Abstract
Background
Disseminated tumor cells (DTCs) in the bone marrow are observed in about 40% at primary diagnosis of breast cancer and predict poor survival. While anti-resorptive therapy with bisphosphonates was shown to eradicate minimal residue disease in the bone marrow, the effect of denosumab on DTCs, particularly in the neoadjuvant setting, is largely unknown. The recent GeparX clinical trial reported that denosumab, applied as an add-on treatment to nab-paclitaxel based neoadjuvant chemotherapy (NACT), did not improve the patient’s pathologic complete response (pCR) rate. Herein, we analyzed the predictive value of DTCs for the response to NACT and interrogated whether neoadjuvant denosumab treatment may eradicate DTCs in the bone marrow.
Methods
A total of 167 patients from the GeparX trial were analyzed for DTCs at baseline by immunocytochemistry using the pan-cytokeratin antibody A45-B/B3. Initially DTC-positive patients were re-analyzed for DTCs after NACT ± denosumab.
Results
At baseline, DTCs were observed in 43/167 patients (25.7%) in the total cohort, however their presence did not predict response to nab-paclitaxel based NACT (pCR rates: 37.1% in DTC-negative vs. 32.6% DTC-positive; p = 0.713). Regarding breast cancer subtypes, the presence of DTCs at baseline was numerically associated with response to NACT in TNBC patients (pCR rates: 40.0% in DTC-positive vs. 66.7% in DTC-negative patients; p = 0.16). Overall, denosumab treatment did not significantly increase the given DTC-eradication rate of NACT (NACT: 69.6% DTC-eradication vs. NACT + denosumab: 77.8% DTC-eradication; p = 0.726). In TNBC patients with pCR, a numerical but statistically non-significant increase of DTC-eradication after NACT + denosumab was observed (NACT: 75% DTC-eradication vs. NACT + denosumab: 100% DTC-eradication; p = 1.00).
Conclusion
This is the first study worldwide, demonstrating that neoadjuvant add-on denosumab over a short-term period of 24 months does not increase the DTC-eradication rate in breast cancer patients treated with NACT.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-023-01619-2.
Keywords: GeparX trial, Denosumab, Disseminated tumor cells, Bone marrow, Neoadjuvant chemotherapy
Introduction
Despite recent advances in early detection and systemic treatment, about 20–30% of patients with early breast cancer experience distant metastatic relapse. Recurrent disease can occur even years after primary treatment and constitutes the predominant cause of breast cancer specific death [1–4]. This is probably due to minimal residue disease, shaped by occult micrometastases, which have been seeded by early hematogenic dissemination [3, 5, 6]. Already at primary diagnosis of breast cancer, about 30–40% of patients have disseminated tumor cells (DTCs) in the bone marrow (BM) [7, 8]. It has been widely accepted that the presence of DTCs at primary diagnosis as well as their persistence after neoadjuvant chemotherapy (NACT) are both predictors of poor survival [7, 9–11].
Anti-resorptive agents, such as bisphosphonates, counteract osteoclast mediated bone-resorption and are widely used to treat patients, which suffer from bone metastasis induced skeletal adverse events or cancer treatment-induced bone loss and osteoporosis [12–15]. It is known that adjuvant bisphosphonates reduce the rate of breast cancer recurrence and improve prognosis in postmenopausal breast cancer patients [16, 17]. Moreover, oral ibandronate treatment of apparently disease-free patients was shown to completely eradicate persisting DTCs after 6–12 months [18], suggesting a direct effect of bisphosphates on micrometastasis in the bone marrow.
The human monoclonal IgG2 antibody denosumab represents a further class of anti-resorptive agents and targets receptor activator of nuclear factor-kappaB ligand (RANKL), [19]. Inactivation of RANKL by denosumab prevents RANKL signalling, which in turn reduces osteoclastic bone-resorption [20]. Comparable to bisphosphonates, denosumab is a well-established therapeutic option in breast cancer patients for the treatment of skeletal adverse events in metastatic bone disease, treatment-induced bone loss and osteoporosis [21, 22]. Moreover, RANK signaling was shown to contribute to the initiation and progression of breast cancer [23, 24]. Accordingly, RANKL and its receptor are highly expressed in breast cancer patients and predict poor prognosis [24–27].
Although there is a great body of pre-clinical evidence that RANK signaling promotes proliferation and (bone) metastatic progression of breast cancer [23, 28, 29], it still controversially discussed, whether targeted inhibition of RANK signalling by denosumab treatment will confer clinical benefit in patients with early breast cancer. While the ABCSG-18 trial showed that the addition of denosumab to adjuvant systemic treatment results in an improved disease-free survival [30], the D-CARE trial did not resolve any improvement of disease-related outcomes for high-risk early breast cancer patients, treated with denosumab [31]. Moreover, the phase IIb prospective randomized GeparX trial reported that denosumab, added to anthracycline/taxane-based NACT, did not improve pCR rates [32].
Serum RANKL levels were shown to be higher in DTC-positive compared to DTC-negative breast cancer patients and were reported to predict clinically manifest bone metastasis [33], suggesting a potential role of RANK-signaling in micrometastasis. However, whether denosumab eradicates DTCs, as it has been reported for bisphosphonates [18], is completely unknown. Using the framework of the recent GeparX study [32], we herein analyzed the clinical relevance of DTCs for predicting response to NACT and interrogated whether neoadjuvant denosumab treatment may eradicate DTCs in the bone marrow.
