Abstract
Purpose
Innovative, efficient treatments are desperately needed for people with glioblastoma (GBM).
Methods
Sixteen patients (median age 65.8 years) with newly diagnosed, small-sized, not safely resectable supratentorial GBM underwent interstitial photodynamic therapy (iPDT) as upfront eradicating local therapy followed by standard chemoradiation. 5-aminolevulinic acid (5-ALA) induced protoporphyrin IX was used as the photosensitizer. The tumors were irradiated with light at 635 nm wavelength via stereotactically implanted cylindrical diffuser fibers. Outcome after iPDT was retrospectively compared with a positively-selected in-house patient cohort (n = 110) who underwent complete tumor resection followed by chemoradiation.
Results
Median progression-free survival (PFS) was 16.4 months, and median overall survival (OS) was 28.0 months. Seven patients (43.8%) experienced long-term PFS > 24 months. Median follow-up was 113.9 months for the survivors. Univariate regression revealed MGMT-promoter methylation but not age as a prognostic factor for both OS (p = 0.04 and p = 0.07) and PFS (p = 0.04 and p = 0.67). Permanent iPDT-associated morbidity was seen in one iPDT patient (6.3%). Patients treated with iPDT experienced superior PFS and OS compared to patients who underwent complete tumor removal (p < 0.01 and p = 0.01, respectively). The rate of long-term PFS was higher in iPDT-treated patients (43.8% vs. 8.9%, p < 0.01).
Conclusion
iPDT is a feasible treatment concept and might be associated with long-term PFS in a subgroup of GBM patients, potentially via induction of so far unknown immunological tumor-controlling processes.
Keywords: 5-aminolevulinic acid, Interstitial photodynamic therapy, Glioblastoma, Overall survival, Postoperative morbidity, Progression-free survival
Introduction
Glioblastoma (GBM) is the most common malignant brain tumor [1]. Current treatment concepts comprise maximal safe resection followed by a combination of radiotherapy and chemotherapy with temozolomide [2], possibly augmented by tumor-treating fields [3]. Despite this aggressive treatment regimen, median survival is limited to 15–20 months [2–4]. Unfavorable outcome must be particularly expected if the tumor cannot be resected completely due to eloquent location [5, 6], and/or if an unmethylated O6-methylguanin-DNA-methyltransferase (MGMT) promoter hampers response to chemotherapy [7]. Thus, alternative treatment concepts need to be evaluated.
Photodynamic therapy (PDT) is a local treatment concept used for a variety of neoplastic [8, 9] and non-neoplastic conditions [10]. It is based on the light-induced activation of a photosensitizer leading to the formation of reactive oxygen species and subsequent apoptosis and necrosis of the affected cells [11]. The photosensitizer protoporphyrin IX is preferentially synthesized within malignant glioma cells after oral application of its precursor 5-aminolevulinic acid (5-ALA). This highly specific accumulation makes 5-ALA a well-suited photosensitizer predrug for PDT [12]. The good tumor-to-background-ratio of protoporphyrin IX synthesis is regularly exploited in fluorescence-guided resection [13]. For tumors unamenable to safe complete resection, interstitial PDT (iPDT) has been explored as a minimally invasive procedure where treatment light is applied through stereotactically implanted optical fibers. IPDT was found to be a feasible salvage treatment option in small malignant glioma case series [14–16]. Recently, a larger series of recurrent malignant gliomas reported a post-recurrence survival longer than 24 months for 25% of the treated patients [17].
Based on these promising results in GBM recurrences, we offered iPDT as an alternative local treatment option upon specific demand to patients with small-sized, unifocal, not safely resectable, newly diagnosed GBM. In here, we share our experiences and outcome data, focusing on progression-free survival (PFS), overall survival (OS), and treatment-associated morbidity in a series of 16 adult patients with untreated GBMs undergoing iPDT as primary treatment. All patients received postoperative standard treatment with radiation therapy plus concomitant and adjuvant temozolomide. Outcome data after iPDT were put into perspective with an in-house cohort of GBM patients having undergone complete tumor resection followed by a complete course of radiochemotherapy according to the EORTC/NCIC protocol [2].
