (Fig. 6). PTEN-induced kinase 1 (PINK1) expression was unchanged in soleus muscles, whereas a significant decrease of ~50 and 25% was detected in HFS and HFSR plantaris muscles, respectively. Parkin expression significantly increased in HFSR soleus muscles compared to CON. Phosphorylated and total TANK binding protein-1 (TBK1) significantly increased in HFS and HFSR soleus muscles; however, the phospho-TBK1-to-total TBK1 ratio was elevated in HFS, but not HFSR soleus muscles. In plantaris muscles, phosphor-TBK1 and total TBK1 protein expression did not significantly differ from CON, although a higher expression of total TBK1 was found in HFSR compared to HFS plantaris muscles. Finally, the microtubule-associated protein 1 light chain 3 (LC3) II:I ratio was significantly lower in HFS soleus compared to CON, and trended lower in the HFS plantaris muscle relative to CON expression values.
Figure 6.

Expression of protein markers associated with mitophagy / autophagy in rhesus macaque soleus (SOL) and plantaris (PLT) muscles. Expression of total and phosphorylated (pTBK1) TBK1 are shown and presented as a ratio. Representative blots for each marker are shown (right). Values are shown as means (± S.E.M) relative to control (CON) conditions. HFS: high fat/sucrose diet; HFSR: high fat/sucrose diet with resveratrol supplementation. *: significantly different from CON (p < 0.05); †: significantly different from HFS (p < 0.05)
Discussion
The goal of this study was to determine the impact of a long-term (2 year) high fat/sucrose diet on mitochondrial content in skeletal muscle and the expression of proteins that regulate mitochondrial dynamics. Given that a HFS diet and RESV can influence shifts in myosin heavy chain isoform expression (Hyatt et al., 2016) and that there is a close relationship between muscle phenotype and mitochondrial volume, we expected that mitochondrial dynamics in slow (soleus) and mixed (plantaris) muscles would parallel the phenotypic shifts induced by HFS and/or RESV treatments. Generally, we found that the HFS diet decreased mitochondrial content ~20–25% and that RESV, when consumed with this diet, ameliorated mitochondrial loss in soleus and, to a lesser degree, in plantaris muscles. The reduction in mitochondria content within HFS soleus muscles is consistent with the slow-to-fast phenotypic shift shown earlier in this muscle (Hyatt et al., 2016) and appears to be the result a greater occurrence of mito/autophagy possibly through an AMPK-TBK-mediated pathway (Mihaylova and Shaw, 2011; Seabright and Lai, 2020; Zhao et al., 2018). Our findings support the notion that RESV administration does not generally enhance mitochondrial biogenesis (Higashida et al., 2013), but influences mitochondrial losses in HFSR muscle by tempering mito/autophagy (Chang et al., 2018). Rev-erb-α influence on mito/autophagy has been shown previously in muscle (Mayeuf-Louchart et al., 2017; Woldt et al., 2013) and non-muscle cell lines (Sulli et al., 2018). The increase in Rev-erb-α expression in RESV muscle suggests that its actions may be associated with blunting mito/autophagic pathways to preserve mitochondrial volume. In concert with our previous study (Hyatt et al., 2016), the soleus and plantaris muscles are responsive to RESV treatment when supplemented with a HFS diet, although the magnitude of the response as reflected in expression profiles of the markers throughout the mitochondrial life cycle is group- and muscle-specific.
