Abstract
Cognitive remediation therapy (CRT) for anorexia nervosa (AN) was developed as an adjuvant treatment to target set-shifting and central coherence inefficiencies important in AN and to ultimately improve clinical outcomes of those with AN. The primary aim of this preliminary systematic review and meta-analysis was to determine the effect of CRT for AN relative to control treatments in randomized-controlled trials (RCTs) on neuropsychological inefficiencies at end-of-treatment. Secondary aims were to assess the effect of CRT for AN on dropout, eating-disorder-related, and other psychological outcomes at end-of-treatment. Systematic review and meta-analytic procedures were conducted in accordance with PRISMA Guidelines. RCTs evaluating CRT for AN compared to a control treatment were identified via ProQuest, PsycINFO, PubMed, and SCOPUS. Seven RCTs and one quasi-RCT of CRT for AN were included. RCT quality ratings ranged from fair (n = 3) to good (n = 4). Random-effects meta-analysis was conducted using Hedge’s g. Study heterogeneity was assessed using I2 and publication bias was assessed with Begg’s adjusted-rank correlation and the trim-and-fill method. CRT was not associated with improvement in central coherence compared to control treatments at end-of-treatment (g = 0.25, 95% CI = −0.35, 0.85, k = 3). Set-shifting outcomes were mixed due to heterogeneity of set-shifting measures across studies. CRT may prevent dropout; yet, more studies are needed to draw conclusions. CRT did not confer advantage over control treatments for eating-disorder-related and other psychological outcomes at end-of-treatment. Future RCTs of CRT for AN should use precise measures to assess constructs (particularly for set shifting), increase sample size, and implement longitudinal follow-up. (Word Count: 247 words).
Keywords: Cognitive remediation therapy, Anorexia nervosa, Randomized-controlled trials, Meta-analysis, Systematic review
1. Introduction
Anorexia nervosa (AN) is characterized by objectively low body weight maintained in response to fear of weight gain and/or due to persistent behaviors (e.g., caloric restriction) that suppress weight (American Psychiatric Association, 2013). AN is associated with serious medical and psychiatric comorbidities, and elevated risk for mortality (Fichter & Quadflieg, 2016). Despite the severity of AN, current treatments have limitations, particularly for adults (Zipfel, Giel, Bulik, Hay, & Schmidt, 2015). Moreover, AN treatment attrition is high, with the majority of dropouts occurring early in treatment (Dejong, Broadbent, & Schmidt, 2012), which reduces the likelihood of weight restoration. Thus, there is a critical need for treatments that improve outcomes and promote retention.
Treatment outcomes may be improved through identifying and targeting core AN mechanisms. Research suggested that two putative neuropsychological mechanisms of AN were central coherence and set shifting. Central coherence is the ability to integrate details into a whole, much like arranging pieces of a puzzle together to make a cohesive picture. Persons with AN have central coherence inefficiencies (weak central coherence), such that persons with AN hyper-focus on detail and experience challenges with integrating details into a whole. A large-scale study suggested that adults with current AN had significantly weaker central coherence than unaffected controls (d = 0.26) (Lang et al., 2016); however, findings for adolescents with AN compared to healthy controls were mixed, with some studies finding differences (Lang, Stahl, Espie, Treasure, & Tchanturia, 2015; Rose, Frampton, & Lask, 2015) and others finding no differences (Herbrich, Kappel, & Noort, 2018). Cross-sectional research showed that weak central coherence was present in unaffected adult family members of persons with AN, suggesting that weak central coherence is heritable (Kanakam, Raoult, Collier, & Treasure, 2015; Lang et al., 2016; Roberts, Tchanturia, & Treasure, 2013; Tenconi et al., 2010).
Set shifting is another putative mechanism of AN that is defined as the ability to flexibly transition between rule sets or different tasks (Miyake, Friedman, Emerson, Witzki, & Howerter, 2000). Meta-analysis suggested that adults with AN (vs. healthy controls) have moderate set-shifting inefficiencies (Wu et al., 2014). Evidence for set-shifting inefficiencies in children and adolescents with AN has been weak, with meta-analysis suggesting small, non-significant differences in set shifting between children and adolescents with AN compared to healthy controls (Herbrich et al., 2018; Lang et al., 2015; Westwood, Stahl, Mandy, & Tchanturia, 2016). Large-scale (Tchanturia et al., 2011, 2012) and smaller-scale (Danner et al., 2012) cross-sectional research found that set-shifting inefficiencies persisted after AN remission. Research also found that women with AN and their unaffected sisters performed significantly worse on set-shifting tasks than unrelated, unaffected women, suggesting that set-shifting inefficiencies are trait-versus state-based (Holliday, Tchanturia, Landau, Collier, & Treasure, 2015; Tenconi et al., 2010).
1.1. Cognitive remediation therapy for AN
Cognitive remediation therapy (CRT) for AN was developed as an adjuvant treatment to target central coherence and set-shifting inefficiencies observed in AN. CRT for AN is comprised of cognitive exercises that focus on looking at the “big picture” versus details (central coherence), reducing cognitive and behavioral rigidity (set shifting), and increasing awareness of one’s own thinking styles (Tchanturia, Davies, Reeder, & Wykes, 2010). CRT for AN does not include discussion of eating-disorder-related themes and, thus, may be a non-threatening adjuvant to treatment-as-usual. Feasibility research showed that CRT for AN was well-received by patients, helped to develop a therapeutic alliance, and had low drop-out rates (10–15%) (Tchanturia, Lloyd, & Lang, 2013). Research also supports the feasibility of CRT for AN across settings (e.g., individual, group) and developmental trajectories (e.g., children, adolescents, adults) (Tchanturia et al., 2013).
Previous systematic literature reviews (without accompanying meta-analyses) have qualitatively described the feasibility and acceptability of CRT for AN and provided preliminary evidence in support of neuropsychological change facilitated by CRT for AN (Dahlgren & Ro, 2014; Danner, Dingemans, & Steinglass, 2015; Juarascio, Manasse, Espel, Kerrigan, & Forman, 2015; Tchanturia et al., 2013; Tchanturia, Lounes, & Holttum, 2014). Moreover, a meta-analysis of pre-post CRT studies in children and adolescents with AN found that CRT facilitated small improvements in set shifting and central coherence (Tchanturia, Giombini, Leppanen, & Kinnaird, 2017). However, a limitation of Tchanturia et al. (2017) is reliance on data from pre-post case series rather than data from randomized-controlled trials (RCTs) to draw conclusions. Although pre-post case series are necessary and important for demonstrating the acceptability and feasibility of novel treatments (such as CRT for AN), there are limitations of this study design, including selection bias. RCTs decrease chance of selection bias with similar patient groups (due to randomization) and allow for direct comparison of the active treatment to a controlled treatment.
1.2. The present study
The primary aim of the present study was to conduct a preliminary systematic review and meta-analysis of RCTs of CRT for AN on neuropsychological (i.e., set shifting, central coherence) outcomes at end-of-treatment. We hypothesized that because CRT for AN was developed to directly target neuropsychological inefficiencies associated with AN, CRT for AN would be associated with improved set shifting and central coherence relative to control treatments at end-of-treatment. The secondary aim of this study was to examine the effect of CRT for AN on patient dropout, eating-disorder-related, and other psychological outcomes at end-of-treatment. We hypothesized that CRT for AN would have lower patient dropout rates compared to control treatments because CRT is a non-threatening intervention that does not directly target body weight. We hypothesized that CRT for AN would be associated with improvement in eating-disorder-related outcomes above-and-beyond control treatments, given that CRT for AN is purported to improve neuropsychological inefficiencies that underpin AN symptom expression. Finally, given the high comorbidity among AN and other mental-health disorders (Hudson, Hiripi, Pope, & Kessler, 2007; Swanson, Crow, Grange, Swendsen, & Merikangas, 2011), we investigated the effect of CRT for AN on other psychological functioning relative to control treatments. We hypothesized that depressive and obsessive compulsive symptoms would improve with CRT, as prior research has documented set-shifting inefficiencies in major depressive disorder (Snyder, 2013) and obsessive compulsive disorder (Lawrence et al., 2006).
2. Method
Procedures for this meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement (Moher, Liberati, Tetzlaff, & Altman, 2009). See Appendix A for a PRISMA checklist.
