Abstract
Simple Summary
The new trends in the research on glioblastomas (GBMs) are focusing on understanding the crosstalk between proper neoplastic tissue and its microenvironment. Against this background, the extracellular matrix (ECM) has been classically linked to a purely structural role. However, it has recently become clear that it actively shapes the functional responses of cells to their environment as well. While many components of the ECM have been isolated and characterized, its modifications in the specific setting of GBMs have only been recently explored in the literature. The aim of this paper is to provide a systematic review on the topic and to assess the ECM’s role in shaping tumoral development.
Abstract
Background and aim: While many components of the ECM have been isolated and characterized, its modifications in the specific setting of GBMs have only been recently explored in the literature. The aim of this paper is to provide a systematic review on the topic and to assess the ECM’s role in shaping tumoral development. Methods: An online literature search was launched on PubMed/Medline and Scopus using the research string “((Extracellular matrix OR ECM OR matrix receptor OR matrix proteome) AND (glioblastoma OR GBM) AND (tumor invasion OR tumor infiltration))”, and a systematic review was conducted in accordance with the PRISMA-P guidelines. Results: The search of the literature yielded a total of 693 results. The duplicate records were then removed (n = 13), and the records were excluded via a title and abstract screening; 137 studies were found to be relevant to our research question and were assessed for eligibility. Upon a full-text review, 59 articles were finally included and were summarized as follows based on their focus: (1) proteoglycans; (2) fibrillary proteins, which were further subdivided into the three subcategories of collagen, fibronectin, and laminins; (3) glycoproteins; (4) degradative enzymes; (5) physical forces; (6) and glioma cell and microglia migratory and infiltrative patterns. Conclusions: Our systematic review demonstrates that the ECM should not be regarded anymore as a passive scaffold statically contributing to mechanical support in normal and pathological brain tissue but as an active player in tumor-related activity.
Keywords: glioblastoma, extracellular matrix, cancer, tumor microenvironment
1. Introduction
The new trends in the research on glioblastomas (GBMs) are focusing more and more on the understanding of the crosstalk between proper neoplastic tissue and its microenvironment (TME). The microenvironmental contribution seems to be critical in tumor development, progression, relapse, and resistance to therapies. A definition of the “seed and soil” approach has recently been introduced to describe the glioblastoma landscape: the TME, mainly constituted by inflammatory cells (microglia, monocytes, and macrophages) and stem cells, acts as a fertile “soil” interacting with the “seed”, which is represented by proper neoplastic glial cells [1]. Against this background, the extracellular matrix (ECM) has been classically linked to a mere structural role. However, it has recently become clear that it actively shapes the functional responses of cells to their environment as well. Therefore, the ECM should not be regarded anymore as a passive scaffold statically contributing to mechanical support but as an active player in tumor-related activity: it shows dynamic “structural” modifications and interactions under the pressure of the dysregulated TME, it performs active crosstalk with the inflammatory and stem compartment, and it is able to influence cellular migration [2,3,4,5]. While many components of the ECM have been isolated and characterized, its modifications in the specific setting of GBMs have only been recently explored in the literature. The aim of this paper is to provide an updated systematic review on the topic and to assess the ECM’s role in shaping tumoral development.
2. Materials and Methods
The study presented herein was conducted in accordance with the PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols) guidelines [2]. An online literature search was launched on PubMed/Medline and Scopus using the research string “((Extracellular matrix OR ECM OR matrix receptor OR matrix proteome) AND (glioblastoma OR GBM) AND (tumor invasion OR tumor infiltration))”; research was last conducted in September 2022. Two authors, S.M. and F.F., independently conducted the abstract screening for eligibility. Any discordance was solved through consensus with a third senior author, G.M.D.P. No restrictions on date of publication were made. Exclusion criteria were as follows: studies published in languages other than English and meta-analyses. A systematic abstract screening of the references (forward search) was performed to identify additional records.
3. Results
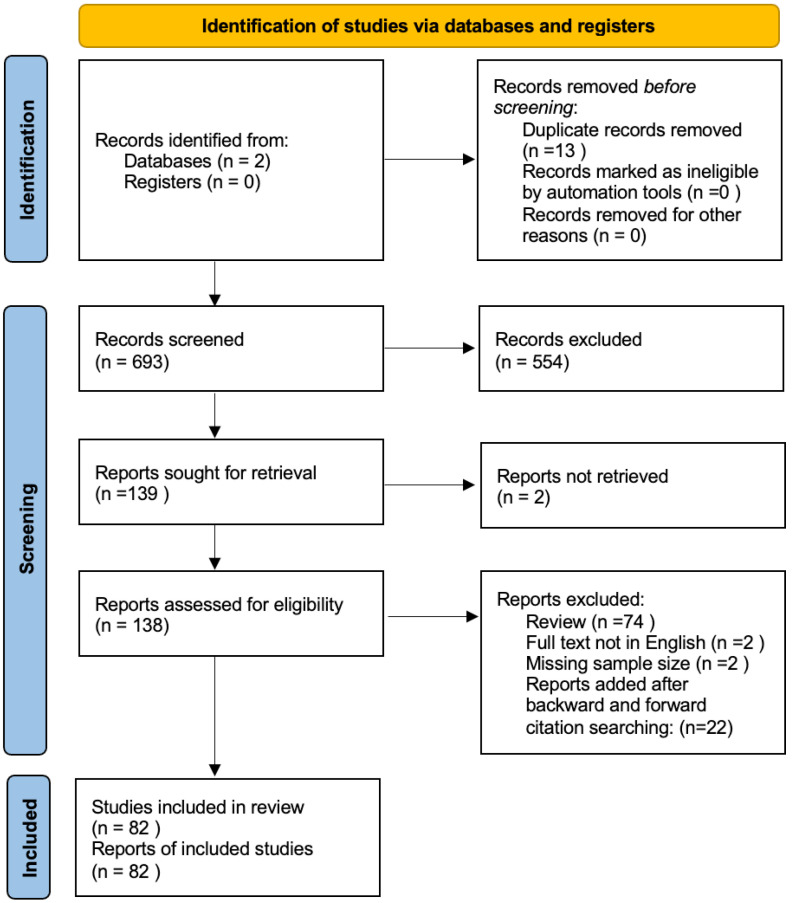
The search of the literature yielded a total of 693 results. The duplicate records were then removed (n = 13), and the records were excluded via a title and abstract screening; 138 studies were found to be relevant to our research question and were assessed for eligibility (Figure 1). Upon a full-text review and forward search, 82 articles were finally included.
Figure 1.
Prisma flowchart summarizing the results of our research.
The reviewed papers were further divided into six categories based on their focus:
-
(1)
Proteoglycans (Table 1);
-
(2)
Fibrillary proteins, which were further subdivided into three subcategories: collagen (Table 2a), fibronectin (Table 2b), and laminins (Table 2c);
-
(3)
Periostin (Table 3);
-
(4)
Glycoproteins (Table 4);
-
(5)
Degradative Enzymes (Table 5);
-
(6)
Physical forces (Table 6);
-
(7)
Glioma cell and microglia migratory and infiltrative patterns (Table 7).
Table 1.
Proteoglycans.
| Author | Year | Type of Study | Sample Size | Main Findings |
|---|---|---|---|---|
| Logun, M.T. [6] | 2017 | In vitro cell culture | 1 human cell line | CS-GAGs directly induce the enhanced cell migration and haptotaxis of glioma cells. |
| Logun, M.T. [7] | 2019 | In vitro cell culture | 1 murine cell line | GAG promotes GBM cell invasion. |
| Schrappe, M. [8] | 1991 | Human GBMs and monoclonal antibodies | 5 human GBMs | Human GBMs specifically express a chondroitin sulfate proteoglycan that is recognized by monoclonal antibodies and is localized on the glioma cell surface. |
| Kim, Y. [9] | 2018 | Mathematical model | High-molecular-weight CSPGs can regulate the exodus of local reactive astrocytes from the main tumor lesion, leading to an encapsulation of noninvasive tumors and an inhibition of tumor invasion. | |
| Silver, D.J. [10] | 2013 | In vitro cell culture and xenograft | 3 human cell lines | Microenvironmental glycosylated chondroitin sulfate proteoglycans inversely correlate with the invasive character of human gliomas. |
| Onken, J. [11] | 2013 | In vitro cell culture and siRNA | 2 human cell lines | Versican isoform V1 is a proliferation-enhancing and promigratory molecule in high-grade glioma in vitro. |
| Tran, V.M. [12] | 2018 | In vitro cell culture | 4 human cell lines and 2 murine cell lines | HPSE has a role in tumor invasion, acting on HSPGs on the cell surface. |
| Su, G. [13] | 2006 | In vitro cell culture | 5 human cell lines | HSPGs have a great capability to promote FGF-2 signaling. The enhanced HSPG activity correlated with a high level of expression of Gpc-1 and structural HSGAG alterations. |
| Watanabe, A. [14] | 2005 | Reverse transcription PCR | 10 glioma cell lines, 2 GBM specimens, and 2 normal brain specimens | Syndecan-1 is a key molecule in the motility of cells; it is crucial in coupling the organization of fascin spikes in response to a physiological extracellular ligand, TSP-1, and an overexpression of syndecan-1 in a heterologous cell type is sufficient for causing a dramatic enhancement of cell spreading and formation of fascin spikes in response to TSP-1. It is significantly expressed by GBM cells. |
| Chen, J.E. [15] | 2018 | matrix-bound HA and xenograft GBM population | 1 patient-derived xenograft GBM population | GBM migration is strongly influenced by HA molecular weight. |
| Hayen, W. [16] | 1999 | hyaluronan-containing fibrin gels and GBM cell lines | 1 GBM cell line | In complex three-dimensional substrates, the predominant effect of hyaluronan on cell migration might be indirect and requires modulation of fibrin polymerization. |
| Chen, J.W.E. [17] | 2018 | In vitro cell culture and hyaluronan synthesis inhibition | 2 cell lines | GBM cells under hypoxia show invasive behavior. The lack of matrix-bound HA affects GBM response by inducing compensatory HA secretion of this essential cell adhesive biomolecule, which is associated with increased GBM invasion. |
| Chen, J.W.E. [18] | 2022 | culture of patient-derived GBM cells | CD133+ GBM subpopulation increases in response to both hypoxia and matrix-bound hyaluronan. | |
| Akiyama, Y. [19] | 2001 | Cell culture and surgical specimen | Antibody blockage | HA-receptors contribute to brain tumor adhesion, proliferation, migration, and biological features. |
| Pibuel, M.A. [20] | 2021 | In vitro cell culture and hyaluronan synthesis inhibition | 2 human cell lines | 4MU markedly inhibits cell migration and induces senescence in human GBM cell lines; 4MU modulates the expression and the distribution of CD44, RHAMM, and MMP-2. |
| Tsatas, D. [21] | 2002 | In vitro cell culture and antibody blockage | 4 glioma cell lines | Hyaluronan induces genes encoding matrix-degrading enzymes (plasminogen cascade). |
| Zhang, H. [22] | 2022 | In vitro cell culture and hyaluronan synthesis inhibition | 3 cell lines | HA was found to mediate glioma proliferation, progression, and invasion; it potentially promoted macrophage recruitment and M2 polarization through the IL-1/CHI3L1 and TGF-b/CHI3L1 axes. |
Table 2.
