Abstract
Advances in understanding the regulatory functions of the nervous system have revealed neural cholinergic signaling as a key regulator of cytokine responses and inflammation. Cholinergic drugs, including the centrally acting acetylcholinesterase inhibitor, galantamine, which are in clinical use for the treatment of Alzheimer’s disease and other neurodegenerative and neuropsychiatric disorders, have been rediscovered as anti-inflammatory agents. Here, we provide a timely update on this active research and clinical developments. We summarize the involvement of cholinergic mechanisms and inflammation in the pathobiology of Alzheimer’s disease, Parkinson’s disease, and schizophrenia, and the effectiveness of galantamine treatment. We also highlight recent findings demonstrating the effects of galantamine in preclinical and clinical settings of numerous conditions and diseases across the lifespan that are characterized by immunological, neurological, and metabolic dysfunction.
Keywords: Alzheimer’s disease, cholinergic signaling, galantamine, inflammation, inflammatory disorders, metabolism, Parkinson’s disease, schizophrenia
1 |. INTRODUCTION
Galantamine (also known as galanthamine) is a plant alkaloid with a long history of being explored in the treatment of cognitive impairment and many other conditions (Plaitakis & Duvoisin, 1983). As elegantly articulated before, this history appears to have started in ancient Greece. In his epic poems Homer describes the mysterious plant ‘Moly’, which is given to Odysseus by Hermes to help his crew to fight against the amnesic effects of Circe’s poison–allegedly a centrally acting anticholinergic drug (Plaitakis & Duvoisin, 1983). Based on empirical evidence it has been suggested that ‘Moly’ might have been the common snowdrop (Galanthus nivalis), a plant native of the Balkan Peninsula, and a major natural source of galantamine–a centrally acting cholinergic compound (Plaitakis & Duvoisin, 1983). Thus, Homer may have described the first example of applying galantamine to stimulate brain cholinergic signaling and prevent severe anticholinergic poisoning and memory loss. Progress in our understanding of the important role of the brain cholinergic system in the regulation of cognition and memory, provides modern scientific highlights of Homer’s descriptions. Brain (forebrain) cholinergic neurodegeneration, and the associated progressive loss of synaptic fields and diminished release of acetylcholine (ACh) are prominent features of Alzheimer’s disease (AD) (Hampel et al., 2018). Galantamine, an acetylcholinesterase (AChE) inhibitor and a centrally acting cholinergic drug is currently used for treating cognitive impairment in patients with mild to moderate AD (Birks, 2006; Prvulovic et al., 2010; Richarz et al., 2014).
In addition to AD, brain cholinergic mechanisms are implicated in the pathogenesis of several other neurological disorders, including Parkinson’s disease (PD) and schizophrenia. Immune dysregulation and aberrant inflammation have been identified as pathobiological features of these diseases. Recent insights have highlighted a key role for cholinergic signaling in the regulation of immunity and inflammation (Pavlov et al., 2018; Pavlov & Tracey, 2017). Importantly, the anti-inflammatory properties of galantamine were also discovered. This knowledge has substantially improved our understanding of the regulatory functions of cholinergic signaling and the therapeutic effects of galantamine for a wide range of disorders across the lifespan. Here, we provide a timely summary of cholinergic signaling in the regulation of inflammation and the growing experimental evidence supporting the therapeutic efficacy of galantamine for targeting cholinergic mechanisms in disorders characterized by immune and metabolic dysregulation. We also outline the current understanding of the role of brain cholinergic dysfunction and inflammation in AD, PD, and schizophrenia and the ongoing therapeutic use and exploration of galantamine for treating these and a growing number of other diseases and conditions.
2 |. THE BRAIN CHOLINERGIC SYSTEM
The cholinergic system in the brain is a major neuromodulatory network. A main component is the basal forebrain cholinergic system (BFCS), which consists of neurons localized in the medial septum, vertical and horizontal limbs of the diagonal band of Broca, nucleus basalis, and substantia innominata. Nucleus basalis of Meynert (NbM) in humans is the equivalent of nucleus basalis magnucellular in the rodent brain. The BFCS innervates mainly the cortex (neocortex), hippocampus, amygdala, and the olfactory bulb, and plays a key role in the regulation of attention, learning, and memory (Ballinger et al., 2016; Hampel et al., 2018). Other main brain cholinergic neuronal localizations are the mesopontine pedunculopontine tegmental (PPT) and laterodorsal tegmental (LDT) nuclei. These nuclei innervate subcortical structures including the thalamus, hypothalamus, and hindbrain (cerebellum, pons, and medulla oblongata). PPT and LDT cholinergic neurons have a documented role in the regulation of sleep-wake cycles and motor function (Woolf, 1991; Woolf & Butcher, 2011). Cholinergic neurons—large interneurons are also localized within the striatum and implicated in the complex regulation of a striatal microcircuitry related to motor function (Abudukeyoumu et al., 2019; Woolf & Butcher, 1981). An important molecular determinant of brain cholinergic signaling is the enzyme choline acetyltransferase (ChAT), which synthesizes acetylcholine (ACh) using choline and acetyl coenzyme A (acetyl-CoA) as precursors. (Figure 1). The vesicular acetylcholine transporter (VAChT) is a critical molecule in ACh release; it loads ACh in vesicles prior to its release in the synaptic cleft (Prado et al., 2013). Acetylcholinesterase (AChE) is an enzyme with mainly a neuronal origin that degrades ACh into choline (which is recycled for ACh synthesis) and acetate, thus terminating cholinergic neurotransmission (Giacobini, 2004; Soreq & Seidman, 2001). Butyrylcholinesterase (BuChE), which is mainly glial, is another enzyme that degrades ACh (Giacobini, 2004) (Figure 1). Muscarinic acetylcholine receptors (mAChRs) predominantly of the M1 subtype, are expressed on postsynaptic, cholinoceptive neurons; mAChRs play a major role in processing cholinergic neurotransmission (Soreq & Seidman, 2001; Thiele, 2013). Presynaptic M2 and M4 mAChRs expressed on cholinergic neurons negatively modulate cholinergic neurotransmission serving as autoreceptors for ACh (Soreq & Seidman, 2001; Thiele, 2013) (Figure 1). Nicotinic acetylcholine receptors (nAChRs) also modulate and mediate cholinergic neurotransmission in the brain (Albuquerque et al., 2009; Zoli et al., 2015). Presynaptic nAChRs, including α7nAChR and α4/β2nAChR are positive regulators of ACh and other neurotransmitters (Albuquerque et al., 2009; Dani & Bertrand, 2007). Postsynaptic nAChRs play a role in processing cholinergic neurotransmission in addition to mAChRs (Albuquerque et al., 2009; Dani & Bertrand, 2007) (Figure 1). Cortex areas, the hippocampus, hypothalamus, and other regions receiving cholinergic innervations, interact directly or through polysynaptic pathways with the dorsal vagal complex, including the dorsal motor nucleus of the vagus (DMN) in the brainstem medulla oblongata (Pavlov & Tracey, 2012, 2017; Travagli et al., 2006). DMN and nucleus ambiguus (NA) are brainstem cholinergic nuclei that provide axonal projections within the vagus nerve regulating peripheral respiratory, cardiovascular, gastrointestinal, and metabolic processes. Recent discoveries have also indicated that brain (forebrain) cholinergic signaling and the vagus nerve are major physiological regulators of inflammation (Lehner et al., 2019; Pavlov et al., 2006, 2009, 2018; Tracey, 2002). These regulatory circuitries can be activated using cholinergic drugs, including galantamine (Figure 2).
FIGURE 1.
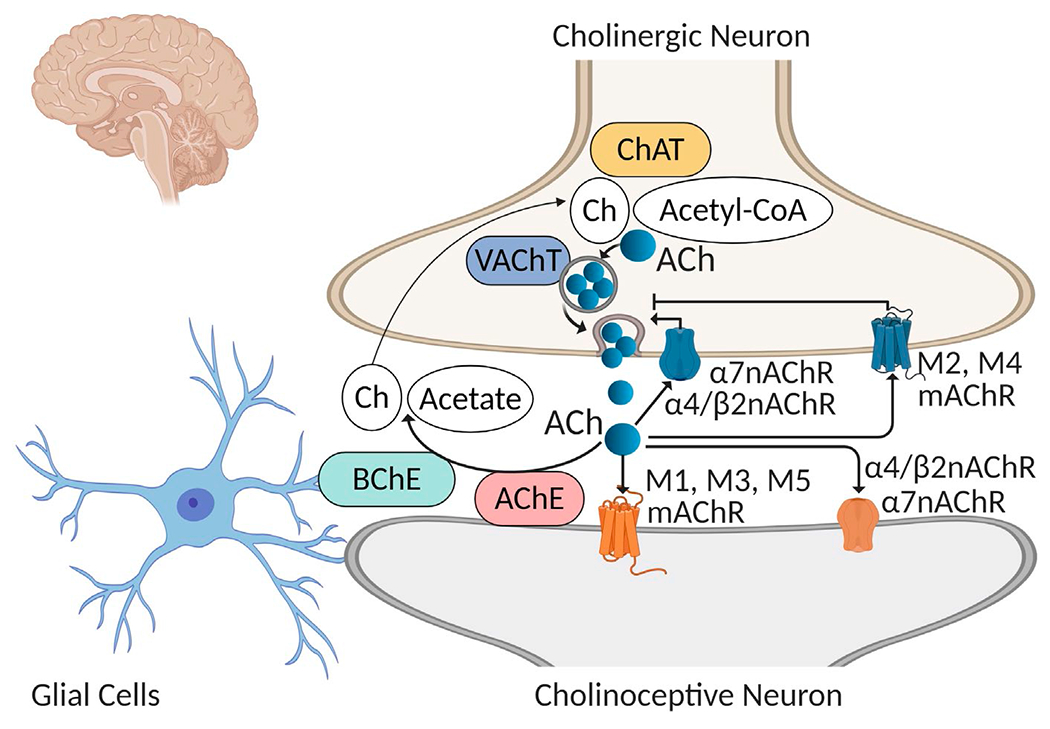
The synthesis, transport, action, and degradation of acetylcholine in the brain. Acetylcholine (ACh) is synthesized from acetyl coenzyme A (Acetyl-CoA) and choline (Ch) by the enzyme choline acetyltransferase (ChAT) in the cytoplasm of the cholinergic neurons. The vesicular acetylcholine transporter (VAChT) loads ACh in vesicles before its release in the synaptic cleft. ACh interacts with postsynaptic muscarinic receptors, including the M1, M3, and M5 subtype and nicotinic receptors, including α4/α2nAChR and α7nAChR, which mediate cholinergic transmission. ACh also interacts with presynaptic receptors, including the M2 and M4 mAChR, which inhibit ACh release (autoinhibition) and α7nAChR and α4/β2nAChR, which stimulate ACh release. (Of note, other presynaptic and postsynaptic nAChRs also play a role in modulating cholinergic transmission in the brain Albuquerque et al., 2009; Zoli et al., 2015)). ACh is degraded by acetylcholinesterase (AChE) and by butyrylcholinesterase (BChE, with predominantly glial origins) to acetate and choline, which is re-utilized for ACh synthesis via re-uptake through the high affinity choline transporter (not shown). (Figure created using Biorender)
FIGURE 2.
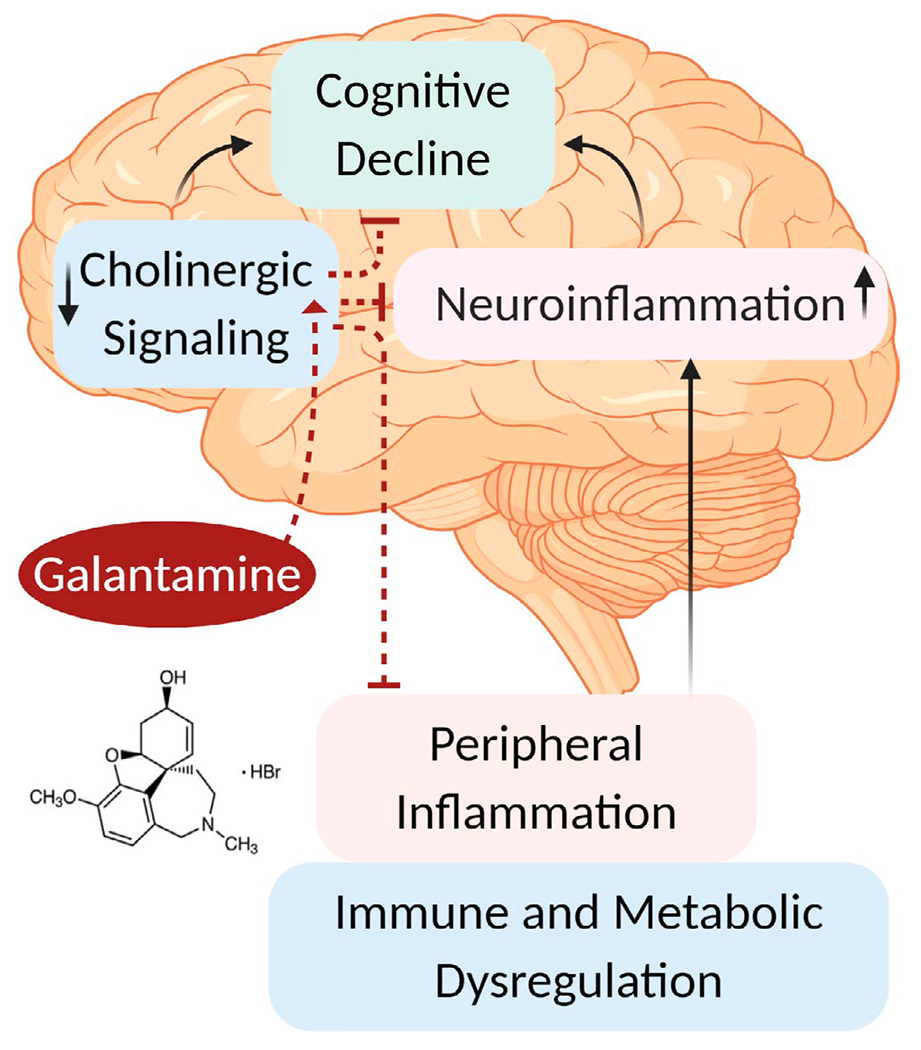
Brain cholinergic signaling and therapeutic efficacy of galantamine. Brain cholinergic signaling plays a key role in the regulation of vital physiological functions, including cognition and inflammation. Brain cholinergic dysfunction and neuroinflammation, documented in Alzheimer’s disease, Parkinson’s disease, schizophrenia, and other disorders have been shown to contribute to cognitive impairment. Peripheral inflammation, driven by immune and metabolic dysregulation reportedly contributes and exacerbates neuroinflammation. Activation of brain cholinergic signaling by administering the centrally acting AChE inhibitor galantamine (galantamine hydrobromide in most pre-clinical studies) helps counteract cognitive decline and inhibits peripheral inflammation (through the inflammatory reflex). Brain cholinergic activation also lowers neuroinflammation. Suppressing aberrant peripheral inflammation may ameliorate metabolic derangements and reduce neuroinflammation. These and possibly other mechanisms mediate galantamine’s beneficial effects in a numerous neurological, inflammatory, and metabolic disorders. See text for details. (Figure created using Biorender)
3 |. GALANTAMINE DRUG DEVELOPMENT AND TIMELINE
Galantamine is an alkaloid phytochemical. In the 1950s this compound, isolated from the bulbs of the common snowdrop (Galanthus nivalis), was shown to alleviate the effect of tubocurarine on skeletal muscles and used under the trade name Nivalin for treating poliomyelitis (infantile paralysis) and myasthenia gravis in Bulgaria and other European countries (Heinrich & Lee Teoh, 2004; Paskov, 1957, 1964; Revelli & Graso, 1962; Stoyanov, 1964; Uzunov, 1966). Galantamine was also isolated from the snowflake plant (Leucojum aestivum) found in Europe and North America (Janssen & Schäfer, 2017). More recently, because of the scarcity of its botanic sources galantamine has been chemically synthesized (Marco-Contelles et al., 2006). In 1960 galantamine was reported to inhibit AChE activity (Irwin & Smith, 1960). In 1965, it was demonstrated that galantamine crossed the blood brain barrier and increased central cholinergic tone (Popova & Bogolepov, 1965). As an AChE inhibitor, galantamine reversibly and competitively blocks its enzymatic activity, extending the half-life of ACh and thus stimulating cholinergic signaling (Figure 1). In addition to its action as an AChE inhibitor, galantamine has been reported to be a positive allosteric modulator of nAChRs, specifically of the human α7, α4β2, α3β4, and α6β4 subtypes; binding of galantamine to an allosteric site of the receptor molecule causes conformational changes that increase receptor responsiveness to ACh (Samochocki et al., 2000, 2003). It should be noted, however, that a more recent study failed to demonstrate galantamine’s positive allosteric modulation of human α7nAChRs or α4β2nAChRs (Kowal et al., 2018), which is in contrast with previous findings (Texidó et al., 2005). Many preclinical and clinical studies of galantamine for treating AD were performed in the 1980s and 1990s, respectively (Heinrich & Lee Teoh, 2004). Galantamine hydrobromide (C17H21NO3·HBr) (Figure 2), also known as Reminyl, was first approved in 2001 by the U.S. Food and Drug Administration (FDA) for the treatment of mild to moderate dementia of AD (Thompson, 2001). Since then, several generic equivalents of galantamine have been approved by the FDA and its trade name was changed to Razadyne™.
