Abstract
Objective
The use and effects of electronic (e)‐cigarettes (e‐cigs) are particularly relevant for otolaryngology providers as tobacco plays a major role in benign and malignant diseases of the upper aerodigestive tract. This review aims to (1) summarize the recent policies regarding e‐cigs and important patterns of use and (2) serve as a comprehensive resource for clinical providers on the known biologic and clinical effects of e‐cigs on the upper aerodigestive tract.
Data Sources
PubMed/MEDLINE.
Review Methods
We conducted a narrative review on (1) general information on e‐cig use and informative findings in the lower respiratory system and a comprehensive review on (2) the effects of e‐cigs on cell and animal models and the clinical implications of these products on human health as is relevant to otolaryngology.
Conclusions
Although e‐cigs are likely less harmful than conventional cigarettes, preliminary research on e‐cigs suggest several deleterious effects including in the upper aerodigestive tract. Due to this, there has been increased interest in restricting e‐cig usage, particularly among the adolescent population, and caution in recommending e‐cigs to current smokers.
Implications for Practice
Chronic e‐cig use is likely to have clinical implications. It is critical for otolaryngology providers to be aware of the rapidly changing regulations and use patterns regarding e‐cigs and how e‐cigs influence human health, particularly with regards to the upper aerodigestive tract, to accurately council patients regarding potential risks and benefits of use.
Keywords: aerodigestive, e‐cigarettes (e‐cigs), electronic nicotine delivery systems, head and neck, otolaryngology, vaping
Tobacco has been implicated in 70% to 80% of head and neck malignancies 1 , 2 as well as benign diseases of the head and neck. 3 The morbidity of tobacco‐related head and neck disorders is immense; patients are often left with deficits in voice and articulation, dysphagia, and disfigurement. There are over 60 well‐established carcinogens in tobacco 4 , 5 and a number of toxins, including ones not seen in combustible cigarettes, have been also found in electronic (e)‐cigarettes (e‐cigs). 6 , 7
In response to the rapid rise in youth nicotine use as a result of e‐cigs, 8 also known as electronic nicotine delivery systems, as well as growing evidence of the substantial harm of e‐cigs, numerous government agencies have initiated policies to reduce the incidence of nicotine addiction including the banning of many flavors in “cartridge‐based” products as well as imposing limitations on nicotine content. 9 , 10 , 11 , 12 , 13 Nevertheless, the prevalence of e‐cig use remains high, 14 in large part due to the exemption of disposable e‐cigs and flavor enhancers from these policies. 15 The growing use of e‐cigs has prompted a commensurate increase in research on their effects. On PubMed alone, the number of published articles on e‐cigs has increased from 372 in 2014 to 1132 in 2021 and there are hundreds of review papers on various aspects of e‐cig use. Significant headway has been made in assessing the effect of e‐cig components on the respiratory and cardiovascular systems, 16 , 17 , 18 , 19 , 20 , 21 , 22 and the number of studies exploring the effects of e‐cigs on other organ systems has also recently grown. 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46
Despite the increase in research on the effect of e‐cigs on cellular biology and human health, reports on e‐cigs as they directly pertain to the upper aerodigestive tract remain limited, particularly for benign disorders. Furthermore, the current rate of relevant research on e‐cigs outpaces the ability of most practicing clinical providers to review. To provide a comprehensive review of e‐cigs as it is most relevant for providers who manage the upper aerodigestive tract, the review serves two main objectives. First, we provide a broad overview on the latest policies regarding e‐cigs, patterns of use in the general and adolescent populations, and highlight important insights on e‐cigs from the lower respiratory system. Second, we detail the current findings on the effects of e‐cigs on preclinical models and clinical outcomes in otolaryngology and identify areas and topics that require further research.
Methods
To address our two distinct goals of (1) providing a broad up‐to‐date overview of e‐cigs and (2) comprehensively reviewing the direct impact of e‐cigs on the upper aerodigestive tract, we performed our review in two phases.
To address our first goal, we evaluated recent statements and resources regarding e‐cigs from the CDC, FDA, and the AAO‐HNS to determine which topics would be of greatest interest and relevance to the otolaryngology practitioner. Based on these, we decided to include in the review information regarding regulatory policies, use patterns, toxicities, and major known biological effects on human health. We then searched PubMed and Ovid Medline for articles published on e‐cigarettes in the last 10 years related to these topics. We additionally searched Google for rulings, policy statements, and news reports regarding e‐cigs.
Subsequently, to provide a comprehensive review of preclinical and clinical studies pertaining specifically to the upper aerodigestive tract and address our second goal, we performed a structured review of the preclinical and clinical studies by using a combination of the following search terms: e‐cigarette and/or otolaryngology and/or ENT and/or head and neck and/or oral and/or mouth and/or hypopharynx and/or oropharynx and/or nasal and/or nose and/or sinus and/or larynx and/or throat and/or voice and/or ear and/or otology. In addition, the reference lists of selected articles as well as review papers resulting from these searches were evaluated for additional studies not originally found. We included all primary literature that utilized cells, animal models, or clinical samples to evaluate the biological effects of e‐cigs in the upper aerodigestive tract. Studies were excluded if one of the following applied: not available in English, did not contain original data, or aims not relevant to biologic consequences of e‐cigs on the upper aerodigestive tract or ear (eg, articles assessing perceptions, policy, behavior, bronchial effects) including studies specific to dental health. All searches were performed in September 2021. Further details regarding searches conducted for goal 1 and goal 2 can be found in Supplemental Tables 1 and 2 and Figure 1, available online.
Discussion
Part One: Overview of E‐cigarettes
History and Marketing of E‐cigs
E‐cigs and other vaping products work by heating nicotine‐containing “e‐juice” or “e‐liquid” into an aerosol which is inhaled by the user. E‐juice is primarily made up of glycerin and propylene glycol into which nicotine, flavoring, and a range of other minor components are dissolved. These products not only go by several names but also come in a wide range of shapes and sizes (Figure 1A). Early “cig‐a‐likes,” introduced to the US market in 2006, simulated the size and shape of conventional cigarettes. Subsequently, cartridge/pod devices became more popular, particularly with the introduction of JUUL in 2015. Over the last few years, partially in response to government regulation on pod devices such as JUUL, disposables (eg, Puff Bar), and high‐powered advanced personal vaporizers or MODs have seen a dramatic increase in use. 47 , 48
Figure 1.

Common e‐cigarette (e‐cig) products and associated toxins. Representation of the (A) range of popular e‐cig devices and (B) known toxins found in e‐cigs.
E‐cigs are produced by both startup companies as well as major international tobacco companies and have been marketed as being different from conventional cigarettes, including with claims that e‐cigs are safe and benign. 49 The perception among the general public is that e‐cigs use results in limited or no health consequences, 50 , 51 , 52 , 53 particularly flavored e‐cigs. 54
Toxic Constituents in E‐cigs
Tobacco is known to have over 60 well‐described carcinogenic agents and hundreds more under study. 4 , 5 Although e‐juice has fewer known carcinogens as compared to combustible cigarettes, studies have proven the presence of toxic constituents in e‐cigs. 55 , 56 These constituents include but are not limited to carbonyl compounds, 57 thiocyanate, 58 heavy metals, 7 , 59 , 60 silicate particles, 60 diacetyl, 61 and the known carcinogens formaldehyde 62 , 63 and N′‐nitrosonornicotine 56 , 64 (Figure 1B). Many of these components of e‐cigs come from the flavoring additives or devices themselves and thus vary widely between products. 65 , 66 , 67 , 68 , 69 , 70 , 71 Several of these toxins have not been notably associated with traditional cigarette smoke. Bystanders can also be exposed to toxins in secondhand and thirdhand smoke from e‐cigs. 72 , 73 , 74 , 75
Regulation of E‐cigs
The largest concern regarding e‐cigs is the rise in new smokers, particularly adolescents, and relapsed former‐smokers. 8 , 76 , 77 , 78 , 79 Once addicted to nicotine via e‐cigs, many adolescents explore other tobacco products and are more likely to use traditional combustible cigarettes. 80 , 81 , 82 Due to the increased prevalence of e‐cig use, there has been progress in regulatory oversight for e‐cigs including the banning of many flavors in pods by the FDA 9 , 10 and capping of nicotine at 20 mg/mL in Europe. 11 Most recently, the FDA has denied market authorization to the largest cartridge‐based producer, JUUL, 12 though this has been stalled by a federal appeals court. 13 Nevertheless, many methods including disposable and refillable e‐cigs remain largely unregulated. 15 There is no production standard among manufacturers and thus the contents and potential toxicities can vary widely. 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 Furthermore, users are increasingly creating their own device modifications, many of which pose an increased risk for elevated nicotine and carcinogen consumption and combustion. 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94
Evaluating Patterns of Use
E‐cigs now make up the majority of nicotine use in teenagers and young adults 95 and the largest portion of non‐cigarette or cigar use in older adults. 96 In order to adequately counsel at‐risk patients about e‐cigs, medical providers rely on patients' self‐reported nicotine use. Studies have shown that the majority of users of alternative nicotine products including e‐cigs will answer “no” if asked about a smoking history but will affirm use of a particular alternative product when asked specifically. 97 , 98 While e‐cigs make up the largest share of alternative nicotine product use, there is a wide range of both traditional and modern alternative nicotine products including heated tobacco (HT) and oral nicotine products (ONP) (Figure 2). 99 , 100 , 101 Use patterns also vary based on nationality and culture. 102 , 103 It is important, then, to ask about specific forms of tobacco consumption, both inhaled and smokeless, when assessing a patient's tobacco history. This is relevant for assessing both disease and surgical risk; an association with e‐cig exposure and poor wound healing and flap failure has been reported in both animal models and surgical patients. 97 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 Unfortunately, due to the variable nicotine content of e‐cig products, it can be difficult to obtain a quantitative estimate of usage (ie, pack year equivalent).
Figure 2.
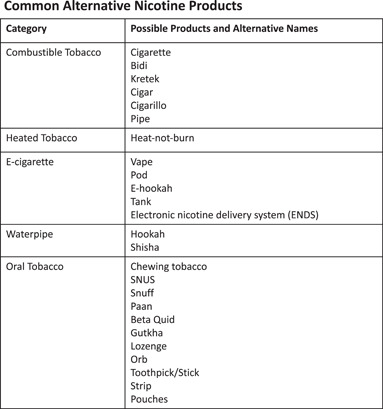
Range of alternative nicotine products. Patients who use alternative forms of nicotine, such as those listed, often deny a smoking history but will report use when asked about specific methods of consumption.
