Summary
Protein phosphorylation is a fundamental element of cell signaling. First discovered as a biochemical switch in glycogenolysis, we now know that this posttranslational modification permeates all aspects of cellular behavior. In humans, over 540 protein kinases attach phosphate to acceptor amino acids, whereas around 160 phosphoprotein phosphatases remove phosphate to terminate signaling. Aberrant phosphorylation underlies disease, and kinase inhibitor drugs are increasingly used clinically as targeted therapies. Specificity in protein phosphorylation is achieved in part because kinases and phosphatases are spatially organized inside cells. A prototypic example is compartmentalization of the cAMP dependent protein kinase A through association with A-kinase anchoring proteins. This configuration creates autonomous signaling islands where the anchored kinase is constrained in proximity to activators, effectors, and selected substates. This article primarily focuses on AKAP signaling in the heart with an emphasis on anchoring proteins that spatiotemporally coordinate excitation-contraction coupling and hypertrophic responses.
Historical Overview
Seminal Discoveries
Phosphorylation is a posttranslational modification that is a cornerstone of many intracellular signaling cascades. This simple chemical cue has evolved into a sophisticated signaling paradigm that controls a myriad of physiological processes. This reaction occurs when the γ-phosphate group of adenosine 5’-triphosphate (ATP) is covalently linked to serine, threonine, tyrosine or histidine side chains1. The forward reaction, carried out by protein kinases, introduces negative charge, or changes the shape of the modified sidechain to alter the biochemical properties of the substrate protein2,3. The reverse reaction is catalyzed by phosphoprotein phosphatases that remove phosphate groups from substrates3. Although protein phosphorylation was first reported in the 1930’s, we’re only now beginning to grasp the full extent to which this covalent modification governs the biology of cells and underlies disease. Hence, pharmacological control of protein phosphorylation is a major therapeutic objective with 76 kinase inhibitor drugs currently approved for use and many more in clinical trials4.
Carl and Gerty Cori laid the groundwork for the study of phosphorylation through their pioneering research into the process of glycogen metabolism undertaken at the University of Washington St. Louis5. They discovered an intermediate compound that assisted in the breakdown of glycogen called glucose-1-phosphate, and identified glycogen phosphorylase as the rate limiting enzyme in glycogen production. They received the Nobel prize for Physiology in 1947 for this discovery.
The next breakthrough was the discovery of cyclic nucleotides as intracellular second messengers of hormone action. Sutherland and Rall, working at Vanderbilt University in 1957, showed that phosphorylase kinase was activated in a cyclic adenosine 3’,5’-monophosphate (cAMP) dependent manner6,7. They showed that the primary messengers epinephrine and glucagon signaled the formation of cAMP by adenylyl cyclase (AC), which led to the activation of phosphorylase kinase6,8. This work won the Nobel Prize in 1971.
Following up on earlier work into glycogen metabolism, biochemists Edmond H. Fischer and Edwin G. Krebs at the University of Washington would expand the study of glycogen phosphorylase. This enzyme exists in active and inactive forms termed phosphorylase a and b respectively. Fischer and Krebs demonstrated that the transfer of a phosphoryl group donated by Mg-ATP was necessary for the phosphorylase b to a transition9,10. We now know that phosphorylation of Ser14 converts dormant phosphorylase b into active phosphorylase a. This phosphoryl group is removed by an enzyme which came to be known as phosphoprotein phosphatase 110. Hence Fischer and Krebs became the first to define the reversible nature of protein phosphorylation. This was followed by the discovery of cAMP dependent protein kinase (also called protein kinase A or PKA) by Krebs, Walsh, and Perkins in 196811. Fischer and Krebs were awarded the Nobel Prize in 1992 for their discovery of protein regulation by reversible phosphorylation12.
The utility of phosphorylation as a commonly used signaling mechanism gained further prominence in 1979 when Tony Hunter and colleagues at the Salk institute revealed phosphorylation on tyrosine while studying the Rous Sarcoma virus13,14. Tony Pawson in Toronto and Joseph Schlessinger, now at Yale, independently defined phosphotyrosine as a recognition motif for Src homology 2 (SH2) domains in the assembly of macromolecular signaling complexes15,16. From then onward, the study of protein phosphorylation has been inextricably linked to research advances related to cancer, diabetes, and the cardiovascular system. A timeline of key developments in our understanding of PKA phosphorylation is presented in figure 1.
Figure 1: Timeline of key discoveries pertaining to cAMP responsive phosphorylation.
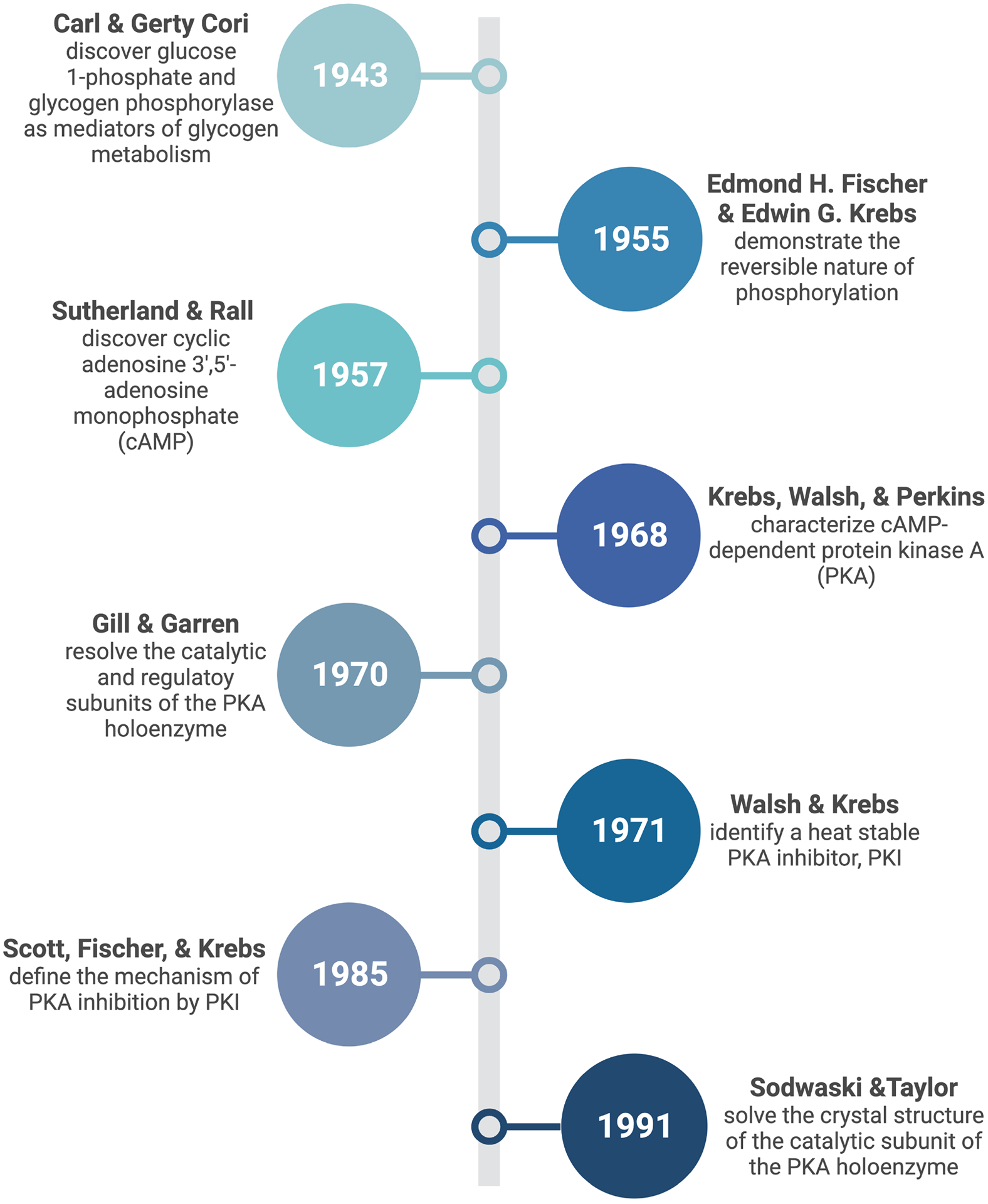
(Figure created in Biorender)
cAMP Dependent Protein Kinase (PKA)
The utility of protein phosphorylation as an adaptable form of cellular regulation is underscored by evidence that 1–2% of most genomes encode protein kinases. This includes over 540 genes in humans17–19. Protein kinase A signaling is often hailed as an archetype to understand the action of eukaryotic protein kinases. Yet, this enzyme is atypical in many respects20. Of the eukaryotic protein kinases, only PKA and casein kinases exist as tetrameric holoenzymes whose catalytic activity is autoinhibited by a separate family of regulatory subunits20. When steroids, neurotransmitters, or hormones engage G-protein coupled receptors (GPCRs), they mobilize adenylyl cyclases to promote a rapid rise in intracellular levels of cAMP21. Importantly, activation of PKA is dependent upon cAMP binding rather than autophosphorylation within the active site of the enzyme22,23. PKA acts a master regulator of diverse cellular processes including (but not limited to) glycogenolysis, transcription, proliferation, hormone synthesis and release, and excitation-contraction coupling20. Moreover, the specificity and spatiotemporal resolution of PKA action is determined by a family of A Kinase Anchoring Proteins (AKAPs) that sequester this key enzyme with preferred substrates24–26
PKA exists as a holoenzyme composed of a dimer of regulatory subunits (R) that bind and constrain catalytic subunits (C)27. In 1970, Gill and Garren were the first to resolve the regulatory and catalytic components of the holoenzyme using bovine adrenal cortical PKA28. At the same time, Tao, Salas and Lipmann used sucrose density gradients to fractionate rabbit reticulocyte PKA holoenzymes into catalytic and regulatory subunits29. Elegant follow-up studies by Susan Taylor, Jackie Corbin and Edwin G. Krebs showed that the R subunits existed in two forms (I and II), and that each protomer was capable of binding two molecules of cAMP30,31–33. We now know that binding of cAMP to regulatory subunits relieves autoinhibitory control over the catalytic subunits; thereby activating the intact holoenzyme34–36. Around the same time Walsh and Krebs uncovered a heat stable protein inhibitor of PKA catalytic activity isolated from rabbit skeletal muscle, termed PKI37. Kinetic studies of PKI and peptide analogues by Scott, Fischer and Krebs defined the mechanism of kinase inhibition38–40. Concurrent studies by Kemp, Walsh and colleagues came to the same conclusions41. PKI peptides proved to be a valuable tool utilized by Sodwaski and Taylor to lock the PKA catalytic subunit into a conformation that allowed it’s successful crystallization42 (Figure 1). The significance of this latter work cannot be understated, as it revealed the bilobal fold that is a recognizable hallmark of all protein kinases43,44.
Several laboratories have amassed considerable evidence that cAMP signaling events are much more spatially controlled than originally considered. Recent work on the boundaries of cAMP nanodomains shows that discrete and autonomous pockets of cAMP are generated upon the mobilization of G-protein coupled receptors45–47. Discerning the structure of AKAP signaling islands provides a molecular basis for how PKA is constrained within second messenger signaling compartments34,35. These studies show that the anchored PKA holoenzyme is maintained within 200–400 angstroms of selected substrates25. An implication of this revised view of cAMP signaling is that local phosphorylation events often occur within the narrow confines of AKAP signaling islands. Consequently, mutations that alter AKAP function or impact the localization of PKA underlie disease48,49.
