Abstract
Coagulase-negative staphylococci (CoNS) are increasingly becoming a public health issue worldwide due to their growing resistance to antibiotics and common involvement in complications related to invasive surgical procedures, and nosocomial and urinary tract infections. Their behavior either as a commensal or a pathogen is a result of strict regulation of colonization and virulence factors. Although functionality of virulence factors and processes involved in their regulation are quite well understood in S. aureus, little is known about them in CoNS species. Therefore, the aim of our studies was to check if clinical CoNS strains may contain virulence factors and genes involved in resistance to methicillin, that are homologous to S. aureus. Moreover, we checked the presence of elements responsible for regulation of genes that encode virulence factors typical for S. aureus in tested isolates. We also investigated whether the regulation factors produced by one CoNS isolate can affect virulence activity of other strains by co-incubation of tested isolates with supernatant from other isolates. Our studies confirmed the presence of virulence factor and regulatory genes attributed to S. aureus in CoNS isolates and indicated that one strain with an active agr gene is able to affect biofilm formation and δ-toxin activity of strains with inactive agr genes. The cognition of prevalence and regulation of virulence factors as well as antibiotic resistance of CoNS isolates is important for better control and treatment of CoNS infections.
Keywords: staphylococcus, genes transfer, virulence, coagulase negative, CoNS virulence
1. Introduction
The ability of staphylococci to occupy numerous host niches, and exist either as a commensal or a pathogen has been attributed to a strict regulation of colonization and virulence factors, e.g., haemolysins, toxins, adhesins [1].
For many years, only S. aureus was considered as pathogenic among the staphylococci group, but lately it is postulated that coagulase-negative staphylococci (CoNS) may produce various virulence factors typically attributed to S. aureus, e.g., haemolysins, enterotoxins, and thus there may be ethological factors of infections. Therefore, CoNS species also need more scientific attention related to their pathological capacity. Although functionality of virulence factors and processes involved in their regulation are quite well understood in S. aureus, little is known about them in CoNS species [2].
Some S. aureus strains often contain in their genomes virulence genes that are not found in all strains and that may be carried on discrete genetic elements. Genome analyses revealed that there are different sets of genes encoding different virulence factors in S. aureus, as many of these genes are located in pathogenicity islands wherein genes are translocated by horizontal genome transfer (HGT) via phage transduction, conjugation or by direct uptake of naked DNA by genetic competence. The presence/absence of certain genes on the pathogenicity island of any strain may be a consequence of unique horizontal gene transfer events that occurred within specific S. aureus lineages; in this way, a non-virulent strain may become pathogenic. As all staphylococci, not only S. aureus, may contain similar genes or ability to acquire genes from related species by a horizontal gene transfer (HGT) process, thus, CoNS also become increasingly infective. Therefore treatment of infections caused by CoNS is becoming more and more difficult due to the growing rates of resistance to antibiotics and virulence [2]
Particularly, S. aureus but also coagulase-negative (CoNS) staphylococci are able to secrete numerous exotoxins (alpha, beta, gamma, and delta) that invade host cells. Most S. aureus strains are able to produce at least three (haemolysins HlgAB, HlgCB, and leukocidins LukAB/HG) of the six known leucocidins, and many highly virulent clinical strains able to affect humans produce five (HlgAB, HlgCB, LukAB/HG, Panton–Valentine leucocidin [PVL], and LukED) toxins. γ-haemolysins are divided into two types of bicomponent elements, namely, HlgAB and HlgCB. Both of them consist of units from class S: HlgA (class S component encodes gamma haemolysin-A-like protein), or HlgC (encodes gamma haemolysin C) respectively and HlgB unit (encodes gamma haemolysin-B) from class F. These proteins, after recognition of their cell targets, undergo conformational changes and form oligomeric complexes. This process causes trans-membrane pore formation, and therefore leads to cell death. HlgAB toxin is involved in blood stream infections, e.g., bacteremia and septic arthritis [3], and pathogenic processes of toxic shock syndrome (TSS) as it affects polymorph nuclear cells, monocytes, macrophages, and erythrocytes. HlgCB is responsible for neutrophils’ degradation via lysis [4].
Leukocidins are pore-forming cytotoxins that help the bacteria invade host cells. The bi-component pore-forming leukocidins (Luk) include LukAB and lukED. LukAB is a surface-associated molecule secreted outside the bacterial cell that leads to lysis of host immune cells. LukED, similarly to γ-haemolysin CB (HlgCB), leads to neutrophil lysis. LukAB and LukED lead to extended attacks on macrophages [5].
In staphylococci, most virulence factors, including surface proteins and toxins, are regulated by transcriptional regulators’ two-component systems (TCSs) and quorum sensing systems (QS) with an accessory gene regulator (agr) [6].
Strains may contain one of four classes of accessory gene regulator (agr) locus (on the basis of polymorphism of agrD and agrC genes) marked as agrI–agrIV. The agr regulation system belongs its effector, called RNAIII. It affects the expression of many virulence factors, e.g., exotoxins, sae, and genes involved in biofilm formation, peptidoglycan and amino acid metabolism, as well as transport pathways [7]. It also encodes for δ-haemolysin and therefore the expression of hld serves as a surrogate marker to assess agr functionality [8]. Another element of regulation of the expression of virulence toxins is the S. aureus exoprotein expression (Sae) locus. Together with agr, it activates production of toxins, thus affecting bacterial virulence [9].
Interestingly, agr genes together with RNAIII positively regulate genes that are expressed postexponentially, while coagulase is inhibited, as its expression may be control positively and negatively by the agr system according to the growth stage [10].
The main problem with staphylococci eradication is their growing resistance to antibiotics. Most genes involved in antibiotic resistance are localized on plasmids, and thus can be transferred from one strain to another quite easily. The most common S. aureus resistance is mediated by the mecA gene element, found in the mobile genetic element—the SCCmec. It can be transferred from one strain to another through horizontal gene transfer, and thus may be detected in various CoNS as well as coagulase-positive staphylococci (CoPS) species, but in S. sciuri and S. vitulinus mecA alleles do not lead to resistance to beta-lactams. The mecA gene encodes PBP2′ (PBP2a), which is responsible for resistance to methicillin with cross-resistance to other drugs from the β-lactam group [11].
The detection and spread of multidrug-resistant (MDR) CoNS in hospital settings is a rising problem. For instance, S. epidermidis, S. haemolyticus, and S. hominis are some of the most prevalent factors in infective endocarditis, bloodstream infections (BSI), and neonatal sepsis in neonatal intensive care units (NICU) [12]. Studies from 49 hospitals in the United States of America indicated that CoNS represented 31% among all cases of nosocomial BSI within a period of 7 years [13], while studies from Germany confirmed CoNS as the second most common factor of nosocomial infections [14]. Additionally, infections caused by CoNS are particularly common in newborns and preterm neonates when compared to healthy children or adults [15,16]. Besides a high frequency of resistant CoNS in hospitals, the frequency of drug resistance of CoNS isolates from the environment is also growing (including resistance to last-resort antibiotics, e.g., oxazolidinones and lipopeptides) [3,17]. It is noteworthy that according to the literature, the prevalence of MRCoNS is lower than that of MRSA in most studies, but it might be a result of the fact that occurrence of infection with CoNS is not as common as that with S. aureus and often CoNS are not properly diagnosed as the factor of disease, considered only as a commensal members of the skin microbiota [18]. Anyway, the evolution of CoNS from commensals into invasive, resistant pathogens is happening [19].
As our initial, already published, studies [20] indicated that some tested CoNS isolates contain virulence factors that originally come from S. aureus, we decided to investigate this phenomenon further. Additionally, the aim of the present studies was to check if clinical CoNS strains that contain chosen virulence factors were also enriched with elements responsible for regulation of genes that encode virulence factors typical for S. aureus. Moreover, we checked weather these regulation factors can affect virulence activity of other strains. For this purpose, we decided to screen some more isolates of S. haemolyticus, S. hominis, S. simulans, S. warneri, and additionally S. epidermidis species that were confirmed as etiological factors of chosen human infections, for presence of virulence factors to find more strains with different combinations of virulence factors and elements involved in cell regulation required for cross-talk studies. For these purposes, we tested phenotypically as well as genotypically the presence and activity of virulence factors that are homologous to S. aureus. In these studies we also analyzed the association between methicillin resistance and virulence factors of CoNS isolates, as in S. aureus strains a significant relationship between antibiotic resistance and haaemolysin genes has been observed.
The knowledge related to the presence and regulation of virulence factors typical for S. aureus in CoNS strains may help to understand their less known, pathogenic nature, and thus help to visualize the rising problem of CoNS as an etiological problem of infection but also find a way to eradicate them.
2. Materials and Methods
2.1. Material Isolation and Identification
2.1.1. Tested Strains
In current studies, 47 new, not presented before, CoNS isolates were analyzed. Among them, 6 isolates were identified as S. haemolyticus, 6 as S. hominis, 5 as S. simulans, 6 as S. warneri, and 6 as S. epidermidis by MALDI-TOFF and genetic analysis [21]. Additionally, 19 isolates that were partially introduced in a previous manuscript [20] were further investigated. Among tested isolates, 4 of them were identified as S. haemolyticus, 8 as S. hominis, 4 as S. simulans, and 3 as S. warneri. All isolates were from human blood, wounds, the peritoneum, urethra, and skin dermatitis, or in some cases from other parts of the body, such as eye or appendix.
As controls, the following strains were used: S. haemolyticus ATCC 29970, S. hominis subsp. hominis ATCC 27844, S. warneri ATCC 27836, and S. simulans ATCC 27848.
All isolates used were acquired from a diagnostic microbiology laboratory (Synevo Sp. z o.o.) of Lodz area, Poland, during 2014–2016.
2.1.2. DNA and RNA Isolation
The DNA and RNA materials were isolated using the Genomic Micro AX Staphylococcus Gravity and Total RNA Prep Plus Minicolumn Kit (A&A Biotechnology, Gdańsk, Poland), respectively, according to the protocol provided by the manufacturer. The DNA and RNA quality and concentration were evaluated using the Spectrophotometer Genova NanoJenway.
2.2. Analysis of the Virulence
2.2.1. Phenotypic Analysis of Haemolytic Activity of Tested Isolates
The phenotypic manifestation of haemolysines activity was determined on an agar medium with 5% sheep blood. Additionally the activity of β-toxin was tested by reverse CAMP test as well as analysis of hot–cold effect. Haemolytic activity of β-toxin is promoted after incubation at temperatures below 10 °C, thus this toxin is often called as the ‘hot-cold’ haemolysin. Production of δ-toxin was detected by the presence of synergism with β-haemolysin of S. aureus ATCC 25923 (CAMP test). δ-haemolysin produced by a test strain enhances the β-haemolysis caused by S. aureus ATCC 25923 strain. Briefly, tested isolates were streaked perpendicularly to an S. aureus ATCC 25923 strain. Plates were incubated at 37 °C for 18 h [22].
