Abstract
In the past decades, many studies have widely examined the effects of dietary polyphenols on human health. Polyphenols are well known for their antioxidant properties and for their chelating abilities, by which they can be potentially employed in cases of pathological conditions, such as iron overload. In this review, we have highlighted the chelating abilities of polyphenols, which are due to their structural specific sites, and the differences for each class of polyphenols. We have also explored how the dietary polyphenols and their iron-binding abilities can be important in inflammatory/immunomodulatory responses, with a special focus on the involvement of macrophages and dendritic cells, and how they might contribute to reshape the gut microbiota into a healthy profile. This review also provides evidence that the axes “polyphenol–iron metabolism–inflammatory responses” and “polyphenol–iron availability–gut microbiota” have not been very well explored so far, and the need for further investigation to exploit such a potential to prevent or counteract pathological conditions.
Keywords: polyphenols, iron metabolism, inflammation, gut microbiota
1. Introduction
Polyphenols are secondary metabolites that are naturally present in many plant-derived food and beverages that are commonly consumed in the human diet [1,2,3]. According to their chemical structure, polyphenols can be divided into the following different sub-groups: phenolic acids (including hydroxycinnamic and hydroxybenzoic acids, commonly present in coffee, tea, cocoa, oats, rice, wheat, and some fruits); flavonoids, which include different subclasses, such as flavones (reported in vegetables and fruits, e.g., citrus fruits), flavanones (citrus fruits), isoflavones (soya-derived products), flavonols (mostly present in vegetables, such as onions, tomatoes, cauliflower, and broccoli), flavanols (wine, tea, and cocoa), and anthocyanins (berries, grapes, and red wine); stilbenoids (grapes, berries, peanuts, and wine); tannins (grapes and wine); and lignans (seeds and grains) [4,5,6,7].
Polyphenol-enriched diets exert several beneficial roles in human health, thus supporting the prevention of many non-communicable diseases that are associated with oxidative stress and inflammation [8,9,10]. Specifically, these bioactive compounds from foods are able to modulate human health at multiple levels, as follows: acting as scavengers of reactive oxygen species (ROS) [11]; imprinting an anti-inflammatory profile to the innate immune cells; downmodulating inflammatory cytokines and chemokine secretion; inhibiting the correct immunological synapse formation and, consequently, the initiation of antigen-specific adaptive immunity [12,13]; modulating some important cellular pathways that are linked to tumorigenesis, such as cellular proliferation [14]; and regulating gut microbiota [15].
One of the possible mechanisms that could connect all of these biological activities is the iron-chelating ability of polyphenols, which can influence iron homeostasis in the human body. In fact, iron is a pivotal microelement for living organisms. Indeed, upon the onset of an infection, one of the primary host responses is related to iron sequestration and accumulation into phagocytes. Vice versa, tissue repair is characterized by the immune cells’ depletion of the cytoplasmic iron content.
Iron chelation can negatively impact iron deficiency conditions, thus resulting in a higher risk of anemia, which impacts the immune system, also inducing metabolic and neurological impairments [16,17]. On the other hand, iron overload and accumulation can lead to other pathological conditions, such as hemochromatosis, metabolism dysfunction, and other complicated symptoms [18]. Excessive iron availability represents a risk factor for cancer development, as a consequence of the increased level of reactive iron, which promotes ROS and DNA damage and mutations. Therefore, the chelating activity of polyphenols can play a positive role in iron-overload conditions by helping to reverse the damages that are caused by iron accumulation and, consequently, can act as a nutritional tool for chronic inflammatory syndrome prevention. Moreover, many polyphenols can favor the selection of beneficial bacteria that can transform them into molecules with increased anti-inflammatory activity [19]. To date, little is known about the microbiota communities that can contribute to polyphenol–bacteria interaction, but further studies will help us to develop new polyphenol-based prebiotic formulations with important health benefits [20].
2. Iron Intake, Absorption, and Homeostasis
Iron is one of the most abundant elements on our planet, constitutive of both Earth’s crust and its inner cores [21]. Living organisms, from prokaryotic to eukaryotic cells, require iron for survival and proliferation. Iron is a constituent of hemoproteins, including those that are involved in oxygen transport and storage (hemoglobin and myoglobin), electron transfer (cytochromes), and iron–sulfur-containing proteins, or proteins which require iron to carry out the essential functions in cellular metabolism [22,23]. The biological importance of iron relies on its chemical properties as a transition metal, since it is involved in redox reactions in both its ferric (Fe3+) and ferrous (Fe2+) states. However, an excess of reactive iron can be toxic for cells, since it can participate in Fenton-type reactions with the production of hydrogen peroxide, or lipid peroxides, and the generation of ROS. H2O2 can be further reduced by Fe2+ in OH•, causing protein modification, nuclear and mitochondrial DNA damage, and the oxidation of other biomolecules, thus triggering genetic mutations and/or cell death. Oxidative stress conditions can also induce iron release from its ligand proteins, resulting in higher concentrations of free iron, which in turn generate an enhanced susceptibility to oxidative DNA damage. Therefore, the level of reactive iron and the balance between cellular iron overload and iron deficiency must be carefully controlled and finely regulated [22].
Most living organisms have developed different strategies to acquire, store, and recycle iron [24]. In plants, two strategies are employed to acquire iron. The first strategy, which is mostly adopted by dicots and non-graminaceous monocots, consists of the acidification of the rhizosphere by excreting protons to reduce Fe3+ to Fe2+ and the promotion of its uptake. In the second strategy, which is carried out by grasses, rice, maize, barley, and wheat, phytosiderophores (PS) and other high-affinity chelating compounds are secreted to chelate and uptake ferric iron [21].
In humans, iron can be assumed in the form of heme or non-heme from the diet or can be derived from cellular turnover or exfoliation [25]. The dietary sources of heme iron are represented by red meat, liver, fish, and shellfish, whilst non-heme iron is mainly present in pulses and vegetables [24]. Furthermore, the presence of dietary components such as ascorbic acid can promote heme and non-heme iron absorption, whereas other compounds, such as phytic acid, can reduce iron absorption [25,26].
In the human body, almost 60–70% of iron is bound to hemoglobin (Hb), 20% is deposited in ferritin (Ft), and the remaining percentage is linked to both myoglobin in the muscle tissues and to transferrin (Tf) [27].
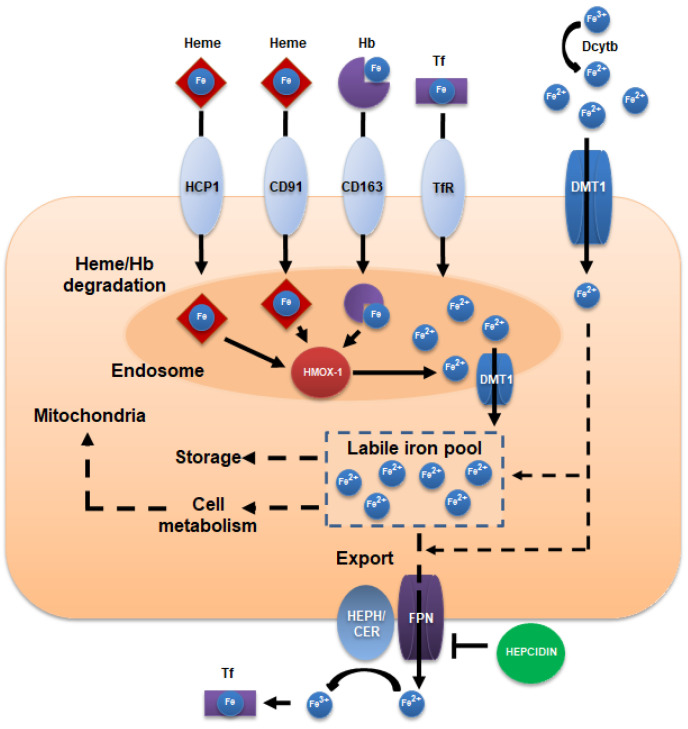
Iron absorption mainly occurs at the duodenal level, where the ferrireductase cytochrome b (DcytB) reduces Fe3+ to Fe2+ in order to allow import by the epithelial divalent metal transporter 1 (DMT1) (Figure 1). Heme iron can be internalized by the low-affinity heme carrier protein HCP1 into the endosome, where it is catabolized by hemeoxygenases (Hmox). Hmox releases free ferrous iron, which is presumably transported to the cytoplasm by divalent metal transporter 1 (DMT1) [22,23]. Here, heme-derived and non-heme iron join the labile iron pool, from which iron can be used by the mitochondria for metabolic reactions, can be incorporated into Ft for cellular storage, or can be exported across the basolateral membrane by ferroportin-1 (FPN-1) into blood circulation. Ferrous iron is subsequently oxidized to ferric iron by the ferroxidases hephaestin, or ceruloplasmin, and bound to Tf [23]. The complex Tf iron interacts with the membrane receptors and is internalized by receptor-mediated endocytosis, which is followed by Tf recycling. Iron uptake also involves the transport of heme–hemopexin complexes, which are internalized via the CD91 receptor (expressed in liver and macrophages), and hemoglobin-bound iron that is complexed with haptoglobin, which is imported via the CD163 receptor (mostly express in monocytes and macrophages).
Figure 1.
Schematic representation of cellular iron metabolism. Divalent metal transporter 1 (DMT1) mediates the cellular import of Fe2+ after its reduction from Fe3+ by the cytochrome-b-like ferrireductase (Dctyb). Transferrin receptor (TfR) binds transferrin–iron complexes before their internalization by receptor-mediated endocytosis. DMT1 function is also implicated in the iron transport from the endosome to the cytoplasm, following the Tf cycle. The hemoglobin scavenger receptor (CD163) and heme-hemopexin receptor (CD91) are implicated in hemoglobin uptake. Once inside the cell, iron joins the labile pool that is stored in ferritin or participates in cell metabolism processes. Ferroportin (FPN) mediates the iron efflux outside the cell, mostly under the regulation of hepcidin. Hephaestin (HEPH) or ceruloplasmin (CER) oxidize Fe2+ to Fe3+ for the binding of iron to transferrin.
At a cellular level, iron regulatory proteins (IRP1 and 2) play an important role in the regulation of iron homeostasis by recognizing the iron-responsive elements (IREs) that are located in the untranslated regions of the mRNA encoding proteins that are involved in iron metabolism, such as DMT1, transferrin receptors, Ft, and FPN1, therefore modulating their translation [28]. At a systemic level, an important protein regulating iron homeostasis is represented by hepcidin, synthetized by the liver, which regulates the iron absorption, distribution, and storage in the different body districts [29]. In this way, increased levels of hepcidin reduce iron transport into the bloodstream, therefore affecting the iron distribution and storage, whereas reduced levels of hepcidin can cause an excess of iron in the bloodstream, Tf saturation, iron accumulation and overload, and hemochromatosis [28,30].
3. Anti/Pro-Oxidant Activities of Polyphenols
Polyphenols are antioxidant compounds that are well known for their radical scavenging activity. By this mechanism, polyphenols behave as reducing agents towards ROS and reactive nitrogen species (RNS), such as OH•, O2•−, and NO•, thus preventing the oxidative stress and DNA damage that are caused by these species. The hydroxyl radical can be generated by different pathways, including the decomposition of peroxynitrous acid [31] or the reduction of peroxides, whereas the production of H2O2, O2•−, and NO• is mostly derived by cellular respiration or cell signaling mechanisms [32]. Polyphenols are able to act as antioxidant compounds by donating an electron or hydrogen atom, thus neutralizing the free radicals. They also act as radical scavengers and chain breakers in lipid peroxidation chain reactions, with the consequent formation of more stable and less reactive species and block of chain reactions before the cell viability can be seriously affected [6,33,34]. Furthermore, polyphenols can induce the expression of antioxidant enzymes, such as glutathione peroxidase, catalase, and superoxide dismutase, which act on hydroxyperoxides, hydrogen peroxide, and superoxide anions, and inhibit the expression of pro-oxidant enzymes, such as cyclooxygenases and lipoxygenases [6,35].
Besides their antioxidant abilities, polyphenols have also been shown to behave as pro-oxidant compounds [35,36,37]. In different cases, the pro-oxidant activity has been related to the structural characteristics of polyphenols, e.g., the flavonol quercetin showed a pronounced pro-oxidative activity, while the flavanones hesperetin and naringenin have displayed milder effects [38]. Some flavonoids containing multiple hydroxyl groups, especially in the B-ring, have been shown to increase the production of hydroxyl radicals [39]. Baicalein, containing a pyrogallol structure in the A-ring, has also been reported to promote hydrogen peroxide production [39,40]. In addition, the pro-oxidant effect can be caused by the autoxidation or enzymatic oxidation (for example, by peroxidases) to which polyphenols can be subjected, causing the production of highly reactive phenoxyl radicals, such as flavonoid quinones. These compounds can be stabilized in vivo by conjugation with nucleophiles, such as GSH, cysteine, or nucleic acids [41].
