Abstract
Acute lymphoblastic leukemia (ALL) is the most common kind of pediatric cancer. Although the cure rates in ALL have significantly increased in developed countries, still 15–20% of patients relapse, with even higher rates in developing countries. The role of non-coding RNA genes as microRNAs (miRNAs) has gained interest from researchers in regard to improving our knowledge of the molecular mechanisms underlying ALL development, as well as identifying biomarkers with clinical relevance. Despite the wide heterogeneity reveled in miRNA studies in ALL, consistent findings give us confidence that miRNAs could be useful to discriminate between leukemia linages, immunophenotypes, molecular groups, high-risk-for-relapse groups, and poor/good responders to chemotherapy. For instance, miR-125b has been associated with prognosis and chemoresistance in ALL, miR-21 has an oncogenic role in lymphoid malignancies, and the miR-181 family can act either as a oncomiR or tumor suppressor in several hematological malignancies. However, few of these studies have explored the molecular interplay between miRNAs and their targeted genes. This review aims to state the different ways in which miRNAs could be involved in ALL and their clinical implications.
Keywords: miRNAs, hematopoiesis, acute lymphoblastic leukemia, miRNA biomarkers, ALL subtypes
1. Introduction
Acute lymphoblastic leukemia (ALL) is the most common cancer type in children under 15 years old [1]. This hematologic malignancy is characterized by a clonal expansion of immature lymphoid progenitor cells [2]. The current treatment for ALL is based on predicted risk of relapse, determined by age and white blood cell count (WBC) at diagnosis, infiltration to other organs, immunophenotype, and presence of cytogenetic and molecular alterations [3]. When comparing developed versus developing countries, mortality is 1–2% and 15–36.1%, 5-year disease-free survival is about 90% and 54.9%, and the proportion of patients with a high risk to relapse is 33% and 50%, respectively [4,5,6]. Relapsed ALL occurs in about 15–20%, but in children from low-income countries, it is up to 35%, which displays high rate of early age mortality [7,8,9].
One of the main goals is to bridge the gap between genotype and clinical prognosis; consequently, a great number of investigations have been developed to achieve a better understanding of each aspect of ALL. In this sense, it is known that epigenetic deregulation plays a key role in leukemogenesis, where non-coding RNAs (ncRNAs) have a pivotal role, acting as epigenetic regulators and enhancers and regulating intra- and inter-chromosomal interactions [10]. ncRNAs are broadly divided into two groups: housekeeping and regulatory (rncRNA). rncRNAs are synthesized at a specific phase of development or in response to some external stimuli and are classified according to their transcript size in small ncRNA (sncRNA) (<200 nucleotides) and long ncRNA (lncRNA) (>200 nucleotides). sncRNAs are very diverse, but micro-RNAs (miRNAs) are among the most studied [11,12,13]. MiRNAs were first described in 1993 by Lee et al., who identified C. elegans genes that control the timing of larval development, the lin-4. The authors discovered that lin-4 gene did not code for a protein, but instead produced a pair of sncRNAs [14]. To date, it is well-known that miRNAs play a relevant role in diverse biological processes and that their expression are disease and tissue specific [15]. Indeed, many efforts have been made to identify miRNA biomarkers in many human diseases.
2. MiRNAs’ Features and Biogenesis
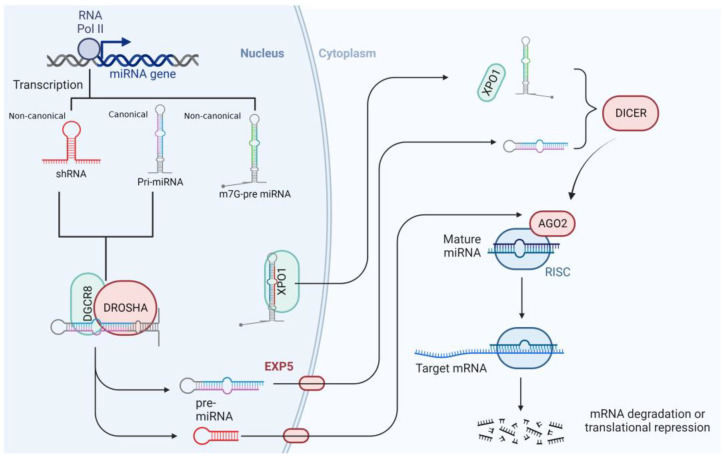
MiRNAs are genomically encoded, untranslated RNA molecules that are, on average, 22 nucleotides in length [16]. Most miRNAs are generated through the canonical miRNA biogenesis pathway that is dependent on Drosha and Dicer; meanwhile, a few of them are processed by non-canonical miRNA pathways [17,18]. Many miRNAs are transcribed from DNA sequences into poly-adenylated and capped primary miRNAs (pri-miRNAs) molecules of >1 kilobase [16,19]. In the canonical miRNA biogenesis pathway, the pri-miRNA is endonucleolytically cleaved by the nuclear microprocessor complex formed by the RNase III enzyme Drosha and the DiGeorge critical region 8 (DGCR8) protein (also called Pasha) into a 60–70 nucleotides long precursor miRNA (pre-miRNA). After that, pre-miRNA can fold back on itself and form a hairpin loop structure that is transported out of the nucleus toward the cytoplasm by exportin-5 (EXP-5) in a complex with Ran-GTP [20]. Once in the cytoplasm, pre-miRNA is separated into two single-stranded molecules (guide and passenger strands), which, together with the RNA-induced silencing complex (RISC), form the RISC loading complex (RLC). This is a multi-protein complex that is composed by the RNase Dicer, the double-stranded RNA-binding domain protein of the transactivation response element RNA-binding protein (TRBP) and Protein kinase RNA (PKR) activator (PACT), and the core component Argonaut (Ago) proteins. Then the passenger strand is released, whereas the guide strand (the functional form) can act as a gene-expression regulator [21,22,23]. MiRNA biogenesis starts with the processing of RNA polymerase II/III transcripts post- or co-transcriptionally (Figure 1) [24].
Figure 1.
MiRNA biogenesis pathways. Schematic diagram of the canonical and non-canonical miRNA biogenesis process. In the canonical pathway, a primary miRNA transcript produced by RNA polymerase II is processed by the Drosha microprocessor in the nucleus. The generated pre-miRNA is transported to the cytoplasm in an EXP5-Ran GT-dependent manner and further processed by the Dicer microprocessor to generate a mature miRNA. The mature miRNA is loaded onto RISC to inhibit the translation of a target mRNA to conduct degradation. The non-canonical pathway is Drosha or Dicer dependent. AGO2, Argonaut protein 2; DGCR8, DiGeorge critical region 8; EXP5, exportin-5; RISC, RNA-induced silencing complex; XPO1, exportin-1.
About half of all identified miRNAs are intragenic and processed mostly from introns, and few of them are located within the exons of protein-coding genes. The remaining are intergenic, transcribed independently of a host gene by RNA polymerase II, and regulated by their promoters [22]. Sometimes miRNAs are transcribed as a family, meaning that they are part of one long transcript, called a cluster, with similar seed regions [23].
Studies have suggested that miRNAs are shuttled between different subcellular compartments to control the rate of translation and even transcription [21]. Most of them can regulate the gene expression by binding the 3′ untranslated regions (UTRs) of targeted mRNAs in an imperfectly complementary sequence manner. However, the interaction between miRNAs with 5′UTR, coding sequence, and gene-promoter regions has also been reported [25]. In addition to the role as transcription repressors, there is a phenomenon called RNA activation (RNAa) that was proven to be a sequence-dependent gene regulation mechanism evolutionarily conserved from C. elegans to mammals [26]. Synthetically designed double-strand RNAs capable of turning on gene expression are termed small/short activation RNAs (saRNAs). RNAa-mediated gene activation seems to depend on Ago protein and correlates with activating chromatin modification at the saRNA-targeted site. In terms of its targeting mechanism(s), saRNA can either act on promoter DNA or nascent transcripts, as there are works in the literature that support both cases [27,28,29].
3. MiRNAs and Hematopoiesis
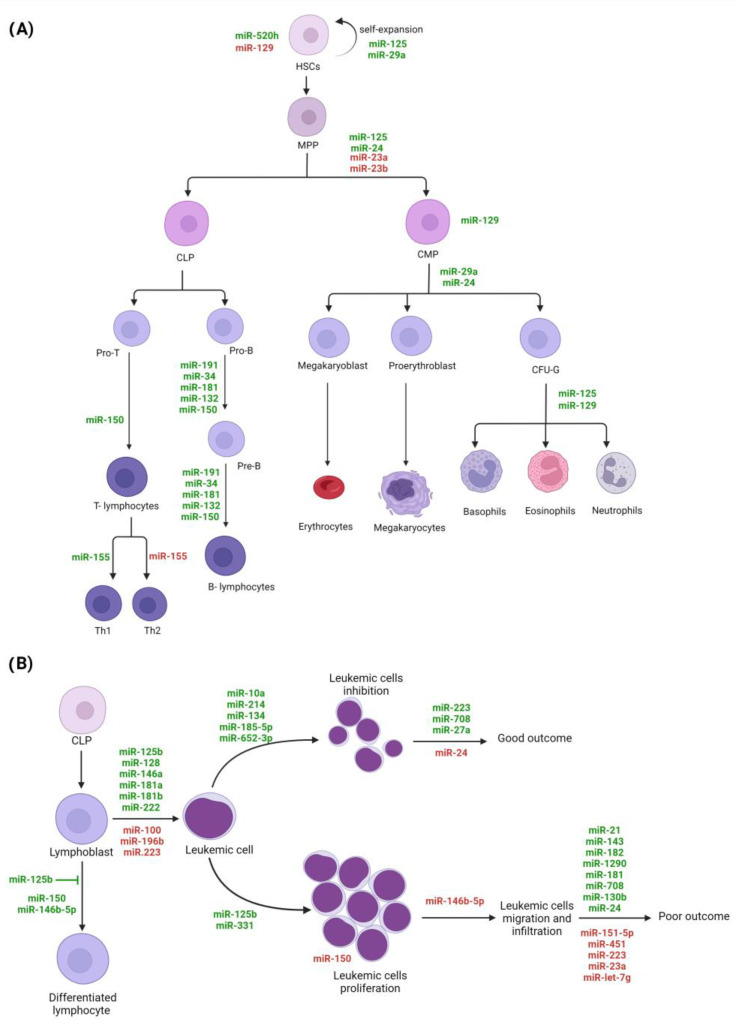
Hematopoiesis is an active, continuous, and highly regulated process by which the production and repopulation of blood cells are carried out [30,31]. Hematopoiesis occurs in the bone marrow (BM), where hematopoietic stem cells (HSCs) with self-regenerative, multipotent, and highly proliferative capacity give rise to multipotent progenitors (MPPs) that can differentiate into the common myeloid (CMP) or lymphoid (CLP) progenitors [30,32]. The CMP differentiation process is called myelopoiesis; it allows for the production of erythrocytes, megakaryocytes/platelets, granulocytes (basophils, eosinophils, neutrophils), and monocytes/macrophages. CLP development, defined as lymphopoiesis, produces B- and T-lymphocytes (B-cells and T-cells, respectively) and natural killer (NK) cells [32]. The final fate of HSCs differentiation is highly regulated by the interaction between this kind of cells with surrounding cellular elements, genomic factors, and microenvironmental conditions that arise in response to hematopoietic demands under normal and pathological circumstances (infectious and inflammatory processes) [30,32,33,34,35]. MiRNAs are among the main modulators of the expression of genes involved in the regulation of cell-differentiation programs [32,36,37,38]. Gene expression analysis in purified hematopoietic populations has shown that specific miRNAs can regulate the differentiation and maturation stages of distinct hematopoietic cell lines [32,36,38,39,40]. For example, under normal conditions, it has been observed that miR-520h is highly expressed only in HSCs, while miR-129 expression is reduced in HSCs but increased in the granulocytic colony-forming unit (CFU-G), positively regulating granulocyte differentiation [32]. Additionally, miR-125 expression has been found to be increased in HSCs, thus indirectly promoting its expansion and compromising the differentiation toward myeloid lineage, particularly to granulocytes and macrophages [32,37,40,41].
Likewise, several miRNAs, such as miR-150, miR-155, miR-181, miR-34a, miR-132, miR-191, miR-24, and the miR-17-92 cluster, have been shown to be important regulators of lymphopoiesis [32,42,43]. Studies indicate that miR-191, miR-34a, miR-181, miR-132, and miR-150 modulate the pro-B- to pre-B-cell stage transition and B-cells’ maturation [32,42,43]. Some miRNAs have shown a tissue-specific effect; for example, while miR-150 expression in BM CLPs regulates pro-B-cell to pre-B-cell transition, in CLPs from thymus, miR-150 suppresses B lineage differentiation and increases the development of T cells (Figure 2A) [42]. Additionally, it has been observed that miRNAs modulate the differentiation of lymphocyte subpopulations; for instance, the presence of miR-155 induces the differentiation of T cells to Th1 subtype, while its absence promotes differentiation to the Th2 subtype [36,42,44,45].
