Abstract
In recent years, Campylobacter has become increasingly resistant to antibiotics, especially those first-choice drugs used to treat campylobacteriosis. Studies in South America have reported cases of antibiotic-resistant Campylobacter in several countries, mainly in Brazil. To understand the current frequency of antibiotic-resistant Campylobacter in humans, farm animals, and food of animal origin in South America, we systematically searched for different studies that have reported Campylobacter resistance. The most commonly reported species were C. jejuni and C. coli. Resistance to ciprofloxacin was found to be ubiquitous in the isolates. Nalidixic acid and tetracycline showed a significantly expressed resistance. Erythromycin, the antibiotic of first choice for the treatment of campylobacteriosis, showed a low rate of resistance in isolates but was detected in almost all countries. The main sources of antibiotic-resistant Campylobacter isolates were food of animal origin and farm animals. The results demonstrate that resistant Campylobacter isolates are disseminated from multiple sources linked to animal production in South America. The level of resistance that was identified may compromise the treatment of campylobacteriosis in human and animal populations. In this way, we are here showing all South American communities the need for the constant surveillance of Campylobacter resistance and the need for the strategic use of antibiotics in animal production. These actions are likely to decrease future difficulties in the treatment of human campylobacteriosis.
Keywords: antimicrobial resistance, food contamination, food of animal origin, meat products, animal husbandry, food-producing animals, antimicrobial susceptibility testing (TSA)
1. Introduction
Campylobacter is a Gram-negative bacterium widely associated with gastroenteritis and enterocolitis in humans worldwide [1]. In the European Union, campylobacteriosis has been the gastrointestinal infection with the highest number of reports in humans since 2005 [2]. In the United States, about 1.3 million cases of the disease are reported annually [2]. The genus Campylobacter comprises 32 species, and 9 subspecies have already been described [3]. Among them, the thermophilic species Campylobacter jejuni (C. jejuni) and Campylobacter coli (C. coli) are frequently isolated from poultry and pig, respectively [4]. Thermophilic Campylobacter is the main cause of bacterial gastroenteritis in humans worldwide [5], mainly C. jejuni [6]. This bacterium composes the microbiota of warm-blooded animals and, in most cases, is associated with asymptomatic infections [7].
Meat products, especially chicken, are often contaminated with C. jejuni, becoming the main vehicle of human campylobacteriosis through the consumption of undercooked meat [6]. Although evisceration plays an essential role in C. jejuni contamination, all steps of the poultry slaughtering process may also have points of contamination. Campylobacter contamination in the meat production chain represents a public health hazard and a challenge for health authorities in terms of surveillance, sub-notification, and control. These public health actions are necessary because the clinical manifestation of campylobacteriosis varies from diarrheal cases to more severe diseases such as Crohn’s disease [8], Miller-Fisher syndrome [9], or neurological sequelae such as Guillain–Barré syndrome [10]. The outcome of the disease depends on the immune status of the host, and the use of antibiotics is necessary for the treatment of severe clinical cases in children, the elderly, and immunocompromised individuals [6].
Regarding the regulatory framework for Campylobacter in food products, especially those of animal origin, the regulations of the U.S., EU, and Australia/New Zealand seem to be the most advanced worldwide. The Food Safety and Inspection Service (FSIS) of the USDA, for example, determines that the maximum acceptable percentage of positive samples can be 15.7% for chicken broiler carcasses, 5.4% for turkey carcasses, 7.7% for chicken parts, and 1.9% for comminuted chicken and comminuted turkey [11]. On the other hand, the EU has a risk assessment framework and a risk assessment model for Campylobacter in broilers that sets a maximum of 1000 cfu/g in 50 carcass samples after chilling [12]. Brazil and other South American countries comply with the regulatory framework announced by the EU. Brazil, in particular, has several slaughterhouses that export products to the European Union. However, the inspection service of the Ministry of Agriculture (MAPA) does not have official limits for Campylobacter in food of animal origin.
Fluoroquinolones have proved to be first-choice antibiotics in the clinical therapy of campylobacteriosis for many years. However, the widespread use of these drugs in clinical and animal husbandry as growth promoters may have created selective pressure for the emergence of fluoroquinolone-resistant Campylobacter in food-producing animals [13,14]. This selection of resistant and multidrug-resistant pathogens represents one of the main challenges for public health actions, mainly in developing countries [15]. Consequently, increasingly frequent multidrug-resistant (MDR) pathogens have emerged worldwide [15]. The World Health Organization (WHO) has included Campylobacter on the list of bacteria for which new antibiotics are urgently needed and has classified it as a high-priority pathogen due to the worldwide emergence of strains with a high level of resistance to fluoroquinolones [16]. This increase in resistance to fluoroquinolones has forced the introduction of a new class of antibiotics. Macrolides are currently first-choice antibiotics in the treatment of campylobacteriosis [17]. Within this class, erythromycin has been the most widely used, demonstrating satisfactory therapeutic results. On the other hand, erythromycin resistance levels have been increasing in recent years [18,19], requiring urgent active surveillance.
C. jejuni is a highly adaptable pathogen with several resistance mechanisms, and it requires intense epidemiological surveillance. While it is widely monitored in the developed countries of the European Union and North America, epidemiological surveillance of resistant Campylobacter in South America is scarce. This is mainly due to underdiagnosed and underreported cases and the lack of existing studies on this pathogen [20,21,22,23,24]. Given these circumstances, this study aimed to explore the frequency of antibiotic-resistant Campylobacter isolates in South American countries among humans, food-producing animals, and food of animal origin, to compile a current distribution of resistant strains and the main sources of contamination.
2. Results
2.1. The Systematic Review Criteria
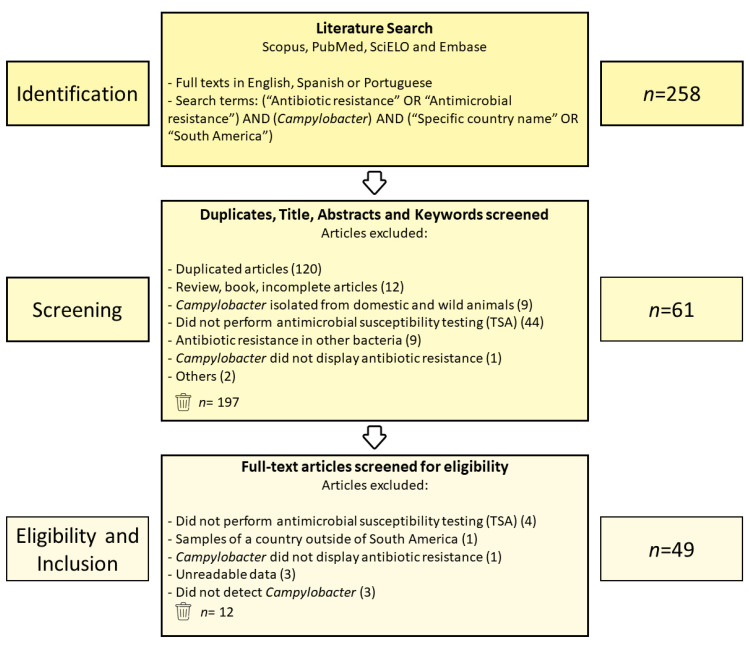
The literature search identified a total of 258 articles from the Scopus (n = 84), PubMed (n = 74), SciELO (n = 11), and Embase (n = 89) databases. First, duplicates were evaluated by reading the titles and abstracts, which resulted in 197 excluded articles. The remaining 61 were subject to a full-text review. Of these, 12 were eliminated based on the eligibility criteria described in the Materials and Methods. Finally, 49 studies were obtained and subsequently used for the review (Figure 1).
Figure 1.
PRISMA flowchart describing the eligibility criteria used in the systematic review process.
2.2. Frequency of Antibiotic-Resistant Campylobacter by South American Countries
Out of 18, nine South American countries had studies showing antibiotic-resistant Campylobacter isolates. Brazil presented 24 of the 49 eligible articles, followed by Peru with 6, Chile and Ecuador with 5, Argentina with 3, Paraguay and Trinidad with 2, and Colombia and Uruguay presented only 1 (Supplementary Table S1).
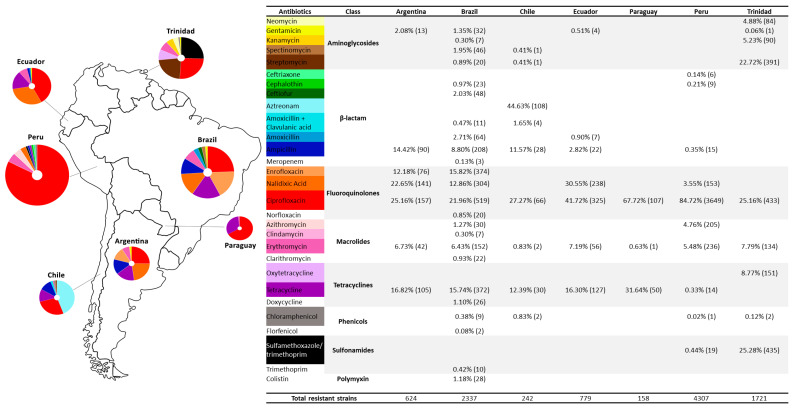
In Argentina, Campylobacter isolates were resistant to seven antibiotics from five classes. Ciprofloxacin was the antibiotic with the higher rate of resistance, followed by nalidixic acid, tetracycline, ampicillin, enrofloxacin, erythromycin, and gentamicin (Figure 2). In Brazil, Campylobacter isolates were resistant to 24 antibiotics from 8 classes. Ciprofloxacin also showed a higher resistance, followed by enrofloxacin, tetracycline, nalidixic acid, ampicillin, erythromycin, amoxicillin, ceftiofur, spectinomycin, gentamicin, azithromycin, colistin, doxycycline, sulfonamide, cephalothin, clarithromycin, streptomycin, norfloxacin, amoxicillin with clavulanic acid, trimethoprim, chloramphenicol, clindamycin, kanamycin, meropenem, and florfenicol. In Chile, Campylobacter isolates showed resistance to eight antibiotics from five classes, with aztreonam being the most resistant, followed by ciprofloxacin, tetracycline, ampicillin, amoxicillin and clavulanic acid, erythromycin and chloramphenicol, and spectinomycin and streptomycin. Ecuador showed isolates resistant to seven antibiotics from five classes. These were ciprofloxacin, nalidixic acid, tetracycline, erythromycin, ampicillin, amoxicillin, and gentamicin. In Paraguay, Campylobacter resistance was shown to three antibiotic classes and three antibiotics: ciprofloxacin, tetracycline, and erythromycin. Peru had Campylobacter isolates resistant to ten antibiotics from seven classes: ciprofloxacin, erythromycin, azithromycin, nalidixic acid, sulfamethoxazole/trimethoprim, ampicillin, tetracycline, cephalothin, ceftriaxone, and chloramphenicol. The Campylobacter strains isolated in Trinidad showed resistance to eight antibiotics from seven classes. They were sulfamethoxazole/trimethoprim, ciprofloxacin, streptomycin, oxytetracycline, erythromycin, kanamycin, neomycin, chloramphenicol, and gentamicin. Finally, Uruguay showed strains with resistance to four antibiotics from three classes. All were resistant to clindamycin, telithromycin, nalidixic acid, and tetracycline (Figure 2). A single study in Colombia did not address the phenotypic resistance of isolates, with the study investigating only resistance genes; thus, it was not taken into account [20].
