Abstract
Lumazine protein from marine luminescent bacteria of Photobacterium species bind with very high affinity to the fluorescent chromophore 6,7-dimethyl-8-ribitylumazine. The light emission of bacterial luminescent systems is used as a sensitive, rapid, and safe assay for an ever-increasing number of biological systems. Plasmid pRFN4, containing the genes encoding riboflavin from the rib operon of Bacillus subtilis, was designed for the overproduction of lumazine. To construct fluorescent bacteria for use as microbial sensors, novel recombinant plasmids (pRFN4-Pp N-lumP and pRFN4-Pp luxLP N-lumP) were constructed by amplifying the DNA encoding the N-lumP gene (luxL) from P. phosphoreum and the promoter region (luxLP) present upstream of the lux operon of the gene by PCR and ligating into the pRFN4-Pp N-lumP plasmid. A new recombinant plasmid, pRFN4-Pp luxLP-N-lumP, was constructed with the expectation that the fluorescence intensity would be further increased when transformed into Escherichia coli. When this plasmid was transformed into E. coli 43R, the fluorescence intensity of transformants was 500 times greater than that of E. coli alone. As a result, the recombinant plasmid in which the gene encoding N-LumP and DNA containing the lux promoter exhibited expression that was so high as to show fluorescence in single E. coli cells. The fluorescent bacterial systems developed in the present study using lux and riboflavin genes can be utilized in the future as biosensors with high sensitivity and rapid analysis times.
Keywords: bioluminescence, fluorescence, lux, Photobacterium, riboflavin
1. Introduction
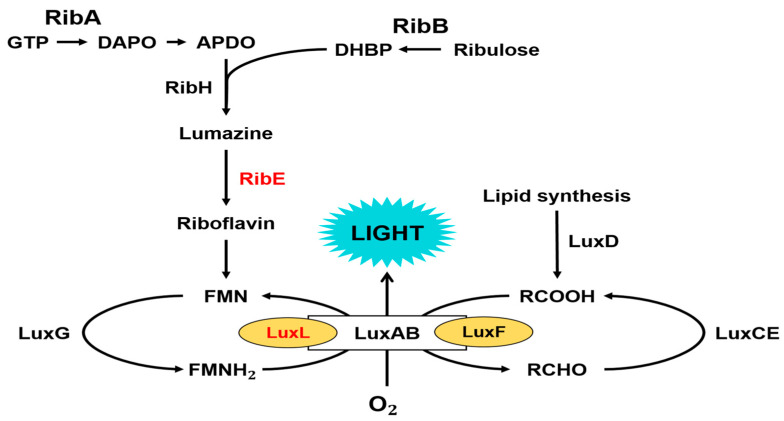
Bioluminescence is the phenomenon of light emission resulting from enzyme-catalyzed oxidation reactions in living organisms. Light emission in marine bacteria catalyzed by bacterial luciferase involves the oxidation of long-chain fatty aldehydes and FMNH2, resulting in the emission of blue–green light [1,2,3] (Figure 1).
| FMNH2 + RCHO + O2 → FMN + H2O + RCOOH + light |
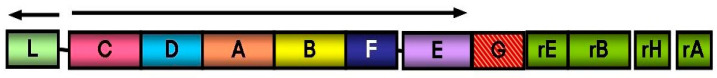
Genes encoding light-emitting enzymes and proteins exist on one operon, forming a group in bioluminescent bacteria. This lux operon consists of the luxAB gene encoding the α and β subunits of luciferase and the luxCDE genes encoding acyl-CoA reductase, acyl-transferase, and acyl-protein synthetase subunits of the fatty acid reductase complex (Figure 2) [4].
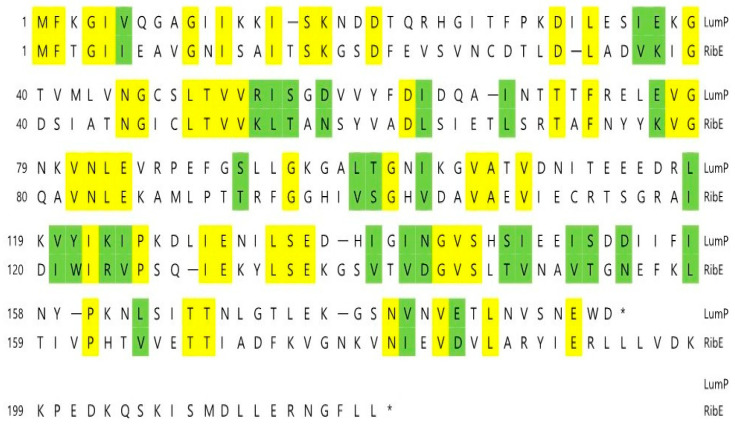
We found that various genes related to riboflavin (vitamin B2) biosynthesis exist downstream of the lux operon in Photobacterium spp. [5]. The biosynthesis of riboflavin is essential in bioluminescent bacteria because it is a precursor of FMNH2 (reduced flavin monophosphate), which is the substrate of the bacterial bioluminescence reaction, as described above. The lumazine protein identified in P. phosphoreum in 1970 [6] is a fluorescent protein that forms non-covalent bonds with 6,7-dimethyl-8-ribityllumazine (lumazine) [7,8]. The luxL gene, which encodes the lumazine protein, exists upstream of the lux operon in Photobacterium spp. (Figure 2), and the fact that the first gene (ribE) adjacent to luxG encodes riboflavin synthase draws molecular genetic interest. As the lumazine protein exhibits approximately 30% amino acid homology with riboflavin synthase [8,9] (Figure 3), it is assumed that the luxL gene was derived from a gene duplication of the riboflavin synthase gene (ribE) as a member of the riboflavin synthase superfamily [9].
Figure 1.
The complete catalytic reactions and proteins involved in light emission by Photobacterium phosphoreum. Proteins are as follows: lumazine protein (LuxL), α and β subunits of luciferase (LuxAB), fatty acid reductase complex (LuxCDE), non-fluorescent flavoprotein (LuxF), flavin reductase (LuxG), GTP cyclohydrolaseII (RibA), dihydroxy-butanone 4-phosphate synthase (RibB), lumazine synthase (RibH), and riboflavin synthase (RibE). Modified from reference [10].
