Abstract
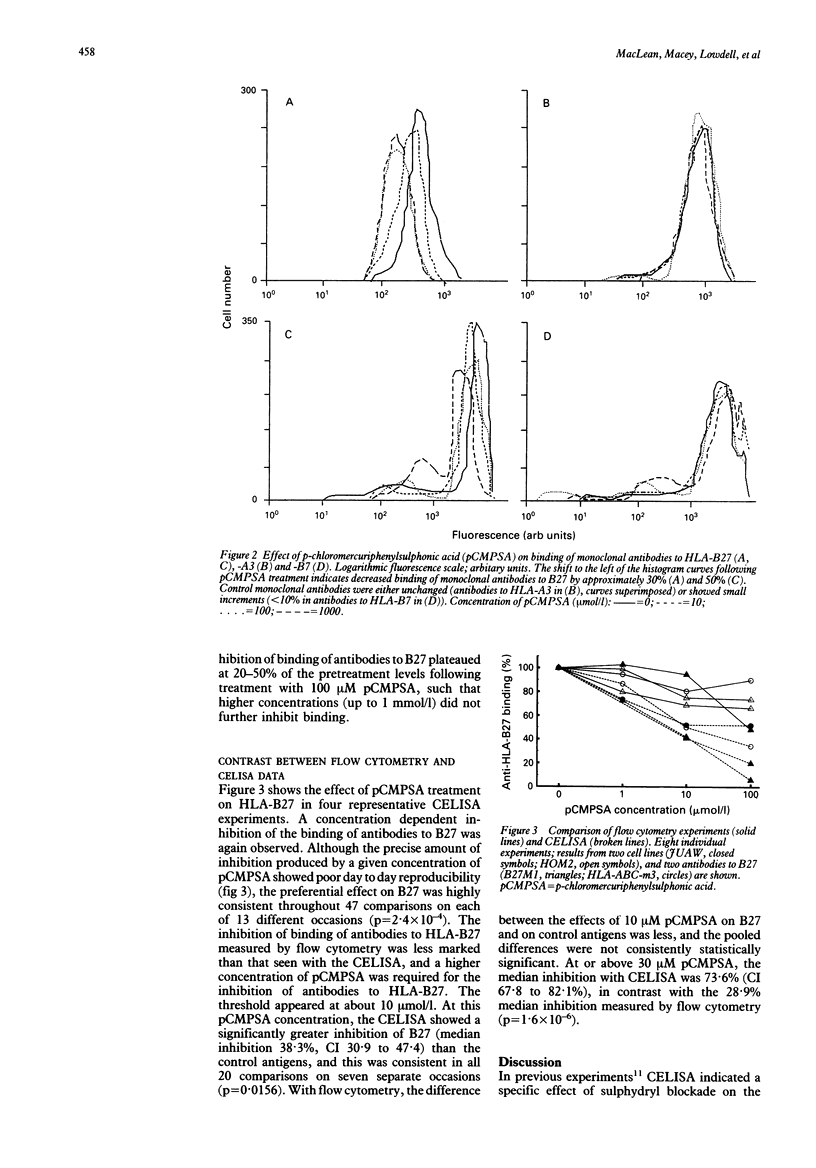
HLA-B27 has an unpaired cysteine on or near its serologically defined spondylitis associated epitope, and it has been argued that its sulphydryl side chain may be chemically reactive. In a previous study it was shown that chemical treatment of HLA-B27 cells with the sulphydryl binding agent p-chloromercuriphenylsulphonic acid (pCMPSA) specifically reduced binding of antibodies to HLA-B27 by up to 80%, as measured in a cellular enzyme linked immunosorbent assay (CELISA). The effect of sulphydryl blockade on intact B27 cells was investigated using flow cytometry. Compared with the CELISA, inhibition required higher concentrations of pCMPSA, and the degree of inhibition produced by a greater than or equal to 30 microM solution of pCMPSA as measured by flow cytometry (median 28.9%) was significantly lower than that measured by CELISA (median 73.6%; p = 1.6 x 10(-6)). Analysis of unfixed, cell surface HLA-B27 by flow cytometry suggests that on most B27 molecules the unpaired sulphydryl site is not available. On the basis of this evidence for modification after translation, a new 'altered self' hypothesis is proposed for the part which HLA-B27 plays in inflammatory disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer J. R., Winrow V. R. HLA-B27 and the causes of arthritis: does molecular biology help? Ann Rheum Dis. 1987 Sep;46(9):713–715. doi: 10.1136/ard.46.9.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Benjamin R., Parham P. Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol Today. 1990 Apr;11(4):137–142. doi: 10.1016/0167-5699(90)90051-a. [DOI] [PubMed] [Google Scholar]
- Berger A. E., Davis J. E., Cresswell P. Monoclonal antibody to HLA-A3. Hybridoma. 1982;1(2):87–90. doi: 10.1089/hyb.1.1982.1.87. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Breur-Vriesendorp B. S., Dekker-Saeys A. J., Ivanyi P. Distribution of HLA-B27 subtypes in patients with ankylosing spondylitis: the disease is associated with a common determinant of the various B27 molecules. Ann Rheum Dis. 1987 May;46(5):353–356. doi: 10.1136/ard.46.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewerton D. A., Hart F. D., Nicholls A., Caffrey M., James D. C., Sturrock R. D. Ankylosing spondylitis and HL-A 27. Lancet. 1973 Apr 28;1(7809):904–907. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- Brown M. J., Allison D. J., Jenner D. A., Lewis P. J., Dollery C. T. Increased sensitivity and accuracy of phaeochromocytoma diagnosis achieved by use of plasma-adrenaline estimations and a pentolinium-suppression test. Lancet. 1981 Jan 24;1(8213):174–177. doi: 10.1016/s0140-6736(81)90058-1. [DOI] [PubMed] [Google Scholar]
