Abstract
Colorectal cancer (CRC) is the third most diagnosed malignancy and a major leading cause of cancer-related deaths worldwide. Despite advances in therapeutic regimens, the number of patients presenting with metastatic CRC (mCRC) is increasing due to resistance to therapy, conferred by a small population of cancer cells, known as cancer stem cells. Targeted therapies have been highly successful in prolonging the overall survival of patients with mCRC. Agents are being developed to target key molecules involved in drug-resistance and metastasis of CRC, and these include vascular endothelial growth factor, epidermal growth factor receptor, human epidermal growth factor receptor-2, mitogen-activated extracellular signal-regulated kinase, in addition to immune checkpoints. Currently, there are several ongoing clinical trials of newly developed targeted agents, which have shown considerable clinical efficacy and have improved the prognosis of patients who do not benefit from conventional chemotherapy. In this review, we highlight recent developments in the use of existing and novel targeted agents against drug-resistant CRC and mCRC. Furthermore, we discuss limitations and challenges associated with targeted therapy and strategies to combat intrinsic and acquired resistance to these therapies, in addition to the importance of implementing better preclinical models and the application of personalized therapy based on predictive biomarkers for treatment selection.
Keywords: Colorectal cancer, Metastatic colorectal cancer, Targeted therapy, Drug-resistance, Personalized medicine
Core Tip: Efforts in cancer research has yielded significant advances in our understanding of the molecular mechanisms underlying colorectal cancer (CRC) resistance and metastasis. Therapeutic strategies centered on targeted molecules involved in CRC progression have been shown to be highly promising in overcoming resistance to conventional treatments. Targeted agents are currently being evaluated in preclinical and clinical studies to identify novel pharmacological targets and to study the efficacy of personalized medicine-based approaches.
INTRODUCTION
Colorectal cancer (CRC) is among the most prevalent malignancies in the world and the third most frequent cause of cancer-related death in the US and Europe[1,2]. Estimates from the American Cancer Society indicate that over 100000 new cases of CRC will be diagnosed in 2022 in the US and 53000 deaths will result from CRC in the same year. In addition to the increased incidence of CRC, the number of patients presenting with advanced, metastatic CRC (mCRC) is increasing[3]. In fact, it has been estimated that 25% of CRC patients have mCRC at the time of diagnosis and 50% of patients subsequently develop mCRC[4].
Lifestyle factors are thought to be a major factor in the increased incidence of CRC, and they include unhealthy diet, lack of physical activity, smoking, and alcohol consumption[3]. Other factors include heredity and family history which contribute to 30% of cases and genetic mutations and variations which contribute to 10% of cases[3]. It is important for health care providers and individuals to understand the causes and risk factors of CRC, in addition to the prevention strategies that could reduce the incidence. Screening can reduce CRC incidence and death through early detection and treatment of disease[3]. Colonoscopy is the standard screening method for CRC[5]. Other imaging-based tests are also available and include computed tomography colonography, colon capsule, and flexible sigmoidoscopy. In addition, screening modalities include stool-based tests, such as fecal immunochemical testing and the multitarget stool DNA test[5].
Conventional therapy for CRC includes surgery, chemotherapy, and radiotherapy[6]. 5-fluorouracil (5-FU) is the standard treatment for mCRC. It is now being combined with other chemotherapeutic drugs to improve patient survival. 5-FU, leucovorin, and irinotecan (FOLFIRI), 5-FU, leucovorin, oxaliplatin, and irinotecan, and 5-FU, oxaliplatin, and leucovorin (FOLFOX4) have been used as multidrug chemotherapy regimens. Although these treatment strategies have improved overall survival (OS), intrinsic and acquired resistance has been a major limitation in the effectiveness of these treatments in 90% of patients with mCRC[6]. Innate resistance is usually noted during early treatment or in early clinical trials. Acquired resistance may occur through different molecular mechanisms, and is specific to each therapy; however, acquired resistance to one drug sometimes results in resistance to other drugs with the same or different mechanism of action. This is known as multidrug resistance and is responsible for multiple cross-resistance towards different drugs[7].
Chemotherapy targets rapidly dividing cells by blocking DNA replication or tubulin assembly, and thus is not specific to cancer cells and is associated with toxicity to healthy tissues[8]. In the last 15 years, major attempts have been made to develop targeted or biological therapies that kill cancer cells by targeting specific pathways implicated in tumor growth. Targeted therapies against cancer cells include mainly monoclonal antibodies (mAbs) that bind membrane growth factor receptors or their ligands, and small molecules that cross the cell membrane and inhibit cell growth and survival[9].
With the development and advancement of next generation sequencing (NGS) and omics technologies[10], it has been possible to determine molecular mechanisms underlying resistance and to develop new strategies to overcome this resistance. Over the past decade, new discoveries in the field of CRC led to the introduction of targeted therapies in clinical practice, which resulted in significant therapeutic efficacy and prolonged survival. New drugs whose action is directed at specific pathways implicated in CRC pathogenesis, including the epidermal growth factor receptor (EGFR) pathway, have been tested in preclinical models and in clinical trials. Yet, the best combination of standard chemotherapy and targeted therapy for the first-line treatment of mCRC has been debated for several years.
Understanding the mechanisms of acquired drug resistance to targeted therapies is critical for the development of novel and effective treatment combinations and will help guide future therapies. In this article, we review mechanisms of resistance to conventional therapy, we discuss the efficacy of novel targeted therapies against drug-resistant and mCRC and challenges associated with them, in addition to strategies to overcome resistance to targeted therapy. We conclude by highlighting lessons learned from molecular studies and their clinical relevance, as well as the importance of employing novel preclinical models to facilitate the development of effective targeted therapy.
RESISTANCE TO THERAPY
Resistance to conventional treatment is one of the most challenging problems in cancer therapy, resulting in poor prognosis, recurrence, and metastasis. It is attributed to several intrinsic and acquired factors in tumor cells and in the microenvironment they reside in.
Cancer stem cells
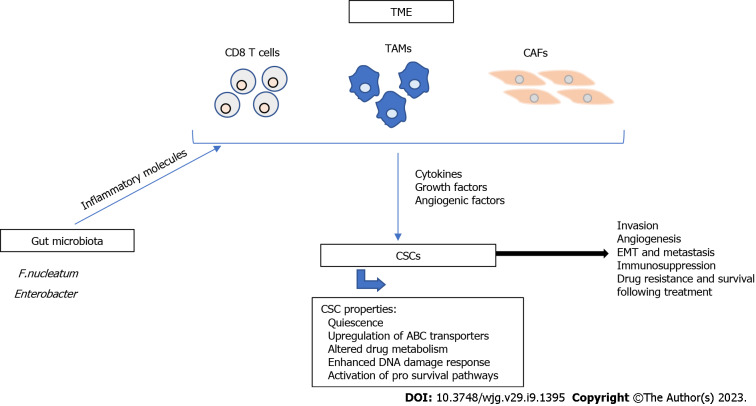
CRC treatment requires surgical intervention, which is accompanied by the application of chemotherapy or radiotherapy, before or after surgery, as neoadjuvant or adjuvant treatment to ensure maximum reduction of tumor size[11]. These treatments are effective against cancer cells but spare the more resistant cancer stem cells (CSCs). Mechanisms of resistance are still unclear, but several factors are known to contribute to it. For example, CSCs are quiescent and do not enter the cell cycle, therefore they are not targeted by conventional therapy that kills highly proliferating cells[12]. Different molecular mechanisms are involved in CRC drug-resistance[13], as shown in Figure 1, and are summarized in this paper.
Figure 1.
Major mechanisms of cancer stem cell resistance. Cancer stem cell (CSC) resistance has been associated with CSC characteristics including quiescence, upregulation of ATP-binding cassette transporters, altered drug metabolism, enhanced DNA damage response, and activation of pro survival pathways. The tumor microenvironment (TME) plays a major role in the resistance of CSCs to therapy. CD8 T cells, tumor associated macrophages, and cancer associated fibroblasts (CAFs) are major components of the TME and contribute to tumor progression and metastasis through the secretion of cytokines, growth factors, and angiogenic factors. Additionally, gut microbiota, such as Fusobacterium nucleatum and Enterobacter secrete inflammatory molecules that modulate the TME and contribute to therapy resistance. All of these mechanisms contribute to tumor invasion, angiogenesis, epithelial-to-mesenchymal transition, immunosuppression, drug resistance and survival following treatment. TAMs: Tumor associated macrophages; CAFs: Cancer associated fibroblasts; EMT: Epithelial-to-mesenchymal transition.
CSCs express high levels of ATP-binding cassette (ABC) transporters that mediate drug efflux and resistance to chemotherapy[14,15]. The first identified ABC member is ABCB1 or P-glycoprotein, which is expressed in normal intestinal cells. The overexpression of ABCB1 has been reported in preclinical and clinical studies of CRC and is associated with resistance to chemotherapy[16,17]. First-, second-, and third-generation inhibitors have been designed against ABCB1 and have been shown to have high affinity; however, their effectiveness is limited and needs further improvement[18]. Other ABC members include ABCC6, ABCC11, ABCF1, and ABCF2 and their upregulation has been documented in CRC tumor tissues[19], suggesting that these transporters may serve as potential targets for reversing drug-resistance in CRC.
The anti-cancer effect of chemotherapeutic drugs can be reduced by impaired drug metabolism. Capecitabine is a chemotherapeutic agent used for the treatment of mCRC. Upon administration, it is converted into 5-FU by thymidine phosphorylase (TP)[20]. It has been shown that methylation of the gene encoding TP inhibits its translation and results in resistance to capecitabine[20]. 5-FU acts by inhibiting thymidylate synthase and incorporating its metabolites into DNA and RNA[21]. Several enzymes, such as orotate phosphoribosyl-transferase and uridine monophosphate synthetase, mediate the conversion of 5-FU into its active metabolites[22]. Interestingly, lower expression of these enzymes is associated with resistance to 5-FU in CRC[23]. Additionally, TP converts 5-FU into 5-fluoro-2’ deoxyuridine and it has been shown to predict good response to 5-FU treatment and is associated with higher progression-free survival (PFS) in patients with high expression of TP[24]. Another enzyme that has been reported to affect response to chemotherapy is carboxylesterase 2. This metabolic enzyme plays a major role in the activation of irinotecan and its high expression and activity improves the efficacy of irinotecan[25]. On the other hand, uridine diphosphate glucuronosyltransferase 1A1 and β-glucuronidase inactivate irinotecan, and their alteration results in reduced irinotecan activity, suggesting that targeting these enzymes may reverse resistance to irinotecan[26,27]. Similarly, dihydropyrimidine dehydrogenase is a metabolic enzyme that mediates the catabolism of 5-FU to its inactive metabolite, and its high expression has been associated with resistance to 5-FU in CRC[28,29].
In cancers, including CRC, the DNA damage response (DDR) is activated and aberrant. This damage response consists of several kinase-dependent signaling pathways and is important for maintaining genome integrity and stability. Damage sensing is usually mediated by DDR sensors, followed by transduction of damage signals to DDR mediators and downstream molecules, leading to either cell cycle arrest, DNA damage repair, or apoptosis[30]. Ataxia telangiectasia mutated and ATM and Rad3-related protein, members of the phosphatidyl-inositol 3-kinase (PI3K) like family of protein kinases, are the main regulators of DDR. They interact with p53 and checkpoint pathways that regulate Cdc25[31]. Several mechanisms attribute to resistance of CSCs to DNA damage and include cell cycle checkpoint alteration and activation of an efficient scavenging system that protects against reactive oxygen species (ROS), which are induced by therapy[32]. Three main pathways that contribute to CRC development are unsensed or repaired by the aberrant DDR. These pathways are chromosomal instability (CIN), CpG island hypermethylation phenotype, and microsatellite instability (MSI) pathways. CIN is common in 80% of CRC cases while MSI results from inactivation of mismatch repair genes (MMR) and is common in sporadic CRC[33]. Notably, DNA repair induced by oxaliplatin is mainly mediated by the nucleotide excision repair pathway[34]. The upregulation of excision repair cross-complementing 1 has been linked to oxaliplatin resistance in CRC[34] and its mRNA expression level is a predictive marker of survival in patients treated with 5-FU and oxaliplatin[35]. These results suggest that the expression levels of DNA repair proteins may serve as treatment response biomarkers, and the reduction of their expression can enhance the effect of DNA-damaging agents, leading to eradication of resistant CSCs.
Intrinsic and acquired resistance to apoptosis is one of the characteristics of CSCs. Apoptosis is regulated by a balance between pro-apoptotic, anti-apoptotic, and pro-survival mechanisms[36], which is frequently altered in cancer, including CRC[34,37]. p53 plays a key role in the induction of apoptosis in response to DNA damage by chemotherapy[34]. However, p53 is mutated in 85% of CRC cases and is linked to resistance to 5-FU and oxaliplatin[38]. In addition, the expression of high levels of anti-apoptotic proteins, including Bcl-2 family members, is a characteristic of CSCs and results in resistance to cell death by apoptosis[39]. Frameshift mutations in the BAX gene result in the loss of expression and activity of the anti-apoptotic protein BAX, leading to chemoresistance[34]. Other anti-apoptotic proteins that are implicated in chemoresistance include Bcl-XL and the FLICE-inhibitory protein[40].
Moreover, several pro survival signaling pathways are activated in CRC. One major pathway is the Wnt/β-catenin pathway, which is important for stemness and resistance. Binding of Wnt ligand to the Frizzled receptor results in activation of β catenin, a key effector in this pathway[41]. Activation of the Wnt pathway induces proliferation and differentiation of CSCs, which is partly mediated by activation of several molecules that are recognized as putative CSC markers and include Lgr5, CD44, CD133, and Epcam[42]. All of these markers are associated with CSC resistance to chemo- and radiotherapy. Other pathways that are involved in stemness include the Notch and Hedgehog pathways[42].
Tumor microenvironment
CRC resistance has been also linked to the tumor microenvironment (TME) that is also involved in the multistep process that encompasses the development of adenomatous polyps from normal colonic epithelium, finally leading to invasive CRC[43,44]. The TME consists mainly of immune cells, endothelial cells, stromal cells, extracellular matrix (ECM), and signaling molecules[45]. Solid tumors, including CRC are infiltrated by different cells, such as dendritic cells, monocytes, neutrophils, CD8 and CD4 T cells, cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), and mesenchymal stem cells. During tumor formation, interactions between tumor and stromal cells and secretion of soluble inflammatory molecules mediate the attraction of immune cells that promote tumor cell survival and metastasis[45,46]. The most important tumor-promoting cells are TAMs and CAFs. These cells facilitate tumor progression through direct contact with other cells or through secretion of cytokines, growth factors, and angiogenic factors, thereby promoting ECM formation, tumor invasion, angiogenesis, epithelial-to-mesenchymal transition (EMT), and immunosuppression[43,45].
Gut microbiota
Strong evidence is emerging to support the role of gut microbiota in the progression and resistance of CRC and interventions made in this regard may hold promises for improving CRC treatment[47]. Fusobacterium nucleatum has been shown to contribute to CRC chemoresistance through activation of innate immune signals that stimulate the autophagy pathway[48]. The use of antibiotics can increase pathogenic bacteria such as Enterobacter and has been shown to reduce the anti-cancer effect of oxaliplatin through modulation of cytokine secretion and ROS production in the TME[49]. On the other hand, the effect of immunotherapy has been shown to be enhanced by intestinal microbiota, such as Faecalibacterium, Clostridiales, and Bifidobacterium spp[50,51]. The exact mechanism of action is still unclear but has been attributed to direct interactions between these bacteria and immune cells[52], in addition to a possible role for microbial metabolites, such as butyrate and propionate[53].
TARGETED THERAPY
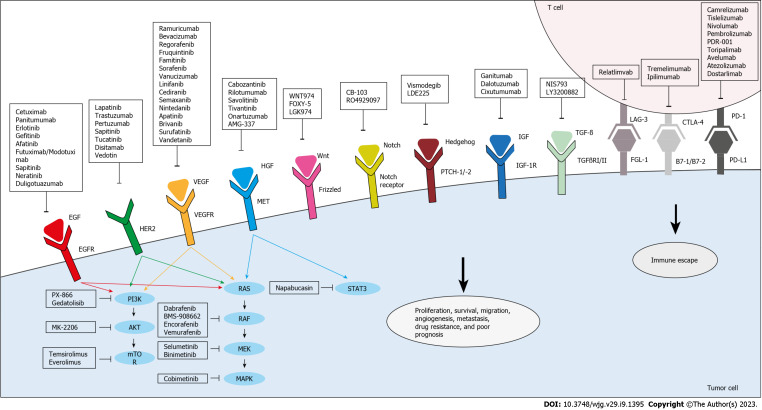
Targeted agents can directly inhibit the proliferation and migration of cancer cells (Figure 2). They could also target the TME, thereby limiting tumor growth and enhancing immune surveillance. Small molecules play a major role in such treatments, as they can penetrate cells to selectively inactivate specific enzymes involved in tumor proliferation induction and apoptosis inhibition[54].
Figure 2.
Targeted therapies under investigation for the treatment of drug-resistant and metastatic colorectal cancer. Anti-epidermal growth factor receptor, anti-vascular endothelial growth factor/vascular endothelial growth factor receptor, and anti-human epidermal growth factor receptor 2 agents inhibit their respective targets and thus, the downstream effector pathways, PI3K/Akt and RAS/RAF. Other agents directly target and inhibit PI3K, AKT, mammalian target of rapamycin, RAF, mitogen-activated extracellular signal-regulated kinase, or mitogen-activated protein kinases. In addition, anti-hepatocyte growth factor/mesenchymal epithelial transition factor receptor agents target this pathway to inhibit signal transducer and activator of transcription, which is also targeted by Napabucasin. Several novel agents that are aimed at other pathways implicated in colorectal cancer proliferation, survival, resistance, and metastasis are also being evaluated. Targeted pathways include Wnt, Notch, Hedgehog, insulin growth factor/insulin growth factor receptor-1, and transforming growth factor beta. Moreover, immune escape can be hindered through immunotherapy which targets co-inhibitory molecules, mainly programmed death-1/programmed death ligand-1, cytotoxic T lymphocyte-associated antigen 4, and lymphocyte activation gene 3. EFGR: Epidermal growth factor receptor; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; HER2: Human epidermal growth factor receptor 2; mTOR: Mammalian target of rapamycin; MEK: Mitogen-activated extracellular signal-regulated kinase; MAPK: Mitogen-activated protein kinases; HGF: Hepatocyte growth factor; MET: Mesenchymal epithelial transition factor receptor; STAT3: Signal transducer and activator of transcription; CRC: Colorectal cancer; IGF: Insulin growth factor; IGF-1R: Insulin growth factor receptor-1; TGF-β: Transforming growth factor beta; PD-1: Programmed death-1; PD-L1: Programmed death ligand-1; CTLA-4: Cytotoxic T lymphocyte-associated antigen 4; LAG-3: Lymphocyte activation gene 3; EGF: Epidermal growth factor; TGFβRI/II: Transforming growth factor-Beta type I/II.