Patients and methods
IPDT patient cohort
All patients were discussed in advance in our local interdisciplinary neurooncological tumor board. The decision to perform iPDT in selected cases was triggered by the patients’ specific demand as well as our prior experiences with iPDT treated malignant glioma recurrences. Eligibility criteria for patients undergoing iPDT on specific demand consisted of (1) small-sized (diameter < 4 cm), circumscribed, untreated GBMs without or moderate midline shift without signs of transtentorial herniation or contact to the ventricular system, (2) unifocal, supratentorial, and (3) patients should rate on the Karnofsky performance scale (KPS) with values ≥ 70. All patients were informed in detail about the procedure and its associated risks, and about the fact that iPDT is not the established standard treatment for newly diagnosed GBMs and is considered an individual treatment attempt. Written informed consent was obtained from all patients. The institutional review board approved the protocol for the retrospective analysis (ethics approval no. 335 − 16, Ludwig-Maximilians-University, Munich, Germany).
Study cohort for comparative analyses
An in-house patient cohort was used for comparative outcome analyses. This cohort included 110 highly selected patients who had received the optimal available first-line treatment for newly-diagnosed GBMs consisting of complete resection (as proven by early post-operative MRI) followed by a full course of adjuvant radiochemotherapy according to the EORTC/NCIC protocol.
Treatment procedure
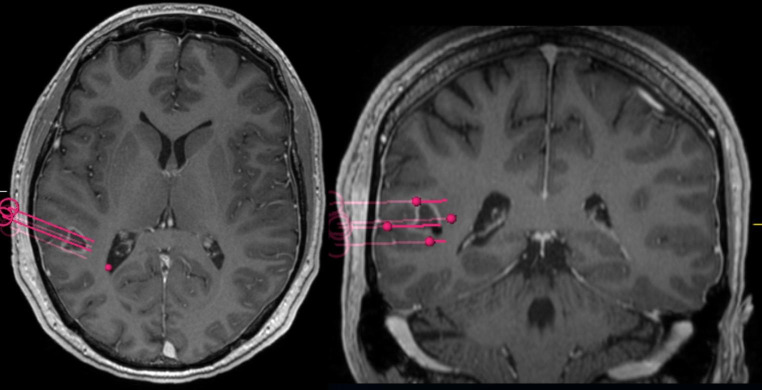
All tumors were diagnosed histologically according to the current WHO classification at the time of treatment [18]. MGMT methylation status, isocitrate dehydrogenase (IDH) mutation status and LOH1p/19q were determined as described previously [19]. Interstitial photodynamic therapy was performed in a standardized fashion as reported in detail before [14, 17]. In brief, a three-dimensional treatment volume was defined using preoperative MRI with contrast-enhanced T1 and, when available, O-2-[18 F]fluoroethyl-L-tyrosine-positron emission tomography (FET-PET). These images, together with T2-weighted images and contrast-enhanced MR-angiography, were fused to the intraoperatively acquired stereotactic computerized tomography (CT) to plan the trajectories of the cylindrical light diffusors (CYD 600, Light Guide Optics, Rheinbach, Germany). Figure 1 shows an implantation schematic. Three hours after systemically administering 5-ALA (medac GmbH, Wedel, Germany) at a standard dose of 20 mg/kg bodyweight (maximum: 30 mg/kg), the light diffusors were implanted stereotactically under general anesthesia. Light irradiation was performed at a wavelength of 635 nm (median total dose: 12,240 J, range 7200–20,520 J; median dose per treatment volume: 2.400 J/cm3, range 969–5760 J/cm3; median light power per diffusor length: 200 mW/cm, range: 100–200 mW/cm; Ceralas PDT Diode Laser, biolitec AG, Jena, Germany). The median duration of irradiation was 1.0 h (range: 1.0 to 2.0 h, elongated irradiation times were compensated by a reduced radiant flux). Prior to and after the irradiation, intraoperative spectral online monitoring measurements were performed to document the transmission of the treatment light through the tissue and the amount of PpIX fluorescence generated therein as well as to monitor for potential treatment-related or treatment-relevant effects [20, 21].
Fig. 1.
Exemplary case illustrating three-dimensional planning of the light diffusor trajectories. The patient (IPDT 02) presented with seizures and aphasia leading to the diagnosis of glioblastoma in the left angular gyrus
Adjuvant treatment and follow-up
In accordance with Stupp protocol [2], all but one patient received concomitant radiochemotherapy and adjuvant cycles of temozolomide chemotherapy. One patient (IPDT 11) was treated by adjuvant radiotherapy only because of an unmethylated MGMT-promoter status in combination with advanced age > 65 years. Adjuvant treatment was initiated within two weeks after iPDT in all patients. Any treatment-associated morbidity was documented. The last clinical follow-up for this specific cohort was January 12th, 2022.