2.1. Study selection
2.1.1. Literature search
An electronic database search was conducted on March 23, 2020 using PsycINFO, ProQuest Dissertations and Theses Global, PubMed, and SCOPUS. The search terms used were anore* combined with CRT or (cognit* and (remed* or train*)). Search terms were abbreviated to permit a variety of spellings and combinations of words. The literature search was extended beyond published, peer-reviewed journal articles to “grey” literature (e.g., book chapters, dissertations, etc.). Reference lists of retrieved manuscripts were manually reviewed for relevant studies not identified through electronic database search. The first and second author independently reviewed manuscripts for inclusion. Discrepancies were resolved with the last author. A PRISMA article selection flow chart is presented in Fig. 1.
Fig. 1.
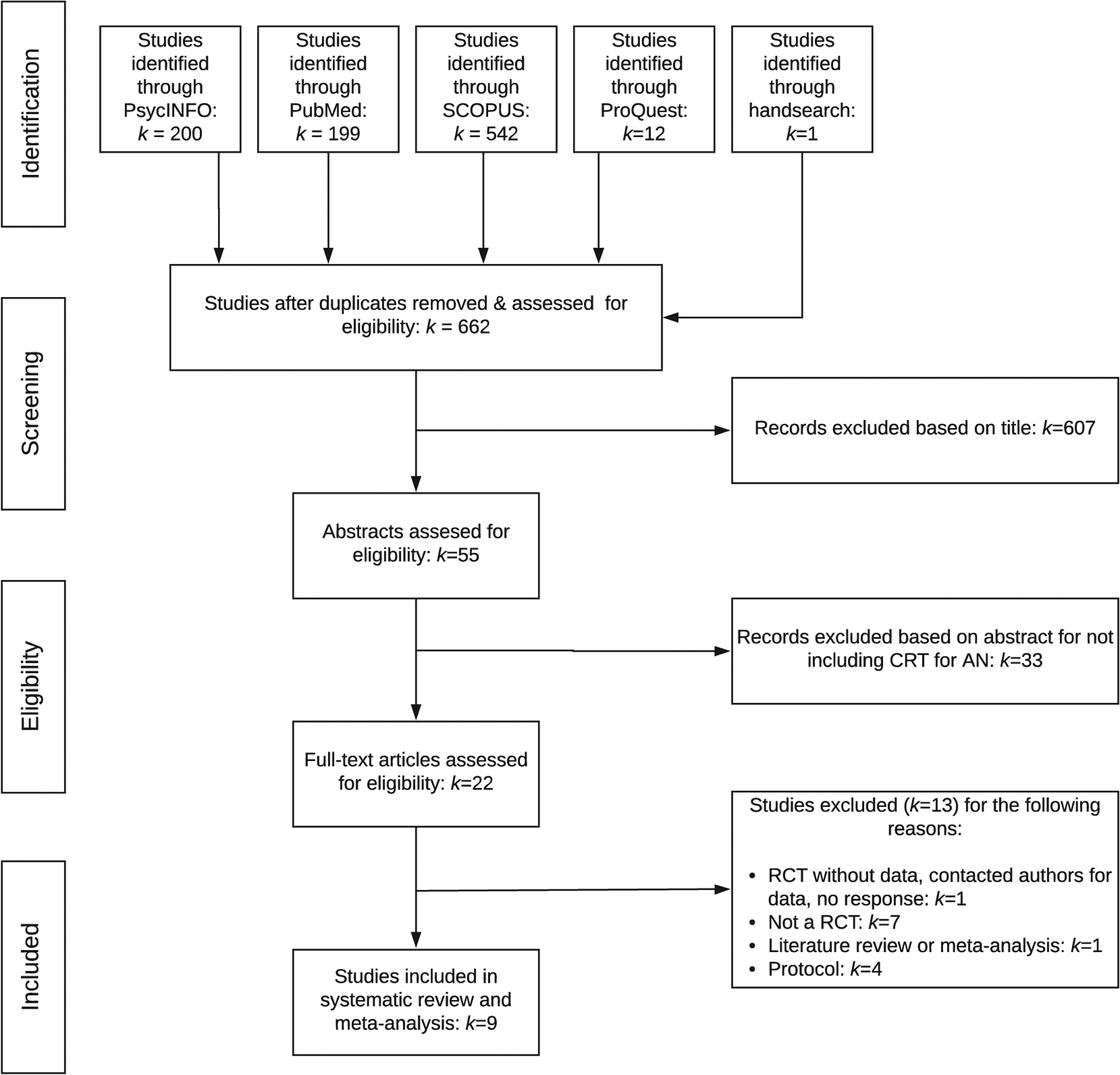
PRISMA flow chart of study selection guidelines.
2.1.2. Eligibility criteria
Studies were included based on the following criteria: 1) examined CRT for AN using an RCT design; and 2) reported sufficient statistics to compute effect sizes or reported effect sizes. Study authors were contacted up to two times if data to compute effect sizes was not provided. The first and second authors extracted means, standard deviations, effect sizes, and other relevant information from included studies.
2.1.3. Study quality assessment
Study quality assessment was conducted in accordance with the National Institutes of Health Quality Assessment of Controlled Trials (National Institutes of Health, 2014). Studies were evaluated on the following criteria: use of a RCT design, randomization method, treatment allocation concealment, blinding of providers and participants to treatment assignment, blinding of assessors, similarity of groups at baseline, dropout rate, adherence to treatment protocols, use of reliable and valid outcome measures, a priori power analysis, a priori analytic plan, and use of intention-to-treat analyses. Studies were assigned ratings of good, fair, or poor based on ratings of the above-mentioned criteria. Studies assigned poor ratings were not included in meta-analysis.
2.2. Statistical analysis
Meta-analysis was conducted if at least three studies provided sufficient data to compute effect sizes. Meta-analysis was conducted using the metafor package (Viechtbauer, 2010) in R Version 3.3.2 (R Core Team, 2013). To estimate effect sizes, standardized mean difference scores (Hedge’s g) were computed and effect sizes were interpreted as follows: 0.2 (small), 0.5 (medium), and 0.8 (large) (Cohen, 1988). We estimated random-effects (vs. fixed-effects) models because random-effects models account for variance within studies due to sampling error and between studies due to variables such as study sites, participant characteristics, and treatment providers (Borenstein, Hedges, Higgins, & Rothstein, 2009). If meta-analysis yielded a significant effect size, we used Rosenthal’s fail-safe N (Rosenthal, 1979) to determine the number of null studies needed to produce a non-significant effect size.
2.2.1. Study heterogeneity
I2 was used to assess study heterogeneity. I2 indicates the percentage of between-study variance due to heterogeneity versus chance. I2 is measured on a scale of 0% (no heterogeneity) to 100% (high heterogeneity) and values of 25%, 50%, and 75% are considered benchmarks of low, moderate, and high heterogeneity, respectively (Borenstein et al., 2009).
2.2.2. Publication bias
Begg’s adjusted-rank correlation test (Begg & Mazumdar, 1994) and the trim-and-fill method (Duval & Tweedie, 2000), which estimates how many studies were missing from meta-analysis and provides adjusted meta-analytic results by imputing missing studies into results, were used to quantitatively assess publication bias.
3. Results
Six hundred sixty-two unique studies were identified, and nine studies met inclusion criteria (see Fig. 1 and Table 1). Two of the nine studies reported on the same RCT (Brockmeyer et al., 2014, 2016); however, these two studies reported on different outcome measures. Thus, outcomes from both Brockmeyer et al. (2014) and Brockmeyer et al. (2016) were included due to non-overlapping information. Herbrich et al. (2017) compared CRT for AN to treatment-as-usual using a quasi-random design, therefore it was not included in meta-analytic computations. A narrative summary of Herbrich et al. (2017) is provided in parallel with RCT findings. All included studies investigated CRT as an adjuvant treatment except Lock et al. (2013) and van Passel et al. (2020), which investigated standalone CRT relative to a control treatment prior to beginning CBT and treatment-as-usual, respectively (see Table 1 for details).
Table 1.
