Abstract
Background
Diabetic retinopathy is a common complication of diabetes and a leading cause of visual impairment and blindness. Research has established the importance of blood glucose control to prevent development and progression of the ocular complications of diabetes. Concurrent blood pressure control has been advocated for this purpose, but individual studies have reported varying conclusions regarding the effects of this intervention.
Objectives
To summarize the existing evidence regarding the effect of interventions to control blood pressure levels among diabetics on incidence and progression of diabetic retinopathy, preservation of visual acuity, adverse events, quality of life, and costs.
Search methods
We searched several electronic databases, including CENTRAL, and trial registries. We last searched the electronic databases on 3 September 2021. We also reviewed the reference lists of review articles and trial reports selected for inclusion.
Selection criteria
We included randomized controlled trials (RCTs) in which either type 1 or type 2 diabetic participants, with or without hypertension, were assigned randomly to more intense versus less intense blood pressure control; to blood pressure control versus usual care or no intervention on blood pressure (placebo); or to one class of antihypertensive medication versus another or placebo.
Data collection and analysis
Pairs of review authors independently reviewed the titles and abstracts of records identified by the electronic and manual searches and the full‐text reports of any records identified as potentially relevant. The included trials were independently assessed for risk of bias with respect to outcomes reported in this review.
Main results
We included 29 RCTs conducted in North America, Europe, Australia, Asia, Africa, and the Middle East that had enrolled a total of 4620 type 1 and 22,565 type 2 diabetic participants (sample sizes from 16 to 4477 participants). In all 7 RCTs for normotensive type 1 diabetic participants, 8 of 12 RCTs with normotensive type 2 diabetic participants, and 5 of 10 RCTs with hypertensive type 2 diabetic participants, one group was assigned to one or more antihypertensive agents and the control group to placebo. In the remaining 4 RCTs for normotensive participants with type 2 diabetes and 5 RCTs for hypertensive type 2 diabetic participants, methods of intense blood pressure control were compared to usual care. Eight trials were sponsored entirely and 10 trials partially by pharmaceutical companies; nine studies received support from other sources; and two studies did not report funding source.
Study designs, populations, interventions, lengths of follow‐up (range less than one year to nine years), and blood pressure targets varied among the included trials.
For primary review outcomes after five years of treatment and follow‐up, one of the seven trials for type 1 diabetics reported incidence of retinopathy and one trial reported progression of retinopathy; one trial reported a combined outcome of incidence and progression (as defined by study authors). Among normotensive type 2 diabetics, four of 12 trials reported incidence of diabetic retinopathy and two trials reported progression of retinopathy; two trials reported combined incidence and progression. Among hypertensive type 2 diabetics, six of the 10 trials reported incidence of diabetic retinopathy and two trials reported progression of retinopathy; five of the 10 trials reported combined incidence and progression.
The evidence supports an overall benefit of more intensive blood pressure intervention for five‐year incidence of diabetic retinopathy (11 studies; 4940 participants; risk ratio (RR) 0.82, 95% confidence interval (CI) 0.73 to 0.92; I2 = 15%; moderate certainty evidence) and the combined outcome of incidence and progression (8 studies; 6212 participants; RR 0.78, 95% CI 0.68 to 0.89; I2 = 42%; low certainty evidence). The available evidence did not support a benefit regarding five‐year progression of diabetic retinopathy (5 studies; 5144 participants; RR 0.94, 95% CI 0.78 to 1.12; I2 = 57%; moderate certainty evidence), incidence of proliferative diabetic retinopathy, clinically significant macular edema, or vitreous hemorrhage (9 studies; 8237 participants; RR 0.92, 95% CI 0.82 to 1.04; I2 = 31%; low certainty evidence), or loss of 3 or more lines on a visual acuity chart with a logMAR scale (2 studies; 2326 participants; RR 1.15, 95% CI 0.63 to 2.08; I2 = 90%; very low certainty evidence). Hypertensive type 2 diabetic participants realized more benefit from intense blood pressure control for three of the four outcomes concerning incidence and progression of diabetic retinopathy.
The adverse event reported most often (13 of 29 trials) was death, yielding an estimated RR 0.87 (95% CI 0.76 to 1.00; 13 studies; 13,979 participants; I2 = 0%; moderate certainty evidence). Hypotension was reported in two trials, with an RR of 2.04 (95% CI 1.63 to 2.55; 2 studies; 3323 participants; I2 = 37%; low certainty evidence), indicating an excess of hypotensive events among participants assigned to more intervention on blood pressure.
Authors' conclusions
Hypertension is a well‐known risk factor for several chronic conditions for which lowering blood pressure has proven to be beneficial. The available evidence supports a modest beneficial effect of intervention to reduce blood pressure with respect to preventing diabetic retinopathy for up to five years, particularly for hypertensive type 2 diabetics. However, there was a paucity of evidence to support such intervention to slow progression of diabetic retinopathy or to affect other outcomes considered in this review among normotensive diabetics. This weakens any conclusion regarding an overall benefit of intervening on blood pressure in diabetic patients without hypertension for the sole purpose of preventing diabetic retinopathy or avoiding the need for treatment for advanced stages of diabetic retinopathy.
Keywords: Humans; Antihypertensive Agents; Antihypertensive Agents/therapeutic use; Blood Pressure; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/complications; Diabetic Retinopathy; Diabetic Retinopathy/complications; Diabetic Retinopathy/epidemiology; Diabetic Retinopathy/prevention & control; Hypertension; Hypertension/complications; Hypertension/drug therapy; Macular Edema; Macular Edema/etiology; Randomized Controlled Trials as Topic
Plain language summary
Blood pressure control for diabetic retinopathy
Review question
Does blood pressure control prevent diabetic retinopathy or slow its progression?
Background
Diabetes is characterized by high levels of blood glucose (sugar circulating in the blood) and is classified as either type 1 or type 2, depending on the underlying cause of increased blood glucose. A common complication in people with diabetes is diabetic retinopathy, often called 'diabetic eye disease,' which affects the blood vessels in the back of the eye. Diabetic retinopathy is a major cause of poor vision and blindness worldwide among adults of working age. Control of blood glucose reduces the risk of diabetic retinopathy and prevents worsening of the condition once it develops. Simultaneous treatment to reduce blood pressure among diabetics has been suggested as another approach to reduce the risks of development and worsening of diabetic retinopathy below the risks achieved by blood glucose control.
Study characteristics
We found 29 randomized controlled trials (a type of study where participants are randomly assigned to one of two or more treatment groups), conducted primarily in North America and Europe, looking at the effects of several methods to lower blood pressure in 4620 type 1 and 22,565 type 2 diabetics, with 16 to 4477 participants in individual trials. The treatment and follow‐up periods in these trials ranged from less than one year to nine years. Eight trials were funded in full by one or more drug companies. Ten other trials had received drug company support, usually in the form of study medications. The remaining 11 studies were conducted with support from government‐sponsored grants and institutional support or did not report funding source. The evidence is current to September 2021.
Key results
Overall, the evidence from 19 trials in which participants were treated for 5 years or longer provided modest support for lowering blood pressure to prevent diabetic retinopathy. However, lowering blood pressure did not keep diabetic retinopathy from worsening once it had developed, or prevent advanced stages of diabetic retinopathy that required treatment of the affected eyes. The evidence favored control of blood pressure for hypertensive type 2 diabetics for more outcomes than favored blood pressure lowering among participants with normal blood pressure. Treatment to reduce the blood pressure of people with diabetes is warranted for other health reasons, but the available evidence does not justify reduction of blood pressure in diabetics with normal blood pressures solely to prevent or slow diabetic retinopathy.
Quality of the evidence
Overall, the quality of the evidence was low to moderate based on the reported information because some studies did not report all of their prespecified outcomes, and results from different studies were not always consistent.
Summary of findings
Summary of findings 1. Blood pressure intervention/intensive blood pressure intervention compared with placebo/standard blood pressure intervention for diabetic retinopathy.
| Blood pressure intervention/intensive blood pressure intervention compared with placebo/standard blood pressure intervention for diabetic retinopathy | |||||
|
Patient or population: type 1 or type 2 diabetics Settings: diabetes and ophthalmology clinics Intervention: blood pressure intervention/intensive blood pressure intervention (antihypertensive medication or intense antihypertensive medication, or intense lifestyle intervention and medication) Comparison: placebo/standard blood pressure intervention (antihypertensive medication) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with no (or less) control by type of diabetes | Risk with blood pressure intervention/intensive blood pressure intervention | ||||
| Incidence of retinopathy by 5 years | Type 1 normotensive | ||||
| 306 per 1000 | 251 per 1000 (173 to 243) | RR 0.82 (0.69 to 0.97) | 1421 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 | |
| Type 2 normotensive | |||||
| 290 per 1000 | 252 per 1000 (189 to 257) | RR 0.87 (0.75 to 1.02) | 1545 (4 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Type 2 hypertensive | |||||
| 209 per 1000 | 157 per 1000 (89 to 154) | RR 0.75 (0.57 to 0.98) | 1974 (6 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Overall | |||||
| 264 per 1000 | 216 per 1000 (193 to 243) | RR 0.82 (0.73 to 0.92) | 4940 (11 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Progression of retinopathy by 5 years | Type 1 normotensive | ||||
| 130 per 1000 | 134 per 1000 (110 to 173) | RR 1.03 (0.82 to 1.29) | 1905 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 | |
| Type 2 normotensive | |||||
| 237 per 1000 | 239 per 1000 (187 to 311) | RR 1.01 (0.78 to 1.30) | 2460 (2 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Type 2 hypertensive | |||||
| 192 per 1000 | 140 per 1000 (71 to 148) | RR 0.73 (0.51 to 1.06) | 779 (2 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Overall | |||||
| 191 per 1000 | 179 per 1000 (149 to 214) | RR 0.94 (0.78 to 1.12) | 5144 (5 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Combined incidence and progression of diabetic retinopathy by 5 years | Type 1 normotensive | ||||
| 378 per 1000 | 227 per 1000 (91 to 207) | RR 0.60 (0.40 to 0.91) | 223 (1 RCT) | ⊕⊕⊝⊝ LOW1,2 | |
| Type 2 normotensive | |||||
| 191 per 1000 | 174 per 1000 (101 to 251) | RR 0.91 (0.58 to 1.44) | 1743 (2 RCTs) | ⊕⊕⊝⊝ LOW1,2 | |
| Type 2 hypertensive | |||||
| 202 per 1000 | 156 per 1000 (104 to 139) | RR 0.77 (0.67 to 0.89) | 4246 (5 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Overall | |||||
| 203 per 1000 | 158 per 1000 (138 to 181) | RR 0.78 (0.68 to 0.89) | 6212 (8 RCTs) | ⊕⊕⊝⊝ LOW1,2 | |
| Incidence of PDR/CSME/VH by 5 years | Type 1 normotensive | ||||
| 105 per 1000 | 109 per 1000 (85 to 139) | RR 1.04 (0.81 to 1.33) | 2054 (2 RCTs) | ⊕⊕⊝⊝ LOW1,3 | |
| Type 2 normotensive | |||||
| 165 per 1000 | 164 per 1000 (137 to 194) | RR 0.99 (0.83 to 1.18) | 2460 (3 RCTs) | ⊕⊕⊝⊝ LOW1,3 | |
| Type 2 hypertensive | |||||
| 95 per 1000 | 73 per 1000 (73 to 90) | RR 0.77 (0.62 to 0.95) | 3723(4 RCTs) | ⊕⊕⊝⊝ LOW1,3 | |
| Overall | |||||
| 82 per 1000 | 75 per 1000 (67 to 85) | RR 0.92 (0.82 to 1.04) | 8237 (9 RCTs) | ⊕⊕⊝⊝ LOW1,3 | |
| Visual acuity (loss of 3 lines or more) by 5 years | 184 per 1000 | 211 per 1000 (116 to 382) | RR 1.15 (0.63 to 2.08) | 2326 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1,2,4 |
| Adverse events—all‐cause mortality | Type 1 normotensive | ||||
| 8 per 1000 | 10 per 1000 (6 to 25) | RR 1.16 (0.57 to 2.36) | 3608 (3 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Type 2 normotensive | |||||
| 57 per 1000 | 48 per 1000 (29 to 58) | RR 0.86 (0.62 to 1.19) | 2599 (3 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Type 2 hypertensive | |||||
| 76 per 1000 | 65 per 1000 (56 to 76) | RR 0.86 (0.74 to 1.01) | 7772 (7 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Overall | |||||
| 54 per 1000 | 45 per 1000 (39 to 53) | RR 0.87 (0.76 to 1.00) | 13,979 (13 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Adverse events—hypotension | 63 per 1000 | 130 per 1000 (103 to 162) | RR 2.04 (1.63 to 2.55) | 3323 (2 RCTs) | ⊕⊕⊝⊝ LOW1,2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CSME: clinically significant macular edema; PDR: proliferative diabetic retinopathy; RCT: randomized controlled trial; RR: risk ratio; VH: vitreous hemorrhage | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded for risk of bias (−1). 2Downgraded for imprecision due to wide confidence intervals (−1). 3Downgraded for heterogeneity (−1). 4Downgraded for inconsistency (−1).
Background
Description of the condition
Introduction and epidemiology
Diabetic retinopathy is a complex disorder of the retinal vasculature that is characterized by increased vascular permeability, retinal ischemia and edema, and new blood vessel formation. The National Eye Institute has reported age‐related macular degeneration, cataracts, glaucoma, and diabetic retinopathy to be the leading causes of visual impairment and blindness among Americans older than 40 years (EDPRG 2004a). Similar findings have been reported for older Americans over the age of 75 years (Desai 2001), and from other epidemiologic studies from Western Europe (Buch 2004; Grey 1989; Krumpaszky 1999; Rosenberg 1996).
Globally, diabetes mellitus is a significant public health problem. Some predictions estimate that the worldwide prevalence of diabetes will exceed 366 million people by 2030 (Wild 2004). Diabetic retinopathy is a common complication among individuals with diabetes and an important cause of loss of vision (Sivaprasad 2012). A diabetic individual has a three‐fold increased risk of blindness compared with the general population (Hayward 2002). The US Centers for Disease Control and Prevention estimates that 25.8 million people in the US were living with diabetes in 2010 (CDC 2011). In the US alone, it is estimated that 4.1 million adults over the age of 40 have diabetic retinopathy (any level of severity), and that 899,000 adults have vision‐threatening diabetic retinopathy (EDPRG 2004b). Among Americans with type 1 diabetes, the prevalence of diabetic retinopathy of any severity is 74.9% and 82.3% in black and white persons respectively; the prevalence of vision‐threatening (severe non‐proliferative and proliferative) retinopathy is 30% and 32.2% (EDPRG 2004c). The prevalence of diabetic retinopathy among type 1 and type 2 diabetics in Wales was recently reported as 56% and 30.3%, respectively (Thomas 2014). People with impaired visual acuity or legal blindness secondary to diabetic retinopathy face enormous challenges in pursuing activities of daily life. Visual impairment is defined as best‐corrected visual acuity worse than 20/40 in the better‐seeing eye; blindness is defined as best‐corrected visual acuity of 20/200 or worse in the better‐seeing eye as measured on the original Bailey‐Lovie or modified Bailey‐Lovie (also known as the Early Treatment Diabetic Retinopathy Study (ETDRS)) visual acuity chart or other charts that use a logMAR scale.
The duration of diabetes and the severity of hyperglycemia are major risk factors associated with the development (incidence) and progression of diabetic retinopathy (DCCT 1993; DRS10 1985; ETDRS18 1998; Harris 1998; Klein 1984a; Klein 1984b; Klein 1988; Krakoff 2003; Kullberg 2002; Leske 2003; Porta 2001; UKPDS33 1998; van Leiden 2003; Zhang 2001). After retinopathy develops, persistent hyperglycemia has been reported to be a more important factor than duration of diabetes for progression of the disease (ETDRS18 1998; Giuffre 2004).
The Diabetes Control and Complications Trial, DCCT, and the United Kingdom Prospective Diabetes Study (UKPDS) demonstrated lower incidence and slower progression of diabetic retinopathy with tight blood glucose control (DCCT 1993; UKPDS33 1998). The Diabetic Retinopathy Study, DRS2 1978; DRS5 1981; DRS8 1981, and the ETDRS, ETDRS1 1985; ETDRS9 1991, demonstrated a decrease in the progression of proliferative diabetic retinopathy and diabetic macular edema with more than 1200 applications of 'pan‐retinal' (for proliferative retinopathy) or with 'focal' (for macular edema) laser photocoagulation. However, the prevalence of diabetic retinopathy observed in recent epidemiologic studies conducted after the DCCT and UKPDS continued to be high (EDPRG 2004b; EDPRG 2004c). Recently, investigators participating in trials conducted by the Diabetic Retinopathy Clinical Research Network (DRCRnet) have reported that other treatments, either alone or in combination with laser treatment, can slow the progression of diabetic retinopathy (DRCR.net 2010). These studies strongly support treatment of diabetic retinopathy to reduce loss of vision. Nevertheless, findings from all studies reinforce the need to evaluate the role of risk factors and to intervene on those that are modifiable in order to decrease the prevalence and severity of diabetic retinopathy.
Risk factors that have been reported for diabetic retinopathy include hypertension (Klein 1989a; Klein 1989b; Leske 2003; Tapp 2003; UKPDS38 1998), hypercholesterolemia (Chew 1996; Klein 2002b; van Leiden 2002), abdominal obesity and elevated body mass index (van Leiden 2002; van Leiden 2003; Zhang 2001), alcohol intake (Giuffre 2004), younger age at onset (Krakoff 2003; Kullberg 2002; Porta 2001), smoking, and ancestry (Keen 2001; Moss 1996). Age and ancestry are not modifiable, but other risk factors suggest possible interventions.
Presentation and diagnosis
Diabetic retinopathy progresses sequentially from a mild non‐proliferative stage to a severe proliferative disorder. Increased retinal vascular permeability occurs early, at the stage of mild non‐proliferative diabetic retinopathy (NPDR). Moderate and severe NPDR are characterized by vascular closure, which results in impaired retinal perfusion (ischemia).
Diabetic retinopathy is typically diagnosed during ophthalmoscopy. Fundus photographs and fluorescein angiograms may be used to monitor progression. Signs of NPDR include microaneurysms, intraretinal hemorrhages, and occasional 'cotton wool spots' caused by closure of small retinal arterioles, resulting in localized ischemia and edema, with consequent damage to nerve fibers leading to reduced axonal transport. Signs of increasing ischemia include extensive intraretinal hemorrhages, venous abnormalities such as wide variations in caliber ('beading') and looping ('reduplication'), capillary non‐perfusion, and intraretinal microvascular abnormalities. Severe NPDR, also known as pre‐proliferative retinopathy, is diagnosed when these changes progress to predefined thresholds.
Proliferative diabetic retinopathy (PDR) is characterized by neovascularization, which is the growth of abnormal blood vessels in response to severe ischemia. The new vessels grow into the vitreous and are often seen at the optic disc (NVD) and elsewhere in the retina (NVE); they are prone to bleeding, which results in vitreous hemorrhage (VH) and vision loss. Furthermore, these vessels may undergo fibrosis and contraction and, along with other fibrous proliferation, may lead to epiretinal membrane formation, vitreoretinal traction bands, retinal tears, and either tractional or rhegmatogenous retinal detachments (i.e. those due to a retinal hole or tear). It is said that PDR is at the high‐risk stage when NVE that occupy a total area of 0.5 optic disc area or more in size throughout the retina are accompanied by pre‐retinal or vitreous hemorrhage, or when NVD occupy an area greater than or equal to about one‐third disc area, even in the absence of VH, or when NVD of any size are accompanied by VH. People in the 'high risk' stage of PDR who do not receive prompt pan‐retinal laser treatment have a 30% to 50% probability of progressing to severe visual acuity loss and blindness (less than 5/200 best‐corrected visual acuity) in three years (DRS8 1981; ETDRS10 1991; ETDRS12 1991).
Increased retinal vascular permeability, which can occur at any stage of diabetic retinopathy, may result in retinal thickening (edema) and lipid deposits (hard exudates). Retinal thickening, hard exudates, or both that occur at or within 500 microns (approximately one‐third an optic disc diameter) of the center of the macula, and which therefore threaten, or actually cause, loss of central visual acuity, are referred to as clinically significant macular edema.
The major reasons for vision loss in diabetic retinopathy include macular edema, macular capillary non‐perfusion (which can be demonstrated by fluorescein angiography), VH, distortion or tractional detachment of the retina (PPP 2019), and neovascular glaucoma (new blood vessels in the iris), which is usually associated with very late‐stage PDR (Fong 2004).
Pathogenesis
Several biochemical pathways have been investigated for the pathogenesis of diabetic retinopathy. Apart from the well‐documented role of chronic hyperglycemia, none of the other biochemical pathways has been shown conclusively to be relevant (Frank 2004).
Although the exact mechanism for the pathogenesis of hypertensive damage in eyes with diabetic retinopathy is unknown, scientists have hypothesized that an increase in blood pressure damages the retinal capillary endothelial cells (Klein 2002a). In eyes with diabetic retinopathy, chronic hyperglycemia leads to endothelial cell damage, pericyte loss, and breakdown of the blood‐retinal barrier. Such changes to the structure of the microvasculature lead to dysregulation of retinal perfusion, thereby making eyes with diabetic retinopathy more susceptible to hyperperfusion damage from hypertension (Gillow 1999).
Description of the intervention
The current standard of care for the prevention and treatment of diabetic retinopathy consists of strict glycemic control and regular ophthalmologic screening for diabetic retinopathy among diabetics, the use of focal laser treatment or intravitreal anti‐vascular endothelial growth factor injections for diabetic macular edema, and the use of pan‐retinal scatter laser photocoagulation for PDR (Smith 2011; Virgili 2014). Strict blood pressure control has been recommended as part of the standard of care for diabetics, because of its known beneficial effect on the prevention of cardiovascular events, stroke, and nephropathy rather than for its effect on diabetic retinopathy (Hansson 1998; HOPESI 2000).
How the intervention might work
Blood pressure control may be beneficial in preventing the development or slowing the progression of diabetic retinopathy by reducing the damage to endothelial cells, blood vessels, and surrounding tissues from hyperperfusion. Diabetic retinopathy leads to endothelial cell dysfunction, loss of pericytes, and breakdown of the blood‐retinal barrier. Hypertension may cause additional vascular damage because of shearing that occurs with hyperperfusion. Blood pressure control may prevent hyperperfusion and decrease the likelihood of shearing damage to the blood vessels from hypertension.
Why it is important to do this review
Diabetic retinopathy remains an important cause of vision loss even with good blood glucose control (ADA 1998; Ferris 1993). At the end of the DCCT, with participant follow‐up of 6.5 ± 1.6 years (mean ± standard error), 10% of type 1 diabetic patients in the intensive glycemic control group had developed diabetic retinopathy despite strict glycemic control (Zhang 2001). Similarly, in the UKPDS, tight blood glucose control decreased but did not eliminate the risk of diabetic retinopathy (UKPDS33 1998). Diabetic retinopathy is a substantial public health problem (Zhang 2010). Because studies of retinal physiology suggest a role for blood pressure in pathological changes in diabetic retinopathy (Sjølie 2011), a systematic review of the effectiveness of blood pressure control with respect to diabetic retinopathy was proposed (Sleilati 2009).
The beneficial health effects on multiple organ systems of control of blood pressure among people with hypertension, including those with diabetes mellitus, has been well‐established during decades of randomized trials. The effects regarding diabetic retinopathy and complications were addressed in the original version of this review (Do 2015a); however, there was limited evidence of effects from randomized trials in which participants had been followed for five years or longer. This update includes several studies that published findings after the original systematic review was completed. The effects of interventions to lower blood pressure among individuals who already have normal or near normal blood pressure and diabetes mellitus were certain. It was thus important to re‐examine the evidence of the effects of interventions to control blood pressure on diabetic retinopathy that has accrued to date.
Objectives
The primary aim of this review was to summarize the existing evidence regarding the effect of interventions to control blood pressure levels among diabetics on incidence and progression of diabetic retinopathy, preservation of visual acuity, adverse events, quality of life, and costs.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs).
Types of participants
We included RCTs in which participants had a diagnosis of either type 1 or type 2 diabetes, irrespective of age, gender, ethnicity, ancestry, status regarding blood pressure or its treatment, or diabetic retinopathy status.
Types of interventions
We included trials in which:
participants assigned to more intense blood pressure control, alone or in combination with other interventions, were compared with participants assigned to less intense blood pressure control;
participants assigned to blood pressure control were compared with participants assigned to usual care or no intervention on blood pressure (placebo);
participants assigned to antihypertensive agents versus placebo;
participants assigned to treatment with one class of antihypertensive agent were compared with participants assigned to another class of antihypertensive agent.
Types of outcome measures
Primary outcomes
Incidence of retinopathy, defined as mild non‐proliferative or more severe diabetic retinopathy, i.e. a retinopathy score on the Early Treatment Diabetic Retinopathy Study (ETDRS) final scale of 35 or greater based on evaluation of stereoscopic color fundus photographs of eyes of participants who did not have retinopathy at baseline (ETDRS10 1991).
Progression of retinopathy, defined as a two‐step or greater progression from baseline on the ETDRS final scale based on evaluation of stereoscopic color fundus photographs of eyes of participants who had diabetic retinopathy at baseline (ETDRS10 1991).
We post hoc added a composite outcome of incidence or progression of retinopathy as reported by several included studies. Some trials used other methods or scales to define retinopathy and its progression; we assessed comparability of other scales with the ETDRS scale and classified changes similar to those measured on the ETDS scale with those given above.
We placed no restrictions on study selection based on the length of follow‐up of participants for primary or secondary outcomes. However, we judged follow‐up for less than one year to be inadequate for the outcomes relevant to this review because of the rate of development and progression of diabetic retinopathy and the time required for antihypertensive agents to affect the microvasculature.
We specified five years as the primary outcome period of interest in the protocol for this review; however, few trials reported outcomes for this exact time interval. We thus analyzed and reported the primary outcomes reported for mean participant follow‐up of four to six years after enrollment to estimate five‐year outcomes. We also analyzed incidence and progression of retinopathy reported at shorter (less than three years) and longer (seven or more years) follow‐up times from a few trials.
Secondary outcomes
We assessed the secondary outcomes at follow‐up times as reported above.
Post hoc: incidence of proliferative diabetic retinopathy (PDR), clinically significant macular edema (CSME), or vitreous hemorrhage (VH) using the criteria described in the included trials.
Decrease in visual acuity by 3 or more lines in both eyes on a logMAR chart. A 3‐line decrease corresponds to a doubling of the minimum angle of resolution.
We also compared classes of antihypertensive medications with respect to these same primary and secondary outcomes.
Adverse effects
We summarized adverse effects related to blood pressure control as reported by the included studies, with a focus on death, systemic effects such as hypotension or hyperkalemia, and adverse ocular events. We did not conduct a search for adverse events reported in RCTs that did not report retinopathy outcomes or in non‐randomized studies (not included in this review).
Quality of life
We summarized vision‐related quality of life data reported by the included studies, as measured using the National Eye Institute Visual Functioning Questionnaire 25, Mangione 2001, or another validated vision‐related scale. When sufficient data become available, comparisons of scores between treatment groups may be of interest.
Economic data
We summarized cost or cost‐effectiveness data reported by the included trials. When sufficient data become available, there may be interest in comparisons of costs of different treatment strategies that yield similar benefits with respect to retinopathy outcomes.
Search methods for identification of studies
Electronic searches
To identify potentially eligible studies for the current version of this review, the Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs. There were no language or publication year restrictions. The electronic databases were last searched on 3 September 2021.
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 9) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 3 September 2021) (Appendix 1).
MEDLINE Ovid (1946 to 3 September 2021) (Appendix 2).
Embase.com (1947 to 3 September 2021) (Appendix 3).
Latin American and Caribbean Health Sciences Literature database (LILACS) (1982 to 3 September 2021) (Appendix 4).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/; searched 3 September 2021) (Appendix 5).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/clinical-trials-registry-platform; searched 3 September 2021) (Appendix 6).
Searching other resources
We searched the reference lists of reports from the included trials and related reviews to find additional potentially eligible studies. We did not conduct manual searches of conference abstracts for this review.
Data collection and analysis
Selection of studies
Pairs of review authors independently assessed the titles and abstracts of all records identified by the electronic and manual searches. We classified each record as 'definitely relevant,' 'possibly relevant,' or 'definitely not relevant.' We obtained the full‐text reports corresponding to records classified as 'definitely relevant' or 'possibly relevant' by at least one review author. Two review authors independently classified studies based on descriptions in the full‐text reports as 'include,' 'exclude,' 'uncertain,' or 'ongoing.' Any disagreements were resolved through discussion. For reports classified as 'uncertain' or 'ongoing,' that is those where information was unclear or insufficient to permit classification, we contacted the trial investigators for additional information or for clarification. We documented studies labeled as 'exclude' and the reasons for their exclusion in the Characteristics of excluded studies table; studies labeled as 'ongoing' in the Characteristics of ongoing studies table; and studies with insufficient publicly available information in the Characteristics of studies awaiting classification table. For reports in languages not read by the review authors, we consulted with translators to assist with screening for eligibility; no full‐text translations were required.
Data extraction and management
Two review authors independently extracted data describing study and participant characteristics, data sufficient to judge risk of bias, and data needed to record outcomes from the included trials onto data collection forms developed by Cochrane Eyes and Vision. We extracted details of study methods, participants, interventions, and outcomes from all included trials. We recorded cost, quality of life, and adverse outcome data when reported. Any discrepancies were resolved through discussion or through consultation with a third review author when members of a pair disagreed. We contacted primary investigators to obtain outcome data not reported. One review author entered data into Review Manager 5 (Review Manager 2020); a second review author verified the entries. We followed the same procedures when we updated the review.
One review author made a final check of the review in June 2014 to confirm that all extracted data had been entered into Review Manager 5 and that entries agreed with the full‐text reports and supplemental information provided by study investigators. During that process, we found additional data regarding outcomes for a few studies. The review author extracted the newly found data; a second review author confirmed all data extracted, entered the data into Review Manager 5, and updated the analyses with the added data included in the original review.
Assessment of risk of bias in included studies
Pairs of review authors independently assessed the included trials for risk of bias according to methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed risk of bias for the following domains.
Generation of allocation sequence
Allocation concealment before randomization
Masking of caregivers, participants, and outcome assessors, separately for primary and secondary outcomes of this review
Methods used to address incomplete or missing outcome data, separately for primary and secondary outcomes of this review
Selective outcome reporting
Other sources of bias
We classified each trial as having 'low,' 'high,' or 'unclear' risk of bias with respect to each domain.
We contacted trial investigators for clarification whenever information relevant to the risk of bias domains was reported unclearly or was missing from the published reports. We attempted to assess evidence of reporting bias by comparing protocols and publications on trial design, where available, with publications of results. We were able to access protocols for only a few of the trials included in the review. For trials without a publicly available protocol, we assessed reporting bias by comparing the results section and the methods sections of published trial reports. Again, any disagreements were resolved through discussion.
Measures of treatment effect
Because all outcomes considered for this review were dichotomous, we estimated and reported risk ratios (RRs) with 95% confidence intervals (CIs) for primary and secondary outcomes (incidence of retinopathy, progression of retinopathy, combined incidence and progression of retinopathy, visual acuity decrease by 3 or more lines, and progression to PDR, CSME, or VH) and for adverse events. Whenever the 95% CI for an RR did not include 1, we interpreted the comparison as yielding a statistically significant result despite the many comparisons reported in this review. We did not estimate treatment effects for quality of life outcomes or costs, but rather summarized these data by study and treatment group as reported by the trial investigators.
Unit of analysis issues
The unit of analysis was the individual trial participant.
Dealing with missing data
We contacted study investigators for missing information or for clarification as needed. When there was no response, we used the available data. We did not include studies that had reported no data for diabetic retinopathy outcomes. We did not impute data for the purposes of this review.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity across included trials using participant characteristics, details of interventions, duration of follow‐up, risk of bias, and definitions of outcomes. We examined statistical heterogeneity using the Chi2 test and I2 values; we considered an I2 greater than 50% as indicative of substantial statistical heterogeneity. We also considered consistency among estimates with respect to direction and magnitude and the extent of overlap among confidence intervals from individual studies.
Assessment of reporting biases
For selective outcome reporting, we compared the protocols of studies, when available, with the primary published report. We compared the outcomes specified in the methods section and the outcomes reported in the results section of published reports when a protocol was not available. We planned to use asymmetry of funnel plots as an indicator of potential publication bias; however, fewer than 10 studies reported most individual outcomes, thereby reducing the utility of this approach. In future updates when meta‐analyses include 10 or more studies, we plan to assess potential publication bias by examining funnel plots.
Data synthesis
Due to methodological, clinical, and statistical heterogeneity among included trials, we used a random‐effects model in meta‐analyses to estimate five‐year outcomes. We based meta‐analyses of outcomes on a fixed‐effect model whenever data were available from only two studies.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses according to baseline level of metabolic control, as determined using glycated hemoglobin, and baseline severity of diabetic retinopathy whenever sufficient data were available. However, none of the publications from the trials reported outcomes by baseline glycated hemoglobin levels or baseline retinopathy. Post hoc, but before retrieval of full‐text reports of studies identified as 'include' or 'uncertain,' we agreed to present outcomes separately for trials that enrolled participants with type 1 and type 2 diabetes because of the different etiologies and characteristics of patients with these conditions. We also decided to present outcomes separately for participants who were hypertensive at baseline for comparison with those who were normotensive or whose hypertension was controlled by treatment at baseline because of potential differences in benefits versus risks of intervention on blood pressure in these subgroups.
Sensitivity analysis
We planned to conduct sensitivity analyses to determine the impact of exclusion of studies with high risk of attrition bias, unpublished studies, and industry‐funded studies. We did not conduct any of the prespecified sensitivity analyses as it was not possible to assess attrition in all included studies; many studies had industry support; and no data were included from unpublished studies.
Summary of findings and assessment of the certainty of the evidence
We prepared a summary of findings table and assessed the overall certainty of the evidence for each outcome using the GRADE approach, which considers five factors (study limitations, indirectness of evidence, inconsistency of results, imprecision, and publication bias). We included the following outcomes at five years in the summary of findings table.
Incidence of retinopathy, defined as mild non‐proliferative or more severe diabetic retinopathy, i.e. a retinopathy score on the ETDRS final scale of 35 or greater based on evaluation of stereoscopic color fundus photographs of eyes of participants who did not have retinopathy at baseline (ETDRS10 1991). Otherwise, we incorporated data based on the methods used by study investigators.
Progression of retinopathy, defined as a two‐step or greater progression from baseline on the ETDRS final scale based on evaluation of stereoscopic color fundus photographs of eyes of participants who had diabetic retinopathy at baseline (ETDRS10 1991).
Combined incidence and progression of diabetic retinopathy based on reports from individual studies, many of which did not report separately on incidence and progression.
Decrease in visual acuity by 3 or more lines in both eyes on a logMAR chart or as reported. A 3‐line decrease (logMAR change of 0.3) corresponds to a doubling of the minimum angle of resolution.
Incidence of PDR, CSME, or VH using the criteria described in the included trials.
Adverse effects: death.
Results
Description of studies
Results of the search
The electronic database searches for the previous version of the review as of 25 April 2014 yielded 15 included RCTs (62 reports), three ongoing studies, and three studies awaiting classification (Do 2015a). We performed three updated searches on 7 June 2019, 21 May 2020, and 3 September 2021; we retrieved a total of 8222 records after removal of duplicates. In total, we screened 8222 records of which 8187 were excluded. We screened 35 full‐text reports of which 21 were excluded; the reasons for exclusion of full‐text reports are provided in the Characteristics of excluded studies table.
In this update, we included four trials that had been excluded from the original review, and two more trials that were awaiting assessment at the time of the original review and from which outcomes subsequently had been published (Figure 1). Overall, we included 85 reports (29 RCTs) and excluded 25 reports. We continue to await classification of one trial from the previous version of the review pending receipt of information from a study investigator (ABCD‐2V (2)).
1.
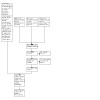
Study flow diagram.
Included studies
A detailed description of each individual included trial is provided in the Characteristics of included studies table. To facilitate comparisons of trials, we subgrouped them into those that enrolled only participants who had type 1 diabetes mellitus; those that enrolled only participants who had type 2 diabetes mellitus and were defined by the study investigators either as normotensive or blood pressure controlled by medication; or those that enrolled only participants who had type 2 diabetes mellitus and were defined as hypertensive by the study investigators. The characteristics of study participants and the design features of individual studies within these subgroups are summarized for comparison in Table 2 and Table 3, Table 4 and Table 5, and Table 6 and Table 7, respectively, with studies in each subgroup ordered by year of initiation or year of the earliest report from the trial.
1. Baseline characteristics of type 1 diabetic participants, by date trial was initiated.
| Characteristic | Larsen | Chase | EUCLID | DIRECT | RASS* | AdDIT | |
| DIRECT Prevent 1 | DIRECT Protect 1 | ||||||
| Number randomized | 20 | 16 | 530 | 1421 | 1905 | 285 | 443 |
| Age, years (mean) | 30 | 21 | 34 | 30 | 32 | 30 | 12.4 |
| Women, % | 20 | 25 | 35 | 43 | 42 | 54 | 46 |
| White, % | na | na | na | 97 | 98 | 98 | na |
| Diabetes duration, years (mean) | 18 | 14.1 | 14.5 | 6.7 | 11.0 | 11.2 | 5 |
| Current smoker, % | na | na | na | 26 | 26 | na | 0.7 |
| Systolic blood pressure, mmHg (mean) | 127 | 115 | 123 | 116 | 117 | 120 | 116 |
| Diastolic blood pressure, mmHg (mean) | 79 | 78 | 81 | 72 | 74 | 70 | 66 |
| Glycated hemoglobin (mean) | 9.2 | 8.4 | 7.1 | 8.1 | 8.5 | 8.5 | 8.4 |
| Body mass index, kg/m2 (mean) | na | na | 24.7 | 24.0 | 24.6 | 25.7 | 21.3 |
| Number assessed for diabetic retinopathy | 20 | 16 | 354 | 1421 | 1905 | 223 | 443 |
| ≤ 20/20 or microaneurysms only, % | 100 | 44 | 38 | 100 | 49 | 34 | 100 |
| 31 to 37, mild NPDR, % | 0 | 25 | 41 | 0 | 42 | 58 | 0 |
| 41 to 53, moderate/severe NPDR, % | 0 | 25 | 11 | 0 | 9 | 9 | 0 |
| > 53, PDR or PRP, % | 0 | 6 | 8 | 0 | 0 | 0 | 0 |
*Data shown for RASS are for participants analyzed for retinopathy outcomes.
na, information not found in study publications NPDR, non‐proliferative diabetic retinopathy PDR, proliferative diabetic retinopathy PRP, panretinal photocoagulation
2. Design features of included trials for type 1 diabetics, by date trial initiated or earliest publication.
| Design feature | Larsen | Chase | EUCLID | DIRECT | RASS | AdDIT | |
| DIRECT Prevent 1 | DIRECT Protect 1 | ||||||
| Year trial initiated | 1985 | 1993a | 1997a | 2001 | 2001 | 2002a | 2009 |
|
Definition of "hypertension," SBP/DBP, mmHg |
> 150/90 | ≥ 141/90 | > 155/90 | > 130/85 | > 130/85 | > 135/85 or antihypertensive medication | na |
| Primary outcome, parent trial | Blood‐retinal barrier leakage |
AER | AER | Diabetic retinopathy | Diabetic retinopathy | Nephropathy |
ACR |
| Type of interventions: | |||||||
| More control | ACEi | ACEi | ACEi | ARB | ARB | ARB or ACEi | ACEi |
| Less control | None | Placebo | Placebo | Placebo | Placebo | Placebo | Placebo |
| Blood pressure target: | |||||||
| More control | Reduce MABP by ≥ 5 mmHg | None | DBP < 75 mmHg | None | None | < 130/80, mmHg | na |
| Less control | None | None | DBP < 75 mmHg | None | None | < 130/80, mmHg | na |
| Other randomized intervention(s) | None | None | None | None | None | None | Statin vs placebo |
| Method of DR diagnosis/classification | Photos read by masked observer. | Fundus exam + photos read by ophthalmologist. | Photos read centrally.b | Photos read centrally.c | Photos read centrally.c | Photos read centrally.d | Photos read centrally. |
aDate of earliest publication. bAarhus, Denmark. cImperial College (London) Retinopathy Grading Centre. dWisconsin Ocular Epidemiology Reading Center.
ACEi, angiotensin converting enzyme inhibitor ACR, albumin/creatinine ratio AER, albumin ejection rate ARB, angiotensin receptor blocker DBP, diastolic blood pressure, mmHg DR, diabetic retinopathy MABP, mean arterial blood pressure, mmHg na, information not found in study publications NPDR, non‐proliferative diabetic retinopathy SBP, systolic blood pressure, mmHg
3. Baseline characteristics of normotensive or treated hypertensive type 2 diabetic participants, by date trial initiated or earliest publication.
| Characteristic | ABCD (1) | Ravid 1993 | Medi‐Cal | ABCD‐2V (1) | Pradhan | ACCORD Eye | DIRECT Protect 2 | J‐EDIT | Knudsen | ROADMAP | Wang | Zhao |
| Number randomized | 480 | 108 | 358 | 129 | 40 | 1590 | 1905 | 1173 | 24 | 4477 | 317 | 224 |
| Age, years (mean) | 59 | 44 | 55 | 56 | 51 | 61 | 57 | 72 | 61 | 58 | 64 | 66 |
| Women, % | 46 | 55 | 72 | 32 | 54 | 46 | 50 | 54 | 42 | 51 | 44 | 65 |
| White, % | 74 | na | 42 | 75 | na | 69 | 96 | 0 | 100 | 100 | 0 | 0 |
| DM duration, years (mean) | 9 | 6.8 | 9.7 | 7.6 | 11 | 10 | 8.8 | 17 | 11 | 6.3 | 10.5 | 7.2 |
| Current smoker, % | 13 | na | 14 | 8 | na | 13 | 15 | 54 | 29 | 16 | na | 8 |
| SBP, mmHg (mean) | 136 | na | 135 | 140 | na | 138 | 133 | 137 | 142 | 137 | 127 | 128 |
| DBP, mmHg (mean) | 84 | na | 78 | 81 | na | 76 | 78 | 76 | 84 | 80 | 77 | 77 |
| Glycated hemoglobin (mean) | 11.6 | 10.4 | 9.5 | 8.4 | 10.6 | 8.3 | 8.2 | 8.4 | 8.4 | 7.6 | 8.2 | 7.6 |
| Body mass index (mean) | 31.5 | 24.4 | 32.3 | 32.0 | 26.9 | 32.4 | 29.4 | 24.2 | 28.9 | 30.7 | 24.3 | 25.2 |
| Number assessed for DR | 463 | 94 | 200 | 119 | 40 | 1261 | 1905 | 940 | 24 | 1758 | 317 | 168 |
| ≤ 20/20 or microaneurysms only, % | 50 | na | 55 | na | 0 | 49 | 28 | 53 | 0 | na | 44 | na |
| 31 to 37, mild NPDR, % | 46 | na | 38 | 42 | 0 | 18 | 54 | 31 | 16 | na | 31 | na |
| 41 to 53, moderate/severe NPDR, % | na | 100 | 36 | 17 | 8 | 84 | na | 26 | na | |||
| > 53, PDR or PRP, % | 4 | na | 7 | 1 | 0 | 2 | 0 | 7 | 0 | na | 0 | na |
| Myocardial infarction, % | 24 | na | na | 2.4 | na | na | 5.2 | 0 | na | 5.7 | na | na |
| Stroke, % | 3.5 | na | na | 0.8 | na | na | 1.4 | 0 | na | 2.3 | na | na |
DBP, diastolic blood pressure, mmHg DM, diabetes mellitus DR, diabetic retinopathy na, information not found in study publications NPDR, non‐proliferative diabetic retinopathy PDR, proliferative diabetic retinopathy PRP, panretinal photocoagulation SBP, systolic blood pressure, mmHg
4. Design features of trials for normotensive or treated hypertensive type 2 diabetic participants, by date initiated or earliest publication.
| Characteristic | ABCD (1) | Ravid 1993 | Medi‐Cal | ABCD‐2V (1) | Pradhan | ACCORD Eye | DIRECT Protect 2 | J‐EDIT | Knudsen | ROADMAP | Wang | Zhao |
| Year trial initiated | 1991 | 1993 | 1995 | 1997 | 1997 | 2001 | 2001 | 2001 | 2003 | 2004 | 2008 | 2008 |
|
Definition of "hypertension," SBP/DBP, mmHg or other |
DBP > 90, not on medication | > 150/90 |
> 135/85 | SBP ≥ 140, DBP ≥ 90, or on medication |
> 140/90 | 1x SBP ≥ 160 or 2x ≥ 140 |
≥ 130/85, ≥ 160/90 on medication |
> 130/85 | na | ≥ 130/80 | na | ≥ 130/80 |
| Primary outcome, parent study | 24‐hour creatinine clearance | Proteinuria, kidney function |
Glycated hemoglobin | 24‐hour creatinine clearance, UAE | DR | Death, MI, stroke | DR | CVD, DM complications | Diabetic maculopathy | ACR | DR | Composite (death, CVD, stroke, retinopathy, etc.) |
| Type of intervention: | ||||||||||||
| More BP control | ACEi or CCA | ACEi | Monthly case management | ARB + | ACEi | Antihypertensive medications by algorithm | ARB | Intense multifactorial intervention.a |
ARB | ARB | ACEi | Intensive management (monthly) |
| Less BP control | Placebo | Placebo | Routine care | Placebo + | Multivitamin | Antihypertensive medications by algorithm | Placebo | Conventional management | Placebo | Placebo | Placebo | Standard care |
| Blood pressure target: | ||||||||||||
| More BP control | DBP decrease ≥ 10 mmHg | < 145/95 mmHg | ≤ 135/85 mmHg | DBP ≤ 75 mmHg | na | SBP < 120 mmHg | na | < 130/85 mmHg | na | < 130/80 mmHg | na | < 130/80 mmHg |
| Less BP control | DBP 80 to 89 mmHg |
< 145/95 mmHg | ≤ 135/85 mmHg | DBP 80 to 89 mmHg |
na | SBP 130 to 139 mmHg |
na | None | na | < 140/90 mmHg | na | < 130/80 mmHg |
| Other randomized intervention(s) | None | None | None | None | None | Glycemic control | None | None | None | None | None | None |
| Method of DR diagnosis | Photos graded centrally.b | Fundus exams |
Photos graded centrally.c |
na | Fundus exams + photos | Photos graded centrally.b | Photos graded centrally.d | Fundus exams + photos | OCTs photos, FAs, by 2 examiners | PRP, vitreous heme | Photos + OCT images | Unclear |
aBody mass index, glycated hemoglobin, triglycerides, high‐density lipoprotein (HDL) cholesterol, total cholesterol. bWisconsin Fudus Photograph Reading Center. c"Santa Barbara", one of the study clinics. dImperial College Retinopathy Grading Centre.
ACEi, angiotensin converting enzyme inhibitor ARB, angiotensin II receptor blocker BP, blood pressure CCA, calcium channel antagonist CVD, cardiovascular disease DBP, diastolic blood pressure, mmHg DM, diabetes mellitus DR, diabetic retinopathy FA, fluorescein angiography na, information not found in study publications OCT, ophthalmic computed tomography PRP, panretinal photocoagulation SBP, systolic blood pressure, mmHg UAE, urinary albumin excretion
5. Baseline characteristics of hypertensive type 2 diabetic participants in included trials, by date trial initiated or earliest publication.
| Characteristic | UKPDS/HDS | ABCD (2) | Steno‐2 | Rachmani 2002 | ADDITION‐Europe | ADVANCE/AdRem | DEMAND | BENEDICT | HINTS | J‐DOIT3 |
| Number randomized | 1148 | 470 | 160 | 141 | 3057 | 2130 | 380 | 1209 | 503 | 2542 |
| Mean age, years | 56 | 58 | 55 | 57 | 60 | 66 | 61 | 62 | 64 | 59 |
| Women, % | 45 | 33 | 26 | 51 | 42 | 39 | 35 | 46 | 5 | 38 |
| White, % | 87 | 66 | na | na | 94 | 48 | na | 100 | 47 | na |
| Mean DM duration, years | 2.6 | 8.6 | 5.8 | 6.2 | 0 | 6.0 | 5.8 | 7.4 | na | 8.6 |
| Current smoker, % | 23 | na | 30 | 0 | 27 | 14 | 13 | 10 | 21 | 23 |
| SBP, mmHg (mean) | 160 | 155 | 148 | 161 | 149 | 143 | 147 | 152 | 145 | 134 |
| DBP, mmHg (mean) | 94 | 98 | 86 | 96 | 86 | 79 | 88 | 89 | na | 80 |
| Glycated hemoglobin (mean) | 6.9 | 11.6 | 8.6 | 9.6 | 7.0 | 7.4 | 5.8 | 5.9 | na | 8.0 |
| Body mass index (mean) | 29.6 | 31.8 | 29.9 | 28.6 | 31.6 | 27.7 | 29.6 | 28.7 | 30.4 | 24.8 |
| Number assessed for DR | 929 | 470 | 160 | 129 | 2190 | 1602 | 237 | 550 | 194 | 2540 |
| ≤ 20/20 or microaneurysms only, % | 67 | 40 | 73 | 15 | na | 82 | 81 | 84 | 70 | na |
| 31 to 37, mild NPDR, % | 25 | 55 | 21 | na | 8 | 19 | 15 | 30 | na | |
| 41 to 53, moderate/severe NPDR, % | 8 | na | 8 | na | ||||||
| > 53, PDR or PRP, % | 5 | 6 | na | 1 | 1 | na | ||||
| Myocardial infarction, % | 0 | 0 | na | 0 | 6.4 | 12 | na | 0 | na | na |
| Stroke, % | 0 | 0 | na | 0 | 2.4 | 9 | na | 0 | na | na |
DBP, diastolic blood pressure, mmHg DM, diabetes mellitus DR, diabetic retinopathy na, information not found in study publications NPDR, non‐proliferative diabetic retinopathy PDR, proliferative diabetic retinopathy PRP, panretinal photocoagulation SBP, systolic blood pressure, mmHg
6. Design features of trials for hypertensive type 2 diabetic participants, by date trial initiated or earliest publication.
| Characteristic | UKPDS/HDS | ABCD (2) | Steno‐2 | Rachmani 2002 | ADDITION‐Europe | ADVANCE/AdRem | DEMAND | BENEDICT | HINTS | J‐DOIT3 |
| Year trial initiated | 1987 | 1991 | 1992 | 1995 | 2001 | 2002 | 2002 | 2003a | 2006 | 2006 |
|
Definition of "hypertension", SBP/DBP, mmHg, or otherb |
≥ 160/95 or antihypertensive medication | DBP ≥ 90 | ≥ 160/95 (≥ 135/85) |
> 140/90 or antihypertensive medication | > 135/85 | > 140/90 or antihypertensive medication | ≥ 130/85 or antihypertensive medication | na | > 140/90 or antihypertensive medication | > 130/80 |
| Primary outcome, parent study | Macro‐ and microvascular complications | 24‐hour creatinine clearance | Nephropathy | eGFR | Death, CVD events | Macro‐ and microvascular complications | GFR | Microalbuminuria | BP control | Macrovascular diabetic complications |
| Type of intervention: | ||||||||||
| More BP control | ACEi or β blocker |
ACEi or CCA | Intensive intervention |
Patient participation in education and care | Stepped antihypertensive medication | ACEi or diuretic | ACEi or CCB | ACEi, ndCCB, or combination | Nurse management |
"Intensive intervention" |
| Less BP control | No ACEi or β blocker |
Placebo | Conventional treatment | Standard care | Routine care | Placebo | CCB or placebo | Placebo | Usual care | Conventional intervention |
| Blood pressure target: | ||||||||||
| More BP control | < 150/85 mmHg | DBP < 75 mmHg | < 140/85 (< 130/80c) |
< 130/85 mmHg | ≤ 120/80 mmHg | na | < 120/80 mmHg | < 120/80 mmHg | ≤ 140/80 mmHg | ≤ 120/75 mmHg |
| Less BP control | < 180/105 mmHg | DBP 80 to 89 mmHg | < 160/95 (< 135/85c) |
na | ≤ 135/85 mmHg | na | < 120/80 mmHg | < 120/80 mmHg | ≤ 140/80 mmHg | ≤ 130/80 mmHg |
| Other randomized intervention(s) | Intense glucose control vs standard | None | None | None | Intense glucose and cholesterol control vs standard | Intense glucose control vs standard | None | ndCCB vs placebo | None | None |
| Method of DR diagnosis | Photos graded centrally. | Photos graded centrally.d | Photos graded centrally.e | Fundus exams | Photos graded centrally.e |
Photos graded centrally.f | Fundus exams + photos | Fundus exams + photos | Diagnosis in electronic chart | Fundus exams + records |
aDate of earliest publication. bAs defined by investigators. cFrom 2000. dWisconsin Fundus Photograph Reading Center. eBaseline photos taken up to 3 months before/after randomization. fAdRem Sub‐study Coordination Centre, Utrecht, the Netherlands.
ACEi, angiotensin converting enzyme inhibitor BP, blood pressure (systolic/diastolic) or other CCA, calcium channel antagonist CCB, calcium channel blocker CVD, cardiovascular disease DBP, diastolic blood pressure, mmHg DR, diabetic retinopathy eGFR, estimated glomerular filtration rate GFR, glomerular filtration rate na, information not found in study publications ndCCBs, non‐dihydropyridine calcium channel blockers SBP, systolic blood pressure, mmHg
Study design
The included trials were conducted in North America, Europe, the Middle East, Asia, Africa, and Australia. The trials varied in size: the largest trial randomized 4477 participants (ROADMAP), but only 1758 were analyzed for diabetic retinopathy outcomes; the next‐largest trial enrolled 3057 participants (ADDITION‐Europe). The smallest trial enrolled 16 participants (Chase). In total, 4620 participants with type 1 diabetes mellitus and 22,565 participants with type 2 diabetes mellitus enrolled in the included trials. Among the trial participants, 4382 type 1 and 16,290 type 2 diabetics had baseline ophthalmic evaluations (Table 2; Table 4; Table 6). Most trials reported diabetic retinopathy outcomes only for participants who had both baseline and follow‐up photographs or examinations. Three trials reported findings from randomized participants at a subset of participating centers where baseline and follow‐up retinal examinations were performed (BENEDICT; DEMAND; Medi‐Cal). Two trials analyzed a subset of participants randomized in a larger parent study who agreed to participate in additional retinal examinations (ACCORD Eye; ADVANCE/AdRem); one study added a trial of blood pressure control during the course of the larger trial (UKPDS/HDS). Three trials were conducted within the Appropriate Blood Pressure Control in Diabetes Trial (ABCD), two for initially normotensive participants, ABCD (1); ABCD‐2V (1), and one for hypertensive participants (ABCD (2)). The ABCD trials and three other trials conducted within another parent study, DIRECT Prevent 1; DIRECT Protect 1; DIRECT Protect 2, were designed, conducted, analyzed, and published as three separate RCTs. Length of study intervention and follow‐up varied among included studies, from less than one year, Knudsen; Pradhan, to nine or more years, UKPDS/HDS; Zhao.
Study participants
Type 1 diabetes
Among the seven trials (4620 participants) that reported outcome data for type 1 diabetics (AdDIT; Chase; DIRECT Prevent 1; DIRECT Protect 1; EUCLID; Larsen; RASS), diabetic retinopathy outcomes were designated by the trial investigators as of primary interest in only two of the trials (DIRECT Prevent 1; DIRECT Protect 1). Except for Chase, diabetic retinopathy was diagnosed and staged by individuals masked to treatment assignment; in five of these six studies the retinal photographs were sent to a reading center for central grading of retinopathy severity.
Participants in these seven trials were young, with mean age per trial of about 30 years, except for AdDIT, and had blood pressures in the normal range at baseline (i.e. were normotensive). Almost all participants in the three trials that reported race/ethnicity were specified to be "white." A large majority of participants had either no retinopathy, microaneurysms only, or mild non‐proliferative retinopathy at baseline. Four trials enrolled a few participants with more severe retinopathy at baseline (Table 2) (Chase; DIRECT Protect 1; EUCLID; RASS). Participants in EUCLID had lower mean glycated hemoglobin levels than participants in the other six trials; mean time since diagnosis of diabetes was shorter for participants in AdDIT, reflecting the younger age of the participants (Table 2). Among trials conducted in the 2000s, hypertension was defined as systolic blood pressure greater than 130 mmHg or diastolic blood pressure greater than 85 mmHg in the absence of antihypertensive medication, which are lower blood pressure values than for trials conducted earlier in this population.
Type 2 diabetes
Individuals with type 2 diabetes enrolled in 22 trials (22,565 participants). The mean ages of participants were primarily in the sixth or seventh decade. Four of the 22 trials were conducted in Asia (J‐DOIT3; J‐EDIT; Wang; Zhao).
In 12 trials (10,825 participants; Table 4), outcome data were reported for participants who at the time of enrollment were defined by the investigators to be either normotensive or hypertensive with blood pressure controlled with antihypertensive medication (ABCD (1); ABCD‐2V (1); ACCORD Eye; DIRECT Protect 2; J‐EDIT; Knudsen; Medi‐Cal; Pradhan; Ravid 1993; ROADMAP; Wang; Zhao). Definitions of normal blood pressure tended to be more restrictive in trials initiated in 1995 or later; blood pressure targets set by the investigators of later trials were correspondingly lower. Diabetic retinopathy was the primary outcome of interest to the investigators of only three of the 10 trials of normotensive participants (DIRECT Protect 2; Pradhan; Wang). In four studies, retinal photographs were sent to a central site for interpretation and grading of diabetic retinopathy severity (ABCD (1); ACCORD Eye; DIRECT Protect 2; Medi‐Cal).
Ten trials (11,740 participants; Table 6) reported outcome data for type 2 diabetics who were hypertensive at baseline as defined by the study investigators (ABCD (2); ADDITION‐Europe; ADVANCE/AdRem; BENEDICT; DEMAND; HINTS; J‐DOIT3; Rachmani 2002; Steno‐2; UKPDS/HDS). None of the 10 trials of type 2 diabetics with hypertension specified diabetic retinopathy outcomes as outcomes of primary interest. In five of the 10 studies in this subgroup, either an angiotensin‐converting enzyme inhibitor or a calcium channel blocker had been given to participants in one arm for comparison with placebo in the other arm (ABCD (2)); ADVANCE/AdRem; BENEDICT; DEMAND; UKPDS/HDS). In five trials, various approaches to the management of hypertension, and often other conditions as well, were compared with "routine," "standard," "conventional," or "usual" care (ADDITION‐Europe; HINTS; J‐DOIT3; Rachmani 2002; Steno‐2). In five of the 10 trials, retinal photographs were assessed centrally to diagnose and/or grade the severity of diabetic retinopathy and to assess outcomes (ABCD (2); ADDITION‐Europe; ADVANCE/AdRem; Steno‐2; UKPDS/HDS).
Study interventions
In all seven trials in which participants with type 1 diabetes mellitus were enrolled, the investigators had randomized participants to an antihypertensive agent (angiotensin‐converting enzyme inhibitor [ACEi] or angiotensin II receptor blocker [ARB]) versus placebo or no treatment for comparison of outcomes. Three of the trials reported blood pressure targets for participants (EUCLID; Larsen; RASS).
In addition to all seven trials of participants with type 1 diabetes, eight of the 12 trials that enrolled normotensive or treated hypertensive participants with type 2 diabetes compared antihypertensive medication to placebo or no treatment (ABCD (1); ABCD‐2V (1); DIRECT Protect 2; Knudsen; Pradhan; Ravid 1993; ROADMAP; Wang). The antihypertensive medication was an ACEi in three trials and an ARB in four trials, while in ABCD (1), participants in the intervention arm were assigned randomly to one of these two types of medication. In the remaining four trials, intensive case management and monitoring by clinical personnel was compared with usual (routine, conventional, or standard) care.
Among the 10 trials enrolling participants with type 2 diabetes mellitus and hypertension, the randomized comparison was between an antihypertensive medication and placebo or another medication in five trials. In two of the 10 trials, a program for patient education and clinical management of hypertension was compared with usual care. In ADDITION‐Europe, ADVANCE/AdRem, and UKPDS/HDS, participants were assigned randomly to different blood glucose control strategies in addition to blood pressure control strategies.
Five trials reported that antihypertensive medications could be added to the assigned intervention at the discretion of the treating clinicians (ABCD (1); ADVANCE/AdRem; BENEDICT; DEMAND; Steno‐2). Flexible dosing schedules for antihypertensive medications were permitted in two trials (ABCD (1); EUCLID). In two trials comparing intensive versus standard blood pressure management and monitoring, the class(es) of antihypertensive prescribed were not specified (HINTS; Zhao). In one of these trials (HINTS), behavioral home blood pressure telemonitoring was also incorporated.
Study outcomes
Incidence and progression of diabetic retinopathy
Methods of diagnosing and classifying diabetic retinopathy varied among the included studies. Masked personnel at central reading centers evaluated photographs submitted for grading in 12 of the 29 included trials, including 5 of the 7 trials of type 1 diabetics (AdDIT; DIRECT Prevent 1; DIRECT Protect 1; EUCLID; RASS), and 9 of the 22 trials of type 2 diabetics (ABCD (1); ABCD (2); ACCORD Eye; ADDITION‐Europe; ADVANCE/AdRem; DIRECT Protect 2; Medi‐Cal; Steno‐2; UKPDS/HDS). The investigators of the remaining two trials of type 1 diabetics reported that diabetic retinopathy was assessed by local ophthalmologists masked to treatment assignment (Chase; Larsen).
Among 11 of the remaining 13 trials of participants with type 2 diabetes that reported the method of ascertaining outcomes, investigators of three trials reported local assessment by a masked examiner (BENEDICT; DEMAND; Pradhan). Six trials based retinopathy outcomes on diagnosis and classification by local ophthalmologists (J‐DOIT3; J‐EDIT; Knudsen; Rachmani 2002; Ravid 1993; Wang); one on retrieval of diagnoses recorded in electronic records (HINTS); and one on documentation of photocoagulation treatment for PDR or macular edema or vitrectomy for VH (ROADMAP). Reports from the remaining trials either did not specify the method used for diagnosing retinopathy or described it unclearly (ABCD‐2V (1); Zhao).
Almost all studies that used central or local grading of photographs to diagnose and monitor progression of diabetic retinopathy employed some version of the severity scale developed for the ETDRS or a modified or simplified version of it. Two trials used the EURODIAB 5‐step scale (EUCLID; Steno‐2).
Regarding the primary outcomes of this review, five‐year incidence of diabetic retinopathy was reported in one trial of type 1 diabetics, DIRECT Prevent 1, and in 10 trials of type 2 diabetics (ABCD (1); ACCORD Eye; ADVANCE/AdRem; BENEDICT; DEMAND; DIRECT Protect 2; J‐EDIT; Rachmani 2002; Ravid 1993; Steno‐2; UKPDS/HDS). Five‐year progression was reported in five trials, one trial of participants with type 1 diabetes, DIRECT Protect 1, and four trials of participants with type 2 diabetes (ADVANCE/AdRem; DIRECT Protect 2; J‐EDIT; UKPDS/HDS).
Trial investigators reported five‐year combined incidence and progression of retinopathy for participants with type 1 diabetes enrolled in one trial (RASS), for normotensive participants with type 2 diabetes in two trials (ABCD (1); ACCORD Eye), and for participants with type 2 diabetes and hypertension in five trials (ABCD (2); ADDITION‐Europe; ADVANCE/AdRem; HINTS; Steno‐2).
Investigators of ACCORD Eye and ROADMAP reported data from observational follow‐up of trial participants. These data from the ROADMAP observational study were the only diabetic retinopathy data reported from that study. We excluded the data from the observational study periods of both studies from analyses of effects of interventions on outcomes for this review.
Incidence of proliferative diabetic retinopathy (PDR), clinically significant macular edema (CSME), or vitreous hemorrhage (VH)
The investigators of nine trials reported five‐year incidence of PDR, CSME, or VH which typically result in treatment of affected eyes (ABCD (1); ADDITION‐Europe; ADVANCE/AdRem; DIRECT Protect 1; DIRECT Protect 2; Rachmani 2002; RASS; Ravid 1993; Steno‐2).
Visual acuity
Only two trials reported moderate loss of visual acuity (loss of 3 to 5 lines on a visual acuity chart with a logMAR scale) (ACCORD Eye; UKPDS/HDS), through four years and eight years, respectively. Investigators in these same trials and in Steno‐2 also reported the incidence of blind eyes among participants.
Other outcomes
Other outcomes, including quality of life, cost‐effectiveness data, and adverse events from individual trials, were reported infrequently, as described in the Effects of interventions section of this updated review.
Sources of funding
Eight trials were sponsored entirely by pharmaceutical companies (ABCD‐2V (1); BENEDICT; DEMAND; DIRECT Prevent 1; DIRECT Protect 1; DIRECT Protect 2; EUCLID; ROADMAP). Ten trials were conducted with partial support from industry and additional support from governmental agencies and foundations (ABCD (1); ABCD (2); ACCORD Eye; BENEDICT; Chase; Knudsen; Medi‐Cal; RASS; Steno‐2; UKPDS/HDS). Partial support from industry was typically in the form of study drugs and supplies or support for conducting specific procedures or analyses. Nine trials were conducted with support exclusively from governmental agencies, foundations, or grants from participating institutions (AdDIT; ADDITION‐Europe; ADVANCE/AdRem; HINTS; J‐EDIT; Pradhan; Ravid 1993; Wang; Zhao). Source of funding to conduct the trial was not reported in Larsen or Rachmani 2002.
Excluded studies
The primary reasons for excluding each of the 21 studies selected during the updated searches are provided in the Characteristics of excluded studies table. We excluded 10 RCTs of blood pressure control because no data were available for diabetic retinopathy outcomes, although diabetic retinopathy was specified as a secondary outcome in a trial publication or registration record. We contacted trial investigators who were first authors of primary reports from the trials to ascertain whether retinopathy outcomes were available. None of the respondents provided data because diabetic retinopathy outcomes had not been evaluated in the trials. We excluded 10 additional studies because they were not RCTs or because they had not investigated blood pressure control interventions. We excluded a report of data from two RCTs because the investigators had reported combined findings from the two trials and did not provide outcome data from the two individual trials in a manner that permitted estimation of effects in each trial.
Risk of bias in included studies
An overall summary of the risk of bias assessments of the included trials with respect to this review is provided in Figure 2. Details for each individual study are presented in the risk of bias section of the Characteristics of included studies table.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Missing cells indicate that study did not measure corresponding review outcomes.
Allocation
We judged 20 trials to be at low risk of bias for random sequence generation (ABCD (1); ABCD (2); ABCD‐2V (1); ACCORD Eye; AdDIT; ADDITION‐Europe; ADVANCE/AdRem; DEMAND; DIRECT Prevent 1; DIRECT Protect 1; DIRECT Protect 2; EUCLID; HINTS; J‐DOIT3; Rachmani 2002; RASS; Ravid 1993; ROADMAP; UKPDS/HDS; Zhao), as reports from these studies indicated that an appropriate randomization method had been used. We assessed the remaining nine trials as having an unclear risk of bias due to insufficient descriptions of random sequence generation in the trial reports (BENEDICT; Chase; J‐EDIT; Knudsen; Larsen; Medi‐Cal; Pradhan; Steno‐2; Wang).
We judged allocation concealment methods as at low risk of bias for 14 trials that employed methods such as assignments made by a central co‐ordinating center; unclear risk of bias for 14 trials for which we could not find a description of the random allocation method employed (AdDIT; ADDITION‐Europe; BENEDICT; Chase; J‐EDIT; Knudsen; Larsen; Medi‐Cal; Pradhan; Rachmani 2002; RASS; Ravid 1993; Wang; Zhao); and high risk of bias for one trial that did not describe an allocation concealment method and did not mask treatments (Steno‐2). Block size in Steno‐2 was also fixed so that later assignments in each block of four could have been known to the investigators.
Consequently, a total of 14 trials were at low risk of selection bias based on both the description of the random allocation process and concealment of the random allocation before assignment (Figure 2).
Masking (performance bias and detection bias)
In 18 of the 28 trials in which one or more of the primary outcomes for this review were reported, it was indicated that assessors of the diabetic retinopathy outcomes were masked to the assigned treatment. We judged these 18 trials to be at low risk, six trials to be at unclear risk (Chase; Medi‐Cal; Rachmani 2002; Ravid 1993; Wang; Zhao), and the remaining four trials to be at high risk of performance and detection bias for retinopathy outcomes (Figure 2) (ABCD‐2V (1); HINTS; J‐DOIT3; J‐EDIT). Of the 19 trials that reported our secondary outcomes, we judged eight trials to be at low risk of performance and detection bias based on proper methods of masking (ABCD (1); DIRECT Protect 1; DIRECT Protect 2; EUCLID; Knudsen; Larsen; RASS; Steno‐2); three trials to be at high risk of performance and detection bias due to lack of masking for visual acuity assessment (Chase; J‐DOIT3; Pradhan); and the remaining seven trials to have an unclear risk of performance and detection bias (ACCORD Eye; ADDITION‐Europe; Pradhan; Rachmani 2002; Ravid 1993; UKPDS/HDS; Wang).
Incomplete outcome data
We judged eight trials to be at high risk of bias due to incomplete primary outcome data, including missing data for roughly 40% of randomized participants in two trials (ABCD (1); ABCD (2)), and the exclusion of participants with missing baseline retinal photographs in one trial (ADVANCE/AdRem). In ADDITION‐Europe, retinal images were taken at a follow‐up visit or extracted from medical records for only 2190 (76.6%) of 2861 participants alive at five years. In AdDIT, there were 15% withdrawals from the trial; 19 participants were excluded from the combined placebo arm and 18 participants from the combined ACEi arm. In J‐EDIT, diabetic retinopathy data were reported for only 940 (80.1%) of 1173 participants due to the inability to see fundus details in photographs; attrition was similar in Zhao and Medi‐Cal. Outcomes for participants with cataract, vitreous heme, or other opacities would thus have been excluded. Furthermore, the dropout rate after six years was 8.9% (104 cases). Investigators of eight trials did not provide sufficient information regarding completeness of follow‐up, thus we judged these trials as being at unclear risk of attrition bias (ABCD‐2V (1); EUCLID; Larsen; Rachmani 2002; RASS; Ravid 1993; ROADMAP; Steno‐2). One trial did not measure our primary outcome of interest; we judged the remaining 12 trials to have a low risk of attrition bias for primary outcome data. Secondary outcomes specified for this review were reported less frequently than primary outcomes; for 11 trials, no single one of our secondary outcomes was reported, so that risk of attrition bias was not applicable for secondary outcomes in those instances.
Selective reporting
We assessed two trials as at high risk of selective outcome reporting. In BENEDICT, a protocol‐specified outcome (progression of retinopathy) was omitted from the published report, and a new outcome (regression of retinopathy) was introduced. Progression and regression are not complementary outcomes because stable retinopathy is not part of either outcome definition. In J‐EDIT, visual acuity outcomes were specified as trial outcomes, but were not reported. We judged two trials to be at unclear risk of reporting bias, as percentages of participants with outcomes were reported without sufficient information to determine numbers of participants in each group (ABCD (1); ABCD (2)). We judged another 12 trials to be at unclear risk of bias, as insufficient information was available from trial reports to permit a judgement regarding reporting bias (ABCD‐2V (1); ACCORD Eye; AdDIT; ADDITION‐Europe; ADVANCE/AdRem; Chase; DEMAND; DIRECT Protect 2; EUCLID; Medi‐Cal; Pradhan; Steno‐2). We deemed the remaining trials to be at low risk of reporting bias.
Other potential sources of bias
We considered 11 trials to have an unclear risk of other bias due to the presence of either partial or complete industry support. We judged three trials to have an unclear risk of other bias due to postrandomization exclusions, ACCORD Eye, and accepting retinal photographs taken after randomization as reflecting the baseline status of the participants' eyes (ADVANCE/AdRem; RASS). We judged seven trials to have a high risk of other bias. One trial without a data monitoring committee was terminated early for futility (Pradhan). Another trial excluded participants from the analyses who discontinued the study medication (DEMAND). In a further trial no retinal images were taken at study entry for assessment of diabetic retinopathy (ADDITION‐Europe). In J‐EDIT the number examined for diabetic retinopathy during follow‐up was not reported by arm. The remaining three trials had members of the data monitoring committee who represented the pharmaceutical company that was funding the trial (ABCD (1); ABCD (2)), or industry support without declarations of interest (ABCD‐2V (1)). No other source of bias was found for the remaining trials.
Effects of interventions
See: Table 1
Subsets of the included trials reported each of the outcomes specified for this review; no single outcome was reported by all 29 included trials. We have provided a summary of data comparing more intensive versus less intensive control of blood pressure (including blood pressure control versus placebo). We have presented overall findings and findings within subgroups of participants defined by type of diabetes (type 1 and type 2) and baseline blood pressure status of trial participants (hypertensive and normotensive or treated hypertensive).
We combined outcomes reported by four to six years of follow‐up to approximate five‐year outcomes. Some outcome data were reported from four trials with a follow‐up period of seven to nine years (J‐DOIT3; Steno‐2; UKPDS/HDS; Zhao). Zhao enrolled type 2 diabetics who had normal blood pressure or blood pressure controlled by medication; the other three trials enrolled participants with type 2 diabetes and hypertension as defined by the investigators. We combined outcome data reported at seven to nine years with five‐year outcomes in analyses of a few outcomes. Whenever outcome data from only three years or less after study entry had been reported, we used those data to estimate approximate two‐year outcomes. These analyses are provided in Appendix 7.
Although different scales and methods were used to assess incidence and progression of retinopathy in the included trials, most were derived from the Airlie House classification (ETDRS10 1991). For the purpose of summarizing evidence in this systematic review, we have considered a change on one retinopathy scale to represent an approximately equal change on another scale. We also have presented the available data comparing outcomes between classes of antihypertensive agents.
Intensive or strict blood pressure control versus less strict blood pressure control
Comparisons in this review focus on intensive or strict blood pressure control versus no or less strict blood pressure control and on effects after five years of intervention and participant follow‐up. Data regarding one or more five‐year outcomes were available from 17 trials (ABCD (1); ABCD (2); ACCORD Eye; ADDITION‐Europe; ADVANCE/AdRem; BENEDICT; DEMAND; DIRECT Prevent 1; DIRECT Protect 1; DIRECT Protect 2; HINTS; J‐EDIT; Rachmani 2002; RASS; Ravid 1993; Steno‐2; UKPDS/HDS). Data regarding seven‐ to nine‐year outcomes were available from J‐DOIT3 and Zhao in addition to Steno‐2 and UKPDS/HDS. Consequently, 19 of the 29 included studies contributed data to effect estimates for trial participants followed for 5 years or longer.
Incidence of retinopathy
Estimated 5‐year rates of incidence of retinopathy among participants without retinopathy at baseline were available from 11 trials (Analysis 1.1; Figure 3): from one trial among 1421 participants with type 1 diabetes and normal blood pressures (DIRECT Prevent 1); from four trials among 1545 participants with type 2 diabetes and normal blood pressures as defined by the trial investigators (ABCD (1); ACCORD Eye; J‐EDIT; Ravid 1993); and from six trials conducted among 1974 participants with type 2 diabetes and hypertension (ADVANCE/AdRem; BENEDICT; DEMAND; Rachmani 2002; Steno‐2; UKPDS/HDS). The estimated risk ratios (RR) and 95% confidence intervals (CI) for intensive intervention on blood pressure compared to less or no intervention based on data from these trials are provided in Figure 3 (Analysis 1.1). The overall estimates of the RR of incident diabetic retinopathy and its 95% CI after five years were 0.82 (0.73 to 0.92; 11 RCTs, 4940 participants; I2 = 15%), which indicate that participants in these 11 trials had realized an overall benefit of blood pressure control of 18% reduction with respect to 5‐year incidence of diabetic retinopathy (moderate certainty evidence). Although the effect estimates were similar for each of the three subgroups of participants (I2 = 0%), they were more heterogeneous in the subgroup of hypertensive type 2 diabetics. This subgroup also had the widest 95% CI on the effect estimate.
1.1. Analysis.

