Abstract
Objective
Patients with rheumatoid arthritis (RA) commonly demonstrate disordered pain processing, associated with high pain sensitization. Pain sensitization is often assessed using quantitative sensory testing (QST), which is burdensome to patients. The self-administered fibromyalgia survey questionnaire (FSQ) has been proposed as a low-burden, surrogate measure of central pain sensitization. We examined the correlation between FSQ and QST in patients with active RA.
Methods
Participants in the Central Pain in Rheumatoid Arthritis (CPIRA) cohort underwent FSQ and QST evaluation at enrollment. QST measures included pressure pain threshold (PPT) at the thumb, trapezius, wrist and knee; temporal summation (TS) at the wrist and arm; and conditioned pain modulation (CPM). Partial Spearman correlation between FSQ and each QST measure was assessed, adjusted for demographic factors, study site, disease characteristics, and pain catastrophizing. Sensitivity analyses included a) stratified analysis by sex and b) evaluation of how each component of FSQ associates with the QST measures.
Results
Among 285 participants with active RA, FSQ was weakly but statistically significantly correlated with PPT (r = −0.21 to −0.31) and TS (r = 0.13 to 0.15) at all sites in unadjusted analyses. After adjustment, statistically significant correlations persisted for PPT at all sites except the thumb, and for TS at the wrist. Sensitivity analyses did not identify differences in association based on sex or with individual FSQ components.
Conclusion
FSQ and QST were correlated among participants with active RA, but the strength of association was weak. QST and FSQ are not interchangeable measures of pain sensitization.
Keywords: Rheumatoid arthritis, Fibromyalgia, Central nervous system sensitization, Pain measurement
Introduction
Patients with rheumatoid arthritis (RA) frequently experience heightened sensitivity to pain in a widespread distribution, suggestive of abnormalities in peripheral and central pain processing.1 Abnormalities in central pain processing, termed central pain sensitization, are associated with worse functional outcomes and reduced response to disease-modifying treatment.2–4 In the research context, quantitative sensory testing (QST) assessments of allodynia, temporal summation, and conditioned pain modulation are often considered proxies for pain sensitization.5 While QST has been used to characterize pain sensitization in RA,1,6,7 it poses a substantial burden to patients and assessors, as it is time-consuming and requires a trained operator to administer the tests in a controlled setting.
The self-administered fibromyalgia survey questionnaire (FSQ) has been proposed as a low-burden surrogate for QST assessment.8–10 The FSQ assesses widespread pain and somatic symptoms like fatigue, poor sleep and cognitive difficulty.11 However, there are limited data evaluating the relationship between the clinical symptoms measured using FSQ and the neurologic abnormalities measured by QST. Previous studies in non-inflammatory pain conditions, and in patients with well-controlled RA, suggest a low-moderate correlation between these measures (|r| = 0.27 – 0.44), which may be limited to certain subpopulations (i.e. female patients).8,12,13 Further, widespread pain and somatic symptoms may be driven by other processes beyond pain sensitization, and therefore may reflect distinct domains contributing to the pain experience in RA.
To our knowledge, no data exist regarding the relationship between pain sensitization (assessed by QST) and the patient-reported symptoms of pain sensitization (assessed by FSQ) among patients with active RA. The assessment of pain sensitization is particularly important in this subgroup because pain sensitization may inflate composite disease activity measures, making it seem as if some patients have active inflammatory disease when they do not.1 Identification of pain sensitization in these patients could impact treatment decisions about escalating disease modifying anti-rheumatic drug (DMARD) therapy and may inform alternative management approaches to target chronic pain.14,15 To address this gap in knowledge, we aim to examine the correlation between FSQ and QST in a cohort of participants who were starting or intensifying DMARD treatment for active RA.
Methods
Study population
Central Pain in Rheumatoid Arthritis (CPIRA) is comprised of participants enrolled prospectively with active RA who are changing DMARD therapy due to uncontrolled disease activity, determined by their treating rheumatologist.1 Between January 2014 and July 2017, 295 participants at five U.S. academic medical centers enrolled in CPIRA. Exclusion criteria included: a) failure to meet 2010 American College of Rheumatology/European League Against Rheumatism criteria for RA diagnosis; b) a coexisting diagnosis of any other systemic autoimmune disease, severe Raynaud phenomenon, peripheral vascular disease, or peripheral neuropathy, and c) use of chronic opiates, changing dose of centrally acting pain medications in the past three months, or prednisone ≥10mg/day. This study complies with the Declaration of Helsinki. The institutional review boards at each site (Boston University H-32334, Brigham and Women’s Hospital 2013P000951, Johns Hopkins University NA_00085841, Northwestern University STU00206528, University of Michigan HUM00081289) approved the study. Informed consent was obtained from all subjects prior to enrollment.