Figure 2.
The miRNAs potentially implicated in hematopoiesis and leukemogenesis. (A) Hematopoiesis process is schematized, and examples of miRNAs expressed involved in the cell differentiation and maturation are indicated. (B) The miRNAs with dysregulated expression that are potentially involved in oncogenic lymphoblast transformation and progression are shown. MiRNAs in red means downregulation, and miRNAs in green means upregulation; HSCs, hematopoietic stem cells; MPP, multipotent progenitors; CLP, common lymphoid progenitors; CMP, common myeloid progenitors; CFU-G, granulocytic colony-forming unit.
Furthermore, it has also been observed that the alteration in the expression of miRNAs can change the fate of cell lineages [46,47]. For example, the deletion of miR-23a and miR-23b in HSCs correlates with a preferential differentiation toward myeloid lineage over B-cell differentiation [36,38]. The overexpression miR-29a contributes to the maintenance of an undifferentiated state and self-renewal capacities of HSCs and increased myelopoiesis; and miR-24 overexpression induces myelopoiesis instead lymphopoiesis (Figure 2A) [36,43].
Oncogenic miR-125b, a microRNA expressed during early T-cell development, enhances proliferation and blocks differentiation in TLX3+ T-ALL by repressing ETS1 and CBFβ genes, critical transcription factors for T-lineage [48]. MiR-125b also participates in metabolic reprogramming in T-ALL, increasing glucose uptake and oxygen consumption in CD4-negative progenitor T cells [49]. MiR-150 exerts a tumor suppressor role in T-ALL by regulating several cell-cycle genes, and its expression is reduced in leukemic T-cells with active mTOR signaling [50]. MiR-146b-5p is another tumor suppressor whose expression is negatively regulated by transcription factor TAL1, leading to enhanced migration and invasion in vitro and lower overall survival in mouse models (Figure 2B) [51].
Due to the central role of miRNAs as regulators of many genes, alterations in these transcripts (mutations, polymorphisms, and expression changes) can modify hematopoietic development and manifest malignant phenotypes [46,47,52,53]. MiRNAs can also regulate genes involved in signaling pathways, such as the Notch, MAPK, and PI3K/AKT pathways, that are common amongst many types of leukemias [54]. For instance, it has been reported that the overexpression of miR-125b and miR-331 is associated with myeloid leukemia and ALL, respectively [36,55]. In addition, it has been observed that the ectopic expression of let-7c promotes granulocytic differentiation in AML [40], the low expression of miR-22 modifies the differentiation of monocytes in normal and leukemic states [40], and changes in the miR-17-92 cluster expression have resulted in the appearance of lymphoproliferative disorders [42]. A recent study identified miRNAs such as miR-125b-5p, miR-155-5p, miR-181a-5p, and miR-19a-3p and coding genes such as BCL2, TP53, KIT, and MYB with the highest number of interactions in several forms of leukemia and other hematological malignancies, including chronic lymphocytic leukemia (CLL) and myelodysplastic syndromes [56].
4. MiRNAs’ Role in Acute Lymphoblastic Leukemia
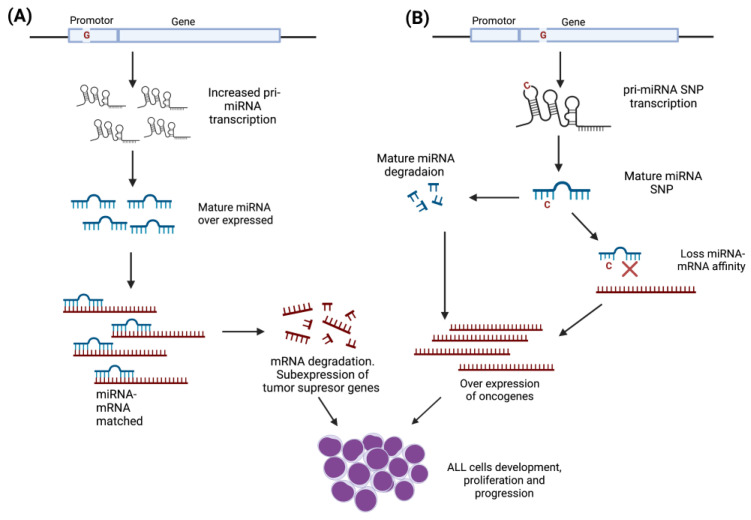
miRNAs have the potential to affect the expression of a large number of genes involved in the origin and evolution of pediatric ALL. The abnormal function of miRNA expressions is one of the main epigenetic mechanisms that plays a relevant role in the development of ALL and other leukemia types [57,58,59]. Mutations and single nucleotide polymorphisms (SNPs) are among the principal factors that can modify miRNAs’ activity (Figure 3), and, in addition to their aberrant expression, they support ALL progression and might modulate the treatment response [60,61,62,63].
Figure 3.
SNPs’ functional effect on miRNAs’ activity. Gene localization of SNPs will determine the effect on miRNA activity. (A) SNPs located in the promoter region could modify the expression levels of the mature miRNA, altering the interaction with mRNA target that codes for transcriptional factors involved in the development and differentiation of lymphoid blasts. The interaction between miRNA–mRNA target could promote the subexpression of tumor-suppressor genes, leading to the development, proliferation, and progression of leukemic cells. (B) SNPs located on the gene structure would give rise to unstable miRNAs that increase their degradation rate or whose sequence change prevents the miRNA–mRNA target interaction, promoting the overexpression of oncogenes and eventually the progression of leukemic cells.
4.1. MiRNAs Polymorphisms as Risk Factors
It is well-known that SNPs located in miRNAs could alter the interaction miRNA-mRNA target or reduce the miRNA gene stability and, hence, its half lifetime. Even though their molecular mechanisms in the ALL pathogenesis is not well understood, several SNPs located in miR-146a, miR-196a-2, miR-499a, and miR-612 have been associated with ALL risk.
MiR-146a. An rs2910164G/C placed in miR-146a has been described as a functional SNP that causes a G:U pair to C:U mismatch in the stem structure of miR-146a precursor, which affects its binding specificity to its targets [64]. The rs2910164G/C was first associated with ALL by Hasani et. al., who reported that the C allele increases the risk for ALL in the Iranian population [65]. Furthermore, Zou et. al. found that the rs2910164 CC genotype increased the ALL risk in children from Asia [63]. Recently, an association in a gender-dependent manner among rs2910164G/C and ALL in Mexican children was published. The rs2910164C allele was identified as a risk factor to ALL in males [66]. However, controversial results have been found in Taiwan by Pei et. al., who documented that miR-146a rs2910164G allele is a protective marker for childhood ALL [62]. Studies in Thai, Indian, and Chinese populations did not observe an association between rs2910164G/C and ALL [61,67,68]. The study performed in patients from India also included rs57095329A/G in miR-146a, but no association with ALL was detected [61].
MiR-196a. The rs11614913 C/T polymorphism, which lies in the mature sequence of miR-196a, negatively impacts endogenous processing of pre-miRNA and its mature form; it has also been associated with various malignancies [69,70,71]. The CC genotype of this variant increases the expression levels of mature miR-196a2 and affects the binding of mature miR-196a2 to its target mRNA when compared with the rs11614913TT genotype [72]. The TC genotype, as well as CC/TC compared with the TT genotype of this variant, was found to be associated with a significantly increased risk for ALL susceptibility in Chinese children [73]. Then, in 2017, these results were replicated by Rakmanee et. al. in the Thai population, and they also reported an association of heterozygote TC genotype, both alone and in combination with CC genotype (TC + CC), increasing the risk of ALL in pediatric patients [74]. In addition, in Mexican cases, it was observed that the miR-196a rs11614913T allele confers risk to ALL in females [66]. Conversely, another study from Taiwan showed no association of the rs11614913C/T variant with ALL, thus highlighting the lack of reproducibility even in studies with populations with a similar genetic background [75].
MiR-499. The miR-499 rs3746444 involves an A/G nucleotide substitution which leads to a change from A:U pair to G:U mismatch in the stem structure of pre-miR-499 [65]. To date, several case-control studies have demonstrated that this variant is implicated in a variety of cancers [76,77,78,79,80]. The rs3746444G allele was found to be associated with a lower risk for ALL in children of European origin [60]. Note that, in Brazilians, it was found that the homozygous mutant genotype GG increases the risk of ALL 17-fold [81], in concordance with the findings described in Mexican children [66]. However, this polymorphism showed no association with ALL in the Iranian population [65].
MiR-612. The rs12803915G/A SNP is located in pre-miR-612. Functional in vitro analyses in different cancer types, such as prostate, colon, and breast, have shown that its effect in mature miR-612 expression might be dependent on the cellular and tissue-specific context [82]. It has been reported as associated with protection against ALL development in Caucasians from Spain, but studies in the Iranian population showed no association [83]. Furthermore, members of the Ikaros family of zinc-finger (IKZF) proteins have previously been associated with ALL susceptibility, and since IKZF2 transcription factor, one of the potential targets of miR-612, is involved in the regulation of lymphocyte development, the effect of the mutant allele rs12803915 A in the regulation of miR-612 could explain an association with ALL risk, but studies in different cohort of patients might be performed to confirm such results [60,84,85].
MiR-34b/c. Associations between the miR-34b/c rs4938723T/C polymorphism (located within the CpG island of the pri-miR-34b/c promoter region) with cancer risk have been studied extensively [86,87,88,89,90], but its influence in ALL was documented till 2016 by Tong et. al. These authors reported that the rs4938723 CC genotype was associated with a reduced ALL risk in Chinese children and showed that the transcription activity of miR-34b/c was increased when T allele transited to C through in vitro luciferase assays in Jurkat and K-562 cell lines [91]. In addition, Hashemi et. al. reported that the C allele significantly decreased the risk of childhood ALL compared to the T allele in the Iranian population [92].
Other miRNAs. MiR-100 (rs543412C/T) and miR-938 (rs2505901T/C) polymorphisms have been explored lately in ALL. Subjects carrying mutant homozygous TT genotype of miR-100 rs543412C/T had a statistically significantly decreased risk of childhood ALL in the Chinese population; in this same study, it was also documented that miR-100 expression was lower in controls than in cases. In addition, it was found that individuals with TT genotype had a significantly lower level of miR-100 compared with the wild CC genotype [68]. Moreover, rs2505901C/T, located in miR-938 and described as a gene responsible for the regulatory pathways of the genes related with cell survival and apoptosis, showed an association between the wild-type homozygous CC genotype and a lower risk of childhood ALL development in a population in Brazil [81].
Genes involved in miRNAs’ processing/maturation. The genetic variants located in genes involved in miRNA processing and maturation may also affect levels of miRNA expression. For instance, rs10035440 in DROSHA, and rs9606248 and rs1640299 in DGCR8, which have been associated with ALL risk, could modify miRNA by altering DROSHA and DGCR8 expression levels [60]. Recently, the genotype AA of the SNP rs3805500 (DROSHA) was documented to be associated with an increased risk of developing ALL when compared to other genotypes. This variant is in linkage disequilibrium with rs640831 (DROSHA), which has been related to an alteration in the maturation of pri-miRNAs and pre-miRNAs [81]. The knowledge about the role of SNPs located in genes involved in miRNAs’ biogenesis is still scarce; thus, future research directions should be focused on this topic.
4.2. MiRNAs’ SNPs and Drug Metabolism
There is no doubt about the relevance of SNPs as modifiers of drug metabolism and toxicity [93,94,95]. A study of SNPs located in miR-5189, miR-595, and miR-6083 genes reported that the rs56292801AA genotype of miR-5189 is associated with protection against methotrexate (MTX) accumulation and toxicity over time. In silico studies revealed that SLC46A1 is the target gene predicted for this miRNA, which encodes for the proton-coupled folate transporter, involved in MTX transport [96,97]. Likewise, the rs4909237TT genotype in miR-595 showed an increased risk of having MTX high plasma levels over time. In addition to SLC46A1, miR-595 target genes include the transporters SLC19A1 and SLCO1A2, which are also involved in MTX uptake [98]. Another miRNA gene associated with MTX concentration is miR-6083, and its rs78790512GG and AG genotypes have been associated with the accumulation of MTX over time. Since no mRNA has been identified as a miR-6083 target, it is speculated that it could have an indirect effect by regulating other ncRNAs [99]. As miRNAs can also regulate genes involved in drug transport, metabolism, and targets, SNPs in miRNA biogenesis proteins are not only related to a predisposition for pediatric ALL but may also participate in drug response or resistance, such as rs639174 in DROSHA, an intronic SNP that has been associated with toxicity during chemotherapeutic treatment [100,101]. Notably, based on the HaploReg v4.1 online tool for variant annotations [102], the rs639174 (DROSHA) variant could modify the binding sites for glucocorticoid receptor (GR) sequence. GR is a critical receptor in ALL, since glucocorticoid (GC) plays an essential role during the treatment of ALL, and mutations in this gene are associated with GC resistance [103].
4.3. MiRNAs’ Expression Profiles and Their Role as Biomarkers in ALL
Several studies have documented that the miRNAs’ expression profiles in ALL could be useful to distinguish between normal and pathological hematopoiesis (diagnostic biomarkers), leukemia lineages, molecular subtypes, relapse, and prognostic risk prediction [104,105,106,107].
