Abstract
To our knowledge, the adoption of Learning Health System (LHS) concepts or approaches for improving stroke care, patient outcomes and value have not previously been summarized. This topical review provides a summary of the published evidence about LHSs applied to stroke, and case examples applied to different aspects of stroke care from high and low-to-middle income countries. Our attempt to systematically identify the relevant literature and obtain real world examples demonstrated the dissemination gaps, the lack of learning and action for many of the related LHS concepts across the continuum of care, but also elucidated the opportunity for continued dialogue on how to study and scale LHS advances. In the field of stroke, we found only a few published examples of LHSs and health systems globally are implementing some selected LHS concepts, but the term is not common. A major barrier to identifying relevant LHS examples in stroke may be the lack of an agreed taxonomy or terminology for classification. We acknowledge that health service delivery settings that leverage many of the LHS concepts do so operationally and the lessons learned are not shared in peer reviewed literature. It is likely that this topical review will further stimulate the stroke community to disseminate related activities and use key words such as learning health system so that the evidence base can be more readily identified.
INTRODUCTION
Despite many advances in stroke treatment and prevention, their implementation into routine clinical practice is suboptimal. To improve the quality, value, safety and equity of patient care, learning health system (LHS) models have been proposed as a means of optimizing healthcare.1 Conceptually, a LHS may represent the evolution of quality improvement (QI) efforts and capabilities within parts of an organization to become unified and operating in concert at the system level. Alignment of specific resources and processes within healthcare organizations can facilitate not only efficient and continuous improvement but also helps to promote innovation and new knowledge through active collaboration.
A LHS is where intelligent automation, clinical decision support, predictive models, positive deviance, surveillance, and comparative effectiveness research is enabled.2 LHS models embed iterative use of routinely collected data within health care to inform clinical decision-making through data infrastructure and interdisciplinary expertise to deliver improved healthcare in consultation with patients.3 Consequently, a well-designed, data-driven LHS can enable iterative adaptations to the process or structure of care delivery and support pragmatic and real-world research. In this way, a LHS can meet the ever changing needs of current and future stakeholders,4 and provides a platform for the generation of new evidence, efficient implementation of evidence, and continuous evaluation of care and outcomes to facilitate QI,5 regardless of the original intention for data collection.6 Core distinguishing features are that a LHS is embedded in healthcare, adaptable, person-focused (defined as patients, families, communities and general public), and not constrained by a time-limited focus (unlike many QI initiatives).
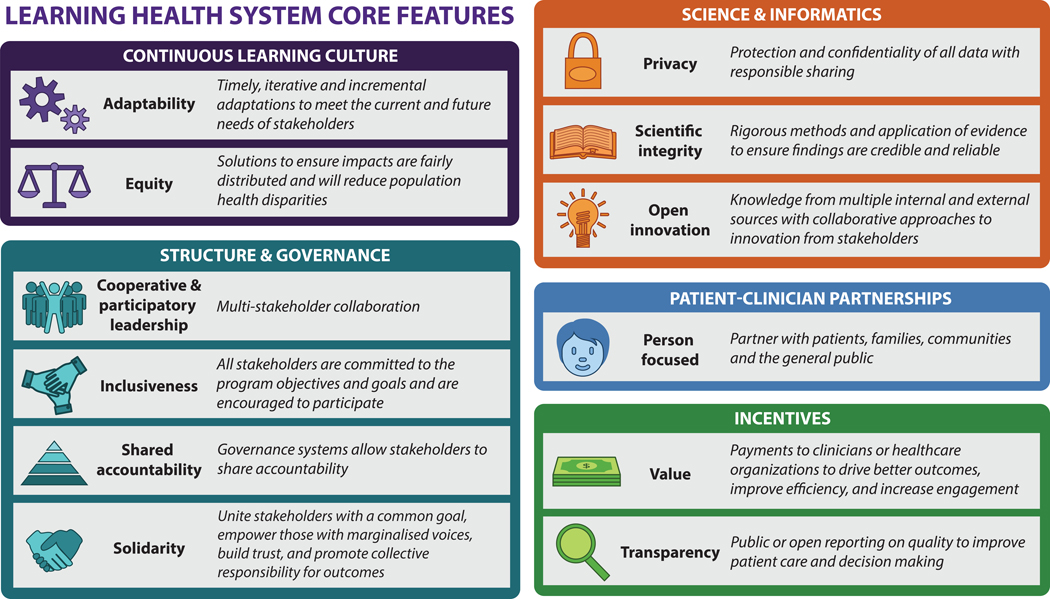
The purpose of this narrative topical review is to summarize relevant published evidence about LHSs applied to stroke, and to provide some case examples from different countries. Because terminology and operationalization of LHS concepts varies,3,4,7,8 and different frameworks and models to describe a LHS with varying complexity and detail have emerged,4,7–11 we employed a general framework to describe the highlighted articles and case studies. The framework is outlined in Figure 1 which provides the commonly reported features of a LHS.4,8,12 These features align with those described in the recent American Heart Association LHS and Cardiovascular Care Scientific Statement which included science and informatics, patient-physician partnerships, continuous learning cultures, and incentives;5 to which we have added structure and governance. Briefly, a LHS requires cooperative, participatory leadership, innovation, and scientific integrity4 that supports a continuous learning culture for improved health care quality and patient-clinician partnerships.8,13 There is a requirement for, and dependence on, technology that supports collection and use of electronic health record data.6 Processes enable both evidence generation and iterative, sustainable improvement with longitudinal benchmarking and feedback to patients, clinicians, and healthcare organization leaders.3 LHSs can operate within an individual healthcare unit or organization, across multiple facilities, or at the healthcare system level.4
Figure 1. Core features of a Learning Health System*.