2.2.2. Phenotypic Analysis of Biofilm Formation
Biofilm formation assay was performed as previously described [23]. CoNS cultures were grown for 24 h at 37 °C with agitation. Next, isolates were grown to the turbidity of a 0.5 McFarland standard in TSB medium and 100 μL of each dilution was loaded into the wells of a non-treated flat-bottom 96-well microtiter plate (Nunc). S. aureus ATCC6538 (biofilm forming) and S. epidermidis ATCC12258 (not biofilm-forming) were used as controls. After 24 h incubation at 37 °C for biofilm production, the supernatants were removed and the adherent cells were stained with an aqueous solution of crystal violet (0.1%, w/v) at room temperature and 2× washed with distilled water. Then, the microtiter plates were dried for a few hours. Bound crystal violet was dissolved by treatment with 30% acetic acid at room temperature and optical density (OD) of each well was measured by using a microplate reader (Biotec) at 550 nm. Wells that contained broth only were used as negative control.
Biofilm density was classified according to the scheme presented by Stepanovic et al. [24]. Three standard deviations above the mean OD of the negative control were calculated as the cutoff value (ODc) for each microtiter plate. According to ODc and average OD of the strain, isolates were described as: strong biofilm producer (4ODc ≤ OD); moderate biofilm producer (2ODc ≤ OD ≤ 4ODc); weak biofilm producer (ODc ≤ OD ≤ 2ODc); and no biofilm producer (OD ≤ ODc). Each test was repeated at least four times.
2.2.3. Detection of Genes Encoded Virulence Factors
CoNS isolates (30), not presented previously, were screened for the presence of the following virulence-associated genes: 1. Involved in biofilm formation, icaA, icaB, icaC, and icaD; 2. Panton–Valentine leukocidin, pvl; 3. staphylococcal enterotoxins, sea, seb, sei seg.; 4. haemolysins: hla, hlb, hld, γ-haemolysin component A (hlgA), γ-haemolysin component B (hlgB). S. aureus ATCC25923 and S. epidermidis ATCC12228 strains were used as positive and negative controls. PCR reactions were performed using the primers and parameters described in Table 1.
Table 1.
Primers used for PCR analysis of genes encoding virulence factors.
| Lp. | Transcript | Author | Sequence | Size [bp] |
|---|---|---|---|---|
| 1. | sea | [25] | 5′-GCAGGGAACAGCTTTAGGCGT-3′ 5′-TCTGTAGAAGTATGAAACACG-3′ |
520 |
| 2. | seb | [25] | 5′-ATGTAATTTTGATATTCGCAGTG-3′ 5′-TGCAGGCATCATATCATACCA-3′ |
643 |
| 3. | sei | [25] | 5′-CAACTCGAATTTTCAACAGGTAC-3′ 5′-CAGGCAGTCCATCTCCTG-3′ |
465 |
| 4. | seg | [25] | 5′-CGTCTCCACCTGTTGAAGG-3′ 5′-CCAAGTGATTGTCTATTGTCG-3′ |
327 |
| 5. | Hla | [26] | 5′-CTTTCCAGCCTACTTTTTTATCAGT-3′ 5′-CTGATTACTATCCAAGAAATTCGATTG-3′ |
209 |
| 6. | Hlb | [26] | -GTTGATGAGTAGCTACCTTCAGT-3′ -GTGCACTTACTGACAATAGTGC-3′ |
309 |
| 7. | hld | [26] | 5′-TTAGTGAATTTGTTCACTGTGTCGA-3′ 5′-AAGAATTTTTATCTTAATTAAGGAAGGAGTG-3′ |
111 |
| 8. | hlg | [26] | 5′-CACCAAATGTATAGCCTAAAGTA-3′ 5′-GTCAYAGAGTCCATAATGCATTTAA-3′ |
535 |
| 9. | hlg2 | [26] | 5′-ATAGTCATTAGGATTAGGTTTCACAAAG-3′ 5′-GACATAGAGTCCATAATGCATTYGT-3′ |
390 |
| 10. | hlgCB | [26] | 5′-GCCAATCCGTTATTAGAAAATGC-3′ 5′-CCATAGAYGTAGCAACGGAT-3′ |
938 |
| 11. | lukED | [26] | 5′-TGAAAAAGGTTCAAAGTTGATACGAG-3′ 5′-TGTATTCGATAGCAAAAGCAGTGCA-3′ |
269 |
| 12. | lukAB | [26] | 5′-TCACTTCTCCACCATACTTC-3′ 5′-TATCAGCAGCAACGACTC-3′ |
638 |
| 13. | pvl | [27] | 5′-ATCATTAGGTAAAATGTCTGGACATGATCCA-3′ 5′-GCATCAASTGTATTGGATAGCAAAAGC-3′ |
433 |
Additionally all 49 tested strains were screened for the presence of virulence-associated leucotoxins lukAB (636bp) and lukCD (269bp), and hlgCB genes according to the literature listed in Table 1. The primer sequences for genes encoding the virulence factors are listed in Table 1.
2.3. Antimicrobial Susceptibility Testing
Methicillin-resistant strains were screened by the disc-diffusion method (using Becton Dickinson discs, cefoxitin FOX-30) in accordance with the EUCAST guidelines. S. aureus ATCC 29213 was used as a control strain.
Polymerase chain reaction (PCR) was executed according to the literature [28]. S. aureus 51625 strain was used as a control.
2.4. Agr Detection and Classification
Agr activity of tested isolates was analyzed by the assessment of δ-haemolysin production. Production of δ-toxin was detected as described above [22].
The classification of agr system groups was based on the hypervariable domain of agr locus according to Soares et al. [29]. Multiplex PCR test was performed to type groups based on their product size according to the literature presented in Table 2. The primers sequences for genes encoding the factors involved in regulation of virulence are listed in Table 2.
Table 2.
Primers used for PCR analysis of genes responsible for regulation of virulence.
| Lp. | Transcript | Author | Sequence | Size [bp] |
|---|---|---|---|---|
| 1. | sarA | [30] | 5′-TGG TCA CTT ATG CTG ACA GAT T-3′ | 313 |
| 5′-TTT GCT TCT GTG ATA CGG TTG-3′ | ||||
| 2. | Sae | [31] | 5′-TGT GGG GTT CAG GAA TTG TT-3′ | 680 |
| 5′-ATT GAT GAG AAG GAT GCC CA-3′ | ||||
| 3. | RNAIII | [32] | 5′-ATGATCACAGAGATGTGA-3′ | 514 |
| 5′-CTGAGTCCTAGGAAACTAACTC-3′ | ||||
| 4. | agrI | [33,34] | 5′-GTCACAAGTACTATAAGCTGCGAT-3′ | 439/441 |
| 5′-ATGCACATGGTGCACATGC-3′ | ||||
| 5. | agrII | [33,34] | 5′-TATTACTAATTGAAAAGTGGCCATAGC-3′ | 572/575 |
| 5′-ATGCACATGGTGCACATGC-3′ | ||||
| 6. | agrIII | [33,34] | 5′-GTAATGTAATAGCTTGTATAATAATACCCAG-3′ | 321/323 |
| 5′-ATGCACATGGTGCACATGC-3′ | ||||
| 7. | agrIV | [33,34] | 5′-ATGCACATGGTGCACATGC-3′ | 657/659 |
| 5′-CGATAATGCCGTAATACCCG3′ |
The genes’ expression was tested by a real-time PCR assay on the Rotor-Gene™ 6000 thermocycler (Corbett Life Science; Qiagen). The primer sequences of used primers are presented in Table 3. Briefly, PCRs were set up using cDNA derived from the input RNA. Reverse transcriptase (RT) reaction was performed using Enhanced Avian HS RT-PCR Kit (Sigma) in accordance with the manufacturer’s protocol. Gene DHFR encoded dihydrofolate reductase was used as a reference.
Table 3.
Primers used for analysis of the expression of genes encoding virulence factors and main genes involved in regulation of virulence in Staphylococcus by RT-PCR.
| Lp. | Transcript | Author | Sequence |
|---|---|---|---|
| 1. | 16S rRNA | [35] | 5′- TGAGATGTTGGGTTAAGTCCCGCA-3′ |
| 5′-CGGTTTCGCTGCCCTTTGTATTGT-3′ | |||
| 2. | hlgA | [36] | 5′-AATCGGAGGCAGTGGCTCATTCAA-3′ |
| 5′-GGACCAGTTGGGTCTTGTGCAAAT-3′ | |||
| 3. | hlgCB | [36] | 5′-TCGGTGGTAATTTCCAATCAGCCC-3′ |
| 5′-CGAATGAATTCGCTTTGACGCCC-3′ | |||
| 4. | lukED | [37] | 5′-GAAATGGGGCGTTACTCAAA-3′ |
| 5′-GAATGGCCAAATCATTCGTT-3′ | |||
| 5. | RNAIII | [38] | 5′-TTTATCTTAATTAAGGAAGGAGTGA-3′ |
| 5′-TGAATTTGTTCACTGTGTCG-3′ | |||
| 6. | lukAB | [39] | 5′-CGT GGA GCG TTA ACT GGA AAT A -3′ |
| 5′-ACA CCT TTA TGT GAC GTA GAT TGA -3′ | |||
| 7. | agrI | [40] | 5′-CCAGCTATAATTAGTGGTATTAAGTACAGTAAACT-3′ |
| 5′-AGGACGCGCTATCAAACATTTT-3′ | |||
| 5′-ATAGGAATTTCGACATTATC-3′ | |||
| 8. | agrII | [40] | 5′-CAATAGTAACAATTTTAGTGACCATGATCA-3′ |
| 5′-GCAGGATCAGTAGTGTATTTTCTTAAAGTT-3′ | |||
| 5′-TTGCAACAGTAGGTTTGTT-3′ | |||
| 9. | agrIII | [40] | 5′-CATTATAACAATTTCACACAGCGTGTT-3′ |
| 5′-GCAAGTGCATAAGAAATTGATACATACA-3′ | |||
| 5′-ATAGTTCTACCAATCTTTTTGG-3′ | |||
| 10. | hld | [41] | 5′-AAGAATTTTTATCTTAATTAAGGAAGGAGTG-3′ |
| 5′-TTAGTGAATTTGTTCACTGTGTGA-3′ | |||
| 11. | sarA | [42] | 5′-GTAATGAGCATGATGAAAGAACTGT-3′ |
| 5′-CGTTGTTTGCTTCAGTGATTCG-3′ | |||
| 12. | sae | [43] | 5′-CAACCATTGCGATTTCTTTACC-3′ |
| 5′-TTAGCTTTAGGTGCTTGTGG-3′ |
2.5. Cross-Inhibition of the Biofilm Formation and the Influence on δ-Toxin Activity among Agr Groups
2.5.1. Supernatant Preparation
The single colonies of CoNS strains with functional agrI, II, or III were grown overnight in 4 mL of TSB (Becton Dickinson) with 100 rpm agitation at 37 °C. After the incubation time, 50 mL of new TSB medium was inoculated with the overnight cultures of each strain and incubated at 37 °C with 100 rpm agitation for 18 h. Next, supernatants were obtained by centrifugation, and cells were eliminated by filtration through a 0.2 μm membrane. The supernatants were stored at 4 °C. The microbiological purity of supernatants was tested on agar medium with 5% sheep blood [44].
