Summary
Background
As of September 2022, nearly 1.3 billion doses of COVID-19 vaccine products have been administered in Latin America and the Caribbean, where 27% of global COVID-19 deaths have occurred. This study aimed to estimate the effectiveness of COVID-19 vaccines against lab-confirmed COVID-19 related hospitalizations and deaths among adults in Argentina, Brazil, Chile, and Colombia.
Methods
Using a test-negative case control design, we evaluated the effectiveness of a primary vaccination series considering six COVID-19 vaccine products (Sputnik V, mRNA-1273, CoronaVac, ChAdOx1, BNT162b2, Ad26.COV2.S) against lab-confirmed COVID-19 hospitalizations and deaths among 83,708 hospitalized adults from February–December, 2021. Data from hospitalization records, COVID surveillance, and vaccination registries were used. Vaccine effectiveness was estimated using logistic regression ((1-OR) x 100).
Findings
The average age of participants was 56.7 (SD = 17.5), and 45,894 (54.8%) were male. Adjusted VE (aVE) estimates for full vaccination against hospitalization were 82% for mRNA-1273 (95% confidence interval (CI) = −30 to 98%), 76% (71%–81%) for BNT162b2, 65% (61–68%) for ChAdOx1, 57% (10–79%) for Sputnik V, 53% (50–56%) for CoronaVac, and 46% (23–62%) for Ad26.COV2.S. Estimates, particularly for CoronaVac, varied by variant. Decreasing aVE was estimated as age increased, particularly for CoronaVac and ChAdOx1. aVE estimates against death were generally higher, with 100% (CI not estimated) for mRNA-1273, 82% (69–90%) for BNT162b2, 73% (69–77%) for ChAdOx1, 65% (60–67%) for CoronaVac, 38% (−75 to 78%) for Sputnik V, 6% (−58 to 44%) for Ad26.COV2.S.
Interpretation
Primary series vaccination with available COVID-19 vaccine products was effective against COVID-19 hospitalization and mortality. Effectiveness varied by product and declined with increasing age.
Funding
This study was funded by the Pan-American Health Organization (PAHO, World Health Organization (WHO)). PAHO convened and led the study implementation.
Keywords: COVID-19 vaccination, Vaccine effectiveness, Case–control, Test-negative, Brazil, Chile, Argentina, Colombia
Research in context.
Evidence before this study
Studies have found COVID-19 vaccines to be effective against hospitalization and mortality. Vaccine effectiveness estimates in these studies have varied by age of the population studied, by vaccine product, and by the predominant variant circulating at the time of study.
Added value of this study
This study is the first multi-country analysis to pool individual-level data in the Americas, aggregating data across four countries and using a single harmonized approach to evaluate the effectiveness of six COVID-19 vaccine products by age and by dominant SARS-CoV-2 circulating variant period. Given the richness of the data sources used in this study, we were able to include detailed information on symptoms as well as information on comorbidities and multiple endpoints. Recognizing the potential benefits of embedding an evaluation of methodological approaches to studying COVID-19 VE and taking advantage of the diverse set of data sources and systems in the countries selected for this study, we also conducted analyses using multiple designs.
Implications of all the available evidence
In this multi-country analysis, we find COVID-19 vaccines prevented hospitalizations and deaths, even among the most elderly population, with estimates varying by age and vaccine product. This study provides further evidence that the primary vaccination series using the COVID-19 vaccine products evaluated in this study is effective in preventing the most severe COVID-19 outcomes. Continued post-introduction observational studies should monitor the optimal combination of products, schedule sequencing, and additional boosting protection to ensure sustained protection.
Introduction
Countries in Latin America and the Caribbean have been among the hardest hit by the COVID-19 pandemic. Approximately 27% of global COVID-19 deaths have occurred in the region despite representing only 8% of the global population, with nearly 1.74 million deaths reported as of September 2022.1 Although younger ages are generally less susceptible to severe disease, and most COVID-19 deaths occur among the elderly and individuals living with health comorbidities, hospitalization and deaths have occurred in all ages.1, 2, 3 Throughout the region, the Pan American Health Organization (PAHO) has worked to increase equitable and affordable access to life-saving vaccines to protect against COVID-19 through its Revolving Fund, in collaboration with the COVID-19 Vaccines Global Access (COVAX) initiative.4,5 As of September 2022, 1.32 billion doses of more than a dozen COVID-19 vaccine products had been administered.6 However, COVID-19 vaccine access and uptake varies widely between countries in the region. For example, as of late July 2022, national coverage per 100 individuals of a COVID-19 vaccination primary series ranged from >90% in Chile to lower than 2% in Haiti.7
Countries in Latin America and the Caribbean began introducing COVID-19 vaccines at the end of December 2020, following emergency authorization of products by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). In the face of supply constraints and the evolving epidemiologic patterns of COVID-19, PAHO's Technical Advisory Group (TAG) on Vaccine-Preventable Diseases urged countries in the region to strictly adhere to the WHO SAGE (Strategic Advisory Group of Experts on Immunization) Roadmap for prioritizing the use of COVID-19 vaccines.8,9 This meant that countries were encouraged to deploy a phased introduction, prioritizing vaccination for frontline and healthcare workers, the elderly, and people living with underlying health conditions. As availability and coverage of COVID-19 vaccines has increased in the region, countries have started vaccinating general population adults and lower priority age groups such as adolescents and children and added booster doses to the primary series vaccination schedule.10
Considering the multiple SARS-CoV-2 variants introduced over time and the demographically diverse composition of the population in Latin America, it is imperative to conduct vaccine effectiveness (VE) studies that assess the performance of COVID-19 vaccines in use for these populations, especially against severe outcomes such as hospitalizations and deaths for which many clinical trials were not sufficiently powered. Here we describe a multi-center study led by PAHO, which established inter-institutional collaborations between academic centers and ministries of health in four Latin American countries - Argentina, Brazil, Chile, and Colombia - to implement harmonized case–control studies to evaluate the effectiveness of COVID-19 vaccines against disease related hospitalizations and deaths. The primary objective of this study was to estimate the effectiveness of COVID-19 vaccines (by vaccine products in use in the select countries in the region) against lab-confirmed COVID-19 related hospitalizations and deaths among general population adults (18+ years old) in Argentina, Brazil, Chile, and Colombia.
Methods
Study design
We conducted a pooled, unmatched test-negative case–control study using data collected by the four country study teams to evaluate the effectiveness of COVID-19 vaccines against hospitalization and death. A test-negative design restricts the source population for cases and controls to individuals who meet symptom criteria for COVID-19 and are tested (Supplementary Section S4). By restricting only to those who fulfill these conditions, the test-negative design minimizes bias related to health-seeking behavior.11,12 Among the eligible population, we defined cases as hospitalized individuals who tested positive for SARS-CoV-2 and controls as hospitalized individuals who tested negative for SARS-CoV-2.
Recognizing the potential benefits of embedding an evaluation of methodological approaches to studying COVID-19 VE and taking advantage of the diverse set of data sources and systems in the countries selected for this study, we also conducted a syndrome-negative case–control study for the primary outcome of hospitalization, using data from Brazil and Chile. In this design, the cases were defined in the same way as in the test-negative design, while the controls were those who were hospitalized, tested negative for SARS-CoV-2, and did not have any COVID-19 symptoms.