A Kinase Anchoring Proteins (AKAPs)
As the utility of cAMP signaling became more apparent, investigators realized that molecular mechanisms must exist to simultaneously coordinate multiple phosphorylation events inside cells. This led to the notion that PKA was compartmentalized50. Three independent observations set the stage for the discovery of AKAPs: PKA was reported to be attached to microtubules via its regulatory subunits51, a calmodulin binding protein was identified as a contaminant with purified R subunits52, and RII-binding proteins were identified by far western blots using phosphorylated RII as the bait53. This technique is commonly referred to as an RII overlay54. Still, a systematic interrogation of localized PKA action was not possible until resolution of the PKA-AKAP binding interface27,55,56 (Figure 2A). Deletion screens of RII carried out by Scott in 1990 identified the first 79 amino acids of RII as necessary and sufficient for binding to microtubule associated protein 2 (MAP2)56. Moreover, removal of the first 14 amino acids of RII not only abolished dimerization, but also prevented interaction with MAP256. By the late 1990’s the structure of the AKAP binding interface with RII was solved. This is now known as the docking and dimerization domain57,58. A hydrophobic cleft is formed by dimerization of the R subunits which fold into an antiparallel x-type helical bundle27. This motif is a common structural feature of all PKA regulatory subunits, as well as a newly identified family of 16 AKAP-interacting factors called R1D2 and R2D2 proteins59–62 (Figure 2A).
Figure 2: AKAPs localize PKA to a variety of intracellular locations.
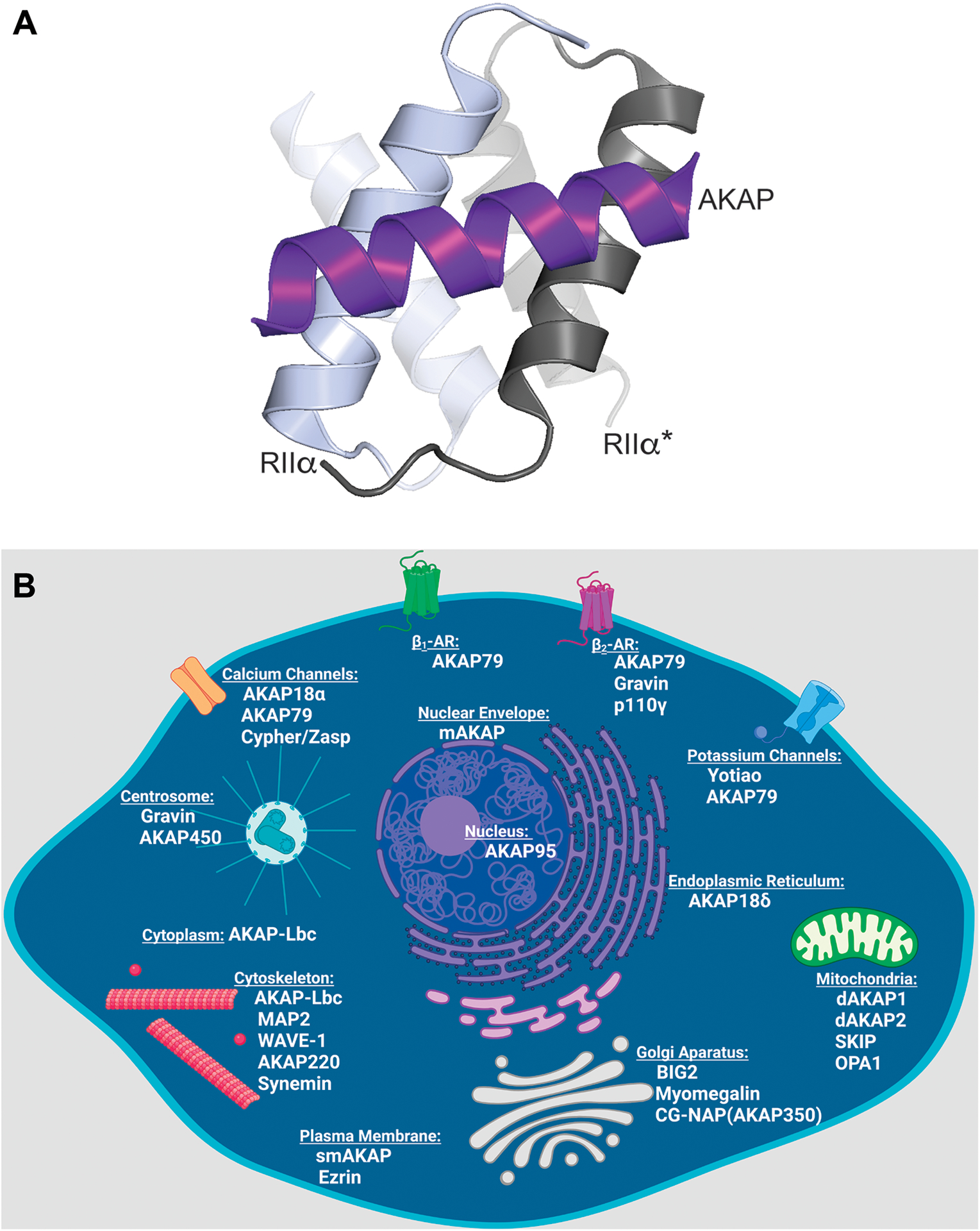
A) Structural model of the AKAP amphipathic helix (purple) in complex with the docking and dimerization domain of RIIα. Both protomers of the RII dimer are depicted in shades of grey. Model made using PYMOL 2.5 and structures from the protein database with accession number 2izx. B) Schematic of a cell indicating the locations of compartment-specific AKAPs (schematic created in Biorender).
Reciprocal studies carried out by Scott and colleagues defined PKA binding motifs on AKAPs. Using human thyroid protein clone 31 (Ht31, now called AKAP-Lbc) as a model, mutational analysis revealed a 14–18 amino acid sequence that was both essential for RII binding and predicted to form an amphipathic α-helix63. Site-directed mutagenesis of Ht31 aimed at disrupting its secondary structure confirmed that AKAPs bound RII via an amphipathic α-helix27,54,63. Cell soluble, stapled, and chemically stabilized peptide disruptors of AKAP/RII binding have subsequently been developed based on this RII binding sequence61,64–67. These reagents and small molecule inhibitors have become valuable tools for investigating anchored PKA function68–72. Acquiring this fundamental understanding of the AKAP/PKA binding interface has provided a molecular toolkit to study all AKAPs and primed the field for significant advancement (Figure 2A).
One angstrom resolution crystal structures of the RIIα docking and dimerization (DD) domain in complex with AKAP peptides add molecular detail to this protein-protein interface27,59,73. Two isoforms exist of each regulatory subunit (RIα, RIβ, RIIα, and RIIβ). The topology of the DD domain varies depending on the isoform of the R subunit present (Figure 2A). The depth of the cleft affects the number of points of contact with the AKAP α-helix. Consequently, AKAPs exhibit different binding affinities for each subtype of PKA, with the majority preferentially binding type II (so named for the R subunit)24,27,59,73. Additionally, RI selective, and dual specificity AKAPs have been defined74–80. Through these high affinity protein-protein interactions, PKA holoenzymes are sequestered at defined locations within cells (Figure 2B). To date, sixty human genes encoding over 150 isoforms have been classified as AKAPs25. As depicted in figure 2B, multiple AKAPs can be targeted to the same subcellular compartment. This does not necessarily reflect a redundancy of function, but rather the exquisite degree of spatial organization provided by AKAPs.
AKAP Signaling Islands
Arguably the most important feature of AKAPs is their ability to organize combinations of enzymes into macromolecular signaling complexes81,82 (Figures 3&4). In this way, these anchoring proteins spatially integrate distinct cell signaling pathways and temporally control activation and termination of cAMP signaling cascades. First evidence for the multivalence of AKAPs came from the observation that the calcium/calmodulin dependent phosphatase Calcineurin/PP2B was an interacting partner for the neuronal anchoring protein AKAP7983. Functional evidence in hippocampal neurons demonstrated that localization of PKA with PP2B by AKAP79 regulated aspects of synaptic transmission84,85. Subsequent studies on the same signaling complex identified protein kinase C (PKC) as an additional binding partner86. Biochemical, genetic, and functional studies have gone on to show how different combinations of AKAP79-anchored enzymes modulate transmembrane receptors and ion channels in neuronal, endocrine and muscle tissues84,87–91.
Figure 3: AKAPs coordinate excitation-contraction coupling.
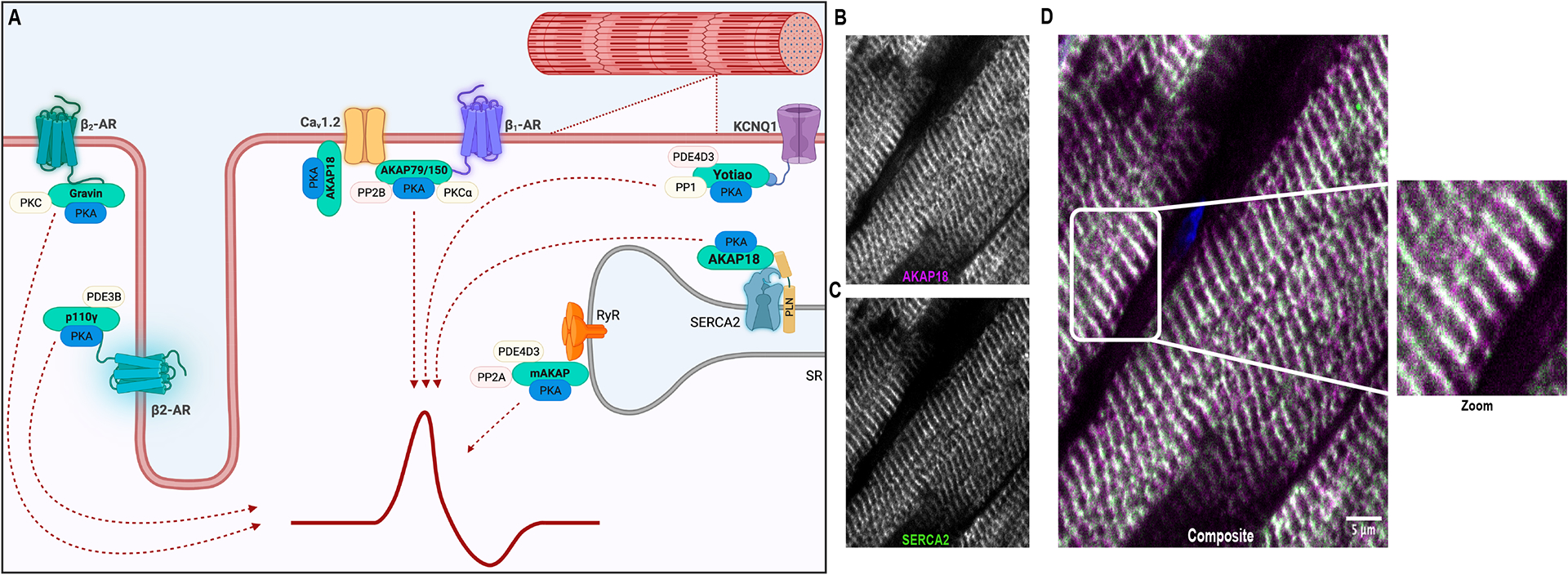
A) Schematic (created in Biorender) depicting AKAP regulation of channel activity, ion flow, contraction, and relaxation of cells. B-D) Confocal images depicting the localization of AKAP18 with SERCA2 ATPase at the Z bands (see zoom panel) within normal human heart (paraffin embedded tissue sections).
