Abstract
Alterations in grey matter volume (GMV) and cortical thickness (CT) in Crohn’s disease (CD) patients has been previously documented. However, the findings are inconsistent, and not a true representation of CD burden, as only CD patients in remission have been studied thus far. We investigate alterations in brain morphometry in patients with active CD and those in remission, and study relationships between brain structure and key symptoms of fatigue, abdominal pain, and extraintestinal manifestations (EIM). Magnetic Resonance Imaging brain scans were collected in 89 participants; 34 CD participants with active disease, 13 CD participants in remission and 42 healthy controls (HCs); Voxel based morphometry (VBM) assessed GMV and white matter volume (WMV), and surface-based analysis assessed cortical thickness (CT). We show a significant reduction in global cerebrospinal fluid (CSF) volume in CD participants compared with HCs, as well as, a reduction in regional GMV, WMV and CT in the left precentral gyrus (motor cortex), and an increase in GMV in the frontal brain regions in CD compared with HCs. Atrophy of the supplementary motor area (SMA) was associated with greater fatigue in CD. We also show alterations in brain structure in multiple regions in CD associated with abdominal pain and extraintestinal inflammations (EIMs). These brain structural alterations likely reflect neuroplasticity to a chronic systemic inflammatory response, abdominal pain, EIMs and fatigue. These findings will aid our understanding of the cross-linking between chronic inflammation, brain structural changes and key unexplained CD symptomatology like fatigue.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11682-022-00742-6.
Keywords: Crohn’s disease, Brain volume, Cortical thickness, Intestinal inflammation, Gut-brain axis, Chronic inflammation, Fatigue
Introduction
Crohn’s disease (CD) patients experience a host of debilitating symptoms, fatigue is a common symptom in active disease, and the second most frequent complaint after extra-intestinal manifestations (EIM) in patients in remission (Singh et al., 2011). Together with abdominal pain and stool frequency (Pariente et al., 2011), fatigue and arthralgia are key variables in the Inflammatory Bowel Disease (IBD) Disability index. These key symptoms are understudied, particularly in relation to their influences on the Central Nervous System (CNS) (Peyrin-Biroulet et al., 2012). Fatigue is mediated via the integration of the CNS and peripheral musculoskeletal systems (Giulio et al., 2006), whereby physiological perturbations occurring in the brain and spinal cord (central fatigue) or at the neuromuscular junction and the skeletal muscle (peripheral fatigue) result in acute and transient decrements in performance. Pro-inflammatory cytokines are involved in symptom generation of central fatigue (Borren et al., 2019), possibly via increasing blood-brain barrier (BBB) permeability, propagating inflammatory signals within the brain via activation of endothelial, glial cells and macrophage, resulting in neuronal cell death (Jones et al., 2006). Systemic inflammation may be linked with demyelinating complications reflected in morphometric changes in the brain of CD patients (Zikou et al., 2014). Intestinal inflammation and abdominal pain may activate central sensitization pathways that convey visceral nociceptive afferent signals from the gut to the brain (Jones et al., 2006; Hubbard et al., 2016), affecting symptom perception and gut function (Jones et al., 2006), with high levels of somatization strongly associated with fatigue severity and impact in inflammatory bowel disease (IBD) patients (Ratnakumaran et al., 2018).
To date, brain morphometry studies relate to CD patients in remission. Using MRI, alterations have been reported in cortical grey matter volume (GMV) (Agostini et al., 2013, 2017; Bao et al., 2015; Erp et al., 2017; Thomann et al., 2020), sub-cortical GMV (Bao et al., 2015; Nair et al., 2016) cortical thickness (CT) (Bao et al., 2015; Nair et al., 2016; Thomann et al., 2016) cortical surface area (CSA) (Nair et al., 2016; Thomann et al., 2016) and cortical folding (Thomann et al., 2016) across multiple brain regions involved in pain, emotion, and homeostasis in CD patients in remission compared to healthy controls (HCs), and GMV has been negatively correlated with disease duration (Agostini et al., 2013; Bao et al., 2015) (see Supplementary Table S1). A recent meta-analysis of voxel based morphometry (VBM) in CD participants in remission showed a significant reduction in GMV in medial frontal gyrus (MFG) compared with HCs (Yeung, 2021). Diffusion Tensor Imaging (DTI) has reported alterations in white matter (WM) fibre integrity in CD patients in remission (Zikou et al., 2014; Hou et al., 2020), suggested to result from cerebral small vessel vasculitis and neurotoxic effects of proinflammatory cytokines (Dolapcioglu & Dolapcioglu, 2015). There are few studies of the relationship of fatigue, abdominal pain and EIM with brain morphometry in CD. CD patients in remission with fatigue are reported to have reduced GMV in left superior frontal gyrus (SFG, a region involved in working memory) compared to HCs without fatigue (du Boisgueheneuc et al., 2006). Abdominal pain in CD participants has been associated with reduced GMV in the insula and anterior cingulate cortex (ACC) compared to CD participants without abdominal pain and HCs (Bao et al., 2017). CD patients with extraintestinal manifestations (EIMs) exhibit altered cortical folding of the ACC and SFG relative to CD without EIMs (Thomann et al., 2016).
This study aims to compare brain morphometry in CD participants with both active disease and in remission with HCs, and to investigate relationships between global/regional GMV, white matter volume (WMV), cerebrospinal fluid (CSF) volume and CT with symptoms of fatigue, abdominal pain, and extraintestinal manifestations (EIM).
Methods and materials
Study design
This study was a case-control study of CD participants against HCs., with approval from the National Research Ethics Service [NRES] Committee East Midlands [14/EM/0192], (clinicaltrials.gov [NCT02772458]). CD participants were identified through a clinical database search, expression of interest list and recruited from IBD Clinics at Nottingham University Hospitals. HCs were recruited through participant databases, study fliers and social media. All CD participants and HCs gave informed consent. CD participants disease activity was defined through objective markers of inflammation: recent ileocolonoscopy (Lamb et al., 2019), CT, magnetic resonance enterography [MRE] showing active inflammatory and uncomplicated disease [not stricturing or penetrating behaviour], else FCP > 250 µg/g or CRP > 5 g/dL (Mosli et al., 2015). CD clinical symptoms were measured at inclusion using the Harvey-Bradshaw Index [HBI] score(Harvey, 1980) Stable doses of immunosuppressive agents or biological agents were permitted. Depression and anxiety symptoms were measured using the Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snaith, 1983). See Supplementary Material for exclusion criteria and additional clinical measures.