2.5.2. Analysis of Cross-Inhibition of Biofilm Formation
CoNS strains with not functional agrI, agr II, or agr III genes were grown for 24 h at 37 °C with agitation. Next, isolates were grown to the turbidity of a 0.5 McFarland standard in TSB medium and 100 μL of each sample was loaded into the wells of a non-treated flat-bottom 96-well microtiter plate (Nunc); then, 100 μL of supernatant was added. The CoNS strains with non-functional agrI, II, or III genes were grown in this TSB supernatant (50–50%) mixture at 37 °C for 24 h. Next, the determination of biofilm formation was executed as mentioned above. S. aureus ATCC6538 (biofilm-forming) and S. epidermidis ATCC12258 (not biofilm-forming) were used as controls [44].
2.5.3. Analysis of the Influence on δ-Toxin Activity
CoNS strains with non-functional agrI, II, or III genes were grown for 24 h at 37 °C with agitation in TSB supernatants (50–50%) mixture. Production of δ-toxin was assessed by CAMP test as described above [22].
2.6. Statistical analysis
To assess correlation of variables, the phi square test was used. The results in phi tests range from 0 to 1, where 1 means a significant association, while 0 means no relationship; thus, results can be interpreted as follows: >0—no or very weak, > 0.05 weak; > 0.10 moderate; > 0.15 strong; > 0.25 very strong.
3. Results
3.1. The Presence of Virulence Factors, mecA Gene, and Susceptibility to Methicillin
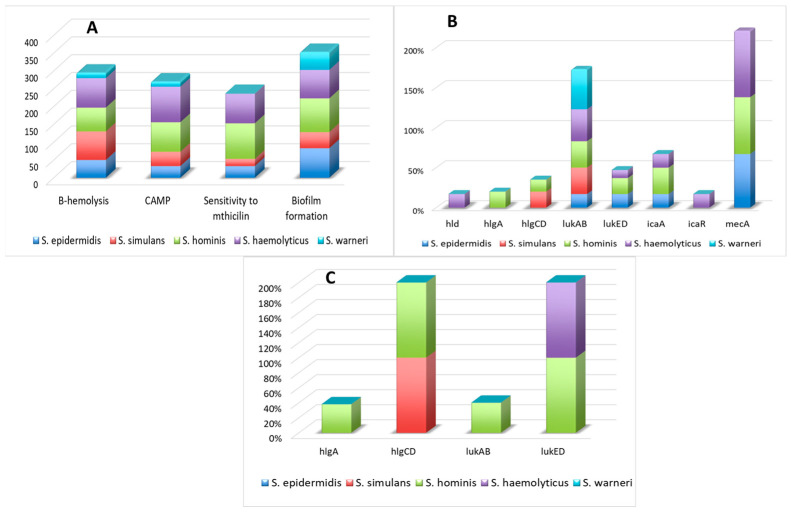
The results of phenotypic manifestation of virulence factors and the presence of their genes in CoNS isolates are presented in Figure 1.
Figure 1.
The presence of virulence factors that are homologous to S. aureus was tested phenotypically (A) and genotypically (B). Figure (C) presents the expression of detected genes.
Most S. haemolyticus and S. simulans isolates indicted haemolytic activity on agar plates supplemented with sheep blood and gave a positive result of the CAMP, which proves that they contained active Β and δ haemolysin genes. Similar haemolytic properties were presented in at least half of tested S. epidermidis and S. hominis isolates, but just a few S. warneri isolates. Moreover, most S. homonis isolates indicated positive CAMP results, while just some isolates from other species presented δ haemolysin activity. None of tested isolates gave a positive result of the CAMP reversed test.
Interestingly, at the same time, the hla, hlb genes that encode haemolysins were not detected in any of the tested isolates, while hld was identified in only one of the tested S. haemolyticus isolates. The hlgA component was detected in less than a quarter of S. hominis isolates but subunit hlgB was not detected in any tested isolates. The hlgCB unit was identified with similar frequency in S. simulans isolates and even less often in S. hominis isolates. The most common gene among all detected genes was lukAB, detected in isolates belonging to all tested species. It was the most prevalent in S. haemolyticus and S. warneri isolates, while in other isolates beside S. epidermidis, its frequency was not lower than 30%.
Most S. epidermidis, S. haemolyticus, and S. homonis isolates were able to produce biofilm, but among all tested elements of icaADBC operon only the icaA gene was detected in three out of five tested species; the icaR transcriptional repressor was found only in a few S. haemolyticus isolates, while other genes were not detected.
Neither the pvl nor any of the enterotoxins genes were detected in any of the tested isolates.
All tested S. hominis and most S. haemolyticus isolates were resistant to methicillin, while all S. warneri isolates indicated sensitivity to the mentioned antibiotic. Phenotypically obtained results were in accordance with results obtained from mecA gene detection studies, as the mecA gene was not detected in any S. warneri isolates, while it was especially common in S. hominis and S. haemolyticus isolates. The mecA gen was not detected in isolates classified as S. simulans, while a few of these isolates were resistant to methicillin in phenotypic studies.
3.2. The Presence of Regulatory Factors
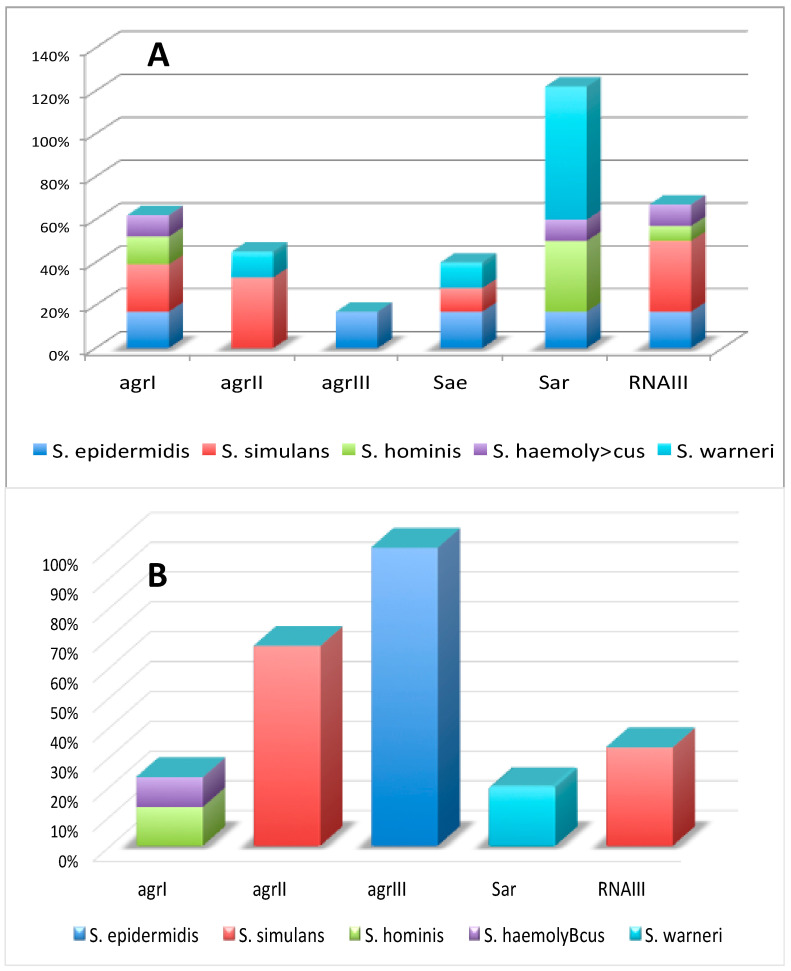
As presented in Figure 2A, among genes that belong to the accessory gene regulatory (agr) system, agrI was detected in isolates assigned to four out of five tested species, but in all cases with quite moderate frequency. The agrII gene was present in more than a quarter of S. simulans isolates and just a few S. warneri isolates. The agrIII gene was found in only several S. epidermidis isolates, while agrIV was not detected in any tested isolates. Other tested genes involved in regulation of the virulence of bacteria were quite common in tested species; sar and sae genes were found in isolates that belonged to all tested species except S. simulans isolates or S. hominis and S. haemolyticus isolates, respectively. The RNAIII gene was not found in any S. warneri isolates, while in isolates that belonged to other species its frequency was quite moderate.
Figure 2.
The presence of genes encoding regulators of virulence factors (the accessory gene regulators, (agr) (A) and their expression (B).
3.3. Statistical Analysis Materials: Będzie Ficant Associa
Statistically, a significant association between the presence of the agrI gene and lukAB, lukED, icaR, and hlgCB genes for all tested isolates from all tested species was observed (for all tested isolates p value = 0.0015–0.04), but at the same time no association between the presence of the agrI gene and any phenotypically demonstrated features was observed (for all tested isolates p value = 0.12–0.7).
The meaningful relationship between the presence of agrII and hlgA/lukAB genes was found for isolates classified as S. simulans (p value = 0.0027 in both cases).
Significant association was also observed for the agrIII gene and lukAB, lukED, and icaR genes in isolates classified as S. epidermidis (p value = 0.014 in all cases).
Interestingly, the association between particular agr genes, the mecA gene, and phenotypically manifested virulence features was observed only between agrII genes and biofilm formation for S. simulans isolates, the agrII gene and results of the CAMP test for S. warneri isolates, the mecA gene and β-haemolysis for S. haemolyticus, and mecA and the result of the CAMP test for S. hominis isolates.
There was also no meaningful correlation between antibiotic resistance manifested phenotypically and the presence of the genes for alpha, delta, and beta haemolysins.
There was no association between the presence of the mecA gene and any genes responsible for virulence or genes involved in regulation of these genes. On the other hand, the association between the presence of the mecA gene and biofilm formation manifested phenotypically (for S. haemolyticus isolates), the mecA gene and β-haemolysis (for S. hominis isolates) was observed.
3.4. Activity of Genes Encoding Virulence and Regulatory Factors
As presented in Figure 1C, the hlgA component presence was detected only in several S. hominis isolates. It has to be noted that activity of the hlgAB gene requires the presence of either active hlgA or hlgB components; thus, as the hlgB gene was not found in any tested isolates, the hlgAB gene was not active in any tested isolates. The expression of hlgCB and lukED was confirmed in all S. simulans and S. hominis as well as S. haemolyticus and S. hominis isolates, respectively. The functional lukAB complex was detected in less than half of S. hominis isolates enriched with this gene. The hld gene was not active in tested isolates. The frequency of isolates with an active agrI gene was marginal in S. hominis and S. haemolyticus, while most agrII genes present in S. simulans isolates were expressed (see Figure 2B). The agrIII genes were active in all S. epidermidis isolates. The sae gene did not exhibit expression in any tested isolates, while sar gene expression was detected in less than a quarter of S. warneri isolates. A few more S. simulans isolates indicted the RNAIII expression.
