Abstract
In early 2020, the COVID-19 pandemic led to substantial disruptions in global activities. The disruptions also included intentional and unintentional reductions in health services, including immunization campaigns against the transmission of wild poliovirus (WPV) and persistent serotype 2 circulating vaccine-derived poliovirus (cVDPV2). Building on a recently updated global poliovirus transmission and Sabin-strain oral poliovirus vaccine (OPV) evolution model, we explored the implications of immunization disruption and restrictions of human interactions (i.e., population mixing) on the expected incidence of polio and on the resulting challenges faced by the Global Polio Eradication Initiative (GPEI). We demonstrate that with some resumption of activities in the fall of 2020 to respond to cVDPV2 outbreaks and full resumption on January 1, 2021 of all polio immunization activities to pre-COVID-19 levels, the GPEI could largely mitigate the impact of COVID-19 to the delays incurred. The relative importance of reduced mixing (leading to potentially decreased incidence) and reduced immunization (leading to potentially increased expected incidence) depends on the timing of the effects. Following resumption of immunization activities, the GPEI will likely face similar barriers to eradication of WPV and elimination of cVDPV2 as before COVID-19. The disruptions from the COVID-19 pandemic may further delay polio eradication due to indirect effects on vaccine and financial resources.
Keywords: Polio, Dynamic modeling, COVID-19 pandemic
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus 2019 disease (COVID-19), in 2020 caused disruptions in human interactions and unprecedented restrictions in population movement that affected both the daily lives of people and the transmission of other diseases. Notably, countries responded to COVID-19 by reducing or shutting down economic and social activities, which led to substantially decreased population mixing and health services, including reduced polio immunization [1]. National immunization programs continue to rely on both Sabin-strain oral poliovirus vaccine (OPV) and inactivated poliovirus vaccine (IPV). The countries supported by the Global Polio Eradication Initiative (GPEI) currently include OPV in their routine immunization (RI) schedules by delivering 3 doses of bivalent OPV (bOPV, containing OPV serotypes 1 and 3) and one dose of IPV (containing serotypes 1, 2, and 3) given with the third bOPV dose. The polio endgame strategy includes ending the use of all OPV after the certification of wild poliovirus (WPV) eradication [2], which the GPEI decided to implement in phases by serotype. The GPEI globally coordinated the cessation of type 2-containing OPV (OPV2) in May 2016 [3], which stopped all use of trivalent OPV (tOPV, containing all three OPV serotypes) in RI and replaced it with bOPV in OPV-using countries. Many GPEI-supported countries also perform periodic supplementary immunization activities (SIAs). The SIAs may include preventive SIAs (pSIAs) using bOPV to increase population immunity for serotypes 1 and 3 or outbreak response SIAs (oSIAs) that use bOPV or a serotype 2 monovalent OPV (mOPV2). As of late 2020, some countries may also potentially use trivalent OPV (tOPV) for some oSIAs based on current GPEI plans.
Two recent reviews discuss the numerous studies related to polio modeling published between 2000 and 2019 by multiple groups and individuals [4] and reflect on nearly 20 years of policy and health economic modeling to support the GPEI [5]. Recent global transmission and evolution modeling [6] suggested that as of the beginning of 2020, the GPEI was not on track to achieve WPV eradication for serotype 1 (WPV1) prior to the end of its 2019–2023 Strategic Plan [7], [8]. Several modeling studies point directly to the challenges of stopping and preventing WPV1 transmission in Pakistan and Afghanistan in the absence of achieving and maintaining high and uniform coverage with OPV in those countries [9], [10], [11], [12], [13]. The cessation of OPV2 did not proceed as smoothly as hoped, and this led the GPEI to release an addendum to its 2019–2023 Strategic plan to manage serotype 2 circulating vaccine-derived polioviruses (cVDPV2s) [8]. A recent modeling study characterized the substantial probability of needing to restart the use of OPV2 in RI in OPV-using countries to manage cVDPV2s [14], which implies complicated logistics associated with OPV2 restart [15]. Building on prior work [14] and efforts to develop an alternative strain of OPV2 [16], another modeling study explored the role of using a stabilized, novel OPV2 (nOPV2) strain for response to cVDPV2 outbreaks [17]. Due to uncertainty about the properties that the new strains would exhibit in widespread use, the analysis considered the bounding characteristics of nOPV2 from no reversion to some reversion and varying its potential to cause vaccine-associated paralytic poliomyelitis (VAPP) from no VAPP to the same frequency of VAPP as OPV [17]. A number of other recent modeling studies estimated the expected health and economic implications of delays in achieving polio eradication and prospective costs for the polio endgame based on the conditions that existed at the end of 2019 (i.e., prior to the COVID-19 pandemic) [18], [19], [20], [21]. The Independent Monitoring Board of the GPEI recommended management and implementation changes needed to reach high coverage with OPV in all critical geographies for WPV1 eradication [22], [23], [24].
Some recent studies explore the impacts of disruptions associated with the COVID-19 pandemic on other maternal and child health interventions [25] and the management of vaccine-preventable diseases [26]. However, to date, no studies report on the likely global impacts of COVID-19 on polio eradication objectives. This analysis seeks to characterize the uncertain impacts on immunization activities and poliovirus transmission using assumptions consistent with information available as of Januray 18, 2021.
