Abstract
Human mammaglobin-A (SCGB2A2) is a secretory protein with an unknown function that is used as a diagnostic marker for breast cancer. However, other tumors can also express mammaglobin-A. To comprehensively study patterns of mammaglobin-A expression, a tissue microarray containing 16,328 samples from 128 different tumor types as well as 608 samples of 76 different normal tissue types was analyzed using immunohistochemistry. Mammaglobin-A positivity was found in only a few normal tissues, including luminal cells of the breast as well as endocervical and endometrial glands. In tumor tissues, 37 of 128 tumor categories showed mamma-globin-A staining, 32 of which were derived from one of four organs: breast (6 tumor categories), endometrium (5 tumor categories), ovary (5 tumor categories), and salivary glands (16 tumor categories). Only five additional tumor types showed occasional weak mammaglobin positivity, including medullary thyroid cancer, teratoma of the testis, squamous cell carcinoma of the skin and pharynx, and prostatic adenocarcinoma. Among 1139 evaluable invasive breast carcinomas of no special type, low mammaglobin-A immunostaining was linked to high BRE grade (p = 0.0011), loss of estrogen and progesterone receptor expression (p < 0.0001 each), and triple-negative status (p < 0.0001) but not to patient survival. In endometrial cancer, mammaglobin-A loss was linked to an advanced tumor stage (p = 0.0198). Our data characterize mammaglobin-A as a highly specific marker for tumors derived from either the breast, female genitals, or salivary gland.
Keywords: mammaglobin-A, immunohistochemistry, tissue microarray, diagnostic marker, human cancers
1. Introduction
Human mammaglobin-A was first described in 1998 as one of several proteins that were differentially expressed between breast cancers and matched normal breast tissues [1]. Together with more than 20 related proteins, termed secretoglobins, mammaglobin-A belongs to the uteroglobin/Clara cell protein family [2,3,4]. The function of secretoglobins is not well understood. They seem to be involved in cell signaling, immune response, and chemotaxis and may also serve as transporters for steroid hormones in humans [5,6,7,8,9,10,11]. The latter may particularly apply to mammaglobin-A as it is capable of binding steroid-like molecules [12,13]. Mammaglobin-A is encoded by the secretoglobin family 2A member 2 (SCGB2A2) gene located on chromosome 11q12 and translates into a protein of 93 amino acids. In normal tissues, mammaglobin-A is expressed almost exclusively by the mammary gland [1]. Due to its frequent upregulation in breast cancers [14], mammaglobin-A has been proposed as a promising target for therapy in these tumors [15]. The impact of mammaglobin overexpression on breast cancer aggressiveness is disputed, with studies reporting a tumor promoting effect [16], tumor suppressive effect [17], or no effect [18].
Due to its preferential expression in breast epithelial cells, mammaglobin-A immunohistochemistry has become an established diagnostic tool for recognizing metastatic breast cancer tissue [19,20]. However, the accumulated data on the prevalence of mammaglobin-A expression in tumors are controversial. For breast cancer, reported mammaglobin-A positivity rates range from 59 to 100% in lobular breast carcinomas [21,22] and from 25 to 94% in invasive breast carcinomas of no special type [23,24]. Immunohistochemical mammaglobin-A positivity has also been found in 11–76% of endometrium carcinomas of the uterus [25,26], 37–100% of ovarian carcinomas [26,27], and in 0–36% of prostatic adenocarcinomas [14,28]. These conflicting data are likely due to the use of different antibodies, immunostaining protocols, and criteria for the categorization of mammaglobin-A staining results in these studies.
To better understand the prevalence and significance of mammaglobin-A expression in cancer, a comprehensive study analyzing large numbers of neoplastic and non-neoplastic tissues under highly standardized conditions is needed. Therefore, we studied mammaglobin-A expression in more than 16,000 tumor tissue samples from 128 different tumor types and subtypes as well as 76 non-neoplastic tissue categories using immunohistochemistry (IHC) in a tissue microarray (TMA) format.
2. Materials and Methods
2.1. Tissue Microarrays (TMAs)
Tissue microarrays composed of normal and tumorous tissues were employed for this study. The normal tissue TMA contained 8 samples from 8 different donors from each of 76 different normal tissue types. The tumor TMAs contained a total of 16,328 primary tumors from 128 tumor types and subtypes. Histopathological data, including grade and pT and pN status, were available from 524 ovarian cancers, 259 endometrium cancers, and 1475 breast cancers. The breast cancer dataset also included molecular information on ER, PR, and HER2 as well as follow-up information on a subset of 877 patients with a median follow-up time of 49 months (range 1–88). The composition of both normal and tumor TMAs is described in Section 3. All samples were from the archives of the Institutes of Pathology, University Hospital of Hamburg, Germany; the Institute of Pathology, Clinical Center Osnabrueck, Germany; and Department of Pathology, Academic Hospital Fuerth, Germany. Tissues were fixed in 4% buffered formalin and then embedded in paraffin. The TMA manufacturing process was described previously in detail [29,30]. A single tissue core measuring 0.6 mm diameter per tumor was used to manufacture the TMA. The use of archived remnants of diagnostic tissues for manufacturing of TMAs and their analysis for research purposes as well as patient data analysis was approved by local laws (HmbKHG, §12) and the local ethics committee (Ethics Commission Hamburg, WF-049/09). All work was carried out in compliance with the Helsinki Declaration.
2.2. Immunohistochemistry (IHC)
Freshly cut TMA sections were immunostained on one day and in one experiment. Two different primary antibodies were used for mammaglobin-A detection in normal tissues: MSVA-457R (rabbit recombinant, MS Validated Antibodies, Hamburg, Germany, #2668-457R-01) and clone 305-1A5 (mouse monoclonal, FLEX RTU; Agilent, Santa Clara, CA, USA, #GA074). For tumor tissue analysis, only one antibody (MSVA-475R) was used. For MSVA-457R, staining was performed manually. Slides were deparaffinized and exposed to heat-induced antigen retrieval for 5 min in an autoclave at 121 °C in pH 9 buffer. Primary antibody was applied at 37 °C for 60 min at a dilution of 1:150. Bound antibody was then visualized using the EnVision Flex kit (Agilent, Santa Clara, CA, USA, #52023) according to the manufacturer’s directions. For 305-1A5 (RTU), slides were stained in a DAKO Autostainer Link 48 after Flex-high (pH 9) antigen retrieval (#GV804) using a protocol recommended by Dako/Agilent. For tumor tissues, the percentage of positive neoplastic cells was estimated, and the staining intensity was semi-quantitatively recorded (0, 1+, 2+, and 3+). For statistical analyses, the staining results were categorized into four groups as described before [31]. Tumors without any staining were considered negative. Tumors with 1+ staining intensity in ≤70% of tumor cells or 2+ intensity in ≤30% of tumor cells were considered weakly positive. Tumors with 1+ staining intensity in >70% of tumor cells, 2+ intensity in 31–70%, or 3+ intensity in ≤30% of tumor cells were considered moderately positive. Tumors with 2+ intensity in >70% or 3+ intensity in >30% of tumor cells were considered strongly positive.
2.3. Statistics
Statistical calculations were performed using JMP 14 software (SAS Institute Inc., Cary, NC, USA). Contingency tables and the chi² test were performed to search for associations between mammaglobin-A and tumor phenotype. Survival curves were calculated according to Kaplan–Meier. The log-rank test was applied to detect significant differences between groups. A p value of ≤0.05 was considered statistically significant.
3. Results
3.1. Technical Issues
A total of 14,232 (87.2%) of 16,328 tumor samples were interpretable in our TMA analysis. The remaining 2096 (12.8%) samples were not interpretable due to the lack of unequivocal tumor cells or a lack of the entire tissue spot. On the normal tissue TMA, enough samples (≥4) were always analyzable per tissue type to determine mammaglobin-A staining patterns.
