Abstract
The Yucca genus encompasses about 50 species native to North America. Species within the Yucca genus have been used in traditional medicine to treat pathologies related to inflammation. Despite its historical use and the popular notion of its antioxidant and anti-inflammatory properties, there is a limited amount of research on this genus. To better understand these properties, this work aimed to analyze phytochemical composition through documentary research. This will provide a better understanding of the molecules and the mechanisms of action that confer such antioxidant and anti-inflammatory properties. About 92 phytochemicals present within the genus have reported antioxidant or anti-inflammatory effects. It has been suggested that the antioxidant and anti-inflammatory properties are mainly generated through its free radical scavenging activity, the inhibition of arachidonic acid metabolism, the decrease in TNF-α (Tumor necrosis factor-α), IL-6 (Interleukin-6), iNOS (Inducible nitric oxide synthase), and IL-1β (Interleukin 1β) concentration, the increase of GPx (Glutathione peroxidase), CAT (Catalase), and SOD (Superoxide dismutase) concentration, and the inhibition of the MAPK (Mitogen-Activated Protein Kinase), and NF-κB (Nuclear factor kappa B), and the activation of the Nrf2 (Nuclear factor erythroid 2–related factor) signaling pathway. These studies provide evidence of its use in traditional medicine against pathologies related to inflammation. However, more models and studies are needed to properly understand the activity of most plants within the genus, its potency, and the feasibility of its use to help manage or treat chronic inflammation.
Keywords: Yucca genus, ethnobotany, phytochemicals, anti-inflammatory, antioxidant
1. Introduction
The Yucca genus belongs to the Agavoideae subfamily, a subfamily that is commonly used in traditional medicine thanks to its anti-inflammatory, antimicrobial, and antiparasitic properties [1]. It encompasses about 40 to 50 species, most of which are native to southern North America. These plants have been used for centuries to treat different ailments [2]. These benefits led to the approval by the FDA (Food and Drug Administration) of the use of Yucca species in various products, especially in dietary supplements [3]. These benefits have attracted research into the genus, which has demonstrated the presence of many biological properties [2].
One of Yucca’s most notable properties is its anti-inflammatory activity. Inflammation is a physiological procedure generated by the immune system in response to tissue injury, stress, pathogens, or toxic compounds [4]. However, in some cases, inflammation can become harmful to the body, such as chronic inflammatory diseases [5], and in those cases, the inflammatory response must be suppressed. The inflammatory process generates reactive oxygen species (ROS) and reactive nitrogen species (RNS), which can cause oxidative stress [5]. Oxidative stress occurs when those oxidative molecules surpass the antioxidant system, and this will damage or affect the function of proteins, lipids, DNA, or RNA [6]; for the same reason, treatment with antioxidants has been shown to help treat inflammatory diseases, such as inflammatory bowel disease [7].
Treatment against these diseases is usually anti-inflammatory. Unfortunately, there are some drug-induced side effects that make some treatments inadequate [7], and there has been a tendency to use traditional plant-based remedies to partially treat inflammatory diseases. Some of these plants have been Yucca species, as they are popularly used to treat arthritis since they counteract some effects of this disease. All of the biological properties of plants are due to the high concentration of phytochemicals [2]. However, to better understand the anti-inflammatory and antioxidant activities within the genus, it is necessary to understand the phytochemical composition and how they act.
Therefore, the objective of this work is to research the phytochemical composition of the species and the anti-inflammatory and antioxidant properties of these molecules. The literature was explored during the period from 2000 to 2022, although the oldest papers were used for the historical context. In this way, it will be possible to know the possible molecules responsible for these effects and their mechanisms of action.
2. Yucca Genus
Yucca species are native from North and Central America [8], and these plants are tolerant to drought, wind, and salt, which is why most of these species thrive in the arid zones of the USA and Mexico [9]. The genus is known for its obligate pollination mutualism with Yucca moths, where the moths provide Yucca with pollen while using the flower to oviposit [8]. Hybridization is common among species, which makes species classification difficult [10].
The literature indicates that the Yucca genus is composed of about 50 species. In particular, “The Plant List” establishes 49 species with an “accepted” status [11]. Although each species has each morphological characteristic, in general, they are long-lived perennials, tree-shaped, with white flowers, and sword-shaped leaves that grow in rosettes [8].
3. Ethnobotanical Use
Since ancient times, Yucca species have been used by natives for many purposes. Some species, such as Yucca schidigera Roezl ex Ortgies, were used for bowstrings, nets, ropes, mats, sandals, and clothing [12]. It is also used as food; an example is that in eastern Costa Rica, there is a tradition where they eat the Itabo flower (Yucca elephantipes Regel) at Easter [13]. Most importantly, these species have been used in traditional medicine. The Cheyenne cultures used Yucca glauca Nutt to stimulate hair growth, and in skin conditions [14]. New Mexico healers use it to treat asthma and headaches [15]. There are also more modern claims of its ethnobotanical use to treat asthma, rheumatism, gonorrhea, sunburns, arthritis, etc. [2,16].
These ethnobotanical uses have led to the emergence of research on their biological activities. Starting in 1975, it was tested as a treatment to manage arthritis [17]. Ever since, hypocholesterolemic activity [18], antimicrobial activity [19], antiprotozoal activity [20], antioxidant activity [21], anti-inflammatory activity [16], and many others have been tested for this genus. The literature especially highlights its anti-inflammatory and antioxidant activity. As mentioned before, all these properties are generated through phytochemicals. Therefore, knowing these molecules allows us to gain a better understanding of their anti-inflammatory and antioxidant activity.
4. Antioxidant and Anti-Inflammatory Activities
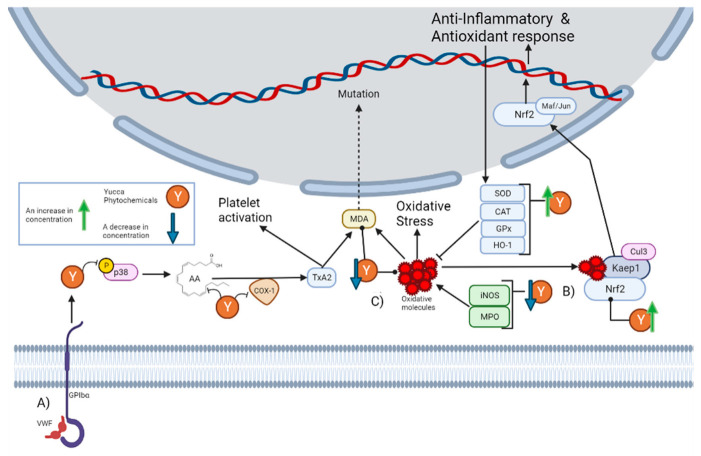
Despite historical ethnobotanical use, its continuous mention in the literature, and the popular notion of its anti-inflammatory properties, a minimal number of current studies have explored these properties. The most mentioned activity in the literature is the anti-platelet effect of Yucca schidigera Roezl ex Ortgies. Platelets are specialized blood cells that play an important role in inflammation. Platelets are capable of upregulating leukocyte functions and releasing proinflammatory cytokines [22]. It has been reported that alcoholic extract from Yucca schidigera Roezl ex Ortgies significantly decreases the various steps of thrombin-platelet activation [23]. The platelet activation pathway begins with stimulation originating from the von Willebrand factor (VWF) to the platelet adhesion receptor glycoprotein (GPIbα), which generates the phosphorylation of P38. This leads to the subsequent release of arachidonic acid (AA), which is then converted into the platelet activator thromboxane A2 (TxA2) by cyclo-oxygenase 1 (COX-1) [24]. As can be seen in Section 5, there are phytochemicals within the genus that can interrupt this pathway. First, n-3 fatty acids can prevent the generation of arachidonic acids [25]. Then, there are molecules that can decrease the concentration of p38, while others inhibit its phosphorylation. There are also molecules that inhibit COX-1 activity and others that decrease its concentration. In fact, it has been reported that the alcoholic extract from Yucca schidigera Roezl ex Ortgies inhibits COX-1 and COX-2 (Cyclooxygenase-2) in vitro [26]. This is graphically represented in Figure 1A.
Figure 1.
This figure illustrates how the Yucca genus phytochemicals influence anti-inflammatory and antioxidant processes through: (A) The platelet activation pathway begins with VWF binding to the GPIbα, which generates the phosphorylation of p38. This leads to the subsequent release of AA, which is converted by COX-1 to the platelet activator TxA2, forming MDA as a residue. MDA can react with DNA-inducing mutations. Yucca genus phytochemicals can interrupt this pathway by preventing the generation of arachidonic acid, decreasing the concentration of p38, or inhibiting its phosphorylation and COX-1 activity. (B) Yucca genus phytochemicals have the capacity to eliminate oxidative stress. This is due to the upregulation of HO-1, GPx, CAT, and SOD. By reducing the concentration of MPO and iNOS. The free radical scavenging activity of many Yucca genus phytochemicals reduces the concentration of oxidative molecules, such as MDA. (C) Nrf2 is regulated by Keap1 and the Cul3 ubiquitin E3 ligase complex. Nrf2 can be activated by oxidative molecules that modify the cysteine residues of Keap1. Once Nrf2 is free, it translocates to the nucleus, and heterodimerizes with small Maf or Jun proteins to generate an antioxidant and anti-inflammatory response. Yucca genus phytochemicals can activate this pathway. The figure was created with BioRender.com.
The anti-inflammatory effect of Yucca gloriosa L., specifically its inhibitory potential against Ovalbumin-Induced Airway Hyperresponsiveness in mice [16] has also been reported. Thus, the alcoholic extract of Yucca gloriosa L. was administered orally at doses of 50, 100, or 200 mg/kg for 7 days 1 h before each sensitization with ovalbumin. Pretreatment with Yucca gloriosa L. significantly decreased the concentrations of TNF-α, IL-6, interleukin-13 (IL-13), and leucocyte count.A similar effect to that reported for various phytochemicals found within the genus, as can be seen in Section 5. Repeated exposure to ovalbumin mimics the symptoms of asthma [27]. Thus, it suggests a possible use of Yucca gloriosa L. against asthma.
Specifically, the decrease in the TNF-α concentration is the effect with the highest incidence within these compounds. This is expected due to the key role that it plays in inflammation. TNF-α is released by a wide variety of immune cells so that it can bind to its receptors and activate different pathways. Once TNF-α has bound to its receptor, it promotes the formation of a complex capable of activating IκBα kinase (IKK) [28]. Once activated, IKK will phosphorylate IκB (Inhibitor of nuclear factor kappa B), which would cause the degradation of IκB and the release of NF-κB [29]. The release of NF-κB will allow its nuclear translocation and the activation of said signaling cascade. There are other pathways that can be induced by TNF-α, such as the c-Jun amino terminal kinase (JNK), p38-MAPK, extracellular signal-regulated kinase 1/2 (ERK1/2) and AKT pathways [28]. Inhibition of all these pathways has also been reported for the phytochemicals described in Section 5. This is graphically represented in Figure 2A.
Figure 2.
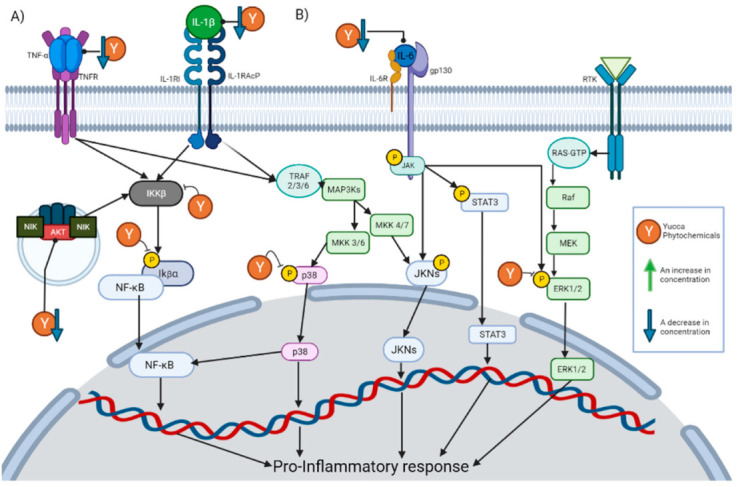
This figure illustrates how the Yucca genus phytochemicals influence anti-inflammatory and antioxidant processes through: (A) The NF-kB pathway can be canonically activated by cytokines, such as TNF-α and IL-1 β, or uncanonically by the endosome complex containing NIK, AKT, and MAC. The pathway begins in the activation of IKK β, which phosphorylate IκBα. This results in the release of NF-kB dimers, translocating to the nucleus and activating the transcription of proinflammatory proteins. Yucca genus phytochemicals can interrupt this pathway by reducing the concentration of TNF-α, IL-1, and AKT, or the inhibition of IKK activation and phosphorylation IκBα. (B) There are 3 MAPK pathways, the ERK1/2, JNK, and p38 MAP kinase. The p38 MAPK pathway begins the activation of receptors, such as TNFR or the IL1R superfamily. This activation will generate the phosphorylation of TRAF 2/3/6 which in turn activates MAP3Ks, which phosphorylates MKK3 or MKK6, and those molecules will activate p38. P38 positively regulates a pro-inflammatory response. In addition, p38 partially modulates the activation of the basal transcription factors that interact with NF-κB. Yucca genus phytochemicals can reduce p38 phosphorylation, inhibiting the signaling pathway. The activation of JNK begins with the phosphorylation of MAP3Ks, which subsequently phosphorylates MKK7 or MKK4, and then phosphorylates the JNK kinases. JNK positively regulates a pro-inflammatory response. IL-6 can activate JNK pathway and STAT3 pathways by activating membrane-bound gp130, which will cause JAK enzymes to phosphorylate proteins such as STAT3. Yucca genus phytochemicals decrease the activation of this pathway. ERK1/2 signaling begins with the binding of a ligand to the RTK; this will activate the G-protein known as Ras. Ras directly binds to Raf and activates it, then Raf activates MEK, and MEK phosphorylates ERK1/2 so it can enter the nucleus and activate transcription factors. Yucca genus phytochemicals have the capacity to suppress this signaling pathway. The figure was created with BioRender.com.
Another of the effects with a high incidence is a decrease in the concentration of IL-6. IL-6 exerts its activity by binding to the IL-6 receptor, thereby activating membrane-bound gp130. This causes JAK enzymes to phosphorylate gp130, thus generating docking sites for proteins, such as STAT3, to bind to and be phosphorylated by JAK enzymes [30]. In this way, signaling pathways, such as MAPK and JAK/STAT3, are initiated. This is graphically represented in Figure 2B.
In addition to these proinflammatory mediators, treatment with Yucca gloriosa L. also decreased the concentration of the oxidative markers nitric oxide (NO), myeloperoxidase (MPO), and malonaldehyde (MDA) [16]. As can be seen in Section 5, the decrease in NO concentration is frequently mentioned, as in Yuccaol A [28]. NO acts as a mediator of inflammatory processes. It is synthesized by the enzymes nitric oxide synthase (NOS) from L-arginine, especially in the context of inflammation; the inducible isoform of NOS (iNOS) is mainly responsible for its production [31]. Although there are several pathways that result in the expression of iNOS, the literature highlights that the transcription factors NF-kB and STAT-1α are essential for its expression in most cases [32]. The main role of NO in inflammation is to react with superoxide anions to form peroxynitrite [31]. However, it is also involved in some regulatory mechanisms since it can react with transition metals or induce nitrosylation within proteins and regulate their activity [31]. Excessive production of NO is present in pathologies, such as hypertension or atherosclerosis [33]. The ability to inhibit iNOS expression stands out due to Yuccaol C, a molecule proper to the genus that has the capacity to reduce the expression of iNOS in J774.A1 macrophages at 1 µM [34]. There are also other phytochemicals within the genus that produce the same effect (Figure 1B).
In addition to NO reduction, many phytochemicals also reported a significant capacity for reducing the concentration of MDA and MPO, known biomarkers of oxidative stress. MPO is a key enzyme in the elimination of pathogens within phagolysosomes of neutrophils, since it generates powerful oxidizing species, such as hypochlorous acid (HOCl), from the catalysis of the reaction of chloride with hydrogen peroxide [35]. HOCl can generate modifications against pathogens’ lipids, DNA, and proteins, but due to this activity, it can also damage host tissue and is involved in inflammatory diseases, such as atherosclerosis [36]. HOCL can react with phosphatidylethanolamines (PEs) and form PE-monochloramine or PE-dichloramine, which are plausible initiators of lipid peroxidation [37]. Lipid peroxidation is the process by which oxidants attack lipids, especially polyunsaturated fatty acids (PUFAs), in the lipid membrane [38]. The peroxidation of PUFAs forms lipid peroxides, which are unstable and decompose to a series of compounds, such as MDA. MDA is formed by the decomposition of arachidonic acid AA during the biosynthesis of TxA2 or by bicyclic endoperoxides during polyunsaturated fatty acid peroxidation [38]. MDA levels are a widely used indicator of lipid peroxidation and oxidative stress, and high levels are associated with various health disorders, such as in lung cancer patients or glaucoma patients [39]. This may be due to the fact that MDA reacts with DNA to form adducts, which have been reported to frequently induce mutations in oncogenes [40]; this is graphically represented in Figure 1A.
There are other reports of the antioxidant activity of the Yucca genus against oxidative stress. A commercially available food additive known as Sarsaponin 30® has been reported to have a protective effect against nitrite-induced oxidative stress in rats [41]. Rats were pretreated with Sarsaponin 30® for 4 weeks prior to the nitrite intoxication in doses of 100 ppm. Said pretreatment reduced the concentrations of MDA and NO in the tissue and in glutathione (Figure 1B).
In addition to rats, dietary supplementation with Yucca schidigera Roezl ex Ortgies a has also shown antioxidant capacity against oxidative stress in fish. In Oreochromis niloticus Biodust®; other food additives from Yucca schidigera Roezl ex Ortgies alleviate growth arrest, intestinal dysfunction, and oxidative damage induced by heat stress [42]. This is done by the downregulation of the ubiquitin-proteasome system, TNF-α, IL-1β, and interleukin 8 (IL-8), as well as by enhancing the Nrf2 signaling pathway. As can be seen in Section 5, the decrease of IL-1β concentration is an effect well represented through phytochemicals within the genus. IL-1 refers to two separate cytokine genes, IL-1α and IL-1β, that bind to the same receptors and stimulate similar proinflammatory signals [43]. For IL-1β to be excreted, its precursor must be processed by caspase-1 from the NALP3 inflammasome and excreted by the ATP/P2X7R influx [44]. In the same way, within the described phytochemicals of the genus, there are reports of the ability to inhibit NALP3 inflammasome formation, which would prevent the excretion of IL-1β. Once excreted, it will exert its activity by binding to the extracellular IL-1 type I receptor (IL-1RI), which will lead to the recruitment of IL-1R accessory protein (IL-1RAcP) and other adapters, and thus activate the NFκB, JNK, ERK, or MPAK signaling pathways [43], and this graphical represented in Figure 2A.
Another case with fish was with Cyprinus carpio, where they were fed an extract of Yucca schidigera Roezl ex Ortgies at doses of 200 or 400 mg/kg for 8 weeks, which improved their growth and intestinal antioxidant status [45]. This is due to an increase in the mRNA levels of GPx, CAT, SOD, and Nrf2, in addition to a reduction in the levels of IL-1β and IL-6. GPx, CAT, and SOD are known as front-line antioxidant defense systems. This is because ROS molecules are the most abundant oxidizing molecules within cells, especially molecules such as superoxide anions. Moreover, SOD can transform these superoxide anion molecules into hydrogen peroxide (H2O2) and O2, so that subsequently CAT or GPx catalyzes the reduction of H2O2 to water, thus eliminating the oxidative danger of these molecules [46]. It should be noted that the increase in these antioxidant enzymes is one of the most reported effects within the phytochemicals reported in Section 5, as many managed to increase the concentration of these 3 enzymes (Figure 1B).
As it can be seen through Section 5 that the reported effect of Yucca extracts, the main pathways involved in its anti-inflammatory and antioxidant effect are the inhibition of MAPK, and NF-κB, and the activation of the Nrf2 signaling pathway.
4.1. Inhibition of NF-κB Signaling Pathway
The NF-κB is a family of transcription factors that coordinate one of the most common proinflammatory signaling pathways. Within the phytochemicals in Section 5, there is constant mention of the inhibition of this pathway (Figure 2A). The family of NF-κB has 5 members: RelA, c-Rel, RelB, p50, and p52. RelA, RelB, and c-Rel share a transactivation domain that makes them capable of promoting transcriptional activation, while p50 and p52 act as coactivators [47]. There are 2 variations of this pathway, the canonical one where RelA and p50 are responsible for promoting the transcription of target genes, and in the non-canonical RelB and p52 [48].
The canonical NF-kB pathway is primarily a response to proinflammatory cytokines, such as TNF-α and IL-1, and it has an important role in chronic inflammatory diseases [49]. The pathway begins with the activation of receptors, such as TNFR and IL-1RI, which will generate a series of steps resulting in the activation of IKKβ [48]. The IKKβ will phosphorylation IκBα, which results in the release of the sequestered RelA-p50 dimers. Once these dimers translocate to the nucleus, they activate the transcription of receptors and proinflammatory cytokines involved in the inflammatory response [48]. As mentioned above, the inhibition of IKK activation and phosphorylation IκBα are an abundant effect between phytochemicals described in Section 5.
The non-canonical NF-kB pathway begins with the activation of the TNFR superfamily members, or the formation of an endosome complex containing NIK, AKT, and MAC, to stabilize and accumulate NIK. The NIK (NF-kB inducing kinase) with IKKα will induce the phosphorylation of the precursor of p52, resulting in the formation of RelB/p52 dimer [49].
4.2. Inhibition of the MAPK Signaling Pathway
The MAPK superfamily is one of the major mechanisms used in signaling pathways and is characterized by its activation through the dual phosphorylation on adjacent threonine and tyrosine residues [50]. In inflammation, the activation of receptors triggers the MAPK pathways, and transcription factors are phosphorylated and activated, such as NF-κB [51]. There are 3 well-known MAPK pathways, the ERK1/2, JNK, and p38 MAP kinase, all of which activate proinflammatory stimuli [52]. ERK1/2 signaling begins with the binding of a ligand to the receptor tyrosine kinase (RTK); this activates G-protein kwon as Ras. Ras directly binds to Raf and activates it, then Raf activates MEK, and MEK phosphorylates ERK1/2 so it can enter the nucleus and activate transcription factors [53]. As can be seen in Section 5, there are molecules within the Yucca genus that have shown the capacity to suppress this signaling pathway.