2. Methods
We use an integrated global economic, risk, and dynamic poliovirus transmission model (see [6] and its technical appendix for full details). The differential equation-based transmission model tracks 8 types of immunity, includes a 20-stage OPV reversion process, and a 5-stage waning process [6]. Briefly, the 20-stage OPV reversion process allows the model to abstractly represent the dynamics that occur in real populations as OPV-related viruses lose their attenuating mutations and in the context of low population immunity become cVDPVs that behave like homotypic WPVs. The model assumes that people mix homogenously overall within the subpopulation, with some heterogeneity in mixing by age [6]. The model allows for stochastic exportations of viruses preferentially from subpopulations into other subpopulations in the same block or to other blocks based on assumptions about preferential mixing areas (e.g., for several blocks that abstractly represent continents) [6]. The model also includes relatively infrequent stochastic long-range exportations (described below) and potential stochastic risks of reintroductions from containment failures and other sources [6]. The model focuses on characterizing population immunity to transmission (i.e., characterization of the ability of populations to sustain poliovirus transmission or not) and all aspects of poliovirus transmission dynamics, including die out and incidence of cases [6], [27]. Overall, the model accounts for variability that exists in the world by abstractly capturing differences in demographic characteristics, transmission potential, and other conditions [6] at the subpopulation level.
The model stratifies the population into blocks by World Bank income level (WBIL) [28]: low-income (LI), lower middle-income (LMI), upper middle-income (UMI), and high-income (HI) [6]. For each block, we characterize the current polio vaccine use according to multiple RI schedules [29]: OPV+IPV (former OPV-only [30], with one added IPV dose given simultaneously with the third OPV dose), IPV/OPV (sequential schedules that give IPV first followed by OPV), and IPV-only [6]. The model includes 72 blocks further divided into 10 subpopulations each, to apportion the 2019 global population of approximately 7.2 billion people [60] into 720 subpopulations with approximately 10.7 million each [6]. The model further groups the blocks into 9 preferential mixing areas of different sizes, which represent larger geographical regions (e.g., Africa, Australasia, Europe) [6]. We use a simplified model structure to capture global dynamics at a high level (i.e., without explicitly considering individual countries of variable sizes), but with sufficient stratification to reproduce the characteristics that exist globally [6]. The model includes the potential need to restart OPV2 in RI and SIAs, which the model triggers upon reaching 5000 cumulative global cVDPV2 cases caused since OPV2 cessation in mid-2016 [6], [15].
We begin with a scenario from a prior analysis that simulated the global immunization practices and epidemiological conditions as of the end of 2019 (i.e., before the COVID-19 pandemic) [6], [14], [17] (Reference Case 2 or “RC2” from prior studies [14], [17], which we refer to here as “pre-COVID”). Recent polio epidemiology provides an opportunity to explore our model assumptions related to exportation of poliovirus from one model subpopulation to another. In a world with all populations well-vaccinated, exportation events play a relatively minor role in overall transmission, because populations maintain high levels of population immunity to transmission such that any exportations die out (i.e., they do not effectively start outbreaks or restart transmission) and the importation events remain unobserved. However, global experience with continued serotype 2 transmission and cases after OPV2 cessation provides an opportunity to explore the role of exportations in the global model [6], because OPV2 cessation led to decreasing population immunity to transmission after May 2016 [31], [32]. For context, we use an exportation threshold (E*) along with preferential mixing assumptions to simulate potential infective interactions of individuals from one subpopulation with individuals in other subpopulations in the same or other blocks [6]. In the model, we track the cumulative number of effective infections (CEI) in each subpopulation for each OPV model reversion stage. Specifically, when the CEI of a given reversion stage reaches an E* of 200,000, the model triggers a potentially effective introduction of the same reversion stage virus into a randomly selected (according to preferential mixing assumptions) subpopulation and resets the CEI to zero. The model assumes that 96%, 3.5%, and 0.5% of exportations go to subpopulations in the same block, subpopulations in a block in the same preferential mixing area (e.g., large country or continent), or anywhere (e.g., on a flight), respectively [6]. The effect of changing the E* value is negligible in highly immunized populations, consistent with the concept that virus introductions do not effectively reintroduce transmission in the subpopulations with high population immunity and the reality that even with sophisticated case-based surveillance, we do not observe most of these events. Recognizing the role of E* in the post-OPV2 cessation world and prior to COVID-19 (i.e., before March 19, 2020), we ran the global model for this time and refer to it as “pre-COVID” (for brevity, dropping the 19). We run the pre-COVID analyses with E* of 100,000 or 125,000 for LI and LMI to compare with the prior reference case that assumed E* of 200,000 [6], [14]. Based on this analysis, we select a revised value for E* (relevant prior to the COVID-19 pandemic) for LI and LMI countries and show the GPEI trajectory for 2019–2023 modeled in the hypothetical absence of the COVID-19 pandemic. Based on analysis of international tourism data that shows dramatically more international travel for individuals in UMI and HI countries [33], we assume an E* of 25,000 for UMI and HI subpopulations. We note that since all transmission occurs in LI and LMI countries, the E* assumption for UMI and HI does not impact the results and we have no epidemiological data to evaluate this assumption.
