Abstract
Background
The COVID-19 pandemic has highlighted health disparities affecting ethnic minority communities. There is growing concern about the lack of diversity in clinical trials. This study aimed to assess the representation of ethnic groups in UK-based COVID-19 randomised controlled trials (RCTs).
Methods
A systematic review and meta-analysis were undertaken. A search strategy was developed for MEDLINE (Ovid) and Google Scholar (1st January 2020–4th May 2022). Prospective COVID-19 RCTs for vaccines or therapeutics that reported UK data separately with a minimum of 50 participants were eligible. Search results were independently screened, and data extracted into proforma. Percentage of ethnic groups at all trial stages was mapped against Office of National Statistics (ONS) statistics. Post hoc DerSimonian-Laird random-effects meta-analysis of percentages and a meta-regression assessing recruitment over time were conducted. Due to the nature of the review question, risk of bias was not assessed. Data analysis was conducted in Stata v17.0. A protocol was registered (PROSPERO CRD42021244185).
Results
In total, 5319 articles were identified; 30 studies were included, with 118,912 participants. Enrolment to trials was the only stage consistently reported (17 trials). Meta-analysis showed significant heterogeneity across studies, in relation to census-expected proportions at study enrolment. All ethnic groups, apart from Other (1.7% [95% CI 1.1–2.8%] vs ONS 1%) were represented to a lesser extent than ONS statistics, most marked in Black (1% [0.6–1.5%] vs 3.3%) and Asian (5.8% [4.4–7.6%] vs 7.5%) groups, but also apparent in White (84.8% [81.6–87.5%] vs 86%) and Mixed 1.6% [1.2–2.1%] vs 2.2%) groups. Meta-regression showed recruitment of Black participants increased over time (p = 0.009).
Conclusions
Asian, Black and Mixed ethnic groups are under-represented or incorrectly classified in UK COVID-19 RCTs. Reporting by ethnicity lacks consistency and transparency. Under-representation in clinical trials occurs at multiple levels and requires complex solutions, which should be considered throughout trial conduct. These findings may not apply outside of the UK setting.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-023-02809-7.
Keywords: Ethnicity, COVID-19, Medical research, Clinical trials
Background
Since the emergence of the Coronavirus disease (COVID-19) pandemic in January 2020 in the United Kingdom (UK), longstanding health disparities affecting ethnic minority communities have come to light [1]. Emerging evidence has shown that ethnic minorities have had the highest rate of diagnosis [2], severe disease requiring advanced respiratory support [3] and mortality [4–6]. Several reasons for the observed differences have been proposed including higher rates of social deprivation; higher rates of pre-existing health conditions (for example type 2 diabetes and cardiovascular disease); greater frequency of living in large or multi-generational households; and poorer access to health services [7–11]. To combat severe acute respiratory distress syndrome 2 (SARS-CoV-2) and limit its transmission and complications, many randomised controlled trials (RCTs) have been conducted globally to determine effective treatments and develop vaccines which have been subsequently rolled out at a population level. Landmark trials have provided compelling evidence for several vaccines such as BNT162b2 messenger ribonucleic acid (mRNA) [12], ChAdOx1 nCoV-19 (AZD1222) [13] and mRNA-1273 [14], and therapies including dexamethasone [15] and sotrovimab [16].
A well-designed and conducted RCT is considered the gold standard (Level I) to evaluate the causal effect of medical interventions. Like other study types, RCTs also depend upon participation of all groups to improve generalisability and validity of the findings. There is growing concern about the lack of diversity in trials across health and clinical research over the last few years [17–20]. This may stem from anxieties around the implications of participation within ethnic minority communities, added costs of participation (such as travel and parking), language barriers, knowledge gaps and lack of diversity within the research team [21–24].
Given the disproportionate impact of COVID-19 on minority individuals, inclusion of ethnic minority populations in COVID-19 trials is vital to understanding differences of interventions in disease severity and outcomes as well as addressing critical gaps in knowledge. Therefore, this systematic review aimed to assess the representation of different ethnic groups in UK-based COVID-19 vaccine and therapeutic trials and compared them to nationally available data on ethnic minority populations in the UK.
Methods
This systematic review and meta-analysis was conducted and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [25] (Supplementary Materials, Appendix 3 and 4). A protocol was registered in advance with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42021244185). Ethical approval was not required.
Search strategy and selection criteria
A search strategy was developed to identify COVID-19 RCTs that were published in MEDLINE (Ovid) and Google Scholar (Supplementary Materials, Appendix 1). This deviated from the search strategy described in the protocol but was considered comprehensive following discussion with a subject librarian and the review team. We included articles available in English, published between 1st January 2020 and 4th May 2022 in peer-reviewed journals. RCTs were included if they (1) explored COVID-19 vaccine or therapies (medical devices and treatments), (2) were conducted in the UK or reported data separately for the UK population and (3) had a minimum sample size of 50 adults (Table 1). The minimum sample size of 50 patients was a pragmatic decision taken by the authors. We restricted to UK-based studies due to the diversity of the population and good quality ethnicity data at the population level.
Table 1.
A summary of the inclusion criteria for the meta-analysis and meta-regression
| PICOS criteria | Inclusion criteria |
|---|---|
| Population | Adults ≥ 18 years |
| Intervention | COVID-19 vaccine or therapeutic treatment |
| Comparator | Any or none |
| Outcomes | Any |
| Study design | Randomised controlled trial (any phase) with a minimum sample size of 50 and conducted in UK or reporting UK data |
| Time period | Published in peer-reviewed journal between 1st January 2020 and 4th May 2022 |
Our main outcome was percentage of each ethnic group at different trial stages, for the following: people approached for inclusion; people screened for inclusion; people determined eligible for inclusion; people determined ineligible for inclusion; people enrolled in the trial; people followed up at primary endpoint; people followed up at longest follow-up. However, on review of the data, it became apparent that we could only assess the percentage of ethnic groups enrolled in the included trials due to lack of data availability, though we could report on the number of trials that reported proportions of ethnic groups at each trial stage. We also investigated the number of trials reporting effect estimates by ethnicity at each trial stage as a secondary outcome.
Search results were saved to Rayyan (Qatar Computing Research Institute), a systematic review web-based application. Abstracts were independently screened for inclusion by four review authors (M.M, L.G, H.J and D.G). An online discussion was held between the authors to compare results and adjudicate any discrepancies. Where discrepancies could not be resolved by discussion, they were referred to a second review author. Following exclusion of studies which did not meet the inclusion criteria and duplicates, full-text screening was carried out independently and in duplicate (by M.M, L.G, H.J and D.G) and data was extracted into piloted proforma. Due to the nature of the review questions, we did not assess risk of bias.
Data analysis
Data were collected on participant demographics (age and sex), type of intervention (vaccine or treatment), total number of participants and general study characteristics. To assess our main outcome, we extracted additional data on the reporting of ethnic diversity of participants at each stage of the trial. We also documented the approach to recruitment, and whether efforts were made to recruit from ethnic minority communities. We documented the enrolment period for each trial to investigate whether recruitment of ethnic minorities changed over time. All studies which reported any ethnicity data were included in the final analysis.
The percentage of each ethnic group within each trial was calculated as a proportion and mapped against national population statistics using Office of National Statistics (ONS) 2011 data for each outcome.