It is noteworthy that in only one isolate that was classified as S. simulans, species agrI gene and hlgCB genes were expressed, while lukAB gene was inactive. All isolates with an active agr gene (agrI or agrIII) carried the inactive lukAB gene.
Among all 49 tested isolates, 11 expressed one of the agr genes; 27% of these isolates came from blood, 45% from wounds, and 18% from foot ulceration. Among 7 of the most expressed isolates (that contained at least 3 genes involved in bacterial virulence), 43% came from blood and 28% came from peritoneum. Among S. epidermidis isolates, only one of them contained inactive agrI and active agrIII genes at the same time, as well as inactive lukAB, sae, sar, and mec genes. It gave a negative result for the CAMP test and to β-type haemolysis. All S. hominis isolates with active agrI genes led to β-type haemolysis and gave positive results for the CAMP test. Additionally, one of these isolates indicated inactive hlgA and hlgCB genes, but an active mecA gene. All S. simulans isolates with active agrII genes led to β-type haemolysis and gave positive results for the CAMP test, but in none of them was the hld gene detected. Only one isolate classified as S. haemolyticus with an active agrI gene was detected. This isolate had also hld, lukED, and RNAIII genes, but none of these genes was active and none of these isolates led to β-type haemolysis and gave positive results for the CAMP test.
3.5. The Cross-Talk Analysis
To check whether the inter- and intra-strain and species cross-inhibition (interaction) may occur, isolates with inactive agr genes were incubated with cell-free supernatants from strains with active agr genes. The influence of one isolate on another one was analyzed by phenotypic manifestation of biofilm formation and activity of δ-toxin in the CAMP test (obtained results are presented in Table 4).
Table 4.
The cross-talk between isolates from the same as well as different species.
| Supernatant | Strain | CAMP | Β-Haemolysis | Biofilm |
|---|---|---|---|---|
| Without supernatant | S. epidermidis ATCC12228 * | + | + | + |
|
S. aureus ATCC 25923 * |
n/a | + | + | |
| S. simulans 01 * | - | + | ++ | |
| S. simulans 02 * | + | + | +++ | |
| S. epidermidis */** | - | + | +++ | |
| S. simulans 03 ** | + | + | +++ | |
| S. hominis 01 * | + | + | ++ | |
| S. haemolyticus * | + | + | ++ | |
| S. hominis 02 * | + | + | ++ | |
| S. simulans 04 ** | + | + | ++ | |
| S. simulans 03 | S. epidermidis ATCC12228 | + | + | + |
| S. simulans 01 | + | + | + | |
| S. simulans 02 | + | + | + | |
| S. epidermidis | + | + | + | |
| S. hominis 01 | S. epidermidis ATCC12228 | - | + | + |
| S. simulans 01 | - | + | + | |
| S. simulans 02 | - | + | + | |
| S. epidermidis | - | + | + | |
| S. haemolyticus | S. epidermidis ATCC12228 | + | + | + |
| S. simulans 01 | + | + | + | |
| S. simulans 02 | + | + | + | |
| S. epidermidis | + | + | + | |
| S. hominis 02 | S. epidermidis ATCC12228 | - | + | + |
| S. simulans 01 | - | + | + | |
| S. simulans 02 | - | + | + | |
| S. epidermidis | - | + | + | |
| S. simulans 04 | S. epidermidis ATCC12228 | + | + | + |
| S. simulans 01 | + | + | + | |
| S. simulans 02 | + | + | + | |
| S. epidermidis | + | + | + | |
| S. epidermidis | S. epidermidis ATCC12228 | + | + | + |
| S. simulans 01 | + | + | + | |
| S. simulans 02 | + | + | + | |
|
S. aureus ATCC 25923 |
S. epidermidis ATCC12228 | n/a | + | + |
| S. simulans 01 | n/a | + | + | |
| S. simulans 02 | n/a | + | + | |
| S. epidermidis | n/a | + | + |
* Inactive agrI Gene, ** Active agrIII or agrII Gene. - No Biofilm Producer, + Weak Biofilm Producer, ++ Moderate Biofilm Producer, +++ Strong Biofilm Producer.
It has been observed that supernatants led to reduction of biofilm formation by isolates with inactive agr genes, except the S. epidermidis ATCC12228 strain, as in this case used supernatants did not affect biofilm production. No changes in the level of inhibition between supernatants from different isolates were observed and there was no difference whether: (1). Supernatants came from isolate of the same species as treated isolates or not, (2). Supernatants came from isolates with strong or weak ability to form biofilm, (3). Treated isolates were strong or weak biofilm producers. As supernatants did not affect bacterial growth, it means that reduction of biofilm formation by tested supernatants was not a result of changes in bacterial growth.
All used supernatants from isolates with an active agrII gene or combination of inactive agrI and active agrIII gene were able to activate previously inactive δ-haemolysin activity of isolates with inactive agr genes, thus giving positive results in the CAMP test. Incubation of all tested isolates with supernatants from S. hominis 01 and S. hominis 02 (with active agrI gene) isolates led to negative results in the CAMP test, while incubation of all tested isolates with supernatant from S. haemolyticus (with active agrI gene) isolates led to positive results in the CAMP test for all tested isolates. Similarly to results from the test of the influence of supernatant on biofilm formation, no differences were observed when supernatants came from the isolate that belonged to the same species as treated isolate.
4. Discussion
Virulence factors including hydrolytic enzymes, leukocidin, enterotoxins, and haemolysins play important roles in staphylococcal escape from both innate and adaptive immune responses, and growth and spread of bacteria in the host. The presence of virulence factors in CoNS may not only increase their pathogenicity, but also make them a reservoir for resistant genes that can be transmitted to other pathogens [45].
Haemolysins produced by staphylococci indicate cytolytic effects on many different types of cells, e.g., erythrocytes, platelets, monocytes, and neutrophils [46]. During chronic pathogenesis, the selective pressures from antibiotic activity and the host immune response can increase expression of α-haemolysin, which is involved in colonization of S. aureus in respiratory tract infections. Beta-haemolysin (Hlb), besides induction of haemolysis on blood agar plates in a limited number of S. aureus strains, increases the host cell susceptibility to α-haemolysin and PVL and leads to lymphotoxicity, and DNA cleavage [47,48]. The hld gene that encodes delta haemolysin is located within the RNAIII locus of accessory gene regulator (agr), thus the expression of delta haemolysin genes can be a useful marker of agr function [49]. Delta haemolysin activates neutrophils leading to the generation of reactive oxygen intermediates (ROI) and induces the release of pro-inflammatory cytokines from keratinocytes [50]. It may lead to intestinal diseases, e.g., acute diarrhea to severe enteritis [51].
δ-Haemolysin in opposition to Γ-haemolysin, leads to destruction of red blood cells only at high concentrations as it is able to create a trans-membrane pore which lyses the cell membrane. It is well documented that HlgAB and HlgCB toxins require the presence of a class S and a class F component for increased activity and the relation between these two elements is synergistic. [52]. Overexpression of HlgAB determines the highly virulent phenotype of Newman strain as it is strongly responsible for haemolytic activity of bacterial strains. Interestingly, in the presence of HlgAB toxin, the hla gene could indicate only marginal activity [53].
In our studies, the hlgB gene was not found in any tested isolates, which suggests that the hlgAB complex was inactive in all cases. It has to be noted that γ-haemolysin components are located in the core genome and are highly conserved in most S. aureus lineages, but the diversity within their coding sequences had been proved [54].
Although lukAB genes, similarly to hlg genes, are located on core genome, and thus should not be as easy a subject of horizontal transfer as genes located on plasmids or pathogenicity islands, they were the most commonly detected in tested isolates. In contrast to lukAB, the lukED gene sequence is highly conserved [55] and unlike lukAB, and hlg, is located on pathogenicity island (SaPI) vSa, which is stable and not relocated via horizontal gene transfer [56]. Additionally, sequence identity between LukED and PVL is around 75% [37]. In our studies, the lukED gene was also detected in S. epidermidis, S. hominis, and S. haemolyticus isolates, but with lower prevalence than lukAB, although higher than pvl gene, and its activity was confirmed in all tested S. hominis and S. haemolyticus isolates. According to the literature, the prevalence of the lukED genes may vary between different strains as in some studies lukED genes were found in 87% of S. aureus strains while in other studies a prevalence of about 30% in human clinical and colonizing isolates was noted [37,57].
The hla, hlb genes were not detected in any tested isolates, although most S. haemolyticus and S. simulans isolates led to haemolysis on agar plates with sheep blood. This means that probably most tested isolates contained genes encoding B haemolysin with a different sequence than that used in our studies’ sequence from S. aureus. Additionally, neither pvl nor any enterotoxins genes were detected in tested isolates, while the hld gene was detected only in one S. hominis isolate. The obtained results are in accordance with our already published results of the characteristic of CoNS isolates from, e.g., blood and wounds, and allow us to conclude that transfer of hla, hlb, as well as pvl and any tested enterotoxins genes from S. aureus into tested CoNS isolates via horizontal transfer did not occur [20]. It is well documented that some toxins, e.g., alpha haemolysin, have been encoded in the genome, while others, e.g., enterotoxins, Panton–Valentine leukocidin (PVL), are encoded on mobile genetic elements such as plasmids, prophages, transposons, or pathogenicity islands [58,59]. As mobile genetic elements are effective vehicles for spreading virulence and drug-resistant genes between S. aureus strains through horizontal gene transfer, we should expect that toxin genes such as sea, pvl should be acquired by tested isolates more easily than hlgAB or hlgCB genes localized on the core genome, but the obtained result show the opposite tendency. Moreover, studies presented by some other researchers indicated the prevalence of these genes in CoNS isolates. For instance, Udo et al. indicated that the seb gene was dominant in CoNS isolated from adult patients [60], while in studies presented by Vasconcelos et al., gene was the most common among tested virulence factors in CoNS isolates obtained from newborns in Brazil [61]. Nasaj et al. indicated that more than 50% of analyzed CoNS isolates from hospitalized patients in Iran showed prevalence of hla, hlb, hlg, and hld genes [62]. Nevertheless, there are suggestions that the presence of virulence genes in CoNS isolates may vary depending on the geographical locations [60,62, 63] and different environmental conditions may cause extensive changes in resistance and pathogenicity of bacteria [64,65].
Many CoNS are able to form biofilms to enhance their virulence as well as to protect themselves from the diffusion of antibiotics into the host cells [66]. Biofilms are able to adhere to various objects, e.g., indwelling medical devices. This process is possible due to surface proteins’ polysaccharide intercellular adhesion (PIA), regulated by icaADBC genes [67]. The most prevalent among ica genes was icaA, detected in S. epidermidis, S. hominis, and S. haemolyticus isolates. Besides icaA, only the icaR gene was found in some S. haemolyticus isolates and no other ica genes were disclosed in tested isolates. Anyway, most tested isolates were classified phenotypically as biofilm producers. Kord et.al. proved that bacterial strains are able to form biofilms in the absence of ica genes, which may suggest the presence of specific mechanisms of biofilm formation independent of ica genes [68]. Interestingly, recent studies have indicated that haemolysins are also involved in biofilm formation in S. aureus [69].
