Abstract
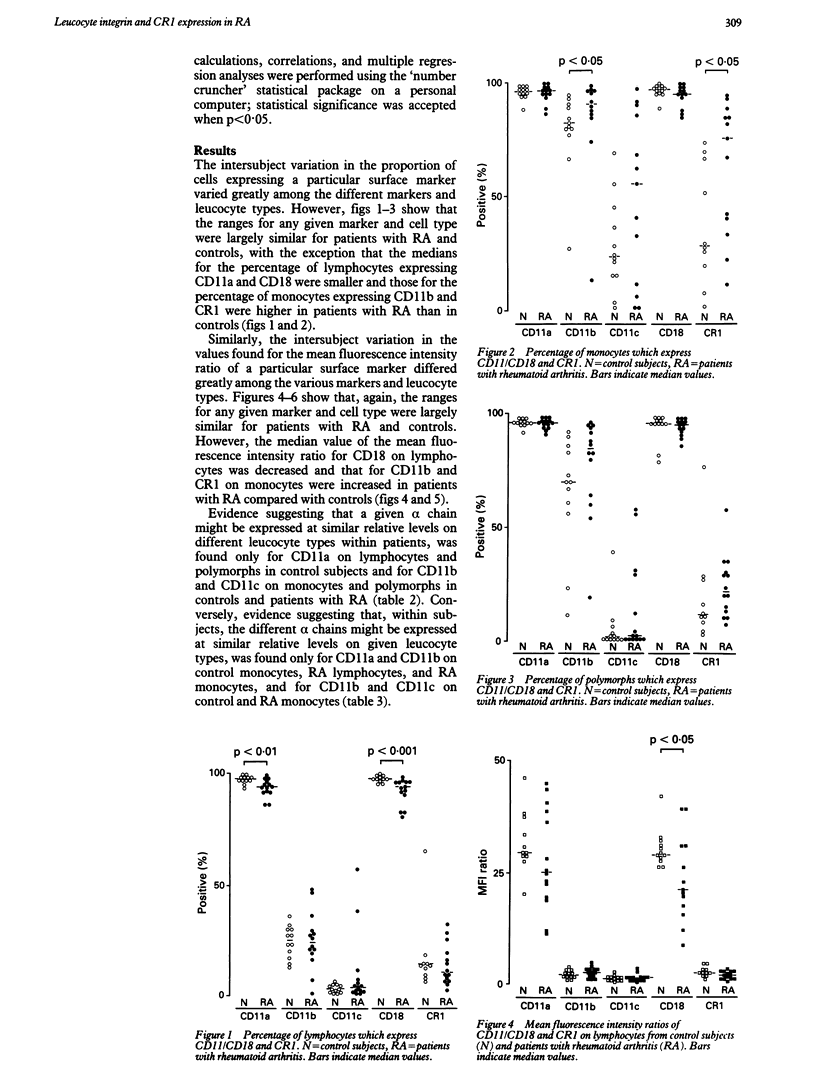
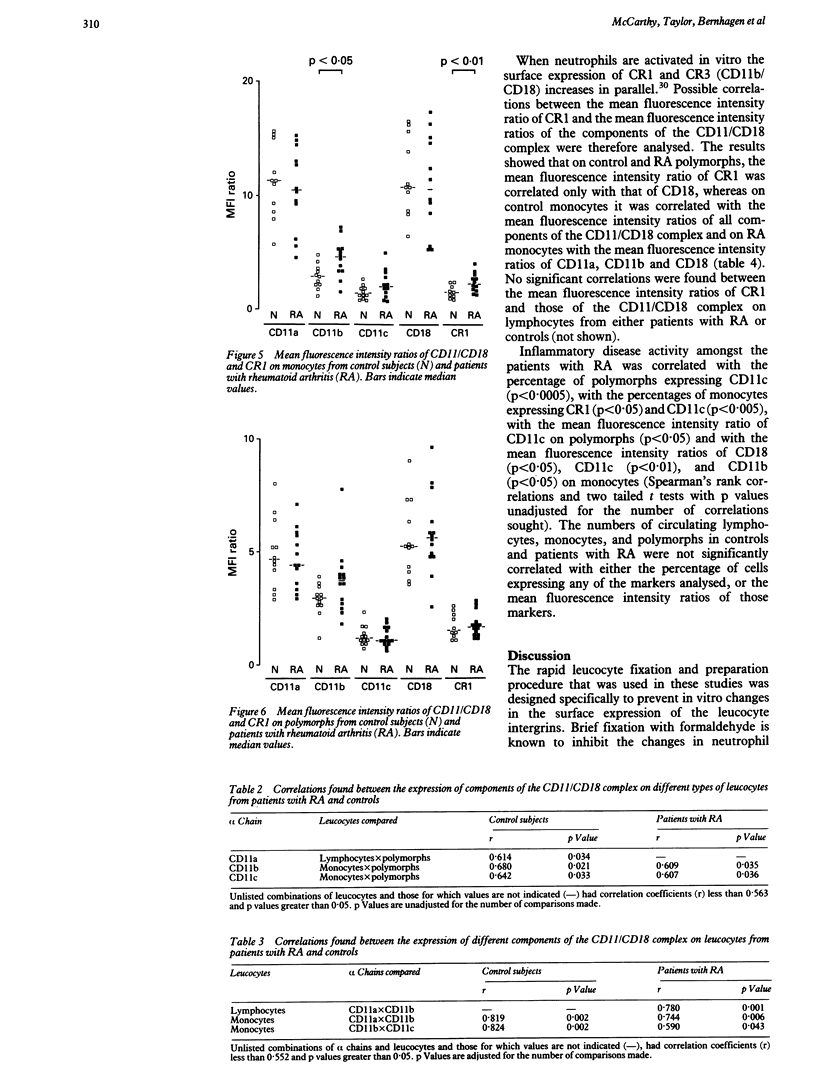
Expression of the leucocyte integrins (CD11a, b, c/CD18) and of CD35 (CR1) on leucocytes from the peripheral blood of patients with rheumatoid arthritis (RA) (n = 14) and control subjects (n = 12) was measured by flow cytometry using a rapid fixation and leucocyte preparation procedure. The mean (SE) percentages of lymphocytes expressing CD11a (RA 93.4 (1.7)%; controls 97.2 (1.8)%) and CD18 (RA 91.3 (2.3)%; controls 97.0 (2.6)%) were lower and the percentage of monocytes expressing CD11b (RA 86.9 (11.4)%; controls 78.4 (11.9)%) and CR1 (RA 62.6 (15.5)%; controls 36.6 (17.6)%) were higher in patients with RA than in controls. In addition, the mean fluorescence intensity of CD18 (RA 22.1 (2.3); controls 30.7 (2.5)) on lymphocytes was decreased and that of CD11b (RA 4.5 (0.8); controls 2.9 (0.9)) and CR1 (RA 2.4 (0.4); controls 1.5 (0.5)) on monocytes was increased in patients with RA compared with controls. The functional importance (if any) of the altered expression of the antigens on lymphocytes is not yet known. Altered expression on monocytes is consistent with activation within the circulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Freeman K. L., Heerdt B., Hughes B. J., Jack R. M., Smith C. W. Abnormal stimulated adherence of neonatal granulocytes: impaired induction of surface Mac-1 by chemotactic factors or secretagogues. Blood. 1987 Sep;70(3):740–750. [PubMed] [Google Scholar]
- Anderson D. C., Miller L. J., Schmalstieg F. C., Rothlein R., Springer T. A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J Immunol. 1986 Jul 1;137(1):15–27. [PubMed] [Google Scholar]
- Arnaout M. A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990 Mar 1;75(5):1037–1050. [PubMed] [Google Scholar]
- Babcock G. F., Alexander J. W., Warden G. D. Flow cytometric analysis of neutrophil subsets in thermally injured patients developing infection. Clin Immunol Immunopathol. 1990 Jan;54(1):117–125. doi: 10.1016/0090-1229(90)90011-e. [DOI] [PubMed] [Google Scholar]