We used baseline data from CPIRA for this study. Analyses were restricted to 285 participants with data in at least one of the seven QST measures as well as complete data in FSQ and covariates. Ten participants were excluded due to missing data in covariates (i.e. race or C-reactive protein [CRP]).
Assessment of clinical variables
Variables including age, sex, RA disease duration, RA serostatus, body mass index (BMI), and enrollment site were assessed at the baseline study visit. Height and weight were measured to calculate BMI [weight (kg)]/[height (m2)]. Presence of rheumatoid factor (>14 IU/ml) and cyclic citrullinated peptide antibody (>17 U) was assessed through serum analysis, performed at a central laboratory. Patient-reported questionnaires provided demographic and RA disease duration information. Pain catastrophizing was assessed using the Pain Catastrophizing Scale.16 An assessment of clinical pain intensity was captured using a 0 – 10 numeric rating scale of overall pain.
Assessment of RA disease activity and inflammation
RA disease activity was assessed through measurement of CRP and calculation of the Clinical Disease Activity Index (CDAI) which includes tender joint count (TJC), swollen joint count (SJC), patient global assessment and assessor global assessment.17,18 Trained study staff members performed standard 28-joint counts and assessor global assessments. Responses for patient global assessment were measured on a 100-point scale.
Assessment of pain sensitization
Quantitative sensory testing (QST)
We evaluated three baseline QST measures: pressure pain threshold (PPT), temporal summation (TS), and conditioned pain modulation (CPM). We performed interrater reliability assessments for both PPT and TS; intraclass correlation coefficients (ICC) for both measures ranged from 0.71 to 0.90, which is considered good to excellent.19 The ICC for CPM was 0.45, which is considered fair.
PPT, which assesses hyperalgesia, was measured using a Wagner Force 10 FDX algometer with a 1cm2 probe placed at the bilateral trapezius muscles, wrists, knees and thumbnails. PPTs assess overall sensitivity to pain. Low PPTs at joint sites represent a combination of peripheral and central mechanisms of sensitization, whereas low PPTs at non-joint sites indicate central mechanisms of sensitization. Pressure was increased by 0.5 kilogram force (kgf) per second until the participant reported pain at each assessment site. PPT was defined as the pressure at which the participant reported pain with lower values suggesting more sensitivity.
TS assesses amplification of painful inputs in response to repeated stimuli and is considered a specific measure of pain facilitation. We measured TS using 6 weighted probes (8 – 256 millinewton (mN)) placed on the participant’s wrist and forearm. Probe weight was increased until the participant reported a pain score of 30 – 40/100, or the heaviest weight was reached. The probe registering a 30 – 40/100 pain score was then tapped against the wrist and dorsal forearm 10 times, with 0.5 seconds for each tap and 1 second between taps. After taps 1, 5, and 10, the participant rated pain on a 0 – 100 scale. We subtracted the participant’s pain score at tap 1 from the score at tap 10, then divided by 10 to provide a TS score from 0 – 10. Higher TS scores represent higher pain amplification.
CPM is believed to be a measure of descending inhibitory pain modulation. The conditioning stimulus engages the descending (inhibitory) analgesic pathway, while the test stimulus assesses the effect of this inhibition. In an appropriately functioning pathway, the inhibition results in a lessened pain response to the second stimulus. Our conditioning stimulus was a cold water bath at 5 – 7 °C, into which participants placed their right hand. We assessed PPT at the left trapezius muscle at two time points: before the cold water bath, and 20 seconds after initiation of the cold water bath. CPM was reported as the ratio of PPT at the second time point to PPT at the first time point, with lower values suggesting inefficient descending analgesic inhibition.