4 |. CHOLINERGIC SIGNALING IN THE REGULATION OF INFLAMMATION AND GALANTAMINE AS AN ANTI-INFLAMMATORY AGENT
Inflammation is a vital protective response to tissue injury and invading pathogens; it involves a complex and regulated series of events underlying innate and adaptive immune responses (Chen & Nunez, 2010; Nathan, 2002; Olofsson et al., 2017). The release of cytokines, including tumor necrosis factor (TNF), from macrophages and other immune cells is a central event mediating innate immune responses (Olofsson et al., 2017). The normal script of inflammation dictates that it is localized, tightly controlled, and resolved in a timely manner, resulting in wound healing and pathogen neutralization (Chen & Nunez, 2010; Tracey, 2009). However, inflammation can deviate from this optimal scenario and various forms of exacerbated, sustained, or non-resolved inflammation are implicated in disease pathogenesis and progression (Olofsson et al., 2017; Tracey, 2009). Inflammation is tightly controlled by immune, humoral, and neuronal mechanisms (Elenkov et al., 2000; Olofsson et al., 2017; Talbot et al., 2016; Webster et al., 2002). Discoveries about 20 years ago have considerably advanced our understanding of the regulation of inflammation by identifying neural cholinergic signaling as a key component (Hoover, 2017; Pavlov et al., 2003; Tracey, 2002, 2007). These and subsequent discoveries also highlighted a physiological mechanism–the inflammatory reflex, which regulates the production of TNF and other pro-inflammatory cytokines and inflammation (Tracey, 2002). In the inflammatory reflex, increasing circulating cytokine levels in the periphery are detected by afferent (sensory) vagal neurons; the resultant signal is sent to the brainstem dorsal vagal complex and efferent (motor) vagus nerve signaling is generated, which leads to ACh release in the periphery (Metz & Pavlov, 2018; Pavlov & Tracey, 2005; Tracey, 2002). Within the inflammatory reflex, activation of neural signaling via the vagus nerve-splenic nerve axis results in the release of ACh by a subset of ChAT-containing T cells (Rosas-Ballina et al., 2011). ACh acting through α7nAChRs expressed on macrophages and other immune cells regulates immune responses and suppresses TNF and other pro-inflammatory cytokine levels (Pavlov et al., 2018; Pavlov & Tracey, 2017). Suppression of NF-κB nuclear translocation (Gallowitsch-Puerta & Pavlov, 2007; Guarini et al., 2003; Parrish et al., 2008), stimulation of the Janus kinase/signal transducer and activator of transcription 3 (JAK2/STAT3) pathway (de Jonge et al., 2005), inhibition of inflammasome activation (Lu et al., 2014), and cyclic adenosine 3′,5′-cyclic monophosphate (cAMP) signaling (Tarnawski et al., 2018) have been indicated as relevant intracellular mechanisms downstream α7nAChR. The inflammatory reflex can be stimulated by electrical vagus nerve stimulation with subsequent ACh release and α7nAChRs agonists (Borovikova et al., 2000; Chatterjee et al., 2017; Pavlov et al., 2007; Pavlov & Tracey, 2015; Sitapara et al., 2020).
Experimental evidence indicates that cholinergic signaling in the brain controls peripheral cytokine responses and inflammation and that the inflammatory reflex provides a brain-to-periphery communication conduit in this regulation (Pavlov & Tracey, 2015) (Figure 2). Targeting brain M1 mAChRs using central administration of the orthosteric agonist, McN-A-343 (4-[[[(3-Chlorophenyl) amino] carbonyl]oxy]-N,N,N-trimethyl-2-butyn-1-aminium chloride), or the selective positive allosteric modulator, BQCA (benzyl quinolone carboxylic acid), results in the suppression of serum TNF and other anti-inflammatory effects in murine endotoxemia and colitis; these effects are abolished following vagotomy (Lehner et al., 2019; Munyaka et al., 2014; Pavlov et al., 2006). Brain mAChR signaling mediates anti-inflammatory effects in neuro-immune circuitries involving the vagus nerve in experimental models of hemorrhagic shock (Guarini et al., 2004; Lee et al., 2010) and in acupuncture (Song et al., 2012). Peripheral (intraperitoneal, i.p.) administration of the centrally acting M1 mAChR orthosteric agonist, xanomeline, or the selective positive allosteric modulator, BQCA, results in reduced TNF production and improved survival in murine endotoxemia (Lehner et al., 2019; Rosas-Ballina et al., 2015). These effects are mediated through brain mAChRs and vagus nerve-splenic nerve signaling (Lehner et al., 2019; Rosas-Ballina et al., 2015). In contrast, administration of anticholinergic drugs results in increased inflammatory responses triggered by endotoxin in a mice with tauopathy (Yoshiyama et al., 2012, 2015). In this model, trihexyphenidyl, a centrally acting M1 mAChR antagonist, causes a greater increase in splenic and brain IL-1β expression compared with an M1 mAChR antagonist that exhibits weaker ability to penetrate the blood–brain barrier (Yoshiyama et al., 2012).
Brain cholinergic signaling can be stimulated by galantamine and other AChE inhibitors (Figure 2). Peripheral administration of galantamine and another centrally acting AChE inhibitor—huperzine A inhibits serum TNF and IL-6 levels and improves survival in mice with lethal endotoxemia, acting through a central brain mAChR-dependent and vagus nerve-mediated mechanism (Pavlov et al., 2009). Very recently, using mice with selective forebrain genetic ablation of VAChT (a key determinant of ACh neural release) revealed the importance of forebrain cholinergic signaling in mediating galantamine’s anti-inflammatory effects in a model of endotoxemia (Lehner et al., 2019). In contrast to the significant suppressive effect of galantamine in control mice, peripheral galantamine administration failed to decrease serum TNF significantly in mice with forebrain VAChT deficiency following LPS injection (Lehner et al., 2019). Galantamine also alleviates colonic inflammation and disease severity in two models of inflammatory bowel disease (IBD)—colitis (Ji et al., 2014). Blockade of brain mAChRs, vagotomy or splenic nerve transection abrogates the effects of galantamine, delineating brain mAChR-triggered cholinergic signaling pathway linked to the neural vagus nerve-splenic nerve as an underlying mechanism (Ji et al., 2014). Galantamine also regulates the vagus nerve-based inflammatory reflex in mice with systemic lupus erythematosus, a model where inflammation drives hypertension (Pham et al., 2018). Chronic galantamine treatment (once daily, for two weeks) increases vagus nerve activity, alleviates splenic and renal inflammation, and decreases hypertension in this model (Pham et al., 2018). Additionally, brain mAChR-mediated action of galantamine linked to the activation of the efferent arm of the inflammatory reflex alleviates acid-induced acute lung injury/acute respiratory distress in rabbits (Yang et al., 2018).
Peripheral AChE inhibition may also generate anti-inflammatory effects. For instance, a reduction of intestinal inflammation by another AChE inhibitor, rivastigmine, is mediated by mechanisms involving both brain and peripheral AChE inhibition (Shifrin et al., 2013). However, experimental evidence indicates that inhibiting CNS AChE activity induces greater anti-inflammatory protection compared with peripheral enzyme inhibition. Treatment with physostigmine, a centrally acting AChE inhibitor produces a greater anti-inflammatory effect in rats with colitis compared with neostigmine, an AChE inhibitor whose activity is restricted to periphery (Miceli & Jacobson, 2003). The centrally acting AChE inhibitor, donepezil, but not the peripherally-acting AChE inhibitor, pyridostigmine, enhances vagal tone, decreases circulating TNF, IL-6, and IFN-γ levels, and alleviates hypertension, and prevents cardiac remodeling in spontaneously hypertensive rats (Lataro et al., 2015). While treatment with either the centrally acting AChE inhibitor, physostigmine, or the peripherally restricted, neostigmine, decreases markers of oxidative stress and CD11b upregulation by polymorphonuclear neutrophils (PMNs) in rats with experimental sepsis, treatment with physostigmine, but not neostigmine, improves survival compared to vehicle treatment (Bitzinger et al., 2019). There are some reports indicating the lack of anti-inflammatory efficacy of peripherally-acting AChE inhibitors. For example, neostigmine treatment fails to ameliorate pro-inflammatory cytokine responses in a murine model of mechanical ventilation-induced lung injury (Kox et al., 2009) and organ injury in endotoxemia (Akinci et al., 2005). AChE-targeted microRNAs are also an important regulatory hub in the cholinergic control of immune function and inflammation (Shaked et al., 2009; Vaknine & Soreq, 2020).
In addition to peripheral inflammation, galantamine and other centrally acting AChE inhibitors such as rivastigmine and donepezil alleviate neuroinflammation (Dasuri et al., 2016; Nizri et al., 2008; Wang et al., 2018) (Figure 2). In a mouse model of neuroinflammation, galantamine reduces hippocampal microglia and astrocyte activation, as well as cytokine production within the hippocampus (Liu, Zhang, et al., 2018). Neuroinflammation is a term used to describe inflammatory processes within the CNS driven by microglial and astrocyte activation (Olofsson et al., 2017). Similar to inflammation in the periphery, neuroinflammation can be a protective event, but when sustained or dysregulated it can contribute to neuronal injury and neurodegeneration (McManus & Heneka, 2017; Olofsson et al., 2017). Neuroinflammation is observed in many conditions characterized by peripheral immune and metabolic dysregulation including, endotoxemia, sepsis, obesity, diabetes, and liver disease (Chang et al., 2019; Pavlov & Tracey, 2015). Peripheral inflammation in these conditions may exacerbate or even lead to neuroinflammation (Hoogland et al., 2015; Olofsson et al., 2017) (Figure 2). There is also evidence for brain cholinergic dysfunction and cognitive impairment in parallel with neuroinflammation (Chang et al., 2019; Scherer et al., 2014). For instance, increased serum levels of TNF, IL-6, and other pro-inflammatory cytokines measured 4 hr following LPS-administration in mice are associated with differential alterations of ChAT, AChE, and M1 mAChRs, and neuroinflammation in various brain regions (Silverman et al., 2015). Neuronal loss and decreased cortical innervations based on VAChT staining, as well as cognitive impairment are indicated in rats surviving endotoxemia (Semmler et al., 2007). In addition, increased levels of pro-inflammatory cytokines and alterations in metabolic molecules, BFCS neuronal deficits, and neuroinflammation are observed in mice that survive experimental sepsis (Zaghloul et al., 2017). Brain cholinergic hypofunction has also been documented in patients with cirrhosis and in animal models of liver failure (Garcia-Ayllon et al., 2008). In this context, it is reasonable to suggest that brain cholinergic deficiency, which may be caused by neuroinflammation, drives further inflammation and neuroinflammation (Figure 2); we propose that administering galantamine and other centrally acting AChE inhibitors will interrupt this vicious cycle.
Clinical translation is the ultimate goal of studying cholinergic signaling in controlling aberrant inflammation. Studies with vagus nerve stimulation in animal models of numerous inflammatory conditions have recently progressed into successful clinical trials with implanted bioelectronic vagus nerve stimulators in patients with IBD and rheumatoid arthritis (Bonaz et al., 2016; Genovese et al., 2020; Koopman et al., 2016). These preclinical and clinical advances have greatly contributed to the emerging field of bioelectronic medicine (Olofsson & Tracey, 2017; Pavlov, 2019; Pavlov et al., 2019; Pavlov & Tracey, 2019a, 2019b). Repurposing cholinergic drugs with anti-inflammatory actions, which are already clinically tested and/or FDA approved provides another viable avenue for clinical translation. Pre-clinical findings also can significantly contribute to clinical translation. Galantamine treatment (4 mg/kg, i.p., once daily for 4 weeks) in mice with high-fat diet-induced obesity and features of metabolic syndrome significantly alleviates inflammation, insulin resistance, and hepatic steatosis (Satapathy et al., 2011). These preclinical insights along with the abundant information regarding galantamine’s safety profile in humans facilitated a recent randomized, double blind, placebo-controlled clinical trial in patients with metabolic syndrome (Consolim-Colombo et al., 2017). In this clinical trial, treatment of 30 patients (15 males and 15 females) with clinically approved doses of galantamine for 8 weeks, compared with 30 placebo-treated patients (15 males and 15 females) significantly alleviated plasma levels of TNF and leptin (pro-inflammatory) and increased plasma levels of IL-10 and adiponectin (anti-inflammatory) (Consolim-Colombo et al., 2017). Galantamine treatment (versus. placebo) also ameliorated oxidative stress in patients with metabolic syndrome (Sangaleti et al., 2020). These effects were accompanied by lower insulin levels and improved insulin resistance in galantamine-treated patients, as well as modulation of the autonomic neural regulation toward vagus nerve predominance (Consolim-Colombo et al., 2017; Sangaleti et al., 2020). Further studies with galantamine are expected to define its use for alleviating metabolic syndrome—an obesity-associated disorder of pandemic proportions with limited treatment options (Grundy, 2008).
5 |. BRAIN CHOLINERGIC SIGNALING, INFLAMMATION, AND GALANTAMINE IN NEURODEGENERATIVE AND NEUROPSYCHIATRIC DISORDERS
5.1 |. Alzheimer’s disease (AD)
AD is a progressive neurodegenerative disease with no curative options. AD is the most prevalent form of dementia and a debilitating and lethal neurodegenerative disorder which is expected to affect 1 in 85 people around the world by 2050 (Brookmeyer et al., 2007). AD has a serious deleterious impact on patients, close relatives, and caregivers and prior to the novel coronavirus disease 2019 (COVID-19) it was considered “the greatest challenge for health and social care in the 21st century” (Livingston et al., 2020). AD is characterized by progressive memory loss and neurocognitive decline leading to behavioral dysfunction and the inability to perform daily activities required for independent living (Hampel et al., 2018; Weller & Budson, 2018). Patients typically progress from normal cognition to mild cognitive impairment, followed by mild-to-moderate-to-severe dementia. Although most cases are sporadic and occur later in life (typically affecting adults 65 years and older), 5%–6% of AD patients experience early onset disease (i.e. prior to 65 years and typically associated with genetic predisposition) (Mendez, 2019). Despite more than 100 years of research, the exact cause(s) of AD are not well understood. Characteristic pathological features include abnormal accumulation of amyloid-β in plaques and hyperphosphorylated-tau in neurofibrillary tangles, which are related to massive losses of synapses, dendrites, and eventually neurons (Livingston et al., 2020).
The series of discoveries that BFCS neurons and axonal projections undergo severe degeneration in the course of AD was a key breakthrough in early studies of the disease (Bartus et al., 1982; Hampel et al., 2018). Significant neurodegeneration in the PPT and LDT in AD (Jellinger, 1988) or no alterations (Woolf et al., 1989) have been reported. In addition, local interneurons representing the cholinergic system within the striatum, also undergo some degree of neurodegeneration in AD (Selden et al., 1994). ‘The cholinergic hypothesis’ of age-related cognitive dysfunction was introduced in the early 1980s (Bartus et al., 1982). This hypothesis is related to the loss or dysfunction of BFCS neurons contributing to the dementia observed in AD patients (Davies & Maloney, 1976; Perry et al., 1978). These discoveries resulted in the development of cholinergic drugs—reversible and competitive AChE inhibitors for the symptomatic treatment of AD patients (Birks, 2006; Hampel et al., 2018). Results from pre-clinical (animal) models of AD also indicate a role for cholinergic dysfunction (e.g. cholinergic neuronal loss and abnormalities in choline transport, ACh secretion, and nAChRs) in cognitive decline, and the effectiveness of galantamine and other AChE inhibitors in improving cognitive, behavioral, and other pathological features (Bales et al., 2006; Bhattacharya et al., 2014; Dong et al., 2005; Watanabe et al., 2009) (Table 1). Brain mAChR-mediated signaling has also been actively explored in the search for treatment options for AD (Scarpa et al., 2020). Because of the predominant postsynaptic expression of M1 mAChR (on cholinoceptive neurons) in brain regions innervated by BFCS and observations that it is preserved in the disease course, M1 mAChR has been a major molecular therapeutic target in AD (Scarpa et al., 2020). Recently, the focus has been on developing M1 mAChR positive allosteric modulators, including BQCA, which bind to allosteric sites of the receptor molecule and facilitate the selective action of ACh on the orthosteric site. These compounds with remarkable M1 mAChR selectivity have shown some encouraging results in improving cognitive impairment and exerting disease-modifying effects in AD, possibly related to reducing pathogenic amyloid processing (Scarpa et al., 2020). Since the mode of action of these compounds requires a certain supply of endogenous ACh, their effectiveness may become limited because of the loss of cholinergic neurons with AD progression. Three AChE inhibitors are currently approved for AD by the U.S. FDA—donepezil, rivastigmine, and galantamine (Haake et al., 2020; Hampel et al., 2018). Although galantamine was the last AChE inhibitor to be approved by the FDA, it has been extensively studied and its efficacy in treating mild to moderate AD patients is indicated (Lilienfeld, 2002; Nakagawa et al., 2017; Prvulovic et al., 2010; Richarz et al., 2014) (Table 2). Galantamine’s safety and tolerability are well-established for the recommended maintenance doses of 16mg and 24mg per day and the higher dose of 32mg per day; the most common adverse effects are gastrointestinal (GI) issues, which are experienced with higher doses (Birks, 2006; Haake et al., 2020; Lilienfeld, 2002; Nakagawa et al., 2017; Prvulovic et al., 2010; Richarz et al., 2014). In a study with 1,024 galantamine- and 1,021 placebo-treated patients with mild to moderate AD, two-year drug treatment significantly reduced mortality and cognitive decline, and improved daily living activities (Hager et al., 2014). In a recent meta-analysis of randomized controlled trials with currently prescribed AChE inhibitors and memantine—an N-methyl-D-aspartate (NMDA) receptor antagonist, galantamine was proposed as the ‘first choice’ for treating AD based on the effects of these compounds on cognitive, functional, behavioral, and global changes in AD patients (Li et al., 2019).
