Abstract
Background
There are few studies on the time to return to activities of daily living (ADL) after craniotomy in patients with brain tumors. This study aimed to investigate the duration before returning to ADLs after craniotomy for brain tumors and present data that can provide information and guidelines on the appropriate time needed.
Methods
Patients (n = 183 of 234) who underwent craniotomy for brain tumors between April 2021 and July 2021 capable of self-care upon discharge were enrolled, and data of 158 were collected. The start time of 85 ADL items was prospectively investigated for 4 months postoperatively, using the self-recording sheet.
Results
Over 89% and 87% of the patients performed basic ADL items within a month and instrumental ADL items within 2 months (medians: within 18 days), except for a few. Regarding work, 50% of the patients returned within 4 months. Washing hair with a wound was performed at 18 days of median value, after 4 months of dyeing/perming hair, 6 days of drinking coffee/tea, after 4 months of air travel, and 40 days of complementary and alternative medicine. In patients with infratentorial tumors or surgical problems, return times were much later for various items.
Conclusions
It is possible to provide practical information and guidelines on the duration to return to ADL after craniotomy in brain tumor patients. These study findings also reduce uncertainty about recovery and daily life and help patients return to their daily life at the appropriate time, thereby maintaining function and daily well-being after surgery.
Keywords: Activity of daily living, Brain tumor, Craniotomy, Daily life, Time to return
Introduction
Primary brain tumor incidence in Korea increased from 11.7 per 100,000 in 2005 to 23.4 in 2013 [3]. Recently, the 5-year survival rate of brain tumors was 41.5% [7], and the survival rate for benign brain tumors after 15 years was over 80% [14]; therefore, it is necessary to focus on patients returning home after treatment. Being diagnosed with a brain tumor is the biggest event of a lifetime, and given the risk associated with brain tumors, it is natural for doctors and patients to focus on treatment in the early stages, emphasizing the choice of treatment method and risks [15]. However, despite the focus being on treatment methods, such as surgery, due to the diagnosis severity and urgency, the information the patient needs is not limited to treatment [15]; therefore, maintaining the patient’s function and daily well-being is as important as extending the survival period [4].
Many patients with brain tumors contemplate the duration before they return to their daily life postoperatively or when they will functionally recover to pre-operative levels. However, there is insufficient information about recovery and daily activity resumption postoperatively, and the patient’s symptoms and the time required for recovery postoperatively differ from the information provided by the medical staff [15]. Reducing disease-related uncertainty in patients with brain tumors improves symptoms and quality of life [8]; however, due to the heterogeneity in diverse and complex brain tumor diagnoses and treatment processes, it is challenging to provide guidelines for the time to return to daily activities which apply to all patients, and evidence is lacking.
Considering studies conducted so far on time to return to activities of daily living (ADL) postoperatively in patients with brain tumors, work contents were most accounted for among various ADL items [5, 10, 17, 21, 23], and there was a study on shampoo timing [12]. Besides that, most studies compared daily activities level or function pre- and postoperatively [19, 20] or grasped the postoperative status of single ADL items, such as complementary and alternative medicine (CAM), sexuality, and air travel [6, 13, 18, 22].
As mentioned above, there are few studies on the time required to return to comprehensive and specific ADL postoperatively in patients with brain tumors. Therefore, it is necessary to prepare a guideline for patients with brain tumors to help them resolve the uncertainties concerning their recovery processes and return to their daily activities in the safest and fastest time (from their point of view) postoperatively. Therefore, in this study, we presented data that can provide information and guidelines on the appropriate return time by investigating the time to return to ADL postoperatively in patients who underwent brain tumor craniotomy.
Methods
Study design and population
A prospective study was conducted to determine the time of return to ADL after craniotomy in patients with brain tumors. The Institutional Review Board approved this study, and patient consent was obtained (study number: 2021–03-113). The inclusion criteria were as follows: (a) age ≥ 18 years; (b) histologically confirmed primary or metastatic brain tumor; (c) patients who underwent tumor removal via craniotomy; (d) Eastern Cooperative Oncology Group-Performance Status (ECOG-PS) ≤ 2 or Karnofsky Performance Status (KPS) ≥ 70 at discharge postoperatively; (e) patients without communication challenges, who understood the study purpose, and consented to participate; (f) performance status not deteriorating below the criterion during the study; (g) no re-craniotomy for tumor progression/recurrence or residual tumor within the study period.
Data collection methods
Demographic and clinical characteristics of the patients were obtained from the electronic medical record. A self-recording sheet was developed to measure the time needed for patients to return to ADL postoperatively, minimizing the recall period. The ADL items in the self-recording sheet reflected the list of basic and instrumental activities presented via clustering by Oort et al. [11] and the detailed list of activities provided by the websites of 27 hospitals or institutions. In addition, the items were confirmed through discussions among clinical field expertise to meet the patients’ information needs. It was designed to enter the time to return for each ADL item. The content validity of the developed self-recording sheet of the ADL items was evaluated by a group consisting of five experts: a neurosurgeon, two head nurses in the neuro ward, a coordinator, and a physician assistant. The scale-level content validity index, universal agreement method (S-CVI/UA), was 0.78, and the scale-level content validity index, averaging method (S-CVI/Ave), was 0.94. The item-level content validity index (I-CVI) was also 0.60–1.00.
ADLs were divided into 15 items of basic (BADL) and 31 instrumental ADL (IADL), and each item was presented in order of frequency. Four items each of BADL and IADL were composed of detailed activities and a total of 85 items (35 and 50 items, respectively). BADL included activities that enable basic survival and well-being, such as bathing, toilet use, dressing, and eating, whereas IADL involved more complex activities, such as financial management, chores, grocery shopping, phone calls, and medication use. The time to return to ADL was when these abilities were recovered postoperatively and ADL before diagnosis was possible, meaning the time elapsed after surgery. If it had not been performed preoperatively and there was no plan to perform it in the future, it was marked as “not applicable.”
The self-recording sheets where the patients directly filled out the time they returned to their ADL were collected between April and November 2021. We checked whether the patients had filled the execution time for each ADL item at discharge, 1, 2, 3, and 4 months postoperatively, and encouraged them to fill in any omissions. Copies of the sheets were kept at discharge and a month postoperatively (at the time of visit) to provide in case of loss, and the final sheets were collected 4 months postoperatively. When there was a scheduled hospital visit, the patient was contacted in advance and reminded to fill and bring a self-recording sheet.