4.3.1. Diagnostic Biomarkers
One of the first studies aimed at identifying miRNAs differentially expressed between ALL and normal samples was performed by Zanette et al. in 2007. The authors assessed the expression of 164 miRNAs in CD19+ B cells from healthy individuals and ALL patients, identifying 45 miRNAs differentially expressed between both groups. The most overexpressed were miR-128b, miR-204, miR-218, miR-331, and miR-181b, and the most underexpressed were and miR-135b, miR-132, miR-199, miR-139, and miR-150 [108]. Furthermore, a study comparing CD34+ B cells from ALL patients and healthy subjects reported 15 miRNAs differentially expressed among groups. In that study, the most upregulated miRNAs were miR-128a, miR-142, miR-150, miR-181, miR-30e-5p, miR-193, miR-34b, miR-365, miR-582, and miR-708, and the highest downregulated miRNAs were miR-100, miR-125b, miR-99a, miR-196b, and miR-let-7e [109]. Other studies contributed to the identification of miRNAs abnormally expressed in ALL cases versus normal samples observing a higher expression of many other miRNAs, as described in Table 1. Nevertheless, only miR-125b-1, miR-128, miR-128(a-c), miR-142-p3, miR-155, miR-181a, miR-181b, miR-181b-5p, and miR-203 are among the most consistently replicated in ALL samples at the time of diagnosis [107,110,111,112,113,114,115,116,117]. Recently, it was reported that miRNAs enriched in exosomes and vesicles of circulating leukemic cells, such as miR-181b-5p, could also be useful as a prognosis biomarker for child ALL [115]. It has been demonstrated that miR-181b-5p contributes to the proliferation, migration, and invasion of leukemic cells [115]. By using small RNA sequencing, some differentially expressed miRNAs have also been found in T-lineage ALL compared to healthy bone marrow, with miR-548a-3p, miR-128-3p, miR-181b-5p, miR-20b-5p, miR-574-5p, miR-10a-5p, miR-582-3p, and miR-143-3p being among the most deregulated [110,118].
Table 1.
Abnormally expressed miRNAs in acute lymphoblastic leukemia patients compared to healthy individuals.
| Upregulated | Downregulated |
|---|---|
| miR-7e [111], miR-9 [111], miR-9* [111], miR-34a [116], miR-92a [113], miR-100 [119], miR-125b-1 [114], miR-128 [111,120,121], miR-130b [111], miR-142-3p [112], miR-146a [115,119,122], miR-155 [115], miR-181 [121], miR-181a [111,117], miR-181b [111,115], miR-210 [123], miR-222 [112,116], miR-339 [112], miR-363 [111], miR-511 [116], miR-638 [113], miR-1943 [111], miR-1841 [111], miR-1931 [111], miR-1987 [111], miR-1890 [111], miR-1902 [111] | let-7e [121], miR-18a [124], miR-26a [116], miR-30a [111], miR-100 [121,123], miR-126 [111], miR-143 [111], miR-145 [115], miR-196a [119], miR-196b [117,121], miR-199b-3p [111], miR-200c [125], miR-203 [114], miR-221 [116], miR-223 [111,116], miR-326 [125], miR-373* [112], miR-451 [112], miR-582-5p [111], miR-1893 [111], miR-1971* [111], miR-1834 [111], miR-1842* [111], miR-1842 [111] |
MiRNAs in bold letters are described in more than one paper.
At this time, many efforts have been made to improve the detection of hematological malignancies by using specific miRNA that could perform better than other markers for disease diagnosis; however, few of them have been validated in more than one study, and none of them has reported a consensus miRNA signature, revealing the complexity of the biology of ALL and the potential involvement of technical and sample-size biases during the studies performed.
4.3.2. Immunophenotype Classification
The identification of miRNAs as potential biomarkers for subtyping linages in ALL has been a constant objective in diverse studies, but the results are still controversial [48,49,51,109,110,126,127].
For instance, several miRNAs were found to be abnormally expressed in T-cell ALL in contrast to B-ALL, as shown in Table 2, but few of them have been replicated in at least two different reports like miR-151, miR-424, miR-29c-5p, and miR-708 have [126,127,128,129]. Moreover, miR-29c-5p has been considered to be the best discriminator between childhood T- and B-ALL, due not only to its upregulation in T-ALL, but also because among the potential targeted genes of this miRNA are some participating in ALL development, such as AFF1, KMT2A, and ELK4 [126].
Table 2.
The miRNAs that are differentially expressed in T-ALL compared with B-ALL.
| Upregulated | Downregulated |
|---|---|
| miR-29c-5p [126], miR-137 [129], miR-148a [127], miR-149* [130], miR-193b* [129], miR-424-5p [126], miR-424 [127], miR-450b-5p [126], miR-450a-5p [126], miR-506 [129], miR-509-5p [129], miR-510 [129], miR-542-5p [126], miR-629-5p [126] | miR-100 [128], miR-151 [127], miR-151a-5p [126], miR-151b [126], miR-195-5p [126], miR-371b-5p [126], miR-383 [129], miR-425-5p [126], miR-455-5p [126], miR-497-5p [126], miR-574-5p [126], miR-708 [128,129], miR-708-5p [126], miR-1266-5p [126] |
MiRNAs in bold letters are described in more than one paper.
4.3.3. Molecular Subtype Identification
The impact of miRNAs on ALL molecular subtype classification is one of the mayor fields to which miRNA expression profiles have contributed. For instance, Schotte et al., when evaluating 397 miRNAs, found that these genes cluster in specific expression patterns, which are useful to differentiate among mixed-lineage leukemia (MLL)-rearrangements, TEL-AML1-positive, E2A-PBX1-positive, and hyperdiploidies, but not BCR-ABL1-positive ALL and “B-other” ALL (lacking recurrent fusion genes) [110]. In addition, a study focused on ALL patients with microdeletions (mainly in IKZF1), and it was demonstrated that the expression level of miR-128 was significantly lower in this group of patients than it was in their counterparts. In addition, cases with CDKN2A/B and miR-31deletions had a low expression of miR-542, and patients who were positive to miR-31 and PAX5 deletion displayed low expression of miR-24, miR-708, and miR-128; meanwhile, high expression of miR-24 and miR-542 was associated with PAR1 deletion, and overexpression of miR-708 was associated with ETV6 deletion [131].
MLL-rearranged leukemias are a group of aggressive leukemias that is often developed in infants. Recently, upregulated miR-130b and miR-128a were identified in MLL-AF4 primary samples [132]. Interestingly, miR-130b and miR-128a recapitulated MLL-AF4 leukemias with unique lineages in murine models, underlining the complexity of the mechanisms that drive leukemogenesis [132]. The loss of expression of other miRNAs, such as miR-432, miR-503, and miR-148a, which are markedly downregulated in MLL-rearranged leukemia, is predicted to target and overdrive the expression of several genes associated with early recurrence and poor outcome [133]. The knowledge of miRNAs in normal and pathological hematopoiesis has been obtained from murine models and cell lines. For instance, studies in murine models positive to MLL-AF4 fusion protein encoded by the chromosomal translocation t(4;11) show that miR-128a and miR-130b can drive the transition from a pre-leukemic to acute leukemia stage and that both miRNAs are needed to maintain the leukemic phenotype. An interesting fact was that miR-130b resulted in a mixed/B-cell precursor (BCP)/myeloid leukemia, while miR-128a was conducted to a pro-B ALL [132]. In cell lines carrying t (4;11), it was observed that miR-142-3p inhibits the MLL-AF4 expression [134].
Other subtypes recently described, such as ERG-altered ALL, have also been associated with miRNAs’ expression signatures, with the upregulation of the known miR-125b-2 cluster being characteristic of this subtype [135]. In another novel subtype that involves gene fusions with the myocyte enhancer factor 2 gene (called MEF2D-rearranged), it was shown that the miR-122 is a critical repressor of MEF2D. The loss of the miR-122 target site by chromosomal translocation leads to MEF2D overexpression, acting cooperatively with the transcriptional activities of MEF2D that include PAX5 inhibition and the arrest of B cells’ differentiation [136].
4.3.4. Disease-Free Survival and Overall Survival
Deregulated miRNAs’ expression has been explored as a diagnostic biomarker associated with disease-free survival (DFS) and overall survival (OS) to identify patients with a high risk of relapse or detect the minimal residual disease (MRD).
Among the miRNAs associated with unfavorable long-term clinical outcome are miRNA-21, miR-33, miR-143, miR-182, miR-215, miR-369-5p, miR-496, miR-518d, miR-599, miRNA-155a, and miR-181a [137,138]. In addition to a poor prognosis, miRNA-21 upregulation has been associated with high-risk clinical features, such as age, lower platelet count, and worse disease-free survival and OS [139]. Moreover, miR-125b, which has a critical role in B-cell development, has been suggested as an independent prognostic factor. The downregulation of this ncRNA correlates with the inferior outcome at diagnosis, but on the other hand, its high expression on day 33 (after induction phase) was associated with short-term survival and worse OS [140]. To note, high levels of miR-143 and miR-182 at the end of induction therapy were associated with a higher risk of short-term relapse and death (Figure 2B) [141].
Overall, miR-10a, miR-134, miR-214, miR-484, miR-572, miR-580, miR-624, and miR-627 have been linked to a favorable outcome, and the first three (miR-10a, miR-134, and miR-214) were previously reported as tumor suppressors in other types of cancers. Moreover, miR-10a and miR-214 work by inhibiting cell proliferation, and miR-134 works by downregulating the SOX2 (sex-determining region Y-box 2) transcription factor that has been identified as an oncogene [110,142,143,144]. Additionally, miR-23a, miR-27a, miR-128b, miR-181, and miR-223, showed a differential expression pattern when comparing samples obtained at complete remission versus diagnostic times. The expression profile was also significantly distinct among the relapse and non-relapsed groups. Since miR-223 has previously been identified as a tumor-suppressor gene and an important factor in leukemogenesis, this can explain its overexpression in complete remission patients [137,145].
Relapse. Both miR-922 and miR-1324 were differentially expressed miRNAs associated with early relapse (relapses that occur within 36 months from initial diagnosis) [146]. These miRNAs were previously identified in other types of cancer but not in ALL. MiR-708, miR-27a, and miR-223 have been proposed as potential biomarkers due to the correlation of a higher relapse free survival rate with high expression of these three miRNAs in ALL pediatric patients. Moreover, their target genes are implicated in leukemic cell development, differentiation, and activation [137].
Another study showed that a gene-expression signature comprising a low expression of miR-151-5p and miR-451 and the high expression of miR-1290 is an independent prognostic biomarker of relapse in B-ALL (10.5-fold increased risk). This signature maintained a significant association with relapse, even when excluding patients with unfavorable cytogenetic markers, and IKZF1 deletion (24.5-fold increased risk) [147]. Han et al. showed a miRNA expression profile in pediatric ALL patients in complete remission that was significantly distinct from that of patients that presented relapse. MiR-223, miR-23a, and let-7g were downregulated, whereas miR-181 family, miR-708, and miR-130b were upregulated in the relapse samples [137]. Moreover, a significant association was observed between miR-24 overexpression and the risk of relapse, as well as a shorter overall survival compared to those with low miR-24 expression [148].
Minimal Residual Disease. In addition, to discriminate between ALL patients versus healthy controls, it was reported that miRNA-155a is a potential biomarker to evaluate MRD [138]. Recently, Rzepiel et al. (2019) reported that circulating miR-128-3p and miR-222-3p in blood predict day 15 flow cytometry MRD results 7 days earlier; thus, these miRNAs may act as biomarkers of residual leukemia [149].
Chemotherapeutic Response and miRNA Expression. The survival rate in ALL has been improved over the last two decades; however, the mortality proportion due to toxicity of chemotherapy still happens. Thus, it is relevant to identify biomarkers that can help to detect those cases that are at a high risk of developing toxicity, especially to the cornerstone of chemotherapy agents (vincristine, asparaginase, daunorubicin, glucocorticoids, cyclophosphamide, cytarabine, methotrexate, and the thiopurines mercaptopurine and thioguanine) [150]. For example, Zhang et al. identified a unique miRNA-expression signature composed of eight miRNAs (miR-18a, miR-532, miR-218, miR-625, miR-193a, miR-638, miR-550, and miR-633) that can differentiate between a good or poor prednisone response in pediatric ALL [151].
In ALL cell lines and prednisone-resistant patients’ samples, it was found that miR-124 and miR-331-3p were upregulated, potentially by repressing the glucocorticoids (GC) receptor expression and by the inhibition of the JNK/MAPK pathway [152,153]. By conducting a microarray analysis of ALL cases, it was found that miR-185-5p is overexpressed; furthermore, it was observed that its overexpression increases cell apoptosis and cycle arrest and decreases cell survival GC resistance cell line by targeting one component of the mammalian target of rapamycin complex, a pathway involved in several types of cancer and associated with GC resistance in hematological malignancies [154].