Figure 2.
Antibiotic resistance in Campylobacter isolates from studies of South American countries. The values in parentheses refer to the amount of Campylobacter isolates resistant to the specific antibiotic. Circle diagrams are limited to the ten antibiotics with the highest frequency.
2.3. The Frequency of Campylobacter Species among Isolates Recovered from South American Eligible Studies
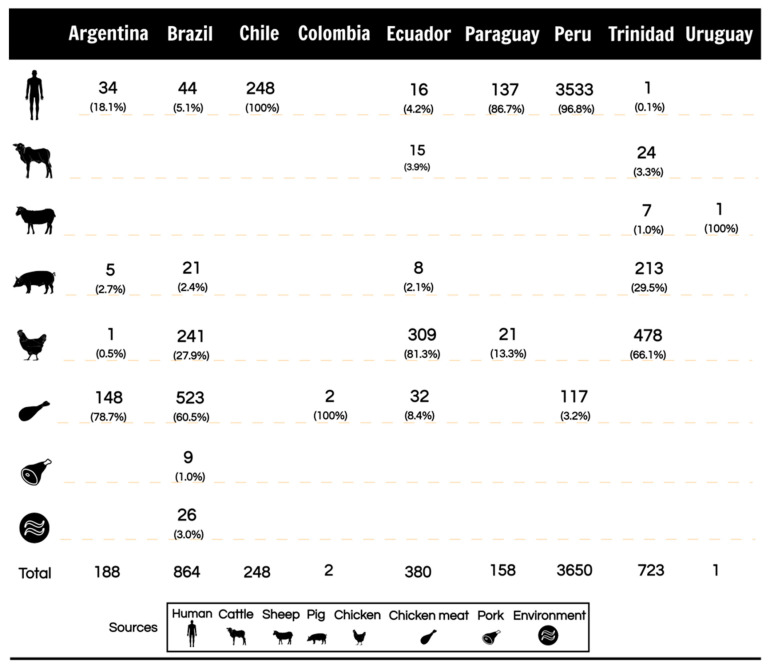
The data showed that C. jejuni, C. coli, and C. lari were the Campylobacter species isolated in eligible studies (Table 1). In Argentina, two species were reported, C. jejuni (75.6%) and C. coli (22.6%), and derived from chicken meat, human, pork, and chicken (Figure 3). In Chile, C. jejuni (95.9%) and C. coli (4.1%) were reported. This report was from studies with antibiotic-resistant strains of human sources (Figure 3). In Brazil, three species were reported: C. jejuni (82.9%), C. coli (13.0%), and C. lari (0.1%). The studies conducted in this country showed a greater diversity of sources with antibiotic-resistant Campylobacter. The sources were chicken meat, chicken, human, environment, pork, and swine (Figure 3). Studies from Colombia and Paraguay displayed one specie each, C. coli (100%) and C. jejuni (95.4%), respectively (Table 1). Colombia also reported one study with isolates from food (Figure 3). Ecuador and Peru showed the detection of C. jejuni (77.1% and 83.5%) and C. coli (22.1% and 15.5%), respectively (Table 1). In Ecuador, studies reported isolated strains from chicken, human, chicken meat, cattle, and pork (Figure 3). In Peru, antibiotic-resistant Campylobacter was isolated in humans and chicken meat (Figure 3). In Trinidad, C. coli (71.7%) and C. jejuni (28.2%) were reported (Table 1). These isolated were reported to be from chicken, pork, cattle, sheep, and human (Figure 3). Finally, Paraguay showed studies with isolates from humans and chickens, and in Uruguay, a single study reported Campylobacter isolated from a sheep (Figure 3).
Table 1.
Number and frequency of species among isolated Campylobacter strains in South America.
| Country | Articles (n) | Sample Analyzed |
Campylobacter Isolated (n) |
Campylobacter Species (n) |
Frequency (%) | Reference |
|---|---|---|---|---|---|---|
| Argentina | 3 | 50 | 11 | C. jejuni (8) | C. jejuni (75.6%) | [25] |
| C. coli (3) | C. coli (22.5%) | |||||
| 327 | 50 | C. jejuni (48) | [22] | |||
| C. coli (2) | ||||||
| 555 | 152 | C. jejuni (105) | [23] | |||
| C. coli (43) | ||||||
| Brazil | 22 | 259 | 9 | C. jejuni (5) | C. jejuni (82.9%) | [24] |
| C. coli (3) | C. coli (13.0%) | |||||
| 92 | 16 | C. jejuni (16) | C. lari (0.1%) | [25] | ||
| 50 | 34 | C. coli (14) | [26] | |||
| 70 | 70 | C. jejuni (69) | [27] | |||
| 24 | 24 | C. jejuni (22) | [28] | |||
| C. coli (1) | ||||||
| C. lari (1) | ||||||
| 1 | 1 | C. jejuni (1) | [29] | |||
| 67 | 67 | C. jejuni (67) | [30] | |||
| 42 | 42 | C. jejuni (14) | [31] | |||
| C. coli (25) | ||||||
| 95 | 20 | C. jejuni (18) | [32] | |||
| C. coli (2) | ||||||
| 78 | 46 | C. jejuni (39) | [33] | |||
| C. coli (7) | ||||||
| 173 | 28 | C. jejuni (28) | [34] | |||
| 120 | 18 | C. jejuni (5) | [35] | |||
| C. coli (13) | ||||||
| 1070 | 99 | C. jejuni (99) | [36] | |||
| 159 | 159 | C. jejuni (81) | [37] | |||
| C. coli (78) | ||||||
| 54 | 54 | C. jejuni (54) | [38] | |||
| 116 | 116 | C. jejuni (116) | [39] | |||
| 442 | 35 | C. jejuni (35) | [40] | |||
| 141 | 141 | C. jejuni (140) | [41] | |||
| C. coli (1) | ||||||
| 50 | 50 | C. jejuni (50) | [42] | |||
| 515 | 80 | C. jejuni (80) | [43] | |||
| 2 | 2 | C. jejuni (2) | [44] | |||
| 48 | 32 | C. jejuni (32) | [45] | |||
| Chile | 5 | 81 | 81 | C. jejuni (69) | C. jejuni (95.9%) | [46] |
| C. coli (12) | C. coli (4.1%) | |||||
| 50 | 50 | C. jejuni (50) | [47] | |||
| 108 | 108 | C. jejuni (108) | [48] | |||
| 73 | 73 | C. jejuni (73) | [49] | |||
| 350 | 28 | C. jejuni (26) | [50] | |||
| C. coli (2) | ||||||
| Colombia | 1 | 2 | 2 | C. coli (2) | C. coli (100%) | [20] |
| Ecuador | 5 | 120 | 50 | C. jejuni (39) | C. jejuni (77.1%) | [51] |
| C. coli (11) | C. coli (22.9%) | |||||
| 51 | 32 | C. jejuni (22) | [52] | |||
| C. coli (10) | ||||||
| 253 | 16 | C. jejuni (13) | [53] | |||
| C. coli (3) | ||||||
| 250 | 64 | C. jejuni (49) | [54] | |||
| C. coli (15) | ||||||
| 379 | 218 | C. jejuni (170) | [55] | |||
| C. coli (48) | ||||||
| Paraguay | 1 | 150 | 22 | C. jejuni (21) | C. jejuni (95.4%) | [56] |
| Peru | 6 | 120 | 117 | C. coli (117) | C. jejuni (80.4%) | [57] |
| 189 | 189 | C. jejuni (189) | C. coli (14.8%) | [58] | ||
| 230 | 19 | C. jejuni (16) | [59] | |||
| C. coli (3) | ||||||
| 4652 | 4652 | C. jejuni (3856) | [60] | |||
| C. coli (554) | ||||||
| 150 | 106 | C. jejuni (30) | [61] | |||
| C. coli (76) | ||||||
| 7 | 7 | C. jejuni (4) | [62] | |||
| C. coli (3) | ||||||
| Trinidad | 1 | 689 | 315 | C. jejuni (89) | C. jejuni (28.2%) | [63] |
| C. coli (226) | C. coli (71.7%) |
Figure 3.
Number and frequency of antibiotic-resistant Campylobacter extracted from the eligible studies displayed by the source of isolation and country.
