Abstract
Synovial tissues from inflamed and noninflamed knee joints of 13 patients with untreated rheumatoid arthritis were examined for vascular proliferation and morphological alteration of endothelial cells. Perivascular mononuclear cell infiltration and increased thickness of the synovial lining layer were noted in tissues from inflamed and noninflamed joints of patients with rheumatoid arthritis; vascular proliferation and morphological alteration of endothelial cells to resemble high endothelial venules were seen only in tissues from inflamed joints of patients with rheumatoid arthritis. These observations suggest that the migration of mononuclear cells from the peripheral blood to the perivascular areas and lining layer occurs before vascular proliferation and morphological alteration of endothelial cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. O., Anderson N. D. Lymphocyte emigration from high endothelial venules in rat lymph nodes. Immunology. 1976 Nov;31(5):731–748. [PMC free article] [PubMed] [Google Scholar]
- Ashida E. R., Johnson A. R., Lipsky P. E. Human endothelial cell-lymphocyte interaction. Endothelial cells function as accessory cells necessary for mitogen-induced human T lymphocyte activation in vitro. J Clin Invest. 1981 May;67(5):1490–1499. doi: 10.1172/JCI110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausprunk D. H., Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977 Jul;14(1):53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Brown R. A., Weiss J. B., Tomlinson I. W., Phillips P., Kumar S. Angiogenic factor from synovial fluid resembling that from tumours. Lancet. 1980 Mar 29;1(8170):682–685. [PubMed] [Google Scholar]
- Cush J. J., Lipsky P. E. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Oct;31(10):1230–1238. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- Dumonde D. C., Pulley M. S., Paradinas F. J., Southcott B. M., O'Connell D., Robinson M. R., den Hollander F., Schuurs A. H. Histological features of skin reactions to human lymphoid cell line lymphokine in patients with advanced cancer. J Pathol. 1982 Dec;138(4):289–308. doi: 10.1002/path.1711380402. [DOI] [PubMed] [Google Scholar]
- Edwards J. C. The origin of type A synovial lining cells. Immunobiology. 1982 Apr;161(3-4):227–231. doi: 10.1016/S0171-2985(82)80078-8. [DOI] [PubMed] [Google Scholar]
- FitzGerald O. M., Hess E. V., Chance A., Highsmith R. F. Quantitative studies of human monokine-induced endothelial cell elongation. J Leukoc Biol. 1987 May;41(5):421–428. doi: 10.1002/jlb.41.5.421. [DOI] [PubMed] [Google Scholar]
- Freemont A. J., Jones C. J., Bromley M., Andrews P. Changes in vascular endothelium related to lymphocyte collections in diseased synovia. Arthritis Rheum. 1983 Dec;26(12):1427–1433. doi: 10.1002/art.1780261203. [DOI] [PubMed] [Google Scholar]