We then focused on modeling the impacts of the COVID-19 pandemic, which remained ongoing at the time of writing. We focus on characterization of the early impacts on polio immunization and mitigation measures for SARS-CoV-2 on poliovirus transmission using the assumptions in Table 1 . Review of the available data related to national restrictions on mixing in response to the COVID-19 pandemic showed some variability in the nature of restrictions and their date of implementation [34]. However, restrictions on international travel to “high risk” or “any” countries globally occurred in most countries around March 20, 2020 [34], and consequently we assumed COVID-19 pandemic impacts start globally on March 20, 2020. Table 1 shows the assumed COVID-19 pandemic impacts on polio immunization for both RI and SIAs. Based on anecdotal information, we assume a reduction in RI coverage with 3 or more nonbirth polio vaccine (POL3) doses focusing on an absolute difference of 10%, which produces higher relative changes for low RI coverage subpopulations and lower relative change for high RI coverage subpopulations (i.e., POL3-0.10). We reduce RI coverage with 1 or 2 nonbirth doses proportional to the reduction in POL3. Consistent with GPEI activities to date, we model the changes in SIAs in non-endemic countries by removing all bOPV pSIAs from March 20, 2020 through December 31, 2020 and removing oSIAs between March 20, 2020 and September 1, 2020. For endemic countries, we assume all pSIAs and oSIAs resume from July 1, 2020 with no change in their intensity. We assume oSIAs in non-endemic countries resume with decreased oSIA intensity (i.e., lower true coverage, higher proportion of repeatedly missed children) from September 1, 2020 through the end of 2020 using mOPV2. For this analysis, we assume full recovery of the GPEI and the return to regular RI and SIA immunization starting on January 1, 2021. With respect to the impacts on poliovirus transmission, we assume a reduction in contacts starting on March 20, 2020. We reproduce population mixing restrictions by assuming a temporary increase in people staying near home, which we model as a temporary increase in E* equal to 1.5 × E* through December 31, 2020 and by temporarily reducing the subpopulation-specific basic reproduction number (R0) by 1 through August 31, 2020. Using a fixed reduction in R0 (i.e., an absolute difference) produces higher relative changes for low R0 blocks (i.e., relatively higher income countries) and lower relative change for high R0 blocks over the duration of these temporary restrictions.
Table 1.
Model assumptions used to characterize the impacts of the COVID-19 pandemic on poliovirus immunization and transmission.
| Input | Value |
|---|---|
| RI related inputs | |
| RI reduction start date | March 20, 2020 |
| RI reduction end date | December 31, 2020 |
| Change in average RI coverage with 3 or more nonbirth doses | |
|
LI LMI UMI HI |
0.77 → 0.67 0.83 → 0.73 0.94 → 0.84 0.95 → 0.85 |
| Change in average RI coverage with 1 or 2 nonbirth doses | |
|
LI LMI UMI HI |
0.20 → 0.17 0.15 → 0.13 0.07 → 0.06 0.23 → 0.20 |
| SIA related inputs | |
| SIA reduction start date | March 20, 2020 |
| SIA reduction end date | December 31, 2020 |
| Subpopulation-specific oSIA impact level (SI Level [6]) change in non-endemic countries | |
|
before September 1, 2020 after September 1, 2020 |
no oSIAs −1 |
| Subpopulation-specific pSIA change in non-endemic countries | no pSIAs |
| Subpopulation-specific SIA impact level (SI Level [39]) change in endemic countries | |
|
before July 1, 2020 after July 1, 2020 |
no SIAs no change |
| Transmission related inputs | |
| Mixing restriction start date | March 20, 2020 |
| Mixing restriction end date | August 31, 2020 |
| Subpopulation-specific R0 decrease | −1 |
| Exportation restriction start date | March 20, 2020 |
| Exportation restriction end date | December 31, 2020 |
| Exportation threshold (E*) factor increase | 1.5 |
Abbreviations: E*, exportation threshold; HI, high-income; LMI, lower middle-income; LI, low-income; oSIA, outbreak response SIA; pSIA, planned, preventive SIA; RC, reference case; RI, routine immunization; R0, basic reproduction number; SIAs, supplementary immunization activities; UMI, upper middle-income.
We focus our prospective analysis on the time horizon of 2020 through the end of 2023, which corresponds to the remainder of the current GPEI strategic plan [7]. We use updated inputs calibrated through 2020 [6], [14], which led to several adjustments to make the model assumptions consistent with the actual immunization and epidemiological experiences through the end of 2020. First, we corrected prior overestimation of mOPV2 use in block representing conditions like DRC by lowering the oSIA impact in one subpopulation of that block compared assumptions made in 2019 [14]. Second, we also corrected the pSIA schedules to make them consistent with the actual vaccines used (i.e., bOPV use in 2019 and tOPV use in 2020 and early 2021 in the endemic block representing conditions like Pakistan and Afghanistan). Third, we revised the dynamics of serotype 2 introductions in blocks representing conditions like Pakistan and Afghanistan based on updated evidence related to (i) unexpected and atypical epidemiological VDPV2 outbreaks Pakistan in 2019 (i.e., manual deterministic 5 point introductions of OPV-related virus at modeled reversion stage consistent with partially reverted virus (stage 5) instead of 9 point introductions of Sabin virus (stage 0) in block 34 subpopulation 1), and (ii) exportation of cVDPV2 from Pakistan to Afghanistan in early 2020 (i.e., manual deterministic point introduction of fully reverted virus (stage 19) in block 32 subpopulation 2). Finally, we updated the outbreak response assumptions in the endemic block to occur in the full block instead of only a single subpopulation. We used the same assumptions from a prior analysis [17] to characterize the bounds of nOPV2 properties consistent with available information. We consider 3 different permutations of the scenario with COVID-19 impacts that differ with respect to the vaccine used for oSIAs between July 1, 2021 and the end of the time horizon [17]. Specifically, we use the same assumptions for the bounding cases of nOPV2 assuming ideal (i.e., no reversion, no VAPP) or less than ideal (i.e., some reversion, some VAPP) properties for nOPV2 [17]. We refer to the COVID-19 pandemic impact scenarios based on the differences in the vaccine used for oSIAs as: (1) “COVID+mOPV2,” (2) “COVID+nOPV2 (ideal)” and (3) “COVID+nOPV2 (not ideal)”). We run the model (coded in the general-purpose programming language JAVATM in the integrated development environment EclipseTM) assuming unconstrained vaccine supplies to support characterization of potential vaccine needs [6]. For each scenario, we perform 100 stochastic iterations using the Amazon Elastic Compute Cloud (Amazon EC2) and we report the expected values, medians, and ranges for cases, including estimates of VAPP cases.
