Summary
Human and murine neutrophils differ with respect to representation in blood, receptors, nuclear morphology, signaling pathways, granule proteins, NADPH oxidase regulation, magnitude of oxidant and hypochlorous acid production, and their repertoire of secreted molecules. These differences often matter and can undermine extrapolations from murine studies to clinical care, as illustrated by several failed therapeutic interventions based on mouse models. Likewise, coevolution of host and pathogen undercuts fidelity of murine models of neutrophil-predominant human infections.
However, murine systems that accurately model the human condition can yield insights into human biology difficult to obtain otherwise. The challenge for investigators who employ murine systems is to distinguish models from pretenders and to know when the mouse provides biologically accurate insights. Testing with human neutrophils observations made in murine systems would provide a safeguard but is not always possible. At a minimum, studies that use exclusively murine neutrophils should have accurate titles supported by data and restrict conclusions to murine neutrophils and not encompass all neutrophils.
For now, the integration of evidence from studies of neutrophil biology performed using valid murine models coupled with testing in vitro of human neutrophils combines the best of both approaches to elucidate the mysteries of human neutrophil biology.
Keywords: human neutrophiis, murine neutrophils, murine models, human infection
Perspective and purpose
Our understanding of many of the biological processes related to human health and disease derives in large part from two investigative approaches, namely clinical observations of patients afflicted with disease, either acquired or inherited, and animal experimental systems intended to model human disorders. Astute clinicians in search of alleviating the suffering of their patients drive basic and translational studies to identify underlying physiology, both normal and aberrant. Particularly powerful because of extensive background genetic information and well-characterized inbred strains, murine experimental systems have served to model a wide spectrum of human diseases, including infections, autoimmune disorders, inflammatory syndromes, and malignancies. Investigators often use murine experimental systems to assess the role of neutrophils in a specific disease, syndrome, or malignancy, either as their exclusive experimental tool or as an adjunct to their studies of human neutrophils in vitro. Enthusiasm for the use of murine systems as surrogates for human neutrophils is occasionally excessive and can distort perspective. For example, in a detailed review of the approaches available to study the contribution of neutrophils in murine model systems 1, the authors enthusiastically note in their concluding comments that “the use of mouse models for the study of neutrophils in vivo represents a milestone in the understanding of the biology of these cells and has enabled the deep interrogation of a number of pathways leading to diverse pathologies” 1. As evidence of the success of this approach, the authors cite the use of DNase I as therapy for lung disease in cystic fibrosis “after the discovery that NETs [neutrophil extracellular traps] play a pivotal role in the disease”. In fact, the Food and Drug Administration (FDA) approved the use of recombinant human DNase I for patients with cystic fibrosis in 1993, more than a decade before the publication of the first report of NETs 2. Multiple studies of the role of the highly viscous, DNA-rich secretions created by the large number of spent and necrotic cells in the airways of patients with cystic fibrosis prompted the FDA’s decision.
There may be many effective ways to manipulate neutrophils in murine experimental systems, but the intention in utilizing such systems is to have a tractable model of neutrophil-dependent biology that both mirrors the appearance of the original (i.e. the human) and recapitulates the same underlying mechanism(s). That is, to be an accurate and informative model, the mouse neutrophil must reproduce the behavior, biology, and biochemistry of human neutrophils in the process under study. In that way, investigators can probe the model to identify key regulatory molecules, critical pathways, and events that fuel pathophysiology caused by human neutrophils. A murine system that phenotypically mirrors the human situation but not the underlying mechanism is not a bona fide model and may prompt misleading conclusions.
One might ask what adverse consequences could arise from the use of experimental systems anchored only on the assumption that the same mechanisms underly a given phenotype for both mouse and man. At least two come to mind. From the perspective of the pursuit of knowledge, yet undiscovered aspects of human biology not represented in mice might be missed when the observations lack data on mechanism. Furthermore, extrapolation of observations from murine studies might fuel clinical trials in humans that prove unsuccessful because of the rationale for a particular intervention is flawed 3-7. Hence, the incentive to know the fidelity of murine (or any animal) models should be great.
This review is neutrophil-centric, addressing only those settings where neutrophils represent critical contributors to the biological process under study. Consequently, my purposes are two-fold: first, to summarize the recognized differences in structure, composition, and biochemistry between murine and human neutrophils. The second goal is to present situations where such differences are such that the murine experimental system fails to represent human biology faithfully and is unlikely to yield sound and clinically relevant insights.
The structure, composition, and biology of human neutrophils
Current understanding of the functional repertoire of human neutrophils, albeit incomplete, includes a wide variety of activities that contribute to host defense against threats, both infectious and inanimate, and, when excessive, can culminate in tissue damage 8-11. Neutrophils are the predominant nucleated cell in human blood (50-70% of leukocytes) and serve as first responders to the invasion by microbes, the presence of foreign bodies, or the sequalae of tissue trauma.
In response to engagement of receptors on their surface, neutrophils adhere to activated endothelial cells, transmigrate into tissue, and crawl towards the origin of the stimulating agent. Neutrophils are phagocytic and respond to particulate stimuli, with or without opsonization with complement, antibody, or both, by ingesting the target or, when too large to eat, by adhering to its surface. Phagocytosis prompts a coordinated response whereby intracellular granules fuse with nascent phagosomes and cytosolic components of the NADPH oxidase assemble on the extraluminal face of the phagosomal membrane. With fusion, granules release their contents into the phagosome, delivering an array of enzymes, antimicrobial peptides, and other active biomolecules (Fig. 1). Activation of the NADPH oxidase promptly follows its assembly and transfers electrons from cytosolic NADPH to molecular oxygen in the phagosome, thereby generating first superoxide ion, which is rapidly converted to hydrogen peroxide that in turn interacts with the azurophilic granule protein myeloperoxidase (MPO) to produce the potently bactericidal agent, hypochlorous acid (HOCl) 12-16. HOCl reacts with the luminal contents of the phagosome, including both the ingested target (e.g. a microbe) and the granule proteins, thereby generating a host of modified proteins that are themselves microbicidal (Fig. 2) 17-19. The synergistic activities among the individual granule proteins and between granule proteins and oxidants create an environment highly toxic to most microbes 20-22.
Figure 1. Microbicidal action in human neutrophil phagosomes.
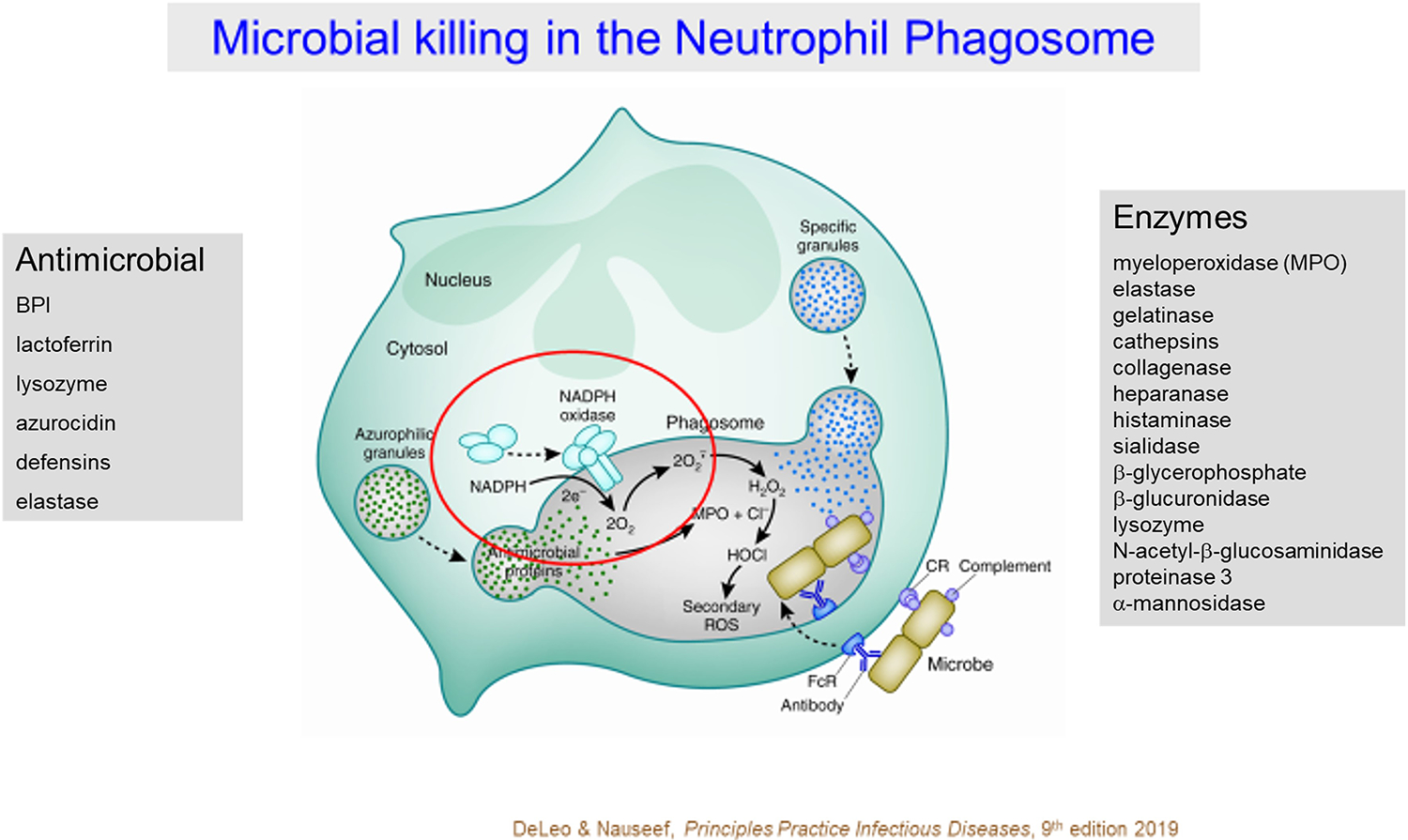
Antimicrobial events in the phagosomes of human neutrophils reflect the synergistic actions of granule proteins and oxidants. Granule proteins synthesized during granulocyte development in the bone marrow are packaged into one of several granule compartments (e.g. azurophilic and specific granules) and released into phagosomes by fusion with the nascent phagosome. Granule proteins include enzymes and proteins with direct bactericidal activity. Coincident with phagocytosis, the multicomponent NADPH oxidase assembles on the nascent phagosome and shuttles electrons from cytoplasmic NADPH to molecular O2 in the lumen of the phagosome, thereby generating superoxide anion (). Dismutation of produces H2O2 that in turn reacts with MPO to form HOCl. Most of the oxygen consumed by stimulated human neutrophils can be recovered as HOCl. Interactions among the granule proteins and between granule proteins and oxidants creates an environment toxic, and often lethal, for a wide variety of microbes [Figure from 8].
Figure 2. MPO-related events in phagosomes of human neutrophils.