- Banga J. P., LeRoy F., Suleyman S., Kasp E., Brown E., Dumonde D. Analysis of antigenic determinants of retinal S-antigen with monoclonal antibodies. Invest Ophthalmol Vis Sci. 1988 Jan;29(1):12–21. [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Buyon J. P., Shadick N., Berkman R., Hopkins P., Dalton J., Weissmann G., Winchester R., Abramson S. B. Surface expression of Gp 165/95, the complement receptor CR3, as a marker of disease activity in systemic Lupus erythematosus. Clin Immunol Immunopathol. 1988 Jan;46(1):141–149. doi: 10.1016/0090-1229(88)90014-1. [DOI] [PubMed] [Google Scholar]
- Cush J. J., Lipsky P. E. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Oct;31(10):1230–1238. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- Emery P., Lopez A. F., Burns G. F., Vadas M. A. Synovial fluid neutrophils of patients with rheumatoid arthritis have membrane antigen changes that reflect activation. Ann Rheum Dis. 1988 Jan;47(1):34–39. doi: 10.1136/ard.47.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Freyer D. R., Morganroth M. L., Rogers C. E., Arnaout M. A., Todd R. F., 3rd Modulation of surface CD11/CD18 glycoproteins (Mo1, LFA-1, p150,95) by human mononuclear phagocytes. Clin Immunol Immunopathol. 1988 Feb;46(2):272–283. doi: 10.1016/0090-1229(88)90189-4. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Senecal F. M., Schwartz B. R., Yee E. K., Taylor R. F., Beatty P. G., Price T. H., Ochs H. D. The role of neutrophil membrane glycoprotein GP-150 in neutrophil adherence to endothelium in vitro. Blood. 1985 Jul;66(1):167–178. [PubMed] [Google Scholar]
- Heurkens A. H., Breedveld F. C., Keur C. V., Brand R., Daha M. R. Degradation of aggregates of activated C3 (C3b) by monocytes of patients with rheumatoid arthritis is related to vasculitis. Clin Exp Immunol. 1990 May;80(2):177–180. doi: 10.1111/j.1365-2249.1990.tb05229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth J. E., Gotch F. M., Hildreth P. D., McMichael A. J. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur J Immunol. 1983 Mar;13(3):202–208. doi: 10.1002/eji.1830130305. [DOI] [PubMed] [Google Scholar]
- Hogg N., MacDonald S., Slusarenko M., Beverley P. C. Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology. 1984 Dec;53(4):753–767. [PMC free article] [PubMed] [Google Scholar]
- Hogg N., Takacs L., Palmer D. G., Selvendran Y., Allen C. The p150,95 molecule is a marker of human mononuclear phagocytes: comparison with expression of class II molecules. Eur J Immunol. 1986 Mar;16(3):240–248. doi: 10.1002/eji.1830160306. [DOI] [PubMed] [Google Scholar]