(a) Collagen, (b) fibronectin, and (c) laminins.
| Author | Year | Type of Study | Sample Size | Main Findings |
|---|---|---|---|---|
| (a) | ||||
| Calori, I.R. [23] | 2022 | GBM cell lines and type I collagen | 4 cell lines | The enzymatic cleavage of collagen affects spheroid morphology and increases cell migration while maintaining cell viability. |
| Wang, Y. [24] | 2022 | GBM cell lines and COL1A2 siRNA | 3 cell lines | COL1A2 plays an important role in driving GBM progression. COL1A2 inhibition attenuates GBM proliferation by promoting cell cycle arrest. |
| Chintala, et al. [25] | 1996 | In vitro cell culture | 4 cell lines | Collagen type II is involved in migration and invasion of glioblastoma cells. |
| Mammoto, T. et al. [26] | 2013 | In vitro cell culture and antibody blockage | 3 cell lines | D-penicillamine decreases collagen expression, disrupts collagen structure in tumors, and inhibits brain tumor growth. |
| Senner, V. [27] | 2008 | In vitro cell culture and siRNA | 5 cell lines | Glioma cell lines can utilize collagen type XVI as a substrate for adhesion. |
| Huijbers, I.J., et al. [28] | 2010 | Human cell culture and antibody blockage | 79 gliomas | Fibrillar collagens are extensively deposited in GBMs; the collagen type I internalization receptor Endo180 is both highly expressed in these tumors and serves to mediate the invasion of tumor cells through collagen-containing matrices. |
| Lin, J. [29] | 2021 | In vitro cell culture and xenograft | 58 gliomas | P4HA2 is a prognostic marker and exerts oncogenic functions to promote the malignancy of gliomas (grade II to grade IV). The underlying mechanism may be regulating the collagen-dependent PI3K/AKT signaling pathway. |
| Jiang, X. [30] | 2017 | In vitro cell culture and xenograft | 2 cell lines | HSP47 promotes GBM stem-like cell survival by modulating tumor microenvironment ECM through TGF-β pathway. |
| (b) | ||||
| Author | Year | Type of Study | Sample Size | Main Findings |
| Ohnishi, T. [31] | 1998 | In vitro human cell culture and antibody blockage | 9 GBM samples | Fibronectin concentration seems to be higher in tumor cells and promotes migration of glioma cells. |
| Chintala, S.K. [32] | 1996 | In vitro human cell culture and antibody blockage | 13 GBM samples | Glioblastoma cells produce collagen type IV, laminins, and fibronectin. |
| Caffo, M. [33] | 2004 | In vitro human cell culture and antibody blockage | 6 GBM samples | Integrins appear to be of great interest in GBM treatment either as targeted therapies, drug-delivering vectors, or diagnostic tools for tumor imaging. |
| Serres, E. [34] | 2013 | In vitro human cell culture and antibody blockage | 3 GBM lines | FN produced by tumor cells has a role in GBM pathophysiology. |
| Sengupta, S. [35] | 2010 | Xenograft and siRNA | Murine glioma cell line | Fibronectin silencing aborts integrin signaling in GL261 cells and fails to initiate Src kinase and STAT3 activity, thus aggressively reducing survivin expression. |
| Huang, J.M. [36] | 2006 | In vitro cell culture and GBM cell line | 1 GBM cell line | The interaction between beta1-integrin and FN may stimulate U251MG cell migration, changing the structure of the microfilament skeleton and the number of pseudopodia. Beta1-integrin may play a role in the LN-mediated in vitro invasion of U251MG cells. |
| Yu, S. [37] | 2020 | Xenograft and siRNA | 3 cell lines | GBP2 dramatically promotes GBM tumor growth and invasion in mice and significantly reduces the survival time of the mice with a tumor. |
| Kabir, F. [38] | 2022 | Mathematical model—bioinformatics model | 7 data sets | FN1 has prognostic value in GBMs. |
| (c) | ||||
| Author | Year | Model Used | Sample Size | Main Findings |
| Tysnes, B.B. [39] | 1999 | Xenograft | 5 GBM samples | Laminins can be produced by GFAP positive cells during glioma cell invasion in humans. |
| Caffo, M. [33] | 2004 | In vitro human cells and antibody blockage | 6 GBM samples | Integrins appear to be of great interest in GB treatment either as targeted therapies, drug-delivering vectors, or diagnostic tools for tumor imaging. |
| Sun, T. [40] | 2022 | Xenograft culture | 107 GBMS samples | Inhibition of the vascular BM component laminin-411, which is produced by tumor cells like many other tumor ECM components, disrupts the perivascular CSC niche, negatively affects CSCs, and may enhance the efficacy of glioma therapy. |
| Khazenzon, N.M. [41] | 2003 | In vitro human cell culture and antibody blockage | 2 cell lines | Laminin-8 may play an important role in glioma invasion. |
| Gamble, J.T. [42] | 2018 | Xenograft culture | 1 cell line | Laminin alpha 5 significantly lowers the invasion of mobile U251MG cells. |
Table 3.
Periostin.
| Author | Year | Model Used | Sample Size | Main Findings |
|---|---|---|---|---|
| Wang, H. [43] | 2013 | Frozen glioma tissue and microarray | 220 frozen glioma tissues | The expression levels of POSTN are relative to glioma grade progression and are inversely correlated with overall survival in high-grade glioma patients. |
| Landré, V. [44] | 2016 | In vitro human cell culture, plasmids, and antibodies | 2 cell lines | TAp73 controls glioblastoma cell invasion by regulating the expression of the matricellular protein POSTN. |
| Ouanouki, A. [45] | 2018 | In vitro human cell culture | 1 cell line | Periostin acts as a central element in TGF-β-induced EMT. |
Table 4.
Glycoprotein—tenascin.
| Author | Year | Model Used | Sample Size | Main Findings |
|---|---|---|---|---|
| Xia, S. [46] | 2016 | Xenograft culture | 2 human cell lines | TNC expression levels or gene copy numbers do not significantly affect patient survival, and TNC knockdown cells are more sensitive to antiproliferative strategies. |
| Hirata, E. [47] | 2009 | Xenograft culture | 1 human cell line | Endogenous tenascin facilitates GBM cell invasion by regulating focal adhesion, and, therefore, GBMs with higher Tenascin C expression have a more aggressive behavior. |
| Zhang, J.F. [48] | 2019 | In vitro human cell culture and siRNA | 2 cell lines | IL-33/NF-κB/TNC supports cancer progression. |
| Sarkar, S. [49] | 2015 | Xenograft culture | 7 human cell lines | TNC is a promoter of the invasiveness of BTICs through a mechanism involving ADAM-9 proteolysis via the c-Jun NH2-terminal kinase pathway. |
| Sarkar, S. [50] | 2006 | In vitro human cell culture | 2 cell lines | Tenascin-C is a favorable substrate for glioma invasiveness; its effect is mediated through MMP-12. |
| Mai, J. [51] | 2002 | In vitro human cell culture | Cathepsin B and Tenascin-C are highly expressed in malignant anaplastic astrocytomas and glioblastomas when compared to normal brain tissues and are associated with tumor neovessels. |
Table 5.
Degradative enzymes.
| Author | Year | Model Used | Sample Size | Main Findings |
|---|---|---|---|---|
| Li, Q. [52] | 2016 Mar | Data set and genome mRNA | 23 types of MMPs and 305 gliomas | Patients expressing MMP9 may have a longer survival and may benefit from temozolomide chemotherapy. |
| Lakka, S.S. [53] | 2004 | In vitro human cell culture and siRNA | 1 cell line | Simultaneous RNAi-mediated targeting of MMP-9 and cathepsin B has potential application in the treatment of human gliomas. |
| Kargiotis, O. [54] | 2008 | In vitro human cell culture and xenograft | 4 cell lines | MMP-2 inhibition induces apoptotic cell death and suppresses tumor growth. |
| Schuler, P.J. [55] | 2012 | Xenograft | 1 cell line | uPA, uPAR, MMP-2, and MMP-9 play an important role in GBM growth. |
| Sun, J. [56] | 2019 | In vitro human cell culture | 12 GBMs | TRAF6 and MMP9 have higher expression in GBMs compared to adjacent tissues. High expression of TRAF6 and MMP9 is significantly associated with unfavorable prognoses. |
| Zhao, Y. [57] | 2008 | In vitro human cell culture and recombinant protein | 1 cell line | uPA directly cleaves the latent form of MMP-9 both at the N- and C-terminus, and this novel activation pathway promotes U1242 GBM cell invasion. |
| Chang, L. [58] | 2015 | In vitro human cell culture | 1 cell line | The hedgehog signaling pathway promotes the invasion and migration of GBM cells by enhancing MMP-2 and MMP-9 expression via the PI3K/AKT pathway. |
| Zheng, Q. [59] | 2019 | In vitro human cell culture | 2 cell lines | IL-17A promotes GBM cell migration and invasion via PI3K/AKT signaling pathway. |
| Das, G. [60] | 2011 | Human cell culture and antibodies | 2 cell lines | Rictor bridges two major pathways—Akt (PKB)/mTOR and Raf-1-MEK-ERK—for regulation of MMP-9 activity and invasion of glioma tumor cells. |
| Djediai, S. [61] | 2021 | Human cell culture and RNA isolation | 1 cell line | MT1-MMP and TGF-β mediate EMT-like induction in glioblastoma cells. |
| Zhai, Y. [62] | 2022 | Immunostaining with rabbit monoclonal antibodies | 214 gliomas | MT1-MMP, β1-integrin, and YAP1 are prognostic biomarkers. |
| Held-Feindt, J. [63] | 2005 | Human GBM samples, GBM cell lines, and RT-PCR | 4 GBM cell lines | In human glioblastomas, secretory proteases, such as ADAMTS4 and ADAMTS5, are expressed at the mRNA and protein levels in considerable amounts. |
| Siney, E.J. [64] | 2017 | Excised high-grade glioma and antibody inhibition | 12 excised GBMs | ADAM10 and ADAM17 inhibition selectively increases GSC migration, and the migrated GSCs exhibit a differentiated phenotype. |
Table 6.