Figure 4: AKAPs mediate the heart’s hypertrophic response.
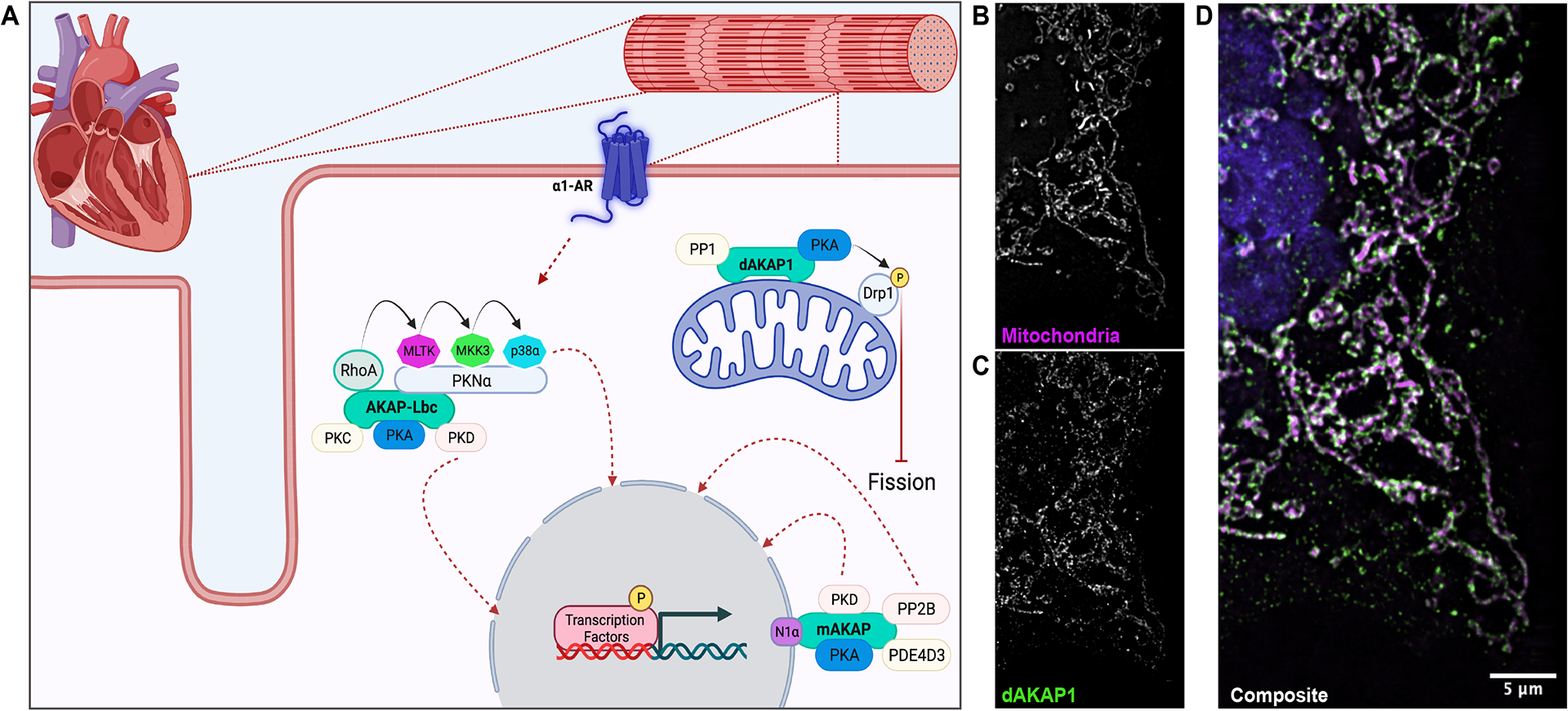
A) Schematic (created in Biorender) depicting AKAP regulation of hypertrophic gene expression, reactive oxygen species generation, and mitochondrial morphology. B-D) Three-dimensional structured illumination microscopy shows the localization of dAKAP1 to the mitochondria of U2OS cells.
Additional interactions with AKAP79 preferentially place PKA in proximity to adenylyl cyclase 5, the enzyme that generates intracellular cAMP21 92–94. This configuration creates an internally constrained negative feedback loop as anchored PKA phosphorylation of adenylyl cyclase 5 inhibits cAMP synthesis. Elegant work by Carmen Dessauer and colleagues has defined AKAP-AC interactions in various cellular systems94–96. AKAPs also associate with GPCRs, as indicated by initial work focused on anchored regulation of β-adrenergic signaling97–99. More recently it has been shown that PKA can anchor to other GPCR’s. For example, Stefan and colleagues report that PKA is directly anchored to orphan receptor Gpr161 in primary cilia100.
Spatiotemporal control of cAMP signal termination is achieved by AKAP association with phosphodiesterases (PDEs). The was first reported in 2001 when it was demonstrated that muscle-selective mAKAP, maintains a cAMP signaling module, including PKA and the cAMP-specific phosphodiesterase (PDE4D3) in the heart101–104. Around the same time, it was noted that AKAP450 constrains PKA and PDE4 at the centrosomes in Sertoli cells105,106. Importantly the long isoforms of PDE4’s are substrates for anchored PKA, and phosphorylation enhances cAMP degradation103,107. These reports suggest that proximity of PKA and PDE’s in AKAP signaling islands sustain a negative feedback loop to restore basal cAMP levels24. Further support for tight spatial control of cAMP signaling was provided when it was shown that mAKAP anchoring of PDE4D3 not only reduces local cAMP concentrations, but also provides spatial control over the cAMP responsive guanine nucleotide exchange factor EPAC-1102,108. Thus, AKAP signaling complexes participate in all aspects of cAMP signaling from the initiation of second messenger responses, to the termination cAMP signals, and bi-directional control of phosphorylation events.
Tissue Specific AKAP Regulation of Physiological Function
AKAPs in the Reproductive and Central Nervous Systems
Upwards of 150 tissue specific AKAP isoforms are transcribed from the human genome24. AKAPs are particularly prevalent in male and female germ cells, where they contribute to sperm development and motility as well as maturation of the female genital tract109. The distinctive morphology of sperm and their specialized intracellular environment favor the local activation of anchored cAMP signaling elements. AKAP110, AKAP220 and sAKAP84 are linked to sperm motility via the clustering of phosphodiesterases alongside PKA110,111. Local cAMP production in sperm is mediated by a soluble adenylyl cyclase called sAC or AC10 to create local nanodomains of second messenger112. Consideration of the aforementioned observations may help to explain the abundance of AKAPs expressed in male germ cells. In the female reproductive system, D-AKAP1 has been linked to oocyte maturation as evidenced by infertility in female knockout mice. In addition to the regulation of germ cell development, the anchoring protein Gravin is required for proper gastrulation in zebra fish113. This AKAP is thought to mediate axis elongation via inhibition of the Rho/ROCK pathway113.
One of the first neuronal AKAPs characterized, AKAP79 (or its murine ortholog AKAP150), facilitates synaptic signaling events71. AKAP79 scaffolds calcineurin (PP2B) with PKA and PKC by associating with both the plasma membrane and postsynaptic density proteins. This affects synaptic plasticity on multiple levels, principally by regulating the activity of various ion channels86,84,114 Other AKAPs also contribute to neuronal functionality. For example, WAVE-1 anchors PKA and the tyrosine kinase Abl to the actin cytoskeleton to mediate synaptic remodeling. Analysis of WAVE-1 knockout mice revealed that loss of this signaling complex underlies defects in axonal guidance, sensory motor function and hippocampal learning and memory formation115–117. As these topics have been extensively reviewed, we have chosen to focus on AKAP signaling in the heart.
AKAPs in the Heart
According to the American Heart Association and National Institutes of Health, cardiovascular diseases in 2020 accounted for nineteen million deaths worldwide. This represents an increase of 18.7% over the previous decade. Hence, the development of molecular therapies for the prevention and treatment of cardiomyopathies is a critical healthcare initiative. The abundance of cardiac AKAPs makes them attractive therapeutic targets. Consequently, a significant body of work has focused on characterizing AKAP signaling in the heart. Cardiac AKAPs identified to date include: AKAP79/150, AKAP18, mAKAP, AKAP-Lbc, dAKAP1, dAKAP2, p110γ, myomegalin, troponin T, AKAP95, AKAP220, gravin, ezrin, synemin, SKIP, BIG2, cypher/zasp, and yotiao. These anchoring proteins coordinate physiological processes including excitation contraction coupling, calcium homeostasis, myogenic tone, contractility, gene transcription, pathological cardiac remodeling and hypertrophy as well as oxidative stress responses24,118
AKAP Regulation of EC Coupling
The recurrent contraction and relaxation of cardiomyocytes requires the synchronized coordination of cAMP and Ca2+ signaling events (Figure 3A). This process, known as excitation-contraction (EC) coupling, occurs when an action potential from the sinoatrial node is transduced into a chemical cue via voltage-gated ion channels localized to introversions of the sarcolemma called the transverse tubules119 (Figure 3B–D). Following membrane depolarization, Ca2+ enters the cell through L-type Ca2+ channels. This stimulates further Ca2+ release from intracellular stores via ryanodine receptors (RyRs) within the membrane of the Sarcoplasmic Reticulum (SR). This transient rise in intracellular Ca2+ triggers contraction of the myofibrils120. The final phase of relaxation is largely dependent upon reuptake of calcium through the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA2)119,121,122 (Figure 3A). EC coupling is a high fidelity process, with one action potential corresponding to one contraction123. Consequently, disruption of signaling events at any level of this tightly controlled cascade (EC uncoupling) can result in cardiac arrhythmias and eventually heart failure.
EC coupling is regulated at many levels by PKA signaling downstream of adrenergic stimulation124. Catecholamines engage β-adrenergic receptors coupled to the stimulatory Gαs pathway. This stimulates adenylyl cyclase to produce cAMP, followed by PKA phosphorylation of substrates125 including L-type calcium channels (primarily Cav1.2)126, phospholamban (PLN)127,128, and ryanodine receptors129. Anchoring of PKA is crucial for maintenance of proper calcium homeostasis and EC coupling. Anchoring proteins, including AKAP79/150, AKAP18, mAKAP, Gravin and Yotiao have been implicated in this process. Each AKAP coordinates distinct elements of this highly choreographed, vital, and repetitive physiological process.
AKAP79/150
Early biochemical and electrophysiological studies demonstrated that AKAP79 localized PKA to L-type calcium channels at the plasma membrane to mediate phosphorylation dependent modulation of ion conductance130,131 (Figure 3A). It has also been shown that PKA inhibition in native cardiomyocytes negatively impacts calcium currents downstream of β-adrenergic stimulation130. Yet, mouse models lacking AKAP5 (the gene encoding AKAP79/150) demonstrate that cardiomyocytes do not respond to β-adrenergic stimulation in the absence of this anchoring protein132. Calcium influx is mediated, in part, by the association of AKAP79 with Cav1.2133. This heteromeric channel is comprised of a transmembrane subunit and variable accessory subunits. AKAP79/150 associates with Cav1.2 via the C-terminus to target kinases and phosphatases capable of modifying the channel134. AKAP5−/−mice display improper trafficking of β-adrenergic receptors, suggesting a role for this AKAP signaling island in receptor resensitization135. Other regulatory events may also participate in modulating Cav1.2 conductance.