- Chen B. P., Parham P. Direct binding of influenza peptides to class I HLA molecules. Nature. 1989 Feb 23;337(6209):743–745. doi: 10.1038/337743a0. [DOI] [PubMed] [Google Scholar]
- Coppin H. L., McDevitt H. O. Absence of polymorphism between HLA-B27 genomic exon sequences isolated from normal donors and ankylosing spondylitis patients. J Immunol. 1986 Oct 1;137(7):2168–2172. [PubMed] [Google Scholar]
- Karr R. W., Hahn Y., Schwartz B. D. Structural identity of human histocompatibility leukocyte antigen-B27 molecules from patients with ankylosing spondylitis and normal individuals. J Clin Invest. 1982 Feb;69(2):443–450. doi: 10.1172/JCI110468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean L., Perrett D., Winrow V. R., Archer J. R. Status of an unpaired thiol group on the HLA-B27 epitope. Clin Exp Immunol. 1989 Sep;77(3):417–421. [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Parham P., Rust N., Brodsky F. A monoclonal antibody that recognizes an antigenic determinant shared by HLA A2 and B17. Hum Immunol. 1980 Sep;1(2):121–129. doi: 10.1016/0198-8859(80)90099-3. [DOI] [PubMed] [Google Scholar]
- Parham P., Bodmer W. F. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature. 1978 Nov 23;276(5686):397–399. doi: 10.1038/276397a0. [DOI] [PubMed] [Google Scholar]
- Parham P., Lomen C. E., Lawlor D. A., Ways J. P., Holmes N., Coppin H. L., Salter R. D., Wan A. M., Ennis P. D. Nature of polymorphism in HLA-A, -B, and -C molecules. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4005–4009. doi: 10.1073/pnas.85.11.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurog J. D. Immunology, genetics, and animal models of the spondyloarthropathies. Curr Opin Rheumatol. 1990 Aug;2(4):586–591. doi: 10.1097/00002281-199002040-00006. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Trapani J. A., Vaughan H. A., Sparrow R. L., Tait B. D., McKenzie I. F. Description of a mouse monoclonal anti-HLA-B27 antibody HLA-ABC-m3. Hum Immunol. 1983 Aug;7(4):205–216. doi: 10.1016/0198-8859(83)90058-7. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., Berlin R. D. Effect of phagocytosis on membrane transport of nonelectrolytes. J Exp Med. 1971 Oct 1;134(4):1016–1035. doi: 10.1084/jem.134.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]