*categories and the descriptions for learning health system features were based on papers by Zurynski Y et al, (2020),8 Menear M et al (2019)4 and Institute of Medicine (2013)12
LITERATURE SEARCH METHODS
From an initial scan of the literature we realized that many aspects of relevant programs that apply LHS models in stroke may not be identified using search terms such as: ‘learning health system*’ (where * indicates a wildcard for searching), ‘learning healthcare,’ ‘learning health-care,’ ‘systems of care,’ ‘care quality,’ ‘quality improvement,’ ‘improv*,’ or ‘implement.*’ Adding terms such as: ‘data-driven,’ ‘hubs’, ‘collaborat*’ ‘stakeholder,’ ‘translation,’ ‘data infrastructure,’ ‘evidence-based,’ ‘informatic*’ or ‘ehealth’ yielded many articles but few of direct relevance to the LHS concepts. In the review by Platt et al assessing the first 10 years of LHS from 2007 to 2017 no eligible articles related to the field of stroke were found, and only two for cardiology.6 Therefore, we used a two-part approach for this topical review.
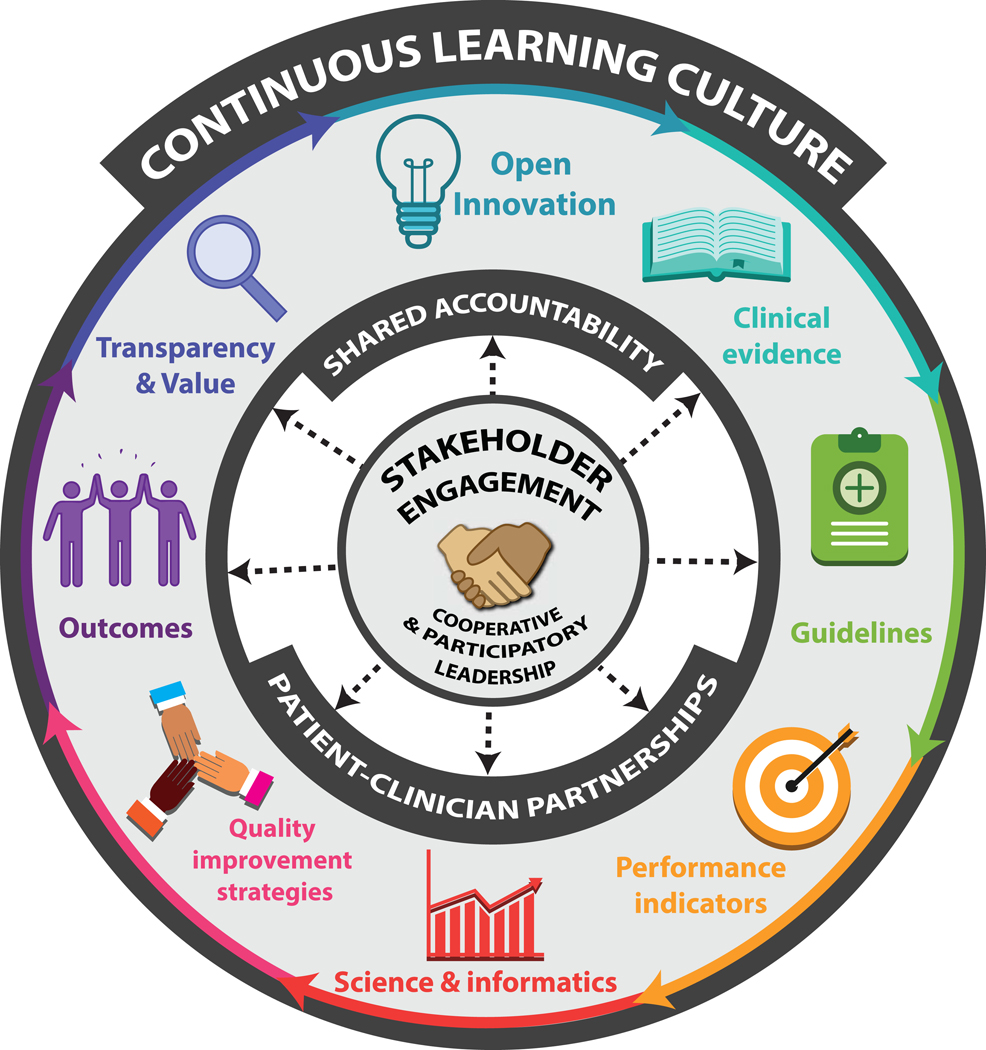
First, we undertook key word searches in Medline and Web-of-science (core collection) to identify relevant articles describing a LHS applied to stroke. Titles and abstracts generated from the searches were checked for relevance, duplicates were deleted, and full articles were retrieved and retained if within scope of this topical review. Reference lists and article citations were manually searched to aid in finding additional articles. The information from the articles was summarized and presented in a narrative format under major themes and subthemes. For this article we report on the features of LHS approaches applied to cerebrovascular disease (ischemic or hemorrhagic stroke and transient ischemic attack [TIA]) from the identified published literature. Figure 2 was developed to describe the core elements of a Stroke Learning System.
Figure 2.
The Stroke Learning System
Second, we designed a case study survey template including 54 closed or open-ended questions/sub-questions to be completed in a Qualtrics survey that mapped onto the Figure 1 framework. We identified senior stroke clinical and policy leaders, involved in World Stroke Organization committees and programs, to provide LHS cases from different regions of the world to enrich our published information with real-world examples. In addition, we identified a lead author who had recently published a quality improvement program for stroke in China to invite them to contribute a case example. We also invited an author to describe a QI project for TIA from the United States Department of Veterans Affairs. Invitations to participate and complete the electronic survey were distributed to 11 individuals by email and each person received one reminder. Invited contributors were requested to describe the LHS approach used in their country, which LHS elements were included, and what component of the stroke continuum of care was the focus (i.e. prevention, treatment, rehabilitation). Respondents were encouraged to provide relevant publications.
