Abstract
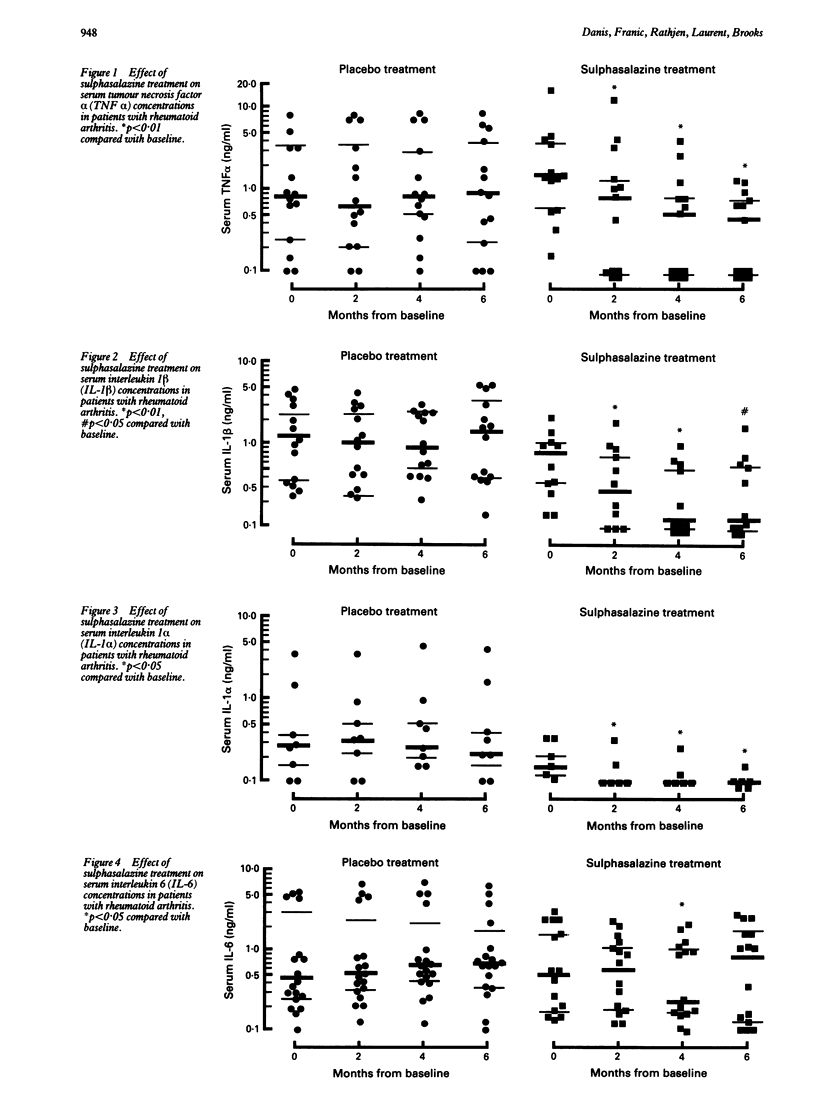
Interleukin 1 (IL-1), IL-6, and tumour necrosis factor (TNF) alpha are pleiotropic cytokines produced predominantly by macrophages which have been implicated in the pathogenesis of rheumatoid arthritis (RA). Sulphasalazine has been shown to have disease modifying properties and to inhibit the production of cytokines in vitro. To evaluate the effect of sulphasalazine on cytokine production in vivo, serum cytokine levels were measured in a group of patients with RA entered into a randomised controlled trial. Serum levels of IL-1 alpha, IL-1 beta, IL-6, and TNF alpha were measured at baseline and at two monthly intervals for six months in 17 patients receiving sulphasalazine and in 22 patients treated with placebo. The two groups of patients had a similar age and sex distribution, had had RA for less than a year, had no joint erosions, and had not been treated previously with any other disease modifying drugs. In the 39 patients studied IL-1 alpha was detected (> 0.1 ng/ml) at baseline in 14 patients (median 0.24 ng/ml), IL-1 beta in 25 patients (median 1.0 ng/ml), TNF alpha in 27 patients (median 1.2 ng/ml), and IL-6 in 33 patients (median 0.44 ng/ml). In the group treated with sulphasalazine there was a progressive and significant decline in serum IL-1 alpha, IL-1 beta, and TNF alpha levels over the six month period (median levels at six months were < 0.1, 0.12, and 0.44 ng/ml respectively). Interleukin 6 levels were significantly reduced only at the four month time point (median level of 0.23 ng/ml). These reductions were associated with improvements in clinical and laboratory measures of disease activity. In contrast patients receiving the placebo showed no changes in serum cytokine levels and no improvement in clinical and laboratory indices of disease activity. These results suggest that sulphasalazine may exert its disease modifying effect partly by suppressing cytokine production in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Dayer J. M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Bax D. E., Amos R. S. Sulphasalazine: a safe, effective agent for prolonged control of rheumatoid arthritis. A comparison with sodium aurothiomalate. Ann Rheum Dis. 1985 Mar;44(3):194–198. doi: 10.1136/ard.44.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borth W., Luger T. A. Identification of alpha 2-macroglobulin as a cytokine binding plasma protein. Binding of interleukin-1 beta to "F" alpha 2-macroglobulin. J Biol Chem. 1989 Apr 5;264(10):5818–5825. [PubMed] [Google Scholar]
- Borth W., Urbanski A., Prohaska R., Susanj M., Luger T. A. Binding of recombinant interleukin-1 beta to the third complement component and alpha 2-macroglobulin after activation of serum by immune complexes. Blood. 1990 Jun 15;75(12):2388–2395. [PubMed] [Google Scholar]