Figure 2.
Gene organization of the lux operon region in bioluminescence bacteria of Photobacterium phosphoreum species. Arrows indicates the direction of transcription and “r” indicates riboflavin genes. The functions of genes are shown in Figure 1.
Figure 3.
Comparison of amino acid sequences of lumazine protein (LumP) and riboflavin synthase (RibE) from Photobacterium phosphoreum. Identical or similar amino acids are shown boxed by yellow and green, respectively. Bars and asterisks indicate amino acid gaps and stop codons, respectively.
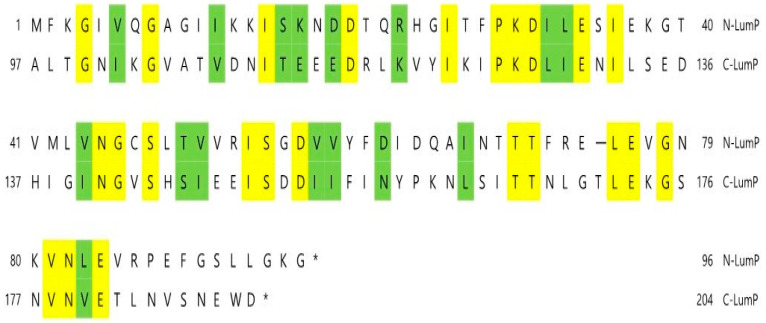
It is inferred that the lumazine protein functions as an optical transponder in light-emitting reactions of bioluminescent bacteria [9,11]. The lumazine protein shifts the wavelength and amplifies the maximum bioluminescence intensity in Photobacterium spp. by binding with lumazine, which is the fluorescent chromophore and substrate of riboflavin synthase [3,4]. The riboflavin synthase possesses the peculiar feature of intramolecular homology of the amino acid sequence between the N-terminal half domain (N-LumP) and C-terminal half domain (C-LumP) of the lumazine protein (Figure 4) [11], and it has been reported that lumazine binds only to N-LumP [12].
Figure 4.
Amino acid sequence homology between the N-terminal half of lumazine protein (N-LumP) and the C-terminal half of lumazine protein (C-LumP) from P. phosphoreum NCMB844. Identical or similar amino acids are shown boxed by yellow and green, respectively. The bar and asterisks indicate amino acid gaps and stop codons, respectively.
In this study, we employed plasmid pRFN4 [13], which contains five riboflavin biosynthesis genes of B. subtilis containing the mutated riboflavin synthase gene (ribB), for overproduction of lumazine. The nomenclature of the riboflavin biosynthetic genes in B. subtilis differs from those in other species. The ribB is the gene coding for riboflavin synthase from B. subtilis in the pRFN4 plasmid, whereas ribE is for riboflavin synthase in marine bioluminescent bacteria [5].
We inserted the gene encoding the N-terminal half domain of lumazine protein (N-LumP) into this plasmid to increase the fluorescence intensity, as lumazine binds to the lumazine protein with high affinity and functions as a chromophore (Figure 5). Moreover, we inserted intergenic DNA between luxL and the luxC promoter region (Figure 6) from P. phosphoreum to increase expression of the gene for lumazine protein (luxL). Intergenic DNA showed an extensive AT-rich region upstream of P. phosphoreum luxC. The GC content of this upstream region is less than half that of the lux-containing region, raising the possibility that the relative ease of melting DNA in AT-rich sequences may be related to the regulation of the lux system. We then transformed E. coli with a series of pRFN4 plasmids and conducted a spectroscopic study of the E. coli transformants, evaluating fluorescence intensity and examining the fluorescence of single cells using confocal microscopy.
Figure 5.
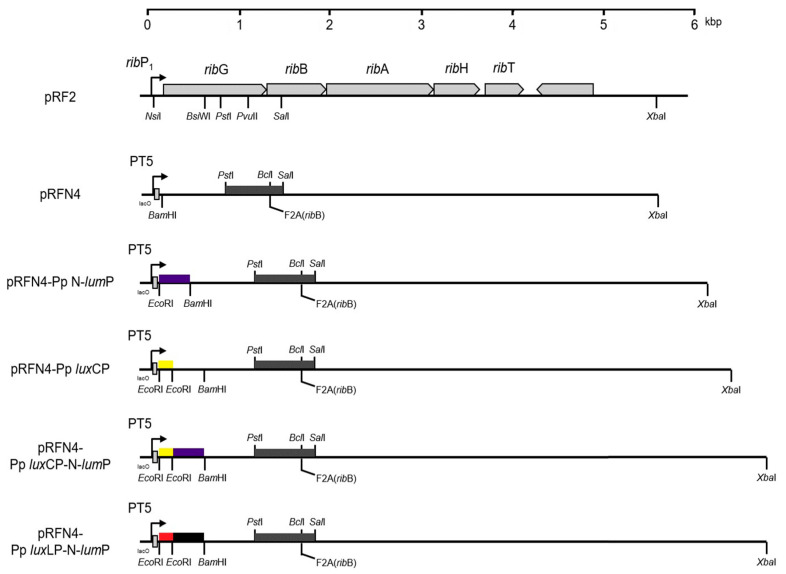
Gene map of recombinant plasmids with inserted P. phosphoreum lux genes. Recombinant pRFN4 plasmids used in this study: pRFN4, pRFN4-Pp N-lumP, pRFN4-Pp luxCP, pRFN4-Pp luxCP-N-lumP, and pRFN4-Pp luxLP-N-lumP plasmids. The nomenclature of riboflavin biosynthetic genes of B. subtilis differs from other species. Therefore, ribE in pRF2 and pRFN4 is the gene coding for riboflavin synthase in B. subtilis [5]. In pRFN4, the ribE gene coding for Phe 2 in riboflavin synthase was replaced with Ala for overproduction of lumazine (F2A in ribB) [13].
Figure 6.
Nucleotide sequences of the intergenic region between luxL (lumP) and luxC genes of P. phosphoreum. PCR primer binding sequences of the luxL and luxC promoters are shown in blue and red colors, respectively. Restriction sites of EcoRI (GAATTC) are underlined. The luxC gene is transcribed by the complementary nucleotide sequences. The nucleotide sequence (accession number L21989) was reported in the author’s previous paper [14].