RESULTS
The Stroke LHS Literature
Overall, relatively few articles were retrieved that described a LHS applied to stroke care despite multiple refinements to the key word searches. We found several articles that outlined a roadmap for achieving the vision a LHS for stroke.14–17 We also identified a scientific statement from the American Heart Association on LHS and cardiovascular care.5 In this emerging field, few articles explicitly described all of the core features of a LHS (e.g., equity, inclusiveness, person focused, privacy, etc; Figure 1) or all elements of a LHS in stroke care (e.g., performance indicators, data systems with benchmarking, continuous learning for improved quality, stakeholder input, etc; Figure 2). It may have been that in some cases all LHS features were included in the actual implementation of the program, but that these components were not explicitly described in the references. The focus of LHSs for stroke ranged from targeting prevention,16,18–20 acute treatment of ischemic stroke,17,21 TIA,18,22 oral care to prevention of complications after intracerebral hemorrhage,23 and systems-level adoption to improve the whole patient journey from symptom onset to life back in the community.15,24,25 Overall, we found more publications from high income than low-to-middle income countries. There was evidence of the desire to adopt and embed stroke LHSs in low-to-middle income countries.14,26
LHS Published Reports from High Income Countries
Within Canada, six of thirteen provinces established an integrated stroke system between 2005 and 2012 based on national guidelines and tools developed by working groups and supported by public awareness campaigns.25 In these provinces there was specific designation of comprehensive stroke centers with regional strategies to guarantee access to recommended interventions such as thrombolysis and stroke unit care, as well as inter-provider collaboration with telemedicine and performance reporting. These provinces formed a provincial committee and committed funding to support the programs. To better understand the impact of stroke systems of care, differences in stroke mortality between provinces with and without these systems were compared as part of a real-world, retrospective cohort analysis.25 The authors reported a sustained decrease in 30-day in-hospital mortality commensurate with an increase in resources to establish the multifactorial stroke system intervention for stroke treatment and prevention.25 Explicit reference to this systems approach being a LHS was not made.
