Abstract
Aroma is a crucial factor determining the market value and consumer satisfaction of fresh oriental melon. However, few studies focus on the volatile flavor of fresh oriental melon, and the effect of forchlorfenuron application on the aroma profile is unclear. This study characterized the volatile profile of fresh oriental melon fruit after forchlorfenuron application by E-nose and HS-GC-IMS. The holistic variation of volatile compounds exhibited evident distinction based on linear discriminant analysis (LDA) with E-nose. Forty-eight volatile compounds were identified from fresh oriental melon via GC-IMS, mainly esters, alcohols, aldehydes, and ketones, along with smaller quantities of sulfides and terpenes. Compared to pollination melon fruits, 13 critical different volatile flavor compounds were screened out in forchlorfenuron application groups by the PLS-DA model, imparting sweet fruity flavor. The results of the current study provide a valuable basis for evaluating the flavor quality of oriental melon after forchlorfenuron treatment.
Keywords: oriental melon, volatile compounds, forchlorfenuron, flavor, headspace-gas chromatography-ion mobility spectrometry, electronic nose
1. Introduction
Oriental melon is a species of thin-pericarp melon [1], and it has the largest plantation in China, accounting for about 51% of the total global production. Oriental melons are often planted in greenhouses to increase the price of fruit. However, the lack of pollinators often affects the fruit-set rate for facilities. Forchlorfenuron is a synthetic cytokinin-like growth regulator, which can act synergistically with endogenous auxins to induce parthenocarpy and promote cell expansion [2]. In recent years, forchlorfenuron has been extensively used in oriental melon cultivation to improve the fruit set.
With the increasing prevalence of forchlorfenuron application, more and more studies have focused on its influence on fruit quality [3,4]. Several studies have shown that the application of forchlorfenuron decreased sucrose and glucose content and increased bitterness in melon [5,6]. In addition to sugar, volatile aroma plays a decisive role in the purchase of oriental melon [7]. The volatile components of melon have been analyzed in previous reports, and approximately 300 compounds have been identified [8,9,10,11]. They produce volatile aldehydes, alcohols, and especially large quantities of esters, likely to be the key contributors to their unique aroma [12,13,14]. However, limited studies have reported the effect of forchlorfenuron on the aroma compounds of oriental melon fruit. Although Li et al. found that the abundance of volatile compounds was decreased after forchlorfenuron application in muskmelon using gas chromatography–mass spectrometry,13 the findings have generally been obtained from frozen samples as an alternative to fresh samples. It has been reported that significant changes in volatiles occurred during the freezing process in fruits and vegetables [15,16,17]. To accurately evaluate the effect of forchlorfenuron on aroma characteristics, a quick and straightforward method to discriminate the variation of aroma volatiles using fresh oriental melon fruit is critical.
Sensory analysis using trained panelists has been employed conventionally to evaluate the variation of fruit aroma, which can directly measure the fruit flavor intensity. However, this method is expensive and time-consuming, with low objectivity and reproducibility [18,19]. Electronic nose (E-nose) and gas chromatography–ion mobility spectrometry (GC-IMS), as emerging techniques for volatile-compound analysis, offer advantages of fast detection speed, high sensitivity, and little sample pretreatment [20,21,22,23,24]. These techniques have been successfully utilized individually or in combination in many fields, mainly involving freshness prediction, adulteration identification, and food composition classification. Ezhilan et al. discriminated the pathogen contamination of apples using E-nose; higher classification accuracy was attained with an accuracy of 99.9% [25]. The Guo et al. study showed the potential of GC-IMS-based approaches to evaluate the volatile compound profiles of fresh-cut yam at different stages in the yellowing period [26].
Therefore, the present study aims to identify the differentiation of flavor changes in oriental melon treated with different concentrations of forchlorfenuron. E-nose and HS-GC-IMS were applied to characterize the volatile compound composition and content when the oriental melon fruits were harvested after maturation. The results will provide new theoretical guidance for the more appropriate use of forchlorfenuron in oriental melon.
2. Materials and Methods
2.1. The Oriental Melon Field Trials
Oriental melons (Cucumis melo var. makuwa) were cultivated in a greenhouse during a summer–autumn cycle with common growing conditions. The temperature of the greenhouse was maintained in the range of 25–30 °C, with 60% average relative humidity throughout the experiment. The oriental melons were divided into three forchlorfenuron application groups and one pollination group. In the treatment groups, doses of forchlorfenuron soluble concentrate (SL) were set from 10 to 20 mg/L according to the recommended dose on the registered label, and the melon ovary was completely dipped with forchlorfenuron solutions (10 mg/L, 15 mg/L, 20 mg/L) for 1–2 s, respectively. In the pollination group, only the chasmogamy of female flowers by male flowers was considered. All fruit-set treatments were performed on the same morning (6–9 AM).
Representative melon fruit samples were harvested with the best edible quality according to the experience of melon farmers (34 days after pollination or forchlorfenuron application). Mature melon fruits were selected using a combination of different harvest indices, including smooth-skinned with sweet and fragrant pulp, aroma emission detected by the human nose, pale yellow skin color, and peduncle suberization (Figure S1) [11]. In addition, melons were selected in this experiment based on uniform size, weight, and color, and at least six fruits were collected in each group. Melon samples were placed in polyethylene bags and transported to the laboratory for the next stage.
2.2. Sample Preparation
The samples were hand cut with a sharp knife into 2 cm slices, from which the blossom ends and the stem were discarded. Tissues were immediately smashed by a pulverizer, and melon samples were placed into headspace vials, sealed until analysis.