Patients and methods
Characterization of study patients and inclusion criteria
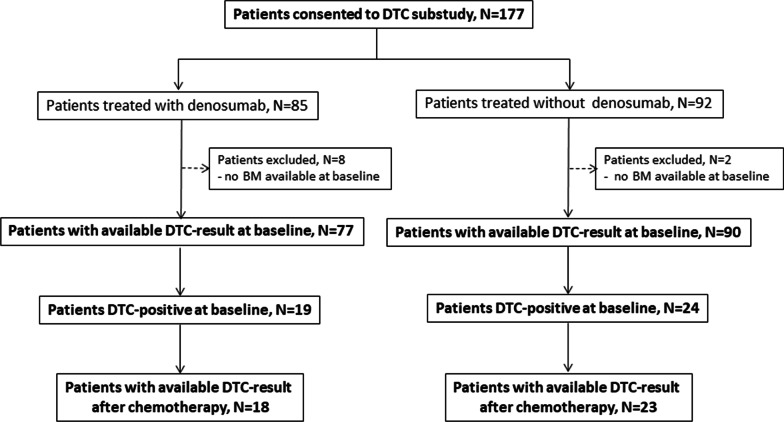
The translational GeparX linked substudy was conducted at the Department of Gynecology and Obstetrics, University Hospital Carl Gustav Carus, TU Dresden, Germany. In total, 177 patients [32] were recruited from the GeparX trial and in 167/177 of these patients, bone marrow aspirates could be obtained (Fig. 1). The study was performed in accordance with good clinical practice guidelines, national laws and the Declaration of Helsinki. Informed written consent for DTC-analysis was obtained from all patients and the study was approved by the Local Research Ethics Committee (ethical vote number 2016315 and EK237082012).
Fig. 1.
Conceptual workflow of the translational DTC substudy. The flow chart gives an overview on the inclusion of patients into the DTC substudy of the GeparX trial and the availability of DTC-results at baseline and after NACT. BM: bone marrow
Collection and processing of bone marrow samples
Bone marrow samples were aspirated at baseline (before the beginning of neoadjuvant chemotherapy). In case of DTC-positivity, patients were subjected to a follow-up bone marrow aspiration during surgery. Isolation of the mononuclear cell (MNC) fraction from bone marrow was performed according to the recommendations for standardized tumor cell detection published by the German Consensus Group of Senology [34, 35]. Briefly, bone marrow was bilaterally aspirated from the anterior iliac crests (between 5–10 ml per site) under local anesthesia (or during surgery), heparinized (5000 U/ml) and processed within 24 h. MNCs were isolated from heparinized bone marrow (5000 U/ml) by Ficoll-Hypaque density gradient centrifugation (density 1.077 g/mol; Pharmacia, Freiburg, Germany) at 400× g for 30 min. Interphase cells were washed (400× g for 15 min) and re-suspended in phosphate buffered saline (PBS). A total of 1.5 × 106 MNCs per area of 240 mm2 were directly spun onto glass slides (400× g for 5 min) coated with poly-L-lysine (Sigma, Deisenhofen, Germany) using a Hettich cytocentrifuge (Tuttlingen, Germany). In total, 9 × 106 MNCs per patient were analyzed. The slides were air-dried overnight at room temperature.
Immunocytochemistry
Immunocytochemical detection of cytokeratin (CK)-positive DTCs was performed, according to the recommendations for standardized tumor cell detection published by the German Consensus Group of Senology [34, 35]. Staining was performed using the murine monoclonal antibody A45-B/B3 (Micromet, Germany), directed against a common epitope of CK polypeptides including the CK heterodimers 8/18 and 8/19. The protocol has already been described in detail elsewhere [34, 35]. Briefly, the method includes (a) permeabilization of the cells with a detergent (5 min), (b) fixation with a formaldehyde-based solution (10 min), (c) binding of a A45-B/B3-alkaline phosphatase conjugate to cytoskeletal CKs (45 min) and (d) formation of an insoluble red reaction product at the site of binding of the CK-specific antibody conjugate (15 min) using the DAKO-APAAP detection kit (DakoCytomation, Denmark). All experimental steps were performed, according to the manufacturer’s instructions. Subsequently, the cells were mounted with Kaiser’s glycerol/gelatin (Merck, Darmstadt, Germany) in Tris–EDTA buffer (Sigma, Deisenhofen, Germany). A Fab-fragment-alkaline phosphatase conjugate (Micromet, Munich, Germany) served as negative control and did not show relevant background staining in human bone marrow samples. Furthermore, a positive control using the A45-B/B3-alkaline phosphatase conjugate and CK-expressing MCF-7 breast cancer cells (ATCC, Rockville, MD) was stained in parallel to each batch of patient samples under identical experimental conditions.
Automated detection and classification of cytokeratin-positive DTCs
Microscopic evaluation of the CK-stained bone marrow samples for DTC-detection was carried out using the ARIOL system (Applied Imaging) according to the International Society for Haematotherapy and Graft Engineering (ISHAGE) evaluation criteria and the DTC consensus [34, 35]. This automated scanning microscope and imaging system consist of a slide loader, camera, computer and software. The software was specifically trained for the automated detection of CK-positive cells, based on particular colour, intensity, size, pattern, and shape. Each detected cell was reviewed and classified according to ISHAGE criteria by an experienced examinator. A patient was categorically considered DTC-positive, if at least one CK-positive cell was detectable in at least one of the two two-sided bone marrow aspirates.
