Abstract
Simple Summary
Hypercoagulability has been demonstrated to have a strong association with cancer. It may result in sterile thrombotic cardiac vegetations known as “non-bacterial thrombotic” or “marantic” endocarditis. While cancer-associated NBTE is a rare entity, lung and pancreatic cancers are the most common tumor sites. Adenocarcinoma is the most common pathology, and embolization is the most common presentation. Survival is better in cases that undergo surgery and in more recent years. To prevent the potentially devastating complication of marantic endocarditis, we recommend conducting more aggressive echocardiography screening for patients with solid tumors, particularly those with adenocarcinoma of the lung and pancreas. This is based on the finding that 88.6% of patients with marantic endocarditis in this meta-analysis initially presented with embolic cerebrovascular events.
Abstract
Hypercoagulability is strongly associated with cancer and may result in non-bacterial thrombotic endocarditis (NBTE). The aim of our meta-analysis was to explore the demographics and characteristics of this condition in cancer. Databases were systematically searched. The outcomes were to identify the annual trend in premortem diagnosis among the entire cohort and different subgroups and to identify differences in characteristics and survival in the considered population. A total of 121 studies with 144 patients were included. The proportion of marantic endocarditis associated with lung cancer was 0.29 (95% CI, 0.21–0.37; p < 0.001), that associated with pancreatic cancer was 0.19 (95% CI, 0.13–0.27; p < 0.001), that associated with advanced cancer stage (metastasis) was 0.69 (95% CI, 0.61–0.76; p < 0.001), and that associated with adenocarcinoma was 0.65 (95% CI, 0.56–0.72; p < 0.001). Median and 6-month overall survival (OS) were 1.3 months and 32.3%, respectively, with 6-month OS of 20.8% vs. 37.0% in lung vs. other cancers, respectively (p = 0.06) and 42.9% vs. 31.1% among those who underwent intervention vs. those who did not (p = 0.07). Cases discovered in recent years had better survival (HR = 0.98 (95% CI, 0.96–0.99; p = 0.003). While cancer-associated NBTE is a rare entity, lung cancers were the most common tumor site and are frequently associated with more advanced and metastatic cancer stages. The prognosis is dismal, especially among lung cancers.
Keywords: marantic endocarditis, anticoagulation, overall survival, meta-analysis, lung cancer
1. Introduction
Hypercoagulability has been demonstrated to be associated with cancer. There is unlikely a single mechanism but rather a diverse set of pathophysiologic mechanisms. These include leukocytosis and thrombocytosis, the expression of tissue factor and/or phospholipid, and enhanced expression of circulating inflammatory markers. Besides patient- and treatment-related factors such as immobility, hospitalization, central venous catheters, surgery, radiation therapy, and vascular toxicity of chemotherapeutic agents may play a role [1,2]. In 1860, Armand Trousseau described the association between thrombotic events and cancer diagnosis, a phenomenon that became known as the “Trousseau sign” [3]. One of the consequences of this hypercoagulable status is the development of sterile thrombotic friable cardiac vegetations, which were first described by Zeigler R et al. in 1888 in cadavers [4] and later termed “non-bacterial thrombotic endocarditis” (NBTE) [5] or “marantic endocarditis” [6] and considered a paraneoplastic phenomenon. It is a rare entity, with an overall incidence estimated at 1.3% based on 82,676 autopsies [7]. Another study also demonstrated a low incidence between 0.9 and 1.6% in adult autopsy populations among all cases of endocarditis [8]. However, the exact prevalence of marantic endocarditis is not well established, as it is often clinically silent or an incidental finding, with most cases diagnosed postmortem [9,10,11]. Autopsy studies suggest that 75% of cases are cancer-associated, and the remainder is associated with non-cancer etiologies that are related to hypercoagulable states such as systemic lupus erythematosus [7,12]. In more recent studies, 19% of patients with solid tumors who were free from any cardiac-related symptoms were found to have sterile cardiac vegetations upon transthoracic echocardiography (TTE) screening [13]. Due to recent advances in cardiac imaging techniques, there have been marked improvements in the diagnosis and management of NBTE. One of the most important developments has been the use of transesophageal echocardiography (TEE) [9,14,15]. Furthermore, cardiac magnetic resonance imaging (MRI) was found to be very accurate in detecting clots on the heart valves, and it may be particularly useful in cases in which TEE cannot be performed or is inconclusive and to differentiate between infective and non-infective endocarditis [16]. Due to these advances, there has been an increase in the rate of the premortem diagnosis of non-bacterial thrombotic endocarditis in cancer, in addition to the monitoring of the effectiveness of treatment [17]. The first case diagnosed premortem was reported in 1976 [18]. To date, all cancer-associated NBTEs have been reported as case reports or case series; notably, there has been no systematic review of the demographics and characteristics of this condition in cancer, apart from the literature review performed by Patel et al., which included only premortem cancer and non-cancer-associated cases [19]. Addressing this knowledge gap was our aim in this systematic review and meta-analysis, which included all reported pre- and postmortem case reports or series of cancer-associated NBTE.
2. Materials and Methods
2.1. Search Strategy and Study Selection
The protocol for this review was registered with the PROSPERO registry of systematic reviews (registration number: CRD42022331513). This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20].
In April 2022, a medical librarian performed comprehensive searches to identify studies that included all post- and premortem case reports and series of cancer-associated non-bacterial thrombotic endocarditis (NBTE) reported in the literature.
Searches were performed on the following databases: Ovid MEDLINE (ALL—1946 to present), Ovid EMBASE (1974 to present), and The Cochrane Library (Wiley). The search strategy included all appropriate controlled vocabulary and keywords for the concepts of “marantic” and “NBTE” (the full search strategy is reported in Supplementary Table S1). To limit publication bias, there were no publication date or article type restrictions on the search strategy. The reference lists of all included studies were searched to identify further articles that could potentially be recruited (i.e., backward snowballing).
Retrieved studies were screened for inclusion. Titles and abstracts were reviewed against predefined inclusion/exclusion criteria by 2 independent reviewers. Discrepancies were resolved by consensus. For final inclusion, the full text was retrieved and screened by 2 independent reviewers (A.D. and I.E.).
Articles considered for inclusion were all case reports and case series of cancer-associated non-bacterial thrombotic endocarditis “marantic” diagnosed either premortem or postmortem that included full patient data. Noncancer NBTE studies were excluded. The PRISMA flow chart is shown in Supplementary Figure S1.
2.2. Data Extraction and Quality Assessment
Two investigators (A.D. and I.E.) performed data extraction independently. All the following variables were obtained: the presence of antiphospholipid antibodies, primary cancer organ, pathology, organ of metastasis (grouped as bone, brain, liver, lung, lymph nodes, multiple, or others), marantic presentation before the diagnosis of cancer (grouped as known cancer case or marantic as a first presentation), interval diagnosis (defined as the time between diagnosis of cancer and diagnosis of NBTE), age, chronic obstructive pulmonary disease (COPD), smoking, diabetes, obesity, dyslipidemia, hypertension, embolization event at presentation, embolic event after diagnosis, postmortem diagnosis of NBTE, position of vegetation, valve insufficiency, valve stenosis, intervention, type of surgery, presenting symptoms, and survival status and time.
In case of missing data, we reported the percentage of available data only; however, for missing survival time data, we used conditional imputation (median of alive or dead cases).
The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Reports was used for the quality assessment of included papers [21]. Studies were assessed using an eight-question checklist about clear description of (1) patient demographic characteristics, (2) patient history and timeline, (3) current clinical condition of the patient upon presentation, (4) diagnostic tests or assessment methods and results, (5) intervention(s) or treatment procedure(s), (6) post-intervention clinical condition, (7) adverse events (harms) or unanticipated events, and (8) takeaway lessons; each question was answered by either yes, no, unclear, or not applicable (Supplementary Table S2).
2.3. Outcomes of Interest
The primary outcome was to identify the annual trend in premortem diagnosis among the entire cohort.
The secondary outcomes were to identify the annual trend in premortem diagnosis among different subgroups (lung, pancreas, other gastrointestinal cancers, gynecological tumors, and others); identify differences in characteristics among (A) females vs. males, (B) lung vs. others, and (C) marantic first presentation vs. known cancer; estimate the survival difference among (A) females vs. males, (B) lung vs. others, (C) marantic first presentation vs. known cancer, (D) intervention vs. no intervention, and (E) metastatic vs. non-metastatic cases; and identify predictors of late mortality.
2.4. Follow-Up and Survival Analysis
Overall survival was defined as the time from diagnosis of marantic endocarditis until death from any cause. The reversed Kaplan–Meier method was used to calculate the median follow-up time.
2.5. Statistical Analysis
Continuous data were presented as median and interquartile range and compared using the Mann–Whitney U test or as mean and standard deviation and compared using a t-test after testing for normality. Categorical data were presented as frequency count and percentages and compared across groups using chi-square or Fisher’s test, as appropriate.
A one-sample proportions test was used to identify the probability of marantic endocarditis being associated with certain categories of certain variables, e.g., adenocarcinoma among histopathology variables.
Conditional imputation was used for cases with missed follow-up time and known follow-up status.
Cox regression was used to identify the predictors of late mortality and reported as hazard ratios (HRs) and their 95% confidence intervals (95% CIs). Variables were selected for multivariate analysis based on their statistical and clinical significance.
Overall survival was estimated using Kaplan–Meier methods and compared among groups using a log-rank test.
A staked bar plot with overlying linear regression was used to assess the annual trend of premortem diagnosis and the annual trend of cases that underwent intervention.
Data were analyzed using R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) within RStudio. Tableone, Survival, and Survminer packages were used.
3. Results
Among 829 searched studies, 121 studies [19,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141] (published from 1962 to 2021) with 144 patients met our inclusion criteria (Table 1). The flow chart of our included studies is shown in Supplementary Figure S1. Around 25% (39) of included studies were from the USA, and only 1 study was reported from China [19,22,23,24,27,29,32,33,34,39,41,43,46,47,48,57,58,59,68,69,73,76,81,84,86,88,92,93,94,100,101,105,108,111,114,115,118,131,132,136]. A list of the included studies and the quality assessment are reported in Supplementary Table S2.
Table 1.