3. Results
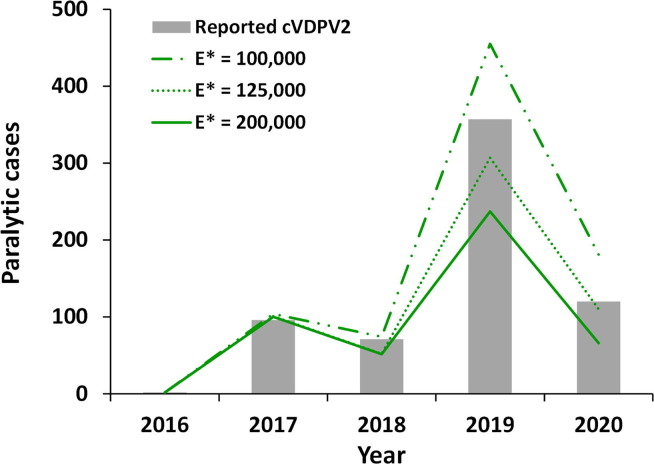
As serotype 2 population immunity to transmission steadily decreased with increased time since OPV2 cessation, the epidemiological experience of increasing observation of cVDPV2 cases allowed us to examine the ability of the model to capture effective cVDPV2 introductions into increasingly vulnerable subpopulations. Fig. 1 shows the impact of changing E* from 200,000 [6], [14], [17] to 100,000 or 125,000 for expected cVDPV2 cases compared to the reported cVDPV2 cases from 2016 through March 19, 2020 (i.e., pre-COVID). The choice of E* does not affect the modeled expected values when population immunity to transmission remains relatively high (e.g., WPV1, WPV3, and cVDPV2 pre-2016, not shown). However, Fig. 1 shows notable divergence of the model results for the different E* values for the expected cVDPV2 cases per year from 2018 through March 19, 2020 and compared to reported cases. Based on this analysis, we changed the default assumption for E* to 125,000, which shows the best agreement between model predictions and the reported cVDPV2 cases. Fig. 1 shows the model results of the analysis that we performed in July 2020, which we calibrated using information available through early 2020, and the actual number of reported cases for January 1- March 19, 2020. We confirmed that the updates to the model that we applied to calibrate through the end of 2020 (see last paragraph of the methods) improved the model performance compared to the 2019 and 2020 model estimates for the E* of 125,000, but we did not repeat the simulations with E* of 200,000 or 100,000.
Fig. 1.
Modeled average annual cVDPV2 cases in 100 stochastic iterations for the global model for 2016 through March 19, 2020, Note: * 2020 only includes January 1-March 19 to model the pre-COVID-19 time. Abbreviations: cVDPV2, serotype 2 circulating vaccine derived polioviruses; E*, Subpopulation exportation threshold (cumulative number of infections within a subpopulation that triggers exportation to another subpopulation).
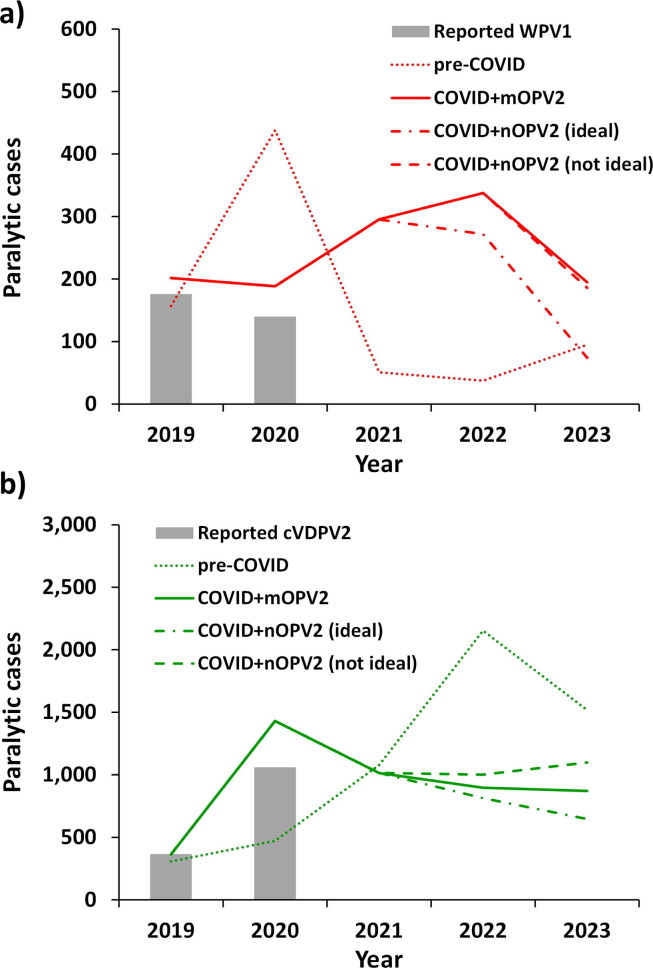
Fig. 2 shows the expected values of (a) WPV1 and (b) cVDPV2 cases based on 100 stochastic iterations of scenarios. The COVID-19 pandemic impact scenarios assume the immunization-related changes in RI and SIAs (top and middle of Table 1) as well as the transmission-related temporary changes in R0 and E* (bottom of Table 1) for the three different post-2020 oSIA vaccine options [17]. The conditions and epidemiology driving the incidence in these figures differ, with the results in Fig. 2(a) reflecting the WPV1 endemic areas of Pakistan and Afghanistan while those in Fig. 2(b) depend both on the situation with cVDPV2 transmission in Africa and contributions from Pakistan and Afghanistan. Consistent with the assumptions of unconstrained vaccine supplies and the resumption of pre-COVID immunization activities and transmission-related inputs by January 2021, the curves in Fig. 2 show estimates consistent with continued high control [6], but not eradication of WPV1 or cVDPV2 by the end of 2023. The results for the COVID-19 pandemic impact scenarios for WPV1 transmission (Fig. 2(a)) shows a substantial expected decrease in WPV1 cases in 2020 compared to the pre-COVID scenario due to the modeled decreased transmission and relatively fast resumption of bOPV and tOPV SIAs