Inclusion and exclusion criteria
Eligible individuals were hospitalized patients aged 18 years and older who were admitted during 2021 but after COVID-19 vaccines had become widely available in each country (Supplementary Section S1), who presented with fever and cough or 3 or more COVID-like illness symptoms, who underwent SARS-CoV-2 RT-PCR testing within 10 days of symptom onset, who were admitted within 14 days of symptom onset, and had no documented history of SARS-CoV-2 infection in the prior 90 days before hospitalization (Supplementary Fig. S1). As previously described, cases were all eligible patients who tested positive, and controls were those individuals who tested negative. While cases could serve as controls prior to their case hospitalization, we excluded controls that had previously been cases. For repeat hospitalizations that occurred within 7 days of each other, we used the date of the first hospitalization occurrence. We also excluded individuals for whom there was incomplete or inconsistent vaccination data (e.g. only a date for a second dose recorded), those with heterologous schedules, and those missing data on covariates included in the analysis (Supplementary Fig. S1).
Study setting and source populations
Argentina, Brazil, Chile, and Colombia were included in this regional study. All four countries initiated COVID-19 vaccination in high-risk groups (healthcare workers, elderly persons, individuals living with comorbidities) by the beginning of 2021, followed by an expansion to general population adults aged 18+ by August 2021. A total of seven vaccine products (AstraZeneca's ChAdOx1, Sinovac's CoronaVac, Janssen's Ad26.COV2.S, Moderna's mRNA-1273, Pfizer's BNT162b2, Sinopharm's Vero Cell, and Russian Direct Investment Fund's Sputnik V)a were used across the four country settings in these populations.
In each country, the study source population was defined based on data availability, either from a sample of hospital sites, secondary administrative data, or surveillance systems (Supplementary Section S1). Hospital sites, where applicable, were selected following three criteria: 1) sites that were involved in COVID-19 surge response; 2) capability of electronic information systems to integrate admission records with other public health surveillance systems; 3) sub-national geographic representation.
For the test-negative design enrollees, hospitalized patients were screened for eligibility, including if they met the symptom criteria outlined above, from a purposive sample of hospitals located in geographically diverse areas in Chile (n = 3) and Argentina (n = 3). Colombia's test-negative source population was defined by pooling two administrative sources, the first from a Caribbean region insurance affiliate with data from hospital sites, and the second from surveillance under the jurisdiction of the Bogota Secretary of Health. For Brazil, the national severe acute respiratory infection (SARI) surveillance system (SIVEP-Gripe) for five states was used, which reports all hospital admissions with COVID-like symptoms.
For the syndrome-negative design enrollees, only Chile and Brazil had data available to identify eligible syndrome-negative controls, defined as hospitalized patients for any non-COVID-19 cause who tested negative for SARS-CoV-2. In Chile, the same three hospital sites used for the test-negative design were used for this secondary design, and in Brazil, a sample of five hospital sites was selected, one from each of the same states that were represented in the test-negative study.
Data sources and procedures
Each study team screened patients for inclusion using admission record data sources, originating from either hospital information or surveillance systems (Supplementary Section S1). COVID-19 testing and symptom data related to the index event were obtained from national surveillance systems and linked to admission records if not readily available in the admission record. Country study teams also obtained individual-level data for demographic characteristics, prior SARS-CoV-2 testing history, comorbidity history in the prior year, and COVID-19 vaccine receipt, extracting data from various sources (e.g., notifiable disease surveillance systems, national immunization information systems, hospital electronic and paper-based information record systems) (Supplementary Section S1).
Additionally, metadata from SARS-CoV-2 genome sequences that were submitted to the EpiCoV platform in the Global Initiative on Sharing Influenza database (GISAID) (https://www.gisaid.org/) were used to derive group-level temporal indicators for dominant variant circulation. These data were downloaded on June 27th, 2022 and extracted using the following criteria: location (South America/Argentina or South America/Brazil or South America/Chile or South America/Colombia), host (human), and collection date (from January to December 2021). Lack of precision in the format of the sample collection date was an exclusion criterion (Supplementary Section S2).
Exposure
We defined partially vaccinated as those who had received only one dose of a two-dose primary series at least 14 days before the hospitalization date, and fully vaccinated as those who received one dose of a one-dose primary series vaccine (i.e., Ad26.COV2.S), or two doses of a two-dose primary series product (i.e., CoronaVac, BNT162b2, mRNA-1273, Sputnik V, or ChAdOx1) at least 14 days before hospitalization. Individuals who had received a first dose of a two-dose primary series product less than 14 days prior to hospitalization were considered unvaccinated. Those who had received an additional dose at least 14 days before hospitalization were considered boosted and excluded from the primary analysis.
Outcomes
The primary outcome of this study was lab-confirmed COVID-19 related hospitalization, and the secondary was lab-confirmed COVID-19 related deaths.
Statistical analysis
A priori, we estimated case and control sample size requirements for the purpose of guiding country teams in the process of screening and selecting sufficient numbers of patients for matched analysis using the two case control designs. Sample size calculations are described in the Technical Appendix (Supplementary Section S3). Due to very small numbers of test-negative controls resulting from minimal non-COVID respiratory virus circulation in each country as well as combined between countries, all analyses were unmatched. All test-negative analyses utilize data pooled from the four countries, and the syndrome-negative analyses utilize data pooled from Brazil and Chile.
We conducted logistic regression analyses separately for each vaccine product of which at least 100 individuals in the study had received. In the vaccine effectiveness adjusted (aVE) analyses, we controlled for continuous age, sex, secondary administrative unit location of residence, date of hospitalization grouped into categorical epiweeks, presence of 1 or more comorbidities (binary variable; see Supplementary Section S1 for details), and study site country. We also conducted separate analyses for four age groups: 18–49, 50–64, 65–79, 80+. To account for differences in VE against specific circulating dominant variants of SARS-CoV-2, we conducted analyses for two separate periods based on trends in variant circulation period across the four countries: February to July when Gamma was most prevalent, and September to December when Delta was most prevalent (Supplementary Figs. S2a and b).
We repeated the main analysis described above with the outcome of death, where cases were those who were hospitalized, met symptom criteria, tested positive for SARS-CoV-2, and died. Controls included those who were hospitalized, met symptom criteria, and tested negative for SARS-CoV-2. In a sensitivity analysis, we included only controls who met this definition and who also died. We also conducted sensitivity analyses around the symptom criteria for participant inclusion, relaxing the criteria to two or more reported symptoms.
The unconditional logistic regression models reported odds ratios, which were reported as vaccine effectiveness (one minus the odds ratio ×100%), with their 95% confidence intervals. All analyses were done in R.
Ethics
Following review of the study protocol (Supplementary Section C), PAHO and partner institution IRBs determined the study exempt from full review as the project exclusively involved secondary analysis of de-identified existing data. The protocol also underwent IRB approval in each country, as individual identifiers were obtained for linkages and then masked for analysis.