Diet composition (high fat-only versus high fat/sucrose) and the duration of consumption are important considerations when assessing changes in skeletal muscle mitochondria (Bonnard et al., 2008; Malik et al., 2019; Wang et al., 2014). The presence of sucrose in a high-fat diet elicits a different mitochondrial response than a diet high in fat alone. The mechanism attributed to this difference is unknown, but not without controversy given that the type and amount of sugar consumed may or may not contribute to the development of insulin resistance, type 2 diabetes, and/or cardiovascular disease (Stanhope, 2016). For example, mitochondrial dysfunction could be the result of morphological, functional deficiencies, or both. Ylikallio et al. (2010) observed that skeletal muscle with abnormally high mtDNA in bitransgenic mice (overexpressing Twinkle and Tfam) exhibited nucleoid enlargement, impaired mtDNA transcription, accumulation of mtDNA deletions, and deficiencies in the electron transport chain. Others have reported dysfunction resulting from a reduction in the capacity to catabolize lipids (Hulver et al., 2003; Kelley et al., 2002; Kim et al., 2000; Simoneau and Kelley, 1997). Chanseaume et al. (2006), who observed lower mitochondrial respiration and ATP production in soleus muscles of rats fed either a high fat or high sucrose diet, maintains that excess energy, irrespective of the dietary source, leads to impairment of skeletal muscle mitochondria. Although we found that RESV ameliorated losses in mitochondrial content, it is unclear whether this was accompanied by a functional restoration and requires additional investigation. At a minimum, a HFS-induced mitochondrial loss in muscles that are important for posture and locomotion would ultimately impair whole-body aerobic capacity and lower the time to fatigue, which are possibly important practical considerations for individuals consuming such a diet.
RESV treatment upregulates Rev-erb-α protein suggesting a new RESV-mediated action on mitochondrial dynamics (Woldt et al., 2013). Our data also suggest that Rev-erb-α is associated more with a faster than a slower muscle phenotype based on the expression profiles revealed in the plantaris and soleus muscles, respectively. Rev-erb-α is closely linked with the circadian clock and has recently been shown to participate in myogenesis (Chatterjee et al, 2019), maintenance of adult muscle mass by inhibiting autophagy (Mayeuf-Louchart et al., 2017; Woldt et al., 2013), and impact mitochondrial health in skeletal muscle through the Lkb1-AMPK-Sirt1-PGC-1α pathway (Woldt et al., 2013). AMPK is well known for its role in energy sensing and activation of glucose/fatty acid metabolism; recently, phosphorylated AMPK has been shown to activate TBK byway of unc-51 like autophagy activating kinase 1 (ULK1) in adipose tissue (Zhao et al., 2018). The group-specific expression pattern of phospho-AMPK and -TBK1 in the present study, particularly in HFS muscles, lend credence to this pathway within skeletal muscle tissue (Seabright and Lai, 2020). Furthermore, our findings support those of Wang et al. (2018) who showed that RESV supplementation blunted the mito/autophagic markers in quadriceps muscles of diabetic mice. Taken together, the observed differences in mitochondrial content between HFS and HFSR muscles appears to be the manifestation of two pathways at play: a AMPK/TBK-mediated augmentation of mito/autophagy in HFS muscles and a Rev-erb-α-mediated inhibition of mito/autophagy in HFSR muscles. It is clear, however, that RESV supplementation cannot completely counter the effects of a long-term HFS diet: in the mixed plantaris muscle, mitochondrial content was not restored to control levels as was observed in the slow soleus muscle.
Stephenson et al. (2012) reported that, unlike in the soleus muscle, the fast extensor digitorum longus muscle exhibited a slight (13–18%), but significant, increase in OXPHOS proteins following a 12-week high fat/sucrose (e.g., Western) diet. Here, no significant increases were observed in HFS muscles, although RESV treatment elicited a modest increase in proteins of complexes I, II, and III in HFSR soleus, but not plantaris, muscles. It is possible that without the additive stimulus of, for example, aerobic exercise, RESV supplementation has minimal impact on OXPHOS protein expression (Cheng et al., 2020; Higashida et al, 2013) and respiration (Beijers et al., 2020; Feillet-Coudray et al., 2009) particularly in a mixed muscle phenotype. Given the emerging role of humanin and MOTS-c in modulating glucose and/or lipid metabolism (Lee et al., 2015; Lee et al., 2016; Muzumdar et al., 2009; Reynolds et al., 2021), we expected expression to increase within HFS and HFSR muscles, but detected no changes in HFS or HFSR groups. It is possible that the expression of these peptides are responsive to more acute perturbations in diet and/or muscle activity than lengthy interventions used in the present study (Reynolds et al., 2021). Likewise, proteins associated with fusion/fission events of the mitochondria were not impacted in the presence of RESV treatment, although we cannot discount the occurrence of molecular habituation to chronic exposure to diet and RESV on gene expression or protein translation, which may differ from acute or short-term consumption (Martinez et al., 2018).