Comparison 1: More blood pressure control versus no (or less) control , Outcome 1: Incidence of retinopathy by 5 years
3.

Incidence of diabetic retinopathy by 5 years: more blood pressure control versus less blood pressure control.
The estimates within pairs of subgroups defined by type of diabetes (Analysis 2.1) and classification of blood pressure at baseline (Analysis 3.1) were similar to overall estimates. We judged the certainty of the evidence for five‐year estimates to be moderate, downgrading for risk of bias.
2.1. Analysis.

Comparison 2: Outcomes by type of diabetes: More blood pressure control versus no (or less) control, Outcome 1: Incidence of diabetic retinopathy by 5 years
3.1. Analysis.

Comparison 3: Outcomes by blood pressure status at entry: More blood pressure control versus no (or less) control, Outcome 1: Incidence of diabetic retinopathy by 5 years
Progression of retinopathy
Data from five trials permitted the estimation of five‐year rates of progression of retinopathy among participants in whom retinopathy already was present at time of trial enrollment (Analysis 1.2; Figure 4). Progression was reported for 1905 type 1 diabetics enrolled in DIRECT Protect 1; 2460 type 2 diabetics defined as normotensive by study investigators and enrolled in two trials (DIRECT Protect 2; J‐EDIT); and 779 hypertensive type 2 diabetics enrolled in two trials (ADVANCE/AdRem; UKPDS/HDS). The estimated overall RR for progression during five years was 0.94 (95% CI 0.78 to 1.12; 5 RCTs, 5144 participants; I2 = 57%), suggesting no evidence of an overall effect on progression of retinopathy as a result of more intensive intervention to control blood pressure (Analysis 1.2, Figure 4). The CIs for the three subgroups overlapped, and all included 1.00, indicating no effect of more intervention to control blood pressure. The effect estimates within subgroups defined by type of diabetes (Analysis 2.2) and the investigators' classification of baseline blood pressure (Analysis 3.2) were similar to the overall estimates. We rated the certainty of evidence for five‐year estimates of progression as moderate, downgrading for risk of bias.
1.2. Analysis.