We initially anticipated that heterogeneity in reporting would preclude statistical synthesis and had planned to only calculate percentage of each ethnic group in each trial and map this against national population statistics using forest plots, as reported in our protocol. However, after data extraction, we found more similarities and detailed reporting than we had anticipated, and hence conducted the following post hoc statistical analyses. A DerSimonian-Laird random-effects meta-analysis and a meta-regression to assess changes in recruitment over time were conducted in Stata v 17.0, following a logit transformation of study-specific nonzero proportions and confidence intervals (obtained with Wilson’s method). The meta-regression was conducted to reflect the change in recruitment practices that may have occurred during the pandemic. A p < 0.05 was deemed indicative of statistical significance.
Role of the funding source
This study was funded by the South Asian Health Foundation. In addition, it was supported by members (K.K, F.Z) of the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC-EM).
Results
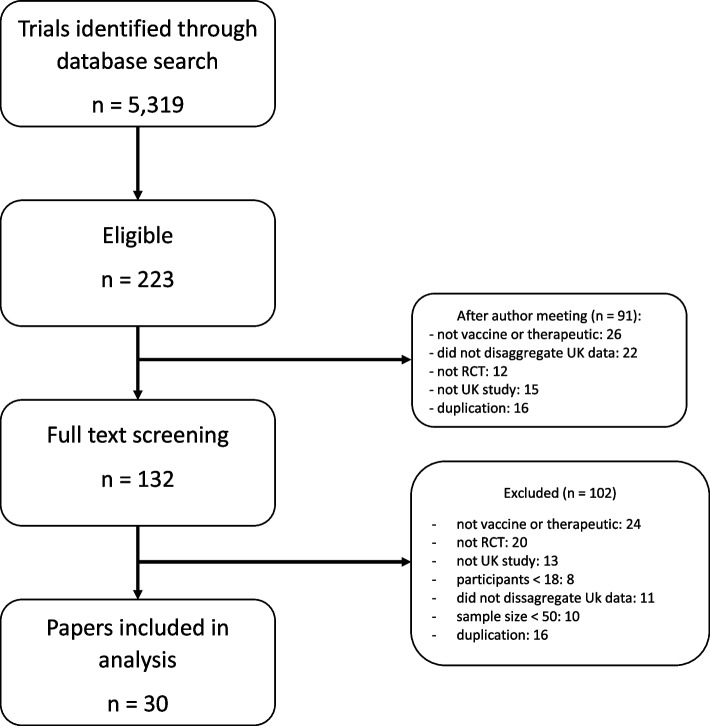
A total of 5319 studies were identified through the database search, and 5096 studies were excluded during the abstract screening phase. After removal of duplicates and those that did not meet the inclusion criteria, we reviewed 132 trials for full-text screening, after which a further 102 trials were excluded (Fig. 1) and a total of 30 were included (see Table 2 for a list of identified articles and their characteristics). The most common reasons for exclusion at full text stage were because data were not reported by country, meaning UK data could not be extracted, or the included paper did not assess a COVID-19 vaccine or therapeutic. Of the included studies, 21 were for therapeutic trials and 9 were COVID-19 vaccine trials. One study that met the inclusion criteria did not include any data on ethnicity.
Fig. 1.
Study selection
Table 2.
A total of 30 studies were included in the meta-analysis and meta-regression following a MEDLINE (Ovid) and Google Scholar search
| No | Title | Authors | Year | Intervention | Total participants | Mean age | % male | Vaccine/ Therapeutic | Enrolment period |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Safety and efficacy of NVX-CoV2373 Covid-19 vaccine | Heath PT et al | 2021 | NVX-CoV2373 | 14,039 | 56 | 51.56% | Vaccine | Sep 2020—Nov 2020 |
| 2 | Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial | Yu LM et al | 2021 | Budesonide | 3006 | 64 | 46.57% | Therapeutic | Nov 2020–Mar 2021 |
| 3 | Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK: a randomised, controlled, open-label, adaptive platform trial | Butler CC et al | 2021 | Doxycycline | 1792 | 61 | 44.16% | Therapeutic | Jul 2020–Dec 2020 |
| 4 | Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial | Butler CC et al | 2021 | Azithromycin | 1388 | 60.7 | 43.22% | Therapeutic | Jul–Nov 2020 |
| 5 | Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial | Monk PD et al | 2020 | Interferon beta-1 | 101 | 57.1 | 59.18% | Therapeutic | Mar–May 2020 |
| 6 | Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK | Voysey M et al | 2021 | ChAdOx1 nCoV-19 (AZD1222) | 7548 | 38.69% | Vaccine | Apr–Nov 2020 | |
| 7 | Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202,012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial | Emary KRW et al | 2021 | ChAdOx1 nCoV-19 (AZD1222) | 8534 | 40.65% | Vaccine | May–Nov 2020 | |
| 8 | Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial | Horby PW et al | 2020 | Lopinavir-ritonavir | 5040 | 66.3 | 61.05% | Therapeutic | Mar–Jun 2020 |
| 9 | Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial | Ramakrishnan S et al | 2021 | Budesonide | 139 | 45 | 42.45% | Therapeutic | Jul–Dec 2020 |
| 10 | Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial | RECOVERY collaborative | 2021 | Convalescent plasma | 11,558 | 63.5 | 64.28% | Therapeutic | May 2020–Jan 2021 |
| 11 | Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial | RECOVERY collaborative | 2021 | Tocilizumab | 4116 | 63.6 | 67.40% | Therapeutic | Apr 2020–Jan 2021 |
| 12 | Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2 | Painter WP et al | 2021 | Molnupiravir | 130 | 38.7 | 83.85% | Therapeutic | |
| 13 | Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial | RECOVERY collaborative | 2021 | Azithromycin | 7763 | 65.2 | 62.08% | Therapeutic | Apr–Nov 2020 |
| 14 | Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials | Voysey M et al | 2021 | ChAdOx1 nCoV-19 (AZD1222) | 8948 | 40.87% | Vaccine | Apr–Dec 2020 | |
| 15 | Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial | Ramasamy MN et al | 2020 | ChAdOx1 nCoV-19 (COV002) | 552 | 60.5 | 50.18% | Vaccine | May–Aug 2020 |
| 16 | Safety and immunogenicity of the ChadOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial | Folegatti PM et al | 2020 | ChadOx1 nCoV-19 | 1077 | 35 | 50.23% | Vaccine | Apr–May 2020 |
| 17 | Dexamethasone in Hospitalized Patients with Covid-19 | RECOVERY collaborative | 2021 | Dexamethasone | 6425 | 66.1 | 63.37% | Therapeutic | Mar–June 2020 |
| 18 | Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial | RECOVERY collaborative | 2020 | Hydroxychloro- quine | 4716 | 65.4 | 62.21% | Therapeutic | Mar–June 2020 |
| 19 | Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxaemic respiratory failure and COVID-19: The RECOVERY-RS randomized clinical trial | Perkins G et al | 2022 | Non-invasive ventilatory strategies | 1273 | 56.7 | 66.30% | Therapeutic | Apr 2020–May 2021 |
| 20 | An online breathing and wellbeing programme (ENO Breathe) for people with persistent symptoms following COVID-19: a parallel group, single blind, randomised controlled trial | Philip KEJ et al | 2022 | Online breathing and wellbeing programme | 150 | 49.5 | 17.69% | Therapeutic | Apr–May 2021 |
| 21 | Colchicine for Covid-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial | Dorward J et al | 2022 | Colchicine | 1381 | 61 | Therapeutic | Mar–May 2021 | |
| 22 | Inspiratory muscle training enhances recovery post COVID-19: a randomised clinical trial | McNarry MA et al | 2022 | Inspiratory muscles | 148 | 46 | 11.49% | Therapeutic | |
| 23 | Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled open-label, platform trial | RECOVERY collaborative | 2022 | Casirivimab and imdevimab | 9785 | 62.63% | Therapeutic | Sep 2020–May 2021 | |
| 24 | Namilumab or inflixmab compared with standard of care in hospitalised patients with COVID-19 (CATALYST): a randomised, multicentre, multi-arm, multistage, open-label, adaptive, phase 2, proof of concept trial | Fisher BA et al | 2022 | Namilumab or inflixmab | 146 | 58.4 | 61.64% | Therapeutic | Jun 2020–Feb 2021 |