TABLE 1.
Therapeutic effects of galantamine in pre-clinical/animal models
| Disease/condition | Study | |
|---|---|---|
| Alzheimer’s disease | (Wu et al., 2015) | • Reduced total area of amyloid load within the hippocampus of transgenic APP/PS1 mice; suppressed astrocyte activation and decreased intracellular TNF and IL-6 expression |
| (Van Dam et al., 2005) | • Symptomatic benefit for cognitive impairment in APP23 mice | |
| (Van Dam & De Deyn, 2006) | • Improved spatial accuracy during probe trial in; disease-modifying effects of galantamine in transgenic APP23 mice | |
| (Bhattacharya et al., 2014) | • Improved cognitive and behavioral symptoms and suggested disease-modifying and neuroprotective effects indicated by delayed Aβ plaque formation and reduced gliosis in 5X Familial AD (5XFAD) mouse model | |
| (Capsoni et al., 2002) | • Protective effects on the basal forebrain cholinergic deficit; no effect on tau hyperphosphorylation; suppressed formation of extracellular deposition of Aβ in AD11 mice | |
|
| ||
| Parkinson’s disease | (Yanagida et al., 2008) | • Neuroprotective effects of a combination of galantamine and nicotine against 6-hydroxydopamine (6-OHDA)-induced dopaminergic neuronal loss through suggested allosteric α7nAChR activation |
|
| ||
| Schizophrenia | (Hohnadel et al., 2007) | • Improved prepulse inhibition (PPI) deficits in three PPI disruption rat models AChE inhibitors, indicating the potential to improve cognition in schizophrenia by improving auditory sensory gating |
| (Wang et al., 2007) | • Alleviated negative symptom of social withdrawal in animals (in synergy with risperidone) mediated by dopamine-D(1) receptors in the medial prefrontal cortex through nAChR activation-increased dopamine release | |
|
| ||
| Endotoxemia | (Pavlov et al., 2009) | • Suppressed serum TNF levels and improved survival through a brain mAChR-, vagus nerve-, and α7nAChR-mediated mechanisms |
| (Liu, Zhang, et al., 2018) | • Improved deficits in special memory and learning; inhibited hippocampal microglial activation and inflammation | |
|
| ||
| IBD (colitis) | (Ji et al., 2014) | • Suppressed inflammation and alleviated disease severity through a brain mAChR-mediated and vagus nerve to spleen signaling mechanisms |
| (Wazea et al., 2018) | • Anti-inflammatory, anti-apoptotic, and anti-oxidant effects with peripheral α7nAChR and JAK2/SOCS3 signaling pathway involvement | |
|
| ||
| Obesity and Metabolic syndrome | (Satapathy et al., 2011) | • Decreased inflammation (lower plasma IL-6 and leptin); reduces glucose and insulin levels, and alleviates insulin resistance; lower cholesterol levels; ameliorated hepatic steatosis |
|
| ||
| Type 1 diabetes | (Hanes et al., 2015) | • Delayed onset of hyperglycemia; attenuated immune cell infiltration in pancreatic islets; decreased serum anti-insulin antibodies |
|
| ||
| Diabetes (n5-STZ and cafeteria diet rat model) | (Ali et al., 2015) | • Anti-inflammatory, anti-oxidative, and anti-apoptotic effects; galantamine effects better than vildagliptin (anti-diabetic drug); best effects with the combination regimen |
|
| ||
| Arthritis (adjuvant-induced) | (Gowayed et al., 2015) | • Suppressed inflammation and alleviated disease severity in a rat model; galantamine effects superior than the reference drug leflunomide |
|
| ||
| Cancer (colon) | (Sammi et al., 2018) | • Decreased aberrant crypt foci count; altered inflammatory and oxidative profiles |
|
| ||
| Traumatic brain injury | (Njoku et al., 2019) | • Alleviated cognitive deficits; improved recovery after experimental brain trauma |
|
| ||
| Postoperative cognitive dysfunction | (Wang et al., 2018) | • Alleviated cognitive dysfunction; suppressed neuroinflammation |
|
| ||
| Neonatal hypoxia ischemia | (Odorcyk et al., 2017) | • Ameliorated brain damage; increased survival of neurons; reduced astrocytic reactivity; increased catalase activity |
|
| ||
| Spinal cord injury | (Sperling et al., 2019) | • Improved functional recovery; reduces lesion size |
|
| ||
| Huntington’s Disease | (Park et al., 2008) | • Attenuated neurodegeneration |
|
| ||
| Substance abuse disorders | (Koseki et al., 2014) | • Suppressed reinstatement of methamphetamine-seeking behavior in rats |
| (Ashare et al., 2016) | • Reduced nicotine reinforcement in rats | |
|
| ||
| Down syndrome | (de Souza et al., 2011) | • Improved olfactory learning in mice |
|
| ||
| Nerve gas (soman, sarin) and organophosphorus insecticide poisoning | (Albuquerque et al., 2006) | • Significant protection against the acute toxicity and lethality of soman, sarin, and paraoxon (the active metabolite of the insecticide parathion) in guinea pigs; higher efficiency than pyridostigmine, a peripherally acting AChE inhibitor |
| (Pereira et al., 2010) | • Counteracted lethality and acute toxicity of soman administration to guinea pigs; 100% survival attained when galantamine and atropine administered 30–45 min after LD50 soman | |
| (Golime et al., 2018) | • Increased levels of neuroprotective genes in brain and alleviated soman toxicity and lethality | |
| (Lane et al., 2020) | • Prevented acute toxicity of supra-lethal doses of soman in non-human primates | |
TABLE 2.
Therapeutic effects of galantamine in clinical (human) settings
| Disease/condition | Study | Effect |
|---|---|---|
| Poliomyelitis | (Revelli & Graso, 1962) | • Symptomatic relief |
|
| ||
| Myasthenia gravis | (Uzunov, 1966) | • Symptomatic relief |
|
| ||
| Alzheimer’s disease | (Tariot et al., 2000) | • Significantly improved cognitive, functional, and behavioral symptoms at 16 and 24 mg/day compared with placebo; dose escalation enhances the drug tolerability |
| (Wilkinson & Murray, 2001) | • Significant improvements of Assessment Scale Cognitive Subscale, Clinical Global Impression of Change, and Progressive Deterioration Scale scores compared with placebo; well-tolerated at 18 and 24 mg/day with mild, transient effects typical of cholinomimetic agents | |
| (Kaufer et al., 2005) | • Decreased caregiver burden | |
|
| ||
| Myocardial Infarction in Alzheimer’s disease | (Nordstrom et al., 2013) | • Reduced risk of myocardial infarction and death in patients with AD treated with galantamine or other AChEs with stronger correlations with higher doses |
|
| ||
| Stroke in Alzheimer’s disease | (Lin et al., 2016) | • Decreased risk of ischemic stroke in patients with dementia without previous ischemic stroke history treated with galantamine or other AChE inhibitors |
| (Tan et al., 2018) | • Reduced risk of ischemic stroke and death in patients with dementia on galantamine and other AChE inhibitors | |
|
| ||
| Type 2 diabetes in Alzheimer’s disease and mix-pathology dementia | (Secnik et al., 2020) | • Decreased mortality in patients with diabetes and AD or mixed-pathology dementia treated with AChE inhibitors; galantamine and donepezil associated with largest benefits |
|
| ||
| Parkinson’s disease with dementia | (Aarsland et al., 2003) | • Improvements of global mental symptoms in most patients (worsening in some); alleviated hallucinations; improved cognitive (clock-drawing) function |
| (Liu, Wile, et al., 2018) | • Improved cognitive functions scores; improved hallucinations, anxiety, sleep disturbance, and apathy; Improvements in gait and decreases in freezing and falls; decreased levels of distress in patients’ relatives and improvements in daily activity | |
|
| ||
| Schizophrenia | (Buchanan et al., 2008) | • Differential benefits for aspects of processing speed and verbal memory |
| (Schubert et al., 2006) | • Improved memory and attention in patients who are stabilized on risperidone | |
| (Conley et al., 2009) | • Benefit on alogia, but not on other negative symptoms | |
| (Choueiry et al., 2019) | • in combination with CDP-choline, an α7nAChR agonist, galantamine improved gaiting indices, and improved impaired inhibition of the testing stimulus; suggested α7nAChR -mediated modulation of speech gaiting indices | |
| (Koola et al., 2020) | • Alleviated cognitive impairments (a meta-analysis of six randomized controlled trials) | |
|
| ||
| Metabolic syndrome | (Consolim-Colombo et al., 2017) | • Suppressed inflammation (decreased pro-inflammatory cytokines, leptin, increased IL-10 and adiponectin); reduced glucose and insulin levels, and alleviated insulin resistance; Improved heart rate variability |
| (Sangaleti et al., 2020) | • Alleviated oxidative stress | |
|
| ||
| Traumatic brain injury | (Tenovuo, 2005) | • Alleviated cognitive deficits; improved vigilance |
|
| ||
| Autism Spectrum Disorders | (Ghaleiha et al., 2014) | • Improvement in the irritability and Lethargy/Social Withdrawal subscales with no significant difference in side effects compared with placebo |
|
| ||
| Huntington’s Disease | (Petrikis et al., 2004) | • Improved motor and psychiatric symptoms |
|
| ||
| Substance abuse disorders | (Ashare et al., 2016) | • Suppressed smoking behavior |
| (Sugarman et al., 2019) | • Modest improvement in cognitive outcomes in patients with cannabis use disorder | |
|
| ||
| Lucid dreaming | (LaBerge et al., 2018) | • Increased frequency in a dose-dependent manner |
Recent studies have provided important insights into the BFCS degenerative alterations in the chronology of AD pathogenesis. Imaging studies have shown that BFCS and especially NbM degeneration occurs very early in the course of the disease, even prior to the onset of cognitive decline (Fernández-Cabello et al., 2020; Schmitz & Spreng, 2016). There is experimental evidence that reduced basal forebrain volume associated with BFCS neurodegeneration occurs before pathological alterations in the entorhinal cortex and predicts the spread of the disease to cortical areas. These observations challenge the dogmatic view that AD is a disease of cortical origin. They also identify forebrain cholinergic signaling as an early therapeutic target for intervention before cognitive deterioration is detected (Fernández-Cabello et al., 2020; Schmitz & Spreng, 2016). In this context, it is intriguing and important to consider administering galantamine or other AChE inhibitory treatments very early, even prior to cognitive decline in AD.
Neuroinflammation characterized by excessive and sustained microglial and astrocyte activation is recognized as the third major feature of AD pathogenesis, which substantially contributes to disease progression and severity (Heneka et al., 2015; Kinney et al., 2018) (Figure 2). Positron emission tomography (PET) scan imaging and studies of post-mortem AD brains show activated microglia and reactive astrocytes around amyloid beta plaques (Heneka et al., 2015). In addition, studies of cerebral spinal fluid (CSF) reveal increasing levels of inflammatory biomarkers (e.g. YKL-40 (tyrosine (Y), lysine (K), and leucine (L) protein-40kDa), ICAM-1 (intercellular adhesion molecule-1), VCAM-1 (vascular cell adhesion molecule-1), IL-15, and Flt-1 (fms-like tyrosine kinase-1)) across the continuum of AD from the subclinical stage to severe dementia, with an elevation in these biomarkers associated with an increased risk of developing dementia (Janelidze et al., 2018). Interestingly, high levels of these CSF markers are associated with cortical thinning in the precuneus and superior parietal regions and with consequent cognitive decline in AD patients who previously had no dementia (Janelidze et al., 2018). A link between BFCS neurodegeneration and an inflammatory phenotype in a tau transgenic mouse model of AD was recently reported (Cranston et al., 2020). Mice expressing a truncated tau fragment exhibit AD-like pathology that follows a Braak-like progression with cognitive impairment (Cranston et al., 2020). Their brains show progressive loss of ChAT-expressing neurons in the BFCS after 3, 6, and 9 months accompanied by decreased AChE staining in the cortex and hippocampus, as well as spatial learning deficits. In parallel, increased microglial IL-1β expression in the hippocampus, and increased microglial staining (Iba1) in the basal forebrain, hippocampus and entorhinal cortex at 6 months were also identified (Cranston et al., 2020). A recent study with presymptomatic AD subjects reported a correlation between loss of basal forebrain cholinergic integrity (manifested as longitudinal decreases of basal forebrain volume) and increased biomarkers of inflammation and abnormal amyloid and tau pathology (Schmitz et al., 2020). These findings suggest the possibility that an age-associated loss of brain cholinergic anti-inflammatory neuromodulation diminishes microglial reactivity to amyloid and tau (Schmitz et al., 2020). Galantamine treatment reduces astrocyte activation and astrocyte TNF and IL-6 levels, and amyloid deposition, and improves memory and learning in the mouse APP/PS1 (amyloid precursor/presenilin 1) model of AD (Wu et al., 2015) (Table 1).
Peripheral inflammation, determined by increased levels of circulating cytokines, including TNF, IL-6, IL-1β, TGF-β, IL-12, and IL-18 and higher TGF-β levels in the CSF have been detected in AD patients (Swardfager et al., 2010). Peripheral inflammation interferes with immune responses in the brain and exacerbates neuroinflammation in AD (Heneka et al., 2015) (Figure 2). There is a growing interest in studying anti-inflammatory strategies, including TNF inhibitors for alleviating brain pathology and cognitive dysfunction in AD, as indicated by preclinical studies and ongoing clinical trials (Decourt et al., 2017). Obesity, metabolic syndrome, and type 2 diabetes also have been associated with increased risk for AD and the characteristic low-grade chronic inflammation interrelated with insulin resistance and other metabolic derangements in these conditions may provide an important link (Chang et al., 2019; Heneka et al., 2015; Secnik et al., 2020). Galantamine suppresses pro-inflammatory cytokine release and inflammation in preclinical models of many conditions, including obesity, metabolic syndrome, and diabetes (Ali et al., 2015; Hanes et al., 2015; Pavlov & Tracey, 2015; Satapathy et al., 2011) (Table 1). Galantamine, in doses clinically approved for AD, also alleviates inflammation, oxidative stress and insulin resistance in patients with metabolic syndrome (Consolim-Colombo et al., 2017; Sangaleti et al., 2020) (Table 2). Anti-inflammatory effects of galantamine, rivastigmine and donepezil in AD patients have also been reported (Blasko et al., 2007; Reale et al., 2004). PBMCs isolated from AD patients treated with the AChE inhibitor, Donepezil, express lower circulating levels of TNF, IL-1β, and IL-6 and higher levels of IL-4 compared with age-matched controls (Reale et al., 2004). A recent study demonstrated that galantamine and donepezil treatments are associated with reduced mortality in AD patients with and without diabetes (Secnik et al., 2020). In 7,073 AD subjects the use of AChE inhibitors, including galantamine, was associated with a 34% lower risk for myocardial infarction and a lower risk of death; patients taking the highest doses of galantamine (24 mg), donepezil (10 mg), and rivastigmine (>6 mg) had the lowest risk of myocardial infarction (Nordstrom et al., 2013) (Table 2). Another study, evaluating the risk of stroke and death in 44,288 patients with dementia reported significantly reduced risks in patients treated with AChE inhibitors, including galantamine, compared with age-matched controls (Tan et al., 2018). Together these findings strongly suggest that the anti-inflammatory effects of galantamine substantially contribute to its overall effectiveness in AD disease (Figure 2). They also justify further therapeutic exploration of galantamine for broader benefits in AD patients.