2. Results
2.1. Construction of pRFN Plasmid Derivatives
It has been reported [12] that Asn 101 and Ile 102 located beyond the N-terminal region are also involved in binding of the lumazine ligand with amino acids such as Ser 48, Thr 50, and Ala 66 at the binding sites in the N-terminal half of the lumazine protein (N-LumP) (Figure 3 and Figure 4). Therefore, the gene (luxL) encoding for N-LumP extending to the amino acid Glu 115 from P. phosphoreum was synthesized by PCR and ligated into the pRFN4 vector to construct the recombinant plasmid pRFN4-Pp N-lumP (Figure 5). We conducted the PCR process with the pT7-5 PpEE plasmid containing the lux genes of P. phosphoreum NCBM 844 using primers for amplifying the DNA of the gene encoding N-LumP, as well as DNA of the promoter regions of luxC and luxL by the PCR (Figure 6). We applied EcoRI and/or BamH1 for restriction endonuclease digestion, purified the DNA product, and used T4 ligase for ligation into the pRFN4 plasmid [13], yielding plasmid pRFN4-Pp N-lumP, which contained the luxL gene coding for N-LumP.
We also performed PCR to insert luxC and luxL promoter regions (Figure 6), whose template was the pT7-5 PpEE plasmid containing the P. phosphoreum lux gene. To clone luxC and luxL promoters from P. phosphoreum, we performed the same PCR procedure as described above and constructed recombinant plasmids pRFN4-Pp luxCP and pRFN4-Pp luxCP-N-lumP. From these experiments, we generated recombinant plasmids pRFN4-Pp luxCP, pRFN4-Pp luxCP N-lumP, and pRFN4-Pp luxLP-N-lumP. The DNA inserts had sizes of approximately 9.0 kb, 9.4 kb, and 9.5 kb, respectively (Figure 5). Insertion of the correct DNA sequence was confirmed by DNA sequencing.
2.2. Fluorescence Intensities in E. coli
First, we examined the fluorescence intensity of E. coli cell extracts harboring the recombinant plasmid containing the gene encoding N-LumP from P. phosphoreum. The supernatant before sonication also exhibited fluorescence, indicating that the fluorophore was present in the liquid media. Fluorescence was monitored in the presence of lumazine with fixed excitation at 410 nm and riboflavin with excitation at 450 nm. To express the recombinant plasmids into which the lux gene and promoter DNA of P. phosphoreum were inserted, we transformed the plasmids into competent E. coli 43R cells and incubated them in LB (Luria–Bertani) medium. The incubated fluid was adjusted to a fixed volume and saturated with buffer solution A ((pH 7.2) consisting of 50 mM Tris-HCl (Tris-hydrochloride), 0.5 mM DTT (dithiothreitol), and 0.5 mM EDTA (ethylenediaminetetraacetic acid)). The supernatant of the mixture was obtained by ultrasonication and centrifugation, and the fluorescence intensity was evaluated using a fluorescence spectrophotometer.
2.2.1. Fluorescence Intensities by Lumazine
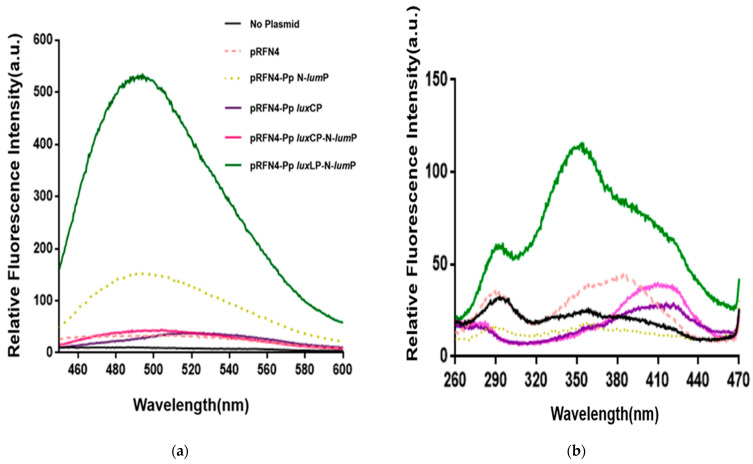
A previous study [15] showed that among different E. coli strains, E. coli 43R [16] exhibited the most significant change in luminescence intensity of >1000-fold when transformed with a plasmid containing whole genes that are necessary for light emission from P. leiognathi. Based on the results of our recent study on the generation of fluorescent bacteria [5], we expected a larger increase in fluorescence intensity in E. coli 43R than in E. coli XL-1 when transformed with pRFN4 plasmids. First, we examined the emission spectrum of the transformed E. coli 43R at a fixed excitation wavelength of 410 nm (Figure 7a). In comparing the fluorescence intensities between E. coli 43R transformed with the pRFN4-Pp N-lumP plasmid and those transformed with the pRFN4 plasmid, the intensities increased by 5-fold. The fluorescence intensity in E. coli 43R transformed with the pRFN4-Pp luxLP-N-lumP plasmid, in which DNA of luxL promoter region was inserted into pRFN4-Pp N-lumP, was the highest among all E. coli transformed with pRFN4 plasmid derivatives (Figure 7a).
Figure 7.
Fluorescence spectra of E. coli 43R transformed with recombinant pRFN4 plasmids containing P. phosphoreum lux DNA. (a) Emission spectrum in fixation at excitation wavelength of 410 nm. (b) Excitation spectrum for emission at 490 nm.
