Abstract
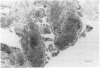
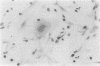
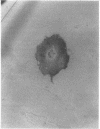
The influx of cells into the synovial intima in rheumatoid joints may include osteoclasts and their precursors. The distribution of osteoclast markers--namely, tartrate resistant acid phosphatase activity and the expression of vitronectin receptor (shown with monoclonal antibodies 13C2 and 23C6)--was therefore examined in synovium obtained from patients with rheumatoid (RA) or degenerative (OA) arthritis. Tartrate resistant acid phosphatase positive cells were found in frozen sections of 60% (n = 30) of RA and 69% (n = 29) of OA synovial membranes. Whereas all synovia tested (four RA, four OA) showed diffuse staining of the lining cells with 13C2, 55% (n = 11) of RA and 57% (n = 14) of OA synovial membranes contained isolated cells stained with 23C6 scattered throughout the tissue. In cultures of synovial cells, tartrate resistant acid phosphatase positive, multinuclear, and 23C6 positive cells were found; these cells did not, however, form resorption pits on bone slices. The results show that fully differentiated osteoclasts are uncommon in synovium from patients with either degenerative or inflammatory arthropathies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash P., Loutit J. F., Townsend K. M. Osteoclasts derive from hematopoietic stem cells according to marker, giant lysosomes of beige mice. Clin Orthop Relat Res. 1981 Mar-Apr;(155):249–258. [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J., Horton M. A., McGee J. O. New sites of cellular vitronectin receptor immunoreactivity detected with osteoclast-reacting monoclonal antibodies 13C2 and 23C6. Bone Miner. 1990 Jan;8(1):7–22. doi: 10.1016/0169-6009(91)90136-n. [DOI] [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum. 1984 Sep;27(9):968–975. doi: 10.1002/art.1780270902. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Davies J., Warwick J., Totty N., Philp R., Helfrich M., Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989 Oct;109(4 Pt 1):1817–1826. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher R. Origin of synovial type A cells during inflammation. An experimental approach. Immunobiology. 1982 Apr;161(3-4):232–245. doi: 10.1016/S0171-2985(82)80079-X. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Willoughby D. A. Demonstration of bone marrow derived cells in synovial lining by means of giant intracellular granules as genetic markers. Ann Rheum Dis. 1982 Apr;41(2):177–182. doi: 10.1136/ard.41.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Generation of osteoclasts from hemopoietic cells and a multipotential cell line in vitro. J Cell Physiol. 1989 Sep;140(3):478–482. doi: 10.1002/jcp.1041400311. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Lewis D., McNulty K., Pringle J. A., Chambers T. J. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985 Nov;45(11 Pt 2):5663–5669. [PubMed] [Google Scholar]
- MacDonald B. R., Takahashi N., McManus L. M., Holahan J., Mundy G. R., Roodman G. D. Formation of multinucleated cells that respond to osteotropic hormones in long term human bone marrow cultures. Endocrinology. 1987 Jun;120(6):2326–2333. doi: 10.1210/endo-120-6-2326. [DOI] [PubMed] [Google Scholar]
- Orcel P., Bielakoff J., de Vernejoul M. C. Formation of multinucleated cells with osteoclast precursor features in human cord monocytes cultures. Anat Rec. 1990 Jan;226(1):1–9. doi: 10.1002/ar.1092260102. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Chenu C., Bird A., Mundy G. R., Roodman G. D. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J Bone Miner Res. 1989 Feb;4(1):113–118. doi: 10.1002/jbmr.5650040116. [DOI] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Revell P. A. Synovial lining cells. Rheumatol Int. 1989;9(2):49–51. doi: 10.1007/BF00270244. [DOI] [PubMed] [Google Scholar]
- Salisbury A. K., Duke O., Poulter L. W. Macrophage-like cells of the pannus area in rheumatoid arthritic joints. Scand J Rheumatol. 1987;16(4):263–272. doi: 10.3109/03009748709102927. [DOI] [PubMed] [Google Scholar]
- Scott D. L., Delamere J. P., Walton K. W. The distribution of fibronectin in the pannus in rheumatoid arthritis. Br J Exp Pathol. 1981 Aug;62(4):362–368. [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Vestal D. J., Irwin S. V., Burke T. A., Cheresh D. A. Purification and functional characterization of integrin alpha v beta 5. An adhesion receptor for vitronectin. J Biol Chem. 1990 Jul 5;265(19):11008–11013. [PubMed] [Google Scholar]
- Snipes R. G., Lam K. W., Dodd R. C., Gray T. K., Cohen M. S. Acid phosphatase activity in mononuclear phagocytes and the U937 cell line: monocyte-derived macrophages express tartrate-resistant acid phosphatase. Blood. 1986 Mar;67(3):729–734. [PubMed] [Google Scholar]
- Tinkler S. M., Williams D. M., Linder J. E., Johnson N. W. Kinetics of osteoclast formation: the significance of blood monocytes as osteoclast precursors during 1 alpha-hydroxycholecalciferol-stimulated bone resorption in the mouse. J Anat. 1983 Sep;137(Pt 2):335–340. [PMC free article] [PubMed] [Google Scholar]
- Udagawa N., Takahashi N., Akatsu T., Sasaki T., Yamaguchi A., Kodama H., Martin T. J., Suda T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989 Oct;125(4):1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- Waller H. A., Butler M. G., McClean J. G., Dowd G. S., Scott D. L. Localisation of fibronectin mRNA in the rheumatoid synovium by in situ hybridisation. Ann Rheum Dis. 1992 Jun;51(6):735–740. doi: 10.1136/ard.51.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]