Abstract
The Asteraceae family is one of the largest families in the plant kingdom with many of them extensively used for significant traditional and medicinal values. Being a rich source of various phytochemicals, they have found numerous applications in various biological fields and have been extensively used for therapeutic purposes. Owing to its potential phytochemicals present and biological activity, these plants have found their way into pharmaceutical industry as well as in various aspects of nanotechnology such as green synthesis of metal oxide nanoparticles. The nanoparticles developed from the plants of Asteraceae family are highly stable, less expensive, non-toxic, and eco-friendly. Synthesized Asteraceae-mediated nanoparticles have extensive applications in antibacterial, antifungal, antioxidant, anticancer, antidiabetic, and photocatalytic degradation activities. This current review provides an opportunity to understand the recent trend to design and develop strategies for advanced nanoparticles through green synthesis. Here, the review discussed about the plant parts, extraction methods, synthesis, solvents utilized, phytochemicals involved optimization conditions, characterization techniques, and toxicity of nanoparticles using species of Asteraceae and their potential applications for human welfare. Constraints and future prospects for green synthesis of nanoparticles from members of the Asteraceae family are summarized.
Keywords: Asteraceae family, green synthesis, nanoparticle, phytochemicals, characterization, biological activity, toxicity
1. Introduction
The Asteraceae (Aster, Compositae, Daisy) family represents around 1600 genera, of which around 80 are reported for nanoparticle synthesis. Asteraceae is a sophisticated and botanically immensely specialized family containing mostly herbs. They are found in the tropics and tropical and warm areas of South, Southeast, and East Asia, Africa, Madagascar, and Central South America. Several of the genera in this family, such as Aster, Helianthus, Chrysanthemum, and Tagetes, are ornamentals, most of which have therapeutic properties. Many members of this family are used in medicine. Some are commonly planted in the field for vegetable and nutritional purposes. The order Asterales is made up of a single family, the sunflower family, which is the biggest of all plant families, with approximately 20,000 species. The Asteraceae is not only a vast and widespread family, but also, as one might assume, a varied one. Evolution has been generated in several directions, and the main developmental pathways are characterized by combining related genera into tribes [1].
Around 300 Asteraceae species have been utilized for medicinal reasons. Phytochemical derivatives from Artemisia annua, for example, are used in the treatment of malaria. The endangered Saussurea involucrata had been used for anti-inflammatory, anti-tumor, and radical scavenging capabilities. Various Asteraceae family species such as Lactuca sativa, Cichorium intybus, Smallanthus sonchifolius, Helianthus tuberosus, etc., have been used as food crops. Seeds of Asteraceae species such as Helianthus annuus and Carthamus tinctorius have been used as cooking oil. Chrysanthemum, Tanacetum, and Pulicaria genera are proven to have insecticidal activities and are commercially significant members of the Asteraceae family [2]. Various ethnobotanical data on the traditional uses of Asteraceae species, particularly for blisters, breathing problems, miscarriage, pain, hypertension, runny nose, whooping cough, bowel problems, constipation, vomiting and diarrhea, metabolic syndrome, skin problems, infections, fracture, headache, cardiovascular disease, itchiness, anemia, menstruation illness, numbness, skin disorders, snake bites, sex issues, and dental illness is reported [3]. Plants present in this family are also used to cure different diseases such as tumors, sleeping sickness, indigestion, hepatotoxicity, epilepsy, etc. It also has antimicrobial, antioxidant, anti-proliferative, anti-inflammatory, and vasodilatory activities [4].
Recent studies reported that plants belonging to the Asteraceae family have an excellent ability to synthesize NPs in non-toxic ways and these NPs have numerous applications. Different NPs such as silver [5], gold [6], copper [7], iron oxide [8], and zinc oxide [9] are successfully synthesized from Asteraceae members. The biosynthesis of NPs using plant extract of Asteraceae members is simple, easily available, low cost, and eco-friendly [10,11]. Numerous studies offering experimental data on the biological impacts of Asteraceae species have grown in recent years. There is, however, no comprehensive systematic review that summarizes existing understanding. With extensive traditional knowledge and application of Asteraceae species, the current study attempted to compile all published research on their phytochemical extraction process for nanoparticle synthesis and pharmacological properties for the first time.
2. Plant-Based Green Synthesis of Nanoparticles
Green synthesis has acquired a lot of importance as a sustainable, economical, feasible, and environment-friendly synthesizing procedure for a variety of bio-inspired materials. Green synthesis helps in decreasing the harmful effects associated with nanoparticle synthesis by physical and chemical methods. Plant phytochemicals involved in green synthesis show greater reduction and stabilization properties. Biologically, the nanoparticle can be synthesized using bacteria, fungi, algae, and plants [12]. Among all these organisms, plants have a higher potential to produce the NPs because the synthesis of NPs using microorganisms is affected by culture contamination, lengthy procedures to produce adequate production of biomass, less control over NP size, and reuse of biomass for the subsequent nanoparticle synthesis. It is also difficult to maintain the microbial culture under aseptic conditions and the cost of isolation of microorganisms is not economically efficient [13]. At the same time, plant synthesis is more beneficial than the other methods due to its high stability, lack of contamination risk, easy preparation, and less time consumption [7,14]. Plants and their extracts act as natural chemicals because they contain phytochemicals such as flavonoids, terpenoids, phenols, polyphenols, amides, aldehydes, and saponins [15]. Reducing and capping the nature of phytochemicals and plant enzymes such as reductase help to reduce the NPs from metal ions. Plants eliminate the usage of expensive instruments, high-pressure, and hazardous chemicals [16].
3. Asteraceae Mediated Nanoparticle Synthesis: The Pursued Routes
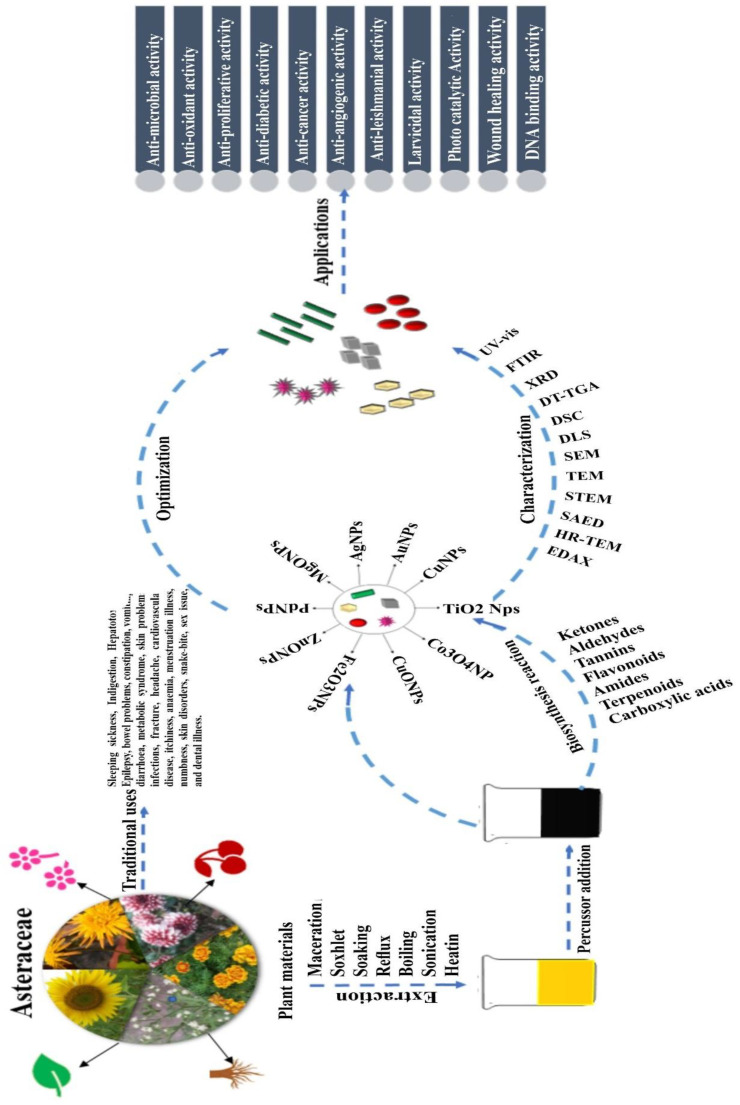
Efficient synthesis, extraction, and identification of nanoparticles require optimization of parameters such as the plant material and solvents used, phytochemicals involved, factors affecting the synthesis, and characterization techniques utilized for identification (Figure 1, Table 1 and Table 2).
Figure 1.
Flow chart showing the steps involved in the extraction, synthesis, optimization, and characterization of nanoparticles from the Asteraceae family and its applications.
Table 1.
Studies carried out to synthesize metal nanoparticles from the Asteraceae family and their biological applications.
| Plant | Part Used | Solvent Used | Extraction Method | Phytochemicals | Characterization Techniques | SPR Peak (nm) | Nanoparticle Size (nm) | Activity | References |
|---|---|---|---|---|---|---|---|---|---|
| Silver NPs | |||||||||
| Acanthospermum hispidum | Leaf | DiW | Reflux | Saponins, coumarins, phenols, flavonoids, volatile oils, tannins, and sterols | UV–Vis, FE-SEM, EDX, TEM, FTIR, Particle size, and zeta potential | 417 | 20–60 | Antibacterial, antifungal, antimalarial, and antimycobacterial activity | [17] |
| Achillea biebersteinii | Flower | DDW | Boiling | Polysaccharides, polyphenols, and proteins | UV–Vis, TEM, zeta potential, and EDX | 460 | 12 ± 2 | Anti-angiogenesis activity | [18] |
| Acroptilon repens | Whole plant | DDW | Reflux | Caryophyllene oxide, α-copaene, β-caryophylene, and β-copaene-4-α-ol | UV–Vis, SEM, and TEM | 420 | 38.89 | Anti-efflux activity | [19] |
| Ageratina adenophora | Leaf | - | - | Carbohydrates, alkaloids, phenols, flavonoids, xanthoprotein, glycosides, tannins, steroids, and terpenoids |
XRD, and FTIR | - | 25 | Antimicrobial activity | [20] |
| Ageratum conyzoides | Leaf | DDW | Boiling | Alkaloids, flavonoids, chromenes, benzofurans, and terpenoids | UV–Vis, FTIR, SEM, TEM, XRD, and EDX | 443 | 14–48 | DNA-binding, antioxidant, H2O2 sensing, and photocatalytic properties | [13] |
| Ambrosia arborescens | Leaf | DW | Stirring | Sesquiterpenic lactones, monoterpenes, terpenoids, and polyacetylenic resins | UV–Vis, FTIR, STEM, and SEM-EDX, | 414 | 14 ± 6 | Larvicidal activity | [21] |
| Anthemis atropatana | Aerial parts | Methanol | Boiling | Flavonoids, and phenolic compounds | UV–Vis, XRD, TEM, SEM, and FTIR | 430 | 38.89 | Antibacterial and cytotoxic activity | [21,22] |
| Arctium lappa | Whole plant | DW | Boiling | Phenolic acids, flavonoids, alkaloids, and terpenoids | UV–Vis, XRD, TEM, HRTEM, FTIR, EDX, TG, and DTA | 435 | 21.3 | Antimicrobial activity and catalyst for degradation of pollutants | [6] |
| Arnicae anthodium | Whole plant | DW | Boiling | Flavonoids. Triterpenes, sesquiterpene lactones and essential oils. | UV–Vis, FTIR, TXRF, and SEM-EDS | 458 | 90–118 | Antimicrobial activity | [23] |
| Artemisia marschalliana | Aerial parts | 50% ethanol | Boiling | Phenolic acids and flavonoids | UV–Vis, XRD, FTIR, TEM, SEM, zeta potential, and EDS | 430 | 5–50 | Antioxidant, anticancer, and antibacterial activity | [24] |
| Artemisia turcomanica | Leaf | 50% ethanol | Boiling | Phenolic acids, flavonoids, alkaloids and terpenoids | UV–Vis, TEM, SEM, XRD, and FTIR | 430 | 22 | Cytotoxic and anti-cancer activity | [25] |
| Artemisia vulgaris | Leaf | Methanol | Maceration | Phenolic acids, flavonoids, and alkaloids | UV–Vis, SEM, EDX, TEM, AFM, and FTIR | 420 | 25 | Antimicrobial, antioxidant, and antiproliferative activities | [26] |
| Aspilia pluriseta | Leaf | DW | Boiling | Flavonoids, phenols, alkaloids, and amino acids | UV–Vis, FTIR, SEM, DLS, TEM, and XRD | 427 | 6 | Antimicrobial and catalytic activity | [27] |
| Bidens frondosa | Whole plant | DW | Boiling | Terpenoids, phenolics and proteins | UV–Vis, FTIR, FESEM, and EDS | 443 | 20–70 | Tyrosinase activity | [28] |
| Bidens pilosa | Leaf, stem, and root | DW | Stirring | Terpenes, essential oils, tannins, polysaccharides, phenols, amino acids, ascorbic acid and organic acids |
UV–Vis, SEM, TEM, EDX, and FTIR | 410 | 17 | Antimicrobial and anticancer activity | [29] |
| Blumea eriantha | Whole plant | Ethanol | Soxhlet | Phenols and flavonoids | UV–Vis, FTIR, SEM, XRD, and TEM | 445 | 10 | Antioxidant, antimicrobial, and cytotoxic activities | [30] |
| Calendula officinalis | Seed | DW | Boiling | Triterpenoids, flavonoids, coumarines, quinones, volatile oil, carotenoids, and amino acids | UV–Vis, TEM, XRD, and FTIR | 440 | 05–10 | - | [31] |
| Carpesium cernuum | Whole plant | Methanol | Reflux | Polyphenols | UV–Vis, and HR-TEM | 430 | 13.0 ± 0.2 | Antioxidant and anticancer activity | [32] |
| Carthamus tinctorius | Stem and Leaf | DW | Boiling | Flavonoids, polyphenols, proteins, sugars and saponins | HR-TEM, FTIR, and SEM | - | 10 | Antibacterial activity | [33] |
| Centaurea virgata | Aerial parts | N-hexane, chloroform, and methanol: water | Soxhlet | Flavonoids, phenolic acids, and terpenes | UV–Vis, FTIR, TEM, SEM, EDX, TGA XRD, and zeta potential | 420 | 25–50 | Antioxidant activity | [34] |
| Centratherum anthalminticum | Whole plant | DW | Heating | Phenolics and flavones | UV–Vis, XRD, SEM, FTIR, Particle size, DLS, and zeta potential | 436 | <50 | Antimicrobial activity | [35] |
| Chamaemelum nobile | Whole plant | DW | Heating | Phenolics and flavones | UV–Vis, DLS, FTIR, XRD, and TEM | 422 | 24.2 ± 3.1 | Antibacterial activity | [36] |
| Chromoleana odorata | Leaf | - | - | - | UV–Vis, FTIR, XRD, SEM, FE-SEM, and EDX | 428 | 20–25 | Antibacterial activity and hydrogen peroxide detection | [37] |
| Chrysanthemum indicum | Flower | DW | Boiling | Flavonoids, terpenoids, and glycosides | UV–Vis, XRD, TEM, and EDX | 435 | 37.71–71.99 | Antibacterial and cytotoxic activity | [38] |
| Chrysanthemum morifolium | Flower | DW | Boiling | Flavonoids, caffeoylquinic acids, chlorogenic acid, phenolic acids | UV–Vis, FTIR, XRD, and TEM | 430 | 20–50 | Antibacterial activity | [39] |
| Cichorium intybus | Leaf | DDW | Boiling | Phenolic acids, triterpenoids, sterols, and hydroxycinnamic acid derivatives | XRD, FTIR, zeta potential, TEM, SEM, and EDS | - | 17.17 | Anticancer activity | [40] |
| Cosmos caudatus | Leaf | DW | Boiling | Phenolic acids, triterpenoids, and sterols | UV–Vis, XRD, FTIR, FESEM-EDX, and TEM | 439 | 21.49 ± 7.43 | - | [41] |
| Cosmos sulphureus | Leaf | DW | Boiling | Phenols, polyphenolic, and flavonoids | UV–Vis, Particle size, zeta potential, DLS, and SEM | 430–440 | 55–80 | Antimicrobial and antioxidant properties | [42] |
| Crassocephalum rubens | Leaf | DW | Boiling | Flavonoids, and polyphenols | UV–Vis, EDX, TEM, SEM, and FTIR | 470 | 15–25 | Antioxidant activity | [43] |
| Cynara cardunculus | Leaf | DW | Boiling | Polyphenols, flavonoids, and terpenoids | TEM, EDS, FTIR, and XPS | 435 | 45 | Antibacterial and electrochemical activity | [44] |
| Cynara scolymus | Leaf | DW | Heating | Alkaloids, polyphenols, flavonoid, and amino acid | UV–Vis, FTIR, SEM, EDX, and zeta sizer | 434 | 98.47 ± 2.04 | Anticancer activity | [45] |
| Dahlia pinnata | Leaf | DW | Boiling | Flavonoids, and phenolics | UV–Vis, XRD, TEM, and FTIR | 460 | 15 | Detection of Hg2+ ion | [46] |
| Dicoma tomentosa | Bark | DW | Boiling | Flavonoids, phenolic acids, and terpenes | UV–Vis | 430–480 | - | Antimicrobial activity | [47] |
| Dittrichia viscosa | Leaf | DW | Boiling | Flavonoids and polyphenols | UV–Vis, XRD, FTIR, and TEM | 406 | 5–25 | Bactericidal effects | [48] |
| Echinacea purpurea | Whole plant | DW | Heating | Caffeic acid derivatives, polysaccharides, alkaloids, alkylamides, and polyphenols | UV–Vis, XRD, SEM, and FTIR | 481 | 68.24 | Antioxidant activity | [49] |
| Echinops sp. | Root | DW | Heating | Carbohydrates, alkaloids, phenols, flavonoids, xanthoprotein, glycosides, tannins, steroids, and terpenoid | UV–Vis, UV-DRS, FTIR, XRD, SEM, EDXA, TEM, HRTEM, and SAED | 454 | 33.86 | Antimicrobial activity | [50] |
| Eclipta alba | Leaf | DW | Boiling | Phenols, flavonoids, and aldehydes | UV–Vis, DLS, FTIR, XRD, and SEM | 433 | 310–400 | Antimicrobial and cytotoxic activity | [51] |
| Elephantopus scaber | Leaf | DW | Boiling | Phenolics, amino acids, aliphatic, and aromatic hydroxyl groups | UV–Vis, NTA, TEM, XRD, and FTIR | 435 | 50 | Antioxidant activity | [52] |
| Erigeron bonariensis | Leaf | DW | Boiling | Terpenoids, flavonoids, and phenol derivatives | UV–Vis, SEM, EDX, TEM, XRD, AFM, and FTIR | 422 | 13 | Catalytic activity | [53] |
| Eupatorium odoratum | Leaf | DW | Boiling | Tannins, saponins, phytates, flavonoids, betacyanins, and alkaloids, steroids, terpenoids, phenols, quinones, and glycosides | UV–Vis, particle size, TEM, and PXRD | 424 | 23.6 | Antimicrobial and mosquito larvicidal activity | [54] |
| Galinsoga formosa | Leaf and Flower | DW | Boiling | Phenolics, amino acids, aliphatic, and aromatic hydroxyl groups | UV–Vis | 350–400 | - | Photocatalytic degradation activity | [55] |
| Gazania rigens | Whole plant | DW | Boiling | Flavonoids, polyphenols, proteins, sugars, and saponins | UV–Vis, XRD, EDX, and SEM | 425–460 | 31.35 | Antioxidant and photocatalytic degradation activity | [56] |
| Gundelia tournefortii | Leaves | DW | Stirring | Scopoletin, chlorogenic acids, terpinen-4-ol, linalool, zingiberene, caffeic acid, cymene, p-cymene, limonene, gallic acid, stigmasterol, aesculin, quercetin, and β-sitosterol. | UV–Vis, FE-SEM, TEM, XRD, and FTIR | 419 | 16.5 | Fungicidal, bactericidal, and cutaneous wound healing effects | [57] |
| Gynura procumbens | Leaves | DiW | Heating | Flavonoid and glycosides | UV–Vis, FTIR, TEM, and zeta potential | 449–471 | 100 | - | [58] |
| Handelia trichophylla | Flower | DiW | Stirring | - | UV–Vis, FESEM, EDX, TEM, FTIR, and XRD | 448 | 20–50 | Cytotoxic and antibacterial activity | [59] |
| Helichrysum graveolens | Shoot | DW | Flavonoid and other secondary metabolites | UV–Vis, FTIR, and TEM | 439 | 11 | Antimicrobial, anticancer, and photocatalytic degradation activity | [60] | |
| Jurinea dolomiaea | Root | DW and methanol | Soaking | Phenols and flavonoids | UV–Vis, XRD, SEM, and FTIR | 444 | 24.58 | Antimicrobial activity | [61] |
| Kleinia grandiflora | Leaf | DiW | Boiling | - | UV–Vis, FTIR, XRD, SEM, TEM, and EDX | 436–448 | 20–50 | Antimicrobial, cytotoxicity, and photocatalytic degradation activity | [62] |
| Lactuca sativa | Leaf | Ultrapure water | Boiling | Polyphenols, flavonoids, sterols, triterpenes, triterpenoid saponins, beta-phenylethylamines, tetrahydroisoquinolines, reducing sugars such as glucose and fructose, amino acids, and proteins | UV–Vis, TEM, SEM, and FTIR | 450 | 40–70 | Antimicrobial activity | [63] |
| Launaea taraxacifolia | Leaf | DW | Heating | Alcohols, amides, and carbohydrates | UV–Vis, SEM, EDX, and TEM | 440 | 9–15.5 | Antibacterial activity | [64] |
| Matricaria recutita | Stem | DW and absolute ethanol | Boiling | Terpenoids, flavonoids, and coumarins | UV–Vis, SAED, HRTEM, and FTIR | 445 | 11 | Mercury ions sensor | [65] |
| Mikania micrantha | Leaf | DW | Boiling | Polyphenols, polyamides, and flavonoids | UV–Vis, FTIR, XRD, EDX, and TEM | 425 | 5–20 | Antibacterial activity | [66] |