Role of the funding source
This study was funded by the Pan-American Health Organization (PAHO)/World Health Organization (WHO). PAHO convened and led the study implementation and was therefore involved in the study design, collection, analysis, and interpretation of data, writing, and submission.
Results
The test-negative case–control study for VE against hospitalization included 83,708 patients (64,178 cases, 19,530 controls). The average age of participants was 56.7 (SD = 17.5), and 45,894 (54.8%) were male. Enrolled individuals were mostly from Brazil (n = 75,151, 90% of events). The other countries contributed to events as follows: Argentina (n = 826, 1%), Chile (n = 1961, 2%), Colombia (n = 5770, 7%) (Supplementary Fig. S1, Supplementary Table S1). Gamma followed by Delta were the predominant circulating variants during the study period (Supplementary Fig. S2a), although variants did vary by country (Supplementary Fig. S2b). Trends in hospitalizations over time also varied by country (Supplementary Fig. S3). In total, 27% of cases and 55% of controls were vaccinated (Supplementary Table S1). The majority of vaccinated individuals had received their most recent dose within 90 days prior to hospitalization.
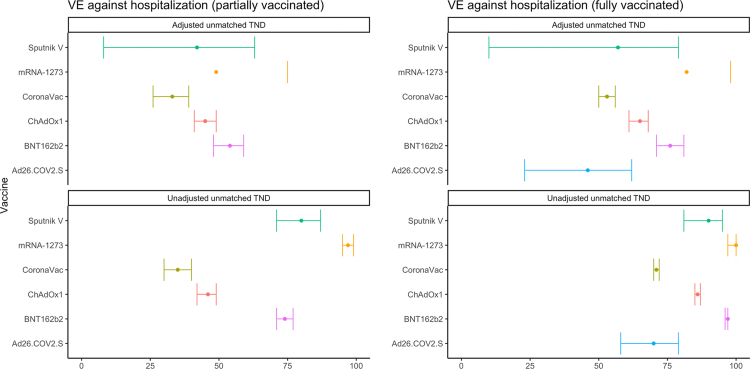
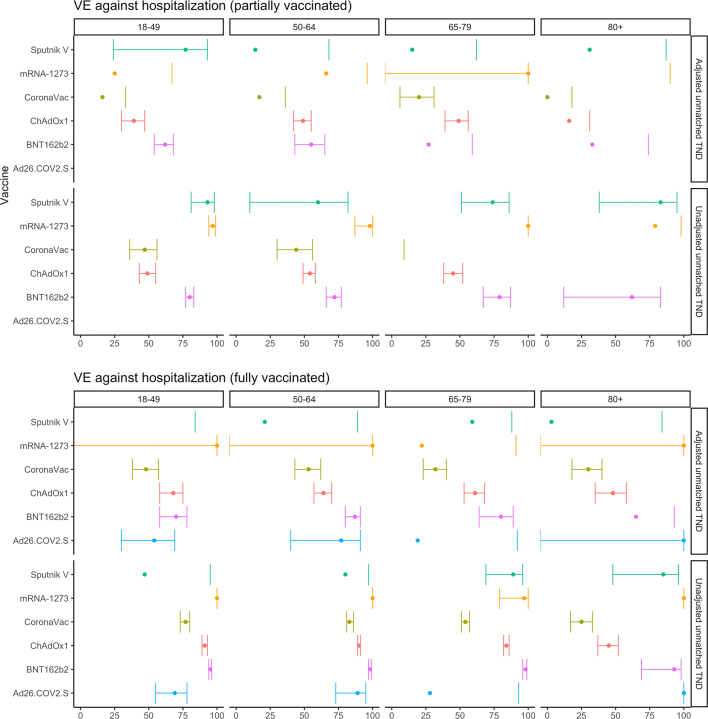
We show that all COVID-19 vaccine products evaluated were effective against hospitalization, although VE varied by product (Fig. 1, Table 1). As expected, estimates for fully vaccinated were higher than partially vaccinated. Of the six vaccine products analyzed in our all age-group primary analysis, adjusted VE (aVE) estimates for full vaccination were 82% for mRNA-1273 (95% confidence interval (CI) = −30 to 98%), 76% (71%–81%) for BNT162b2, 65% (61–68%) for ChAdOx1, 57% (10–79%) for Sputnik V, 53% (50–56%) for CoronaVac, and 46% (23–62%) for Ad26.COV2.S. The estimates varied for the different periods analyzed when Gamma and then Delta were the predominant variants (Supplementary Fig. S4a, and b and Supplementary Table S2a,and b). When stratifying by age groups, we observed decreasing aVE as age increases, particularly for CoronaVac and ChAdOx1 (Fig. 2, Table 2). Sensitivity analysis to assess the influence of relaxing the symptom criteria to two or more symptoms yielded similar findings to our primary analysis (results not shown).
Fig. 1.
Pooled VE against lab-confirmed COVID-19 hospitalization, by vaccine product in use. Adjusted for continuous age, sex, secondary administrative unit location of residence, date of hospitalization grouped into categorical epiweeks, presence of 1 or more comorbidities, and study site country. Error bars show the 95% confidence intervals. Some estimates and/or 95% confidence intervals fall outside of the range 0–100% shown on the graph. These values can be found in Table 1. TND = test-negative design.
Table 1.
Pooled COVID-19 Vaccine Effectiveness against lab-confirmed COVID-19 hospitalization for partially and fully vaccinated study participants, by vaccine product in use. Latin America, 2021.
| Vaccine | Analytic Method | # Vaccinated | # Unvaccinated | Partially vaccinated | Fully vaccinated |
|---|---|---|---|---|---|
| Sputnik V | Adjustedb unmatched TND | 145 | 55,174 | 42 (8, 63) | 57 (10, 79) |
| Sputnik V | Unadjusted unmatched TND | 145 | 55,174 | 80 (71, 87) | 90 (81, 95) |
| mRNA-1273 | Adjusted unmatched TND | 114 | 55,174 | 49 (−5, 75) | 82 (−30, 98) |
| mRNA-1273 | Unadjusted unmatched TND | 114 | 55,174 | 97 (95, 99) | 100 (97, 100) |
| CoronaVac | Adjusted unmatched TND | 14,897 | 55,174 | 33 (26, 39) | 53 (50, 56) |
| CoronaVac | Unadjusted unmatched TND | 14,897 | 55,174 | 35 (30, 40) | 71 (70, 72) |
| ChAdOx1 | Adjusted unmatched TND | 10,884 | 55,174 | 45 (41, 49) | 65 (61, 68) |
| ChAdOx1 | Unadjusted unmatched TND | 10,884 | 55,174 | 46 (42, 49) | 86 (85, 87) |
| BNT162b2 | Adjusted unmatched TND | 2262 | 55,174 | 54 (48, 59) | 76 (71, 81) |
| BNT162b2 | Unadjusted unmatched TND | 2262 | 55,174 | 74 (71, 77) | 97 (96, 97) |
| Ad26.COV2.Sa | Adjusted unmatched TND | 150 | 55,174 | 46 (23, 62) | |
| Ad26.COV2.Sa | Unadjusted unmatched TND | 150 | 55,174 | 70 (58, 79) |
Primary series is one-dose, so there are no partially vaccinated estimates.
