Abstract
Over 1014 symbiotic microorganisms are present in a healthy human body and are responsible for the synthesis of vital vitamins and amino acids, mediating cellular pathways and supporting immunity. However, the deregulation of microbial dynamics can provoke diverse human diseases such as diabetes, human cancers, cardiovascular diseases, and neurological disorders. The human gastrointestinal tract constitutes a hospitable environment in which a plethora of microbes, including diverse species of archaea, bacteria, fungi, and microeukaryotes as well as viruses, inhabit. In particular, the gut microbiome is the largest microbiome community in the human body and has drawn for decades the attention of scientists for its significance in medical microbiology. Revolutions in sequencing techniques, including 16S rRNA and ITS amplicon sequencing and whole genome sequencing, facilitate the detection of microbiomes and have opened new vistas in the study of human microbiota. Especially, the flourishing fields of metagenomics and metatranscriptomics aim to detect all genomes and transcriptomes that are retrieved from environmental and human samples. The present review highlights the complexity of the gastrointestinal tract microbiome and deciphers its implication not only in cellular homeostasis but also in human diseases. Finally, a thorough description of the widely used microbiome detection methods is discussed.
Keywords: gut microbiome, human gastrointestinal tract, oral microbiome, metagenomics, amplicon sequencing, 16S rRNA sequencing, metatranscriptomics
1. Introduction
Recent revolutions in sequencing techniques have transformed traditional microbiology into modern microbiology. To date, microbiology constitutes a flourishing biological field that involves the study of both microbiome and microbiota. To begin with, although these terms are regularly used interchangeably, microbiome, as it was first defined in the late 1980s, is a microbial community that includes the microorganisms that cohabit in a well-defined microenvironment, whereas the living members of the microbiome that can be observed microscopically are known as the microbiota [1,2]. However, breakthroughs in molecular microbiology have given birth to the study of metagenomics which comprises all genomes and genetic products that are harbored in living samples, incorporating humans, or retrieved from environmental samples, such as water and soil, whereas metatransriptomics refers to the study of transcriptomes of microorganisms [3].
Over 1014 symbiotic microorganisms are living in the human body and constitute the human microbiota. Multiple projects aim to decipher all the microbiome communities that are present in the human body, and most of them are focused on the gut microbiome, which constitutes the largest group of habitants [4]. Microbiome studies support that gut inhabitants are physiological endogenous factors that produce vital vitamins and amino acids, which cannot be synthesized by the organism, and mediate cellular mechanisms, thus influencing the immune system and health [5,6]. The composition of the gut microbiome is critical for maintaining homeostasis, and the deregulation of microbial dynamics can provoke diverse human diseases including diabetes, human cancers, allergic diseases, and neurological disorders [7,8,9,10]. Nowadays, microbial studies aim to characterize all the organisms that are harbored in the human body by identifying their DNA sequences. Notably, studies have been focused on the detection of marker genes, such as the 16S rRNA gene, which is conserved in bacteria and archaea, utilizing the amplicon sequencing methodology [4]. On the contrary, the newly introduced metagenomics approach is based on whole genome sequencing techniques, hence identifying all microbial genomes that are retrieved from a sample and aims both to classify the microorganisms and reveal functional information about their contribution to human homeostasis. A plethora of microbiome projects, incorporating the Human Microbiome Project (HMP), the Integrative Human Microbiome Project (iHMP), and the European MetaHIT, have been launched worldwide in order to both decipher the human microbiome and understand the impact of these symbionts in human health and disease [11,12,13].
In the present review, we focused on deciphering the complexity of the human gastrointestinal tract microbiome that plays a critical role in homeostasis and interplays between inflammation, disease, and cancer. Moreover, the current review aims to provide a thorough description of the detection methods that are widely used for the characterization of the microbiome in the era of modern microbiology. Notably, the classic culturing techniques for identifying microbes, namely culturome, and the newest amplicon-based sequencing methods and culture-free metagenomic sequencing approaches are sufficiently depicted. Finally, the contribution of the newly introduced metatranscriptomics sequencing is also highlighted.
2. The Role of the Gastrointestinal Tract Microbiota in Human Health and Disease
The human body constitutes a natural habitat for microbial communities, such as diverse species of Archaea, Bacteria, Fungi, and microeukaryotes as well as Viruses, which have been detected in multiple anatomical body sites and tissues including the skin surface, the respiratory tract, the gastrointestinal or alimentary tract, the mammary gland, and the urogenital tract [14]. Notably, the core microbiome is comprised of predominant aerobe microorganisms that inhabit the skin, the nasal cavity, and the respiratory tract, whereas in the gastrointestinal tract, the anaerobes dominate (Table 1) [14]. More precisely, the alimentary tract is made up of various organs that swallow, digest and absorb food, which are the oral cavity, pharynx, esophagus, stomach, small and large intestine, and accessory organs, and is a unique environment that harbors plenty microorganisms [15].
Table 1.
Common bacteria species found in human tracts and their oxygen requirements.
| Bacteria | Oxygen Requirement |
|---|---|
| Mycobacterium tuberculosis | Obligate aerobe |
| Micrococcus luteus | |
| Neisseria meningitidis | |
| Neisseria gonorrhoeae | |
| Bacteroidetes | Obligate anaerobe |
| Porphyromonas sp. | |
| Prevotella sp. | |
| Clostridium spp. | |
| Staphylococci | Facultative anaerobes |
| Gemella sp. | |
| Enterobacteriaceae | |
| Lactobacilli | Aerotolerant anaerobes |
| Campylobacter jejuni | Microaerophile |
To begin with, commensal bacteria are dominant in the oral cavity. Especially, more than 1000 species have been detected and studies have shown that they mediate cellular processes and maintain homeostasis [16,17]. It is worth mentioning that Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, Streptococcus, Spirochaetes, Synergistetes, and Tenericutes are representative phyla that have been found in this highly complex bacteria community (Figure 1). More precisely, streptococci constitute the first bacteria inhabitants of the oral cavity and under physiological conditions; they are responsible for the generation of acids by catalyzing the metabolism of carbohydrates [18]. Both Streptococcus salivarius and Streptococcus gordonii produce great amounts of alkali; hence, they contribute to human homeostasis by regulating the levels of acids in the oral cavity. On the contrary, the levels of Streptococcus mutans and Porphyromonas gingivalis are influenced by carbonic anhydrases, pH, and ions in the oral cavity; hence, they are implicated in diseases such as dental caries and periodontitis [19].
The class of Archaea is restricted since only some species of methanogens have been found, containing Methanobrevibacter oralis, Methanobacterium curvum, and Methanosarcina mazeii [20,21]. Various studies support that methanogens facilitate hydrogen transfer, and thus influence the growth of bacteria that are responsible for periodontal diseases. Consequently, increased levels of methanogens such as Methanobrevibacter oralis are related to periodontitis [20]. As for Fungi, Candida is in abundance in the oral cavity, whereas various species of the Aspergillus, Cladosporium, Cryptococcus, Aureobasidium, Saccharomycetales, and Fusarium genera are also present [21]. Adaptive immune responses and the host’s innate reflexes allow the cohabitation of Candida albicans and other microorganisms in the oral cavity [22]. These symbiotic relationships between the host and the microorganisms restrict the colonization and the growth of pathogens and promote homeostasis. However, disruptions of the physiological parameters such as temperature, nutrients, and pH that contribute to the establishment of these resident microbes, influence human pathophysiology [22]. The commensal oral microbiota colonizes all surfaces of the mouth leaving little space for pathogenic invaders, thus protecting the cavity and maintaining systemic health [23,24]. For instance, the health-associated Bacteria Streptococcus salivarius produces the toxin bacteriocin which prevents the growth and activity of Gram-negative bacterial species that cause periodontitis and halitosis [25]. Additionally, other Streptococcus species have been related to type 1 diabetes [26]. Chronic kidney diseases are also influenced by oral microbiota. In patients with chronic kidney disease, higher levels of Candida albicans and Porphyromonas gingivalis are responsible for chronic periodontitis [27]. On the contrary, the oral virome contains different types of viruses, including Human Papilloma Virus (HPV), hepatitis, and mumps viruses, as well as Herpes simplex and Rabies lyssavirus, which all have been found in saliva and are usually disease-associated. For instance, Herpes simplex is responsible for gingivostomatitis, whereas HPV causes several oral conditions such as focal epithelial hyperplasia, oral papillomatosis or even neck squamous cell carcinoma [28].
