Abstract
MicroRNAs (miRNAs), small noncoding RNAs, are post-transcriptional gene regulators that can promote the degradation or decay of coding mRNAs, regulating protein synthesis. Many experimental studies have contributed to clarifying the functions of several miRNAs involved in regulatory processes at the cardiac level, playing a pivotal role in cardiovascular disease (CVD). This review aims to provide an up-to-date overview, with a focus on the past 5 years, of experimental studies on human samples to present a clear background of the latest advances to summarize the current knowledge and future perspectives. SCOPUS and Web of Science were searched using the following keywords: (miRNA or microRNA) AND (cardiovascular diseases); AND (myocardial infarction); AND (heart damage); AND (heart failure), including studies published from 1 January 2018 to 31 December 2022. After an accurate evaluation, 59 articles were included in the present systematic review. While it is clear that miRNAs are powerful gene regulators, all the underlying mechanisms remain unclear. The need for up-to-date data always justifies the enormous amount of scientific work to increasingly highlight their pathways. Given the importance of CVDs, miRNAs could be important both as diagnostic and therapeutic (theranostic) tools. In this context, the discovery of “TheranoMIRNAs” could be decisive in the near future. The definition of well-setout studies is necessary to provide further evidence in this challenging field.
Keywords: MicroRNAs (miRNAs), cardiovascular diseases (CVD), myocardial infarction, heart damage, heart failure, TheranoMIRNAs
1. Introduction
The function and development of the human heart are tightly regulated at multiple levels, starting from the differentiation from multipotent cells to embryonic cardiovascular progenitors, and then suppressing progenitor characteristics to allow differentiation and terminal maturation to arrive at aging processes [1]. MicroRNAs (miRNAs), which are small noncoding RNAs, are post-transcriptional gene regulators that can promote the degradation or decay of coding mRNAs, regulating protein synthesis [2]. In this way, these molecules are involved in various developmental, physiological, and pathological processes [3].
Over the past 15 years, a large number of experimental studies have contributed to clarifying the functions of several miRNAs involved in regulatory processes at the cardiac level [4,5]. Various studies have highlighted the pivotal role of certain miRNAs in hypertrophy, heart failure, and myocardial infarction, considering that they may be involved both in degenerative and reparative processes in cardiomyocytes [6,7,8,9,10,11]. Furthermore, given the intrinsic nature of the heart which is a muscle, several experimental studies focused on the involvement of the so-called myo-miRNAs as mediators and markers of cardiac damage [12,13]. Finally, other studies have shown that other miRNAs, not heart tissue or muscle-specific, may play a pivotal role in cardiovascular disease (CVD) [14,15,16,17,18].
In addition, therapeutic applications of miRNAs in the field of cardiovascular disease have been numerous in recent years, also considering that the death of cardiac cells in the aging heart cannot be replaced because of the arrest of cell division, so new therapeutic applications are needed to prevent cardiac damage. In recent years, attempts have been made to use miRNAs to control the cell cycle, thus trying to restore the regenerative potential of the mature myocardium [19,20,21].
In this context, this research field remains challenging for the international scientific community. This review aims to provide an up-to-date overview, with a focus on the past 5 years, of experimental studies in human samples to present a clear background of the latest advances in order to summarize our current knowledge and future directions.
2. Methods
A systematic review was conducted according to the PRISMA guidelines [22].
SCOPUS and Web of Science were used as the search engines, and searching was conducted from 1 January 2018 to 31 December 2022 to evaluate the present research on the association between miRNA dysregulation and cardiovascular diseases. The following keywords were used: (miRNA or microRNA) AND (cardiovascular diseases); AND (myocardial infarction); AND (heart damage); AND (heart failure).
2.1. Inclusion and Exclusion Criteria
The following exclusion criteria were used: (1) review, 531; (2) Book Chapter, 41; (3) Letter, 17; (4) Conference Paper, 8; (5) Editorial, 7; (6) Note, 5; (7) Retracted, 5; (8) Short Survey, 3; (9) Erratum, 2; articles not in English, 37.
The inclusion criteria were as follows: (1) Original Article, (2) Case Report, (3) Articles in English, (4) Human studies.
2.2. Quality Assessment and Data Extraction
F.S. and M.E. initially evaluated all the articles, evaluating the title, the abstract, and the whole text. M.S. then reanalyzed the articles chosen independently. In cases of conflicting opinions between the articles, they were submitted to C.P.
2.3. Characteristics of Eligible Studies
A total of 2379 articles were collected. Of these, 499 duplicates were removed. A total of 619 articles did not meet the inclusion criteria and 37 articles were not in English. Of a total of 1224 eligible studies, through an electronic function of the SCOPUS database, 640 articles were excluded based on the following keywords: “Animal”; “Animals”; “Animal Experiment”; “Animal Model”; “Animal Tissue”; “Mouse”; “Animal Cell”; “Rat”; “Mice”; “In Vitro Study”; “Cell Proliferation”; “Rats”; “Disease Models, Animal”; “Mice, Inbred C57BL”; “Cells, Cultured”; “Sprague Dawley Rat”; “Rats, Sprague-Dawley”. In conclusion, 584 articles were further screened. After an accurate evaluation, 59 articles were included in the present systematic review (Figure 1).
Figure 1.
Flow diagram illustrating included and excluded studies in this systematic review.
3. Results and Discussion
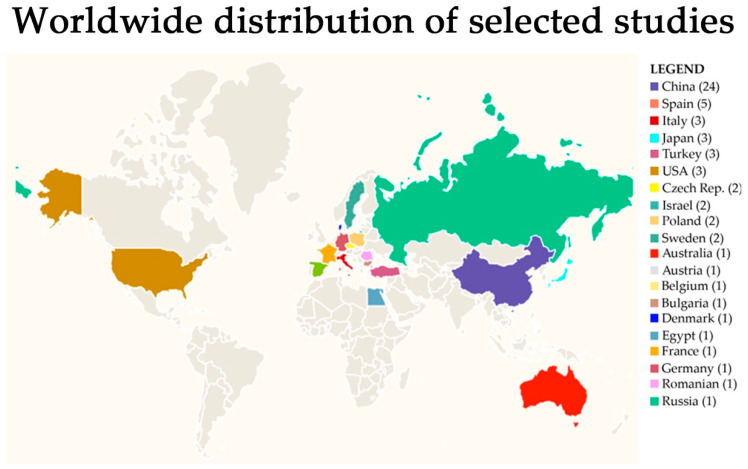
Analyzing the nationality of the first author, as summarized in Figure 2, the research groups that contributed to the selected 59 articles were from China (24), Spain (5), Italy, Japan, Turkey, and the USA (3), Czech Republic, Israel, Poland, and Sweden (2), Australia, Austria, Belgium, Bulgaria, Denmark, Egypt, France, Germany, Romania, and Russia (1). Temporarily, the distribution of published articles by year of publication was 2018 (3), 2019 (6), 2020 (14), 2021 (22), and 2022 (14).
Figure 2.
Distribution of selected studies by country.
Focusing on the different miRNAs investigated, the roles of 223 miRNAs were tested. Among these, the miRNAs investigated in at least five studies were miR-133a-3p (10), miR-21, miR-499a-5p (8 times each), miR-1 (6), and miR-126 (5). This number refers only to those miRNAs that had been tested singularly, while a lot of studies used a screening approach based on microarray technologies in order to select the miRNAs to test in the second phase of their studies.
All selected articles are summarized in Table 1, following a chronological criterion.
Table 1.
Main findings of the systematic review. The results are divided into columns: author, type of study, miRNAs involved, target (genetic, pathway, cellular), type of regulation (up/down), and clinical consequences. HCM—Hypertrophic cardiomyopathy, MI/R—myocardial ischemic reperfusion, ACS—acute coronary syndrome, MVD—multivessel disease, HF—Heart failure, AF—Atrial fibrillation, CAD—coronary artery disease, DCM—idiopathic dilated cardiomyopathy, CVE—Cardiovascular event, CVD—Cardiovascular disease, ACAS—Asymptomatic carotid artery stenosis, ARVC—Arrhythmogenic right ventricular cardiomyopathy, SCD—Sudden cardiac death, MI—Myocardial infarction, CTEPH—Chronic thromboembolic pulmonary hypertension, FGFR1—Fibroblast growth factor receptor 1.
