Abstract
The specificity protein (Sp) transcription factors (TFs) Sp1, Sp2, Sp3 and Sp4 exhibit structural and functional similarities in cancer cells and extensive studies of Sp1 show that it is a negative prognostic factor for patients with multiple tumor types. In this review, the role of Sp1, Sp3 and Sp4 in the development of cancer and their regulation of pro-oncogenic factors and pathways is reviewed. In addition, interactions with non-coding RNAs and the development of agents that target Sp transcription factors are also discussed. Studies on normal cell transformation into cancer cell lines show that this transformation process is accompanied by increased levels of Sp1 in most cell models, and in the transformation of muscle cells into rhabdomyosarcoma, both Sp1 and Sp3, but not Sp4, are increased. The pro-oncogenic functions of Sp1, Sp3 and Sp4 in cancer cell lines were studied in knockdown studies where silencing of each individual Sp TF decreased cancer growth, invasion and induced apoptosis. Silencing of an individual Sp TF was not compensated for by the other two and it was concluded that Sp1, Sp3 and Sp4 are examples of non-oncogene addicted genes. This conclusion was strengthened by the results of Sp TF interactions with non-coding microRNAs and long non-coding RNAs where Sp1 contributed to pro-oncogenic functions of Sp/non-coding RNAs. There are now many examples of anticancer agents and pharmaceuticals that induce downregulation/degradation of Sp1, Sp3 and Sp4, yet clinical applications of drugs specifically targeting Sp TFs are not being used. The application of agents targeting Sp TFs in combination therapies should be considered for their potential to enhance treatment efficacy and decrease toxic side effects.
Keywords: Sp1, Sp3, Sp4, non-oncogene addiction, prognostic, pro-oncogenic
1. Background
Specificity protein 1 (Sp1) was among the first transcription factors (TFs) identified and is a member of the Sp/Kruppel-like factor (Sp/KLF) family. Members of this family exhibit variable structural domains and functions but all contain conserved zinc fingers in their DNA binding domains that bind GC-rich (Sps) and CACC (KLFs) boxes [1,2,3,4,5,6,7]. Not surprisingly, within the Sp and KLF sub-families there can be some overlap and competition for the same cis-elements, although for many Sp-regulated genes, differences in cell context and levels of expression dictate which Sp transcription factor is active. Among the 9 Sp genes, Sp1-Sp4 are most similar in terms of both structure and function (Figure 1), and they are the prime focus of this review. It should also be pointed out that among Sp1-Sp4, most research has focused on Sp1 and to a lesser extent Sp3 and it is possible that for some genes and pathways, the potential contributions of Sp2 and Sp4 have been understudied. There has been extensive research on the mechanisms of Sp-regulated gene expression, which frequently is observed in genes that lack a TATA box. Many Sp-regulated genes bind and activate gene expression through one or more GC-rich sequences proximal to the start sites where there are ordered assemblies of nuclear cofactors to form a transcriptionally active complex that includes DNA-bound Sp1, Sp3 or Sp4. The composition of transcription complexes includes polymerase II, transcription factor IID (TFIID), TATA box binding protein (TBP) and associated factors (TAFs) and members of the cofactor required for Sp1 activation/mediator (CRISP/MED) complexes [8]. The overall complex is highly variable and both gene- and cell-context-dependent. Moreover, there is also evidence that Sp TFs bind imperfect/variable GC-rich sequences and also interact with distal enhancer sequences, as described for the Topoisomerase IIa promoter [9]. In this review, there is a focus on the interactions of Sp TFs with non-coding RNAs and their functions; however, it should also be noted that Sp1 physically interacts with over 55 other proteins [2]. Sp1 function is also influenced by post-transcriptional modifications that include phosphorylation, acetylation, glycosylation and cleavage, and these changes can enhance or inhibit protein stability. Unfortunately, data for Sp3-Sp4 in terms of transcriptional function, post-transcriptional modifications and interactions with other factors have not been extensively investigated.
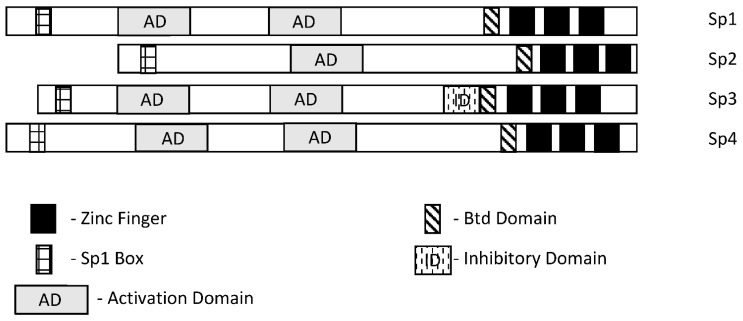
Figure 1.
Schematic structures of Sp1, Sp2, Sp3 and Sp4 [1,2]. These transcription factors exhibit several common structural features; however, Sp3 expresses an inhibitory domain that results in gene-specific decreased expression in some cell lines.
Several excellent reviews on Sp transcription factors and their role on genes and pathways associated with cancer and non-cancer endpoints have been published [1,2,3,4,5,6,7] and this article primarily focuses on Sp TFs and cancer findings from studies published within the last 5 years, more recent studies and their significance. It will become apparent that while Sp TFs are not oncogenes, their designation as non-oncogene addiction genes is highly appropriate [7].
2. Sp TFs as Cancer Prognostic Factors
Extensive analysis of tumor and non-tumor tissues has identified many prognostic factors that can be used to predict patient outcomes. Moreover, in some cases, the results dictate the application of specific treatment regimens, and this is particularly true of early-stage breast cancer where expression of estrogen receptor α (ERα, ESR1) in mammary tumors usually results in treatment with endocrine therapies [10]. Table 1 illustrates the important role of Sp1 as a negative prognostic factor for multiple cancers where Sp1 is generally more highly expressed in tumors compared to normal tissue and overexpression is correlated with decreased disease-free patient survival or another negative outcome. With the exception of highly variable results for lung cancer, most tumors overexpress Sp1 (or Sp3) compared to non-tumor tissue and poorer outcomes are observed in patients with tumors overexpressing this TF. In liver cancer, both Sp1 and Sp2 are negative prognostic factors for survival [11,12,13]. In many cases, manuscripts reporting the role of Sp1 as a diagnostic factor are accompanied by laboratory studies showing the pro-oncogenic functional activities of Sp1.
Table 1.
Clinical/prognostic Significance of Sp transcription factors.
| Tumor | Sp TF | Prognosis | Refs. |
|---|---|---|---|
| Prostate | Sp1/Sp3/FLIP | Overexpression correlated with a high Gleason score and predicted recurrence | [14] |
| Esophageal squamous cell carcinoma | Sp1 | High Sp1 predicts poor patient survival | [15] |
| Astrocytoma | Sp1 | Poor patient prognosis | [16] |
| Bladder urothelial carcinoma | Sp | Poor clinical outcomes | [17] |
| Glioma | Sp1 | Poor outcomes, higher expression in higher grades, immune invasion | [18,19,20] |
| Head and Neck | Sp3 | Predicted poor survival | [21] |
| Pancreatic | Sp1 (Sp1/LOXL2) | Decreased survival, higher grade, dual prognostic factor (with LOXL2) | [22,23,24] |
| Oral squamous cell carcinoma | Sp1 | Overexpressed and prometastatic | [25] |
| Gastric cancer | Sp1 | Overexpressed, poor prognosis, increased in higher stages | [26,27,28,29,30] |
| Liver cancer | Sp1 | Overexpressed, poor prognosis | [11,12] |
| Colin cancer | Sp1/Sp3 | Overexpressed, decreased survival | [31,32] |
| Breast cancer | Sp1/Par3 | Lower levels/advanced stage, poor prognosis | [33,34,35,36] |
| Lung cancer | Sp1 | Variable prognosis, decreased Sp1 with increasing stage | [37,38,39] |
| Ovarian cancer | Sp1/DANCR | Sp1 overexpression in tumor, correlates with DANCR | [40] |
| Liver cancer | Sp2 | Decreased survival | [13] |
Meta-analysis of multiple studies has also been used to probe the role of Sp1 in gastric cancer, and higher Sp1 expression is correlated with increased depth of invasion and lymph node metastasis, increased TNM staging and Lauren’s classification [41]. A similar meta-analysis approach was used to examine multiple tumor types [42] and similar associations were observed as reported for gastric cancer.
3. Role of Sp in Cell Transformation
Sp1 is clearly a negative prognostic factor for multiple cancers, and this is accompanied by increased expression of Sp transcription factors in tumors compared to non-tumor tissues. These observations suggest that the process that drives the transformation of a normal cell to a tumor cell may also involve Sp transcription factors. This was investigated in a classical study that examined the effects of carcinogen or oncogene-induced transformation of human fibroblasts into fibrosarcoma cells in which the fibrosarcoma, but not the fibroblasts, had the ability to form tumors in athymic nude mice [43,44]. This dramatic change in the phenotype of fibrosarcoma cells compared to the fibroblasts was accompanied by an 8- to 18-fold increased expression of Sp1 protein, which is enhanced during fibroblast cell transformation. Moreover, it was also demonstrated that knockdown of Sp1 in the fibrosarcomas resulted in cells that did not form tumors in athymic nude mice. Other studies show that EGF-induced transformation of bladder epithelial cells and Kras induced transformation of MCFI0A cells also involved Sp1 or an Sp1-regulated gene [45,46]. CYP1B1 also enhanced the proliferation, migration and invasion of MCFI0A and MCF7 cells and this was also accompanied by increased expression of Sp1 and Sp1 regulated genes and silencing or inhibition of Sp1 inhibited CYP1B1-mediated transformation [47].
Arsenic is a carcinogen and considered to be a public health hazard. Exposures of human bronchial epithelial Beas-2B cells to arsenic over a period of several months lead to cell transformation and this was due, in part, to induction DNA methyltransferase 1 (DNMT1) [48]. However, further examination found that arsenic induced Sp1, which in part enhanced DNMT1 expression and loss of miR-199a-5p, which was critical for arsenic-induced transformation. The proposed mechanism involves arsenic-induced Sp1, which in turn activates DNMT1 and suppresses miR-199a-5p. These results demonstrate a role for Sp1 in arsenic-induced transformation of Beas-2B cells; however, the direct effect of Sp1-mediated suppression of miR-199a-5p is unexpected and needs further investigation. Rhabdomyosarcomas (RMS) express high levels of Sp1 compared to non-transformed muscle tissue and RMS cell lines express high levels of Sp1, Sp3 and Sp4. Transformation of human smooth muscles with telomerase, the PAX3-FOXO1 oncogene and NMyc transforms these muscle cell lines; however, expression of only one or two of these factors is not sufficient for transformation [49]. Interestingly, transfection of one or two of these genes dramatically induces expression of Sp1 and Sp3 but not Sp4. This suggests that the process of cell transformation is accompanied by early induction of Sp1 and Sp3 prior to conversion of the muscle cell into a cancer cell [50].
The role of Sp TFs in the process of transformation has also been investigated in cancer stem cells, where they directly regulate genes associated with “stemness” or cooperate with other genes and non-coding RNAs to enhance stemness. At present, there is strong evidence for the role of Sp1 in inducing stemness, and the cooperating factors vary with tumor type. Stemness in breast cancer is maintained by the long non-coding RNA408 (Lnc408)—dependent recruitment of Sp3 to CBY1 gene promoters to inhibit expression of CBY1, which indirectly enhances levels of nuclear β-catenin and β-catenin regulated cancer stem cell-related genes [51]. In gastric cancer, Sp1 regulates expression of leucine-rich repeat-containing receptor 5 (LGR5), a key stem cell factor [52], and in hepatocellular carcinoma, Sp1 induced LncRNA DPPA2 upstream binding RNA (DUBR) [53]. DUBR not only promotes stemness, but also oxaliplatin resistance through an Sp1/DUBR/E2F1-CIP2A axis. The cancer stem-cell-related protein BMI1 is overexpressed in lung cancer and is important for maintaining this phenotype and resistance to pemetrexed [54]. BMI1 also regulates Sp1 expression and knockdown of Sp1 or treatment mithramycin reverses many of the effects of BMI1, including chug resistance. The pro-oncogenic LncRNA HOTAIR interacts with and upregulates Sp1, which induces DNMI1, and transcriptional repression of miR-199a-5p and targeting downregulation of Sp1 or DNMI1 was found to decrease stemness and progression of cutaneous squamous cell carcinoma [55]. In papillary thyroid carcinoma, the LncRNA DOCK9-AS2 interacts with and induces Sp1, which in turn induces β-catenin, which is further induced by DOCK9-AS2 interacting with miR-1972, resulting in increased β-catenin and Wnt signaling [56]. Sp1 is overexpressed in glioblastoma cells [18,19,20] and plays a role in maintaining stemness and drug resistance in this tumor type. It was also reported that ANGPTL4 and Sp4 were overexpressed in GBM and predicted poor patient prognosis [57]. Sp4 also regulates ANGPTL4 and downstream EGFR/AKT/4E-BP1, which is associated with temozolomide resistance and expression of cancer stem cell markers. Drug resistance and stemness in GBM were also associated with Sp1 in another study [58] and in glioma, HDAC/Sp1 regulation of BMI1 enhanced stemness [59]; this exhibited some overlap with lung cancer cells and BMI1 [54].
4. Sp TFs and Regulation of Protein-Encoding Genes in Cancer Cells
In 1983–1984, Tjian and coworkers initially identified Sp1 as a factor that stimulated SV40 early promoter transcription by 40-fold and bound to GC-rich elements in target gene promoters [60,61]. This same group also identified Sp2 as another TF that bound SV40 [60], and approximately a decade later, Sp3 and Sp4 were also characterized [62,63,64,65,66] as a structurally related sub-class of the Sp/KLF family. Subsequent research has demonstrated that Sp1-Sp4 TFs directly regulate or co-regulate thousands of protein-encoding genes associated with cell proliferation, survival, migration and invasion [7]. A detailed study of the role of Sp1, Sp3 and Sp4 in cancer was investigated in multiple cancer cell lines by individual knockdown of the three genes and their combination coupled with analysis of the resulting functional and genomic effects and their overlap [66]. Knockdown of Sp1 (siSp1), Sp3 (siSp3) and Sp4 (siSp4) and their combination (siSp1, 3, 4) decreased growth, increased Annexin V staining (apoptosis) and decreased invasion in A549 lung, MiaPaca2 (pancreatic), SW480 (colon), 786-0 (kidney), SKBR3 (breast), MDA-MB231 (breast), Panc1 (pancreatic) and L3.6 pL (pancreatic) cancer cells. Knockdown efficiencies were high and cell context-dependent differences in functional response potencies were < three-fold for most responses. For most responses, cells deficient in Sp1, Sp3 and Sp4 (triple knockdown) exhibited the highest effect on growth inhibition, induction of Annexin V staining and inhibition of invasion; however, the magnitude of the differences between single and triple knockdown was relatively modest. These results indicate that Sp1, Sp3 and Sp4 individually regulate proliferation, survival and invasion of cancer cells and the loss of one of these TFs is not compensated or rescued by the other two. One possible explanation is that Sp1, Sp3 and Sp4 cooperatively regulate many of the same pro-oncogenic genes and loss of a single TF compromises any possible rescue by the other two.
