Abstract
Background:
Over 57 million people in Bangladesh have been chronically exposed to arsenic-contaminated drinking water. They also face environmental exposure to elevated levels of cadmium (Cd), manganese (Mn), and lead (Pb), all of which have been previously observed in environmental and biological samples for this population. These metals have been linked to adverse neurocognitive outcomes in adults and children, though their effects on adolescents are not yet fully characterized. Additionally, previous studies have linked selenium (Se) to protective effects against the toxicity of these other metals.
Objectives:
To examine the associations between mixed metals exposure and cognitive function in Bangladeshi adolescents.
Methods:
The Metals, Arsenic, & Nutrition in Adolescents study (MANAs) is a cross-sectional study of 572 Bangladeshi adolescents aged 14–16 years, whose parents were enrolled in the Health Effects of Arsenic Longitudinal Study (HEALS). Biosamples were collected from these adolescents for measurement of whole blood metalloid/metal levels of As, Cd, Mn, Pb, and Se. Participants also completed an abbreviated version of The Cambridge Neuropsychological Test Automated Battery (CANTAB), a cognitive function test designed to measure performance across several aspects of executive function. Linear regression was used to examine associations for each metal while controlling for the other metals. Bayesian Kernel Machine Regression (BKMR) assessed the overall mixture effect in addition to confirming the effects of individual metal components observed via linear regression.
Results:
Linear regression revealed negative associations for Spatial Working Memory and both As and Mn (As B=−2.40, Mn B=−5.31, p < 0.05). We also observed negative associations between Cd and Spatial Recognition Memory (B=−2.77, p < 0.05), and Pb and Delayed Match to Sample, a measure of visual recognition and memory (B=−3.67, p < 0.05). Finally, we saw a positive association for Se and Spatial Span Length (B=0.92, p < 0.05). BKMR results were largely consistent with the regression analysis, showing meaningful associations for individual metals and CANTAB subtests, but no overall mixture effect. Via BKMR, we observed negative associations between Pb and Delayed Match to Sample, and Cd and Spatial Recognition Memory; this analysis also showed positive associations for Se and the Planning, Reaction Time, and Spatial Span subtests. BKMR posterior inclusion probability consistently reported that Se, the only component of the mixture to show a positive association with cognition, was the most important member of the mixture.
Conclusions:
Overall, we found Se to be positively associated with cognition, while Mn and As were linked to poorer working memory, and Cd and Pb were associated with poorer visual recognition and memory. Our observations are consistent with previous reports on the effects of these metal exposures in adults and children. Our findings also suggest agreement between linear regression and BKMR methods for analyzing metal mixture exposures. Additional studies are needed to evaluate the impact of mixed metals exposure on adverse health and poorer cognition later in life for those exposed during adolescence. Findings also suggest that metal exposure mitigation efforts aimed at adolescents might influence lifelong cognitive outcomes in regions where environmental exposure to metals is endemic.
Keywords: Environmental metal exposures, Metal mixtures, Arsenic, Manganese, Lead, Cadmium, Selenium, Cognitive function, Adolescents, The Cambridge Neuropsychological Test Automated Battery (CANTAB)
1. Introduction
Arsenic-contaminated drinking water affects more than 140 million people worldwide and up to 57 million people in Bangladesh alone. (World Bank, 2015; Government of the People’s Republic of Bangladesh et al., 2001) Arsenic (As) exposure has been linked to adverse neurocognitive outcomes in children and adults, (Tyler and Allan, 2014) with domain-specific deficits reported in memory, (Rosado et al., 2007) attention, perceptual reasoning, visual processing, and spatial problem-solving, (von Ehrenstein et al., 2007).
In addition to As, many metals are known to adversely affect neurocognitive health. In recent years, public health research has focused on the potential adverse effects of metal mixtures, with an emphasis on mixtures containing cadmium (Cd), manganese (Mn), lead (Pb), and the metalloid As. (Claus Henn et al., 2014) This mixture of metalloid/metals has previously been found in Bangladeshi street dust, (Safiur Rahman et al., 2019) drinking water, and in urine samples collected from participants of the Health Effects of Arsenic Longitudinal Study (HEALS), (Sanchez et al., 2018) whose offspring were recruited for participation in this study of adolescents, known as the Metals, Arsenic, & Nutrition in Adolescents study (MANAs). Additionally, this investigation includes selenium (Se) in its metalloid/metal mixture, as it has been observed to attenuate the adverse effects of As. (Aschner, 1997).
Though the effects of metal exposures on neurocognitive function have been examined in adults and children, they have not been fully characterized in adolescents. Adolescence is an important neurodevelopmental period, during which brain regions linked to executive function develop and mature. (Pohl et al., 2019) As a result, previously published studies in adults and children cannot provide adequate insight into the executive function capabilities that rapidly emerge during adolescence, such as planning and goal-oriented behaviors. (Pohl et al., 2019) Tests designed to assess adolescent executive function should examine memory, attention, and planning as these tasks are controlled by brain regions that continue maturing throughout adolescence and early adulthood. (Anderson et al., 2001) This study examines the impact of exposure to a metal mixture on these aspects of executive function associated with adolescent frontal and prefrontal cortex development. (Green et al., 2019).