| Oedera genistifolia | Leaf | DW | Heating | Phenolic, flavonoids, carbohydrates, terpenoids, and proteins | UV–Vis, FTIR, SEM, EDX, TEM, XRD, and TGA | 400–500 | 34.2 | Cytotoxic and antibacterial activity | [67] |
| Parthenium hysterophorus | Leaf | DW | Boiling | Alkaloids, glycoside, proteins, terpenoids, flavonoids, saponins, and tannins | UV–Vis, DLS, zeta potential, SEM, TEM, and FTIR | 432 | 20–25 | Anti-bacterial and antioxidant activity | [68] |
| Pechuelloeschea leubnitziae | Root | Hexane, dichloromethane, and methanol | Rotary evaporator | Saponins, anthraquinones, flavonoids, and polyphenols | UV–Vis, FTIR, XRD, EDX, and TEM | 400 | 100 | Anti-proliferative activity | [69] |
| Pluchea sericea | Leaf | DW | Heating | Flavonoids and phenolic compounds | UV–Vis, EDS, zeta potential, DLS, and EDS | 487 | 59.2 | Antibacterial activity | [70] |
| Pulicaria glutinosa | Whole plant | DiW | Reflux | Flavonoids and polyphenols | UV–Vis, XRD, TEM, EDX, and FTIR | 422–459 | 40–60 | - | [71] |
| Rhanterium epapposum | Flower | 70% Methanol | Heating | - | UV–Vis, XRD, TEM, and FTIR | 423 | 16.3 | Antifungal and cytotoxic activities | [72] |
| Sanvitalia procumbens | Whole plant | DW | Heating | Flavonoids, phenolic groups, organic acids, and proteins | UV–Vis, FTIR, XRD, EDX, and SEM | 438 | 46 | Photocatalytic degradation activity | [73] |
| Saussurea costus | Root | - | - | - | UV–Vis, SEM, TEM, EDX, and FTIR | 420 | 5–15 | Photocatalytic degradation activity | [74] |
| Scorzonera calyculata | Aerial part | Ethanol and water | Stirring | Phenolic acid, flavonoids, alkaloids, and terpenoids | UV–Vis, TEM, SEM, FTIR, and XRD | 420 | 25.28 | Antibacterial, anticancer, and antioxidant activity | [75] |
| Seripheidium quettense | Aerial part | DW | Boiling | Phenols and flavonoids | UV–Vis, FTIR, XRD, SEM, TEM, and EDX | 428 | 48.40–55.35 | Antibacterial, antifungal, and cytotoxic activity | [76] |
| Silybum marianum | Seed | DW | Boiling | Proteins, polysaccharides, and flavonoids | UV–Vis, XRD, and TEM | 425 | 1–25 | - | [77] |
| Solidago altissima | Leaf | Millipore water | Boiling | - | UV–Vis, FTIR, EDS, SEM, TEM, and XRD | 462 | 111 | Antibacterial and photocatalytic activity | [78] |
| Solidago canadensis | Leaf | DW | Boiling | - | UV–Vis, and TEM | - | 180.6 | Cytotoxic activity | [79] |
| Spilanthes calva | Leaf | DW | Boiling | - | UV–Vis, SEM, EDAX, and FTIR | 448.5 | 5–50 | - | [80] |
| Stevia rebaudiana | Leaf | 70% Ethanol | Heating | Flavonoids, phenolic acids, fatty acids, proteins, and vitamins | UV–Vis, and SEM | 450 | 16–25 | - | [81] |
| Synedrella nodiflora | Leaf | - | - | - | UV–Vis, FTIR, and XRD | 460 | - | Antimicrobial activity | [82] |
| Tagetes erecta | Flower | DiW | Boiling | - | UV–Vis, FTIR, XRD, SEM, and EDAX | 420 | 24–49 | Photocatalytic degradation activity | [83] |
| Tanacetum vulgare | Fruit | Ultrapure water | Boiling | - | UV–Vis, TEM, XRD, EDX, and FTIR | 452 | 10–40 | - | [84] |
| Taraxacum officinale | Leaf | Milli-Q water | Boiling | Flavonoid and phenolics acids (caffeic acid, and chlorogenic acid) | UV–Vis, XRD, FTIR, and HR-TEM | 435 | 15 | Antimicrobial, antioxidant, and anticancer activity | [85] |
| Tithonia diversifolia | Leaf | DW | Boiling | Proteins, polysaccharides, and terpenoids | UV–Vis, TEM, EDX, TG-DTA, and FT-IR | 435 | 25 | Antimicrobial activity | [86] |
| Tragopogon buphthalmoides | Whole plant | DW | Boiling | - | UV–Vis, XRD, FESEM, TEM and FTIR | 420 | - | Photocatalytic degradation activity | [87] |
| Tragopogon collinus | Leaf | Ethanol and methanol | Soaking and boiling | - | UV–Vis, TEM, XRD, and FT-IR | 400 | 7 | Antibacterial activity | [88] |
| Verbesina encelioides | Leaf and stem | DiW | Boiling | Sesquiterpenes, flavonoids, galegine, triterpenoids friedelin, epifriedelin, lupeol, a-, b-amyrin, stigmasterol, botulin, and bsitosterol | UV–Vis, FTIR, SEM, and XRD | 430 | 54.6 | Antimicrobial activity | [89] |
| Vernonia amygdalina | Leaf | Ethanol, 50% ethanol, DiW | Sonication | - | SEM, TEM, EDX, and FTIR | - | 41.555 ± 2.488 | Anticancer activity | [90] |
| Vernonia cinerea | Leaf | DDW | Boiling | - | UV–Vis, TEM, XRD, and FTIR | 430 | 5–50 | Antibacterial activity | [91] |
| Wedelia chinensis | Leaf | Milli-Q water | Boiling | Flavonoids and polyphenols | UV–Vis, TEM, EDX, XRD, XPS, and FTIR | 408 | 31.68 | Antioxidant, antibacterial and cytotoxic activity | [92] |
| Xanthium strumarium | Leaf | DiW | Boiling | Alkaloids, flavonoids, triterpenoids, terpenoids, tannin, saponin, quinone, protein, and sugars | HRTEM, SAED, FESEM, EDX, XRD, AFM, and FTIR | 436 | - | Antibacterial and antileishmanial activity | [15] |
| Zinnia elegans | Seed | - | - | - | UV–Vis, and DLS | 439 | 79.5 | Antioxidant activity | [93] |
| Gold NPs | |||||||||
| Arctium lappa | Whole plant | DDW | Heating | - | UV, SEM, TEM, FTIR, and AFM | 580 | 10–40 | Cytotoxic activity | [94] |
| Centaurea behen | Leaf | DiW | Boiling | Flavonoids, alkaloids, sesquiterpene lactones, lignans, chlorogenic, caffeic, ferulic, p-coumaric acids, isoquercitrin, and coumarin | UV–Vis, FTIR, XRD, EDX, and TEM | 538 | 50 | Antioxidant and anticancer activity | [95] |
| Cichorium intybus | Seed | DDW | Reflux | Alkaloids, inulin, sesquiterpene lactones, coumarins, vitamins, chlorophyll pigments, unsaturated sterols, flavonoids, saponins, tannins, and polyphenols. | UV–Vis, DLS, TEM, zeta potential, XRD, and FTIR | 540 | 10–30 | Antiproliferative, antioxidant, and photocatalytic activities | [96] |
| Crassocephalum rubens | Leaf | DW | Boiling | Flavonoids and polyphenols | UV–Vis, TEM, SEM, and FTIR | 540 | 15–25 | Antioxidant activity | [43] |
| Echinacea angustifolia | Flower | DW | Heating and stirring | Flavonoids, phenolics, flavones, and terpenoid | UV–Vis, FTIR and SEM | 560 | 80–120 | Antibacterial activity | [97] |
| Eclipta alba | Whole plant | Methanol | Soxhlet | - | UV–Vis, XRD, FTIR, DLS, TEM, SEM, and AFM | 536 | 26 | Antibacterial, antidiabetic, and anti-apoptotic activity | [98] |
| Elephantopus scaber | Leaf | - | - | - | UV–Vis, FTIR, SEM, and TEM | 540 | 20–40 | Anticancer activity | [99] |
| Erigeron annuus | Flower | - | - | - | UV–Vis, HR-TEM, XRD, EDS, FTIR and zeta potential | 537 | 20–100 | Catalytic activity | [100] |
| Eupatorium odoratum | Leaf | DiW | Heating | - | UV–Vis, DLS, FTIR, and TEM | 528 | 10–20 | Catalytic activity | [101] |
| Gundelia tournefortii | Leaf | DW | Soxhlet | - | UV–Vis, FTIR, FESEM, and EDS | 528 | 40–45 | Cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing activity | [102] |
| Rhanterium epapposum | Flower | Methanol | Heating | - | UV–Vis, XRD, TEM, and FTIR | 525 | 17.9 | Antifungal and cytotoxic activities | [72] |
| Solidago canadensis | Leaf | DDW | Maceration | Flavonoids, phenolic acids, glucosides, polysaccharides, diterpenes, triterpenoid saponosides, saponins, tannins, and essential oils | UV–Vis, ATR-FTIR, XRD, TEM, EDX, SAED, and SEM | 530 | 8–200 | - | [103] |
| Stevia rebaudiana | Leaf | Methanol | Soxhlet | - | UV–Vis, FTIR, XRD, SEM, and TEM | 500–550 | 17 | - | [104] |
| Taraxacum officinale | Whole plant | DW | Heating | - | UV–Vis, SEM, TEM, and XRD | 500–600 | 15 | - | [105] |
| Xanthium strumarium | Leaf | DiW | Heating | - | UV–Vis, FTIR, XRD, SEM, and TEM | - | 9.60–11.70 | Antibacterial and antifungal activity | [106] |
| Copper NPs | |||||||||
| Achillea biebersteinii | Leaf | DW | Stirring | Phenolics, anthraquinone, alkaloids, steroids, flavonoids, saponin, and tannin | UV–Vis, FTIR, EDS, TEM, and FESEM | 577 | 16.8 | Cytotoxic activity | [107] |
| Ageratum houstonianum | Leaf | DDW | Heating | Flavonoids, alkaloids, tannins, terpenes, steroid, and saponins, | UV–Vis, XRD, SEM, FTIR, TEM, and particle size analyzer | - | ~80 | Photocatalytic and antibacterial activity | [13] |
| Blumea balsamifera | Leaf | Ethyl acetate, n-hexane, and acetate | Rotary evaporator | Flavonoids and terpenoids | UV–Vis, SEM, and EDX | 540 | 30–55 | Antioxidant and cytotoxicity activity | [108] |
| Eclipta prostrata | Leaf | DW | Boiling | Thiophene-derivatives, steroids, triterpenes, flavonoids, polyacetylenes, polypeptides, and coumestans | UV–Vis, XRD, SEM, FTIR, EDX and HRTEM | 695 | 31 ± 1.2 | Antioxidant and cytotoxicity activity | [109] |
| Pluchea sericea | Leaf | DDW | Boiling | Phenols, flavonoids, and proteins | FTIR, EDS, and SEM | - | 68.1 | Insecticide activity | [110] |
| Tridax procumbens | Leaf | DW | Boiling | Alkaloid, carbohydrates, phenols, flavonoids, protein, amino acids, and phytosterol | UV–Vis, FTIR, SEM and XRD | 320 | 71 | Antioxidant, antibacterial, photocatalytic degradation activity | [111] |
| Palladium NPs | |||||||||
| Pulicaria glutinosa | Whole plant | DiW | Reflux | Polyphenolic and flavonoidic groups | UV–Vis, XRD, TEM, EDX, and FTIR | 415 | 20–25 | Catalytic activity | [112] |
Note: UV–Vis: UV–Visible spectrophotometry; SEM: scanning electron microscopy; TEM: transmission electron microscopy; HRTEM: high resolution transmission electron microscopy; STEM: scanning transmission electron microscopy; SAED: selected area electron diffraction; XRD: X-ray crystallography; EDAX: energy dispersive X-ray analysis; DT-TGA: differential thermo gravimetric analysis; FTIR: Fourier transform infrared spectroscopy; TGA: thermal gravimetric analysis; DSC: differential scanning calorimetry; DTA: differential thermal analysis; TXRF: total reflection X-ray fluorescence; PPMS: physical property measurement system; VSM: vibrating sample temperature; EDXRF: energy dispersive X-ray fluorescences; BET: Brunau–Emmet–Teller analysis; XPS: X-ray photoelectron spectroscopy; AFM: atomic force microscopy; DLS: dynamic light scattering method; nm: nanometer; DW: distilled water; DDW: double distilled water; DiW: deionized water; SPR: surface plasmon resonance—: not available.
Table 2.
Studies carried out to synthesize metal oxide nanoparticles from Asteraceae family and their biological applications.
| Plant | Part Used | Solvent Used | Extraction Method | Phytochemicals | Characterization Techniques | SPR Peak (nm) | Nanoparticle Size (nm) | Activity | References |
|---|---|---|---|---|---|---|---|---|---|
| Zinc oxide NPs | |||||||||
| Arctium lappa | Whole plant | DDW | Heating and stirring | Polyacetylenes, arctinol, arctinal, arctinon, guaiane lactones, lignans, flavonoids, phenolic acids, inulin phytosterols, essential oil potassium, magnesium, and calcium salts, sesquiterpene bitter | UV, SEM, TEM, FTIR, and AFM | 350 | 10 to 40 | Cytotoxic activity | [94] |
| Artemisia annua | Whole plant | - | Heating and stirring | - | UV, FTIR, XRD, and TEM | 330 | 20 | Cytotoxic activity | [113] |
| Artemisia pallens | Whole plant | DDW | Distillation | - | UV, FTIR, XRD, SEM, and TEM | 370 | 50–100 | Antimicrobial activity | [114] |
| Artemisia scoparia | Whole plant | - | - | - | UV, FT-IR, XRD, TEM, FESEM, EDX, DLS, and zeta potential | 370 | 9.00 ± 4.00 | Anticancer activity | [115] |
| Cynara scolymus | Leaf | DW | Boiling | Phenolics acids, bitter sesquiterpenes lactones, and flavonoids | UV, FTIR, SEM, TEM, EDXA, and XRD | 371 | 65 | Antimicrobial, antiproliferative, and photocatalytic activity | [116] |
| Dicoma anomala | - | - | - | Alkaloids, flavonoids, tannins, and saponins | UV–Vis, TEM, FTIR, EDS, and XRD | 386 | - | Antidiabetic activity | [117] |
| Dittrichia graveolens | Whole plant | - | - | - | UV–Vis, FTIR, and FESEM | 285–320 | 100 | - | [118] |
| Echinacea angustifolia | Flower | DW | Heating and stirring | Flavonoids, phenolics, flavones, and terpenoids | UV–Vis, FTIR, and SEM | 368 | 90–170 | Antibacterial activity | [97] |
| Lactuca sativa | Whole plant | - | - | - | SEM, zeta potential, and DLS | - | 90 | - | [119] |
| Parthenium hysterophorus | Leaf | DDW | Heating | - | UV–Vis, SEM, TEM, and SEM-EDX, | 400 | 16–45 | Antibacterial activity | [120] |
| Saussurea lappa | Root | Methanol | Soaking | - | UV–Vis, FTIR, XRD, FESEM, and EDX | 430 | 26 ± 1 | Cytotoxic, antibacterial, and antifungal activities | [121] |
| Silybum marianum | Whole plant | DW | Heating and stirring | Polyphenols and flavonoids | UV–Vis, FTIR, XRD, HRSEM, and HRTEM | 374 | 25 | Antibacterial, antifungal, cytotoxicity, antileishmanial, antioxidant, and enzyme inhibition activity. | [122] |
| Tagetes erecta | Flower | - | - | Alkaloids, flavonoids, carbohydrates, amino acids, tannins, and proteins | UV, XRD, and SEM | 364.15 | 30–50 | Antioxidant, antimicrobial, and cytotoxic activities | [123] |
| Tithonia diversifolia | Leaf | DDW | Heating and stirring | Flavonoid, tannin, glycoside, alkaloids, saponin, steroids, and phenol. | UV–Vis, FTIR, XRD, SEM, EDX, and TEM | 385 | 9.83–28.85 | Dye degradation activity | [124] |
| Tragopogon collinus | Leaf | Ethanol | Boiling | Phenols | UV–Vis, TEM, XRD, and FT-IR | 369 | 21 | Antibacterial activity | [125] |
| Vernonia amygdalina | Leaf | Ethanol | Heating and stirring | - | UV–Vis, SEM, FTIR, XRD, and EDX | 347 | 9.5 | - | [126] |
| Zinnia elegans | Seed | - | - | - | UV–Vis, and DLS | 350 | 82.6 | Antioxidant activity | [93] |
| Iron Oxide NPs | |||||||||
| Ageratum conyzoides | Whole | DW | Boiling | Phenols and flavonoids | UV–Vis, FTIR, XRD, SEM, and SEM-EDX | 390 | 85.9 | Antimicrobial and photocatalytic activity | [8] |
| Artemisia vulgaris | Leaf | DiW | Heating | - | TEM, PSA, XRD, FTIR, VSM, and TGA | - | 30 | Photocatalytic degradation activity | [127] |
| Bidens pilosa | Leaf | DW | Heating | Phenols and flavonoids | UV–Vis, FTIR, EDXRF, XRD, and SEM | 288 | - | Photocatalytic degradation activity | [128] |
| Centaurea cyanus | Whole | DDW | Heating | Polyphenols, phenols, and flavonoids | XRD, BET, FTIR, and FE-SEM | - | 24 | Photocatalytic degradation activity | [129] |
| Galinsoga parviflora | Leaf | DW | Heating | Phenols and flavonoids | UV–Vis, FTIR, EDXRF, XRD, and SEM | 267 | - | Photocatalytic degradation activity | [128] |
| Mikania mikrantha | Leaf | DDW | Boiling | - | UV–Vis, XRD, SEM, TEM, and FTIR | - | 20.27 | Antimicrobial activity | [130] |
| Stevia | Whole | DiW | - | - | XRD, FESEM, HRTEM, TGA, XPS, VSM, and zeta potential | - | 20 | Antioxidant activity | [131] |
| Vernonia amygdalina | Leaf | DiW | Boiling | - | UV, FTIR, XRD, and SEM | 396 | - | - | [132] |
| Wedelia urticifolia | Leaf | DDW | Heating | - | UV, FTIR, XRD, TEM, and PPMS. | 320 | 70 | Photocatalytic degradation activity | [133] |
| Copper Oxide NPs | |||||||||
| Acanthospermum hispidum | Leaf | DiW | Reflux | Coumarins, tannins, saponins, phenols, flavonoids, sterols, and volatile oils | FESEM, EDX, TEM, XRD, and FTIR | - | 9–21 | Antimicrobial, antimalarial and antimycobacterial activity | [134] |
| Anthemis nobilis | Flower | DDW | Reflux | Luteolin-7-O-glucoside, apigenin-7-O-apioglucoside, and apigenin-7-O-glucoside. | UV–Vis, SEM, EDS, XRD, and FTIR | 250 | - | Catalytic activity | [135] |
| Eupatorium odoratum | Leaf | DW | Boiling | Flavonoids, phenolic compounds, and triterpenoids | UV–Vis, FTIR, XRD, SEM, TEM, and EDAX | 211 and 305 | - | Antibacterial activity | [136] |
| Titanium oxide NPs | |||||||||
| Ageratina altissima | Leaf | DDW | Boiling | - | UV–Vis, FTIR, XRD, and FESEM | 332 | 60–100 | Photocatalytic degradation activity | [137] |
| Echinacea purpurea | Whole plant | DDW | Boiling | Alkamides, cichroic acid, and polysaccharides | UV–Vis, SEM, TXRF, and FTIR | 280 | 120 | - | [138] |
| Sonchus asper | Leaf | DW | Soxhlet | - | XRD, FTIR, and FESEM | - | 9–15 | Antimicrobial activity | [139] |
| Nickel oxide NPs | |||||||||
| Ageratum conyzoides | Leaf | Methanol | Maceration | Alkaloids, tannins, phenols, saponin, and flavonoids | UV–Vis, FTIR, particle size, XRD, and TEM | 324 | 8–15 | Photocatalytic activity | [140] |
| Tagetes erecta | Leaf | DDW | Boiling | Flavonoids and phenols | XRD, SEM-EDX, TEM, and XPS | 266–285 | 18.2 | Photocatalytic, electrochemical sensing, and antibacterial activity | [141] |
| Cobalt Oxide NPs | |||||||||
| Taraxacum Officinale | Leaf | DiW | Soaking | Flavonoids and phenols | UV–Vis, FTIR, SEM, and TEM | 319 | 50–100 | Catalytic activity | [142] |
| Magnesium oxide NPs | |||||||||
| Artemisia abrotanum | Whole plant | DW | Magnetic stirring | Polyphenols, flavonoids (aglycones and glycosylates), and hydroxycinnamic derivatives | UV–Vis, FTIR, XRD, SEM, and TEM | 300 | 10 | Antioxidant and photocatalytic activity | [143] |
| Chromolaena odorata | Leaf | DiW | Water bath | Alkaloids, flavonoids, tannins, and other phenolic compounds | UV–Vis, FTIR, SEM, EDX, TEM, XRD, TGA, and DTA | 270 | 12.3 | Antimicrobial and catalytic activity | [144] |
| Saussurea costus | Root | Methanol | Reflux | Sesquiterpenes, alkaloid, triterpenes, lignans, and tannins | UV–Vis, FTIR, XRD, SEM, zeta potential, and DLS | 250 and 320 | 34 | Antimicrobial, anticancer, and photocatalytic activity | [145] |
Note: UV–Vis: UV–Visible spectrophotometry; SEM: scanning electron microscopy; TEM: transmission electron microscopy; HRTEM: high resolution transmission electron microscopy; STEM: scanning transmission electron microscopy; SAED: selected area electron diffraction; XRD:X-ray crystallography; EDAX: energy dispersive X-ray analysis; DT-TGA: differential thermo gravimetric analysis; FTIR: Fourier transform infrared spectroscopy; TGA: thermal gravimetric analysis; DSC: differential scanning calorimetry; DTA: differential thermal analysis; TXRF: total reflection X-ray fluorescence; PPMS: physical property measurement system; VSM: vibrating sample temperature; EDXRF: energy dispersive X-ray fluorescence; BET: Brunau–Emmet–Teller analysis; XPS: X-ray photoelectron spectroscopy; AFM: atomic force microscopy; DLS: dynamic light scattering method; nm: nanometer; DW: distilled water; DDW: double distilled water; DiW: deionized water; SPR: surface plasmon resonance—: not available.