The pro-oxidant activity of polyphenols could also be associated with their ability to reduce Fe3+ (or other transition metal ions) and the prevention of their binding to other chelating ligands, such as EDTA. In fact, the pro-oxidant properties of polyphenols have been experimentally observed in the presence of metal chelators, such as EDTA, and the oxidized form of the metal ion Fe3+ [36,42,43,44,45]. Increased levels of OH• have been observed following the reduction in Fe3+ complexed with EDTA in the presence of myricetin, quercetin, or catechin [42]. Some phenolics (quercetin, phloretin, phloridzin, phloroglucinol, gallic acid, ferulic acid, and 3,4-dihydroxyphenylacetic acid) have been found to enhance OH• generation under the Fe2+-EDTA-H2O2 system [35]. However, an important consideration that should be taken into account is that the intracellular environment is prevalently reducing, due to the presence of reductant agents such as NADH, glutathione, ascorbic acid, etc., [46,47,48]. Therefore, most of the metal ions that are not bound to proteins would be mainly in their reduced forms in vivo [49]. Polyphenols can display both antioxidant and pro-oxidant activities in very similar conditions, even though their pro-oxidant activity may increase in the presence of very strong chelators (such as EDTA, or medications with chelating abilities, e.g., bleomycin) or high concentrations of H2O2 [35,49]. Furthermore, the pro-oxidative potential of polyphenols can also differ within the same class, dependent on their concentration (low levels of polyphenols may have antioxidant activity, whereas higher concentrations may display pro-oxidant effects), pH conditions, or stereochemistry, which might partly explain the controversy among their antioxidant and pro-oxidant effects [37].
4. Iron-Chelating Abilities of Polyphenols
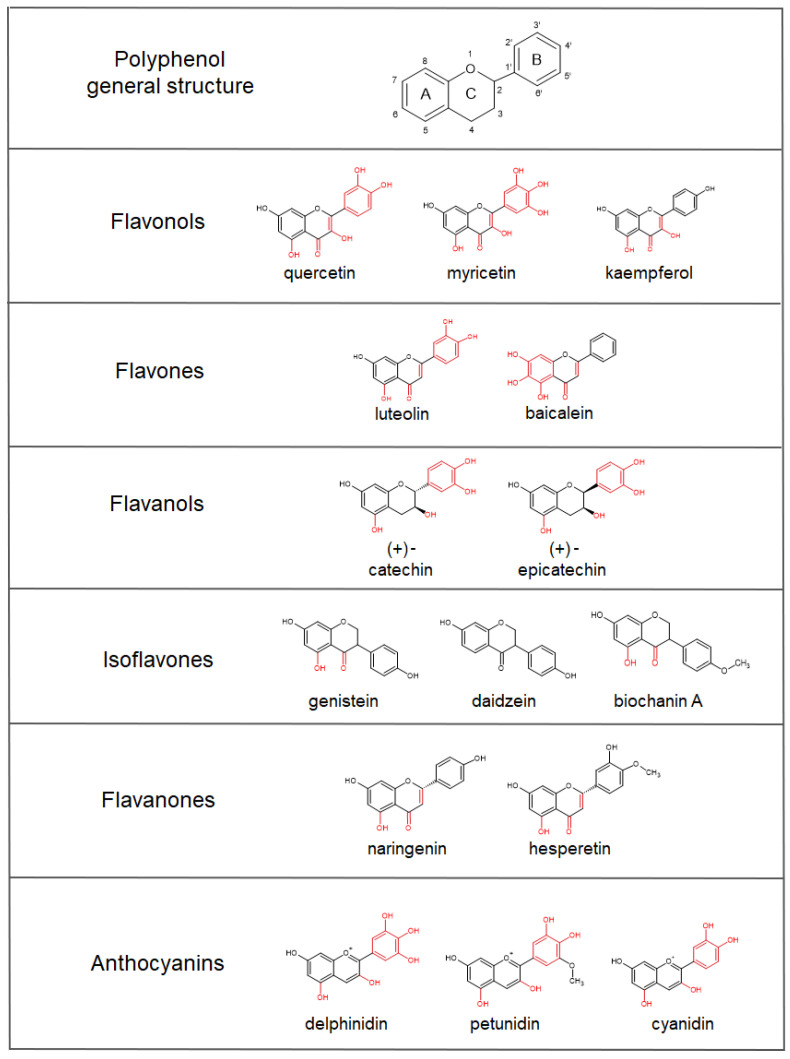
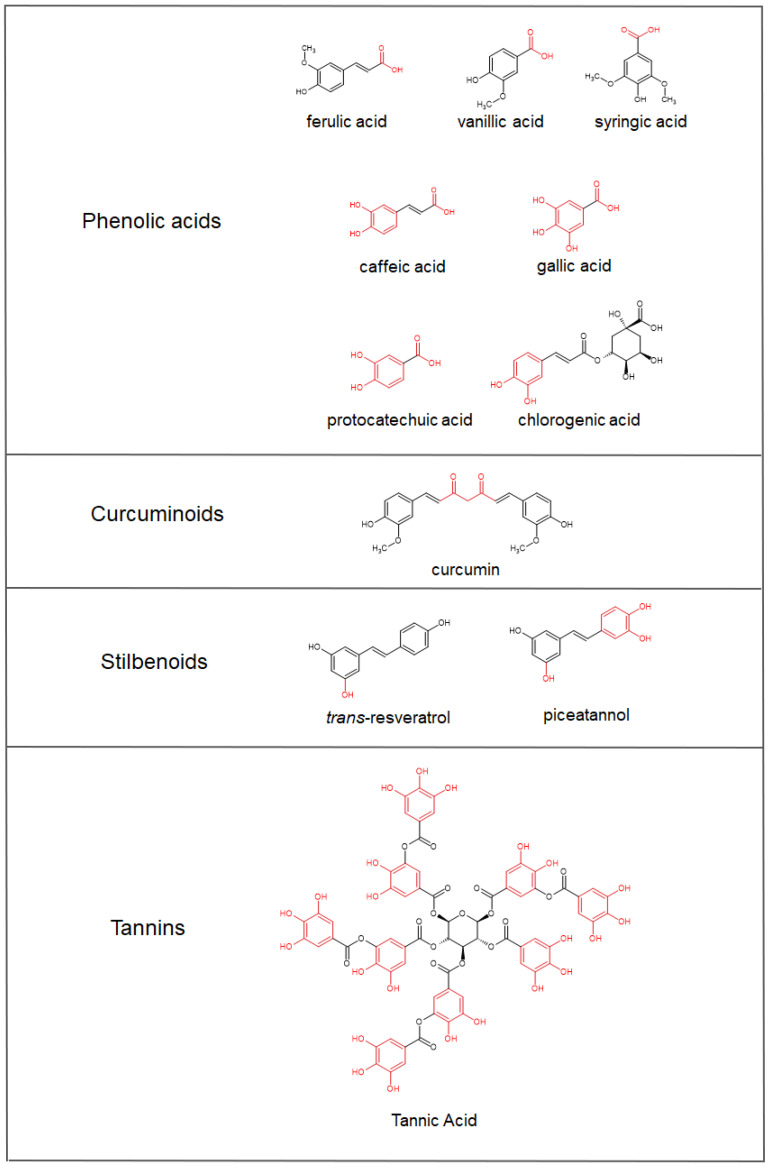
Besides their radical scavenging activities, another mechanism by which polyphenols can exert their antioxidant activity involves iron binding. Iron, and transition metals in general, can be involved in the generation of oxygen free radicals, the reduction of peroxides, or reactions with superoxide anions [50,51,52], and, subsequently, oxidative stress. Polyphenols have been shown to have iron-binding abilities, which are mainly related to the presence of catechol and galloyl groups. Some studies have shown that the 6,7-dihydroxy structure, B-ring catechol, the galloyl groups, the 2,3-double bond, and the 3- and 5-hydroxylic groups in co-presence with the 4-keto group are associated with chelation properties, and therefore are eligible as iron-binding sites [49,53,54,55,56] (Figure 2). For example, baicalein and baicalin, containing 6,7-dihydroxy groups, have strong iron-binding activities [57]. Flavonoids, such as quercetin and rutin, with 3- and 5-hydroxy-4-keto groups, or flavones and flavonols with 2,3-double bonds in general, are also important metal chelators [58,59]. Ellagic acid, with its four hydroxyl groups, shows metal-transition-chelating abilities, with the possibility to participate in antioxidant redox reactions, resulting in an efficient free radical scavenger [60,61]. Interestingly, when it is incubated with iron–EDTA or iron–citrate complexes, ellagic acid is able to remove iron from those ligands by forming an iron–ellagic acid complex, which reduces the levels of iron ions in the solution that catalyze free radical formation, and therefore showing an antioxidant mechanism that is different from “classical” OH• radical scavenging [45]. In the case of curcumin, the β-diketone group has been suggested to be responsible for iron chelation, even though it does not affect or block iron cellular uptake [62,63]. In in vivo systems, curcumin’s iron-chelating abilities show the ability to affect the systemic iron metabolism (e.g., a decline in serum iron and transferrin saturation, decreased iron levels in the spleen and bone marrow, IRPs activation, repressed ferritin levels, and hepcidin hepatic synthesis), thus suggesting possible effects in patients with both a marginal and a high iron status [64,65]. Furthermore, the iron-chelating abilities of curcumin have also been suggested to contribute to anticancer activities through the formation of redox-active iron complexes and iron depletion in cancer cells [63,64,65].
Figure 2.
Representative classes of polyphenols and examples of compounds belonging to each group, showing different iron-chelating abilities. Polyphenol general structure is formed by two aromatic rings, indicated as A and B, linked together by three carbon atoms forming an oxygenated heterocycle, the C ring. The 6,7-dihydroxy structure, B-ring catechol, galloyl groups, 2,3-double bond, 3- and 5-hydroxylic groups, β-diketone group, and carboxylic groups associated with iron-binding properties are highlighted in red.
In the case of isoflavones, the 5-hydroxy-4-keto group has been suggested to chelate ferric and ferrous ions, even though the affinity towards these ions was lower than those of the other iron-chelating flavonoids [66]. In particular, genistein and biochanin A, but not daidzein, show chelating abilities of Fe3+, indicating that isoflavones bind the metals at the 4-keto and the 5-OH sites [67].
Lakey-Beitia and co-workers [68] propose the following three groups of polyphenols based on the binding sites: a group with one metal binding site, to which belong the curcuminoids, some stilbenoids, isoflavones, and flavanones; the group with two binding sites that includes some flavones and some anthocyanins; and the group with three metal binding sites that includes flavonols, flavanols, some anthocyanins, and tannins.
In general, the polyphenolic compounds with catechol moieties on the B-ring are more potent inhibitors of the Fenton reaction than those without catechol groups [69]. In addition, the presence of a large number of catechol/galloyl groups (as in the case of tannic acid) contributes to enhanced iron chelation. Perron and co-workers [70] have shown that compounds with galloyl groups have a higher antioxidant activity compared to those with only catechol groups. Phenolic acids bearing catechol or galloyl groups (caffeic acid, gallic acid, protocatechuic acid, and chlorogenic acid) have shown more intriguing iron-binding properties compared to the other polyphenols lacking these groups (ferulic acid, syringic acid, and vanillic acid) [53]. For these, the carboxylate group has been proposed as the most eligible group for iron complexation [71]. In addition, the structures with galloyl moiety, as in the case of gallic acid within the group of hydroxybenzoic acids, scored better than those of the catechol type (as in the case of protocatechuic acid), which could be attributed to the number and position of the hydroxyl groups. It is worthy of notice that gallic acid alone exhibits a reduced iron-chelating capacity, which is probably because of the third hydroxyl group in position three. Indeed, this third OH group can stabilize the flavonoid ring structure and has radical scavenging abilities [72,73], but reduces the iron-chelation ability [53]. Similarly, the presence of methoxy groups (as in the case of vanillic acid, syringic acid, and ferulic acid) increases the radical scavenging activities but hinders the chelation abilities of polyphenols [53,74].
5. Polyphenols’ Bioavailability
The bioavailability of dietary nutrients and compounds usually designates the quantity or fraction of the ingested dose that is absorbed from the gastrointestinal tract [75]. In the case of polyphenols, the bioavailability is strongly influenced by their physical properties (e.g., molecular mass, polarity, and hydrophobic moieties) and the presence of proteins, lipids, and fibers in the ingested food matrixes [76]. For instance, poor anthocyanin bioavailability can be improved by their binding to dietary fibers, which are able to protect them from degradation due to the pH of the intestinal environment and allow them to reach the large intestine and remain there for a longer time [76]. Once they are ingested, polyphenols reach the intestine, where they are first deconjugated by enzymes such as lactase phlorizin hydrolase (LPH, located on the enterocytes membrane) or β-glucosidase (CBG, cytosolic), in order to facilitate the absorption by the epithelial cells. Following this first process of absorption, the polyphenols are transported through the portal vein to the liver, where they undergo phase I metabolism, which implies hydrolysis and oxidation reactions, and phase II metabolism, prevalently including glucuronidation, methylation, and sulfonation reactions [77,78]. Such metabolites can have markedly changed iron-chelating and anti/pro-oxidant properties [79,80]. They can be detected in systemic circulation; however, such modifications (in particular, sulfation and glucuronidation) also facilitate their efflux into the intestinal lumen. Once they are released into the intestinal lumen, the polyphenols are then subjected to microbial biotransformation, which can produce smaller and structurally simpler compounds [81,82]. Several studies report the presence of phase II metabolites (glucuronides or sulfated forms) in plasma samples after the ingestion of polyphenols, or foods that are enriched in polyphenols, indicating that the intestines and the liver are the main sites of polyphenol metabolism [83], whereas polyphenolic aglycones are not commonly found there [84,85]. Table 1 reports some examples from published studies describing the bioavailability and the excretion paths of representative classes of polyphenols. The maximal plasma concentration (Cmax) and the time to reach it (tmax) strongly depend on the diverse classes of compounds, the type and amount of the food source, the studied species, and the interindividual variability [86,87]. In some cases, such as for quercetin, the type of bound sugar moiety can also influence the efficiency of the intestinal absorption and, therefore, the polyphenol bioavailability [83].