Evaluation and statistical analyses
The two study endpoints, PFS and OS, were calculated from the date of iPDT or tumor resection (reference cohort). Long-term survival was defined as a PFS of > 24.0 months as calculated by Kaplan-Meier method. Follow-up was assessed by the reverse Kaplan-Meier method. Survival was evaluated with the Kaplan-Meier method and compared by the log-rank test. As potential prognostic factors, MGMT methylation status and age were assessed by univariate regression because of the lesser number of events in the iPDT cohort. Categorical variables were analyzed using the χ2 test and age with Student’s t-test. A p-value ≤ 0.05 was considered significant. All calculations were performed using SPSS Statistics 25 (IBM, Armonk, New York, USA).
Results
Patient population
Sixteen patients were consecutively treated with iPDT between 2008 and 2014. Their median age at iPDT was 65.8 years (range 29.7–76.5), and all had a KPS of 90. Clinically, epilepsy (n = 12), aphasia (n = 3), and hemiparesis (n = 1) led to the tumor diagnosis. The treated tumors were localized in the temporal lobe (n = 8), the frontal lobe (n = 3), the parietal lobe (n = 4), and central gyrus and subcentral lobe (n = 1). Fourteen tumors were located in the dominant hemisphere. A methylated MGMT-promoter status was seen in 8/16 (50.0%) and an IDH mutation in 2/16 (12.5%) of iPDT patients. No tumor had a 1p/19q codeletion (LOH1p/19q). The median tumor volume was 6.1 cm3 (range: 1.4–21.8 cm3); the number of implanted light diffusors ranged from three to ten per tumor. Table 1 details histopathological profiles at the time of iPDT and outcome data for the iPDT patient cohort.
Table 1.
Patient characteristics including biomarker status and follow-up
| Patient number | sex | Age at iPDT* (years) | MGMT promoter methylated | IDH mutation | Ki67 proliferation index | PFS (months) | OS (months) | status |
|---|---|---|---|---|---|---|---|---|
| IPDT 01 | m | 29,7 | yes | yes | ? | 64,7 | 102,4 | deceased |
| IPDT 02 | m | 40,6 | no | no | 10% | 59,2 | 95,0 | deceased |
| IPDT 03 | f | 50,3 | yes | no | 10% | 127,1 | 127,5 | alive |
| IPDT 04 | m | 69,9 | no | no | 30% | 8,3 | 15,0 | deceased |
| IPDT 05 | m | 68,2 | no | no | 15% | 12,0 | 16,1 | deceased |
| IPDT 06 | m | 63,7 | no | no | 20% | 4,3 | 9,0 | deceased |
| IPDT 07 | m | 70,1 | yes | no | 25% | 110,1 | 110,3 | alive |
| IPDT 08 | f | 74,1 | no | no | 25% | 60,6 | 66,4 | deceased |
| IPDT 09 | m | 33,3 | partially | yes | 85% | 113,6 | 113,9 | alive |
| IPDT 10 | f | 74,3 | no | no | 30% | 16,4 | 28,0 | deceased |
| IPDT 11 | m | 68,8 | no | no | 21% | 6,0 | 8,5 | deceased |
| IPDT 12 | m | 68,0 | yes | no | 7% | 6,5 | 8,0 | deceased |
| IPDT 13 | f | 57,3 | partially | no | 15% | 9,5 | 25,2 | deceased |
| IPDT 14 | m | 54,3 | yes | no | 15% | 35,7 | 43,9 | deceased |
| IPDT 15 | m | 76,5 | no | no | 10% | 7,4 | 9,2 | deceased |
| IPDT 16 | m | 53,4 | yes | no | 28% | 17,8 | 36,4 | deceased |
* iPDT = interstitial photodynamic therapy; MGMT = O6-methylguanin-DNA-methyltransferase; IDH = isocitrate dehydrogenase; PFS = progression-free survival; OS = overall survival; m = male; f = female.
MGMT promoter methylation status was determined as described before [22].