A regional ‘learning collaborative’ example from Canada is the Saskatchewan Acute Stroke Pathway. This was developed in 2014 by a multidisciplinary team of experts and included representatives from the Ministry of Health, the Saskatchewan Health Quality Council, the individual Saskatchewan Health Regions, Emergency Medical Services (EMS), and patient representatives.21 Process mapping was used to define the hyperacute stroke patient pathway and enabled continued study and refinement that led to a culture of continuous improvement in Saskatchewan. Between June 2015 and December 2016, five different centers trialed the proposed pathway using a series of Plan-Do-Study-Act (PDSA) cycles with the final pathway deployed province-wide across nine centers in January 2017. Stakeholder engagement, including stroke caregivers, at multiple sites as well as the establishment of stroke champions were identified as major success factors. In addition, facility-based stroke leadership teams were essential for knowledge translation and implementation of the new protocols, achieving engagement of local medical and health system leaders, providing local education of EMS partners, and supporting local data collection processes. The provincial Acute Stroke Pathway committee provided medical leadership and practical support and feedback to the facility-based stroke teams in supporting their commitment to change.21 The collaborative nature of the program allowed for process improvements to be discussed regularly with multidisciplinary teams across different centers supporting a shared learning culture. From the successes of this experience, the province committed to developing a centralized dashboard to provide access to real-time data. The Saskatchewan Health Authority planned to expand to other aspects of stroke care as part of a new comprehensive stroke strategy.21
There is also evidence for a single site LHS approach from Canada described as a ‘quality improvement framework.’ Kappor et al demonstrated the advantages of inter-departmental approaches to optimizing acute neurovascular imaging for patients with TIA or minor stroke.27 Specifically, they engaged stakeholders in all aspects of the intervention development including establishing and visibly promoting an agreed memorandum of understanding between the departments involved. They also designed and implemented an electronic decision support tool integrated into the electronic health system to guide physicians at the time of test ordering and provided education in various formats. This reinforced the intervention for the first 15 weeks of implementation through daily active surveillance, and contacting the physicians involved in protocol deviations via email to ensure they were aware of the protocol. The benefits of the intervention, assessed using an interrupted time series analyses, included an increase in the proportion of eligible ‘high-risk’ patients receiving appropriate care and enhanced efficiency given a reduction in duplicate vascular imaging.27
In the United States (US) there were also several stroke LHS examples. A methods paper described the development and optimization of a regional systems of stroke care with the primary objective to increase use of reperfusion therapies and reduce door-to-needle (DNT) times.17 Ehrich et al proposed use of a ‘systems-of-care engineering approach’ to be implemented for nine hub hospitals and their spoke sites, across four US states, for the IMPROVE stroke care project.17 The stakeholders included the public, emergency medical services (EMS), and acute stroke hospitals. The system was based on establishing a manual of operations as a living document that could be updated as new knowledge emerged from the consortium or as published evidence for stroke changed. They proposed access to near-real time data to evaluate effectiveness by working with health information technology groups to create a seamless data collection system that can harness mobile technology/equipment and can allow self-reported patient data to be captured.17 Explicit reference to this systems approach being a LHS was not made.
In a second example, a LHS systems approach to support clinical teams to improve the quality of care for patients with TIA was evaluated within six Department of Veteran Affairs medical centers.22 The program was designed on the basis of stakeholder interviews, the existing literature, validated electronic quality measures, and baseline quality of care data identifying opportunities for improvement.28 The five component TIA LHS (referred to as the PREVENT program) included: multi-media professional education; a digital ‘hub’ that included a dashboard for dynamic quality of care data displays;18 QI support, tools and resources, and a virtual collaborative29 to share lessons learned across teams; clinical programs (e.g., pharmacy-based medication protocol, ED TIA protocol), and electronic health record tools (e.g., patient identification tools, templated notes, order menus).28 External facilitators supported program implementation.30 The program improved care quality at the six active implementation sites. Sites with the greatest implementation success were those with champions whose teams engaged in planning and goal setting, and regularly reflected upon their performance data and evaluated their progress against their QI plans.31 Based on the strength of the quality improvement at participating sites, the PREVENT program was deployed across the national Veteran Affairs system which is the largest integrated healthcare system in the US (see below for the case example).