Statistical analysis
Data were processed using SPSS version 25.0 (IBM Corporation, Armonk, NY, USA). Frequency and percentage or mean and standard deviation of demographic and clinical characteristics were obtained. The Kaplan–Meier survival analysis and percentile were performed to evaluate the time to return to ADL. Additionally, the number and percentage of each period were calculated to assess the ADL return rate postoperatively. The difference in the time to return to ADL according to the demographic and clinical characteristics was analyzed using the Mann–Whitney U test, Kruskal–Wallis test, independent samples t-test, ANOVA, Spearman’s correlation, and multivariable regression after normality test using the Kolmogorov–Smirnov test and Shapiro–Wilk test. A statistician guided the statistical analyses.
Results
Participant characteristics
Consecutive patients (n = 234) underwent craniotomy for brain tumors between April and July 2021 at the Samsung Medical Center in Seoul, Korea. Finally, 183 of the 234 patients were enrolled in the study, and 158 with complete data were included in the final analysis (Fig. 1). Table 1 presents the demographic and clinical characteristics of patients who underwent craniotomy for brain tumors. The mean age of the patients during surgery was 53.5 years (19–80 years), and there were 71 males (44.9%) and 87 females (55.1%). At the time before surgery, 10 patients (6.3%) were smoking and 31 patients (19.6%) were drinking alcohol. Nine (5.7%) of the patients have received medication at an outpatient clinic for psychological problems such as depression or panic disorder and 49 patients (31.0%) had religions such as Christianity or Buddhism. Of the patients, 78 (49.4%) were employed preoperatively, and 46 (29.1%) were homemakers or students. Histologically, meningioma was the most common in 64 patients (40.5%), followed by glioma in 36 (22.8%), and low and high grade were in 94 (59.5%) and 64 (40.5%) patients, respectively. Regarding the tumor location, 120 patients (75.9%) had tumors in the supratentorium and 38 (24.1%) in the infratentorium. Thirty patients (19.0%) had challenges such as facial palsy, hearing impairment, and swallowing difficulty postoperatively. Additionally, 35 (22.2%), 47 (29.8%), and 3 (1.9%) patients received postoperative radiation therapy, chemotherapy, and ventriculoperitoneal shunt (VPS), respectively. Eight patients (5.1%) took steroids intermittently or continuously during the postoperative follow-up period (regardless of immediately after surgery), and used dexamethasone 1–8 mg per day, mainly around 2 months. Sixty patients (38.0%) mostly continued to take antiepileptic drug during the postoperative follow-up period, and mainly used levetiracetam 1000 mg per day. Of the 52 patients (32.9%) who visited the hospital after discharge, about 1/3 were hospitalized within 1 month postoperatively, about 1/3 within 2 months, and the rest after 2 months, for an average of 6 days.
Fig. 1.
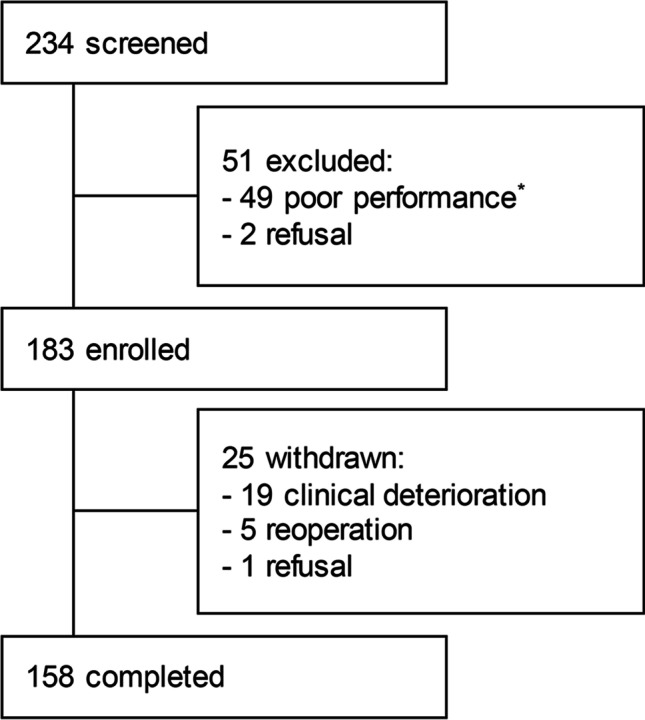
Study enrollment. Schematic overview of the screening, enrollment, and follow-up of the study participants. *Poor performance: Eastern Cooperative Oncology Group-Performance Status (ECOG-PS) > 2 or Karnofsky Performance Status (KPS) < 70
Table 1.