3. Discussion
Campylobacter antibiotic resistance can be developed through spontaneous mutations and the acquisition of resistance determinants can be through natural transformation, transduction, or conjugation [64], according to their different mechanisms of evasion against each antibiotic. Treatment in humans is performed with fluoroquinolones due to their broad spectrum of action, and efficacy against both Gram-negative and Gram-positive bacteria [25]. In food-producing animals, fluoroquinolones are often used to treat infections and as a feed additive indiscriminately. This systematic review showed that studies investigating antibiotic resistance in Campylobacter in South America are limited and underexplored. Nevertheless, the results showed that antibiotic resistance in Campylobacter had recently increased with concerns regarding resistance against the drugs used as the first choice to treat human campylobacteriosis. Our data compilation showed high levels of ciprofloxacin resistance in seven South American countries (Argentina, Brazil, Chile, Ecuador, Paraguay, Peru, and Trinidad). Peru reached more than 80% of the resistance rate among all antibiotics (Figure 2). High levels of resistance to fluoroquinolone (75–90%) in clinical Campylobacter strains have also been reported in countries on other continents [65,66,67], demonstrating the existence of a global health problem. The fast ability of Campylobacter to acquire resistance to fluoroquinolones was demonstrated experimentally with only one or two administrations of these antibiotics [13]. The main target of fluoroquinolones in Campylobacter is the enzyme DNA gyrase (Topoisomerase II) [68]. This enzyme comprises two subunits, A and B, respectively, encoded by gyrA and gyrB genes. Its action consists of the catalysis of the ATP-dependent negative supercoiling of DNA and is involved in DNA replication, recombination, and transcription [69]. When fluoroquinolones bind to these enzymes, a stable complex is formed, trapping the enzymes in DNA, leading to DNA double-stranded breaks and bacterial death [69,70]. In Campylobacter, the primary mechanism of the development of fluoroquinolone resistance is a single-point mutation in the quinolone resistance-determining region (QRDR) of gyrA [17], leading to the substitution of isoleucine for threonine at codon 86 of the gyrA gene, which confers a high-level resistance and inhibits bacterial DNA synthesis [70]. Because ciprofloxacin is one of the first options to treat campylobacteriosis, resistance against this drug may compromise antibiotic therapy, posing a public health risk.
Erythromycin was introduced as a substitute in the human clinical treatment of Campylobacter infections due to increased resistance to ciprofloxacin [14]. This drug belongs to the macrolide class and, so far, it is the first choice antibiotic for the treatment of human campylobacteriosis [64,71]. Our results showed that erythromycin-resistant Campylobacter was isolated in all South American countries except Uruguay (Figure 2). Although rare, the results indicated that erythromycin-resistant Campylobacter is spreading, creating a new warning for the use of this drug in animals and humans. In Brazil, macrolides such as tylosin were widely used both as a feed additive [72] and to prevent clostridiosis in swine production. In parallel, an increase in erythromycin-resistant Clostridium difficile, which coexists with Campylobacter in the intestinal tract of poultry and pigs [72], was observed in several farms using erythromycin. Therefore, the use of macrolides to prevent clostridiosis may have contributed to the selection of macrolide-resistant Campylobacter [72]. Tylosin was recently banned as a food additive in Brazil; however, it is still used to treat and prevent animal infections [71]. Macrolides such as erythromycin and tylosin are bacteriostatic. They act by binding to the P site of the 50S ribosomal subunit and inhibit protein synthesis [71]. Campylobacter can evade macrolide binding by accumulating mutations in 23S rRNA at position 2074 or 2075 and through other mechanisms such as an efflux pump and altered membrane permeability [14]. The data showed that Campylobacter is poorly resistant to macrolides. However, the main concern with macrolide resistance is the compromised treatment of human infections, as erythromycin is currently the main human antibiotic [71].
Another treatment option for campylobacteriosis is tetracycline, which is an antibiotic with broad activity against Gram-negative and Gram-positive bacteria for human and animal treatment and has been used since 1948. The absence of significant adverse side effects has contributed to its widespread use in the treatment of human and animal infections [73]. However, it did not take long before the first case of tetracycline resistance appeared. In 1953, the first tetracycline-resistant bacterium, Shigella dysenteriae, was isolated [74]. Currently, resistance to tetracycline has been reported in several bacteria [75,76,77]. Campylobacter is becoming increasingly resistant [78,79]. According to our results, seven out of eight South American countries showed Campylobacter resistance to tetracycline. Their resistance rate was the second, third, and even the most frequent in these countries (Figure 2). Tetracycline-resistant Campylobacter is related to the tet(O) gene that encodes the tet(O) protein [73]. This protein protects the ribosome from the inhibitory effect of tetracycline and is usually associated with conjugative plasmids [73,80]. Plasmid-mediated resistance spreads faster than chromosomal resistance, thus increasing the emergence of resistant strains. Several studies have reported the appearance of plasmids conferring tetracycline resistance in C.jejuni and C. coli [80,81,82]. The presence of tet(O) in the conjugative plasmids may explain the high distribution of tetracycline resistance found in our results (Figure 2). Tetracycline is widely used to treat animal and human infections [17,83]. Moreover, it is also used as a prophylactic and growth-promoting agent in food-producing animals [84]. The extensive use of tetracycline is due to its broad-spectrum activity and low cost [85]. However, the indiscriminate use of these drugs may put selective pressure on bacteria, pushing the emergence of antibiotic-resistant bacteria that can be transmitted to humans through environment, food, and agricultural workers by direct contact [84].
We observed ampicillin resistance in isolates from Argentina, Brazil, and Chile. In addition, ampicillin-resistant Campylobacter were also detected in Ecuador and Peru, but they were in a smaller proportion (Figure 2). Ampicillin belongs to the β-lactam class comprising penicillins, cephalosporins, carbapenems, and monobactams, known by the β-lactam ring in their structures [86]. Their action consists of binding to the penicillin-binding proteins of the bacteria cell wall, inhibiting peptidoglycan synthesis and causing cell lysis [87]. Most Campylobacter strains are inherently resistant to many beta-lactams, mainly first- and second-generation penicillins and cephalosporins [17]. They also possess mechanisms that potentiate resistance to this class of antibiotics [17]. The production of beta-lactamases (similar to penicillinases) is the most common and essential resistance mechanism [88]. These enzymes can be encoded by the blaOXA-61 gene, a chromosomal gene present in most Campylobacter strains that confers resistances to β-lactams [89] amoxicillin, ampicillin, and ticarcillin [90]. Therefore, the ampicillin-resistant Campylobacter exhibited here may be related to the production of β-lactamases. The expression of penicillinase-type β-lactamase in Campylobacter can overcome the β-lactamase inhibitors tazobactam, clavulanic acid, and sulbactam [91]. Here, we found resistance to amoxicillin and amoxicillin with clavulanic acid in Brazil, Chile, and Ecuador, which showed low levels of resistance (Figure 2). The mechanisms of resistance to beta-lactam are not yet fully consolidated but are usually related to the presence of the blaOXA-61 gene [92]. However, some strains harbor the blaOXA-61 gene and are not resistant to β-lactams, demonstrating that there may be other mechanisms involved that have not been revealed [92]. In addition, other mechanisms have been described as mediators of β-lactams resistance, such as modifications in outer membrane porins and efflux pumps [86]. Most of the studies performed here in South America have not performed a molecular analysis to identify the genes related to beta-lactam resistance, assessing only phenotypic resistance. The absence of molecular analysis made it difficult to understand the mechanisms of resistance to these antibiotics in South American strains. Therefore, in addition to complement phenotypic tests, molecular analysis should be performed in future studies. Interestingly, a high resistance to aztreonam was found in Chile (Figure 2). Aztreonam is a synthetic monocyclic β-lactam antimicrobial agent belonging to the monobactam family [93]; therefore, its action consists of interfering with the biosynthesis of bacterial cell walls [93], showing an excellent efficacy against Gram-negative bacteria and, due to its poor oral absorption, it is administered intramuscularly or intravenously [94]. However, one of the eligible studies raised the debate that aztreonam was not very efficacious against microaerophilic and aerobic bacteria due to its weak binding to penicillin-binding protein sites in these microorganisms [95]. Therefore, it was initially thought that Campylobacter could naturally resist aztreonam. Later, Campylobacter upsaliensis strains with sensitivity to aztreonam were found, demonstrating that this resistance did not apply to all species [95]. Still, in general, most Campylobacter species are expected to show resistance to this drug.
Resistance to gentamicin was infrequently detected in Argentina, Brazil, and Ecuador. This is probably related to the occasional use of this antibiotic in food-producing animals, mainly because the route of administration is intramuscular, making it difficult to use on a large scale [96]. Furthermore, in specific cases of human infections, severe bacteremia may develop, requiring the intravenous administration of aminoglycosides [97]. Generally, Campylobacter exhibits a low resistance to gentamicin [98]. In the United States, gentamicin resistance in Campylobacter was rare; the first detection was in 2000 from a human sample and subsequently in 2007, isolated from retail chicken [99]. However, since then, gentamicin resistance in Campylobacter has been increasing, presenting higher levels of resistance in isolates detected in 2011 [99]. The main mechanisms of aminoglycoside resistance among Gram-positive and Gram-negative bacteria are an enzymatic modification and antibiotic inactivation [99]. In Campylobacter, resistance to gentamicin is not very clear; however, several related genes, such as aacA4, aac(6’)-Ie/aph(2’)-Ia (also called aacA/aphD and encoding a bifunctional enzyme), aph(2”)-If, and aph(2″)-Ig have been reported in Campylobacter [100,101,102,103]. aph(2″)-Ig represents the most recently identified gentamicin resistance gene and encodes a phosphotransferase [100].
Resistance to sulfamethoxazole with trimethoprim was only detected in Paraguay and Peru (Figure 2). Strains from Peru presented a low rate of resistance; however, in Paraguay, sulfamethoxazole was the primary antibiotic causing resistance in Campylobacter. These results are similar to some European studies in which a high rate of resistance was detected [104,105,106]. Resistance to sulfamethoxazole and trimethoprim was long considered to be intrinsic [107]. However, some studies have shown that it can be acquired through mobile genetic elements by a horizontal gene transfer [108], which should be investigated because of the high spread of these elements.