The fluorescence intensity of E. coli 43R transformed with the pRFN4-Pp luxLP-N-lumP plasmid was 500-fold greater than that of E. coli 43R transformed with the pRFN4 plasmid, which may be due to non-covalent binding between the fluorescent lumazine protein and the lumazine chromophore. However, the insertion of the luxC promoter (pRFN4-Pp luxCP) or N-lumP (pRFN-Pp N-lumP) into pRFN4 did not increase the fluorescence intensity in E. coli cells transformed with these plasmids (Figure 7a). The fluorescence intensity of E. coli 43R transformed with recombinant plasmid pRFN4-Pp luxCP-N-lumP was lower than that of E. coli 43R transformed with pRFN4-Pp N-lumP plasmid (Figure 7a). Initially, we expected that the luxC promoter of P. phosphoreum would increase the fluorescence intensity by promoting the expression of the lumazine protein gene and by increasing binding of the lumazine ligand to N-LumP. However, the results showed that insertion of the P. phosphoreum luxC promoter did not increase the fluorescence intensity, suggesting that it failed to promote the expression of lumazine protein, as otherwise expected.
We also measured the excitation spectrum (Figure 7b) at a fixed emission wavelength of 490 nm by lumazine, in contrast to the previous process (Figure 7a). In accord with the results of the emission spectrum, the increase in the fluorescence intensity of the excitation spectrum was the highest in E. coli 43R transformed with the pRFN4-Pp luxLP-N-lumP plasmid among the transformed E. coli 43R with the pRFN4 plasmid derivatives. In particular, cell extract luminescence from 43R cells transformed with recombinant pRFN4-Pp luxLP-N-lumP was 100 times higher than that of E. coli 43R. Upon binding to lumazine, N-LumP had an absorbance maximum at 418 nm [12]; however, the excitation spectrum for emission at 490 nm of E. coli 43R cell extract transformed with pRFN4-Pp luxLP-N-lumP shows a maximum peak at approximately 360 nm. The different shapes of the excitation spectra compared to those of cells transfected with other plasmids, which have a maximum peak at approximately 418 nm, reflected that N-LumP binds to riboflavin as well as lumazine.
Paulus et al. reported [17] that riboflavin, which possesses maximum absorption peaks at 450 and 370 nm, undergoes a substantial bathochromic shift (up to 20 nm) upon binding to N-LumP. The maximum absorption band at 410 nm originating from lumazine undergoes a bathochromic shift of approximately 5 nm upon binding of the protein. Moreover, the protein-bound ligand exhibited a broad, relatively weak absorbance band extending beyond 500 nm, whereas the free ligand was devoid of absorption [12].
2.2.2. Fluorescence Intensities by Riboflavin
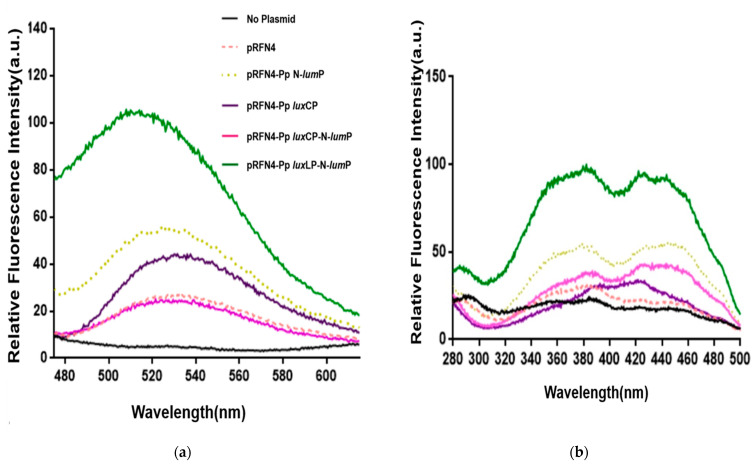
It is known that riboflavin competes with lumazine for binding to the lumazine protein [17]. Therefore, we examined the pattern and relative intensity of the emission spectra using the excitation wavelength of riboflavin at 450 nm from the cell extract transformed with pRFN4 plasmids (Figure 8a) and observed that the emission spectrum was similar to that of lumazine. In accord with the emission spectrum of the lumazine at an excitation wavelength of 410 nm, as shown in Figure 7a, it was observed that E. coli 43R transformed with the pRFN4-Pp luxLP-N-lumP plasmid has the strongest fluorescence intensity among the E. coli transformed with pRFN4 plasmid derivatives when the excitation wavelength was fixed at 450 nm (Figure 8a). E. coli 43R transformed with the pRFN4-Pp N-lumP plasmid showed approximately 2~3 times higher intensity than E. coli 43R transformed with the pRFN4 plasmid. Furthermore, the fluorescence intensity of E. coli 43R transformed with pRFN4-Pp luxLP-N-lumP was approximately 2~5 times greater than that of E. coli 43R transformed with the pRFN4 plasmid, yielding the strongest intensity among all transformed E. coli 43R (Figure 8 and Figure 9).
Figure 8.
Fluorescence spectra of E. coli 43R transformed with recombinant pRFN4 plasmids containing P. phosphoreum lux DNA. (a) Emission spectra in fixation at excitation wavelength of 450 nm. (b) Excitation spectra for emission at 530 nm.
Figure 9.
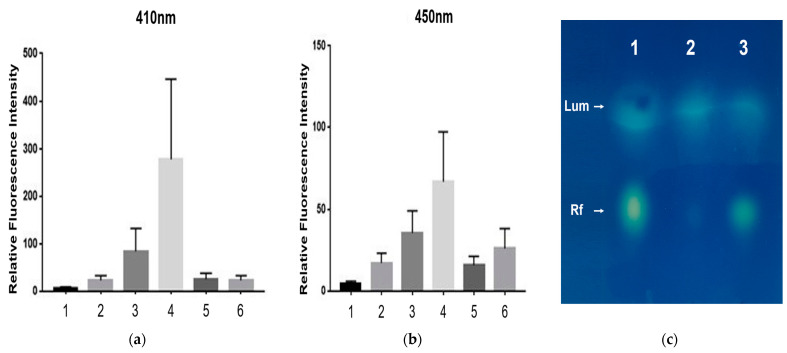
Relative fluorescence intensities. 1, E. coli 43R itself; 2, pRFN4 in E. coli 43R; 3, pRFN4-Pp N-lumP in E. coli 43R, 4, pRFN4-Pp luxLP-N-lumP in E. coli 43R; 5, pRFN4-Pp luxCP-N-lumP in E. coli 43R; 6, pRFN4-Pp luxCP in E. coli 43R. (a) Excitation wavelength was fixed at 410 nm for lumazine. (b) Excitation wavelength was fixed at 450 nm for riboflavin. (c) Identification of lumazine and riboflavin in TLC (thin layer chromatography) panel under UV illumination. 1, Mixtures of lumazine and riboflavin; 2, cell free extract of pRFN4 in E. coli 43R; 3, cell free extract of pRFN4-Pp luxLP-N-lumP in E. coli 43R. Lum, lumazine; Rf, riboflavin.
