Abstract
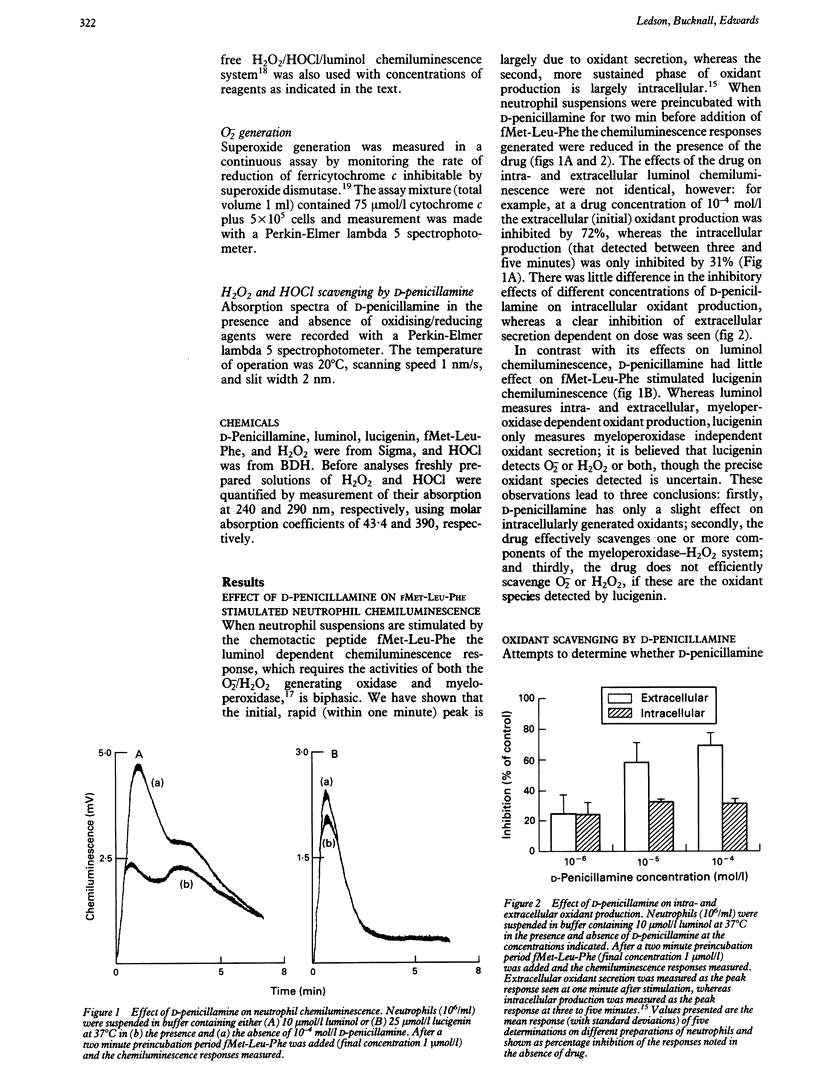
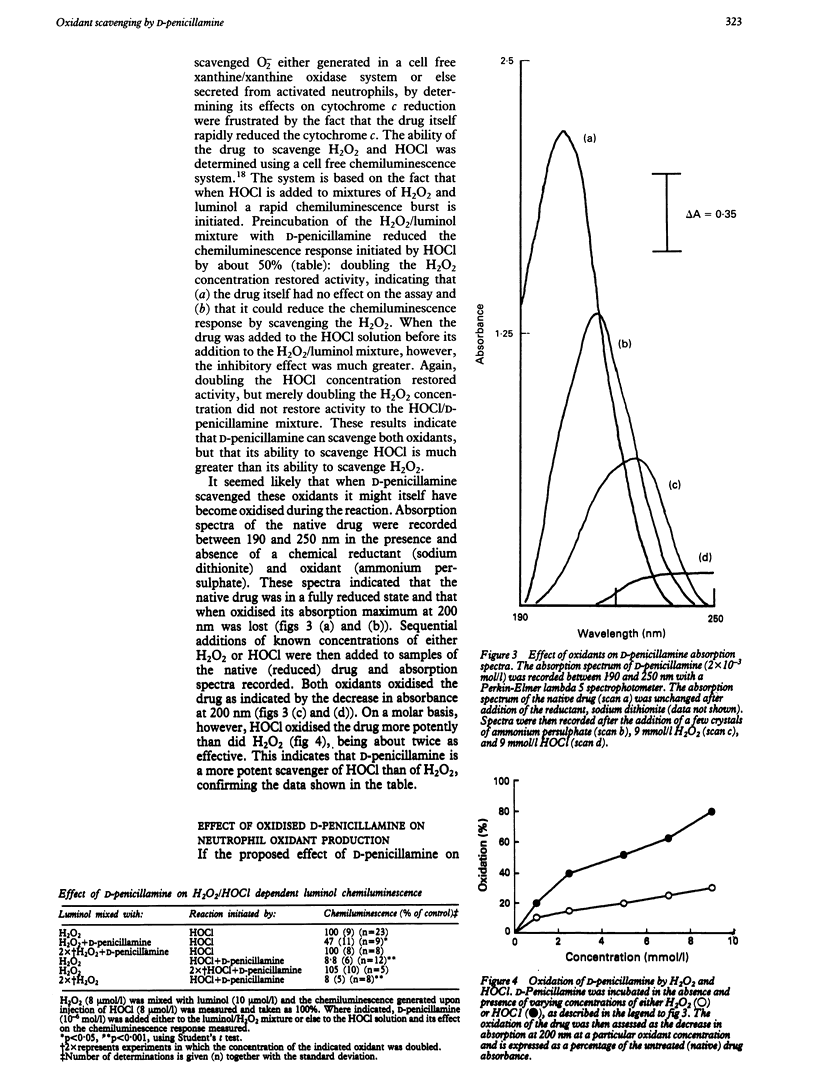
D-Penicillamine inhibited oxidant secretion from human neutrophils after activation by the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (fMet-Leu-Phe), as assessed by luminol dependent chemiluminescence. In contrast, this drug had little effect on either intracellular oxidant production or lucigenin dependent chemiluminescence activated by the same agonist. The drug was shown to scavenge both H2O2 and HOCl in a cell free luminol chemiluminescence system, though its ability to scavenge HOCl was greater than that for H2O2. Both these oxidants could oxidise the drug, but again HOCl was more potent than H2O2. When D-penicillamine was oxidised by exposure to H2O2 it could no longer serve as a scavenger of secreted oxidants from neutrophils. These data suggest that in vivo the preferential scavenging of HOCl may be important under pathological conditions where secreted myeloperoxidase may be functional.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender J. G., Van Epps D. E., Searles R., Williams R. C., Jr Altered function of synovial fluid granulocytes in patients with acute inflammatory arthritis: evidence for activation of neutrophils and its mediation by a factor present in synovial fluid. Inflammation. 1986 Dec;10(4):443–453. doi: 10.1007/BF00915828. [DOI] [PubMed] [Google Scholar]
- Brestel E. P. Co-oxidation of luminol by hypochlorite and hydrogen peroxide implications for neutrophil chemiluminescence. Biochem Biophys Res Commun. 1985 Jan 16;126(1):482–488. doi: 10.1016/0006-291x(85)90631-x. [DOI] [PubMed] [Google Scholar]
- Cuperus R. A., Hoogland H., Wever R., Muijsers A. O. The effect of D-penicillamine on myeloperoxidase: formation of compound III and inhibition of the chlorinating activity. Biochim Biophys Acta. 1987 Mar 18;912(1):124–131. doi: 10.1016/0167-4838(87)90255-x. [DOI] [PubMed] [Google Scholar]
- Dularay B., Elson C. J., Dieppe P. A. Enhanced oxidative response of polymorphonuclear leukocytes from synovial fluids of patients with rheumatoid arthritis. Autoimmunity. 1988;1(3):159–169. doi: 10.3109/08916938808997161. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Hughes V., Barlow J., Bucknall R. Immunological detection of myeloperoxidase in synovial fluid from patients with rheumatoid arthritis. Biochem J. 1988 Feb 15;250(1):81–85. doi: 10.1042/bj2500081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W. Luminol- and lucigenin-dependent chemiluminescence of neutrophils: role of degranulation. J Clin Lab Immunol. 1987 Jan;22(1):35–39. [PubMed] [Google Scholar]