| First Author, Year, and Country | Study Model | miRNAs Investigated | Biological Sample/Target | Dysregulation (Up/Down) | Effect of Dysregulation |
|---|---|---|---|---|---|
| Bayés-Genis et al., 2018, Spain [23] | Observational prospective study | miR-22-3p, miR-133a-3p, miR-133b, miR-208a-3p, miR-320a, miR-345-5p, MiR-378a-3p, miR-423-5p, miR-499a-5p, MiR-622, miR-1254, MiR-1306-5p | Serum/CCAR1 (cell division and apoptosis regulator protein 1) | up (miR-1254, miR-1306-5p) | Significantly associated with increased in-hospital heart failure (HF) death. |
| Guo et al., 2018, China [24] | Case-control | miR-133a, miR-221 | Plasma/Heart Failure with Reduced Ejection Fraction (HFrEF) gene | down | HF diagnostic biomarkers in elderly patients |
| Masson et al., 2018, Italy [25] | Randomized clinical trial | miR-132 | Plasma/anti-hypertrophic transcription factor FoxO3 | down | Prediction of HF severity |
| Li et al., 2019, China [26] | Case-control | miR-208, miR-494, miR-499, miR-1303 | Plasma/PTEN, ROCK1 | down | miR-208, miR-494, miR-499 and miR-1303 can be used as markers of myocardial infarction (MI), however, they do not have a higher value than traditional troponins |
| Liu et al., 2019, China [27] | Cohort | miR-150-3p, miR-197-5p, miR-320a, miR-494-3p, miR-939-5p, miR-1268a, miR-1275, | Plasma/Cardiomyocytes | up | Increase in HF adverse events |
| Mayer et al., 2019, Czech Republic [28] | Observational prospective study | miR-1, miR-19a, miR-21, miR-34a, miR-126, miR-133a, miR-197, miR-208b, miR-214, miR-223, miR-499 | Serum/Inflammatory cells (increased fibrosis and apoptosis) | down (miR-19a) | In patients with carotid artery stenosis (CAS), a low level of miR-19a is an independent risk factor for mortality |
| Pinchi et al., 2019, Italy [29] | Case-control | miR-1-3p, miR-133a-3p, miR-208a-3p, miR-499a-5p, | Heart tissue/Ion channels | down (miR-1, miR-208) up (all in SCD) | Biomarkers of sudden cardiac death (SCD) due to early acute MI |
| Zhang et al., 2019, China [30] | Case-control | miR-155 | Serum/Genes involved in pulmonary fibrosis and CAD (coronary artery disease). | down | Patients with HF after MI have elevated levels of these miRNAs |
| Zhu et al., 2019, China [31] | Retrospective cohort | miR-182-5p miR-5187-5p | Plasma/VEGF (vascular endothelial growth factor). | up | Diagnostic biomarkers for CAD |
| Asulin et al., 2020, Israel [32] | Case-control | miR-145-5p, miR-199a-5p, miR-5701 | Human genes of development, cell growth, differentiation, proliferation, apoptosis, metabolism, and tissue remodeling | up (rheumatic valvulopathy) down (idiopathic valvulopathy) | Useful for differentiating the etiology of rheumatic from idiopathic valvulopathies |
| Barbalata et al., 2020, Romania [33] | Original article | miR-92a-3p miR-142-3p, miR-155-5p, miR-223-3p, | Plasma/TGF-beta2 | (miR-92a) Down (miR-142, miR-155, miR-223) up |
Prediction of CVD in patients with peripheral arterial disease |
| Ben-Zvi et al., 2020, Israel [34] | Case-control | miR-21-5p, miR-92b-3p, miR-125b-5p, miR- 133a-3p | Serum/cardiomyocytes | up (miR-125b-5p, miR-133-3p), down (miR-21-5p, miR-92b-3p) | Increased incidence of HF |
| Elbaz et al., 2020, France [35] | Case-control | miR-16, miR-92a, miR-122, miR-150, miR-186, miR-195, miR-223-5p, | Serum/Inflammatory cells (increased fibrosis and apoptosis) | up | Biomarker risk of ACS (acute coronary syndrome). |
| Ling et al., 2020, China [36] | Case-control | miR-21, miR-126 | Serum/PTEN | (miR-21) down (miR-126) up |
Biomarker of ACS |
| Liu et al., 2020, China [37] | Case-control | miR-1-3p, miR-20b-5p, miR-30b-5p, miR-142-3p, miR-1273g-3p, miR-6515-3p, miR-6793-5p, miR-7109-3p, | Serum/MAPK signaling pathway | up | Involvement in the pathogenesis of angina (stable and unstable) |
| Nie et al., 2020, China [38] | Case-control | miR-4281 miR-4763-3p | Plasma/KEGG related to apoptosis (TGF-β, mTOR, insulin, MAPK, p53) | up | Potential biomarker of fulminant myocarditis |
| Santos et al., 2020, Denmark [39] | Case-control | miR-130b-3p, miR-208a-3p, miR-338-5p | Heart biopsy/ion channel genes, extracellular matrix genes | down (ion channel genes) up (extracellular matrix genes) | Involvement in the development of AF |
| Silverman et al., 2020, USA [40] | Case-control | miR-29a-3p, miR-30a-5p, miR-150-5p | Plasma/Inflammatory cells (increased fibrosis and apoptosis) | up | Risk of increased SCD in patients with CAD |
| Su et al., 2020, China [41] | Case-control | miR-1 | Serum/Endothelial function, angiogenesis and cell apoptosis | up | miR-1 within 3 h of acute chest pain is an independent risk factor for mortality in patients with MI |
| Turky et al., 2020, Egypt [42] | Observational prospective study | miR-133a | Plasma/FGFR1 | up | Biomarker for early identification of stable CAD |
| Wakabayashi, 2020, Japan [43] | Case-control | miR-16-5p, miR-17-5p, miR-92a-3p miR-106a-5p, miR-135a-3p, miR-150-3p, miR-191-5p, mR-320b, miR-451a, miR-486-5p, miR-663b, | Serum/pro-inflammatory cytokines in foam cells and collagen synthesis in vascular smooth muscle cells | up | Increased incidence of ischemic heart disease |
| Wang et al., 2020, China [44] | Case-control | miR-22, miR-499 | Serum/Cardiac myosin heavy chain gene | up | Sensitive and specific biomarkers for the diagnosis of MI |
| Weldy et al., 2020, USA [45] | Observational prospective study | miR-28- 3p, miR-371b-3p, miR-433-3p | Plasma/SMAD3 and 4, TGF-β1 and 2, E2F family transcription factors | up | Increasing right ventricular (RV) size and decreasing RV systolic function |
| Brundin et al., 2021, Sweden [46] | Case-control | miR-16-5p, miR-21-5p, miR-29-5p, miR-133a-3p, miR-191-5p, miR-320a, miR-423-5p | Serum/extracellular matrix proteins | up | Seven miRNAs were upregulated both in subjects suffering from idiopathic dilated cardiomyopathy (DCM) and ischemic heart disease (IHD) |
| Chen et al., 2021, China [47] | Case-control | miR-4329, miR-6718-5p | Plasma/MAPK, PI3K-Akt, Ras, Rap1 signaling pathway | down | Biomarkers for acute MI |
| Coban et al., 2021, Turkey [48] | Original article | miR-18a-3p, miR-130b-5p | Serum/SPP1 and TNFRSF11B genes | up | Biomarkers of CAD development |
| Elgebaly et al., 2021, USA [49] | Case-control | miR-106b miR-137, | Serum/Genes of Nourin | up | Biomarkers for early diagnosis of myocardial ischemia in patients suspected of CAD |
| Garcia-Elias et al., 2021, Spain [50] | Case-control | miR-22-5p, miR-199a-5p | Plasma/L-type Ca2+ channel, NCX and connexin-40 | up | Decreased cardiac ejection fraction and increased incidence of AF |
| Gevaert et al., 2021, Belgium [51] | Observational prospective study | miR-181c | MAPK1, DNM2, and CDH1 (HFpEF pathophysiology) | up | Predicts response to exercise training in patients with HF |
| He et al., 2021, China [52] | Observational prospective study | miR-29b | Plasma/Inflammatory cells (increased fibrosis and apoptosis) | down | Independent risk factor for coronary artery calcification in patients with renal disease |
| Hromadka et al., 2021, Czech Republic [53] | Randomized clinical trial | miR-126-3p, miR-223-3p | VEGF, VCAM-1, SPRED1, PIK3R2/p85- beta, P2Y12, RPS6KB1/HIF1a | up | Independent risk stratification biomarkers for thrombotic events after MI |
| Lu et al., 2021, China [54] | Observational prospective study | miR-27b | Serum/Vascular smooth muscle cells |
up | Prediction of the occurrence of ACS |
| Mihaleva et al., 2021, Bulgaria [55] | Case-control | miR-16-5p, miR-155-3p, miR-155-5p, miR-210, miR-221-3p, miR-424-5p | Serum/HIF1A (transcriptional regulator of the adaptive response to hypoxia) | up | Biomarker of cardiovascular complications in diabetic patients |
| Moric-Janiszewska et al., 2021, Poland [56] | Case-control | miR-1, miR-133a, miR-133b | Genes involved in the regulation of ion channels | up | Diagnostic biomarkers of arrhythmia in pediatric patient |
| Neiburga et al., 2021, China [57] | Original article | miR-10-5p, miR-10b-3p, miR-17-3p, miR-21-5p, miR-151a-5p, miR-181a-5p, miR-185-5p, miR-194-5p, miR-199a-3p, miR-199b-3p, miR-212-3p, miR-363-3p, miR-548d-5p, miR-744-5p, miR-3117-3p, miR-5683, miR-5701 | Serum/AKT, PTEN and IRS1 | down | Biomarkers of CVD |
| Sacchetto et al., 2021, Italy [58] | Case-control | miR-185-5p | Plasma/Inflammatory cells | up | Diagnostic biomarkers for ARVC (arrhythmogenic right ventricular cardiomyopathy) |
| Shen et al., 2021, China [59] | Case-control | Let-7b-3p, miR-21-3p, miR-28-3p, miR-99b-5p, miR-181c-3p, miR-133b, miR-320a, miR-500a-3p, miR-574-5p, miR-940, miR-1268b, miR-1307-3p, miR-4286, miR-4485-3p, | Serum/PI3K/AKT pathway | up (miR-4286) | Biomarker for increased risk of ACS |
| Suzuki, 2021, Japan [60] | Case-control | miR-126, miR-221, miR-222 | Serum/NF-κB pathway | down | Increased incidence of hypertension |
| Szelenberger et al., 2021, Poland [61] | Case-control | miR-130b-3p, miR-142-3p, miR-146a-3p, miR-197-5p, miR-301a-3p, miR-338-3p, miR-3162-5p, miR-3656, miR-4299, miR-8069 | Platelet/ARHGEF12, (regulation of actin cytoskeleton), AKT3 (focal adhesion), ARHGEF12 (vascular smooth muscle contraction) | 5 miRNAs were upregulated (miR-301a-3p, miR-142-3p, miR-146a-3p, miR-130b-3p, miR-338-3p) and 5 miRNAs were downregulated (miR-8069, miR-4299, miR-3656, miR-197-5p, miR-3162-5p) | Potential platelet biomarker of ACS |
| Thottakara, 2021, Germany [62] | Case-control | miR-1, miR-495-3p, miR-499a-5p, miR-627-3p, miR-3144, miR-4454, | Plasma/Sarcomeric genes | up | Increased incidence of hypertrophic cardiomyopathy (HCM) |
| Tong et al., 2021, China [63] | Observational prospective study | miR-222 | Serum/PI3K/AKT pathway | down | Increased incidence of MI/R |
| Xiao et al., 2021, China [64] | Case-control | miR-146a | Serum/S100A12 | up | Biomarker for adverse prognosis of ST-Segment Elevation MI |
| Yamada et al., 2021, Japan [65] | Retrospective cohort | miR-21, miR-29a, miR-126 | Serum/Inflammatory cells | up (miR-21 and miR-19a) down (miR-126) |
Risk of premature death from cancer and CVD |
| Yan et al., 2021, China [66] | Case-control | miR-133a-3p, miR-223-3p, miR-499a-5p, miR-3113-5p, | Heart tissue/Inflammatory cells (increased fibrosis and apoptosis) | up | Sensitive biomarkers of SCD |
| Zhelankin et al., 2021, Russia [67] | Case-control | miR-21-5p, miR-17-5p, miR-146a-5p, | Plasma/cardiomyocytes | up (miR-21-5p, miR-146a-5p) down (miR-17-5p) | An increase in miR-146a-5p and miR-21-5p is an indicator of ACS, a decrease in miR-17-5p could be considered a general biomarker of CAD. |
| Eikelis et al., 2022, Australia [68] | Original article | miR-132 | Serum/PTEN, SIRT1 | down | Biomarker of hypertension in obese patients |
| Eyyupkoca et al., 2022, Turkey [69] | Case-control | miR-23b-3p, miR-26b-5p, miR-199a-5p, miR-301a-3p, miR-374a-5p, miR-423-5p, miR-483-5p, miR-652-3p | Plasma/Gene expression and remodeling of extracellular matrix | down (miR-301a-3p, miR-374a-5p) up (miR-423-5p) | Biomarker of adverse left ventricular remodeling after MI |