Antibiotic resistance of staphylococci including CoNS, especially against betalactams over the years [70]. It is well documented that when methicillin-sensitive S. aureus (MSSA) strains receive SCCmec complex, they become resistant to methicillin [71]. Nowadays, the presence of the mecA gene is quite common in staphylococci. For instance, it has been detected in 80% of the CoNS isolates causing late-onset sepsis in neonates [69]. Additionally, some strains may contain also another mec gene, a SCCmec element type XI called mecC [72]. The mecC gene is not as common in staphylococcal strains as the mecA gene, but slowly the frequency of its presence in staphylococcal strains has become ubiquitous. In our studies, although the mecA gen was not detected in any isolates classified as S. simulans and in only around 30% of S. haemolyticus isolates, respectively, almost a quarter of all these isolates were resistant to methicillin in phenotypic studies. The obtained results suggest that these isolates may harbor the mecC gene, which is responsible for resistance to cefoxitin, giving in our case MRSS and MRSH phenotypes, respectively [73]. Moreover, only around 50% of S. haemolyticus isolates with the mecA gene present indicated resistance to methicillin phenotypically, which suggests that in only around half of these isolates was the mecA gene active. It should be noted that the obtained results come from a limited number of isolates classified as particular species but also that many different factors may lead to antibiotic resistance, including age, gender, climatic conditions, food type, and especially regional culture [74]. According to the literature, prevalence of methicillin-resistant CoNS may vary depending on the geographic regions, with MR-CoNS rates ranging between 16 and 50% [75,76]. Community-acquired commensal CoNS in Europe indicated low prevalence of mecA. For instance, in studies that were carried out in Germany, mecA detection among the CoNS isolates was low (i.e., 7%) and only 10% of tested patients were colonized by at least one MR-CoNS isolate. Studies from Etiopia confirmed high frequency (17%) of the presence of resistant CoNS strains (more than 50% were resistant to penicillin, ampicillin, tetracycline, or sulfamethoxazole-trimethoprim) [17,77,78].
The staphylococcal agr-QS system upregulates α, β, γ, and δ–haemolysins, leukotoxins, lipases, while it represses the transcription of cell wall-associated proteins [79], thus the loss of activity of agr genes reduces expression of genes encoded haemolytic activity and the isolate without functional agr should produce only one type of haemolysin (usually a-haemolysin) [80]. This system also coordinates the expression of its effector molecule RNAIII, which as was already mentioned modulates the expression of virulence factors at transcriptional and post-transcriptional levels [81]. There are suggestions that enterotoxins and leukocidins are upregulated also by sae (accessory element) and sar [82]. Interestingly, the agr system upregulates toxin genes’ expression during the late phase of growth and downregulates the cell surface factor by responding to auto-inducing peptides (AIP) produced by S. aureus [81].
In our studies isolates from wounds and blood showed the most prevalence of agr genes; isolates from peritoneum were also well equipped with genes from the agr regulatory system. Li et al. indicated that S. epidermidis isolates from catheters, blood cultures, urine, wounds, sputa, cerebrospinal fluid, and dialysate were prevalent in agr group I genes, while agr group III genes were rarely detected [83]. Our analysis, based on polymorphisms of the agr gene, indicated that S. epidermidis, S. hominis, and S. haemolyticus isolates were prevalent in agrI gene similarly to MRSA isolates from many already published studies [84,85], while S. simulans and S warneri isolates contained mainly agrII genes. The agrIII genes were detected only in some S. epidermidis isolates, but all of these genes were active. Moreover, S. simulans isolates were the most frequently equipped with active agr genes (55% of tested isolates in total), while in isolates denoted as other CoNS species, the presence of agr genes was not very common. It is postulated that agr group I is strongly associated with CA-MRSA genotypes, while agr group II is more correlated with HA-MRSA in human isolates [86]. In addition, another study reported that methicillin resistance of bovine isolates is more prevalent in agr group I than other groups [87]. The mecA genes are found in agr groups I, II, and III, but group IV strains have not acquired a SCCmec element [88].
Beside agr genes, the most frequently detected gene responsible for regulation of virulence was the sar gene, but its activity was limited to only a few isolates denoted as S. simulans and S. warneri. The presence of sae and RNAIII genes was rather marginal and in only a few cases were RNAIII genes active, while in all tested isolates enriched in the sae gene it was inactive. These genes were detected only in S. epidermidis, S. simulans, and S. warneri isolates. S. warneri isolates and S. hominis isolates were enriched in the sar gene, while in isolates assigned to other CoNS species we detected similarly marginal frequency of the sae gene. The agrII genes in most S. simulans and all agrIII genes in S epidermidis isolates were active, while only in some S. simulans and S. warneri isolates were active RNAIII and sar genes found. This means that in any of tested isolates, the complete set of functional agr regulatory system homologous to S. aureus was not found. It is noteworthy that the identity in the agr locus among different Staphylococcus spp. was high, especially in the first 50 and last 150 nucleotides, but also many insertion/deletions, especially in the 5’ end may be found [89]. For instance, the agr locus of S. epidermidis is nearly 68% homologous to S. aureus locus [90].
It is postulated that superantigens production in S. aureus are directly correlated to the agr type of isolates. Many studies presented in the literature indicated that isolates prevalent in superantigens usually contained agr type I or III genes, which is in accordance with our studies where isolates with detected toxin genes were prevalent in the agrI gene. However, in some other reports, agrII isolates were predominant. Additionally, significant association between many virulence factors and agrI was indicated, but biofilm, hlb, hlg and hld were significantly associated with agrIII. Other studies also confirmed that staphylococcal strains enriched in agrIII and agrII more often produce biofilm than strains contain other types of agr [91,92,93], while in our studies most isolates enriched with genes encoding haemolysins or leukocidines belonged to agrI.
In nature, most microorganisms grow in multispecies communities where inter- and intraspecies relations take a place. For instance, Todd et.al. indicated that C. albicans when grown with S. aureus shows effects its virulence factors’ protein expression [94]. Cross-communication involving agr interference between particular strains of S. aureus and chosen other species, e.g., S. epidermidis and P. aeruginosa, within the same ecological niche has previously been observed. Additionally, some staphylococcal isolates belonging to S. epidermidis species via autoinducing peptide (AIP) molecules inhibit agr activity of S. aureus groups I, II and III, but not IV, while only AIP secreted by S. aureus with active agrIV gene are able to inhibit S. epidermidis agr activity [95]. It is postulated that the intra- and inter-cross-talk via the agr quorum sensing system may affect the virulence as well as colonization ability of staphylococci similarly to other bacteria [96]. In studies described by Martínez-García et al., AgrI and AgrII culture supernatants from S. epidermidis significantly reduced the biofilm formation of AgrI, II, and III S. epidermidis strains, while the AgrIII supernatant did not affect the biofilm formation of the AgrII strain [44]. Canovas et al. also proved that staphylococci, e.g., S. schleiferi from varying environmental niches, can affect virulence and colonization of S. aureus [97]. The observation was that S. schleiferi is a potent inhibitor of S. aureus agr genes and from all agr types (from I to IV). It offers a potential new avenue for exploring staphylococci as sources for quorum sensing inhibitors that can be used to target S. aureus agr-related infections. S. schleiferi is also able to inhibit S. aureus strains by affecting its agr gen activity, especially strains with the agrI gene [98].
Although the agr cross-inhibition between the different S. aureus agr groups and between S. aureus and some other staphylococcal species (especially S. epidermidis) is quite well documented, little is known about the relations between CoNS species, especially about the influence of agr genes on the activity of virulence factors [33,99]. Our results indicated that CoNS isolates, similarly to S. aureus strains with active agr genes, are able to inhibit biofilm formation of other CoNS isolates with inactive agr genes and there was no difference whether isolates contained the same type of agr or not. Moreover, there was no distinction in the obtained results as to whether isolates were assigned to the same or different species, which means that there was no cross-species barrier for agr system activity. Our findings are in agreement with data provided by other groups; it is well documented that there is downregulation of agr facilitate biofilm development in staphylococci [100,101]. The agr-deficient variants produce much higher but worse-organized biofilm mass when compared to strains with active agr genes. This might be related to the fact that the lack of agr-dependent production of PSMs involved in functional biofilm architecture is dependent on the agr system [102]. The level of biofilm production was changed only for S. epidermidis ATCC 12228 strain. This might be related to the fact that the S. epidermidis ATCC12228 strain is generally a weak biofilm producer.
It is postulated that an active agr system is necessary to induce synergistic haemolysis that can be observed by the CAMP test [103]. Our studies all used supernatants from isolates with active agrII genes, or a combination of inactive agrI and active agrIII genes were able to activate previously inactive δ-haemolysin activity of isolates with inactive agr genes, suggesting that strains with active agrII and III genes are able to affect activity of virulence factors of other strains. A more complex situation was observed when isolates with inactive agrI genes were incubated with supernatants from isolates with active agrI genes as supernatant from some isolates led to inhibition of δ-haemolysin (and negative results of the CAMP test) of some isolates, while others activated expression of inactive δ-haemolysin genes in other tested isolates. This might be a result of the fact that Hld is encoded by RNAIII, the effector of agr [32], thus the virulence factor of isolates with active RNAIII gene (S. simulans 02 and S. epidermidis ATCC 12228) might be muted by Agr from supernatant from isolates with active agrI genes, while virulence genes from isolates with inactive RNAIII genes (S. simulans 01, S. epidermidis) will not be affected by Agr from these supernatants. On the other hand, incubation of all tested isolates with supernatant from isolate without RNAIII gene (S. haemolyticus) isolates led to positive results of CAMP tests for all tested isolates. The obtained result might be associated with the fact that supernatants from both used S. hominis isolates also contained active mecA genes, while S. haemolyticus isolate was sensitive to methicillin phenotypically and the presence of the mecA gene was not found. Additionally, one of these isolates indicated an inactive hld gene, but an active mecA gene. It has been proved that in MRSA strains, the mecA gene leads to some changes in virulence factors of the organism [104]. The activity of this gene affects some proteins such as agr, and agr-regulated enterotoxins and PVL [105]. This finding is in accordance with our results as all S. hominis isolates with active agrI genes led to β-type haemolysis and gave positive results in the CAMP test.
There are suggestions that anti-virulence therapy and treatment targeting the agr system might be a good alternative to common antibiotics in antimicrobial therapy [106].
Bacterial adaptation to a changing environment is often associated with the mutation in genes involved in their virulence. For instance, it is postulated that staphylococcal resistant to methicillin with agr variations due to their defects in activity of agr-depending quorum sensing system may contain additional agr-independent virulence factors as a likely part of their survival strategy. This strategy may lead to pleiotropic effects and the generation of phenotypic heterogeneity [107], enabling success of certain S. aureus strains, e.g., the CA-MRSA USA300 clone that strongly expresses toxins under control of the agr system, thus inducing itchy skin infections, and is pandemic in the USA [108,109].