| 25 | Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector and protein-adjuvant vaccines in the UK (Com-COV2): a single blind, randomised, phase 2, non-inferiority trial | Stuart ASV et al | 2022 | mRNA, viral-vector and protein-adjuvant vaccines | 532 | 62 | 39.47% | Vaccine | Apr–May 2021 |
| 26 | Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial | Munro APS et al | 2021 | ChAdOx1 nCov-19 or BNT162b2 | 2883 | 49.84% | Vaccine | Jun 2021 | |
| 27 | Safety and immunogenicity of heterologous versus homologous prime-boost schedules with adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial | Liu X et al | 2021 | heterologous v homologous prime-boost with adenoviral vectored and mRNA COVID-19 vaccine | 463 | 57.8 | 54.21% | Vaccine | Feb 2021 |
| 28 | Azithromycin versus standard care in patients with mild-to-moderate COVID-19 (ATOMIC2): an open-label, randomised trial | Hinks TSC et al | 2021 | Azithromycin | 295 | 45.9 | 51.53% | Therapeutic | Jun 2020–Jan 2021 |
| 29 | Favipiravir, lopinavir-ritonavir or combination therapy (FLARE): a randomised, double blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19 | Lowe DM et al | 2022 | Favipiravir, lopinavir-ritonavir or combination therapy | 240 | 40 | 51.25% | Therapeutic | Oct 2020–Nov 2021 |
| 30 | Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial | RECOVERY collaborative | 2022 | Aspirin | 14,892 | 59.2 | 61.78% | Therapeutic | Nov 2020–Mar 2021 |
The total number of patients included in the meta-analysis was 118,912. The mean age of participants was 61.1 (range 35–66.3) years, and 55.03% (range 11.49–83.85%) were male. The trials enrolled patients between March 2020 and November 2021.
Reporting of ethnicity through different stages of the trial was limited and inconsistent (Table 3). Of the 30 trials in the review, none reported data on those approached for inclusion by ethnicity. No trials reported data on those screened for inclusion by individual ethnicity, though seven trials, all from the RECOVERY (Randomised Evaluation of COVID-19 Therapy) group (https://www.recoverytrial.net/), reported these data as “White”, “BAME” (Black, Asian and minority ethnic) or “Unknown”, which was not further disaggregated. The percentage of “White” participants was under-represented in all 7 trials compared with ONS population statistics at this stage, with approximately a quarter of participants documented as “BAME” or “Unknown” in all trials (Table 4). Similarly, no trials reported data on those either deemed eligible for inclusion or deemed ineligible for inclusion by individual ethnicity, though the same seven trials from the RECOVERY group reported these data as “White”, “BAME” or “Unknown”. The proportion of those deemed eligible was similar to that at the screening stage, while the proportion of those deemed ineligible compared to the screening stage was more varied, with no clear pattern indicating whether “White” or “BAME” participants were more likely to be ineligible.
Table 3.
A summary of the reporting by ethnicity through different stages of COVID-19 clinical trials, including those approached, screened and deemed eligible or ineligible for inclusion; those enrolled in the trial; those followed up at the primary end point and longest follow-up; and reporting of effect estimates
| Stage of trial | No. of trials reporting by ethnicity (no. participants) | References | No. of trials reporting as “White”, “BAME”, “non-white”, “Other” (no. participants) | References |
|---|---|---|---|---|
| Approached for inclusion | 0 | 0 | ||
| Screened for inclusion | 0 | 7 (60,179) | [8, 10, 13, 17, 18, 23, 26] | |
| Eligible for inclusion | 0 | 7 (60,179) | [8, 10, 13, 17, 18, 23, 26] | |
| Ineligible for inclusion | 0 | 7 (60,179) | [8, 10, 13, 17, 18, 23, 26] | |
| Enrolled in trial | 17 (52,747) | [1–4, 6, 7, 12, 14–16, 19, 20, 24, 25, 27–29] | 11 (64,722) | [5, 8–11, 13, 17, 18, 23, 26, 30] |
| Followed up at primary endpoint | 0 | 9 (64,434) | [8–11, 13, 17, 18, 23, 26] | |
| Followed up at longest follow-up | 0 | 0 | ||
| Effect estimates | 0 | 11 (79,740) | [1, 8–11, 13, 17–19, 23, 26] |
Table 4.
A summary of the RECOVERY group’s reporting of ethnicity at different trial stages, including those approached for inclusion; those deemed eligible or ineligible for inclusion; and those followed up at the primary endpoint. The RECOVERY trials reported ethnicity as “White”, “BAME” or “non-white”, and did not disaggregate this data further
| Trial no | No. of people approached for inclusion reported by ethnicity | No. of people deemed eligible for inclusion reported by ethnicity | No. of people deemed ineligible for inclusion reported by ethnicity | No. of people followed up at primary endpoint reported by ethnicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White (%) | BAME (%) | Unknown (%) | White (%) | BAME (%) | Unknown (%) | White (%) | BAME (%) | Unknown (%) | White (%) | BAME (%) | Unknown (%) | ||
| 8 | 6060 (75) | 1351 (17) | 692 (9) | 3781 (75) | 865 (17) | 394 (8) | 2279 (74) | 486 (16) | 298 (10) | 3781 | 865 | ||
| 10 | 10,810 (75) | 2271 (16) | 1241 (9) | 8914 (77) | 1720 (15) | 924 (8) | 1896 (69) | 551 (20) | 317 (11) | 8914 | 1720 | ||
| 11 | 3127 (76) | 732 (18) | 257 (6) | ||||||||||
| 13 | 9301 (75) | 1852 (15) | 1180 (10) | 5939 (77) | 1109 (14) | 715 (9) | 3362 (74) | 743 (16) | 465 (10) | 5939 | 1109 | ||
| 17 | 6015 (74) | 1420 (17) | 697 (9) | 4689 (73) | 1147 (18) | 589 (9) | 1326 (78) | 273 (16) | 108 (6) | 4689 (73) | 1147 (18) | 589 (9) | |
| 18 | 5860 (73) | 1365 (18) | 690 (9) | 3479 (74) | 857 (18) | 380 (8) | 2381 (74) | 508 (16) | 310 (10) | 3479 | 857 | ||
| 23 | 10,039 (77) | 1809 (14) | 1184 (9) | 7601 (78) | 1293 (13) | 891 (9) | 2438 (75) | 516 (16) | 293 (9) | 7601 | 1293 | ||
| 30 | 16,189 (76) | 3260 (15) | 1900 (9) | 11,129 (75) | 2378 (16) | 1385 (9) | 5690 (80) | 882 (12) | 515 (7) | 11,129 | 2378 | ||
Seventeen trials reported the number of people enrolled in the trial by individual ethnicity, though one of these only reported participants as “White”, “Black” or “Other”. A further eleven grouped enrolled participants into “White” vs “BAME”, “White” vs “non-white” or “White” vs “Other” (in this case meaning all non-White participants). The percentage of those enrolled in these studies is discussed in detail in the meta-analysis. In the seven RECOVERY trials for which there is complete data, there were no loss of participants from those deemed eligible for inclusion and subsequently enrolled in the study.