3.1. Plant Material Used
Roots, stems, leaves, fruits, flowers, and seeds of Asteraceae members were used for the synthesis of NPs. The parts which are used to synthesize the nanoparticle could be washed and heated using a solvent. Researchers reported that both fresh and dried samples could be used to synthesize NPs. Dried samples at room temperature go through the process of weighing and crushing. Using Whatman filter paper, solutions are filtered, and clear solutions are used for synthesis [107]. Several studies report NPs being successfully synthesized from leaves of Acanthospermum hispidum, [17], the stem of Matricaria recutita [65], roots of Pechuelloeschea leubnitziae [69], the flower of Rhanterium epapposum [72], and seeds of Silybum marianum [77], etc., of Asteraceae members.
3.2. Extraction Methods
Extraction is the first and crucial step in the production of NPs. It happens when the solvent is diffused into plant tissues and solubilized phytochemicals with similar polarity and also these phytochemicals in the plant extract function as biocatalysts. The plant extract can be extracted using different methods such as maceration, soaking, soxhlet, reflux, sonication, heating, and boiling methods. Maceration was used to prepare an aqueous extract of Solidago canadensis to synthesize gold NPs [103]. Leaf extracts of Spilanthes calva were prepared using a boiling method to synthesize silver NPs [146]. To synthesize silver NPs from the leaf extract of Tragopogon collinus both the soaking and boiling methods were used [88].
3.3. Solvents Used
The solvent-free synthesis is not achievable in nanoparticle synthesis, since solvents have a crucial role in transferring the heat, dissolving solids, purification, and isolation steps, and altering viscosity. In green synthesis, the solvent is used in large amounts when compared to other materials, so the choice of solvent is essential, and also the types of solvents used during extraction significantly affect the amount of reducing agents extracted. Benzene is proven to be the best solvent but cannot be used in the synthesis of NPs due to its carcinogenic nature. Predominantly distilled water in addition to organic solvents such as ethanol and methanol are used to prepare extracts for the NPs synthesis from Asteraceae members. Among all the solvents, water is the best choice for the synthesis of NPs as it is non-toxic, eco-friendly, non-flammable, and economically feasible [147]. Tagetes erecta aqueous extracts were used for nickel NPs synthesis [141]. However, methanol and ethanol extract is used to synthesize silver nanoparticles from Tragopogon collinus [88].
3.4. Phytochemicals Involved
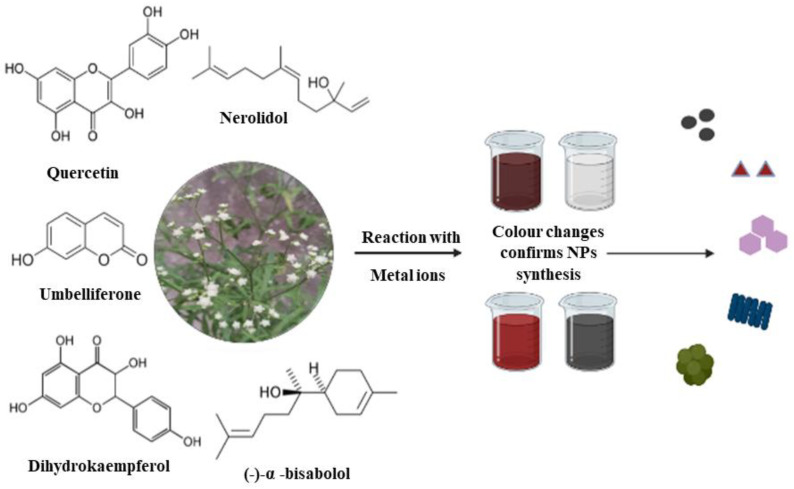
It is reported that the phytochemicals such as ketones, aldehydes, tannins, flavonoids, amides, terpenoids, and carboxylic acids in the plant are in charge of metal ion reduction (Figure 2). The compounds in the plant extract components, are capable of electron donation, causing metal ion reduction to NPs. Plant extract concentration also depends on the solvent used for the extraction process. Tannins help in the production of silver NPs by acting as reducing and capping agents and also water-soluble secondary metabolites such as proteins, amino acids, and phenol control the biosynthesis of silver NPs. Several studies report plant extract and phytochemical concentrations affecting the size, shape, and application of the nanoparticle [148]. Tragopogon collinus extract containing phenolic compounds play a prominent role in the production of NPs [88]. In UV spectrum analysis, the aqueous leaf extract of Bidens pilosa, Galinsoga parviflora, and Conyza bonariensis showed absorbance peaks at 288 nm, 267 nm, and 286 nm, respectively. These peaks confirmed the presence of sugars, polyphenols, and amino acids, helpful in Fe ions reduction to Fe NPs [128].
Figure 2.
Summary of the role of phytochemicals present in Asteraceae family in reduction of metal ions to various nanostructured materials.
3.5. Nanoparticle Synthesis from Asteraceae Species
A large number of Asteraceae members have been utilized for the synthesis of various nanoparticles such as gold, silver, iron, copper, etc. For example, silver NPs synthesized from Asteraceae family members have significant catalytic action, atomic behavior, and biochemical reactivity due to large surface area. Recognition, reduction, limited nucleation, and growth help in the formation of silver NPs. In the recognition stage, the metal ions will be trapped on the surface protein of the plant extract by electrostatic interaction. Thereafter, proteins present in the extract reduce the Ag+ ions to Ag0 by changing the secondary structure of the protein. This causes Ag ions reduction and accumulation in nuclei. The linkage of protein and a large number of biomolecules in the solution may lead to isotropic growth and the production of stable NPs [149].
The NPs synthesized by the addition of silver solution to the extract via. the green method is detected by color change. The color change is an indicator of the Ag ions reduction to Ag NPs by the plant extract phytochemicals. Briefly, the phytochemical compounds such as polyphenols, terpenoids, etc., present in the extract of Asteraceae members, donate electrons to reduce metal ions and form zero-valent metal atoms. Eventually, the collision of metal atoms with these atoms in the mixture results in the formation of several atoms with a stable core. These atoms will perform as nucleation regions and will form clusters that will continue to grow till an active supply of atoms, results in NPs formation. The process is carried out by the reduction of metal ions into metal NPs [33]. Similarly, other metal ions are converted to respective metal NPs as plant extracts are capable of forming NPs by adding metal salt to the solution. The color change in metal NPs varies from each other for example, dark brown, wine red, reddish-brown, and white color for silver, gold, iron, and zinc oxide NPs, respectively.
3.5.1. Factors Affecting the Synthesis of Asteraceae NPs
The green synthesis of metal nanoparticle formation is by metal ion reduction caused by phytochemical compounds present in Asteraceae members. Several factors affect the synthesis of NPs such as plant extract concentration, metal ions concentration, temperature, pH, and reaction time. These factors affect the size, shape, and distribution of NPs.
Temperature
During nanoparticle synthesis, temperature plays a crucial role in metal ion reduction to metal NPs. Normally, the reaction is carried out at room temperature, but it is also reported that some members of the Asteraceae family need a higher temperature to reduce the metal ions to metal NPs. Studies report silver NPs synthesized at room temperature (Acanthospermum hispidum and Anthemis atropatana), 40 °C (Achillea biebersteinii), 60 °C (Centratherum anthelmminticum), and 80 °C (Arnicae anthodium) [17,18,22,23,35]. UV–Vis spectroscopy explains that Ag NPs synthesized from leaves of Arctium lappa and Eupatorium odartum at 90 °C give an intense surface plasmon resonance (SPR) band and this intense SPR band indicates synthesis of NPs on a large scale [6,54]. At room temperature, gold NPs could be synthesized from Centaurea behens leaf extract when it is mixed with chloroauric acid [95]. Rectangular, cubic, and hexagonal-shaped Cu NPs can be synthesized using Ageratum houstonianum leaf extract at room temperature [13]. At 55 °C, a dark brown colored copper nanoparticle solution was formed from copper (II) nitrate trihydrate solution and aqueous leaf extract of Calendula officinalis [150]. In copper nanoparticle formation, when time increases the surface plasmon resonance decreases due to the oxidation of Cu NPs [151]. Metal oxide NPs such as iron oxide NPs and ZnO NPs were formed from Artemisia species and metal precursors at room temperature [114,127].
pH
pH is a significant parameter during the synthesis of NPs. NPs’ size, shape, and stability are affected by the reacting solutions’ nature, i.e., acidic and alkaline medium. Reports suggest large-sized NPs are formed in an acidic medium and small-sized NPs are formed in an alkaline medium. However, the conversion efficiency of NPs is high in an alkaline medium [152]. Studies on the pH effect on AgNPs’ formation using Tithonia diversifolia showed absorbance intensity increased gradually with an increase in pH range. However, in basic and neutral pH, the Ag NPs formation was very fast which was evident from the color change in the reaction mixture. However, at basic pH, there is a possibility of Ag ions precipitating as AgOH. Studies support pH 7 as the optimal pH to synthesize silver NPs [86]. pH was maintained at 5.4 to synthesize gold NPs from an aqueous extract of Sphaeranthus indicus and hydrogen tetrachloroaurate (II) trihydrate [153]. Different pH levels such as 9, 10, 11, and 12 were also used for the synthesis of zinc oxide NPs from Tragopogon collinus extract, and a broad peak was observed in pH 9 and a narrow peak showed in pH 12 solution. The broad peak could be due to the large particle size and the narrow peak due to the nanosized material. Therefore, pH 12 was concluded as best for zinc oxide NPs formation using Tragopogon collinus [125].
Reaction Time
Reaction time is a major factor in the synthesis of NPs. In Asteraceae-mediated nanoparticle synthesis, the formation of NPs takes place immediately after adding the metal precursor. Interestingly, the reduction and synthesis of silver NPs using Bidens frondosa extract were observed using UV–visible spectroscopic analysis. Silver nitrate solution addition to B. frondosa extract, Ag NPs synthesis started immediately and maximum production of AgNPs occurred at ambient temperature on 5 h of incubation [28]. The size, shape, and stability of the nanoparticle are also dependent upon the reaction time. The reaction time varies based on factors such as the concentration of metal ions, phytochemicals present, temperature, and pH of the plant extract [154]. Initially, the mixture of Sphaeranthus indicus extract and AuCl4 solution was light yellow color, it changed to a wine-red color after 30 min of stirring [153].
Metal Ion Concentration
Metal ion concentration depends upon which metal is being used to synthesize NPs. Studies reported that for silver nanoparticle synthesis, the frequently used concentration is 1 mM, and other concentrations (1, 2, 3, 5, 8, 10, 53, 100, and 200 mM) of metal NPs are synthesized [148]. Varying concentrations of zinc acetate dihydrate (0.05 to 0.25 M) were taken to synthesize zinc oxide NPs from the mixture of zinc acetate dihydrate and aqueous extract of Tragopogon collinus. Results showed that the absorption intensity was low at 0.2 M and high at 0.05 M. When metal ion concentration is increased beyond the threshold then gradually the nanoparticle synthesis will be decreased, and also higher concentration can lead to the agglomeration of the NPs [125]. Metal ions concentration also varies based on the presence of phytochemicals. The concentration of metal ions will also affect the size, shape, and uses of NPs [148].
Plant Extract Concentration
Concentration of plant extract depends upon the number of phytochemicals present in the plant. The concentration of phytochemicals varies among plants and within plant families. Studies revealed that 0.1 g to 10 g of plant parts were utilized to synthesize NPs [128,133]. An amount of 10 g of Wedelia urticifolia leaves was utilized to synthesize magnetic iron oxide nanorods. Similar studies revealed that 20 g dried powder of Bidens pilosa, Galinsoga parviflora, and Conyza bonariensis was utilized to synthesize iron NPs [128]. Investigation of the production of zinc oxide nanoparticles using Tragopogon collinus, different amounts of extracts (0.25, 0.5, 1, and 2 mL) were utilized. The result showed that 1 ml of the extract van reduce 50 mL of 0.01 M of zinc acetate dihydrate solution, for the synthesis of a large quantity of zinc oxide NPs. The optimum quantity or higher amount of the extract increases the intensity of the absorbance peak. The quantity of synthesized NPs increases when the phytochemical present in the extract is more. Hence, large quantities of extract increase the production of NPs with improved absorption intensity [125].
3.6. Separation of NPs
The centrifugation approach for purifying NPs is frequently used to remove residual components and byproducts. Apart from centrifugation, NPs can be separated using chromatography and electrophoresis techniques [155]. Appropriate separation and purification are critical for nanoparticle characterization and applications. As noted in the reviewed publications, the green synthesis produced a variety of forms and sizes, the majority of which were spherical and polydisperse, and was proven to be efficient for the creation of silver NPs. Green synthesis, compared to physical and chemical synthesis processes results in less controlled morphologies, which may be related to several reducing/capping phytochemicals, which cause multiple redox reaction rates and growth of the NPs [16].
3.7. Characterization
Characterization techniques are utilized for the determination of NPs’ form, shape, surface, and dispersion. UV–visible spectrophotometry (UV–Vis), dynamic light scattering (DLS), zeta potential, Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), differential scanning calorimetry (DSC) energy dispersive spectroscopy (EDS), selected area electron diffraction (SAED), thermogravimetric analysis (TGA), scanning electron microscopy (SEM), transmission electron microscopy (TEM), scanning transmission mode (STEM), etc., are some of the commonly used methods [156].
3.7.1. UV–Visible Spectroscopy
UV–Vis is a relatively easier technique that permits rapid identification and characterization of NPs. Because of the interaction of light with movable surface electrons of NPs produces a significant absorbance band in the 400–500 nm range known as surface plasmon resonance (SPR) [157].
The UV–Vis absorbance peaks were observed in a range of 414 to 460 nm, 530 to 580 nm, 320 to 690 nm, 415 nm, 330 to 430 nm, 260 to 496 nm, 211 to 305 nm, 282 to 322 nm, 266 to 324 nm, and 250 to 320 nm for Ag NPs, Au NPs, Cu NPs, Pd NPs, ZnO NPs, Fe2O3 NPs, CuO NPs, TiO2 NPs, CO3O4 NPs, MgO NPs, respectively [8,17,94,107,112,134,137,140,142,143].
The copper NPs synthesized using Achillea biebersteinii leaf aqueous extract peaked at 577 nm [107]. Iron NPs synthesized using Ageratum conyzoides extracts were observed at 390 nm [8]. Biosynthesized titanium dioxide NPs by using Echinacea purpurea Herba extract that showed a peak at 280 nm [138]. Gold NPs synthesized using flower extract of Carthamus tinctorius showed a peak at 560 nm [158].
3.7.2. Fourier Transforms Infrared Spectroscopy
The FT-IR reveals the surface properties of nanomaterials. This method aids in the identification of functional groups in both phytoconstituents and the resultant NPs. The FT-IR analysis of plant phytochemicals in free form or attached to NPs occasionally predicts minor band changes. There have been few studies on the use of pure phytochemical substances in the manufacture and use of NPs [45]. The list of nanoparticles synthesized from Asteraceae family, which characterized through FT-IR spectra described in Table 3.
Table 3.