AKAP18
AKAP18 is concentrated at the Z bands of cardiomyocytes (Figure 3B–D). Initial studies characterized the association of AKAP18 (also called AKAP15) with cardiac L-type calcium channels136,137. AKAP7, the gene encoding AKAP18, is subject to alternative splicing. The resultant AKAP18 isoforms vary in length, localization, tissue specific distribution, and function138,139. Splice variation results in the formation of at least five major AKAP18 isoforms, two short and three long (AKAP18α, AKAP18β, AKAP18γ, AKAP18δ and AKAP18ε)138,140,141. These isoforms are all expressed in the heart. AKAP18α is targeted to plasma membranes by lipid modification of its N-terminus in the form of myristoylation and palmitoylation136. Once at the membrane, AKAP18α associates with L-type calcium channels via a conserved leucine zipper motif on the C-terminus of the channels142,143 (Figure 3A). Numerous studies have shown that Cav1.2 is phosphorylated by PKA on multiple sites (Ser1700, Thr1704, Ser1928, and Ser1518) and that this modification increases calcium channel conductance and contractile force126,142,144–149. Although anchoring of PKA with Cav1.2 is required for the maintenance of calcium homeostasis, it remains unclear exactly how channel activity is regulated from a molecular standpoint150. For example, PKA phosphorylation of the monomeric G protein Rad has recently been shown to contribute to channel activation151.
As previously mentioned, the long isoforms of AKAP18 lack membrane targeting sequences. AKAP18γ and AKAP18δ contain unique localization sequences which direct them to organelles such as the sarcoplasmic reticulum (Figure 3A). It’s well established that SERCA2 activity is mediated by its association with a small transmembrane protein called phospholamban. Depending on its phosphorylation state, PLN modifies the activity of SERCA2. Unphosphorylated PLN binds SERCA2 and inhibits calcium transport activity. Phosphorylated PLN releases its inhibitory hold on SERCA2, thus allowing for calcium reuptake into the SR and cardiomyocyte relaxation. Previous studies have shown that AKAP18δ scaffolds PKA with SERCA2 and PLN152. PKA is thus positioned to phosphorylate PLN, facilitating SERCA2 function, cardiomyocyte relaxation and maintenance of excitation-contraction coupling152 (Figures 3B–D).
The localized activity of other kinases and phosphatases also participate in maintaining EC coupling. For example, calcium/calmodulin-dependent protein kinase II (CAMKII) has been linked to the regulation of calcium homeostasis. As described above, adrenergic stimulation elicits cellular calcium entry through L-type calcium channels. This influx triggers further release of calcium from intracellular stores within the sarcoplasmic reticulum into the cytosol via ryanodine receptors. Recent work has demonstrated an additional role for AKAP18δ in scaffolding CAMKII to ryanodine receptors153. Researchers have employed the use of peptide arrays, surface plasmon resonance, structural modeling, and advanced microscopy to show that different regions of AKAP18δ differentially regulate CAMKII activity. The N-terminal region of AKAP18δ binds and inhibits activation of CAMKII. Paradoxically, binding at the C-terminal region of AKAP18δ enhances CAMKII activation and promotes PLN phosphorylation. The net effect being faster Ca2+ release by RyRs and reuptake into the SR153,154. This represents a more nuanced AKAP-mediated mechanism of regulating calcium homeostasis.
Gravin and Yotiao
Gravin scaffolds protein kinase A and protein kinase C with β2-adrenergic receptors (β2-ARs)155,156 (Figure 3A). This product of the AKAP12 gene positively regulates cardiac contractility. Gravin influences positive ionotropic effects by mediating β2-AR desensitization157. Interestingly, contractility is increased with and without β-adrenergic stimulation in mouse models lacking gravin157,158. In fact, the phosphorylation state of PLN and SERCA2 remains unchanged in these animals as compared with wild type controls158. While details of this molecular mechanism need to be clarified, gravin signaling islands are thought to maintain receptor sensitivity.
PKA-mediated phosphorylation of slow acting potassium channels is also important for the maintenance of EC coupling. These outward potassium currents (IKs) help to repolarize the membrane and contribute to myocardial relaxation or lusitropy. Elegant studies have shown that Yotiao scaffolds PKA and protein phosphatase 1 (PP1) with the KCNQ1 subunit of the channel131 (Figure 3A). Interestingly, yotiao itself is phosphorylated on Serine43 by PKA following adrenergic stimulation131. Mutational analysis revealed that substitution of Ser43 with alanine diminished channel conductance but did not alter channel phosphorylation, or the association of Yotiao with KCNQ1. Additionally, allosteric interactions that proceed through AC9 may prime KCNQ1 for phosphorylation by PKA95. Therefore, the yotiao macromolecular complex is allosterically modulating the activity of the potassium channel159.
AKAP coordination of Cardiac Hypertrophy
Cardiac hypertrophy is the heart’s primary adaptive response to pressure or volume stressors, and/or damage to the overall contractile machinery (Figure 4A). Damage to the heart can result from a variety of factors including injury and disease. Hypertrophy describes either the concentric enlargement or elongation of cardiomyocytes as a compensatory mechanism in place of mitotic proliferation. Acute hypertrophic signaling can be beneficial for the heart. For example, the muscle cells in the heart can enlarge in response to exercise in order to compensate for the increased demand for cardiac output160. In contrast, chronic hypertrophic signaling underlies cardiomyopathies such as ischemia and hypertension that eventually lead to heart failure118. This pathological hypertrophy comprises a thickening of the myocardioum, reduction in chamber volume, interstitial fibrosis and reduced cardiac output. These systemic changes are accompanied by alterations in gene expression and cellular metabolism118 (Figure 4A). Adult cardiomyocytes are largely terminally differentiated and are therefore particularly susceptible to pathological hypertophy. Cardiac AKAPs can elicit pro or anti-hypertrophic effects depending upon the composition of binding partners within each signaling island (Figure 4A).
AKAP-Lbc
Multiple signaling elements converge to regulate the heart’s complex hypertrophic response. These include cytokines, MAP kinases, protein phosphatases, adenylyl cyclases, phosphodiesterases and transcription factors. The ability of AKAPs to coordinate these disparate enzymes make them key molecular platforms for the integration and propagation of intracellular signals. Activation of hypertrophic gene regulatory programs results in changes in cellular metabolism and cytoskeletal remodeling associated with disease. AKAP-Lbc signaling islands interface with the myocyte enhancer factor (MEF) pathway that is turned on in response to elevated catecholamines to promote pathological remodeling113,161. In particular, the transcription factor MEF2 is dephosphorylated by calcineurin in complex with AKAP-Lbc, allowing for its translocation to the nucleus and upregulation of hypertrophic gene expression (Figure 4A). AKAP-Lbc is also associated with class II histone deacetylases (HDACs 4 and 5)161. These enzymes repress the transcription of certain genes. AKAP-Lbc positions PKC to phosphorylate PKD, allowing for the latter to be translocated into the nucleus161. Once there, PKD phosphorylates HDACs, eliciting their nuclear export. This anchored signaling event facilitates MEF-mediated activation of gene regulatory pathways associated with pathological hypertrophy113,131,161.
AKAP-Lbc signaling islands mediate other aspects of hypertrophic signaling. This anchoring protein also encodes a guanine nucleotide exchange factor for Rho (Rho GEF), which then induces p38 MAPK signaling downstream of α-adrenergic receptor stimulation in cardiomyocytes to induce hypertrophy162–164 (Figure 4A). AKAP-Lbc therefore acts as an intermediate, linking α-adrenergic signaling to the mobilization of MAP kinase activity. Oxidative stress, a scenario in which the generation of reactive oxygen species (ROS) exceeds the cell’s natural clearing capacity by antioxidant enzymes, has also been linked with the onset of hypertrophy165,166. This imbalance can derive from physical and chemical stressors; and may lead to impaired contractility and apoptosis in addition to hypertrophy118,167. AKAP-Lbc protects against ROS induced damage by several mechanisms. In response to α1-adrenergic stimulation, AKAP-Lbc associated protein kinase D (PKD) phosphorylates and inactivates the phosphatase slingshot 1L (SSH1L). This circumvents the activation of a cofilin2/Bax subcomplex at the mitochondria168. This protects the cell from the increased ROS production that is emblematic of hypertrophy. In this way, AKAP-Lbc elicits a cardioprotective effect. AKAP-Lbc mediated activation of PKD also results in the phosphorylation of CREB which in turn upregulates expression of the anti-apoptotic gene Bcl2118,168. Hence, the pleiotropic effects elicited by AKAP-Lbc signaling make it a complex therapeutic target for the treatment of heart disease.
dAKAP1
Mitochondrial AKAPs affect cellular respiration, ROS production, and survival169,170. For example, the dual specificity anchoring protein dAKAP1 (a product of the AKAP1 gene, also called S-AKAP84, AKAP121 or AKAP149) has been linked to cardiac hypertrophy via its regulation of mitochondrial ROS generation74,171–173. One isoform, AKAP121 acts in a cardioprotective manner by controlling mitochondrial morphology via the phosphorylation of Drp1 (Figure 4A–D). Further support for the cardioprotective role of AKAP121 is provided by evidence that these signaling islands repress nuclear translocation of the nuclear factor of activated T-cells NFATc3 that would otherwise enable hypertrophic gene expression174. In vivo studies consolidate this notion by showing that under conditions mimicking pressure overload, AKAP121 expression is depleted. This results in mitochondrial dysfunction, oxidative stress, and cell death172. Thus, cardiac function at the level of the mitochondria may proceed through dAKAP1/AKAP121 (Figures 4B–D). However, other anchoring proteins may also participate in this process. For example, SKIP, an entirely RI-selective AKAP, is located at the inner mitochondrial membrane where it regulates metabolic processes80. Consequently, local control of the hypertrophic response undoubtedly involves the concerted influence of multiple AKAP signaling islands (Figure 4A).
mAKAP
mAKAP is also associated with hypertrophic gene expression. This anchoring protein, originally called AKAP100, was identified in the original RII overlay screen for AKAPs conducted in the Scott laboratory175. Due to splice variation, there are two main isoforms of mAKAP (mAKAPα and mAKAPβ). Full length mAKAPα is a 255 kDa protein mainly localized to neurons, with mAKAPβ (230 kDa) being the predominant isoform in the heart. mAKAPβ associates with the nuclear envelope in cardiomyocytes through direct binding to an integral membrane protein called nesprin1-α176. Biochemical analyses have identified numerous mAKAP binding partners including PKA177, PDE4D3101, adenylyl cyclases94, PP2A and calcineurin178,179, RyRs129,178,180,181, Rap1 and EPAC1108, and the MAP kinases MEK and ERK108,182 (Figure 4A).