after the disruptions of immunization caused by the COVID-19 pandemic, followed by substantial expected increase in WPV1 cases from 2021 due to vaccination schedule changes caused by increased mOPV2 use in 2020 to combat cVDPV2 outbreaks leading to displacement and/or cancelation of some bOPV pSIAs. Compared to the pre-COVID model estimates [14], the results for the COVID-19 pandemic impact scenarios on cVDPV2 transmission (Fig. 2(b)) show an initial increase in cVDPV2 cases relative to the pre-COVID estimates in 2020 due to temporary gaps in vaccination. After this initial impact, all three COVID-19 pandemic impact scenarios begin to diverge, but all show cVDPV2 cases decreasing in 2021 below the pre-COVID scenario followed by a further but slight decrease in 2022. The results for the COVID+nOPV2 (ideal) scenario show a decrease in cVDPV2 cases relative to the pre-COVID scenario due to the implementation of non-reverting nOPV2 use from 2021. The results for the COVID+mOPV2 scenario show a decrease in cVDPV2 cases relative to the pre-COVID scenario, but an increased cVDPV2 case count relative to the COVID+nOPV2 (ideal) scenario due to the continued mOPV2 use from 2021. The results for the COVID+nOPV2 (not ideal) scenario show the smallest impact with respect to the decrease in cVDPV2 cases relative to the pre-COVID scenario. This occurs due to the assumptions that some reversion of nOPV2 occurs and thus may result in increased secondary immunization and possibly seed new outbreaks, but at an assumed decreased ability for secondary spread compared to mOPV2 leading to less population immunity. If we had modeled the disruptions in immunization without the offsetting reductions in transmission and exportation risks, then the model would show higher number of cases and OPV2 restarts within the time horizon (results not shown).
Fig. 2.
Modeled average annual WPV1 and cVDPV2 cases in 100 stochastic iterations for 2019–2023 for the modeled scenarios: (a) WPV1 and (b) cVDPV2, Abbreviations: COVID, coronavirus disease 2019; cVDPV2, serotype 2 circulating vaccine-derived poliovirus; mOPV2, serotype 2-containing monovalent Sabin-strain oral poliovirus vaccine; nOPV2, serotype 2 containing stabilized, novel oral poliovirus vaccine; WPV1, serotype 1 wild poliovirus.
Table 2 summarizes the expected values, medians, and ranges for the 100 iterations of the scenarios shown in Fig. 2. For the pre-COVID scenario, 46 of the 100 iterations trigger an OPV2 restart (i.e. reaches 5000 cumulative cVDPV2 cases since OPV2 cessation) by the end of 2023, and many more go on to trigger OPV2 restart in later years in prior analyses that considered longer time horizons [6], [14], [17]. For the COVID-19 pandemic impact scenarios, 33, 27, and 38 of the 100 iterations trigger an OPV2 restart by the end of 2023 for COVID+mOPV2, COVID+nOPV2 (ideal) and COVID+nOPV2 (not ideal), respectively. Overall, these results suggest substantial probabilities of needing to restart OPV2 in RI similar to prior analyses [6], [14], [17]. The results show that while disruptions in mixing may temporarily reduce the transmission of cVDPV2s, once population mixing resumes, the transmission will rebound if the speed and quality of outbreak responses are not improved. The actual results will depend on the timing and extent of disruption and resumption of immunization activities.
Table 2.