Fourier transform infrared (FT-IR) spectra of the nanoparticles synthesized from Asteraceae family.
| Plant Name | FTIR Absorption Bands (cm−1) | Possible Functional Group | References | |
|---|---|---|---|---|
| Plant Extract | NPs | |||
| Silver NPs | ||||
| Acanthospermum hispidum | 3786 | - | -OH | [17] |
| 2964 | - | C-H | ||
| 1706 | - | C=O | ||
| 1601 | - | C=C | ||
| 1016 | - | C-O | ||
| Ageratum conyzoides | - | 3440.29 | N-H stretching | [13] |
| - | 2358.95 | C-H | ||
| 1383.98 | - | Alcohol, ethers, esters, carboxylic acids, and amino acids | ||
| 1613.99 | - | C=O | ||
| 1074.83 | - | C-OH | ||
| Ambrosia arborescens | - | 1570 | C=C | [21] |
| - | 1050 | CO | ||
| 1337.47 | - | O-H | ||
| 3280 | - | OH | ||
| - | - | |||
| Anthemis atropatana | - | 1014 | C-O | [22] |
| - | 1048 | C-O stretching | ||
| 3344 | 1595 | N-H bending | ||
| - | 2368 | Cyanide | ||
| Arctium lappa | 596 | 632 | C-Cl stretching | [6] |
| 1033 | 1036 | C-N | ||
| 1336 | 1384 | N-H | ||
| 2870 | 2853 | C-H stretching | ||
| 3375 | 3375 | O-H stretching | ||
| Arnicae anthodium | 3284 | - | -OH stretching | [23] |
| 2853 | - | -C-H stretching | ||
| 1735 | - | C=C | ||
| 1622 | - | C=O | ||
| 1370 | - | -C-O | ||
| 1027 | - | -C-O-C | ||
| - | 430, 395 | -OH | ||
| Artemisia marschalliana | 3463 | - | O-H | [24] |
| 3510 | - | Protein binding | ||
| 2962, 2823 | - | C-H | ||
| 1624 | - | C-O | ||
| - | 1398 | C-N | ||
| 1049 | 1038 | C-O-C | ||
| Artemisia turcomanica | 13,429 | 3429–3473 | O-H | [25] |
| 3029 | - | C-H | ||
| 2929 | - | Aliphatic group | ||
| 1635 | - | C=O | ||
| 1459 | - | CH2 | ||
| 1273 | - | C-O-C phenolic stretching | ||
| 1064, 1119, 1168 | - | C-O-C | ||
| 1201 | - | C-O-C stretching | ||
| 1000 | - | C=C-H | ||
| - | 1635–1624 | Carbonyl amide group | ||
| - | 1382 | N=O | ||
| Artemisia vulgaris | 3419, 3151 | - | O=H | [26] |
| 1619 | - | -C=O | ||
| 1400 | - | -C-N | ||
| 1069 | - | -C-O | ||
| Carthamus tinctorius | 3293 | - | -OH | [33] |
| 2932 | - | C-H | ||
| 1725 | - | C=O | ||
| 1599 | 1533 | C=C | ||
| 1414 | - | C=C aromatic | ||
| 1053 | - | C-O | ||
| 860 | - | C-H | ||
| 818 | - | #ERROR! | ||
| 776 | 323 | N-H | ||
| Chrysanthemum indicum | 3293 | - | –OH | [38] |
| 2932 | - | C–H | ||
| 1725 | - | C=O | ||
| 1053 | - | C=C,C–O–H | ||
| 1599 | - | C=C | ||
| - | 1288 to 1299 | Ag | ||
| Chrysanthemum morifolium | 1406 | - | C=C group | [39] |
| 1078 | - | C–O stretch | ||
| 2921 | - | C–H | ||
| 3384 | - | O–H | ||
| Cichorium intybus | 3413.05 | - | O–H alcoholic group | [40] |
| 2922.98 | - | Aliphatic C–H group | ||
| 1619.08 | - | C=C | ||
| 1384.6 | - | C–H | ||
| 1114.28 | - | C–O–C | ||
| - | 874.47 | N–H | ||
| Cosmos caudatus | 3364.81 | - | O–H | [41] |
| 2925.49 | - | C–H | ||
| 1650.59 | - | C=O | ||
| 1384.67 | - | C-N | ||
| 1067.62 | - | O–H secondary alcohols | ||
| Cosmos Sulphureus | - | 1643.35 | ––C==C–– | [42] |
| - | 2980.02 | C––H | ||
| - | 3421.72 | O––H | ||
| 1637.56 | - | ––C==C–– | ||
| 2981.95, 3748.2 | - | C––H | ||
| Cynara scolymus | - | 538 | Ag+ to Ag | [45] |
| Dahlia pinnata | 1064 and 3265 | - | Aromatic compounds | [46] |
| 2916 | - | C-H stretching of aldehydes | ||
| 673 and 1595 | - | Halo-alkanes and bending of C-H bonds | ||
| Echinacea purpurea | 3,203 | - | OH stretching | [49] |
| 2929 and 2829 | - | C–H bonds | ||
| Echinops sp. | 3395 | - | OH stretching frequencies | [50] |
| 1718 | - | C=O vibration of ketonic groups | ||
| 2925 | - | C−H stretching mode | ||
| 601 | - | Ag–O bond | ||
| Eclipta alba | 3603 and 3471 | - | O–H stretch | [51] |
| 3379 and 3278 | - | Primary and secondary amines and amides | ||
| 2931 | - | C–H stretch | ||
| 1064 | - | C–N stretch represents aliphatic amines | ||
| Elephantopus scaber | 1611 to 1400 | - | Presence of aromatic rings in the leaf extract. | [52] |
| 1109 | - | Presence of OH groups | ||
| Erigeron bonariensis | 3376 | - | -OH groups of phenolic compounds and -NH stretching of the proteins | [53] |
| - | 3434 | Intensity of Ag | ||
| Helichrysum graveolens | 2927 | - | C–O stretching, free | [60] |
| 1608 | - | C=O stretching | ||
| 1035 | - | C–N stretching | ||
| 1417 | - | O–H bend | ||
| - | 820 | C–O stretching | ||
| - | 606 | C–X stretching vibration | ||
| 2358 | - | C–H asymmetric stretching | ||
| Oedera genistifolia | 1117 | - | Plant extract | [67] |
| 1118 | - | NP synthesized | ||
| Spilanthes calva | 3919.31 | - | O-H-stretch | [80] |
| 3435 | - | O-H-stretch | ||
| 1412.79 | - | C-F stretch | ||
| 1257.83 | - | C-F stretch | ||
| Tagetes erecta | 3401 | - | O–H group | [83] |
| 2940 | - | Aromatic compounds | ||
| 1673 | - | –C=C– bond | ||
| 1104 | - | C–N bond | ||
| Taraxacum officinale | 3360 to 3400 | - | -NH2 in primary aromatic amines and -OH groups | [85] |
| 2300 to 2990 | - | C-H | ||
| 1421 | - | C=C | ||
| 1610 | - | C=O | ||
| 1063 | - | C-OH | ||
| Tithonia diversifolia | 3398 | - | O–H stretching vibrations of polyols | [86] |
| 1641 | - | Stretching vibration of (NH) C O group | ||
| - | 672 | N–H | ||
| Tragopogon Collinus | 3385 | - | OH | [88] |
| 2921 | - | NH | ||
| - | 1640 | C–O in amide I | ||
| - | 1413 | NH2 group in amide II | ||
| Vernonia cinerea | 1633 | - | Amide I, C=O groups | [91] |
| 3431 | - | O–H stretching | ||
| 1515 and 1540 | - | –C=C (aromatic ring) | ||
| 1380 | - | O–H in-plane bend of phenol | ||
| Wedelia chinensis | 1022 | - | C–O | [92] |
| 1326 | - | C-O-C stretching | ||
| 1696 | - | C=O | ||
| Gold NPs | ||||
| Arctium lappa | 3307 | - | -OH stretching and the aliphatic methylene group -C-H stretching | [94] |
| 2151 | - | Alkynes group | ||
| 1634 | - | Carboxyl stretching | ||
| - | 415, 406, 394 and 383 | Metal biomolecules found in the extract | ||
| Erigeron annuus | 3100, 2850, 2620, 1300, 1100, and 620 | - | Extract | [100] |
| 2900 | - | C-H stretching vibration in methylene group | ||
| 1405 | - | Hydrocarbons of methylene group | ||
| Rhanterium epapposum | 1622 to 1630 | - | C=O stretching of carbonyl groups | [72] |
| - | 925 to 553 | Stretching of haloalkanes | ||
| Stevia rebaudiana | 1078 | - | Nitrogen–carbon C-N bond stretching of aliphatic amine groups | [104] |
| 240 and 1634 | - | Amides III and II bands of proteins | ||
| - | 1629 | Amide I | ||
| Copper NPs | ||||
| Ageratum houstonianum | 3264.96 | - | O–H stretch | [159] |
| 2916.19 | - | N+–H stretch | ||
| 2359.9 | - | C–H stretching | ||
| - | 1074.64 | O-C stretching | ||
| - | 667.81 | Aromatic H bending | ||
| - | 597.86 | |||
| Blumea balsamifera | 3378 | - | OH bond of phenolic compound such as flavonoids, tannins, and glycoside | [108] |
| 1100 and 1700 | C-O and C=O | |||
| 610 | Cu NPs | |||
| Eclipta prostrata | 3333 | - | Hydroxy group | [109] |
| 2917 | - | Methylene C-H asym./sym. stretch | ||
| 1615 | - | Aromatic ring stretch | ||
| - | 1610 | NH C=O to metals CuNPs | ||
| Pluchea sericea | 3341 | - | O-H stretching | [110] |
| 2935 | - | C-H and N-H bonds | ||
| 1623–1410 | - | C=N stretching vibrations | ||
| 1046 | - | C=O | ||
| - | 622 | Cu NPs | ||
| Titanium oxide NPs | ||||
| Ageratina altissima | 3287 | - | Alcohol, phenols with O-H stretches | [137] |
| 2922 | - | Ammonium ions with N-H stretching | ||
| 1645 | - | Acyclic compound with C-C stretching | ||
| 1537 | - | Aliphatic of the nitro compound with stretching of N-O | ||
| 1238 | - | C-O stretching | ||
| 1150 | - | Alcohol compound with C-O stretching | ||
| Echinacea purpurea | 1024 | - | C-O stretching alcohols | [138] |
| 1385 | - | C-H rock alkenes | ||
| 1590 | - | C=C characteristic of saturated hydrocarbons | ||
| 3320 | - | O-H | ||
| Sonchus asper | 3937 | - | OH stretching vibrations | [139] |
| 3190 | - | N-H stretching | ||
| 2851 | - | Symmetric CH2 stretching bands | ||
| 2600 | - | H bonded NH vibrations | ||
| - | 1000 and 500 | Ti-O-Ti linkage in TiO2 | ||
| Copper oxide NPs | ||||
| Eupatorium odoratum | 3976 | - | Adsorbed water molecules | [136] |
| 3406 | - | -OH stretching vibrations of phenolic group | ||
| 1520 | - | C‚ C stretch in aromatic rings | ||
| 1420 | - | O-H bend of polyphenol | ||
| - | 1121 | Cu-OH vibrations | ||
| - | 815 and 613 | -CH bending vibrations | ||
| - | 653 and 610 | Cu-O signals | ||
Note: NPs: nanoparticles;—: not applicable.
The IR spectrum of silver Ag NPs synthesized from Ageratum conyzoides showed absorption bands at 3444.29, 2358.95, 1613.99, 1383.98, 1074.83, and 699.38 cm−1. The peak at 3440.29 cm−1 corresponds to amide N-H stretching. The peak observed at 2358.95 cm−1 may be due to the C-H stretching of the methylene group. The band at 1383.98 cm−1 corresponds to the presence of stretching vibrations of alcohol, esters, ethers, carboxylic acids, and amino acids [13]. The AuNPs peaks were observed at 415, 406, 394, 383, and 1629, which detect metal oxide bonds. The Cu NPs represent broad peaks at 3378 cm−1 and can be assigned to the phenolic compounds with OH bonds such as flavonoids, tannins, and glycoside derivatives. In addition, peaks at 1100 and 1700 cm−1 depict C-O and C=O stretching, respectively, of Blumea balsamifera leaf extracts [108]. The peaks 1264 and 1077 indicate the presence of C–O stretching of alcohols, carboxylic acids, and ester and ether groups in Pd NPs [112].
3.7.3. X-ray Diffraction
XRD offers chemical information for both elemental and phase research. XRD is beneficial for measuring stress and analysis of texture, in addition to chemical characterization. XRD analysis requires crystalline samples, however, the technique can determine the degree of crystallinity in polymers. XRD has typically been used for bulk sample analysis. However, with the introduction of new optical techniques, the thin-film examination may now be performed [160].
The XRD pattern of CuNPs was synthesized from Eclipta prostrata leaves extract, showing the formation of a face-centered cubic (FCC) arrangement ranging from 23 to 57 nm, with an average size of 31±1.2 nm [109]. Peaks for AgNPs were observed at 38.2°, 44.1°, 64.1°, and 77.0° [18]. The 2θ values 38°, 44°, 64°, and 77° correspond to AuNPs [95]. The crystallinity of Pd NPs from P. glutinosa plant extract was confirmed by XRD analysis. Five distinct reflections in the diffractogram at 40.02° (111), 46.49° (200), 68.05° (220), 81.74° (311), and 86.24° (222) were observed, which predicts to FCC shape of palladium NPs [112]. The XRD pattern for ZnO NPs was 31.61°, 34.26°, 36.10°, 47.37°, 56.40°, 62.68°, and 67.72° [113]. The peaks appearing at 2 thetas of 19.86, 25.90, 26.11, 28.31, 29.82, 29.99, and 30.04 correspond to Fe2O3 NPs [8].
3.7.4. Zeta Potential
The zeta potential indicates a nanoparticle’s charge concerning its surroundings. The zeta potential, however, is not a measurement of the molecule’s surface charge; rather, it is a measurement of the electric double layer formed by the surrounding ions in the solution. Zeta potential between 10 and +10 mV are essentially considered neutral, but zeta potential greater than +30 mV or less than 30 mV are strong cations and strong anions, respectively [161].
The zeta potential of synthesized AgNPs from Centratherum anthalminticum (L.) Kuntze was measured at −25.75 mV [35]. The zeta potential was observed at −31 mV suggesting the stability of AgNPs synthesized from Artemisia marschalliana [24]. The super-paramagnetic Fe2O3 NPs synthesized using the Stevia plant showed a magnitude of zeta potential observed at −41.1 mV [131]. The AuNPs synthesized by Cichorium intybus L. showed a zeta potential of −19.7 eV. Zeta potential measurement was performed to predict the surface charge and stability of NPs [96].
3.7.5. Dynamic Light Scattering (DLS)
The sizing of NPs by DLS uses temporal variation of scattered light from suspended particles in Brownian motion to calculate their hydrodynamic size distribution [161,162]. It measures the hydrodynamic size, direct study of retention periods (also offers a hydrodynamic size), and differential refractometry or viscometry to assess macromolecular components’ molecular weight. [163]. The particle size of copper NPs synthesized by using Ageratum houstonianum Mill leaf extract was observed to be approx. 80 nm. The size of dispersed NPs was also confirmed by DLS analysis [13]. AuNPs synthesized by Cichorium intybus L. and Elephantopus scaber (Linn.) leaf extract showed the particle size 1.7–3.2 nm and 20–40 nm, respectively [96].
3.7.6. Differential Scanning Calorimetry (DSC)
Melting characteristics and dependent melting temperature depression of synthesized nanomaterials are determined using DSC. The Gibbs–Thomson equation is utilized to study the size-dependent melting temperature property of alloy NPs, yielding a satisfactory prediction of melting temperature depression [163,164].
3.7.7. Thermogravimetric Analysis (TGA)
In a controlled environment, the change in mass of a sample as a function of temperature and/or time is measured by TGA. A high-precision thermobalance is coupled to a pan/crucible holder within a temperature-controlled furnace to form the thermogravimetric analyzer used for TGA studies. The sample environment is controlled by a purge gas supplied into the furnace, such as nitrogen gas for an inert atmosphere or air/oxygen for an oxidizing atmosphere. Temperatures ranging from room temperature to 1000 °C are ideal for TGA studies [165].
After heating to 900 °C, the biosynthesized Ag/AgCl NPs using aqueous leaf extract of Oedera genistifolia preserved more than 70% of their original weight. Initial weight loss between 30–200 °C might be attributed to Ag/AgCl NPs moisture loss, and subsequent weight loss was detected. At 900 °C, the Ag/AgCl NPs preserved around 70% of their weight, indicating their resilience [67]. TGA provides the measure of biosynthesized IONPs from Artemisia vulgaris leaf extract weight as temperature varies over time. At temperatures below 200 °C, the mass of NPs varies by about 100%, indicating that the substance is related to water. At temperatures of up to 200 °C, IONPs begin to lose mass, indicating the breakdown of NPs coated biomolecule compounds [127].
3.7.8. Selected Area Electron Diffraction (SAED)
SAED patterns were utilized to determine the typical morphological characteristics, framework, crystal structure, and chemical properties to identify the particles studied. For TiO2 rutile nano-size granules, a series of field examinations were carried out at various time frames and weather conditions to demonstrate the preliminary capability of these collecting and analysis methods [166]. SAED pattern for AgNPs synthesized using Matricaria recutita (Babunah) plant extract confirmed a spot pattern with XRD peak values <311>, <220>, and <111> planes [65].
3.7.9. Scanning Electron Microscopy (SEM)
SEM pictures were captured in secondary electron mode (accelerating voltage of 10 kV) and processed with Image Tool software. The granule sizes were measured and compared to the Feret diameters. As metal sputtering sources, Pt/Pd and Cr targets (99.99% purity) were used, which create a configuration of distinct nanomaterials. A conducting sample of 6 m thick aluminum foil was used. Within the resolution range of the electron microscope utilized (1–3 nm), no NPs were found on its surface. Silica gel on chromatograms was used as a 2D nonconducting sample. Molecular sieves with well-developed 3D surface morphology were used as samples [167]. The investigation of NPs produced by magnet iron sputtering is also of interest to enhance experimental processes. SEM investigations of nonconducting materials are made more informative by the deposition of a metal onto a sample surface through magnetron sputtering [168].
The size and form of the Ag NPs produced from Eclipta alba leaf extract were measured, having a range of sizes from 310 to 400 nm [51]. The formation of AuNPs with Gundelia tournefortii L. possessed a spherical shape with an average diameter of 40–45 nm [102]. The Cu NPs size was confirmed to be 30–55 nm [108]. CuO NPs synthesized using Anthemis nobilis flowers show morphology-like rectangular structures ranging from 8–20 nm [135]. ZnO NPs synthesized using Artemisia aucheri are depicted as seabeds consisting of spherical and granular shapes in the range of 15–40 nm [169]. The nanoparticle sizes were observed in a range of 10 to 180 nm, 10 to 200 nm, 16 to 71 nm, 20 to 25 nm, 10 to 170 nm, 20 to 86 nm, 9 to 21 nm, 9 to 120 nm, 8 to 20 nm, 10 to 34 nm for AgNPs, AuNPs, CuNPs, Pd NPs, ZnO NPs, Fe2O3 NPs, CuO NPs, TiO2 NPs, CO3O4 NPs, MgO NPs, respectively [24,51,114,137,145,159].
3.7.10. Transmission Electron Microscopy (TEM)
An electron beam imaging method for visualizing nanostructured samples that provide considerably higher resolution than light-based imaging techniques. Transmission electron microscopy is the best method for directly measuring nanoparticle size, grain boundaries, diameter, and morphological characteristics. The particle size range is wide, spanning from 1 nm to 5 nm. There is, however, a strong predilection for very tiny agglomeration. We divided them into four categories: FCC, icosahedral, decahedral, and twinned particles. It should be noted that our approach produces particles with an alkyl–thiol molecule passivating the surface [170].
The zinc oxide NPs synthesized using the Artemisia pallens plant extract showed a TEM result that shows a homogenous wurtzite structure [114]. The NiO NPs biosynthesized using Tagetes erecta L leaf extract revealed irregular forms of NPs [141]. The particle size ranges from 5 to 25 nm spherical particles for CuO NPs synthesized by Acanthospermum hispidum L. extract [134]. The spherical shape of AgNPs from Erigeron bonariensis with a particle size of 13 nm [53]. Gold NPs synthesized by Solidago canadensis L. extract showed a combination of single crystals and twinned particles [103]. The nanoparticle sizes were observed in a range of 10 to 100 nm, 10 to 50 nm, 20 to 50 nm, 5 to 50 nm, 20 to 70 nm, 5 to 60 nm, 12 to 50 nm, 5 to 50 nm, 8 to 20 nm, 5 to 25 nm for Ag, Au, Cu, Pd, ZnO, Fe2O3, CuO, TiO2, CO3O4, and MgO NPs, respectively [17,94,114,128,137,145,159].
3.7.11. Scanning Transmission Mode (STEM)
The STEM can approach atomic resolution, enabling direct imaging of smaller dimensions previously unobservable using traditional electron microscopy techniques. Combining this model with a high-angle annular dark-field detector, where the contrast on the picture is generally proportional to Z (where n is near 2), it is possible to identify elements on materials just solely on their atomic weight difference. This direct interpretation is of particular importance in the catalysis sector since bimetallic NPs are utilized in a variety of processes, including CO oxidation, hydrocarbon hydrogenation, and vinyl acetate production, among others. Probes as small as one can now be made, single molecules can be photographed, and the structure and form of microscopic NPs as small as a few nanometers may be detected [171]. The silver NPs synthesized using Ambrosia arborescens were observed as spherical and dispersed in solution with an average particle size of 14 ± 6 nm [21].