Criteria of included patients.
| Level | Overall | |
|---|---|---|
| n | 144 | |
| Antiphospholipid antibodies (%) | No | 141 (97.9) |
| Yes | 3 (2.1) | |
| Primary cancer organ (%) | Gynecological | 23 (16.0) |
| Lung | 41 (28.5) | |
| Other GIT cancers | 19 (13.2) | |
| Others | 33 (22.9) | |
| Pancreas | 28 (19.4) | |
| Lung vs. other cancers (%) | Others | 103 (71.5) |
| Lung cancer | 41 (28.5) | |
| Pathology (%) | Adenocarcinoma | 93 (64.6) |
| Hematopoietic | 6 (4.2) | |
| Others | 38 (26.4) | |
| Sarcoma | 1 (0.7) | |
| SCC | 6 (4.2) | |
| Adenocarcinoma vs. other pathologies (%) | Other pathologies | 51 (35.4) |
| Adenocarcinoma | 93 (64.6) | |
| Organ of metastasis (n = 97) (%) | Bone | 5 (5.2) |
| Brain | 3 (3.1) | |
| Liver | 23 (23.7) | |
| Lung | 7 (7.2) | |
| Lymph nodes | 22 (22.7) | |
| Multiple | 25 (25.8) | |
| Others | 12 (12.4) | |
| Metastasis (n = 141) (%) | Non-metastatic | 43 (30.7) |
| Metastatic | 97 (69.3) | |
| Marantic presentation before diagnosis of cancer (n = 125) (%) | Known cancer case | 42 (33.6) |
| Marantic as first presentation | 83 (66.4) | |
| Interval diagnosis (months) (mean (SD) | 6.24 (25.51) | |
| Age (median (IQR)) | 60.00 (49.75, 66.00) | |
| Sex (%) | Females | 81 (56.25) |
| Males | 63 (43.75) | |
| COPD (n = 52) (%) | No | 49 (94.2) |
| Yes | 3 (5.8) | |
| Smoking (n = 56) (%) | No | 43 (76.8) |
| Yes | 13 (23.2) | |
| Diabetes (n = 52) (%) | No | 43 (82.7) |
| Yes | 9 (17.3) | |
| Obesity (n = 53) (%) | No | 52 (98.1) |
| Yes | 1 (1.9) | |
| Dyslipidemia (n = 54) (%) | No | 46 (85.2) |
| Yes | 8 (14.8) | |
| Hypertension (n = 56) (%) | No | 36 (64.3) |
| Yes | 20 (35.7) | |
| Embolization event at presentation (n = 132) (%) | No | 15 (11.4) |
| Yes | 117 (88.6) | |
| Embolic event after diagnosis (n = 125) (%) | No | 76 (60.8) |
| Yes | 49 (39.2) | |
| Incidental finding of NBTE (%) | No | 132 (91.7) |
| Yes | 12 (8.3) | |
| Postmortem diagnosis of NBTE (%) | No | 95 (66.0) |
| Yes | 49 (34.0) | |
| Position of vegetation (n = 132) (%) | Aortic | 48 (36.4) |
| Left atrium | 1 (0.8) | |
| Mitral | 62 (47.0) | |
| Multiple | 21 (15.9) | |
| Valve insufficiency (n = 89) (%) | No | 28 (31.5) |
| Yes | 61 (68.5) | |
| Valve stenosis (n = 90) (%) | No | 81 (90.0) |
| Yes | 9 (10.0) | |
| Underwent intervention (%) | No | 123 (85.4) |
| Yes | 21 (14.6) | |
| Type of surgery (%) | Excision of vegetation | 5 (3.5) |
| None | 123 (85.4) | |
| Replacement | 16 (11.1) | |
| Presenting symptoms (n = 139) (%) | Cardiological | 6 (4.5) |
| Neurological | 96 (72.7) | |
| Others | 7 (5.3) | |
| Respiratory | 13 (9.8) | |
| Vascular | 10 (7.6) | |
| Death (%) | No | 47 (32.6) |
| Yes | 97 (67.4) | |
| Time to death (days) (median (IQR)) | 15.40 (2.73, 56.00) |
According to a one-proportion test, the proportion of marantic endocarditis associated with lung cancer was 0.29 (95% CI, 0.21–0.37; p < 0.001), pancreatic cancer was 0.19 (95% CI, 0.13–0.27; p < 0.001), advanced stage (metastatic tumor) was 0.69 (95% CI, 0.61–0.76; p < 0.001), isolated liver metastasis was 0.23 (95% CI, 0.16–0.33; p ≤ 0.001), and adenocarcinoma was 0.65 (95% CI, 0.56–0.72; p < 0.001).
Differences in characteristics among lung vs. other cancers are reported in Table 2.
Table 2.
Different criteria among lung vs. others.
| Level | Non-Lung Cancers | Lung Cancer | p | |
|---|---|---|---|---|
| n | 103 | 41 | ||
| Antiphospholipid antibodies (%) | No | 100 (97.1) | 41 (100.0) | 0.647 |
| Yes | 3 (2.9) | 0 (0.0) | ||
| Primary cancer organ (%) | Gynecological | 23 (22.3) | 0 (0.0) | <0.001 |
| Lung | 0 (0.0) | 41 (100.0) | ||
| Other GIT cancers | 19 (18.4) | 0 (0.0) | ||
| Others | 33 (32.0) | 0 (0.0) | ||
| Pancreas | 28 (27.2) | 0 (0.0) | ||
| Pathology (%) | Adenocarcinoma | 60 (58.3) | 33 (80.5) | 0.004 |
| Hematopoietic | 6 (5.8) | 0 (0.0) | ||
| Others | 34 (33.0) | 4 (9.8) | ||
| Sarcoma | 1 (1.0) | 0 (0.0) | ||
| SCC | 2 (1.9) | 4 (9.8) | ||
| Adenocarcinoma vs. other pathologies (%) | Other pathologies | 43 (41.7) | 8 (19.5) | 0.02 |
| Adenocarcinoma | 60 (58.3) | 33 (80.5) | ||
| Organ of metastasis (n = 97) (%) | Bone | 3 (4.8) | 2 (5.9) | 0.258 |
| Brain | 2 (3.2) | 1 (2.9) | ||
| Liver | 20 (31.7) | 3 (8.8) | ||
| Lung | 5 (7.9) | 2 (5.9) | ||
| Lymph nodes | 11 (17.5) | 11 (32.4) | ||
| Multiple | 15 (23.8) | 10 (29.4) | ||
| Others | 7 (11.1) | 5 (14.7) | ||
| Metastasis (n = 141) (%) | Non-metastatic | 38 (37.6) | 5 (12.8) | 0.008 |
| Metastatic | 63 (62.4) | 34 (87.2) | ||
| Marantic presentation before diagnosis of cancer (n = 125) (%) | Known cancer case | 34 (36.2) | 8 (25.8) | 0.401 |
| Marantic as a first presentation | 60 (63.8) | 23 (74.2) | ||
| Interval diagnosis (months) (mean (SD)) | 7.95 (29.56) | 1.54 (3.81) | 0.257 | |
| Age (median (IQR)) | 59.00 (49.00, 65.00) | 61.00 (55.00, 67.00) | 0.389 | |
| Sex (%) | F | 63 (61.2) | 18 (45.0) | 0.118 |
| M | 40 (38.8) | 22 (55.0) | ||
| COPD (n = 52) (%) | No | 39 (97.5) | 10 (83.3) | 0.254 |
| Yes | 1 (2.5) | 2 (16.7) | ||
| Smoke (n = 56) (%) | No | 37 (92.5) | 6 (37.5) | <0.001 |
| Yes | 3 (7.5) | 10 (62.5) | ||
| Diabetes (n = 52) (%) | No | 31 (77.5) | 12 (100.0) | 0.17 |
| Yes | 9 (22.5) | 0 (0.0) | ||
| Obesity (n = 53) (%) | No | 40 (97.6) | 12 (100.0) | 1 |
| Yes | 1 (2.4) | 0 (0.0) | ||
| Dyslipidemia (n = 54) (%) | No | 35 (85.4) | 11 (84.6) | 1 |
| Yes | 6 (14.6) | 2 (15.4) | ||
| Hypertension (n = 56) (%) | No | 27 (64.3) | 9 (64.3) | 1 |
| Yes | 15 (35.7) | 5 (35.7) | ||
| Embolization event at presentation (n = 132) (%) | No | 12 (12.4) | 3 (8.6) | 0.767 |
| Yes | 85 (87.6) | 32 (91.4) | ||
| Embolic event after diagnosis (n = 125) (%) | No | 55 (59.1) | 21 (65.6) | 0.661 |
| Yes | 38 (40.9) | 11 (34.4) | ||
| Incidental finding of NBTE (%) | No | 95 (92.2) | 37 (90.2) | 0.956 |
| Yes | 8 (7.8) | 4 (9.8) | ||
| Postmortem diagnosis of NBTE (%) | No | 74 (71.8) | 21 (51.2) | 0.031 |
| Yes | 29 (28.2) | 20 (48.8) | ||
| Position of vegetation (n = 132) (%) | Aortic | 29 (31.2) | 19 (48.7) | 0.008 |
| Left atrium | 1 (1.1) | 0 (0.0) | ||
| Mitral | 42 (45.2) | 20 (51.3) | ||
| Multiple | 21 (22.6) | 0 (0.0) | ||
| Valve insufficiency (n = 89) (%) | No | 23 (35.4) | 5 (20.8) | 0.292 |
| yes | 42 (64.6) | 19 (79.2) | ||
| Valve stenosis (n = 90) (%) | No | 57 (86.4) | 24 (100.0) | 0.131 |
| Yes | 9 (13.6) | 0 (0.0) | ||
| Underwent intervention (%) | No | 84 (81.6) | 39 (95.1) | 0.069 |
| Yes | 19 (18.4) | 2 (4.9) | ||
| Type of surgery (%) | Excision of vegetation | 4 (3.9) | 1 (2.4) | 0.096 |
| None | 84 (81.6) | 39 (95.1) | ||
| Replacement | 15 (14.6) | 1 (2.4) | ||
| Presenting symptoms (n = 139) (%) | Cardiological | 4 (4.2) | 2 (5.6) | 0.467 |
| Neurological | 68 (70.8) | 28 (77.8) | ||
| Others | 4 (4.2) | 3 (8.3) | ||
| Respiratory | 11 (11.5) | 2 (5.6) | ||
| Vascular | 9 (9.4) | 1 (2.8) | ||
| Death (144) (%) | No | 37 (36.3) | 8 (20.0) | 0.094 |
| Yes | 65 (63.7) | 32 (80.0) | ||
| Time to death (days) (median (IQR)) | 19.60 (1.05, 56.00) | 10.99 (3.50, 35.28) | 0.64 |
Differences in characteristics among (A) females vs. males and (B) marantic first presentation vs. known cancer are reported in Supplementary Table S3.