Summary of included studies.
| Study | Study design | Sample size (% female)a | Age, Mean (SD) | Duration of Illness, Mean (SD) | % AN-BPb | Dropout, n (%) | Location | Level of care | Dose | Key outcome measures |
|---|---|---|---|---|---|---|---|---|---|---|
| Lock et al. (2013) | CRT + CBT: | n = 23 (91%) | 22.5 (4.9) | 6.8 (5.4) years | 76% | 3 (13%) | USA | Outpatient | 8–45 min weekly sessions over 2 months | RCFT DKEFS CWIT BMI EDE BDI |
| CBT only: | n = 23 (87%) | 23.0 (6.8) | 5.9 (6.2) years | 77% | 7 (33%) | |||||
| Brockmeyer et al. (2014) and Brockmeyer et al. (2016) | CRT + TAUc: | n = 20 (100%) | 23.63 (6.73) | 7.89 (7.60) years | 21% | 2 (10%) | Germany | Inpatient or outpatient | 9 face-to-face sessions; 21 computer-assisted sessions over 3 weeks | Cued task-switching paradigm (2014) Visual target-detection task (2016) |
| NNTd + TAU: | n = 20 (100%) | 26.65 (8.95) | 14.68 (1.76) years | 5% | 0 (0%) | |||||
| Dingemans et al. (2014) | CRT + TAU: | n = 41 (100%) | 27.0 (8.6) | 9.3 (7.4) years | AN or BNe | 0 (0%) | Netherlands | Inpatient | 10–45 min sessions over 6 weeks | RCFT WCST BMI EDEQ EDQOL BDI |
| TAU: | n = 41 (100%) | 26.9 (8.0) | 9.8 (7.7) years | 0 (0%) | ||||||
| Steinglass et al. (2014) | CRT: | n = 15 (87%) | 29.1 (7.8) | 10.5 (6.3) years | 53% | 0 (0%) | USA | Inpatient | 12–45 min sessions 3 times per week over 4 weeks | BMI Calories consumed in test meal |
| AN-EXRPf: | n = 15 (100%) | 26.5 (8.8) | 9.5 (10.1) years | 20% | 0 (0%) | |||||
| Herbrich et al. (2017) g | CRT + TAU: | n = 24 (100%) | 15.6 (1.3) | 18.21 (11.66) months | 16.7% | 21.8% | Germany | Inpatient & outpatient | Ten 45–60 min sessions; twice weekly | RCFT BRIEF-SR BMI ChEDEQ DIKJ STAI(C)-T |
| TAU only: | n = 24 (100%) | 15.3 (0.9) | 12.92 (15.85) months | 16.7% | 41.7% | |||||
| Lock, Fitzpatrick, Agras, Weinbach, and Jo (2018) | CRT + FBT: | n = 15 (86.7%) | 14.42 (1.83) | 12.43 (17.59) months | N.R. | 1 (6.7%) | USA | Outpatient | 15–30 min sessions for 9 months adjuvant to FBT | RCFT WCST BMI EDEQ YBC-EDS BDI CY-BOCS |
| AT + FBT: | n = 15 (93.3%) | 14.55 (1.48) | 8.47 (5.46) months | N.R. | 4 (26.7%) | |||||
| Sproch, Anderson, Sherman, Crawford, and Brandt (2019) | CRT + TAU: | n = 135 (89.6%) | 23.9 (12.8) | 8.0 (10.3) years | N.R. | 0 (0%) | USA | Inpatient | 5–1 h sessions twice per week | WCST CTMT BMI CBT thought records |
| TAU only: | n = 140 (92.1%) | 22.2 (12.8) | 6.4 (9.6) years | N.R. | 0 (0%) | |||||
| van Passel et al. (2020) | CRT + TAU: | n = 30 (97%) | 25.19 (7.11) | 5.10 (7.50) years | N.R. | N.R. | Netherlands | N.R. | 10–45 min sessions twice per week | EDEQ EDQOL |
| SATg + TAU: | n = 30 (90%) | 24.60 (6.98) | 4.66 (4.35) years | N.R. | N.R. |
Note. AN = anorexia nervosa; AN-EXRP = Exposure and Response Prevention for AN; AN-BP = AN-Binge eating/purging type; AN-R = AN restricting type; AT = art therapy; BDI=Beck Depression Inventory; BRIEF-SR = Behavioral Rating Inventory for Executive Functioning – Self Report; BMI = body mass index; CBT cognitive behavioral therapy; ChEDEQ = Child Eating Disorder Examination Questionnaire; CTMT = comprehensive trail making test; CRT = cognitive remediation therapy; CY-BOCS=Children’s Yale-Brown Obsessive-Compulsive Scale; DIKJ = Depression Inventory for Children and Adolescents; D-KEFS CWIT = Delis-Kaplan Executive Function System Color-Word Interference Test; EDE = Eating Disorder Examination; EDEQ = Eating Disorder Examination Questionnaire; EDQoL = Eating Disorder Quality of Life Questionnaire; FBT = Family-Based Treatment; NNT = non-specific neurocognitive therapy; N.R. = not reported; RCFT = Rey-Osterrieth Complex Figure Test; RCT = randomized-controlled trial; SAT = specialized attention therapy; SD = standard deviation; STAI(C)-T = State-Trait Anxiety Inventory for Children; TMT = Trail Making Test; TAU = treatment-as-usual; TMT = Trail Making Test; WCST = Wisconsin Card Sorting Test; YBCEDS=Yale-Brown-Cornell Eating Disorder Severity Scale.
Information provided if gender reported.
Information provided if subtypes reported.
TAU reflects standard care received during inpatient care, including weight restoration, medical management, and group and/or individual counseling.
NNT targets attention, deductive reasoning, and memory instead of cognitive flexibility.
Participant diagnoses were: BN (n = 4; 26.7%), AN-BP (n = 7; 46.7%), or AN-R (n = 4; 26.7%).
AN-EXRP is a manualized adaptation of exposure and response prevention that aims to reduce fear and anxiety associated with eating and body dissatisfaction in AN.
SAT aims to help increase positive experiences through various exercises, such as playing board games.
RCT quality assessment ratings are detailed in Table 2. RCT quality assessment ratings ranged from fair to good. An overall summary of meta-analytic results from RCTs is presented in Table 3.
Table 2.
Article quality assessment.
| Studya | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Total scoreb | Overall study quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lock et al. (2013) | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 9 | Fair |
| Brockmeyer et al. (2014 & 2016)c | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 10 | Fair |
| Dingemans et al. (2014) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 12 | Good |
| Steinglass et al. (2014) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 11 | Good |
| Lock et al. (2018) | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 10 | Fair |
| Sproch et al. (2019) | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 12 | Good |
| van Passel et al. (2020) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 12 | Good |
Note. 1 = randomized-control trial design; 2 = adequate randomization method (i.e., computer-generated random assignments); 3 = treatment allocation concealment to patients; 4 = providers blinded to treatment group assignment; 5 = blinding of assessors; 6 = similarity of groups at baseline; 7 = overall drop-out rate less than or equal to 20% of patients allocated to treatment; 8 = difference between drop-out rates between treatment groups 15% or less; 9 = therapist adherence to treatment protocol described; 10 = other interventions similar between groups; 11 = outcome measures reliable and valid; 12 = authors reported sufficient sample size to detect differences between main outcomes with at least 80% power; 13 = a priori specified outcomes and subgroup analyses; 14 = use of intention-to-treat analysis. Individual assessment items rated as 0 or 1, for no or yes, respectively.
Herbrich et al. (2017) was not included in article quality assessment because it was not included in meta-analysis because is not a randomized-controlled trial (it is a quasi-randomized controlled trial).
Total scores were calculated by summing scores from each item. Total score values were used to assign quality rankings: 1–5 = poor, 6–10 = fair, and 11–14 = good.
Brockmeyer et al. (2014) and Brockmeyer et al. (2016) report on different outcomes from the same RCT.
Table 3.
Summary of meta-analytic results from randomized-controlled trials.
| Measure | g | 95% CI | SE | I 2 |
|---|---|---|---|---|
| Neuropsychological measures | ||||
| RCFT CCI (k = 3) | 0.247 | −0.353, 0.847 | 0.306 | 61.08% |
| Eating-disorder-related measures | ||||
| Body mass index (k = 5) | −0.109 | −0.316, 0.098 | 0.106 | 0.00% |
| EDE Global score (k = 4) | −0.005 | −0.287, 0.278 | 0.144 | 0.00% |
| Non-eating-disorder measures | ||||
| Depression (k = 3) | −0.137 | −0.479, 0.205 | 0.174 | 0.00% |
Note. CI = confidence interval; CRT = cognitive remediation therapy; EDE = Eating Disorder Examination; ES = effect size; RCFT CCI = Rey-Osterrieth Complex Figure Test Central Coherence Index; SE = standard error.