Extensive in vitro and in vivo studies indicate that the primary directive of the mAKAP signaling complex is to alter gene expression coincident with myocyte remodeling. The transcripton factors NFATc and MEF2 are dephosphorylated by mAKAP associated calcineurin. This triggers their translocation to the nucleus and the upregulation of gene expression associated with hypertrophic cardiac remodeling183–185. PKD is also activated in complex with mAKAP, leading to phosphorylation of HDAC4 and the subsequent derepression of hypertophic genes183,186. Interestingly, knockout of mAKAPβ expression has been shown to elicit a cardioprotective effect in mouse models of pressure overload and hypertrophy183. Conversely, it has been shown that mAKAP scaffolding of E3 ubiquitin ligases with hypoxia-inducible factor 1 (HIF-1α) mediates the stability of HIF-1α. In response to hypoxia, HIF-1α is stabilized so that it can translocate to the nucleus and promote the transcription of cell survival genes187. These studies demonstrate that mAKAP provides a multipurpose molecular platform to coordinate enzyme activities necessary for the processing of multiple hypertrophic signals.
Conclusions and Perspectives
From its humble beginnings as a regulatory element of glycogen metabolism, we now recognize that protein phosphorylation is a universal biochemical regulatory mechanism on par with protein ubiquitination, acetylation and proteolysis4. Many pivotal studies on phosphorylation were conducted in the heart, illustrating how this mode of covalent modification controls force generation and contractility. Spatiotemporal control of protein phosphorylation by AKAPs may be particularly important in this context because of the speed and repetition required for EC coupling. Likewise, modulation of adaptive responses such as physiological and pathological cardiac hypertrophy require the relay of signals from the plasma membrane to the transcriptional machinery embedded in the nucleus. Thus, mechanisms that confer local control of protein phosphorylation events have evolved to accommodate the specialized function of cardiomyocytes (Figures 3A & 4A).
Elegant fluorescent spectroscopy studies show that physiological accumulation of cAMP occurs within nanometer-sized domains45–47. This new model is consistent with our own molecular evidence that anchored, intact, and active PKA holoenzymes operate within a 200 to 400-A° radius34,35. This creates discrete and autonomous pockets of activity where anchored PKA phosphorylates preferred substrates; and could explain why multiple AKAPs are located at the SR, plasma membranes, mitochondria, and nuclear envelope in cardiomyocytes. For example, AKAP79, AKAP18α and Cypher/Zasp control phosphorylation of L-type Ca2+ channels to initiate EC coupling. Close by, mAKAP modulates ryanodine receptors to promote further calcium release, while AKAP18γ facilitates calcium reuptake by SERCA2. This highlights the remarkable degree of spatial organization provided by AKAPs that is necessary to ensure processive implementation of distinct phosphorylation events (Figure 3A).
Perhaps not surprisingly, aberrant AKAP signaling can have pathological ramifications. As previously mentioned, depletion of mitochondrial AKAP121 occurs under conditions of pressure overload. Polymorphisms in AKAP18α that abolish PKA anchoring alter regulation of cardiac L-type Ca2+ channels and are linked with increased susceptibility to febrile seizures138. Likewise, polymorphisms in d-AKAP2/AKAP10 are linked to arrythmias and may be related to increased cardiac dysfunction within the aging population188. In another pathological context, Cushing’s syndrome, mutations in PKAc that prevent its incorporation into AKAP signaling islands underlie hypertension, arrythmia and other cardiovascular complications49. Taken together, these examples underscore the exquisite degree of subcellular organization provided by AKAPs. Thus, targeting drugs to AKAPs offers an appealing therapeutic strategy to exploit this sophisticated signaling terrain. Such precision pharmacological regimens would endeavor to minimize off-target effects. While local kinase inhibition is currently effective at tethering modified drug adducts to their sites of action189,190, there is hope that this strategy can be refined to deliver small molecules that allosterically inhibit anchored kinases. In closing, Eddy Fischer and Ed Krebs would be astonished at the advances made in understanding reversible protein phosphorylation. They would be equally enthusiastic and intrigued to find out what the next generation of researchers will discover.
Acknowledgments:
This article is dedicated to the memory of Edmond H Fischer and Edwin G Krebs. One of us (JDS) had the privilege and pleasure to work with Eddy and Ed for several years and is eternally grateful for all the encouragement, insights, and mentorship that they provided. The authors also wish to thank Katherine Forbush and members of the Scott lab for editing and valuable discussions during the preparation of this manuscript. Work was supported by National Institutes of Health (NIH) Grants R01DK119186 (J.D.S.), R01DK119192 (J.D.S.), T32 HL007828 (K.B.C.), and F32 HL160558 (K.B.C.).
Abbreviations
- PKA
protein kinase A
- AKAPs
A-Kinase Anchoring proteins
- ATP
adenosine 5’-triphosphate
- cAMP
cyclic adenosine 3’,5’-monophosphate
- AC
adenylyl cyclase
- SH2
Src homology 2 domain
- GPCR
G protein coupled receptor
- R subunit
PKA regulatory subunit
- C subunit
PKA catalytic subunit
- Ht31
human thyroid protein clone 31
- PKI
heat stable peptide inhibitor of PKA
- DD
docking and dimerization domain of PKA holoenzyme
- PKC
protein kinase C
- PDE
phopsphodiesterase
- SAC
soluble adenylyl cyclase
- PP2B
protein phosphatase 2B, calcineurin
- CaN
calcineurin
- EC coupling
excitation-contraction coupling
- Ca2+
calcium
- RYR
ryanodine receptors
- SR
sarcoplasmic reticulum
- SERCA2
sarcoplasmic/endoplasmic reticulum Ca2+ ATPase
- PLN
phospholamban
- CAMKII
calcium/calmodulin-dependent protein kinase II
- β2–AR
β2-adrenergic receptors
- I Ks
outward potassium currents
- PPI
protein phosphatase 1
- MEF
myocyte enhancer factor
- HDACs 4 and 5
histone deacetylases 4 and 5
- Rho GEF
guanine nucleotide exchange factor for Rho
- ROS
reactive oxygen species
- SSH1L
slingshot 1L
- PKD
protein kinase D
- NFAT
nuclear factor of activated T-cells
- HIF-1a
hypoxia-inducible factor 1a
- MAP2
microtubule associated protein 2
References
- 1.Ubersax JA & Ferrell JE Jr Mechanisms of specificity in protein phosphorylation. Nature Reviews Molecular Cell Biology 8, 530–541, doi: 10.1038/nrm2203 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Ardito F, Giuliani M, Perrone D, Troiano G & Muzio LL The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). International Journal of Molecular Medicine 40, 271–280, doi: 10.3892/ijmm.2017.3036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishi H, Shaytan A & Panchenko AR Physicochemical mechanisms of protein regulation by phosphorylation. Front Genet 5, 270, doi: 10.3389/fgene.2014.00270 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen P, Cross D & Jänne PA Kinase drug discovery 20 years after imatinib: progress and future directions. Nature Reviews Drug Discovery 20, 551–569, doi: 10.1038/s41573-021-00195-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cori GT The Enzymatic Conversion of Phosphorylase b to a Journal of Biological Chemistry, 321–3321943). [Google Scholar]
- 6.Rall TW, Sutherland EW & Berthet J The relationships of epinephrine and glucagon to liver phosphorylase. J. Biol. Chem 224, 463–475 (1957). [PubMed] [Google Scholar]
- 7.Rall TW & Sutherland EW Formation of cyclic adenine ribonucleotide by tissue particles. J. Biol. Chem 232, 1065–1076 (1958). [PubMed] [Google Scholar]
- 8.Sutherland EW Studies on the mechanism of hormone action. Science 171, 401–408 (1972). [DOI] [PubMed] [Google Scholar]
- 9.Fischer EH & Krebs EG Conversion of phosphorylase b to phosphorylase a in muscle extracts. J. Biol. Chem 216, 121–132 (1955). [PubMed] [Google Scholar]
- 10.Krebs EG & Fischer EH The phosphorylase b to a converting enzyme of rabbit skeletal muscle. Biochimica et Biophysica Acta 20, 150–157, doi: 10.1016/0006-3002(56)90273-6 (1956). [DOI] [PubMed] [Google Scholar]
- 11.Walsh DA, Perkins JP & Krebs EG An adenosine 3’,5’-monophosphate-dependent protein kinase from rabbit skeletal muscle. J. Biol. Chem 243, 3763–3765 (1968). [PubMed] [Google Scholar]
- 12.Cohen P Protein kinases--the major drug targets of the twenty-first century? Nat Rev Drug Discov 1, 309–315 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Sefton BM, Hunter T & Beemon K Product of in vitro translation of the Rous sarcoma virus src gene has protein kinase activity. J Virol 30, 311–318, doi: 10.1128/jvi.30.1.311-318.1979 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckhart W, Hutchinson MA & Hunter T An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell 18, 925–933 (1979). [DOI] [PubMed] [Google Scholar]
- 15.Sadowski I, Stone JC & Pawson T A non-catalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps. Mol. Cell. Biol 6, 4396–4408 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemmon MA & Schlessinger J Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134, doi: 10.1016/j.cell.2010.06.011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehti-Shiu MD & Shiu S-H Diversity, classification and function of the plant protein kinase superfamily. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 2619–2639, doi: 10.1098/rstb.2012.0003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manning G, Whyte DB, Martinez R, Hunter T & Sudarsanam S The protein kinase complement of the human genome. Science 298, 1912–1934 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Manning G, Plowman GD, Hunter T & Sudarsanam S Evolution of protein kinase signaling from yeast to man. Trends Biochem. Sci 27, 514–520 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Turnham RE & Scott JD Protein kinase A catalytic subunit isoform PRKACA; History, function and physiology. Gene 577, 101–108, doi: 10.1016/j.gene.2015.11.052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dessauer CW Adenylyl cyclase--A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol. Pharmacol 76, 935–941, doi:mol.109.059345 [pii] 10.1124/mol.109.059345 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbin JD & Keely SL Characterization and regulation of heart adenosine 3’: 5’- monophosphate-dependent protein kinase isozymes. J. Biol. Chem 252, 910–918 (1977). [PubMed] [Google Scholar]
- 23.Potter RL & Taylor SS Correlation of the cAMP binding domain with a site of autophosphorylation on the regulatory subunit of cAMP-dependent protein kinase II from porcine skeletal muscle. J Biol Chem 254, 9000–9005 (1979). [PubMed] [Google Scholar]
- 24.Langeberg LK & Scott JD Signalling scaffolds and local organization of cellular behaviour. Nat Rev Mol Cell Biol 16, 232–244, doi: 10.1038/nrm3966 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omar MH & Scott JD AKAP Signaling Islands: Venues for Precision Pharmacology. Trends in Pharmacological Sciences 41, 933–946, doi: 10.1016/j.tips.2020.09.007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tasken K & Aandahl EM Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev 84, 137–167, doi: 10.1152/physrev.00021.2003 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Gold MG et al. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell 24, 383–395 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Gill GN & Garren LD A cyclic-3′,5′-adenosine monophosphate dependent protein kinase from the adrenal cortex: Comparison with a cyclic AMP binding protein. Biochemical and Biophysical Research Communications 39, 335–343, doi: 10.1016/0006-291x(70)90581-4 (1970). [DOI] [PubMed] [Google Scholar]