Targeting EGFR
EGFR belongs to the ErbB family of receptor tyrosine kinases and is involved in cellular proliferation, survival, migration, adhesion, and angiogenesis[55,56]. 80% of CRCs express or upregulate the EGFR gene[57,58], and this expression is associated with a risk of metastasis[59], therefore inhibiting EGFR could be a possible strategy to reduce cellular proliferation.
EGFR activation can be blocked by mAbs or tyrosine kinase inhibitors (TKIs). EGFR mAbs include cetuximab and panitumumab, which are currently used in parallel with FOLFOX or FOLFIRI regimens in the treatment of patients with KRAS or NRAS wild-type (WT) tumors[60]. In RAS-mutant tumors, constitutive activation of signaling pathways downstream of EGFR limits the effectiveness of EGFR inhibitors[61].
Cetuximab is a chimeric murine human IgG1 mAb that binds to the extracellular domain of EGFR and inhibits its pro-oncogenic action in cancer cells[62,63] (Table 1 and Figure 2). It also binds to natural killer cells and induces antibody-dependent cell-mediated cytotoxicity[62]. In a study that involved patients with advanced CRC after treatment with irinotecan, treatment with cetuximab alone or in combination with irinotecan showed significant clinical activity, with an enhanced rate of response and median survival time in the combination groups[64]. Combining cetuximab with FOLFIRI reduced the risk of progression of mCRC by 15% in first-line treatment of patients with KRAS WT tumors, when compared to FOLFIRI alone[65]. Complete or partial tumor responses were observed in 46.9% of patients treated with combination therapy and in 38.7% of patients treated with FOLFIRI alone[65]. Another treatment regimen that was tested in the first-line treatment of mCRC included FOLFOX4 and cetuximab[66]. Results from this randomized study showed an increased chance of response and lower risk of disease progression in the combination-treated group when compared to FOLFOX4 alone in KRAS WT patients[66]. A more recent randomized phase 3 Medical Research Council COIN trial showed that adding cetuximab to oxaliplatin-based chemotherapy increased the response rate in patients with advanced CRC; yet no enhancement of PFS or OS was shown[67].
Table 1.
Agents targeting epidermal growth factor receptors and downstream molecules under clinical investigation for the treatment of drug-resistant and metastatic colorectal cancer
|
Agent
|
Targeted molecule
|
Condition
|
Study phase
|
Clinical trial identifier
|
| Erlotinib | EGFR | First-line treatment for mCRC | Phase III | NCT01229813 |
| Futuximab/Modotuximab (Sym-004) | EGFR | mCRC | Phase II | NCT02083653 |
| Gefitinib | EGFR | Refractory CRC | Phase I/II | NCT00242788 |
| Afatinib | EGFR | Refractory mCRC | Phase II | NCT01919879 |
| Advanced CRC | Phase II | NCT00801294 | ||
| mCRC | Phase II | NCT01152437 | ||
| Dabrafenib (GSK2118436) | BRAF | mCRC | Phase II | NCT03668431 |
| mCRC | Phase II | NCT03428126 | ||
| BMS-908662 | BRAF | K-RAS/BRAF-mutated CRC | Phase I/II | NCT01086267 |
| Encorafenib | Wild-type and BRAF V600E | Previously untreated BRAF-mutant mCRC | Phase II | NCT03693170 |
| Vemurafenib | Mutated BRAF V600E | BRAF V600E mutated advanced CRC | Phase II | NCT03727763 |
| PX-866 | PI3K | mCRC | Phase I/II | NCT01252628 |
| Gedatolisib | PI3K/mTOR | KRAS/NRAS-wild-type mCRC | Phase II | NCT01925274 |
| mCRC | Phase I/II | NCT01937715 | ||
| Temsirolimus CCI-770 | mTOR | KRAS-mutated mCRC | Phase II | NCT00827684 |
| Cetuximab-refractory CRC | Phase I | NCT00593060 | ||
| Everolimus (RAD001) | mTOR | mCRC | Phase II | NCT01387880 |
| mCRC | Phase I/II | NCT01058655 | ||
| Advanced mCRC | Phase I/II | NCT01139138 | ||
| Refractory mCRC | Phase I | NCT01154335 | ||
| MK-2206 | AKT | Advanced CRC | Phase II | NCT01333475 |
| Napabucasin (BBI608) | STAT3 | Previously treated mCRC | Phase III | NCT03522649 |
| Cobimetinib | MAPK | mCRC | Phase III | NCT02788279 |
| Selumetinib | MEK | mCRC | Phase II | NCT00514761 |
| Binimetinib | MEK | Previously untreated BRAF-mutant mCRC | Phase II | NCT03693170 |
| Neratinib | EGFR/HER2/4 | KRAS/NRAS/BRAF/PIK3CA-wild-type mCRC | Phase II | NCT03457896 |
| Sapitinib (AZD-8931) | EGFR/HER2/3 | mCRC | Phase II | NCT01862003 |
| Duligotuzumab (MEHD7945A) | EGFR/HER3 | KRAS-mutated mCRC | Phase II | NCT01652482 |
| Trastuzumab | HER2 | First-line HER2-positive mCRC | Phase III | NCT05253651 |
| Tucatinib | HER2 | First-line HER2-positive mCRC | Phase III | NCT05253651 |
| Disitamab Vedotin | HER2 | HER2-positive advanced CRC | Phase II | NCT05493683 |
| HER2-expressing mCRC | Phase II | NCT05333809 | ||
| Trastuzumab-emtansine | HER2 | HER2-positive mCRC progressing after trastuzumab and lapatinib | Phase II | NCT03418558 |
EGFR: Epidermal growth factor receptor; mCRC: Metastatic colorectal cancer; PI3K: Phosphoinositide 3-kinases; mTOR: Mammalian target of rapamycin; STAT3: Signal transducer and activator of transcription 3; AKT: Protein kinase B; MAPK: Mitogen-activated protein kinases; MEK: Mitogen-activated extracellular signal-regulated kinase; HER2: Human epidermal growth factor receptor 2.
Similar to cetuximab, treatment with panitumumab alone or in combination with standard chemotherapy has shown promising results in several clinical trials[60,68]. Panitumumab monotherapy was effective in CRC patients with KRAS WT tumors, with a response rate of 17%[69]. In an open-label phase III trial that involved patients with chemotherapy-refractory mCRC, panitumumab plus best supportive care (BSC) significantly prolonged PFS when compared to BSC alone. Response rates were 10% for panitumumab and 0% for BSC, with no difference observed in OS[70]. Several clinical trials were conducted to compare the efficacy of panitumumab and FOLFOX4 in comparison to FOLFOX4 alone[60,68]. Results from a phase III trial showed that combination treatment significantly improved PFS whereas the increase in OS was insignificant when compared to FOLFOX4 alone in KRAS WT tumors[60]. Except for the toxicities that are usually associated with EGFR inhibitors, adverse event rates were comparable between these treatments[60]. The very recent PARLIM trial showed that PFS and OS were improved upon the addition of panitumumab to FOLFOX in KRAS WT CRC patients with R0/1-resected liver metastases. Importantly, no new adverse events were observed in the combination-treated group[71].
The most common side effects observed in trials of these EGFR mAbs were skin toxicity, abdominal pain, nausea, diarrhea, infusion reactions, fatigue, and hypomagnesemia. Rare adverse events included pulmonary fibrosis, sepsis, severe skin toxicity, and anaphylaxis[72].
EGFR TKIs are small molecules derived from quinazolines that block the tyrosine kinase domain of different receptors, including EGFR. Erlotinib is specific to EGFR alone and is used to block ligand-induced EGFR receptor phosphorylation[73]. Gefitinib is another EGFR TKI that has a similar mechanism of action to erlotinib, but also targets other pathways, such as the extracellular signal-related kinases 1/2 (ERK1/2) pathway in mesothelioma cell lines[73].
It is important to note that studies investigating the efficacy of EGFR targeted therapy vary widely in clinical context, and some focus on the effect of EGFR monotherapy while others compare it to a combination of various chemotherapy regimens. One important factor to be taken into consideration is KRAS status, which could be used as a biomarker to predict the effectiveness of a treatment. Several inhibitors targeting EGFR or downstream molecules are currently under clinical investigation and are summarized in Table 1.
Targeting HER
Human EGFR 2 (HER2) is emerging as a key driver in CRC. It acts similar to EGFR, as they both share common downstream pathways, such as RAS/RAF/MEK and PI3K/AKT, which explains the link between HER2 overexpression and resistance to EGFR inhibitors[74,75]. The HER2/neu oncogene encodes a receptor with intrinsic tyrosine kinase activity[76]. HER2 lacks an endogenous ligand unlike other members of the HER/EGFR/ERBB system[77]. Homodimerization or heterodimerization with other EGFR family receptors, HER3 and EGFR, results in transphosphorylation of tyrosine residues within the cytoplasmic domain of HER2, thus leading to its activation[77,78]. HER2-HER3 heterodimers activate the PI3K/AKT pathway which is implicated in cancer cell growth and survival[79].
Different rates of HER2 amplification have been reported in CRC[80-82], with rates of membranous expression ranging from 2.1% to 11%[80,83,84], and that of cytoplasmic expression ranging from 47.4 to 68.5%[80,85,86]. Several factors may account for this variability, including small sample size, different antibodies used for immunohistochemistry (IHC), and analysis of different subgroups of patients with multiple clinical characteristics[87]. The efficacy of targeted agents against HER2-expressing CRC was determined in several clinical trials. Ramanathan et al[88] reported the detection of HER2/neu overexpression in only 8% of screened tumors in patients with advanced CRC and this low overexpression rate limited the study of irinotecan and trastuzumab, a humanized mAb targeting the HER2/neu receptor, in a phase II clinical study. Yet, partial response was observed in some patients, and the response was maintained for approximately six wk[88]. In a proof-of-concept study that exploited patient-derived xenografts (PDX), HER2 was identified as an effective therapeutic target in cetuximab-resistant mCRC[89]. HER2 amplification was detected in clinically unresponsive KRAS WT patients, and the combination of lapatinib (a dual EGFR/HER2 TKI) and pertuzumab induced an increase in response rate and tumor regression, in agreement with clinical studies in patients with similar clinicopathological characteristics[89]. The synergic antiproliferative effect of HER2 and EGFR blockade was also demonstrated in cetuximab-resistant CRC cell lines[74,90]. Interestingly, HER2 activating mutations were identified in CRC PDX and were shown to be highly sensitive to HER2/EGFR TKIs neratinib and afatinib and resulted in tumor regression when subjected to dual HER2 targeted therapy with trastuzumab plus TKIs[91]. It was also reported that these mutations cause oncogenic transformation of colon epithelial cells and resistance to anti-EGFR monotherapy[91]. Various clinical trials targeting HER2 alterations in combination with chemotherapeutic therapies in patients with mCRC have validated findings from preclinical studies. High toxicity[92] and poor accrual[88,93] were the reasons behind halting earlier clinical studies evaluating the addition of HER2 mAbs (trastuzumab or pertuzumab) to cetuximab or chemotherapy (i.e., irinotecan, 5-FU, and oxaliplatin). In a phase I trial involving patients with HER2-positive refractory tumors, none of the CRC patients responded to the combination of trastuzumab, paclitaxel, and interleukin (IL)-12[94]. More recently, a study that followed the stringent HERACLES criteria reported that the combination of trastuzumab and lapatinib achieved an objective response rate of 30% and was well tolerated in KRAS codon 12/13 WT, HER2-positive mCRC patients[95]. Within the same project, HERACLES-B phase II trial assessed the efficacy of pertuzumab and trastuzumab emtansine; however, it did not reach its primary endpoint of response rate. Yet, this combination can be considered a potential therapeutic strategy for HER2-positive mCRC, based on the high disease control achieved, in addition to the enhanced PFS and low toxicity[96]. The MyPathway trial assessed the combination of pertuzumab and trastuzumab in pretreated HER2-amplified mCRC patients and further supported the efficacy of the dual blockage of HER2[97,98]. Several agents targeting HER and EGFR are currently under clinical investigation (Table 1).
Targeting VEGF
Angiogenesis is the formation of new blood vessels from endothelial cells. It is mediated by vascular endothelial growth factor (VEGF), together with platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF)[99]. Angiogenesis plays an important role in tumor initiation, growth, and metastasis. The VEGF system consists of six ligands and three receptors known as VEGF receptors (VEGFR). VEGF-A is secreted by multiple cell types, including cancer cells, and plays a major role in survival, growth, differentiation, and migration of endothelial cells[100]. VEGF-A mediates its effect by binding to VEGFR2, which is the major signal transducer of angiogenesis and is expressed by endothelial cells. On the other hand, VEGFR1 is a strong VEGF inhibitor[101]. Hypoxia is a key regulator of angiogenesis in cancer through hypoxia-inducible factors, which induce transcription of several genes, including VEGF-A[102].
VEGF levels and VEGFR activity are elevated in patients with CRC and are associated with poor prognosis[103]. The activation of this system is important both in local sites to support tumor progression and in metastatic sites to support neovascularization and tumor survival; therefore, a targeted therapy against VEGF/VEGFR might be developed at all stages of tumor progression and metastasis. Like EGFR, targeted therapy against angiogenesis consists of mAbs and TKIs. mAbs bind to VEGF-A or block the extracellular domain of its receptor. mAbs that bind VEGF-A include bevacizumab and aflibercept, thereby preventing activation of their receptors. Ramucirumab binds to the VEGFR2 extracellular domain, inhibiting the binding of VEGF ligands, thereby inhibiting receptor activation[104].
Bevacizumab as a monotherapy has a limited effect and is therefore used in combination with chemotherapy in first- and later-lines of mCRC treatment[105]. It is the first Food And Drug Administration (FDA)-approved VEGF-targeted agent for mCRC[105]. The first randomized clinical trial showed that bevacizumab improves response rate, PFS, and OS, thereby enhancing chemotherapy efficacy[106]. Combining bevacizumab (5 mg per kg of body weight every two wk) with irinotecan, 5-FU, and leucovorin (IFL) enhanced median duration of survival and PFS, as compared to IFL treatment alone, corresponding to a hazard ratio for death of 0.66 and for disease progression of 0.54, respectively[106]. The results also showed that median duration of the response to combination treatment was 10.4 mo as compared to 7.1 mo in the group treated with IFL and placebo[106]. A major adverse event was grade 3 hypertension which was more common in the group treated with IFL and bevacizumab but was easily managed. More recent trials showed that modern combination regimens were better substitutes for IFL; however, the efficacy of combining bevacizumab with first-line treatment of mCRC has been controversial. Several recent clinical trials demonstrated the promising efficiency of combining bevacizumab with trifluridine/tipiracil, which is usually better tolerated than capecitabine, especially in elderly patients with mCRC[107-109]. Notably, promising results were reported in the phase II TASCO study that assessed the effectiveness of combining bevacizumab with trifluridine/tipiracil as first-line treatment in untreated patients with unresectable mCRC[110]. This combination treatment achieved better median PFS and OS when compared to patients receiving bevacizumab plus capecitabine. On the other hand, Chen et al[111] carried out a meta-analysis that showed no improvement in OS upon the addition of bevacizumab to FOLFOX/FOLFIRI/capecitabine plus oxaliplatin (XELOX) regimens when compared to chemotherapy alone, unless PFS is considered, specifically in capecitabine-based regimens. This exception was established based on two trials, the NO16966 study[112] and ITACA trial[113], which used PFS as an endpoint measurement. These studies showed that adding bevacizumab to oxaliplatin-based therapy (XELOX or FOLFOX4) significantly improved PFS in patients with mCRC[112]. OS and response rate were not changed by the addition of bevacizumab, suggesting that prolonged treatment may be needed for optimal combination efficacy[112]. Interestingly, it has been documented that both patients with KRAS mutations and with WT KRAS may benefit from adding bevacizumab to chemotherapy[114,115]. The efficacy of the second-line application of bevacizumab has also been validated in several trials that showed longer PFS and OS, and a better response rate, compared with standard chemotherapy alone in the E3200 study[116] and III ML18147 trial[117].
The addition of aflibercept to FOLFIRI enhanced the survival of patients progressing who were previously given oxaliplatin-based regimens[118]. Combination treatment resulted in a 9% increase in response rate, accompanied by an improvement in PFS from 4.7 to 6.9 mo and OS from 12.1 to 13.5 mo[118].
Ramucirumab was approved by the FDA for second-line treatment of mCRC based on the phase III RAISE trial[119]. Data from this study showed that the addition of ramucirumab significantly prolonged PFS and OS but not response rate, following first-line treatment with 5-FU, oxaliplatin, and bevacizumab[119].
Few VEGF TKIs have been proven to be effective in patients with mCRC. These include regorafenib, which was approved by FDA for the treatment of mCRC[120]. Yet, regorafenib has multiple targets, other than VEGF, whereby it also inhibits PDGF receptor, FGF receptor, and BRAF[120]. Notably, treatment of mCRC patients with regorafenib was associated with enhanced OS[121]. A more significant OS benefit was observed when combining regorafenib with its major metabolites, M-2 and M-5, in concentrations ranging between 2.5 and 5.5 mg/L[121]. While no improvement in the response rate was shown upon adding regorafenib to FOLFOX in mCRC patients as compared to chemotherapy alone[120], better median OS and PFS were achieved using regorafenib alone than placebo for refractory mCRC treatment in the phase III CORRECT trial[122]. These results were also validated in an Asian population in the CONCUR trial[123]. Anlotinib, a novel TKI that inhibits VEGFR1/2/3, among other kinases, showed an enhanced overall rate response and PFS when combined with capecitabine and oxaliplatin in the first-line treatment of mCRC[124]. Other TKI agents have been developed in the last few years, these include fruquintinib[125] and famitinib[126], in addition to other agents that are under clinical investigation and are summarized in Table 2.