3.2. Mammaglobin-A in Normal Tissue
The normal tissues were analyzed using both antibodies. Using the antibody MSVA-457R, mammaglobin-A immunostaining was only seen in a few normal tissue types, including luminal cells of the breast (not in all cells and with variable intensity), endocervical glands (mostly intense but not all glands in all patients), endometrium (not in all cells and with variable intensity), scattered epithelial cells in the fallopian tube (moderate staining intensity), eccrine glands of the skin (weak to moderate), a few scattered cells in the salivary glands (weak to moderate), and in some principal and clear cells of the epididymis (weak to moderate). Mammaglobin staining was found to be most intense in endocervical and endometrial glands, where the staining often also involved stroma components. Such stroma staining may reflect a diffusion/contamination artifact caused by a “spill-over” of the highly abundant mammaglobin-A protein into adjacent structures, which is potentially facilitated by pre-fixation tissue damage. Examples of mammaglobin-A-positive normal tissues are shown in Figure 1. Mammaglobin-A staining was completely lacking in the muscle, myometrium, ovary, fat, non-keratinizing squamous epithelium of the lip, oral cavity, tonsil, ectocervix, esophagus, urothelium, transitional epithelium of the anal canal, decidua, placenta, lymph node, spleen, thymus, stomach, duodenum, ileum, appendix, colon, rectum, gall bladder, liver, Brunner gland, bronchial gland, kidney, prostate, seminal vesicle, testis, respiratory epithelium, lung, adrenal gland, parathyroid gland, brain, and pituitary gland. Using the antibody 305-1A5, all staining seen using MSVA-457R was confirmed except for the staining of specific cells of the epididymis (Supplementary Figure S1). At the selected conditions, the staining with 305-1A5 also resulted in strong staining of the thyroid (colloid staining), matrix proteins in the aortic wall, and the cytoplasm of intestinal epithelial cells and excretory ducts of salivary glands.
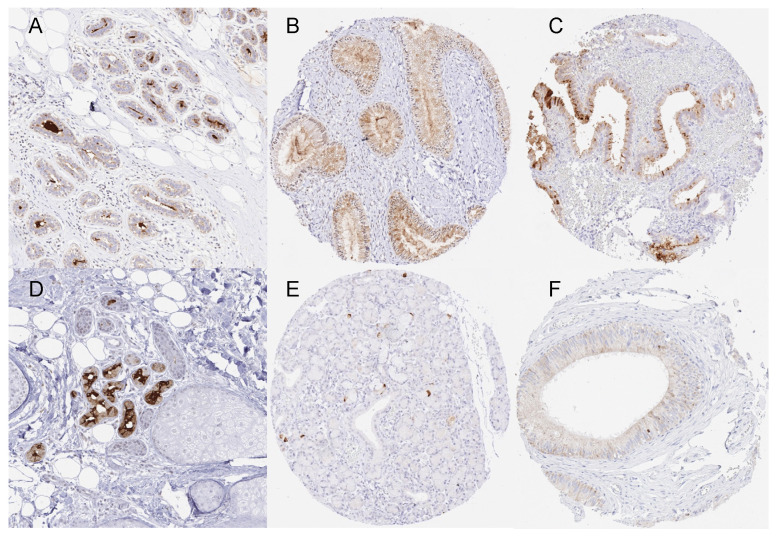
Figure 1.
Mammaglobin-A immunostaining of normal tissues. The panels show an apical membranous and cytoplasmic staining of variable intensities in luminal cells of the breast ((A), magnification from a TMA spot), endocervical glands (B), endometrial glands (C), eccrine glands of the skin ((D), magnification from a TMA spot), scattered epithelial cells of submandibular gland (E), and some chief cells in the corpus epididymis (F).
3.3. Mammaglobin-A in Neoplastic Tissues
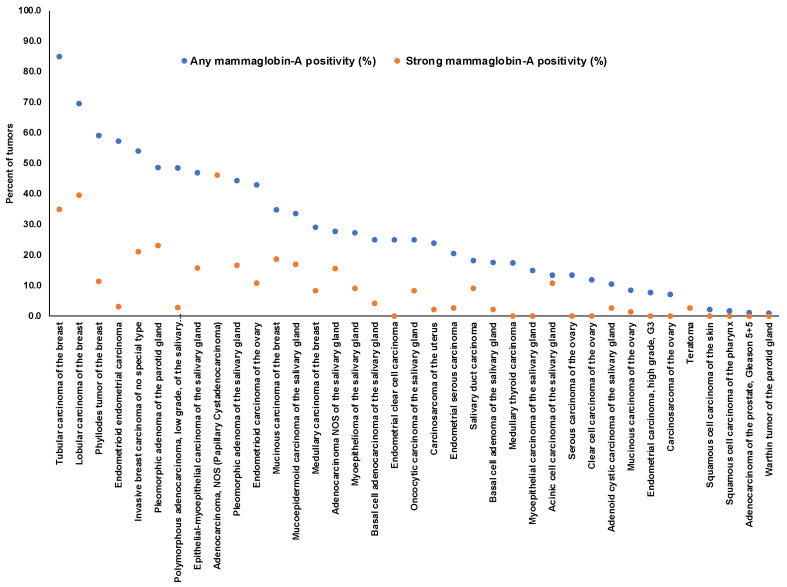
Positive mammaglobin-A immunostaining was detectable in 1450 (10.2%) of the 14,232 analyzable tumors, including 659 (4.6%) with weak, 275 (1.9%) with moderate, and 516 (3.6%) with strong immunostaining. Overall, 37 (28.9%) of 128 tumor categories showed detectable mammaglobin-A staining with 26 (20.3%) tumor categories showing, at least in one case, strong positivity (Table 1). A total of 1437 of 1450 (99%) of all mammaglobin-A-positive tumors were derived from four organs, including the salivary glands (16 tumor categories), breast (6 tumor categories), endometrium (5 tumor categories), and ovary (5 tumor categories). Only five additional tumor types showed occasional mammaglobin-A-positive cases. These tumor categories included medullary thyroid cancer, testicular teratoma, squamous cell carcinoma of the skin, squamous cell carcinoma of the pharynx, and prostatic adenocarcinoma (Gleason 5 + 5 = 10). In these tumors, mammaglobin-A positivity was mostly considered to be weak. Representative images of mammaglobin-A-positive tumors are shown in Figure 2. A graphical representation of a ranking order of mammaglobin-A-positive and strongly positive tumors is given in Figure 3.
Table 1.
Mammaglobin-A immunostaining in human tumors.