Limitations
The sample size in each group was small, which was partially due to the limitations of resources related to working with nonhuman primates as well as the length of the study. In addition, the present study and analyses was conceptualized post-hoc through collaborative efforts which did not allow for the option of isolating viable mitochondria for direct measures of, for example, functionality after a long-term high-fat diet. Furthermore, we assessed the expression of the markers / regulators of mitochondrial content or pathways, but we did not directly assess mitochondrial content / volume through, for example, electron microscopy. Lastly, the inclusion of a key group (normal diet with RESV) could help generalize our findings to a wider populous.
In conclusion, the slow-to-fast phenotypic change observed within the soleus muscle following a long-term high fat/sugar diet (Hyatt et al., 2016) is accompanied by a 20–25% reduction in mitochondrial content that was blunted by resveratrol supplementation. Similar mitochondrial losses in the mixed plantaris muscles were observed although RESV could not completely prevent a depletion in mitochondria to the level observed in soleus muscles. It is clear that the presence of sucrose and duration of high fat/sugar feeding (2 years) have deleterious impacts the mitochondria content and RESV is a viable counter-strategy to a long-term unhealthy diet.
Supplementary Material
Acknowledgements:
The authors would like to thank Mr. Beau Pullman for technical expertise and insightful discussions. This study was supported in part by the Intramural Research Program, National Institutes on Aging, NIH.
Footnotes
Conflict of Interest:
None
References
- Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol 88(1): 99–107, 2003. doi: 10.1113/eph8802505 [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Klinkerfuss GH, Terjung RL, Mole PA & Holloszy JO. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol 222: 373–378,1972. doi: 10.1152/ajplegacy.1972.222.2.373 [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444(7117):337–42, 2006. doi: 10.1038/nature05354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijers RJ, Gosker HR, Sanders KJ, de Theije C, Kelders M, Clarke G, Cryan JF, van den Borst B, Schols AM. Resveratrol and metabolic health in COPD: A proof-of-concept randomized controlled trial. Clin Nutr 39(10): 2989–2997, 2020. doi: 10.1016/j.clnu.2020.01.002 [DOI] [PubMed] [Google Scholar]
- Bernier M, Wahl D, Ali A, Allard J, Faulkner S, Wnorowski A, Sanghvi M, Moaddel R, Alfaras I, Mattison JA, Tarantini S, Tucsek Z, Ungvari Z, Csiszar A, Pearson KJ, de Cabo R. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging 8(5): 899–916, 2016. doi: 10.18632/aging.100942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118(2): 789–800, 2008. doi: 10.1172/JCI32601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Liu HW, Chen YT, Chen YA, Chen YJ, Chang SJ. Resveratrol protects muscle cells against palmitate-induced cellular senescence and insulin resistance through ameliorating autophagic flux. J Food Drug Anal 26(3): 1066–1074, 2018. doi: 10.1016/j.jfda.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanseaume E, Malpuech-Brugère C, Patrac V, Bielicki G, Rousset P, Couturier K, Salles J, Renou JP, Boirie Y, Morio B. Diets high in sugar, fat, and energy induce muscle type-specific adaptations in mitochondrial functions in rats. J Nutr 136(8): 2194–200, 2006. doi: 10.1093/jn/136.8.2194 [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Yin H, Li W, Lee J, Yechoor VK, Ma K. The Nuclear Receptor and Clock Repressor Rev-erbα Suppresses Myogenesis. Sci Rep 9(1): 4585, 2019. doi: 10.1038/s41598-019-41059-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Zhang HH, Zheng J, et al. Resveratrol attenuates high-fat diet-induced insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial β-oxidation. Metabolism 60(11): 1598–1609, 2011. doi: 10.1016/j.metabol.2011.04.002 [DOI] [PubMed] [Google Scholar]