Image acquisition
Structural brain MRI images were collected as part of a wider MRI protocol including functional MRI brain responses to a test meal. Participants were scanned on a 3T Philips Achieva scanner (Philips Medical Systems, Best, Netherlands) with a 32-channel receive head coil. Brain scans were acquired with a T1-weighted MPRAGE sequence orientated along the AC-PC line (1mm3,TE/TR = 8.3/3.8ms,flip angle = 8°,SENSE = 2,160 slices,256 × 256 matrix).
Imaging data analysis
Voxel-based morphometry (VBM) to assess GMV, WMV and CSF volume, and surface-based analysis (SBA) to assess CT were conducted. Preprocessing for both analyses was conducted in CAT12 (Computational Anatomy Toolbox) (version 12.6;http://www.neuro.uni-jena.de/cat/) within SPM12 (version 7771;http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) using MATLAB version 9.7 (R2019b,MathWorks) (See Supplementary Material).
A first level analysis was performed using a general linear model (GLM) implemented in SPM12. An independent t-test was performed to evaluate differences in regional GMV, WMV and CT between CD and HCs, CD with versus without abdominal pain, and CD with versus without EIM. A correlation analysis was performed between IBD fatigue scores, disease duration, HBI and regional GMV, WMV and CT. For VBM analysis, TIV, sex, and age were included as normalized covariates-of-no-interest in the GLM to remove the effects of brain size, sex and age from the data. For CT analysis, age and sex were included as nuisance variables.
Uncorrected analyses were performed at P < 0.001, cluster size k > 10, and family wise error (FWE) correction for multiple comparisons was performed with clusters considered significant at P < 0.05. Statistical inferences were deduced using nonparametric permutations (5,000) and a Threshold-Free Cluster Enhancement (TFCE) (Smith & Nichols, 2009) correction applied to t-statistic maps (https://www.fil.ion.ucl.ac.uk/spm/ext/#TFCE).
Non-imaging data analysis
Analysis was performed using SPSS Statistics version 27.0. Variables were tested for normality using a Shapiro-Wilk test. Normal data are expressed as mean ± standard error of mean (SEM) and non-parametric data as median (interquartile range, IQR). Correlation between variables were evaluated using a Spearman for non-parametric data and Pearson’s correlation for parametric data.
Results
Participant characteristics
47 CD participants, 34(72%) with active disease and 13(28%) in remission, and 42 HCs were studied (See Table 2 and S3). A consort diagram of recruitment is shown in Fig. 1. Time between the evaluation of active CD and study visit was 2 (1–8) months. Across all CD participants, age was 31.0(18–68)years (median,range) with a disease duration of 7.5(1–40)years, and C-reactive protein (CRP) of 5(5-224)mg/dl, faecal calprotectin (FCP) 434(18-1800)µg/g, Harvey-Bradshaw Index (HBI) 3(0–9), IBD fatigue score 12.0 (0–15), abdominal pain score 2.0(0–50), TNFα 3.96(0-1234)pg/ml, IL-6 34.8(0-259)pg/ml and IL-1β 1.25(0-1955)pg/ml. HCs were age-matched (30.5(19–65) years). As expected, age and disease duration were significantly positively correlated in CD participants (P = 0.022) and IBDF, abdominal pain, and HBI were significantly intercorrelated (P < 0.001). There was no significant difference in circulating cytokines IL-6, IL-1β, or TNF-α serum levels between CD and HC groups. Twelve CD participants had EIMs. No participants had high or severe depression scores, or significant history of psychiatric disorders.
Table 2.
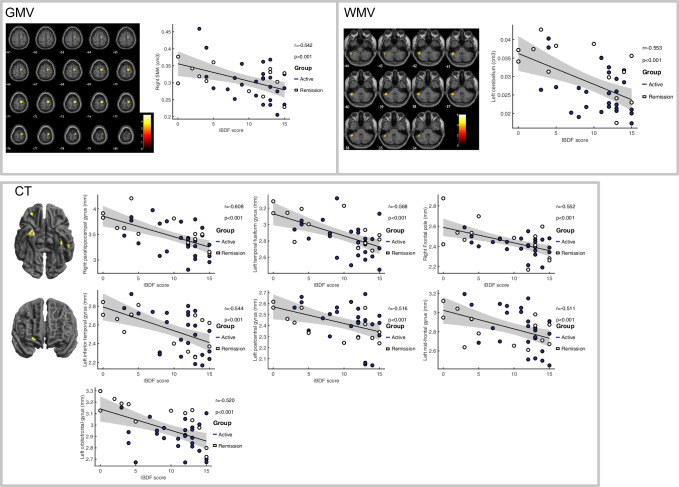
Anatomical regions showing an association between GMV and CT with IBD fatigue
| Measure | MNI regions | MNI coordinates | Uncorrected p (peak level) | T value (peak level) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| GMV | Right SMA | 10 | -12 | 66 | < 0.0001 | 4.1 |
| WMV | Left cerebellum | -52 | -42 | -39 | < 0.0001 | 6.01 |
| CT | Right para-hippocampal gyrus | 27 | -1 | -37 | < 0.0001 | 4.54 |
| Left temporal fusiform gyrus | -38 | -21 | -25 | < 0.0001 | 4.33 | |
| Right frontal pole | 23 | 53 | -5 | < 0.0001 | 4.08 | |
| Left inferior temporal gyrus | -53 | -27 | -32 | < 0.0001 | 3.81 | |
| Left postcentral gyrus | -12 | -38 | 54 | < 0.0001 | 3.58 | |
| Left midfrontal gyrus | -42 | 22 | 39 | < 0.0001 | 3.5 | |
| Right frontal pole | 30 | 43 | -13 | < 0.0001 | 3.58 | |
| Left orbitofrontal cortex | -30 | 30 | 1 | < 0.0001 | 3.63 | |
| Left temporal fusiform gyrus | -35 | -5 | -44 | < 0.0001 | 3.38 | |
Fig. 1.