4. Application of Asteraceae-Based Nanoparticles
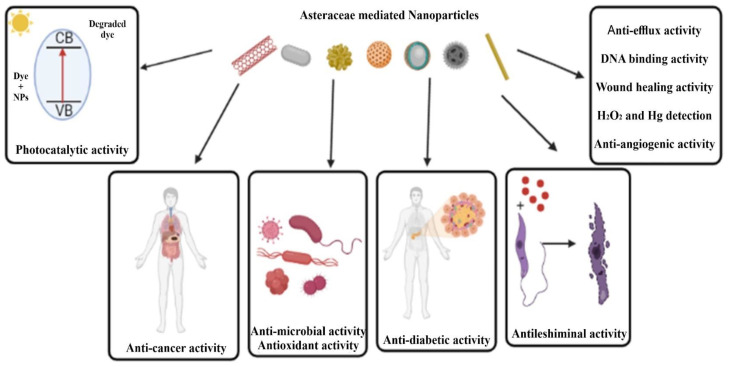
Unlike the traditional application of plants from the Asteraceae family, green synthesized nanoparticles have shown highly significant biological responses. These may be attributed to the small size of these particles which can be targeted specifically for biological applications such as antimicrobial, anticancer, photocatalytic, etc. (Figure 1 and Figure 3, Table 1 and Table 2).
Figure 3.
Role of Asteraceae mediated nanostructured materials in effluent treatment, drug delivery, antimicrobial, antioxidant, and other medical diagnoses.
4.1. Antimicrobial Activity
Researchers have reported numerous antimicrobial activities by green synthesized NPs using Asteraceae members. NPs such as silver, copper, gold, iron oxide, zinc oxide, titanium oxide, nickel oxide, and copper oxides synthesized from different members of Asteraceae exhibited great antimicrobial activity. Most commonly, Ag NPs are synthesized from Asteraceae members as Ag is a safe non-toxic metal. Ag NPs have great potential because of their antimicrobial properties and were also used in the treatment of contaminated groundwater. Ag NPs are good antibiotics and preservatives [172], thus used in the food industry. The Ag NPs synthesized from Carthamus tinctorius showed antibacterial activity against toxic pathogens such as Pseudomonas fluorescens (ATCC 13867) and Staphylococcus aureus (ATCC 25923) in the food industry [33]. Ag NPs synthesized from leaf extract of Eupatorium odaratum exhibited a broad spectrum of antibacterial and antifungal potential against Escherichia coli, Bacillus subtilis, S. aureus, Salmonella typhi, and Candida albicans, respectively [54]. Leaves of Tagetes erecta were capable of synthesizing Ag NPs and showed antibacterial activity against E. coli (DH5-Alpha) and Staphylococcus aureus (ATCC9144™) [173]. Quasi-spherical shaped Ag NPs synthesized from Acanthospermum hispidum have antibacterial, antifungal, and antimycobacterial activity [17].
Similarly, Tragopogon collinus synthesized ZnO NPs exhibited antibacterial properties against E. coli (PTCC 1270) and Staphylococcus aureus (PTCC 1112) [125]. Synthesized Cynara scolymus ZnO NPs from leaf extract exhibit antimicrobial properties against Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922) Pseudomonas aeruginosa (ATCC 27853) Candida tropicalis (IFM 46521), and Candida albicans (IFM 40009) [116]. Parthenium hysterophorus-mediated ZnO NPs (25 µL/mL) have good antifungal activity against Aspergillus flavus (MTCC-7589), and Aspergillus niger (MTCC-2587) [174]. Ageratum conyzoides can reduce iron metal to Fe NPs which have moderate antimicrobial activity against Escherichia coli (ATCC25922), Bacillus subtilis, Staphylococcus aureus (ATCC-25923), Pseudomonas aeruginosa (ATCC-27853), and Candida albicans (ATCC 90028) [8]. Recent studies also reported that CuO NPs synthesized from Acanthospermum hispidum showed antibacterial, and antimycobacterial activity against Escherichia coli (MTCC 443), Pseudomonas aeruginosa (MTCC 1688), Staphylococcus aureus (MTCC 96) and Streptococcus pyogenus (MTCC 442) and Mycobacterium tuberculosis H37RV [134].
4.2. Antioxidant Activity
Antioxidants are substances that may remove, prevent, or delay cell damage caused by free radicals including reactive oxygen species (ROS), reactive nitrogen species (RNS), and other unstable molecules. DPPH (2,2-diphenyl-1-picryl-hydrazyl) assay is a commonly used method for the determination of antioxidant capacity [175]. Many researchers report that Asteraceae-mediated NPs have high antioxidant activity and can be used to treat diseases caused by oxidative stress and free radical-related disease. High antioxidant properties of Asteraceae members are accounted for by a large amount of phenolic and flavonoid content.
Studies report that synthesized Ag NPs from the leaf extract of Ageratum conyzoides has high antioxidant properties [13]. Ag NPs synthesized from Calendula officinalis are a good source of antioxidants because of their high antioxidant activity and can be used in the production of medicines and cosmetics [176]. Recent research proved that Au NPs synthesized from leaves of Centaurea behen, Crassocephalum rubens, Gundelia tournefortii, and seeds of Cichorium intybus can act as antioxidants [43,95,102]. Antioxidants were also produced from ZnO NPs synthesized from the flower of Tagetes erecta and seeds of Zinnia elegans [93,123]. Aqueous extract of Silybum marianum synthesized ZnO NPs showed antioxidant properties [122]. Cu NPs synthesized from Blumea balsamifera, and Eclipta prostrata, also showed antioxidant activities [108,109].
4.3. Anticancer Activity
NPs synthesized from the Asteraceae family have a higher potential for controlling the growth and multiplication of tumor cells. Ag NPs synthesized from Artemisia marschalliana and A. turcomanica exhibit anticancer activity in the human gastric cancer AGS cell line [24,25]. ZnO NPs from Achillea millefolium are highly stable and biocompatible. They showed cytotoxic activity on lung and colon cancer cells [177]. ZnO NPs from leaf extract of Costus pictus have cytotoxic activity against Dalton lymphoma ascites cells [9]. Au NPs from leaf extract of Centaurea behen showed anticancer activity against leukemia cell line [95]. ZnO NPs using leaf extract of Cynara scolymus were found to possess anti-proliferative activity against the human breast cancer cell line [116].
4.4. Antidiabetic Activity
Diabetes is a metabolic disorder that is developed due to glucose intolerance and hyperglycemia. It is also caused due to changes in food and lifestyle. A recent investigation reported that silver NPs synthesized from Phagnalon niveum methanol extract demonstrated antidiabetic activity by reducing the blood glucose level and also reduced the body weight of rats in 1 to 21 days [178]. Spherical shaped-CuO NPs synthesized from Silybum marianum seed extract displayed great enzymatic inhibitory activity against ureases, alpha-amylase, and lipases so it was concluded that they can act as antidiabetic agents [179]. ZnO NPs and Au NPs which are synthesized using Dicoma anomala and Eclipta alba, respectively, are good alternative sources for antidiabetic medicine [98,117].
4.5. Antileishmanial Activity
Leishmania is a parasitic protozoan that is a causative organism for oropharyngeal mucosa inflammation, cutaneous lesions, and visceral infections. Antileishmanial drugs are usually antimonial compounds, they are highly toxic. Pentavalent antimony drugs such as meglumine antimoniate and sodium stibogluconate are used in the initial treatment of leishmaniasis [180]. A recent study in green synthesis proved that zinc oxide NPs synthesized using Silybum marianum can replace toxic antimonial drugs to destroy Leishmania tropica (KMH23) which causes Leishmaniasis [122].
4.6. Anti-Angiogenic Activity
Angiogenesis has a major role in atherosclerosis, tumor growth, myocardial infarction, carcinogenesis, limb ischemia, and cardiac ischemia. Recent studies report Ag NPs synthesized from flower extract of Achillea biebersteinii can reduce angiogenesis. The anti-angiogenic activity of the silver nanoparticle was studied in the rat aortic ring model [18].
4.7. Photocatalytic Activity
Nanoparticles have been utilized for the degradation of various anionic, catatonic, and neutral dyes [181]. Dyes, commonly used in paper, plastic, food, cosmetics, leather, textile, and pharmaceutical industries and have proven to be harmful to both aquatic and human life due to their toxic, mutagenic, carcinogenic, and teratogenic effects. Research supports NPs synthesized from Asteraceae members as good catalysts to degrade the toxic dyes to non-toxic compounds. Ag NPs synthesized from leaf extract of Ageratum conyzoides showed photocatalytic degradation properties [13]. ZnO NPs formed from Cynara scolymus leaf extract were able to degrade 94.3% of methyl violet and 89.5% of malachite green dyes after 120 min of UV exposure [116]. A total of 83% of methylene blue was degraded by NiO NPs synthesized from leaf extract of Ageratum conyzoides [140]. Under solar light, TiO2 NPs synthesized from leaf extract of Ageratina altissima had the potential to degrade dyes such as crystal violet, methylene blue, alizarin red, and methyl orange [137]. FeO NPs synthesized from Wedelia urticifolia leaf extract and Centaurea cyanus, showed photocatalytic degradation activity and were used for the removal of toxic chemicals or dyes from the aquatic environment [129,133].
4.8. Other Activities
Nanoparticles synthesized from plants of the Asteraceae family revealed other applications such as anti-efflux activity, DNA binding, detection of mercury ions, cutaneous wound healing effect, electrochemical sensing activity, hydrogen peroxide detection, and tyrosinase inhibitory activity. Silver NPs synthesized from Acroptilon repens have been shown to have anti-efflux activity against clinical isolates such as Acinetobacter bumanni [19,129]. DNA binding and Hydrogen peroxide sensing properties have been found in Agertum conyzoides Ag NPs [13]. Ag NPs formed by the reduction of Bidens frondosa and Ag salt precursor showed tyrosinase inhibitory activity [28]. NiO NPs from the leaf extracts of Tagetes erecta have electrochemical sensing properties [141]. Au and Ag NPs synthesized from Gundelia tournefortii showed cutaneous wound healing activity [57,102] and Ag NPs synthesized from Dahlia pinnata were utilized for mercury ion detection [46].
5. Toxicity of Asteraceae Mediated Nanoparticles
NPs are highly toxic to the cells in comparison to large particles of the same chemicals. Studies concluded that the toxicity of NPs is inversely proportional to the size of the particles [182]. Several studies with the NPs synthesized from Asteraceae members have looked into how this toxicity can be used as an application to better suit the environment. Even with their toxicity to humans, low levels of NPs can still be used with an apt efficiency rate to reduce pollution as well as kill out several harmful living agents within our environment [183].
Ag NPs synthesized from the flower extracts of Chrysanthemum indicum have been proven to have lethal activity. These NPs can bring about the maximum mortality rate of Anopheles stephensi mosquitoes regardless of whether it is larvae or pupae [184].
The Cd NPs synthesized from Tagetes sp. showed a similar type of maximum mortality rate against Aedes albopictus at 72 h incubation while normal incubation only yielded 65 to 70% mortality. This showed that not only concentration but also incubation time can affect the toxicity that NPs have on particular mosquitos or other organisms. So, the ideal way to use NPs would be to use less concentration with high incubation time [185]. The leaf extract of Ambrosia arborescens and subsequent Ag NPs produced from the same plant extracts NPs had a dose-dependent toxic effect against Aedes aegypti larvae. However, no mortality rate was observed in the control groups [21]. Gold NPs from Sphaeranthus indicus extract did not have any particular toxic effect on the plant cells or aquatic invertebrates such as Artemia nupulii when tested with a particular similar dose-dependent concentration. However, it was shown to prompt the mitotic division of the root tip cells in Allium cepa, and also promoted the germination of pollen grains in Gloriosa superba [153].
While in the case of humans, toxicity is a bit different compared to the other fauna that has been characterized. NPs of size below 10 nm behave the same as gases, so they can easily enter through human tissue. After inhalation, NPs spread to the heart, lungs, spleen, liver, brain, and gastrointestinal tract and may disrupt the function of normal cells [182].
6. Constraints of Asteraceae-Mediated Nanoparticle Synthesis
Asteraceae-mediated nanoparticles have significant activities and applications, however, there are limitations in plant selection, synthesis process, nanoparticle quality assurances, and their applications. These limitations challenge the production of nanoparticles in large-scale and industrial production. Several plants in the Asteraceae family have been used to synthesize locally available nanoparticles. Yet, industrial production of Asteraceae-mediated nanoparticles is very hard to achieve due to the varying effects of climatic conditions, growing seasons, and large-scale cultivation of plants used for synthesis. Some of the very important concerns in the process of synthesis are long reaction time, pH, temperature, the use of chemicals, and excessive energy consumption. The challenge in the separation and purification of nanoparticles due to the interference of other phytochemicals in plants is another obstacle faced during the process of synthesis. The quality of obtained nanoparticles could be affected due to agglomeration, irregular shape, size, and low yield. Another limitation of Asteraceae-mediated nanoparticles is in their application, the efficiency of activities will be low, and time-consuming and large amounts of nanoparticles should be utilized for the same to achieve activities more efficiently.
7. Conclusions and Prospects
Extracts from plant parts such as leaves, roots, flowers, peels, stems, bark, and biological modifications were effectively employed for the synthesis of NPs under ambient circumstances under extremely moderate reaction conditions due to the clear potential efficacy and eco-friendliness of biogenic synthesis. UV–Vis, SEM, TEM, HR-TEM, STEM, SAED, XRD, EDAX, DT-TGA, FTIR, TGA, DSC, and DLS techniques, etc., were utilized for characterization. Biogenic NPs have shown remarkable anti-cancer, anti-diabetic, antibacterial, antifungal, and antioxidant properties. Under different temperature and pH conditions, the NPs remained stable for a longer amount of time. Phytochemicals in the plant extracts, such as polyphenols, polyphenolics, flavonoids, and other functional groups, different nanomaterial frameworks, and morphological characteristics were formed. Asteraceae is a large family with a vast number of beneficial plants. Silver, gold, copper, iron oxide, and zinc oxide NPs are successfully synthesized using Asteraceae members. NPs synthesized using Asteraceae members have huge applications such as antibacterial, antifungal, antiparasitic, antioxidant, photocatalytic degradation, and cytotoxic activities and thus need significant attention to be an important area of research in phyto-nanotechnology that provides new avenues towards the eco-friendly and economical synthesis of nanostructured materials. The mechanism involved in the synthesis of NPs, which is briefly through phytochemicals present in plants, aids in the reduction of metal NPs, but the exact mechanism remains unknown as to which phytochemicals play an important role in synthesis. It is said that collectively bioactive compounds aid in synthesis. It would be fascinating to learn which phytochemical molecule is responsible for green nanoparticle production.
Acknowledgments
All the authors are thankful to their respective universities and institutes for their support.