Demographic and clinical characteristics (N = 158)
| Variables n (%) / Mean ± SD | Variables n (%) / Mean ± SD | ||
|---|---|---|---|
| Demographic data | Clinical characteristics | ||
| Age (years) | Histological type | ||
| Mean ± SD | 53.5 ± 14.26 | Glioma | 36 (22.8) |
| 19–29 | 10 (6.3) | Meningioma | 64 (40.5) |
| 30–39 | 14 (8.9) | Schwannoma | 16 (10.1) |
| 40–49 | 34 (21.5) | Metastasis | 31 (19.6) |
| 50–59 | 41 (25.9) | Other | 11 (7.0) |
| 60–69 | 38 (24.1) | WHO grade | |
| 70–80 | 21 (13.3) | I | 79 (50.0) |
| Sex | II | 15 (9.5) | |
| Male | 71 (44.9) | III | 12 (7.6) |
| Female | 87 (55.1) | IV | 52 (32.9) |
| Smoking status | Hemispheric location (mainly) | ||
| Smoker | 10 (6.3) | Right | 79 (50.0) |
| Non-smoker | 148 (93.7) | Left | 71 (44.9) |
| Alcohol consumption | Bilateral or Midline | 8 (5.1) | |
| Drinker | 31 (19.6) | Location (mainly) | |
| Non-drinker | 127 (80.4) | Supratentorial | 120 (75.9) |
| Psychological challenges | Infratentorial | 38 (24.1) | |
| w/ a history of psychological problem | 9 (5.7) | Preexisting craniotomy | 20 (12.7) |
| w/o a history of psychological problem | 149 (94.3) | Surgical complications | 30 (19.0) |
| Occupation | Postoperative seizure | 8 (5.1) | |
| Employed | 78 (49.4) | Adjuvant treatment | |
| Homemaker | 42 (26.6) | Radiation therapy | 35 (22.2) |
| Student | 4 (2.5) | Chemotherapy | 47 (29.8) |
| Not employed | 34 (21.5) | Gamma knife radiosurgery | 28 (17.7) |
| Marital status | Ventriculoperitoneal shunt | 3 (1.9) | |
| Married | 128 (81.0) | Wound revision | 1 (0.6) |
| Not married | 27 (17.1) | None | 88 (55.7) |
| Unknown | 3 (1.9) | Postoperative steroid use | 8 (5.1) |
| Religion | Postoperative AED use | 60 (38.0) | |
| Religious | 49 (31.0) | Hospital revisit | 52 (32.9) |
| Non-religious | 83 (52.5) | Postoperative hospital stay (days) | 7.5 ± 3.21 |
| Unknown | 26 (16.5) | Postoperative ICU stay (days) | 1.2 ± 0.99 |
AED antiepileptic drug, ICU intensive care unit, WHO World Health Organization
*Surgical complications: facial palsy, hearing impairment, swallowing difficulty, and motor weakness
Time to return to ADL after surgery
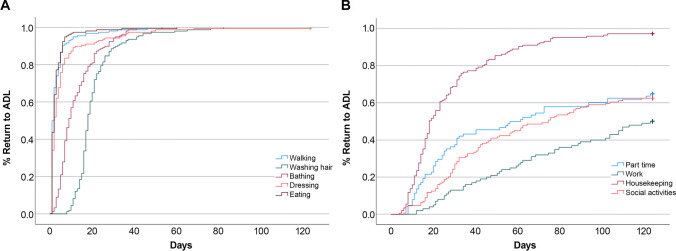
Regarding the BADL items, 90% of the patients required < 6 days to walk and have a meal, < 1 month to wash their hair with a wound and take a shower, and < 13 days to dress (Fig. 2 and Table 2). Among the representative BADL items, over 89% of the patients returned within a month, except for rubbing an incision when shampooing, lifting/carrying/moving objects, and sexual activity, and the median values were within 18 days. However, regarding rubbing an incision when shampooing, lifting/carrying/moving objects, and sexual activity, 3.2%, 1.9%, and 55.6% of the patients, respectively, did not return until 4 months postoperatively (Table 2).
Fig. 2.
Percentage of return to ADL after surgery. The graph illustrates the return rates according to the time elapsed after surgery for representative BADL (A) and IADL (B) items
Table 2.
Time to return to ADL postoperatively (N = 158)
| Activities | N/A (n, %) |
Percentile of time to return (days) | Postoperative period (n, %) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 25 | 50 | 75 | 90 | 100 | 1 m | 2 m | 3 m | 4 m | 4 m- | |||
| Basic ADL domains | ||||||||||||||
| Mobility | Walking |
0 (0.0) |
1 | 1 | 1 | 2 | 4 | 6 | 53 |
155 (98.1) |
3 (1.9) |
- | - | - |
| Bathing/washing | Washing hair with a wound |
1 (0.6) |
8 | 12 | 16 | 18 | 22 | 31 | 82 |
141 (89.8) |
13 (8.3) |
3 (1.9) |
- | - |
| Rubbing an incision when shampooing |
4 (2.5) |
8 | 16 | 18 | 25 | 41 | 62 | 123+ |
93 (60.4) |
46 (29.9) |
6 (3.9) |
4 (2.6) |
5 (3.2) |
|
| Dyeing/perming hair |
57 (36.1) |
5 | 76 | 113 | 123+ | 123+ | 123+ | 123+ |
3 (3.0) |
5 (4.9) |
7 (6.9) |
15 (14.9) |
71 (70.3) |
|
| Taking a shower |
0 (0.0) |
1 | 4 | 6 | 9 | 16 | 27 | 53 |
150 (94.9) |
8 (5.1) |
- | - | - | |
| Dressing |
0 (0.0) |
1 | 1 | 1 | 2 | 5 | 13 | 123+ |
149 (94.3) |
7 (4.4) |
1 (0.6) |
0 (0.0) |
1 (0.6) |
|
| Feeding/eating/drinking | Having a meal |
0 (0.0) |
1 | 1 | 1 | 2 | 3 | 6 | 60 |
156 (98.7) |
2 (1.3) |
- | - | - |
| Coffee/tea |
8 (5.1) |
1 | 2 | 3 | 6 | 15 | 38 | 123+ |
133 (88.7) |
10 (6.7) |
1 (0.7) |
2 (1.3) |
4 (2.7) |
|
| Climbing the stairs |
0 (0.0) |
1 | 5 | 7 | 11 | 21 | 33 | 123+ |
140 (88.6) |
12 (7.6) |
3 (1.9) |
1 (0.6) |
2 (1.3) |
|
| Toilet use |
0 (0.0) |
1 | 1 | 1 | 2 | 4 | 7 | 56 |
155 (98.1) |
3 (1.9) |
- | - | - | |
| General self-care |
0 (0.0) |
1 | 1 | 2 | 6 | 15 | 26 | 123+ |
147 (93.1) |
9 (5.7) |
1 (0.6) |
0 (0.0) |
1 (0.6) |
|
| Grooming |
0 (0.0) |
1 | 2 | 4 | 8 | 16 | 27 | 123+ |
144 (91.1) |
11 (7.0) |
2 (1.3) |
0 (0.0) |
1 (0.6) |
|
| Transferring (bed/chair/wheelchair/toilet/tub/shower/car) |
0 (0.0) |
1 | 1 | 1 | 3 | 6 | 14 | 56 |
154 (97.5) |
4 (2.5) |
- | - | - | |
| Lifting/carrying/moving objects over 2 kg |
1 (0.6) |
