Monkeypox (mpox) virus is a zoonotic viral infection endemic to some parts of Africa that re-emerged in May 2022 as an international outbreak.1 Although ocular involvement had been reported in previous endemic and exported cases,2 , 3 information associated with the current outbreak is scarce.4 Although most cases of mpox present with mild, self-limited disease, some individuals show severe manifestations including pneumonitis, encephalitis, or secondary infections. Ocular infections can result in corneal scarring and permanent vision loss.2 Tecovirimat, cidofovir, and its prodrug brincidofovir are potential options for severe cases, but only tecovirimat has market authorization in the European Union for the treatment of orthopoxvirus infection, including mpox5 To date, no specific treatment approach for patients with ocular involvement resulting from mpox has been established.2 , 3 Although the efficacy of tecovirimat (600 mg twice daily for 14 days) has not been tested properly for the treatment of mpox in studies in humans and is not approved by the Food and Drug Administration for this disease, it has been recommended by the Centers for Disease Control and Prevention for its use in patients with severe disease, including ocular and periorbital infections.6 We assessed the incidence, clinical spectrum, and therapeutic response after institution of a multidisciplinary protocol in patients with ophthalmic manifestation of mpox infection.
From June 20 through August 3, 2022, all patients with mpox disease and ocular manifestations whose disease was diagnosed in the mpox clinic of the Sexually Transmitted Infections Program at the Hospital Clínic of Barcelona, Barcelona, Spain, were included in a prospective study and were referred for assessment to a specific mpox clinic led by an ophthalmologist. Ophthalmic follow-up evaluations were performed until resolution of disease. Ocular involvement was considered severe if any of the following were present: corneal ulcerations with thinning or scarring, visually significant corneal edema, extensive conjunctival inflammation with risk of symblepharon, or significant preseptal or orbital involvement. Treatment was stratified according to potential ocular complications of eyelids, conjunctiva, or cornea, including corticosteroids and tecovirimat in patients with severe disease (Table S1, available at www.aaojournal.org). This study was approved by the local ethics committee, and patients gave written informed consent (identifier, HCB/2022/0608).
Nine of 880 patients (1%) demonstrated ocular manifestations. All were men who have sex with men with a mean ± standard deviation age of 38 ± 11 years. Two showed HIV infection and were receiving antiretroviral therapy and two showed negative HIV results with pre-exposure prophylaxis. Two had received smallpox vaccination in childhood.
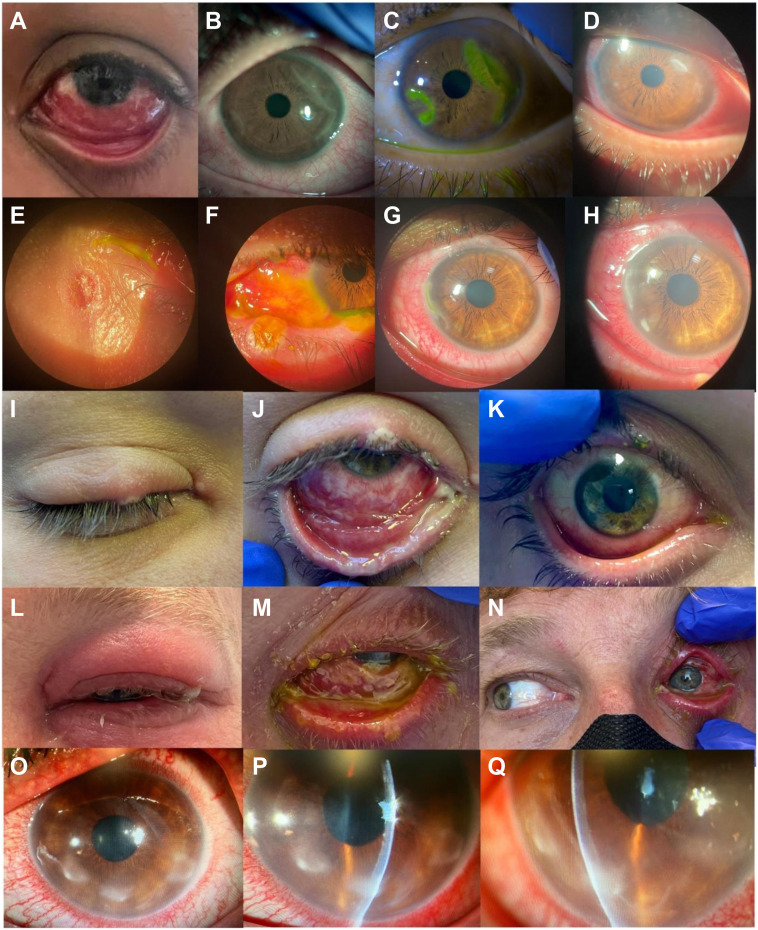
Ocular manifestations (Fig 1 ) included conjunctivitis (n = 9), blepharitis (n = 7), papular rash involving the eyelid (n = 5), corneal ulcerations (n = 4), focal conjunctival erosions (n = 3), corneal stromal edema (n = 2), corneal endothelitis, anterior uveitis, and preseptal cellulitis (n = 1 each). Five patients (55.6%) had severe cases that were treated with tecovirimat: 2 patients showed corneal ulcerations and thinning (1 patient also showed corneal stromal edema), 1 patient showed scarring and persistent corneal edema, 1 patient showed preseptal cellulitis and corneal ulcerations, and 1 patient showed severe membranous conjunctivitis and extensive conjunctival inflammation at risk of symblepharon (Fig 1).