- 29.Tao M, Salas ML & Lipmann F Mechanism of activation by adenosine 3’:5’-cyclic monophosphate of a protein phosphokinase from rabbit reticulocytes. Proc Natl Acad Sci U S A 67, 408–414, doi: 10.1073/pnas.67.1.408 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canaves JM & Taylor SS Classification and Phylogenetic Analysis of the cAMP-Dependent Protein Kinase Regulatory Subunit Family. Journal of Molecular Evolution 54, 17–29, doi: 10.1007/s00239-001-0013-1 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Corbin JD et al. Studies on the properties and mode of action of the purified regulatory subunit of bovine heart adenosine 3’:5’-monophosphate-dependent protein kinase. J. Biol. Chem 253, 3997–4003 (1978). [PubMed] [Google Scholar]
- 32.Krebs EG, Blumenthal DK, Edelman AM & Hales CN in Mechanisms of Receptor Regulation (eds Crooke ST & Poste G) 324–367 (Plenum, 1985). [Google Scholar]
- 33.Hofmann F, Beavo JA, Bechtel PJ & Krebs EG Comparison of adenosine 3’:5’-monophosphate-dependent protein kinases from rabbit skeletal and bovine heart muscle. J. Biol. Chem 250, 7795–7801 (1975). [PubMed] [Google Scholar]
- 34.Smith FD et al. Intrinsic disorder within an AKAP-protein kinase A complex guides local substrate phosphorylation. Elife 2, e01319, doi: 10.7554/eLife.01319 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith FD et al. Local protein kinase A action proceeds through intact holoenzymes. Science 356, 1288–1293, doi: 10.1126/science.aaj1669 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Scott JD, McKnight GS & Krebs EG A constitutively active holoenzyme from the cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 88, 2446–2450 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh DA et al. Purification and characterization of a protein inhibitory of adenosine 3’,5’-monophosphate-dependent protein kinases. J. Biol. Chem 246, 1977–1985 (1971). [PubMed] [Google Scholar]
- 38.Scott JD, Fischer EH, DeMaille JG & Krebs EG Identification of an inhibitory region of the heat-stable protein inhibitor of the cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A 82, 4379–4383 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott JD, Fischer EH & Krebs EG The inhibitory region of the heat-stable protein inhibitor of the cAMP-dependent protein kinase. Proc. Natl.Acad. Sci. USA 84, 703–708 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott JD, Glaccum MB, Fischer EH & Krebs EG Primary-structure requirements for inhibition by the heat-stable inhibitor of the cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A 83, 1613–1616 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glass DB, Cheng H-C, Kemp BE & Walsh DA Differential and common recognition sites of the catalytic sites of the cGMP-dependent and cAMP-dependent protein kinases by the inhibitory peptides derived from the heat-stable inhibitor protein. J. Biol. Chem 261, 121611–112171 (1986). [PubMed] [Google Scholar]
- 42.Knighton DR et al. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253, 414–420 (1991). [DOI] [PubMed] [Google Scholar]
- 43.Taylor SS, Ilouz R, Zhang P & Kornev AP Assembly of allosteric macromolecular switches: lessons from PKA. Nat Rev Mol Cell Biol 13, 646–658, doi: 10.1038/nrm3432 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang P, Kornev AP, Wu J & Taylor SS Discovery of Allostery in PKA Signaling. Biophys Rev 7, 227–238, doi: 10.1007/s12551-015-0170-x (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anton SE et al. Receptor-associated independent cAMP nanodomains mediate spatiotemporal specificity of GPCR signaling. Cell 185, 1130–1142 e1111, doi: 10.1016/j.cell.2022.02.011 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Zaccolo M & Pozzan T Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295, 1711–1715. (2002). [DOI] [PubMed] [Google Scholar]
- 47.Bock A et al. Optical Mapping of cAMP Signaling at the Nanometer Scale. Cell 184, 2793, doi: 10.1016/j.cell.2021.04.043 (2021). [DOI] [PubMed] [Google Scholar]
- 48.Turnham RE et al. An acquired scaffolding function of the DNAJ-PKAc fusion contributes to oncogenic signaling in fibrolamellar carcinoma. eLife 8, e44187, doi: 10.7554/eLife.44187 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omar MH et al. Mislocalization of protein kinase A drives pathology in Cushing’s syndrome. Cell Reports 40, 111073–111091, doi: 10.1016/j.celrep.2022.111073 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buxton ILO & Brunton LL Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. JBC 258, 10233–10239 (1983). [PubMed] [Google Scholar]
- 51.Theurkauf WE & Vallee RB Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J. Biol. Chem 257, 3284–3290 (1982). [PubMed] [Google Scholar]
- 52.Sarkar D, Erlichman J & Rubin CS Identification of a calmodulin-binding protein that co-purifies with the regulatory subunit of brain protein kinase II. J. Biol. Chem 259, 9840–9846 (1984). [PubMed] [Google Scholar]
- 53.Lohmann SM, DeCamili P, Enig I & Walter U High-affinity binding of the regulatory subunit (RII) of cAMP-dependent protein kinase to microtubule-associated and other cellular proteins. Proc. Natl. Acad. Sci. U.S.A 81, 6723–6727 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carr DW, Hausken ZE, Fraser ID, Stofko-Hahn RE & Scott JD Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. Cloning and characterization of the RII-binding domain. J. Biol. Chem 267, 13376–13382 (1992). [PubMed] [Google Scholar]
- 55.Colledge M & Scott JD AKAPs: from structure to function. Trends Cell Biol 9, 216–221. (1999). [DOI] [PubMed] [Google Scholar]
- 56.Scott JD et al. Type II regulatory subunit dimerization determines the subcellular localization of the cAMP-dependent protein kinase. J. Biol. Chem 265, 21561–21566 (1990). [PubMed] [Google Scholar]
- 57.Newlon MG et al. A novel mechanism of PKA anchoring revealed by solution structures of anchoring complexes. Embo J 20, 1651–1662. (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newlon MG et al. The molecular basis for protein kinase A anchoring revealed by solution NMR. Nat. Struct. Biol 6, 222–227. (1999). [DOI] [PubMed] [Google Scholar]
- 59.Sarma GN et al. Structure of D-AKAP2:PKA RI complex: insights into AKAP specificity and selectivity. Structure 18, 155–166, doi:S0969–2126(10)00007–9 [pii] 10.1016/j.str.2009.12.012 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dahlin HR, Zheng N & Scott JD Beyond PKA: Evolutionary and structural insights that define a docking and dimerization domain superfamily. J Biol Chem 297, 100927, doi: 10.1016/j.jbc.2021.100927 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gotz F et al. AKAP18:PKA-RIIalpha structure reveals crucial anchor points for recognition of regulatory subunits of PKA. Biochem J 473, 1881–1894, doi: 10.1042/BCJ20160242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carr DW et al. Identification of sperm-specific proteins that interact with A-kinase anchoring proteins in a manner similar to the type II regulatory subunit of PKA. J Biol Chem 276, 17332–17338, doi: 10.1074/jbc.M011252200 (2001). [DOI] [PubMed] [Google Scholar]
- 63.Carr DW et al. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J Biol Chem 266, 14188–14192 (1991). [PubMed] [Google Scholar]
- 64.Alto NM et al. Bioinformatic design of A-kinase anchoring protein-in silico: A potent and selective peptide antagonist of type II protein kinase A anchoring. Proc. Natl. Acad. Sci. U.S.A 100, 4445–4450 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carlson CR et al. Delineation of Type I Protein Kinase A-selective Signaling Events Using an RI Anchoring Disruptor. J Biol Chem 281, 21535–21545 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Wang Y et al. PKA-Type I Selective Constrained Peptide Disruptors of AKAP Complexes. ACS Chem Biol 10, 1502–1510, doi: 10.1021/acschembio.5b00009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kennedy EJ & Scott JD Selective disruption of the AKAP signaling complexes. Methods Mol Biol 1294, 137–150, doi: 10.1007/978-1-4939-2537-7_11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burns-Hamuro LL et al. Designing isoform-specific peptide disruptors of protein kinase A localization. Proc. Natl. Acad. Sci. U.S.A 100, 4072–4077 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deak VA & Klussmann E Pharmacological Interference With Protein-protein Interactions of Akinase Anchoring Proteins as a Strategy for the Treatment of Disease. Curr Drug Targets 17, 1147–1171, doi: 10.2174/1389450116666150416114247 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Stefan E et al. Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J Am Soc Nephrol 18, 199–212, doi:ASN.2006020132 [pii] 10.1681/ASN.2006020132 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Rosenmund C et al. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature 368, 853–856 (1994). [DOI] [PubMed] [Google Scholar]
- 72.Tavalin SJ, Westphal RS, Colledge M, Langeberg LK & Scott JD The molecular architecture of neuronal kinase/phosphatase signalling complexes. Biochem Soc Trans 27, 539–542 (1999). [DOI] [PubMed] [Google Scholar]
- 73.Kinderman FS et al. A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol Cell 24, 397–408 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang LJ, Durick K, Weiner JA, Chun J & Taylor SS Identification of a novel dual specificity protein kinase A anchoring protein, D-AKAP1. J. Biol. Chem 272, 8057–8064 (1997). [DOI] [PubMed] [Google Scholar]
- 75.Huang LJ, Durick K, Weiner JA, Chun J & Taylor SS D-AKAP2, a novel protein kinase A anchoring protein with a putative RGS domain. Proc. Natl. Acad. Sci. USA 94, 11184–11189 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miki K & Eddy EM Identification of tethering domains for protein kinase A type Ialpha regulatory subunits on sperm fibrous sheath protein FSC1. J Biol Chem 273, 34384–34390 (1998). [DOI] [PubMed] [Google Scholar]
- 77.Miki K & Eddy EM Single amino acids determine specificity of binding of protein kinase A regulatory subunits by protein kinase A anchoring proteins [In Process Citation]. J Biol Chem 274, 29057–29062 (1999). [DOI] [PubMed] [Google Scholar]
- 78.Burgers PP et al. Structure of smAKAP and its regulation by PKA-mediated phosphorylation. FEBS J 283, 2132–2148, doi: 10.1111/febs.13726 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kovanich D et al. Sphingosine kinase interacting protein is an A-kinase anchoring protein specific for type I cAMP-dependent protein kinase. Chembiochem 11, 963–971, doi: 10.1002/cbic.201000058 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Means CK et al. An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proceedings of the National Academy of Sciences of the United States of America 108, E1227–1235, doi: 10.1073/pnas.1107182108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scott JD & Pawson T Cell signaling in space and time: where proteins come together and when they’re apart. Science 326, 1220–1224, doi: 10.1126/science.1175668 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fraser ID & Scott JD Modulation of ion channels: a “current” view of AKAPs. Neuron 23, 423–426 (1999). [DOI] [PubMed] [Google Scholar]
- 83.Coghlan VM et al. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science 267, 108–112 (1995). [DOI] [PubMed] [Google Scholar]