A single study from Uruguay reported a multidrug-resistant strain of Campylobacter fetus (C. fetus) isolated from a sheep abortion [109]. It was the only article in which C. fetus was detected. This species is found in the intestinal tract of sheep, cattle, and many other species, causing reproductive disease after reaching the uterus via bacteremia [110]. In sheep, C. fetus causes late abortions, stillbirths, and the birth of weak lambs [109] and is recognized as a significant cause of abortions in sheep worldwide [110]. In humans, the main suspected route of transmission of C. fetus is the consumption of contaminated animal products or contact with farm animals, causing diarrhea, bacteremia, abortion, and perinatal mortality [111]. We found no studies on C. fetus isolated from humans in South America. However, samples tested in this single study from Uruguay showed resistance to four classes of antibiotics: quinolone (nalidixic acid), tetracycline (tetracycline), ketolide (telithromycin), and lincosamide (clindamycin), all at a low frequency. Unfortunately, data on antibiotic resistance in Campylobacter in this country are scarce, as is information on antibiotic use, making it difficult to better understand the current situation. This is probably because tetracycline resistance is rarely reported in C. fetus, but since it is an antibiotic used to treat campylobacteriosis, more attention should be paid to these findings.
Regarding the origin of Campylobacter isolates, we collected studies that detected antibiotic-resistant Campylobacter in humans, food-producing animals, food of animal origin, and environmental samples. We found a significant rate of antibiotic-resistant Campylobacter isolated from food of animal origin and food-producing animals in most South American countries (Figure 3). Five countries (Argentina, Brazil, Colombia, Ecuador, and Peru) detected a high frequency of antibiotic-resistant Campylobacter in food samples. Resistant strains in the food chain are of concern due to the high capacity of human infection through the consumption of contaminated food [112]. Although the in vitro culture of Campylobacter is difficult, in the environment, these bacteria can survive under adverse conditions such as acid and oxidative stress [113] and in modified atmosphere packaging [114]. Some species, such as C. jejuni, can develop biofilms on abiotic surfaces as a survival mechanism to resist different environmental conditions, thus promoting their permanence in the food production chain and reaching the final product [20,23]. Poultry is the main reservoir of Campylobacter, which is usually found in contaminated chicken meat [115]. Campylobacter can also be found in other matrices, such as pork and beef [116]. Detections and research are extensive in chickens because the body temperature of a chicken presents the optimal growth temperature for Campylobacter (42 °C) [117]. The misuse of antibiotics in poultry selects antibiotic-resistant mutants, which can spread throughout the meat production chain [118]. The presence of Campylobacter in food demonstrates the critical role of raw meat in the risk of human exposure. Eligible studies from Trinidad, for example, showed more antibiotic-resistant Campylobacter isolated from animal sources than from human infections. One of the Trinidad studies discussed the indiscriminate use of antibiotics in poultry farming, which are widely used as growth promoters and therapeutic agents, often without veterinary guidance. This fact explains the high rate of antibiotic resistance in this country [96]. Brazil presented a great diversity of sources contaminated with antibiotic-resistant Campylobacter compared to other countries. They were detected in animals, food of animal origin, environment, and humans (Figure 3). The multiple sources of contamination observed may be related to the more significant number of studies available. On the other hand, it may also indicate an alert for the misuse of antibiotics in this country. The most frequent source was food-producing animals; most isolates were derived from chickens. Food of animal origin also showed a high frequency of chicken meat, the most common source and route of Campylobacter infection in humans [6]. Brazil has a significant importance in the world market of chicken meat, being the largest exporter in the world and the third largest producer of chicken meat [116]. However, Brazilian authorities do not set standards for this food pathogen and do not have surveillance programs to control and prevent campylobacteriosis, generating underreporting cases [119].
In parallel to the frequency of resistance, we compile the distribution of Campylobacter in terms of the frequency of its isolated species. The results showed that Argentina, Brazil, Chile, Ecuador, Paraguay, and Peru had a higher detection of C. jejuni, except Colombia and Trinidad, which found higher rates or only the detection of C. coli (Table 1). This high frequency of C. jejuni is related to the frequent isolation of this species of chicken [4], a matrix susceptible to contamination and the primary source of human contamination through the consumption of contaminated chicken meat [120]. In the United Kingdom (UK) and the United States, C. jejuni is detected in 90% of human illness [121], often due to the consumption of contaminated chicken meat. Consequently, chicken and chicken meat represent the main source of infection for humans [23,120,122]. According to Suzuki and Yamamoto (2009), although C. jejuni is the most widespread, the proportion of C. jejuni and C. coli varies in some countries [123]. For example, we showed that Colombia detected only C. coli, and Trinidad exhibited a more significant detection of C. coli than C. jejuni (Table 1). This information is divergent in some situations, as studies show C. jejuni strains with higher levels of resistance than C. coli [122], and others that find no significant differences between them [23].
4. Materials and Methods
4.1. Search Criteria
A systematic literature search was performed in the Scopus, PubMed, Scielo, and Embase databases following the guidelines of the PRISMA group (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [124] from January to July 2022. The study protocol was publicly registered at the study’s initiation (PROSPERO CRD 42023389096). The main eligibility criteria were studies published in English, Spanish, or Portuguese, with no publication date limit. The string used the following predetermined groups of keywords that were set individually or in combination:
Search component 1. “Antibiotic resistance” OR “Antimicrobial resistance”.
Search component 2. “Campylobacter or Campylobacter spp.”.
Search component 3. “Specific country name” OR “South America”.
The string “specific country name” was represented by the following South American countries: Argentina, Aruba, Bolivia, Brazil, Caribbean Netherlands, Chile, Colombia, Curaçao, Ecuador, Falkland Islands, Guiana, French Guiana, Paraguay, Peru, Suriname, Trinidad and Tobago, Uruguay, and Venezuela. The content of the studies’ bibliography were used to search for other relevant studies that met the eligibility criteria.
4.1.1. Inclusion Criteria
Studies must address the detection of antimicrobial resistance in Campylobacter spp.
Research must be conducted in South American countries.
Campylobacter isolates must be derived from humans, food-producing animals, or are divergent.
The study must report the total number of samples analyzed and the number of Campylobacter isolated from them.
Confirmatory testing for Campylobacter should be addressed with biochemical and/or PCR/sequencing tests.
4.1.2. Exclusion Criteria
Incomplete books, reviews, and articles.
Studies written in a language other than English, Spanish, or Portuguese.
Studies in which Campylobacter was not detected or was detected in sources other than humans, food-producing animals, or food of animal origin.
Studies that did not perform antibiotic susceptibility testing or showed 100% antimicrobial sensibility.
4.2. Focus Questions
The following questions were developed according to the Population, Intervention, Comparison, and Outcome (PICO) method: in which countries in South America have cases of antibiotic-resistant Campylobacter been detected again? Which antibiotic has the lowest and highest resistance level against Campylobacter? Which sources are most related to the detection of resistant Campylobacter? Which Campylobacter species are most frequent?
4.3. Assessment of the Risk of Bias
Possible sources of bias include the inclusion/exclusion criteria of the study, the database chosen, the language, the number of articles, and the type of article selected for this review. Another essential assessment of bias concerns the different methodologies to evaluate antimicrobial susceptibility. Some studies utilized the minimum inhibitory concentration (MIC), while others used the disc diffusion method.
4.4. Frequency Calculations
The frequency of antibiotic-resistant Campylobacter in each country was calculated by the ratio of strains exhibiting resistance to a specific antibiotic over the sum of all resistant strains as follows:
The frequency of antibiotic-resistant Campylobacter by the isolation source was calculated by the ratio of all antibiotic-resistant Campylobacter in each source over the total number of Campylobacter isolates as follows:
The frequency of Campylobacter species was measured considering all isolates regardless of whether they were antibiotic-resistant or not.
5. Conclusions
Studies regarding antibiotic resistance in Campylobacter isolated from South American countries need to be better explored. The need for more studies and the lack of reporting of human infection cases prevent the realization of a complete picture, making it challenging to analyze the primary sources related to human infection and the incidence of resistance associated with antibiotic misuse in food-producing animals. Our study alerts all communities to the need for a close surveillance, investigation, and controlled use of ciprofloxacin and tetracycline in South American animal production. These actions will decrease the higher frequency of resistance in Campylobacter and reduce the hazard of infection by this pathogen for various populations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12030548/s1, Table S1: Metadata from the eligible studies collected in the systematic review process.
Author Contributions
Conceptualization, A.B.P. and P.P.; methodology, A.B.P. and P.P.; data curation, A.B.P. and P.P.; writing—original draft preparation, A.B.P., P.P. and A.M.P.d.S.; writing—review and editing, A.B.P., P.P., A.M.P.d.S. and C.A.C.J.; visualization, P.P.; supervision, C.A.C.J.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors are thankful for the financial support provided by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) Brazil—grantnumber [E26/202.227/2018] and [E26/204.078/2022], the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—grantnumber [313119/2020-1], and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Brazil—FinanceCode001.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Igwaran A., Okoh A.I. Human Campylobacteriosis: A Public Health Concern of Global Importance. Heliyon. 2019;5:e02814. doi: 10.1016/j.heliyon.2019.e02814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L., Zhang J., Wang J., Butaye P., Kelly P., Li M., Yang F., Gong J., Yassin A.K., Guo W., et al. Newly Identified Colistin Resistance Genes, Mcr-4 and Mcr-5, from Upper and Lower Alimentary Tract of Pigs and Poultry in China. PLoS ONE. 2018;13:e0193957. doi: 10.1371/journal.pone.0193957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa D., Iraola G. Pathogenomics of Emerging Campylobacter Species. Clin. Microbiol. Rev. 2019;32 doi: 10.1128/CMR.00072-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang M., Zhou Q., Zhang X., Zhou S., Zhang J., Tang X., Lu J., Gao Y. Antibiotic Resistance Profiles and Molecular Mechanisms of Campylobacter from Chicken and Pig in China. Front. Microbiol. 2020;11:592496. doi: 10.3389/fmicb.2020.592496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Man S.M. The Clinical Importance of Emerging Campylobacter Species. Nat. Rev. Gastroenterol. Hepatol. 2011;8:669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 6.Bolton D.J. Campylobacter Virulence and Survival Factors. Food Microbiol. 2015;48:99–108. doi: 10.1016/j.fm.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Indikova I., Humphrey T.J., Hilbert F. Survival with a Helping Hand: Campylobacter and Microbiota. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.01266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen H.L., Dalager-Pedersen M., Nielsen H. Risk of Inflammatory Bowel Disease after Campylobacter jejuni and Campylobacter concisus Infection: A Population-Based Cohort Study. Scand. J. Gastroenterol. 2019;54:265–272. doi: 10.1080/00365521.2019.1578406. [DOI] [PubMed] [Google Scholar]
- 9.Leonhard S.E., Mandarakas M.R., Gondim F.A.A., Bateman K., Ferreira M.L.B., Cornblath D.R., van Doorn P.A., Dourado M.E., Hughes R.A.C., Islam B., et al. Diagnosis and Management of Guillain–Barré Syndrome in Ten Steps. Nat. Rev. Neurol. 2019;15:671–683. doi: 10.1038/s41582-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez Y., Rojas M., Pacheco Y., Acosta-Ampudia Y., Ramírez-Santana C., Monsalve D.M., Gershwin M.E., Anaya J.-M. Guillain–Barré Syndrome, Transverse Myelitis and Infectious Diseases. Cell. Mol. Immunol. 2018;15:547–562. doi: 10.1038/cmi.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukted N., Tuitemwong P., Tuitemwong K., Poonlapdecha W., Erickson L.E. Inactivation of Campylobacter during Immersion Chilling of Chicken Carcasses. J. Food Eng. 2017;202:25–33. doi: 10.1016/j.jfoodeng.2017.02.007. [DOI] [Google Scholar]
- 12.COMMISSION REGULATION (EU) 2017/ 1495—of 23 August 2017—Amending Regulation (EC) No 2073/2005 as Regards Campylobacter in Broiler Carcases. [(accessed on 10 January 2023)]; Available online: https://www.legislation.gov.uk/eur/2017/1495/data.pdf.