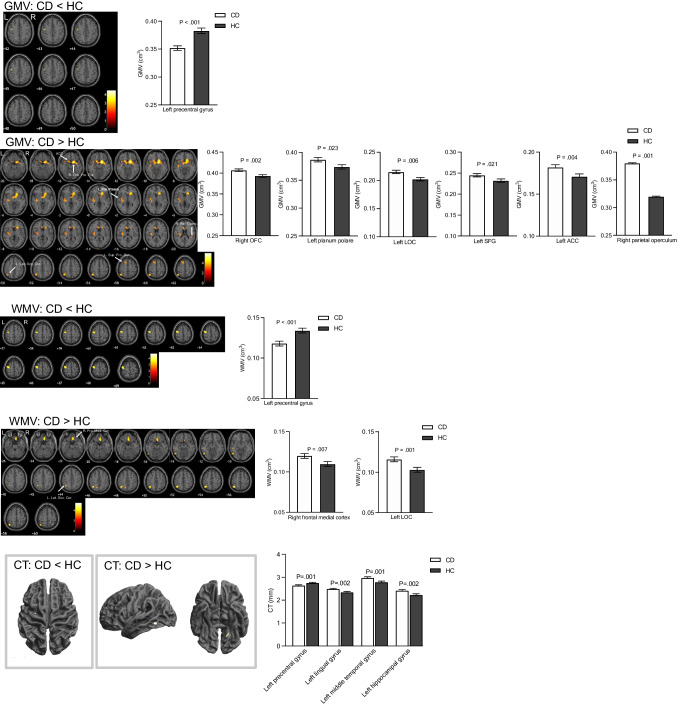
Altered grey matter volume (GMV), white matter volume (WMV) and cortical thickness (CT) in CD compared to HCs. GMV and WMV data assessed using age, TIV and sex, CT data assessed using age and sex as covariates of no interest. All data displayed at P < 0.001, uncorrected on a T1-weighted normalized anatomical image. OFC = orbital frontal cortex, LOC = Lateral occipital cortex, SFG = Superior frontal gyrus, ACC = anterior cingulate cortex. Note: left superior frontal gyrus, left planum polare, left lateral occipital, and right orbital frontal gyrus survived TFCE FWE corrections (P < 0.05) – see Table 1
Altered global structural morphometry in CD compared with HC
No significant difference between CD participants and HCs was found in absolute total intracranial volume (TIV), GMV or WMV, or GMV and WMV when adjusted for TIV alone or TIV, age and sex. A significant reduction in CSF volume was evident in CD compared to HCs (CD:231 ± 4.9 ml (mean ± SEM), HC:258 ± 5.3ml, P < 0.001) which persisted after adjusting for TIV, age and sex. No significant difference was found in global CT or age and sex adjusted CT between CD participants and HCs (Supplementary Table S4). Absolute global CT negatively correlated with abdominal pain (Spearman rho=-0.35,p = 0.013) and IBDF (Spearman rho=-0.34,p = 0.034). After controlling for age and sex, correlation between abdominal pain and CT remained significant (P = 0.025), correlation between IBDF and CT was not (P = 0.067). GMV, WMV, and CSF were not associated with fatigue or abdominal pain.
Altered regional structural morphometry in CD compared with HC
A significant reduction in GMV, WMV and CT was evident in left precentral gyrus in CD compared to HCs. Conversely, CD participants had significantly greater GMV in left lateral occipital cortex (LOC), left superior frontal gyrus (SFG), left planum polare, right orbital frontal cortex (OFC), left ACC and left parietal operculum cortex, as well as greater WMV in right frontal medial cortex and greater CT in the left middle temporal gyrus, left lingual gyrus and left hippocampus in CD compared to HCs. No significant differences were found in any GMV, WMV regions of interest (ROI) between active and remission CD groups before or after adjusting for age, TIV and sex, similarly no significant differences were found in CT (ROI) between active and remission CD groups. Figure 1 shows brain regions with significantly altered regional GMV and WMV, and CT in CD (n = 47) compared to HCs (n = 42), Table 1.
Table 1.
Regions showing GMV, WMV and CT alterations in CD participants compared to HCs
| Metric | MNI regions | Hemisphere | MNI coordinates | T value (peak level) | Uncorrected p (peak level) | Uncorrected cluster size | TFCE FWE corrected P | TFE Cluster size | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||||
| CD < HC | GMV | precentral gyrus | L | -46 | -9 | 44 | 3.46 | < 0.001 | 56 | ||
| WMV | precentral gyrus | L | -48 | -10 | 68 | 4.73 | < 0.001 | 797 | |||
| CT | precentral gyrus | L | -28 | -10 | 48 | 3.25 | 0.001 | 34 | |||
| CD > HC | GMV | superior frontal gyrus | L | -14 | 14 | 60 | 4.15 | < 0.001 | 799 | 0.035 | 1012 |
| planum polare | L | -42 | -36 | 6 | 4.03 | < 0.001 | 2237 | 0.048 | 10 | ||
| lateral occipital | L | -44 | -62 | 62 | 4.6 | < 0.001 | 829 | 0.04 | 196 | ||
| orbital frontal cortex | R | 14 | 4 | -15 | 5.96 | < 0.001 | 5782 | < 0.001 | 34,316 | ||
| anterior cingulate | L | -10 | 26 | 12 | 4.13 | < 0.001 | 444 | ||||
| parietal operculum | R | 45 | -36 | 21 | 3.61 | <0.001 | 129 | ||||
| WMV | lateral occipital | L | -40 | -63 | 62 | 5.41 | < 0.0001 | 734 | |||
| frontal medial | R | 3 | 36 | -22 | 3.92 | < 0.0001 | 1069 | ||||
| lateral occipital | L | -60 | -69 | -14 | 4.18 | < 0.0001 | 272 | ||||
| CT | mid temporal gyrus | L | -57 | -55 | -10 | 3.5 | < 0.0001 | 75 | |||
| lingual gyrus | L | -19 | -59 | -7 | 3.37 | 0.001 | 102 | ||||
| hippocampus | L | -13 | -39 | -5 | 3.26 | 0.001 | 32 | ||||
Negative association of fatigue with regional brain volume loss and cortical thinning
Higher fatigue scores were associated with a reduction in GMV in right supplementary motor area (SMA) as well as in WMV in left cerebellum. Higher fatigue scores were associated with cortical thinning in multiple regions (right para-hippocampal gyrus, frontal pole, left temporal fusiform gyrus, OFC, inferior temporal gyrus, post central gyrus and MFG). Figure 2 shows brain regions with significantly reduced GMV, WMV and CT with greater fatigue in CD (n = 39), Table 2.