To identify if miRNA is associated with prednisolone, vincristine, L-asparaginase, and daunorubicin resistance, Schotte et al. studied 81 ALL cases and found that sixteen miRNAs were discriminative for resistance to one or more drugs. MiR-454 has been expressed at a 1.9-fold-lower level in L-asparaginase-resistant cases in comparison to their counterparts, whereas miR-125b, miR-99a, and miR-100 were the most upregulated in patients resistant to vincristine and daunorubicin [110]. Otherwise, experimental studies revealed that the co-expression of miR-125b and miR-99a or miR-100 increases the cellular viability of REH cell lines treated with vincristine [155].
Han et al. found that miR-708 was significantly upregulated in prednisone good-response patients, indicating that the miR-708 level before chemotherapy could be highly predictive of prednisone response [137]. MiR-125b has been also reported as being associated with chemotherapy resistance in B-cell malignant multiple myeloma, for which the expression can prevent cell death through suppression of p53 and the p53/miR-34a/SIRT regulatory network [140,156].
In addition, specific miRNAs have been studied in vitro to better understand their participation and function in drug response; for example, the relevance of the level of expression of miR-210 was studied by Mei et al. Their results outlined that increasing/decreasing miR-210 expression could enhance or reduce the response of leukemic cell lines to daunorubicin–dexamethasone–L-asparaginase and daunorubicin–dexamethasone–vincristine, respectively. These findings suggest that an increased intracellular level of miR-210 enhances the sensitivity of leukemic cells to common chemotherapeutic drugs and decreases their viability [157].
Another relevant miRNA is miR-652-3p because it has been documented that increased levels of its expression might assist in suppressing lymphoblastic leukemia cells. Jiang et. al. suggest that the expression of miR-652-3p is markedly lower in the lymphoblastic leukemia cell lines compared with the normal B-cell lines and implied that the increase in apoptosis induced by the overexpression of miR652-3p might, to some extent, contribute to the increased sensitivity of lymphoblastic leukemia cells to chemotherapeutic drugs, particularly vincristine and cytarabine [158].
In addition to the in vitro and in vivo models and ALL samples’ findings, computational tools have also been useful to predict miRNAs’ deregulation and their implications for prognosis and therapy resistance. Regarding this, a semantics-based search approach predicted that miR-142-3p and miR-17-5p potentially regulate GC resistance, being some of the most promising and validated markers across different studies found in the literature [159].
ALL cells co-cultured with bone-marrow stromal cells or primary human osteoblasts lead to the downregulation of miR-221 and miR-222 and G0 cell-cycle arrest; conversely, induced expression of miR-221 sensitized leukemic cells to chemotherapy agents and allowed cell-cycle progression [160]. The crucial role of the bone-marrow niche for both HSCs and leukemic cells is known. The bone-marrow niche provides cues that ensure survival and quiescence of HSCs, a context that leukemic cells with stem-like features benefit from, often leading to resistance to therapy and relapse.
There are encouraging findings in miRNA research for pediatric leukemias. Despite concerns that sncRNAs’ stability could limit their accurate detection, there is a high correlation between miRNAs’ expression in body fluids, such as plasma, serum, urine, bile, and feces, and their tissues of origin, highlighting their potential benefit as clinical biomarkers for disease-specific monitoring [161,162,163]. Comprehensive tools are still needed to find the most accurate miRNAs for prognostic purposes in ALL since accuracy and reproducibility are still important caveats in this research area.
5. Molecular Mechanisms of miRNAs in ALL
The most straightforward approach of miRNA functional mechanisms is through mice models, cell-lines studies, and functional enrichment analysis (FEA) using the miRNA-target genes. FEA use databases that have been developed to infer miRNA functions and identify targeted genes (miRGator, miRDB, miRò, MAGIA and FAME, miRanda, PicTar, and TargetScanS); thus, it can give us a large amount of knowledge of the potential biological role of them [164]. Nevertheless, few studies have been conducted to increase our understanding of the molecular mechanisms of miRNAs underlying ALL development [155].
Among the most replicated miRNAs, miR-125b is considered to be a diagnostic and prognostic biomarker, and it is a key miRNA that is implicated in T-cell maturation. This ncRNA is overexpressed in undifferentiated T-cells and induces glucose metabolic switch in T cells via targeting TNF-α-induced protein 3, which is frequently found to be inactivated, mutated, or deleted in leukemia. It also increases cell growth and in vivo invasiveness, contributing to T-ALL development [48,49]. Furthermore, miR-125b acts as a critical tumor suppressor since its abnormal expression impairs the exit of immature B cells from the bone marrow, and its silencing is necessary for normal B-cell development [46].
Moreover, miR-128 has been reported to show dysregulation in several tumors that results in inhibition or promotion of tumor proliferation. The transcriptional factor and oncogene proto-oncogene polycomb ring finger, which is important in hematopoietic stem cells and leukemia stem-cell self-renewal, has been predicted to be one of its putative targets, and in silico predictions suggest that it is one of the major regulators of lymphoid differentiation. It was proposed as a potential biomarker to discriminate between AML and ALL, as well as to predict MRD [107,120,149,165].
Experimental studies have suggested that miR-26b, miR-20b-5p, and hsa-miR-363-3p could be relevant to T-ALL development [128,166]. For instance, miR-101, which is frequently downregulated in primary T-ALL samples, plays a tumor-suppressor role, as it suppresses the expression of Notch1 and the proto-oncogene TAL1 [167,168].
When comparing with normal marrow cells, miR-196b was found to be upregulated in ALL patients positive to MLL-rearrangements and in those with T-ALL. Evidence shows that the presence of MLL fusion proteins leads to aberrant mir-196b expression, causing increased proliferation and abnormal hematopoietic differentiation and contributing to leukemogenesis. MLL regulates the expression of the homeobox domain (HOX) gene family that is involved in the regulation of normal hematopoiesis. Aberrant expression of HOXA genes is not restricted to MLL-rearranged precursor B-ALL cases; it has also been reported in T-ALL patients. MiR-196b is mapped between the HOXA9 and HOXA10 genes on chromosome 7p15.2, so the level of expression of miR-196b may be linked to HOXA gene transcription [169,170].
A bioinformatic analysis of public gene-expression datasets revealed that miR-124-1, miR-124-2, and miR-124-3 are overexpressed in ALL patients with early recurrence. Interestingly, it was predicted that the tumor-suppressor gene, interferon-induced protein-44-like gene is targeted by miR-124-3p, which displays the potential therapeutic implications of this ncRNA [171].
6. Closing Remarks and Conclusions
It has been widely documented that miRNAs’ deregulation in cancer is often due to structural alterations (chromosomal translocations, deletions, and amplifications) in regions that contain them, or perturb genes that control their expression. Particularly in hematological malignancies, there are some examples, such as loss of 13q14 region in CLL, that contain miR-15 and miR-16, which are important regulators of BCL2-mediated apoptosis, leading to a reduced expression or loss of expression [172,173]; the proto-oncogene MYC, frequently altered by chromosomal rearrangements and amplifications, regulates several miRNAs, such as the miR-17-92 cluster, miR-34a, and miR-15a/16-1, and its expression is also controlled by miR-17-92 in a feedback-loop manner [174]. Furthermore, miRNAs are involved in most aspects of ALL biology, observed as differential expression profiles associated with the disease, molecular subtypes, prognosis, and drug response. Although the studies reveal a great heterogeneity on the miRNAs involved in any clinical aspect of ALL, the findings that miR-125 and miR-142 have been detected in several studies point to the fact that these miRNAs have potential for applications in the clinical field. Proper validation to ensure reproducibility across different technologies and methodologies is key for their implementation into the clinical setting and for patients’ benefit.
Overall, miRNAs’ therapeutic potential is encouraging but still faces major challenges. The fact that miRNAs can regulate numerous cancer-related pathways makes them attractive therapeutic targets, and many technical approaches are currently evaluated for the purpose of restoring miRNAs’ function in diverse diseases, namely as miRNA sponges, antisense antagomers, small molecule inhibitors, miRNA editing, and non-viral delivery systems [175,176,177,178]. Unfortunately, only a few efforts have progressed into clinical trials, and miRNAs have been mostly proposed as diagnosis or prognosis markers. The improvement of delivery systems could enhance their therapeutic application by diminishing the off-target effects seen in preclinical studies and by increasing their stability for tissue or disease-specific activity.
Thus, the knowledge stated so far here and the continuous interest of many research groups in understanding the biological effect of miRNAs expression are of great relevance not only because they could be implemented as non-invasive diagnostic biomarkers, but they could also help in regard to ALL diagnosis, risk stratification, and relapse and toxicity prediction. This could be translated into a better personalized therapy for specific groups of patients that share specific expression miRNA profiles, which could help to improve survival and reduce the risk of secondary adverse effects.
Author Contributions
D.K.M.-S., D.A.B.-L., C.J.P.-A. and G.M.C.-M. reviewed the literature and wrote the first draft of the manuscript; D.K.M.-S. and S.J.-M. were involved in the conception of the review; J.M.M.-A., J.R.-B., A.H.-M. and S.J.-M. critically reviewed the final version. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This study was funded by the Instituto Nacional de Medicina Genómica (01/2018/I). Diana Karen Mendiola-Soto was supported by CONACyT (CVU 706961).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Fernandes M.R., Souza Vinagre L.W.M., Rodrigues J.C.G., Wanderley A.V., Fernandes S.M., Gellen L.P.A., Alcantara A.L., Sousa B.B., Burbano R.M.R., Assumpcao P.P., et al. Correlation of Genetic Variants and the Incidence, Prevalence and Mortality Rates of Acute Lymphoblastic Leukemia. J. Pers. Med. 2022;12:370. doi: 10.3390/jpm12030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jammal N., Chew S., Jabbour E., Kantarjian H. Antibody based therapy in relapsed acute lymphoblastic leukemia. Best Pract. Res. Clin. Haematol. 2020;33:101225. doi: 10.1016/j.beha.2020.101225. [DOI] [PubMed] [Google Scholar]
- 3.De Oliveira J.C., Brassesco M.S., Scrideli C.A., Tone L.G., Narendran A. MicroRNA expression and activity in pediatric acute lymphoblastic leukemia (ALL) Pediatr. Blood Cancer. 2012;59:599–604. doi: 10.1002/pbc.24167. [DOI] [PubMed] [Google Scholar]
- 4.Jaime-Perez J.C., Jimenez-Castillo R.A., Pinzon-Uresti M.A., Cantu-Rodriguez O.G., Herrera-Garza J.L., Marfil-Rivera L.J., Gomez-Almaguer D. Real-world outcomes of treatment for acute lymphoblastic leukemia during adolescence in a financially restricted environment: Results at a single center in Latin America. Pediatr. Blood Cancer. 2017;64:e26396. doi: 10.1002/pbc.26396. [DOI] [PubMed] [Google Scholar]