- 13.Adler-Mosca H., Lüthy-Hottenstein J., Martinetti Lucchini G., Burnens A., Altwegg M. Development of Resistance to Quinolones in Five Patients with Campylobacteriosis Treated with Norfloxacin or Ciprofloxacin. Eur. J. Clin. Microbiol. Infect. Dis. 1991;10:953–957. doi: 10.1007/BF02005451. [DOI] [PubMed] [Google Scholar]
- 14.Bolinger H., Kathariou S. The Current State of Macrolide Resistance in Campylobacter Spp.: Trends and Impacts of Resistance Mechanisms. Appl. Environ. Microbiol. 2017;83:e00416-17. doi: 10.1128/AEM.00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ata Z., Dinc G., Yibar A., Müştak H.K., Sahan O. Extended Spectrum Beta-Lactamase Activity and Multidrug Resistance of Salmonella Serovars Isolated from Chicken Carcasses from Different Regions of Turkey. Vet. Fakültesi Derg. 2015;62:119–123. doi: 10.1501/Vetfak_0000002668. [DOI] [Google Scholar]
- 16.Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., Ouellette M. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 17.Wieczorek K., Osek J. Antimicrobial Resistance Mechanisms among Campylobacter. BioMed Res. Int. 2013;2013:1–12. doi: 10.1155/2013/340605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore J.E., Barton M.D., Blair I.S., Corcoran D., Dooley J.S.G., Fanning S., Kempf I., Lastovica A.J., Lowery C.J., Matsuda M., et al. The Epidemiology of Antibiotic Resistance in Campylobacter. Microbes Infect. 2006;8:1955–1966. doi: 10.1016/j.micinf.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Vlieghe E.R., Jacobs J.A., Van Esbroeck M., Koole O., Van Gompel A. Trends of Norfloxacin and Erythromycin Resistance of Campylobacter jejuni/Campylobacter coli Isolates Recovered from International Travelers, 1994 to 2006. J. Travel Med. 2008;15:419–425. doi: 10.1111/j.1708-8305.2008.00236.x. [DOI] [PubMed] [Google Scholar]
- 20.Bernal J.F., Donado-Godoy P., Valencia M.F., León M., Gómez Y., Rodríguez F., Agarwala R., Landsman D., Mariño-Ramírez L. Whole-Genome Sequences of Two Campylobacter coli Isolates from the Antimicrobial Resistance Monitoring Program in Colombia. Genome Announc. 2016;4:e00131-16. doi: 10.1128/genomeA.00131-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pantozzi F.L., Moredo F.A., Vigo G.B., Giacoboni G.I. Resistencia a los antimicrobianos en bacterias indicadoras y zoonóticas aisladas de animales domésticos en Argentina. Rev. Argent. de Microbiol. 2010;42:49–52. doi: 10.1590/S0325-75412010000100011. [DOI] [PubMed] [Google Scholar]
- 22.Tamborini A.L., Casabona L.M., Viñas M.R., Asato V., Hoffer A., Farace M.I., Lucero M.C., Corso A., Pichel M. Campylobacter spp.: Prevalencia y caracterización feno-genotípica de aislamientos de pacientes con diarrea y de sus mascotas en la provincia de La Pampa, Argentina. Rev. Argent. de Microbiol. 2012;44:266–271. [PubMed] [Google Scholar]
- 23.Zbrun M.V., Olivero C., Romero-Scharpen A., Rossler E., Soto L.P., Astesana D.M., Blajman J.E., Berisvil A., Signorini M.L., Frizzo L.S. Antimicrobial Resistance in Thermotolerant Campylobacter Isolated from Different Stages of the Poultry Meat Supply Chain in Argentina. Food Control. 2015;57:136–141. doi: 10.1016/j.foodcont.2015.03.045. [DOI] [Google Scholar]
- 24.Biasi R.S., Freitas de Macedo R.E., Scaranello Malaquias M.A., Franchin P.R. Prevalence, Strain Identification and Antimicrobial Resistance of Campylobacter spp. Isolated from Slaughtered Pig Carcasses in Brazil. Food Control. 2011;22:702–707. doi: 10.1016/j.foodcont.2010.10.005. [DOI] [Google Scholar]
- 25.de Moura H.M., Silva P.R., da Silva P.H.C., Souza N.R., Racanicci A.M.C., Santana Â.P. Antimicrobial Resistance of Campylobacter jejuni Isolated from Chicken Carcasses in the Federal District, Brazil. J. Food Prot. 2013;76:691–693. doi: 10.4315/0362-028X.JFP-12-485. [DOI] [PubMed] [Google Scholar]
- 26.Dias T.S., Machado L.S., Vignoli J.A., Cunha N.C., Nascimento E.R., Pereira V.L.A., Aquino M.H.C. Phenotypic and Molecular Characterization of Erythromycin Resistance in Campylobacter jejuni and Campylobacter coli Strains Isolated from Swine and Broiler Chickens. Pesq. Vet. Bras. 2020;40:598–603. doi: 10.1590/1678-5150-pvb-6466. [DOI] [Google Scholar]
- 27.Dias T.S., Nascimento R.J., Machado L.S., Abreu D.L.C., do Nascimento E.R., Pereira V.L.A., de Aquino M.H.C. Comparison of Antimicrobial Resistance in Thermophilic Campylobacter Strains Isolated from Conventional Production and Backyard Poultry Flocks. Br. Poult. Sci. 2021;62:188–192. doi: 10.1080/00071668.2020.1833302. [DOI] [PubMed] [Google Scholar]
- 28.Ferro I.D., Benetti T.M., Oliveira T.C.R.M., Abrahão W.M., Farah S.M.S.S., Luciano F.B., Macedo R.E.F. Evaluation of Antimicrobial Resistance of Campylobacter spp. Isolated from Broiler Carcasses. Br. Poult. Sci. 2015;56:66–71. doi: 10.1080/00071668.2014.981796. [DOI] [PubMed] [Google Scholar]
- 29.Fonseca B.B., Rossi D.A., Maia C.A., Nalevaiko P.C., Melo R.T., Cuccato L.P., Beletti M.E. Characterization of the Virulence, Growth Temperature and Antibiotic Resistance of the Campylobacter jejuni IAL 2383 Strain Isolated from Humans. Braz. J. Microbiol. 2014;45:271–274. doi: 10.1590/S1517-83822014000100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frazão M.R., Cao G., Medeiros M.I.C., da Silva Duque S., Allard M.W., Falcão J.P. Antimicrobial Resistance Profiles and Phylogenetic Analysis of Campylobacter jejuni Strains Isolated in Brazil by Whole Genome Sequencing. Microb. Drug Resist. 2021;27:660–669. doi: 10.1089/mdr.2020.0184. [DOI] [PubMed] [Google Scholar]
- 31.Gomes C.N., Frazão M.R., Passaglia J., Duque S.S., Medeiros M.I.C., Falcão J.P. Molecular Epidemiology and Resistance Profile of Campylobacter jejuni and Campylobacter coli Strains Isolated from Different Sources in Brazil. Microb. Drug Resist. 2020;26:1516–1525. doi: 10.1089/mdr.2019.0266. [DOI] [PubMed] [Google Scholar]
- 32.Hungaro H.M., Mendonça R.C.S., Rosa V.O., Badaró A.C.L., Moreira M.A.S., Chaves J.B.P. Low Contamination of Campylobacter spp. on Chicken Carcasses in Minas Gerais State, Brazil: Molecular Characterization and Antimicrobial Resistance. Food Control. 2015;51:15–22. doi: 10.1016/j.foodcont.2014.11.001. [DOI] [Google Scholar]
- 33.Kim J.M., Hong J., Bae W., Koo H.C., Kim S.H., Park Y.H. Prevalence, Antibiograms, and Transferable Tet(O) Plasmid of Campylobacter jejuni and Campylobacter coli Isolated from Raw Chicken, Pork, and Human Clinical Cases in Korea. J. Food Prot. 2010;73:1430–1437. doi: 10.4315/0362-028X-73.8.1430. [DOI] [PubMed] [Google Scholar]
- 34.Kleinubing N.R., Ramires T., de Fátima Rauber Würfel S., Haubert L., Scheik L.K., Kremer F.S., Lopes G.V., da Silva W.P. Antimicrobial Resistance Genes and Plasmids in Campylobacter Jejuni from Broiler Production Chain in Southern Brazil. LWT. 2021;144:111202. doi: 10.1016/j.lwt.2021.111202. [DOI] [Google Scholar]
- 35.Lopes G.V., Landgraf M., Destro M.T. Occurrence of Campylobacter in Raw Chicken and Beef from Retail Outlets in São Paulo, Brazil. J. Food Saf. 2018;38:e12442. doi: 10.1111/jfs.12442. [DOI] [Google Scholar]
- 36.Melo R.T., Grazziotin A.L., Júnior E.C.V., Prado R.R., Mendonça E.P., Monteiro G.P., Peres P.A.B.M., Rossi D.A. Evolution of Campylobacter jejuni of Poultry Origin in Brazil. Food Microbiol. 2019;82:489–496. doi: 10.1016/j.fm.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Nascimento R.J., Frasão B.S., Dias T.S., Nascimento E.R., Tavares L.S.B., Almeida V.L., Aquino M.H.C. Detection of Efflux Pump CmeABC in Enrofloxacin Resistant Campylobacter spp. Strains Isolated from Broiler Chickens (Gallus Gallus Domesticus) in the State of Rio de Janeiro, Brazil. Pesq. Vet. Bras. 2019;39:728–733. doi: 10.1590/1678-5150-pvb-6004. [DOI] [Google Scholar]
- 38.Paravisi M., Laviniki V., Bassani J., Kunert Filho H., Carvalho D., Wilsmann D., Borges K., Furian T., Salle C., Moraes H., et al. Antimicrobial Resistance in Campylobacter jejuni Isolated from Brazilian Poultry Slaughterhouses. Braz. J. Poult. Sci. 2020;22:eRBCA-2020-1262. doi: 10.1590/1806-9061-2020-1262. [DOI] [Google Scholar]