3.1. Primary outcome: neuropsychological functioning
3.1.1. Central coherence
Meta-analysis revealed a small, statistically non-significant improvement in central coherence outcomes measured by the Rey-Osterrieth Complex Figures Test at end-of-treatment in persons who completed CRT versus a control treatment (see Table 3 and Fig. 2). Begg’s adjusted-rank correlation test and the trim-and-fill method did not suggest publication bias (p’s > 0.05). Herbrich et al. (2017) found no differences in central coherence assessed by the Rey-Osterrieth Complex Figures Test at six-month follow-up in adolescents with AN who received CRT adjuvant to treatment-as-usual versus treatment-as-usual alone.
Fig. 2.
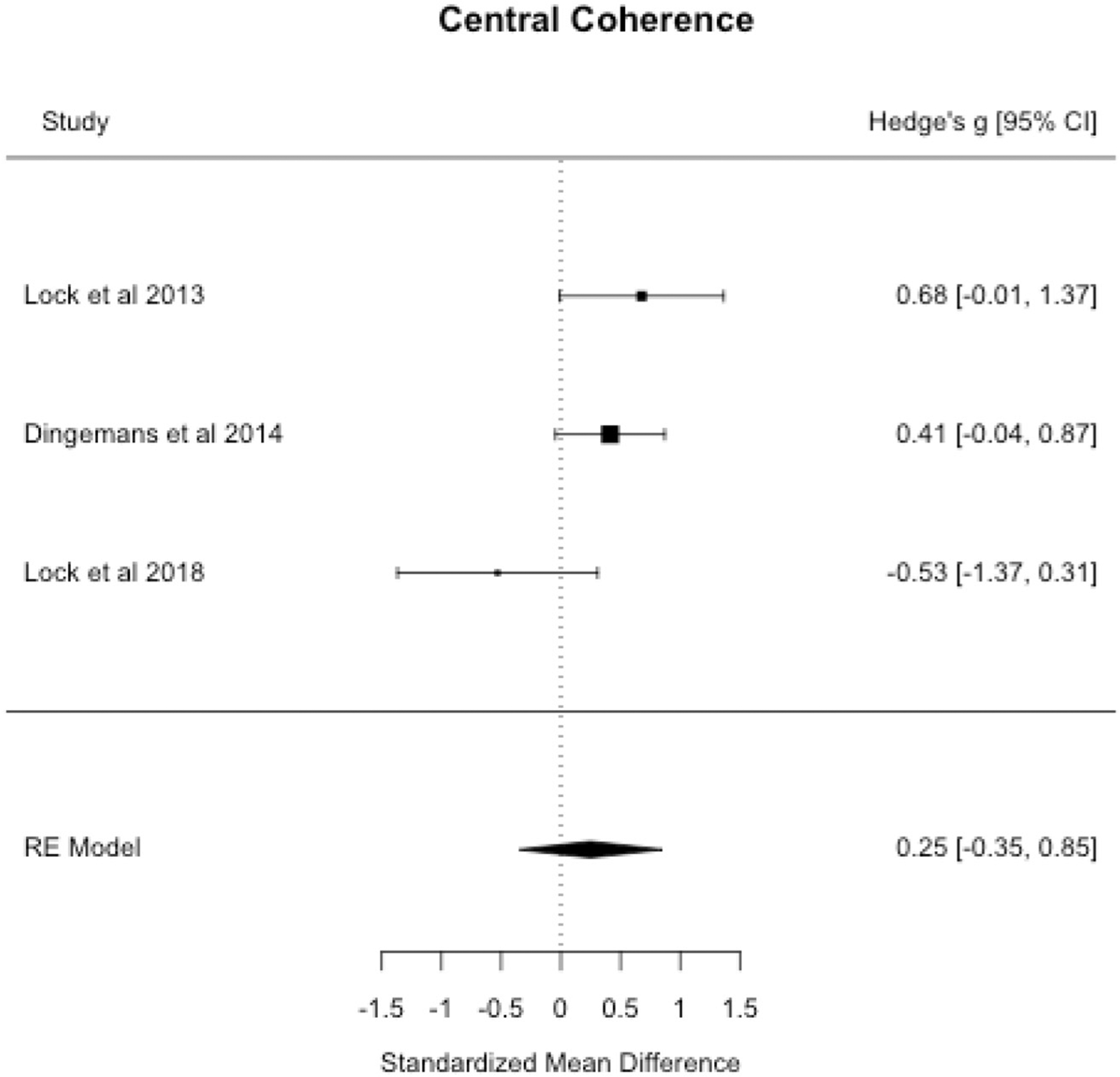
Central coherence forest plot.
3.1.2. Set shifting
Set shifting was operationalized with a variety of measures, including the Wisconsin Cart Sorting Task (k = 3), Trail Making Test (k = 1), Comprehensive Trail Making Test (k = 1), Delis-Kaplan Executive Function System Color-Word Interference Test (k = 1), a computerized cued task-switching paradigm (k = 1), and a visual target-detection task (k = 1). Due to the heterogeneity of set-shifting measures, meta-analysis was not carried out and instead results are qualitatively described below, by measure.
Using the Wisconsin Card Sorting Task, Dingemans et al. (2014) found no differences in set-shifting changes at end-of-treatment in persons with a severe or enduring eating disorder who received CRT adjuvant to treatment-as-usual compared to treatment-as-usual only. Similarly, Lock et al. (2018) did not find set-shifting differences at end-of-treatment in adolescents with AN who received CRT (vs. art therapy) adjuvant to family-based treatment (FBT) using the Wisconsin Card Sorting Task. Sproch et al. (2019) also found no end-of-treatment differences in set-shifting outcomes measured by the Wisconsin Card Sorting Task in persons with AN who received CRT (vs. a control treatment) adjuvant to treatment-as-usual.
Using the Trail Making Test, Dingemans et al. (2014) found that set-shifting inefficiencies showed small-to-moderate improvements at six-weeks follow-up in persons with a severe or enduring eating disorder who received CRT adjunctive to treatment-as-usual versus treatment-as-usual alone (d = 0.41). However, Herbrich et al. (2017) found no differences in set-shifting measured by the Delis-Kaplan Executive Function System Trail Making Test at six-month follow-up between adolescents with AN who received CRT versus treatment-as-usual. Sproch et al. (2019) found no differences in set shifting measured by the Comprehensive Trail Making Test between persons with AN who received CRT versus a control treatment adjunctive to treatment-as-usual.
Two studies used other set-shifting measures. Lock et al. (2013) found moderate-to-large improvements in set shifting measured by the Delis-Kaplan Executive Function System Color-Word Interference Test in persons with AN who received CRT versus CBT (effect size = 0.79). Brockmeyer et al. (2014) used a computerized cued task-switching paradigm to measure changes in set shifting over treatment and found that persons with AN receiving CRT plus treatment-as-usual (vs. non-specific neurocognitive therapy plus treatment-as-usual) demonstrated moderate improvements in set shifting at end-of-treatment (d = 0.62). Brockmeyer et al. (2016) [an additional report on the findings of Brockmeyer et al., 2014] found that persons with AN who received CRT improved set-shifting ability on a visual target-detection task, whereas persons who received non-specific neurocognitive therapy did not show the same improvements in set shifting at end-of-treatment (d = 0.58).
3.1.3. Cognitive thought records
Sproch et al. (2019) examined changes in thought records to test “clinically relevant” changes set shifting. Sproch et al. (2019) examined changes in self-reported believability of the original thought (reported as percentage), number of alternative thoughts identified, and believability of most believable alternative thought (reported as percentage) over the course of CRT compared to a control treatment adjuvant to treatment-as-usual. Sproch et al. (2019) found no differences in original thought believability, number of alternative thoughts identified, and believability of most believable alternative thought between persons with AN who received CRT versus a control treatment adjuvant to treatment-as-usual.
3.2. Secondary outcome: treatment dropout
Lock et al. (2013) investigated dropout rates for standalone outpatient CRT versus outpatient CBT after eight sessions and dropout rates after all patients were allocated to 16 sessions of outpatient CBT following the aforementioned an eight-session course of CRT or CBT. Lock et al. (2013) found that outpatients with AN had a lower dropout rate at the end of eight sessions of CRT versus CBT (three versus seven dropouts, respectively). However, Lock et al. (2013) found similar end-of-treatment dropout rates following 16 weeks of CBT, suggesting CRT did not promote long-term retention. Lock et al. (2018) found that CRT adjuvant to FBT was associated with fewer dropouts than art therapy adjuvant to FBT (one versus four dropouts, respectively). Herbrich et al. (2017) found that adolescents receiving CRT had increased treatment completion rates (79.2%) compared to adolescents receiving treatment-as-usual (58.3%). Herbrich et al. (2017) found no significant differences in BMI increases based on treatment type at six-months follow-up.