E‐cigs for Current Smokers: Weighing Benefits Against Risk of Dual Usage
One popularly cited benefit of e‐cigs use is that they can reduce or eliminate the use of traditional cigarettes in current smokers. This potential benefit is the main reported driving factor for increased e‐cig use among cancer patients. 112 There is indeed evidence that e‐cigs are not as harmful as conventional cigarettes. 113 , 114 , 115 , 116 However, the clinical impact of e‐cig use in current smokers is complicated by additional factors. Although e‐cig use has been shown to modestly reduce cigarette consumption, 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 about 40% to 50% of smokers who do try e‐cigs become “dual‐users” who continue to smoke cigarettes but use e‐cigs in places smoking is prohibited, thus facilitating their addiction. 128 , 129 , 130 , 131 , 132 Dual users often have a higher consumption of nicotine and toxic byproducts and lower rates of smoking cessation. 122 , 133 , 134 Furthermore, a large portion of e‐cig users believe they are not addicted to nicotine despite regular use 52 , 135 which can limit motivation to quit. Therefore, while e‐cigs may be a useful tool for harm reduction in current smokers who are unwilling to otherwise reduce the use of combustible cigarettes or have failed other forms of smoking cessation, care should be taken to caution these patients about the risk of dual usage. This is reflected in the position statement by the AAO‐HNS regarding e‐cigs. 136
Biological Impact of E‐cigarettes: Insights from the Lower Respiratory System
E‐cigs have been studied extensively with regards to the lower respiratory system. Components of e‐cig aerosol have been shown to cause oxidative damage, 137 , 138 , 139 DNA breaks, 140 and inhibit DNA repair 6 , 141 in human‐derived lung cells which theoretically could lead to the development of oncogenic mutations (Figure 3A), though the effect size of mutagenic activity from e‐cigs is still unclear. 142 , 143 Several studies in lung‐derived cell lines demonstrate that e‐cig vapor results in gene expression changes similar to cigarette smoke and increases the risk of malignant transformation and metastasis. 6 , 141 , 144 , 145 E‐cigs have also been shown to cause mitochondrial dysfunction, 140 , 146 alter the cellular metabolome and proteome, 147 , 148 , 149 and reduce cellular viability, 150 , 151 indicating that e‐cigs may not only increase the risk of cancer but also lead to the development of benign disorders. Finally, e‐juice and e‐cig aerosols have been demonstrated to potentially increase the risk of infection by promoting inflammation while reducing innate immune activity and the production of antimicrobial proteins. 152 , 153 , 154
Figure 3.
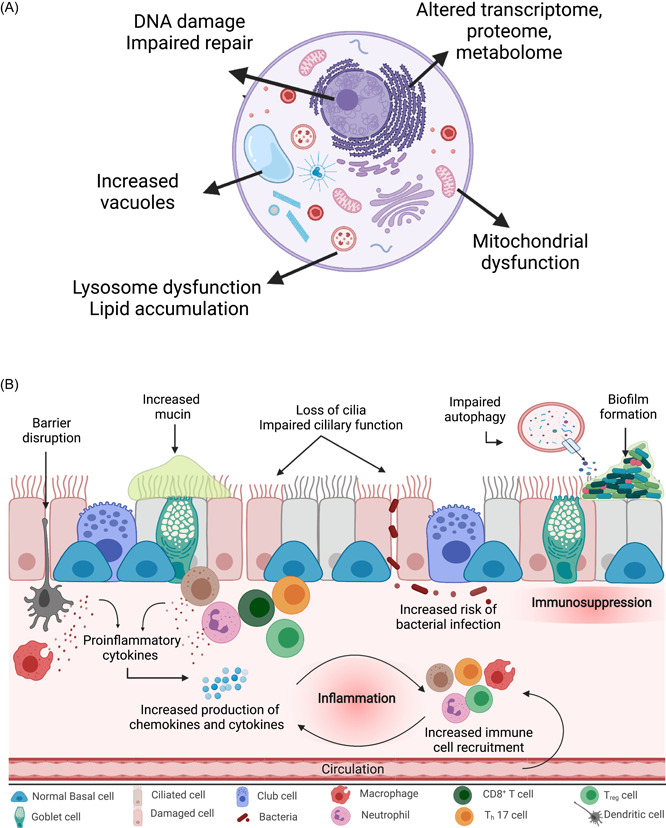
Cellular toxicity from e‐cigarettes (e‐cigs). (A) Example of pathways through which e‐cigs cause cytotoxicity. (B) Changes noted cell and animal models after e‐juice and e‐cig aerosol exposure.
The effect e‐cigs have on cellular and tissue function has been confirmed in organoid and animal models (Figure 3B). Exposure to e‐cig aerosol and e‐juice has been shown to cause a wide variety of biological changes in the airway including increased production and decreased clearance of mucin, 155 , 156 , 157 reduction in ciliary function, 157 , 158 increase in cytokines, 153 and impaired immune cell function. 154 , 159 In respiratory models, e‐cigs have additionally been shown to increase alveolar‐capillary barrier permeability 160 and increase airway hyperreactivity. 156 , 157 Finally, early data in mouse lung models demonstrates that prolonged e‐cig exposure can directly induce malignancy. 161
Clinically, e‐cigs have been shown to directly impact lower airway epithelial cells in a similar manner as conventional cigarettes resulting in functional changes such as increased airway resistance and airway obstruction, symptomatic immune suppression, mucin production, irritation and inflammation, and lung disease exacerbation. 16 , 21 , 22 , 162 , 163 While the risk of cancer in e‐cig users has not been fully studied due to the relatively short time e‐cigs have been on the market, preliminary experimental in vivo human data has demonstrated epigenetic changes in the airway after even brief e‐cig aerosol exposure including changes in the p53 pathway. 164 In addition, over 2600 cases of e‐cig or vaping product use associated lung injury have been reported secondary to vaping in the United States alone, 18 , 19 largely due to the presence of vitamin E acetate in aerosolized cannabis oils. 165
Part Two: Biological and Clinical Endpoints in the Upper Aerodigestive Tract
Basic Research on E‐cigs in Otolaryngology
Several studies have evaluated the effect of e‐cigs in cell, organoid, and animal models of the upper aerodigestive tract (Table 1). The largest volume of primary literature on the effects of e‐cigs on the upper aerodigestive tract is in relation to oral and dental health. 26 , 27 , 28 , 29 , 166 , 167 , 168 , 169 As expected, e‐cigs do not appear to be as harmful as conventional cigarette smoke in cell and animal models. 170 , 171 Nevertheless, numerous studies have demonstrated biological disruption as a result of even brief e‐cig exposure. 29 Liquid nicotine has been shown to promote the migration of dysplastic oral keratinocytes, suggesting a contribution of nicotine alone to oral carcinogenesis. 172 , 173 In the larynx, rats exposed to e‐cig aerosols showed early evidence of hyperplasia and metaplasia of the mucosa, 174 although the results were not statistically significant in this limited study, and increases in IL‐4. 175 In addition, both nicotine‐containing and nicotine‐free e‐juice have been found to cause biologic disruptions including oxidative stress, DNA breakage, metanuclear anomalies, liposomal dysfunction, and solvent and lipid accumulation, and cytotoxicity in human gingival fibroblasts, 67 , 176 , 177 , 178 , 179 , 180 vocal fold epithelial cells and fibroblasts, 181 , 182 head and neck squamous cell carcinoma cell lines, 67 , 183 , 184 , 185 noncancerous oral and oropharyngeal epithelial cells, 139 , 178 , 186 , 187 , 188 , 189 , 190 , 191 , 192 middle ear epithelial cells, 193 , 194 , 195 nasal epithelial cells, 196 , 197 and organotypic cultures/organoids. 178 , 182 , 197 Interestingly, a recent study found that oral cancer cells exposed to e‐cig aerosols increased cell resistance to cisplatin through changes in drug transporters, suggesting a mechanism for e‐cig‐induced chemotherapy resistance. 198 Therefore, the potential mutagenic and functional effects of e‐cigs and other noncombustible nicotine products on various cell types throughout the head and neck should not be underestimated.
Table 1.
Primary Studies on the Effects of E‐Cigarettes on the Upper Aerodigestive Tract and Ear in Cell and Animal Models
| References | Subject of interest | Study design | Exposure time (method) | Outcome(s) |
|---|---|---|---|---|
| Alanazi et al 177 | Primary human gingival fibroblasts | Ex vivo | 1 hour a day for 1, 3, 5, and 7 days (extract in media) | Changes in morphology, decreased viability, increased apoptosis, delayed migration/wound closure |
| Carson et al 197 | Primary human nasal epithelial cell 3D model | Ex vivo | 100 seconds (puffs over air‐liquid interface) | Reduction in ciliary beat frequency, development of fibrillar mesh‐like debris |
| Duggar et al 187 | Oral keratinocytes | In vitro | 24 hours a day for 1, 2, and 3 days (extract in media) | Decreased growth, increase in cytotoxin expression |
| Ganapathy et al 139 | Human oral keratinocyte cell line (POE9n), human oral SCC cell line (UM‐SCC‐1) | In vitro | 1 hour every other day for 2 weeks (extract in media) | Increased oxidative DNA damage |
| Go et al 185 | Human middle ear epithelial cell line (HMEEC) | In vitro | 24 hours (extract in media) | Decreased viability, increased autophagy and apoptosis, increased cytokines, increased mucin production, dysregulation of water channels |
| Ha et al 175 | Murine model (C57BL/6) | In vivo | 31 minutes and 40 seconds a day, 5 days a week, for 16 weeks (whole body chamber) | Differential inflammatory cytotoxin levels |
| Iskandar et al 170 | Human buccal epithelial (EpiOral) 3D model | In vitro | 28 minutes (puffs over culture) | No significant histopathologic changes, significant differential gene expression (especially inflammatory pathways) |
| Ji et al 188 | Human oral epithelial cell line (NHOK) | In vitro | 24 hours (extract in media) | Increased cytotoxicity, increased oxidative stress |
| Ji et al 189 | Human oral epithelial cell line (NHOK) | In vitro | 4 hours (extract in media) | Differential gene expression (especially unfolded protein response) |
| Lungova et al 182 | Human induced pluripotent stem cell (hiPSC)—derived engineered vocal fold mucosae | In vitro | 24 hours a day for 7 days (extract in media) | Changes in mucosal structure, increased mucin clots, dysregulated cytokine production, lipid deposition within cytoplasm and intercellular spaces causing epithelial injury and remodeling |
| Martinez et al 181 | Human vocal fold fibroblast cell line (hVFF) | In vitro | 24 hours (extract in media) | Increased cytotoxicity, no significant differences in gene expression |
| Manyanga et al 198 | Human oral and pharyngeal epithelial cell lines (UM‐SCC‐1, WSU‐HN6, WSU‐HN30) | In vitro | 24 hours a day for 2 and 4 days (extract in media) | Increased viability in setting of cisplatin exposure due to increased cisplatin resistance |
| Pushalkar et al 185 | Human hypopharyngeal SCC (FaDu) and leukoplakia (MSK‐Leuk‐1) cell lines | In vitro | 40 minutes (puffed over culture) | Increased inflammatory cytokines in setting of infection, increased infection efficiency |
| Rouabhia et al 190 | Primary human gingival epithelial cells | Ex vivo | 15 minutes a day for 1, 2, and 3 days (puffs over culture) | Change in morphology, increased apoptosis, increased DNA fragmentation |
| Rouabhia et al 196 | Primary human nasal epithelial cells and 3D model | Ex vivo | 15 minutes twice a day for 1, 2, and 3 days (puffs over culture) | Changes in morphology, decreased viability, changes in mucosal structure, increased inflammatory cytokines |
| Sancilio et al 176 | Primary human gingival fibroblasts | Ex vivo | 6 and 24 hours a day for 1, 2, and 3 days (extract in media) | Changes in morphology, decreased viability, increased apoptosis, increased ROS formation |
| Sancilio et al 179 | Primary human gingival fibroblasts | Ex vivo | 3 and 24 hours a day for 1 and 2 days (extract in media) | Changes in morphology, increased cytotoxicity, changes in collagen release, increase in lysosomes, increased LC3 IIB/LC3 I expression |
| Salturk et al 174 | Murine model (Wistar albino, female) | In vivo | 60 minutes a day for 4 weeks (whole body chamber) | Nonsignificant trend toward increased squamous metaplasia and hyperplasia, no difference in Ki67 expression |
| Song et al 193 | Human middle ear epithelial cell line (HMEEC‐1) | In vitro | 24 hours (extract in media) | Decreased viability |
| Song et al 194 | Human middle ear epithelial cell line (HMEEC‐1) | In vitro | 24 hours (extract in media) | Decreased viability, increased inflammatory cytokines, differential gene expression |