Physical forces.
| Author | Year | Model Used | Sample Size | Main Findings |
|---|---|---|---|---|
| Herrera-Perez, M. [65] | 2015 | In vitro human cell culture in 3D matrix | Migration of glioblastoma stem cells is reduced by the presence of hyaluronan. | |
| Ulrich, T.A. [66] | 2009 | In vitro human cell culture | 2 cell lines | Increasing ECM rigidity can induce a cascade of phenotypic changes in human glioma cells, which includes increased cell spreading, faster motility, and enhanced proliferation. |
| Kaufman, L.J. [67] | 2005 | Human cells encapsulated in 3D hydrogel | 1 cell line | GBM tumors are affected significantly by the total collagen concentration in the gel, and there are distinct growth patterns in low- and high-concentration collagen type I gels. Specifically, increasing concentrations of collagen type I correlate positively with invasion but negatively with MTS growth. |
| Wang, C. [68] | 2014 | In vitro human cell culture | 15 primer sequences | Matrix stiffness modulates GBM progression. |
| Lim, E.J. [69] | 2018 | In vitro cell culture and siRNA | 1 cell line | tMSLCs, as stromal cells, provide force-mediated proinvasive ECM remodeling in the GBM microenvironment. |
| Pu, W. [70] | 2020 | In vitro human cell culture | 2 cell lines | MPs play pivotal roles in the invasiveness of GBMs by degrading the surrounding tissue, activating signal transduction, and releasing ECM-bound growth factors. |
Table 7.
Glioma cell and microglia migratory and infiltrative patterns.
| Author | Year | Model Used | Sample Size | Main Findings |
|---|---|---|---|---|
| Koh, I. [71] | 2018 | In vitro human cell culture | 1 cell line | MMP9 and HAS2 are highly upregulated in pdGCs cultured within the pdECM. In fact, both MMPs and HASs have been implicated in playing crucial roles in GBM invasiveness. |
| Herrera-Perez, M. [65] | 2015 | In vitro human cell culture | GSC migration is not limited to a unique migration mode that is usually observed in in vitro studies but is able to concomitantly exhibit multiple migration modes (collective and single) as a response to the heterogeneity of the environment. | |
| Rao, S.S. [72] | 2013 | In vitro human cell culture and 3D matrix | 1 cell line | GBM migration is an inverse function of HA concentration, with HA impeding and eventually stopping cell movement. |
| Cui, Y. [73] | 2020 | In vitro human cell culture and 3D matrix | 1 cell line | HA addition to the collagen culture environment induces many changes consistent with the amoeboid migratory phenotype, including rounded morphology, squeezing or gliding motility, cortical actin expression, reduced cell–fiber interactions, and reduced integrin expression. |
| Hirata, E. [74] | 2012 | Xenograft and shRNA | 3 cell lines | Zizimin1 appears to play an important role in the formation of multiple pseudopodia and invasion of the brain parenchyma. |
| Lively, S. [75] | 2013 | Cultured rat microglial cells | 1 cell line | Microglial cells migrate during CNS development and after CNS damage or disease. |
| de Vrij, J. [76] | 2015 | Human cell culture | 2 cell lines | EVs are mechanisms for GBMs to use to induce MT1-MMP expression in GBM associated microglia, supporting tumor growth. |
| Gabrusiewicz, K. [77] | 2011 | Murine glioma cells and xenograft | 1 cell line | Resident microglia and blood-derived macrophages contribute to a pool of glioma-infiltrating immune cells and regulate tumor angiogenesis and invasion, which are essential for glioma progression. |
| Bettinger, I. [78] | 2002 | Murine glioma cell and mouse microglial cell cultures | 1 cell line | Microglial cells promote the invasive phenotype of diffuse astrocytoma cells. |
| Markovic, D.S. [79] | 2005 | Murine glioma cell and microglial cell cultures | The presence of microglia in a GBM has a protumorigenic effect. | |
| Markovic, D.S. [80] | 2009 | Murine glioma cells and shRNA | 1 cell line | Protumorigenic role of microglial cells is substantial and may put microglial cells into focus as a target for new brain tumor therapies. Therapeutic TLR blockade, which may be achieved with TLR subtype-specific antagonists, could serve as a future tool to attenuate microglia-promoted tumor invasion. |
| Wu, C.Y.J. [81] | 2020 | Human cell culture and poli- and monoclonal antibodies | 3 cell lines | Chemokine axis in the glioma microenvironment is subject to CCL5-mediated invasion, and such regulation is facilitated by GAM activation. Restriction of calcium-dependent pathways may be pivotal in eliminating CCL5/GAM-regulated glioma invasion. |
| Kulla, A. [82] | 2000 | Human cell culture | 90 gliomas | Higher numbers of tumor-infiltrating macrophagic/microglial cells are present in TN-positive areas of human gliomas; TN serves as a permissive substrate for macrophage migration and may have a certain role in modulating and possibly promoting the trafficking of cells of monocyte lineage in malignant human gliomas. |
| Xia, S. [46] | 2016 | Xenograft culture and shRNA | 2 cell lines | TNC knockdown cells are more sensitive to antiproliferative strategies, which could ultimately lead to novel combinatory antitumor strategies that can target both tumor invasion and proliferation. |
| Hu, F. [83] | 2015 | Murine and human cells and animal xenograft | 5 cell lines | Versican, released from gliomas, promotes tumor expansion through glioma-associated microglial/macrophage TLR2 signaling and subsequent expression of MT1-MMP. |
| Juliano, J. [84] | 2018 | Animal model and retrovirus injection | Increased density of glioma cells is correlated with increased activation of microglia. | |
3.1. Proteoglycans
Proteoglycans are a heterogeneous group of complex extracellular and cell surface macromolecules composed of a central core protein with covalently linked glycosaminoglycan (GAG) chains. Through interactions with chemokines, neurotrophins, growth factors, and the other components of the ECM, proteoglycans (PGs) play a critical role in many basic processes of the CNS, including cellular proliferation, migration, specification, synaptogenesis, plasticity, and regeneration [6]. For these reasons, it has been proposed that PGs could be involved in several aspects of tumor biology, including cell proliferation, tumor cell adhesion and migration, inflammation, and angiogenesis. Indeed, recent studies have proven that heparan sulfate proteoglycans (HSPGs) and chondroitin sulfate proteoglycans (CSPGs) are largely upregulated in GBM samples relative to normal brain tissue [7].
Chondroitin sulfate proteoglycans (CSPGs) consist of a protein core and covalently attached chondroitin sulfate side chains. It has been noticed that CSPGs and related enzymes are upregulated in the GBM microenvironment relative to normal tissue [8]. Similarly, in vitro studies have shown an upregulation of focal adhesion proteins, such as FAK and Vinculin, and a faster migration of glioma cells in oversulfated hydrogel matrices when compared to nonsulfated hydrogels. Moreover, these data suggest that CSPGs can modulate glioma invasiveness in a GAG-sulfation-dependent manner. Indeed, CSPGs show a different affinity to chemokines and chemokine receptors according to the sulfatation rate of CS-GAG [7]. Beyond the sulfatation rate, even the pure concentration of CSPGs in the microenvironment has been identified as a major parameter in the regulation of glioma cell invasiveness. In fact, low-CSPG levels in the microenvironment are associated with the downregulation of the LAR (leukocyte common antigen-related)-CSGAG complex, while high-CSPG levels induce its upregulation. LAR is a CS-GAG receptor that regulates cell adhesion to the ECM components. When LAR-CSGAG is downregulated, adhesion between the tumor cells and the ECM components is weak, therefore allowing the tumor cells to spread. On the other hand, high levels of CSPGs and, consequently, an upregulation of LAR-CSGAG induces strong adhesion, preventing the dispersion of the glioma cells. Moreover, LAR-CSGAG seems to influence the activation and migration of the microglia toward the tumor periphery [9,10].
Versican is one of the most represented proteins among the CSPGs in the ECM. An in vitro cell culture study revealed an important role of Versican in the regulation of glioma cell migration and adhesion. Indeed, the downregulation of Versican in the isoform V1 by siRNAs is associated with a significant reduction in proliferation and migration in glioblastoma cell lines, and TGF-beta 2, a well-known modulator of glioma cell invasion, was identified as the primary inductor of Versican 1 [11].
HSPGs consist of a core protein and covalently attached heparan sulfate (HS) glycosaminoglycan chains. Extensive co- and posttranslational enzymatic modifications, particularly involving the 6-O-sulfate (6OS) of glucosamine, generate great structural heterogeneity. An analysis conducted on human GBM cell lines and murine GBM cell lines demonstrated great heterogeneity in the content and in the structure of HS glycosaminoglycans between different glioma cell lines, suggesting a role in tumorigenesis and subtype differentiation. Heparanase is an enzyme involved in the biological regulation of HSPGs since it cleaves HS chains to reduce the HS chain length and to release smaller biologically active oligosaccharides. Heparanase induces the modification of the HS content and structure in the microenvironment and, therefore, is thought to be involved in GBM genesis. Indeed, a study on cell invasion into a three-dimensional matrix showed that clones with heterozygous deletions in HPSE and reduced HPSE expression exhibit a marked decrease in tumor cell invasion and cell adhesion to laminins [12].
Glypicans and Syndecans represent the most expressed families of heparan sulfate proteoglycans in the brain. Glypicans are overexpressed in the glioma microenvironment when compared to normal brain tissue and, according to the literature, can stimulate glioma growth by inducing the upregulation of the FGF-2 signal [13,14]. Similarly, the Syndecan family is overexpressed in the glioma microenvironment. Particularly, Syndecan-1 is overexpressed in almost all glioma cell lines studied and is poorly expressed in normal specimens. It has been suggested that the overexpression of Syndecan-1 in the glioma microenvironment induces tumor invasion through the upregulation of thrombospondin-1 [14].