2.3. E-Nose Analysis
The volatile profile of fresh oriental melon fruit was detected by PEN 3 E-nose (AIRSENSE Company, Schwerin, Germany). The E-nose consists of ten different metal oxide sensors. Each sensor has its corresponding sensitive substances: sensor 1 W1C is sensitive to aromatic compounds; sensor 2 W5S is sensitive to oxynitride; sensor 3 W3C is sensitive to ammonia and aromatic compounds; sensor 4 W6S is sensitive to hydrogen; sensor 5 W5C is sensitive to alkanes and aromatic compounds; sensor 6 W1S is sensitive to methane; sensor 7 W1W is sensitive to sulfur compounds; sensor 8 W2S is sensitive to ethanol; sensor 9 W2W is sensitive to aromatic and organic sulfur compounds; and sensor 10 W3S is sensitive to long-chain alkanes [27,28]. Samples (10 g) were placed in a 100 mL beaker and sealed with tin foil for 60 min. The determination conditions were as follows: the flow rate of carrier gas (pure dry air) was 400 mL/min, pre-injection time was 5 s, sample measurement time was 100 s, reset time was 5 s, and cleaning time was 100 s.
2.4. HS-GC-IMS Analysis
The identification of the characteristic volatile compounds of fresh oriental melon fruit was performed using a FlavourSpec® ion mobility spectrometry (IMS) instrument (G.A.S., Dortmund, Germany) equipped with an auto-sampler unit, a syringe, a heated splitless injector, and a radioactive ionization source for headspace (HS) analysis.
The detection processes of HS-GC-IMS were conducted as described by Guo et al. [26] and adjusted slightly according to fresh oriental melon fruit characteristics. A homogenized oriental melon sample (2 g) was transferred to a 20 mL headspace bottle and was incubated at 40 °C for 20 min. Then, 200 μL was sampled from the headspace and was automatically injected into the heated injector at a temperature of 45 °C. After injection, GC was performed with a 15 m standard capillary column (FS-SE-54-CB capillary column, 15 m × 0.53 mm) to separate volatile compounds. The flow of carrier gas (nitrogen gas, 99.99% purity) was set at 2.0 mL/min. The analytes were ionized by a tritium source (6.5 keV) at atmospheric pressure and then transferred to the drift tube (98 mm length). Four groups of melon samples were detected in sequence by GC-IMS (repeated four times), which takes 25 min per sample. The retention index (RI) of volatile compounds was calculated using standardized n-ketones (Sinopharm Chemical Reagent Beijing Co., Ltd., Beijing, China) whose RI was linear. Compounds were identified by comparing RI and drift time (Dt, the time required for ions to reach the collector through the drift tube, in milliseconds) to the standard in the GC × IMS Library supplied by G.A.S. (Dortmund, Germany). The GC-IMS fingerprint analysis was conducted by comparing GC retention time and IMS drift time.
2.5. Multivariate Analysis
Linear discriminant analysis (LDA) was performed using the E-nose software system. The instrumental analysis software includes the Laboratory Analytical Viewer (LAV, G.A.S., Dortmund, Germany) and three plug-ins, as well as the GC × IMS Library Search, which can be used for sample analysis from different angles.
OriginPro 9.1 (Origin Lab Corporation, Northampton, MA, USA) was used to draw the radar chart. Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were conducted using SIMCA-P software v14.1 (Umetrics, Umea, Sweden).
The odor type and odor strength were obtained from The Good Scents Company Information System. (http://www.thegoodscentscompany.com/index.html (accessed on 9 March 2023)).
3. Results and Discussion
3.1. Evaluation of the Volatile Compounds of Fresh Oriental Melon Fruit by E-Nose
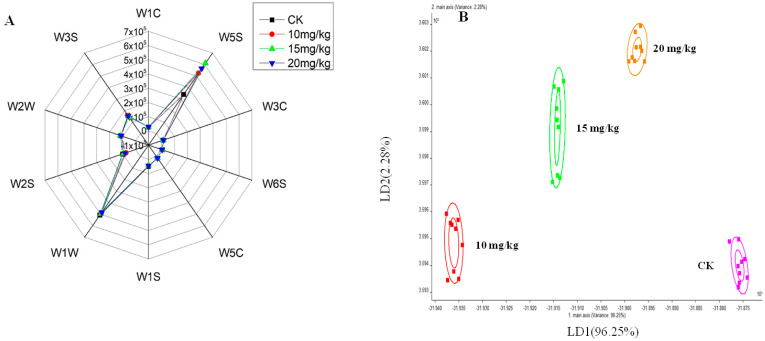
E-nose analysis was conducted on fresh oriental melon from different treatment groups to monitor shifts in aroma composition. The odor radar map of volatile compounds in fresh oriental melon is presented in Figure 1A, following detection using 10 odor sensors. It was found that the W5S and W1W sensors had stronger responses to the volatiles of melon samples, indicating that fresh oriental melon might have higher abundances of nitrogen oxides and terpene compounds. Especially, the response values of the W5S sensor were 1.3–2.0 times higher in all three forchlorfenuron application groups (10 mg/kg, 15 mg/kg, and 20 mg/kg) than in the pollination group (CK), suggesting that oriental melon in forchlorfenuron application groups may have high abundances of nitrogen oxides. For strawberries and avocados, more nitric oxide was detected in unripe fruits [29]. Previous studies reported that applying forchlorfenuron can result in a prolonged ripening process and delayed fruit maturity [30,31], which may be one reason for the higher concentrations of nitrogen oxides being detected in forchlorfenuron application groups.
Figure 1.
(A) Radar chart and (B) linear discriminant analysis (LDA) of fresh oriental melon after forchlorfenuron application obtained by E-nose measurement. CK: pollination group; 10 mg/kg: low-dose forchlorfenuron application group; 15 mg/kg: mid-dose forchlorfenuron application group; 20 mg/kg: high-dose forchlorfenuron application group.
Based on the responses of the E-nose sensors, the LDA method was used to reduce the differences within the classification and expand the differences between different groups in this study. As shown in Figure 1B, the variance contribution rates of LD1 and LD2 were 96.25% and 2.28%, respectively. Moreover, the LDA analysis showed the variation of each group along the abscissa (LD1) with a trend. The distance between the four groups was relatively far, especially between the pollination group (CK) and the other three forchlorfenuron application groups (10 mg/kg, 15 mg/kg, and 20 mg/kg), indicating that the three forchlorfenuron application groups were significantly different from the pollination group. These findings show that the E-nose could be applied to monitor the changes of volatile compounds in fresh oriental melon after forchlorfenuron application. However, none of the sensors of the E-nose were sensitive to esters in fresh oriental melon, which are the important volatile compounds of oriental melon. Therefore, more precise instruments (i.e., GC-IMS) were used in the subsequent experiments.
