Abstract
BACKGROUND
Lenalidomide plus dexamethasone is a standard treatment for patients with newly diagnosed multiple myeloma who are ineligible for autologous stem-cell transplantation. We sought to determine whether the addition of daratumumab would significantly reduce the risk of disease progression or death in this population.
METHODS
We randomly assigned 737 patients with newly diagnosed multiple myeloma who were ineligible for autologous stem-cell transplantation to receive daratumumab plus lenalidomide and dexamethasone (daratumumab group) or lenalidomide and dexamethasone alone (control group). Treatment was to continue until the occurrence of disease progression or unacceptable side effects. The primary end point was progression-free survival.
RESULTS
At a median follow-up of 28.0 months, disease progression or death had occurred in 240 patients (97 of 368 patients [26.4%] in the daratumumab group and 143 of 369 patients [38.8%] in the control group). The estimated percentage of patients who were alive without disease progression at 30 months was 70.6% (95% confidence interval [CI], 65.0 to 75.4) in the daratumumab group and 55.6% (95% CI, 49.5 to 61.3) in the control group (hazard ratio for disease progression or death, 0.56; 95% CI, 0.43 to 0.73; P<0.001). The percentage of patients with a complete response or better was 47.6% in the daratumumab group and 24.9% in the control group (P<0.001). A total of 24.2% of the patients in the daratumumab group, as compared with 7.3% of the patients in the control group, had results below the threshold for minimal residual disease (1 tumor cell per 105 white cells) (P<0.001). The most common adverse events of grade 3 or 4 were neutropenia (50.0% in the daratumumab group vs. 35.3% in the control group), anemia (11.8% vs. 19.7%), lymphopenia (15.1% vs. 10.7%), and pneumonia (13.7% vs. 7.9%).
CONCLUSIONS
Among patients with newly diagnosed multiple myeloma who were ineligible for autologous stem-cell transplantation, the risk of disease progression or death was significantly lower among those who received daratumumab plus lenalidomide and dexamethasone than among those who received lenalidomide and dexamethasone alone. A higher incidence of neutropenia and pneumonia was observed in the daratumumab group. (Funded by Janssen Research and Development; MAIA ClinicalTrials.gov number, NCT02252172.)
Multiple myeloma is a hematologic cancer in which clonal plasma-cell proliferation leads to complications and death.1 Initial treatment for newly diagnosed multiple myeloma depends on whether a patient may have unacceptable toxic effects from high-dose chemotherapy and may be unable to undergo autologous stem-cell transplantation.1 Younger patients without substantial coexisting conditions usually receive an induction regimen followed by high-dose chemotherapy and autologous stem-cell transplantation.2 For patients who are ineligible for stem-cell transplantation, multiagent regimens, including alkylating agents, glucocorticoids, immunomodulatory drugs, proteasome inhibitors, and new agents, are the standard of care.2–5
Daratumumab, a human IgGκ monoclonal antibody that targets CD38, has direct antitumor and immunomodulatory activity.6–10 Initial approval of daratumumab as monotherapy for patients with heavily pretreated myeloma was based on the phase 1/2 GEN501 and SIRIUS trials.11,12 Subsequently, daratumumab in combination with standard-of-care therapy showed clinical benefit across phase 3 trials involving patients with newly diagnosed myeloma (the ALCYONE trial) and patients with relapsed or refractory myeloma (the CASTOR and POLLUX trials).13–15 In the POLLUX trial, treatment with daratumumab plus lenalidomide and dexamethasone resulted in a risk of disease progression or death that was 63% lower than the risk with lenalidomide and dexamethasone alone.15 After a median follow-up of 44.3 months, the median progression-free survival was 44.5 months in the daratumumab group, as compared with 17.5 months in the control group, with no new safety concerns observed.16
In the phase 3 Frontline Investigation of Revlimid and Dexamethasone versus Standard Thalidomide (FIRST) trial5,17 involving patients with newly diagnosed multiple myeloma who were ineligible for autologous stem-cell transplantation, treatment with lenalidomide and dexamethasone administered until disease progression resulted in significantly longer overall survival than treatment with melphalan, prednisone, and thalidomide; these findings established the regimen of lenalidomide and dexamethasone as standard of care. Here, we report the results of a prespecified interim analysis of a phase 3 trial (MAIA) in which we assessed the efficacy and safety of daratumumab plus lenalidomide and dexamethasone as compared with lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma who were ineligible for autologous stem-cell transplantation.
Methods
Trial Design and Oversight
In this randomized, open-label, phase 3 trial, patients were enrolled from March 2015 through January 2017 at 176 sites in 14 countries across North America, Europe, the Middle East, and the Asia–Pacific region. The independent ethics committee or institutional review board at each site approved the protocol, available with the full text of this article at NEJM.org. The trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All the patients provided written informed consent. The trial was designed by the authors in collaboration with the sponsor, Janssen Research and Development. The sponsor compiled and maintained the data and funded professional medical writers to prepare the manuscript for submission. The authors reviewed and approved the manuscript. The sponsor and the authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
PATIENTS
Eligible patients had documented newly diagnosed multiple myeloma,18 had an Eastern Cooperative Oncology Group performance status score of 0 to 2 (on a 5-point scale, with higher numbers indicating greater disability), and were ineligible for high-dose chemotherapy with stem-cell transplantation owing to age (≥65 years) or to the presence of coexisting conditions that were likely to result in the development of unacceptable side effects associated with high-dose chemotherapy with stem-cell transplantation. Other inclusion criteria were a hemoglobin level of 7.5 g or more per deciliter, an absolute neutrophil count of 1000 or more per cubic millimeter, a platelet count of 70,000 or more per cubic millimeter (>50,000 per cubic millimeter if ≥50% of nucleated bone marrow cells were plasma cells), aspartate aminotransferase and alanine aminotransferase levels no more than 2.5 times the upper limit of the normal range, a total bilirubin level no more than 2.0 times the upper limit of the normal range, creatinine clearance of 30 ml or more per minute, and a corrected serum calcium level of 14 mg or less per deciliter (3.5 mmol or less per liter). Additional eligibility criteria are listed in the Supplementary Appendix, available at NEJM.org.