Statistical methods
Data analysis was performed using SAS® (Statistical Analysis Software; version 9.4 under SAS Enterprise Guide 7.1 on Microsoft Windows 7 Enterprise). DTC presence at baseline, DTC-eradication after NACT and pCR rates (stratified by baseline DTCs or by DTC-eradication) were presented as descriptive bar charts. Groupwise comparisons were performed using the Fisher's Exact Test.
Results
The presence of DTCs at baseline and their eradication after NACT ± denosumab
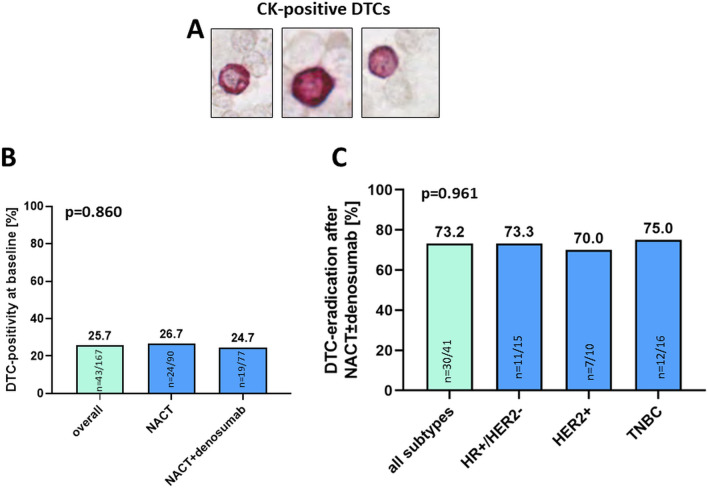
A total of 167 patients from the GeparX clinical trial were available for DTC-analysis (Fig. 1). Patient characteristics are shown in Supplementary Table 1. At baseline, the overall DTC-positivity was 25.7% (43/167 patients) with a median of 1 DTC per patient (range 1–9 DTCs per patient, Fig. 2A). The distribution of baseline parameters with regard to DTC-positivity is shown in Supplementary Table 2. Notably, DTC-positivity at baseline did not significantly differ in NACT vs. NACT + denosumab treated patients (26.7% vs. 24.7%; p = 0.860; Fig. 2B).
Fig. 2.
DTC-positivity at baseline and DTC-eradication after NACT ± denosumab. A Representative images of CK-positive DTCs in the bone marrow, stained by immunocytochemistry with the antibody A45-B/B3. B The bar chart shows the percentage of patients, being positive for DTCs in the bone marrow among the total cohort of the substudy and in the different study arm, i.e. in patients with NACT only and in patients with NACT + denosumab. C Bar charts showing the percentage of DTC-eradication in the bone marrow among the total cohort of the substudy and the different subtypes of breast cancer. P values according to the Fisher's Exact Test are indicated. HR: hormone receptor; HER2: human epidermal growth receptor 2; TNBC: triple-negative breast cancer
To monitor the rate of DTC-eradiation in response to NACT ± denosumab, we subsequently performed bone marrow re-puncture and DTC follow-up analysis in patients with a DTC-positive status at baseline. A total of 41/43 DTC-positive patients were available for this purpose. A patient was considered “DTC-eradicated”, if DTCs were initially present at baseline, followed by a negative DTC-status after NACT ± denosumab. We observed a high rate of DTC-eradication among baseline DTC-positive breast cancer patients after NACT ± denosumab (73.2%), which was consistent across the different subtypes of breast cancer (73.3% in HR + /HER2-; 70.0% in HER2 + ; 75.0% in TNBC; p = 0.961; Fig. 2C).
To sum up, we report a high rate of DTC-eradication (> 70%) in the total study population after NACT ± denosumab among baseline DTC-positive breast cancer patients.
Predictive value of DTCs for response to nab-paclitaxel based NACT
A total of 60/167 (35.9%) patients had a pCR. Of those, HR+/HER2− patients had the lowest pCR rate (15.3%), followed by HER2+ patients (43.3%). The highest pCR rate was observed in TNBC patients (55.4%).
To investigate the predictive value of DTCs for response to NACT, we compared baseline DTC-status with the patient’s pCR rate after NACT ± denosumab. Overall, the pCR rate in DTC-positive patients was 32.6% versus 37.1% in DTC-negative patients (p = 0.713; Fig. 3). Thus, no statistically significant association between pCR rate and baseline DTC-positivity was reported. The same result was evident for HR+/HER2− and HER2+ subtypes, in which also no significant differences between the pCR rates in DTC-positive vs. DTC-negative patients were observed (HR+/HER2−: 18.8% vs. 14.3%, p = 0.699; HER2+: 40% vs. 45%, p = 1.000). Interestingly, TNBC patients with DTC-positivity at baseline had a numerically lower pCR rate than patients without evidence of DTCs (41.2% vs. 60.4%; 19.2% difference in pCR; p = 0.256, Fig. 3). Due to the limited number of patients, a further stratification regarding the NACT vs. NACT + denosumab arm was not reasonable at this point.
Fig. 3.
