Abstract
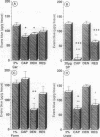
This study was performed to investigate whether different models of acute joint inflammation showed a neurogenic component and to establish whether this is mediated through sensory afferent or sympathetic efferent nerve fibres. Intra-articular injection of 2% carrageenan, 20 micrograms substance P, 1% formalin, and 2% urate all produced an inflammatory response. Prior surgical denervation of the joint significantly inhibited this response in the carrageenan and formalin models, but not the others. Pretreatment of the joint with 1% capsaicin (about one week previously) significantly reduced the inflammatory response in all models except formalin. In animals pretreated long term with reserpine (to deplete sympathetic nerve endings of their neurotransmitters) significant reductions occurred in the inflammatory responses to substance P and urate. Intraarticular injection of compound 48/80 produced a marked inflammatory response, which was only significantly reduced by capsaicin pretreatment. These results suggest that both the formalin and carrageenan models of inflammation depend to some extent on the integrity of the sensory innervation of the joint, and thus have a neurogenically mediated component to the inflammatory process they generate. In these models there seems to be little contribution from sympathetic efferent fibres. Each model of inflammation showed a different pattern of response to the pretreatments, suggesting that the mediators of the inflammatory process may differ in each case.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Goldman R., Stabinsky Y., Gottlieb P., Fridkin M., Teichberg V. I., Blumberg S. Enhancement of phagocytosis - a newly found activity of substance P residing in its N-terminal tetrapeptide sequence. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1445–1451. doi: 10.1016/0006-291x(80)90581-1. [DOI] [PubMed] [Google Scholar]
- Bayliss W. M. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J Physiol. 1901 Feb 28;26(3-4):173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D. The influence of the sympathetic nervous system on capillary permeability. Res Exp Med (Berl) 1978 Jul 24;173(1):1–8. doi: 10.1007/BF01851368. [DOI] [PubMed] [Google Scholar]
- Engel D. The influence of the sympathetic nervous system on capillary permeability. J Physiol. 1941 Jan 14;99(2):161–181. doi: 10.1113/jphysiol.1941.sp003891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell W. R., Russell N. J. Extravasation in the knee induced by antidromic stimulation of articular C fibre afferents of the anaesthetized cat. J Physiol. 1986 Oct;379:407–416. doi: 10.1113/jphysiol.1986.sp016260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C. Peptides and neurogenic inflammation. Br Med Bull. 1987 Apr;43(2):386–400. doi: 10.1093/oxfordjournals.bmb.a072189. [DOI] [PubMed] [Google Scholar]
- GASSER H. S. Unmedullated fibers originating in dorsal root ganglia. J Gen Physiol. 1950 Jul 20;33(6):651–690. doi: 10.1085/jgp.33.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse R., Holzer P., Lembeck F. Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol. 1980 Feb;68(2):207–213. doi: 10.1111/j.1476-5381.1980.tb10409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. L. Role of endogenous catecholamines in the anti-inflammatory activity of alpha-adrenoceptor blocking agents. Br J Pharmacol. 1974 May;51(1):45–53. doi: 10.1111/j.1476-5381.1974.tb09630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gözsy B., Kátó L. Role of norepinephrine and 5-hydroxytryptamine in the delayed phase of the inflammatory reaction in rats. Int Arch Allergy Appl Immunol. 1966;30(6):553–560. doi: 10.1159/000229842. [DOI] [PubMed] [Google Scholar]
- Harada M., Takeuchi M., Fukao T., Katagiri K. A simple method for the quantitative extraction of dye extravasated into the skin. J Pharm Pharmacol. 1971 Mar;23(3):218–219. doi: 10.1111/j.2042-7158.1971.tb08647.x. [DOI] [PubMed] [Google Scholar]
- Helme R. D., Andrews P. V. The effect of nerve lesions on the inflammatory response to injury. J Neurosci Res. 1985;13(3):453–459. doi: 10.1002/jnr.490130311. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenins P. Identification of the unmyelinated sensory nerves which evoke plasma extravasation in response to antidromic stimulation. Neurosci Lett. 1981 Sep 1;25(2):137–141. doi: 10.1016/0304-3940(81)90321-9. [DOI] [PubMed] [Google Scholar]
- Kimball E. S., Persico F. J., Vaught J. L. Substance P, neurokinin A, and neurokinin B induce generation of IL-1-like activity in P388D1 cells. Possible relevance to arthritic disease. J Immunol. 1988 Nov 15;141(10):3564–3569. [PubMed] [Google Scholar]
- Lam F. Y., Ferrell W. R. Capsaicin suppresses substance P-induced joint inflammation in the rat. Neurosci Lett. 1989 Oct 23;105(1-2):155–158. doi: 10.1016/0304-3940(89)90028-1. [DOI] [PubMed] [Google Scholar]
- Lam F. Y., Ferrell W. R. Inhibition of carrageenan induced inflammation in the rat knee joint by substance P antagonist. Ann Rheum Dis. 1989 Nov;48(11):928–932. doi: 10.1136/ard.48.11.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Linde B., Chisolm G., Rosell S. The influence of sympathetic activity and histamine on the blood-tissue exchange of solutes in canine adipose tissue. Acta Physiol Scand. 1974 Oct;92(2):145–155. doi: 10.1111/j.1748-1716.1974.tb05731.x. [DOI] [PubMed] [Google Scholar]
- Lotz M., Carson D. A., Vaughan J. H. Substance P activation of rheumatoid synoviocytes: neural pathway in pathogenesis of arthritis. Science. 1987 Feb 20;235(4791):893–895. doi: 10.1126/science.2433770. [DOI] [PubMed] [Google Scholar]
- Marasco W. A., Showell H. J., Becker E. L. Substance P binds to the formylpeptide chemotaxis receptor on the rabbit neutrophil. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1065–1072. doi: 10.1016/0006-291x(81)90727-0. [DOI] [PubMed] [Google Scholar]
- Mazurek N., Pecht I., Teichberg V. I., Blumberg S. The role of the N-terminal tetrapeptide in the histamine releasing action of substance P. Neuropharmacology. 1981 Nov;20(11):1025–1027. doi: 10.1016/0028-3908(81)90091-5. [DOI] [PubMed] [Google Scholar]
- Nilsson J., von Euler A. M., Dalsgaard C. J. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985 May 2;315(6014):61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- PARRATT J. R., WEST G. B. Release of 5-hydroxytryptamine and histamine from tissues of the rat. J Physiol. 1957 Jul 11;137(2):179–192. doi: 10.1113/jphysiol.1957.sp005805. [DOI] [PMC free article] [PubMed] [Google Scholar]