Adjusted for continuous age, sex, secondary administrative unit location of residence, date of hospitalization grouped into categorical epiweeks, presence of 1 or more comorbidities, and study site country.
Fig. 2.
COVID-19 vaccine effectiveness against lab-confirmed COVID-19 hospitalization, by vaccine product in use and by age group, Latin America, 2021. Adjusted for continuous age, sex, secondary administrative unit location of residence, date of hospitalization grouped into categorical epiweeks, presence of 1 or more comorbidities, and study site country. Error bars show the 95% confidence intervals. Some estimates and/or 95% confidence intervals fall outside of the range 0–100% shown on the graph. These values can be found in Table 2. TND = test-negative design.
Table 2.
Pooled COVID-19 Vaccine Effectiveness against lab-confirmed COVID-19 hospitalization for partially and fully vaccinated study participants, by vaccine product in use and by age group. Latin America, 2021.
| Vaccine | Age group | Analytic Method | # Vaccinated | # Unvaccinated | Partially vaccinated | Fully vaccinated |
|---|---|---|---|---|---|---|
| Sputnik V | 80+ | Adjusteda unmatched TND | 30 | 2701 | 31 (−269, 87) | 3 (−474, 84) |
| Sputnik V | 80+ | Unadjusted unmatched TND | 30 | 2701 | 83 (38, 95) | 85 (48, 96) |
| Sputnik V | 65–79 | Adjusted unmatched TND | 56 | 9337 | 15 (−90, 62) | 59 (−40, 88) |
| Sputnik V | 65–79 | Unadjusted unmatched TND | 56 | 9337 | 74 (51, 86) | 89 (69, 96) |
| Sputnik V | 50–64 | Adjusted unmatched TND | 37 | 18,198 | 14 (−129, 68) | 21 (−472, 89) |
| Sputnik V | 50–64 | Unadjusted unmatched TND | 37 | 18,198 | 60 (10, 82) | 80 (−21, 97) |
| Sputnik V | 18–49 | Adjusted unmatched TND | 22 | 24,938 | 77 (24, 93) | −65 (−1627, 84) |
| Sputnik V | 18–49 | Unadjusted unmatched TND | 22 | 24,938 | 93 (81, 98) | 47 (−407, 95) |
| mRNA-1273 | 80+ | Adjusted unmatched TND | 6 | 2701 | −37 (−1733, 90) | 100 (NE) |
| mRNA-1273 | 80+ | Unadjusted unmatched TND | 6 | 2701 | 79 (−104, 98) | 100 (NE) |
| mRNA-1273 | 65–79 | Adjusted unmatched TND | 16 | 9337 | 100 (-Inf, 100) | 22 (−550, 91) |
| mRNA-1273 | 65–79 | Unadjusted unmatched TND | 16 | 9337 | 100 (NE) | 97 (79, 100) |
| mRNA-1273 | 50–64 | Adjusted unmatched TND | 17 | 18,198 | 66 (−188, 96) | 100 (NE) |
| mRNA-1273 | 50–64 | Unadjusted unmatched TND | 17 | 18,198 | 98 (87, 100) | 100 (NE) |
| mRNA-1273 | 18–49 | Adjusted unmatched TND | 75 | 24,938 | 25 (−74, 67) | 100 (NE) |
| mRNA-1273 | 18–49 | Unadjusted unmatched TND | 75 | 24,938 | 97 (94, 99) | 100 (NE) |
| CoronaVac | 80+ | Adjusted unmatched TND | 4161 | 2701 | 0 (−23, 18) | 30 (18, 40) |
| CoronaVac | 80+ | Unadjusted unmatched TND | 4161 | 2701 | −33 (−57, −12) | 25 (17, 33) |
| CoronaVac | 65–79 | Adjusted unmatched TND | 8044 | 9337 | 20 (6, 31) | 32 (23, 40) |
| CoronaVac | 65–79 | Unadjusted unmatched TND | 8044 | 9337 | −3 (−16, 9) | 54 (51, 57) |
| CoronaVac | 50–64 | Adjusted unmatched TND | 1307 | 18,198 | 17 (−9, 36) | 53 (43, 62) |
| CoronaVac | 50–64 | Unadjusted unmatched TND | 1307 | 18,198 | 44 (30, 56) | 83 (81, 86) |
| CoronaVac | 18–49 | Adjusted unmatched TND | 1385 | 24,938 | 16 (−5, 33) | 48 (38, 57) |
| CoronaVac | 18–49 | Unadjusted unmatched TND | 1385 | 24,938 | 47 (36, 56) | 77 (73, 80) |
| ChAdOx1 | 80+ | Adjusted unmatched TND | 2292 | 2701 | 16 (−2, 31) | 48 (35, 58) |
| ChAdOx1 | 80+ | Unadjusted unmatched TND | 2292 | 2701 | −52 (−76, −31) | 45 (37, 52) |
| ChAdOx1 | 65–79 | Adjusted unmatched TND | 2109 | 9337 | 49 (39, 56) | 61 (53, 68) |
| ChAdOx1 | 65–79 | Unadjusted unmatched TND | 2109 | 9337 | 45 (38, 52) | 84 (82, 86) |
| ChAdOx1 | 50–64 | Adjusted unmatched TND | 4384 | 18,198 | 49 (42, 55) | 64 (57, 70) |
| ChAdOx1 | 50–64 | Unadjusted unmatched TND | 4384 | 18,198 | 54 (49, 58) | 90 (89, 91) |
| ChAdOx1 | 18–49 | Adjusted unmatched TND | 2099 | 24,938 | 39 (30, 47) | 68 (58, 75) |
| ChAdOx1 | 18–49 | Unadjusted unmatched TND | 2099 | 24,938 | 49 (43, 55) | 91 (89, 93) |
| BNT162b2 | 80+ | Adjusted unmatched TND | 44 | 2701 | 33 (−76, 74) | 65 (−77, 93) |
| BNT162b2 | 80+ | Unadjusted unmatched TND | 44 | 2701 | 62 (12, 83) | 93 (69, 98) |
| BNT162b2 | 65–79 | Adjusted unmatched TND | 228 | 9337 | 27 (−30, 59) | 80 (64, 89) |
| BNT162b2 | 65–79 | Unadjusted unmatched TND | 228 | 9337 | 79 (67, 87) | 98 (96, 99) |
| BNT162b2 | 50–64 | Adjusted unmatched TND | 810 | 18,198 | 55 (43, 65) | 87 (80, 91) |
| BNT162b2 | 50–64 | Unadjusted unmatched TND | 810 | 18,198 | 72 (66, 77) | 98 (97, 99) |
| BNT162b2 | 18–49 | Adjusted unmatched TND | 1180 | 24,938 | 62 (54, 68) | 70 (58, 78) |
| BNT162b2 | 18–49 | Unadjusted unmatched TND | 1180 | 24,938 | 80 (77, 83) | 95 (94, 96) |
| Ad26.COV2.S | 80+ | Adjusted unmatched TND | 1 | 2701 | 100 (NE) | |
| Ad26.COV2.S | 80+ | Unadjusted unmatched TND | 1 | 2701 | 100 (NE) | |
| Ad26.COV2.S | 65–79 | Adjusted unmatched TND | 4 | 9337 | 19 (−714, 92) | |
| Ad26.COV2.S | 65–79 | Unadjusted unmatched TND | 4 | 9337 | 28 (−592, 93) | |
| Ad26.COV2.S | 50–64 | Adjusted unmatched TND | 20 | 18,198 | 77 (40, 91) | |
| Ad26.COV2.S | 50–64 | Unadjusted unmatched TND | 20 | 18,198 | 89 (73, 95) | |
| Ad26.COV2.S | 18–49 | Adjusted unmatched TND | 125 | 24,938 | 54 (30, 69) | |
| Ad26.COV2.S | 18–49 | Unadjusted unmatched TND | 125 | 24,938 | 69 (55, 78) |
NE = not estimated.