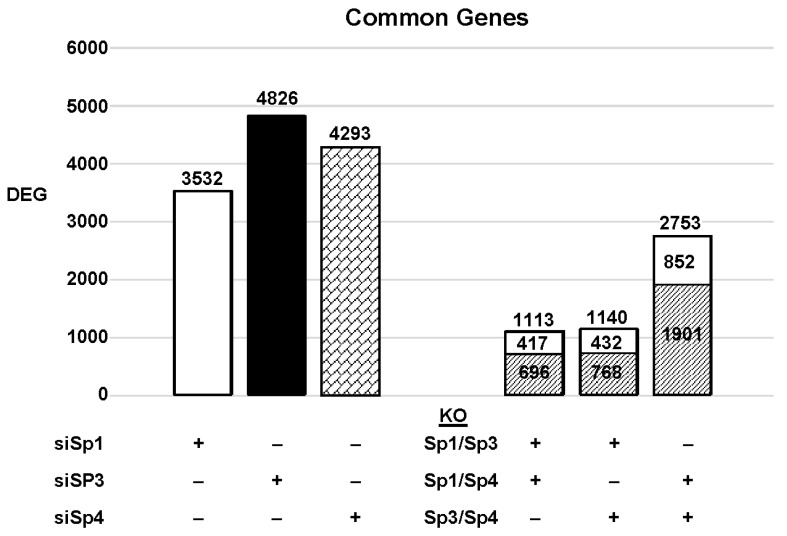
The highly invasive Panc1 pancreatic cancer cell line was used as a model to investigate the differential expression of genes after knockdown of Sp1, Sp3 and Sp4. Figure 2 illustrates the number of DEGs after knockdown of Sp1, Sp3 and Sp4, including 3532, 4826 and 4232 genes, respectively. Further analysis shows that the common DEGs after knockdown of Sp1/Sp3, Sp1/Sp4 and Sp3/Sp4 were 1113, 1140 and 2753, respectively, indicating that pairs of the three Sp TFs regulated a relatively high percentage of genes in common. This was particularly true for Sp3/Sp4, in which 2753 genes were commonly regulated by both transcription factors, which includes 57 and 64% of all Sp3 and Sp4 regulated genes, respectively. This would suggest that particularly for Sp3 and Sp4 and also the other pairs (Sp1/Sp3, Sp1/Sp4), there may be significant cooperative regulation of genes that requires more than one Sp TF. As demonstrated in Figure 2 and Figure 3, Sp1, Sp3 and Sp4 regulate expression of several thousand genes, with many of them associated with cancer proliferation, survival, and migration/invasion. Moreover, the three transcription factors also regulate genes in common and also genes that are Sp- specific and vary with cell context. Sp (Sp1, Sp3 and Sp4) regulated genes include epidermal growth factor receptor 1 (EGFR), other tyrosine kinases, cMyc, bcl2, survivin, vascular endothelial growth factor receptors (VEGFR1 and VEGFR2), matrix metalloproteinases and many other genes.
Figure 2.
Sp knockdown and changes in gene expression [7]. Panc1 cells were transfected with siRNAs, and after Sp knockdown, the changes in gene expression and the genes commonly induced/repressed by siSp1/siSp3, siSp1/siSp4, and siSp4/siSp3 were determined ( : decreased and
: decreased and  : increased expression in the double knockout groups).
: increased expression in the double knockout groups).
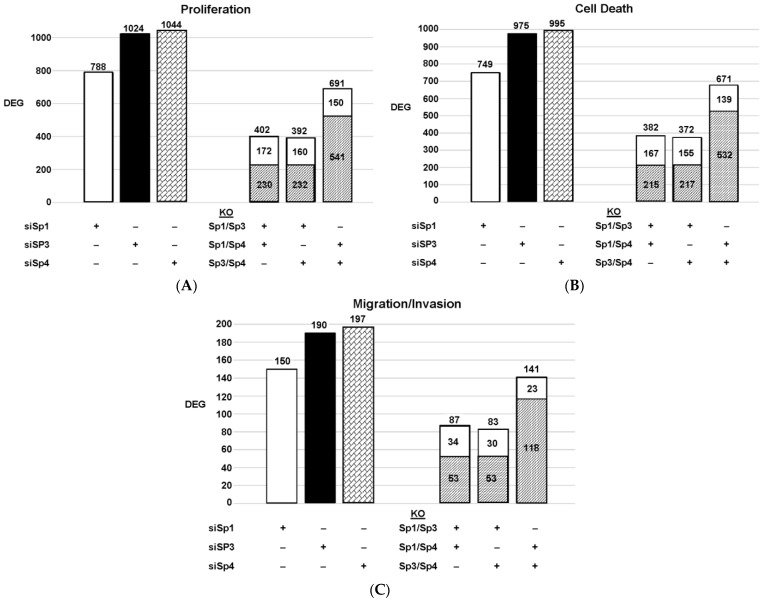
Figure 3.
Effects of Sp knockdown by RNAi [7]: IPA analysis of the differentially expressed genes in Panc1 cells associated with cell proliferation (A), Annexin V staining (B), and invasion (C). In these same samples, the common genes observed after knockdown of Sp1/Sp3, Sp1/Sp4 and Sp3/Sp4 are given. ( : decreased and
: decreased and  : increased expression in the double knockout group).
: increased expression in the double knockout group).
Since Sp TF regulate genes associated with cell proliferation, survival, and invasion, we used ingenuity pathway analysis (IPA) to analyze DEGs for each pathway after knockdown of individual Sps and their combination. The relative expressions of DEGs were determined and the results are illustrated in Figure 3. The patterns of DEGs associated with Panc1 cell proliferation, survival, and invasion after knockdown of Sp1, Sp3 and Sp4 were similar; however, the number of genes involved followed the order of proliferation ≥cell death > invasion. In addition, the pattern of the number of DEGs commonly expressed by Sp1/Sp3, Sp1/Sp4 and Sp3/Sp4 associated with cell proliferation, survival and invasion was higher than that observed for the total genes. The percentage of common genes/total genes was the highest for Sp3/Sp4, where the percentages were 67%, 68% and 74% (Sp3), and 66%, 67% and 72% (Sp4) for cell proliferation, survival, and invasion respectively. Casual IPA analysis also confirmed by their z scores (>2.0 or <−2.0) that the DEGs in each group were strongly associated with the functional responses.
There is evidence from the large number of publications that not only do Sp1, Sp3 and Sp4 regulate pro-oncogenic pathways and genes, but there are also reports that Sp2 performs similar functions [13,67,68]. For example, Sp2 knockdown in hepatocellular carcinoma cells decreases cell migration, proliferation and survival of hepatocellular carcinoma cells and this is due, in part, to decreasing the expression of the TRIB3 gene [13]. Additionally, Sp2-dependent suppression carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) [67] and overexpression of Sp2 increase susceptibility to wound- and carcinogen-induced tumorigenesis [68]. Thus, Sp1-Sp4 regulation of protein-encoding genes plays an important role in cell transformation and tumorigenesis.
5. Sp TFs-MicroRNA (miRNA) Interactions in Cancer Cells
Although noncoding RNAs have been described long before the sequence of the human genome was published, it became evident from the sequencing data that only a small faction (1–2%) of the human genome encodes for proteins [69]. Subsequent studies have identified many different types of non-coding RNAs (ncRNAs), including housekeeping and regulatory ncRNAs, which have been linked to many functions, some of which include interactions with Sp TFs [69,70,71,72,73]. Mature miRNAs have a length ≤20 nucleotides and are processed from pri-miRNA; one of their major functions involves interactions of the seed sequences of these miRNAs with complementary 6-8 base pair sites in the 3′-region of target genes to inhibit transcription [70]. There is a sub-set of miRNAs that directly inhibit Sp1 expression and the resulting inverse expression of these miRNAs with Sp1 is sometimes also associated with their use as a positive prognostic value for cancer patients. MiRNAs that repress expression of Sp1, Sp3 and Sp4 are illustrated in Table 2, and it is clear that several miRNAs are key regulators of Sp expression in multiple tumor types and Sp1 is preferentially targeted in cancer cells. It is also evident that multiple miRNAs target Sp in the same tumor cell type. For example, miRNA-375, miRNA-375-3p, miRNA-1224-5p, miRNA-382, and miRNA-149 target Sp1 and decrease expression of Sp1 in colorectal cancer and eight miRNAs decrease Sp1 expression in gastric cancer. Some of the miRNAs in Table 2 and others are also regulated by Sp TF in cancer cells. For example, Sp1 induces expression of multiple miRNAs in lung cancer cells (miRNA-3194-5p, miRNA-218-5p, miRNA-193-5p, miRNA-182-5p and miRNA-135-5p), [74] miRNA-200 in breast cancer cells [75], and miRNA-365 in Hela cells [76]. In contrast, Sp1 decreases miR-335 expression in ovarian cancer cells, and this is one of the rare reported examples of Sp1 as a transcriptional receptor [77].
Table 2.
MiRNA-Dependent inhibition of Sp. TFs.
| miRNA | Sp TF | Tumor | Refs. |
|---|---|---|---|
| miRNA-29b | Sp1 | Myeloid leukemia | [78,79] |
| miRNA-29b | Sp1 | Multiple myeloma | [80] |
| miRNA-23b | Sp1 | Multiple myeloma | [81] |
| miRNA-377 | Sp1 | Glioblastoma | [82] |
| miRNA-380-3p | Sp1 | Neuroblastoma | [83] |
| miRNA-29b | Sp1 | Tongue squamous cell carcinoma | [84] |
| miRNA-429 | Sp1 | Esophageal carcinoma | [85] |
| miRNA-506 | Sp1/Sp3 | Breast cancer cells | [86] |
| miRNA-27b | Sp1 | Non-small cell lung cancer | [87] |
| miRNA-324-5p | Sp1 | Hepatocellular carcinoma | [88] |
| miRNA-491-3p | Sp1 | Hepatocellular carcinoma | [89] |
| miRNA-200b/200c | Sp1 | Gastric cancer | [90] |
| miRNA-22 | Sp1 | Gastric cancer | [91] |
| miRNA-223 | Sp1 | Gastric cancer | [92] |
| miRNA-638 | Sp1 | Gastric cancer | [93] |
| miRNA-145/133a/133b | Sp1 | Gastric cancer | [94] |
| miRNA-335 | Sp1 | Gastric cancer | [95] |
| miRNA-375 | Sp1 | Pancreatic adenocarcinoma | [96] |
| miRNA-375 | Sp1 | Colorectal cancer | [97] |
| miRNA-375-3p | Sp1 | Colorectal cancer | [98] |
| miRNA-1224-5p | Sp1 | Colorectal cancer | [99] |
| miRNA-382 | Sp1 | Colorectal cancer | [100] |
| miRNA-149 | Sp1 | Colorectal cancer | [32] |
| miRNA-429 | Sp1 | Renal cell adenocarcinoma | [101] |
| miRNA-137 | Sp1 | Bladder cancer | [102] |
| miRNA-375 | Sp1 | Squamous cervical cancer | [103] |
| miRNA-34a | Sp1 | Hela cells | [104] |
| miRNA-330 | Sp1 | Prostate cancer | [105] |
6. Sp TFs-LncRNA Interactions in Cancer Cells
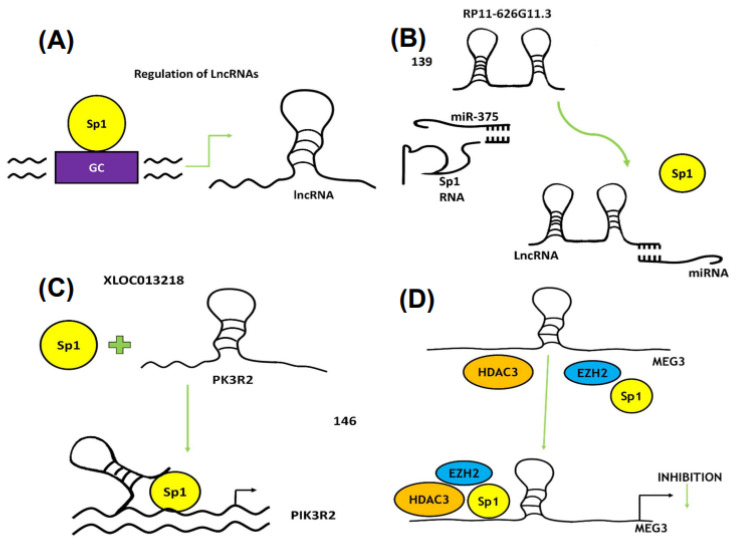
Long non-coding RNAs (lncRNAs) are another class of ncRNAs that are > 200 nucleotides long, and it is estimated that the human genome encodes more than 28,000 lncRNAs. LncRNAs have multiple functions, including both tumor suppressor and tumor promoter-like activities [106,107,108,109]. These activities are the result of their diverse mechanisms of action that act via signaling, decoys, guides and scaffolds [110]. Sp1 plays a varied role in regulating LncRNA since Sp1 and various LncRNAs regulate each other individually or reciprocally and also cooperate with other gene products and miRNAs in cancer cells. Since Sp1 is a negative prognostic factor for many tumors, it is not surprising that Sp1 regulates expression of several LncRNA, many of which are also pro-oncogenic. Table 3 summarizes a number of lncRNAs that are directly regulated by Sp1 and some of these ncRNAs are also regulated by Sp3 and Sp4 [86,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143]. Sp TFs also interact with lncRNA/miRNA where there is not a direct modulation of lncRNA/Sp expression [144,145,146,147,148]. In addition, there is also evidence that lncRNA LOC90024 promotes an RNA splicing step that results in formation of a long pro-oncogenic form of Sp4 [148,149]. Examples of mechanisms involving Sp TFs and lncRNAs include the following; direct transcriptional activation of lncRNAs by Sp1 (Figure 4A); sponging of miR-375 by RP11-626G11-3 to enhance Sp1 levels (Figure 4B); formation of an Sp1/XLOC013218 complex on the PIK3R2 promoter to activate gene expression (Figure 4C); and formation of an HDAC3/Sp1/EZH2 complex on the MEG3 promoter to inhibit gene expression (Figure 4D). The physical and functional interactions of Sp1, Sp3 and Sp4 with non-coding RNAs have primarily been observed for Sp1, as indicated from Table 2 and Table 3. However, it is apparent from the current available data that Sp interactions with ncRNAs are highly variable and cell-context-dependent. The emergence of dominant Sp-miRNA and Sp-lncRNA complexes that modulate critical pathways in cancer will be dependent on the results of future research. Thus, many functional effects of lncRNAs are Sp1 dependent and these are often in association with other genes involved in the complex. With few exceptions, lncRNA/miRNA pathways that lead to higher expression of Sp1, Sp3 and Sp4 result in downstream activation of pro-oncogenic genes/pathways, indicating that drugs targeting ncRNAs or Sp TFs should be highly effective anti-cancer agents.