Figure 1.
Eye appearance and follow-up evolution of the 5 patients with more severe ocular manifestations. A–D, Patient 1, who demonstrated significant conjunctivitis at presentation (A) and showed geographical corneal ulcers (B) that evolved to severe thinning 8 days later (C). One week after Tecovirimat administration, the corneal lesions started to improve, with healing 1 month after presentation (D). E–H, Patient 2, who demonstrated eyelid papules (E) and membranous conjunctivitis with focal conjunctival erosions in the left eye (F) that extended over time and closed after 2 weeks of treatment with povidone–iodine and oxytetracycline plus polymyxin plus hydrocortisone ointment. Twenty days after initially seeking treatment, he demonstrated corneal involvement with semilunar corneal ulceration and Dellen (G) that infiltrated in the ensuing 5 days with stromal corneal edema (H). I–K, Patient 3, who showed eyelid papules (I) and membranous and mucopurulent conjunctivitis and conjunctival erosions (J) that were resolved 28 days after presentation (K). L–N, Patient 6, who demonstrated severe unilateral eyelid edema (L) and membranous conjunctivitis (M). Three days after presentation, he demonstrated cellulitis with restriction of the medium rectus and was admitted (N). O–Q, Patient 9 with peripheral corneal semilunar infiltrates (O, P) that evolved to corneal thinning with stromal edema (Q).
Monkeypox virus DNA was detected in conjunctival samples from all patients at presentation, and polymerase chain reaction results usually were positive for more than 1 month’s duration. No relationship was found between mpox polymerase chain reaction quantification and severity of ocular manifestations (Table S2, available at www.aaojournal.org).
Our systematic assessment detected an incidence of mpox ocular involvement of 1% (95% confidence interval, 0.005–0.019), which is 5-fold higher than that reported among 1955 cases published to date in major case series in the United Kingdom,7 Spain,8 the United States,9 and worldwide4; in these series, detailed ocular manifestations and management were not reported. In previous endemic mpox outbreaks in African countries, ocular manifestations were common (> 20%),10 with cultural, social, health care access, and age at presentation suggested as contributing factors for the difference in rates.4 , 10 We did not find more common or severe ocular involvement in individuals with HIV infection, although the 2 patients with HIV infection showed a CD4 T-cell count of more than 500 cells/μL. It is important to note that ocular involvement in the 2 individuals who had received the smallpox vaccine in childhood was mild (Table S2).
All 5 individuals with severe ocular disease were treated with tecovirimat. No patients reported any side effects, and ocular symptoms improved significantly 1 week after the start of tecovirimat with a complete resolution in a median of 29 days (interquartile range, 25–39 days). According to our results, tecovirimat should be considered if corneal involvement is present because of the risk of vision loss, as well as in cases of severe conjunctivitis, as reported in one of the recently communicated case reports.11 Our protocol also included 5% topical povidone–iodine drops 4 times daily because of its broad-spectrum microbicidal activity. A short course of systemic steroids (prednisone 30–40 mg daily for 3 days followed by 15–20 mg for an additional 3 days) was administered as part of the institutional multidisciplinary protocol when severe mucositis was present and showed excellent results in the control of inflammation and pain without exacerbation of skin lesions. This treatment is similar to that used for other DNA viral keratitis types (e.g., herpes virus) in which a combination of antiviral and corticosteroid drugs is used depending on the extent of epithelial and stromal involvement. Although corticosteroids are avoided in epithelial keratitis because of potential virus activation, we included topical dexamethasone 1 drop (1 mg/1 ml) 4 times daily as part of the treatment when severe conjunctivitis was present without epithelial involvement or in cases of stromal, endothelial keratitis and always with a concomitant administration of tecovirimat. It is important to highlight: (1) that we did not observe any worsening of the patients’ skin lesions whenever corticosteroids were used and (2) that although monkeypox DNA could be detected for long periods in ocular samples, it decreased steadily over time to undetectable levels, indicating full viral clearance. After an average follow-up of 3 months, complete resolution was observed in 7 patients, and we have detected peripheral corneal scarring without significant vision loss in only 2 patients with previous corneal involvement. All patients showed a very good evolution with our protocol of treatment, although longer follow-up may be needed to detect further improvement.