Around one-third (39) of the included studies was from the United States; the other third was from Japan (18), the United Kingdom (12), and Australia (8); while the last third was from the remaining 26 countries, with fewer than 4 studies each. Thus, two-thirds of the studies come from just 4 of the 30 different countries that published case reports (Supplementary Figure S3).
3.1. Trend Analysis
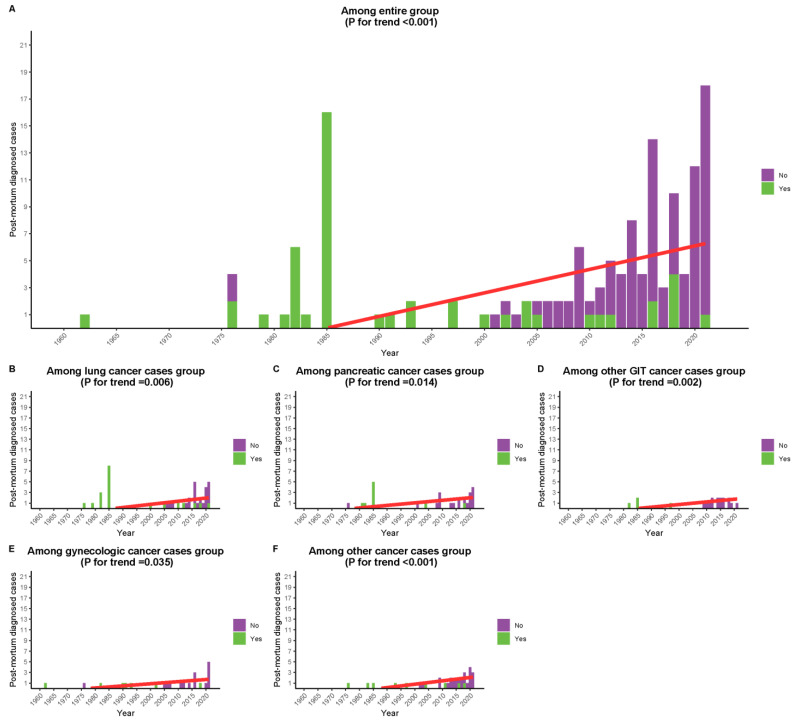
There was a decrease in postmortem diagnosis with a corresponding increase in premortem diagnosis in the recent era compared to the previous era among the (A) entire group (p < 0.001), (B) lung cancer group (p = 0.006), (C) pancreatic cancer group (p = 0.014), (D) other gastrointestinal tract (GIT) cancers (p = 0.002), (E) gynecologic cancer (0.035), and (F) other cancers (p < 0.001) (Figure 1A–F).
Figure 1.
Trend of premortem diagnosis of marantic cases over the included study years among (A) the entire group (p < 0.001), (B) lung cancer group (p = 0.006), (C) pancreatic cancer group (p = 0.014), (D) other GIT cancers (p = 0.002), (E) gynecologic cancer (0.035), and (F) other cancers (p < 0.001).
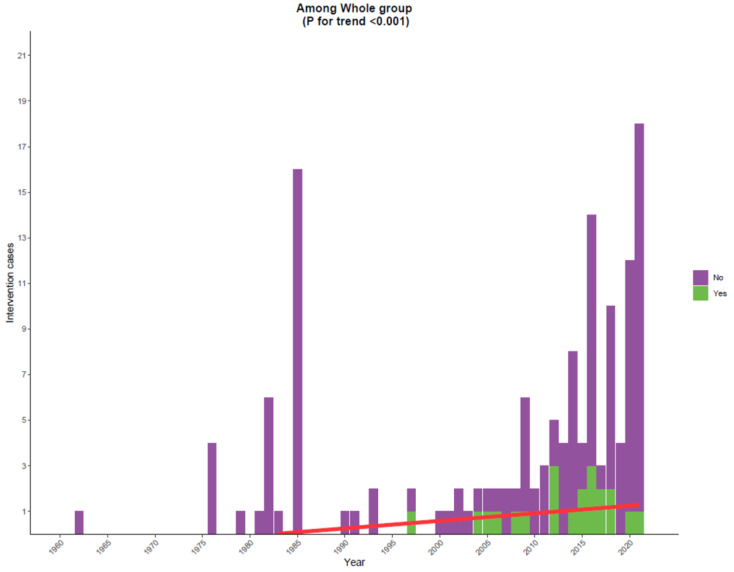
Similarly, while the rate of intervention is still low (21/144, 14.6%), there was a progressive increase over the years (p < 0.001) (Figure 2).
Figure 2.
Stacked bar plot showing the number of cases that underwent intervention; while the rate of intervention is still low (21/144), there has been a progressive increase over the years.
3.2. Survival Analysis
Median follow-up time among the entire group was 5 months (95% CI: 5–8). The maximum follow-up time was 27 months. One-third of patients were alive at the end of the follow-up period.
The 1-, 3-, and 6-month overall survival among the entire group were 74.3% (95% CI: 67.4–82), 39.4% (95% CI: 31.9–48.6), and 32.3% (95% CI: 25–41.7), respectively. Median OS was 1.3 months (95% CI: 1.2–2.3). There was a trend toward poor OS among lung cancer vs. other cancers, with 1-, 3-, and 6-month overall survival of 70.1% (95% CI: 57.3–85.9), 26.0% (95% CI: 15.3–44.2), and 20.8% (95% CI: 11.2–38.4), respectively, in lung cancer vs. 76.0% (95% CI: 68.1–84.9), 44.9% (95% CI: 36–56.2), and 37.0% (95% CI: 28.0–48.9), respectively, in other cancers (log-rank p = 0.06). There was a trend toward better OS among those who underwent intervention vs. those who did not undergo intervention, with 1-, 3-, and 6-month overall survival of 85.7% (95% CI: 72.0–100.0), 57.1% (95% CI: 38.4–85.1), and 42.9% (95% CI: 24.1–76.1) vs. 72.6% (95% CI: 65.0–81.0), 36.7% (95% CI: 28.9–46.6), and 31.1% (95% CI: 23.6–40.9) respectively (log-rank p value = 0.07). There was no significant difference in OS among (A) females vs. males, (B) marantic first presentation vs. known cancer, and (C) metastatic vs. non-metastatic cases (Supplementary Figure S2).
Cox multivariate analysis revealed that cases discovered in recent years were associated with better survival (HR = 0.98 (95% CI: 0.96–0.99; p = 0.003)), and there was a trend toward worse survival among elderly patients (HR = 1.02 (95% CI: 1.00–1.04; p = 0.061)) (Table 3).
Table 3.
Predictors of late mortality using Cox regression analysis.
| Variables | Hazard Ratio, 95% CI, p Value |
|---|---|
| Age | 1.021 [0.999; 1.043], 0.06157 |
| Year | 0.975 [0.959; 0.991], 0.00289 |
| Underwent intervention | 1.235 [0.472; 3.229], 0.66705 |
| Pathology (adenocarcinoma vs. others) | 0.994 [0.560; 1.764], 0.98430 |
| Cancer organ (ref: lung) | |
|
0.928 [0.477; 1.806], 0.82582 |
|
0.555 [0.210; 1.463], 0.23369 |
|
0.578 [0.180; 1.855], 0.35703 |
|
0.724 [0.338; 1.548], 0.40459 |
| Organ affected by metastasis (liver vs. others) | 1.235 [0.644; 2.368], 0.52555 |
4. Discussion
Marantic endocarditis is a type of non-bacterial endocarditis, which is an inflammation of the inner lining of the heart (endocardium) that is not due to bacterial infection. It is also called “noninfective endocarditis” or “aseptic endocarditis“. This condition is most commonly associated with cancer, particularly malignant melanoma, lung, pancreas, and breast carcinomas [17,27,57,142,143]. Marantic endocarditis can also occur in patients with other types of malignancies, as well as in patients with chronic inflammatory conditions such as rheumatoid arthritis and systemic lupus erythematosus [144].
Marantic endocarditis is characterized by the formation of small, friable, and non-bacterial vegetations on the heart valves. These vegetations are composed of fibrin, and platelets and are usually asymptomatic, but they can cause valve dysfunction and embolization if they become large [145]. In some cases, marantic endocarditis can lead to heart failure, stroke, or other serious embolic complications. The prevalence of this condition is not accurately determined. The aim of this study was to address this condition in cancer patients in order to provide a precise and conclusive recommendation regarding screening and follow-up of cancer patients for this condition.
4.1. Demographic, Clinical, and Pathologic Characteristics
This meta-analysis included all postmortem and premortem case reports and series of cancer-associated NBTE reported in the literature. Among 144 cases, the median age was 60 years, and 81 patients (56.6%) were females. The most common associated cancer was lung cancer (28.5%, p < 0.001) followed by pancreas cancer (19.4%, p < 0.001), gynecological malignancies (16%), and others (36.1%). This can be explained by the higher hypercoagulability state of these cancers, with the highest rates reported for venous thromboembolism and Trousseau’s syndrome [146]. Adenocarcinoma histology predominated (64.6% vs. 35.4% for other pathologies, p < 0.001). Marantic endocarditis was usually associated with a more advanced stage (69.3% were metastatic at initial presentation, p < 0.001), and the lung cancer group was the most common in subgroup analysis (87.2% (lung) vs. 62.4% (others), p = 0.008). Mitral and aortic valves were the most frequently affected valves (47% vs. 36.4%, respectively), with 15.9% of cases affecting more than one valve. These results are in line with the largest autopsy series to date, demonstrating more involvement of left-sided valves (mitral 43.4% vs. aortic 36% vs. multiple 13.1%). A study by Lopez et al. also demonstrated a higher prevalence of non-bacterial thrombotic endocarditis among adenocarcinoma of the lung and pancreas [7].
4.2. Presentation and Diagnosis
We found that there has been a decrease in the rate of postmortem diagnoses in the recent era and that NBTE is no longer considered merely a postmortem pathologic curiosity. The diagnosis of marantic endocarditis was the initial incident leading to cancer diagnosis in 66.4% of cases. In our meta-analysis, embolization events were the most common presentation (88.6%), with cerebral embolization (stroke) predominating (72.7%). This is in line with the observation by Lopez et al. that most of marantic vegetations are located on the atrial surface of the valves, with frequent embolization due to loose attachments to the endothelial surface [7]. Vegetations can also disrupt valve function [147,148], and our analysis showed that the mode of valve dysfunction was more commonly insufficiency (68.5%) than valve stenosis (10%).
