Abstract
The aim of the study was to identify genetic variants associated with personal best scores in Turkish track and field athletes and to compare allelic frequencies between sprint/power and endurance athletes and controls using a whole-exome sequencing (WES) approach, followed by replication studies in independent cohorts. The discovery phase involved 60 elite Turkish athletes (31 sprint/power and 29 endurance) and 20 ethnically matched controls. The replication phase involved 1132 individuals (115 elite Russian sprinters, 373 elite Russian endurance athletes (of which 75 athletes were with VO2max measurements), 209 controls, 148 Russian and 287 Finnish individuals with muscle fiber composition and cross-sectional area (CSA) data). None of the single nucleotide polymorphisms (SNPs) reached an exome-wide significance level (p < 2.3 × 10−7) in genotype–phenotype and case–control studies of Turkish athletes. However, of the 53 nominally (p < 0.05) associated SNPs, four functional variants were replicated. The SIRT1 rs41299232 G allele was significantly over-represented in Turkish (p = 0.047) and Russian (p = 0.018) endurance athletes compared to sprint/power athletes and was associated with increased VO2max (p = 0.037) and a greater proportion of slow-twitch muscle fibers (p = 0.035). The NUP210 rs2280084 A allele was significantly over-represented in Turkish (p = 0.044) and Russian (p = 0.012) endurance athletes compared to sprint/power athletes. The TRPM2 rs1785440 G allele was significantly over-represented in Turkish endurance athletes compared to sprint/power athletes (p = 0.034) and was associated with increased VO2max (p = 0.008). The AGRN rs4074992 C allele was significantly over-represented in Turkish sprint/power athletes compared to endurance athletes (p = 0.037) and was associated with a greater CSA of fast-twitch muscle fibers (p = 0.024). In conclusion, we present the first WES study of athletes showing that this approach can be used to identify novel genetic markers associated with exercise- and sport-related phenotypes.
Keywords: athletic performance, athletics, track and field, athletes, aerobic capacity, muscle hypertrophy, sports genetics, WES, EWAS, GWAS
1. Introduction
Whether pure talent or long-term experiences promotes athletic performance is one of the questionable issues [1]. Progression in the sport sciences has underlined that athletic performance was a phenomenon affected by lots of factors including physiology and environment [2]. Recent studies have also figured out the possible association of the genetic background of the athletes in their high personal performances, resulting in the rise of a novel scientific branch, called sport genetics [3,4].
Sport genetics could be defined as the investigation of the genes and their molecular mechanisms affecting athletic performance and the determination of the possible association of the variants, especially single nucleotide polymorphisms (SNPs), with diverse athletic parameters including branch or personal performances [5]. According to the studies on sport genetics, 66% of athletic performance has been linked to the genetic background [6]. Moreover, physical parameters were also associated with the genetic background. For instance, 44–68% of endurance and 49–56% of muscular force were shown to be affected by genetic variations [7,8]. Thus, both genetics and the environment, which would influence each other, have key roles in athletic performance [9,10]. For example, training periods to reach a performance level were proved to be linked to the genetic background of the athletes [9].
Recently, identification of candidate genes and/or variants associated with sports parameters has greatly attracted scientists. Until now, more than 235 genetic variants have been linked to the athletic parameters [10,11]. However, the results of the single-gene and/or variant approach may mislead, owing to the ignorance of the other related genes and/or variants. Consequently, it was realized that the results for the associations of each gene and/or variant were controversial [12]. Hence, multigenetic factors should be targeted to totally explore the possible associations. In parallel, several genome-wide association studies (GWAS) have been conducted on sports genetics. GWAS is a powerful technique to cover all known or unknown SNPs [13,14,15]. GWAS has proposed novel associated genes and/or SNPs for the athletic parameters such as endurance, aerobic capacity, metabolism, and muscle fiber composition [16,17]. However, the complexity and cost of GWAS limit such studies, and pilot experiments are suggested [18]. Exome-wide association studies (EWAS) could be an alternative to overcome the problems with GWAS. EWAS has also been previously chosen as a strategy to find the possible associations in the sports genetics [19].
The aim of the present study was to identify genetic variants associated with personal best scores in Turkish track and field athletes and to compare allelic frequencies between sprint/power and endurance athletes and controls using a whole-exome sequencing approach, followed by replication studies in independent cohorts of athletes and controls.
2. Materials and Methods
2.1. Ethical Approval
The study was carried out in accordance with the Declaration of Helsinki, and approval was obtained from the Gazi University Non-Interventional Clinical Research Ethics Committee (with the decision dated 5 April 2021 and numbered 09) and from the Ethics Committee of the Federal Research and Clinical Center of Physical-Chemical Medicine of the Federal Medical and Biological Agency of Russia (Approval number 2017/04).
2.2. Participants
2.2.1. The Turkish Cohorts
The Turkish study involved 60 elite athletes (sprint/power: 11 females (35.5%) and 20 males (64.5%); endurance: 10 females (34.5%) and 19 males (65.5%); mean age ± SD: 25.1 ± 4.8; height (cm): 174.97 ± 7.9; body weight (kg) 72.5 ± 22.4; sport experience (year) = 9.4 ± 4.8; personal best (PB) = 1005.63 ± 94.55) licensed in different clubs and affiliated with the Turkish Athletics Federation. The number of controls (non-athletes) was 20 (6 females (30.0%) and 14 males (70.0%); mean age ± SD: 23.5 ± 7.1), and they were healthy unrelated citizens of Turkish descent without any competitive sports experience.