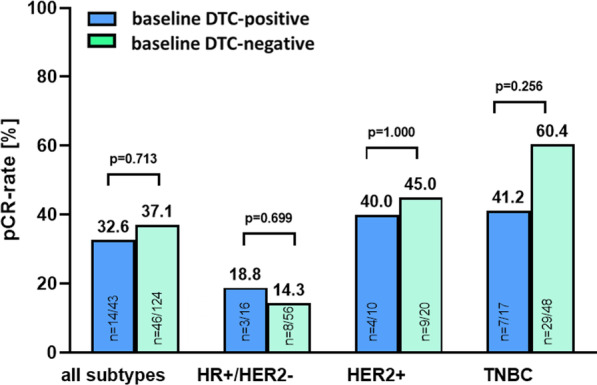
Predictive value of baseline DTCs. Bar chart showing pCR rate after NACT ± denosumab among the total cohort and the different subtypes of breast cancer with regard to DTC-status at baseline. P values according to the Fisher's Exact Test are indicated. HR: hormone receptor; HER2: human epidermal growth receptor 2; TNBC: triple-negative breast cancer
We further analyzed, whether a pCR to NACT ± denosumab may parallel DTC-eradication in the bone marrow. Across all subtypes, there was no statistical significance between pCR rate and DTC-eradication. Thus, the pCR rate in DTC-persistent patients was 27.3% versus 36.7% in DTC-eradicated patients (p = 0.719; Fig. 4). The same trend was observed for HR+/HER2− and HER2+ subtypes, in which no significant differences between the pCR rates in DTC-persistent vs. DTC-eradicated patients were observed (25.0% vs. 18.2% in HR+ /HER2− , p = 1.000; 33.3% vs. 42.9% in HER2+ , p = 1.000). Again, in patients with TNBC, there was a numerical trend towards a possible association between DTC-persistence and decreased pCR rate (25.0% vs. 50.0%; 25% difference in pCR; p = 0.585, Fig. 4). Due to the limited number of patients, a further stratification regarding the NACT versus NACT + denosumab arm was not reasonable at this point.
Fig. 4.
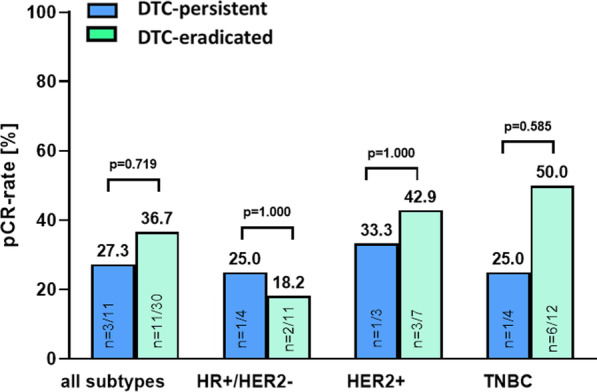
Association between pCR rate and DTC-eradication. Bar chart showing pCR rate after NACT ± denosumab among the total cohort and the different subtypes of breast cancer with regard to DTC-eradication. P values according to the Fisher's Exact Test are indicated. HR: hormone receptor; HER2: human epidermal growth receptor 2; TNBC: triple-negative breast cancer
We conclude that the presence of DTCs at baseline does not predict overall response to NACT ± denosumab. Moreover, there was no association between pCR and DTC-eradication. However, subtype analysis showed some numerical trends for a possible association between DTCs and pCR in TNBC.
The effect of denosumab on DTCs
We inquired, whether neoadjuvant add-on denosumab treatment, framed by the GeparX study [32], may eradicate DTCs in the bone marrow. Therefore, we analyzed, whether denosumab may increase the given DTC-eradication rate by NACT. DTC-eradication in the NACT + denosumab arm (77.8%) was numerically higher compared to the NACT arm without denosumab (69.6%). However, this difference did not reach statistical significance (p = 0.726; Fig. 5). In TNBC patients with a pCR, 0/3 patients (0%) were DTC-positive after NACT + denosumab (p = 0.429), whereas 1/4 patients (25%) were DTC-positive after NACT alone (p = 1.00).
Fig. 5.
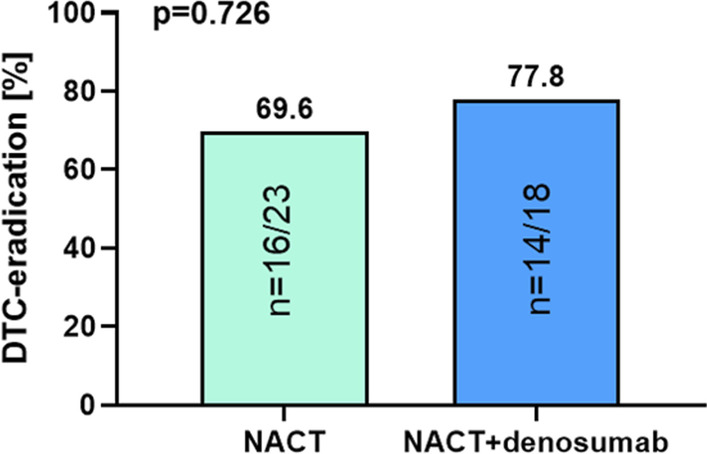
Effect of denosumab on DTC-eradication. Bar chart showing DTC-eradication rates in patients with NACT vs. patients with NACT + denosumab. P values according to the Fisher's Exact Test are indicated
Taken together, denosumab did not increase the overall DTC-eradication rate by NACT. However, subtype analysis shows a numerical trend towards a possible effect of denosumab on DTCs in TNBC.