5.2 |. Parkinson’s disease (PD)
Parkinson’s disease (PD) is a chronic neurodegenerative disorder characterized by the loss of dopamine-producing neurons in the substantia nigra (Armstrong & Okun, 2020; Conti et al., 2018; Ztaou & Amalric, 2019). This leads to tremors, reduced limb mobility, slowed movement and changes in gait and balance, as well as non-motor symptoms, including depression, anxiety, visual hallucinations, REM (rapid eye movement) sleep behavior disorder and dysphagia, as well as cognitive decline; many symptoms are typically observed in the later stages of PD (Armstrong & Okun, 2020). PD affects more men than women and age is a risk factor—the average age of diagnosis is approximately 62 years (Pagano et al., 2016). There are numerous disease variants, including genetic and idiopathic variants, with variable motor and non-motor pathologies and prognoses (Armstrong & Okun, 2020). Substantia nigra dopaminergic neurons innervate the striatum, a major input structure of the basal ganglia. Striatal cholinergic interneurons integrate a complex microcircuitry regulating movement by receiving neurotransmitter input via dopamine, glutamate, and serotonin receptors and then relaying resultant signals to postsynaptic mAChRs and nAChRs (Conti et al., 2018; Ztaou & Amalric, 2019). Degeneration of substantia nigra neurons innervating the striatum was linked to insufficient input to striatal cholinergic interneurons and increased local cholinergic tone (Conti et al., 2018). The ‘dopamine-ACh balance hypothesis’ describes the decreased ratio of dopaminergic neurons to cholinergic interneurons within the striatum as a major contributing factor to the motor and non-motor pathologies of PD (Conti et al., 2018). Prior to the discovery of L-Dopa, anticholinergic drugs were used in the treatment of PD patients (Ztaou & Amalric, 2019). However, anticholinergics do not improve akinesia or bradykinesia and may increase the risk of dementia and neuropsychiatric complications (Conti et al., 2018; Ferreira et al., 2013). This consideration is in line with imaging studies demonstrating cholinergic dysfunction and moderate to severe deficits in cortical ACh levels associated with cognitive impairment in PD. Structural MRI and cognitive testing reveals that a decreased volume of BFCS nucleus basalis of Meynert area, followed by a significant decrease in the medial septum/diagonal band volume, precedes and predicts future dementia in patients with PD (Pereira et al., 2020) (Figure 2). PD is related with gait dysfunction and an associated risk for falls remains, and dopamine replacement therapy provides incomplete beneficial effects. A recent study utilizing selective chemogenetic modulation of striatal cholinergic interneurons indicated their key role in turning behavior and traversing dynamic surfaces; stimulation of these interneurons in a rat model of PD improves turning performance and reduced fall rates (Avila et al., 2020).
Animal studies, postmortem brain evaluation, brain imaging, and epidemiological studies highlight the role of neuroinflammation in PD pathogenesis (Gelders et al., 2018). Post-mortem studies of PD brains dating back to the late 1980s reveal microglial activation (McGeer et al., 1988). In accordance, studies of CSF from patients with PD show elevated IL-1β, IL-6, IFNγ, and TNF levels (Gelders et al., 2018). The presence of activated microglia in living PD patients has been confirmed using PET imaging with tracers specific for activated microglia cells (Gerhard et al., 2006). The presence of neuroinflammation, as determined using PET imaging for microglial cell activation, was also indicated in patients with idiopathic REM sleep behavior, a condition experienced by patients who eventually develop PD with synucleinopathies (Stokholm et al., 2017). This study suggests that neuroinflammation precedes PD, in at least some patients, pointing to neuroinflammation as a potential therapeutic target for preventing or delaying onset and/or progression. The relationship between neuroinflammation and neurodegeneration in PD is an area of active research; improved understanding of the causality of this relationship and its chronology will likely lead to more targeted therapies. The genetic form of PD known as LRRK2 (Leucine Rich Repeat Kinase 2 (gene))-associated PD is accompanied by increased ACh hydrolysis in the cortical region (Liu, Wile, et al., 2018). In addition, the LRRK2 variant is proposed to be accompanied by neuroinflammation, as LRRK2 is highly expressed in activated microglial cells and LRRK2 modulates microglial cell inflammatory responses (Brockmann et al., 2016). Thus, treatments with galantamine or other centrally acting AChE inhibitors may specifically benefit this subset of PD patients.
A few small clinical trials support the beneficial effects of galantamine in PD patients (Table 2). In an open-label trial 16 women with PD plus dementia treated with galantamine (16mg per day maintenance dose) for 8 weeks showed improved cognition (in 62% of patients), hallucinations (in 78%), and motor symptoms (in 46%) when compared to pre-treatment levels (Aarsland et al., 2003). The safety and efficacy of galantamine for treating dementia in PD patients was also assessed in a small open-controlled trial with 21 patients and 20 controls (Litvinenko et al., 2008). A maximum dose of 16mg of galantamaine per day led to improved cognitive, neuropsychiatric, and motor symptoms over 24 weeks. When compared to their pre-treatment levels, patients receiving galantamine showed improvements in hallucinations, anxiety levels, sleep disturbances, daily activities, and gait. However, drug treatment did not significantly alter tremors. A Cochrane review (meta-analysis) of six randomized, double-blind, placebo-controlled trials testing AChE inhibitors in PD patients with dementia or cognitive impairment concluded that AChE inhibitor treatment led to statistically significant improvements in global assessment, cognitive function, behavioral changes, and daily living activities (Rolinski et al., 2012). A more recent systematic review (published in mid-2014) of prospective, randomized controlled trials of cholinesterase inhibitors in 941 PD patients reported significant cognitive improvements and reduced death rates among patients taking AChE inhibitors; of note, AChE inhibitor treatment did not affect tremors or fall rates (Pagano et al., 2015). PD and other neurological conditions have been linked to alterations in gut microbiota as very recently summarized (Santos et al., 2019). Preclinical studies have demonstrated that alterations in gut microbiota mediate motor deficits, microglia activation, and brain pathology consistent with neuroinflammation, suggesting that targeting the microbiome via probiotics may reduce neuroinflammation (Sampson et al., 2016). It is intriguing to relate these findings to regulation of the gut microbiome as a therapeutic option for altering PD brain pathology. In this respect vagus nerve cholinergic regulation of GI permeability and inflammation and galantamine efficacy in alleviating pathology in the metabolic syndrome—a disorder also linked to dysregulated gut microbiota, indicate novel therapeutic approaches (Consolim-Colombo et al., 2017; Pavlov & Tracey, 2012). Better understanding of cholinergic dysfunction in the various subtypes of PD and mild to moderate disease may lead to earlier treatment with galantamine and other AChE inhibitors and more effective therapeutic strategies. Furthermore, combination therapies that target pathologies should be explored for PD and other complex chronic conditions.
5.3 |. Schizophrenia
Schizophrenia is a complex and chronic neuropsychiatric illness characterized by hallucinations and delusions (also known as positive symptoms), social withdrawal and flat affect (also known as negative symptoms), disorganized speech and alogia (or poverty of speech), as well as cognitive dysfunction (Kahn et al., 2015; Marder & Cannon, 2019). Although the onset of schizophrenia can occur at any time during one’s life, onset typically occurs in the late teens to early 20’s in men and somewhat later in women (Gogtay et al., 2011). Consistent with its early onset, schizophrenia is proposed to result from a complex interplay of genetic, sex, environmental, and other factors (e.g. paternal age, migration status, drug abuse, and social adversity) that are proposed to impact early brain development (Kahn et al., 2015; Marder & Cannon, 2019). In the absence of understanding the underlying causes of this disease, patients continue to be treated with therapies that target symptoms rather than mechanisms of pathology. Numerous studies support ‘neurotransmitter-based hypotheses’, including alterations in the glutamatergic, dopaminergic, and cholinergic systems in schizophrenia. Abnormalities of the cholinergic system in schizophrenia were first indicated during the 1950s and 1960s, when it was discovered that administration of irreversible AChE inhibitors could activate psychotic-like episodes in healthy controls and patients with schizophrenia (Gershon & Shaw, 1961; Rowntree et al., 1950). The ‘cholinergic imbalance hypotheses’ were proposed in the etiology of schizophrenia, tardive dyskinesia, and Huntington’s chorea in 1975 (Davis et al., 1975). Post-mortem analyses of schizophrenic brains revealed a negative correlation between ChAT activity in the parietal cortex and severity of cognitive deficits (Powchik et al., 1998). A role for altered brain cholinergic signaling in cognitive impairment observed in patients with schizophrenia has been described (Berman et al., 2007). Results of postmortem brain studies and neuroimaging of schizophrenia patients reveal global reductions in cholinergic signaling and a decrease in M1 mAChR and M4 mAChR expression in the hippocampus, striatum and cortex (Dean & Scarr, 2020; Scarr et al., 2018). Consistent with these observations, centrally acting M1 mAChR and M4 mAChR agonists have been proposed for treating cognitive impairment and other core features of schizophrenia (Dean & Scarr, 2020; Jones et al., 2012). Of note, in a double-blind, placebo-controlled trial with 20 schizophrenia patients, treatment with xanomeline, an M1/M4 mAChR agonist, significantly alleviated the positive and negative symptoms, and improved cognition—more specifically verbal learning and short-term memory (Anantha Shekhar et al., 2008). It is important to note that xanomeline was recently shown to significantly suppress inflammation within the inflammatory reflex (Rosas-Ballina et al., 2015). As elaborated later in this section, accumulating evidence indicates a role for inflammation as a driver of pathogenesis in schizophrenia and thus, presents a reasonable therapeutic target (Kirkpatrick & Miller, 2013; Meyer et al., 2011). Therefore, it is interesting and important to consider that the anti-inflammatory effects of xanomeline may contribute to its remarkable effectiveness in treating schizophrenia. Targeting nAChRs and specifically α7nAChRs via small molecule agonists in schizophrenia is also currently being explored (Gibbons & Dean, 2016; Jones et al., 2012). As in AD, the focus has now shifted from orthosteric mAChR and nAChR agonists to newer generations of positive allosteric modulators with improved selectivity and fewer side effects (Jones et al., 2012). Inhibition of AChE activity (by AChE inhibitors, such as galantamine) to effectively raise ACh levels at the synaptic cleft is another approach to improve cholinergic signaling and cognitive function in schizophrenia (Gibbons & Dean, 2016).
Immune dysfunction, inflammation, and neuroinflammation in schizophrenia have been described for decades (Müller, 2018) and elevated levels of cytokines in blood and cerebrospinal fluid have been demonstrated in schizophrenia patients (Müller, 2018). In addition, viral infections and autoimmunity have been described in the setting of schizophrenia since the 1980s (Ganguli et al., 1987; Torrey & Peterson, 1973). It is now recognized that peripheral inflammation and neuroinflammation significantly contribute to the pathogenesis of schizophrenia and can be therapeutically targeted for alleviating disease pathology (Buckley, 2019; Meyer et al., 2011). Similar to other conditions, it has been proposed that inflammatory alterations may be early indicators of schizophrenia-related pathology (Feigenson et al., 2014; Kahn & Sommer, 2015; Monji et al., 2013). Neuroinflammation and specifically increased microglial activity in schizophrenia have been identified in post-mortem studies (Trépanier et al., 2016). However, a review of four studies using PET imaging with tracers specific for ligands expressed on activated microglial cells summarizes the failure to reliably detect neuroinflammation in living schizophrenia patients (Pasternak et al., 2016). Developing more refined neuroimaging approaches in the future may shed light on this discrepancy.
The first-line of treatment in schizophrenia are antipsychotic medications. Several case reports support the potential beneficial effects of adding galantamine for treating the negative symptoms of schizophrenia (Arnold et al., 2004; Ochoa & Clark, 2006). However, clinical trials have shown mixed results. For example, treatment of schizophrenic subjects (n = 24) with either placebo or galantamine (16 mg per day maintenance dose) in addition to their antipsychotic medication for 12 weeks resulted in slight, but not significant improvements in cognitive function (Lee et al., 2007). Treating stable adult schizophrenia patients with galantamine (32 mg per day maintenance dose) versus, placebo for eight weeks (n = 20) did not significantly alter attention performance or motor speed and actually worsened performance on executive function and memory tasks (Dyer et al., 2008). In contrast, treatment of 16 male and 1 female subjects with schizophrenia, who were stabilized on risperidone, with galantamine (24 mg per day maintenance dose versus, placebo) for two months significantly improved short-term memory and attention (Schubert et al., 2006) (Table 2). Galantamine treatment of subjects with chronic schizophrenia with stable positive and negative symptoms (n = 42, 24 mg per day maintenance dose) versus. placebo (n = 38) for 12 weeks significantly improved alogia (poverty of speech), which has been linked to cognitive impairment (Conley et al., 2009). A recent meta-analysis assessed the results of nine randomized controlled trials for the efficacy of AChE inhibitors as an add-on treatment to antipsychotic medication on cognition in subjects with schizophrenia (Santos et al., 2018). Of note, only three of the nine studies employed galantamine; five studies used donepezil; and one study used rivastigmine. While six trials (with 219 subjects) reported that AChE inhibitors improved speed of processing, eight trials (with 252 subjects) found that placebo was better than AChE inhibitors for attention, and eight trials (n = 273) found no differences in placebo versus AChE inhibitor on working memory. However, in a meta-analysis of six randomized controlled trials, galantamine was shown to significantly alleviate cognitive impairment in patients with schizophrenia and to have substantial (although not statistically significant) beneficial effects on positive and negative symptoms (Koola et al., 2020). Recent randomized, double-blind, and placebo-controlled study demonstrated significantly reduced sensory gating impairments in 20 schizophrenia patients who received combined therapy of galantamine (16 mg) with CDP (cytidine diphosphate)-choline (500 mg) (Choueiry et al., 2019) (Table 2). These findings highlight the enhanced therapeutic utility of combining galantamine (an AChE inhibitor and a positive allosteric modulator of α7nAChR) with an α7nAChR agonist, that is, CDP-choline. They also point to the role of α7nAChR in this modulation that can be employed in future therapeutic strategies for schizophrenia. Combining galantamine with memantine, both FDA approved for treating AD, may also be a promising therapeutic approach in schizophrenia (Koola, 2018). However, it should also be considered that in addition to being an NMDA antagonist (designated as its principle mode of action), memantine has α7nAChR antagonistic properties (Aracava et al., 2005). Antipsychotic therapies, the first-line treatment for schizophrenia, have serious and significant side effects, including weight gain and obesity, dyslipidemia, hypercholesterolemia, hyperglycemia, insulin resistance, metabolic syndrome, diabetes, and cardiovascular disease (American Diabetes Association et al., 2004; Citrome & Volavka, 2004, 2005). It is reasonable to suggest combining antipsychotic therapy with galantamine for at risk patients based on previous findings demonstrating that galantamine improves lipid profiles in diet-induced obesity and insulin resistance in mice (Satapathy et al., 2011), diabetes in rodents (Ali et al., 2015; Hanes et al., 2015) and alleviates insulin resistance in obese patients with the metabolic syndrome (Consolim-Colombo et al., 2017; Sangaleti et al., 2020). Future multi-center controlled trials with uniform criteria, treatment strategies, and assessments should provide further insights into the effectiveness of galantamine, perhaps in combination with other drugs, in schizophrenia.
6 |. BROADENING THE SCOPE OF THE THERAPEUTIC UTILITY OF GALANTAMINE
Anti-inflammatory and disease-alleviating effects of galantamine have been reported in a growing number of conditions in both pre-clinical (Table 1) and clinical (Table 2) settings. Most of these conditions affect people across their entire lifespan and are not simply disorders of the aged.
The effectiveness of galantamine has been evaluated in animal models of diabetes and other diseases (Table 1). In non-obese mice with type 1 diabetes, galantamine treatment significantly delays the onset of hyperglycemia, decreases immune cell infiltration in the pancreatic islets, and lowers serum anti-insulin antibody levels (Hanes et al., 2015). Galantamine also exerts anti-inflammatory, anti-oxidative, anti-apoptotic and beneficial metabolic effects in a rat model of n5-STZ- induced diabetes (produced by injecting streptozotocin (STZ) on neonatal day 5 (n5)) (Ali et al., 2015). Of note, most of the effects of this cholinergic drug are of higher magnitude when compared with the anti-diabetic drug, vildagliptin, and the combined treatment results in additional synergistic improvements. In rats with experimental arthritis, galantamine treatment significantly alleviates inflammation (serum levels of anti-cyclic citrullinated peptide antibodies, TNF, and monocyte chemoattractant protein-1 (MCP-1)) and hind paw swelling (Gowayed et al., 2015). These effects are even more pronounced than the effect of the reference drug, leflunomide (Gowayed et al., 2015). In a model of 1, 2-dimethylhydrazine-induced colon cancer in rats, galantamine treatment decreases the number of aberrant crypt foci, and suppresses oxidative stress and inflammatory indices (Sammi et al., 2018).
In a model of traumatic brain injury in rats, galantamine treatment causes a significant reduction in the cortical lesion volume and restored behavioral flexibility (Njoku et al., 2019). Treatments with centrally acting AChE inhibitors, including galantamine of patients with chronic traumatic brain injury result in better vigilance and attention associated with improved general function (Tenovuo, 2005). In neonatal rats with hypoxia ischemia, pretreatment with galantamine protects against brain neuronal loss, decreases astrocyte hypertrophy and exerts anti-oxidative effects, which is associated with decreased brain damage (Odorcyk et al., 2017). In a rat model of acute spinal cord injury, galantamine treatment (5 mg/kg, i.p.) for five days significantly decreases lesion size, increases tissue survival, and accelerates hind limb motor function recovery (Sperling et al., 2019).