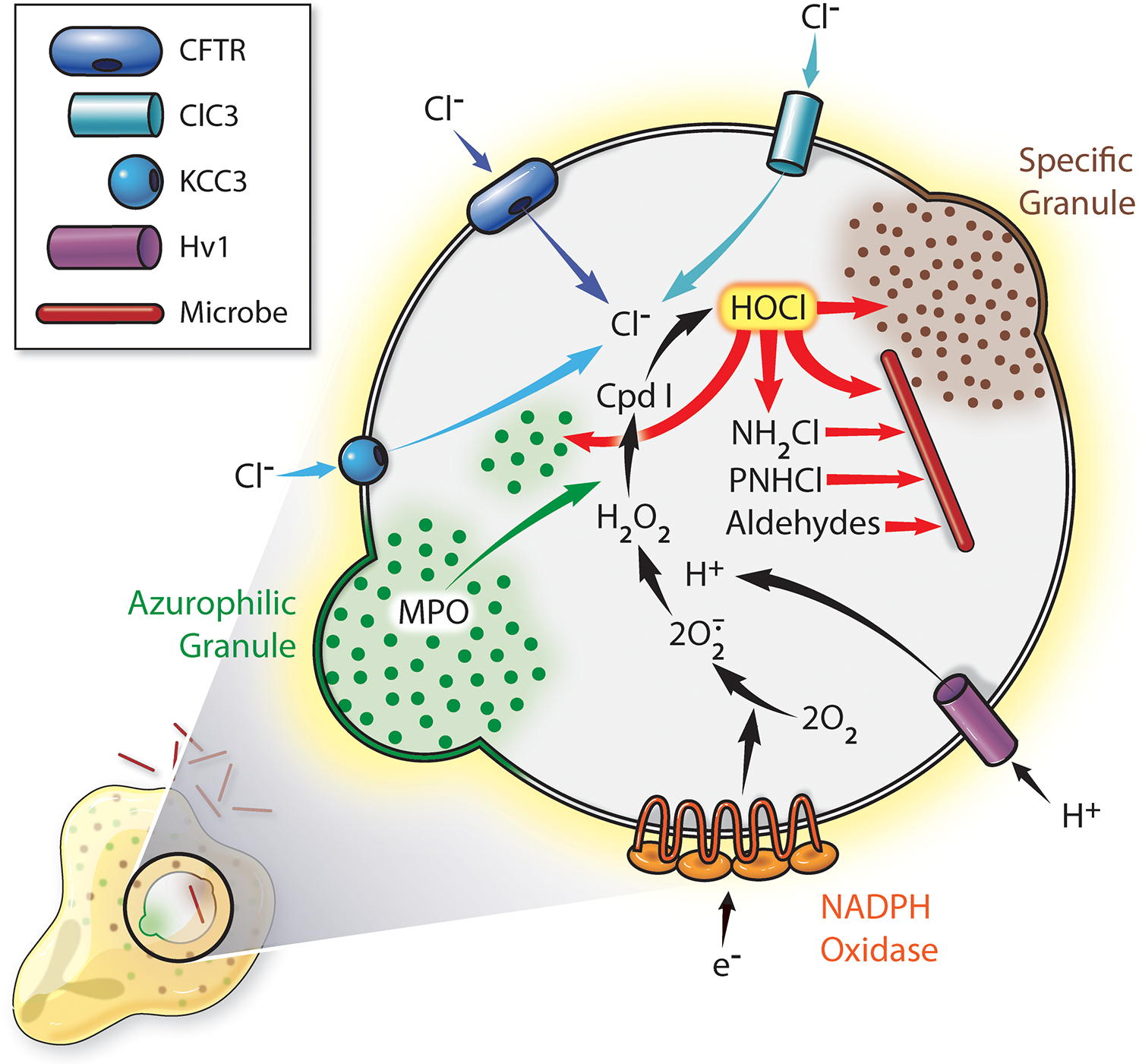
At the onset of phagocytosis, granules and secretory vesicles fuse with nascent phagosomes, delivery granule proteins into the phagosomal lumen and inserting functionally important membrane proteins, including the voltage-gated proton channel Hv1 and chloride channels ClC3, KCC3, and cystic fibrosis transmembrane conductance regular (CFTR), into the membrane of the phagosomes. Granule fusion delivers MPO, and CFTR supports transport of Cl− from cytoplasm into the phagosome. H2O2 generated by the NADPH oxidase, reacts with MPO to form Compound I (Cpd I), which mediates the two-electron oxidation of Cl− to Cl+ and the production of HOCl. HOCl reacts with a wide variety of biomolecules, including proteins, lipids, carbohydrates, histones, and nucleic acids 162-165. With respect to proteins, HOCl reacts most readily with sulfur-containing residues, notably methionine and cysteine, and generates monochloramines (NH4Cl), protein chloramines (PNHCl), aldehydes, and other products, some of which exert sustained antimicrobial action [reviewed in 166]. It is important to recognize that HOCl does not react preferentially with microbial proteins rather than host targets; all susceptible substrates are attacked regardless of their origin. Given that host proteins predominate in the phagosomes, most of the chlorinated and oxidized proteins are of host origin. [Figure from 96]
The contribution of neutrophils to human innate immunity extends beyond the ingestion and killing of microbes 9-11. Activated neutrophils release a host of chemokines and cytokines that recruit additional cells to the site and condition the milieu of the local inflammatory response 10. Furthermore, under optimal conditions, activation prompts neutrophils to engage transcriptional pathways that trigger apoptosis and initiate events that culminate in termination of the inflammatory response 23-25. Sometimes, as with interactions with particular bacteria, such as Staphylococcus aureus, or when stimulation is excessive, neutrophils undergo a necrotic cell death 24,26 and thereby release cytoplasmic contents that provide danger signals to drive additional inflammation. In some settings, neutrophils, as well as other cells, release extracellular traps (aka ETs), whose DNA may entrap microbes, contribute to autoimmune disease, or promote further inflammation 27,28.
In summary, human neutrophils play a prominent role in every stage of the inflammatory response, as first responders to many different types of threats, as sources of some of the secreted immunomodulators released at the site, and as active participants in the termination of inflammation and the tissue repair needed to restore homeostasis 29.
Human vs Murine Neutrophils
Morphology and distribution
It may be news but not a surprise that differences in cellular organization, contents, and activities exist between species 30,31, largely reflecting customization to the lifestyles and needs of the individual species during evolution. Overall, however, human and murine neutrophils share many structural features
As noted earlier, neutrophils are the predominant nucleated cell in circulating human blood, at a concentration of 3-11 X 103/μL, and account for 57-75% of the leukocytes. The neutrophil predominance is typical for many, but not all, mammals 32. Mice, like ruminants, have a lymphocyte-dominant hemogram, with lymphocytes at 75 to 90% of circulating leukocytes 33. The maturation of neutrophils in humans and mice differs significantly, as CXCR4 binding retains human cells in bone marrow until they mature 9,34, whereas murine neutrophils appear to complete maturation outside of the bone marrow 35. Whether these variables would differentially alter the kinetics of normal neutrophil turnover or influence the emergency granulopoiesis that operates during acute inflammation in humans 9 is unknown.
Mature human neutrophils possess lobed nuclei, typically with two to five lobes and 50% with three lobes. Certain drugs and some vitamin deficiencies (e.g. folic acid, vitamin B12) can cause hypersegmentation of the nuclei of human neutrophils. The presence of multi-lobed nuclei is considered by some as an attribute that allows neutrophils to traverse tight spaces, as occurs during transmigration through vascular endothelium, because of the flexibility of the nucleus 36,37. Apparently, high deformability allows human neutrophils to migrate rapidly and to accommodate the variety of vessel sizes encountered. Hypolobulated neutrophils exhibit reduced migratory behavior 38. The multiple indentations in the nuclei of murine neutrophils can give an appearance of lobulation, although many cells possess nuclei with a ring form. The half-life of circulating neutrophils differs between humans and mice as well, with that for human neutrophils ~ 6.5 hours 39 and for murine cells 11.4 hours 40.
Receptors
In general, receptors on the cell surface engage ligands, soluble and particle-bound, to transmit signals intracellularly and initiate specific cellular responses. Consequently, the repertoire of receptors on a cell dictates the agonists that will be detected and the responsive pathways that will be activated. The interactions and crosstalk among signaling pathways sculpt the phenotype observed.
Although murine and human neutrophils share many receptors, significant differences have been identified in specific settings. In the case of the colony-stimulating factor receptor family (CSF1R/IL34/CSF1), studies of specific receptor knock-out mice demonstrate that CSF1R/CSF1 and CSF3R/CSF3 are required for normal development of monocytes and neutrophils, but their loss does not result in absolute neutropenia [reviewed in 41]. In fact, loss of CSF1 results in increased numbers of circulating murine neutrophils 42-44. In contrast, mutations in the human gene encoding CSF3 and CSF3R result in severe congenital neutropenia, with affected children experiencing severe and life-threatening infections due to the absence of circulating neutrophils 45-47.
The balance between pro- and anti-inflammatory activities in neutrophils is maintained, in part, by the interplay between different types of immunoreceptors, which are defined as “transmembrane structure(s) containing an extracellular immunoglobulin-like domains and intracellular signaling via conserved immunoreceptor tyrosine-based activations (ITAMs) or immunoreceptor tyrosine-based inhibitory motifs (ITIMS)”[reviewed in 48. The interplay between these receptors provides neutrophils with the functional plasticity necessary to adapt to the wide variety of situations they encounter. Notable differences in the array of immunoreceptors in human and murine neutrophils have been identified and were comprehensively reviewed by van Rees et al. 48 (Figure 3), although a few merit brief mention here.
Figure 3. Immunoreceptors on human and murine neutrophils.