| Sun et al 191 | Human oral leukoplakia cell line (MSK‐Leuk1) | In vitro | 16 hours (extract in media) | Increased metabolism of polycyclic aromatic hydrocarbons to genotoxic products |
| Sundar et al 178 | Human gingival epithelium cell line (HGEPp) and 3D model (EpiGingival) | In vitro | 15 minutes (puffed over culture) | Increased protein carbonylation, increased inflammatory cytokines, increased DNA damage |
| Tellez et al 186 | Human oral epithelial (MOE1A, MOE1B) and leukoplakia (MSK‐Leuk1) cell lines | In vitro | 20 minutes (puffed over culture) | Increased cytotoxicity, increased DNA damage |
| Tsai et al 184 | Human oral SCC cell lines (Ca9‐22, CAL‐27) | In vitro | 24 hours (extract in media) | Altered cell invasion, increased RAGE expression, differential cytokine expression |
| Ureña et al 67 | Human oral SCC (SCC‐25) and gingival fibroblast cell lines (HGF‐1) | In vitro | 3 minutes total—1 minute every 3 hours (puffed over culture) | Decreased viability, increased oxidative stress |
| Vermehren et al 180 | Human gingival fibroblast cell line (HFIB‐G) | In vitro | 15 minutes (puffed over culture) | Decreased proliferation, increased metabolic activity, no significant difference in apoptosis or ROS formation |
| Welz et al 192 | Primary human pharyngeal organoid | Ex vivo | 24 and 2.5 hours per day for 5 days (extract in media) | Increased cytotoxicity, increased DNA fragmentation |
| Yu et al 183 | Human oral SCC cell lines (HN30, UMSCC10B) | In vitro | 24 hours a day for 1 week (extract in media) | Increased DNA strand breaks, increased cell arrest, increased apoptosis and necrosis |
Translational Research on E‐cigs in Otolaryngology
A smaller number of studies have looked at the effects of e‐cigs in human users (Table 2). In a preliminary study looking at the oral transcriptome of e‐cig users and cigarette smokers as compared to nonsmokers, e‐cig users showed a disruption of multiple molecular pathways that have been implicated in carcinogenesis, many of which overlapped with smokers but some of which were unique. 199 Consistent with previous research in cells, an increase in inflammatory markers and changes in the gene expression and metabolome can be detected from saliva and mucosa collected from e‐cig users compared to nonsmokers. 200 , 201 Another study found evidence of inflammation and immune suppression in the oral mucosa of e‐cig users 185 which is consistent with separate studies with similar findings in the nasal mucosa. 202 , 203 Finally, analysis of the saliva of e‐cig users has revealed the presence of carcinogens, 58 , 64 metanuclear abnormalities in oral cells, 204 changes in antimicrobial properties, 205 and possibly the oral microbiome. 185 , 206 The generalizability and clinical applicability of these results will become more evident as additional studies are performed.
Table 2.
Primary Studies on the Biologic Effects of E‐Cigarettes on the Upper Aerodigestive Tract and Ear in Human E‐Cig Users
| References | Subject of interest | Study design | Number of e‐cig users | Outcome(s) |
|---|---|---|---|---|
| Alqahtani et al 200 | Saliva | Cross‐sectional | 14 | Elevated inflammatory cytokines, differential expression of metabolites |
| Bustamante et al 64 | Saliva | Cross‐sectional | 16 | Elevated levels of carcinogen N′‐nitrosonornicotine (NNN) |
| Cichońska et al 205 | Saliva | Cross‐sectional | 40 | Decreased lysozyme and increased lactoferrin, no difference in IgA |
| Flieger et al 58 | Saliva | Cross‐sectional | 8 | Elevated levels of thiocyanate |
| Franco et al 171 | Oral epithelial cells | Cross‐sectional | 22 | No significant increase in micronuclei |
| Hamad et al 201 | Buccal cells and blood | Self‐controlled | 3 | Differential gene expression |
| Martin et al 202 | Nasal epithelial cells | Cross‐sectional | 12 | Differential gene expression (especially suppression of immune‐related genes) |
| Pushalkar et al 185 | Saliva | Cross‐sectional | 40 | Altered oral microbiome, nonsignificant but considerable alterations in inflammatory cytokines |
| Rebuli et al 203 | Nasal epithelial cell | Clinical trial | 15 | Suppression of IgA in setting of viral inoculation, differential gene expression, differential cytokine levels |
| Schwarzmeier et al 204 | Oral epithelial cells | Cross‐sectional | 20 | Increased metanuclear abnormalities |
| Stewart et al 206 | Saliva and buccal swab | Cross‐sectional | 10 | No significant differences in oral microbiome |
| Tommasi et al 199 | Oral epithelial cells | Cross‐sectional | 42 | Differential gene expression |
Clinical Research on E‐cigs in Otolaryngology
E‐cig use has also been associated with a wide range of self‐reported upper aerodigestive symptoms including throat irritation and discomfort, cough, tongue pain, nasal congestion, and sinus infections. 53 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 Although research on the effects of e‐cigs on human oral, oropharyngeal, and laryngeal health is still in its early stages, findings thus far are consistent with effects of e‐cigs in other organ systems and include immune suppression and inflammation (Table 3). Due to their effect on the immune system and oral microbiome, e‐cigs may additionally promote the risk of oral infection. 218 Consistent with this, are multiple reports of inflammatory and infectious processes in the mouth and throat including uvulitis and epiglottitis which appear to be secondary to e‐cig use, 219 , 220 although interestingly there is a single case study reporting resolution of recurrent tonsillitis in a nonsmoker after e‐cig use. 221 Finally, there are multiple reports of gingival and dental disease resulting from e‐cig use. 26 , 27 , 28 , 29 , 209 , 222 , 223 , 224
Table 3.
Primary Studies on Clinical Symptoms and Findings in Patients Who Report E‐Cig Usage
| References | Study design | Number of e‐cig users | Outcomes(s) |
|---|---|---|---|
| Andresen et al 233 | Case report | 1 | Fall with e‐cig in mouth resulting in pharyngeal and esophageal burns, diffuse supraglottic edema requiring tracheostomy and long‐term percutaneous feeding |
| Bardellini et al 225 | Case‐control | 45 | Similar total prevalence of oral mucosal lesions in e‐cig users compared to former smokers; increase in nicotine stomatitis, hairy tongue, and angular cheilitis compared to former smokers |
| Bartram et al 226 | Case report | 1 | Lichenoid eruption after switching from cigarettes to e‐cig with high propylene glycol content with near‐resolution after switching to e‐cig with low propylene glycol content |
| Bozzella et al 220 | Case report | 1 | Epiglottitis in e‐cig user without other risk factors or identifiable etiology; focal erosions/ulcerations and reactive/inflammatory changes on biopsy of arytenoid, soft palate, tongue |
| Brooks et al 90 | Case report | 1 | E‐cig explosion resulting in extensive intraoral and facial injuries |
| Brownson et al 85 | Case series | 15 | E‐cig explosion resulting in flame burns, chemical burns, and blast injuries |
| Cant et al 229 | Case report | 1 | Necrotic palatal ulcer in setting of e‐cig use |
| Cason et al 91 | Case report | 1 | E‐cig explosion resulted in extensive intraoral and facial injuries and inhalational injuries |
| Chen et al 215 | Observational | 138,448 | Wide range of self‐reported symptoms from e‐cig users, most commonly related to the respiratory system and mouth |
| Cho et al 209 | Cross‐sectional | 216 | Adolescent daily e‐cig users report increased rate of oral pain and dental concerns compared to never users |
| Demir et al 241 | Case report | 1 | Pediatric sudden sensorineural hearing loss after accidental ingestion of e‐liquid |
| Farinha et al 227 | Case report | 1 | Lingua villosa nigra after switching from cigarettes to e‐cig and resolution after switching back to cigarettes |
| Farsalinos et al 53 | Observational | 19,414 | Wide range of self‐reported symptoms from e‐cig users, most commonly sore/dry mouth and throat |
| Frossard et al 219 | Case report | 1 | Acute uvulitis after e‐cig use without other risk factors or known etiology requiring intubation |
| Gill et al 235 | Case report | 1 | Accidental ingestion of e‐liquid resulting in nicotine poisoning |
| Harrison et al 88 | Case report | 1 | E‐cig explosion resulting in extensive intraoral injuries |
| Huilgol et al 222 | Cross‐sectional | 4957 | Self‐reported daily e‐cig use associated with poor oral health compared to intermittent or no use |
| Hua et al 207 | Observational | 481 | Wide range of self‐reported symptoms from e‐cig users, most commonly related to the respiratory system and mouth/throat |
| Hua et al 208 | Observational | NA (41,216 posts) | Wide range of self‐reported symptoms from e‐cig users, most commonly related to the neurologic and respiratory symptoms and mouth/throat |
| Hughes et al 236 | Observational | 256 | Of 256 calls to a poison center regarding e‐cigs, majority involved children and the refill containers or fluid. Of pediatric patients who ingested e‐liquid, initial symptoms present in 32% |
| Jankowski et al 216 | Observational | 61 | Wide range of self‐reported symptoms from e‐cig users, most commonly cough and sore throat |
| King et al 210 | Observational | 1624 | Wide range of self‐reported symptoms from e‐cig users, most commonly cough and dry/irritated mouth/throat |
| Klawinski et al 231 | Case report | 1 | Stage IV SCC in 19‐year‐old with no other risk factors |
| Kumral et al 211 | Randomized control | 42 | SNOT‐22 scores worse in patients who used e‐cigs to quit smoking than those who quit without the use of e‐cigs |
| Kumetz et al 92 | Case series | 2 | E‐cig explosion resulting in extensive intraoral injury and burns |
| Li et al 213 | Cross‐sectional | 641 | Adult e‐cig users have increased wheezing and related respiratory symptoms compared to nonusers but less than current smokers |
| Luo et al 214 | Observational | NA (7927 posts) | Wide range of self‐reported symptoms from e‐cig users, most commonly respiratory and throat symptoms |
| Miler et al 221 | Case report | 1 | Resolution of chronic tonsillitis in a never‐smoker after vaping |
| Mokeem et al 218 | Cross‐sectional | 30 | Oral Candida albicans burden in e‐cig users is significantly higher than never‐smokers and similar to cigarette and waterpipe smokers |
| Moore et al 93 | Case report | 1 | E‐cig explosion resulting in intraoral injuries |
| Morse et al 84 | Case report | 1 | E‐cig explosion result in extensive oropharyngeal and palatal burns and associated right ear pain requiring intubation, Dobhoff tube, right SLN blocks, and extensive swallow and speech therapy |
| Nguyen et al 230 | Case series | 2 | Oral basaloid SCC in two patients with no other risk factors |
| Norii et al 94 | Case report | 1 | E‐cig explosion resulting in pharyngeal injury and C1 and C2 fracture |
| Reuther et al 104 | Quasi‐experimental | 10 | 5 minutes of vaping in prior nonusers resulted in temporary increase in capillary perfusion of buccal mucosa |
| Richmond et al 237 | Observational | 220 | Of 220 cases of e‐cig inhalation and ingestion in children presenting to pediatricians, majority of inhalation in male adolescents while majority of ingestion was in male children. Both inhalation and ingestion most commonly resulted in nausea/vomiting, cough, throat irritation, or acute toxicity |