Assessment of fibromyalgia severity
All participants completed the Fibromyalgia Survey Questionnaire (FSQ), which is based on the 2010/2011 ACR Preliminary Diagnostic Criteria for Fibromyagia.11 This instrument is composed of a widespread pain index (WPI) which assesses self-reported pain at 19 pre-specified sites, and a 0 – 12 symptom severity scale (SSS). SSS measures the sum of self-reported fatigue, nonrestorative sleep, and cognitive symptoms on a 1 – 3 point Likert scale and the presence of headache, abdominal pain, and depression assessed as binary variables. This questionnaire has been previously used to measure severity of fibromyalgia, the prototypical centralized pain condition, in the general population as well as in disease-specific cohorts, including the CPIRA cohort.1,20 Previous studies have suggested that a FSQ score ≥ 12 be considered the threshold for diagnosis of fibromyalgia.21 However, recent studies suggest that the concept of fibromyalgia is more appropriately viewed as a continuum rather than a discrete entity.22–24
Statistical analysis
Descriptive statistics were used to evaluate demographic and clinical data. The primary analysis evaluated Spearman correlations between each QST measure (PPT, TS, CPM) and overall FSQ score. Partial correlations were adjusted for age, sex, race, BMI, study site, seropositivity, CRP, SJC, and pain catastrophizing. We performed a sex-stratified sensitivity analysis to examine the possibility suggested from literature that sex may modify the correlation between FSQ and QST measures.8 A second sensitivity analysis evaluated the correlation between QST and each FSQ component: WPI which assesses the extent of pain, and SSS which assesses the severity of comorbid non-pain symptoms. We did not adjust for multiple testing because the objective of this study was only to describe the relationship between various QST measures and FSQ, as opposed to confirming a specific hypothesis about the relationship between QST measures in general and FSQ.
Results
We describe the characteristics of the 285 participants included in this study in Table 1. Mean age was 54.70 (standard deviation (SD) 13.74) years, 82.1% were female, 74.7% were Caucasian, and 78.3% were seropositive. Mean (SD) baseline CDAI score was 24.56 (14.25), representing high RA disease activity.18 Mean (SD) baseline FSQ score was 11.22 (6.08) out of 31 total possible, with 32% of the study population meeting the American College of Rheumatology 2011 Modified Diagnostic Criteria for Fibromyalgia.25
Table 1:
Baseline characteristics (N = 285)*
| Variable | Mean (SD) or % |
|---|---|
| Age (years) | 54.70 (13.74) |
| Female | 82.1% |
| Caucasian | 74.7% |
| BMI (kg/m2) | 28.58 (6.62) |
| Seropositive, % | 78.3% |
| RA duration (years) | 9.97 (11.88) |
| Biologic DMARD use | 24.9% |
| Site, % of enrolled | |
| Brigham/MGH | 51.9% |
| Boston University | 10.2% |
| Michigan University | 19.3% |
| Johns Hopkins | 18.6% |
| Pain Catastrophizing Scale | 18.67 (13.56) |
| Pain Intensity (NRS 0–10) | 5.25 (2.29) |
| CDAI | 24.56 (14.25) |
| Patient global | 4.23 (2.44) |
| Physician global | 3.68 (2.28) |
| Swollen joint count | 5.26 (5.25) |
| Tender joint count | 10.89 (8.60) |
| CRP (mg/L) | 8.15 (12.45) |
| FSQ score | 11.22 (6.08) |
| WPI score | 5.95 (4.32) |
| SSS score | 5.27 (2.65) |
| QST | |
| Thumbnail PPT (kgf) | 3.67 (1.95) |
| Trapezius PPT (kgf) | 2.93 (1.65) |
| Wrist PPT (kgf) | 2.93 (1.59) |
| Knee PPT (kgf) | 5.41 (2.84) |
| Wrist TS | 13.06 (14.78) |
| Arm TS | 12.54 (14.63) |
| CPM | 1.40 (0.35) |
CDAI n=243; patient global n=243; thumbnail PPT, trapezius PPT, wrist PPT n=284; knee PPT n=283; wrist TS n=282; arm TS n=281; CPM n=279.