3.2. Qualitative Analysis of the Volatile Compounds by HS-GC-IMS
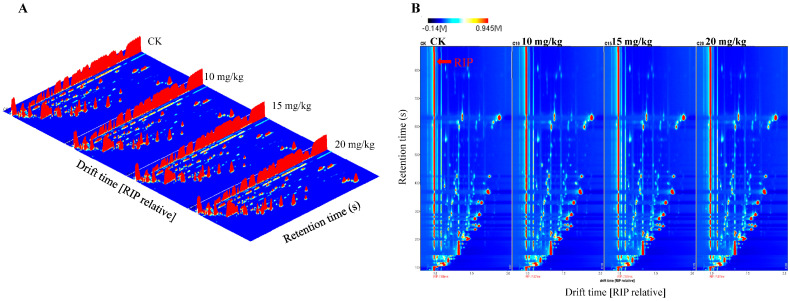
Figure 2 shows the three-dimensional (Figure 2A) and two-dimensional (Figure 2B) spectra obtained by HS-GC-IMS relying on chemical morphology. Different colors indicate different concentrations of the individual compounds, with white dots indicating a lower concentration and red dots indicating a higher concentration (Tian et al., 2020). Several single compounds might produce multiple signals or spots (dimers or even trimers), which are attributed to their varying concentrations [26].
Figure 2.
Volatile flavor compounds in fresh oriental melon after pollination or forchlorfenuron application: (A) a three-dimensional spectrum of the HS-GC-IMS response data; (B) a two-dimensional spectrum of the HS-GC-IMS response data.
It could be seen that volatile compounds were effectively separated from signal dots. Most of the signals appeared in the retention time of 100–700 s and the drift time of 1.0–2.0. A total of 48 volatiles were identified from the GC-IMS library (Table 1), including esters (27), alcohols (7), aldehydes (7), ketones (4), sulfides (2), and terpenes (1). These volatiles’ primary descriptive odor dimensions are fruity and ethereal, which have high odor strength. Among the 48 volatiles, esters, including 9 acetates and 18 nonacetate esters, were dominant quantitatively, accounting for 56.25%. The other quantity-predominant compounds were alcohols (14.58%), aldehydes (14.58%), and ketones (8.33%); sulfides and terpenes together accounted for only 6.25%. Consistent with the previous study, ethyl hexanoate, ethyl 2-methylbutyrate, and ethyl butanoate were considered key odorants in various melon fruits, having fruity, floral, and sweet odor [4,12]. Alcohols and aldehydes with nine carbon atoms, dominated by (Z)-non-6-enal, (E)-2-nonenal, and (3Z,6Z)-nona-3,6-dien-1-ol, which smelled “cantaloupe-like, cucumber-like”, were identified by many researchers as the characteristic components of the family Cucurbitaceae [4,11,12]. In this study, only n-Nonanal with nine carbon atoms was identified in oriental melon fruits. These differences could be attributed to geographical and cultivar variations as well as the different aroma extraction methods used. Compared with the oriental melon, the muskmelon has a different distribution profile of volatile compounds as has been reported, with aldehydes being the dominant compounds (33.33%), followed by esters (27.45%), alcohols (25.49%), and ketones (13.76%) [32].
Table 1.
Volatile flavor compounds in oriental melon identified by HS-GC-IMS.
| Count | Compound | CAS# | Formula | MW | RI | Rt [sec] | Dt [RIPrel] | Comment | Odor Type | Odor Strength |
|---|---|---|---|---|---|---|---|---|---|---|
| Esters (27) | ||||||||||
| 1 | Ethyl hexanoate | 123-66-0 | C8H16O2 | 144.2 | 1007.3 | 598.713 | 13.415 | monomer | fruity | high |
| 2 | Ethyl hexanoate | 123-66-0 | C8H16O2 | 144.2 | 1007.3 | 598.713 | 17.987 | dimer | fruity | high |
| 3 | Amyl acetate | 628-63-7 | C7H14O2 | 130.2 | 916.2 | 424.211 | 13.134 | monomer | fruity | / |
| 4 | Amyl acetate | 628-63-7 | C7H14O2 | 130.2 | 916.2 | 424.211 | 17.643 | dimer | fruity | / |
| 5 | 3-Methylbutyl acetate | 123-92-2 | C7H14O2 | 130.2 | 880.9 | 368.989 | 12.962 | monomer | fruity | high |
| 6 | 3-Methylbutyl acetate | 123-92-2 | C7H14O2 | 130.2 | 882.4 | 371.198 | 17.425 | dimer | fruity | high |
| 7 | Ethyl 2-methylbutanoate | 7452-79-1 | C7H14O2 | 130.2 | 852.4 | 332.543 | 12.448 | monomer | fruity | medium |
| 8 | Ethyl 2-methylbutanoate | 7452-79-1 | C7H14O2 | 130.2 | 850.6 | 330.334 | 16.536 | dimer | fruity | medium |
| 9 | Butyl acetate | 123-86-4 | C6H12O2 | 116.2 | 810.3 | 287.261 | 1237 | monomer | ethereal | high |
| 10 | Butyl acetate | 123-86-4 | C6H12O2 | 116.2 | 813.7 | 290.574 | 16.208 | dimer | ethereal | high |
| 11 | Ethyl butanoate | 105-54-4 | C6H12O2 | 116.2 | 796.7 | 274.007 | 12.058 | monomer | fruity | high |