Adjusted for continuous age, sex, secondary administrative unit location of residence, date of hospitalization grouped into categorical epiweeks, presence of 1 or more comorbidities, and study site country.
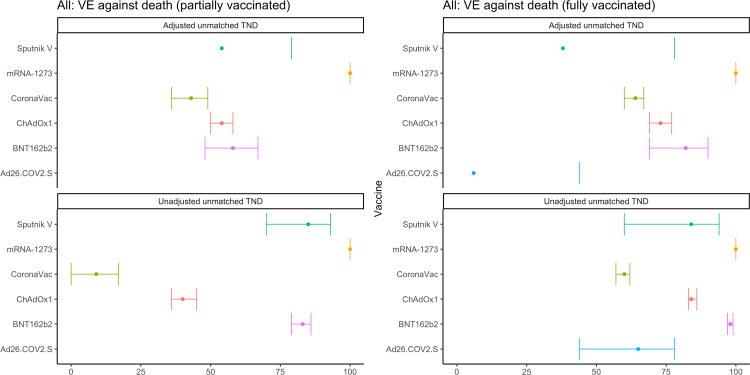
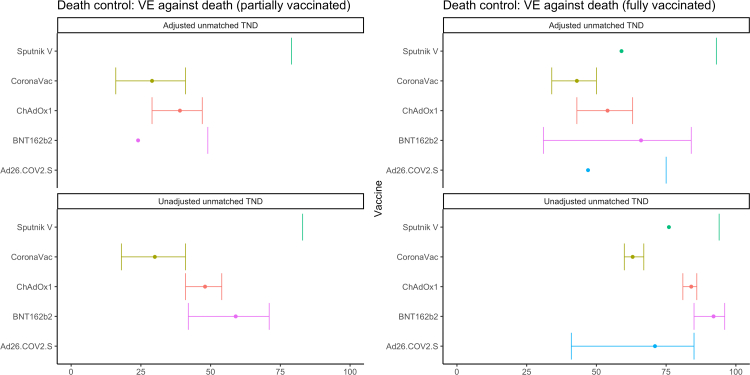
We find similar trends when analyzing VE against death using our primary definition of controls, with the aVE against death generally higher than aVE against hospitalization (Fig. 3, Table 3): 100% (CI not estimated) for mRNA-1273, 82% (69–90%) for BNT162b2, 73% (69–77%) for ChAdOx1, 64% (60–67%) for CoronaVac, 38% (−74 to 78%) for Sputnik V, and 6% (−58 to 44%) for Ad26.COV2.S. When restricting to controls who died, the aVE are lower for most vaccines (Fig. 4, Table 4): 66% (31–84%) for BNT162b2, 43% (34–50%) for CoronaVac, and 54% (43–63%) for ChAdOx1 (mRNA-1273 estimates could not be calculated with this method due to no deaths among those with this vaccine); we find higher estimates for two vaccines but note the wide and overlapping confidence intervals: 47% (−12 to 75%) for Ad26.COV2.S and 59% (−136 to 93%) for Sputnik V.
Fig. 3.
COVID-19 vaccine effectiveness against lab-confirmed COVID-19 death using all controls, by vaccine product in use. Latin America, 2021. Adjusted for continuous age, sex, secondary administrative unit location of residence, date of hospitalization grouped into categorical epiweeks, presence of 1 or more comorbidities, and study site country. Error bars show the 95% confidence intervals. Some estimates and/or 95% confidence intervals fall outside of the range 0-100% shown on the graph. These values can be found in Table 3. TND = test-negative design.
Table 3.
COVID-19 Vaccine Effectiveness against lab-confirmed COVID-19 death using all controls, by vaccine product in use. Latin America, 2021.
| Vaccine | Analytic method | # Vaccinated | # Unvaccinated | Partially vaccinated | Fully vaccinated |
|---|---|---|---|---|---|
| Sputnik V | Adjusteda unmatched TND | 92 | 19,188 | 54 (−1, 79) | 38 (−74, 78) |
| Sputnik V | Unadjusted unmatched TND | 92 | 19,188 | 85 (70, 93) | 84 (60, 94) |
| mRNA-1273 | Adjusted unmatched TND | 104 | 19,188 | 100 (NE) | 100 (NE) |
| mRNA-1273 | Unadjusted unmatched TND | 104 | 19,188 | 100 (NE) | 100 (NE) |
| CoronaVac | Adjusted unmatched TND | 8242 | 19,188 | 43 (36, 49) | 64 (60, 67) |
| CoronaVac | Unadjusted unmatched TND | 8242 | 19,188 | 9 (0, 17) | 60 (57, 62) |
| ChAdOx1 | Adjusted unmatched TND | 5702 | 19,188 | 54 (50, 58) | 73 (69, 77) |
| ChAdOx1 | Unadjusted unmatched TND | 5702 | 19,188 | 40 (36, 45) | 84 (83, 86) |
| BNT162b2 | Adjusted unmatched TND | 1461 | 19,188 | 58 (48, 67) | 82 (69, 90) |
| BNT162b2 | Unadjusted unmatched TND | 1461 | 19,188 | 83 (79, 86) | 98 (97, 99) |
| Ad26.COV2.S | Adjusted unmatched TND | 82 | 19,188 | 6 (−58, 44) | |
| Ad26.COV2.S | Unadjusted unmatched TND | 82 | 19,188 | 65 (44, 78) |
NE = not estimated.
Adjusted for continuous age, sex, secondary administrative unit location of residence, date of hospitalization grouped into categorical epiweeks, presence of 1 or more comorbidities, and study site country.
Fig. 4.
COVID-19 vaccine effectiveness against lab-confirmed COVID-19 death, by vaccine product use, restricting to controls who died. Latin America, 2021. Adjusted for continuous age, sex, secondary administrative unit location of residence, date of hospitalization grouped into categorical epiweeks, presence of 1 or more comorbidities, and study site country. Error bars show the 95% confidence intervals. Some estimates and/or 95% confidence intervals fall outside of the range 0-100% shown on the graph. These values can be found in Table 4. TND = test-negative design.
Table 4.