The main route of elimination of polyphenols is urinary excretion, even though biliary excretion should be considered for some compounds, such as quercetin or curcumin [87,88,89,90].
Polyphenols’ intestinal absorption is mainly mediated by epithelial glucose or monocarboxylates transporters (MCTs) [91]. Quercetin glucosides can be directly transported via SGLT1 (sodium-dependent glucose transporter 1) or hydrolyzed to quercetin before their absorption by passive diffusion in the small intestine [83]. Rutin is metabolized by intestinal bacteria into phenolic compounds prior to being absorbed via MCT, or through the paracellular pathway. The uptake transporters OATPs (organic anion transport polypeptides) and OATs (organic anion transporter) contributes to the uptake of quercetin and its metabolites into the liver and the kidneys [83]. A hesperetin derivative, MTBH (8-methylene-tert-butylamine-3’,5,7-trihydroxy-4’-methoxyflavanone), has been shown to be mainly absorbed by the transcellular passive diffusion mechanism and to use MCT carriers to enter the cells [92]. Trans-resveratrol has been reported to use a passive transport to cross the apical membrane of the intestinal cells, whereas the transport of its trans-piceid derivative is likely to be active, involving SGLT1 [93]. On the other hand, the main transporters that are implicated in polyphenol efflux are MRP2 (multi-drug resistance protein 2), BCPR (breast cancer resistance protein), and P-gp (P-glycoprotein transporters) [91]. MPR2 and BCPR mediate the excretion of quercetin and its metabolites through bile and urine, eliminating them from the body [83]. MRP2 has been also shown to be involved in stilbene efflux [93]. P-gp and BCPR are used for MTBH efflux transport [92]. However, Teng and co-workers (2012) [91] reported different affinities for SLGT1, MRP2, and P-gp transporters among the different classes of polyphenols, indicating that their influx or efflux may be dependent on their chemical structures.
Table 1.
Examples of studies reporting polyphenols’ bioavailability in humans.
| Polyphenol Compound—Food Source | Dosage | Cmax 1 of the Main Compounds in Plasma (tmax 2) | Main Compounds Excreted in Urine (Time) | Reference |
|---|---|---|---|---|
| Quercetin-4’-O-glucoside | 100 mg | 2.12 µg·mL−1 of quercetin-4’-O-glucoside (0.70 h) | NA 3 | [94] |
| Quercetin-3-O-rutinoside | 200 mg | 0.32 µg·mL−1 of quercetin-3-O-rutinoside (6.98 h) | NA | [94] |
| Fried onions (containing 275 µmol flavonols, principally quercetin-4’-glucoside and quercetin-3,4’-diglucoside) | 270 g | 665 nM of quercetin-3’-sulfate (0.75 h) 351 nM of quercetin-3-glucuronide (0.60 h) 63 nM of quercetin diglucuronide (0.80 h) |
274 nM quercetin diglucuronide (8–24 h) | [95] |
| Fresh blackcurrant (containing 897 mg total anthocyanins) | 100 g | Anthocyanins not detected |
339 µg total anthocyanins (48 h) 17.3 mg hippuric acid (0–24 h) |
[84] |
| Elderberry concentrate (containing 1.9 g anthocyanins and equivalent to 235 mL fresh juice) | 11 g | NA | 15.8 µg cyanidin-3-glucoside (1 h) 29.8 µg cyanidin-3-sambubioside (2 h) |
[96] |
| Homogenized raspberries (containing 292 µmol anthocyanins, 6.3 µmol ellagic acids, 251 µmol ellagitannins, and 2.5 µmol phenolic acids) | 300 g | 180 nmol·L−1 of 3’,4’-dihydroxyphenylaceticacid (6 h) 78 nmol·L−1 of 4’-Hydroxyhippuricacid (1 h) 47 nmol·L−1 of ferulic acid-4′-sulfate (1.5 h) 18 nmol·L−1 of ferulic acid-4′-O-glucuronide (1.5 h) 14 nmol of isoferulic acid-3’-O-glucuronide (1.5 h) |
19.9 nmol cyanidin-3-O-glucoside (0–48 h) 6.4 nmol 4-Hydroxybenzoic acid (0–48 h) 6.5 nmol ferulic acid-4-sulfate (0–48 h) 16.1 nmol 4-hydroxyhippuricacid (0–48 h) 239 nmol hippuric acid (0–48 h) |
[97] |
| Evelor 500 mg tablets (containing trans-resveratrol) | 500 g | 71.18 mg·mL−1 of trans-resveratrol (1.339 h) 1516.014 mg·mL−1 of sulfated resveratrol 4083.900 mg·mL−1 of glucuronated resveratrol |
NA | [98] |
| Coffee beverage, containing various doses of chlorogenic acid: 412 µmol (A) 635 µmol (B) 795 µmol (C) |
200 mL | 808 µmol of total chlorogenic acid derivatives (0.5–6 h) (A) 1242 µmol of total chlorogenic acid derivatives (0.5–6 h) (B) 1164 µmol of total chlorogenic acid derivatives (0.5–6 h) (C) |
100.7 µmol of total chlorogenic acid derivatives (0–24 h) (A) 160.0 µmol of total chlorogenic acid derivatives (0–24 h) (B) 125.2 µmol of total chlorogenic acid derivatives (0–24 h) (C) |
[99] |
| Curcuminoids as native powder, micronized powder, or liquid micelles (containing 410 mg curcumin, 80 mg demethoxycurcumin, and 10 mg bis-demethoxycurcumin) |
500 mg | 7.1 nmol curcumin (7.5 h) (native powder) 41.6 nmol curcumin (8.8 h) (micronized powder) 3228 nmol curcumin (1.1 h) (liquid micelles) |
5.1 nmol 4 curcumin (0–24 h) (native powder) 70.6 nmol 4 curcumin (0–24 h) (micronized powder) 753 nmol 4 curcumin (0–24 h) (liquid micelles) |
[100] |
1 Cmax: maximal plasma concentration. 2 tmax: time to reach Cmax. 3 NA: not available. 4 data expressed as nmol·g−1 creatinine.
6. Polyphenol-Mediated Iron Sequestration Affects the Inflammatory Response
6.1. The Host Level
Polyphenols display remarkable anti-inflammatory and modulatory activities on the immune system, at both the intestinal and the systemic level. Such activities involve cellular mediators—such as macrophages, lymphocytes, and dendritic cells—and protein mediators, such as cytokines and interleukins. Polyphenols can impair the release of interleukins, such as IL1β, IL-6, and IL-8, the tumor necrosis factor (TNF) [101,102,103], and chemokines [12]. They can also affect the signaling systems that are involved in inflammatory processes, as in the case of T-cell proliferation and B lymphocyte activation [34]. Polyphenols can also modulate the NFκB signaling and MAP kinase pathways [104,105] and the production of mediators of inflammation through phospholipase A2 and cyclooxygenase 2 (COX-2), which are enzymes that are involved in the arachidonic acid metabolism [106,107,108]. Another important anti-inflammatory mechanism concerns nitric oxide (NO) production at the vascular level, since polyphenols can inhibit NO release, which is involved in the inflammatory responses that are triggered by free radicals [5]. Polyphenols also inhibit extracellular-matrix-degrading enzymes, such as the matrix metalloproteinase-2 and 9 [109,110,111].
Among these important modulation mechanisms on the inflammatory and immune-related pathways, another interesting route concerns cellular iron homeostasis. The iron-chelating properties of polyphenols can play important roles in systemic iron regulation and during the inflammatory processes at the gut level and/or in iron overload conditions, such as hemochromatosis.
Under homeostatic conditions, iron undergoes systemic recycling to supply the needs of the body metabolism. Dietary iron intestinal absorption compensates the body losses only in part (for example, through intestinal epithelium desquamation or menstrual bleeding [112]). Hence, senescent red blood cells (sRBC) represent an important supply of iron for hemeproteins de novo synthesis (e.g., hemoglobin) and erythropoiesis [112]. Heme deriving from sRBC is mainly recovered by macrophages in the red pulp of the spleen, the liver, and the small intestine, and phagocytized and degraded by Hmox-1 to release iron into the cytoplasm, which is subsequently exported by ferroportin. Such iron release from macrophages is important, not only for iron recycling, but also for tissue iron availability in homeostatic and pathological conditions, as well as in wound healing [113]. In this context, liver hepcidin exhibits an important systemic regulatory role, acting as a ferroportin gene expression inhibitor or representing negative feedback for iron release, based on the iron plasmatic concentrations [114]. The systemic release of soluble inflammatory mediators, mainly IL-6, induces the hepatocytes to release hepcidin, which, in turn, downregulates ferroportin activity, resulting in the inhibition of iron release from the cells and iron absorption under inflammatory conditions [115,116].
It is worth noting that iron homeostasis dictates the polarization of innate immune cells, with an indirect effect on the adaptive immune response. This is especially true for macrophages exerting different immune functions, ranging from pathogen recognition, antigen processing, phagocytic clearance, and positive/negative immune regulation in the resolution of the immune response and tissue repair [117]. These immune functions depend on the macrophages’ functional states; in fact, based on the local environment, macrophage plasticity identifies two phenotypes: the M1 macrophage phenotype, or the “classically” activated phenotype, with pro-inflammatory activities, and the M2 macrophage phenotype, or the “alternatively” activated phenotype, with anti-inflammatory activities and tissue repair properties [118]. The two macrophage phenotypes are characterized by marked differences in the effector functions and the gene expression profile. However, the identification of M1 and M2 largely oversimplifies the plasticity of the macrophages, which rather shows a continuum of the functional activation states that are modulated by the environmental stimuli, such as the iron content in a given cell or tissue [119]. Specifically, macrophages represent one of the major sources of the available iron in our body, thus becoming crucial in iron homeostasis regulation. During inflammation, the macrophages sequester iron, while during tissue repair, the macrophages release iron, also contributing to cell proliferation [120]. Furthermore, macrophage polarization could affect the systemic iron balance that modulates the expression of the hepcidin and ferroportin genes [121]. While M1 polarization induces the up-modulation of hepcidin, promoting iron sequestration and retention, M2 polarization upregulates ferroportin gene expression, enhancing iron release [113,122,123].
Moreover, the balance between iron influx/efflux also modulates other important cells of innate immunity, i.e., the dendritic cells (DCs), which are activated to support inflammation, tissue healing, and host tolerance [124]. DCs maturation is inhibited by iron depletion, since inflammation favors iron influx and the tolerance iron efflux [124]. On the other hand, in in vitro iron-overload-mimicking conditions, bone-marrow derived DCs (BMDCs) have been found to differentiate into MHCIIlowCD11c+CD11b+F4/80+ cells, with a reduced ability to release inflammatory cytokines [125].
Another environmental stimulus that is able to influence the polarization of macrophages is diet. The vast majority of immune cells are located in mucosal tissues, and macrophages and DCs in particular patrol the intestinal epithelium from the basolateral side, sometimes projecting dendrites into the intestinal lumen. Thus, it is not surprising that the luminal content can influence the immune cells’ response. In fact, the beneficial role of the numerous bioactive compounds that are obtained from foods in the modulation of the inflammatory response has been widely demonstrated, with a focus on immune cells from the innate response, such as macrophages and dendritic cells [12,13,126,127,128,129,130]. Thus, the link between polyphenols, iron metabolism, and the inflammatory/immune response can easily come up, even if it has not been extensively studied until now. To explain polyphenols’ potential to act on iron homeostasis and inflammatory conditions, we can start to consider different in vitro and in vivo studies that have investigated the effects of polyphenols on iron homeostasis (Table 2). Specifically, in the presence of polyphenols, macrophages change their polarization and, therefore, the iron availability, in order to reduce the inflammatory processes [131,132,133,134,135]. In C57BL/6 mice that were subjected to a high-fat diet, the administration of a purple, red corn extract that was enriched in anthocyanins resulted in the upregulation of M2 markers in the adipose tissue macrophages, with a downregulation of inflammatory mediators and an increased expression of iron-metabolism genes that were associated with an iron storage reduction [132]. In vitro experiments have shown that quercetin reduces ferroptosis by inhibiting M1 macrophage polarization and ameliorating the inflammatory response [134]. Following quercetin exposure, liposaccharide (LPS)-treated BMDCs change their polarization into an M2-like profile, increasing the ferroportin expression level and, subsequently, the iron efflux, therefore reducing their inflammatory abilities [124,125].
Importantly, when investigating the link between dietary polyphenols and iron metabolism, the modulation of the oxidative pathways has to be taken into account. In iron-overloaded rats, curcumin administration reduced iron accumulation in the spleen and the liver and subsequent oxidative stress by increasing the endogenous antioxidant and anti-inflammatory abilities [136], as also reported in Table 2. Myricetin also reduces iron uptake in Caco-2 cells [137] and the iron content in vivo by inhibiting the expression of transferrin receptor 1 (TFR1) in an Alzheimer’s disease mouse model [138]. Baicalein and quercetin attenuate iron-overload lipid peroxidation and protein oxidation in mouse liver injuries [139]. Low dosages of resveratrol have been shown to reduce ineffective erythropoiesis [140]. Such studies therefore suggest that the iron-chelating abilities of polyphenols can be useful in the reduction in iron overload and the consequent oxidative cellular stress, thus indicating a valuable nutritional strategy to prevent hemolytic disorders.