- Førre O., Thoen J., Dobloug J. H., Egeland T., Kvien T. K., Mellbye O. J., Natvig J. B. Detection of T-lymphocyte subpopulation in the peripheral blood and the synovium of patients with rheumatoid arthritis and juvenile rheumatoid arthritis using monoclonal antibodies. Scand J Immunol. 1982 Feb;15(2):221–226. doi: 10.1111/j.1365-3083.1982.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Hale L. P., Martin M. E., McCollum D. E., Nunley J. A., Springer T. A., Singer K. H., Haynes B. F. Immunohistologic analysis of the distribution of cell adhesion molecules within the inflammatory synovial microenvironment. Arthritis Rheum. 1989 Jan;32(1):22–30. doi: 10.1002/anr.1780320105. [DOI] [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Haskard D., Cavender D., Ziff M. Phorbol ester-stimulated T lymphocytes show enhanced adhesion to human endothelial cell monolayers. J Immunol. 1986 Sep 1;137(5):1429–1434. [PubMed] [Google Scholar]
- Henderson B., Pettipher E. R. The synovial lining cell: biology and pathobiology. Semin Arthritis Rheum. 1985 Aug;15(1):1–32. doi: 10.1016/0049-0172(85)90007-1. [DOI] [PubMed] [Google Scholar]
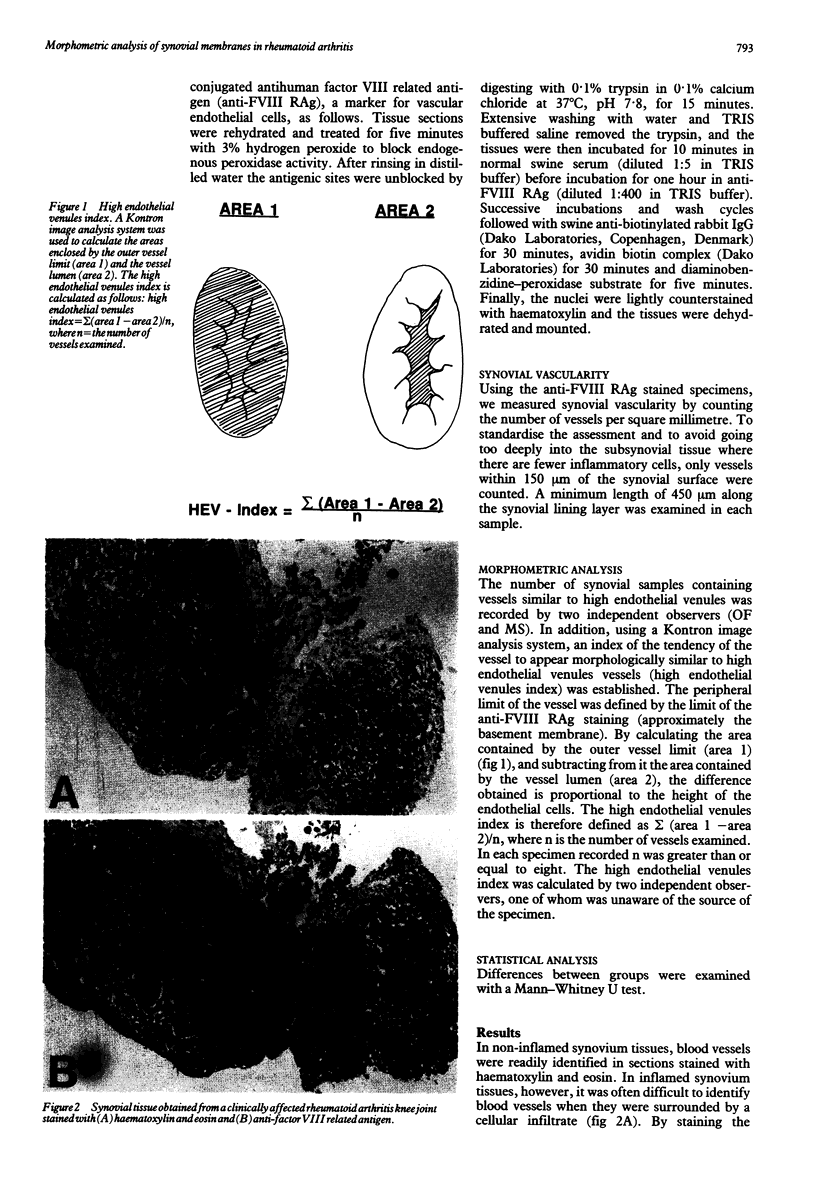
- Iguchi T., Ziff M. Electron microscopic study of rheumatoid synovial vasculature. Intimate relationship between tall endothelium and lymphoid aggregation. J Clin Invest. 1986 Feb;77(2):355–361. doi: 10.1172/JCI112312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Ziff M. Electron microscopic observations of immunoreactive cells in the rheumatoid synovial membrane. Arthritis Rheum. 1976 Jan-Feb;19(1):1–14. doi: 10.1002/art.1780190101. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Steere A. C., Fox R. I., Butcher E. C. A distinct endothelial cell recognition system that controls lymphocyte traffic into inflamed synovium. Science. 1986 Aug 1;233(4763):556–558. doi: 10.1126/science.3726548. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Wigren A., Wigzell H. Relationship between HLA-DR-expressing cells and T lymphocytes of different subsets in rheumatoid synovial tissue. Scand J Immunol. 1981 May;15(5):501–507. doi: 10.1111/j.1365-3083.1982.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Konttinen Y. T., Reitamo S., Ranki A., Häyry P., Kankaanapä U., Wegelius O. Characterization of the immunocompetent cells of rheumatoid synovium from tissue sections and eluates. Arthritis Rheum. 1981 Jan;24(1):71–79. doi: 10.1002/art.1780240112. [DOI] [PubMed] [Google Scholar]
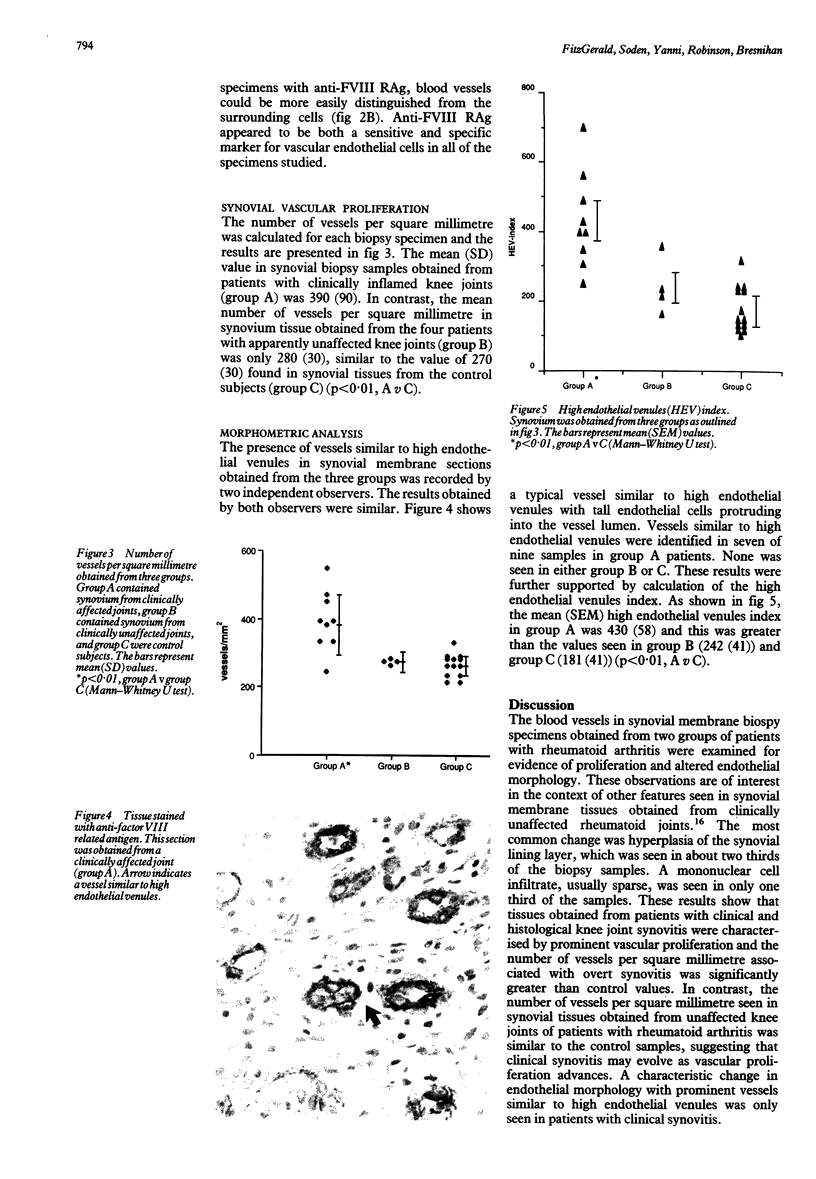
- Kurosaka M., Ziff M. Immunoelectron microscopic study of the distribution of T cell subsets in rheumatoid synovium. J Exp Med. 1983 Oct 1;158(4):1191–1210. doi: 10.1084/jem.158.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalor P. A., Mapp P. I., Hall P. A., Revell P. A. Proliferative activity of cells in the synovium as demonstrated by a monoclonal antibody, Ki67. Rheumatol Int. 1987;7(5):183–186. doi: 10.1007/BF00541375. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Basement membrane thickening of postcapillary venules and capillaries in rheumatoid synovium. Immunoelectron microscopic and electron microscopic morphometric analysis. Arthritis Rheum. 1987 Jan;30(1):18–30. doi: 10.1002/art.1780300103. [DOI] [PubMed] [Google Scholar]