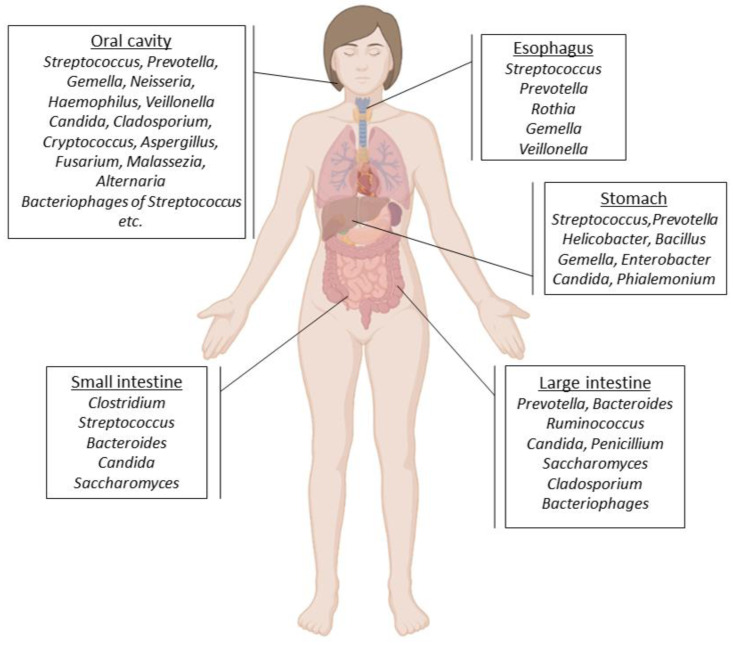
Figure 1.
Microbes that inhabit the oral cavity [29], esophagus [30], stomach [31] as well as small and large intestines [32,33,34] in humans.
Although the dynamic of microbial colonies enhances oral homeostasis, a range of microorganisms has also been related to oral diseases such as dental caries, periodontitis, and cancer (Table 2). Notably, numerous pathological alterations that occur within the microbial environment can affect bacterial growth and activity, hence initiating disease. More precisely, reductions in the pH of the saliva and the increase in the lactic acid that is produced by oral bacteria such as Streptococcus mutans, Bifidobacterium, Propionibacterium, and Lactobacillus lead to dental caries [35]. In the case of periodontitis, a periodontal pocket is formed due to a gap between the teeth and the gingivae. The periodontal pocket is colonized with various bacteria species, including Porphyromonas gingivalis, Porphyromonas endodontalis, Treponema denticola, Anaeroglobus geminatus, Eubacterium saphenum, and Prevotella denticola and finally, the tissue is damaged [36]. The oral microbiome can also cause infections at different body sites leading to serious diseases. For example, clinical studies in patients with cystic fibrosis have shown that oral bacterial species have been found in the lung [37].
The human pharyngeal microbiome comprises the phyla Actinobacteria, Firmicutes, Bacteroidetes, Proteobacteria, and Fusobacteria, among which Bacteroidetes is the most abundant, while Prevotella, Neisseria, Streptococcus, Campylobacter, and Haemophilus are the most prevalent genera [38]. More precisely, the gram-positive Streptococcus pyogenes as well as Prevotella melaninogenica are responsible for pharyngitis, while pharyngeal colonization by Neisseria species such as N. meningitidis and N. gonorrhoeae have been detected in patients with gonococcal infections [39]. Moreover, although the pharyngeal cavity harbors a variety of pathogenic species such as Haemophilus influenza, Staphylococcus aureus, Streptococcus pneumonia, and Mycoplasma pneumonia, in many cases these residents are not attacking the immune system and the host is characterized as asymptomatic [38]. For instance, Streptococcus pneumonia, which is responsible for deathly pneumococcal diseases, is normally found in the human pharynx of healthy individuals but it can migrate to different tissues and cause serious infections [40]. Additionally, the human oropharyngeal virome includes a plethora of respiratory viruses such as DNA Chloroviruses [41]. For example, Chlorovirus Acanthocystis turfacea chlorella virus 1 (ATCV-1) inhabits human mucosal surfaces such as the pharynx and produces a variety of enzymes that either enhance or impair the host’s immunity [41].
In the same manner, the esophageal microbiome is similar to the pharyngeal microbiome and is comprised of six bacteria phyla among which Firmicutes is overexpressed. Accordingly, Bacteroidetes, Actinobacteria, Fusobacteria Proteobacteria, and TM7 are also present (Figure 1). Streptococcus constitutes a highly abundant genus, but Prevotella and Veillonella are also in great abundance [42]. Changes in the esophageal microbiome levels can cause a plethora of esophageal-related diseases such as Barrett’s esophagus, esophageal adenocarcinoma, and eosinophilic esophagitis. For instance, the progression of esophageal adenocarcinoma can be induced by rare and abundant phage communities [43]. Recent studies support that significant variations in the abundance of microorganisms, including Streptococcus, Prevotella, and Treponema, have been detected in esophageal carcinoma tissues indicating that they constitute markers for the prognosis and diagnosis of esophageal squamous cell carcinoma [44]. Additionally, fungal esophagitis is an infection that is caused by Candida species or Filamentous Fungi [45].
For many years scientists believed that the stomach is a sterile organ, hence it cannot harbor any bacterial community. However, this dogma was demolished in 1982, when Helicobacter pylori was detected. Of note, Helicobacter pylori is a key player in gastric homeostasis, since it modulates the acid levels in the stomach, hence affecting the gastric microbiome. Helicobacter pylori infection can cause serious diseases such as chronic gastritis and carcinogenesis [31]. In gastric mucosa, the phylum of Firmicutes dominates, while Proteobacteria, Actinobacteria, Bacteroidetes, and Fusobacteria are also present [46]. Moreover, the gastric microbiota is also comprised of additional acid-resistant bacterial strains that either are grown into the stomach, or migrate from the oral cavity. Namely, these bacteria species are Streptococcus, Neisseria, Veillonella, Clostridium, and Lactobacillus [31].
Table 2.
Human diseases that have been related to imbalance of the normal gastrointestinal tract microbiome.
| Human Disease | Related Microorganisms | Reference |
|---|---|---|
| Atopic dermatitis | Staphylococcucus aureus, Cutibacterium, Streptococcus, Acinetobacter, Gemella | [47,48] |
| Cystic fibrosis | Streptococcus species | [49] |
| Depression | Coprococcus, Sellimonas, Clostridium, Hungatella | [50,51] |
| Autism | Clostridium bolteae | [52] |
| Asthma | Clostridia, Proteobacteria | [53,54] |
| Obesity | Actinobacteria, Bacteroidetes | [55,56] |
| Tuberculosis | Mycobacterium tuberculosis, Bacteroides fragilis, Prevotella, Enterococcus | [57] |
| Periodontal diseases | Spirochaetes, Synergistetes, Bacteroidetes | [58] |
| Dental caries | Streptococcus mutans, Lactobacillus spp., Candida albicans | [35] |
| Oral cancer | Streptococcus species | [59] |
| Esophageal cancer | Tannerella forsythia, Porphyromonas gingivalis | [60] |
| Cardiovascular disease | Campylobacter rectus, Porphyromonas gingivalis, Porphyromonas endodontalis, Prevotella intermedia | [61] |
| Rheumatoid arthritis | Veillonella, Atopobium, Prevotella, Leptotrichia | [62,63,64] |
| Parkinson’s disease | Lachnospiraceae, Faecalibacterium, Lactobacillus, Akkermansia, Bifidobacterium | [65] |
| Alzheimer’s disease | Spirochaetes | [66] |
| Diabetes | Aggregatibacter, Neisseria, Gemella, Selenomonas, Actinomyces, Fusobacterium, Streptococcus | [67,68,69] |
Especially, gut microbiota or gut microbiome is the largest microbiome community in the human body and has drawn for decades the attention of scientists for its significance in medical microbiology. Of note, the gut microbial community constitutes a dynamic and complex collection of all microorganisms that are accommodated in the gastrointestinal tract, including Bacteria, Archaea, Fungi, and Viruses [2]. Physiologically, these populations cooperate in different ways to provide immune defense against pathogenic organisms, regulate metabolic processes, and support cellular homeostasis, being characterized as the host barriers to infections [70]. More precisely, the intestinal microbiome is basically composed of multiple bacterial species (~50 bacterial phyla) in which Bacteroidetes and Firmicutes are the dominant phyla in a healthy human gut. Additionally, Proteobacteria and Actinobacteria are found in abundance, whereas the genera Bifidobacterium, Escherichia, Clostridium, and Akkermansia are also detected at lower levels [9,71]. Moreover, most studies support that the human gut archaeome includes two methanogenic classes of Archaea: the Methanobacteriales and the Methanomassiliicoccales. Methanobrevibacter smithii and Methanosphaera stadtmanae are the main representative species of Methanobacteriales, whereas in the case of Methanomassiliicoccales, Candidatus Methanomassiliicoccus intestinalis, Methanomethylophilus alvus, Methanomassiliicoccus luminyensis and the strains Mx02, Mx03, and Mx06 are dominant [72]. Additional studies have also reported that members of Haloarchaea, including Haloferax and Halorubrum spp., are also present in the human gut [73,74]. To continue with, fungal communities inhabit the gastrointestinal tract and support the immune system, and hence maintain cellular homeostasis [75]. The phylum of Ascomycota is overrepresented in gut mycobiome, whereas Basidiomycota and Mucoromycota exhibit lower expression levels [76,77].