COVID-19 Vaccine Effectiveness against lab-confirmed COVID-19 death, by vaccine product use, restricting to controls who died. Latin America, 2021.
| Vaccine | Analytic method | # Vaccinated | # Unvaccinated | Partially vaccinated | Fully vaccinated |
|---|---|---|---|---|---|
| Sputnik V | Adjusteda unmatched TND | 18 | 11,977 | −111 (−2037, 79) | 59 (−136, 93) |
| Sputnik V | Unadjusted unmatched TND | 18 | 11,977 | −32 (−942, 83) | 76 (−2, 94) |
| CoronaVac | Adjusted unmatched TND | 4073 | 11,977 | 29 (16, 41) | 43 (34, 50) |
| CoronaVac | Unadjusted unmatched TND | 4073 | 11,977 | 30 (18, 41) | 63 (60, 67) |
| ChAdOx1 | Adjusted unmatched TND | 2453 | 11,977 | 39 (29, 47) | 54 (43, 63) |
| ChAdOx1 | Unadjusted unmatched TND | 2453 | 11,977 | 48 (41, 54) | 84 (81, 86) |
| BNT162b2 | Adjusted unmatched TND | 206 | 11,977 | 24 (−12, 49) | 66 (31, 84) |
| BNT162b2 | Unadjusted unmatched TND | 206 | 11,977 | 59 (42, 71) | 92 (85, 96) |
| Ad26.COV2.S | Adjusted unmatched TND | 36 | 11,977 | 47 (−12, 75) | |
| Ad26.COV2.S | Unadjusted unmatched TND | 36 | 11,977 | 71 (41, 85) |
Adjusted for continuous age, sex, secondary administrative unit location of residence, date of hospitalization grouped into categorical epiweeks, presence of 1 or more comorbidities, and study site country.
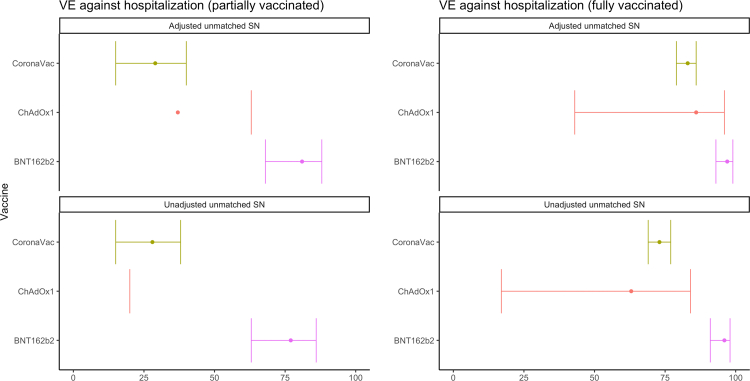
We also conducted a syndrome-negative analysis, using data from Brazil (n = 602, 8%) and Chile (n = 6702, 92%). In this analysis, we found an aVE against hospitalization of 97% (93–99%) for BNT162b2, 83% (79–86%) for CoronaVac, and 86% (43–96%) for ChAdOx1 (Fig. 5, Table 5).
Fig. 5.
COVID-19 vaccine effectiveness against lab-confirmed COVID-19 hospitalization using syndrome negative (SN) controls, by vaccine product, in Chile and Brazil. Latin America, 2021. Adjusted for continuous age, sex, secondary administrative unit location of residence, date of hospitalization grouped into categorical epiweeks, presence of 1 or more comorbidities, and study site country. Error bars show the 95% confidence intervals. TND = test-negative design.
Table 5.
COVID-19 Vaccine Effectiveness against lab-confirmed COVID-19 hospitalization using syndrome negative (SN) controls, by vaccine product, in Chile and Brazil. Latin America, 2021.
| Vaccine | Analytic method | # Vaccinated | # Unvaccinated | Partially vaccinated | Fully vaccinated |
|---|---|---|---|---|---|
| CoronaVac | Adjusteda unmatched SN | 3026 | 3685 | 29 (15, 40) | 83 (79, 86) |
| CoronaVac | Unadjusted unmatched SN | 3026 | 3685 | 28 (15, 38) | 73 (69, 77) |
| ChAdOx1 | Adjusted unmatched SN | 152 | 3685 | 37 (−8, 63) | 86 (43, 96) |
| ChAdOx1 | Unadjusted unmatched SN | 152 | 3685 | −18 (−75, 20) | 63 (17, 84) |
| BNT162b2 | Adjusted unmatched SN | 430 | 3685 | 81 (68, 88) | 97 (93, 99) |
| BNT162b2 | Unadjusted unmatched SN | 430 | 3685 | 77 (63, 86) | 96 (91, 98) |
Adjusted for continuous age, sex, secondary administrative unit location of residence, date of hospitalization grouped into categorical epiweeks, presence of 1 or more comorbidities, and study site country.
Discussion
This is the first multi-country analysis for the Americas, to our knowledge, to evaluate the effectiveness of multiple COVID-19 vaccines by age and by dominant SARS-CoV-2 circulating variant, leveraging readily available data sources from four demographically diverse countries and applying a single harmonized methodological approach. We find that COVID-19 vaccines prevented hospitalizations and deaths, even among the most elderly population. Because older populations were vaccinated first, it is possible that VE may have begun to wane; however, the majority of individuals even among the oldest age groups were vaccinated less than five months prior to hospitalization (Supplementary Fig. S5).
The VE estimates from our analysis generally fall within the range reported in other peer-reviewed studies, with mRNA vaccines having the highest VE estimates.13,14 Previously published studies from Argentina,15 Brazil,16 Chile,17 and Colombia18,19 reported COVID-19 VE estimates that were similar or slightly higher than ours. These VE estimates were against hospitalization or death for the primary vaccination series among general population adults aged 60 years and older during periods when Gamma and Delta variants were predominantly circulating (Argentina: Sputnik V against death: 93% (95% CI: 93–95%), ChAdOx1 against death: 94% (93–94%); Brazil: CoronaVac against hospitalization: 56% (47–63%); Chile: CoronaVac against hospitalization: 85% (84–86%)).13,14
It is challenging to make direct comparisons between studies due to differences in the study inclusion criteria, endpoint definitions, specific analytic time periods, and vaccine products and age groups included in analysis. However, the VE estimates for mRNA vaccines in our analysis are consistent with the previously published literature.14 Further, differences between our study estimates and other reports for CoronaVac are likely driven by the specific time periods and places included in our analysis. For example, CoronaVac VE estimates using the syndrome-negative design from our analysis, which relied primarily on data from Chile, are closer to estimates reported by Chile previously during a time period in the country's outbreak predominantly affected by the gamma variant.17 In contrast, the CoronaVac VE estimates using the TN design were lower than estimates reported in Chile but closer to estimates previously reported in Brazil.16 This is potentially due to the substantial number of contributing hospitalization events to the analysis from Brazil during the delta period, when we see lower effectiveness for CoronaVac. We explored the dominant circulating variant specific effects observed across pooled data from all four countries in sub-analyses, and indirectly controlled for other country-specific heterogeneity in our pooled main analysis model. Future studies could more precisely control for differences between country settings in healthcare quality, access to care, the timing and distributional equity of COVID-19 vaccination, and other potential confounding factors like nutritional status.
