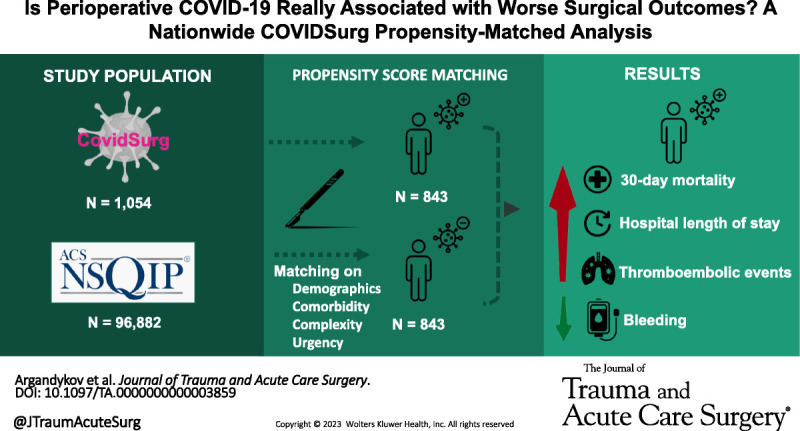
This nationwide analysis shows that patients undergoing surgery with perioperative COVID-19 had higher risks of 30-day mortality & morbidity, especially thromboembolic events, compared to matched patients undergoing surgeries of similar urgency & complexity.
KEY WORDS: COVID-19, COVIDSurg, mortality, postoperative complications
BACKGROUND
Patients undergoing surgery with perioperative COVID-19 are suggested to have worse outcomes, but whether this is COVID-related or due to selection bias remains unclear. We aimed to compare the postoperative outcomes of patients with and without perioperative COVID-19.
METHODS
Patients with perioperative COVID-19 diagnosed within 7 days before or 30 days after surgery between February and July 2020 from 68 US hospitals in COVIDSurg, an international multicenter database, were 1:1 propensity score matched to patients without COVID-19 undergoing similar procedures in the 2012 American College of Surgeons National Surgical Quality Improvement Program database. The matching criteria included demographics (e.g., age, sex), comorbidities (e.g., diabetes, chronic obstructive pulmonary disease, chronic kidney disease), and operation characteristics (e.g., type, urgency, complexity). The primary outcome was 30-day hospital mortality. Secondary outcomes included hospital length of stay and 13 postoperative complications (e.g., pneumonia, renal failure, surgical site infection).
RESULTS
A total of 97,936 patients were included, 1,054 with and 96,882 without COVID-19. Prematching, COVID-19 patients more often underwent emergency surgery (76.1% vs. 10.3%, p < 0.001). A total of 843 COVID-19 and 843 non–COVID-19 patients were successfully matched based on demographics, comorbidities, and operative characteristics. Postmatching, COVID-19 patients had a higher mortality (12.0% vs. 8.1%, p = 0.007), longer length of stay (6 [2–15] vs. 5 [1–12] days), and higher rates of acute renal failure (19.3% vs. 3.0%, p < 0.001), sepsis (13.5% vs. 9.0%, p = 0.003), and septic shock (11.8% vs. 6.0%, p < 0.001). They also had higher rates of thromboembolic complications such as deep vein thrombosis (4.4% vs. 1.5%, p < 0.001) and pulmonary embolism (2.5% vs. 0.4%, p < 0.001) but lower rates of bleeding (11.6% vs. 26.1%, p < 0.001).
CONCLUSION
Patients undergoing surgery with perioperative COVID-19 have higher rates of 30-day mortality and postoperative complications, especially thromboembolic, compared with similar patients without COVID-19 undergoing similar surgeries. Such information is crucial for the complex surgical decision making and counseling of these patients.
LEVEL OF EVIDENCE
Prognostic and Epidemiologic; Level IV.
The COVID-19 pandemic has caused significant disruptions in health care systems globally. Reallocation of vital medical resources to provide critical care to a rapidly increasing number of patients afflicted by SARS-CoV-2 infection resulted in many surgeries getting canceled or postponed.1 However, emergency surgery and some elective surgery (e.g., cancer surgery) services continued to operate, providing care for patients whose surgical disease required immediate or timely intervention.2 Perioperative infection with SARS-CoV-2 could increase the risk of adverse outcomes due to a synergistic effect of dysregulated systemic inflammation and hypercoagulability associated with SARS-CoV-2.3,4 In addition, a broad spectrum of concomitant pulmonary and extrapulmonary manifestations of SARS-CoV-2 in surgical patients could make them particularly prone to serious postoperative complications.5–8
Earlier reports of surgical patients infected with SARS-CoV-2 revealed high postoperative mortality and morbidity rates.2,9–11 However, these results should be cautiously interpreted, as these studies did not have control patients without SARS-CoV-2 infection to estimate the additive effect of SARS-CoV-2 infection on postoperative outcomes. Specifically, many suggest that the majority of the patients with SARS-CoV-2 infection that underwent surgery had a larger number of comorbid conditions and a poorer clinical condition at presentation. It remained unclear whether the increased morbidity and mortality in surgical patients with SARS-CoV-2 infection are attributable to the viral disease and its surrounding system challenges or are a selection bias of whom we operated on during the pandemic. To provide careful benchmarking, several recent studies compared risk-adjusted postoperative outcomes between patients with and without SARS-CoV-2 infection during the pandemic and pre-pandemic periods.12–14 These studies suggested that the presence of SARS-CoV-2 in the perioperative period may be associated with worse surgical outcomes, although the data remains nondefinite. Despite the current guidelines regarding the timing of elective surgery following SARS-CoV-2 infection,15 many surgeons worldwide continue to face the challenging dilemma of whether to pursue surgery or opt for often less effective but potentially safer nonoperative alternatives in emergency surgery patients with SARS-CoV-2 and to balance safety and negative impact of delayed elective surgery.10,16,17
To further address selection bias associated with the COVID-19 pandemic, our study aimed to compare postoperative mortality and major complications between patients with perioperative SARS-CoV-2 infection and propensity score–matched historical controls without SARS-CoV-2 infection undergoing elective and emergency surgeries of similar complexity.
PATIENTS AND METHODS
Study Population and Setting
The data for this study were collected as a part of the international multicenter COVIDSurg Collaborative. We included all patients 17 years or older undergoing any type of surgery with a perioperative SARS-CoV-2 infection (diagnosed within 7 days before or 30 days after surgery) in 1 of the 68 US hospitals between February and July 2020. Only US patients from the COVIDSurg database were used to minimize any country-level variation of care, as they were propensity score matched to patients from the 2012 American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) registry. To maximize matching success, the year 2012 was chosen because of the availability of a broader spectrum of variables that were omitted in the subsequent years of NSQIP registries but were present in the COVIDSurg database (e.g., history of myocardial infarction, history of transient ischemic attack or stroke, peripheral vascular disease, coronary artery disease).
The ACS-NSQIP methodology of systematic data collection has been well established and validated.18,19 The NSQIP Participant Use Data File details the full list of variables provided by the NSQIP.20 The following variables were used in the current study to perform propensity score matching: demographics (age, sex), body mass index (BMI), American Society of Anesthesiologists (ASA) classification, comorbidities (e.g., diabetes, chronic obstructive pulmonary disease, cancer), the preoperative requirement of ventilator-assisted respiration, and hemoglobin and white blood cell count. In addition, the NSQIP variables of “EMERGNCY” or “ELECTSURG” were used to categorize a principal operative procedure as emergent or elective, respectively. All operative procedures were further categorized based on a surgical specialty defined by the NSQIP variable “SURGSPEC” (e.g., general surgery, orthopedics, vascular).
Study Variables and Outcomes
COVIDSurg Database
Patient characteristics included age, sex, BMI, and ASA physical status classification grade. Comorbidity variables included the following: smoking status, cancer, chronic kidney disease, chronic obstructive pulmonary disease, congestive heart failure, diabetes, coronary artery disease, peripheral vascular disease, history of stroke, or transient ischemic attack. Operative variables included urgency of surgery (elective vs. emergency surgery), the principal anesthesia mode (local, regional, and general), a primary procedure performed (grouped by surgical specialty), and grade of surgery (minor vs. major) based on the Bupa schedule of procedures.
Patients with SARS-CoV-2 infection were diagnosed by one of the following criteria: (1) laboratory confirmation of SARS-CoV-2 infection based on the presence of viral RNA using quantitative reverse-transcription polymerase chain reaction, (2) pathognomonic radiographic findings based on chest computed tomography or chest x-ray, or (3) clinical diagnosis made by a treatment team following constellation of symptoms that were consistent with SARS-CoV-2 infection (cough, dyspnea, fever, and myalgia). The timing of SARS-CoV-2 infection was categorized as preoperative or postoperative. A corresponding local principal investigator of each participating hospital was asked to verify the completeness of the records and that all eligible patients were appropriately entered into the database.
Assigning a Grade of Surgery Complexity to CPT Codes
The COVIDSurg Database includes a variable defining the complexity of each operation as “minor” or “major”, based on the Bupa schedule of procedures.21 The NSQIP database defines the principal and most complex operative procedure among all the procedures performed during the index hospitalization, with the “PRNCPTX” variable, using Current Procedural Terminology (CPT) codes. Still, a classification of operative complexity is not readily available. To measure (and match for) the equivalent grade (minor vs. major) of surgery in NSQIP, the following methodology was applied: (1) we identified all CPT codes that correspond to operative procedures listed in the COVIDSurg database, and (2) we linked these CPT codes with the complexity value (minor vs. major) of the corresponding procedure in the COVIDSurg database. The list of CPT codes corresponding to surgical procedures from the COVIDSurg Database and the associated procedural complexity values is provided in Supplementary Table 1S, http://links.lww.com/TA/C844.