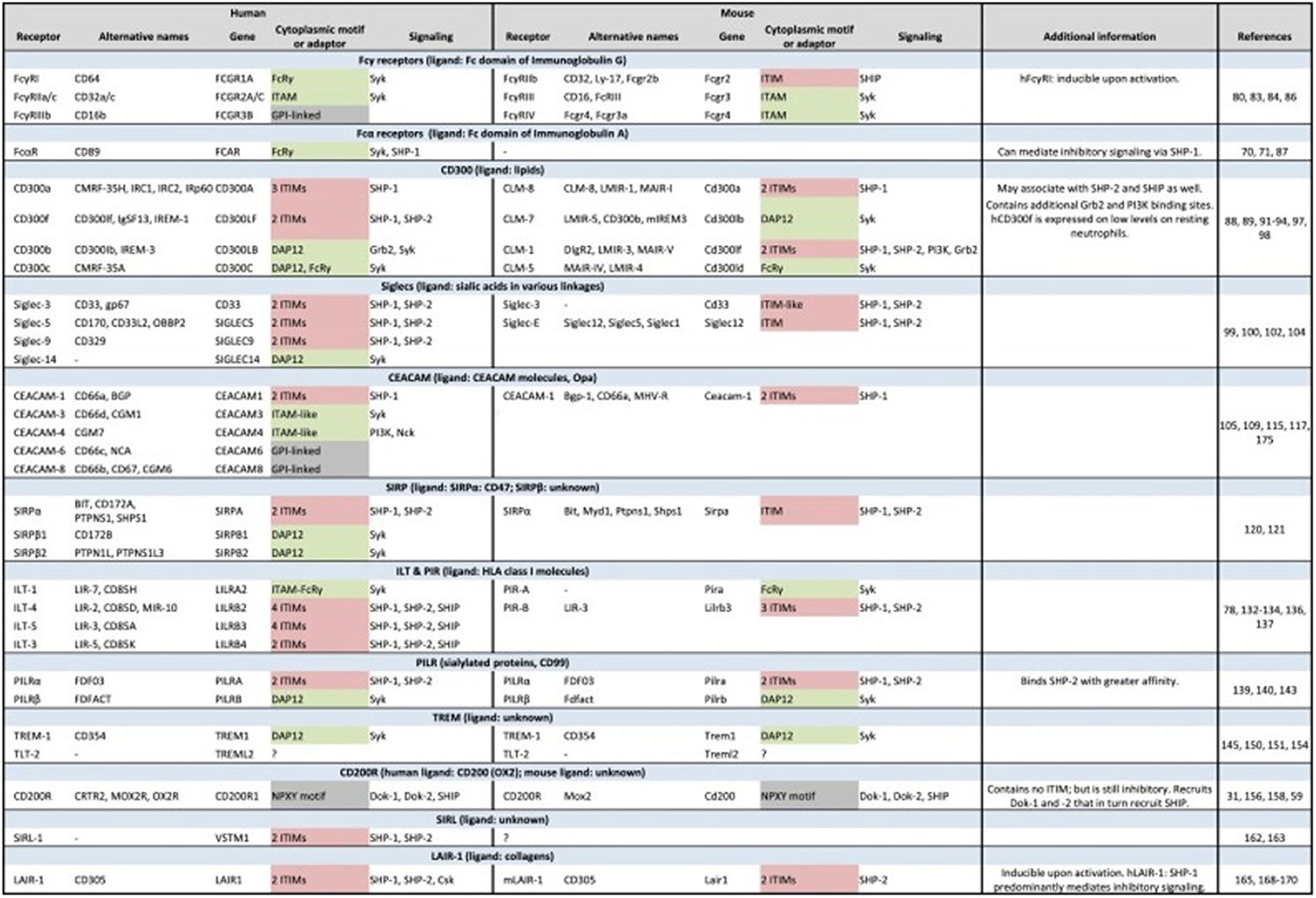
Expression of members of the immunoreceptor family on human and murine neutrophils, including those with ITAM (green), ITIM (red), or other (gray) signaling pathways. Table originally published in 48
Neutrophils in both species express receptors for Fc domains of aggregated IgG (FcγR) and participate in the ingestion of IgG-coated particles and microbes. Human neutrophils have three distinct Fcγ receptors, FcγRI (aka CD64), FcγRIIa (CD32a), and FcγRIIIb (CD16b), each with different affinities and behavior, and a receptor for IgA (FcαR). Both FcγRI and FcγRIIa have activating cytoplasmic motifs and signal via Syk, whereas FcγRIIIb is glycophosphatidylinositol-linked and lacks signaling motifs. Murine neutrophils lack FcRα, despite possessing IgA, and express an inhibitory FcγRIIb and activating FcγRIII and FcγRIV, the first signaling through SHIP and the latter two via Syk. Such differences in ligand recognition and signaling attributes (activating vs inhibitory) extend to other immunoreceptor types, including CD300 receptors, Siglec receptors, SIRP receptors and CEACAM receptors [see 48 for detailed discussion].
The differential expression of CEACAM receptors has significant implications relevant to human disease. The CEACAM receptors have a critical role in host defense against the exclusively human pathogen, Neisseria gonorrhea (Ngc). Clinically, a robust neutrophilic inflammatory response characterizes infections with Ngc 49. Human neutrophils express three CEACAMs (CEACAM 1, 3, and 6) that bind colony opacity-associated (opa) outer membrane proteins on Ngc and mediate direct binding and uptake of Ngc by neutrophils 50-52. Murine neutrophils lack the opa-binding CEACAMs and do not bind Ngc. Genetic manipulation to express the necessary human proteins in murine neutrophils corrects this specific short-coming 53.
In addition to the immunoreceptors, others differ between man and mouse, most notably the formyl peptide receptors (FPRs). N-formylated proteins from bacteria and mitochondria are the proteins first recognized as agonists for FPRs, but the list of ligands has grown significantly over time 54. FPRs are G protein-coupled transmembrane proteins whose engagement by suitable ligands stimulates adhesion, degranulation, NADPH oxidase activation, and chemotaxis in neutrophils The human genome includes three genes that encode FPRs (FPR1, FPR2, FPR3), and human neutrophils express FPR1 and FPR2 on their plasma membrane. There are eight members of the murine FPR gene family, with the orthologue for human FPR1, mFPR1, sharing many of the structural and functional features of the human receptor. One receptor, mFpr-rs1, is expressed on murine neutrophils and initially thought to share functional properties with human FPR2. However, studies in transfected cell lines demonstrate that the response profile of mFPR-rs1 does not parallel that of human FPR2 55. Other studies have identified differences in the FPR-dependent, agonist-induced activation of the NADPH oxidase in human and murine neutrophils [e.g. 56]. The variety of specific ligands for the individual FPRs and, in the case of mouse, tissue expression in non-leukocytes likely reflect differential expansion of the FPR gene cluster between mouse and man during evolution 55,57,58 and may undermine the fidelity of murine experimental systems that aim to mirror FPR-dependent human disease. Such differences have prompted an effort to identify FPR1- and FPR2-specific ligands that operate in both species [e.g. 59] and thus provide informative experimental tools
Signaling
Downstream of receptors are the biochemical pathways that relay signals to the intracellular machinery that elicits cellular responses. The Rho family GTPase Rac2 is a component required for activation of the phagocyte NADPH oxidase 60,61 and represents the predominant isoform in human neutrophils, with 80-95% Rac2 and the remainder Rac1 62,63. Murine neutrophils, on the other hand, express Rac1 and Rac2 in equal amounts. Mice deficient in Rac2 exhibit markedly depressed formyl peptide-stimulated F-actin polymerization, chemotaxis, and NADPH oxidase activity despite the presence of Rac1, which shares ~92% amino acid identity with Rac2 64. The Bokoch lab exploited these differences in Rac isoform expression between murine and human neutrophils to identify differential regulation of Rac1 and Rac2 over the gradient of fMLF concentration encountered as neutrophils crawl toward the source of chemoattractant 65. Such studies illustrate how an appreciation of the differences between murine and human neutrophils can enable the dissection of complex human physiology.
Exposure to specific cytokines, chemokines, and other proinflammatory molecules converts neutrophils from their resting state to one in which they have a heightened responsiveness to stimuli, a process referred to as priming. The primed phenotype can include enhanced adhesion, chemotaxis, phagocytosis, degranulation, apoptosis, and NADPH oxidase activity, depending on the priming agent and the context [reviewed in 66]. Typically, the priming agent does not itself activate neutrophils but instead prompts neutrophils to have an augmented sensitivity or response to subsequent exposure to a known agonist. Approximately 10% of circulating human and murine neutrophils exhibit a phenotype with increased expression of adhesion and activation markers and are “primed for recruitment” to a nascent inflammatory site 67.
Priming of the NADPH oxidase in human neutrophils depends on selective phosphorylation of a subset of serine residues whose phosphorylation controls oxidase assembly and activation [reviewed in 68]. Critical to priming by GM-CSF, TNFα, and LPS, for formyl peptide-activation of the NADPH oxidase is MAPK phosphorylation of SER345 in p47phox 69. Following its phosphorylation, proline-isomerase Pin1 binds to PRO346 and promotes conformational changes in p47phox that render other serine residues accessible for PKC-dependent phosphorylation 70-72], which then exposes motifs in p47phox that mediate assembly of a complete and functional oxidase. Thus, the phosphorylation of SER345 and binding of Pin1 serve as essential early steps in the priming activity of GM-CSF, TNFα, and LPS on formyl peptide activation of NADPH oxidase in human neutrophils 71-73.
Exposure of murine neutrophils to LPS, TNFα, or cytochalasin B primes NADPH oxidase stimulation by FPR agonists 56,74, although it is unknown if the underlying mechanism is the same as that for the human phagocyte NADPH oxidase. In general, the phosphorylation sites in murine p47phox differ from those in the human protein (although rat p47phox is identical to human), and SER345 is not conserved in murine p47phox. Although murine p47phox has SER346, the required adjacent residue is not proline, making it unlikely that its phosphorylation by MAPK would mediate priming in a Pin1-dependent fashion 71,75. However, THR356 in murine p47phox is phosphorylated by p38MAPK and has an adjacent proline residue, making it possible that the same mechanism, albeit with different residues, supports murine and human neutrophil priming [personal communication, Jamel El Benna, Ph.D., Research Director, Centre de Recherche sur l’Inflammation (CRI), Laboratoire d’Excellence Inflamex, Unversité de Paris, INSERM-U11499, Paris, France].
As part of the signaling pathway in TNFα-primed formyl peptide activation of the NADPH oxidase, there is sequential activation of different classes of phosphoinositide-3-kinases (PI3Ks), with the first phase dependent on PI3Kγ and the second on PI3Kδ 76. TNFα primes murine neutrophils as well but less robustly than human neutrophils and with dependence on only PI3Kγ 76. Although the authors offered no explanation for the differences in the observed PI3K isoform-dependent TNFα priming between human and murine neutrophils, they recommended caution when using murine experimental systems to predict the efficacy of PI3K inhibitors on responses of human neutrophils.
Secreted proteins
Granule proteins
Proteomic analyses of human 77,78 and murine 79,80 neutrophlls reveal many similarities, as expected, but some important differences [reviewed in 10]. Neutrophils possess intracellular compartments, granules, in which antimicrobial proteins, enzymes, and other bioactive molecules are stored and ready for release during degranulation into nascent phagosomes or the extracellular space [reviewed 81]. The azurophilic granules of human neutrophils possess three proteins (at least) that are absent from the granules of murine neutrophils: α-defensins, azurocidin (aka CAP37, aka heparin-binding protein, HBP), and bactericidal permeability increasing protein (BPI aka CAP57). Prominent contributors to host defense against infection and inflammation, these cationic antimicrobial peptides both damage or kill microbes as well as neutralize pathogen-associated factors that promote or exacerbate sepsis 82.
The α-defensins comprise nearly 50% of the protein in human azurophilic granules 83, exert broad antimicrobial action against viruses, bacteria (Gram-positive and Gram-negative), and fungi 84-86, and are chemotactic for T cell subtypes and immature dendritic cells 87. Concentrations of α-defensins in plasma increase from ~ 42 ng/ml at rest to 900-170,000 ng/ml in sepsis, and α-defensins appear in pulmonary secretions during inflammatory lung disease 88. The cationic antimicrobial peptide azurocidin meets infectious threats by directly damaging ingested microbes and by summoning additional host defenses, thereby serving both as a chemoattractant and as an activator of monocytes and macrophages 89. Furthermore, azurocidin acts on endothelial cells and fibroblasts, thereby contributing to tissue damage in some settings 89. BPI, the third of the azurophilic granule proteins present in human but absent from murine neutrophils, binds avidly to the surface of Gram-negative bacteria, thereby compromising microbial integrity 90, and to endotoxin, thus neutralizing its proinflammatory action 91,92 and its contribution to the pathophysiology of many infectious and inflammatory diseases 93. Taken together, the absence of these three antimicrobial proteins from murine neutrophils represents a substantial difference in the relative abilities of human and murine neutrophils to damage microbes, recruit additional host cells to the site of infection, and neutralize proinflammatory microbial components such as LPS.
But in addition to the absence from murine neutrophils of agents of host defense, there are differences in the relative amounts of some antimicrobial granule proteins, as is the case for MPO. Depending on the strain of mouse, murine neutrophils express 20 to 50% of the MPO present in human neutrophils 85,94, and the NADPH oxidase activity of murine neutrophils is 2- to 4-fold less than that of human neutrophils in response to conventional stimuli such as opsonized zymosan or phorbol myristate acetate (personal communication, Dr. Mary Dinauer, Department of Pediatrics and Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO, USA). Thus, the capacity of murine neutrophils to generate HOCl and its reaction products (vide infra) is much less than that of human neutrophils. Because the MPO-H2O2-chloride system provides a potent and perhaps the most efficient oxygen-dependent antimicrobial activity in human neutrophil phagosomes 13,18,19,21,95,96, its absence would compromise intraphagosomal killing.