- 39.Ramires T., de Oliveira M.G., Kleinubing N.R., de Fátima Rauber Würfel S., Mata M.M., Iglesias M.A., Lopes G.V., Dellagostin O.A., da Silva W.P. Genetic Diversity, Antimicrobial Resistance, and Virulence Genes of Thermophilic Campylobacter Isolated from Broiler Production Chain. Braz. J. Microbiol. 2020;51:2021–2032. doi: 10.1007/s42770-020-00314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossi D.A., Dumont C.F., de Souza Santos A.C., de Lourdes Vaz M.E., Prado R.R., Monteiro G.P., da Silva Melo C.B., Stamoulis V.J., dos Santos J.P., de Melo R.T. Antibiotic Resistance in the Alternative Lifestyles of Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2021;11:535757. doi: 10.3389/fcimb.2021.535757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sierra-Arguello Y.M., Morgan R.B., Perdoncini G., Lima L.M., Gomes M.J.P., Nascimento V.P. do Resistance to β-Lactam and Tetracycline in Campylobacter spp. Isolated from Broiler Slaughterhouses in Southern Brazil. Pesq. Vet. Bras. 2015;35:637–642. doi: 10.1590/S0100-736X2015000700006. [DOI] [Google Scholar]
- 42.Sierra-Arguello Y.M., Perdoncini G., Morgan R.B., Salle C.T.P., Moraes H.L.S., Gomes M.J.P., do Nascimento V.P. Fluoroquinolone and Macrolide Resistance in Campylobacter jejuni Isolated from Broiler Slaughterhouses in Southern Brazil. Avian Pathol. 2016;45:66–72. doi: 10.1080/03079457.2015.1120272. [DOI] [PubMed] [Google Scholar]
- 43.Vaz C.S.L., Voss-Rech D., Lopes L.S., Silva V.S. Applied Research Note: Pulsed-Field Gel Electrophoresis and Antimicrobial Resistance Profiles of Campylobacter jejuni Isolated from Brazilian Broiler Farms. J. Appl. Poult. Res. 2021;30:100168. doi: 10.1016/j.japr.2021.100168. [DOI] [Google Scholar]
- 44.de Fátima Rauber Würfel S., Jorge S., de Oliveira N.R., Kremer F.S., Sanchez C.D., Campos V.F., da Silva Pinto L., da Silva W.P., Dellagostin O.A. Campylobacter jejuni Isolated from Poultry Meat in Brazil: In Silico Analysis and Genomic Features of Two Strains with Different Phenotypes of Antimicrobial Susceptibility. Mol. Biol. Rep. 2020;47:671–681. doi: 10.1007/s11033-019-05174-y. [DOI] [PubMed] [Google Scholar]
- 45.de Fátima Rauber Würfel S., da Fontoura Prates D., Kleinubing N.R., Vecchia J.D., Vaniel C., Haubert L., Dellagostin O.A., da Silva W.P. Comprehensive Characterization Reveals Antimicrobial-Resistant and Potentially Virulent Campylobacter Isolates from Poultry Meat Products in Southern Brazil. LWT. 2021;149:111831. doi: 10.1016/j.lwt.2021.111831. [DOI] [Google Scholar]
- 46.Bravo V., Katz A., Porte L., Weitzel T., Varela C., Gonzalez-Escalona N., Blondel C.J. Genomic Analysis of the Diversity, Antimicrobial Resistance and Virulence Potential of Clinical Campylobacter jejuni and Campylobacter coli Strains from Chile. PLoS Negl. Trop. Dis. 2021;15:e0009207. doi: 10.1371/journal.pntd.0009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collado L., Muñoz N., Porte L., Ochoa S., Varela C., Muñoz I. Genetic Diversity and Clonal Characteristics of Ciprofloxacin-Resistant Campylobacter jejuni Isolated from Chilean Patients with Gastroenteritis. Infect. Genet. Evol. 2018;58:290–293. doi: 10.1016/j.meegid.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 48.Fernández H., Mansilla M., González V. Antimicrobial Susceptibility of Campylobacter jejuni subsp. Jejuni Assessed by E-Test and Double Dilution Agar Method in Southern Chile. Mem. Inst. Oswaldo Cruz. 2000;95:247–249. doi: 10.1590/S0074-02762000000200020. [DOI] [PubMed] [Google Scholar]
- 49.García C.P., Valenzuela S.N., Rodríguez L.M.V., León C.E., Fernández J.H. Susceptibilidad antimicrobiana de Campylobacter jejuni aislado de coprocultivos en Santiago de Chile. Rev. Chil. Infectol. 2009;26 doi: 10.4067/S0716-10182009000700004. [DOI] [PubMed] [Google Scholar]
- 50.Levican A., Flores O., Sanchez S., Bascuñan M.G., Lopez R., Ojeda K., Hernandez E., Salah P. Faecal Shedding of Campylobacteria among Domestic and Wild Animals from an Urban Coastal Área. Austral J. Vet. Sci. 2019;51:83–90. doi: 10.4067/S0719-81322019000200083. [DOI] [Google Scholar]
- 51.Ochoa S., Simaluiza R.J., Toledo Z., Fernández H. Frequency and Antimicrobial Behaviour of Thermophilic Campylobacter Species Isolated from Ecuadorian Backyard Chickens. Arch. Med. Vet. 2016;48:311–314. doi: 10.4067/S0301-732X2016000300011. [DOI] [Google Scholar]
- 52.Simaluiza R.J., Toledo Z., Ochoa S., Fernandez H. The Prevalence and Antimicrobial Resistance of Campylobacter jejuni and Campylobacter coli in Chicken Livers Used for Human Consumption in Ecuador. J. Anim. Vet. Adv. 2015;14:6–9. [Google Scholar]
- 53.Simaluiza R., Toledo Z., Fernández H. Prevalencia y caracterización del perfil de susceptibilidad antimicrobiana de Campylobacter jejuni y Campylobacter coli en niños con diarrea de la ciudad de Loja, Ecuador. Rev. Chil. Infectol. 2018;35:213–215. doi: 10.4067/s0716-10182018000200213. [DOI] [PubMed] [Google Scholar]
- 54.Toledo Z., Simaluiza R.J., Fernández H. Occurrence and Antimicrobial Resistance of Campylobacter jejuni and Campylobacter coli Isolated from Domestic Animals from Southern Ecuador. Cienc. Rural. 2018;48 doi: 10.1590/0103-8478cr20180003. [DOI] [Google Scholar]
- 55.Vinueza-Burgos C., Wautier M., Martiny D., Cisneros M., Van Damme I., De Zutter L. Prevalence, Antimicrobial Resistance and Genetic Diversity of Campylobacter coli and Campylobacter jejuni in Ecuadorian Broilers at Slaughter Age. Poult. Sci. 2017;96:2366–2374. doi: 10.3382/ps/pew487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardozo L., Castro L., Zarate N., Torres C., Stavis S. Presence of Campylobacter spp. and Antimicrobial Resistance to Ciprofloxacin and Erythromycin in One Laying Hens Production Stablishment of THE Central Department, Paraguay. Compend. Cienc. Vet. 2017;7:7–11. doi: 10.18004/compend.cienc.vet.2017.07.02.07-11. [DOI] [Google Scholar]
- 57.Anampa D., Benites C., Lázaro C., Espinoza J., Angulo P., Díaz D., Manchego A., Rojas M. Detección del gen ermB asociado a la resistencia a macrólidos en cepas de Campylobacter aisladas de pollos comercializados en Lima, Perú. Rev. Panam. de Salud Pública. 2020;44:1. doi: 10.26633/RPSP.2020.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Espinoza N., Rojas J., Pollett S., Meza R., Patiño L., Leiva M., Camiña M., Bernal M., Reynolds N.D., Maves R., et al. Validation of the T86I Mutation in the GyrA Gene as a Highly Reliable Real Time PCR Target to Detect Fluoroquinolone-Resistant Campylobacter jejuni. BMC Infect. Dis. 2020;20:518. doi: 10.1186/s12879-020-05202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jennings M.C., Tilley D.H., Ballard S.-B., Villanueva M., Costa F.M., Lopez M., Steinberg H.E., Giannina Luna C., Meza R., Silva M.E., et al. Case–Case Analysis Using 7 Years of Travelers’ Diarrhea Surveillance Data: Preventive and Travel Medicine Applications in Cusco, Peru. Am. J. Trop. Med. Hyg. 2017:16–0633. doi: 10.4269/ajtmh.16-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pollett S., Rocha C., Zerpa R., Patiño L., Valencia A., Camiña M., Guevara J., Lopez M., Chuquiray N., Salazar-Lindo E., et al. Campylobacterantimicrobial Resistance in Peru: A Ten-Year Observational Study. BMC Infect. Dis. 2012;12:193. doi: 10.1186/1471-2334-12-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moya-Salazar J., Terán-Vásquez A., Salazar-Hernández R. Alta Resistencia Antimicrobiana a Fluoroquinolonas por Campylobacter en Pacientes Pediátricos de un Hospital Peruano. Rev. Peru Med. Exp. Salud Public. 2018;35:155–156 . doi: 10.17843/rpmesp.2018.351.3607. [DOI] [PubMed] [Google Scholar]
- 62.Taitt C.R., Leski T.A., Prouty M.G., Ford G.W., Heang V., House B.L., Levin S.Y., Curry J.A., Mansour A., Mohammady H.E., et al. Tracking Antimicrobial Resistance Determinants in Diarrheal Pathogens: A Cross-Institutional Pilot Study. Int. J. Mol. Sci. 2020;21:5928. doi: 10.3390/ijms21165928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adesiyun A.A., Kaminjolo J.S., Loregnard R., Kitson-Piggott W. Campylobacter Infections in Calves, Piglets, Lambs and Kids in Trinidad. Br. Vet. J. 1992;148:547–556. doi: 10.1016/0007-1935(92)90011-O. [DOI] [PubMed] [Google Scholar]