| Gager, 2022, Austria [70] | Observational prospective study | miR-125a (miR-125b, miR-223) |
Plasma/Cardiomyocytes | up | Reduction of survival for ACS |
| James et al., 2022, Sweden [71] | Case-control | miR-224-5p | Extracellular Vesicles (EVs)/SMAD unit (TGF-beta pathway) | up | Biomarker of endothelial dysfunction in patients with low coronary flow reserve |
| J. Li et al., 2022, China [72] | Case-control | miR-203 | Serum/Inflammatory cells (increased fibrosis and apoptosis) | up | Biomarker for early prediction of ST-Segment Elevation MI |
| Miao et al., 2022, China [73] | Retrospective study | miR-17-5p, miR-20a-5p, miR-93-5p, miR-665, miR-3202 | Serum/Pulmonary artery smooth muscle cells and pulmonary artery endothelial cells (proliferation and apoptosis) | miR-20a-5p, miR-93-5p, miR-17-5p downregulated | Useful parameter in the diagnosis of chronic thromboembolic pulmonary hypertension (CTEPH) |
| Mompeón et al., 2022, Spain [74] | Observational prospective study | let-7g-5p, let-7e-5p, miR-26a-5p | Plasma/Involvement in the production of cytokines and chemokines | down | Potential biomarker of MI prognosis |
| Moscoso et al., 2022, Spain [75] | Observational prospective study | miR-125b, miR-499a, | Serum/KEGG related to apoptosis (TGF-β, mTOR, insulin, MAPK, p53) | up | Improvement of left ventricular ejection fraction after cardiac resynchronization therapy |
| de los Reyes-García et al., 2022, Spain [76] | Original article | miR-146a | Serum/TLR/NF-kB pathway | down | Contribution to thrombo-inflammation and MI recurrence in young patients |
| Wang et al., 2022, China [77] | Observational prospective study | miR-29 | Serum/PI3K/mTOR/HIF1α/VEGF pathway | down | Development of MI |
| Yang et al., 2022, China [78] | Case-control | miR-29b (miR-let-7b) |
Serum/Osteogenic transcription factors | down | Increased incidence of coronary artery calcification |
| Yu et al., 2022, China [79] | Case-control | miR-221, miR-222 | Plasma/c-Raf/MEK/ERK pathway | up | Severity of ACS |
| Zhang et al., 2022, China [80] | Case-control | miR-21, miR-208b | Plasma/TGF-β1/Smad-3 Signaling Pathway | up | Cardiac fibrosis progression through activation of the TGF-β1/Smad-3 signaling pathway |
| Zhou et al., 2022, China [81] | Observational prospective study | miR-133a | Serum/FGFR1 | up | Biomarker for early identification of stable CAD |
3.1. miRNA Dysregulation and Heart Failure
The first study published in the included period was by Bayés-Genis et al. [23]; these authors investigated the role of 12 miRNAs, concluding that the levels of miR-1254 and miR-1306-5p were significantly associated with a risk of mortality and heart failure (HF) hospitalization. In this scenario, hypertrophic cardiomyopathy (HCM), a disease in which the heart muscle becomes thickened, is most often inherited and is the most common form of genetic heart disease. The main hallmarks of HCM are left ventricular hypertrophy, myocardial disarray, and interstitial fibrosis [82,83,84].
Wakabayashi et al. [43] demonstrated that different miRNAs could be involved in proinflammatory cytokine production, suggesting their involvement in ischemic heart disease (IHD). Moreover, the same authors demonstrated that the upregulation is significantly higher in Caucasians compared to Asian subjects, justifying the data that mortality from IHD is significantly lower in Japan than in other developed countries. Brundin et al. [46] reported that seven miRNAs were upregulated both in subjects suffering from idiopathic dilated cardiomyopathy (DCM) and IHD. Moreover, they described that miR-29-5p was significantly higher in DCM compared with IHD. Weldy et al. [45] reported that the high plasma levels of miR-28-3p, miR-433-3p, and miR-371b-3p are related to increased right ventricular size. Moscoso et al. [75] described the prognostic value of miR-499a and miR-125b in response to cardiac resynchronization therapy: the patients who positively responded to therapy showed lower plasma levels of these miRNAs. Eyyupkoca et al. [69] described that eight miRNAs (miR-23b-3p, miR-26b-5p, miR-199a-5p, miR-301a-3p, miR-374a-5p, miR-423-5p, miR-483-5p, and miR-652-3p) were associated with adverse left ventricular remodeling (ALVR), although only three, miR-301a-3p, miR-374a-5p, and miR-423-5p, in a significative manner. James et al. [71] performed a multiplex array profiling, demonstrating that miR-224-5p is more expressed in circulating vesicles of patients with reduced coronary flow reserve. Barbalata et al. [33] concluded that plasma levels of miR-142, miR-223, and miR-155 were higher in peripheral artery disease (PAD) patients with cardiovascular events (CVEs) compared with those without CVEs, while miR-92a is lower. Based on the results of this study, these miRNAs could be used as predictive factors of CVEs among PAD patients with good accuracy.
Tong et al. [63] demonstrated that miR-222 is more expressed in the first phase after MI. Moreover, these authors performed an in vitro study, demonstrating that MI damage could be attenuated by the inhibition of miR-222, considering that it may be involved in the regulation of the PI3K/AKT pathway during myocardial hypoxia-reoxygenation (HR) injury. Liu et al. [27] reported that different miRNAs were over-expressed in subjects with HF; in particular, they associated high plasma levels of miR-197-5p with adverse events after HF in patients under 50 years old. Garcia-Elias et al. [50] reported that plasma levels of miR-199a-5p and miR-22-5p are higher in patients with heart failure combined with reduced ejection fraction (HFrEF) and atrial fibrillation (AF), suggesting that they could be involved in arrhythmogenic mechanisms. Ben-Zvi et al. [34] demonstrated that various miRNAs may be involved in HF, showing upregulation of miR-125b-5p and miR-133-3p, and downregulation of miR-21-5p and miR-92b-3p. Masson et al. [25] highlighted a pivotal role for miR-132; in particular, miR-132 may be helpful to intensify strategies aimed at reducing re-hospitalization. Based on their results, higher levels of miR-132 were associated with more severe HF symptoms, suggesting that an anti-miR could be useful to reduce the risk for these patients. In the same year, Guo et al. [24] published a paper that highlighted the importance of miR-133a and miR-221 as potential HF diagnostic biomarkers, particularly when their levels were high in elderly patients. Zhang et al. [30] reported that patients with HF after MI had elevated miRNA-155 levels and poor cardiac function; for this reason, they hypothesized the use of this miRNA as a biomarker to assess the severity of the disease.
The identification of new molecular biomarkers in the early detection of HCM is a challenging objective in the research field. As reported by Thottakara et al. [62], the levels of miR-1, miR-3144, miR-4454, miR-495-3p, miR-499a-5p, and miR-627-3p were tested in the plasma samples of patients affected by HCM, and were higher than in healthy individuals. Interestingly, these authors described for the first time the correlation between high miR-4454 plasma levels and cardiac fibrosis in HCM, suggesting that miR-4454 could be used as a potential biomarker for cardiac fibrosis.
Nie et al. [38] reported that at the onset, miR-4763-3p and miR-4281 were upregulated in the plasma samples of patients suffering from fulminant myocarditis (FM), while their levels were restored as the clinical symptoms recovered. In this way, these miRNAs could be used as predictor factors of therapy-responsive patients.
3.2. miRNA Dysregulation and Acute Coronary Syndrome (ACS)
Acute coronary syndrome (ACS) is a term used to describe a series of conditions that are associated with a sudden reduction in blood flow to the heart. ACS includes three different pathological conditions: ST-segment elevation myocardial infarction (STEMI); non-ST-segment myocardial infarction (NSTEMI); and unstable angina (UA). A wide range of miRNAs have been investigated to clarify their role in ACS, considering that it is multifactorial and occurs in response to inflammation [85,86]. In this scenario, Mompeón et al. [74] reported that let-7g-5p, let-7e-5p, and miR-26a-5p plasma expression was inversely associated with serum levels of pro-inflammatory cytokines; this research group concluded that these biomarkers could be used as therapeutic/target biomarkers for ischemic heart disease.
Yu et al. [79] demonstrated that plasma levels of circulating miR-221 and miR-222 are upregulated in ACS patients, showing elevated levels which are positively associated with the severity of the coronary artery lesions. Elbaz et al. [35] concluded that seven miRNAs were significantly differentially expressed comparing ACS cases versus controls; in this way, miR-16, miR-92a, miR-122, miR-150, miR-186, miR-195, and miR-223-5p could be used as novel biomarkers for ACS. Shen et al. [59] investigated the role of 14 candidate miRNAs, demonstrating that the higher plasma levels of miR-4286 could be associated with an increased risk of ACS. Szelenberger et al. [61] analyzed miRNA expression starting from platelet samples in order to identify new molecular biomarkers of ACS. These authors analyzed the expression levels of 10 miRNAs, identifying 5 miRNAs overexpressed and 5 downregulated. Moreover, combining other clinical data, they concluded that in platelet samples, miR-142-3p could be used as a marker of modeling for ACS risk. Gager et al. [70] reported that high levels of miR-125b are related to a low survival rate after an ACS, suggesting that lower values could be related to improved long-term survival in patients with ACS and multivessel disease (MVD).
Coronary artery calcification (CAC) is an indicator of coronary artery disease (CAD), providing information about the cardiovascular risks of the patients involved in ACS. CAC is frequently associated with different chronic inflammatory diseases, such as diabetes, chronic kidney disease, and aging, that are widely diffused in developed countries; for this reason, the identification of theranostic markers could be very useful. Yang et al. [78] reported that the under-regulation of miR-29 in serum samples may be considered an important signal of early identification of CAC. In agreement with this study, He et al. [52] concluded that lower plasma levels of miRNA-29b may be used as independent risk factors for the incidence of cardiovascular events (CVEs) and CAC. Lu et al. [54] focused their study on the role of miR-27b: high levels of this miRNA may predict the occurrence of asymptomatic carotid artery stenosis (ACAS), with a high risk of generating subsequent cerebral ischemia events (CIEs).