BMI body mass index; RA rheumatoid arthritis; DMARD disease-modifying anti-rheumatic drug; MGH Massachusetts General Hospital, NRS numeric rating scale; CDAI clinical disease activity index; CRP C-reactive protein; FSQ fibromyalgia survey questionnaire; WPI widespread pain index; SSS symptom severity score; QST quantitative sensory index; PPT pressure pain threshold; kgf kilogram force; TS temporal summation; CPM conditioned pain modulation
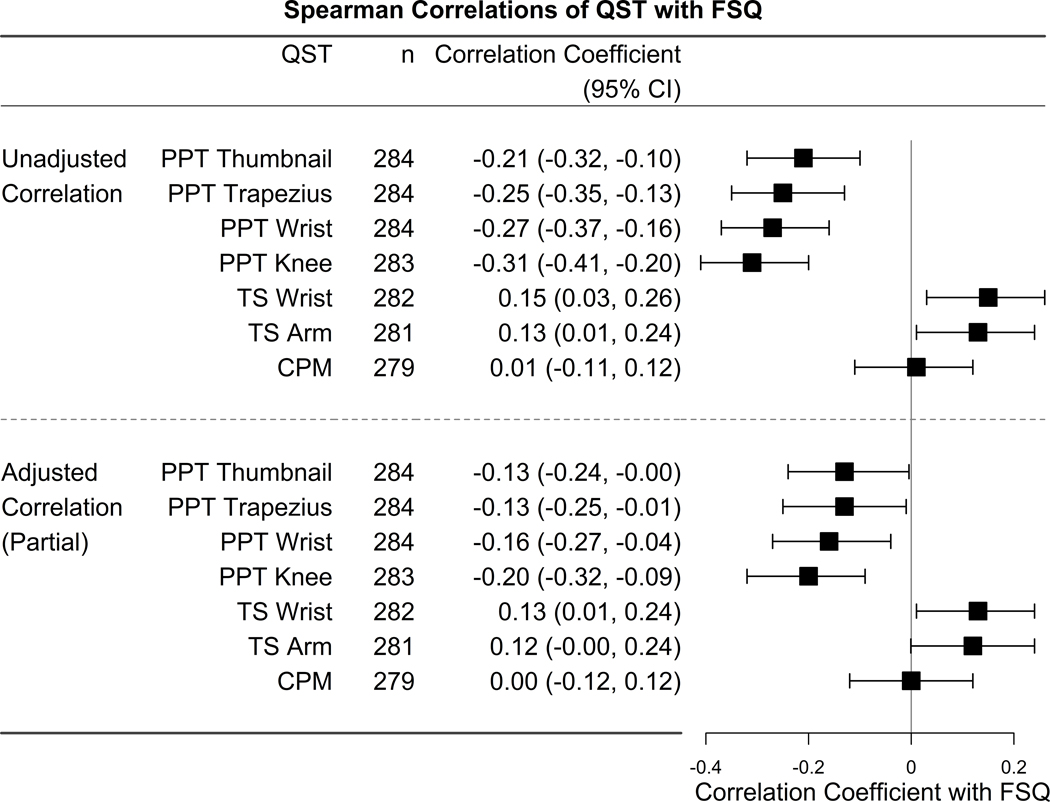
In unadjusted analyses, FSQ had a statistically significant, but weak inverse correlation between FSQ and PPT at all sites, including the thumb (r = −0.21 (95% confidence interval [CI] −0.32, −0.10)), trapezius (r = −0.25 (95% CI −0.35, −0.13)), wrist (r = −0.27 (95% CI −0.37, −0.16)), and knee (r = −0.31 (95% CI −0.41, −0.20)). Negative correlation coefficient values indicate that increasing FSQ score is associated with a decrease in pain threshold (measured by PPT), representing higher pain sensitization. Weak correlations were also found between FSQ and TS at the wrist (r = 0.15 (95% CI 0.03, 0.26)), and arm (r = 0.13 (95% CI 0.01, 0.24)). Adjusting for covariates reduced the magnitude of these correlations, but correlations between FSQ and PPT at the trapezius (r = −0.13 (95% CI −0.25, −0.01)), wrist (r = −0.16 (95% CI −0.27, −0.04)), and knee (r = −0.20 (95% CI −0.32, −0.09)), as well as TS at the wrist (r = 0.13 (95% CI 0.01, 0.24)) remained statistically significant. No significant correlation was found between FSQ and CPM (Figure 1).
Figure 1:
Unadjusted and adjusted correlations between QST measures and FSQ
Adjusted for age, sex, race, BMI, seropositivity, swollen joint count, CRP, pain catastrophizing, and site.
QST quantitative sensory testing; FSQ fibromyalgia survey questionnaire; PPT pressure pain threshold; TS temporal summation; CPM conditioned pain modulation
To examine the previously reported effect of sex on the relationship between FSQ and QST, we examined Spearman correlations of FSQ and QST by sex.8 Individually, correlations for men and women were similar in magnitude and statistical significance to the overall analysis. The largest difference occurred in the correlations of FSQ with PPT of the trapezius, but no meaningful pattern related to sex was observed (Table 2).