The Cambridge Neuropsychological Test Automated Battery (CANTAB) is a cognitive function test designed to assess performance across executive function tasks. (Cambridge Cognition Ltd., 2006) The CANTAB presents abstract images to participants using a non-linguistic computerized test paradigm that automatically scores non-verbal responses. Consequently, the CANTAB may be more culturally neutral than language-based cognitive tests. (Luciana and Nelson, 2002).
This study builds upon a prior investigation of intellectual function in the MANAs adolescents. (Wasserman et al., 2018) We aim to examine the effects of an As, Cd, Mn, Pb, and Se metal mixture on cognitive function as measured by the CANTAB.
2. Materials and methods
HEALS was established in 2000 as a prospective cohort study in a 35 km2 region in Araihazar, Bangladesh; since its inception, HEALS has recruited over 30,000 participants ages 18–75. In 2012, for a study designed to assess the effects of As exposure on cognitive function in adolescents, a subset of age-appropriate (14–16 years old) HEALS participants’ children were recruited for MANAs.
During MANAs recruitment, from 2012 to 2016, field staff approached 927 households of HEALS study participants in 51 villages, each expected to have a 14–16 year old child. (Wasserman et al., 2018) Among the 927 households approached, 12 home visits found no age-appropriate child within the home, 12 home visits found that the child had passed away, and 29 households had moved away from the study site. From the remaining 874 households, 25 were excluded for other reasons – 14 because a child with a severe deficit was noted during the home visit (e.g., autism), 2 due to the presence of twins, and 9 because children had aged out of the target recruitment range. Of the 849 remaining households, 111 refused participation, resulting in a final sample size of 738 participants who consented to joining the study, donated biological samples, and completed the structured interview. From these 738 participants initially recruited into the study, data cleaning systematically removed 59 participants based on previously reported criteria, (Saxena et al., 2021) and an additional 107 participants were excluded for missing or incomplete CANTAB data. This yielded an overall sample size of 572 participants for all analyses reported here.
Household characteristics were assessed via structured parental interview. Collected data included parental age, education, occupation, wall type (a proxy for socioeconomic status [SES]), and other relevant demographics.
Less than a week after this home visit, mother-child pairs visited the field clinic. During these visits, adolescent participants underwent a physical exam, completed the selected CANTAB subtests, and donated urine and blood samples. A brief test of cognitive function (Wechsler Abbreviated Scale of Intelligence [WASI], see below for details) was administered to mothers, since maternal intelligence was being considered as a potential covariate for this study. Participants were given a small, non-monetary, age-appropriate gift as a token of appreciation. Parental informed consent and adolescent assent were obtained by staff physicians in Bangladesh. The Columbia University Medical Center IRB and the Bangladesh Medical Research Council IRB approved this study protocol.
Seven CANTAB subtests selected to measure aspects of executive function were administered to study participants (Supplemental Table 1). The subtest Stockings of Cambridge (PLAN) assessed participants’ planning abilities; Spatial Span (SSP) and Spatial Working Memory (SWM) subtests assessed working memory; Reaction Time (RTI) and Rapid Visual Information Processing (RVP) subtests assessed attention; finally, Delayed Match to Sample (DMS) and Spatial Recognition Memory (SRM) subtests assessed visual recognition memory. To meet model assumptions and facilitate interpretation, all CANTAB scores have been normalized and transformed such that higher scores are indicative of better performance.
The WASI, a short reliable intelligence test that can be used across many groups, was administered to participants’ mothers. (The Psychological Corporation, 1999) The full WASI consists of two performance subtests (Block Design and Matrix Reasoning) and two verbal subtests (Vocabulary and Similarities); however, this study’s WASI battery only included the Vocabulary and Matrix Reasoning subtests. (Wasserman et al., 2018).
Whole blood samples were collected in our field laboratory in Araihazar, where they were immediately processed, frozen, stored at −80 °C, and shipped on dry ice to Columbia University. Whole blood was analyzed for blood arsenic (bAs), manganese (bMn), lead (bPb), cadmium (bCd), and selenium (bSe) concentrations using a Perkin-Elmer NexION 350S equipped Elemental Scientific autosampler 4DX. ICP-MS-DRC methods for metals in whole blood were developed according to published procedures. (Pruszkowski et al., 1998; Stroh, 1988) As previously reported, (Wasserman et al., 2018) intraprecision coefficients of variation for these blood metals were bMn= 3.2%, bAs= 3.7%, bSe= 2.0%, bPb= 1.6%, bCd= 7.8%, and the interprecision coefficients were bMn= 5.9%, bAs= 7.3%, bSe= 5.3%, bPb= 3.9%, and bCd= 16.0%. Normal mean blood levels of these metals for unexposed individuals are < 1 μg/L for As, (ATSDR, 2007) ~0.315 μg/L for Cd, (ATSDR, 2012b) 4–15 μg/L for Mn, (ATSDR, 2012a) 5 μg/L for Pb, (Centers for Disease Control, 2015) 98 μg/L for Se (note that Se deficiency is extremely rare and occurs at <40 μg/L). (Mayo Clinic, 2020)
Bivariate analysis was used to assess associations between potential covariates and the exposures and outcomes. Selected covariates have significant associations to at least one metal/metalloid biomarker and at least one CANTAB subtest score. Covariate selection was informed by previously identified covariates for this sample of adolescents. (Wasserman et al., 2018) The final control variables were child’s sex, child’s years of education, child’s head circumference, child’s BMI, maternal intelligence as measured by WASI, paternal education, and wall type.