Hyaluronan is a linear and nonsulfated GAG which can bind ECM proteins and proteoglycans, building a three-dimensional network. HA serves as a ligand for the membrane receptor CD-44 and the RHAMM and MEK/ERK signaling pathways, participating in cellular growth, cellular proliferation, and cellular differentiation. In vitro studies have demonstrated that glioma cell behavior differs on the base of the HA structure in the tumor microenvironment since hydrogel matrices containing high-molecular-weight HA (500K) showed significantly reduced invasion when compared to all other hydrogel groups (-HA, 10, and 60K) [15]. Moreover, gels resulting from fibrin polymerization in the presence of HA stimulate glioma cell migration, suggesting that HA could regulate glioma cell invasiveness by modulating the fibrin fiber architecture [16]. The recent data show that hypoxia could enhance endogenous HA production by glioblastoma cells [17] and that HA could stimulate glioblastoma growth by upregulating CD133+ GBM cell fractions [18]. Concerning HA downstream signaling inducing glioma cell migration, receptor CD-44 and RHAMM appear to be involved [19]. CD44-HA mediated cell invasion can be modulated by EGFR, a well-known receptor overexpressed in gliomas. In fact, CD-44 binds to EGFR, leading to an upregulation of urokinase-type plasminogen activator (uPA), urokinase-type plasminogen activator receptor (uPAR), and plasminogen activator inhibitor-1 (PAI-1) in response to HA [20,21]. Moreover, recent studies have suggested that HA potentially promotes macrophage recruitment and M2 polarization through the IL-1/CHI3L1 and TGF-b/CHI3L1 axes and that it also regulates the expression of PD-L1 [22].
3.2. Fibrillar Proteins
3.2.1. Collagen
Normal brain tissue ECMs are poor in collagen, whereas its content in the glioma microenvironment and especially around vessels presents a large increase. Actually, different subtypes of collagen have been investigated over the years and are related to GBM invasiveness. Recent findings have shown that GBM cell lines form tight spheroids in the presence of type I collagen and that the enzymatic cleavage of collagen affects spheroid morphology and increases cell migration [23]. In particular, the collagen alpha-2(I) chain (COL1A2) was found to be upregulated in GBMs compared with normal brain tissue, and it is related to poor progression-free survival and overall survival [24]. Similarly, collagen type III was found to play a role in the modulation of migration and the invasion of glioblastoma cell lines in a dose-related manner; moreover, migration and invasion were inhibited in the presence of monoclonal type III collagen antibodies [25]. In vitro studies revealed that, under the specific conditions of physical compaction, human glioblastoma cell lines induce the expression of collagen type IV and type VI. Moreover, collagen disruptors such as β-aminopropionitrile induced the inhibition of glioblastoma growth in the mouse orthotopic brain tumor model [26]. Collagen type XVI mRNA was also found to be upregulated in glioblastoma cell lines. It seems to play a role in glioma cell adhesion to the ECM since a SiRNA knockdown resulted in decreased cell adhesion, although migration remained unchanged [27].
Different families of receptors and different pathways have been supposed to be involved in collagen-related GBM invasion. Functionally, Endo180 (CD280), a collagen-binding receptor overexpressed in GBMs, serves as the major collagen internalization receptor in GBMs and is critical in glioma cell invasion into the ECM [28]. Prolyl-4-hydroxylase subunit 2 (P4HA2) is a member of the collagen modification enzymes involved in the remodeling of the extracellular matrix (ECM). The main transcriptional P4HA2 level was found to be higher in glioma samples compared to normal brain tissue, and this correlates with glioma grading and patient survival; moreover, a P4HA2 knockdown significantly decreased cellular invasion and migration in Matrigel. An in vivo subcutaneous xenograft assay in a nude mouse model led to the same conclusions. Since P4HA2 overexpression correlates with higher levels of collagen types I, IV, and VI, a pathway was hypothesized in which P4HA4 promotes an overexpression of the collagen content, which serves as a major ligand for the activation of PI3K/AKT signaling [29]. HSP47 serves as a human chaperone protein for collagen. Recent findings suggest that HSP47 is significantly overexpressed in GBMs and that it promotes GBM stem-like cell survival by modulating the tumor microenvironment through the TGF-β pathway [30].
3.2.2. Fibronectin
Fibronectin (FN) is a high-molecular-weight glycoprotein poorly represented in normal brain tissue ECMs that binds cellular receptors such as integrins and the other components of the ECM, playing an important role in cellular growth, cellular differentiation, migration, and embryonic development. In vitro and in vivo immunohistochemical studies revealed that fibronectin, especially the isoform containing the ED-A and ED-B sequences, and fibronectin cellular receptors are present in almost all glioblastoma microenvironments, especially around the vessels [31,32,33]. In cell cultures, glioblastoma cells showed chemotactic migration towards fibronectin in a dose-dependent manner; moreover, cell adhesion to fibronectin appeared to be dose-related and dependent on glioma invasiveness [34]. In 3D matrix cultures, the depletion of FN by targeted short hairpin RNA expression compromised collective invasion. Similarly, in orthotopic grafts, FN depletion significantly reduced tumor growth and angiogenesis [35,36].
It has been hypothesized that FN-related GBM invasion downstream involves the Integrin B1 fibronectin receptor and the Src kinase/STAT3 signaling pathways [36]. Moreover, recent findings suggest that GBP2, an interferon-inducible large GTPase, is essential in inducing FN expression via the Stat3-pathway [37]. These data suggest the role of fibronectin in regulating in vivo and in vitro glioblastoma cell invasion; moreover, it has been recently proposed as a prognostic biomarker since high FN1 expression appears to be related to poor prognoses [38].
3.2.3. Laminins
Laminins are a large group of glycoproteins consisting of three long polypeptides (the alpha, beta, and gamma chains present in different isoforms) that are not abundant in normal brain tissue and that are mainly present in the basal lamina. The proteins are multifunctional and play roles in development, differentiation, and cell migration, as they can interact with many cell surface proteins. Laminins are abundant in the glioma microenvironment and are mostly associated with the basal lamina of blood vessels, especially in the brain/tumor confrontation zone. In vitro studies revealed that, in the presence of laminins, glioma cell lines form F-actins, form strong and dense stress fibers, and increase the number of pseudopodia on the cell surface, stimulating cell adhesion and invasion. Moreover, during the progression of glial tumors, laminin-9 (alpha4beta2gamma1) is switched to laminin-8 (alpha4beta1gamma1), which is, therefore, considered to be primarily involved in glioblastoma invasion [39]. According to these findings, the overexpression of Laminin isoform-411 (Laminin 8) has been identified to be correlated with higher recurrence rates and the shorter survival of GBM patients. As expected, the depletion of laminin-411 with CRISPR/Cas9 in human GBM cells led to the reduced growth of the resultant intracranial tumors in mice and significantly increased the survival of the host animals by suppressing the Notch pathways compared to mice with untreated cells [40]. Similar in vitro studies have shown that antisense oligonucleotides against both the alpha4 and beta1 chains of laminin-8 are able to significantly block the invasion of cocultures in Matrigel [41]. The debate is on the role of Laminin alpha-5. Recent zebrafish xenograft models with knocked-down Laminin alpha-5 indicate that lama5 discourages glioblastoma cell dispersal and decreases invasion despite previous evidence indicating laminin alpha-5 to be promigratory in in vitro settings [42].
3.2.4. Periostin
The implication of PRO in GBM growth has been enquired in the last decade. Indeed, it was found that the expression of PRO is related to glioma grading and is inversely related to OS. Moreover, in cases of the overexpression of PRO, the genes related to cell migration and proliferation, such as MMP-9, were significantly enriched [43]. Recent evidence suggests p73 to be a main inductor of glioblastoma cell invasion through the direct activation of PRO [44]. It is thought that PRO could stimulate the transforming growth factor β (TGF-β)-induced epithelial–mesenchymal transition via the Akt and Fak signaling pathways [45].
3.3. Glycoproteins
Tenascin
Tenascins are large hetero- or homohexameric glycoproteins in which the subunits are held together with disulfide bonds. Four members are known in this family: Tenascin-C, -R, -X, and -W. Tenascins are involved in the modulation of cell adhesion, migration, and growth. Tenascin–C is found to be overexpressed in the glioblastoma microenvironment. In vitro, TNC knockdown glioblastoma cell lines were characterized by increased adhesion to the ECM components mediated through the upregulation of the FAK-pathway [46]. Moreover, in the presence of endogenous or exogenous TNC, glioblastoma cell lines expressed an increase in cell migration in a dose-related manner, while no differences in cellular growth were detected. Similarly, mouse xenograft models showed that tumors derived from TNC knockdown cells were less invasive, with tumor cells confined to better-defined tumor borders compared to the control tumors, while tumor size was approximately equal [47]. These data suggest the role of tenascin-C in modulating glioma cell invasion and migration through the ECM without interfering with cell proliferation.
Recently, it has been proposed that TNC expression in glioma tissue may be promoted by the IL-33-ST2-NFkB pathway [48]. Although the details of the role of downstream Tenascin-C in GBM invasion are not clear up to date, proteases, Cathepsin-B, MMP-12, and the ADAM-9-MAPK8 pathway were found to play a pivotal role in TNC-mediated GBM invasion [49,50,51].
3.4. Degradative Enzymes
MMPs are a group of zinc-dependent endopeptidases that degrade several components of the ECM via integrin mediation, participating in tissue structural changes, cell proliferation, and cell migration. At least 23 members of the human MMP family have been identified. GBM cells are known to secrete various MMPs through which they degrade various ECM proteins, including fibronectin, laminins, collagen, and gelatin, promoting cell migration and releasing activated proteins through cleavage [85]. Specifically, a specific subgroup of MMPs (including MMP-1, -2, -7, -9, -11, -12, -14, -15, and -25) was shown to be strictly related to glioma grading and glioblastoma development. In particular, high levels of MMP-9 and MMP-2 were found to be associated with a higher tumor grade, a lesser response to chemotherapy, and a worse survival outcome [52,53,54].