Fig. 2.
Areas of a GMV loss, b WMV loss and c cortical thinning correlated with increased IBD fatigue score in CD participants displayed on a T1-weighted normalized anatomical image (P < 0.001, uncorrected), no regions survived TFCE FWE corrections (P < 0.05). GMV and WMV data assessed using age, TIV and sex, CT data assessed using age and sex as covariates of no interest. SMA = supplementary motor area
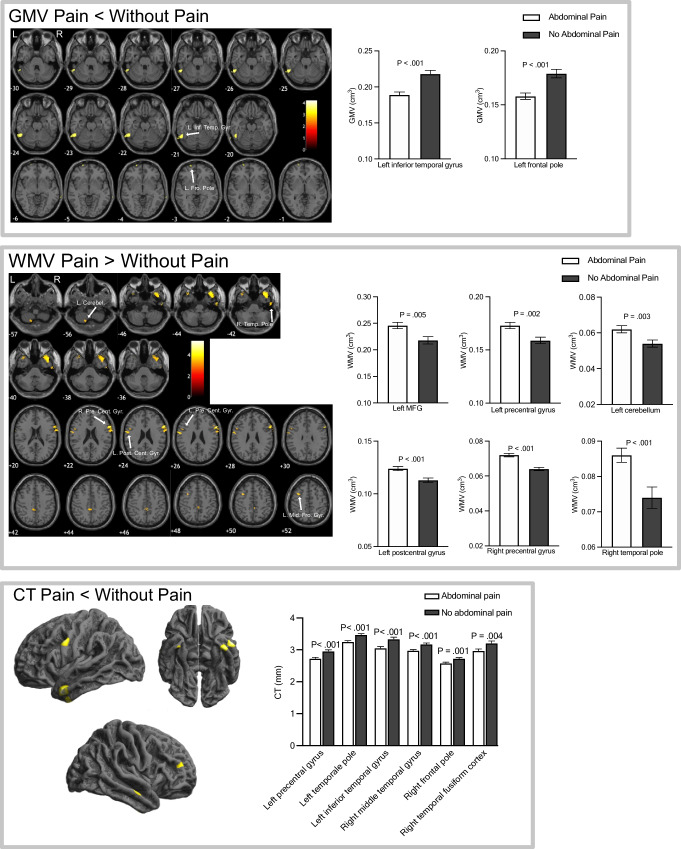
Comparison of regional GMV, WMV, and CT between CD participants with and without abdominal pain
CD participants with abdominal pain showed a reduction in GMV in left inferior temporal gyrus and frontal pole, as well as cortical thinning in the left precentral gyrus, left temporal pole, left inferior temporal gyrus, right middle temporal gyrus, right frontal pole and right temporal fusiform cortex compared with CD participants without abdominal pain. Conversely, CD participants with abdominal pain showed greater WMV in right temporal pole, right precentral gyrus, left postcentral gyrus, left MFG, left cerebellum and left precentral gyrus compared with CD participants without abdominal pain. There was a significant group effect between pain vs. no pain groups across all GMV, WMV and CT ROIs. Additionally, a group effect between active vs. remission CD groups in the WMV left postcentral gyrus, WMV left precentral gyrus and CT right MFG was present. There were no significant interaction effects between the (CD abdominal pain vs. CD no abdominal pain) (active vs. remission) CD groups, indicating differences observed between the CD abdominal pain group were not influenced by disease status. Figure 3 shows areas with significant alterations in GMV, WMV, and CT in CD participants with (n = 27) compared to without abdominal pain (n = 20), Table 3.
Fig. 3.
Areas with GMV, WMV and CT alterations in CD participants with abdominal pain compared with CD without abdominal pain. GMV and WMV data assessed using age, TIV and sex, CT data assessed using age and sex as covariates of no interest. Maps displayed on a T1-weighted normalized anatomical image at uncorrected P < 0.001, no regions survived TFCE FWE corrections (P < 0.05)
Table 3.