| 12 | Ethyl butanoate | 105-54-4 | C6H12O2 | 116.2 | 796.7 | 274.007 | 15.615 | dimer | fruity | high |
| 13 | Ethyl 2-methylpropanoate | 97-62-1 | C6H12O2 | 116.2 | 756.0 | 236.253 | 11.927 | monomer | fruity | high |
| 14 | Ethyl 2-methylpropanoate | 97-62-1 | C6H12O2 | 116.2 | 755.3 | 235.641 | 15.619 | dimer | fruity | high |
| 15 | Ethyl propanoate | 105-37-3 | C5H10O2 | 102.1 | 709.0 | 198.307 | 1148 | monomer | fruity | high |
| 16 | Ethyl propanoate | 105-37-3 | C5H10O2 | 102.1 | 709.0 | 198.307 | 14.528 | dimer | fruity | high |
| 17 | Methyl 2-methylbutanoate | 868-57-5 | C6H12O2 | 116.2 | 776.1 | 254.614 | 11.927 | monomer | fruity | / |
| 18 | Methyl 2-methylbutanoate | 868-57-5 | C6H12O2 | 116.2 | 774.8 | 253.39 | 1533 | dimer | fruity | / |
| 19 | Ethyl Acetate | 141-78-6 | C4H8O2 | 88.1 | 590.9 | 145.231 | 10.968 | monomer | ethereal | high |
| 20 | Ethyl Acetate | 141-78-6 | C4H8O2 | 88.1 | 599.6 | 148.029 | 13.349 | dimer | ethereal | high |
| 21 | Methyl isobutyrate | 547-63-7 | C5H10O2 | 102.1 | 687.5 | 184.409 | 11.419 | monomer | fruity | / |
| 22 | Methyl isobutyrate | 547-63-7 | C5H10O2 | 102.1 | 688.1 | 184.759 | 14.424 | dimer | fruity | / |
| 23 | Isobutyl acetate | 110-19-0 | C6H12O2 | 116.2 | 767.5 | 246.674 | 16.135 | fruity | medium | |
| 24 | Ethyl pentanoate | 539-82-2 | C7H14O2 | 130.2 | 901.4 | 399.717 | 12.764 | monomer | fruity | high |
| 25 | Ethyl pentanoate | 539-82-2 | C7H14O2 | 130.2 | 901.4 | 399.717 | 16.829 | dimer | fruity | high |
| 26 | Methyl hexanoate | 106-70-7 | C7H14O2 | 130.2 | 925.4 | 440.483 | 12.895 | monomer | fruity | medium |
| 27 | Methyl hexanoate | 106-70-7 | C7H14O2 | 130.2 | 926.1 | 441.667 | 16.845 | dimer | fruity | medium |
| Alcohols (7) | ||||||||||
| 28 | Ethanol | 64-17-5 | C2H6O | 46.1 | 483.8 | 110.843 | 10.485 | monomer | alcoholic | medium |
| 29 | Ethanol | 64-17-5 | C2H6O | 46.1 | 484.8 | 111.146 | 11.285 | dimer | alcoholic | medium |
| 33 | 1-Octen-3-ol | 3391-86-4 | C8H16O | 128.2 | 985.1 | 555.176 | 11.583 | monomer | earthy | high |
| 31 | 1-Octene-3-ol | 3391-86-4 | C8H16O | 128.2 | 986.2 | 557.384 | 15.954 | dimer | earthy | high |
| 32 | 1-Octen-3-ol | 3391-86-4 | C8H16O | 128.2 | 985.1 | 555.176 | 17.302 | polymer | earthy | high |
| 33 | 2-Methylbutanol | 137-32-6 | C5H12O | 88.1 | 736.3 | 219.252 | 12.326 | monomer | ethereal | medium |
| 34 | 2-Methylbutanol | 137-32-6 | C5H12O | 88.1 | 738.5 | 221.168 | 1472 | dimer | ethereal | medium |
| Aldehydes (7) | ||||||||||
| 35 | Benzaldehyde | 100-52-7 | C7H6O | 106.1 | 957.5 | 500.837 | 11.488 | fruity | high | |
| 36 | 3-Methylbutanal | 590-86-3 | C5H10O | 86.1 | 665.8 | 173.049 | 11.584 | monomer | aldehydic | high |
| 37 | 3-Methylbutanal | 590-86-3 | C5H10O | 86.1 | 661.3 | 170.97 | 14.065 | dimer | aldehydic | high |
| 38 | n-Nonanal | 124-19-6 | C9H18O | 142.2 | 1106.9 | 783.669 | 14.789 | monomer | aldehydic | high |
| 39 | n-Nonanal | 124-19-6 | C9H18O | 142.2 | 1106.9 | 783.669 | 19.299 | dimer | aldehydic | high |
| 40 | (E)-Hept-2-enal | 18829-55-5 | C7H12O | 112.2 | 956.9 | 499.653 | 16.611 | green | high | |
| 41 | Pentanal | 110-62-3 | C5H10O | 86.1 | 697.5 | 190.514 | 1183 | fermented | / | |
| Ketones (4) | ||||||||||
| 42 | Acetone | 67-64-1 | C3H6O | 58.1 | 500.2 | 116.093 | 11.249 | solvent | high | |
| 43 | 2,3-Butanedione | 431-03-8 | C4H6O2 | 86.1 | 582.4 | 142.503 | 11.657 | buttery | high | |
| 44 | 2-Butanone | 78-93-3 | C4H8O | 72.1 | 584.4 | 143.132 | 10.575 | monomer | ethereal | / |
| 45 | 2-Butanone | 78-93-3 | C4H8O | 72.1 | 583.3 | 142.782 | 12.482 | dimer | ethereal | / |
| Sulfides (2) | ||||||||||
| 46 | Butyl sulfide | 544-40-1 | C8H18S | 146.3 | 1072.9 | 721.533 | 12.851 | alliaceous | high | |
| 47 | Dimethyl trisulfide | 3658-80-8 | C2H6S3 | 126.3 | 947.6 | 481.593 | 13.084 | alliaceous | / | |
| Terpenes (1) | ||||||||||
| 48 | Alpha-Pinene | 80-56-8 | C10H16 | 136.2 | 938.3 | 464.062 | 12.175 | herbal | high |
The odor type and odor strength were obtained from The Good Scents Company Information System.