Restarts triggered, estimated expected value ((median) and [range]) of cVDPV2 cases and expected value ((median) and [range]) of vaccine use for outbreak response in 100 stochastic iterations for 2019–2023 for the scenarios modeled (see main text for descriptions).
| Scenario | Restarts triggered | Estimated expected cVDPV2 cases (median) [range] |
Estimated expected VAPP2 cases (median) [range] |
Estimated expected total cases* (median) [range] |
Estimated expected millions of oSIA doses used by vaccine type** (median) [range] |
||
|---|---|---|---|---|---|---|---|
| mOPV2 | IPV | nOPV2 | |||||
| Pre-COVID | 46 | 5526 (4454) [778–15,142] |
12 (11) [6–24] |
6605 (5480) [1312–15,544] |
545 (497) [283–950] |
0.48 (0) [0–48] |
NA |
| COVID + mOPV2 | 33 | 4572 (3807) [1028–11,549] |
16 (15) [10–27] |
537 (4300) [1494–12,737] | 692 (679) [359–1219] |
0.65 (0) [0–65] |
NA |
| COVID + nOPV2 (ideal) | 27 | 4266 (3501) [897–11,677] |
9 (9) [8–13] |
4657 (3836) [1070–11,862] |
187 (182) [137–274] |
0.65 (0) [0–65] |
358 (328) [175–737] |
| COVID + nOPV2 (not ideal) | 38 | 4907 (3885) [1039–12,114] |
16 (15) [10–25] |
5557 (4364) [1344–13,731] |
187 (182) [137–274] |
0.65 (0) [0–65] |
492 (504) [189–958] |
*Includes all type 2 cases (i.e., totals from all infections with live polioviruses, including WPV, VDPVs, and VAPP, which sums to more than the prior two columns due to cases associated with OPV-related viruses and cases associated with rare, but non-zero stochastic risks such as containment breaches), ** Includes doses for the entire time horizon such that all scenarios include mOPV2 use through June 30, 2021., Abbreviations: COVID, coronavirus disease 2019; cVDPV2, serotype 2 circulating vaccine-derived poliovirus; IPV, inactivated poliovirus vaccine; mOPV2, serotype 2-containing monovalent Sabin-strain oral poliovirus vaccine; nOPV2, serotype 2 containing stabilized, novel oral poliovirus vaccine; VAPP2, serotype 2 vaccine-associated paralytic polio
4. Discussion
Although prior global modeling did not show sensitivity to the assumed exportation threshold (E*), the growing gap in immunity to infection after OPV2 cessation changed its relative importance in many high-risk areas. The continued transmission of viruses related to OPV2 (but not fully reverted) and cVDPV2s after OPV2 cessation provided an opportunity to explore the calibration of between-population mixing using polio epidemiological data that did not exist prior to OPV2 cessation. If OPV2 cessation led to die out of the transmission of all serotype 2 live polioviruses, as hoped and as occurred in many countries [35], [36], then our need to adjust the global model assumptions for exportations from subpopulations with cVDPV2 cases and mOPV2 use to other subpopulation would have remained limited. This analysis confirms that in global models in which population immunity remains relatively low, the assumptions about the frequency of exportations between subpopulations will substantially impact the results, and that when population immunity remains high, exportations may show negligible impact and may largely occur unobserved.
The competing impacts of reductions in immunization, which all else equal would tend to increase expected incidence, and decreases in population mixing, which all else equal would tend to decrease expected incidence, lead to challenges with respect to forecasting. Overall, these results suggest that a relatively short-term (on the order of months) disruption on polio eradication activities will not substantially change the overall trajectory (i.e., no WPV1 eradication or end of cVDPV2 transmission by 2023) from the pre-COVID period. However, the disruptions could indirectly affect an already fragile OPV vaccine supply with few manufacturers and limited capacity and may lead to substantial cost impacts for vaccine manufacturers and donors. Specifically, by disrupting RI and pSIAs, the COVID-19 pandemic substantially decreased demand for bOPV that vaccine manufacturers produced and filled in anticipation of 2020 demand, and some of these vaccine doses may get wasted. The management of OPV orders and excess supplies of bOPV in 2020 could potentially send signals to manufacturers to stop and reduce production now for future sales. The indirect consequences of delayed demand could lead to scarcity later, and geo-political challenges related to accessing scarce supplies due to supply chain dynamics [37], [38]. Although these impacts on expected incidence may not seem large, the indirect effects on vaccine and financial resources could lead to substantial impacts on the ability to achieve WPV1 eradication and to successfully end all OPV use.
As with our prior studies, the global model comes with limitations (see appendix of [6]). The limitations include uncertainty about all of the assumptions in Table 1, since the true impacts of the COVID-19 pandemic on decreased RI activities, missed outbreak SIAs, reduced population mixing, and transmission dynamics remain uncertain. In a separate recent analysis, we explored the characterization of E* and variability in responses to pandemic threats by countries with different income levels [39]. Although we use 100 stochastic iterations for each scenario, this may not capture relatively rare events. However, our focus on direct comparisons using conserved seed values for the iterations across all scenarios mitigates some concerns about the impacts of rare events. The intrinsic uncertainty underlying our projections underscore the importance of focusing on the generic insights and not the specific values of the estimates. Uncertainty about the future of the GPEI and national immunization activities remain unaddressed by this analysis and will emerge over time. Clearly if immunization activities remain restricted for longer than modeled here and/or with decreased effectiveness, the situation would be worse, and conversely if transmission and exportations remain restricted longer than modeled here, the expected outcomes may improve. In this context, future modeling will need to explore the impacts of any disruptions caused by the continuing global experience with COVID-19 and the roll out of vaccines. In addition, the real constraints that exist for the OPV supply may also imply worse outcomes than modeled.
These results support the need for increased supplies of OPV2 consistent with the GPEI plans [8]. We expect the disruptions from the COVID-19 pandemic may further delay polio eradication, but with full resumption of GPEI activities to pre-COVID-19 levels in January 2021, the GPEI could return to its pre-COVID-19 behavior, which would still remain off-track for WPV1 eradication and successful OPV2 cessation by 2023.
Data statement
All of the data that the authors can share is available in the public domain and appropriate citations are provided.