Table 3.
Sp TFs regulate LncRNA expression in cancer cell.
| Sp TF | LncRNA | Tumor | Ref. |
|---|---|---|---|
| Sp1 | MIR155HG | Glioblastoma | [111] |
| Sp1 | HOTTIP | Osteosarcoma | [112] |
| Sp1 | Lnc00152 | Retinoblastoma | [113] |
| Sp1 | RNA TINCR | Gastric cancer | [114] |
| Sp1 | LINC01638 | Non-small cell lung cancer | [115] |
| Sp1 | THAP7-AS1 | Gastric cancer | [116] |
| Sp1 | MELTF-AS1 | Non-small cell lung cancer | [117] |
| Sp1 | DUBR | Hepatocellular carcinoma | [53] |
| Sp1 | HOTAIR | Hepatitis B virus | [118] |
| Sp1 a | PCAT6 | Breast cancer | [119] |
| Sp1 a | PCAT19 | Gastric cancer | [120] |
| Sp1 a | LINC00659 | Gastric cancer | [121] |
| Sp1 a | LINC00520 | Non-small cell lung cancer | [122] |
| Sp1 a | MIR155HG | Melanoma | [123] |
| Sp1a | CTBP1-AS2 | Hepatocellular carcinoma | [124] |
| Sp1 a | HOXD-AS1 | Cholangiocarcinoma | [125] |
| Sp1 a | LMCD-AS1 | Osteosarcoma | [126] |
| Sp1 a | LINC00689 | Osteosarcoma | [127] |
| Sp1 a | SNHG4 | Prostate | [128] |
| Sp1/Sp3/Sp4 | MALAT-1 | Pancreatic cancer | [129] |
| Sp1 ab | MEG3 | Pancreatic cancer | [130] |
| Sp1 b | SAMMSON | Thyroid carcinoma | [131] |
| Sp1 b | MALAT1 | Lung adenocarcinoma | [132] |
| Sp1 b | HOTAIR | Hepatocellular carcinoma | [133] |
| Sp1 b | HOTAIR | NSCLC | [134] |
| Sp1 | CRNDE | Hepatocellular carcinoma | [135] |
| Sp1 b | HOTAIR | Cutaneous squamous cell carcinoma | [55] |
| Sp1 ab | HOTAIRM1bc | Glioblastoma | [136] |
| Sp1 | TUG1 | Colorectal cancer | [137] |
| Sp1 | POU3F3 | Cervical cancer | [138] |
| Sp1 a | LINC00511 | Glioma | [139] |
| Sp1 a | TINCR | Colorectal cancer | [140] |
| Sp1 a | RP11-626G113bc | Glioma | [141] |
| Sp1 a | MIR31HG | NSCLC | [142] |
| Sp1 a | SNHG22 | Ovarian cancer | [143] |
a: miR involved; c: +Line—Sp1; b: reciprocal.
Figure 4.
Interactions of Sp1 and lncRNAs. (A) Sp1 binds to the GC-rich promoter of a lncRNA to induce gene expression. (B) Silencing of Sp1 by miR-375 is reversed by competitive binding of a lncRNA to a miRNA. (C) Sp1 interacts with lncRNA XLOC013218 and forms an activation complex on the PIK3R2 gene promoter, whereas (D) MEG3 expression is inhibited by the EZH2/HDAC3/Sp1 complex.
7. Sp Transcription Factors as Drug Targets
Sp transcription factors are prognostic indicators for multiple cancers (Table 1) and interact with both miRNAs and lncRNAs (Table 2 and Table 3) to facilitate cancer cell proliferation, survival, migration and invasion. These pro-oncogenic activities correlate with the results of knockdown studies that are consistent with their designation as non-oncogene addiction genes [66]. Despite these facts, anticancer agents that specifically target SpTFs are not being developed currently for clinical applications, even though several small molecules that are used for cancer and other chemotherapies also downregulate/degrade Sp1, Sp3 and Sp4. These include HDAC inhibitors, metformin, bardoxolone methyl, bortezomib and some non-steroidal anti-inflammatory drugs (NSAIDs). Two review articles from this laboratory have previously outlined compounds that downregulate or induce degradation of Sp TFs [4,150], and these include drug-induced ROS, proteasome-dependent degradation, cannabinoid receptor (CBR) induced responses, zinc depletion and kinase/phosphatase pathways. Studies in this laboratory have investigated drugs that activate most of these pathways [4,150] and result in coordinated downregulation of Sp1, Sp3 and Sp4. Most other studies have focused on drug-induced downregulation of only Sp1, and it can be assumed that in many cases, downregulation of Sp1 is accompanied by parallel decreases in Sp3 and Sp4. Multiple classes of compounds decrease expression of Sp TFs in cancer cells, and these include structurally diverse ROS inducers, non-steroidal anti-inflammatory drugs (NSAIDs), cannabinoids and other drugs including retinoids, α—tocopherol thiazolidinediones, bortezomib, flavonoids and structurally diverse natural products and synthetic analogs [4,150].
7.1. ROS Pathway
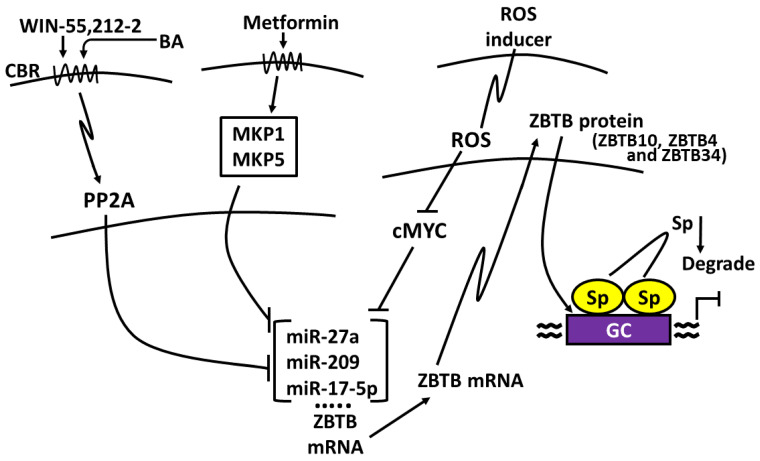
ROS inducers are among the most well-characterized compounds that decrease levels of Sp TFs in cancer cells, and this response contributes to their overall anticancer activities. ROS inducers include phenethyl isothiocyanate (PEITC), benzyl isothiocyanate (BITC), celastrol, curcumin, betulinic acid, piperlongumine, penfluridol, the nitro aspirin GT-094, histone deacetylase (HDAC) inhibitors, hydrogen peroxide, ascorbic acid, arsenic trioxide, and t-butyl hydroperoxide [151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166]. In addition, several other compounds that target Sp1 downregulation including phloretin, honokiol, triptolide baicalin, quercetin, licochalcone, 7,8-dihydroxyflavone [167,168,169,170,171,172,173,174,175,176,177,178,179,180] also induce ROS [181,182,183,184,185,186] and may act in some cell lines through the ROS-Sp (downregulation) pathway. The mechanism associated with ROS-dependent downregulation of Sp TFs was determined over several years and was in part dependent on two separate and independent studies. Firstly, O’Hagan and coworkers reported that ROS induced genome-wide chromatin shifts of complexes containing CpG islands and this resulted in the downregulation of c-Myc [187]. A second study reported that treatment of breast cancer cells with an HDAC inhibitor induced expression of an Sp repressor gene ZBTB10 and this was accompanied by downregulation of miR-27b, which is part of the miRNA-23a-27a-24-2 cluster [188]. These results, coupled with extensive studies on various ROS inducers, conclude that the overall mechanism of Sp downregulation is linked to ROS-dependent downregulation of Myc and Myc-dependent miRNAs (including miR-27a) (Figure 5). This results in the induction of ZBTB10, which in turn competitively binds GC-rich promoters and displaces Sp TFs. ZBTB10 and related ZBTB genes do not have a transactivation domain, and this results in gene silencing at GC-rich sites at the expense of Sp TFs. Subsequent studies show that miR-27a also regulates expression of ZBTB34 [151,152,162,165,166]. This also results in decreased expression of Sp1, Sp3 and Sp4, which are self-regulated genes. In addition, cMyc also regulates miRNA-20a and miRNA-17-5p expression, which are part of the miRNA-17-92 cluster, and this results in induction of ZBTB4, which also represses expression of Sp TFs [189]. The results of these studies were confirmed by both overexpression and rescue experiments and also demonstrate that knockdown of cMyc also decreases levels of Sp1, Sp3 and Sp4.
Figure 5.
Mechanisms of Sp downregulation [149,150,160,162,164]: ROS-inducers target Myc [149,150] whereas Metformin and WIN target kinases [189,190,191] to activate ZBTB (via miRNA downregulation), which displace Sp TFs from GC-rich sites. ZBTB genes induced via these pathways include ZBTB10, ZBTB34 and ZBTB4.
7.2. Kinase/Phosphatase Pathway
Interestingly, the role of ZBTB-induced suppression was also observed downstream from drug-induced activation of kinases through the cannabinoid receptor. The synthetic CB receptor ligand WIN55,212-activates protein phosphatase 2A, resulting in miRNA-27a downregulation and activation of ZBTB10 in colon cancer cells [190] (Figure 5). Moreover, it was reported in that in breast cancer cells, betulinic acid also targeted the miRNA-279-ZBTB10 pathway through betulinic acid acting as a cannabinoid receptor ligand [191]. The antidiabetic drug metformin also induced Sp downregulation and like many other agents noted above, the mechanism was cell-context-dependent. In Panc1 cells, metformin-dependent downregulation of Sp TFs was due to mitogen-activated protein kinase phosphatase 1 (MKP-1) and MKP-5, which targeted miR-27a-ZBTB10, whereas in Panc28 and L3.6pL cells, metformin induced proteasome-dependent degradation of Sp1, Sp3 and Sp4 [192,193] (Figure 5). In addition, several other studies found that phosphatases induced Sp1 downregulation. For example, progesterone activation of progesterone receptor induced MKP1 and Sp1 downregulation [193] and both α-tocopherol succinate and hydrogen peroxide activated a phosphatase-JNK1 pathway that also decreased expression of Sp1 [194,195].
7.3. Proteasome-Dependent Degradation
Several studies have reported proteasome-dependent degradation of Sp1, Sp3 and Sp4 by a number of anticancer agents, including tolfenamic acid and related NSAIDs, betulinic acid and celecoxib [196,197,198,199,200,201]. The mechanisms of drug-induced degradation of Sp TFs by proteasomes has been investigated in other studies, which suggest that multiple pathways are involved. Sumoylated Sp1 can recruit the E3 ubiquitin ligase RING Finger protein 4 (RNF4) which undergo proteasome degradation; a similar pathway has also been observed for betulinic acid, which induces degradation of Sp1 and Sp3 [202,203,204,205]. Further studies on the role of sumoylation and other cofactors on degradation of Sp1, Sp3 and Sp4 need to be further investigated. Activation of caspases also plays a role in Sp degradation, and this pathway has been observed for several drugs including aspirin, retinoids, tolfenamic acid, and bortezomib; these effects are also cell-context-dependent [206,207,208,209,210,211,212]. Many of these studies have focused on the mechanisms associated with only one of the Sp proteins (usually Sp1), and the results clearly demonstrate that several mechanisms are operative.
7.4. Activation of Caspases
For example, cleavage of Sp1 by a retinoid in liver cancer cells involves induction of caspase-3 and transglutaminase [210] and caspases-2 and 3 in leukemia cells [209]. A role for caspase-3 activation in Sp1 degradation has been observed in other studies [211,212], whereas bortezomib was found to decrease Sp1, Sp3 and Sp4 in leukemia cells, and this was dependent on caspase-8 [179]. The zinc chelator N,N,N′,N′-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN) sequesters zinc, and this results in activation of caspases-3,8, and 9 and downregulation of Sp1 [207]. Similar results were observed in colon cancer cells treated with aspirin [206], which also induced degradation of Sp1, Sp3 and Sp4, and treatment with tolfenamic acid [208] gave results similar to that observed for aspirin. This was confirmed in studies showing that activation of caspases and Sp downregulation by TPEN, aspirin and tolfenamic acid was reversed in cancer cells after cotreatment with zinc sulfate. Figure 6 illustrates the structurally diverse agents that downregulate Sp transcription factors via mechanisms outlined in Figure 5. These studies indicate that drugs targeting the pro-oncogenic Sp1, Sp3 and Sp4 act through multiple pathways that are cell-context-dependent.
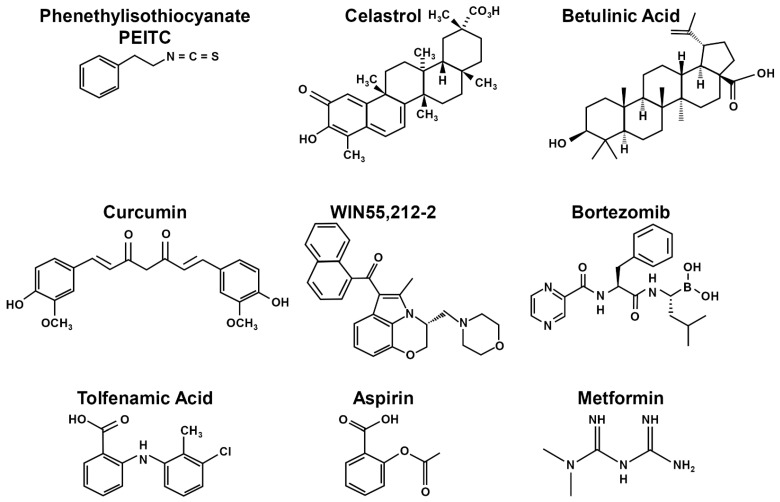
Figure 6.