Discussion
This is the first study worldwide, analyzing the effect of neoadjuvant denosumab on breast cancer DTCs. Overall DTC-positivity in our study cohort (25.7%) was comparable to that reported in a comprehensive meta-analysis of 4703 patients with stage I-III breast cancer (30.6%) [7], excluding any bias in our study towards methodology and patient selection. Moreover, DTC-positivity at baseline was well balanced between the denosumab and non-denosumab treated study arm, indicating that there is no additional selection bias between the different study arms, which may have confounded our results.
The fact that (1) anti-resorptive therapy with bisphosphonates is already known to eradicate DTCs in the BM [18] and that (2) circulating levels of serum RANKL are elevated in DTC-positive breast cancer patients [33], provided a strong rationale for us to hypothesize that anti-resorptive denosumab likewise promotes the eradication of DTCs in the BM. Therefore, we re-analyzed patients with baseline DTC-positivity for the presence of DTCs after NACT ± denosumab, in order to distinguish between DTC-eradication vs. DTC-persistence. We observed a substantial DTC-eradication rate after NACT ± denosumab (73%). So far, only little is known about the effectiveness of NACT in eradicating DTCs in the BM, since previous reports primarily focused on the prognostic significance of DTCs after NACT [9–11]. In a previous study on breast cancer, DTC-positivity in the overall study population (adjuvant and neoadjuvant treatment) was 29%. Interestingly, in those patients with neoadjuvant treatment, DTC-positivity after NACT was still 25%, suggesting that overall DTC-positivity was not substantially decreased by NACT [36]. In the adjuvant setting, inconclusive results with regard to DTC-eradication after treatment have been reported [37–41]. Those opposing results could likely be due to use to confounding biases, with regard to therapy regimes, patient selection or different DTC-detection methods. Moreover, our study is not directly comparable to others, since we conceptually assessed the DTC-eradication rate by follow-up analysis of a pre-selected cohort of 100% DTC-baseline positive patients, so that “negative to positive switchers”, which also influence the overall DTC-frequency after NACT, could not be considered.
We report for the overall cohort that (1) DTC-positivity at baseline is non-predictive for response to NACT and that (2) DTC-eradication does not parallel the pCR rate. These finding are supported by a previous study to show that there is no overall association between pCR and DTC-status after NACT [10]. It could be hypothesized that DTCs undergo an independent metastatic progression in parallel to the primary tumor [42], so that their chemosensitivity could be different to that of the primary tumor mass. This may explain that DTCs in our study neither predicted response to NACT nor their eradication reflected a pCR. Nevertheless, we observed a numerical trend towards a predictive value of DTCs in TNBC. This could be of high clinical interest and requires further investigation, since the pCR rate is higher and the association between pCR and outcome is more pronounced in TNBC compared to the HR+/HER2− subtype [43].
The underlying GeparX trial showed that the addition of denosumab to NACT did not increase pCR [32]. In line with these findings, we could not observe an overall effect of denosumab on DTCs in the GeparX study cohort. However, this result refers to short-term denosumab treatment (24 months, 6 applications), as it was framed by the GeparX trial design [32]. Long-term follow-up data of the GeparX study will be awaited in the next years, in order to analyze the long-term effect of denosumab on DTCs and on the patient’s survival. Nonetheless, we observed again in TNBC patients, that there was a numerical trend towards an increase of DTC-eradication by denosumab. This trend is in line with the previous observation, that TNBC, in comparison to the other intrinsic subtypes, is generally more likely to show DTC-eradication after NACT [36]. We hypothesize that DTCs of TNBC could possibly be more sensitive to anti-resorptive therapy with denosumab, since RANK is overexpressed in this breast cancer subtype [44]. Moreover, RANKL, which is expressed in response to progesterone in progesterone receptor (PR)-positive luminal epithelial cells, has a paracrine proliferative effect on neighboring PR-negative basal cells [24], suggesting a potential dependency of basal-like cancer, which is enriched in TNBC [45], to RANK signaling. Due to the limited number of TNBC patients, our statistical analysis was of limited information value, however, our results encourage further investigation of the denosumab effect on DTCs in TNBC patients. Considering the prognostic impact and the potentially (dormant) stem-like state of persisting DTCs after NACT [9–11, 46], further studies should address, whether denosumab could possibly be used as a cell cycle-independent drug for DTC-eradication in TNBC.
Conclusion
This is the first study worldwide, demonstrating that neoadjuvant add-on denosumab over a short-term period of 24 months does not increase the DTC-eradication rate in breast cancer patients treated with NACT. Nevertheless, our results suggest a trend towards a potential predictive effect of DTCs and an increased DTC-eradication by denosumab in TNBC, which warrants further investigation.
Supplementary Information
Additional file 1: Table S1. Baseline Characteristics of the DTC substudy.
Additional file 2: Table S2. Baseline Characteristics in DTC-positive vs. DTC-negative patients
Acknowledgements
The authors want to thank Heike Hilbrich, Katja Wilsch and Katrin Jäger for excellent technical assistance in the context of DTC-analysis in the bone marrow.