We also measured the excitation spectrum for emission at a wavelength of 530 nm by riboflavin (Figure 8b), in contrast to the previous process of Figure 8a. This result is similar to that observed in results shown in Figure 7b, as the strength of the fluorescence intensities caused by riboflavin from the cell extracts transformed with the pRFN4-Pp luxLP-N-lumP plasmid were much stronger than that of E. coli 43R transformed with the pRFN4, pRFN4-Pp N-lumP, or pRFN4-Pp luxCP-N-lumP plasmids. However, in contrast to the fluorescence excitation spectrum of lumazine shown in Figure 7b, the excitation spectrum of riboflavin from E. coli 43R transformed with the pRFN4-Pp N-lumP plasmid showed the same pattern of fluorescence as E. coli 43R transformed with other pRFN4 plasmids, as shown in Figure 8b.
A comparison of fluorescence intensities from E. coli cell extracts transformed with different derivatives of pRFN plasmids is shown in Figure 9. The fluorescence intensity at 410 nm from the cell extract of E. coli 43R gradually increased by approximately 2∼6-fold after transformation with pRFN4 and insertion of the pRFN4-Pp N-lumP plasmid. Moreover, the increase in intensity of E. coli 43R was sharp and varied approximately 5∼6-fold when transformed with pRFN4-Pp luxLP-N-lumP (Figure 9). Therefore, the highest fluorescence intensity of cells containing recombinant plasmid pRFN4-Pp luxLP-N-lumP was 500 times higher compared to the intensity of E. coli 43R itself (Figure 9a).
However, the fluorescence spectroscopic results for E. coli 43R transformed with the recombinant plasmid which carried the luxC promoter of P. phosphoreum NCMB 844 was different from that of E. coli 43R transformed with the pRFN4 plasmid harboring the lux promoter of P. leiognathi described in our previous study [5]. The fluorescence intensity of E. coli 43R transformed with pRFN4-Pp luxCP or pRFN4-Pp luxCP-Pp N-lumP plasmids was higher than that of E. coli 43R transformed with the pRFN4 plasmid, but significantly lower than that of E. coli 43R transformed with the pRFN4-Pp N-lumP plasmid (Figure 9a,b).
The identities of lumazine and riboflavin in cell free extracts were finally confirmed by silica gel of TLC developed with 3% ammonium chloride. Lumazine and riboflavin were visualized by fluorescence upon excitation using a long-wavelength UV lamp. As shown in Figure 9c, lumazine emitted greenish–blue and riboflavin emitted yellowish–green light, respectively.
2.3. Single-Cell Fluorescence Imaging by Confocal Microscopy
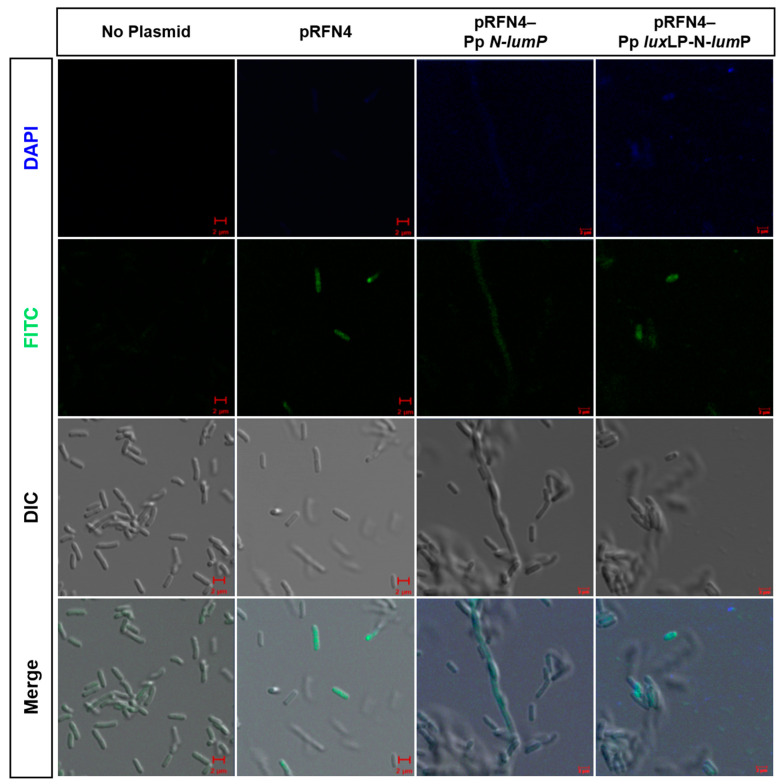
We tested whether a single fluorescent E. coli cell could be detected using confocal microscopy. E. coli 43R cells transformed with the recombinant plasmid containing lux DNA from Photobacterium spp. was loaded into a small amount of LB medium. We examined single-cell images of fluorescent E. coli with DAPI (excitation 405 nm) and FITC (excitation 458 nm) filter sets in the super-resolution confocal laser scanning microscope LSM880 and additionally performed differential interference contrast (DIC) imaging and merged images. E. coli 43R itself without any plasmids did not show any fluorescence, whereas we observed fluorescence in single E. coli 43R cells transformed with derivatives of pRFN4 that contained DNA for the gene and its promoter. We inferred that the overproduction of lumazine ligand caused the transformed E. coli 43R to express fluorescence. The fluorescence of E. coli 43R transformed with recombinant pRFN4-Pp luxLP-N-lumP plasmid was greater than that of E. coli 43R transformed with the pRFN4 plasmid. Furthermore, in E. coli 43R cells transformed with the recombinant plasmid pRFN4-Pp luxLP-N-lumP that contained DNA of the P. phosphoreum luxL promoter region, the fluorescence was more intense, and more cells expressed green light, as shown in Figure 10.