Structures of compounds that induce Sp downregulation. Compounds that induce downregulation of Sp1, Sp3 and Sp4 are structurally diverse and include PEITC [151], celastrol [153], betulinic acid [155,165,191], curcumin [154,158], WIN55,212-2 [190], bortezomib [179], tolfenamic acid [198,208], aspirin [206] and metformin [192,213].
8. Summary and Conclusions
There is increasing evidence that Sp1, Sp3 and Sp4 play an important role in multiple cancers and their prognostic importance spans their functional pro-oncogenic activities alone and in combination with miRNAs and lncRNAs. Genomic studies on these transcription factors and genes/pathways regulated by Sp1, Sp3 and Sp4 demonstrate their role in the growth, survival and migration/invasion of cancer cells and tumors, and this is consistent with their designation as non-oncogene addiction genes. Moreover, this designation is supported by interaction of Sp TFs with ncRNAs where their role is associated with enhancing pro-oncogenic pathways. There is also extensive evidence that multiple compounds, including approved drugs that are used for other diseases, induce downregulation or degradation of Sp1, Sp3 and Sp4, which is accompanied by inhibition of cell/tumor growth and invasion and induction of apoptosis. Anticancer agents that target Sp TFs are not yet clinically used for cancer chemotherapy and the clinical applications of these agents, including repurposed drugs, need to be evaluated in combination therapies.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This is a review article summarizing published data.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
The research was funded by the Syd Kyle Chair endowment and the National Institutes of Health P30-ES029067.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kim C.-K., He P., Bialkowska A.B., Yang V.W. SP and KLF Transcription Factors in Digestive Physiology and Diseases. Gastroenterology. 2017;152:1845–1875. doi: 10.1053/j.gastro.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beishline K., Azizkhan-Clifford J. Sp1 and the ‘hallmarks of cancer’. FEBS J. 2015;282:224–258. doi: 10.1111/febs.13148. [DOI] [PubMed] [Google Scholar]
- 3.Li L., Davie J.R. The role of Sp1 and Sp3 in normal and cancer cell biology. Ann. Anat. Anat. Anzeiger. 2010;192:275–283. doi: 10.1016/j.aanat.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Safe S., Abbruzzese J., Abdelrahim M., Hedrick E. Specificity Protein Transcription Factors and Cancer: Opportunities for Drug Development. Cancer Prev. Res. 2018;11:371–382. doi: 10.1158/1940-6207.CAPR-17-0407. [DOI] [PubMed] [Google Scholar]
- 5.Orzechowska-Licari E.J., LaComb J.F., Mojumdar A., Bialkowska A.B. SP and KLF Transcription Factors in Cancer Metabolism. Int. J. Mol. Sci. 2022;23:9956. doi: 10.3390/ijms23179956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vizcaíno C., Mansilla S., Portugal J. Sp1 transcription factor: A long-standing target in cancer chemotherapy. Pharmacol. Ther. 2015;152:111–124. doi: 10.1016/j.pharmthera.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Hedrick E., Cheng Y., Jin U.-H., Kim K., Safe S. Specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 are non-oncogene addiction genes in cancer cells. Oncotarget. 2016;7:22245–22256. doi: 10.18632/oncotarget.7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Alessio J.A., Ng R., Willenbring H., Tjian R. Core promoter recognition complex changes accompany liver development. Proc. Natl. Acad. Sci. USA. 2011;108:3906–3911. doi: 10.1073/pnas.1100640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams A.O., Isaacs R.J., Stowell K.M. Down-regulation of human topoisomerase IIalpha expression correlates with relative amounts of specificity factors Sp1 and Sp3 bound at proximal and distal promoter regions. BMC Mol. Biol. 2007;8:36. doi: 10.1186/1471-2199-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis P.A., Pagani O., Fleming G.F., Walley B.A., Colleoni M., Láng I., Gómez H.L., Tondini C., Ciruelos E., Burstein H.J., et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N. Engl. J. Med. 2018;379:122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L., Ji P., Qu N., Pu W.L., Jiang D.W., Liu W.Y., Li Y.-Q., Shi R.-L. The impact of high co-expression of Sp1 and HIF1α on prognosis of patients with hepatocellular cancer. Oncol. Lett. 2016;12:504–512. doi: 10.3892/ol.2016.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong L.-M., Yao L., Lu N., Dong Y.-L., Zhang J., Wang Y.-Q., Liu L., Zhang H.-L., Huang J.-G., Liao C.-G. Interaction of KLF6 and Sp1 regulates basigin-2 expression mediated proliferation, invasion and metastasis in hepatocellular carcinoma. Oncotarget. 2016;7:27975. doi: 10.18632/oncotarget.8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yue Z., Jie C., Jiatao L., Wei H., Wei W., Guoping S. Sp2 promotes invasion and metastasis of hepatocellular carcinoma by targeting TRIB3 protein. Cancer Med. 2020;9:3592–3603. doi: 10.1002/cam4.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedolla R.G., Gong J., Prihoda T.J., Yeh I.T., Thompson I.M., Ghosh R., Kumar A.P. Predictive Value of Sp1/Sp3/FLIP Signature for Prostate Cancer Recurrence. PLoS ONE. 2012;7:e44917. doi: 10.1371/journal.pone.0044917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu L., Sang M., Li J., Liu F., Wu Y., Liu S., Wang P., Shan B. Expression and prognostic significance of MAGE-A11 and transcription factors (SP1,TFCP2 and ZEB1) in ESCC tissues. Pathol. Res. Pract. 2019;215:152446. doi: 10.1016/j.prp.2019.152446. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y.-T., Tsai H.-P., Wu C.-C., Chen C.-Y., Chai C.-Y., Kwan A.-L. High-level Sp1 is Associated with Proliferation, Invasion, and Poor Prognosis in Astrocytoma. Pathol. Oncol. Res. 2019;25:1003–1013. doi: 10.1007/s12253-018-0422-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J., Lu Z., Ke M., Cai X. Sp1 is overexpressed and associated with progression and poor prognosis in bladder urothelial carcinoma patients. Int. Urol. Nephrol. 2022;54:1505–1512. doi: 10.1007/s11255-022-03212-6. [DOI] [PubMed] [Google Scholar]
- 18.Guan H., Cai J., Zhang N., Wu J., Yuan J., Li J., Li M. Sp1 is upregulated in human glioma, promotes MMP-2-mediated cell invasion and predicts poor clinical outcome. Int. J. Cancer. 2012;130:593–601. doi: 10.1002/ijc.26049. [DOI] [PubMed] [Google Scholar]
- 19.Dong Q., Cai N., Tao T., Zhang R., Yan W., Li R., Zhang J., Luo H., Shi Y., Luan W., et al. An Axis Involving SNAI1, microRNA-128 and SP1 Modulates Glioma Progression. PLoS ONE. 2014;9:e98651. doi: 10.1371/journal.pone.0098651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y., Cao F., Xiong Y., Zhou H. SP1 transcriptionally activates NLRP6 inflammasome and induces immune evasion and radioresistance in glioma cells. Int. Immunopharmacol. 2021;98:107858. doi: 10.1016/j.intimp.2021.107858. [DOI] [PubMed] [Google Scholar]
- 21.Essafi-Benkhadir K., Grosso S., Puissant A., Robert G., Essafi M., Deckert M., Chamorey E., Dassonville O., Milano G., Auberger P., et al. Dual Role of Sp3 Transcription Factor as an Inducer of Apoptosis and a Marker of Tumour Aggressiveness. PLoS ONE. 2009;4:e4478. doi: 10.1371/journal.pone.0004478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang N.Y., Woda B.A., Banner B.F., Whalen G.F., Dresser K.A., Lu D. Sp1, a New Biomarker That Identifies a Subset of Aggressive Pancreatic Ductal Adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2008;17:1648–1652. doi: 10.1158/1055-9965.EPI-07-2791. [DOI] [PubMed] [Google Scholar]
- 23.Hu J., Hu H., Hang J-j Yang H-y Wang Z-y Wang L., Chen D.-H., Wang L.-W. Simultaneous high expression of PLD1 and Sp1 predicts a poor prognosis for pancreatic ductal adenocarcinoma patients. Oncotarget. 2016;7:78557–78565. doi: 10.18632/oncotarget.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim I.-k., Lee Y.S., Kim H.S., Dong S.M., Park J.S., Yoon D.S. Specific protein 1(SP1) regulates the epithelial-mesenchymal transition via lysyl oxidase-like 2(LOXL2) in pancreatic ductal adenocarcinoma. Sci. Rep. 2019;9:5933. doi: 10.1038/s41598-019-42501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X.-b., Wang J., Li K., Fan X.-N. Sp1 promotes cell migration and invasion in oral squamous cell carcinoma by upregulating Annexin A2 transcription. Mol. Cell. Probes. 2019;46:101417. doi: 10.1016/j.mcp.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Wang L., Wei D., Huang S., Peng Z., Le X., Wu T.T., Yao J., Ajani J., Xie K. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin. Cancer Res. 2003;9:6371–6380. [PubMed] [Google Scholar]
- 27.Lee H.S., Park C.-K., Oh E., Erkin Ö.C., Jung H.S., Cho M.-H., Kwon M.J., Chae S.W., Kim S.-H., Wang L.-H., et al. Low SP1 Expression Differentially Affects Intestinal-Type Compared with Diffuse-Type Gastric Adenocarcinoma. PLoS ONE. 2013;8:e55522. doi: 10.1371/journal.pone.0055522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao J.C., Wang L., Wei D., Gong W., Hassan M., Wu T.-T., Mansfield P., Ajani J., Xie K. Association between Expression of Transcription Factor Sp1 and Increased Vascular Endothelial Growth Factor Expression, Advanced Stage, and Poor Survival in Patients with Resected Gastric Cancer. Clin. Cancer Res. 2004;10:4109–4117. doi: 10.1158/1078-0432.CCR-03-0628. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J., Zhu Z.-G., Ji J., Yuan F., Yu Y.-Y., Liu B.-Y., Lin Y.-Z. Transcription factor Sp1 expression in gastric cancer and its relationship to long-term prognosis. World J. Gastroenterol. 2005;11:2213–2217. doi: 10.3748/wjg.v11.i15.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J.-J., Ren Y.-L., Shu C.-J., Zhang Y., Chen M.-J., Xu J., Li J., Li A.-P., Chen D.-Y., He J.-D., et al. JP3, an antiangiogenic peptide, inhibits growth and metastasis of gastric cancer through TRIM25/SP1/MMP2 axis. J. Exp. Clin. Cancer Res. 2020;39:118. doi: 10.1186/s13046-020-01617-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maurer G.D., Leupold J.H., Schewe D.M., Biller T., Kates R.E., Hornung H.-M., Lau-Werner U., Post S., Allgayer H. Analysis of Specific Transcriptional Regulators as Early Predictors of Independent Prognostic Relevance in Resected Colorectal Cancer. Clin. Cancer Res. 2007;13:1123–1132. doi: 10.1158/1078-0432.CCR-06-1668. [DOI] [PubMed] [Google Scholar]
- 32.Wang F., Ma Y.-L., Zhang P., Shen T.-Y., Shi C.-Z., Yang Y.-Z., Moyer M.-P., Zhang H.-Z., Chen H.-Q., Liang Y., et al. SP1 mediates the link between methylation of the tumour suppressor miR-149 and outcome in colorectal cancer. J. Pathol. 2013;229:12–24. doi: 10.1002/path.4078. [DOI] [PubMed] [Google Scholar]
- 33.Li L., Gao P., Li Y., Shen Y., Xie J., Sun D., Xue A., Zhao Z., Xu Z., Zhang M., et al. JMJD2A-dependent silencing of Sp1 in advanced breast cancer promotes metastasis by downregulation of DIRAS3. Breast Cancer Res. Treat. 2014;147:487–500. doi: 10.1007/s10549-014-3083-7. [DOI] [PubMed] [Google Scholar]
- 34.Wang X.B., Peng W.Q., Yi Z.J., Zhu S.L., Gan Q.H. Expression and prognostic value of transcriptional factor sp1 in breast cancer. Ai Zheng. 2007;26:996–1000. [PubMed] [Google Scholar]
- 35.Kim J.-Y., Jung H.H., Ahn S., Bae S., Lee S.K., Kim S.W., Lee J.E., Nam S.J., Ahn J.S., Im Y.-H., et al. The relationship between nuclear factor (NF)-κB family gene expression and prognosis in triple-negative breast cancer (TNBC) patients receiving adjuvant doxorubicin treatment. Sci. Rep. 2016;6:31804. doi: 10.1038/srep31804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y., Yao D., Li Y., Zhang S., Tao Z., Zhang L., Hu X., Wang B., Chen S. Loss of polarity protein Par3 is mediated by transcription factor Sp1 in breast cancer. Biochem. Biophys. Res. Commun. 2021;561:172–179. doi: 10.1016/j.bbrc.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Hsu T.I., Wang M.C., Chen S.Y., Yeh Y.M., Su W.C., Chang W.C., Hung J.-J. Sp1 expression regulates lung tumor progression. Oncogene. 2012;31:3973–3988. doi: 10.1038/onc.2011.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong L.-M., Liao C.-G., Fei F., Guo X., Xing J.-L., Chen Z.-N. Transcription factor Sp1 regulates expression of cancer-associated molecule CD147 in human lung cancer. Cancer Sci. 2010;101:1463–1470. doi: 10.1111/j.1349-7006.2010.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H.-W., Wang E.-W., Li L.-X., Yi S.-H., Li L.-C., Xu F.-L., Wang D.-L., Wu Y.-Z., Nian W.-Q. A regulatory loop involving miR-29c and Sp1 elevates the TGF-β1 mediated epithelial-to-mesenchymal transition in lung cancer. Oncotarget. 2016;7:85905–85916. doi: 10.18632/oncotarget.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui P.-H., Li Z.-Y., Li D.-H., Han S.-Y., Zhang Y.-J. SP1-induced lncRNA DANCR contributes to proliferation and invasion of ovarian cancer. Kaohsiung J. Med. Sci. 2021;37:371–378. doi: 10.1002/kjm2.12316. [DOI] [PubMed] [Google Scholar]