RANDOMIZATION AND TREATMENT
Using an interactive Web-response system, we randomly assigned patients, in a 1:1 ratio, to receive daratumumab plus lenalidomide and dexamethasone (daratumumab group) or lenalidomide and dexamethasone alone (control group) (Fig. S1 in the Supplementary Appendix). Randomization was stratified according to International Staging System disease stage (I vs. II vs. III, with higher stages indicating more severe disease) (see Table S1 in the Supplementary Appendix for details on the staging criteria), geographic region (North America vs. other), and age (<75 vs. ≥75 years).
During each 28-day cycle, all the patients received oral lenalidomide (25 mg on days 1 through 21) and oral dexamethasone (40 mg on days 1, 8, 15, and 22) until disease progression or unacceptable toxic effects. For patients who had a creatinine clearance between 30 and 50 ml per minute, a reduced dose of lenalidomide (10 mg) was recommended. Adjustment of the dose of lenalidomide was recommended in the case of neutropenia and thrombocytopenia (Table S2 in the Supplementary Appendix). Patients who were older than 75 years of age or who had a body-mass index (the weight in kilograms divided by the square of the height in meters) of less than 18.5 received dexamethasone at a dose of 20 mg once weekly. Patients in the daratumumab group received intravenous daratumumab at a dose of 16 mg per kilogram of body weight once weekly during cycles 1 and 2, every 2 weeks during cycles 3 through 6, and every 4 weeks thereafter; preinfusion medications were administered approximately 1 hour before each daratumumab dose (details are provided in the Supplementary Appendix).
END POINTS AND ASSESSMENTS
The primary end point was progression-free survival, which was defined as the time from randomization to either disease progression or death. Secondary efficacy end points included the time to progression; the percentage of patients with a complete response (undetectable M-protein level on two consecutive serum and urine immunofixation tests and <5% plasma cells in bone marrow), a stringent complete response (complete response plus a normal free light-chain ratio and absence of clonal plasma cells, as assessed by immunofluorescence or immunohistochemical analysis or by two-color to four-color flow cytometry), negative status for minimal residual disease (at a threshold of 1 tumor cell per 105 white cells), overall response (including partial response, very good partial response, complete response, and stringent complete response), and a very good partial response (defined by a ≥90% reduction in serum M protein plus a urinary M-protein level of <100 mg per 24 hours) or better; overall survival; the time to response; the duration of response; efficacy in the subgroup of patients with a high-risk cytogenic profile (defined by a del17p, t[14;16], or t[4;14] abnormality [or a combination of these] on fluorescence in situ hybridization or karyotype analysis); and safety. Progressive disease was defined according to International Myeloma Working Group criteria (Table S3 in the Supplementary Appendix).19,20 Complete definitions of these end points are provided in the Supplementary Appendix.
Serum samples and 24-hour urine samples were obtained for efficacy assessment every 28 days for 2 years and then every 8 weeks thereafter until disease progression; all samples were evaluated at a central laboratory. In the case of patients who had positive serum immunofixation and daratumumab interference, complete responses were confirmed with the use of reflex assays.21 Minimal residual disease was evaluated by means of the Adaptive Biotechnologies clonoSEQ next-generation sequencing assay (version 2.0) with the use of bone marrow aspirate obtained at baseline, at the time of suspected complete or stringent complete response, and at 12, 18, 24, and 30 months after the first dose in patients who had a complete response or better. Safety assessments included the evaluation of adverse events, which were graded according to version 4 of the National Cancer Institute Common Terminology Criteria for Adverse Events22; electrocardiography; clinical laboratory testing; physical examinations; and vital signs.
STATISTICAL ANALYSIS
The primary analysis was performed in the intention-to-treat population, which included all patients who underwent randomization. The safety population included all patients who received at least one dose of the trial treatment. The primary end point of progression-free survival was compared between the treatment groups with the use of a stratified log-rank test, and the treatment effect (hazard ratio) and corresponding 95% confidence interval were estimated with the use of a stratified Cox regression model, with treatment as the sole explanatory variable. Other time-to-event efficacy end points were analyzed similarly. Response to trial treatment and progressive disease were evaluated with the use of a validated computer algorithm.14,15 Continuous variables were summarized with the use of descriptive statistics, and categorical variables were summarized as numbers and percentages. Time-to-event variables were summarized with the use of the Kaplan–Meier method. We analyzed binary end points using a stratified Cochran–Mantel–Haenszel test. If the risk of the primary end point was found to be significantly lower in the daratumumab group than in the control group, the following secondary end points, as ordered here, were to be tested sequentially: complete response or better, very good partial response or better, negative status for minimal residual disease, overall response, and overall survival. The significance level was determined according to the alpha-spending function specific to each end point (see the Supplementary Appendix). For the evaluation of overall survival, a modified linear alpha-spending function was used to determine the alpha level at the time of each of three analyses (the second interim analysis, the primary progression-free survival analysis, and the final overall survival analysis). The alpha level was 0.0001 at the time of this first analysis.