A comparison of how human and murine neutrophils interact with Candida albicans illustrates the biological consequences of generating too little HOCl. Human neutrophils kill C.albicans, a common human commensal and one of the predominant fungal pathogens for humans 97, and prevent fungal filamentation in an MPO-dependent fashion 98. Neutrophils from individuals with an inherited deficiency of MPO fail to kill C.albicans in vitro or to damage hyphal elements in C.albicans and Aspergillus fumigatus 99-104. The defective candidacidal activity in vitro due to insufficient HOCl production has clinical manifestations, as four of the first six MPO-deficient humans had severe infection with disseminated or visceral candidiasis 101-103,105. In many ways, the interaction of murine neutrophils with C.albicans resembles that of MPO-deficient human neutrophils. Murine neutrophils kill C.albicans less effectively than do human neutrophils and fail to prevent Candida germ tube formation 98. In fact, hyphal growth of ingested C.albicans results in the death of the murine neutrophil. Ineffective fungal killing occurs in murine blood as well as in isolated neutrophils, with significantly greater fungal survival and filamentation in murine blood than in human blood 106. Although the defective candidacidal action of murine neutrophils likely reflects both the absence of α-defensins and the lesser amounts of MPO, the depressed activity of whole blood suggests that that soluble factors that act against C.albicans in mice are relatively ineffective as well. Together these inadequacies in cellular and soluble factor defenses constitute a serious short-coming in the use of murine experimental systems to model human immune response to C.albicans.
Cytokines
Secreted biomolecules that are immunoregulatory and serve to tune the immune response, both at the site of acute inflammation and systemically, cytokines were once not considered part of the repertoire of human neutrophil activity. However, since the early 1990s when investigators demonstrated that human neutrophils produced IL-8 and TNFα in response to phagocytosis 107,108, subsequent studies have identified additional cytokines produced by human neutrophils in response to a variety of agonists and in diverse experimental settings [reviewed in10,109,110 111. Neutrophil-derived cytokines include both those stored intracellularly [e.g. TNFα-related apoptosis-inducing ligand (TRAIL) 112,113] as well as those synthesized de novo and secreted in response to in vitro stimulation. The list includes both pro- and anti-inflammatory cytokines, chemokines, colony stimulating factors, TNFα family members, and factors that promote angiogenesis and fibrogenesis 111. Although neutrophils express only ~10 to 20% the RNA of other leukocytes 114 and therefore produce and secrete relatively less cytokine per cell, the enormous number of neutrophils at an inflammatory site enables neutrophil-derived agents to influence significantly local events. Secreting such bioactive agents, stimulated neutrophils communicate with other cells, both near and far, to modulate the inflammatory response 9.
Human and murine neutrophils differ in the cytokines expressed and secreted, summarized in 111. (Figure 4). Human neutrophils do not produce IFNβ, IL-10, or IL-17 115 116, for example. Considering the far-reaching and multiple immunomodulatory properties of cytokines, one anticipates that the inflammatory milieu in many contexts would differ in mice and humans and, more importantly, that it would be difficult to predict the ways in which events in the two species would differ. It is possible that the cytokines and granule proteins act in concert to recruit and activate immune cells in an inflammatory process 10, thus adding to both the complexity of the biology and to the uncertainty in predicting species’ differences and their consequences.
Figure 4. Chemokines and cytokines.
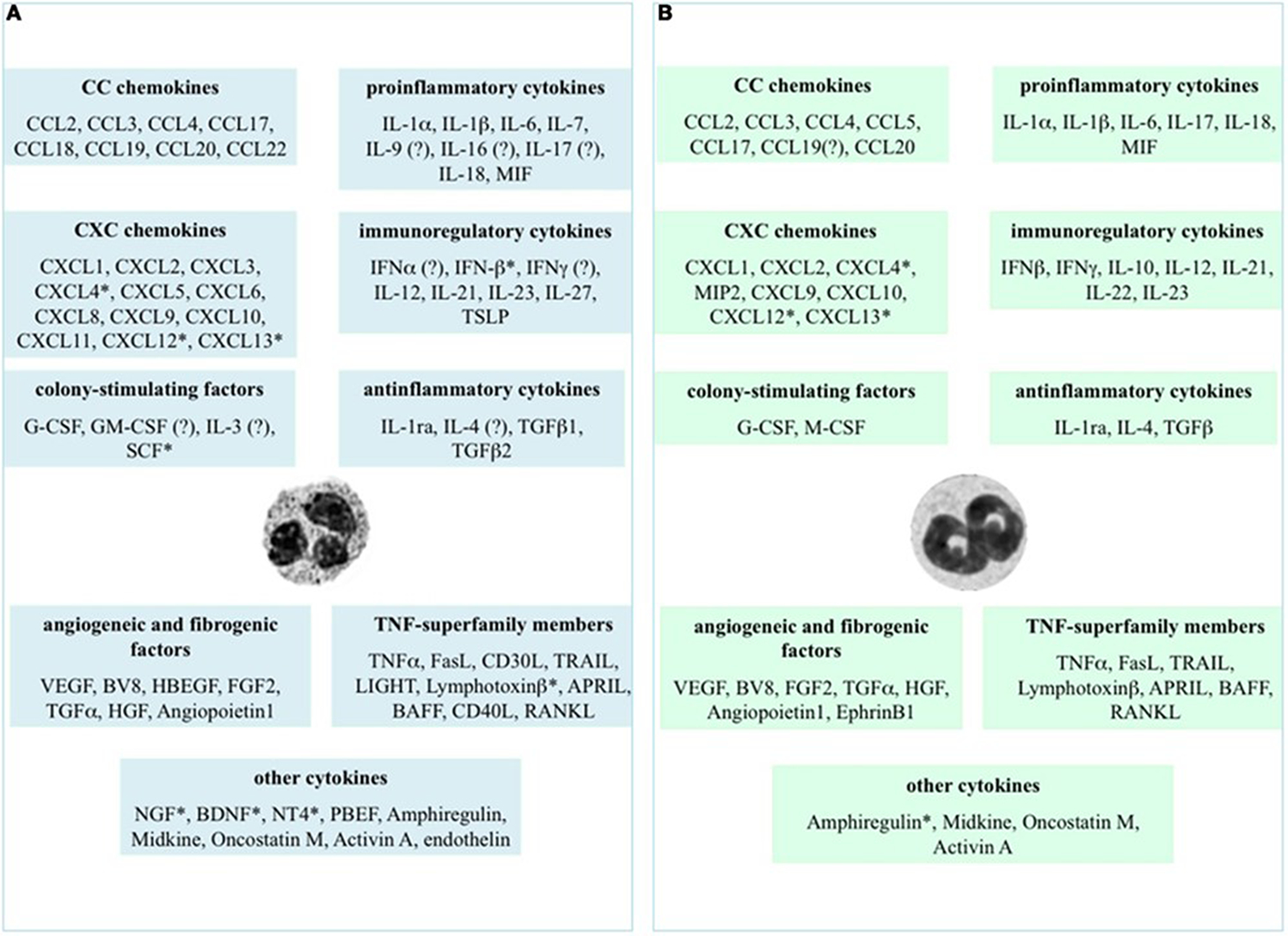
Chemokines and cytokines potentially expressed or produced by human (panel A) and murine (panel B) neutrophils. The evidence for expression came from assessing gene expression, immunochemical staining, protein quantitation by enzyme-linked immunoabsorbant assays as well as some biological assays. The * indicates that the evidence was limited to mRNA detection and ? indicates data that are controversial at the time of this publication (2014) Table originally published in 111
Experimental studies using mice
The significant differences in the genetic composition of mice and humans comes as no surprise, given the divergence of the two species during evolution 65 to 75 million years ago. Although the mouse genome contains more genes than does that of humans (20,210 vs 19,042 6), a large majority of genes (75% of murine, 80% of human) are closely related functionally [reviewed in 6]. However, mice and humans differ markedly in their genomic responses to proinflammatory stimuli such as trauma, burns, and endotoxemia 117. In part, these differences may reflect the contrasting strategies that mice and humans have developed to optimize their odds of survival in an environment replete with infectious threats.
In general, multicellular organisms can adopt one of two distinct strategies to encounters with infectious threats: resistance or tolerance 6,118-120. Resistance mechanisms include those targeted to kill (or at least damage) and eliminate invading microbes. Alternatively, tolerance allows the host to maintain function despite the presence of large numbers of potential pathogens. Although neither mice nor man utilizes one strategy at the expense of the other, resistance mechanisms predominate in human response to infection, particularly with respect to the innate immune system, whereas mice rely more on tolerance to endure infectious threats. Detailed discussions of these alternative approaches to infection have been presented more extensively elsewhere 119,120, but the contrasting sensitivities of murine and human innate immune responses to lipopolysaccharide (LPS) illustrate the differences between resistance and tolerance. Simply put, mice tolerate endotoxemia and are highly resistant to LPS, with a lethal dose ~ 10 mg/kg. In contrast, humans mount a robust immune response to LPS and are exquisitely sensitive, with ~15 μg/kg triggering shock 6,7. These diametrically opposite responses to endotoxin reflect differences in the determinants of sentinel and reactive immune cells and in the soluble factors that contribute critically to detection of LPS 121. Such genetic and phenotypic differences in the innate immune responses to a prototypical microbial proinflammatory agonist such as LPS may explain, at least in part, the legacy of failed therapies to target endotoxemia and sepsis: the murine experimental systems used to mimic the human response to endotoxin were imposters, not models. Consequently, the extrapolations from mouse to man fell short of expectations 5-7.
The differences in genomic responses of mice and humans include proinflammatory stimuli in addition to those to endotoxin 117. Many investigators working with murine experimental systems to explore physiologic or pathophysiologic mechanisms that relate to human neutrophil biology or innate immune responses have identified some of the limitations of such systems that approximate phenomenology but do not mirror the human condition with respect to underlying mechanism. Several examples illustrate this important point.
Neutrophils are critical contributors to the pathophysiology of many autoimmune diseases and targets for therapy with intravenous immunoglobulin G (IVIG), which is frequently used clinically in many inflammatory and autoimmune diseases 122. IVIG contains antibodies to the death receptor Fas (aka CD95) and to Siglec-9, thereby prompting cytokine-dependent neutrophil death when infused into humans and mediating a beneficial clinical response 123-126. In contrast to the effects on human neutrophils, IVIG, F(ab’)2 or Fc fragments of IgG, pooled murine IgG, or agonistic monoclonal antibodies to Fas or Siglec-9 have no effect on murine neutrophils. These contrasting results prompted the authors to underscore “the need to establish experimental systems that take into account the divergent species-related effects of IVIG on neutrophils” 127.
The α-defensins released from activated human neutrophils promotes damage in acute lung injury by disrupting capillary-epithelial cell barriers, and the absence of α-defensins from murine neutrophils disqualifies unmodified murine systems as potential models of acute human lung injury. The creation of transgenic mice expressing α-defensins in their neutrophils was required to provide a better approximation of acute lung injury in humans for study 88.
Although both human and murine neutrophils generate extracellular traps in response to larval Strongyloides stercoralis, the extracellular traps from human, but not murine, neutrophils are required for killing of larvae 128.