- 84.Colledge M et al. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron 27, 107–119 (2000). [DOI] [PubMed] [Google Scholar]
- 85.Samelson BK et al. A-kinase Anchoring Protein 79/150 Recruits Protein Kinase C to Phosphorylate Roundabout Receptors. J Biol Chem 290, 14107–14119, doi: 10.1074/jbc.M115.637470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klauck TM et al. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science 271, 1589–1592 (1996). [DOI] [PubMed] [Google Scholar]
- 87.Hoshi N, Langeberg LK & Scott JD Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat Cell Biol 7, 1066–1073 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oliveria SF, Dell’Acqua ML & Sather WA AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron 55, 261–275 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murphy JG et al. AKAP-anchored PKA maintains neuronal L-type calcium channel activity and NFAT transcriptional signaling. Cell Rep 7, 1577–1588, doi: 10.1016/j.celrep.2014.04.027 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoshi N, Langeberg LK, Gould CM, Newton AC & Scott JD Interaction with AKAP79 modifies the cellular pharmacology of PKC. Mol Cell 37, 541–550, doi:S1097–2765(10)00043–2 [pii] 10.1016/j.molcel.2010.01.014 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tunquist BJ et al. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proc Natl Acad Sci U S A 105, 12557–12562 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scott JD, Dessauer CW & Tasken K Creating order from chaos: cellular regulation by kinase anchoring. Annu Rev Pharmacol Toxicol 53, 187–210, doi: 10.1146/annurev-pharmtox-011112-140204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piggott LA, Bauman AL, Scott JD & Dessauer CW The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc Natl Acad Sci U S A 105, 13835–13840, doi:0712100105 [pii] 10.1073/pnas.0712100105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kapiloff MS et al. An adenylyl cyclase-mAKAPbeta signaling complex regulates cAMP levels in cardiac myocytes. J. Biol. Chem 284, 23540–23546, doi:M109.030072 [pii] 10.1074/jbc.M109.030072 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y, Chen L, Kass RS & Dessauer CW The A-kinase anchoring protein Yotiao facilitates complex formation between adenylyl cyclase type 9 and the IKs potassium channel in heart. J Biol Chem 287, 29815–29824, doi: 10.1074/jbc.M112.380568 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bavencoffe A et al. Persistent Electrical Activity in Primary Nociceptors after Spinal Cord Injury Is Maintained by Scaffolded Adenylyl Cyclase and Protein Kinase A and Is Associated with Altered Adenylyl Cyclase Regulation. J Neurosci 36, 1660–1668, doi: 10.1523/jneurosci.0895-15.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fraser ID et al. Assembly of an A kinase-anchoring protein-beta(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr Biol 10, 409–412 (2000). [DOI] [PubMed] [Google Scholar]
- 98.Tao J, Wang HY & Malbon CC Protein kinase A regulates AKAP250 (gravin) scaffold binding to the beta2-adrenergic receptor. EMBO J. 22, 6419–6429 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Havekes R et al. Gravin orchestrates protein kinase A and beta2-adrenergic receptor signaling critical for synaptic plasticity and memory. J Neurosci 32, 18137–18149, doi: 10.1523/JNEUROSCI.3612-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bachmann VA et al. Gpr161 anchoring of PKA consolidates GPCR and cAMP signaling. Proc Natl Acad Sci U S A 113, 7786–7791, doi: 10.1073/pnas.1608061113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dodge KL et al. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 20, 1921–1930. (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dodge-Kafka KL, Langeberg L & Scott JD Compartmentation of cyclic nucleotide signaling in the heart: the role of A-kinase anchoring proteins. Circ Res 98, 993–1001 (2006). [DOI] [PubMed] [Google Scholar]
- 103.Carlisle Michel JJ et al. PKA phosphorylation of PDE4D3 facilitates recruitment of the mAKAP signaling complex. Biochem. J 381, 587–592 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Michel JJ et al. Spatial restriction of PDK1 activation cascades by anchoring to mAKAPalpha. Mol Cell 20, 661–672 (2005). [DOI] [PubMed] [Google Scholar]
- 105.Tasken KA et al. Phosphodiesterase 4D and protein kinase a type II constitute a signaling unit in the centrosomal area. J. Biol. Chem 276, 21999–22002. (2001). [DOI] [PubMed] [Google Scholar]
- 106.Pidoux G & Tasken K Specificity and spatial dynamics of protein kinase A signaling organized by A-kinase-anchoring proteins. J. Mol. Endocrinol 44, 271–284, doi:JME-10–0010 [pii] 10.1677/JME-10-0010 [doi] (2010). [DOI] [PubMed] [Google Scholar]
- 107.Conti M et al. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J. Biol. Chem 278, 5493–5496 (2003). [DOI] [PubMed] [Google Scholar]
- 108.Dodge-Kafka KL et al. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 437, 574–578 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krahling AM et al. CRIS-a novel cAMP-binding protein controlling spermiogenesis and the development of flagellar bending. PLoS Genet 9, e1003960, doi: 10.1371/journal.pgen.1003960 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schillace RV, Voltz JW, Sim AT, Shenolikar S & Scott JD Multiple interactions within the AKAP220 signaling complex contribute to protein phosphatase 1 regulation. J Biol Chem 276, 12128–12134, doi: 10.1074/jbc.M010398200 [doi] M010398200 [pii] (2001). [DOI] [PubMed] [Google Scholar]
- 111.Bajpai M et al. AKAP3 selectively binds PDE4A isoforms in bovine spermatozoa. Biol Reprod 74, 109–118, doi: 10.1095/biolreprod.105.043588 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rahman N, Buck J & Levin LR pH sensing via bicarbonate-regulated “soluble” adenylyl cyclase (sAC). Front Physiol 4, 343, doi: 10.3389/fphys.2013.00343 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carnegie GK & Burmeister BT A-Kinase Anchoring Proteins That Regulate Cardiac Remodeling. Journal of Cardiovascular Pharmacology 58, 451–458, doi: 10.1097/fjc.0b013e31821c0220 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang J, Bal M, Bierbower S, Zaika O & Shapiro MS AKAP79/150 signal complexes in G-protein modulation of neuronal ion channels. J Neurosci 31, 7199–7211, doi: 10.1523/JNEUROSCI.4446-10.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Soderling SH et al. The WRP component of the WAVE-1 complex attenuates Rac-mediated signalling. Nature Cell Biol. 4, 970–975. (2002). [DOI] [PubMed] [Google Scholar]
- 116.Soderling SH et al. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J. Neurosci 27, 355–365 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Soderling SH et al. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc. Natl. Acad. Sci. U.S.A 100, 1723–1728 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Diviani D, Osman H, Delaunay M & Kaiser S The role of A-kinase anchoring proteins in cardiac oxidative stress. Biochemical Society Transactions 47, 1341–1353, doi: 10.1042/bst20190228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bers DM Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70, 23–49, doi: 10.1146/annurev.physiol.70.113006.100455 (2008). [DOI] [PubMed] [Google Scholar]
- 120.Fabiato A Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol 245, C1–14, doi: 10.1152/ajpcell.1983.245.1.C1 (1983). [DOI] [PubMed] [Google Scholar]
- 121.Brini M & Carafoli E Calcium pumps in health and disease. Physiol Rev 89, 1341–1378, doi: 10.1152/physrev.00032.2008 (2009). [DOI] [PubMed] [Google Scholar]
- 122.Anderson DM et al. Widespread control of calcium signaling by a family of SERCA-inhibiting micropeptides. Sci Signal 9, ra119, doi: 10.1126/scisignal.aaj1460 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vega AL, Yuan C, Votaw VS & Santana LF Dynamic Changes in Sarcoplasmic Reticulum Structure in Ventricular Myocytes. Journal of Biomedicine and Biotechnology 2011, 1–14, doi: 10.1155/2011/382586 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lou Q, Janardhan A & Efimov IR in Advances in Experimental Medicine and Biology 1145–1174 (Springer Netherlands, 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Colombe A-S & Pidoux G Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Infarction. Cells 10, 922, doi: 10.3390/cells10040922 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hulme JT, Westenbroek RE, Scheuer T & Catterall WA Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac Cav1.2 channels during β-adrenergic regulation. Proceedings of the National Academy of Sciences 103, 16574–16579, doi:doi: 10.1073/pnas.0607294103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tada M, Kirchberger MA & Katz AM Regulation of calcium transport in cardiac sarcoplasmic reticulum by cyclic AMP-dependent protein kinase. Recent Adv Stud Cardiac Struct Metab 9, 225–239 (1976). [PubMed] [Google Scholar]
- 128.Ha KN et al. Lethal Arg9Cys phospholamban mutation hinders Ca2+-ATPase regulation and phosphorylation by protein kinase A. Proceedings of the National Academy of Sciences 108, 2735–2740, doi: 10.1073/pnas.1013987108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marx SO et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101, 365–376 (2000). [DOI] [PubMed] [Google Scholar]
- 130.Gao T et al. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron 19, 185–196 (1997). [DOI] [PubMed] [Google Scholar]
- 131.McConnachie G, Langeberg LK & Scott JD AKAP signaling complexes: getting to the heart of the matter. Trends Mol Med 12, 317–323 (2006). [DOI] [PubMed] [Google Scholar]
- 132.Nichols CB et al. Sympathetic Stimulation of Adult Cardiomyocytes Requires Association of AKAP5 With a Subpopulation of L-Type Calcium Channels. Circulation Research 107, 747–756, doi: 10.1161/circresaha.109.216127 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Catterall WA Structure and Regulation of Voltage-Gated Ca2+ Channels. Annual Review of Cell and Developmental Biology 16, 521–555, doi: 10.1146/annurev.cellbio.16.1.521 (2000). [DOI] [PubMed] [Google Scholar]
- 134.Navedo MF & Santana LF CaV1.2 sparklets in heart and vascular smooth muscle. Journal of Molecular and Cellular Cardiology 58, 67–76, doi: 10.1016/j.yjmcc.2012.11.018 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li X, Nooh MM & Bahouth SW Role of AKAP79/150 Protein in β1-Adrenergic Receptor Trafficking and Signaling in Mammalian Cells. Journal of Biological Chemistry 288, 33797–33812, doi: 10.1074/jbc.m113.470559 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fraser ID et al. A novel lipid-anchored A-kinase Anchoring Protein facilitates cAMP- responsive membrane events. Embo J 17, 2261–2272 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gray PC, Tibbs VC, Catterall WA & Murphy BJ Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J. Biol. Chem 272, 6297–6302 (1997). [DOI] [PubMed] [Google Scholar]
- 138.Smith FD et al. Single nucleotide polymorphisms alter kinase anchoring and the subcellular targeting of A-kinase anchoring proteins. Proceedings of the National Academy of Sciences 115, E11465–E11474, doi: 10.1073/pnas.1816614115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trotter KW et al. Alternative splicing regulates the subcellular localization of A-kinase anchoring protein 18 isoforms. J. Cell Biol 147, 1481–1492 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.J Johnson KR, Nicodemus-Johnson J, Carnegie GK & Danziger RS Molecular evolution of A-kinase anchoring protein (AKAP)-7: implications in comparative PKA compartmentalization. BMC Evol Biol 12, 125, doi: 10.1186/1471-2148-12-125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Henn V et al. Identification of a novel A-kinase anchoring protein 18 isoform and evidence for its role in the vasopressin-induced aquaporin-2 shuttle in renal principal cells. J Biol Chem 279, 26654–26665, doi: 10.1074/jbc.M312835200 (2004). [DOI] [PubMed] [Google Scholar]