None of the trials reported those followed up at the primary endpoint by individual ethnicity, though eight trials, all from the RECOVERY group, reported this data as “White”, “BAME” or “Unknown”, with none reporting loss to follow-up from those enrolled in the trial. One further trial reported the primary outcome for White participants only. No trials reported by ethnicity at the longest follow-up, though all the RECOVERY trials made reference to “further analyses specified at 6 months”. None of the trials reported effect estimates by ethnicity, though eleven trials report this data for “White”, “BAME” or “Other” groups, and do not disaggregate this further.
Three studies documented specific strategies to improve recruitment of ethnic minority groups, and three studies mentioned recruiting from ethnic minority communities, though no details were provided (Table 5). None of these trials recruited a higher proportion of participants from ethnic minority communities compared to those not reporting recruitment strategies.
Table 5.
A summary of the strategies used to improve recruitment of ethnic minority groups in COVID-19 trials
| Trial no | Strategies to improve recruitment |
|---|---|
| 2. Yu LM er al | “To increase recruitment from ethnic minority and socially deprived communities, which have been disproportionately affected by COVID-19, we used several outreach strategies, including the appointment in September, 2020, of an expert working with ethnic minorities; active collaboration with community, religious and health organisations; and promotion in multiple languages through a range of media.” |
| 4. Butler CC et al | “Given the increased risk from COVID-19 among Black, Asian, and minority ethnic communities, we actively reached out to a range of religious and community organisations at national and regional levels to increase participation from diverse backgrounds.” |
| 19. Perkins G et al.* | “Collection and reporting of race and ethnicity based on fixed categories and mandated by funder due to disproportionate effect of COVID-19 infection on non-white population.” – no specific recruitment strategy described |
| 21. Doward J et al | “Several community outreach strategies were implemented aiming to increase recruitment of those from ethnically diverse communities and socioeconomically deprived backgrounds, who have been disproportionally affected by COVID-19.” |
| 26. Munro APS et al.* | “Recruitment of those identifying as black or minority ethnic was particularly encouraged.”—no specific recruitment strategy described |
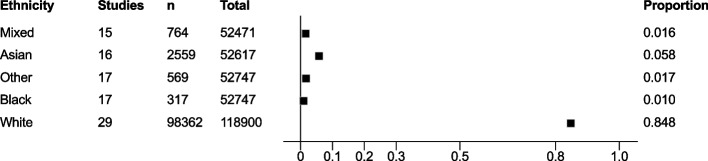
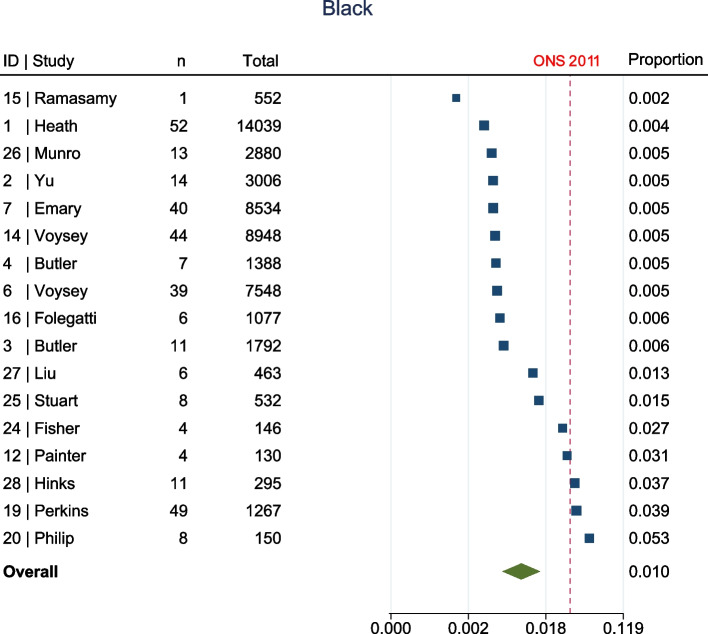
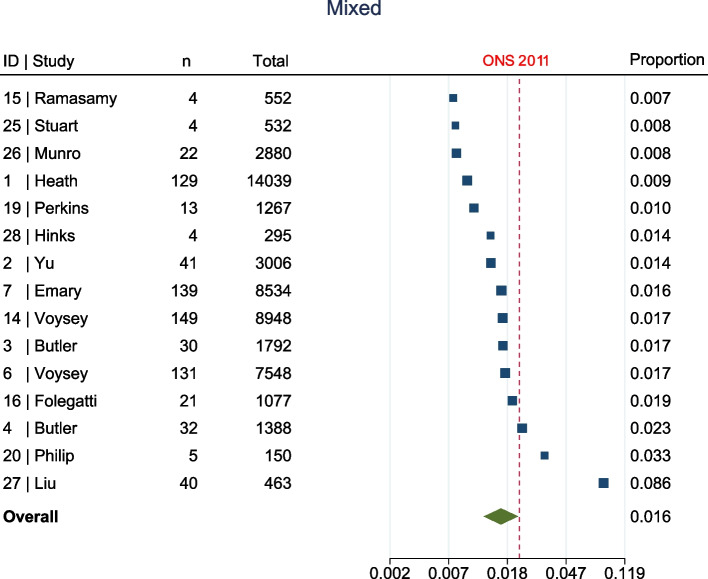
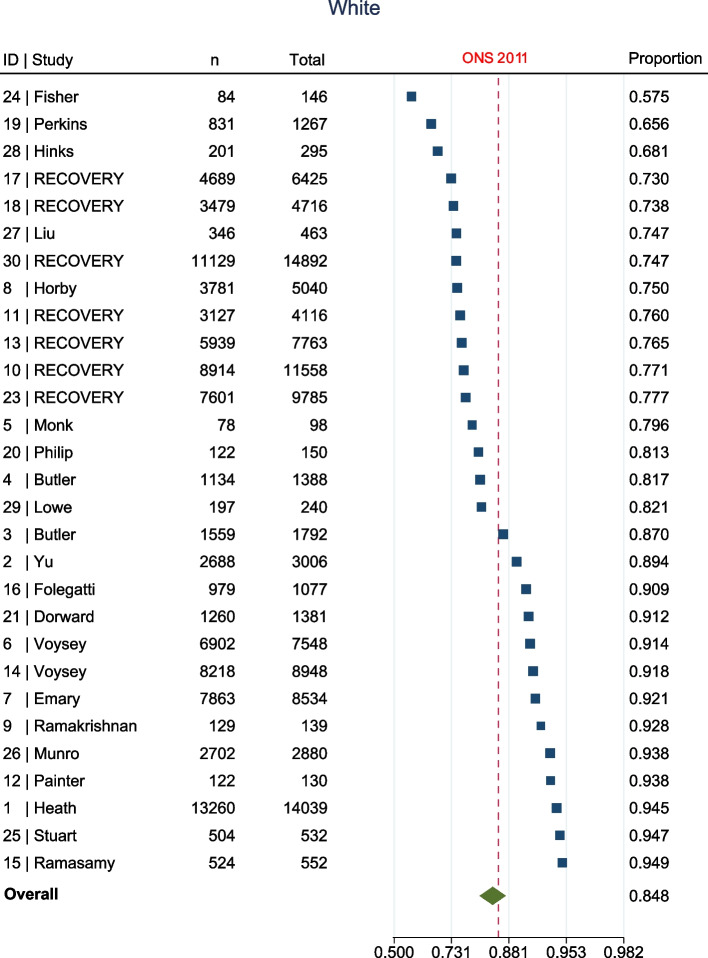
The meta-analysis, summarised in Fig. 2, shows that at enrolment to the trial, all ethnic groups, apart from Other (1.7% [95% confidence interval (CI) 1.1–2.8%] vs ONS 1%, Fig. 3), were represented to a lesser extent than that suggested by 2011 ONS statistics. This was most marked in the Asian (5.8% [95% CI 4.4–7.6%] vs ONS 7.5%, Fig. 4) and Black (1% [95% CI 0.6–1.5%] vs ONS 3.3%, Fig. 5) groups, though also apparent in the Mixed (1.6% [95% CI1.2–2.1%] vs ONS 2.2%, Fig. 6) and White (84.8% [95% CI 81.6–87.5%] vs ONS 86%, Fig. 7) ethnic groups. The meta-analysis shows significant variation across studies, in relation to census-expected proportions of patients recruited from different ethnic groups.