1 | 6 | 9 | 18 | 34 | 57 | 123+ |
115 (73.3) |
28 (17.8) |
9 (5.7) |
2 (1.3) |
3 (1.9) |
|
| Sexual activity |
50 (31.6) |
8 | 25 | 46 | 123+ | 123+ | 123+ | 123+ |
16 (14.8) |
18 (16.7) |
9 (8.3) |
5 (4.6) |
60 (55.6) |
|
| Passive mobility (sitting, standing, bending, etc.) |
0 (0.0) |
1 | 1 | 1 | 2 | 6 | 12 | 123+ |
153 (96.9) |
3 (1.9) |
1 (0.6) |
0 (0.0) |
1 (0.6) |
|
| Bladder management |
0 (0.0) |
0 | 1 | 1 | 2 | 4 | 6 | 27 |
158 (100.0) |
- | - | - | - | |
| Bowel management |
0 (0.0) |
0 | 2 | 3 | 4 | 6 | 8 | 31 |
158 (100.0) |
- | - | - | - | |
| Undertaking a single simple task |
0 (0.0) |
1 | 1 | 3 | 6 | 10 | 25 | 96 |
147 (93.1) |
10 (6.3) |
1 (0.6) |
- | - | |
| Instrumental ADL domains | ||||||||||||||
| Work (also studying/volunteering/homemaking) | Part time |
70 (44.3) |
8 | 11 | 20 | 59 | 123+ | 123+ | 123+ |
36 (40.9) |
10 (11.4) |
5 (5.7) |
6 (6.8) |
31 (35.2) |
| Work (8 h/day) |
58 (36.7) |
12 | 26 | 58 | 123+ | 123+ | 123+ | 123+ |
13 (13.0) |
16 (16.0) |
10 (10.0) |
11 (11.0) |
50 (50.0) |
|
| Non-physical work |
79 (50.0) |
8 | 13 | 23 | 51 | 123+ | 123+ | 123+ |
31 (39.3) |
15 (19.0) |
5 (6.3) |
5 (6.3) |
23 (29.1) |
|
| Physical work |
83 (52.5) |
8 | 11 | 21 | 57 | 123+ | 123+ | 123+ |
29 (38.7) |
12 (16.0) |
5 (6.7) |
2 (2.7) |
27 (36.0) |
|
| Chores |
13 (8.2) |
4 | 8 | 13 | 18 | 33 | 60 | 123+ |
105 (72.4) |
27 (18.6) |
7 (4.8) |
2 (1.4) |
4 (2.8) |
|
| Social activities |
14 (8.9) |
6 | 17 | 30 | 74 | 123+ | 123+ | 123+ |
41 (28.5) |
28 (19.4) |
15 (10.4) |
6 (4.2) |
54 (37.5) |
|
| Leisure time (hobby’s, sports, vacation) | Hobbies (non-sports) |
50 (31.6) |
2 | 8 | 13 | 27 | 78 | 123+ | 123+ |
61 (56.5) |
16 (14.8) |
6 (5.5) |
6 (5.5) |
19 (17.6) |
| Climbing mountains |
58 (36.7) |
5 | 26 | 52 | 123+ | 123+ | 123+ | 123+ |
16 (16.0) |
12 (12.0) |
11 (11.0) |
6 (6.0) |
55 (55.0) |
|
| Jogging/running |
85 (53.8) |
9 | 40 | 80 | 123+ | 123+ | 123+ | 123+ |
6 (8.2) |
10 (13.7) |
5 (6.8) |
5 (6.8) |
47 (64.4) |
|
| Vacation |
4 (2.5) |
7 | 41 | 75 | 123+ | 123+ | 123+ | 123+ |
9 (5.9) |
21 (13.6) |
29 (18.8) |
15 (9.7) |
80 (52.0) |
|
| Use of transport/travel around | Riding as a passenger in a vehicle/car |
0 (0.0) |
1 | 5 | 6 | 8 | 13 | 21 | 61 |
151 (95.6) |
7 (4.4) |
- | - | - |
| Air travel |
15 (9.5) |
8 | 123+ | 123+ | 123+ | 123+ | 123+ | 123+ |
1 (0.7) |
0 (0.0) |
2 (1.4) |
1 (0.7) |
139 (97.2) |
|
| Communicating/expressing |
0 (0.0) |
0 | 1 | 1 | 2 | 4 | 8 | 31 |
158 (100) |
- | - | - | - | |
| Shopping (grocery, clothing or other products) |
1 (0.6) |
1 | 3 | 5 | 11 | 26 | 69 | 123+ |
127 (80.9) |
11 (7.0) |
10 (6.4) |
2 (1.3) |
7 (4.4) |
|
| Finances and administration (handling money, filling in forms) |
10 (6.3) |
2 | 6 | 10 | 17 | 32 | 86 | 123+ |
109 (73.6) |
21 (14.2) |
4 (2.7) |
2 (1.4) |
12 (8.1) |
|
| Driving a car |
57 (36.1) |
7 | 13 | 20 | 61 | 123+ | 123+ | 123+ |
34 (33.7) |
18 (17.8) |
12 (11.9) |
7 (6.9) |
30 (29.7) |
|
| Modern appliances ((mobile) phone, computer, laptop, tablet, Satnav) |
3 (1.9) |
1 | 1 | 2 | 4 | 7 | 15 | 123+ |
146 (94.2) |
4 (2.6) |
1 (0.6) |
1 (0.6) |
3 (1.9) |
|
| Managing own medication | Taking medicine |
0 (0.0) |
0 | 1 | 1 | 2 | 5 | 8 | 37 |
155 (98.1) |
3 (1.9) |
- | - | - |
| CAM therapy |
7 (4.4) |
1 | 7 | 12 | 40 | 85 | 123+ | 123+ |
64 (42.4) |
35 (23.2) |
20 (13.2) |
6 (4.0) |
26 (17.2) |
|
| Able to live independently |
0 (0.0) |
1 | 6 | 9 | 21 | 54 | 123+ | 123+ |
101 (63.9) |
26 (16.5) |
7 (4.4) |
7 (4.4) |
17 (10.8) |
|
| Learning new things |
8 (5.1) |
1 | 11 | 26 | 123+ | 123+ | 123+ | 123+ |
41 (27.3) |
18 (12.0) |
9 (6.0) |
3 (2.0) |
79 (52.7) |
|
123+ after 123 days, ADL activities of daily living, CAM complementary and alternative medicine, N/A not applicable
Regarding the IADL items, approximately 2 months postoperatively, 50% of the patients returned to part-time work, and 25% returned to work 8 h/day. After approximately 1 month, 75% of the patients returned to chores, and 25% returned to social activities (Fig. 2 and Table 2). Among the representative IADL items, over 69% of the patients returned within a month, and over 87% returned within 2 months except for work, social activities, leisure, driving a car, living independently, and learning new things (median: within 18 days). In the case of working 8 h/day, 50.0% of the patients returned within 4 months. Regarding the items of part-time/full-time work (8 h/day), social activities, and leisure, 35.2%/50.0%, 37.5%, and 17.6–100.0% of the patients, respectively, did not return until 4 months postoperatively. Also, regarding driving a car, living independently, and learning new things, 29.7%, 10.8%, and 52.7% of patients, respectively, did not return until 4 months postoperatively. In addition, dyeing/perming hair was performed after a median of 4 months, 6 days of drinking coffee/tea, after 4 months of air travel, and 40 days of CAM therapy (Table 2).