Given the richness of the data sources, we were able to include detailed information on symptoms as well as information on comorbidities and multiple endpoints. We also conducted analyses using multiple designs, although it is challenging to make direct comparisons between the designs given the differences in the populations in each analysis. As previously discussed, the higher estimates in the syndrome-negative analysis likely occurred because most patients in this analysis were from Chile where most hospitalizations occurred during the earlier part of the study period during which Gamma predominated. From a study design implementation perspective, the syndrome-negativedesign is a good choice in settings where there are small numbers of TN controls available for the comparator population, as was the case for our multi-country analysis. However, testing of all patients regardless of symptoms is required for unbiased estimates from the syndrome-negative design. Other studies have assessed the use of both TN and syndrome-negative control in case control studies evaluating the effectiveness of COVID-19 vaccines and found no significant differences between the two types of controls.20,21 The lower estimates for VE against death in the test negative analyses when restricting to controls who died are consistent with a lower VE in the older age groups who comprise a larger proportion of those who died (Supplementary Fig. S6). Choosing a control group that represents the source population from which the cases arose for the outcome of death is challenging. We have included two plausible options for control groups for this outcome, which may help provide bounds for estimates of VE against death.
Using a single harmonized methodological approach, our analysis provides a unique understanding of COVID-19 VE across four countries in adults 18 years and older for two periods of time. Our study is subject to limitations. The sample size was largely driven by Brazil; the results may therefore not be as generalizable to places that had different predominant variants. We indirectly controlled for other potential unmeasured confounding by using a country term in our models, but the results as a whole for the test-negative design specific to vaccines in use in Brazil are more representative of Brazil than the other country contexts, and there is potential for residual confounding. While the study included several sites for each country, we did not have nationally representative samples from each country, which may also limit the generalizability beyond the study site locations in settings where hospitals were used. In addition, the data came from a combination of patient data sources, including insurance claims databases, surveillance databases, and electronic and paper-based health records. Using date of hospitalization as the index date could bias the VE estimates towards the null if vaccinated individuals were hospitalized later on in infection; however, vaccinated individuals were hospitalized sooner or at similar times relative to testing than unvaccinated individuals (Supplementary Fig. S7). We also had limited viral genomic sequencing available at the individual level for the cases included in the study and thus relied on time as a proxy. Despite the richness of the data sources, we did not have completely consistent data on all potential confounders (e.g., socioeconomic status) between countries, and prior infection data was missing for most individuals. Bias indicator analyses, using those vaccinated in the past 0–13 days as a comparison instead of the unvaccinated, suggest the presence of unmeasured confounding (Supplementary Table S3); although we note that the assumption of time-invariancy in exposure, susceptibility, and testing required to validly use this method is likely not met in our data.22 Finally, sample size for some vaccines and some age groups was limited, leading to imprecise estimates, and VE for some products (e.g. Sinopharm) or heterologous schedules could not be estimated due to small sample sizes e.g., Sputnik V + mRNA-1273) (Supplementary Table S4).
This study provides further evidence that the primary vaccination series using the COVID-19 vaccine products evaluated in this study is effective against preventing the most severe COVID-19 outcomes. These findings underscore the importance of widespread and equitable access to these life-saving vaccines. Continued post-introduction observational studies should monitor the optimal combination of products, schedule sequencing, and additional boosting protection to ensure sustained protection.
Contributors
LHdO, CMT, MCC conceived of the study. LHdO, CMT, MCC, RK, CBJ led the development of the protocol. AdRM, GRB, CBJ, CD participated in the data collection. RK and CBJ led the analysis of the data and wrote the first draft of the manuscript. All authors participated in the writing and editing of the manuscript. The Regional Group collected the data and participated in the design, analysis, writing, and editing. All authors approved submission and agreed to be accountable for the work.
Data sharing statement
Data access will be considered after reviewing proposed research aims.
Declaration of interests
CMT received research suppport fees to support this work. CJ and RK were hired consultant to support study design, implementation, and analysis. CD has received payment or honoraria from Merck, Sanofi, Janssen, Pfizer.
Acknowledgements
This study was supported by funding from PAHO/WHO. We acknowledge the originating and submitting laboratories of the sequences from GISAID's EpiCoV Database. We especially thank the individuals from the country teams who supported data collection and quality checks.
Regional COVID-19 VE in Adults Study Working Group ✦
∗Indicates country study PI(s)
Argentina
Analia Rearte∗, Ministerio de Salud de la Nación Argentina; Escuela Superior de Medicina Universidad Nacional de Mar del Plata
Ignacio Leandro Uriarte, Escuela Superior de Medicina Universidad Nacional de Mar del Plata
Elsa Baumester, Servicio Virosis Respiratorias, Laboratorio Nacional de Referencias de SARS-CoV-2/COVID-19, Influenza, y Sarampion, INEI-ANLIS Malbran
Maria Elena Borda, Hospital Nacional Profesor Alejandro Posadas
Miguel Diaz Cordoba, Hospital Rawson de Córdoba
Juan Facundo Petrina, Director de la Dirección de Epidemiología, Ministerio de Salud, Tierra del Fuego
Ezequiel Consiglio, Instituto de Salud Comunitaria, Universidad Nacional de Hurlingham
Carla Vizzotti, Ministerio de Salud de la Nación Argentina
Brazil
Tatiana Guimarães de Noronha∗,Instituto de Tecnologia em Imuniobiológicos Bio-Manguinhos at Oswaldo Cruz Foundation, Rio de Janeiro; School of Medicine at Universidade Federal Fluminense] , Niterói, Rio de Janeiro
Maria Paula Gomes Mourão, FMT-HVD/UEA - Manaus, Amazonas
Jeova Keny Baima Colares, UNIFOR and Ceará Public Health School, Fortaleza, Ceará
Sonia Mara Raboni, UFPR-Curitiba, Paraná
Tazio Vanni, UNB-Brasilia, Federal District
Lely Guzman, Pan American Health Organization - Brazil, Brasilia, Federal District.
Adriana Regina Farias Pontes Lucena, National Immunization Program, Ministry of Health.
Chile
Maria Elena Santolaya∗, Hospital Dr. Luis Calvo Mackenna and School of Medicine at the Universidad de Chile
Cinthya Urquidi, School of Medicine at the Universidad de los Andes, Santiago, Chile
Claudia P. Cortes, School of Medicine at the Universidad de Chile, Santiago, Chile
Pedro Pablo Usedo Lopez, Infectious Disease Unit/IAAS Epidemiology, Hospital Regional de Antofagasta
Rosana Benitez, Clinica Dávila, Santiago, Chile
Veronica Menares Latorre, Hospital Regional Libertador Bernardo O’Higgins
Andrea Moller Roth, Regional Ministry Health Secretary of O’Higgins Region, Subsecretary of Public Health, Ministry of Health
Iván Brstilo Cerda, Department of Immunization, Division of Disease Prevention and Control, Ministry of Health, Government of Chile
Solange Santillana, Pan American Health Organization - Chile, Santiago.