3.3. Different Profiles of Volatile Flavor Compounds in Fresh Oriental Melon after Forchlorfenuron Application by HS-GC-IMS
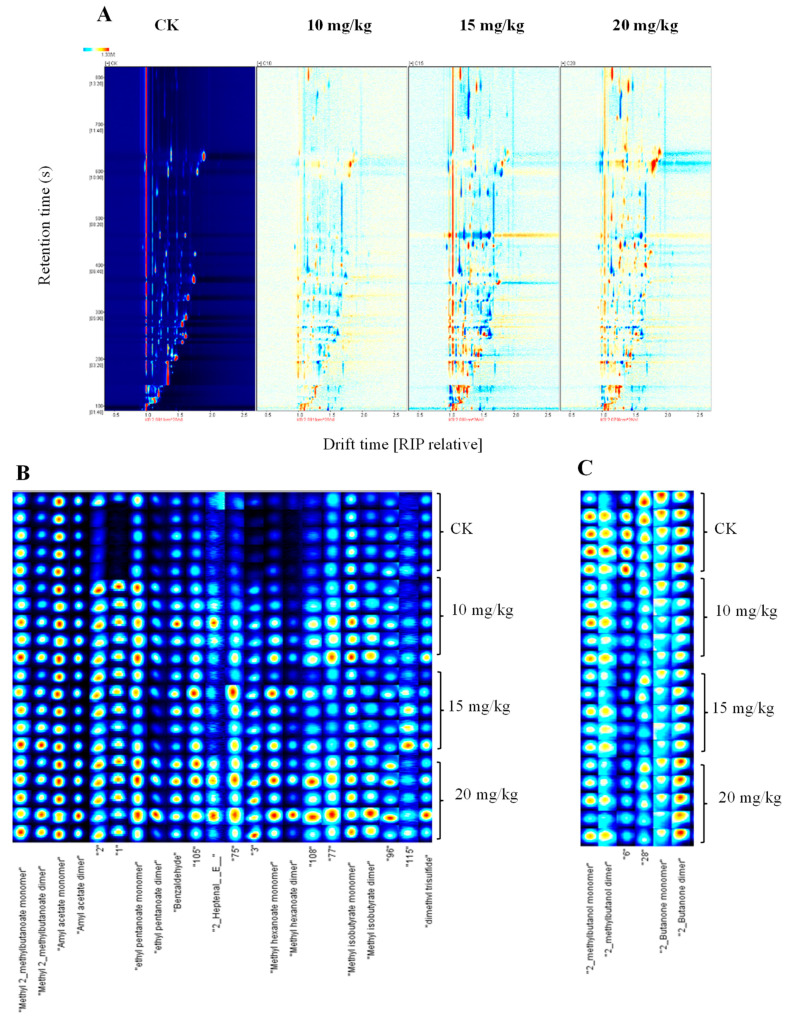
To better understand the effect of volatile compounds in fresh oriental melon after forchlorfenuron application, the difference comparison model was applied to compare the differences between the different treatment groups. The topographic plot of fresh oriental melon from the pollination group (CK) was selected as a reference, and the spectrum of the other samples deducted the reference. If the volatile compounds were consistent, the background after deduction was white; red and blue indicated that the concentration of volatile compounds was higher and lower than the reference, respectively. As shown in Figure 3A, the number of red dots increased gradually with the increase in concentration of the forchlorfenuron application (10–20 mg/kg). Moreover, more red spots were observed for the mid-dose (15 mg/kg) and high-dose (20 mg/kg) groups. Further, several blue spots can be observed in the three forchlorfenuron application groups, indicating that the concentration of several volatile compounds in the three forchlorfenuron application groups was lower than in the pollination group (CK).
Figure 3.
Differences of volatile flavor compounds of fresh oriental melon after pollination or forchlorfenuron application: (A) two-dimensional spectrum of the HS-GC-IMS response data; (B,C) gallery plot of the HS-GC-IMS response data.
The fingerprint was used to make an accurate judgment regarding the dense material on the topographic plot. As shown in Figure 3B,C, the signal intensities of methyl 2-methyl butanoate, amyl acetate, ethyl propanoate, (E)-hept-2-enal, methyl hexanoate, methyl isobutyrate, and dimethyl trisulfide were found to be higher in three forchlorfenuron application groups but were lower in the pollinated group (CK). These volatile compounds impart sweet, fruity, and sulfureous flavor. On the contrary, 2-methylbutanol imparts an alcoholic flavor, and 2-butanone possesses an ethereal fruity odor, which were significantly lower in the three forchlorfenuron application groups. A previous study showed that forchlorfenuron application could significantly influence the volatile flavor compounds in muskmelon [14]. The relative abundances of 14 volatile compounds emitted by the forchlorfenuron-treated fruits declined, including six ethyl esters, four aldehydes, three alcohols, and one ketone. By contrast, the relative abundance of 1-hexanol was on average higher in all the forchlorfenuron-treated fruits [11].
3.4. PCA and OPLS-DA Analysis
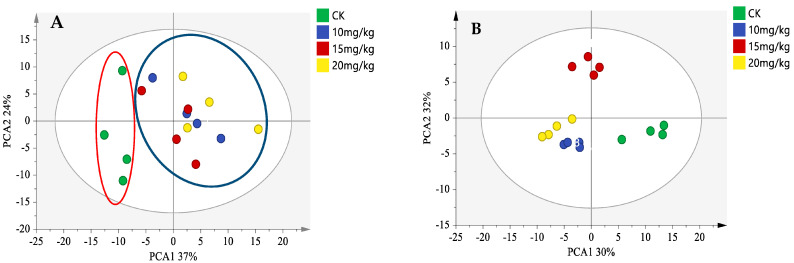
To better visualize the variability of volatile flavor compounds in fresh oriental melon after forchlorfenuron application, multivariate analyses (PCA and OPLS-DA) were attempted in this study. As shown in Figure 4A, all volatile compounds obtained from HS-GC-IMS were subjected to PCA analysis, with PC1 and PC2 accounting for 61% of the total variance. All samples from the pollination group (CK) are positioned on the left side of the score plot, and all melon samples from the forchlorfenuron application groups (10 mg/kg, 15 mg/kg, and 20 mg/kg) are positioned on the right side. Moreover, the proximity of the forchlorfenuron application groups indicates they have similar volatile compounds. The analysis results were consistent with those of the E-nose.