- 41.Shi S., Zhang Z.G. Role of Sp1 expression in gastric cancer: A meta-analysis and bioinformatics analysis. Oncol. Lett. 2019;18:4126–4135. doi: 10.3892/ol.2019.10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen M., Gao Y., Gan K., Liu K., Xu B. SP1 Expression and the Clinicopathological Features of Tumors: A Meta-Analysis and Bioinformatics Analysis. Pathol. Oncol. Res. 2021;27:581998. doi: 10.3389/pore.2021.581998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lou Z., O’Reilly S., Liang H., Maher V.M., Sleight S.D., McCormick J.J. Down-regulation of overexpressed sp1 protein in human fibrosarcoma cell lines inhibits tumor formation. Cancer Res. 2005;65:1007–1017. doi: 10.1158/0008-5472.1007.65.3. [DOI] [PubMed] [Google Scholar]
- 44.McCormick J.J., Maher V.M. Malignant Transformation of Human Skin Fibroblasts by Two Alternative Pathways. In: Rhim J.S., Kremer R., editors. Human Cell Transformation: Role of Stem Cells and the Microenvironment. Springer; New York, NY, USA: 2012. pp. 191–207. [DOI] [PubMed] [Google Scholar]
- 45.Jin H., Xu J., Guo X., Huang H., Li J., Peng M., Zhu J., Tian Z., Wu X.-R., Tang M.-S., et al. XIAP RING domain mediates miR-4295 expression and subsequently inhibiting p63α protein translation and promoting transformation of bladder epithelial cells. Oncotarget. 2016;7:56540–56557. doi: 10.18632/oncotarget.10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong X., Zheng L., Shen J., Zhang D., Xiong M., Zhang Y., He X., Tanyi J.L., Yang F., Montone K.T., et al. Suppression of MicroRNA 200 Family Expression by Oncogenic KRAS Activation Promotes Cell Survival and Epithelial-Mesenchymal Transition in KRAS-Driven Cancer. Mol. Cell. Biol. 2016;36:2742–2754. doi: 10.1128/MCB.00079-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon Y.-J., Baek H.-S., Ye D.-J., Shin S., Kim D., Chun Y.-J. CYP1B1 Enhances Cell Proliferation and Metastasis through Induction of EMT and Activation of Wnt/β-Catenin Signaling via Sp1 Upregulation. PLoS ONE. 2016;11:e0151598. doi: 10.1371/journal.pone.0151598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He J., Liu W., Ge X., Wang G.-C., Desai V., Wang S., Mu W., Bhardwaj V., Seifert E., Liu L.-Z., et al. Arsenic-induced metabolic shift triggered by the loss of miR-199a-5p through Sp1-dependent DNA methylation. Toxicol. Appl. Pharmacol. 2019;378:114606. doi: 10.1016/j.taap.2019.114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naini S., Etheridge K.T., Adam S.J., Qualman S.J., Bentley R.C., Counter C.M., Linardic C.M. Defining the Cooperative Genetic Changes That Temporally Drive Alveolar Rhabdomyosarcoma. Cancer Res. 2008;68:9583–9588. doi: 10.1158/0008-5472.CAN-07-6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chadalapaka G., Jutooru I., Sreevalsan S., Pathi S., Kim K., Chen C., Crose L., Linardic C., Safe S. Inhibition of rhabdomyosarcoma cell and tumor growth by targeting specificity protein (Sp) transcription factors. Int. J. Cancer. 2013;132:795–806. doi: 10.1002/ijc.27730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wen S., Qin Y., Wang R., Yang L., Zeng H., Zhu P., Li Q., Qiu Y., Chen S., Liu Y., et al. A novel Lnc408 maintains breast cancer stem cell stemness by recruiting SP3 to suppress CBY1 transcription and increasing nuclear β-catenin levels. Cell Death Dis. 2021;12:437. doi: 10.1038/s41419-021-03708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilhelm F., Simon E., Böger C., Behrens H.-M., Krüger S., Röcken C. Novel Insights into Gastric Cancer: Methylation of R-spondins and Regulation of LGR5 by SP1. Mol. Cancer Res. 2017;15:776–785. doi: 10.1158/1541-7786.MCR-16-0472. [DOI] [PubMed] [Google Scholar]
- 53.Liu S., Bu X., Kan A., Luo L., Xu Y., Chen H., Lin X., Lai Z., Wen D., Huang L., et al. SP1-induced lncRNA DUBR promotes stemness and oxaliplatin resistance of hepatocellular carcinoma via E2F1-CIP2A feedback. Cancer Lett. 2022;528:16–30. doi: 10.1016/j.canlet.2021.12.026. [DOI] [PubMed] [Google Scholar]
- 54.Shen H.-T., Chien P.-J., Chen S.-H., Sheu G.-T., Jan M.-S., Wang B.-Y., Chang W.-W. BMI1-Mediated Pemetrexed Resistance in Non-Small Cell Lung Cancer Cells Is Associated with Increased SP1 Activation and Cancer Stemness. Cancers. 2020;12:2069. doi: 10.3390/cancers12082069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J., Hou S.-F., Tang F.-J., Liu D.-S., Chen Z.-Z., Zhang H.-L., Wang S.-H. HOTAIR/Sp1/miR-199a critically regulates cancer stemness and malignant progression of cutaneous squamous cell carcinoma. Oncogene. 2022;41:99–111. doi: 10.1038/s41388-021-02014-x. [DOI] [PubMed] [Google Scholar]
- 56.Dai W., Jin X., Han L., Huang H., Ji Z., Xu X., Tang M., Jiang B., Chen W. Exosomal lncRNA DOCK9-AS2 derived from cancer stem cell-like cells activated Wnt/β-catenin pathway to aggravate stemness, proliferation, migration, and invasion in papillary thyroid carcinoma. Cell Death Dis. 2020;11:743. doi: 10.1038/s41419-020-02827-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai Y.-T., Wu A.-C., Yang W.-B., Kao T.-J., Chuang J.-Y., Chang W.-C., Hsu T.-I. ANGPTL4 Induces TMZ Resistance of Glioblastoma by Promoting Cancer Stemness Enrichment via the EGFR/AKT/4E-BP1 Cascade. Int. J. Mol. Sci. 2019;20:5625. doi: 10.3390/ijms20225625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang K.-Y., Huang C.-T., Hsu T.-I., Hsu C.-C., Liu J.-J., Chuang C.-K., Hung J.-J., Chang W.-C., Tsai K.K., Chuang J.-Y. Stress stimuli induce cancer-stemness gene expression via Sp1 activation leading to therapeutic resistance in glioblastoma. Biochem. Biophys. Res. Commun. 2017;493:14–19. doi: 10.1016/j.bbrc.2017.09.095. [DOI] [PubMed] [Google Scholar]
- 59.Tsai Y.-T., Wu C.-C., Ko C.-Y., Hsu T.-I., Chang W.-C., Lo W.-L., Chuang J.-Y. Correlation between the expression of cancer stem cell marker BMI1 and glioma prognosis. Biochem. Biophys. Res. Commun. 2021;550:113–119. doi: 10.1016/j.bbrc.2021.02.140. [DOI] [PubMed] [Google Scholar]
- 60.Dynan W.S., Tjian R. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell. 1983;32:669–680. doi: 10.1016/0092-8674(83)90053-3. [DOI] [PubMed] [Google Scholar]
- 61.Gidoni D., Dynan W.S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. Nature. 1984;312:409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- 62.Kingsley C., Winoto A. Cloning of GT box-binding proteins: A novel Sp1 multigene family regulating T-cell receptor gene expression. Mol. Cell. Biol. 1992;12:4251–4261. doi: 10.1128/mcb.12.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagen G., Müller S., Beato M., Suske G. Sp1-mediated transcriptional activation is repressed by Sp3. EMBO J. 1994;13:3843–3851. doi: 10.1002/j.1460-2075.1994.tb06695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hagen G., Dennig J., Preiß A., Beato M., Suske G. Functional Analyses of the Transcription Factor Sp4 Reveal Properties Distinct from Sp1 and Sp3. J. Biol. Chem. 1995;270:24989–24994. doi: 10.1074/jbc.270.42.24989. [DOI] [PubMed] [Google Scholar]
- 65.Kalff-Suske M., Kunz J., Grzeschik K.H., Suske G. Human Sp4 transcription factor gene (SP4) maps to chromosome 7p15. Genomics. 1995;26:631–633. doi: 10.1016/0888-7543(95)80191-N. [DOI] [PubMed] [Google Scholar]
- 66.Kalff-Suske M., Kunz J., Grzeschik K.H., Suske G. Human Sp3 transcriptional regulator gene (SP3) maps to chromosome 2q31. Genomics. 1996;37:410–412. doi: 10.1006/geno.1996.0582. [DOI] [PubMed] [Google Scholar]
- 67.Phan D., Cheng C.-J., Galfione M., Vakar-Lopez F., Tunstead J., Thompson N.E., Burgess R.R., Najjar S.M., Yu-Lee L.-Y., Lin S.-H. Identification of Sp2 as a Transcriptional Repressor of Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 in Tumorigenesis. Cancer Res. 2004;64:3072–3078. doi: 10.1158/0008-5472.CAN-03-3730. [DOI] [PubMed] [Google Scholar]
- 68.Kim T.-H., Chiera S.L., Linder K.E., Trempus C.S., Smart R.C., Horowitz J.M. Overexpression of Transcription Factor Sp2 Inhibits Epidermal Differentiation and Increases Susceptibility to Wound- and Carcinogen-Induced Tumorigenesis. Cancer Res. 2010;70:8507–8516. doi: 10.1158/0008-5472.CAN-10-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dunham I., Kundaje A., Aldred S.F., Collins P.J., Davis C.A., Doyle F., Epstein C.B., Frietze S., Harrow J., Kaul R., et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ebert Margaret S., Sharp Phillip A. Roles for MicroRNAs in Conferring Robustness to Biological Processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leung A.K.L., Sharp P.A. MicroRNA Functions in Stress Responses. Mol. Cell. 2010;40:205–215. doi: 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loganathan T., Doss C.G.P. Non-coding RNAs in human health and disease: Potential function as biomarkers and therapeutic targets. Funct. Integr. Genom. 2023;23:33. doi: 10.1007/s10142-022-00947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Safe S. MicroRNA-Specificity Protein (Sp) Transcription Factor Interactions and Significance in Carcinogenesis. Curr. Pharmacol. Rep. 2015;1:73–78. doi: 10.1007/s40495-014-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Young M.-J., Chen Y.-C., Wang S.-A., Chang H.-P., Yang W.-B., Lee C.-C., Liu C.-Y., Tseng Y.-L., Wang Y.-C., Sun H.S., et al. Estradiol-mediated inhibition of Sp1 decreases miR-3194-5p expression to enhance CD44 expression during lung cancer progression. J. Biomed. Sci. 2022;29:3. doi: 10.1186/s12929-022-00787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kolesnikoff N., Attema J.L., Roslan S., Bert A.G., Schwarz Q.P., Gregory P.A., Goodall G.J. Specificity Protein 1 (Sp1) Maintains Basal Epithelial Expression of the miR-200 Family: IMPLICATIONS FOR EPITHELIAL-MESENCHYMAL TRANSITION. J. Biol. Chem. 2014;289:11194–11205. doi: 10.1074/jbc.M113.529172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Z., Xiao S.-B., Xu P., Xie Q., Cao L., Wang D., Luo R., Zhong Y., Chen H.-C., Fang L.-R. miR-365, a Novel Negative Regulator of Interleukin-6 Gene Expression, Is Cooperatively Regulated by Sp1 and NF-κB. J. Biol. Chem. 2011;286:21401–21412. doi: 10.1074/jbc.M110.198630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang S., Li Y., Sun S., Cai J., Cao J. Sp1 promotes ovarian cancer cell migration through repressing miR-335 expression. Biochem. Biophys. Res. Commun. 2020;524:211–216. doi: 10.1016/j.bbrc.2020.01.063. [DOI] [PubMed] [Google Scholar]
- 78.Liu S., Wu L.-C., Pang J., Santhanam R., Schwind S., Wu Y.-Z., Hickey C.J., Yu J., Becker H., Maharry K., et al. Sp1/NFκB/HDAC/miR-29b Regulatory Network in KIT-Driven Myeloid Leukemia. Cancer Cell. 2010;17:333–347. doi: 10.1016/j.ccr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang X., Schwind S., Yu B., Santhanam R., Wang H., Hoellerbauer P., Mims A., Klisovic R., Walker A.R., Chan K.K., et al. Targeted Delivery of microRNA-29b by Transferrin-Conjugated Anionic Lipopolyplex Nanoparticles: A Novel Therapeutic Strategy in Acute Myeloid Leukemia. Clin. Cancer Res. 2013;19:2355–2367. doi: 10.1158/1078-0432.CCR-12-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amodio N., Di Martino M.T., Foresta U., Leone E., Lionetti M., Leotta M., Gullà A.M., Pitari M.R., Conforti F., Rossi M., et al. miR-29b sensitizes multiple myeloma cells to bortezomib-induced apoptosis through the activation of a feedback loop with the transcription factor Sp1. Cell Death Dis. 2012;3:e436. doi: 10.1038/cddis.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fulciniti M., Amodio N., Bandi R.L., Cagnetta A., Samur M.K., Acharya C., Prabhala R., D’Aquila P., Bellizzi D., Passarino G., et al. miR-23b/SP1/c-myc forms a feed-forward loop supporting multiple myeloma cell growth. Blood Cancer J. 2016;6:e380. doi: 10.1038/bcj.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang R., Luo H., Wang S., Chen W., Chen Z., Wang H.-W., Chen Y., Yang J., Zhang X., Wu W., et al. MicroRNA-377 inhibited proliferation and invasion of human glioblastoma cells by directly targeting specificity protein 1. Neuro-Oncol. 2014;16:1510–1522. doi: 10.1093/neuonc/nou111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cai Z., Zheng F., Ding Y., Zhan Y., Gong R., Li J., Aschner M., Zhang Q., Wu S., Li H. Nrf2-regulated miR-380-3p Blocks the Translation of Sp3 Protein and Its Mediation of Paraquat-Induced Toxicity in Mouse Neuroblastoma N2a Cells. Toxicol. Sci. 2019;171:515–529. doi: 10.1093/toxsci/kfz162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jia L.-F., Huang Y.-P., Zheng Y.-F., Lyu M.-Y., Wei S.-B., Meng Z., Gan Y.-H. miR-29b suppresses proliferation, migration, and invasion of tongue squamous cell carcinoma through PTEN–AKT signaling pathway by targeting Sp1. Oral. Oncol. 2014;50:1062–1071. doi: 10.1016/j.oraloncology.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y., Li M., Zang W., Ma Y., Wang N., Li P., Wang T., Zhao G. MiR-429 up-regulation induces apoptosis and suppresses invasion by targeting Bcl-2 and SP-1 in esophageal carcinoma. Cell. Oncol. 2013;36:385–394. doi: 10.1007/s13402-013-0144-6. [DOI] [PubMed] [Google Scholar]