Notably, a plethora of intestinal protozoan helminthic parasites that belong to micro-eukaryotes have been detected in the human gut and are responsible for various infections such as giardiasis, amoebiasis, and cryptosporidiosis [78]. More precisely, infections by Giardia intestinalis result in giardiasis, Cryptosporidium spp. causes cryptosporidiosis, Entamoeba histolytica is responsible for invasive amoebic infections, whereas infections by Cyclospora cayetanenensis lead to cyclosporiasis [79,80]. On the contrary, infections by helminthic parasites including Ascaris lumbricoides, Ancylostoma duodenale, Trichiuris trichiuria, and Necator americanicus affect human health since they disturb mental and physical growth but there is no evidence that they are deathly [81]. Finally, the gut also harbors viruses which contribute to homeostasis in human physiology. Briefly, DNA bacteriophages, such as Caudovirales and members of other families such as Myoviridae and Siphoviridae, dominate in the gut virome, while Circovirus and a small number of other eukaryotic and archaea viruses are present [71,82,83,84].
Furthermore, the high complexity of the gut microbiome contributes to the development of the immune system and gut homeostasis and elicits effective immune responses against invasive pathogens, including viruses. Microbial balance in the gut can ensure immunity by blocking invading pathogens not only in the gastrointestinal tract but also in multiple human organs. For instance, many studies support that gut microbes can eliminate infections by lung-associated viruses, such as the flu virus and SARS-CoV-2 by producing antiviral proteins [85]. This crucial role of gut microbiota depends on cellular mechanisms that regulate microbial metabolites and molecular pathways both in the host and their inhabitants [86].
The gut microbiota has been related to multiple diseases including brain disorders, neuropsychiatric disorders, cardiovascular diseases, type 2 diabetes, asthma, and human malignancies (Table 2). Firstly, recent studies support that the gut microbial community affects communication and interactions between the central and enteric nervous systems, namely the gut–brain axis [87]. Psychiatric disorders such as depression and anxiety have been connected to gut microbiome composition, since various bacteria genera, including Coprococcus, Sellimonas, Clostridium, and Hungatella, are involved in the synthesis of the key neurotransmitters serotonin, GABA, and glutamate [88]. In the same manner, increased levels of Actinobacteria, Bacteroidetes, and Protobacteria are also responsible for the emergence of depressive disorders (Table 2). Alterations in the expression levels of gut bacteria species are responsible for the progression of Parkinson’s disease. More precisely, a decrease in the levels of Lachnospiraceae and Faecalibacterium and an increase in the levels of Lactobacillus, Akkermansia, and Bifidobacterium have been associated with poor prognosis [65]. As far as type 2 diabetes is concerned, multiple studies have reported that the levels of Firmicutes and Clostridia are significantly lower in patients with type 2 diabetes [89]. Gut-microbiota-derived molecules such as short-chain fatty acids (SCFA), trimethylamine-N-oxide (TMAO), and uremic toxins are implicated in the development of cardiovascular diseases (Table 2). Changes in microbiota profile can lead to an increased risk of cardiovascular diseases. For instance, in adipose tissues, the abundance of Akkermansia has been correlated with inflammation and lipid metabolism [90], whereas obesity is affected by the lower ratio of Bacteroidetes to Firmicutes [91]. Moreover, in the case of asthma, the Proteobacteria are overexpressed, whereas the levels of Firmicutes and Bacteroidetes are decreased [92]. Finally, cancer-associated studies suggest that the human microbiome has a huge impact on carcinogenesis by influencing the proliferation of the host cells, mediating host metabolism, and affecting cellular immunity [93,94].
3. Detecting Microbes
Undoubtedly, identifying microbes and studying their functional role as well as their great diversity and complexity in the human body constitutes an attractive research field. However, the identification and quantification of the gut microbiome remain incomplete tasks due to its dynamic [95]. In the last decades, technological innovations in molecular microbiology have enabled the characterization of microbiomes and multiple studies have attempted to decipher the complex features of microorganisms and their role in human physiology. Up to date, four strategies for studying the human microbiome are widely used: a. the traditional culturomics approach that is based on the type of microbes, b. the amplicon-based DNA sequencing method, c. the modern whole genome metagenomics strategy, and d. the quite new metatranscriptomics approach. In this section, all the available strategies are thoroughly discussed.
3.1. Culturomics
More than a hundred years have passed since the first technique for identifying microorganisms was introduced. More precisely, in the early 1880s the plating method for culturing and detecting microbes, based on their biochemical features, was established by Robert Koch. Although classical microbiome studies are based on cultivation techniques, they enable the detection of only half of the gut bacteria. Over the years, improvements in culture conditions, the development of molecular technologies, and the advent of both Mass Spectrometry and Sanger sequencing have led to culturomics, a recently adapted technique that combines culture-dependent approaches and high-throughput methods for mapping the microbiome [96,97].
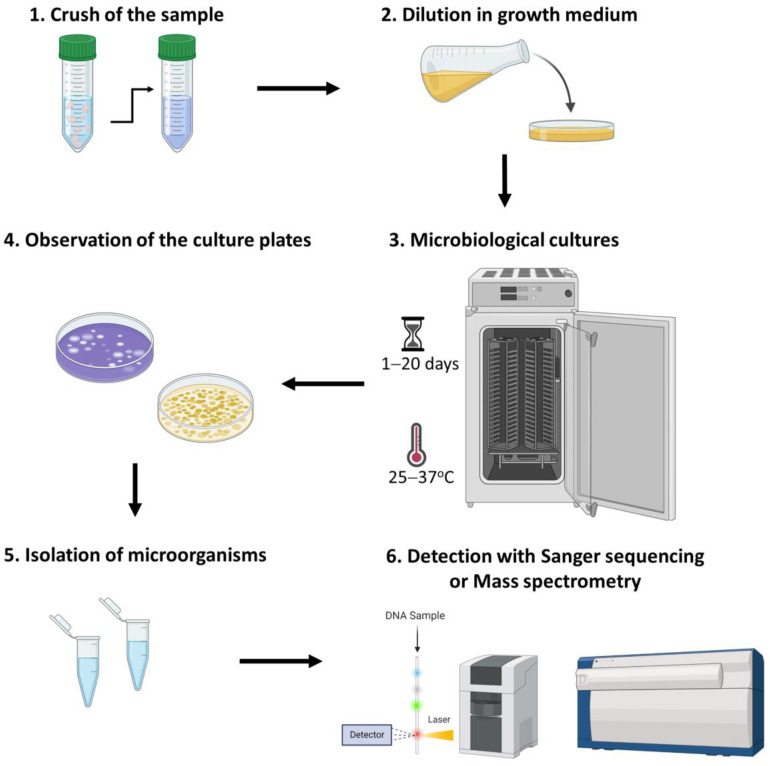
The culturomics approach includes distinct and sufficient steps (Figure 2). Briefly, the first step involves the crushing of the living or environmental samples and their dilution in a liquid growth medium. The selection of the appropriate culture media depends on the type of microbial species that are cultured since each microorganism has different nutritional requirements (Table 3). More precisely, based on the agar concentration growth media are classified into solid, semisolid, and liquid [98]. In addition, culture plates are incubated for 1–20 days at 25–37 °C. Of note, incubating oxygen levels vary among different microbial communities since most of them are aerobic, whereas the gastrointestinal tract harbors anaerobic species [99,100,101]. In addition, culture plates are incubated for 1–20 days at 25–37 °C. Accordingly, the culture plates are observed, and different phenotypes are isolated and grown individually. For the detection of the isolated bacterial species, cultures are harvested, and extracts are purified [70,102]. The lysates can be detected by either 16S rRNA/rDNA sequencing or matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) [103].
Figure 2.
Culturomics method for the detection of microbiome.
Table 3.