Survival after iPDT
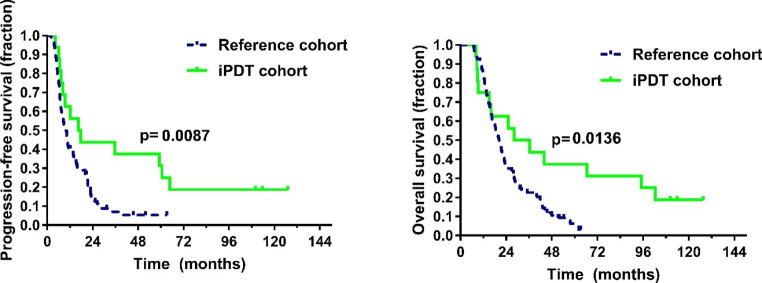
Median follow-up for the survivors was 113.9 months. Within this follow-up, 13 patients succumbed to their disease. Median PFS was 16.4 months; median OS was 28.0 months (see Fig. 2). One-year and two-year PFS rates were 56.3% and 43.8%, one-year and two-year OS rates were 75.0% and 62.5%, respectively. Univariate regression revealed MGMT-promoter methylation but not age as a prognostic factor for both OS (p = 0.04 and p = 0.07) and PFS (p = 0.04 and p = 0.67). At the time of recurrence, the patients received bevacizumab/irinotecan (n = 5), re-radiotherapy (n = 3), brachytherapy (n = 3), temozolomide rechallenge (n = 3), procarbazine/lomustine chemotherapy (n = 2) or a combination thereof. Four patients presented with cognitive deterioration at first recurrence and received best supportive care; no patient underwent open tumor resection.
Fig. 2.
Progression-free survival and overall survival were significantly longer in the interstitial photodynamic therapy (iPDT) cohort compared to the reference cohort
Comparison to reference population
Patients in the reference cohort resembled iPDT patients with respect to age (median 56.1 years (range: 17.2–86.6 years) vs. 65.9 years, p = 0.21), MGMT promoter methylation (methylation rate: 48/99 (48.5%) vs. 8/16 (50.0%), p = 0.91), and IDH mutation rate (3/40 (7.5%) vs. 2/16 (12.5%), p = 0.55). KPS was higher in the iPDT treated group (90 for all iPDT patients, median 80, range 60–90 in the reference cohort, p < 0.01). MGMT methylation, but not age were associated with a prolonged OS (p < 0.01 and p = 0.12) and PFS (p < 0.01 and p = 0.07).
Patients treated with iPDT experienced superior progression-free and overall survival compared to patients who had undergone complete tumor resection (p < 0.01 and p = 0.01, respectively). The rate of patients with long-term PFS (> 24 months) was higher in iPDT treated patients (43.8% vs. 8.9%, p < 0.01, see also Table 2).
Table 2.
Comparison between iPDT and reference cohort
| iPDT cohort | Reference cohort | p-value | ||
|---|---|---|---|---|
|
Median age (range) |
65.8 years (29.7–76.5 years) |
56.1 years (17.2–86.6 years) |
0.21 | |
| MGMT promoter methylated |
8/16 50.0% |
48/99* 48.5% |
0.91 | |
| IDH mutant |
2/16 12.5% |
3/40* 7.5% |
0.55 | |
|
Median PFS (95% CI) |
16.4 months | 9.9 months | < 0.01 | |
|
Median OS (95% CI) |
28.0 months (6.0–50.0) |
20.4 months (17.9–22.9) |
0.01 | |
| Long-term PFS (> 24 months) |
7/16 (43.8%) |
11/110 (8.9%) |
< 0.01 |
* Molecular analyses were unavailable for some patients in the reference cohort.
MGMT = O6-methylguanin-DNA-methyltransferase; IDH = isocitrate dehydrogenase; PFS = progression-free survival; CI = confidence interval; OS = overall survival.
Among patients with MGMT-methylated tumors, 62.5% of iPDT treated patients experienced long-term PFS (> 24 months), compared to 16.7% in the reference group (p < 0.01).