Moreover, in order to investigate the interaction of ubiquitous polyphenols, such as quercetin and related flavonoids with iron supplements, Mazhar and coworkers administered a low-iron diet to female Sprague Dawley rats for 20 days to induce iron-deficiency anemia [141]. After that, a 30-day treatment with 50 mg kg−1 ferrous sulfate, combined in an equal ratio of quercetin, quercetagetin, and patuletin, was administered. The combined administration of flavonoids and ferrous sulfate ameliorates some of the hematological parameters and increases the splenic tissue availability of iron, as well as the expression of ferroportin in this animal model of iron-deficiency anemia. Importantly, this effect seems to be variable with alterations in structural features of flavonoids, even though these functional differences need further investigation. It is important to note that the authors have also proposed that the increase in the serum and splenic stores could be based on the formation of a metal-ion–chelate complex with organic ligands and that could improve their transport across the membrane and the absorption. The positive metallic ion charge could be shielded by polyphenols, thus preventing the interaction with the negatively charged layer of mucin, and thereby increasing their lipophilicity and their absorption by the intestinal enterocytes. Finally, the iron–quercetin complex may provide an alternative pathway for iron absorption through the glucose transporter [141].
Table 2.
Studies reporting the effects of structurally different polyphenols in iron homeostasis, oxidative stress, and inflammatory conditions.
| Compound | Model | Effect | Reference |
|---|---|---|---|
| Anthocyanins | C57BL/6 mice fed with a high-fat diet supplemented with purple corn anthocyanins in drinking water | Downregulation of inflammatory mediators, increased expression of iron genes metabolism, and upregulation M2 markers in adipose tissue macrophages | [132] |
| Quercetin | In vitro RAW 264.7 cells; in vivo lipopolysaccharide (LPS)/ovalbumin (OVA)-induced neutrophilic asthma mouse model | Reduction in ferroptosis and inhibition of M1 macrophage polarization. The effect was ferrostatin-like | [134] |
| Quercetin | In vitro bone marrow dendritic cells (BMDCs) | Changes in M2-like phenotype, increased expression in ferroportin, and reduction in inflammatory abilities | [124] |
| Quercetin and baicalin | Kunming mice fed with a diet supplemented with iron-dextran and baicalin 1% or quercetin 1% | Inhibition of iron-overload induced lipid peroxidation and protein oxidation in the liver | [139] |
| Resveratrol | Kunming mice with iron-overload induced liver fibrosis | Regulation of iron homeostasis by reducing the expression of hepcidin, ferritin, TfR, and DMT1, and raising the expression of FPN-1 | [142] |
| Resveratrol | In vitro human β-thalassemic-erythroid cells; in vivo in β-thalassemic mice (Hbbth3/+) | Reduction in ineffective erythropoiesis, increase in hemoglobin levels, and reduction in oxidative stress in circulating red cells | [140] |
| Myricetin | In vitro HepG2 cells; HEK293 cells; C57BL/6 mice | Inhibition of hepcidin expression in vitro; and reduced hepatic hepcidin expression, decreased splenic iron levels, and increased serum iron levels in vivo | [143] |
| Curcumin | C3H/HeNCrl mice fed with different amounts of iron and curcumin | Decreased iron levels in blood, liver, bone marrow, and spleen, induced TfR1, and repressed ferritin and hepcidin | [65] |
| Quercetin, quercetagetin, and patuletin |
Sprague Dawley rats fed with low-iron diet for 20 days, followed by a 30-day treatment with 50 mg kg−1 ferrous sulfate supplement combined with quercetin, quercetagetin, and patuletin | Improved hematological parameters and increased splenic tissue availability of iron and ferroportin expression | [141] |
| Curcumin | In vitro T51B cells | Repression of iron-dependent generation of ROS and inhibition of intracellular iron toxicity | [62] |
| Curcumin | Sprague Dawley rats receiving iron and curcumin supplementation in drinking water | Reduction in iron overload-induced lipid peroxidation and reduction in oxidative stress in the liver and spleen | [136] |
| Curcumin | In vitro LLC-PK and NRK52E cells | Stimulation of heme-oxygenase1 expression | [144] |
6.2. The Gut Microbiota Level
The data that have been obtained from clinical practice indicate that oral iron supplements may have a deleterious effect that is related to inflammatory bowel disease patients (IBD, i.e., ulcerative colitis and Crohn’s disease) with increased intestinal inflammation, thus, intravenous infusion is the recommended strategy [145,146]. This observation can be explained in light of the knowledge that iron availability is an important limiting factor for gut bacterial proliferation, and that less than 20% of orally supplemented iron is absorbed in the duodenum, while the rest becomes available for the intestinal microorganisms. As mentioned previously, the host response to inflammation accounts for numerous strategies that are aimed at limiting iron availability. Among these, an important role is displayed by the iron-chelating Lipocalin 2 (LCN2), which is a protein that is produced by enterocytes, hepatocytes, macrophages, neutrophiles, and myeloid cells that can limit microbial overgrowth.
At the intestinal level, LCN2 is mostly expressed by epithelial and myeloid cells and shows a very strong affinity toward the bacterial siderophores and a strong bacteriostatic function, since its binding to siderophores, such as enterobactin, inhibits iron acquisition by the bacteria, therefore limiting their growth. A lack of LCN2 results in gut dysbiosis in Lcn2−/− mice [147], and, under intestinal inflammation conditions, it facilitates the growth of pathogenic bacteria and a severe colonic inflammation status. LCN2 may therefore shift the microbial community towards a beneficial milieu in the case of chronic intestinal inflammation [148,149]. However, the pathogens that do not strictly rely on enterobactin-mediated iron acquisition can gain an advantage from LCN2-mediated commensal growth [25]. Furthermore, increased levels of LCN2 have also been associated with pro-inflammatory conditions, insulin resistance, and obesity-related disorders [148]. Iron availability at the intestinal level can be affected by both the commensal bacteria and by the dietary components. In fact, with their abilities to capture iron, the commensal bacteria share iron among themselves and with the human host [150,151]. Furthermore, the bacteria can indirectly improve the iron availability by enzymatically degrading the iron chelators, such as tannins or phytates [152]. In addition, dietary components such as ascorbic acid and organic acids (citric acid, malic acid, etc.), both deriving from the diet or as by-products of bacterial metabolism (lactic acid and propionic acid), can improve the iron bioavailability and bio-accessibility [153].
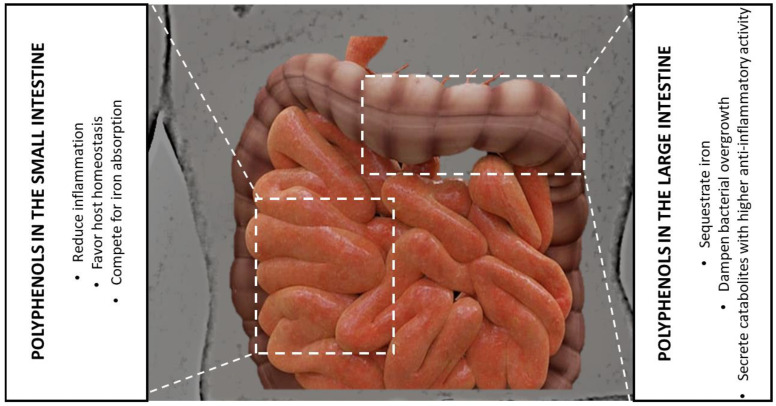
Polyphenols, which are characterized by well-known iron-chelating abilities, can play either the role of antimicrobial agents [154] or as growth promoters of some bacterial species [155,156]. This could be also explained by the very limited bioavailability of polyphenols; in fact, while only a small percentage of the total intake is absorbed in the small intestine, the vast majority of it can reach the lumen of the large intestine and can interact with the gut microbiota [157]. Considering the relationship between the polyphenols and iron, DMT1 fails to transport the polyphenol–iron complex into the epithelial cells, and, in this way, the polyphenol–iron complex reduces the iron availability in the intestinal lumen, impairing the gut microbiota growth. This aspect should be considered during intestinal inflammatory events that are associated with microbial dysbiosis, because the iron sequestration that is mediated by iron–polyphenol complexes could be an effective strategy to deprive the gut microbial species of a crucial supply. Furthermore, polyphenols can be the substrate for the growth of some microbiota species, and not others, and the by-products deriving from the microbial metabolism and biotransformation can have effects on the host [5,158] (Figure 3).
Figure 3.
Schematic representation of the polyphenols’ role at the level of the small and large intestines, being important in the reduction in inflammation, iron metabolism, bacterial growth, and metabolite production with anti-inflammatory activities.
In in vitro fermentation and in vivo experiments, increased dietary iron reshapes the gut microbiota composition, with reduced inflammatory responses, increased toxic metabolites, and bacterial-virulence-associated pathways. Such changes therefore predispose the system to an increased risk of infections, the development of a dysbiotic phenotype, and a predisposition to disease [159,160]. Commensal species, such as lactobacilli and Bifidobacterium, have been shown to have lower iron requirements compared to other pathogenic strains belonging to Enterobacteriaceae (e.g., Salmonella), Yersinia, Pseudonomas, E. coli, and Clostridium (e.g., C. difficile) [161,162]. Some lactobacilli species, such as Lactobacillus plantarum, have been found to be able to grow in iron-restricted media [163]. Lactobacillus sakei has shown a complete machinery for iron sequestration and metabolism but does not require complete dependence on iron to grow; although, the iron sources enhance its survival rate [164]. Some Bifidobacteria strains antagonize Salmonella Typhimurium and pathogenic E. coli in vitro by sequestering and subtracting the iron from their growth [165]. The non-pathogenic E. coli strain Nissle utilizes an LCN2-resistant salmochelin to acquire iron and in such a way that it is able to compete with Salmonella [166,167]. Despite these observations, the studies on the changes in the composition of gut microbiota following iron supplementation are insufficient and do not allow for whole generalization. In many studies, iron supplementation has been reported to either to increase, reduce, or have no effect on the different microbiota genera [168,169,170], according to the previously used models. However, consistent data are available to date regarding mostly Lactobacillaceae and Bifidobacteriaceae, which decrease upon iron supplementation [159,160,169,171]. In this context, the presence of polyphenols in the gut environment could change the iron availability for the gut bacterial species; although, in an iron-deprived environment, some pathogenic strains producing siderophores could benefit and scavenge from the polyphenol–iron complexes [171]. Apart from this possibility, polyphenols may act to prevent the iron uptake of bacteria, therefore dampening the overgrowth of the intestinal bacteria. This could be particularly important in the case of inflammatory conditions that are affected by dysbiosis, as in IBD; however, future studies will be required in order to understand if a polyphenol-enriched diet may be integrated with probiotic supplements to better support the growth of the correct microbiota. In this regard, it is important to underline that microbial metabolites have a crucial role in intestinal pathogenesis; however, recent evidence has shown that they are also involved in host iron absorption and metabolism. Butyrate, diaminopropionate, and reuterin can act on the duodenal enterocytes to reduce iron absorption through HIF-2α activity. Moreover, these metabolites reduce the expression of intracellular iron storage proteins, such as ferritin. These metabolites are known to be produced by bacteria of the Lactobacilli genus, and others, and are part of the short-chain fatty acids (SCFAs) class. Thus, they can have a dual role in the host. They can reduce and modulate excessive inflammatory responses and, at the same time, affect iron absorption. Interestingly, this can potentially be employed in probiotic formulations that are used to treat iron overload, or in hemochromatosis to reduce body iron concentrations and excessive inflammation [172].
Iron might have a role in the modulating effects of bacterial-derived SCFAs [173]. The use of an iron-deficient diet in rats reduced butyrate- and propionate-producing bacteria compared to the control animals [174], which might be due to the need for iron by the enzymes that are involved in SCFAs synthesis. Even though the iron levels need to be kept low in order to not incur excessive inflammation, it is also necessary to feed enough of this microelement to the “good” bacteria, since they can also chelate it and reduce its availability to the pathogenic species. Streptococcaceae, Pesteurellaceae, and Methanobacteriaceae were found to be involved in the metabolism of histidine from imidazole propionate, and this, in turn, impairs the insulin signaling in the liver and the adipose tissue [175].
In general, iron and microbiota share a role in modulating the host’s immune system and physiology; microbes fight over iron ions to grow, stealing them from each other and from the enterocytes, while becoming deprived of the host through LCN2 and lactoferrin. While an excess in iron concentration can favor the growth of pathogenic bacteria and induce the onset of several diseases, the right amount of this ion is necessary for eubiotic bacteria for their production of metabolites that are beneficial to the host. In this interplay, polyphenols intervene to chelate more iron and reduce its bioavailability to the pathobionts. The iron is then metabolized by other bacteria that produce health-promoting molecules.