Different pathways of MMP activation have been investigated. The role of the uPA-uPAR pathway in the activation of MMP-9 and MMP-2 in GBMs is well established [55]. uPA is a protease, which is overexpressed in high-grade gliomas, that converts plasminogen to plasmin with a better efficacy when anchored to its receptor, uPAR. Both uPA and plasmin are responsible for MMP activation [56,57]. Moreover, uPA/uPAR, through an interaction with the integrin receptor, has been proven to activate downstream signaling through the activation of FAK, ERK, and Src, which lead to F-Actin assembly, membrane protrusion, and cell migration. Recently, other signaling pathways have been identified. The activation of Sonic Hedgehog signaling is related to an increase in the migration and invasion of GBM cells, which is mediated through the overexpression of MMP-9/-2 via the PI3K/AKT pathway [58]. In a similar way, it has been suggested that even IL-17A might control glioma cell invasiveness by inducing the overexpression of MMP-9/-2 via PI3K/AKT [59]. However, Rictor, a component of the mTOR complex, induces glioma cell migration, increasing MMP-9 expression through the Raf-1-MEK-ERK signaling pathway [60].
Membrane-type MMPs are a subgroup of metalloproteinases that are membrane-associated and have cytoplasmic domains, which may be important in cellular signaling. It has been proven that MT-MMP plays a role in the cleavage of pro-MMP to the active form of MMP-2 [86]. MT1-MMP was found to be involved in the epithelial-to-mesenchymal-transition of glioblastoma cells through pathway signaling, which involves transforming growth factor beta and SNAIL [61]. Similarly, other studies found a correlation between the expression of the MT1-MMP, Beta1-integrin, YAP1 pathways and the grading of gliomas [62].
The subfamily of adamalysins (ADAM proteases) was shown to be overexpressed in glioblastoma cell lines in vitro and in glioblastoma patients and may contribute to cell invasion. ADAM-10 and ADAM-17 are overexpressed in the glioblastoma microenvironment. In vitro studies reported that ADAM10 and ADAM17 inhibition selectively increases glioma sphere-forming cells but not neural stem cell migration and that the migrated GSCs exhibit a differentiated phenotype, suggesting a role in retaining the cells in the tumorigenic environment in an undifferentiated state [63,64].
3.5. Physical Forces
Many studies have proven that ECM mechanical changes influence glioma cell invasion, migration, and morphology [65]. Particularly, in vitro studies revealed that, in highly rigid ECMs, tumor cells spread extensively and migrate rapidly, whereas, in lower rigidity ECMs, comparable to normal brain tissue, tumor cells appear rounded and fail to migrate productively [66,67]. Moreover, a variation in matrix stiffness induced the differential expression of enzymes in which HA-synthases and MMP-1 were upregulated in the stiff condition [68]. It has been proposed that tumor-associated mesenchymal stem-like cells could play an important role in glioblastoma ECM remodeling through CCL2/JAK1/MLC2 signaling [69].
Other in vitro studies reported that, in response to hyperosmolarity and hydrostatic pressure, GBM cell lines upregulated the expression of urokinase-type plasminogen activator (uPA) and matrix metalloproteinases (MMPs), promoting cell invasion [70].
3.6. Glioma Cell and Microglia Migration and Invasion Patterns
Carcinoma cell invasion is a complex reciprocal process in which cells induce the reorganization of the structure and composition of the ECM, and, in turn, the microenvironment influences cancer cell function, migration pathways, and cell morphology [71].
GBM cells are regulated through several environmental mechanisms that facilitate the spread of these tumors. For example, the invasion pattern of malignant GBMs is associated with the distinct anatomic pathways following the myelinated fiber tracts and blood vessels. In addition to the anatomical and physical aspects, there is accumulating evidence that specific ECM components (such as hyaluronan, vitronectin, and tenascin-C) are unregulated at the border of the spreading GBMs, and this may alter cellular invasiveness. Molecular guidance during cell invasion is often dependent on the ECM, and the underlying mechanism of glioblastoma invasion and the GBM-specific ECM microenvironment represent interesting and potentially meaningful fields of research [65].
In vitro studies, using patient-tissue-derived decellularized ECMs and glioblastoma cell lines, revealed that cancer cells that move through the ECM can be distinguished by their invasion mode. The mesenchymal mode is based on the MMP proteolytic degradation of the matrix, and, in this mode, cells have an elongated morphology and show a polarized extension of the leading edge; additionally, in the ameboid mode, rounded cells tend to migrate in the absence of proteolytic ECM degradation and squeeze through the ECM space [71]. Different ECM compositions have been proven to play a pivotal role in regulating the cell morphology and the migration pathway. For example, in 3D collagen matrices, glioma cells typically show mesenchymal-like migration, whereas, in the presence of HA cells, they assume an ameboid-like pattern [72]. Moreover, GBM cells treated with MMP2/9 inhibitors have a rounded-ameboid mode of invasion, whereas the inhibition of HA synthases (HASs) promotes the morphological transition from a rounded-amoeboid to an elongated-mesenchymal morphology [73]. These findings, together, suggest that the ECM-cell interaction could lead to a switch between these two patterns of migration, enhancing glioma cells’ ability for invasion and representing a mechanism of target therapy escaping.
According to the literature, glioma cell invasion develops preferentially along preexisting tracks such as myelinated axons and blood vessels. Indeed, in vivo studies have shown that the glioma cells that spread along blood vessels and those directly invading the brain parenchyma exhibit different morphological features, the former being spindle-shaped with a single pseudopodium towards the direction of movement and the latter exhibiting multiple pseudopodia with random invasion directions. Moreover, the former exhibits an overexpression of Rho family GTPase activity in contrast with the latter, which exhibits an overexpression of Rac1 and Cdc42 activity [74]. In support of these findings, recent in vitro studies showed that the glioma neurospheres located close to the rods tend to assume a collective strand and to perform fast migration along this physical support, maintaining cell–cell contact, whereas the cells facing the matrix directly exhibit single-cell and random migration in 3D matrices with pseudo vessels recreated using sterile microrods coated with Matrigel [75].
According to recent findings, the nontumoral cells in the glioma microenvironment may reciprocally interact with the ECM components and with glioma cells themselves, representing a further cell migration and invasiveness modulation system. Macrophages/microglia account for up to 30% of the cells in the glioma microenvironment. It is known that macrophages can assume two different forms in tissue repair and, most of all, in ECM remodeling: classical M1-activation, in which the macrophages present an ameboid or round shape and which has been supposed to sustain a proinflammatory role, and alternative M2-activation, in which unipolar macrophages are present and which has a role in antagonizing proinflammatory mediators [76]. In vitro studies showed that microglia can degrade and migrate through the ECM by using a wide range of degradative enzymes. Interestingly, M2-activated macrophages present a higher rate of migration compared to M1-activated macrophages, which is sustained, most of all, by the overexpression of MMP2, Cat-k, and Cat-s [77]. Glioma cells may directly influence the activity of surrounding nontumoral cells with extracellular vesicles, leading, in this instance, to a differentiation of macrophages toward the M2-activated form [78], which, indeed, is the most represented in the glioma microenvironment among the macrophage phenotypes, suggesting a role of activated macrophages/microglia in tumor growth.
Previous in vitro studies have shown that, in presence of microglia, glioma cells show a higher rate of migration and invasiveness. Moreover, such a phenomenon seems to be microglial-specific since replacing microglial with nonmicroglial cells, such as oligodendrocytes or endothelial cells, did not show any significant impact on glioma cell migration. It has been proposed that macrophages/microglia directly affect glioma cell migration through the ECM by secreting MMPs and that they indirectly affect it by promoting the activation of pro-MMP secreted in the microenvironment by glioma cells by means of membrane-type metalloproteases (MT-MMP), which has been proven to be overexpressed in tumor-associated microglia. Among all the different forms of degradative enzymes, MMP-2 seems to play a pivotal role. In fact, in organotypical brain slice models, MMP-2 activity was found to be much higher when glioma cells were cultured in the presence of microglia. Altogether, these data suggest that microglia could play an important role in tumor cell invasion by cooperating with glioma cells themselves in the remodeling of the extracellular matrix, providing a favorable substrate for migration [79]. In support of the data, in vitro studies revealed that glioma cells tend to migrate, in a heterogeneous shape, toward activated microglia-conditioned media and that such migration is sustained through an overexpression of MMP-2 [80,81].
Moreover, not only may the microglia modify the structure of the ECM, but the latter can also have an effect on the former: it has been proven that different components of the ECM could influence microglial activity. Indeed, tumor specimen studies revealed a close relation between the number of macrophages and the Tenascin-C content in the glioma extracellular matrix, suggesting that Tenascin-C may represent a permissive substrate for macrophagic migration in gliomas [82]. Moreover, microglia expressed different morphologies in the Tenascin knockdown glioma xenograft when compared to the control group, exhibiting the first ameboid-like morphology, resembling activated microglia, and the latter resembling inactivated microglia with long and thin processes, pointing out once again the reciprocal interaction between microglial cells and the extracellular matrix. Similarly, mouse xenograft studies revealed that Versican acts as a major ligand for the Toll-like receptors expressed on the macrophage surface, inducing the activation of the latter through an overexpression of MT-MMP [46,83].
In a recent study, the migratory behavior of the microglia and tumor glioma cells at the tumor infiltrative edge was studied and compared to better understand the dynamics of tumor infiltration and, eventually, the reciprocal interactions between the microglia and glioma cells. As reported, in a mouse brain slice model, microglial cells exhibited a migration pattern termed “simple diffusive” characterized by a random walk in a nonrestricted environment, whereas glioma cells exhibited a migration pattern termed “super diffusive” characterized by a persistent directionality of cell migration. At the infiltrative edge, microglial cells present a higher migration speed and a lower directionality compared to the microglial cells located in the peritumoral area, whereas glioma cells present, on average, a higher speed and directionality compared to microglial cells. Moreover, both the microglial and glioma cells exhibit little motility when located further away from the tumor infiltrative edge. Considering these findings, it has been proposed that glioma cells stimulate the activity and motility of microglial cells towards the infiltrative edge and that, in turn, activated microglial cells condition the infiltrative edge microenvironment by modifying the extracellular matrix to reduce the impedance to migration, allowing efficacious glioma cell invasion [84].
4. Discussion
The ECM forms a critical and dynamic scaffold that supports the normal brain architecture. It is a complex nonhomogenous structure which physiologically displays a vast and complex interaction with neural and supporting cells. Normal brain tissue has unique components that are not expressed in other tissues. In fact, the brain ECM presents small amounts of fibrous proteins, such as collagen, fibronectin, and laminins, and high amounts of glycosaminoglycans (either bound to proteins, chondroitin sulfate, dermatan sulfate, heparan sulfate, and keratan sulfate, or unbounded in the form of hyaluronan); proteoglycans, called lecticans (versican, aggrecan, neurocan, brevican, and decorin); and glycoproteins, such as tenascin-C.