List of the growth media that are used for the culture of the most prevalent gut microbial communities.
| Related Microorganisms | Culture Media |
|---|---|
| Gram-positive Staphylococci | Baird-Parker agar |
| Streptococcus pyogenes | Crystal Violet Blood Agar |
| Gram-negative bacterial species | Hektoen Enteric Agar |
| Mycobacterium species | Lowenstein Jensen Medium |
| Gram-negative bacterial species | MacConkey’s Agar |
| Gram-positive bacterial species | Mannitol Salt Agar |
| Enterococcus species | Potassium Tellurite Medium |
| Pseudomonas aeruginosa | Pseudosel Agar |
| Neisseria gonorrhoeae | Thayer Martin Agar |
| Vibrio species | Thiosulfate Citrate Bile Salts Sucrose Agar |
| Salmonella & Shigella species | Salmonella-Shigella Agar |
| Salmonella species | Wilson and Blair’s Agar |
| Ectomycorrhizal fungi | BAF Medium |
| Ectomycorrhizal fungi | Modified Melin-Norkrans Medium |
| Certain fungi | Sabouraud Agar |
As for the advantages of culturomics approaches, they are high-throughput methods that are between the culture-dependent and the culture-independent strategies and can identify a high number of novel species that are visible in bacterial colonies. Moreover, due to the growing conditions, culturome enables the selection of the desired target and efficiently provides microbial isolates [104,105]. As for its drawbacks, culturome is a time-consuming and cost-ineffective approach that also requires accurate experimental manipulations. Furthermore, culturome results are highly influenced by the quality of culture media and environmental conditions such as temperature. Lastly, an additional limitation is that uncultured microbiota cannot be observed and hence detected (Table 4).
Table 4.
Advantages and drawbacks of microbiome high-throughput detection methods.
| Detection Method | Advantages | Drawbacks | Reference |
|---|---|---|---|
| Culturome | Visible colonies | Time-consuming and expensive | [106] |
| Microbial isolates | Sterile environmental conditions | ||
| Selection of the target | Laborious | ||
| Amplicon sequencing | Cost-effective | PCR biases | [107,108] |
| Easy and quick analysis | False-positive and false-negative samples | ||
| Selection of the target | No discrimination between dead and live microbes | ||
| Low-biomass requirement | Limited genus-level taxonomic resolution | ||
| Metagenomics | Culture-independent | Expensive | [109] |
| Species or strain level taxonomic resolution | Complex and time-consuming analysis | ||
| Captures all genomes present in a sample | No discrimination between dead and live microbes | ||
| Culture-independent | Host-derived contamination | ||
| Metatranscriptomics | Transcript level resolution | Expensive | [110] |
| Assessment of gene expression | Complex and time-consuming analysis | ||
| Discrimination between dead and live microbes | Snapshot of protein expression levels Host RNA contamination |
3.2. Amplicon Sequencing Analysis
Molecular biology research has been dramatically enhanced due to the introduction of high-throughput sequencing techniques that enabled a plethora of applications including molecular microbiota studies. More precisely, microbiome analysis has been radically transformed due to advances in sequencing applications including amplicon and whole genome approaches. Especially, amplicon sequencing constitutes a well-established method that includes the amplification and the detection of target genes that harbor characteristic and conserved motifs. Notably, studying microbiomes using amplicon sequencing can be divided into two approaches: a. 16S rRNA amplicon sequencing for identifying the prokaryotic sequences of Bacteria and Archaea and b. internal transcribed spacers (ITS) sequencing for the detection of the eukaryotic Fungi microcommunities. The prokaryotic 16S rRNA gene has conserved regions which are disrupted by nine variable regions that are used for the phylogenetic classification of genera in various microbes within a sample [111,112]. On the contrary, the rRNA cistron has the ITS region that is used as a DNA marker for the detection of fungal species [113].
Multiple studies support that amplicon sequencing has numerous advantages as compared to culturing methods [114]. To begin with, the 16S and the ITS rRNA amplicon sequencing method is a cost-effective technique to identify strains that may not be found using culturing methods. Moreover, amplicon sequencing is based on PCR amplification and thus requires low biomass. Additionally, next-generation sequencing (NGS) techniques enable parallel high-throughput generation of reads, the amount of the produced data is relatively small, and the bioinformatics analysis is quite simple [115]. However, PCR amplification can introduce biases such as false-positive and false-negative samples. Furthermore, amplicon sequencing cannot discriminate dead from living bacteria.
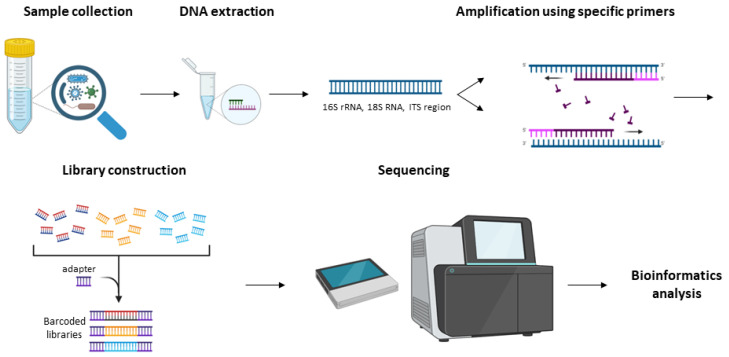
Notably, the benefits of amplicon sequencing and its wide usage in the scientific community have led to a great number of available protocols and workflows for studying the microbiome [114,116,117]. Especially, the NGS platforms, Illumina and Ion Torrent, utilize consensus adapters that bind specifically to conserved regions for amplifying and sequencing the targets of interest [118,119,120,121]. Additionally, NGS technology enables a high quality of generated data. Both approaches share well-defined steps (Figure 3) that include the following: 1. The collection of the sample; 2. Optional culturing of the bacterial or fungal samples in plates at 37 °C for 18–72 h; 3. Isolation of unique bacterial or fungal colonies; 4. Genomic DNA extraction; 5. 16S rRNA gene or ITS amplification procedure is divided into two PCR steps for amplifying and adding the barcodes; 6. Library construction based on the sequencing platform that is used; 7. Amplicon sequencing; and 8. Bioinformatics analysis [122,123]. On the contrary, PacBio and ONT platforms perform full-length 16S sequencing and provide real-time sequencing and direct analysis of the microbiome with greater taxonomic resolution compared to NGS results [124].
Figure 3.
Demonstration of the workflow for the 16S rRNA and ITS amplicon sequencing.
3.3. Metagenomics
Metagenomics aims to sequence the whole DNA that is available in a particular sample/environment, providing more information compared to amplicon sequencing. This method not only detects all the species that inhabit an environment, such as the human gut, but also generates information about the genomic profile of the sample. Metagenomics can be divided into two approaches: taxonomic metagenomics and functional metagenomics. Moreover, taxonomic applications aim to investigate the phylogenetic relationships between the detected sequences and the known microorganisms. Taxonomic profiling is widely used for identifying all the microbes (rare and abundant) included in a sample [125]. On the contrary, functional metagenomics techniques are focused on the identification of functional genes and novel proteins that contribute to the microbial population’s activity. Functional metagenomics can give answers to different biological issues such as how the microbes affect the functional pathways of their hosts and how they are implicated in various pathologies [126].
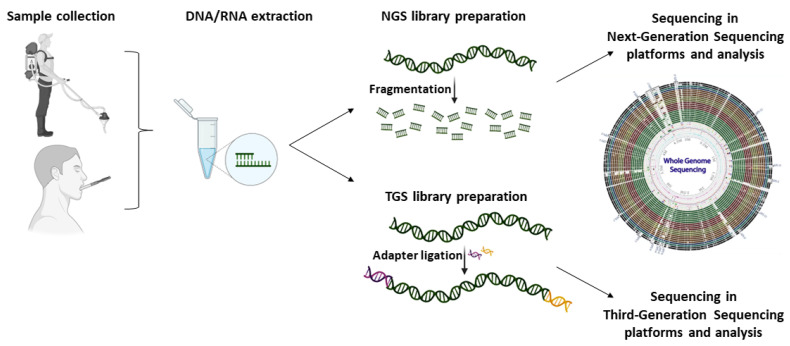
Both NGS and TGS approaches enable metagenomics sequencing; however, due to their chemistries, the library preparation steps, and the bioinformatics analysis procedure differ. Briefly, a metagenomics experiment is comprised of fundamental steps including the sample collection, the isolation of the DNA, the construction of either the NGS or TGS library, and, finally, the DNA sequencing (Figure 4). Of note, NGS libraries require the fragmentation of the DNA sample, whereas TGS enables the direct detection of full-length DNA sequences. In contrast to amplicon sequencing, metagenomics is more expensive and requires a greater amount of output data to perform accurate bioinformatics analysis and detect all the microorganisms that are present in a sample. Metagenomics are not only culture-independent methods but also PCR-free protocols that enable the absolute quantification of all genomes that are present in a sample without introducing PCR biases. However, the samples are often contaminated with host-derived DNA and the high complexity of the generated data can incommode the analysis (Table 4).