| Rogér et al 89 | Case report | 1 | E‐cig explosion resulting in extensive intraoral injuries and burns |
| Sample 239 | Cross‐sectional | 7 | E‐cig users had raised vocal shimmer compared to nonusers and use was associated with abnormal mucosal wave, free edge, phase closure, vocal fold varices, and vocal fold edema |
| Seo et al 234 | Case report | 1 | Death of a 15‐month‐old child from accidental ingestion of e‐liquid |
| Soule et al 212 | Observational | 49 | Wide range of self‐reported symptoms from e‐cig users, most commonly dry throat/mouth |
| Tsiouma et al 228 | Case report | 1 | Palatal ulceration in e‐cig user which resolved with treatment after switching to conventional cigarettes |
| Tuhanioğlu et al 238 | Cross‐sectional | 21 | No significant difference in VHI‐10 values or vocal quality between e‐cig users and nonusers |
| Vaught et al 86 | Case report | 1 | E‐cig explosion resulting in significant facial trauma |
| Walele et al 217 | Clinical trial | 102 | Prior smokers who switched from conventional cigarettes to e‐cig noted headache, nasopharyngitis, sore throat as the most common adverse events, most of which were transient |
A few studies have demonstrated an increase in mucosal lesions in e‐cig users as compared to controls and former smokers. 225 , 226 , 227 Two case studies have linked necrotic palatal ulcers with e‐cig use. 228 , 229 Importantly, multiple cases of oral carcinoma in the setting of prolonged e‐cig use in the absence of other risk factors have been reported. 230 , 231 Aside from effects of the toxic and carcinogenic contents of aerosolized e‐juice on the upper aerodigestive tract, e‐cig devices have also been implicated in multiple reports of burns and trauma of the upper aerodigestive tract and maxillofacial structures 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 232 , 233 and toxic ingestion. 234 , 235 , 236 , 237
Studies pertaining to the clinical effect of e‐cigs on the larynx and ear are particularly limited. One study evaluating acoustic voice changes in e‐cig users found no significant difference between e‐cig users and controls, however this study was limited by the small cohort size, short duration of e‐cig use (1‐3 years), and explicit exclusion of laryngeal pathologies for which cigarette and e‐cig exposure is known to be a significant risk factor such laryngeal inflammation and irritation. 238 A second limited study also showed no change in acoustic measures between e‐cig users and nonsmokers but did find significant reduction in the vocal fold mucosal wave, irregularity of the free vocal fold edge, and abnormal phase closure as evaluated by videostroboscopy. 239 While traditional cigarette smoke has been implicated in a number of otologic disorders, 240 there is currently only one report regarding a case of sudden sensorineural hearing loss after ingestion of e‐juice 241 with regards to the clinical effect of e‐cigs on the ear.
Future Directions
Although there has been an exponential rise in basic and translational work regarding e‐cigs in multiple organ systems, there are fewer studies pertaining specifically to the upper aerodigestive tract. Meta‐analyses on existing studies are needed but complicated by significant variations between and within studies in e‐cig brands, devices, and flavors used; exposure method; extract concentrations; and exposure durations; among other factors. Furthermore, the potential long‐term health consequences remain unknown; many consequences of cigarette smoking develop only after chronic use, and no current laboratory experiment can truly emulate decades of exposure. Even less is known about other alternative nicotine products including HTs and ONPs, which have been gaining market share and will likely play an increasing role in tobacco‐ and nicotine‐associated health concerns. While e‐cigs appear less carcinogenic than conventional cigarettes, the data thus far suggests a negative impact of e‐cigs across multiple organ systems including the upper aerodigestive tract. Given the outsized impact of smoking on diseases in otolaryngology, further work on e‐cigs and other alternative nicotine products is urgently needed.
Researchers in otolaryngology can benefit from building upon the research performed in models of other organ systems, particularly the lower respiratory system, to determine how e‐cig exposure interacts with the unique anatomy, physiology, and function of the various areas of the head and neck. In addition, the current reports on clinical diseases in e‐cig users pertaining to the upper aerodigestive tract are largely case studies and case series, thus necessitating further investigation to determine if e‐cigs are truly the causal agent. Finally, there are several avenues specific to otolaryngology that have been minimally explored, particularly the effects of e‐cigs on the sinonasal cavity, larynx, and ear.
Implications for Practice
Otolaryngology providers are among the first clinicians that nicotine users encounter with a smoking‐related disease process. Despite recent enhancement of policies to reduce youth nicotine use, youth use of and addiction to nicotine products has increased dramatically as compared to the decade prior, and medical practitioners should expect to see the health effect of this increase in the coming decades. In current smokers, the evidence for the utility of e‐cigs for overall risk reduction is heterogenous. While e‐cigs have been demonstrated to have fewer carcinogenic agents than their traditional counterparts—a fact often advertised by tobacco companies—there is evidence that these products remain a significant factor in the development of disease. Therefore, it is critical for every otolaryngology provider to stay abreast regarding the potential health consequences of e‐cigs, particularly with regards to the upper aerodigestive tract.
Author Contributions
Joanne Soo, study design, literature search and analysis, manuscript preparation, revisions, approval for final version; Meena Easwaran, literature analysis, manuscript preparation, revisions, approval for final version; Elizabeth Erickson‐DiRenzo: study design, manuscript preparation, revisions, approval for final version.
Disclosures
Competing interests
None.
Funding sources
None.
Supporting information
Supplemental Figure 1.
Supporting information.
Supporting information.
Acknowledgments
We would like to thank Dr Robert Jackler for his comments and the Stanford Research into the Impact of Tobacco Advertising (SRITA) collection for providing the images for Figure 1 (tobacco.stanford.edu). Figure 3 was created with BioRender.com.
References
- 1. Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jethwa AR, Khariwala SS. Tobacco‐related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017;36(3):411‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tavaluc R, Tan‐Geller M. Reinke's edema. Otolaryngol Clin North Am. 2019;52(4):627‐635. [DOI] [PubMed] [Google Scholar]
- 4. Hecht SS. Tobacco carcinogens, their biomarkers and tobacco‐induced cancer. Nat Rev Cancer. 2003;3(10):733‐744. [DOI] [PubMed] [Google Scholar]
- 5. NTP (National Toxicology Program) . Report on carcinogens, fifteenth edition. 2021. Accessed February 10, 2022. https://ntp.niehs.nih.gov/go/roc15
- 6. Canistro D, Vivarelli F, Cirillo S, et al. E‐cigarettes induce toxicological effects that can raise the cancer risk. Sci Rep. 2017;7(1):2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hess CA, Olmedo P, Navas‐Acien A, Goessler W, Cohen JE, Rule AM. E‐cigarettes as a source of toxic and potentially carcinogenic metals. Environ Res. 2017;152:221‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soneji S, Barrington‐Trimis JL, Wills TA, et al. Association between initial use of e‐cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta‐analysis. JAMA Pediatr. 2017;171(8):788‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Food and Drug Administration (FDA), HHS . Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the family smoking prevention and tobacco control act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. 2016. Accessed September 7, 2021. https://www.govinfo.gov/content/pkg/FR-2016-05-10/pdf/2016-10685.pdf [PubMed]
- 10.111th Congress. Family smoking prevention and tobacco control act, H.R. 1256, Pub. L. No. 111–31, 123, 1776 Stat. 2009. Accessed September 7, 2021. https://www.govinfo.gov/content/pkg/PLAW-111publ31/pdf/PLAW-111publ31.pdf
- 11. European Commission . Directive 2014/40/EU of the European Parliament and of the Council of 4 April 2014 on the approximation of the laws, regulations and administrative provisions of the Member States concerning the manufacture, presentation and sale of tobacco and related products and repealing directive 2001/37/EC. 2014. Accessed September 7, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014L0040
- 12. Food and Drug Administration (FDA), HHS . FDA denies authorization to market JUUL products. 2022. Accessed July 2, 2022. https://www.fda.gov/news-events/press-announcements/fda-denies-authorization-market-juul-products
- 13. The Washington Post . Court temporarily halts FDA ban on Juul e‐cigarettes. 2022. Accessed July 2, 2022. https://www.washingtonpost.com/business/2022/06/25/juul-vaping-cigarettes-fda-ban-appeal/
- 14. Yang Y, Lindblom EN, Salloum RG, Ward KD. The impact of a comprehensive tobacco product flavor ban in San Francisco among young adults. Addict Behav Rep. 2020;11:100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaiha SM, Lempert LK, McKelvey K, Halpern‐Felsher B. E‐cigarette devices, brands, and flavors attract youth: informing FDA's policies and priorities to close critical gaps. Addict Behav. 2022;126:107179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gotts JE, Jordt SE, McConnell R, Tarran R. What are the respiratory effects of e‐cigarettes? BMJ. 2019;366:l5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalininskiy A, Kittel J, Nacca NE, Misra RS, Croft DP, McGraw MD. E‐cigarette exposures, respiratory tract infections, and impaired innate immunity: a narrative review. Pediatr Med. 2021;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cherian SV, Kumar A, Estrada‐Y‐Martin RM. E‐cigarette or vaping product‐associated lung injury: a review. Am J Med. 2020;133(6):657‐663. [DOI] [PubMed] [Google Scholar]
- 19. Kalininskiy A, Bach CT, Nacca NE, et al. E‐cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kennedy CD, van Schalkwyk MCI, McKee M, Pisinger C. The cardiovascular effects of electronic cigarettes: a systematic review of experimental studies. Prev Med. 2019;127:105770. [DOI] [PubMed] [Google Scholar]
- 21. Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e‐cigarettes. Am J Physiol Lung Cell Mol Physiol. 2017;313(2):L193‐L206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thirión‐Romero I, Pérez‐Padilla R, Zabert G, Barrientos‐Gutiérrez I. Respiratory impact of electronic cigarettes and “low‐risk” tobacco. Rev Invest Clin. 2019;71(1):17‐27. [DOI] [PubMed] [Google Scholar]
- 23. Martheswaran T, Shmunes MH, Ronquillo YC, Moshirfar M. The impact of vaping on ocular health: a literature review. Int Ophthalmol. 2021;41(8):2925‐2932. [DOI] [PubMed] [Google Scholar]