Whereas binding of Shiga toxin to murine neutrophils depends on the presence of globotriaosylceramide 3 (Gb3) on the plasma membrane, its association with human neutrophils does not. Consequently, human serum amyloid P protects mice but not humans from the deleterious effects of Shiga toxin 2, an important virulence factor in hemolytic uremic syndrome caused by enteropathogenic E.coli O157:H7 129.
As discussed earlier, the reduced capacity of murine neutrophils to generate HOCl undermines the accuracy, at the mechanistic level, of studies to elucidate the role of neutrophils in human infections with C.albicans. Similarly, murine neutrophils do not respond to Mycobacterium tuberculosis in the same MPO-dependent fashion as do human neutrophils 130. Furthermore, MPO activity figures prominently in sterile settings of chronic inflammation, including atherosclerosis 131. Whereas MPO-dependent biochemistry has been implicated in the initiation and progression of human atherosclerosis, some murine systems used to study atherosclerosis lack evidence for a contribution from MPO 132,133.
In many settings, the behavior of tumors in mice fails to mirror critical aspects of human disease 134. In part, the discrepancies likely reflect the absence of immunoediting, a fundamental determinant in the selection and development of human malignancies 135. Many human tumors are extensively infiltrated with neutrophils136, and the presence of tumor-associated neutrophils has been implicated in tumor angiogenesis 137. Neutrophil infiltration of human tumors has been assigned prognostic significance, and some investigators in the field have advocated for neutrophil-targeted therapy as part of patient management 135,137-139, which together underscore the clinical importance of having valid animal models.
Modeling human infections in which neutrophils play a central role
The examples mentioned above demonstrate that in some biological settings where neutrophils serve a defining role, the murine experimental system does not mirror the human condition with sufficient accuracy to provide informative insights into the mechanism(s) underlying human disease. However, many investigators have utilized murine models successfully to explore the pathogenesis of human clinical disorders, thus providing evidence that in the right setting, a murine model can serve as a powerful and informative experimental tool. Unfortunately, the identification of the right situation is a challenge often unrecognized by reviewers of grants or manuscripts who greet data derived from in vitro studies of human neutrophils with a request for the applicant or authors to test the hypothesis in question in a mouse model or knock-out. Unfortunately, such requests often prompt investigators to comply and thereby generate data that may be compelling but too often lack a connection with the human biology intended to be explicated.
To suggest using a mouse system to model human infections in which neutrophils figure prominently fails to consider the coevolution of humans and human-adapted microbes. Humans and their pathogens have coevolved to the extent that reciprocal adaptive changes have occurred in each and have thereby exerted selective pressures on each. This coevolution of host and pathogen serves as a potent agent in the biology of human infection 140. Accordingly, the human immune system has specific strategies to target certain microbes, and, on the other hand, microbes that are successful pathogens employ adaptive responses to resist, evade, or counteract host responses. The coadaptation of microbes to humans and of human innate immunity to microbes has fostered the development of reciprocal constructs and responses that, in some cases, are so finely tuned that the introduction of a given microbe into a non-human species yields no valuable or valid insights, regardless of how similar the phenotype or entertaining the result of the murine infection. Some examples of the disconnect between mouse and man are obvious. Exclusively human pathogens such as Neisseria gonorrhoeae cannot be studied in a mouse model without significant genetic retooling of the mouse, as the organism is exquisitely and exclusively adapted to infect humans. For other microbes, it may be less readily apparent why a mouse model is in many ways inappropriate, as is the case for Staphylococcus aureus.
Staphylococcal infections in murine experimental systems
S.aureus is a human commensal that causes frequent and serious infections in humans, often complicated by local tissue necrosis, distant metastatic spread, and commonly death 141. Early host defense against S.aureus is neutrophil-mediated and oxidant-dependent 142, convincingly demonstrated by the morbidity and mortality of staphylococcal disease in individuals with chronic granulomatous disease, who lack a functional NADPH oxidase 143 and are particularly susceptible to infection with S.aureus 144. S.aureus has adapted successfully to man and become a prominent cause of infection in even the immunocompetent host.
Evidence from in vitro studies of normal human and of MPO-deficient neutrophils demonstrates that optimal killing of ingested S.aureus by human neutrophils is MPO-dependent 95,145. However, neutrophils fail to kill all ingested staphylococci, and surviving staphylococci within neutrophils often trigger necrotic cell death of the host cell 146, thereby releasing proinflammatory agonists that promote more inflammation and tissue destruction locally and metastatic infection to bone, heart, lungs and other vital organs. Adaptations during co-evolution with humans have brought S.aureus success as a human pathogen. In contrast to its relationship with humans, S.aureus is not an organism that naturally infects mice, although strains may be adapted to cause transmissible disease among mice 147.
Strategies that S.aureus has employed to gain foothold in humans include those that block chemotaxis, ingestion, and killing by neutrophils [reviewed in 148]. For example, the chemotaxis inhibitory protein of S.aureus (CHIPS) binds the C5a and formyl peptide receptors of human neutrophils better than murine receptors to inhibit human neutrophil chemotaxis 149. Secreted staphylococcal proteins such as staphylococcal complement inhibitor (SCIN) and its homologues block steps in complement activation in the cascade that culminates in opsonization of the organism and phagocytosis by neutrophils 150,151. S.aureus secretes a variety of leukotoxins, including Panton Valentine Leukocidin (PVL), which target specifically human cells to promote their lytic cell death 152-155. Some have little or /no binding to murine leukocytes [reviewed 153] (Figure 5). Even within the phagosome, S.aureus targets specifically human neutrophil attack by secreting SPIN (staphylococcal peroxidase inhibitor), a protein that binds to and inhibits the activity of human MPO [reviewed 156]. S.aureus increase expression of SPIN within the phagosomes of human neutrophils, and SPIN inhibits MPO activity and the generation of HOCl 157,158. SPIN does not bind or inhibit the MPO from rabbit, mouse, horse, or cow neutrophils, providing evidence of the remarkable human adaptation achieved by S.aureus 156,157.
Figure 5. Staphylococcal bicomponent leukotoxins.
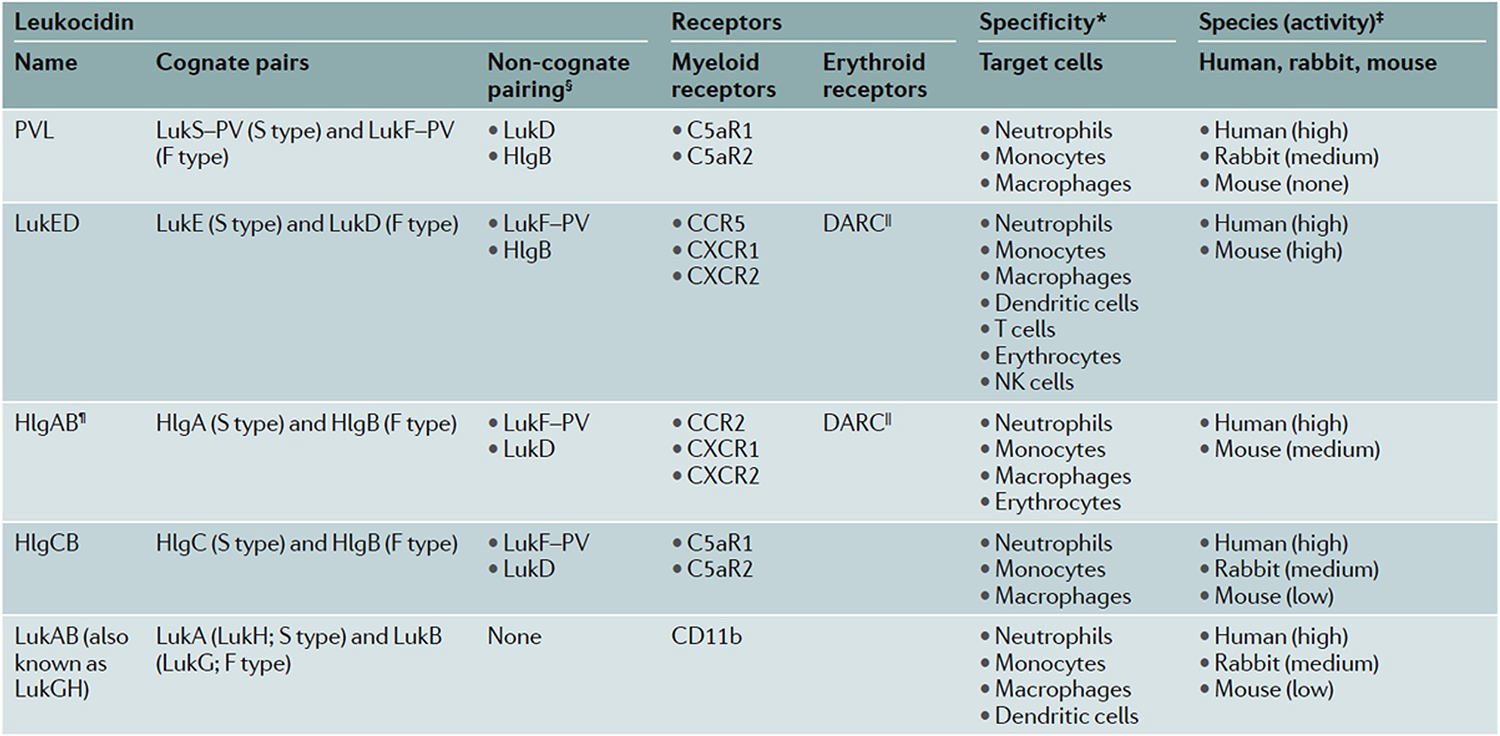
Human-adapted S.aureus produce distinct leukocidins that target myeloid and erythroid receptors with species-specific differences in susceptibility as demonstrated by experimental data (*). § designates potential pairs of the S component with an F component from another leukocidin; II indicates that DARC makes erythrocytes susceptible to the actions of LukED and HlgAB; ¶ HlgAB engages CCR2 and DARC in both human and murine cells, but only human CXCR1 and CXCR2. C5aR1, C5a anaphylatoxin chemotactic receptor 1; CCR2, CC-chemokine receptor 2; CXCR1, CXC chemokine receptor 1; DARC, Duffy antigen receptor for chemokines; HlgA, γ-haemolysin A; LukA, leukocidin A; NK cells, natural killer cells; PVL, Panton–Valentine leukocidin. Table originally published in 153
*Shown are the leukocytes for which there are experimental data in the literature. ‡On the basis of published susceptibility of tested primary cells. §Potential non-cognate pairing of the S component with an F component of another leukocidin. which results in functional mixed pores or inactive hybrid complexes, depending on the pair. ∥DARC renders erythrocytes susceptible to the haemolytic activity of LukED and HlgAB. ¶HlgAB targets both human and murine CCR2 and DARC, but only human CXCRl and CXCR2. CSaRl. C5a anaphylatoxin chemotactic receptor 1; CCR2, CC-chemokine receptor 2; CXCRl. CXC chemokine receptor 1; DARC. Duffy antigen receptor for chemokines: HlgA, y-haemolysin A: LukA. leukocidin A; NK cells, natural killer cells: PVL. Panton-Valentine leukocidin.
Thus, during its coevolution with man, human-adapted strains of S.aureus have acquired attributes and behaviors finely tuned to elements of the human innate immune system in ways that cannot be precisely mimicked in unmodified murine experimental systems.