Comparison 1: More blood pressure control versus no (or less) control , Outcome 2: Progression of retinopathy by 5 years
4.

Progression of diabetic retinopathy by 5 years: more blood pressure control versus less blood pressure control.
2.2. Analysis.

Comparison 2: Outcomes by type of diabetes: More blood pressure control versus no (or less) control, Outcome 2: Progression of diabetic retinopathy by 5 years
3.2. Analysis.

Comparison 3: Outcomes by blood pressure status at entry: More blood pressure control versus no (or less) control, Outcome 2: Progression of diabetic retinopathy by 5 years
Combined incidence and progression of retinopathy
We used data from reports from eight RCTs to estimate five‐year rates of combined incidence and progression of diabetic retinopathy (ABCD (1); ABCD (2); ACCORD Eye; ADDITION‐Europe; ADVANCE/AdRem; HINTS; RASS; Steno‐2). The overall five‐year effect estimate of more intervention on blood pressure for this combined outcome (Analysis 1.3, Figure 5) yielded an estimated overall RR of 0.78 (95% CI 0.68 to 0.89; 8 RCTs, 6212 participants; I2 = 42%), supporting a five‐year reduction of 22%. The estimated effect from ACCORD Eye favored less intensive intervention on blood pressure; however, the evidence from the remaining trials supports more intensive intervention. A sensitivity analysis that excluded ACCORD Eye yielded estimates similar to the overall estimates (RR 0.76, 95% CI 0.68 to 0.85; 7 RCTs, 4949 participants; I2 =9%). We rated the certainty of evidence for the overall estimates as low, owing to risk of bias and imprecision of estimates for the normotensive subgroups.
1.3. Analysis.

Comparison 1: More blood pressure control versus no (or less) control , Outcome 3: Combined incidence and progression of retinopathy by 5 years
5.

Combined incidence and progression of diabetic retinopathy by 5 years: more blood pressure control versus less blood pressure control.
We also investigated the effects of excluding data from all or individual studies that reported either incidence or progression as well as their combination. Those exclusions resulted in little or no difference in effect estimates from those calculated for all eight trials.
Incidence of PDR, CSME, or VH
Data suitable for estimating five‐year incidence of progression to PDR, CSME, or VH, regardless of retinopathy status at trial entry, were available from nine trials (ABCD (1); ADDITION‐Europe; ADVANCE/AdRem; DIRECT Protect 1; DIRECT Protect 2; Rachmani 2002; RASS; Ravid 1993; Steno‐2). These outcomes were in some instances reported separately, but were more often reported together because the presence of any of the three indicates the need for treatment. There was no evidence of an overall beneficial effect of intense blood pressure intervention for this outcome: estimated RR 0.92 (95% CI 0.82 to 1.04; 9 RCTs, 8237 participants; I2 = 31%) (Analysis 1.4; Figure 6). However, the estimated effects differed between participants who were classified by trial investigators as hypertensive at time of trial enrollment (RR 0.52, 95% CI 0.33 to 0.84; 4 RCTs, 4279 participants; I2 = 3%) and those who were not (RR 1.00, 95% CI 0.83 to 1.19; 5 RCTs, 4588 participants; I2 = 10%), as shown in Analysis 3.4. These estimates support a beneficial effect among participants who had type 2 diabetes and hypertension at trial enrollment, but no effect among participants with either type 1 or type 2 diabetes who were not defined as being hypertensive by trial investigators. We rated the certainty of evidence for these estimates as low, owing to risk of bias and heterogeneity.
1.4. Analysis.

Comparison 1: More blood pressure control versus no (or less) control , Outcome 4: Incidence of PDR, CSME, or VH by 5 years
6.

Progression to PDR, CSME, or VH by 5 years: more blood pressure control versus less blood pressure control.
3.4. Analysis.

Comparison 3: Outcomes by blood pressure status at entry: More blood pressure control versus no (or less) control, Outcome 4: Incidence of PDR, CSME, or VH by 5 years
Visual acuity loss of 3 or more lines
Investigators from J‐DOIT3 reported that no participant had experienced a visual acuity loss of 3 or more lines during a mean of 8.5 years of follow‐up. Investigators from two trials, ACCORD Eye and UKPDS/HDS, reported such visual acuity losses from baseline levels to permit an estimation of the five‐year effect on this outcome of intervention on blood pressure (RR 1.15, 95% CI 0.63 to 2.08; 2 RCTs, 2326 participants; I2 = 90%), with the data from ACCORD Eye indicative of an adverse effect of intense blood pressure control on loss of visual acuity, but the data from UKPDS/HDS suggesting no effect on this outcome (Analysis 1.5; Figure 7). The certainty of evidence for either a beneficial or detrimental effect of intervention to control blood pressure on moderate loss of visual acuity was very low, irrespective of how findings from the two trials were combined, due to risk of bias, imprecision, and inconsistency.
1.5. Analysis.

Comparison 1: More blood pressure control versus no (or less) control , Outcome 5: Loss of visual acuity
7.

Loss of visual acuity: more blood pressure control versus less blood pressure control.
Investigators from ACCORD Eye, Steno‐2, and UKPDS/HDS also reported on blind eyes observed by four, eight, and nine years, respectively, among participants with type 2 diabetes. The effect estimate for ACCORD Eye was RR 1.13 (95% CI 0.77 to 1.66; 3758 participants) for normotensive participants, which differs from the estimate from UKPDS/HDS of RR 0.77 (95% CI 0.38 to 1.59; 1148 participants) for hypertensive participants. The UKPDS/HDS investigators also reported that no participant had become blind in both eyes by the end of the follow‐up period. The Steno‐2 investigators reported blindness in one eye for 1 of 80 hypertensive participants in the intensive group and 7 of 80 participants in the standard group during the first four years of follow‐up (estimated RR 0.14, 95% CI 0.02 to 1.13; 160 participants). The CIs on the estimates from all three trials included 1.0, indicating neither benefit nor harm with respect to this outcome of intense intervention to control blood pressure.
Treatment with one class of antihypertensive medication versus another class of antihypertensive medication
A secondary goal of this review was to compare outcomes for different classes of antihypertensive agents to identify which is most effective. In seven trials (ABCD (1); ABCD (2); AdDIT; BENEDICT; DEMAND; RASS; UKPDS/HDS), different classes of antihypertensive agents or combinations were compared to an ACEi. The trial identifiers, test antihypertensive medication compared to an ACEi, outcomes for which the medications were compared, and the relative effectiveness of the test medications are shown in Table 8. Another class of antihypertensive agent was compared to an ACEi in more than one trial for only one outcome, five‐year combined incidence and progression of diabetic retinopathy. In comparison to an ACEi, no other class of antihypertensive agent, alone or in combination with an ACEi, was clearly superior to an ACEi based on data reported in the included trials.
7. Effects of angiotensin converting enzyme inhibitors (ACEi) versus other classes of antihypertensive medication.
| Outcome | Trial identifier | Comparator class | RR (95% CI) |
| Incidence of DR | DEMAND | ACEi + CCB | 0.48 (0.13, 1.86) |
| Progression of DR | UKPDS/HDS | Beta blocker | 1.01 (0.75, 1.35) |
| Combined incidence and progression of DR | ABCD (1) | CCB | 1.33 (0.93, 1.92) |
| ABCD (2) | CCB | 0.93 (0.71, 1.21) | |
| RASS | ARB | 0.89 (0.49, 1.87 | |
| Regression of DR | BENEDICT | Non‐ACEi | 0.44 (0.22, 0.87) |
| All‐cause mortality | UKPDS/HDS | Beta blocker | 0.88 (0.64, 1.20) |
ACEi, angiotensin‐converting enzyme inhibitor ARB, angiotensin receptor blocker CCB, calcium channel blocker DR, diabetic retinopathy RR, risk ratio CI, confidence interval
Adverse events
Reporting of adverse events associated with blood pressure control was inconsistent among the included trials. The investigators of most trials reported adverse events observed during the entire study period. The most frequently reported adverse event for participants in these trials was death from all causes, reported in 13 trials with follow‐up periods of 4 to 9 years (ABCD (1); ABCD (2); ADDITION‐Europe; DEMAND; DIRECT Prevent 1; DIRECT Protect 1; DIRECT Protect 2; J‐DOIT3; Rachmani 2002; RASS; Steno‐2; UKPDS/HDS; Zhao). The J‐EDIT investigators reported the total number of deaths that occurred during six years of follow‐up, but did not report the number of deaths in each intervention arm. As shown in Analysis 1.6 (Figure 8), the estimated RR for death was 0.87 (95% CI 0.76 to 1.00; 13 RCTs, 13,979 participants; I2 = 0%), indicating no important effect on all‐cause mortality by intervention to control blood pressure. The effect of blood pressure intervention on death from all causes was similar among type 1 and type 2 diabetics (Analysis 2.5): estimated RR 1.20 (95% CI 0.58 to 2.45) and RR 0.86 (95% CI 0.75 to 0.99; 10 RCTs, 10,371 participants; I2 = 0%), respectively.
1.6. Analysis.

Comparison 1: More blood pressure control versus no (or less) control , Outcome 6: Adverse effect: death, 4 to 9 years
8.

Death from all causes, 4 to 9 years: more blood pressure control versus less blood pressure control.
2.5. Analysis.

Comparison 2: Outcomes by type of diabetes: More blood pressure control versus no (or less) control, Outcome 5: Adverse effect: death, 4 to 9 years
Two trials that followed participants for four years or longer reported hypotension (DIRECT Prevent 1; DIRECT Protect 1). Among participants with type 1 diabetes who were not hypertensive at trial entry (DIRECT Prevent 1; DIRECT Protect 1), significantly more hypotension was reported with more intense intervention on blood pressure: estimated RR 2.04 (95% CI 1.63 to 2.55; 2 RCTs, 3323 participants; I2 = 37%). Of note, the majority of participants were normotensive type 1 diabetics.
RASS investigators reported no case of hyperkalemia in the more intense intervention arm (94 participants) and one case in the less intense intervention arm (96 participants). The J‐DOIT3 investigators reported 39 cases of hyperkalemia in their 9‐year trial; cases were nearly evenly divided between the two intervention arms: 18 of 1269 participants in the more intensive intervention arm and 21 of 1271 participants in the less intensive intervention arm.
The UKPDS/HDS investigators reported cataract surgery among 36 of 758 participants in the tight blood pressure control arm versus 14 of 390 participants in the less tight blood pressure control arm.
Quality of life
The UKPDS/HDS investigators reported a cross‐sectional quality of life assessment conducted at eight centers (UKPDS 37). A subset of participants completed a "domain specific" questionnaire (n = 636) and/or the EQ‐5D questionnaire (n = 747) at mean times since randomization to tight or less tight blood pressure control of five and eight years, respectively. Of the specific measures (work satisfaction, mood state, symptoms, cognitive mistakes), median scores for the tight blood pressure control group differed from those of the less tight control group by 1 or 2 points for anger, symptoms, and both patient‐ and relative‐reported cognitive mistakes; however, interquartile ranges were similar. Total scores on the mood state scale differed by 3.5 points, with better scores among the tight control group. The authors' statement that the difference was not statistically significant was supported by substantial overlap of the CIs. EQ‐5D scores in the two groups were similar except for the anxiety dimension, for which respondents in the tight blood pressure control group had worse scores (P < 0.05). Within the tight blood glucose control arm, scores for respondents assigned to an ACEi were compared to scores of those assigned to a beta‐blocker. The authors stated that "a lower proportion of patients who were allocated to β‐blockers reported anxiety, on the generic questionnaire, than those allocated to ACE inhibitors (28% vs. 40%, Fisher's exact test, P = 0.010)."
In ADDITION‐Europe, 2217 participants completed EQ‐5D, EQ‐VAS, 36‐item Short Form Health Survey (SF‐36), and well‐being questionnaires. Health status, well‐being, diabetes‐specific quality of life, and treatment satisfaction did not differ between groups assigned to more intervention on blood pressure and routine care.
The ACCORD Study Group reported the rationale and design of health‐related quality of life and cost‐effectiveness components; however, we have not found any published data after an intensive search and queries to ACCORD Eye investigators. Investigators of other trials in which quality of life was assessed or was planned to be assessed have not yet reported findings.
Cost‐effectiveness
The UKPDS/HDS investigators evaluated the cost‐effectiveness of tight blood pressure control in participants with hypertension and type 2 diabetes (UKPDS 40 and UKPDS 63). The trial investigators performed an incremental cost‐effectiveness analysis in which they calculated the net costs and net effectiveness of tight control versus less tight control based on outcome data from their trial. Costs included treatment fees for blood pressure control, visits to doctors at diabetes centers, tests obtained at medical visits, and the costs of treating diabetic complications. Investigators estimated that the total costs for less tight blood pressure control was GBP 6145 per patient over an 8.4‐year period compared to GBP 6381 per patient with tight control, which represented a difference of GBP 237. Although there was a higher cost of antihypertensive treatment in the tight blood pressure control group than in the less tight control group, the analysis based on UKPDS/HDS data indicated that this increment was offset by reduced complication costs, longer survival, and greater interval without complications in the tight blood pressure control group than in the less tight blood pressure control group. The value of this finding is limited by outdated definitions of hypertension and its control that were used by the UKPDS investigators.
The UKPDS investigators also compared the cost‐effectiveness of an ACEi (captopril) to a beta‐blocker (atenolol) (UKPDS/HDS report 54). Although the two agents used to treat hypertensive type 2 diabetics were found to have similar effects, atenolol was associated with lower overall costs due to lower drug costs and fewer and shorter hospitalizations.
Data from the Steno‐2 study were used to evaluate the cost‐effectiveness of intensive versus conventional multifactorial interventions in type 2 diabetics (Gaede et al. 2008). However, the costs of photography, ophthalmologic examinations, or other clinical procedures were not considered part of the "annual cost of consultation." Two later reports were published in 2016 and 2019; progression of retinopathy and blind eyes were considered as outcomes in the 2016 publication. However, the cost models continued to ignore the costs of photocoagulation, vitrectomy, or other interventions for diabetic retinopathy. ADDITION‐Europe assessed cost‐effectiveness in a health technology assessment report; the investigators indicated that the intervention cost GBP 981 per patient and was not cost‐effective at cost ≥ GBP 631 per patient.
Summary
The available evidence from randomized trials of intensive or more intervention to control blood pressure supports a beneficial overall effect on the five‐year incidence of diabetic retinopathy in comparison to no or less intervention based on an estimated reduction in incidence of 22% among participants initially free of retinopathy. This effect was consistent among participants with type 1 or type 2 diabetes mellitus as well as those who had normal or elevated blood pressures at the time of enrollment.
Our overall findings suggest no reduction in the risk of five‐year progression of diabetic retinopathy attributable to interventions to control blood pressure among participants with retinopathy at baseline, either overall or within subgroups based on type of diabetes or blood pressure at trial enrollment.
The effect estimate for the five‐year combined outcome of incidence and progression of diabetic retinopathy was similar to that for incidence, with an estimated reduction in the risk of this outcome with blood pressure intervention of about 22%.
We did not find an overall benefit for intervention to control blood pressure with respect to incidence of PDR, CSME, or VH or for moderate or more severe loss of visual acuity. However, the data for trial participants with type 2 diabetes and hypertension were consistent with a beneficial effect of more intensive blood pressure control in reducing the incidence of PDR, CSME, or VH.
Few studies reported on loss of visual acuity. Estimates from three trials suggest neither benefit nor harm with respect to visual acuity attributable to more intervention to control blood pressure.
Discussion
Summary of main results
We found 29 RCTs that evaluated the use of blood pressure‐lowering interventions in participants with either type 1 or type 2 diabetes and that had reported one or more diabetic retinopathy outcomes. The 29 trials included 15 from which data were available for our original review of this topic, and 14 more added as a result of recent searches for trials. There was considerable variability among the trials with respect to sample size, blood pressure control interventions, eligibility criteria, diabetic retinopathy outcomes reported, years during which trials had been conducted, and length of follow‐up of participants. In accordance with the original study protocol, we focused our analyses on five‐year effect estimates for primary and secondary outcomes specified in this review.
Among the 11 trials that reported the incidence of diabetic retinopathy among 4940 participants, findings supported intensive blood pressure intervention, with an estimated reduction in 5‐year incidence of 18%. Five trials including 5144 participants with diabetes reported data on progression of retinopathy. Although we observed some statistical heterogeneity, as well as clinical and methodologic heterogeneity, among the findings of the five trials, overall the evidence indicated no benefit of more intense intervention on blood pressure with respect to progression of diabetic retinopathy. The overall effect estimate from the eight trials (6212 participants) that reported combined incidence and progression of retinopathy was similar to that for incidence alone, that is a 22% reduction in risk with more intense intervention on blood pressure.
Nine trials with a total of 8237 reported data regarding the five‐year incidence of PDR, CSME, or VH. The overall effect estimate was consistent with no difference between intervention arms. Investigators of two trials reported both moderate loss of visual acuity (among 2380 participants); investigators of three trials reported more severe loss leading to blind eyes (among 5060 participants). Again, the findings for loss of visual acuity were similar after more intervention to control blood pressure versus less intervention.
Regarding the six outcomes that were the focus of this review, too few trials reported information to permit an estimate of the effect of intense intervention on moderate loss of visual acuity. Overall, the evidence supported modest benefits of intense intervention for three of the remaining five outcomes. Among the trials that enrolled hypertensive type 2 diabetics, the evidence for four of the five outcomes supported benefits of intensive intervention on blood pressure compared to less intervention, with reductions in detrimental outcomes by 23% to 48%.
The investigators of the clinical trials included in this review did not systematically report on adverse events related to tight blood pressure control apart from death from any cause, one of the six outcomes analyzed. The incidence of serious ocular adverse events cannot be estimated reliably from the data provided by the included trials.
Overall completeness and applicability of evidence
Although we limited this review to the consideration of findings reported from RCTs, the included trials differed from one another in several important respects. We thus must consider a number of sources of variability when interpreting the available evidence.
Baseline laboratory data differed in the participant cohorts that participated in the various trials (Table 2; Table 4; Table 6). In some trials, baseline glycated hemoglobin values were quite high, approximately 11% in the intensively treated and control groups. In most other studies that provided five‐year data, glycated hemoglobin values were approximately 8% or lower (Table 2; Table 4; Table 6). Although the target of intervention in these studies was blood pressure, the separate influence of blood glucose may have had a greater effect on the retinopathy outcome.
Clinical centers in these trials were established in numerous geographic locations. For example, ADVANCE/AdRem was carried out in 20 countries, and the Diabetic Retinopathy Candesartan Trials (DIRECT) program had centers in 30 countries. While this dispersion resulted in considerable ethnic and genetic variation among participants, specific racial or ethnic distributions were not reported in the descriptions of most of the trial populations. Four of the included trials were conducted in Asia (J‐DOIT3; J‐EDIT; Wang; Zhao). Centers in India and China participated in ADVANCE/AdRem, and centers in South Africa participated in the three DIRECT trials. Although ADVANCE/AdRem reported a comparison of retinal lesions at baseline among white and Asian participants, none of the trials provided data to suggest that diabetic individuals from different racial or ethnic groups differed in their responses to blood pressure control with respect to the development or progression of retinopathy or to adverse events.
Trials that reported five‐year outcomes and thus contributed data to this review varied widely in size, from as few as 100 participants, Ravid 1993, to about 3000, ADDITION‐Europe. Rates of incidence and progression of diabetic retinopathy were sometimes not reported in a form amenable to the methods of analysis suitable for a systematic review. When necessary, we derived data from graphical displays and contacted study investigators.
Although most trials used the Early Treatment Diabetic Retinopathy Study (ETDRS) or the EURODIAB scale for grading retinopathy, the different trials had different definitions for progression of diabetic retinopathy (from one to three steps on a scale, counting progression in one or both eyes). Of the 19 trials that reported one or more outcomes by 5 years, only 11 clearly distinguished participants without retinopathy at baseline, in whom incidence could be assessed, from those with retinopathy detected at baseline; the other eight trials reported a combination of incidence and progression of retinopathy. It is possible that the effect of hypertension or its control on progression varies with the level of severity of retinopathy initially present or that had been selected to define outcomes for individual trials.
The blood pressure goals of several of the early trials included in this review do not reflect current practice. For example, in UKPDS/HDS, the tight blood pressure control group aimed for a blood pressure less than 150/85 mmHg, with the less tight control group aiming for a blood pressure of 180/105 mmHg or less. However, in the ACCORD Eye blood pressure intervention trial, the goal was a systolic blood pressure of less than 140 mmHg for the less intensive control group, and less than 120 mmHg for the intensive control group. We noted that most of the included trials had met or had come close to meeting blood pressure goals based on the blood pressure outcomes reported. However, mean blood pressure achieved during several trials was nearly the same in the group that had received more intense intervention and the group that had received less intervention.
Quality of the evidence
Eleven of the 19 studies that provided data we used to estimate 5‐year outcome rates evaluated the presence and severity of retinopathy using standard photographic protocols that provided objective documentation of the status of the retina, evaluation by observers masked to treatment status of participants, and reproducible evaluation by separate graders when necessary. In EUCLID, there was only a single grader, thus providing no mechanism to check for consistency or reproducibility of the grading. Methods for diagnosing and monitoring progression of diabetic retinopathy varied among the other eight trials, but typically relied on fundus examinations, together with photography and other imaging, with interpretation by one or more local ophthalmologists.
Two of the early trials, UKPDS/HDS and RASS, did not have a true baseline for assessing incidence or progression of diabetic retinopathy during follow‐up. In these studies, "baseline" retinal photographs taken as much as three years before, UKPDS/HDS, or one year after, RASS, randomization to the study arm were accepted as documenting the status of the eyes of participants at study entry.
Several studies embedded in parent studies with different goals restricted the analysis and reporting of retinopathy data to participants who had both baseline and follow‐up assessments of retinopathy, without any attempt to impute data for missing assessments or to account for missing data otherwise, so as to provide or estimate outcomes for the full participant population studied. Missing follow‐up assessments are common in trials with clinical outcomes, particularly those conducted among participants who are elderly or who have multiple medical problems. No totally satisfactory method has been identified to account for missing clinical outcome data due to deaths of trial participants.
Most of the included studies did not assess retinopathy at multiple times after participant enrollment. Photographs were typically taken only at baseline and a single follow‐up time or when needed to document retinal status before treatment. Exceptions were the three DIRECT trials, J‐EDIT, and UKPDS/HDS.
The trials used various strategies for intervening on blood pressure. For this review, we pooled data from the more intense strategy in each trial for comparison with the less intense strategy. A single antihypertensive medication versus placebo was the most frequent approach among trials for normotensive participants. Half of the trials on hypertensive participants compared various multifactorial interventions with standard care. Too few data from too few trials were available to estimate the effects of different interventions on review outcomes.
Attrition was an issue in some trials. Denominators for percentages were often not reported. In the case of insufficient information about deaths and other losses to follow‐up, we used as denominators the number of participants randomly assigned to each arm.
We assessed the quality of evidence provided for outcomes evaluated in this updated review in Table 1.
Potential biases in the review process
In only 19 of the 29 included RCTs were participants treated and followed up long enough to permit an estimation of 5‐year outcomes. The remaining 10 trials provided data for one or more outcomes observed by 3 years or less, including 1 trial that reported outcomes at less than 1 year.
A major difficulty we encountered in our efforts to identify adverse events of blood pressure intervention was that several of the 19 included trials from which data were available to estimate 5‐year outcome rates and effect estimates were ancillary to a larger parent study (ACCORD Eye; ADVANCE/AdRem; BENEDICT; HINTS; RASS; UKPDS/HDS), and the adverse events reported in study publications were cardiovascular or renal events that were outcomes of the parent trial. Another difficulty was that participants in several trials were also randomized to concurrent interventions, primarily on blood glucose (ACCORD Eye; ADDITION‐Europe; ADVANCE/AdRem; UKPDS/HDS), but also on lipids (AdDIT; RASS). Several multifactorial interventions also had components aimed at weight control and other health goals.
The protocol for this review did not specify subgroup analyses for participants with type 1 and type 2 diabetes and for those characterized by blood pressure levels at baseline (Sleilati 2009). We made the decision to examine findings in these subgroups after we began screening the results of the electronic searches for the original version of this review. Our familiarity with diabetic retinopathy and randomized trials for this condition suggested that it would be inappropriate to conduct analyses specified in the protocol without considering the effects of blood pressure intervention in these different subgroups of participants. Although we examined the available data for these subgroups, subgroup findings were usually consistent with the overall findings.
We searched extensively for all trials that evaluated blood pressure control for diabetic retinopathy in individuals with and without retinopathy at baseline. We included trials where the effect of blood pressure control on diabetic retinopathy was evaluated in a subset of all participants randomized into the study (e.g. ACCORD Eye; ADVANCE/AdRem; RASS). We excluded 10 trials of blood pressure control in people with diabetes that reported that retinopathy was an outcome of interest because data regarding diabetic retinopathy outcomes were not available. We designed our searches to identify all trials of blood pressure control in people with diabetes, and screened the search results for any mention of diabetic retinopathy or other ocular outcomes in publications and registry records. We also contacted the authors of reports from trials that suggested that data regarding ocular outcomes might be available.
Of the 19 trials that reported 5‐year rates of either incidence or progression (or a combination of incidence and progression) (ABCD (1); ABCD (2); ACCORD Eye; ADDITION‐Europe; ADVANCE/AdRem; BENEDICT; DEMAND; DIRECT Prevent 1; DIRECT Protect 1; DIRECT Protect 2; HINTS; J‐DOIT3; J‐EDIT; Rachmani 2002; RASS; Ravid 1993; Steno‐2; UKPDS/HDS; Zhao), 6 trials reported data for 2‐step changes from baseline on the ETDRS final scale or a modification of it that provided comparable data. Three trials reported 3‐step changes as progression; in the absence of data for 2‐step changes, we analyzed these data together with 2‐step changes. Similarly, for the few trials that used more condensed scales for grading diabetic retinopathy, we used the data as reported, that is 1‐step or 2‐step changes. One trial reported incidence of diabetic retinopathy only as change from no retinopathy to any retinopathy. We elected to pool the available data, reasoning that the same definition was used in trial arms compared within individual RCTs, and that the goal was to estimate relative effects of blood pressure intervention rather than absolute risks. Furthermore, some 1‐step changes on the condensed grading scales undoubtedly were equivalent to 2‐step changes on the more detailed ETDRS scale.
We could not obtain sufficient data from all included trials to incorporate their findings into all of our calculations of effect estimates, particularly for adverse events and complications. Although some large parent studies of some included trials reported adverse events, we usually were not able to identify outcomes among diabetic participants in the trials of blood pressure intervention or in the subgroup participating in a retinopathy substudy.
Agreements and disagreements with other studies or reviews
A previous qualitative review of current therapy for the prevention and treatment of diabetic retinopathy reached many of the same conclusions as the current report (Mohamed 2007). However, this earlier review did not include results of the ADVANCE/AdRem, DIRECT Prevent 1, DIRECT Protect 1, DIRECT Protect 2, RASS, Steno‐2, and ACCORD Eye trials, which were still in progress at the time of publication of that review. No quantitative synthesis was described in Mohamed 2007; data from the included trials were reported without distinction between outcomes at different follow‐up times and between incidence and progression of diabetic retinopathy. The authors of Arguedas 2013 reviewed data from randomized trials and other studies to estimate the effects of blood pressure control for hypertension in people with diabetes mellitus with respect to death and serious systemic effects; they did not consider diabetic retinopathy. They noted the absence of information regarding effects in people with type 1 diabetes, which was also the smallest subgroup in our review.
Authors' conclusions
Implications for practice.
Among the outcomes examined, benefits with respect to diabetic retinopathy after five years of intervention with tight or intensive blood pressure control were restricted primarily to the subgroup of trial participants with type 2 diabetes whom trial investigators had defined as hypertensive at trial entry. For this subgroup of nearly 9000 diabetics, beneficial effects, although modest, were estimated for four of the six outcomes evaluated in our analysis of the available evidence. On the other hand, among nearly 11,000 trial participants with type 2 diabetes and normal blood pressures, no outcome suggested a benefit regarding efforts to reduce blood pressure to lower levels after five years of intervention. Three trials conducted for participants with type 1 diabetes, all of whom were judged to have normal blood pressure at time of enrollment, provided inconsistent evidence regarding the effects of tight blood pressure control to reduce blood pressures to lower levels. The available evidence indicates that administration of antihypertensive agents does not decrease progression of retinopathy.
Insufficient evidence was available regarding the adverse effects of interventions to achieve current blood pressure targets to permit comparison of benefits and risks. Some physicians use angiotensin‐converting enzyme inhibitors to prevent or delay the development or to slow progression of diabetic nephropathy and anticipate benefit regarding diabetic retinopathy. However, physicians should be aware that data on adverse events related to tight blood pressure control in diabetics are sparse; patients on these medications require close monitoring because they may be at risk for serious adverse events. A few trials reported incidents of hypotension and hyperkalemia experienced by trial participants. Of note, the cost of many of the multifactorial interventions used in the completed randomized trials may be unjustifiable for use among diabetics who are not hypertensive.
Treatment of hypertension in diabetics has been demonstrated to have substantial benefit on survival and other outcomes. Our investigation shows that it also has some benefits with respect to diabetic retinopathy. However, the currently available evidence does not support blood pressure lowering among diabetics without hypertension for the sole purpose of preventing or slowing progression of diabetic retinopathy or for avoiding the need for treatment for advanced stages of diabetic retinopathy.
Implications for research.
The 10 trials that enrolled type 2 diabetics with hypertension and followed them for five or more years after randomization permitted the estimation of intervention effects for this subgroup of 9000 participants. It is unlikely that additional trials conducted for this subgroup of diabetics would modify our effect estimates to a meaningful degree, apart from providing more precise estimates. However, additional randomized controlled trials may help to define subgroups, if any exist, among type 1 diabetics or among type 2 diabetics who have normal blood pressures but who might benefit from medication or other interventions to achieve lower blood pressure targets with respect to reduced incidence and progression of retinopathy. If future trials are undertaken, the designers should specify not only the type of diabetics they wish to enroll (type 1 or type 2), but also whether the goal is prevention of diabetic retinopathy (i.e. only diabetics without retinopathy eligible for enrollment) or slowing the progression of existing retinopathy; the blood pressure status of diabetics eligible to participate (normotensive without treatment, hypertensives with blood pressure controlled with treatment, untreated hypertensives); the blood pressure goals of each trial arm; and vision outcomes, which have been reported rarely in completed trials. Furthermore, target sample sizes should be large enough and follow‐up intervals long enough to provide precise estimates of outcomes, both overall and within subgroups of interest. Although we found reports of hypotension from only three trials and of hyperkalemia from only two other trials among the 29 included trials, the designers of future trials may wish to monitor and report these and other adverse events that are possibly related to blood pressure control interventions.
The evidence from 29 randomized controlled trials conducted with the participation of thousands of diabetics may be interpreted by some researchers and sponsors to mean that additional trials designed to address this issue are not justifiable. An argument can be made that it would be more cost‐effective to focus on research to explain why blood pressure control affects incidence of retinopathy but not progression among hypertensive type 2 diabetics by analyzing individual participant data from completed trials. In the absence of more consistent and convincing data regarding the subgroup of diabetics likely to benefit from intervention on blood pressure, it may be more useful to conduct trials that focus on interventions that influence other modifiable risk factors for diabetic retinopathy.
What's new
| Date | Event | Description |
|---|---|---|
| 23 March 2023 | New search has been performed | Searches updated to remove unrelated list of drugs. |
| 23 March 2023 | New citation required but conclusions have not changed | Fourteen new trials included. |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 1, 2015
| Date | Event | Description |
|---|---|---|
| 8 February 2016 | Amended | ACCORD Eye entries corrected and necessary analyses corrected. |
| 19 August 2008 | Amended | Converted to new review format |
Acknowledgements
We thank Lori Rosman at Cochrane Eyes and Vision Group for devising and running the search strategies for the electronic searches. We also thank Tianjing Li and Gianni Virgili for commenting on early versions of the draft. We are grateful to Robert N Frank (RNF) for his help with the original version of this review and to Kristina Lindsley (KL) for her assistance with screening records retrieved by recent searches of the electronic databases. We acknowledge the Cochrane Eyes and Vision Group editorial team for their advice and assistance during the preparation of this update.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Diabetic Retinopathy] explode all trees #2 diabet* near retinopath* #3 proliferat* near retinopath* #4 vitre* near detach* #5 retina* near detach* #6 diabet* near maculopath* #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Blood Pressure] explode all trees #9 MeSH descriptor: [Blood Glucose] explode all trees #10 MeSH descriptor: [Hypertension] explode all trees #11 (pressure or glucose or glycem*) near/3 blood* #12 hypertens* #13 #8 or #9 or #9 or #10 or #11 or #12 #14 MeSH descriptor: [Antihypertensive Agents] explode all trees #15 MeSH descriptor: [Angiotensin‐Converting Enzyme Inhibitors] explode all trees #16 ACE next inhibitor* #17 antihypertensive near agent* or drug* or medicat* #18 MeSH descriptor: [Calcium Channel Blockers] explode all trees #19 calcium next channel next blocker* #20 calcium next channel next antagonist* #21 MeSH descriptor: [Diuretics] explode all trees #22 diuretic* #23 MeSH descriptor: [Adrenergic beta‐Agonists] explode all trees #24 adrenergic next beta next antagonist* #25 beta next blocker* #26 MeSH descriptor: [Adrenergic alpha‐Agonists] explode all trees #27 adrenergic next alpha next antagonist* #28 MeSH descriptor: [Vasodilator Agents] explode all trees #29 MeSH descriptor: [Angiotensin II Type 1 Receptor Blockers] explode all trees #30 angiotensin near blocker* #31 MeSH descriptor: [Acebutolol] explode all trees #32 acebutolol #33 MeSH descriptor: [Amiloride] explode all trees #34 amiloride #35 MeSH descriptor: [Amlodipine] explode all trees #36 amlodipine #37 atorvastin #38 benazepril #39 MeSH descriptor: [Atenolol] explode all trees #40 atenolol #41 MeSH descriptor: [Bendroflumethiazide] explode all trees #42 bendroflumethiazide #43 MeSH descriptor: [Nadolol] explode all trees #44 nadolol #45 MeSH descriptor: [Betaxolol] explode all trees #46 betaxolol #47 MeSH descriptor: [Bisoprolol] explode all trees #48 bisoprolol #49 bosentan #50 bucindolol #51 MeSH descriptor: [Bumetanide] explode all trees #52 bumetanide #53 candesartan #54 MeSH descriptor: [Captopril] explode all trees #55 captopril #56 MeSH descriptor: [Carteolol] explode all trees #57 carteolol #58 carvedilol #59 MeSH descriptor: [Chlorothiazide] explode all trees #60 chlorothiazide #61 MeSH descriptor: [Chlorthalidone] explode all trees #62 chlorthalidone #63 MeSH descriptor: [Clonidine] explode all trees #64 clonidine #65 MeSH descriptor: [Diazoxide] explode all trees #66 diazoxide #67 MeSH descriptor: [Diltiazem] explode all trees #68 diltiazem #69 MeSH descriptor: [Doxazosin] explode all trees #70 doxazosin #71 MeSH descriptor: [Enalapril] explode all trees #72 MeSH descriptor: [Felodipine] explode all trees #73 felodipine #74 eperenone #75 MeSH descriptor: [Epoprostenol] explode all trees #76 epoprostenol #77 eprosartan #78 esmolol #79 MeSH descriptor: [Ethacrynic Acid] explode all trees #80 ethacrynic next acid #81 MeSH descriptor: [Fenoldopam] explode all trees #82 fenoldopam #83 MeSH descriptor: [Fosinopril] explode all trees #84 fosinopril #85 MeSH descriptor: [Furosemide] explode all trees #86 furosemide #87 MeSH descriptor: [Guanabenz] explode all trees #88 guanabenz #89 guanadrel #90 MeSH descriptor: [Guanfacine] explode all trees #91 guanfacine #92 MeSH descriptor: [Hydralazine] explode all trees #93 hydralazine #94 irbesartan #95 MeSH descriptor: [Lisinopril] explode all trees #96 lisinopril #97 MeSH descriptor: [Losartan] explode all trees #98 losartan #99 methyldopa #100 MeSH descriptor: [Metoprolol] explode all trees #101 metoprolol #102 moexipril #103 olmesartan #104 MeSH descriptor: [Propranolol] explode all trees #105 propranolol #106 quinapril #107 MeSH descriptor: [Spironolactone] explode all trees #108 spironlactone #109 telmisartan #110 MeSH descriptor: [Timolol] explode all trees #111 timolol #112 MeSH descriptor: [Triamterene] explode all trees #113 triamterene #114 valsartan #115 MeSH descriptor: [Hydrochlorothiazide] explode all trees #116 hydrochlorothiazide #117 MeSH descriptor: [Iloprost] explode all trees #118 iloprost #119 MeSH descriptor: [Indapamide] explode all trees #120 indapamide #121 isoxsurpine #122 MeSH descriptor: [Isradipine] explode all trees #123 isradipine #124 MeSH descriptor: [Labetalol] explode all trees #125 labetalol #126 lercanidipine #127 MeSH descriptor: [Mecamylamine] explode all trees #128 mecamylamine #129 MeSH descriptor: [Methyclothiazide] explode all trees #130 methyclothiazide #131 MeSH descriptor: [Metolazone] explode all trees #132 metolazone #133 MeSH descriptor: [Mibefradil] explode all trees #134 mibefradil #135 MeSH descriptor: [Minoxidil] explode all trees #136 minoxidil #137 MeSH descriptor: [Nicardipine] explode all trees #138 nicardipine #139 MeSH descriptor: [Nifedipine] explode all trees #140 nifedipine #141 MeSH descriptor: [Nisoldipine] explode all trees #142 nisoldipine #143 MeSH descriptor: [Nitric Oxide] explode all trees #144 MeSH descriptor: [Nitroprusside] explode all trees #145 nitroprusside #146 omapatrilat #147 MeSH descriptor: [Penbutolol] explode all trees #148 penbutolol #149 MeSH descriptor: [Perindopril] explode all trees #150 perindopril #151 MeSH descriptor: [Phenylbutazone] explode all trees #152 phenylbutazone #153 MeSH descriptor: [Phenoxybenzamine] explode all trees #154 phenoxybenzamine #155 MeSH descriptor: [Phentolamine] explode all trees #156 phentolamine #157 MeSH descriptor: [Pindolol] explode all trees #158 pindolol #159 pindolol #160 MeSH descriptor: [Polythiazide] explode all trees #161 polythiazide #162 MeSH descriptor: [Prazosin] explode all trees #163 prazosin #164 prostacyclin #165 MeSH descriptor: [Ramipril] explode all trees #166 ramipril #167 MeSH descriptor: [Reserpine] explode all trees #168 reserpine #169 sildenafil #170 spirapril #171 terazosin #172 torsemide #173 trandolapril #174 MeSH descriptor: [Verapamil] explode all trees #175 verapamil #176 trepostinil #177 MeSH descriptor: [Triamterene] explode all trees #178 triamterene #179 MeSH descriptor: [Trichlormethiazide] explode all trees #180 trichlormethiazide #181 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 #182 #41 or #42 or (#43 and or#44) or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 #183 #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79 or #80 or #81 or #82 or #83 or #84 or #85 or #86 or #87 or #88 or #89 or #90 or #91 or #92 or #93 or #94 or #95 or #96 or #97 or #98 or #99 or #100 or #101 or #102 or #103 or #104 or #105 or #106 or #107 or #108 or #109 or #110 #184 #111 or #112 or #113 or #114 or #115 or #116 or #117 or #118 or #119 or #120 or #121 or #122 or #123 or #124 or #125 or #126 or #127 or #128 or #129 or #130 or #131 or #132 or #133 or #134 or #135 or #136 or #137 or #138 or #139 or #140 #185#141 or #142 or #143 or #144 or #145 or #146 or #147 or #148 or #149 or #150 or #151 or #152 or #153 or #154 or #155 or #156 or #157 or #158 or #159 or #160 or #161 or #162 or #163 or #164 or #165 or #166 or #167 or #168 or #169 or #170 or #171 or #172 or #173 or #174 or #175 or #176 or #177 or #178 or #179 or #180 #18 6#181 or #182 or #183 or #184 or #185 #187 #7 and #13 #188 #7 and #186 #189 #187 or #188
Appendix 2. MEDLINE (OvidSP) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp diabetic retinopathy/ 14. retinopath$.tw. 15. (vitre$ adj3 detach$).tw. 16. (retina$ adj3 detach$).tw. 17. (diabet$ adj3 maculopath$).tw. 18. or/13‐17 19. exp blood pressure/ 20. exp blood glucose/ 21. exp hypertension/ 22. ((pressure or glucose or glycem$) adj3 blood$).tw. 23. hypertens$.tw. 24. or/19‐23 25. exp antihypertensive agents/ 26. exp angiotensin converting enzyme inhibitors/ 27. (ACE adj1 inhibitor$).tw. 28. (antihypertensive adj2 (agent$ or drug$ or medicat$)).tw. 29. exp calcium channel blockers/ 30. (calcium adj1 channel adj1 blocker$).tw. 31. (calcium adj1 channel adj1 antagonist$).tw. 32. exp diuretics/ 33. diuretic$.tw. 34. exp adrenergic beta antagonists/ 35. (adrenergic adj1 beta adj1 antagonist$).tw. 36. (beta adj1 blocker$).tw. 37. exp adrenergic alpha antagonist/ 38. (adrenergic adj1 alpha adj1 antagonist$).tw. 39. exp vasodilator agents/ 40. exp angiotensin II type 1 receptor blockers/ 41. (angiotensin adj3 blocker$).tw. 42. acebutolol.tw. 43. amiloride.tw. 44. amlodipine.tw. 45. atorvastin.tw. 46. benazepril.tw. 47. atenolol.tw. 48. bendroflumethiazide.tw. 49. nadolol.tw. 50. betaxolol.tw. 51. bisoprolol.tw. 52. bosentan.tw. 53. bucindolol.tw. 54. bumetanide.tw. 55. candesartan.tw. 56. captopril.tw. 57. carteolol.tw. 58. carvedilol.tw. 59. chlorothiazide.tw. 60. chlorthalidone.tw. 61. clonidine.tw. 62. diazoxide.tw. 63. diltiazem.tw. 64. diltiazem.tw. 65. doxazosin.tw. 66. enalapril.tw. 67. enalapril$.tw. 68. felodipine.tw. 69. eplerenone.tw. 70. epoprostenol.tw. 71. eprosartan.tw. 72. esmolol.tw. 73. (ethacrynic adj1 acid).tw. 74. fenoldopam.tw. 75. fosinopril.tw. 76. furosemide.tw. 77. guanabenz.tw. 78. guanadrel.tw. 79. guanfacine.tw. 80. hydralazine.tw. 81. irbesartan.tw. 82. lisinopril.tw. 83. losartan.tw. 84. methyldopa.tw. 85. metoprolol.tw. 86. moexipril.tw. 87. olmesartan.tw. 88. propranolol.tw. 89. quinapril.tw. 90. spironolactone.tw. 91. telmisartan.tw. 92. timolol.tw. 93. triamterene.tw. 94. valsartan.tw. 95. hydrochlorothiazide.tw. 96. iloprost.tw. 97. indapamide.tw. 98. isoxsuprine.tw. 99. isradipine.tw. 100. labetalol.tw. 101. lercanidipine.tw. 102. mecamylamine.tw. 103. methyclothiazide.tw. 104. metolazone.tw. 105. mibefradil.tw. 106. minoxidil.tw. 107. nicardipine.tw. 108. nifedipine.tw. 109. nisoldipine.tw. 110. exp nitric oxide/ 111. exp nitroprusside/ 112. nitroprusside.tw. 113. omapatrilat.tw. 114. penbutolol.tw. 115. perindopril.tw. 116. phenylbutazone.tw. 117. phenoxybenzamine.tw. 118. phentolamine.tw. 119. pindolol.tw. 120. polythiazide.tw. 121. prazosin.tw. 122. prostacyclin.tw. 123. ramipril.tw. 124. reserpine.tw. 125. sildenafil.tw. 126. spirapril.tw. 127. terazosin.tw. 128. torsemide.tw. 129. trandolapril.tw. 130. verapamil.tw. 131. trepostinil.tw. 132. triamterene.tw. 133. trichlormethiazide.tw. 134. or/25‐133 135. 18 and 24 and 12 136. 18 and 134 and 12 137. 135 or 136
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. Embase (OvidSP) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. diabetic retinopathy/ 34. retinopath$.tw. 35. (vitre$ adj3 detach$).tw. 36. (retina$ adj3 detach$).tw. 37. (diabet$ adj3 maculopath$).tw. 38. or/33‐37 39. exp blood pressure/ 40. glucose blood level/ 41. exp hypertension/ 42. ((pressure or glucose or glycem$) adj3 blood$).tw. 43. hypertens$.tw. 44. or/39‐43 45. exp antihypertensive agent/ 46. exp dipeptidyl carboxypeptidase inhibitor/ 47. (ACE adj1 inhibitor$).tw. 48. (antihypertensive adj2 (agent$ or drug$ or medicat$)).tw. 49. exp calcium channel blocking agent/ 50. (calcium adj1 channel adj1 blocker$).tw. 51. (calcium adj1 channel adj1 antagonist$).tw. 52. exp diuretic agent/ 53. diuretic$.tw. 54. exp beta adrenergic receptor blocking agent/ 55. (adrenergic adj1 beta adj1 antagonist$).tw. 56. (beta adj1 blocker$).tw. 57. exp alpha adrenergic receptor blocking agent/ 58. (adrenergic adj1 alpha adj1 antagonist$).tw. 59. exp vasodilator agent/ 60. exp angiotensin 1 receptor antagonist/ 61. (angiotensin adj3 blocker$).tw. 62. acebutolol.tw. 63. amiloride.tw. 64. amlodipine.tw. 65. atorvastin.tw. 66. benazepril.tw. 67. atenolol.tw. 68. bendroflumethiazide.tw. 69. nadolol.tw. 70. betaxolol.tw. 71. bisoprolol.tw. 72. bosentan.tw. 73. bucindolol.tw. 74. bumetanide.tw. 75. candesartan.tw. 76. captopril.tw. 77. carteolol.tw. 78. carvedilol.tw. 79. chlorothiazide.tw. 80. chlorthalidone.tw. 81. clonidine.tw. 82. diazoxide.tw. 83. diltiazem.tw. 84. diltiazem.tw. 85. doxazosin.tw. 86. enalapril.tw. 87. enalapril$.tw. 88. felodipine.tw. 89. eplerenone.tw. 90. epoprostenol.tw. 91. eprosartan.tw. 92. esmolol.tw. 93. (ethacrynic adj1 acid).tw. 94. fenoldopam.tw. 95. fosinopril.tw. 96. furosemide.tw. 97. guanabenz.tw. 98. guanadrel.tw. 99. guanfacine.tw. 100. hydralazine.tw. 101. irbesartan.tw. 102. lisinopril.tw. 103. losartan.tw. 104. methyldopa.tw. 105. metoprolol.tw. 106. moexipril.tw. 107. olmesartan.tw. 108. propranolol.tw. 109. quinapril.tw. 110. spironolactone.tw. 111. telmisartan.tw. 112. timolol.tw. 113. triamterene.tw. 114. valsartan.tw. 115. hydrochlorothiazide.tw. 116. iloprost.tw. 117. indapamide.tw. 118. isoxsuprine.tw. 119. isradipine.tw. 120. labetalol.tw. 121. lercanidipine.tw. 122. mecamylamine.tw. 123. methyclothiazide.tw. 124. metolazone.tw. 125. mibefradil.tw. 126. minoxidil.tw. 127. nicardipine.tw. 128. nifedipine.tw. 129. nisoldipine.tw. 130. exp nitric oxide/ 131. nitroprusside sodium/ 132. nitroprusside.tw. 133. omapatrilat.tw. 134. penbutolol.tw. 135. perindopril.tw. 136. phenylbutazone.tw. 137. phenoxybenzamine.tw. 138. phentolamine.tw. 139. pindolol.tw. 140. polythiazide.tw. 141. prazosin.tw. 142. prostacyclin.tw. 143. ramipril.tw. 144. reserpine.tw. 145. sildenafil.tw. 146. spirapril.tw. 147. terazosin.tw. 148. torsemide.tw. 149. trandolapril.tw. 150. verapamil.tw. 151. trepostinil.tw. 152. triamterene.tw. 153. trichlormethiazide.tw. 154. or/45‐153 155. 38 and 44 156. 38 and 154 157. or/155‐156 158. 32 and 157
Appendix 4. LILACS search strategy
retinopath$ and blood pressure$ or antihypertensive or angiotensin$ or ACE
Appendix 5. ClinicalTrials.gov search strategy
Diabetic Retinopathy AND (Blood Pressure OR Antihypertensive OR Angiotensin OR ACE)
Appendix 6. WHO ICTRP search strategy
Diabetic Retinopathy AND Blood Pressure
Appendix 7. More blood pressure control versus less blood pressure control: outcomes by 3 years or less
Abbreviations: CI, confidence interval; CSME, clinically significant macular edema; M‐H, Mantel‐Haenszel; PDR, proliferative diabetic retinopathy; VH, vitreous hemorrhage
8.1 Incidence of retinopathy by 3 years or less
8.2 Progression of retinopathy by 3 years or less
8.3 Combined incidence and progression of retinopathy by 3 years or less
| Subgroup | Studies | Participants | Risk ratio (M‐H, Random) [95% CI] |
| Normotensive, type 1 | 1 (EUCLID) | 134 | 0.31 [0.09, 1.08] |
| Normotensive, type 2 | 1 (ABCD‐2V (1)) | 129 | 0.95 [0.06, 14.93] |
| Total (95% CI) | 2 | 263 | 0.37 [0.12, 1.13], I2 = 0% |
8.4 Progression to PDR, CSME, or VH by 3 years or less
8.5 Adverse effect: death, 3 years or less
| Subgroup | Studies | Participants | Risk ratio (M‐H, Random) [95% CI] |
| Normotensive, type 1 | 0 | 0 | Not estimable |
| Normotensive, type 2 | 2 (ABCD‐2V (1); Medi‐Cal) | 369 | 2.27 [0.34, 15.22] |
| Total (95% CI) | 2 | 369 | 2.27 [0.34, 15.22], I2 = 0% |
8.6 Adverse effect: hypotension, 3 years or less
| Subgroup | Studies | Participants | Risk ratio (M‐H, Random) [95% CI] |
| Normotensive, type 1 | 1 (AdDIT) | 443 | 2.99 [0.12, 72.92] |
| Normotensive, type 2 | 0 | 0 | Not estimable |
| Total (95% CI) | 1 | 443 | 2.99 [0.12, 72.92] |
Data and analyses
Comparison 1. More blood pressure control versus no (or less) control .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incidence of retinopathy by 5 years | 11 | 4940 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 1.1.1 Normotensive type 1 | 1 | 1421 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.69, 0.97] |
| 1.1.2 Normotensive type 2 | 4 | 1545 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.75, 1.02] |
| 1.1.3 Hypertensive type 2 | 6 | 1974 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.57, 0.98] |
| 1.2 Progression of retinopathy by 5 years | 5 | 5144 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.12] |
| 1.2.1 Normotensive type 1 | 1 | 1905 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.82, 1.29] |
| 1.2.2 Normotensive type 2 | 2 | 2460 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.30] |
| 1.2.3 Hypertensive type 2 | 2 | 779 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.51, 1.06] |
| 1.3 Combined incidence and progression of retinopathy by 5 years | 8 | 6212 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.68, 0.89] |
| 1.3.1 Normotensive type 1 | 1 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.40, 0.91] |
| 1.3.2 Normotensive type 2 | 2 | 1743 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.44] |
| 1.3.3 Hypertensive type 2 | 5 | 4246 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.67, 0.89] |
| 1.4 Incidence of PDR, CSME, or VH by 5 years | 9 | 8237 | Risk Ratio (IV, Fixed, 95% CI) | 0.92 [0.82, 1.04] |
| 1.4.1 Normotensive Type 1 | 2 | 2054 | Risk Ratio (IV, Fixed, 95% CI) | 1.04 [0.81, 1.33] |
| 1.4.2 Normotensive Type 2 | 3 | 2460 | Risk Ratio (IV, Fixed, 95% CI) | 0.99 [0.83, 1.18] |
| 1.4.3 Hypertensive Type 2 | 4 | 3723 | Risk Ratio (IV, Fixed, 95% CI) | 0.77 [0.62, 0.95] |
| 1.5 Loss of visual acuity | 3 | 7392 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.68, 1.49] |
| 1.5.1 Loss of ≥ 3 lines | 2 | 2326 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.63, 2.08] |
| 1.5.2 Unilateral blindness | 3 | 5066 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.42, 1.59] |
| 1.6 Adverse effect: death, 4 to 9 years | 13 | 13979 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.76, 1.00] |
| 1.6.1 Normotensive type 1 | 3 | 3608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.57, 2.36] |
| 1.6.2 Normotensive type 2 | 3 | 2599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.62, 1.19] |
| 1.6.3 Hypertensive type 2 | 7 | 7772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.74, 1.01] |
| 1.7 Adverse effect: hypotension | 2 | 3323 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.63, 2.55] |
| 1.8 Adverse effect: hyperkalemia | 2 | 2825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.54] |
| 1.9 Progression to PDR, CSME, or VH by 4 to 9 years | 10 | 9759 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 1.9.1 Normotensive type 1 | 2 | 2128 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.80, 1.32] |
| 1.9.2 Normotensive type 2 | 3 | 2460 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.18, 2.23] |
| 1.9.3 Hypertensive type 2 | 5 | 5171 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.43, 0.82] |
1.7. Analysis.