A large number of studies have proven that the ECM does not only have a mechanical supporting role in the regulation of neural stem cell behavior, neuronal migration, the formation of axonal processes and their myelin sheaths, synapse formation, etc. [4]. Indeed, cells interact with the components of the ECM through membrane receptors (integrins, CD44, BEHAB/brevican, and N-CAM), producing molecular responses and establishing mutual interactions in which cells can modify the microenvironment and vice versa.
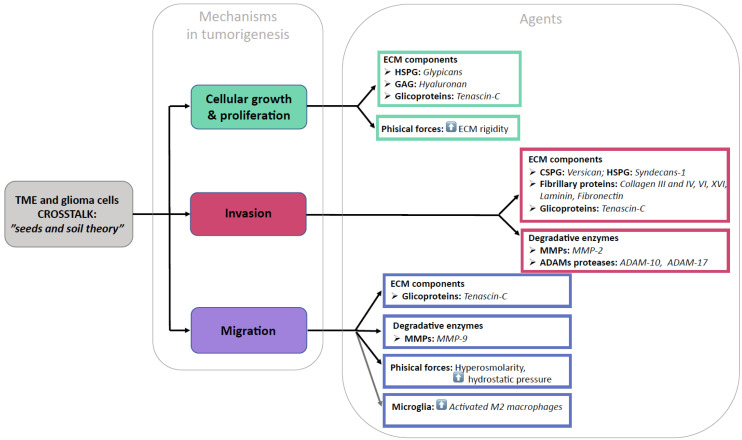
Functional studies in vitro and genetic studies in mice have provided evidence that the ECM affects virtually all the aspects of nervous system development and function and that it ultimately plays a significant role in all the phases of GBM development and progression. The ECM in GBMs shows significant remodeling compared to the ECM in a healthy brain. Recent evidence highlights that the three-dimensional ECM architecture and its mechanical properties affect the cell behavior in the TME both at the tumoral core and in its periphery. The ECM elements have been shown to display either an attractant or repellant action respective to the glioma cells, microglia, monocytes, macrophages, and stem cells that constitute the TME. We will herein examine the different ECM structural component modifications in GBMs (Figure 2).
Figure 2.
Pictorial summary of our findings.
5. Conclusions
Our systematic review demonstrates that the ECM should not be regarded anymore as a passive scaffold statically contributing to mechanical support in normal and pathological brain tissue but as an active player in tumor-related activity. Further research is necessary to fully understand the clinical implications of these preliminary findings.
Abbreviations
| BEHAB | Brain-enriched hyaluronan-binding protein |
| CD44 | Cluster of differentiation 44 |
| CD280 | Endo180 |
| CHI3L1 | Chitinase-3-like protein 1 |
| CNS | Central nervous system |
| COL1A2 | Collagen alpha-2(I) chain |
| CSPG | Chondroitin sulfate proteoglycan |
| ECM | Extracellular matrix |
| ED-A | Extra domain A |
| ED-B | Extra domain B |
| EGFR | Epidermal growth factor receptor |
| FAK | Focal adhesion kinase |
| GAG | Glycosaminoglycan |
| GBM | Glioblastoma |
| HSPG | Heparan sulfate proteoglycan |
| IL-1 | Interleukin-1 |
| LAR | Leukocyte common antigen-related |
| MEK/ERK | Mitogen-activated protein kinase/extracellular signal-regulated kinase |
| MMP | Matrix metalloproteinase |
| NFkB | Nuclear factor kappa-light-chain enhancer of activated B cells |
| N-CAM | Neural cell adhesion molecule |
| PAI-1 | Plasminogen activator inhibitor-1 |
| P4HA2 | Prolyl-4-hydroxylase subunit 2 |
| PRISMA-P | Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols |
| PRO | Periostin |
| RHAMM | Receptor for hyaluronan-mediated motility |
| siRNA | Small interfering RNA |
| TGF-β | Transforming growth factor beta |
| TME | Tumor microenvironment |
| uPA | Urokinase-type plasminogen activator |
| uPAR | Urokinase-type plasminogen activator receptor |
Author Contributions
Conceptualization, G.M.D.P. and S.M.; methodology, G.M. and G.M.D.P.; investigation, F.F. and S.M.; writing—original draft preparation, S.M.; writing—review and editing, G.M., L.B., Q.G.D.A., P.M., L.L. and R.D.B.; supervision, A.O. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Menna G., Manini I., Cesselli D., Skrap M., Olivi A., Ius T., Della Pepa G.M. Immunoregulatory effects of glioma-associated stem cells on the glioblastoma peritumoral microenvironment: A differential PD-L1 expression from core to periphery? Neurosurg. Focus. 2022;52:E4. doi: 10.3171/2021.11.FOCUS21589. [DOI] [PubMed] [Google Scholar]
- 2.Ruoslahti E. Brain extracellular matrix. Glycobiology. 1996;6:489–492. doi: 10.1093/glycob/6.5.489. [DOI] [PubMed] [Google Scholar]
- 3.Novak U., Kaye A.H. Extracellular matrix and the brain: Components and function. J. Clin. Neurosci. 2000;7:280–290. doi: 10.1054/jocn.1999.0212. [DOI] [PubMed] [Google Scholar]
- 4.Barros C.S., Franco S.J., Müller U. Extracellular matrix: Functions in the nervous system. Cold Spring Harb. Perspect. Biol. 2011;3:a005108. doi: 10.1101/cshperspect.a005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D., Liberati A., Tetzlaff J., Altman D.G., the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logun M.T., Bisel N.S., Tanasse E.A., Zhao W., Gunasekera B., Mao L., Karumbaiah L. Glioma cell invasion is significantly enhanced in composite hydrogel matrices composed of chondroitin 4- and 4,6-sulfated glycosaminoglycans. J. Mater. Chem. 2016;4:6052–6064. doi: 10.1039/C6TB01083K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logun M.T., Wynens K.E., Simchick G., Zhao W., Mao L., Zhao Q., Mukherjee S., Brat D.J., Karumbaiah L. Surfen-mediated blockade of extratumoral chondroitin sulfate glycosaminoglycans inhibits glioblastoma invasion. FASEB J. 2019;33:11973–11992. doi: 10.1096/fj.201802610RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrappe M., Klier F.G., Spiro R.C., Waltz T.A., Reisfeld R.A., Gladson C.L. Correlation of chondroitin sulfate proteoglycan expression on proliferating brain capillary endothelial cells with the malignant phenotype of astroglial cells. Cancer Res. 1991;51:4986–4993. [PubMed] [Google Scholar]
- 9.Kim Y., Kang H., Powathil G., Kim H., Trucu D., Lee W., Lawler S., Chaplain M. Role of extracellular matrix and microenvironment in regulation of tumor growth and LAR-mediated invasion in glioblastoma. PLoS ONE. 2018;13:e0204865. doi: 10.1371/journal.pone.0204865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silver D.J., Siebzehnrubl F.A., Schildts M.J., Yachnis A.T., Smith G.M., Smith A.A., Scheffler B., Reynolds B.A., Silver J., Steindler D.A. Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment. J. Neurosci. 2013;33:15603–15617. doi: 10.1523/JNEUROSCI.3004-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onken J., Moeckel S., Leukel P., Leidgens V., Baumann F., Bogdahn U., Vollmann-Zwerenz A., Hau P. Versican isoform V1 regulates proliferation and migration in high-grade gliomas. J. Neuro-Oncol. 2014;120:73–83. doi: 10.1007/s11060-014-1545-8. [DOI] [PubMed] [Google Scholar]
- 12.Tran V.M., Wade A., McKinney A., Chen K., Lindberg O.R., Engler J.R., Persson A.I., Phillips J.J. Heparan sulfate glycosaminoglycans in glioblastoma promote tumor invasion. Mol. Cancer Res. 2017;15:1623–1633. doi: 10.1158/1541-7786.MCR-17-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su G., Meyer K., Nandini C.D., Qiao D., Salamat S., Friedl A. Glypican-1 is frequently overexpressed in human gliomas and enhances FGF-2 signaling in glioma cells. Am. J. Pathol. 2006;168:2014–2026. doi: 10.2353/ajpath.2006.050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe A., Mabuchi T., Satoh E., Furuya K., Zhang L., Maeda S., Naganuma H. Expression of syndecans, a heparan sulfate proteoglycan, in malignant gliomas: Participation of nuclear factor-κB in upregulation of syndecan-1 expression. J. Neuro-Oncol. 2006;77:25–32. doi: 10.1007/s11060-005-9010-3. [DOI] [PubMed] [Google Scholar]
- 15.Chen J.-W.E., Pedron S., Shyu P., Hu Y., Sarkaria J.N., Harley B.A.C. Influence of hyaluronic acid transitions in tumor microenvironment on glioblastoma malignancy and invasive behavior. Front. Mater. 2018;5:39. doi: 10.3389/fmats.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayen W., Goebeler M., Kumar S., Riessen R., Nehls V. Hyaluronan stimulates tumor cell migration by modulating the fibrin fiber architecture. J. Cell Sci. 1999;112:2241–2251. doi: 10.1242/jcs.112.13.2241. [DOI] [PubMed] [Google Scholar]
- 17.Chen J.-W.E., Lumibao J., Blazek A., Gaskins H.R., Harley B. Hypoxia activates enhanced invasive potential and endogenous hyaluronic acid production by glioblastoma cells. Biomater. Sci. 2018;6:854–862. doi: 10.1039/C7BM01195D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J.-W.E., Leary S., Barnhouse V., Sarkaria J.N., Harley B.A. Matrix hyaluronic acid and hypoxia influence a CD133+ subset of patient-derived glioblastoma cells. Tissue Eng. 2022;28:330–340. doi: 10.1089/ten.tea.2021.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akiyama Y., Jung S., Salhia B., Lee S., Hubbard S., Taylor M., Mainprize T., Akaishi K., van Furth W., Rutka J.T. Hyaluronate receptors mediating glioma cell migration and proliferation. J. Neuro-Oncol. 2001;53:115–127. doi: 10.1023/A:1012297132047. [DOI] [PubMed] [Google Scholar]