Summary
I chose this topic with great trepidation, since I have previously written about the functionally relevant differences between human and murine neutrophils and their potential consequences with respect to the use of murine experimental systems to model human neutrophil-dependent biology 133. Furthermore, others have contrasted human and murine immune systems in great detail 6,31,106,159,160, including a recently published thought-provoking perspective 161. However, I decided that the exercise has merit, but neither as an indictment of murine models, since that would be unjustified and not my intention, nor as a tribute to in vitro studies of human neutrophils.
Of course, the in vitro study of isolated human neutrophils, the experimental approach that has anchored my scientific career, has many shortcomings. The process of isolating neutrophils from venous blood itself imposes stresses on cells that are non-physiologic (exposure to plastics, dilute buffers, anticoagulants, centrifugal forces, density gradient media etc) and likely perturb neutrophils. Are isolated neutrophils ever “resting”? Some investigators keep isolated neutrophils at 4°C (or colder in an ice bath) before starting experiments in order to avoid inadvertent stimulation. What changes in membrane behavior or neutrophil metabolism accompany such cold exposure? Most consequential to the behavior of isolated neutrophils however is the absence from such studies of soluble factors and other cells, circulating as well as tissue-bound, with which neutrophils communicate. In the absence of such trophic factors, neutrophils isolated from venous blood only approximate neutrophils in vivo. Animal models avoid all these shortcomings (and likely more).
How best to proceed? Without question, human and murine neutrophils differ in many ways: differences in relative abundance in circulation blood, in receptors, signaling pathways, granule proteins, NADPH oxidase regulation, magnitude of oxidant and HOCl production, and repertoire of cytokines and other secreted molecules they produce. Consequently, authors should use and editors should demand accurate titles for manuscripts. If all the data in a report derive from the study of murine neutrophils or a murine model exclusively, then the correct and scientifically accurate title of the manuscript should include mouse/murine to modify the word neutrophils. Sometimes, but not always, the differences matter greatly and can derail extrapolations from murine studies to clinical care, as illustrated by several failed clinical interventions based on presumed murine models, such as therapy for sepsis.
However, when bona fide models, murine experimental systems can provide insights into human biology difficult to obtain in any other way. Furthermore, when murine models may fall short of explicating mechanisms underlying certain human diseases, the novel insights into general immune mechanisms have been remarkable. The challenge for investigators who employ murine experimental systems, however, is to distinguish models from pretenders and to know when the mouse provides accurate insights into the human biology. Testing in humans those observations made in the murine systems would provide a safeguard, but such experiments are not always possible. The expense and the housing requirements can preclude the use of animal models that better mimic human neutrophils, such as rabbit or pig. For now, the integration of evidence from studies of neutrophil biology performed using sound murine models coupled with testing in vitro of human neutrophils will combine the best of both approaches to elucidate the mysteries of human neutrophil biology.
Acknowledgements
Thanks to Jamel El Benna, Ph.D., [Research Director, Centre de Recherche sur l’Inflammation (CRI), Laboratoire d’Excellence Inflamex, Université de Paris, INSERM-U11499, Paris, France] and to Mary C. Dinauer, M.D., Ph.D. [Department of Pediatrics and Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO] for sharing their personal insights on the priming and activity, respectively, of the NADPH oxidase in murine neutrophils. The Nauseef lab is supported by NIH grant AI132335 and use of facilities at the Iowa City Department of Veterans Affairs Medical Center, Iowa City, IA.
Abbreviations:
- fMLF
formyl-methionyl-leucyl-phenylalanine
- FPR
formyl peptide receptor
- HOCl
hypochlorous acid
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- NETs
neutrophil extracellular traps
- TNFα
tumor necrosis factor α
Footnotes
Conflict of Interest The author has no conflicts of interest.
REFERENCES
- 1.Stackowicz J, Jönsson F, Reber LL. Mouse Models and Tools for the in vivo Study of Neutrophils. Front Immunol. 2019;10:3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 3.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1(8538):893–895. [DOI] [PubMed] [Google Scholar]
- 4.Monaco AP. Chimerism in organ transplantation: conflicting experiments and clinical observations. Transplantation. 2003;75(9 Suppl):13s–16s. [DOI] [PubMed] [Google Scholar]
- 5.Munford RS. Murine responses to endotoxin: another dirty little secret? JInfectDis. 2010;201:175–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol. 2014;34(5):433–454. [PubMed] [Google Scholar]
- 7.Cavaillon JM, Singer M, Skirecki T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol Med. 2020;12(4):e10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLeo FR, Nauseef WM. Granulocytic Phagocytes. In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 9 ed. Philadelphia, PA: Elsevier; 2020:83–98. [Google Scholar]
- 9.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15(7):602–611. [DOI] [PubMed] [Google Scholar]
- 10.Cassatella MA, Ostberg NK, Tamassia N, Soehnlein O. Biological Roles of Neutrophil-Derived Granule Proteins and Cytokines. Trends Immunol. 2019;40(7):648–664. [DOI] [PubMed] [Google Scholar]
- 11.Ley K, Hoffman HM, Kubes P, et al. Neutrophils: New insights and open questions. Science immunology. 2018;3(30). [DOI] [PubMed] [Google Scholar]
- 12.Nauseef WM. The Neutrophil NADPH Oxidase. In: Vissers M, Hampton MB, Kettle AJ, ed. Hydrogen Peroxide Metabolism in Health and Disease. Boca Raton, FL: CRC Press; 2018:237–277. [Google Scholar]
- 13.Forbes LV, Kettle AJ. Myeloperoxidase: Unleashing the Power of Hydrogen Peroxide. In: Vissers MCM, Hampton MB, Kettle AJ, eds. Hydrogen Peroxide Metabolism in Health and Disease. Boca Rotan: CRC Press; 2018:281–304. [Google Scholar]
- 14.Albrett AM, Ashby LV, Dickerhof N, Kettle AJ, Winterbourn CC. Heterogeneity of hypochlorous acid production in individual neutrophil phagosomes revealed by a rhodamine-based probe. J Biol Chem. 2018;293:15715–15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kettle AJ, Anderson RF, Hampton MB, Winterbourn CC. Reactions of superoxide with myeloperoxidase. Biochemistry. 2007;46:4888–4897. [DOI] [PubMed] [Google Scholar]
- 16.Kettle AJ, Winterbourn CC. Superoxide modulates the activity of myeloperoxidase and optimizes the production of hypochlorous acid. Biochem J. 1988;252:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green JN, Chapman ALP, Bishop CJ, Winterbourn CC, Kettle AJ. Neutrophil granule proteins generate bactericidal ammonia chloramine on reaction with hydrogen peroxide. Free Radic Biol Med. 2017;113:363–371. [DOI] [PubMed] [Google Scholar]
- 18.Winterbourn CC, Kettle AJ, Hampton MB. Reactive Oxygen Species and Neutrophil Function. Annu Rev Biochem. 2016;85:765–792. [DOI] [PubMed] [Google Scholar]
- 19.Kettle AJ, Winterbourn CC. Myeloperoxidase: Structure and Function of the Green Heme Peroxidase of Neutrophils. In: Heme Peroxidases. 4 ed.: the Royal Society of Chemistry; 2016:272–308. [Google Scholar]
- 20.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. [DOI] [PubMed] [Google Scholar]
- 21.Nauseef WM. The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling. Curr Opin Immunol. 2019;60:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odeberg H, Olsson I. Microbicidal mechanisms of human granulocytes: synergistic effects of granulocyte elastase and myeloperoxidase or chymotrypsin-like cationic protein. Infect Immun. 1976;14:1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi SD, DeLeo FR. Role of neutrophils in innate immunity: a systems biology-level approach. Wiley Interdiscip Rev Syst Biol Med. 2009;1(3):309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi SD, Malachowa N, DeLeo FR. Influence of Microbes on Neutrophil Life and Death. Frontiers in cellular and infection microbiology. 2017;7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res. 2009;43:25–61. [DOI] [PubMed] [Google Scholar]
- 26.Rungelrath V, Porter AR, Malachowa N, et al. Further Insight into the Mechanism of Human PMN Lysis following Phagocytosis of Staphylococcus aureus. Microbiology spectrum. 2021;9(2):e0088821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Ishikawa T, Lai Y, Nallapothula D, Singh RR. Diverse Roles of NETosis in the Pathogenesis of Lupus. Front Immunol. 2022;13:895216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hidalgo A, Libby P, Soehnlein O, Aramburu IV, Papayannopoulos V, Silvestre-Roig C. Neutrophil extracellular traps: from physiology to pathology. Cardiovasc Res. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shim HB, Deniset JF, Kubes P. Neutrophils in homeostasis and tissue repair. Int Immunol. 2022;34(8):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Styrt B Species variation in neutrophil biochemistry and function. J Leukoc Biol. 1989;46(1):63–74. [DOI] [PubMed] [Google Scholar]
- 31.Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. [DOI] [PubMed] [Google Scholar]
- 32.Fingerhut L, Dolz G, de Buhr N. What Is the Evolutionary Fingerprint in Neutrophil Granulocytes? International journal of molecular sciences. 2020;21(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doeing DC, Borowicz JL, Crockett ET. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin Pathol. 2003;3(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deniset JF, Surewaard BG, Lee WY, Kubes P. Splenic Ly6G(high) mature and Ly6G(int) immature neutrophils contribute to eradication of S. pneumoniae. J Exp Med. 2017;214(5):1333–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann K, Sperling K, Olins AL, Olins DE. The granulocyte nucleus and lamin B receptor: avoiding the ovoid. Chromosoma. 2007;116(3):227–235. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Chen M, Chang W. Roles of the nucleus in leukocyte migration. J Leukoc Biol. 2022. [DOI] [PubMed] [Google Scholar]
- 38.Gaines P, Tien CW, Olins AL, et al. Mouse neutrophils lacking lamin B-receptor expression exhibit aberrant development and lack critical functional responses. Exp Hematol. 2008;36(8):965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mauer AM, Athens JW, Ashenbrucker H, Cartwright GE, Wintrobe MM. LEUKOKINETIC STUDIES. II. A METHOD FOR LABELING GRANULOCYTES IN VITRO WITH RADIOACTIVE DIISOPROPYLFLUOROPHOSPHATE (DFP). J Clin Invest. 1960;39(9):1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100(3):854–861. [DOI] [PubMed] [Google Scholar]
- 41.Pinheiro D, Mawhin MA, Prendecki M, Woollard KJ. In-silico analysis of myeloid cells across the animal kingdom reveals neutrophil evolution by colony-stimulating factors. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai XM, Ryan GR, Hapel AJ, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99(1):111–120. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida H, Hayashi S, Kunisada T, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345(6274):442–444. [DOI] [PubMed] [Google Scholar]