A third example from the US stems from the states funded by the Centers for Disease Control and Prevention to participate in the Paul Coverdell National Acute Stroke Registry.32 Several states transitioned from surveillance and retrospective analysis of data in the registry to active programs aimed to improve quality and outcomes.33–35 For example, a quality improvement initiative for stroke was designed by the Wisconsin Coverdell Stroke Program within a single ‘model’ hospital. Developmental evaluation approach was used whereby the evaluators collaborated with the project stakeholders to address process issues within dynamic contexts to co-design interventions.15 Process mapping and stakeholder interviews were undertaken to provide in-depth detail on the local challenges and successes associated with their patient and data flow. Patient and caregivers provided feedback, as well as EMS and hospital clinical staff. Accountability, communication and partnerships were the main themes that emerged from the interviews and the evaluation provided a method for stakeholders to identify opportunities to work more effectively by agreeing on shared strategies.15
The Coverdell program partnered with the Joint Commission and the American Heart/Stroke Associations Get With The Guidelines®-Stroke (GWTG-Stroke) programs to align performance measures, reduce duplication and encourage hospitals to participate in one or more programs. Although networks in these programs employ different LHS concepts, explicit reference to LHS is not made. GWTG-Stroke is an example of a hospital registry and quality improvement program that has leveraged real world clinical data to advance knowledge and drive evidence into practice. The hospital-based GWTG-Stroke program focuses on performance measurement and the use of Plan Do Study Act cycles for ongoing, rapid quality improvement. Learning sessions provide a forum for sharing across participating facilities and to disseminating emerging evidence. Hospital awards offer public acknowledgement of stroke QI achievement.36 The GWTG-Stroke program has been successfully implemented at over 2000 US hospitals, which accounted for approximately half of index stroke patient admissions annually.36 Participation in the GWTG-Stroke program has been associated with improvements in care quality (e.g., increased use of antithrombotics, thrombolysis, smoking cessation education) in the US.36–40 While significant in the overall improvements made in stroke care and outcomes, reports to date do not explicitly describe components of LHSs at the individual participating institutions. More specifically, the capacity at local hospitals to examine their data, partner with informaticists and integrate GWTG-Stroke data with other hospital or community data is not addressed in publications. It is also unclear if individual hospitals engage patients locally to guide generation of evidence, innovation and improvements to care. The use of incentives in GWTG-Stroke is also an area for further investigation.
In some regions and countries, networks have tested specific strategies for improvement including those from China, South America and Australia.41–44 Each exemplifies one or two LHS components, but none address how the participating hospitals and health systems operationalized these beyond the study for long-term evidence generation, innovation and improvements to care and outcomes. Even among the most robust studies is a dissemination gap in how participating hospitals and healthcare systems were subsequently able to sustain clinical decision support, predictive models, surveillance, and comparative effectiveness, implementation or quality improvement research.
A single stroke center LHS in Japan used data to drive improvements to clinical pathways and prevent complications following intracerebral hemorrhage associated with poor oral care.23 The authors described the use of machine learning to identify risk factors specific to patients with stroke to create a risk-stratified clinical pathway to improve the quality of oral care. This LHS evolved between 2012 and 2018 and, consequently, a decrease in stroke-associated pneumonia was found.23
In the United Kingdom a co-designed clinical decision support system integrated with the electronic medical record for the management of risk factors for stroke secondary prevention among patients with multimorbidity has been described; a future feasibility trial is planned.16,19 The comprehensive stakeholder engagement process used in designing, prototyping and evaluating their decision aid for use in general practice settings was undertaken using qualitative interviews. The authors state that their comprehensive approach sets the standard for delivering LHS interventions for clinical practice.16
In the Czech Republic, LHS approach was used to dramatically shorten national DNT time for intravenous thrombolysis (IVT). In a population of around 10 million, there are 13 comprehensive and 32 primary stroke centers in the Czech Republic, all of them certified by Ministry of Health. Referral of patients by EMS is legally mandated only within certified network. More details about development and organization of national stroke network were published.45
Based on the evidence that shorter onset-to-treatment time improves outcome after IVT, Czech Stroke Society facilitated collection of relevant logistical data in Registry of Stroke Care Quality and provided monthly feedback to every stroke center. Using other supplementary activities such as simulation training and with involvement of Angels Initiative, national DNT time for IVT was shortened to national median 25 minutes in 201845 and 22 minutes in 2019.46 Next, analysis of impact of shortening of DNT on outcome documented that dramatic shortening of DNT improved outcome without any negative impact on e.g. risk of bleeding (actually it was opposite = less bleeding).46 Based on the evidence, that ultrashort DNT is feasible to be achieved in any type of the hospital and that such shortening is improving outcome, Czech Stroke Society modified national stroke guidelines and since 2021 recommend that target median DNT time should be below 20 minutes.