- 86.Wang X.X., Guo G.C., Qian X.K., Dou D.W., Zhang Z., Xu X.D., Duan X., Pei X.-H. miR-506 attenuates methylation of lncRNA MEG3 to inhibit migration and invasion of breast cancer cell lines via targeting SP1 and SP3. Cancer Cell Int. 2018;18:171. doi: 10.1186/s12935-018-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang J., Lv X., Fan L., Huang G., Zhan Y., Wang M., Lu H. MicroRNA-27b suppresses growth and invasion of NSCLC cells by targeting Sp1. Tumor Biol. 2014;35:10019–10023. doi: 10.1007/s13277-014-2294-1. [DOI] [PubMed] [Google Scholar]
- 88.Cao L., Xie B., Yang X., Liang H., Jiang X., Zhang D., Xue P., Chen D., Shao Z. MiR-324-5p Suppresses Hepatocellular Carcinoma Cell Invasion by Counteracting ECM Degradation through Post-Transcriptionally Downregulating ETS1 and SP1. PLoS ONE. 2015;10:e0133074. doi: 10.1371/journal.pone.0133074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Y., Qi X., Chen J., Wei W., Yu C., Yan H., Pu M., Li Y., Miao L., Li C., et al. The miR-491-3p/Sp3/ABCB1 axis attenuates multidrug resistance of hepatocellular carcinoma. Cancer Lett. 2017;408:102–111. doi: 10.1016/j.canlet.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 90.Tang H., Deng M., Tang Y., Xie X., Guo J., Kong Y., Ye F., Su Q., Xie X. miR-200b and miR-200c as Prognostic Factors and Mediators of Gastric Cancer Cell Progression. Clin. Cancer Res. 2013;19:5602–5612. doi: 10.1158/1078-0432.CCR-13-1326. [DOI] [PubMed] [Google Scholar]
- 91.Guo M.-M., Hu L.-H., Wang Y.-Q., Chen P., Huang J.-G., Lu N., He J.-H., Liao C.-G. miR-22 is down-regulated in gastric cancer, and its overexpression inhibits cell migration and invasion via targeting transcription factor Sp1. Med. Oncol. 2013;30:542. doi: 10.1007/s12032-013-0542-7. [DOI] [PubMed] [Google Scholar]
- 92.Hu J., Shan Z., Hu K., Ren F., Zhang W., Han M., Li Y., Feng K., Lei L., Feng Y. miRNA-223 inhibits epithelial-mesenchymal transition in gastric carcinoma cells via Sp1. Int. J. Oncol. 2016;49:325–335. doi: 10.3892/ijo.2016.3533. [DOI] [PubMed] [Google Scholar]
- 93.Zhao L.Y., Yao Y., Han J., Yang J., Wang X.F., Tong D.D., Song T.S., Huang C., Shao Y. miR-638 Suppresses Cell Proliferation in Gastric Cancer by Targeting Sp2. Dig. Dis. Sci. 2014;59:1743–1753. doi: 10.1007/s10620-014-3087-5. [DOI] [PubMed] [Google Scholar]
- 94.Qiu T., Zhou X., Wang J., Du Y., Xu J., Huang Z., Zhu W., Shu Y., Liu P. MiR-145, miR-133a and miR-133b inhibit proliferation, migration, invasion and cell cycle progression via targeting transcription factor Sp1 in gastric cancer. FEBS Lett. 2014;588:1168–1177. doi: 10.1016/j.febslet.2014.02.054. [DOI] [PubMed] [Google Scholar]
- 95.Xu Y., Zhao F., Wang Z., Song Y., Luo Y., Zhang X., Jiang L., Sun Z., Miao Z., Xu H. MicroRNA-335 acts as a metastasis suppressor in gastric cancer by targeting Bcl-w and specificity protein 1. Oncogene. 2012;31:1398–1407. doi: 10.1038/onc.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu W., Lou W., Mei L. A key regulatory loop AK4P1/miR-375/SP1 in pancreatic adenocarcinoma. Epigenetics. 2022;18:2148433. doi: 10.1080/15592294.2022.2148433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cui F., Wang S., Lao I., Zhou C., Kong H., Bayaxi N., Li J., Chen Q., Zhu T., Zhu H. miR-375 inhibits the invasion and metastasis of colorectal cancer via targeting SP1 and regulating EMT-associated genes. Oncol. Rep. 2016;36:487–493. doi: 10.3892/or.2016.4834. [DOI] [PubMed] [Google Scholar]
- 98.Xu X., Chen X., Xu M., Liu X., Pan B., Qin J., Xu T., Zeng K., Pan Y., He B., et al. miR-375-3p suppresses tumorigenesis and partially reverses chemoresistance by targeting YAP1 and SP1 in colorectal cancer cells. Aging. 2019;11:7357–7385. doi: 10.18632/aging.102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li J., Peng W., Yang P., Chen R., Gu Q., Qian W., Ji D., Wang Q., Zhang Z., Tang J., et al. MicroRNA-1224-5p Inhibits Metastasis and Epithelial-Mesenchymal Transition in Colorectal Cancer by Targeting SP1-Mediated NF-κB Signaling Pathways. Front. Oncol. 2020;10:294. doi: 10.3389/fonc.2020.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ren Y., Zhang H., Jiang P. MicroRNA-382 inhibits cell growth and migration in colorectal cancer by targeting SP1. Biol. Res. 2018;51:51. doi: 10.1186/s40659-018-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu D., Niu X., Pan H., Zhou Y., Zhang Z., Qu P., Zhou J. Tumor-suppressing effects of microRNA-429 in human renal cell carcinoma via the downregulation of Sp1. Oncol. Lett. 2016;12:2906–2911. doi: 10.3892/ol.2016.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng X., Xu Z., Gu J., Huang H., Gao G., Zhang X., Li J., Jin H., Jiang G., Sun H., et al. Induction of miR-137 by Isorhapontigenin (ISO) Directly Targets Sp1 Protein Translation and Mediates Its Anticancer Activity Both In Vitro and In Vivo. Mol. Cancer Ther. 2016;15:512–522. doi: 10.1158/1535-7163.MCT-15-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang F., Li Y., Zhou J., Xu J., Peng C., Ye F., Shen Y., Lu W., Wan X., Xie X. miR-375 Is Down-Regulated in Squamous Cervical Cancer and Inhibits Cell Migration and Invasion via Targeting Transcription Factor SP1. Am. J. Pathol. 2011;179:2580–2588. doi: 10.1016/j.ajpath.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guennewig B., Roos M., Dogar A.M., Gebert L.F., Zagalak J.A., Vongrad V., Metzner K.J., Hall J. Synthetic pre-microRNAs reveal dual-strand activity of miR-34a on TNF-α. Rna. 2014;20:61–75. doi: 10.1261/rna.038968.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mao Y., Chen H., Lin Y., Xu X., Hu Z., Zhu Y., Wu J., Xu X., Zheng X., Xie L. microRNA-330 inhibits cell motility by downregulating Sp1 in prostate cancer cells. Oncol. Rep. 2013;30:327–333. doi: 10.3892/or.2013.2452. [DOI] [PubMed] [Google Scholar]
- 106.Bhat S.A., Ahmad S.M., Mumtaz P.T., Malik A.A., Dar M.A., Urwat U., Shah R.A., Ganai N.A. Long non-coding RNAs: Mechanism of action and functional utility. Non-Coding RNA Res. 2016;1:43–50. doi: 10.1016/j.ncrna.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bhan A., Soleimani M., Mandal S.S. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mattick J.S., Amaral P.P., Carninci P., Carpenter S., Chang H.Y., Chen L.-L., Chen R., Dean C., Dinger M.E., Fitzgerald K.A., et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023:1–17. doi: 10.1038/s41580-022-00566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yao R.-W., Wang Y., Chen L.-L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019;21:542–551. doi: 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]
- 110.Wang Kevin C., Chang Howard Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu X., Wan Q., Wang J., Hou P., Zhang Q., Wang Q., Lu X. Epigenetic Activation of lncRNA MIR155HG Mediated by Promoter Hypomethylation and SP1 is Correlated with Immune Infiltration in Glioma. Onco Targets Ther. 2022;15:219–235. doi: 10.2147/OTT.S349078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu K., Ni J.-D., Li W.-Z., Pan B.-Q., Yang Y.-T., Xia Q., Huang J. The Sp1/FOXC1/HOTTIP/LATS2/YAP/β-catenin cascade promotes malignant and metastatic progression of osteosarcoma. Mol. Oncol. 2020;14:2678–2695. doi: 10.1002/1878-0261.12760. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Gao Y., Luo X., Zhang J. Sp1-mediated up-regulation of lnc00152 promotes invasion and metastasis of retinoblastoma cells via the miR-30d/SOX9/ZEB2 pathway. Cell. Oncol. 2021;44:61–76. doi: 10.1007/s13402-020-00522-8. [DOI] [PubMed] [Google Scholar]
- 114.Xu Tp Liu Xx Xia R., Yin L., Kong R., Chen W.-M., Huang M.-D., Shu Y.-Q. SP1-induced upregulation of the long noncoding RNA TINCR regulates cell proliferation and apoptosis by affecting KLF2 mRNA stability in gastric cancer. Oncogene. 2015;34:5648–5661. doi: 10.1038/onc.2015.18. [DOI] [PubMed] [Google Scholar]
- 115.Guo L., Fang L., Liu Y. SP1-regulated LINC01638 promotes proliferation and inhibits apoptosis in non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2019;23:8913–8920. doi: 10.26355/eurrev_201910_19287. [DOI] [PubMed] [Google Scholar]
- 116.Liu H.-T., Zou Y.-X., Zhu W.-j., Sen L., Zhang G.-h., Ma R.-R., Guo X.-Y., Gao P. lncRNA THAP7-AS1, transcriptionally activated by SP1 and post-transcriptionally stabilized by METTL3-mediated m6A modification, exerts oncogenic properties by improving CUL4B entry into the nucleus. Cell. Death Differ. 2022;29:627–641. doi: 10.1038/s41418-021-00879-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu X., Wang J., Wang W., Lu C., Qu T., He X., Liu X., Guo R., Zhang E. Copy number amplification and SP1-activated lncRNA MELTF-AS1 regulates tumorigenesis by driving phase separation of YBX1 to activate ANXA8 in non-small cell lung cancer. Oncogene. 2022;41:3222–3238. doi: 10.1038/s41388-022-02292-z. [DOI] [PubMed] [Google Scholar]
- 118.Ren F., Ren J.H., Song C.L., Tan M., Yu H.B., Zhou Y.J., Qin Y.-P., Cheng S.-T., Zhang Y., Huang A.-L., et al. LncRNA HOTAIR modulates hepatitis B virus transcription and replication by enhancing SP1 transcription factor. Clin. Sci. 2020;134:3007–3022. doi: 10.1042/CS20200970. [DOI] [PubMed] [Google Scholar]
- 119.Zhu Q., Wang S., Shi Y. LncRNA PCAT6 activated by SP1 facilitates the progression of breast cancer by the miR-326/LRRC8E axis. Anti-Cancer Drugs. 2022;33:178–190. doi: 10.1097/CAD.0000000000001253. [DOI] [PubMed] [Google Scholar]
- 120.Xiao L., Yuan W., Huang C., Luo Q., Xiao R., Chen Z.-H. LncRNA PCAT19 induced by SP1 and acted as oncogene in gastric cancer competitively binding to miR429 and upregulating DHX9. J. Cancer. 2022;13:102–111. doi: 10.7150/jca.61961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y., Guo Y., Zhuang T., Xu T., Ji M. SP1-Induced Upregulation of lncRNA LINC00659 Promotes Tumour Progression in Gastric Cancer by Regulating miR-370/AQP3 Axis. Front. Endocrinol. 2022;13:936037. doi: 10.3389/fendo.2022.936037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang J.-F., Xi Z.-N., Su H.-J., Bao Z., Qiao Y.-H. SP1-induced overexpression of LINC00520 facilitates non-small cell lung cancer progression through miR-577/CCNE2 pathway and predicts poor prognosis. Hum. Cell. 2021;34:952–964. doi: 10.1007/s13577-021-00518-y. [DOI] [PubMed] [Google Scholar]
- 123.Huo J., Wang Y., Zhang Y., Wang W., Yang P., Zhao W., Zhang M., Cui L., Zhang D. The LncRNA MIR155HG is Upregulated by SP1 in Melanoma Cells and Drives Melanoma Progression via Modulating the MiR-485-3p/PSIP1 Axis. Anticancer. Agents Med. Chem. 2022;22:152–159. doi: 10.2174/1871520621666210322092906. [DOI] [PubMed] [Google Scholar]
- 124.Liu L.-x., Liu B., Yu J., Zhang D.-y., Shi J.-h., Liang P. SP1-induced upregulation of lncRNA CTBP1-AS2 accelerates the hepatocellular carcinoma tumorigenesis through targeting CEP55 via sponging miR-195-5p. Biochem. Biophys. Res. Commun. 2020;533:779–785. doi: 10.1016/j.bbrc.2020.09.080. [DOI] [PubMed] [Google Scholar]
- 125.Li J., Jiang X., Li Z., Huang L., Ji D., Yu L., Zhou Y., Cui Y. SP1-induced HOXD-AS1 promotes malignant progression of cholangiocarcinoma by regulating miR-520c-3p/MYCN. Aging. 2020;12:16304–16325. doi: 10.18632/aging.103660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.He J.-w., Li D.-j., Zhou J.-h., Zhu Y.-l., Yu B.-q. SP1-mediated upregulation of lncRNA LMCD1-AS1 functions a ceRNA for miR-106b-5p to facilitate osteosarcoma progression. Biochem. Biophys. Res. Commun. 2020;526:670–677. doi: 10.1016/j.bbrc.2020.03.151. [DOI] [PubMed] [Google Scholar]
- 127.Xing W., Xu W.Y., Chang L., Zhang K., Wang S.R. SP1-induced lncRNA LINC00689 overexpression contributes to osteosarcoma progression via the miR-655/SOX18 axis. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2205–2217. doi: 10.26355/eurrev_202003_20486. [DOI] [PubMed] [Google Scholar]
- 128.Wang Z.-Y., Duan Y., Wang P. SP1-mediated upregulation of lncRNA SNHG4 functions as a ceRNA for miR-377 to facilitate prostate cancer progression through regulation of ZIC5. J. Cell. Physiol. 2020;235:3916–3927. doi: 10.1002/jcp.29285. [DOI] [PubMed] [Google Scholar]