Partial Spearman correlations controlling for covariates were used to examine associations between variables. Additionally, we fit linear regression models to explore associations between blood metal concentrations and CANTAB scores, controlling for potential confounding by other metals, and log-transforming and centering blood metal measurements to reduce the impact of extreme values and improve model fitting.
To explore non-linear and non-additive associations, we utilized Bayesian Kernel Machine Regression (BKMR). This allowed us to further investigate the effect of the metal mixture on CANTAB scores. BKMR is a semi-parametric model that accounts for the correlated structure of the exposure mixture with a flexible kernel function, allowing for the estimation of complex exposure-response relationships between metals and CANTAB scores, while considering high-order effects. (Bobb et al., 2015) BKMR includes a component-specific variable selection process to identify important mixture members. This paper’s analysis method parallels our research group’s previously published work, which utilized regression analysis and BKMR to describe the effects of this metal mixture on adolescent intellectual function as measured by the Wechsler Intelligence Scale for Children (WISC-IV). (Wasserman et al., 2018) Analyses were conducted using SAS 9.4 or R 3.5.1, using a significance level of 0.05.
3. Results
Study participants were aged 14–16 years, with BMI 11.8–30.8 (Table 1). Among the 572 participants, 99% had elevated bAs (>1 μg/L), 87% had elevated bCd (>0.315 μg/L), 12% (N = 70) had elevated bMn (>15 μg/L), all had elevated bPb (>20 μg/L), and 98% had bSe in the higher end of the normal range (>98 μg/L). Table 1 shows study sample descriptive characteristics.
Table 1.
Characteristics and CANTAB Scores of Study Participants.
| Characteristic (N = 572) | Mean (SD)a |
|---|---|
| Demographics and Covariates | |
| Age (yrs.) | 14.6 (0.7) |
| BMI | 18.3 (2.9) |
| Education (yrs.) | 6.9 (2.2) |
| Father’s Education (yrs.) | 4.3 (4.1) |
| Home construction (concrete wall) | 27.8 (159) |
| Head Circumference (cm) | 51.3 (3.7) |
| Maternal WASI Score | 33.4 (8.9) |
| Male: % (N) | 47.2 (270) |
| Blood Metals | |
| Arsenic (μg/L) | 4.8 (4.3) |
| Cadmium (μg/L) | 0.62 (0.32) |
| Manganese (μg/L) | 11.4 (3.6) |
| Lead (μg/L) | 98.7 (43.6) |
| Selenium (μg/L) | 133.3 (17.8) |
| CANTAB Subscale Scores | |
| Delayed Match to Sample | 73.2 (14.5) |
| Planning | 7.1 (1.8) |
| Rapid Visual Processing | −0.2 (0.4) |
| Reaction Time | −6.1 (0.2) |
| Spatial Recognition Memory | 74.5 (13.7) |
| Spatial Span | 5.8 (1.4) |
| Spatial Working Memory | −44.9 (18.4) |
Mean (SD) except where otherwise noted.
Controlled partial Spearman correlations for blood metal/metalloid levels and CANTAB scores are presented in Table 2. We observed a negative correlation for As and Cd (r = −0.14, p < 0.05), and positive correlations for Cd and Mn (r = 0.17, p < 0.05), Cd and Pb (r = 0.21, p < 0.05), and Pb and Se (r = 0.14, p < 0.05). Correlations among CANTAB scores varied widely (Table 2). We observed weak negative correlations for concentrations of As, Cd, Mn, Pb and CANTAB scores; in contrast, we saw weak positive correlations between Se and CANTAB scores (Table 2).
Table 2.
Partial Spearman Correlations (rho) for Blood Metal Concentrations and CANTAB Subtest Scores.
| Cd | Mn | Pb | Se | Delayed Match to Sample |
Planning | Rapid Visual Processing |
Reaction Time |
Spatial Recognition Memory |
Spatial Span |
Spatial Working Memory |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| As | −0.14 * * | −0.07† | −0.01 | −0.02 | −0.03 | 0.08† | −0.03 | 0.08† | −0.03 | −0.02 | −0.05 |
| Cd | 0.17 * * | 0.21 * * | 0.08† | −0.03 | 0.02 | 0.01 | 0.07† | −0.06 | 0.00 | 0.04 | |
| Mn | 0.03 | 0.02 | −0.03 | 0.02 | 0.01 | −0.04 | 0.00 | −0.10 * | −0.13 * * | ||
| Pb | 0.14 * * | −0.12 * * | 0.04 | −0.05 | 0.13 * * | 0.07 | 0.00 | 0.09 * | |||
| Se | 0.01 | 0.03 | −0.04 | 0.10 * | −0.03 | 0.07† | 0.07 | ||||
| Delayed Match to Sample | 0.13 * * | 0.16 * * | −0.02 | 0.17 * * | 0.26 * * | 0.15 * * | |||||
| Planning | 0.13 * * | 0.03 | 0.12 * * | 0.16 * * | 0.09 * | ||||||
| Rapid Visual Processing | 0.02 | 0.11 * * | 0.18 * * | 0.08† | |||||||
| Reaction Time | −0.02 | 0.06 | 0.11 * * | ||||||||
| Spatial Recognition Memory | 0.30 * * | 0.20 * * | |||||||||
| Spatial Span | 0.25 * * |
N = 572. Covariates: BMI, head circumference, child’s years of education, maternal intelligence (WASI), paternal years of education, wall type, and sex. Superscript indicates significant correlations at the following levels:
0.05 < p < 0.10
p < 0.05.
p < 0.01.