Author Contributions
This review article was produced through collaboration between the authors. Conceptualization, J.K.S., B.B., W.-C.L.; writing—original draft preparation, J.P.J., B.B.; selected bibliographic sources, J.G., M.P., A.V.A., N.A.A.-D., M.V.A., N.J.; writing—review and editing, J.K.S., B.B., W.-C.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors hereby declare that they have no conflict of interest and have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
No external funding was received for conducting this study.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rahman H.M.M., Parvin M.I.A. Taxonomic Studies on the Family Fabaceae (weeds) at Rajshahi University Campus. Plant. 2015;3:20. doi: 10.11648/j.plant.20150303.11. [DOI] [Google Scholar]
- 2.Gao T., Yao H., Song J., Zhu Y., Liu C., Chen S. Evaluating the Feasibility of Using Candidate DNA Barcodes in Discriminating Species of the Large Asteraceae Family. BMC Evol. Biol. 2010;10:324. doi: 10.1186/1471-2148-10-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konovalov D.A. Polyacetylene Compounds of Plants of the Asteraceae Family (Review) Pharm. Chem. J. 2014;48:613–631. doi: 10.1007/s11094-014-1159-7. [DOI] [Google Scholar]
- 4.Michel J., Abd Rani N.Z., Husain K. A Review on the Potential Use of Medicinal Plants From Asteraceae and Lamiaceae Plant Family in Cardiovascular Diseases. Front. Pharmacol. 2020;11:852. doi: 10.3389/fphar.2020.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arokiyaraj S., Saravanan M., Badathala V. Green Synthesis of Silver Nanoparticles Using Aqueous Extract of Taraxacum Officinale and Its Antimicrobial Activity. South Indian J. Biol. Sci. 2015;1:115. doi: 10.22205/sijbs/2015/v1/i2/100433. [DOI] [Google Scholar]
- 6.Nguyen T.T.-N., Vo T.-T., Nguyen B.N.-H., Nguyen D.-T., Dang V.-S., Dang C.-H., Nguyen T.-D. Silver and Gold Nanoparticles Biosynthesized by Aqueous Extract of Burdock Root, Arctium Lappa as Antimicrobial Agent and Catalyst for Degradation of Pollutants. Environ. Sci. Pollut. Res. 2018;25:34247–34261. doi: 10.1007/s11356-018-3322-2. [DOI] [PubMed] [Google Scholar]
- 7.Kilic A., Altınkaynak C., Ildiz N., Ozdemir N., Yilmaz V., Ocsoy I. A New Approach for Green Synthesis and Characterization of Artemisia L. (Asteraceae) Genotype Extracts -Cu2 Nanocomplexes (nanoflower) and Their Effecitve Antimicrobial Activity. Med. Sci. Int. Med. J. 2020;9:191. doi: 10.5455/medscience.2019.08.9165. [DOI] [Google Scholar]
- 8.Madivoli E.S., Kareru P.G., Maina E.G., Nyabola A.O., Wanakai S.I., Nyang’au J.O. Biosynthesis of Iron Nanoparticles Using Ageratum Conyzoides Extracts, Their Antimicrobial and Photocatalytic Activity. SN Appl. Sci. 2019;1:500. doi: 10.1007/s42452-019-0511-7. [DOI] [Google Scholar]
- 9.Suresh J., Pradheesh G., Alexramani V., Sundrarajan M., Hong S.I. Green Synthesis and Characterization of Zinc Oxide Nanoparticle Using Insulin Plant ( Costus Pictus D. Don ) and Investigation of Its Antimicrobial as Well as Anticancer Activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018;9:015008. doi: 10.1088/2043-6254/aaa6f1. [DOI] [Google Scholar]
- 10.Awwad A.M., Salem N.M., Abdeen A.O. Green Synthesis of Silver Nanoparticles Using Carob Leaf Extract and Its Antibacterial Activity. Int. J. Ind. Chem. 2013;4:29. doi: 10.1186/2228-5547-4-29. [DOI] [Google Scholar]
- 11.Wangkheirakpam S.D., Devi W.R., Singh C.B., Laitonjam W.S. Green Synthesis of Silver Nanoparticles Using Strobilanthes Flaccidifolius Nees. Leaf Extract and Its Antibacterial Activity. J. Adv. Chem. 2016;8:1523–1532. doi: 10.24297/jac.v8i1.4033. [DOI] [Google Scholar]
- 12.Rao B., Tang R.-C. Green Synthesis of Silver Nanoparticles with Antibacterial Activities Using Aqueous Eriobotrya Japonica Leaf Extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017;8:015014. doi: 10.1088/2043-6254/aa5983. [DOI] [Google Scholar]
- 13.Chandraker S.K., Lal M., Shukla R. DNA-Binding, Antioxidant, H2O2 Sensing and Photocatalytic Properties of Biogenic Silver Nanoparticles Using Ageratum Conyzoides L. Leaf Extract. RSC Adv. 2019;9:23408–23417. doi: 10.1039/C9RA03590G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar P. Nanomaterial’s Synthesis, Types and Their Use in Bioremediation and Agriculture. Nat. Resour. Hum. Health. 2022;2:349–365. doi: 10.53365/nrfhh/144289. [DOI] [PubMed] [Google Scholar]
- 15.Kumar V., Gundampati R.K., Singh D.K., Jagannadham M.V., Sundar S., Hasan S.H. Photo-Induced Rapid Biosynthesis of Silver Nanoparticle Using Aqueous Extract of Xanthium Strumarium and Its Antibacterial and Antileishmanial Activity. J. Ind. Eng. Chem. 2016;37:224–236. doi: 10.1016/j.jiec.2016.03.032. [DOI] [Google Scholar]
- 16.Elemike E.E., Onwudiwe D.C., Fayemi O.E., Botha T.L. Green Synthesis and Electrochemistry of Ag, Au, and Ag–Au Bimetallic Nanoparticles Using Golden Rod (Solidago Canadensis) Leaf Extract. Appl. Phys. A. 2019;125:42. doi: 10.1007/s00339-018-2348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghotekar S., Pansambal S., Pawar S.P., Pagar T., Oza R., Bangale S. Biological Activities of Biogenically Synthesized Fluorescent Silver Nanoparticles Using Acanthospermum Hispidum Leaves Extract. SN Appl. Sci. 2019;1:1342. doi: 10.1007/s42452-019-1389-0. [DOI] [Google Scholar]
- 18.Baharara J., Namvar F., Ramezani T., Hosseini N., Mohamad R. Green Synthesis of Silver Nanoparticles Using Achillea Biebersteinii Flower Extract and Its Anti-Angiogenic Properties in the Rat Aortic Ring Model. Molecules. 2014;19:4624–4634. doi: 10.3390/molecules19044624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behdad R., Mirzaie A., Zare Karizi S. Green Synthesis of Silver Nanoparticle Using Acroptilon Repens Extract and Evaluation of Its Anti-Efflux Activity against Acinetobacter Bumanni Clinical Isolates. J. Microb. World. 2017;10:210–221. [Google Scholar]
- 20.Gautam S.K., Baid Y., Magar P.T., Binadi T.R., Regmi B. Antimicrobial Study of Green Synthesized Silver Nanoparticles (AgNPs) by Using Ageratina Adenophora and Its Characterization. Int. J. Appl. Sci. Biotechnol. 2021;9:128–132. doi: 10.3126/ijasbt.v9i2.37822. [DOI] [Google Scholar]
- 21.Morejón B., Pilaquinga F., Domenech F., Ganchala D., Debut A., Neira M. Larvicidal Activity of Silver Nanoparticles Synthesized Using Extracts of Ambrosia arborescens (Asteraceae) to Control Aedes aegypti L. (Diptera: Culicidae) J. Nanotechnol. 2018;2018:6917938. doi: 10.1155/2018/6917938. [DOI] [Google Scholar]
- 22.Dehghanizade S., Arasteh J., Mirzaie A. Green Synthesis of Silver Nanoparticles Using Anthemis Atropatana Extract: Characterization and in Vitro Biological Activities. Artif. Cells Nanomed. Biotechnol. 2018;46:160–168. doi: 10.1080/21691401.2017.1304402. [DOI] [PubMed] [Google Scholar]
- 23.Dobrucka R., Długaszewska J. Antimicrobial Activities of Silver Nanoparticles Synthesized by Using Water Extract of Arnicae Anthodium. Indian J. Microbiol. 2015;55:168–174. doi: 10.1007/s12088-015-0516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salehi S., Shandiz S.A.S., Ghanbar F., Darvish M.R., Ardestani M.S., Mirzaie A., Jafari M. Phytosynthesis of Silver Nanoparticles Using Artemisia Marschalliana Sprengel Aerial Part Extract and Assessment of Their Antioxidant, Anticancer, and Antibacterial Properties. Int. J. Nanomed. 2016;11:1835–1846. doi: 10.2147/IJN.S99882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mousavi B., Tafvizi F., Bostanabad S.Z. Green Synthesis of Silver Nanoparticles Using Artemisia turcomanica Leaf Extract and the Study of Anti-Cancer Effect and Apoptosis Induction on Gastric Cancer Cell Line (AGS) Artif. Cells Nanomed. Biotechnol. 2018;46:499–510. doi: 10.1080/21691401.2018.1430697. [DOI] [PubMed] [Google Scholar]
- 26.Rasheed T., Bilal M., Iqbal H.M.N., Li C. Green Biosynthesis of Silver Nanoparticles Using Leaves Extract of Artemisia Vulgaris and Their Potential Biomedical Applications. Colloids Surf. B Biointerfaces. 2017;158:408–415. doi: 10.1016/j.colsurfb.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Nyabola A.O., Kareru P.G., Madivoli E.S., Wanakai S.I., Maina E.G. Formation of Silver Nanoparticles via Aspilia Pluriseta Extracts Their Antimicrobial and Catalytic Activity. J. Inorg. Organomet. Polym. Mater. 2020;30:3493–3501. doi: 10.1007/s10904-020-01497-7. [DOI] [Google Scholar]
- 28.Abbas Q., Saleem M., Phull A.R., Rafiq M., Hassan M., Lee K.-H., Seo S.-Y. Green Synthesis of Silver Nanoparticles Using Extract and Their Tyrosinase Activity. Iran J Pharm Res. 2017;16:763–770. [PMC free article] [PubMed] [Google Scholar]
- 29.Mtambo S.E., Krishna S.B.N., Sershen, Govender P. Physico-Chemical, Antimicrobial and Anticancer Properties of Silver Nanoparticles Synthesised from Organ-Specific Extracts of Bidens pilosa L. S. Afr. J. Bot. 2019;126:196–206. doi: 10.1016/j.sajb.2019.07.046. [DOI] [Google Scholar]
- 30.Rohankumar R.C., Somnath D.B., Mangesh A.B., Dheeraj S.R., Ganesh H.W., Sachin S.T., Mukund N.U. Characterization, Antioxidant, Antimicrobial and Cytotoxic Activities of Green Synthesized Silver and Iron Nanoparticles Using Alcoholic Blumea Eriantha DC Plant Extract. Mater. Today Commun. 2020;24:101320. [Google Scholar]
- 31.Baghizadeh A., Ranjbar S., Gupta V.K., Asif M., Pourseyedi S., Karimi M.J., Mohammadinejad R. Green Synthesis of Silver Nanoparticles Using Seed Extract of Calendula officinalis in Liquid Phase. J. Mol. Liq. 2015;207:159–163. doi: 10.1016/j.molliq.2015.03.029. [DOI] [Google Scholar]
- 32.Ahn E.-Y., Jin H., Park Y. Green Synthesis and Biological Activities of Silver Nanoparticles Prepared by Carpesium Cernuum Extract. Arch. Pharm. Res. 2019;42:926–934. doi: 10.1007/s12272-019-01152-x. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Félix F., López-Cota A.G., Moreno-Vásquez M.J., Graciano-Verdugo A.Z., Quintero-Reyes I.E., Del-Toro-Sánchez C.L., Tapia-Hernández J.A. Sustainable-Green Synthesis of Silver Nanoparticles Using Safflower (Carthamus Tinctorius L.) Waste Extract and Its Antibacterial Activity. Heliyon. 2021;7:e06923. doi: 10.1016/j.heliyon.2021.e06923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tüzün B.S., Hohmann J., Kivcak B. Green Bio-Inspired Synthesis, Characterization and Activity of Silver Nanoparticle Forms of Centaurea Virgata Lam. and the Isolated Flavonoid Eupatorin. Green Process. Synth. 2018;7:372–379. doi: 10.1515/gps-2017-0027. [DOI] [Google Scholar]
- 35.Sadiqa A., Gilani S.R., Anwar A., Mehboob A., Saleem A., Rubab S. Biogenic Fabrication, Characterization and Drug Loaded Antimicrobial Assay of Silver Nanoparticles Using Centratherum Anthalminticum (L.) Kuntze. J. Pharm. Sci. 2021;110:1969–1978. doi: 10.1016/j.xphs.2021.01.034. [DOI] [PubMed] [Google Scholar]
- 36.Erjaee H., Rajaian H., Nazifi S. Synthesis and Characterization of Novel Silver Nanoparticles Using Chamaemelum Nobile Extract for Antibacterial Application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017;8:025004. doi: 10.1088/2043-6254/aa690b. [DOI] [Google Scholar]
- 37.Jayeoye T.J., Eze F.N., Olatunde O.O., Benjakul S., Rujiralai T. Synthesis of Silver and Silver@zero Valent Iron Nanoparticles Using Chromolaena odorata Phenolic Extract for Antibacterial Activity and Hydrogen Peroxide Detection. J. Environ. Chem. Eng. 2021;9:105224. doi: 10.1016/j.jece.2021.105224. [DOI] [Google Scholar]
- 38.Arokiyaraj S., Arasu M.V., Vincent S., Prakash N.U., Choi S.H., Oh Y.-K., Choi K.C., Kim K.H. Rapid Green Synthesis of Silver Nanoparticles from Chrysanthemum indicum L. and Its Antibacterial and Cytotoxic Effects: An in Vitro Study. Int. J. Nanomed. 2014;9:379–388. doi: 10.2147/IJN.S53546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Y., Du Z., Lv H., Jia Q., Tang Z., Zheng X., Zhang K., Zhao F. Green Synthesis of Silver Nanoparticles by Chrysanthemum Morifolium Ramat. Extract and Their Application in Clinical Ultrasound Gel. Int. J. Nanomed. 2013;8:1809–1815. doi: 10.2147/IJN.S43289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Behboodi S., Baghbani-Arani F., Abdalan S., Sadat Shandiz S.A. Green Engineered Biomolecule-Capped Silver Nanoparticles Fabricated from Cichorium intybus Extract: In Vitro Assessment on Apoptosis Properties Toward Human Breast Cancer (MCF-7) Cells. Biol. Trace Elem. Res. 2019;187:392–402. doi: 10.1007/s12011-018-1392-0. [DOI] [PubMed] [Google Scholar]
- 41.Mohamad R. Ph.D. Thesis. Universiti Teknologi Malaysia; Johor Bahru, Malaysia: 2013. Biosynthesis of Au, Ag and Bimetallic Au-Ag Nanoparticles Using Aqueous Leaf Extract of Cosmos Caudatus. [Google Scholar]
- 42.Malaka R., Hema J.A., Muthukumarasamy N.P., Sambandam A., Subramanian S., Sevanan M. Green Synthesis of Silver Nanoparticles Using Cosmos Sulphureus and Evaluation of Their Antimicrobial and Antioxidant Properties. Nano Biomed. Eng. 2016;7:160–168. doi: 10.5101/nbe.v7i4.p160-168. [DOI] [Google Scholar]
- 43.Adewale O.B., Egbeyemi K.A., Onwuelu J.O., Potts-Johnson S.S., Anadozie S.O., Fadaka A.O., Osukoya O.A., Aluko B.T., Johnson J., Obafemi T.O., et al. Biological Synthesis of Gold and Silver Nanoparticles Using Leaf Extracts of Crassocephalum Rubens and Their Comparative in Vitro Antioxidant Activities. Heliyon. 2020;6:e05501. doi: 10.1016/j.heliyon.2020.e05501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Ruíz-Baltazar Á.J., de Jesús Ruíz-Baltazar Á., Reyes-López S.Y., de Lourdes Mondragón-Sánchez M., Estevez M., Hernández-Martinez A.R., Pérez R. Biosynthesis of Ag Nanoparticles Using Cynara Cardunculus Leaf Extract: Evaluation of Their Antibacterial and Electrochemical Activity. Results Phys. 2018;11:1142–1149. doi: 10.1016/j.rinp.2018.11.032. [DOI] [Google Scholar]
- 45.Erdogan O., Abbak M., Demirbolat G.M., Birtekocak F., Aksel M., Pasa S., Cevik O. Green Synthesis of Silver Nanoparticles via Cynara Scolymus Leaf Extracts: The Characterization, Anticancer Potential with Photodynamic Therapy in MCF7 Cells. PLoS ONE. 2019;14:e0216496. doi: 10.1371/journal.pone.0216496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy K., Sarkar C.K., Ghosh C.K. Rapid Colorimetric Detection of Hg2+ Ion by Green Silver Nanoparticles Synthesized Using Dahlia Pinnata Leaf Extract. Green Process. Synth. 2015;4:455–461. doi: 10.1515/gps-2015-0052. [DOI] [Google Scholar]
- 47.Arya G., Malav A.K., Gupta N., Kumar A., Nimesh S. Biosynthesis and in Vitro Antimicrobial Potential of Silver Nanoparticles Prepared Using Dicoma Tomentosa Plant Extract. Nanosci. Nanotechnol.-Asia. 2018;8:240–247. doi: 10.2174/2210681207666170613095203. [DOI] [Google Scholar]
- 48.Sedira S., Sobti N. Silver Nanoparticles Bioreduction by Dittrichia Viscosa Leaves Extract and Its Bactericidal Effects. Int. J. Nanoparticles. 2016;9:19. doi: 10.1504/IJNP.2016.078519. [DOI] [Google Scholar]
- 49.Gecer E.N., Erenler R., Temiz C., Genc N., Yildiz I. Green Synthesis of Silver Nanoparticles from Echinacea Purpurea (L.) Moench with Antioxidant Profile. Part. Sci. Technol. 2022;40:50–57. doi: 10.1080/02726351.2021.1904309. [DOI] [Google Scholar]
- 50.Murthy H.C. Green Silver Nanoparticles Synthesised Using Medicinal Plant Echinops Sp. Root Extract for Antimicrobial Applications. Nanochemistry Res. 2020;5:128–140. doi: 10.22036/ncr.2020.02.003. [DOI] [Google Scholar]
- 51.Premasudha P., Venkataramana M., Abirami M., Vanathi P., Krishna K., Rajendran R. Biological Synthesis and Characterization of Silver Nanoparticles Using Eclipta Alba Leaf Extract and Evaluation of Its Cytotoxic and Antimicrobial Potential. Bull. Mater. Sci. 2015;38:965–973. doi: 10.1007/s12034-015-0945-5. [DOI] [Google Scholar]
- 52.Kharat S.N., Mendhulkar V.D. Synthesis, Characterization and Studies on Antioxidant Activity of Silver Nanoparticles Using Elephantopus Scaber Leaf Extract. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;62:719–724. doi: 10.1016/j.msec.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 53.Kumar V., Singh D.K., Mohan S., Hasan S.H. Photo-Induced Biosynthesis of Silver Nanoparticles Using Aqueous Extract of Erigeron Bonariensis and Its Catalytic Activity against Acridine Orange. J. Photochem. Photobiol. B. 2016;155:39–50. doi: 10.1016/j.jphotobiol.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Elemike E., Onwudiwe D., Ekennia A., Sonde C., Ehiri R. Green Synthesis of Ag/Ag2O Nanoparticles Using Aqueous Leaf Extract of Eupatorium Odoratum and Its Antimicrobial and Mosquito Larvicidal Activities. Molecules. 2017;22:674. doi: 10.3390/molecules22050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahmod M., Junayed A., Bhowmick C., Sompa S., Sultana T., Akter T., Abedin M., Zubair M., Islam M., Mogal M., et al. Antibacterial Activity of Silver Nanoparticles Synthesized from Leaf and Flower Extracts of Galinsoga Formosa. J. Adv. Biotechnol. Exp. Ther. 2021;4:178. doi: 10.5455/jabet.2021.d118. [DOI] [Google Scholar]
- 56.Shahzadi T., Kanwal A., Jabeen H., Riaz T., Zaib M. Eco-Friendly synthesis of silver nanopartricles using gazania rigens and evaluation of activities. J. Environ. Eng. Landsc. Manage. 2021;20:43–52. doi: 10.30638/eemj.2021.005. [DOI] [Google Scholar]
- 57.Han S., Ahmeda A., Jalalvand A.R., Lu W., Zangeneh M.M., Zangeneh A. Application of Silver Nanoparticles Containing Gundelia tournefortii L. Leaf Aqueous Extract in the Treatment of Microbial Diseases and Cutaneous Wound Healing. Appl. Organomet. Chem. 2022;36:e5491. doi: 10.1002/aoc.5491. [DOI] [Google Scholar]
- 58.Nadzir M.M., Idris F.N., Hat K. Green Synthesis of Silver Nanoparticle Using Gynura procumbens Aqueous Extracts; Proceedings of the 6th International Conference on Environment (ICENV2018): Empowering Environment and Sustainable Engineering Nexus Through Green Technology; Penang, Malaysia. 11–13 December 2018; [DOI] [Google Scholar]
- 59.Yazdi M.E.T., Amiri M.S., Hosseini H.A., Oskuee R.K., Mosawee H., Pakravanan K., Darroudi M. Plant-Based Synthesis of Silver Nanoparticles in Handelia trichophylla and Their Biological Activities. Bull. Mater. Sci. 2019;42:155. doi: 10.1007/s12034-019-1855-8. [DOI] [Google Scholar]
- 60.Yazdi M.E.T., Amiri M.S., Akbari S., Sharifalhoseini M., Nourbakhsh F., Mashreghi M., Yousefi E., Abbasi M.R., Modarres M., Es-haghi A. Green Synthesis of Silver Nanoparticles Using Helichrysum graveolens for Biomedical Applications and Wastewater Treatment. BioNanoScience. 2020;10:1121–1127. doi: 10.1007/s12668-020-00794-2. [DOI] [Google Scholar]
- 61.Riaz M., Altaf M., Khan M.Q., Manzoor S., Shekheli M.A., Shah M.A., Ilyas S.Z., Hussain Z. Green Synthesis of Silver Nanoparticles Using Jurinea Dolomiaea and Biological Activities. J. Nanosci. Nanotechnol. 2018;18:8386–8391. doi: 10.1166/jnn.2018.16401. [DOI] [PubMed] [Google Scholar]
- 62.Kanagamani K., Muthukrishnan P., Shankar K., Kathiresan A., Barabadi H., Saravanan M. Antimicrobial, Cytotoxicity and Photocatalytic Degradation of Norfloxacin Using Kleinia Grandiflora Mediated Silver Nanoparticles. J. Clust. Sci. 2019;30:1415–1424. doi: 10.1007/s10876-019-01583-y. [DOI] [Google Scholar]