Table 2:
Unadjusted correlations between QST measures and FSQ after stratification by sex
| Men | Women | |
|---|---|---|
| QST | Correlation Coefficient (95% Confidence Limits) | Correlation Coefficient (95% Confidence Limits) |
| PPT thumb | −0.15 (−0.41, 0.13) | −0.22 (−0.34, −0.09) |
| PPT trapezius | −0.36 (−0.57, −0.09) | −0.20 (−0.32, −0.08) |
| PPT wrist | −0.23 (−0.48, 0.05) | −0.26 (−0.38, −0.14) |
| PPT knee | −0.31 (−0.54, −0.03) | −0.30 (−0.41, −0.18) |
| TS wrist | 0.08 (−0.20, 0.35) | 0.15 (0.02, 0.27) |
| TS arm | 0.07 (−0.21, 0.34) | 0.14 (0.01, 0.27) |
| CPM | 0.00 (−0.28, 0.28) | −0.01 (−0.14, 0.12) |
QST quantitative sensory testing; PPT pressure pain threshold; TS temporal summation; CPM conditioned pain modulation
To evaluate for differences in the strength of relationship between QST and each component of the FSQ, we examined how each QST measure correlated with WPI and SSS (Table 3). For PPT, the magnitude of the observed correlations for SSS (range r = −0.25 to −0.31) was similar to those seen in the primary analysis, while those for WPI were lower than those seen in the primary analysis (range r = −0.13 to −0.24). Weak correlations were found between SSS and TS of wrist (r = 0.16 (95% CI 0.04, 0.27)) and TS of the arm (r = 0.13 (95% CI 0.01, 0.24)) while no significant correlations were found between WPI and TS. No significant correlations were found with either FSQ component and CPM.
Table 3:
Unadjusted correlations between QST measures and the individual components of FSQ, the WPI and SSS
| WPI | SSS | |
|---|---|---|
| QST | Correlation Coefficient (95% Confidence Limits) | Correlation Coefficient (95% Confidence Limits) |
| PPT thumb | −0.13 (−0.24, −0.01) | −0.26 (−0.36, −0.14) |
| PPT trapezius | −0.18 (−0.29, −0.07) | −0.25 (−0.36, −0.14) |
| PPT wrist | −0.20 (−0.31, −0.08) | −0.29 (−0.39, −0.18) |
| PPT knee | −0.24 (−0.34, −0.12) | −0.31 (−0.41, −0.20) |
| TS wrist | 0.12 (0.00, 0.23) | 0.16 (0.04, 0.27) |
| TS arm | 0.10 (−0.02, 0.22) | 0.13 (0.01, 0.24) |
| CPM | 0.01 (−0.11, 0.13) | 0.01 (−0.11, 0.13) |
QST quantitative sensory testing; PPT pressure pain threshold; TS temporal summation; CPM conditioned pain modulation
Discussion
In a cohort of patients with RA escalating DMARD therapy due to uncontrolled disease activity, FSQ was weakly correlated with PPT and TS, and not correlated with CPM. These relationships did not differ by sex. In a sensitivity analysis, the correlations between both components of FSQ (SSS and WPI) and QST measures were minimally different. These results indicate that, among patients with active RA, the patient-reported symptoms measured by FSQ are not strongly associated with quantitative measurements of pain sensitization assessed by QST. Thus, while the FSQ may reflect severity of fibromyalgia in terms of symptoms, it may not provide additional insights into altered nociceptive signal processing.
The relationship between patient-reported outcome measures like FSQ, and quantitative assessments like QST, may be influenced by a patient’s underlying disease state and associated type of pain pathology. Prior work has shown moderate correlations between PPT and self-reported pain measures (i.e. McGill Pain Questionnaire) among patients with non-inflammatory conditions like fibromyalgia and chronic fatigue syndrome.26,27 In contrast, reported widespread pain was not associated with PPT in a study of patients with knee osteoarthritis.28 Our work shows that patients with active RA, a highly inflammatory condition, demonstrate weak correlations between FSQ and QST. One explanation for this finding may be that the FSQ, in addition to detecting widespread muscle pain typical of central pain sensitization, is capturing inflammatory joint pain in patients with active RA. This explanation is supported by our group’s previous finding that swollen joint count and CRP increase with increasing FSQ.29
In our secondary analysis, we did not see differences in the correlation between QST and FSQ when stratified by sex. This is in contrast to a prior study of patients with knee osteoarthritis, where there was a strong correlation between FSQ and PPT among female patients, but no correlation among male patients.8 The authors hypothesized that this finding may be related to sex differences in pain characteristics because females in their study had higher FSQ scores, higher pain hypersensitivity measured by PPT, as well as higher rates of depression, anxiety, and pain catastrophizing. While our analysis is limited by the small percentage of men (17.9%), prior work has revealed mixed results regarding the influence of sex on experimental pain models.30 It is also possible that the role of sex as a modifier of the relationship between FSQ and QST depends on other factors, such as disease type (e.g., osteoarthritis vs. RA).