- Meijer C. J., de Graaff-Reitsma C. B., Lafeber G. J., Cats A. In situ localization of lymphocyte subsets in synovial membranes of patients with rheumatoid arthritis with monoclonal antibodies. J Rheumatol. 1982 May-Jun;9(3):359–365. [PubMed] [Google Scholar]
- Nightingale G., Hurley J. V. Relationship between lymphocyte emigration and vascular endothelium in chronic inflammation. Pathology. 1978 Jan;10(1):27–44. doi: 10.3109/00313027809063477. [DOI] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Ziff M. Binding of normal human mononuclear cells to blood vessels in rheumatoid arthritis synovial membrane. Arthritis Rheum. 1986 Jun;29(6):789–792. doi: 10.1002/art.1780290613. [DOI] [PubMed] [Google Scholar]
- Parrott D. V., De Sousa M. A., East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J Exp Med. 1966 Jan 1;123(1):191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Schoefl G. I. The migration of lymphocytes across the vascular endothelium in lymphoid tissue. A reexamination. J Exp Med. 1972 Sep 1;136(3):568–588. doi: 10.1084/jem.136.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden M., Rooney M., Cullen A., Whelan A., Feighery C., Bresnihan B. Immunohistological features in the synovium obtained from clinically uninvolved knee joints of patients with rheumatoid arthritis. Br J Rheumatol. 1989 Aug;28(4):287–292. doi: 10.1093/rheumatology/28.4.287. [DOI] [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolpen A. H., Guinan E. C., Fiers W., Pober J. S. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986 Apr;123(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Vracko R. Basal lamina layering in diabetes mellitus. Evidence for accelerated rate of cell death and cell regeneration. Diabetes. 1974 Feb;23(2):94–104. [PubMed] [Google Scholar]
- Wagner C. R., Vetto R. M., Burger D. R. Expression of I-region-associated antigen (Ia) and interleukin 1 by subcultured human endothelial cells. Cell Immunol. 1985 Jun;93(1):91–104. doi: 10.1016/0008-8749(85)90391-0. [DOI] [PubMed] [Google Scholar]
- Young C. L., Adamson T. C., 3rd, Vaughan J. H., Fox R. I. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1984 Jan;27(1):32–39. doi: 10.1002/art.1780270106. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Haskard D. O., Cavender D., Johnson A. R., Ziff M. Human gamma interferon increases the binding of T lymphocytes to endothelial cells. Clin Exp Immunol. 1985 Dec;62(3):554–560. [PMC free article] [PubMed] [Google Scholar]
- Yu C. L., Haskard D., Cavender D., Ziff M. Effects of bacterial lipopolysaccharide on the binding of lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(2):569–573. [PubMed] [Google Scholar]