3.3. Secondary outcome: eating-disorder-related measures
3.3.1. Body mass index (BMI)
Meta-analytic results suggested no differences in BMI for persons receiving CRT versus a control treatment at end-of-treatment (see Table 3 and Fig. 3). Begg’s adjusted-rank correlation test and the trim-and-fill method did not indicate publication bias (p’s > 0.05).
Fig. 3.
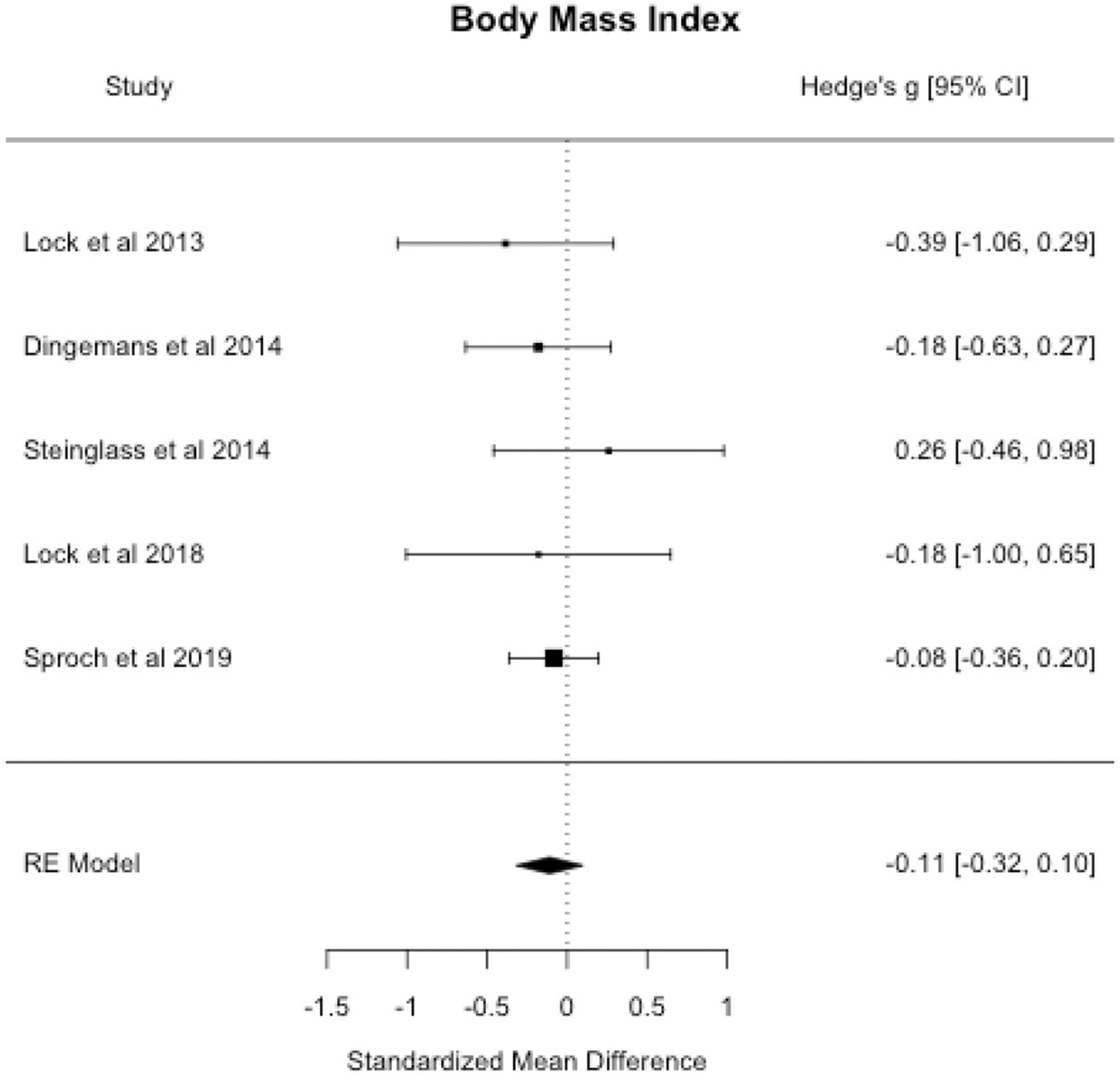
Body mass index forest plot.
3.3.2. Eating-disorder symptoms
Meta-analysis suggested no differences in eating-disorder symptoms measured by Eating Disorders Examination (EDE) or Eating Disorders Examination Questionnaire (EDE-Q) global scores at end-of-treatment (see Table 3 and Fig. 4). There was no indication of publication bias, per Begg’s adjusted-rank correlation test and the trim-and-fill method (p’s > 0.05). Similarly, Herbrich et al. (2017) found that adolescents receiving CRT versus treatment-as-usual did not differ on EDE-Q global scores at six-months follow-up.
Fig. 4.
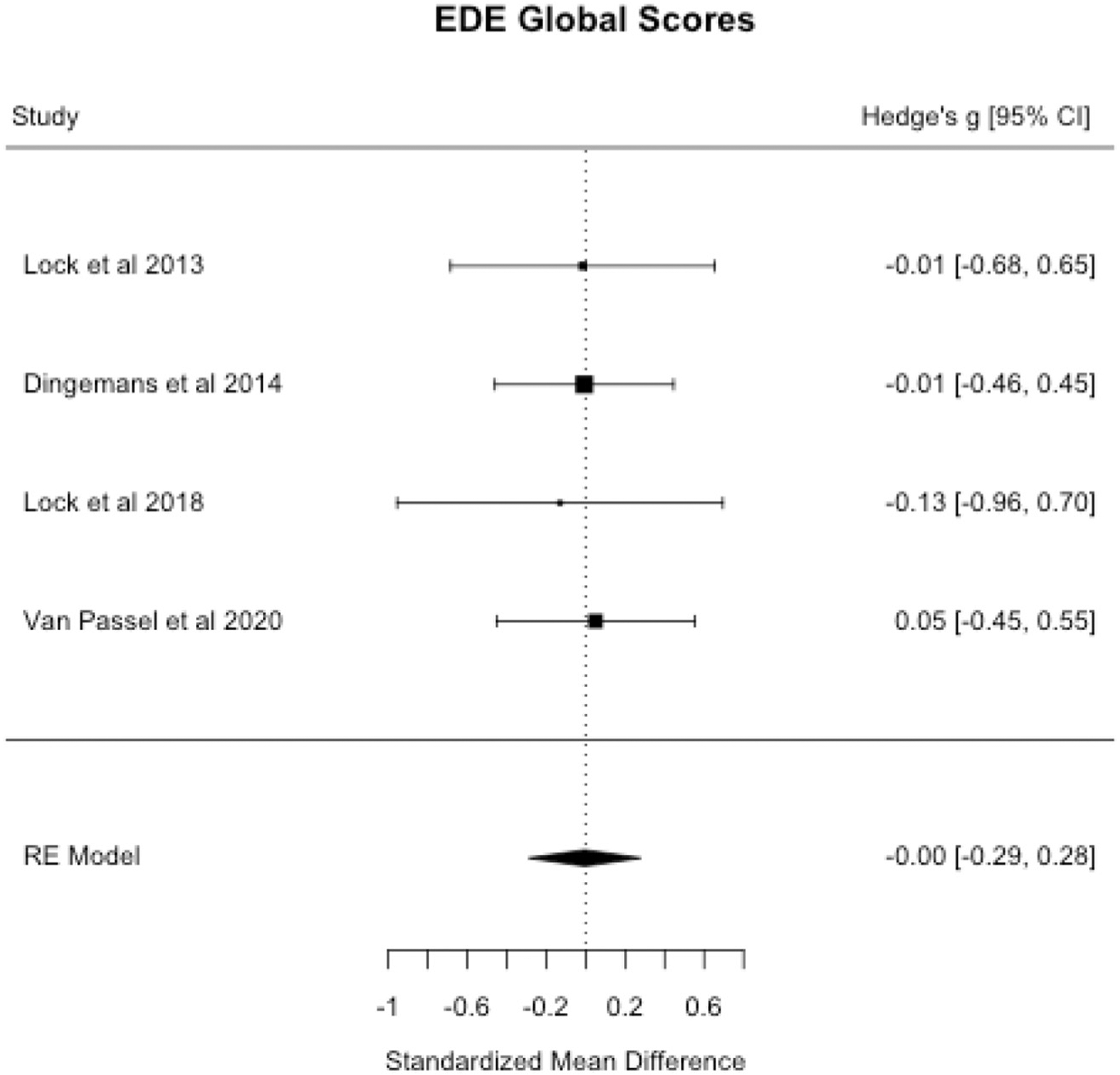
EDE global scores forest plot.