CRediT authorship contribution statement
Dominika A. Kalkowska: Conceptualization, Methodology, Formal analysis, Visualization, Writing - original draft. Arie Voorman: Investigation, Writing - review & editing. Mark A. Pallansch: Investigation, Writing - review & editing. Steven G.F. Wassilak: Investigation, Writing - review & editing. Stephen L. Cochi: Investigation, Writing - review & editing. Kamran Badizadegan: Visualization, Writing - review & editing. Kimberly M. Thompson: Conceptualization, Supervision, Writing - original draft, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the Bill and Melinda Gates Foundation for supporting the completion of this work [INV-009333]. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Footnotes
This article was published as part of a supplement supported by Centers for Disease Control and Prevention Global Immunization Division. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or World Health Organization or UNICEF or Bill and Melinda Gates Foundation. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors.
References
- 1.World Health Organization Global Polio Eradication Initiative. Call to action to support COVID-19 response. https://polioeradication.org/news-post/call-to-action-to-support-covid-19-response/; 2020 [accessed August 20, 2020].
- 2.World Health Organization Global Polio Eradication Initiative. Polio eradication and endgame Strategic Plan (2013-2018). http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf; 2013 [accessed Jun 4, 2019].
- 3.Hampton L.M., Farrell M., Ramirez-Gonzalez A., Menning L., Shendale S., Lewis I., et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine - Worldwide, 2016. MMWR. 2016;65:934–938. doi: 10.15585/mmwr.mm6535a3. [DOI] [PubMed] [Google Scholar]
- 4.Thompson K.M., Kalkowska D.A. Review of poliovirus modeling performed from 2000–2019 to support global polio eradication. Expert Rev Vaccines. 2020;19:661–686. doi: 10.1080/14760584.2020.1791093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson K.M., Kalkowska D.A. Reflections on modeling poliovirus transmission and the polio eradication endgame. Risk Anal. 2020;41:229–247. doi: 10.1111/risa.13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalkowska D.A., Wassilak S.G.F., Cochi S.L., Pallansch M.A., Thompson K.M. Global transmission of live polioviruses: updated integrated dynamic modeling of the polio endgame. Risk Anal. 2021;41:248–265. doi: 10.1111/risa.13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Global Polio Eradication Initiative. Polio eradication and endgame strategic plan (2019-2023). http://polioeradication.org/wp-content/uploads/2019/05/polio-endgame-strategy-2019-2023.pdf; 2019 [accessed Jun 4, 2019].
- 8.World Health Organization Global Polio Eradication Initiative. Strategy for the response to type 2 circulating vaccine-derived poliovirus 2020–2021: Addendum to the Polio eradication and endgame strategic plan (2019-2023). http://polioeradication.org/wp-content/uploads/2020/04/Strategy-for-the-response-to-type-2-circulating-Vaccine-Derived-Poliovirus-20200406.pdf; 2020 [accessed Mar 10, 2020].
- 9.Duintjer Tebbens R.J., Pallansch M.A., Cochi S.L., Ehrhardt D.T., Farag N.H., Hadler S.C., et al. Modeling poliovirus transmission in Pakistan and Afghanistan to inform vaccination strategies in undervaccinated subpopulations. Risk Anal. 2018;38:1701–1717. doi: 10.1111/risa.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duintjer Tebbens RJ, Thompson KM. Evaluation of proactive and reactive proactive strategies for polio eradication activities in Pakistan and Afghanistan. Risk Anal 2019;39:389-401. [DOI] [PMC free article] [PubMed]
- 11.Kalkowska D.A., Duintjer Tebbens R.J., Thompson K.M. Environmental surveillance system characteristics and impacts on confidence about no undetected serotype 1 wild poliovirus circulation. Risk Anal. 2019;39:414–425. doi: 10.1111/risa.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalkowska D.A., Duintjer Tebbens R.J., Pallansch M.A., Thompson K.M. Modeling undetected live poliovirus circulation after apparent interruption of transmission: Pakistan and Afghanistan. Risk Anal. 2019;39:402–413. doi: 10.1111/risa.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalkowska D.A., Thompson K.M. Insights from modeling preventive supplemental immunization activities as a strategy to eliminate wild poliovirus transmission in Pakistan and Afghanistan. Risk Anal. 2021;41:266–272. doi: 10.1111/risa.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalkowska D.A., Pallansch M.A., Cochi S.L., Kovacs S.D., Wassilak S.G.F., Thompson K.M. Updated characterization of post-OPV cessation risks: Lessons from 2019 serotype 2 outbreaks and implications for the probability of OPV restart. Risk Anal. 2021;41:320–328. doi: 10.1111/risa.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson K.M., Kalkowska D.A. Logistical challenges and assumptions for modeling the failure of global cessation of oral poliovirus vaccine (OPV) Expert Rev Vaccines. 2019;18:725–736. doi: 10.1080/14760584.2019.1635463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Damme P., De Coster I., Bandyopadhyay A.S., Revets H., Withanage K., De Smedt P., et al. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. Lancet. 2019;394:148–158. doi: 10.1016/S0140-6736(19)31279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalkowska D.A., Pallansch M.A., Wilkinson A., Bandyopadhyay A.S., Konopka-Anstadt J.L., Burns C.C., et al. Updated characterization of poliovirus outbreak response strategies for 2019–2029: Impacts of the use of novel OPV2 strains. Risk Anal. 2021;41:329–348. doi: 10.1111/risa.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson KM, Kalkowska DA. Potential future use, costs, and value of poliovirus vaccines. Risk Anal 2020; online Jul 9. doi: 10.1111/risa.13557. [DOI] [PMC free article] [PubMed]
- 19.Kalkowska D.A., Thompson K.M. Health and economic outcomes associated with polio vaccine policy options: 2019–2029. Risk Anal. 2021;41:364–375. doi: 10.1111/risa.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson K.M., Kalkowska D.A. An updated economic analysis of the Global Polio Eradication Initiative. Risk Anal. 2021;41:393–406. doi: 10.1111/risa.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann M., Hagedorn B., Lyons H. Projection of costs of polio eradication compared to permanent control. J Infect Dis. 2020;221:561–565. doi: 10.1093/infdis/jiz488. [DOI] [PubMed] [Google Scholar]
- 22.Independent Monitoring Board of the Global Polio Eradication Initiative. How to cut a long story short, 16th Report, October 2018. Available from: http://polioeradicationorg/wp-content/uploads/2018/11/20181105-16th-IMB-Report-FINALpdf, accessed 27 August 2020; 2018.