- 63.Kanchana A., Agarwal I., Sunkar S., Nellore J., Namasivayam K. Biogenic Silver Nanoparticles From Spinacia Oleracea And Lactuca sativa And Their Potential Antimicrobial Activity. Dig. J. Nanomater. Biostructures. 2011;6:1741–1750. [Google Scholar]
- 64.Essien E.R., Atasie V.N., Udobang E.U., Umanu G. Preparation of Monodispersed and Cytotoxic Silver Nanoparticles Using Launaea Taraxacifolia Leaf Extract. J. Nanostructure Chem. 2019;9:259–268. doi: 10.1007/s40097-019-00316-x. [DOI] [Google Scholar]
- 65.Uddin I., Ahmad K., Khan A.A., Kazmi M.A. Synthesis of Silver Nanoparticles Using Matricaria Recutita (Babunah) Plant Extract and Its Study as Mercury Ions Sensor. Sens. Bio-Sens. Res. 2017;16:62–67. doi: 10.1016/j.sbsr.2017.11.005. [DOI] [Google Scholar]
- 66.Biswas A., Vanlalveni C., Adhikari P.P., Lalfakzuala R., Rokhum L. Biosynthesis, Characterisation and Antibacterial Activity of Mikania Micrantha Leaf Extract-mediated AgNPs. Micro Nano Lett. 2019;14:799–803. doi: 10.1049/mnl.2018.5661. [DOI] [Google Scholar]
- 67.Okaiyeto K., Ojemaye M.O., Hoppe H., Mabinya L.V., Okoh A.I. Phytofabrication of Silver/Silver Chloride Nanoparticles Using Aqueous Leaf Extract of Oedera Genistifolia: Characterization and Antibacterial Potential. Molecules. 2019;24:4382. doi: 10.3390/molecules24234382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahsan A., Farooq M.A., Ahsan Bajwa A., Parveen A. Green Synthesis of Silver Nanoparticles Using Parthenium Hysterophorus: Optimization, Characterization and In Vitro Therapeutic Evaluation. Molecules. 2020;25:3324. doi: 10.3390/molecules25153324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mofolo M.J., Kadhila P., Chinsembu K.C., Mashele S., Sekhoacha M. Green Synthesis of Silver Nanoparticles from Extracts of Pechuel-Loeschea Leubnitziae: Their Anti-Proliferative Activity against the U87 Cell Line. Inorg. Nano-Met. Chem. 2020;50:949–955. doi: 10.1080/24701556.2020.1729191. [DOI] [Google Scholar]
- 70.Abdelmoteleb A., Valdez-Salas B., Carrillo-Beltran M., Hernandez D.D., González-Mendoza D. Green Synthesis of Silver Nanoparticles Using Pluchea Sericea a Native Plants from Baja California, Mexico and Their Potential Application as Antimicrobials. Iran. J. Sci. Technol. Trans. A Sci. 2018;42:457–463. doi: 10.1007/s40995-016-0019-6. [DOI] [Google Scholar]
- 71.Khan M., Khan M., Adil S.F., Tahir M.N., Tremel W., Alkhathlan H.Z., Al-Warthan A., Siddiqui M.R.H. Green Synthesis of Silver Nanoparticles Mediated by Pulicaria Glutinosa Extract. Int. J. Nanomed. 2013;8:1507. doi: 10.2147/IJN.S43309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qhtani M.S.J.A., Al Qhtani M.S.J., El-Debaiky S.A., Sayed M. Antifungal and Cytotoxic Activities of Biosynthesized Silver, Zinc and Gold Nanoparticles by Flower Extract of Rhanterium Epapposum. Open J. Appl. Sci. 2020;10:663–674. [Google Scholar]
- 73.Aslam M., Fozia F., Gul A., Ahmad I., Ullah R., Bari A., Mothana R.A., Hussain H. Phyto-Extract-Mediated Synthesis of Silver Nanoparticles Using Aqueous Extract of Sanvitalia Procumbens, and Characterization, Optimization and Photocatalytic Degradation of Azo Dyes Orange G and Direct Blue-15. Molecules. 2021;26:6144. doi: 10.3390/molecules26206144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abd El-Aziz A.R.M., Gurusamy A., Alothman M.R., Shehata S.M., Hisham S.M., Alobathani A.A. Silver Nanoparticles Biosynthesis Using Saussurea Costus Root Aqueous Extract and Catalytic Degradation Efficacy of Safranin Dye. Saudi J. Biol. Sci. 2021;28:1093–1099. doi: 10.1016/j.sjbs.2020.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ayromlou A., Masoudi S., Mirzaie A. Scorzonera Calyculata Aerial Part Extract Mediated Synthesis of Silver Nanoparticles: Evaluation of Their Antibacterial, Antioxidant and Anticancer Activities. J. Clust. Sci. 2019;30:1037–1050. doi: 10.1007/s10876-019-01563-2. [DOI] [Google Scholar]
- 76.Qasim Nasar M., Zohra T., Khalil A.T., Saqib S., Ayaz M., Ahmad A., Shinwari Z.K. Seripheidium Quettense Mediated Green Synthesis of Biogenic Silver Nanoparticles and Their Theranostic Applications. Green Chem. Lett. Rev. 2019;12:310–322. doi: 10.1080/17518253.2019.1643929. [DOI] [Google Scholar]
- 77.Gopalakrishnan R., Loganathan B., Raghu K. Green Synthesis of Au–Ag Bimetallic Nanocomposites Using Silybum Marianum Seed Extract and Their Application as a Catalyst. RSC Adv. 2015;5:31691–31699. doi: 10.1039/C5RA03571F. [DOI] [Google Scholar]
- 78.Kumar V.A., Uchida T., Mizuki T., Nakajima Y., Katsube Y., Hanajiri T., Maekawa T. Synthesis of Nanoparticles Composed of Silver and Silver Chloride for a Plasmonic Photocatalyst Using an Extract from a Weed Solidago altissima (goldenrod) Adv. Nat. Sci. Nanosci. Nanotechnol. 2016;7:015002. doi: 10.1088/2043-6262/7/1/015002. [DOI] [Google Scholar]
- 79.Botha T.L., Elemike E.E., Horn S., Onwudiwe D.C., Giesy J.P., Wepener V. Cytotoxicity of Ag, Au and Ag-Au Bimetallic Nanoparticles Prepared Using Golden Rod (Solidago canadensis) Plant Extract. Sci. Rep. 2019;9:4169. doi: 10.1038/s41598-019-40816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rethinam R., Jeyachandran R. Green Synthesis of Silver Nanoparticles Using Aqueous Leaf Extract of Spilanthes Calva Dc. World J. Pharm. Res. 2016;5:822–828. [Google Scholar]
- 81.Laguta I., Stavinskaya O., Kazakova O., Fesenko T., Brychka S. Green Synthesis of Silver Nanoparticles Using Stevia Leaves Extracts. Appl. Nanosci. 2018;9:755–765. doi: 10.1007/s13204-018-0680-5. [DOI] [Google Scholar]
- 82.Ogunsile B.O., Labulo A.H., Fajemilehin A.M. Green Synthesis of Silver Nanoparticles from Leaf Extracts of Parquetina Nigrescens and Synedrella Nodiflora and Their Antimicrobial Activity. Ife J. Sci. 2016;18:245–254. [Google Scholar]
- 83.Katta V.K.M., Dubey R.S. Green Synthesis of Silver Nanoparticles Using Tagetes Erecta Plant and Investigation of Their Structural, Optical, Chemical and Morphological Properties. Mater. Today. 2021;45:794–798. doi: 10.1016/j.matpr.2020.02.809. [DOI] [Google Scholar]
- 84.Dubey S.P., Lahtinen M., Sillanpää M. Tansy Fruit Mediated Greener Synthesis of Silver and Gold Nanoparticles. Process Biochem. 2010;45:1065–1071. doi: 10.1016/j.procbio.2010.03.024. [DOI] [Google Scholar]
- 85.Saratale R.G., Benelli G., Kumar G., Kim D.S., Saratale G.D. Bio-Fabrication of Silver Nanoparticles Using the Leaf Extract of an Ancient Herbal Medicine, Dandelion (Taraxacum officinale), Evaluation of Their Antioxidant, Anticancer Potential, and Antimicrobial Activity against Phytopathogens. Environ. Sci. Pollut. Res. Int. 2018;25:10392–10406. doi: 10.1007/s11356-017-9581-5. [DOI] [PubMed] [Google Scholar]
- 86.Tran T.T.T., Vu T.T.H., Nguyen T.H. Biosynthesis of Silver Nanoparticles Using Tithonia Diversifolia Leaf Extract and Their Antimicrobial Activity. Mater. Lett. 2013;105:220–223. doi: 10.1016/j.matlet.2013.04.021. [DOI] [Google Scholar]
- 87.Jabbari R., Ghasemi N. Investigating Methylene Blue Dye Adsorption Isotherms Using Silver Nano Particles Provided by Aqueous Extract of Tragopogon Buphthalmoides. Chem. Methodol. 2020;5:21–29. doi: 10.22034/chemm.2021.118446. [DOI] [Google Scholar]
- 88.Seifipour R., Nozari M., Pishkar L. Green Synthesis of Silver Nanoparticles Using Tragopogon Collinus Leaf Extract and Study of Their Antibacterial Effects. J. Inorg. Organomet. Polym. Mater. 2020;30:2926–2936. doi: 10.1007/s10904-020-01441-9. [DOI] [Google Scholar]
- 89.Kushwaha H.B., Malik C.P. Nanofabrication of Silver Nanoparticles from the Stem and Leaf Extract of Verbesina Encelioides. Natl. Acad. Sci. Lett. 2012;35:555–563. doi: 10.1007/s40009-012-0097-8. [DOI] [Google Scholar]
- 90.Joseph J., Khor K.Z., Moses E.J., Lim V., Aziz M.Y., Abdul Samad N. In Vitro Anticancer Effects of Leaf Extract and Green-Synthesised Silver Nanoparticles. Int. J. Nanomed. 2021;16:3599–3612. doi: 10.2147/IJN.S303921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sahayaraj K., Roobadevi M., Rajesh S., Azizi S. Vernonia cinerea (L.) Less. Silver Nanocomposite and Its Antibacterial Activity against a Cotton Pathogen. Res. Chem. Intermed. 2015;41:5495–5507. doi: 10.1007/s11164-014-1676-8. [DOI] [Google Scholar]
- 92.Paul Das M., Rebecca Livingstone J., Veluswamy P., Das J. Exploration of Wedelia Chinensis Leaf-Assisted Silver Nanoparticles for Antioxidant, Antibacterial and in Vitro Cytotoxic Applications. J. Food Drug Anal. 2018;26:917–925. doi: 10.1016/j.jfda.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh Y., Gaur S., Singhal A., Chauhan D.K. Phytotoxic Assessment Of Agno3 And Znso4 Vis À Vis Agnps And Znonps In Tagetes Erecta L. And Zinnia Elegans Jacq. Plant Arch. 2021;21:724–730. doi: 10.51470/PLANTARCHIVES.2021.v21.S1.109. [DOI] [Google Scholar]
- 94.Dobrucka R., Romaniuk-Drapała A., Kaczmarek M. Biologically Synthesized of Au/Pt/ZnO Nanoparticles Using Arctium Lappa Extract and Cytotoxic Activity against Leukemia. Biomed. Microdevices. 2020;22:72. doi: 10.1007/s10544-020-00526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abdoli M., Arkan E., Shekarbeygi Z., Khaledian S. Green Synthesis of Gold Nanoparticles Using Centaurea Behen Leaf Aqueous Extract and Investigating Their Antioxidant and Cytotoxic Effects on Acute Leukemia Cancer Cell Line (THP-1) Inorg. Chem. Commun. 2021;129:108649. doi: 10.1016/j.inoche.2021.108649. [DOI] [Google Scholar]
- 96.Torabi N., Nowrouzi A., Ahadi A., Vardasbi S., Etesami B. Green Synthesis of Gold Nanoclusters Using Seed Aqueous Extract of Cichorium intybus L. and Their Characterization. SN Appl. Sci. 2019;1:981. doi: 10.1007/s42452-019-1035-x. [DOI] [Google Scholar]
- 97.Attar A., Yapaoz M.A. Biomimetic Synthesis, Characterization and Antibacterial Efficacy of ZnO and Au Nanoparticles Using Echinacea Flower Extract Precursor. Mater. Res. Express. 2018;5:055403. doi: 10.1088/2053-1591/aac05f. [DOI] [Google Scholar]
- 98.Vijayakumar S., Vinayagam R., Anand M.A.V., Venkatachalam K., Saravanakumar K., Wang M.-H., Sangeetha C.C., Gothandam K.M., David E. Green Synthesis of Gold Nanoparticle Using Eclipta Alba and Its Antidiabetic Activities through Regulation of Bcl-2 Expression in Pancreatic Cell Line. J. Drug Deliv. Sci. Technol. 2020;58:101786. doi: 10.1016/j.jddst.2020.101786. [DOI] [Google Scholar]
- 99.Mendhulkar V., Shinde A. Anticancer Activity of Gold Nanobioconjugates Synthesized from Elephantopus Scaber (linn.) Leaf Extract. J. Cancer Res. Ther. 2021;10 doi: 10.4103/jcrt.JCRT_1043_20. [DOI] [PubMed] [Google Scholar]
- 100.Velmurugan P., Cho M., Lee S.-M., Park J.-H., Lee K.-J., Myung H., Oh B.-T. Phyto-Crystallization of Silver and Gold by Erigeron Annuus (L.) Pers Flower Extract and Catalytic Potential of Synthesized and Commercial Nano Silver Immobilized on Sodium Alginate Hydrogel. J. Saudi Chem. Soc. 2016;20:313–320. doi: 10.1016/j.jscs.2014.09.004. [DOI] [Google Scholar]
- 101.Punnoose M.S., Bijimol D., Mathew B. Microwave Assisted Green Synthesis of Gold Nanoparticles for Catalytic Degradation of Environmental Pollutants. Environ. Nanotechnol. Monit. Manag. 2021;16:100525. [Google Scholar]
- 102.Zhaleh M., Zangeneh A., Goorani S., Seydi N., Zangeneh M.M., Tahvilian R., Pirabbasi E. In Vitro and in Vivo Evaluation of Cytotoxicity, Antioxidant, Antibacterial, Antifungal, and Cutaneous Wound Healing Properties of Gold Nanoparticles Produced via a Green Chemistry Synthesis Using Gundelia tournefortii L. as a Capping and Reducing Agent. Appl. Organomet. Chem. 2019;33:e5015. doi: 10.1002/aoc.5015. [DOI] [Google Scholar]
- 103.Mariychuk R., Grulova D., Grishchenko L.M., Linnik R.P., Lisnyak V.V. Green Synthesis of Non-Spherical Gold Nanoparticles Using Solidago Canadensis L. Extract. Appl. Nanosci. 2020;10:4817–4826. doi: 10.1007/s13204-020-01406-x. [DOI] [Google Scholar]
- 104.Sadeghi B., Mohammadzadeh M., Babakhani B. Green Synthesis of Gold Nanoparticles Using Stevia Rebaudiana Leaf Extracts: Characterization and Their Stability. J. Photochem. Photobiol. B. 2015;148:101–106. doi: 10.1016/j.jphotobiol.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 105.Del Moral A., Borjas-Garcia S.E., Rosas G. Green Synthesis of Gold Nanoparticles Using Taraxacum officinale Extract. Microsc. Microanal. 2018;24:1740–1741. doi: 10.1017/S1431927618009182. [DOI] [Google Scholar]
- 106.Vijaya Kumar P., Mary Jelastin Kala S., Prakash K.S. Synthesis of Gold Nanoparticles Using Xanthium Strumarium Leaves Extract and Their Antimicrobial Studies: A Green Approach. Rasayan J. Chem. 2018;11:1544–1551. doi: 10.31788/RJC.2018.1144044. [DOI] [Google Scholar]
- 107.Wang G., Ahmeda A., Malek Z., Mansooridara S., Zangeneh A., Zangeneh M.M. Chemical Characterization and Therapeutic Properties of Achillea Biebersteinii Leaf Aqueous Extract Synthesized Copper Nanoparticles against Methamphetamine-induced Cell Death in PC12: A Study in the Nanotechnology and Neurology Fields. Appl. Organomet. Chem. 2020;34:e5488. doi: 10.1002/aoc.5488. [DOI] [Google Scholar]
- 108.Binawati G., Ilham M., Ida K. Biosynthesis Copper Nanoparticles Using Blumea Balsamifera Leaf Extracts: Characterization of Its Antioxidant and Cytotoxicity Activities. Surf. Interfaces. 2020;21:100799. [Google Scholar]
- 109.Chung I.-M., Abdul Rahuman A., Marimuthu S., Kirthi A.V., Anbarasan K., Padmini P., Rajakumar G. Green Synthesis of Copper Nanoparticles Using Leaves Extract and Their Antioxidant and Cytotoxic Activities. Exp. Ther. Med. 2017;14:18–24. doi: 10.3892/etm.2017.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.León-Jimenez E., California B., Valdéz-Salas B., González-Mendoza D., Tzintzun-Camacho O., Gutiérrez T. Synthesis and Insecticide Activity of Cu-Nanoparticles from Prosopis Juliflora (Sw) DC and Pluchea Sericea (Nutt.) on Phenacoccus Solenopsis Tinsley (Hemiptera: Pseudococcidae) Rev. De La Soc. EntomolÓGica Argent. 2019;78:12–21. doi: 10.25085/rsea.780202. [DOI] [Google Scholar]
- 111.Kalpana V.N., Chakraborthy P., Palanichamy V., Rajeswari V.D. Synthesis and Characterization of Copper Nanoparticles Using Tridax Procumbens and Its Application in Degradation of Bismarck Brown. Analysis. 2016;10:17. [Google Scholar]
- 112.Khan M., Khan M., Kuniyil M., Adil S.F., Al-Warthan A., Alkhathlan H.Z., Tremel W., Tahir M.N., Siddiqui M.R.H. Biogenic Synthesis of Palladium Nanoparticles Using Pulicaria Glutinosa Extract and Their Catalytic Activity towards the Suzuki Coupling Reaction. Dalton Trans. 2014;43:9026–9031. doi: 10.1039/C3DT53554A. [DOI] [PubMed] [Google Scholar]
- 113.Wang D., Cui L., Chang X., Guan D. Biosynthesis and Characterization of Zinc Oxide Nanoparticles from Artemisia Annua and Investigate Their Effect on Proliferation, Osteogenic Differentiation and Mineralization in Human Osteoblast-like MG-63 Cells. J. Photochem. Photobiol. B. 2020;202:111652. doi: 10.1016/j.jphotobiol.2019.111652. [DOI] [PubMed] [Google Scholar]
- 114.Gomathi R., Suhana H. Green Synthesis, Characterization and Antimicrobial Activity of Zinc Oxide Nanoparticles Using Artemisia Pallens Plant Extract. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2021;51:1663–1672. doi: 10.1080/24701556.2020.1852256. [DOI] [Google Scholar]
- 115.Mohammadi Shivyari A., Tafvizi F., Noorbazargan H. Anti-Cancer Effects of Biosynthesized Zinc Oxide Nanoparticles Using Artemisia Scoparia in Huh-7 Liver Cancer Cells. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2022;52:375–386. [Google Scholar]
- 116.Rajapriya M., Sharmili S.A., Baskar R., Balaji R., Alharbi N.S., Kadaikunnan S., Khaled J.M., Alanzi K.F., Vaseeharan B. Correction to: Synthesis and Characterization of Zinc Oxide Nanoparticles Using Cynara Scolymus Leaves: Enhanced Hemolytic, Antimicrobial, Antiproliferative, and Photocatalytic Activity. J. Clust. Sci. 2020;31:803. doi: 10.1007/s10876-019-01734-1. [DOI] [Google Scholar]
- 117.Balogun F.O., Ashafa A.O.T. Green-Synthesized Zinc Oxide Nanoparticles from Aqueous Root Extract of Dicoma Anomala (Sond.) Mitigates Free Radicals and Diabetes-Linked Enzymes. Nanosci. Nanotechnol.-Asia. 2020;10:918–929. doi: 10.2174/2210681210666200117150727. [DOI] [Google Scholar]
- 118.Hoseinpour V., Souri M., Ghaemi N., Shakeri A. Optimization of green synthesis of ZnO nanoparticles by Dittrichia graveolens (L.) aqueous extract. Health Biotechnol. Biopharma. 2017;1:39–49. [Google Scholar]
- 119.Xu J., Luo X., Wang Y., Feng Y. Evaluation of Zinc Oxide Nanoparticles on Lettuce (Lactuca sativa L.) Growth and Soil Bacterial Community. Environ. Sci. Pollut. Res. Int. 2018;25:6026–6035. doi: 10.1007/s11356-017-0953-7. [DOI] [PubMed] [Google Scholar]
- 120.Datta A., Patra C., Bharadwaj H., Kaur S., Khajuria R. Green Synthesis of Zinc Oxide Nanoparticles Using Parthenium Hysterophorus Leaf Extract and Evaluation of Their Antibacterial Properties. J. Biotechnol. Biomater. 2017;7:271–276. doi: 10.4172/2155-952X.1000271. [DOI] [Google Scholar]
- 121.Kolahalam L.A., Prasad K.R.S., Murali Krishna P., Supraja N. Plant Rhizome Extract-Based Zinc Oxide Nanoparticles: Synthesis, Characterization and Its Antibacterial, Antifungal Activities and Cytotoxic Studies against Chinese Hamster Ovary (CHO) Cell Lines. Heliyon. 2021;7:e07265. doi: 10.1016/j.heliyon.2021.e07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hameed S., Khalil A.T., Ali M., Numan M., Khamlich S., Shinwari Z.K., Maaza M. Greener Synthesis of ZnO and Ag-ZnO Nanoparticles Using Silybum Marianum for Diverse Biomedical Applications. Nanomedicine. 2019;14:655–673. doi: 10.2217/nnm-2018-0279. [DOI] [PubMed] [Google Scholar]
- 123.Ilangovan A., Venkatramanan A., Thangarajan P., Saravanan A., Rajendran S., Kaveri K. Green Synthesis of Zinc Oxide Nanoparticles (ZnO NPs) Using Aqueous Extract of Tagetes Erecta Flower and Evaluation of Its Antioxidant, Antimicrobial, and Cytotoxic Activities on HeLa Cell Line. Curr. Biotechnol. 2021;10:61–76. doi: 10.2174/2211550109999201202123939. [DOI] [Google Scholar]
- 124.Obayomi K.S., Oluwadiya A.E., Lau S.Y., Dada A.O., Akubuo-Casmir D., Adelani-Akande T.A., Fazle Bari A.S.M., Temidayo S.O., Rahman M.M. Biosynthesis of Tithonia Diversifolia Leaf Mediated Zinc Oxide Nanoparticles Loaded with Flamboyant Pods (Delonix Regia) for the Treatment of Methylene Blue Wastewater. Arab. J. Chem. 2021;14:103363. doi: 10.1016/j.arabjc.2021.103363. [DOI] [Google Scholar]
- 125.Seifipour R., Nozari M., Pishkar L. Preparation of ZnO Nanoparticles Using Tragopogon Collinus Leaf Extract and Study of Its Antibacterial Effects for Therapeutic Applications. J. Plant Biochem. Biotechnol. 2021;30:586–595. doi: 10.1007/s13562-020-00638-w. [DOI] [Google Scholar]
- 126.Ossai A.N., Ezike S.C., Dikko A.B. Bio-Synthesis of Zinc Oxide Nanoparticles from Bitter Leaf (vernonia Amygdalina) Extract for Dye-Sensitized Solar Cell Fabrication. [(accessed on 31 July 2022)]. Available online: https://www.jmaterenvironsci.com/Document/vol11/vol11_N3/JMES-2020-11-38-Ossai.pdf.