Abbreviations
- DTCs:
Disseminated tumor cells
- BC:
Breast cancer
- NACT:
Neoadjuvant chemotherapy
- pts:
Patients
- TNBC:
Triple-negative breast cancer
- pCR:
Pathologic complete response
- BM:
Bone marrow
- RANKL:
Receptor activator of NFĸB
- RANKL:
Receptor activator of NFĸB ligand
- PBS:
Phosphate buffered saline
- MNCs:
Mononuclear cells
- ISHAGE:
International Society for Hematotherapy and Graft Engineering
- PR:
Progesterone receptor
Author contributions
PW: conception and design of the study, patient recruitment, interpretation of the results revising the manuscript. JUB: patient recruitment, conception and design of the GeparX study. PK: patient recruitment. TL: patient recruitment, interpretation of the results, revising the manuscript. MJ: patient recruitment. BVS: patient recruitment. ES: patient recruitment. CS: conception and design of the GeparX study, patient recruitment. TF: patient recruitment. CD: conception and design of the GeparX study, acquisition of data and to the analysis/interpretation of the results. CK patient recruitment. KR: conception and design of the GeparX study. HT: patient recruitment. SK: patient recruitment. AP: patient recruitment, performance of analytical bone marrow aspirations for DTC-analysis. OS: patient recruitment. CM: patient recruitment, performance of analytical bone marrow aspirations for DTC-analysis. JDK: contribution to the experimental work for DTC-analysis, acquisition of data and to the analysis/interpretation of the results, drafting and revising the manuscript. VN: statistician of the the GeparX study, analysis/interpretation of the results. SL: conception and design of the GeparX study, interpretation of the results. All authors read, revised and approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. GeparX and the DTC substudy were financially supported by Amgen and BMS.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The study was performed in accordance with good clinical practice guidelines, national laws and the Declaration of Helsinki. Informed written consent for DTC-analysis was obtained from all patients and the study was approved by the Local Research Ethics Committee (ethical vote number 2016315 and EK237082012).
Consent for publication
Not applicable.
Competing interests
CD reports personal fees from Novartis, Roche, MSD Oncology, Daiichi Sankyo, Molecular Health, Astra Zeneca, Merck; grants from Myriad Genetic and Roche; Stock and Other Ownership Interests from Sividon Diagnostics (until 2016); and has patent applications WO2015114146A1 and WO2010076322A1- therapy response, and WO2020109570A1 - cancer immunotherapy pending, and a patent Software (VMscope digital pathology). HT reports other from Pierre Fabre, other from Pfizer Pharma, other from Mundipharma, other from ClinSol, other from Novartis, other from Lilly, other from AMGEN, other from Grünenthal, other from Vifor, other from AstraZeneca, other from Mylan, other from BMS, during the conduct of the study. JUB reports personal fees from Amgen, Astra Zeneca, Exact Science, MSD Oncology, Lilly, Novartis, Pfizer, Sysmex, Roche, and Sonoscape. KR reports personal fees from AstraZeneca, personal fees from Pfizer, personal fees from MSD, outside the submitted work. PW reports honoraria from AstraZeneca, MSD Oncology, Eisai, Novartis/ Pfizer, Roche Pharma AG, Amgen, Pfizer; Consulting or Advisory Role from Amgen, Astra Zeneca, MSD Oncology, Novartis, Roche Pharma AG, Tesaro, PharmaMar; Travel, Accommodations, Expenses from RochePharma AG, outside the submitted work. SK reports personal fees from Lilly, Roche, Genomic Health, Novartis, Amgen, Celgene, Daiichi Sankyo, Astra Zeneca, Somatex, MSD, Pfizer, Puma Technology, PFM medical, non-financial support from Roche, Daiichi Sankyo, Lilly, Sonoscape outside the submitted work, and a relationship with the WSG study group as co-director. SL reports grants and other from Abbvie, grants and other from Amgen, grants and other from AstraZeneca, grants and other from Celgene, grants, personal fees and other from Daiichi-Sankyo, grants and other from Novartis, grants and other from Pfizer, grants and other from Roche, other from BMS, other from Eirgenix, other from Lilly, other from Merck, other from MSD, other from SeaGen, other from Prime/Medscape, other from Puma, other from Samsung, other from Pierre Fabre, grants from Teva, grants from Vifor, grants from Immunomedics, personal fees from Chugai, outside the submitted work; In addition, Dr. Loibl has a patent EP14153692.0 pending. TL reports scientific talks from MSD, Pfizer, Novartis, Teva, Tesaro, Roche, Amgen, Clovis, Lilly, GSK, Gilead; Travel/Kongress-Support from Pharmamar, Roche, Pfizer, MSD, Celgene, Clovis, Daiichi Sankyo; Consulting or Advisory Role from Amgen, Roche, Tesaro, MSD, Pfizer, Lilly, Myriad, Esai, GSK, Gilead, Daiichi Sankyo outside the submitted work. No other potential conflict of interest relevant to this article was reported.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Muller V, Fehm T, Janni W, Gebauer G, Solomayer E, Pantel K. Clinical relevance of disseminated tumor cells in the bone marrow and circulating tumor cells in the blood of breast cancer patients. Breast Care (Basel) 2009;4(5):333–338. doi: 10.1159/000235888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riethdorf S, Pantel K. Disseminated tumor cells in bone marrow and circulating tumor cells in blood of breast cancer patients: current state of detection and characterization. Pathobiology. 2008;75(2):140–148. doi: 10.1159/000123852. [DOI] [PubMed] [Google Scholar]