- 24. Miglio F, Naroo S, Zeri F, Tavazzi S, Ponzini E. The effect of active smoking, passive smoking, and e‐cigarettes on the tear film: an updated comprehensive review. Exp Eye Res. 2021;210:108691. [DOI] [PubMed] [Google Scholar]
- 25. Nicholson T, Scott A, Newton Ede M, Jones SW. The impact of E‐cigarette vaping and vapour constituents on bone health. J Inflamm. 2021;18(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Almeida‐da‐Silva CLC, Matshik Dakafay H, O'Brien K, Montierth D, Xiao N, Ojcius DM. Effects of electronic cigarette aerosol exposure on oral and systemic health. Biomed J. 2021;44(3):252‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sultan AS, Jessri M, Farah CS. Electronic nicotine delivery systems: oral health implications and oral cancer risk. J Oral Pathol Med. 2021;50(3):316‐322. [DOI] [PubMed] [Google Scholar]
- 28. Ebersole J, Samburova V, Son Y, et al. Harmful chemicals emitted from electronic cigarettes and potential deleterious effects in the oral cavity. Tob Induc Dis. 2020;18:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang I, Sandeep S, Rodriguez J. The oral health impact of electronic cigarette use: a systematic review. Crit Rev Toxicol. 2020;50(2):97‐127. [DOI] [PubMed] [Google Scholar]
- 30. Ruszkiewicz JA, Zhang Z, Gonçalves FM, Tizabi Y, Zelikoff JT, Aschner M. Neurotoxicity of e‐cigarettes. Food Chem Toxicol. 2020;138:111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGrath‐Morrow SA, Gorzkowski J, Groner JA, et al. The effects of nicotine on development. Pediatrics. 2020;145(3):e20191346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jamshed L, Perono GA, Jamshed S, Holloway AC. Early life exposure to nicotine: postnatal metabolic, neurobehavioral and respiratory outcomes and the development of childhood cancers. Toxicol Sci. 2020;178(1):3‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tzortzi A, Kapetanstrataki M, Evangelopoulou V, Behrakis P. A systematic literature review of e‐cigarette‐related illness and injury: not just for the respirologist. Int J Environ Res Public Health. 2020;17(7):2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eltorai AE, Choi AR, Eltorai AS. Impact of electronic cigarettes on various organ systems. Respir Care. 2019;64(3):328‐336. [DOI] [PubMed] [Google Scholar]
- 35. Arany I, Taylor M, Fülöp T, Dixit M. Adverse effects of chronic nicotine exposure on the kidney: potential human health implications of experimental findings. Int J Clin Pharmacol Ther. 2018;56(11):501‐506. [DOI] [PubMed] [Google Scholar]
- 36. Tweed JO, Hsia SH, Lutfy K, Friedman TC. The endocrine effects of nicotine and cigarette smoke. Trends Endocrinol Metab. 2012;23(7):334‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Münzel T, Hahad O, Kuntic M, Keaney JF, Deanfield JE, Daiber A. Effects of tobacco cigarettes, e‐cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J. 2020;41(41):4057‐4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benowitz NL, Fraiman JB. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol. 2017;14(8):447‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bergstrom HC, McDonald CG, French HT, Smith RF. Continuous nicotine administration produces selective, age‐dependent structural alteration of pyramidal neurons from prelimbic cortex. Synapse. 2008;62(1):31‐39. [DOI] [PubMed] [Google Scholar]
- 40. Biyani S, Derkay CS. E‐cigarettes: considerations for the otolaryngologist. Int J Pediatr Otorhinolaryngol. 2015;79(8):1180‐1183. [DOI] [PubMed] [Google Scholar]
- 41. Biyani S, Derkay CS. E‐cigarettes: an update on considerations for the otolaryngologist. Int J Pediatr Otorhinolaryngol. 2017;94:14‐16. [DOI] [PubMed] [Google Scholar]
- 42. Stobbs N, Lillis A, Kumar N. E‐cigarettes in ENT: what do we need to know? J Laryngol Otol. 2016;130(6):512‐515. [DOI] [PubMed] [Google Scholar]
- 43. Flach S, Maniam P, Manickavasagam J. E‐cigarettes and head and neck cancers: a systematic review of the current literature. Clin Otolaryngol. 2019;44(5):749‐756. [DOI] [PubMed] [Google Scholar]
- 44. Kar M, Emre IE, Bayar Muluk N, Cingi C. Effect of electronic cigarettes on the inner mucosa of the craniofacial region. J Craniofac Surg. 2019;30(3):e235‐e238. [DOI] [PubMed] [Google Scholar]
- 45. Born H, Persky M, Kraus DH, Peng R, Amin MR, Branski RC. Electronic cigarettes: a primer for clinicians. Otolaryngol Head Neck Surg. 2015;153(1):5‐14. [DOI] [PubMed] [Google Scholar]
- 46. Harrell PT, Simmons VN, Correa JB, Padhya TA, Brandon TH. Electronic nicotine delivery systems (“e‐cigarettes”): review of safety and smoking cessation efficacy. Otolaryngol Head Neck Surg. 2014;151(3):381‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bold KW, Kong G, Morean M, et al. Trends in various e‐cigarette devices used by high school adolescents from 2017‐2019. Drug Alcohol Depend. 2021;219:108497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. The Wall Street Journal . Puff Bar has overtaken Juul as the favorite e‐cigarette for teens. 2021. Accessed February 27, 2022. https://www.wsj.com/articles/puff-bar-has-overtaken-juul-as-the-favorite-e-cigarette-for-teens-11633021209
- 49. Henningfield JE, Zaatari GS. Electronic nicotine delivery systems: emerging science foundation for policy. Tob Control. 2010;19(2):89‐90. [DOI] [PubMed] [Google Scholar]
- 50. Choi K, Forster JL. Beliefs and experimentation with electronic cigarettes. Am J Prev Med. 2014;46(2):175‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sharma A, McCausland K, Jancey J. Adolescent's health perceptions of e‐cigarettes: a systematic review. Am J Prev Med. 2021;60(5):716‐725. [DOI] [PubMed] [Google Scholar]
- 52. Russell C, Katsampouris E, Mckeganey N. Harm and addiction perceptions of the JUUL e‐cigarette among adolescents. Nicotine Tob Res. 2020;22(5):713‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Farsalinos K, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. Int J Environ Res Public Health. 2014;11(4):4356‐4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Strombotne K, Buckell J, Sindelar JL. Do JUUL and e‐cigarette flavours change risk perceptions of adolescents? Evidence from a national survey. Tob Control. 2021;30(2):199‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tegin G, Mekala HM, Sarai SK, Lippmann S. E‐cigarette toxicity. South Med J. 2018;111(1):35‐38. [DOI] [PubMed] [Google Scholar]
- 56. Eshraghian E, Al‐Delaimy W. A review of constituents identified in e‐cigarette liquids and aerosols. Tob Prev Cessat. 2021;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Qu Y, Kim KH, Szulejko JE. The effect of flavor content in e‐liquids on e‐cigarette emissions of carbonyl compounds. Environ Res. 2018;166:324‐333. [DOI] [PubMed] [Google Scholar]
- 58. Flieger J, Kawka J, Tatarczak‐Michalewska M. Levels of the thiocyanate in the saliva of tobacco smokers in comparison to e‐cigarette smokers and nonsmokers measured by HPLC on a phosphatidylcholine column. Molecules. 2019;24(20):3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fels Elliott DR, Shah R, Hess CA, et al. Giant cell interstitial pneumonia secondary to cobalt exposure from e‐cigarette use. Eur Respir J. 2019;54(6):1901922. [DOI] [PubMed] [Google Scholar]
- 60. Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8(3):e57987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. White AV, Wambui DW, Pokhrel LR. Risk assessment of inhaled diacetyl from electronic cigarette use among teens and adults. Sci Total Environ. 2021;772:145486. [DOI] [PubMed] [Google Scholar]
- 62. Salamanca JC, Meehan‐Atrash J, Vreeke S, Escobedo JO, Peyton DH, Strongin RM. E‐cigarettes can emit formaldehyde at high levels under conditions that have been reported to be non‐averse to users. Sci Rep. 2018;8(1):7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e‐cigarette aerosols. N Engl J Med. 2015;372(4):392‐394. [DOI] [PubMed] [Google Scholar]
- 64. Bustamante G, Ma B, Yakovlev G, et al. Presence of the carcinogen N′‐nitrosonornicotine in saliva of e‐cigarette users. Chem Res Toxicol. 2018;31(8):731‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kosmider L, Sobczak A, Prokopowicz A, et al. Cherry‐flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax. 2016;71(4):376‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon‐flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28(2):198‐208. [DOI] [PubMed] [Google Scholar]
- 67. Ureña JF, Ebersol LA, Silakov A, Elias RJ, Lambert JD. Impact of atomizer age and flavor on in vitro toxicity of aerosols from a third‐generation electronic cigarette against human oral cells. Chem Res Toxicol. 2020;33(10):2527‐2537. [DOI] [PubMed] [Google Scholar]
- 68. Lisko JG, Tran H, Stanfill SB, Blount BC, Watson CH. Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in e‐cigarette cartridges and refill solutions. Nicotine Tob Res. 2015;17(10):1270‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Varlet V, Farsalinos K, Augsburger M, Thomas A, Etter JF. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12(5):4796‐4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhou Y, Irshad H, Dye WW, Wu G, Tellez CS, Belinsky SA. Voltage and e‐liquid composition affect nicotine deposition within the oral cavity and carbonyl formation. Tob Control. 2021;30(5):485‐491. [DOI] [PubMed] [Google Scholar]
- 71. Brown CJ, Cheng JM. Electronic cigarettes: product characterisation and design considerations. Tob Control. 2014;23:ii4‐ii10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e‐cigarettes) impairs indoor air quality and increases FeNO levels of e‐cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628‐637. [DOI] [PubMed] [Google Scholar]
- 73. Czogala J, Goniewicz ML, Fidelus B, Zielinska‐Danch W, Travers MJ, Sobczak A. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob Res. 2014;16(6):655‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hess I, Lachireddy K, Capon A. A systematic review of the health risks from passive exposure to electronic cigarette vapour. Public Health Res Pract. 2016;26(2):2621617. [DOI] [PubMed] [Google Scholar]
- 75. Goniewicz ML, Lee L. Electronic cigarettes are a source of thirdhand exposure to nicotine. Nicotine Tob Res. 2015;17(2):256‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McMillen R, Klein JD, Wilson K, Winickoff JP, Tanski S. E‐cigarette use and future cigarette initiation among never smokers and relapse among former smokers in the PATH study. Public Health Rep. 2019;134(5):528‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Barufaldi L, Guerra R, de Albuquerque RC, et al. Risk of smoking relapse with the use of electronic cigarettes: a systematic review with meta‐analysis of longitudinal studies. Tob Prev Cessat. 2021;29:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Löhler J, Wollenberg B. Are electronic cigarettes a healthier alternative to conventional tobacco smoking? Eur Arch Otrhinolaryngol. 2019;276(1):17‐25. [DOI] [PubMed] [Google Scholar]