Of two planned interim analyses, the first evaluated only safety after 100 patients had received at least 8 weeks of treatment or had discontinued treatment. The second, reported here, assessed safety and efficacy after 240 events of disease progression or death had occurred (i.e., 62% of the 390 planned events for the primary analysis). The final overall survival analysis is planned to be performed after 330 deaths have been reported. We estimated that a sample of 730 patients would provide the trial with 80% power to detect a risk of disease progression or death that was 25% lower with daratumumab plus lenalidomide and dexamethasone than with lenalidomide and dexamethasone alone, using a log-rank test at a two-sided alpha level of 0.05.
Results
PATIENTS AND TREATMENT
A total of 737 patients were randomly assigned — 368 to the daratumumab group and 369 to the control group. Demographic and clinical characteristics of the two groups were well balanced at baseline (Table 1). The median age was 73 years (range, 45 to 90), and 14.3% of patients had a high-risk cytogenetic profile. The median time since the diagnosis of multiple myeloma was 0.9 months (range, 0 to 14.5).
Table 1.
Demographic and Baseline Disease Characteristics in the Intention-to-Treat Population.*
| Characteristic | Daratumumab Group (N = 368) | Control Group (N = 369) |
|---|---|---|
| Median age (range) — yr | 73.0 (50–90) | 74.0 (45–89) |
| Age category — no. (%) | ||
| <65 yr | 4 (1.1) | 4 (11) |
| 65 to <70 yr | 74 (20.1) | 73 (19.8) |
| 70 to <75 yr | 130 (35.3) | 131 (35.5) |
| ≥75 yr | 160 (43.5) | 161 (43.6) |
| ECOG performance status — no. (%)† | ||
| 0 | 127 (34.5) | 123 (33.3) |
| 1 | 178 (48.4) | 187 (50.7) |
| 2‡ | 63 (17.1) | 59 (16.0) |
| ISS disease stage — no. (%)§ | ||
| I | 98 (26.6) | 103 (27.9) |
| II | 163 (44.3) | 156 (42.3) |
| III | 107 (29.1) | 110 (29.8) |
| Type of measurable disease — no. (%) | ||
| IgG | 225 (61.1) | 231 (62.6) |
| IgA | 65 (17.7) | 66 (17.9) |
| Other¶ | 9 (2.4) | 10 (2.7) |
| Detected in urine only | 40 (10.9) | 34 (9.2) |
| Detected as serum free light-chain only | 29 (7.9) | 28 (7.6) |
| Cytogenetic profile — no./total no. (%)‖ | ||
| Standard risk | 271/319 (85.0) | 279/323 (86.4) |
| High risk | 48/319 (15.0) | 44/323 (13.6) |
| Median time since initial diagnosis of multiple myeloma (range) — mo | 0.95 (0.1–13.3) | 0.89 (0–14.5) |
The intention-to-treat population included all patients who underwent randomization. Post hoc analyses showed no significant differences between the two groups in the characteristics evaluated at baseline.
Eastern Cooperative Oncology Group (ECOG) performance status is scored on a scale from 0 to 5, with 0 indicating no symptoms and higher scores indicating increasing disability.
Two patients had a score of greater than 2 (one patient had a score of 3, and another patient had a score of 4).
The International Staging System (ISS) disease stage, which is derived on the basis of the combination of serum β2-microglobulin and albumin levels, consists of three stages. Higher stages indicate more severe disease.
This category includes IgD, IgE, IgM, and biclonal.
Cytogenetic risk was based on fluorescence in situ hybridization or karyotype analysis; patients who had a high-risk cytogenetic profile had at least one high-risk abnormality (del17p, t[14;16], or t[4;14]).
Among the patients who underwent randomization, 729 patients (364 in the daratumumab group and 365 in the control group) received at least one dose of the trial treatment (Fig. S2 in the Supplementary Appendix). At the time of the clinical data cutoff for the primary analysis (September 24, 2018), a total of 118 patients (32.4%) in the daratumumab group and 207 patients (56.7%) in the control group had discontinued treatment, most commonly because of progressive disease (14.6% in the daratumumab group and 23.8% in the control group) and adverse events (7.4% and 16.2%, respectively). Patients who discontinued treatment for reasons other than disease progression and remained in the trial were followed for the primary end point.
The median duration of treatment was 25.3 months (range, 0.1 to 40.4) in the daratumumab group and 21.3 months (range, 0.03 to 40.6) in the control group, and the median number of treatment cycles was 27 (range, 1 to 44) in the daratumumab group and 22 (range, 1 to 43) in the control group (Table S4 in the Supplementary Appendix). The median relative dose intensity (see the Supplementary Appendix for definitions) for daratumumab was 98.4%. The median relative dose intensity for lenalidomide was 76.2% in the daratumumab group and 91.4% in the control group; 112 patients (30.8%) in the daratumumab group and 83 patients (22.7%) in the control group received 10 mg or less of the starting dose of lenalidomide. In addition, a higher percentage of patients in the daratumumab group than in the control group had dose modifications of lenalidomide owing to adverse events that occurred after the start of treatment, including lenalidomide discontinuations (20.9% and 17.0%, respectively) or dose delays, reductions, reescalations, or skipping (combined, 77.5% and 64.7%, respectively). The median relative dose intensity for dexamethasone was 84.2% in the daratumumab group and 90.7% in the control group.