Outcomes
The primary outcome was 30-day mortality. Secondary outcomes were hospital length of stay (LOS) and 13 postoperative complications such as pneumonia, sepsis, septic shock, acute kidney injury, myocardial infarction, stroke or transient ischemic attack, cardiac arrest requiring cardiopulmonary resuscitation, thromboembolic outcomes (deep vein thrombosis, pulmonary embolism), surgical site infections (SSIs) (superficial, deep and organ space SSIs), and bleeding necessitating transfusion.
Statistical Analyses
Descriptive statistics were computed for patient characteristics and postoperative outcomes. Categorical and continuous variables were compared using the χ2 test and Wilcoxon rank-sum test, respectively. Patients with SARS-CoV-2 infection were propensity score matched (1:1) to the nearest neighbor without SARS-CoV-2, with a caliper width of 0.2 of the standard deviation of the probit of the propensity score.22 Propensity scores for each patient were derived from a multivariable logistic regression model based on patient characteristics (age, sex, BMI, ASA physical status), SARS-CoV-2 exposure, comorbidities (smoking status, cancer, chronic kidney disease, chronic obstructive pulmonary disease, congestive heart failure, diabetes, coronary artery disease, peripheral vascular disease, history of stroke or transient ischemic attack), preoperative invasive ventilation, hemoglobin and white blood cell count, anesthesia type, urgency (emergent vs. elective), and the complexity of surgery (major vs. minor). To assess the overall distribution of propensity score before and after matching, kernel densities were plotted. Matched patients were compared using the χ2 test for categorical variables and Wilcoxon rank-sum test for continuous outcomes.
Patients who had missing data related to inclusion and exclusion criteria, assigned grade of surgery, and mortality were excluded. The remaining missing data were handled using multiple imputations by chained equations, computing 10 imputations for each missing data point. Descriptive statistics were calculated based on unimputed data.
Four sensitivity analyses were performed, each with a new and separate propensity score match. The first one included only patients with SARS-CoV-2 infection with preoperative respiratory findings (defined by the presence of dyspnea, cough, hemoptysis, or sputum) and/or radiologic evidence associated with SARS-CoV2 infection. The second sensitivity analysis included only asymptomatic patients with SARS-CoV-2 infection. The third sensitivity analysis only included patients who underwent emergency surgery. The last sensitivity analysis included only patients who underwent elective surgery. Categorical variables and outcomes were reported as the number of patients (percentage), whereas continuous variables and outcomes were reported as median (interquartile range [IQR]). Clinical outcomes were adjusted for multiple comparisons using the Benjamini-Hochberg method to control for false discovery rate.23 The level of statistical significance was set at a two-sided p value of <0.05. All analyses were performed using STATA v.17 (StataCorp 2021, College Station, TX).
Ethical Oversight and Reporting
This study was submitted to and exempted from approval by the local institutional review board. The results of this study are reported following the Strengthening the Reporting of Observational Studies in Epidemiology and Reporting of Studies Conducted Using Observational Routinely Collected Health Data recommendations (Supplementary Data 1, http://links.lww.com/TA/C843).24,25
RESULTS
Patient Characteristics Pre-Matching
We identified 1,054 patients with SARS-CoV-2 infection from the COVIDSurg database and 96,882 patients from the 2012 NSQIP database undergoing similar surgeries based on the corresponding CPT codes and assigned grade of surgery definition. Baseline demographic and clinical characteristics of the patients before propensity score matching are shown in Table 1. The two cohorts of patients with and without perioperative SARS-CoV-2 infection were significantly different in baseline characteristics. Patients with SARS-CoV-2 infection more often underwent emergency surgery (76.1% vs. 10.3%, p < 0.001) but less often underwent complex operations (54.7% vs. 73.4%, p < 0.001).
TABLE 1.
Demographic and Clinical Characteristics of Patients Without SARS-CoV-2 Infection (NSQIP) and Patients With SARS-CoV-2 Infection (COVIDSurg)
| Characteristics | Patients Without SARS-CoV-2 (n = 96,882) | Patients With SARS-CoV-2 (n = 1,054) | p |
|---|---|---|---|
| Age group, n (%) | 0.62 | ||
| 17–49 y | 32,608 (33.7) | 374 (35.5) | |
| 50–59 y | 21,327 (22.0) | 226 (21.4) | |
| 60–69 y | 21,556 (22.2) | 217 (20.6) | |
| 70–79 y | 14,341 (14.8) | 157 (14.9) | |
| 80+ y | 7,050 (7.3) | 80 (7.6) | |
| Sex, n (%) | <0.001 | ||
| Female | 53,951 (55.7) | 454 (43.1) | |
| Male | 42,931 (44.3) | 600 (56.9) | |
| BMI, n (%) | 0.096 | ||
| Underweight | 1,486 (1.5) | 23 (2.4) | |
| Normal | 23,232 (24.0) | 247 (25.4) | |
| Overweight | 30,584 (31.6) | 288 (29.7) | |
| Obese | 41,580 (42.9) | 413 (42.5) | |
| ASA status, n (%) | <0.001 | ||
| 1 | 10,170 (10.5) | 41 (3.9) | |
| 2 | 46,805 (48.3) | 214 (20.3) | |
| 3 | 35,229 (36.4) | 450 (42.7) | |
| 4 | 4,542 (4.7) | 322 (30.6) | |
| 5 | 136 (0.1) | 27 (2.6) | |
| Comorbidities, n (%) | |||
| Current smoker | 17,258 (17.8) | 98 (9.3) | <0.001 |
| Cancer | 1,888 (1.9) | 88 (8.3) | <0.001 |
| Chronic kidney disease | 778 (0.8) | 184 (17.5) | <0.001 |
| COPD | 3,874 (4.0) | 61 (5.8) | 0.003 |
| Congestive heart failure | 487 (0.5) | 102 (9.7) | <0.001 |
| Diabetes | 13,659 (14.1) | 380 (36.1) | <0.001 |
| Coronary artery disease | 967 (1.0) | 83 (7.9) | <0.001 |
| Peripheral vascular disease | 2,033 (2.1) | 106 (10.1) | <0.001 |
| Stoke or transient ischemic attack | 1,902 (2.0) | 87 (8.3) | <0.001 |
| Preoperative invasive ventilation, n (%) | 312 (0.3) | 165 (15.7) | <0.001 |
| Hemoglobin, median (IQR), g/L | 13.3 (12.3–14.3) | 11.1 (8.7–13.1) | <0.001 |
| WBC count, median (IQR), ×109/L | 7.1 (5.8–9) | 9 (6.6–13.2) | <0.001 |
| Urgency of surgery, n (%) | <0.001 | ||
| Elective | 86,890 (89.7) | 252 (23.9) | |
| Emergency | 9,992 (10.3) | 802 (76.1) | |
| Anesthesia, n (%) | 0.68 | ||
| Local | 40 (4.7) | 43 (5.1) | |
| Regional | 54 (6.4) | 46 (5.5) | |
| General | 749 (88.8) | 754 (89.4) | |
| Grade of surgery, n (%) | <0.001 | ||
| Minor | 25,811 (26.6) | 477 (45.3) | |
| Major | 71,071 (73.4) | 577 (54.7) | |
| Surgical specialty, n (%) | <0.001 | ||
| Neurosurgery | 4,972 (5.1) | 82 (7.8) | |
| General surgery | 53,170 (54.9) | 355 (33.7) | |
| Thoracic | 984 (1.0) | 22 (2.1) | |
| Plastics | 1,571 (1.6) | 5 (0.5) | |
| Gynecology | 7,466 (7.7) | 59 (5.6) | |
| ENT surgery | 877 (0.9) | 171 (16.2) | |
| Cardiac surgery | 1,388 (1.4) | 24 (2.3) | |
| Urology | 5,047 (5.2) | 65 (6.2) | |
| Orthopedics | 17,946 (18.5) | 105 (10.0) | |
| Vascular surgery | 3,461 (3.6) | 153 (14.5) |
COPD, chronic obstructive pulmonary disease; ENT, ear, nose, and throat surgery; WBC, white blood cell.
Among patients with SARS-CoV-2 infection, 569 of 1,054 patients (53.98%) were diagnosed preoperatively, whereas 395 patients (37.48%) were diagnosed postoperatively; 90 patients (8.54%) had a missing time of diagnosis. Among patients with a preoperative diagnosis of SARS-CoV-2 infection, 372 patients (65.38%) did not have any constellation of symptoms associated with SARS-CoV-2 infection.
Patient Characteristics Post-Matching
After 1:1 propensity score matching, a total of 843 SARS-CoV-2 positive and 843 SARS-CoV-2 negative patients were included in the analysis (Table 2). In brief, the two cohorts were similar in all preoperative characteristics, including demographics, ASA, comorbidities, grade of surgery, and type of surgery. Figure 1 shows the prematching and postmatching kernel density plots, demonstrating a similar distribution of propensity scores postmatching.
TABLE 2.