The p38 MAPK pathway begins the activation of receptors, such as toll-like receptors, TNFR, or the IL1R superfamily, to inflammatory stimuli. This activation generates the phosphorylation of TRAF 2/3/6 (TNF receptor-associated factor), which in turn activates MAP3Ks, such as TAK1. Then, MAP3K phosphorylates MKK3 or MKK6, and those molecules activate p38 [54]. Many of the pro-inflammatory responses, such as TNF-α, IL-1β, IL-6, IL-8, and COX-2, are positively regulated by p38 [51]. This pathway can regulate the NF-κB-dependent gene expression because p38 partially modulates the activation of basal transcription factors that interact with NF-κB [51]. Phytochemicals within the Yucca genus can reduce p38 phosphorylation and inhibit the signaling pathway.
There are 3 types of JNK proteins JNK1 (encoded by MAPK8), JNK2 (encoded by MAPK9), and JNK3 (encoded by MAPK10), where JNK1 and JNK2 are found in almost all cells [55]. This signaling pathway, as the other two can be triggered by proinflammatory cytokines. The activation of JNK begins with the phosphorylation of MAP3Ks, which subsequently phosphorylates MKK7 or MKK4, and then phosphorylates the JNK kinases [56]. JNK regulates the activity and maturation of T cells, as well as pro-inflammatory cytokines such as IL-6 and TNF-α, and therefore this pathway is related to chronic inflammatory disorders [57]. Within the genus, there are molecules that decrease the activation of this pathway (Figure 2B).
4.3. Activation of the Nrf2 Signaling Pathway
Nrf2 is a transcription factor that regulates the expression of antioxidant and anti-inflammatory proteins, and it is considered a modulator of species longevity [58]. Its anti-inflammatory effect is due to an indirect control of NF-kB activity and a direct control of IL-6 and IL-1β expression [59]. In fact, under normal inflammatory conditions, Nrf2 expression is activated by NF-kB to initiate a slow response that can stop the NF-kB inflammatory response [60]. Nrf2 is considered the major regulator against oxidative stress, as it regulates the expression of antioxidant response element genes, such as SOD, GPx, NADP(H) quinone oxidoreductase (NQO1), and heme oxygenase (HO-1) [61]. It also regulates Phase II of xenobiotic metabolism, where it transforms carcinogenic intermediates, generated by Phase I of xenobiotic metabolism, into less toxic metabolites [62].
Nrf2 is regulated by Kelch-like ECH-associated protein 1 (Keap1) and the Cullin 3 (Cul3) ubiquitin E3 ligase complex. Keap1 sequester Nrf2 and functions as an adaptor, so the Cul3 complex ubiquitinates Nrf2 to facilitate its proteasomal degradation [63]. Nrf2 can be activated by oxidative molecules modifying the cysteine residues of Keap1, stabilizing Nrf2-Keap1 interaction, and preventing Nrf2 ubiquitination [61,64]. Therefore, new Nrf2 could be synthetized without Kaep1 being able to sequester it. Nrf2 binds to Keap1 through a high-affinity ETGE motif, so proteins with this motif can interact with Keap1 and prevent Nrf2 sequestering [64]. Once Nrf2 is free, it translocates to the nucleus, and heterodimerizes with small Maf or Jun proteins to upregulate or inhibit target genes [61]. The activation of this pathway is one of the most reported effects throughout this genus of phytochemicals, by increasing the concentration of Nrf2 or inhibiting Keap1 (Figure 1B).
One gene regulated by Nrf2 is HO-1 (Heme oxygenase 1). The main function of HO-1 is to catalyze Haem (an iron-containing porphyrin) degradation; it uses cytochrome P450 reductase to transform Haem, NADPH, and O2 to biliverdin, carbon monoxide, ferrous iron (Fe2+), NADP+, and H2O [65]. However, it has also been shown to have anti-inflammatory properties. They have been shown to help chronic inflammation, along with Nrf2, to inhibit the adhesion of inflammatory cells by downregulating the expression of cell adhesion molecules, such as vascular cell adhesion molecule 1 (VCAM1) [60]. This could explain the ability of some reported phytochemicals to decrease the expression of VCAM1.
4.4. Free Radical Scavenging Activity
Finally, there are relatively abundant reports in the literature on extracts from the Yucca genus with free radical scavenging activity in vitro. Yucca aloifolia L. leaf extracts with MeOH, CHCl3, EtOAc, nBuOH, and n-hexane solvent were tested for their radical scavenging activity [66]. Were Yucca aloifolia L. MeOH showed the highest potential by having an activity versus control of 74% in the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay and an inhibition of 64% in the linoleic acid peroxidation assay. Yucca schidigera Roezl ex Ortgies radical scavenging activity was tested with TEAC (Trolox Equivalent Antioxidant Capacity) assay and had trolox equivalents (TE) values of 1.78 [67,68] and 5.78 mM [69], respectively. Yucca baccata Torr. butanolic extract showed a 29.18 (μg TE/mg) in DPPH assay, 121.8 (μg TE/mg) in TEAC assay, 33.41 (μg TE/mg) in ferric reducing antioxidant power (FRAP) assay, and 156.84 in oxygen radical absorbance capacity (ORAC) assay [70]. These reports are consistent with the radical scavenging activity observed in phytochemicals observed in Section 5 (Figure 1B).
5. Phytochemistry
For thousands of years, mankind has used plants to treat various ailments. This knowledge has been passed down through hundreds of generations and remains the main form of health care for more than 4 billion people today [71]. Phytochemicals naturally protect the plant from environmental hazards, pathogenic attacks, or grant characteristics, such as its aroma and flavor. Due to these functions’, plants have the capability to produce a wide range of molecules, where factors such as soil pH, light, temperature, or stress will change its chemical composition [71]. Many of these molecules will have similar proprieties, especially those that are closely related.
Due to that, in recent years, there has been a trend in countries such as China where plants are being used to generate new drugs. Some phytochemicals are able to modulate inflammation and oxidative stress at the same time, since these two physiological phenomena often share the same pathways and intensify each other. An example of this is that ROS can act as an inflammatory signaling molecule, and in turn, inflammation can induce oxidative stress and reduce cellular antioxidant capacity [72].
Out of the documentary research, 365 molecules were found in the literature, of which 92 had antioxidant or anti-inflammatory reported activity. Of these molecules, 51 can be classified as Phenolic Compounds, 13 as Glycosides, 7 as Saponins, 9 as Fatty acids, 5 as Terpenes, 3 as Tocopherol, 2 as Dicarboxylic acid, 1 as Phytosterol, and Xanthones. The antioxidant and anti-inflammatory activities reported in the literature of the phytochemicals found in the Yucca genus can be seen through Section 5.1, Section 5.2, Section 5.3, Section 5.4 and Section 5.5
5.1. Phenolic Compounds
Phenolic compounds are phytochemicals that are characterized as containing an aromatic ring bonded to some hydroxyl groups in their structure. Plants can produce a wide variety of phenolic compounds [73]. These compounds play an important role in defense mechanisms against pathogens and stress conditions, such as drought, salinity, and UV [74]. This role is due, in part, to the structural capacity to capture free radicals and chelate metals, which protect the plant from oxidizing molecules [73]. These molecules maintain this antioxidant capacity when consumed, but as can be seen in Table 1, this is not the only reason behind their antioxidant or anti-inflammatory properties. Many of these molecules can downregulate inflammatory pathways, such as NF-kB, and upregulate antioxidant pathways, such as Nrf-2. A behavior that has been described similarly to non-steroidal anti-inflammatory drugs, the most commonly used drugs against inflammation [75].
Table 1.
Some of the antioxidant and anti-inflammatory effects of the phenolic compounds of the Yucca genus reported in the literature.
| Metabolite | Species Where It Has Been Founded | Effect | Administrated Doses | References | |
|---|---|---|---|---|---|
| Effect | Metabolite Screening | ||||
| Resveratrol |
Yucca schidigera Yucca gloriosa
Yucca periculosa Yucca elephantipes |
A Resveratrol supplement significantly reduced the concentrations of MDA and increased the levels of GPx, CAT, and SOD in horses | Horses were fed with 30 g (2 g of resveratrol) for 10 days and then 15 g (1 g of resveratrol) for 18 days. | [76] | [67,77,78,79] |
| Resveratrol increased the expression of SIRT1 (Sirtuin 1) and decreased ROS and p38 levels in human umbilical endothelial cells with TNF-α-induced injury. By inhibiting the MAPK/NF-κB pathway. | The cells were treated with doses of 0, 5, 10, and 20 μM. | [80] | |||
| Resveratrol inhibited PGE2 (Prostaglandin E2) synthesis in murine embryonic fibroblasts ex vivo. This was found to be due to resveratrol’s ability to bind directly with COX-2. | The IC50 (Half maximal inhibitory concentration) was 60 μM. | [81] | |||
| 4,4’-Dihydroxystilbene (DHS) | Yucca periculosa | DHS decreased levels of MDA and 8-oxo-dG (8-Oxo-2’-deoxyguanosine), TNF-α, COX-2, MMP-9 (Matrix metalloproteinase 9), and p65 in mice with cigarette smoke-induced lung failure. It also activates Nrf2 by suppressing its ubiquitylation. | Dose of 2 and 4 mg/kg. | [82] | [77] |
| DHS showed good antioxidant activity in vitro against the galvinoxyl radical, Cu (II) ions, and Fe2+/VC-induced lipid peroxidation in human erythrocyte ghosts. | Doses of 1, 2, and 3 μM were used for Fe2+/VC-induced lipid peroxidation. | [83] | |||
| 3,3’,5,5’-tetrahydroxy-4-methoxystilbene |
Yucca periculosa
Yucca schidigera Yucca gloriosa |
The TEAC assay showed that 3,3’,5,5’-tetrahydroxy-4-methoxystilbene has the highest scavenging activity of all phenols in Yucca schidigera. It also reduced the effects of oxidative stress on blood platelets. | TEAC assay value was 2.252 mM | [67] | [67,77,78] |
| It inhibited the carbonylation, nitration, and oxidation of proteins. Reduced peroxynitrite-induced platelet lipid peroxidation. | A concentration of 0.1 mM was used | [84] |
|||
| Yuccaol A | Yucca schidigera Yucca gloriosa | Yuccaol A significantly decreases the NO release in LPS-stimulated J774.A1 macrophages. | At a concentration of 100 µM | [34,78] | |
| Yuccaol C | Yucca schidigera Yucca gloriosa | Yuccaol C reduces iNOS expression by inhibiting the transcription factor NF-kB. | From 0.1 µM it reduces the expression of iNOS in J774.A1 macrophages induced by LPS (Lipopolysaccharide). | [34,78] |
|
| Gloriosaol A | Yucca gloriosa | Showed an Antioxidant Activity higher than quercetin with the TEAC assay. | TEAC assay value was 5.55 mM | [69,78] | |
| Gloriosaol B | Yucca gloriosa | Showed an Antioxidant Activity higher than quercetin with the TEAC assay. | TEAC assay value was 3 mM | [69,78] | |
| Gloriosaol C | Yucca gloriosa | Showed an Antioxidant Activity higher than quercetin with the TEAC assay. | TEAC assay value was 5.6 mM | [69,78] | |
| Gloriosaol D | Yucca gloriosa | A combination of Gloriosaol D and E Showed an Antioxidant Activity higher than quercetin with the TEAC assay. | TEAC assay value was 4.91 mM | [69,78] | |
| Gloriosaol E | Yucca gloriosa | ||||
| Gallic acid | Yucca elephantipes Yucca aloifolia variegata Yucca aloifolia | Gallic acid increased Nrf2 expression, which suppresses ROS and IL-1β levels and blocked the activation of the inflammasome NLRP3 (Nucleotide-binding oligomerization domain-like receptor containing domain 3 of pyrin) in mice. | A dose of 100 mg/kg was injected intra-articular. | [85] |
[86,87] |
| Gallic acid significantly inhibited inflammation in a rat model of arthritis in a dose-dependent manner. This could be due to an inhibition of PDE4 (Phosphodiesterase 4) activity, and a decrease in TNF-α concentration. | Doses of 1, 5, and 10 μg/g. | [88] |
|||
| Chlorogenic acid | Yucca elephantipes Yucca aloifolia variegata Yucca aloifolia | Chlorogenic acid inhibited the production of TNF-α in and attenuated arthritis progression in collagen-induced arthritis mice. All of these are probably possible through the suppression of NF-κB pathways. | Doses of 30 mg/kg and 60 mg/kg for the attenuation of arthritis. | [89] | [79,86,87] |
| Cinnamic acid | Yucca elephantipes Yucca aloifolia variegata Yucca aloifolia | Cinnamic acid reduced the concentrations of MDA, TNF-α, and increased CAT activity in cisplatin-induced splenotoxicity in rats. | A dose of 50 mg/kg per 7 days | [90] | [86,87] |
| Hesperetin |
Yucca elephantipes
Yucca aloifolia variegata |
Hesperetin decreased the levels of ROS, LPO (Lipid Peroxidation), TLR4 Toll-like receptor 4, p-NF-κB, and TNF-α and IL-1β, and increased the level of Nrf2 and HO-1 in mice with Aβ-induced neurodegeneration. | Doses of 50 mg/kg were injected for 6 weeks | [91] | [79,86,87] |
| Hesperetin decreased the expression of NO, IL-1β, IL-6, and TNF-α in LPS-stimulated BV-2 microglial cells. This is done by inhibiting the activation of the ERK pathway. | Cells were pretreated with doses of 0, 5, 25, 50, 100, and 200 µM | [92] | |||
| Naringenin |
Yucca elephantipes
Yucca aloifolia variegata |
Mice with induced rheumatoid arthritis treated with naringenin significantly reduced inflammation and arthritis. It also decreased MPO and NO levels even more than those treated with methotrexate, a common treatment for arthritis. | The treatment against Rheumatoid Arthritis lasted three weeks with a dose of 40 mg/kg daily and orally. | [93] | [79,86,87] |
| Naringenin pretreatment reduced the concentration of NO, PGE2, iNOS, COX-2, IL-1β, TNF-α, and MCP-1 (Monocyte chemoattractant protein 1) in BV2 microglial cells treated with LPS. Attenuation could be due to the suppression of NF-κB and MAPK pathways. | Cells were treated with 80 µM | [94] | |||
| Luteolin |
Yucca elephantipes
Yucca aloifolia variegata |
A clinical trial with a dietary formulation containing luteolin was administered to children with autism spectrum disorders (ASD) and it was found that this formulation significantly decreased IL-6 and TNF-α. | The dietary formulation contained 100 mg of luteolin, 70 mg of quercetin, and 30 mg of quercetin glycoside rutin, for 26 weeks. | [95] | [79,86,87] |
| Luteolin decreased MPO, H2O2, MDA, and NF-κB levels, while increasing GPx, SOD, CAT activity, and GSH (Glutathione), and vitamin C levels in rats exposed to sodium fluoride. | Doses of 100 and 200 mg/kg/day orally for one week. | [96] | |||
| Caffeic acid |
Yucca elephantipes
Yucca aloifolia variegata |
Treatment with caffeic acid decreased the expression of NF-κB and IL-1β in Wistar rats with hearing loss | A dose of 30 mg/kg | [97] | [79,86,87] |
| Caffeic acid showed effective antioxidant properties in vitro compared to standard antioxidants. | At 10 and 30 μg/mL, caffeic acid showed 68.2 and 75.8% inhibition of lipid peroxidation. | [98] | |||
| Acacetin |
Yucca elephantipes
Yucca aloifolia variegata |
Acacetin blocked the activation of NF-κB, decreased the activity of MPO, the expression of iNOS and COX-2, and increased the expression of SOD and HO-1 in sepsis-induced Acute lung injury mice. | Oral administration of 80 mg/kg showed the best results. | [99] | [86,87] |
| Epicatechin | Yucca elephantipes | Epicatechin decreased the levels of TNF-a, IL-6, NO, MPO, and MDA and inhibited NF-kB activation in mice with induced ulcerative colitis. | Doses of 100, 200, or 300 mg/kg were administered orally. | [100] | [79] |
| Epicatechin has peroxyl radical scavenging activity. | At a concentration of 20.53 µmol. | [101] | |||
| Protocatechuic acid | Yucca elephantipes | Protocatechuic acid showed higher antioxidant activity than Trolox in DPPH, ABTS, reducing power (Fe3+), reducing power (Cu2+), superoxide anion radical scavenging, hydroxyl radical scavenging, chelating capacity (Fe2+), and chelating capacity (Cu2+). | The ratio of IC50 Trolox/Protocatechuic acid was: 2.8, 2.3, 3.7, 6.1, 4.2, 1.0, 2.7, and 1.5, respectively. |
[102] | [79] |
| Protocatechial acid treatment decreased the levels of NO and LPO and increased GSH, CAT, and SOD in different rat models. | Doses of 25, 50, and 100 mg/kg orally | [103] | |||
| Shikimic acid |
Yucca carnerosana
Yucca elephantipes |
Shikimic acid treatment decreased TNF-α and IL-1β levels and inhibited of ERK 1/2 and p38 phosphorylation in LPS-stimulated RAW 264.7 macrophages. | Cells were cultured with 10 mM | [104] | [79,105] |
| Eriodictyol | Yucca carnerosana | Eriodictyol decreased NO production, prevented induced oxidation, and increased CAT activity in murine macrophages. | The mean effective concentrations of antioxidant activity were 14 μM. | [106] | [105] |
| Pre-treatment with eriodictyol induced the Nrf2 pathway in mice with acute lung injury. This decreased the levels of TNF-α, IL-6, IL-1β, MIP-2 (Macrophage inflammatory protein 2), H2O2, and MDA | 30 mg/kg was given orally 2 days before the acute lung injury. | [107] | |||
| Scopoletin | Yucca elephantipes | Scopoletin had scavenging activity against superoxide anion in the xanthine/xanthine oxidase reaction system. | At concentration of 12.5, 25, 50, and 100 µm. | [108] | [79] |
| Scopoletin reduced PGE2 and TNF-α expression, MPO activity, and MDA levels in carrageenan-induced mice. | A dose of 100 mg/kg | [109] | |||
| Nordihydrocapsiate | Yucca carnerosana | Nordihydrocapsiate inhibits NF-κB activation by inhibiting IκBα degradation and IKK activation. | At a concentration of 100 μM | [110] | [105] |
| Myricetin | Yucca elephantipes | Myricetin protects cells against H2O2-induced cell damage by scavenging radicals and restoring the expression of SOD, CAT, and GPx. | At a concentration of 10μg/ml | [111] | [79] |
| Myricetin suppresses activation of the NF-κB pathway; by inhibiting AKT and NF-κB p65 phosphorylation; and the MAPK signaling pathway; by inhibiting the phosphorylation of JNK, p-ERK, and p38. | At a concentration of 12.5 and 25 μM | [112] | |||
| Ellagic acid | Yucca elephantipes | Ellagic acid showed high radical scavenging capacity against DPPH, inhibits lipid peroxidation, and increases SOD, CAT, and GPX activity in V79-4 cells. | 4, 20, and 100 µg/ml | [113] | [79] |
| Ellagic acid intake decreased the expression of IL-1beta, IL-6, TNF-α, and MCP-1 and increased that of GPx, SOD, and CAT in diabetic mice. | Ellagic acid at 2% | [114] | |||
| Nordihydroguaiaretic acid | Yucca elephantipes | Nordihydroguaiaretic acid activates the Nrf2/HO-1 antioxidant pathway in cerebellar granule neurons. | At a concentration of 20 µM | [115] | [79] |
| Nordihydroguaiaretic acid suppressed ERK activation in RANKL-treated mouse RAW-D and BMM cells. | At a concentration of 5 µM | [116] | |||
| Sinapic acid |
Yucca carnerosana
Yucca elephantipes |
Sinapic acid has a good antioxidant capacity in vitro. | It had an IC50 of 32.2 μM in the DPPH and for the Ferric Reducing Antioxidant Power assay of 482.6 μM/mol | [117] | [79,105] |
| Sinapic acid inhibited the expression of iNOS, COX-2, TNF-α, and IL-1β in RAW 264.7 cells induced with LPS. These observations are due to the suppression of the nuclear translocation of the p65 and p50 NF-κB subunits and the degradation of IκBα. | The reported inhibition was with pretreatment of 30 mg/kg orally 1 h before the induction of paw edema. | [118] | |||
| Cirsimarin | Yucca elephantipes | Cirsimarin in vitro inhibited lipid peroxidation and induced a 36% reduction in H2O2 production in adipose tissue. | IC50 = 370 µM in lipid peroxidation and 15 µM in H2O2 production. | [119] | [79] |
| Rosmarinic acid |
Yucca elephantipes
Yucca aloifolia variegata |
Rosmarinic acid reduced the levels of MPO, NO, IL-6, IL-1β, TNF-α, iNOS, and COX-2 in mice with dextran sodium sulfate-induced colitis. | The dose range of 25–100 mg/kg | [120] | [79,87] |
| Rosmarinic acid showed protective activity against peroxynitrite-mediated damage and inhibited NO and iNOS in RAW264.7. This is done by inhibiting the serine phosphorylation of IκBα. | At a concentration of 10, 50 mm | [121] | |||
| Rosmarinic increased the activity of SOD, CAT, and GPx in aged mice. | Results were seen at a dose of 200 mg/kg once daily for 30 days. | [122] | |||
| Psoralen | Yucca elephantipes | Psoralens inhibit the expression of TNF-α, IL-1β, IL-6, and IL-8, and proteins involved in the TLR4-IRAK4-NF-κb pathway in LPS-stimulated cells. The anti-inflammatory activity could be due to an agonist effect on the estrogen receptor. | At a concentration of 12.5 micrograms/mL | [123] | [79] |
| Psoralen inhibits the expression of MMP-1, -2, -3, -9, -12, -13 and IL-1β, -6, -12 in human synoviocytes cultured with TNF-α. | At a concentration of 1,10 and 20 µM | [124] | |||
| 4-Hydroxyphenylacetic acid | Yucca elephantipes | 4-Hydroxyphenylacetic acid increased Nrf2 translocation to the nucleus, which enhanced antioxidant enzymes in mice with induced acute liver failure. | The mice were treated with 4-hydroxyphenylacetic acid at doses of 6, 12, or 25 mg/kg for 3 days. | [120] | [79] |
| Angelicin | Yucca elephantipes | Angelicin inhibited IL-4, IL-5, and IL-13 production and NF-kB activation in mice with ovalbumin-induced asthma. | Pretreatment of 2.5, 5, and 10 mg/kg was administered intraperitoneally | [125] | [79] |
| Angelicin blocked the phosphorylation of IκB-α, NF-κBp65, p38 MAPK, and JNK in mice with LPS-induced acute lung injury. | Doses of 1, 5, and 10 mg/kg were injected intraperitoneally. | [126] | |||
| Ferulic acid |
Yucca carnerosana
Yucca elephantipes Yucca aloifolia variegata |
Ferulic acid demonstrated an anti-inflammatory effect and reduced IL-1β and TNF-α levels in mice with collagen-induced arthritis. | 1.28 μg/g/d were administered intragastrically twice a day. | [107] | [79,87,105] |
| Ferulic acid reduced superoxide radicals and ICAM-1 (Intercellular adhesion molecule) and NF-κB expression in mice with cerebral artery occlusion. | Dose of 100 mg/kg, intravenously | [127] | |||
| 6-Methylcoumarin | Yucca aloifolia variegata | 6-methylcoumarin reduced NO, PGE2, iNOS, COX-2 levels, and MAPK phosphorylation and degradation of RAW 264.7 cells stimulated by IκB-α LPS | Cells were pretreated with doses of 300, 400, and 500 μM for 1 h | [128] | [87] |