Anatomical regions showing GMV, WMV and CT alterations in CD participants with abdominal pain compared with CD without abdominal pain. MFG = middle frontal gyrus
| Measure | Hemisphere | MNI regions | MNI coordinates | Uncorrected p (peak level) | T value (peak level) | Cluster size | |||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Abdominal pain < No abdominal pain | GMV | L | inferior temporal gyrus | -63 | -54 | -30 | < 0.0001 | 4.18 | 385 |
| L | frontal pole | -21 | 74 | -9 | < 0.0001 | 4.13 | 36 | ||
| CT | L | precentral gyrus | -56 | 2 | 36 | < 0.0001 | 4.1 | 433 | |
| L | temporal pole | -53 | 5 | -31 | < 0.0001 | 4.68 | 358 | ||
| L | inferior temporal gyrus | -42 | -3 | -44 | < 0.0001 | 4.07 | 317 | ||
| R | mid temporal gyrus | 59 | -12 | -18 | < 0.0001 | 4 | 285 | ||
| R | frontal pole | 40 | 47 | 19 | < 0.0001 | 3.57 | 109 | ||
| R | temporal fusiform cortex | 34 | -4 | -44 | 0.001 | 3.44 | 62 | ||
| Abdominal pain > No abdominal pain | WMV | R | temporal pole | 40 | 21 | -48 | < 0.0001 | 5.21 | 1037 |
| R | precentral gyrus | 60 | 16 | 28 | < 0.0001 | 5.31 | 489 | ||
| L | postcentral gyrus | -64 | -2 | 27 | < 0.0001 | 4.84 | 145 | ||
| L | midfrontal gyrus | -38 | 8 | 51 | < 0.0001 | 3.79 | 142 | ||
| L | cerebellum | -9 | -72 | -62 | < 0.0001 | 3.99 | 126 | ||
| L | precentral gyrus | -52 | 9 | 26 | < 0.0001 | 3.63 | 57 | ||
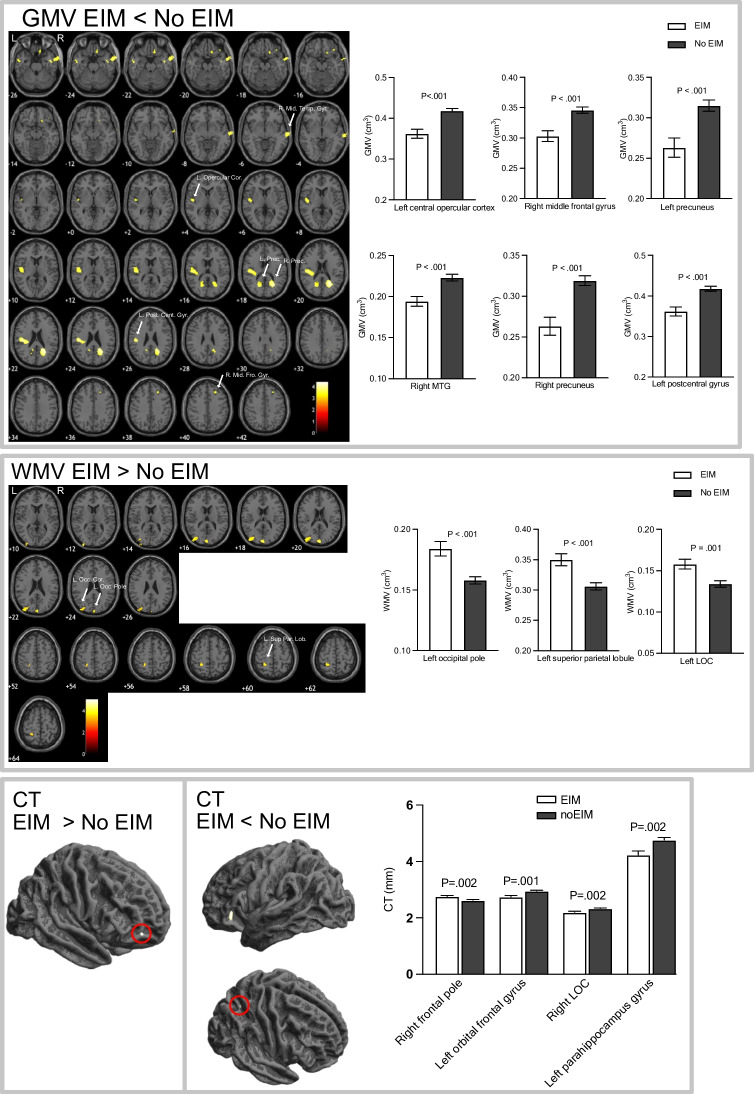
Comparison of regional GMV and CT between CD with and without EIM
CD participants with EIMs had lower GMV in the left postcentral gyrus, left central opercular cortex, bilateral precuneus, right MFG, right middle temporal gyrus, as well as cortical thinning in the left OFC, right LOC and left para-hippocampal gyrus compared to CD participants without EIMs. Conversely, CD participants with EIM had greater WMV in the left LOC, left superior parietal lobule, left occipital pole as well as greater CT in the right frontal pole compared with CD participants without EIM. Figure 4 shows those areas with significant alterations in GMV, WMV and CT in CD participants without EIM (n = 35) compared with CD participants with EIM (n = 12), Table 4.
Fig. 4.
Regions showing alternations in GMV, WMV and CT in CD participants with EIM compared with CD participants without EIM. GMV and WMV data assessed using age, TIV and sex, CT data assessed using age and sex as covariates of no interest. Maps displayed on a T1- weighted normalized anatomical image at uncorrected P < 0.001, no regions survived TFCE FWE corrections (P < 0.05). MTG = middle temporal gyrus, LOC = lateral occipital cortex, MFG = midfrontal gyrus
Table 4.
Regions showing alterations in GMV, WMV and CT in CD participants with EIM compared to those without EIM
| Measure | Hemisphere | MNI regions | MNI coordinates | T value (peak level) | Uncorrected p (peak level) | Cluster uncorrected size | TFCE FWE corrected P | Cluster size TFCE corrected | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||||
| EIM < no EIM | GMV | L | postcentral gyrus | -48 | -22 | 26 | 4.23 | < 0.001 | 1602 | 0.04 | 447 |
| L | central opercular | -48 | -10 | 8 | 3.83 | < 0.001 | 47 | 0.049 | 47 | ||
| R | precuneus | 27 | -60 | 21 | 4.36 | < 0.001 | 1113 | ||||
| L | precuneus | -20 | -63 | 18 | 3.83 | < 0.001 | 333 | ||||
| R | midfrontal gyrus | 30 | 26 | 50 | 3.91 | < 0.001 | 292 | ||||
| R | middle temporal gyrus | 78 | -15 | -6 | 3.74 | < 0.001 | 274 | ||||
| CT | L | orbital frontal cortex | -34 | 29 | -3 | 3.59 | < 0.001 | 134 | |||
| R | lateral occipital cortex | 25 | -59 | 49 | 3.61 | < 0.001 | 32 | ||||
| L | Para-hippocampal gyrus | -25 | -8 | -30 | 3.35 | < 0.001 | 15 | ||||
| EIM > no EIM | WMV | L | lateral occipital cortex | -45 | -88 | 20 | 4.39 | < 0.001 | 542 | ||
| L | superior parietal lobule | -26 | -44 | 60 | 3.96 | < 0.001 | 249 | ||||
| L | occipital pole | 0 | -93 | 21 | 4.6 | < 0.001 | 245 | ||||
| CT | R | frontal pole | 40 | 53 | -4 | 3.41 | < 0.001 | 21 | |||
Discussion
Brain CSF volume was significantly reduced in CD compared with HCs. CSF flow to the brain is essential for protein clearance to prevent accumulation of toxic protein aggregates (Puy et al., 2016). Impaired CSF flow is suggested to result in cognitive deficits in the elderly (Attier-Zmudka et al., 2019). In other systemic inflammatory diseases such as rheumatoid arthritis, pro-inflammatory cytokines in CSF have positively correlated with fatigue (Lampa et al., 2012), and TNF blockade shown to reduce CSF protein levels (Estelius et al., 2019). In chronic fatigue syndrome (CFS), higher fatigue scores associate with reduced CSF volume (Finkelmeyer et al., 2018). The reduced CSF in CD participants may be attributed to systemic inflammation leading to fatigue symptoms. However, we did not show a significant correlation between reduced CSF volume and fatigue scores, or any significant differences in serum cytokine levels between CD and HCs, although prior studies suggest cytokines increase in CSF during systemic inflammation (Engler et al., 2017; Herrick & Tansey, 2021).