- 20.Pibuel M.A., Poodts D., Díaz M., Molinari Y.A., Franco P.G., Hajos S.E., Lompardía S.L. Antitumor effect of 4MU on glioblastoma cells is mediated by senescence induction and CD44, RHAMM and p-ERK modulation. Cell Death Discov. 2021;7:280. doi: 10.1038/s41420-021-00672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsatas D., Kanagasundaram V., Kaye A.H., Novak U. EGF receptor modifies cellular responses to hyaluronan in glioblastoma cell lines. J. Clin. Neurosci. 2002;9:282–288. doi: 10.1054/jocn.2001.1063. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H., Zhang N., Dai Z., Wang Z., Zhang X., Liang X., Zhang L., Feng S., Wu W., Ye W., et al. Hyaluronic acids mediate the infiltration, migration, and M2 polarization of macrophages: Evaluating metabolic molecular phenotypes in gliomas. Mol. Oncol. 2022;16:3927–3948. doi: 10.1002/1878-0261.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calori I.R., Alves S.R., Bi H., Tedesco A.C. Type-I collagen/collagenase modulates the 3D structure and behavior of glioblastoma spheroid models. ACS Appl. Bio Mater. 2022;5:723–733. doi: 10.1021/acsabm.1c01138. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Sakaguchi M., Sabit H., Tamai S., Ichinose T., Tanaka S., Kinoshita M., Uchida Y., Ohtsuki S., Nakada M. COL1A2 inhibition suppresses glioblastoma cell proliferation and invasion. J. Neurosurg. 2022;138:639–648. doi: 10.3171/2022.6.JNS22319. [DOI] [PubMed] [Google Scholar]
- 25.Chintala S.K., Sawaya R., Gokaslan Z.L., Rao J.S. The effect of type III collagen on migration and invasion of human glioblastoma cell lines in vitro. Cancer Lett. 1996;102:57–63. doi: 10.1016/0304-3835(96)04163-8. [DOI] [PubMed] [Google Scholar]
- 26.Mammoto T., Jiang A., Jiang E., Panigrahy D., Kieran M.W., Mammoto A. Role of collagen matrix in tumor angiogenesis and glioblastoma multiforme progression. Am. J. Pathol. 2013;183:1293–1305. doi: 10.1016/j.ajpath.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senner V., Ratzinger S., Mertsch S., Grässel S., Paulus W. Collagen XVI expression is upregulated in glioblastomas and promotes tumor cell adhesion. FEBS Lett. 2008;582:3293–3300. doi: 10.1016/j.febslet.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Huijbers I.J., Iravani M., Popov S., Robertson D., Al-Sarraj S., Jones C., Isacke C.M. A role for fibrillar collagen deposition and the collagen internalization receptor Endo180 in glioma invasion. PLoS ONE. 2010;5:e9808. doi: 10.1371/journal.pone.0009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin J., Jiang L., Wang X., Wei W., Song C., Cui Y., Wu X., Qiu G. P4HA2 promotes epithelial-to-mesenchymal transition and glioma malignancy through the collagen-dependent PI3K/AKT pathway. J. Oncol. 2021;2021:1406853. doi: 10.1155/2021/1406853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang X., Zhou T., Wang Z., Qi B., Xia H. HSP47 promotes glioblastoma stemlike cell survival by modulating tumor microenvironment extracellular matrix through TGF-β pathway. ACS Chem. Neurosci. 2017;8:128–134. doi: 10.1021/acschemneuro.6b00253. [DOI] [PubMed] [Google Scholar]
- 31.Ohnishi T., Hiraga S., Izumoto S., Matsumura H., Kanemura Y., Arita N., Hayakawa T. Role of fibronectin-stimulated tumor cell migration in glioma invasion in vivo: Clinical significance of fibronectin and fibronectin receptor expressed in human glioma tissues. Clin. Exp. Metastasis. 1998;16:729–741. doi: 10.1023/A:1006532812408. [DOI] [PubMed] [Google Scholar]
- 32.Chintala S.K., Sawaya R., Gokaslan Z.L., Fuller G., Rao J.S. Immunohistochemical localization of extracellular matrix proteins in human glioma, both in vivo and in vitro. Cancer Lett. 1996;101:107–114. doi: 10.1016/0304-3835(96)04124-9. [DOI] [PubMed] [Google Scholar]
- 33.Caffo M., Caruso G., Meli F., Galatioto S., Sciacca M.P., Tomasello F., Germano A. An immunohistochemical study of extracellular matrix proteins laminin, fibronectin and type IV collagen in paediatric glioblastoma multiforme. Acta Neurochir. 2004;146:1113–1118. doi: 10.1007/s00701-004-0344-y. [DOI] [PubMed] [Google Scholar]
- 34.Serres E., Debarbieux F., Stanchi F., Maggiorella L., Grall D., Turchi L., Burel-Vandenbos F., Figarella-Branger D., Virolle T., Rougon G., et al. Fibronectin expression in glioblastomas promotes cell cohesion, collective invasion of basement membrane in vitro and orthotopic tumor growth in mice. Oncogene. 2013;33:3451–3462. doi: 10.1038/onc.2013.305. [DOI] [PubMed] [Google Scholar]
- 35.Sengupta S., Nandi S., Hindi E.S., Wainwright D.A., Han Y., Lesniak M.S. Short hairpin RNA-mediated fibronectin knockdown delays tumor growth in a mouse glioma model. Neoplasia. 2010;12:837–847. doi: 10.1593/neo.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J.M., Tian X.X., Zhong Y.F., Ma D.L., Ma Y., You J.F., Zhang Y. Effects of beta1-integrin, fibronectin and laminin on invasive behavior of human gliomas. Zhonghua Bing Li Xue Za Zhi. 2006;35:478–482. (In Chinese) [PubMed] [Google Scholar]
- 37.Yu S., Yu X., Sun L., Zheng Y., Chen L., Xu H., Jin J., Lan Q., Chen C.C., Li M. GBP2 enhances glioblastoma invasion through Stat3/fibronectin pathway. Oncogene. 2020;39:5042–5055. doi: 10.1038/s41388-020-1348-7. [DOI] [PubMed] [Google Scholar]
- 38.Kabir F., Apu M.N.H. Multi-omics analysis predicts fibronectin 1 as a prognostic biomarker in glioblastoma multiforme. Genomics. 2022;114:110378. doi: 10.1016/j.ygeno.2022.110378. [DOI] [PubMed] [Google Scholar]
- 39.Tysnes B.B., Mahesparan R., Thorsen F., Haugland H.K., Porwol T., Enger P.Ø., Lund-Johansen M., Bjerkvig R. Laminin expression by glial fibrillary acidic protein positive cells in human gliomas. Int. J. Dev. Neurosci. 1999;17:531–539. doi: 10.1016/S0736-5748(99)00055-6. [DOI] [PubMed] [Google Scholar]
- 40.Sun T., Patil R., Galstyan A., Klymyshyn D., Ding H., Chesnokova A., Cavenee W.K., Furnari F.B., Ljubimov V.A., Shatalova E.S., et al. Blockade of a Laminin-411-notch axis with CRISPR/Cas9 or a nanobioconjugate inhibits glioblastoma growth through tumor-microenvironment cross-talk targeting tumor microenvironment to treat glioblastoma. Cancer Res. 2019;79:1239–1251. doi: 10.1158/0008-5472.CAN-18-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khazenzon N.M., Ljubimov A.V., Lakhter A.J., Fujita M., Fujiwara H., Sekiguchi K., Sorokin L.M., Petäjäniemi N., Virtanen I., Black K.L., et al. Antisense inhibition of laminin-8 expression reduces invasion of human gliomas in vitro. Mol. Cancer Ther. 2003;2:985–994. [PubMed] [Google Scholar]
- 42.Gamble J.T., Reed-Harris Y., Barton C.L., La Du J., Tanguay R., Greenwood J.A. Quantification of glioblastoma progression in zebrafish xenografts: Adhesion to laminin alpha 5 promotes glioblastoma microtumor formation and inhibits cell invasion. Biochem. Biophys. Res. Commun. 2018;506:833–839. doi: 10.1016/j.bbrc.2018.10.076. [DOI] [PubMed] [Google Scholar]
- 43.Wang H., Wang Y., Jiang C. Stromal protein periostin identified as a progression associated and prognostic biomarker in glioma via inducing an invasive and proliferative phenotype. Int. J. Oncol. 2013;42:1716–1724. doi: 10.3892/ijo.2013.1847. [DOI] [PubMed] [Google Scholar]
- 44.Landré V., Antonov A., Knight R., Melino G. p73 promotes glioblastoma cell invasion by directly activating POSTN (periostin) expression. Oncotarget. 2016;7:11785–11802. doi: 10.18632/oncotarget.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ouanouki A., Lamy S., Annabi B. Periostin, a signal transduction intermediate in TGF-β-induced EMT in U-87MG human glioblastoma cells, and its inhibition by anthocyanidins. Oncotarget. 2018;9:22023–22037. doi: 10.18632/oncotarget.25153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia S., Lal B., Tung B., Wang S., Goodwin C.R., Laterra J. Tumor microenvironment tenascin-C promotes glioblastoma invasion and negatively regulates tumor proliferation. Neuro Oncol. 2016;18:507–517. doi: 10.1093/neuonc/nov171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirata E., Arakawa Y., Shirahata M., Yamaguchi M., Kishi Y., Okada T., Takahashi J.A., Matsuda M., Hashimoto N. Endogenous tenascin-C enhances glioblastoma invasion with reactive change of surrounding brain tissue. Cancer Sci. 2009;100:1451–1459. doi: 10.1111/j.1349-7006.2009.01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J.-F., Tao T., Wang K., Zhang G.-X., Yan Y., Lin H.-R., Li Y., Guan M.-W., Yu J.-J., Wang X.-D. IL-33/ST2 axis promotes glioblastoma cell invasion by accumulating tenascin-C. Sci. Rep. 2019;9:20276. doi: 10.1038/s41598-019-56696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarkar S., Zemp F.J., Senger D., Robbins S.M., Yong V.W. ADAM-9 is a novel mediator of tenascin-C-stimulated invasiveness of brain tumor—Initiating cells. Neuro-Oncol. 2015;17:1095–1105. doi: 10.1093/neuonc/nou362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarkar S., Nuttall R.K., Liu S., Edwards D.R., Yong V.W. Tenascin-C stimulates glioma cell invasion through matrix metalloproteinase-12. Cancer Res. 2006;66:11771–11780. doi: 10.1158/0008-5472.CAN-05-0470. [DOI] [PubMed] [Google Scholar]
- 51.Mai J., Sameni M., Mikkelsen T., Sloane B. Degradation of extracellular matrix protein tenascin-C by cathepsin B: An interaction involved in the progression of gliomas. Biol. Chem. 2002;383:1407–1413. doi: 10.1515/BC.2002.159. [DOI] [PubMed] [Google Scholar]