3.3.3. Eating disorder-related quality of life
Dingemans et al. (2014) found that CRT adjunctive to treatment-as-usual (compared to treatment-as-usual alone) resulted in large improvements in eating disorder-related quality of life (d = 1.36) at end-of-treatment. In contrast, van Passel et al. (2020) found a small-to-moderate difference in eating disorder-related quality of life at end-of-treatment, such that patients receiving CRT reported increased eating disorder-related quality of life than those receiving specialized attention therapy (d = 0.48).
3.3.4. Caloric intake
Steinglass et al. (2014) found that persons who received Exposure and Response Prevention for AN (AN-EXRP) significantly increased food intake in a laboratory test meal from baseline to end-of-treatment compared to persons who received CRT (d = 0.92). Additionally, changes in pre-test meal anxiety did not differ between AN-EXRP and CRT over the course of treatment; however, improvements in anxiety were associated with increased test meal caloric intake in persons with AN who received AN-EXRP (but not CRT).
3.4. Secondary outcome: other psychological measures
3.4.1. Anxious symptoms
Dingemans et al. (2014), Herbrich et al. (2017), and Lock et al. (2018) did not find differences in state anxiety at end-of-treatment among treatment groups.
3.4.2. Depressive symptoms
Meta-analysis revealed no effect of treatment on depressive symptoms at end-of-treatment. There was no indication of publication bias (p’s > 0.05). Results are presented in Table 3 and Fig. 5.
Fig. 5.
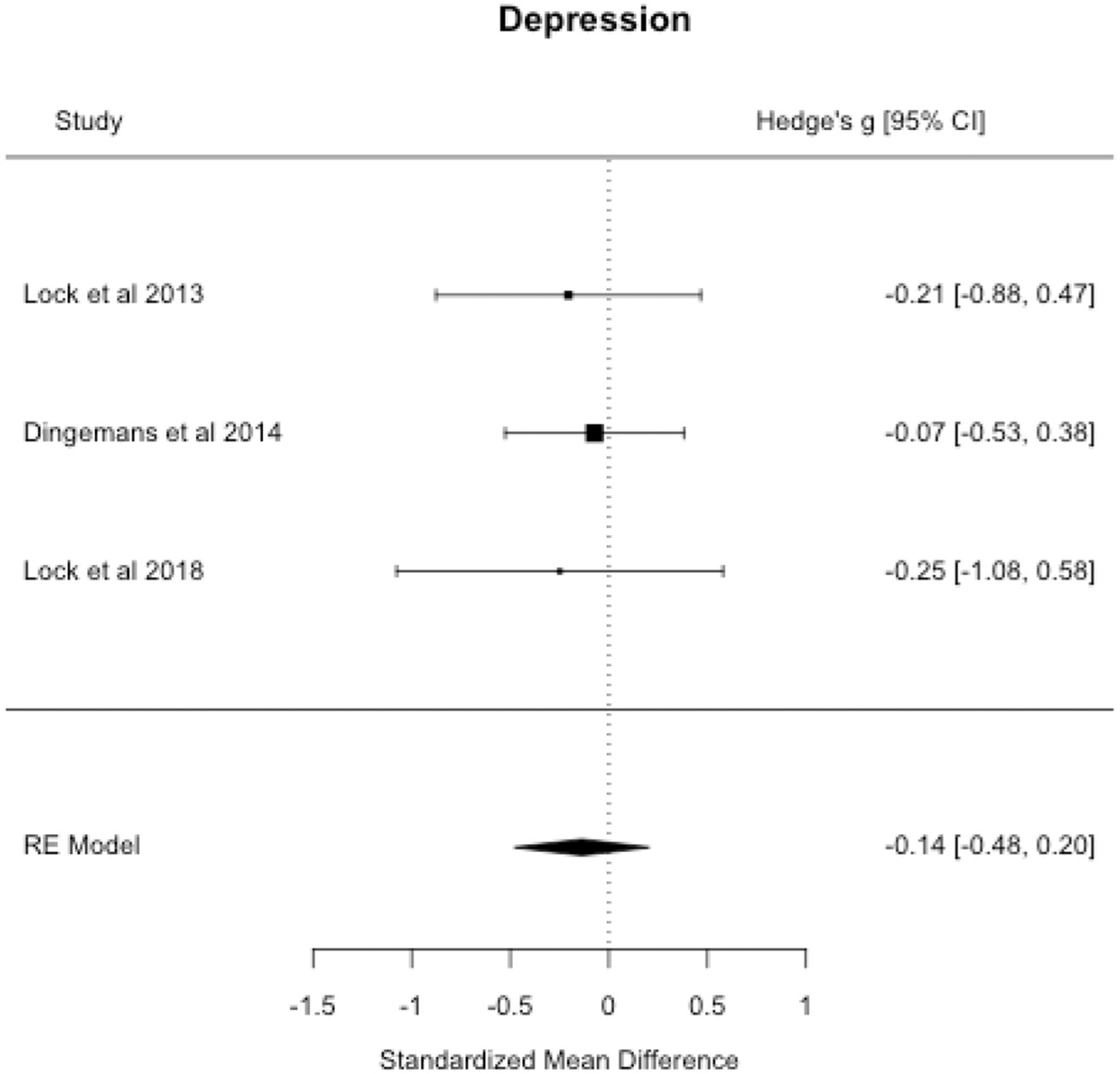
Depressive symptoms forest plot.
3.4.3. Obsessive-compulsive symptoms
Lock et al. (2018) found that adolescents with AN who received art therapy (vs. CRT) adjuvant to FBT had moderate-to-large reductions in general obsessive-compulsive symptoms assessed by the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS; d = 0.74). Additionally, Lock et al. (2018) found that art therapy (vs. CRT) adjuvant to FBT facilitated small reductions eating-related obsessions and compulsions measured by the Yale-Brown-Cornell Eating Disorder Scale (YBC-EDS; d = 0.26).
3.4.4. Perfectionism
Dingemans et al. (2014) found no differences in perfectionism at end-of-treatment for CRT adjuvant to treatment-as-usual compared to treatment-as-usual alone. Herbrich et al. (2017) found that adolescents with AN who received CRT adjuvant to treatment-as-usual versus treatment-as-usual alone showed increases in perfectionism at six-month follow-up.
4. Discussion
The primary aim of this preliminary systematic review and meta-analysis was to describe and test the effect of CRT for AN versus control treatments in RCTs on neuropsychological outcomes. The secondary aim was to assess the effect of CRT for AN versus control treatments on dropout, eating-disorder-related, and other psychological outcomes. First, meta-analytic results suggested that CRT for AN was associated with small, statistically non-significant, improvements in central coherence at end-of-treatment compared to control treatments. This finding is congruent with the only previous meta-analysis of CRT for AN for children and adolescents, which suggested small improvements in central coherence in pre-post studies of CRT for AN (Tchanturia et al., 2017), though a key difference is that our result was statistically nonsignificant. Taken together with Tchanturia et al. (2017)’s findings, our findings raise the interesting question of whether CRT for AN in its current form is sufficient to strengthen central coherence in persons with AN. Instead, CRT for AN may need to be modified to increase the intensity and dose of exercises to facilitate robust increases in central coherence. Improvements in set shifting for CRT versus a control treatment ranged from no difference to large differences; unfortunately, we could not perform a meta-analysis of set-shifting outcomes due to variability in set-shifting assessments across studies. Thus, when future research is published on this topic, it will be important to revisit set-shifting effects. Second, a qualitative review of findings suggested that CRT (vs. control treatments) may be associated with fewer dropouts; however, we were unable to conduct meta-analysis. Third, we found that CRT did not confer advantage over control treatments for increasing BMI and decreasing eating-disorder symptoms. Fourth, end-of-treatment outcomes for depressive symptoms were similar for CRT and control conditions. We were unable to conduct meta-analysis for obsessive-compulsive and anxious symptoms and perfectionism, as fewer than three studies reported outcomes for each of these measures.