Based on experimental evidence from animal models and clinical observations it has been suggested that dysfunctional brain cholinergic signaling mediates some autism-associated behavioral manifestations (Kemper & Bauman, 1998; Mukaetova-Ladinska et al., 2010; Rossignol & Frye, 2014). Galantamine and other centrally acting AChE inhibitors have been studied in the treatment of children with autism spectrum disorder (Ghaleiha et al., 2014; Rossignol & Frye, 2014). Galantamine treatment improves some of the core and associated disease manifestations (Ghaleiha et al., 2014; Rossignol & Frye, 2014). Drug treatment (up to 24 mg per day, for 10 weeks) does not result in significant side effects compared with placebo in a randomized, double-blind, placebo-controlled study in children with autism spectrum disorder (Ghaleiha et al., 2014). There is some, albeit limited, evidence that galantamine may benefit patients with Huntington’s disease (Park et al., 2008; Petrikis et al., 2004). In a rat model of Huntington’s disease, galantamine reduces 3-nitropropionic (3NP) acid-induced neurologic deficits and brain neurodegeneration (Park et al., 2008). Galantamine treatment of a patient with the disease improved motor and psychiatric indices (Petrikis et al., 2004). Studies in substance abuse and addiction have also indicated beneficial effects of galantamine. Galantamine reduces nicotine reinforcement in rats and this drug treatment for two weeks significantly lowers smoking rates and inhibits smoking satisfaction and reward compared with placebo in humans (Ashare et al., 2016). Galantamine inhibits the reinstatement of methamphetamine-seeking behavior in mice, a finding that suggests that this drug may be useful in the treatment of relapses of methamphetamine-seeking behavior (Koseki et al., 2014). Brain cholinergic dysfunction has also been indicated in Down syndrome (Chen et al., 2009; Granholm et al., 2000). Galantamine treatment significantly improves olfactory learning in Ts(1716)65Dn mice with segmental trisomy for distal mouse Chr 16 (a mouse model of Down syndrome known as Ts65Dna), which suggests the potential of this cholinergic drug to improve cognition in Down syndrome patients with dementia (de Souza et al., 2011; Prasher, 2004). To our knowledge, no randomized controlled trials of galantamine in people with Down syndrome have been undertaken (Mohan et al., 2009), supporting the need for further research.
Brain cholinergic signaling is implicated in the neurobiology of lucid dreaming—a state of consciousness of being aware that one is dreaming while dreaming (Baird et al., 2019; LaBerge et al., 2018). As previously reviewed, lucid dreaming can be explored in the treatment of persistent nightmares, narcolepsy, to establish brain activity markers of self-awareness, and to provide unique insights into neuropsychiatric conditions (Baird et al., 2019). Pre-sleep treatment with galantamine (4 mg or 8 mg per day) dose-dependently increases the frequency of lucid dreams compared with placebo in a double blind study with 121 participants (LaBerge et al., 2018). While the exact mechanism(s) by which galantamine stimulates lucid dreams are unclear, activating of brain cholinergic mechanisms that results in stimulation of REM sleep intensity and phasic activation, and enhanced cognitive processing may play a role (Baird et al., 2019; LaBerge et al., 2018).
Exposure to organophosphorus compounds, including nerve agents such soman, sarin, VX, and tabun, insecticides, and pesticides can be lethal, but effective antidotal treatments are not available. Most of the toxicity of these compounds is because of their irreversible inhibition of AChE. Galantamine has been shown to significantly counteract soman, sarin and paraoxon (the active metabolite of the insecticide parathion) acute toxicity and lethality (Albuquerque et al., 2006). Galantamine exerts greater efficacy than the peripherally acting AChE inhibitor pyridostigmine, and is less toxic than huperzine, another centrally acting AChE inhibitor (Albuquerque et al., 2006). Galantamine administration to guinea pigs just prior to or even up to 15 min after exposure to these nerve gas components significantly alleviates toxicity and improves survival (Pereira et al., 2010). A direct comparison of galantamine and other centrally acting AChE inhibitors, including donepezil, rivastigmine, and huperzine A identified galantamine as a superior antidotal therapy for soman toxicity in guinea pigs (Aracava et al., 2009). A very recent study reported that a clinically relevant oral dose of galantamine prevented soman-induced lethality and alleviated neuropathology in Cynomolgus monkeys post-treated with conventional antidotes (Lane et al., 2020). Of note, these effects were superior to the effects of the peripherally acting AChE inhibitor pyridostigmine, approved as pretreatment for military personnel at risk of exposure to soman. These and other reported findings in rodents and non-human primates (Golime et al., 2018; Hamilton et al., 2017; Hilmas et al., 2009) are of considerable interest for developing galantamine as an antidote for organophosphate poisoning.
7 |. CONCLUSIONS
During the last decade our understanding of the regulatory functions of neural cholinergic signaling has substantially evolved and improved. Cholinergic mechanisms have been indicated in the regulation of immunity, inflammation and metabolism and galantamine and other cholinergic drugs in current clinical use for the treatment of neurological conditions have been characterized as anti-inflammatory agents. Galantamine is a centrally acting AChE inhibitor and proposed positive allosteric modulator of nAChRs that has been used for decades in the treatment of diseases characterized by cholinergic dysfunction. Galantamine was approved by the U.S. FDA in 2001 for treating mild to moderate AD and accumulating evidence indicates its efficacy in slowing the cognitive decline in patients with AD. Galantamine has also been used to treat patients with PD and shown to improve cognitive decline in some patients and to reduce death rates. Clinical trials with galantamine in patients with schizophrenia reveal mixed results, with benefits in short-term memory and attention in some patients. While patients diagnosed with AD and PD are usually above the age of 60 years, schizophrenia is typically diagnosed in late adolescence and early adulthood. In all three diseases, there is evidence for brain cholinergic dysfunction and cognitive deterioration, interrelated with neuroinflammation and peripheral inflammation. Relatively recently—about a decade ago studying the vagus nerve-based inflammatory reflex and cholinergic signaling in the regulation of immunity and inflammation led to the discovery of the anti-inflammatory properties of galantamine. This has now provided new perspectives for better understanding the effectiveness of galantamine in AD, PD, schizophrenia, and a growing number of other neurodegenerative and neurological conditions, including autism spectrum disorder. Active ongoing research continues to indicate the utility of galantamine in the treatment of conditions characterized by aberrant inflammation and metabolic dysregulation. This research progressed into recent clinical developments supporting the anti-inflammatory and other beneficial effects of galantamine in metabolic syndrome—a disorder of pandemic proportions. Future studies will continue evaluating galantamine as a single treatment or in combination with other pharmacological or device-generated therapeutic modalities (within the scope of bioelectronic medicine) in preventive and treatment strategies for conditions with neurological, inflammatory and metabolic etiology.
ACKNOWLEDGEMENTS
The authors thank Dr Kevin J. Tracey for his comments on this review. This work was supported in part by the following grants: R01GM128008, and R01GM121102
Funding information
National Institute of General Medical Sciences, Grant/Award Number: R01GM121102 and R01GM128008
Abbreviations:
- 3NP
3-nitropropionic acid
- 6-OHDA
6-hydroxydopamine
- acetyl-CoA
acetyl coenzyme A
- ACh
acetylcholine
- AChE
acetylcholinesterase
- AD
Alzheimer’s disease
- APP/PS1
amyloid precursor/presenilin 1
- α7nAChR
alpha 7 nicotinic acetylcholine receptor
- BFCS
basal forebrain cholinergic system
- BQCA
benzyl quinolone carboxylic acid
- BuChE
butyrylcholinesterase
- cAMP
adenosine 3′,5′-cyclic monophosphate
- CDP
cytidine diphosphate
- ChAT
choline acetyltransferase
- CNS
central nervous system
- CSF
cerebral spinal fluid
- DMN
dorsal motor nucleus
- FDA
Food and Drug Administration
- Flt-1
fms-like tyrosine kinase-1
- GI
gastrointestinal
- i.p.
intraperitoneal
- IBD
inflammatory bowel disease
- ICAM-1
intercellular adhesion molecule-1
- IFN-γ
interferon gamma
- IL
interleukin
- JAK/STAT3
Janus kinase/signal transducer and activator of transcription 3
- JAK2/SOC3
Janus kinase 2/suppressor of cytokine signaling-3
- L-dopa
levorotatory form of dopa
- LDT
laterodorsal tegmental
- LRRK2
Leucine Rich Repeat Kinase 2 (gene)
- mAChR
muscarinic acetylcholine receptor
- McN-A-343
4-[[[(3-Chlorophenyl}amino] carbonyl]oxy]-N,N,N-trimethyl-2-butyn-1-aminium chloride
- MCP-1
monocyte chemoattractant protein-1
- MRI
magnetic resonance imaging
- n5-STZ
streptozotocin (STZ) injected on neonatal day 5 (n5)
- NAA
nucleus ambiguous
- nAChR
nicotinic acetylcholine receptor
- NbM
Nucleus basalis of Meynert
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NMDA
N-methyl-D-aspartate
- PBMCs
peripheral blood mononuclear cells
- PD
Parkinson’s disease
- PET
positron emission tomography
- PMNs
polymorphonuclear neutrophils
- PPI
prepulse inhibition
- PPT
pedunculopontine tegmental
- REM
rapid eye movement
- TGF-β
transforming growth factor beta
- TNF
tumor necrosis factor
- Ts65Dn
Ts(1716)65Dn line of mice with segmental trisomy for distal mouse Chr 16
- VAChT
vesicular acetylcholine transporter
- VCAM-1
vascular cell adhesion molecule-1
- YKL-40
tyrosine (Y), lysine (K), and leucine (L) protein-40kDa
Footnotes
CONFLICT OF INTEREST
Valentin A. Pavlov is an author on patents broadly related to the content of this review and has assigned his rights to the Feinstein Institutes for Medical Research. Christine N. Metz declares that the work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Aarsland D, Hutchinson M, & Larsen JP (2003). Cognitive, psychiatric and motor response to galantamine in Parkinson’s disease with dementia. International Journal of Geriatric Psychiatry, 18, 937–941. 10.1002/gps.949 [DOI] [PubMed] [Google Scholar]
- Abudukeyoumu N, Hernandez-Flores T, Garcia-Munoz M, & Arbuthnott GW (2019). Cholinergic modulation of striatal microcircuits. European Journal of Neuroscience, 49, 604–622. 10.1111/ejn.13949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinci SB, Ulu N, Yondem OZ, Firat P, Guc MO, Kanbak M, & Aypar U (2005). Effect of neostigmine on organ injury in murine endotoxemia: Missing facts about the cholinergic antiinflammatory pathway. World Journal of Surgery, 29, 1483–1489. 10.1007/s00268-005-0073-2 [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EFR, Alkondon M, & Rogers SW (2009). Mammalian nicotinic acetylcholine receptors: from structure to function. Physiological Reviews, 89, 73–120. 10.1152/physrev.00015.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EFR, Aracava Y, Fawcett WP, Oliveira M, Randall WR, Hamilton TA, Kan RK, Romano JA, & Adler M (2006). Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents. Proceedings of the National Academy of Sciences of the United States of America, 103, 13220–13225. 10.1073/pnas.0605370103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MA, El-Abhar HS, Kamel MA, & Attia AS (2015). Antidiabetic effect of galantamine: Novel effect for a known centrally acting drug. PLoS One, 10, e0134648. 10.1371/journal.pone.0134648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, & North American Association for the Study of Obesity. (2004). Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care, 27, 596–601. 10.2337/diacare.27.2.596 [DOI] [PubMed] [Google Scholar]
- Aracava Y, Pereira EF, Akkerman M, Adler M, & Albuquerque EX (2009). Effectiveness of donepezil, rivastigmine, and (+/−)huperzine A in counteracting the acute toxicity of organophosphorus nerve agents: Comparison with galantamine. The Journal of Pharmacology and Experimental Therapeutics, 331, 1014–1024. 10.1124/jpet.109.160028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracava Y, Pereira EF, Maelicke A, & Albuquerque EX (2005). Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. The Journal of Pharmacology and Experimental Therapeutics, 312, 1195–1205. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, & Okun MS (2020). Diagnosis and treatment of parkinson disease: A review. JAMA, 323, 548–560. 10.1001/jama.2019.22360 [DOI] [PubMed] [Google Scholar]
- Arnold DS, Rosse RB, Dickinson D, Benham R, Deutsch SI, & Nelson MW (2004). Adjuvant therapeutic effects of galantamine on apathy in a schizophrenia patient. The Journal of Clinical Psychiatry, 65, 1723–1724. 10.4088/JCP.v65n1219e [DOI] [PubMed] [Google Scholar]
- Ashare RL, Kimmey BA, Rupprecht LE, Bowers ME, Hayes MR, & Schmidt HD (2016). Repeated administration of an acetylcholinesterase inhibitor attenuates nicotine taking in rats and smoking behavior in human smokers. Translational Psychiatry, 6, e713. 10.1038/tp.2015.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila C, Kucinski A, & Sarter M (2020). Complex movement control in a rat model of Parkinsonian falls: Bidirectional control by striatal cholinergic interneurons. The Journal of Neuroscience, 40(31), 6049–6067. 10.1523/JNEUROSCI.0220-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B, Mota-Rolim SA, & Dresler M (2019). The cognitive neuroscience of lucid dreaming. Neuroscience & Biobehavioral Reviews, 100, 305–323. 10.1016/j.neubiorev.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Tzavara ET, Wu S, Wade MR, Bymaster FP, Paul SM, & Nomikos GG (2006). Cholinergic dysfunction in a mouse model of Alzheimer disease is reversed by an anti-A beta antibody. The Journal of Clinical Investigation, 116, 825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger EC, Ananth M, Talmage DA, & Role LW (2016). Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron, 91, 1199–1218. 10.1016/j.neuron.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL 3rd, Beer B, & Lippa AS (1982). The cholinergic hypothesis of geriatric memory dysfunction. Science (New York, N.Y.), 217,408–414. [DOI] [PubMed] [Google Scholar]
- Berman JA, Talmage DA, & Role LW (2007). Cholinergic circuits and signaling in the pathophysiology of schizophrenia. International Review of Neurobiology, 78, 193–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Haertel C, Maelicke A, & Montag D (2014). Galantamine slows down plaque formation and behavioral decline in the 5XFAD mouse model of Alzheimer’s disease. PLoS One, 9, e89454. 10.1371/journal.pone.0089454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks J (2006) Cholinesterase inhibitors for Alzheimer’s disease. The Cochrane database of systematic reviews, Cd005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzinger DI, Gruber M, Tümmler S, Malsy M, Seyfried T, Weber F, Redel A, Graf BM, & Zausig YA (2019). In vivo effects of neostigmine and physostigmine on neutrophil functions and evaluation of acetylcholinesterase and butyrylcholinesterase as inflammatory markers during experimental sepsis in rats. Mediators of Inflammation, 2019, 8274903. 10.1155/2019/8274903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasko I, Knaus G, Weiss E, Kemmler G, Winkler C, Falkensammer G, Griesmacher A, Würzner R, Marksteiner J, & Fuchs D (2007). Cognitive deterioration in Alzheimer’s disease is accompanied by increase of plasma neopterin. Journal of Psychiatric Research, 41, 694–701. 10.1016/j.jpsychires.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Bonaz B, Sinniger V, Hoffmann D, Clarençon D, Mathieu N, Dantzer C, Vercueil L, Picq C, Trocmé C, Faure P, Cracowski J-L, & Pellissier S (2016). Chronic vagus nerve stimulation in Crohn’s disease: A 6-month follow-up pilot study. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society, 28, 948–953. 10.1111/nmo.12792 [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, & Tracey KJ (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature, 405, 458–462. 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- Brockmann K, Apel A, Schulte C, Schneiderhan-Marra N, Pont-Sunyer C, Vilas D, Ruiz-Martinez J, Langkamp M, Corvol J-C, Cormier F, Knorpp T, Joos TO, Gasser T, Schüle B, Aasly JO, Foroud T, Marti-Masso JF, Brice A, Tolosa E, … Maetzler W (2016). Inflammatory profile in LRRK2-associated prodromal and clinical PD. Journal of Neuroinflammation, 13, 122. 10.1186/s12974-016-0588-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, & Arrighi HM (2007). Forecasting the global burden of Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 3, 186–191. 10.1016/j.jalz.2007.04.381 [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Conley RR, Dickinson D, Ball MP, Feldman S, Gold JM, & McMahon RP (2008). Galantamine for the treatment of cognitive impairments in people with schizophrenia. The American Journal of Psychiatry, 165, 82–89. 10.1176/appi.ajp.2007.07050724 [DOI] [PubMed] [Google Scholar]
- Buckley PF (2019). Neuroinflammation and Schizophrenia. Current Psychiatry Reports, 21,72. 10.1007/s11920-019-1050-z [DOI] [PubMed] [Google Scholar]