A total of 35 patients in the daratumumab group discontinued treatment with lenalidomide and dexamethasone completely but continued to receive daratumumab monotherapy. The median duration of single-agent daratumumab treatment was 7.3 months (range, 0.03 to 31.2) among these patients.
EFFICACY
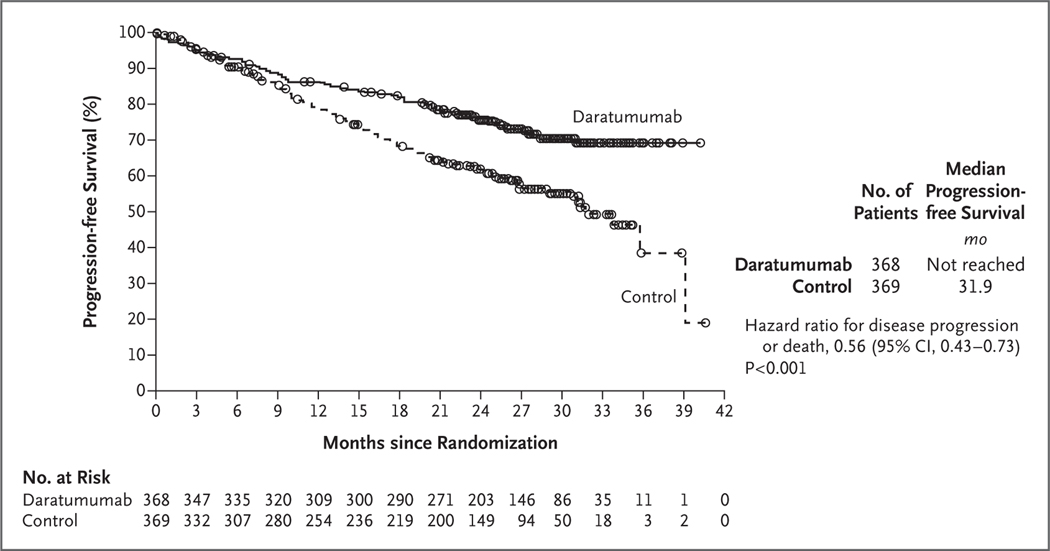
At a median follow-up of 28.0 months (range, 0 to 41.4), disease progression or death had occurred in 240 patients (97 of 368 patients [26.4%] in the daratumumab group and 143 of 369 patients [38.8%] in the control group). The Kaplan–Meier estimate of the percentage of patients who were alive without disease progression at 30 months was 70.6% (95% confidence interval [CI], 65.0 to 75.4) in the daratumumab group and 55.6% (95% CI, 49.5 to 61.3) in the control group. The median progression-free survival was not reached in the daratumumab group and was 31.9 months (95% CI, 28.9 to not reached) in the control group. The hazard ratio for disease progression or death in the daratumumab group as compared with the control group was 0.56 (95% CI, 0.43 to 0.73; P<0.001) (Fig. 1).
Figure 1. Progression-free Survival.
Shown are the results of the Kaplan–Meier estimates of progression-free survival among patients in the intention-to-treat population. The daratumumab group received treatment with daratumumab, lenalidomide, and dexamethasone; the control group received treatment with lenalidomide and dexamethasone. The interim analysis of progression-free survival was performed after 240 events of disease progression or death had occurred (62% of the planned 390 events for the final analysis).
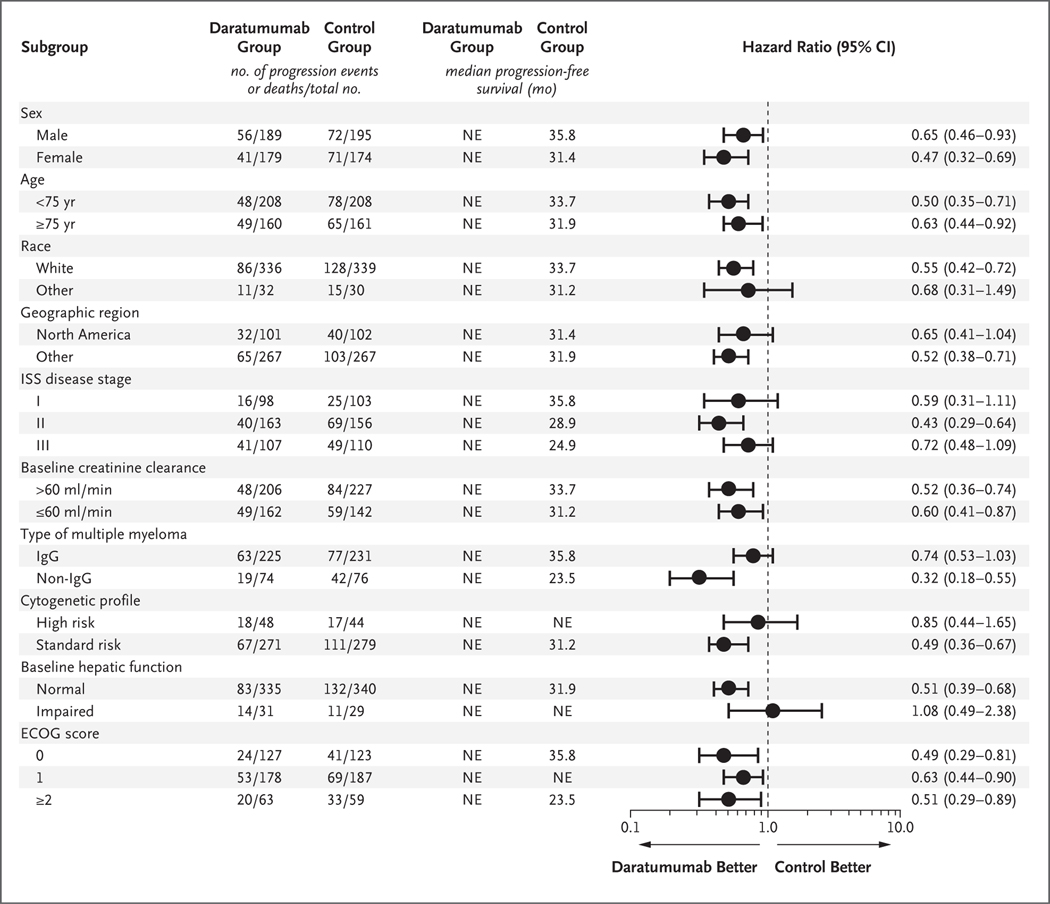
Prespecified subgroup analyses of progression-free survival confirmed the superiority of the daratumumab regimen over the control regimen across all subgroups, except in the subgroup of patients who had hepatic impairment at baseline (Fig. 2). The progression-free survival benefit was maintained among patients 75 years of age or older (hazard ratio, 0.63; 95% CI, 0.44 to 0.92).