Zohra Abaakouk, Pan American Health Organization - Chile, Santiago.
Colombia
Angel Paternina Caicedo, Fundación Alzak
Nelson Alvis Guzman∗, Fundación Alzak/Universidad de Cartagena
Juan Carlos Fernandez Mercado, Mutual SER.
Fernando de la Hoz Restrepo∗, Universidad Nacional de Colombia
David Santiago Quevedo, Secretaria Distrital de Salud de Bogotá
Sofia Rios Oliveros, Secretaria Distrital de Salud de Bogotá
Diane Moyano Romero, Secretaria Distrital de Salud de Bogotá.
Footnotes
Sputnik V was only used in Argentina; CoronaVac was only used in Brazil, Chile, Colombia; mRNA-1273 was only used in Colombia; the proportion of doses administered in eligible populations in the country settings combined was highest for CoronaVac and ChAdOx1.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100474.
Contributor Information
Rebecca Kahn, Email: rek160@mail.harvard.edu.
Cara B. Janusz, Email: cjanusz@umich.edu.
Marcia C. Castro, Email: mcastro@hsph.harvard.edu.
Carla Domingues, Email: cmasdomingues@gmail.com.
Jamie Ponmattam, Email: jponmattam@hsph.harvard.edu.
Lucia Helena de Oliveira, Email: oliveirl@paho.org.
Regional COVID-19 VE in Adults Study Working Group:
Analia Rearte, Ignacio Leandro Uriarte, Elsa Baumester, Maria Elena Borda, Miguel Diaz Cordoba, Juan Facundo Petrina, Ezequiel Consiglio, Carla Vizzotti, Tatiana Guimarães de Noronha, Maria Paula Gomes Mourão, Jeova Keny Baima Colares, Sonia Mara Raboni, Tazio Vanni, Lely Guzman, Adriana Regina Farias Pontes Lucena, Maria Elena Santolaya, Cinthya Urquidi, Claudia P. Cortes, Pedro Pablo Usedo Lopez, Rosana Benitez, Veronica Menares Latorre, Andrea Moller Roth, Iván Brstilo Cerda, Solange Santillana, Zohra Abaakouk, Angel Paternina Caicedo, Nelson Alvis Guzman, Juan Carlos Fernandez Mercado, Fernando de la Hoz Restrepo, David Santiago Quevedo, Sofia Rios Oliveros, and Diane Moyano Romero
Appendix A. Supplementary data
References
- 1.COVID-19 - PAHO/WHO response. 2022. https://www.paho.org/en/documents/covid-19-pahowho-response-report-79-july-1-2022 Jul 14. Report 79 (July 1, 2022)
- 2.O'Driscoll M., Ribeiro Dos Santos G., Wang L., et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 3.Viner R.M., Mytton O.T., Bonell C., et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults. JAMA Pediatr. 2021;175:143. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PAHO revolving Fund, 2020. https://www.paho.org/en/revolvingfund. Accessed July 14, 2022.
- 5.Feb 15. PAHO delivers 100 million COVAX vaccine doses to Latin America and the Caribbean, 2022. https://www.paho.org/en/news/15-2-2022-paho-delivers-100-million-covax-vaccine-doses-latin-america-and-caribbean. Accessed Aug 1, 2022.
- 6.COVID-19 vaccine doses administered in the Americas, 2020. https://ais.paho.org/imm/IM_DosisAdmin-Vacunacion.asp. Accessed July 14, 2022.
- 7.COVID-19 vaccine doses administered in the Americas, 2020. https://ais.paho.org/imm/IM_DosisAdmin-Vacunacion.asp. Accessed Aug 1, 2022.
- 8.The Pan American Health Organization’s (PAHO) Technical Advisory Group (TAG) on Vaccine-preventable Diseases Fifth ad hoc meeting of the technical advisory group (TAG) on vaccine-preventable diseases. 2020. https://www.paho.org/en/documents/fifth-ad-hoc-meeting-technical-advisory-group-tag-vaccine-preventable-diseases-4-august 4 August 2020, USA (virtual meeting).
- 9.WHO SAGE Roadmap for prioritizing uses of COVID-19 vaccines. 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Prioritization-2022.1 published online Jan 21.
- 10.Pan American Health Organization . PAHO; 2022. XXVII meeting of PAHO's technical advisory group (TAG) on vaccine-preventable diseases: tailoring the SAGE Roadmap to the requirements of the Americas and the strategic use of COVID-19 booster doses, 27 January 2022 (virtual)https://iris.paho.org/handle/10665.2/55781 [Google Scholar]
- 11.Evaluation of COVID-19 vaccine effectiveness. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-measurement-2021.1 published online March 17. [Google Scholar]
- 12.Dean N.E., Hogan J.W., Schnitzer M.E. Covid-19 vaccine effectiveness and the test-negative design. N Engl J Med. 2021;385:1431–1433. doi: 10.1056/NEJMe2113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IVAC. Website. COVID-19 data 2020. https://view-hub.org/covid-19/effectiveness-studies
- 14.Higdon M.M., Wahl B., Jones C.B., et al. A systematic review of Coronavirus disease 2019 vaccine efficacy and effectiveness against severe acute respiratory syndrome Coronavirus 2 infection and disease. Open Forum Infect Dis. 2022;9:ofac138. doi: 10.1093/ofid/ofac138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rearte A., Castelli J.M., Rearte R., et al. Effectiveness of rAd26-rAd5, ChAdOx1 nCoV-19, and BBIBP-CorV vaccines for risk of infection with SARS-CoV-2 and death due to COVID-19 in people older than 60 years in Argentina: a test-negative, case-control, and retrospective longitudinal study. Lancet. 2022;399:1254–1264. doi: 10.1016/S0140-6736(22)00011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranzani O.T., Hitchings M.D.T., Dorion M., et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021:374. doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jara A., Undurraga E.A., González C., et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paternina-Caicedo A., Jit M., Alvis-Guzmán N., et al. Effectiveness of CoronaVac and BNT162b2 COVID-19 mass vaccination in Colombia: a population-based cohort study. Lancet Reg Health Am. 2022;12 doi: 10.1016/j.lana.2022.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arregocés-Castillo L., Fernández-Niño J., Rojas-Botero M., et al. Effectiveness of COVID-19 vaccines in older adults in Colombia: a retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longev. 2022;3:e242–e252. doi: 10.1016/S2666-7568(22)00035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenforde M.W., Patel M.M., Ginde A.A., et al. Effectiveness of severe acute respiratory syndrome Coronavirus 2 messenger RNA vaccines for preventing Coronavirus disease 2019 hospitalizations in the United States. Clin Infect Dis. 2021;74:1515–1524. doi: 10.1093/cid/ciab687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tenforde M.W. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults — United States, March–July 2021. MMWR Morb Mortal Wkly Rep. 2021:70. doi: 10.15585/mmwr.mm7034e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hitchings M.D.T., Lewnard J.A., Dean N.E., et al. Use of recently vaccinated individuals to detect bias in test-negative case-control studies of COVID-19 vaccine effectiveness. Epidemiology. 2022;33:450–456. doi: 10.1097/EDE.0000000000001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.