- Hogg N. The leukocyte integrins. Immunol Today. 1989 Apr;10(4):111–114. doi: 10.1016/0167-5699(89)90238-7. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kornblihtt A. R., Gutman A. Molecular biology of the extracellular matrix proteins. Biol Rev Camb Philos Soc. 1988 Nov;63(4):465–507. doi: 10.1111/j.1469-185x.1988.tb00668.x. [DOI] [PubMed] [Google Scholar]
- Malhotra V., Hogg N., Sim R. B. Ligand binding by the p150,95 antigen of U937 monocytic cells: properties in common with complement receptor type 3 (CR3). Eur J Immunol. 1986 Sep;16(9):1117–1123. doi: 10.1002/eji.1830160915. [DOI] [PubMed] [Google Scholar]
- McCarthy D. A., Bernhagen J., Liu Y. C., Perry J. D. A rapid preparation technique for leucocytes. J Microsc. 1990 Apr;158(Pt 1):63–72. doi: 10.1111/j.1365-2818.1990.tb02977.x. [DOI] [PubMed] [Google Scholar]
- McCarthy D. A., Bernhagen J., Taylor M. J., Hamblin A. S., James I., Thompson P. W., Perry J. D. Morphological evidence that activated polymorphs circulate in the peripheral blood of patients with rheumatoid arthritis. Ann Rheum Dis. 1992 Jan;51(1):13–18. doi: 10.1136/ard.51.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore F. D., Jr, Davis C., Rodrick M., Mannick J. A., Fearon D. T. Neutrophil activation in thermal injury as assessed by increased expression of complement receptors. N Engl J Med. 1986 Apr 10;314(15):948–953. doi: 10.1056/NEJM198604103141503. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Hasslen S. R., Ahrenholz D. H., Haus E., Solem L. D. Influence of minor thermal injury on expression of complement receptor CR3 on human neutrophils. Am J Pathol. 1986 Dec;125(3):563–570. [PMC free article] [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Seligmann B. E., Metcalf J. A., Frank M. M., Gallin J. I. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985 Apr;134(4):2580–2587. [PubMed] [Google Scholar]
- Patarroyo M., Makgoba M. W. Leucocyte adhesion to cells in immune and inflammatory responses. Lancet. 1989 Nov 11;2(8672):1139–1142. doi: 10.1016/s0140-6736(89)91498-0. [DOI] [PubMed] [Google Scholar]
- Philips M. R., Buyon J. P., Winchester R., Weissmann G., Abramson S. B. Up-regulation of the iC3b receptor (CR3) is neither necessary nor sufficient to promote neutrophil aggregation. J Clin Invest. 1988 Aug;82(2):495–501. doi: 10.1172/JCI113623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichyangkul S., Schick D., Schober W., Dixon G., Khan A. Increased expression of adhesive proteins on leukocytes by TNF alpha. Exp Hematol. 1988 Aug;16(7):588–593. [PubMed] [Google Scholar]