Figure 2. Prespecified Subgroup Analysis of Progression-free Survival.
Shown are the results of an analysis of progression-free survival in prespecified subgroups in the intention-to-treat population. The daratumumab group received treatment with daratumumab, lenalidomide, and dexamethasone; the control group received treatment with lenalidomide and dexamethasone. The International Staging System (ISS) disease stage, which is derived on the basis of the combination of serum β2-microglobulin and albumin levels, consists of three stages, with higher stages indicating more advanced disease. The subgroup analysis for the type of myeloma was performed on data from patients who had measurable disease in serum. A high-risk cytogenetic profile was defined by the detection of a del17p, t(14;16), or t(4;14) cytogenetic abnormality (or a combination of these) on fluorescence in situ hybridization or karyotype analysis. Impaired baseline hepatic function includes mild impairment (total bilirubin level less than or equal to the upper limit of the normal range [ULN] and aspartate aminotransferase level higher than the ULN, or total bilirubin level higher than the ULN and ≤1.5 times the ULN), moderate impairment (total bilirubin level >1.5 times and ≤3 times the ULN), and severe impairment (total bilirubin level >3 times the ULN). Eastern Cooperative Oncology Group (ECOG) performance status is scored on a scale from 0 to 5, with 0 indicating no symptoms and higher scores indicating increasing disability. NE denotes could not be estimated.
In the intention-to-treat population, the percentage of patients with a complete response or better was significantly higher in the daratumumab group than in the control group (47.6% vs. 24.9%), as was the percentage with very good partial response or better (79.3% vs. 53.1%) (P<0.001 for both comparisons) (Table 2). The percentage of patients with an overall response was 92.9% in the daratumumab group and 81.3% in the control group (P<0.001).
Table 2.
Summary of Response Rates and Minimal Residual Disease Status in the Intention-to-Treat Population.*
| Variable | Daratumumab Group (N = 368) | Control Group (N = 369) | P Value |
|---|---|---|---|
| Overall response — no. (% [95% CI]) | 342 (92.9 [89.8–95.3]) | 300 (81.3 [76.9–85.1]) | <0.001† |
| Best overall response — no. (%) | |||
| Complete response or better | 175 (47.6) | 92 (24.9) | <0.001† |
| Stringent complete response‡ | 112 (30.4) | 46 (12.5) | — |
| Complete response | 63 (17.1) | 46 (12.5) | — |
| Very good partial response or better | 292 (79.3) | 196 (53.1) | <0.001† |
| Very good partial response | 117 (31.8) | 104 (28.2) | — |
| Partial response | 50 (13.6) | 104 (28.2) | — |
| Stable disease | 11 (3.0) | 56 (15.2) | — |
| Progressive disease | 1 (03) | 0 | — |
| Response could not be evaluated | 14 (3.8) | 13 (3.5) | — |
| Negative status for minimal residual disease — no. (%)§ | 89 (24.2) | 27 (7.3) | <0.001¶ |
Response was assessed on the basis of International Myeloma Working Group recommendations (details on the criteria for disease responses are provided in the protocol). The following secondary end points were tested sequentially, each with an overall two-sided alpha level of 0.05, with the use of a hierarchical testing approach: complete response or better, very good partial response or better, negative status for minimal residual disease, and overall response.
The P value was calculated with the use of the Cochran–Mantel–Haenszel chi-square test.
Criteria for a stringent complete response include the criteria for a complete response plus a normal free light-chain ratio and absence of clonal plasma cells, as assessed by immunofluorescence or immunohistochemical analysis or by two-color to four-color flow cytometry.
The threshold for minimal residual disease was defined as 1 tumor cell per 105 white cells. Status regarding minimal residual disease is based on a postrandomization assessment performed on bone marrow samples with the use of a validated next-generation sequencing assay (clonoSEQ Assay, version 2.0; Adaptive Biotechnologies) in accordance with International Myeloma Working Group guidelines on assessment of minimal residual disease.23
The P value was calculated with the use of the Fisher’s exact test.
The percentage of patients who were negative for minimal residual disease (at a threshold of 1 tumor cell per 105 white cells) was more than 3 times as high in the daratumumab group as in the control group (24.2% vs. 7.3%, P<0.001) (Table 2). Negative status for minimal residual disease was associated with longer progression-free survival than positive status, regardless of the trial treatment (Fig. S3 in the Supplementary Appendix). All the patients who were negative for minimal residual disease had a complete response or better.