| Mammaglobin-A Immunostaining | |||||||
|---|---|---|---|---|---|---|---|
| Tumor Entity | on TMA (n) | Anal. (n) | Neg. (%) | Weak (%) | Mod. (%) | Str. (%) | |
| Tumors of the skin | Pilomatricoma | 35 | 32 | 100.0 | 0.0 | 0.0 | 0.0 |
| Basal cell carcinoma | 88 | 83 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Benign nevus | 29 | 29 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Squamous cell carcinoma of the skin | 90 | 90 | 97.8 | 2.2 | 0.0 | 0.0 | |
| Malignant melanoma | 48 | 45 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Merkel cell carcinoma | 46 | 41 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the head and neck | Squamous cell carcinoma of the larynx | 110 | 105 | 100.0 | 0.0 | 0.0 | 0.0 |
| Squamous cell carcinoma of the pharynx | 60 | 59 | 98.3 | 1.7 | 0.0 | 0.0 | |
| Oral squamous cell carcinoma (floor of the mouth) | 130 | 129 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Pleomorphic adenoma of the parotid gland | 50 | 39 | 51.3 | 10.3 | 15.4 | 23.1 | |
| Warthin tumor of the parotid gland | 104 | 98 | 99.0 | 0.0 | 1.0 | 0.0 | |
| Adenocarcinoma, NOS (papillary cystadenocarcinoma) | 14 | 13 | 53.8 | 0.0 | 0.0 | 46.2 | |
| Salivary duct carcinoma | 15 | 11 | 81.8 | 9.1 | 0.0 | 9.1 | |
| Acinic cell carcinoma of the salivary gland | 181 | 148 | 86.5 | 1.4 | 1.4 | 10.8 | |
| Adenocarcinoma NOS of the salivary gland | 109 | 83 | 72.3 | 6.0 | 6.0 | 15.7 | |
| Adenoid cystic carcinoma of the salivary gland | 180 | 114 | 89.5 | 7.9 | 0.0 | 2.6 | |
| Basal cell adenocarcinoma of the salivary gland | 25 | 24 | 75.0 | 20.8 | 0.0 | 4.2 | |
| Basal cell adenoma of the salivary gland | 101 | 91 | 82.4 | 9.9 | 5.5 | 2.2 | |
| Epithelial–myoepithelial carcinoma of the salivary gland | 53 | 51 | 52.9 | 23.5 | 7.8 | 15.7 | |
| Mucoepidermoid carcinoma of the salivary gland | 343 | 259 | 66.4 | 9.7 | 6.9 | 17.0 | |
| Myoepithelial carcinoma of the salivary gland | 21 | 20 | 85.0 | 5.0 | 10.0 | 0.0 | |
| Myoepithelioma of the salivary gland | 11 | 11 | 72.7 | 18.2 | 0.0 | 9.1 | |
| Oncocytic carcinoma of the salivary gland | 12 | 12 | 75.0 | 16.7 | 0.0 | 8.3 | |
| Polymorphous adenocarcinoma, low grade, of the salivary gland | 41 | 35 | 51.4 | 34.3 | 11.4 | 2.9 | |
| Pleomorphic adenoma of the salivary gland | 53 | 36 | 55.6 | 13.9 | 13.9 | 16.7 | |
| Tumors of the lung, pleura, and thymus | Adenocarcinoma of the lung | 246 | 176 | 100.0 | 0.0 | 0.0 | 0.0 |
| Squamous cell carcinoma of the lung | 130 | 69 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Small cell carcinoma of the lung | 20 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Mesothelioma, epithelioid | 39 | 28 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Mesothelioma, biphasic | 76 | 63 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Thymoma | 29 | 29 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the female genital tract | Squamous cell carcinoma of the vagina | 78 | 73 | 100.0 | 0.0 | 0.0 | 0.0 |
| Squamous cell carcinoma of the vulva | 130 | 124 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Squamous cell carcinoma of the cervix | 130 | 125 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Endometrioid endometrial carcinoma | 236 | 225 | 42.7 | 43.6 | 10.7 | 3.1 | |
| Endometrial serous carcinoma | 82 | 73 | 79.5 | 17.8 | 0.0 | 2.7 | |
| Carcinosarcoma of the uterus | 48 | 46 | 76.1 | 21.7 | 0.0 | 2.2 | |
| Endometrial carcinoma, high grade, G3 | 13 | 13 | 92.3 | 7.7 | 0.0 | 0.0 | |
| Endometrial clear cell carcinoma | 8 | 8 | 75.0 | 25.0 | 0.0 | 0.0 | |
| Endometrioid carcinoma of the ovary | 110 | 93 | 57.0 | 25.8 | 6.5 | 10.8 | |
| Serous carcinoma of the ovary, high grade | 559 | 479 | 86.6 | 13.2 | 0.2 | 0.0 | |
| Mucinous carcinoma of the ovary | 96 | 71 | 91.5 | 5.6 | 1.4 | 1.4 | |
| Clear cell carcinoma of the ovary | 50 | 42 | 88.1 | 11.9 | 0.0 | 0.0 | |
| Carcinosarcoma of the ovary | 47 | 42 | 92.9 | 4.8 | 2.4 | 0.0 | |
| Brenner tumor | 9 | 9 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the breast | Invasive breast carcinoma of no special type | 1391 | 1214 | 45.9 | 20.6 | 12.4 | 21.2 |
| Lobular carcinoma of the breast | 294 | 260 | 30.4 | 21.2 | 8.8 | 39.6 | |
| Medullary carcinoma of the breast | 26 | 24 | 70.8 | 12.5 | 8.3 | 8.3 | |
| Tubular carcinoma of the breast | 27 | 20 | 15.0 | 35.0 | 15.0 | 35.0 | |
| Mucinous carcinoma of the breast | 58 | 43 | 65.1 | 9.3 | 7.0 | 18.6 | |
| Phyllodes tumor of the breast | 50 | 44 | 40.9 | 34.1 | 13.6 | 11.4 | |
| Tumors of the digestive system | Adenomatous polyp, low-grade dysplasia | 50 | 50 | 100.0 | 0.0 | 0.0 | 0.0 |
| Adenomatous polyp, high-grade dysplasia | 50 | 49 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Adenocarcinoma of the colon | 1932 | 1808 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Gastric adenocarcinoma, diffuse type | 226 | 161 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Gastric adenocarcinoma, intestinal type | 224 | 167 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Gastric adenocarcinoma, mixed type | 62 | 56 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Adenocarcinoma of the esophagus | 133 | 82 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Squamous cell carcinoma of the esophagus | 124 | 71 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Squamous cell carcinoma of the anal canal | 91 | 85 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Cholangiocarcinoma | 50 | 49 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Hepatocellular carcinoma | 50 | 50 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Ductal adenocarcinoma of the pancreas | 662 | 593 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Pancreatic/ampullary adenocarcinoma | 119 | 86 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Acinar cell carcinoma of the pancreas | 14 | 13 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Gastrointestinal stromal tumor (GIST) | 50 | 49 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the urinary system | Non-invasive papillary urothelial carcinoma, pTa G2 low grade | 177 | 141 | 100.0 | 0.0 | 0.0 | 0.0 |
| Non-invasive papillary urothelial carcinoma, pTa G2 high grade | 141 | 117 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Non-invasive papillary urothelial carcinoma, pTa G3 | 187 | 113 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Urothelial carcinoma, pT2-4 G3 | 1207 | 825 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Small cell neuroendocrine carcinoma of the bladder | 18 | 18 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Sarcomatoid urothelial carcinoma | 25 | 21 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Clear cell renal cell carcinoma | 858 | 824 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Papillary renal cell carcinoma | 255 | 232 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Clear cell (tubulo) papillary renal cell carcinoma | 21 | 20 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Chromophobe renal cell carcinoma | 131 | 122 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Oncocytoma | 177 | 162 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the male genital organs | Adenocarcinoma of the prostate, Gleason 3 + 3 | 83 | 83 | 100.0 | 0.0 | 0.0 | 0.0 |
| Adenocarcinoma of the prostate, Gleason 4 + 4 | 80 | 80 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Adenocarcinoma of the prostate, Gleason 5 + 5 | 85 | 85 | 98.8 | 0.0 | 1.2 | 0.0 | |
| Adenocarcinoma of the prostate (recurrence) | 261 | 254 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Small cell neuroendocrine carcinoma of the prostate | 17 | 15 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Seminoma | 621 | 611 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Embryonal carcinoma of the testis | 50 | 44 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Yolk sac tumor | 50 | 43 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Teratoma | 50 | 38 | 97.4 | 0.0 | 0.0 | 2.6 | |
| Squamous cell carcinoma of the penis | 80 | 79 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of endocrine organs | Adenoma of the thyroid gland | 50 | 47 | 100.0 | 0.0 | 0.0 | 0.0 |
| Papillary thyroid carcinoma | 50 | 48 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Follicular thyroid carcinoma | 49 | 49 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Medullary thyroid carcinoma | 50 | 46 | 82.6 | 13.0 | 4.3 | 0.0 | |
| Anaplastic thyroid carcinoma | 26 | 24 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Adrenal cortical adenoma | 50 | 44 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Adrenal cortical carcinoma | 26 | 25 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Phaeochromocytoma | 50 | 48 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Pancreas, neuroendocrine tumor (NET) | 46 | 42 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Pancreas, neuroendocrine carcinoma (NEC) | 3 | 3 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of hematopoietic and lymphoid tissues | Hodgkin lymphoma | 103 | 100 | 100.0 | 0.0 | 0.0 | 0.0 |
| Non-Hodgkin lymphoma | 62 | 61 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Small lymphocytic lymphoma, B-cell type (B-SLL/B-CLL) | 50 | 50 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Diffuse large B-cell lymphoma (DLBCL) | 114 | 114 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Follicular lymphoma | 88 | 88 | 100.0 | 0.0 | 0.0 | 0.0 | |
| T-cell non-Hodgkin lymphoma | 24 | 24 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Mantle cell lymphoma | 18 | 18 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Marginal zone lymphoma | 16 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Diffuse large B-cell lymphoma (DLBCL) in the testis | 16 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Burkitt lymphoma | 5 | 3 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of soft tissue and bone | Tendosynovial giant cell tumor | 45 | 43 | 100.0 | 0.0 | 0.0 | 0.0 |
| Granular cell tumor | 53 | 43 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Leiomyoma | 50 | 47 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Leiomyosarcoma | 87 | 87 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Liposarcoma | 132 | 121 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Malignant peripheral nerve sheath tumor (MPNST) | 13 | 11 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Myofibrosarcoma | 26 | 26 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Angiosarcoma | 73 | 67 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Angiomyolipoma | 91 | 88 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Dermatofibrosarcoma protuberans | 21 | 18 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Ganglioneuroma | 14 | 14 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Kaposi sarcoma | 8 | 5 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Neurofibroma | 117 | 116 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Sarcoma, not otherwise specified (NOS) | 75 | 71 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Paraganglioma | 41 | 41 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Primitive neuroectodermal tumor (PNET) | 23 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Rhabdomyosarcoma | 7 | 7 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Schwannoma | 121 | 118 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Synovial sarcoma | 12 | 11 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Osteosarcoma | 43 | 36 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Chondrosarcoma | 38 | 17 | 100.0 | 0.0 | 0.0 | 0.0 | |
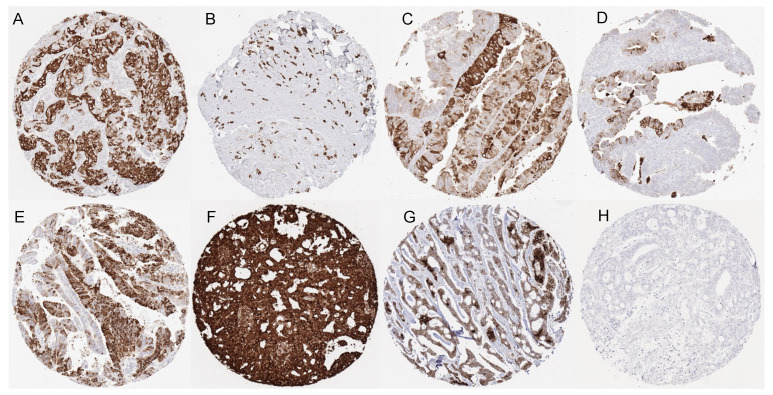
Figure 2.