| Citropten | Yucca elephantipes | Citropten Reduces NFκB and MAPK Signaling Pathway, Alleviates Colon Inflammation and Effector T Cell Activity in Mice with DSS-Induced Colitis. | Oral administration of 10 mg/kg and 40 mg/kg | [129] | [79] |
| Emodin | Yucca elephantipes | Emodin decreased levels of amylase, lipase, TNF-α, IL-18, and ROS in rats with severe acute pancreatitis. | At a concentration of 6 mg/mL | [130] | [79] |
| Emodin reduced the levels of TNF-α, IL-6, MPO, MDA, Cytokine-induced neutrophil chemoattractant, MIP-2, ICAM-1, and NF-κB nuclear translocation in cholestatic-induced hepatitis rats. | 20 mg/kg/d were administered intragastrically for 4 days. | [131] | |||
| Chrysin | Yucca elephantipes | Chrysin treatment inhibits p65 NF-κB unit, TNF-α, IL-1β, and IL-6 levels, where 100 mg/kg had the same effect as 100 mg/kg methylprednisolone in rats with spinal cord injury. It also reduced the concentration of iNOS and NO. | Doses of 30 and 100 mg/kg/day for 26 days. | [132] | [79] |
| Chrysin treatment decreased lipid peroxidation and increased the activities of SOD, CAT GPx, GSH, vitamin C, and vitamin E in hepatitis-induced rats. | Doses of 25, 50, and 100 mg/kg/day orally for 6 days. | [133] | |||
| Alpinetin | Yucca carnerosana | Alpinetin decreased levels of ROS, MDA, NLRP3 inflammasome activation, and IL-1β, IL-6, TNF-α, COX-2, and iNOS in mice with induced liver fibrosis. This could be because the nuclear expression of Nrf2 increased. | Mice were injected intraperitoneally with doses of 15 and 60 mg/kg. | [134] | [105] |
| Alpinetin repressed p65 nuclear translocation, and IκB phosphorylation, and stimulated ERK phosphorylation in TNF-α-stimulated rat chondrocytes for 48 h. | The chondrocytes were treated with doses of 10 μM and 20 μM, | [135] | |||
| Vanillin | Yucca elephantipes | Vanillin treatment in ICR mice increased plasma antioxidant activity as demonstrated by the ORAC assay. It also showed good antioxidant capacity in vitro ith: DPPH radical, galvinoxyl radical, ABTS• scavenging assays, and Oxidative Hemolysis Inhibition Assay. | Doses of 25 mg/mL dissolved were administered orally. | [136] | [79] |
| Vanillin pretreatment inhibited hepatic lipid peroxidation, protein carbonyl formation, expression of TNF-α, IL-6, IL-1β, and increased CAT, SOD, and GSH levels in rats with induced hepatotoxicity. | Doses of 150 mg/kg were injected intraperitoneally for 3 days before induction of hepatotoxicity. | [137] | |||
| Quercetin |
Yucca aloifolia variegata
Yucca elephantipes |
Quercetin pretreatment decreased the expression of NF-κB, VCAM-1, ICAM-1, E-selectin, and AP-1 in human umbilical vein endothelial cells induced with TNF-α. | The cells were cultured with quercetin for 18 h at a dose of 30 μg/mL. | [138] | [79,87] |
| Quercetin significantly increased CAT, SOD, and GPx activity in rats with induced diabetes. | A dose of 15 mg/kg/day was injected intraperitoneally. | [139] | |||
| Quercetin decreased IL-1β, TNF-a levels, and IκBα expression in patients with coronary artery disease. | A daily dose of 120 mg for 2 months. | [140] | |||
| Kaempferide | Yucca elephantipes | Kaempferol decreased levels of TNF-α, iNOS, IL-12, p38, and IκBα phosphorylation, p65 nuclear translocation, and increased HO-1 and Nrf2 levels in mice with nephrotoxicity. | Doses of 50, 100, 150 and 200 mg/kg. | [141] | [79] |
| Procyanidin B2 |
Yucca aloifolia variegata
Yucca elephantipes |
Procyanidin B2 decreased the expression of p65, COX2, iNOS, IL-6, IL-1β, NO production, and blocked NLRP3 inflammasome activation in LPS-treated THP-1 macrophages. | Cells were treated with 10 μM. | [142] | [79,87] |
| Procyanidin B2 decreased PSA, TNF-α, IL-6, and IL-10 levels and increased SOD, CAT, GSH, Nrf2, and HO-1 levels in rats with chronic induced prostatitis. | Doses of 20 and 40 mg/kg were administered for 4 weeks. | [143] | |||
| Umbelliferone | Yucca elephantipes | Umbelliferone decreased lipid peroxidation and increased vitamin C, vitamin E, GSH levels, and SOD, CAT, and GPx activity in rats with induced diabetes. | Dose of 30 mg/kg for 45 days. | [144] | [79] |
| Umbelliferone reduced the levels of MPO, MDA, COX-2, PGE2, NF-kB, VEGF (Vascular endothelial growth factor), TNF-α, IL-1β, IL-6, IL-10, and IL-17 in rats treated with complete Freund’s adjuvant. | Doses of 10, 20, 40 mg/kg for 28 days | [145] | |||
| Catechin | Yucca elephantipes | Catechin decreased NO production and iNOS, COX2, TNF-α, and IL-1β expression in LPS-stimulated RAW 264.7 cells. Combined treatment with quercetin and catechin was also shown to have synergistic anti-inflammatory effects. | At a concentration of 150μM | [146] | [79] |
| Catechin decreased MDA level and increased SOD, GST (Glutathione-S-transferase), and CAT activities in diabetic-induced rats. | Doses of 20, 40 or 80 mg/kg/day | [147] | |||
| Scopolin | Yucca elephantipes | Scopolin reduced IL-6, VEGF, and FGF-2 in rats with adjuvant-induced arthritis. | Doses of 50 or 100 mg/kg were injected intraperitoneally for 10 days | [148] | [79] |
| Kaempferol |
Yucca aloifolia variegata
Yucca elephantipes |
Kaempferol reduced iNOS, COX-2, and CRP (Reactive C-protein) expression and inhibits NF-κB activation by inhibiting IκBα degradation and IKK activation in the human hepatocyte-derived Chang Liver cell line. | Concentration from 5 to 200 μmol/L | [149] | [79,87] |
| Kaempferol pretreatment decreased ROS excess and increased SOD, CAT, and GPx levels in human erythrocytes with ROS-induced hemolysis. | A dose of 100 μg/mL suppressed 87.4% of hemolysis | [150] | |||
| Hesperidin | Yucca elephantipes Yucca aloifolia variegata Yucca aloifolia Yucca flamentosa | Hesperidin decreased the levels of MDA, NO, NF-kB and p-Akt, while it increased the levels of GSH in rats with acute cisplatin-induced hepatotoxicity. | Dose of 100 or 200 mg/kg/day for 7 days | [151] | [79,87] |
| Hesperidin showed good scavenging potential against DPPH and protected yeast cells against stressors. | 25 and 50 µM were used in all tests | [152] | |||
| Apigenin | Yucca elephantipes Yucca aloifolia variegata | Apigenin pretreatment decreased NF-kB and COX-2 expression, and IL-6, IL-1β, and TNF-α levels in ALI mice with LPS-induced inflammation. | Doses of 10 or 20 mg/kg were injected intraperitoneally. | [153] | [79,87] |
| Apigenin decreased LPO levels and increased SOD, CAT, GPx, vitamin C, vitamin E, and GSH levels in rats with oxidative stress caused by the carcinogen. | A dose of 25 mg/kg/day was administered intraperitoneally for 14 days. | [154] | |||
| Vanillic acid | Yucca elephantipes | Vanillic acid pretreatment inhibited oxidative stress, IL1β, TNF-α, and IL-33 expression and suppressed NF-κB activation in inflammation-induced mice. | Doses of 3–30 mg/kg injected intraperitoneally. | [155] | [79] |
| Vanillic acid pretreatment reduced lipid peroxidation and decreased IL-1β, IL-6, and TNF-α expression in rats with induced cardiotoxicity. It also showed good radical scavenging ability in vitro. | Dose of 5 mg or 10 mg/kg/day for 10 days. | [156] | |||
Table 1 abbreviations: MDA = Malondialdehyde, GPx = Glutathione peroxidase, CAT = Catalase, SOD = Superoxide dismutase, SIRT1 = Sirtuin 1, ROS = Reactive oxygen species, MAPK = Mitogen-Activated Protein Kinase, NF-κB = Nuclear transcription factor-κB, TNF-α = Tumor necrosis factor α, PGE2 = Prostaglandin E2, COX-2 = Cyclooxygenase-2, IC50 = Half maximal inhibitory concentration, 8-oxo-dG = 8-Oxo-2’-deoxyguanosine, MMP-9 = Matrix metalloproteinase-9, Nrf2 = Nuclear factor erythroid 2–related factor 2, TEAC = Trolox equivalent antioxidant capacity, NLRP3 = Nucleotide-binding oligomerization domain-like receptor containing domain 3 of pyrin, PDE4 = Phosphodiesterase 4, IL = Interleukin, NO = Nitric oxide, ERK = Extracellular signal-regulated kinase, LPO = Lipid Peroxidation, TLR4 = Toll-like receptor 4, iNOS = Inducible nitric oxide synthase, MCP-1 = Monocyte chemoattractant protein-1, MPO = Myeloperoxidase, PCO = Protein carbonyl, GSH = Glutathione, Kim-1 = Kidney injury marker 1, CTnI = Cardiac troponin I, ALI = Acute lung injury, HO- 1 = Heme oxygenase 1, IKB-α = Inhibitor of nuclear factor kappa B, LPS = Lipopolysaccharide, MIP-2 = Macrophage inflammatory protein-2, IKK = IkB kinase, JNK = Jun N-terminal kinase, IRAK-4 = Interleukin-1 receptor-associated kinase 4, ICAM-1 = Intercellular adhesion molecule, CINC-1 = Cytokine-induced neutrophil chemoattractant, VEGF = Vascular endothelial growth factor, GST = Glutathione-S-transferase, FGF2 = Fibroblast growth factor 2, CRP = Reactive C-protein.
Within the Yucca genus, there is great diversity and concentration of phenolic compounds. Specifically, unique phenolic derivatives with potent antioxidant activity have been found in Yucca gloriosa L. (gloriosaols) and Yucca schidigera Roezl ex Ortgies (yuccaols) [34,69]. Among these unique molecules of the genus, Yuccaol C stands out because it prevents NF-kB activation and inhibits iNOS expression and NO release in a dose-dependent manner [34].
5.2. Saponins
Saponins are amphiphilic compounds that have a saccharide chain attached to a steroid or triterpenoid [157]. These compounds are involved in plant development and protection, where they are synthesized in response to pathogens, insects, or herbivores [158]. They are found in legume seeds in the human diet, and various positive effects on health are attributed to them [157]. In fact, since 1950, these molecules have been used to produce steroidal hormones and drugs [159]. As can be seen in Table 2, saponins have the capacity to decrease the levels of proinflammatory cytokines, especially steroidal saponins, due to their similarity to steroid hormones. This similarity allows some saponins to act as agonists to the glucocorticoid receptor, which generates glucocorticoid-like effects [160]. These types of molecules are found in a high content within the Yucca genus and are widely used in the food, pharmaceutical, and cosmetic industries [161]. As with the phenolic compounds, in Yucca schidigera Roezl ex Ortgies, new saponins have been found: Yucca spirostanosides [162].
Table 2.
Some of the antioxidant and anti-inflammatory effects of saponins of the Yucca genus reported in the literature.
| Metabolite | Species Where It Has Been Founded | Effect | Administrated Doses | References | |
|---|---|---|---|---|---|
| Effect | Metabolite Screening | ||||
| Sarsasapogenin |
Yucca schidigera
Yucca aloifolia variegata |
Sarsasapogenin suppressed NLRP3 inflammasome activation and the NF-κB pathway by downregulating PAR-1 (Protease-activated receptor 1) expression in rats with induced diabetes. | Dose 20 or 60 mg/kg/day by intragastric tube for 10 weeks. | [163] | [87] |
| Sarsapogenin decreases the levels of MDA, MPO, NO, TNF-α, IL-12, IL-6, TXB2 (Thromboxane B2), and IgE (Immunoglobulin E) in mice with induced asthma. | A dose of 50 µg/mouse | [164] | |||
| Hecogenin | Yucca elephantipes Yucca aloifolia variegata Yucca glauca | Hecogenin decreased NF-κB and p53 expression levels, decreased lipid peroxidation, and increased SOD, GPx, GST, CAT, vitamin E, vitamin C, and GSH levels in rats with induced myocardial infarction. | 50 µg/kg dose orally for 21 days | [165] | [86,87] |
| Hecogenin reduced the levels of MDA, MPO, TNF-α, IL-6, IL12, TXB2, IgE and NO, and increased SOD, GSH in rats with induced colitis | Dose of 50 µg/rat transrectally at 72, 48, 24 and 2 h before colitis induction. | [166] | |||
| Hecogenin decreases the levels of MPO, IL-6, IL-2, TBX2, and COX-2 expression in rats treated with complete Freund’s adjuvant. | At a concentration of 50 µg/kg dose, orally | [167] | |||
| Diosgenin | Yucca aloifolia variegata | Diosgenin increased SOD, GPx levels, and decreased ROS concentration, TBARS (Thiobarbituric acid-reactive substances), caspase-3 activation, NF-κB expression, and modulated PKA (Protein kinase A) and p38 activation. | Oral feeding at a dose of 130 mg/kg daily for 4 weeks. | [168] | [87] |
| Timosaponin BII | Yucca glauca | Timosaponin BII decreased IL-6, TNF-α, MDA levels and increased SOD, HO-1 and Nrf-2 levels in rats with induced myocardial infarction. | Dose of 50 or 100 mg/kg/day by gastric tube for 5 days. | [169] | [170] |
| Timosaponin BII decreased phosphorylation of mTOR (Mammalian target of rapamycin) and NF-κB. | 100, 300, and 500 mg/kg/day orally for 3 months | [171] | |||
| Timosaponin AIII |
Yucca gloriosa
Yucca macrocarpa |
Thymosaponin AIII decreased IL-1β, TNF-α, IL-6, MPO levels, NF-κB activation, and increased IL-10 concentration in mice with induced colonic shortening. It also inhibited the binding of LPS to the Toll-like receptor 4 of macrophages and could be metabolized to sarsasapogenin by intestinal microbiota, a molecule that has also shown anti-inflammatory activity. | Oral administration of 5 or 10 mg/kg/day for 3 days. | [172] | [170] |
| Degalactotigonin | Yucca gloriosa | Degalactotigonin inhibited the phosphorylation of the EGFR (Epidermal growth factor receptor) in human pancreatic cancer cell lines PANC1. | At a concentration of 100 ng/mL for 24 h | [173] | [78,170] |
| Sweroside | Yucca aloifolia variegata | Sweroside pretreatment inhibited Keap1, induced Nrf2 nuclear translocation, inhibited ROS, and increased SOD and GPx activity in hypoxia/reoxygenation-induced H9c2 cells. | At a concentration of 50 μM for 24 h | [174] | [87] |
| Sweroside activated SIRT1, suppressed NF-κB, and promoted Forkhead O1 transcription factor signaling pathways in LPS-induced RAW264.7 cells. 20, | At a concentration of 40 and 80 µM | [175] | |||
Table 2 abbreviations: NLRP3 = Nucleotide-binding oligomerization domain-like receptor containing domain 3 of pyrin, NF-κB = Nuclear transcription factor-κB, PAR-1 = Protease-activated receptor 1, MDA = Malondialdehyde, GPx = Glutathione peroxidase, CAT = Catalase, SOD = Superoxide dismutase, MPO = Myeloperoxidase, NO = Nitric oxide, TNF-α = Tumor necrosis factor α IL = Interleukin, TXB2 = Thromboxane B2, IgE = Immunoglobulin E, GST = Glutathione-S-transferase, GSH = Glutathione, COX-2 = Cyclooxygenase-2, TBARS = Thiobarbituric acid-reactive substances, PKA = Protein kinase A, ROS = Reactive oxygen species, HO- 1 = Heme oxygenase 1, Nrf2 = Nuclear factor erythroid 2–related factor, mTOR = Mammalian target of rapamycin, LPS = Lipopolysaccharide.
5.3. Glycosides
Glycosides are a large structurally diverse group of phytochemicals; they have 2 units a small metabolite (aglycone) and a sugar (glycone) [176]. When plants add sugar to small metabolites, it improves their biodistribution, metabolism, and storage [177]. Most of its biological activities come from the “small metabolite”, but the addition of sugar will change the magnitude of the activity. An example of this is that rutin (Quercetin 3-rutinoside) has higher anti-inflammatory activity than its aglycone part, quercetin [178]. This difference may be due to its absorption and metabolism, where glycosides are mostly absorbed in the small intestine after deglycosylation, which allows the metabolite to enter the liver and then be excreted to the blood [179]. As can be seen in Table 3, its antioxidant and anti-inflammatory activity is well known.
Table 3.
Some of the antioxidant and anti-inflammatory effects of Glycosides of the Yucca genus reported in the literature.
| Metabolite | Species Where It Has Been Founded | Effect | Administrated Doses | References | |
|---|---|---|---|---|---|
| Effect | Metabolite Screening | ||||
| Vitexin-2″-O-rhamnoside | Yucca elephantipes | Vitexin-2″-O-rhamnoside had an antioxidant effect against tert-butyl-hydroperoxide-induced lipid peroxidation in ECV304 cell culture. | The optimal concentration in ECV304 cell culture was 62.5 μmol/L | [180] | [86] |
| Isosakuranetin-7-O-neohesperidoside (Poncirin) | Yucca elephantipes | A treatment with poncirin showed improvement in the liver in male albino mice with Carbon tetra chloride-induced liver injury. This is done by neutralizing nitric oxide production, enhancing the concentration of GST, GSH, CAT, SOD, and attenuating the activity of lipid peroxidase, MPO, and the levels of IL-1β, IL-6 and TNF-α. | At a concentration of 30 mg/kg. | [181] | [86] |
| Poncirin improved symptoms associated in mice with liver injury induced by paracetamol. This is done by decreasing the expression of NF-κB, JNK, and COX-2. | At a concentration of 30 mg/kg. | [182] | |||
| Apigenin-7-O-β-d-glucoside | Yucca elephantipes | Apigenin-7-O-β-d-glucoside showed antioxidant activity in vitro. | Where its IC50 (μg/mL) for ABTS was 36.5 ± 0.76, O•2− 86.4 ± 0.21, DPPH• 34.2 ± 0.98 and •OH 155.2 ± 0.25. | [100] | [86] |
| Myricitrin | Yucca elephantipes | Myricitrin treatment increased GSH level and Cytochrome P450 2E1 expression, and decreased lipid peroxidation, and COX-2 and TNF-a expression in mice with tetrachloride intoxication. carbon (CCl4) | Doses of 10, 30 and 100 mg/kg by oral tube, once a day for 2 days after poisoning. | [183] | [79] |
| Myricitrin can effectively scavenge free radicals in vitro and decrease acrylamide toxicity by inhibiting ROS generation in human gastrointestinal Caco-2 cells. | At a concentration of 2.5–10 μg | [184] | |||
| Kaempferol dirhamnoside | Yucca aloifolia variegata | In vitro kaempferol dirhamnoside showed high reactivity with DPPH, inhibited MPO activity and decreased ascorbyl radical-induced lipid peroxidation. | The IC50s were: 84.0 ± 7.8 μM, 86 ± 9.9 μM, 320 ± 14.1 μM, respectively | [185] | [87] |
| Phloridzin | Yucca elephantipes | Phloridzin increased SOD, GSH levels, and decreased TNF-α, IL-6, and MDA levels in LPS-treated mice. | 10–20 mg/kg, orally | [186] | [79] |
| Sweroside | Yucca aloifolia variegata | Sweroside pretreatment inhibited Keap1, induced Nrf2 nuclear translocation, inhibited ROS, and increased SOD and GPx activity in hypoxia/reoxygenation-induced H9c2 cells. | At a concentration of 50 μM for 24 h | [174] | [87] |
| Sweroside activated SIRT1, suppressed NF-κB, and promoted Forkhead O1 transcription factor signaling pathways in LPS-induced RAW264.7 cells. 20, | At a concentration of 40 and 80 µM | [175] | |||
| Kaempferol-3-O-glucoside | Yucca elephantipes | Kaempferol-3-O-glucoside had a good antioxidant activity with the DPPH radical scavenging method. | The mean IC50 of 1.25 ± 0.09 µg/mL | [187] | [79] |
| Quercitrin |
Yucca aloifolia variegata
Yucca elephantipes |
Quercitrin decreased lipid peroxidative products and increased SOD, CAT, GPx, activity in rats with induced diabetes. | Oral administration of 30 mg/kg for 30 days | [188] | [79,87] |
| Quercitrin decreased iNOS expression in rats with induced colitis. This was due to the inhibition of NF-κB activity. | Oral administration of 1 or 5 mg/kg/day | [189] | |||
| Quercetin reduced MPO activity, TNF-α and IL-1β expression, iNOS, and reduced NF-κB activation in rats with colitis. | Oral administration of 1 mg/kg/day | [190] | |||
| Rutin |
Yucca aloifolia variegata
Yucca elephantipes |
Rutin decreased MDA, ROS, NF-kBp65, TNF-α, and caspase-3 levels, as well as increased GSH levels, CAT activity, and Nrf-2 and HO-1 expression in rats with diabetic neuropathy. | Doses of 100 or 200 mg/kg were injected intraperitoneally for 8 weeks. | [191] | [79,87] |
| Rutin showed good radical scavenging activity in DPPH assay, total antioxidant activity, reducing power, hydroxyl radical scavenging assay, superoxide radical scavenging assay and lipid peroxidation assay. | At the concentration of 0.05 mg/mL it showed an inhibition of 90.4% in DPPH and the IC50 was 24 mg/mL for the lipid peroxidation assay. | [192] | |||
| Naringin |
Yucca aloifolia variegata
Yucca elephantipes |
Naringenin decreased the levels of iNOS, ICAM-1, COX-2, MCP-1, TNF-α, TLR4, IL-6, p65, and p-IkBa in mice with induced colitis. | Dosage of 50 mg/kg/day orally for 10 days. | [193] | [79,87] |
| Naringenin supplementation increased SOD and CAT activities in hypercholesterolemic patients. | 400 mg/capsule/day for 8 weeks | [194] | |||
| Hyperoside | Yucca aloifolia variegata | Hyperoside decreased levels of TNF-α, IL-6, NO, NF-κB, and IκB-α degradation in lipopolysaccharide-stimulated mouse peritoneal macrophages. | The maximum rate of inhibition by hyperoside 5 μM. | [195] | [87] |
| Hyperoside decreased the levels of TNF-α, and the activation of AKT, NF-κB and ERK 1/2 in HUVEC. | Cells were treated with doses of 5, 10, 20 or 50 µM | [196] | |||
| Hyperoside scavenged ROS, which prevented lipid peroxidation, protein carbonyl, and cellular DNA damage in cells with H2O2-induced damage. | Cells were treated with 5 µM. | [197] | |||
| Esculin | Yucca aloifolia variegata | Esculin decreased levels of TNF-α, IL-6, MDA, MCP-1, Intracellular adhesion molecule 1, and p38, and increased SOD activity in C57BL/6J mice. | Dose of 5, 10, or 20 mg/kg for 2 weeks. | [198] | [87] |
| Esculin decreased NO, MPO, and MDA levels while increasing SOD activity in mice with acute gastric injury. | Doses of 12.5, 25 and 50 mg/kg | [199] | |||
| Kaempferol-3-O-rhamnoside | Yucca aloifolia variegata | Kaempferol-3-O-rhamnoside decreased the levels of IL-4, IL-5 and IL-13, TNF-α, IgE, this through the inhibition of Akt phosphorylation. | Doses of 26 mg/kg/day were administered orally for 6 days. | [200] | [87] |
Table 3 abbreviations: EGFR = Epidermal growth factor receptor, NF-κB = Nuclear transcription factor-κB, MDA = Malondialdehyde, GPx = Glutathione peroxidase, CAT = Catalase, SOD = Superoxide dismutase, MPO = Myeloperoxidase, NO = Nitric oxide, TNF-α = Tumor necrosis factor α IL = Interleukin, = Immunoglobulin E, GST = Glutathione-S-transferase, GSH = Glutathione, COX-2 = Cyclooxygenase-2, ROS = Reactive oxygen species, HO- 1 = Heme oxygenase 1, Nrf2 = Nuclear factor erythroid 2–related factor, LPS = Lipopolysaccharide, JNK = Jun N-terminal kinase, CYP2E1 = Cytochrome P450 2E1, Keap1 = Kelch-like ECH-associated protein 1, SIRT1 = Sirtuin 1, iNOS = Inducible nitric oxide synthase, MDA = Malondialdehyde, MPO = Myeloperoxidase, ICAM-1 = Intracellular adhesion molecule 1, MCP-1 = monocyte chemoattractant protein 1, TLR4 = Toll-like receptor 4, IKB-α = Inhibitor of nuclear factor kappa B, ERK = Extracellular signal-regulated kinase, IgE = Immunoglobulin.