- 64.Devi A., Mahony T.J., Wilkinson J.M., Vanniasinkam T. Antimicrobial Susceptibility of Clinical Isolates of Campylobacter jejuni from New South Wales, Australia. J. Glob. Antimicrob. Resist. 2019;16:76–80. doi: 10.1016/j.jgar.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 65.Schiaffino F., Colston J.M., Paredes-Olortegui M., François R., Pisanic N., Burga R., Peñataro-Yori P., Kosek M.N. Antibiotic Resistance of Campylobacter Species in a Pediatric Cohort Study. Antimicrob. Agents Chemother. 2019;63:e01911-18. doi: 10.1128/AAC.01911-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Signorini M.L., Rossler E., Díaz David D.C., Olivero C.R., Romero-Scharpen A., Soto L.P., Astesana D.M., Berisvil A.P., Zimmermann J.A., Fusari M.L., et al. Antimicrobial Resistance of Thermotolerant Campylobacter Species Isolated from Humans, Food-Producing Animals, and Products of Animal Origin: A Worldwide Meta-Analysis. Microb. Drug Resist. 2018;24:1174–1190. doi: 10.1089/mdr.2017.0310. [DOI] [PubMed] [Google Scholar]
- 67.Sproston E.L., Wimalarathna H.M.L., Sheppard S.K. Trends in Fluoroquinolone Resistance in Campylobacter. Microb. Genom. 2018;4 doi: 10.1099/mgen.0.000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Changkwanyeun R., Usui M., Kongsoi S., Yokoyama K., Kim H., Suthienkul O., Changkaew K., Nakajima C., Tamura Y., Suzuki Y. Characterization of Campylobacter jejuni DNA Gyrase as the Target of Quinolones. J. Infect. Chemother. 2015;21:604–609. doi: 10.1016/j.jiac.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Han J., Wang Y., Sahin O., Shen Z., Guo B., Shen J., Zhang Q. A Fluoroquinolone Resistance Associated Mutation in GyrA Affects DNA Supercoiling in Campylobacter jejuni. Front. Cell. Inf. Microbio. 2012;2 doi: 10.3389/fcimb.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aksomaitiene J., Ramonaite S., Olsen J.E., Malakauskas M. Prevalence of Genetic Determinants and Phenotypic Resistance to Ciprofloxacin in Campylobacter jejuni from Lithuania. Front. Microbiol. 2018;9:203. doi: 10.3389/fmicb.2018.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Engberg J. Quinolone and Macrolide Resistance in Campylobacter jejuni and C. coli: Resistance Mechanisms and Trends in Human Isolates. Emerg. Infect. Dis. 2001;7:24–34. doi: 10.3201/eid0701.010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rychlik I. Composition and Function of Chicken Gut Microbiota. Animals. 2020;10:103. doi: 10.3390/ani10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Connell S.R., Tracz D.M., Nierhaus K.H., Taylor D.E. Ribosomal Protection Proteins and Their Mechanism of Tetracycline Resistance. Antimicrob. Agents Chemother. 2003;47:3675–3681. doi: 10.1128/AAC.47.12.3675-3681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chopra I., Roberts M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001;65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Francesco A., Salvatore D., Sakhria S., Catelli E., Lupini C., Abbassi M.S., Bessoussa G., Ben Yahia S., Ben Chehida N. High Frequency and Diversity of Tetracycline Resistance Genes in the Microbiota of Broiler Chickens in Tunisia. Animals. 2021;11:377. doi: 10.3390/ani11020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poirel L., Madec J.-Y., Lupo A., Schink A.-K., Kieffer N., Nordmann P., Schwarz S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018;6:6.4.14. doi: 10.1128/microbiolspec.ARBA-0026-2017. [DOI] [PubMed] [Google Scholar]
- 77.Vilela F.P., Gomes C.N., Passaglia J., Rodrigues D.P., Costa R.G., Tiba Casas M.R., Fernandes S.A., Falcão J.P., Campioni F. Genotypic Resistance to Quinolone and Tetracycline in Salmonella Dublin Strains Isolated from Humans and Animals in Brazil. Microb. Drug Resist. 2019;25:143–151. doi: 10.1089/mdr.2017.0329. [DOI] [PubMed] [Google Scholar]
- 78.García-Fernández A., Dionisi A.M., Arena S., Iglesias-Torrens Y., Carattoli A., Luzzi I. Human Campylobacteriosis in Italy: Emergence of Multi-Drug Resistance to Ciprofloxacin, Tetracycline, and Erythromycin. Front. Microbiol. 2018;9:1906. doi: 10.3389/fmicb.2018.01906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woźniak-Biel A., Bugla-Płoskońska G., Kielsznia A., Korzekwa K., Tobiasz A., Korzeniowska-Kowal A., Wieliczko A. High Prevalence of Resistance to Fluoroquinolones and Tetracycline Campylobacter Spp. Isolated from Poultry in Poland. Microb. Drug Resist. 2018;24:314–322. doi: 10.1089/mdr.2016.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sougakoff W., Papadopoulou B., Nordmann P., Courvalin P. Nucleotide Sequence and Distribution of Gene TetO Encoding Tetracycline Resistance in Campylobacter coli. FEMS Microbiol. Lett. 1987;44:153–159. doi: 10.1111/j.1574-6968.1987.tb02260.x. [DOI] [Google Scholar]
- 81.Sagara H., Mochizuki A., Okamura N., Nakaya R. Antimicrobial Resistance of Campylobacter jejuni and Campylobacter coli with Special Reference to Plasmid Profiles of Japanese Clinical Isolates. Antimicrob. Agents Chemother. 1987;31:713–719. doi: 10.1128/AAC.31.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tenover F.C., Bronsdon M.A., Gordon K.P., Plorde J.J. Isolation of Plasmids Encoding Tetracycline Resistance from Campylobacter Jejuni Strains Isolated from Simians. Antimicrob. Agents Chemother. 1983;23:320–322. doi: 10.1128/AAC.23.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hormeño L., Campos M.J., Vadillo S., Quesada A. Occurrence of Tet(O/M/O) Mosaic Gene in Tetracycline-Resistant Campylobacter. Microorganisms. 2020;8:1710. doi: 10.3390/microorganisms8111710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., Teillant A., Laxminarayan R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Granados-Chinchilla F., Rodríguez C. Tetracyclines in Food and Feedingstuffs: from Regulation to Analytical Methods, Bacterial Resistance, and Environmental and Health Implications. J. Anal. Methods Chem. 2017;2017:1–24. doi: 10.1155/2017/1315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iovine N.M. Resistance Mechanisms in Campylobacter jejuni. Virulence. 2013;4:230–240. doi: 10.4161/viru.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin S.I., Kaye K.M. Beta-Lactam Antibiotics: Newer Formulations and Newer Agents. Infect. Dis. Clin. North Am. 2004;18:603–619. doi: 10.1016/j.idc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 88.Casagrande Proietti P., Guelfi G., Bellucci S., De Luca S., Di Gregorio S., Pieramati C., Franciosini M.P. Beta-Lactam Resistance in Campylobacter coli and Campylobacter jejuni Chicken Isolates and the Association between BlaOXA-61 Gene Expression and the Action of β-Lactamase Inhibitors. Vet. Microbiol. 2020;241:108553. doi: 10.1016/j.vetmic.2019.108553. [DOI] [PubMed] [Google Scholar]
- 89.Hadiyan M., Momtaz H., Shakerian A. Prevalence, Antimicrobial Resistance, Virulence Gene Profile and Molecular Typing of Campylobacter Species Isolated from Poultry Meat Samples. Vet. Med. Sci. 2022;8:2482–2493. doi: 10.1002/vms3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alfredson D.A., Korolik V. Isolation and Expression of a Novel Molecular Class D β-Lactamase, OXA-61, from Campylobacter jejuni. Antimicrob. Agents Chemother. 2005;49:2515–2518. doi: 10.1128/AAC.49.6.2515-2518.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lachance N., Gaudreau C., Lamothe F., Larivière L.A. Role of the Beta-Lactamase of Campylobacter Jejuni in Resistance to Beta-Lactam Agents. Antimicrob. Agents Chemother. 1991;35:813–818. doi: 10.1128/AAC.35.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeng X., Brown S., Gillespie B., Lin J. A Single Nucleotide in the Promoter Region Modulates the Expression of the -Lactamase OXA-61 in Campylobacter jejuni. J. Antimicrob. Chemother. 2014;69:1215–1223. doi: 10.1093/jac/dkt515. [DOI] [PubMed] [Google Scholar]
- 93.Sykes R.B., Bonner D.P. Aztreonam: The First Monobactam. Am. J. Med. 1985;78:9. doi: 10.1016/0002-9343(85)90196-2. [DOI] [PubMed] [Google Scholar]
- 94.Hellinger W.C., Brewer N.S. Carbapenems and Monobactams: Imipenem, Meropenem, and Aztreonam. Mayo Clin. Proc. 1999;74:420–434. doi: 10.4065/74.4.420. [DOI] [PubMed] [Google Scholar]
- 95.Thomas G.D. Pilot Study for the Development of a New Campylobacter Selective Medium at 37 °C Using Aztreonam. J. Clin. Pathol. 2005;58:413–416. doi: 10.1136/jcp.2004.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rodrigo S., Adesiyun A., Asgarali Z., Swanston W. Antimicrobial Resistance of Campylobacter spp. Isolated from Broilers in Small Poultry Processing Operations in Trinidad. Food Control. 2007;18:321–325. doi: 10.1016/j.foodcont.2005.10.011. [DOI] [Google Scholar]