Table 2.
Agents targeting vascular endothelial growth factor/vascular endothelial growth factor receptor under clinical investigation for the treatment of drug-resistant and metastatic colorectal cancer
|
Agent
|
Targeted molecule
|
Condition
|
Study phase
|
Clinical trial identifier
|
| Vanucizumab | VEGF-A/angiopoietin-2 | mCRC | Phase II | NCT02141295 |
| Sorafenib | VEGFR | mCRC | Phase II | NCT03251612 |
| Previously treated mCRC | Phase II | NCT01471353 | ||
| mCRC | Phase II | NCT00826540 | ||
| KRAS-mutated mCRC | Phase II | NCT01715441 | ||
| Bevacizumab | VEGF | Untreated mCRC | Phase II | NCT02141295 |
| Advanced CRC | Phase II | NCT02487992 | ||
| Linifanib ABT-869 | VEGFR | Advanced CRC | Phase II | NCT00707889 |
| Vatalanib | VEGFR | mCRC | Phase III | NCT00056446 |
| mCRC | Phase III | NCT00056459 | ||
| Famitinib | VEGFR2/3 | Advanced CRC | Phase II | NCT01762293 |
| Cediranib | VEGFR2 | First-line mCRC | Phase III | NCT00399035 |
| Semaxanib | VEGFR | mCRC | Phase III | NCT00004252 |
| Advanced CRC | Phase I/II | NCT00005818 | ||
| Nintedanib | VEGFR | Refractory mCRC | Phase III | NCT02149108 |
| Ramucirumab | VEGFR2 | Chemotherapy refractory mCRC | Phase III | NCT03520946 |
| Apatinib | VEGFR2 | Refractory CRC | Phase II | NCT03190616 |
| mCRC | NA | NCT03743428 | ||
| End-stage CRC | Phase II | NCT02829385 | ||
| Brivanib | VEGFR2 | KRAS-wild-type mCRC | Phase III | NCT00640471 |
| Regorafenib | VEGFR1/2/3 | Later-lines treatment of mCRC | Phase III | NCT05328908 |
| mCRC | Phase III | NCT05425940 | ||
| Surufatinib | VEGFR1/2/3 | Advanced CRC | Phase II | NCT05372198 |
| Lenvatinib | VEGFR1/2/3 | mCRC | Phase III | NCT04776148 |
| Fruquitinib | VEGFR tyrosine kinase | Non-MSI-H/dMMR mCRC | Phase II | NCT04866862 |
| Vandetanib | VEGF/VEGFR | mCRC | Phase I | NCT00532090 |
| mCRC | Phase II | NCT00500292 | ||
| Advanced CRC | Phase I | NCT00496509 |
VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; mCRC: Metastatic colorectal cancer; MSI: Microsatellite instability; dMMR: mismatch repair deficient.
Targeting MEK and mutant BRAF
BRAF mutations are found in 8% to 12% of mCRC cases, and the V600E-activating mutations, which are the most prevalent mutations, are most commonly located in right-colon tumors, and confer a worse prognosis for mCRC[127,128]. BRAF mutations are generally mutually exclusive with KRAS and NRAS mutations. Notably, BRAF and RAS are the only available biomarkers for advanced CRC that are used in clinical practice[129].
BRAF is a downstream effector of RAS in the EGFR pathway and several preclinical studies have shown that BRAF inhibition may induce EGFR overactivation and that EGFR inhibition is important for sensitizing resistant cell lines to anti-BRAF agents[130]. In fact, BRAF inhibitor monotherapy in CRCs harboring V600E-activating mutations is ineffective with a response rate of only 5%[131]. Capalbo et al[132] reported the first clinical evidence that combining anti-EGFR (panitumumab) and an inhibitor of BRAF V600 kinase (vemurafenib) achieves strong disease control and is well tolerated in patients with mCRC that progressed on standard lines of treatment. However, this is only achieved in RAS and BRAF WT tumors, as RAS and BRAF mutations lead to the constitutive activation of downstream transducers of EGFR, circumventing EGFR inhibition, resulting in failure of anti-EGFR therapy[133-135]. A very recent randomized trial reported that the addition of vemurafenib to irinotecan combined with cetuximab improved PFS (hazard ratio of 0.50) in patients with BRAF-mutated, RAS WT mCRC. The response rate was 17% upon addition of vemurafenib and 4% without vemurafenib[136]. Disease control rate was also improved by 44%, suggesting that blocking signaling activity of EGFR using cetuximab prevents its feedback upregulation by vemurafenib. Interestingly, treatment with EGFR and BRAF inhibitors led to a decline in circulating tumor DNA (ctDNA) BRAF V600E variant allele frequency in 87% of the studied population[136]. In the phase III BEACON CRC trial, twenty-nine patients with BRAF V600E-mutant mCRC who had experienced treatment failure with chemotherapy were selected to assess the safety of the encorafenib, binimetinib, and cetuximab regimen. The results showed that the tolerability of this treatment regimen was acceptable, with an overall response rate of 48%, median PFS of 8.0 mo, and median OS of 15.3 mo[137].
BRAF V600E mutations result in constitutive activation of BRAF kinase, which results in activation of mitogen-activated protein kinase (MAPK) kinases MEK1 and MEK2. The latter phosphorylates and activates ERK kinases, resulting in phosphorylation and activation of key molecules involved in proliferation and survival[138].
Studies have shown that combination therapies targeting RAF and EGFR or RAF and MEK can inhibit feedback reactivation of the MAPK signaling pathway, resulting in more robust inhibition and improved efficacy of the treatment in BRAF-mutant CRC[139,140]. Combining RAF and MEK inhibitors produced a 12% partial response and 2% complete response, with a more than 36 mo duration of response, whereby 56% of the patients achieved stable disease. Interestingly, 9 patients who remained in the study for more than 6 mo had reduced levels of phosphorylated ERK during treatment, relative to pretreatment biopsies[141]. A clinical trial of combined inhibition of BRAF, EGFR, and MEK with dabrafenib, panitumumab, and trametinib, respectively, showed improved efficacy in patients with BRAF V600E-mutant CRC[140]. Interestingly, the triplet regimen achieved a response rate of 21% that was higher than dabrafenib and panitumumab (10%) or panitumumab and trametinib (0%)[140]. The BEACON trial reported similar results, whereby a triple treatment consisting of cetuximab, encorafenib, and binimetinib (a MEK inhibitor) significantly prolonged OS and achieved a higher response rate than standard chemotherapy, with a comparable rate of adverse events[142]. Few agents targeting mutant BRAF or MEK have been tested in clinical settings in the context of mCRC (Table 1).
The most common adverse events associated with BRAF inhibition include rash, fatigue, arthralgia, and diarrhea. When combined with MEK inhibitors, toxicities include pulmonary toxicities and ophthalmic changes[143].
Targeting c-MET and HGF
MET is activated by hepatocyte growth factor (HGF) that is secreted by cells of mesenchymal lineage[144]. The MET pathway is frequently aberrantly activated in CRC, in which its overexpression has been reported in up to 70% of cases[144]. MET has been proposed to be a major contributor to resistance to anti-angiogenic therapy and is associated with progression, metastasis, and poor prognosis[145,146], due to c-MET activation of several proteins, such as surviving and x-linked inhibitor of apoptosis protein[147]. In fact, inhibition of the VEGF pathway results in upregulation of MET. A study reported that resistance to cetuximab was caused by MET locus amplification in CRC PDX and that treatment with a MET inhibitor led to an anti-tumor effect[148].
Various mAbs and small molecules with different mechanisms of action have been developed to target the HGF-MET pathway in mCRC[9]. Some drugs are directed at blocking HGF activation and production, while other drugs inhibit the binding of HGF to MET receptors. Agents that interfere with the binding of HGF to MET can be classified as MET antagonists, which competitively bind to MET receptors or as MET TKIs, which inhibit intracellular tyrosine kinase activity[9].
Cabozantinib is a multi-kinase inhibitor that targets MET and VEGFR2, in addition to other kinases[146]. A study reported a potent growth inhibitory effect of cabozantinib in 80% of tumors treated using a CRC PDX model and this inhibition was mostly observed in tumors with PIK3CA mutation. Mechanistically, cabozantinib inhibited Akt activation and decreased the expression of genes involved in the PI3K pathway[146]. Several clinical trials assessed the efficacy of agents that neutralize HGF and block its ability to bind to the MET receptor. A randomized phase Ib/II trial of panitumumab in combination with rilotumumab (a human mAb against HGF), ganitumab (a human mAb against insulin-like growth factor 1 receptor), or placebo in patients with KRAS WT mCRC showed a significant increase in overall response rate of 10% when combining panitumumab with rilotumumab[149]. However, the enhancement in response rate did not translate into significant improvement in OS and PFS. Agents, such as onartuzumab, that compete with HGF for binding to MET have been developed and tested in various solid tumors, including CRC. A phase II randomized trial of first-line FOLFOX plus bevacizumab with or without onartuzumab (MET inhibitor) reported an improvement in PFS in the MET IHC-negative population with mCRC, as compared to those receiving treatment without onartuzumab[150]. However, the addition of onartuzumab did not improve OS or response rate in this population[150]. Tivantinib is an oral small molecule allosteric receptor TKI that selectively keeps MET in the inactive state[151]. In the case of mCRC, clinical trials of tivantinib are insufficient to evaluate its efficacy. A phase I/II trial involving CRC patients with WT KRAS receiving tivantinib or placebo plus cetuximab and irinotecan found no PFS improvement[152]. A recent phase II trial of tivantinib and cetuximab in patients with MET-high KRAS WT mCRC did not meet its primary endpoint; yet, results suggested some efficacy of the combination, with approximately 10% of patients achieving an objective response[153]. Merestinib, an oral multikinase inhibitor, demonstrated an acceptable safety profile and potential anti-tumor effect in a recent first-in-human phase I study involving patients with advanced cancer, including CRC[154]. Findings from this study warrant further investigation to determine the efficacy of this agent in patients with KRAS WT mCRC.
Mild adverse events have been reported for the above-mentioned agents, including fatigue, poor appetite, allergic reactions, edema, skin rash, and neutropenia[155,156].
AMG-337, an oral ATP-competitive TKI specific to MET, is being investigated in a CRC phase I trial (Table 3). Crizotinib targets TKIs of MET, in addition to macrophage-stimulating 1 receptor and ROS proto-oncogene[157]. Although there is a lack of clinical evidence for crizotinib in CRC, a series of trials are in progress[158] (Table 3). The use of crizotinib might enhance the response to radiation therapy in KRAS-mutant CRC cell lines, and a combination of crizotinib with mitomycin C seemed to have a synergistic effect against CRC in preclinical results, which showed promise for future anti-CRC treatments[159]. Few MET inhibitors are under clinical investigation for the treatment of mCRC, and several new agents are being tested in patients with CRC (Table 3).
Table 3.
Agents targeting mesenchymal epithelial transition factor receptor under clinical investigation for the treatment of colorectal cancer and metastatic colorectal cancer
|
Agent
|
Targeted molecule
|
Condition
|
Study phase
|
Clinical trial identifier
|
| Savolitinib | MET | mCRC | Phase II | NCT03592641 |
| Tivantinib | MET | mCRC | Phase I/II | NCT01075048 |
| Onartuzumab | MET | CRC | Phase II | NCT01418222 |
| Cabozantinib | MET/RET/VEGFR-2 | CRC | Phase I | NCT02008383 |
| mCRC | Phase I | NCT03798626 | ||
| Refractory mCRC | Phase II | NCT03542877 | ||
| Rilotumumab | HGF | KRAS wild-type mCRC | Phase I/II | NCT00788957 |
MET: Mesenchymal epithelial transition factor receptor; mCRC: Metastatic colorectal cancer; CRC: Colorectal cancer; VEGFR: Vascular endothelial growth factor receptor; HGF: Hepatocyte growth factor; RET: Rearranged during transfection.
Immune checkpoint inhibitors
In addition to developing agents to directly target pathways involved in tumor growth and metastasis, there is great interest in modulating other pathways involved in immune recognition and responses against cancer cells (Table 4). Immune escape has been frequently identified in various cancers, including CRC[160]. Underlying mechanisms include secretion of immunosuppressive cytokines (transforming growth factor beta (TGFβ), IL-6, CXCL3, CXCL4, and high mobility group box-1), recruitment of regulatory T cells, and loss of immunogenicity via downregulation of major histocompatibility complex-I (MHC-I)[161,162]. Tumor activation of co-inhibitory receptors, also known as immune checkpoint receptors, on the surface of T cells results in T cell inactivation and exhaustion[163]. These receptors include programmed death-1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4)[164]. PD-1 is expressed on peritumoral lymphocytes and is activated by its ligands [programmed death ligand-1 (PD-L1) or PD-L2], which are expressed on tumor cells, to suppress immune functions[165]. mCRC lesions express higher levels of PD-L1 than primary lesions[166], paving the way for promising clinical benefits. Six antibodies against PD-1 or PD-L1 have been approved by the FDA as an anti-cancer treatment, among which some have been evaluated in mCRC patients[167,168]. Metastatic DNA mismatch repair-deficient (dMMR)/MSI-high (MSI-H) CRC has a poor prognosis and is less responsive to conventional chemotherapy, which could be linked to BRAF mutation[169,170]. Importantly, patients who have high mutational tumor burden, with dMMR or MSI-H, respond to immune checkpoint targeted therapy[171-173], most probably due to the fact that mutations result in tumor neoantigens that attract T cell infiltration[174]. Pembrolizumab was the first PD-1 inhibitor to be approved by the FDA for the treatment of mCRC. The KEYNOTE-016 study showed that MSI-H mCRC patients responded to pembrolizumab treatment and showed a response rate of 40% and PFS of 78%[168]. The efficacy of pembrolizumab for the treatment of MSI-H mCRC was also validated in another phase I clinical trial[175]. The more recent trial, KEYNOTE-164, showed that when given in the second-line setting, pembrolizumab resulted in an objective response rate of 33%, PFS of 2.3 mo, and OS of 31.4 mo[176]. The clinical benefit of PD-1 blockade in dMMR mCRC was also documented in the CheckMate 142 phase I trial of nivolumab in patients with refractory solid tumors, 14 of whom had mCRC. A durable complete response was achieved in one patient with mCRC, after receiving five doses of 3 mg/kg nivolumab[177]. This study led to the FDA approval of nivolumab for dMMR or MSI-H mCRC. Combined therapy with nivolumab and the CTLA-4 inhibitor ipilimumab produced durable clinical benefits and helped previously treated patients who had MSI-H or dMMR reach high PFS and OS rates[178,179]. The potential of PD-1 blockade using the single-agent dostarlimab was also evaluated in a very recent phase II study in patients selected for having dMMR stage II or III rectal adenocarcinoma. Administration of dostarlimab every three wk for six mo in twelve patients, who had not received chemoradiotherapy or undergone surgery, resulted in a clinical complete response in all patients with no evidence of progression or recurrence during the six to twenty-five mo follow-up[180]. Several preclinical studies are evaluating other potential immunotherapy agents. A novel antibody (LBL-007), recently characterized by Yu et al[181], targets lymphocyte activation gene 3 (LAG-3) expressed on activated T cells, natural killer cells, and B cells, and functions to negatively regulate these cells. This antibody was found to bind activated T cells and prevent LAG-3 binding to MHC class II molecules, blocking downstream signaling induction in vitro. In vivo results showed that treating mice bearing CRC with LBL-007 significantly delayed tumor growth and combining it with an anti-PD-1 antibody led to a more effective inhibition. Serum LBL-007 levels were high in monkeys injected with LBL-007 at 3, 10, or 30 mg/kg[181]. Another negative regulator of the immune system, T cell immunoglobulin and mucin domain 3, has been shown to be expressed in mCRC and plays an important role in cancer progression[181], and therefore might be a potential target for immunotherapy.
Table 4.
Agents targeting immune checkpoints under clinical investigation for the treatment of drug-resistant and metastatic colorectal cancer
|
Agent
|
Targeted molecule
|
Condition
|
Study phase
|
Clinical trial identifier
|
| Camrelizumab | PD-1 | Non-MSI-H/dMMR mCRC | Phase II | NCT04866862 |
| mCRC | Phase II | NCT03912857 | ||
| Tislelizumab | PD-1 | HER2-Positive Advanced CRC | Phase II | NCT05493683 |
| Nivolumab | PD-1 | Later-lines treatment of mCRC | Phase III | NCT05328908 |
| Advanced CRC | Phase I | NCT02991196 | ||
| Metastatic MSS CRC | Phase I | NCT03993626 | ||
| mCRC | Phase II | NCT04166383 | ||
| Pembrolizumab (MK-3475) | PD-1 | MSI-H/dMMR CRC | Phase III | NCT05239741 |
| mCRC | Phase III | NCT04776148 | ||
| MMR-proficient mCRC | Phase II | NCT03519412 | ||
| HER2-expressing mCRC | Phase II | NCT03631407 | ||
| HER2-expressing mCRC | Phase II | NCT05333809 | ||
| PDR-001 | PD-1/PD-L1 | mCRC | Phase I | NCT03081494 |
| First-line mCRC | Phase I | NCT03176264 | ||
| Toripalimab | PD-1/PD-L1 | mCRC | Phase II | NCT03927898 |
| Avelumab | PD-1/PD-L1 | mCRC | Phase II | NCT03150706 |
| mCRC | Phase II | NCT03258398 | ||
| Atezolizumab | PD-L1 | mCRC | Phase III | NCT05425940 |
| mCRC | Phase III | NCT02788279 | ||
| First-line mCRC | Phase II | NCT02291289 | ||
| Refractory CRC | Phase II | NCT02873195 | ||
| Relatlimab | LAG-3 | Later-lines treatment of mCRC | Phase III | NCT05328908 |
| Tremelimumab | CTLA-4 | mCRC | Phase I/II | NCT03202758 |
| mCRC | Phase II | NCT03122509 | ||
| mCRC | Phase II | NCT03428126 | ||
| mCRC | Phase II | NCT03435107 |
PD-1: Programmed death-1; PD-L1: Programmed death ligand-1; mCRC: metastatic colorectal cancer; MSI-H/dMMR: Microsatellite instability-high/mismatch repair deficient; MSS: Microsatellite stable; HER2: Human epidermal growth factor receptor 2; LAG-3: Lymphocyte activation gene 3; CTLA-4: Cytotoxic T lymphocyte-associated antigen 4.