- Pizzolo G., Sloane J., Beverley P., Thomas J. A., Bradstock K. F., Mattingly S., Janossy G. Differential diagnosis of malignant lymphoma and nonlymphoid tumors using monoclonal anti-leucocyte antibody. Cancer. 1980 Dec 15;46(12):2640–2647. doi: 10.1002/1097-0142(19801215)46:12<2640::aid-cncr2820461218>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schleiffenbaum B., Moser R., Patarroyo M., Fehr J. The cell surface glycoprotein Mac-1 (CD11b/CD18) mediates neutrophil adhesion and modulates degranulation independently of its quantitative cell surface expression. J Immunol. 1989 May 15;142(10):3537–3545. [PubMed] [Google Scholar]
- Schwartz B. R., Harlan J. M. Neutrophil membrane sulfhydryl groups are involved in stimulated neutrophil adherence to endothelium. J Leukoc Biol. 1989 Mar;45(3):177–182. doi: 10.1002/jlb.45.3.177. [DOI] [PubMed] [Google Scholar]
- Setiadi H., Wautier J. L., Courillon-Mallet A., Passa P., Caen J. Increased adhesion to fibronectin and Mo-1 expression by diabetic monocytes. J Immunol. 1987 May 15;138(10):3230–3234. [PubMed] [Google Scholar]
- Tonnesen M. G., Anderson D. C., Springer T. A., Knedler A., Avdi N., Henson P. M. Adherence of neutrophils to cultured human microvascular endothelial cells. Stimulation by chemotactic peptides and lipid mediators and dependence upon the Mac-1, LFA-1, p150,95 glycoprotein family. J Clin Invest. 1989 Feb;83(2):637–646. doi: 10.1172/JCI113928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Bender J. G., Simpson S. J., Chenoweth D. E. Relationship of chemotactic receptors for formyl peptide and C5a to CR1, CR3, and Fc receptors on human neutrophils. J Leukoc Biol. 1990 Jun;47(6):519–527. doi: 10.1002/jlb.47.6.519. [DOI] [PubMed] [Google Scholar]
- Vedder N. B., Harlan J. M. Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest. 1988 Mar;81(3):676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M. Neutrophil adherence to human endothelium in vitro occurs by CDw18 (Mo1, MAC-1/LFA-1/GP 150,95) glycoprotein-dependent and -independent mechanisms. J Clin Invest. 1988 Feb;81(2):531–537. doi: 10.1172/JCI113351. [DOI] [PMC free article] [PubMed] [Google Scholar]