The difference in time to return to ADL based on participant characteristics
Considering the differences in the time to return to ADL postoperatively based on the demographic and clinical characteristics of patients who underwent craniotomy for brain tumors (Tables 3 and 4), patients with supratentorial tumors were faster than those with infratentorial tumors in taking a shower (p = 0.004); however, they were slower in jogging/running (p = 0.029). Patients with surgical complications were slower than those without in having a meal (p = 0.019), general self-care (p = 0.005), grooming (p = 0.024), passive mobility (p = 0.003), chores (p = 0.014), and climbing mountains (p = 0.004).
Table 3.
Difference in time to return to basic ADL based on participant characteristics (including significant variables in univariable analysis)
| Variables | Median (Q1, Q3)/Mean ± SD/r | p-value | Variables | Median (Q1, Q3)/Mean ± SD/r | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | ||||||
| Basic ADL domains | VPS/wound revision | Yes | 11.0 (4.0, 16.5) | 0.019 | 0.057 | ||||
| Walking | No | 2.0 (1.0, 4.0) | |||||||
| Age | − 0.188 | 0.018 | 0.562 | General self-care | |||||
| Location II | Supratentorial | 1.0 (1.0, 2.0) | 0.001 | 0.932 | Location I | Right | 7.0 (2.0, 16.0) | 0.033 | 0.025* |
| Infratentorial | 3.0 (1.0, 5.0) | Left | 6.0 (3.0, 15.5) | ||||||
| Surgical complications | Yes | 4.5 (1.0, 6.0) | < 0.001 | 0.288 | Midline/bilateral | 2.5 (1.0, 3.5) | |||
| No | 1.0 (1.0, 3.0) | Location II | Supratentorial | 5.0 (2.0, 14.0) | 0.018 | 0.412 | |||
| VPS/wound revision | Yes | 11.5 (4.0, 17.0) | 0.009 | 0.056 | Infratentorial | 9.5 (5.0, 17.0) | |||
| No | 1.0 (1.0, 3.0) | Surgical complications | Yes | 13.0 (7.0, 20.0) | 0.002 | 0.005* | |||
| Washing hair with a wound | No | 5.0 (2.0, 13.0) | |||||||
| Preexisting craniotomy | Yes | 21.5 (17.5, 29.5) | 0.006 | 0.002* | Grooming | ||||
| No | 18.0 (16.0, 21.0) | Sex | Male | 5.0 (3.0, 10.0) | < 0.001 | 0.031* | |||
| VPS/wound revision | Yes | 31.0 (25.0, 42.0) | 0.008 | 0.010* | Female | 11.0 (5.0, 18.5) | |||
| No | 18.0 (16.0, 22.0) | Occupation | Yes | 9.0 (5.0, 16.5) | 0.008 | 0.459 | |||
| Taking a shower | No | 5.0 (3.0, 10.0) | |||||||
| Sex | Male | 11.0 (7.0, 17.5) | 0.003 | 0.111 | Tumor type II | Benign | 9.5 (4.0, 18.0) | 0.033 | 0.851 |
| Female | 8.0 (5.0, 13.5) | Malignant | 5.0 (3.0, 11.0) | ||||||
| Location II | Supratentorial | 8.0 (5.0, 15.0) | 0.002 | 0.004* | Location II | Supratentorial | 7.0 (3.5, 14.0) | 0.030 | 0.705 |
| Infratentorial | 12.0 (8.0, 21.0) | Infratentorial | 11.0 (5.0, 18.0) | ||||||
| Adjuvant treatment | Yes | 10.5 (7.0, 21.0) | 0.029 | 0.021* | Surgical complications | Yes | 14.0 (7.0, 19.0) | 0.005 | 0.024* |
| No | 8.0 (5.0, 14.0) | No | 6.5 (4.0, 14.0) | ||||||
| Dressing | Transferring (bed/chair/wheelchair/toilet/tub/shower/car) | ||||||||
| Age | − 0.208 | 0.009 | 0.450 | Location II | Supratentorial | 2.0 (1.0, 5.0) | 0.007 | 0.664 | |
| Location II | Supratentorial | 2.0 (1.0, 5.0) | 0.002 | 0.525 | Infratentorial | 5.0 (3.0, 6.0) | |||
| Infratentorial | 4.5 (2.0, 7.0) | Surgical complications | Yes | 6.0 (3.0, 10.0) | 0.001 | 0.125 | |||
| Surgical complications | Yes | 5.0 (3.0, 7.0) | 0.001 | 0.157 | No | 2.0 (1.0, 5.0) | |||
| No | 2.0 (1.0, 4.5) | Passive mobility (sitting/standing/bending etc.) | |||||||
| VPS/wound revision | Yes | 11.5 (4.0, 42.5) | 0.043 | 0.001* | Age | 0.196 | 0.014 | 0.483 | |
| No | 2.0 (1.0, 5.0) | Location II | Supratentorial | 2.0 (1.0, 5.0) | 0.001 | 0.560 | |||
| Having a meal | Infratentorial | 5.5 (3.0, 10.0) | |||||||
| Age | − 0.338 | < 0.001 | 0.470 | Surgical complications | Yes | 6.5 (3.0, 11.0) | < 0.001 | 0.003* | |
| Occupation | Yes | 2.0 (1.0, 4.0) | 0.015 | 0.667 | No | 2.0 (1.0, 5.0) | |||
| No | 1.0 (1.0, 2.0) | Bladder management | |||||||
| Location II | Supratentorial | 1.0 (1.0, 3.0) | < 0.001 | 0.177 | Age | − 0.237 | 0.003 | 0.133 | |
| Infratentorial | 3.0 (2.0, 6.0) | Location II | Supratentorial | 2.0 (1.0, 2.0) | < 0.001 | 0.181 | |||
| Surgical complications | Yes | 3.5 (2.0, 6.0) | < 0.001 | 0.019* | Infratentorial | 4.0 (1.0, 6.0) | |||
| No | 2.0 (1.0, 3.0) | Surgical complications | Yes | 4.0 (1.0, 6.0) | 0.001 | 0.058 | |||
| VPS/wound revision | Yes | 11.5 (4.0, 19.5) | 0.010 | 0.016* | No | 2.0 (1.0, 2.5) | |||