Mammaglobin-A immunostaining in cancer. The panels show distinct, diffuse, or focal mammaglobin-A immunostaining of breast cancer of no special type (A), a lobular breast cancer (B), an endometrioid (C) and a high-grade serous carcinoma (D) of the ovary, an endometrioid endometrium carcinoma (E) as well as a mucoepidermoid (F) and an adenoid cystic (G) carcinoma of the salivary gland. Mammaglobin-A staining is absent in the colorectal adenocarcinoma (H).
Figure 3.
Ranking order of mammaglobin-A immunostaining in tumors. Both the frequency of positive cases (blue dots) and strongly positive cases (orange dots) are shown.
3.4. Mammaglobin-A Expression, Tumor Phenotype, and Prognosis
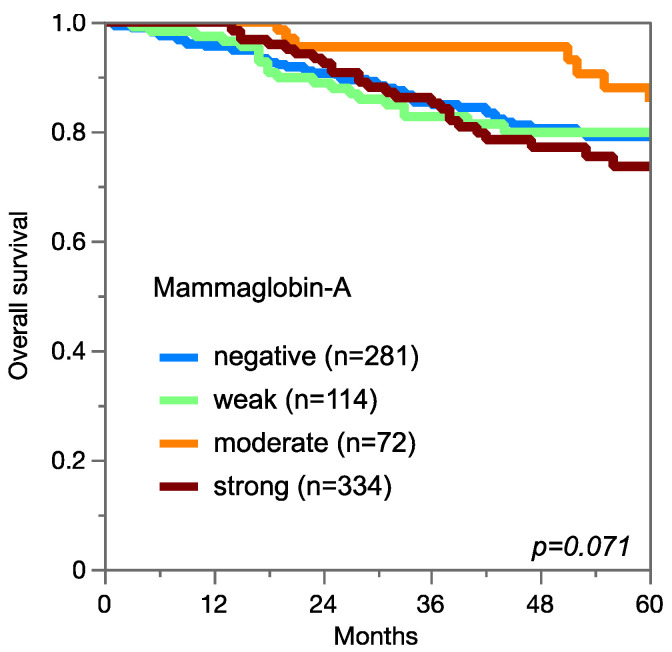
Among 1139 evaluable invasive breast carcinomas of NST, low or absent mammaglobin-A immunostaining was linked to a high BRE grade (p = 0.0011; Table 2), loss of estrogen receptor and progesterone receptor expression, and triple-negative status (p < 0.0001 each) but not to overall survival (Figure 4). Absent or low mammaglobin-A immunostaining was linked to an advanced tumor stage in endometroid endometrium carcinoma (p = 0.0198). Although a similar trend was seen for endometroid and serous high-grade carcinomas of the ovary, these associations did not reach statistical significance.
Table 2.
Mammaglobin-A immunostaining and tumor phenotypes in breast cancers of no special type, endometroid endometrium carcinoma, as well as endometroid and serous high-grade ovarian cancers.
| Mammaglobin-A IHC | ||||||||
|---|---|---|---|---|---|---|---|---|
| n | Negative (%) | Weak (%) | Moderate (%) | Strong (%) | p | |||
| Breast cancer of no special type (NST) | Tumor stage | pT1 | 601 | 44.3 | 21.5 | 14.1 | 20.1 | 0.1421 |
| pT2 | 411 | 46.5 | 18.5 | 11.7 | 23.4 | |||
| pT3–4 | 84 | 54.8 | 16.7 | 6.0 | 22.6 | |||
| Grade | G1 | 176 | 37.5 | 21.6 | 13.6 | 27.3 | 0.0011 | |
| G2 | 590 | 41.9 | 21.7 | 13.7 | 22.7 | |||
| G3 | 372 | 54.3 | 19.4 | 10.8 | 15.6 | |||
| Nodal stage | pN0 | 515 | 44.1 | 21.6 | 14.6 | 19.8 | 0.3017 | |
| pN1 | 223 | 44.8 | 19.7 | 13.0 | 22.4 | |||
| pN2 | 69 | 53.6 | 15.9 | 5.8 | 24.6 | |||
| pN3 | 56 | 50.0 | 14.3 | 8.9 | 26.8 | |||
| HER2 status | neg | 851 | 47.1 | 20.8 | 11.2 | 20.9 | 0.4501 | |
| pos | 118 | 43.2 | 22.0 | 16.1 | 18.6 | |||
| ER status | neg | 200 | 63.5 | 14.5 | 9.5 | 12.5 | <0.0001 | |
| pos | 717 | 41.6 | 23.0 | 12.8 | 22.6 | |||
| PR status | neg | 390 | 55.4 | 17.7 | 10.5 | 16.4 | <0.0001 | |
| pos | 568 | 40.0 | 23.2 | 13.2 | 23.6 | |||
| Triple negative | no | 756 | 41.7 | 22.6 | 13.4 | 22.4 | <0.0001 | |
| yes | 134 | 75.4 | 10.4 | 5.2 | 9.0 | |||
| Endometrioid endometrial cancers | Tumor stage | pT1 | 113 | 33.6 | 48.7 | 14.2 | 3.5 | 0.0198 |
| pT2 | 24 | 50.0 | 37.5 | 12.5 | 0.0 | |||
| pT3–4 | 35 | 48.6 | 51.4 | 0.0 | 0.0 | |||
| pN0 | 50 | 36.0 | 56.0 | 6.0 | 2.0 | 0.0986 | ||
| Nodal stage | pN+ | 30 | 63.3 | 33.3 | 3.3 | 0.0 | ||
| Endometrioid ovarian cancer | Tumor stage | pT1 | 22 | 40.9 | 31.8 | 9.1 | 18.2 | 0.1686 |
| pT2 | 4 | 100.0 | 0.0 | 0.0 | 0.0 | |||
| pT3 | 5 | 80.0 | 20.0 | 0.0 | 0.0 | |||
| Nodal stage | pN0 | 20 | 40.0 | 35.0 | 10.0 | 15.0 | 0.3835 | |
| pN1 | 7 | 71.4 | 14.3 | 0.0 | 14.3 | |||
| Serous ovarian cancers | Tumor stage | pT1 | 31 | 77.4 | 22.6 | 0.0 | 0.0 | 0.2 |
| pT2 | 40 | 92.5 | 7.5 | 0.0 | 0.0 | |||
| pT3 | 245 | 88.2 | 11.8 | 0.0 | 0.0 | |||
| Nodal stage | pN0 | 78 | 83.3 | 16.7 | 0.0 | 0.0 | 0.3767 | |
| pN1 | 153 | 87.6 | 12.4 | 0.0 | 0.0 | |||
Figure 4.