- Capsoni S, Giannotta S, & Cattaneo A (2002). Nerve growth factor and galantamine ameliorate early signs of neurodegeneration in anti-nerve growth factor mice. Proceedings of the National Academy of Sciences of the United States of America, 99, 12432–12437. 10.1073/pnas.192442999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EH, Chavan SS, & Pavlov VA (2019). Cholinergic control of inflammation, metabolic dysfunction, and cognitive impairment in obesity-associated disorders: mechanisms and novel therapeutic opportunities. Frontiers in Neuroscience, 13, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee PK, Yeboah MM, Solanki MH, Kumar G,Xue X, Pavlov VA, Al-Abed Y, & Metz CN (2017). Activation of the cholinergic anti-inflammatory pathway by GTS-21 attenuates cisplatin-induced acute kidney injury in mice. PLoS One, 12, e0188797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, & Nunez G (2010). Sterile inflammation: Sensing and reacting to damage. Nature Reviews. Immunology, 10, 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dyakin VV, Branch CA, Ardekani B, Yang D, Guilfoyle DN, Peterson J, Peterhoff C, Ginsberg SD, Cataldo AM,& Nixon RA (2009). In vivo MRI identifies cholinergic circuitry deficits in a Down syndrome model. Neurobiology of Aging, 30, 1453–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiry J, Blais CM,Shah D, Smith D, Fisher D, Labelle A,& Knott V (2019). Combining CDP-choline and galantamine, an optimized alpha7 nicotinic strategy, to ameliorate sensory gating to speech stimuli in schizophrenia. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 145, 70–82. 10.1016/j.ijpsycho.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Citrome L, & Volavka J (2004). Consensus development conference on antipsychotic drugs and obesity and diabetes: Response to consensus statement. Diabetes Care, 27, 2087–2088. [DOI] [PubMed] [Google Scholar]
- Citrome L, & Volavka J (2005). Consensus development conference on antipsychotic drugs and obesity and diabetes: Response to consensus statement. The Journal of Clinical Psychiatry, 66, 1073–1074. [DOI] [PubMed] [Google Scholar]
- Conley RR, Boggs DL, Kelly DL, McMahon RP, Dickinson D, Feldman S, Ball MP, & Buchanan RW (2009). The effects of galantamine on psychopathology in chronic stable schizophrenia. Clinical Neuropharmacology, 32, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consolim-Colombo FM, Sangaleti CT, Costa FO, Morais TL, Lopes HF, Motta JM, Pavlov VA (2017). Galantamine alleviates inflammation and insulin resistance in patients with metabolic syndrome in a randomized trial. JCI Insight, 2, 1–13. 10.1172/jci.insight.93340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti MM, Chambers N, & Bishop C (2018). A new outlook on cholinergic interneurons in Parkinson’s disease and L-DOPA-induced dyskinesia. Neuroscience & Biobehavioral Reviews, 92, 67–82. [DOI] [PubMed] [Google Scholar]
- Cranston AL, Wysocka A, Steczkowska M, Zadrozny M, Palasz E, Harrington CR, Theuring F, Wischik CM, Riedel G, & Niewiadomska G (2020). Cholinergic and inflammatory phenotypes in transgenic tau mouse models of Alzheimer’s disease and frontotemporal lobar degeneration. Brain Communications, 2(1), 1–17. 10.1093/braincomms/fcaa033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, & Bertrand D (2007). Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annual Review of Pharmacology and Toxicology, 47, 699–729. 10.1146/annurev.pharmtox.47.120505.105214 [DOI] [PubMed] [Google Scholar]
- Dasuri K, Zhang L, Kim SO, Bruce-Keller AJ, & Keller JN (2016). Dietary and donepezil modulation of mTOR signaling and neuroinflammation in the brain. Biochimica Et Biophysica Acta, 1862, 274–283. 10.1016/j.bbadis.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P, & Maloney AJ (1976). Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet (London, England), 2, 1403. 10.1016/S0140-6736(76)91936-X [DOI] [PubMed] [Google Scholar]
- Davis KL, Hollister LE, Berger PA,& Barchas JD(1975).Cholinergic imbalance hypotheses of psychoses and movement disorders: Strategies for evaluation. Psychopharmacology Communications, 1, 533–543. [PubMed] [Google Scholar]
- de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud H-R, Uematsu S, Akira S, van den Wijngaard RM, & Boeckxstaens GE (2005). Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nature immunology, 6, 844–851. 10.1038/ni1229 [DOI] [PubMed] [Google Scholar]
- de Souza FM, Busquet N, Blatner M, Maclean KN, & Restrepo D (2011). Galantamine improves olfactory learning in the Ts65Dn mouse model of Down syndrome. Scientific Reports, 1, 137. 10.1038/srep00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, & Scarr E(2020). Muscarinic M1 and M4 receptors: Hypothesis driven drug development for schizophrenia. Psychiatry Research, 288, 112989. 10.1016/j.psychres.2020.112989 [DOI] [PubMed] [Google Scholar]
- Decourt B, Lahiri DK, & Sabbagh MN (2017). Targeting tumor necrosis factor alpha for Alzheimer’s disease. Current Alzheimer Research, 14, 412–425. 10.2174/1567205013666160930110551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Csernansky CA, Martin MV, Bertchume A, Vallera D, & Csernansky JG (2005). Acetylcholinesterase inhibitors ameliorate behavioral deficits in the Tg2576 mouse model of Alzheimer’s disease. Psychopharmacology (Berl), 181, 145–152. 10.1007/s00213-005-2230-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer MA, Freudenreich O, Culhane MA, Pachas GN, Deckersbach T, Murphy E, Goff DC, & Evins AE (2008). High-dose galantamine augmentation inferior to placebo on attention, inhibitory control and working memory performance in nonsmokers with schizophrenia. Schizophrenia Research, 102, 88–95. 10.1016/j.schres.2007.12.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, & Vizi ES (2000). The sympathetic nerve–an integrative interface between two supersystems: The brain and the immune system. Pharmacological Reviews, 52, 595–638. [PubMed] [Google Scholar]
- Feigenson KA, Kusnecov AW,& Silverstein SM(2014). Inflammation and the two-hit hypothesis of schizophrenia. Neuroscience and Biobehavioral Reviews, 38, 72–93. 10.1016/j.neubiorev.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Cabello S, Kronbichler M, Van Dijk KRA, Goodman JA, Spreng RN, & Schmitz TW(2020). Basal forebrain volume reliably predicts the cortical spread of Alzheimer’s degeneration. Brain: A Journal of Neurology, 143, 993–1009. 10.1093/brain/awaa012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JJ, Katzenschlager R, Bloem BR, Bonuccelli U, Burn D, Deuschl G, Dietrichs E, Fabbrini G, Friedman A, Kanovsky P, Kostic V, Nieuwboer A, Odin P, Poewe W, Rascol O, Sampaio C, Schiipbach M, Tolosa E, Trenkwalder C, Oertel WH (2013). Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. European Journal of Neurology, 20, 5–15. 10.1111/j.1468-1331.2012.03866.x [DOI] [PubMed] [Google Scholar]
- Gallowitsch-Puerta M, & Pavlov VA (2007). Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sciences, 80, 2325–2329. 10.1016/j.lfs.2007.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli R, Rabin BS, Kelly RH, Lyte M, & Ragu U (1987). Clinical and laboratory evidence of autoimmunity in acute schizophrenia. Annals of the New York Academy of Sciences, 496, 676–685. 10.1111/j.1749-6632.1987.tb35829.x [DOI] [PubMed] [Google Scholar]
- Garcia-Ayllon M-S, Cauli O, Silveyra M-X, Rodrigo R, Candela A, Compan A, Jover R, Perez-Mateo M, Martinez S, Felipo V, & Saez-Valero J (2008). Brain cholinergic impairment in liver failure. Brain: A Journal of Neurology, 131, 2946–2956. 10.1093/brain/awn209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelders G, Baekelandt V, & Van der Perren A (2018). Linking neuroinflammation and neurodegeneration in Parkinson’s disease. Journal of Immunology Research, 2018, 4784268. 10.1155/2018/4784268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese M, Gaylis N, Sikes D, Kivitz A, Horowitz D, Peterfy C, Glass E, Levine Y, & Chernoff D (2020). Safety and efficacy of neurostimulation with a miniaturised vagus nerve stimulation device in patients with multidrug-refractory rheumatoid arthritis: A two-stage multicentre, randomised pilot study. The Lancet Rheumatology, 2(9), e527–e538. 10.1016/S2665-9913(20)30172-7 [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, & Brooks DJ (2006). In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiology of Disease, 21, 404–412. 10.1016/j.nbd.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Gershon S, & Shaw FH (1961). Psychiatric sequelae of chronic exposure to organophosphorus insecticides. Lancet (London, England), 1, 1371–1374. [DOI] [PubMed] [Google Scholar]
- Ghaleiha A,Ghyasvand M, Mohammadi MR, Farokhnia M,Yadegari N, Tabrizi M, Hajiaghaee R, Yekehtaz H, & Akhondzadeh S (2014). Galantamine efficacy and tolerability as an augmentative therapy in autistic children: A randomized, double-blind, placebo-controlled trial. Journal of Psychopharmacology (Oxford, England), 28, 677–685. 10.1177/0269881113508830 [DOI] [PubMed] [Google Scholar]
- Giacobini E (2004). Cholinesterase inhibitors: New roles and therapeutic alternatives. Pharmacological Research, 50, 433–440. 10.1016/j.phrs.2003.11.017 [DOI] [PubMed] [Google Scholar]
- Gibbons A, & Dean B (2016). The cholinergic system: An emerging drug target for schizophrenia. Current Pharmaceutical Design, 22, 2124–2133. 10.2174/1381612822666160127114010 [DOI] [PubMed] [Google Scholar]
- Gogtay N, Vyas NS, Testa R, Wood SJ, & Pantelis C (2011). Age of onset of schizophrenia: Perspectives from structural neuroimaging studies. Schizophrenia Bulletin, 37, 504–513. 10.1093/schbul/sbr030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golime R, Palit M, Acharya J, & Dubey DK (2018). Neuroprotective effects of galantamine on nerve agent-induced neuroglial and biochemical changes. Neurotoxicity Research, 33, 738–748. 10.1007/s12640-017-9815-9 [DOI] [PubMed] [Google Scholar]
- Gowayed MA, Refaat R, Ahmed WM, & El-Abhar HS (2015). Effect of galantamine on adjuvant-induced arthritis in rats. European Journal of Pharmacology, 764, 547–553. 10.1016/j.ejphar.2015.07.038 [DOI] [PubMed] [Google Scholar]
- Granholm A-C-E, Sanders LA, & Crnic LS (2000). Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down’s syndrome. Experimental Neurology, 161, 647–663. 10.1006/exnr.1999.7289 [DOI] [PubMed] [Google Scholar]
- Grundy SM (2008). Metabolic syndrome pandemic. Arteriosclerosis, Thrombosis, and Vascular Biology, 28, 629–636. 10.1161/ATVBAHA.107.151092 [DOI] [PubMed] [Google Scholar]
- Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini A, Marini R, Adamo EB, Venuti FS, & Squadrini F (2003). Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation, 107, 1189–1194. [DOI] [PubMed] [Google Scholar]
- Guarini S, Cainazzo MM, Giuliani D, Mioni C, Altavilla D, Marini H, Bigiani C,Ghiaroni V, Passaniti M, Leone S, Bazzani C,Caputi AP, Squadrito F, & Bertolini A (2004). Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovascular Research, 63, 357–365. 10.1016/j.cardiores.2004.03.029 [DOI] [PubMed] [Google Scholar]
- Haake A, Nguyen K, Friedman L, Chakkamparambil B, & Grossberg GT (2020). An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opinion on Drug Safety, 19, 147–157. 10.1080/14740338.2020.1721456 [DOI] [PubMed] [Google Scholar]
- Hager K, Baseman AS, Nye JS, Brashear HR, Han J, Sano M, Davis B, & Richards HM (2014). Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer’s disease. Neuropsychiatric Disease and Treatment, 10, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LR, Schachter SC, & Myers TM (2017). Time course, behavioral safety, and protective efficacy of centrally active reversible acetylcholinesterase inhibitors in cynomolgus macaques. Neurochemical Research, 42, 1962–1971. 10.1007/s11064-016-2120-9 [DOI] [PubMed] [Google Scholar]
- Hampel H, Mesulam M-M, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ, & Khachaturian ZS (2018). The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain: A Journal of Neurology, 141,1917–1933. 10.1093/brain/awy132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes WM, Olofsson PS, Kwan K, Hudson LK, Chavan SS, Pavlov VA, & Tracey KJ (2015). Galantamine attenuates type 1 diabetes and inhibits anti-insulin antibodies in non-obese diabetic mice. Molecular Medicine, 21(1), 702–708. 10.2119/molmed.2015.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich M, & Lee Teoh H (2004). Galanthamine from snowdrop-the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. Journal of Ethnopharmacology, 92, 147–162. 10.1016/j.jep.2004.02.012 [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Kummer MP (2015). Neuroinflammation in Alzheimer’s disease. The Lancet. Neurology, 14, 388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmas CJ, Poole MJ, Finneran K, Clark MG, & Williams PT (2009). Galantamine is a novel post-exposure therapeutic against lethal VX challenge. Toxicology and Applied Pharmacology, 240, 166–173. 10.1016/j.taap.2009.07.029 [DOI] [PubMed] [Google Scholar]
- Hohnadel E, Bouchard K, & Terry AV Jr (2007). Galantamine and donepezil attenuate pharmacologically induced deficits in prepulse inhibition in rats. Neuropharmacology, 52, 542–551. 10.1016/j.neuropharm.2006.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, & van de Beek D (2015). Systemic inflammation and microglial activation: Systematic review of animal experiments. Journal of Neuroinflammation, 12, 114. 10.1186/s12974-015-0332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DB (2017). Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacology & Therapeutics, 179, 1–16. 10.1016/j.pharmthera.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RL, & Smith HJ (1960). Cholinesterase inhibition by galanthamine and lycoramine. Biochemical Pharmacology, 3, 147–148. 10.1016/0006-2952(60)90030-7 [DOI] [PubMed] [Google Scholar]
- Janelidze S, Mattsson N, Stomrud E, Lindberg O, Palmqvist S, Zetterberg H, Blennow K, & Hansson O (2018). CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology, 91, e867–e877. 10.1212/WNL.0000000000006082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen B, & Schäfer B (2017). Galantamine. Chemtexts, 3, 7. 10.1007/s40828-017-0043-y [DOI] [Google Scholar]
- Jellinger K (1988). The pedunculopontine nucleus in Parkinson’s disease, progressive supranuclear palsy and Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 51, 540–543. 10.1136/jnnp.51.4.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, & Ghia JE (2014). Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunology, 7, 335–347. 10.1038/mi.2013.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Byun N, & Bubser M (2012). Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 37, 16–42. 10.1038/npp.2011.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RS, & Sommer IE (2015). The neurobiology and treatment of first-episode schizophrenia. Molecular Psychiatry, 20, 84–97. 10.1038/mp.2014.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, O’Donovan M, Correll CU, Kane JM, van Os J, & Insel TR (2015). Schizophrenia. Nature Reviews Disease Primers, 1, 15067. 10.1038/nrdp.2015.67 [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Borson S, Kershaw P, & Sadik K (2005). Reduction of caregiver burden in Alzheimer’s disease by treatment with galantamine. CNS Spectrums, 10,481–488. 10.1017/S1092852900023178 [DOI] [PubMed] [Google Scholar]
- Kemper TL, & Bauman M (1998). Neuropathology of infantile autism. Journal of Neuropathology and Experimental Neurology, 57, 645–652. 10.1097/00005072-199807000-00001 [DOI] [PubMed] [Google Scholar]
- Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, & Lamb BT (2018). Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s & Dementia (New York, N. Y.), 4, 575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, & Miller BJ (2013). Inflammation and schizophrenia. Schizophrenia Bulletin, 39, 1174–1179. 10.1093/schbul/sbt141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koola MM (2018). Potential role of antipsychotic-galantamine-memantine combination in the treatment of positive, cognitive, and negative symptoms of schizophrenia. Complex Psychiatry, 4, 134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koola MM, Looney SW, Hong H, Pillai A, & Hou W (2020). Meta-analysis of randomized controlled trials of galantamine in schizophrenia: Significant cognitive enhancement. Psychiatry Research, 291, 113285. 10.1016/j.psychres.2020.113285 [DOI] [PubMed] [Google Scholar]
- Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, & Tak PP (2016). Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America, 113, 8284–8289. 10.1073/pnas.1605635113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki T, Mouri A, Suzuki S, Nakajima A, Mamiya T, Yan Y, & Nabeshima T (2014). Galantamine attenuates reinstatement of cue-induced methamphetamine-seeking behavior in mice. Addiction Biology, 19, 1–4. 10.1111/j.1369-1600.2011.00425.x [DOI] [PubMed] [Google Scholar]
- Kowal NM, Ahring PK, Liao VWY, Indurti DC, Harvey BS, O’Connor SM, Chebib M, Olafsdottir ES, & Balle T (2018). Galantamine is not a positive allosteric modulator of human α4β2 or α7 nicotinic acetylcholine receptors. British Journal of Pharmacology, 175, 2911–2925. 10.1111/bph.14329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox M, van Velzen JF, Pompe JC, Hoedemaekers CW, van der Hoeven JG, & Pickkers P (2009). GTS-21 inhibits pro-inflammatory cytokine release independent of the Toll-like receptor stimulated via a transcriptional mechanism involving JAK2 activation. Biochemical Pharmacology, 78, 863–872. 10.1016/j.bcp.2009.06.096 [DOI] [PubMed] [Google Scholar]