Postmatching Demographic and Clinical Characteristics of Patients Without SARS-CoV-2 Infection (NSQIP) and Patients With SARS-CoV-2 Infection (COVIDSurg)
| Characteristics | Patients Without SARS-CoV-2 (n = 843) | Patients With SARS-CoV-2 (n = 843) | p |
|---|---|---|---|
| Age group, n (%) | 0.54 | ||
| 17–49 y | 298 (35.3) | 317 (37.6) | |
| 50–59 y | 177 (21.0) | 181 (21.5) | |
| 60–69 y | 169 (20.0) | 165 (19.6) | |
| 70–79 y | 115 (13.6) | 115 (13.6) | |
| 80+ y | 84 (10.0) | 65 (7.7) | |
| Sex, n (%) | 1 | ||
| Female | 378 (44.8) | 378 (44.8) | |
| Male | 465 (55.2) | 465 (55.2) | |
| BMI, n (%) | 0.83 | ||
| Underweight | 18 (2.1) | 20 (2.6) | |
| Normal | 234 (27.8) | 204 (26.3) | |
| Overweight | 246 (29.2) | 224 (28.8) | |
| Obese | 345 (40.9) | 329 (42.3) | |
| ASA status, n (%) | 0.38 | ||
| 1 | 53 (6.3) | 41 (4.9) | |
| 2 | 167 (19.8) | 191 (22.7) | |
| 3 | 369 (43.8) | 375 (44.5) | |
| 4 | 223 (26.5) | 211 (25.0) | |
| 5 | 31 (3.7) | 25 (3.0) | |
| Comorbidities, n (%) | |||
| Current smoker | 86 (10.2) | 83 (9.8) | 0.81 |
| Cancer | 80 (9.5) | 71 (8.4) | 0.44 |
| Chronic kidney disease | 115 (13.6) | 106 (12.6) | 0.52 |
| COPD | 51 (6.0) | 42 (5.0) | 0.34 |
| Congestive heart failure | 71 (8.4) | 54 (6.4) | 0.11 |
| Diabetes | 263 (31.2) | 270 (32.0) | 0.71 |
| Coronary artery disease | 55 (6.5) | 53 (6.3) | 0.84 |
| Peripheral vascular disease | 77 (9.1) | 76 (9.0) | 0.93 |
| Stoke or transient ischemic attack | 63 (7.5) | 55 (6.5) | 0.45 |
| Preoperative invasive ventilation, n (%) | 78 (9.3%) | 78 (9.3%) | 1 |
| Hemoglobin, median (IQR), g/L | 11.7 (9.9–13.3) | 11.7 (9.25–13.4) | 0.7 |
| WBC count, median (IQR), ×109/L | 9.4 (6.9–13.1) | 8.8 (6.4–12.9) | 0.054 |
| Urgency of surgery, n (%) | 0.17 | ||
| Elective | 213 (25.3) | 238 (28.2) | |
| Emergency | 630 (74.7) | 605 (71.8) | |
| Anesthesia, n (%) | 0.68 | ||
| Local | 40 (4.7) | 43 (5.1) | |
| Regional | 54 (6.4) | 46 (5.5) | |
| General | 749 (88.8) | 754 (89.4) | |
| Grade of surgery, n (%) | 0.92 | ||
| Minor | 310 (36.8) | 312 (37.0) | |
| Major | 533 (63.2) | 531 (63.0) | |
| Surgical specialty, n (%) | 0.6 | ||
| Neurosurgery | 65 (7.7) | 76 (9.0) | |
| General surgery | 377 (44.7) | 346 (41.0) | |
| Thoracic | 22 (2.6) | 20 (2.4) | |
| Plastics | 4 (0.5) | 5 (0.6) | |
| Gynecology | 42 (5.0) | 54 (6.4) | |
| ENT surgery | 42 (5.0) | 53 (6.3) | |
| Cardiac surgery | 29 (3.4) | 23 (2.7) | |
| Urology | 39 (4.6) | 50 (5.9) | |
| Orthopedics | 104 (12.3) | 98 (11.6) | |
| Vascular surgery | 119 (14.1) | 118 (14.0) |
COPD, chronic obstructive pulmonary disease; ENT, ear, nose, and throat surgery; WBC, white blood cell.
Figure 1.
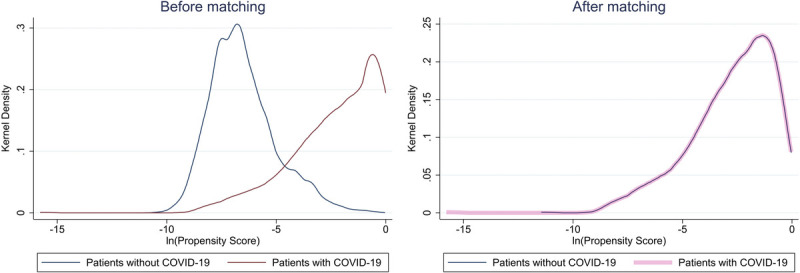
Prematching and postmatching kernel density plots of the natural logarithm of propensity scores.
Outcomes of Matched COVID-19 Versus Non–COVID-19 Patients
After propensity score matching, patients with SARS-CoV-2 infection had a higher rate of 30-day mortality (12.0% vs. 8.1%, p = 0.012) and longer hospital LOS (median [IQR], 6 [2–15] vs. 5 [1–12] days; p = 0.035) compared with patients without SARS-CoV-2 (Fig. 2 and Table 3). In addition, patients with perioperative SARS-CoV-2 infection had higher rates of acute renal failure (19.3% vs. 3.0%, p < 0.001), pneumonia (24.2% vs. 4.6%, p < 0.001), sepsis (13.5% vs. 9.0%, p = 0.006), septic shock (11.8% vs. 6.0%, p < 0. 001), wound dehiscence (2.1% vs. 0.7, p = 0.019), and cardiac arrest (4.4% vs. 1.4%, p < 0.001) but similar rates of superficial/deep (4.6% vs. 3.8, p = 0.4) and organ space SSIs (1.4% vs. 2.3, p = 0.214). The presence of SARS-CoV-2 in the perioperative period was also associated with higher rates of postoperative thromboembolic complications such as deep vein thrombosis (4.4% vs. 1.5%, p < 0.001), pulmonary embolism (2.5% vs. 0.4%, p < 0.001), and stroke (1.8% vs. 0.5%, p = 0.017) but lower rate of bleeding requiring transfusions (11.6% vs. 26.1%, p < 0.001).
Figure 2.
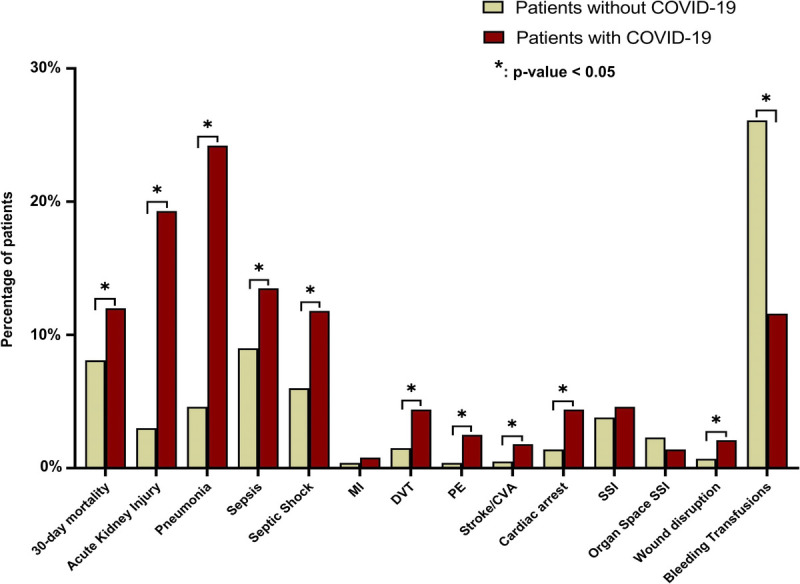
Thirty-day mortality and postoperative complications of propensity score–matched patients with and without SARS-CoV-2 infections. CVA, cerebrovascular accident; DVT, deep venous thrombosis; MI, myocardial infarction; PE, pulmonary embolism.
TABLE 3.
30-Day Mortality and Postoperative Complications of Propensity Score–Matched Patients With and Without SARS-CoV-2 Infections
| Outcome | Patients Without SARS-CoV-2 (n = 843) | Patients With SARS-CoV-2 (n = 843) | p | Risk Difference (CI 95%) |
|---|---|---|---|---|
| 30-d Mortality | 68 (8.1%) | 101 (12.0%) | 0.012 | 0.04 (0.01–0.07) |
| Hospital LOS, median (IQR) | 5 (1–12) | 6 (2–15) | 0.035 | — |
| Pneumonia | 39 (4.6%) | 204 (24.2%) | <0.001 | 0.20 (0.16–0.23) |
| Sepsis | 76 (9.0%) | 114 (13.5%) | 0.006 | 0.05 (0.01–0.08) |
| Septic shock | 51 (6.0%) | 99 (11.8%) | <0.001 | 0.06 (0.03–0.08) |
| Acute kidney injury | 25 (3.0%) | 163 (19.3%) | <0.001 | 0.16 (0.13–0.19) |
| Myocardial infarction | 3 (0.4%) | 7 (0.8%) | 0.214 | 0.00 (−0.00 to 0.01) |
| Cardiac arrest | 12 (1.4%) | 37 (4.4%) | <0.001 | 0.03 (0.01–0.05) |
| Deep venous thrombosis | 13 (1.5%) | 37 (4.4%) | <0.001 | 0.03 (0.01–0.04) |
| Pulmonary embolism | 3 (0.4%) | 21 (2.5%) | <0.001 | 0.02 (0.01–0.03) |
| Stroke/CVA | 4 (0.5%) | 15 (1.8%) | 0.017 | 0.01 (0.00–0.02) |
| SSI | 32 (3.8%) | 39 (4.6%) | 0.4 | 0.01 (−0.01 to 0.03) |
| Organ space SSI | 19 (2.3%) | 12 (1.4%) | 0.214 | −0.01 (−0.02 to 0.00) |
| Wound disruption | 6 (0.7%) | 18 (2.1%) | 0.019 | 0.01 (0.00–0.03) |
| Bleeding transfusions | 220 (26.1%) | 98 (11.6%) | <0.001 | −0.14 (0.00 to 0.03) |
p Values were adjusted for multiple comparisons using the Benjamini-Hochberg correction.
CI, confidence interval; CVA, cerebrovascular accident; DVT, deep venous thrombosis; MI, myocardial infarction; PE, pulmonary embolism.