Mammaglobin-A immunostaining and overall survival in breast carcinoma of no special type.
4. Discussion
The data of this study identify mammaglobin-A as an oligospecific marker that is expressed in only a few non-vital normal tissues and corresponding tumors.
The International Working Group for Antibody Validation (IWGAV) had proposed that assay validation for immunohistochemistry on formalin fixed tissues should include either a comparison with expression data obtained by another independent method or a comparison with an independent second antibody [32]. Our normal tissue analysis revealed mammaglobin-A immunostaining in only seven organs, including all four organs (breast, uterine cervix, sebaceous glands (skin), and salivary glands) for which RNA expression data have been described in databases resulting from the Human Protein Atlas (HPA) RNA-seq tissue dataset [33], the FANTOM5 project [34,35], and the Genotype-Tissue Expression (GTEx) project [36]. The fact that mammaglobin-A RNA expression was not described for the endometrium, fallopian tube, and epididymis may potentially be due to the small fraction of the total cells of these organs expressing mammaglobin-A, which would result in a marked under-representation of these cells in RNA analyses. The confirmation of positive staining in the endometrium and fallopian tube using the independent mammaglobin-A antibody 305-1A5 identifies these organs as true mammaglobin-A expressors. The positive staining using MSVA-457R in the epididymis were not confirmed using 305-1A5 and may, therefore, reflect a specific cross-reactivity of this antibody. Positive staining in the aortic wall, small intestine, and thyroid were limited to 305-1A5 and may, therefore, constitute specific cross-reactivities of this antibody.
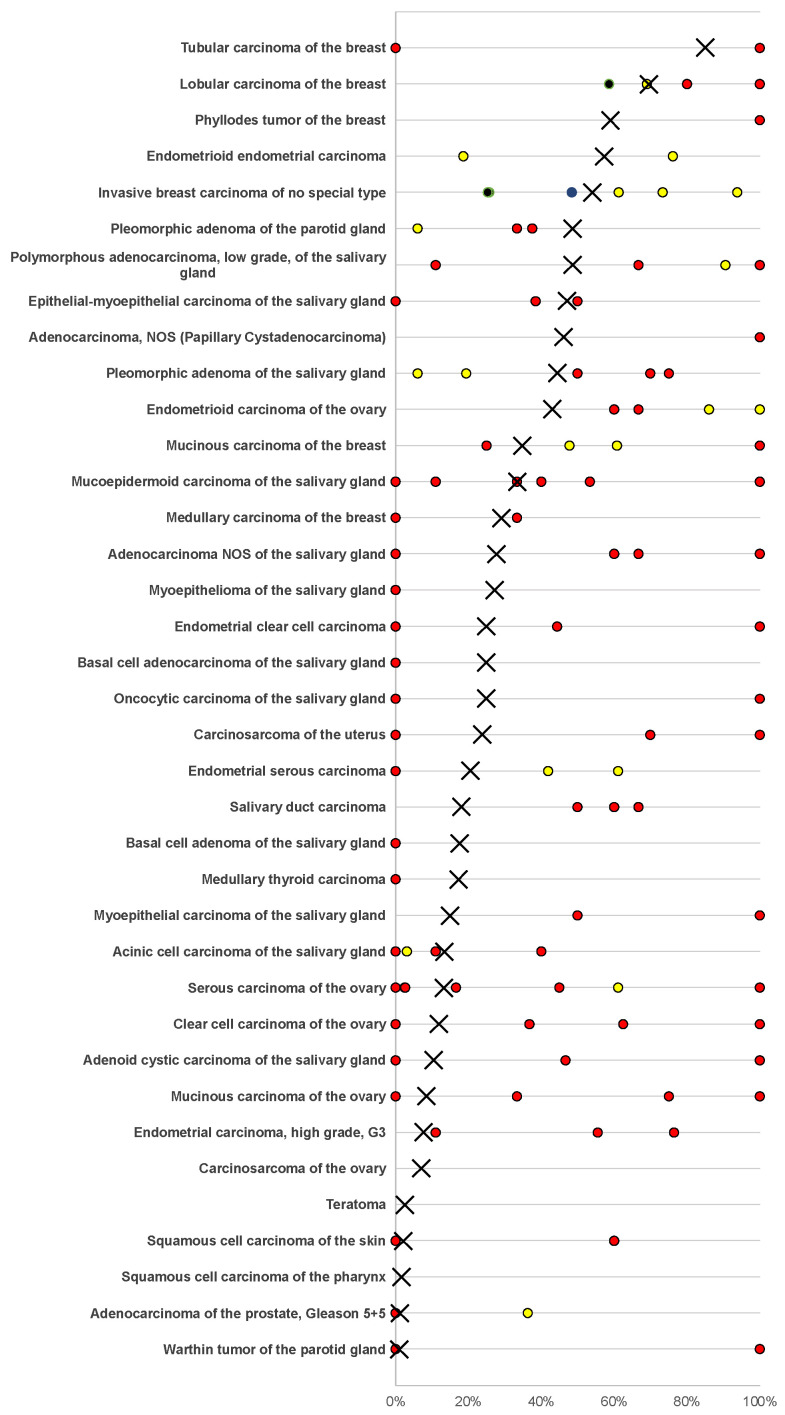
The standardized analysis of 14,232 tumors provided data that were largely reflective of the mammaglobin-A expression pattern in normal tissues. The fact that mammaglobin-A can be expressed in neoplasms derived from the breast [21], ovary [37], uterus [26], and salivary glands [38] was already known. More than 130 studies have analyzed mammaglobin-A expression in these tumors using immunohistochemistry (Figure 5). The diversity of the results of these studies mirrors the usual variability of IHC data that are a logical result of the use of different antibodies, staining protocols, and thresholds for defining “positive” cases [39]. A pivotal result of this study is a ranking order of human tumors according to the prevalence and intensity of mammaglobin-A expression. This enables an assessment of the relative importance of mammaglobin-A across tumor entities. Although the absolute positivity rates described in this study are specific to the reagents and the protocol used in our laboratory, a similar ranking order would be expected if other specific antibodies or different protocols were used. The complete absence of mammaglobin-A immunostaining in several important non-breast and non-gynecological cancer types that share histologic similarities with these tumors, such as adenocarcinomas from the gastrointestinal tract, pancreas, and lung, and cholangiocellular carcinomas, emphasizes the high diagnostic utility of mammaglobin-A immunohistochemistry if the clinical and morphological differential diagnosis includes gynecological tumors. It is also of note that mammaglobin-A immunostaining was not observed in any of 1235 urothelial tumors in this study. This emphasizes the utility of mammaglobin-A in combination with GATA3, a marker that primarily recognizes breast and urothelial neoplasms [40].
Figure 5.
Mammaglobin-A positivity in the literature. An “×” indicates the fraction of mammaglobin-A-positive tumors in the present study. Dots indicate the reported frequencies from the literature for comparison: red dots mark studies with ≤10 tumors, yellow dots mark studies with 11–25 tumors, and black dots mark studies with >25 tumors. All studies are quoted in a list of references in Supplementary Table S1.