Zhelankin et al. [67] reported that an increase in miR-146a-5p and miR-21-5p represents an indicator of ACS; at the same time, a downregulation of miR-17-5p could be considered a general biomarker of CAD. Zhou et al. [81] concluded that the circulating level of miR-133a was significantly higher in patients with periprocedural myocardial injury before and after the procedure, concluding that this biomarker may be used for early identification of stable CAD patients. Coban et al. [48] reported miR-18a-3p and miR-130b-5p as biomarkers of CAD, indicating its development and progression. The role of miR-150-5p, miR-29a-3p, and miR-30a-5 has been investigated by Silverman et al. [40] who highlighted the high plasma levels of these miRNAs as being related to SCD in subjects suffering from CAD. These results are confirmed by Yamada et al. [65] who reported that levels of miR-21 and miR-29a are higher in subjects suffering from CVD, while a low level of miR-126 seems to be related to an increased risk of death. Zhu et al. [31] summarized that serum levels of miR-182-5p and miR-5187-5p could be used as diagnostic biomarkers for CVD, providing diagnostic information for discriminating unprotected left main coronary artery disease patients from non-coronary artery disease. Asulin et al. [32] reported that the expression values of different miRNAs could be related to the etiology of rheumatic from idiopathic valvulopathies, improving our knowledge of the mechanisms underlining aortic valve (AV) disease. Sacchetto et al. [58] performed a case-control study, concluding that the upregulation in the plasma sample of miR-185-5p could be used as a promising biomarker in arrhythmogenic right ventricular cardiomyopathy (ARVC) patients.
Liu et al. [37] reported that different miRNAs are upregulated both in stable and unstable angina (UA) compared with subjects with normal coronary arteries; in particular, they suggest that these circulating miRNAs could be used as potential biomarkers of UA. Ling et al. [36] reported that miR-21 (less expressed compared with controls) and miR-126 (more expressed compared to controls) could be useful markers for predicting AMI and UA. Elgebaly et al. [49] reported that the expression levels of miR-137 and miR-106b-5p could be used as reliable biomarkers for UA and acute STEMI patients. Turky et al. [42] confirmed the importance of testing miR-133 in the plasma of subjects suffering from CVD. Particularly, this miRNA was higher in NSTEMI than UA patients; moreover, a significant upregulation is reported in the STEMI group compared to the NSTEMI group. Xiao et al. [64] concluded that miR-146a could be used as a new biomarker for adverse prognosis of STEMI; in particular, it may exert its function and its pathogenesis by targeting S100A12, a protein that is involved in the inflammatory response. J. Li et al. [72] analyzed the predictive value of serum miR-203 for acute STEMI, concluding that this miRNA represents a potential new biomarker in the early prediction of STEMI. Finally, Li et al. [26] demonstrated that the plasma levels of miR-208, miR-494, miR-499, and miR-1303 were higher in the tested group compared to controls; at the same time, the authors concluded that their predictive value is not superior to high-sensitivity cardiac troponin T (hs-cTnT) as myocardial markers in the diagnosis of early acute myocardial infarction (MI).
Neiburga et al. [57] examined the role of 17 miRNAs, identifying different risk factors: in particular, miR-629-5p and miR-98-5p were indicated as risk factors for acute MI. Gevaert et al. [51] investigated the role of miR-181c in response to exercise training in patients after MI: the circulating levels of this miRNA could be useful to identify low responders (LR) prior to training, giving the opportunity to plan a specific recovery activity. Su et al. [41] reported that circulating miR-1 within 3 h of acute chest pain could be of potential diagnostic value for AMI and could be classified as an independent risk factor for the prognosis of AMI. Hromadka et al. [53] identified two miRNAs that could be considered predictor markers for thrombotic events; in particular, miR-126-3p and miR-223-3p may be used for ischemic risk stratification after AMI. de los Reyes-García et al. [76] reported that miR-146 could be considered a promotor of thromboinflammation and recurrence in young patients with AMI. Chen et al. [47] reported that the expression levels of miR-4329 and miR-6718 were significantly lower in patients with myocardial infarction compared with controls. Based on these data, the authors concluded that these miRNAs could be used as potential biomarkers for AMI. Zhang et al. [80] demonstrated that miR-21 and MiR-208b promote cardiac fibrosis in AMI patients, activating the TGF- β1/Smad-3 signaling pathway. Wang et al. [44] investigated the role of miR-22 and miR-499 as sensitive biomarkers in the diagnosis of MI, confirming that these miRNAs could play an important role in early diagnosis. Wang et al. [77] investigated the role of miR-29: it was expressed less in subjects after MI. These authors demonstrated that the inhibition of this miRNA promoted angiogenesis, reduced fibrosis, and alleviated MI damage. Finally, Moric-Janiszewska et al. [56] identified miR-1, miR-133a, and miR-133b as potential diagnostic biomarkers of arrhythmia in pediatric patients.
3.3. miRNA Dysregulation in Heart Tissue Samples
Based on the literature review data, in the last five years, only three studies investigated miRNA dysregulation in heart tissue samples. These studies may be considered important because they were performed on human samples obtained after autopsy or tissue biopsies.
Yan et al. [66] investigated, in heart tissue, the role of four miRNAs (miR-133a-3p, miR-223-3p, miR-499a-5p, miR-3113-5p) as potential biomarkers for the diagnosis of sudden cardiac death (SCD). Based on their results, all miRNAs were significantly upregulated in the SCD group, suggesting their use in the post-mortem investigation in cases of SCD.
In agreement with this study, Pinchi et al. [29] investigated the role of miR-1, miR-133, miR-208, and miR-499, concluding that all miRNAs were higher in subjects who died from SCD compared with control. Moreover, the same authors compared the SCD group with the acute myocardial infarction (AMI) group, concluding that the expression levels of miR-1, miR-208, and miR-499 could distinguish between SCD and AMI.
Santos et al. [39] analyzed the miRNA profile of heart biopsies from atrial fibrillation (AF) patients, reporting that miR-130b-3p, miR-208a-3p, and miR-338-5p were differentially expressed in AF tissue samples.
3.4. miRNA Dysregulation and Increased Risks of Hypertension
Hypertension is a major health problem worldwide, considering that it is an important risk factor for heart disease and stroke; early identification could be very useful in order to prevent severe consequences in patients.
Suzuki et al. [60] reported that levels of miR-126, miR-221, and miR-222 were lower in subjects suffering from high blood pressure, suggesting that these miRNAs could be good candidates for hypertension prediction.
Miao et al. [73] concluded that miR-20a-5p, miR-93-5p, and miR-17-5p could have a potential value for chronic thromboembolic pulmonary hypertension with right ventricular dysfunction and injury.
Eikelis et al. [68], in their study, investigated the role of miR-132, describing a negative correlation between cardiovascular and metabolic diseases. Particularly, the reduction of miR-132 levels could be considered a target for the regulation of liver lipid homeostasis, with the possibility to act on the control of obesity-related blood pressure.
Mihaleva et al. [55] investigated the role of six miRNAs, demonstrating that their levels are higher in the tested group compared to controls. In particular, the expression levels of miR-155-5p and miR-424-5p were significantly higher, suggesting their pivotal role as promising biomarkers for cardiovascular damage in patients with type 2 diabetes mellitus. Mayer et al. [28], in their experimental study, demonstrated that a low expression of circulating miR-19a reflected a substantial additional mortality risk in stable cardiovascular patients.
3.5. The Most Investigated miRNAs: The Role of miR-133a-3p, miR-21, miR-499a-5p, miR-1, and miR-126
As previously described, the most studied miRNA is miR-133a-3p. Based on a recent in vitro study, considering that cardiac hypertrophy was induced by Ang II, the expression of miR-133a-3p was repressed in Ang II-treated HCM cells; contrariwise, its overexpression may be a promising strategy for cardiac hypertrophy treatment [87]. Its role has been investigated as an MI biomarker, but it has been concluded that it is not more specific when compared with troponin [88]. Moreover, this miRNA has been investigated as a regulator of angiogenesis, obtaining conflicting results. Nevertheless, its role could be functional in the regulation of diseased endothelial cells, leading to new therapeutic interventions in the treatment of patients suffering from cardiovascular pathologies that occur with excessive or insufficient angiogenesis [89]. Interestingly, this miRNA has been identified as a predictor biomarker for familial hypercholesterolemia as a substrate of CVD, considering that its elevated plasma levels anticipate these values within the following 2 years (average). This miRNA acts directly through lipid and inflammatory signaling in key cells for atherosclerosis progression [90].
MiR-21 is an essential regulator for cardiac homeostasis, acting as a cell–cell messenger with diverse functions. Considering its pivotal role in cardiac function regulation, it has been frequently investigated, taking into account its prospects in clinical therapy. Particularly, it exhibits fundamental functions in the cardiovascular system by targeting different mRNAs [91]. MiR-21 is involved in various cardiomyopathies (such as heart failure, dilated cardiomyopathy, myocardial infarction, and diabetic cardiomyopathy). Its levels notably change in both heart tissue and blood circulation, providing cardiac protection after heart injury [92]. It is noteworthy that in various papers, this miRNA was found more expressed in the infarct zone in mouse heart tissue exposed to AMI; this miRNA is involved in myocardial fibrosis post-MI, promoting the transforming growth factor-beta 1 (TGF-β1)-induced fibroblast activation, with the subsequent increased expression of Collagen-1, alpha-smooth muscle actin (α-SMA), and F-actin; on the other hand, miR-21 attenuated fibrotic processes [93].
In 2015, miR-499a-5p was described as a novel and sensitive biomarker of AMI. Based on this study, this miRNA may be used as a useful marker for early diagnosis of AMI [94]. The same miRNA seems to be involved in the prevention of DOX cardiotoxicity: as recently described, it could directly target p21, attenuating DOX-induced mitochondrial fission and apoptosis [95]. Similar results were reported by Shi et al., as based on their results, this miRNA is expressed in cardiomyocytes, and its expression was increased after AMI. These authors concluded that it plays a pivotal role in cardiomyocyte injury induced by hypoxia/reoxygenation (H/R); however, its pathway remains unclear [96].
One of the first studies that ascertained the pivotal role of miR-1 on cardiac contractile function and the potential molecular mechanisms was published in 2012. This study provides the first evidence that this miRNA dysregulation can cause adverse structural remodeling of the heart [97]. Later, it was reported that MirR-1 expression may induce atrophy, imparting fine-tuning of gene expression, as a part of redox signaling that leads to phenotypic alterations on heart tissue [98]. This miRNA was expressed in a different manner, allowing the differentiation between hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM), considering that it was downregulated in HCM. Moreover, miR-1-3p levels are correlated with the left ventricular end-diastolic diameter (LVEDD) and left ventricular ejection fraction (LVEF), which reflect the cardiac function in HCM [99]. One of the first studies that ascertained the pivotal role of miR-1 on cardiac contractile function and the potential molecular mechanisms demonstrated that the knockdown of miR-1 could mitigate the adverse changes in cardiac function associated with the overexpression of miR-1.