Fig. 2.
Summary of meta-analysis of enrolment to trials
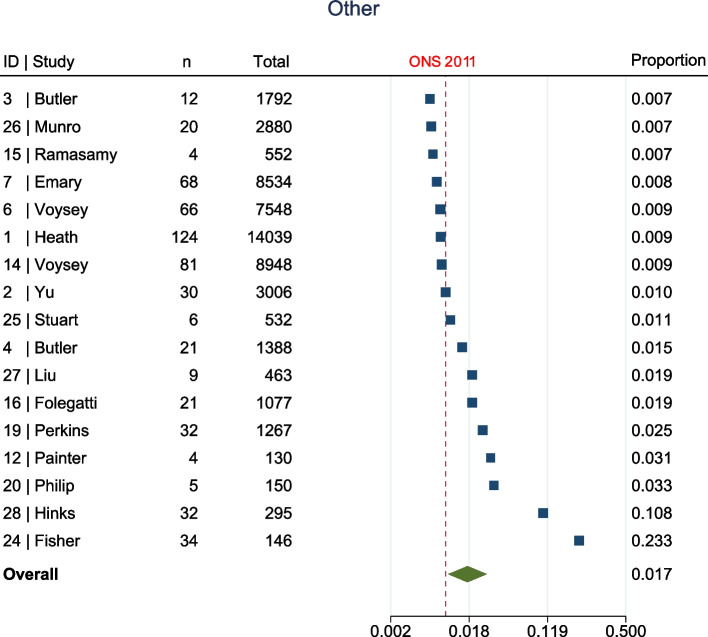
Fig. 3.
Seventeen trials documented enrolment of Other participants. Overall effect shows Other participants were over-represented when compared to ONS statistics (1.7% [95% CI 1.1–2.8%] vs ONS 1%)
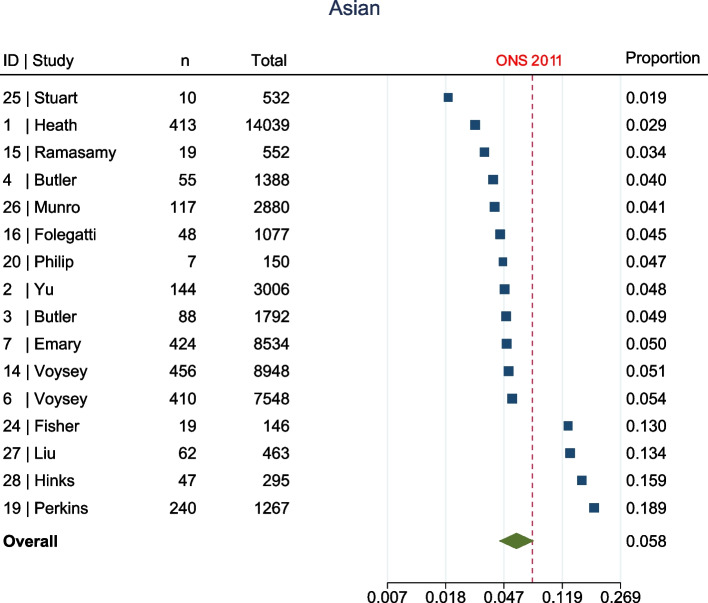
Fig. 4.
Sixteen trials documented enrolment of Asian participants. Overall effect shows Asian participants were under-represented when compared to ONS statistics (5.8% [95% CI 4.4–7.6%] vs ONS 7.5%)
Fig. 5.
Seventeen trials documented enrolment of Black participants. Overall effect shows Black participants were under-represented when compared to ONS statistics (1% [95% CI 0.6–1.5%] vs ONS 3.3%)
Fig. 6.
Fifteen trials documented enrolment of Mixed participants. Overall effect shows Mixed participants were under-represented when compared to ONS statistics (1.6% [95% CI1.2–2.1%] vs ONS 2.2%)
Fig. 7.
Twenty-nine trials documented enrolment of White participants. Overall effect shows White participants were under-represented when compared to ONS statistics (84.8% [95% CI 81.6–87.5%] vs ONS 86%)
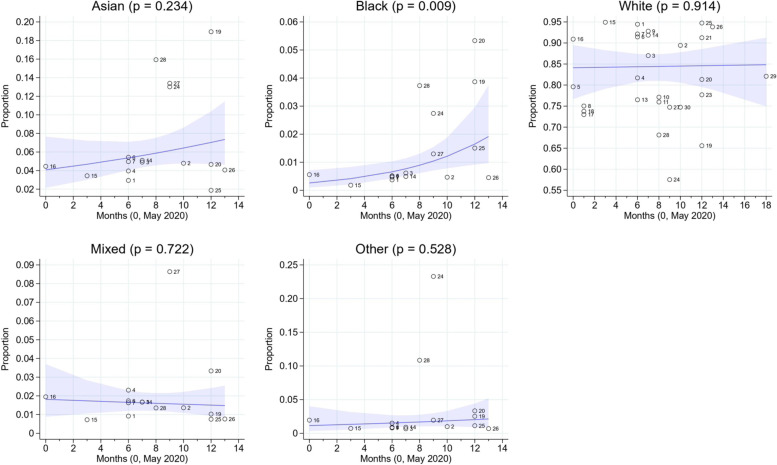
Figure 8 illustrates the results of the meta-regression, which shows that recruitment in the Black ethnic group improved from May 2020 to June 2021 (from an estimated 0.26 to 1.92%, p = 0.009). There were no statistically significant temporal trends in the other groups (Asian (p = 0.234), White (p = 0.914), Other (p = 0.528) and Mixed (p = 0.722).