The athletes were categorized as either sprint/power or endurance athletes as determined by the distance, duration, and energy requirements of their events. All athletes were nationally ranked in the top ten in their sports discipline and had participated in international competitions such as the Olympic Games, European Championships, Universiade, Mediterranean Games, and Balkan Championship. The sprint/power group included sprint and power athletes whose events demand predominantly anaerobic energy production. The athletes in this group (n = 31) were 100–400 m runners (n = 9), jumpers (n = 3), and throwers (n = 19). The endurance athlete group (n = 29) included athletes competing in long-distance events demanding predominantly aerobic energy production. This group included 3000 m (n = 12), 5000 m (n = 5), 10,000 m (n = 4), and marathon (n = 8) runners. The informed voluntary consent and demographic information forms were obtained from the participants before the measurements. The International Association of Athletics Federations (IAAF) score scale was used to determine the performance levels of the athletes, depending on their personal best/competitive performance [20]. For instance, the IAAF score scale of a male athlete who runs 100 m in 10.05 sec is 1189, while that of a marathon runner who completes the race in 2 h 20 min 11 sec is 997. Thus, the performance scale of the marathon runner is less than that of the 100 m runner. The IAAF scales are useful for the determination of performances of athletes from diverse athletics events and genders.
2.2.2. The Russian Cohorts
The Russian case–control study involved 488 elite athletes (293 males and 195 females), of whom 115 were elite sprint/power athletes (29 100–400 m runners, 38 500–1000 m speed skaters, 22 sprint cyclists, 26 50 m swimmers), and 373 were elite endurance athletes (52 rowers, 32 biathletes, 7 long-distance cyclists, 30 kayakers and canoers, 37 middle- and long-distance speed skaters, 92 cross-country skiers, 63 middle- and long-distance runners, 31 middle- and long-distance swimmers, 8 race walkers, and 21 triathletes). The athletes were Russian national team members (participants and prize winners in international competitions) who had never tested positive for doping. Of 373 endurance athletes, 46 male endurance athletes (rowers, kayakers, speed skaters, biathletes, and cross-country skiers) and 29 female endurance athletes (rowers, kayakers, speed skaters, biathletes, and cross-country skiers) participated in the study of aerobic performance. Controls were 209 healthy and unrelated citizens of Russia without any competitive sport experience.
The Russian muscle biopsy study involved 148 physically active participants of Russian origin (99 males: mean age ± SD: 30.4 ± 7.9 years; 49 females: mean age ± SD: 27.1 ± 7.3 years).
2.2.3. The Finnish Cohort
The Finnish muscle biopsy study (replication phase) involved 287 individuals (167 males, age 59.5 ± 8.1 years; 120 females, age 60.7 ± 7.4 years) from the FUSION study as previously described [21].
2.3. Evaluation of Muscle Fiber Composition by Immunohistochemistry
2.3.1. Russian Study
Vastus lateralis samples were obtained from the left legs of the participants using the modified Bergström needle procedure with aspiration under local anesthesia using 2% lidocaine solution. Serial cross-sections (7 μm) were obtained from frozen samples. The sections were then incubated at RT in primary antibodies against slow or fast isoforms of the myosin heavy chains, as previously described [17,22].
2.3.2. Finnish Study
Muscle fiber composition in 287 Finnish individuals was estimated based on the expression of the myosin heavy chain 1 (MYH1), myosin heavy chain 2 (MYH2), myosin heavy chain 7 (MYH7), Ca2+ ATPase A1, and Ca2+ ATPase A2 genes, as previously described [21].
2.4. VO2max Measurement
Maximal oxygen consumption rate (VO2max) in rowers, kayakers, speed skaters and biathletes was determined using an incremental test to exhaustion on specific ergometers. VO2max was determined breath-by-breath using a MetaLyzer II (Cortex Bio-physik, Leipzig, Germany), MetaMax 3B (Cortex Biophysik, Leipzig, Germany) or MetaMax 3B-R2 gas analysis systems (Cortex Biophysik, Leipzig, Germany), as previously described [23].
2.5. Whole-Exome Sequencing (WES)
The peripheral blood obtained from the participants was processed to isolate total DNA by DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Next, qualities of isolated DNA were checked by 1% agarose gel, and the concentrations were determined by a NanoDrop (NanoDrop 1000 Spectrophotometer V3.8; Thermo Scientific, Waltham, MA, USA). WES was performed after library preparation by the Twist Human Comprehensive Exome Panel (Twist Biosciences, San Francisco, CA, USA) according to the supplier’s instructions. Briefly, enzymatic DNA fragmentation was performed, and Twist Hybridization probes and Dynabeads™ MyOne™ Streptavidin T1 (Invitrogen, Carlsbad, CA, USA) were used for the hybridization. After the steps of library enrichment and determination of the library sizes, the samples were uploaded to the flow cells and the run was performed by Illumina NextSeq500 (Illumina Inc., San Diego, CA, USA). Average read depth was aimed as minimum 200×. Raw data were processed to by the Genome Analysis Toolkit (GATK)’s [24]. The HaplotypeCaller program was used to obtain Binary Alignment Map (BAM) files and subsequently produce an output Variant Call Format (VCF) file via the GRCh38/hg38 reference genome. Finally, variants were annotated by ANNOVAR [25].
2.6. Data Extraction
As the primary evaluation of the data, the VCF files were combined, and 511,061 variants were detected. Only SNPs were analyzed in the context of the present study. The variants with a minor allele frequency (MAF) < 0.01, incorrectly annotated, and non-autosomal were eliminated, and 219,232 SNPs were further evaluated.