At least 11 studies involving 30–1017 patients have earlier analyzed the prognostic relevance of mammaglobin-A expression in breast cancer. Among these, three found a poor prognosis of tumors with high mammaglobin-A expression [20,41,42], five reported a poor prognosis of tumors with low mammaglobin-A expression [25,43,44,45,46], and three did not find a link between mammaglobin expression and patient outcome [47,48,49]. The fact that we were unable to find a significant association between mammaglobin-A expression and patient outcome in 1139 cases of invasive breast cancer of homogeneous histologic subtype (all NST) might suggest that mammaglobin-A expression is not a critical prognostic feature in breast cancer. A complex role of mammaglobin-A in breast cancer is suggested by the documented occurrence of both increased and reduced or even lost mammaglobin-A expression in subsets of breast cancers. A tumor-relevant role for mammaglobin-A upregulation in neoplastic breast epithelium has earlier been proposed by functional studies showing that mammaglobin-A can both promote [16] as well as reduce [17] cell proliferation, migration, and invasion capacities of breast cancer cells. The functional mechanism(s) by which mammaglobin upregulation could influence cancer aggressiveness are not clear. Mammaglobin-A downregulation has been documented in 49% of breast cancers by Zafrakas et al. [14] comparing normal and tumor tissues, and our data shows the complete absence of mammaglobin-A staining in 46% of NST cancers, while normal breast epithelium usually showed detectable staining. A reduced expression of proteins that normally occur in the cells of tumor origin is a sign of tumor cell dedifferentiation, which is often related to unfavorable tumor features [50,51]. This concept may explain associations between reduced mammaglobin-A expression and high tumor grades or unfavorable molecular parameters in breast cancer and an advanced tumor stage in endometrium cancer. In our study, the best patient outcome was seen in breast cancer patients with a moderate mammaglobin-A staining of their tumors, while the prognosis was slightly worse in tumors with weak or negative staining and worst in tumors with strong staining. Based on these—statistically insignificant—data, it could be considered that moderate staining reflects the normal status and that both upregulation and downregulation could be linked to tumor progression.
The fact that mammaglobin-A expression was only found in non-vital tissues in our near complete normal tissue screening involving 76 different tissue categories identifies mammaglobin-A as a potentially attractive therapeutic target. Several studies have indeed evaluated the utility of mammaglobin-A as a drug target and investigated multiple therapeutic approaches. Two studies used adoptive CD8 cytotoxic T-cell transfer and engineered dendritic cells as proof of principle to induce immune responses against mammaglobin-A-positive breast cancer cells in mice [52] and cell cultures [53]. Other studies successfully generated mammaglobin-A-specific CD4 and CD8 T-cell cultures and identified candidate mammaglobin-A epitopes that could serve as antitumor vaccines [54,55,56,57]. One of them led to a clinical phase 1 trial where the safety of a mammaglobin-A DNA vaccine was demonstrated in patients with metastatic breast cancer [58]. At present (last updated January 2023), the authors are recruiting patients for a phase 1b trial (NCT02204098). Recently, novel mammaglobin-A epitopes were reported which could be employed for a specific mammaglobin-A-targeting nanoparticle-conjugated antibody therapy [15].
In summary, our data show that mammaglobin-A can be highly expressed in various tumors derived from the breast, ovary, uterus, and salivary glands. The fact that mammaglobin-A expression was only rarely found in tumors derived from other organs makes mammaglobin-A immunohistochemistry a useful tool to determine the origin of adenocarcinomas, especially in female patients.
Acknowledgments
We are grateful to Melanie Witt, Inge Brandt, Maren Eisenberg, Laura Behm, and Sünje Seekamp for their excellent technical assistance. The mammaglobin-A antibody clone MSVA-457R was provided from MS Validated Antibodies GmbH (owned by a family member of GS).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics13061202/s1. Figure S1: IHC validation by comparison of antibodies. The panels show a concordance of immunostaining results obtained by two independent mammaglobin-A antibodies. Using MSVA-457R, a membranous and cytoplasmic mammaglobin-A positivity was seen in a fraction of endometrium glands (A), epithelial cells of the fallopian tube (B), scattered epithelial cells of the submandibulary gland (C), and in a fraction of epithelial cells of the endocervix (D), while staining was absent in the thyroid (E), aortic wall (F), and small intestine (G). Using clone 305-1A5 (RTU), identical cell types were stained in the endometrium (I), fallopian tube (K), submandibulary gland (L), and the endocervix (M). Additional staining, only seen by clone 305-1A5, occurred in the thyroid (colloid; N), aortic wall (intercellular matrix; O), and the small intestine (cytoplasmic; P). Apical staining of chief cells of the epididymis was only seen using MSVA-457R (H) and not by clone 305-1A5 (Q). The images A–H and I–Q are from consecutive tissue sections. Table S1: List of studies used to generate Figure 5.
Author Contributions
N.G., D.H., C.B., R.S. and G.S. contributed to the conception, design, data collection, data analysis, and manuscript writing. P.T., D.D., C.V., A.M.L., C.F., R.U., S.S., A.M., M.L., F.B., A.H., C.B., E.B., A.H.M., T.K., R.S., S.M., T.S.C., F.J. and P.L. participated in the pathology data analysis, data interpretation, and collection of samples. R.S. and C.H.-M. participated in data analysis. G.S., R.S., D.H. and E.B. performed study supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The use of archived remnants of diagnostic tissues for the manufacturing of TMAs and their analysis for research purposes as well as patient data analysis has been approved by local laws (HmbKHG, §12) and the local ethics committee (Ethics Commission Hamburg, WF-049/09). All work was carried out in compliance with the Helsinki Declaration.
Informed Consent Statement
Patient consent was waived due to local laws (HmbKHG, §12 (2)) that permit research with anonymized diagnostic left-over tissue samples.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Patrick Timm was awarded a scholarship from the Hanns-Seidel-Stiftung.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Watson M.A., Darrow C., Zimonjic D.B., Popescu N.C., Fleming T.P. Structure and transcriptional regulation of the human mammaglobin gene, a breast cancer associated member of the uteroglobin gene family localized to chromosome 11q13. Oncogene. 1998;16:817–824. doi: 10.1038/sj.onc.1201597. [DOI] [PubMed] [Google Scholar]
- 2.Watson M.A., Watson M.A., Fleming T.P. Mammaglobin, a mammary-specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer. Res. 1996;56:860–865. [PubMed] [Google Scholar]
- 3.Ni J., Kalff-Suske M., Gentz R., Schageman J., Beato M., Klug J. All human genes of the uteroglobin family are localized on chromosome 11q12.2 and form a dense cluster. Ann. N. Y. Acad. Sci. 2000;923:25–42. doi: 10.1111/j.1749-6632.2000.tb05517.x. [DOI] [PubMed] [Google Scholar]
- 4.Becker R.M., Darrow C., Zimonjic D.B., Popescu N.C., Watson M.A., Fleming T.P. Identification of mammaglobin B, a novel member of the uteroglobin gene family. Genomics. 1998;54:70–78. doi: 10.1006/geno.1998.5539. [DOI] [PubMed] [Google Scholar]