- 5.Nunez-Enriquez J.C., Barcenas-Lopez D.A., Hidalgo-Miranda A., Jimenez-Hernandez E., Bekker-Mendez V.C., Flores-Lujano J., Solis-Labastida K.A., Martinez-Morales G.B., Sanchez-Munoz F., Espinoza-Hernandez L.E., et al. Gene Expression Profiling of Acute Lymphoblastic Leukemia in Children with Very Early Relapse. Arch. Med. Res. 2016;47:644–655. doi: 10.1016/j.arcmed.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez-Hernandez E., Jaimes-Reyes E.Z., Arellano-Galindo J., Garcia-Jimenez X., Tiznado-Garcia H.M., Duenas-Gonzalez M.T., Martinez Villegas O., Sanchez-Jara B., Bekker-Mendez V.C., Ortiz-Torres M.G., et al. Survival of Mexican Children with Acute Lymphoblastic Leukaemia under Treatment with the Protocol from the Dana-Farber Cancer Institute 00-01. Biomed. Res. Int. 2015;2015:576950. doi: 10.1155/2015/576950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jimenez-Hernandez E., Duenas-Gonzalez M.T., Arellano-Galindo J., Medrano-Ortiz-De-Zarate M.E., Bekker-Mendez V.C., Berges-Garcia A., Solis-Labastida K., Sanchez-Jara B., Tiznado-Garcia H.M., Jaimes-Reyes E.Z., et al. Survival of Mexican children with acute myeloid leukaemia who received early intensification chemotherapy and an autologous transplant. Biomed. Res. Int. 2015;2015:940278. doi: 10.1155/2015/940278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko R.H., Ji L., Barnette P., Bostrom B., Hutchinson R., Raetz E., Seibel N.L., Twist C.J., Eckroth E., Sposto R., et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: A Therapeutic Advances in Childhood Leukemia Consortium study. J. Clin. Oncol. 2010;28:648–654. doi: 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C., Li W. Genomics and pharmacogenomics of pediatric acute lymphoblastic leukemia. Crit. Rev. Oncol. Hematol. 2018;126:100–111. doi: 10.1016/j.critrevonc.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Nana-Sinkam S.P., Croce C.M. MicroRNAs as therapeutic targets in cancer. Transl. Res. 2011;157:216–225. doi: 10.1016/j.trsl.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Palazzo A.F., Lee E.S. Non-coding RNA: What is functional and what is junk? Front. Genet. 2015;6:2. doi: 10.3389/fgene.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P., Wu W., Chen Q., Chen M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019;16 doi: 10.1515/jib-2019-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatti G.K., Khullar N., Sidhu I.S., Navik U.S., Reddy A.P., Reddy P.H., Bhatti J.S. Emerging role of non-coding RNA in health and disease. Metab. Brain Dis. 2021;36:1119–1134. doi: 10.1007/s11011-021-00739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 15.Croce C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee H., Han S., Kwon C.S., Lee D. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell. 2016;7:100–113. doi: 10.1007/s13238-015-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elshazli R.M., Toraih E.A., Hussein M.H., Ruiz E.M., Kandil E., Fawzy M.S. Pan-Cancer Study on Variants of Canonical miRNA Biogenesis Pathway Components: A Pooled Analysis. Cancers. 2023;15:338. doi: 10.3390/cancers15020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee Y.S., Nakahara K., Pham J.W., Kim K., He Z., Sontheimer E.J., Carthew R.W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/S0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 20.Yi R., Qin Y., Macara I.G., Cullen B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes. Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makarova J.A., Shkurnikov M.U., Wicklein D., Lange T., Samatov T.R., Turchinovich A.A., Tonevitsky A.G. Intracellular and extracellular microRNA: An update on localization and biological role. Prog. Histochem. Cytochem. 2016;51:33–49. doi: 10.1016/j.proghi.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 22.De Rie D., Abugessaisa I., Alam T., Arner E., Arner P., Ashoor H., Astrom G., Babina M., Bertin N., Burroughs A.M., et al. An integrated expression atlas of miRNAs and their promoters in human and mouse. Nat. Biotechnol. 2017;35:872–878. doi: 10.1038/nbt.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanzer A., Stadler P.F. Molecular evolution of a microRNA cluster. J. Mol. Biol. 2004;339:327–335. doi: 10.1016/j.jmb.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 24.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 25.Broughton J.P., Lovci M.T., Huang J.L., Yeo G.W., Pasquinelli A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell. 2016;64:320–333. doi: 10.1016/j.molcel.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang V., Qin Y., Wang J., Wang X., Place R.F., Lin G., Lue T.F., Li L.C. RNAa is conserved in mammalian cells. PLoS ONE. 2010;5:e8848. doi: 10.1371/journal.pone.0008848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng X., Jiang Q., Chang N., Wang X., Liu C., Xiong J., Cao H., Liang Z. Small activating RNA binds to the genomic target site in a seed-region-dependent manner. Nucleic Acids Res. 2016;44:2274–2282. doi: 10.1093/nar/gkw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portnoy V., Lin S.H., Li K.H., Burlingame A., Hu Z.H., Li H., Li L.C. saRNA-guided Ago2 targets the RITA complex to promoters to stimulate transcription. Cell Res. 2016;26:320–335. doi: 10.1038/cr.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasudevan S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip. Rev. 2012;3:311–330. doi: 10.1002/wrna.121. [DOI] [PubMed] [Google Scholar]
- 30.Comazzetto S., Shen B., Morrison S.J. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev. Cell. 2021;56:1848–1860. doi: 10.1016/j.devcel.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Man Y., Yao X., Yang T., Wang Y. Hematopoietic Stem Cell Niche During Homeostasis, Malignancy, and Bone Marrow Transplantation. Front. Cell Dev. Biol. 2021;9:621214. doi: 10.3389/fcell.2021.621214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim M., Civin C.I., Kingsbury T.J. MicroRNAs as regulators and effectors of hematopoietic transcription factors. Wiley Interdiscip. Rev. 2019;10:e1537. doi: 10.1002/wrna.1537. [DOI] [PubMed] [Google Scholar]
- 33.Levesque J.P., Winkler I.G. Hierarchy of immature hematopoietic cells related to blood flow and niche. Curr. Opin. Hematol. 2011;18:220–225. doi: 10.1097/MOH.0b013e3283475fe7. [DOI] [PubMed] [Google Scholar]
- 34.Kovtonyuk L.V., Fritsch K., Feng X., Manz M.G., Takizawa H. Inflamm-Aging of Hematopoiesis, Hematopoietic Stem Cells, and the Bone Marrow Microenvironment. Front. Immunol. 2016;7:502. doi: 10.3389/fimmu.2016.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frobel J., Landspersky T., Percin G., Schreck C., Rahmig S., Ori A., Nowak D., Essers M., Waskow C., Oostendorp R.A.J., et al. The Hematopoietic Bone Marrow Niche Ecosystem. Front. Cell Dev. Biol. 2021;9:705410. doi: 10.3389/fcell.2021.705410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghafouri-Fard S., Niazi V., Taheri M. Role of miRNAs and lncRNAs in hematopoietic stem cell differentiation. Noncoding RNA Res. 2021;6:8–14. doi: 10.1016/j.ncrna.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bissels U., Bosio A., Wagner W. MicroRNAs are shaping the hematopoietic landscape. Haematologica. 2012;97:160–167. doi: 10.3324/haematol.2011.051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luinenburg D.G., de Haan G. MicroRNAs in hematopoietic stem cell aging. Mech. Ageing Dev. 2020;189:111281. doi: 10.1016/j.mad.2020.111281. [DOI] [PubMed] [Google Scholar]
- 39.Edginton-White B., Bonifer C. The transcriptional regulation of normal and malignant blood cell development. FEBS J. 2022;289:1240–1255. doi: 10.1111/febs.15735. [DOI] [PubMed] [Google Scholar]
- 40.Neaga A., Bagacean C., Tempescul A., Jimbu L., Mesaros O., Blag C., Tomuleasa C., Bocsan C., Gaman M., Zdrenghea M., et al. MicroRNAs Associated with a Good Prognosis of Acute Myeloid Leukemia and Their Effect on Macrophage Polarization. Front. Immunol. 2020;11:582915. doi: 10.3389/fimmu.2020.582915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alemdehy M.F., Erkeland S.J. MicroRNAs: Key players of normal and malignant myelopoiesis. Curr. Opin. Hematol. 2012;19:261–267. doi: 10.1097/MOH.0b013e328353d4e9. [DOI] [PubMed] [Google Scholar]
- 42.Luan C., Yang Z., Chen B. The functional role of microRNA in acute lymphoblastic leukemia: Relevance for diagnosis, differential diagnosis, prognosis, and therapy. Onco Targets Ther. 2015;8:2903–2914. doi: 10.2147/OTT.S92470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Attaway M., Chwat-Edelstein T., Vuong B.Q. Regulatory Non-Coding RNAs Modulate Transcriptional Activation During B Cell Development. Front. Genet. 2021;12:678084. doi: 10.3389/fgene.2021.678084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallaert A., Durinck K., Taghon T., van Vlierberghe P., Speleman F. T-ALL and thymocytes: A message of noncoding RNAs. J. Hematol. Oncol. 2017;10:66. doi: 10.1186/s13045-017-0432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winter S.J., Krueger A. Development of Unconventional T Cells Controlled by MicroRNA. Front. Immunol. 2019;10:2520. doi: 10.3389/fimmu.2019.02520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li G., So A.Y., Sookram R., Wong S., Wang J.K., Ouyang Y., He P., Su Y., Casellas R., Baltimore D. Epigenetic silencing of miR-125b is required for normal B-cell development. Blood. 2018;131:1920–1930. doi: 10.1182/blood-2018-01-824540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kretov D.A., Shafik A.M., Cifuentes D. Assessing miR-451 Activity and Its Role in Erythropoiesis. Methods Mol. Biol. 2018;1680:179–190. doi: 10.1007/978-1-4939-7339-2_12. [DOI] [PubMed] [Google Scholar]
- 48.Renou L., Boelle P.Y., Deswarte C., Spicuglia S., Benyoucef A., Calvo J., Uzan B., Belhocine M., Cieslak A., Landman-Parker J., et al. Homeobox protein TLX3 activates miR-125b expression to promote T-cell acute lymphoblastic leukemia. Blood Adv. 2017;1:733–747. doi: 10.1182/bloodadvances.2017005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z., Smith K.R., Khong H.T., Huang J., Ahn E.E., Zhou M., Tan M. miR-125b regulates differentiation and metabolic reprogramming of T cell acute lymphoblastic leukemia by directly targeting A20. Oncotarget. 2016;7:78667–78679. doi: 10.18632/oncotarget.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Podshivalova K., Wang E.A., Hart T., Salomon D.R. Expression of the miR-150 tumor suppressor is restored by and synergizes with rapamycin in a human leukemia T-cell line. Leuk. Res. 2018;74:1–9. doi: 10.1016/j.leukres.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Correia N.C., Fragoso R., Carvalho T., Enguita F.J., Barata J.T. MiR-146b negatively regulates migration and delays progression of T-cell acute lymphoblastic leukemia. Sci. Rep. 2016;6:31894. doi: 10.1038/srep31894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss C.N., Ito K. A Macro View of MicroRNAs: The Discovery of MicroRNAs and Their Role in Hematopoiesis and Hematologic Disease. Int. Rev. Cell Mol. Biol. 2017;334:99–175. doi: 10.1016/bs.ircmb.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sole C., Larrea E., Manterola L., Goicoechea I., Armesto M., Arestin M., Caffarel M., Araujo A., Fernandez M., Araiz M., et al. Aberrant expression of MicroRNAs in B-cell lymphomas. Microrna. 2016;5:87–105. doi: 10.2174/2211536605666160825150830. [DOI] [PubMed] [Google Scholar]
- 54.Guo J.R., Li W., Wu Y., Wu L.Q., Li X., Guo Y.F., Zheng X.H., Lian X.L., Huang H.F., Chen Y.Z., et al. Hepatocyte growth factor promotes proliferation, invasion, and metastasis of myeloid leukemia cells through PI3K-AKT and MAPK/ERK signaling pathway. Am. J. Transl. Res. 2016;8:3630–3644. [PMC free article] [PubMed] [Google Scholar]
- 55.Tapeh B.E.G., Alivand M.R., Solali S. The role of microRNAs in acute lymphoblastic leukaemia: From biology to applications. Cell Biochem. Funct. 2020;38:334–346. doi: 10.1002/cbf.3466. [DOI] [PubMed] [Google Scholar]