| No | 2.0 (1.0, 3.0) | VPS/wound revision | Yes | 11.0 (4.0, 16.5) | 0.008 | < 0.001* | |||
| Climbing the stairs | No | 2.0 (1.0, 3.0) | |||||||
| Location II | Supratentorial | 10.0 (7.0, 19.0) | 0.049 | 0.914 | Bowel management | ||||
| Infratentorial | 14.0 (9.0, 24.0) | Location II | Supratentorial | 4.0 (3.0, 6.0) | 0.010 | 0.265 | |||
| Surgical complications | Yes | 16.0 (10.0, 29.0) | 0.008 | 0.087 | Infratentorial | 5.0 (4.0, 6.0) | |||
| No | 10.0 (7.0, 18.5) | VPS/wound revision | Yes | 10.5 (5.5, 16.0) | 0.017 | 0.004* | |||
| Toilet use | No | 4.0 (3.0, 6.0) | |||||||
| Age | − 0.233 | 0.003 | 0.748 | Undertaking a single simple task | |||||
| Location II | Supratentorial | 2.0 (1.0, 3.0) | < 0.001 | 0.897 | Location II | Supratentorial | 5.0 (2.0, 9.0) | 0.010 | 0.844 |
| Infratentorial | 4.0 (2.0, 6.0) | Infratentorial | 8.5 (5.0, 13.0) | ||||||
| Surgical complications | Yes | 4.5 (2.0, 6.0) | < 0.001 | 0.354 | Surgical complications | Yes | 8.5 (5.0, 17.0) | 0.030 | 0.745 |
| No | 2.0 (1.0, 3.0) | No | 5.0 (2.0, 9.0) | ||||||
ADL activities of daily living, VPS ventriculoperitoneal shunt; *p < 0.05
Table 4.
Difference in time to return to instrumental ADL based on participant characteristics (including significant variables in univariable analysis)
| Variables | Median (Q1, Q3)/Mean ± SD/r | p-value | Variables | Median (Q1, Q3)/Mean ± SD/r | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | ||||||
| Instrumental ADL domains | Location II | Supratentorial | 2.0 (1.0, 3.0) | 0.014 | 0.568 | ||||
| Non-physical work | Infratentorial | 2.5 (1.0, 6.0) | |||||||
| Preexisting craniotomy | Yes | 62.0 (52.0, 75.0) | 0.039 | Surgical complications | Yes | 4.5 (1.0, 6.0) | 0.013 | 0.051 | |
| No | 27.5 (18.0, 52.5) | No | 2.0 (1.0, 3.0) | ||||||
| Chores | Shopping | ||||||||
| Surgical complications | Yes | 35.5 (14.0, 56.5) | 0.014 | Age | 0.245 | 0.003 | |||
| No | 18.0 (12.0, 28.0) | Finances and administration | |||||||
| Climbing mountains | Age | 0.200 | 0.020 | 0.068 | |||||
| Surgical complications | Yes | 81.0 (76.5, 103.0) | 0.004 | Occupation | Yes | 15.0 (9.0, 24.5) | 0.049 | 0.029* | |
| No | 34.5 (26.0, 67.0) | No | 23.0 (12.0, 47.0) | ||||||
| Jogging/running | Modern appliances ((mobile) phone, computer, laptop etc.) | ||||||||
| Location II | Supratentorial | 64.33 ± 32.16 | 0.029 | Location II | Supratentorial | 3.0 (1.0, 6.0) | 0.008 | 0.505 | |
| Infratentorial | 40.38 ± 19.35 | Infratentorial | 5.0 (3.0, 11.0) | ||||||
| Vacation | Preexisting craniotomy | Yes | 5.0 (3.5, 9.0) | 0.044 | 0.143 | ||||
| Location I | Right | 62.59 ± 28.02 | 0.046 | No | 3.0 (1.0, 6.0) | ||||
| Left | 76.38 ± 30.37 | Surgical complications | Yes | 5.0 (3.0, 8.0) | 0.028 | 0.655 | |||
| Midline/bilateral | 41.67 ± 40.38 | No | 3.0 (1.0, 6.0) | ||||||
| Riding as a passenger in a vehicle/car | Adjuvant treatment | Yes | 5.0 (2.0, 7.0) | 0.030 | 0.060 | ||||
| Age | − 0.175 | 0.028 | 0.646 | No | 3.0 (1.0, 5.0) | ||||
| Surgical complications | Yes | 11.5 (7.0, 20.0) | 0.006 | 0.063 | Managing their medications | ||||
| No | 7.0 (6.0, 12.0) | Location II | Supratentorial | 2.0 (1.0, 4.0) | 0.006 | 0.912 | |||
| Communicating/self-expression | Infratentorial | 4.0 (2.0, 6.0) | |||||||
| Occupation | Yes | 2.0 (1.0, 4.0) | 0.028 | 0.899 | Surgical complications | Yes | 5.0 (1.0, 6.0) | 0.044 | 0.091 |
| No | 1.0 (1.0, 2.0) | No | 2.0 (1.0, 4.0) | ||||||
ADL activities of daily living; *p < 0.05
Among the BADL items, the timing of hair washing with a wound was later in patients with a previous craniotomy than in those without (p = 0.002) and in patients with VPS/wound revision than in those without (p = 0.010). Furthermore, the grooming time was earlier in men than in women (p = 0.031), and later in patients with surgical complications than those without (p = 0.024). Among the IADL items, the timing of non-physical work was later in patients with a previous craniotomy than in those without (p = 0.039), and the time taken for climbing mountains was later in patients with surgical complications than in those without (p = 0.004).
Discussion
This study attempted to present basic data to provide information and guidelines to medical staff and patients on the appropriate return period by prospectively investigating the time to return to ADL postoperatively in patients who underwent craniotomy for brain tumors.