The circulating levels of miR-126 could be indicative of CVD; indeed, in several studies, it has been described that reduced levels of this miRNA are found in patients with HF compared with healthy controls. For these reasons, high levels of miR-126 may be related to better clinical conditions of patients affected by CVD [100]. In the same way, other authors described that low serum levels of circulating miR-126 were associated with an increased risk of premature death, suggesting that changes in serum levels of circulating miRNAs could be related to risk factors of premature death events [100,101]. In agreement with these studies, other authors described that low levels of this mRNA are related to increased amounts of inflammatory factors that could mediate the insurgence of CAD and atherosclerosis. These characteristics may suggest that this miRNA could be a future therapeutic target to reduce endothelial inflammation, thus decreasing the chances of developing CAD [102].
4. Conclusions
To date, CVD is one of the most important causes of mortality worldwide, thus the need for effective preventive strategies is imperative. Moreover, especially in developed countries, given that the average age of life tends to be higher, and considering that aging is associated with significant changes both in cardiovascular structure and function, the identification of new molecular markers remains one of the most important fields to be studied [103,104,105]. Particularly, the identification of markers capable of being sensitive in order to identify clinical signs and symptoms early to reduce morbidity and mortality from CVD remains a challenging research topic.
Analyzing the results of this review, most papers focused on miRNAs as promising therapeutic targets and biomarkers of drug responses and/or therapeutic approaches. The majority of the selected studies focused on circulating miRNAs, analyzing their expression levels in serum or plasma samples.
Only three studies were performed evaluating miRNA expression in human cardiac tissue after myocardial infarction. Two studies were performed in post-mortem samples: the investigation performed in post-mortem samples is an important research field considering the strengths of using autoptic tissues [29,66]. As demonstrated in other contexts [106,107], thanks to post-mortem findings, it is possible to perform an experimental investigation in tissues sampled from subjects who had died with an exact cause of death. In our opinion, the evaluation of expression values of the selected miRNAs could be carried out considering that it is performed on tissue lesions and that miRNAs are stable in FFPE samples [108]. For example, if the goal of the experimental study is monitoring heart tissue after MI, during autopsy, it is possible to select the exact area affected by infarction. In this way, it is possible to evaluate the expression values of the miRNAs by comparing the values in damaged tissue with normal tissue without the variability among subjects. We believe that in the near future, the use of post-mortem samples could be important to identify promising biomarkers of organ damage [109]. Moreover, thanks to the opportunity to analyze FFPE tissue, it could be possible to use post-mortem samples collected for other purposes, such as histological investigation to establish organ damage. In this way, forensic institutes are an invaluable “magic box” to obtain samples, underlying the importance of respect for ethical issues, such as the Declaration of Helsinki. Notably, the concept of a Biobank is not completely new: to date, population-based biobanks have been used to obtain extensive phenotypic and genotypic data; nevertheless, to date, the miRNA tools on biological samples stored in biobanks are still poorly investigated. Moreover, usually, only a few biobanks are based on tissue samples, usually stored blood samples. Furthermore, the biobanks that store tissue samples work predominantly in the cancer research field. Finally, it is important to note that several developed countries have not instituted national tissue biobanks [110,111,112].
The international scientific community has worked hard to elucidate the mechanisms by which miRNAs exert regulatory effects on gene expression, regulating physiological and pathological mechanisms. While it is clear that miRNAs are powerful gene regulators, all the underlying mechanisms remain unclear. The need for up-to-date data always justifies the enormous amount of scientific work in order to increasingly highlight their pathways. Given the importance of CVDs, miRNAs could be important both as diagnostic and therapeutic (theranostic) tools. In this context, the discovery of “TheranoMIRNAs” (new terms to describe the miRNAs that may be used both for diagnostic and therapeutic purposes) could be decisive in the near future. The definition of well set out studies is necessary in order to provide further evidence in this challenging field.
Acknowledgments
The authors thank the Scientific Bureau of the University of Catania for language support.
Author Contributions
Author Contributions: Conceptualization, F.S. and C.P.; methodology, F.S., M.S., G.C., M.E. and C.P.; software, F.S.; validation, C.P.; formal analysis, F.S., M.S., G.C., M.E. and C.P.; investigation, F.S.; resources, F.S.; data curation, F.S., M.S., G.C., M.E. and C.P.; writing—original draft preparation, F.S.; writing—review and editing, F.S., M.S. and C.P.; visualization, F.S.; supervision, C.P.; project administration, F.S.; funding acquisition, F.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was supported by the Research Grant “PIA.CE.RI., PIAno di inCEntivi per la RIcerca di Ateneo 2020/2022” from the Department of Medical, Surgical and Advanced Technologies “G.F. Ingrassia”, University of Catania, STARTING GRANT (prot. n. 468677/22-F.S.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sturzu A.C., Wu S.M. Developmental and Regenerative Biology of Multipotent Cardiovascular Progenitor Cells. Circ. Res. 2011;108:353–364. doi: 10.1161/CIRCRESAHA.110.227066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catalanotto C., Cogoni C., Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016;17:1712. doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H., Cai J. The Role of MicroRNAs in Heart Failure. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:2019–2030. doi: 10.1016/j.bbadis.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Gorabi A.M., Bianconi V., Pirro M., Banach M., Sahebkar A. Regulation of cardiac stem cells by microRNAs: State-of-the-art. Biomed. Pharmacother. 2019;120:109447. doi: 10.1016/j.biopha.2019.109447. [DOI] [PubMed] [Google Scholar]
- 6.Samra M., Srivastava K. Non-coding RNA and their potential role in cardiovascular diseases. Gene. 2023;851:147011. doi: 10.1016/j.gene.2022.147011. [DOI] [PubMed] [Google Scholar]
- 7.Ramos J.T.G.D.S., Pereira A.G., Ferrari F.S., Andrade M.F., de Melo C.S., Boas P.J.F.V., Felix T.F., de Carvalho M., Dorna M.S., Azevedo P.S., et al. Circulating miRNAs are associated with frailty and ST-elevation myocardial infarction pathways. Arch. Gerontol. Geriatr. 2023;106:104870. doi: 10.1016/j.archger.2022.104870. [DOI] [PubMed] [Google Scholar]
- 8.Qian L., Zhao Q., Yu P., Lü J., Guo Y., Gong X., Ding Y., Yu S., Fan L., Fan H., et al. Diagnostic potential of a circulating miRNA model associated with therapeutic effect in heart failure. J. Transl. Med. 2022;20:267. doi: 10.1186/s12967-022-03465-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilella-Figuerola A., Gallinat A., Escate R., Mirabet S., Padró T., Badimon L. Systems Biology in Chronic Heart Failure—Identification of Potential miRNA Regulators. Int. J. Mol. Sci. 2022;23:15226. doi: 10.3390/ijms232315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klimczak-Tomaniak D., Haponiuk-Skwarlińska J., Kuch M., Pączek L. Crosstalk between microRNA and Oxidative Stress in Heart Failure: A Systematic Review. Int. J. Mol. Sci. 2022;23:15013. doi: 10.3390/ijms232315013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolodziej F., McDonagh B., Burns N., Goljanek-Whysall K. MicroRNAs as the Sentinels of Redox and Hypertrophic Signalling. Int. J. Mol. Sci. 2022;23:14716. doi: 10.3390/ijms232314716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao Y., Zhao J., Tuazon J.P., Borlongan C.V., Yu G. MicroRNA-133a and Myocardial Infarction. Cell Transplant. 2019;28:831–838. doi: 10.1177/0963689719843806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horak M., Novak J., Bienertova-Vasku J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016;410:1–13. doi: 10.1016/j.ydbio.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Sessa F., Esposito M., Salerno M. Experimental studies on androgen administration in animal models: Current and future perspectives. Curr. Opin. Endocrinol. Diabetes. 2022;29:566–585. doi: 10.1097/MED.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 15.Li D., Wang P., Wei W., Wang C., Zhong Y., Lv L., Wang J. Serum MicroRNA Expression Patterns in Subjects After the 5-km Exercise Are Strongly Associated With Cardiovascular Adaptation. Front. Physiol. 2021;12:755656. doi: 10.3389/fphys.2021.755656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inzulza-Tapia A., Alarcón M. Role of Non-Coding RNA of Human Platelet in Cardiovascular Disease. Curr. Med. Chem. 2021;29:3420–3444. doi: 10.2174/0929867329666211230104955. [DOI] [PubMed] [Google Scholar]
- 17.dos Santos J.A.C., Veras A.S.C., Batista V.R.G., Tavares M.E.A., Correia R.R., Suggett C.B., Teixeira G.R. Physical exercise and the functions of microRNAs. Life Sci. 2022;304:120723. doi: 10.1016/j.lfs.2022.120723. [DOI] [PubMed] [Google Scholar]