Comparison 1: More blood pressure control versus no (or less) control , Outcome 7: Adverse effect: hypotension
1.8. Analysis.

Comparison 1: More blood pressure control versus no (or less) control , Outcome 8: Adverse effect: hyperkalemia
1.9. Analysis.

Comparison 1: More blood pressure control versus no (or less) control , Outcome 9: Progression to PDR, CSME, or VH by 4 to 9 years
Comparison 2. Outcomes by type of diabetes: More blood pressure control versus no (or less) control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Incidence of diabetic retinopathy by 5 years | 11 | 4940 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 2.1.1 Type 1 diabetes | 1 | 1421 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.69, 0.97] |
| 2.1.2 Type 2 diabetes | 10 | 3519 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 2.2 Progression of diabetic retinopathy by 5 years | 5 | 5144 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.12] |
| 2.2.1 Type 1 | 1 | 1905 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.82, 1.29] |
| 2.2.2 Type 2 | 4 | 3239 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.71, 1.14] |
| 2.3 Combined incidence and progression by 5 years | 8 | 6212 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.68, 0.89] |
| 2.3.1 Type 1 | 1 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.40, 0.91] |
| 2.3.2 Type 2 | 7 | 5989 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.69, 0.92] |
| 2.4 Incidence of PDR, CSME, or VH by 5 years | 9 | 8612 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.57, 1.07] |
| 2.4.1 Type 1 | 2 | 2128 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.80, 1.32] |
| 2.4.2 Type 2 | 7 | 6484 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.33, 1.04] |
| 2.5 Adverse effect: death, 4 to 9 years | 13 | 13884 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 2.5.1 Type 1 | 3 | 3513 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.58, 2.45] |
| 2.5.2 Type 2 | 10 | 10371 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 0.99] |
2.3. Analysis.

Comparison 2: Outcomes by type of diabetes: More blood pressure control versus no (or less) control, Outcome 3: Combined incidence and progression by 5 years
2.4. Analysis.

Comparison 2: Outcomes by type of diabetes: More blood pressure control versus no (or less) control, Outcome 4: Incidence of PDR, CSME, or VH by 5 years
Comparison 3. Outcomes by blood pressure status at entry: More blood pressure control versus no (or less) control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Incidence of diabetic retinopathy by 5 years | 11 | 4940 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 3.1.1 Normotensive or treated hypertensive | 5 | 2966 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.76, 0.95] |
| 3.1.2 Hypertensive | 6 | 1974 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.57, 0.98] |
| 3.2 Progression of diabetic retinopathy by 5 years | 5 | 5144 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.12] |
| 3.2.1 Normotensive or treated hypertensive | 3 | 4365 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.87, 1.18] |
| 3.2.2 Hypertensive | 2 | 779 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.51, 1.06] |
| 3.3 Combined incidence and progression of diabetic retinopathy by 5 years | 8 | 6212 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.68, 0.89] |
| 3.3.1 Normotensive or treated hypertensive | 3 | 1966 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.57, 1.15] |
| 3.3.2 Hypertensive | 5 | 4246 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.67, 0.89] |
| 3.4 Incidence of PDR, CSME, or VH by 5 years | 9 | 8867 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.59, 1.08] |
| 3.4.1 Normotensive or treated hypertensive | 5 | 4588 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.19] |
| 3.4.2 Hypertensive | 4 | 4279 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.33, 0.84] |
| 3.5 Adverse effect: death, 4 to 9 years | 13 | 13979 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 3.5.1 Normotensive or treated hypertensive | 6 | 6207 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.67, 1.21] |
| 3.5.2 Hypertensive | 7 | 7772 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.74, 1.01] |
3.3. Analysis.

Comparison 3: Outcomes by blood pressure status at entry: More blood pressure control versus no (or less) control, Outcome 3: Combined incidence and progression of diabetic retinopathy by 5 years
3.5. Analysis.

Comparison 3: Outcomes by blood pressure status at entry: More blood pressure control versus no (or less) control, Outcome 5: Adverse effect: death, 4 to 9 years
Comparison 4. Outcomes by 7 to 9 years: More blood pressure control versus no (or less) control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Incidence of retinopathy | 3 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.56, 0.88] |
| 4.1.1 Normotensive type 1 | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.1.2 Normotensive type 2 | 1 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.34] |
| 4.1.3 Hypertensive type 2 | 2 | 369 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.89] |
| 4.2 Progression of retinopathy | 2 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.49] |
| 4.2.1 Normotensive type 1 | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.2.2 Normotensive type 2 | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.2.3 Hypertensive type 2 | 2 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.49] |
| 4.3 Combined incidence and progression of retinopathy | 2 | 2700 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.75, 0.97] |
| 4.3.1 Normotensive type 1 | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.3.2 Normotensive type 2 | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.3.3 Hypertensive type 2 | 2 | 2700 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.75, 0.97] |
| 4.4 Incidence of PDR, CSME, or VH | 1 | 1148 | Odds Ratio (IV, Fixed, 95% CI) | 0.64 [0.43, 0.95] |
| 4.4.1 Normotensive type 1 | 0 | 0 | Odds Ratio (IV, Fixed, 95% CI) | Not estimable |
| 4.4.2 Normotensive type 2 | 0 | 0 | Odds Ratio (IV, Fixed, 95% CI) | Not estimable |
| 4.4.3 Hypertensive type 2 | 1 | 1148 | Odds Ratio (IV, Fixed, 95% CI) | 0.64 [0.43, 0.95] |
| 4.5 Blind eyes | 2 | 1306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.11] |
4.1. Analysis.

Comparison 4: Outcomes by 7 to 9 years: More blood pressure control versus no (or less) control, Outcome 1: Incidence of retinopathy
4.2. Analysis.

Comparison 4: Outcomes by 7 to 9 years: More blood pressure control versus no (or less) control, Outcome 2: Progression of retinopathy
4.3. Analysis.

Comparison 4: Outcomes by 7 to 9 years: More blood pressure control versus no (or less) control, Outcome 3: Combined incidence and progression of retinopathy
4.4. Analysis.

Comparison 4: Outcomes by 7 to 9 years: More blood pressure control versus no (or less) control, Outcome 4: Incidence of PDR, CSME, or VH
4.5. Analysis.