Concerning time to return to BADL, over 89% of patients returned within a month for most items except for rubbing an incision when shampooing, lifting/carrying/moving objects, and sexual activity. The overall functional capacity of patients at discharge postoperatively was maintained in a relatively good condition compared to that preoperatively [19, 20]. Moreover, in this study, the performance status at discharge was good; nonetheless, it required a long time for most patients to return to BADL items. A previous study was conducted to shorten the hair-washing period of neurosurgical patients to 3 days postoperatively [22]; however, in this study, it took a median of 18 days for patients to wash their hair with a wound by applying usual shampoo, and 90% of them took up to a month. Besides, after 4 months, 3.2% of the patients did not rub an incision when shampooing. Regarding sexual activity, 55.6% of the patients did not return until 4 months postoperatively. Considering that sexual changes were observed in 53% of patients 3 years postoperatively for low-grade glioma [22], it is necessary to ensure that access to and management of sexual health is not neglected.
Regarding IADL, it took up to 2 months for over 87% of the patients to return to most items except for work, social activities, leisure, driving a car, living independently, and learning new things. Of the work items, an important patient-centered outcome parameter, 25% of patients returned within approximately 2 months and 50% within 4 months for 8-h work daily. In previous studies, based on follow-up for at least 9 months after glioma surgery, 18–97% of patients returned to work, and the higher the tumor’s malignancy, the lower the return rate to work [9, 10, 17, 21]. Furthermore, in the case of meningioma, 57% of the patients returned to work 2 years postoperatively [23]. Therefore, it is necessary to increase the follow-up period in the future. Regarding social activities, 25% of the patients returned after about a month, whereas 37.5% did not return within 4 months. Key themes of life disrupted, navigating the new reality of life, and social survivorship versus separation were derived from the metasynthesis of qualitative research on the effects of brain tumors on social networks [2]; therefore, various changes can occur in the social network after a brain tumor surgery. For leisure, 17.6–100% of the patients did not return until 4 months postoperatively. Among them, regarding mountain climbing, there was a difference in return time depending on a complication postoperatively. The time to return to sports differed by type; however, most required over 4 months to return. Considering the results of another study [16], where 50% of athletes with cranial lesions, including those without surgery, returned after 6 months, it is necessary to extend the follow-up period.
According to the results of this study, consistent with previous studies [1, 5, 17, 19–21, 23], the return period differed for each item depending on the patient. Particularly, there was a difference in the time to return postoperatively depending on factors such as the supra- or infratentorial tumor location and the presence or absence of surgical complications. Although the supra- or infratentorial tumor location became less significant after applying multivariable analysis, it was considered an important variable because it was significant for many items of ADL in univariable analysis, was related to both BADL and IADL, and was clinically significant factor. Analyzing these factors in depth makes it possible to suggest an appropriate return period. In addition, it will provide useful information about the possible return period to daily activities, including items that patients are curious about in the clinical field, such as driving a car, dyeing/perming hair, drinking coffee/tea, air travel, and CAM therapy.
This study had the following limitations. First, although performance status did not significantly deteriorate below the criterion during the study, return times may have been somewhat delayed because some patients who were in various statuses and displayed slow recovery and temporary worsening of their condition were included. Second, each patient had a different understanding of the item. Third, it seems that the measurements were more conservative than the actual possible return time because we asked patients to record the time when it was executed, not the time when it was judged that it could be done, and patients tend to hesitate or be hindered by caregivers. Fourth, responses may be limited due to the influence of time limit, seasonal factors, and the COVID-19 situation and may not have been completed, especially for items such as sports or air travel. This should be considered when interpreting the results, and it is recommended to extend the postoperative research period in the future. Considering the research period extension, it is necessary to improve the research method, such as reorganizing the tool and computerizing the data collection process. Finally, the participants in this study were recruited from a single medical institution in Korea; it is difficult to generalize the findings from this study results. Nevertheless, this study was relevant because it focused on the return times for comprehensive, specific, and practical ADL, used a validated tool, approached it from the patient’s point of view, and was conducted prospectively. This research activity was an initial attempt to understand the recovery process after craniotomy in patients with brain tumors. Therefore, the results of this study can be used as basic data to provide daily life management guidelines for patients with brain tumors after craniotomy.
Conclusion
Return time to daily life after craniotomy in brain tumor patients was measured for various ADL items, and the return time differed for each patient and varied based on several factors. Therefore, it is possible to provide practical information and guidelines regarding the time of ADL return after craniotomy in patients with brain tumors. It will also reduce uncertainty about recovery and daily life and help patients return to their daily life at the appropriate time, thereby maintaining function and well-being of daily life postoperatively. Furthermore, the results of this study are suggested to be used in developing interventions related to daily life recovery.
Author contribution
Conception or design of the work: Jeong-A Lee, Ae Ran Kim, Ho Jun Seol. Data acquisition: Jeong-A Lee, Eun-Young Tak, Yumin Kim, Hyun-ju Shin, Gyeong-won Mun, Sook-Jin Kim. Statistical analysis: Jeong-A Lee. Drafting the work: Jeong-A Lee. Revising the work for valuable intellectual content: Jeong-A Lee, Ae Ran Kim, Ho Jun Seol. Discussion of the results and final approval of the revision: Jeong-A Lee, Ae Ran Kim, Eun-Young Tak, Yumin Kim, Hyun-ju Shin, Gyeong-won Mun, Sook-Jin Kim, Ho Jun Seol.
Funding
This research was supported by grants from the department of nursing, Samsung Medical Center, Seoul, Republic of Korea in 2021.
Data availability
Data are available upon reasonable request.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Samsung Medical Center (study number: 2021–03-113) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Brain Tumors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jeong-A Lee and Ae Ran Kim contributed equally to this work.