- 5.Brown N.M., Stenzel T.T., Friedman P.N., Henslee J., Huper G., Marks J.R. Evaluation of expression based markers for the detection of breast cancer cells. Breast Cancer Res. Treat. 2006;97:41–47. doi: 10.1007/s10549-005-9085-8. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee A.B., Kundu G.C., Mantile-Selvaggi G., Yuan C.J., Mandal A.K., Chattopadhyay S., Zheng F., Pattabiraman N., Zhang Z. Uteroglobin: A novel cytokine? Cell Mol. Life Sci. 1999;55:771–787. doi: 10.1007/s000180050331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukherjee A.B., Kundu G.C., Mandal A.K., Pattabiraman N., Yuan C.J., Zhang Z. Uteroglobin: Physiological role in normal glomerular function uncovered by targeted disruption of the uteroglobin gene in mice. Am. J. Kidney. Dis. 1998;32:1106–1120. doi: 10.1016/S0272-6386(98)70093-9. [DOI] [PubMed] [Google Scholar]
- 8.Miele L. Antiflammins Bioactive peptides derived from uteroglobin. Ann. N. Y. Acad. Sci. 2000;923:128–140. doi: 10.1111/j.1749-6632.2000.tb05524.x. [DOI] [PubMed] [Google Scholar]
- 9.Miele L., Cordella-Miele E., Mukherjee A.B. Uteroglobin: Structure, molecular biology, and new perspectives on its function as a phospholipase A2 inhibitor. Endocr. Rev. 1987;8:474–490. doi: 10.1210/edrv-8-4-474. [DOI] [PubMed] [Google Scholar]
- 10.Vasanthakumar G., Manjunath R., Mukherjee A.B., Warabi H., Schiffmann E. Inhibition of phagocyte chemotaxis by uteroglobin, an inhibitor of blastocyst rejection. Biochem. Pharmacol. 1988;37:389–394. doi: 10.1016/0006-2952(88)90204-3. [DOI] [PubMed] [Google Scholar]
- 11.Levin S.W., Butler J.D., Schumacher U.K., Wightman P.D., Mukherjee A.B. Uteroglobin inhibits phospholipase A2 activity. Life Sci. 1986;38:1813–1819. doi: 10.1016/0024-3205(86)90135-9. [DOI] [PubMed] [Google Scholar]
- 12.Al Joudi F.S. Human mammaglobin in breast cancer: A brief review of its clinical utility. Indian J. Med. Res. 2014;139:675–685. [PMC free article] [PubMed] [Google Scholar]
- 13.Carter D., Douglass J.F., Cornellison C.D., Retter M.W., Johnson J.C., Bennington A.A., Fleming T.P., Reed S.G., Houghton R.L., Diamond D.L., et al. Purification and characterization of the mammaglobin/lipophilin B complex, a promising diagnostic marker for breast cancer. Biochemistry. 2002;41:6714–6722. doi: 10.1021/bi0159884. [DOI] [PubMed] [Google Scholar]
- 14.Zafrakas M., Petschke B., Donner A., Fritzsche F., Kristiansen G., Knuchel R., Dahl E. Expression analysis of mammaglobin A (SCGB2A2) and lipophilin B (SCGB1D2) in more than 300 human tumors and matching normal tissues reveals their co-expression in gynecologic malignancies. BMC Cancer. 2006;6:88. doi: 10.1186/1471-2407-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z., Yang X., Duan C., Li J., Tong R., Fan Y., Feng J., Cao R., Zhong W., Feng X., et al. Identification and characterization of mammaglobin-A epitope in heterogenous breast cancers for enhancing tumor-targeting therapy. Signal. Transduct. Target. 2020;5:82. doi: 10.1038/s41392-020-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picot N., Guerrette R., Beauregard A.P., Jean S., Michaud P., Harquail J., Benzina S., Robichaud G.A. Mammaglobin 1 promotes breast cancer malignancy and confers sensitivity to anticancer drugs. Mol. Carcinog. 2016;55:1150–1162. doi: 10.1002/mc.22358. [DOI] [PubMed] [Google Scholar]
- 17.Koh E.H., Cho Y.W., Mun Y.J., Ryu J.H., Kim E.J., Choi D.S., Maeng K.Y., Han J., Kang D. Upregulation of human mammaglobin reduces migration and invasion of breast cancer cells. Cancer. Investig. 2014;32:22–29. doi: 10.3109/07357907.2013.861473. [DOI] [PubMed] [Google Scholar]
- 18.Sjodin A., Ljuslinder I., Henriksson R., Hedman H. Mammaglobin and lipophilin B expression in breast tumors and their lack of effect on breast cancer cell proliferation. Anticancer Res. 2008;28:1493–1498. [PubMed] [Google Scholar]
- 19.De Lara S., Parris T.Z., Werner R.E., Helou K., Kovacs A. GATA3 as a putative marker of breast cancer metastasis-A retrospective immunohistochemical study. Breast J. 2018;24:184–188. doi: 10.1111/tbj.12863. [DOI] [PubMed] [Google Scholar]
- 20.Talaat I.M., Hachim M.Y., Hachim I.Y., Ibrahim R.A.E., Ahmed M., Tayel H.Y. Bone marrow mammaglobin-1 (SCGB2A2) immunohistochemistry expression as a breast cancer specific marker for early detection of bone marrow micrometastases. Sci. Rep. 2020;10:13061. doi: 10.1038/s41598-020-70012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaoxian T., Baohua Y., Xiaoli X., Yufan C., Xiaoyu T., Hongfen L., Rui B., Xiangjie S., Ruohong S., Wentao Y. Characterisation of GATA3 expression in invasive breast cancer: Differences in histological subtypes and immunohistochemically defined molecular subtypes. J. Clin. Pathol. 2017;70:926–934. doi: 10.1136/jclinpath-2016-204137. [DOI] [PubMed] [Google Scholar]
- 22.Dyhdalo K.S., Booth C.N., Brainard J.A., Croyle M.C., Kolosiwsky A.M., Goyal A., Gildea T.R., Almeida F.A., Nassar A., Reynolds J.P. Utility of GATA3, mammaglobin, GCDFP-15, and ER in the detection of intrathoracic metastatic breast carcinoma. J. Am. Soc. Cytopathol. 2015;4:218–224. doi: 10.1016/j.jasc.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Huo L., Zhang J., Gilcrease M.Z., Gong Y., Wu Y., Zhang H., Resetkova E., Hunt K.K., Deavers M.T. Gross cystic disease fluid protein-15 and mammaglobin A expression determined by immunohistochemistry is of limited utility in triple-negative breast cancer. Histopathology. 2013;62:267–274. doi: 10.1111/j.1365-2559.2012.04344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cimino P.J., Jr., Perrin R.J. Mammaglobin-A immunohistochemistry in primary central nervous system neoplasms and intracranial metastatic breast carcinoma. Appl. Immunohistochem. Mol. Morphol. 2014;22:442–448. doi: 10.1097/PAI.0b013e318294ca46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki E., Tsunoda N., Hatanaka Y., Mori N., Iwata H., Yatabe Y. Breast-specific expression of MGB1/mammaglobin: An examination of 480 tumors from various organs and clinicopathological analysis of MGB1-positive breast cancers. Mod. Pathol. 2007;20:208–214. doi: 10.1038/modpathol.3800731. [DOI] [PubMed] [Google Scholar]
- 26.Hagemann I.S., Pfeifer J.D., Cao D. Mammaglobin expression in gynecologic adenocarcinomas. Hum. Pathol. 2013;44:628–635. doi: 10.1016/j.humpath.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Bellone S., Tassi R., Betti M., English D., Cocco E., Gasparrini S., Bortolomai I., Black J.D., Todeschini P., Romani C., et al. Mammaglobin B (SCGB2A1) is a novel tumour antigen highly differentially expressed in all major histological types of ovarian cancer: Implications for ovarian cancer immunotherapy. Br. J. Cancer. 2013;109:462–471. doi: 10.1038/bjc.2013.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kandalaft P.L., Simon R.A., Isacson C., Gown A.M. Comparative Sensitivities and Specificities of Antibodies to Breast Markers GCDFP-15, Mammaglobin A, and Different Clones of Antibodies to GATA-3: A Study of 338 Tumors Using Whole Sections. Appl. Immunohistochem. Mol. Morphol. 2016;24:609–614. doi: 10.1097/PAI.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 29.Kononen J., Bubendorf L., Kallioniemi A., Barlund M., Schraml P., Leighton S., Torhorst J., Mihatsch M.J., Sauter G., Kallioniemi O.P. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat. Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 30.Mirlacher M., Simon R. Recipient block TMA technique. Methods Mol. Biol. 2010;664:37–44. doi: 10.1007/978-1-60761-806-5_4. [DOI] [PubMed] [Google Scholar]
- 31.Simon R., Mirlacher M., Sauter G. Immunohistochemical analysis of tissue microarrays. Methods Mol. Biol. 2010;664:113–126. doi: 10.1007/978-1-60761-806-5_12. [DOI] [PubMed] [Google Scholar]