Reports from low-to-middle income countries
With the transition to a universal health care in the Philippines, policy has been established to build capacity for acute stroke management and establish Acute Stroke Ready Hospitals.14 The authors detailing the current state of stroke care in the Philippines included recommendations for leadership and governance, financing, service delivery and the health workforce capacity, and the need for more integrated data reporting systems to address recognized gaps.14 Explicit reference to the systems approach being recommended as a LHS was not made.
In 2015 there was a call to build a LHS for stroke and heart disease for China to increase accessibility to high quality care, and underpin it with the capacity to monitor performance to learn about what works best for particular subgroups of patients.26 Subsequently, the Chinese Stroke Center Alliance emerged to support uptake of national stroke guidelines, provide quality improvement tools including a monitoring and feedback system for performance measures.24 By 2017, 1576 hospitals had contributed data and the alliance committed to provide ongoing training workshops of stroke center development and stroke care to improve stroke center expansion and stroke care quality.24 Many reports have emerged and efforts are ongoing.47
Quality improvement collaboratives: a stepping stone for learning health systems in stroke
A systematic review of ‘quality improvement collaboratives (QIC)’ included 20 papers which described 12 QICs.48 The individual QICs ranged from having 10–24 sites and covered different aspects of stroke care including pre-hospital care, hospital care, rehabilitation, and primary care settings.48 The methods covered shared learning lessons, local QI activities in various formats with the majority having access to web-based/electronic data systems to receive performance feedback. The authors of this review found that the greater the complexity of the QIC the more challenging is was to implement and achieve positive outcomes. In some organizations, little experience with QI initiatives or the lack of familiarity with national data registries led to poor uptake or limited improvements being reported. Other barriers included lack of organizational or local leader support, limited access to resources, or the sole reliance on one clinical champion. It was also found that when QIC support and resources were no longer available that continued improvement might not be sustained. Limitations identified within this body of research was lack of involvement of patients or caregivers in providing their perspectives of QI and only half of the QIC measured patient outcomes.48 The authors call for future research to focus on methods to sustain the benefits of short-term QIC and that engagement, communication and access to best-practice examples could enhance QIC success. Explicit reference to adoption QIC components within a LHS was not made. In this review, the majority of included QICs were from high income countries including US, England, Netherlands and Australia, with one example from Taiwan.49
International LHS Case Examples
We provide seven case examples of LHS from Nigeria, Africa (Af), Australia (Aus1; Aus2), the Czech Republic (CZ), New Zealand (NZ), Sweden (SW) and the United States (US). The focus of the LHS examples included pre-hospital care (Aus1, SW), acute stroke care (Af, Aus1, Aus2, CZ, NZ, SW, US), TIA care (US), prevention (Af, CZ, SW), telemedicine (Af, Aus1, NZ, SW), rehabilitation care (CZ, SW), and long-term care (SW). Three of the examples represented a national approach while the others were based at the regional level. Full details are provided in the Supplemental file.
Use of Science and Informatics differed in terms of the sources of data (periodic audit, use of administrative data, continuous collection via registries) and ranged from having real-time access to data reports (n=6) using dashboards and benchmarking. In terms of Patient-Clinician partnerships, 4/7 case examples included patient representatives on their governance committees. In several case examples it was possible for patients to access their health records or update their own data (US) or receive a copy of their data (NZ, SW). The Continuous Learning Culture described included interdisciplinary engagement with leaders from academic, clinical, government/non-government and patient organizations. National clinical guidelines were used for developing or updating LHS priorities for monitoring and QI. Multiple strategies were used to update best practice approaches including education sessions; webinars; community advisory board meetings; peer engagement workshops; reports to government, collaborative communities/networks, advocacy with non-government bodies, journal articles, media and communication via emails, websites and conferences.
All case examples used QI initiatives or strategies to improve stroke care. The strategies most often used were education, hospital/clinician engagement, audit and feedback including use of regular PDSA cycles, quality metrics, workshops (virtual/face-to-face), hospital visits/external facilitation, webinars/virtual collaboratives, reminders, staff meetings protocols or clinical pathways, or systems redesign-based kick-offs. In terms of Structure and Governance, each LHS example had a leadership group with clinical experts for oversight and direction with varying membership of community representatives, government of broader stroke society networks. There were only two case examples that used financial incentives directed at health services providers as part of their LHS approach (Aus2, NZ). While in 2/7 public reporting was used as an incentive or open peer reporting as the basis for motivating care improvements.