7. Polyphenols as Prebiotics—Chelating Iron to Select Beneficial Bacteria
The iron-chelating activity of polyphenols shapes the intestinal microbiota and reduces dysbiosis. The new populations that arise are beneficial to the host, as they can further boost the polyphenol anti-inflammatory effects through the production of polyphenol derivatives [176]. An example of this is the production of bacteria-derived metabolites, e.g., succinate by some genera of Lachnoclostridium, and this process has shown positive improvements in the healing of inflamed mucosa; additionally, polyunsaturated fatty acids can be produced by the intestinal bacteria [177]. These bacteria genera were often seen to increase after treatment with polyphenol-enriched diets.
To this extent, polyphenols and iron-chelating molecules can be employed as prebiotics, using their iron-depriving ability to reduce pathogenic bacteria, while, at the same time, favoring the thriving of beneficial species that use the unabsorbed polyphenols to produce other anti-inflammatory compounds [178].
Over time, many bacteria genera have been studied in order to observe how they metabolize polyphenols and what they can produce. Lactobacilli can use mulberry polyphenols to produce chlorogenic, caffeic, and ferulic acid metabolites [179]; Bacteroides can convert rutin to quercetin [180]; and Bifidobacteria can also use sea buckthorn to synthetize caffeic acid [181]. Firmicutes of the Eubacterium, Clostridium, and Flavonifractor genera can metabolize flavonols to obtain SCFAs and their derivatives [182]. Many other bacteria genera have already been tested in vitro in order to observe what pathways are involved in the metabolism of polyphenols and what enzymes could be employed directly to convert the natural molecules into more powerful bioactive compounds. This research area is critical in the production of new, healthier prebiotic formulations that can be commercialized in the next years [183,184]. Other bacteria of the Akkermansia genus were found to be able to metabolize anthocyanins and increase insulin sensitivity [185], and fecal microbial enzymes are able to catalyze polyphenol digestion. Bacteria can break phenolic rings and demethylate and dehydroxylate polyphenols in order to obtain smaller acids and aldehydes.
Furthermore, the gut microbiota transforms ellagic acid into urolithins [186]. They can also act on dietary lignans to produce molecules such as enterodiol and enterolactone through a series of reactions that are orchestrated by several bacterial communities [187]. While this is only the tip of the iceberg, new studies are needed in order to discover more bacteria, ideally at a species or strain level, that can transform and boost polyphenol activity and suppress dysregulated inflammation.
Finally, polyphenols’ anti-inflammatory role has been demonstrated by numerous reports, with most of them reporting their ability to suppress IL-6 secretion. The axis between IL-6 [129,130,188,189] and the hepatic production of several iron-related acute-phase proteins has been previously discussed [29]. Thus, polyphenols may have a dual role, supporting homeostasis during healthy periods and dampening inflammation and dysbiosis during chronic inflammation.
8. Conclusions
The iron-chelating abilities of polyphenols can help to explain, at least in part, their antioxidant and anti-inflammatory properties. Such iron-binding properties can also be involved in anti-tumorigenic activities, since polyphenols can reduce the iron availability for cancer cells and have cytotoxic activity towards cancer cells by behaving as pro-oxidants through iron–Fenton reactions [63,65,190,191,192]. Although the presence of specific chelating sites in the polyphenol structure has been reported to be important to explicate their iron-chelation activity, we should take into account that these phytochemicals are deeply transformed (i.e., glycosylated, methylated, acylated, hydroxylated, etc.) before their storage in the plant tissues. Therefore, the iron-chelating properties require in vivo conditions that are favorable to set free the chelating sites. Our need of further studies to clarify the axis between polyphenol–iron-metabolism-inflammatory mediators, and the interplay between polyphenols, iron availability, and microbiota, is clear from this review. Such studies are complicated by the study design and the data interpretation since they are strongly dependent from the adopted model, the iron status, and the iron form (non-heme/heme), and because the interaction between the host, the iron metabolism, and the bacterial iron regulation is not always predictable. Targeting bacteria-specific iron uptake, promoting commensal bacteria, and optimizing iron availability for the host may improve therapeutic and nutritional approaches in the context of gut inflammatory diseases.
Author Contributions
Conceptualization, A.S. (Aurelia Scarano), B.L., F.B., S.D.S., G.V., A.S. (Angelo Santino) and M.C.; writing—original draft preparation, A.S. (Aurelia Scarano), B.L., F.B., S.D.S., G.V., A.S. (Angelo Santino) and M.C.; writing—review and editing, A.S. (Aurelia Scarano), B.L., F.B., S.D.S., G.V., A.S. (Angelo Santino) and M.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Laddomada B., Colella G., Tufariello M., Durante M., Angiuli M., Salvetti G., Mita G. Application of a simplified calorimetric assay for the evaluation of extra virgin olive oil quality. Food Res. Int. 2013;54:2062–2068. doi: 10.1016/j.foodres.2013.05.035. [DOI] [Google Scholar]
- 2.Pasqualone A., Summo C., Laddomada B., Mudura E., Coldea T.E. Effect of processing variables on the physico-chemical characteristics and aroma of borş, a traditional beverage derived from wheat bran. Food Chem. 2018;265:242–252. doi: 10.1016/j.foodchem.2018.05.095. [DOI] [PubMed] [Google Scholar]
- 3.Bonanno A., Di Grigoli A., Todaro M., Alabiso M., Vitale F., Di Trana A., Giorgio D., Settanni L., Gaglio R., Laddomada B., et al. Improvement of oxidative status, milk and cheese production, and food sustainability indexes by addition of durum wheat bran to dairy cows’ diet. Animals. 2019;9:698. doi: 10.3390/ani9090698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarano A., Chieppa M., Santino A. Looking at flavonoid biodiversity in horticultural crops: A colored mine with nutritional benefits. Plants. 2018;7:98. doi: 10.3390/plants7040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarano A., Chieppa M., Santino A. Plant polyphenols-biofortified foods as a novel tool for the prevention of human gut diseases. Antioxidants. 2020;9:1225. doi: 10.3390/antiox9121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laddomada B., Blanco A., Mita G., D’Amico L., Singh R.P., Ammar K., Crossa J., Guzmán C. Drought and Heat Stress Impacts on Phenolic Acids Accumulation in Durum Wheat Cultivars. Foods. 2021;10:2142. doi: 10.3390/foods10092142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speer H., D’Cunha N.M., Botek M., McKune A.J., Sergi D., Georgousopoulou E., Mellor D.D., Naumovski N. The effects of dietary polyphenols on circulating cardiovascular disease biomarkers and iron status: A systematic review. Nutr. Metab. Insight. 2019;12:1–12. doi: 10.1177/1178638819882739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch W. Dietary Polyphenols-Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients. 2019;11:1039. doi: 10.3390/nu11051039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Santis S., Clodoveo M.L., Cariello M., D’Amato G., Franchini C., Faienza M.F., Corbo F. Polyphenols and obesity prevention: Critical insights on molecular regulation, bioavailability and dose in preclinical and clinical settings. Crit. Rev. Food Sci. Nutr. 2021;61:1804–1826. doi: 10.1080/10408398.2020.1765736. [DOI] [PubMed] [Google Scholar]
- 11.Rudrapal M., Khairnar S.J., Khan J., Dukhyil A.B., Ansari M.A., Alomary M.N., Alshabrmi F.M., Palai S., Deb P.K., Devi R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022;13:806470. doi: 10.3389/fphar.2022.806470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delvecchio F.R., Vadrucci E., Cavalcanti E., De Santis S., Kunde D., Vacca M., Myers J., Allen F., Bianco G., Huang A.Y., et al. Polyphenol administration impairs T-cell proliferation by imprinting a distinct dendritic cell maturational profile. Eur. J. Immunol. 2015;45:2638–2649. doi: 10.1002/eji.201545679. [DOI] [PubMed] [Google Scholar]
- 13.Shakoor H., Feehan J., Apostolopoulos V., Platat C., Al Daheri A.S., Ali H.I., Ismail L.C., Bosevski M., Stojanovska L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients. 2021;13:728. doi: 10.3390/nu13030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patra S., Pradha B., Nayak R., Behera C., Das S., Patra S.K., Efferth T., Jena M., Bhutia S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine. 2021;90:153554. doi: 10.1016/j.phymed.2021.153554. [DOI] [PubMed] [Google Scholar]
- 15.Wan M.L.Y., Co V.A., El-Nezami H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021;61:690–711. doi: 10.1080/10408398.2020.1744512. [DOI] [PubMed] [Google Scholar]
- 16.Hare D., Ayton S., Bush A., Lei P. A delicate balance: Iron metabolism and diseases of the brain. Front. Aging Neurosci. 2013;5:34. doi: 10.3389/fnagi.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan F.S. Iron deficiency anemia due to excessive green tea drinking. Clin. Case Rep. 2016;4:1053–1056. doi: 10.1002/ccr3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mobarra N., Shanaki M., Ehteram H., Nasiri H., Sahmani M., Saeidi M., Goudarzi M., Pourkarim H., Azad M. A review on iron chelators in treatment of iron overload syndromes. Int. J. Hematol. Oncol. Stem Cell Res. 2016;10:239–247. [PMC free article] [PubMed] [Google Scholar]
- 19.Aravind S.M., Wichienchot S., Tsao R., Ramakrishnan S., Chakkaravarthi S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021;142:110189. doi: 10.1016/j.foodres.2021.110189. [DOI] [PubMed] [Google Scholar]
- 20.Nazzaro F., Fratianni F., De Feo V., Battistelli A., Gomes Da Cruz A., Coppola R. Polyphenols, the new frontiers of prebiotics. In: Gomes da Cruz A., Prudencio Schwinden E., Almeida Esmerino E., da Silva M.C., editors. Advances in Food and Nutrition Research. 1st ed. Volume 94. Elsevier; Amsterdam, The Netherlands: 2020. pp. 35–89. [DOI] [PubMed] [Google Scholar]
- 21.Naranjo-Arcos M.A., Bauer P. Iron Nutrition, Oxidative Stress, and Pathogen Defense. In: Erkekoglu P., Kocer-Gumusel B., editors. Nutritional Deficiency. InTechOpen; London, UK: 2016. pp. 63–98. [DOI] [Google Scholar]
- 22.Hentze M.W., Muckenthaler M.U., Andrews N.C. Balancing Acts: Molecular Control Of Mammalian Iron Metabolism. Cell. 2004;117:285–297. doi: 10.1016/S0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 23.Ma Q., Kim E.-Y., Lindsay A., Han O. Bioactive dietary polyphenols inhibit heme iron absorption in a dose-dependent manner in human intestinal Caco-2 cells. J. Food Sci. 2011;76:H143–H150. doi: 10.1111/j.1750-3841.2011.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verna G., Sila A., Liso M., Mastronardi M., Chieppa M., Cena H., Campiglia P. Iron-enriched nutritional supplements for the 2030 pharmacy shelves. Nutrients. 2021;13:378. doi: 10.3390/nu13020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chieppa M., Giannelli G. Immune cells and microbiota response to iron starvation. Front. Med. 2018;5:109. doi: 10.3389/fmed.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck K.L., Conlon C.A., Kruger R., Coad J. Dietary determinants of and possible solutions to iron deficiency for young women living in industrialized countries: A review. Nutrients. 2014;6:3747–3776. doi: 10.3390/nu6093747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briguglio M., Hrelia S., Malaguti M., Lombardi G., Riso P., Porrini M., Perazzo P., Banfi G. The central role of iron in human nutrition: From folk to contemporary medicine. Nutrients. 2020;12:1761. doi: 10.3390/nu12061761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Paola A., Tortora C., Argenziano M., Marrapodi M.M., Rossi F. Emerging roles of the iron chelators in inflammation. Int. J. Mol. Sci. 2022;23:7977. doi: 10.3390/ijms23147977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nemeth E., Ganz T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 30.Anderson G.J. Mechanisms of iron loading and toxicity. Am. J. Hematol. 2007;82((Suppl. 12)):1128–1131. doi: 10.1002/ajh.21075. [DOI] [PubMed] [Google Scholar]
- 31.Beckman J.S., Beckman T.W., Chen J., Marshall P.A., Freeman B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C.B., Rahu N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Graft-Johnson J., Nowak D. Effect of selected plant phenolics on Fe2+-EDTA-H2O2 system mediated deoxyribose oxidation: Molecular structure-derived relationships of anti- and pro-oxidant actions. Molecules. 2017;22:59. doi: 10.3390/molecules22010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laughton M.J., Halliwell B., Evans P.J., Hoult J.R. Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin: Effects on lipid peroxidation, hydroxyl radical generation and bleomycin-dependent damage to DNA. Biochem. Pharmacol. 1989;38:2859–2865. doi: 10.1016/0006-2952(89)90442-5. [DOI] [PubMed] [Google Scholar]