5. Conclusions
Our results, similarly to already published data, indicate that CoNS may soon become significant pathogens. The variety of properties among isolates, which is the result of HGT, confirms the formation and selection of methicillin-resistant strains as well as strains with new combinations of virulence features among CoNS. Our results confirmed the intra- and inter-cross-talk between chosen staphylococcal isolates mediated via the agr quorum sensing system and suggest that the agr quorum sensing system may play an important role for inter-species communication and that this may be involved in the changes in the virulence of staphylococci. These studies broaden our understanding of antibiotic resistance and carriage of virulence determinants in CoNS isolates confirmed as etiological factors of human infection. As bacterial virulence and antibiotic resistance have a significant influence on disease severity and treatment, further studies of CoNS virulence, including the species tested in this manuscript, are necessary.
Author Contributions
Conceptualization, M.G.; methodology, M.G.; software, M.G. and E.B.; validation, M.G., E.B. and M.S.; formal analysis, M.G.; investigation, M.G. and E.B.; resources, M.G.; data curation, M.G.; writing—original draft preparation, M.G.; writing—review and editing, M.G., M.S. and E.B.; visualization, M.G.; supervision, M.G.; project administration, M.G.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yarwood J.M., Schlievert P.M. Quorum sensing in Staphylococcus infections. J. Clin. Investig. 2003;112:1620. doi: 10.1172/JCI200320442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du Toit A. Increasing virulence factors. Nat. Rev. Microbiol. 2018;16:658. doi: 10.1038/s41579-018-0091-3. [DOI] [PubMed] [Google Scholar]
- 3.Spaan A.N., Vrieling M., Wallet P., Badiou C., Reyes-Robles T., Ohneck E.A., Yvonne Benito Y., de Haas C.J.C., Day C.J., Jennings M.P., et al. The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat. Commun. 2014;11:5438. doi: 10.1038/ncomms6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandenesch F., Lin G., Henry T. Staphylococcus aureus haemolysins, bicomponent leukocidins, and cytolytic peptides: A redundant arsenal of membrane-damaging virulence factors? Front. Cell. Infect. Microbial. 2012;2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventura C.L., Malachowa N., Hammer C.H., Nardone G.A., Robinson M.A., Kobayashi S.D., DeLeo F.R. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS ONE. 2010;5:e11634. doi: 10.1371/journal.pone.0011634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawada-Matsuo M., Yoshida Y., Nakamura N., Komatsuzawa H. Role of two-component systems in the resistance of Staphylococcus aureus to antibacterial agents. Virulence. 2011;2:427. doi: 10.4161/viru.2.5.17711. [DOI] [PubMed] [Google Scholar]
- 7.Novick R.P. Autoinduction and Signal Transduction in the Regulation of Staphylococcal Virulence. Mol. Microbiol. 2003;48:1429. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 8.Divyakolu S., Chikkala R., Ratnakar K.S., Sritharan V. Haemolysins of Staphylococcus aureus—An Update on Their Biology, Role in Pathogenesis and as Targets for Anti-Virulence Therapy. Adv. Inf. Dis. 2019;9:80. [Google Scholar]
- 9.Liu Q., Cho H., Yeo W.S., Bae T. The extracytoplasmic linker peptide of the sensor protein SaeS tunes the kinase activity required for staphylococcal virulence in response to host signals. PLoS Pathog. 2015;11:e1004799. doi: 10.1371/journal.ppat.1004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebeau C., Vandenesch F., Greenland T., Novick R.P., Etienne J. Coagulase expression in Staphylococcus aureus is positively and negatively modulated by an agr-dependent mechanism. J. Bacteriol. 1994;17:5534. doi: 10.1128/jb.176.17.5534-5536.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chovanová R., Mikulášová M., Vaverková S. In vitro antibacterial and antibiotic resistance modifying effect of bioactive plant extracts on methicillin-resistant Staphylococcus epidermidis. Int. J. Microbiol. 2013;9:760969. doi: 10.1155/2013/760969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldman M.H., Rasmussen M., Olaison L., Påhlman L.I. Endocarditis due to Staphylococcus lugdunensis-A retrospective national registry-based study. Eur. J. Clin. Microbiol. Infect. Dis. 2020;40:1103. doi: 10.1007/s10096-020-04134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wisplinghoff H., Bischoff T., Tallent S.M., Seifert H., Wenzel R.P., Edmond M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004;39:309. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 14.Ott E., Saathoff S., Graf K., Schwab F., Chaberny I.F. The prevalence of nosocomial and community acquired infections in a university hospital: An observational study. Dtsch. Aerzteblatt Int. 2013;110:533. doi: 10.3238/arztebl.2013.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papan C., Karremann M., Weis M., Petzold A., Zahn K., Schroten H., Weichert S., Tenenbaum T. A 28-Day-Old Boy with Multifocal Osteomyelitis Mimicking Non-Accidental Injury. Klin. Padiatr. 2021;233:91. doi: 10.1055/a-1219-8053. [DOI] [PubMed] [Google Scholar]
- 16.Eichel V., Papan C., Mutters N.T. Update Hygiene: Prevention of Vascular Catheter-Associated Infections in Premature and Newborn Infants. Klin. Padiatr. 2019;231:177–182. doi: 10.1055/a-0883-5350. [DOI] [PubMed] [Google Scholar]
- 17.Marincola G., Liong O., Schoen C., Abouelfetouh A., Hamdy A., Wencker F.D.R., Marciniak T., Becker K., Köck R., Ziebuhr W. Antimicrobial Resistance Profiles of Coagulase-Negative Staphylococci in Community-Based Healthy Individuals in Germany. Front. Public Health. 2021;17:684456. doi: 10.3389/fpubh.2021.684456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deyno S., Fekadu S., Seyfe S. Prevalence and antimicrobial resistance of coagulase negative staphylococci clinical isolates from Ethiopia: A meta-analysis. BMC Microbiol. 2018;18:43. doi: 10.1186/s12866-018-1188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu W., Kim H.K., Rauch S., Schneewind O., Missiakas D. Pathogenic conversion of coagulase-negative staphylococci. Microbes Infect. 2017;19:101. doi: 10.1016/j.micinf.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szemraj M., Grazul M., Balcerczak E., Szewczyk E.M. Staphylococcal species less frequently isolated from human clinical specimens-are they a threat for hospital patients? BMC Infect. Dis. 2020;20:128. doi: 10.1186/s12879-020-4841-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirotaki S., Sasaki T., Kuwahara-Arai K., Hiramatsu K. Rapid and accurate identification of human-associated staphylococci by use of multiplex PCR. J. Clin. Microbiol. 2011;49:3627. doi: 10.1128/JCM.00488-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hébert G.A., Hancock G.A. Synergistic haemolysis exhibited by species of Staphylococci. J. Clin. Microbiol. 1985;22:409. doi: 10.1128/jcm.22.3.409-415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Tool G.A. Microtiter dish biofilm formation assay. JoVE. 2011;47:2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stepanović S., Vuković D., Hola V., Bonaventura G.D., Djukić S., Ćirković I., Ruzicka F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis. 2007;115:891. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 25.Monday S.R., Bohach G.A. Use of multiplex PCR to detect classical and newly described pyrogenic toxin genes in staphylococcal isolates. J. Clin. Microbiol. 1999;37:3411. doi: 10.1128/JCM.37.10.3411-3414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarraud S., Mougel C., Thioulouse J., Lina G., Meugnier H., Forey F., Nesme X., Etienne J., Vandenesch F. Relationships between Staphylococcus aureus Genetic Background, Virulence Factors, agr Groups (Alleles), and Human Disease. Infect. Immun. 2002;70:631. doi: 10.1128/IAI.70.2.631-641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ünal N., Çinar O. Detection of stapylococcal enterotoxin, methicillin-resistant and Panton–Valentine leukocidin genes in coagulase-negative staphylococci isolated from cows and ewes with subclinical mastitis. Trop. Anim. Health Prod. 2012;44:369. doi: 10.1007/s11250-011-0032-x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang K., McClure J.A., Elsayed S., Louie T., Conly J.M. Novel Multiplex PCR Assay for Characterization and Concomitant Subtyping of Staphylococcal Cassette Chromosome. J. Clin. Microbiol. 2005;43:5026. doi: 10.1128/JCM.43.10.5026-5033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soares B.S., Melo D.A., Motta C.C., Marques V.F., Barreto N.B., Coelho S.M.O., Coelho S., Souza M.M.S. Characterization of virulence and antibiotic profile and agr typing of Staphylococcus aureus from milk of subclinical mastitis bovine in State of Rio de Janeiro. Arq. Bras. Med. Vet. Zootec. 2017;69:843–850. doi: 10.1590/1678-4162-9260. [DOI] [Google Scholar]
- 30.Frebourg N.B., Lefebvre S., Baert S., Lemeland J.F. PCR-Based Assay for Discrimination between Invasive and Contaminating Staphylococcus epidermidis Strains. J. Clin. Microbiol. 2000;38:877. doi: 10.1128/JCM.38.2.877-880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giraudo A., Calzolari A., Cataldi A., Bogni C., Nagel R. The sae locus of Staphylococcus aureus encodes a two-component regulatory system. FEMS Microbiol. Lett. 1999;177:15–22. doi: 10.1111/j.1574-6968.1999.tb13707.x. [DOI] [PubMed] [Google Scholar]
- 32.Novick R.P., Ross H.F., Projan S.J., Kornblum J., Kreiswirth B., Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lina G., Boutite F., Tristan A., Bes M., Etienne J., Vandenesch F. Bacterial competition for human nasal cavity colonization: Role of staphylococcal agr alleles. Appl. Environ. Microbiol. 2003;69:18. doi: 10.1128/AEM.69.1.18-23.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilot P., Lina G., Cochard T., Poutrel B. Analysis of the Genetic Variability of Genes Encoding the RNA III-Activating Components Agr and TRAP in a Population of Staphylococcus aureus Strains Isolated from Cows with Mastitis. J. Clin. Microbiol. 2002;40:4060. doi: 10.1128/JCM.40.11.4060-4067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attia A.S., Benson M.A., Stauff D.L., Torres V.J., Skaar E.P. Membrane Damage Elicits an Immunomodulatory Program in Staphylococcus aureus. PLoS Pathog. 2010;6:e1000802. doi: 10.1371/journal.ppat.1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benson M.A., Ohneck E.A., Ryan C., Alonzo F., Smith H., Narechania A., Kolokotronis S.O., Satola S.W., Uhlemann A.C., Sebra R., et al. Evolution of hypervirulence by a MRSA clone through acquisition of a transposable element. Mol. Microbiol. 2014;93:664. doi: 10.1111/mmi.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonzo F., Benson M.A., Chen J., Novick R.P., Shopsin B., Torres V.J. Staphylococcus aureus leukocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol. Microbiol. 2012;83:423. doi: 10.1111/j.1365-2958.2011.07942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bezar I.F., Mashruwala A.A., Boy J.M., Stock A.M. Drug-like Fragments Inhibit agr-Mediated Virulence Expression in Staphylococcus aureus. Sci. Rep. 2019;9:6786. doi: 10.1038/s41598-019-42853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kessel C.I. Master’s Thesis. Northern Michigan University; Marquette, MI, USA: 2017. Molecular Subtyping of Staphylococcus aureus Isolates from the u.p. Community for the Presence of Toxin-Encoding Genes, Nmu Commons All Nmu; p. 133. [Google Scholar]