Comparison 4: Outcomes by 7 to 9 years: More blood pressure control versus no (or less) control, Outcome 5: Blind eyes
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ABCD (1).
| Study characteristics | ||
| Methods |
Study design: 1 of 2 parallel‐group RCTs conducted within ABCD Unit of randomization and analysis: individual Number randomized, total: 480 normotensive participants Per group: 237 to intensive therapy and 243 to moderate therapy; of intensive therapy group, 118 to nisoldipine, 119 to enalapril Sample size calculation: sample size calculation was based on glomerular filtration rate; power was not reported |
|
| Participants |
Country: USA Study period: accrual: March 1991 to May 1993; follow‐up planned for 5 to 7 years after March 1991 Type of diabetes: type 2 Participants’ status at baseline:
Equivalence of intervention groups at baseline: well balanced Inclusion criteria: type 2 diabetes diagnosed by WHO criteria, age 40 to 74 years at study enrollment, DBP 80 to 89 mmHg, willing to participate in study, and likely to complete 5 to 7 years of study Exclusion criteria: pregnancy or lactation (women), allergies to study medications, heart disease including uncorrected heart block, myocardial infarction, angina or heart failure, malignant hypertension, peripheral vascular disease, aortic dissection, on dialysis or other kidney disease, liver disease |
|
| Interventions |
Intervention 1: intensive blood pressure control
Goal DBP 10 mmHg below baseline
Participants were randomized again to either nisoldipine 10 mg/day, titrated to 20, 40, and then 60 mg/day (plus placebo for enalapril), or enalapril 5 mg/day titrated to 10, 20, and then 40 mg/day (plus placebo for nisoldipine) as the initial antihypertensive medication. Intervention 2: moderate blood pressure control Goal DBP 80 to 89 mmHg; randomized to nisoldipine or enalapril if DBP ≥ 90 mmHg Both interventions: additional antihypertensive medications were added in an open‐label, step‐wise manner, initially with metoprolol, then hydrochlorothiazide, and then until the target blood pressure was achieved. Addition of medications was at the discretion of the medical director, but the additional medications could not include calcium channel blockers or ACEi. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of retinopathy: progression of retinopathy defined as 2‐step worsening from baseline based on 7‐field stereoscopic fundus photographs Secondary outcomes, as specified for this review: progression to PDR Other diabetic retinopathy outcomes: none reported Retinopathy diagnosis and monitoring: "Retinopathy was staged ... by the Fundus Photograph Reading Center at the University of Wisconsin ... All retinal films were interpreted and graded ... without knowledge of the treatment arm. The graders used the protocol of the Early Treatment Diabetic Retinopathy Study (ETDRS)." Participants (eyes) examined for the outcomes: 480 Intervals at which outcomes were assessed: retinal photographs taken at baseline and 2‐ and 5‐year exams Cost of interventions: not reported Quality of life: not reported Adverse outcomes: death, CV events Other outcomes reported from the study: glomerular filtration rate, urinary albumin excretion, left ventricular hypertrophy, neuropathy, stroke, and cardiovascular events |
|
| Notes |
Source of funding: industry (Bayer) and government (NIDDK) Declaration of interest: not reported Run‐in length: 7 to 11 weeks on placebo Class(es) of antihypertensive agents assigned: calcium channel blocker, ACEi Degrees of blood pressure control achieved: final 4 years: SBP 128 (12) mmHg in intensive group, 137 (11) mmHg in moderate group; DBP 75 (5) mmHg intensive group, 81 (5) mmHg moderate group Trial registration: none reported and none found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization using permuted block randomization within strata was used to ensure equal sample sizes within all arms of the study.” |
| Allocation concealment (selection bias) | Low risk | “The random assignment to intensive versus moderate treatment with either active nisoldipine coat‐core or enalapril medication was made by telephone by the Data Coordinating Center and the Clinical Coordinating Center.” |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | “The drugs and placebos were administered in a double‐blind manner. If the single study medication assigned did not achieve the target blood pressure, then open‐label antihypertensive medications were added in a stepwise fashion until the target blood pressure was achieved.” "Study nurses were blinded [sic] to the use of enalapril versus nisoldipine but could not be blinded with respect to intensive versus moderate blood pressure control." “All retinal films are interpreted and staged at the Wisconsin Retinal Reading Center without knowledge of the group to which the patient has been randomized.” |
| Masking (performance bias and detection bias) Secondary outcomes | Low risk | “The drugs and placebos were administered in a double‐blind manner. If the single study medication assigned did not achieve the target blood pressure, then open‐label antihypertensive medications were added in a stepwise fashion until the target blood pressure was achieved.” "Study nurses were blinded [sic] to the use of enalapril versus nisoldipine but could not be blinded with respect to intensive versus moderate blood pressure control." “All retinal films are interpreted and staged at the Wisconsin Retinal Reading Center without knowledge of the group to which the patient has been randomized.” |
| Incomplete outcome data (attrition bias) Primary outcome | High risk | 83 participants did not complete 5‐year follow‐up exam, 28 of whom had died (i.e. 20% of remainder not examined). Numerators and denominators of retinopathy outcomes not given; only percentages reported. No analysis to account for attrition |
| Selective reporting (reporting bias) | Unclear risk | Unclear with available information |
| Other bias | High risk | “Financial support was provided by Bayer Pharmaceutical Company.” “Supported by the Bayer Pharmaceutical Company and by a grant (DK50298‐02) from the National Institute of Diabetes and Digestive and Kidney Diseases." “We are indebted to the members of the Data and Safety Monitoring Committee for their guidance: Paul W. Whelton, M.D., Tulane University, New Orleans; . . . and Kevin Higgins, M.D., Bayer Pharmaceuticals, West Haven, Conn.” |
ABCD (2).
| Study characteristics | ||
| Methods |
Study design: 1 of 2 parallel‐group RCTs conducted within ABCD Unit of randomization and analysis: individual Number randomized, total: 470 hypertensive participants Per group: 237 to intensive therapy and 233 to moderate therapy; second randomization to nisoldipine or enalapril Sample size calculation: sample size calculation was based on glomerular filtration rate; power was not reported |
|
| Participants |
Country: USA Study period: accrual: March 1991 to May 1993; follow‐up planned for 5 to 7 years after March 1991 Type of diabetes: type 2 Participants’ status at baseline:
Equivalence of intervention groups at baseline: well balanced Inclusion criteria: type 2 diabetes diagnosed by WHO criteria, aged 40 to 74 years at study enrollment, DBP ≥ 90 mmHg and off all antihypertensive medication, willing to participate in study, and likely to complete 5 to 7 years of study Exclusion criteria: pregnancy or lactation (women), allergies to study medications, heart disease including uncorrected heart block, myocardial infarction, angina or heart failure, malignant hypertension, peripheral vascular disease, aortic dissection, on dialysis or other kidney disease, liver disease |
|
| Interventions |
Intervention 1: intensive blood pressure control
Goal DBP 75 mmHg
Participants were randomized again to either nisoldipine 10 mg/day, titrated to 20, 40, and then 60 mg/day (plus placebo for enalapril), or enalapril 5 mg/day titrated to 10, 20, and then 40 mg/day (plus placebo for nisoldipine) as the initial antihypertensive medication. Additional antihypertensive medications were added in an open‐label, step‐wise manner initially with metoprolol, then hydrochlorothiazide, and then until the target blood pressure was achieved. Addition of medications was at the discretion of the medical director, but the additional medications could not include calcium channel blockers or ACEi. Intervention 2: moderate blood pressure control Goal DBP 89 mmHg Both interventions: additional antihypertensive medications were added in open‐label, step‐wise manner, initially with metoprolol, hydrochlorothiazide, and then until the target blood pressure was achieved. Addition of medications was at the discretion of the medical director, but the additional medications could not include calcium channel blockers or ACEi. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of retinopathy by 2 and 5 years follow‐up, progression of retinopathy by 2‐step and 3‐step worsening from baseline, based on photo gradings by the Wisconsin Retinal Reading Center Secondary outcomes, as specified for this review: none reported Other diabetic retinopathy outcomes: none reported Retinopathy diagnosis and monitoring: "All retinal films were interpreted and graded by the Fundus Photograph Reading Center at the University of Wisconsin without knowledge of the treatment arm. The graders used the protocol of the Early Treatment Diabetic Retinopathy Study (ETDRS)." Participants (eyes) examined for the outcomes: 470 Intervals at which outcomes were assessed: based on retinal photographs taken at 2‐ and 5‐year follow‐up Cost of interventions: not reported Quality of life: not reported Adverse outcomes: death Other outcomes reported from the study: glomerular filtration rate, urinary albumin excretion, left ventricular hypertrophy, neuropathy, and cardiovascular events |
|
| Notes |
Source of funding: industry (Bayer) and government (NIDDK) Declaration of interest: not reported Run‐in length: 7 to 11 weeks on placebo Class(es) of antihypertensive agents assigned: calcium channel blocker, ACEi Degrees of blood pressure control achieved:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization using permuted block randomization within strata was used to ensure equal sample sizes within all arms of the study.” |
| Allocation concealment (selection bias) | Low risk | “The random assignment to intensive versus moderate treatment with either active nisoldipine coat‐core or enalapril medication was made by telephone by the Data Coordinating Center and the Clinical Coordinating Center.” |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | “The drugs and placebos were administered in a double‐blind manner. If the single study medication assigned did not achieve the target blood pressure, then open‐label antihypertensive medications were added in a stepwise fashion until the target blood pressure was achieved.” “All retinal films are interpreted and staged at the Wisconsin Retinal Reading Center without knowledge of the group to which the patient has been randomized.” |
| Incomplete outcome data (attrition bias) Primary outcome | High risk | No specific information on withdrawals, exclusions, or losses to follow‐up was reported. However, figures in each report of outcomes show numbers of participants at each examination through 5 years that suggest ~40% of those enrolled were lost to follow‐up sometime during the 5‐year period. No analysis to account for attrition |
| Selective reporting (reporting bias) | Unclear risk | Unclear with available information. Percentages reported without explicit denominators for outcomes during 5 years. |
| Other bias | High risk | “Financial support was provided by Bayer Pharmaceutical Company.” “Supported by the Bayer Pharmaceutical Company and by a grant (DK50298‐02) from the National Institute of Diabetes and Digestive and Kidney Diseases." “We are indebted to the members of the Data and Safety Monitoring Committee for their guidance: Paul W. Whelton, M.D., Tulane University, New Orleans; ... and Kevin Higgins, M.D., Bayer Pharmaceuticals, West Haven, Conn.” |
ABCD‐2V (1).
| Study characteristics | ||
| Methods |
Study design: single‐center randomized controlled trial Unit of randomization and analysis: individual Number randomized:
Sample size calculation: former participants in ABCD (2); i.e. convenience sample |
|
| Participants |
Country: USA Study period: not reported Type of diabetes: type 2 Participants' status at baseline:
Equivalence of groups at baseline: more retinopathy in moderate group; otherwise well balanced Inclusion criteria: type 2 diabetes, 40 to 81 years of age, SBP < 140 mmHg, DBP 80 to 89 mmHg and without evidence of overt albuminuria (< 200 μg/min) Exclusion criteria: "pregnant or lactating women, need for any antihypertensive medications, documented myocardial infarction or cerebrovascular accident within the past 6 months, severe peripheral vascular disease, history of bilateral renal artery stenosis or stenosis in a solitary kidney, evidence of severe liver disease, hyperkalemia, or history of active cancer." |
|
| Interventions |
Intervention 1: intensive treatment: valsartan 80 mg/day with target DBP 75 mmHg; medications increased in step‐wise manner to achieve target (valsartan 160 mg/day, hydrochlorothiazide 12.5 mg/day, 25 mg/day, metoprolol 50 mg/day, 100 mg twice a day, additional medication at discretion of medical director) Intervention 2: moderate treatment: placebo while DBP 80 to 89 mmHg; if SBP ≥ 140 mmHg, or DBP ≥ 90 mmHg, antihypertensive medications added step‐wise as described for intensive treatment Length of follow‐up:
|
|
| Outcomes |
Primary outcomes for this review: progression of retinopathy Secondary outcomes for this review: none mentioned Other diabetic retinopathy outcomes: regression of diabetic retinopathy Retinopathy diagnosis and monitoring: methods not described; possibly similar to those used in ABCD (1) and ABCD (2) Intervals at which outcomes were assessed: every 6 months Participants (eyes) examined for outcomes: 119 (?) Cost of interventions: not reported Quality of life: not reported Adverse outcomes: death Other outcomes: change in creatinine clearance from baseline, proportion of participants with doubling of serum creatinine, and change in log urinary excretion from baseline, progression/regression of neuropathy, incidence of cardiovascular events |
|
| Notes |
Source of funding: Novartis Pharmaceutical Company Declaration of interest: not reported Run‐in length: 5 weeks on placebo Class(es) of antihypertensive agents assigned: ARB (valsartan) Degree of blood pressure control achieved: "average over follow‐up"; 118 (10.9)/75 (5.7) mmHg intensive arm, 124 (10.9)/80 (6.5) mmHg moderate arm (SBP [SD]/DBP [SD]) Trial registration: not reported and not found in registers consulted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The Colorado Prevention Center (CPC) statistician generated the randomization list using SAS version 6.12 ... Randomization was stratified by sex and baseline serum creatinine concentration." |
| Allocation concealment (selection bias) | Low risk | "No copies of the original randomization list were distributed. The original randomization list was secured within the Data Coordinating Center of the CPC." |
| Masking (performance bias and detection bias) Primary outcomes | High risk | "To achieve the study BP goals, the study nurses by necessity knew the BP treatment categories for their individual patients." |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | "Five [7.5%] participants in the intensive BP control group and five [7.9%] in the moderate BP control group discontinued the study before study termination." One of the five participants in the intensive group died before the end of the study. No analysis to account for losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or design paper found for this trial that described outcomes assessed for comparison to those reported. |
| Other bias | High risk | Industry support and no declaration of interest |
ACCORD Eye.
| Study characteristics | ||
| Methods |
Study design: substudy of ACCORD – a 2 x 2 factorial RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: target of 4065, which would have given 80% power to detect a 20% relative reduction with intensive blood pressure control as compared with standard blood pressure control (power of 77% for the blood pressure question for ACCORD Eye with 1263 enrolled participants, per ad hoc calculation) |
|
| Participants |
Country: USA and Canada Study period: randomization in ACCORD began January 2001 (for Vanguard centers), was restarted February 2003, and ended October 2005; follow‐up completed June 2009; 8‐year follow‐up of ACCORD Eye survivors 2010 to 2014 Type of diabetes: type 2 Participants’ status at baseline inACCORD Eyeblood pressure trial except as noted:
Equivalence of groups at baseline: imbalances on gender, cholesterol level, blood pressure, diabetic retinopathy Inclusion criteria: type 2 diabetes (> 3 months); glycated hemoglobin level of ≥ 7.5%; and high risk for cardiovascular disease (established cardiovascular disease or had known risk factors); retinal photographs at baseline (for ACCORD Eye) Exclusion criteria: history of PDR that had been treated with laser photocoagulation or vitrectomy |
|
| Interventions |
Intervention 1: intensive blood pressure control (intervention) arm targeted SBP < 120 mmHg. The recommendation was to start with a combination of a diuretic and either an ACEi or a beta‐blocker at randomization. Drug doses to be increased or additional antihypertensive medications added, or both, at "milepost" visits in the intensive group until goal was reached. Intervention 2: standard blood pressure control arm targeted SBP 130 to 139 mmHg. Medication dose titration or the addition of another drug was indicated whenever SBP was ≥ 160 mmHg at a single visit or ≥ 140 mmHg at 2 successive visits. Treatment algorithms for both arms described in Cushman 2007. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: progression of diabetic retinopathy Secondary outcomes, as specified for this review: development of PDR necessitating photocoagulation therapy or vitrectomy; macular edema; moderate vision loss from baseline to 4 years (i.e. loss of ≥ 3 lines on logMAR visual acuity charts) Other diabetic retinopathy outcomes: legal blindness (20/160 or worse), severe vision loss (5/200) Retinopathy diagnosis and monitoring: "The fundus photographs were evaluated by trained graders, who were unaware of the treatment assignments, at the Fundus Photograph Reading Center (University of Wisconsin, Madison), on the basis of the photographic standards defined for the Early Treatment Diabetic Retinopathy Study (ETDRS) and graded according to an abbreviated and modified version of the ETDRS Final Retinopathy Severity Scale for Persons, which combines the severity levels from both eyes for each person." Participants (eyes) examined for the outcomes: 1263 for retinopathy, 1746 for visual acuity Intervals at which outcomes were assessed: visual acuity assessed at baseline, years 2, 4, 6, and 8 or at the study close‐out visit; retinopathy assessed at baseline, 4 and 8 years Cost of the interventions: not reported Quality of life: not reported Adverse outcomes: death, CV events, renal failure, hypotension Other outcomes reported: none from ACCORD Eye |
|
| Notes |
Sources of funding: government; industry‐donated study medications, equipment, and supplies Declaration of interest: several authors declared interests related to pharmaceutical companies Class(es) of antihypertensive agents assigned: not specified Degree of blood pressure control achieved: intensive: median SBP < 120 mmHg (115 to 120); standard: median SBP < 140 mmHg (135 to 140) Trial registration: NCT00542178 Other: participants were also randomized to intensive (maintenance of glycated hemoglobin < 6.0%) or standard (glycated hemoglobin 7.0% to 7.9%) glycemic control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “An Internet‐based, web browser randomization procedure will be employed in ACCORD” (protocol). Algorithm used to generate random sequence was not described clearly. |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed centrally on the study's web site with the use of permuted blocks to maintain concealment of future study‐group assignments" |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | The blood pressure component of ACCORD was an unmasked, open‐label randomized trial. However: "The fundus photographs were evaluated by trained graders, who were unaware of the treatment assignments ..." |
| Masking (performance bias and detection bias) Secondary outcomes | Unclear risk | Not reported whether visual acuity assessors and ophthalmologists were masked to interventions |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | 291 of 1263 participants (23%), 155 assigned to intensive blood pressure control and 136 assigned to standard blood pressure control, had missing 4‐year outcome data; number of deaths included in 291 not found. "there was no evidence of significant differences regarding the amount of missing data, and the results of sensitivity analyses supported those of the primary analyses." Used logistic regression methods for multiple imputation |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | See above |
| Selective reporting (reporting bias) | Unclear risk | Progression to PDR or CSME, or both, not reported. |
| Other bias | Unclear risk | 65 of 3537 (1.8%) of randomized participants enrolled were subsequently excluded from the trial due to detection of exclusion criteria after randomization. "... baseline fundus photographs could not be obtained within four months of randomization." Conflicts of interest for the Data and Safety Monitoring Board members are unclear. "For the Eye Substudy (in a subset of 4065 participants), the baseline eye exam/fundus photography can be performed up to 2 months post‐randomization." (protocol) |
AdDIT.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Unit of randomization and analysis: individual Number randomized:
Sample size calculation: a sample size of 400 (100 participants in each group) would provide a power of 93% to detect a 25% lower albumin‐to‐creatinine ratio among the participants receiving the ACEi (ACEi plus placebo and ACEi plus statin [200 participants]) than among those receiving placebo (placebo plus placebo and statin plus placebo [200 participants]) or among the participants receiving the statin (statin plus placebo and ACEi plus statin [200 participants]) than among those receiving placebo (placebo plus placebo and ACEi plus placebo [200 participants]) |
|
| Participants |
Country: UK, Canada, Australia Study period: accrual May 2009 to August 2013; treatment until March 2016 Type of diabetes: type 1 Participants’ status at baseline:
Equivalence at baseline: participant characteristics similar in all 4 groups Inclusion criteria: 10 to 16 years of age and had received a diagnosis of diabetes at least 1 year earlier (or diagnosis within the past year with an undetectable C‐peptide level) Exclusion criteria: not type 1 diabetes, pregnancy or unwillingness to adhere to contraceptive advice and pregnancy testing, severe hyperlipidemia or a family history suggesting familial hypercholesterolemia, hypertension unrelated to diabetic nephropathy, previous exposure to the investigational drugs, unwillingness or inability to adhere to the trial protocol, the presence of coexisting conditions (excluding treated hypothyroidism and celiac disease), proliferative retinopathy, and the presence of renal disease that was not associated with type 1 diabetes |
|
| Interventions |
Intervention 1: ACEi + placebo or ACEi + statin (ACEi = quinapril; statin = atorvastatin) Intervention 2: placebo: statin + placebo and placebo + placebo (placebos matched to quinapril and atorvastatin) Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: progression of diabetic retinopathy, 2‐step, and 3‐step Secondary outcome, as specified for this review: none Other diabetic retinopathy outcome: not reported Retinopathy diagnosis and monitoring: "Diabetic retinopathy was graded centrally accordingly [sic] to the Early Treatment Diabetic Retinopathy [Study] (ETDRS) adaptation of the modified Airlie House classification of diabetic retinopathy at the Centre for Eye Research, Melbourne, Australia. The level of severity was assigned from a 15‐step diabetic retinopathy severity scale modified from the ETDRS grade." Participants (eyes) examined for the outcome: ACEi 190; placebo 187; 377 total Intervals at which outcomes assessed: annually for diabetic retinopathy Cost of interventions: not yet reported Quality of life: assessed at baseline and annually; not yet reported Adverse outcomes: symptomatic hypotension Other outcomes reported from the study: primary: change in albumin secretion rate; secondary: changes in IMT, blood pressure, lipids, and GFR Adverse outcomes: ocular: none reported; systemic: 4 cases of symptomatic hypotension |
|
| Notes |
Source of funding: DRF, Diabetes UK, British Heart Foundation, JDRF Canadian Clinical Trials Network, Canadian Diabetes Association, Heart and Stroke Fund of Canada; for retinopathy gradings, National Health and Research Council Australia Declaration of interest: many declared Run‐in length: not applicable Class(es) of antihypertensive agents assigned: ACEi (quinapril) Degrees of blood pressure control achieved: "non‐significant lower systolic blood pressure z scores"; little change from baseline over 4 years of follow‐up Trial registration: ClinicalTrials.gov NCT01581476; ISRCTN91419926; EudraCT2007‐001039‐72 Notes: 5‐year follow‐up study underway; diabetic retinopathy specified as outcome #2 Note that DR progression was measured by 2‐step and 3‐step. The 2‐step results were recorded for 3 years, and the 3‐step changes were recorded for 4 years. ACEi versus placebo: 2‐step change (HR 1.12, 95% CI 0.70 to1.60); 3‐step change (HR 1.03, 95% CI 0.71 to 1.50) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomized by the PI [principal investigator] using a secure internet‐based service http://www.sealedenvelope.org . . . provides randomization with minimization. . . on baseline HbA1c, gender, age, duration of diabetes and total cholesterol” |
| Allocation concealment (selection bias) | Unclear risk | Randomized by the Principal Investigator using a secure internet‐based service (www.sealedenvelope.com). |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | Central review and grading of digital images of both eyes for diabetic retinopathy |
| Incomplete outcome data (attrition bias) Primary outcome | High risk | > 15% withdrawals from the trial; 19 participants were excluded from the combined placebo arms, and 18 participants were excluded from the combined ACEi arms |
| Selective reporting (reporting bias) | Unclear risk | Quality of life data and economic data not yet reported. |
| Other bias | Low risk | No evidence of bias due to other sources |
ADDITION‐Europe.
| Study characteristics | ||
| Methods |
Study design: parallel‐group cluster‐RCT Unit of randomization and analysis: clinical practices Number randomized:
Sample size calculation: "We calculated that a patient‐level randomised trial would have required enrolment of 2700 individuals (1350 per treatment group) to detect a 30% reduction in the risk of the primary endpoint at a 5% significance level, and with 90% power. This calculation allowed for 10% loss to follow‐up and assumed an event rate in the routine care group of 3% per year, on the basis of the results of the UK Prospective Diabetes Study Group (UKPDS). We expected a minimum effect of clustering within general practice, with the estimated within‐cluster correlation coefficient being 0.01. We assumed that the average number of participants per general practice would be 10 and, therefore, the design effect was 1·09. Thus, we inflated the estimated sample size for this cluster trial to 3000 patients in total." |
|
| Participants |
Country: Denmark, UK, the Netherlands Study period: September 2001 to December 2009 Type of diabetes: type 2 Participants' status at baseline:
Equivalence of groups at baseline: small but statistically significant differences between groups in favor of intensive group for SBP, DBP, glycated hemoglobin, and total and LDL cholesterol Inclusion criteria: newly diagnosed type 2 diabetes; for retinopathy, digital images of fovea and macula of each eye at 5‐year follow‐up exam Exclusion criteria: cancer or other illness with a life expectancy of < 12 months, psychological or psychiatric problems that limit participation, housebound, pregnancy, lactation, contraindication to study medications, history of alcoholism or drug abuse |
|
| Interventions |
Intervention 1: intensive treatment: step‐wise treatment of glycated hemoglobin, blood pressure, and cholesterol to achieve goals: < 7.0%, ≲ 135/75 mmHg, < 5.0 mmol/dL without IHD or < 4.5 mmol/dL with IHD Intervention 2: routine care: "Patients received standard care according to the recommendations applicable to each centre." Length of follow‐up:
|
|
| Outcomes |
Primary outcomes for this review: progression of retinopathy Secondary outcomes for this review: not reported Other diabetic retinopathy outcomes: blindness, macular edema; incidence of severe DR vs no/less DR reportedly assessed but not reported Retinopathy diagnosis and monitoring: "Three certified graders unaware of the participants' study group allocation used a quantitative method to grade retinal images, which were subsequently categorized according to the Early Treatment Diabetic Retinopathy Study (ETDRS) semiquantitative scale. Two binary endpoints were then defined: 1) any retinopathy versus no retinopathy and 2) severe or proliferative retinopathy versus none, mild, or moderate retinopathy." Participants (eyes) examined for outcome: 2190 participants (< 80% of survivors at 5 years) Intervals at which outcomes were assessed: 5 years after enrollment Cost of interventions: intensive intervention cost GBP 981 per patient, and was not cost‐effective at cost > GBP 631 per patient Quality of life: assessed for 2861 participants (with imputation) (and 2217 with complete data in a separate report). Health status, well‐being, diabetes‐specific quality of life, and treatment satisfaction did not differ between groups. Adverse outcomes: death Other outcomes: composite CVD event (primary): MI, death, stroke, revascularization, amputation individual events (secondary) |
|
| Notes |
Sources of funding: multiple sources from government agencies, foundations, etc. Declaration of interest: many declarations of interest regarding multiple sources including grants, speaking and travel expenses, advisory board memberships, etc. Run‐in length: not applicable Classes of antihypertensive agents assigned: none Degree of blood pressure control achieved: 5‐year follow‐up, intensive 134.8/79.5 mmHg, routine 137.1/80.7 mmHg Trial registration: NCT00237549 Other: author's contact information: Annelli Sandbaek, annelli.sandbaek@alm.au.dk. Study website: www.addition.au.dk. ADDITION‐Leicester (NCT00318032) reported 5‐year rates of outcomes from 1 participating site and used local data to model outcome rates and to make 10‐ and 20‐year projections. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The practices were randomly assigned by statisticians independent of measurement teams to provide intensive multifactorial treatment or routine diabetes care according to national guidelines in a 1:1 ratio. Randomization included statification by [different factors by country]." |
| Allocation concealment (selection bias) | Unclear risk | Practices were randomized before the screening phase began; some dropped out before the treatment phase. |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | Low risk of bias for retinopathy assessment: "Retinopathy was assessed using gradable digital images (two from each eye; one with the fovea in the center and one with the macula in the center)." "Three certified graders unaware of the participants' study group allocation used a quantitative method to grade retinal images, which were subsequently categorized according to the [ETDRS] semiquantitative scale." |
| Masking (performance bias and detection bias) Secondary outcomes | Unclear risk | No information available for visual acuity |
| Incomplete outcome data (attrition bias) Primary outcome | High risk | Retinal images available (taken at follow‐up visit or extracted from medical records) for 2190 (76.6%) of 2861 participants alive at 5 years. |
| Selective reporting (reporting bias) | Unclear risk | "Two binary endpoints were then defined: 1) any retinopathy versus no retinopathy and 2) severe or proliferative retinopathy versus none, mild, or moderate retinopathy." The first of these 2 outcomes was reported, but not the second. |
| Other bias | High risk | No retinal images were taken at study entry for DR assessment at the time of diagnosis. Also, follow‐up images could have been taken 3 to 5 years after baseline. |
ADVANCE/AdRem.
| Study characteristics | ||
| Methods |
Study design: substudy of ADVANCE – a 2 x 2 factorial RCT Unit of randomization and analysis: individual Number randomized, total: 11,140 in ADVANCE; 2130 enrolled in ADVANCE/AdRem. 1602 participants of 1982 with gradable baseline photographs and had not had laser treatment Number analyzed: 1241 participants who had gradable baseline and final photographs Per group: 623 assigned to perindopril‐indapamide, 618 to placebo Sample size calculation: target of 2000 was estimated to provide 85% power to detect an absolute reduction of 6.2% in the primary outcome event rate, assuming an event rate of 27.8% over 6 years and a type I error of 0.05. "The main limitation of this study is the lower than planned sample size." |
|
| Participants |
Country: 14 countries in Asia, Australia, Europe, and North America (39 centers) Study period: baseline photographs: August 2002 to January 2004 Type of diabetes: type 2 Participants’ status at baseline:
Inclusion criteria: all participants in ADVANCE (55 years of age or older at recruitment; diagnosed with type 2 diabetes at age 30 years or older; history of at least 1 of the following conditions: major cardiovascular disease, risk factors including history of major microvascular disease, current cigarette smoking, elevated total cholesterol (> 6.0 mmol/L), low HDL cholesterol (< 1.0 mmol/L), microalbuminuria, diagnosed with type 2 diabetes 10 years or more preceding entry into the study or age 65 years or older at recruitment; an indication for an ACEi) who enrolled at centers with retinal cameras were eligible to participate in ADVANCE/AdRem Exclusion criteria: for ADVANCE: definite indication or contraindication for the active study treatments or a definite indication for a glycated hemoglobin target of ≤ 6.5%, long‐term insulin therapy at study entry, or participating in a different clinical trial; in addition for ADVANCE/AdRem: previous ophthalmological intervention or inability to obtain good‐quality photographs due to either severe cataract or inadequate pupil dilation (< 4 mm) |
|
| Interventions |
Intervention 1: perindopril (2 mg) plus indapamide (0.625 mg) daily at randomization and doubled to perindopril (4 mg) plus indapamide (1.25 mg) after 3 months Intervention 2: placebo Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: progression of diabetic retinopathy ≥ 2 steps by ETDRS classification Secondary outcome, as specified for this review: none reported Other diabetic retinopathy outcomes: ≥ 1 and ≥ 3 steps of progression and individual diabetic retinopathy lesion development; individual diabetic retinopathy lesion development and progression; distortion of retinal vasculature geometry Retinopathy diagnosis and monitoring: "The ETDRS classification was slightly modified in the UKPDS study, and this modified classification is used in the AdRem study. ... Detected lesions were graded in comparison with the ETDRS final scale standard photographs. ... All images were graded centrally (at the University Medical Center Utrecht) by two independent readers trained by the Retinopathy Grading Center, Hammersmith Hospital, Imperial College London." Participants (eyes) examined for outcome: 1241 Intervals at which outcomes were assessed: baseline (photographs taken within 3 months following ADVANCE randomization were considered “baseline” photographs), at 2 years, and at "final follow‐up" examination; retinal photographs taken at 2‐year follow‐up used when photographs at final follow‐up were not available Cost of the interventions: not reported Quality of life: not reported Adverse outcomes: not mentioned Other outcomes: composite macrovascular and microvascular disorders |
|
| Notes |
Source of funding: institutional and government support Declaration of interest: 2 authors declared interests related to pharmaceutical companies Run‐in length: 6 weeks on “active blood pressure lowering treatment and usual glucose lowering treatment” Class(es) of antihypertensive agents assigned: ACEi plus diuretic Degree of blood pressure control achieved: perindopril‐indapamide treatment yielded greater mean decrease than placebo of 6.1 mmHg in SBP and 2.3 mmHg in DBP Other: concomitant treatment with standard and intensive glucose therapy as randomized |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was stratified by study centre, history of macrovascular disease, and background use of perindopril at baseline." "A central computer‐based randomization service will assign patients to treatments ..." |
| Allocation concealment (selection bias) | Low risk | "Study treatments were allocated using a central, computer‐based randomization service accessible by internet, telephone, and facsimile." |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | “The two main effects comparisons were a double‐blind comparison of blood pressure‐lowering vs placebo ... blinded endpoint evaluation design.” |
| Incomplete outcome data (attrition bias) Primary outcome | High risk | "All analyses were based on the intention‐to‐treat principle (1,241 patients with gradable baseline and final photographs)." However, participants with missing or ungradable retinal photographs at baseline or follow‐up were excluded from the analyses. "In the absence of final retinal photographs, available 2 year photographs were used." |
| Selective reporting (reporting bias) | Unclear risk | Visual acuity and adverse events, which were specified in design paper, were not reported. Other outcomes reported on in the results were consistent with prespecified outcomes. |
| Other bias | Unclear risk | Baseline photographs were taken after the randomization visit, "preferably within 3 months after"; median of 2 months (IQR 1 to 6 months); these participants were scheduled for biennial photographs during follow‐up. However, participants who had had their first photographs taken more than 3 months following ADVANCE randomization had no biennial photographs taken routinely during follow‐up, only at the final ADVANCE visit (Stolk et al. 2007). |
BENEDICT.
| Study characteristics | ||
| Methods |
Study design: retinopathy substudy of parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: No sample size estimate for retinopathy substudy: “at the time the present analyses were planned, no data were available on the regression of retinopathy in hypertensive patients with type 2 diabetes on intensified BP and metabolic control ... ." For the BENEDICT trial, based on time to persistent albuminuria at 3‐year follow‐up with evidence of 9.5% in the placebo group and 3.1% in the treatment group, estimated 225 per group to provide 80% power at 2‐sided alpha = 0.05; adjusted for attrition to 300 per group, 1200 total. |
|
| Participants |
Country: Italy (2 of 9 BENEDICT centers in retinopathy substudy) Study period: not reported; design and number enrolled published in 2003 Type of diabetes: type 2 Participants’ status at baseline:
Inclusion criteria: hypertensive type 2 diabetics < 25 years duration; urinary albumin excretion rate < 20 μg/min in 2 of 3 overnight urine collections; serum creatinine < 1.5 mg/dL; hypertension defined as previous antihypertensive therapy or SBP/DBP > 130/85 mmHg; for retinopathy substudy, fundus exam/photos at baseline Exclusion criteria: concomitant non‐diabetic renal disease; glycated hemoglobin > 11%; specific indications or contraindications for study drugs; concomitant CV disease included MI and stroke |
|
| Interventions |
Intervention 1: ACEi: trandolapril 2 mg/day Intervention 2: non‐dihydropyridine CCB verapamil SR, 240 mg/day Intervention 3: fixed‐dose combination of verapamil SR, 180 mg/day, plus trandolapril 2 mg/day (VeraTran) Intervention 4: placebo All intervention groups: "Other antihypertensive drugs (with the exception of RAS inhibitors and ndCCBs different from the study drugs) could be used to achieve and maintain target BP according to predefined guidelines." Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of retinopathy, progression of retinopathy Secondary outcomes, as specified for this review: none Other diabetic retinopathy outcomes: regression of retinopathy; regression of retinal changes in participants with baseline retinopathy defined as "a persistent (up to the final visit) change in the stage of retinal involvement from proliferative to pre‐proliferative retinopathy, or from pre‐proliferative retinopathy to no retinal involvement" Retinopathy diagnosis and monitoring: "Retinal evaluation by ophthalmoscopy and photography (in a subgroup) were scheduled at baseline, every year thereafter, at final visit in all patients ... they were evaluated independently by two ophthalmologists ... blinded to the clinical and laboratory data of the patients." Retinopathy was classified as none, pre‐proliferative, or proliferative ("simplified classification"), but it is unclear whether based on photographs only or combination of examination and photographs. Participants (eyes) examined for the outcome: 460 for incident DR, 90 for DR progression Intervals at which outcomes were assessed: baseline; yearly thereafter; and at the final visit Cost of the interventions: mentioned, not reported Quality of life: not reported Adverse outcomes: CV mortality and morbidity, endstage renal failure, death Other outcomes reported from the study: progression to micro‐ and macroalbuminuria, increase in albuminuria, rate of glomerular filtration rate decline, incidence of major cardiovascular events, overall and cardiovascular mortality |
|
| Notes |
Source of funding: partial support from industry Declaration of interest: not reported; sponsor represented on Steering Committee which acts on safety committee recommendations Run‐in length: "At the first visit of the study, patients on ACE inhibitor and conventional antihypertensive treatment withdraw previous antihypertensive medications and are randomized after at least 6‐ and 3‐week washout, respectively." Classes of antihypertensive agents assigned: ACEi, CCB Degree of blood pressure control achieved:retinopathy substudy: "blood pressure control was similar across different treatment groups" In BENEDICT, similar for all arms at 3 years, SBP/DBP about 135/75 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The unblinded data centre receives the randomization code from the sponsor." |
| Allocation concealment (selection bias) | Unclear risk | No description provided. |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | "evaluated independently by two ophthalmologists blinded to clinical and laboratory data of the patients" |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | "Of the 1209 patients randomized in the original BENEDICT cohort, 583 patients were referred to the two centers involved in the present study. Five hundred‐fifty patients had a baseline funduscopy evaluation" 1209 randomized, while 1204 were analyzed in parent study: 4 never took study medication, 1 ineligible. |
| Selective reporting (reporting bias) | High risk | "The retinopathy grading score used makes it possible to quantify the progression of the retinal changes. Worsening of the disease is defined as progression from a less‐severe to a more‐severe class during follow‐up." However, the results were reported as: "... regression of retinopathy ..." Progression cannot be derived from regression without data regarding stabilization. |
| Other bias | Low risk | Support in part from Abbott (pharmaceutical company). "The Authors' work was independent and the funding source had no involvement in study design[,], in the collection, analysis, and [sic] interpretation of data in the writing of the report and [sic] in the decision to submit the article for publication." |
Chase.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: none reported |
|
| Participants |
Country: USA Study period: not reported Type of diabetes: type 1 Participants’ status at baseline:
Equivalence of groups at baseline: retinopathy worse in captopril group; otherwise fair, given small sample size Inclusion criteria: insulin‐dependent type 1 diabetes, with an albumin excretion rate of 20 to 200 µg/min on 3 of 4 overnight urine collections Exclusion criteria: not otherwise reported |
|
| Interventions |
Intervention 1: captopril 50 mg twice a day Intervention 2: placebo Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as defined for this review: progression of DR by at least 1 step on the modified Airlie House classification based on 7‐field color photographs Secondary outcomes, as specified for this review: progression to PDR Other diabetic retinopathy outcomes: improvement of diabetic retinopathy by 1 or more grades Retinopathy diagnosis and monitoring: "Initially, all subjects underwent pupil dilation followed by direct ophthalmoscopy by two observers (a pediatrician and an ophthalmologist), slit‐lamp examination, color photography of the seven standard fields, and fluorescein angiography ... at six‐ to 12‐month intervals ... fluorescein angiograms were done only if verification of a change was necessary." Grading of retinal changes was done using the modified Airlie House system" (Grades 1 to 6 [PDR]). "The final eye grade for the worst [sic] eye was determined by the ophthalmologist ... ." Participants (eyes) examined for the outcome: 16 Intervals at which outcomes were assessed: 6‐ to 12‐month intervals Cost of the interventions: not reported Quality of life: not reported Adverse outcomes: not reported Other outcomes reported from the study: albumin excretion rate, creatinine clearance rate, protein intake |
|
| Notes |
Sources of funding: partial support from industry, foundation, and government Declaration of interest: not reported Class(es) of antihypertensive agents compared: ACEi Degree of blood pressure control achieved: no target blood pressure reported; 2‐year follow‐up blood pressure was similar to baseline blood pressure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The subjects were randomized in a double‐blind study design.” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Masking (performance bias and detection bias) Primary outcomes | Unclear risk | “double‐blind study design” |
| Masking (performance bias and detection bias) Secondary outcomes | High risk | Apparently the investigator assessed DR on photos and was also aware of treatment. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | Data reported for all 16 participants. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Outcome reported for all participants. |
| Selective reporting (reporting bias) | Unclear risk | Unclear with available information |
| Other bias | Unclear risk | “Supported in part by Bristol‐Myers Squibb Company.” Too little information reported regarding methods to classify as low risk. |
DEMAND.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized
Sample size calculation: target of 342 would give 80% power with 20% "nonassessable" |
|
| Participants |
Country: Italy (7 centers) and Slovenia (1 center) Study period: May 2002 to June 2005 Type of diabetes: type 2 Participants’ status at baseline:
Equivalence of groups at baseline: placebo group had more DR, fewer women, longer DM duration, and poorer glycemic control than drug groups Inclusion criteria: > 40 years of age with hypertension and known history of type 2 diabetes (WHO criteria); < 25 years duration, with urinary albumin excretion < 200 µg/min in ≳ 2 of 3 consecutive, sterile overnight samples, and serum creatinine < 1.5 mg/dL; hypertension defined as untreated SBP/DBP > 130/85 mmHg or concomitant antihypertensive therapy Exclusion criteria: glycated hemoglobin > 11%, ischemic kidney disease, urinary tract obstruction, or urinary abnormalities suggestive of primary glomerular disease, or specific indications or contraindications to ACEi or calcium channel blocker therapy |
|
| Interventions |