- 37.Makácová K., Mladěnka P., Filipský T., Říha M., Jahodár L., Trejtnar F., Bovicelli P., Proietti Silvestri I., Hrdina R., Saso L. Iron reduction potentiates hydroxyl radical formation only in flavonols. Food Chem. 2012;135:2584–2592. doi: 10.1016/j.foodchem.2012.06.107. [DOI] [PubMed] [Google Scholar]
- 38.Yen G.C., Duh P.D., Tsai H.L., Huang S.L. Pro-oxidative properties of flavonoids in human lymphocytes. Biosci. Biotechnol. Biochem. 2003;67:1215–1222. doi: 10.1271/bbb.67.1215. [DOI] [PubMed] [Google Scholar]
- 39.Heim K.E., Tagliaferro A.R., Bobilya D.J. Flvaonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002;13:572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 40.Hodnick W.F., Kung F.S., Bohmont C.W., Pardini R.S. Inhibition of mitochondrial respiration and production of toxic oxygen radicals by flavonoids. A structure-activity study. Biochem. Pharmacol. 1986;35:2345–2357. doi: 10.1016/0006-2952(86)90461-2. [DOI] [PubMed] [Google Scholar]
- 41.Procházková D., Boušová I., Wilhemová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Puppo A. Effect of flavonoids on hydroxyl radical formation by Fenton-type reactions; influence of the iron chelator. Phytochemistry. 1992;31:85–88. doi: 10.1016/0031-9422(91)83011-9. [DOI] [Google Scholar]
- 43.Moran J.F., Klucas R.V., Grayer R.J., Abian J., Becana M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic. Biol. Med. 1997;22:861–870. doi: 10.1016/S0891-5849(96)00426-1. [DOI] [PubMed] [Google Scholar]
- 44.Inoue S., Ito K., Yamamoto K., Kawanishi S. Caffeic acid causes metal-dependent damage to cellular and isolated DNA through H2O2 formation. Carcinogenesis. 1992;13:1497–1502. doi: 10.1093/carcin/13.9.1497. [DOI] [PubMed] [Google Scholar]
- 45.Dalvi L.T., Moreira D.C., Andrade Jr R., Ginani J., Alonso A., Hermes-Lima M. Ellagic acid inhibits iron-mediated free radical formation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017;173:910–917. doi: 10.1016/j.saa.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 46.Bradshaw P.C. Cytoplasmic and mitochondrial NADPH-coupled redox systems in the regulation of aging. Nutrients. 2019;11:504. doi: 10.3390/nu11030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marì M., de Gregorio E., de Dios E., Roca-Agujetas V., Cucarull B., Tutusaus A., Morales A., Colell A. Mitochondrial glutathione: Recent insights and role in disease. Antioxidants. 2020;9:909. doi: 10.3390/antiox9100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhitkovich A. Nuclear and cytoplasmic functions of vitamin C. Chem. Res. Toxicol. 2020;33:2515–2526. doi: 10.1021/acs.chemrestox.0c00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perron N.R., Brumaghim J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 50.Romano G., Del Coco L., Milano F., Durante M., Palombieri S., Sestili F., Visioni A., Abderrazek J., Fanizzi F.P., Laddomada B. Phytochemical Profiling and Untargeted Metabolite Fingerprinting of the MEDWHEALTH Wheat, Barley and Lentil Whole-Meal Flours. Foods. 2022;11:4070. doi: 10.3390/foods11244070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kehrer J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/S0300-483X(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 52.Henle E.S., Han Z., Tang N., Rai P., Luo Y., Linn S. Sequence-specific DNA cleavage by Fe2+-mediated Fenton reaction has possible biological implications. J. Biol. Chem. 1999;274:962–971. doi: 10.1074/jbc.274.2.962. [DOI] [PubMed] [Google Scholar]
- 53.Andjelković M., Camp J.V., De Meulenaer B., Depaemelaere G., Socaciu C., Verloo M., Verhe R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006;98:23–31. doi: 10.1016/j.foodchem.2005.05.044. [DOI] [Google Scholar]
- 54.Braakhuis A. Evidence on the health benefits of supplemental propolis. Nutrients. 2019;11:2705. doi: 10.3390/nu11112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X., Li Y., Han L., Li J., Liu C., Sun C. Role of flavonoids in the treatment of iron overload. Front. Cell Dev. Biol. 2021;9:685364. doi: 10.3389/fcell.2021.685364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kasprzak M.M., Erxleben A., Ochocki J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015;5:45853–45877. doi: 10.1039/C5RA05069C. [DOI] [Google Scholar]
- 57.Borges A., Abreu A., Dias C., Saavedra M., Borges F., Simões M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules. 2016;21:877. doi: 10.3390/molecules21070877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khalili M., Ebrahimzadeh M., Kosaryan M. In vivo iron-chelating activity and phenolic profiles of the Angel’s wings mushroom, Pleurotus porrigens (Higher Basidiomycetes) Int. J. Med. Mushrooms. 2015;17:847–856. doi: 10.1615/IntJMedMushrooms.v17.i9.50. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez M.T., Mira M.L., Florêncio M.H., Jennings K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002;92:105–111. doi: 10.1016/S0162-0134(02)00511-1. [DOI] [PubMed] [Google Scholar]
- 60.Galano A., Marquez M.F., Pérez-González A. Ellagic acid: An unusually versatile protector against oxidative stress. Chem. Res. Toxicol. 2014;27:904–918. doi: 10.1021/tx500065y. [DOI] [PubMed] [Google Scholar]
- 61.Alfei S., Marengo B., Zuccari G. Oxidative stress, antioxidant capabilities and bioavailability: Ellagic acid or urolithins? Antioxidants. 2020;9:707. doi: 10.3390/antiox9080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Messner D.J., Sivam G., Kowdley K.V. Curcumin reduces the toxic effects of iron loading in rat liver epithelial cells. Liver Int. 2009;29:63–72. doi: 10.1111/j.1478-3231.2008.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rainey N.E., Moustapha A., Saric A., Nicolas G., Sureau F., Petit P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019;5:150. doi: 10.1038/s41420-019-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiao Y., Wilkinson IV J., Pietsch E.C., Buss J.L., Wan W., Planalp R., Torti F.M., Torti S.V. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006;40:1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Jiao Y., Wilkinson IV J., Di X., Wang W., Hatcher H., Kock N.D., D’Agostino Jr R., Knovich M.A., Torti F.M., Torti S.V. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood. 2009;113:462–469. doi: 10.1182/blood-2008-05-155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karlíčková J., Macáková K., Říha M., Teixeira Pinheiro L.M., Filipský T., Horňasová V., Hrdina R., Mladěnka P. Isoflavones reduce copper with minimal impact on iron in vitro. Oxid. Med. Cell Longev. 2015;2015:437381. doi: 10.1155/2015/437381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dowling S., Regan F., Hughes H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010;104:1091–1098. doi: 10.1016/j.jinorgbio.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Lakey-Beitia J., Burillo A.M., La Penna G., Hedge M.L., Rao K.S. Polyphenols as potential metal chelation compounds against Alzeimer’s disease. J. Alzheimer’s Dis. 2021;82:S335–S357. doi: 10.3233/JAD-200185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng I.F., Breen K. On the ability of four flavonoids, baicilein, luteolin, naringenin, and quercetin, to suppress the Fenton reaction of the iron-ATP complex. BioMetals. 2000;13:77–83. doi: 10.1023/A:1009229429250. [DOI] [PubMed] [Google Scholar]
- 70.Perron N.R., Hodges J.N., Jenkins M., Brumaghim J.L. Predicting how polyphenol antioxidants prevent DNA damage by binding to iron. Inorg. Chem. 2008;47:6153–6161. doi: 10.1021/ic7022727. [DOI] [PubMed] [Google Scholar]
- 71.Malacaria L., Corrente G.A., Beneduci A., Furia E., Marino T., Mazzone G. A review on coordination properties of Al(III) and Fe(III) toward natural antioxidant molecules: Experimental and theoretical insights. Molecules. 2021;26:2603. doi: 10.3390/molecules26092603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pannala A.S., Chan T.S., O’Brien P.J., Rice-Evans C.A. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001;282:1161–1168. doi: 10.1006/bbrc.2001.4705. [DOI] [PubMed] [Google Scholar]
- 73.Fiuza S.M., Van Besien E., Milhazes N., Borges F., Marques M.P.M. Conformational analysis of a trihydroxylated derivative of cinnamic acid—A combined Raman spectroscopy and ab initio study. J. Mol. Struct. 2004;693:103–118. doi: 10.1016/j.molstruc.2004.02.019. [DOI] [Google Scholar]
- 74.Mladěnka P., Macáková K., Filipský T., Zatloukalová L., Jahodář L., Bovicelli P., Silvestri I.P., Hrdina R., Saso L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011;105:693–701. doi: 10.1016/j.jinorgbio.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 75.McGhie T.K., Walton M.C. the bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007;51:702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- 76.Scarano A., Chieppa M., Santino A. 2022. Microbiota as a metabolic organ processing dietary polyphenols. In: Glibetic M., editor. Comprehensive Gut Microbiota. Volume 1. Elsevier; Amsterdam, The Netherlands: 2022. pp. 20–26. [DOI] [Google Scholar]
- 77.Marín L., Miguélez E.M., Villar C.J., Lombó F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015;2015:95215. doi: 10.1155/2015/905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hussain M.B., Hassan S., Waheed M., Javed A., Farooq M.A., Tahir A. Bioavailability and metabolic pathway of phenolic compounds. In: Soto-Hernández M., García-Mateos R., Palma-Tenango M., editors. Plant Physiological Aspects of Phenolic Compounds. InTechOpen; London, UK: 2019. [DOI] [Google Scholar]
- 79.Lomozová Z., Catapano M.C., Hrubša M., Karlíčkova J., Macáková K., Kučera R., Mladěnka P. Chelation of iron and copper by quercetin B-ring methyl metabolites, isorhamnetin and tamarixetin, and their effect on metal-based Fenton chemistry. J. Agric. Food Chem. 2021;69:5926–5937. doi: 10.1021/acs.jafc.1c01729. [DOI] [PubMed] [Google Scholar]
- 80.Saito A., Sugisawa A., Umegaki K., Sunagawa H. Protective effects of quercetin and its metabolites on H2O2-induced chromosomal damage to WIL2-NS cells. Biosci. Biotechnol. Biochem. 2004;68:271–276. doi: 10.1271/bbb.68.271. [DOI] [PubMed] [Google Scholar]
- 81.Mosele J.I., Macià A., Motilva M.J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules. 2015;20:17429–17468. doi: 10.3390/molecules200917429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Catalkava G., Venema K., Lucini L., Rocchetti G., Delmas D., Daglia M., De Filippis A., Xiao H., Quiles J.L., Capanoglu E. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020;1:109–133. doi: 10.1002/fft2.25. [DOI] [Google Scholar]
- 83.Hai Y., Zhang Y., Liang Y., Ma X., Qi X., Xiao J., Xue W., Luo Y., Yue T. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives. Food Front. 2020;1:420–434. doi: 10.1002/fft2.50. [DOI] [Google Scholar]
- 84.Holland W., Brett G.M., Radreau P., Saha S., Teucher B., Bennet R., Kroon P. Processing blackcurrants dramatically reduces the content and does not enhance urinary yield of anthocyanins in human subjects. Food Chem. 2008;108:869–878. doi: 10.1016/j.foodchem.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 85.Li H., Li M., Fu J., Ao H., Wang W., Wang X. Enhancement of oral bioavailability of quercetin by metabolic inhibitory nanosuspensions compared to conventional nanosuspensions. Drug Deliv. 2021;28:1226–1236. doi: 10.1080/10717544.2021.1927244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kasikci M.B., Bagdatlioglu N. Bioavailability of quercetin. Curr. Res. Nutr. Food Sci. 2016;4:146–151. doi: 10.12944/CRNFSJ.4.Special-Issue-October.20. [DOI] [Google Scholar]
- 87.Crespy V., Morand C., Manach C., Besson C., Demigne C., Remesy C. Part of quercetin absorbed in the small intestine is conjugated and further secreted in the intestinal lumen. Am. J. Physiol. 1999;277:G120–G126. doi: 10.1152/ajpgi.1999.277.1.G120. [DOI] [PubMed] [Google Scholar]
- 88.Matsukawa N., Matsumoto M., Hara H. High biliary excretion levels of quercetin metabolites after administration of a quercetin glycoside in conscious bile duct cannulated rats. Biosci. Biotechnol. Biochem. 2009;73:1863–1865. doi: 10.1271/bbb.90031. [DOI] [PubMed] [Google Scholar]
- 89.Lee J.H., Oh J.-H., Lee Y.-J. Biliary excretion of curcumin is mediated by multidrug resistance-associated protein 2. Biol. Pharm. Bull. 2012;35:777–780. doi: 10.1248/bpb.35.777. [DOI] [PubMed] [Google Scholar]
- 90.Prasad S., Tyagi A.K., Aggarwal B.B. Recent development in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014;46:2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Teng Z., Yuan C., Zhang F., Huan M., Cao W., Li K., Yang J., Cao D., Zhou S., Mei Q. Intestinal absorption and first-pass metabolism of polyphenol compounds in rat and their transport dynamics in Caco-2 cells. PLoS ONE. 2012;7:e29647. doi: 10.1371/journal.pone.0029647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen C., Chen R., Qian Z., Meng X., Hu T., Li Y., Chen Z., Huang C., Hu C., Li J. Intestinal absorption mechanisms of MTBH, a novel hesperetin derivative, in Caco-2 cells, and potential involvement of monocarboxylate transporter 1 and multidrug resistance protein 2. Eur. J. Pharm. Sci. 2015;78:214–224. doi: 10.1016/j.ejps.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 93.Henry C., Vitrac X., Decendit A., Ennamany R., Krisa S., Merillon J.-M. Cellular uptake and efflux of trans-piceid and its aglycone trans-resveratrol on the apical membrane of human intestinal Caco-2 cells. J. Agric. Food Chem. 2005;53:798–803. doi: 10.1021/jf048909e. [DOI] [PubMed] [Google Scholar]