- 40.Francois P., Koessler T., Huyghe A., Harbarth S., Bento M., Lew D., Etienne J., Pittet D., Schrenzel J. Rapid Staphylococcus aureus agr Type Determination by a Novel Multiplex Real-Time Quantitative PCR Assay. J. Clin. Microbiol. 2006;44:1892. doi: 10.1128/JCM.44.5.1892-1895.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan X., Qin N., Wu C., Sheng J., Yang R., Zheng B., Ma Z., Liu L., Peng X., Jia A. Transcriptome analysis of the biofilm formed by methicillin-susceptible Staphylococcus aureus. Sci. Rep. 2015;5:11997. doi: 10.1038/srep11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iqbal Z., Seleem M.N., Hussain H.I., Huang L., Hao H., Yuan Z. Comparative virulence studies and transcriptome analysis of Staphylococcus aureus strains isolated from animals. Sci. Rep. 2016;6:35442. doi: 10.1038/srep35442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho H., Jeong D.W., Liu Q., Yeo W.S., Vogl T., Skaar E.P., Chazin W.J., Bae T. Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Staphylococcus aureus Infections. PLoS Pathog. 2015;11:e1005026. doi: 10.1371/journal.ppat.1005026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez-García S., Ortiz-García C.I., Cruz-Aguilar M., Zenteno J.C., Murrieta-Coxca J.M., Pérez-Tapia S.M., Rodríguez-Martínez S., Cancino-Diaz M.E., Cancino-Diaz J.C. Competition/antagonism associations of biofilm formation among Staphylococcus epidermidis Agr groups I, II, and III. J. Microbiol. 2019;57:143. doi: 10.1007/s12275-019-8322-5. [DOI] [PubMed] [Google Scholar]
- 45.May L., Klein E.Y., Rothman R.E., Laxminarayan R. Trends in antibiotic resistance in coagulase-negative staphylococci in the United States, 1999 to 2012. Antimicrob. Agents Chemother. 2014;58:1404. doi: 10.1128/AAC.01908-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reyes-Robles T., Torres V.J. Staphylococcus aureus Pore-Forming Toxin. Curr. Top. Microbiol. Immunol. 2016;409:121. doi: 10.1007/82_2016_16. [DOI] [PubMed] [Google Scholar]
- 47.Goerke C., Köller J., Wolz C. Ciprofloxacin and trimethoprim cause phage induction and virulence modulation in Staphylococcus aureus. Antimicrob. Agents Chemother. 2006;50:171. doi: 10.1128/AAC.50.1.171-177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huseby M., Shi K., Brown C.K., Digre J., Mengistu F., Seo K.S., Bohach G.A., Schlievert P.M., Ohlendorf D.H., Earhart C.A. Structure and Biological Activities of β Toxin from Staphylococcus aureus. J. Bacteriol. 2007;189:8719. doi: 10.1128/JB.00741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Traber K., Novick R. A Slipped-Mispairing Mutation in AgrA of Laboratory Strains and Clinical Isolates Results in Delayed Activation of agr and Failure to Translate δ- and α-haemolysins. Mol. Microbiol. 2006;59:1519. doi: 10.1111/j.1365-2958.2006.04986.x. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura Y., Oscherwitz J., Cease K.B., Chan S.M., Munoz-Planillo R., Hasegawa M., Villaruz A.E., Cheung G.Y.C., McGavin M.J., Travers J.B., et al. Staphylococcus δ-Toxin Induces Allergic Skin Disease by Activating Mast Cells. Nature. 2013;503:397. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnside K., Lembo A., de los Reyes M., Iliuk A., BinhTran N.T., Connelly J.E., Lin W.J., Schmidt B.Z., Richardson A.R., Fang F.C., et al. Regulation of haemolysin expression and virulence of Staphylococcus aureus by a serine/threonine kinase and phosphatase. PLoS ONE. 2010;5:e11071. doi: 10.1371/journal.pone.0011071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooney J., Kienle Z., Foster T.J., O’Toole P.W. The gammahaemolysin locus of Staphylococcus aureus comprises three linked genes, two of which are identical to the genes for the F and S components of leukocidin. Infect. Immun. 1993;61:768. doi: 10.1128/iai.61.2.768-771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geiger T., Goerke C., Mainiero M., Kraus D., Wolz C. The virulence regulator Sae of Staphylococcus aureus: Promoter activities and response to phagocytosiFs-related signals. J. Bacteriol. 2008;190:3419. doi: 10.1128/JB.01927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCarthy A.J., Lindsay J.A. Staphylococcus aureus innate immune evasion is lineage-specific: A bioinfomatics study. Infect. Genet. Evol. 2013;19:7. doi: 10.1016/j.meegid.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Yoong P., Torres V.J. The effects of Staphylococcus aureus leukotoxins on the host: Cell lysis and beyond. Curr. Opin. Microbiol. 2013;16:63. doi: 10.1016/j.mib.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baba T., Bae T., Schneewind O., Takeuchi F., Hiramatsu K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: Polymorphism and evolution of two major pathogenicity islands. J. Bacteriol. 2008;190:300. doi: 10.1128/JB.01000-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gravet A., Couppie P., Meunier O., Clyti E., Moreau B., Pradinaud R., Monteil H., Prevost G. Staphylococcus aureus isolated in cases of impetigo produces both epidermolysin A or B and LukE-LukD in 78% of 131 retrospective and prospective cases. J. Clin. Microbiol. 2001;39:4349. doi: 10.1128/JCM.39.12.4349-4356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foster T.J., Geoghegan J.A., Ganesh V.K., Höök M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014;12:49. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y., Yeh A.J., Cheung G.Y., Villaruz A.E., Tan V.Y., Joo H.S., Chatterjee S.S., Yu Y., Otto M. Basis of virulence in a panton-valentine leukocidin-negative community-associated methicillin-resistant Staphylococcus aureus strain. J. Infect. Dis. 2015;2011:472. doi: 10.1093/infdis/jiu462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Udo E.E., Al-Bustan M.A., Jacob L.E., Chugh T.D. Enterotoxin production by coagulase-negative staphylococci in restaurant workers from Kuwait City may be a potential cause of food poisoning. J. Med. Microbiol. 1999;48:819. doi: 10.1099/00222615-48-9-819. [DOI] [PubMed] [Google Scholar]
- 61.Vasconcelos N.G., Pereira V.C., Araujo J.P., da Cunha M.d.L.R.S. Molecular detection of enterotoxins E, G, H and I in Staphylococcus aureus and coagulase-negative staphylococci isolated from clinical samples of newborns in Brazil. J. Appl. Microbiol. 2011;111:749. doi: 10.1111/j.1365-2672.2011.05076.x. [DOI] [PubMed] [Google Scholar]
- 62.Nasaj M., Saeidi Z., Asghari B., Roshanaei G., Arabestani M.R. Identifcation of haemolysin encodin genes and their association with antimicrobial resistance pattern among clinical isolates of coagulase-negative Staphylococci. BMC Res. Notes. 2020;13:68. doi: 10.1186/s13104-020-4938-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.da Silva Sdos S., Cidral T.A., Soares M.J., de Melo M.C. Enterotoxin-Encoding Genes in Staphylococcus spp. from Food Handlers in a University Restaurant. Foodborne Pathog. Dis. 2015;12:921. doi: 10.1089/fpd.2015.1941. [DOI] [PubMed] [Google Scholar]
- 64.MacFadden D.R., McGough S.F., Fisman D., Santillana M., Brownstein J.S. Antibiotic resistance increases with local temperature. Nat. Clim. Chang. 2018;8:510. doi: 10.1038/s41558-018-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singer A.C., Shaw H., Rhodes V., Hart A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front. Microbiol. 2016;7:1728. doi: 10.3389/fmicb.2016.01728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klingenberg C., Aarag E., Ronnestad A., Sollid J.E., Abrahamsen T.G., Kjeldsen G., Flaegstad T. Coagulase-negative staphylococcal sepsis in neonates. Association between antibiotic resistance, biofilm formation and the host inflammatory response. Pediatr Infect. Dis. J. 2005;24:817–822. doi: 10.1097/01.inf.0000176735.20008.cd. [DOI] [PubMed] [Google Scholar]
- 67.Hira V., Sluijter M., Goessens W.H., Ott A., de Groot R., Hermans P.W., Kornelisse R.F. Coagulase-negative staphylococcal skin carriage among neonatal intensive care unit personnel: From population to infection. J. Clin. Microbiol. 2010;48:3876. doi: 10.1128/JCM.00967-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kord M., Ardebili A., Jamalan M., Jahanbakhsh R., Behnampour N., Ghaemia E.A. Evaluation of Biofilm Formation and Presence of Ica Genes in Staphylococcus epidermidis Clinical Isolates. Osong Public Health Res. Perspect. 2018;9:160. doi: 10.24171/j.phrp.2018.9.4.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.den Reijer P.M., Haisma E.M., Lemmens-den Toom N.A., Willemse J., Koning R.A., Demmers J.A., Dekkers D.H.W., Rijkers E., El Ghalbzouri A., Nibbering P.H., et al. Detection of alpha-toxin and other virulence factors in biofilms of Staphylococcus aureus on polystyrene and a human epidermal model. PLoS ONE. 2016;11:e0145722. doi: 10.1371/journal.pone.0145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma X.X., Wang E.H., Liu Y., Luo E.J. Antibiotic susceptibility of coagulase-negative staphylococci (CoNS): Emergence of teicoplanin-non-susceptible CoNS strains with inducible resistance to vancomycin. J. Med. Microbiol. 2011;60:1661. doi: 10.1099/jmm.0.034066-0. [DOI] [PubMed] [Google Scholar]