- 97.Aarestrup F.M., Engberg J. Antimicrobial Resistance of Thermophilic Campylobacter. Vet. Res. 2001;32:311–321. doi: 10.1051/vetres:2001127. [DOI] [PubMed] [Google Scholar]
- 98.Lehtopolku M., Nakari U.-M., Kotilainen P., Huovinen P., Siitonen A., Hakanen A.J. Antimicrobial Susceptibilities of Multidrug-Resistant Campylobacter jejuni and C. coli Strains: In Vitro Activities of 20 Antimicrobial Agents. Antimicrob. Agents Chemother. 2010;54:1232–1236. doi: 10.1128/AAC.00898-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao S., Mukherjee S., Chen Y., Li C., Young S., Warren M., Abbott J., Friedman S., Kabera C., Karlsson M., et al. Novel Gentamicin Resistance Genes in Campylobacter Isolated from Humans and Retail Meats in the USA. J. Antimicrob. Chemother. 2015;70:1314–1321. doi: 10.1093/jac/dkv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Y., Mukherjee S., Hoffmann M., Kotewicz M.L., Young S., Abbott J., Luo Y., Davidson M.K., Allard M., McDermott P., et al. Whole-Genome Sequencing of Gentamicin-Resistant Campylobacter coli Isolated from U.S. Retail Meats Reveals Novel Plasmid-Mediated Aminoglycoside Resistance Genes. Antimicrob. Agents Chemother. 2013;57:5398–5405. doi: 10.1128/AAC.00669-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee M.D., Sanchez S., Zimmer M., Idris U., Berrang M.E., McDermott P.F. Class 1 Integron-Associated Tobramycin-Gentamicin Resistance in Campylobacter jejuni Isolated from the Broiler Chicken House Environment. Antimicrob. Agents Chemother. 2002;46:3660–3664. doi: 10.1128/AAC.46.11.3660-3664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qin S., Wang Y., Zhang Q., Chen X., Shen Z., Deng F., Wu C., Shen J. Identification of a Novel Genomic Island Conferring Resistance to Multiple Aminoglycoside Antibiotics in Campylobacter coli. Antimicrob. Agents Chemother. 2012;56:5332–5339. doi: 10.1128/AAC.00809-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Toth M., Frase H., Antunes N.T., Vakulenko S.B. Novel Aminoglycoside 2″-Phosphotransferase Identified in a Gram-Negative Pathogen. Antimicrob. Agents Chemother. 2013;57:452–457. doi: 10.1128/AAC.02049-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.El-Adawy H., Hotzel H., Düpre S., Tomaso H., Neubauer H., Hafez H.M. Determination of Antimicrobial Sensitivities of Campylobacter jejuni Isolated from Commercial Turkey Farms in Germany. Avian Dis. 2012;56:685–692. doi: 10.1637/10135-031912-Reg.1. [DOI] [PubMed] [Google Scholar]
- 105.Giacomelli M., Salata C., Martini M., Montesissa C., Piccirillo A. Antimicrobial Resistance of Campylobacter jejuni and Campylobacter coli from Poultry in Italy. Microb. Drug Resist. 2014;20:181–188. doi: 10.1089/mdr.2013.0110. [DOI] [PubMed] [Google Scholar]
- 106.Nayak R., Stewart T., Nawaz M., Cerniglia C. In Vitro Antimicrobial Susceptibility, Genetic Diversity and Prevalence of UDP-Glucose 4-Epimerase (GalE) Gene in Campylobacter coli and Campylobacter jejuni from Turkey Production Facilities. Food Microbiol. 2006;23:379–392. doi: 10.1016/j.fm.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 107.Karmali M.A., De Grandis S., Fleming P.C. Antimicrobial Susceptibility of Campylobacter jejuni with Special Reference to Resistance Patterns of Canadian Isolates. Antimicrob. Agents Chemother. 1981;19:593–597. doi: 10.1128/AAC.19.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gibreel A., Sköld O. High-Level Resistance to Trimethoprim in Clinical Isolates of Campylobacter jejuni by Acquisition of Foreign Genes (Dfr1 and Dfr9) Expressing Drug-Insensitive Dihydrofolate Reductases. Antimicrob. Agents Chemother. 1998;42:3059–3064. doi: 10.1128/AAC.42.12.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dorsch M.A., Casaux M.L., Calleros L., Aráoz V., Caffarena R.D., Monesiglio C., Barcellos M., da Silva Silveira C., Perdomo Y., Banchero G., et al. Placentitis and Abortion Caused by a Multidrug Resistant Strain of Campylobacter fetus Subspecies Fetus in a Sheep in Uruguay. Rev. Argent. de Microbiol. 2022;54:25–30. doi: 10.1016/j.ram.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 110.Sahin O., Yaeger M., Wu Z., Zhang Q. Campylobacter—Associated Diseases in Animals. Annu. Rev. Anim. Biosci. 2017;5:21–42. doi: 10.1146/annurev-animal-022516-022826. [DOI] [PubMed] [Google Scholar]
- 111.Wagenaar J.A., van Bergen M.A.P., Blaser M.J., Tauxe R.V., Newell D.G., van Putten J.P.M. Campylobacter Fetus Infections in Humans: Exposure and Disease. Clin. Infect. Dis. 2014;58:1579–1586. doi: 10.1093/cid/ciu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Founou L.L., Founou R.C., Essack S.Y. Antibiotic Resistance in the Food Chain: A Developing Country-Perspective. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gomes C.N., Passaglia J., Vilela F.P., Pereira da Silva F.M.H.S., Duque S.S., Falcão J.P. High Survival Rates of Campylobacter coli under Different Stress Conditions Suggest That More Rigorous Food Control Measures Might Be Needed in Brazil. Food Microbiol. 2018;73:327–333. doi: 10.1016/j.fm.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 114.Meredith H., Valdramidis V., Rotabakk B.T., Sivertsvik M., McDowell D., Bolton D.J. Effect of Different Modified Atmospheric Packaging (MAP) Gaseous Combinations on Campylobacter and the Shelf-Life of Chilled Poultry Fillets. Food Microbiol. 2014;44:196–203. doi: 10.1016/j.fm.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 115.Sibanda N., McKenna A., Richmond A., Ricke S.C., Callaway T., Stratakos A.C., Gundogdu O., Corcionivoschi N. A Review of the Effect of Management Practices on Campylobacter Prevalence in Poultry Farms. Front. Microbiol. 2018;9:2002. doi: 10.3389/fmicb.2018.02002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Silva W.C., Targino B.N., Mendonça R.S., Sant’Ana A.S., Hungaro H.M. Campylobacter: An Overview of Cases, Occurrence in Food, Contamination Sources, and Antimicrobial Resistance in Brazil. Food Rev. Int. 2018;34:364–389. doi: 10.1080/87559129.2017.1298125. [DOI] [Google Scholar]
- 117.Frirdich E., Biboy J., Pryjma M., Lee J., Huynh S., Parker C.T., Girardin S.E., Vollmer W., Gaynor E.C. The Campylobacter jejuni Helical to Coccoid Transition Involves Changes to Peptidoglycan and the Ability to Elicit an Immune Response. Mol. Microbiol. 2019;112:280–301. doi: 10.1111/mmi.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Desmonts M.-H., Dufour-Gesbert F., Avrain L., Kempf I. Antimicrobial Resistance in Campylobacter Strains Isolated from French Broilers before and after Antimicrobial Growth Promoter Bans. J. Antimicrob. Chemother. 2004;54:1025–1030. doi: 10.1093/jac/dkh473. [DOI] [PubMed] [Google Scholar]
- 119.Gomes B.C., de Melo Franco B.D.G., De Martinis E.C.P. Microbiological Food Safety Issues in Brazil: Bacterial Pathogens. Foodborne Pathog. Dis. 2013;10:197–205. doi: 10.1089/fpd.2012.1222. [DOI] [PubMed] [Google Scholar]
- 120.Silva J., Leite D., Fernandes M., Mena C., Gibbs P.A., Teixeira P. Campylobacter spp. as a Foodborne Pathogen: A Review. Front. Microbio. 2011;2 doi: 10.3389/fmicb.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cody A.J., Bray J.E., Jolley K.A., McCarthy N.D., Maiden M.C.J. Core Genome Multilocus Sequence Typing Scheme for Stable, Comparative Analyses of Campylobacter jejuni and C. coli Human Disease Isolates. J. Clin. Microbiol. 2017;55:2086–2097. doi: 10.1128/JCM.00080-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nobile C.G.A., Costantino R., Bianco A., Pileggi C., Pavia M. Prevalence and Pattern of Antibiotic Resistance of Campylobacter spp. in Poultry Meat in Southern Italy. Food Control. 2013;32:715–718. doi: 10.1016/j.foodcont.2013.02.011. [DOI] [Google Scholar]
- 123.Suzuki H., Yamamoto S. Campylobacter Contamination in Retail Poultry Meats and By-Products in the World: A Literature Survey. J. Vet. Med. Sci. 2009;71:255–261. doi: 10.1292/jvms.71.255. [DOI] [PubMed] [Google Scholar]
- 124.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.