Sensitivity Analyses: Patients With and Without Preoperative Respiratory/Radiologic Findings of SARS-CoV-2 Infection
In a sensitivity analysis including only patients with preoperative respiratory or radiologic findings, 408 patients with SARS-CoV-2 infection and 408 patients without SARS-CoV-2 infection were de novo propensity score matched in a similar fashion (Supplementary Table 2S, http://links.lww.com/TA/C844). The overall 30-day mortality rate in symptomatic patients with SARS-CoV2 infection was significantly higher than in a matched cohort of patients without SARS-CoV-2 infection (19.1% vs. 11.3%, p = 0.004). Similar to the overall cohort, the preoperative diagnosis of SARS-CoV2 infection, along with the presence of respiratory or radiologic findings of COVID-19 disease, was associated with a longer hospital LOS (median [IQR], 9 [4–23] vs. 8 [3–15] days; p = 0.035) and higher rates of eight postoperative complications shown in Figure 3A and Supplementary Table 3S, http://links.lww.com/TA/C844. In contrast, the rate of postoperative bleeding events that required transfusions was still higher in patients without SARS-CoV-2 infection.
Figure 3.
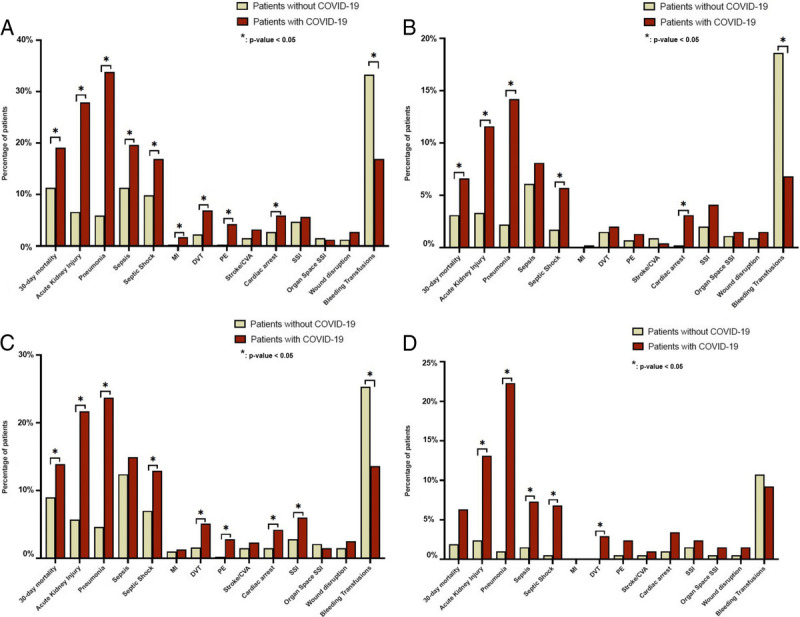
Sensitivity analyses (A–D) for 30-day mortality and postoperative complications of propensity score–matched patients without SARS-CoV-2 infection and (A, upper left corner) symptomatic patients with SARS-CoV-2 infection and preoperative respiratory/radiologic findings, (B, upper right corner) asymptomatic patients with SARS-CoV-2 infection, (C, lower left corner) patients with SARS-CoV-2 infection who had emergency surgery, and (D, lower right corner) patients with SARS-CoV-2 infection who had elective surgery. CVA, cerebrovascular accident; DVT, deep venous thrombosis, MI, myocardial infarction; PE, pulmonary embolism.
An additional sensitivity analysis considering only asymptomatic patients with SARS-CoV-2 infection resulted in 458 patients with SARS-CoV-2 infection propensity score matched with 458 patients without SARS-CoV-2 infection (Supplementary Table 4S, http://links.lww.com/TA/C844). Overall, 30-day mortality among asymptomatic patients was still significantly higher when compared with matched patients without SARS-CoV-2 infection (6.6% vs. 3.1%, p = 0.033). Similarly, when compared with patients without SARS-CoV-2 infection, the rates of acute kidney injury, cardiac arrest, pneumonia, and septic shock were higher in patients with SARS-CoV-2 infection despite the absence of preoperative symptoms and radiologic findings associated with COVID-19 infection upon admission (Fig. 3B and Supplementary Table 5S, http://links.lww.com/TA/C844).
Sensitivity Analyses: Emergency Versus Elective Surgery
In the sensitivity analysis of patients who underwent emergency surgery, patients with SARS-CoV-2 infection had a significantly higher 30-day mortality rate (13.9% vs. 9.0%, p = 0.012) compared with propensity score–matched patients undergoing emergency surgeries of similar complexity but without SARS-CoV-2 infection (Supplementary Table 6S, http://links.lww.com/TA/C844). Despite no significant difference in hospital LOS between the two cohorts of patients undergoing emergency surgeries (7 days [IQR, 2–17 days] vs. 6 days [IQR, 3–12.5 days], p = 0.18), perioperative diagnosis of SARS-CoV-2 infection was associated with higher rates of pneumonia (23.7% vs. 4.6%, p < 0.001), septic shock (12.9% vs. 7.0%, p < 0.001), acute kidney injury (21.7% vs. 5.7%, p < 0.001), cardiac arrest (4.2% vs. 1.5%, p = 0.009), deep venous thrombosis (5.1% vs. 1.6%, p < 0.001) and pulmonary embolism (2.8% vs. 0.2%, p < 0.001), and SSIs (6.0% vs. 2.8%, p = 0.009) (Fig. 3C and Supplementary Table 7S, http://links.lww.com/TA/C844). In contrast, patients without SARS-CoV-2 infection experienced a higher rate of postoperative bleeding that required transfusion (25.3% vs. 13.6%, p < 0.001).
In the sensitivity analysis, including only patients undergoing elective surgery, patients with SARS-CoV-2 infection were similarly matched to patients without SARS-CoV-2 infection (Supplementary Table 8S, http://links.lww.com/TA/C844). Figure 3D and Supplementary Table 9S, http://links.lww.com/TA/C844, show the postoperative outcomes of these two cohorts of patients. Perioperative SARS-CoV-2 infection in elective procedures was not associated with a statistically different 30-day mortality rate (6.3% vs. 1.9%, p = 0.06) and hospital LOS (3 days [IQR, 1–9 days] vs. 2 days [IQR, 0–5 days]; p = 0.06). In contrast, patients undergoing elective surgeries with perioperative SARS-CoV-2 infection had higher rates of pneumonia (22.3% vs. 1.0%, p < 0.001), sepsis (7.3% vs. 1.5%, p = 0.014), septic shock (6.8% vs. 0.5%, p < 0.001), acute kidney injury (13.1% vs. 2.4%, p < 0.001), and deep venous thrombosis (2.9% vs. 0.0%, p = 0.04). All missing data values were imputed using multiple imputation by chained equations (Supplementary Table 10S, http://links.lww.com/TA/C844).
DISCUSSION
This nationwide study compares the rates of mortality and 13 postoperative complications between patients with SARS-CoV-2 infection from the COVIDSurg database and propensity score matched non–SARS-CoV-2 patients from the ACS-NSQIP database undergoing surgeries of similar urgency and complexity. Our study found that perioperative SARS-CoV-2 infection is associated with a significantly higher rate of 30-day mortality, longer hospital LOS, and a higher risk of severe postoperative complications when compared with matched patients without SARS-CoV-2 infection. The increased risk was observed even in asymptomatic patients who did not exhibit signs and symptoms of SARS-CoV-2.
While comparing patients with SARS-CoV-2 infection with the prepandemic historical controls, our findings provide further insight into the combined effect of SARS-CoV-2 infection and the impact caused by stressed health care systems on surgical patients operated during the first wave of the COVID-19 pandemic in the United States. Lal et al.26 used the Veterans Affairs database and similarly reported increased rates of pulmonary, septic, and ischemic complications but did not find increased 30-day mortality in propensity score–matched patients with SARS-CoV-2 infection. In contrast, a more recent study using the same database but examining midterm surgical outcomes in matched patients with and without SARS-CoV-2 found a significantly higher risk of mortality, pulmonary, thrombotic, and septic complications in patients, particularly during the first 4 weeks following SARS-CoV-2 infection.13 However, despite the decent sample size and the use of propensity score matching, both studies have limited generalizability because of the inherent patient characteristics specific to the Veterans Affairs population. Nevertheless, the elevated risk of postoperative mortality and morbidity also supports the findings from another large international study, which reported increased rates of 30-day mortality up to 7 weeks following SARS-CoV-2 infection.14 Although data collection was done in October 2020, the results provided early evidence for the subsequent recommendation regarding the timing of surgery in unvaccinated patients. To address this gap, a recent study examining the role of COVID-19 vaccination in elective surgery did not find an increased risk of perioperative complications in vaccinated patients compared with prepandemic controls.27
In our study, the SARS-CoV-2 positive cohort consisted of both symptomatic and asymptomatic patients. Both groups had a significantly higher 30-day mortality when compared as separate cohorts with matched non–SARS-CoV-2 patients in the sensitivity analyses. Early studies reported no difference in mortality among symptomatic and asymptomatic SARS-CoV-2–infected patients, which might be attributed to limited testing capacity at the beginning of the pandemic.28,29 In contrast, another study focusing on patients undergoing select emergency general surgery procedures found that the higher risk of 30-day mortality and pulmonary complications is only notable in patients who had preoperative respiratory clinical or radiological findings of SARS-CoV-2 infection.30
One particular finding of our study also worth discussing is the markedly higher proportion of postoperative thromboembolic complications among patients with perioperative SARS-CoV-2 infection compared with matched controls. This is consistent with early evidence suggesting that an inflammatory coagulopathy plays a role in the development of thromboembolic events in SARS-CoV-2–infected patients.4,31 Perioperative or recent SARS-CoV-2 infection was similarly found to be an independent risk factor for postoperative venous thromboembolism in a recent large, international COVIDSurg study.32 As interestingly, the matched cohort of patients without SARS-CoV-2 infection in our study even experienced a higher rate of postoperative bleeding that required transfusion compared with patients with SARS-CoV-2 infection, a finding that might also indicate the potential hypercoagulable state associated with COVID-19.33,34
The majority of our SARS-CoV-2 patients underwent emergent operations, which was not surprising given that many academic and government organizations recommended limiting or postponing elective surgeries during pandemic peaks.2,35,36 Considering that most patients in the NSQIP registry had elective procedures, we performed two additional sensitivity analyses comparing separately elective cases in SARS-CoV-2–positive patients with historical controls undergoing surgeries of similar complexity. Patients with SARS-CoV-2 who had elective procedures compared with historical controls had a tendency toward a higher rate of 30-day mortality, but this did not reach statistical significance. In our opinion, the failure of finding a difference in the outcome should be evaluated with caution, as it is possibly due to a lower sample size (type II error), the fact that elective procedures performed during the COVID-19 pandemic might not be representative of elective surgery done before the pandemic because of prioritization of more critical elective procedures, and considering the prior findings reported in the first COVIDSurg study. That study demonstrated a substantially high risk of postoperative mortality (23.8%) and pulmonary complications (51.2%) in patients with perioperative COVID-19 and that increase was also notable in elective surgery patients.9 In addition, a recent study examining postoperative complications in 5,479 patients undergoing elective surgeries in the United States compared SARS-CoV-2–positive patients with the prepandemic cohort of surgical patients undergoing similar surgeries. Although Deng et al.12 reported a markedly elevated risk of 30-day postoperative complications associated with SARS-CoV-2 infection, the study lacked mortality information and information on whether patients with a confirmed SARS-CoV-2 infection were symptomatic or asymptomatic. One study with a prepandemic control group was performed by the Dutch Surgical COVID-19 Research Collaborative and similarly aimed to dissect the independent role of perioperative SARS-CoV-2 infection in amplifying the surgical risk of postoperative outcomes. Their matched-cohort analysis of 123 SARS-CoV-2–positive and 196 SARS-CoV-2–negative control patients similarly showed increased risks of 30-day mortality (12.2%) and postoperative complications (20.3%).29 Interestingly, the mortality rate of 4.6% in the control SARS-CoV-2 negative group was twice as high compared with the prepandemic rate of 2% reported in a Dutch national surgical registry. A prospective cohort study focusing on surgical patients with a perioperative SARS-CoV-2 infection in the United States, performed as a part of the COVIDSurg collaborative, found the rates of mortality and pulmonary complications to be 11.0% and 39.5%, respectively.37 Although the mortality rate in the United States was lower compared with the initial international COVIDSurg data, it was still higher when compared with the prepandemic rates of mortality.38 Those studies lacked control comparison groups.