Figure 4.
Workflow of whole genome and whole transcriptome sequencing approaches for metagenomics and metatranscriptomics studies using next- and third-generation sequencing platforms.
3.4. Metatranscriptomics
The newly introduced metatranscriptomics approaches enable the study of the transcriptomic profile of microorganisms and have the potential to gain deeper insights into the composition of the human microbiota. Of note, whole RNA sequencing strategies can offer the quantification of the expression levels of active genes in complex microbial communities and total mRNA sequencing unravels the expression patterns of microbes within a host, such as the human gut [127]. Moreover, metatranscriptomic data are used for the determination of transcriptionally active microbial populations that interact with hosts, including humans, and for the detection of the active metabolic pathways that are connected to human diseases [110]. In healthy individuals, metatranscriptomics approaches are used to determine the activity of microbial communities that contribute to homeostasis by producing vital vitamins and amino acids or being involved in carbohydrate metabolism [128].
In the same manner as metagenomics, metatranscriptomics includes two distinct steps: the wet lab procedures and the bioinformatics analysis. As for the wet lab step, it contains the isolation and purification of the microbiome RNA, the construction of the metatranscriptomic sequencing libraries, and, finally, the RNA seq in the NGS sequencing systems (Figure 4). The second step of metatranscriptomics analysis comprises the analysis of the generated data. Although a number of bioinformatics algorithms have been developed for the study of the metagenome, the design of computational methods for analyzing metatranscriptomic data remains a challenging process [129]. It should be mentioned that, except for the NGS platforms that have already been established in the field of microbiology for the study of metatranscriptome through RNA-seq, the brand-new direct RNA nanopore sequencing approach constitutes a highly promising strategy. In brief, the method enables the characterization of the RNA without the reverse transcription and PCR amplification steps, and performs absolute quantification of RNA, and, thus, is expected to be a milestone in modern metatranscriptomics [130]. Ultimately, metatranscriptomics has the ability to change our perception of the biological function of microbes in the human body and will undoubtedly enhance our efforts in understanding their involvement in cellular mechanisms that disrupt human homeostasis and promote carcinogenesis.
4. Conclusions
Overall, the human body is a host for different types of microorganisms among which bacteria are dominant. The human microbiota benefits the body by synthesizing vital vitamins and amino acids, thus stimulating the immune system. Especially, the gastrointestinal tract accommodates a plethora of microbes that contribute to cellular homeostasis. However, multiple studies report that immune deregulation is correlated with changes in gut microbiome leading to the development of various diseases. To date, different detection methods have been used to decipher the characteristics of the human microbiome and clarify the relationship between microbes and human health and disease. The selection of the detection method is based on the type of experiment and the issues that need to be addressed. Notably, the type and the quality of the samples, the cost, and the needed time for each approach should always be taken into consideration. Undoubtedly, metagenomics will provide novel insights into human microbiome and health and will enhance not only modern microbiological research but also clinical microbiology and epidemiology through the establishment of novel approaches for preventing, improving, and reversing microbiome-related diseases [70].
Author Contributions
K.A. drafted the text of the article and designed all figures and tables; P.G.A. provided additional text and important discussions for the enhancement of the text; P.G.A. and A.S. critically reviewed the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Berg G., Rybakova D., Fischer D., Cernava T., Verges M.C., Charles T., Chen X., Cocolin L., Eversole K., Corral G.H., et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome. 2020;8:103. doi: 10.1186/s40168-020-00875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thursby E., Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garrido-Cardenas J.A., Manzano-Agugliaro F. The metagenomics worldwide research. Curr. Genet. 2017;63:819–829. doi: 10.1007/s00294-017-0693-8. [DOI] [PubMed] [Google Scholar]
- 4.Ursell L.K., Metcalf J.L., Parfrey L.W., Knight R. Defining the human microbiome. Nutr. Rev. 2012;70:S38–S44. doi: 10.1111/j.1753-4887.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings J.H., Macfarlane G.T. Colonic microflora: Nutrition and health. Nutrition. 1997;13:476–478. doi: 10.1016/S0899-9007(97)00114-7. [DOI] [PubMed] [Google Scholar]
- 6.Gomaa E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 7.Shreiner A.B., Kao J.Y., Young V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015;31:69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J., Wang K., Wang X., Pang Y., Jiang C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell. 2021;12:360–373. doi: 10.1007/s13238-020-00814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peroni D.G., Nuzzi G., Trambusti I., Di Cicco M.E., Comberiati P. Microbiome Composition and Its Impact on the Development of Allergic Diseases. Front. Immunol. 2020;11:700. doi: 10.3389/fimmu.2020.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boddy S.L., Giovannelli I., Sassani M., Cooper-Knock J., Snyder M.P., Segal E., Elinav E., Barker L.A., Shaw P.J., McDermott C.J. The gut microbiome: A key player in the complexity of amyotrophic lateral sclerosis (ALS) BMC Med. 2021;19:13. doi: 10.1186/s12916-020-01885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Integrative HMP (iHMP) Research Network Consortium The Integrative Human Microbiome Project. Nature. 2019;569:641–648. doi: 10.1038/s41586-019-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogunrinola G.A., Oyewale J.O., Oshamika O.O., Olasehinde G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020;2020:8045646. doi: 10.1155/2020/8045646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogobuiro I., Gonzales J., Tuma F. StatPearls. StatPerals Publishing; Treasure Island, FL, USA: 2022. Physiology, Gastrointestinal. [PubMed] [Google Scholar]
- 16.Gao L., Xu T., Huang G., Jiang S., Gu Y., Chen F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell. 2018;9:488–500. doi: 10.1007/s13238-018-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita Y., Takeshita T. The oral microbiome and human health. J. Oral. Sci. 2017;59:201–206. doi: 10.2334/josnusd.16-0856. [DOI] [PubMed] [Google Scholar]
- 18.Abranches J., Zeng L., Kajfasz J.K., Palmer S.R., Chakraborty B., Wen Z.T., Richards V.P., Brady L.J., Lemos J.A. Biology of Oral Streptococci. Microbiol. Spectr. 2018;6:5–6. doi: 10.1128/microbiolspec.GPP3-0042-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capasso C., Supuran C.T. An Overview of the Carbonic Anhydrases from Two Pathogens of the Oral Cavity: Streptococcus mutans and Porphyromonas gingivalis. Curr. Top Med. Chem. 2016;16:2359–2368. doi: 10.2174/1568026616666160413135522. [DOI] [PubMed] [Google Scholar]
- 20.Lepp P.W., Brinig M.M., Ouverney C.C., Palm K., Armitage G.C., Relman D.A. Methanogenic Archaea and human periodontal disease. Proc. Natl. Acad. Sci. USA. 2004;101:6176–6181. doi: 10.1073/pnas.0308766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wade W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013;69:137–143. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Patel M. Oral Cavity and Candida albicans: Colonisation to the Development of Infection. Pathogens. 2022;11:335. doi: 10.3390/pathogens11030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarco M.F., Vess T.J., Ginsburg G.S. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral. Dis. 2012;18:109–120. doi: 10.1111/j.1601-0825.2011.01851.x. [DOI] [PubMed] [Google Scholar]
- 24.Atarashi K., Suda W., Luo C., Kawaguchi T., Motoo I., Narushima S., Kiguchi Y., Yasuma K., Watanabe E., Tanoue T., et al. Ectopic colonization of oral bacteria in the intestine drives T(H)1 cell induction and inflammation. Science. 2017;358:359–365. doi: 10.1126/science.aan4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wescombe P.A., Heng N.C., Burton J.P., Chilcott C.N., Tagg J.R. Streptococcal bacteriocins and the case for Streptococcus salivarius as model oral probiotics. Future Microbiol. 2009;4:819–835. doi: 10.2217/fmb.09.61. [DOI] [PubMed] [Google Scholar]