- 44.Hibbs ML, Quilici C, Kountouri N, et al. Mice lacking three myeloid colony-stimulating factors (G-CSF, GM-CSF, and M-CSF) still produce macrophages and granulocytes and mount an inflammatory response in a sterile model of peritonitis. J Immunol. 2007;178(10):6435–6443. [DOI] [PubMed] [Google Scholar]
- 45.Lakshman R, Finn A. Neutrophil disorders and their management. J Clin Pathol. 2001;54(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia J, Miller CA, Baty J, et al. Somatic mutations and clonal hematopoiesis in congenital neutropenia. Blood. 2018;131(4):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walkovich K, Connelly JA. Congenital Neutropenia and Rare Functional Phagocyte Disorders in Children. Hematol Oncol Clin North Am. 2019;33(3):533–551. [DOI] [PubMed] [Google Scholar]
- 48.van Rees DJ, Szilagyi K, Kuijpers TW, Matlung HL, van den Berg TK. Immunoreceptors on neutrophils. Semin Immunol. 2016;28(2):94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unemo M, Seifert HS, Hook EW 3rd, Hawkes S, Ndowa F, Dillon JR. Gonorrhoea. Nat Rev Dis Primers. 2019;5(1):79. [DOI] [PubMed] [Google Scholar]
- 50.Swanson J, Sparks E, Zeligs B, Siam MA, Parrott C. Studies on gonococcus infection. V. Observations on in vitro interactions of gonococci and human neutrophils. Infect Immun. 1974;10(3):633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virji M, Watt SM, Barker S, Makepeace K, Doyonnas R. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol. 1996;22(5):929–939. [DOI] [PubMed] [Google Scholar]
- 52.Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 1997;16(12):3435–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarantis H, Gray-Owen SD. Defining the roles of human carcinoembryonic antigen-related cellular adhesion molecules during neutrophil responses to Neisseria gonorrhoeae. Infect Immun. 2012;80(1):345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He HQ, Ye RD. The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition. Molecules. 2017;22(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang ZG, Ye RD. Characterization of two new members of the formyl peptide receptor gene family from 129S6 mice. Gene. 2002;299(1-2):57–63. [DOI] [PubMed] [Google Scholar]
- 56.Bylund J, Samuelsson M, Collins LV, Karlsson A. NADPH-oxidase activation in murine neutrophils via formyl peptide receptors. Exp Cell Res. 2003;282(2):70–77. [DOI] [PubMed] [Google Scholar]
- 57.Li L, Chen K, Xiang Y, et al. New development in studies of formyl-peptide receptors: critical roles in host defense. J Leukoc Biol. 2016;99(3):425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao JL, Chen H, Filie JD, Kozak CA, Murphy PM. Differential expansion of the N-formylpeptide receptor gene cluster in human and mouse. Genomics. 1998;51(2):270–276. [DOI] [PubMed] [Google Scholar]
- 59.Skovbakke SL, Winther M, Gabl M, et al. The peptidomimetic Lau-(Lys-βNSpe)(6)-NH(2) antagonizes formyl peptide receptor 2 expressed in mouse neutrophils. Biochem Pharmacol. 2016;119:56–65. [DOI] [PubMed] [Google Scholar]
- 60.Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21 rac1 Nature. 1991;353:668–670. [DOI] [PubMed] [Google Scholar]
- 61.Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254:1512–1515. [DOI] [PubMed] [Google Scholar]
- 62.Heyworth PG, Bohl BP, Bokoch GM, Curnutte JT. Rac translocates independently of the neutrophil NADPH oxidase components p47 phox and p67 phox . Evidence for its interaction with flavocytochrome b 558 J Biol Chem. 1994;269:30749–30752. [PubMed] [Google Scholar]
- 63.Quinn MT, Evans T, Loetterle LR, Jesaitis AJ, Bokoch GM. Translocation of Rac correlates with NADPH oxidase activation. Evidence for equimolar translocation of oxidase components. J Biol Chem. 1993;268:20983–20987. [PubMed] [Google Scholar]
- 64.Li S, Yamauchi A, Marchal CC, Molitoris JK, Quilliam LA, Dinauer MC. Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J Immunol. 2002;169(9):5043–5051. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Sun C, Glogauer M, Bokoch GM. Human neutrophils coordinate chemotaxis by differential activation of Rac1 and Rac2. J Immunol. 2009;183(4):2718–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miralda I, Uriarte SM, McLeish KR. Multiple Phenotypic Changes Define Neutrophil Priming. Frontiers in cellular and infection microbiology. 2017;7:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fine N, Barzilay O, Sun C, et al. Primed PMNs in healthy mouse and human circulation are first responders during acute inflammation. Blood advances. 2019;3(10):1622–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009;41(4):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dang PMC, Stensballe A, Boussetta T, et al. A specific p47 phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. JClinInvest. 2006;116 (7 ):2033–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Makni-Maalej K, Boussetta T, Hurtado-Nedelec M, Belambri SA, Gougerot-Pocidalo MA, El-Benna J. The TLR7/8 agonist CL097 primes N-formyl-methionyl-leucyl-phenylalanine-stimulated NADPH oxidase activation in human neutrophils: critical role of p47phox phosphorylation and the proline isomerase Pin1. J Immunol. 2012;189(9):4657–4665. [DOI] [PubMed] [Google Scholar]
- 71.Boussetta T, Gougerot-Pocidalo MA, Hayem G, et al. The prolyl isomerase Pin1 acts as a novel molecular switch for TNF-α-induced priming of the NADPH oxidase in human neutrophils. Blood. 2010;116(26):5795–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev. 2016;273(1):180–193. [DOI] [PubMed] [Google Scholar]
- 73.Liu M, Bedouhene S, Hurtado-Nedelec M, et al. The Prolyl Isomerase Pin1 Controls Lipopolysaccharide-Induced Priming of NADPH Oxidase in Human Neutrophils. Front Immunol. 2019;10:2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Onnheim K, Bylund J, Boulay F, Dahlgren C, Forsman H. Tumour necrosis factor (TNF)-alpha primes murine neutrophils when triggered via formyl peptide receptor-related sequence 2, the murine orthologue of human formyl peptide receptor-like 1, through a process involving the type I TNF receptor and subcellular granule mobilization. Immunology. 2008;125(4):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liou YC, Zhou XZ, Lu KP. Prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem Sci. 2011;36(10):501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Condliffe AM, Davidson K, Anderson KE, et al. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood. 2005;106(4):1432–1440. [DOI] [PubMed] [Google Scholar]
- 77.Rieckmann JC, Geiger R, Hornburg D, et al. Social network architecture of human immune cells unveiled by quantitative proteomics. Nat Immunol. 2017;18(5):583–593. [DOI] [PubMed] [Google Scholar]
- 78.Grabowski P, Hesse S, Hollizeck S, et al. Proteome Analysis of Human Neutrophil Granulocytes From Patients With Monogenic Disease Using Data-independent Acquisition. Mol Cell Proteomics. 2019;18(4):760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, Zhao J, Cai C, et al. A Label-Free Quantitative Proteomic Analysis of Mouse Neutrophil Extracellular Trap Formation Induced by Streptococcus suis or Phorbol Myristate Acetate (PMA). Front Immunol. 2018;9:2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdel Malik R, Zippel N, Frömel T, et al. AMP-Activated Protein Kinase α2 in Neutrophils Regulates Vascular Repair via Hypoxia-Inducible Factor-1α and a Network of Proteins Affecting Metabolism and Apoptosis. Circ Res. 2017;120(1):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol Rev. 2016;273(1):11–28. [DOI] [PubMed] [Google Scholar]
- 82.Winstel V, Schneewind O, Missiakas D. Staphylococcus aureus Exploits the Host Apoptotic Pathway To Persist during Infection. mBio. 2019;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gabay JE, Almeida RP. Antibiotic peptides and serine protease homologs in human polymorphonuclear leukocytes: defensins and azurocidin. Curr Opin Immunol. 1993;5(1):97–102. [DOI] [PubMed] [Google Scholar]
- 84.Ganz T, Lehrer RI. Antimicrobial peptides of leukocytes. Curr Opin Hematol. 1997;4:53–58. [DOI] [PubMed] [Google Scholar]
- 85.Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opin Immunol. 2002;14(1):96–102. [DOI] [PubMed] [Google Scholar]
- 86.Xu D, Lu W. Defensins: A Double-Edged Sword in Host Immunity. Front Immunol. 2020;11:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang D, Chertov O, Oppenheim JJ. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37). J Leukoc Biol. 2001;69(5):691–697. [PubMed] [Google Scholar]
- 88.Bdeir K, Higazi AA, Kulikovskaya I, et al. Neutrophil alpha-defensins cause lung injury by disrupting the capillary-epithelial barrier. Am J Respir Crit Care Med. 2010;181(9):935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soehnlein O, Lindbom L. Neutrophil-derived azurocidin alarms the immune system. J Leukoc Biol. 2009;85(3):344–351. [DOI] [PubMed] [Google Scholar]
- 90.Weiss J Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defense against gram-negative bacteria. Biochem Soc. 2003;31:785–790. [DOI] [PubMed] [Google Scholar]
- 91.Gioannini TL, Teghanemt A, Zarember K, Weiss JP. Regulation of endotoxin with host cells. JEndotoxin Research. 2003;9:401–408. [DOI] [PubMed] [Google Scholar]
- 92.Levy O Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. JLeukocBiol. 2004;76:909–925. [DOI] [PubMed] [Google Scholar]
- 93.Schultz H, Weiss J. The bactericidal / permeability-increasing protein (BPI) in infection and inflammatory disease. Clin Chim Acta. 2007;384:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rausch PG, Moore TG. Granule enzymes of polymorphonuclear neutrophils: a phylogenetic comparison. Blood. 1975;46:913–919. [PubMed] [Google Scholar]
- 95.Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. JLeukBiol. 2013;93:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nauseef WM. Myeloperoxidase in human neutrophil host defence. Cell Microbiol. 2014;16(8):1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol. 2015;15(10):630–642. [DOI] [PubMed] [Google Scholar]
- 98.Ermert D, Niemiec MJ, Rohm M, Glenthoj A, Borregaard N, Urban CF. Candida albicans escapes from mouse neutrophils. J Leukoc Biol. 2013;94(2):223–236. [DOI] [PubMed] [Google Scholar]
- 99.Cech P, Papathanassiou A, Boreux G, Roth P, Miescher PA. Hereditary myeloperoxidase deficiency. Blood. 1979;53:403–411. [PubMed] [Google Scholar]
- 100.Larrocha C, de Castro MF, Fontan G, Vitoria A, Fernandez-Chacon JL, Jimenez C. Hereditary myeloperoxidase deficiency: study of 12 cases. Scand J Haematol. 1982;29:389–397. [DOI] [PubMed] [Google Scholar]
- 101.Lehrer RI, Cline MJ. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. JClinInvest. 1969;48:1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moosmann K, Bojanowsky A. Rezidivierende candidiosis bei myeloperoxydasemangel. MonatsschrKinderheilkd. 1975;123:407–409. [PubMed] [Google Scholar]
- 103.Parry MF, Root RK, Metcalf JA, Delaney KK, Kaplow LS, Richar NJ. Myeloperoxidase deficiency: prevalence and clinical significance. AnnInternMed. 1981;95:293–301. [DOI] [PubMed] [Google Scholar]
- 104.Diamond RD, Clark RA, Haudenschild CC. Damage to Candida albicans hyphae and pseudohyphae by the myeloperoxidase system and oxidative products of neutrophil metabolism in vitro. JClinInvest. 1980;66:908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cech P, Stalder H, Widmann JJ, Rohner A, Miescher PA. Leukocyte myeloperoxidase deficiency and diabetes mellitus associated with Candida albicans liver abscess. Am J Med. 1979;66(1):149–153. [DOI] [PubMed] [Google Scholar]