Another multicenter study that included 2,132 patients from 25 Spanish hospitals found similar rates of 30-day mortality among surgical patients with and without SARS-CoV-2 infection (12.6% vs. 4.6%).39 However, despite their propensity score–matched analysis not revealing a statistically significant difference in 30-day mortality (12.0% vs. 8.1%; p = 0.163), SARS-CoV-2 infection was still associated with higher rates of 90-day mortality and 30-day postoperative complications. Several factors potentially limit the generalizability of their findings, including a small number of patients with SARS-CoV-2 infection (175 patients) and considering only emergency cases confined to a single surgical specialty. In view of our findings, as well as those in the aforementioned US, Spanish, and Dutch studies, we suggest that the increased morbidity and mortality of patients with COVID-19 are potentially inherent to the disease and its systemic and pulmonary complications as discussed previously. Nevertheless, we cannot exclude system-related factors during the pandemic (e.g., staff shortage, bed capacity issues) that could have contributed.
Our study still has a few limitations. First, the study included patients from the 2012 ACS-NSQIP registry as historical matched controls, and surgical practice and definitions of specific postoperative outcomes may have changed over time. Second, our propensity score–matched analysis might have residual confounding bias because of unaccounted confounders not being available in the databases used in this study. Specifically, there was no information about reasons for surgery, which could contribute to residual confounding by indication, especially because the patient population included those diagnosed preoperatively and postoperatively. Similarly, the COVIDSurg database did not have data regarding COVID-19 severity. Third, since the data were collected during the first wave of the pandemic, our analysis did not include patients who received vaccination or COVID-19 therapeutics and those who could have been infected by a different variant of SARS-CoV-2 (Delta, Omicron), thereby limiting the generalizability of our findings. A recently launched international, multicenter study, COVIDSurg-3 by the COVIDSurg Collaborative, aims to examine postoperative surgical outcomes in the era of vaccination and Omicron. Fourth, the low sample size in subgroups might have limited ability to detect a statistically significant difference in outcomes between the groups. In addition, the grade of surgery was defined based on the Bupa Schedule, which might not entirely reflect a surgical procedure's complexity when mapped onto the NSQIP-defined principal procedure variable. Nevertheless, using CPT codes corresponding to procedures performed in the COVIDSurg database provided consistency and minimized variation in labeling the grade of surgery in the NSQIP dataset.
CONCLUSION
Using a nationwide propensity score–matched cohort, we demonstrated significantly higher rates of mortality and postoperative complications, especially thromboembolic events, among patients with perioperative SARS-CoV-2 infection compared with a matched cohort of patients without SARS-CoV-2 infection. Moreover, matched SARS-CoV-2–infected patients had a higher risk of mortality even if they were asymptomatic at presentation. Such information is crucial for the complex surgical decision making and counseling of patients presenting with positive SARS-CoV-2 infection status. Further studies are required to understand the impact of new variants, large-scale vaccination, and new therapeutics on the postoperative outcomes of COVID-19 patients.
AUTHORSHIP
D.A., A.D.-G., M.E., A.G., J.A.P.-Z., M.B., A.M.R., D.N., A.B., and H.M.A.K. performed review and study design. D.A. and H.M.A.K. performed data analysis. D.A., A.D.-G., M.E., A.G., J.A.P.-Z., M.B., A.M.R., D.N., A.B., and H.M.A.K. performed the interpretation of analytical results. D.A. and H.M.A.K. performed the writing of the manuscript draft. A.D.-G., M.E., A.G., J.A.P.-Z., M.B., A.M.R., D.N., and A.B. performed the critical revision of the manuscript.
ACKNOWLEDGMENTS
We thank Xiao Liu, Alistair Denniston, and Royal College of Surgeons of England COVID-19 Research Group.
This study was funded by the National Institute for Health Research, Association of Coloproctology of Great Britain and Ireland, Bowel and Cancer Research, Bowel Disease Research Foundation, Association of Upper Gastrointestinal Surgeons, British Association of Surgical Oncology, British Gynecological Cancer Society, European Society of Coloproctology, NIHR Academy, Sarcoma UK, Vascular Society for Great Britain and Ireland, and Yorkshire Cancer Research. The funding bodies have no rule design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
DISCLOSURE
H.M.A.K. receives royalties from UpToDate related to a COVID-19 published article. The rest of the authors declare no conflicts of interest.
COVIDSurg Authorship List
Operations Committee: Kwabena Siaw-Acheampong, Leah Argus, Daoud Chaudhry, Brett E. Dawson, James C. Glasbey, Rohan R. Gujjuri, Conor S. Jones, Sivesh K. Kamarajah, Chetan Khatri, James M. Keatley, Samuel Lawday, Elizabeth Li, Harvinder Mann, Ella J. Marson, Kenneth A Mclean, Maria Picciochi, Elliott H. Taylor, Abhinav Tiwari, Joana F.F. Simoes, Isobel M. Trout, Mary L. Venn, Richard J.W. Wilkin, Aneel Bhangu*, Dmitri Nepogodiev*
*Co-chairs
Dissemination Committee: Irida Dajti (Albania), Arben Gjata (Albania), Luis Boccalatte (Argentina), Maria Marta Modolo (Argentina), Daniel Cox (Australia), Peter Pockney (Australia), Philip Townend (Australia), Felix Aigner (Austria), Irmgard Kronberger (Austria), Kamral Hossain (Bangladesh), Gabrielle VanRamshorst (Belgium), Ismail Lawani (Benin), Gustavo Ataide (Brazil), Glauco Baiocchi (Brazil), Igor Buarque (Brazil), Muhammad Gohar (Bulgaria), Mihail Slavchev (Bulgaria), Arnav Agarwal (Canada), Amanpreet Brar (Canada), Janet Martin (Canada), Maria Marta Modolo (Chile), Maricarmen Olivos (Chile), Jose Calvache (Colombia), Carlos Jose Perez Rivera (Colombia), Ana Danic Hadzibegovic (Croatia), Tomislav Kopjar (Croatia), Jakov Mihanovic (Croatia), Jaroslav Klat (Czech Republic), René Novysedlak (Czech Republic), Peter Christensen (Denmark), Alaa El-Hussuna (Denmark), Sylvia Batista (Dominican Republic), Eddy Lincango (Ecuador), Sameh H. Emile (Egypt), Mengistu Gebreyohanes Mengesha (Ethiopia), Dr. Samuel Hailu (Ethiopia), Hailu Tamiru (Ethiopia), Joonas Kauppila (Finland), Johanna Laukkarinen (Finland), Alexis Arnaud (France), Markus Albertsmeiers (Germany), Hans Lederhuber (Germany), Markus Loffler (Germany), Stephen Tabiri (Ghana), Symeon Metallidis (Greece), Georgios Tsoulfas (Greece), Maria Aguilera Lorena (Guatemala), Gustavo Grecinos (Guatemala), Tamas Mersich (Hungary), Daniel Wettstein (Hungary), Dhruv Ghosh (India), Gabriele Kembuan (Indonesia), Peiman Brouk (Iran), Mohammad Khosravi (Iran), Masoud Mozafari (Iran), Ahmed Adil (Iraq), Helen M. Mohan (Ireland), Oded Zmora (Israel), Marco Fiore (Italy), Gaetano Gallo (Italy), Francesco Pata (Italy), Gianluca Pellino (Italy), Sohei Satoi (Japan), Faris Ayasra (Jordan), Mohammad Chaar (Jordan), Ildar R. Fakhradiyev (Kazakhstan), Mohammad Jamal (Kuwait), Muhammed Elhadi (Libya), Aiste Gulla (Lithuania), April Roslani (Malaysia), Laura Martinez (Mexico), Antonio Ramos De La Medina (Mexico), Oumaima Outani (Morocco), Pascal Jonker (Netherlands), Schelto Kruijff (Netherlands), Milou Noltes (Netherlands), Pieter Steinkamp (Netherlands), Willemijn van der Plas (Netherlands), Adesoji Ademuyiwa (Nigeria), Babatunde Osinaike (Nigeria), Justina Seyi-olajide (Nigeria), Emmanuel Williams (Nigeria), Sofija Pejkova (North Macedonia), Knut Magne Augestad (Norway), Zainab Al Balushi (Oman), Ahmad Qureshi (Pakistan), Raza Sayyed (Pakistan), Mustafa Abu Mohsen Daraghmeh (Palestine), Sadi Abukhalaf (Palestine), Moises Cukier (Panama), Hugo Gomez (Paraguay), Sebastian Shu (Peru), Ximena Vasquez (Peru), Marie Dione Parreno-Sacdalan (Philippines), Piotr Major (Poland), José Azevedo (Portugal), Miguel Cunha (Portugal), Irene Santos (Portugal), Ahmad Zarour (Qatar), Eduard-Alexandru Bonci (Romania), Ionut Negoi (Romania), Sergey Efetov (Russia), Andrey Litvin (Russia), Faustin Ntirenganya (Rwanda), Ehab AlAmeer (Saudi Arabia), Dejan Radenkovic (Serbia), Frederick Koh Hong Xiang (Singapore), Chew Min Hoe (Singapore), James Ngu Chi Yong (Singapore), Rachel Moore (South Africa), Ncamsile Nhlabathi (South Africa), Ruth Blanco Colino (Spain), Ana Minaya Bravo (Spain), Ana Minaya-Bravo (Spain), Umesh Jayarajah (Sri Lanka), Dakshitha Wickramasinghe (Sri Lanka), Mohammed Elmujtaba (Sudan), William Jebril (Sweden), Martin Rutegård (Sweden), Malin Sund (Sweden), Arda Isik (Turkey), Sezai Leventoğlu (Turkey), Tom E.F. Abbott (UK), Ruth Benson (UK), Ed Caruna (UK), Sohini Chakrabortee (UK), Andreas Demetriades (UK), Anant Desai (UK), Thomas D. Drake (UK), John G. Edwards (UK), Jonathan P. Evans (UK), Samuel Ford (UK), Christina Fotopoulou (UK), Ewen Griffiths (UK), Peter Hutchinson (UK), Michael D. Jenkinson (UK), Tabassum Khan (UK), Stephen Knight (UK), Angelos Kolias (UK), Elaine Leung (UK), Siobhan McKay (UK), Lisa Norman (UK), Riinu Ots (UK), Vidya Raghavan (UK), Keith Roberts (UK), Andrew Schache (UK), Richard Shaw (UK), Katie Shaw (UK), Neil Smart (UK), Grant Stewart (UK), Sudha Sundar (UK), Dale Vimalchandran (UK), Naomi Wright (UK), Sattar Alshryda (United Arab Emirates), Osaid Alser (United States), Kerry Breen (United States), Ian Ganly (United States), Haytham Kaafarani (United States), Brittany Kendall (United States), Hassan Mashbari (United States), Hamza Al Naggar (Yemen), Dennis Mazingi (Zimbabwe), EuroSurg, European Society of Coloproctology (ESCP), GlobalSurg, GlobalPaedSurg, ItSURG, PTSurg, SpainSurg, Italian Society of Colorectal Surgery (SICCR), Association of Surgeons in Training (ASiT), Irish Surgical Research Collaborative (ISRC), Joana F.F. Simoes (Chair).