We assessed blood metal concentration and CANTAB score associations in controlled linear regression models (Table 3). We observed negative associations between SWM and both As (B=−2.40, p < 0.05) and Mn (B=−5.31, p < 0.05). We also observed negative associations for Cd and SRM (B=−2.77, p < 0.05), and Pb and DMS (B=−3.67, p < 0.05). Finally, we found Se and SSP to be positively associated (B=0.92, p < 0.05). Sensitivity analysis confirmed no interactions between metals in this mixture.
Table 3.
Estimated regression coefficient B (SE) for the association between blood metals predictors and CANTAB subscale score.
| Delayed match to sample | Planning | Rapid visual processing | Reaction time | Spatial recognition memory | Spatial span | Spatial working memory | |
|---|---|---|---|---|---|---|---|
| As | −0.99 (0.89) | 0.12 (0.13) | −0.01 (0.01) | 0.01 (0.02) | −0.88 (0.85) | −0.13 (0.08) | −2.40 * (1.10) |
| Cd | 0.00 (1.26) | 0.07 (0.16) | 0.00 (0.01) | 0.01 (0.02) | −2.77 * (1.20) | 0.04 (0.12) | 0.17 (1.56) |
| Mn | −2.51 (2.13) | 0.27 (0.28) | 0.01 (0.01) | 0.02 (0.03) | 3.50 (2.03) | −0.25 (0.19) | −5.31 * (2.64) |
| Pb | −3.67 * (1.49) | −0.05 (0.19) | −0.01 (0.01) | 0.03 (0.02) | 1.90 (1.42) | −0.16 (0.14) | 1.09 (1.85) |
| Se | 4.62 (4.52) | 0.89 (0.58) | 0.00 (0.03) | 0.16 * (0.07) | −3.20 (4.31) | 0.92 * (0.41) | 5.96 (5.61) |
N = 572. Covariates in the linear models: BMI, head circumference, child’s years of education, maternal intelligence (WASI), paternal years of education, wall type, sex, and other blood metals. Metals are centered and log-transformed (i.e.log(metal/median); medians: As = 3.52 μg/L, Cd= 0.55 μg/L, Mn = 10.79 μg/L, Pb = 91.29 μg/L, Se = 133.65 μg/L. Superscript indicates significant differences at the following levels:
p < 0.05
p < 0.01.
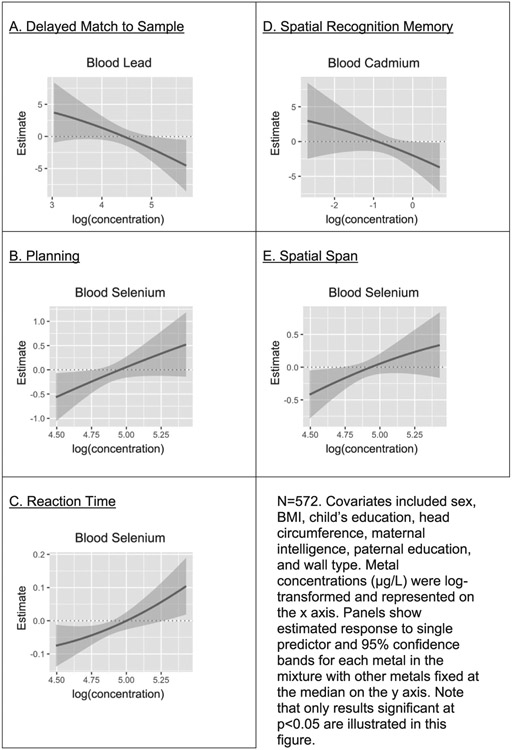
Our BKMR analysis results were consistent with the linear regression. The BKMR did not detect an association between the overall metal mixture and any of the CANTAB subtests, and it did not detect any metals interactions; however, it did show associations for individual metals and CANTAB variables. Exposure-response functions and 95% credible bands for each metal in the mixture with all other metals fixed at their median are shown in Fig. 1. Panel A shows Pb to be negatively associated with DMS. Panels B, C, and E show Se to be positively associated with PLAN, RTI, and SSP, respectively. Finally, Panel D shows Cd to be negatively associated with SRM. BKMR showed no significant effects of individual metals for RVP or SWM; the remaining associations between components in the metal mixture and CANTAB subtests were not statistically significant. The top two posterior inclusion probabilities (PIP) of the metals in the mixture for each of the CANTAB subtests are shown in Table 4. We observed that for DMS, PLAN, RTI, SSP, and SRM, the metal shown to be associated with each subtest score in the 95% credible bands had relatively higher PIP. For example, for DMS, we saw in the 95% credible bands that Pb had a significant negative association with test score (Fig. 1; Panel A), and in the PIP we observed that Pb was the most influential metal in the mixture for DMS (Table 4).
Fig. 1.
BKMR (Bayesian Kernel Machine Regression) univariate predictor-response functions showing associations between metal mixture components and CANTAB subtest scores.