- 26.Hou K., Wu Z.X., Chen X.Y., Wang J.Q., Zhang D., Xiao C., Zhu D., Koya J.B., Wei L., Li J., et al. Microbiota in health and diseases. Signal Transduct. Target Ther. 2022;7:135. doi: 10.1038/s41392-022-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastos J.A., Diniz C.G., Bastos M.G., Vilela E.M., Silva V.L., Chaoubah A., Souza-Costa D.C., Andrade L.C. Identification of periodontal pathogens and severity of periodontitis in patients with and without chronic kidney disease. Arch Oral Biol. 2011;56:804–811. doi: 10.1016/j.archoralbio.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Andrei E.C., Banita I.M., Munteanu M.C., Busuioc C.J., Mateescu G.O., Malin R.D., Pisoschi C.G. Oral Papillomatosis: Its Relation with Human Papilloma Virus Infection and Local Immunity-An Update. Medicina. 2022;58:1103. doi: 10.3390/medicina58081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deo P.N., Deshmukh R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019;23:122–128. doi: 10.4103/jomfp.JOMFP_304_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park C.H., Lee S.K. Exploring Esophageal Microbiomes in Esophageal Diseases: A Systematic Review. J. Neurogastroenterol Motil. 2020;26:171–179. doi: 10.5056/jnm19240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nardone G., Compare D. The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases? United Eur. Gastroenterol. J. 2015;3:255–260. doi: 10.1177/2050640614566846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Guryn K., Hubert N., Frazier K., Urlass S., Musch M.W., Ojeda P., Pierre J.F., Miyoshi J., Sontag T.J., Cham C.M., et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe. 2018;23:458–469.e455. doi: 10.1016/j.chom.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kastl A.J., Jr., Terry N.A., Wu G.D., Albenberg L.G. The Structure and Function of the Human Small Intestinal Microbiota: Current Understanding and Future Directions. Cell Mol. Gastroenterol. Hepatol. 2020;9:33–45. doi: 10.1016/j.jcmgh.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dieterich W., Schink M., Zopf Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018;6:116. doi: 10.3390/medsci6040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Struzycka I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014;63:127–135. doi: 10.33073/pjm-2014-018. [DOI] [PubMed] [Google Scholar]
- 36.Mombelli A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol 2000. 2018;76:85–96. doi: 10.1111/prd.12147. [DOI] [PubMed] [Google Scholar]
- 37.Rogers G.B., Carroll M.P., Serisier D.J., Hockey P.M., Jones G., Kehagia V., Connett G.J., Bruce K.D. Use of 16S rRNA gene profiling by terminal restriction fragment length polymorphism analysis to compare bacterial communities in sputum and mouthwash samples from patients with cystic fibrosis. J. Clin. Microbiol. 2006;44:2601–2604. doi: 10.1128/JCM.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Z., Kang Y., Yu J., Ren L. Human pharyngeal microbiome may play a protective role in respiratory tract infections. Genom. Proteom. Bioinform. 2014;12:144–150. doi: 10.1016/j.gpb.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kononen E., Gursoy U.K. Oral Prevotella Species and Their Connection to Events of Clinical Relevance in Gastrointestinal and Respiratory Tracts. Front. Microbiol. 2021;12:798763. doi: 10.3389/fmicb.2021.798763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooks L.R.K., Mias G.I. Streptococcus pneumoniae’s Virulence and Host Immunity: Aging, Diagnostics, and Prevention. Front. Immunol. 2018;9:1366. doi: 10.3389/fimmu.2018.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yolken R.H., Jones-Brando L., Dunigan D.D., Kannan G., Dickerson F., Severance E., Sabunciyan S., Talbot C.C., Jr., Prandovszky E., Gurnon J.R., et al. Chlorovirus ATCV-1 is part of the human oropharyngeal virome and is associated with changes in cognitive functions in humans and mice. Proc. Natl. Acad. Sci. USA. 2014;111:16106–16111. doi: 10.1073/pnas.1418895111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May M., Abrams J.A. Emerging Insights into the Esophageal Microbiome. Curr. Treat Options Gastroenterol. 2018;16:72–85. doi: 10.1007/s11938-018-0171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma T., Ru J., Xue J., Schulz S., Mirzaei M.K., Janssen K.P., Quante M., Deng L. Differences in Gut Virome Related to Barrett Esophagus and Esophageal Adenocarcinoma. Microorganisms. 2021;9:1701. doi: 10.3390/microorganisms9081701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y., Lin Z., Lin Y., Chen Y., Peng X.E., He F., Liu S., Yan S., Huang L., Lu W., et al. Streptococcus and Prevotella are associated with the prognosis of oesophageal squamous cell carcinoma. J. Med. Microbiol. 2018;67:1058–1068. doi: 10.1099/jmm.0.000754. [DOI] [PubMed] [Google Scholar]
- 45.Dhar A., Haboubi H.N., Attwood S.E., Auth M.K.H., Dunn J.M., Sweis R., Morris D., Epstein J., Novelli M.R., Hunter H., et al. British Society of Gastroenterology (BSG) and British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) joint consensus guidelines on the diagnosis and management of eosinophilic oesophagitis in children and adults. Gut. 2022;71:1459–1487. doi: 10.1136/gutjnl-2022-327326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J., Zhou X., Liu X., Ling Z., Ji F. Role of the Gastric Microbiome in Gastric Cancer: From Carcinogenesis to Treatment. Front. Microbiol. 2021;12:641322. doi: 10.3389/fmicb.2021.641322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paller A.S., Kong H.H., Seed P., Naik S., Scharschmidt T.C., Gallo R.L., Luger T., Irvine A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019;143:26–35. doi: 10.1016/j.jaci.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park D.H., Kim J.W., Park H.J., Hahm D.H. Comparative Analysis of the Microbiome across the Gut-Skin Axis in Atopic Dermatitis. Int. J. Mol. Sci. 2021;22:4228. doi: 10.3390/ijms22084228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott J.E., O’Toole G.A. The Yin and Yang of Streptococcus Lung Infections in Cystic Fibrosis: A Model for Studying Polymicrobial Interactions. J. Bacteriol. 2019;201:e00115-19. doi: 10.1128/JB.00115-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster J.A., McVey Neufeld K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Peirce J.M., Alvina K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019;97:1223–1241. doi: 10.1002/jnr.24476. [DOI] [PubMed] [Google Scholar]
- 52.Pequegnat B., Sagermann M., Valliani M., Toh M., Chow H., Allen-Vercoe E., Monteiro M.A. A vaccine and diagnostic target for Clostridium bolteae, an autism-associated bacterium. Vaccine. 2013;31:2787–2790. doi: 10.1016/j.vaccine.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 53.Frati F., Salvatori C., Incorvaia C., Bellucci A., Di Cara G., Marcucci F., Esposito S. The Role of the Microbiome in Asthma: The Gut(-)Lung Axis. Int. J. Mol. Sci. 2018;20:123. doi: 10.3390/ijms20010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barcik W., Boutin R.C.T., Sokolowska M., Finlay B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity. 2020;52:241–255. doi: 10.1016/j.immuni.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu B.N., Liu X.T., Liang Z.H., Wang J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021;27:3837–3850. doi: 10.3748/wjg.v27.i25.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aoun A., Darwish F., Hamod N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev. Nutr. Food Sci. 2020;25:113–123. doi: 10.3746/pnf.2020.25.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y., Wang J., Wu C. Microbiota and Tuberculosis: A Potential Role of Probiotics, and Postbiotics. Front. Nutr. 2021;8:626254. doi: 10.3389/fnut.2021.626254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lourenvarsigmao T.G.B., Spencer S.J., Alm E.J., Colombo A.P.V. Defining the gut microbiota in individuals with periodontal diseases: An exploratory study. J. Oral Microbiol. 2018;10:1487741. doi: 10.1080/20002297.2018.1487741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chocolatewala N., Chaturvedi P., Desale R. The role of bacteria in oral cancer. Indian J. Med. Paediatr. Oncol. 2010;31:126–131. doi: 10.4103/0971-5851.76195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baba Y., Iwatsuki M., Yoshida N., Watanabe M., Baba H. Review of the gut microbiome and esophageal cancer: Pathogenesis and potential clinical implications. Ann. Gastroenterol. Surg. 2017;1:99–104. doi: 10.1002/ags3.