In summary, using a standardized ophthalmologic diagnostic assessment and a protocolized management, we observed that ocular involvement is uncommon, but may be severe, in the current mpox outbreak. In cases of severe eye involvement, tecovirimat with or without is a treatment option to consider.
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s):
M.P.: Consultant – Abbvie Allergan, Carl Zeiss Meditec, Abbvie, Thea; Financial support – Abbvie Allergan; Lecturer – Abbvie Allergan, Carl Zeiss Meditec, Thea; Executive Committee Member – European Glaucoma Society.
A.V.: Lecturer – GSK.
B.S.-D.: Consultant and Lecturer – Chiesi, Gensight; Financial support – Bausch & Lomb; Data safety and monitoring committee – Rewind Therapeutics; Equity owner – Bionure.
Supported by CIBER (Consorcio Centro de Investigación Biomédica en Red; CB 2021), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea—NextGenerationEU; and Acción Estratégica “Impacto clínico y microbiológico del brote por el virus de la viruela del mono en pacientes en España (2022): proyecto multicéntrico MONKPOX-ESP22” (CIBERINFEC).
HUMAN SUBJECTS: Human subjects were included in this study. The study was conducted according to the tenets of the Declaration of Helsinki and ethical approval was obtained from the Human Research Ethics Committee (HREC) of the Hospital Clínic of Barcelona, Spain (HCB/2022/0608). Patients gave written informed consent (HCB/2022/0608).
No animal subjects were included in this study.
Author Contributions:
Conception and design: Blanco
Analysis and interpretation: Pazos, Riera, Blanco
Data collection: Blanco, Pazos, Riera, Moll-Udina, Catala, Narvaez, Fuertes, Dotti-Boada, Petiti, Izquierdo-Serra, Maldonado, Chang-Sotomayor, Garcia, Camós-Carreras, Gilera, De Loredo, Peraza-Nieves, Ventura-Abreu, Spencer, Del Carlo, Torras, Nicolas, Adán, Vilella, Puig, Martinez, Martinez, Sánchez-Dalmau, Riera
Obtained funding: N/A
Overall responsibility: Pazos, Blanco
Supplementary Data
References
- 1.World Health Organization https://www.who.int/news-room/fact-sheets/detail/monkeypox Available at:
- 2.Jezek Z., Szczeniowski M., Paluku K.M., Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156:293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 3.Ogoina D., Iroezindu M., James H.I., et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis. 2020;71:e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 4.Thornhill J.P., Barkati S., Walmsley S., et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 5.European Center for Disease Prevention and Control https://www.ecdc.europa.eu/en/all-topics-z/monkeypox/factsheet-health-professionals Available at:
- 6.Centers for Disease Control and Prevention https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html Available at:
- 7.Girometti N., Byrne R., Bracchi M., et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;22:1321–1328. doi: 10.1016/S1473-3099(22)00411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Català A., Clavo-Escribano P., Riera-Monroig J., et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br J Dermatol. 2022;187:765–772. doi: 10.1111/bjd.21790. [DOI] [PubMed] [Google Scholar]
- 9.Philpott D.H.C., Alroy K.A., et al. Epidemiologic and clinical characteristics of monkeypox cases—United States, May 17–July 22, 2022. MMWR Morbid Mortal Wkly Rep. 2022 doi: 10.15585/mmwr.mm7132e3. ePub: August 5, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelaal A., Serhan H.A., Mahmoud M.A., et al. Ophthalmic manifestations of monkeypox virus. Eye (Lond) 2023;37(3):383–385. doi: 10.1038/s41433-022-02195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scandale P., Raccagni A.R., Nozza S. Unilateral blepharoconjunctivitis due to monkeypox virus infection. Ophthalmology. 2022;129:1274. doi: 10.1016/j.ophtha.2022.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.