Table 4.
BKMR (Bayesian Kernel Machine Regression) posterior inclusion probabilities (PIP) showing the two most influential metal predictors for each CANTAB subtest.
| Rank | Exposure | PIP |
|---|---|---|
| Delayed Match to Sample | ||
| 1 | Pb* | 0.48 |
| 2 | Se | 0.34 |
| Planning | ||
| 1 | Se* | 0.63 |
| 2 | Mn | 0.17 |
| Rapid Visual Processing | ||
| 1 | Pb | 0.46 |
| 2 | Se | 0.22 |
| Reaction Time | ||
| 1 | Se* | 0.73 |
| 2 | Cd | 0.02 |
| Spatial Span | ||
| 1 | Se* | 0.63 |
| 2 | Mn | 0.44 |
| Spatial Recognition Memory | ||
| 1 | Se | 0.27 |
| 2 | Cd* | 0.19 |
| Spatial Working Memory | ||
| 1 | Se | 0.44 |
| 2 | Mn | 0.43 |
N = 572. Covariates included sex, BMI, child’s education, head circumference, maternal intelligence, paternal education, and wall type. Metal concentrations (μg/L) were log-transformed. Panels show influence-rank for each metal in the mixture, and the posterior inclusion probability (PIP) for each metal in the mixture.
Significant association at p < 0.05. No significant effects were seen for the overall mixture.
4. Discussion
In this study of adolescents with environmental exposure to a metalloid/metal mixture, blood concentrations of As, Cd, Mn, and Pb were consistently associated with poorer cognitive function, while blood concentrations of Se were associated with better cognitive function. BKMR analysis reinforced these findings, confirming negative associations between cognitive function and blood concentrations of Pb and Cd, and positive associations between cognitive function and Se.
Several blood mental concentrations were correlated, e.g., positive correlations between Cd and Mn, Cd and Pb, Pb and Se; negative correlations for As and Cd. These correlations are consistent with previously observed environmental exposure to metalloid/metal mixtures in Bangladesh. (Islam et al., 2018).
CANTAB subtests are intended to measure related but subtly different aspects of cognitive function. As expected, we only observed weak positive correlations between these measures, due to differences in the specific aspects of cognition being assessed. Thus, correlations among CANTAB subtest scores do not indicate issues with construct validity.
We found negative associations for Spatial Working Memory (SWM) score and blood concentrations of both As and Mn. This subtest evaluates ability to remember and manipulate spatial information over a brief period of time. Mild deficits in spatial working memory might be reflected in difficulty recalling where a familiar object had recently been placed (e.g., lost keys). Our observed negative associations for As, Mn, and SWM are consistent with previous reports linking As and Mn, alone (Nahar et al., 2014; Torres-Agustín et al., 2013) and jointly, (Yorifuji et al., 2011) to poorer memory function in children. Our results are also consistent with studies in adults that link As to poorer executive function (O’Bryant et al., 2011) and Mn to deficits in working memory, (Bowler et al., 2015) visuospatial memory (Bowler et al., 2015), and visual short-term memory. (Ellingsen et al., 2008).
Additionally, we found Cd to be associated with lower Spatial Recognition Memory (SRM), a test of visual recognition and memory. SRM assesses ability to determine if an object has been previously seen in a specific location. Deficits in SRM might be reflected in difficulty noticing a change in the position of a familiar object, or in recalling where familiar objects were previously observed. (MRC Centre for Synaptic Plasticity, 2011) Our results are consistent with prior studies in children (Rodriguez-Barranco et al., 2014) and adults (Li et al., 2018) that show negative associations between Cd and cognition, though these studies did not specifically identify negative associations with spatial recognition and memory.
We also observed Pb to be negatively associated with Delayed Match to Sample (DMS), a subtest of visual recognition and memory. DMS tests ability to recall a complex visual pattern over a brief period of time, evaluating working memory and visual recognition/memory. Deficits in this type of task might include difficulty recognizing recently seen patterns. The observed negative association between Pb and DMS is consistent with previous studies reporting Pb to be negatively associated with child cognition (Lucchini et al., 2012) and memory; (Yorifuji et al., 2011) it is also consistent with studies in adults that link Pb to deficits in overall memory, (Payton et al., 1998) working memory, (Seo et al., 2014) and executive function. (Bandeen-Roche et al., 2009).
Finally, we observed positive associations for Se and both RTI and SSP. Reaction time (RTI) is a test of attention that assesses speed in responding to a visual cue, including the time taken to detect, process, and respond. Deficits in RTI might include difficulty responding rapidly to novel or unexpected visual stimuli. Spatial Span (SSP) is a test of working memory that assesses ability to briefly recall a spatial sequence. Deficits in tasks related to SSP might include difficulty remembering spatial patterns, e.g., navigational directions, or difficulty remembering ordered object locations, e.g., putting a series of objects back in the spaces they were moved from. Positive associations between Se and cognition have been previously observed in children (Skröder et al., 2017) and adults. (Akbaraly and Hininger-Favier, 2007) Our findings are also consistent with studies that report a protective effect of Se against As exposure, (Aschner, 1997) and with studies in neonates that report a protective effect of Se against Mn neurotoxicity. (Yang et al., 2014).