- 52.Li Q., Chen B., Cai J., Sun Y., Wang G., Li Y., Li R., Feng Y., Han B., Li J., et al. Comparative analysis of matrix metalloproteinase family members reveals that MMP9 predicts survival and response to temozolomide in patients with primary glioblastoma. PLoS ONE. 2016;11:e0151815. doi: 10.1371/journal.pone.0151815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lakka S.S., Gondi C.S., Yanamandra N., Olivero W.C., Dinh D.H., Gujrati M., Rao J.S. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23:4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- 54.Kargiotis O., Chetty C., Gondi C.S., Tsung A.J., Dinh D.H., Gujrati M., Lakka S.S., Kyritsis A.P., Rao J.S. Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in impaired invasion and tumor-induced angiogenesis, induces apoptosis in vitro and inhibits tumor growth in vivo in glioblastoma. Oncogene. 2008;27:4830–4840. doi: 10.1038/onc.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Schuler P.J., Bendszus M., Kuehnel S., Wagner S., Hoffmann T.K., Goldbrunner R., Vince G.H. Urokinase plasminogen activator, uPAR, MMP-2, and MMP-9 in the C6-glioblastoma rat model. Vivo. 2012;26:571–576. [PubMed] [Google Scholar]
- 56.Sun J., Zhao B., Du K., Liu P. TRAF6 correlated to invasion and poor prognosis of glioblastoma via elevating MMP9 expression. Neuroreport. 2019;30:127–133. doi: 10.1097/WNR.0000000000001171. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y., Lyons C.E., Xiao A., Templeton D.J., Sang Q.A., Brew K., Hussaini I.M. Urokinase directly activates matrix metalloproteinases-9: A potential role in glioblastoma invasion. Biochem. Biophys. Res. Commun. 2008;369:1215–1220. doi: 10.1016/j.bbrc.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang L., Zhao D., Liu H.-B., Wang Q.-S., Zhang P., Li C.-L., DU W.-Z., Wang H.-J., Liu X., Zhang Z.-R., et al. Activation of sonic hedgehog signaling enhances cell migration and invasion by induction of matrix metalloproteinase-2 and -9 via the phosphoinositide-3 kinase/AKT signaling pathway in glioblastoma. Mol. Med. Rep. 2015;12:6702–6710. doi: 10.3892/mmr.2015.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng Q., Diao S., Wang Q., Zhu C., Sun X., Yin B., Zhang X., Meng X., Wang B. IL-17A promotes cell migration and invasion of glioblastoma cells via activation of PI3K/AKT signalling pathway. J. Cell Mol. Med. 2019;23:357–369. doi: 10.1111/jcmm.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Das G., Shiras A., Shanmuganandam K., Shastry P. Rictor regulates MMP-9 activity and invasion through Raf-1-MEK-ERK signaling pathway in glioma cells. Mol. Carcinog. 2011;50:412–423. doi: 10.1002/mc.20723. [DOI] [PubMed] [Google Scholar]
- 61.Djediai S., Suarez N.G., El Cheikh-Hussein L., Torres S.R., Gresseau L., Dhayne S., Joly-Lopez Z., Annabi B. MT1-MMP cooperates with TGF-β receptor-mediated signaling to trigger snail and induce epithelial-to-mesenchymal-like transition in U87 glioblastoma cells. Int. J. Mol. Sci. 2021;22:13006. doi: 10.3390/ijms222313006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhai Y., Sang W., Su L., Shen Y., Hu Y., Zhang W. Analysis of the expression and prognostic value of MT1-MMP, β1-integrin and YAP1 in glioma. Open Med. 2022;17:492–507. doi: 10.1515/med-2022-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Held-Feindt J., Paredes E.B., Blömer U., Seidenbecher C., Stark A.M., Mehdorn H.M., Mentlein R. Matrix-degrading proteases ADAMTS4 and ADAMTS5 (disintegrins and metalloproteinases with thrombospondin motifs 4 and 5) are expressed in human glioblastomas. Int. J. Cancer. 2006;118:55–61. doi: 10.1002/ijc.21258. [DOI] [PubMed] [Google Scholar]
- 64.Siney E.J., Holden A., Casselden E., Bulstrode H., Thomas G.J., Willaime-Morawek S. Metalloproteinases ADAM10 and ADAM17 mediate migration and differentiation in glioblastoma sphere-forming cells. Mol. Neurobiol. 2017;54:3893–3905. doi: 10.1007/s12035-016-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herrera-Perez M., Voytik-Harbin S.L., Rickus J.L. Extracellular matrix properties regulate the migratory response of glioblastoma stem cells in three-dimensional culture. Tissue Eng. 2015;21:2572–2582. doi: 10.1089/ten.tea.2014.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ulrich T.A., de Juan Pardo E.M., Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69:4167–4174. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaufman L., Brangwynne C., Kasza K., Filippidi E., Gordon V., Deisboeck T., Weitz D. Glioma expansion in collagen I matrices: Analyzing collagen concentration-dependent growth and motility patterns. Biophys. J. 2005;89:635–650. doi: 10.1529/biophysj.105.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang C., Tong X., Yang F. Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using peg-based hydrogels. Mol. Pharm. 2014;11:2115–2125. doi: 10.1021/mp5000828. [DOI] [PubMed] [Google Scholar]
- 69.Lim E.-J., Suh Y., Kim S., Kang S.-G., Lee S.-J. Force-mediated proinvasive matrix remodeling driven by tumor-associated mesenchymal stem-like cells in glioblastoma. BMB Rep. 2018;51:182–187. doi: 10.5483/BMBRep.2018.51.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pu W., Qiu J., Riggins G.J., Parat M.-O. Matrix protease production, epithelial-to-mesenchymal transition marker expression and invasion of glioblastoma cells in response to osmotic or hydrostatic pressure. Sci. Rep. 2020;10:2634. doi: 10.1038/s41598-020-59462-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koh I., Cha J., Park J., Choi J., Kang S.-G., Kim P. The mode and dynamics of glioblastoma cell invasion into a decellularized tissue-derived extracellular matrix-based three-dimensional tumor model. Sci. Rep. 2018;8:4608. doi: 10.1038/s41598-018-22681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rao S., DeJesus J., Short A.R., Otero J.J., Sarkar A., Winter J.O. Glioblastoma behaviors in three-dimensional collagen-hyaluronan composite hydrogels. ACS Appl. Mater. Interfaces. 2013;5:9276–9284. doi: 10.1021/am402097j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cui Y., Cole S., Pepper J., Otero J.J., Winter J.O. Hyaluronic acid induces ROCK-dependent amoeboid migration in glioblastoma cells. Biomater. Sci. 2020;8:4821–4831. doi: 10.1039/D0BM00505C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirata E., Yukinaga H., Kamioka Y., Arakawa Y., Miyamoto S., Okada T., Sahai E., Matsuda M. In vivo fluorescence resonance energy transfer imaging reveals differential activation of Rho-family GTPases in glioblastoma cell invasion. J. Cell Sci. 2012;125:858–868. doi: 10.1242/jcs.089995. [DOI] [PubMed] [Google Scholar]
- 75.Lively S., Schlichter L.C. The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion. J. Neuroinflamm. 2013;10:843. doi: 10.1186/1742-2094-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Vrij J., Maas S.N., Kwappenberg K.M., Schnoor R., Kleijn A., Dekker L., Luider T.M., de Witte L.D., Litjens M., van Strien M.E., et al. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int. J. Cancer. 2015;137:1630–1642. doi: 10.1002/ijc.29521. [DOI] [PubMed] [Google Scholar]
- 77.Gabrusiewicz K., Ellert-Miklaszewska A., Lipko M., Sielska M., Frankowska M., Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS ONE. 2011;6:e23902. doi: 10.1371/journal.pone.0023902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bettinger I., Thanos S., Paulus W. Microglia promote glioma migration. Acta Neuropathol. 2002;103:351–355. doi: 10.1007/s00401-001-0472-x. [DOI] [PubMed] [Google Scholar]
- 79.Markovic D.S., Glass R., Synowitz M., van Rooijen N., Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J. Neuropathol. Exp. Neurol. 2005;64:754–762. doi: 10.1097/01.jnen.0000178445.33972.a9. [DOI] [PubMed] [Google Scholar]
- 80.Markovic D.S., Vinnakota K., Chirasani S., Synowitz M., Raguet H., Stock K., Sliwa M., Lehmann S., Kälin R., van Rooijen N., et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc. Natl. Acad. Sci. USA. 2009;106:12530–12535. doi: 10.1073/pnas.0804273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu C.Y.-J., Chen C.-H., Lin C.-Y., Feng L.-Y., Lin Y.-C., Wei K.-C., Huang C.-Y., Fang J.-Y., Chen P.-Y. CCL5 of glioma-associated microglia/macrophages regulates glioma migration and invasion via calcium-dependent matrix metalloproteinase 2. Neuro-Oncol. 2020;22:253–266. doi: 10.1093/neuonc/noz189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kulla A., Liigant A., Piirsoo A., Rippin G., Asser T. Tenascin expression patterns and cells of monocyte lineage: Relationship in human gliomas. Mod. Pathol. 2000;13:56–67. doi: 10.1038/modpathol.3880010. [DOI] [PubMed] [Google Scholar]
- 83.Hu F., Dzaye O.D., Hahn A., Yu Y., Scavetta R.J., Dittmar G., Kaczmarek A.K., Dunning K.R., Ricciardelli C., Rinnenthal J.L., et al. Glioma-derived versican promotes tumor expansion via glioma-associated microglial/macrophages toll-like receptor 2 signaling. Neuro-Oncol. 2015;17:200–210. doi: 10.1093/neuonc/nou324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Juliano J., Gil O., Hawkins-Daarud A., Noticewala S., Rockne R.C., Gallaher J., Massey S.C., Sims P.A., Anderson A.R.A., Swanson K.R., et al. Comparative dynamics of microglial and glioma cell motility at the infiltrative margin of brain tumours. J. R. Soc. Interface. 2018;15:20170582. doi: 10.1098/rsif.2017.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sternlicht M.D., Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fillmore H.L., VanMeter T.E., Broaddus W.C. Membrane-type matrix metalloproteinases (MT-MMP)s: Expression and function during glioma invasion. J. Neuro-Oncol. 2001;53:187–202. doi: 10.1023/A:1012213604731. [DOI] [PubMed] [Google Scholar]