Collaborators (*asterisk indicates principle investigator): Wong J.J., Napolitano L.*, Hemmila M. (Michigan Medicine, United States); Amin D.*, Abramowicz S., Roser S.M. (Emory University, United States); Olson K.A., Riley C., Heron C., Cardenas T.*, Leede E., Thornhill M., Haynes A.B.*, McElhinney K., Roward S., Trust M.D.*, Hill C.E., Teixeira P.G.* (Dell Seton Medical Center at the University of Texas at Austin, United States); Etchill E., Stevens K.*, Ladd M.R., Long C., Rose J., Kent A., Yesantharao P., Vervoort D., Jenny H., Gabre-Kidan A., Margalit A., Tsai L., Malapati H., Yesantharao L. (Johns Hopkins Hospital, United States); Abdou H., Diaz J.*, Richmond M., Clark J., O'Meara L., Hanna N. (University of Maryland Medical Center, United States); Ying Y.*, Fleming J., Ovaitt A., Gigliotti J., Fuson A. (University of Alabama Birmingham, United States); Cooper Z.*, Salim A.*, Hirji S.A., Brown A., Chung C., Hansen L., Okafor B.U., Roxo V., Raut C.P., Jolissaint J.S., Mahvi D.A. (Brigham and Women's Hospital, United States); Kaafarani H.*, Breen K., Bankhead-Kendall B., Alser O., Mashbari H., Velmahos G., Maurer L.R., El Moheb M., Gaitanidis A., Naar L., Christensen M.A., Kapoen C., Langeveld K., El Hechi M., Mokhtari A. (Massachusetts General Hospital, United States); Haqqani M.H., Drake F.T.* (Boston Medical Center, United States); Goldenberg-Sandau A.*, Galbreath B. (Cooper University Hospital, United States); Reinke C.*, Ross S., Thompson K., Manning D., Perkins, R. (Atrium Health Carolinas Medical Center, United States); Eriksson E.*, Evans H. (Medical University of South Carolina, United States); Masrur M., Giulianotti P., Benedetti E. (University of Illinois at Chicago, Chicago, IL, United States); Chang G.*, Ourieff J., Dehart D. (Mount Sinai Hospital, United States); Dorafshar A., Price T., Bhama A.R., Torquati A.*, Cherullo E., Kennedy R., Myers J. (Rush University Medical Centre, United States); Rubin K.* (University Hospitals of Cleveland, United States); Ban V.S.*, Aoun S.G., Batjer H.H., Caruso J. (University of Texas Southwestern, United States); Carmichael H., Velopulos C.G.*, Wright F.L., Urban S., McIntyre Jr. R.C., Schroeppel T.J.*, Hennessy E.A., Dunn J.*, Zier L. (University of Colorado Hospital/Memorial Hospital/Medical Center of the Rockies, United States); Burlew C.*, Coleman J.* (Denver Health, United States); Colling K.P.* (Saint Mary's Medical Center-Essentia Health, United States); Hall B., Rice H.E.*, Hwang E.S., Olson S.A., Moris D. (Duke University Medical Center, United States); Verma R.*, Hassan R. (Nassau University Medical Center, United States); Volpe A., Merola, S. (NewYork Presbyterian Queens, Flushing, United States); O'Banion L.A.*, Lilienstein J., Dirks R. (University of California San Francisco-Fresno, United States); Marwan H.*, Almasri M.*, Kulkarni G., Mehdi M., Abouassi A., Abdallah M., San Andrés M., Eid J., Aigbivbalu E., Sundaresan J., George B. (University of Texas Medical Branch, United States); Ssentongo A., Ssentongo P., Oh J.S., Hazelton J.*, Maines J., Gusani N., Garner M., Horvath S. (Pennsylvania State University, United States); Zheng F.* (Houston Methodist Hospital, United States); Ujiki M. (Northshore University Healthsystem, United States); Kinnaman G., Meagher A.*, Sharma I., Holler E. (Iu Health Methodist Hospital, United States); McKenzie K.*, Chan J., Fretwell K., Nugent III W., Khalil A., Chen D., Post N., Rostkowski T., Brahmbhatt D. (Jamaica Hospital, United States); Huynh K., Hibbard M.L. (Kaiser Permanente West Los Angeles, United States); Schellenberg M.* (LAC+USC Medical Center, United States); Martin RCG*, Bhutiani N. (University of Louisville Hospital and Norton Hospital, Louisville, United States); Giorgakis E.*, Laryea J., Bhavaraju A., Sexton K., Roberts M., Kost M., Kimbrough M., Burdine L., Kalkwarf K., Robertson R. (University of Arkansas for Medical Sciences, United States); Gosain A.*, Camp L., Lewit R. (Le Bonheur Children's Hospital, United States); Kronenfeld J.P., Urrechaga E., Goel N., Rattan R., Hart V.*, Allen M., Gilna G. (Jackson Memorial Hospital, United States); Cioci A., Ruiz G.*, Allen M., Rakoczy K., Pavlis W., Saberi R. (University of Miami Hospital, United States); Morris R.*, Karam B.S. (Froedtert Hospital, United States); Brathwaite CEM*, Liu H., Petrone P., Hakmi H., Sohail A.H., Baltazar G., Heckburn R. (NYU Langone Health-NYU Winthrop Hospital, United States); Nygaard R.M.*, Colonna E.T., Endorf F.W., Hill M.J. (Hennepin Healthcare, Minneapolis, United States); Maiga A., Dennis B.*, Levin J.H., Lallemand M. (Vanderbilt University Medical Center, United States); Choron R., Peck G.*, Soliman F., Rehman S. (Robert Wood Johnson University Hospital, United States); Glass N.*, Juthani B., Deisher D. (The University Hospital, United States); Ruzgar N.M., Ullrich S.J.*, Sion M.* (Yale New Haven Hospital, United States); Paranjape C.*, El Moheb M., Kar A.R. (Newton Wellesley Hospital, United States); Gillezeau C., Rapp J., Taioli E., Miles B.A.*, Alpert N. (Mount Sinai Hospital, United States); Podolsky D.*, Coleman N.L., Callahan M.P. (NewYork-Presbyterian/Columbia University Medical Center, United States); Ganly I.*, Brown L. (Memorial Sloan Kettering Cancer Center, United States); Monson JRT (AdventHealth Orlando, Orlando, United States); Dehal A.* (Kaiser Permanente Panorama City Medical Center, United States); Abbas A., Soliman A., Kim B., Jones C., Dauer E., Renza-Stingone E., Hernandez E., Gokcen E., Kropf E., Sufrin H., Hirsch H., Ross H., Engel J., Sewards J., Diaz J., Poggio J., Sanserino K., Rae L., Philp M.*, Metro M., McNelis P., Petrov R., Rehman S., Pazionis T. (Temple University Hospital, United States); Till B., Lamm R., Rios-Diaz A.J., Palazzo F.* (Thomas Jefferson University Hospital, United States); Rosengart M.*, Nicholson K. (University of Pittsburgh Medical Center, United States); Carrick M.M.*, Rodkey K. (Medical City Plano, United States); Suri A., Callcut R.* (UC Davis Medical Center, United States); Nicholson S.*, Talathoti N. (UT Health San Antonio [UTHSA], University Hospital [UHS], San Antonio, United States); Klaristenfeld D.* (Kaiser Permanente San Diego Medical Center, United States); Biffl W.*, Marsh C., Schaffer K. (Scripps Memorial Hospital, United States); Berndtson A.E.*, Averbach S., Curry T. (University of California San Diego, United States); Kwan-Feinberg R.*, Consorti E., Gonzalez R., Grolman R., Liu T., Merzlikin O. (Alta Bates Summit Medical Center [Sutter Health], United States); Abel M.K., Ozgediz D., Boeck M., Kornblith L.Z.*, Nunez-Garcia B., Robinson B., Park P. (University of California San Francisco [UCSF], United States); Utria A.F., Rice-Townsend S.E.*, Javid P., Hauptman J., Kieran K. (Seattle Children's Hospital, United States); Nehra D.*, Walters A., Cuschieri J., Davidson G.H. (Harborview Medical Center, United States); Nunez J.*, Cosker R. (University of Utah Healthcare, Salt Lake City, United States); Eckhouse S.* (Washington University School of Medicine, United States); Choudhry A., Marx W.* (Suny Upstate University Hospital, United States); Jamil T.*, Seegert S., Al-Embideen S. (ProMedica Toledo Hospital, United States); Quintana M.*, Jackson H. (The George Washington University Hospital, United States); Wexner S.D., Kent I. (Cleveland Clinic Florida, Weston, United States); Martins P.N.* (University of Massachusetts, UMass Memorial Hospital, United States).