Our BKMR analysis generated an exposure-response function for each of the metals while controlling for the others and assessed the effect of the overall mixture. Additionally, while the non-parametric kernel did not constrain associations and linear relationships were not assumed, the BKMR exposure-response functions supported the assumptions of the linear regression models. BKMR revealed no overall effect of the mixture on any of the seven CANTAB subtests. These results may be due to the CANTAB’s design: it is intended to measure cognitive functions that closely map to specific brain regions, each of which may be differentially affected by the metals in the mixture. BKMR analyses of individual metals were consistent with regression results. BKMR identified Se as the most important independent component of the mixture, since it had the highest PIP in the model, and had positive associations with SOC, RTI, and SSP. Positive associations between Se and the RTI and SSP subtests were also seen in the linear regression analysis. BKMR showed negative associations for Pb and DMS, and for Cd and SRM, indicating Pb and Cd were important components of the mixture for these respective subtests, which is consistent with linear regression results. Arsenic and Mn were negatively associated with SWM in regression analyses, while similar findings in the BKMR did not achieve statistical significance. This stems from differences in these two approaches — BKMR has less power than linear regression due to its non-parametric nature. Finally, BKMR did not reveal any interactions between the metals; this is consistent with our linear regression analyses, which also did not show metal interactions.
The results we present in this paper echo findings reported in a previously published analysis of the association between this metal mixture and WISC-IV scores in the MANAs adolescents. In that analysis, As was found to be negatively associated with overall intelligence, and with subscale measures of processing speed, verbal comprehension, and working memory. (Wasserman et al., 2018) Similarly, Cd was found to be negatively associated with overall intelligence and verbal comprehension, while Pb was negatively associated with working memory and verbal comprehension. Finally, that study reported Se to be positively associated with overall intelligence function. (Wasserman et al., 2018).
The results we report in this paper suggest that our previously observed association between Pb and working memory may be specific to spatial working memory. (Wasserman et al., 2018) The identification of a specific aspect of working memory provides a more refined interpretation of the association reported in our prior study. Additionally, this paper identifies several novel associations between these metals and cognitive tasks that could not be parsed using the WISC-IV. These include the observed negative associations between Mn and spatial working memory, Cd and spatial recognition memory, and Pb and visual recognition memory (i.e., DMS), as well as the observed positive association for Se and spatial working memory (i.e., SSP). These findings imply that spatial working memory may be the cognitive construct most impacted by exposure to this mixture of metals. Since spatial awareness and working memory are both strongly associated with the frontoparietal brain regions, (Ackerman and Courtney, 2012; Chai et al., 2018) this also suggests that frontoparietal brain regions may be especially vulnerable to this mixed metal environmental exposure. Additionally, our observed association between Cd and SRM indicates that cognitive function related to spatial aspects of broader cognitive domains, i.e., spatial aspects of working memory or recognition memory, may be more vulnerable to the harmful effects of Cd exposure. These findings also suggest that interventions to mitigate the cognitive effects of these metal exposures may include cognitive coaching or training that focuses specifically on the spatial aspects of working memory and recognition memory.
This study has several strengths, including its focus on adolescence, an important neurodevelopmental period during which brain structures linked to executive function continue to mature. In addition, the study uses a culturally neutral non-linguistic cognitive test, and employs both linear regression and BKMR to focus on the effect of the overall metal mixture. Major limitations include the study’s cross-sectional nature, which precludes inferences regarding temporality and causality. Furthermore, while the study is not sufficiently powered for sex-stratified analyses, which is a limitation, exploratory analyses did not indicate that any of our findings differed by sex (data not shown). Also, given this study’s epidemiological approach and its focus exclusively on neurocognitive outcomes, this analysis may not entirely represent all physiological and biological interactions that may be associated with the body burden of exposure to a mixture of metals. Moreover, metal levels measured via venous blood draws, such as those analyzed in this paper, may not accurately represent metal levels in neurological tissue, since metals are known to accumulate in the brain and central nervous system (CNS) throughout the course of a prolonged exposure. Additional limitations include potential unmeasured confounding from pre or perinatal developmental exposures, potential unaccounted for selection bias, and potential unaccounted for measurement error in biomarkers quantification. This study’s generalizability to other populations is limited, as environmental exposure to this metal mixture is location specific. While the study maintains internal validity, we are unable to make inferences about the magnitude of these effect sizes as compared to other studies.
In conclusion, we found that exposure to As, Cd, Mn, and Pb was negatively associated with cognitive function related to working memory, spatial working memory, and visual recognition and memory. In contrast, we found Se to have a positive association with working memory, attention, and planning for the observed blood concentration range of approximately 85.88 μg/L–188.4 μg/L. These results are consistent with our previous findings, (Wasserman et al., 2018) and provide additional insight into the impact of metals exposure on cognitive tasks. This work suggests that previously characterized harmful effects of metals exposures on children’s cognition may persist in adolescence, and it contributes to the body of literature examining metal/metalloid exposures and cognitive effects across the life course. Reducing exposure to metals should continue to be a public health priority, as these environmental exposures can have widespread adverse impacts on executive function across the exposed population, compromising the health, well-being, and possibly even the earning power of individuals living in highly afflicted regions. Interventions limiting metal exposure and/or mitigating the harmful cognitive effects of these exposures have the potential to make a serious and meaningful impact on the lives of exposed individuals.