2.7. Genotyping
DNA samples from Russian individuals were obtained from leukocytes (venous blood). DNA extraction and purification from blood samples were performed using commercial kits (Techno-sorb), according to the manufacturer’s instructions (Techno-clon, Moscow, Russia). Genotyping of the candidate SNPs from the discovery phase was performed using microarray technology [26].
DNA samples from Finnish individuals were extracted from the blood, and the polymorphisms were genotyped using the HumanOmni2.5–4v1_H BeadChip array (Illumina, San Diego, CA, USA), as previously described [21].
2.8. Statistical Analyses
Association analyses of Turkish data were performed by a Chi-square test using thet R program [27]. During the EWAS, the unified mixed-model method [27] was used.
| y = Xβ + Sτ + e | (1) |
where y is the phenotypic observation; Xβ is the fixed effect; and Sτ is the SNP effect [27]. The statistical significance probabilities of the SNP effects were converted to −log10p. The results of EWAS analyses were presented as a Manhattan Plot. The exome-wide significance level was set at p < 2.3 × 10−7 (i.e., 0.05/219,232 SNPs).
Statistical analyses of Russian and Finnish data were conducted using GraphPad InStat Version 3.05 (GraphPad Software, Inc., San Diego, CA, USA) software. The PLINK 1.9 program (National Institutes of Health, Bethesda, MD, USA) was used to perform genetic data quality control, and PLINK 2.0 was used to perform principal component analysis and association testing via generalized linear models. Bcftools was used for vcf file conversion. The phasing and imputation of genotypes were completed using the shapeit2 and impute2 programs. Differences in phenotypes between groups were analyzed using regression analysis adjusted for covariates. The chi-square test (χ2) was used to test for the presence of the Hardy–Weinberg equilibrium (HWE). Thereafter, the frequencies of genotypes or alleles were compared between sprint/power and endurance athletes and controls using Fisher’s exact test. All data are presented as means (SD). The p-values < 0.05 were considered statistically significant.
3. Results
3.1. Discovery Phase
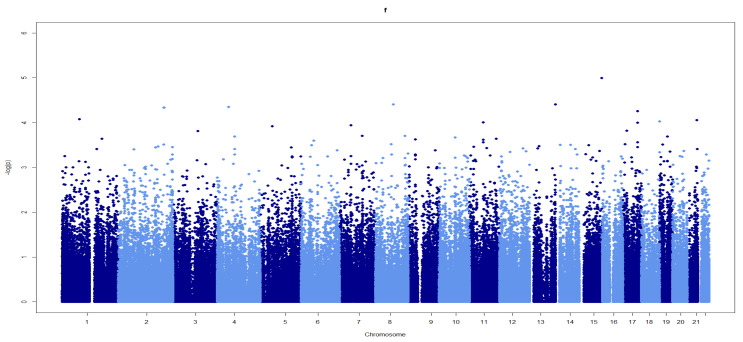
None of the SNPs reached an exome-wide significance level (p < 2.3 × 10−7) in genotype–phenotype and case–control studies of Turkish athletes (Figure 1). The only SNP that was close to the threshold (p = 1.0 × 10−5) was rs8037843 in the Pyroglutamyl-Peptidase I Like (PGPEP1L) gene (Figure 1). Although rs8037843 correlated with personal bests in athletes, there were no allelic differences between the Turkish and Russian endurance and sprint/power athletes and controls with respect to this SNP (p > 0.05).
Figure 1.
Manhattan plot showing associations between SNPs across chromosomes and personal bests in elite Turkish athletes. Light and dark blue: the illustration separating the consecutive chromosomes for comprehensibility.
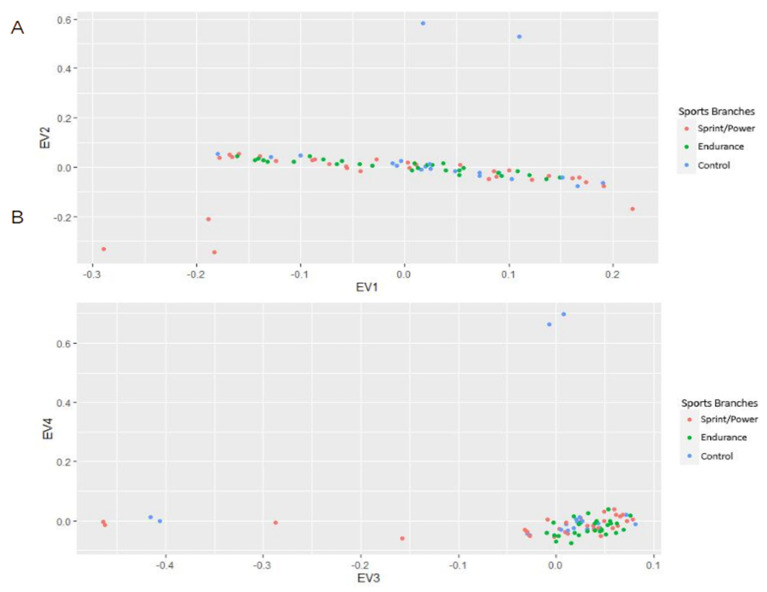
The genotypic differences between the groups were evaluated by principal component analysis on an SNP matrix (PCA). PCA of the genotyping data pointed out no significant influence of sport disciplines (Figure 2) on genotype distributions.
Figure 2.
Principal component analysis on the SNP matrix showing genotype distributions across different groups in EV1−EV2 (A) and EV3−EV4 (B) planes.