Clinicians must be vigilant observers, with a high index of suspicion required to make a premortem diagnosis. Based on our results, embolic events without evidence of atherosclerotic disease, atrial fibrillation, infective endocarditis, and/or new or changed cardiac murmur should raise clinical suspicion. In three large autopsy series, 75% of marantic vegetations were less than 3 mm, and 70% were multiple [149,150,151]. Although TTE is an excellent initial modality, it has a limited ability to detect small vegetations (<3 mm) [152]; therefore transesophageal echocardiography (TEE) may be required. Additional clues for diagnosis can be found with brain MRI, which can be used to differentiate the stroke pattern of NBTE and infective endocarditis. The former usually exhibits a pattern of numerous lesions in multiple territories with variation in size, as compared to single or disseminated punctate lesions for the latter [16].
4.3. Management
Although the rate of intervention is still low (only 21 cases), there has been a progressive increase over years (Figure 2). As a rule, the treatment of the underlying etiology is the mainstay of treatment for non-bacterial thrombotic endocarditis. Long-term heparin therapy has been shown to be effective in preventing progression and decreasing the risk of embolic events [153,154], which stands to reason, given the hypercoagulable etiology of marantic endocarditis compared to the histological composition of platelets and fibrin. Nevertheless, other studies have demonstrated no benefit of oral anticoagulation with warfarin [155,156]. The role of surgery is unclear, with no available guidelines to guide management in this regard. Consideration of surgery should be individualized, and patients should be selected based on their performance status, degree of valve dysfunction, tumor stage, and overall prognosis. Patients with an early cancer stage with and valve dysfunction and/or recurrent embolic events, despite anticoagulation, may be considered for vegetation excision or valve replacement. In our study, among the 21 patients who underwent interventions, 16 underwent replacement, and 5 underwent excision of vegetations.
4.4. Prognosis
The prognosis of marantic endocarditis depends on several factors, including the underlying cause, the extent and location of the vegetations, and the patient’s overall health [15,157]. In general, the prognosis of marantic endocarditis is good, as the vegetations are usually small and do not typically cause significant damage to the heart valves. Many patients with marantic endocarditis do not experience symptoms, and the condition may be discovered incidentally on an echocardiogram or in other imaging studies [118,158]. However, as we mentioned before, in some cases, the vegetations can cause emboli that can result in serious complications such as stroke, heart attack, or organ damage [31,69,74]. These complications can lead to mortality have been reported more commonly in cancer patients [13,69,114].
Almost seventy percent of cases in this meta-analysis were associated with advanced metastatic disease, portending a poor prognosis, in line with a study by Edoute et al., who reported a higher incidence among metastatic adenocarcinoma patients [13]. Two-thirds of cases were dead at the end of the follow-up period. We could not accurately detect the exact cause of death among the included studies. However, there was a trend toward poor OS among lung cancer cases, with a poor 5-month OS of 20.8%. There was also a trend toward better OS among those who underwent interventions for NBTE treatment. This might be attributed to the removal of the embolic source, thereby preventing further embolic events and avoiding the potential risk of long-lasting anticoagulation therapy. Multivariate analysis revealed diagnosis in the recent era and younger patient age as predictors of better survival.
4.5. Strengths and Limitations
Our meta-analysis is the first to analyze all reported cases of cancer-associated non-bacterial thrombotic endocarditis diagnosed either pre- or postmortem and highlight the demographic and clinical characteristics, treatment outcomes, and prognosis of this rare condition using individual patients data, as all cases were reported as case reports or case series with a near-full description of the most relevant data. Unfortunately, the cause of death could not be identified in most cases. We also excluded non-English studies, which might be a source of selection bias.
5. Conclusions
Non-bacterial thrombotic endocarditis is a rare entity. Most of the reported cases included in this meta-analysis were cancer-associated. Lung and pancreatic cancers were the most common tumor sites. Adenocarcinoma was the most frequently associated pathology. Embolization events were the most common presentation, especially stroke. NBTE was an incidental finding, leading to cancer diagnosis in 66.4% of the reported cases, and was frequently associated with more advanced and metastatic cancer stages. Anticoagulation was the main treatment and was infrequently managed with surgery. The prognosis was dismal, especially among lung cancer cases, with a 6-month OS of 20.8%.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15061848/s1, Table S1: Searched keywords table; Table S2: Quality assessment of the included studies; Table S3: Different criteria among (A) females vs males, and (B) marantic first presentation vs known cancer; Figure S1: PRISMA flowchart of included studies (N = 121) /cases (N = 144); Figure S2: Kaplan Meier curves with Estimated survival time among (A) Entire cohort, (B) females vs ales, (C) lung vs others, and (D) marantic 1st presentation vs known cancer, (E) intervention vs no-intervention, (F) metastatic vs non-metastatic cases; Figure S3. Number of cases reported per each country.
Author Contributions
Conceptualization, S.K. and M.R.; methodology, M.R. and A.D. (Arnaldo Dimagli); software, A.D. (Anas Dabsha) and M.R.; validation, I.A.M.H.E. and M.G.; formal analysis, A.D. (Anas Dabsha) and M.R.; investigation, O.M.K., I.A.M.H.E., and A.D. (Anas Dabsha); resources, A.I., O.M.K., M.B., and M.H.; data curation, M.R., I.A.M.H.E., A.D. (Ana Dabsha), M.H., and M.B.; writing—original draft preparation, S.K., O.M.K. and A.I.; writing—review and editing, S.L.M., L.N.G., and M.G.; visualization, A.I. and M.H.; supervision, M.R., S.L.M. and L.N.G.; project administration, M.R. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Baron J.A., Gridley G., Weiderpass E., Nyren O., Linet M. Venous thromboembolism and cancer. Lancet. 1998;351:1077–1080. doi: 10.1016/S0140-6736(97)10018-6. [DOI] [PubMed] [Google Scholar]
- 2.Schmaier A.A., Ambesh P., Campia U. Venous thromboembolism and cancer. Curr. Cardiol. Rep. 2018;20:1–10. doi: 10.1007/s11886-018-1034-3. [DOI] [PubMed] [Google Scholar]
- 3.Trousseau A. Lectures on Clinical Medicine. Volume 2 Lindsay & Blakiston; Pennsylvania, PA, USA: 1873. [Google Scholar]
- 4.Ziegler E. Ueber den Bau und die Entstehung der endocaridtis chen Efflorescenzen. Ver. Kong Inn. Med. 1888;7:339–343. [Google Scholar]
- 5.Gross L., FRIEDBERG C.K. Nonbacterial thrombotic endocarditis: Classification and general description. Arch. Intern. Med. 1936;58:620–640. doi: 10.1001/archinte.1936.00170140045004. [DOI] [Google Scholar]
- 6.Libman E. Characterization of various forms of endocarditis. J. Am. Med. Assoc. 1923;80:813–818. doi: 10.1001/jama.1923.02640390001001. [DOI] [Google Scholar]
- 7.Lopez J.A., Ross R.S., Fishbein M.C., Siegel R.J. Nonbacterial thrombotic endocarditis: A review. Am. Heart J. 1987;113:773–784. doi: 10.1016/0002-8703(87)90719-8. [DOI] [PubMed] [Google Scholar]
- 8.Bussani R., De-Giorgio F., Pesel G., Zandonà L., Sinagra G., Grassi S., Baldi A., Abbate A., Silvestri F. Overview and comparison of infectious endocarditis and non-infectious endocarditis: A review of 814 autoptic cases. In Vivo. 2019;33:1565–1572. doi: 10.21873/invivo.11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joffe I.I., Jacobs L.E., Owen A.N., Ioli A., Kotler M.N. Noninfective valvular masses: Review of the literature with emphasis on imaging techniques and management. Am. Heart J. 1996;131:1175–1183. doi: 10.1016/S0002-8703(96)90094-0. [DOI] [PubMed] [Google Scholar]
- 10.Deppisch L.M., Fayemi A.O. Non-bacterial thrombotic endocarditis: Clinicopathologic correlations. Am. Heart J. 1976;92:723–729. doi: 10.1016/S0002-8703(76)80008-7. [DOI] [PubMed] [Google Scholar]
- 11.Llenas-García J., Guerra-Vales J.M., Montes-Moreno S., López-Ríos F., Castelbón-Fernández F.J., Chimeno-García J. Nonbacterial thrombotic endocarditis: Clinicopathologic study of a necropsy series. Rev. Española De Cardiol. (Engl. Ed.) 2007;60:493–500. doi: 10.1016/S0300-8932(07)75066-2. [DOI] [PubMed] [Google Scholar]
- 12.Candela M., Vidal C., Roman J., Aramburo P. Non-bacterial thrombotic endocarditis in cancer patients. Acta Cardiol. 1991;46:1–9. [PubMed] [Google Scholar]
- 13.Edoute Y., Haim N., Rinkevich D., Brenner B., Reisner S.A. Cardiac valvular vegetations in cancer patients: A prospective echocardiographic study of 200 patients. Am. J. Med. 1997;102:252–258. doi: 10.1016/S0002-9343(96)00457-3. [DOI] [PubMed] [Google Scholar]