Fig. 8.
Results of the meta-regression. Recruitment in the Black ethnic group improved from May 2020 to June 2021 (from an estimated 0.26% to 1.92%, p = 0.009). There were no statistically significant temporal trends in the other groups
Discussion
We conducted an extensive review of literature to determine any disparities in the representation of ethnic minority groups in UK COVID-19 clinical trials. Our meta-analysis findings demonstrate that in 30 trials of over 100,000 participants, the Asian, Black, Mixed and White ethnic groups were represented to a lesser extent than that suggested by 2011 ONS statistics. There is significant heterogeneity in the proportions of participants recruited from different ethnic groups across studies, though the Asian and Black groups demonstrate the greatest proportion of studies below the percentages demonstrated by the 2011 census. These results might indicate one of two things: first, that Asian and Black groups were enrolled at lower percentages than population averages in more studies than White or Other groups, though whether this interpretation withstands scrutiny is unclear, as the White ethnic group were also not over-represented in the majority of the data. This may lead us to a second conclusion: that Asian, Black and Mixed ethnicities were more likely to be classified as “Other”, grouped into problematic “non-White” or “BAME” categories, or not recorded at all (for example, as “Unknown”).
If the first interpretation is correct, it suggests that ethnic minority groups are more likely to be under-represented in COVID-19 trials. This continues a trend of poor recruitment from European trials when compared to North American trials, though neither have shown a temporal improvement in representation of ethnic minority participants [27]. There is also a large body of evidence which has identified previous racial and ethnic enrolment disparities in other types of medical research including trials on cancer, diabetes [20, 28] and cardiovascular disease [29] over the last decade. This may be particularly concerning as the hospital population during the pandemic did not reflect ONS population statistics, with a higher proportion of inpatients from ethnic minority communities [30], in theory providing a larger pool for research teams to recruit from. This raises the important consideration of what population triallists should aspire to map to. Should trial recruitment aim to be representative of the general population, or of the population to whom the interventions are most relevant? If the latter, we might expect vaccine trials, which are designed for whole populations, to map to ONS data, but to use a different reference point for treatments for severe disease which affect a greater proportion of ethnic minority groups, such as tocilizumab for severe COVID-19 infection.
If the second interpretation is true, it raises questions about data accuracy and reporting. There is a distinct lack of consistency in the reporting of results by ethnicity, with many studies continuing to use the term “BAME”, which is no longer favoured due to its emphasis on certain ethnic groups, to the exclusion of others [26]. Harmonisation of data is made difficult by differences in ethnicity coding internationally [31], and there have been calls for more detailed and consistent ethnicity coding [32]. Moreover, one could legitimately question the utility of presenting results for genetically, phenotypically, and culturally heterogenous groups under one umbrella (“BAME” or “non-white”), and indeed the authors suggest that in many cases this is to bolster numbers, increasing the likelihood that subgroup analyses are statistically significant.
It is important to clarify why all groups, apart from “Other” appear to be under-represented in the data. The weighted averages do not include individuals grouped under terms such as “BAME”, “non-White” or “Other” (referencing non-White), as this disaggregated data was not available. Therefore, all groups appear to be under-represented as a proportion of the total.
The meta-regression showed that over the course of the pandemic, recruitment in the Black group improved over the study period (p = 0.009), while no significant temporal trends were seen in the other ethnic groups. Improved recruitment amongst the Black ethnicity could be due to the recognition that COVID-19 disproportionately affected ethnic minorities, leading to calls to increase and encourage recruitment from these communities [33], as illustrated in Table 5.
Recruitment to trials, however, is far from the only issue. Our findings show limited reporting by ethnicity at all stages. Enrolment to trials was best reported, with seventeen of the 30 studies breaking participants down by individual ethnic groups. The meta-analysis highlighted that while twenty-nine reported participant enrolment for the White ethnicity, only 17, 16 and 15 studies reported participant enrolment for the Black, Asian and Mixed ethnicity groups respectively. Some of the other studies grouped individuals from minority communities as “BAME” and in these studies “BAME” representation was higher than UK ONS data (if including Asian, Black, Mixed and Other groups from ONS statistics, though such definitions in these studies were not always clear). It is important to highlight that no information was available on the representation of individual ethnicities within these studies, or indeed the “non-White” grouping used by other studies. The over-representation of this grouping of ethnicities is in direct contrast to the studies that reported enrolment data by individual ethnic groups, where a lower proportion of Black, Asian and Mixed participants were enrolled when compared to ONS data in the majority of the studies (14/17, 12/16 and 12/15, respectively).
Understanding enrolment disparities and data absenteeism in RCTs is vital as a lack of diversity can bias the results and limit generalisability to underrepresented populations. It is acknowledged that genetic polymorphisms can affect responses to vaccines [34] and medicinal therapeutics, such as antihypertensives, heart failure medications and warfarin [35]. Interventions which have been predominantly tested in White populations may not be as effective in other ethnicities [20, 36], and indeed a lack of representation in trials for vaccines and therapeutics fuels mistrust and vaccine hesitancy amongst minority communities [22].
A variety of reasons for the underrepresentation of ethnic minorities have been proposed. These can broadly be grouped into three categories: those occurring at system, individual and interpersonal levels. Barriers at the healthcare system and hospital level include restrictive study designs, financial costs associated with running trials and lack of community engagement. Commonly reported individual barriers revolve around lack of comfort, lack of knowledge on the research process and the study, logistics and time and resource constraints [37]. Doctor-patient relationships, including a lack of support if problems arose [22], language barriers [23], and mistrust (particularly suspicions of a hidden agenda [22]) play an important role at an interpersonal level [38, 39]. Overcoming these barriers is key to improving recruitment and participation in medical research, and tailored strategies will need to be implemented to improve participation in research of ethnic minority groups. A one-size fits all approach is inadequate as barriers identified vary between community groups [22]. These issues need to be approached at the conceptualisation of a trial, and inclusion of ethnic minorities should be considered at all stages of the research process [23].
Previous studies have shown that community-based [40, 41] and multimedia interventions [42] can be effective in increasing participation in research. Despite this, we found only three studies designed specific strategies to improve recruitment of ethnic minority groups (Table 5). None of these trials recruited a higher proportion of participants from ethnic minority communities, suggesting the issue requires a more complex solution. Two further studies made mention of recruiting from Black, Asian or minority ethnic communities, one mandated by the funder; however, no details were made available for how this was pursued.
The Consolidated Standard of Reporting Trials (CONSORT) statement provides guidance to researchers on reporting findings from RCTs. It advocates providing baseline demographic and clinical features of participants [43]. However, it does not specify which sociodemographic characteristics should be captured or presented. In order to combat underrepresentation, the National Institute for Health and Care Research recently developed the Innovations in Clinical Trial Design and Delivery for the Under-served (INCLUDE) framework, a tool that provides specific guidance to researchers on improving recruitment of minority groups [44]. Such an approach appears to have borne fruit in North America, where the Food and Drug Administration (FDA)-published guidance emphasises collecting racial and ethnic data in clinical trials, and appears to have improved the collection of such data [27].