Intervention 1: manidipine (10 mg/day) + delapril (30 mg/day) Intervention 2: delapril (30 mg/day) Intervention 3: placebo Treatment target, all groups: SBP/DBP 120/80 mmHg Antihypertensive drugs added step‐wise to achieve blood pressure target: 1) hydrochlorothiazide, indapamide, or furosemide; 2) beta‐ or alpha‐blockers; 3) doxazosis, prazosin, clonidine, or alpha‐methyldopa. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of retinopathy and progression of retinopathy Secondary outcome, as specified for this review: none Other diabetic retinopathy outcomes: regression of retinopathy, both arms combined; regression defined as no retinal changes or improvements were observed at 2 consecutive evaluations in eyes with retinopathy at baseline Retinopathy diagnosis and monitoring: "Fundus changes were assessed at the ophthalmology unit ... by 2 ophthalmologists ... blinded to study data with indirect binocular ophthalmoscopy ... . Photographs ... were taken ... . Retinal involvement was graded from no apparent retinopathy to mild, moderate, or severe preproliferative retinopathy and to proliferative retinopathy ... . New‐onset retinopathy was diagnosed when any grade of retinopathy was observed in 2 consecutive evaluations in eyes with no retinopathy at baseline" Participants (eyes) examined for the outcome: 208 Intervals at which outcomes were assessed: baseline and every year after randomization Cost of the interventions: not reported Quality of life: not reported Adverse outcomes: fatal CV event, fatal cancer, non‐fatal major CV event, non‐fatal cancer, other Other outcomes reported from the study: rate of glomerular filtration rate decline, amputation, peripheral neuropathy, other CV events |
|
| Notes |
Source of funding: partial support from industry: "Chiesi Farmaceutici SpA (Parma, Italy) funded the study and provided the experimental drugs but had no involvement in the study conduct and data handling, analyses, and reporting." Declaration of interest: authors declared that they had nothing to disclose Run‐in length: 12 weeks wash‐out Classes of antihypertensive agents assigned: combination of calcium channel blocker (manidipine) and ACEi (delapril), ACEi alone Degree of blood pressure control achieved: means during follow‐up 137.2 (10)/80.5 (6) mmHg Trial registration: NCT00157586 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The Chiesi Farmaceutici Statistical Unit created a computer generated randomization list with a block of 6 patients assigned to each therapy with a 1:1:1 ratio." |
| Allocation concealment (selection bias) | Low risk | "Randomization numbers were blindly assigned by the treatment assignment secretariat at the Mario Negri Institute (Ranica, Italy)... . Individual sealed envelopes containing the randomized treatment code were provided to each centre and could be broken for safety reasons after discussion with the study coordinator." |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | "Study treatments were externally nondistinguishable orange, rounded tablets containing either delapril 15 mg plus manidipine 5 mg, delapril 15 mg, or placebo ... Patients and investigators were all blinded throughout the study." "All of the end points were adjudicated at the blind review by a clinical end point committee unaware of the treatment assignments." |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | Participants who discontinued medication (8%) were excluded from the final analysis. |
| Selective reporting (reporting bias) | Unclear risk | Retinopathy incidence and progression not reported separately. |
| Other bias | High risk | Excluded participants from the analyses who discontinued the study medication |
DIRECT Prevent 1.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT conducted as part of DIRECT Unit of randomization and analysis: individual Number randomized
Sample size calculation: target of 1300, which would give 80% power to detect a 5% significance for treatment effect of 23% |
|
| Participants |
Country: 30 countries: Australia, Austria, Belgium, Bulgaria, Canada, Croatia, the Czech Republic, Denmark, Estonia, France, Georgia, Germany, Greece, Hungary, Ireland, Israel, Italy, Latvia, Lithuania, Luxembourg, New Zealand, Poland, Portugal, Romania, Russian Federation, South Africa, Spain, Sweden, Turkey, the UK Study period: August 2001 to February 2004 (end of randomization) with follow‐up through March 2008 Type of diabetes: type 1 Participants’ status at baseline:
Inclusion criteria: type 1 diabetics without retinopathy: age 18 to 50 years, no restriction on gender, younger than 36 years of age at diagnosis of diabetes, 5 (1?) to 15 years duration, continuous insulin use within a year of diagnosis, no microalbuminuria, SBP ≤ 130 mmHg and DBP ≤ 85 mmHg and a retinal grading level of 10/10 on the ETDRS scale with 7‐field stereo retinal photographs Exclusion criteria: eye conditions precluding capture of gradable retinal photographs (open‐angle glaucoma, cataracts obscuring view of retina); valvular stenosis; history of heart attack or stroke; pregnant or lactating women; renal impairment defined as serum creatinine ≥ 110 µmol/L for women and ≥ 130 µmol/L for men |
|
| Interventions |
Intervention 1: candesartan cilexetil 16 mg (ARB) Intervention 2: placebo In both arms, dose doubled after 1 month, based on tolerability; adjustable to 8, 16, or 32 mg/day by physician. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of retinopathy defined as ≳ 2‐step progression from 10/10 on the ETDRS scale Secondary outcomes, as specified for this review: none mentioned or reported Other diabetic retinopathy outcomes: none Retinopathy diagnosis and monitoring: retinal photographs graded independently by 2 masked graders at the Imperial College, London, according to 11‐step ETDRS grading scale Participants (eyes) examined for the outcome: 1421 Intervals at which outcomes were assessed: yearly until at least 4 years for diabetic retinopathy outcomes Cost of the interventions: not reported Quality of life: not reported Adverse outcomes: death, hypotension, hypoglycemia, others Other outcomes reported from the study: change in albumin excretion rate, serum total and HDL cholesterol, and glycemic control |
|
| Notes |
Source of funding: industry Declaration of interest: all authors declared interests related to pharmaceutical companies Class(es) of antihypertensive agents assigned: angiotensin receptor antagonist only Degree of blood pressure control achieved: difference in mean (SD) between cadesartan and placebo "at last visit": SBP 2.6 mmHg lower, DBP 2.7 mmHg lower Trial registration: NCT00252733 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation is performed centrally by a computerized system.” |
| Allocation concealment (selection bias) | Low risk | “Random assignment was done centrally, using an interactive voice‐response system. Both investigators and participants were unaware of the treatment allocation status.” |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | “Participants were initially assigned to candesartan 16 mg once a day or matching placebo.” “Two independent observers, a primary and secondary grader, constituting a team, were assigned to each patient for the duration of the study at the Retinopathy Grading Centre, Imperial College, London, UK. The team assessed the photographs for diabetic retinopathy and clinically significant macular oedema; they were unaware of the treatment the patients were assigned to.” |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | Intention‐to‐treat analysis for all participants randomized. Reasons and per cent discontinued similar in both treatment groups. Imputation used from previous photographs when photographs missed. |
| Selective reporting (reporting bias) | Low risk | Low risk for retinopathy outcomes. CSME and final visual acuity not reported. |
| Other bias | Unclear risk | "This study was jointly funded by AstraZeneca and Takeda." "The sponsors did the statistical analysis, with validation by an independent statistician.” No further details on independent validation |
DIRECT Protect 1.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT conducted as part of DIRECT Unit of randomization and analysis: individual Number randomized
Sample size calculation: target of 1850, which would give 80% power to detect a 5% significance for treatment effect of 25% |
|
| Participants |
Country: 30 countries; Australia, Austria, Belgium, Bulgaria, Canada, Croatia, the Czech Republic, Denmark, Estonia, France, Georgia, Germany, Greece, Hungary, Ireland, Israel, Italy, Latvia, Lithuania, Luxembourg, New Zealand, Poland, Portugal, Romania, Russian Federation, South Africa, Spain, Sweden, Turkey, the UK Study period: August 2001 to February 2004 (end of randomization) with follow‐up through March 2008 Type of diabetes at baseline: type 1 Participants’ status at baseline:
Inclusion criteria: age 18 to 55 years, no restriction on gender, younger than 36 years of age when type 1 diabetes diagnosed, duration of 1 to 20 years, continuously used insulin within a year of diagnosis, no microalbuminuria, SBP ≤ 130 mmHg and DBP ≤ 85 mmHg and a diabetic retinopathy grading ≥ 20/10 (mild, non‐proliferative), up to ≤ 47/47 (moderately severe non‐proliferative) on the ETDRS scale based on 7‐field stereo retinal photographs Exclusion criteria: eye conditions precluding capture of gradable retinal photographs (open‐angle glaucoma, cataracts obscuring view of retina), patients with valvular stenosis, history of heart attack or stroke, pregnant or lactating women, patients with renal impairment defined as serum creatinine ≥ 110 µmol/L for women and ≥ 130 µmol/L for men |
|
| Interventions |
Intervention 1: candesartan cilexetil 16 mg (ARB) Intervention 2: placebo Daily dose doubled or halved after 1 month; then doubled or halved based on tolerability. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: progression of retinopathy Secondary outcomes, as specified for this review: progression to CSME or PDR, or both, per the ETDRS protocol Other diabetic retinopathy outcomes: overall change in retinopathy from baseline to final visit, regression of retinopathy Retinopathy diagnosis and monitoring: retinal photographs sent for central grading by 2 masked independent graders at Imperial College London; 11‐step ETDRS scale used Participants (eyes) examined for the outcome: 1905 Intervals at which outcomes were assessed: 6 months and yearly thereafter for at least 4 years for retinopathy outcomes Cost of the interventions: not reported Quality of life: not reported Adverse outcomes: death, hypotension, hyperglycemia, other Other outcomes reported from the study: change in albumin excretion rate, serum total and HDL cholesterol, glycemic control |
|
| Notes |
Sources of funding: industry Declaration of interest: all authors declared interests related to pharmaceutical companies Run‐in length: none Class(es) of antihypertensive agents assigned: angiotensin receptor antagonist only Degree of blood pressure control: candesartan lower than placebo "at last visit": SBP 3.6 mmHg lower, DBP 2.5 mmHg lower Trial registration: NCT00252720 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation is performed centrally by a computerized system.” |
| Allocation concealment (selection bias) | Low risk | “Random assignment was done centrally, using an interactive voice‐response system. Both investigators and participants were unaware of the treatment allocation status.” |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | “Participants were initially assigned to candesartan 16 mg once a day or matching placebo.” “Two independent observers, a primary and secondary grader, constituting a team, were assigned to each patient for the duration of the study at the Retinopathy Grading Centre, Imperial College, London, UK. The team assessed the photographs for diabetic retinopathy and clinically significant macular oedema; they were unaware of the treatment the patients were assigned to.” |
| Masking (performance bias and detection bias) Secondary outcomes | Low risk | Same as above |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | Intention‐to‐treat analysis reported for all participants randomized. Reasons and per cent discontinued similar in both treatment groups. Imputation for missed photographs based on previous photograph. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Intention‐to‐treat analysis with imputation for all participants randomized. Reasons and per cent discontinued similar in both treatment groups. |
| Selective reporting (reporting bias) | Low risk | 'Low risk' for retinopathy outcomes, but final visual acuity not reported |
| Other bias | Unclear risk | "This study was jointly funded by AstraZeneca and Takeda." "The sponsors did the statistical analysis, with validation by an independent statistician.” No further details on independent validation |
DIRECT Protect 2.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT conducted as part of DIRECT Unit of randomization and analysis: individual Number randomized
Sample size calculation: target of 1700 followed at least 3 years, which would give 80% power to detect a 5% significance for treatment effect of 27% for 3‐step or more progression |
|
| Participants |
Country: 30 countries: Australia, Austria, Belgium, Bulgaria, Canada, Croatia, the Czech Republic, Denmark, Estonia, France, Georgia, Germany, Greece, Hungary, Ireland, Israel, Italy, Latvia, Lithuania, Luxembourg, New Zealand, Poland, Portugal, Romania, Russian Federation, South Africa, Spain, Sweden, Turkey, the UK Study period: August 2001 to February 2004 (end of randomization) and March 2008 (end of follow‐up) Type of diabetes: type 2 Participants’ status at baseline:
Equivalence of groups at baseline: well balanced Inclusion criteria: age 37 to 75 years, no restriction on gender, onset at 36 years of age or older, type 2 diabetes for 1 to 20 years, no microalbuminuria, SBP ≤ 130 mmHg and DBP ≤ 85 mmHg or treated for hypertension with SBP ≤ 160 mmHg and DBP ≤ 90 mmHg, and a retinopathy grading level from ≥ 20/10 (mild non‐proliferative) up to ≤ 47/47 (moderately severe non‐proliferative) on the ETDRS scale based on 7‐field stereo retinal photographs Exclusion criteria: eye conditions precluding capture of gradable retinal photographs (angle closure glaucoma, dense cataracts), valvular stenosis, recent heart attack or stroke, pregnant or lactating women, renal impairment (serum creatinine ≥ 110 µmol/L for women and ≥ 130 µmol/L for men, or taking renin angiotensin system inhibitors) |
|
| Interventions |
Intervention 1: candesartan cilexetil 16 mg (ARB) daily Intervention 2: placebo Dose increased up to 32 mg once a day after 1 month based on tolerability; dose adjustable by physician to 8, 16, or 32 mg/day as needed in each group. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: progression of retinopathy Secondary outcomes, as specified for this review: incidence of CSME or PDR, or both per the ETDRS protocol; change in visual acuity from baseline to "final visit" Other diabetic retinopathy outcomes: overall change in retinopathy from baseline to final visit Retinopathy diagnosis and monitoring: retinal photographs taken per ETDRS protocol and sent to Retinopathy Grading Centre, Imperial College London for grading (11‐point ETDRS scale) Participants (eyes) examined for the outcome: 1905 Intervals at which outcomes were assessed: retinal photographs taken at 6 months, 1 year, and every year thereafter Cost of interventions: not reported Quality of life: not reported Adverse outcomes: death, hypertension, other Other outcomes reported from the study: change in albumin excretion rate, serum total and HDL cholesterol, and glycemic control |
|
| Notes |
Sources of funding: industry Declaration of interest: all authors declared interests related to pharmaceutical companies Run‐in length: not applicable Class(es) of antihypertensive agents assigned: angiotensin receptor antagonist only Degree of blood pressure control achieved: reduction in blood pressure in both groups; mean changes were 4.3/2.5 mmHg in the candesartan group and 2.9/1.3 mmHg in the placebo group Trial registration: NCT00252694 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation is performed centrally by a computerized system.” |
| Allocation concealment (selection bias) | Low risk | “Random assignment was done centrally, using an interactive voice‐response system. Both investigators and participants were unaware of the treatment allocation status.” |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | “Participants were initially assigned to candesartan 16 mg once a day or matching placebo.” “Two grading teams of independent observers, each consisting of a primary and a secondary grader, were assigned to each patient for the duration of the study at the Retinopathy Grading Centre, Imperial College London, UK. Graders, who were unaware of treatment allocation, assessed all photographs for retinopathy." |
| Masking (performance bias and detection bias) Secondary outcomes | Low risk | Same as for primary outcome |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | Intention‐to‐treat analysis for all randomized participants. Reasons for discontinuation and per cent discontinued were similar in the 2 groups. Time‐to‐event analysis. Missed photograph grades imputed by study investigators from previous photograph grades. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Intention‐to‐treat analysis included all randomized participants. Reasons for discontinuation and per cent discontinued appear to be similar in the 2 groups. Time‐to‐event analysis. Imputation by study investigators for retinopathy outcomes |
| Selective reporting (reporting bias) | Unclear risk | Low risk for retinopathy outcomes. Progression to PDR and CSME not reported; visual acuity not reported. |
| Other bias | Unclear risk | "This study was jointly funded by AstraZeneca and Takeda." “The sponsors did the statistical analysis, with validation by an independent statistician." The authors had full access to all data and were free to interpret data and draw conclusions. |
EUCLID.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized
Sample size calculation: target of 580 (500 with follow‐up) to give 80% power to detect a 28% reduction in retinopathy progression |
|
| Participants |
Countries: Austria, Belgium, Croatia, Finland, Greece, Hungary, Ireland, Italy, Luxembourg, Poland, Romania, the UK Study period: not reported Type of diabetes: type 1 Participants’ status at baseline:
Inclusion criteria: men and women (on contraception or postmenopausal) aged 20 to 59 years; IDDM defined as diagnosis before 36 years of age and continuous insulin required within 1 year of diagnosis, resting DBP 75 to 90 mmHg, SBP ≤ 155 mmHg Exclusion criteria: renal artery stenosis, cardiac valve obstruction, accelerated hypertension, recent myocardial infarction, CABG, stroke, CHF, abnormal renal function (creatinine > 1.8 mg/dL), postural hypotension, or idiosyncratic reactions to ACEi |
|
| Interventions |
Intervention 1: 10 mg/day lisinopril Intervention 2: placebo In both groups, dose could be increased to 20 mg/day at 3 months and at subsequent visits to achieve a target DBP < 75 mmHg. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of retinopathy and retinopathy progression by at least 2 levels; retinal photographs at baseline and 24 months; classification was on a 5‐level scale, using the EURODIAB diabetic retinopathy classification from photos Secondary outcomes, as specified for this review: progression to PDR Other diabetic retinopathy outcomes: retinopathy progression by 1 level; regression of retinopathy Retinopathy diagnosis and monitoring: retinal photographs classified centrally by masked ophthalmologist (Aarhus, Denmark) according to 5‐point EURODIAB scale (modification of Airlie House scale) Participants (eyes) examined for the outcome: 323 for progression; 134 for incidence Intervals at which outcomes were assessed: retinal photographs taken at baseline and at 24 months; unclear whether photographs were taken at other follow‐up visits Cost of interventions: not reported Quality of life: not reported Adverse outcomes: hypoglycemia, others Other outcomes reported from the study: change in albumin excretion rate and glycated hemoglobin |
|
| Notes |
Sources of funding: industry Declaration of interest: not reported Run‐in length: 1 month run‐in on placebo; 70% adherence required for eligibility Class(es) of antihypertensive agents assigned: ACEi only Degree of blood pressure control achieved: mean DBP near target in lisinopril group and 3 mmHg lower than placebo Trial registration: not reported and not found in registers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was stratified by centre and albuminuric status.” “Patients were randomly assigned to lisinopril or placebo with a block size of four. Separate schemes were created for each stratum (microalbuminuric and normoalbuminuric), with a FORTRAN computer program validated against the SAS RANUNI random‐number generator. This scheme was generated by Zeneca Pharmaceuticals, so that both the coordinating centre and the local investigators were unaware of the allocation.” |
| Allocation concealment (selection bias) | Low risk | “Local investigators telephoned the coordinating centre with the provisional albuminuric status, and were given an identification number that matched numbers on pill boxes.” “This scheme was generated by Zeneca Pharmaceuticals, so that both the coordinating centre and the local investigators were unaware of the allocation. Sealed envelopes were supplied to each centre and the coordinating centre so that the code could be broken in an emergency.” |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | “AKS assessed all photographs according to the EURODIAB protocol, based on the modified Airlie House classification. She had no access to information about patients, except study number.” |
| Masking (performance bias and detection bias) Secondary outcomes | Low risk | Same as above |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | Analysis based on 325 of 354 participants who had 2‐year photographs of 409 randomized participants. Reasons for missed photographs were similar in the 2 groups. Not all photographs gradable for all outcomes |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Same as above |
| Selective reporting (reporting bias) | Unclear risk | CSME and visual acuity not reported. |
| Other bias | Unclear risk | “This study was supported by a grant from Zeneca Pharmaceuticals, who also provided the lisinopril and placebo tablets.” Also, the company generated the randomization scheme. |
HINTS.
| Study characteristics | ||
| Methods |
Study design: parallel, 4‐arm RCT; randomization stratified by diabetic status Unit of randomization and analysis: individual Number randomized: total: 252 diabetics of 593 participants (diabetics and non‐diabetics) Per group: (diabetics only) 49 usual care, 49 medication management, 50 behavioral management, 47 medication and behavioral management Number analyzed: 48 usual care, 45 medication management, 50 behavioral management, 43 medication and behavioral management Sample size calculation: "We estimated the necessary sample size empirically through a simulation study. We expect approximately 15% of the enrolled sample to dropout by the end of the study based on our prior studies. For a type‐I error of 0.05 and 80% power, we require a total of 600 patients to be able to detect a 15% increase in the probability of BP control" for the overall cohort. Length of follow‐up (planned and actual): 18 months Number of arms: parallel, 4‐arm RCT; randomization stratified by diabetic status |
|
| Participants |
Country: USA Study period: May 2006 to August 2010 Type of diabetes: not reported; however, given age of participants probably type 2 Participants’ status at baseline:
Equivalence at baseline: groups appear similar at baseline Inclusion criteria: military veterans diagnosed with uncontrolled blood pressure (defined as average > 140/80 mmHg), prescribed medicine to lower BP, regular primary care physician at Duke VAMC. For the DM subgroup, dilated examinations of the eyes had to have been performed at baseline (before or within 2 months after) and at 1 or more follow‐up visits 365 days or more after enrollment. Exclusion criteria: hospitalization for stroke in past 3 months; heart attack (unclear whether ever or past 3 months); surgery for blocked arteries (same comment); diagnosed with metastatic cancer or treated with dialysis, diagnosed with dementia or hearing impairment that prevents hearing/speaking by telephone; creatinine serum lab value > 2.5 mg/dL |
|
| Interventions |
Intervention 1: usual care (no intervention) Intervention 2: behavioral: nurse behavioral intervention with home BP telemonitoring Intervention 3: medication: nurse medication management with home BP telemonitoring Intervention 4: combined behavioral and medication: nurse administered tailored behavioral and medication management Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: progression of retinopathy from absence to presence or less severe to more severe from the time of enrollment to the most recent follow‐up 365 days or later (progression at 1 to 2 years after randomization) Secondary outcome, as specified for this review: none reported for DM subgroup Other diabetic retinopathy outcomes: none mentioned Retinopathy diagnosis and monitoring: chart review by single abstractor Participants (eyes) examined for the outcome: 194 total (numbers reported by arm sum to 196) Intervals at which outcomes were assessed: baseline and 6‐month intervals from enrollment; for DR analysis, at least once at 365 days or longer after enrollment Cost of interventions: not reported Quality of life: not reported Adverse events: not reported Other outcomes reported from the study: the primary study reported change in BP from baseline at 6‐month intervals |
|
| Notes |
Source of funding: US Department of Veterans Affairs Declaration of interest: none reported Run‐in length: none Class(es) of antihypertensive agents: not reported in available information Degrees of blood pressure control achieved: in parent trial, investigators reported “moderate reduction in systolic BP achieved HINTS via the nurse‐administered telemedicine program (8 mmHg lower in the combined intervention group vs the control group" Trial registration number: NCT00237692 Other: after controlling for duration of follow‐up, the odds of diabetic retinopathy progression were significantly greater among participants receiving usual care than among participants receiving medication management, either alone or in combination with behavioral management (OR 2.16, 95% CI 1.03 to 4.52; P = 0.04), but did not differ from the group receiving behavioral management alone (OR 0.88, 95% CI 0.40 to 1.95; P = 0.84). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Consenting patients are then randomized to 1 of the 4 arms using consecutively numbered envelopes; randomization is stratified by diabetic status" |
| Allocation concealment (selection bias) | Low risk | “consecutively numbered envelopes, stratified by diabetic status” |
| Masking (performance bias and detection bias) Primary outcomes | High risk | Per ClinicalTrials.gov: “Masking: None (Open Label)” |
| Masking (performance bias and detection bias) Secondary outcomes | High risk | Per ClinicalTrials.gov: “Masking: None (Open Label)” |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | Diabetic retinopathy was not specified as an outcome of the parent trial. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | No secondary outcomes reported for the DM group. |
| Selective reporting (reporting bias) | Low risk | Diabetic retinopathy prevalence at baseline and progression during follow‐up reported only for diabetics in study population. |
| Other bias | Unclear risk | “a single chart abstractor determined the presence and severity of diabetic retinopathy at baseline and the most recent follow‐up, as recorded in the electronic chart” |
J‐DOIT3.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized: 2542 enrolled and randomly assigned; total: 2542 (2328 completed the study) Number per group: intensive therapy 1271 (1269 analyzed); conventional therapy 1271 Sample size calculation: original: 90% power, α = 0.05 (2‐sided), 30% risk reduction in conventional group. Recalculated: 80% power after first year of accrual |
|
| Participants |
Country: Japan (81 centers) Study period: June 2006 to March 2016 Type of diabetes: type 2 Participants' status at baseline:
Equivalence at groups at baseline: characteristics similar between groups apart from smoking status, with a higher proportion of current smokers in the intensive therapy group than in the conventional therapy group (Table 1) Inclusion criteria: age 45 to 70; type 2 DM; glycated hemoglobin ≥ 6.9%, despite diet, exercise, oral antidiabetic; SBP ≥ 140 mmHg or DBP ≥ 90 mmHg and not on antihypertensive agent or SBP ≥ 130 mmHg or DBP ≥ 80 mmHg on ARB, ACEi, or long‐acting CCB; LDL cholesterol ≥ 120 mg/dL, triglycerides ≥ 150 mg/dL, HDL cholesterol ≤ 40 mg/dL Exclusion criteria: poor BP control with medication (SBP ≥ 200 mmHg or DBP ≥ 120 mmHg); on insulin therapy; non‐diabetic renal disease; type 1 DM strongly suspected; anti‐GAD antibody‐positive; LDL cholesterol ≥ 200 mg/dL; secondary hypertension suspected; DR worse than PDR; judged ineligible by the study physician |
|
| Interventions |
Intervention 1: intensive BP monitoring: step‐wise treatment of blood glucose, blood pressure, and lipids to achieve goals: BMI < 22 and glycated hemoglobin < 6.2%, SBP ≤ 120 mmHg, DBP ≤ 75 mmHg, LDL cholesterol < 80 mg/dL Intervention 2: conventional BP monitoring: goals of BMI < 24 and glycated hemoglobin < 6.9%, SBP ≤ 130 mmHg, DBP ≤ 80 mmHg, LDL cholesterol < 120 mg/dL Participants in both groups had education in diet, exercise, and smoking cessation, but there was more intensive education in the intensive arm. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: combined incidence and progression of diabetic retinopathy Secondary outcome, as specified for this review: loss of vision presumed to be due to DR Other diabetic retinopathy outcomes: none mentioned Retinopathy diagnosis and monitoring: "Retinopathy was assessed by ophthalmologist at each institution once per year and reported through the electronic data capturing system." Intervals at which outcomes were assessed: baseline and annually for DR; every 1 to 3 months for CV outcomes Participants (eyes) examined for the outcome: 1540 Cost of interventions: not reported Quality of life: not reported Adverse outcomes: death, "lower limb vascular events," "severe side effects" (127 in intensive, 121 in conventional, i.e. 10% in both), "severe hypoglycemia" (7 in intensive, 4 in conventional), fluid retention Other outcomes: any of MI, CABG, PTCA, stroke, carotid endarterectomy, carotid artery stenting, death from any cause (primary outcome for the trial); onset or progression of nephropathy, lower limb amputation, or revascularization |
|
| Notes |
Source of funding: Ministry of Health, Labour, and Welfare of Japan, Japan Foundation for the Promotion of International Medical Research Cooperation, Japan Diabetes Foundation, and multiple pharmaceutical companies Declaration of interest: many among the investigators at the 81 participating sites (described in both manuscripts published in English) Run‐in length: not applicable Class(es) of antihypertensive agents assigned: none; ARB, ACEi, long‐acting CCB, diuretic, β blocker, or α blocker used in stepped fashion to achieve BP goals Degrees of blood pressure control achieved:
Trial registration: NCT00300976 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomly assigned (1:1) by the dynamic balancing minimisation method with four stratification factors (sex, age, HbA1c, and history of cardiovascular disease ... .” “Assignments were made by the electronic data capturing system using computer‐generative random numbers and minimization software for group allocation.” |
| Allocation concealment (selection bias) | Low risk | "Assignments were made by the electronic data capturing system using computer‐generated random numbers and minimization software for group allocation" |
| Masking (performance bias and detection bias) Primary outcomes | High risk | “open‐label” trial |
| Masking (performance bias and detection bias) Secondary outcomes | High risk | “open‐label” trial |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | Generalized estimating equations (GEE) for repeated measures and Cox regression analysis (unadjusted and adjusted) used to estimate HRs and CIs. 2 participants in the intensive group “were removed from all analyses" after “the blinded review of all patients” because "ineligible after randomisation". |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | See above |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes and adverse systemic events reported, either individually or in combinations. |
| Other bias | Low risk | No evidence of any other source of bias. Investigators' involvement with various pharmaceutical companies could have affected the brands of medications prescribed, but the step‐wise treatment algorithm was quite specific about the classes of individual agents. |
J‐EDIT.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: based on diabetes‐related death and complications; P < 0.001 to detect or rule out a difference between arms Number analyzed: total for DR: 940; total for diabetes outcomes: 1173 |
|
| Participants |
Country: Japan (39 centers) Study period: accrual—March 2001 to February 2002; end of trial? (before April 2012) Type of diabetes: type 2 Participants' status at baseline:
Baseline equivalence of groups: well balanced Inclusion criteria: age 65 to 85 years; glycated hemoglobin ≥ 7.9% OR 7.4% to < 7.9% AND BP > 130/85 mmHg; other criteria related to cholesterol, etc. Exclusion criteria: VA < 20/200 and history of glaucoma; if grading of the ocular fundus was not possible, eye was excluded from analysis of DR (“940 eyes of 940 participants met the inclusion criteria”); if MI or stroke in < 6 months, excluded |
|
| Interventions |
Intervention 1: intensive BP monitoring: goals: glycated hemoglobin < 6.9%; BMI < 25 kg/m2; BP < 130/85 mmHg; HDL cholesterol > 40 mg/dL; serum triglycerides < 150 mg/dL, serum total cholesterol < 120 mg/dL for participants without CHD. For participants with CHD: LDL cholesterol < 100 mg/dL. Physicians prescribed oral hypoglycemic drugs or insulin and atorvastatin to achieve targets. Intervention 2: conventional BP monitoring: usual baseline treatment for diabetes, hypertension, and dyslipidemia without special treatment goals, i.e. no intervention Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as defined for this review: incidence of DR, progression of DR Secondary outcome, as specified for this review: none Other diabetic retinopathy outcomes: risk factors for DR Retinopathy diagnosis and monitoring: "The severity of the retinopathy and maculopathy was based on the fundus findings of ophthalmologists, and fundus photographs taken when they were available." 5‐stage scale for retinopathy (modified International scale), 4‐stage scale for maculopathy similar to International scale Participants (eyes) examined for the outcome: 940, reported Intervals at which outcomes were assessed: annually Cost of interventions: not reported Quality of life: “Standardized questionnaires were used to obtain self‐reported data on smoking, alcohol, hypoglycemia frequency, nutritional status, dietary habits and adherence, self‐efficacy, activities of daily living (ADLs), physical activities, comprehensive cognitive function, and psychological status including diabetes burden and depressive symptoms.” Adverse outcomes: "We did not observe any significant differences in fatal or non‐fatal cardiovascular events and composite events, including diabetes‐related mortality, between the two treatment groups over the follow‐up period. All‐cause mortality (total = 95/1173)" Other outcomes: multiple, including fatal and non‐fatal events, risk factors for DR |
|
| Notes |
Sources of funding: "this study was supported by Longevity Sciences from the Ministry of Health and Labour, and Welfare (H12‐Choju‐016, H15‐Chojyu‐016, H17‐Choju‐Ordinal‐013) and the Japan Foundation for Aging and Health" Declaration of interest: "There is no conflict of interest. The Japanese Elderly Diabetes Intervention Trial (J‐EDIT) Study Group has not cleared [sic] any potential conflicts" Class(es) of antihypertensive agents assigned: none; ACEi, ARB, calcium blockers, β blockers, α blockers, diuretics, antihyperlipidemic drugs used in both arms Degree of blood pressure control achieved: (Figure 4): intensive 136/71 mmHg (estimated); conventional 135/72 mmHg (“no significant differences ... were observed between the two groups during follow up”) Trial registration: VMIN 000000890 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly allocated,” but no details reported. Unclear what is meant by “randomized factors”; stratification factors? |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Masking (performance bias and detection bias) Primary outcomes | High risk | Participants in the intensive group must have been aware of frequent telephone contacts by study personnel. |
| Incomplete outcome data (attrition bias) Primary outcome | High risk | DR data reported for only 940 of 1173 participants due to the inability to grade the fundus, thus participants with cataract, vitreous heme, or other opacities would have been excluded. The dropout rate after 6 years was 8.9% (104 cases), so did not account for others. |
| Selective reporting (reporting bias) | High risk | For outcomes pertaining to this review, VA change from baseline or VA at any follow‐up time was not reported, although VA was measured at each annual examination. |
| Other bias | High risk | Inadequate information on numerators and denominators for percentages; in particular, the number examined for DR during follow‐up not reported by arm. |
Knudsen.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: none mentioned |
|
| Participants |
Country: Denmark Study period: not reported Type of diabetes: type 2 Participants' status at baseline:
Equivalence of groups at baseline: fair, given small sample size Inclusion criteria: (a) type 2 diabetes, i.e. onset after age 30 years, no need for insulin for at least 1 year after diagnosis of DM, no history of ketoacidosis; (b) diabetic maculopathy, i.e. macular edema defined as retinal hemorrhages and/or microaneurysms combined with hard exudates and/or retinal edema in macula; (c) no evidence of CSME requiring macular laser treatment; (d) no previous laser photocoagulation treatment; (e) no evidence of other ocular diseases apart from mild age‐related cataract that could affect visual acuity and retinal function; (f) no past or present treatment with ACEi or angiotensin II receptor antagonists; (g) SBP 110 to 175 mmHg and DBP 60 to 95 mmHg, p‐creatinine < 200 μmol/L Exclusion criteria: none reported except as implied by inclusion criteria above |
|
| Interventions |
Intervention 1 (intensive): losartan 50 mg/day Intervention 2 (standard): placebo Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: progression of retinopathy Secondary outcome(s), as specified for this review: visual acuity Other diabetic retinopathy outcomes: retinal thickness ("central retinal thickness") measured on OCT images Retinopathy diagnosis and monitoring: "assigned an overall retinopathy score from 1 to 6 according to the principles used in the [WESDR]"; ETDRS report 12 cited Participants (eyes) examined for outcomes: 24 Intervals at which outcomes were assessed: not specified; findings at baseline and "follow‐up" given without other information Cost of interventions: not reported Quality of life: not reported Adverse outcomes: worsening of ME, laser photocoagulation Other outcomes reported from the study: UAE rate, transcapillary escape of albumin rate |
|
| Notes |
Source of funding: Danish Association of the Blind, Jochum Jensen Memorial Grant, Merck Declaration of interest: reported Run‐in length: not applicable Class(es) of antihypertensive agents assigned: angiotensin II receptor antagonist only Degree of blood pressure control achieved: per abstract, BP decreased from 144 (17)/83 (10) mmHg to 138 (20)/78 (11) mmHg in losartan group, while BP in placebo group remained unchanged: 140 (14)/78 (5) mmHg at baseline, 139 (13)/82 (9) mmHg at follow‐up. However, baseline BP values not exactly those given in Table 1. Trial registration: none reported or found by searching registers Other: losartan "significantly increased retinal thickness in the central macular area compared with placebo treated patients, where no change in retinal thickness was observed" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | "The ophthalmologist performing the eye examinations had no knowledge of the patients' randomization status." Retinopathy was graded independently from photographs and fluorescein angiograms by 2 graders. |
| Masking (performance bias and detection bias) Secondary outcomes | Low risk | Same as above |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | Outcome reported for all 24 participants. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Outcome reported for all 24 participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned were reported on. |
| Other bias | Unclear risk | Partial support from industry |
Larsen.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: none mentioned |
|
| Participants |
Country: Denmark Study period: 1985 to ? Type of diabetes: type 1 Participants' status at baseline: (reported for 9 captopril, 6 no treatment)
Equivalence of groups at baseline: difficult to assess because of imbalance in number of eyes for which data were reported Inclusion criteria: age < 50 years, diabetes onset < 40 years old, blood pressure < 150/90 mmHg, background DR, serum creatinine < 130 μM or GFR > 6 mL/min Exclusion criteria: arterial hypertension by WHO criteria (> 160/95 mmHg), medication other than oral contraception, PDR, ME or previous laser photocoagulation |
|
| Interventions |
Intervention 1 (intensive): captopril 1.5 mg morning and evening; adjusted up to 50 mg twice daily Intervention 2 (standard): no treatment Both groups maintained "normal diabetes diet." Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: progression of DR Secondary outcome(s), as specified for this review: change in visual acuity, progression to PDR or laser photocoagulation Other diabetic retinopathy outcomes: regression of DR Retinopathy diagnosis and monitoring: "The progression of morphological changes was evaluate by the observer, who was given the fundus photographs and retinal angiograms obtained for each patient at entry and after 18 months but was unaware of their chronological order. The observer was then asked to rank the pictures according to the severity of retinopathy." Participants (eyes) examined for DR outcomes: 9 captopril, 6 no treatment (15 total) Intervals at which outcomes assessed: 18 months after baseline Cost of interventions: not reported Quality of life: not reported Adverse outcomes: not reported Other outcomes reported from the study: stabilization of arterial blood pressure by > 5 mmHg, blood retinal barrier permeability |
|
| Notes |
Source of funding: not reported Declaration(s) of interest: "The authors have no commercial or priprietary interest in the drugs or instruments used in this study." Run‐in length: not applicable Class(es) of antihypertensive agents assigned: ACEi (captopril) Degree of blood pressure control achieved: after 18 months, mean (SD) BP was 124 (13)/74 (9) mmHg in captopril group (n = 9) and 132 (9)/79 (7) mmHg in no treatment group. Mean changes from baseline were −1/−3 mmHg in captopril group and +3/−2 mmHg in no treatment group. Trial registration: none reported and none found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | "The color fundus photographs were quantitated by a masked observer ... . The progression of morphological changes was evaluated by the observer, who was given the fundus photographs and retinal angiograms obtained for each patient at entry and after 18 months but was unaware of the chronological order. The observer was then asked to rank the pictures according to the severity of retinopathy." |
| Masking (performance bias and detection bias) Secondary outcomes | Unclear risk | No information reported regarding measurement of visual acuity. |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | DR outcomes reported for 3 of 5 participants without 18‐month photographs. 1 of 10 participants in the captopril group and 2 of 10 participants in the no treatment group were excluded after photocoagulation. 18‐month outcome available for 10 participants in the captopril group and 8 participants in the no treatment group. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Same as above for primary outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes assessed were reported. |
| Other bias | Unclear risk | Only "quantitated" outcomes reported for progression of DR and visual acuity; interpretation not straightforward. |
Medi‐Cal.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization: individual Number randomized, total: 358 Number analyzed: 240 for DR Per group: 121 intensive, 119 standard Sample size calculation: based on 1% difference between groups in glycated hemoglobin and 30% dropout rate, power > 95%, 2‐sided probability of 0.05, yielding 150 participants per arm in parent study |
|
| Participants |
Country: USA (2 centers) Study period: enrollment July 1995 to June 1999 Type of diabetes: type 2 Participants' status at baseline:in retinopathy substudy except as noted
Equivalence of groups at baseline: well balanced Inclusion criteria: glycated hemoglobin > 7.5% Exclusion criteria: not reported |
|
| Interventions |
Intervention 1: intensive case management + primary care, including home glucose monitor, individualized education regarding diabetes, diet, exercise, and self‐care Intervention 2: standard, primary care only Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of DR Secondary outcome, as specified for this review: none Other diabetic retinopathy outcome(s): combined incidence and progression of DR Retinopathy diagnosis and monitoring: "All photographs were labeled with only the patient's identification number and were sent for reading in Santa Barbara ... and graded by an experienced endocrinologist who, before the study, had readings verified by and ophthalmologist until agreement was virtually 100%." Participants (eyes) examined for retinopathy outcomes: 82 for incidence, 100 for combined incidence and progression Intervals at which DR outcomes assessed: 6 months and yearly for fundus photos Cost of interventions: not reported Quality of life: not reported Adverse outcomes: hypoglycemia Other outcomes reported from the study: change in glycated hemoglobin, body weight, lipids, blood pressure |
|
| Notes |
Source(s) of funding: State of California Medi‐Cal Managed Care Division and Center for Disease Control, Prevention, CTRC at Harbor‐UCLA Medical Center, Lifescan (meters and supplies) Declaration of interest: not reported Length of run‐in period: not applicable Class(es) of antihypertensive agents assigned: none Degree of blood pressure control achieved: SBP and DBP each decreased by about 3 mmHg from baseline in intensive arm compared to < 1 mmHg change in standard arm Trial registration: not reported and not found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Masking (performance bias and detection bias) Primary outcomes | Unclear risk | Not possible to mask caregivers or participants to intervention arm. Photographs read centrally by an endocrinologist for DR outcomes. |
| Incomplete outcome data (attrition bias) Primary outcome | High risk | Of 240 participants at 2 centers with fundus cameras, 200 had at least 1 follow‐up photo. Of 61 participants without DR at baseline in intensive arm, incidence reported for 48 (79%); of 49 participants without DR in standard arm, incidence reported for 34 (69%). |
| Selective reporting (reporting bias) | Unclear risk | Combined incidence and progression of DR not reported in an analyzable form. Otherwise, all outcomes mentioned in 2 publications were reported. |
Pradhan.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: not reported |
|
| Participants |
Country: USA Study period: April 1997 through June 1998 Type of diabetes: type 2 Participants’ status at baseline:
Equivalence of groups at baseline: fairly well balanced, considering small sample size Inclusion criteria: type 2 diabetes, normotensive (< 140/90 mmHg), not on any ACEi or antihypertensive agent, moderate or severe NPDR identified and graded as ETDRS 40 to 50 Exclusion criteria: abnormal serum creatinine, visual acuity < 20/50, dipstick proteinuria more than trace, treatment with ACEi or other antihypertensive medications |
|
| Interventions |