- 14.Dutta T., Karas M.G., Segal A.Z., Kizer J.R. Yield of transesophageal echocardiography for nonbacterial thrombotic endocarditis and other cardiac sources of embolism in cancer patients with cerebral ischemia. Am. J. Cardiol. 2006;97:894–898. doi: 10.1016/j.amjcard.2005.09.140. [DOI] [PubMed] [Google Scholar]
- 15.Zmaili M.A., Alzubi J.M., Kocyigit D., Bansal A., Samra G.S., Grimm R., Griffin B.P., Xu B. A contemporary 20-year Cleveland Clinic experience of nonbacterial thrombotic endocarditis: Etiology, echocardiographic imaging, management, and outcomes. Am. J. Med. 2021;134:361–369. doi: 10.1016/j.amjmed.2020.06.047. [DOI] [PubMed] [Google Scholar]
- 16.Singhal A.B., Topcuoglu M.A., Buonanno F.S. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis: A diffusion-weighted magnetic resonance imaging study. Stroke. 2002;33:1267–1273. doi: 10.1161/01.STR.0000015029.91577.36. [DOI] [PubMed] [Google Scholar]
- 17.Mazokopakis E.E., Syros P.K., Starakis I.K. Nonbacterial thrombotic endocarditis (marantic endocarditis) in cancer patients. Cardiovasc. Haematol. Disord.-Drug Targets (Former. Curr. Drug Targets-Cardiovasc. Hematol. Disord.) 2010;10:84–86. doi: 10.2174/187152910791292484. [DOI] [PubMed] [Google Scholar]
- 18.Estevez C.M., Corya B.C. Serial echocardiographic abnormalities in nonbacterial thrombotic endocarditis of the mitral valve. Chest. 1976;69:801–804. doi: 10.1378/chest.69.6.801. [DOI] [PubMed] [Google Scholar]
- 19.Patel M.J., Elzweig J. Non-bacterial thrombotic endocarditis: A rare presentation and literature review. BMJ Case Rep. 2020;13:e238585. doi: 10.1136/bcr-2020-238585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., Currie M., Qureshi R., Mattis P., Lisy K. Joanna Briggs Institute Reviewer’s Manual. Volume 5. The Joanna Briggs Institute; Adelaide, Australia: 2020. [(accessed on 12 March 2023)]. Chapter 7: Systematic reviews of etiology and risk. Available online: https://synthesismanual.jbi.global. [Google Scholar]
- 22.Ahmed S., Jani P., Yamani M.H., Ailawadhi S., Alegria V.R., Ailawadhi M. Marantic endocarditis associated with T-cell large granular lymphocytic leukemia: First report of its occurrence with a lymphoproliferative malignancy in adults. J. Oncol. Pract. 2018;14:625–627. doi: 10.1200/JOP.18.00168. [DOI] [PubMed] [Google Scholar]
- 23.Alaiti M.A., Hoit B.D. Nonbacterial thrombotic endocarditis. Echocardiography. 2015;32:1051–1052. doi: 10.1111/echo.12915. [DOI] [PubMed] [Google Scholar]
- 24.Albright B.B., Black J.D., Vilardo N., Schwartz P.E. Correction of coagulopathy associated with non-bacterial thrombotic endocarditis (NBTE) by surgical debulking in a case of ovarian clear cell carcinoma. Gynecol. Oncol. Rep. 2016;17:13. doi: 10.1016/j.gore.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali N., Konstantinov I., Heath J.A., Bhagwat K., Cheung M. Nonbacterial thrombotic endocarditis in a child with non-Hodgkin’s lymphoma. Pediatr. Cardiol. 2012;33:843–845. doi: 10.1007/s00246-012-0230-y. [DOI] [PubMed] [Google Scholar]
- 26.Ali M. Non-bacterial thrombotic endocarditis and subclinical myopericarditis in a patient with advanced rectal cancer. Case Rep. 2015;2015:bcr2015212820. doi: 10.1136/bcr-2015-212820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arvold N.D., Hsu L., Chen W.Y., Benzaquen L.R., Weiss S.E. Marantic endocarditis with cardioembolic strokes mimicking leptomeningeal metastases in breast cancer. J. Clin. Oncol. 2011;29:e743–e746. doi: 10.1200/JCO.2011.36.4190. [DOI] [PubMed] [Google Scholar]
- 28.Ashenhurst E., Chertkow G. Cerebral embolism from nonbacterial thrombotic endocarditis. Can. Med. Assoc. J. 1962;86:313. [PMC free article] [PubMed] [Google Scholar]
- 29.Bhardwaj B., Bajwa A., Sharma A., Towheed A., Sanghani B.V., McGhie A.I. Nonbacterial thrombotic endocarditis involving both the tricuspid and aortic valves. Echocardiography. 2016;33:1916–1918. doi: 10.1111/echo.13390. [DOI] [PubMed] [Google Scholar]
- 30.Binet Q., Goffinet C., Etogo-Asse F.-E., Shaza L. Nonbacterial thrombotic endocarditis in a patient with gastric cancer and SARS-CoV-2 infection. Clin. J. Gastroenterol. 2021;14:1031–1035. doi: 10.1007/s12328-021-01412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borowski A., Ghodsizad A., Cohnen M., Gams E. Recurrent embolism in the course of marantic endocarditis. Ann. Thorac. Surg. 2005;79:2145–2147. doi: 10.1016/j.athoracsur.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Chen L., Li Y., Gebre W., Lin J.H. Myocardial and cerebral infarction due to nonbacterial thrombotic endocarditis as an initial presentation of pancreatic adenocarcinoma. Arch. Pathol. Lab. Med. 2004;128:1307–1308. doi: 10.5858/2004-128-1307-MACIDT. [DOI] [PubMed] [Google Scholar]
- 33.Cheung B., Shivkumar A., Ahmed A.S. Embolic showering from non-bacterial thrombotic endocarditis and adenocarcinoma of the lung. Eur. J. Case Rep. Intern. Med. 2020;7:001798. doi: 10.12890/2020_001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chisholm J.C., Jr., Ireland C.S., Scott R.N. Bronchogenic carcinoma, leukemoid reaction, marantic endocarditis, and consumptive thrombocytopathy. J. Natl. Med. Assoc. 1982;74:447. [PMC free article] [PubMed] [Google Scholar]
- 35.Clough H., George J., Duncan A. Psychosis due to non-bacterial thrombotic endocarditis. Age Ageing. 2010;39:276–277. doi: 10.1093/ageing/afp245. [DOI] [PubMed] [Google Scholar]
- 36.Detremerie C., Timmermans F., De Pauw M., Gheeraert P., Hemelsoet D., Toeback J., Bové T., Vandecasteele E. Stroke due to non-bacterial thrombotic endocarditis as initial presentation of breast invasive ductal carcinoma. Acta Clin. Belg. 2017;72:268–273. doi: 10.1080/17843286.2016.1219012. [DOI] [PubMed] [Google Scholar]
- 37.Dewey N., Mughal L.H., Houghton A.R., Khoo J. Right ventricular marantic endocarditis. Echo Res. Pract. 2015;2:I3. doi: 10.1530/ERP-14-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douin C., Marchetta S., Dulgheru R., Bruyère P.-J., Moonen M., Lancellotti P. Case report: Aortic bioprosthesis marantic endocarditis. Acta Cardiol. 2021;76:1143–1144. doi: 10.1080/00015385.2020.1813995. [DOI] [PubMed] [Google Scholar]
- 39.Elboudwarej O., Wei J., Siegel R. Diagnosis of an aortic valvular lesion. Heart. 2015;101:719. doi: 10.1136/heartjnl-2014-306723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fanale M.A., Zeldenrust S.R., Moynihan T.J. Case 2. Marantic endocarditis in advanced cancer. J. Clin. Oncol. 2002;20:4111–4114. doi: 10.1200/JCO.2002.20.19.4111. [DOI] [PubMed] [Google Scholar]
- 41.Farooqui A.A., Ashraf R., D’Ornellas R., Aslam A., Marcelin M., Shetty V. Tricuspid Valve Vegetation Secondary to Ovarian Cancer Leading to Acute Stroke via Pulmonary Arteriovenous Malformation. Cureus. 2021;13:e17136. doi: 10.7759/cureus.17136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreira T.L., Alves R., Judas T., Delerue M.F. Marantic endocarditis and paraneoplastic pulmonary embolism. Case Rep. 2017;2017:bcr2017220217. doi: 10.1136/bcr-2017-220217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fournier J.B., Testa E.J. Nonbacterial thrombotic endocarditis. N. Engl. J. Med. 2019;380:e48. doi: 10.1056/NEJMicm1804137. [DOI] [PubMed] [Google Scholar]
- 44.Frazer G., Laing R., Lamont D. Non-bacterial thrombotic endocarditis with a negative transesophageal echocardiogram. N. Z. Med. J. (Online) 2005;118:U1589. [PubMed] [Google Scholar]
- 45.Fujimoto D., Mochizuki Y., Nakagiri K., Shite J. Unusual rapid progression of non-bacterial thrombotic endocarditis in a patient with bladder cancer despite undergoing intensification treatment with rivaroxaban for acute venous thromboembolism. Eur. Heart J. 2018;39:3907. doi: 10.1093/eurheartj/ehy569. [DOI] [PubMed] [Google Scholar]
- 46.Garcia I., Fainstein V., Rios A., Luna M., Mansell P., Reuben J., Hersh E. Nonbacterial thrombotic endocarditis in a male homosexual with Kaposi’s sarcoma. Arch. Intern. Med. 1983;143:1243–1244. doi: 10.1001/archinte.1983.00350060175027. [DOI] [PubMed] [Google Scholar]
- 47.Glass J.P. The diagnosis and treatment of stroke in a patient with cancer: Nonbacterial thrombotic endocarditis (NBTE): A case report and review. Clin. Neurol. Neurosurg. 1993;95:315–318. doi: 10.1016/0303-8467(93)90108-S. [DOI] [PubMed] [Google Scholar]
- 48.Gray K.M., Nguyen B., Baker L., Ahmad M. Non-bacterial thrombotic endocarditis and coronary thrombectomy in a patient with metastatic small cell lung carcinoma. BMJ Case Rep. 2021;14:e239893. doi: 10.1136/bcr-2020-239893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green S. Two unusual cardiac complications of gynaecological malignancy: Myocardial metastases and non-infective thrombotic endocarditis. Br. J. Clin. Pract. 1990;44:74–75. [PubMed] [Google Scholar]
- 50.Gundersen H., Moynihan B. An uncommon cause of stroke: Non-bacterial thrombotic endocarditis. J. Stroke Cerebrovasc. Dis. 2016;25:e163–e164. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Harris A.Z., Ternouth I., Lallu B.D. Case report: Marantic endocarditis in renal cell carcinoma: Nephrectomy a treatment. Eur. Heart J.-Case Rep. 2021;5:ytab437. doi: 10.1093/ehjcr/ytab437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hofstra J., Timmer J., Breeman A., Havenith M. Non-bacterial thrombotic endocarditis in metastatic caecal adenocarcinoma. Neth. Heart J. 2009;17:349–350. doi: 10.1007/BF03086282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holt J.N. A case of multifocal stroke—The first presentation of underlying ovarian malignancy. J. Surg. Case Rep. 2021;2021:rjaa550. doi: 10.1093/jscr/rjaa550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ilyas S., Lang W., Belani N., Philip Stockwell M. Non-bacterial thrombotic endocarditis as a Cause of Cryptogenic Stroke in Malignancy. Rhode Isl. Med. J. 2021;104:63–66. [PubMed] [Google Scholar]