- 106.Machata S, Sreekantapuram S, Hünniger K, et al. Significant Differences in Host-Pathogen Interactions Between Murine and Human Whole Blood. Front Immunol. 2020;11:565869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bazzoni F, Cassatella MA, Rossi F, Ceska M, Dewald B, Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991;173:771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bazzoni F, Cassatella MA, Laudanna C, Rossi F. Phagocytosis of opsonized yeast induces tumor necrosis factor-α mRNA accumulation and protein release by human polymorphonuclear leukocytes. J Leukoc Biol. 1991;50:223–228. [DOI] [PubMed] [Google Scholar]
- 109.Conlon BP, Rowe SE, Gandt AB, et al. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nature microbiology. 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tamassia N, Cassatella MA, Bazzoni F. Fast and Accurate Quantitative Analysis of Cytokine Gene Expression in Human Neutrophils by Reverse Transcription Real-Time PCR. Methods Mol Biol. 2020;2087:243–260. [DOI] [PubMed] [Google Scholar]
- 111.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cassatella MA. On the production of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2L) by human neutrophils. JLeukBiol. 2006;79:1140–1149. [DOI] [PubMed] [Google Scholar]
- 113.Simons MP, Leidal KG, Nauseef WM, Griffith TS. TNF-related apoptosis-inducing ligand (TRAIL) is expressed throughout myeloid development, resulting in a broad distribution among neutrophil granules. JLeukocBiol. 2008;83(3):621–629. [DOI] [PubMed] [Google Scholar]
- 114.Scapini P, Calzetti F, Cassatella MA. On the detection of neutrophil-derived vascular endothelial growth factor (VEGF). J Immunol Methods. 1999;232(1-2):121–129. [DOI] [PubMed] [Google Scholar]
- 115.Tamassia N, Cassatella MA, Bazzoni F. Fast and Accurate Quantitative Analysis of Cytokine Gene Expression in Human Neutrophils by Reverse Transcription Real-Time PCR. In: Quinn MT, DeLeo FR, eds. Neutrophil Methods and Protocols. Vol 2087. Third ed. New York, NY: Humana Press; 2020:243–261. [DOI] [PubMed] [Google Scholar]
- 116.Tamassia N, Bianchetto-Aguilera F, Arruda-Silva F, et al. Cytokine production by human neutrophils: Revisiting the "dark side of the moon". Eur J Clin Invest. 2018;48 Suppl2:e12952. [DOI] [PubMed] [Google Scholar]
- 117.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol. 2012;30:271–294. [DOI] [PubMed] [Google Scholar]
- 119.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8(11):889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Råberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318(5851):812–814. [DOI] [PubMed] [Google Scholar]
- 121.Warren HS, Fitting C, Hoff E, et al. Resilience to bacterial infection: difference between species could be due to proteins in serum. J Infect Dis. 2010;201(2):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Norris PAA, Kaur G, Lazarus AH. New insights into IVIg mechanisms and alternatives in autoimmune and inflammatory diseases. Curr Opin Hematol. 2020;27(6):392–398. [DOI] [PubMed] [Google Scholar]
- 123.Schaub A, von Gunten S, Vogel M, et al. Dimeric IVIG contains natural anti-Siglec-9 autoantibodies and their anti-idiotypes. Allergy. 2011;66(8):1030–1037. [DOI] [PubMed] [Google Scholar]
- 124.von Gunten S, Schaub A, Vogel M, Stadler BM, Miescher S, Simon HU. Immunologic and functional evidence for anti-Siglec-9 autoantibodies in intravenous immunoglobulin preparations. Blood. 2006;108(13):4255–4259. [DOI] [PubMed] [Google Scholar]
- 125.Casulli S, Topçu S, Fattoum L, et al. A differential concentration-dependent effect of IVIg on neutrophil functions: relevance for anti-microbial and anti-inflammatory mechanisms. PLoS One. 2011;6(10):e26469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Altznauer F, von Gunten S, Späth P, Simon HU. Concurrent presence of agonistic and antagonistic anti-CD95 autoantibodies in intravenous Ig preparations. J Allergy Clin Immunol. 2003;112(6):1185–1190. [DOI] [PubMed] [Google Scholar]
- 127.Schneider C, Wicki S, Graeter S, et al. IVIG regulates the survival of human but not mouse neutrophils. Sci Rep. 2017;7(1):1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bonne-Année S, Kerepesi LA, Hess JA, et al. Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microbes and infection / Institut Pasteur. 2014;16(6):502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Griener TP, Mulvey GL, Marcato P, Armstrong GD. Differential binding of Shiga toxin 2 to human and murine neutrophils. J Med Microbiol. 2007;56(Pt 11):1423–1430. [DOI] [PubMed] [Google Scholar]
- 130.Linnemann LC, Schaible UE, Dallenga TK. Evaluation of Myeloperoxidase as Target for Host-Directed Therapy in Tuberculosis In Vivo. International journal of molecular sciences. 2022;23(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davies MJ, Hawkins CL. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid Redox Signal. 2020;32(13):957–981. [DOI] [PubMed] [Google Scholar]
- 132.Brennan ML, Anderson MM, Shih DM, et al. Increased atherosclerosis in myeloperoxidase-deficient mice. JClinInvest. 2001;107(4):419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nauseef WM. The proper study of mankind. JClinInvest. 2001;107(4):401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eruslanov EB, Singhal S, Albelda SM. Mouse versus Human Neutrophils in Cancer: A Major Knowledge Gap. Trends in cancer. 2017;3(2):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. [DOI] [PubMed] [Google Scholar]
- 136.Quail DF, Amulic B, Aziz M, et al. Neutrophil phenotypes and functions in cancer: A consensus statement. J Exp Med. 2022;219(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ozel I, Duerig I, Domnich M, Lang S, Pylaeva E, Jablonska J. The Good, the Bad, and the Ugly: Neutrophils, Angiogenesis, and Cancer. Cancers (Basel). 2022;14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bui TM, Yalom LK, Sumagin R. Tumor-associated neutrophils: orchestrating cancer pathobiology and therapeutic resistance. Expert Opin Ther Targets. 2021;25(7):573–583. [DOI] [PubMed] [Google Scholar]
- 139.Que H, Fu Q, Lan T, Tian X, Wei X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim Biophys Acta Rev Cancer. 2022;1877(5):188762. [DOI] [PubMed] [Google Scholar]
- 140.Woolhouse ME, Webster JP, Domingo E, Charlesworth B, Levin BR. Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nat Genet. 2002;32(4):569–577. [DOI] [PubMed] [Google Scholar]
- 141.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu T, Porter AR, Kennedy AD, Kobayashi SD, Deleo FR. Phagocytosis and Killing of Staphylococcus aureus by Human Neutrophils. J Innate Immun. 2014;6(5):639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dinauer MC. Neutrophil Defects and Diagnosis Disorders of Neutrophil Function: An Overview. Methods Mol Biol. 2020;2087:11–29. [DOI] [PubMed] [Google Scholar]
- 144.Marciano BE, Spalding C, Fitzgerald A, et al. Common severe infections in chronic granulomatous disease. Clin Infect Dis. 2015;60(8):1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Albrich JM, Hurst JK. Oxidative inactivation of Escherichia coli by hypochlorous acid. Rates and differentiation of respiratory from other reaction sites. FEBS Lett. 1982;144:157–161. [DOI] [PubMed] [Google Scholar]
- 146.Greenlee-Wacker M, DeLeo FR, Nauseef WM. How methicillin-resistant Staphylococcus aureus evade neutrophil killing. Curr Opin Hematol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Holtfreter S, Radcliff FJ, Grumann D, et al. Characterization of a mouse-adapted Staphylococcus aureus strain. PLoS One. 2013;8(9):e71142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nature reviews Microbiology. 2015;13(9):529–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.de Haas CJC, Veldkamp KE, Peschel A, et al. Chemotaxis inhibitory protein of Staphylococcus aureus , a bacterial antiinflammatory agent. J Exp Med. 2005;199:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rooijakkers SHM, Ruyken M, Roos A, et al. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol. 2005;6(9):920–927. [DOI] [PubMed] [Google Scholar]
- 151.Jongerius I, Köhl J, Pandey MK, et al. Staphylococcal complement evasion by various convertase-blocking molecules. J Exp Med. 2007;204(10):2461–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Spaan AN, Henry T, van Rooijen WJ, et al. The staphylococcal toxin panton-valentine leukocidin targets human c5a receptors. Cell Host Microbe. 2013;13(5):584–594. [DOI] [PubMed] [Google Scholar]
- 153.Spaan AN, van Strijp JAG, Torres VJ. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nature reviews Microbiology. 2017;15(7):435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fromageau A, Gilbert FB, Prévost G, Rainard P. Binding of the Staphylococcus aureus leucotoxin LukM to its leucocyte targets. Microb Pathog. 2010;49(6):354–362. [DOI] [PubMed] [Google Scholar]
- 155.Spaan AN, Schiepers A, de Haas CJ, et al. Differential Interaction of the Staphylococcal Toxins Panton-Valentine Leukocidin and γ-Hemolysin CB with Human C5a Receptors. J Immunol. 2015;195(3):1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Allison M, Mishra N, Geisbrecht BV. Structure, function, and mechanistic insights into a novel family of myeloperoxidase inhibitory proteins expressed by staphylococci. In: Hawkins CL, Nauseef WM, eds. Mammalian Heme Peroxidases. Boca Raton, FL: CRC Press; 2022:307–321. [Google Scholar]
- 157.de Jong NWM, Ramyar KX, Guerra FE, et al. Immune evasion by a staphylococcal inhibitor of myeloperoxidase. Proc Natl Acad Sci U S A. 2017;114(35):9439–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.de Jong NWM, Ploscariu NT, Ramyar KX, et al. A structurally dynamic N-terminal region drives function of the staphylococcal peroxidase inhibitor (SPIN). J Biol Chem. 2018;293(7):2260–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rosen H Editorial: of mice and men--yet again. J Leukoc Biol. 2013;94(2):210–212. [DOI] [PubMed] [Google Scholar]
- 160.Davis MM. A prescription for human immunology. Immunity. 2008;29:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Medetgul-Ernar K, Davis MM. Standing on the shoulders of mice. Immunity. 2022;55(8):1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Davies MJ. Reactivity of peroxidase-derived oxidants with proteins, glycoproteins, and proteoglycans. In: Hawkins CL, Nauseef WM, eds. Mammalian Heme Peroxidases. Boca Raton, FL: CRC Press; 2022:53–78. [Google Scholar]
- 163.Hawkins CL. Reactivity of hypochlorous acid (HOCl) with nucleic acids, RNA, and DNA. In: Hawkins CL, Nauseef WM, eds. Mammalian Heme Peroxidases. Boca Raton, FL: CRC Press; 2022:79–94. [Google Scholar]
- 164.Pike DP, Ford DA. Reactivity of peroxidase oxidants with lipids: the generation of biologically important modified lipids. In: Hawkins CL, Nauseef WM, eds. Mammalian Heme Peroxidases. Boca Raton: CRC Press; 2022:95–114. [Google Scholar]
- 165.Hallberg LAE, Thorsen NW, Hartsema EA, Hägglund PM, Hawkins CL. Mapping the modification of histones by the myeloperoxidase-derived oxidant hypochlorous acid (HOCl). Free Radic Biol Med. 2022. [DOI] [PubMed] [Google Scholar]
- 166.Shearer HL, Hampton MB, Dickerhof N. Bactericidal Activity of the Oxidants Derived from Mammalian Heme Peroxidases. In: Hawkins CL, Nauseef WM, eds. Mammalian Heme Peroxidases. Boca Raton, London, New York: CRC Press; 2022:171–188. [Google Scholar]