- 71.Chlebowicz M.A., Nganou K., Kozytska S., Arends J.P., Engelmann S., Grundmann H., Ohlsen K., van Dijl J.M. Recombination between ccrC genes in a type V (5C2&5) Staphylococcal Cassette Chromosome mec (SCCmec) of Staphylococcus aureus ST398 leads to conversion from methicillin resistance to methicillin susceptibility in vivo. Antimicrob. Agents Chemother. 2010;54:783. doi: 10.1128/AAC.00696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kriegeskorte A., Idelevich E.A., Schlattmann A., Layer F., Strommenger B., Denis O., Paterson G.K., Holmes M.A., Werner G., Becker K. Comparison of different phenotypic approaches to screen and detect mecC-harboring methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2018;56:e00826. doi: 10.1128/JCM.00826-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia-Alvarez L. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: A descriptive study. Lancet Infect. Dis. 2011;11:595. doi: 10.1016/S1473-3099(11)70126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wushouer H., Zhang Z.X., Wang J.H., Ji P., Zhu Q.F., Aishan R., Shi L.W. Trends and relationship between antimicrobial resistance and antibiotic use in Xinjiang Uyghur Autonomous Region, China: Based on a 3 year surveillance data, 2014–2016. J. Infect. Public Health. 2018;11:339. doi: 10.1016/j.jiph.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 75.Kateete D.P., Asiimwe B.B., Mayanja R., Najjuka C.F., Rutebemberwa E. Species and drug susceptibility profiles of staphylococci isolated from healthy children in Eastern Uganda. PLoS ONE. 2020;15:e0229026. doi: 10.1371/journal.pone.0229026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He S., Lin J., Li Y., Zhang W., Zhou J., Han Z., Yao Z. Insights into the epidemiology of methicillin-resistant coagulase-negative Staphylococci carriage in community-based drug users. J. Infect. Public Health. 2020;13:1742. doi: 10.1016/j.jiph.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 77.Budri P.E., Shore A.C., Coleman D.C., Kinnevey P.M., Humpreys H., Fitzgerald-Hughes D. Observational cross-sectional study of nasal staphylococcal species of medical students of diverse geographical origin, prior to healthcare exposure: Prevalence of SCCmec, fusC, fusB and the arginine catabolite mobile element (ACME) in the absence of selective antibiotic pressure. BMJ Open. 2018;8:e020391. doi: 10.1136/bmjopen-2017-020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cavanagh J.P., Wolden R., Heise P., Esaiassen E., Klingenberg C., Aarag Fredheim E.G. Antimicrobial susceptibility and body site distribution of community isolates of coagulase-negative staphylococci. Apmis. 2016;124:973. doi: 10.1111/apm.12591. [DOI] [PubMed] [Google Scholar]
- 79.Bronner S., Monteil H., Prevost G. Regulation of virulence determinants in Staphylococcus aureus: Complexity and applications. FEMS Microbiol. Rev. 2004;28:183. doi: 10.1016/j.femsre.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Viedma E., Perez-Montarelo D., Villa J., Munoz-Gallego I., Larrosa N., Fernandez-Hidalgo N., Gavaldà J., Almirante B., Chaves F. Sub-inhibitory concentrations of oxacillin modify the expression of agr locus in Staphylococcus aureus clinical strains belonging to different clonal complexes. BMC Infect. Dis. 2018;16:177. doi: 10.1186/s12879-018-3088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gray B., Hall P., Gresham H. Targeting agrand agr-like quorum sensing systems for development of common therapeutics to treat multiple Gram-positive bacterial infections. Sensors. 2013;13:5130. doi: 10.3390/s130405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan L., Li S.R., Jiang B., Hu X.M., Li S. Therapeutic Targeting of the Staphylococcus aureus Accessory Gene Regulator (agr) System. Front. Microbiol. 2018;9:55. doi: 10.3389/fmicb.2018.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li M., Guan M., Jiang X.F., Yuan F.Y., Xu M., Zhang W.Z., Lu Y. Genetic polymorphism of the accessory gene regulator (agr) locus in Staphylococcus epidermidis and its association with pathogenicity. J. Med. Microbiol. 2004;53:545. doi: 10.1099/jmm.0.05406-0. [DOI] [PubMed] [Google Scholar]
- 84.Wang S., Chiueh W.Y., Sun J.R., Tsao S.M., Lu J.J. Molecular typing and phenotype characterization of methicillin-resistant Staphylococcus aureus isolates from blood in Taiwan. PLoS ONE. 2012;7:e30394. doi: 10.1371/journal.pone.0030394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neetu J.P., Murugan S. Genotyping of methicillin resistant Staphylococcus aureus from tertiary care hospitals in Coimbatore, South India. J. Glob. Infect. Dis. 2016;8:68. doi: 10.4103/0974-777X.182119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nichol K.A., Adam H.J., Hussain Z., Mulvey M.R., McCracken M., Mataseje L.F., Thompson K., Kost S., Lagacé-Wiens P.R.S., Hoban D.J., et al. Comparison of community-associated and health care-associated methicillin-resistant Staphylococcus aureus in Canada: Results of the CANWARD 2007–2009 study. Diagn. Microbiol. Infect. Dis. 2011;69:320. doi: 10.1016/j.diagmicrobio.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 87.Mohsenzadeh M., Ghazvini K., Azimian A. Frequency of specific agr groups and antibiotic resistance in Staphylococcus aureus isolated from bovine mastitis in the northeast of Iran. Vet. Res. Forum. 2015;6:295. [PMC free article] [PubMed] [Google Scholar]
- 88.Wright J.S., Traber K.E., Corrigan R., Benson S.A., Musser J.M., Novick R.P. The agr radiation: An early event in the evolution of staphylococci. J. Bacteriol. 2005;187:5585. doi: 10.1128/JB.187.16.5585-5594.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tegmark K., Morfeldt E., Arvidson S. Regulation of agr-dependent virulence genes in Staphylococcus aureus by RNAIII from coagulase-negative staphylococci. J. Bacteriol. 1998;180:3181. doi: 10.1128/JB.180.12.3181-3186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Wamel W.J., Van Rossum G., Verhoef J., Vandenbroucke-Grauls C.M., Fluit A.C. Cloning and characterization of an accessory gene regulator (agr)-like locus from Staphylococcus epidermidis. FEMS Microbiol. Lett. 1998;163:1. doi: 10.1016/S0378-1097(98)00132-3. [DOI] [PubMed] [Google Scholar]
- 91.Khoramrooz S.S., Mansouri F., Marashifard M., Hosseini S.A.A.M., Chenarestane-Olia F.A., Ganavehei B., Gharibpour F., Shahbazi A., Mirzaii M., Darban-Sarokhalil D. Detection of biofilm related genes, classical enterotoxin genes and agr typing among Staphylococcus aureus isolated from bovine with subclinical mastitis in southwest of Iran. Microb. Pathog. 2016;97:45. doi: 10.1016/j.micpath.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 92.Ikonomidis A., Vasdeki A., Kristo I., Maniatis A.N., Tsakris A., Malizos K.N., Pournaras S. Association of biofilm formation and methicillin-resistance with accessory gene regulator (agr) loci in Greek Staphylococcus aureus clones. Microb. Pathog. 2009;47:341. doi: 10.1016/j.micpath.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 93.Khelissa S.O., Jama C., Abdallah M., Boukherroub R., Faille C., Chihib N.E. Efect of incubation duration, growth temperature, and abiotic surface type on cell surface properties, adhesion and pathogenicity of biofilm detached Staphylococcus aureus cells. AMB Express. 2017;7:191. doi: 10.1186/s13568-017-0492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Todd O.A., Fidel P.L., Harro J.M., Hilliard J.J., Tkaczyk C., Sellman B.R., Noverr M.C., Peters B.M. Candida albicans Augments Staphylococcus aureus Virulence by Engaging the Staphylococcal agr Quorum Sensing System. mBio. 2019;10:e00910-19. doi: 10.1128/mBio.00910-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Otto M., Echner H., Voelter W., Gotz F. Pheromone cross-inhibition between Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 2001;69:1957. doi: 10.1128/IAI.69.3.1957-1960.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.LaSarre B., Federle M.J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 2013;77:73. doi: 10.1128/MMBR.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Canovas J., Baldry M., Bojer M.S., Andersen P.S., Gless B.H., Grzeskowiak P.K., Stegger M., Damborg P., Olsen C.A., Ingmer H. Cross-Talk between Staphylococcus aureus and Other Staphylococcal Species via the agr Quorum Sensing System. Front. Microbiol. 2016;8:1949. doi: 10.3389/fmicb.2017.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ji G., Pei W., Zhang L., Qiu R., Lin J., Benito Y., Lina G., Novick R.P. Staphylococcus intermedius Produces a Functional agr Autoinducing Peptide Containing a Cyclic Lactone. J. Bacteriol. 2005;187:3139. doi: 10.1128/JB.187.9.3139-3150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toledo-Silva B., Nogueira de Souza F., Piepers S., Mertens K., Haesebrouck F., De Vliegher S. Metabolites of bovine-associated non-aureus staphylococci influence expression of Staphylococcus aureus agr-related genes in vitro. Vet. Res. 2021;52:62. doi: 10.1186/s13567-021-00933-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ferreira F.A., Rodrigues Souza R., de Sousa Moraes B., de Amorim Ferreira A.M., Américo M.A., Longo Fracalanzza S.E., dos Santos Silva Couceiro J.N., Sá Figueiredo A.M. Impact of agr dysfunction on virulence profiles and infections associated with a novel methicillin-resistant Staphylococcus aureus (MRSA) variant of the lineage ST1-SCCmec IV. BMC Microbiol. 2013;13:93. doi: 10.1186/1471-2180-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dai L., Yang L., Parsons C., Findlay V.J., Molin S., Qin Z. Staphylococcus epidermidis recovered from indwelling catheters exhibit enhanced biofilm dispersal and “selfrenewal” through downregulation of agr. BMC Microbiol. 2012;12:102. doi: 10.1186/1471-2180-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Periasamy S., Joo H.S., Duong A.C., Otto M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA. 2012;109:1281. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheung G.Y.C., Duong A.C., Otto M. Direct and synergistic haemolysis caused by Staphylococcus phenol-soluble modulins: Implications for diagnosis and pathogenesis. Microbes Infect. 2012;14:380. doi: 10.1016/j.micinf.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suryadevara M., Clark A.E., Wolk D.M., Carman A., Rosenbaum P.F., Shaw J. Molecular characterization of invasive Staphylococcus aureus infection in central New York children: Importance of two clonal groups and inconsistent presence of selected virulence determinants. J. Pediatr. Infect. Dis. Soc. 2013;2:30. doi: 10.1093/jpids/pis087. [DOI] [PubMed] [Google Scholar]
- 105.Rudkin J.K., Edwards A.M., Bowden M.G., Brown E.L., Pozzi C., Waters E.M., Chan W.C., Williams P., O’Gara J.P., Massey R.C. Methicillin resistance reduces the virulence of healthcare-associated methicillin-resistant Staphylococcus aureus by interfering with the agr quorum sensing system. J. Infect. Dis. 2012;205:798. doi: 10.1093/infdis/jir845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rasko D.A., Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010;9:117. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 107.Huber C., Stamm I., Ziebuhr W., Marincola G., Bischoff M., Strommenger B., Jaschkowitz G., Marciniak T., Cuny C., Witte W., et al. Silence as a way of niche adaptation: mecC-MRSA with variations in the accessory gene regulator (agr) functionality express kaleidoscopic phenotypes. Sci. Rep. 2020;10:14787. doi: 10.1038/s41598-020-71640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cheung G.Y., Wang R., Khan B.A., Sturdevant D.E., Otto M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 2011;79:1927. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.DeLeo F.R., Otto M., Kreiswirth B.N., Chambers H.F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.