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jie Z., Xia H., Zhong S.L., Feng Q., Li S., Liang S., Zhong H., Liu Z., Gao Y., Zhao H., et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017;8:845. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao T., Wei Y., Zhu Y., Xie Z., Hai Q., Li Z., Qin D. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front. Immunol. 2022;13:1007165. doi: 10.3389/fimmu.2022.1007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maeda Y., Takeda K. Role of Gut Microbiota in Rheumatoid Arthritis. J. Clin. Med. 2017;6:60. doi: 10.3390/jcm6060060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen B., Zhao Y., Li S., Yang L., Wang H., Wang T., Bin S., Gai Z., Heng X., Zhang C., et al. Variations in oral microbiome profiles in rheumatoid arthritis and osteoarthritis with potential biomarkers for arthritis screening. Sci. Rep. 2018;8:17126. doi: 10.1038/s41598-018-35473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romano S., Savva G.M., Bedarf J.R., Charles I.G., Hildebrand F., Narbad A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Park. Dis. 2021;7:27. doi: 10.1038/s41531-021-00156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varesi A., Pierella E., Romeo M., Piccini G.B., Alfano C., Bjorklund G., Oppong A., Ricevuti G., Esposito C., Chirumbolo S., et al. The Potential Role of Gut Microbiota in Alzheimer’s Disease: From Diagnosis to Treatment. Nutrients. 2022;14:668. doi: 10.3390/nu14030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Z., Sun B., Yu D., Zhu C. Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus. Front. Cell Infect. Microbiol. 2022;12:834485. doi: 10.3389/fcimb.2022.834485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saad E., Tummala A., Agab M., Rodriguez-Nava G. Gemella morbillorum as the Culprit Organism of Post-Colonoscopy Necrotizing Perineal Soft Tissue Infection in a Diabetic Patient With Crohn’s Disease. J. Med. Cases. 2022;13:99–103. doi: 10.14740/jmc3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keogh R.A., Doran K.S. Group B Streptococcus and diabetes: Finding the sweet spot. PLoS Pathog. 2023;19:e1011133. doi: 10.1371/journal.ppat.1011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W.L., Xu S.Y., Ren Z.G., Tao L., Jiang J.W., Zheng S.S. Application of metagenomics in the human gut microbiome. World J. Gastroenterol. 2015;21:803–814. doi: 10.3748/wjg.v21.i3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tiamani K., Luo S., Schulz S., Xue J., Costa R., Khan Mirzaei M., Deng L. The role of virome in the gastrointestinal tract and beyond. FEMS Microbiol. Rev. 2022;46:fuac027. doi: 10.1093/femsre/fuac027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chibani C.M., Mahnert A., Borrel G., Almeida A., Werner A., Brugere J.F., Gribaldo S., Finn R.D., Schmitz R.A., Moissl-Eichinger C. A catalogue of 1,167 genomes from the human gut archaeome. Nat. Microbiol. 2022;7:48–61. doi: 10.1038/s41564-021-01020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nam Y.D., Chang H.W., Kim K.H., Roh S.W., Kim M.S., Jung M.J., Lee S.W., Kim J.Y., Yoon J.H., Bae J.W. Bacterial, archaeal, and eukaryal diversity in the intestines of Korean people. J. Microbiol. 2008;46:491–501. doi: 10.1007/s12275-008-0199-7. [DOI] [PubMed] [Google Scholar]
- 74.Seck E.H., Senghor B., Merhej V., Bachar D., Cadoret F., Robert C., Azhar E.I., Yasir M., Bibi F., Jiman-Fatani A.A., et al. Salt in stools is associated with obesity, gut halophilic microbiota and Akkermansia muciniphila depletion in humans. Int. J. Obes. 2019;43:862–871. doi: 10.1038/s41366-018-0201-3. [DOI] [PubMed] [Google Scholar]
- 75.Hallen-Adams H.E., Suhr M.J. Fungi in the healthy human gastrointestinal tract. Virulence. 2017;8:352–358. doi: 10.1080/21505594.2016.1247140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forbes J.D., Bernstein C.N., Tremlett H., Van Domselaar G., Knox N.C. A Fungal World: Could the Gut Mycobiome Be Involved in Neurological Disease? Front. Microbiol. 2018;9:3249. doi: 10.3389/fmicb.2018.03249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Belvoncikova P., Splichalova P., Videnska P., Gardlik R. The Human Mycobiome: Colonization, Composition and the Role in Health and Disease. J. Fungi. 2022;8:1046. doi: 10.3390/jof8101046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haque R. Human intestinal parasites. J. Health Popul. Nutr. 2007;25:387–391. [PMC free article] [PubMed] [Google Scholar]
- 79.Petri W.A., Jr., Haque R., Lyerly D., Vines R.R. Estimating the impact of amebiasis on health. Parasitol Today. 2000;16:320–321. doi: 10.1016/S0169-4758(00)01730-0. [DOI] [PubMed] [Google Scholar]
- 80.Davis A.N., Haque R., Petri W.A., Jr. Update on protozoan parasites of the intestine. Curr. Opin. Gastroenterol. 2002;18:10–14. doi: 10.1097/00001574-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Wright J.E., Werkman M., Dunn J.C., Anderson R.M. Current epidemiological evidence for predisposition to high or low intensity human helminth infection: A systematic review. Parasit Vectors. 2018;11:65. doi: 10.1186/s13071-018-2656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao Z., Sugimura N., Burgermeister E., Ebert M.P., Zuo T., Lan P. The gut virome: A new microbiome component in health and disease. EBioMedicine. 2022;81:104113. doi: 10.1016/j.ebiom.2022.104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoyles L., McCartney A.L., Neve H., Gibson G.R., Sanderson J.D., Heller K.J., van Sinderen D. Characterization of virus-like particles associated with the human faecal and caecal microbiota. Res. Microbiol. 2014;165:803–812. doi: 10.1016/j.resmic.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 84.Spencer L., Olawuni B., Singh P. Gut Virome: Role and Distribution in Health and Gastrointestinal Diseases. Front. Cell Infect. Microbiol. 2022;12:836706. doi: 10.3389/fcimb.2022.836706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Samuelson D.R., Welsh D.A., Shellito J.E. Regulation of lung immunity and host defense by the intestinal microbiota. Front. Microbiol. 2015;6:1085. doi: 10.3389/fmicb.2015.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sencio V., Machado M.G., Trottein F. The lung-gut axis during viral respiratory infections: The impact of gut dysbiosis on secondary disease outcomes. Mucosal. Immunol. 2021;14:296–304. doi: 10.1038/s41385-020-00361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sorboni S.G., Moghaddam H.S., Jafarzadeh-Esfehani R., Soleimanpour S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022;35:e0033820. doi: 10.1128/CMR.00338-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Radjabzadeh D., Bosch J.A., Uitterlinden A.G., Zwinderman A.H., Ikram M.A., van Meurs J.B.J., Luik A.I., Nieuwdorp M., Lok A., van Duijn C.M., et al. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022;13:7128. doi: 10.1038/s41467-022-34502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma S., Tripathi P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019;63:101–108. doi: 10.1016/j.jnutbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Schneeberger M., Everard A., Gomez-Valades A.G., Matamoros S., Ramirez S., Delzenne N.M., Gomis R., Claret M., Cani P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 92.Hilty M., Burke C., Pedro H., Cardenas P., Bush A., Bossley C., Davies J., Ervine A., Poulter L., Pachter L., et al. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou P., Hu Y., Wang X., Shen L., Liao X., Zhu Y., Yu J., Zhao F., Zhou Y., Shen H., et al. Microbiome in cancer: An exploration of carcinogenesis, immune responses and immunotherapy. Front. Immunol. 2022;13:877939. doi: 10.3389/fimmu.2022.877939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sepich-Poore G.D., Zitvogel L., Straussman R., Hasty J., Wargo J.A., Knight R. The microbiome and human cancer. Science. 2021;371:eabc4552. doi: 10.1126/science.abc4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gotschlich E.C., Colbert R.A., Gill T. Methods in microbiome research: Past, present, and future. Best Pract. Res. Clin. Rheumatol. 2019;33:101498. doi: 10.1016/j.berh.2020.101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang J., Liu Y.X., Guo X., Qin Y., Garrido-Oter R., Schulze-Lefert P., Bai Y. High-throughput cultivation and identification of bacteria from the plant root microbiota. Nat. Protoc. 2021;16:988–1012. doi: 10.1038/s41596-020-00444-7. [DOI] [PubMed] [Google Scholar]
- 97.Liu Y.X., Qin Y., Chen T., Lu M., Qian X., Guo X., Bai Y. A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell. 2021;12:315–330. doi: 10.1007/s13238-020-00724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lagier J.C., Dubourg G., Million M., Cadoret F., Bilen M., Fenollar F., Levasseur A., Rolain J.M., Fournier P.E., Raoult D. Culturing the human microbiota and culturomics. Nat. Rev. Microbiol. 2018;16:540–550. doi: 10.1038/s41579-018-0041-0. [DOI] [PubMed] [Google Scholar]