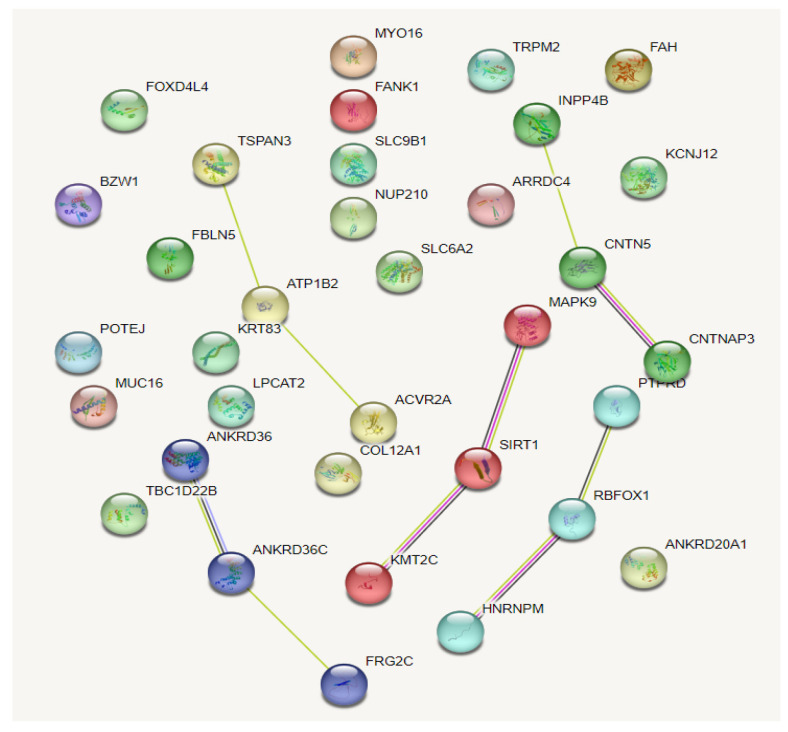
Comparisons of allelic frequencies between three groups (endurance vs. sprint/power athletes; endurance athletes vs. controls; sprint/power athletes vs. controls) showed 53 SNPs whose frequencies were significantly differentiated between the sprint/power and endurance group (but not in the separate sub-groups of female and male athletes due to low sample sizes) (Supplementary Table S1). The genes in which these SNPs were located were further analyzed by the String database (v.11.5; https://string-db.org/, accessed on 10 December 2022) for the functional interaction and pathway analyses. The results showed minimal interactions between the proteins, and the Markov Cluster Algorithm (MCL) option in the database demonstrated five clusters (Figure 3).
Figure 3.
Interaction of and clustering of the protein-coding genes on which deviated frequencies of the SNPs were realized between the sprint/power and endurance groups.
3.2. Replication Studies
Of the 53 nominally (p < 0.05) associated SNPs, four variants were replicated in the following studies involving Russian and Finnish individuals. More specifically, the SIRT1 rs41299232 G allele was significantly over-represented in Turkish (44.0 vs. 4.0%; p = 0.047) and Russian (63.5 vs. 55.4%; p = 0.018) endurance athletes compared to sprint/power ones and was associated with increased relative VO2max (C/C (n = 9): 63.2 (8.0) mL/min/kg, C/G (n = 35): 64.3 (7.0) mL/min/kg, G/G (n = 31): 66.2 (6.4) mL/min/kg; p = 0.037 adjusted for sex), and a greater proportion of slow-twitch muscle fibers in Finnish subjects (C/C (n = 45): 42.8 (12.7)%, C/G (n = 147): 44.4 (16.0)%, G/G (n = 95): 47.0 (13.7)%; p = 0.035 adjusted for age and sex).
The NUP210 rs2280084 A allele was significantly predominant in Turkish (68.0 vs. 34.0%, p = 0.044) and Russian (59.2 vs. 50.0%, p = 0.012) endurance athletes compared to the sprint/power group. In addition, the rs2280084 A allele was over-represented in highly elite Russian endurance athletes (n = 119) compared to controls (64.1 vs. 52.9%, p = 0.003).
The frequency of the TRPM2 rs1785440 G allele was significantly higher in Turkish endurance athletes compared to sprint/power athletes (57.0 vs. 18.0%, p = 0.034) and was associated with increased VO2max (A/A (n = 1): 58.3 (0) mL/min/kg, A/G (n = 16): 63.2 (6.9) mL/min/kg, G/G (n = 58): 65.6 (6.8) mL/min/kg; p = 0.008).
The AGRN rs4074992 C allele was significantly over-represented in Turkish sprint/power athletes compared to endurance athletes (83.0 vs. 44.0%, p = 0.037) and was associated with a greater CSA of fast-twitch muscle fibers in physically active Russian individuals (p = 0.024 adjusted for sex, age, type, and level of physical activity).
4. Discussion
Athletic performance and branches have widely been proved to be a result of the combination of environmental and genetic factors [1,28]. The latter, named as sports genetics, has attracted sports scientists since it was relatively a new branch [10]. The studies on sport genetics have focused on single-gene and/or SNP alteration between the sport branches, which may mislead [12]. Hence, studies aiming at the involvement of multigenetic factors are needed. Limited studies, but not on the Turkish population, have reported GWAS results in sports genetics [10,13,14,16,29]. Thus, the present study focused on the assessment of the multigenetic factors in the elite sprint/power and endurance athletes using the WES approach. Although WES is not a cumulative approach compared to whole-genome sequencing (WGS), it may be advantageous for a pilot study such as the presented one to eliminate the analysis efforts and cost problems.