- Devarshi PP, McNabney SM, Henagan TM. Skeletal Muscle Nucleo-Mitochondrial Crosstalk in Obesity and Type 2 Diabetes. Int J Mol Sci 18(4): 831, 2017. doi: 10.3390/ijms18040831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feillet-Coudray C, Sutra T, Fouret G, Ramos J, Wrutniak-Cabello C, Cabello G, Cristol JP, Coudray C. Oxidative stress in rats fed a high-fat high-sucrose diet and preventive effect of polyphenols: Involvement of mitochondrial and NAD(P)H oxidase systems. Free Radic Biol Med 46(5): 624–32, 2009. doi: 10.1016/j.freeradbiomed.2008.11.020 [DOI] [PubMed] [Google Scholar]
- Fiori JL, Shin YK, Kim W, Krzysik-Walker SM, González-Mariscal I, Carlson OD, Sanghvi M, Moaddel R, Farhang K, Gadkaree SK, Doyle ME, Pearson KJ, Mattison JA, de Cabo R, Egan JM. Resveratrol prevents β-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes 62(10): 3500–13, 2013. doi: 10.2337/db13-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Roves P, Huss JM, Han DH, Hancock CR, Iglesias-Gutierrez E, Chen M, Holloszy JO. Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci USA 04(25): 10709–13, 2007. doi: 10.1073/pnas.0704024104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidlund EK, von Walden F, Venojärvi M, Risérus U, Heinonen OJ, Norrbom J, Sundberg CJ. Humanin skeletal muscle protein levels increase after resistance training in men with impaired glucose metabolism. Physiol Rep 4(23): e13063, 2016. doi: 10.14814/phy2.13063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, Subramaniam S, Balaban RS. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523(7562): 617–20, 2015. doi: 10.1038/nature14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Pérez Y, Capllonch-Amer G, Gianotti M, Lladó I, Proenza AM. Long-term high-fat-diet feeding induces skeletal muscle mitochondrial biogenesis in rats in a sex-dependent and muscle-type specific manner. Nutr Metab 9: 15, 2012. doi: 10.1186/1743-7075-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Su K, Cui L, Tas E, Zhang T, Dong HH, Yakar S, Muzumdar RH. Central effects of humanin on hepatic triglyceride secretion. Am J Physiol Endocrinol Metab 309(3): E283–92, 2015. doi: 10.1152/ajpendo.00043.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureev AP, Shaforostova EA, Popov VN. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front Genet 10: 435, 2019. doi: 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd BJ. Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab 36(5): 589–97, 2011. doi: 10.1139/h11-070 [DOI] [PubMed] [Google Scholar]
- Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105(22): 7815–20, 2008. doi: 10.1073/pnas.0802057105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: a reevaluation. PLoS Biol 11(7): e1001603, 2013. doi: 10.1371/journal.pbio.1001603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou CY, Tain YL, Yu HR, Huang LT. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int J Mol Sc. 20(3): 535, 2019. doi: 10.3390/ijms20030535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ, MacDonald KG, Cline GW, Shulman GI, Dohm GL, Houmard JA. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab 284(4): E741–7, 2003. doi: 10.1152/ajpendo.00514.2002 [DOI] [PubMed] [Google Scholar]
- Hyatt JPK, Nguyen L, Hall AE, Huber AM, Kocan JC, Mattison JA, de Cabo R, LaRocque JR, Talmadge RJ. Muscle-specific myosin heavy chain shifts in response to a long-term high fat / high sugar diet and resveratrol treatment in nonhuman primates. Front Physiol 7: 77, 2016. doi: 10.3389/fphys.2016.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana BA, Chintamaneni PK, Krishnamurthy PT, Wadhwani A, Mohankumar SK. Cytosolic lipid excess-induced mitochondrial dysfunction is the cause or effect of high fat diet-induced skeletal muscle insulin resistance: a molecular insight. Mol Biol Rep 46(1): 957–963, 2019. doi: 10.1007/s11033-018-4551-7 [DOI] [PubMed] [Google Scholar]
- Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, Ward TM, Younts CM, Lewis K, Allard JS, Longo DL, Belman JP, Malagon MM, Navas P, Sanghvi M, Moaddel R, Tilmont EM, Herbert RL, Morrell CH, Egan JM, Baur JA, Ferrucci L, Bogan JS, Bernier M, de Cabo R. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab 18(4): 533–45, 2013. doi: 10.1016/j.cmet.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen DL, Ravussin E. The role of mitochondria in health and disease. Curr Opin Pharmacol 9(6): 780–6, 2009. doi: 10.1016/j.coph.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51(10): 2944–50, 2002. doi: 10.2337/diabetes.51.10.2944 [DOI] [PubMed] [Google Scholar]
- Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279(5): E1039–44, 2000. doi: 10.1152/ajpendo.2000.279.5.E1039 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Xiao J, Wan J, Cohen P, Yen K. Mitochondrially derived peptides as novel regulators of metabolism. J Physiol 595(21): 6613–6621, 2017. doi: 10.1113/JP274472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alpha. Cell 127:1109–1122, 2006. doi: 10.1016/j.cell.2006.11.013 [DOI] [PubMed] [Google Scholar]
- Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab 21(3): 443–54, 2015. doi: 10.1016/j.cmet.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Kang YC, Park WH, Jeong JH, Pak YK. Negative transcriptional regulation of mitochondrial transcription factor A (TFAM) by nuclear TFAM. Biochem Biophys Res Commun 450(1): 166–71, 2014. doi: 10.1016/j.bbrc.2014.05.082 [DOI] [PubMed] [Google Scholar]
- Lee C, Kim KH, Cohen P. MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free Radic Biol Med 100: 182–187, 2016. doi: 10.1016/j.freeradbiomed.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Higashida K, Kawamura T, Higuchi M. Time Course of Decrease in Skeletal Muscle Mitochondrial Biogenesis after Discontinuation of High-Fat Diet. I 64(3): 233–238, 2018. doi: 10.3177/jnsv.64.233 [DOI] [PubMed] [Google Scholar]
- Malik AN, Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 13(5): 481–92, 2013. doi: 10.1016/j.mito.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Malik AN, Simões ICM, Rosa HS, Khan S, Karkucinska-Wieckowska A, Wieckowski MR. A Diet Induced Maladaptive Increase in Hepatic Mitochondrial DNA Precedes OXPHOS Defects and May Contribute to Non-Alcoholic Fatty Liver Disease. Cells 8(10): 1222, 2019. doi: 10.3390/cells8101222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Guimera A, Welsh CM, Proctor CJ, McArdle A, Shanley DP. ‘Molecular habituation’ as a potential mechanism of gradual homeostatic loss with age. Mech Ageing Dev 169: 53–62, 2018. doi: 10.1016/j.mad.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab 20(1): 183–90, 2014. doi: 10.1016/j.cmet.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeuf-Louchart A, Thorel Q, Delhaye S, Beauchamp J, Duhem C, Danckaert A, Lancel S, Pourcet B, Woldt E, Boulinguiez A, Ferri L, Zecchin M, Staels B, Sebti Y, Duez H. Rev-erb-α regulates atrophy-related genes to control skeletal muscle mass. Sci Rep 7(1): 14383, 2017. doi: 10.1038/s41598-017-14596-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memme JM, Erlich AT, Phukan G, Hood DA. Exercise and mitochondrial health. J Physiol 2019. doi: 10.1113/JP278853 [DOI] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13(9): 1016–23, 2011. doi: 10.1038/ncb2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto PM, LeBlanc PJ, Holloway GP. High-Fat Diet Causes Mitochondrial Dysfunction as a Result of Impaired ADP Sensitivity. Diabetes 67(11): 2199–2205, 2018. doi: 10.2337/db18-0417 [DOI] [PubMed] [Google Scholar]
- Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, Budagov T, Cui L, Einstein FH, Poduval A, Hwang D, Barzilai N, Cohen P. Humanin: a novel central regulator of peripheral insulin action. PLoS One 4(7): e6334, 2009. doi: 10.1371/journal.pone.0006334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. (1997–2020). ImageJ. U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/ [Google Scholar]