- 56.Turk A., Calin G.A., Kunej T. MicroRNAs in Leukemias: A Clinically Annotated Compendium. Int. J. Mol. Sci. 2022;23:3469. doi: 10.3390/ijms23073469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mardani R., Jafari Najaf Abadi M.H., Motieian M., Taghizadeh-Boroujeni S., Bayat A., Farsinezhad A., Gheibi Hayat S.M., Motieian M., Pourghadamyari H. MicroRNA in leukemia: Tumor suppressors and oncogenes with prognostic potential. J. Cell Physiol. 2019;234:8465–8486. doi: 10.1002/jcp.27776. [DOI] [PubMed] [Google Scholar]
- 58.Rawoof A., Swaminathan G., Tiwari S., Nair R.A., Dinesh Kumar L. LeukmiR: A database for miRNAs and their targets in acute lymphoblastic leukemia. Database. 2020;2020:baz151. doi: 10.1093/database/baz151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie Y., Xu Z., Wang K., Wang N., Wang Y., Bao J. Networks of micrornas and genes in acute lymphoblastic leukemia. Mol. Med. Rep. 2015;12:5361–5368. doi: 10.3892/mmr.2015.4116. [DOI] [PubMed] [Google Scholar]
- 60.Gutierrez-Camino A., Lopez-Lopez E., Martin-Guerrero I., Pinan M.A., Garcia-Miguel P., Sanchez-Toledo J., Carbone Baneres A., Uriz J., Navajas A., Garcia-Orad A., et al. Noncoding RNA-related polymorphisms in pediatric acute lymphoblastic leukemia susceptibility. Pediatr. Res. 2014;75:767–773. doi: 10.1038/pr.2014.43. [DOI] [PubMed] [Google Scholar]
- 61.Jemimah Devanandan H., Venkatesan V., Scott J.X., Magatha L.S., Durairaj Paul S.F., Koshy T. MicroRNA 146a Polymorphisms and Expression in Indian Children with Acute Lymphoblastic Leukemia. Lab. Med. 2019;50:249–253. doi: 10.1093/labmed/lmy074. [DOI] [PubMed] [Google Scholar]
- 62.Pei J.S., Chang W.S., Hsu P.C., Chen C.C., Chin Y.T., Huang T.L., Hsu Y.N., Kuo C.C., Wang Y.C., Tsai C.W., et al. Significant Association Between the MiR146a Genotypes and Susceptibility to Childhood Acute Lymphoblastic Leukemia in Taiwan. Cancer Genom. Proteom. 2020;17:175–180. doi: 10.21873/cgp.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zou D., Yin J., Ye Z., Zeng Q., Tian C., Wang Y., Chen Q., Chen R. Association Between the miR-146a Rs2910164 Polymorphism and Childhood Acute Lymphoblastic Leukemia Susceptibility in an Asian Population. Front. Genet. 2020;11:886. doi: 10.3389/fgene.2020.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu T., Zhu Y., Wei Q.K., Yuan Y., Zhou F., Ge Y.Y., Yang J.R., Su H., Zhuang S.M. A functional polymorphism in the miR-146a gene is associated with the risk for hepatocellular carcinoma. Carcinogenesis. 2008;29:2126–2131. doi: 10.1093/carcin/bgn195. [DOI] [PubMed] [Google Scholar]
- 65.Hasani S.S., Hashemi M., Eskandari-Nasab E., Naderi M., Omrani M., Sheybani-Nasab M. A functional polymorphism in the miR-146a gene is associated with the risk of childhood acute lymphoblastic leukemia: A preliminary report. Tumour Biol. 2014;35:219–225. doi: 10.1007/s13277-013-1027-1. [DOI] [PubMed] [Google Scholar]
- 66.Jimenez-Morales S., Nunez-Enriquez J.C., Cruz-Islas J., Bekker-Mendez V.C., Jimenez-Hernandez E., Medina-Sanson A., Olarte-Carrillo I., Martinez-Tovar A., Flores-Lujano J., Ramirez-Bello J., et al. Association Analysis Between the Functional Single Nucleotide Variants in miR-146a, miR-196a-2, miR-499a, and miR-612 with Acute Lymphoblastic Leukemia. Front. Oncol. 2021;11:762063. doi: 10.3389/fonc.2021.762063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chansing K., Pakakasama S., Hongeng S., Thongmee A., Pongstaporn W. Lack of Association between the MiR146a Polymorphism and Susceptibility to Thai Childhood Acute Lymphoblastic Leukemia. Asian Pac. J. Cancer Prev. 2016;17:2435–2438. [PubMed] [Google Scholar]
- 68.Xue Y., Yang X., Hu S., Kang M., Chen J., Fang Y. A genetic variant in miR-100 is a protective factor of childhood acute lymphoblastic leukemia. Cancer Med. 2019;8:2553–2560. doi: 10.1002/cam4.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffman A.E., Zheng T., Yi C., Leaderer D., Weidhaas J., Slack F., Zhang Y., Paranjape T., Zhu Y. microRNA miR-196a-2 and breast cancer: A genetic and epigenetic association study and functional analysis. Cancer Res. 2009;69:5970–5977. doi: 10.1158/0008-5472.CAN-09-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu W., Xu J., Liu S., Chen B., Wang X., Li Y., Qian Y., Zhao W., Wu J. Effects of common polymorphisms rs11614913 in miR-196a2 and rs2910164 in miR-146a on cancer susceptibility: A meta-analysis. PLoS ONE. 2011;6:e20471. doi: 10.1371/journal.pone.0020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tian T., Xu Y., Dai J., Wu J., Shen H., Hu Z. Functional polymorphisms in two pre-microRNAs and cancer risk: A meta-analysis. Int. J. Mol. Epidemiol. Genet. 2010;1:358–366. [PMC free article] [PubMed] [Google Scholar]
- 72.Hu Z., Chen J., Tian T., Zhou X., Gu H., Xu L., Zeng Y., Miao R., Jin G., Ma H., et al. Genetic variants of miRNA sequences and non-small cell lung cancer survival. J. Clin. Invest. 2008;118:2600–2608. doi: 10.1172/JCI34934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tong N., Xu B., Shi D., Du M., Li X., Sheng X., Wang M., Chu H., Fang Y., Li J., et al. Hsa-miR-196a2 polymorphism increases the risk of acute lymphoblastic leukemia in Chinese children. Mutat. Res. 2014;759:16–21. doi: 10.1016/j.mrfmmm.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 74.Rakmanee S., Pakakasama S., Hongeng S., Sanguansin S., Thongmee A., Pongstaporn W. Increased Risk of Thai Childhood Acute Lymphoblastic Leukemia with the MiR196a2 T>C Polymorphism. Asian Pac. J. Cancer Prev. 2017;18:1117–1120. doi: 10.22034/APJCP.2017.18.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen C.C., Hsu P.C., Shih L.C., Hsu Y.N., Kuo C.C., Chao C.Y., Chang W.S., Tsai C.W., Bau D.T., Pei J.S., et al. MiR-196a-2 Genotypes Determine the Susceptibility and Early Onset of Childhood Acute Lymphoblastic Leukemia. Anticancer Res. 2020;40:4465–4469. doi: 10.21873/anticanres.14451. [DOI] [PubMed] [Google Scholar]
- 76.Liu Z., Li G., Wei S., Niu J., El-Naggar A.K., Sturgis E.M., Wei Q. Genetic variants in selected pre-microRNA genes and the risk of squamous cell carcinoma of the head and neck. Cancer. 2010;116:4753–4760. doi: 10.1002/cncr.25323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu Z., Liang J., Wang Z., Tian T., Zhou X., Chen J., Miao R., Wang Y., Wang X., Shen H., et al. Common genetic variants in pre-microRNAs were associated with increased risk of breast cancer in Chinese women. Hum. Mutat. 2009;30:79–84. doi: 10.1002/humu.20837. [DOI] [PubMed] [Google Scholar]
- 78.Xiang Y., Fan S., Cao J., Huang S., Zhang L.P. Association of the microRNA-499 variants with susceptibility to hepatocellular carcinoma in a Chinese population. Mol. Biol. Rep. 2012;39:7019–7023. doi: 10.1007/s11033-012-1532-0. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y., Yang B., Ren X. Hsa-miR-499 polymorphism (rs3746444) and cancer risk: A meta-analysis of 17 case-control studies. Gene. 2012;509:267–272. doi: 10.1016/j.gene.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 80.Wang L., Qian S., Zhi H., Zhang Y., Wang B., Lu Z. The association between hsa-miR-499 T>C polymorphism and cancer risk: A meta-analysis. Gene. 2012;508:9–14. doi: 10.1016/j.gene.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 81.de Souza T.P., de Carvalho D.C., Wanderley A.V., Fernandes S.M., Rodrigues J.C.G., Cohen-Paes A., Fernandes M.R., Mello Junior F.A.R., Pastana L.F., Vinagre L., et al. Influence of variants of the drosha, mir499a, and mir938 genes on susceptibility to acute lymphoblastic leukemia in an admixed population from the brazilian amazon. Am. J. Transl. Res. 2020;12:8216–8224. [PMC free article] [PubMed] [Google Scholar]
- 82.Kim H.K., Prokunina-Olsson L., Chanock S.J. Common genetic variants in miR-1206 (8q24.2) and miR-612 (11q13.3) affect biogenesis of mature miRNA forms. PLoS ONE. 2012;7:e47454. doi: 10.1371/journal.pone.0047454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siyadat P., Ayatollahi H., Barati M., Sheikhi M., Shahidi M. High Resolution Melting Analysis for Evaluation of mir-612 (Rs12803915) Genetic Variant with Susceptibility to Pediatric Acute Lymphoblastic Leukemia. Rep. Biochem. Mol. Biol. 2021;9:385–393. doi: 10.52547/rbmb.9.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trevino L.R., Yang W., French D., Hunger S.P., Carroll W.L., Devidas M., Willman C., Neale G., Downing J., Raimondi S.C., et al. Germline genomic variants associated with childhood acute lymphoblastic leukemia. Nat. Genet. 2009;41:1001–1005. doi: 10.1038/ng.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Papaemmanuil E., Hosking F.J., Vijayakrishnan J., Price A., Olver B., Sheridan E., Kinsey S.E., Lightfoot T., Roman E., Irving J.A., et al. Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat. Genet. 2009;41:1006–1010. doi: 10.1038/ng.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Son M.S., Jang M.J., Jeon Y.J., Kim W.H., Kwon C.I., Ko K.H., Park P.W., Hong S.P., Rim K.S., Kwon S.W., et al. Promoter polymorphisms of pri-miR-34b/c are associated with hepatocellular carcinoma. Gene. 2013;524:156–160. doi: 10.1016/j.gene.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 87.Ji T.X., Zhi C., Guo X.G., Zhou Q., Wang G.Q., Chen B., Ma F.F. MiR-34b/c rs4938723 Polymorphism Significantly Decreases the Risk of Digestive Tract Cancer: Meta-analysis. Asian Pac. J. Cancer Prev. 2015;16:6099–6104. doi: 10.7314/APJCP.2015.16.14.6099. [DOI] [PubMed] [Google Scholar]
- 88.Yuan F., Sun R., Chen P., Liang Y., Ni S., Quan Y., Huang J., Zhang L., Gao L. Combined analysis of pri-miR-34b/c rs4938723 and TP53 Arg72Pro with cervical cancer risk. Tumor Biol. 2016;37:6267–6273. doi: 10.1007/s13277-015-4467-y. [DOI] [PubMed] [Google Scholar]
- 89.Sanaei S., Hashemi M., Rezaei M., Hashemi S.M., Bahari G., Ghavami S. Evaluation of the pri-miR-34b/c rs4938723 polymorphism and its association with breast cancer risk. Biomed. Rep. 2016;5:125–129. doi: 10.3892/br.2016.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hashemi M., Moazeni-Roodi A., Bahari G., Taheri M., Ghavami S. Association between miR-34b/c rs4938723 polymorphism and risk of cancer: An updated meta-analysis of 27 case-control studies. J. Cell Biochem. 2019;120:3306–3314. doi: 10.1002/jcb.27598. [DOI] [PubMed] [Google Scholar]
- 91.Tong N., Chu H., Wang M., Xue Y., Du M., Lu L., Zhang H., Wang F., Fang Y., Li J., et al. Pri-miR-34b/c rs4938723 polymorphism contributes to acute lymphoblastic leukemia susceptibility in Chinese children. Leuk. Lymphoma. 2016;57:1436–1441. doi: 10.3109/10428194.2015.1092528. [DOI] [PubMed] [Google Scholar]
- 92.Hashemi M., Bahari G., Naderi M., Sadeghi-Bojd S., Taheri M. Pri-miR-34b/c rs4938723 polymorphism is associated with the risk of childhood acute lymphoblastic leukemia. Cancer Genet. 2016;209:493–496. doi: 10.1016/j.cancergen.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 93.Amstutz U., Offer S.M., Sistonen J., Joerger M., Diasio R.B., Largiader C.R. Polymorphisms in MIR27A Associated with Early-Onset Toxicity in Fluoropyrimidine-Based Chemotherapy. Clin. Cancer Res. 2015;21:2038–2044. doi: 10.1158/1078-0432.CCR-14-2817. [DOI] [PubMed] [Google Scholar]
- 94.Meulendijks D., Henricks L.M., Amstutz U., Froehlich T.K., Largiader C.R., Beijnen J.H., de Boer A., Deenen M.J., Cats A., Schellens J.H., et al. Rs895819 in MIR27A improves the predictive value of DPYD variants to identify patients at risk of severe fluoropyrimidine-associated toxicity. Int. J. Cancer. 2016;138:2752–2761. doi: 10.1002/ijc.30014. [DOI] [PubMed] [Google Scholar]
- 95.Zhan X., Wu W., Han B., Gao G., Qiao R., Lv J., Zhang S., Zhang W., Fan W., Chen H., et al. Hsa-miR-196a2 functional SNP is associated with severe toxicity after platinum-based chemotherapy of advanced nonsmall cell lung cancer patients in a Chinese population. J. Clin. Lab. Anal. 2012;26:441–446. doi: 10.1002/jcla.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qiu A., Min S.H., Jansen M., Malhotra U., Tsai E., Cabelof D.C., Matherly L.H., Zhao R., Akabas M.H., Goldman I.D., et al. Rodent intestinal folate transporters (SLC46A1): Secondary structure, functional properties, and response to dietary folate restriction. Am. J. Physiol. Cell Physiol. 2007;293:C1669–C1678. doi: 10.1152/ajpcell.00202.2007. [DOI] [PubMed] [Google Scholar]