At a median follow-up of 28.0 months, 138 patients had died — 62 (16.8%) in the daratumumab group and 76 (20.6%) in the control group (Fig. S4 in the Supplementary Appendix). The median overall survival was not reached in either group, and follow-up for long-term survival is ongoing.
Among the patients who had a response (partial response or better), 80.3% (95% CI, 75.1 to 84.5) in the daratumumab group and 65.7% (95% CI, 58.6 to 71.8) in the control group sustained the response for 30 months. The median time to the first response was 1.05 months in both groups, and the median time to a complete response or better was 10.4 months in the daratumumab group and 11.2 months in the control group. The percentage of patients who were negative for minimal residual disease increased over time at a higher rate in the daratumumab group than in the control group (Fig. S5 in the Supplementary Appendix).
SAFETY
Table 3 summarizes the most common adverse events of any grade (in >30% of patients in either group) and the most common adverse events of grade 3 or 4 (in >10% of patients in either group) during treatment in the safety population. The most common adverse events of grade 3 or 4 were neutropenia (50.0% in the daratumumab group and 35.3% in the control group), anemia (11.8% and 19.7%), lymphopenia (15.1% and 10.7%), pneumonia (13.7% and 7.9%), and leukopenia (11.0% and 4.9%). The incidence of infections of any grade was 86.3% in the daratumumab group and 73.4% in the control group; the incidence of grade 3 or 4 infections was 32.1% in the daratumumab group and 23.3% in the control group.
Table 3.
Most Common Adverse Events and Second Primary Cancers Reported during Treatment in the Safety Population.*
| Event | Daratumumab Group (N = 364) | Control Group (N = 365) | ||
|---|---|---|---|---|
| Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | |
| number of patients (percent) | ||||
| Hematologic adverse events | ||||
| Neutropenia | 207 (56.9) | 182 (50.0) | 154 (42.2) | 129 (35.3) |
| Anemia | 126 (34.6) | 43 (11.8) | 138 (37.8) | 72 (19.7) |
| Leukopenia | 68 (18.7) | 40 (11.0) | 34 (9.3) | 18 (4.9) |
| Lymphopenia | 66 (18.1) | 55 (15.1) | 45 (12.3) | 39 (10.7) |
| Nonhematologic adverse events | ||||
| Infections | 314 (86.3) | 117 (32.1) | 268 (73.4) | 85 (23.3) |
| Pneumonia | 82 (22.5) | 50 (13.7) | 46 (12.6) | 29 (7.9) |
| Diarrhea | 207 (56.9) | 24 (6.6) | 168 (46.0) | 15 (4.1) |
| Constipation | 149 (40.9) | 6 (1.6) | 130 (35.6) | 1 (03) |
| Fatigue | 147 (40.4) | 29 (8.0) | 104 (28.5) | 14 (3.8) |
| Peripheral edema | 140 (38.5) | 7 (19) | 107 (29.3) | 2 (0.5) |
| Back pain | 123 (33.8) | 11 (3.0) | 96 (26.3) | 11 (3.0) |
| Asthenia | 117 (32.1) | 16 (4.4) | 90 (24.7) | 13 (3.6) |
| Nausea | 115 (31.6) | 5 (14) | 84 (23.0) | 2 (0.5) |
| Second primary cancer† | 32 (8.8) | NA | 26 (7.1) | NA |
| Invasive second primary cancer | 12 (3.3) | NA | 13 (3.6) | NA |
| Any infusion-related reaction | 149 (40.9) | 10 (2.7) | NA | NA |
The safety population included all patients who received at least one dose of the trial treatment. Adverse events of any grade that were reported in more than 30% of patients in either treatment group and grade 3 or 4 adverse events that were reported in more than 10% of patients in either treatment group are listed. NA denotes not applicable.
The presence of a second primary cancer was prespecified in the statistical analysis plan as an adverse event of clinical interest.
Serious adverse events were reported in 62.9% of the patients in the daratumumab group and in 62.7% of the patients in the control group. Pneumonia was the most common serious adverse event, occurring in 13.2% of the patients in the daratumumab group and in 7.4% of the patients in the control group. The percentage of patients who had adverse events that led to discontinuation of the trial treatment was 7.1% in the daratumumab group and 15.9% in the control group. Discontinuation of the trial treatment owing to an infection occurred in 0.5% of the patients in the daratumumab group and in 1.4% of the patients in the control group; no patients in the daratumumab group, as compared with 1 patient (0.3%) in the control group, discontinued treatment because of neutropenia.
Adverse events that resulted in death were observed in 25 patients (6.9%) in the daratumumab group and in 23 patients (6.3%) in the control group; the most common such event was pneumonia, which resulted in death in 0.5% and 0.8% of the patients, respectively. Invasive second primary cancers occurred in 12 patients (3.3%) in the daratumumab group (solid tumors in 2.7% and hematologic cancers in 0.5%) and in 13 patients (3.6%) in the control group (solid tumors in 3.0% and hematologic cancers in 0.5%).
Infusion-related reactions associated with daratumumab were reported in 40.9% of the patients in the daratumumab group; 2.7% of the patients had events of grade 3 or 4, including one patient who had grade 4 hypertension, and no grade 5 events were reported (Table S5 in the Supplementary Appendix). Infusion-related reactions usually occurred during administration of the first dose (in 98.0% of the patients who had such reactions), and only one patient (the patient who had grade 4 hypertension) discontinued daratumumab treatment after an infusion-related reaction.