5.4. Fatty Acids
Fatty acids are lipid structures composed of a long carbon chain with a carboxyl group at one end and a methyl group at the other end [201]. If this structure has a double bond, it is classified as “Unsaturated fatty acids”. Plants mainly produce unsaturated fatty acids. These can be synthesized by plants as part of the various defense systems against biotic and abiotic stresses [202]. Fatty acids also function as modulators of cell membranes, as energy reserves, as extracellular barriers, and as precursors of signaling molecules [203]. As can be seen in Table 4, their anti-inflammatory and antioxidant properties are well known. This effect depends on the position of the first double bond within the carbon chain. If it occurs in the sixth (n-6), it will be considered pro-inflammatory because it is a precursor of arachidonic acid [25]. If it occurs in the third (n-3), it will be considered anti-inflammatory because it will compete as a substrate for n-6 metabolism [25]. On the other hand, these structures are susceptible to oxidize, and for the same reason, they work as antioxidants. Within the Yucca genus, it has been reported that Yucca aloifolia variegate L. contains more saturated fatty acids than unsaturated, constituted mainly by palmitic acid and palmitoleic acid [87].
Table 4.
Some of the antioxidant and anti-inflammatory effects of fatty acids of the Yucca genus reported in the literature.
| Metabolite | Species Where It Has Been Founded | Effect | Administrated Doses | References | |
|---|---|---|---|---|---|
| Effect | Metabolite Screening | ||||
| Gamma-Linolenic acid |
Yucca elephantipes
Yucca aloifolia variegata |
Treatment with α-linolenic acid inhibited the production of PGE2 in on synovial membrane explant cultures with LPS-induced inflammation. | At a concentration of 300 µg/mL | [204] | [86,87] |
| α-linolenic acid inhibited PGE2 production in Aβ-stimulated PC12 cells. It also suppressed IκB-α degradation, which blocked NF-κB activation. | At a concentration of 100 µM | [205] | |||
| Caproic acid | Yucca aloifolia variegata | Capric acid suppressed MAPK phosphorylation and NF-kB activation in THP-1 cells treated with P. acnes. | 100 μM | [206] | [87] |
| Lauric acid | Yucca aloifolia variegata | Lauric acid suppressed MAPK phosphorylation and NF-kB activation in THP-1 cells treated with P. acnes. | 100 μM | [206] | [87] |
| Lauric acid had inhibitory activity against COX-1 and COX-2 | Inhibitory activity against COX-1 and COX-2 at 100 µg/mL. | [207] | |||
| Pentadecanoic acid |
Yucca elephantipes
Yucca aloifolia variegata |
Pentadecanoic acid decreased the levels of MCP-1 and IL-6 in C57BL/6 J mice | 5 mg/kg for 12 weeks | [208] | [79,87] |
| Gama-linoleic acid | Yucca aloifolia variegata | Linoleic acid inhibited NO production and decreased p50 subunit expression of NF-κB, TNF-α, IL-6, IL-1β, and nitric oxide synthase 2 in LPS-treated RAW 264.7 cells. This could be due to the activation of the peroxisome proliferator-activated receptor-α. | 150 µM | [209] | [21,87] |
| Stearic acid | Yucca aloifolia variegata | Stearic acid inhibits lipid peroxidation and increases SOD and CAT activity in cultured rat cortical neurons. | 3,10 and 30 µmol/L | [210] | [21,87] |
| Stearic acid attenuated NF-κB activation in rats with cholestasis and leukocyte accumulation. | Intraperitoneal administration of 1000 nmol/kg/day was started 2 weeks before injury and continued for 5 weeks. | [211] | |||
| Oleic acid | Yucca aloifolia variegata | Oleic acid decreases the concentration of IL-1β, IL-6, and TNF-α and NF-κβ in rats with cadmium-induced lesions in heart and liver tissues. | 10 mg/kg, orally | [212] | [21,87] |
| Cis-5,8,11,14,17-Eicosapentaenoic acid | Yucca aloifolia variegata | Pretreatment with 5,8,11,14,17-eicosapentaenoic acid decreased TNF-α expression by preventing IκB-α phosphorylation in LPS-induced THP-1 cells. | Cells were incubated with 60 μM | [213] | [87] |
| 9,12-Octadecadienoic acid | Yucca elephantipes | 9,12-octadecadienoic acid had antioxidant activity with an IC50 value of 45.65 µg/mL by the DPPH Radical Scavenging Method. Anti-inflammatory activity was also tested in rats with carrageenan-induced edema, where treatment with 9,12-Octadecadienoic acid showed a significant reduction in edema paw volume. | 150μg/kg | [214] | [79] |
Table 4 abbreviations: PGE2 = Prostaglandin E2, LPS = Lipopolysaccharide, IKB-α = Inhibitor of nuclear factor kappa B, NF-κB = Nuclear transcription factor-κB, MAPK = Mitogen-Activated Protein Kinase, COX-1 = Cyclooxygenase-1, COX-2 = Cyclooxygenase-2, MCP-1 = Monocyte chemoattractant protein 1, IL = interleukin, PPARα = Peroxisome proliferator-activated receptor-α, CAT = Catalase, SOD = Superoxide dismutase, TNF-α = Tumor necrosis factor α.
5.5. Other Phytochemicals
Among the phytochemicals that were found at a lower frequency, the terpenes stand out with the anti-inflammatory effect. Terpenes are the most abundant and diverse class of phytochemicals; structurally, they are made up of isoprene molecules (Table 5). They have a wide range of functions, from primarily being part of plant structures to being quinones in electron transfer [215]. On the other hand, tocopherols stand out for their antioxidant activity. These molecules are exclusively synthesized in photosynthetic organisms and consist of a chromanol head group with one, two, or three methyl groups, and an isoprenoid [216]. α-Tocopherol is the major vitamin E component and one of the most important antioxidant regulatory mechanisms [217].
Table 5.
Some of the antioxidant and anti-inflammatory effects of other phytochemicals of the Yucca genus reported in the literature.
| Metabolite | Group | Species Where It Has Been Founded | Effect | Administrated Doses | References | |
|---|---|---|---|---|---|---|
| Effect | Metabolite Screening | |||||
| Stigmasterol | Phytosterol | Yucca aloifolia variegata | In chondrocytes from newborn mice or human osteoarthritis treated with IL-1β, preincubation with stigmasterol decreased the expression of Matrix Metallopeptidase 3, Matrix Metallopeptidase 13, ADAMTS-4 and PGE2, this by counteracting the effect of IL-1β on the NF-κB pathway. | Cells were pre-incubated for 48 h with 20 μg/ml | [218] | [87] |
| Stigmasterol treatment improved clinical severity by reducing joint destruction and decreasing the expression of TNF-α, IL-6, IL-1β, iNOS and COX-2, p65 and p38 by inhibiting the activation of p- IκBα in collagen-induced arthritic rats. | 200 mg/kg orally daily for 20 days | [219] | ||||
| In rats with ischemia/reperfusion brain injury, stigmasterol treatment decreased COX-2 and p65 expressions. In addition to significantly increasing the expression of Nrf2, HO-1, SOD, CAT, and GPx. | Dosis de 20, 40 y 80 mg/kg | [220] | ||||
| Phytol | Terpenes | Yucca aloifolia variegata | Phytol treatment reduced MPO activity and the concentration of TNF-α, IL-6 and COX-2. By downregulating p38 and NF-κB signaling pathways in a mouse model of arthritis induced by complete Freund’s adjuvant. | Oral administration of 50 mg/kg | [221] | [87] |
| Phytol exhibits a dose-dependent anti-inflammatory effect in formalin-induced paw edema by decreasing the levels of COX-1, COX-2, NF-κB and IL-1 β. | 100 mg/kg of phytol | [222] | ||||
| Phytol demonstrated a strong antioxidant effect in vitro. Eliminating hydroxyl radicals and nitric oxide and preventing the formation of TBARS | The concentration of 0.9, 1.8, 3.6, 5.4 and 7.2 ng/mL was used in the in vitro tests. | [223] | ||||
| Malic acid | Dicarboxylic acid | Yucca aloifolia variegata | Malic acid decreases TNF-α levels and inhibits platelet aggregation in rats with myocardial ischemia/reperfusion injury. | Doses of 500 mg/kg and 250 mg/kg | [224] | [87] |
| Loliolide | Terpenes | Yucca aloifolia variegata | Loliolide was found to exert dose-dependent positive effects on the protective effects against peroxide-induced cell damage. | Cells were pretreated with 250 and 500 μM of loliode | [225] | [87] |
| Loliolide treatment in LPS-stimulated RAW 264.7 macrophages decreased TNF-α and IL-6 production. | 100 µM | [226] | ||||
| Loliolide treatment in LPS-stimulated RAW 264.7 macrophages downregulated IκBα and p65 in a dose-dependent manner, thereby inhibiting NF-κB nuclear translocation. In addition, it downregulates the proteins involved in the MAPK pathway (p38, ERK, and JNK), thus inhibiting MAPK phosphorylation. | 15.6, 31.2, 62.5 µm/mL | [227] | ||||
| α-Tocopherol | Tocopherol | Yucca aloifolia | Oral α-tocopherol supplementation maintains cellular redox status in polychlorinated biphenyl-induced toxicity in rat liver, lung, and kidney. Therefore, the activities of SOD, CAT, GPx, and glutathione reductase are maintained, and the levels of lipid peroxides, hydroxyl radicals, and hydrogen peroxides are kept low. | 50 mg/kg body weight/day | [228] | [21] |
| α-Tocopherol treatment significantly reduced IL-6 levels, thereby inhibiting NF-κB-mediated gene transcription in cancerous mice. | 150 mg/kg per day orally for 15 consecutive days | [229] | ||||
| γ-Tocotrienol | Tocotrienols | Yucca aloifolia | γ-tocotrienol an inhibits NF-κB pathaway in RAW 264.7 macrophages. | 20 µM | [230] | [21] |
| γ-tocotrienol treatment in TNF-α-induced inflammation in adipocytes suppressed IκB-α phosphorylation and NF-κB activation. | Cells were pretreated with 0.024, 0.24, and 2.4 μM γ-tocotrienol for 6 h. | [231] | ||||
| In rats with induced arthritis, treatment with γ-tocotrienol reduced arthritis-induced changes in the C-reactive protein, TNF-α, SOD, and GSH levels. | The rats were treated orally with 5 mg/kg body weight of γ-tocotrienol between days 21 and 45. | [232] | ||||
| δ-Tocotrienol | Tocotrienols | Yucca aloifolia | δ-tocotrienol inhibits NO production and expression of IL-1β, IL-1α, IL-6, TNF-α, IL-12, iNOS, VCAM1 (Vascular cell adhesion protein 1), ICAM1, COX2, IL-1RA, TRAF1 and CD40 in mice. | Mice were fed 100 ppm tocotrienol | [233] | [21] |
| Succinic acid | Dicarboxylic acid | Yucca carnerosana | The injection of succinic acid in Wistar rats showed a high inhibitory capacity of LPO. | 25 mg/kg intraperitoneally injected into rats | [234] | [105] |
| Neophytadiene | Terpenes | Yucca aloifolia variegata | Neophytadiene was treated with LPS-stimulated RAW 264.7 and pretreated in rats injected with 10 mg/kg LPS. Neophytadiene inhibited NO production and the expression of TNF-α, IL-6, and IL-10, | 25, 50, 100 μM/mL in vitro 12, 25, 50 mg/kg pretreated for 7 days in vivo |
[235] | [87] |
| Mangiferin | Xanthones | Yucca elephantipes | Mangiferin pretreatment increases GST, GPx, SOD, and CAT levels in rats with induced myocardial infarction. | 100 mg/kg suspended in 2 mL dimethyl sulfoxide administered intraperitoneally for 28 days | [236] | [79] |
| Mangiferin pretreatment prevented apoptosis and decreased levels of MDA, IL-1F, and IL-18 in mice subjected to cecal ligation and puncture. This is due to the inhibition of the NLRP3 inflammasome and the upregulation of Nrf2. | 15 mg/kg | [237] | ||||
| Mangiferin pretreatment increases HO-1 expression and activity in sepsis-induced mice with acute lung injury. This inhibited MAPK and NF-κB signaling. | 10, 30, and 100 mg/kg once daily for 7 days before cecal ligation and puncture. | [238] | ||||
| Cinnamaldehyde | Terpenes | Yucca aloifolia variegata | Cinnamaldehyde increased antioxidant enzymes such as SOD, GPx, and GST. | Rats were administered orally by gavage at dose levels of 2.14, 6.96, 22.62, and 73.5 mg/kg body weight/day for a period of 10, 30, and 90 days. | [239] | [87] |
| Cinnamaldehyde reduced levels of proinflammatory cytokines and increased levels of antioxidant enzymes in patients with rheumatoid arthritis. Molecular docking indicated that cinnamaldehyde interacts with the key residues of TNF-α and IL-6. | 20 or 40 µM cinnamaldehyde | [240] | ||||
| Nerolidol | Terpenes |
Yucca elata
Yucca filamentosa |
Nerolidol pretreatment increases SOD, CAT, and GPx, activity by enhancing MP-activated protein kinase phosphorylation. | A significant effect started at 30 μmol/kg | [241] | [59] |
| Nerolidol decreased the expression of TNF-α, IL-1β, IL-6, NF-kB, PGE-2, and COX-2 and increased the level of IL-10, IL-4, and serum, antioxidant activity in rats with arthritis induced. | Doses of 200, 400, and 800 m,g/kg were administered for 28 days. | [242] | ||||
| Chrysophanol | Anthraquinone | Yucca elephantipes | Chrysophanol reduced protein expression of NF-κBp65, p-NF-κB p65, IκBα, p-IκBα, TNF-α, and IL-1β, decreased MPO and MDA levels, and increased SOD activity in mice with acute lung injury (LPS). This by regulating HMGB1/NF-κB signaling through histone deacetylase 3. | Doses of 7.5, 15 and 30 mg/kg | [243] | [79] |
| Chrysophanol increased Nrf2 levels and decreased HO-1, glutamate-cysteine ligase, glutamate cysteine ligase, IKKa, IkBa, NF-kB, and ROS generation in H9C2 cells stimulated with LPS. Chrysophanol is suggested to act as an activator of Nrf2. |
Cells were cultured at doses of 0, 10, 20, 40, 80, 160, and 320 uM. | [159] | ||||
Table 5 abbreviations: MDA = Malondialdehyde, GPx = Glutathione peroxidase, CAT = Catalase, SOD = Superoxide dismutase, MAPK = Mitogen-Activated Protein Kinase, NF-κB = Nuclear transcription factor-κB, TNF-α = Tumor necrosis factor α, PGE2 = Prostaglandin E2, COX-2 = Cyclooxygenase-2, COX-1 = Cyclooxygenase-1, ROS = Reactive Oxygen, Nrf2 = Nuclear factor erythroid 2–related factor 2, IL = Interleukin, NO = Nitric oxide, ERK = Extracellular signal-regulated kinase, LPO = Lipid Peroxidation, iNOS = Inducible nitric oxide synthase, MPO = Myeloperoxidase, GSH = Glutathione, HO- 1 = Heme oxygenase 1, IKB-α = Inhibitor of nuclear factor kappa B, LPS = Lipopolysaccharide, MIP-2 = Macrophage inflammatory protein-2, IKK = IkB kinase, JNK = Jun N-terminal kinase, ICAM-1 = Intercellular adhesion molecule, GST = Glutathione-S-transferase, FGF2 = Fibroblast growth factor 2, MMP-3 = Matrix Metallopeptidase 3, MMP-13 = Matrix Metallopeptidase 13, ADAMTS- 4 = ADAM Metallopeptidase with Thrombospondin Type 1 Motif 4, TBARS = Thiobarbituric acid reactive substances, ROS = Reactive oxygen species, VCAM1 = Vascular cell adhesion protein 1.
5.6. Availability of Reported Phytochemicals
It is worth noting that the presence by itself of phytochemicals does not guarantee that it will generate the desired biological effect. As with drugs, the quantity of the phytochemical dictates its efficacy. There are many factors that could alter the quantity of phytochemicals. Phytochemicals are mostly generated in response to external stimuli [71]. Thus, all external stimuli alter the synthesis of phytochemicals. In the same way, there will be differences depending on the tissue. In addition, plant tissue may undergo postharvest changes [244]. Then, the extraction of phytochemicals will alter the availability. Here, factors such as the solvent, temperature, time, and pH, among others, will influence the type and amount of phytochemicals obtained [245]. In general, there are a small number of reports assessing the quantity of Yucca phytochemicals in the literature. The same is true regarding the difference between extraction and improvement in phytochemical concentration. Within the reports included here, the great variability caused by the factors previously described is notorious. This can be seen in Table 6. Specifically, the difference can be seen when comparing the quantity of resveratrol, 3,3’,5,5’-tetrahydroxy-4-methoxystilbene, Yuccaol A, and Yuccaol C obtained between both extraction methods. Despite the differences in concentrations and types of phytochemicals, the presence of multiple phytochemicals with similar biological effects would suggest a robustness that would allow for the prevalence of their antioxidant and anti-inflammatory activity.
Table 6.
Quantity of reported phytochemicals in the Yucca genus.
| Metabolite | Yucca Specie | Plant Material | Extraction | Concentration | Reference |
|---|---|---|---|---|---|
| Resveratrol | Yucca schidigera | Bark was obtained from Desert King Int., Chula Vista, CA, USA |
Ultrasonic | 9.11 ± 2.11 mg/g plant | [77] |
| Yucca schidigera | Yucca bark was collected in October 2001 in San Diego, CA. | Soxhlet | 2.5 mg/300 g of Yucca powder | [68] | |
| 3,3’,5,5’-tetrahydroxy-4-methoxystilbene | Yucca schidigera | 5.1 mg/300 g of Yucca powder | |||
| Yucca schidigera | Bark was obtained from Desert King Int., Chula Vista, CA, USA |
Ultrasonic | 9.10 ± 1.10 mg/g plant | [68] | |
| Yuccaol A | Yucca schidigera | 14.72 ± 2.10 mg/g plant | |||
| Yucca gloriosa roots | The roots and the bark were collected in December, in Tbilisi, Georgia. | MeOH at room temperature | 0.02 ± 0.01 mg/g plant | ||
| Yucca gloriosa | 2.64 ± 0.80 mg/g plant | ||||
| Yucca schidigera | Yucca bark was collected in October 2001 in San Diego, CA. | Soxhlet | 3.1 mg/300 g of Yucca powder | [68] | |
| Yuccaol C | Yucca schidigera | 8.0 mg/300 g of Yucca powder | |||
| Yucca gloriosa roots | The roots and the bark were collected in December, in Tbilisi, Georgia. | MeOH at room temperature | 10.68 ± 1.01 | [77] | |
| Yucca gloriosa | 13.85 ± 1.21 mg/g plant | ||||
| Yucca schidigera | Bark was obtained from Desert King Int., Chula Vista, CA, USA |
Ultrasonic | 12.02 ± 2.28 mg/g plant | ||
| Gloriosaol A | Yucca gloriosa roots | The roots and the bark were collected in December, in Tbilisi, Georgia | MeOH at room temperature | 3.02 ± 1.50 mg/g plant | [77] |
| Yucca gloriosa | 11.87 ± 1.32 mg/g plant | ||||
| Gloriosaol B | Yucca gloriosa roots | 5.30 ± 1.80 mg/g plant | |||
| Yucca gloriosa | 29.73 ± 2.15 mg/g plant | ||||
| Gloriosaol C | Yucca gloriosa roots | 3.33 ± 0.9 mg/g plant | |||
| Yucca gloriosa | 4.53 ± 1.42 mg/g plant | ||||
| Gloriosaol D & Gloriosaol E |
Yucca gloriosa roots | 4.96 ± 1.71 mg/g plant | |||
| Yucca gloriosa | 10.85 ± 1.58 mg/g plant | ||||
| Gallic acid | Yucca elephantipes flowers | Collected during summer in the city of Xalapa, Veracruz, México | MeOH at room temperature | 5.28 μg/g of dry sample | [79] |
| Protocatechuic acid | 21.14 μg/g of dry sample | ||||
| Vanillic acid | 205.28 μg/g of dry sample | ||||
| Chlorogenic acid | 47.52 μg/g of dry sample | ||||
| Caffeic acid | 74.1 μg/g of dry sample | ||||
| Ferulic acid | 140.59 μg/g of dry sample | ||||
| Rutin | 393.2 μg/g of dry sample | ||||
| Quercetin | 9.54 μg/g of dry sample | ||||
| Kaempferol | 37.16 μg/g of dry sample |
Finally, another important factor related to the availability of phytochemicals is microbiota. The gut microbiota metabolizes most molecules consumed, including drugs or phytochemicals. In the intestine, phytochemicals are degraded by microbes and absorbed by tissues [246]. Some phytochemicals need to be metabolized by the gut microbiota in order to generate its biological effect [247]. Poorly absorptive phytochemicals can undergo structural modifications that improve their bioavailability [246,247]. This especially applies to glycosides, as mentioned above. Glycosides have low bioavailability and bioactivity until their aglycone is deglycosylated by gut microbiota [246,247]. This modification through gut microbiota has been reported to have a role in some antioxidant and anti-inflammatory effects. This is especially true through Nrf2, as the genus Lactobacillus capable of stimulating its activation through small molecules [248]. One example of this is the biotransformation of caffeic acid, a phytochemical that can be found in the Yucca, into 4-vinyl-catechol. This is an activator of Nrf2 [249]. It has also been reported that treatment with pre-fermented Angelica sinensis activates Nrf2 signaling better than treatment with non-fermented Angelica sinensis in mice [250]. It also increases the level of bacteria related to Nrf2 signaling, such as Lactobacillus. Thus, the fermentation of phytochemicals through bacteria, such as Lactobacillus, is key for the efficient activation of Nfr2. From the abundance of reports on Yucca phytochemicals activating Nrf2, it could be assumed that other phytochemicals follow the pathway of phytochemicals and are metabolized by bacteria into Nrf2 activators.