In addition to using such frameworks, we call for consistency in the reporting of ethnicity in randomised controlled trials; first, in the terminology used to describe ethnic groups, both in the UK and internationally, to enable comparison of data across trials [45]. Second, to avoid grouping multiple ethnic groups under one term, ensuring better reporting of effect estimates by different ethnic groups. And third, collecting and reporting ethnicity data at every stage of a trial, increasing transparency and improving our understanding of where failures to recruit or retain participants occur.
Our study had several strengths. Our search strategy was robust, and our data collection methods were piloted and rigorous. Each full-text article was reviewed by two authors. We performed a meta-analysis and meta-regression, which provided a better estimate of the percentage and improved the generalisability of our findings. Limitations include that our systematic review assessed only UK-based studies, thus our findings are not generalisable to other countries. However, this was to allow comparison of our findings with high-quality data on proportion of patients from different ethnic groups in our national datasets. Second, some multinational studies that recruited from UK centres did not break down their results by individual country, meaning these were excluded, which may have biased the results. Third, the least biased pooled estimate for the analyses was for the White ethnicity, which was available for all studies; for other ethnicities, missing data may have impacted on the pooled estimate (for example, where ethnicities have been grouped into “BAME” rather than reported separately). Fourth, the population statistics we used were based on 2011 ONS data, which may not reflect today’s population statistics. Data from the 2021 census is awaited, but it is likely that changes to the demographic composition of the UK in this time will shine a harsher light on poor representation from ethnic minority communities. A further sub-analysis of interest is an examination of the characteristics of the organisation hosting the trial, and the region where the study took place, as there is significant heterogeneity in ethnic diversity in the UK. However, many of the studies we included were national or multi-site and did not disaggregate their results by region or site, making such an analysis impossible. This represents an area for future research. Finally, we have used one approach to the analysis of proportions, while others are available.
Conclusions
This systematic review of 30 trials with over 100,000 participants shows that Asian, Black and Mixed ethnic groups are either under-represented to a greater extent or incorrectly documented as “BAME”, “Other”, “non-white” or “Unknown” in UK COVID-19 RCTs. Underrepresentation in clinical trials occur at the system, individual and interpersonal level and require complex solutions, which should be approached at trial conception and considered throughout the research process. Reporting of trials by ethnicity lacks consistency, with obsolete terminology in common use, and grouping of multiple ethnicities commonplace despite genetic and phenotypic differences. Few trials report specific methods for recruiting participants from ethnic minorities. Those conducting trials need to make use of available frameworks for recruiting patients and report data that are consistent in terminology and have greater transparency.
Supplementary Information
Additional file 1: Appendix 1. Search strategy. Appendix 2. Numbers used for the analysis. Appendix 3. PRISMA abstract checklist. Appendix 4. PRISMA checklist. Appendix 5. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registries only.
Acknowledgements
We would like to thank Nia Roberts, Bodleian Health Care Libraries, for her advice on databases and invaluable help in putting together the search strategy for this review. We would like to offer our sincere thanks to the Trustees of the South Asian Health Foundation, Professor Kiran Patel, Professor Vinod Patel and Professor Wasim Hanif, for their support with this project.
Abbreviations
- ARC-EM
Applied Research Collaboration East Midlands
- BAME
Black, Asian and minority ethnic
- CI
Confidence interval
- CONSORT
Consolidated Standards of Reporting Trials
- COVID-19
Coronavirus disease
- FDA
Food and Drug Administration
- INCLUDE
Innovations in Clinical Trial Design and Delivery for the Under-served
- mRNA
Messenger ribonucleic acid
- NIHR
National Institute for Health and Care Research
- ONS
Office for National Statistics
- PRISMA
Preferred Reporting Items for Systematic Review and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
- RECOVERY
Randomised Evaluation of COVID-19 Therapy
- RCT
Randomised controlled trial
- SARS-CoV-2
Severe acute respiratory distress syndrome 2
- UK
United Kingdom
Authors’ contributions
MM was responsible for conceptualisation, investigation, methodology, formal analysis, writing the original draft, reviewing and editing subsequent drafts and project administration. LG was responsible for conceptualisation, investigation, methodology, writing the original draft and reviewing and editing subsequent drafts. HJ was responsible for conceptualisation, investigation, methodology and reviewing and editing the paper. DG was responsible for conceptualisation, investigation and reviewing and editing the paper. JHB was responsible for methodology, supervision and reviewing and editing the paper. FZ was responsible for formal analysis, visualisation and reviewing and editing the paper. HS was responsible for reviewing and editing the paper. KK was responsible for methodology, supervision and reviewing and editing the paper. All authors read and approved the final manuscript.
Funding
This study was funded by the South Asian Health Foundation. In addition, it was supported by members (K.K, F.Z) of the NIHR Applied Research Collaboration East Midlands (ARC-EM).
Availability of data and materials
Data collected for this study and the study protocol will be made available with publication of the manuscript and can be obtained by emailing the corresponding author, after investigator support is obtained.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare no additional support from any organisation for the submitted work, other than that declared in the manuscript; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
MM has received grants from the NIHR as part of an academic clinical fellowship, received travel grants in 2019 to support a voluntary placement in Zambia and receives royalties from Cambridge University Press.
JHB has received grants from the WHO, NIHR, Cancer Research UK and the British Heart Foundation. She has received honoraria for presenting at the University of Vermont and Boston Children’s Hospital, and payment for peer review for the Tryg Foundation.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khunti K, Singh AK, Pareek M, et al. Is ethnicity linked to incidence or outcomes of covid-19? BMJ. 2020;369:m1548. [DOI] [PubMed]
- 2.Mulholland RH, Sinha IP. Ethnicity and COVID-19 infection: are the pieces of the puzzle falling into place? BMC Med. 2020;18:206. doi: 10.1186/s12916-020-01669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Intensive Care National Audit & Research Centre (ICNARC). ICNARC report on COVID-19 in critical care: England, Wales & Northern Ireland. 23 October 2020. ICNARC Case Mix Programme Database, Intensive Care National Audit & Research Centre. 2020.
- 4.Public Health England. COVID-19: review of disparities in risks and outcomes. 2020. https://www.gov.uk/government/publications/covid-19-review-of-disparities-in-risks-and-outcomes. Accessed 27 Jan 2022.
- 5.Williams DR, Cooper LA. COVID-19 and Health Equity—A New Kind of “Herd Immunity”. JAMA. 2020;323:2478. doi: 10.1001/jama.2020.8051. [DOI] [PubMed] [Google Scholar]
- 6.Pan D, Sze S, Minhas JS, et al. The impact of ethnicity on clinical outcomes in COVID-19: A systematic review. EClinicalMedicine. 2020;23:100404. doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JT, Krieger N. Revealing the Unequal Burden of COVID-19 by Income, Race/Ethnicity, and Household Crowding: US County Versus Zip Code Analyses. J Public Health Manag Pract. 2021;27:S43–S56. doi: 10.1097/PHH.0000000000001263. [DOI] [PubMed] [Google Scholar]
- 8.Razai MS, Kankam HKN, Majeed A, et al. Mitigating ethnic disparities in covid-19 and beyond. BMJ. 2021;372:m4921. [DOI] [PubMed]
- 9.New Policy Institute. Accounting for the Variation in the Confirmed Covid-19 Caseload across England: An analysis of the role of multi-generation households, London and time. New Policy Institute. 2020. https://www.npi.org.uk/publications/housing-and-homelessness/accounting-variation-confirmed-covid-19-caseload-across-england-analysis-role-multi-generation-households-london-and-time. Accessed 27 Jan 2022.