In our present study, we could not detect any SNPs whose frequencies reached an exome-wide significance. The primary problem with such studies would be the limitations with the number of participants [28]. Sport genetics has been established on a population- and sport-branch-specific manner. However, the restricted number of elite athletes would be a challenge to conclude exact findings [5]. Still, by the fact that the number of participants in such studies could affect the results according to the literature [30], such studies are still needed as a pilot comprehensive report to guide both the geneticists and sport scientists.
By the lack of associations with a threshold of p < 2.3 × 10−7, we further compared the frequencies of the SNPs between the sprint/power and endurance groups with p < 0.05 using the Chi square test. The results pointed out 53 SNPs whose frequencies significantly differentiated between the sport groups (p < 0.05; Supplementary Table S1). Of the 53 SNPs, four functional (i.e., affecting gene expression) variants located on the (or near) SIRT1, NUP210, TRPM2, and AGRN genes were replicated in Russian and Finnish individuals with consistent effects.
The SIRT1 gene encodes the sirtuin 1 protein which is considered as a functional regulator (through the deacetylation and activation) of peroxisome proliferator-activated receptor-γ coactivator (PGC-1α) that induces a metabolic gene transcription program of mitochondrial fatty acid oxidation (one of the positive factors of aerobic capacity) [31]. In our study, we found that the SIRT1 rs41299232 G allele was significantly over-represented in Turkish and Russian endurance athletes compared to sprint/power ones and was associated with increased VO2max and a greater proportion of slow-twitch muscle fibers. Both phenotypes are considered advantageous for endurance athletes. According to the GTEx portal [32], the SIRT1 rs41299232 (intronic variant) is significantly (p = 4.2 × 10−33) associated with the altered expression of the SIRT1 gene in the whole blood. Previously, the rs41299232 G allele was reported to be associated with an increased red blood cell count (p = 0.0000015), higher hemoglobin concentration (p = 0.0032), and higher physical activity (p = 0.0031) in the UK Biobank cohort [33], which is in line with our findings.
The NUP210 gene encodes nucleoporin 210 (a membrane-spanning glycoprotein), which is a major component of the nuclear pore complex. Previously, the NUP210 has been shown as a critical regulator of muscular and neuronal differentiation [34]. Muscle function experiments in mice have shown that Nup210 is required for muscle endurance during voluntary running and muscle repair after injury [35]. In our study, we found that the frequency of the NUP210 rs2280084 A allele was significantly higher in Turkish and Russian endurance athletes compared to sprint/power athletes, as well as in highly elite Russian endurance athletes compared to controls. According to the GTEx portal [32], the NUP210 rs2280084 (missense variant) is significantly associated with changed expression of the NUP210 gene in the brain (p = 3.9 × 10−11) and the whole blood (p = 0.000032).
The TRPM2 gene encodes the transient receptor potential cation channel subfamily M member 2 protein. TRPM2 plays an important role in a variety of cellular functions, including cell proliferation, insulin release, cell motility, and cell death [36,37]. Recently, it has been shown that TRPM2-mediated Ca2+ signaling is required for training-induced improvement in skeletal muscle mitochondrial functions and fiber-type transition in mice [38]. In our study, we found that the TRPM2 rs1785440 G allele was significantly over-represented in Turkish endurance athletes compared to sprint/power ones and was associated with increased VO2max in Russian athletes. According to the GTEx portal [32], the TRPM2 rs1785440 (intronic variant) is significantly (p = 2.5 × 10−12) associated with an altered expression of the TRPM2 gene in the skeletal muscle.
The AGRN gene encodes the agrin protein, which regulates the maintenance of the neuromuscular junction [39]. Previous studies have linked the AGRN gene variants with sarcopenia-related traits (muscle mass and strength) and congenital myasthenia [39,40]. Furthermore, Agrn gene expression has been shown to be upregulated after progressive weighted wheel running in mice [41]. In our study, we found that the AGRN rs4074992 C allele was significantly over-represented in Turkish sprint/power athletes compared to endurance athletes and was associated with a greater CSA of fast-twitch muscle fibers in physically active Russian individuals. Muscle fiber size is a surrogate indicator of muscle mass and is positively associated with power and strength [42,43,44]. According to the GTEx portal [32], the AGRN rs4074992 (intergenic variant) is significantly (p ≥ 3.8 × 10−8) associated with altered expression of the AGRN gene in multiple tissues. Previously, the rs4074992 C allele has been reported to be associated with increased appendicular lean mass (p = 0.0031) in the UK Biobank cohort [33], which is in line with our findings.
Like the present study, a study in diverse populations conducted in the literature reported that none of the SNPs reached genome-wide significance with the endurance athlete status [14]. Nonetheless, others reported the associations of the specific SNPs with different exercise-related parameters [15,16,17,45,46,47,48]. However, the number of participants in those studies was increased by the involvement of the athletes from close countries. Importantly, only one study was able to present a clear association between Tatar wrestlers and a specific SNP in an athletic group with limited participants [5]. Therefore, we can also underline that such studies are critically influenced by the populations, number of the participants, and sport branches.
The present study had some limitations that may be common in other sport genetics studies. These limitations could be the restricted number of participants in the discovery phase (n = 80), heterogeneity in the branches, diverse ethnicity in the Turkish population, lack of controllability of environmental factors, and ignorance of the epigenetic mechanisms. On the other hand, we regard that we were able to reduce the probability of obtaining false-positive results by replicating our initial findings—a widely used approach in sports genetics—in the larger cohorts of Russian (n = 845) and Finnish individuals (n = 287) [14,23,49]. Still, the present study figured out four important SNPs that would further be analyzed in the next studies.