4.1. Limitations and future directions
There are certain limitations of past research that should be considered alongside findings of this preliminary systematic review and meta-analysis. One limitation of the current literature is that the quality of some of the RCTs included in this systematic review was lower (i.e., fair versus good ratings). Two common reasons that RCTs were assigned a fair versus good rating were that the RCT was underpowered (due to its stated pilot nature). Moreover, most current RCTs of CRT for AN did not use intention-to-treat analyses, which include all participants in analyses, regardless of how much (if any) treatment received and reduce bias in results. Thus, future RCTs of CRT for AN should take care to ensure adequate power by increasing sample size and using intention-to-treat analyses. Another limitation is increased heterogeneity due to variability in control treatments, which varied from treatment-as-usual, non-specific neurocognitive therapy, cognitive behavioral therapy, to art therapy. Similarly, there was variability in treatment length and length of follow-up across studies. Future RCTs should implement long-term follow-ups so that the field can better understand the long-term effects of CRT for AN. Additionally, the effects of CRT for AN are not well understood in adolescents – despite promising preliminary case studies and case series – and only one RCT included in this meta-analysis was comprised of an adolescent-only sample. Indeed, two RCT protocols of CRT for AN in adolescents have been published (Giombini et al., 2018; Timko, Goulazian, Fitzpatrick, & Rodriguez, 2018) and dissemination of results of these studies into the literature may lead to improved CRT research.
A further limitation of the current CRT for the AN literature is heterogeneity of tasks and measures used to operationalize constructs and, for neuropsychological measures, use of measures that confound constructs. Tasks that confound neuropsychological constructs render identification of specific neuropsychological functions that improve with CRT unclear and difficult. For example, Wildes, Forbes, and Marcus (2014) noted that the Wisconsin Card Sorting Task – a task commonly used to measure set shifting in CRT studies – assesses both set shifting and reversal learning, which have distinct behavioral and neural correlates. Set shifting is associated with neural regions implicated in cognitive control, including the dorsolateral prefrontal cortex and the anterior cingulate cortex (Bissonette, Powell, & Roesch, 2013). Reversal learning is the ability to modulate behavior to obtain a reward when reward contingencies (rules) change. Reversal learning is associated with reward-based neural circuits, including dopaminergic pathways in the mesolimbic area (Izquierdo, Brigman, Radke, Rudebeck, & Holmes, 2017). Future studies of CRT could benefit from using precise measures that do not confound constructs, such as the computerized cued task-switching paradigm used by Brockmeyer et al. (2014) or, as suggested by Wildes et al. (2014), the Cambridge Neuropsychological Test Automated Battery.
A final limitation is that studies of CRT have not tested differences in outcomes between AN-BP and AN-R subtypes. Wu et al. (2014)’s meta-analytic results indicated that compared to healthy controls, set shifting was impaired in AN-R (Hedge’s g = −0.51), but not in AN-BP (Hedge’s g = −0.18). Functional magnetic resonance imaging research showed that persons with AN-BP had similar patterns of brain activation to healthy controls during a set-shifting task, yet persons with AN-R showed increased activity in the precuneus and insula (brain regions implicated in self-awareness) (Van Autreve, Aeken, Heeringen, Vancayseele, & Vervaet, 2016). Future research is needed to understand whether CRT in its current form is helpful for those with AN-BP. Results from such research may be leveraged to inform a CRT tailored to improve neuropsychological function and outcomes for AN-BP.
4.2. Conclusions
In this preliminary systematic review and meta-analysis of RCTs of CRT for AN, we found that CRT for AN conferred a small but statistically non-significant effect for central coherence at end-of-treatment relative to control treatments. We were unable to determine the effect of CRT on set shifting due to variability in set-shifting measurement across studies. There is preliminary evidence that CRT promotes retention in treatment, though more research is needed. CRT did not improve eating-disorder-related symptoms or other psychological symptoms. Recommendations for future RCTs of CRT for AN include use of precise measures to assess constructs (particularly for set shifting), standardization of control treatments, longitudinal follow-up, and investigating whether CRT in its current form affects change similarly across AN subtypes. Additionally, future research could explore how varied intensity and dose of exercises used to strengthen central coherence effect outcomes. Moreover, adequately powered RCTs of CRT for AN that utilize intention-to-treat analyses are needed to determine if CRT improves outcomes for the deadliest form of mental illness.
Role of funding sources
Funding for this study was provided by the 2017 University of Kansas Graduate Research Summer Scholarship (awarded to Dr. Hagan). The University of Kansas had no role in the study design, literature search, data analysis, data interpretation, manuscript preparation/writing, nor the decision to submit this manuscript for publication.
Footnotes
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eatbeh.2020.101391.
References
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Begg CB, & Mazumdar M (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 1088–1101. [PubMed] [Google Scholar]
- Bissonette GB, Powell EM, & Roesch MR (2013). Neural structures underlying set-shifting: Roles of medial prefrontal cortex and anterior cingulate cortex. Behavioural Brain Research, 250, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, & Rothstein HR (2009). Introduction to meta-analysis. Wiley. [Google Scholar]
- Brockmeyer T, Ingenerf K, Walther S, Wild B, Hartmann M, Herzog W, … Friederich HC (2014). Training cognitive flexibility in patients with anorexia nervosa: A pilot randomized controlled trial of cognitive remediation therapy. The International Journal of Eating Disorders, 47(1), 24–31. 10.1002/eat.22206. [DOI] [PubMed] [Google Scholar]
- Brockmeyer T, Walther S, Ingenerf K, Wild B, Hartmann M, Weisbrod M, … Friederich HC (2016). Brain effects of computer-assisted cognitive remediation therapy in anorexia nervosa: A pilot fMRI study. Psychiatry Research, 249, 52–56. 10.1016/j.pscychresns.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Academic Press. [Google Scholar]
- Dahlgren CL, & Ro O (2014). A systematic review of cognitive remediation therapy for anorexia nervosa—Development, current state and implications for future research and clinical practice. Journal of Eating Disorders, 2(1), 26. 10.1186/s40337-014-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner UN, Dingemans AE, & Steinglass J (2015). Cognitive remediation therapy for eating disorders. Current Opinion in Psychiatry, 28(6), 468–472. 10.1097/yco.0000000000000192. [DOI] [PubMed] [Google Scholar]
- Danner UN, Sanders N, Smeets PAM, van Meer F, Adan RAH, Hoek HW, & van Elburg AA (2012). Neuropsychological weaknesses in anorexia nervosa: Set-shifting, central coherence, and decision making in currently ill and recovered women. International Journal of Eating Disorders, 45(5), 685–694. 10.1002/eat.22007. [DOI] [PubMed] [Google Scholar]
- Dejong H, Broadbent H, & Schmidt U (2012). A systematic review of dropout from treatment in outpatients with anorexia nervosa. The International Journal of Eating Disorders, 45(5), 635–647. 10.1002/eat.20956. [DOI] [PubMed] [Google Scholar]
- Dingemans AE, Danner UN, Donker JM, Aardoom JJ, van Meer F, Tobias K, … van Furth EF (2014). The effectiveness of cognitive remediation therapy in patients with a severe or enduring eating disorder: A randomized controlled trial. Psychotherapy and Psychosomatics, 83(1), 29–36. 10.1159/000355240. [DOI] [PubMed] [Google Scholar]
- Duval S, & Tweedie R (2000). A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association, 95(449), 89–98. [Google Scholar]
- Fichter MM, & Quadflieg N (2016). Mortality in eating disorders-results of a large prospective clinical longitudinal study. International Journal of Eating Disorders, 49(4), 391–401. 10.1002/eat.22501. [DOI] [PubMed] [Google Scholar]
- Giombini L, Nesbitt S, Cox H, Foxall A, Sharia T, Easter A, & Tchanturia K (2018). Cognitive remediation therapy (CRT) in a specialist inpatient eating disorder service for children and adolescents: CAN-CRT study protocol for a pilot randomised controlled trial. European Eating Disorders Review, 26(5), 438–446. [DOI] [PubMed] [Google Scholar]
- Herbrich L, Kappel V, van Noort BM, & Winter S (2018). Differences in set-shifting and central coherence across anorexia nervosa subtypes in children and adolescents. European Eating Disorders Review. [DOI] [PubMed] [Google Scholar]