- 3.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative G: Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. [DOI] [PubMed]
- 5.Hosseini H, Obradovic MMS, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C, Maneck M, et al. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540(7634):552–558. doi: 10.1038/nature20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banys M, Krawczyk N, Fehm T. The role and clinical relevance of disseminated tumor cells in breast cancer. Cancers (Basel) 2014;6(1):143–152. doi: 10.3390/cancers6010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353(8):793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 8.Domschke C, Diel IJ, Englert S, Kalteisen S, Mayer L, Rom J, Heil J, Sohn C, Schuetz F. Prognostic value of disseminated tumor cells in the bone marrow of patients with operable primary breast cancer: a long-term follow-up study. Ann Surg Oncol. 2013;20(6):1865–1871. doi: 10.1245/s10434-012-2814-4. [DOI] [PubMed] [Google Scholar]
- 9.Janni W, Vogl FD, Wiedswang G, Synnestvedt M, Fehm T, Juckstock J, Borgen E, Rack B, Braun S, Sommer H, et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse—a European pooled analysis. Clin Cancer Res. 2011;17(9):2967–2976. doi: 10.1158/1078-0432.CCR-10-2515. [DOI] [PubMed] [Google Scholar]
- 10.Hall C, Krishnamurthy S, Lodhi A, Bhattacharyya A, Anderson A, Kuerer H, Bedrosian I, Singh B, Lucci A. Disseminated tumor cells predict survival after neoadjuvant therapy in primary breast cancer. Cancer. 2012;118(2):342–348. doi: 10.1002/cncr.26202. [DOI] [PubMed] [Google Scholar]
- 11.Mathiesen RR, Borgen E, Renolen A, Lokkevik E, Nesland JM, Anker G, Ostenstad B, Lundgren S, Risberg T, Mjaaland I, et al. Persistence of disseminated tumor cells after neoadjuvant treatment for locally advanced breast cancer predicts poor survival. Breast Cancer Res. 2012;14(4):R117. doi: 10.1186/bcr3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleisch H. Bisphosphonates: a new class of drugs in diseases of bone and calcium metabolism. Recent Results Cancer Res. 1989;116:1–28. doi: 10.1007/978-3-642-83668-8_1. [DOI] [PubMed] [Google Scholar]
- 13.Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97(12):2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, Wheeler H, Simeone JF, Seaman J, Knight RD. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335(24):1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 15.Brown SA, Guise TA. Cancer treatment-related bone disease. Crit Rev Eukaryot Gene Expr. 2009;19(1):47–60. doi: 10.1615/CritRevEukarGeneExpr.v19.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Early Breast Cancer Trialists' Collaborative G: Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–61. [DOI] [PubMed]
- 17.Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D, Kaufmann M, Bastert G. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339(6):357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann O, Aktas B, Goldnau C, Heubner M, Oberhoff C, Kimmig R, Kasimir-Bauer S. Effect of ibandronate on disseminated tumor cells in the bone marrow of patients with primary breast cancer: a pilot study. Anticancer Res. 2011;31(10):3623–3628. [PubMed] [Google Scholar]
- 19.Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11(5):401–419. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 20.Casas A, Llombart A, Martin M. Denosumab for the treatment of bone metastases in advanced breast cancer. Breast. 2013;22:585–592. doi: 10.1016/j.breast.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Hadji P, Aapro MS, Body JJ, Gnant M, Brandi ML, Reginster JY, Zillikens MC, Gluer CC, de Villiers T, Baber R, et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017;7:1–12. doi: 10.1016/j.jbo.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzoli R, Body JJ, Brandi ML, Cannata-Andia J, Chappard D, El Maghraoui A, Gluer CC, Kendler D, Napoli N, Papaioannou A, et al. Cancer-associated bone disease. Osteoporos Int. 2013;24(12):2929–2953. doi: 10.1007/s00198-013-2530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468(7320):103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 24.Infante M, Fabi A, Cognetti F, Gorini S, Caprio M, Fabbri A. RANKL/RANK/OPG system beyond bone remodeling: involvement in breast cancer and clinical perspectives. J Exp Clin Cancer Res. 2019;38(1):12. doi: 10.1186/s13046-018-1001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Poznak C, Cross SS, Saggese M, Hudis C, Panageas KS, Norton L, Coleman RE, Holen I. Expression of osteoprotegerin (OPG), TNF related apoptosis inducing ligand (TRAIL), and receptor activator of nuclear factor kappaB ligand (RANKL) in human breast tumours. J Clin Pathol. 2006;59(1):56–63. doi: 10.1136/jcp.2005.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santini D, Perrone G, Roato I, Godio L, Pantano F, Grasso D, Russo A, Vincenzi B, Fratto ME, Sabbatini R, et al. Expression pattern of receptor activator of NFkappaB (RANK) in a series of primary solid tumors and related bone metastases. J Cell Physiol. 2011;226(3):780–784. doi: 10.1002/jcp.22402. [DOI] [PubMed] [Google Scholar]
- 27.Park HS, Lee A, Chae BJ, Bae JS, Song BJ, Jung SS. Expression of receptor activator of nuclear factor kappa-B as a poor prognostic marker in breast cancer. J Surg Oncol. 2014;110(7):807–812. doi: 10.1002/jso.23737. [DOI] [PubMed] [Google Scholar]