Figure 10.
Fluorescence microscopic images of E. coli 43R containing the recombinant plasmids of pRFN4. E. coli 43R itself (No plasmid), transformed with pRFN4, with pRFN4-Pp N-lumP, and with pRFN4 Pp luxLP N-lumP. DAPI, Diamidino-2-phenylindol; FITC, Fluorescein isothiocynate; DIC, Differential interference contrast. Scale bar; 2 µm.
3. Discussion
There has been a significant interest in luminescence and fluorescence for cellular and molecular imaging, and fluorescence is now the dominant methodology applied in biotechnology, flow cytometry, DNA sequencing, and genetic analysis [18,19]. The light emission of the bacterial luminescent system is being applied as sensitive, rapid, and safe assays in an ever-increasing number of biological systems and bioluminescence-based imaging of living cells has become an important tool in biological and medical research [20].
Our previous study [15] showed that transformed mutant E. coli RR1 emitted a drastically high level of light, which was strong enough to identify high luciferase enzyme activities in cell extracts, as well as to readily measure the fatty acid reductase activity responsible for supplying fatty aldehyde substrates. The light intensity was approximately 1000 times higher than that of the other transformed E. coli strains. This result is consistent with that observed for the V. harveyi lux system [16], indicating that a repressor or inhibitor may have been eliminated in the mutant E. coli.
In a series of studies regarding the generation of fluorescent bacteria, we initially started to work with pRFN4 plasmids by inserting the lumazine protein gene from P. leiognathi to test fluorescence intensities and single-cell imaging [10]. By binding one molecule of lumazine to the protein, the fluorescence intensity of N-LumP is increased because of its lumazine chromophore [12]. In this study, we selected P. phosphoreum, which is the brightest strain among bioluminescent bacteria, to construct pRFN4 derivatives containing the N-lumP gene and/or its promoter region from P. phosphoreum. Accordingly, we expected that increased binding of the lumazine ligand with the lumazine protein would yield higher fluorescence, as the luxL promoter from P. phosphoreum promotes the expression of the N-terminal half of luxL gene encoding N-LumP. The fluorescence intensity was significantly increased by insertion of the lux promoter when E. coli was transformed with the recombinant plasmid pRFN4-Pp luxLP-N-lumP. Remarkably, when this plasmid was transformed into E. coli 43R, the fluorescence intensity of the transformant was 500 times higher than that of E. coli itself.
Additionally, we conducted an imaging study with E. coli 43R transformed with a plasmid that harbored the lumazine protein gene of P. phosphoreum and a plasmid that was also inserted containing the luxL promoter region of P. phosphoreum. As shown in confocal microscopy images in Figure 10, the fluorescence of a single cell showed a similar tendency to the results from the fluorescence spectrophotometer. The single-cell image is in line with data from the fluorescence spectrophotometer, showing that the fluorescence of a single cell became stronger when E. coli was transformed with the recombinant plasmid containing the gene coding for N-LumP. As a result, cells transformed with the recombinant plasmid containing the gene coding for N-LumP as well as the lux promoter exhibited expression so high as to show fluorescence of single E. coli cells. Thus, the bacterial system with lux and riboflavin genes described in the present study can be utilized in the near future as a biosensor with high sensitivity and short analysis time.
Recently, bacterial bioluminescence has been used as a reporter system in plant protoplasts and single-cell imaging systems [20,21]. Microbial studies have many possibilities, such as mass production through incubation and improved selectivity by modulating incubation conditions to restrict unwanted metabolic pathways and induce adaptation to certain environmental conditions [20,21,22,23]. In addition, the lux biosensor based on B. subtillis for DNA-tropic and oxidative stress-causing agents was constructed [24]. Therefore, if future research continues to emphasize the possibilities for microbial studies and applications, the results presented in this paper will provide a solid basis for the development of microbial biosensors.
4. Materials and Methods
4.1. Strains and Vectors
We used E. coli XL-1 Blue as the cloning strain and measured fluorescence in E. coli 43R, an isogenic strain of E. coli RR1 [15]. The pRFN4 plasmid containing B. subtilis riboflavin biosynthetic genes was used as a cloning vector [13] (Table 1).
Table 1.
Bacterial strains and plasmids used in this study.
| Strains | Characteristics | Source |
| E. coli XL-1 Blue | Cloning strain | Real Biotech Corporation |
| E. coli 43R | Mutant of E. coli RR1 strain | Miyamoto.et al. (1987) J. Bacteriol. [16] |
| Plasmids | Characteristics | Source |
| pRFN4 | Recombinant plasmid containing the riboflavin genes from Bacillus subtilis | Illarionov et al. (2004) J. Org. Chem. [13] |
| pT7-5 PpEE | Recombinant pT7-5 plasmid containing the luxL and luxC genes, as well as their intergenic DNA from P. phosphoreum NCBM 844 | Woo et al. (2005) Kor. J. Microbiol. [14] |
| pRFN4- Pp N-lumP |
pRFN4 plasmid inserting the gene of 5′-terminal half of luxL for N-LumP from P. phosphoreum | This study |
| pRFN4- Pp luxCP |
pRFN4 plasmid inserting the promoter region for luxC from P. phosphoreum | This study |
| pRFN4- Pp luxCP-N-lumP |
pRFN4-Pp N-lumP plasmid inserting the promoter region for luxC from P. phosphoreum | This study |
| pRFN4- Pp luxLP-N-lumP |
pRFN4-Pp N-lumP plasmid inserting the DNA for promoter region for luxL from P. phosphoreum | This study |
4.2. Preparation of Recombinant Plasmids by PCR
DNA was amplified via PCR using the pT7-5 PpEE plasmid as a template. This plasmid contained the DNA of luxL and luxC and their promoter DNAs from P. phosphoreum NCBM 844 (Table 1). To amplify DNA containing the gene coding for the N-terminal half domain (N-LumP) up to the 115th amino acid (glutamic acid), as well as the luxL and luxC promoter regions shown in Figure 6, we used forward and reverse primers (Table 2). The PCR process consisted of 5 stages to duplicate pRFN4-Pp luxCP and pRFN4-Pp luxCP-N-lumP plasmids. The conditions of each stage were: pre-denaturation 95 °C, 5 min; denaturation 95 °C, 20 s; annealing 50 °C, 20 s; extension 72 °C, 30 s; and final extension 72 °C, 3 min. We performed the same PCR amplification and restriction endonuclease reactions using the same enzymes. After transformation, the colonies on LB agar plate were picked and incubated in a small amount of LB medium, with a small amount of DNA extracted using a miniprep kit.