- 55.Iranpour A., Mahmoodian R., Haghighi A., Vakili M., Shahriari-Ahmadi A., Hajsadeghi S., Arabi M. A 34 Year Old Man with Purple Discoloration and Paresthesia. Int. J. Hematol.-Oncol. Stem Cell Res. 2014;8:41. [PMC free article] [PubMed] [Google Scholar]
- 56.Ito S., Yoshitomi H., Pak M., Kawahara H., Oshima T., Ito S., Watanabe N., Sato H., Adachi T., Takeda M. Trousseau syndrome with nonbacterial thrombotic endocarditis in a patient with uterine cancer. Intern. Med. 2013;52:1353–1358. doi: 10.2169/internalmedicine.52.9384. [DOI] [PubMed] [Google Scholar]
- 57.Jameson G.S., Ramanathan R.K., Borad M.J., Downhour M., Korn R., Von Hoff D. Marantic endocarditis associated with pancreatic cancer: A case series. Case Rep. Gastroenterol. 2009;3:67–71. doi: 10.1159/000207195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joshi S.B., Richards M.J., Holt D.Q., Yan B.P., Aggarwal A. Marantic endocarditis presenting as recurrent arterial embolisation. Int. J. Cardiol. 2009;132:e14–e16. doi: 10.1016/j.ijcard.2007.07.106. [DOI] [PubMed] [Google Scholar]
- 59.Julson J.R., Weiland T., Kemp W.L. Acute subdural hemorrhage associated with both metastatic adenocarcinoma of the dura and minor head trauma: A case report and review of the literature. Acad. Forensic Pathol. 2018;8:769–776. doi: 10.1177/1925362118797754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalangos A., Pretre R., Girardet C., Ricou F., Faidutti B. An atypical aortic valve non-bacterial thrombotic endocarditis in the course of multiple myeloma. Eur. Heart J. 1997;18:351–352. doi: 10.1093/oxfordjournals.eurheartj.a015243. [DOI] [PubMed] [Google Scholar]
- 61.Kaneyuki D., Matsuura K., Ueda H., Kohno H., Kanbe M., Matsumiya G. Surgical management of nonbacterial thrombotic endocarditis in malignancy. Surg. Case Rep. 2017;3:1–4. doi: 10.1186/s40792-017-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanthasamy V., Natarajan I. Rare and challenging case of stroke as a manifestation of non bacterial thrombotic endocarditis in an underlying ovarian clear cell carcinoma. JRSM Open. 2016;7:2054270416669304. doi: 10.1177/2054270416669304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaufmann C.C., Wessely E., Huber K. Non-bacterial thrombotic endocarditis in the context of pulmonary adenocarcinoma: A case report. Eur. Heart J. Case Rep. 2020;4:1. doi: 10.1093/ehjcr/ytaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kearsley J.H., Tattersall M.H. Cerebral embolism in cancer patients. Q. J. Med. 1982;51:279–291. [PubMed] [Google Scholar]
- 65.Khan O.A., Rogers V., Sharma R., Ohri S.K. Lung cancer masquerading as prosthetic valve endocarditis. Heart Lung Circ. 2008;17:161–163. doi: 10.1016/j.hlc.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Khan K.A., Wahid K., Qureshi S.U. Nonbacterial thrombotic endocarditis as the initial presentation of prostate cancer- a case report. J. Pak. Med. Assoc. 2019;69:1737–1740. doi: 10.5455/JPMA.9385. [DOI] [PubMed] [Google Scholar]
- 67.Kijpaisalratana N., Chutinet A., Travanichakul S., Kitjawijit T., Yokumporn P., Duangjino K., Suwanwela N.C. Nonbacterial thrombotic endocarditis related to adenocarcinoma of the uterine cervix. Case Rep. Neurol. 2020;12:183–188. doi: 10.1159/000507277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimyai-Asadi A., Usman A., Milani F. Cutaneous manifestations of marantic endocarditis. Int. J. Dermatol. 2000;39:290–292. doi: 10.1046/j.1365-4362.2000.00935.x. [DOI] [PubMed] [Google Scholar]
- 69.Kooiker J.C., MacLean J.M., Sumi S.M. Cerebral embolism, marantic endocarditis, and cancer. Arch. Neurol. 1976;33:260–264. doi: 10.1001/archneur.1976.00500040044006. [DOI] [PubMed] [Google Scholar]
- 70.Kuipers R.S., Berghuis M.A., Ogilvie A.C., van Wissen S.A., Riezebos R.K. Non-bacterial thrombotic endocarditis manifested by ventricular fibrillation in a patient with low grade ovarian carcinoma: Case report and literature review. Eur. Heart J.-Case Rep. 2021;5:ytab120. doi: 10.1093/ehjcr/ytab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurdi M., Beanlands D.S., Chan K.L., Veinot J.P. Nonbacterial thrombotic endocarditis presenting as aortic stenosis with suspected infective endocarditis: Clinicopathological correlation. Can. J. Cardiol. 2004;20:549–552. [PubMed] [Google Scholar]
- 72.Kwon S., Jeon J., Kim J., Kim M.-J., Yoon J.S. Neoplastic non-bacterial endocarditis of the aortic valve. Eur. Heart J.–Cardiovasc. Imaging. 2017;18:605. doi: 10.1093/ehjci/jew296. [DOI] [PubMed] [Google Scholar]
- 73.Lal G., Brennan T.V., Hambleton J., Clark O.H. Coagulopathy, marantic endocarditis, and cerebrovascular accidents as paraneoplastic features in medullary thyroid cancer—Case report and review of the literature. Thyroid. 2003;13:601–605. doi: 10.1089/105072503322238872. [DOI] [PubMed] [Google Scholar]
- 74.Lee V., Gilbert J.D., Byard R.W. Marantic endocarditis–a not so benign entity. J. Forensic Leg. Med. 2012;19:312–315. doi: 10.1016/j.jflm.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 75.Lee J.M., Lim J.H., Kim J.-S., Park J.S., Memon A., Lee S.-K., Nam H.-S., Cho J.-H., Kwak S.-M., Lee H.L. Multiple hypercoagulability disorders at presentation of non-small-cell lung cancer. Tuberc. Respir. Dis. 2014;77:34–37. doi: 10.4046/trd.2014.77.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Makhdumi M., Meyer D.M., Roberts W.C. Malignancy-Associated Non-Bacterial Thrombotic Endocarditis Causing Aortic Regurgitation and Leading to Aortic Valve Replacement. Am. J. Cardiol. 2021;154:120–122. doi: 10.1016/j.amjcard.2021.05.032. [DOI] [PubMed] [Google Scholar]
- 77.Mantovani F., Navazio A., Barbieri A., Boriani G. A first described case of cancer-associated non-bacterial thrombotic endocarditis in the era of direct oral anticoagulants. Thromb. Res. 2017;149:45–47. doi: 10.1016/j.thromres.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 78.Marglani O., Al-Herabi A., Odell P. Marantic endocarditis as an unusual paraneoplastic syndrome of head and neck squamous cell carcinoma. J. Otolaryngol.-Head Neck Surg. 2009;38:E76. [PubMed] [Google Scholar]
- 79.Markides V., Nihoyannopoulos P. Non-bacterial thrombotic endocarditis. Eur. J. Echocardiogr. 2000;1:291–294. doi: 10.1053/euje.2000.0027. [DOI] [PubMed] [Google Scholar]
- 80.Martín-Martorell P., Insa-Molla A., Chirivella-González M.I., Cervera-Miguel J.I. Nonbacterial thrombotic endocarditis associated with lung adenocarcinoma. Clin Transl Oncol. 2007;9:744–746. doi: 10.1007/s12094-007-0133-1. [DOI] [PubMed] [Google Scholar]
- 81.Mitma A.A., Varghese J.G., Witt D., Zarich S.W. Stroke and a valvular lesion in a patient with stage IV non-small cell lung cancer. Case Rep. 2016;2016:bcr2016215317. doi: 10.1136/bcr-2016-215317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morimoto S., Tanaka J., Saito Y., Tsuyama N., Nishimura T., Komiya T., Kyo S., Arai T., Kanemaru A., Kanemaru K., et al. Non-bacterial thrombotic endocarditis in a Trousseau syndrome patient with stomach cancer: A case report. Geriatr. Gerontol. Int. 2016;16:1171–1172. doi: 10.1111/ggi.12694. [DOI] [PubMed] [Google Scholar]
- 83.Moţăţăianu A., Maier S., Gothard A., Bajkó Z., Bălaşa R. Severe Fatal Systemic Embolism Due to Non-Bacterial Thrombotic Endocarditis as the Initial Manifestation of Gastric Adenocarcinoma: Case Report. J. Crit. Care Med. (Targu Mures) 2018;4:68–73. doi: 10.2478/jccm-2018-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nadkarni N., Lee Y.J., Hoefen R., Alweis R. Cholangiocarcinoma manifesting as non-bacterial thrombotic endocarditis in a young patient. Am. J. Med. 2020;133:e396–e398. doi: 10.1016/j.amjmed.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 85.Nakashima K., Mori M., Haraki T., Hirase H., Yoshimuta T., Ichida F., Okeie K., Konno T., Hayashi K., Ino H., et al. Non-bacterial thrombotic endocarditis associated with Trousseau’s syndrome. J. Echocardiogr. 2012;10:115–116. doi: 10.1007/s12574-012-0125-1. [DOI] [PubMed] [Google Scholar]
- 86.Neilan T.G., Price M.C., Sanborn D.Y., Gainor J.F., Chen A. Case 33-2018: A 57-Year-Old Man with Confusion, Fever, Malaise, and Weight Loss. N. Engl. J. Med. 2018;379:1658–1669. doi: 10.1056/NEJMcpc1802830. [DOI] [PubMed] [Google Scholar]
- 87.Al Nidawi F., Mohamed M.W., Taha F., Alarab D., Hussein A.E.M. Recurrent Strokes as the First Presentation of Occult Pancreatic Cancer; Trousseau Syndrome: A Case Report. Case Rep. Oncol. 2021;14:1741–1747. doi: 10.1159/000520759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Numnum T.M., Leath III C.A., Straughn Jr M.J. Synchronous primary endometrial and ovarian carcinoma in a patient with marantic endocarditis. Obstet. Gynecol. 2006;108:748–750. doi: 10.1097/01.AOG.0000190220.13074.87. [DOI] [PubMed] [Google Scholar]