Footnotes
Published online: January 19, 2023.
This study was presented at the 81st Annual Meeting of the American Association for the Surgery of Trauma and Clinical Congress of Acute Care Surgery, September 21–24, 2022, in Chicago, Illinois.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Contributor Information
Dias Argandykov, Email: dargandykov@mgh.harvard.edu.
Ander Dorken-Gallastegi, Email: adorkengallastegi@gmail.com.
Mohamad El Moheb, Email: elmoheb.m@gmail.com.
Anthony Gebran, Email: anthony.gebran@gmail.com.
Jefferson A. Proaño-Zamudio, Email: jproanozamudio@mgh.harvard.edu.
Mary Bokenkamp, Email: mbokenkamp@me.com.
Angela M. Renne, Email: arenne@mgh.harvard.edu.
Dmitri Nepogodiev, Email: nepogodiev@gmail.com.
Aneel Bhangu, Email: A.A.Bhangu@bham.ac.uk.
Collaborators: Kwabena Siaw-Acheampong, Leah Argus, Daoud Chaudhry, Brett E. Dawson, James C. Glasbey, Rohan R. Gujjuri, Conor S. Jones, Sivesh K. Kamarajah, Chetan Khatri, James M. Keatley, Samuel Lawday, Elizabeth Li, Harvinder Mann, Ella J. Marson, Kenneth A. Mclean, Maria Picciochi, Elliott H. Taylor, Abhinav Tiwari, Joana F.F. Simoes, Isobel M. Trout, Mary L. Venn, Richard J.W. Wilkin, Aneel Bhangu, Dmitri Nepogodiev, Irida Dajti, Arben Gjata, Luis Boccalatte, MariaMarta Modolo, Daniel Cox, Peter Pockney, Philip Townend, Felix Aigner, Irmgard Kronberger, Kamral Hossain, Gabrielle VanRamshorst, Ismail Lawani, Gustavo Ataide, Glauco Baiocchi, Igor Buarque, Muhammad Gohar, Mihail Slavchev, Arnav Agarwal, Amanpreet Brar, Janet Martin, Maria Marta Modolo, Maricarmen Olivos, Jose Calvache, Carlos Jose Perez Rivera, Ana Danic Hadzibegovic, Tomislav Kopjar, Jakov Mihanovic, Jaroslav Klat, René Novysedlak, Peter Christensen, Alaa El-Hussuna, Sylvia Batista, Eddy Lincango, Sameh H. Emile, Mengistu Gebreyohanes Mengesha, Samuel Hailu, Hailu Tamiru, Joonas Kauppila, Johanna Laukkarinen, Alexis Arnaud, Markus Albertsmeiers, Hans Lederhuber, Markus Loffler, Stephen Tabiri, Symeon Metallidis, Georgios Tsoulfas, Maria Aguilera Lorena, Gustavo Grecinos, Tamas Mersich, Daniel Wettstein, Dhruv Ghosh, Gabriele Kembuan, Peiman Brouk, Mohammad Khosravi, Masoud Mozafari, Ahmed Adil, Helen M. Mohan, Oded Zmora, Marco Fiore, Gaetano Gallo, Francesco Pata, Gianluca Pellino, Sohei Satoi, Faris Ayasra, Mohammad Chaar, Ildar R. Fakhradiyev, Mohammad Jamal, Muhammed Elhadi, Aiste Gulla, April Roslani, Laura Martinez, Antonio Ramos De La Medina, Oumaima Outani, Pascal Jonker, Schelto Kruijff, Milou Noltes, Pieter Steinkamp, Willemijn van der Plas, Adesoji Ademuyiwa, Babatunde Osinaike, Justina Seyi-olajide, Emmanuel Williams, Sofija Pejkova, Knut Magne Augestad, Zainab Al Balushi, Ahmad Qureshi, Raza Sayyed, Mustafa Abu Mohsen Daraghmeh, Sadi Abukhalaf, Moises Cukier, Hugo Gomez, Sebastian Shu, Ximena Vasquez, Marie Dione Parreno-Sacdalan, Piotr Major, José Azevedo, Miguel Cunha, Irene Santos, Ahmad Zarour, Eduard-Alexandru Bonci, Ionut Negoi, Sergey Efetov, Andrey Litvin, Faustin Ntirenganya, Ehab AlAmeer, Dejan Radenkovic, Frederick Koh Hong Xiang, Chew Min Hoe, James Ngu Chi Yong, Rachel Moore, Ncamsile Nhlabathi, Ruth Blanco Colino, Ana Minaya Bravo, Ana Minaya-Bravo, Umesh Jayarajah, Dakshitha Wickramasinghe, Mohammed Elmujtaba, William Jebril, Martin Rutegård, Malin Sund, Arda Isik, Sezai Leventoğlu, Tom E.F. Abbott, Ruth Benson, Ed Caruna, Sohini Chakrabortee, Andreas Demetriades, Anant Desai, Thomas D. Drake, John G. Edwards, Jonathan P. Evans, Samuel Ford, Christina Fotopoulou, Ewen Griffiths, Peter Hutchinson, Michael D. Jenkinson, Tabassum Khan, Stephen Knight, Angelos Kolias, Elaine Leung, Siobhan McKay, Lisa Norman, Riinu Ots, Vidya Raghavan, Keith Roberts, Andrew Schache, Richard Shaw, Katie Shaw, Neil Smart, Grant Stewart, Sudha Sundar, Dale Vimalchandran, Naomi Wright, Sattar Alshryda, Osaid Alser, Kerry Breen, Ian Ganly, Haytham Kaafarani, Brittany Kendall, Hassan Mashbari, Hamza Al Naggar, Dennis Mazingi, Joana F.F. Simoes, Wong J.J., Napolitano L., Hemmila M., Amin D., Abramowicz S., Roser S.M., Olson K.A, Riley C., Heron C., Cardenas T., Leede E., Thornhill M., Haynes A.B., McElhinney K., Roward S., Trust M.D., Hill C.E., Teixeira P.G., Etchill E., Stevens K., Ladd M.R., Long C., Rose J., Kent A., Yesantharao P., Vervoort D., Jenny H., Gabre-Kidan A., Margalit A., Tsai L., Malapati H., Yesantharao L., H. Abdou, J. Diaz, M. Richmond, J. Clark, L. O'Meara, N. Hanna, Y. Ying, J. Fleming, A. Ovaitt, J. Gigliotti, A. Fuson, Z. Cooper, A. Salim, S.A. Hirji, A. Brown, C. Chung, L. Hansen, B.U. Okafor, V. Roxo, C.P. Raut, J.S. Jolissaint, D.A. Mahvi, Kaafarani H., Breen K., Bankhead-Kendall B., Alser .o, Mashbari H., Velmahos G., Maurer L.R., El Moheb M., Gaitanidis A., Naar L., Christensen M.A., Kapoen C., Langeveld K., El Hechi M., Mokhtari A., M.H. Haqqani, F.T. Drake, A. Goldenberg-Sandau, B. Galbreath, Reinke C, Ross S, Thompson K, Manning D, Perkins R., E. Eriksson, H. Evans, M. Masrur, P. Giulianotti, E. Benedetti, G. Chang, J. Ourieff, D. Dehart, A. Dorafshar, T. Price, A.R. Bhama, A. Torquati, E. Cherullo, R. Kennedy, J. Myers, K. Rubin, V.S. Ban, S.G. Aoun, H.H. Batjer, J. Caruso, H. Carmichael, C.G. Velopulos, F.L. Wright, S. Urban, R.C. McIntyre, Jr., T.J. Schroeppel, E.A. Hennessy, J. Dunn, L. Zier, C. Burlew, J. Coleman, K.P. Colling, B. Hall, H.E. Rice, E.S. Hwang, S.A. Olson, D. Moris, R. Verma, R. Hassan, A. Volpe, S. Merola, L.A. O'Banion, J. Lilienstein, R. Dirks, H. Marwan, M. Almasri, G. Kulkarni, M. Mehdi, A. Abouassi, M. Abdallah, M. San Andrés, J. Eid, E. Aigbivbalu, J. Sundaresan, B. George, A. Ssentongo, P. Ssentongo, J.S. Oh, J. Hazelton, J. Maines, N. Gusani, M. Garner, S. Horvath, F. Zheng, M. Ujiki, G. Kinnaman, A. Meagher, I. Sharma, E. Holler, McKenzie K., J. Chan, K. Fretwell, W. Nugent, III, A. Khalil, D. Chen, N. Post, T. Rostkowski, D Brahmbhatt, K. Huynh, M.L. Hibbard, M. Schellenberg, Martin RCG, N. Bhutiani, E. Giorgakis, J. Laryea, A. Bhavaraju, K. Sexton, M. Roberts, M. Kost, M. Kimbrough, L. Burdine, K. Kalkwarf, R. Robertson, A. Gosain, L. Camp, R. Lewit, J.P. Kronenfeld, E. Urrechaga, N. Goel, R. Rattan, V. Hart, M. Allen, G. Gilna, A. Cioci, G. Ruiz, M. Allen, K. Rakoczy, W. Pavlis, R. Saberi, R. Morris, B.S. Karam, Brathwaite CEM, H. Liu, P. Petrone, H. Hakmi, A.H. Sohail, G. Baltazar, R. Heckburn, R.M. Nygaard, E.T. Colonna, F.W. Endorf, M.J. Hill, A. Maiga, B. Dennis, J.H. Levin, M. Lallemand, R. Choron, G. Peck, F. Soliman, S. Rehman, N. Glass, B. Juthani, D. Deisher, N.M. Ruzgar, S.J. Ullrich, M. Sion, C. Paranjape, M. El Moheb, A.R. Kar, C. Gillezeau, J. Rapp, E. Taioli, B.A. Miles, N. Alpert, D. Podolsky, N.L. Coleman, M.P. Callahan, I. Ganly, L. Brown, Monson JRT, A. Dehal, A. Abbas, A. Soliman, B. Kim, C. Jones, E. Dauer, E. Renza-Stingone, E. Hernandez, E. Gokcen, E. Kropf, H. Sufrin, H. Hirsch, H. Ross, J. Engel, J. Sewards, J. Diaz, J. Poggio, K. Sanserino, L. Rae, M. Philp, M. Metro, McNelis P., Petrov R., Rehman S., Pazionis T., B. Till, R. Lamm, A.J. Rios-Diaz, F. Palazzo, M. Rosengart, K. Nicholson, M.M. Carrick, K. Rodkey, A. Suri, R. Callcut, S. Nicholson, N. Talathoti, D. Klaristenfeld, W. Biffl, C. Marsh, K. Schaffer, A.E. Berndtson, S. Averbach, T. Curry, R. Kwan-Feinberg, E. Consorti, R. Gonzalez, R. Grolman, T. Liu, O. Merzlikin, M.K. Abel, D. Ozgediz, M. Boeck, L.Z. Kornblith, B. Nunez-Garcia, B. Robinson, P. Park, A.F. Utria, S.E. Rice-Townsend, P. Javid, J. Hauptman, K. Kieran, D. Nehra, A. Walters, J. Cuschieri, G.H. Davidson, J. Nunez, R. Cosker, S. Eckhouse, A. Choudhry, W. Marx, T. Jamil, S. Seegert, S. Al-Embideen, M. Quintana, H. Jackson, S.D. Wexner, I. Kent, and P.N. Martins