- LaBerge S, LaMarca K, & Baird B (2018). Pre-sleep treatment with galantamine stimulates lucid dreaming: A double-blind, placebo-controlled, crossover study. PLoS One, 13, e0201246. 10.1371/journal.pone.0201246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane M, Carter D, Pescrille JD, Aracava Y, Fawcett WP, Basinger GW, Pereira EFR, & Albuquerque EX (2020). Oral pretreatment with galantamine effectively mitigates the acute toxicity of a supralethal dose of soman in cynomolgus monkeys posttreated with conventional antidotes. The Journal of Pharmacology and Experimental Therapeutics, 375, 115–126. 10.1124/jpet.120.265843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lataro RM, Silva CA, Tefe-Silva C, Prado CM, & Salgado HC (2015). Acetylcholinesterase Inhibition Attenuates the Development of Hypertension and Inflammation in Spontaneously Hypertensive Rats. American Journal of Hypertension, 28, 1201–1208. 10.1093/ajh/hpv017 [DOI] [PubMed] [Google Scholar]
- Lee S-T, Chu K, Jung K-H, Kang K-M, Kim J-H, Bahn J-J, Jeon D, Kim M, Lee SK, & Roh J-K (2010). Cholinergic anti-inflammatory pathway in intracerebral hemorrhage. Brain Research, 1309, 164–171. 10.1016/j.brainres.2009.10.076 [DOI] [PubMed] [Google Scholar]
- Lee SW, Lee JG, Lee BJ, & Kim YH (2007). A 12-week, double-blind, placebo-controlled trial of galantamine adjunctive treatment to conventional antipsychotics for the cognitive impairments in chronic schizophrenia. International Clinical Psychopharmacology, 22, 63–68. 10.1097/YIC.0b013e3280117feb [DOI] [PubMed] [Google Scholar]
- Lehner KR, Silverman HA, Addorisio ME, Roy A, Al-Onaizi MA, Levine Y, Olofsson PS, Chavan SS, Gros R, Nathanson NM, Al-Abed Y, Metz CN, Prado VF, Prado MAM, Tracey KJ, & Pavlov VA (2019). Forebrain cholinergic signaling regulates innate immune responses and inflammation. Frontiers in Immunology, 10, 585. 10.3389/fimmu.2019.00585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DD, Zhang YH, Zhang W, & Zhao P (2019). Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of alzheimer’s disease. Frontiers in Neuroscience, 13, 472. 10.3389/fnins.2019.00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld S (2002). Galantamine–a novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer’s disease. CNS Drug Reviews, 8, 159–176. 10.1111/j.1527-3458.2002.tb00221.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Wu PH, Chen CS, Yang YH, & Yang YH (2016). Association between acetylcholinesterase inhibitors and risk of stroke in patients with dementia. Scientific Reports, 6, 29266. 10.1038/srep29266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinenko IV, Odinak MM, Mogil’naya VI, & Emelin AY (2008). Efficacy and safety of galantamine (reminyl) for dementia in patients with Parkinson’s disease (an open controlled trial). Neuroscience and Behavioral Physiology, 38, 937–945. 10.1007/s11055-008-9077-3 [DOI] [PubMed] [Google Scholar]
- Liu SY, Wile DJ, Fu JF, Valero J, Shahinfard E, McCormick S, Mabrouk R, Vafai N, McKenzie J, Neilson N, & Stoessl AJ (2018). The effect of LRRK2 mutations on the cholinergic system in manifest and premanifest stages of Parkinson’s disease: A cross-sectional PET study. The Lancet. Neurology, 17, 309–316. 10.1016/S1474-4422(18)30032-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang Y, Zheng X, Fang T, Yang X, Luo X, … Yu Y (2018). Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. Journal of Neuroinflammation, 15(1), 112. 10.1186/s12974-018-1141-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, … Mukadam N (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet, 396(10248), 413–446. 10.1016/s0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Kwan K, Levine YA, Olofsson PS, Yang H, Li J, … Tracey KJ (2014). α7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Molecular Medicine, 20(1), 350–358. 10.2119/molmed.2013.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Contelles J, do Carmo Carreiras M, Rodríguez C, Villarroya M, & García AG(2006). Synthesis and pharmacology of galantamine. Chemical Reviews, 106, 116–133. [DOI] [PubMed] [Google Scholar]
- Marder SR, & Cannon TD (2019). Schizophrenia. The New England Journal of Medicine, 381, 1753–1761. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, & McGeer E (1988). Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology, 38, 1285. [DOI] [PubMed] [Google Scholar]
- McManus RM, & Heneka MT (2017). Role of neuroinflammation in neurodegeneration: New insights. Alzheimer’s Research & Therapy, 9, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF (2019). Early-onset Alzheimer disease and its variants. Continuum (Minneapolis, Minn.), 25, 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz CN, & Pavlov VA (2018). Vagus nerve cholinergic circuitry to the liver and the gastrointestinal tract in the neuroimmune communicatome. American journal of physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Schwarz MJ, & Müller N (2011). Inflammatory processes in schizophrenia: A promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacology & Therapeutics, 132, 96–110. 10.1016/j.pharmthera.2011.06.003 [DOI] [PubMed] [Google Scholar]
- Miceli PC, & Jacobson K (2003). Cholinergic pathways modulate experimental dinitrobenzene sulfonic acid colitis in rats. Autonomic Neuroscience: Basic & Clinical, 105, 16–24. 10.1016/S1566-0702(03)00023-7 [DOI] [PubMed] [Google Scholar]
- Mohan M, Bennett C, & Carpenter PK (2009). Galantamine for dementia in people with Down syndrome. Cochrane Database of Systematic Reviews, (1), 1–17. 10.1002/14651858.CD007656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M, Yamauchi Y, Yamada S, & Kanba S (2013). Neuroinflammation in schizophrenia especially focused on the role of microglia. Progress in neuro-psychopharmacology & Biological Psychiatry, 42, 115–121. 10.1016/j.pnpbp.2011.12.002 [DOI] [PubMed] [Google Scholar]
- Mukaetova-Ladinska E, Westwood J, & Perry E (2010) Cholinergic component of autism spectrum disorder, pp. 129–161. [Google Scholar]
- Müller N (2018). Inflammation in schizophrenia: Pathogenetic aspects and therapeutic considerations. Schizophrenia Bulletin, 44, 973–982. 10.1093/schbul/sby024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyaka P, Rabbi MF, Pavlov VA, Tracey KJ, Khafipour E, & Ghia JE (2014). Central muscarinic cholinergic activation alters interaction between splenic dendritic cell and CD4+CD25− T cells in experimental colitis. PLoS One, 9, e109272. 10.1371/journal.pone.0109272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa R, Ohnishi T, Kobayashi H, Yamaoka T, Yajima T, Tanimura A, Kato T, & Yoshizawa K (2017). Long-term effect of galantamine on cognitive function in patients with Alzheimer’s disease versus a simulated disease trajectory: An observational study in the clinical setting. Neuropsychiatric Disease and Treatment, 13, 1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C (2002). Points of control in inflammation. Nature, 420, 846–852. 10.1038/nature01320 [DOI] [PubMed] [Google Scholar]
- Nizri E, Irony-Tur-Sinai M, Faranesh N, Lavon I, Lavi E, Weinstock M, & Brenner T (2008). Suppression of neuroinflammation and immunomodulation by the acetylcholinesterase inhibitor rivastigmine. Journal of Neuroimmunology, 203, 12–22. 10.1016/j.jneuroim.2008.06.018 [DOI] [PubMed] [Google Scholar]
- Njoku I, Radabaugh HL, Nicholas MA, Kutash LA, O’Neil DA, Marshall IP, Cheng JP, Kline AE, & Bondi CO (2019). Chronic treatment with galantamine rescues reversal learning in an attentional set-shifting test after experimental brain trauma. Experimental Neurology, 315, 32–41. 10.1016/j.expneurol.2019.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom P, Religa D, Wimo A, Winblad B, & Eriksdotter M (2013). The use of cholinesterase inhibitors and the risk of myocardial infarction and death: A nationwide cohort study in subjects with Alzheimer’s disease. European Heart Journal, 34, 2585–2591. 10.1093/eurheartj/eht182 [DOI] [PubMed] [Google Scholar]
- Ochoa EL, & Clark E (2006). Galantamine may improve attention and speech in schizophrenia. Human Psychopharmacology, 21, 127–128. 10.1002/hup.751 [DOI] [PubMed] [Google Scholar]
- Odorcyk FK, Nicola F, Duran-Carabali LE, Figueiró F, Kolling J, Vizuete A, Konrath EL, Gonçalves CA, Wyse A, & Netto CA (2017). Galantamine administration reduces reactive astrogliosis and upregulates the anti-oxidant enzyme catalase in rats submitted to neonatal hypoxia ischemia. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 62, 15–24. 10.1016/j.ijdevneu.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Olofsson PS, Metz CN, & Pavlov VA (2017). The Neuroimmune Communicatome in Inflammation. In Cavaillon J, & Singer M (Eds.), Inflammation: From Molecular and Cellular Mechanisms to the Clinic, Vol. 4 (pp. 1485–1516). Wiley-VCH Verlag GmbH & Co.. [Google Scholar]
- Olofsson PS, & Tracey KJ (2017). Bioelectronic medicine: Technology targeting molecular mechanisms for therapy. Journal of Internal Medicine, 282, 3–4. 10.1111/joim.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano G, Ferrara N, Brooks DJ, & Pavese N (2016). Age at onset and Parkinson disease phenotype. Neurology, 86,1400–1407. 10.1212/WNL.0000000000002461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano G, Rengo G, Pasqualetti G, Femminella GD, Monzani F, Ferrara N, & Tagliati M (2015). Cholinesterase inhibitors for Parkinson’s disease: A systematic review and meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry, 86, 767–773. 10.1136/jnnp-2014-308764 [DOI] [PubMed] [Google Scholar]
- Park JE, Lee ST, Im WS, Chu K, & Kim M (2008). Galantamine reduces striatal degeneration in 3-nitropropionic acid model of Huntington’s disease. Neuroscience Letters, 448, 143–147. 10.1016/j.neulet.2008.10.020 [DOI] [PubMed] [Google Scholar]
- Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, … Pavlov VA (2008). Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Molecular Medicine, 14(9–10), 567–574. 10.2119/2008-00079.parrish [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskov D (1957) Effect of galanthamine on the skeletal muscles. Proceedings of the Department of Biological and Medical Sciences at the Bulgarian Academy of Sciences. Series Experimental Biology and Medicine (Sofia) 1, 29–35. [Google Scholar]
- Paskov D (1964). Nivalin: Pharmacology and clinical application. Pharmachim. [Google Scholar]
- Pasternak O, Kubicki M, & Shenton ME (2016). In vivo imaging of neuroinflammation in schizophrenia. Schizophrenia Research, 173, 200–212. 10.1016/j.schres.2015.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA (2019). Collateral benefits of studying the vagus nerve in bioelectronic medicine. Bioelectronic Medicine, 5, 5. 10.1186/s42234-019-0021-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Chavan SS, & Tracey KJ (2018). Molecular and functional neuroscience in immunity. Annual Review of Immunology, 36, 783–812. 10.1146/annurev-immunol-042617-053158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Chavan SS, & Tracey KJ (2019). Bioelectronic medicine: From preclinical studies on the inflammatory reflex to new approaches in disease diagnosis and treatment. Cold Spring Harbor Perspectives in Medicine, 10(3), a034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, Al-Abed Y, & Tracey KJ (2006). Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proceedings of the National Academy of Sciences of the United States of America, 103, 5219–5223. 10.1073/pnas.0600506103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, LaRosa GJ, Miller EJ, Tracey KJ, & Al-Abed Y (2007). Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis*. Critical Care Medicine, 35(4), 1139–1144. 10.1097/01.ccm.0000259381.56526.96 [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, & Tracey KJ (2009). Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain, Behavior, and Immunity, 23, 41–45. 10.1016/j.bbi.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, & Tracey KJ (2005). The cholinergic anti-inflammatory pathway. Brain, Behavior, and Immunity, 19, 493–499. 10.1016/j.bbi.2005.03.015 [DOI] [PubMed] [Google Scholar]
- Pavlov VA, & Tracey KJ (2012). The vagus nerve and the inflammatory reflex–linking immunity and metabolism. Nature Reviews. Endocrinology, 8, 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, & Tracey KJ (2015). Neural circuitry and immunity. Immunologic Research, 63, 38–57. 10.1007/s12026-015-8718-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, & Tracey KJ (2017). Neural regulation of immunity: Molecular mechanisms and clinical translation. Nature Neuroscience, 20, 156–166. 10.1038/nn.4477 [DOI] [PubMed] [Google Scholar]
- Pavlov VA, & Tracey KJ (2019a). Bioelectronic Medicine. Cold Spring Harbor Laboratory Press. [Google Scholar]
- Pavlov VA, & Tracey KJ (2019b). Bioelectronic medicine: Updates, challenges and paths forward. Bioelectronic Medicine, 5, 1. 10.1186/s42234-019-0018-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Wang H, Czura CJ, Friedman SG, & Tracey KJ (2003). The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Molecular Medicine, 9, 125–134. 10.1007/BF03402177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira EF, Aracava Y, Alkondon M, Akkerman M, Merchenthaler I, & Albuquerque EX (2010). Molecular and cellular actions of galantamine: Clinical implications for treatment of organophosphorus poisoning. Journal of Molecular Neuroscience: MN, 40, 196–203. 10.1007/s12031-009-9234-3 [DOI] [PubMed] [Google Scholar]
- Pereira JB, Hall S, Jalakas M, Grothe MJ, Strandberg O, Stomrud E, Westman E, van Westen D, & Hansson O (2020). Longitudinal degeneration of the basal forebrain predicts subsequent dementia in Parkinson’s disease. Neurobiology of Disease, 139, 104831. 10.1016/j.nbd.2020.104831 [DOI] [PubMed] [Google Scholar]
- Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, & Perry RH (1978). Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. British Medical Journal, 2, 1457–1459. 10.1136/bmj.2.6150.1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrikis P, Andreou C, Piachas A, Bozikas VP, & Karavatos A (2004). Treatment of Huntington’s disease with galantamine. International Clinical Psychopharmacology, 19, 49–50. 10.1097/00004850-200401000-00010 [DOI] [PubMed] [Google Scholar]
- Pham GS, Wang LA, & Mathis KW (2018). Pharmacological Potentiation of the Efferent Vagus Nerve Attenuates Blood Pressure and Renal Injury in a Murine Model of Systemic Lupus Erythematosus. American journal of physiology. Regulatory, integrative and comparative physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaitakis A, & Duvoisin RC (1983). Homer’s moly identified as Galanthus nivalis L.: Physiologic antidote to stramonium poisoning. Clinical Neuropharmacology, 6, 1–5. [DOI] [PubMed] [Google Scholar]
- Popova EN, & Bogolepov NN (1965). Changes in the neurons of some regions of the brain under the effect of nivaline. Biulleten’ Eksperimental’noi Biologii I Meditsiny, 59, 104–109. [PubMed] [Google Scholar]
- Powchik P, Davidson M, Haroutunian V, Gabriel SM, Purohit DP, Perl DP, Harvey PD, & Davis KL (1998). Postmortem studies in schizophrenia. Schizophrenia Bulletin, 24, 325–341. [DOI] [PubMed] [Google Scholar]
- Prado VF, Roy A, Kolisnyk B, Gros R, & Prado MA (2013). Regulation of cholinergic activity by the vesicular acetylcholine transporter. The Biochemical Journal, 450, 265–274. [DOI] [PubMed] [Google Scholar]
- Prasher VP (2004). Review of donepezil, rivastigmine, galantamine and memantine for the treatment of dementia in Alzheimer’s disease in adults with Down syndrome: Implications for the intellectual disability population. International Journal of Geriatric Psychiatry, 19, 509–515. [DOI] [PubMed] [Google Scholar]
- Prvulovic D, Hampel H, & Pantel J (2010). Galantamine for Alzheimer’s disease. Expert Opinion on Drug Metabolism & Toxicology, 6, 345–354. [DOI] [PubMed] [Google Scholar]
- Reale M, larlori C, Gambi F, Feliciani C, Salone A, Toma L, DeLuca G, Salvatore M, Conti P, & Gambi D (2004). Treatment with an acetylcholinesterase inhibitor in Alzheimer patients modulates the expression and production of the pro-inflammatory and anti-inflammatory cytokines. Journal of Neuroimmunology, 148(1–2), 162–171. 10.1016/j.jneuroim.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Revelli U, & Graso E (1962). Nivaline (Galanthus nivalis) therapy in polimyelitis. Minerva Medico, 53, 881–884. [PubMed] [Google Scholar]
- Richarz U, Gaudig M, Rettig K, & Schauble B (2014). Galantamine treatment in outpatients with mild Alzheimer’s disease. Acta Neurologica Scandinavica, 129, 382–392. 10.1111/ane.12195 [DOI] [PubMed] [Google Scholar]