- 142.Hulme JT, Lin TW-C, Westenbroek RE, Scheuer T & Catterall WA β-Adrenergic regulation requires direct anchoring of PKA to cardiac Cav1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proceedings of the National Academy of Sciences 100, 13093–13098, doi: 10.1073/pnas.2135335100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hulme JT, Ahn M, Hauschka SD, Scheuer T & Catterall WA A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca(2+) channel and modulates its function. J. Biol. Chem 277, 4079–4087 (2002). [DOI] [PubMed] [Google Scholar]
- 144.Cserne Szappanos H et al. Identification of a novel cAMP dependent protein kinase A phosphorylation site on the human cardiac calcium channel. Scientific Reports 7, doi: 10.1038/s41598-017-15087-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hofmann F, Flockerzi V, Kahl S & Wegener JW L-Type CaV1.2 Calcium Channels: From In Vitro Findings to In Vivo Function. Physiological Reviews 94, 303–326, doi: 10.1152/physrev.00016.2013 (2014). [DOI] [PubMed] [Google Scholar]
- 146.Kumari N, Gaur H & Bhargava A Cardiac voltage gated calcium channels and their regulation by β-adrenergic signaling. Life Sciences 194, 139–149, doi: 10.1016/j.lfs.2017.12.033 (2018). [DOI] [PubMed] [Google Scholar]
- 147.Fuller MD, Emrick MA, Sadilek M, Scheuer T & Catterall WA Molecular Mechanism of Calcium Channel Regulation in the Fight-or-Flight Response. Science Signaling 3, ra70–ra70, doi:doi: 10.1126/scisignal.2001152 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fu Y, Westenbroek RE, Scheuer T & Catterall WA Phosphorylation sites required for regulation of cardiac calcium channels in the fight-or-flight response. Proceedings of the National Academy of Sciences 110, 19621–19626, doi:doi: 10.1073/pnas.1319421110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fu Y, Westenbroek RE, Scheuer T & Catterall WA Basal and β;-adrenergic regulation of the cardiac calcium channel Cav1.2 requires phosphorylation of serine 1700. Proceedings of the National Academy of Sciences 111, 16598–16603, doi:doi: 10.1073/pnas.1419129111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weiss S, Oz S, Benmocha A & Dascal N Regulation of Cardiac L-Type Ca2+ Channel Cav1.2 Via the β-Adrenergic-cAMP-Protein Kinase A Pathway. Circulation Research 113, 617–631, doi: 10.1161/circresaha.113.301781 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Liu G et al. Mechanism of adrenergic CaV1.2 stimulation revealed by proximity proteomics. Nature 577, 695–700, doi: 10.1038/s41586-020-1947-z (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lygren B et al. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep 8, 1061–1067 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Carlson CR et al. AKAP18δ Anchors and Regulates CaMKII Activity at Phospholamban-SERCA2 and RYR. Circulation Research 130, 27–44, doi: 10.1161/circresaha.120.317976 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gonano LA & Vila Petroff M AKAP18δ Puts CaMKII in the Right Place at the Right Time: Implications for Cardiac Ca2+ Handling. Circulation Research 130, 45–47, doi: 10.1161/circresaha.121.320537 (2022). [DOI] [PubMed] [Google Scholar]
- 155.Nauert JB, Klauck TM, Langeberg LK & Scott JD Gravin, an autoantigen recognized by serum from myasthenia gravis patients, is a kinase scaffold protein. Curr. Biol 7, 52–62. (1997). [DOI] [PubMed] [Google Scholar]
- 156.Shih M, Lin F, Scott JD, Wang HY & Malbon CC Dynamic complexes of beta2-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J. Biol. Chem 274, 1588–1595 (1999). [DOI] [PubMed] [Google Scholar]
- 157.Li Z et al. Force development and intracellular Ca2+ in intact cardiac muscles from gravin mutant mice. European Journal of Pharmacology 807, 117–126, doi: 10.1016/j.ejphar.2017.04.020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guillory AN et al. Enhanced Cardiac Function in Gravin Mutant Mice Involves Alterations in the β-Adrenergic Receptor Signaling Cascade. PLoS ONE 8, e74784, doi: 10.1371/journal.pone.0074784 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen L, Kurokawa J & Kass RS Phosphorylation of the A-kinase-anchoring protein Yotiao contributes to protein kinase A regulation of a heart potassium channel. J Biol Chem 280, 31347–31352 (2005). [DOI] [PubMed] [Google Scholar]
- 160.Wakatsuki T, Schlessinger J & Elson EL The biochemical response of the heart to hypertension and exercise. Trends Biochem Sci 29, 609–617, doi: 10.1016/j.tibs.2004.09.002 (2004). [DOI] [PubMed] [Google Scholar]
- 161.Carnegie GK et al. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell 32, 169–179 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Appert-Collin A, Cotecchia S, Nenniger-Tosato M, Pedrazzini T & Diviani D The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates alpha1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U.S.A 104, 10140–10145 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Diviani D, Soderling J & Scott JD AKAP-Lbc anchors protein kinase A and nucleates Galpha 12-selective Rho-mediated stress fiber formation. J. Biol. Chem 276, 44247–44257. (2001). [DOI] [PubMed] [Google Scholar]
- 164.Cariolato L, Cavin S & Diviani D A-kinase anchoring protein (AKAP)-Lbc anchors a PKN-based signaling complex involved in alpha1-adrenergic receptor-induced p38 activation. J Biol Chem 286, 7925–7937, doi: 10.1074/jbc.M110.185645 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Takimoto E & Kass DA Role of Oxidative Stress in Cardiac Hypertrophy and Remodeling. Hypertension 49, 241–248, doi: 10.1161/01.hyp.0000254415.31362.a7 (2007). [DOI] [PubMed] [Google Scholar]
- 166.Ramachandra CJA et al. Oxidative stress in cardiac hypertrophy: From molecular mechanisms to novel therapeutic targets. Free Radical Biology and Medicine 166, 297–312, doi: 10.1016/j.freeradbiomed.2021.02.040 (2021). [DOI] [PubMed] [Google Scholar]
- 167.Van Der Pol A, Van Gilst WH, Voors AA & Van Der Meer P Treating oxidative stress in heart failure: past, present and future. European Journal of Heart Failure 21, 425–435, doi: 10.1002/ejhf.1320 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Taglieri DM et al. The C-terminus of the long AKAP13 isoform (AKAP-Lbc) is critical for development of compensatory cardiac hypertrophy. J Mol Cell Cardiol 66, 27–40, doi: 10.1016/j.yjmcc.2013.10.010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Czachor A, Failla A, Lockey R & Kolliputi N Pivotal role of AKAP121 in mitochondrial physiology. American Journal of Physiology-Cell Physiology 310, C625–C628, doi: 10.1152/ajpcell.00292.2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gabrovsek L et al. A-kinase-anchoring protein 1 (dAKAP1)-based signaling complexes coordinate local protein synthesis at the mitochondrial surface. Journal of Biological Chemistry 295, 10749–10765, doi: 10.1074/jbc.ra120.013454 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Abrenica B, AlShaaban M & Czubryt MP The A-kinase anchor protein AKAP121 is a negative regulator of cardiomyocyte hypertrophy. J Mol Cell Cardiol 46, 674–681, doi: 10.1016/j.yjmcc.2009.01.018 (2009). [DOI] [PubMed] [Google Scholar]
- 172.Perrino C et al. AKAP121 downregulation impairs protective cAMP signals, promotes mitochondrial dysfunction, and increases oxidative stress. Cardiovascular Research 88, 101–110, doi: 10.1093/cvr/cvq155 (2010). [DOI] [PubMed] [Google Scholar]
- 173.Lin RY, Moss SB & Rubin CS Characterization of S-AKAP84, a novel developmentally regulated A kinase anchor protein of male germ cells. J Biol Chem 270, 27804–27811, doi: 10.1074/jbc.270.46.27804 (1995). [DOI] [PubMed] [Google Scholar]
- 174.Kim H et al. Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia. Mol Cell 44, 532–544, oi: 10.1016/j.molcel.2011.08.045 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McCartney S, Little BM, Langeberg LK & Scott JD Cloning and characterization of A-kinase anchor protein 100 (AKAP100): a protein that targets A-kinase to the sarcoplasmic reticulum. J. Biol. Chem 270, 9327–9333 (1995). [DOI] [PubMed] [Google Scholar]
- 176.Pare GC, Easlick JL, Mislow JM, McNally EM & Kapiloff MS Nesprin-1alpha contributes to the targeting of mAKAP to the cardiac myocyte nuclear envelope. Exp Cell Res 303, 388–399 (2005). [DOI] [PubMed] [Google Scholar]
- 177.Kapiloff MS, Schillace RV, Westphal AM & Scott JD mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J. Cell Sci 112, 2725–2736 (1999). [DOI] [PubMed] [Google Scholar]
- 178.Kapiloff MS, Jackson N & Airhart N mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci 114, 3167–3176. (2001). [DOI] [PubMed] [Google Scholar]
- 179.Passariello CL, Li J, Dodge-Kafka K & Kapiloff MS mAKAP—A Master Scaffold for Cardiac Remodeling. Journal of Cardiovascular Pharmacology 65, 218–225, doi: 10.1097/fjc.0000000000000206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pare GC et al. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J Cell Sci 118, 5637–5646 (2005). [DOI] [PubMed] [Google Scholar]
- 181.Ruehr ML et al. Targeting of protein kinase A by muscle A kinase-anchoring protein (mAKAP) regulates phosphorylation and function of the skeletal muscle ryanodine receptor. J Biol Chem 278, 24831–24836 (2003). [DOI] [PubMed] [Google Scholar]
- 182.Paruchuri S & Thodeti CK Form follows function: polymorphisms in mAKAP alter cardiac cAMP/PKA signaling. American Journal of Physiology-Heart and Circulatory Physiology 315, H626–H628, doi: 10.1152/ajpheart.00248.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kritzer MD et al. The scaffold protein muscle A-kinase anchoring protein beta orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure. Circ Heart Fail 7, 663–672, doi: 10.1161/circheartfailure.114.001266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li J et al. The mAKAPbeta scaffold regulates cardiac myocyte hypertrophy via recruitment of activated calcineurin. J Mol Cell Cardiol 48, 387–394, doi: 10.1016/j.yjmcc.2009.10.023 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li J, Vargas MAX, Kapiloff MS & Dodge-Kafka KL Regulation of MEF2 transcriptional activity by calcineurin/mAKAP complexes. Experimental Cell Research 319, 447–454, doi: 10.1016/j.yexcr.2012.12.016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang L et al. Phospholipase Cε Hydrolyzes Perinuclear Phosphatidylinositol 4-Phosphate to Regulate Cardiac Hypertrophy. Cell 153, 216–227, doi: 10.1016/j.cell.2013.02.047 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wong W, Goehring AS, Kapiloff MS, Langeberg LK & Scott JD mAKAP compartmentalizes oxygen-dependent control of HIF-1alpha. Sci Signal 1, ra18, doi:1/51/ra18 [pii] 10.1126/scisignal.2000026 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Neumann SA et al. AKAP10 (I646V) functional polymorphism predicts heart rate and heart rate variability in apparently healthy, middle-aged European-Americans. Psychophysiology 46, 466–472 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bucko PJ et al. Subcellular drug targeting illuminates local kinase action. Elife 8, doi: 10.7554/eLife.52220 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bucko PJ et al. Gravin-associated kinase signaling networks coordinate gamma-tubulin organization at mitotic spindle poles. J Biol Chem 295, 13784–13797, doi: 10.1074/jbc.RA120.014791 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