- 18.Pomara C., D’Errico S., Riezzo I., De Cillis G.P., Fineschi V. Sudden cardiac death in a child affected by Prader-Willi syndrome. Int. J. Leg. Med. 2005;119:153–157. doi: 10.1007/s00414-004-0513-9. [DOI] [PubMed] [Google Scholar]
- 19.Shah V., Shah J. Restoring Ravaged Heart: Molecular Mechanisms and Clinical Application of miRNA in Heart Regeneration. Front. Cardiovasc. Med. 2022;9:835138. doi: 10.3389/fcvm.2022.835138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pang J.K.S., Phua Q.H., Soh B.-S. Applications of miRNAs in cardiac development, disease progression and regeneration. Stem Cell Res. Ther. 2019;10:336. doi: 10.1186/s13287-019-1451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerretani D., Riezzo I., Fiaschi A.I., Centini F., Giorgi G., D’Errico S., Fiore C., Karch S.B., Neri M., Pomara C., et al. Cardiac Oxidative Stress Determination and Myocardial Morphology after a Single Ecstasy (MDMA) Administration in a Rat Model. Int. J. Legal Med. 2008;122:461–469. doi: 10.1007/s00414-008-0262-2. [DOI] [PubMed] [Google Scholar]
- 22.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayés-Genis A., Lanfear D.E., de Ronde M.W.J., Lupón J., Leenders J.J., Liu Z., Zuithoff N.P.A., Eijkemans M.J.C., Zamora E., De Antonio M., et al. Prognostic Value of Circulating MicroRNAs on Heart Failure-Related Morbidity and Mortality in Two Large Diverse Cohorts of General Heart Failure Patients. Eur. J. Heart Fail. 2018;20:67–75. doi: 10.1002/ejhf.984. [DOI] [PubMed] [Google Scholar]
- 24.Guo M., Luo J., Zhao J., Shang D., Lv Q., Zang P. Combined Use of Circulating MiR-133a and NT-ProBNP Improves Heart Failure Diagnostic Accuracy in Elderly Patients. Med. Sci. Monit. 2018;24:8840–8848. doi: 10.12659/MSM.911632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masson S., Batkai S., Beermann J., Bär C., Pfanne A., Thum S., Magnoli M., Balconi G., Nicolosi G.L., Tavazzi L., et al. Circulating MicroRNA-132 Levels Improve Risk Prediction for Heart Failure Hospitalization in Patients with Chronic Heart Failure. Eur. J. Heart Fail. 2018;20:78–85. doi: 10.1002/ejhf.961. [DOI] [PubMed] [Google Scholar]
- 26.Li P., Li S.Y., Liu M., Ruan J.W., Wang Z.D., Xie W.C. Value of the Expression of MiR-208, MiR-494, MiR-499 and MiR-1303 in Early Diagnosis of Acute Myocardial Infarction. Life Sci. 2019;232:116547. doi: 10.1016/j.lfs.2019.116547. [DOI] [PubMed] [Google Scholar]
- 27.Liu W., Zheng J., Dong J., Bai R., Song D., Ma X., Zhao L., Yao Y., Zhang H., Liu T. Association of MiR-197-5p, a Circulating Biomarker for Heart Failure, with Myocardial Fibrosis and Adverse Cardiovascular Events among Patients with Stage C or D Heart Failure. Cardiology. 2019;141:212–225. doi: 10.1159/000493419. [DOI] [PubMed] [Google Scholar]
- 28.Mayer O., Seidlerová J., Černá V., Kučerová A., Vaněk J., Karnosová P., Bruthans J., Wohlfahrt P., Cífková R., Pešta M., et al. The Low Expression of Circulating MicroRNA-19a Represents an Additional Mortality Risk in Stable Patients with Vascular Disease. Int. J. Cardiol. 2019;289:101–106. doi: 10.1016/j.ijcard.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Pinchi E., Frati P., Aromatario M., Cipolloni L., Fabbri M., La Russa R., Maiese A., Neri M., Santurro A., Scopetti M., et al. MiR-1, MiR-499 and MiR-208 Are Sensitive Markers to Diagnose Sudden Death Due to Early Acute Myocardial Infarction. J. Cell Mol. Med. 2019;23:6005–6016. doi: 10.1111/jcmm.14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B., Li B., Qin F., Bai F., Sun C., Liu Q. Expression of Serum MicroRNA-155 and Its Clinical Importance in Patients with Heart Failure after Myocardial Infarction. J. Int. Med. Res. 2019;47:6294–6302. doi: 10.1177/0300060519882583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu L., Chen T., Ye W., Wang J.Y., Zhou J.P., Li Z.Y., Li C.C. Circulating MiR-182-5p and MiR-5187-5p as Biomarkers for the Diagnosis of Unprotected Left Main Coronary Artery Disease. J. Thorac. Dis. 2019;11:1799–1808. doi: 10.21037/jtd.2019.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asulin N., Volinsky N., Grosman-Rimon L., Kachel E., Sternik L., Raanani E., Altshuler R., Magen I., Ben-Zvi I., Margalit N., et al. Differential MicroRNAs Expression in Calcified versus Rheumatic Aortic Valve Disease. J. Card Surg. 2020;35:1508–1513. doi: 10.1111/jocs.14636. [DOI] [PubMed] [Google Scholar]
- 33.Barbalata T., Moraru O.E., Stancu C.S., Devaux Y., Simionescu M., Sima A.V., Niculescu L.S. Increased Mir-142 Levels in Plasma and Atherosclerotic Plaques from Peripheral Artery Disease Patients with Post-Surgery Cardiovascular Events. Int. J. Mol. Sci. 2020;21:9600. doi: 10.3390/ijms21249600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Zvi I., Volinsky N., Grosman-Rimon L., Haviv I., Rozen G., Andria N., Asulin N., Margalit N., Marai I., Amir O. Cardiac-Peripheral Transvenous Gradients of MicroRNA Expression in Systolic Heart Failure Patients. ESC Heart Fail. 2020;7:835–843. doi: 10.1002/ehf2.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elbaz M., Faccini J., Laperche C., Grousset E., Roncalli J., Ruidavets J.B., Vindis C. Identification of a MiRNA Based-Signature Associated with Acute Coronary Syndrome: Evidence from the FLORINF Study. J. Clin. Med. 2020;9:1674. doi: 10.3390/jcm9061674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling H., Guo Z., Shi Y., Zhang L., Song C. Serum Exosomal MicroRNA-21, MicroRNA-126, and PTEN Are Novel Biomarkers for Diagnosis of Acute Coronary Syndrome. Front. Physiol. 2020;11:654. doi: 10.3389/fphys.2020.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S., Guo X., Zhong W., Weng R., Liu J., Gu X., Zhong Z. Circulating Microrna Expression Profiles in Patients with Stable and Unstable Angina. Clinics. 2020;75:e1546. doi: 10.6061/clinics/2020/e1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nie X., He M., Wang J., Chen P., Wang F., Lai J., Li C., Yu T., Zuo H., Cui G., et al. Circulating MiR-4763-3p Is a Novel Potential Biomarker Candidate for Human Adult Fulminant Myocarditis. Mol. Ther. Methods Clin. Dev. 2020;17:1079–1087. doi: 10.1016/j.omtm.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos J.L., Rodríguez I., Olesen M.S., Bentzen B.H., Schmitt N. Investigating Gene-MicroRNA Networks in Atrial Fibrillation Patients with Mitral Valve Regurgitation. PLoS ONE. 2020;15:e0232719. doi: 10.1371/journal.pone.0232719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silverman M.G., Yeri A., Moorthy M.V., Camacho Garcia F., Chatterjee N.A., Glinge C.S.A., Tfelt-Hansen J., Salvador A.M., Pico A.R., Shah R., et al. Circulating MiRNAs and Risk of Sudden Death in Patients with Coronary Heart Disease. JACC Clin. Electrophysiol. 2020;6:70–79. doi: 10.1016/j.jacep.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su T., Shao X., Zhang X., Yang C., Shao X. Value of Circulating MiRNA-1 Detected within 3 h after the Onset of Acute Chest Pain in the Diagnosis and Prognosis of Acute Myocardial Infarction. Int. J. Cardiol. 2020;307:146–151. doi: 10.1016/j.ijcard.2019.09.050. [DOI] [PubMed] [Google Scholar]
- 42.Turky H.F.E.S., Mohammed W.A.L.E., Shalaby S.M., Etewa R.L., Kandil N.T., Galal I. Plasma MicroRNA-133a as a Potential Biomarker for Acute Coronary Syndrome. Jordan J. Biol. Sci. 2020;13:191–196. [Google Scholar]
- 43.Wakabayashi I., Eguchi R., Sotoda Y., von Lewinski D., Sourij H., Daimon T., Groschner K., Rainer P.P. Blood Levels of MicroRNAs Associated with Ischemic Heart Disease Differ between Austrians and Japanese: A Pilot Study. Sci. Rep. 2020;10:13628. doi: 10.1038/s41598-020-69332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X., Tian L., Sun Q. Diagnostic and Prognostic Value of Circulating MiRNA-499 and MiRNA-22 in Acute Myocardial Infarction. J. Clin. Lab. Anal. 2020;34:2410–2417. doi: 10.1002/jcla.23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weldy C.S., Syed S.A., Amsallem M., Hu D.Q., Ji X., Punn R., Taylor A., Navarre B., Reddy S. Circulating Whole Genome MiRNA Expression Corresponds to Progressive Right Ventricle Enlargement and Systolic Dysfunction in Adults with Tetralogy of Fallot. PLoS ONE. 2020;15:e0241476. doi: 10.1371/journal.pone.0241476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brundin M., Wågsäter D., Alehagen U., Carlhäll C.J. Circulating MicroRNA-29-5p Can Add to the Discrimination between Dilated Cardiomyopathy and Ischaemic Heart Disease. ESC Heart Fail. 2021;8:3865–3874. doi: 10.1002/ehf2.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen S., Fang H., Liu R., Fang Y., Wu Z., Xie P. MiR-6718-5p and MiR-4329 Can Be Used as Potential Biomarkers for Acute Myocardial Infarction. J. Card Surg. 2021;36:3721–3728. doi: 10.1111/jocs.15868. [DOI] [PubMed] [Google Scholar]
- 48.Coban N., Ozuynuk A.S., Erkan A.F., Guclu-Geyik F., Ekici B. Levels of MiR-130b-5p in Peripheral Blood Are Associated with Severity of Coronary Artery Disease. Mol. Biol. Rep. 2021;48:7719–7732. doi: 10.1007/s11033-021-06780-5. [DOI] [PubMed] [Google Scholar]