- 99.Schwerdtfeger L.A., Nealon N.J., Ryan E.P., Tobet S.A. Human colon function ex vivo: Dependence on oxygen and sensitivity to antibiotic. PLoS ONE. 2019;14:e0217170. doi: 10.1371/journal.pone.0217170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diakite A., Dubourg G., Dione N., Afouda P., Bellali S., Ngom I.I., Valles C., Tall M.L., Lagier J.C., Raoult D. Optimization and standardization of the culturomics technique for human microbiome exploration. Sci. Rep. 2020;10:9674. doi: 10.1038/s41598-020-66738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim R., Attayek P.J., Wang Y., Furtado K.L., Tamayo R., Sims C.E., Allbritton N.L. An in vitro intestinal platform with a self-sustaining oxygen gradient to study the human gut/microbiome interface. Biofabrication. 2019;12:015006. doi: 10.1088/1758-5090/ab446e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaspar U., Kriegeskorte A., Schubert T., Peters G., Rudack C., Pieper D.H., Wos-Oxley M., Becker K. The culturome of the human nose habitats reveals individual bacterial fingerprint patterns. Environ. Microbiol. 2016;18:2130–2142. doi: 10.1111/1462-2920.12891. [DOI] [PubMed] [Google Scholar]
- 103.Matar G., Bilen M. Culturomics, A potential approach paving the way toward bacteriotherapy. Curr. Opin. Microbiol. 2022;69:102194. doi: 10.1016/j.mib.2022.102194. [DOI] [PubMed] [Google Scholar]
- 104.Greub G. Culturomics: A new approach to study the human microbiome. Clin. Microbiol. Infect. 2012;18:1157–1159. doi: 10.1111/1469-0691.12032. [DOI] [PubMed] [Google Scholar]
- 105.Naud S., Khelaifia S., Mbogning Fonkou M.D., Dione N., Lagier J.C., Raoult D. Proof of Concept of Culturomics Use of Time of Care. Front. Cell Infect Microbiol. 2020;10:524769. doi: 10.3389/fcimb.2020.524769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kellenberger E. Exploring the unknown. The silent revolution of microbiology. EMBO Rep. 2001;2:5–7. doi: 10.1093/embo-reports/kve014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rezasoltani S., Ahmadi Bashirzadeh D., Nazemalhosseini Mojarad E., Asadzadeh Aghdaei H., Norouzinia M., Shahrokh S. Signature of Gut Microbiome by Conventional and Advanced Analysis Techniques: Advantages and Disadvantages. Middle East J. Dig. Dis. 2020;12:5–11. doi: 10.15171/mejdd.2020.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wensel C.R., Pluznick J.L., Salzberg S.L., Sears C.L. Next-generation sequencing: Insights to advance clinical investigations of the microbiome. J. Clin. Investig. 2022;132:e154944. doi: 10.1172/JCI154944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang L., Chen F., Zeng Z., Xu M., Sun F., Yang L., Bi X., Lin Y., Gao Y., Hao H., et al. Advances in Metagenomics and Its Application in Environmental Microorganisms. Front. Microbiol. 2021;12:766364. doi: 10.3389/fmicb.2021.766364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shakya M., Lo C.C., Chain P.S.G. Advances and Challenges in Metatranscriptomic Analysis. Front. Genet. 2019;10:904. doi: 10.3389/fgene.2019.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Janda J.M., Abbott S.L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: Pluses, perils, and pitfalls. J. Clin. Microbiol. 2007;45:2761–2764. doi: 10.1128/JCM.01228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Woo P.C., Lau S.K., Teng J.L., Tse H., Yuen K.Y. Then and now: Use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin. Microbiol. Infect. 2008;14:908–934. doi: 10.1111/j.1469-0691.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- 113.Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L., Levesque C.A., Chen W., Fungal Barcoding C., Fungal Barcoding Consortium Author L. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gupta S., Mortensen M.S., Schjorring S., Trivedi U., Vestergaard G., Stokholm J., Bisgaard H., Krogfelt K.A., Sorensen S.J. Amplicon sequencing provides more accurate microbiome information in healthy children compared to culturing. Commun. Biol. 2019;2:291. doi: 10.1038/s42003-019-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rozas M., Brillet F., Callewaert C., Paetzold B. MinION Nanopore Sequencing of Skin Microbiome 16S and 16S-23S rRNA Gene Amplicons. Front. Cell Infect Microbiol. 2021;11:806476. doi: 10.3389/fcimb.2021.806476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Callahan B.J., Grinevich D., Thakur S., Balamotis M.A., Yehezkel T.B. Ultra-accurate microbial amplicon sequencing with synthetic long reads. Microbiome. 2021;9:130. doi: 10.1186/s40168-021-01072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kinoshita Y., Niwa H., Uchida-Fujii E., Nukada T. Establishment and assessment of an amplicon sequencing method targeting the 16S-ITS-23S rRNA operon for analysis of the equine gut microbiome. Sci. Rep. 2021;11:11884. doi: 10.1038/s41598-021-91425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kameoka S., Motooka D., Watanabe S., Kubo R., Jung N., Midorikawa Y., Shinozaki N.O., Sawai Y., Takeda A.K., Nakamura S. Benchmark of 16S rRNA gene amplicon sequencing using Japanese gut microbiome data from the V1-V2 and V3-V4 primer sets. BMC Genom. 2021;22:527. doi: 10.1186/s12864-021-07746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alcon-Giner C., Dalby M.J., Caim S., Ketskemety J., Shaw A., Sim K., Lawson M.A.E., Kiu R., Leclaire C., Chalklen L., et al. Microbiota Supplementation with Bifidobacterium and Lactobacillus Modifies the Preterm Infant Gut Microbiota and Metabolome: An Observational Study. Cell Rep. Med. 2020;1:100077. doi: 10.1016/j.xcrm.2020.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fehlner-Peach H., Magnabosco C., Raghavan V., Scher J.U., Tett A., Cox L.M., Gottsegen C., Watters A., Wiltshire-Gordon J.D., Segata N., et al. Distinct Polysaccharide Utilization Profiles of Human Intestinal Prevotella copri Isolates. Cell Host Microbe. 2019;26:680–690.e685. doi: 10.1016/j.chom.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Allali I., Arnold J.W., Roach J., Cadenas M.B., Butz N., Hassan H.M., Koci M., Ballou A., Mendoza M., Ali R., et al. A comparison of sequencing platforms and bioinformatics pipelines for compositional analysis of the gut microbiome. BMC Microbiol. 2017;17:194. doi: 10.1186/s12866-017-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mortensen M.S., Brejnrod A.D., Roggenbuck M., Abu Al-Soud W., Balle C., Krogfelt K.A., Stokholm J., Thorsen J., Waage J., Rasmussen M.A., et al. The developing hypopharyngeal microbiota in early life. Microbiome. 2016;4:70. doi: 10.1186/s40168-016-0215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanschagrin S., Yergeau E. Next-generation sequencing of 16S ribosomal RNA gene amplicons. J. Vis. Exp. 2014;90:51709. doi: 10.3791/51709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nygaard A.B., Tunsjo H.S., Meisal R., Charnock C. A preliminary study on the potential of Nanopore MinION and Illumina MiSeq 16S rRNA gene sequencing to characterize building-dust microbiomes. Sci. Rep. 2020;10:3209. doi: 10.1038/s41598-020-59771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kumar Awasthi M., Ravindran B., Sarsaiya S., Chen H., Wainaina S., Singh E., Liu T., Kumar S., Pandey A., Singh L., et al. Metagenomics for taxonomy profiling: Tools and approaches. Bioengineered. 2020;11:356–374. doi: 10.1080/21655979.2020.1736238. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Lam K.N., Cheng J., Engel K., Neufeld J.D., Charles T.C. Current and future resources for functional metagenomics. Front. Microbiol. 2015;6:1196. doi: 10.3389/fmicb.2015.01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leigh D.M., Schefer C., Cornejo C. Determining the Suitability of MinION’s Direct RNA and DNA Amplicon Sequencing for Viral Subtype Identification. Viruses. 2020;12:801. doi: 10.3390/v12080801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bashiardes S., Zilberman-Schapira G., Elinav E. Use of Metatranscriptomics in Microbiome Research. Bioinform. Biol. Insights. 2016;10:19–25. doi: 10.4137/BBI.S34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Terron-Camero L.C., Gordillo-Gonzalez F., Salas-Espejo E., Andres-Leon E. Comparison of Metagenomics and Metatranscriptomics Tools: A Guide to Making the Right Choice. Genes. 2022;13:2280. doi: 10.3390/genes13122280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brown B.L., Watson M., Minot S.S., Rivera M.C., Franklin R.B. MinION nanopore sequencing of environmental metagenomes: A synthetic approach. Gigascience. 2017;6:1–10. doi: 10.1093/gigascience/gix007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.