Assessment of regional brain volumes showed reduced GMV, WMV and CT in CD participants compared with HCs in left precentral gyrus, the primary motor cortex implicated in motor function, which is in line with previously reported studies in CD (Zikou et al., 2014; Bao et al., 2015). Reduced WMV in the precentral gyrus has been reported in patients with CFS (Finkelmeyer et al., 2018). Atrophy of the left precentral gyrus, evidenced by reduced GMV, WMV and cortical thinning, may be related to fatigue symptoms observed in CD patients. CD patients also showed significantly greater GMV relative to HCs in left SFG, a region implicated in working memory, with alterations likely resulting in cognitive deficits (du Boisgueheneuc et al., 2006). We show increased GMV in the left planum temporale within the superior temporal gyrus (STG), an area implicated in language function (Shapleske et al., 1999) and left ACC in CD compared with HCs. Structural, functional and metabolic alterations in ACC have been reported in CD, and attributed to stress, pain, negative emotions and changes in gut microbiota (Bao et al., 2017; Liu et al., 2018; Lv et al., 2018; Kong et al., 2021, 2022; Li et al., 2021). Further, we show increased GMV in the right parietal operculum, a region implicated in pain (Horing et al., 2019), as well as cortical thickening in the left middle temporal gyrus in CD participants relative to HCs, which is in agreement with Nair et al. (Nair et al., 2016).
Significant alterations were also seen in the sensorimotor network where greater fatigue scores correlated with reduced GMV in right SMA. GM atrophy of SMA may be linked with symptoms of fatigue due to an attenuation of a central drive to peripheral neuromuscular activity. Repetitive transcranial magnetic stimulation of SMA has been shown to increase the recovery rate from central fatigue (Sharples et al., 2016). We show that increased fatigue was also associated with decreased CT in left postcentral gyrus, a somatosensory region, as well as reduced WMV in left cerebellum, a region involved in sensory-motor processing, cognitive and emotional functioning (Schmahmann, 2019), also implicated in chronic fatigue syndrome (CFS) (Barnden et al., 2011). Further, we show a negative correlation between fatigue and CT in right para-hippocampal gyrus, a region showing reduced functional connectivity with greater fatigue scores in CFS patients (Boissoneault et al., 2016). Increase in fatigue also associated with reduced CT in left temporal fusiform gyrus and left inferior temporal gyrus, right frontal pole (anterior part of prefrontal cortex), left MFG and left orbitofrontal cortex (OFC). In patients with CFS, frontal regions are related to attentional resources (i.e. exertion of extra mental effort to improve task performance) (Mizuno et al., 2015). Atrophy of frontal regions associated with increased fatigue may result in attentional deficits and cognitive fatigue leading to enhanced perception of fatigue in CD.
A decrease in global CT was associated with an increase in abdominal pain. Further, CD with abdominal pain had reduced regional GMV in left inferior temporal gyrus and left frontal pole compared to CD without abdominal pain. Cortical thinning was found in CD with abdominal pain compared with those without in temporal regions, left precentral gyrus and the right frontal pole, in contrast to regions previously reported by Bao et al. (Bao et al., 2017). Notably, GMV alterations implicated in pain processing are not solely limited to regions of the pain matrix, (Smallwood et al., 2013; Torta et al., 2014)., with controversy regarding the direction of change (increase or decrease) (Smallwood et al., 2013; Torta et al., 2014). Brain structural alterations associated with pain maybe linked to an imbalance in neurotransmitters (Lv et al., 2018), ongoing nociceptive inputs, heightened attention to nociceptive and unpleasant sensory stimuli leading to use-dependent plasticity effects (May, 2008; Pomares et al., 2017).
The presence of EIM in CD is an indicator of greater inflammatory burden and systemic disease. CD participants with EIMs had reduced GMV in sensorimotor regions of left postcentral gyrus, left central operculum and bilateral precuneus, right middle frontal and right middle temporal gyrus, as well as cortical thinning in the left orbital frontal gyrus, right LOC compared with CD without EIM. We also show greater WMV and CT in the left occipital regions and right frontal pole respectively. Our findings are in contrast to a previous study examining brain structure in relation to EIMs in CD, where no difference in CT was reported (Thomann et al., 2016). EIM-associated brain structural alterations are possibly linked to a chronic inflammatory response and disease burden.
This study has some limitations. There is a variation in disease duration and severity of inflammation and medication use across the CD group. The cross-sectional nature of this study means chronic symptoms are only assessed at a single time point, longitudinal studies are warranted to assess the time course of brain structural changes in CD. Our structural differences may represent neural correlates of different disease courses (e.g. mild vs. complicated), however we were underpowered to study brain structural differences based on disease course.
Conclusion
This is the largest study to date in patients with active CD. We show a significant reduction in global CSF volume, and regional GMV, WMV and CT in the motor cortex, and an increase in GMV in frontal brain regions in CD compared with HCs. Alterations in brain structure in multiple regions in CD associated with fatigue, abdominal pain and EIMs, may reflect neuroplasticity effects to a chronic systemic inflammatory response and chronic symptom stimuli, explaining the persistence of fatigue symptoms in CD patients in remission.