The lessons learned from the implementation of stroke LHSs based on these case examples are described in Table 150–55 and included: the critical role of quality of care data (CR, NZ, SW, US), the importance of adequate staffing (Aus1), the effectiveness of financial incentives (Aus2), the value of leadership involvement (CR), the benefits of a multi-pronged approach (NZ), the imperative to include everyone to reduce inequity (NZ), and the difficulty with program sustainability (US).
Table 1:
Case examples of Stroke Learning Health Systems and lessons learnt
| ID | Country Name of program References | Description of program and relevant websites | Lessons learnt from implementation of a stroke LHS |
|---|---|---|---|
| Af | Africa ARISES50 | A mobile-health based stroke information and surveillance system to improve stroke awareness, recognition, and presentation at an appropriate facility. https://grantome.com/grant/NIH/R01-NS115944–01 | Huge proportion of patients do not present promptly in appropriate facilities after a stroke. Inadequate knowledge and financial constrains were identified as barriers. |
| Aus1 | Australia Victorian Stroke Telemedicine program | Equitable access to acute reperfusion therapies in regional hospitals: the Victorian Stroke Telemedicine program. https://www.vst.org.au/ | Staffing requirements to provide the service with increasing demand. Training and education needs Proved the feasibility and effectiveness of a state-wide program based on patient-level data, clinician feedback and cost-effectiveness evidence.51,52 |
| Aus2 | Australia Stroke12353 | Multi-component quality improvement program to improve care for patients with stroke/TIA in acute hospitals in Queensland. https://auscr.com.au/ | Use of financial incentives and externally facilitated audit and feedback workshops led to increased adherence to quality indicators for acute stroke care. National registry data permitted assessment against hospitals outside of Queensland that were not offered the program. |
| CZ | Czech Republic RES-Q | Leadership-Czech stroke society. Partners: all certified stroke centers. Other partners: Ministry of Health, Insurance companies. Data collection on stroke care quality through the Registry of Stroke Care Quality (RES-Q). Audit and feedback through RES-Q online + through communication between Society and Hospitals. https://qualityregistry.eu/ | Leadership by Stroke Society and collection of data on stroke care quality in RES-Q are the most important components. |
| NZ | New Zealand | Data driven service improvement programmes (e.g. National REGIONS audit,54 Stroke volume estimates to support service planning55). QI programs to support reperfusion or Telestroke registers. | (1) Good data are essential (2) Government imperative is very helpful (3) A multi-pronged approach to meet different learning styles is important (4) A collaborative approach is most effective (shared goal with a little competition rather than the reverse) (5) Involving everyone (not just the willing and motivated) and making it feasible for everyone is the only way to address inequity |
| SW | Sweden Riksstroke | The Swedish Stroke Register (Riksstroke) Multi-component quality improvement program to improve care for patients with stroke/TIA in acute hospitals and follow up at 3 months and 1 year including PROMS and PREMS. On-line dashboard for core variables. Target values for several items have been set. Register items closely aligned to national guidelines recommendations. https://www.riksstroke.org/eng/ | Some quality metrics are in the hands of clinicians and possible to adapt to (e g changes in preventive drug therapies). Other quality domains are related to structure and staffing, and have been very difficult to change. |
| US | United States PREVENT18,22,28,29 | Multi-component quality improvement program to improve care for patients with TIA at US Department of Veterans Affairs facilities. | The challenge for this program was sustainability; specifically, moving from research at six sites to national deployment was successful, but the transition from national deployment to ongoing clinical operations was problematic. |
LHS: learning health system; QI: quality improvement; PROMS: patient-reported outcome measures; PREMS: patient-reported experience measures; TIA: transient ischemic attack
DISCUSSION
In the field of stroke, we found only a few published examples of LHSs and health systems globally are implementing some selected LHS concepts, but the term is not common. A major barrier to identifying relevant LHS examples in stroke may be the lack of an agreed taxonomy or terminology for classification. Related, it is quite possible that health service delivery settings that leverage many of the LHS concepts do so operationally and the lessons learned are not shared in peer reviewed literature. Although the field of LHSs applied to stroke care seems to be in its infancy, it is quite possible that related advancements and high functioning systems have not yet been examined through this lens. The evolution of quality improvement programs such as GWTG-Stroke or national clinical quality registries into fully realized LHS can be advanced through technology and greater involvement in patient-clinician partnerships. This will increase the capabilities within organizations to embed intelligent automation and clinical decision support. It will also support greater involvement of their patients to provide input on clinical and research matters or engage with their health data to understand their health, care management, and personal actions that support it.5 It is also acknowledged that health system leaders seeking to transform their organization toward an LHS needs to make significant monetary investments, hire staff with specialized skills, and re-allocate effort toward continuous learning and knowledge generation.1
It is likely that this topical review will further stimulate the stroke community to disseminate related activities. Easterling, et al evaluated 79 LHS publications across various clinical domains and identified 94 LHS elements which they organized into a LHS consolidated framework.1 The four most common elements were: the system generates knowledge or evidence, QI activities are standard practice, patients and family members are actively engaged, and there is a learning culture. Similar trends were identified in this topical review and it is expected that the adoption of LHS terminology in publications on stroke will grow. In 2017 the AHA published a scientific statement with recommendations for next steps in the development of a LHS for cardiovascular disease.5 Recent enhancements to data infrastructure and informatics tools (e.g., validated electronic quality measures,56,57 natural language processes to extract radiology information58) and acceptance of patient-provider partnerships and continuous learning will further support the transformation needed for stroke care. Specifically, this AHA Scientific Statement5 provides an important outline for the framework elements of Science and Informatics, Patient-Clinician partnerships, Leadership, culture and incentives related to what success would look like as a useful road map for continued movement toward functional LHSs. Our paper extends these concepts to provide additional guidance on structure and governance with examples for readers.