- 23.Independent Monitoring Board of the Global Polio Eradication Initiative. The art of survival: independent monitoring board of the Global Polio Eradication Initiative, 17th Report, November 2019. Available from: http://wwwpolioeradicationorg/Portals/0/Document/Aboutus/Governance/IMB/10IMBMeeting/10IMB_Report_ENpdf, accessed 27 August 2020; 2019.
- 24.Independent Monitoring Board of the Global Polio Eradication Initiative. The new normal: finding the path back to eradication in the time of coronavirus, 18th Report, December 2020. Available from: http://polioeradicationorg/wp-content/uploads/2020/08/20200816-IMB-18th-Report-FINALpdf, accessed 27 August 2020; 2020.
- 25.Roberton T., Carter E.D., Chou V.B., Stegmuller A.R., Jackson B.D., Tam Y., et al. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Global Health. 2020;8(7):e901–e908. doi: 10.1016/S2214-109X(20)30229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbas K, Procter SR, van Zandvoort K, Clark A, Funk S, Mengistu T, et al. Routine childhood immunisation during the COVID-19 pandemic in Africa: a benefit–risk analysis of health benefits versus excess risk of SARS-CoV-2 infection. Lancet Glob Health 2020, 8,(10):e1264 - e1272. [DOI] [PMC free article] [PubMed]
- 27.Thompson K.M., Pallansch M.A., Duintjer Tebbens R.J., Wassilak S.G.F., Cochi S.L. Modeling population immunity to support efforts to end the transmission of live polioviruses. Risk Anal. 2013;33:647–663. doi: 10.1111/j.1539-6924.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Bank. World Bank analytical classifications - calendar year 2017 GNI per capita in US$ (Atlas methodology). Available from: http://databank.worldbank.org/data/download/site-content/OGHIST.xls; 2019 [accessed June 4, 2019].
- 29.World Health Organization. World schedule as of 2018/July/11. Available from: http://apps.who.int/immunization_monitoring/globalsummary/schedules; 2019 [accessed July 19, 2019].
- 30.Duintjer Tebbens RJ, Pallansch MA, Wassalik SGF, Cochi SL, Thompson KM. An economic analysis of poliovirus risk management policy options for 2013-2052. BMC Infectious Dis 2015;15. doi: 10.1186/s12879-015-1112-8. [DOI] [PMC free article] [PubMed]
- 31.Duintjer Tebbens R.J., Hampton L.M., Thompson K.M. Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: Risks of potential non-synchronous cessation. BMC Infect Dis. 2016;16:237. doi: 10.1186/s12879-016-1537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duintjer Tebbens R.J., Hampton L.M., Thompson K.M. Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of inadvertent trivalent oral poliovirus vaccine use. BMC Infect Dis. 2016;16:231. doi: 10.1186/s12879-016-1537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Bank. International tourism number of departures. Available from: https://data.worldbank.org/indicator/ST.INT.DPRT; 2020 [accessed Oct 5, 2020].
- 34.University of Oxford. Our World in Data: International travel controls during the COVID-19 pandemic. Available from: https://ourworldindata.org/grapher/international-travel-covid; 2020 [accessed July 17, 2020].
- 35.Thompson K.M., Duintjer Tebbens R.J. Lessons from globally-coordinated cessation of serotype 2 oral poliovirus vaccine for the remaining serotypes. J Infect Dis. 2017;216:S168–S175. doi: 10.1093/infdis/jix128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duintjer Tebbens R.J., Thompson K.M. Polio endgame risks and the possibility of restarting the use of oral poliovirus vaccine. Expert Rev Vaccines. 2018;17:739–751. doi: 10.1080/14760584.2018.1506333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duintjer Tebbens R.J., Pallansch M.A., Alexander J.P., Thompson K.M. Optimal vaccine stockpile design for an eradicated disease: application to polio. Vaccine. 2010;28:4312–4327. doi: 10.1016/j.vaccine.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Duintjer Tebbens R.J., Thompson K.M. Poliovirus vaccination during the endgame: insights from integrated modeling. Expert Rev Vaccines. 2017;16:577–586. doi: 10.1080/14760584.2017.1322514. [DOI] [PubMed] [Google Scholar]
- 39.Thompson K.M., Kalkowsa D.A., Badizadegan K. Hypothetical emergence of poliovirus in 2020: 1. Consequences of policy decisions to respond using nonpharmaceutical interventions. Expert Rev Vaccines. 2021 doi: 10.1080/14760584.2021.1891888. [DOI] [PubMed] [Google Scholar]