We also considered the hypothesis that the separate components of the FSQ may be differentially associated with QST measures. The SSS-component of the FSQ assesses symptoms (fatigue, waking unrefreshed, cognitive symptoms, headaches, lower abdominal pain, depression), which are a part of the syndrome of fibromyalgia but may not be directly related to pain sensitivity and may also be due to other causes. In contrast, the WPI-component of the FSQ focuses specifically on pain distribution. Thus, we performed a sensitivity analysis to separately examine associations between QST and the two sub-components of the FSQ (WPI and SSS). However, the strength of the correlations between QST and each component of the FSQ were not meaningfully different (Table 3).
Our study has notable strengths. To our knowledge, this is the first study to evaluate the relationship between examiner-derived and patient-reported measurements of pain sensitization in patients with an active inflammatory condition. Patients with co-existing inflammatory pain have historically been under-studied in pain research,31 despite the high prevalence and well-documented morbidity caused by disorders of central pain sensitization in this population.32,33 This study uses data from CPIRA, one of the only cohorts to systematically collect examiner-derived and patient-reported pain measures in a population with a systemic inflammatory condition.
There are several limitations to our work. First, the study is cross-sectional, and causation cannot be determined from these observational data. While our correlations were statistically significant, they reflect weak to moderate associations. The clinical significance of these associations relies on how well we understand the mechanism of the phenomenon being measured, how well the measures capture that phenomenon, and the similarities and differences between the corelated measures. Second, the goal of this study was to assess the correlation between FSQ and QST-assessed pain sensitization in patients with active RA. Thus, our results do not necessarily extend to patients with well controlled inflammatory arthritis. Understanding how these measures may perform in different patient populations may help researchers in judging the performance of their own studies. Third, although QST is commonly used to assess pain sensitivity and, thereby, yield inferences about peripheral and central pain pathways, there is no gold standard for assessing pain sensitization. Prior work has questioned the use of QST as a reference standard. For example, in patients with low back pain, QST had limited prognostic value for predicting the development of chronic symptoms or treatment failure after surgery.34,35 Both QST and FSQ measures typically correlate only modestly with functional neuroimaging techniques that are considered by some experts to be superior to either measure.36,37 These results do not mean that FSQ or QST do not provide useful information, only that the two measures are capturing different concepts. QST may be most useful when used in conjunction with other measures of pain which may include patient reported questionnaires and neuroimaging. The idea of using different diagnostic tools to capture specific aspects of the fibromyalgia experience is highlighted by the recently proposed Nociplastic-Based Fibromyalgia Features (NFF) tool.38 While its psychometric properties have not yet been established, this tool is interesting in that it de-emphasizes the somatic symptoms included in the 2016 diagnostic criteria in favor of specific features of pain, such as aggravation with physical or emotional stress, pain migration, and the description of pain as excruciating. Fourth, while a cut-off value for characterizing patients with fibromyalgia using FSQ scores has been published, such cutoffs have not yet been established for QST measures. Some have argued that it is, in fact, not appropriate to establish these cut-offs given that pain sensitization is a continuum, as opposed to a condition defined by a clinically meaningful cut-point.
In conclusion, these results do not support the use of FSQ as a proxy measurement for QST among patients with active RA. The difference between our results and results from non-inflammatory pain conditions suggests that population-specific characteristics may impact the performance of these measures. While FSQ and QST each provide valuable information, they do not appear to assess the same construct in this population with high levels of inflammatory pain.