Supplementary Material
Acknowledgements
We thank our staff, the fieldworkers at our study site, and the study participants in Bangladesh, whose contributions made this work possible. This work was supported by the National Institutes of Health and the National Institute of Environmental Health Sciences grants: SRP P42 ES010349, P30ES009089, T32ES007322, F31ES029370-01A1:RS.
Footnotes
CRediT authorship contribution statement
Roheeni Saxena: Writing – original draft, Writing – review & editing, Visualization, Methodology, Software, Formal analysis. Mary V. Gamble: Conceptualization, Supervision, Resources, Validation, Writing – review & editing. Gail A. Wasserman: Conceptualization, Supervision, Resources, Validation, Writing – review & editing. Xinhua Liu: Supervision, Methodology, Validation, Writing – review & editing. Faruque Parvez: Project administration, Writing – review & editing. Ana Navas-Acien: Supervision, Validation, Writing – review & editing. Tariqul Islam: Resources, Writing – review & editing. Pam Factor-Litvak: Conceptualization, Validation, Writing – review & editing. Mohammed Nasir Uddin: Resources, Writing – review & editing. Marianthi-Anna Kioumourtzoglou: Supervision, Methodology, Validation, Writing – review & editing. Elizabeth A. Gibson: Methodology, Validation, Writing – review & editing. Hasan Shahriar: Resources, Writing – review & editing. Vesna Slavkovic: Investigation, Writing – review & editing. Vesna Illiveski: Investigation, Writing – review & editing. Nancy J. LoIacono: Resources, Data curation, Writing – review & editing. Olgica Balac: Investigation, Writing – review & editing. Joseph H. Graziano: Conceptualization, Funding acquisition, Methodology, Resources, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ecoenv.2022.113229.
References
- Ackerman CM, Courtney SM, 2012. Spatial relations and spatial locations are dissociated within prefrontal and parietal cortex. J. Neurophysiol 108 (9), 2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Toxic Substances Disease Registry (ATSDR), In: US Department of Health & Human Services PHS, (Ed.), Toxicological Profile for Arsenic, ⟨https://www.atsdr.cdc.gov/toxguides/toxguide-2.pdf⟩. 2007. [Google Scholar]
- , 2012Agency for Toxic Substances and Disease Registry (ATSDR), 2012a. In: U.S. Department of Health and Human Services PHS, (Ed.), Toxicological Profile for Manganese, Atlanta, GA. [Google Scholar]
- Agency for Toxic Substances Disease Registry (ATSDR), In: US Department of Health & Human Services PHS, (Ed.), Toxicological Profile for Cadmium. ⟨https://www.atsdr.cdc.gov/toxguides/toxguide-5.pdf⟩. 2012b. [Google Scholar]
- Akbaraly TN, Hininger-Favier I, Carri, et al. , 2007. Plasma selenium over time andcognitive decline in the elderly. Epidemiology 18 (1), 52–58. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C, 2001. Development of executive functions through late childhood and adolescence in an Australian sample. Dev. Neuropsychol 20 (1), 385–406. [DOI] [PubMed] [Google Scholar]
- Aschner M, 1997. Astrocyte metallothioneins (MTs) and their neuroprotective role. Ann. N. Y Acad. Sci 825, 334–347. [DOI] [PubMed] [Google Scholar]
- Bandeen-Roche K, Glass TA, Bolla KI, Todd AC, Schwartz BS, 2009. Cumulative lead dose and cognitive function in older adults. Epidemiology 20 (6), 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, et al. , 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16 (3), 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RM, Kornblith ES, Gocheva VV, 2015. Environmental exposure to manganese in air: associations with cognitive functions. Neurotoxicology 49, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- , 2006Cambridge Cognition Ltd., 2006. CANTAB Eclipse Administration Manual, Cambridge, England. [Google Scholar]
- , 2015Centers for Disease Control, 2015. Reference Blood Lead Levels (BLL) for Adults in the U.S. In: National Institute for Occupational Safetyand Health, (Ed.), Department of Health and Human Services CfDCaP. [Google Scholar]
- Chai WJ, Abd Hamid AI, Abdullah JM, 2018. Working memory from the psychological and neurosciences perspectives: a review. Front Psychol. 9, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Coull BA, Wright RO, 2014. Chemical mixtures and children’s health. Curr. Opin. Pedia 26 (2), 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ehrenstein OS, Poddar S, Yuan Y, et al. , 2007. Children’s intellectual function in relation to arsenic exposure. Epidemiology 18 (1), 44–51. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Konstantinov R, Bast-Pettersen R, et al. , 2008. A neurobehavioral study of current and former welders exposed to manganese. Neurotoxicology 29 (1), 48–59. [DOI] [PubMed] [Google Scholar]
- Government of the People’s Republic of Bangladesh, Department for International Development, British Geological Survey, (Eds.), 2001. Arsenic Contamination of Groundwater in Bangladesh. Vol 1: Summary. In: Kinniburgh D, Smedley P, (Eds.), UK: British Geological Survey, BGS Technical Report Keyworth. [Google Scholar]