Figure 4.
Score plots of the PCA model (A) and OPLS-DA mode (B) of volatile flavor compounds of fresh oriental melon after pollination or forchlorfenuron application.
Supervised OPLS-DA was then conducted on the volatile compounds of fresh oriental melon samples to test the validity of PCA clustering and to further clarify the critical different aroma-active compounds of oriental melon after forchlorfenuron application. As shown in Figure 4B, a clear discrimination was achieved between the fresh oriental melon samples from different treatment groups. The OPLS-DA model explained a cumulative 62% of the total variance with high-quality performance parameters (R2Y = 0.874, Q2 = 0.894, and CV-ANOVA p-value < 0.05) [33]. The results indicate the goodness-of-fit and predictability of the PLS-DA model and confirm the excellent performance of GC-IMS combined with PLS-DA for the discrimination of oriental melon after forchlorfenuron application. Meanwhile, the VIP method of PLS-DA was used to screen the critical different aroma-active compounds of oriental melon after forchlorfenuron application. As shown in Table 2, the VIP scores of 13 volatile compounds were more than 1, indicating that these compounds might be used as critical markers in determining discrimination in the HS-GC-IMS PLS-DA model.
Table 2.
The 13 difference markers of the volatile flavor compounds of oriental melon under different treatment groups.
| Compound | CAS# | Formula | Odor Descriptor | Peak Area | |||
|---|---|---|---|---|---|---|---|
| CK | 10 mg/kg | 15 mg/kg | 20 mg/kg | ||||
| Esters | |||||||
| Ethyl acetate dimer | 141-78-6 | C4H8O2 | ethereal fruity sweet weedy green | 33,030.65 | 31,044.70 | 31,555.70 | 31,832.88 |
| 3_Methylbutyl acetate dimer | 123-92-2 | C7H14O2 | sweet fruity banana solvent | 9685.77 | 10,484.30 | 10,242.62 | 10,906.42 |
| Ethyl propanoate dimer | 105-37-3 | C5H10O2 | sweet fruity rum juicy fruit grape pineapple | 9154.11 | 9455.36 | 9616.75 | 9970.14 |
| Isobutyl acetate | 110-19-0 | C6H12O2 | sweet fruity ethereal banana tropical | 5429.39 | 4908.41 | 4740.68 | 4901.22 |
| Ethyl hexanoate dimer | 123-66-0 | C8H16O2 | sweet fruity pineapple waxy green banana | 1320.87 | 3116.89 | 2774.75 | 3979.39 |
| Ethyl hexanoate monome | 123-66-0 | C8H16O2 | sweet fruity pineapple waxy green banana | 2109.47 | 3067.76 | 2806.68 | 3155.46 |
| Amyl acetate dime | 628-63-7 | C7H14O2 | ethereal fruity banana pear banana apple | 1403.12 | 2094.79 | 1673.21 | 2559.41 |
| Ethyl 2-methylbutanoate dime | 7452-79-1 | C7H14O2 | sharp sweet green apple fruity | 6161.62 | 6653.40 | 6602.08 | 7472.25 |
| Ethyl 2-methylbutanoate monome | 7452-79-1 | C7H14O2 | sharp sweet green apple fruity | 1103.21 | 1326.94 | 1325.19 | 1380.20 |
| Ethyl pentanoate monomer | 539-82-2 | C7H14O2 | sweet fruity apple pineapple green tropical | 492.25 | 889.20 | 661.18 | 972.12 |
| Methyl 2-methylbutanoate dime | 868-57-5 | C6H12O2 | ethereal estery fruity tutti frutti green apple lily of the valley powdery fatty | 491.19 | 679.60 | 770.23 | 900.93 |
| Methyl hexanoate monomer | 106-70-7 | C7H14O2 | fruity pineapple ether | 137.25 | 379.01 | 441.57 | 604.47 |
| Aldehydes | |||||||
| Benzaldehyde | 100-52-7 | C7H6O | strong sharp sweet bitter almond cherry | 241.09 | 389.94 | 335.86 | 425.24 |
The 13 difference markers contain 12 esters, imparting a sweet fruity flavor in fresh oriental melon. Except for ethyl acetate and isobutyl acetate, the signal intensities of the other 10 esters in the pollination group (CK) were much lower than that of the three forchlorfenuron application groups (10 mg/kg, 15 mg/kg, and 20 mg/kg). Among them, ethyl 2-methyl butanoate was decreased appreciably in overripe cantaloupe as has been reported [34]. Consistent with the result of E-nose, applying forchlorfenuron could delay the fruit maturity of oriental melon [30], which may be one of the reasons for the decrease of these esters in the pollination group (CK).
The major limitation of this work includes the lack of data regarding the specific contribution of each compound to the aroma of fresh oriental fruits. Further studies are being designed to identify key aroma-active compounds in fresh oriental melon. The concentration and odor activity value (OAV) of critical volatile flavor compounds will be further calculated to verify the obtained results from this study. Furthermore, the mechanism of the changes in the types and concentrations of volatile compounds in oriental melon fruit after forchlorfenuron application is still unknown, and this needs further study.