6. Future Perspectives
Although there is research on the anti-inflammatory and antioxidant properties of the Yucca genus, there are still many unknowns to be resolved. First, most of the research related to the anti-inflammatory and antioxidant properties has only focused on Yucca schidigera Roezl ex Ortgies and Yucca gloriosa L. [22,23,27,42]. It is worth noting that, within these 2 species, phytochemicals endemic to the genus have been found. Some of these have shown a particular effect against inflammation and oxidative stress [31]. Therefore, it could be expected to find other phytochemicals with similar structures among the other species. It has been reported that metabolomics can be used in taxonomical classifications [251]. If there are molecules that have a similar structure, it is very possible that they have a quite similar effect. This is based on the similarity principle, where similar molecules exhibit similar biological activity [252]. Thus, within the 50 species, there could be molecules with greater anti-inflammatory and antioxidant potential. Thus, there are unexplored unknowns related to most Yucca species. More specifically, to its anti-inflammatory and antioxidant properties and phytochemicals.
However, even with the favorable results of the research done with Yucca schidigera Roezl ex Ortgies and Yucca gloriosa L., further study of its use against inflammatory diseases is still needed. In particular, in vitro studies rarely cope with the complexity of human diseases [253]. From what could be found in the literature, only a few disease models have been used to study the therapeutic potential of Yucca, such as ovalbumin-induced airway hyperresponsiveness in mice [27]. Similarly, the efficacy and potency of different Yucca extracts have not been compared. Nor has its effect been compared with that of known treatments. Therefore, the study of Yucca genus against established models of inflammatory diseases is another field without much exploration. Finally, since the discovery of Yuccaol C and its mechanism of action against NO synthesis [31]. There has not been much research done on this topic. This is surprising since it is found in relatively large proportions within Yucca schidigera Roezl ex Ortgies and Yucca gloriosa L., as can be seen in Table 1. In addition to its reported efficiency, against the NF-kB pathway [31]. Thus, there is another unexplored Yucca topic. For the same reason, although there is favorable evidence on the anti-inflammatory and antioxidant capacity of Yucca genus. There is still a lot of research to be done before being able to describe the genus or its phytochemicals as an alternative to treat inflammatory diseases.
7. Conclusions
Yucca genus encompasses about 40 to 50 species, natives of southern North America. For centuries, it has been used to treat pathologies such as asthma, rheumatism, gonorrhea, sunburns, arthritis, etc. The ethnobotanical use led to the testing of many biological activities, where its antioxidant and anti-inflammatory excels. Unfortunately, there are a limited number of studies, so knowing its composition will provide a better understanding of the molecules responsible for these properties. This is because it is known that the medicinal use of plants is due to its phytochemicals. The documentary research found 92 phytochemicals with reported antioxidant and anti-inflammatory activities. Most of these molecules can be classified as phenolic compounds, glycosides, saponins, or fatty acids. Within these molecules, phytochemicals, such as Yuccaol C, stand out because they are original to the genus and have significant anti-inflammatory and antioxidant activity. The antioxidant and anti-inflammatory properties are mainly generated through free radical scavenging activity, the inhibition of arachidonic acid metabolism, the inhibition of MAPK and NF-κB, and the activation of Nrf2 signaling pathway. The NF-kB pathway is mainly inhibited by phytochemicals through the inhibition of IKK activation and phosphorylation IκBα, and the decrease of NF-kB concentration. The MAPK pathways are mainly inhibited by reducing p38, JNK, and ERK1/2 phosphorylation. Nrf2 is activated by increasing its concentration or inhibiting Keap1. However, there is evidence of the antioxidant and anti-inflammatory activity of some species within the genus, and although it is not abundant, the fact that a great variety of the phytochemicals that compose it present the same activities allows us to assess these properties.
Abbreviations
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| IL-1β | Interleukin 1β |
| GPx | Glutathione peroxidase |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| MAPK | Mitogen-Activated Protein Kinase |
| NF-κB | Nuclear factor kappa B |
| Nrf2 | Nuclear factor erythroid 2–related factor |
| FDA | Food and Drug Administration |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| MDA | Malonaldehyde |
| SIRT1 | Sirtuin 1 |
| PGE2 | Prostaglandin E2 |
| COX-2 | Cyclooxygenase-2 |
| IC50 | Half maximal inhibitory concentration |
| 8-oxo-dG | 8-Oxo-2’-deoxyguanosine |
| MMP-9 | Matrix metalloproteinase 9 |
| TEAC | Trolox equivalent antioxidant capacity |
| NLRP3 | Nucleotide-binding oligomerization domain-like receptor containing domain 3 of pyrin |
| PDE4 | Phosphodiesterase 4 |
| NO | Nitric oxide |
| ERK | Extracellular signal-regulated kinase |
| LPO | Lipid Peroxidation |
| TLR4 | Toll-like receptor 4 |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MPO | Myeloperoxidase |
| GSH | Glutathione |
| HO-1 | Heme oxygenase 1 |
| IκBα | Inhibitor of nuclear factor kappa B |
| LPS | Lipopolysaccharide |
| MIP-2 | Macrophage inflammatory protein 2 |
| IKK | IkB kinase |
| JNK | c-Jun amino terminal kinase |
| ICAM-1 | Intercellular adhesion molecule |
| VEGF | Vascular endothelial growth factor |
| GST | Glutathione-S-transferase |
| CRP | Reactive C-protein |
| PAR-1 | Protease-activated receptor 1 |
| TXB2 | Thromboxane B2 |
| IgE | Immunoglobulin E |
| TBARS | Thiobarbituric acid-reactive substances |
| PKA | Protein kinase A |
| mTOR | Mammalian target of rapamycin |
| EGFR | Epidermal growth factor receptor |
| Keap1 | Kelch-like ECH-associated protein 1 |
| COX-1 | Cyclooxygenase-1 |
| VCAM1 | Vascular cell adhesion protein 1 |
| VWF | von Willebrand factor |
| GPIbα | platelet adhesion receptor glycoprotein |
| AA | arachidonic acid |
| TxA2 | Thromboxane A2 |
| IL-13 | Interleukin-13 |
| NOS | Nitric oxide synthase |
| PUFAs | Polyunsaturated fatty acids |
| PEs | Phosphatidylethanolamines |
| IL-8 | Interleukin 8 |
| IL-1RI | IL-1 type I receptor |
| IL-1RAcP | IL-1R accessory protein |
| H2O2 | Hydrogen peroxide |
| NIK | NF-kB inducing kinase |
| TRAF | TNF receptor-associated factor |
| RTK | Receptor tyrosine kinase |
| Cul3 | Cullin 3 |
| NQO1 | NADP(H) quinone oxidoreductase |
| ORAC | Oxygen radical absorbance capacity |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
Author Contributions
Conceptualization, E.B.C. and A.Z.M.S.; A.M. and M.M.M.Y.E.; writing—original draft preparation, E.B.C., M.M.M.Y.E. and A.Z.M.S.; A.M.; writing—review and editing, A.Z.M.S., A.M. and P.D.P.; visualization, A.Z.M.S.; supervision, M.M.M.Y.E. and A.Z.M.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data is contained and referenced within the article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Karamova N., Gumerova S., Hassan G.O., Abdul-Hafeez E.Y., Ibrahim O.H.M., Orabi M.A.A., Ilinskaya O. Antioxidant and Antimutagenic Potential of Extracts of Some Agavaceae Family Plants. Bionanoscience. 2016;6:591–593. doi: 10.1007/s12668-016-0286-x. [DOI] [Google Scholar]
- 2.Patel S. Yucca: A medicinally significant genus with manifold therapeutic attributes. Nat. Prod. Bioprospecting. 2012;2:231–234. doi: 10.1007/s13659-012-0090-4. [DOI] [Google Scholar]
- 3.Sahoo S., Kaur D., Sethi A., Chandrahas, Saini A., Chandra M. Effect of Dietary Supplementation of Yucca schidigera Extract on the Performance and Litter Quality of Broilers in Winter Season. Anim. Nutr. Feed. Technol. 2016;16:475–484. doi: 10.5958/0974-181X.2016.00043.3. [DOI] [Google Scholar]
- 4.Borges R.S., Ortiz B.L.S., Pereira A.C.M., Keita H., Carvalho J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2018;229:29–45. doi: 10.1016/j.jep.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory Responses and Inflamma-tion-Associated Diseases in Organs. Oncotarget. 2018;9:7204. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia E.I.C., Elghandour M.M.M.Y., Khusro A., Alcala-Canto Y., Tirado-González D.N., Barbabosa-Pliego A., Salem A.Z.M. Dietary Supplements of Vitamins E, C, and β-Carotene to Reduce Oxidative Stress in Horses: An Overview. J. Equine Vet. Sci. 2022;110:103863. doi: 10.1016/j.jevs.2022.103863. [DOI] [PubMed] [Google Scholar]
- 7.Debnath T., Kim D.H., Lim B.O. Natural Products as a Source of Anti-Inflammatory Agents Associated with Inflammatory Bowel Disease. Molecules. 2013;18:7253–7270. doi: 10.3390/molecules18067253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Ramírez Y., Palomeque-Carlín A., Chávez Ortiz L.I., de la Rosa-Carrillo M.D.L., Pérez-Molphe-Balch E. Micropropagation of Yucca Species. Plant Cell Cult. Protoc. 2018;1815:171–177. doi: 10.1007/978-1-4939-8594-4_10. [DOI] [PubMed] [Google Scholar]
- 9.Pellmyr O. Yuccas, Yucca Moths, and Coevolution: A Review. Ann. Mo. Bot. Gard. 2003;90:35–55. doi: 10.2307/3298524. [DOI] [Google Scholar]
- 10.Eguiarte L.E., Leebens-Mack J., Heyduk K. Editorial: Recent Advances and Future Perspectives for Agavoideae Research: Agave, Yucca and Related Taxa. Front. Plant Sci. 2021;12:687596. doi: 10.3389/fpls.2021.687596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Plant List. [(accessed on 5 October 2022)]. Available online: http://www.theplantlist.org/
- 12.Anderson M.K., Keeley J.E. Valuing Chaparral. Springer; Berlin/Heidelberg, Germany: 2018. Native Peoples’ Relationship to the California Chaparral; pp. 79–121. [Google Scholar]
- 13.Corrales V.M.S. Léxico Relativo al Ámbito Del Tamal En Costa Rica. Káñina Rev. Artes Letras. 2006;30:191–197. [Google Scholar]
- 14.Bernier G.R. Masters Thesis. University of Montana; Missoula, MT, USA: 2004. Ethnobotany of the Northern Cheyenne: Medicinal Plants. [Google Scholar]
- 15.Lim T.K., Lim T.K. Yucca Filamentosa. Edible Medicinal and Non-Medicinal Plants. Flowers. 2014;7:134–137. [Google Scholar]
- 16.Gupta S., Duraiswamy B., Muthureddy Nataraj S.K., Rama Satyanarayana Raju K., Babu U.V. Inhibitory Potential of Yucca Gloriosa, L. Extract and Isolated Gloriosaol Isomeric Mixture on Ovalbumin Induced Airway Hyperresponsiveness in Balb/C Mice. Clin. Pharm. Biopharm. 2014;2:2. doi: 10.4172/2167-065X.S2-002. [DOI] [Google Scholar]
- 17.Bingham R., Bellew B.A., Bellew J.G. Yucca Plant Saponin in the Management of Arthritis. J. Appl. Nutr. 1975;17:45–51. [Google Scholar]
- 18.Kim S.-W., Park S.-K., Kang S., Kang H.-C., Oh H.-J., Bae C.-Y., Bae D.-H. Hypocholesterolemic Property OfYucca Schidigera AndQuillaja Saponaria Extracts in Human Body. Arch. Pharm. Res. 2003;26:1042–1046. doi: 10.1007/BF02994756. [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez-García G.J., Quintana-Romero L.A., Morales-Figueroa G.G., Esparza-Romero J., Pérez-Morales R., López-Mata M.A., Juárez J., Sánchez-Escalante J.J., Peralta E., Quihui-Cota L. Effect of Yucca Baccata Butanolic Extract on the Shelf Life of Chicken and Development of an Antimicrobial Packaging for Beef. Food Control. 2021;127:108142. doi: 10.1016/j.foodcont.2021.108142. [DOI] [Google Scholar]
- 20.Del Trujillo R.C.L. Doctoral Thesis. Centro de Investigación en Alimentación y Desarrollo; Mazatlán Mexico: 2017. Characterization and Evaluation of yucca baccata saponins against giardia intestinalis trophozoites in vitro. [Google Scholar]
- 21.Mokbli S., Nehdi I.A., Sbihi H.M., Tan C.P., Al-Resayes S.I., Rashid U. Yucca Aloifolia Seed Oil: A New Source of Bioactive Compounds. Waste Biomass Valorization. 2018;9:1087–1093. doi: 10.1007/s12649-017-9892-2. [DOI] [Google Scholar]
- 22.Thomas M.R., Storey R.F. The Role of Platelets in Inflammation. Thromb. Haemost. 2015;114:449–458. doi: 10.1160/TH14-12-1067. [DOI] [PubMed] [Google Scholar]
- 23.Olas B., Wachowicz B., Stochmal A., Oleszek W. Inhibition of Blood Platelet Adhesion and Secretion by Different Phenolics from Yucca Schidigera Roezl. Bark. Nutrition. 2005;21:199–206. doi: 10.1016/j.nut.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Driver B., Marks D.C., van der Wal D.E. Not All (N) SAID and Done: Effects of Nonsteroidal Anti-inflammatory Drugs and Paracetamol Intake on Platelets. Res. Pr. Thromb. Haemost. 2020;4:36–45. doi: 10.1002/rth2.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marion-Letellier R., Savoye G., Ghosh S. Polyunsaturated Fatty Acids and Inflammation. IUBMB Life. 2015;67:659–667. doi: 10.1002/iub.1428. [DOI] [PubMed] [Google Scholar]
- 26.Wenzig E.M., Oleszek W., Stochmal A., Kunert O., Bauer R. Influence of Phenolic Constituents from Yucca Schidigera Bark on Arachidonate Metabolism in Vitro. J. Agric. Food Chem. 2008;56:8885–8890. doi: 10.1021/jf801289m. [DOI] [PubMed] [Google Scholar]
- 27.Casaro M., Souza V.R., Oliveira F.A., Ferreira C.M. OVA-Induced Allergic Airway Inflammation Mouse Model. In: Guest P.C., editor. Pre-Clinical Models: Techniques and Protocols. Volume 1916. Springer New York; New York, NY, USA: 2019. pp. 297–301. [DOI] [PubMed] [Google Scholar]
- 28.Iqbal M., Verpoorte R., Korthout H.A.A.J., Mustafa N.R. Phytochemicals as a Potential Source for TNF-α Inhibitors. Phytochem. Rev. 2013;12:65–93. doi: 10.1007/s11101-012-9251-7. [DOI] [Google Scholar]
- 29.Israël A. The IKK Complex: An Integrator of All Signals That Activate NF-ΚB? Trends Cell. Biol. 2000;10:129–133. doi: 10.1016/S0962-8924(00)01729-3. [DOI] [PubMed] [Google Scholar]
- 30.Johnson D.E., O’Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 Signalling Axis in Cancer. Nat. Rev. Clin. Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korhonen R., Lahti A., Kankaanranta H., Moilanen E. Nitric Oxide Production and Signaling in Inflammation. Curr. Drug Targets Inflamm. Allergy. 2005;4:471–479. doi: 10.2174/1568010054526359. [DOI] [PubMed] [Google Scholar]
- 32.Pautz A., Art J., Hahn S., Nowag S., Voss C., Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 2010;23:75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Rochette L., Lorin J., Zeller M., Guilland J.-C., Lorgis L., Cottin Y., Vergely C. Nitric Oxide Synthase Inhibition and Oxi-dative Stress in Cardiovascular Diseases: Possible Therapeutic Targets? Pharmacol. Ther. 2013;140:239–257. doi: 10.1016/j.pharmthera.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Marzocco S., Piacente S., Pizza C., Oleszek W., Stochmal A., Pinto A., Sorrentino R., Autore G. Inhibition of inducible nitric oxide synthase expression by yuccaol C from Yucca schidigera roezl. Life Sci. 2004;75:1491–1501. doi: 10.1016/j.lfs.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins C.L., Davies M.J. Role of Myeloperoxidase and Oxidant Formation in the Extracellular Environment in Inflamma-tion-Induced Tissue Damage. Free. Radic. Biol. Med. 2021;172:633–651. doi: 10.1016/j.freeradbiomed.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Malle E., Furtmüller P.G., Sattler W., Obinger C. Myeloperoxidase: A Target for New Drug Development? Br. J. Pharmacol. 2007;152:838–854. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawai Y., Kiyokawa H., Kimura Y., Kato Y., Tsuchiya K., Terao J. Hypochlorous Acid-Derived Modification of Phospho-lipids: Characterization of Aminophospholipids as Regulatory Molecules for Lipid Peroxidation. Biochemistry. 2006;45:14201–14211. doi: 10.1021/bi0610909. [DOI] [PubMed] [Google Scholar]
- 38.Ayala A., Muñoz M.F., Argüelles S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondial-dehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh Z., Karthigesu I.P., Singh P., Rupinder K. Use of Malondialdehyde as a Biomarker for Assessing Oxidative Stress in Different Disease Pathologies: A Review. Iran J. Public Health. 2014;43:7–16. [PMC free article] [PubMed] [Google Scholar]
- 40.Marnett L.J. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181:219–222. doi: 10.1016/S0300-483X(02)00448-1. [DOI] [PubMed] [Google Scholar]
- 41.Cigerci H., Fidan A.F., Konuk M., Yuksel H., Kucukkurt I., Eryavuz A., Sozbilir N.B. The protective potential of Yucca schidigera (Sarsaponin 30®) against nitrite-induced oxidative stress in rats. J. Nat. Med. 2009;63:311–317. doi: 10.1007/s11418-009-0338-4. [DOI] [PubMed] [Google Scholar]
- 42.Li S., Wang R., Dai Z., Wang C., Wu Z. Dietary supplementation with Yucca schidigera extract alleviated heat stress-induced unfolded protein response and oxidative stress in the intestine of Nile tilapia (Oreochromis niloticus) Ecotoxicol. Environ. Saf. 2021;219:112299. doi: 10.1016/j.ecoenv.2021.112299. [DOI] [PubMed] [Google Scholar]
- 43.Gabay C., Lamacchia C., Palmer G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 44.Weber A., Wasiliew P., Kracht M. Interleukin-1β (IL-1β) Processing Pathway. Sci. Signal. 2010;3:cm2. doi: 10.1126/scisignal.3105cm2. [DOI] [PubMed] [Google Scholar]
- 45.Wang L., Wu D., Fan Z., Li H., Li J., Zhang Y., Xu Q., Wang G., Zhu Z. Effect of Yucca schidigera extract on the growth performance, intestinal antioxidant status, immune response, and tight junctions of mirror carp (Cyprinus carpio) Fish Shellfish. Immunol. 2020;103:211–219. doi: 10.1016/j.fsi.2020.05.039. [DOI] [PubMed] [Google Scholar]
- 46.Culhuac E.B., Elghandour M.M.M.Y., Adegbeye M.J., Barbabosa-Pliego A., Salem A.Z.M. Influence of Dietary Selenium on the Oxidative Stress in Horses. Biol. Trace Element Res. 2022;201:1695–1703. doi: 10.1007/s12011-022-03270-y. [DOI] [PubMed] [Google Scholar]
- 47.Smale S.T. Dimer-specific Regulatory Mechanisms within the NF-κB Family of Transcription Factors. Immunol. Rev. 2012;246:193–204. doi: 10.1111/j.1600-065X.2011.01091.x. [DOI] [PubMed] [Google Scholar]
- 48.Yu H., Lin L., Zhang Z., Zhang H., Hu H. Targeting NF-ΚB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. 2020;5:1–23. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawrence T. The Nuclear Factor NF-ΚB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arbabi S., Maier R. v Mitogen-Activated Protein Kinases. Crit. Care Med. 2002;30:S74–S79. doi: 10.1097/00003246-200201001-00010. [DOI] [PubMed] [Google Scholar]