- Green R, Till C, Al-Hakeem H, et al. , 2019. Assessment of neuropsychological performance in Mexico City youth using the Cambridge Neuropsychological Test Automated Battery (CANTAB). J. Clin. Exp. Neuropsychol 41 (3), 246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MM, Karim MR, Zheng X, Li X, 2018. Heavy metal and metalloid pollution of soil, water and foods in Bangladesh: a critical review. Int. J. Environ. Res. Public Health 15 (12), 2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang Z, Fu Z, et al. , 2018. Associations between blood cadmium levels and cognitive function in a cross-sectional study of US adults aged 60 years or older. BMJ Open 8 (4), e020533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Zoni S, Guazzetti S, et al. , 2012. Inverse association of intellectual function with very low blood lead but not with manganese exposure in Italian adolescents. Environ. Res 118, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Nelson CA, 2002. Assessment of neuropsychological function through use of the Cambridge neuropsychological testing automated battery: performance in 4- to 12-year-old children. Dev. Neuropsychol 22 (3), 595–624. [DOI] [PubMed] [Google Scholar]
- Mayo Clinic. Selenium: Clinical and Interpretive Mayo Clinic Laboratories. Published 2020. Accessed May 2020, 2020. [Google Scholar]
- MRC Centre for Synaptic Plasticity, 2011. Brain Basics: the fundamentals of neuroscience. University of Bristol, School of Medical Sciences. ⟨http://www.bris.ac.uk/synaptic/⟩. (Accessed 2020). [Google Scholar]
- Nahar MN, Inaoka T, Fujimura M, 2014. A consecutive study on arsenic exposure and intelligence quotient (IQ) of children in Bangladesh. Environ. Health Prev. Med 19 (3), 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Edwards M, Menon CV, Gong G, Barber R, 2011. Long-term low-level arsenic exposure Is associated with poorer neuropsychological functioning: a project FRONTIER study. Int. J. Environ. Res. Public Health 8 (3), 861–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton M, Riggs KM, Spiro A 3rd, Weiss ST, Hu H, 1998. Relations of bone and blood lead to cognitive function: the VA Normative Aging Study. Neurotoxicol. Teratol 20 (1), 19–27. [DOI] [PubMed] [Google Scholar]
- Pohl KM, Thompson WK, Adeli E, Linguraru MG, 2019. Adolescent brain cognitive development neurocognitive prediction. In: Proceedings of the First Challenge, ABCD-NP 2019, Held in Conjunction with MICCAI 2019, first ed., Shenzhen, China October 13, 2019. [Google Scholar]
- Pruszkowski E, Neubauer K, Thomas R, 1998. An overview of clinical applications by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). At. Spectrosc 19 (4), 111–115. [Google Scholar]
- Rodriguez-Barranco M, Lacasana M, Gil F, 2014. Cadmium exposure and neuropsychological development in school children in southwestern Spain. Environ. Res 134, 66–73. [DOI] [PubMed] [Google Scholar]
- Rosado JL, Ronquillo D, Kordas K, 2007. Arsenic exposure and cognitive performance in Mexican schoolchildren. Environ. Health Perspect 115 (9), 1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safiur Rahman M, Khan MDH, Jolly YN, Kabir J, Akter S, Salam A, 2019. Assessing risk to human health for heavy metal contamination through street dust in the Southeast Asian Megacity: Dhaka, Bangladesh. Sci. Total Environ 660, 1610–1622. [DOI] [PubMed] [Google Scholar]
- Sanchez TR, Slavkovich V, LoIacono N, 2018. Urinary metals and metal mixtures in Bangladesh: exploring environmental sources in the Health Effects of Arsenic Longitudinal Study (HEALS). Environ. Int 121 (Pt 1), 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Liu X, Navas-Acien A, 2021. Nutrition, one-carbon metabolism and arsenic methylation in Bangladeshi adolescents. Environ. Res 195, 110750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Lee B-K, Jin S-U, et al. , 2014. Lead-induced impairments in the neural processes related to working memory function. PLos One 9 (8), e105308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skröder H, Kippler M, Tofail F, Vahter M, 2017. Early-Life selenium status and cognitive function at 5 and 10 years of age in Bangladeshi children. Environ. Health Perspect 125 (11), 117003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroh A, 1988. Determination of Pb and Cd in whole blood using isotope dilution ICP-MS. At. Spectrosc 14 (5), 141–143. [Google Scholar]
- The Psychological Corporation, 1999. Wechsler Abbreviated Scale of Intelligence Manual. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Torres-Agustín R, Rodríguez-Agudelo Y, Schilmann A, et al. , 2013. Effect of environmental manganese exposure on verbal learning and memory in Mexican children. Environ. Res 121, 39–44. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Allan AM, 2014. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr. Environ. Health Rep 1 (2), 132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, 2018. A cross-sectional study of water arsenic exposure and intellectual function in adolescence in Araihazar, Bangladesh. Environ. Int 118, 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank. Bangladesh Multiple Indicator Cluster Survey 2012–2013. 2015. [Google Scholar]
- Yang X, Bao Y, Fu H, Li L, Ren T, Yu X, 2014. Selenium protects neonates against neurotoxicity from prenatal exposure to manganese. PLoS One 9 (1), e86611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Debes F, Weihe P, Grandjean P, 2011. Prenatal exposure to lead and cognitive deficit in 7- and 14-year-old children in the presence of concomitant exposure to similar molar concentration of methylmercury. Neurotoxicol. Teratol 33 (2), 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.