- 10.Katikireddi SV, Lal S, Carrol ED, et al. Unequal impact of the COVID-19 crisis on minority ethnic groups: a framework for understanding and addressing inequalities. J Epidemiol Community Health. 2021;75:970. doi: 10.1136/jech-2020-216061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nafilyan V, Islam N, Ayoubkhani D, et al. Ethnicity, household composition and COVID-19 mortality: a national linked data study. J R Soc Med. 2021;114:182–211. doi: 10.1177/0141076821999973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The RECOVERY Collaborative Group Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 17.Shaw AR, Perales-Puchalt J, Johnson E, et al. Representation of Racial and Ethnic Minority Populations in Dementia Prevention Trials: A Systematic Review. J Prev Alzheimers Dis. 2021;9:1–6. doi: 10.14283/jpad.2021.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoel AW, Kayssi A, Brahmanandam S, et al. Under-representation of women and ethnic minorities in vascular surgery randomized controlled trials. J Vasc Surg. 2009;50:349–354. doi: 10.1016/j.jvs.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger JS, Melloni C, Wang TY, et al. Reporting and representation of race/ethnicity in published randomized trials. Am Heart J. 2009;158:742–747. doi: 10.1016/j.ahj.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Khunti K, Bellary S, Karamat MA, et al. Representation of people of South Asian origin in cardiovascular outcome trials of glucose-lowering therapies in Type 2 diabetes. Diabet Med. 2017;34:64–68. doi: 10.1111/dme.13103. [DOI] [PubMed] [Google Scholar]
- 21.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]
- 22.Ekezie W, Czyznikowska BM, Rohit S, et al. The views of ethnic minority and vulnerable communities towards participation in COVID-19 vaccine trials. J Public Health. 2021;43:e258–e260. doi: 10.1093/pubmed/fdaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodicoat DH, Routen AC, Willis A, et al. Promoting inclusion in clinical trials—a rapid review of the literature and recommendations for action. Trials. 2021;22:880. doi: 10.1186/s13063-021-05849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willis A, Isaacs T, Khunti K. Improving diversity in research and trial participation: the challenges of language. Lancet Public Health. 2021;6:e445–e446. doi: 10.1016/S2468-2667(21)00100-6. [DOI] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khunti K, Routen A, Pareek M, et al. The language of ethnicity. BMJ. 2020;m4493. [DOI] [PubMed]
- 27.Zhang J, Van Spall HGC, Wang Y, et al. Enrollment of black, indigenous, and other people of color in multicountry randomized controlled trials of diabetes conducted in North America and Europe. Diabetes Care. 2022;45(7):e116–e117. doi: 10.2337/dc22-0261. [DOI] [PubMed] [Google Scholar]
- 28.Bowe T, Salabati M, Soares RR, et al. Racial, Ethnic, and gender disparities in diabetic macular edema clinical trials ophthalmol. Retina. 2022;6(6):531–533. doi: 10.1016/j.oret.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Tahhan AS, Vaduganathan M, Greene SJ, et al. Enrollment of older patients, women, and racial/ethnic minority groups in contemporary acute coronary syndrome clinical trials: a systematic review. JAMA Cardiol. 2020;5:714. doi: 10.1001/jamacardio.2020.0359. [DOI] [PubMed] [Google Scholar]
- 30.Gardiner T, Cooke G, Fidler S, et al. The under-representation of BAME patients in the COVID-19 Recovery trial at a major London NHS Trust. J Infect. 2020;82(4);84-123. [DOI] [PMC free article] [PubMed]
- 31.Routen A, Akbari A, Banerjee A, et al. Strategies to record and use ethnicity information in routine health data. Nat Med. 2022;28:1338–1342. doi: 10.1038/s41591-022-01842-y. [DOI] [PubMed] [Google Scholar]
- 32.Khunti K, Routen A, Banerjee A, et al. The need for improved collection and coding of ethnicity in health research. J Public Health. 2021;43:e270–e272. doi: 10.1093/pubmed/fdaa198. [DOI] [PubMed] [Google Scholar]
- 33.Treweek S, Forouhi NG, Narayan KMV, et al. COVID-19 and ethnicity: who will research results apply to? The Lancet. 2020;395:1955–1957. doi: 10.1016/S0140-6736(20)31380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jethwa H, Wong R, Abraham S. Covid-19 vaccine trials: Ethnic diversity and immunogenicity. Vaccine. 2021;39:3541–3543. doi: 10.1016/j.vaccine.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson JA. Ethnic Differences in Cardiovascular Drug Response: Potential Contribution of Pharmacogenetics. Circulation. 2008;118:1383–1393. doi: 10.1161/CIRCULATIONAHA.107.704023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Van Spall HGC, Wang Y, et al. Twenty-year trends in racial and ethnic enrollment in large diabetes randomized controlled trials. BMC Med. 2022;20:294. doi: 10.1186/s12916-022-02501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark LT, Watkins L, Piña IL, et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Curr Probl Cardiol. 2019;44:148–172. doi: 10.1016/j.cpcardiol.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Hamel LM, Penner LA, Albrecht TL, et al. Barriers to Clinical Trial Enrollment in Racial and Ethnic Minority Patients With Cancer. Cancer Control J Moffitt Cancer Cent. 2016;23:327–337. doi: 10.1177/107327481602300404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark LT, Watkins L, Piña IL, et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Curr Probl Cardiol. 2019;44:148–172. doi: 10.1016/j.cpcardiol.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 40.McCaskill-Stevens W, McKinney MM, Whitman CG, et al. Increasing minority participation in cancer clinical trials: the minority-based community clinical oncology program experience. J Clin Oncol. 2005;23:5247–5254. doi: 10.1200/JCO.2005.22.236. [DOI] [PubMed] [Google Scholar]
- 41.Duda C, Mahon I, Chen MH, et al. Impact and costs of targeted recruitment of minorities to the National Lung Screening Trial. Clin Trials. 2011;8:214–223. doi: 10.1177/1740774510396742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30:2516–2521. doi: 10.1200/JCO.2011.39.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 Explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869–c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treweek S, Banister K, Bower P, et al. Developing the INCLUDE Ethnicity Framework—a tool to help trialists design trials that better reflect the communities they serve. Trials. 2021;22:337. doi: 10.1186/s13063-021-05276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Routen A, Akbari A, Banerjee A, et al. Strategies to record and use ethnicity information in routine health data. Nat Med. Epub ahead of print 31 May 2022. 10.1038/s41591-022-01842-y. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix 1. Search strategy. Appendix 2. Numbers used for the analysis. Appendix 3. PRISMA abstract checklist. Appendix 4. PRISMA checklist. Appendix 5. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registries only.
Data Availability Statement
Data collected for this study and the study protocol will be made available with publication of the manuscript and can be obtained by emailing the corresponding author, after investigator support is obtained.