Table 2.
Oligonucleotide primers for PCR amplification of the lux promoter region. Restriction sites for EcoRI (GAATTC) and BamHI (GGATCC) are underlined.
| Species | Primer | Sequence |
|---|---|---|
| P. phosphoreum | N-lumP forward | 5′-CAAATGAAGAAGAATTCCATTTTAAATATG-3′ |
| P. phosphoreum | N-lumP reverse | 5′-AAACTTTAAGAGGATCCTCTTCTTC-3′ |
| P. phosphoreum | luxC promoter forward | 5′-CTTTATCATAGAATTCCCTTTAAATTCC-3′ |
| P. phosphoreum | luxC promoter reverse | 5′-CTTGTTATTTTTAGAATTCTCTTATTCTTTC-3′ |
| P. phosphoreum | luxL promoter forward | 5′-GTTTATCGAATTCCATTTTAAATCATC-3′ |
| P. phosphoreum | luxL promoter reverse | 5′-CATATTTAAAATGGAATTCTTCATTTGG-3′ |
We then loaded and electrophoresed the products on 1.0% agarose gels. After electrophoresis, we identified the recombinant plasmids with the pRFN4-Pp luxCP and the pRFN4-Pp luxCP-N-lumP plasmids. The sizes of the inserts were approximately 9.0 kb and 9.4 kb, respectively.Among the 5 PCR stages, the processes of denaturation, annealing, and extension were repeated for 30 cycles. After PCR, the amplified DNA was purified via gel extraction. The purified DNA products and vectors cleaved by the same restriction endonuclease enzymes (EcoRI/BamH1) were then ligated into pRFN4 plasmids by T4 DNA ligase.
The ligated DNA was transferred into competent E. coli XL-1 cells. We incubated these colonies and extracted a small amount of DNA. We checked for transformation by performing PCR and applying restriction enzymes. Finally, we confirmed the orientation and insertion of the correct sequences using DNA sequencing. E. coli 43R cells were transformed with the pRFN4 derivative plasmids pRFN4-Pp N-lumP, pRFN4-Pp luxCP, pRFN4-Pp luxCP-N-lumP, and pRFN4-Pp luxLP-N-lumP (Table 1 and Figure 5), into which the N-lumP gene and/or lux promoters had been inserted in the previous process. The cells were then incubated under the following conditions: E. coli XL-1 Blue and E. coli 43R transformed with appropriate plasmids were incubated in LB medium containing 100 μg/mL of ampicillin at 37 °C. Incubated recombinant E. coli cells were adjusted to a fixed volume (A660 × Volume = 30 mL). Each strain was centrifuged at 2,500 rpm for 20 min to sediment the cells.
4.3. Buffer Solutions
We dissolved each cell extract in 25 mL of buffer solution A (pH (7.2) consisting of 50 mM Tris-HCl (Tris-hydrochloride), 0.5 mM DTT (dithiothreitol), and 0.5 mM EDTA (ethylenediaminetetraacetic acid)). The supernatant from each solution was collected via ultrasonication (3 × 30 s) and centrifugation.
4.4. Fluorescence Analysis
Using a LS 45 fluorescence spectrophotometer (PerkinElmer, Llantrisant, UK), we scanned the emission spectrum of each supernatant at fixed excitation wavelengths of 410 and 450 nm, and scanned the excitation spectra at emission wavelengths of 490 and 530 nm, respectively. Lumazine and riboflavin in cell free extracts were confirmed by silica gel (Baker, NJ, USA) of TLC developed with 3% ammonium chloride. The authentic lumazine was a gift from Prof. Fischer’s lab at the University of Hamburg and riboflavin was purchased from Sigma-Aldrich. The reference compounds of lumazine and riboflavin, as well as those from E. coli, were visualized by fluorescence upon excitation using a long-wavelength UV lamp.
4.5. Imaging of Fluorescent E. coli under Confocal Microscopy
Poly-L-lysine was smeared onto glass slides and incubated for at least 1 h. We then placed the E. coli on the prepared glass slides and fixed them by placing a cover-slip on top. Single-cell images of fluorescent E. coli were examined using a super-resolution confocal laser scanning microscope LSM 880 with Airyscan (Carl Zeiss, Jena, Germany).
5. Conclusions
Using modified pRFN4 plasmids containing the lux gene promoter DNA from the brightest strain of bioluminescent bacteria, P. phosphoreum, and with the gene coding for the minimum version of the amino-terminal half of lumazine protein (N-LumP), as well as the mutant strain of E. coli 43R, we developed fluorescent bacteria with 500 times the fluorescence intensity of normal E. coli. We can acquire single-cell images of fluorescent bacteria, and this can be used for a microbial sensor which can detect heavy metals, environmental pollutants, and antibiotics by checking their effects on bacterial fluorescence intensities.