- 89.O’Boyle C.P., Dempsey J., Otridge B., Barniville H. Malignant islet-cell tumour of the pancreas presenting with non-bacterial thrombotic endocarditis and eosinophilia. Postgrad. Med. J. 1981;57:457–458. doi: 10.1136/pgmj.57.669.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ojeda V.J., Frost F., Mastaglia F.L. Non-bacterial thrombotic endocarditis associated with malignant disease: A clinicopathological study of 16 cases. Med. J. Aust. 1985;142:629–631. doi: 10.5694/j.1326-5377.1985.tb113555.x. [DOI] [PubMed] [Google Scholar]
- 91.Okuchi K., Fujioka M., Iwanaga H., Koshimae N., Sakaki T. Fulminant cerebral infarctions caused by nonbacterial thrombotic endocarditis due to gallbladder cancer. Acta Neurochir. 1997;139:995–996. doi: 10.1007/BF01411313. [DOI] [PubMed] [Google Scholar]
- 92.Olney B.A., Schattenberg T.T., Campbell J.K., Okazaki H., Lie J. The consequences of the inconsequential: Marantic (nonbacterial thrombotic) endocarditis. Am. Heart J. 1979;98:513–522. doi: 10.1016/0002-8703(79)90259-X. [DOI] [PubMed] [Google Scholar]
- 93.Orfanelli T., Sultanik E., Shell R., Gibbon D. Nonbacterial thrombotic endocarditis: A rare manifestation of gynecologic cancer. Gynecol. Oncol. Rep. 2016;17:72. doi: 10.1016/j.gore.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oueida Z., Scola M. Ovarian clear cell carcinoma presenting as non-bacterial thrombotic endocarditis and systemic embolization. World J. Oncol. 2011;2:270. doi: 10.4021/wjon367e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Panicucci E., Bruno C., Ferrari V., Suissa L. Recurrence of ischemic stroke on direct oral anticoagulant therapy in a patient with marantic endocarditis related to lung cancer. J. Cardiol. Cases. 2021;23:242–245. doi: 10.1016/j.jccase.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perrone F., Biagi A., Facchinetti F., Bozzetti F., Ramelli A., Vezzani A., Manca T., Gnetti L., Majori M., Alfieri V., et al. Systemic thromboembolism from a misdiagnosed non-bacterial thrombotic endocarditis in a patient with lung cancer: A case report. Oncol. Lett. 2020;20:194. doi: 10.3892/ol.2020.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Piovanelli B., Rovetta R., Bonadei I., Vizzardi E., D’Aloia A., Metra M. Nonbacterial thrombotic endocarditis in pancreatic cancer. Monaldi Arch. Chest Dis. 2013;80:189–192. doi: 10.4081/monaldi.2013.5236. [DOI] [PubMed] [Google Scholar]
- 98.Polo J., Raufast D., Cornand D., Elias A. Acute ischaemia of the lower limb due to non-bacterial thrombotic endocarditis with recent venous thrombo-embolic disease as the initial manifestation of lung adenocarcinoma: A case report. Eur. Heart J. Case Rep. 2021;5:ytab426. doi: 10.1093/ehjcr/ytab426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramirez-Escudero Ugalde G., Codina Prat M., Candina Urizar R., Garcia Ibarrondo N., Manzanal Rey A., Ruiz Gomez L., Ugedo Alzaga K., Castellanos Alcalde M., Lambarri Izaguirre A., Asla Ormaza C. P691 New onset severe mitral regurgitation in oncological patient: When the disease progresses. Eur. Heart J.-Cardiovasc. Imaging. 2020;21:jez319.366. doi: 10.1093/ehjci/jez319.366. [DOI] [Google Scholar]
- 100.Randhawa G., Aslam A., Suarez M.J., Lin Y.S., Kuhn-Basti M. Non-Bacterial Thrombotic Endocarditis: A Case of Metastatic Pancreatic Cancer Masquerading as Infective Endocarditis. Cureus. 2020;12:e9103. doi: 10.7759/cureus.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Royter V., Cohen S.N. Recurrent embolic strokes and cardiac valvular disease in a patient with non-small cell adenocarcinoma of lung. J. Neurol. Sci. 2006;241:99–101. doi: 10.1016/j.jns.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 102.Sakima H., Isa K., Kokuba K., Nakachi K., Ikemiyagi H., Shiroma K., Ishihara S., Tokashiki T., Yasu T., Ohya Y. Recurrent embolic stroke due to nonbacterial thrombotic endocarditis followed by transesophageal echocardiography. Arch. Neurol. 2011;68:1604–1605. doi: 10.1001/archneurol.2011.687. [DOI] [PubMed] [Google Scholar]
- 103.Sánchez Quirós B., Ruiz López N., López Herrero R., Bartolomé Bartolomé C. Marantic endocarditis. Rev. Esp. Anestesiol. Reanim. 2020;67:208–211. doi: 10.1016/j.redar.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 104.Sánchez-Enrique C., Vilacosta I., Moreno H.G., Delgado-Bolton R., Pérez-Alonso P., Martínez A., Vivas D., Ferrera C., Olmos C. Infected marantic endocarditis with leukemoid reaction. Circ. J. 2014;78:2325–2327. doi: 10.1253/circj.CJ-14-0079. [DOI] [PubMed] [Google Scholar]
- 105.Savarapu P., Abdelazeem B., Isa S., Baral N., Hassan M. Cancer-Related Non-Bacterial Thrombotic Endocarditis Presenting as Acute Ischemic Stroke. Cureus. 2021;13:e14953. doi: 10.7759/cureus.14953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sawai T., Ikezawa M., Hirota A., Yamamoto S., Nakajima H., Makino K., Takase K., Ito M. Large Non-Bacterial Vegetation Causing Acute Aortic Regurgitation—Unexpected Finding at Autopsy. Circ. J. 2018;82:2378–2379. doi: 10.1253/circj.CJ-17-1039. [DOI] [PubMed] [Google Scholar]
- 107.Scalia G.M., Tandon A.K., Robertson J.A. Stroke, aortic vegetations and disseminated adenocarcinoma—A case of marantic endocarditis. Heart Lung Circ. 2012;21:234–236. doi: 10.1016/j.hlc.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 108.Sekulic M., Gupta A., Patterson A., Oliveira G., Rajagopalan S. Chemotherapy-associated nonbacterial thrombotic endocarditis: A radiological mimicker of cardiac amyloidosis requiring histopathologic examination for definitive diagnosis. Cardiovasc. Pathol. 2020;47:107210. doi: 10.1016/j.carpath.2020.107210. [DOI] [PubMed] [Google Scholar]
- 109.Shatila W., Rizkallah A., Aldin E.S., Tfayli A. Nonbacterial thrombotic endocarditis as the sole manifestation of stage IV gastric cancer: A case report. J. Med. Case Rep. 2014;8:267. doi: 10.1186/1752-1947-8-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shibata N., Matsumoto K., Kitamura S., Sakashita A., Kizawa Y., Hirata K.I. Nonbacterial Thrombotic Endocarditis Concomitant with Repeated Systemic Embolization That Received Palliative Care Based on the Antemortem Diagnosis. Intern. Med. 2018;57:3559–3563. doi: 10.2169/internalmedicine.1381-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shoji M.K., Kim J.-H., Bakshi S., Govea N., Marukian N., Wang S.J. Nonbacterial thrombotic endocarditis due to primary gallbladder malignancy with recurrent stroke despite anticoagulation: Case report and literature review. J. Gen. Intern. Med. 2019;34:1934–1940. doi: 10.1007/s11606-019-05166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shuaib A. Stroke from other etiologies masquerading as migraine-stroke. Stroke. 1991;22:1068–1074. doi: 10.1161/01.STR.22.8.1068. [DOI] [PubMed] [Google Scholar]
- 113.Sia C.H., Lim J.S., Poh K.K., Chin T.M. A classical case of non-bacterial thrombotic endocarditis from pancreatic adenocarcinoma presenting as multiple strokes, myocardial infarction and acute limb ischaemia. Oxf. Med. Case Rep. 2016;2016:omw084. doi: 10.1093/omcr/omw084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singh V., Bhat I., Havlin K. Marantic endocarditis (NBTE) with systemic emboli and paraneoplastic cerebellar degeneration: Uncommon presentation of ovarian cancer. J. Neuro-Oncol. 2007;83:81–83. doi: 10.1007/s11060-006-9306-y. [DOI] [PubMed] [Google Scholar]
- 115.Smeglin A., Ansari M., Skali H., Oo T.H., Maysky M. Marantic endocarditis and disseminated intravascular coagulation with systemic emboli in presentation of pancreatic cancer. J. Clin. Oncol. 2008;26:1383–1385. doi: 10.1200/JCO.2007.12.9148. [DOI] [PubMed] [Google Scholar]
- 116.Soga Y., Taira K., Sugimoto A., Kurosawa M., Kira H., Su T., Doi K., Nakano A., Himura Y. Mitral valve nonbacterial thrombotic endocarditis: A rare multi-surgery-tolerant survivor of Trousseau’s syndrome. Surg Case Rep. 2018;4:104. doi: 10.1186/s40792-018-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spurgeon L., Ispoglou S. Non-bacterial thrombotic endocarditis in pancreatic cancer and other high-risk malignancies: The case for prophylactic treatment. Oxf. Med. Case Rep. 2021;2021:omab110. doi: 10.1093/omcr/omab110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Starobinska E., Robinson E.A., Brucks E., Scott S. Marantic endocarditis: Incidental infarcts leading to diagnosis of pancreatic cancer. Case Rep. 2018;2018:bcr2018224529. doi: 10.1136/bcr-2018-224529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Studdy P., Willoughby J.M. Non-bacterial thrombotic endocarditis in early cancer. Br. Med. J. 1976;1:752. doi: 10.1136/bmj.1.6012.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sugawara M., Nakao T., Yatomi Y., Daimon M. Development of aortic regurgitation due to non-bacterial thrombotic endocarditis. J. Med. Ultrason. 2021;48:363–364. doi: 10.1007/s10396-021-01105-2. [DOI] [PubMed] [Google Scholar]
- 121.Suzuki S., Tanaka K., Nogawa S., Umezawa A., Hata J., Fukuuchi Y. Expression of interleukin-6 in cerebral neurons and ovarian cancer tissue in Trousseau syndrome. Clin. Neuropathol. 2002;21:232–235. [PubMed] [Google Scholar]