- 94.Graefe E.U., Witting J., Pharm D., Mueller S., Riethling A.-K., Uehleke B., Drewelow B., Pforte H., Jacobasch G., Derendorf H. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001;41:492–499. doi: 10.1177/00912700122010366. [DOI] [PubMed] [Google Scholar]
- 95.Mullen W., Edwards C.A., Crozier A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006;96:107–116. doi: 10.1079/BJN20061809. [DOI] [PubMed] [Google Scholar]
- 96.Mülleder U., Murkovic M., Pfannhauser W. Urinary excretion of cyanidin glycosides. J. Biochem. Biophys. Methods. 2002;53:61–66. doi: 10.1016/S0165-022X(02)00093-3. [DOI] [PubMed] [Google Scholar]
- 97.Ludwig I.A., Mena P., Calani L., Borges G., Pereira-Caro G., Bresciani L., Del Rio D., Lean M.E., Crozier A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015;89:758–769. doi: 10.1016/j.freeradbiomed.2015.10.400. [DOI] [PubMed] [Google Scholar]
- 98.Sergides C., Chirilă M., Silvestro L., Pitta D., Pittas A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016;11:164–170. doi: 10.3892/etm.2015.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stalmach A., Williamson G., Crozier A. Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct. 2014;5:1727–1737. doi: 10.1039/C4FO00316K. [DOI] [PubMed] [Google Scholar]
- 100.Schiborr C., Kocher A., Behnam D., Jandasek J., Toelstede S., Frank J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014;58:516–527. doi: 10.1002/mnfr.201300724. [DOI] [PubMed] [Google Scholar]
- 101.Larrosa M., Yanez-Gascon M.J., Selma V.M., González-Sarrías A., Toti S., Cerón J.J., Tomás-Barberán F., Dolara P., Espín J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009;57:2211–2220. doi: 10.1021/jf803638d. [DOI] [PubMed] [Google Scholar]
- 102.Cavalcanti E., Vadrucci E., Delvecchio F.R., Addabbo F., Bettini S., Liou R., Monsurrò V., Huang A.Y.C., Pizzaro T.T., Santino A., et al. Administration of reconstituted polyphenol oil bodies efficiently suppresses dendritic cell inflammatory pathways and acute intestinal inflammation. PLoS ONE. 2013;9:e88898. doi: 10.1371/journal.pone.0088898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liso M., Sila A., Verna G., Scarano A., Donghia R., Castellana F., Cavalcanti E., Pesole P.L., Sommella E.M., Lippolis A., et al. Nutritional regimes enriched with antioxidants as an efficient adjuvant for IBD patients under infliximab administration, a pilot study. Antioxidants. 2022;11:1380. doi: 10.3390/antiox11010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-kappaB activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 105.Vendrame S., Klimis-Zacas D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-kB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015;73:348–358. doi: 10.1093/nutrit/nuu066. [DOI] [PubMed] [Google Scholar]
- 106.D’Introno A., Paradiso A., Scoditti E., D’Amico L., De Paolis A., Carluccio M.A., Nicoletti N., De Gara L., Santino A., Giovinazzo G. Antioxidant and anti-inflammatory properties of tomato fruit synthesizing different amounts of stilbenes. Plant Biotech. J. 2009;7:422–429. doi: 10.1111/j.1467-7652.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- 107.Serra D., Paixão J., Nunes C., Dinis T.C.P., Almeida L.M. Cyanidin-3-glucoside suppresses cytokine-induced inflammatory response in human intestinal cells: Comparison with 5- aminosalicylic acid. PLoS ONE. 2013;8:e73001-10. doi: 10.1371/journal.pone.0073001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Calabriso N., Massaro M., Scoditti E., Pasqualone A., Laddomada B., Carluccio M.A. Phenolic extracts from whole wheat biofortified bread dampen overwhelming 1 inflammatory response in human endothelial cells and monocytes: Major role of VCAM-1 and CXCL-10. Eur. J. Nutr. 2020;59:2603–2615. doi: 10.1007/s00394-019-02109-y. [DOI] [PubMed] [Google Scholar]
- 109.Calabriso N., Massaro M., Scoditti E., Pellegrino M., Ingrosso I., Giovinazzo G., Carluccio M.A. Red grape skin polyphenols blunt matrix metalloproteinase-2 and -9 activity and expression in cell models of vascular inflammation: Protective role in degenerative and inflammatory diseases. Molecules. 2016;21:1147. doi: 10.3390/molecules21091147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Calabriso N., Scoditti E., Massaro M., Pellegrino M., Storelli C., Ingrosso I., Giovinazzo G., Carluccio M.A. Multiple anti-inflammatory and anti-atherosclerotic properties of red wine polyphenolic extracts: Different role of hydroxycinnamic acids, flavonols and stilbenes on endothelial inflammatory gene expression. Eur. J. Nutr. 2016;55:477–489. doi: 10.1007/s00394-015-0865-6. [DOI] [PubMed] [Google Scholar]
- 111.Calabriso N., Scoditti E., Massaro M., Maffia M., Chieppa M., Laddomada B., Carluccio M.A. Non-Celiac Gluten Sensitivity and Protective Role of Dietary Polyphenols. Nutrients. 2022;14:2679. doi: 10.3390/nu14132679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nairz M., Therl I., Swirski F.K., Weiss G. “Pumping iron”—How macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflugers. Arch. 2017;469:397–418. doi: 10.1007/s00424-017-1944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Recalcati S., Cairo G. Macrophages and iron: A special relationship. Biomedicines. 2021;9:1585. doi: 10.3390/biomedicines9111585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ganz T. Macrophages and systemic iron homeostasis. J. Innate Immun. 2012;4:446–453. doi: 10.1159/000336423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drakesmith H., Nemeth E., Ganz T. Ironing out Ferroportin. Cell Metab. 2015;22:777–787. doi: 10.1016/j.cmet.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gammella E., Correnti M., Cairo G., Recalcati S. Iron Availability in Tissue Microenvironment: The Key Role of Ferroportin. Int. J. Mol. Sci. 2021;22:2986. doi: 10.3390/ijms22062986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sica A., Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lawrence T., Natoli G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 119.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nairz M., Schroll A., Sonnweber T., Weiss G. The struggle for iron—A metal at the host-pathogen interface. Cell Microbiol. 2010;12:1691–1702. doi: 10.1111/j.1462-5822.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 121.Agoro R., Taleb M., Quesniaux V.F.J., Mura C. Cell iron status influences macrophage polarization. PLoS ONE. 2018;13:e0196921. doi: 10.1371/journal.pone.0196921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ni S., Yuan Y., Kuang Y., Li X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022;13:816282. doi: 10.3389/fimmu.2022.816282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Agoro R., Mura C. Inflammation-induced up-regulation of hepcidin and down-regulation of ferroportin transcription are dependent on macrophage polarization. Blood Cells Mol. Dis. 2016;61:16–25. doi: 10.1016/j.bcmd.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 124.Galleggiante V., De Santis S., Cavalcanti E., Scarano A., De Benedictis M., Serino G., Caruso M.L., Mastronardi M., Pinto A., Campiglia P., et al. Dendritic cells modulate iron homeostasis and inflammatory abilities following quercetin exposure. Curr. Pharm. Des. 2017;23:2139–2146. doi: 10.2174/1381612823666170112125355. [DOI] [PubMed] [Google Scholar]
- 125.Verna G., Liso M., De Santis S., Dicarlo M., Cavalcanti E., Crovace A., Sila A., Campiglia P., Santino A., Lippolis A., et al. Iron Overload Mimicking Conditions Skews Bone Marrow Dendritic Cells Differentiation into MHCIIlowCD11c+CD11b+F4/80+ Cells. Int. J. Mol. Sci. 2020;21:1353. doi: 10.3390/ijms21041353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De Santis S., Kunde D., Serino G., Galleggiante V., Caruso M.L., Mastronardi M., Cavalcanti E., Ranson N., Pinto A., Campiglia P., et al. Secretory leukoprotease inhibitor is required for efficient quercetin-mediated suppression of TNFα secretion. Oncotarget. 2016;7:75800–75809. doi: 10.18632/oncotarget.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Santis S., Galleggiante V., Scandiffio L., Liso M., Sommella E., Sobolewski A., Spilotro V., Pinto A., Campiglia P., Serino G., et al. Secretory Leukoprotease Inhibitor (Slpi) Expression Is Required for Educating Murine Dendritic Cells Inflammatory Response Following Quercetin Exposure. Nutrients. 2017;9:706. doi: 10.3390/nu9070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Du P., Song J., Qiu H., Liu H., Zhang L., Zhou J., Jiang S., Liu J., Zheng Y., Wang M. Polyphenols Extracted from Shanxi-Aged Vinegar Inhibit Inflammation in LPS-Induced RAW264.7 Macrophages and ICR Mice via the Suppression of MAPK/NF-κB Pathway Activation. Molecules. 2021;26:2745. doi: 10.3390/molecules26092745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Santis S., Liso M., Verna G., Curci F., Milani G., Faienza M.F., Franchini C., Moschetta A., Chieppa M., Clodoveo M.L., et al. Extra Virgin Olive Oil Extracts Modulate the Inflammatory Ability of Murine Dendritic Cells Based on Their Polyphenols Pattern: Correlation between Chemical Composition and Biological Function. Antioxidants. 2021;10:1016. doi: 10.3390/antiox10071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Santis S., Crupi P., Piacente L., Mestice A., Colabufo N.A., Amodio L., Pontrelli P., Gesualdo L., Moschetta A., Clodoveo M.L., et al. Extra virgin olive oil extract rich in secoiridoids induces an anti-inflammatory profile in peripheral blood mononuclear cells from obese children. Front. Nutr. 2022;9:1017090. doi: 10.3389/fnut.2022.1017090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen L., Yang S., Zumbrun E.E., Guan H., Nagarkatti P.S., Nagarkatti M. Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages. Mol. Nutr. Food Res. 2015;59:853–864. doi: 10.1002/mnfr.201400819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tomay F., Marinelli A., Leoni V., Caccia C., Matros A., Mock H.-P., Tonelli C., Petroni K. Purple corn extract induces long-lasting reprogramming and M2 phenotypic switch of adipose tissue macrophages in obese mice. J. Transl. Med. 2019;17:237. doi: 10.1186/s12967-019-1972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shabani M., Sadeghi A., Hosseini H., Teimouri M., Khorzoughi R.B., Pasalar P., Meshkani R. Resveratrol alleviates obesity-induced skeletal muscle inflammation via decreasing M1 macrophage polarization and increasing the regulatory T cell population. Sci. Rep. 2020;10:3791. doi: 10.1038/s41598-020-60185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Y., Wan R., Peng W., Zhao X., Bai W., Hu C. Quercetin alleviates ferroptosis accompanied by reducing M1 macrophage polarization during neutrophilic airway inflammation. Eur. J. Pharmacol. 2022;938:175407. doi: 10.1016/j.ejphar.2022.175407. [DOI] [PubMed] [Google Scholar]
- 135.Peréz S., Rius-Peréz S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants. 2022;11:1394. doi: 10.3390/antiox11071394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Badria F.A., Ibrahim A.S., Badria A.F., Elmarakby A.A. Curcumin attenuates iron accumulation and oxidative stress on the liver and spleen of chronic iron-overload rats. PLoS ONE. 2015;10:e134156. doi: 10.1371/journal.pone.0134156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hart J., Tako E., Kochian L., Glahn R. Identification of Black Bean (Phaseolus vulgaris L.) Polyphenols that inhibit and promote iron uptake by Caco-2 cells. J. Agric. Food Chem. 2015;63:5950–5956. doi: 10.1021/acs.jafc.5b00531. [DOI] [PubMed] [Google Scholar]
- 138.Wang B., Zhong Y., Gao C., Li J. Myricetin ameliorates scopolamine induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem. Biophys. Res. Commun. 2017;490:336–342. doi: 10.1016/j.bbrc.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Y., Li H., Zhao Y., Gao Z. Dietary supplementation of baicalin and quercetin attenuates iron overload induced mouse liver injury. Eur. J. Pharmacol. 2006;535:263–269. doi: 10.1016/j.ejphar.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 140.Franco S., De Falco L., Ghaffari S., Brugnara C., Sinclair D.A., Mattè A., Iolascon A., Mohandas N., Bertoldi M., An X., et al. Resveratrol accelerates erythroid maturation by activation of Foxo3 and ameliorates anemia beta-thalassemic mice. Haematologica. 2014;99:267–275. doi: 10.3324/haematol.2013.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mazhar M., Faizi S., Gul A., Kabir N., Simjee S.U. Effects of naturally occurring flavonoids on ferroportin expression in the spleen in iron deficiency anemia in vivo. RSC Adv. 2017;7:23238–23245. doi: 10.1039/C7RA02138K. [DOI] [Google Scholar]