- 51.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Soares-Silva M., Diniz F.F., Gomes G.N., Bahia D. The Mitogen-Activated Protein Kinase (MAPK) Pathway: Role in Immune Evasion by Trypanosomatids. Front. Microbiol. 2016;7:183. doi: 10.3389/fmicb.2016.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong T., Liu M., Ji B., Bai B., Cheng B., Wang C. Role of the Extracellular Signal-Regulated Kinase 1/2 Signaling Pathway in Ischemia-Reperfusion Injury. Front. Physiol. 2019;10:1038. doi: 10.3389/fphys.2019.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beamer E., Corrêa S.A.L. The P38MAPK-MK2 Signaling Axis as a Critical Link Between Inflammation and Synaptic Trans-mission. Front. Cell. Dev. Biol. 2021;9:635636. doi: 10.3389/fcell.2021.635636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner E.F., Nebreda Á.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 56.Park J.G., Aziz N., Cho J.Y. MKK7, the essential regulator of JNK signaling involved in cancer cell survival: A newly emerging anticancer therapeutic target. Ther. Adv. Med Oncol. 2019;11 doi: 10.1177/1758835919875574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roy P.K., Rashid F., Bragg J., A Ibdah J. Role of the JNK signal transduction pathway in inflammatory bowel disease. World J. Gastroenterol. 2008;14:200–202. doi: 10.3748/wjg.14.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Svensson G.P., Pellmyr O., Raguso R.A. Strong Conservation of Floral Scent Composition in Two Allopatric Yuccas. J. Chem. Ecol. 2006;32:2657–2665. doi: 10.1007/s10886-006-9189-6. [DOI] [PubMed] [Google Scholar]
- 60.Cuadrado A., Manda G., Hassan A., Alcaraz M.J., Barbas C., Daiber A., Ghezzi P., León R., López M.G., Oliva B., et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018;70:348–383. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 61.Liu Q., Gao Y., Ci X. Role of Nrf2 and Its Activators in Respiratory Diseases. Oxidative Med. Cell. Longev. 2019;2019:7090534. doi: 10.1155/2019/7090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Y., Li W., Su Z., Kong A.-N.T. The Complexity of the Nrf2 Pathway: Beyond the Antioxidant Response. J. Nutr. Biochem. 2015;26:1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akhter S., Irfan H.M., Jahan S., Shahzad M., Latif M.B. Nerolidol: A Potential Approach in Rheumatoid Arthritis through Reduction of TNF-α, IL-1β, IL-6, NF-KB, COX-2 and Antioxidant Effect in CFA-Induced Arthritic Model. Inflammopharmacology. 2022;30:537–548. doi: 10.1007/s10787-022-00930-2. [DOI] [PubMed] [Google Scholar]
- 64.Silva-Islas C.A., Maldonado P.D. Canonical and Non-Canonical Mechanisms of Nrf2 Activation. Pharm. Res. 2018;134:92–99. doi: 10.1016/j.phrs.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 65.Campbell N.K., Fitzgerald H.K., Dunne A. Regulation of Inflammation by the Antioxidant Haem Oxygenase. Nat. Rev. Immunol. 2021;21:411–425. doi: 10.1038/s41577-020-00491-x. [DOI] [PubMed] [Google Scholar]
- 66.Muhammad Z., Nasir R., Asim M., Fozia A., Munawar I., Muhammad M., Muhammad S. Antioxidant, Antibacterial, Antifungal Activities and Phytochemical Analysis of Dagger (Yucca Aloifolia) Leaves Extracts. J. Med. Plants Res. 2013;7:243–249. [Google Scholar]
- 67.Piacente S., Pizza C., Oleszek W. Saponins and Phenolics of Yucca Schidigera Roezl: Chemistry and Bioactivity. Phytochem. Rev. 2005;4:177–190. doi: 10.1007/s11101-005-1234-5. [DOI] [Google Scholar]
- 68.Piacente S., Montoro P., Oleszek W., Pizza C. Yucca s Chidigera Bark: Phenolic Constituents and Antioxidant Activity. J. Nat. Prod. 2004;67:882–885. doi: 10.1021/np030369c. [DOI] [PubMed] [Google Scholar]
- 69.Bassarello C., Bifulco G., Montoro P., Skhirtladze A., Benidze M., Kemertelidze E., Pizza C., Piacente S. Yucca Gloriosa: A Source of Phenolic Derivatives with Strong Antioxidant Activity. J. Agric. Food Chem. 2007;55:6636–6642. doi: 10.1021/jf071131n. [DOI] [PubMed] [Google Scholar]
- 70.Morales-Figueroa G.-G., Pereo-Vega G.D., Reyna-Murrieta M.E., Pérez-Morales R., López-Mata M.A., Sánchez-Escalante J.J., Tapia-Rodriguez M.R., Ayala-Zavala J.F., Juárez J., Quihui-Cota L. Antibacterial and Antioxidant Properties of Extracts of Yucca Baccata, a Plant of Northwestern Mexico, against Pathogenic Bacteria. Biomed. Res. Int. 2022;2022:9158836. doi: 10.1155/2022/9158836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cordell G.A. Phytochemistry and Traditional Medicine–A Revolution in Process. Phytochem. Lett. 2011;4:391–398. doi: 10.1016/j.phytol.2011.05.005. [DOI] [Google Scholar]
- 72.Xiao L., Sun Y., Tsao R. Paradigm Shift in Phytochemicals Research: Evolution from Antioxidant Capacity to Anti-Inflammatory Effect and to Roles in Gut Health and Metabolic Syndrome. J. Agric. Food Chem. 2022;70:8551–8568. doi: 10.1021/acs.jafc.2c02326. [DOI] [PubMed] [Google Scholar]
- 73.Vuolo M.M., Lima V.S., Junior M.R.M. Bioactive Compounds. Elsevier; Amsterdam, The Netherlands: 2019. Phenolic Compounds: Structure, Classification, and Antioxidant Power; pp. 33–50. [Google Scholar]
- 74.Kumar S., Abedin M.M., Singh A.K., Das S. Role of Phenolic Compounds in Plant-Defensive Mechanisms. Plant Phenolics Sustain. Agric. 2020;1:517–532. [Google Scholar]
- 75.Ambriz-Pérez D.L., Leyva-López N., Gutierrez-Grijalva E.P., Heredia J.B. Phenolic Compounds: Natural Alternative in Inflammation Treatment. A Review. Cogent Food Agric. 2016;2:1131412. [Google Scholar]
- 76.Ememe M.U., Mshelia W.P., Ayo J.O. Ameliorative Effects of Resveratrol on Oxidative Stress Biomarkers in Horses. J. Equine Vet. Sci. 2015;35:518–523. doi: 10.1016/j.jevs.2015.02.006. [DOI] [Google Scholar]
- 77.Torres P., Avila J.G., de Vivar A.R., García A.M., Marín J.C., Aranda E., Céspedes C.L. Antioxidant and Insect Growth Regulatory Activities of Stilbenes and Extracts from Yucca Periculosa. Phytochemistry. 2003;64:463–473. doi: 10.1016/S0031-9422(03)00348-0. [DOI] [PubMed] [Google Scholar]
- 78.Montoro P., Skhirtladze A., Bassarello C., Perrone A., Kemertelidze E., Pizza C., Piacente S. Determination of Phenolic Compounds in Yucca Gloriosa Bark and Root by LC–MS/MS. J. Pharm. Biomed. Anal. 2008;47:854–859. doi: 10.1016/j.jpba.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 79.Juarez-Trujillo N., Monribot-Villanueva J.L., Jiménez-Fernández V.M., Suárez-Montaño R., Aguilar-Colorado Á.S., Guerrero-Analco J.A., Jiménez M. Phytochemical Characterization of Izote (Yucca Elephantipes) Flowers. J. Appl. Bot. Food Qual. 2018;91:202–210. [Google Scholar]
- 80.Pan W., Yu H., Huang S., Zhu P. Resveratrol Protects against TNF-α-Induced Injury in Human Umbilical Endothelial Cells through Promoting Sirtuin-1-Induced Repression of NF-KB and P38 MAPK. PLoS ONE. 2016;11:e0147034. doi: 10.1371/journal.pone.0147034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zykova T.A., Zhu F., Zhai X., Ma W., Ermakova S.P., Lee K.W., Bode A.M., Dong Z. Resveratrol Directly Targets COX-2 to Inhibit Carcinogenesis. Molecular Carcinogenesis: Published in cooperation with the University of Texas MD Anderson Cancer Center. 2008;47:797–805. doi: 10.1002/mc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang T., Dai F., Li G.-H., Chen X.-M., Li Y.-R., Wang S.-Q., Ren D.-M., Wang X.-N., Lou H.-X., Zhou B. Trans-4, 4′-Dihydroxystilbene Ameliorates Cigarette Smoke-Induced Progression of Chronic Obstructive Pulmonary Disease via Inhibiting Oxidative Stress and Inflammatory Response. Free. Radic Biol. Med. 2020;152:525–539. doi: 10.1016/j.freeradbiomed.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 83.Fan G.-J., Liu X.-D., Qian Y.-P., Shang Y.-J., Li X.-Z., Dai F., Fang J.-G., Jin X.-L., Zhou B. 4, 4′-Dihydroxy-Trans-Stilbene, a Resveratrol Analogue, Exhibited Enhanced Antioxidant Activity and Cytotoxicity. Bioorg. Med. Chem. 2009;17:2360–2365. doi: 10.1016/j.bmc.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 84.Olas B., Wachowicz B., Nowak P., Stochmal A., Oleszek W., Glowacki R., Bald E. Comparative Studies of the Antioxidant Effects of a Naturally Occurring Resveratrol Analogue–Trans-3, 3′, 5, 5′-Tetrahydroxy-4′-Methoxystilbene and Resveratrol–against Oxidation and Nitration of Biomolecules in Blood Platelets. Cell. Biol. Toxicol. 2008;24:331–340. doi: 10.1007/s10565-007-9045-7. [DOI] [PubMed] [Google Scholar]
- 85.Lin Y., Luo T., Weng A., Huang X., Yao Y., Fu Z., Li Y., Liu A., Li X., Chen D. Gallic Acid Alleviates Gouty Arthritis by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis through Enhancing Nrf2 Signaling. Front. Immunol. 2020;11:580593. doi: 10.3389/fimmu.2020.580593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Attallah N.G.M., El-Sherbeni S.A., El-Kadem A.H., Elekhnawy E., El-Masry T.A., Elmongy E.I., Altwaijry N., Negm W.A. Elucidation of the Metabolite Profile of Yucca Gigantea and Assessment of Its Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities. Molecules. 2022;27:1329. doi: 10.3390/molecules27041329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.El Sayed A.M., Basam S.M., Marzouk H.S., El-Hawary S. LC–MS/MS and GC–MS Profiling as Well as the Antimicrobial Effect of Leaves of Selected Yucca Species Introduced to Egypt. Sci. Rep. 2020;10:1–15. doi: 10.1038/s41598-020-74440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.JIANG D., ZHANG M., ZHANG Q., CHEN Y., WU W., Xiang M.U., Wu C. Influence of Gallic Acid on Porcine Neutrophils Phosphodiesterase 4, IL-6, TNF-α and Rat Arthritis Model. J. Integr. Agric. 2015;14:758–764. doi: 10.1016/S2095-3119(14)60824-8. [DOI] [Google Scholar]
- 89.Fu X., Lyu X., Liu H., Zhong D., Xu Z., He F., Huang G. Chlorogenic Acid Inhibits BAFF Expression in Collagen-Induced Arthritis and Human Synoviocyte MH7A Cells by Modulating the Activation of the NF-ΚB Signaling Pathway. J. Immunol. Res. 2019;2019:8042097. doi: 10.1155/2019/8042097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abd El-Raouf O.M., El-Sayed E.M., Manie M.F. Cinnamic Acid and Cinnamaldehyde Ameliorate Cisplatin-induced Splenotoxicity in Rats. J. Biochem. Mol. Toxicol. 2015;29:426–431. doi: 10.1002/jbt.21715. [DOI] [PubMed] [Google Scholar]
- 91.Ikram M., Muhammad T., Rehman S.U., Khan A., Jo M.G., Ali T., Kim M.O. Hesperetin Confers Neuroprotection by Regulating Nrf2/TLR4/NF-ΚB Signaling in an Aβ Mouse Model. Mol. Neurobiol. 2019;56:6293–6309. doi: 10.1007/s12035-019-1512-7. [DOI] [PubMed] [Google Scholar]
- 92.Jo S.H., Kim M.E., Cho J.H., Lee Y., Lee J., Park Y.-D., Lee J.S. Hesperetin Inhibits Neuroinflammation on Microglia by Suppressing Inflammatory Cytokines and MAPK Pathways. Arch. Pharm. Res. 2019;42:695–703. doi: 10.1007/s12272-019-01174-5. [DOI] [PubMed] [Google Scholar]
- 93.Hajizadeh A., Azizi S. Effects of Naringenin on Experimentally Induced Rheumatoid Arthritis in Wistar Rats. Arch. Razi. Inst. 2021;76:903. doi: 10.22092/ari.2020.351612.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park H.Y., Kim G.-Y., Choi Y.H. Naringenin Attenuates the Release of Pro-Inflammatory Mediators from Lipopolysaccharide-Stimulated BV2 Microglia by Inactivating Nuclear Factor-ΚB and Inhibiting Mitogen-Activated Protein Kinases. Int. J. Mol. Med. 2012;30:204–210. doi: 10.3892/ijmm.2012.979. [DOI] [PubMed] [Google Scholar]
- 95.Tsilioni I., Taliou A., Francis K., Theoharides T.C. Children with Autism Spectrum Disorders, Who Improved with a Luteolin-Containing Dietary Formulation, Show Reduced Serum Levels of TNF and IL. Transl. Psychiatry. 2015;5:e647. doi: 10.1038/tp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oyagbemi A.A., Omobowale T.O., Ola-Davies O.E., Asenuga E.R., Ajibade T.O., Adejumobi O.A., Afolabi J.M., Ogunpolu B.S., Falayi O.O., Saba A.B. Luteolin-mediated Kim-1/NF-kB/Nrf2 Signaling Pathways Protects Sodium Fluoride-induced Hypertension and Cardiovascular Complications. Biofactors. 2018;44:518–531. doi: 10.1002/biof.1449. [DOI] [PubMed] [Google Scholar]
- 97.Paciello F., di Pino A., Rolesi R., Troiani D., Paludetti G., Grassi C., Fetoni A.R. Anti-Oxidant and Anti-Inflammatory Effects of Caffeic Acid: In Vivo Evidences in a Model of Noise-Induced Hearing Loss. Food Chem. Toxicol. 2020;143:111555. doi: 10.1016/j.fct.2020.111555. [DOI] [PubMed] [Google Scholar]
- 98.Gülçin İ. Antioxidant Activity of Caffeic Acid (3, 4-Dihydroxycinnamic Acid) Toxicology. 2006;217:213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 99.Sun L.-C., Zhang H.-B., Gu C.-D., Guo S.-D., Li G., Lian R., Yao Y., Zhang G.-Q. Protective Effect of Acacetin on Sepsis-Induced Acute Lung Injury via Its Anti-Inflammatory and Antioxidative Activity. Arch. Pharm. Res. 2018;41:1199–1210. doi: 10.1007/s12272-017-0991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang H., Li X., Wu K., Wang M., Liu P., Wang X., Deng R. Antioxidant Activities and Chemical Constituents of Flavonoids from the Flower of Paeonia Ostii. Molecules. 2016;22:5. doi: 10.3390/molecules22010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yilmaz Y., Toledo R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic. Acid. J. Agric. Food Chem. 2004;52:255–260. doi: 10.1021/jf030117h. [DOI] [PubMed] [Google Scholar]
- 102.Li X., Wang X., Chen D., Chen S. Antioxidant Activity and Mechanism of Protocatechuic Acid in Vitro. Funct. Foods Health Dis. 2011;1:232–244. doi: 10.31989/ffhd.v1i7.127. [DOI] [Google Scholar]
- 103.Lende A.B., Kshirsagar A.D., Deshpande A.D., Muley M.M., Patil R.R., Bafna P.A., Naik S.R. Anti-Inflammatory and Analgesic Activity of Protocatechuic Acid in Rats and Mice. Inflammopharmacology. 2011;19:255–263. doi: 10.1007/s10787-011-0086-4. [DOI] [PubMed] [Google Scholar]
- 104.Rabelo T.K., Guimaraes A.G., Oliveira M.A., Gasparotto J., Serafini M.R., de Souza Araújo A.A., Quintans-Junior L.J., Moreira J.C.F., Gelain D.P. Shikimic Acid Inhibits LPS-Induced Cellular pro-Inflammatory Cytokines and Attenuates Mechanical Hyperalgesia in Mice. Int. Immunopharmacol. 2016;39:97–105. doi: 10.1016/j.intimp.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 105.López-Ramírez Y., Cabañas-García E., Areche C., Trejo-Tapia G., Pérez-Molphe-Balch E., Gómez-Aguirre Y.A. Callus Induction and Phytochemical Profiling of Yucca Carnerosana (Trel.) McKelvey Obtained from in Vitro Cultures. Rev. Mex. Ing. Quim. 2021;20:823–837. doi: 10.24275/rmiq/Bio2209. [DOI] [Google Scholar]
- 106.Mokdad-Bzeouich I., Mustapha N., Sassi A., Bedoui A., Ghoul M., Ghedira K., Chekir-Ghedira L. Investigation of Immunomodulatory and Anti-Inflammatory Effects of Eriodictyol through Its Cellular Anti-Oxidant Activity. Cell Stress Chaperones. 2016;21:773–781. doi: 10.1007/s12192-016-0702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu G., Guo H., Huang Y.A.N., Wu C., Zhang X. Eriodictyol, a Plant Flavonoid, Attenuates LPS induced Acute Lung Injury through Its Antioxidative and Anti inflammatory Activity. Exp. Ther. Med. 2015;10:2259–2266. doi: 10.3892/etm.2015.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shaw C., Chen C., Hsu C., Chen C., Tsai Y. Antioxidant Properties of Scopoletin Isolated from Sinomonium Acutum. Phytother. Res. 2003;17:823–825. doi: 10.1002/ptr.1170. [DOI] [PubMed] [Google Scholar]
- 109.Ding Z., Dai Y., Hao H., Pan R., Yao X., Wang Z. Anti-Inflammatory Effects of Scopoletin and Underlying Mechanisms. Pharm. Biol. 2008;46:854–860. doi: 10.1080/13880200802367155. [DOI] [Google Scholar]
- 110.Sancho R., Lucena C., Macho A., Calzado M.A., Blanco-Molina M., Minassi A., Appendino G., Muñoz E. Immunosuppressive Activity of Capsaicinoids: Capsiate Derived from Sweet Peppers Inhibits NF-κB Activation and Is a Potent Antiinflammatory Compound in Vivo. Eur. J. Immunol. 2002;32:1753–1763. doi: 10.1002/1521-4141(200206)32:6<1753::AID-IMMU1753>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 111.Wang Z.H., Kang K.A., Zhang R., Piao M.J., Jo S.H., Kim J.S., Kang S.S., Lee J.S., Park D.H., Hyun J.W. Myricetin Suppresses Oxidative Stress-Induced Cell Damage via Both Direct and Indirect Antioxidant Action. Env. Toxicol. Pharm. 2010;29:12–18. doi: 10.1016/j.etap.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 112.Hou W., Hu S., Su Z., Wang Q., Meng G., Guo T., Zhang J., Gao P. Myricetin Attenuates LPS-Induced Inflammation in RAW 264.7 Macrophages and Mouse Models. Future Med. Chem. 2018;10:2253–2264. doi: 10.4155/fmc-2018-0172. [DOI] [PubMed] [Google Scholar]
- 113.Han D.H., Lee M.J., Kim J.H. Antioxidant and Apoptosis-Inducing Activities of Ellagic Acid. Anticancer. Res. 2006;26:3601–3606. [PubMed] [Google Scholar]
- 114.Chao P., Hsu C., Yin M. Anti-Inflammatory and Anti-Coagulatory Activities of Caffeic Acid and Ellagic Acid in Cardiac Tissue of Diabetic Mice. Nutr. Metab. 2009;6:1–8. doi: 10.1186/1743-7075-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guzmán-Beltrán S., Espada S., Orozco-Ibarra M., Pedraza-Chaverri J., Cuadrado A. Nordihydroguaiaretic Acid Activates the Antioxidant Pathway Nrf2/HO-1 and Protects Cerebellar Granule Neurons against Oxidative Stress. Neurosci. Lett. 2008;447:167–171. doi: 10.1016/j.neulet.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 116.Li Y.-J., Kukita A., Watanabe T., Takano T., Qu P., Sanematsu K., Ninomiya Y., Kukita T. Nordihydroguaiaretic Acid Inhibition of NFATc1 Suppresses Osteoclastogenesis and Arthritis Bone Destruction in Rats. Lab. Investig. 2012;92:1777–1787. doi: 10.1038/labinvest.2012.134. [DOI] [PubMed] [Google Scholar]
- 117.Gaspar A., Martins M., Silva P., Garrido E.M., Garrido J., Firuzi O., Miri R., Saso L., Borges F. Dietary Phenolic Acids and Derivatives. Evaluation of the Antioxidant Activity of Sinapic Acid and Its Alkyl Esters. J. Agric. Food Chem. 2010;58:11273–11280. doi: 10.1021/jf103075r. [DOI] [PubMed] [Google Scholar]
- 118.Yun K.-J., Koh D.-J., Kim S.-H., Park S.J., Ryu J.H., Kim D.-G., Lee J.-Y., Lee K.-T. Anti-Inflammatory Effects of Sinapic Acid through the Suppression of Inducible Nitric Oxide Synthase, Cyclooxygase-2, and Proinflammatory Cytokines Expressions via Nuclear Factor-ΚB Inactivation. J. Agric. Food Chem. 2008;56:10265–10272. doi: 10.1021/jf802095g. [DOI] [PubMed] [Google Scholar]
- 119.Soulage C., Soares A.F., Girotti C., Zarrouki B., Demarne F.E., Lagarde M., Géloën A. Antioxidant Effect of Cirsimarin, a Flavonoid Extracted from Microtea Debilis. Phytopharm. Ther. Values II. 2008;20:55–63. [Google Scholar]
- 120.Zhao H., Jiang Z., Chang X., Xue H., Yahefu W., Zhang X. 4-Hydroxyphenylacetic Acid Prevents Acute APAP-Induced Liver Injury by Increasing Phase II and Antioxidant Enzymes in Mice. Front. Pharmacol. 2018;9:653. doi: 10.3389/fphar.2018.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qiao S., Li W., Tsubouchi R., Haneda M., Murakami K., Takeuchi F., Nisimoto Y., Yoshino M. Rosmarinic Acid Inhibits the Formation of Reactive Oxygen and Nitrogen Species in RAW264. 7 Macrophages. Free Radic. Res. 2005;39:995–1003. doi: 10.1080/10715760500231836. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y., Chen X., Yang L., Zu Y., Lu Q. Effects of Rosmarinic Acid on Liver and Kidney Antioxidant Enzymes, Lipid Peroxidation and Tissue Ultrastructure in Aging Mice. Food Funct. 2015;6:927–931. doi: 10.1039/C4FO01051E. [DOI] [PubMed] [Google Scholar]