Author Contributions
Experiments design, C.-Y.L.; cell biology experiments, S.-J.L. and M.C.; molecular biology work, I.Y., S.L., N.C., and S.-J.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by a research grant from Chungnam National University.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Brodl E., Winkler A., Macheroux P. Molecular Mechanisms of Bacterial Bioluminescence. Comput. Struct. Biotechnol. J. 2018;16:551–564. doi: 10.1016/j.csbj.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee J., Müller F., Visser A.J. The Sensitized Bioluminescence Mechanism of Bacterial Luciferase. Photochem. Photobiol. 2019;95:679–704. doi: 10.1111/php.13063. [DOI] [PubMed] [Google Scholar]
- 3.Love A.C., Precher J.A. Seeing (and Using) the Light: Recent Developments in Bioluminescence Technology. Cell Chem. Biol. 2020;27:904–920. doi: 10.1016/j.chembiol.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meighen E.A. Genetics of Bacterial of Bioluminescence. Annu. Rev. Genet. 1994;28:117–139. doi: 10.1146/annurev.ge.28.120194.001001. [DOI] [PubMed] [Google Scholar]
- 5.Lee C.Y., O’Kane D.J., Meighen E.A. Riboflavin Synthesis Genes are Linked with the lux Operon of Photobacterium phosphoreum. J. Bacteriol. 1994;176:2100–2104. doi: 10.1128/jb.176.7.2100-2104.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gast R., Lee J. Isolation of in vivo Emitter in Bacterial Bioluminescence. Proc. Natl. Acad Sci. USA. 1978;75:833–837. doi: 10.1073/pnas.75.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Kane D.J., Karle A.J., Lee J. Chemical Characterization of Lumazine Protein from Photobacterium leiognathi: Comparison with lumazine protein from Photobacterium phosphoreum. Biochemistry. 1985;24:1467–1475. doi: 10.1021/bi00327a027. [DOI] [PubMed] [Google Scholar]
- 8.O’Kane D.J., Woodward B., Lee J., Prasher D.C. Borrowed Proteins in Bacterial Bioluminescence. Proc. Natl. Acad. Sci. USA. 1991;88:1100–1104. doi: 10.1073/pnas.88.4.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J. Bioluminescence. In: Smith K.C., editor. The Science of Photobiology. Springer; New York, NY, USA: 1989. pp. 391–417. [Google Scholar]
- 10.Lim S., Oh E., Choi M., Lee E., Lee C.Y. Generation of Fluorescent Bacteria with the Genes Coding for Lumazine Protein and Riboflavin Biosynthesis. Sensors. 2021;21:4506. doi: 10.3390/s21134506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatwell L., Illarionova V., Illarionov B., Eisenreich W., Huber R., Skerra A., Bacher A., Fischer M. Structure of Lumazine Protein, an Optical Transponder of Luminescent Bacteria. J. Mol. Biol. 2008;382:44–55. doi: 10.1016/j.jmb.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 12.Illarionov B., Eisenreich W., Wirth M., Lee C.Y., Woo Y.E., Bacher A., Fischer M. Lumazine proteins from Photobacteria: Localization of the Single Ligand Binding Site to the N-terminal domain. Biol. Chem. 2007;388:1313–1323. doi: 10.1515/BC.2007.134. [DOI] [PubMed] [Google Scholar]
- 13.Illarionov B., Fischer M., Lee C.Y., Bacher A., Eisenreich W. Rapid Preparation of Isotopolog Libraries by in vivo Transformation of 13C-Glucose. Studies on 6,7-Dimetyl-8-ribityllumazine, a Biosynthetic Precursor of Vitamin B2. J. Org. Chem. 2004;69:5588–5594. doi: 10.1021/jo0493222. [DOI] [PubMed] [Google Scholar]
- 14.Woo Y.E., Kim S.Y., Lee C.Y. Generation and Expression of Amino-Terminal Domain of the Gene Coding for the Lumazine Protein from Photobacterium phosphoreum. Kor. J. Microbiol. 2005;41:306–311. [Google Scholar]
- 15.Lee C.Y., Szittner R., Meighen E.A. The lux Genes of the Luminous Bacterial Symbiont, Photobacterium leiognathi, of the ponyfish. Eur. J. Biochem. 1991;201:161–167. doi: 10.1111/j.1432-1033.1991.tb16269.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto C.M., Byers D., Graham A.F., Meighen E.A. Expression of Bioluminescence in Escherichia coli by Recombinant Vibrio harveyi DNA. J. Bacteriol. 1987;169:247–253. doi: 10.1128/jb.169.1.247-253.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulus B., Illarionov B., Nohr D., Roellinger G., Kacprzak S., Fischer M., Weber S., Bacher A., Schleicher E. One Protein, Two Chromophores: Comparative Spectroscopic Characterization of 6,7-Dimethyl-8-ribityllumazine and Riboflavin Bound to Lumazine Protein. J. Phys. Chem. 2014;118:13092–13105. doi: 10.1021/jp507618f. [DOI] [PubMed] [Google Scholar]
- 18.Klose A.D., Paragas N. Automated Quantification of Bioluminescence Images. Nat. Commun. 2018;9:4262. doi: 10.1038/s41467-018-06288-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe L., Dikici E., Daunert S. Engineering Bioluminescent Proteins: Expanding their Analytical Potential. Anal. Chem. 2009;81:8662–8668. doi: 10.1021/ac9007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregor C., Pape J.K., Gwosch K.C., Gilat T., Sahl S.J., Hell S.W. Autonomous Bioluminescence Imaging of Single Mammalian Cells with the Bacterial Bioluminescence System. Proc. Natl. Acad. Sci. USA. 2019;116:26491–26496. doi: 10.1073/pnas.1913616116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui B., Zhang I., Song Y., Wei J., Li C., Wang T., Wang Y., Zhao T., Shen X. Engineering an Enhanced, Thermostable, Monomeric Bacterial Luciferase Gene as a Reporter in Plant Protoplast. PLoS ONE. 2014;9:e107885. doi: 10.1371/journal.pone.0107885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei Y., Chen W., Mulchandani A. Microbial Biosensors. Anal. Chim. Acta. 2006;568:200–210. doi: 10.1016/j.aca.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 23.Gregor C., Gwosch K., Sahl S.J., Hell S.W. Strongly Enhanced Bacterial Bioluminescence with the Ilux Operon for Single-Cell Imaging. Proc. Natl. Acad. Sci. USA. 2018;115:962–967. doi: 10.1073/pnas.1715946115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessenikh A.G., Novoyatlova U.S., Bazhenov S.V., Stepanova E.S., Khrulnova S.A., Gnuchikh E.Y., Kotova V.Y., Kudryavtseva, Bermeshev M.V., Manukhov I.N. Constructing of Bacillus subtillis-Based Lux Biosensors with the Use of Stress-Inducible Promoters. Int. J. Mol. Sci. 2021;22:9571. doi: 10.3390/ijms22179571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.