- 97.Zhao R., Qiu A., Tsai E., Jansen M., Akabas M.H., Goldman I.D. The proton-coupled folate transporter: Impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Mol. Pharmacol. 2008;74:854–862. doi: 10.1124/mol.108.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Desmoulin S.K., Hou Z., Gangjee A., Matherly L.H. The human proton-coupled folate transporter: Biology and therapeutic applications to cancer. Cancer Biol. Ther. 2012;13:1355–1373. doi: 10.4161/cbt.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iparraguirre L., Gutierrez-Camino A., Umerez M., Martin-Guerrero I., Astigarraga I., Navajas A., Sastre A., Garcia de Andoin N., Garcia-Orad A. MiR-pharmacogenetics of methotrexate in childhood B-cell acute lymphoblastic leukemia. Pharmacogenet. Genomics. 2016;26:517–525. doi: 10.1097/FPC.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 100.Yokoi T., Nakajima M. microRNAs as mediators of drug toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:377–400. doi: 10.1146/annurev-pharmtox-011112-140250. [DOI] [PubMed] [Google Scholar]
- 101.Lopez-Lopez E., Gutierrez-Camino A., Pinan M.A., Sanchez-Toledo J., Uriz J.J., Ballesteros J., Garcia-Miguel P., Navajas A., Garcia-Orad A. Pharmacogenetics of microRNAs and microRNAs biogenesis machinery in pediatric acute lymphoblastic leukemia. PLoS ONE. 2014;9:e91261. doi: 10.1371/journal.pone.0091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ward L.D., Kellis M. HaploReg: A resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–D934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paugh S.W., Bonten E.J., Savic D., Ramsey L.B., Thierfelder W.E., Gurung P., Malireddi R.K., Actis M., Mayasundari A., Min J., et al. NALP3 inflammasome upregulation and CASP1 cleavage of the glucocorticoid receptor cause glucocorticoid resistance in leukemia cells. Nat. Genet. 2015;47:607–614. doi: 10.1038/ng.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pui C.H., Schrappe M., Ribeiro R.C., Niemeyer C.M. Childhood and adolescent lymphoid and myeloid leukemia. Hematol. Am. Soc. Hematol. Educ. Program. 2004;2004:118–145. doi: 10.1182/asheducation-2004.1.118. [DOI] [PubMed] [Google Scholar]
- 105.Randolph T.R. Advances in acute lymphoblastic leukemia. Clin. Lab. Sci. 2004;17:235–245. [PubMed] [Google Scholar]
- 106.Ultimo S., Martelli A.M., Zauli G., Vitale M., Calin G.A., Neri L.M. Roles and clinical implications of microRNAs in acute lymphoblastic leukemia. J. Cell Physiol. 2018;233:5642–5654. doi: 10.1002/jcp.26290. [DOI] [PubMed] [Google Scholar]
- 107.Longjohn M.N., Squires W.R.B., Christian S.L. Meta-analysis of microRNA profiling data does not reveal a consensus signature for B cell acute lymphoblastic leukemia. Gene. 2022;821:146211. doi: 10.1016/j.gene.2022.146211. [DOI] [PubMed] [Google Scholar]
- 108.Zanette D.L., Rivadavia F., Molfetta G.A., Barbuzano F.G., Proto-Siqueira R., Silva W.A., Jr., Falcao R.P., Zago M.A. miRNA expression profiles in chronic lymphocytic and acute lymphocytic leukemia. Braz. J. Med. Biol. Res. 2007;40:1435–1440. doi: 10.1590/S0100-879X2007001100003. [DOI] [PubMed] [Google Scholar]
- 109.Schotte D., Chau J.C., Sylvester G., Liu G., Chen C., van der Velden V.H., Broekhuis M.J., Peters T.C., Pieters R., den Boer M.L., et al. Identification of new microRNA genes and aberrant microRNA profiles in childhood acute lymphoblastic leukemia. Leukemia. 2009;23:313–322. doi: 10.1038/leu.2008.286. [DOI] [PubMed] [Google Scholar]
- 110.Schotte D., de Menezes R.X., Akbari Moqadam F., Khankahdani L.M., Lange-Turenhout E., Chen C., Pieters R., Den Boer M.L. MicroRNA characterize genetic diversity and drug resistance in pediatric acute lymphoblastic leukemia. Haematologica. 2011;96:703–711. doi: 10.3324/haematol.2010.026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang H., Yang J.H., Zheng Y.S., Zhang P., Chen X., Wu J., Xu L., Luo X.Q., Ke Z.Y., Zhou H., et al. Genome-wide analysis of small RNA and novel MicroRNA discovery in human acute lymphoblastic leukemia based on extensive sequencing approach. PLoS ONE. 2009;4:e6849. doi: 10.1371/journal.pone.0006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ju X., Li D., Shi Q., Hou H., Sun N., Shen B. Differential microRNA expression in childhood B-cell precursor acute lymphoblastic leukemia. Pediatr. Hematol. Oncol. 2009;26:1–10. doi: 10.1080/08880010802378338. [DOI] [PubMed] [Google Scholar]
- 113.Fayed D., Donia T., El-Shanshory M., Ali E.M.M., Mohamed T.M. Evaluation of MicroRNA92, MicroRNA638 in Acute Lymphoblastic Leukemia of Egyptian Children. Asian Pac. J. Cancer Prev. 2021;22:1567–1572. doi: 10.31557/APJCP.2021.22.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Swellam M., Hashim M., Mahmoud M.S., Ramadan A., Hassan N.M. Aberrant Expression of Some Circulating miRNAs in Childhood Acute Lymphoblastic Leukemia. Biochem. Genet. 2018;56:283–294. doi: 10.1007/s10528-018-9844-y. [DOI] [PubMed] [Google Scholar]
- 115.Yan W., Song L., Wang H., Yang W., Hu L., Yang Y. Extracellular vesicles carrying miRNA-181b-5p affects the malignant progression of acute lymphoblastic leukemia. J. Transl. Med. 2021;19:511. doi: 10.1186/s12967-021-03174-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luna-Aguirre C.M., de la Luz Martinez-Fierro M., Mar-Aguilar F., Garza-Veloz I., Trevino-Alvarado V., Rojas-Martinez A., Jaime-Perez J.C., Malagon-Santiago G.I., Gutierrez-Aguirre C.H., Gonzalez-Llano O., et al. Circulating microRNA expression profile in B-cell acute lymphoblastic leukemia. Cancer Biomark. 2015;15:299–310. doi: 10.3233/CBM-150465. [DOI] [PubMed] [Google Scholar]
- 117.Shafik R.E., Abd El Wahab N., Mokhtar M.M., El Taweel M.A., Ebeid E. Expression of microRNA-181a and microRNA-196b in Egyptian Pediatric acute Lymphoblastic Leukemia. Asian Pac. J. Cancer. Prev. 2020;21:3429–3434. doi: 10.31557/APJCP.2020.21.11.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dawidowska M., Jaksik R., Drobna M., Szarzynska-Zawadzka B., Kosmalska M., Sedek L., Machowska L., Lalik A., Lejman M., Ussowicz M., et al. Comprehensive Investigation of miRNome Identifies Novel Candidate miRNA-mRNA Interactions Implicated in T-Cell Acute Lymphoblastic Leukemia. Neoplasia. 2019;21:294–310. doi: 10.1016/j.neo.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Swellam M., El-Khazragy N. Clinical impact of circulating microRNAs as blood-based marker in childhood acute lymphoblastic leukemia. Tumor Biol. 2016;37:10571–10576. doi: 10.1007/s13277-016-4948-7. [DOI] [PubMed] [Google Scholar]
- 120.Shafik R.E., Abd El Wahab N., Senoun S.A., Ebeid E., El Taweel M.A. Expression of Micro-RNA 128 and Let-7b in Pediatric Acute Lymphoblastic Leukemia Cases. Asian Pac. J. Cancer Prev. 2018;19:2263–2267. doi: 10.22034/APJCP.2018.19.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Oliveira J.C., Scrideli C.A., Brassesco M.S., Morales A.G., Pezuk J.A., Queiroz Rde P., Yunes J.A., Brandalise S.R., Tone L.G. Differential miRNA expression in childhood acute lymphoblastic leukemia and association with clinical and biological features. Leuk. Res. 2012;36:293–298. doi: 10.1016/j.leukres.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 122.Shahid S., Shahid W., Shaheen J., Akhtar M.W., Sadaf S. Circulating miR-146a expression as a non-invasive predictive biomarker for acute lymphoblastic leukemia. Sci. Rep. 2021;11:22783. doi: 10.1038/s41598-021-02257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hassan N.M., Refaat L.A., Ismail G.N., Abdellateif M., Fadel S.A., AbdelAziz R.S. Diagnostic, prognostic and predictive values of miR-100 and miR-210 in pediatric acute lymphoblastic Leukemia. Hematology. 2020;25:405–413. doi: 10.1080/16078454.2020.1843753. [DOI] [PubMed] [Google Scholar]
- 124.Mosakhani N., Missiry M.E., Vakkila E., Knuutila S., Vakkila J. Low Expression of miR-18a as a Characteristic of Pediatric Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2017;39:585–588. doi: 10.1097/MPH.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 125.Ghodousi E.S., Rahgozar S. MicroRNA-326 and microRNA-200c: Two novel biomarkers for diagnosis and prognosis of pediatric acute lymphoblastic leukemia. J. Cell Biochem. 2018;119:6024–6032. doi: 10.1002/jcb.26800. [DOI] [PubMed] [Google Scholar]
- 126.Almeida R.S., Costa E.S.M., Coutinho L.L., Garcia Gomes R., Pedrosa F., Massaro J.D., Donadi E.A., Lucena-Silva N. MicroRNA expression profiles discriminate childhood T- from B-acute lymphoblastic leukemia. Hematol. Oncol. 2019;37:103–112. doi: 10.1002/hon.2567. [DOI] [PubMed] [Google Scholar]
- 127.Fulci V., Colombo T., Chiaretti S., Messina M., Citarella F., Tavolaro S., Guarini A., Foa R., Macino G. Characterization of B- and T-lineage acute lymphoblastic leukemia by integrated analysis of MicroRNA and mRNA expression profiles. Genes Chromosomes Cancer. 2009;48:1069–1082. doi: 10.1002/gcc.20709. [DOI] [PubMed] [Google Scholar]
- 128.Gebarowska K., Mroczek A., Kowalczyk J.R., Lejman M. MicroRNA as a Prognostic and Diagnostic Marker in T-Cell Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2021;22:5317. doi: 10.3390/ijms22105317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nair R.A., Verma V.K., Beevi S.S., Rawoof A., Alexander L.E., Prasad E.R., Kumari P.K., Kumar P., Dinesh Kumar L. MicroRNA Signatures in Blood or Bone Marrow Distinguish Subtypes of Pediatric Acute Lymphoblastic Leukemia. Transl. Oncol. 2020;13:100800. doi: 10.1016/j.tranon.2020.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fan S.J., Li H.B., Cui G., Kong X.L., Sun L.L., Zhao Y.Q., Li Y.H., Zhou J. miRNA-149* promotes cell proliferation and suppresses apoptosis by mediating JunB in T-cell acute lymphoblastic leukemia. Leuk. Res. 2016;41:62–70. doi: 10.1016/j.leukres.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 131.Krzanowski J., Madzio J., Pastorczak A., Tracz A., Braun M., Tabarkiewicz J., Pluta A., Mlynarski W., Zawlik I. Selected miRNA levels are associated with IKZF1 microdeletions in pediatric acute lymphoblastic leukemia. Oncol. Lett. 2017;14:3853–3861. doi: 10.3892/ol.2017.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Malouf C., Antunes E.T.B., O’Dwyer M., Jakobczyk H., Sahm F., Landua S.L., Anderson R.A., Soufi A., Halsey C., Ottersbach K. miR-130b and miR-128a are essential lineage-specific codrivers of t(4;11) MLL-AF4 acute leukemia. Blood. 2021;138:2066–2092. doi: 10.1182/blood.2020006610. [DOI] [PubMed] [Google Scholar]
- 133.Mousavian Z., Nowzari-Dalini A., Stam R.W., Rahmatallah Y., Masoudi-Nejad A. Network-based expression analysis reveals key genes related to glucocorticoid resistance in infant acute lymphoblastic leukemia. Cell Oncol. 2017;40:33–45. doi: 10.1007/s13402-016-0303-7. [DOI] [PubMed] [Google Scholar]
- 134.Dou L., Li J., Zheng D., Li Y., Gao X., Xu C., Gao L., Wang L., Yu L. MicroRNA-142-3p inhibits cell proliferation in human acute lymphoblastic leukemia by targeting the MLL-AF4 oncogene. Mol. Biol. Rep. 2013;40:6811–6819. doi: 10.1007/s11033-013-2798-6. [DOI] [PubMed] [Google Scholar]
- 135.Vendramini E., Giordan M., Giarin E., Michielotto B., Fazio G., Cazzaniga G., Biondi A., Silvestri D., Valsecchi M.G., Muckenthaler M.U., et al. High expression of miR-125b-2 and SNORD116 noncoding RNA clusters characterize ERG-related B cell precursor acute lymphoblastic leukemia. Oncotarget. 2017;8:42398–42413. doi: 10.18632/oncotarget.16392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hirano D., Hayakawa F., Yasuda T., Tange N., Yamamoto H., Kojima Y., Morishita T., Imoto N., Tsuzuki S., Mano H., et al. Chromosomal translocation-mediated evasion from miRNA induces strong MEF2D fusion protein expression, causing inhibition of PAX5 transcriptional activity. Oncogene. 2019;38:2263–2274. doi: 10.1038/s41388-018-0573-9. [DOI] [PubMed] [Google Scholar]