Postoperative morbidity
Perioperative transient morbidity within the first 30 days after iPDT included new aphasia (n = 2), worsening of a preexisting aphasia (n = 2), new hemiparesis (n = 2), worsening of a preexisting hemiparesis (n = 1), and pulmonary embolism (n = 1). Since two patients were affected by multiple postoperative symptoms/complications, a patient-based analysis resulted in a total treatment-associated morbidity rate of 37.5% (6/16 patients). Most neurological symptoms were edema-related and improved with oral dexamethasone treatment except for one patient with a permanent new aphasia after iPDT. No long-term steroid treatment or delay of adjuvant treatment was necessary. Thus, a permanent morbidity rate of 6.3% (1/16 of iPDT patients) was recorded over the course of follow-up.
Discussion
Adjuvant radiochemotherapy according to the EORTC/NCIC protocol has shown to improve the outcome of glioblastoma patients with a methylated MGMT-promoter status; however, overall survival remains limited [23]. Besides the incremental focus on biomarker profiling leading to more personalized treatment strategies, there remains an increasing need for the evaluation of more effective treatment concepts. One of these possible novel treatment concepts is iPDT for which this study provides a detailed outcome data analysis in comparison to the best available conventional treatment option. It is shown that iPDT, although requiring a careful planning procedure, is a feasible concept with an acceptable side effect profile for patients with untreated, small-sized, unresectable GBMs. A remarkable number of patients, in particular those with a methylated MGMT-promoter status, experienced long-term survival after iPDT. Furthermore, iPDT did not interfere with any further adjuvant treatment options.
One iPDT treated patient suffered a permanent treatment-associated morbidity. All other side effects were edema-associated and could be sufficiently treated by oral dexamethasone administration. Whenever possible, dexamethasone was waived so as to not impair potential immune-modulatory effects. No patient had to undergo long-term anti-edematous therapy and no delay of adjuvant treatment due to treatment-associated complications was recorded.
When assessing the resulting Kaplan-Meier survival curves for PFS and OS, the observed patterns for the iPDT patient cohort were found to reach a plateau indicating long-term PFS and OS for a subgroup of iPDT patients. This favorable course of disease cannot be attributed to additional adjuvant treatment since all patients, with the exception of one case merely receiving radiotherapy, only underwent initial radiochemotherapy in analogy to the EORTC/NCIC protocol with up to nine cycles of TMZ at most. Thereafter, a treatment-free period up to the first sign of tumor recurrence was initiated in all patients. Thus, the burden of adjuvant treatment could be kept to a minimum for a good proportion of these iPDT patients in the initial stages of the disease. No exceptionally aggressive recurrence treatment was noted, either. In this patient series very promising PFS and OS rates one year and two years after iPDT treatment could be recorded. Most surprisingly, a significant proportion of seven patients (43.8%) experienced a PFS > 24.0 months not linked to very aggressive salvage treatment.
Two iPDT-treated patients harbored IDH-mutant tumors and would therefore, under the current WHO classification [24], be grouped as astrocytoma, IDH mutant, CNS WHO grade 4. Possibly, this molecular profile is in part responsible for the favorable outcome in these cases, although the value of iPDT for this subgroup is not clear. IDH mutant tumors were found in the reference group in comparable numbers. Another limitation of our study cohort is the selection of small tumor volumes, so as to minimize the risk of patient harm due to space-occupying increase in edema. Whether this treatment may also benefit the many cases of larger tumors cannot be answered at this point. The patients’ excellent clinical status and prompt initiation of adjuvant therapy may have also contributed to the positive result.
A methylated MGMT-promoter status was associated with improved outcome parameters in both treatment groups, as is also seen in other treatment settings [7, 25]. The remarkably large survival benefit of iPDT-treated patients with positive MGMT-promoter methylation status, however, can most likely not only be explained by the known increased chemosensitivity by itself. Patients with an unmethylated MGMT-promoter profile showed outcomes similar to resected patients. Meanwhile the subgroup with methylated MGMT-promoter responded even better than resected patients. This response might be mediated through an immune-modulatory effect. The observed outcome improvement may thus be seen as a surrogate marker of biologically different inflammatory/immunological re-sponses to iPDT.
The precise mechanisms of tumor inactivation by iPDT, reflected in the recorded favorable PFS rate, are not entirely understood yet. Ex vivo experimental data have shown that PDT does have the ability to induce ROS in glioma cell spheroids causing consecutive necrosis [26]. It is now believed that through these events PDT does not only cause a local effect on the directly treated tumor volume but may also trigger a systemic immunological/inflammatory response which significantly contributes to the observed long-term tumor control [11, 27]. In mouse models, following PDT, an immediate infiltration of the tumor tissue by neutrophil granulocytes, mast cells, monocytes, and macrophages has been observed [28]. Preliminary ex vivo studies also suggest that iPDT impacts the adaptive immune system by increasing the cytotoxic potential of human CD8 + T-cells by alterating the cells’ mRNA and protein expression profiles [29]. These findings point to a potential iPDT-related induction of so far unknown tumor-controlling processes possibly overcoming the limitations of other local treatment concepts.