Pathways offering potential for targeted therapy
Several clinical trials have been initiated to evaluate the efficacy of agents targeting other pathways, yet no meaningful results have been presented so far. RO4929097 is a selective inhibitor of γ-secretase, a proteolytic enzyme that produces an activated intracellular Notch[182]. Notch is an attractive drug target as it is involved in CRC progression; however, a study of RO4929097 showed that no objective radiographic responses were observed and only a few mCRC patients had stable disease, although positive staining for intracellular Notch and its receptor was demonstrated in tissues[182]. A randomized phase II trial of vismodegib, a Hedgehog pathway inhibitor, reported no added benefit in combination with FOLFIRI or FOLFOX, and was instead associated with increased toxicity in mCRC patients[183]. The expression of morphogenetic protein 4 (BMP-4) has been shown to be upregulated in human CRC tissue and inhibition of BMP-4 by BMP type I receptor inhibitor, LDN-193189, induced apoptosis and inhibited tumor formation in mice injected with CRC cells[184]. The progress in the development of agents targeting TGF-β, Wnt, and ATP-binding cassette member B5 is still limited and needs further investigation[185-187]. Limitations in targeted therapy against these pathways are attributed to the existence of crosstalk between pathways, in addition to difficulty selecting patients, identifying predictive biomarkers, and specifically blocking targeted molecules. However, several clinical trials are investigating novel agents, which are summarized in Table 5.
Table 5.
Agents targeting other pathways under clinical investigation for the treatment of drug-resistant and metastatic colorectal cancer
|
Agent
|
Targeted molecule
|
Condition
|
Study phase
|
Clinical trial identifier
|
| CB-103 | Notch | Resistant to oxaliplatin or irinotecan-based therapy advanced or mCRC | Phase I/II | NCT03422679 |
| RO4929097 | Notch | mCRC | Phase II | NCT01116687 |
| WNT974 | Wnt | BRAF-mutant mCRC | Phase I/II | NCT02278133 |
| FOXY-5 | Wnt | mCRC | Phase I | NCT02020291 |
| LGK974 | Wnt | BRAF mutant CRC | Phase I | NCT01351103 |
| Vismodegib (GDC-0449) | Hedgehog | First-line therapy mCRC | Phase II | NCT00636610 |
| mCRC | Phase II | NCT00959647 | ||
| LDE225 | Hedgehog | mCRC | Phase I | NCT01576666 |
| NIS793 | TGF | Advanced CRC | Phase I | NCT02947165 |
| LY3200882 | TGF | Advanced chemotherapy -resistant CRC with an activated TGF-beta Signature | Phase I/II | NCT04031872 |
| Ganitumab | IGF-1R | KRAS wild-type mCRC | Phase I/II | NCT00788957 |
| KRAS-mutant mCRC | Phase II | NCT00813605 | ||
| Dalotuzumab (MK-0646) | IGF-1R | mCRC | Phase II | NCT00614393 |
| Cixutumumab (IMC-A12) | IGF-1R | mCRC resistant to EGFR therapy | Phase II | NCT00503685 |
Wnt: Wingless-related integration site; mCRC: Metastatic colorectal cancer; TGF: Transforming growth factor; IGF-1R: Insulin growth factor receptor-1; EGFR: Epidermal growth factor receptor.
BEATING RESISTANCE TO TARGETED THERAPY
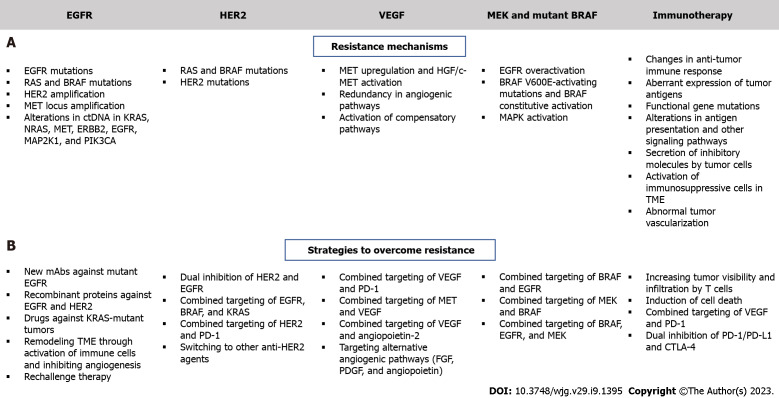
Although multiple targeted therapy agents have demonstrated significant potency in mCRC patients, several challenges hinder the effectiveness of these therapies. Such therapies are associated with intrinsic and acquired resistance and a thorough understanding of resistance mechanisms is essential for developing effective drugs (Figure 3). For example, EGFR inhibitors are effective against KRAS WT mCRC but not KRAS mutated mCRC and there is a need for effective agents in this poor prognosis group. Several clinical trials have assessed the combination of VEGF and chemotherapy, but no attractive results have been shown[9,188].
Figure 3.
Mechanisms of resistance to targeted therapy and strategies to overcome resistance in colorectal cancer. A: Resistance mechanisms; B: Strategies to overcome resistance. EGFR: Epidermal growth factor receptor; HER2: Human epidermal growth factor receptor 2; VEGF: Vascular endothelial growth factor; MEK: Mitogen-activated extracellular signal-regulated kinase; MAP2K1: Mitogen-Activated Protein Kinase 1; PI3KCA: Phosphoinositide 3-kinases catalytic subunit alpha; MAP2K1: Mitogen-activated protein kinase 1; HGF: Hepatocyte growth factor; MET: Mesenchymal epithelial transition; ERBB2: Erb-B2 Receptor Tyrosine Kinase 2; TME: Tumor microenvironment; FGF: Fibroblast growth factor; PDGF: Platelet-derived growth factor; PD-1: Programmed death-1; PD-L1: Programmed death ligand-1; CTLA-4: cytotoxic T lymphocyte-associated antigen 4.
Overcoming resistance to EGFR
Administration of EGFR antibodies with MEK inhibitors has been tested in preclinical models, but clinical data are still limited[189]. Alterations in ctDNA in the following genes: KRAS, NRAS, MET, ERBB2, FLT3, EGFR, and MAP2K1 have been identified in patients with primary or secondary resistance to EGFR inhibition[190]. Thus, determining the ctDNA profiles of patients with mCRC might help predict patient response[191]. Güttlein et al[192] recently tested NRAS, KRAS, and BRAF mutations in liquid plasma biopsies of patients with mCRC and reported a 12- and 4-mo median PFS of RAS/BRAF WT and RAS/BRAF mutated patients, respectively. The frequency of plasma mutations was highest for KRAS (34%). This study suggested that analysis of these mutations in the plasma of mCRC patients can be used to predict OS. The REVEAL study identified multiple actionable targets by performing NGS and transcriptional analysis of tumor and liquid biopsies during and after standard first-line chemotherapy treatment of patients with mCRC[193]. Differentially identified genes reported by this study were associated with EMT, ECM modulation, metabolism regulation, and several oncogenic pathways, such as PI3K/AKT and MAPK[193]. This study also reported the secreted phosphoprotein 1/osteopontin gene as a potentially druggable target whose inhibition also modulates the previously mentioned oncogenic pathways. Interestingly, the approach devised in this study aids in identifying mutations and transcriptional changes following first-line treatment, and thus can be used to predict novel resistance mechanisms and manage them by administering the appropriate targeted agents. Several clinical studies are underway to determine patient subsets who can benefit from anti-EGFR therapy[194,195]; however, sensitivity thresholds in PCR should be taken into consideration since they can affect the genotyping of KRAS, NRAS, BRAF and PIK3C. This would improve the selection of treatment for mCRC with anti-EGFR therapy, as shown by the ULTRA trial[196]. A prospective-retrospective cohort study documented that ctDNA KRAS tested using Digital PCR showed consistency with tumor tissues obtained from mCRC patients and predicted responses to EGFR inhibition[197]. Notably, recent studies have demonstrated that while left-sided KRAS WT mCRC should be preferentially treated with anti-EGFR agents, right-sided tumors might respond better to bevacizumab plus chemotherapy; however, optimization of treatment for these subsets of tumors is yet to be achieved[198-200]. Reversal strategies have emerged to overcome intrinsic resistance, and these include development of new EGFR inhibitors, combination of anti-EGFR with multitargeted inhibitors, development of small molecules that enhance the effect of anti-EGFR agents, and the implementation of metabolic regulators[201]. The development of EGFR mAbs that bind to mutated extracellular domains may enhance the efficacy of these treatments. A study involving CRC patients showed that MM-151, a mAb that binds to different regions of EGFR, significantly inhibits EGFR signaling and decreases mutations in ctDNA[202]. The FDA-approved anti-EGFR agent, necitumumab, was developed to bind to EGFR that harbors the most common cetuximab-resistant variant[203]. The first-in-class anti-EGFR non-overlapping mAbs mixture Sym004 has been documented to suppress mutant EGFR signaling in cetuximab-resistant cell lines and in xenograft models, contrary to cetuximab and panitumumab[204]. Interestingly, Sym004 is currently under clinical investigation for the treatment of mCRC (Table 1 and Figure 1). Notably, recombinant protein-based therapeutics have become an interesting therapeutic option for the treatment of resistant mCRC. A very recent study showed that PEPDG278D, a recombinant human protein that induces the degradation of both EGFR and HER2, exerts strong anti-tumor activity and overcomes resistance to anti-EGFR therapy in CRC PDX[205]. As for patients with KRAS-mutant CRC, a fully humanized EGFR mAb (GC1118) showed significant inhibitory effects against KRAS-mutant CRC PDX[206] and hopes are now placed on the use of this novel compound for better targeting of these tumors.
Extrinsic resistance is mainly mediated by changes in the TME, specifically immune cells and CAFs, in addition to novel development of KRAS mutations and activation of angiogenesis[207,208]. Strategies to remodel the TME are usually beneficial to increase the efficacy of anti-EGFR antibodies and they may include activation of T cells and natural killer cells, suppression of CAFs, and inhibition of angiogenesis through VEGF blockade[201].
Interestingly, rechallenge and reintroduction strategies have been implemented in recent years and have been tested on patients with mCRC who have received an anti-EGFR therapy and whose treatment was halted[209]. Rechallenge refers to anti-EGFR re-treatment of KRAS WT mCRC patients who have initially received and benefited from first-line anti-EGFR therapy before disease progression and receiving a different treatment. Reintroduction refers to re-exposure after prior discontinuation of anti-EGFR therapy due to toxicity, intolerance, and other factors[209,210]. Very recently, Schulz et al[210] reported real-world evidence supporting the benefits of anti-EGFR treatment re-exposure in patients with mCRC, regardless of the reason for discontinuation of anti-EGFR therapy. The reintroduction or rechallenge of this treatment was associated with high OS and PFS[210], suggesting that the administration of more than one-line of treatment with anti-EGFR could be a promising tool to manage disease progression, given the limitations in the current treatment options.
Overcoming resistance to anti-HER2 therapy
Several strategies have been tested to combat resistance to anti-HER2 therapy (Figure 3). These include dual HER2 and EGFR inhibition in the first-line setting and increasing sensitivity to HER2 blockade following resistance to trastuzumab-based therapy[190,211]. Patients with HER2-amplified mCRC that harbor RAS, BRAF, or PIK3CA mutations show limited response to HER2 inhibitors[211], and therefore require a novel therapeutic strategy that would concomitantly block feedback loops involving EGFR, BRAF, and KRAS in mutated mCRC. In terms of the first strategy, several compounds are currently under clinical investigation and new drugs are being proposed as candidates to inhibit both molecules and improve efficacy of CRC targeted therapy, particularly in HER2-positive mCRC[212,213] (Table 1). In fact, HER2 amplification has been linked with resistance to EGFR inhibition[214] and thus, may serve as a biomarker for these treatment regimens. Moreover, combinations of HER2 and PD-1 inhibitors are also being investigated in HER2 expressing advanced CRC or mCRC (Table 6). As for patients with trastuzumab-refractory disease, a possible strategy would be to switch to another anti-HER2 agent. A novel antibody-drug conjugate (T-DM1) consisting of a mAb covalently linked to the cytotoxic agent DM1 has shown robust activity in patients with trastuzumab-resistant HER2-positive breast cancer[215]. A clinical trial is currently evaluating the efficacy of this new compound in HER2-positive mCRC progressing after trastuzumab and lapatinib (Table 1).
Table 6.
Combination of targeted therapies under clinical investigation for the treatment of drug-resistant and metastatic colorectal cancer
|
Agents
|
Targeted molecule (s)
|
Condition
|
Study phase
|
Clinical trial identifier
|
| Encorafenib + Binimetinib + Cetuximab | Wild type plus BRAF V600E and MEK, EGFR | Previously untreated BRAF-mutant mCRC | Phase II | NCT03693170 |
| Tucatinib + Trastuzumab | HER2 | First-line HER2-positive mCRC | Phase III | NCT05253651 |
| Disitamab + Vedotin + Tislelizumab | HER2 and PD-1 | HER2-positive advanced CRC | Phase II | NCT05493683 |
| Vanucizumab + Bevacizumab | VEGF-A/angiopoietin-2 and VEGF | mCRC | Phase II | NCT02141295 |
| Regorafenib + Nivolumab | VEGFR1/2/3 and PD-1 | Later-lines treatment of mCRC | Phase III | NCT05328908 |
| Lenvatinib + Pembrolizumab | VEGFR1/2/3 and PD-1 | mCRC | Phase III | NCT04776148 |
| Fruquitinib + Camrelizumab | VEGFR tyrosine kinase and PD-1 | Non-MSI-H/dMMR mCRC | Phase II | NCT04866862 |
| Disitamab + Vedotin + Pembrolizumab | HER2 and PD-1 | HER2-expressing mCRC | Phase II | NCT05333809 |
| Cobimetinib + Atezolizumab | MAPK and PD-L1 | mCRC | Phase III | NCT02788279 |
| Cetuximab + Vemurafenib | EGFR and mutated BRAF V600E | BRAF V600E Mutated Advanced CRC | Phase II | NCT03727763 |
| Penpulimab + Anlotinib | PD-1 and VEGFR1/2/3 | Refractory mCRC | Phase II | NCT04970914 |
| Favezelimab | LAG-3 and PD-1 | Previously treated metastatic PD-L1 positive CRC | Phase III | NCT05064059 |
| MEN1611 + Cetuximab | PI3K and EGFR | mCRC | Phase I/II | NCT04495621 |
| Encorafenib + Cetuximab + Pembrolizumab | BRAF V600E, as well as wild-type BRAF, EGFR, andPD-1 | Previously untreated mCRC | Phase II | NCT05217446 |
| RXC004 + Nivolumab | Porcupine (wnt activator) and PD1 | RNF43 or RSPO aberrated, metastatic, MSS CRC after progression on SOC | Phase II | NCT04907539 |
| Regorafenib + Pembrolizumab | VEGFR1/2/3PD1 | Advanced or mCRC | Phase I/II | NCT03657641 |
| Isatuximab + Atezolizumab | Epitope on CD38, and PD-L1 | mCRC | Phase I/II | NCT03555149 |
| Atezolizumab + Selicrelumab + Bevacizumab | PD-L1, CD40 antigen, and VEGF | mCRC | Phase I/II | NCT03555149 |
| Atezolizumab + Idasanutlin | PD-L1 and MDM2 | mCRC | Phase I/II | NCT03555149 |
| Atezolizumab + Regorafenib | PD-L1 andVEGFR1/2/3 | mCRC | Phase I/II | NCT03555149 |
| Olaparib (MK-7339) + Bevacizumab | PARP and VEGF | Unresectable or mCRC | Phase III | NCT04456699 |
| Nivolumab + Ipilimumab | PD-1 andCTLA-4 | dMMR and/or MSI mCRC resistant to anti-PD1 monotherapy | Phase II | NCT05310643 |
| Nivolumab + Ipilimumab | PD-1 and CTLA-4 | dMMR and/or MSI mCRC | Phase II | NCT04730544 |
| Surufatinib + Sintilimab | VEGFR1/2/3 and PD-1 | Advanced MSS-Type CRC | Phase II | NCT04764006 |
| Camrelizumab + Apatinib | PD-1 and VEGFR-2 | Advanced CRC | Phase I/II | NCT04067986 |
| Fruquintinib + Tislelizumab + Stereotactic ablative radiotherapy | VEGFR1/2/3 and PD-1 | mCRC | Phase II | NCT04948034 |
| Avelumab + Cetuximab + mFOLFOXIRI | PD-1/PD-L1 and EGFR | Unresectable mCRC | Phase II | NCT04513951 |
| Geptanolimab (GB226) + Fruquintinib | PD-1 and VEGFR1/2/3 | mCRC | Phase I | NCT03977090 |
| Selinexor + Pembrolizumab | Exportin 1 and PD-1 | Previously treated mCRC with RAS mutations | Phase II | NCT04854434 |
| Panitumumab + Rilotumumab | EGFR and HGF | wild-type KRAS mCRC | Phase I/II | NCT00788957 |
| Panitumumab + Ganitumab | EGFR and IGF-1R | wild-type KRAS mCRC | Phase I/II | NCT00788957 |
MEK: Mitogen-activated extracellular signal-regulated kinase; EGFR: Epidermal growth factor receptor; mCRC: Metastatic colorectal cancer; HER2: Human epidermal growth factor receptor 2; PD-1: Programmed death-1; PD-L1: Programmed death ligand-1; VEGF-A: Vascular endothelial growth factor-A; VEGFR: Vascular endothelial growth factor receptor; MSI-H/dMMR: Microsatellite instability-high/mismatch repair deficient; MSS: Microsatellite stable; MAPK: mitogen-activated protein kinases; LAG-3 Lymphocyte activation gene 3; PI3K: Phosphoinositide 3-kinases; RNF43: Ring Finger Protein 43; RSPO: R-spondin; SOC: Standard of Care; PARP: Poly ADP ribose polymerase; CTLA-4: Cytotoxic T lymphocyte-associated antigen 4; HGF: Hepatocyte growth factor; IGF-1R: Insulin growth factor receptor-1.