4. Conclusions
In this study, the influence of forchlorfenuron on the volatile flavor compounds in fresh oriental melon was studied. E-nose can effectively separate the oriental melon from different application groups. The volatile profile of fresh oriental melon was characterized by establishing the fingerprint with GC-IMS. Forty-eight volatile components, including esters, alcohols, aldehydes, and ketones, along with smaller quantities of sulfides and terpenes, were identified from flesh oriental melon. Esters are the main substance of volatile compounds in fresh oriental melon. Compared to pollination melon fruits, the application of forchlorfenuron could significantly influence the content of esters in fresh oriental melon, which may be related to the prolonged ripening process of oriental melon after forchlorfenuron application. This work could be conducive to a better understanding of the characteristic aroma differences of fresh oriental melon after forchlorfenuron application and provide new theoretical guidance for the more rational use of forchlorfenuron.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12061272/s1, Figure S1:Representative oriental melon fruits at mature period in different application groups.
Author Contributions
Conceptualization, methodology, validation, and formal analysis, Q.W.; methodology and validation, X.C. and C.Z.; visualization, X.L. and N.Y.; visualization and investigation, H.S.; supervision, J.W.; conceptualization, supervision, project administration, and funding acquisition, F.J. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
This research was funded by National Natural Science Foundation of China (No. 31871890) and the Central Public-interest Scientific Institution Basal Research Fund (No. Y2022XK29).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lija M., Beevy S.S. A Review on the diversity of Melon. Plant Sci. Today. 2021;8:995–1003. doi: 10.14719/pst.1300. [DOI] [Google Scholar]
- 2.Wang Q., Su H., Yue N., Li M., Li C., Wang J., Jin F. Dissipation and risk assessment of forchlorfenuron and its major metabolites in oriental melon under greenhouse cultivation. Ecotoxicol. Environ. Saf. 2021;225:112700. doi: 10.1016/j.ecoenv.2021.112700. [DOI] [PubMed] [Google Scholar]
- 3.Ainalidou A., Karamanoli K., Menkissoglu-Spiroudi U., Diamantidis G., Matsi T. CPPU treatment and pollination: Their combined effect on kiwifruit growth and quality. Sci. Hortic. 2015;193:147–154. doi: 10.1016/j.scienta.2015.07.011. [DOI] [Google Scholar]
- 4.Fredes A., Sales C., Barreda M., Valcárcel M., Roselló S., Beltrán J. Quantification of prominent volatile compounds responsible for muskmelon and watermelon aroma by purge and trap extraction followed by gas chromatography–mass spectrometry determination. Food Chem. 2016;190:689–700. doi: 10.1016/j.foodchem.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Fu Q., Zhang X., Zhu H., Wang H. Effects of different pollination methods on fruit quality of melon cultured in greenhouse. China Vegtables. 2014;11:31–36. doi: 10.3969/j.issn.1000-6346.2014.11.009. [DOI] [Google Scholar]
- 6.Luo F., Li Q., Yu L., Wang C., Qi H. High concentrations of CPPU promotes cucurbitacin B accumulation in melon (Cucumis melo var. makuwa Makino) fruit by inducing transcription factor CmBt. Plant Physiol. Bioch. 2020;154:770–781. doi: 10.1016/j.plaphy.2020.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Niu Q., Hui Y., Jin H., Chen S.J.S. Assessment of taste attributes of peanut meal enzymatic-hydrolysis hydrolysates using an electronic tongue. Sensors. 2015;15:11169–11188. doi: 10.3390/s150511169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kourkoutas D., Elmore J.S., Mottram D.S. Comparison of the volatile compositions and flavour properties of cantaloupe, Galia and honeydew muskmelons. Food Chem. 2006;97:95–102. doi: 10.1016/j.foodchem.2005.03.026. [DOI] [Google Scholar]
- 9.Shi J., Wu H., Xiong M., Chen Y., Chen J., Zhou B., Wang H., Li L., Fu X., Bie Z., et al. Comparative analysis of volatile compounds in thirty-nine melon cultivars by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Food Chem. 2020;316:126342. doi: 10.1016/j.foodchem.2020.126342. [DOI] [PubMed] [Google Scholar]
- 10.Lignou S., Parker J.K., Oruna-Concha M.J., Mottram D.S. Flavour profiles of three novel acidic varie-ties of muskmelon (Cucumis melo L.) Food Chem. 2013;139:1152–1160. doi: 10.1016/j.foodchem.2013.01.068. [DOI] [PubMed] [Google Scholar]
- 11.Pang X., Guo X., Qin Z., Yao Y., Hu X., Wu J. Identification of aroma-active compounds in Jiashi muskmelon juice by GC-O-MS and OAV calculation. J. Agric. Food Chem. 2012;60:4179–4185. doi: 10.1021/jf300149m. [DOI] [PubMed] [Google Scholar]
- 12.Mayobre C., Pereira L., Eltahiri A., Bar E., Lewinsohn E., Garcia-Mas J., Pujol M. Genetic dissection of aroma biosynthesis in melon and its relationship with climacteric ripening. Food Chem. 2021;353:129484. doi: 10.1016/j.foodchem.2021.129484. [DOI] [PubMed] [Google Scholar]
- 13.Li X.X., Hayata Y., Sakamoto T., Maneerat C., Osajima Y. Influence of the Seeds on Aroma of Muskmelon (Cucumis melo L.) J. Jpn. Soc. Hortic. Sci. 2002;71:532–534. doi: 10.2503/jjshs.71.532. [DOI] [Google Scholar]
- 14.Li J., Lin T., Ren D., Wang T., Tang Y., Wang Y., Xu L., Zhu P., Ma G. Transcriptomic and Metabolomic Studies Reveal Mechanisms of Effects of CPPU-Mediated Fruit-Setting on Attenuating Volatile Attributes of Melon Fruit. Agronomy. 2021;11:1007. doi: 10.3390/agronomy11051007. [DOI] [Google Scholar]