- Danis V. A., Franic G. M., Rathjen D. A., Brooks P. M. Effects of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, interferon-gamma (IFN-gamma), tumour necrosis factor-alpha (TNF-alpha) and IL-6 on the production of immunoreactive IL-1 and TNF-alpha by human monocytes. Clin Exp Immunol. 1991 Jul;85(1):143–150. doi: 10.1111/j.1365-2249.1991.tb05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougados M., Boumier P., Amor B. Sulphasalazine in ankylosing spondylitis: a double blind controlled study in 60 patients. Br Med J (Clin Res Ed) 1986 Oct 11;293(6552):911–914. doi: 10.1136/bmj.293.6552.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastgate J. A., Symons J. A., Wood N. C., Capper S. J., Duff G. W. Plasma levels of interleukin-1-alpha in rheumatoid arthritis. Br J Rheumatol. 1991 Aug;30(4):295–297. doi: 10.1093/rheumatology/30.4.295. [DOI] [PubMed] [Google Scholar]
- Eastgate J. A., Symons J. A., Wood N. C., Grinlinton F. M., di Giovine F. S., Duff G. W. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988 Sep 24;2(8613):706–709. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Farr M., Tunn E., Crockson A. P., Bacon P. A. The long term effects of sulphasalazine in the treatment of rheumatoid arthritis and a comparative study with penicillamine. Clin Rheumatol. 1984 Dec;3(4):473–481. doi: 10.1007/BF02031270. [DOI] [PubMed] [Google Scholar]
- Fries J. F., Spitz P. W., Young D. Y. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982 Sep-Oct;9(5):789–793. [PubMed] [Google Scholar]
- Fries J. F., Spitz P., Kraines R. G., Holman H. R. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980 Feb;23(2):137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- Helle M., Boeije L., de Groot E., de Vos A., Aarden L. Sensitive ELISA for interleukin-6. Detection of IL-6 in biological fluids: synovial fluids and sera. J Immunol Methods. 1991 Apr 8;138(1):47–56. doi: 10.1016/0022-1759(91)90063-l. [DOI] [PubMed] [Google Scholar]
- Hovdenes J., Kvien T. K., Hovdenes A. B. IL-6 in synovial fluids, plasma and supernatants from cultured cells of patients with rheumatoid arthritis and other inflammatory arthritides. Scand J Rheumatol. 1990;19(3):177–182. doi: 10.3109/03009749009095040. [DOI] [PubMed] [Google Scholar]
- Jensen H. S., Tvede N., Diamant M., Mogensen H. H., Hansen M. B., Thomsen B. S., Pedersen P. B., Bendtzen K. Elastolytic activity of human monocytes from synovial fluid and blood of patients with arthritis. Relations to levels of interleukin 6 and soluble interleukin 2 receptor. Scand J Rheumatol. 1991;20(2):83–90. doi: 10.3109/03009749109165281. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Hirano T., Nagasawa S., Kishimoto T. Identification of alpha 2-macroglobulin as a carrier protein for IL-6. J Immunol. 1989 Jan 1;142(1):148–152. [PubMed] [Google Scholar]