Based on our experiences, we do believe that iPDT is an appealing treatment concept for patients with newly diagnosed small-sized GBMs and deserves further evaluation in prospective clinical trials such as NCT03897491.
Conclusion
We here show that iPDT is a promising local treatment concept for patients with newly diagnosed small-sized GBMs. Notably, despite eloquent tumor localizations, a tolerable risk profile was seen. A considerable proportion of patients, especially those with methylated MGMT-promoter status, experienced long-term PFS after iPDT. This might point to the induction of so far, at least in detail, unknown tumor-controlling processes, e.g. inflammatory/immunological responses. Patients treated by iPDT compared favorably in terms of PFS and OS to the cohort of patients who received the optimal conventional treatment, which is an especially impressive finding as the iPDT-treated tumors were not safely resectable. Future clinical and experimental studies should be performed to improve the understanding of the underlying cellular and serological mechanisms and, consequently, to help to identify the subset of patients most suitable for iPDT.
Author contributions
SQ, RS, HS, AR, and NT conceptualized the study; CS, MA, MS, SK, RE, RS, and AR investigated the data; SQ, CS, MF, and ME curated the data and aided with methodology; SQ, CS, MA, MF performed the formal analysis; RF, KB, and RE added resources; MF and AR validated the results; SQ and AR visualized the data; SQ and CS wrote the original draft; RS, HS, AR, and NT edited the draft; AR and NT supervised and administered the project; all authors reviewed the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study received no external funding. Maximilian Aumiller, Marco Foglar and Mohamed El Fahim have received funding by the Deutsche Forschungsgemeinschaft (GRK2274).
Data Availability
Clinical and molecular data on all patients are anonymized and stored in local data bases secured by passwords.
Code Availability
Not applicable.
Declarations
Conflicts of interest/Competing interests
Stefanie Quach - No disclosures. Christoph Schwartz - No disclosures. Maximilian Aumiller - No disclosures. Marco Foglar - No disclosures. Michael Schmutzer - No disclosures. Sophie Katzendobler - No disclosures. Mohamed El Fahim - No disclosures. Robert Forbrig - No disclosures. Katja Bochmann - No disclosures. Rubert Egensperger - No disclosures. Ronald Sroka - No disclosures. Herbert Stepp - No disclosures. Adrian Rühm - No disclosures. Niklas Thon - No disclosures.
Consent to participate
Consent to participate in retrospective studies is given prospectively by all patients treated at the Department of Neurosurgery of the Ludwig Maximilian University of Munich through a local prospective tumor registry.
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals
The present study was conducted retrospectively.
Ethics approval
Ethics approval was obtained by the ethics committee of the Ludwig Maximilian University of Munich (project number 335-16).
Consent for publication
All authors have consented in submitting this manuscript for publication in the Journal of Neuro-Oncology.