- 142.Wang H., Jiang C., Yang Y., Li J., Wang Y., Wang C., Gao Y. Resveratrol ameliorates iron overload induced liver fibrosis in mice by regulating iron homeostasis. PeerJ. 2022;10:e13592. doi: 10.7717/peerj.13592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mu M., An P., Wu Q., Shen X., Shao D., Wang H., Zhang Y., Zhang S., Yao H., Min J., et al. The dietary flavonoid myricetin regulates iron homeostasis by suppressing hepcidin expression. J. Nutr. Biochem. 2016;30:53–61. doi: 10.1016/j.jnutbio.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 144.Balogun E., Hoque M., Gong P., Killeen E., Green C., Foresti R., Alam J., Motterlini R. Curcumin activates the haem ovygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moretti D., Goede J.S., Zeder C., Jiskra M., Chatzinakou V., Tjalsma H., Melse-Boonstra A., Brittenham G., Swinkels D., Zimmermann M. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126:1981–1989. doi: 10.1182/blood-2015-05-642223. [DOI] [PubMed] [Google Scholar]
- 146.Ponce M.C., Polman K., Roos N., Wieringa F.T., Berger J., Doak C.M. What Approaches are Most Effective at Addressing Micronutrient Deficiency in Children 0-5 Years? A Review of Systematic Reviews. Matern. Child. Health J. 2019;23((Suppl. 1)):4–17. doi: 10.1007/s10995-018-2527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Singh V., Galla S., Golonka R.M., Patterson A.D., Chassaing B., Joe B., Vijay-Kumar M. Lipocalin 2 deficiency-induced gut microbiota dysbiosis evokes metabolic syndrome in aged mice. Physiol. Genom. 2020;52:314–321. doi: 10.1152/physiolgenomics.00118.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moschen A.R., Adolph T.E., Gerner R.R., Wieser V., Tilg H. Lipocalin-2: A master mediator of intestinal and metabolic inflammation. Trends Endocr. Metab. 2017;28:388–397. doi: 10.1016/j.tem.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 149.Lu F., Inoue K., Kato J., Minamishima S., Morisaki H. Functions and regulation of lipocalin-2 in gut-origin sepsis: A narrative review. Crit. Care. 2019;23:269. doi: 10.1186/s13054-019-2550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pi H., Helmann J.D. Ferrous iron efflux systems in bacteria. Metallomics. 2017;9:840–851. doi: 10.1039/C7MT00112F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kramer J., Özkaya Ö., Kümmerli R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020;18:152–163. doi: 10.1038/s41579-019-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gibson R.S.J.F. The role of diet- and host-related factors in nutrient bioavailability and thus in nutrient-based dietary requirement estimates. Food Nutr. Bull. 2007;28:S77–S100. doi: 10.1177/15648265070281S108. [DOI] [PubMed] [Google Scholar]
- 153.Seyoum Y., Baye K., Humblot C. Iron homeostasis in host and gut bacteria—A complex interrelationship. Gut Microbes. 2021;13:e1874855. doi: 10.1080/19490976.2021.1874855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012;23:174. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 155.De Santis S., Scarano A., Liso M., Calabrese F.M., Verna G., Cavalcanti E., Sila A., Lippolis A., De Angelis M., Santino A., et al. Polyphenols enriched diet administration during pregnancy and lactation prevents dysbiosis in ulcerative colitis predisposed littermates. Front. Cell Infect. Microbiol. 2021;11:622327. doi: 10.3389/fcimb.2021.622327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vacca M., Celano G., Lenucci M.S., Fontana S., Forgia F.M., Minervini F., Scarano A., Santino A., Dalfino G., Gesualdo L., et al. In vitro selection of probiotics, prebiotics, and antioxidants to develop an innovative symbiotic (NatuREN G) and testing its effect in reducing uremic toxins in fecal batches from CKD patients. Microorganisms. 2021;9:1316. doi: 10.3390/microorganisms9061316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kawabata K., Yoshioka Y., Terao J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules. 2019;24:370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Santino A., Scarano A., De Santis S., De Benedictis M., Giovinazzo G., Chieppa M. Gut microbiota modulation and anti-inflammatory properties of dietary polyphenols in IBD: New and consolidated perspectives. Curr. Pharm. Des. 2017;23:2344–2351. doi: 10.2174/1381612823666170207145420. [DOI] [PubMed] [Google Scholar]
- 159.Kortman G., Mulder M., Richters T., Shanmugam N., Trebicka E., Boekhorst J., Timmerman H., Roelofs R., Wiegerinck E., Laarakkers C., et al. Low dietary iron intake restrains the intestinal inflammatory response and pathology of enteric infection by food-borne bacterial pathogens. Eur. J. Immunol. 2015;45:2553–2567. doi: 10.1002/eji.201545642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kortman G., Dutilh B., Maathuis A., Engelke U., Boekhorst J., Keegan K., Nielsen F., Betley J., Weir J., Kingsbury Z., et al. Microbial metabolism shifts towards an adverse profile with supplementary iron in the TIM-2 in vitro model of the human colon. Front. Microbiol. 2016;6:1481. doi: 10.3389/fmicb.2015.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Runyen-Janecky L.J. Role and regulation of heme iron acquisition in gram-negative pathogens. Front. Cell Infect. Microbiol. 2013;3:55. doi: 10.3389/fcimb.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sousa Gerós A., Simmons A., Drakesmith H., Aulicino A., Frost J.N. The battle for iron in enteric infections. Immunology. 2020;161:186–199. doi: 10.1111/imm.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Archibald F. Lactobacillus Plantarum, an Organism Not Requiring Iron. FEMS Microbiol. Lett. 1983;19:29–32. doi: 10.1111/j.1574-6968.1983.tb00504.x. [DOI] [Google Scholar]
- 164.Duhutrel P., Bordat C., Wu T.-D., Zagorec M., GuerquinKern J.-L., Champomier-Vergès M.-C. Iron sources used by the nonpathogenic lactic acid bacterium Lactobacillus sakei as revealed by electron energy loss spectroscopy and secondary-ion mass spectrometry. Appl. Environ. Microbiol. 2010;76:560–565. doi: 10.1128/AEM.02205-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vazquez-Gutierrez P., de Wouters T., Werder J., Chassard C., Lacroix C. High iron-sequestrating bifidobacteria inhibit enteropathogen growth and adhesion to intestinal epithelial cells in vitro. Front. Microbiol. 2016;7:1480. doi: 10.3389/fmicb.2016.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Deriu E., Liu J., Pezeshki M., Edwards R., Ochoa R., Contreras H., Libby S., Fang F., Raffatellu M. Probiotic bacteria reduce Salmonella Typhimurium intestinal colonization by competing for iron. Cell Host Microbe. 2013;14:26–37. doi: 10.1016/j.chom.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Valdebenito M., Crumbliss A.L., Winkelmann G., Hantke K. Environmental factors influence the production of enterobactin, salmochelin, aerobactin, and yersiniabactin in Escherichia coli strain Nissle 1917. Int. J. Med. Microbiol. 2006;296:513–520. doi: 10.1016/j.ijmm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 168.Nitert M.D., Gomez-Arango L.F., Barrett H.L., McIntyre H.D., Anderson G.J., Frazer D.M., Callaway L. Iron supplementation has minor effects on gut microbiota composition in overweight and obese women in early pregnancy. Br. J. Nutr. 2018;120:283–289. doi: 10.1017/S0007114518001149. [DOI] [PubMed] [Google Scholar]
- 169.Jaeggi T., Kortman G.A., Moretti D., Chassard C., Holding P., Dostal A., Boekhorst J., Timmerman H.M., Swinkels D.W., Tjalsma H.J.G. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in kenyan infants. Gut. 2015;64:731–742. doi: 10.1136/gutjnl-2014-307720. [DOI] [PubMed] [Google Scholar]
- 170.Dostal A., Baumgartner J., Riesen N., Chassard C., Smuts C.M., Zimmermann M.B., Lacroix C. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: A randomised, placebo-controlled intervention trial in south African children. Br. J. Nutr. 2014;112:547–556. doi: 10.1017/S0007114514001160. [DOI] [PubMed] [Google Scholar]
- 171.Kortman G.A.M., Raffatellu M., Swinkels D.W., Tjalsma H. Nutritional iron turned inside out: Intestinal stress from a gut microbial perspective. FEMS Microbiol. Rev. 2014;38:1202–1234. doi: 10.1111/1574-6976.12086. [DOI] [PubMed] [Google Scholar]
- 172.Das N.K., Schwartz A.J., Barthel G., Inohara N., Liu Q., Sankar A., Hill D., Ma X., Lamberg O., Schnizlein M.K., et al. Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab. 2020;31:115–130.e6. doi: 10.1016/j.cmet.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mayneris-Perxachs J., Moreno-Navarrete J.M., Fernández-Real J. The role of iron in host–microbiota crosstalk and its effects on systemic glucose metabolism. Nat. Rev. Endocrinol. 2022;18:683–698. doi: 10.1038/s41574-022-00721-3. [DOI] [PubMed] [Google Scholar]
- 174.Dostal A., Chassard C., Hilty F.M., Zimmermsann M.B., Jaeggi T., Rossi S., Lacroix C. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J. Nutr. 2012;142:271–277. doi: 10.3945/jn.111.148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Koh A., Molinaro A., Stahlman M., Khan M.T., Schmidt C., Mannerås-Holm L., Wu H., Carreras A., Jeong H., Olofsson L.E., et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell. 2018;175:947–961.e17. doi: 10.1016/j.cell.2018.09.055. [DOI] [PubMed] [Google Scholar]
- 176.Li H., Christmas L.M., Li R., Gu L. Synergic interactions between polyphenols and gut microbiota in mitigating inflammatory bowel diseases. Food Funct. 2020;11:4878–4891. doi: 10.1039/D0FO00713G. [DOI] [PubMed] [Google Scholar]
- 177.Druart C., Bindels L.B., Schmaltz R., Neyrinck A.M., Cani P.D., Walter J., Ramer-Tait A.E., Delzenne N.M. Ability of the gut microbiota to produce PUFA-derived bacterial metabolites: Proof of concept in germ-free versus conventionalized mice. Mol. Nutr. Food Res. 2015;59:1603–1613. doi: 10.1002/mnfr.201500014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lavefve L., Howard L.R., Carbonero F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020;11:45–65. doi: 10.1039/C9FO01634A. [DOI] [PubMed] [Google Scholar]
- 179.Cheng J.-R., Liu X.-M., Chen Z.-Y., Zhang Y.-S., Zhang Y.-H. Mulberry anthocyanin biotransformation by intestinal probiotics. Food Chem. 2016;213:721–727. doi: 10.1016/j.foodchem.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 180.Riva A., Kolimár D., Spittler A., Wisgrill L., Herbold C.W., Abrankó L., Berry D. Conversion of Rutin, a Prevalent Dietary Flavonol, by the Human Gut Microbiota. Front. Microbiol. 2020;11:585428. doi: 10.3389/fmicb.2020.585428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Attri S., Goel G. Influence of polyphenol rich seabuckthorn berries juice on release of polyphenols and colonic microbiota on exposure to simulated human digestion model. Food Res. Int. 2018;111:314–323. doi: 10.1016/j.foodres.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 182.Koudoufio M., Desjardins Y., Feldman F., Spahis S., Delvin E., Levy E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants. 2020;9:982. doi: 10.3390/antiox9100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Corrêa T.A.F., Rogero M.M., Hassimotto N.M.A., Lajolo F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019;6:188. doi: 10.3389/fnut.2019.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rodríguez-Daza M.C., Pulido-Mateos E.C., Lupien-Meilleur J., Guyonnet D., Desjardins Y., Roy D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021;8:689456. doi: 10.3389/fnut.2021.689456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Alves-Santos A.M., Sugizaki C.S.A., Carielo Lima G., Veloso Naves M.M. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods. 2020;74:104169. doi: 10.1016/j.jff.2020.104169. [DOI] [Google Scholar]
- 186.Kosmala M., Jurgoński A., Juśkiewicz J., Karlińska E., Macierzyński J., Rój E., Zduńczyk Z. Chemical Composition of Blackberry Press Cake, Polyphenolic Extract, and Defatted Seeds, and Their Effects on Cecal Fermentation, Bacterial Metabolites, and Blood Lipid Profile in Rats. J. Agric. Food Chem. 2017;65:5470–5479. doi: 10.1021/acs.jafc.7b01876. [DOI] [PubMed] [Google Scholar]
- 187.Plamada D., Vodnar D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients. 2022;14:137. doi: 10.3390/nu14010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sommella E., Verna G., Liso M., Salviati E., Esposito T., Carbone D., Pecoraro C., Chieppa M., Campiglia P. Hop-derived fraction rich in beta acids and prenylflavonoids regulates the inflammatory response in dendritic cells differently from quercetin: Unveiling metabolic changes by mass spectrometry-based metabolomics. Food Funct. 2021;12:12800–12811. doi: 10.1039/D1FO02361F. [DOI] [PubMed] [Google Scholar]
- 189.Verna G., Liso M., Cavalcanti E., Bianco G., Di Sarno V., Santino A., Campiglia P., Chieppa M. Quercetin Administration Suppresses the Cytokine Storm in Myeloid and Plasmacytoid Dendritic Cells. Int. J. Mol. Sci. 2021;22:8349. doi: 10.3390/ijms22158349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hatcher H.C., Singh R.N., Torti F.M., Torti S.V. Synthetic and natural iron chelators: Therapeutic potential and clinical use. Future Med. Chem. 2009;1:1643–1670. doi: 10.4155/fmc.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ohara T., Tomono Y., Boyi X., Yingfu S., Omori K., Matsukawa A. A novel, nontoxic iron chelator, super-polyphenol, effectively induces apoptosis in human cancer cell lines. Oncotarget. 2018;9:32751–32760. doi: 10.18632/oncotarget.25973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Leon-Gonzalez A.J., Auger C., Schini-Kerth V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015;98:371–380. doi: 10.1016/j.bcp.2015.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.