Supplementary Information
Below is the link to the electronic supplementary material.
(DOCX 139 KB)
Acknowledgements
We would like to thank staff at the NIHR Nottingham Biomedical Research Centre and MRI operators at the Sir Peter Mansfield Imaging Centre for their time and support.
Author contribution
GT collected the data, carried out the initial analyses drafted the initial manuscript, and reviewed and revised the manuscript. SE supervised data collection, and reviewed and revised the manuscript, and critically reviewed the manuscript for important intellectual content. MA carried out further analyses and reviewed and revised the manuscript, and critically reviewed the manuscript for important intellectual content. JM collected CD in remission data. SR recruited patients, coordinated and collected data. STF and GWM conceptualized and designed the study, coordinated and supervised data collection, and reviewed and revised the manuscript, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
Crohn’s & Colitis Foundation of America (336416), Medical Research Council (MRC), Doctoral Training Programme (DTP), 2015, Crohn’s and Colitis UK Medical Research Award (M2017/6), Joan Browne Legacy PhD scholarship.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
This study received research ethics committee approval from National Research Ethics Service [NRES] Committee East Midlands [REC reference 14/EM/0192 as of 10/07/2015].
Consent to participate
Informed consent was obtained from all individual participants included in this study.
Consent to publish
All authors have approved the manuscript and agree with its publication.
Competing interests
Authors of this paper have no conflict of interests to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gita Thapaliya, Email: gthapal2@jhmi.edu.
Gordon William Moran, Email: gordon.moran@nottingham.ac.uk.
References
- Agostini A, et al. New insights into the brain involvement in patients with Crohn’s disease: a voxel-based morphometry study. Neurogastroenterology & Motility. 2013;25(2):147–153. doi: 10.1111/nmo.12017. [DOI] [PubMed] [Google Scholar]
- Agostini, A., et al. (2017). Stress and brain functional changes in patients with Crohn’s disease: A functional magnetic resonance imaging study. Neurogastroenterology and Motility, 1–10. 10.1111/nmo.13108 [DOI] [PubMed]
- Attier-Zmudka, J., et al. (2019). Decreased cerebrospinal fluid flow is associated with cognitive deficit in elderly patients. Frontiers in Aging Neuroscience, 11(APR), 1–8. 10.3389/fnagi.2019.00087 [DOI] [PMC free article] [PubMed]
- Bao C, et al. Differences in brain gray matter volume in patients with Crohn’s disease with and without abdominal pain. Oncotarget. 2017;8(55):93624–93632. doi: 10.18632/oncotarget.21161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao CH, et al. Alterations in brain gray matter structures in patients with Crohn’s disease and their correlation with psychological distress. Journal of Crohn’s & Colitis. 2015;9(7):532–540. doi: 10.1093/ecco-jcc/jjv057. [DOI] [PubMed] [Google Scholar]
- Barnden LR, et al. A brain MRI study of chronic fatigue syndrome: Evidence of brainstem dysfunction and altered homeostasis. NMR in Biomedicine. 2011;24(10):1302–1312. doi: 10.1002/nbm.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissoneault J, Letzen J, Lai Song SR. Abnormal resting state functional connectivity in patients with chronic fatigue syndrome: An arterial spin-labeling fMRI study. Magnetic Resonance Imaging. 2016;34(4):603–608. doi: 10.1016/j.mri.2015.12.008.Abnormal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borren NZ, van der Woude CJ, Ananthakrishnan AN. Fatigue in IBD: epidemiology, pathophysiology and management. Nature Reviews Gastroenterology and Hepatology Springer US. 2019;16(4):247–259. doi: 10.1038/s41575-018-0091-9. [DOI] [PubMed] [Google Scholar]
- Dolapcioglu C, Dolapcioglu H. Structural brain lesions in inflammatory bowel disease. World Journal of Gastrointestinal Pathophysiology. 2015;6(4):124–130. doi: 10.4291/wjgp.v6.i4.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Boisgueheneuc F, et al. Functions of the left superior frontal gyrus in humans: a lesion study. Brain: a Journal of Neurology England. 2006;129(Pt 12):3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- Engler H, et al. Selective increase of cerebrospinal fluid IL-6 during experimental systemic inflammation in humans: association with depressive symptoms. Molecular Psychiatry. 2017;22(10):1448–1454. doi: 10.1038/mp.2016.264. [DOI] [PubMed] [Google Scholar]
- Estelius J, et al. Mass spectrometry-based analysis of cerebrospinal fluid from arthritis patients - Immune-related candidate proteins affected by TNF blocking treatment. Arthritis Research & Therapy. 2019;21(1):1–11. doi: 10.1186/s13075-019-1846-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelmeyer, A., et al. (2018). Grey and white matter differences in Chronic Fatigue Syndrome – A voxel-based morphometry study’, NeuroImage: Clinical. Elsevier, 17(September 2017), pp.24–30. 10.1016/j.nicl.2017.09.024. [DOI] [PMC free article] [PubMed]
- Giulio C, Di, Daniele F, Tipton CM. Angelo Mosso and muscular fatigue: 116 years after the first congress of physiologists: IUPS commemoration. Advances in Physiology Education. 2006;30(2):51–57. doi: 10.1152/advan.00041.2005. [DOI] [PubMed] [Google Scholar]
- Harvey B. Index of Crohn’s disease activity. The Lancet. 1980;315(8170):711. doi: 10.1016/S0140-6736(80)92858-5. [DOI] [PubMed] [Google Scholar]
- Herrick, M. K., & Tansey, M. G. (2021). Is LRRK2 the missing link between inflammatory bowel disease and Parkinson’s disease? NPJ Parkinson’s Disease, Springer US, 7(1). 10.1038/s41531-021-00170-1. [DOI] [PMC free article] [PubMed]
- Horing B, Sprenger C, Büchel C. The parietal operculum preferentially encodes heat pain and not salience. PLoS Biology. 2019;17(8):e3000205. doi: 10.1371/journal.pbio.3000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, et al. Alterations in brain white matter microstructural properties in patients with Crohn’s disease in remission. Scientific Reports Springer US. 2020;10(1):1–9. doi: 10.1038/s41598-020-59098-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CS, et al. Abdominal pain, the adolescent and altered brain structure and function. PLoS One. 2016;11(5):1–30. doi: 10.1371/journal.pone.0156545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MP, et al. Brain-gut connections in functional GI disorders: Anatomic and physiologic relationships. Neurogastroenterology and Motility. 2006;18(2):91–103. doi: 10.1111/j.1365-2982.2005.00730.x. [DOI] [PubMed] [Google Scholar]