Intervention 1: 5 mg enalapril daily Intervention 2: multivitamin placebo daily Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: none Secondary outcomes, as specified for this review: progression to PDR or macular edema requiring laser treatment based on slit‐lamp examination; PDR when detected ophthalmoscopically and documented by 7‐field photos; visual acuity measured but not reported Other diabetic retinopathy outcomes: none mentioned Retinopathy diagnosis and monitoring: if a slit‐lamp examination by the masked ophthalmologist showed either PDR or CSME, photographs were taken to confirm the diagnosis Intervals at which outcomes were assessed: all participants scheduled to have retinal photographs taken at 1 and 2 years; slit‐lamp assessment every 3 months Participants (eyes) examined for outcome: 35 Cost of interventions: not reported Quality of life: not reported Adverse outcomes: not mentioned Other outcomes reported: proteinuria |
|
| Notes |
Source of funding: government agency Declaration of interest: not reported Class(es) of antihypertensive agents assigned: ACEi only Length of run‐in period: none Degree of blood pressure control achieved: "mean arterial pressures were similar at last visit"; 90.4 (1.4) mmHg in enalapril group, 93.6 (2.0) mmHg in multivitamin group, and did not differ from each other Trial registration: not reported and not found Other: terminated with mean follow‐up less than 1 year for “unlikelihood” of demonstrating a significant difference between ACEi and placebo in the study cohort |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “After randomization to either a multivitamin (MVI) placebo or an ACE‐I ...”; details of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Masking (performance bias and detection bias) Secondary outcomes | Unclear risk | PDR and CSME reported at 3‐month intervals based on slit‐lamp examination by ophthalmologist for whom masking was not mentioned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Visual acuity was measured but not reported. "One woman had an allergic reaction and was dropped from the study. Four patients did not return for the 3‐month evaluation and were also dropped from the study since they could not be contacted." Dropouts not specified for treatment arm. Also, study terminated early for futility. |
| Selective reporting (reporting bias) | Unclear risk | Unclear from available information, but visual acuity measured at 3‐month intervals and not reported |
| Other bias | High risk | Terminated prematurely for futility; less than 1‐year follow‐up inadequate to assess diabetic retinopathy outcomes in so few participants. |
Rachmani 2002.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: type I error = 0.05, 0.80 power, 5% difference in GFR or 20 difference in ACR between groups yielded 45 participants per group |
|
| Participants |
Country: Israel Study period: 1995 to 2000? Type of diabetes: type 2 Participants' status at baseline:
Equivalence of groups a baseline: well balanced Inclusion criteria: type 2 diabetes mellitus, hypertension, and hyperlipidemia Exclusion criteria: age < 45 or > 70 years, smoking, duration of diabetes > 10 years, BMI > 35, serum creatinine > 2 mg/dL, ACR ≥ 200 mg/g, history of stroke, acute MI, unstable angina, vascular surgery, malignance, liver disease, autoimmune disease, life‐threatening condition, life expectancy < 5 years |
|
| Interventions |
Intervention 1: intensive: patient participation program (supported self‐management): measurement of seated BP at 9 a.m. daily (given portable mercury manometer); education, targets for BP (≤ 130/85 mmHg), glycated hemoglobin (≤ 7.0), BMI (< 25 men, < 24 women), lipids, exercise, medication compliance Intervention 2: standard: explanation to participant of problems related to diabetes, letter for primary care physician with examination and laboratory findings Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of DR Secondary outcome(s), as specified for this review: progression to PDR Other diabetic retinopathy outcomes: none Retinopathy diagnosis and monitoring: "The data available at each visit included ... a written report of funduscopy by an ophthalmologist." Participants (eyes) examined for outcomes: 129 Intervals at which outcomes were assessed: annually Cost of interventions: not reported; intensive group of participants averaged 2 extra visits per year, 486 total for group Quality of life: not reported Adverse outcomes: death Other outcomes reported from the study: changes in kidney function parameters, BP, glycated hemoglobin, lipid levels |
|
| Notes |
Source of funding: not reported Declaration(s) of interest: not reported Run‐in length: not applicable Class(es) of antihypertensive agents assigned: none (by end of study. 100% of intensive group on ACEi or ARB and 97% on hydrochlorothiazide vs 54% and 37% in standard care group) Degree of blood pressure control achieved: at 4 years, intensive group BP 142 (5.8)/84 (1.8), standard group 148 (6.1)/88 (1.7), i.e. decrease of 20/12 mmHg from baseline in intensive group, 12/8 mmHg in standard group Trial registration: not reported or found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was carried out on a 1:1 basis by using a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Masking (performance bias and detection bias) Primary outcomes | Unclear risk | Personal primary care physicians were masked, but study personnel and participants were not. No information about masking of non‐study ophthalmologist who performed fundoscopy |
| Masking (performance bias and detection bias) Secondary outcomes | Unclear risk | Same rationale as for primary outcome |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | 13 (9.2%) of 141 participants enrolled missed 1 or more of 4 annual examinations and were excluded from analysis of outcomes. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Same as for primary outcome |
| Selective reporting (reporting bias) | Low risk | No protocol or registry record available; all outcomes mentioned were reported. |
RASS.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: target of 95 participants per each of 3 arms, which would give 80% power to detect a 2‐sided 5% significance for treatment effect of 50% decrease in rate of change in mesangial fractional volume per glomerulus with 10% dropout; "RASS trial may not have adequate statistical power to detect small effects of RAS blockade on DR" |
|
| Participants |
Country: Canada (2 centers) and USA (1 center) Study period: not reported; design published in 2002 Type of diabetes: type 1 Participants’ status at baseline:
Equivalence of groups at baseline: well balanced Inclusion criteria: age 18 years or older; type 1 diabetes defined as onset before the patient's 45th birthday; BMI < 26 kg/m2 along with a positive glutamate decarboxylase or islet cell antibody test at time of diagnosis if between 31 and 40 years of age; no evidence of renal disease; participants who did not have PDR at baseline and who had fundus photographs at both baseline (defined as within 1 year after randomization) and 5 years were included in analyses for diabetic retinopathy Exclusion criteria: evidence of renal disease; blood pressure > 135/85 mmHg or requiring antihypertensive medications; albumin excretion rate > 20 μg/min; pregnancy; failure to take at least 85% of placebo pills during a 2‐week run‐in period; and glomerular filtration rate < 90 mL/min/1.73 m2 of body surface area |
|
| Interventions |
Intervention 1: enalapril (ACEi) 10 mg + 'losartan' placebo daily Intervention 2: losartan (ARB) 50 mg + 'enalapril' placebo daily Intervention 3: 'enalapril' and 'losartan' placebos daily 40 months after first randomization, doses for the interventions were doubled owing to evidence suggesting that the reduction in proteinuria was greater with higher doses of study treatments; the treatments were increased to 20 mg enalapril and 100 mg losartan by doubling the number of pills taken each day. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of diabetic retinopathy by 2 steps and progression by 3 steps on ETDRS scale Secondary outcomes, as specified for this review: as above for PDR and CSME; visual acuity not reported Other diabetic retinopathy outcomes: combined incidence and progression of diabetic retinopathy Retinopathy diagnosis and monitoring: "Stereoscopic fundus photographs ... were graded by observers, unaware of the study‐drug assignments, at the university of Wisconsin Ocular Epidemiology Reading Center who used the modified Airlie House Classification and the ETDRS severity scale ..." Participants (eyes) examined for outcome: 223 Intervals at which outcomes were assessed: retinal photographs taken at baseline, midpoint, and conclusion of the study Cost of interventions: not reported Quality of life: not reported Adverse outcomes: death, hypokalemia, ketoacidosis, hyperkalemia, chronic cough, others Other outcomes reported from the study: change in mesangial fractional volume, albumin excretion rate, and glomerular filtration rate |
|
| Notes |
Sources of funding: industry and government Declaration of interest: several authors declared interests related to pharmaceutical companies Run‐in length: 2 weeks on placebo Class(es) of antihypertensive agents assigned: ACEi and ARB Degree of blood pressure control achieved: < 120/80 during follow‐up; enalapril and losartan groups 2 to 4 mmHg lower than placebo group Trial registration: NCT001439949 Other: of 285 participants, only 223 were analyzed for retinopathy outcomes; 28 with no baseline photographs, 4 with baseline PDR, and 30 with no 5‐year photographs were excluded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to one of three groups with the use of computer‐generated blocks of six and stratified according to centre and sex ..." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | "These [stereoscopic fundus photographs] were graded by observers, unaware of the study‐drug assignments, at the University of Wisconsin Ocular Epidemiology Reading Center who used the modified Airlie House ..." |
| Masking (performance bias and detection bias) Secondary outcomes | Low risk | Same as above for primary outcome |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | "... use of multiple imputation techniques to assess effects of patients excluded for not having both the baseline and 5‐year ... diabetic retinopathy grades, respectively." Participants without baseline photographs were excluded from the analyses. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | See above for details of multiple imputation for missing retinal photographs at 5‐year follow‐up. |
| Selective reporting (reporting bias) | Low risk | Unclear from available information. Retinopathy assessment is the only pertinent outcome described in Mauer 2002 paper. |
| Other bias | Unclear risk | Partially supported by industry. Baseline retinal photographs were taken after randomization for 45.3% of the participants (mean time to baseline photographs was 4.8 ± 4.8 months after randomization for these participants). 32 participants were excluded: 4 with baseline PDR and 28 (of 285) without baseline photographs within 1 year after randomization. Not stated, but 30 more were not analyzed for retinopathy, presumably because follow‐up photographs were not taken |
Ravid 1993.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: none reported |
|
| Participants |
Country: Israel Study period: not reported Type of diabetes: type 2 Participants' status at baseline:
Equivalence of groups at baseline: well balanced Inclusion criteria: type 2 diabetes by WHO criteria, age < 50 years, duration of diabetes < 10 years, no evidence of systemic, renal, cardiac, or hepatic disease; BMI < 27 kg/m2; normal BP on 2 consecutive exams (SBP ≤ 150 mmHg, DBP ≤ 90 mmHg); serum creatinine < 1.4 mg/dL, microalbuminuria on 2 visits without evidence of UTI Exclusion criteria: not otherwise specified |
|
| Interventions |
Intervention 1: intensive: enalapril 10 mg daily Intervention 2: standard: placebo In either group, if SBP ≥ 145 mmHg or DBP ≥ 95 mmHg on 2 consecutive occasions, long‐acting nifedipine initiated. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of diabetic retinopathy Secondary outcome, as specified for this review: progression to PDR Other diabetic retinopathy outcomes: none Retinopathy diagnosis and monitoring: "Fundoscopy was done yearly by an ophthalmologist, and the presence of diabetic retinopathy was recorded." Participants (eyes) examined for the outcome(s): 94 Intervals at which outcomes assessed: yearly Cost of interventions: not reported Quality of life: not reported Adverse outcomes: cough that resulted in discontinuation of medication Other outcomes reported from the study: creatinine clearance, albuminuria, blood pressure |
|
| Notes |
Source(s) of funding: Nissensun‐Tyomkin medical research grant Declaration of interest: none mentioned Run‐in length: "2‐month pretreatment period" Classes of antihypertensives assigned: ACEi only Degree of blood pressure control achieved: mean BP change from baseline to 5 years was 99 (2.1) to 100 (3.2) mmHg in enalapril group, 97 (3.2) to 102 (3.4) mmHg in placebo group Trial registration: not reported and not found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done using a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Masking (performance bias and detection bias) Primary outcomes | Unclear risk | "The placebo tablets were similar to but not identical to enalapril." No mention made regarding whether the ophthalmologist who performed yearly funscopic examinations was masked to treatment assignment. |
| Masking (performance bias and detection bias) Secondary outcomes | Unclear risk | Same as above for primary outcome |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | 7 (12.5%) enalapril participants and 5 (9.7%) placebo participants without 5‐year data. Fewer missed earlier exams, but: "The final analysis was therefore done on 94 patients ... " (i.e. without outcomes for these 12 participants). |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Same as for primary outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes corresponding to measurements and exams during the trial were reported. |
| Other bias | Unclear risk | Retinopathy status at baseline not reported. |
ROADMAP.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: the study can "detect a 30% reduction in the risk of microalbuminuria (hazard ratio of 1.433) with 90% power at the 5% significance level ... . Thus, at least 2043 subjects are needed in each treatment arm and 328 events of microalbuminuria are expected to be observed. To compensate for withdrawals, 2200 patients are being recruited and randomized to each of the two treatment arms of the study." |
|
| Participants |
Country: 19 European countries (262 centers): Austria, Belgium, Bulgaria, the Czech Republic, Estonia, France, Germany, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Russia, Slovak Republic, Spain, the Netherlands, the UK, and Ukraine (142 centers in ROADMAP‐OFU) Study period: October 2004 to July 2009 Type of diabetes: type 2 Participants' status at baseline:881 olmesartan, 877 placebo, 1758 overall
Equivalence of groups at baseline: well balanced Inclusion criteria: type 2 diabetics free of signs of urinary albumin excretion who have 1 additional cardiovascular risk factor; if hypertensive, not taking ACEi or ARBs; 18 to 75 years of age; glycated hemoglobin ≥ 6.5% or are on treatment; hypertensive (SBP ≥ 130 mmHg or DBP ≥ 80 mmHg, or both) Exclusion criteria: renal and/or renal‐vascular disease (including malignant or severe renal disease); a history of nephrectomy and/or renal transplantation, or if they require dialysis; a recent history (within 6 months of starting the study) of myocardial infarction, stroke, transient ischemic attack, myocardial revascularization or reperfusion; recent use of (within 6 months of starting the study) ARBs or ACEi or if they have severe hypertension, defined as SBP > 200 mmHg or DBP > 110 mmHg, or both; severe uncontrolled hyperlipidemia, severe heart failure, bradycardia (< 50 beats/minute at rest), a significant narrowing of the aortic bicuspid valve, a severe obstruction of cardiac outflow (hypertrophic cardiomyopathy, New York Heart Association (NYHA) stage 3 to 4) |
|
| Interventions |
Intervention 1: 40 mg olmesartan twice daily (target BP < 130/< 80 mmHg) Intervention 2: placebo tablet twice daily (target BP < 140/< 90 mmHg) In both groups, diuretics, α or β blockers, calcium channel agonists (but not ARBs or ACEi) until target blood pressure achieved. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of retinopathy Secondary outcomes for this review: none mentioned Other diabetic retinopathy outcomes reported: none Retinopathy diagnosis and monitoring: " ... all available data were collected from routine visits at the study center and from the primary physician" "Occurrence or progression of retinopathy as assessed by laser treatment (photocoagulation) and/or onset of vitreous haemorrhage." Intervals at which outcomes were assessed: 1.4 to 5.1 years (mean 2.3 year) and 2.1 to 6.7 years (mean 3.3 years) after last ROADMAP visit Participants (eyes) examined for outcome: 1758 Cost of interventions: not reported Quality of life: not reported Adverse outcomes: hypotension, hyperkalemia; serious events; drug‐related events, hypertension, headache; others Other outcomes: time to albuminuria, cardiovascular mortality, stroke, cardiovascular morbidity, serum creatinine, hospitalization for various bad outcomes (endstage renal disease, worsening glomerular filtration rate) |
|
| Notes |
Source of funding: Daiichi Sankyo Declaration of interest: "All steering committee members are consultants for Sankyo for the ROADMAP study." Run‐in length: 4 weeks Class(es) of antihypertensive agents assigned: ARB Degree of blood pressure control achieved:
Trial registration: NCT00185159 Other: could not find DR data in ROADMAP publications; limited to data in ROADMAP‐OFU |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients are given a sequential number and assigned in 1:1 ratio to olmesartan medoxomil or placebo from a list produced using SAS statistical software (version 8.2; SAS Institute, Cary, North Carolina, USA) by PRA International (Reston, Virginia, USA)." |
| Allocation concealment (selection bias) | Low risk | "The original randomization list is kept at Kankyo Pharma GMBH (Munchen, Germany), but details of the treatment assigned to each patient are provided in a sealed envelope that is kept in a secure area and can be made available to investigators if required. The study medication, olmesartan medoxomil, and matching placebo are supplied as tablets in boxes that have been labelled and packaged for individual patients by Sankyo Pharma GmbH." |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | Per ClinicalTrials.gov record, participants and investigators were masked to treatment assignment. |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | Analyzed as randomized, but withdrawals and deaths excluded from analysis. No imputation or accounting for censoring mentioned for ROADMAP. May have included/reported only participants with at least 1 follow‐up examination. Fewer than half the ROADMAP participants were reported as included in ROADMAP‐OFU, a subsequent observational follow‐up study in which the only data regarding incidence of DR were reported. |
| Selective reporting (reporting bias) | High risk | Neither incidence nor progression of retinopathy reported for main trial period, although specified as outcomes. |
Steno‐2.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: target of 128 to give 90% power to detect a significant treatment effect of 23% difference in urinary albumin excretion rate with 0.05 probability |
|
| Participants |
Country: Denmark Study period: enrollment 1992 to 1993; total study period 1992 to 2001 Type of diabetes: type 2 Participants’ status at baseline:
Equivalence of groups at baseline: fairly well balanced Inclusion criteria: albumin excretion rate 30 mg to 300 mg in 4 of 6 24‐hour overnight samples, type 2 diabetes diagnosed by 1985 WHO criteria, age between 40 and 65 years Exclusion criteria: age < 40 or > 65, stimulated serum C‐peptide concentration < 600 pmol/L 6 minutes after 1‐minute intravenous glucagon injection, pancreatic insufficiency, diabetes secondary to pancreatitis, alcohol abuse, non‐diabetic kidney disease, malignancy or life‐threatening disease probable within 4 years |
|
| Interventions |
Intervention 1: multifactorial (intensive); all participants received ACEi equivalent to captopril 50 mg 2 times a day irrespective of blood pressure with additional intensive therapy for hypertension for a goal SBP < 140 mmHg and DBP < 85 mmHg; vitamin C 250 mg/day for non‐smokers and 1250 mg/day for smokers; vitamin E 100 mg/day for non‐smokers and 500 mg/day for smokers; aspirin 150 mg/day; instructions to limit daily fat intake to < 30% and saturated fat intake to < 10%; light to moderate exercise for 30 minutes, 5 times a week; smoking cessation; metformin 1 g twice a day if BMI > 25; NPH insulin if glycated hemoglobin > 7%. Intervention 2: standard treatment by participants' medical practitioners based on 1998 Danish guidelines with goal SBP < 160 mmHg and DBP < 95 mmHg Antihypertensive therapy was initiated in a step‐wise fashion starting with ACEi, progressing to angiotensin II receptor blockers, thiazides, calcium antagonists, and beta blockers as needed to achieve BP treatment goals. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of retinopathy; progression of retinopathy defined as at least 1 level in either eye; retinal photographs graded according to EURODIAB 6‐level grading scale Secondary outcomes, as specified for this review: progression to PDR or maculopathy Other diabetic retinopathy outcomes: blindness in 1 eye by WHO criteria or worse than 6/60 Retinopathy diagnosis and monitoring: "Retinal photographs ... were graded by two independent ophthalmologists, masked to treatment allocation, according to the EURODIAB six‐level grading scale." Participants (eyes) examined for outcome: 160 in 1999; 130 in 2001; 66 in 2014 Intervals at which outcomes were assessed: retinopathy assessed at baseline and every 2 years Cost of interventions: EUR2538 per quality‐adjusted life expectancy with multifactorial intensive treatment (2008); intensive intervention "cost‐neutral" over 20 years (2019) Quality of life: not reported Adverse outcomes: death, non‐fatal CV events, hypoglycemia Other outcomes reported from the study: urinary albumin excretion |
|
| Notes |
Sources of funding: unclear; the cost‐effectiveness analysis was supported by the Steno Diabetes Center and Novo Nordisk A/S Declaration of interest: interests reported by several investigators, but funding source unknown Lenth of run‐in: not applicable Class(es) of antihypertensive agents assigned: not applicable Degree of blood pressure control achieved: blood pressure in intensive therapy group dropped by 14 (2)/12 (2) mmHg by "end of study" compared with 3 (3)/8 (2) mmHg in standard therapy group Other: denominators for retinopathy analysis unclear given some PDR and photocoagulation at baseline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Concealed randomisation was done in groups of four with two in each treatment arm and thus allowed a maximum difference of two patients per group per stratum." |
| Allocation concealment (selection bias) | High risk | "Concealed randomisation ... ", but treatments not masked, so 1 or 2 assignments in some blocks of 4 could be known |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | Interventions compared in the trial do not permit the masking of participants and personnel; however, the "photographs were graded by two independent ophthalmologists, masked to treatment allocation ...". |
| Masking (performance bias and detection bias) Secondary outcomes | Low risk | “The photographs were graded by two independent ophthalmologists, masked to treatment allocation ...” |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | Analyzed as randomized, but withdrawals and deaths excluded from analyses. No imputation or accounting for censoring |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Analyzed as randomized, but withdrawals and deaths excluded from analyses. No imputation or accounting for censoring |
| Selective reporting (reporting bias) | Unclear risk | Unclear with available information |
| Other bias | Unclear risk | Source(s) of funding not reported. |
UKPDS/HDS.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: none found in more than 50 study publications for TBP control versus LTBP control or captopril versus atenolol |
|
| Participants |
Country: UK Study period: enrollment 1987 to 1991 Type of diabetes: type 2 Participants’ status at baseline:
Equivalence of groups at baseline: well balanced Inclusion criteria: type 2 diabetes and participating in the UKPDS, mean of blood pressure readings from 3 consecutive visits SBP > 160 mmHg or DBP > 90 mmHg, or both, when not receiving treatment for hypertension or SBP > 150 mmHg or DBP > 85 mmHg, or both, on treatment for hypertension; patients with SBP ≥ 200 mmHg or DBP ≥ 105 mmHg, or both, on any single occasion were eligible for randomization Exclusion criteria: requirement for strict blood pressure control due to a previous stroke, accelerated hypertension, ketonuria > 3 mmol/L; cardiac or renal failure; those who required beta‐blockade (myocardial infarction in the previous year or current angina); severe vascular disease with more than 1 major vascular episode; contraindication to beta‐blockade (with conditions such as asthma, intermittent claudication, foot ulcers or amputations); and severe concurrent illness |
|
| Interventions |
Intervention 1: TBP control policy aiming for blood pressure < 150/85 mmHg; random allocation to either ACEi or a beta blocker
Intervention 2: LTBP control policy aiming for blood pressure ≤ 180/105 mmHg but avoiding therapy with ACEi or beta blockers. In both groups, if blood pressure targets were not met, other agents were added; recommended sequence: furosemide 20 mg (maximum 40 mg) twice a day, slow‐release nifedipine 10 mg (maximum 40 mg) twice a day, methyldopa 250 mg (maximum 500 mg) twice a day, and prazosin 1 mg (maximum 5 mg) 3 times a day. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of diabetic retinopathy, progression of retinopathy defined as a 2‐step or greater change by ETDRS grading Secondary outcomes, as specified for this review: visual loss defined as the best vision in either eye, deteriorating by 3 lines or more on the ETDRS chart (clinical records); progression to PDR or photocoagulation Other diabetic retinopathy outcomes: vitreous hemorrhage; blindness in either eye Retinopathy diagnosis and monitoring: "Retinal photographs were masked to avoid any patient identification prior to being assessed at a central grading center. Assessment involved an initial review by 2 independent assessors for ... the presence or absence of diabetic retinopathy. Any eyes [sic] with retinopathy were then graded by 2 independent senior assessors ... . Retinopathy lesions were assessed against corresponding Early Treatment Diabetic Retinopathy Study (ETDRS) standard photographs or measurements." Participants (eyes) examined for outcome: 929 Intervals at which outcomes were assessed: baseline (retinal photographs taken up to 3 years prior to randomization within the hypertension study component of UKPDS) and annual direct ophthalmoscopy with photos to document changes; analyses reported at mean intervals of 1.5, 4.5, and 7.5 years from randomization Cost of interventions: reported in UKPDS 40, UKPDS 54, and UKPDS 84 publications Quality of life: UKPDS 37: cross‐sectional comparison of TBP control with LTBP control Adverse outcomes: death, hypoglycemia, others Other outcomes reported from the study: occurrence of 1) first clinical endpoint related to diabetes (sudden death, death from hyper‐ or hypoglycemia, fatal or non‐fatal myocardial infarction, angina, heart failure, stroke, renal failure, amputation, vitreous hemorrhage, retinal photocoagulation, blindness in 1 eye, or cataract extraction, 2) death related to diabetes due to myocardial infarction, sudden death, stroke, peripheral vascular disease, renal disease, hyper‐ or hypoglycemia, (3) death from all causes |
|
| Notes |
Sources of funding: government agencies, industry, and foundations Declaration of interest: not reported Run‐in period: 5 weeks on diet alone (UKPDS) Class(es) of antihypertensive agents assigned: ACEi (captopril), beta blocker (atenolol) Degree of blood pressure control achieved: over 9 years, mean (SD) BP 144 (14)/82 (7) mmHg in TBP group, 154 (16)/87 (7) mmHg in LTBP group; differences for captopril vs atenolol (95% CI): 1 (−1 to +3)/1 (0 to +2) mmHg Trial registration: none reported or found Other: all participants also taking part in UKPDS RCT of control of blood glucose levels |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was described in UKPDS 33, as follows. “Randomisation was by means of centrally produced, computer‐generated therapy allocations in sealed, opaque envelopes which were opened in sequence.” “The trial was open once patients were randomised.” “The randomization was stratified for those with or without previous therapy for hypertension.” |
| Allocation concealment (selection bias) | Low risk | “Sealed opaque envelopes were used and checked as described for the UK Prospective Diabetes Study [i.e. in UKPDS 33].” |
| Masking (performance bias and detection bias) Primary outcomes | Low risk | “Retinal photographs, masked for all patient‐identifying details and assigned a unique identification number, were assessed initially by two independent, experienced readers for quality and adherence to protocol as well as the presence of any diabetic retinal lesions.” “Retinopathy requiring photocoagulation or vitreous hemorrhage was independently assessed and recorded throughout the study” (UKPDS 69) |
| Masking (performance bias and detection bias) Secondary outcomes | Unclear risk | As above for PDR and CSME. It is unclear from report for UKPDS 69 and UKPDS VIII whether visual acuity examiners were masked. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | “Survival function estimates were calculated using the product limit (Kaplan‐Meier) method.” Participants were kept in assigned groups, but outcomes reported only for available cases. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Same as above |
| Selective reporting (reporting bias) | Low risk | All retinopathy outcomes reported. |
| Other bias | Unclear risk | “To form the baseline data set, we used the retinal photograph taken up to 3 years prior to hypertension randomization.” |
Wang.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization: individual Number randomized:
Sample size calculation: not reported |
|
| Participants |
Country: China Study period: January 2008 to ? Type of diabetes: type 2 Participants' status at baseline:
Equivalence of groups at baseline: well balanced Inclusion criteria: age 18 to 80 years, "male or nonpregnant female", "one or both eyes without or with mild and moderate NPDR; patients received health education; no history of laser photocoagulation or vitrectomy; BCVA of 0.5 or better" Exclusion criteria: "severe NPDR patients and PDR patients; poor general condition including high blood pressure, poor blood glucose control and renal failure; severe cataract cases; pregnancy; patients with poor compliance" |
|
| Interventions |
Intervention 1: captopril 12.5 mg twice daily, increased after 3 months to 3 times daily Intervention 2: placebo Not allowed: "other renin‐angiotensin system (RAS) blockers, aldosterone inhibitors, renin inhibitors, potassium‐sparing diuretics, monoamine oxidase inhibitor antidepressants, cholestyramine and colestipol resins". No BP target given for either intervention. Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: progression of DR Secondary outcome(s), as specified for this review: change in visual acuity, DME Other diabetic retinopathy outcomes: BCVA improvement by 2 lines, regression of macular edema based on OCT Retinopathy diagnosis and monitoring: "The DR grading [of color fundus photographs] and the DME staging were performed according to the diagnosis code of the American Academy of Ophthalmology (AAO) 2001 Annual Meeting." No additional information reported. Intervals at which outcomes were assessed: 6, 12, 18, and 24 months Participants (eyes) examined for outcomes: 317 Cost of interventions: not reported Quality of life: not reported Adverse outcomes: hyperkalemia, clinically significant hypotension, others Other outcomes reported from the study: foveal thickness on OCT images |
|
| Notes |
Source of funding: Key Project of the National Eleventh‐Five Year Research Program of China (No. 2007BAi181307) Declaration of interest: none Run‐in length: none Class(es) of antihypertensive agents assigned: ACEi Degree of blood pressure control achieved: mean (SD) BP < 130/80 mmHg with both interventions; "blood pressure in both groups was not significantly different in the follow‐up" Trial registration: not reported or found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Masking (performance bias and detection bias) Primary outcomes | Unclear risk | No mention of masking |
| Masking (performance bias and detection bias) Secondary outcomes | Unclear risk | No mention of masking |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | Outcomes reported for total number of participants enrolled. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Outcomes reported for total number of participants enrolled. |
| Selective reporting (reporting bias) | Low risk | Data reported for all outcomes stated in publication; no protocol or registry record available. |
Zhao.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Unit of randomization and analysis: individual Number randomized:
Sample size calculation: not reported |
|
| Participants |
Country: China Study period: enrollment August to December 2008; follow‐up to December 2017 Type of diabetes: type 2 Participants' baseline status:
Equivalence of groups at baseline: well balanced Inclusion criteria: 1) patients with T2DM diagnostic criteria as established by the WHO; 2) > 30 years of age when diagnosed, residents of the community; 3) 30 to 80 years old; 4) signed informed consent Exclusion criteria: 1) residential mobility, difficult to be regular follow‐up; 2) severe movement disorder; 3) poor compliance; 4) long‐term oral corticosteroids; 5) 2.5 times greater than normal ALT; 6) serum creatinine greater than 200 μmol/L; 7) moderate and severe schizophrenia; 8) those who are participating in other clinical trials; 9) those who are considered by the doctors as inappropriate to participate |
|
| Interventions |
Intervention 1: intensive monitoring: examined every month; glycated hemoglobin re‐examined every 3 months Intervention 2: standard group: followed every 2 months; glycated hemoglobin re‐examined every 6 months Target control, per guidelines: " ... defined as HbA1c < 7.0 mmol/L, systolic blood pressure (SBP) < 130 mm Hg, diastolic blood pressure (DPB) < 80 mm Hg, and low‐density lipoprotein cholesterol (LDL‐C), 2.6 mmol/L ...". However, BP target stated to have been < 140/90 mmHg (Discussion). Different for intensive and standard groups? Length of follow‐up:
|
|
| Outcomes |
Primary outcome, as specified for this review: incidence of diabetic retinopathy Secondary outcome, as specified for this review: "diabetic retinopathy (fundus photocoagulation and vitrectomy", i.e. PDR Other diabetic retinopathy outcomes: none Retinopathy diagnosis and monitoring: "The patients were examined once a year, including ... fundus examination ... . Microvascular complications include ... diabetic retinopathy (fundus photocoagulation and vitrectomy). The photographs were uploaded to the data center and checked." Intervals at which outcomes were assessed: every 1 or 2 months; glycated hemoglobin was re‐examined every 3 or 6 months, for intensive and standard groups respectively. Frequency of fundus photography or examinations not mentioned: "photographs were uploaded to the data center and checked". Participants (eyes) examined for the outcome: 168: intensive 92, standard 76 Cost of interventions: not reported Quality of life: not reported ("benefit of quality of life") Adverse effects outcomes: "As far as death caused by cardiovascular events, cerebrovascular events, and newly onset coronary heart disease are concerned, there were no significant differences on the aforementioned endpoint events between the two groups based on target control achieved more than 3 times or not. There was less incidence of new onset cerebrovascular events, stenosis or occlusion of large arteries, and diabetic microvascular complications in patients who achieved target control (HbA1c and LDL‐C) and the joint target control more than 3 times than those less than 3 times" Other outcomes: MI, stroke, CHD, nephropath, CVD/TIA, CABG, peripheral vascular disease, angina, hospitalization for various events; diabetic foot, amputation |
|
| Notes |
Source of funding: Special Scientific Research on Capital Health Development, Beijing Municipal Science & Technology Commission, and International Diabetes Federation Declaration of interest: reported no conflict of interest Run‐in length: not applicable Class(es) of antihypertensive agents assigned: not applicable Degrees of blood pressure control achieved, mean SBP/DBP: intensive: 127.0 (9.9)/71.5 (7.9) mmHg; standard: 127.8 (11.3)/71.0 (7.4) mmHg; "there was no statistical difference in the control of blood pressure between groups ... " Trial registration: registered with ChiCTR‐TRC13003978 and ChiCTR‐OOC‐15006090 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Multistage random sampling approach (completed by the statistical expert) |
| Allocation concealment (selection bias) | Unclear risk | Treatment allocation concealment not reported. |
| Masking (performance bias and detection bias) Primary outcomes | Unclear risk | No mention of masking |
| Incomplete outcome data (attrition bias) Primary outcome | High risk | Outcomes reported for 81.4% (86.8% of survivors) of intensive group and 68.5% (78.4% of survivors) of standard group. |
| Incomplete outcome data (attrition bias) Secondary outcomes | High risk | Outcomes reported for 81.4% (86.8% of survivors) of intensive group and 68.5% (78.4% of survivors) of standard group. |
| Selective reporting (reporting bias) | Low risk | Outcomes specified in trial registry were reported in study. |
| Other bias | Low risk | None identified. |
Note: we sought information from all reports from each individual study whenever an included trial was reported in more than one publication, technical report, protocol, or registry record.
Abbreviations:
AAO, American Academy of Ophthalmology ABCD (1), Appropriate Blood Pressure Control in Diabetes (3 RCTs) ACCORD Eye, Action to Control Cardiovascular Risk in Diabetes ‐ Eye Study ACEi, angiotensin‐converting enzyme inhibitors ACR, albumin to creatinine ratio AdDIT, Adolescent type 1 Diabetes cardio‐renal Intervention Trial ADDITION‐Europe, Anglo‐Danish‐Dutch Study of Intensive Treatment in People with Screen‐Detected Diabetes in Primary Care ADVANCE/AdRem, Action in Diabetes and Vascular Disease Retinal Measurements Study ALT, alanine transaminase ARB, angiotensin receptor blocker BCVA, best‐corrected visual acuity BENEDICT, BErgamo NEphrologic DIabetes Complications Trial BMI, body mass index, kg/m2 BP, blood pressure, usually expressed as SBP/DBP, each in mmHg C‐peptide, connecting peptide CABG, coronary artery bypass graft surgery CCB, calcium channel blocker CHD, coronary heart disease CHF, congestive heart failure CI, confidence interval CSME, clinically significant macular edema CTRC at Harbor‐UCLA Medical Center, Clinical and Translational Research Center at Harbor‐University of California, Los Angeles Medical Center CV, cardiovascular CVD, cardiovascular disease CWS, cotton‐wool spots DBP, diastolic blood pressure DCCT, Diabetes Control and Complications Trial DEMAND, Delapril and Manidipine for Nephroprotection in Diabetes DIRECT Prevent 1, 1 of 3 Diabetic Retinopathy Candesartan Trials Programme RCTs: Prevent 1, Protect 1, Protect 2 DM, diabetes mellitus DME, diabetic macular edema DR, diabetic retinopathy ETDRS, Early Treatment Diabetic Retinopathy Study EUCLID, EURODIAB Controlled Trial of Lisinopril in Insulin‐Dependent Diabetes Mellitus study EURODIAB, European Diabetes GAD, glutamic acid decarboxylase GFR, glomerular filtration rate HbA1c, glycated hemoglobin HDL, high‐density lipoprotein HR, hazard ratio IDDM, insulin‐dependent diabetes mellitus IHD, ischemic heart disease IMT, intima‐media thickness IQR, interquartile range IRMA, intraretinal microvascular abnormalities LDL, low‐density lipoprotein LDL‐C, low‐density lipoprotein cholesterol LTBP, less tight blood pressure logMAR, logarithm of the minimum angle of resolution of an eye ME, macular edema Medi‐Cal, California Medi‐Cal Type 2 Diabetes Study MI, myocardial infarction ndCCBs, non‐dihydropyridine calcium channel blockers NEJM, New England Journal of Medicine NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases, US National Institutes of Health NPDR, non‐proliferative diabetic retinopathy NPH, Neutral Protamine Hagedorn NV, neovascularization OCT, optical coherence tomography OFU, observational follow‐up OR, odds ratio p‐creatinine, plasma creatinine PDR, proliferative diabetic retinopathy PTCA, percutaneous transluminal coronary angioplasty PVD, peripheral vascular disease RAS, renin‐angiotensin system RASS, Renin‐Angiotensin System Study RCT, randomized controlled trial RD, retinal detachment ROADMAP, Randomized Olmesartan and Diabetes Microalbuminuria Prevention SBP, systolic blood pressure SD, standard deviation SR, sustained release Steno‐2, Steno type 2 randomised study T2DM, type 2 diabetes mellitus TBP, tight blood pressure TIA, transient ischemic attack UAE, urinary albumin excretion UKPDS/HDS, United Kingdom Prospective Diabetes Study/Hypertension in Diabetes Study UTI, urinary tract infection VA, visual acuity WESDR, Wisconsin Epidemiologic Study of Diabetic Retinopathy WHO, World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aro 2019_new | Not an intervention of interest |
| Auyanet 2010 | Not an RCT; case‐control study to evaluate whether people with diabetic retinopathy who had or had not received photocoagulation had been treated with carvedilol |
| CALM‐II | No data on diabetic retinopathy were available; the investigator's response to our query indicated that retinopathy data from this trial had not been analyzed. |
| Chang 2011 | Not an RCT; commentary on an included trial, no new trial data |
| DCCT | Not an RCT of blood pressure control |
| Durruty 2000 | No data on diabetic retinopathy were available; we did not receive a response to multiple requests for information regarding outcomes on diabetic retinopathy; RCT of 57 participants with type 2 diabetes and normal blood pressure randomized to enalapril or placebo. |
| Faguer de Moustier 1989 | No data on diabetic retinopathy were available; RCT of 20 participants with type 2 diabetes and slight hypertension randomized to nicardipine or placebo. |
| Harrold 1969 | Not an RCT of blood pressure control; RCT of 56 participants with diabetic retinopathy randomized to clofibrate (a lipid‐lowering agent) or placebo |
| Jackson 1992 | Not an RCT; retrospective cohort study evaluating people with diabetic retinopathy and use of angiotensin‐converting enzyme (ACE) inhibitors |
| JDCS 2011 | Not an RCT of blood pressure control; RCT of 2205 participants with type 2 diabetes randomized to intensive lifestyle intervention or conventional diabetes treatment; data on diabetic retinopathy outcomes were reported for the entire RCT cohort and not by treatment group |
| Lehsten 1996 | Not an RCT; case‐control study to compare the prevalence of hypertension in people with or without diabetic retinopathy |
| Malik 1998 | No data on diabetic retinopathy were available; we did not receive a response to our request for information regarding outcomes for diabetic retinopathy; RCT of 41 participants with type 1 or type 2 diabetes and normal blood pressure randomized to trandolapril or placebo |
| MCSG 1995 | Not an RCT of blood pressure control; RCT of 70 participants with insulin‐dependent diabetes randomized to intensive or conventional diabetes treatment |
| Mehlsen 2011 | No data on diabetic retinopathy were available; cross‐over RCT of 25 participants with type 1 diabetes, mild retinopathy, and normal blood pressure randomized to amlodipine followed by lisinopril, or vice versa |
| Newsom 1991 | No data on diabetic retinopathy were available; RCT of 8 participants with insulin‐dependent diabetes and normal blood pressure randomized to propanolol, dilevalol, salbutamol, or placebo. |
| OSCAR | Not population (diabetics) of interest |
| Patel 1998 | No data on diabetic retinopathy were available; RCT of 45 participants with type 1 or type 2 diabetes and hypertension randomized to perindopril or atenolol. |
| Porush 2000 | Not an RCT; narrative review of multiple RCTs of blood pressure control in diabetic participants with or without diabetic retinopathy; most of the RCTs discussed focused on renal and cardiovascular outcomes and were not included in this review |
| Rachmani 2000 | Report of 2 RCTs of combined total of 250 participants with type 2 diabetes and normal blood pressure randomized to enalapril or placebo. Unable to obtain numerator and denominator to permit calculation of incidence rates for each arm of each RCT |
| Rassam 1997 | No data on diabetic retinopathy were available; RCT of 42 participants with mild diabetic retinopathy and normal blood pressure randomized to perindopril or placebo. |
| Schwartz 1998 | No data on diabetic retinopathy were available; RCT of 1715 participants with type 2 diabetes and hypertension randomized to irbesartan or amlodipine. |
RCT, randomized controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
ABCD‐2V (2).
| Methods |
Study design: randomized controlled trial, single center Unit of randomization and analysis: individual Number randomized:
Sample size calculation: not reported |
| Participants |
Country: USA Study period: not reported Age:mean (SD) 56.7 (7.7) years in intensive treatment group, 55.5 (7.7) in moderate treatment group Gender, % women: 33.3% in intensive treatment group, 31.7% in moderate treatment group Race/ethnicity: 71.2% white, 19.7% Hispanic, 9.1% African‐American in intensive treatment group; 76.2% white, 11.1% Hispanic, 6.4% African‐American, and 3.2% Asian in moderate treatment group Inclusion criteria: type 2 diabetes, 40 to 81 years of age, with a SBP < 140 mmHg, a DBP between 80 and 90 mmHg, and without evidence of overt albuminuria (< 200 µg/min) Exclusion criteria: "pregnant or lactating women, need for any antihypertensive medications, documented myocardial infarction or cerebrovascular accident within the past 6 months, severe peripheral vascular disease, history of bilateral renal artery stenosis or stenosis in a solitary kidney, evidence of severe liver disease, hyperkalemia, or history of active cancer" Type of diabetes: type 2 Glycated hemoglobin categories/levels: not reported Retinopathy status: both non‐proliferative and proliferative |
| Interventions |
Intervention 1: intensive treatment Intervention 2: moderate treatment Length of follow‐up:
|
| Outcomes |
Primary outcomes for this review: progression of diabetic retinopathy Secondary outcomes for this review: none mentioned Other diabetic retinopathy outcomes: regression of diabetic retinopathy Other outcomes: change in creatinine clearance from baseline, proportion with doubling of serum creatinine, and change in log urinary albumin excretion from baseline; progression/regression of neuropathy; incidence of cardiovascular events Intervals at which outcomes were assessed: every 6 months Eyes examined for outcome: not reported Cost of interventions: not reported Quality of life: not reported |
| Notes |
Source of funding: Novartis Pharmaceutical Company Declaration of interest: not reported Awaiting information from study investigators |
DBP, diastolic blood pressure SBP, systolic blood pressure SD, standard deviation
Differences between protocol and review
The authors of the original version of this review added a post hoc secondary outcome (i.e. incidence of proliferative retinopathy or clinically significant macular edema); for the updated review, we added vitreous hemorrhage to this list as also requiring treatment. As in the original review, we retained the combined outcome of incidence or progression of diabetic retinopathy, which was not specified in the protocol. We reported findings from the included trials separately for participants with type 1 and type 2 diabetes and by baseline hypertension status (hypertensive or normotensive), although the authors of the review protocol did not anticipate analyses by these subgroups when they developed the protocol for the review.
Contributions of authors