Discussion
The results of this phase 3 trial showed that among patients with newly diagnosed multiple myeloma who were ineligible for stem-cell transplantation, treatment with daratumumab plus lenalidomide and dexamethasone resulted in significantly longer progression-free survival than lenalidomide and dexamethasone alone; the risk of disease progression or death was 44% lower in the daratumumab group than in the control group. These findings can be added to those from a growing list of trials that support the use of daratumumab-based regimens across patient populations with multiple myeloma.13–16,24,25 In our trial, the percentage of patients with a complete response or better was nearly twice as high and the percentage of patients who were negative for minimal residual disease was more than 3 times as high in the daratumumab group as in the control group; these findings are consistent with those of previous trials. We anticipate that responses in individual patients, including negative status for minimal residual disease, will deepen over time, as has been observed with other daratumumab-containing regimens.26,27
The results of this interim analysis showed that the benefit of daratumumab with respect to progression-free survival was not as high in the subgroup of patients who had a high-risk cytogenetic profile as it was in the subgroup of patients who had a standard-risk cytogenetic profile. However, in the CASTOR and POLLUX trials, among patients with relapsed or refractory multiple myeloma who had a high-risk cytogenetic profile, substantial benefits with respect to progression-free survival and minimal residual disease with daratumumab plus standard-of-care regimens were observed after a longer follow-up period.16,25
Cross-trial comparisons are limited by differences in patient populations and trial designs but are important to contextualize our findings. The phase 3 Southwest Oncology Group (SWOG) S0777 trial of lenalidomide plus dexamethasone with or without bortezomib in patients with newly diagnosed myeloma for whom immediate autologous stem-cell transplantation was not indicated showed that the triplet combination resulted in longer progression-free survival (median, 41 months vs. 29 months; hazard ratio, 0.74), including among patients who were 65 years of age or older (43% of the trial population; median, 34 months vs. 24 months) and among patients who were older than 75 years of age (median, 34 months vs. 17 months).4,28 Our trial population was notably older; 99% of the patients were 65 years of age or older, and 44% were 75 years of age or older. The median progression-free survival benefit in the daratumumab group in our trial was maintained for patients 75 years of age or older (hazard ratio, 0.63); the hazard ratio observed in the FIRST trial — in which lenalidomide and dexamethasone administered until disease progression was compared with melphalan, prednisone, and thalidomide — was 0.78 in the subgroup of patients older than 75 years of age.17 Progression-free survival was longer in the control group of our trial than in the similarly treated group in the FIRST trial, a finding that may be attributed to the longer median duration of treatment observed in the control group in our trial (21.3 months in our trial vs. 18.4 months in the FIRST trial).5
The median duration of treatment was longer in the daratumumab group than in the control group (25.3 months vs. 21.3 months). Although patients in the daratumumab group received treatment for a longer period of time, they received less lenalidomide than the control group, possibly owing to a higher incidence of adverse events that led to dose discontinuations or dose modifications in this group. Nevertheless, the efficacy of the daratumumab-based regimen was not affected by the lower dose of lenalidomide, as shown by the consistent progression-free survival benefit in the subgroup of patients who had a baseline creatinine clearance level of 60 ml or less per minute.
The daratumumab group had a higher incidence of neutropenia and infections (including pneumonia) than the control group. However, the incidence of grade 3 or 4 infections was 29% with lenalidomide and dexamethasone in the primary analysis of the FIRST trial,5 which was consistent with the incidence in our trial (32.1% in the daratumumab group and 23.3% in the control group). The percentage of patients who discontinued treatment because of these adverse events was low in our trial and was consistent with the safety profile observed in the POLLUX and ALCYONE trials, the latter of which represents a similar population of patients with newly diagnosed myeloma who were ineligible for stem-cell transplantation.
In this trial involving patients with newly diagnosed multiple myeloma who were ineligible for stem-cell transplantation, the addition of daratumumab to lenalidomide and dexamethasone resulted in significantly longer progression-free survival, a higher response rate, an increased depth of response, and a longer duration of response than lenalidomide and dexamethasone alone. Daratumumab plus lenalidomide and dexamethasone was associated with a higher incidence of neutropenia and infections.
Supplementary Material
Acknowledgments
Supported by Janssen Research and Development.
We thank the patients who volunteered to participate in this trial, their families, and the staff members at the trial sites who cared for them; the members of the data and safety monitoring committee (Nikhil C. Munshi, M.D. [chair], Mario DiCato, M.D., and Weichung Shih, Ph.D.); representatives of the sponsor who were involved in data collection and analyses; Intergroupe Francophone du Myélome for their strong support and guidance during the trial start-up; and Jason Jung, Ph.D., and Melissa Brunckhorst, Ph.D., of MedErgy, for editorial assistance in the development of an earlier draft of the manuscript.