- Kong, N., et al. (2021). Changes in the anterior cingulate cortex in Crohn’s disease: A neuroimaging perspective. Brain and Behavior, 11(3), e02003. 10.1002/brb3.2003 [DOI] [PMC free article] [PubMed]
- Kong N, et al. Neurophysiological effects of the anterior cingulate cortex on the exacerbation of Crohn’s disease: A combined fMRI-MRS study. Frontiers in Neuroscience. 2022;16:840149. doi: 10.3389/fnins.2022.840149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb CA, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampa J, et al. Peripheral inflammatory disease associated with centrally activated IL-1 system in humans and mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(31):12728–12733. doi: 10.1073/pnas.1118748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. Brain functional changes in patients with Crohn’s disease: A resting-state fMRI study. Brain and Behavior. 2021;11(8):1–10. doi: 10.1002/brb3.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, et al. Altered topological patterns of brain functional networks in Crohn’s disease. Brain Imaging and Behavior, Springer US. 2018;12(5):1466–1478. doi: 10.1007/s11682-017-9814-8. [DOI] [PubMed] [Google Scholar]
- Lv K, et al. Neurotransmitter alterations in the anterior cingulate cortex in Crohn’s disease patients with abdominal pain: A preliminary MR spectroscopy study. NeuroImage: Clinical. 2018;20(September):793–799. doi: 10.1016/j.nicl.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137(1):7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Mizuno K, et al. Less efficient and costly processes of frontal cortex in childhood chronic fatigue syndrome. NeuroImage: Clinical the Authors. 2015;9:355–368. doi: 10.1016/j.nicl.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosli, M. H., et al. (2015). C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: A systematic review and meta-analysis. The American Journal of Gastroenterology, United States, 110(6), 802–819; quiz 820. 10.1038/ajg.2015.120 [DOI] [PubMed]
- Nair, V. A., et al. (2016). Structural imaging changes and behavioral correlates in patients with Crohn’s disease in remission. Frontiers in Human Neuroscience, 10(SEP2016), 1–11. 10.3389/fnhum.2016.00460 [DOI] [PMC free article] [PubMed]
- Pariente B, et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflammatory Bowel Diseases. 2011;17(6):1415–1422. doi: 10.1002/ibd.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin-Biroulet L, et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut. 2012;61(2):241–247. doi: 10.1136/gutjnl-2011-300049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomares FB, et al. Histological underpinnings of grey matter changes in fibromyalgia investigated using multimodal brain imaging. The Journal of Neuroscience. 2017;37(5):1090–1101. doi: 10.1523/jneurosci.2619-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puy V, et al. Interactions between flow oscillations and biochemical parameters in the cerebrospinal fluid. Frontiers in Aging Neuroscience. 2016;8:154. doi: 10.3389/fnagi.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnakumaran R, et al. Fatigue in inflammatory bowel disease reflects mood and symptom-reporting behavior rather than biochemical activity or anemia. Clinical Gastroenterology and Hepatology, AGA Institute. 2018;16(7):1165–1167. doi: 10.1016/j.cgh.2017.11.030. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The cerebellum and cognition. Neuroscience Letters. 2019;688(July):62–75. doi: 10.1016/j.neulet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Shapleske J, et al. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Research Brain Research Reviews Netherlands. 1999;29(1):26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Sharples SA, et al. Cortical mechanisms of central fatigue and sense of effort. PLoS One. 2016;11(2):1–21. doi: 10.1371/journal.pone.0149026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S., et al. (2011). Common symptoms and stressors among individuals with inflammatory bowel diseases. Clinical Gastroenterology and Hepatology (9 vol., pp. 769–775). Elsevier Inc. 9. 10.1016/j.cgh.2011.05.016. [DOI] [PubMed]
- Smallwood RF, et al. Structural brain anomalies and chronic pain: A quantitative meta-analysis of gray matter volume. Journal of Pain Elsevier Ltd. 2013;14(7):663–675. doi: 10.1016/j.jpain.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M., & Nichols, T. E. (2009). Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed]
- Thomann AK, et al. Altered markers of brain development in Crohn’s disease with extraintestinal manifestations - A pilot study. PLoS One. 2016;11(9):1–14. doi: 10.1371/journal.pone.0163202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomann, A. K., et al. (2020). Exploring joint patterns of brain structure and function in inflammatory bowel diseases using multimodal data fusion. Neurogastroenterology and Motility, 1–10. 10.1111/nmo.14078 [DOI] [PubMed]
- Torta, D. M. E., et al. (2014). Gray matter alterations in chronic pain: A network-oriented meta-analytic approach’. NeuroImage: Clinical (4 vol., pp. 676–686). Elsevier Ltd. 10.1016/j.nicl.2014.04.007. [DOI] [PMC free article] [PubMed]
- van Erp S, et al. Cerebral magnetic resonance imaging in quiescent Crohn’s disease patients with fatigue. World Journal of Gastroenterology. 2017;23(6):1018–1029. doi: 10.3748/wjg.v23.i6.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, A. W. K. (2021). Structural and functional changes in the brain of patients with Crohn’s disease: an activation likelihood estimation meta-analysis. Brain Imaging and Behavior, 1–12. 10.1007/s11682-020-00291-w [DOI] [PubMed]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Traduction française: J.F. Lépine. Acta Psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Zikou AK, et al. Brain involvement in patients with inflammatory bowel disease: a voxel-based morphometry and diffusion tensor imaging study. European Radiology. 2014;24:2499–2506. doi: 10.1007/s00330-014-3242-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 139 KB)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.