- 123.Li H., Xu J., Li X., Hu Y., Liao Y., Zhou W., Song Z. Anti-Inflammatory Activity of Psoralen in Human Periodontal Ligament Cells via Estrogen Receptor Signaling Pathway. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-85145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang C., Al-Ani M.K., Sha Y., Chi Q., Dong N., Yang L., Xu K. Psoralen Protects Chondrocytes, Exhibits Anti-Inflammatory Effects on Synoviocytes, and Attenuates Monosodium Iodoacetate-Induced Osteoarthritis. Int. J. Biol. Sci. 2019;15:229. doi: 10.7150/ijbs.28830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wei D., Guo X., Lin L., Lin M., Gong Y., Ying B., Huang M. Effects of Angelicin on Ovalbumin (OVA)-Induced Airway Inflammation in a Mouse Model of Asthma. Inflammation. 2016;39:1876–1882. doi: 10.1007/s10753-016-0423-2. [DOI] [PubMed] [Google Scholar]
- 126.Liu F., Sun G., Gao H., Li R., Soromou L.-W., Chen N., Deng Y.-H., Feng H. Angelicin Regulates LPS-Induced Inflammation via Inhibiting MAPK/NF-ΚB Pathways. J. Surg. Res. 2013;185:300–309. doi: 10.1016/j.jss.2013.05.083. [DOI] [PubMed] [Google Scholar]
- 127.Cheng C.-Y., Ho T.-Y., Lee E.-J., Su S.-Y., Tang N.-Y., Hsieh C.-L. Ferulic Acid Reduces Cerebral Infarct through Its Antioxidative and Anti-Inflammatory Effects Following Transient Focal Cerebral Ischemia in Rats. Am. J. Chin. Med. 2008;36:1105–1119. doi: 10.1142/S0192415X08006570. [DOI] [PubMed] [Google Scholar]
- 128.Kang J.-K., Chung Y.-C., Hyun C.-G. Anti-Inflammatory Effects of 6-Methylcoumarin in LPS-Stimulated RAW 264.7 Macrophages via Regulation of MAPK and NF-ΚB Signaling Pathways. Molecules. 2021;26:5351. doi: 10.3390/molecules26175351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee H.-S., Kim E.-N., Jeong G.-S. Ameliorative Effect of Citropten Isolated from Citrus Aurantifolia Peel Extract as a Modulator of T Cell and Intestinal Epithelial Cell Activity in DSS-Induced Colitis. Molecules. 2022;27:4633. doi: 10.3390/molecules27144633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xia S., Ni Y., Zhou Q., Liu H., Xiang H., Sui H., Shang D. Emodin Attenuates Severe Acute Pancreatitis via Antioxidant and Anti-Inflammatory Activity. Inflammation. 2019;42:2129–2138. doi: 10.1007/s10753-019-01077-z. [DOI] [PubMed] [Google Scholar]
- 131.Ding Y., Zhao L., Mei H., Zhang S.-L., Huang Z.-H., Duan Y.-Y., Ye P. Exploration of Emodin to Treat Alpha-Naphthylisothiocyanate-Induced Cholestatic Hepatitis via Anti-Inflammatory Pathway. Eur. J. Pharmacol. 2008;590:377–386. doi: 10.1016/j.ejphar.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 132.Jiang Y., Gong F.-L., Zhao G.-B., Li J. Chrysin Suppressed Inflammatory Responses and the Inducible Nitric Oxide Synthase Pathway after Spinal Cord Injury in Rats. Int. J. Mol. Sci. 2014;15:12270–12279. doi: 10.3390/ijms150712270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pushpavalli G., Kalaiarasi P., Veeramani C., Pugalendi K.V. Effect of Chrysin on Hepatoprotective and Antioxidant Status in D-Galactosamine-Induced Hepatitis in Rats. Eur. J. Pharmacol. 2010;631:36–41. doi: 10.1016/j.ejphar.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 134.Zhu Z., Hu R., Li J., Xing X., Chen J., Zhou Q., Sun J. Alpinetin Exerts Anti-Inflammatory, Anti-Oxidative and Anti-Angiogenic Effects through Activating the Nrf2 Pathway and Inhibiting NLRP3 Pathway in Carbon Tetrachloride-Induced Liver Fibrosis. Int. Immunopharmacol. 2021;96:107660. doi: 10.1016/j.intimp.2021.107660. [DOI] [PubMed] [Google Scholar]
- 135.Gao Y., Wang S., He L., Wang C., Yang L. Alpinetin Protects Chondrocytes and Exhibits Anti-Inflammatory Effects via the NF-ΚB/ERK Pathway for Alleviating Osteoarthritis. Inflammation. 2020;43:1742–1750. doi: 10.1007/s10753-020-01248-3. [DOI] [PubMed] [Google Scholar]
- 136.Tai A., Sawano T., Yazama F., Ito H. Evaluation of Antioxidant Activity of Vanillin by Using Multiple Antioxidant Assays. Biochim. Biophys. Acta. 2011;1810:170–177. doi: 10.1016/j.bbagen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 137.Makni M., Chtourou Y., Fetoui H., Garoui E.M., Boudawara T., Zeghal N. Evaluation of the Antioxidant, Anti-Inflammatory and Hepatoprotective Properties of Vanillin in Carbon Tetrachloride-Treated Rats. Eur. J. Pharmacol. 2011;668:133–139. doi: 10.1016/j.ejphar.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 138.Chen T., Zhang X., Zhu G., Liu H., Chen J., Wang Y., He X. Quercetin Inhibits TNF-α Induced HUVECs Apoptosis and Inflammation via Downregulating NF-KB and AP-1 Signaling Pathway in Vitro. Medicine. 2020;99:e22241. doi: 10.1097/MD.0000000000022241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Abdelmoaty M.A., Ibrahim M.A., Ahmed N.S., Abdelaziz M.A. Confirmatory Studies on the Antioxidant and Antidiabetic Effect of Quercetin in Rats. Indian J. Clin. Biochem. 2010;25:188–192. doi: 10.1007/s12291-010-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chekalina N., Burmak Y., Petrov Y., Borisova Z., Manusha Y., Kazakov Y., Kaidashev I. Quercetin Reduces the Transcriptional Activity of NF-KB in Stable Coronary Artery Disease. Indian Heart J. 2018;70:593–597. doi: 10.1016/j.ihj.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Z., Sun W., Sun X., Wang Y., Zhou M. Kaempferol Ameliorates Cisplatin Induced Nephrotoxicity by Modulating Oxidative Stress, Inflammation and Apoptosis via ERK and NF-ΚB Pathways. AMB Express. 2020;10:1–11. doi: 10.1186/s13568-020-00993-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martinez-Micaelo N., González-Abuín N., Pinent M., Ardévol A., Blay M. Procyanidin B2 Inhibits Inflammasome-mediated IL-1β Production in Lipopolysaccharide-stimulated Macrophages. Mol. Nutr. Food Res. 2015;59:262–269. doi: 10.1002/mnfr.201400370. [DOI] [PubMed] [Google Scholar]
- 143.Wang W., Chen R., Wang J. Procyanidin B2 Ameliorates Carrageenan-Induced Chronic Nonbacterial Prostatitis in Rats via Anti-Inflammatory and Activation of the Nrf2 Pathway. Biochem. Biophys. Res. Commun. 2017;493:794–799. doi: 10.1016/j.bbrc.2017.08.089. [DOI] [PubMed] [Google Scholar]
- 144.Ramesh B., Pugalendi K. v Antioxidant Role of Umbelliferone in STZ-Diabetic Rats. Life Sci. 2006;79:306–310. doi: 10.1016/j.lfs.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 145.Wu G., Nie W., Wang Q., Hao Y., Gong S., Zheng Y., Lv H. Umbelliferone Ameliorates Complete Freund Adjuvant–Induced Arthritis via Reduction of NF-ΚB Signaling Pathway in Osteoclast Differentiation. Inflammation. 2021;44:1315–1329. doi: 10.1007/s10753-021-01418-x. [DOI] [PubMed] [Google Scholar]
- 146.Li T., Li F., Liu X., Liu J., Li D. Synergistic Anti-inflammatory Effects of Quercetin and Catechin via Inhibiting Activation of TLR4–MyD88-mediated NF-κB and MAPK Signaling Pathways. Phytother. Res. 2019;33:756–767. doi: 10.1002/ptr.6268. [DOI] [PubMed] [Google Scholar]
- 147.Samarghandian S., Azimi-Nezhad M., Farkhondeh T. Catechin Treatment Ameliorates Diabetes and Its Complications in Streptozotocin-Induced Diabetic Rats. Dose Response. 2017;15:1559325817691158. doi: 10.1177/1559325817691158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pan R., Dai Y., Gao X., Xia Y. Scopolin Isolated from Erycibe Obtusifolia Benth Stems Suppresses Adjuvant-Induced Rat Arthritis by Inhibiting Inflammation and Angiogenesis. Int. Immunopharmacol. 2009;9:859–869. doi: 10.1016/j.intimp.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 149.García-Mediavilla V., Crespo I., Collado P.S., Esteller A., Sánchez-Campos S., Tuñón M.J., González-Gallego J. The Anti-Inflammatory Flavones Quercetin and Kaempferol Cause Inhibition of Inducible Nitric Oxide Synthase, Cyclooxygenase-2 and Reactive C-Protein, and down-Regulation of the Nuclear Factor KappaB Pathway in Chang Liver Cells. Eur. J. Pharmacol. 2007;557:221–229. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 150.Liao W., Chen L., Ma X., Jiao R., Li X., Wang Y. Protective Effects of Kaempferol against Reactive Oxygen Species-Induced Hemolysis and Its Antiproliferative Activity on Human Cancer Cells. Eur. J. Med. Chem. 2016;114:24–32. doi: 10.1016/j.ejmech.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 151.Omar H.A., Mohamed W.R., Arafa E.-S.A., Shehata B.A., el Sherbiny G.A., Arab H.H., Elgendy A.N.A.M. Hesperidin Alleviates Cisplatin-Induced Hepatotoxicity in Rats without Inhibiting Its Antitumor Activity. Pharmacol. Rep. 2016;68:349–356. doi: 10.1016/j.pharep.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 152.Wilmsen P.K., Spada D.S., Salvador M. Antioxidant Activity of the Flavonoid Hesperidin in Chemical and Biological Systems. J. Agric. Food Chem. 2005;53:4757–4761. doi: 10.1021/jf0502000. [DOI] [PubMed] [Google Scholar]
- 153.Wang J., Liu Y.-T., Xiao L., Zhu L., Wang Q., Yan T. Anti-Inflammatory Effects of Apigenin in Lipopolysaccharide-Induced Inflammatory in Acute Lung Injury by Suppressing COX-2 and NF-KB Pathway. Inflammation. 2014;37:2085–2090. doi: 10.1007/s10753-014-9942-x. [DOI] [PubMed] [Google Scholar]
- 154.Singh J.P.V., Selvendiran K., Banu S.M., Padmavathi R., Sakthisekaran D. Protective Role of Apigenin on the Status of Lipid Peroxidation and Antioxidant Defense against Hepatocarcinogenesis in Wistar Albino Rats. Phytomedicine. 2004;11:309–314. doi: 10.1078/0944711041495254. [DOI] [PubMed] [Google Scholar]
- 155.Calixto-Campos C., Carvalho T.T., Hohmann M.S.N., Pinho-Ribeiro F.A., Fattori V., Manchope M.F., Zarpelon A.C., Baracat M.M., Georgetti S.R., Casagrande R. Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, Cytokine Production, and NFκB Activation in Mice. J. Nat. Prod. 2015;78:1799–1808. doi: 10.1021/acs.jnatprod.5b00246. [DOI] [PubMed] [Google Scholar]
- 156.Prince P.S.M., Rajakumar S., Dhanasekar K. Protective Effects of Vanillic Acid on Electrocardiogram, Lipid Peroxidation, Antioxidants, Proinflammatory Markers and Histopathology in Isoproterenol Induced Cardiotoxic Rats. Eur. J. Pharmacol. 2011;668:233–240. doi: 10.1016/j.ejphar.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 157.Singh B., Singh J.P., Singh N., Kaur A. Saponins in Pulses and Their Health Promoting Activities: A Review. Food Chem. 2017;233:540–549. doi: 10.1016/j.foodchem.2017.04.161. [DOI] [PubMed] [Google Scholar]
- 158.Faizal A., Geelen D. Saponins and Their Role in Biological Processes in Plants. Phytochem. Rev. 2013;12:877–893. doi: 10.1007/s11101-013-9322-4. [DOI] [Google Scholar]
- 159.Passos F.R.S., Araújo-Filho H.G., Monteiro B.S., Shanmugam S., de Souza Araújo A.A., da Silva Almeida J.R.G., Thangaraj P., Júnior L.J.Q., Quintans J. de S.S. Anti-Inflammatory and Modulatory Effects of Steroidal Saponins and Sapogenins on Cytokines: A Review of Pre-Clinical Research. Phytomedicine. 2021;96:153842. doi: 10.1016/j.phymed.2021.153842. [DOI] [PubMed] [Google Scholar]
- 160.Zhang T., Zhong S., Li T., Zhang J. Saponins as Modulators of Nuclear Receptors. Crit. Rev. Food Sci. Nutr. 2020;60:94–107. doi: 10.1080/10408398.2018.1514580. [DOI] [PubMed] [Google Scholar]
- 161.Tenon M., Feuillère N., Roller M., Birtić S. Rapid, Cost-Effective and Accurate Quantification of Yucca Schidigera Roezl. Steroidal Saponins Using HPLC-ELSD Method. Food Chem. 2017;221:1245–1252. doi: 10.1016/j.foodchem.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 162.Qu L., Wang J., Ruan J., Yao X., Huang P., Wang Y., Yu H., Han L., Zhang Y., Wang T. Spirostane-Type Saponins Obtained from Yucca Schidigera. Molecules. 2018;23:167. doi: 10.3390/molecules23010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tang Z.-Z., Zhang Y.-M., Zheng T., Huang T.-T., Ma T.-F., Liu Y.-W. Sarsasapogenin Alleviates Diabetic Nephropathy through Suppression of Chronic Inflammation by Down-Regulating PAR-1: In Vivo and in Vitro Study. Phytomedicine. 2020;78:153314. doi: 10.1016/j.phymed.2020.153314. [DOI] [PubMed] [Google Scholar]
- 164.Ingawale D.K., Mandlik S.K., Patel S.S. Combination of Sarsasapogenin and Fluticasone Attenuates Ovalbumin-Induced Airway Inflammation in a Mouse Asthma Model. Immunopharmacol. Immunotoxicol. 2020;42:128–137. doi: 10.1080/08923973.2020.1728541. [DOI] [PubMed] [Google Scholar]
- 165.Pei R., Duan C., Vijayalakshmi A. Hecogenin Attenuates Isoproterenol-Induced Myocardial Infarction through Nuclear Factor-Kappa B-Mediated Signaling Pathway in Rats. Pharmacogn. Mag. 2022;18:463. [Google Scholar]
- 166.Ingawale D.K., Mandlik S.K., Patel S.S. Hecogenin and Fluticasone Combination Attenuates TNBS-Induced Ulcerative Colitis in Rats via Downregulation of pro-Inflammatory Mediators and Oxidative Stress. Immunopharmacol. Immunotoxicol. 2021;43:160–170. doi: 10.1080/08923973.2021.1872617. [DOI] [PubMed] [Google Scholar]
- 167.Ingawale D.K., Patel S.S. Hecogenin Exhibits Anti-Arthritic Activity in Rats through Suppression of pro-Inflammatory Cytokines in Complete Freund’s Adjuvant-Induced Arthritis. Immunopharmacol. Immunotoxicol. 2018;40:59–71. doi: 10.1080/08923973.2017.1405439. [DOI] [PubMed] [Google Scholar]
- 168.Chen C.-T., Wang Z.-H., Hsu C.-C., Lin H.-H., Chen J.-H. In Vivo Protective Effects of Diosgenin against Doxorubicin-Induced Cardiotoxicity. Nutrients. 2015;7:4938–4954. doi: 10.3390/nu7064938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Deng X.-Y., Chen J.-J., Li H.-Y., Ma Z.-Q., Ma S.-P., Fu Q. Cardioprotective Effects of Timosaponin B II from Anemarrhenae Asphodeloides Bge on Isoproterenol-Induced Myocardial Infarction in Rats. Chem. Biol. Interact. 2015;240:22–28. doi: 10.1016/j.cbi.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 170.Jiménez G.G., Durán A.G., Macías F.A., Simonet A.M. Structure, Bioactivity and Analytical Methods for the Determination of Yucca Saponins. Molecules. 2021;26:5251. doi: 10.3390/molecules26175251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang N., Xu P., Wu R., Wang X., Wang Y., Shou D., Zhang Y. Timosaponin BII Improved Osteoporosis Caused by Hyperglycemia through Promoting Autophagy of Osteoblasts via Suppressing the MTOR/NFκB Signaling Pathway. Free Radic. Biol. Med. 2021;171:112–123. doi: 10.1016/j.freeradbiomed.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 172.Lim S.-M., Jeong J.-J., Kang G.-D., Kim K.-A., Choi H.-S., Kim D.-H. Timosaponin AIII and Its Metabolite Sarsasapogenin Ameliorate Colitis in Mice by Inhibiting NF-ΚB and MAPK Activation and Restoring Th17/Treg Cell Balance. Int. Immunopharmacol. 2015;25:493–503. doi: 10.1016/j.intimp.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 173.Tuan Anh H.L., Tran P.T., Thao D.T., Trang D.T., Dang N.H., van Cuong P., van Kiem P., van Minh C., Lee J.-H. Degalactotigonin, a Steroidal Glycoside from Solanum Nigrum, Induces Apoptosis and Cell Cycle Arrest via Inhibiting the EGFR Signaling Pathways in Pancreatic Cancer Cells. Biomed. Res. Int. 2018;2018:3120972. doi: 10.1155/2018/3120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li J., Zhao C., Zhu Q., Wang Y., Li G., Li X., Li Y., Wu N., Ma C. Sweroside Protects Against Myocardial Ischemia–Reperfusion Injury by Inhibiting Oxidative Stress and Pyroptosis Partially via Modulation of the Keap1/Nrf2 Axis. Front. Cardiovasc. Med. 2021;8:650368. doi: 10.3389/fcvm.2021.650368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang R., Dong Z., Lan X., Liao Z., Chen M. Sweroside Alleviated LPS-Induced Inflammation via SIRT1 Mediating NF-ΚB and FOXO1 Signaling Pathways in RAW264. 7 Cells. Molecules. 2019;24:872. doi: 10.3390/molecules24050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bartnik M., Facey P.C. Pharmacognosy. Elsevier; Amsterdam, The Netherlands: 2017. Glycosides; pp. 101–161. [Google Scholar]
- 177.Kytidou K., Artola M., Overkleeft H.S., Aerts J.M.F.G. Plant Glycosides and Glycosidases: A Treasure-Trove for Therapeutics. Front. Plant. Sci. 2020;11:357. doi: 10.3389/fpls.2020.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guardia T., Rotelli A.E., Juarez A.O., Pelzer L.E. Anti-Inflammatory Properties of Plant Flavonoids. Effects of Rutin, Quercetin and Hesperidin on Adjuvant Arthritis in Rat. Il Farm. 2001;56:683–687. doi: 10.1016/S0014-827X(01)01111-9. [DOI] [PubMed] [Google Scholar]
- 179.Yang B., Liu H., Yang J., Gupta V.K., Jiang Y. New Insights on Bioactivities and Biosynthesis of Flavonoid Glycosides. Trends Food Sci. Technol. 2018;79:116–124. doi: 10.1016/j.tifs.2018.07.006. [DOI] [Google Scholar]
- 180.Ying X., Li H., Xiong Z., Sun Z., Cai S., Zhu W., Bi Y., Li F. LC Determination of Malondialdehyde Concentrations in the Human Umbilical Vein Endothelial Cell Culture Medium: Application to the Antioxidant Effect of Vitexin-2 ″-O-Rhamnoside. Chromatographia. 2008;67:679–686. doi: 10.1365/s10337-008-0569-7. [DOI] [Google Scholar]
- 181.Ullah H., Khan A., Baig M.W., Ullah N., Ahmed N., Tipu M.K., Ali H., Khan S. Poncirin Attenuates CCL4-Induced Liver Injury through Inhibition of Oxidative Stress and Inflammatory Cytokines in Mice. BMC Complement Med. 2020;20:1–14. doi: 10.1186/s12906-020-02906-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ullah H., Khan A., Bibi T., Ahmad S., Shehzad O., Ali H., Seo E.K., Khan S. Comprehensive in Vivo and in Silico Approaches to Explore the Hepatoprotective Activity of Poncirin against Paracetamol Toxicity. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022;395:195–215. doi: 10.1007/s00210-021-02192-1. [DOI] [PubMed] [Google Scholar]
- 183.Domitrović R., Rashed K., Cvijanović O., Vladimir-Knežević S., Škoda M., Višnić A. Myricitrin Exhibits Antioxidant, Anti-Inflammatory and Antifibrotic Activity in Carbon Tetrachloride-Intoxicated Mice. Chem. Biol. Interact. 2015;230:21–29. doi: 10.1016/j.cbi.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 184.Chen W., Feng L., Shen Y., Su H., Li Y., Zhuang J., Zhang L., Zheng X. Myricitrin Inhibits Acrylamide-Mediated Cytotoxicity in Human Caco-2 Cells by Preventing Oxidative Stress. Biomed. Res. Int. 2013;2013:724183. doi: 10.1155/2013/724183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.De Sousa E., Zanatta L., Seifriz I., Creczynski-Pasa T.B., Pizzolatti M.G., Szpoganicz B., Silva F.R.M.B. Hypoglycemic Effect and Antioxidant Potential of Kaempferol-3, 7-O-(α)-Dirhamnoside from Bauhinia f Orficata Leaves. J. Nat. Prod. 2004;67:829–832. doi: 10.1021/np030513u. [DOI] [PubMed] [Google Scholar]
- 186.Kamdi S.P., Raval A., Nakhate K.T. Phloridzin Attenuates Lipopolysaccharide-Induced Cognitive Impairment via Antioxidant, Anti-Inflammatory and Neuromodulatory Activities. Cytokine. 2021;139:155408. doi: 10.1016/j.cyto.2020.155408. [DOI] [PubMed] [Google Scholar]
- 187.Taiwo F.O., Oyedeji O., Osundahunsi M.T. Antimicrobial and Antioxidant Properties of Kaempferol-3-O-Glucoside and 1-(4-Hydroxyphenyl)-3-Phenylpropan-1-One Isolated from the Leaves of Annona Muricata (Linn.) J. Pharm. Res. Int. 2019;26:1–13. doi: 10.9734/jpri/2019/v26i330138. [DOI] [Google Scholar]