Dr. Facon reports receiving fees for serving on a board of directors, advisory committee, and speakers bureau from Celgene, Janssen, and Takeda, and fees for serving on a board of directors and advisory committee from Amgen, Sanofi, Karyopharm, Oncopeptides, and Roche; Dr. Kumar, receiving grant support and fees for serving on a board of directors and advisory committee from AbbVie, Celgene, and Kite Pharma; Dr. Plesner, serving on an independent response assessment committee for Celgene; Dr. Orlowski, receiving consulting fees and fees for serving on a board of directors and advisory committee from Bristol-Myers Squibb, Celgene, Kite Pharma, Sanofi, and Amgen, consulting fees from Takeda, and grant support from BioTheryX and Spectrum Pharma; Dr. Moreau, receiving honoraria and fees for serving on a board of directors, advisory committee, and speakers bureau from Amgen, Celgene, Janssen, AbbVie, and Takeda; Dr. Bahlis, receiving grant support, consulting fees, and honoraria from Amgen and Celgene; Dr. Hulin, receiving grant support and honoraria from Celgene and Janssen, and honoraria from Amgen and Takeda; Dr. Goldschmidt, receiving consulting fees and fees for serving on a board of directors and advisory committee from Adaptive Biotechnologies, grant support, consulting fees, and fees for serving on a board of directors and advisory committee from Amgen, Sanofi, and Takeda, grant support, consulting fees, honoraria, and fees for serving on a board of directors and advisory committee from Bristol-Myers Squibb, Celgene, and Janssen, honoraria and research funding from Chugai, grant support from Mundipharma, grant support and honoraria from Novartis, and honoraria from Art Tempi; Dr. O’Dwyer, receiving consulting fees from Janssen; Dr. Perrot, receiving consulting fees, honoraria, and equity from Janssen; Dr. Venner, receiving grant support and honoraria from Janssen and Celgene, and honoraria from Amgen and Takeda; Dr. Weisel, receiving grant support, consulting fees, and honoraria from Janssen, consulting fees from Celgene and Juno, consulting fees, honoraria, and research funding from Amgen, consulting and honoraria from Bristol-Myers Squibb and Takeda, and grant support and consulting fees from Sanofi; Dr. Raje, receiving consulting fees from Amgen, Celgene, Bristol-Myers Squibb, Janssen, and Takeda, and grant support from Astra-Zeneca; Dr. Macro, receiving honoraria and travel support from Celgene and Amgen, and grant support, honoraria, and travel support from Janssen and Takeda; Dr. Leleu, receiving honoraria from Amgen, Takeda, Janssen, Bristol-Myers Squibb, Novartis, Roche, Merck, Mundipharma, Gilead, AbbVie, Karyopharm, Celgene, and Incyte; Dr. Ahmadi, being employed by and owning equity in Genmab; Dr. Chiu, being employed by and owning equity in Janssen; Dr. Wang, Dr. van Rampelbergh, Dr. Uhlar, Dr. Kobos, and Dr. Qi, being employed by Janssen; and Dr. Usmani, receiving consulting fees from AbbVie, Genmab, and Mundipharma, grant support and consulting fees from Amgen, Celgene, Merck, Janssen, and Seattle Genetics, and grant support from Bristol-Myers Squibb, Pharmacyclics, and Sanofi. No other potential conflict of interest relevant to this article was reported.
Footnotes
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A complete list of investigators in the MAIA trial is provided in the Supplementary Appendix, available at NEJM.org.
REFERENCES
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011; 364: 1046–60. [DOI] [PubMed] [Google Scholar]
- 2.Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet 2015; 385:2197–208. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Rajkumar SV, San Miguel JF, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol 2014; 32: 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet 2017; 389: 519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med 2014; 371: 906–17. [DOI] [PubMed] [Google Scholar]
- 6.Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016; 128: 384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams HC III, Stevenaert F, Krejcik J, et al. High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytometry A 2019;95: 279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 2011; 186:1840–8. [DOI] [PubMed] [Google Scholar]
- 9.Overdijk MB, Verploegen S, Bögels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 2015; 7: 311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overdijk MB, Jansen JH, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol 2016; 197: 807–13. [DOI] [PubMed] [Google Scholar]
- 11.Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 2015; 373: 1207–19. [DOI] [PubMed] [Google Scholar]
- 12.Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 2016; 387: 1551–60. [DOI] [PubMed] [Google Scholar]
- 13.Mateos M-V, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 2018; 378: 518–28. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 754–66. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 1319–31. [DOI] [PubMed] [Google Scholar]
- 16.Bahlis N, Dimopoulos MA, White DJ, et al. Three-year follow up of the phase 3 POLLUX study of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) alone in relapsed or refractory multiple myeloma (RRMM). Blood 2018;132: Suppl 1: 1996. abstract. [Google Scholar]
- 17.Facon T, Dimopoulos MA, Dispenzieri A, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood 2018;131: 301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15(12): e538–e548. [DOI] [PubMed] [Google Scholar]
- 19.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 2011; 117: 4691–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durie BGM, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia 2006;20: 1467–73. [DOI] [PubMed] [Google Scholar]
- 21.McCudden C, Axel AE, Slaets D, et al. Monitoring multiple myeloma patients treated with daratumumab: teasing out monoclonal antibody interference. Clin Chem Lab Med 2016; 54: 1095–104. [DOI] [PubMed] [Google Scholar]
- 22.Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. May 28, 2009. (https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5×7.pdf). [Google Scholar]
- 23.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016; 17(8):e328–e346. [DOI] [PubMed] [Google Scholar]
- 24.Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017; 130: 974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mateos M-V, Sonneveld P, Hungria VTM, et al. Efficacy and safety of daratumumab, bortezomib, and dexamethasone (D-Vd) versus bortezomib and dexamethasone (Vd) in first relapse patients: two-year update of CASTOR. Blood 2018; 132: Suppl 1: 3270. abstract. [Google Scholar]
- 26.Dimopoulos MA, San-Miguel J, Belch A, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica 2018;103: 2088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spencer A, Lentzsch S, Weisel K, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica 2018; 103: 2079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durie BGM, Hoering A, Sexton R, et al. Longer term follow up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood 2018; 132: Suppl 1: 1992. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.