- Herbrich L, Noort B, Pfeiffer E, Lehmkuhl U, Winter S, & Kappel V (2017). Follow-up assessment of cognitive remediation therapy in adolescent anorexia nervosa: A pilot study. European Eating Disorders Review, 25(2), 104–113. [DOI] [PubMed] [Google Scholar]
- Holliday J, Tchanturia K, Landau S, Collier D, & Treasure J (2015). Is impaired set-shifting an endophenotype of anorexia nervosa? The American Journal of Psychiatry, 162(12), 2269–2275. 10.1176/appi.ajp.162.12.2269. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, & Kessler RC (2007). The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry, 61(3), 348–358. 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, & Holmes A (2017). The neural basis of reversal learning: An updated perspective. Neuroscience, 345, 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarascio AS, Manasse SM, Espel HM, Kerrigan SG, & Forman EM (2015). Could training executive function improve treatment outcomes for eating disorders? Appetite, 90, 187–193. 10.1016/j.appet.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakam N, Raoult C, Collier D, & Treasure J (2015). Set shifting and central coherence as neurocognitive endophenotypes in eating disorders: A preliminary investigation in twins. The World Journal of Biological Psychiatry, 14(6), 464–475. 10.3109/15622975.2012.665478. [DOI] [PubMed] [Google Scholar]
- Lang K, Roberts M, Harrison A, Lopez C, Goddard E, Khondoker M, … Tchanturia K (2016). Central coherence in eating disorders: A synthesis of studies using the rey osterrieth complex figure test. PLoS One, 11(11), e0165467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang K, Stahl D, Espie J, Treasure J, & Tchanturia K (2015). Set shifting in children and adolescents with anorexia nervosa: An exploratory systematic review and meta-analysis. International Journal of Eating Disorders, 47(4), 394–399. https://doi.org/10.1002/erv.2172 doi:10.1002/eat.22235. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Wooderson S, Mataix-Cols D, David R, Speckens A, & Phillips ML (2006). Decision making and set shifting impairments are associated with distinct symptom dimensions in obsessive-compulsive disorder. Neuropsychology, 20(4), 409. [DOI] [PubMed] [Google Scholar]
- Lock J, Agras WS, Fitzpatrick KK, Bryson SW, Jo B, & Tchanturia K (2013). Is outpatient cognitive remediation therapy feasible to use in randomized clinical trials for anorexia nervosa? International Journal of Eating Disorders, 46(6), 567–575. 10.1002/eat.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J, Fitzpatrick KK, Agras WS, Weinbach N, & Jo B (2018). Feasibility study combining art therapy or cognitive remediation therapy with family-based treatment for adolescent anorexia nervosa. European Eating Disorders Review, 26(1), 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, & Howerter A (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ, 339. 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health (2014). Study quality assessment tools. National Heart, Lung, and Blood Institute (NHLBI)https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. [Google Scholar]
- van Passel B, Danner UN, Dingemans AE, Aarts E, Sternheim LC, Becker ES, … Cath DC (2020). Cognitive remediation therapy does not enhance treatment effect in obsessive-compulsive disorder and anorexia nervosa: A randomized controlled trial. Psychotherapy and Psychosomatics, 1–14. 10.1159/000505733. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing.
- Roberts ME, Tchanturia K, & Treasure JL (2013). Is attention to detail a similarly strong candidate endophenotype for anorexia nervosa and bulimia nervosa? The World Journal of Biological Psychiatry, 14(6), 452–463. 10.3109/15622975.2011.639804. [DOI] [PubMed] [Google Scholar]
- Rose M, Frampton IJ, & Lask B (2015). Central coherence, organizational strategy, and visuospatial memory in children and adolescents with anorexia nervosa. Applied Neuropsychology: Child, 3(4), 284–296. 10.1080/21622965.2013.775064. [DOI] [PubMed] [Google Scholar]
- Rosenthal R (1979). The file drawer problem and tolerance for null results. Psychological Bulletin, 86(3), 638. [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139(1), 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproch LE, Anderson KP, Sherman MF, Crawford SF, & Brandt HA (2019). A randomized controlled trial of group cognitive remediation therapy for anorexia nervosa: Effects on set-shifting tasks for inpatient adults and adolescents. International Journal of Eating Disorders, 52(9), 1004–1014. 10.1002/eat.23143. [DOI] [PubMed] [Google Scholar]
- Steinglass JE, Albano AM, Simpson HB, Wang Y, Zou J, Attia E, & Walsh BT (2014). Confronting fear using exposure and response prevention for anorexia nervosa: A randomized controlled pilot study. The International Journal of Eating Disorders, 47(2), 174–180. 10.1002/eat.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson SA, Crow SJ, Grange DL, Swendsen J, & Merikangas KR (2011). Prevalence and correlates of eating disorders in adolescents: Results from the National Comorbidity Survey Replication Adolescent Supplement. Archives of General Psychiatry, 68(7), 714–723. 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchanturia K, Davies H, Reeder C, & Wykes T (2010). Cognitive remediation therapy for anorexia nervosa. King’s College London: University of London. [Google Scholar]
- Tchanturia K, Davies H, Roberts M, Harrison A, Nakazato M, Schmidt U, … Morris R (2012). Poor cognitive flexibility in eating disorders: Examining the evidence using the Wisconsin Card Sorting Task. PLoS One, 7(1), e28331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchanturia K, Giombini L, Leppanen J, & Kinnaird E (2017). Evidence for cognitive remediation therapy in young people with anorexia nervosa: Systematic review and meta-analysis of the literature. European Eating Disorders Review, 25(4), 227–236. [DOI] [PubMed] [Google Scholar]
- Tchanturia K, Harrison A, Davies H, Roberts M, Oldershaw A, Nakazato M, … Treasure J (2011). Cognitive flexibility and clinical severity in eating disorders. PLoS One, 6(6), e20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchanturia K, Lloyd S, & Lang K (2013). Cognitive remediation therapy for anorexia nervosa: Current evidence and future research directions. The International Journal of Eating Disorders, 46(5), 492–495. 10.1002/eat.22106. [DOI] [PubMed] [Google Scholar]
- Tchanturia K, Lounes N, & Holttum S (2014). Cognitive remediation in anorexia nervosa and related conditions: A systematic review. European Eating Disorders Review, 22(6), 454–462. 10.1002/erv.2326. [DOI] [PubMed] [Google Scholar]
- Tenconi E, Santonastaso P, Degortes D, Bosello R, Titton F, Mapelli D, & Favaro A (2010). Set-shifting abilities, central coherence, and handedness in anorexia nervosa patients, their unaffected siblings and healthy controls: Exploring putative endophenotypes. The World Journal of Biological Psychiatry, 11(6), 813–823. 10.3109/15622975.2010.483250. [DOI] [PubMed] [Google Scholar]
- Timko CA, Goulazian TJ, Fitzpatrick KK, & Rodriguez D (2018). Cognitive remediation therapy (CRT) as a pretreatment intervention for adolescents with anorexia nervosa during medical hospitalization: A pilot randomized controlled trial protocol. Pilot and Feasibility Studies, 4(1), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Autreve S, De Baene W, Baeken C, Heeringen K, Vancayseele N, & Vervaet M (2016). Differential neural correlates of set-shifting in the bingeing–purging and restrictive subtypes of anorexia nervosa: An fmri study. European Eating Disorders Review. doi: 10.1002/erv.2437. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W (2010). Conducting meta-analyses in R with the meta for package. Journal of Statistical Software, 36(3), 1–48. [Google Scholar]
- Westwood H, Stahl D, Mandy W, & Tchanturia K (2016). The set-shifting profiles of anorexia nervosa and autism spectrum disorder using the Wisconsin Card Sorting Test: A systematic review and meta-analysis. Psychological Medicine, 46(9), 1809–1827. [DOI] [PubMed] [Google Scholar]
- Wildes JE, Forbes EE, & Marcus MD (2014). Advancing research on cognitive flexibility in eating disorders: The importance of distinguishing attentional set-shifting and reversal learning. International Journal of Eating Disorders, 47(3), 227–230. [DOI] [PubMed] [Google Scholar]
- Wu M, Brockmeyer T, Hartmann M, Skunde M, Herzog W, & Friederich HC (2014). Set-shifting ability across the spectrum of eating disorders and in overweight and obesity: A systematic review and meta-analysis. Psychological Medicine, 44(16), 3365–3385. https://doi.org/10.1002/erv.2172 10.1017/S0033291714000294. [DOI] [PubMed] [Google Scholar]
- Zipfel S, Giel KE, Bulik CM, Hay P, & Schmidt U (2015). Anorexia nervosa: Aetiology, assessment, and treatment. The Lancet Psychiatry, 2(12), 1099–1111. 10.1016/S2215-0366(15)00356-9. [DOI] [PubMed] [Google Scholar]