- 32.Uhlen M., Bandrowski A., Carr S., Edwards A., Ellenberg J., Lundberg E., Rimm D.L., Rodriguez H., Hiltke T., Snyder M., et al. A proposal for validation of antibodies. Nat. Methods. 2016;13:823–827. doi: 10.1038/nmeth.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thul P.J., Akesson L., Wiking M., Mahdessian D., Geladaki A., Ait Blal H., Alm T., Asplund A., Bjork L., Breckels L.M., et al. A subcellular map of the human proteome. Science. 2017;356:6340. doi: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- 34.Lizio M., Harshbarger J., Shimoji H., Severin J., Kasukawa T., Sahin S., Abugessaisa I., Fukuda S., Hori F., Ishikawa-Kato S., et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome. Biol. 2015;16:22. doi: 10.1186/s13059-014-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lizio M., Abugessaisa I., Noguchi S., Kondo A., Hasegawa A., Hon C.C., de Hoon M., Severin J., Oki S., Hayashizaki Y., et al. Update of the FANTOM web resource: Expansion to provide additional transcriptome atlases. Nucleic. Acids Res. 2019;47:D752–D758. doi: 10.1093/nar/gky1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Consortium G.T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tassi R.A., Bignotti E., Falchetti M., Calza S., Ravaggi A., Rossi E., Martinelli F., Bandiera E., Pecorelli S., Santin A.D. Mammaglobin B expression in human endometrial cancer. Int. J. Gynecol. Cancer. 2008;18:1090–1096. doi: 10.1111/j.1525-1438.2007.01137.x. [DOI] [PubMed] [Google Scholar]
- 38.Soares C.D., de Lima Morais T.M., Carlos R., Martins M.D., de Almeida O.P., Mariano F.V., Altemani A. Immunohistochemical expression of mammaglobin in salivary duct carcinomas de novo and salivary duct carcinoma ex pleomorphic adenoma. Hum. Pathol. 2019;92:59–66. doi: 10.1016/j.humpath.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Sauter G., Simon R., Hillan K. Tissue microarrays in drug discovery. Nat. Rev. Drug Discov. 2003;2:962–972. doi: 10.1038/nrd1254. [DOI] [PubMed] [Google Scholar]
- 40.Miettinen M., McCue P.A., Sarlomo-Rikala M., Rys J., Czapiewski P., Wazny K., Langfort R., Waloszczyk P., Biernat W., Lasota J., et al. GATA3: A multispecific but potentially useful marker in surgical pathology: A systematic analysis of 2500 epithelial and nonepithelial tumors. Am. J. Surg. Pathol. 2014;38:13–22. doi: 10.1097/PAS.0b013e3182a0218f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C., Zhang T. Human mammaglobin: A specific marker for breast cancer prognosis. J. BUON. 2016;21:35–41. [PubMed] [Google Scholar]
- 42.Lee G.W., Kim J.Y., Koh E.H., Kang D., Choi D.S., Maeng K.Y., Lee J.S. Plasma human mammaglobin mRNA associated with poor outcome in patients with breast cancer. Genet. Mol. Res. 2012;11:4034–4042. doi: 10.4238/2012.November.28.2. [DOI] [PubMed] [Google Scholar]
- 43.Nunez-Villar M.J., Martinez-Arribas F., Pollan M., Lucas A.R., Sanchez J., Tejerina A., Schneider J. Elevated mammaglobin (h-MAM) expression in breast cancer is associated with clinical and biological features defining a less aggressive tumour phenotype. Breast Cancer Res. 2003;5:R65–R70. doi: 10.1186/bcr587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raica M., Cimpean A.M., Meche A., Alexa A., Suciu C., Muresan A. Analysis of the immunohistochemical expression of mammaglobin A in primary breast carcinoma and lymph node metastasis. Rom. J. Morphol. Embryol. 2009;50:341–347. [PubMed] [Google Scholar]
- 45.Span P.N., Waanders E., Manders P., Heuvel J.J., Foekens J.A., Watson M.A., Beex L.V., Sweep F.C. Mammaglobin is associated with low-grade, steroid receptor-positive breast tumors from postmenopausal patients, and has independent prognostic value for relapse-free survival time. J. Clin. Oncol. 2004;22:691–698. doi: 10.1200/JCO.2004.01.072. [DOI] [PubMed] [Google Scholar]
- 46.Guan X.F., Hamedani M.K., Adeyinka A., Walker C., Kemp A., Murphy L.C., Watson P.H., Leygue E. Relationship between mammaglobin expression and estrogen receptor status in breast tumors. Endocrine. 2003;21:245–250. doi: 10.1385/ENDO:21:3:245. [DOI] [PubMed] [Google Scholar]
- 47.Baker E., Whiteoak N., Hall L., France J., Wilson D., Bhaskar P. Mammaglobin-A, VEGFR3, and Ki67 in Human Breast Cancer Pathology and Five Year Survival. Breast Cancer Auckl. 2019;13:1178223419858957. doi: 10.1177/1178223419858957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni Y.B., Tsang J.Y., Chan S.K., Tse G.M. GATA-binding protein 3, gross cystic disease fluid protein-15 and mammaglobin have distinct prognostic implications in different invasive breast carcinoma subgroups. Histopathology. 2015;67:96–105. doi: 10.1111/his.12625. [DOI] [PubMed] [Google Scholar]
- 49.Fritzsche F.R., Thomas A., Winzer K.J., Beyer B., Dankof A., Bellach J., Dahl E., Dietel M., Kristiansen G. Co-expression and prognostic value of gross cystic disease fluid protein 15 and mammaglobin in primary breast cancer. Histol. Histopathol. 2007;22:1221–1230. doi: 10.14670/HH-22.1221. [DOI] [PubMed] [Google Scholar]
- 50.Friedmann-Morvinski D., Verma I.M. Dedifferentiation and reprogramming: Origins of cancer stem cells. EMBO Rep. 2014;15:244–253. doi: 10.1002/embr.201338254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jopling C., Boue S., Izpisua Belmonte J.C. Dedifferentiation, transdifferentiation and reprogramming: Three routes to regeneration. Nat. Rev. Mol. Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- 52.Lerret N.M., Rogozinska M., Jaramillo A., Marzo A.L. Adoptive transfer of Mammaglobin-A epitope specific CD8 T cells combined with a single low dose of total body irradiation eradicates breast tumors. PLoS ONE. 2012;7:e41240. doi: 10.1371/journal.pone.0041240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui H., Zhang W., Hu W., Liu K., Wang T., Ma N., Liu X., Liu Y., Jiang Y. Recombinant mammaglobin A adenovirus-infected dendritic cells induce mammaglobin A-specific CD8+ cytotoxic T lymphocytes against breast cancer cells in vitro. PLoS ONE. 2013;8:e63055. doi: 10.1371/journal.pone.0063055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viehl C.T., Frey D.M., Phommaly C., Chen T., Fleming T.P., Gillanders W.E., Eberlein T.J., Goedegebuure P.S. Generation of mammaglobin-A-specific CD4 T cells and identification of candidate CD4 epitopes for breast cancer vaccine strategies. Breast Cancer Res. Treat. 2008;109:305–314. doi: 10.1007/s10549-007-9657-x. [DOI] [PubMed] [Google Scholar]
- 55.Tiriveedhi V., Sarma N.J., Subramanian V., Fleming T.P., Gillanders W.E., Mohanakumar T. Identification of HLA-A24-restricted CD8(+) cytotoxic T-cell epitopes derived from mammaglobin-A, a human breast cancer-associated antigen. Hum. Immunol. 2012;73:11–16. doi: 10.1016/j.humimm.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ilias Basha H., Tiriveedhi V., Fleming T.P., Gillanders W.E., Mohanakumar T. Identification of immunodominant HLA-B7-restricted CD8+ cytotoxic T cell epitopes derived from mammaglobin-A expressed on human breast cancers. Breast Cancer Res. Treat. 2011;127:81–89. doi: 10.1007/s10549-010-0975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soysal S.D., Muenst S., Kan-Mitchell J., Huarte E., Zhang X., Wilkinson-Ryan I., Fleming T., Tiriveedhi V., Mohanakumar T., Li L., et al. Identification and translational validation of novel mammaglobin-A CD8 T cell epitopes. Breast Cancer Res. Treat. 2014;147:527–537. doi: 10.1007/s10549-014-3129-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim S.W., Goedegebuure P., Gillanders W.E. Mammaglobin-A is a target for breast cancer vaccination. Oncoimmunology. 2016;5:e1069940. doi: 10.1080/2162402X.2015.1069940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.