Overcoming resistance to anti-VEGF therapy
The major mechanisms of resistance to anti-VEGF therapy are still not fully elucidated. Redundancy in angiogenic signaling pathways and compensation through activation of other pathways may contribute to this resistance (Figure 3). Several agents are currently under development for the purpose of improving anti-angiogenic therapy efficacy (Table 2). Importantly, it has recently been shown that the location of the primary tumor affects the choice of targeted therapy for the treatment of mCRC, whereby left-sided tumors benefit more than right-sided tumors from EGFR inhibition[198,200]. As mentioned before, combining anti-angiogenic agents with immune checkpoint inhibitors has been shown to restore vascular-immune crosstalk to establish a strong anti-tumor immune response[216]. In addition to VEGF/VEGFR, targeting alternative angiogenic pathways such as FGF, PDGF, and angiopoietins can inhibit VEGF-independent angiogenic pathways that are activated in response to VEGF blockade[217]. In mCRC patients, increased plasma levels of FGF, PDGF, and placental growth factor were linked to disease progression during bevacizumab-based therapy[217]. The clinical efficacy of the dual inhibition of VEGF-A and angiopoietin-2 using vanucizumab is still under phase II clinical trials, though with promising results[218] (Table 2). It is important to note that additional factors, including hypoxia and the limited blood supply restrict the delivery of drugs to the tumor site, resulting in resistance. In addition, cancer resistance to anti-VEGF therapy has been linked to activation of the HGF/c-MET pathway[219]. The latter activates key pathways involved in CRC metastasis and drug-resistance, including MAPK/ERK, STAT3, NF-κB, and PI3K/Akt[219]. Several MET inhibitors are being evaluated in the clinic for the purpose of blocking MET to overcome resistance to anti-VEGFR treatment (Table 3). This approach has produced effective results in other types of cancer, including advanced renal cell carcinoma[220]. Dual inhibition of MET and VEGFR2 using cabozantinib showed a strong anti-tumor effect in a preclinical CRC PDX model and the effect was greatest in tumors that possessed a mutation in the PIK3CA gene[146]. Several trials have been initiated to evaluate the efficacy of this compound in mCRC (Table 3).
Overcoming resistance to immunotherapy
Evading the immune system is an important hallmark of cancer, including CRC and is linked to immunotherapy and targeted therapy resistance[221]. Intrinsic resistance to immunotherapy is mainly conferred by changes in anti-tumor immune response, aberrant expression of tumor antigens, functional gene mutations, alterations in antigen presentation and other signaling pathways in tumor cells, in addition to secretion of inhibitory molecules by tumor cells[222] (Figure 3). Extrinsic mechanisms include activation of immunosuppressive cells in the TME and abnormal tumor vascularization[222]. One of the most effective strategies to deal with resistance to immunotherapy involves increasing tumor visibility and infiltration by T cells, through induction of immunogenic cell death by targeted agents and other therapies. The success of combining anti-angiogenic agents with immunotherapy has been shown in several cancers and is being evaluated in phase III clinical studies involving patients with advanced or metastatic and/or refractory CRC (Table 6). In addition, the efficacy of combining immune checkpoint inhibitors with chemokines that mediate the recruitment of T cells into the TME warrants investigation in mCRC. This could also be achieved by the administration of VEGF inhibitors that would normalize tumor vasculature and permit T-cell infiltration[223].
Enhancing the immune system function is also a good strategy to activate effector T cells and inhibit immunosuppressive immune cells. An emerging approach is the dual or combinatory inhibition of PD-1/PD-L1 and CTLA-4 to concomitantly block immune system inhibitory pathways and has shown promising results in preclinical[224,225] and clinical[226] (Table 6) models of mCRC. Ongoing trials are also addressing genomic and epigenetic alterations by evaluating the efficacy of anti-PD-1 agents in combination with VEGFR or CTLA-4 inhibitors in dMMR and/or MSI mCRC (Table 6).
Implementation of better preclinical models
The importance of preclinical models has been highlighted in the case of mCRC. The rapidly emerging role of patient-derived tumor samples may be considered one of the revolutionizing approaches to improve treatment strategies. Such samples can be propagated in mice to produce PDXs or in three-dimensional cultures to produce patient-derived organoids (PDOs)[227-230]. These models are important for understanding and predicting treatment responses in drug-resistant CRC and mCRC. Molecular response predictors are usually identified in clinical trials by employing a statistically significant enrichment for a genetic mutation and correlating it with a clinical outcome in responsive and non-responsive patients. A major limitation of this approach is the inability to elucidate the mechanisms underlying this correlation and to validate whether these predictors influence response to treatment. Cancer cell line cultures have made it possible to gain insight into the functional processes; however, they do not recapitulate the in vivo structure, in addition to the genomic and functional heterogeneity of mCRC. Therefore, patient-derived models are ideal platforms with clinical fidelity and good reflection of disease diversity. These models are being used for target discovery and for characterization of response biomarkers to combat drug-resistance and to predict treatment response[228]. For example, PDX were used to validate the correlation between KRAS mutations in exon 2 and de novo resistance to EGFR inhibition and to identify HER2 as a potential target in cetuximab-resistant mCRC[89,231]. Additionally, these models were the first to identify KRAS exon 3 and 4 mutations as predictors of resistance to EGFR mAbs[89]. Both PDX and PDOs have clinical relevance; however, PDOs are easier to cultivate and are useful for high-throughput drug screening[232]. Subsequently, PDOs have been used to model CRC and study mechanisms of resistance. In addition, the newly emerging CRISPR/Cas9 genome-editing tool has been applied to introduce mutations in normal human colorectal organoids and has confirmed the role of these mutations in CSC maintenance, in addition to metastasis and resistance to therapy[233,234]. The association between KRAS mutation and lack of response to EGFR blockade has been also validated in organoids derived from mCRC[235]. Importantly, results from PDOs have been shown to recapitulate clinical response to targeted therapies, including cetuximab and regorafenib[236]. Notably, PDO-based drug screening has been used to improve the accuracy and effectiveness of precision medicine, paving the way for PDO-based personalized therapy[237]. CRC PDOs can be also used to identify patients that benefit from a specific targeted therapy.
CONCLUSION
Given the high molecular heterogeneity associated with CRC, different mechanisms of resistance may develop. A multi-targeted approach to therapy and the use of combination targeted therapy as a first-line treatment, rather than after the patients demonstrate drug-resistance and progress on treatment, have been an active area of research based on the efficacy of these strategies in preclinical models. Several clinical trials have investigated the efficacy of combination therapies targeted at two or three pathways; however, the high toxicity levels associated with these therapies is a limitation to bear in mind as it represents a critical challenge to the development of effective therapies for the treatment of drug-resistant and mCRC. Nevertheless, data from clinical studies are showing promising signs of efficacy. This has been made possible through targeting adaptive feedback pathways and the discovery and implementation of predictive biomarkers for targeted therapy, which are critical in identifying patients that could benefit from combination targeted therapy. Biomarker detection computational algorithms and tools are being designed for this purpose and should be followed by clinical validation and approval. Importantly, personalized treatment could be developed to promote survival and prognosis of CRC patients without causing adverse events. With the advancement of NGS and genome profiling, it has been possible to decipher predictive responses to anti-cancer treatments and to select the appropriate treatment for each individual, depending on the genetic characteristics and clinical tumor features. Strategic planning of treatment regimens is essential to enhance the effectiveness of targeted agents and to decrease the possibility of side effects. Conjugation of inhibitory molecules using Nanoparticle technology is an attractive approach in this case. Nanoparticles are being used for the targeted delivery of drugs to the affected tissues and optimization methods can be applied to increase their uptake efficiency.
Other tools that could help improve personalized medicine include the triphasic enhanced computed tomography radiomics signature that was recently tested by Cao et al[238] and has been shown to be effective in predicting CRC MSI status with 0.837 and 0.821 accuracy and sensitivity, respectively. Moreover, whole genome sequencing, multi-region whole exome sequencing, simultaneous single-cell RNA-sequencing, and single-cell targeted cDNA Sanger sequencing are being used to obtain single-cell genomic and transcriptomic landscapes of adjacent normal tissues, primary tumors, and metastatic tumors[239], which could also improve individualized treatment.
Given the importance of the gut microbiota in the progression of CRC, microbiome profiles can be integrated with other genomic and epigenomic profiles to enhance personalized targeted therapies against CRC, resulting in better clinical outcomes. Nonetheless, this adds another level of complexity to the application of this approach. Interestingly, modification of the gut microbiota through targeted inhibition of pathogenic bacteria can be used to prepare patients for CRC treatment by augmenting the host immune system.
Changes in mutations or transcription should be monitored during administration of treatment, in addition to changes in immune responses and inflammatory molecules that can influence the choice of treatment. These immune signatures may be indispensable for improving clinical outcome. Interestingly, it has been reported that the peripheral blood repertoire of T cell receptor changes during the course of chemotherapy in patients with mCRC, and thus could have a prognostic value[12].
In summary, the application of personalized medicine requires the integration of tumor mutations and epigenetic modifications, TME gene expression, host immune proficiency, and their changes during disease progression and treatment. The constant search for novel targets involved in drug-resistance and metastasis will lead to the identification of interesting molecular traits that can be modulated using biomarker-driven treatments to overcome resistance to therapy.
Footnotes
Conflict-of-interest statement: All authors declare no conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 13, 2022
First decision: September 29, 2022
Article in press: November 16, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Lebanon
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cheng H, China; Luo Y, China; Peng XC, China S-Editor: Liu GL L-Editor: Webster JR P-Editor: Liu GL
Contributor Information
Samar Al Bitar, Department of Anatomy, Cell Biology and Physiological Sciences, American University of Beirut, Beirut 1107-2020, Lebanon.
Marwan El-Sabban, Department of Anatomy, Cell Biology and Physiological Sciences, American University of Beirut, Beirut 1107-2020, Lebanon.
Samer Doughan, Department of Surgery, American University of Beirut Medical Center, Beirut 1107-2020, Lebanon.
Wassim Abou-Kheir, Department of Anatomy, Cell Biology and Physiological Sciences, American University of Beirut, Beirut 1107-2020, Lebanon. wa12@aub.edu.lb.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Advani S, Kopetz S. Ongoing and future directions in the management of metastatic colorectal cancer: Update on clinical trials. J Surg Oncol. 2019;119:642–652. doi: 10.1002/jso.25441. [DOI] [PubMed] [Google Scholar]
- 5.Shaukat A, Levin TR. Current and future colorectal cancer screening strategies. Nat Rev Gastroenterol Hepatol. 2022;19:521–531. doi: 10.1038/s41575-022-00612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 8.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mardis ER. The Impact of Next-Generation Sequencing on Cancer Genomics: From Discovery to Clinic. Cold Spring Harb Perspect Med. 2019;9 doi: 10.1101/cshperspect.a036269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messersmith WA. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J Natl Compr Canc Netw. 2019;17:599–601. doi: 10.6004/jnccn.2019.5014. [DOI] [PubMed] [Google Scholar]
- 12.Chen K, Zhang C, Ling S, Wei R, Wang J, Xu X. The metabolic flexibility of quiescent CSC: implications for chemotherapy resistance. Cell Death Dis. 2021;12:835. doi: 10.1038/s41419-021-04116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 14.Begicevic RR, Falasca M. ABC Transporters in Cancer Stem Cells: Beyond Chemoresistance. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18112362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinbichler TB, Dudás J, Skvortsov S, Ganswindt U, Riechelmann H, Skvortsova II. Therapy resistance mediated by cancer stem cells. Semin Cancer Biol. 2018;53:156–167. doi: 10.1016/j.semcancer.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 2006;580:998–1009. doi: 10.1016/j.febslet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 17.Du J, He Y, Li P, Wu W, Chen Y, Ruan H. IL-8 regulates the doxorubicin resistance of colorectal cancer cells via modulation of multidrug resistance 1 (MDR1) Cancer Chemother Pharmacol. 2018;81:1111–1119. doi: 10.1007/s00280-018-3584-x. [DOI] [PubMed] [Google Scholar]
- 18.Wu CP, Ohnuma S, Ambudkar SV. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12:609–620. doi: 10.2174/138920111795163887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hlavata I, Mohelnikova-Duchonova B, Vaclavikova R, Liska V, Pitule P, Novak P, Bruha J, Vycital O, Holubec L, Treska V, Vodicka P, Soucek P. The role of ABC transporters in progression and clinical outcome of colorectal cancer. Mutagenesis. 2012;27:187–196. doi: 10.1093/mutage/ger075. [DOI] [PubMed] [Google Scholar]
- 20.Kosuri KV, Wu X, Wang L, Villalona-Calero MA, Otterson GA. An epigenetic mechanism for capecitabine resistance in mesothelioma. Biochem Biophys Res Commun. 2010;391:1465–1470. doi: 10.1016/j.bbrc.2009.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 22.Blondy S, David V, Verdier M, Mathonnet M, Perraud A, Christou N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020;111:3142–3154. doi: 10.1111/cas.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith M, Mwenifumbo JC, Cheung PY, Paul JE, Pugh TJ, Tang MJ, Chittaranjan S, Morin RD, Asano JK, Ally AA, Miao L, Lee A, Chan SY, Taylor G, Severson T, Hou YC, Griffith OL, Cheng GS, Novik K, Moore R, Luk M, Owen D, Brown CJ, Morin GB, Gill S, Tai IT, Marra MA. Novel mRNA isoforms and mutations of uridine monophosphate synthetase and 5-fluorouracil resistance in colorectal cancer. Pharmacogenomics J. 2013;13:148–158. doi: 10.1038/tpj.2011.65. [DOI] [PubMed] [Google Scholar]
- 24.Lindskog EB, Derwinger K, Gustavsson B, Falk P, Wettergren Y. Thymidine phosphorylase expression is associated with time to progression in patients with metastatic colorectal cancer. BMC Clin Pathol. 2014;14:25. doi: 10.1186/1472-6890-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchino J, Takayama K, Harada A, Sone T, Harada T, Curiel DT, Kuroki M, Nakanishi Y. Tumor targeting carboxylesterase fused with anti-CEA scFv improve the anticancer effect with a less toxic dose of irinotecan. Cancer Gene Ther. 2008;15:94–100. doi: 10.1038/sj.cgt.7701100. [DOI] [PubMed] [Google Scholar]
- 26.de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S. Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Clin Pharmacokinet. 2018;57:1229–1254. doi: 10.1007/s40262-018-0644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Innocenti F, Undevia SD, Ramírez J, Mani S, Schilsky RL, Vogelzang NJ, Prado M, Ratain MJ. A phase I trial of pharmacologic modulation of irinotecan with cyclosporine and phenobarbital. Clin Pharmacol Ther. 2004;76:490–502. doi: 10.1016/j.clpt.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman L, Diasio RB, Danenberg PV. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322–1327. [PubMed] [Google Scholar]
- 29.Soong R, Shah N, Salto-Tellez M, Tai BC, Soo RA, Han HC, Ng SS, Tan WL, Zeps N, Joseph D, Diasio RB, Iacopetta B. Prognostic significance of thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase protein expression in colorectal cancer patients treated with or without 5-fluorouracil-based chemotherapy. Ann Oncol. 2008;19:915–919. doi: 10.1093/annonc/mdm599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maréchal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell. 2007;11:175–189. doi: 10.1016/j.ccr.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peitzsch C, Kurth I, Kunz-Schughart L, Baumann M, Dubrovska A. Discovery of the cancer stem cell related determinants of radioresistance. Radiother Oncol. 2013;108:378–387. doi: 10.1016/j.radonc.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Mirza-Aghazadeh-Attari M, Darband SG, Kaviani M, Mihanfar A, Aghazadeh Attari J, Yousefi B, Majidinia M. DNA damage response and repair in colorectal cancer: Defects, regulation and therapeutic implications. DNA Repair (Amst) 2018;69:34–52. doi: 10.1016/j.dnarep.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Marin JJ, Sanchez de Medina F, Castaño B, Bujanda L, Romero MR, Martinez-Augustin O, Moral-Avila RD, Briz O. Chemoprevention, chemotherapy, and chemoresistance in colorectal cancer. Drug Metab Rev. 2012;44:148–172. doi: 10.3109/03602532.2011.638303. [DOI] [PubMed] [Google Scholar]