- 49.Elgebaly S.A., Christenson R.H., Kandil H., Ibrahim M., Rizk H., El-Khazragy N., Rashed L., Yacoub B., Eldeeb H., Ali M.M., et al. Nourin-Dependent Mir-137 and Mir-106b: Novel Biomarkers for Early Diagnosis of Myocardial Ischemia in Coronary Artery Disease Patients. Diagnostics. 2021;11:703. doi: 10.3390/diagnostics11040703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Elias A., Tajes M., Yañez-Bisbe L., Enjuanes C., Comín-Colet J., Serra S.A., Fernández-Fernández J.M., Aguilar-Agon K.W., Reilly S., Martí-Almor J., et al. Atrial Fibrillation in Heart Failure Is Associated with High Levels of Circulating MicroRNA-199a-5p and 22–5p and a Defective Regulation of Intracellular Calcium and Cell-to-Cell Communication. Int. J. Mol. Sci. 2021;22:10377. doi: 10.3390/ijms221910377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gevaert A.B., Witvrouwen I., Van Craenenbroeck A.H., Van Laere S.J., Boen J.R.A., Van de Heyning C.M., Belyavskiy E., Mueller S., Winzer E., Duvinage A., et al. MiR-181c Level Predicts Response to Exercise Training in Patients with Heart Failure and Preserved Ejection Fraction: An Analysis of the OptimEx-Clin Trial. Eur. J. Prev. Cardiol. 2021;28:1722–1733. doi: 10.1093/eurjpc/zwab151. [DOI] [PubMed] [Google Scholar]
- 52.He J., Pan M., Xu M., Chen R. Circulating MiRNA-29b and Sclerostin Levels Correlate with Coronary Artery Calcification and Cardiovascular Events in Maintenance Hemodialysis Patients. Cardiol. Res. Pract. 2021;2021:9208634. doi: 10.1155/2021/9208634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hromadka M., Motovska Z., Hlinomaz O., Kala P., Tousek F., Jarkovsky J., Beranova M., Jansky P., Svoboda M., Krepelkova I., et al. Mir-126-3p and Mir-223-3p as Biomarkers for Prediction of Thrombotic Risk in Patients with Acute Myocardial Infarction and Primary Angioplasty. J. Pers. Med. 2021;11:508. doi: 10.3390/jpm11060508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu T., Li X., Long C., Ji W., Jiang L., Tian J. Circulating MiR-27b as a Biomarker of the Development and Progression of Carotid Artery Stenosis. Clin. Appl. Thromb. Hemost. 2021:27. doi: 10.1177/10760296211057903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mihaleva I., Kyurkchiyan S., Dodova R., Nikolov R., Markova T., Gateva P., Dimova I. MiRNA Expression Analysis Emphasized the Role of MiR-424 in Diabetic Cardiovascular Complications. Int. J. Diabetes. Dev. Ctries. 2021;41:579–585. doi: 10.1007/s13410-021-00934-8. [DOI] [Google Scholar]
- 56.Moric-Janiszewska E., Smolik S., Morka A., Szydłowski L., Kapral M. Expression Levels of Serum Circulating MicroRNAs in Pediatric Patients with Ventricular and Supraventricular Arrhythmias. Adv. Med. Sci. 2021;66:411–417. doi: 10.1016/j.advms.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Neiburga K.D., Vilne B., Bauer S., Bongiovanni D., Ziegler T., Lachmann M., Wengert S., Hawe J.S., Güldener U., Westerlund A.M., et al. Vascular Tissue Specific Mirna Profiles Reveal Novel Correlations with Risk Factors in Coronary Artery Disease. Biomolecules. 2021;11:1683. doi: 10.3390/biom11111683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sacchetto C., Mohseni Z., Colpaert R.M.W., Vitiello L., De Bortoli M., Vonhögen I.G.C., Xiao K., Poloni G., Lorenzon A., Romualdi C., et al. Circulating Mir-185-5p as a Potential Biomarker for Arrhythmogenic Right Ventricular Cardiomyopathy. Cells. 2021;10:2578. doi: 10.3390/cells10102578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen M., Xu X., Liu X., Wang Q., Li W., You X., Peng R., Yuan Y., Long P., Niu R., et al. Prospective Study on Plasma Microrna-4286 and Incident Acute Coronary Syndrome. J. Am. Heart Assoc. 2021;10:e018999. doi: 10.1161/JAHA.120.018999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki K., Yamada H., Fujii R., Munetsuna E., Ando Y., Ohashi K., Ishikawa H., Yamazaki M., Maeda K., Hashimoto S., et al. Association between Circulating Vascular-Related MicroRNAs and an Increase in Blood Pressure: A 5-Year Longitudinal Population-Based Study. J. Hypertens. 2021;39:84–89. doi: 10.1097/HJH.0000000000002606. [DOI] [PubMed] [Google Scholar]
- 61.Szelenberger R., Karbownik M.S., Kacprzak M., Maciak K., Bijak M., Zielińska M., Czarny P., Śliwiński T., Saluk-Bijak J. Screening Analysis of Platelet MiRNA Profile Revealed MiR-142-3p as a Potential Biomarker in Modeling the Risk of Acute Coronary Syndrome. Cells. 2021;10:3526. doi: 10.3390/cells10123526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thottakara T., Lund N., Krämer E., Kirchhof P., Carrier L., Patten M. A Novel MiRNA Screen Identifies MiRNA-4454 as a Candidate Biomarker for Ventricular Fibrosis in Patients with Hypertrophic Cardiomyopathy. Biomolecules. 2021;11:1718. doi: 10.3390/biom11111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tong X., Chen J., Liu W., Liang H., Zhu H. LncRNA LSINCT5/MiR-222 Regulates Myocardial Ischemia-reperfusion Injury through PI3K/AKT Pathway. J. Thromb. Thrombolysis. 2021;52:720–729. doi: 10.1007/s11239-021-02506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao S., Xue T., Pan Q., Hu Y., Wu Q., Liu Q., Wang X., Liu A., Liu J., Zhu H., et al. MicroRNA-146a Serves as a Biomarker for Adverse Prognosis of ST-Segment Elevation Myocardial Infarction. Cardiovasc. Ther. 2021;2021:2923440. doi: 10.1155/2021/2923441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamada H., Suzuki K., Fujii R., Kawado M., Hashimoto S., Watanabe Y., Iso H., Fujino Y., Wakai K., Tamakoshi A. Circulating MiR-21, MiR-29a, and MiR-126 Are Associated with Premature Death Risk Due to Cancer and Cardiovascular Disease: The JACC Study. Sci. Rep. 2021;11:5298. doi: 10.1038/s41598-021-84707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan F., Chen Y., Ye X., Zhang F., Wang S., Zhang L., Luo X. MiR-3113-5p, MiR-223-3p, MiR-133a-3p, and MiR-499a-5p Are Sensitive Biomarkers to Diagnose Sudden Cardiac Death. Diagn. Pathol. 2021;16:67. doi: 10.1186/s13000-021-01127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhelankin A.V., Stonogina D.A., Vasiliev S.V., Babalyan K.A., Sharova E.I., Doludin Y.V., Shchekochikhin D.Y., Generozov E.V., Akselrod A.S. Circulating Extracellular Mirna Analysis in Patients with Stable Cad and Acute Coronary Syndromes. Biomolecules. 2021;11:962. doi: 10.3390/biom11070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eikelis N., Dixon J.B., Lambert E.A., Hanin G., Tzur Y., Greenberg D.S., Soreq H., Marques F.Z., Fahey M.T., Head G.A., et al. MicroRNA-132 May Be Associated with Blood Pressure and Liver Steatosis—Preliminary Observations in Obese Individuals. J. Hum. Hypertens. 2022;36:911–916. doi: 10.1038/s41371-021-00597-2. [DOI] [PubMed] [Google Scholar]
- 69.Eyyupkoca F., Ercan K., Kiziltunc E., Ugurlu I.B., Kocak A., Eyerci N. Determination of MicroRNAs Associated with Adverse Left Ventricular Remodeling after Myocardial Infarction. Mol. Cell Biochem. 2022;477:781–791. doi: 10.1007/s11010-021-04330-y. [DOI] [PubMed] [Google Scholar]
- 70.Gager G.M., Eyileten C., Postula M., Gasecka A., Jarosz-Popek J., Gelbenegger G., Jilma B., Lang I., Siller-Matula J. Association Between the Expression of MicroRNA-125b and Survival in Patients with Acute Coronary Syndrome and Coronary Multivessel Disease. Front. Cardiovasc. Med. 2022;9:948006. doi: 10.3389/fcvm.2022.948006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.James K., Bryl-Gorecka P., Olde B., Gidlof O., Torngren K., Erlinge D. Increased Expression of MiR-224-5p in Circulating Extracellular Vesicles of Patients with Reduced Coronary Flow Reserve. BMC Cardiovasc. Disord. 2022;22:321. doi: 10.1186/s12872-022-02756-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J., Wang N., Wen X., Huang L.Y., Cui R.Q., Zhang J. Serum MiRNA-203 as a Novel Biomarker for the Early Prediction of Acute ST-Elevation Myocardial Infarction. J. Cardiovasc. Transl. Res. 2022;15:1406–1413. doi: 10.1007/s12265-022-10269-2. [DOI] [PubMed] [Google Scholar]
- 73.Miao R., Gong J., Guo X., Guo D., Zhang X., Hu H., Zhong J., Yang Y., Li Y. Diagnostic Value of MiRNA Expression and Right Ventricular Echocardiographic Functional Parameters for Chronic Thromboembolic Pulmonary Hypertension with Right Ventricular Dysfunction and Injury. BMC Pulm. Med. 2022;22:171. doi: 10.1186/s12890-022-01962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mompeón A., Pérez-cremades D., Paes A.B., Sanchis J., Ortega-paz L., Andrea R., Brugaletta S., Sabate M., Novella S., Dantas A.P., et al. Circulating MiRNA Fingerprint and Endothelial Function in Myocardial Infarction: Comparison at Acute Event and One-Year Follow-Up. Cells. 2022;11:1823. doi: 10.3390/cells11111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moscoso I., Cebro-Márquez M., Martínez-Gómez Á., Abou-Jokh C., Martínez-Monzonís M.A., Martínez-Sande J.L., González-Melchor L., García-Seara J., Fernández-López X.A., Moraña-Fernández S., et al. Circulating MiR-499a and MiR-125b as Potential Predictors of Left Ventricular Ejection Fraction Improvement after Cardiac Resynchronization Therapy. Cells. 2022;11:271. doi: 10.3390/cells11020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de los Reyes-García A.M., Rivera-Caravaca J.M., Zapata-Martínez L., Águila S., Véliz-Martínez A., García-Barberá N., Gil-Perez P., Guijarro-Carrillo P.J., Orenes-Piñero E., López-García C., et al. MiR-146a Contributes to Thromboinflammation and Recurrence in Young Patients with Acute Myocardial Infarction. J. Pers. Med. 2022;12:1185. doi: 10.3390/jpm12071185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X., Liu Y., Hou H., Shao W., Huang D., Hao Z., Xue H., Ye Y. MiRNA-29 Aggravates Myocardial Infarction via Inhibiting the PI3K/MTOR/HIF1α/VEGF Pathway. Aging. 2022;14:3129–3142. doi: 10.18632/aging.203997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang N., Song Y., Dong B., Yang J., Li Y., Qin Q., Guo Z. Analysis of MiRNA Associated with Coronary Artery Calcification. Comput. Math. Methods Med. 2022;2022:3708547. doi: 10.1155/2022/3708547. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Yu X., Xu J.F., Song M., Zhang L., Li Y.H., Han L., Tang M.X., Zhang W., Zhong M., Wang Z.H. Associations of Circulating MicroRNA-221 and 222 With the Severity of Coronary Artery Lesions in Acute Coronary Syndrome Patients. Angiology. 