- Maury C. P., Andersson L. C., Teppo A. M., Partanen S., Juvonen E. Mechanism of anaemia in rheumatoid arthritis: demonstration of raised interleukin 1 beta concentrations in anaemic patients and of interleukin 1 mediated suppression of normal erythropoiesis and proliferation of human erythroleukaemia (HEL) cells in vitro. Ann Rheum Dis. 1988 Dec;47(12):972–978. doi: 10.1136/ard.47.12.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissilä M., Lehtinen K., Leirisalo-Repo M., Luukkainen R., Mutru O., Yli-Kerttula U. Sulfasalazine in the treatment of ankylosing spondylitis. A twenty-six-week, placebo-controlled clinical trial. Arthritis Rheum. 1988 Sep;31(9):1111–1116. doi: 10.1002/art.1780310905. [DOI] [PubMed] [Google Scholar]
- Podor T. J., Jirik F. R., Loskutoff D. J., Carson D. A., Lotz M. Human endothelial cells produce IL-6. Lack of responses to exogenous IL-6. Ann N Y Acad Sci. 1989;557:374–387. [PubMed] [Google Scholar]
- Porter D. R., Capell H. A. The use of sulphasalazine as a disease modifying antirheumatic drug. Baillieres Clin Rheumatol. 1990 Dec;4(3):535–551. doi: 10.1016/s0950-3579(05)80006-8. [DOI] [PubMed] [Google Scholar]
- Pullar T., Hunter J. A., Capell H. A. Sulphasalazine in rheumatoid arthritis: a double blind comparison of sulphasalazine with placebo and sodium aurothiomalate. Br Med J (Clin Res Ed) 1983 Oct 15;287(6399):1102–1104. doi: 10.1136/bmj.287.6399.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullar T., Hunter J. A., Capell H. A. Sulphasalazine in the treatment of rheumatoid arthritis: relationship of dose and serum levels to efficacy. Br J Rheumatol. 1985 Aug;24(3):269–276. doi: 10.1093/rheumatology/24.3.269. [DOI] [PubMed] [Google Scholar]
- Saxne T., Palladino M. A., Jr, Heinegård D., Talal N., Wollheim F. A. Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 1988 Aug;31(8):1041–1045. doi: 10.1002/art.1780310816. [DOI] [PubMed] [Google Scholar]
- Shanahan W. R., Jr, Hancock W. W., Korn J. H. Expression of IL-1 and tumor necrosis factor by endothelial cells: role in stimulating fibroblast PGE2 synthesis. J Exp Pathol. 1989;4(1):17–27. [PubMed] [Google Scholar]
- Symons J. A., McDowell T. L., di Giovine F. S., Wood N. C., Capper S. J., Duff G. W. Interleukin 1 in rheumatoid arthritis: potentiation of immune responses within the joint. Lymphokine Res. 1989 Fall;8(3):365–372. [PubMed] [Google Scholar]
- Tetta C., Camussi G., Modena V., Di Vittorio C., Baglioni C. Tumour necrosis factor in serum and synovial fluid of patients with active and severe rheumatoid arthritis. Ann Rheum Dis. 1990 Sep;49(9):665–667. doi: 10.1136/ard.49.9.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Kaufmann C., Espevik T., Husby G. Interleukin-6 in synovial fluid from patients with arthritis. Clin Immunol Immunopathol. 1989 Mar;50(3):394–398. doi: 10.1016/0090-1229(89)90146-3. [DOI] [PubMed] [Google Scholar]
- van der Heijde D. M., van Riel P. L., Nuver-Zwart I. H., Gribnau F. W., vad de Putte L. B. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet. 1989 May 13;1(8646):1036–1038. doi: 10.1016/s0140-6736(89)92442-2. [DOI] [PubMed] [Google Scholar]