References
- 1.Al-Shudifat AR, Kahlon B, Hoglund P, Soliman AY, Lindskog K, Siesjo P. Age, gender and tumour size predict work capacity after surgical treatment of vestibular schwannomas. J Neurol Neurosurg Psychiatry. 2014;85:106–111. doi: 10.1136/jnnp-2013-305168. [DOI] [PubMed] [Google Scholar]
- 2.Cubis L, Ownsworth T, Pinkham MB, Chambers S. The social trajectory of brain tumor: a qualitative metasynthesis. Disabil Rehabil. 2018;40:1857–1869. doi: 10.1080/09638288.2017.1315183. [DOI] [PubMed] [Google Scholar]
- 3.Dho YS, Jung KW, Ha J, Seo Y, Park CK, Won YJ, Yoo H. An updated nationwide epidemiology of primary brain tumors in Republic of Korea, 2013. Brain Tumor Res Treat. 2017;5:16–23. doi: 10.14791/btrt.2017.5.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efficace F, Taphoorn M. Methodological issues in designing and reporting health-related quality of life in cancer clinical trials: the challenge of brain cancer studies. J Neurooncol. 2012;108:221–226. doi: 10.1007/s11060-012-0819-2. [DOI] [PubMed] [Google Scholar]
- 5.Gzell C, Wheeler H, Guo L, Kastelan M, Back M. Employment following chemoradiotherapy in glioblastoma: a prospective case series. J Cancer Surviv. 2014;8:108–113. doi: 10.1007/s11764-013-0311-9. [DOI] [PubMed] [Google Scholar]
- 6.Heng S, Hughes B, Hibbert M, Khasraw M, Lwin Z. Traveling with cancer: a guide for oncologists in the modern world. J Glob Oncol. 2019;5:1–10. doi: 10.1200/JGO.19.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korea Central Cancer Registry, National Cancer Center (2021) Annual report of cancer statistics in Korea in 2019. Ministry of Health and Welfare. https://ncc.re.kr/cancerStatsList.ncc?searchKey=total&searchValue=&pageNum=1. Accessed 17 January 2022
- 8.Lin L, Chiang HH, Acquaye AA, Vera-Bolanos E, Gilbert MR, Armstrong TS. Uncertainty, mood states, and symptom distress in patients with primary brain tumors: analysis of a conceptual model using structural equation modeling. Cancer. 2013;119:2796–2806. doi: 10.1002/cncr.28121. [DOI] [PubMed] [Google Scholar]
- 9.Mandonnet E, De Witt Hamer P, Poisson I, Whittle I, Bernat AL, Bresson D, Madadaki C, Bouazza S, Ursu R, Carpentier AF, George B, Froelich S. Initial experience using awake surgery for glioma: oncological, functional, and employment outcomes in a consecutive series of 25 cases. Neurosurgery. 2015;76:382–389. doi: 10.1227/NEU.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 10.Ng S, Herbet G, Moritz-Gasser S, Duffau H. Return to work following surgery for incidental diffuse low-grade glioma: a prospective series with 74 patients. Neurosurgery. 2020;87:720–729. doi: 10.1093/neuros/nyz513. [DOI] [PubMed] [Google Scholar]
- 11.Oort Q, Taphoorn MJB, Sikkes SAM, Uitdehaag BMJ, Reijneveld JC, Dirven L. Evaluation of the content coverage of questionnaires containing basic and instrumental activities of daily living (ADL) used in adult patients with brain tumors. J Neurooncol. 2019;143:1–13. doi: 10.1007/s11060-019-03136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palese A, Moreale R, Noacco M, Pistrino F, Mastrolia I, Sartor A, Scarparo C, Skrap M. Post-operative shampoo effects in neurosurgical patients: a pilot experimental study. Surg Infect (Larchmt) 2015;16:133–138. doi: 10.1089/sur.2014.028. [DOI] [PubMed] [Google Scholar]
- 13.Phillips M, Saria M, Eisenberg A, Kelly DF, Barkhoudarian G. Safety of commercial airflight in patients with brain tumors: a case series. J Neurooncol. 2018;139:617–623. doi: 10.1007/s11060-018-2905-6. [DOI] [PubMed] [Google Scholar]
- 14.Porter KR, McCarthy BJ, Berbaum ML, Davis FG. Conditional survival of all primary brain tumor patients by age, behavior, and histology. Neuroepidemiology. 2011;36:230–239. doi: 10.1159/000327752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozmovits L, Khu KJ, Osman S, Gentili F, Guha A, Bernstein M. Information gaps for patients requiring craniotomy for benign brain lesion: a qualitative study. J Neurooncol. 2010;96:241–247. doi: 10.1007/s11060-009-9955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saigal R, Batjer HH, Ellenbogen RG, Berger MS. Return to play for neurosurgical patients. World Neurosurg. 2014;82:485–491. doi: 10.1016/j.wneu.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Senft C, Behrens M, Lortz I, Wenger K, Filipski K, Seifert V, Forster MT. The ability to return to work: a patient-centered outcome parameter following glioma surgery. J Neurooncol. 2020;149:403–411. doi: 10.1007/s11060-020-03609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin YS, Lee JA, Bae SH, Lee SY, Jang MK. Investigation into the use of complementary and alternative medicine and factors affecting use in Korean patients with brain tumors. J Korean Acad Fundam Nurs. 2013;20:147–156. doi: 10.7739/jkafn.2013.20.2.147. [DOI] [Google Scholar]
- 19.Slusarz R, Biercewicz M, Rosinczuk J, Lorencowicz R. A multicenter study on the early assessment of functional capacity of patients with brain tumor after surgery. J Neurosci Nurs. 2019;51:221–226. doi: 10.1097/JNN.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 20.Ślusarz R, Królikowska A, Jabłońska R, Haor B, Antczak-Komoterska A, Śniegocki M, Szewczyk MT. Evaluation of everyday activities in patients after brain tumor surgery. J Neurol Neurosurg Nurs. 2018;7:111–117. [Google Scholar]
- 21.Starnoni D, Berthiller J, Idriceanu TM, Meyronet D, d'Hombres A, Ducray F, Guyotat J. Returning to work after multimodal treatment in glioblastoma patients. Neurosurg Focus. 2018;44:E17. doi: 10.3171/2018.3.FOCUS1819. [DOI] [PubMed] [Google Scholar]
- 22.Surbeck W, Herbet G, Duffau H. Sexuality after surgery for diffuse low-grade glioma. Neuro Oncol. 2015;17:574–579. doi: 10.1093/neuonc/nou326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thurin E, Corell A, Gulati S, Smits A, Henriksson R, Bartek J, Jr, Salvesen O, Jakola AS. Return to work following meningioma surgery: a Swedish nationwide registry-based matched cohort study. Neurooncol Pract. 2020;7:320–328. doi: 10.1093/nop/npz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.