2022;73:579–587. doi: 10.1177/00033197211034286. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y., Yuan B., Xu Y., Zhou N., Zhang R., Lu L., Feng Z. MiR-208b/MiR-21 Promotes the Progression of Cardiac Fibrosis Through the Activation of the TGF-Β1/Smad-3 Signaling Pathway: An in Vitro and in Vivo Study. Front. Cardiovasc. Med. 2022;9:924629. doi: 10.3389/fcvm.2022.924629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Y., Chen Z., Chen A., Ma J., Qian J., Ge J. Elevated Serum MiR-133a Predicts Patients at Risk of Periprocedural Myocardial Injury after Elective Percutaneous Coronary Intervention. Cardiol. J. 2022;29:284–292. doi: 10.5603/CJ.a2020.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fineschi V., Silver M.D., Karch S.B., Parolini M., Turillazzi E., Pomara C., Baroldi G. Myocardial Disarray: An Architectural Disorganization Linked with Adrenergic Stress? Int. J. Cardiol. 2005;99:277–282. doi: 10.1016/j.ijcard.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 83.Sessa F., Esposito M., Messina G., di Mizio G., di Nunno N., Salerno M. Sudden Death in Adults: A Practical Flow Chart for Pathologist Guidance. Healthcare. 2021;9:870. doi: 10.3390/healthcare9070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Argo A., Zerbo S., Buscemi R., Trignano C., Bertol E., Albano G.D., Vaiano F. A Forensic Diagnostic Algorithm for Drug-Related Deaths: A Case Series. Toxics. 2022;10:152. doi: 10.3390/toxics10040152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fineschi V., Karch S.B., D’Errico S., Pomara C., Riezzo I., Turillazzi E. Cardiac Pathology in Death from Electrocution. Int. J. Legal Med. 2006;120:79–82. doi: 10.1007/s00414-005-0011-8. [DOI] [PubMed] [Google Scholar]
- 86.Sessa F., Anna V., Messina G., Cibelli G., Monda V., Marsala G., Ruberto M., Biondi A., Cascio O., Bertozzi G., et al. Heart Rate Variability as Predictive Factor for Sudden Cardiac Death. Aging. 2018;10:166–177. doi: 10.18632/aging.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Y.-F., Wang R., Chen W., Cao Y.-D., Li L.-P., Chen X. MiR-133a-3p Attenuates Cardiomyocyte Hypertrophy through Inhibiting Pyroptosis Activation by Targeting IKKε. Acta Histochem. 2021;123:151653. doi: 10.1016/j.acthis.2020.151653. [DOI] [PubMed] [Google Scholar]
- 88.Peters L.J.F., Biessen E.A.L., Hohl M., Weber C., van der Vorst E.P.C., Santovito D. Small Things Matter: Relevance of MicroRNAs in Cardiovascular Disease. Front. Physiol. 2020;11:793. doi: 10.3389/fphys.2020.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmed S., Kurusamy S., David E.L.S., Khan K., Kalyanakrishnan K., Ian-Gobo M., Kola T.M., Wilkinson R.N., Kannappan V., Wang W., et al. Aberrant Expression of MiR-133a in Endothelial Cells Inhibits Angiogenesis by Reducing pro-Angiogenic but Increasing Anti-Angiogenic Gene Expression. Sci. Rep. 2022;12:14730. doi: 10.1038/s41598-022-19172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Escate R., Padró T., Suades R., Camino S., Muñiz O., Diaz-Diaz J.L., Sionis A., Mata P., Badimon L. High MiR-133a Levels in the Circulation Anticipates Presentation of Clinical Events in Familial Hypercholesterolaemia Patients. Cardiovasc. Res. 2021;117:109–122. doi: 10.1093/cvr/cvaa039. [DOI] [PubMed] [Google Scholar]
- 91.Dai B., Wang F., Nie X., Du H., Zhao Y., Yin Z., Li H., Fan J., Wen Z., Wang D.W., et al. The Cell Type–Specific Functions of MiR-21 in Cardiovascular Diseases. Front. Genet. 2020;11:563166. doi: 10.3389/fgene.2020.563166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Surina S., Fontanella R.A., Scisciola L., Marfella R., Paolisso G., Barbieri M. MiR-21 in Human Cardiomyopathies. Front. Cardiovasc. Med. 2021;8:767064. doi: 10.3389/fcvm.2021.767064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kura B., Kalocayova B., Devaux Y., Bartekova M. Potential Clinical Implications of Mir-1 and Mir-21 in Heart Disease and Cardioprotection. Int. J. Mol. Sci. 2020;21:700. doi: 10.3390/ijms21030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L., Chen X., Su T., Li H., Huang Q., Wu D., Yang C., Han Z. Circulating MiR-499 Are Novel and Sensitive Biomarker of Acute Myocardial Infarction. J. Thorac. Dis. 2015;7:303–308. doi: 10.1016/j.jacc.2015.06.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wan Q., Xu T., Ding W., Zhang X., Ji X., Yu T., Yu W., Lin Z., Wang J. MiR-499-5p Attenuates Mitochondrial Fission and Cell Apoptosis via P21 in Doxorubicin Cardiotoxicity. Front. Genet. 2019;10:734. doi: 10.3389/fgene.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi Y., Han Y., Niu L., Li J., Chen Y. MiR-499 Inhibited Hypoxia/Reoxygenation Induced Cardiomyocytes Injury by Targeting SOX6. Biotechnol. Lett. 2019;41:837–847. doi: 10.1007/s10529-019-02685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ai J., Zhang R., Gao X., Niu H.-F., Wang N., Xu Y., Li Y., Ma N., Sun L.-H., Pan Z.-W., et al. Overexpression of MicroRNA-1 Impairs Cardiac Contractile Function by Damaging Sarcomere Assembly. Cardiovasc. Res. 2012;95:385–393. doi: 10.1093/cvr/cvs196. [DOI] [PubMed] [Google Scholar]
- 98.Seok H., Lee H., Lee S., Ahn S.H., Lee H.-S., Kim G.-W.D., Peak J., Park J., Cho Y.K., Jeong Y., et al. Position-Specific Oxidation of MiR-1 Encodes Cardiac Hypertrophy. Nature. 2020;584:279–285. doi: 10.1038/s41586-020-2586-0. [DOI] [PubMed] [Google Scholar]
- 99.Li M., Chen X., Chen L., Chen K., Zhou J., Song J. MiR-1-3p That Correlates with Left Ventricular Function of HCM Can Serve as a Potential Target and Differentiate HCM from DCM. J. Transl. Med. 2018;16:161. doi: 10.1186/s12967-018-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pordzik J., Eyileten-Postuła C., Jakubik D., Czajka P., Nowak A., De Rosa S., Gasecka A., Cieślicka-Kapłon A., Sulikowski P., Filipiak K.J., et al. Mir-126 Is an Independent Predictor of Long-Term All-Cause Mortality in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. 2021;10:2371. doi: 10.3390/jcm10112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Czajka P., Fitas A., Jakubik D., Eyileten C., Gasecka A., Wicik Z., Siller-Matula J.M., Filipiak K.J., Postula M. MicroRNA as Potential Biomarkers of Platelet Function on Antiplatelet Therapy: A Review. Front. Physiol. 2021;12:498. doi: 10.3389/fphys.2021.652579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arnab B., Debabrata R., Hindol M., Rudranil B., Sanmoy K., Abhishek R., Kaushik M., Navanil B. Microrna 126 as a surrogate marker for primary prevention of atherosclerotic cardiovascular disease across different age groups in susceptible population. J. Am. Coll. Cardiol. 2022;79:3466. doi: 10.1016/S0735-1097(22)04457-6. [DOI] [Google Scholar]
- 103.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sessa F., Salerno M., di Mizio G., Bertozzi G., Messina G., Tomaiuolo B., Pisanelli D., Maglietta F., Ricci P., Pomara C. Anabolic Androgenic Steroids: Searching New Molecular Biomarkers. Front. Pharmacol. 2018;9:1321. doi: 10.3389/fphar.2018.01321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neri M., Othman S.M., Cantatore S., de Carlo D., Pomara C., Riezzo I., Turillazzi E., Fineschi V. Sudden Infant Death in an 8-Month-Old Baby with Dengue Virus Infection: Searching for Virus in Postmortem Tissues by Immunohistochemistry and Western Blotting. Pediatr. Infect. Dis. J. 2012;31:878–880. doi: 10.1097/INF.0b013e31825c4a08. [DOI] [PubMed] [Google Scholar]
- 106.Sessa F., Salerno M., Bertozzi G., Cipolloni L., Messina G., Aromatario M., Polo L., Turillazzi E., Pomara C. MiRNAs as Novel Biomarkers of Chronic Kidney Injury in Anabolic-Androgenic Steroid Users: An Experimental Study. Front. Pharmacol. 2020;11:563756. doi: 10.3389/fphar.2020.563756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sessa F., Salerno M., Cipolloni L., Bertozzi G., Messina G., di Mizio G., Asmundo A., Pomara C. Anabolic-Androgenic Steroids and Brain Injury: MiRNA Evaluation in Users Compared to Cocaine Abusers and Elderly People. Aging. 2020;12:15314–15327. doi: 10.18632/aging.103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kakimoto Y., Kamiguchi H., Ochiai E., Satoh F., Osawa M. MicroRNA Stability in Postmortem FFPE Tissues: Quantitative Analysis Using Autoptic Samples from Acute Myocardial Infarction Patients. PLoS ONE. 2015;10:e0129338. doi: 10.1371/journal.pone.0129338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sessa F., Salerno M., Pomara C. Autopsy Tool in Unknown Diseases: The Experience with Coronaviruses (SARS-CoV, MERS-CoV, SARS-CoV-2) Medicina. 2021;57:309. doi: 10.3390/medicina57040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Annaratone L., de Palma G., Bonizzi G., Sapino A., Botti G., Berrino E., Mannelli C., Arcella P., di Martino S., Steffan A., et al. Basic Principles of Biobanking: From Biological Samples to Precision Medicine for Patients. Virchows Arch. 2021;479:233–246. doi: 10.1007/s00428-021-03151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cojocneanu R., Braicu C., Raduly L., Jurj A., Zanoaga O., Magdo L., Irimie A., Muresan M.-S., Ionescu C., Grigorescu M., et al. Plasma and Tissue Specific MiRNA Expression Pattern and Functional Analysis Associated to Colorectal Cancer Patients. Cancers. 2020;12:843. doi: 10.3390/cancers12040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Speirs V., Cox A., Chelala C., James J.A., Adams R.A., Jones J.L. A Biobank Perspective on Use of Tissue Samples Donated by Trial Participants. Lancet Oncol. 2022;23:e205. doi: 10.1016/S1470-2045(22)00186-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable as no new data were created or analyzed in this study.