- 129.Cheng Y., Imanirad P., Jutooru I., Hedrick E., Jin U.-H., Rodrigues Hoffman A., Leal de Araujo J., Morpurgo B., Golovko A., Safe S. Role of metastasis-associated lung adenocarcinoma transcript-1 (MALAT-1) in pancreatic cancer. PLoS ONE. 2018;13:e0192264. doi: 10.1371/journal.pone.0192264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han T., Zhuo M., Yuan C., Xiao X., Cui J., Qin G., Wang L., Jiao F. Coordinated silencing of the Sp1-mediated long noncoding RNA MEG3 by EZH2 and HDAC3 as a prognostic factor in pancreatic ductal adenocarcinoma. Cancer Biol. Med. 2020;17:953–969. doi: 10.20892/j.issn.2095-3941.2019.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shao L., Sun W., Wang Z., Dong W., Qin Y. Long noncoding RNA SAMMSON promotes papillary thyroid carcinoma progression through p300/Sp1 axis and serves as a novel diagnostic and prognostic biomarker. IUBMB Life. 2020;72:237–246. doi: 10.1002/iub.2158. [DOI] [PubMed] [Google Scholar]
- 132.Li S., Ma F., Jiang K., Shan H., Shi M., Chen B. Long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 promotes lung adenocarcinoma by directly interacting with specificity protein 1. Cancer Sci. 2018;109:1346–1356. doi: 10.1111/cas.13587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu J., Tang X., Shi Y., Ma C., Zhang H., Zhang J., Lu Y., Wei J., Li L., Han L. Crosstalk of LncRNA HOTAIR and SP1-mediated repression of PDK1 contributes to β-Elemene-inhibited proliferation of hepatocellular carcinoma cells. J. Ethnopharmacol. 2022;283:114456. doi: 10.1016/j.jep.2021.114456. [DOI] [PubMed] [Google Scholar]
- 134.Wu J., Tang Q., Ren X., Zheng F., He C., Chai X., Li L., Hann S.S. Reciprocal interaction of HOTAIR and SP1 together enhance the ability of Xiaoji decoction and gefitinib to inhibit EP4 expression. J. Ethnopharmacol. 2019;237:128–140. doi: 10.1016/j.jep.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 135.Chen K.Y., Zhu S.G., He J.W., Duan X.P. LncRNA CRNDE is involved in radiation resistance in hepatocellular carcinoma via modulating the SP1/PDK1 axis. Neoplasma. 2022;69:918–930. doi: 10.4149/neo_2022_211230N1853. [DOI] [PubMed] [Google Scholar]
- 136.Hao Y., Li X., Chen H., Huo H., Liu Z., Chai E. Over-expression of long noncoding RNA HOTAIRM1 promotes cell proliferation and invasion in human glioblastoma by up-regulating SP1 via sponging miR-137. NeuroReport. 2020;31:109–117. doi: 10.1097/WNR.0000000000001380. [DOI] [PubMed] [Google Scholar]
- 137.Liu W., Meng J., Su R., Shen C., Zhang S., Zhao Y., Liu W., Du J., Zhu S., Li P., et al. SP1-mediated up-regulation of lncRNA TUG1 underlines an oncogenic property in colorectal cancer. Cell Death Dis. 2022;13:433. doi: 10.1038/s41419-022-04805-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chang S., Sun L., Feng G. SP1-mediated long noncoding RNA POU3F3 accelerates the cervical cancer through miR-127-5p/FOXD1. Biomed. Pharm. 2019;117:109133. doi: 10.1016/j.biopha.2019.109133. [DOI] [PubMed] [Google Scholar]
- 139.Li C., Liu H., Yang J., Yang J., Yang L., Wang Y., Yan Z., Sun Y., Sun X., Jiao B. Long noncoding RNA LINC00511 induced by SP1 accelerates the glioma progression through targeting miR-124-3p/CCND2 axis. J. Cell. Mol. Med. 2019;23:4386–4394. doi: 10.1111/jcmm.14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yu S., Wang D., Shao Y., Zhang T., Xie H., Jiang X., Deng Q., Jiao Y., Yang J., Cai C., et al. SP1-induced lncRNA TINCR overexpression contributes to colorectal cancer progression by sponging miR-7-5p. Aging. 2019;11:1389–1403. doi: 10.18632/aging.101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Y., Mou C., Shang M., Jiang M., Xu C. Long noncoding RNA RP11-626G11.3 promotes the progression of glioma through miR-375-SP1 axis. Mol. Carcinog. 2020;59:492–502. doi: 10.1002/mc.23173. [DOI] [PubMed] [Google Scholar]
- 142.Dandan W., Jianliang C., Haiyan H., Hang M., Xuedong L. Long noncoding RNA MIR31HG is activated by SP1 and promotes cell migration and invasion by sponging miR-214 in NSCLC. Gene. 2019;692:223–230. doi: 10.1016/j.gene.2018.12.077. [DOI] [PubMed] [Google Scholar]
- 143.Guan N., Zheng H., Wu X., Xie L., Tong X. SP1-Regulated Non-Coding RNA SNHG22 Promotes Ovarian Cancer Growth and Glycolysis. Cancer Manag. Res. 2021;13:7299–7309. doi: 10.2147/CMAR.S318378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li J., Li S., Chen Z., Wang J., Chen Y., Xu Z., Jin M., Yu W. miR-326 reverses chemoresistance in human lung adenocarcinoma cells by targeting specificity protein 1. Tumor Biol. 2016;37:13287–13294. doi: 10.1007/s13277-016-5244-2. [DOI] [PubMed] [Google Scholar]
- 145.Yang C., Han S. The circular RNA circ0005654 interacts with specificity protein 1 via microRNA-363 sequestration to promote gastric cancer progression. Bioengineered. 2021;12:6305–6317. doi: 10.1080/21655979.2021.1971031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu X., Zhang B., Jia Y., Fu M. SNHG17 enhances the malignant characteristics of tongue squamous cell carcinoma by acting as a competing endogenous RNA on microRNA-876 and thereby increasing specificity protein 1 expression. Cell Cycle. 2020;19:711–725. doi: 10.1080/15384101.2020.1727399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang X., Yao J., Shi H., Gao B., Zhou H., Zhang Y., Zhao D., Gao S., Wang C., Zhang L. Hsa_circ_0026628 promotes the development of colorectal cancer by targeting SP1 to activate the Wnt/β-catenin pathway. Cell Death Dis. 2021;12:802. doi: 10.1038/s41419-021-03794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou J., Xu N., Liu B., Wang C., He Z., Lenahan C., Tang W., Zeng H., Guo H. lncRNA XLOC013218 promotes cell proliferation and TMZ resistance by targeting the PIK3R2-mediated PI3K/AKT pathway in glioma. Cancer Sci. 2022;113:2681–2692. doi: 10.1111/cas.15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meng N., Chen M., Chen D., Chen X.H., Wang J.Z., Zhu S., He Y.-T., Zhang X.-L., Lu R.-X., Yan G.-R. Small Protein Hidden in lncRNA LOC90024 Promotes “Cancerous” RNA Splicing and Tumorigenesis. Adv. Sci. 2020;7:1903233. doi: 10.1002/advs.201903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Safe S., Kasiappan R. Natural Products as Mechanism-based Anticancer Agents: Sp Transcription Factors as Targets. Phytother. Res. 2016;30:1723–1732. doi: 10.1002/ptr.5669. [DOI] [PubMed] [Google Scholar]
- 151.Jutooru I., Guthrie A.S., Chadalapaka G., Pathi S., Kim K., Burghardt R., Jin U.-H., Safe S. Mechanism of Action of Phenethylisothiocyanate and Other Reactive Oxygen Species-Inducing Anticancer Agents. Mol. Cell. Biol. 2014;34:2382–2395. doi: 10.1128/MCB.01602-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kasiappan R., Jutooru I., Karki K., Hedrick E., Safe S. Benzyl Isothiocyanate (BITC) Induces Reactive Oxygen Species-dependent Repression of STAT3 Protein by Down-regulation of Specificity Proteins in Pancreatic Cancer. J. Biol. Chem. 2016;291:27122–27133. doi: 10.1074/jbc.M116.746339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chadalapaka G., Jutooru I., Safe S. Celastrol decreases specificity proteins (Sp) and fibroblast growth factor receptor-3 (FGFR3) in bladder cancer cells. Carcinogenesis. 2012;33:886–894. doi: 10.1093/carcin/bgs102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gandhy S.U., Kim K., Larsen L., Rosengren R.J., Safe S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer. 2012;12:564. doi: 10.1186/1471-2407-12-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chintharlapalli S., Papineni S., Ramaiah S.K., Safe S. Betulinic Acid Inhibits Prostate Cancer Growth through Inhibition of Specificity Protein Transcription Factors. Cancer Res. 2007;67:2816–2823. doi: 10.1158/0008-5472.CAN-06-3735. [DOI] [PubMed] [Google Scholar]
- 156.Jutooru I., Chadalapaka G., Sreevalsan S., Lei P., Barhoumi R., Burghardt R., Safe S. Arsenic trioxide downregulates specificity protein (Sp) transcription factors and inhibits bladder cancer cell and tumor growth. Exp. Cell Res. 2010;316:2174–2188. doi: 10.1016/j.yexcr.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jutooru I., Chadalapaka G., Abdelrahim M., Basha M.R., Samudio I., Konopleva M., Andreeff M., Safe S. Methyl 2-Cyano-3,12-dioxooleana-1,9-dien-28-oate Decreases Specificity Protein Transcription Factors and Inhibits Pancreatic Tumor Growth: Role of MicroRNA-27a. Mol. Pharmacol. 2010;78:226–236. doi: 10.1124/mol.110.064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jutooru I., Chadalapaka G., Lei P., Safe S. Inhibition of NFκB and Pancreatic Cancer Cell and Tumor Growth by Curcumin Is Dependent on Specificity Protein Down-regulation. J. Biol. Chem. 2010;285:25332–25344. doi: 10.1074/jbc.M109.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pathi S.S., Lei P., Sreevalsan S., Chadalapaka G., Jutooru I., Safe S. Pharmacologic doses of ascorbic acid repress specificity protein (Sp) transcription factors and Sp-regulated genes in colon cancer cells. Nutr. Cancer. 2011;63:1133–1142. doi: 10.1080/01635581.2011.605984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pathi S.S., Jutooru I., Chadalapaka G., Sreevalsan S., Anand S., Thatcher G.R., Safe S. GT-094, a NO-NSAID, Inhibits Colon Cancer Cell Growth by Activation of a Reactive Oxygen Species-MicroRNA-27a: ZBTB10-Specificity Protein Pathway. Mol. Cancer Res. 2011;9:195–202. doi: 10.1158/1541-7786.MCR-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hedrick E., Li X., Safe S. Penfluridol Represses Integrin Expression in Breast Cancer through Induction of Reactive Oxygen Species and Downregulation of Sp Transcription Factors. Mol. Cancer Ther. 2017;16:205–216. doi: 10.1158/1535-7163.MCT-16-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Karki K., Hedrick E., Kasiappan R., Jin U.-H., Safe S. Piperlongumine Induces Reactive Oxygen Species (ROS)-Dependent Downregulation of Specificity Protein Transcription Factors. Cancer Prev. Res. 2017;10:467–477. doi: 10.1158/1940-6207.CAPR-17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Taoka R., Jinesh G.G., Xue W., Safe S., Kamat A.M. CF3DODA-Me induces apoptosis, degrades Sp1, and blocks the transformation phase of the blebbishield emergency program. Apoptosis. 2017;22:719–729. doi: 10.1007/s10495-017-1359-1. [DOI] [PubMed] [Google Scholar]
- 164.Kasiappan R., Jutooru I., Mohankumar K., Karki K., Lacey A., Safe S. Reactive Oxygen Species (ROS)-Inducing Triterpenoid Inhibits Rhabdomyosarcoma Cell and Tumor Growth through Targeting Sp Transcription Factors. Mol. Cancer Res. 2019;17:794–805. doi: 10.1158/1541-7786.MCR-18-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lo W.-L., Hsu T.-I., Yang W.-B., Kao T.-J., Wu M.-H., Huang Y.-N., Yeh S.-H., Chuang J.-Y. Betulinic Acid-Mediated Tuning of PERK/CHOP Signaling by Sp1 Inhibition as a Novel Therapeutic Strategy for Glioblastoma. Cancers. 2020;12:981. doi: 10.3390/cancers12040981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hedrick E., Crose L., Linardic C.M., Safe S. Histone Deacetylase Inhibitors Inhibit Rhabdomyosarcoma by Reactive Oxygen Species–Dependent Targeting of Specificity Protein Transcription Factors. Mol. Cancer Ther. 2015;14:2143–2153. doi: 10.1158/1535-7163.MCT-15-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li W., Liu M., Xu Y.-F., Feng Y., Che J.-P., Wang G.-C., Zheng J.-H. Combination of quercetin and hyperoside has anticancer effects on renal cancer cells through inhibition of oncogenic microRNA-27a. Oncol. Rep. 2014;31:117–124. doi: 10.3892/or.2013.2811. [DOI] [PubMed] [Google Scholar]
- 168.Long C., Wang J., Guo W., Wang H., Wang C., Liu Y., Sun X. Triptolide inhibits transcription of hTERT through down-regulation of transcription factor specificity protein 1 in primary effusion lymphoma cells. Biochem. Biophys. Res. Commun. 2016;469:87–93. doi: 10.1016/j.bbrc.2015.11.076. [DOI] [PubMed] [Google Scholar]
- 169.Banerjee S., Sangwan V., McGinn O., Chugh R., Dudeja V., Vickers S.M., Saluja A.K. Triptolide-induced Cell Death in Pancreatic Cancer Is Mediated by O-GlcNAc Modification of Transcription Factor Sp1. J. Biol. Chem. 2013;288:33927–33938. doi: 10.1074/jbc.M113.500983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ma W., Liu X., Du W. Baicalin induces apoptosis in SW480 cells through downregulation of the SP1 transcription factor. Anti-Cancer Drugs. 2019;30:153–158. doi: 10.1097/CAD.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Arora N., Alsaied O., Dauer P., Majumder K., Modi S., Giri B., Dudeja V., Banerjee S., Von Hoff D., Saluja A. Downregulation of Sp1 by Minnelide leads to decrease in HSP70 and decrease in tumor burden of gastric cancer. PLoS ONE. 2017;12:e0171827. doi: 10.1371/journal.pone.0171827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang G., Yin X., Ma D., Su Z. Anticancer activity of Phloretin against the human oral cancer cells is due to G0/G1 cell cycle arrest and ROS mediated cell death. J. Buon. 2020;25:344–349. [PubMed] [Google Scholar]