Conceiving the review: GS Designing the review: GS, SV, RNF Co‐ordinating the updated review: SA, GH, BSH Undertaking manual searches for the updated review: SA, GH, BSH Screening recent search results: SA, GH, BSH, KL Organizing retrieval of reports and records: SA, GH Screening retrieved reports and records against the inclusion criteria: SA, GH, BSH, KL Appraising the quality of studies: SA, GH, BSH Abstracting data from studies: SA, GH, BSH Writing to study investigators for additional information: BSH Data management for the updated review: SA, GH Entering data into Review Manager 5: SA, GH Interpretation of data: DVD, BSH, GS, RNF Writing the updated review: BSH, DVD, SA, GH Performing previous work that was the foundation of the current study: DVD, GS, RNF
Guarantor of the review: BSH
RNF: Robert N Frank; KL: Kristina Lindsley
Sources of support
Internal sources
-
New Source of support, Other
None
External sources
-
Cochrane Eyes and Vision Group US Project, USA
Funded by Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health (PI: Tianjing Li, MD, MHS, PhD)
-
Research to Prevent Blindness, USA
The Wilmer Eye Institute is the recipient of an unrestricted research grant from Research to Prevent Blindness. As a faculty member, Barbara S Hawkins benefits from that grant.
-
Queen's University Belfast, UK
Gianni Virgili, Co‐ordinating Editor for Cochrane Eyes and Vision’s work is funded by the Centre for Public Health, Queen’s University Belfast, Northern Ireland.
Declarations of interest
Diana V Do: no conflicts to declare
Genie Han: no conflicts to declare
Samuel A Abariga: no conflicts to declare
Gina Sleilati: no conflicts to declare
S Swaroop Vedula: no conflicts to declare
Barbara S Hawkins: no conflicts to declare
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
ABCD (1) {published data only}
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney International 2002;61(3):1086-97. [DOI] [PubMed] [Google Scholar]
- Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia 1996;39(12):1646-54. [DOI] [PubMed] [Google Scholar]
- Schrier RW, Estacio RO, Mehler PS, Hiatt WR. Appropriate blood pressure control in hypertensive and normotensive type 2 diabetes mellitus: a summary of the ABCD trial. Nature Clinical Practice Nephrology 2007;3(8):428-38. [DOI] [PubMed] [Google Scholar]
- Schrier RW, Savage S. Appropriate Blood Pressure Control in Type II Diabetes (ABCD Trial): implications for complications. American Journal of Kidney Diseases 1992;20(6):653-7. [DOI] [PubMed] [Google Scholar]
ABCD (2) {published data only}
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000;23(Suppl 2):B54-64. [PubMed] [Google Scholar]
- Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin dependent diabetes and hypertension. New England Journal of Medicine 1998;338(10):645-52. [DOI] [PubMed] [Google Scholar]
- Savage S, Nagel NJ, Estacio RO, Feig PU, MacCarthy EP, Lukken NJ, et al. The ABCD (Appropriate Blood Pressure Control in Diabetes) Trial. Rationale and design of a trial of hypertension control (moderate or intensive) in type II diabetes. Online Journal of Current Clinical Trials 1993 Nov:Doc No 104. [PubMed] [Google Scholar]
- Schrier RW, Estacio RO, Mehler PS, Hiatt WR. Appropriate blood pressure control in hypertensive and normotensive type 2 diabetes mellitus: a summary of the ABCD trial. Nature Clinical Practice Nephrology 2007;3(8):428-38. [DOI] [PubMed] [Google Scholar]
ABCD‐2V (1) {published data only}
- Estacio RO, Coll JR, Tran ZV, Schrier RW. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. American Journal of Hypertension 2006;19:1241-8. [DOI] [PubMed] [Google Scholar]
ACCORD Eye {published data only}
- Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Eye Study Group and Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Study Group. Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Follow-On Study. Diabetes Care 2016;39:10889-1100. [DOI: 10.2337/dc16-0024] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosius WT, Danis RP, Goff DC Jr, Greven CM, Gerstein HC, Cohen RM, et al. Lack of association between thiazolidinediones and macular edema in type 2 diabetes: the ACCORD eye substudy. Archives of Ophthalmology 2010;128(3):312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial: design and methods. American Journal of Cardiology 2007;99(12A):21-33i. [DOI] [PubMed] [Google Scholar]
- Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. New England Journal of Medicine 2010;363(3):233-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, Ambrosius WT, Howard LT, Greven CM, Johnson S, Danis RP, et al. Rationale, design, and methods of the Action to Control Cardiovascular Risk in Diabetes Eye Study (ACCORD-EYE). American Journal of Cardiology 2007;99(12A):103-11i. [DOI] [PubMed] [Google Scholar]
- Chew EY, Ambrosius WT. Update of the ACCORD Eye Study. New England Journal of Medicine 2011;364(2):188-9. [DOI] [PubMed] [Google Scholar]
- Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, et al for the Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology 2014;121:2443-51. [DOI: 10.1016/j.ophtha.2014.07.019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman WC, Grimm RH, Cutler JA, Evan GW, Capes S, Corson MA. Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. American Journal of Cardiology 2007;99(12A):44-55i. [DOI: 10.1016/j.amjcard.2007.03.005] [DOI] [PubMed] [Google Scholar]
- Gangaputra S, Lovato JF, Hubbard L, Davis MD, Esser BA, Ambrosius WT, et al for the ACCORD Eye Research Group. Comparison of standardized clinical classification with fundus photograph grading for the assessment of diabetic retinopathy and diabetic macular edema severity. Retina 2013;33:1393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein HC, Ambrosius WT, Danis R, Ishail-Beigi R, Cushman W, Calles J, et al for the ACCORD Study Group. Diabetic retinopathy, its progression, and incident cardiovascular events in the ACCORD trial. Diabetes Care 2013;36:1266-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower EW, Lovato JF, Ambrosius WT, Chew EY, Danis RP, Davis MD, et al for the ACCORD Study Group. Lack of association between thiazolidinediones and incidence and progression of diabetic eye disease: The ACCORD Eye Study. American Journal of Ophthalmology 2018;187:138-47. [DOI: 10.1016/j.ajo.2017.12.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376(9739):419-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail-Beigi F, Craven TE, O'Connor PJ, Karl D, Calles-Escandon J, Hramiak I, et al. Combined intensive blood pressure and glycemic control does not produce an additive benefit on microvascular outcomes in type 2 diabetic patients. Kidney International 2012;81(6):586-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MD, Anderson RT, Aron D, Atkinson HH, Bastien A, Chen J, et al. Health-related quality of life and cost-effectiveness components of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: rationale and design. American Journal of Cardiology 2007;99(12A):90-102i. [DOI] [PubMed] [Google Scholar]
AdDIT {published data only}
- Adolescent type 1 Diabetes cardio-renal Intervention Trial Research Group. Adolescent type 1 diabetes cardio-renal intervention trial (AdDIT). BMC Pediatrics 2009;9(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Aguirre PZ, Wong TY, Craig ME, Davis EA, Cotterill A, Couper JJ, et al. The Adolescent Cardio-Renal Intervention Trial (AdDIT): retinal vascular geometry and renal function in adolescents with type 1 diabetes. Diabetologia 2018;61(4):968-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunger DB. Banting Memorial Lecture 2016 Reducing lifetime risk of complications in adolescents with Type 1 diabetes. Diabetic Medicine 2017;34(4):460-6. [DOI] [PubMed] [Google Scholar]
- Inman M, Daneman D, Curtis J, Sochett E, Clarke A, Dunger D, et al. Social determinants of health are associated with modifiable risk factors for cardiovascular disease and vascular function in pediatric type 1 diabetes. Journal of Pediatrics 2016;177:167-72. [DOI] [PubMed] [Google Scholar]
- Inman M, Daneman D, Curtis J, Sochett E, Elia Y, Dunger D, et al. Assessing social determinants of health in a pediatric diabetes clinical research trial: are recruited subjects representative of the larger clinical population. Diabetes Research and Clinical Medicine 2016;113:41-3. [DOI] [PubMed] [Google Scholar]
- Maftei O, Pena A, Sullivan T, Jones T, Donaghue K, Cameron F, et al. Early atherosclerosis relates to urinary albumin excretion and cardiovascular risk factors in adolescents with type 1 diabetes: Adolescent type 1 Diabetes cardio-renal Intervention Trial (AdDIT). Diabetes Care 2014;37(11):3069-75. [DOI] [PubMed] [Google Scholar]
- Marcovecchio M, Chiesa S, Bond S, Daneman D, Dawson S, Donaghue K, et al. ACE inhibitors and statins in adolescents with type 1 diabetes. New England Journal of Medicine 2017;377(18):1733-45. [DOI] [PubMed] [Google Scholar]
ADDITION‐Europe {published data only}
- Griffin SJ, Borch-Johnson K, Davies MJ, Khunti K, Rutten GEHM, Sandbaek A, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet 2011;9(378):156-67. [DOI: 10.1016./S0140-6736(11)606-98-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin SJ, Rutten GEHM, Khunti K, Witte DR, Lauritzen T, Sharp SJ, et al. Long-term effects of intensive multifactorial therapy in individuals with screen-detected type 2 diabetes in primary care: 10-year follow-up of the ADDITION-Europe cluster randomised trial. Lancet Diabetes and Endocrinology 2019;7:925-37. [DOI] [PubMed] [Google Scholar]
- Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BHR, Tutten G for the ADDITION Study Group. The ADDITION Study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with Type 2 diabetes detected by screening. International Journal of Obesity 2000;Suppl 3:S6-11. [DOI] [PubMed] [Google Scholar]
- Sandbaek A, Griffin SJ, Sharp SJ, Simmons RK, Borch-Johnsen K, Rutten GE, et al. Effect of early multifactorial therapy compared with routine care on microvascular outcomes at 5 years in people with screen-detected diabetes: a randomized controlled trial: The ADDITION-Europe Study. Diabetes Care 2014;37(7):2015-23. [DOI] [PubMed] [Google Scholar]
- Simmons RK, Borch-Johnson K, Lauritzen T, Rutten GEHM, Sandbaek A, den Donk M, et al. A randomised trial of the effect and cost-effectiveness of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with screen-detected type 2 diabetes: the Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen-Detected Diabetes in Primary Care (ADDITION-Europe) study. Health Technology Assessment 2016;20(64):1-?. [ISSN: 1366-5278] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Donk M, Griffin SJ, Stellato RK, Simmons RK, Sandbaek A, Lauritzen T, et al. Effect of early intensive multifactorial therapy compared with routine care on self-reported health status, general well-being, diabetes-specific quality of life and treatment satisfaction in screen-detected type 2 diabetes mellitus patients (ADDITION-Europe): a cluster-randomised trial. Diabetologia 2013;56:2367-77. [DOI: 10.1007/s0125-013-3011-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D, Dales J, Zaccardo F, Hill S, Moore C, Farooqi A, et al. Intensive versus standard multifactorial cardiovascular risk factor control in screen-detected type 2 diabetes: 5-year and longer-term modelled outcomes of the ADDITION-Leicester study. Diabetes and Metabolism Research Review 2019;35:e3111. [DOI: 10.1002/dmrr.3111] [DOI] [PubMed] [Google Scholar]
ADVANCE/AdRem {published data only}
- ADVANCE Collaborative Group. ADVANCE - Action in Diabetes and Vascular Disease: patient recruitment and characteristics of the study population at baseline. Diabetic Medicine 2005;22(7):882-8. [DOI] [PubMed] [Google Scholar]
- ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370(9590):829-40. [DOI] [PubMed] [Google Scholar]
- ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England Journal of Medicine 2008;358(24):2560-72. [DOI] [PubMed] [Google Scholar]
- ADVANCE Management Committee. Study rationale and design of ADVANCE: Action in Diabetes and Vascular Disease - preterax and diamicron MR controlled evaluation. Diabetologia 2001;44(9):1118-20. [DOI] [PubMed] [Google Scholar]
- Beulens JWJ, Patel A, Vingerling JR, Cruickshank JK, Hughes AD, Stanton A, et al. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetologia 2009;52(10):2027-46. [DOI] [PubMed] [Google Scholar]
- Patel A, Chalmers J, Neal B, Chapman N, Girgis S, MacMahon S. Blood pressure lowering in diabetes: a brief review of current evidence and description of a new trial. Clinical and Experimental Pharmacology and Physiology 2001;28(12):1108-11. [DOI] [PubMed] [Google Scholar]
- Poulter NR. Blood pressure and glucose control in subject with diabetes: new analysis from ADVANCE. Journal of Hypertension 2009;27(1):S3-8. [DOI] [PubMed] [Google Scholar]
- Stolk RP, Schooneveld MJ, Cruickshank JK, Hughes AD, Stanton A, Lu J, et al. Retinal vascular lesions in patients of Caucasian and Asian origin with type 2 diabetes: baseline results from the ADVANCE Retinal Measurements (AdRem) study. Diabetes Care 2008;31(4):708-13. [DOI] [PubMed] [Google Scholar]
- Stolk RP, Vingerling JR, Cruickshank JK, Hughes AD, Stanton A, Juming L, et al. Rationale and design of the AdRem study: evaluating the effects of blood pressure lowering and intensive glucose control on vascular retinal disorders in patients with type 2 diabetes mellitus. Contemporary Clinical Trials 2007;28(1):6-17. [DOI] [PubMed] [Google Scholar]
BENEDICT {published data only}
- BENEDICT Group. The BErgamo NEphrologic DIabetes Complications Trial (BENEDICT). Controlled Clinical Trials 2003;24(4):442-61. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Fassi A, Parvanova I, Bruno S, Iliev IP, Brusegan V, et al. Preventing microalbuminuria in type 2 diabetes. New England Journal of Medicine 2004;351(19):1941-51. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Iliev I, Filipponi M, Tadini S, Perna A, Ganeva M. Effect of trandolapril on regression of retinopathy in hypertensive patients with type 2 diabetes: a prespecified analysis of the Benedict trial. Journal of Ophthalmology 2010;2010:Article ID 106384. [DOI: 10.1155/2010/106384] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chase {published data only}
- Chase HP, Garg SK, Harris S, Hoops S, Jackson WE, Holmes DL. Angiotensin-converting enzyme inhibitor treatment for young normotensive diabetic subjects: a two-year trial. Annals of Ophthalmology 1993;25(8):284-9. [PubMed] [Google Scholar]
DEMAND {published data only}
- Ruggenenti P, Lauria G, Iliev IP, Fassi A, Ilieva AP, Rota S. Effects of manidipine and delapril in hypertensive patients with type 2 diabetes mellitus: the Delapril and Manidipine for Nephroprotection in Diabetes (DEMAND) randomized clinical trial. Hypertension 2011;58(5):776-83. [DOI] [PubMed] [Google Scholar]
DIRECT Prevent 1 {published data only}
- Chaturvedi N, DIRECT Programme Study Group. The DIabetic REtinopathy Candesartan Trials (DIRECT) Programme, rationale and study design. Journal of the Renin-Angiotensin-Aldosterone System 2002;3(4):255-61. [DOI] [PubMed] [Google Scholar]
- Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet 2008;372(9647):1394-402. [DOI] [PubMed] [Google Scholar]
- DIRECT Programme Study Group. The DIabetic REtinopathy Candesartan Trials (DIRECT) Programme: baseline characteristics. Journal of the Renin-Angiotensin-Aldosterone System 2005;6(1):25-32. [DOI] [PubMed] [Google Scholar]
- Sjolie AK, Klien R, Portat M, Orchard T, Fuller J, Parving HH, et al. Retinal microaneurysm count predicts progression and regression of diabetic retinopathy. Post-hoc results from the DIRECT Programme. Diabetic Medicine 2011;28(3):345-51. [DOI] [PubMed] [Google Scholar]
DIRECT Protect 1 {published data only}
- Chaturvedi N, DIRECT Programme Study Group. DIabetic REtinopathy Candesartan Trials (DIRECT) Programme, rationale and study design. Journal of the Renin-Angiotensin-Aldosterone System 2002;3(4):255-61. [DOI] [PubMed] [Google Scholar]
- Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet 2008;372(9647):1394-402. [DOI] [PubMed] [Google Scholar]
- DIRECT Programme Study Group. DIabetic REtinopathy Candesartan Trials (DIRECT) Programme: baseline characteristics. Journal of the Renin-Angiotensin-Aldosterone System 2005;6(1):25-32. [DOI] [PubMed] [Google Scholar]
DIRECT Protect 2 {published data only}
- Chaturvedi N, DIRECT Programme Study Group. DIabetic REtinopathy Candesartan Trials (DIRECT) Programme, rationale and study design. Journal of the Renin-Angiotensin-Aldosterone System 2002;3(4):255-61. [DOI] [PubMed] [Google Scholar]
- DIRECT Programme Study Group. DIabetic REtinopathy Candesartan Trials (DIRECT) Programme: baseline characteristics. Journal of Renin-Angiotensin-Aldosterone System 2005;6(1):25-32. [DOI] [PubMed] [Google Scholar]
- Sjolie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet 2008;372(9647):1385-93. [DOI] [PubMed] [Google Scholar]
EUCLID {published data only}
- Chaturvedi N, Fuller JH, Pokras F, Rottiers R, Papazoglou N, Aiello LP, et al. Circulating plasma vascular endothelial growth factor and microvascular complications of type 1 diabetes mellitus: the influence of ACE inhibition. Diabetes Medicine 2001;18(4):288-94. [DOI] [PubMed] [Google Scholar]
- Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, et al. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. Lancet 1998;351(9095):28-31. [DOI] [PubMed] [Google Scholar]
- EUCLID Study Group. Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. Lancet 1997;349(9068):1787-92. [PubMed] [Google Scholar]
- Sjolie AK, Chaturvedi N, Fuller J, EUCLID Study Group. Effect of lisinopril on progression of retinopathy and microalbuminuria in normotensive subjects with insulin-dependent diabetes mellitus [Effekt af lisinopril på progression af retinopati og mikroalbuminuri hos normotensive personer med insulinafhaengig diabetes mellitus]. Ugeskrift for Laeger 1999;161(7):949-52. [PubMed] [Google Scholar]
- Sjolie AK, Stephenson J, Aldington S, Kohner E, Janka H, Stevens L, et al. Retinopathy and vision loss in insulin-dependent diabetes in Europe. The EURODIAB IDDM Complications Study. Ophthalmology 1997;104(2):252-60. [DOI] [PubMed] [Google Scholar]
HINTS {published data only}
- Bosworth H, Powers B, Olsen M, McCant F, Grubber J, Smith V, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Archives of Internal Medicine 2011;171(13):1173-80. [DOI] [PubMed] [Google Scholar]
- Bosworth HB, Olsen M K, McCant F, Harrelson Ml, Gentry P, Rose C, et al. Hypertension Intervention Nurse Telemedicine Study (HINTS): testing a multifactorial tailored behavioral/educational and a medication management intervention for blood pressure control. American Heart Journal 2007;153(6):918-24. [DOI] [PubMed] [Google Scholar]
- Muir K, Grubber J, Mruthyunjaya P, McCant F, Bosworth H. Progression of diabetic retinopathy in the Hypertension Intervention Nurse Telemedicine Study. JAMA Ophthalmology 2013;131(7):957-8. [DOI] [PubMed] [Google Scholar]
J‐DOIT3 {published data only}
- Ueki, Sasako T, Kato M, Okazaki Y, Okahata S, Katsuyama H, et al. Design of and rationale for the Japan Diabetes Optimal Integrated Treatment study for 3 major risk factors of cardiovascular diseases (J-DOIT3): a multicenter, open-label, randomized, parallel-group trial. BMJ Open Diabetes Research and Care 2016;4(1):e000123. [DOI: 10.1136/bmjdrc-2015-000123] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki K, Sasako T, Okazaki Y, Kato M, Okahata S, Katsuyama H, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5(12):951-64. [DOI: 10.1016/s2213-8587(17)30327-3] [DOI] [PubMed] [Google Scholar]
J‐EDIT {published data only}
- Araki A, Iimuro S, Sakurai T, Umegaki H, Iijima K, Nakano H, et al. Long‐term multiple risk factor interventions in Japanese elderly diabetic patients: The Japanese Elderly Diabetes Intervention Trial–study design, baseline characteristics and effects of intervention. Geriatrics & Gerontology International 2012;12:7-17. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Yoshimura Y, Kawasaki R, Kamada C, Tanaka S, Horikawa C, et al. Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology 2013;24(2):204-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Iimuro S, Ohashi Y Sone H, Yamashita H, Ito H, Japanese Elderly Intervention Trial Research Group. Prevalence and risk factors for diabetic maculopathy, and its relationship to diabetic retinopathy in elderly Japanese patients with type 2 diabetes mellitus. Geriatrics and Gerontology International 2012;12:134-40. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Iimuro S Ohashi Y, Sone H, Ito H, Yamashita H, Japanese Elderly Diabetes Intervention Trial Study Group. Long‐term risk factors for diabetic retinopathy and diabetic maculopathy in elderly Japanese patients with type 2 diabetes mellitus. Geriatrics and Gerontology International 2012;12:141-4. [DOI] [PubMed] [Google Scholar]
Knudsen {published data only}
- Knudsen ST, Bek T, Poulsen PL, Hove MN, Rehling M, Mogensen CE. Effects of losartan on diabetic maculopathy in type 2 diabetic patients: a randomized, double-masked study. Journal of Internal Medicine 2003;254:147-58. [DOI] [PubMed] [Google Scholar]
Larsen {published data only}
- Larsen M, Hommel E, Parving HH, Lund-Andersen H. Protective effect of captopril on the blood-retina barrier in normotensive insulin-dependent diabetic patients with nephropathy and background retinopathy. Graefe's Archive of Clinical and Experimental Ophthalmology 1990;228(6):505-9. [DOI] [PubMed] [Google Scholar]
Medi‐Cal {published data only}
- California Medi-Cal Type 2 Diabetes Study Group. Closing the gap: effect of diabetes care management on glycemic control among low-income ethnic minority populations.. Diabetes Care 2004;27(1):95-103. [DOI] [PubMed] [Google Scholar]
- Pettitt DJ, Wollitzer AO, Jovanovic L, He G, Ipp E, California Medi-Cal Type 2 Diabetes Study Group. Decreasing the risk of diabetic retinopathy in a study of case management. Diabetes Care 2005;28(12):2819-22. [DOI] [PubMed] [Google Scholar]
Pradhan {published data only}
- Pradhan R, Fong D, March C, Jack R, Rezapour G, Norris K, et al. Angiotensin-converting enzyme inhibition for the treatment of moderate to severe diabetic retinopathy in normotensive type 2 diabetic patients. A pilot study. Journal of Diabetes and Its Complications 2002;16(6):377-81. [DOI] [PubMed] [Google Scholar]
Rachmani 2002 {published data only}
- Rachmani R, Levi Z, Slavachevski I, Avin M, Ravid M. Teaching patients to monitor their risk factors retards the progression of vascular complications in high-risk patients with type 2 diabetes mellitus—a randomized prospective study. Diabetic Medicine 2002;19(5):385-92. [DOI] [PubMed] [Google Scholar]
RASS {published data only}
- Klein R, Moss SE, Sinaiko AR, Zinman B, Gardiner R, Suissa S, et al. The relation of ambulatory blood pressure and pulse rate to retinopathy in type 1 diabetes mellitus. The Renin-Angiotensin System Study. Ophthalmology 2006;113(12):2231-6. [DOI] [PubMed] [Google Scholar]
- Klein R, Zinman B, Gardiner R, Suissa S, Donnelly SM, Sinaiko AR, et al. The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients. The Renin-Angiotensin System Study. Diabetes 2005;54(2):527-33. [DOI] [PubMed] [Google Scholar]
- Mauer M, Zinman B, Gardiner R, Drummond KN, Suissa S, Donnelly SM, et al. ACE-I and ARBs in early diabetic retinopathy. Journal of the Renin-Angiotensin-Aldosterone System 2002;3(4):262-9. [DOI] [PubMed] [Google Scholar]
- Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. New England Journal of Medicine 2009;361(1):40-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ravid 1993 {published data only}
- Ravid M, Savin H, Jutrin I, Bental T, Katz B, Lishner M. Long-term stabilizing effect of angiotensin-converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Annals of Internal Medicine 1993;118(8):577-81. [DOI] [PubMed] [Google Scholar]
ROADMAP {published data only}
- Haller H, Ito S, Izzo JL, Januszewica A, Katayama S, Menne J, et al for the ROADMAP Investigators. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. New England Journal of Medicine 2011;364(10):907-17 [with Supplementary Appendix]. [CTG: ] [DOI] [PubMed] [Google Scholar]
- Haller H, Viberti GC, Mimran A, Remuzzi G, Rabelink AJ, Ritz E, et al. Preventing microalbuminuria in patients with diabetes: rationale and design of the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study. Journal of Hypertension 2006;24(2):403-8. [DOI] [PubMed] [Google Scholar]
- Menne J, Fitz E, Ruilope LM, Chatzikyrkou C, Viberti G, Haller H. The Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) observational follow-up study: benefits of RAS blockade with olmesartan treatment are sustained after study discontinuation. Journal of the American Heart Association 2014;3:e000810. [DOI: 10.1161/JAHA.114.000810] [CLINICALTRIALS.GOV: NCT00185159] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menne J, Izzo JL, Ito S, Januszewicz A, Katayama S, Chatzykirkou C, et al for the ROADMAP Investigators. Prevention of microalbuminuria in patients with type 2 diabetes and hypertension. Journal of Hypertension 2012;30:811-8. [DOI: 10.1097/HJH.0b013e328351856d] [DOI] [PubMed] [Google Scholar]
Steno‐2 {published data only}
- Gaede J, Oellgaard J, Ibsen R, Gaede P, Nortoft E, Parving H-H, et al. A cost analysis of intensified vs conventional multifactorial therapy in individuals with type 2 diabetes: a post hoc analysis of the Steno-2 study. Diabetologia 2019;62:147-55. [CTG: ] [DOI: 10.1007/s00125-017-4739-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaede P, Lammert M, Valentine WJ, Parving H-H, Palmer AJ, Pedersen O, et al. Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes. Diabetes Care 2008;31(8):1510-5. [CTG: ] [DOI: 10.2337/dc07-2452] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaede P, Oellgaard J, Carstensen B, Rossing P, Lund-Andersen H, Parving H-H, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia 2016;59:2298-307. [CTG: ] [DOI: 10.1007/s00125-016-4065-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaede P, Vedel P, Larsen N, Jensen GVH, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New England Journal of Medicine 2003;348(5):383-93. [DOI] [PubMed] [Google Scholar]
- Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 1999;353(9153):617-22. [DOI] [PubMed] [Google Scholar]
- Vaag AA. Glycemic control and prevention of microvascular and macrovascular disease in the Steno 2 study. Endocrine Practice 2006;12(Suppl 1):89-92. [DOI] [PubMed] [Google Scholar]
UKPDS/HDS {published data only}
- Clarke PM, Gray AM, Briggs A, Stevens RJ, Matthews DR, Holman RR, et al. Cost-utility analyses of intensive blood glucose and tight blood pressure control in type 2 diabetes (UKPDS 72). Diabetologia 2005;48(5):868-77. [DOI] [PubMed] [Google Scholar]
- Gray A, Clarke P, Farmer A, Holman R. Implementing intensive control of blood glucose concentration and blood pressure in type 2 diabetes in England: cost analysis (UKPDS 63). BMJ 2002;325(7369):860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A, Clarke P, Raikou M, Adler A, Stevens R, Neil A, et al. An economic evaluation of atenolol vs. captopril in patients with type 2 diabetes (UKPDS 54). Diabetes Medicine 2001;18(6):438-44. [DOI] [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Andrew H, Neil W, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. New England Journal of Medicine 2008;359(15):1565-76. [DOI] [PubMed] [Google Scholar]
- Hypertension in Diabetes Study Group. Hypertension in Diabetes Study III. Prospective study of therapy of hypertension in type 2 diabetic patients: efficacy of ACE inhibition and β-blockade. Diabetic Medicine 1994;11(8):773-82. [PubMed] [Google Scholar]
- Hypertension in Diabetes Study Group. Hypertension in Diabetes Study IV. Therapeutic requirements to maintain tight blood pressure control. Diabetologia 1996;39(12):1554-61. [DOI] [PubMed] [Google Scholar]
- Kohner EM, Stratton IM, Aldington SJ, Turner RC, Matthews DR, UK Prospective Diabetes Study Group. Microaneurysms in the development of diabetic retinopathy (UKPDS 42). Diabetologia 1999;42(9):1107-12. [DOI] [PubMed] [Google Scholar]
- lva ML, Gray A, Mihaylova B, Leal J, Holman RR. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabetic Medicine 2015;32:459-66. [DOI: 10.1111/dme-12647] [DOI] [PubMed] [Google Scholar]
- Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, et al. UKPDS 50: Risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia 2001;44(2):156-63. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352(9131):837-53. [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Cost effectiveness analysis of improved blood pressure control in hypertensive patients with type 2 diabetes: UKPDS 40. BMJ 1998;317(7160):720-6. [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998;317(7160):713-20. [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). Diabetes Care 1999;22(7):1125-36. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus. UKPDS 69. Archives of Ophthalmology 2004;122(11):1631-40. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317(7160):703-13. [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia 1991;34(12):877-90. [PubMed] [Google Scholar]
Wang {published data only}
- Wang N, Zheng Z, Jin HY, Xu X. Treatment effects of captopril on non-proliferative diabetic retinopathy. Chinese Medical Journal 2012;125(2):287-92. [PubMed] [Google Scholar]
Zhao {published data only}
- Zhao C-M, Cui X-L, Wan G, Lu Y-Z, Niu Y-Q, Su C-Y, et al. Analysis of the effect of nine consecutive years’ intensive management and number of times achieving the target control on endpoint events in T2DM patients in Sanlitun Community Health Service Center in Beijing. International Journal of Endocrinology 2020;2020:Article ID 3646342. [DOI: 10.1155/2020/3646342] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aro 2019_new {published data only}
- Aro A, Kauppinen A, Kivinen N, Selander T, Kinnunen K, Tuomilehto J, et al. Life style intervention improves retinopathy status—The Finnish Diabetes Prevention Study. Nutrients 2019;11(7):1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Auyanet 2010 {published data only}
- Auyanet I, Rodriguez LJ, Esparza N, Cabrera F, Rossique P, Suria S, et al. Does carvedilol minimize the requirements for laser photocoagulation in diabetic retinopathy? [Minimiza el carvedilol los requerimientos de fotocoagulacion laser en la retinopatia diabetica?]. Nefrologia 2010;30(4):473-4. [DOI] [PubMed]
CALM‐II {published data only}
- Andersen NH, Knudsen ST, Poulsen PL, Poulsen SH, Helleberg K, Eiskjaer H, et al. Dual blockade with candesartan cilexetil and lisinopril in hypertensive patients with diabetes mellitus: rationale and design. Journal of the Renin-Angiotensin-Aldosterone System 2003;4(2):96-9. [DOI] [PubMed] [Google Scholar]
- Andersen NH, Poulsen PL, Knudsen ST, Poulsen SH, Eiskjaer H, Hansen KW, et al. Long-term dual blockade with candesartan and lisinopril in hypertensive patients with diabetes: the CALM II study. Diabetes Care 2005;28(2):273-7. [DOI] [PubMed] [Google Scholar]
Chang 2011 {published data only}
- Chang CH, Chuang LM. Effects of medical therapies on retinopathy progression in type 2 diabetes: is blood pressure control the lower the better? Journal of Diabetes Investigation 2011;2(2):101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
DCCT {published data only}
- Genuth S, Lipps J, Lorenzi G, Nathan DM, Davis MD, Lachin JM, et al. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287(19):2563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22(1):99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. American Journal of Cardiology 1995;75(14):894-903. [DOI] [PubMed] [Google Scholar]
Durruty 2000 {published data only}
- Durruty P, Carpentier C, Krause P, Garcia de los Rios M. Evaluation of retinal involvement in type 2 diabetics with microalbuminuria [Evaluacion del compromiseo retinal en diabeticos tipo 2 microalbuminuricos]. Revista Medica de Chile 2000;128(10):1085-92. [PubMed] [Google Scholar]
Faguer de Moustier 1989 {published data only}
- Faguer de Moustier B, Paoli V, Tchobroutsky G. Metabolic controlled trial of nicardipine in type 2 diabetic patients with slight hypertension. Current Therapeutic Research, Clinical and Experimental 1989;45(4):690-704. [Google Scholar]
Harrold 1969 {published data only}
- Harrold BP, Marmion VJ, Gough KR. A double-blind controlled trial of clofibrate in the treatment of diabetic retinopathy. Diabetes 1969;18(5):285-91. [DOI] [PubMed] [Google Scholar]
Jackson 1992 {published data only}
- Jackson WE, Holmes DL, Garg SK, Harris S, Chase HP. Angiotensin-converting enzyme inhibitor therapy and diabetic retinopathy. Annals of Ophthalmology 1992;24(3):99-103. [PubMed] [Google Scholar]
JDCS 2011 {published data only}
- Kawasaki R, Tanaka S, Tanaka S, Yamamoto T, Sone H, Ohashi Y, et al. Incidence and progression of diabetic retinopathy in Japanese adults with type 2 diabetes: 8 year follow-up study of the Japan Diabetes Complications Study (JDCS). Diabetologia 2011;54(9):2288-94. [DOI] [PubMed] [Google Scholar]
- Sone H, Tanaka S, Iimuro S, Tanaka S, Oida K, Yamasaki Y, et al. Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study). Diabetologia 2010;53(3):419-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehsten 1996 {published data only}
- Lehsten K, Becker B, Michaelis D, Eggert HJ, Rjasanowski I, Salzsieder E. On the prospective significance of age at onset of diabetes, metabolic control, and hypertension for the development of diabetic retinopathy in patients with IDDM. Diabetes und Stoffwechsel 1996;5(1):18-25. [Google Scholar]
Malik 1998 {published data only}
- Malik RA, Williamson S, Abbott C, Carrington AL, Iqbal J, Schady W, et al. Effect of angiotensin-converting-enzyme (ACE) inhibitor trandolapril on human diabetic neuropathy: randomised double-blind controlled trial. Lancet 1998;352(9145):1978-81. [DOI] [PubMed] [Google Scholar]
MCSG 1995 {published data only}
- Microalbuminuria Collaborative Study Group. Intensive therapy and progression to clinical albuminuria in patients with insulin dependent diabetes mellitus and microalbuminuria. BMJ 1995;311(7011):973-7. [PMC free article] [PubMed] [Google Scholar]
Mehlsen 2011 {published data only}
- Mehlsen J, Jeppesen P, Erlandsen M, Poulsen PL, Bek T. Lack of effect of short-term treatment with amlodipine and lisinopril on retinal autoregulation in normotensive patients with type 1 diabetes and mild diabetic retinopathy. Acta Ophthalmologica 2011;89(8):764-8. [DOI] [PubMed] [Google Scholar]
Newsom 1991 {published data only}
- Newsom RS, Rassam SM, Kohner EM. The effect of beta blockers on retinal blood flow in diabetic patients. European Journal of Ophthalmology 1991;1(3):131-6. [DOI] [PubMed] [Google Scholar]
OSCAR {published data only}
- Ogawa H, Mitsuyama K, Jinnouchi T, Matsui K, Arakawa K for the OSCAR Study Group. Rationale, design, and patient baseline characteristics of OlmeSartan and Calcium Antagonists randomized (OSCAR) study: a study comparing the incidence of cardiovascular events between high-dose angiotensin II receptor blocker (ARB) monotherapy and combination therapy of ARB with calcium channel blocker in Japanese elderly high-risk hypertensive patients. Hypertension Research 2009;32:575-80. [CTG: ] [DOI: 10.1038/2009.60] [DOI] [PubMed] [Google Scholar]
Patel 1998 {published data only}
- Patel V, Rassam SM, Chen HC, Jones M, Kohner EM. Effect of angiotensin-converting enzyme inhibition with perindopril and beta-blockade with atenolol on retinal blood flow in hypertensive diabetic subjects. Metabolism: Clinical and Experimental 1998;47(12 Suppl 1):28-33. [DOI] [PubMed] [Google Scholar]
Porush 2000 {published data only}
- Porush JG. The benefits of angiotensin II receptor antagonists in high-risk hypertensive patients with diabetes. European Heart Journal Supplements 2000;2(B):B22-7. [Google Scholar]
Rachmani 2000 {published data only}
- Rachmani R, Lidar M, Levy Z, Ravid M. Effect of enalapril on the incidence of retinopathy in normotensive patients with type 2 diabetes. European Journal of Internal Medicine 2000;11(1):48-50. [Google Scholar]
Rassam 1997 {published data only}
- Rassam SMB, Patel VJ, Kohner E. Effect of ACE-inhibitors on retinopathy score and retinal hemodynamics in normotensive diabetic subjects. American Academy of Ophthalmology 1997;38:S207. [Google Scholar]
Schwartz 1998 {published data only}
- Schwartz MM, Lewis EJ, Leonard-Martin T, Lewis JB, Batlle D, The Collaborative Study Group. Renal pathology patterns in type II diabetes mellitus: relationship with retinopathy. Nephrology Dialysis Transplantation 1998;13(10):2547-52. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ABCD‐2V (2) {published data only}
- Bedigian M. Improving the prognosis of diabetic patients: evaluating the role of intensive versus moderate blood pressure control with selective angiotensin II receptor block (ARB) therapy. Journal of the Renin-Angiotensin-Aldosterone System 2000;1(Suppl 2):25-8. [DOI] [PubMed] [Google Scholar]
- Bedigian MP, Estacio RO, Jefferes BW, Biggerstaff S, Schrier RW. Baseline characteristics of the hypertensive cohort of the appropriate blood pressure control in diabetes trial—part 2 with valsartan (ABCD-2V). American Journal of Hypertension 2000;13(4 Pt 2):56-7A. [Google Scholar]
- Estacio RO, Coll JR, Tran ZV, Schrier RW. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. American Journal of Hypertension 2006;19(12):1241-8. [DOI] [PubMed] [Google Scholar]
- Shrier RW, Estacio RO, Jeffers BW, Biggerstaff S, Krinsk E, Pincus JR, et al. ABCD-2V: Appropriate blood pressure control in diabetes—part 2 with valsartan. American Journal of Hypertention 1999;12(4 Pt 2):141A. [Google Scholar]
Additional references
ADA 1998
- American Diabetes Association. Diabetic retinopathy. Diabetes Care 1998;21(1):157-9. [PubMed] [Google Scholar]
Arguedas 2013
- Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD008277. [DOI: 10.1002/14651858.CD008277.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buch 2004
- Buch H. Prevalence and causes of visual impairment and blindness among Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology 2004;111(1):53-61. [DOI] [PubMed] [Google Scholar]
CDC 2011
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011.
Chew 1996
- Chew E. Association of elevated serum lipid levels with retinal hard exudates in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Archives of Ophthalmology 1996;114(9):1079-84. [DOI] [PubMed] [Google Scholar]
Cushman 2007
- Cushman WC, Grimm RH Jr, Cutler JA, Evans GW, Capes S, Corson MA, et al. Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. American Journal of Cardiology 2007;99(12A):44-55i. [DOI] [PubMed] [Google Scholar]
DCCT 1993
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine 1993;329(14):977-86. [DOI] [PubMed] [Google Scholar]
Desai 2001
Do 2015a
- Do DV, Wang X, Vedula SS, Marrone M, Sleilati G, Hawkins BS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD006127. [DOI: 10.1002/14651858.CD006127.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
DRCR.net 2010
- Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, et al, Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117(6):1064-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
DRS10 1985
- Rand LI, Prud'homme GJ, Ederer F, Canner PL. Factors influencing the development of visual loss in advanced diabetic retinopathy. Diabetic Retinopathy Study (DRS) report number 10. Investigative Ophthalmology and Visual Science 1985;26(7):983-91. [PubMed] [Google Scholar]
DRS2 1978
- The Diabetes Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy: the second report of Diabetic Retinopathy Study findings. Ophthalmology 1978;85(1):82-106. [DOI] [PubMed] [Google Scholar]
DRS5 1981
- The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy: relationship of adverse treatment effects to retinopathy severity. Diabetic Retinopathy Study report number 5. Developments in Ophthalmology 1981;2:248-56. [PubMed] [Google Scholar]
DRS8 1981
- The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings. DRS report number 8. Ophthalmology 1981;88(7):583-600. [PubMed] [Google Scholar]
EDPRG 2004a
- The Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Archives of Ophthalmology 2004;122(4):477-85. [DOI] [PubMed] [Google Scholar]
EDPRG 2004b
- The Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adults in the United States. Archives of Ophthalmology 2004;122(4):552-63. [DOI] [PubMed] [Google Scholar]
EDPRG 2004c
- The Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Archives of Ophthalmology 2004;122(4):546-51. [DOI] [PubMed] [Google Scholar]
ETDRS10 1991
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991;98(5 Suppl):786-806. [PubMed] [Google Scholar]
ETDRS1 1985
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Archives of Ophthalmology 1985;103(12):1796-806. [PubMed] [Google Scholar]
ETDRS12 1991
- Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98(5 Suppl):823-33. [PubMed] [Google Scholar]
ETDRS18 1998
- Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, et al. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study report number 18. Investigative Ophthalmology and Visual Science 1998;39(2):233-52. [PubMed] [Google Scholar]
ETDRS9 1991
- Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology 1991;98(5 Suppl):766-85. [PubMed] [Google Scholar]
Ferris 1993
- Ferris FL 3rd. How effective are treatments for diabetic retinopathy? JAMA 1993;269(10):1290-1. [PubMed] [Google Scholar]
Fong 2004
- Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Retinopathy in diabetes. Diabetes Care 2004;27(Suppl 1):S84-7. [DOI] [PubMed] [Google Scholar]
Frank 2004
- Frank RN. Medical progress: diabetic retinopathy. New England Journal of Medicine 2004;350(1):48-58. [DOI] [PubMed] [Google Scholar]
Gillow 1999
- Gillow JT, Gibson JM, Dodson PM. Hypertension and diabetic retinopathy—what's the story? British Journal of Ophthalmology 1999;83(9):1083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giuffre 2004
- Giuffre G, Lodato G, Dardanoni G. Prevalence and risk factors of diabetic retinopathy in adult and elderly subjects: The Castedaccia Eye Study. Graefe's Archives of Clinical and Experimental Ophthalmology 2004;242(7):535-40. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
Grey 1989
- Grey RHB. Blind and partial sight registration in Avon. British Journal of Ophthalmology 1989;73(2):88-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansson 1998
- Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351(9118):1755-62. [DOI] [PubMed] [Google Scholar]
Harris 1998
- Harris MI, Klein R, Cowie CC, Rowland M, Byrd-Holt DD. Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type-2 diabetes? A US population study. Diabetes Care 1998;21(8):1230-5. [DOI] [PubMed] [Google Scholar]
Hayward 2002
- Hayward LM. What is the prevalence of visual impairment in the general and diabetic populations: are there ethnic and gender differences? Diabetic Medicine 2002;19(1):27-34. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
HOPESI 2000
- Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE sub-study. Lancet 2000;355(9200):253-9. [PubMed] [Google Scholar]
Keen 2001
- Keen H. The appearance of retinopathy and progression to proliferative retinopathy: the WHO multinational study of vascular disease in diabetes. Diabetologia 2001;44(Suppl 2):S22-30. [DOI] [PubMed] [Google Scholar]
Klein 1984a
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Archives of Ophthalmology 1984;102(4):520-6. [DOI] [PubMed] [Google Scholar]
Klein 1984b
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Archives of Ophthalmology 1984;102(4):527-32. [DOI] [PubMed] [Google Scholar]
Klein 1988
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA 1988;260(19):2864-71. [PubMed] [Google Scholar]
Klein 1989a
- Klein R, Moss SE, Klein BE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XI. The incidence of macular edema. Ophthalmology 1989;96(10):1501-10. [DOI] [PubMed] [Google Scholar]
Klein 1989b
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Is blood pressure a predictor of the incidence or progression of diabetic retinopathy? Archives of Internal Medicine 1989;149(11):2427-32. [PubMed] [Google Scholar]
Klein 2002a
- Klein R, Klein BE. Blood pressure control and diabetic retinopathy. British Journal of Ophthalmology 2002;86(4):365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 2002b
- Klein R. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology 2002;109(7):1225-34. [DOI] [PubMed] [Google Scholar]
Krakoff 2003
- Krakoff J, Lindsay RS, Looker HC, Nelson RG, Hanson RL, Knowler WC. Incidence of retinopathy and nephropathy in youth-onset compared with adult-onset type 2 diabetes. Diabetes Care 2003;26(1):76-81. [DOI] [PubMed] [Google Scholar]
Krumpaszky 1999
- Krumpaszky HG. Blindness incidence in Germany. A population-based study from Wurttemberg-Hohenzollern. Ophthalmologica 1999;213(3):176-82. [DOI] [PubMed] [Google Scholar]
Kullberg 2002
- Kullberg CE, Abrahamsson M, Arnqvist HJ, Finnstrom K, Ludvigsson J, VISS Study Group. Prevalence of retinopathy differs with age at onset of diabetes in a population of patients with type 1 diabetes. Diabetes Medicine 2002;19(11):924-31. [DOI] [PubMed] [Google Scholar]
Leske 2003
- Leske C. Incidence of diabetic retinopathy in the Barbados Eye Studies. Ophthalmology 2003;110(5):941-7. [DOI] [PubMed] [Google Scholar]
Mangione 2001
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Archives of Ophthalmology 2001;119(7):1050-8. [DOI] [PubMed] [Google Scholar]
Mohamed 2007
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy. A systematic review. JAMA 2007;298(8):902-16. [DOI] [PubMed] [Google Scholar]
Moss 1996
- Moss S, Klein R, Klein BE. Cigarette smoking and ten-year progression in diabetic retinopathy. Ophthalmology 1996;103(9):1438-42. [DOI] [PubMed] [Google Scholar]
Porta 2001
- Porta M, Sjoelie AK, Chaturvedi N, Stevens L, Rottiers R, Veglio M, et al. Risk factors for progression to proliferative diabetic retinopathy in the EURODIAB Prospective Complications Study. Diabetologia 2001;44(12):2203-9. [DOI] [PubMed] [Google Scholar]
PPP 2019
- American Academy of Ophthalmology Retina Panel. Preferred Practice Patterns® Guidelines. Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology, 2019. Available at: www.aao.org/ppp. [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: Nordic Cochrane Center, The Cochrane Collaboration, 2020.
Rosenberg 1996
- Rosenberg T, Klie F. Current trends in newly registered blindness in Denmark. Acta Ophthalmologica Scandinavica 1996;74(4):395-8. [DOI] [PubMed] [Google Scholar]
Sivaprasad 2012
- Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Survey of Opthalmology 2012;57(4):347-70. [DOI] [PubMed] [Google Scholar]
Sjølie 2011
- Sjølie AK, Dodson P, Hobbs FR. Does renin-angiotensin system blockade have a role in preventing diabetic retinopathy? A clinical review. International Journal of Clinical Practice 2011;65(2):148-53. [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith JM, Steel DHW. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database of Systematic Reviews 2011, Issue 5. Art. No: CD008214. [DOI: 10.1002/14651858.CD008214.pub2] [DOI] [PubMed] [Google Scholar]
Tapp 2003
- Tapp RJ, Shaw JE, Harper CA, Courten MP, Balkau B, McCarty DJ, et al. The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care 2003;26(6):1731-7. [DOI] [PubMed] [Google Scholar]
Thomas 2014
- Thomas RL, Dunstan FD, Luzio SD, Chowdhury SR, North RV, Hale SL, et al. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. British Journal of Ophthalmology 2015;99(1):64-8. [DOI] [PubMed] [Google Scholar]
UKPDS33 1998
- UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352(9131):837-53. [PubMed] [Google Scholar]
UKPDS38 1998
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes (UKPDS 38). BMJ 1998;317(7160):703-13. [PMC free article] [PubMed] [Google Scholar]
van Leiden 2002
- Leiden HA. Blood pressure, lipids, and obesity are associated with retinopathy: The Hoorn Study. Diabetes Care 2002;25(8):1320-5. [DOI] [PubMed] [Google Scholar]
van Leiden 2003
- Leiden HA. Risk factors for incident retinopathy in a diabetic and non-diabetic population: The Hoorn Study. Archives of Ophthalmology 2003;121(2):245-51. [DOI] [PubMed] [Google Scholar]
Virgili 2014
- Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD007419. [DOI: 10.1002/14651858.CD007419.pub4] [DOI] [PubMed] [Google Scholar]
Wild 2004
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27(5):1047-53. [DOI] [PubMed] [Google Scholar]
Zhang 2001
- Zhang L, Krzentowski G, Albert A, Lefebvre PJ. Risk of developing retinopathy in Diabetes Control and Complication Trial type 1 diabetic patients with good or poor metabolic control. Diabetes Care 2001;24(7):1275-9. [DOI] [PubMed] [Google Scholar]
Zhang 2010
- Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010;304(6):649-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Do 2015b
- Do DV, Wang X, Vedula SS, Marrone M, Sleilati G, Hawkins BS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD006127. [DOI: 10.1002/14651858.CD006127.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sleilati 2009
- Sleilati G, Frank RN, Mathew MC. Blood pressure control for diabetic retinopathy. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD006127. [DOI: 10.1002/14651858.CD006127] [DOI] [PMC free article] [PubMed] [Google Scholar]