REFERENCES
- 1.COVIDSurg Collaborative . Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107(11):1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knisely A Zhou ZN Wu J, et al. Perioperative morbidity and mortality of patients with COVID-19 who undergo urgent and emergent surgical procedures. Ann Surg. 2021;273(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M Verleden SE Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaafarani HMA el Moheb M Hwabejire JO, et al. Gastrointestinal complications in critically ill patients with COVID-19. Ann Surg. 2020;272(2):e61–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.el Moheb M Naar L Christensen MA, et al. Gastrointestinal complications in critically ill patients with and without COVID-19. JAMA. 2020;324(18):1899–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naar L Langeveld K el Moheb M, et al. Acute kidney injury in critically-ill patients with COVID-19: a single-center experience of 206 consecutive patients. Ann Surg. 2020;272(4):e280–e281. [DOI] [PubMed] [Google Scholar]
- 8.Alser O Mokhtari A Naar L, et al. Multisystem outcomes and predictors of mortality in critically ill patients with COVID-19: demographics and disease acuity matter more than comorbidities or treatment modalities. J Trauma Acute Care Surg. 2021;90(5):880–890. [DOI] [PubMed] [Google Scholar]
- 9.COVIDSurg Collaborative . Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doglietto F Vezzoli M Gheza F, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155(8):691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madrazo Z, Osorio J, Otero A, Biondo S, Videla S. Postoperative complications and mortality following emergency digestive surgery during the COVID-19 pandemic: a multicenter collaborative retrospective cohort study protocol (COVID-CIR). Medicine (Baltimore). 2021;100(5):e24409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng JZ Chan JS Potter AL, et al. The risk of postoperative complications after major elective surgery in active or resolved COVID-19 in the United States. Ann Surg. 2022;275(2):242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad NK Mayorga-Carlin M Sahoo S, et al. Mid-term surgery outcomes in patients with COVID-19: results from a nationwide analysis. Ann Surg. 2022; Published online 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.COVIDSurg Collaborative; GlobalSurg Collaborative . Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2021;76(6):748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Society of Anesthesiologists and Anesthesia Patient Safety Foundation Joint Statement on Elective Surgery/Procedures and Anesthesia for Patients after COVID-19 Infection; 2021. Available at: https://www.asahq.org/about-asa/newsroom/news-releases/2022/02/asa-and-apsf-joint-statement-on-elective-surgery-procedures-and-anesthesia-for-patients-after-covid-19-infection. Accessed September 30, 2022.
- 16.Kibbe MR. Surgery and COVID-19. JAMA. 2020;324(12):1151–1152. [DOI] [PubMed] [Google Scholar]
- 17.Hogan A. COVID-19 and emergency surgery. Br J Surg. 2020;107(7):e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khuri SF Henderson WG Daley J, et al. The patient safety in surgery study: background, study design, and patient populations. J Am Coll Surg. 2007;204(6):1089–1102. [DOI] [PubMed] [Google Scholar]
- 19.Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250(3):363–376. [DOI] [PubMed] [Google Scholar]
- 20.American College of Surgeons National Surgical Quality Improvement Program . User Guide for the 2012 ACS NSQIP Participant Use Data File (PUF). Available at: https://www.facs.org/media/r23m4qap/acsnsqip2012ptpuf-userguide.pdf. Published January 2014. Accessed June 22, 2021.
- 21.BUPA . Schedule of Procedures. Available at: https://codes.bupa.co.uk/procedures. Accessed July 7, 2021.
- 22.Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Published online 2018. Available at: https://EconPapers.repec.org/RePEc:boc:bocode:s432001. Accessed August 7, 2021.
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995;57(1):289–300. Available at: http://www.jstor.org.ezp-prod1.hul.harvard.edu/stable/2346101. Accessed August 7, 2021. [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. [DOI] [PubMed] [Google Scholar]
- 25.Nicholls SG Quach P von Elm E, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement: Methods for arriving at consensus and developing reporting guidelines. PLoS One. 2015;10(5):e0125620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lal BK Prasad NK Englum BR, et al. Periprocedural complications in patients with SARS-CoV-2 infection compared to those without infection: a nationwide propensity-matched analysis. Am J Surg. 2021;222(2):431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le ST, Kipnis P, Cohn B, Liu VX. Covid-19 vaccination and the timing of surgery following Covid-19 infection. Ann Surg. 2022;276(5):e265–e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrier FM Amzallag É Lecluyse V, et al. Postoperative outcomes in surgical COVID-19 patients: a multicenter cohort study. BMC Anesthesiol. 2021;21(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonker PKC van der Plas WY Steinkamp PJ, et al. Perioperative SARS-CoV-2 infections increase mortality, pulmonary complications, and thromboembolic events: a Dutch, multicenter, matched-cohort clinical study. Surgery. 2021;169(2):264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.COVIDSurg Collaborative . Mortality and pulmonary complications in emergency general surgery patients with COVID-19: a large international multicenter study. J Trauma Acute Care Surg. 2022;93(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wichmann D Sperhake JP Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.COVIDSurg Collaborative; GlobalSurg Collaborative . SARS-CoV-2 infection and venous thromboembolism after surgery: an international prospective cohort study. Anaesthesia. 2022;77(1):28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators Goligher EC Bradbury CA, et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Eng J Med. 2021;385(9):777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American College of Surgeons . COVID-19 Guidelines for Triage of Emergency General Surgery Patients. Updated December 8, 2020. Available at:https://www.facs.org/for-medical-professionals/covid-19/clinical-guidance/elective-case/emergency-surgery/. Accessed September 11, 2021.
- 36.Moletta L Pierobon ES Capovilla G, et al. International guidelines and recommendations for surgery during Covid-19 pandemic: a systematic review. Int J Surg. 2020;79:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.COVIDSurg Collaborative . Outcomes and their state-level variation in patients undergoing surgery with perioperative SARS-CoV-2 infection in the USA: a prospective multicenter study. Ann Surg. 2022;275(2):247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.GlobalSurg Collaborative . Mortality of emergency abdominal surgery in high-, middle- and low-income countries. Br J Surg. 2016;103(8):971–988. [DOI] [PubMed] [Google Scholar]
- 39.Osorio J Madrazo Z Videla S, et al. Analysis of outcomes of emergency general and gastrointestinal surgery during the COVID-19 pandemic. Br J Surg. 2021;108(12):1438–1447. [DOI] [PubMed] [Google Scholar]