- 137.Han B.W., Feng D.D., Li Z.G., Luo X.Q., Zhang H., Li X.J., Zhang X.J., Zheng L.L., Zeng C.W., Lin K.Y., et al. A set of miRNAs that involve in the pathways of drug resistance and leukemic stem-cell differentiation is associated with the risk of relapse and glucocorticoid response in childhood ALL. Hum. Mol. Genet. 2011;20:4903–4915. doi: 10.1093/hmg/ddr428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.El-Khazragy N., Noshi M.A., Abdel-Malak C., Zahran R.F., Swellam M. miRNA-155 and miRNA-181a as prognostic biomarkers for pediatric acute lymphoblastic leukemia. J. Cell. Biochem. 2019;120:6315–6321. doi: 10.1002/jcb.27918. [DOI] [PubMed] [Google Scholar]
- 139.Labib H.A., Elantouny N.G., Ibrahim N.F., Alnagar A.A. Upregulation of microRNA-21 is a poor prognostic marker in patients with childhood B cell acute lymphoblastic leukemia. Hematology. 2017;22:392–397. doi: 10.1080/10245332.2017.1292204. [DOI] [PubMed] [Google Scholar]
- 140.Piatopoulou D., Avgeris M., Marmarinos A., Xagorari M., Baka M., Doganis D., Kossiva L., Scorilas A., Gourgiotis D. miR-125b predicts childhood acute lymphoblastic leukaemia poor response to BFM chemotherapy treatment. Br. J. Cancer. 2017;117:801–812. doi: 10.1038/bjc.2017.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Piatopoulou D., Avgeris M., Drakaki I., Marmarinos A., Xagorari M., Baka M., Pourtsidis A., Kossiva L., Gourgiotis D., Scorilas A., et al. Clinical utility of miR-143/miR-182 levels in prognosis and risk stratification specificity of BFM-treated childhood acute lymphoblastic leukemia. Ann. Hematol. 2018;97:1169–1182. doi: 10.1007/s00277-018-3292-y. [DOI] [PubMed] [Google Scholar]
- 142.Ovcharenko D., Kelnar K., Johnson C., Leng N., Brown D. Genome-scale microRNA and small interfering RNA screens identify small RNA modulators of TRAIL-induced apoptosis pathway. Cancer Res. 2007;67:10782–10788. doi: 10.1158/0008-5472.CAN-07-1484. [DOI] [PubMed] [Google Scholar]
- 143.Agirre X., Jimenez-Velasco A., San Jose-Eneriz E., Garate L., Bandres E., Cordeu L., Aparicio O., Saez B., Navarro G., Vilas-Zornoza A., et al. Down-regulation of hsa-miR-10a in chronic myeloid leukemia CD34+ cells increases USF2-mediated cell growth. Mol. Cancer. Res. 2008;6:1830–1840. doi: 10.1158/1541-7786.MCR-08-0167. [DOI] [PubMed] [Google Scholar]
- 144.Hussenet T., Dali S., Exinger J., Monga B., Jost B., Dembele D., Martinet N., Thibault C., Huelsken J., Brambilla E., et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS ONE. 2010;5:e8960. doi: 10.1371/journal.pone.0008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stamatopoulos B., Meuleman N., Haibe-Kains B., Saussoy P., van den Neste E., Michaux L., Heimann P., Martiat P., Bron D., Lagneaux L., et al. microRNA-29c and microRNA-223 down-regulation has in vivo significance in chronic lymphocytic leukemia and improves disease risk stratification. Blood. 2009;113:5237–5245. doi: 10.1182/blood-2008-11-189407. [DOI] [PubMed] [Google Scholar]
- 146.Amankwah E.K., Devidas M., Teachey D.T., Rabin K.R., Brown P.A. Six Candidate miRNAs Associated with Early Relapse in Pediatric B-Cell Acute Lymphoblastic Leukemia. Anticancer Res. 2020;40:3147–3153. doi: 10.21873/anticanres.14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Avigad S., Verly I.R., Lebel A., Kordi O., Shichrur K., Ohali A., Hameiri-Grossman M., Kaspers G.J., Cloos J., Fronkova E., et al. miR expression profiling at diagnosis predicts relapse in pediatric precursor B-cell acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2016;55:328–339. doi: 10.1002/gcc.22334. [DOI] [PubMed] [Google Scholar]
- 148.Organista-Nava J., Gomez-Gomez Y., Illades-Aguiar B., del Carmen Alarcon-Romero L., Saavedra-Herrera M.V., Rivera-Ramirez A.B., Garzon-Barrientos V.H., Leyva-Vazquez M.A. High miR-24 expression is associated with risk of relapse and poor survival in acute leukemia. Oncol. Rep. 2015;33:1639–1649. doi: 10.3892/or.2015.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rzepiel A., Kutszegi N., Gezsi A., Sagi J.C., Egyed B., Peter G., Butz H., Nyiro G., Muller J., Kovacs G.T., et al. Circulating microRNAs as minimal residual disease biomarkers in childhood acute lymphoblastic leukemia. J. Transl. Med. 2019;17:372. doi: 10.1186/s12967-019-2114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Inaba H., Greaves M., Mullighan C.G. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943–1955. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang H., Luo X.Q., Zhang P., Huang L.B., Zheng Y.S., Wu J., Zhou H., Qu L.H., Xu L., Chen Y.Q., et al. MicroRNA patterns associated with clinical prognostic parameters and CNS relapse prediction in pediatric acute leukemia. PLoS ONE. 2009;4:e7826. doi: 10.1371/journal.pone.0007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liang Y.N., Tang Y.L., Ke Z.Y., Chen Y.Q., Luo X.Q., Zhang H., Huang L.B. MiR-124 contributes to glucocorticoid resistance in acute lymphoblastic leukemia by promoting proliferation, inhibiting apoptosis and targeting the glucocorticoid receptor. J. Steroid Biochem. Mol. Biol. 2017;172:62–68. doi: 10.1016/j.jsbmb.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 153.Lucafo M., Sicari D., Chicco A., Curci D., Bellazzo A., Di Silvestre A., Pegolo C., Autry R., Cecchin E., De Iudicibus S., et al. miR-331-3p is involved in glucocorticoid resistance reversion by rapamycin through suppression of the MAPK signaling pathway. Cancer Chemother. Pharmacol. 2020;86:361–374. doi: 10.1007/s00280-020-04122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen P., Shen T., Wang H., Ke Z., Liang Y., Ouyang J., Jiang T. MicroRNA-185-5p restores glucocorticoid sensitivity by suppressing the mammalian target of rapamycin complex (mTORC) signaling pathway to enhance glucocorticoid receptor autoregulation. Leuk. Lymphoma. 2017;58:2657–2667. doi: 10.1080/10428194.2017.1296143. [DOI] [PubMed] [Google Scholar]
- 155.Akbari Moqadam F., Lange-Turenhout E.A., Aries I.M., Pieters R., den Boer M.L. MiR-125b, miR-100 and miR-99a co-regulate vincristine resistance in childhood acute lymphoblastic leukemia. Leuk. Res. 2013;37:1315–1321. doi: 10.1016/j.leukres.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 156.El-Khazragy N., Elshimy A.A., Hassan S.S., Matbouly S., Safwat G., Zannoun M., Riad R.A. Dysregulation of miR-125b predicts poor response to therapy in pediatric acute lymphoblastic leukemia. J. Cell. Biochem. 2018;120:7428–7438. doi: 10.1002/jcb.28017. [DOI] [PubMed] [Google Scholar]
- 157.Mei Y., Gao C., Wang K., Cui L., Li W., Zhao X., Liu F., Wu M., Deng G., Ding W., et al. Effect of microRNA-210 on prognosis and response to chemotherapeutic drugs in pediatric acute lymphoblastic leukemia. Cancer Sci. 2014;105:463–472. doi: 10.1111/cas.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jiang Q., Lu X., Huang P., Gao C., Zhao X., Xing T., Li G., Bao S., Zheng H. Expression of miR-652-3p and Effect on Apoptosis and Drug Sensitivity in Pediatric Acute Lymphoblastic Leukemia. Biomed. Res. Int. 2018;2018:5724686. doi: 10.1155/2018/5724686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen H., Zhang D., Zhang G., Li X., Liang Y., Kasukurthi M.V., Li S., Borchert G.M., Huang J. A semantics-oriented computational approach to investigate microRNA regulation on glucocorticoid resistance in pediatric acute lymphoblastic leukemia. BMC Med. Inform. Decis. Mak. 2018;18:57. doi: 10.1186/s12911-018-0637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moses B.S., Evans R., Slone W.L., Piktel D., Martinez I., Craig M.D., Gibson L.F. Bone Marrow Microenvironment Niche Regulates miR-221/222 in Acute Lymphoblastic Leukemia. Mol. Cancer Res. 2016;14:909–919. doi: 10.1158/1541-7786.MCR-15-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cui C., Cui Q. The relationship of human tissue microRNAs with those from body fluids. Sci. Rep. 2020;10:5644. doi: 10.1038/s41598-020-62534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Su F., Gao Z., Liu Y., Zhou G., Cui Y., Deng C., Liu Y., Zhang Y., Ma X., Wang Y., et al. Integrated Tissue and Blood miRNA Expression Profiles Identify Novel Biomarkers for Accurate Non-Invasive Diagnosis of Breast Cancer: Preliminary Results and Future Clinical Implications. Genes. 2022;13:1931. doi: 10.3390/genes13111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sharifi Z., Talkhabi M., Taleahmad S. Identification of potential microRNA diagnostic panels and uncovering regulatory mechanisms in breast cancer pathogenesis. Sci. Rep. 2022;12:20135. doi: 10.1038/s41598-022-24347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu B., Li J., Cairns M.J. Identifying miRNAs, targets and functions. Brief. Bioinform. 2014;15:1–19. doi: 10.1093/bib/bbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bhatia P., Singh M., Singh A., Sharma P., Trehan A., Varma N. Epigenetic analysis reveals significant differential expression of miR-378C and miR-128-2-5p in a cohort of relapsed pediatric B-acute lymphoblastic leukemia cases. Int. J. Lab. Hematol. 2021;43:1016–1023. doi: 10.1111/ijlh.13477. [DOI] [PubMed] [Google Scholar]
- 166.Drobna M., Szarzynska B., Jaksik R., Sedek L., Kuchmiy A., Taghon T., van Vlierberghe P., Szczepanski T., Witt M., Dawidowska M., et al. hsa-miR-20b-5p and hsa-miR-363-3p Affect Expression of PTEN and BIM Tumor Suppressor Genes and Modulate Survival of T-ALL Cells In Vitro. Cells. 2020;9:1137. doi: 10.3390/cells9051137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qian L., Zhang W., Lei B., He A., Ye L., Li X., Dong X. MicroRNA-101 regulates T-cell acute lymphoblastic leukemia progression and chemotherapeutic sensitivity by targeting Notch1. Oncol. Rep. 2016;36:2511–2516. doi: 10.3892/or.2016.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Correia N.C., Melao A., Povoa V., Sarmento L., Gomez de Cedron M., Malumbres M., Enguita F.J., Barata J.T. microRNAs regulate TAL1 expression in T-cell acute lymphoblastic leukemia. Oncotarget. 2016;7:8268–8281. doi: 10.18632/oncotarget.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Popovic R., Riesbeck L.E., Velu C.S., Chaubey A., Zhang J., Achille N.J., Erfurth F.E., Eaton K., Lu J., Grimes H.L., et al. Regulation of mir-196b by MLL and its overexpression by MLL fusions contributes to immortalization. Blood. 2009;113:3314–3322. doi: 10.1182/blood-2008-04-154310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schotte D., Lange-Turenhout E.A., Stumpel D.J., Stam R.W., Buijs-Gladdines J.G., Meijerink J.P., Pieters R., Den Boer M.L. Expression of miR-196b is not exclusively MLL-driven but is especially linked to activation of HOXA genes in pediatric acute lymphoblastic leukemia. Haematologica. 2010;95:1675–1682. doi: 10.3324/haematol.2010.023481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huang Y., Li J., Chen Y., Jiang P., Wang L., Hu J. Identification of Early Recurrence Factors in Childhood and Adolescent B-Cell Acute Lymphoblastic Leukemia Based on Integrated Bioinformatics Analysis. Front. Oncol. 2020;10:565455. doi: 10.3389/fonc.2020.565455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lin S., Gregory R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cimmino A., Calin G.A., Fabbri M., Iorio M.V., Ferracin M., Shimizu M., Wojcik S.E., Aqeilan R.I., Zupo S., Dono M., et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jackstadt R., Hermeking H. MicroRNAs as regulators and mediators of c-MYC function. Biochim. Biophys. Acta. 2015;1849:544–553. doi: 10.1016/j.bbagrm.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 175.Simonson B., Das S. MicroRNA Therapeutics: The Next Magic Bullet? Mini Rev. Med. Chem. 2015;15:467–474. doi: 10.2174/1389557515666150324123208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang Y., Liang H. When MicroRNAs Meet RNA Editing in Cancer: A Nucleotide Change Can Make a Difference. Bioessays. 2018;40:1700188. doi: 10.1002/bies.201700188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moraes F.C., Pichon C., Letourneur D., Chaubet F. miRNA Delivery by Nanosystems: State of the Art and Perspectives. Pharmaceutics. 2021;13:1901. doi: 10.3390/pharmaceutics13111901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Correia de Sousa M., Gjorgjieva M., Dolicka D., Sobolewski C., Foti M. Deciphering miRNAs’ Action through miRNA Editing. Int. J. Mol. Sci. 2019;20:6249. doi: 10.3390/ijms20246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.