5. Conclusions
In conclusion, by conducting the first comprehensive WES study on elite athletes, we showed that the SIRT1 rs41299232 G, NUP210 rs2280084 A, and TRPM2 rs1785440 G alleles are associated with endurance athlete status, whereas the AGRN rs4074992 C allele is linked with sprint/power athlete status and muscle fiber hypertrophy. Our data indicate that the WES approach followed by replication studies can be used to identify novel genetic markers associated with exercise- and sport-related phenotypes.
Acknowledgments
The authors are grateful to all participants who kindly provided their samples for DNA analysis. The Turkish part of the study was supported by Gazi University Rectorate (Scientific Research Projects Coordination’s Unit, project number: TCD-2021-7116).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14030660/s1, Table S1: SNPs were significantly associated with athlete status in the Turkish cohorts of endurance athletes, sprint/power athletes, and controls.
Author Contributions
Conceptualization, C.B. and M.A.E.; formal analysis, A.K., H.H.K., S.K., E.A.S. and I.I.A.; investigation, C.B., A.K., H.H.K., R.M., E.Z., O.A., I.B., R.E., K.U., E.A.S., A.K.L., N.A.K., E.V.G., L.B., G.B., I.I.A. and M.A.E.; writing—original draft preparation, C.B., H.H.K., A.K. and I.I.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee from the Gazi University Non-Interventional Clinical Research Ethics Committee (with the decision dated April 05, 2021, and numbered 09) and the Ethics Committee of the Federal Research and Clinical Center of Physical-Chemical Medicine of the Federal Medical and Biological Agency of Russia (approval number 2017/04).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gineviciene V., Utkus A., Pranckevičienė E., Semenova E.A., Hall E.C.R., Ahmetov I.I. Perspectives in Sports Genomics. Biomedicines. 2022;10:298. doi: 10.3390/biomedicines10020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varillas-Delgado D., Del Coso J., Gutiérrez-Hellín J., Aguilar-Navarro M., Muñoz A., Maestro A., Morencos E. Genetics and sports performance: The present and future in the identification of talent for sports based on DNA testing. Eur. J. Appl. Physiol. 2022;122:1811–1830. doi: 10.1007/s00421-022-04945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Zaken S., Eliakim A., Nemet D., Rabinovich M., Kassem E., Meckel Y. ACTN3 polymorphism: Comparison between elite swimmers and runners. Sports Med. Open. 2015;1:1–8. doi: 10.1186/s40798-015-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yıldırım D.S., Erdoğan M., Dalip M., Bulğay C., Cerit M. Evaluation of the soldier’s physical fitness test results (strength endurance) ın relation to genotype: Longitudinal study. Egypt. J. Med. Hum. Genet. 2022;23:114. doi: 10.1186/s43042-022-00325-6. [DOI] [Google Scholar]
- 5.Boulygina E.A., Borisov O.V., Valeeva E.V., Ahmetov I.I. Whole genome sequencing of elite athletes. Biol. Sport. 2020;37:295–304. doi: 10.5114/biolsport.2020.96272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Moor M.H.M., Spector T.D., Cherkas L.F., Falchi M., Hottenga J.J., Boomsma D.I., de Geus E.J.C. Genome-wide linkage scan for athlete status in 700 British female DZ twin pairs. Twin Res. Hum. Genet. 2007;10:812–820. doi: 10.1375/twin.10.6.812. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto-Mikami E., Zempo H., Fuku N., Kikuchi N., Miyachi M., Murakami H. Heritability estimates of endurance-related phenotypes: A systematic review and meta-analysis. Scand. J. Med. Sci. Sports. 2018;28:834–845. doi: 10.1111/sms.12958. [DOI] [PubMed] [Google Scholar]
- 8.Zempo H., Miyamoto-Mikami E., Kikuchi N., Fuku N., Miyachi M., Murakami H. Heritability estimates of muscle strength-related phenotypes: A systematic review and meta-analysis. Scand. J. Med. Sci. Sports. 2017;27:1537–1546. doi: 10.1111/sms.12804. [DOI] [PubMed] [Google Scholar]
- 9.Bulgay C., Çetin E., Orhan O., Ergün M.A. The Effects of The ACTN3 and ACE Genes on the Sportıve Performance of Athletes. Inonu Univ. J. Phys. Educ. Sport Sci. 2020;7:1–12. [Google Scholar]
- 10.Ahmetov I.I., Hall E.C., Semenova E.A., Pranckevičienė E., Ginevičienė V. Advances in sports genomics. Adv. Clin. Chem. 2022;107:215–263. doi: 10.1016/bs.acc.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Guilherme J.P.L.F., Semenova E.A., Larin A.K., Yusupov R.A., Generozov E.V., Ahmetov I.I. Genomic Predictors of Brisk Walking Are Associated with Elite Sprinter Status. Genes. 2022;13:1710. doi: 10.3390/genes13101710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulgay C., Ergun M.A. Egzersiz Fizyolojisi ve Temel Kavramlar. Academic Press; New York, NY, USA: 2022. Athletic Performance, Genetics and Epigenetics; pp. 43–56. [Google Scholar]