- Edwards S. W., Say J. E., Hart C. A. Oxygen-dependent killing of Staphylococcus aureus by human neutrophils. J Gen Microbiol. 1987 Dec;133(12):3591–3597. doi: 10.1099/00221287-133-12-3591. [DOI] [PubMed] [Google Scholar]
- Lengfelder E., Fuchs C., Younes M., Weser U. Functional aspects of the superoxide dismutative action of Cu-penicillamine. Biochim Biophys Acta. 1979 Apr 12;567(2):492–502. doi: 10.1016/0005-2744(79)90135-9. [DOI] [PubMed] [Google Scholar]
- Matheson N. R. The effect of antiarthritic drugs and related compounds on the human neutrophil myeloperoxidase system. Biochem Biophys Res Commun. 1982 Sep 16;108(1):259–265. doi: 10.1016/0006-291x(82)91860-5. [DOI] [PubMed] [Google Scholar]
- Müller-Peddinghaus R., Wurl M. The amplified chemiluminescence test to characterize antirheumatic drugs as oxygen radical scavengers. Biochem Pharmacol. 1987 Apr 1;36(7):1125–1132. doi: 10.1016/0006-2952(87)90423-0. [DOI] [PubMed] [Google Scholar]
- Nurcombe H. L., Bucknall R. C., Edwards S. W. Activation of the neutrophil myeloperoxidase-H2O2 system by synovial fluid isolated from patients with rheumatoid arthritis. Ann Rheum Dis. 1991 Apr;50(4):237–242. doi: 10.1136/ard.50.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe H. L., Bucknall R. C., Edwards S. W. Neutrophils isolated from the synovial fluid of patients with rheumatoid arthritis: priming and activation in vivo. Ann Rheum Dis. 1991 Mar;50(3):147–153. doi: 10.1136/ard.50.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe H. L., Edwards S. W. Role of myeloperoxidase in intracellular and extracellular chemiluminescence of neutrophils. Ann Rheum Dis. 1989 Jan;48(1):56–62. doi: 10.1136/ard.48.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staite N. D., Messner R. P., Zoschke D. C. In vitro production and scavenging of hydrogen peroxide by D-penicillamine. Relationship to copper availability. Arthritis Rheum. 1985 Aug;28(8):914–921. doi: 10.1002/art.1780280811. [DOI] [PubMed] [Google Scholar]
- Staite N. D., Zoschke D. C., Messner R. P. Scavenging of hydrogen peroxide--a new mechanism of action for D-penicillamine in rheumatoid arthritis? N Engl J Med. 1984 Aug 23;311(8):538–539. doi: 10.1056/nejm198408233110817. [DOI] [PubMed] [Google Scholar]
- Wasil M., Halliwell B., Moorhouse C. P., Hutchison D. C., Baum H. Biologically-significant scavenging of the myeloperoxidase-derived oxidant hypochlorous acid by some anti-inflammatory drugs. Biochem Pharmacol. 1987 Nov 15;36(22):3847–3850. doi: 10.1016/0006-2952(87)90448-5. [DOI] [PubMed] [Google Scholar]