- Rolinski M, Fox C, Maidment I, & McShane R (2012). Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database of Systematic Reviews, (3), 1–36. 10.1002/14651858.CD006504.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, … Tracey KJ (2011). Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science, 334(6052), 98–101. 10.1126/science.1209985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Valdés-Ferrer SI, Dancho ME, Ochani M, Katz D, Cheng KF, … Pavlov VA (2015). Xanomeline suppresses excessive pro-inflammatory cytokine responses through neural signal-mediated pathways and improves survival in lethal inflammation. Brain, Behavior, and Immunity, 44, 19–27. 10.1016/j.bbi.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, & Frye RE (2014). The use of medications approved for Alzheimer’s disease in autism spectrum disorder: A systematic review. Frontiers in Pediatrics, 2, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowntree DW, Nevin S, & Wilson A (1950). The effects of diisopropylfluorophosphonate in schizophrenia and manic depressive psychosis. Journal of Neurology, Neurosurgery, and Psychiatry, 13, 47–62. 10.1136/jnnp.13.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammi SR, Rawat JK, Raghav N, Kumar A, Roy S, Singh M, Gautam S, Yadav RK, Devi U, Pandey R, & Kaithwas G (2018). Galantamine attenuates N, N-dimethyl hydrazine induced neoplastic colon damage by inhibiting acetylcholinesterase and bimodal regulation of nicotinic cholinergic neurotransmission. European Journal of Pharmacology, 818, 174–183. 10.1016/j.ejphar.2017.10.036 [DOI] [PubMed] [Google Scholar]
- Samochocki M, Höffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EFR, Lübbert H, Albuquerque EX, & Maelicke A (2003). Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. The Journal of Pharmacology and Experimental Therapeutics, 305,1024–1036. 10.1124/jpet.102.045773 [DOI] [PubMed] [Google Scholar]
- Samochocki M, Zerlin M, Jostock R, Groot Kormelink PJ, Luyten WH, Albuquerque EX, & Maelicke A (2000). Galantamine is an allosterically potentiating ligand of the human alpha4/beta2 nAChR. Acta Neurologica Scandinavica. Supplementum, 176, 68–73. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet M-F, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, & Mazmanian SK (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell, 167, 1469–1480.e1412. 10.1016/j.cell.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangaleti CT, Katayama KY, De Angelis K, de Moraes TL, Aruajo AA, Lopes HF, … Consolim-Colombo F (2020) Galantamine alleviates oxidative stress alongside anti-inflammatory and cardio-metabolic effects in subjects with the metabolic syndrome in a randomized trial. medRxiv, 1–24. 10.1101/2019.12.30.19016105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos B, González-Fraile E, Zabala A, Guillé V, Rueda JR, & Ballesteros J (2018). Cognitive improvement of acetylcholinesterase inhibitors in schizophrenia. Journal of Psychopharmacology (Oxford, England), 32, 1155–1166. 10.1177/0269881118805496 [DOI] [PubMed] [Google Scholar]
- Santos SF, de Oliveira HL, Yamada ES, Neves BC, & Pereira A Jr (2019). The gut and Parkinson’s Disease-A bidirectional pathway. Frontiers in Neurology, 10, 574. 10.3389/fneur.2019.00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satapathy SK, Ochani M, Dancho M, Hudson LQK, Rosas-Ballina M, Valdes-Ferrer SI, … Pavlov VA (2011). Galantamine alleviates inflammation and other obesity-associated complications in high-fat diet-fed mice. Molecular Medicine, 17, 599–606. 10.2119/molmed.2011.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa M, Hesse S,& Bradley SJ (2020). Chapter Ten - M1 muscarinic acetylcholine receptors: A therapeutic strategy for symptomatic and disease-modifying effects in Alzheimer’s disease? In Langmead CJ (Ed.), Advances in Pharmacology, Vol. 88 (pp. 277–310). Academic Press. [DOI] [PubMed] [Google Scholar]
- Scarr E, Udawela M, Thomas EA, & Dean B (2018). Changed gene expression in subjects with schizophrenia and low cortical muscarinic M1 receptors predicts disrupted upstream pathways interacting with that receptor. Molecular Psychiatry, 23, 295–303. 10.1038/mp.2016.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer EBS, Loureiro SO, Vuaden FC, da Cunha AA, Schmitz F, Kolling J, Savio LEB, Bogo MR, Bonan CD, Netto CA, & Wyse ATS (2014). Mild hyperhomocysteinemia increases brain acetylcholinesterase and proinflammatory cytokine levels in different tissues. Molecular Neurobiology, 50, 589–596. 10.1007/s12035-014-8660-6 [DOI] [PubMed] [Google Scholar]
- Schmitz TW, & Spreng RN (2016). Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer’s pathology. Nature Communications, 7, 13249. 10.1038/ncomms13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Soreq H, Poirier J, & Spreng RN (2020). Longitudinal basal forebrain degeneration interacts with TREM2/C3 biomarkers of inflammation in presymptomatic Alzheimer’s disease. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 40, 1931–1942. 10.1523/JNEUROSCI.1184-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert MH, Young KA, & Hicks PB (2006). Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biological Psychiatry, 60, 530–533. 10.1016/j.biopsych.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Secnik J, Schwertner E, Alvarsson M, Hammar N, Fastbom J, Winblad B, Garcia-Ptacek S, Religa D, & Eriksdotter M (2020). Cholinesterase inhibitors in patients with diabetes mellitus and dementia: An open-cohort study of ~23 000 patients from the Swedish Dementia Registry. BMJ Open Diabetes Research & Care, 8, e000833. 10.1136/bmjdrc-2019-000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden N, Geula C, Hersh L, & Mesulam MM (1994). Human striatum: Chemoarchitecture of the caudate nucleus, putamen and ventral striatum in health and Alzheimer’s disease. Neuroscience, 60, 621–636. 10.1016/0306-4522(94)90491-X [DOI] [PubMed] [Google Scholar]
- Semmler A, Frisch C, Debeir T, Ramanathan M, Okulla T, Klockgether T, & Heneka MT (2007). Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Experimental Neurology, 204, 733–740. 10.1016/j.expneurol.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, & Soreq H(2009). MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity, 31, 965–973. 10.1016/j.immuni.2009.09.019 [DOI] [PubMed] [Google Scholar]
- Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dubé S, Mallinckrodt C, Bymaster FP, McKinzie DL, & Felder CC (2008). Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. American Journal of Psychiatry, 165, 1033–1039. 10.1176/appi.ajp.2008.06091591 [DOI] [PubMed] [Google Scholar]
- Shifrin H, Nadler-Milbauer M, Shoham S, & Weinstock M (2013). Rivastigmine alleviates experimentally induced colitis in mice and rats by acting at central and peripheral sites to modulate immune responses. PLoS One, 8, e57668. 10.1371/journal.pone.0057668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman HA, Dancho M, Regnier-Golanov A, Nasim M, Ochani M, Olofsson PS, … Pavlov VA (2015). Brain region-specific alterations in the gene expression of cytokines, immune cell markers and cholinergic system components during peripheral endotoxin-induced inflammation. Molecular Medicine, 20, 601–611. 10.2119/molmed.2014.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitapara RA, A. G. G, Valdés-Ferrer SI, Lin M, Patel V, Wang M, Martino AT, … Mantel LL (2020) The α7 nicotinic acetylcholine receptor agonist, GTS-21, attenuates hyperoxiainduced acute inflammatory lung injury by alleviating the accumulation of HMGB1 in the airways and the circulation. Molecular Medicine, 26(3), 1–12. 10.1186/s10020-020-00177-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JG, Li HH, Cao YF, Lv X, Zhang P, Li YS, Zheng YJ, Li Q, Yin PH, Song SL, Wang HY, & Wang XR (2012). Electroacupuncture improves survival in rats with lethal endotoxemia via the autonomic nervous system. Anesthesiology, 116, 406–414. [DOI] [PubMed] [Google Scholar]
- Soreq H, & Seidman S (2001). Acetylcholinesterase-new roles for an old actor. Nature Reviews. Neuroscience, 2, 294–302. [DOI] [PubMed] [Google Scholar]
- Sperling LE, Pires Reis K, Nicola F, Euzebio Teixeira C, Gulielmin Dido G, Dos Santos MG, … Pranke P (2019). Galantamine improves functional recovery and reduces lesion size in a rat model of spinal cord injury. Brain Research, 1724, 146424. 10.1016/j.brainres.2019.146424 [DOI] [PubMed] [Google Scholar]
- Stokholm MG, Iranzo A, Østergaard K, Serradell M, Otto M, Svendsen KB, … Pavese N (2017). Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: A case-control study. The Lancet Neurology, 16(10), 789–796. 10.1016/s1474-4422(17)30173-4 [DOI] [PubMed] [Google Scholar]
- Stoyanov E (1964). Galanthaminum hydrobromicum “Nivalin”, ein neues Antidot der nichtdepolarisierenden Muskelrelaxantien. Pharmakologie Und Klinische Anwendung. Der Anaesthesist, 13, 217–220. [PubMed] [Google Scholar]
- Sugarman DE, De Aquino JP, Poling J, & Sofuoglu M (2019). Feasibility and effects of galantamine on cognition in humans with cannabis use disorder. Pharmacology, Biochemistry, and Behavior, 181, 86–92. 10.1016/j.pbb.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, & Herrmann N (2010). A meta-analysis of cytokines in Alzheimer’s disease. Biological Psychiatry, 68, 930–941. 10.1016/j.biopsych.2010.06.012 [DOI] [PubMed] [Google Scholar]
- Talbot S, Foster SL, & Woolf CJ (2016). Neuroimmunity: Physiology and pathology. Annual Review of Immunology, 34, 421–447. 10.1146/annurev-immunol-041015-055340 [DOI] [PubMed] [Google Scholar]
- Tan ECK, Johnell K, Garcia-Ptacek S, Haaksma ML, Fastbom J, Bell JS, & Eriksdotter M (2018). Acetylcholinesterase inhibitors and risk of stroke and death in people with dementia. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 14, 944–951. 10.1016/j.jalz.2018.02.011 [DOI] [PubMed] [Google Scholar]
- Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, & Ding C (2000). A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology, 54, 2269–2276. 10.1212/WNL.54.12.2269 [DOI] [PubMed] [Google Scholar]
- Tarnawski L, Reardon C, Caravaca AS, Rosas-Ballina M, Tusche MW, Drake AR, … Olofsson PS (2018). Adenylyl cyclase 6 mediates inhibition of TNF in the inflammatory reflex. Frontiers in Immunology, 9, 2648. 10.3389/fimmu.2018.02648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenovuo O (2005). Central acetylcholinesterase inhibitors in the treatment of chronic traumatic brain injury-clinical experience in 111 patients. Progress in neuro-psychopharmacology & Biological Psychiatry, 29, 61–67. 10.1016/j.pnpbp.2004.10.006 [DOI] [PubMed] [Google Scholar]
- Texidó L, Ros E, Martín-Satué M, López S, Aleu J, Marsal J, & Solsona C (2005). Effect of galantamine on the human alpha7 neuronal nicotinic acetylcholine receptor, the Torpedo nicotinic acetylcholine receptor and spontaneous cholinergic synaptic activity. British Journal of Pharmacology, 145, 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A (2013). Muscarinic signaling in the brain. Annual Review of Neuroscience, 36, 271–294. 10.1146/annurev-neuro-062012-170433 [DOI] [PubMed] [Google Scholar]
- Thompson CA (2001). FDA approves galantamine for Alzheimer’s disease. American Journal of health-system Pharmacy: AJHP: Official Journal of the American Society of Health-System Pharmacists, 58, 649. 10.1093/ajhp/58.8.649a [DOI] [PubMed] [Google Scholar]
- Torrey EF, & Peterson MR (1973). Slow and latent viruses in schizophrenia. Lancet (London, England), 2, 22–24. 10.1016/S0140-6736(73)91952-1 [DOI] [PubMed] [Google Scholar]
- Tracey KJ (2002). The inflammatory reflex. Nature, 420, 853–859. 10.1038/nature01321 [DOI] [PubMed] [Google Scholar]
- Tracey KJ (2007). Physiology and immunology of the cholinergic antiinflammatory pathway. The Journal of Clinical Investigation, 117, 289–296. 10.1172/JCI30555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ (2009). Reflex control of immunity. Nature Reviews. Immunology, 9, 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, & Rogers RC (2006). Brainstem circuits regulating gastric function. Annual Review of Physiology, 68, 279–305. 10.1146/annurev.physiol.68.040504.094635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, & Bazinet RP (2016). Postmortem evidence of cerebral inflammation in schizophrenia: A systematic review. Molecular Psychiatry, 21, 1009–1026. 10.1038/mp.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunov N (1966). The effect of nivalin in myasthenia gravis pseudoparalytica. In Kuhn E (Ed.), Progressive Muskeldystrophie Myotonie · Myasthenie. Springer, Berlin. [Google Scholar]
- Vaknine S, & Soreq H (2020). Central and peripheral anti-inflammatory effects of acetylcholinesterase inhibitors. Neuropharmacology, 168, 108020. 10.1016/j.neuropharm.2020.108020 [DOI] [PubMed] [Google Scholar]
- Van Dam D, Abramowski D, Staufenbiel M, & De Deyn PP (2005). Symptomatic effect of donepezil, rivastigmine, galantamine and memantine on cognitive deficits in the APP23 model. Psychopharmacology (Berl), 180,177–190. 10.1007/s00213-004-2132-z [DOI] [PubMed] [Google Scholar]
- Van Dam D, & De Deyn PP (2006). Cognitive evaluation of disease-modifying efficacy of galantamine and memantine in the APP23 model. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 16, 59–69. 10.1016/j.euroneuro.2005.06.005 [DOI] [PubMed] [Google Scholar]
- Wang D, Noda Y, Zhou Y, Nitta A, Furukawa H, & Nabeshima T (2007). Synergistic effect of galantamine with risperidone on impairment of social interaction in phencyclidine-treated mice as a schizophrenic animal model. Neuropharmacology, 52, 1179–1187. 10.1016/j.neuropharm.2006.12.007 [DOI] [PubMed] [Google Scholar]
- Wang T, Zhu H, Hou Y, Gu W, Wu H, Luan Y, Xiao C, & Zhou C (2018). Galantamine reversed early postoperative cognitive deficit via alleviating inflammation and enhancing synaptic transmission in mouse hippocampus. European Journal of Pharmacology, 846, 63–72. 10.1016/j.ejphar.2018.12.034 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yamagata N, Takasaki K, Sano K, Hayakawa K, Katsurabayashi S, Egashira N, Mishima K, Iwasaki K, & Fujiwara M (2009). Decreased acetylcholine release is correlated to memory impairment in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain Research, 1249, 222–228. 10.1016/j.brainres.2008.10.029 [DOI] [PubMed] [Google Scholar]
- Wazea SA, Wadie W, Bahgat AK, & El-Abhar HS (2018). Galantamine anti-colitic effect: Role of alpha-7 nicotinic acetylcholine receptor in modulating Jak/STAT3, NF-ºB/HMGB1/RAGE and p-AKT/Bcl-2 pathways. Scientific Reports, 8, 5110. 10.1038/s41598-018-23359-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, & Sternberg EM (2002). Neuroendocrine regulation of immunity. Annual Review of Immunology, 20, 125–163. [DOI] [PubMed] [Google Scholar]
- Weller J, & Budson A (2018). Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research, 7,1161. 10.12688/f1000research.14506.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D, & Murray J (2001). Galantamine: A randomized, double-blind, dose comparison in patients with Alzheimer’s disease. International Journal of Geriatric Psychiatry, 16, 852–857. 10.1002/gps.409 [DOI] [PubMed] [Google Scholar]
- Woolf NJ (1991). Cholinergic systems in mammalian brain and spinal cord. Progress in Neurobiology, 37, 475–524. 10.1016/0301-0082(91)90006-M [DOI] [PubMed] [Google Scholar]
- Woolf NJ, & Butcher LL (1981). Cholinergic neurons in the caudate-putamen complex proper are intrinsically organized: A combined Evans blue and acetylcholinesterase analysis. Brain Research Bulletin, 7, 487–507. 10.1016/0361-9230(81)90004-6 [DOI] [PubMed] [Google Scholar]
- Woolf NJ, & Butcher LL (2011). Cholinergic systems mediate action from movement to higher consciousness. Behavioural Brain Research, 221, 488–498. 10.1016/j.bbr.2009.12.046 [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Jacobs RW, & Butcher LL (1989). The pontomesencephalotegmental cholinergic system does not degenerate in Alzheimer’s disease. Neuroscience Letters, 96, 277–282. 10.1016/0304-3940(89)90391-1 [DOI] [PubMed] [Google Scholar]
- Wu Z, Zhao L, Chen X, Cheng X, & Zhang Y (2015). Galantamine attenuates amyloid-beta deposition and astrocyte activation in APP/PS1 transgenic mice. Experimental Gerontology, 72, 244–250. [DOI] [PubMed] [Google Scholar]
- Yanagida T, Takeuchi H, Kitamura Y, Takata K, Minamino H, Shibaike T, … Shimohama S (2008). Synergistic effect of galantamine on nicotine-induced neuroprotection in hemiparkinsonian rat model. Neuroscience Research, 62, 254–261. 10.1016/j.neures.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Yang YI, Peng Y, & Yang J (2018). Galantamine protects against hydrochloric acid aspirationinduced acute respiratory distress syndrome in rabbits. Tropical Journal of Pharmaceutical Research, 17, 669. 10.4314/tjpr.v17i4.15 [DOI] [Google Scholar]
- Yoshiyama Y, Kojima A, Itoh K, Isose S, Koide M, Hori K, & Arai K (2015). Does anticholinergic activity affect neuropathology? Implication of neuroinflammation in Alzheimer’s disease. Neurodegenerative Diseases, 15, 140–148. 10.1159/000381484 [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Kojima A, Itoh K, Uchiyama T, & Arai K (2012). Anticholinergics boost the pathological process of neurodegeneration with increased inflammation in a tauopathy mouse model. Neurobiology of Disease, 45, 329–336. 10.1016/j.nbd.2011.08.017 [DOI] [PubMed] [Google Scholar]
- Zaghloul N, Addorisio ME, Silverman HA, Patel HL, Valdés-Ferrer SI, Ayasolla KR, … Pavlov VA (2017). Forebrain cholinergic dysfunction and systemic and brain inflammation in murine sepsis survivors. Frontiers in Immunology, 8, 1673. 10.3389/fimmu.2017.01673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Pistillo E, & Gotti C (2015). Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology, 96, 302–311. 10.1016/j.neuropharm.2014.11.003 [DOI] [PubMed] [Google Scholar]
- Ztaou S, & Amalric M (2019). Contribution of cholinergic interneurons to striatal pathophysiology in Parkinson’s disease. Neurochemistry International, 126, 1–10. 10.1016/j.neuint.2019.02.019 [DOI] [PubMed] [Google Scholar]