- 35.Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg PV, Lenz HJ. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001;19:4298–4304. doi: 10.1200/JCO.2001.19.23.4298. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J. Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacol Ther. 2016;160:145–158. doi: 10.1016/j.pharmthera.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25:20. doi: 10.1186/s12929-018-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu T, Li Z, Gao CY, Cho CH. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J Gastroenterol. 2016;22:6876–6889. doi: 10.3748/wjg.v22.i30.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell KJ, Tait SWG. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018;8 doi: 10.1098/rsob.180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das PK, Islam F, Lam AK. The Roles of Cancer Stem Cells and Therapy Resistance in Colorectal Carcinoma. Cells. 2020;9 doi: 10.3390/cells9061392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gout S, Huot J. Role of cancer microenvironment in metastasis: focus on colon cancer. Cancer Microenviron. 2008;1:69–83. doi: 10.1007/s12307-008-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front Immunol. 2020;11:940. doi: 10.3389/fimmu.2020.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peddareddigari VG, Wang D, Dubois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010;3:149–166. doi: 10.1007/s12307-010-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prieto-Vila M, Takahashi RU, Usuba W, Kohama I, Ochiya T. Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18122574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, Lee HK. Potential Role of the Gut Microbiome In Colorectal Cancer Progression. Front Immunol. 2021;12:807648. doi: 10.3389/fimmu.2021.807648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, Chen Y, Chen H, Hong J, Zou W, Fang JY. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 2017;170:548–563.e16. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 52.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayase E, Jenq RR. Role of the intestinal microbiome and microbial-derived metabolites in immune checkpoint blockade immunotherapy of cancer. Genome Med. 2021;13:107. doi: 10.1186/s13073-021-00923-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17:33–48. doi: 10.1038/s41571-019-0268-3. [DOI] [PubMed] [Google Scholar]
- 55.Chan DLH, Segelov E, Wong RS, Smith A, Herbertson RA, Li BT, Tebbutt N, Price T, Pavlakis N. Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer. Cochrane Database Syst Rev. 2017;6:CD007047. doi: 10.1002/14651858.CD007047.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:15–31. doi: 10.1517/14728222.2011.648617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Messa C, Russo F, Caruso MG, Di Leo A. EGF, TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta Oncol. 1998;37:285–289. doi: 10.1080/028418698429595. [DOI] [PubMed] [Google Scholar]
- 58.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 59.Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71:2454–2460. doi: 10.1002/1097-0142(19930415)71:8<2454::aid-cncr2820710805>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 60.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) vs FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 61.Wee P, Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel) 2017;9 doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yarom N, Jonker DJ. The role of the epidermal growth factor receptor in the mechanism and treatment of colorectal cancer. Discov Med. 2011;11:95–105. [PubMed] [Google Scholar]
- 63.Loft M, Shapiro J, Lee M, Wong R, Tie J, Kosmider S, Wong V, Jalali A, Lee B, Ananda SS, Gibbs P. Compliance with Therapeutic Goods Association prescribing information: weekly or second weekly cetuximab for the treatment of metastatic colorectal cancer. Intern Med J. 2022 doi: 10.1111/imj.15835. [DOI] [PubMed] [Google Scholar]
- 64.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 65.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 66.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 67.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Tian Y, Xu F, Sidhu R. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 69.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 70.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 71.Modest DP, Karthaus M, Kasper S, Moosmann N, Keitel V, Kiani A, Uhlig J, Jacobasch L, Fischer V Weikersthal L, Fuchs M, Kaiser F, Lerchenmüller C, Sent D, Junghanß C, Held S, Lorenzen S, Kaczirek K, Jung A, Stintzing S, Heinemann V. FOLFOX plus panitumumab or FOLFOX alone as additive therapy following R0/1 resection of RAS wild-type colorectal cancer liver metastases - The PARLIM trial (AIO KRK 0314) Eur J Cancer. 2022;173:297–306. doi: 10.1016/j.ejca.2022.07.012. [DOI] [PubMed] [Google Scholar]
- 72.Janani B, Vijayakumar M, Priya K, Kim JH, Prabakaran DS, Shahid M, Al-Ghamdi S, Alsaidan M, Othman Bahakim N, Hassan Abdelzaher M, Ramesh T. EGFR-Based Targeted Therapy for Colorectal Cancer-Promises and Challenges. Vaccines (Basel) 2022;10 doi: 10.3390/vaccines10040499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Favoni RE, Pattarozzi A, Lo Casto M, Barbieri F, Gatti M, Paleari L, Bajetto A, Porcile C, Gaudino G, Mutti L, Corte G, Florio T. Gefitinib targets EGFR dimerization and ERK1/2 phosphorylation to inhibit pleural mesothelioma cell proliferation. Curr Cancer Drug Targets. 2010;10:176–191. doi: 10.2174/156800910791054130. [DOI] [PubMed] [Google Scholar]
- 74.Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda M, Fujisaka Y, Philips J, Shimizu T, Maenishi O, Cho Y, Sun J, Destro A, Taira K, Takeda K, Okabe T, Swanson J, Itoh H, Takada M, Lifshits E, Okuno K, Engelman JA, Shivdasani RA, Nishio K, Fukuoka M, Varella-Garcia M, Nakagawa K, Jänne PA. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3:99ra86. doi: 10.1126/scitranslmed.3002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohan S, Heitzer E, Ulz P, Lafer I, Lax S, Auer M, Pichler M, Gerger A, Eisner F, Hoefler G, Bauernhofer T, Geigl JB, Speicher MR. Changes in colorectal carcinoma genomes under anti-EGFR therapy identified by whole-genome plasma DNA sequencing. PLoS Genet. 2014;10:e1004271. doi: 10.1371/journal.pgen.1004271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Popescu NC, King CR, Kraus MH. Localization of the human erbB-2 gene on normal and rearranged chromosomes 17 to bands q12-21.32. Genomics. 1989;4:362–366. doi: 10.1016/0888-7543(89)90343-1. [DOI] [PubMed] [Google Scholar]
- 77.Martin V, Cappuzzo F, Mazzucchelli L, Frattini M. HER2 in solid tumors: more than 10 years under the microscope; where are we now? Future Oncol. 2014;10:1469–1486. doi: 10.2217/fon.14.19. [DOI] [PubMed] [Google Scholar]
- 78.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richman SD, Southward K, Chambers P, Cross D, Barrett J, Hemmings G, Taylor M, Wood H, Hutchins G, Foster JM, Oumie A, Spink KG, Brown SR, Jones M, Kerr D, Handley K, Gray R, Seymour M, Quirke P. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol. 2016;238:562–570. doi: 10.1002/path.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Valtorta E, Martino C, Sartore-Bianchi A, Penaullt-Llorca F, Viale G, Risio M, Rugge M, Grigioni W, Bencardino K, Lonardi S, Zagonel V, Leone F, Noe J, Ciardiello F, Pinto C, Labianca R, Mosconi S, Graiff C, Aprile G, Frau B, Garufi C, Loupakis F, Racca P, Tonini G, Lauricella C, Veronese S, Truini M, Siena S, Marsoni S, Gambacorta M. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol. 2015;28:1481–1491. doi: 10.1038/modpathol.2015.98. [DOI] [PubMed] [Google Scholar]
- 82.Schuell B, Gruenberger T, Scheithauer W, Zielinski Ch, Wrba F. HER 2/neu protein expression in colorectal cancer. BMC Cancer. 2006;6:123. doi: 10.1186/1471-2407-6-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kavanagh DO, Chambers G, O'Grady L, Barry KM, Waldron RP, Bennani F, Eustace PW, Tobbia I. Is overexpression of HER-2 a predictor of prognosis in colorectal cancer? BMC Cancer. 2009;9:1. doi: 10.1186/1471-2407-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marx AH, Burandt EC, Choschzick M, Simon R, Yekebas E, Kaifi JT, Mirlacher M, Atanackovic D, Bokemeyer C, Fiedler W, Terracciano L, Sauter G, Izbicki JR. Heterogenous high-level HER-2 amplification in a small subset of colorectal cancers. Hum Pathol. 2010;41:1577–1585. doi: 10.1016/j.humpath.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 85.Osako T, Miyahara M, Uchino S, Inomata M, Kitano S, Kobayashi M. Immunohistochemical study of c-erbB-2 protein in colorectal cancer and the correlation with patient survival. Oncology. 1998;55:548–555. doi: 10.1159/000011911. [DOI] [PubMed] [Google Scholar]
- 86.Caruso ML, Valentini AM. Immunohistochemical p53 overexpression correlated to c-erbB-2 and cathepsin D proteins in colorectal cancer. Anticancer Res. 1996;16:3813–3818. [PubMed] [Google Scholar]
- 87.Siena S, Sartore-Bianchi A, Marsoni S, Hurwitz HI, McCall SJ, Penault-Llorca F, Srock S, Bardelli A, Trusolino L. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol. 2018;29:1108–1119. doi: 10.1093/annonc/mdy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramanathan RK, Hwang JJ, Zamboni WC, Sinicrope FA, Safran H, Wong MK, Earle M, Brufsky A, Evans T, Troetschel M, Walko C, Day R, Chen HX, Finkelstein S. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Invest. 2004;22:858–865. doi: 10.1081/cnv-200039645. [DOI] [PubMed] [Google Scholar]
- 89.Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, Corà D, Di Nicolantonio F, Buscarino M, Petti C, Ribero D, Russolillo N, Muratore A, Massucco P, Pisacane A, Molinaro L, Valtorta E, Sartore-Bianchi A, Risio M, Capussotti L, Gambacorta M, Siena S, Medico E, Sapino A, Marsoni S, Comoglio PM, Bardelli A, Trusolino L. A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1:508–523. doi: 10.1158/2159-8290.CD-11-0109. [DOI] [PubMed] [Google Scholar]
- 90.Luca T, Barresi V, Privitera G, Musso N, Caruso M, Condorelli DF, Castorina S. In vitro combined treatment with cetuximab and trastuzumab inhibits growth of colon cancer cells. Cell Prolif. 2014;47:435–447. doi: 10.1111/cpr.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kavuri SM, Jain N, Galimi F, Cottino F, Leto SM, Migliardi G, Searleman AC, Shen W, Monsey J, Trusolino L, Jacobs SA, Bertotti A, Bose R. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015;5:832–841. doi: 10.1158/2159-8290.CD-14-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rubinson DA, Hochster HS, Ryan DP, Wolpin BM, McCleary NJ, Abrams TA, Chan JA, Iqbal S, Lenz HJ, Lim D, Rose J, Bekaii-Saab T, Chen HX, Fuchs CS, Ng K. Multi-drug inhibition of the HER pathway in metastatic colorectal cancer: results of a phase I study of pertuzumab plus cetuximab in cetuximab-refractory patients. Invest New Drugs. 2014;32:113–122. doi: 10.1007/s10637-013-9956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang AY, Lopes G, Hsin KW, Lim R, Fong FK, Wong J. Phase II trial of 5-fluorouracil/Leucovorin/gemcitabine/cisplatin as second-line treatment in patients with metastatic or recurrent colorectal carcinoma: a cancer therapeutics research group study. Clin Colorectal Cancer. 2007;6:646–651. doi: 10.3816/CCC.2007.n.033. [DOI] [PubMed] [Google Scholar]
- 94.Bekaii-Saab TS, Roda JM, Guenterberg KD, Ramaswamy B, Young DC, Ferketich AK, Lamb TA, Grever MR, Shapiro CL, Carson WE 3rd. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol Cancer Ther. 2009;8:2983–2991. doi: 10.1158/1535-7163.MCT-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E, Troiani T, Ciardiello F, Racca P, Bertotti A, Siravegna G, Torri V, Amatu A, Ghezzi S, Marrapese G, Palmeri L, Valtorta E, Cassingena A, Lauricella C, Vanzulli A, Regge D, Veronese S, Comoglio PM, Bardelli A, Marsoni S, Siena S. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738–746. doi: 10.1016/S1470-2045(16)00150-9. [DOI] [PubMed] [Google Scholar]
- 96.Sartore-Bianchi A, Lonardi S, Martino C, Fenocchio E, Tosi F, Ghezzi S, Leone F, Bergamo F, Zagonel V, Ciardiello F, Ardizzoni A, Amatu A, Bencardino K, Valtorta E, Grassi E, Torri V, Bonoldi E, Sapino A, Vanzulli A, Regge D, Cappello G, Bardelli A, Trusolino L, Marsoni S, Siena S. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO Open. 2020;5:e000911. doi: 10.1136/esmoopen-2020-000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, Swanton C, Kurzrock R, Burris H, Sweeney C, Bose R, Spigel DR, Beattie MS, Blotner S, Stone A, Schulze K, Cuchelkar V, Hainsworth J. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518–530. doi: 10.1016/S1470-2045(18)30904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, Burris H, Bose R, Yoo B, Stein A, Beattie M, Kurzrock R. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J Clin Oncol. 2018;36:536–542. doi: 10.1200/JCO.2017.75.3780. [DOI] [PubMed] [Google Scholar]
- 99.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 100.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 101.Shibuya M. VEGFR and type-V RTK activation and signaling. Cold Spring Harb Perspect Biol. 2013;5:a009092. doi: 10.1101/cshperspect.a009092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lopez A, Harada K, Vasilakopoulou M, Shanbhag N, Ajani JA. Targeting Angiogenesis in Colorectal Carcinoma. Drugs. 2019;79:63–74. doi: 10.1007/s40265-018-1037-9. [DOI] [PubMed] [Google Scholar]
- 104.Javle M, Smyth EC, Chau I. Ramucirumab: successfully targeting angiogenesis in gastric cancer. Clin Cancer Res. 2014;20:5875–5881. doi: 10.1158/1078-0432.CCR-14-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosen LS, Jacobs IA, Burkes RL. Bevacizumab in Colorectal Cancer: Current Role in Treatment and the Potential of Biosimilars. Target Oncol. 2017;12:599–610. doi: 10.1007/s11523-017-0518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 107.Pfeiffer P, Yilmaz M, Möller S, Zitnjak D, Krogh M, Petersen LN, Poulsen LØ, Winther SB, Thomsen KG, Qvortrup C. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21:412–420. doi: 10.1016/S1470-2045(19)30827-7. [DOI] [PubMed] [Google Scholar]
- 108.Oki E, Makiyama A, Miyamoto Y, Kotaka M, Kawanaka H, Miwa K, Kabashima A, Noguchi T, Yuge K, Kashiwada T, Ando K, Shimokawa M, Saeki H, Akagi Y, Baba H, Maehara Y, Mori M. Trifluridine/tipiracil plus bevacizumab as a first-line treatment for elderly patients with metastatic colorectal cancer (KSCC1602): A multicenter phase II trial. Cancer Med. 2021;10:454–461. doi: 10.1002/cam4.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sommerhäuser G, Kurreck A, Stintzing S, Heinemann V, von Weikersthal LF, Dechow T, Kaiser F, Karthaus M, Schwaner I, Fuchs M, König A, Roderburg C, Hoyer I, Quante M, Kiani A, Fruehauf S, Müller L, Reinacher-Schick A, Ettrich TJ, Stahler A, Modest DP. Study protocol of the FIRE-8 (AIO-KRK/YMO-0519) trial: a prospective, randomized, open-label, multicenter phase II trial investigating the efficacy of trifluridine/tipiracil plus panitumumab vs trifluridine/tipiracil plus bevacizumab as first-line treatment in patients with metastatic colorectal cancer. BMC Cancer. 2022;22:820. doi: 10.1186/s12885-022-09892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Cutsem E, Danielewicz I, Saunders MP, Pfeiffer P, Argilés G, Borg C, Glynne-Jones R, Punt CJA, Van de Wouw AJ, Fedyanin M, Stroyakovskiy D, Kroening H, Garcia-Alfonso P, Wasan H, Falcone A, Kanehisa A, Egorov A, Aubel P, Amellal N, Moiseenko V. Trifluridine/tipiracil plus bevacizumab in patients with untreated metastatic colorectal cancer ineligible for intensive therapy: the randomized TASCO1 study. Ann Oncol. 2020;31:1160–1168. doi: 10.1016/j.annonc.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 111.Chen YX, Yang Q, Kuang JJ, Chen SY, Wei Y, Jiang ZM, Xie DR. Efficacy of adding bevacizumab in the first-line chemotherapy of metastatic colorectal cancer: evidence from seven randomized clinical trials. Gastroenterol Res Pract. 2014;2014:594930. doi: 10.1155/2014/594930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 113.Passardi A, Nanni O, Tassinari D, Turci D, Cavanna L, Fontana A, Ruscelli S, Mucciarini C, Lorusso V, Ragazzini A, Frassineti GL, Amadori D. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: final results for first-line treatment from the ITACa randomized clinical trial. Ann Oncol. 2015;26:1201–1207. doi: 10.1093/annonc/mdv130. [DOI] [PubMed] [Google Scholar]