- 13.Ahmetov I.I., Kulemin N.A., Popov D.V., Naumov V.A., Akimov E.B., Bravy Y.R., Egorova E., Galeeva A., Generozov E., Kostryukova E., et al. Genome-wide association study identifies three novel genetic markers associated with elite endurance performance. Biol. Sport. 2015;32:3–9. doi: 10.5604/20831862.1124568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rankinen T., Fuku N., Wolfarth B., Wang G., Sarzynski M.A., Alexeev D.G., Ahmetov I.I., Boulay M.R., Cieszczyk P., Eynon N., et al. No evidence of a common DNA variant profile specific to world class endurance athletes. PLoS ONE. 2016;11:e0147330. doi: 10.1371/journal.pone.0147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willems S.M., Wright D.J., Day F.R., Trajanoska K., Joshi P.K., Morris J.A., Matteini A.M., Garton F.C., Grarup N., Oskolkov N., et al. Large-scale GWAS identifies multiple loci for hand grip strength providing biological insights into muscular fitness. Nat. Commun. 2017;8:16015. doi: 10.1038/ncomms16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Khelaifi F., Yousri N.A., Diboun I., Semenova E.A., Kostryukova E.S., Kulemin N.A., Borisov O.V., Andryushchenko L.B., Larin A.K., Generozov E.V., et al. Genome-Wide Association Study Reveals a Novel Association Between MYBPC3 Gene Polymorphism, Endurance Athlete Status, Aerobic Capacity and Steroid Metabolism. Front. Genet. 2020;11:595. doi: 10.3389/fgene.2020.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semenova E.A., Zempo H., Miyamoto-Mikami E., Kumagai H., Larin A.K., Sultanov R.I., Ahmetov I.I. Genome-Wide Association Study Identifies CDKN1A as a Novel Locus Associated with Muscle Fiber Composition. Cells. 2022;11:3910. doi: 10.3390/cells11233910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirschhorn J.N., Daly M.J. Genome-wide association studies for common diseases and complex traits. Nat. Rev. Genet. 2005;6:95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka M., Fuku N., Tanisawa K., Higuchi M., Miyamoto-Mikami E., Wang G., Padmanabhan S., Cheng Y.C., Mitchell B.D., Austin K.G., et al. Exome-wide association study of elite Jamaican and African-American sprint athletes. Sport Sci. Res. 2014;11:68. [Google Scholar]
- 20.Spiriev B. IAAF Scoring Tables of Athletics. [(accessed on 5 November 2022)]. Available online: https://www.worldathletics.org/news/iaaf-news/scoring-tables-2017.
- 21.Taylor D.L., Jackson A.U., Narisu N., Hemani G., Erdos M.R., Chines P.S., Swift A., Idol J., Didion J.P., Welch R.P., et al. Integrative analysis of gene expression, DNA methylation, physiological traits, and genetic variation in human skeletal muscle. Proc. Natl. Acad. Sci. USA. 2019;116:10883–10888. doi: 10.1073/pnas.1814263116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhelankin A.V., Iulmetova L.N., Ahmetov I.I., Generozov E.V., Sharova E.I. Diversity and Differential Expression of MicroRNAs in the Human Skeletal Muscle with Distinct Fiber Type Composition. Life. 2023;13:659. doi: 10.3390/life13030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semenova E.A., Miyamoto-Mikami E., Akimov E.B., Al-Khelaifi F., Murakami H., Zempo H., Kostryukova E.S., Kulemin N.A., Larin A.K., Borisov O.V., et al. The association of HFE gene H63D polymorphism with endurance athlete status and aerobic capacity: Novel findings and a meta-analysis. Eur. J. Appl. Physiol. 2020;120:665–673. doi: 10.1007/s00421-020-04306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van der Auwera G.A., Carneiro M.O., Hartl C., Poplin R., Del Angel G., Levy-Moonshine A., Jordan T., Shakir K., Roazen D., Thibault J., et al. From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013;43:11.10.1–11.10.33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K., Li M., Hakonarson H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikuchi N., Moreland E., Homma H., Semenova E.A., Saito M., Larin A.K., Kobatake N., Yusupov R.A., Okamoto T., Nakazato K., et al. Genes and weightlifting performance. Genes. 2022;13:25. doi: 10.3390/genes13010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J., Pressoir G., Briggs W.H., Vroh Bi I., Yamasaki M., Doebley J.F., McMullen M.D., Gaut B.S., Nielsen D.M., Holland J.B., et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006;38:203–208. doi: 10.1038/ng1702. [DOI] [PubMed] [Google Scholar]
- 28.Wang G., Tanaka M., Eynon N., North K.N., Williams A.G., Collins M., Moran C.N., Britton S.L., Fuku N., Ashley E.A., et al. The Future of Genomic Research in Athletic Performance and Adaptation to Training. Med. Sport Sci. 2016;61:55–67. doi: 10.1159/000445241. [DOI] [PubMed] [Google Scholar]
- 29.Mıyamoto-Mikami E., Fuku N. Genetics and Genomics in Sports. Juntendo Med. J. 2020;66:72–77. doi: 10.14789/jmj.2020.66.JMJ19-P12. [DOI] [Google Scholar]
- 30.Visscher P.M., Hemani G., Vinkhuyzen A.A.E., Chen G.-B., Lee S.H., Wray N.R., Goddard M.E., Yang J. Statistical Power to Detect Genetic (Co)Variance of Complex Traits Using SNP Data in Unrelated Samples. PLoS Genet. 2014;10:e1004269. doi: 10.1371/journal.pgen.1004269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerhart-Hines Z., Rodgers J.T., Bare O., Lerin C., Kim S.H., Mostoslavsky R., Alt F.W., Wu Z., Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.GTEx Portal. [(accessed on 13 February 2023)]. Available online: https://gtexportal.org/home/index.html.
- 33.Open Targets Genetics. [(accessed on 13 February 2023)]. Available online: https://genetics.opentargets.org.