- 28.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440(7084):692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 29.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470(7335):548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gnant M, Pfeiler G, Steger GG, Egle D, Greil R, Fitzal F, Wette V, Balic M, Haslbauer F, Melbinger-Zeinitzer E, et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):339–351. doi: 10.1016/S1470-2045(18)30862-3. [DOI] [PubMed] [Google Scholar]
- 31.Coleman R, Finkelstein DM, Barrios C, Martin M, Iwata H, Hegg R, Glaspy J, Perianez AM, Tonkin K, Deleu I, et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(1):60–72. doi: 10.1016/S1470-2045(19)30687-4. [DOI] [PubMed] [Google Scholar]
- 32.Blohmer JU, Link T, Reinisch M, Just M, Untch M, Stotzer O, Fasching PA, Schneeweiss A, Wimberger P, Seiler S, et al. Effect of denosumab added to 2 different nab-paclitaxel regimens as neoadjuvant therapy in patients with primary breast cancer: the GeparX 2 × 2 randomized clinical trial. JAMA Oncol. 2022;8:1010–1018. doi: 10.1001/jamaoncol.2022.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rachner TD, Kasimir-Bauer S, Gobel A, Erdmann K, Hoffmann O, Browne A, Wimberger P, Rauner M, Hofbauer LC, Kimmig R, et al. Prognostic value of RANKL/OPG serum levels and disseminated tumor cells in nonmetastatic breast cancer. Clin Cancer Res. 2019;25(4):1369–1378. doi: 10.1158/1078-0432.CCR-18-2482. [DOI] [PubMed] [Google Scholar]
- 34.Fehm T, Braun S, Muller V, Janni W, Gebauer G, Marth C, Schindlbeck C, Wallwiener D, Borgen E, Naume B, et al. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer. 2006;107(5):885–892. doi: 10.1002/cncr.22076. [DOI] [PubMed] [Google Scholar]
- 35.Borgen E, Naume B, Nesland JM, Kvalheim G, Beiske K, Fodstad O, Diel I, Solomayer EF, Theocharous P, Coombes RC, et al. Standardization of the immunocytochemical detection of cancer cells in BM and blood: I. Establishment of objective criteria for the evaluation of immunostained cells. Cytotherapy. 1999;1(5):377–388. doi: 10.1080/0032472031000141283. [DOI] [PubMed] [Google Scholar]
- 36.Hall C, Krishnamurthy S, Lodhi A, Mosalpuria K, Kuerer HM, Meric-Bernstam F, Bedrosian I, Hunt KK, Lucci A. Disseminated tumor cells in biologic subtypes of stage I–III breast cancer patients. Ann Surg Oncol. 2010;17(12):3252–3258. doi: 10.1245/s10434-010-1160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker S, Becker-Pergola G, Wallwiener D, Solomayer EF, Fehm T. Detection of cytokeratin-positive cells in the bone marrow of breast cancer patients undergoing adjuvant therapy. Breast Cancer Res Treat. 2006;97(1):91–96. doi: 10.1007/s10549-005-9095-6. [DOI] [PubMed] [Google Scholar]
- 38.Braun S, Kentenich C, Janni W, Hepp F, de Waal J, Willgeroth F, Sommer H, Pantel K. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol. 2000;18(1):80–86. doi: 10.1200/JCO.2000.18.1.80. [DOI] [PubMed] [Google Scholar]
- 39.Drageset V, Nesland JM, Erikstein B, Skovlund E, Sommer H, Anker G, Wist E, Lundgren S, Bergh J, Kvalheim G. Monitoring of disseminated tumor cells in bone marrow in high-risk breast cancer patients treated with high-dose chemotherapy. Int J Cancer. 2006;118(11):2877–2881. doi: 10.1002/ijc.21709. [DOI] [PubMed] [Google Scholar]
- 40.Janni W, Hepp F, Rjosk D, Kentenich C, Strobl B, Schindlbeck C, Hantschmann P, Sommer H, Pantel K, Braun S. The fate and prognostic value of occult metastatic cells in the bone marrow of patients with breast carcinoma between primary treatment and recurrence. Cancer. 2001;92(1):46–53. doi: 10.1002/1097-0142(20010701)92:1<46::AID-CNCR1290>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Janni W, Rack B, Schindlbeck C, Strobl B, Rjosk D, Braun S, Sommer H, Pantel K, Gerber B, Friese K. The persistence of isolated tumor cells in bone marrow from patients with breast carcinoma predicts an increased risk for recurrence. Cancer. 2005;103(5):884–891. doi: 10.1002/cncr.20834. [DOI] [PubMed] [Google Scholar]
- 42.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9(4):302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 43.Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48(18):3342–3354. doi: 10.1016/j.ejca.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Reyes ME, Fujii T, Branstetter D, Krishnamurthy S, Masuda H, Wang X, Reuben JM, Woodward WA, Edwards BJ, Hortobagyi GN, et al. Poor prognosis of patients with triple-negative breast cancer can be stratified by RANK and RANKL dual expression. Breast Cancer Res Treat. 2017;164(1):57–67. doi: 10.1007/s10549-017-4233-5. [DOI] [PubMed] [Google Scholar]
- 45.Alluri P, Newman LA. Basal-like and triple-negative breast cancers: searching for positives among many negatives. Surg Oncol Clin N Am. 2014;23(3):567–577. doi: 10.1016/j.soc.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bragado P, Sosa MS, Keely P, Condeelis J, Aguirre-Ghiso JA. Microenvironments dictating tumor cell dormancy. Recent Results Cancer Res. 2012;195:25–39. doi: 10.1007/978-3-642-28160-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Baseline Characteristics of the DTC substudy.
Additional file 2: Table S2. Baseline Characteristics in DTC-positive vs. DTC-negative patients
Data Availability Statement
All data generated or analysed during this study are included in this published article.