- Rasool S, Geetha T, Broderick TL, Babu JR. High Fat With High Sucrose Diet Leads to Obesity and Induces Myodegeneration. Front Physiol 9: 1054, 2018. doi: 10.3389/fphys.2018.01054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JC, Lai RW, Woodhead JST, Joly JH, Mitchell CJ, Cameron-Smith D, Lu R, Cohen P, Graham NA, Benayoun BA, Merry TL, Lee C. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat Commun 12(1): 470, 2021. doi: 10.1038/s41467-020-20790-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 54(1): 8–14, 2005. doi: 10.2337/diabetes.54.1.8 [DOI] [PubMed] [Google Scholar]
- Ritov VB, Menshikova EV, Azuma K, Wood R, Toledo FG, Goodpaster BH, Ruderman NB, Kelley DE. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab 298(1): E49–58, 2010. doi: 10.1152/ajpendo.00317.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabright AP, Lai YC. Regulatory Roles of PINK1-Parkin and AMPK in Ubiquitin-Dependent Skeletal Muscle Mitophagy. Front Physiol 11: 608474, 2020. doi: 10.3389/fphys.2020.608474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 83(1): 166–71, 1997. doi: 10.1152/jappl.1997.83.1.166 [DOI] [PubMed] [Google Scholar]
- Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54(7): 1926–33, 2005. doi: 10.2337/diabetes.54.7.1926 [DOI] [PubMed] [Google Scholar]
- Stanhope KL. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit Rev Clin Lab Sci 53(1): 52–67, 2016. doi: 10.3109/10408363.2015.1084990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson EJ, Camera DM, Jenkins TA, Kosari S, Lee JS, Hawley JA, Stepto NK. Skeletal muscle respiratory capacity is enhanced in rats consuming an obesogenic Western diet. Am J Physiol Endocrinol Metab 302(12): E1541–9, 2012. doi: 10.1152/ajpendo.00590.2011 [DOI] [PubMed] [Google Scholar]
- Strauss WM (1998) in Current Protocols in Molecular Biology, ed Ausubel FM (Wiley, New York: ), pp 2.2.1–2.2.3. [Google Scholar]
- Sulli G, Rommel A, Wang X, Kolar MJ, Puca F, Saghatelian A, Plikus MV, Verma IM, Panda S. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553(7688): 351–355, 2018. doi: 10.1038/nature25170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryon LD, Crilly MJ, Hood DA. Effect of denervation on the regulation of mitochondrial transcription factor A expression in skeletal muscle. Am J Physiol Cell Physiol 309(4): C228–C238, 2015. doi: 10.1152/ajpcell.00266.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PW, Kuo HM, Huang HT, Chang AY, Weng SW, Tai MH, Chuang JH, Chen IY, Huang SC, Lin TK, Liou CW. Biphasic response of mitochondrial biogenesis to oxidative stress in visceral fat of diet-induced obesity mice. Antioxid Redox Signal 20(16): 2572–88. 2014. doi: 10.1089/ars.2013.5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sun H, Song G, Yang Y, Zou X, Han P, Li S. Resveratrol Improves Muscle Atrophy by Modulating Mitochondrial Quality Control in STZ-Induced Diabetic Mice. Mol Nutr Food Res 62(9): e1700941, 2018. doi: 10.1002/mnfr.201700941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CB, Hughes MC, Edgett BA, Scribbans TD, Simpson CA, Perry CG, Gurd BJ. An examination of resveratrol’s mechanisms of action in human tissue: impact of a single dose in vivo and dose responses in skeletal muscle ex vivo. PLoS One 9(7): e102406, 2014. doi: 10.1371/journal.pone.0102406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, Kamenecka TM, Schaart G, Lefebvre P, Nevière R, Burris TP, Schrauwen P, Staels B, Duez H. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med 19(8): 1039–46, 2013. doi: 10.1038/nm.3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikallio E, Tyynismaa H, Tsutsui H, Ide T, Suomalainen A. High mitochondrial DNA copy number has detrimental effects in mice. Hum Mol Genet 19(13): 2695–2705, 2010. doi: 10.1093/hmg/ddq163 [DOI] [PubMed] [Google Scholar]
- Zhao P, Wong KI, Sun X, Reilly SM, Uhm M, Liao Z, Skorobogatko Y, Saltiel AR. TBK1 at the Crossroads of Inflammation and Energy Homeostasis in Adipose Tissue. Cell 172(4): 731–743.e12, 2018. doi: 10.1016/j.cell.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