- 122.Tai M.L., Tan E.C., Ang C.C., Liam C.K. Recurrent cerebral infarcts secondary to marantic endocarditis in a patient with adenocarcinoma of the lung. Singap. Med. J. 2016;57:524–525. doi: 10.11622/smedj.2016157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takeshita S., Ogata T., Mera H., Tsugawa J., Aoki M., Takeshita M., Tsuboi Y. Multiple Thrombi in the Heart in Trousseau Syndrome Caused by Pancreatic Carcinoma. J. Stroke Cereb. Dis. 2018;27:e75–e77. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 124.Tamura Y., Sakata K., Terada K., Usui S., Kawashiri M.A., Takamura M. Treatment with a Direct Oral Anticoagulant for Nonbacterial Thrombotic Endocarditis. Intern. Med. 2021;60:1881–1885. doi: 10.2169/internalmedicine.6368-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tiong I.S., Williams M.J., Perez D.J. Nonbacterial thrombotic endocarditis with ST-elevation myocardial infarction treated with percutaneous coronary aspiration thrombectomy. Heart Lung Circ. 2013;22:386–389. doi: 10.1016/j.hlc.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 126.Tsai C.C., Wu M.N. Frequent Ischemic Stroke as First Manifestation of Occult Colon Cancer: A Rare Case. Am. J. Case Rep. 2015;16:723–727. doi: 10.12659/AJCR.895130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Umeojiako W.I., Kasouridis I., Sargent R., Ghani S. Atypical marantic endocarditis. BMJ Case Rep. 2019;12:e232057. doi: 10.1136/bcr-2019-232057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vaideeswar P., Pandit M.J., Deshpande J.R., Sivaraman A., Vora I.M. Fibrolamellar carcinoma of the liver--an unusual presentation. J. Postgrad. Med. 1993;39:159–161. [PubMed] [Google Scholar]
- 129.Van Herck J., Thoen H., Delens C., Voet J. Multi-territory stroke preceded by pulmonary embolism with asymptomatic coronavirus disease 2019: A case report. Eur. Heart J. Case Rep. 2021;5:ytab471. doi: 10.1093/ehjcr/ytab471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vlachostergios P.J., Daliani D.D., Dimopoulos V., Patrikidou A., Voutsadakis I.A., Papandreou C.N. Nonbacterial thrombotic (marantic) endocarditis in a patient with colorectal cancer. Onkologie. 2010;33:456–459. doi: 10.1159/000317342. [DOI] [PubMed] [Google Scholar]
- 131.Wang J., Monga N., Mopala P., Husnain M. Development of nonbacterial thrombotic endocarditis while on systemic anticoagulation in pancreatic cancer: A case report. Cureus. 2020;12:e10967. doi: 10.7759/cureus.10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Washburn E.R., Weyant G.W., Yang X.J., Yang Z. A rare case of prostatic ductal adenocarcinoma presenting as papillary metastatic carcinoma of unknown primary: A case report and review of the literature. Hum. Pathol. Case Rep. 2016;6:26–31. doi: 10.1016/j.ehpc.2015.12.004. [DOI] [Google Scholar]
- 133.Wigger O., Windecker S., Bloechlinger S. Nonbacterial thrombotic endocarditis presenting as intracerebral hemorrhage. Wien. Klin. Wochenschr. 2016;128:922–924. doi: 10.1007/s00508-016-1020-y. [DOI] [PubMed] [Google Scholar]
- 134.Wild J., Distelmeier S., Keil P., Weinmann A., Münzel T., Weinmann-Menke J., Kraus D. Non-bacterial thrombotic endocarditis in a patient with pancreatic carcinoma. Echocardiography. 2021;38:1455–1458. doi: 10.1111/echo.15140. [DOI] [PubMed] [Google Scholar]
- 135.Wong S.F., Seow J., Profitis K., Johns J., Barnett S., John T. Marantic endocarditis presenting with multifocal neurological symptoms. Intern. Med. J. 2013;43:211–214. doi: 10.1111/imj.12018. [DOI] [PubMed] [Google Scholar]
- 136.Woo P., Chan D., Cheung T., Zhu X., Poon W. Middle cerebral artery infarction in a cancer patient: A fatal case of Trousseau’s syndrome. Hong Kong Med. J. 2014;20:74–77. doi: 10.12809/hkmj133780. [DOI] [PubMed] [Google Scholar]
- 137.Yagi T., Takahashi K., Tanikawa M., Seki M., Abe T., Suzuki N. Fatal intracranial hemorrhage after intravenous thrombolytic therapy for acute ischemic stroke associated with cancer-related nonbacterial thrombotic endocarditis. J. Stroke Cereb. Dis. 2014;23:e413–e416. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 138.Yamane A., Sadahiro H., Goto H., Inamura A., Ishihara H., Oka F., Oku T., Kondo T., Suzuki M. Multiple ischemic strokes caused by nonbacterial thrombotic endocarditis because of gallbladder cancer: A case report. J. Stroke Cereb. Dis. 2014;23:1727–1729. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 139.Yasutake H., Sugano Y., Ikeda Y., Ohara T., Hasegawa T., Kanzaki H., Anzai T. First Case Report of the Antemortem Diagnosis of Nonbacterial Thrombotic Endocarditis of a Mechanical Prosthetic Valve. Intern. Med. 2016;55:255–257. doi: 10.2169/internalmedicine.55.5470. [DOI] [PubMed] [Google Scholar]
- 140.Yoshii Y., Numata T., Ishitobi W., Takahashi N., Wakui H., Kojima J., Shimizu K., Hara H., Ishikawa T., Kawaishi M., et al. Lung adenocarcinoma complicated by Trousseau’s syndrome successfully treated by a combination of anticoagulant therapy and chemotherapy. Intern. Med. 2014;53:1835–1839. doi: 10.2169/internalmedicine.53.1315. [DOI] [PubMed] [Google Scholar]
- 141.Zhou Y., Yee Y., Qin Y. Non-bacterial thrombotic endocarditis and metastatic lung adenocarcinoma. BMJ Case Rep. 2021;14:e242948. doi: 10.1136/bcr-2021-242948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schlittler L.A., Dallagasperina V.W., Schavinski C., Baggio A.P., Lazaretti N.S., Villaroel R.U. Marantic endocarditis and adenocarcinoma of unknown primary site. Arq. Bras. De Cardiol. 2011;96:e73–e75. [PubMed] [Google Scholar]
- 143.Glancy D.L., Roberts W.C. The heart in malignant melanoma: A study of 70 autopsy cases. Am. J. Cardiol. 1968;21:555–571. doi: 10.1016/0002-9149(68)90289-0. [DOI] [PubMed] [Google Scholar]
- 144.Lee J.L., Naguwa S.M., Cheema G.S., Gershwin M.E. Revisiting Libman–Sacks endocarditis: A historical review and update. Clin. Rev. Allergy Immunol. 2009;36:126–130. doi: 10.1007/s12016-008-8113-y. [DOI] [PubMed] [Google Scholar]
- 145.Eiken P.W., Edwards W.D., Tazelaar H.D., McBane R.D., Zehr K.J. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985–2000. Mayo Clin. Proc. 2001;76:1204–1212. doi: 10.4065/76.12.1204. [DOI] [PubMed] [Google Scholar]
- 146.Caine G.J., Stonelake P.S., Lip G.Y., Kehoe S.T. The hypercoagulable state of malignancy: Pathogenesis and current debate. Neoplasia. 2002;4:465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rosen P., Armstrong D. Nonbacterial thrombotic endocarditis in patients with malignant neoplastic diseases. Am. J. Med. 1973;54:23–29. doi: 10.1016/0002-9343(73)90079-X. [DOI] [PubMed] [Google Scholar]
- 148.Bryan C.S. Nonbacterial thrombotic endocarditis with malignant tumors. Am. J. Med. 1969;46:787–793. doi: 10.1016/0002-9343(69)90029-1. [DOI] [PubMed] [Google Scholar]
- 149.MACDONALD R.A., ROBBINS S.L. The significance of nonbacterial thrombotic endocarditis: An autopsy and clinical study of 78 cases. Ann. Intern. Med. 1957;46:255–273. doi: 10.7326/0003-4819-46-2-255. [DOI] [PubMed] [Google Scholar]
- 150.Rohner R.F., Prior J.T., Sipple J.H. Mucinous malignancies, venous thrombosis and terminal endocarditis with emboli: A syndrome. Cancer. 1966;19:1805–1812. doi: 10.1002/1097-0142(196612)19:12<1805::AID-CNCR2820191207>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 151.Eliakim M., Pinchas S. Degenerative verrucous endocardiosis. A clinical-pathological study of 45 cases with reference to a protracted form of the disease. Isr. J. Med. Sci. 1966;2:42–51. [PubMed] [Google Scholar]
- 152.Mügge A., Daniel W.G., Frank G., Lichtlen P.R. Echocardiography in infective endocarditis: Reassessment of prognostic implications of vegetation size determined by the transthoracic and the transesophageal approach. J. Am. Coll. Cardiol. 1989;14:631–638. doi: 10.1016/0735-1097(89)90104-6. [DOI] [PubMed] [Google Scholar]
- 153.Sack G.H., Jr., Levin J., Bell W.R. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: Clinical, pathophysiologic, and therapeutic features. Medicine. 1977;56:1–37. doi: 10.1097/00005792-197756010-00001. [DOI] [PubMed] [Google Scholar]
- 154.Rogers L.R., Cho E.-s., Kempin S., Posner J.B. Cerebral infarction from non-bacterial thrombotic endocarditis: Clinical and pathological study including the effects of anticoagulation. Am. J. Med. 1987;83:746–756. doi: 10.1016/0002-9343(87)90908-9. [DOI] [PubMed] [Google Scholar]
- 155.Mosesson M.W., Colman R.W., Sherry S. Chronic intravascular coagulation syndrome: Report of a case with special studies of an associated plasma cryoprecipitate (cryofibrinogen) N. Engl. J. Med. 1968;278:815–821. doi: 10.1056/NEJM196804112781503. [DOI] [PubMed] [Google Scholar]
- 156.Owen C.A., Bowie E.W. Chronic intravascular coagulation and fibrinolysis (ICF) syndromes (DIC) Semin. Thromb. Hemost. 1977;3:268–290. doi: 10.1055/s-0028-1087121. [DOI] [Google Scholar]
- 157.Quintero-Martinez J.A., Hindy J.-R., El Zein S., Michelena H.I., Nkomo V.T., DeSimone D.C., Baddour L.M. Contemporary demographics, diagnostics and outcomes in non-bacterial thrombotic endocarditis. Heart. 2022;108 doi: 10.1136/heartjnl-2022-320970. [DOI] [PubMed] [Google Scholar]
- 158.Marin G., Margineanu C., Penes D., Maresiu C., Boiangiu S., Stoica E., Radu R., Antohi E., Chioncel O. P1243 Nonbacterial thrombotic endocarditis (NBTE) of the aortic valve: Incidental finding in hospitalized patients with advanced heart failure. Eur. Heart J.-Cardiovasc. Imaging. 2020;21:jez319.697. doi: 10.1093/ehjci/jez319.697. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.