Financial Support
The CPIRA study was funded by the National Institute of Arthritis and Musculoskeletal and Skin at the National Institutes of Health [R01 AR064850]. This work was also supported by the National Institute of Arthritis and Musculoskeletal and Skin at the National Institutes of Health [P30 AR072579 to YCL, LNM and JS and T32 AR07080 to MNM]; the National Center for Advancing Translational Sciences at the National Institutes of Health [5KL2TR002241-04 to BIW] and the National Institutes of Health [P30 AR072571 and K24 AR070892 to TN].
Conflict of Interest
YCL has received consulting fees, speaking fees, and/or honoraria from Eli Lilly (less than $10,000 each), research support from Pfizer, and stock ownership in Cigna. MB has received consulting fees and/or honoraria from Gilead Sciences (< $10,000) and stock ownership in Johnson and Johnson.
DJC has received consultant fees from Pfizer Inc (> $10,000) and Aptinyx, Daiichi Sankyo, Eli Lilly, Intec Pharma, Samumed, Theravance, Tonix, Zynerba (< $10,000), and expert testimony for Eli Lily, Pfizer Inc, Nix Patterson LLP, Williams & Connolly LLP.
Contributor Information
Meriah N. Moore, Clinical Instructor, Internal Medicine/Rheumatology, University of Michigan Medical School.
Beth I. Wallace, Internal Medicine/Rheumatology, University of Michigan Medical School and VA Ann Arbor Healthcare System, Center for Clinical Management Research.
Jing Song, Internal Medicine/Rheumatology, Northwestern University Feinberg School of Medicine.
Lutfiyya N. Muhammad, Preventive Medicine/Biostatistics, Northwestern University Feinberg School of Medicine.
Andrew C. Heisler, Internal Medicine/Rheumatology, Northwestern University Feinberg School of Medicine.
Daniel J. Clauw, Anesthesiology, Internal Medicine/Rheumatology, Psychiatry, University of Michigan Medical School.
Marcy Bolster, Internal Medicine/Rheumatology, Allergy and Immunology, Massachusetts General Hospital.
Wendy Marder, Internal Medicine/Rheumatology, University of Michigan Medical School.
Tuhina Neogi, Internal Medicine/Rheumatology, Boston University School of Medicine, Evans Biomedical Research Center.
Alyssa Wohlfahrt, Tufts University School of Medicine.
Dorothy D. Dunlop, Internal Medicine/Rheumatology, Northwestern University Feinberg School of Medicine.
Yvonne C. Lee, Internal, Medicine/Rheumatology, Preventive Medicine/Biostatistics, Northwestern University Feinberg School of Medicine.
References
- 1.Lee YC, Bingham CO 3rd, Edwards RR, et al. Association between pain sensitization and disease activity in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Care Res (Hoboken) 2018;70:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clauw DJ, Crofford LJ. Chronic widespread pain and fibromyalgia: what we know, and what we need to know. Best Pract Res Clin Rheumatol 2003;17:685–701. [DOI] [PubMed] [Google Scholar]
- 3.Laursen BS, Bajaj P, Olesen AS, Delmar C, Arendt-Nielsen L. Health related quality of life and quantitative pain measurement in females with chronic non-malignant pain. Eur J Pain 2005;9:267–75. [DOI] [PubMed] [Google Scholar]
- 4.Heisler AC, Song J, Muhammad LN, et al. Association of dysregulated central pain processing and response to disease-modifying anti-rheumatic drug therapy in rheumatoid arthritis. Arthritis Rheumatol 2020;72:2017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med 2014;15:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leffler AS, Kosek E, Lerndal T, Nordmark B, Hansson P. Somatosensory perception and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from rheumatoid arthritis. Eur J Pain 2002;6:161–76. [DOI] [PubMed] [Google Scholar]
- 7.Edwards RR, Wasan AD, Bingham CO 3rd, et al. Enhanced reactivity to pain in patients with rheumatoid arthritis. Arthritis Res Ther 2009;11:R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neville SJ, Clauw AD, Moser SE, et al. Association between the 2011 fibromyalgia survey criteria and multisite pain sensitivity in knee osteoarthritis. Clin J Pain 2018;34:909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum 2013;65:3285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoyagi K, He J, Nicol AL, et al. A subgroup of chronic low back pain patients with central sensitization. Clin J Pain 2019;35:869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011;38:1113–22. [DOI] [PubMed] [Google Scholar]
- 12.Moore RL, Clifford AM, Moloney N, Doody C, Smart KM, O’Leary H. The relationship between clinical and quantitative measures of pain sensitization in knee osteoarthritis. Clin J Pain 2020;36:336–43. [DOI] [PubMed] [Google Scholar]