- 79. Osibogun O, Bursac Z, Maziak W. E‐cigarette use and regular cigarette smoking among youth: population assessment of tobacco and health study (2013‐2016). Am J Prev Med. 2020;58(5):657‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Khouja JN, Suddell SF, Peters SE, Taylor AE, Munafò MR. Is e‐cigarette use in non‐smoking young adults associated with later smoking? A systematic review and meta‐analysis. Tob Control. 2020;30(1):8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. US Department of Health and Human Services . Surgeon general releases advisory on e‐cigarette epidemic among youth. 2018. Accessed August 4, 2021. https://www.hhs.gov/about/news/2018/12/18/surgeon-general-releases-advisory-e-cigarette-epidemic-among-youth.html
- 82. Food and Drug Administration (FDA), HHS . Statement from FDA Commissioner Scott Gottlieb, M.D., on new enforcement actions and a youth tobacco prevention plan to stop youth use of, and access to, JUUL and other e‐cigarettes. 2018. Accessed September 7, 2021. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-new-enforcement-actions-and-youth-tobacco-prevention
- 83. Seitz CMS, Kabir Z. Burn injuries caused by e‐cigarette explosions: a systematic review of published cases. Tob Prev Cessat. 2018;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Morse J, Tittman S, Gelbard A. Oropharyngeal injury from spontaneous combustion of a lithium‐ion battery: a case report. Laryngoscope. 2019;129(1):45‐48. [DOI] [PubMed] [Google Scholar]
- 85. Brownson EG, Thompson CM, Goldsberry S, et al. Explosion injuries from e‐cigarettes. N Engl J Med. 2016;375(14):1400‐1402. [DOI] [PubMed] [Google Scholar]
- 86. Vaught B, Spellman J, Shah A, Stewart A, Mullin D. Facial trauma caused by electronic cigarette explosion. Ear Nose Throat J. 2017;96(3):139‐142. [DOI] [PubMed] [Google Scholar]
- 87. Dekhou A, Oska N, Partiali B, Johnson J, Chung MT, Folbe A. E‐cigarette burns and explosions: what are the patterns of oromaxillofacial injury? J Oral Maxillofac Surg. 2021;79(8):1723‐1730. [DOI] [PubMed] [Google Scholar]
- 88. Harrison R, Hicklin D. Electronic cigarette explosions involving the oral cavity. J Am Dent Assoc. 2016;147(11):891‐896. [DOI] [PubMed] [Google Scholar]
- 89. Rogér JM, Abayon M, Elad S, Kolokythas A. Oral trauma and Tooth avulsion following explosion of e‐cigarette. J Oral Maxillofac Surg. 2016;74(6):1181‐1185. [DOI] [PubMed] [Google Scholar]
- 90. Brooks JK, Kleinman JW, Brooks JB, Reynolds MA. Electronic cigarette explosion associated with extensive intraoral injuries. Dent Traumatol. 2017;33(2):149‐152. [DOI] [PubMed] [Google Scholar]
- 91. Cason DE, Morgan DE, Pietryga JA. Injuries from an exploding e‐cigarette: a case report. Ann Intern Med. 2016;165(9):678‐679. [DOI] [PubMed] [Google Scholar]
- 92. Kumetz EA, Hurst ND, Cudnik RJ, Rudinsky SL. Electronic cigarette explosion injuries. Am J Emerg Med. 2016;34(11):2252.e1‐2252.e3. [DOI] [PubMed] [Google Scholar]
- 93. Moore J, Mihalache G, Messahel A. “Exploding” electronic cigarette: a case report. Br J Oral Maxillofac Surg. 2016;54(9):1056‐1057. [DOI] [PubMed] [Google Scholar]
- 94. Norii T, Plate A. Electronic cigarette explosion resulting in a C1 and C2 fracture: a case report. J Emerg Med. 2017;52(1):86‐88. [DOI] [PubMed] [Google Scholar]
- 95. Creamer MR, Everett Jones S, Gentzke AS, Jamal A, King BA. Tobacco product use among high school students—youth risk behavior survey, United States, 2019. MMWR Suppl. 2020;69(1):56‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(45):1013‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fracol M, Dorfman R, Janes L, et al. The surgical impact of e‐cigarettes: a case report and review of the current literature. Arch Plast Surg. 2017;44(6):477‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hrywna M, Bover Manderski MT, Delnevo CD. Prevalence of electronic cigarette use among adolescents in New Jersey and association with social factors. JAMA Netw Open. 2020;3(2):e1920961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Food and Drug Administration (FDA), HHS . Products, ingredients, & components. 2020. Accessed September 2, 2021. https://www.fda.gov/tobacco-products/products-guidance-regulations/products-ingredients-components
- 100. Centers for Disease Control and Prevention . Smoking & tobacco use: fast facts and fact sheets. 2022. Accessed September 2, 2021. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Ftobacco%2Fdata_statistics%2Ffact_sheets%2Findex.htm
- 101. Jackler RK. What every otolaryngologist should know about electronic cigarettes, especially JUUL. American Academy of Otolaryngology–Head and Neck Surgery. 2019. Accessed September 2, 2021. https://bulletin.entnet.org/home/article/21247521/what-every-otolaryngologist-should-know-about-electronic-cigarettes-especially-juul
- 102. Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncol. 2008;9(7):667‐675. [DOI] [PubMed] [Google Scholar]
- 103. GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990‐2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1123‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Reuther WJ, Hale B, Matharu J, Blythe JN, Brennan PA. Do you mind if I vape? Immediate effects of electronic cigarettes on perfusion in buccal mucosal tissue—a pilot study. Br J Oral Maxillofac Surg. 2016;54(3):338‐341. [DOI] [PubMed] [Google Scholar]
- 105. Page F, Hamnett N, Wearn C, Hardwicke J, Moiemen N. The acute effects of electronic cigarette smoking on the cutaneous circulation. J Plast Reconstr Aesthet Surg. 2016;69(4):575‐577. [DOI] [PubMed] [Google Scholar]
- 106. Lei W, Lerner C, Sundar IK, Rahman I. Myofibroblast differentiation and its functional properties are inhibited by nicotine and e‐cigarette via mitochondrial OXPHOS complex III. Sci Rep. 2017;7:43213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jaleel Z, Blasberg E, Troiano C, et al. Association of vaping with decreased vascular endothelial growth factor expression and decreased microvessel density in cutaneous wound healing tissue in rats. Wound Repair Regen. 2021;29(6):1024‐1034. [DOI] [PubMed] [Google Scholar]
- 108. Rau AS, Reinikovaite V, Schmidt EP, Taraseviciene‐Stewart L, Deleyiannis FWB. Electronic cigarettes are as toxic to skin flap survival as tobacco cigarettes. Ann Plast Surg. 2017;79(1):86‐91. [DOI] [PubMed] [Google Scholar]
- 109. Krishnan NM, Han KD, Nahabedian MY. Can e‐cigarettes cause free flap failure? A case of arterial vasospasm induced by electronic cigarettes following microsurgical breast reconstruction. Plast Reconstr Surg Glob Open. 2016;4(1):e596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Troiano C, Jaleel Z, Spiegel JH. Association of electronic cigarette vaping and cigarette smoking with decreased random flap viability in rats. JAMA Facial Plast Surg. 2019;21(1):5‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Agochukwu N, Liau JY. Debunking the myth of e‐cigarettes: a case of free flap compromise due to e‐cigarette use within the first 24 hours. J Plast Reconstr Aesthet Surg. 2018;71(3):451‐453. [DOI] [PubMed] [Google Scholar]
- 112. Sanford NN, Sher DJ, Xu X, Aizer AA, Mahal BA. Trends in smoking and e‐cigarette use among US patients with cancer, 2014‐2017. JAMA Oncol. 2019;5(3):426‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dautzenberg B, Garelik D. Patients with lung cancer: are electronic cigarettes harmful or useful? Lung Cancer. 2017;105:42‐48. [DOI] [PubMed] [Google Scholar]
- 114. Hecht SS, Carmella SG, Kotandeniya D, et al. Evaluation of toxicant and carcinogen metabolites in the urine of e‐cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17(6):704‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Polosa R, Rodu B, Caponnetto P, Maglia M, Raciti C. A fresh look at tobacco harm reduction: the case for the electronic cigarette. Harm Reduct J. 2013;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Beaglehole R, Bates C, Youdan B, Bonita R. Nicotine without smoke: fighting the tobacco epidemic with harm reduction. Lancet. 2019;394(10200):718‐720. [DOI] [PubMed] [Google Scholar]
- 117. Hajek P, Phillips‐Waller A, Przulj D, et al. A randomized trial of e‐cigarettes versus nicotine‐replacement therapy. N Engl J Med. 2019;380(7):629‐637. [DOI] [PubMed] [Google Scholar]
- 118. Chan GCK, Stjepanović D, Lim C, et al. A systematic review of randomized controlled trials and network meta‐analysis of e‐cigarettes for smoking cessation. Addict Behav. 2021;119:106912. [DOI] [PubMed] [Google Scholar]
- 119. Hartmann‐Boyce J, McRobbie H, Lindson N, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2020;10:CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629‐1637. [DOI] [PubMed] [Google Scholar]
- 121. Wang RJ, Bhadriraju S, Glantz SA. E‐cigarette use and adult cigarette smoking cessation: a meta‐analysis. Am J Public Health. 2021;111(2):230‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kalkhoran S, Glantz SA. E‐cigarettes and smoking cessation in real‐world and clinical settings: a systematic review and meta‐analysis. Lancet Respir Med. 2016;4(2):116‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hartmann‐Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2016;9:CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ghosh S, Drummond MB. Electronic cigarettes as smoking cessation tool: are we there? Curr Opin Pulm Med. 2017;23(2):111‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Patil S, Arakeri G, Patil S, et al. Are electronic nicotine delivery systems (ENDs) helping cigarette smokers quit?—current evidence. J Oral Pathol Med. 2020;49(3):181‐189. [DOI] [PubMed] [Google Scholar]
- 126. Pound CM, Zhang JZ, Kodua AT, Sampson M. Smoking cessation in individuals who use vaping as compared with traditional nicotine replacement therapies: a systematic review and meta‐analysis. BMJ Open. 2021;11(2):e044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ibrahim S, Habiballah M, Sayed IE. Efficacy of electronic cigarettes for smoking cessation: a systematic review and meta‐analysis. Am J Health Promot. 2021;35(3):442‐455. [DOI] [PubMed] [Google Scholar]
- 128. Wang JB, Olgin JE, Nah G, et al. Cigarette and e‐cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS One. 2018;13(7):e0198681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kim CY, Paek YJ, Seo HG, et al. Dual use of electronic and conventional cigarettes is associated with higher cardiovascular risk factors in Korean men. Sci Rep. 2020;10(1):5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Martinez U, Simmons VN, Sutton SK, et al. Targeted smoking cessation for dual users of combustible and electronic cigarettes: a randomised controlled trial. Lancet Public Health. 2021;6(7):e500‐e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Owusu D, Huang J, Weaver SR, et al. Patterns and trends of dual use of e‐cigarettes and cigarettes among U.S. adults, 2015‐2018. Prev Med Rep. 2019;16:101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010‐2013. Nicotine Tob Res. 2015;17(2):219‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Martínez Ú, Martínez‐Loredo V, Simmons VN, et al. How does smoking and nicotine dependence change after onset of vaping? A retrospective analysis of dual users. Nicotine Tob Res. 2020;22(5):764‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Glantz SA, Bareham DW. E‐cigarettes: use, effects on smoking, risks, and policy implications. Annu Rev Public Health. 2018;39:215‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Camara‐Medeiros A, Diemert L, O'Connor S, Schwartz R, Eissenberg T, Cohen JE. Perceived addiction to vaping among youth and young adult regular vapers. Tob Control. 2021;30(3):273‐278. [DOI] [PubMed] [Google Scholar]