- 114.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S. FOLFIRI plus cetuximab vs FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 115.Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH, Atkins JN, Berry S, Polite BN, O'Reilly EM, Goldberg RM, Hochster HS, Schilsky RL, Bertagnolli MM, El-Khoueiry AB, Watson P, Benson AB 3rd, Mulkerin DL, Mayer RJ, Blanke C. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA. 2017;317:2392–2401. doi: 10.1001/jama.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 117.Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C, Steffens CC, Alonso-Orduña V, Schlichting C, Reyes-Rivera I, Bendahmane B, André T, Kubicka S ML18147 Study Investigators. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 118.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 119.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Van Cutsem E, Grothey A, Prausová J, Garcia-Alfonso P, Yamazaki K, Clingan PR, Lonardi S, Kim TW, Simms L, Chang SC, Nasroulah F RAISE Study Investigators. Ramucirumab vs placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 120.Argilés G, Saunders MP, Rivera F, Sobrero A, Benson A 3rd, Guillén Ponce C, Cascinu S, Van Cutsem E, Macpherson IR, Strumberg D, Köhne CH, Zalcberg J, Wagner A, Luigi Garosi V, Grunert J, Tabernero J, Ciardiello F. Regorafenib plus modified FOLFOX6 as first-line treatment of metastatic colorectal cancer: A phase II trial. Eur J Cancer. 2015;51:942–949. doi: 10.1016/j.ejca.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 121.Rousseau B, Boukerma AK, Henriques J, Cohen R, Lucidarme O, Borg C, Tournigand C, Kim S, Bachet JB, Mazard T, Louvet C, Chibaudel B, Vernerey D, Andre T, Hulin A. Impact of trough concentrations of regorafenib and its major metabolites M-2 and M-5 on overall survival of chemorefractory metastatic colorectal cancer patients: Results from a multicentre GERCOR TEXCAN phase II study. Eur J Cancer. 2022;168:99–107. doi: 10.1016/j.ejca.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 123.Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L, Yeh KH, Bi F, Cheng Y, Le AT, Lin JK, Liu T, Ma D, Kappeler C, Kalmus J, Kim TW CONCUR Investigators. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 124.Liu Y, Xiao Q, He J, Hu H, Du J, Zhu Y, Chen J, Liu Z, Wang J, Sun L, Xu D, Li J, Liao X, Cai Y, Cai C, Jin Z, Wang L, Yuan Y, Ding K. Phase II study of anlotinib in combination with oxaliplatin and capecitabine for patients with RAS/BRAF wild-type metastatic colorectal adenocarcinoma as the first-line therapy. BMC Med. 2022;20:155. doi: 10.1186/s12916-022-02357-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, Yang L, Deng Y, Chen ZD, Zhong H, Pan H, Guo W, Shu Y, Yuan Y, Zhou J, Xu N, Liu T, Ma D, Wu C, Cheng Y, Chen D, Li W, Sun S, Yu Z, Cao P, Chen H, Wang J, Wang S, Wang H, Fan S, Hua Y, Su W. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319:2486–2496. doi: 10.1001/jama.2018.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu RH, Shen L, Wang KM, Wu G, Shi CM, Ding KF, Lin LZ, Wang JW, Xiong JP, Wu CP, Li J, Liu YP, Wang D, Ba Y, Feng JP, Bai YX, Bi JW, Ma LW, Lei J, Yang Q, Yu H. Famitinib vs placebo in the treatment of refractory metastatic colorectal cancer: a multicenter, randomized, double-blinded, placebo-controlled, phase II clinical trial. Chin J Cancer. 2017;36:97. doi: 10.1186/s40880-017-0263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Roock W, De Vriendt V, Normanno N, Ciardiello F, Tejpar S. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011;12:594–603. doi: 10.1016/S1470-2045(10)70209-6. [DOI] [PubMed] [Google Scholar]
- 128.Sanz-Garcia E, Argiles G, Elez E, Tabernero J. BRAF mutant colorectal cancer: prognosis, treatment, and new perspectives. Ann Oncol. 2017;28:2648–2657. doi: 10.1093/annonc/mdx401. [DOI] [PubMed] [Google Scholar]
- 129.Pentheroudakis G, Kotoula V, De Roock W, Kouvatseas G, Papakostas P, Makatsoris T, Papamichael D, Xanthakis I, Sgouros J, Televantou D, Kafiri G, Tsamandas AC, Razis E, Galani E, Bafaloukos D, Efstratiou I, Bompolaki I, Pectasides D, Pavlidis N, Tejpar S, Fountzilas G. Biomarkers of benefit from cetuximab-based therapy in metastatic colorectal cancer: interaction of EGFR ligand expression with RAS/RAF, PIK3CA genotypes. BMC Cancer. 2013;13:49. doi: 10.1186/1471-2407-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao B, Wang L, Qiu H, Zhang M, Sun L, Peng P, Yu Q, Yuan X. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget. 2017;8:3980–4000. doi: 10.18632/oncotarget.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yaeger R, Saltz L. BRAF mutations in colorectal cancer: clinical relevance and role in targeted therapy. J Natl Compr Canc Netw. 2012;10:1456–1458. doi: 10.6004/jnccn.2012.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Capalbo C, Marchetti P, Coppa A, Calogero A, Anastasi E, Buffone A, Belardinilli F, Gulino M, Frati P, Catalano C, Cortesi E, Giannini G, Gulino A. Vemurafenib and panitumumab combination tailored therapy in BRAF-mutated metastatic colorectal cancer: a case report. Cancer Biol Ther. 2014;15:826–831. doi: 10.4161/cbt.28878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen J, Ye Y, Sun H, Shi G. Association between KRAS codon 13 mutations and clinical response to anti-EGFR treatment in patients with metastatic colorectal cancer: results from a meta-analysis. Cancer Chemother Pharmacol. 2013;71:265–272. doi: 10.1007/s00280-012-2005-9. [DOI] [PubMed] [Google Scholar]
- 134.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C, Aranda E, Nordlinger B, Cisar L, Labianca R, Cunningham D, Van Cutsem E, Bosman F. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 135.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R Cancer Genome Project. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 136.Kopetz S, Guthrie KA, Morris VK, Lenz HJ, Magliocco AM, Maru D, Yan Y, Lanman R, Manyam G, Hong DS, Sorokin A, Atreya CE, Diaz LA, Allegra C, Raghav KP, Wang SE, Lieu CH, McDonough SL, Philip PA, Hochster HS. Randomized Trial of Irinotecan and Cetuximab With or Without Vemurafenib in BRAF-Mutant Metastatic Colorectal Cancer (SWOG S1406) J Clin Oncol. 2021;39:285–294. doi: 10.1200/JCO.20.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Van Cutsem E, Huijberts S, Grothey A, Yaeger R, Cuyle PJ, Elez E, Fakih M, Montagut C, Peeters M, Yoshino T, Wasan H, Desai J, Ciardiello F, Gollerkeri A, Christy-Bittel J, Maharry K, Sandor V, Schellens JHM, Kopetz S, Tabernero J. Binimetinib, Encorafenib, and Cetuximab Triplet Therapy for Patients With BRAF V600E-Mutant Metastatic Colorectal Cancer: Safety Lead-In Results From the Phase III BEACON Colorectal Cancer Study. J Clin Oncol. 2019;37:1460–1469. doi: 10.1200/JCO.18.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Holderfield M, Deuker MM, McCormick F, McMahon M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer. 2014;14:455–467. doi: 10.1038/nrc3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mizukami T, Izawa N, Nakajima TE, Sunakawa Y. Targeting EGFR and RAS/RAF Signaling in the Treatment of Metastatic Colorectal Cancer: From Current Treatment Strategies to Future Perspectives. Drugs. 2019;79:633–645. doi: 10.1007/s40265-019-01113-0. [DOI] [PubMed] [Google Scholar]
- 140.Corcoran RB, André T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, Hollebecque A, McRee AJ, Siena S, Middleton G, Muro K, Gordon MS, Tabernero J, Yaeger R, O'Dwyer PJ, Humblet Y, De Vos F, Jung AS, Brase JC, Jaeger S, Bettinger S, Mookerjee B, Rangwala F, Van Cutsem E. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAFV600E-Mutant Colorectal Cancer. Cancer Discov. 2018;8:428–443. doi: 10.1158/2159-8290.CD-17-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Corcoran RB, Atreya CE, Falchook GS, Kwak EL, Ryan DP, Bendell JC, Hamid O, Messersmith WA, Daud A, Kurzrock R, Pierobon M, Sun P, Cunningham E, Little S, Orford K, Motwani M, Bai Y, Patel K, Venook AP, Kopetz S. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J Clin Oncol. 2015;33:4023–4031. doi: 10.1200/JCO.2015.63.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Pfeiffer P, Orlov S, Lonardi S, Elez E, Kim TW, Schellens JHM, Guo C, Krishnan A, Dekervel J, Morris V, Calvo Ferrandiz A, Tarpgaard LS, Braun M, Gollerkeri A, Keir C, Maharry K, Pickard M, Christy-Bittel J, Anderson L, Sandor V, Tabernero J. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 143.Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7:122–136. doi: 10.1177/1758834014566428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 145.Takeuchi H, Bilchik A, Saha S, Turner R, Wiese D, Tanaka M, Kuo C, Wang HJ, Hoon DS. c-MET expression level in primary colon cancer: a predictor of tumor invasion and lymph node metastases. Clin Cancer Res. 2003;9:1480–1488. [PubMed] [Google Scholar]
- 146.Song EK, Tai WM, Messersmith WA, Bagby S, Purkey A, Quackenbush KS, Pitts TM, Wang G, Blatchford P, Yahn R, Kaplan J, Tan AC, Atreya CE, Eckhardt G, Kelley RK, Venook A, Kwak EL, Ryan D, Arcaroli JJ. Potent antitumor activity of cabozantinib, a c-MET and VEGFR2 inhibitor, in a colorectal cancer patient-derived tumor explant model. Int J Cancer. 2015;136:1967–1975. doi: 10.1002/ijc.29225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.García-Aranda M, Redondo M. Targeting Receptor Kinases in Colorectal Cancer. Cancers (Basel) 2019;11 doi: 10.3390/cancers11040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bardelli A, Corso S, Bertotti A, Hobor S, Valtorta E, Siravegna G, Sartore-Bianchi A, Scala E, Cassingena A, Zecchin D, Apicella M, Migliardi G, Galimi F, Lauricella C, Zanon C, Perera T, Veronese S, Corti G, Amatu A, Gambacorta M, Diaz LA Jr, Sausen M, Velculescu VE, Comoglio P, Trusolino L, Di Nicolantonio F, Giordano S, Siena S. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 2013;3:658–673. doi: 10.1158/2159-8290.CD-12-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Van Cutsem E, Eng C, Nowara E, Swieboda-Sadlej A, Tebbutt NC, Mitchell E, Davidenko I, Stephenson J, Elez E, Prenen H, Deng H, Tang R, McCaffery I, Oliner KS, Chen L, Gansert J, Loh E, Smethurst D, Tabernero J. Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab vs panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer. Clin Cancer Res. 2014;20:4240–4250. doi: 10.1158/1078-0432.CCR-13-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bendell JC, Hochster H, Hart LL, Firdaus I, Mace JR, McFarlane JJ, Kozloff M, Catenacci D, Hsu JJ, Hack SP, Shames DS, Phan SC, Koeppen H, Cohn AL. A Phase II Randomized Trial (GO27827) of First-Line FOLFOX Plus Bevacizumab with or Without the MET Inhibitor Onartuzumab in Patients with Metastatic Colorectal Cancer. Oncologist. 2017;22:264–271. doi: 10.1634/theoncologist.2016-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao S, Wu W, Jiang H, Ma L, Pan C, Jin C, Mo J, Wang L, Wang K. Selective Inhibitor of the c-Met Receptor Tyrosine Kinase in Advanced Hepatocellular Carcinoma: No Beneficial Effect With the Use of Tivantinib? Front Immunol. 2021;12:731527. doi: 10.3389/fimmu.2021.731527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Eng C, Bessudo A, Hart LL, Severtsev A, Gladkov O, Müller L, Kopp MV, Vladimirov V, Langdon R, Kotiv B, Barni S, Hsu C, Bolotin E, von Roemeling R, Schwartz B, Bendell JC. A randomized, placebo-controlled, phase 1/2 study of tivantinib (ARQ 197) in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with wild-type KRAS who have received first-line systemic therapy. Int J Cancer. 2016;139:177–186. doi: 10.1002/ijc.30049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rimassa L, Bozzarelli S, Pietrantonio F, Cordio S, Lonardi S, Toppo L, Zaniboni A, Bordonaro R, Di Bartolomeo M, Tomasello G, Dadduzio V, Tronconi MC, Piombo C, Giordano L, Gloghini A, Di Tommaso L, Santoro A. Phase II Study of Tivantinib and Cetuximab in Patients With KRAS Wild-type Metastatic Colorectal Cancer With Acquired Resistance to EGFR Inhibitors and Emergence of MET Overexpression: Lesson Learned for Future Trials With EGFR/MET Dual Inhibition. Clin Colorectal Cancer. 2019;18:125–132.e2. doi: 10.1016/j.clcc.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 154.He AR, Cohen RB, Denlinger CS, Sama A, Birnbaum A, Hwang J, Sato T, Lewis N, Mynderse M, Niland M, Giles J, Wallin J, Moser B, Zhang W, Walgren R, Plimack ER. First-in-Human Phase I Study of Merestinib, an Oral Multikinase Inhibitor, in Patients with Advanced Cancer. Oncologist. 2019;24:e930–e942. doi: 10.1634/theoncologist.2018-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Safaie Qamsari E, Safaei Ghaderi S, Zarei B, Dorostkar R, Bagheri S, Jadidi-Niaragh F, Somi MH, Yousefi M. The c-Met receptor: Implication for targeted therapies in colorectal cancer. Tumour Biol. 2017;39:1010428317699118. doi: 10.1177/1010428317699118. [DOI] [PubMed] [Google Scholar]
- 156.Otte JM, Schmitz F, Kiehne K, Stechele HU, Banasiewicz T, Krokowicz P, Nakamura T, Fölsch UR, Herzig K. Functional expression of HGF and its receptor in human colorectal cancer. Digestion. 2000;61:237–246. doi: 10.1159/000007764. [DOI] [PubMed] [Google Scholar]
- 157.Chen RL, Zhao J, Zhang XC, Lou NN, Chen HJ, Yang X, Su J, Xie Z, Zhou Q, Tu HY, Zhong WZ, Yan HH, Guo WB, Wu YL, Yang JJ. Crizotinib in advanced non-small-cell lung cancer with concomitant ALK rearrangement and c-Met overexpression. BMC Cancer. 2018;18:1171. doi: 10.1186/s12885-018-5078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.He X, Jiao XD, Liu K, Qin BD, Wu Y, Ling Y, Liu J, Xu AQ, Song K, Zang YS. Clinical Responses to Crizotinib, Alectinib, and Lorlatinib in a Metastatic Colorectal Carcinoma Patient With ALK Gene Rearrangement: A Case Report. JCO Precis Oncol. 2021;5 doi: 10.1200/PO.20.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cuneo KC, Mehta RK, Kurapati H, Thomas DG, Lawrence TS, Nyati MK. Enhancing the Radiation Response in KRAS Mutant Colorectal Cancers Using the c-Met Inhibitor Crizotinib. Transl Oncol. 2019;12:209–216. doi: 10.1016/j.tranon.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Markman JL, Shiao SL. Impact of the immune system and immunotherapy in colorectal cancer. J Gastrointest Oncol. 2015;6:208–223. doi: 10.3978/j.issn.2078-6891.2014.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kalyan A, Kircher S, Shah H, Mulcahy M, Benson A. Updates on immunotherapy for colorectal cancer. J Gastrointest Oncol. 2018;9:160–169. doi: 10.21037/jgo.2018.01.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Boukouris AE, Theochari M, Stefanou D, Papalambros A, Felekouras E, Gogas H, Ziogas DC. Latest evidence on immune checkpoint inhibitors in metastatic colorectal cancer: A 2022 update. Crit Rev Oncol Hematol. 2022;173:103663. doi: 10.1016/j.critrevonc.2022.103663. [DOI] [PubMed] [Google Scholar]
- 165.Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10:727–742. [PMC free article] [PubMed] [Google Scholar]
- 166.Wang HB, Yao H, Li CS, Liang LX, Zhang Y, Chen YX, Fang JY, Xu J. Rise of PD-L1 expression during metastasis of colorectal cancer: Implications for immunotherapy. J Dig Dis. 2017;18:574–581. doi: 10.1111/1751-2980.12538. [DOI] [PubMed] [Google Scholar]
- 167.Lee HT, Lee SH, Heo YS. Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology. Molecules. 2019;24 doi: 10.3390/molecules24061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Venderbosch S, Nagtegaal ID, Maughan TS, Smith CG, Cheadle JP, Fisher D, Kaplan R, Quirke P, Seymour MT, Richman SD, Meijer GA, Ylstra B, Heideman DA, de Haan AF, Punt CJ, Koopman M. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20:5322–5330. doi: 10.1158/1078-0432.CCR-14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, Agarwal A, Maru DM, Sieber O, Desai J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M, Sukawa Y, Stewart C, Rosenberg M, Mima K, Inamura K, Nosho K, Nowak JA, Lawrence MS, Giovannucci EL, Chan AT, Ng K, Meyerhardt JA, Van Allen EM, Getz G, Gabriel SB, Lander ES, Wu CJ, Fuchs CS, Ogino S, Garraway LA. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;15:857–865. doi: 10.1016/j.celrep.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, Miller VA, Lim D, Amanam I, Chao J, Catenacci D, Cho M, Braiteh F, Klempner SJ, Ali SM, Fakih M. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019;30:1096–1103. doi: 10.1093/annonc/mdz134. [DOI] [PubMed] [Google Scholar]
- 174.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TB, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, Van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.O'Neil BH, Wallmark JM, Lorente D, Elez E, Raimbourg J, Gomez-Roca C, Ejadi S, Piha-Paul SA, Stein MN, Abdul Razak AR, Dotti K, Santoro A, Cohen RB, Gould M, Saraf S, Stein K, Han SW. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12:e0189848. doi: 10.1371/journal.pone.0189848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, Burge M, O'Neil B, Kavan P, Yoshino T, Guimbaud R, Taniguchi H, Elez E, Al-Batran SE, Boland PM, Crocenzi T, Atreya CE, Cui Y, Dai T, Marinello P, Diaz LA Jr, André T. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol. 2020;38:11–19. doi: 10.1200/JCO.19.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, André T. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 179.Morse MA, Overman MJ, Hartman L, Khoukaz T, Brutcher E, Lenz HJ, Atasoy A, Shangguan T, Zhao H, El-Rayes B. Safety of Nivolumab plus Low-Dose Ipilimumab in Previously Treated Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer. Oncologist. 2019;24:1453–1461. doi: 10.1634/theoncologist.2019-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA Jr. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386:2363–2376. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yu X, Huang X, Chen X, Liu J, Wu C, Pu Q, Wang Y, Kang X, Zhou L. Characterization of a novel anti-human lymphocyte activation gene 3 (LAG-3) antibody for cancer immunotherapy. MAbs. 2019;11:1139–1148. doi: 10.1080/19420862.2019.1629239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Strosberg JR, Yeatman T, Weber J, Coppola D, Schell MJ, Han G, Almhanna K, Kim R, Valone T, Jump H, Sullivan D. A phase II study of RO4929097 in metastatic colorectal cancer. Eur J Cancer. 2012;48:997–1003. doi: 10.1016/j.ejca.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Berlin J, Bendell JC, Hart LL, Firdaus I, Gore I, Hermann RC, Mulcahy MF, Zalupski MM, Mackey HM, Yauch RL, Graham RA, Bray GL, Low JA. A randomized phase II trial of vismodegib vs placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin Cancer Res. 2013;19:258–267. doi: 10.1158/1078-0432.CCR-12-1800. [DOI] [PubMed] [Google Scholar]
- 184.Yokoyama Y, Watanabe T, Tamura Y, Hashizume Y, Miyazono K, Ehata S. Autocrine BMP-4 Signaling Is a Therapeutic Target in Colorectal Cancer. Cancer Res. 2017;77:4026–4038. doi: 10.1158/0008-5472.CAN-17-0112. [DOI] [PubMed] [Google Scholar]