- 34.D’Angelo M.A., Gomez-Cavazos J.S., Mei A., Lackner D.H., Hetzer M.W. A change in nuclear pore complex composition regulates cell differentiation. Dev. Cell. 2012;22:446–458. doi: 10.1016/j.devcel.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakuma S., Zhu E.Y., Raices M., Zhang P., Murad R., D’Angelo M.A. Loss of Nup210 results in muscle repair delays and age-associated alterations in muscle integrity. Life Sci. Alliance. 2021;5:e202101216. doi: 10.26508/lsa.202101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sumoza-Toledo A., Penner R. TRPM2: A multifunctional ion channel for calcium signalling. J. Physiol. 2011;589:1515–1525. doi: 10.1113/jphysiol.2010.201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto S., Shimizu S., Kiyonaka S., Takahashi N., Wajima T., Hara Y., Negoro T., Hiroi T., Kiuchi Y., Okada T., et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S.H., Kim B.J., Park D.R., Kim U.H. Exercise induces muscle fiber type switching via transient receptor potential melastatin 2-dependent Ca2+ signaling. J. Appl. Physiol. 2018;124:364–373. doi: 10.1152/japplphysiol.00687.2017. [DOI] [PubMed] [Google Scholar]
- 39.Huzé C., Bauché S., Richard P., Chevessier F., Goillot E., Gaudon K., Ben Ammar A., Chaboud A., Grosjean I., Lecuyer H.A., et al. Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. Am. J. Hum. Genet. 2009;85:155–167. doi: 10.1016/j.ajhg.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pratt J., Whitton L., Ryan A., Juliusdottir T., Dolan J., Conroy J., Narici M., De Vito G., Boreham C. Genes encoding agrin (AGRN) and neurotrypsin (PRSS12) are associated with muscle mass, strength and plasma C-terminal agrin fragment concentration. Geroscience. 2023;2:1–14. doi: 10.1007/s11357-022-00721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dungan C.M., Brightwell C.R., Wen Y., Zdunek C.J., Latham C.M., Thomas N.T., Zagzoog A.M., Brightwell B.D., VonLehmden G.L., Keeble A.R., et al. Muscle-Specific Cellular and Molecular Adaptations to Late-Life Voluntary Concurrent Exercise. Function. 2022;3:zqac027. doi: 10.1093/function/zqac027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guilherme J.P.L., Semenova E.A., Borisov O.V., Larin A.K., Moreland E., Generozov E.V., Ahmetov I.I. Genomic predictors of testosterone levels are associated with muscle fiber size and strength. Eur. J. Appl. Physiol. 2022;122:415–423. doi: 10.1007/s00421-021-04851-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semenova E.A., Pranckevičienė E., Bondareva E.A., Gabdrakhmanova L.J., Ahmetov I.I. Identification and Characterization of Genomic Predictors of Sarcopenia and Sarcopenic Obesity Using UK Biobank Data. Nutrients. 2023;15:758. doi: 10.3390/nu15030758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seaborne R.A., Hughes D.C., Turner D.C., Owens D.J., Baehr L.M., Gorski P., Semenova E.A., Borisov O.V., Larin A.K., Popov D.V., et al. UBR5 is a novel E3 ubiquitin ligase involved in skeletal muscle hypertrophy and recovery from atrophy. J. Physiol. 2019;597:3727–3749. doi: 10.1113/JP278073. [DOI] [PubMed] [Google Scholar]
- 45.Bojarczuk A., Boulygina E.A., Dzitkowska-Zabielska M., Łubkowska B., Leońska-Duniec A., Egorova E.S., Semenova E.A., Andryushchenko L.B., Larin A.K., Generozov E.V., et al. Genome-Wide Association Study of Exercise-Induced Fat Loss Efficiency. Genes. 2022;13:1975. doi: 10.3390/genes13111975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickering C., Suraci B., Semenova E.A., Boulygina E.A., Kostryukova E.S., Kulemin N.A., Borisov O.V., Khabibova S.A., Larin A.K., Pavlenko A.V., et al. A genome-wide association study of sprint performance in elite youth football players. J. Strength Cond. Res. 2019;33:2344–2351. doi: 10.1519/JSC.0000000000003259. [DOI] [PubMed] [Google Scholar]
- 47.Guilherme J., Semenova E.A., Zempo H., Martins G.L., Lancha Junior A.H., Miyamoto-Mikami E., Kumagai H., Tobina T., Shiose K., Kakigi R., et al. Are genome-wide association study identified single-nucleotide polymorphisms associated with sprint athletic status? A replication study with 3 different cohorts. Int. J. Sport. Physiol. Perform. 2021;16:489–495. doi: 10.1123/ijspp.2019-1032. [DOI] [PubMed] [Google Scholar]
- 48.Zani A.L.S., Gouveia M.H., Aquino M.M., Quevedo R., Menezes R.L., Rotimi C., Lwande G.O., Ouma C., Mekonnen E., Fagundes N.J.R. Genetic differentiation in East African ethnicities and its relationship with endurance running success. PLoS ONE. 2022 17:e0265625. doi: 10.1371/journal.pone.0265625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eynon N., Nasibulina E.S., Banting L.K., Cieszczyk P., Maciejewska-Karlowska A., Sawczuk M., Bondareva E.A., Shagimardanova R.R., Raz M., Sharon Y., et al. The FTO A/T polymorphism and elite athletic performance: A study involving three groups of European athletes. PLoS ONE. 2013;8:e60570. doi: 10.1371/journal.pone.0060570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.