- 13.Joharatnam N, McWilliams DF, Wilson D, Wheeler M, Pande I, Walsh DA. A cross-sectional study of pain sensitivity, disease-activity assessment, mental health, and fibromyalgia status in rheumatoid arthritis. Arthritis Res Ther 2015;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lage-Hansen PR, Chrysidis S, Lage-Hansen M, Hougaard A, Ejstrup L, Amris K. Concomitant fibromyalgia in rheumatoid arthritis is associated with the more frequent use of biological therapy: a cross-sectional study. Scand J Rheumatol 2016;45:45–8. [DOI] [PubMed] [Google Scholar]
- 15.da Chakr RM, Brenol SC, Ranzolin A, et al. Rheumatoid arthritis seems to have DMARD treatment decision influenced by fibromyalgia. Rev Bras Reumatol 2016;57:403–11. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- 17.Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum 1993;36:729–40. [DOI] [PubMed] [Google Scholar]
- 18.England BR, Tiong BK, Bergman MJ, et al. 2019 update of the American College of Rheumatology recommended rheumatoid arthritis disease activity measures. Arthritis Care Res 2019;71:1540–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 1994;6:284–90. [Google Scholar]
- 20.Shresher NM, Mohamed AE, Elshahaly MH. Performance of 2016 revised fibromyalgia diagnostic criteria in patients with rheumatoid arthritis. Rheumatol Int 2019;39:1703–10. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46:319–29. [DOI] [PubMed] [Google Scholar]
- 22.Brummett CM, Janda AM, Schueller CM, et al. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiology 2013;119:1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfe F, Walitt BT, Rasker JJ, Katz RS, Häuser W. The use of polysymptomatic distress categories in the evaluation of fibromyalgia (FM) and FM severity. J Rheumatol 2015;42:1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fibromyalgianess Wolfe F. Arthritis Rheum 2009;61:715–6. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011;38:1113–22. [DOI] [PubMed] [Google Scholar]
- 26.Geisser ME, Gracely RH, Giesecke T, Petzke FW, Williams DA, Clauw DJ. The association between experimental and clinical pain measures among persons with fibromyalgia and chronic fatigue syndrome. Eur J Pain 2007;11:202–7. [DOI] [PubMed] [Google Scholar]
- 27.Melzack R. The short-form McGill Pain Questionnaire. Pain 1987;30:191–7. [DOI] [PubMed] [Google Scholar]
- 28.Neogi T, Frey-Law L, Scholz J, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis 2015;74:682–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace BI, Moore MN, Heisler AC, et al. Fibromyalgianess and glucocorticoid persistence among patients with rheumatoid arthritis. Rheumatology (Oxford) 2021. Jul 22 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 30.Riley JL 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain 1998;74:181–7. [DOI] [PubMed] [Google Scholar]
- 31.Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther 2011;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfe F, Michaud K. Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemographic disadvantage characterize RA patients with fibromyalgia. J Rheumatol 2004;31:695–700. [PubMed] [Google Scholar]
- 33.Duffield SJ, Miller N, Zhao S, Goodson NJ. Concomitant fibromyalgia complicating chronic inflammatory arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2018;57:1453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeResche L, Turner JA, Saunders K, Shortreed SM, Von Korff M. Psychophysical tests as predictors of back pain chronicity in primary care. J Pain 2013;14:1663–70. [DOI] [PubMed] [Google Scholar]
- 35.Müller M, Limacher A, Agten CA, et al. Can quantitative sensory tests predict failed back surgery?: A prospective cohort study. Eur J Anaesthesiol 2019;36:695–704. [DOI] [PubMed] [Google Scholar]
- 36.Schweinhardt P, Kalk N, Wartolowska K, Chessell I, Wordsworth P, Tracey I. Investigation into the neural correlates of emotional augmentation of clinical pain. Neuroimage 2008;40:759–66. [DOI] [PubMed] [Google Scholar]
- 37.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheumatol 2010;62:2545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghavidel-Parsa B, Bidari A, Atrkarroushan Z, Khosousi MJ. Implication of the nociplastic features for clinical diagnosis of fibromyalgia: development of the preliminary nociplastic-based fibromyalgia features (NFF) tool. ACR Open Rheumatol 2021. Dec 22 (Epub ahead of print). [DOI] [PMC free article] [PubMed]