- 185.Villalba M, Evans SR, Vidal-Vanaclocha F, Calvo A. Role of TGF-β in metastatic colon cancer: it is finally time for targeted therapy. Cell Tissue Res. 2017;370:29–39. doi: 10.1007/s00441-017-2633-9. [DOI] [PubMed] [Google Scholar]
- 186.Ghosh N, Hossain U, Mandal A, Sil PC. The Wnt signaling pathway: a potential therapeutic target against cancer. Ann N Y Acad Sci. 2019;1443:54–74. doi: 10.1111/nyas.14027. [DOI] [PubMed] [Google Scholar]
- 187.Guo Q, Grimmig T, Gonzalez G, Giobbie-Hurder A, Berg G, Carr N, Wilson BJ, Banerjee P, Ma J, Gold JS, Nandi B, Huang Q, Waaga-Gasser AM, Lian CG, Murphy GF, Frank MH, Gasser M, Frank NY. ATP-binding cassette member B5 (ABCB5) promotes tumor cell invasiveness in human colorectal cancer. J Biol Chem. 2018;293:11166–11178. doi: 10.1074/jbc.RA118.003187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tan AC, Bagley SJ, Wen PY, Lim M, Platten M, Colman H, Ashley DM, Wick W, Chang SM, Galanis E, Mansouri A, Khagi S, Mehta MP, Heimberger AB, Puduvalli VK, Reardon DA, Sahebjam S, Simes J, Antonia SJ, Berry D, Khasraw M. Systematic review of combinations of targeted or immunotherapy in advanced solid tumors. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, Mussolin B, Kwak EL, Buscarino M, Lazzari L, Valtorta E, Truini M, Jessop NA, Robinson HE, Hong TS, Mino-Kenudson M, Di Nicolantonio F, Thabet A, Sartore-Bianchi A, Siena S, Iafrate AJ, Bardelli A, Corcoran RB. Tumor Heterogeneity and Lesion-Specific Response to Targeted Therapy in Colorectal Cancer. Cancer Discov. 2016;6:147–153. doi: 10.1158/2159-8290.CD-15-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, Ponzetti A, Cremolini C, Amatu A, Lauricella C, Lamba S, Hobor S, Avallone A, Valtorta E, Rospo G, Medico E, Motta V, Antoniotti C, Tatangelo F, Bellosillo B, Veronese S, Budillon A, Montagut C, Racca P, Marsoni S, Falcone A, Corcoran RB, Di Nicolantonio F, Loupakis F, Siena S, Sartore-Bianchi A, Bardelli A. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:795–801. doi: 10.1038/nm.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Benavides M, Alcaide-Garcia J, Torres E, Gil-Calle S, Sevilla I, Wolman R, Durán G, Álvarez M, Reyna-Fortes C, Ales I, Pereda T, Robles M, Kushnir M, Odegaard J, Faull I, Alba E. Clinical utility of comprehensive circulating tumor DNA genotyping compared with standard of care tissue testing in patients with newly diagnosed metastatic colorectal cancer. ESMO Open. 2022;7:100481. doi: 10.1016/j.esmoop.2022.100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Güttlein L, Luca MR, Esteso F, Fresno C, Mariani J, Otero Pizarro M, Brest E, Starapoli S, Kreimberg K, Teves P, Mendoza Bertelli A, R Girotti M, Salanova R, O'Connor JM. Liquid biopsy for KRAS, NRAS and BRAF mutation testing in advanced colorectal cancer patients: the Argentinean experience. Future Oncol. 2022;18:3277–3287. doi: 10.2217/fon-2022-0329. [DOI] [PubMed] [Google Scholar]
- 193.Kumbrink J, Bohlmann L, Mamlouk S, Redmer T, Peilstöcker D, Li P, Lorenzen S, Algül H, Kasper S, Hempel D, Kaiser F, Michl M, Bartsch H, Neumann J, Klauschen F, von Bergwelt-Baildon M, Modest DP, Stahler A, Stintzing S, Jung A, Kirchner T, Schäfer R, Heinemann V, Holch JW. Serial Analysis of Gene Mutations and Gene Expression during First-Line Chemotherapy against Metastatic Colorectal Cancer: Identification of Potentially Actionable Targets within the Multicenter Prospective Biomarker Study REVEAL. Cancers (Basel) 2022;14 doi: 10.3390/cancers14153631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sartore-Bianchi A, Pietrantonio F, Lonardi S, Mussolin B, Rua F, Crisafulli G, Bartolini A, Fenocchio E, Amatu A, Manca P, Bergamo F, Tosi F, Mauri G, Ambrosini M, Daniel F, Torri V, Vanzulli A, Regge D, Cappello G, Marchiò C, Berrino E, Sapino A, Marsoni S, Siena S, Bardelli A. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat Med. 2022;28:1612–1618. doi: 10.1038/s41591-022-01886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chennamadhavuni A, Kasi PM. Circulating Tumor DNA in Identifying Resistant Sub-Clones Post EGFR Blockade: Implications for EGFR Rechallenge. Front Oncol. 2022;12:847299. doi: 10.3389/fonc.2022.847299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Santos C, Azuara D, Viéitez JM, Páez D, Falcó E, Élez E, López-López C, Valladares M, Robles-Díaz L, García-Alfonso P, Bugés C, Durán G, Salud A, Navarro V, Capellá G, Aranda E, Salazar R. Phase II study of high-sensitivity genotyping of KRAS, NRAS, BRAF and PIK3CA to ultra-select metastatic colorectal cancer patients for panitumumab plus FOLFIRI: the ULTRA trial. Ann Oncol. 2019;30:796–803. doi: 10.1093/annonc/mdz082. [DOI] [PubMed] [Google Scholar]
- 197.Grasselli J, Elez E, Caratù G, Matito J, Santos C, Macarulla T, Vidal J, Garcia M, Viéitez JM, Paéz D, Falcó E, Lopez Lopez C, Aranda E, Jones F, Sikri V, Nuciforo P, Fasani R, Tabernero J, Montagut C, Azuara D, Dienstmann R, Salazar R, Vivancos A. Concordance of blood- and tumor-based detection of RAS mutations to guide anti-EGFR therapy in metastatic colorectal cancer. Ann Oncol. 2017;28:1294–1301. doi: 10.1093/annonc/mdx112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98. doi: 10.1016/j.ejca.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 199.Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP, Tabernero J, Cervantes A, Ciardiello F. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713–1729. doi: 10.1093/annonc/mdx175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Moretto R, Cremolini C, Rossini D, Pietrantonio F, Battaglin F, Mennitto A, Bergamo F, Loupakis F, Marmorino F, Berenato R, Marsico VA, Caporale M, Antoniotti C, Masi G, Salvatore L, Borelli B, Fontanini G, Lonardi S, De Braud F, Falcone A. Location of Primary Tumor and Benefit From Anti-Epidermal Growth Factor Receptor Monoclonal Antibodies in Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer. Oncologist. 2016;21:988–994. doi: 10.1634/theoncologist.2016-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhou J, Ji Q, Li Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: underlying mechanisms and reversal strategies. J Exp Clin Cancer Res. 2021;40:328. doi: 10.1186/s13046-021-02130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Arena S, Siravegna G, Mussolin B, Kearns JD, Wolf BB, Misale S, Lazzari L, Bertotti A, Trusolino L, Adjei AA, Montagut C, Di Nicolantonio F, Nering R, Bardelli A. MM-151 overcomes acquired resistance to cetuximab and panitumumab in colorectal cancers harboring EGFR extracellular domain mutations. Sci Transl Med. 2016;8:324ra14. doi: 10.1126/scitranslmed.aad5640. [DOI] [PubMed] [Google Scholar]
- 203.Bagchi A, Haidar JN, Eastman SW, Vieth M, Topper M, Iacolina MD, Walker JM, Forest A, Shen Y, Novosiadly RD, Ferguson KM. Molecular Basis for Necitumumab Inhibition of EGFR Variants Associated with Acquired Cetuximab Resistance. Mol Cancer Ther. 2018;17:521–531. doi: 10.1158/1535-7163.MCT-17-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sánchez-Martín FJ, Bellosillo B, Gelabert-Baldrich M, Dalmases A, Cañadas I, Vidal J, Martinez A, Argilés G, Siravegna G, Arena S, Koefoed K, Visa L, Arpí O, Horak ID, Iglesias M, Stroh C, Kragh M, Rovira A, Albanell J, Tabernero J, Bardelli A, Montagut C. The First-in-class Anti-EGFR Antibody Mixture Sym004 Overcomes Cetuximab Resistance Mediated by EGFR Extracellular Domain Mutations in Colorectal Cancer. Clin Cancer Res. 2016;22:3260–3267. doi: 10.1158/1078-0432.CCR-15-2400. [DOI] [PubMed] [Google Scholar]
- 205.Yang L, Bhattacharya A, Li Y, Sexton S, Ling X, Li F, Zhang Y. Depleting receptor tyrosine kinases EGFR and HER2 overcomes resistance to EGFR inhibitors in colorectal cancer. J Exp Clin Cancer Res. 2022;41:184. doi: 10.1186/s13046-022-02389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee HW, Son E, Lee K, Lee Y, Kim Y, Lee JC, Lim Y, Hur M, Kim D, Nam DH. Promising Therapeutic Efficacy of GC1118, an Anti-EGFR Antibody, against KRAS Mutation-Driven Colorectal Cancer Patient-Derived Xenografts. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20235894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sidaway P. Microenvironment plasticity confers cetuximab resistance. Nat Rev Clin Oncol. 2019;16:527. doi: 10.1038/s41571-019-0259-4. [DOI] [PubMed] [Google Scholar]
- 208.Parseghian CM, Napolitano S, Loree JM, Kopetz S. Mechanisms of Innate and Acquired Resistance to Anti-EGFR Therapy: A Review of Current Knowledge with a Focus on Rechallenge Therapies. Clin Cancer Res. 2019;25:6899–6908. doi: 10.1158/1078-0432.CCR-19-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Martinelli E, Ciardiello D, Martini G, Troiani T, Cardone C, Vitiello PP, Normanno N, Rachiglio AM, Maiello E, Latiano T, De Vita F, Ciardiello F. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: challenges and future perspectives. Ann Oncol. 2020;31:30–40. doi: 10.1016/j.annonc.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 210.Schulz MS, Wolf S, Struck V, Thomas N, Husman G, Zeuzem S, Koch C, Trojan J, Schnitzbauer AA, Bechstein WO, Waidmann O. Anti-EGFR Reintroduction and Rechallenge in Metastatic Colorectal Cancer (mCRC): A Real-World Analysis. Cancers (Basel) 2022;14 doi: 10.3390/cancers14071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen C, Di Bartolomeo M, Corallo S, Strickler JH, Goyal L. Overcoming Resistance to Targeted Therapies in Gastrointestinal Cancers: Progress to Date and Progress to Come. Am Soc Clin Oncol Educ Book. 2020;40:161–173. doi: 10.1200/EDBK_280871. [DOI] [PubMed] [Google Scholar]
- 212.de Angelo RM, Almeida MO, de Paula H, Honorio KM. Studies on the Dual Activity of EGFR and HER-2 Inhibitors Using Structure-Based Drug Design Techniques. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19123728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Reid A, Vidal L, Shaw H, de Bono J. Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu) Eur J Cancer. 2007;43:481–489. doi: 10.1016/j.ejca.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 214.Ahcene Djaballah S, Daniel F, Milani A, Ricagno G, Lonardi S. HER2 in Colorectal Cancer: The Long and Winding Road From Negative Predictive Factor to Positive Actionable Target. Am Soc Clin Oncol Educ Book. 2022;42:1–14. doi: 10.1200/EDBK_351354. [DOI] [PubMed] [Google Scholar]
- 215.Burris HA 3rd, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, Tan-Chiu E, Krop IE, Michaelson RA, Girish S, Amler L, Zheng M, Chu YW, Klencke B, O'Shaughnessy JA. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 216.Lopes-Coelho F, Martins F, Pereira SA, Serpa J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22073765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lieu CH, Tran H, Jiang ZQ, Mao M, Overman MJ, Lin E, Eng C, Morris J, Ellis L, Heymach JV, Kopetz S. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. PLoS One. 2013;8:e77117. doi: 10.1371/journal.pone.0077117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Heil F, Babitzki G, Julien-Laferriere A, Ooi CH, Hidalgo M, Massard C, Martinez-Garcia M, Le Tourneau C, Kockx M, Gerber P, Rossomanno S, Krieter O, Lahr A, Wild N, Harring SV, Lechner K. Vanucizumab mode of action: Serial biomarkers in plasma, tumor, and skin-wound-healing biopsies. Transl Oncol. 2021;14:100984. doi: 10.1016/j.tranon.2020.100984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 220.Escudier B, Powles T, Motzer RJ, Olencki T, Arén Frontera O, Oudard S, Rolland F, Tomczak P, Castellano D, Appleman LJ, Drabkin H, Vaena D, Milwee S, Youkstetter J, Lougheed JC, Bracarda S, Choueiri TK. Cabozantinib, a New Standard of Care for Patients With Advanced Renal Cell Carcinoma and Bone Metastases? J Clin Oncol. 2018;36:765–772. doi: 10.1200/JCO.2017.74.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, Kwon BS. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35 Suppl:S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 222.Bai R, Chen N, Li L, Du N, Bai L, Lv Z, Tian H, Cui J. Mechanisms of Cancer Resistance to Immunotherapy. Front Oncol. 2020;10:1290. doi: 10.3389/fonc.2020.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30:507–519. doi: 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fiegle E, Doleschel D, Koletnik S, Rix A, Weiskirchen R, Borkham-Kamphorst E, Kiessling F, Lederle W. Dual CTLA-4 and PD-L1 Blockade Inhibits Tumor Growth and Liver Metastasis in a Highly Aggressive Orthotopic Mouse Model of Colon Cancer. Neoplasia. 2019;21:932–944. doi: 10.1016/j.neo.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rupp T, Genest L, Babin D, Legrand C, Hunault M, Froget G, Castagné V. Anti-CTLA-4 and anti-PD-1 immunotherapies repress tumor progression in preclinical breast and colon model with independent regulatory T cells response. Transl Oncol. 2022;20:101405. doi: 10.1016/j.tranon.2022.101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Monjazeb AM, Giobbie-Hurder A, Lako A, Thrash EM, Brennick RC, Kao KZ, Manuszak C, Gentzler RD, Tesfaye A, Jabbour SK, Alese OB, Rahma OE, Cleary JM, Sharon E, Mamon HJ, Cho M, Streicher H, Chen HX, Ahmed MM, Mariño-Enríquez A, Kim-Schulze S, Gnjatic S, Maverakis E, Marusina AI, Merleev AA, Severgnini M, Pfaff KL, Lindsay J, Weirather JL, Ranasinghe S, Spektor A, Rodig SJ, Hodi SF, Schoenfeld JD. A Randomized Trial of Combined PD-L1 and CTLA-4 Inhibition with Targeted Low-Dose or Hypofractionated Radiation for Patients with Metastatic Colorectal Cancer. Clin Cancer Res. 2021;27:2470–2480. doi: 10.1158/1078-0432.CCR-20-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Byrne AT, Alférez DG, Amant F, Annibali D, Arribas J, Biankin AV, Bruna A, Budinská E, Caldas C, Chang DK, Clarke RB, Clevers H, Coukos G, Dangles-Marie V, Eckhardt SG, Gonzalez-Suarez E, Hermans E, Hidalgo M, Jarzabek MA, de Jong S, Jonkers J, Kemper K, Lanfrancone L, Mælandsmo GM, Marangoni E, Marine JC, Medico E, Norum JH, Palmer HG, Peeper DS, Pelicci PG, Piris-Gimenez A, Roman-Roman S, Rueda OM, Seoane J, Serra V, Soucek L, Vanhecke D, Villanueva A, Vinolo E, Bertotti A, Trusolino L. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17:254–268. doi: 10.1038/nrc.2016.140. [DOI] [PubMed] [Google Scholar]
- 228.Azar J, Bahmad HF, Daher D, Moubarak MM, Hadadeh O, Monzer A, Al Bitar S, Jamal M, Al-Sayegh M, Abou-Kheir W. The Use of Stem Cell-Derived Organoids in Disease Modeling: An Update. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22147667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Al Bitar S, Ballout F, Monzer A, Kanso M, Saheb N, Mukherji D, Faraj W, Tawil A, Doughan S, Hussein M, Abou-Kheir W, Gali-Muhtasib H. Thymoquinone Radiosensitizes Human Colorectal Cancer Cells in 2D and 3D Culture Models. Cancers (Basel) 2022;14 doi: 10.3390/cancers14061363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Monzer A, Wakimian K, Ballout F, Al Bitar S, Yehya A, Kanso M, Saheb N, Tawil A, Doughan S, Hussein M, Mukherji D, Faraj D, Gali-Muhtasib H, Abou-Kheir W. Novel therapeutic diiminoquinone exhibits anticancer effects on human colorectal cancer cells in two-dimensional and three-dimensional in vitro models. World J Gastroenterol. 2022;28:4787–4811. doi: 10.3748/wjg.v28.i33.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 232.Yoshida GJ. Applications of patient-derived tumor xenograft models and tumor organoids. J Hematol Oncol. 2020;13:4. doi: 10.1186/s13045-019-0829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Takeda H, Kataoka S, Nakayama M, Ali MAE, Oshima H, Yamamoto D, Park JW, Takegami Y, An T, Jenkins NA, Copeland NG, Oshima M. CRISPR-Cas9-mediated gene knockout in intestinal tumor organoids provides functional validation for colorectal cancer driver genes. Proc Natl Acad Sci U S A. 2019;116:15635–15644. doi: 10.1073/pnas.1904714116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, Watanabe T, Kanai T, Sato T. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med. 2015;21:256–262. doi: 10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- 235.van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L, McLaren-Douglas A, Blokker J, Jaksani S, Bartfeld S, Volckman R, van Sluis P, Li VS, Seepo S, Sekhar Pedamallu C, Cibulskis K, Carter SL, McKenna A, Lawrence MS, Lichtenstein L, Stewart C, Koster J, Versteeg R, van Oudenaarden A, Saez-Rodriguez J, Vries RG, Getz G, Wessels L, Stratton MR, McDermott U, Meyerson M, Garnett MJ, Clevers H. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford I, Burke R, Rata M, Koh DM, Tunariu N, Collins D, Hulkki-Wilson S, Ragulan C, Spiteri I, Moorcraft SY, Chau I, Rao S, Watkins D, Fotiadis N, Bali M, Darvish-Damavandi M, Lote H, Eltahir Z, Smyth EC, Begum R, Clarke PA, Hahne JC, Dowsett M, de Bono J, Workman P, Sadanandam A, Fassan M, Sansom OJ, Eccles S, Starling N, Braconi C, Sottoriva A, Robinson SP, Cunningham D, Valeri N. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:920–926. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cho YW, Min DW, Kim HP, An Y, Kim S, Youk J, Chun J, Im JP, Song SH, Ju YS, Han SW, Park KJ, Kim TY. Patient-derived organoids as a preclinical platform for precision medicine in colorectal cancer. Mol Oncol. 2022;16:2396–2412. doi: 10.1002/1878-0261.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cao Y, Zhang G, Zhang J, Yang Y, Ren J, Yan X, Wang Z, Zhao Z, Huang X, Bao H, Zhou J. Predicting Microsatellite Instability Status in Colorectal Cancer Based on Triphasic Enhanced Computed Tomography Radiomics Signatures: A Multicenter Study. Front Oncol. 2021;11:687771. doi: 10.3389/fonc.2021.687771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang R, Li J, Zhou X, Mao Y, Wang W, Gao S, Gao Y, Chen K, Yu S, Wu X, Wen L, Ge H, Fu W, Tang F. Single-cell genomic and transcriptomic landscapes of primary and metastatic colorectal cancer tumors. Genome Med. 2022;14:93. doi: 10.1186/s13073-022-01093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]