- 15.Ma Y., Hu X., Chen J., Chen F., Wu J., Zhao G., Liao X., Wang Z. The Effect of Freezing Modes and Frozen Storage on Aroma, Enzyme and Micro-organism in Hami Melon. Food Sci. Technol. Int. 2007;13:259–267. doi: 10.1177/1082013207081776. [DOI] [Google Scholar]
- 16.Jung K., Fastowski O., Poplacean I., Engel K.-H. Analysis and Sensory Evaluation of Volatile Constituents of Fresh Blackcurrant (Ribes nigrum L.) Fruits. J. Agric. Food Chem. 2017;65:9475–9487. doi: 10.1021/acs.jafc.7b03778. [DOI] [PubMed] [Google Scholar]
- 17.Kjeldsen F., Christensen L.P., Edelenbos M. Changes in Volatile Compounds of Carrots (Daucus carota L.) During Refrigerated and Frozen Storage. J. Agric. Food Chem. 2003;51:5400–5407. doi: 10.1021/jf030212q. [DOI] [PubMed] [Google Scholar]
- 18.Wang S., Chen H., Sun B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS) Food Chem. 2020;315:126158. doi: 10.1016/j.foodchem.2019.126158. [DOI] [PubMed] [Google Scholar]
- 19.Qian K., Bao Y., Zhu J., Wang J., Wei Z. Development of a portable electronic nose based on a hybrid filter-wrapper method for identifying the Chinese dry-cured ham of different grades. J. Food Eng. 2021;290:110250. doi: 10.1016/j.jfoodeng.2020.110250. [DOI] [Google Scholar]
- 20.Zhang T., Ayed C., Fisk I.D., Pan T., Wang J., Yang N., Sun Q. Evaluation of volatile metabolites as potential markers to predict naturally-aged seed vigour by coupling rapid analytical profiling techniques with chemometrics. Food Chem. 2022;367:130760. doi: 10.1016/j.foodchem.2021.130760. [DOI] [PubMed] [Google Scholar]
- 21.Arthur K.L., Eiceman G.A., Reynolds J.C., Creaser C.S. Analysis of Supramolecular Complexes of 3-Methylxanthine with Field Asymmetric Waveform Ion Mobility Spectrometry Combined with Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2016;27:800. doi: 10.1007/s13361-016-1351-y. [DOI] [PubMed] [Google Scholar]
- 22.Gallegos J., Garrido-Delgado R., Arce L., Medina L.M. Volatile Metabolites of Goat Cheeses Determined by Ion Mobility Spectrometry. Potential Applications in Quality Control. Food Anal. Methods. 2015;8:1699–1709. doi: 10.1007/s12161-014-0050-1. [DOI] [Google Scholar]
- 23.Chen X., Chen H., Xiao J., Liu J., Tang N., Zhou A. Variations of volatile flavour compounds in finger citron (Citrus medica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2020;138:109717. doi: 10.1016/j.foodres.2020.109717. [DOI] [PubMed] [Google Scholar]
- 24.Garrido-Delgado R., Mercader-Trejo F., Sielemann S., de Bruyn W., Arce L., Valcarcel M. Direct classification of olive oils by using two types of ion mobility spectrometers. Anal. Chim. Acta. 2011;696:108–115. doi: 10.1016/j.aca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Ezhilan M., Nesakumar N., Jayanth Babu K., Srinandan C.S., Rayappan J.B.B. An Electronic Nose for Royal Delicious Apple Quality Assessment—A Tri-layer Approach. Food Res. Int. 2018;109:44–51. doi: 10.1016/j.foodres.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Guo S., Zhao X., Ma Y., Wang Y., Wang D. Fingerprints and changes analysis of volatile compounds in fresh-cut yam during yellowing process by using HS-GC-IMS. Food Chem. 2022;369:130939. doi: 10.1016/j.foodchem.2021.130939. [DOI] [PubMed] [Google Scholar]
- 27.Feng X., Wang H., Wang Z., Huang P., Kan J. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2022;375:131671. doi: 10.1016/j.foodchem.2021.131671. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Yang Y., Zhu Y., Ben A., Qi J. A novel strategy for discriminating different cultivation and screening odor and taste flavor compounds in Xinhui tangerine peel using E-nose, E-tongue, and chemometrics. Food Chem. 2022;384:132519. doi: 10.1016/j.foodchem.2022.132519. [DOI] [PubMed] [Google Scholar]
- 29.Cheng G., Yang E., Lu W., Jia Y., Jiang Y., Duan X. Effect of Nitric Oxide on Ethylene Synthesis and Softening of Banana Fruit Slice during Ripening. J. Agric. Food Chem. 2009;57:5799–5804. doi: 10.1021/jf901173n. [DOI] [PubMed] [Google Scholar]
- 30.United States Environmental Protection Agency United States Environmental Protection Agency Washington, DC 20460. [(accessed on 31 January 2023)]; Available online: https://www3.epa.gov/pesticides/chem_search/ppls/071049-00004-20180119.pdf.
- 31.Senthilkumar S., Vijayakumar R.M., Soorianathasundaram K. Pre-harvest implications and utility of plant bioregulators on grape: A review. Plant Arch. 2018;18:19–27. [Google Scholar]
- 32.Zhou D., Zhang Q., Wu C., Li T., Tu K. Change of soluble sugars, free and glycosidically bound volatile compounds in postharvest cantaloupe fruit response to cutting procedure and storage. Sci. Hortic. 2022;295:110863. doi: 10.1016/j.scienta.2021.110863. [DOI] [Google Scholar]
- 33.Rivera-Pérez A., Romero-González R., Garrido Frenich A. Fingerprinting based on gas chromatography-Orbitrap high-resolution mass spectrometry and chemometrics to reveal geographical origin, processing, and volatile markers for thyme authentication. Food Chem. 2022;393:133377. doi: 10.1016/j.foodchem.2022.133377. [DOI] [PubMed] [Google Scholar]
- 34.Beaulieu J.C., Grimm C.C. Identification of Volatile Compounds in Cantaloupe at Various Developmental Stages Using Solid Phase Microextraction. J. Agric. Food Chem. 2001;49:1345–1352. doi: 10.1021/jf0005768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.