- 136.American Academy of Otolaryngology–Head and Neck Surgery. Position statements on e‐cigarettes. 2021. Accessed September 2, 2021. https://www.entnet.org/resource/position-statements-on-e-cigarettes/
- 137. Bahmed K, Lin CR, Simborio H, et al. The role of DJ‐1 in human primary alveolar type II cell injury induced by e‐cigarette aerosol. Am J Physiol Lung Cell Mol Physiol. 2019;317(4):L475‐L485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e‐juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ganapathy V, Manyanga J, Brame L, et al. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS One. 2017;12(5):e0177780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lerner CA, Rutagarama P, Ahmad T, Sundar IK, Elder A, Rahman I. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem Biophys Res Commun. 2016;477(4):620‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lee HW, Park SH, Weng M, et al. E‐cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc Natl Acad Sci USA. 2018;115(7):E1560‐E1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Thorne D, Crooks I, Hollings M, Seymour A, Meredith C, Gaca M. The mutagenic assessment of an electronic‐cigarette and reference cigarette smoke using the Ames assay in strains TA98 and TA100. Mutat Res Genet Toxicol Environ Mutagen. 2016;812:29‐38. [DOI] [PubMed] [Google Scholar]
- 143. Tommasi S, Bates SE, Behar RZ, Talbot P, Besaratinia A. Limited mutagenicity of electronic cigarettes in mouse or human cells in vitro. Lung Cancer. 2017;112:41‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Schaal CM, Bora‐Singhal N, Kumar DM, Chellappan SP. Regulation of Sox2 and stemness by nicotine and electronic‐cigarettes in non‐small cell lung cancer. Mol Cancer. 2018;17(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zahedi A, Phandthong R, Chaili A, Remark G, Talbot P. Epithelial‐to‐mesenchymal transition of A549 lung cancer cells exposed to electronic cigarettes. Lung Cancer. 2018;122:224‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Muthumalage T, Lamb T, Friedman MR, Rahman I. E‐cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci Rep. 2019;9(1):19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Aug A, Altraja S, Kilk K, Porosk R, Soomets U, Altraja A. E‐cigarette affects the metabolome of primary normal human bronchial epithelial cells. PLoS One. 2015;10(11):e0142053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Madison MC, Landers CT, Gu BH, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest. 2019;129(10):4290‐4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gellatly S, Pavelka N, Crue T, et al. Nicotine‐free e‐cigarette vapor exposure stimulates IL6 and mucin production in human primary small airway epithelial cells. J Inflamm Res. 2020;13:175‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhang S, Zhang J, Chen H, et al. Combined cytotoxicity of co‐exposure to aldehyde mixtures on human bronchial epithelial BEAS‐2B cells. Environ Pollut. 2019;250:650‐661. [DOI] [PubMed] [Google Scholar]
- 151. Rowell TR, Reeber SL, Lee SL, et al. Flavored e‐cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2017;313(1):L52‐L66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One. 2014;9(9):e108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med. 2016;94(6):667‐679. [DOI] [PubMed] [Google Scholar]
- 154. Corriden R, Moshensky A, Bojanowski CM, et al. E‐cigarette use increases susceptibility to bacterial infection by impairment of human neutrophil chemotaxis, phagocytosis, and NET formation. Am J Physiol Cell Physiol. 2020;318(1):C205‐C214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Chung S, Baumlin N, Dennis JS, et al. Electronic cigarette vapor with nicotine causes airway mucociliary dysfunction preferentially via TRPA1 receptors. Am J Respir Crit Care Med. 2019;200(9):1134‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Glynos C, Bibli SI, Katsaounou P, et al. Comparison of the effects of e‐cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2018;315(5):L662‐L672. [DOI] [PubMed] [Google Scholar]
- 157. Garcia‐Arcos I, Geraghty P, Baumlin N, et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine‐dependent manner. Thorax. 2016;71(12):1119‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Clapp PW, Lavrich KS, van Heusden CA, Lazarowski ER, Carson JL, Jaspers I. Cinnamaldehyde in flavored e‐cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am J Physiol Lung Cell Mol Physiol. 2019;316(3):L470‐L486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti‐bacterial and anti‐viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Crotty Alexander LE, Drummond CA, Hepokoski M, et al. Chronic inhalation of e‐cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol. 2018;314(6):R834‐R847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Tang M, Wu XR, Lee HW, et al. Electronic‐cigarette smoke induces lung adenocarcinoma and bladder urothelial hyperplasia in mice. Proc Natl Acad Sci USA. 2019;116(43):21727‐21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. McAlinden KD, Eapen MS, Lu W, Sharma P, Sohal SS. The rise of electronic nicotine delivery systems and the emergence of electronic‐cigarette‐driven disease. Am J Physiol Lung Cell Mol Physiol. 2020;319(4):L585‐L595. [DOI] [PubMed] [Google Scholar]
- 163. Reidel B, Radicioni G, Clapp PW, et al. E‐cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med. 2018;197(4):492‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Staudt MR, Salit J, Kaner RJ, Hollmann C, Crystal RG. Altered lung biology of healthy never smokers following acute inhalation of E‐cigarettes. Respir Res. 2018;19(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar‐lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Isik Andrikopoulos G, Farsalinos K, Poulas K. Electronic nicotine delivery systems (ENDS) and their relevance in oral health. Toxics. 2019;7(4):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Figueredo CA, Abdelhay N, Figueredo CM, Catunda R, Gibson MP. The impact of vaping on periodontitis: a systematic review. Clin Exp Dent Res. 2021;7(3):376‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Briggs K, Bell C, Breik O. What should every dental health professional know about electronic cigarettes? Aust Dent J. 2021;66(3):224‐233. [DOI] [PubMed] [Google Scholar]
- 169. Couch ET, Chaffee BW, Gansky SA, Walsh MM. The changing tobacco landscape. J Am Dent Assoc. 2016;147(7):561‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Iskandar AR, Zanetti F, Kondylis A, et al. A lower impact of an acute exposure to electronic cigarette aerosols than to cigarette smoke in human organotypic buccal and small airway cultures was demonstrated using systems toxicology assessment. Intern Emerg Med. 2019;14(6):863‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Franco T, Trapasso S, Puzzo L, Allegra E. Electronic cigarette: role in the primary prevention of oral cavity cancer. Clin Med Insights Ear Nose Throat. 2016;9:7‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Grando SA. Connections of nicotine to cancer. Nat Rev Cancer. 2014;14(6):419‐429. [DOI] [PubMed] [Google Scholar]
- 173. Wisniewski DJ, Ma T, Schneider A. Nicotine induces oral dysplastic keratinocyte migration via fatty acid synthase‐dependent epidermal growth factor receptor activation. Exp Cell Res. 2018;370(2):343‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Salturk Z, Çakır Ç, Sünnetçi G, et al. Effects of electronic nicotine delivery system on larynx: experimental study. J Voice. 2015;29(5):560‐563. [DOI] [PubMed] [Google Scholar]
- 175. Ha TAN, Madison MC, Kheradmand F, Altman KW. Laryngeal inflammatory response to smoke and vape in a murine model. Am J Otolaryngol. 2019;40(1):89‐92. [DOI] [PubMed] [Google Scholar]
- 176. Sancilio S, Gallorini M, Cataldi A, di Giacomo V. Cytotoxicity and apoptosis induction by e‐cigarette fluids in human gingival fibroblasts. Clin Oral Investig. 2016;20(3):477‐483. [DOI] [PubMed] [Google Scholar]
- 177. Alanazi H, Park HJ, Chakir J, Semlali A, Rouabhia M. Comparative study of the effects of cigarette smoke and electronic cigarettes on human gingival fibroblast proliferation, migration and apoptosis. Food Chem Toxicol. 2018;118:390‐398. [DOI] [PubMed] [Google Scholar]
- 178. Sundar IK, Javed F, Romanos GE, Rahman I. E‐cigarettes and flavorings induce inflammatory and pro‐senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget. 2016;7(47):77196‐77204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Sancilio S, Gallorini M, Cataldi A, Sancillo L, Rana RA, di Giacomo V. Modifications in human oral fibroblast ultrastructure, collagen production, and lysosomal compartment in response to electronic cigarette fluids. J Periodontol. 2017;88(7):673‐680. [DOI] [PubMed] [Google Scholar]
- 180. Vermehren MF, Wiesmann N, Deschner J, Brieger J, Al‐Nawas B, Kämmerer PW. Comparative analysis of the impact of e‐cigarette vapor and cigarette smoke on human gingival fibroblasts. Toxicol In Vitro. 2020;69:105005. [DOI] [PubMed] [Google Scholar]
- 181. Martinez JD, Easwaran M, Ramirez D, Erickson‐DiRenzo E. Effects of electronic (e)‐cigarette vapor and cigarette smoke in cultured vocal fold fibroblasts. Laryngoscope. 2022;133:139‐146. [DOI] [PubMed] [Google Scholar]
- 182. Lungova V, Wendt K, Thibeault SL. Exposure to e‐cigarette vapor extract induces vocal fold epithelial injury and triggers intense mucosal remodeling. Dis Model Mech. 2022;15(8):dmm049476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yu V, Rahimy M, Korrapati A, et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016;52:58‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Tsai KYF, Hirschi Budge KM, Lepre AP, et al. Cell invasion, RAGE expression, and inflammation in oral squamous cell carcinoma (OSCC) cells exposed to e‐cigarette flavoring. Clin Exp Dent Res. 2020;6(6):618‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Pushalkar S, Paul B, Li Q, et al. Electronic Cigarette Aerosol Modulates the Oral Microbiome and Increases Risk of Infection. iScience. 2020;23(3):100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Tellez CS, Juri DE, Phillips LM, et al. Cytotoxicity and genotoxicity of e‐cigarette generated aerosols containing diverse flavoring products and nicotine in oral epithelial cell lines. Toxicol Sci. 2021;179(2):220‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Duggar M, Swanson H, Hill‐Odom M. Determining the effects of e‐cigarette vapor on oral epithelial cells in a cultured cell model. FASEB J. 2018;32:692.3. [Google Scholar]
- 188. Ji EH, Sun B, Zhao T, et al. Characterization of electronic cigarette aerosol and its induction of oxidative stress response in oral keratinocytes. PLoS One. 2016;11(5):e0154447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ji EH, Elzakra N, Chen W, et al. E‐cigarette aerosols induce unfolded protein response in normal human oral keratinocytes. J Cancer. 2019;10(27):6915‐6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Rouabhia M, Park HJ, Semlali A, Zakrzewski A, Chmielewski W, Chakir J. E‐cigarette vapor induces an apoptotic response in human gingival epithelial cells through the caspase‐3 pathway. J Cell Physiol. 2017;232(6):1539‐1547. [DOI] [PubMed] [Google Scholar]