Footnotes
Adrian Rühm and Niklas Thon shared last authorship
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ostrom QT, et al. CBTRUS Statistical Report: primary brain and other Central Nervous System Tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 2022;24(Suppl 5):v1–v95. doi: 10.1093/neuonc/noac202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, et al. Effect of Tumor-Treating Fields Plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a Randomized Clinical Trial. JAMA. 2017;318(23):2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinot OL, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 5.Thon N, Tonn JC, Kreth FW. The surgical perspective in precision treatment of diffuse gliomas. Onco Targets Ther. 2019;12:1497–1508. doi: 10.2147/OTT.S174316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stummer W, van den Bent MJ, Westphal M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochir (Wien) 2011;153(6):1211–1218. doi: 10.1007/s00701-011-1001-x. [DOI] [PubMed] [Google Scholar]
- 7.Thon N, et al. Outcome in unresectable glioblastoma: MGMT promoter methylation makes the difference. J Neurol. 2017;264(2):350–358. doi: 10.1007/s00415-016-8355-1. [DOI] [PubMed] [Google Scholar]
- 8.Li X, et al. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Reviews Clin Oncol. 2020;17(11):657–674. doi: 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- 9.Shafirstein G et al (2017)Interstitial Photodynamic Therapy-A Focused Review. Cancers (Basel), 9(2) [DOI] [PMC free article] [PubMed]
- 10.Pérez C, Zúñiga T, Palavecino CE. Photodynamic therapy for treatment of Staphylococcus aureus infections. Photodiagnosis Photodyn Ther. 2021;34:102285. doi: 10.1016/j.pdpdt.2021.102285. [DOI] [PubMed] [Google Scholar]
- 11.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6(7):535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stepp H, Stummer W. 5-ALA in the management of malignant glioma. Lasers Surg Med. 2018;50(5):399–419. doi: 10.1002/lsm.22933. [DOI] [PubMed] [Google Scholar]
- 13.Stummer W, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 14.Beck TJ, et al. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg Med. 2007;39(5):386–393. doi: 10.1002/lsm.20507. [DOI] [PubMed] [Google Scholar]
- 15.Stummer W, et al. Long-sustaining response in a patient with non-resectable, distant recurrence of glioblastoma multiforme treated by interstitial photodynamic therapy using 5-ALA: case report. J Neurooncol. 2008;87(1):103–109. doi: 10.1007/s11060-007-9497-x. [DOI] [PubMed] [Google Scholar]
- 16.Johansson A, et al. Protoporphyrin IX fluorescence and photobleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Lasers Surg Med. 2013;45(4):225–234. doi: 10.1002/lsm.22126. [DOI] [PubMed] [Google Scholar]
- 17.Lietke S, et al. Interstitial photodynamic therapy using 5-ALA for malignant glioma recurrences. Cancers. 2021;13(8):1767. doi: 10.3390/cancers13081767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louis DN, et al. The 2007 WHO classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katzendobler S et al (2022) Diagnostic yield and complication rate of stereotactic biopsies in Precision Medicine of Gliomas. Frontiers in Neurology, 13 [DOI] [PMC free article] [PubMed]
- 20.Heckl C, et al. Fluorescence and treatment light monitoring for interstitial photodynamic therapy. Photochem Photobiol. 2020;96(2):388–396. doi: 10.1111/php.13203. [DOI] [PubMed] [Google Scholar]
- 21.Aumiller M et al (2021) Interrelation between Spectral Online monitoring and postoperative T1-Weighted MRI in interstitial photodynamic therapy of malignant gliomas. Cancers (Basel), 14(1) [DOI] [PMC free article] [PubMed]
- 22.Eigenbrod S, et al. Molecular stereotactic biopsy technique improves diagnostic accuracy and enables personalized treatment strategies in glioma patients. Acta Neurochir (Wien) 2014;156(8):1427–1440. doi: 10.1007/s00701-014-2073-1. [DOI] [PubMed] [Google Scholar]
- 23.McFaline-Figueroa JR, Wen PY. Negative trials over and over again: how can we do better? Neurooncology. 2023;25(1):1–3. doi: 10.1093/neuonc/noac226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis DN, et al. The 2021 WHO classification of tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weller M, et al. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified Temozolomide Rechallenge in Progressive Glioblastoma: the DIRECTOR trial. Clin Cancer Res. 2015;21(9):2057–2064. doi: 10.1158/1078-0432.CCR-14-2737. [DOI] [PubMed] [Google Scholar]
- 26.Madsen SJ, et al. Photodynamic therapy of human glioma spheroids using 5-aminolevulinic acid. Photochem Photobiol. 2000;72(1):128–134. doi: 10.1562/0031-8655(2000)072<0128:PTOHGS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Wachowska M, Muchowicz A, Demkow U. Immunological aspects of antitumor photodynamic therapy outcome. Cent Eur J Immunol. 2015;40(4):481–485. doi: 10.5114/ceji.2015.56974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krosl G, Korbelik M, Dougherty GJ. Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy. Br J Cancer. 1995;71(3):549–555. doi: 10.1038/bjc.1995.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hübner M, et al. IMPS-15PDT-TREATED GBM CELLS INCREASE EFFECTOR FUNCTIONS OF HUMAN CD8 + T-CELLS. Neurooncology. 2015;17(suppl5):v116–v116. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Clinical and molecular data on all patients are anonymized and stored in local data bases secured by passwords.
Not applicable.