- 173.Cho J.J., Chae J.-I., Yoon G., Kim K.H., Cho J.H., Cho S.-S., Cho Y.S., Shim J.-H. Licochalcone A, a natural chalconoid isolated from Glycyrrhiza inflata root, induces apoptosis via Sp1 and Sp1 regulatory proteins in oral squamous cell carcinoma. Int. J. Oncol. 2014;45:667–674. doi: 10.3892/ijo.2014.2461. [DOI] [PubMed] [Google Scholar]
- 174.Kim D.-W., Ko S.M., Jeon Y.-J., Noh Y.-W., Choi N.-J., Cho S.-D., Moon H.S., Cho Y.S., Shin J.-C., Park S.-M., et al. Anti-proliferative effect of honokiol in oral squamous cancer through the regulation of specificity protein 1. Int. J. Oncol. 2013;43:1103–1110. doi: 10.3892/ijo.2013.2028. [DOI] [PubMed] [Google Scholar]
- 175.Cho J.H., Lee R.H., Jeon Y.-J., Shin J.-C., Park S.-M., Choi N.-J., Seo K.S., Yoon G., Cho S.-S., Kim K.H., et al. Role of transcription factor Sp1 in the 4-O-methylhonokiol-mediated apoptotic effect on oral squamous cancer cells and xenograft. Int. J. Biochem. Cell Biol. 2015;64:287–297. doi: 10.1016/j.biocel.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 176.Lee R.H., Shin J.-C., Kim K.-H., Choi Y.H., Chae J.-I., Shim J.-H. Apoptotic effects of 7,8-dihydroxyflavone in human oral squamous cancer cells through suppression of Sp1. Oncol. Rep. 2015;33:631–638. doi: 10.3892/or.2014.3632. [DOI] [PubMed] [Google Scholar]
- 177.Lee R.H., Cho J.H., Jeon Y.-J., Bang W., Cho J.-J., Choi N.-J., Seo K.S., Shim J.-H., Chae J.-I. Quercetin Induces Antiproliferative Activity Against Human Hepatocellular Carcinoma (HepG2) Cells by Suppressing Specificity Protein 1 (Sp1) Drug Dev. Res. 2015;76:9–16. doi: 10.1002/ddr.21235. [DOI] [PubMed] [Google Scholar]
- 178.Cho J.H., Shin J.-C., Cho J.-J., Choi Y.H., Shim J.-H., Chae J.-I. Esculetin (6,7-dihydroxycoumarin): A potential cancer chemopreventive agent through suppression of Sp1 in oral squamous cancer cells. Int. J. Oncol. 2015;46:265–271. doi: 10.3892/ijo.2014.2700. [DOI] [PubMed] [Google Scholar]
- 179.Karki K., Harishchandra S., Safe S. Bortezomib Targets Sp Transcription Factors in Cancer Cells. Mol. Pharmacol. 2018;94:1187–1196. doi: 10.1124/mol.118.112797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kang D., Zuo W., Wu Q., Zhu Q., Liu P. Inhibition of Specificity Protein 1 Is Involved in Phloretin-Induced Suppression of Prostate Cancer. BioMed Res. Int. 2020;2020:1358674. doi: 10.1155/2020/1358674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Slika H., Mansour H., Wehbe N., Nasser S.A., Iratni R., Nasrallah G., Shaito A., Ghaddar T., Kobeissy F., Eid A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharm. 2022;146:112442. doi: 10.1016/j.biopha.2021.112442. [DOI] [PubMed] [Google Scholar]
- 182.Biswas P., Dey D., Biswas P.K., Rahaman T.I., Saha S., Parvez A., Khan D.A., Lily N.J., Saha K., Sohel M., et al. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int. J. Mol. Sci. 2022;23:11746. doi: 10.3390/ijms231911746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu X., Chen S., Huang K., Lin G. Triptolide promotes ferroptosis by suppressing Nrf2 to overcome leukemia cell resistance to doxorubicin. Mol. Med. Rep. 2023;27:17. doi: 10.3892/mmr.2022.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hong Y.S., Hong S.-W., Kim S.-M., Jin D.-H., Shin J.-S., Yoon D.H., Kim K.-P., Lee J.-L., Heo D.S., Lee J.S., et al. Bortezomib induces G2-M arrest in human colon cancer cells through ROS-inducible phosphorylation of ATM-CHK1. Int. J. Oncol. 2012;41:76–82. doi: 10.3892/ijo.2012.1448. [DOI] [PubMed] [Google Scholar]
- 185.Huang K., Chen Y., Zhang R., Wu Y., Ma Y., Fang X., Shen S. Honokiol induces apoptosis and autophagy via the ROS/ERK1/2 signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2018;9:157. doi: 10.1038/s41419-017-0166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li S., Chen J., Fan Y., Wang C., Wang C., Zheng X., Chen F., Li W. Liposomal Honokiol induces ROS-mediated apoptosis via regulation of ERK/p38-MAPK signaling and autophagic inhibition in human medulloblastoma. Signal Transduct. Target. Ther. 2022;7:49. doi: 10.1038/s41392-021-00869-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.O’Hagan H.M., Wang W., Sen S., DeStefano Shields C., Lee S.S., Zhang Y.W., Clements E.G., Cai Y., Van Neste L., Easwaran H., et al. Oxidative Damage Targets Complexes Containing DNA Methyltransferases, SIRT1, and Polycomb Members to Promoter CpG Islands. Cancer Cell. 2011;20:606–619. doi: 10.1016/j.ccr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Scott G.K., Mattie M.D., Berger C.E., Benz S.C., Benz C.C. Rapid Alteration of MicroRNA Levels by Histone Deacetylase Inhibition. Cancer Res. 2006;66:1277–1281. doi: 10.1158/0008-5472.CAN-05-3632. [DOI] [PubMed] [Google Scholar]
- 189.Kim K., Chadalapaka G., Lee S.O., Yamada D., Sastre-Garau X., Defossez P.A., Park Y.Y., Lee J.S., Safe S. Identification of oncogenic microRNA-17-92/ZBTB4/specificity protein axis in breast cancer. Oncogene. 2012;31:1034–1044. doi: 10.1038/onc.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sreevalsan S., Safe S. The Cannabinoid WIN 55,212-2 Decreases Specificity Protein Transcription Factors and the Oncogenic Cap Protein eIF4E in Colon Cancer Cells. Mol. Cancer Ther. 2013;12:2483–2493. doi: 10.1158/1535-7163.MCT-13-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu X., Jutooru I., Lei P., Kim K., Lee S.-o., Brents L.K., Prather P.L., Safe S. Betulinic Acid Targets YY1 and ErbB2 through Cannabinoid Receptor-Dependent Disruption of MicroRNA-27a:ZBTB10 in Breast Cancer. Mol. Cancer Ther. 2012;11:1421–1431. doi: 10.1158/1535-7163.MCT-12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nair V., Sreevalsan S., Basha R., Abdelrahim M., Abudayyeh A., Rodrigues Hoffman A., Safe S. Mechanism of Metformin-dependent Inhibition of Mammalian Target of Rapamycin (mTOR) and Ras Activity in Pancreatic Cancer: Role of specificity protein (Sp) Transcription Factors. J. Biol. Chem. 2014;289:27692–27701. doi: 10.1074/jbc.M114.592576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen C.-C., Hardy D.B., Mendelson C.R. Progesterone Receptor Inhibits Proliferation of Human Breast Cancer Cells via Induction of MAPK Phosphatase 1 (MKP-1/DUSP1) J. Biol. Chem. 2011;286:43091–43102. doi: 10.1074/jbc.M111.295865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huang P.-H., Wang D., Chuang H.-C., Wei S., Kulp S.K., Chen C.-S. α-Tocopheryl succinate and derivatives mediate the transcriptional repression of androgen receptor in prostate cancer cells by targeting the PP2A-JNK-Sp1-signaling axis. Carcinogenesis. 2009;30:1125–1131. doi: 10.1093/carcin/bgp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chu S., Ferro T.J. Identification of a hydrogen peroxide-induced PP1-JNK1-Sp1 signaling pathway for gene regulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L983–L992. doi: 10.1152/ajplung.00454.2005. [DOI] [PubMed] [Google Scholar]
- 196.Basha R., Baker C.H., Sankpal U.T., Ahmad S., Safe S., Abbruzzese J.L., Abdelrahim M. Therapeutic applications of NSAIDS in cancer: Special emphasis on tolfenamic acid. FBS. 2011;3:797–805. doi: 10.2741/s188. [DOI] [PubMed] [Google Scholar]
- 197.Wei D., Wang L., He Y., Xiong H.Q., Abbruzzese J.L., Xie K. Celecoxib Inhibits Vascular Endothelial Growth Factor Expression in and Reduces Angiogenesis and Metastasis of Human Pancreatic Cancer via Suppression of Sp1 Transcription Factor Activity. Cancer Res. 2004;64:2030–2038. doi: 10.1158/0008-5472.CAN-03-1945. [DOI] [PubMed] [Google Scholar]
- 198.Abdelrahim M., Baker C.H., Abbruzzese J.L., Safe S. Tolfenamic Acid and Pancreatic Cancer Growth, Angiogenesis, and Sp Protein Degradation. JNCI J. Natl. Cancer Inst. 2006;98:855–868. doi: 10.1093/jnci/djj232. [DOI] [PubMed] [Google Scholar]
- 199.Chintharlapalli S., Papineni S., Lei P., Pathi S., Safe S. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors. BMC Cancer. 2011;11:371. doi: 10.1186/1471-2407-11-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang Y.-T., Yang W.-B., Chang W.-C., Hung J.-J. Interplay of Posttranslational Modifications in Sp1 Mediates Sp1 Stability during Cell Cycle Progression. J. Mol. Biol. 2011;414:1–14. doi: 10.1016/j.jmb.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 201.Hsu T.-I., Wang M.-C., Chen S.-Y., Huang S.-T., Yeh Y.-M., Su W.-C., Chang W.-C., Hung J.-J. Betulinic Acid Decreases Specificity Protein 1 (Sp1) Level via Increasing the Sumoylation of Sp1 to Inhibit Lung Cancer Growth. Mol. Pharmacol. 2012;82:1115–1128. doi: 10.1124/mol.112.078485. [DOI] [PubMed] [Google Scholar]
- 202.Wang Y.-T., Chuang J.-Y., Shen M.-R., Yang W.-B., Chang W.-C., Hung J.-J. Sumoylation of Specificity Protein 1 Augments Its Degradation by Changing the Localization and Increasing the Specificity Protein 1 Proteolytic Process. J. Mol. Biol. 2008;380:869–885. doi: 10.1016/j.jmb.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 203.Spengler M.L., Kennett S.B., Moorefield K.S., Simmons S.O., Brattain M.G., Horowitz J.M. Sumoylation of internally initiated Sp3 isoforms regulates transcriptional repression via a Trichostatin A-insensitive mechanism. Cell. Signal. 2005;17:153–166. doi: 10.1016/j.cellsig.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 204.Sapetschnig A., Rischitor G., Braun H., Doll A., Schergaut M., Melchior F., Suske G. Transcription factor Sp3 is silenced through SUMO modification by PIAS1. EMBO J. 2002;21:5206–5215. doi: 10.1093/emboj/cdf510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wei S., Chuang H.-C., Tsai W.-C., Yang H.-C., Ho S.-R., Paterson A.J., Kulp S.K., Chen C.-S. Thiazolidinediones Mimic Glucose Starvation in Facilitating Sp1 Degradation through the Up-Regulation of β-Transducin Repeat-Containing Protein. Mol. Pharmacol. 2009;76:47–57. doi: 10.1124/mol.109.055376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pathi S., Jutooru I., Chadalapaka G., Nair V., Lee S.-O., Safe S. Aspirin Inhibits Colon Cancer Cell and Tumor Growth and Downregulates Specificity Protein (Sp) Transcription Factors. PLoS ONE. 2012;7:e48208. doi: 10.1371/journal.pone.0048208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chimienti F., Seve M., Richard S., Mathieu J., Favier A. Role of cellular zinc in programmed cell death: Temporal relationship between zinc depletion, activation of caspases, and cleavage of Sp family transcription factors11Abbreviations: Chx, cycloheximide; PARP, poly(ADP-ribose) polymerase; TNFα, tumor necrosis factor alpha; and TPEN: N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine. Biochem. Pharmacol. 2001;62:51–62. doi: 10.1016/s0006-2952(01)00624-4. [DOI] [PubMed] [Google Scholar]
- 208.Pathi S., Li X., Safe S. Tolfenamic acid inhibits colon cancer cell and tumor growth and induces degradation of specificity protein (Sp) transcription factors. Mol. Carcinog. 2014;53:E53–E61. doi: 10.1002/mc.22010. [DOI] [PubMed] [Google Scholar]
- 209.Piedrafita F.J., Pfahl M. Retinoid-induced apoptosis and Sp1 cleavage occur independently of transcription and require caspase activation. Mol. Cell. Biol. 1997;17:6348–6358. doi: 10.1128/MCB.17.11.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tatsukawa H., Sano T., Fukaya Y., Ishibashi N., Watanabe M., Okuno M., Moriwaki H., Kojima S. Dual induction of caspase 3- and transglutaminase-dependent apoptosis by acyclic retinoid in hepatocellular carcinoma cells. Mol. Cancer. 2011;10:4. doi: 10.1186/1476-4598-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rickers A., Peters N., Badock V., Beyaert R., Vandenabeele P., Dörken B., Bommert K. Cleavage of transcription factor SP1 by caspases during anti-IgM-induced B-cell apoptosis. Eur. J. Biochem. 1999;261:269–274. doi: 10.1046/j.1432-1327.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 212.Torabi B., Flashner S., Beishline K., Sowash A., Donovan K., Bassett G., Azizkhan-Clifford J. Caspase cleavage of transcription factor Sp1 enhances apoptosis. Apoptosis. 2018;23:65–78. doi: 10.1007/s10495-017-1437-4. [DOI] [PubMed] [Google Scholar]
- 213.Nair V., Pathi S., Jutooru I., Sreevalsan S., Basha R., Abdelrahim M., Samudio I., Safe S. Metformin inhibits pancreatic cancer cell and tumor growth and downregulates Sp transcription factors. Carcinogenesis. 2013;34:2870–2879. doi: 10.1093/carcin/bgt231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review article summarizing published data.