- 191. Sun YW, Kosinska W, Guttenplan JB. E‐cigarette aerosol condensate enhances metabolism of benzo(a)pyrene to genotoxic products, and induces CYP1A1 and CYP1B1, likely by activation of the aryl hydrocarbon receptor. Int J Environ Res Public Health. 2019;16(14):2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Welz C, Canis M, Schwenk‐Zieger S, et al. Cytotoxic and genotoxic effects of electronic cigarette liquids on human mucosal tissue cultures of the oropharynx. J Environ Pathol Toxicol Oncol. 2016;35(4):343‐354. [DOI] [PubMed] [Google Scholar]
- 193. Song JJ, Go YY, Mun JY, et al. Effect of electronic cigarettes on human middle ear. Int J Pediatr Otorhinolaryngol. 2018;109:67‐71. [DOI] [PubMed] [Google Scholar]
- 194. Song JJ, Go YY, Lee JK, et al. Transcriptomic analysis of tobacco‐flavored e‐cigarette and menthol‐flavored e‐cigarette exposure in the human middle ear. Sci Rep. 2020;10(1):20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Go YY, Mun JY, Chae SW, Chang J, Song JJ. Comparison between in vitro toxicities of tobacco‐ and menthol‐flavored electronic cigarette liquids on human middle ear epithelial cells. Sci Rep. 2020;10(1):2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Rouabhia M, Piché M, Corriveau MN, Chakir J. Effect of e‐cigarettes on nasal epithelial cell growth, Ki67 expression, and pro‐inflammatory cytokine secretion. Am J Otolaryngol. 2020;41(6):102686. [DOI] [PubMed] [Google Scholar]
- 197. Carson JL, Zhou L, Brighton L, et al. Temporal structure/function variation in cultured differentiated human nasal epithelium associated with acute single exposure to tobacco smoke or E‐cigarette vapor. Inhal Toxicol. 2017;29(3):137‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Manyanga J, Ganapathy V, Bouharati C, et al. Electronic cigarette aerosols alter the expression of cisplatin transporters and increase drug resistance in oral cancer cells. Sci Rep. 2021;11(1):1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Tommasi S, Caliri A, Caceres A, et al. Deregulation of biologically significant genes and associated molecular pathways in the oral epithelium of electronic cigarette users. Int J Mol Sci. 2019;20(3):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Alqahtani S, Cooper B, Spears CA, Wright C, Shannahan J. Electronic nicotine delivery system‐induced alterations in oral health via saliva assessment. Exp Biol Med. 2020;245(15):1319‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Hamad SH, Brinkman MC, Tsai YH, et al. Pilot study to detect genes involved in DNA damage and cancer in humans: potential biomarkers of exposure to e‐cigarette aerosols. Genes. 2021;12(3):448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Martin EM, Clapp PW, Rebuli ME, et al. E‐cigarette use results in suppression of immune and inflammatory‐response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2016;311(1):L135‐L144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Rebuli ME, Glista‐Baker E, Hoffman JR, et al. Electronic‐cigarette use alters nasal mucosal immune response to live‐attenuated influenza virus. A clinical trial. Am J Respir Cell Mol Biol. 2021;64(1):126‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Schwarzmeier LÂT, da Cruz BS, Ferreira CCP, et al. E‐cig might cause cell damage of oral mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(4):435‐443. [DOI] [PubMed] [Google Scholar]
- 205. Cichońska D, Kusiak A, Kochańska B, Ochocińska J, Świetlik D. Influence of electronic cigarettes on selected antibacterial properties of saliva. Int J Environ Res Public Health. 2019;16(22):4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Stewart CJ, Auchtung TA, Ajami NJ, et al. Effects of tobacco smoke and electronic cigarette vapor exposure on the oral and gut microbiota in humans: a pilot study. PeerJ. 2018;6:e4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Hua M, Alfi M, Talbot P. Health‐related effects reported by electronic cigarette users in online forums. J Med Internet Res. 2013;15(4):e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Hua M, Sadah S, Hristidis V, Talbot P. Health effects associated with electronic cigarette use: automated mining of online forums. J Med Internet Res. 2020;22(1):e15684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Cho JH. The association between electronic‐cigarette use and self‐reported oral symptoms including cracked or broken teeth and tongue and/or inside‐cheek pain among adolescents: a cross‐sectional study. PLoS One. 2017;12(7):e0180506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. King JL, Reboussin BA, Wiseman KD, et al. Adverse symptoms users attribute to e‐cigarettes: results from a national survey of US adults. Drug Alcohol Depend. 2019;196:9‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kumral TL, Saltürk Z, Yildirim G, et al. How does electronic cigarette smoking affect sinonasal symptoms and nasal mucociliary clearance? B‐ENT. 2016;12(1):17‐21. [PubMed] [Google Scholar]
- 212. Soule EK, Bode KM, Desrosiers AC, Guy M, Breland A, Fagan P. User‐perceived negative respiratory symptoms associated with electronic cigarette use. Nicotine Tob Res. 2020;22(suppl 1):S45‐S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Li Q, Zhan Y, Wang L, Leischow SJ, Zeng DD. Analysis of symptoms and their potential associations with e‐liquids' components: a social media study. BMC Public Health. 2016;16:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Luo J, Chen L, Lu X, Yuan J, Xie Z, Li D. Analysis of potential associations of JUUL flavours with health symptoms based on user‐generated data from Reddit. Tob Control. 2021;30(5):534‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Chen L, Lu X, Yuan J, et al. A social media study on the associations of flavored electronic cigarettes with health symptoms: observational study. J Med Internet Res. 2020;22(6):e17496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Jankowski M, Brożek G, Zejda J, Kurowski T, Knura M, Idzik A. The impact of cigarette and e‐cigarette smoking on human health. Eur Respir J. 2017;50(suppl 61):PA4491. [Google Scholar]
- 217. Walele T, Bush J, Koch A, Savioz R, Martin C, O'Connell G. Evaluation of the safety profile of an electronic vapour product used for two years by smokers in a real‐life setting. Regul Toxicol Pharmacol. 2018;92:226‐238. [DOI] [PubMed] [Google Scholar]
- 218. Mokeem SA, Abduljabbar T, Al‐Kheraif AA, et al. Oral candida carriage among cigarette‐ and waterpipe‐smokers, and electronic cigarette users. Oral Dis. 2019;25(1):319‐326. [DOI] [PubMed] [Google Scholar]
- 219. Frossard S, Volansky P, Endara‐Bravo AS. Acute uvulitis secondary to electronic cigarette use. Am J Respir Crit Care Med. 2015;191:A1556. [Google Scholar]
- 220. Bozzella MJ, Magyar M, DeBiasi RL, Ferrer K. Epiglottitis associated with intermittent e‐cigarette use: the vagaries of vaping toxicity. Pediatrics. 2020;145(3):e20192399. [DOI] [PubMed] [Google Scholar]
- 221. Miler JA, Hajek P. Resolution of recurrent tonsillitis in a non‐smoker who became a vaper. A case study and new hypothesis. Med Hypotheses. 2017;109:17‐18. [DOI] [PubMed] [Google Scholar]
- 222. Huilgol P, Bhatt SP, Biligowda N, Wright NC, Wells JM. Association of e‐cigarette use with oral health: a population‐based cross‐sectional questionnaire study. J Public Health (Bangkok). 2019;41(2):354‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Akinkugbe AA. Cigarettes, e‐cigarettes, and adolescents' oral health: findings from the population assessment of tobacco and health (PATH) study. JDR Clin Transl Res. 2019;4(3):276‐283. [DOI] [PubMed] [Google Scholar]
- 224. Ralho A, Coelho A, Ribeiro M, et al. Effects of electronic cigarettes on oral cavity: a systematic review. J Evid Based Dent Pract. 2019;19(4):101318. [DOI] [PubMed] [Google Scholar]
- 225. Bardellini E, Amadori F, Conti G, Majorana A. Oral mucosal lesions in electronic cigarettes consumers versus former smokers. Acta Odontol Scand. 2018;76(3):226‐228. [DOI] [PubMed] [Google Scholar]
- 226. Bartram A, Jones N, Endersby S. Lichenoid eruption associated with use of an e‐cigarette. Br J Oral Maxillofac Surg. 2016;54(4):475. [DOI] [PubMed] [Google Scholar]
- 227. Farinha H, Martins V. Lingua villosa nigra associated with the use of electronic cigarette. Acta Med Port. 2015;28(3):393. [DOI] [PubMed] [Google Scholar]
- 228. Tsiouma O, Andreou A, Sklavounou‐Andrikopoulou A. E‐cigarette and palatal ulcer: a possible relationship? Oral Surg. 2021;14(1):59‐64. [Google Scholar]
- 229. Cant A, Collard B, Cunliffe D. Electronic cigarettes: necrotic ulcer. Br Dent J. 2017;222(4):226. [DOI] [PubMed] [Google Scholar]
- 230. Nguyen H, Kitzmiller JP, Nguyen KT, Nguyen CD, Chi Bui T. Oral carcinoma associated with chronic use of electronic cigarettes. Otolaryngology. 2017;7(2):304. [Google Scholar]
- 231. Klawinski D, Hanna I, Breslin NK, Katzenstein HM, Indelicato DJ. Vaping the venom: oral cavity cancer in a young adult with extensive electronic cigarette use. Pediatrics. 2021;147(5):e2020022301. [DOI] [PubMed] [Google Scholar]
- 232. Jones CD, Ho W, Gunn E, Widdowson D, Bahia H. E‐cigarette burn injuries: comprehensive review and management guidelines proposal. Burns. 2019;45(4):763‐771. [DOI] [PubMed] [Google Scholar]
- 233. Andresen NS, Lee DJ, Kowalski CE, Bayon R. Fall with e‐cigarette in mouth resulting in pharyngeal and esophageal burns. JAMA Otolaryngol Head Neck Surg. 2018;144(4):385‐386. [DOI] [PubMed] [Google Scholar]
- 234. Seo AD, Kim DC, Yu HJ, Kang MJ. Accidental ingestion of e‐cigarette liquid nicotine in a 15‐month‐old child: an infant mortality case of nicotine intoxication. Korean J Pediatr. 2016;59(12):490‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Gill N, Sangha G, Poonai N, Lim R. E‐cigarette liquid nicotine ingestion in a child: case report and discussion. CJEM. 2015;17(6):699‐703. [DOI] [PubMed] [Google Scholar]
- 236. Hughes A, Hendrickson RG. An epidemiologic and clinical description of e‐cigarette toxicity. Clin Toxicol. 2019;57(4):287‐293. [DOI] [PubMed] [Google Scholar]
- 237. Richmond SA, Pike I, Maguire JL, Macpherson A. E‐cigarettes: a new hazard for children and adolescents. Paediatr Child Health. 2018;23(4):255‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Tuhanioğlu B, Erkan SO, Özdaş T, Derici Ç, Tüzün K, Şenkal ÖA. The effect of electronic cigarettes on voice quality. J Voice. 2019;33(5):811.e13‐811.e17. [DOI] [PubMed] [Google Scholar]
- 239. Sample HG. The effect of electronic nicotine delivery systems on the vocal folds. Master's thesis. Cleveland State University; 2019. Accessed September 2, 2021. https://engagedscholarship.csuohio.edu/cgi/viewcontent.cgi?article=2148%26context=etdarchive [Google Scholar]
- 240. Patel S, Wooles N, Martin T. A systematic review of the impact of cigarettes and electronic cigarettes in otology. J Laryngol Otol. 2020;134:951‐956. [DOI] [PubMed] [Google Scholar]
- 241. Demir E, Topal S. Sudden sensorineural hearing loss associated with electronic cigarette liquid: the first case in the literature. Int J Pediatr Otorhinolaryngol. 2018;114:26‐28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1.
Supporting information.
Supporting information.