- 188.Babujanarthanam R., Kavitha P., Mahadeva Rao U.S., Pandian M.R. Quercitrin a Bioflavonoid Improves the Antioxidant Status in Streptozotocin: Induced Diabetic Rat Tissues. Mol. Cell. Biochem. 2011;358:121–129. doi: 10.1007/s11010-011-0927-x. [DOI] [PubMed] [Google Scholar]
- 189.Camuesco D., Comalada M., Rodríguez-Cabezas M.E., Nieto A., Lorente M.D., Concha A., Zarzuelo A., Gálvez J. The Intestinal Anti-inflammatory Effect of Quercitrin Is Associated with an Inhibition in INOS Expression. Br. J. Pharmacol. 2004;143:908–918. doi: 10.1038/sj.bjp.0705941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Comalada M., Camuesco D., Sierra S., Ballester I., Xaus J., Gálvez J., Zarzuelo A. In Vivo Quercitrin Anti-inflammatory Effect Involves Release of Quercetin, Which Inhibits Inflammation through Down-regulation of the NF-κB Pathway. Eur. J. Immunol. 2005;35:584–592. doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- 191.Mittal R., Kumar A., Singh D.P., Bishnoi M., Nag T.C. Ameliorative Potential of Rutin in Combination with Nimesulide in STZ Model of Diabetic Neuropathy: Targeting Nrf2/HO-1/NF-KB and COX Signalling Pathway. Inflammopharmacology. 2018;26:755–768. doi: 10.1007/s10787-017-0413-5. [DOI] [PubMed] [Google Scholar]
- 192.Yang J., Guo J., Yuan J. In Vitro Antioxidant Properties of Rutin. LWT Food Sci. Technol. 2008;41:1060–1066. doi: 10.1016/j.lwt.2007.06.010. [DOI] [Google Scholar]
- 193.Dou W., Zhang J., Sun A., Zhang E., Ding L., Mukherjee S., Wei X., Chou G., Wang Z.-T., Mani S. Protective Effect of Naringenin against Experimental Colitis via Suppression of Toll-like Receptor 4/NF-ΚB Signalling. Br. J. Nutr. 2013;110:599–608. doi: 10.1017/S0007114512005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jung U.J., Kim H.J., Lee J.S., Lee M.K., Kim H.O., Park E.J., Kim H.K., Jeong T.S., Choi M.S. Naringin Supplementation Lowers Plasma Lipids and Enhances Erythrocyte Antioxidant Enzyme Activities in Hypercholesterolemic Subjects. Clin. Nutr. 2003;22:561–568. doi: 10.1016/S0261-5614(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 195.Kim S.-J., Um J.-Y., Hong S.-H., Lee J.-Y. Anti-Inflammatory Activity of Hyperoside through the Suppression of Nuclear Factor-ΚB Activation in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2011;39:171–181. doi: 10.1142/S0192415X11008737. [DOI] [PubMed] [Google Scholar]
- 196.Ku S.-K., Zhou W., Lee W., Han M.-S., Na M., Bae J.-S. Anti-Inflammatory Effects of Hyperoside in Human Endothelial Cells and in Mice. Inflammation. 2015;38:784–799. doi: 10.1007/s10753-014-9989-8. [DOI] [PubMed] [Google Scholar]
- 197.Piao M.J., Kang K.A., Zhang R., Ko D.O., Wang Z.H., You H.J., Kim H.S., Kim J.S., Kang S.S., Hyun J.W. Hyperoside Prevents Oxidative Damage Induced by Hydrogen Peroxide in Lung Fibroblast Cells via an Antioxidant Effect. Biochim. Biophys. Acta. 2008;1780:1448–1457. doi: 10.1016/j.bbagen.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 198.Song Y., Wang X., Qin S., Zhou S., Li J., Gao Y. Esculin Ameliorates Cognitive Impairment in Experimental Diabetic Nephropathy and Induces Anti-Oxidative Stress and Anti-Inflammatory Effects via the MAPK Pathway. Mol. Med. Rep. 2018;17:7395–7402. doi: 10.3892/mmr.2018.8727. [DOI] [PubMed] [Google Scholar]
- 199.Rios E.R.V., Rocha N.F.M., Venâncio E.T., Moura B.A., Feitosa M.L., Cerqueira G.S., Soares P.M.G., Woods D.J., de Sousa F.C.F., Leal L.K.A.M. Mechanisms Involved in the Gastroprotective Activity of Esculin on Acute Gastric Lesions in Mice. Chem. Biol. Interact. 2010;188:246–254. doi: 10.1016/j.cbi.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 200.Chung M.J., Pandey R.P., Choi J.W., Sohng J.K., Choi D.J., Park Y. il Inhibitory Effects of Kaempferol-3-O-Rhamnoside on Ovalbumin-Induced Lung Inflammation in a Mouse Model of Allergic Asthma. Int. Immunopharmacol. 2015;25:302–310. doi: 10.1016/j.intimp.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 201.Lund J., Rustan A.C. eLS. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2022. Fatty Acids: Structures and Properties. [Google Scholar]
- 202.Weber H. Fatty Acid-Derived Signals in Plants. Trends Plant Sci. 2002;7:217–224. doi: 10.1016/S1360-1385(02)02250-1. [DOI] [PubMed] [Google Scholar]
- 203.He M., Ding N.-Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020;11:562785. doi: 10.3389/fpls.2020.562785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Munsterman A.S., Bertone A.L., Zachos T.A., Weisbrode S.E. Effects of the Omega-3 Fatty Acid, α-Linolenic Acid, on Lipopolysaccharide-Challenged Synovial Explants from Horses. Am. J. Vet. Res. 2005;66:1503–1508. doi: 10.2460/ajvr.2005.66.1503. [DOI] [PubMed] [Google Scholar]
- 205.Youn K., Lee S., Jun M. Gamma-Linolenic Acid Ameliorates Aβ-Induced Neuroinflammation through NF-ΚB and MAPK Signalling Pathways. J. Funct. Foods. 2018;42:30–37. doi: 10.1016/j.jff.2017.12.065. [DOI] [Google Scholar]
- 206.Huang W.-C., Tsai T.-H., Chuang L.-T., Li Y.-Y., Zouboulis C.C., Tsai P.-J. Anti-Bacterial and Anti-Inflammatory Properties of Capric Acid against Propionibacterium Acnes: A Comparative Study with Lauric. Acid. J. Dermatol. Sci. 2014;73:232–240. doi: 10.1016/j.jdermsci.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 207.Henry G.E., Momin R.A., Nair M.G., Dewitt D.L. Antioxidant and Cyclooxygenase Activities of Fatty Acids Found in Food. J. Agric. Food Chem. 2002;50:2231–2234. doi: 10.1021/jf0114381. [DOI] [PubMed] [Google Scholar]
- 208.Venn-Watson S., Lumpkin R., Dennis E.A. Efficacy of Dietary Odd-Chain Saturated Fatty Acid Pentadecanoic Acid Parallels Broad Associated Health Benefits in Humans: Could It Be Essential? Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-64960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Saiki P., Kawano Y., van Griensven L.J.L.D., Miyazaki K. The Anti-Inflammatory Effect of Agaricus Brasiliensis Is Partly Due to Its Linoleic Acid Content. Food Funct. 2017;8:4150–4158. doi: 10.1039/C7FO01172E. [DOI] [PubMed] [Google Scholar]
- 210.Wang Z., Liang C., Li G., Yu C., Yin M. Stearic Acid Protects Primary Cultured Cortical Neurons against Oxidative Stress. Acta Pharmacol. Sin. 2007;28:315–326. doi: 10.1111/j.1745-7254.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 211.Pan P.-H., Lin S.-Y., Ou Y.-C., Chen W.-Y., Chuang Y.-H., Yen Y.-J., Liao S.-L., Raung S.-L., Chen C.-J. Stearic Acid Attenuates Cholestasis-Induced Liver Injury. Biochem. Biophys. Res. Commun. 2010;391:1537–1542. doi: 10.1016/j.bbrc.2009.12.119. [DOI] [PubMed] [Google Scholar]
- 212.Bhattacharjee B., Pal P.K., Chattopadhyay A., Bandyopadhyay D. Oleic Acid Protects against Cadmium Induced Cardiac and Hepatic Tissue Injury in Male Wistar Rats: A Mechanistic Study. Life Sci. 2020;244:117324. doi: 10.1016/j.lfs.2020.117324. [DOI] [PubMed] [Google Scholar]
- 213.Zhao Y., Joshi-Barve S., Barve S., Chen L.H. Eicosapentaenoic Acid Prevents LPS-Induced TNF-α Expression by Preventing NF-ΚB Activation. J. Am. Coll. Nutr. 2004;23:71–78. doi: 10.1080/07315724.2004.10719345. [DOI] [PubMed] [Google Scholar]
- 214.Mathur A., Verma S.K., Singh S.K., Prasad G., Dua V.K. Investigation of the Antimicrobial, Antioxidant and Anti-Inflammatory Activity of Compound Isolated from Murraya Koenigii. Int. J. Appl. Biol. Pharm. 2011;2:470–477. [Google Scholar]
- 215.Oldfield E., Lin F. Terpene Biosynthesis: Modularity Rules. Angew. Chem. Int. Ed. 2012;51:1124–1137. doi: 10.1002/anie.201103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Munné-Bosch S., Falk J. New Insights into the Function of Tocopherols in Plants. Planta. 2004;218:323–326. doi: 10.1007/s00425-003-1126-0. [DOI] [PubMed] [Google Scholar]
- 217.Niki E., Traber M.G. A History of Vitamin, E. Ann. Nutr. Metab. 2012;61:207–212. doi: 10.1159/000343106. [DOI] [PubMed] [Google Scholar]
- 218.Gabay O., Sanchez C., Salvat C., Chevy F., Breton M., Nourissat G., Wolf C., Jacques C., Berenbaum F. Stigmasterol: A Phytosterol with Potential Anti-Osteoarthritic Properties. Osteoarthr. Cartil. 2010;18:106–116. doi: 10.1016/j.joca.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 219.Khan M.A., Sarwar A.H.M.G., Rahat R., Ahmed R.S., Umar S. Stigmasterol Protects Rats from Collagen Induced Arthritis by Inhibiting Proinflammatory Cytokines. Int. Immunopharmacol. 2020;85:106642. doi: 10.1016/j.intimp.2020.106642. [DOI] [PubMed] [Google Scholar]
- 220.Liang Q., Yang J., He J., Chen X., Zhang H., Jia M., Liu K., Jia C., Pan Y., Wei J. Stigmasterol Alleviates Cerebral Ischemia/Reperfusion Injury by Attenuating Inflammation and Improving Antioxidant Defenses in Rats. Biosci. Rep. 2020;40:BSR20192133. doi: 10.1042/BSR20192133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Carvalho A.M.S., Heimfarth L., Pereira E.W.M., Oliveira F.S., Menezes I.R.A., Coutinho H.D.M., Picot L., Antoniolli A.R., Quintans J.S.S., Quintans-Júnior L.J. Phytol, a Chlorophyll Component, Produces Antihyperalgesic, Anti-Inflammatory, and Antiarthritic Effects: Possible NFκB Pathway Involvement and Reduced Levels of the Proinflammatory Cytokines TNF-α and IL. J. Nat. Prod. 2020;83:1107–1117. doi: 10.1021/acs.jnatprod.9b01116. [DOI] [PubMed] [Google Scholar]
- 222.Islam M.T., Ayatollahi S.A., Zihad S.M.N.K., Sifat N., Khan M.R., Paul A., Salehi B., Islam T., Mubarak M.S., Martins N. Phytol Anti-Inflammatory Activity: Pre-Clinical Assessment and Possible Mechanism of Action Elucidation. Cell. Mol. Biol. 2020;66:264–269. doi: 10.14715/cmb/2020.66.4.31. [DOI] [PubMed] [Google Scholar]
- 223.Santos C.C.D.M.P., Salvadori M.S., Mota V.G., Costa L.M., de Almeida A.A.C., de Oliveira G.A.L., Costa J.P., de Sousa D.P., de Freitas R.M., de Almeida R.N. Antinociceptive and Antioxidant Activities of Phytol in Vivo and in Vitro Models. Neurosci. J. 2013;2013:949452. doi: 10.1155/2013/949452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tang X., Liu J., Dong W., Li P., Li L., Lin C., Zheng Y., Hou J., Li D. The Cardioprotective Effects of Citric Acid and L-Malic Acid on Myocardial Ischemia/Reperfusion Injury. Evid. Based Complement. Altern. Med. 2013;2013:820695. doi: 10.1155/2013/820695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yang X., Kang M.-C., Lee K.-W., Kang S.-M., Lee W.-W., Jeon Y.-J. Antioxidant Activity and Cell Protective Effect of Loliolide Isolated from Sargassum Ringgoldianum Subsp. Coreanum. Algae. 2011;26:201–208. doi: 10.4490/algae.2011.26.2.201. [DOI] [Google Scholar]
- 226.Silva J., Alves C., Martins A., Susano P., Simões M., Guedes M., Rehfeldt S., Pinteus S., Gaspar H., Rodrigues A. Loliolide, a New Therapeutic Option for Neurological Diseases? In Vitro Neuroprotective and Anti-Inflammatory Activities of a Monoterpenoid Lactone Isolated from Codium Tomentosum. Int. J. Mol. Sci. 2021;22:1888. doi: 10.3390/ijms22041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jayawardena T.U., Kim H.-S., Sanjeewa K.K.A., Han E.J., Jee Y., Ahn G., Rho J.-R., Jeon Y.-J. Loliolide, Isolated from Sargassum Horneri; Abate LPS-Induced Inflammation via TLR Mediated NF-ΚB, MAPK Pathways in Macrophages. Algal Res. 2021;56:102297. doi: 10.1016/j.algal.2021.102297. [DOI] [Google Scholar]
- 228.Banudevi S., Krishnamoorthy G., Venkataraman P., Vignesh C., Aruldhas M.M., Arunakaran J. Role of α-Tocopherol on Antioxidant Status in Liver, Lung and Kidney of PCB Exposed Male Albino Rats. Food Chem. Toxicol. 2006;44:2040–2046. doi: 10.1016/j.fct.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 229.Sharma R., Vinayak M. α-Tocopherol Attenuates NF-ΚB Activation and pro-Inflammatory Cytokine IL-6 Secretion in Cancer-Bearing Mice. Biosci. Rep. 2011;31:421–428. doi: 10.1042/BSR20100137. [DOI] [PubMed] [Google Scholar]
- 230.Wang Y., Park N.-Y., Jang Y., Ma A., Jiang Q. Vitamin E γ-Tocotrienol Inhibits Cytokine-Stimulated NF-ΚB Activation by Induction of Anti-Inflammatory A20 via Stress Adaptive Response Due to Modulation of Sphingolipids. J. Immunol. 2015;195:126–133. doi: 10.4049/jimmunol.1403149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Matsunaga T., Shoji A., Gu N., Joo E., Li S., Adachi T., Yamazaki H., Yasuda K., Kondoh T., Tsuda K. γ-Tocotrienol Attenuates TNF-α-Induced Changes in Secretion and Gene Expression of MCP-1, IL-6 and Adiponectin in 3T3-L1 Adipocytes. Mol. Med. Rep. 2012;5:905–909. doi: 10.3892/mmr.2012.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Radhakrishnan A., Tudawe D., Chakravarthi S., Chiew G.S., Haleagrahara N. Effect of Γ tocotrienol in Counteracting Oxidative Stress and Joint Damage in Collagen induced Arthritis in Rats. Exp. Ther. Med. 2014;7:1408–1414. doi: 10.3892/etm.2014.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Qureshi A.A., Tan X., Reis J.C., Badr M.Z., Papasian C.J., Morrison D.C., Qureshi N. Inhibition of Nitric Oxide in LPS-Stimulated Macrophages of Young and Senescent Mice by δ-Tocotrienol and Quercetin. Lipids Health Dis. 2011;10:1–22. doi: 10.1186/1476-511X-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zarubina I.V., Lukk M.V., Shabanov P.D. Antihypoxic and Antioxidant Effects of Exogenous Succinic Acid and Aminothiol Succinate-Containing Antihypoxants. Bull. Exp. Biol. Med. 2012;153:336–339. doi: 10.1007/s10517-012-1709-5. [DOI] [PubMed] [Google Scholar]
- 235.Bhardwaj M., Sali V.K., Mani S., Vasanthi H.R. Neophytadiene from Turbinaria Ornata Suppresses LPS-Induced Inflammatory Response in RAW 264.7 Macrophages and Sprague Dawley Rats. Inflammation. 2020;43:937–950. doi: 10.1007/s10753-020-01179-z. [DOI] [PubMed] [Google Scholar]
- 236.Prabhu S., Jainu M., Sabitha K.E., Devi C.S.S. Role of Mangiferin on Biochemical Alterations and Antioxidant Status in Isoproterenol-Induced Myocardial Infarction in Rats. J. Ethnopharmacol. 2006;107:126–133. doi: 10.1016/j.jep.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 237.He L., Peng X., Zhu J., Chen X., Liu H., Tang C., Dong Z., Liu F., Peng Y. Mangiferin Attenuate Sepsis-Induced Acute Kidney Injury via Antioxidant and Anti-Inflammatory Effects. Am. J. Nephrol. 2014;40:441–450. doi: 10.1159/000369220. [DOI] [PubMed] [Google Scholar]
- 238.Gong X., Zhang L., Jiang R., Ye M., Yin X., Wan J. Anti-Inflammatory Effects of Mangiferin on Sepsis-Induced Lung Injury in Mice via up-Regulation of Heme Oxygenase. J. Nutr. Biochem. 2013;24:1173–1181. doi: 10.1016/j.jnutbio.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 239.Gowder S.J.T., Devaraj H. Effect of the Food Flavour Cinnamaldehyde on the Antioxidant Status of Rat Kidney. Basic Clin. Pharm. Toxicol. 2006;99:379–382. doi: 10.1111/j.1742-7843.2006.pto_560.x. [DOI] [PubMed] [Google Scholar]
- 240.Mateen S., Rehman M.T., Shahzad S., Naeem S.S., Faizy A.F., Khan A.Q., Khan M.S., Husain F.M., Moin S. Anti-Oxidant and Anti-Inflammatory Effects of Cinnamaldehyde and Eugenol on Mononuclear Cells of Rheumatoid Arthritis Patients. Eur. J. Pharmacol. 2019;852:14–24. doi: 10.1016/j.ejphar.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 241.Ni Y.-L., Shen H.-T., Su C.-H., Chen W.-Y., Huang-Liu R., Chen C.-J., Chen S.-P., Kuan Y.-H. Nerolidol Suppresses the Inflammatory Response during Lipopolysaccharide-Induced Acute Lung Injury via the Modulation of Antioxidant Enzymes and the AMPK/Nrf-2/HO-1 Pathway. Oxidative Med. Cell. Longev. 2019;2019:9605980. doi: 10.1155/2019/9605980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wen Q., Lau N., Weng H., Ye P., Du S., Li C., Lv J., Li H. Chrysophanol Exerts Anti-Inflammatory Activity by Targeting Histone Deacetylase 3 through the High Mobility Group Protein 1-Nuclear Transcription Factor-Kappa B Signaling Pathway in Vivo and in Vitro. Front. Bioeng. Biotechnol. 2021;8:623866. doi: 10.3389/fbioe.2020.623866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lian Y., Xia X., Zhao H., Zhu Y. The Potential of Chrysophanol in Protecting against High Fat-Induced Cardiac Injury through Nrf2-Regulated Anti-Inflammation, Anti-Oxidant and Anti-Fibrosis in Nrf2 Knockout Mice. Biomed. Pharmacother. 2017;93:1175–1189. doi: 10.1016/j.biopha.2017.05.148. [DOI] [PubMed] [Google Scholar]
- 244.Sivakumar D., Sultanbawa Y., Cooperstone J.L., Ziv C. Editorial: Phytochemical changes in vegetables during post-harvest storage and processing, and implications for consumer benefits. Front. Nutr. 2022;9:1025361. doi: 10.3389/fnut.2022.1025361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Xu D.P., Li Y., Meng X., Zhou T., Zhou Y., Zheng J., Li H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017;18:96. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Dey P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019;147:104367. doi: 10.1016/j.phrs.2019.104367. [DOI] [PubMed] [Google Scholar]
- 247.Chen F., Wen Q., Jiang J., Li H.L., Tan Y.F., Li Y.H., Zeng N.K. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? J. Ethnopharmacol. 2016;179:253–264. doi: 10.1016/j.jep.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 248.Saeedi B.J., Liu K.H., Owens J.A., Hunter-Chang S., Camacho M.C., Eboka R.U., Neish A.S. Gut-resident lactobacilli activate hepatic Nrf2 and protect against oxidative liver injury. Cell Metab. 2020;31:956–968. doi: 10.1016/j.cmet.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Aboulgheit A., Karbasiafshar C., Zhang Z., Sabra M., Shi G., Tucker A., Sellke F.W. Lactobacillus plantarum probiotic induces Nrf2-mediated antioxidant signaling and eNOS expression resulting in improvement of myocardial diastolic function. Am. J. Physiol. Heart Circ. Physiol. 2021;321:H839–H849. doi: 10.1152/ajpheart.00278.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lu X., Li J., Ma Y., Khan I., Yang Y., Li Y., Zhang C. Fermented Angelica sinensis activates Nrf2 signaling and modulates the gut microbiota composition and metabolism to attenuate d-gal induced liver aging. Food Funct. 2023;14:215–230. doi: 10.1039/D2FO01637K. [DOI] [PubMed] [Google Scholar]
- 251.Martucci M.E.P., De Vos R.C., Carollo C.A., Gobbo-Neto L. Metabolomics as a potential chemotaxonomical tool: Application in the genus Vernonia Schreb. PLoS ONE. 2014;9:e93149. doi: 10.1371/journal.pone.0093149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zoete V., Daina A., Bovigny C., Michielin O. SwissSimilarity: A web tool for low to ultra-high throughput ligand-based virtual screening. J. Chem. Inf. Model. 2016;56:1399–1404. doi: 10.1021/acs.jcim.6b00174. [DOI] [PubMed] [Google Scholar]
- 253.Leonti M., Casu L. Traditional medicines and globalization: Current and future perspectives in ethnopharmacology. Front. Pharmacol. 2013;4:92. doi: 10.3389/fphar.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is contained and referenced within the article.