- 127.Kouhbanani M.A.J., Beheshtkhoo N., Amani A.M., Taghizadeh S., Beigi V., Bazmandeh A.Z., Khalaf N. Green Synthesis of Iron Oxide Nanoparticles Using Artemisia Vulgaris Leaf Extract and Their Application as a Heterogeneous Fenton-like Catalyst for the Degradation of Methyl Orange. Mater. Res. Express. 2018;5:115013. doi: 10.1088/2053-1591/aadde8. [DOI] [Google Scholar]
- 128.Wanakai S.I., Kareru P.G., Makhanu D.S., Madivoli E.S., Maina E.G., Nyabola A.O. Catalytic Degradation of Methylene Blue by Iron Nanoparticles Synthesized Using Galinsoga parviflora, Conyza bonariensis and Bidens pilosa Leaf Extracts. SN Appl. Sci. 2019;1:1148. doi: 10.1007/s42452-019-1203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davarnejad R., Azizi A., Mohammadi M., Mansoori S. A Green Technique for Synthesising Iron Oxide Nanoparticles by Extract of Centaurea Cyanus Plant: An Optimised Adsorption Process for Methylene Blue. Int. J. Environ. Anal. Chem. 2022;102:2379–2393. doi: 10.1080/03067319.2020.1756273. [DOI] [Google Scholar]
- 130.Biswas A., Vanlalveni C., Lalfakzuala R., Nath S., Rokhum L. Mikania Mikrantha Leaf Extract Mediated Biogenic Synthesis of Magnetic Iron Oxide Nanoparticles: Characterization and Its Antimicrobial Activity Study. Mater. Today Proc. 2021;42:1366–1373. doi: 10.1016/j.matpr.2021.01.108. [DOI] [Google Scholar]
- 131.Khatami M., Alijani H.Q., Fakheri B., Mobasseri M.M., Heydarpour M., Farahani Z.K., Khan A.U. Super-Paramagnetic Iron Oxide Nanoparticles (SPIONs): Greener Synthesis Using Stevia Plant and Evaluation of Its Antioxidant Properties. J. Clean. Prod. 2019;208:1171–1177. doi: 10.1016/j.jclepro.2018.10.182. [DOI] [Google Scholar]
- 132.Habtemariam A.B. Biosynthesis of Magnetite (Fe3O4) Nanostructures Using Vernonia Amygdalina Leaves Extract. Lett. Appl. NanoBioScience. 2021;10:2777–2783. [Google Scholar]
- 133.Rather M.Y., Sundarapandian S. Magnetic Iron Oxide Nanorod Synthesis by Wedelia Urticifolia (Blume) DC. Leaf Extract for Methylene Blue Dye Degradation. Appl. Nanosci. 2020;10:2219–2227. doi: 10.1007/s13204-020-01366-2. [DOI] [Google Scholar]
- 134.Pansambal S. Phytosynthesis and Biological Activities of Fluorescent CuO Nanoparticles Using Acanthospermum Hispidum L. Extract. J. Nanostructures. 2017;7:165–174. doi: 10.18502/jns.v7i3.1. [DOI] [Google Scholar]
- 135.Nasrollahzadeh M., Mohammad Sajadi S., Rostami-Vartooni A. Green Synthesis of CuO Nanoparticles by Aqueous Extract of Anthemis Nobilis Flowers and Their Catalytic Activity for the A3 Coupling Reaction. J. Colloid Interface Sci. 2015;459:183–188. doi: 10.1016/j.jcis.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 136.Gowri M., Latha N., Rajan M. Copper Oxide Nanoparticles Synthesized Using Eupatorium Odoratum, Acanthospermum Hispidum Leaf Extracts, and Its Antibacterial Effects Against Pathogens: A Comparative Study. Bionanoscience. 2019;9:545–552. doi: 10.1007/s12668-019-00655-7. [DOI] [Google Scholar]
- 137.Ganesan S., Ganesh Babu I., Mahendran D., Indra Arulselvi P., Elangovan N., Geetha N., Venkatachalam P. Green Engineering of Titanium Dioxide Nanoparticles Using Ageratina Altissima (L.) King & H.E. Robines. Medicinal Plant Aqueous Leaf Extracts for Enhanced Photocatalytic Activity. Ann. Phytomedicine Int. J. 2016;5:69–75. [Google Scholar]
- 138.Dobrucka R. Synthesis of Titanium Dioxide Nanoparticles Using Herba. Iran J Pharm. Res. 2017;16:756–762. [PMC free article] [PubMed] [Google Scholar]
- 139.Babu N., Pathak V.M., Singh A., Navneet A. Navneet Sonchus Asper Leaves Aqueous Extract Mediated Synthesis of Titanium Dioxide Nanoparticles. Pharma Innov. 2019;8:817–822. [Google Scholar]
- 140.Wardani M., Yulizar Y., Abdullah I., Apriandanu D.O.B. Synthesis of NiO Nanoparticles via Green Route Using Ageratum Conyzoides L. Leaf Extract and Their Catalytic Activity. IOP Conf. Ser. Mater. Sci. Eng. 2019;509:012077. doi: 10.1088/1757-899X/509/1/012077. [DOI] [Google Scholar]
- 141.Likasari I.D., Astuti R.W., Yahya A., Isnaini N., Purwiandono G., Hidayat H., Wicaksono W.P., Fatimah I. NiO Nanoparticles Synthesized by Using Tagetes Erecta L Leaf Extract and Their Activities for Photocatalysis, Electrochemical Sensing, and Antibacterial Features. Chem. Phys. Lett. 2021;780:138914. doi: 10.1016/j.cplett.2021.138914. [DOI] [Google Scholar]
- 142.Rasheed T., Nabeel F., Bilal M., Iqbal H.M.N. Biogenic Synthesis and Characterization of Cobalt Oxide Nanoparticles for Catalytic Reduction of Direct Yellow-142 and Methyl Orange Dyes. Biocatal. Agric. Biotechnol. 2019;19:101154. doi: 10.1016/j.bcab.2019.101154. [DOI] [Google Scholar]
- 143.Dobrucka R. Synthesis of MgO Nanoparticles Using Artemisia Abrotanum Herba Extract and Their Antioxidant and Photocatalytic Properties. Iran. J. Sci. Technol. Trans. A Sci. 2016;42:547–555. doi: 10.1007/s40995-016-0076-x. [DOI] [Google Scholar]
- 144.Essien E.R., Atasie V.N., Oyebanji T.O., Nwude D.O. Biomimetic Synthesis of Magnesium Oxide Nanoparticles Using Chromolaena odorata (L.) Leaf Extract. Chem. Pap. 2020;74:2101–2109. doi: 10.1007/s11696-020-01056-x. [DOI] [Google Scholar]
- 145.Amina M., Al Musayeib N.M., Alarfaj N.A., El-Tohamy M.F., Oraby H.F., Al Hamoud G.A., Bukhari S.I., Moubayed N.M.S. Biogenic Green Synthesis of MgO Nanoparticles Using Saussurea Costus Biomasses for a Comprehensive Detection of Their Antimicrobial, Cytotoxicity against MCF-7 Breast Cancer Cells and Photocatalysis Potentials. PLoS ONE. 2020;15:e0237567. doi: 10.1371/journal.pone.0237567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.R R., Ranjithkumar R., Chandar S.B., Senthil Kumaran C.K., Sharmila C., Simi V. Green Synthesis Of Silver Nanoparticles Using Graviola Leaf Aqueous Extract At Room Temperature. Kongunadu Res. J. 2015;2:6–10. doi: 10.26524/krj86. [DOI] [Google Scholar]
- 147.Shanker U., Jassal V., Rani M., Kaith B.S. Towards Green Synthesis of Nanoparticles: From Bio-Assisted Sources to Benign Solvents. A Review. Int. J. Environ. Anal. Chem. 2016;96:801–835. [Google Scholar]
- 148.Ahmed R.H., Mustafa D.E. Green Synthesis of Silver Nanoparticles Mediated by Traditionally Used Medicinal Plants in Sudan. Int. Nano Lett. 2020;10:1–14. doi: 10.1007/s40089-019-00291-9. [DOI] [Google Scholar]
- 149.Li S., Shen Y., Xie A., Yu X., Qiu L., Zhang L., Zhang Q. Green Synthesis of Silver Nanoparticles Using Capsicum Annuum L. Extract. Green Chem. 2007;9:852–858. doi: 10.1039/b615357g. [DOI] [Google Scholar]
- 150.Gu J., Aidy A., Goorani S. Anti-Human Lung Adenocarcinoma, Cytotoxicity, and Antioxidant Potentials of Copper Nanoparticles Green-Synthesized by Calendula Officinalis. J. Exp. Nanosci. 2022;17:285–296. doi: 10.1080/17458080.2022.2066082. [DOI] [Google Scholar]
- 151.Rai A., Lall R. Antimicrobial, Antioxidant and Cytotoxic Activity of Green Synthesized Copper Nanoparticle of Parthenium Hysterophorus L. Int. J. Multidiscip. Res. Anal. 2021;4:101–116. doi: 10.47191/ijmra/v4-i2-01. [DOI] [Google Scholar]
- 152.Khalil M.M.H., Ismail E.H., El-Baghdady K.Z., Mohamed D. Green Synthesis of Silver Nanoparticles Using Olive Leaf Extract and Its Antibacterial Activity. Arab. J. Chem. 2014;7:1131–1139. doi: 10.1016/j.arabjc.2013.04.007. [DOI] [Google Scholar]
- 153.Balalakshmi C., Gopinath K., Govindarajan M., Lokesh R., Arumugam A., Alharbi N.S., Kadaikunnan S., Khaled J.M., Benelli G. Green Synthesis of Gold Nanoparticles Using a Cheap Sphaeranthus Indicus Extract: Impact on Plant Cells and the Aquatic Crustacean Artemia Nauplii. J. Photochem. Photobiol. B. 2017;173:598–605. doi: 10.1016/j.jphotobiol.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 154.AlSalhi M., Devanesan S., Alfuraydi A., Vishnubalaji R., Munusamy M.A., Murugan K., Nicoletti M., Benelli G. Green Synthesis of Silver Nanoparticles Using Pimpinella Anisum Seeds: Antimicrobial Activity and Cytotoxicity on Human Neonatal Skin Stromal Cells and Colon Cancer Cells. Int. J. Nanomed. 2016;11:4439–4449. doi: 10.2147/IJN.S113193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Baker S., Rakshith D., Kavitha K.S., Santosh P., Kavitha H.U., Rao Y., Satish S. Plants: Emerging as Nanofactories towards Facile Route in Synthesis of Nanoparticles. Bioimpacts. 2013;3:111–117. doi: 10.5681/bi.2013.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bhushan I., Singh V.K., Tripathi D.K. Nanomaterials and Environmental Biotechnology. Springer Nature; Berlin/Heidelberg, Germany: 2020. [Google Scholar]
- 157.Bell J.E., Ellis Bell J., Hall C. Spectroscopy in Biochemistry. CRC Press; Boca Raton, FL, USA: 2018. UV and Visible Absorbance Spectroscopy; pp. 3–62. [Google Scholar]
- 158.Nagaraj B., Malakar B., Divya T.K., Krishnamurthy N., Liny P., Dinesh R., Iconaru S., Ciobanu C. Synthesis of Plant Mediated Gold Nanoparticles Using Flower Extracts of Carthamus Tinctorius L. (safflower) and Evaluation of Their Biological Activities. Dig. J. Nanomater. Biostruct. 2012;7:1289–1296. doi: 10.4172/2155-952X.S1.020. [DOI] [Google Scholar]
- 159.Chandraker S.K., Lal M., Ghosh M.K., Tiwari V., Ghorai T.K., Shukla R. Green Synthesis of Copper Nanoparticles Using Leaf Extract of Ageratum Houstonianum Mill. and Study of Their Photocatalytic and Antibacterial Activities. Nano Express. 2020;1:010033. doi: 10.1088/2632-959X/ab8e99. [DOI] [Google Scholar]
- 160.Nasrazadani S., Hassani S. Handbook of Materials Failure Analysis with Case Studies from the Oil and Gas Industry. Elsevier; Amsterdam, The Netherlands: 2016. Modern Analytical Techniques in Failure Analysis of Aerospace, Chemical, and Oil and Gas Industries; pp. 39–54. [Google Scholar]
- 161.Berg J.M., Romoser A., Banerjee N., Zebda R., Sayes C.M. The Relationship between pH and Zeta Potential of ∼ 30 Nm Metal Oxide Nanoparticle Suspensions Relevant Toin Vitrotoxicological Evaluations. Nanotoxicology. 2009;3:276–283. doi: 10.3109/17435390903276941. [DOI] [Google Scholar]
- 162.Xu R. Progress in Nanoparticles Characterization: Sizing and Zeta Potential Measurement. Particuology. 2008;6:112–115. doi: 10.1016/j.partic.2007.12.002. [DOI] [Google Scholar]
- 163.Cho T.J., Hackley V.A. Fractionation and Characterization of Gold Nanoparticles in Aqueous Solution: Asymmetric-Flow Field Flow Fractionation with MALS, DLS, and UV-Vis Detection. Anal. Bioanal. Chem. 2010;398:2003–2018. doi: 10.1007/s00216-010-4133-6. [DOI] [PubMed] [Google Scholar]
- 164.Zou C., Gao Y., Yang B., Zhai Q. Synthesis and DSC Study on Sn3.5Ag Alloy Nanoparticles Used for Lower Melting Temperature Solder. J. Mater. Sci. Mater. Electron. 2010;21:868–874. doi: 10.1007/s10854-009-0009-y. [DOI] [Google Scholar]
- 165.Dongargaonkar A.A., Clogston J.D. Quantitation of Surface Coating on Nanoparticles Using Thermogravimetric Analysis. Methods Mol. Biol. 2018;1682:57–63. doi: 10.1007/978-1-4939-7352-1_6. [DOI] [PubMed] [Google Scholar]
- 166.Bajpai O.P., Panja S., Chattopadhyay S., Setua D.K. Manufacturing of Nanocomposites with Engineering Plastics. Elsevier; Amsterdam, The Netherlands: 2015. Process–structure–property Relationships in Nanocomposites Based on Piezoelectric-Polymer Matrix and Magnetic Nanoparticles; pp. 255–278. [Google Scholar]
- 167.Polshettiwar V., Asefa T. Nanocatalysis: Synthesis and Applications. John Wiley & Sons; Hoboken, NJ, USA: 2013. [Google Scholar]
- 168.Pogrebnjak A.D., Novosad V. Advances in Thin Films, Nanostructured Materials, and Coatings: Selected Papers from the 2018 International Conference on “Nanomaterials: Applications & Properties”. Springer; Berlin/Heidelberg, Germany: 2019. [Google Scholar]
- 169.Nezamabadi V., Akhgar M.R., Tahamipour B., Rajaei P. Biosynthesis and Antibacterial Activity of ZnO Nanoparticles by Extract. Iran. J. Biotechnol. 2020;18:e2426. doi: 10.30498/IJB.2020.151379.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.José-Yacamán M., Marín-Almazo M., Ascencio J.A. High Resolution TEM Studies on Palladium Nanoparticles. J. Mol. Catal. A Chem. 2001;173:61–74. doi: 10.1016/S1381-1169(01)00145-5. [DOI] [Google Scholar]
- 171.Mayoral A., Mejía-Rosales S., Mariscal M.M., Pérez-Tijerina E., José-Yacamán M. The Co-Au Interface in Bimetallic Nanoparticles: A High Resolution STEM Study. Nanoscale. 2010;2:2647–2651. doi: 10.1039/c0nr00498g. [DOI] [PubMed] [Google Scholar]
- 172.Karthik L., Vishnu Kirthi A., Ranjan S., Mohana Srinivasan V. Biological Synthesis of Nanoparticles and Their Applications. CRC Press; Boca Raton, FL, USA: 2019. [Google Scholar]
- 173.Maji A., Beg M., Das S., Aktara M.N., Nayim S., Patra A., Islam M.M., Hossain M. Study on the Antibacterial Activity and Interaction with Human Serum Albumin of Tagetes Erecta Inspired Biogenic Silver Nanoparticles. Process Biochem. 2020;97:191–200. doi: 10.1016/j.procbio.2020.07.017. [DOI] [Google Scholar]
- 174.Rajiv P., Rajeshwari S., Venckatesh R. Bio-Fabrication of Zinc Oxide Nanoparticles Using Leaf Extract of Parthenium Hysterophorus L. and Its Size-Dependent Antifungal Activity against Plant Fungal Pathogens. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013;112:384–387. doi: 10.1016/j.saa.2013.04.072. [DOI] [PubMed] [Google Scholar]
- 175.Bedlovičová Z., Strapáč I., Baláž M., Salayová A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules. 2020;25:3191. doi: 10.3390/molecules25143191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Khshan K.T., Alkafaje H.A. Biosynthesis of Silver Nanoparticles Using Calendula officinalis (L.) Extract and Evaluating Their Antioxidant Activity. IOP Conf. Ser. Earth Environ. Sci. 2021;735:012073. doi: 10.1088/1755-1315/735/1/012073. [DOI] [Google Scholar]
- 177.ACAR Ç.A. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Achiella Millefolium L.: In Vitro Anti-Cancer Potential On Lung And Colon Cancer Cells. Turk. J. Health Sci. Life. 2021;4:40–45. [Google Scholar]
- 178.Ul Haq M.N., Shah G.M., Gul A., Foudah A.I., Alqarni M.H., Yusufoglu H.S., Hussain M., Alkreathy H.M., Ullah I., Khan A.M., et al. Biogenic Synthesis of Silver Nanoparticles Using and Its In Vivo Anti-Diabetic Effect against Alloxan-Induced Diabetic Wistar Rats. Nanomaterials. 2022;12:830. doi: 10.3390/nano12050830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Iqbal J., Andleeb A., Ashraf H., Meer B., Mehmood A., Jan H., Zaman G., Nadeem M., Drouet S., Fazal H., et al. Potential Antimicrobial, Antidiabetic, Catalytic, Antioxidant and ROS/RNS Inhibitory Activities of Mediated Biosynthesized Copper Oxide Nanoparticles. RSC Adv. 2022;12:14069–14083. doi: 10.1039/D2RA01929A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.De Queiroz A.C., de Dias T.L.M.F., Da Matta C.B.B., Cavalcante Silva L.H.A., de Araújo-Júnior J.X., de Araújo G.B., de Moura F.B.P., Alexandre-Moreira M.S. Antileishmanial Activity of Medicinal Plants Used in Endemic Areas in Northeastern Brazil. Evid. Based. Complement. Alternat. Med. 2014;2014:478290. doi: 10.1155/2014/478290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gangwar J., Sebastian J.K. Unlocking the Potential of Biosynthesized Zinc Oxide Nanoparticles for Degradation of Synthetic Organic Dyes as Wastewater Pollutants. Water Sci. Technol. 2021;84:3286–3310. doi: 10.2166/wst.2021.430. [DOI] [PubMed] [Google Scholar]
- 182.Bahadar H., Maqbool F., Niaz K., Abdollahi M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016;20:1–11. doi: 10.7508/ibj.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yashveer S., Redhu N., Singh V., Sangwan S., Laxman H., Tokas J., Malhotra S., Khurana S., Sindhu A. Nanoparticles in Agriculture: Characterization, Uptake and Role in Mitigating Heat Stress. Nat. Resour. Hum. Health. 2022;2:160–181. doi: 10.53365/nrfhh/144175. [DOI] [Google Scholar]
- 184.Arokiyaraj S., Dinesh Kumar V., Elakya V., Kamala T., Park S.K., Ragam M., Saravanan M., Bououdina M., Arasu M.V., Kovendan K., et al. Biosynthesized Silver Nanoparticles Using Floral Extract of Chrysanthemum indicum L.—Potential for Malaria Vector Control. Environ. Sci. Pollut. Res. Int. 2015;22:9759–9765. doi: 10.1007/s11356-015-4148-9. [DOI] [PubMed] [Google Scholar]
- 185.Hajra A., Dutta S., Mondal N.K. Mosquito Larvicidal Activity of Cadmium Nanoparticles Synthesized from Petal Extracts of Marigold (Tagetes sp.) and Rose (Rosa sp.) Flower. J. Parasit. Dis. 2016;40:1519–1527. doi: 10.1007/s12639-015-0719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.