Learning is fundamental to the LHS model.59 As facilities, systems, and countries develop and implement stroke LHSs as outline in Figure 2, they should explicitly consider how to optimize ongoing learning and adaption. That is from 1) evidence generation and synthesis (including mechanisms to share evidence and guidelines as they emerge); 2) engagement with stakeholders (including patients, front-line clinicians, and hospital or health system administrators); 3) standardized data (including access to real-time, high-quality performance data); and 4) shared learning (specifically from other participants in the LHS, where the learning includes both stroke-specific information as well as topics in quality improvement and implementation science). Both the literature and case examples highlighted in this topical review underscore the importance of developing cultures that embrace learning and QI. There is a tremendous opportunity for greater learning across the stroke community and also for learning from more established LHSs with non-stroke conditions including pediatric liver transplantation,60 inflammatory bowel disease,61 and lung cancer care.62 In the future, it may be a requirement that QI articles in stroke describe how each of the LHS framework elements have been applied.
Health care delivery is often perceived as a set of resources and processes decided upon by health care providers, administrators, payors and politicians. However, it is important to recognize that a high quality LHS will have clear and active engagement of patients, families, clinicians and external stakeholders. Stakeholders and partners are integral to every LHS core feature and they should have a role in every component. This level of inclusion may require a shift for some work cultures; only half of our case examples included patients as partners. A study of 16 LHSs in the US found that the level of involvement may change over time and research is need on how to consistently involve patients in LHSs.63
Principles from several types of research could advance the concepts of LHSs in the stroke community. In addition to the opportunity for leveraging community-based participatory research to increase patient, family and stakeholder engagement in health care transformation, experts in community-based participatory research can also facilitate the more equitable involvement, collaboration and integration of knowledge and ideas.64 This level of engagement can support a more resilient and sustainable model of partnership between healthcare and non-healthcare stakeholders and build trust as a learning healthcare community.65 Several references to learning and QI emphasize the natural fit for improvement science but also the undeniable need for implementation science. Involving experts trained in implementation science can rapidly improve the rigor and translation of the cycles of learning, disseminating and scaling sustainable value-based strategies for evidence-based care delivery.66 The measures of translation, change and improvement can only be possible with a firm foundation in data and informatics. Experts in the UK called on policy makers and funders of research and education to invest in the interdisciplinarity of clinical and bioinformatics as these LHSs depend on the often siloed field of experts that could be transformative if working together.67 community-based participatory research, implementation science, research translation, data science and informatics are not new to stroke research but could all be explored differently in this context of implementing a LHS to optimize stroke care quality, outcomes and costs.
We acknowledge that this topical narrative review may have missed important published examples including from the QI literature especially given the heterogeneity in terms used to describe programs that may adhere to the majority of LHS concepts, articles not published in English or those disseminated as grey literature such as online or organizational reports. There may also be publication bias if negative studies of stroke LHSs have not been afforded a high enough priority by editors or reviewers. Our attempt to systematically identify the relevant literature and obtain real world examples demonstrated the dissemination gaps, the lack of learning and action for many of the related LHS concepts across the continuum of care, and the opportunity for continued dialogue on how to study and scale LHS advances.
Conclusions
The stroke LHS literature primarily includes programs from high income countries which focus most commonly on acute stroke management and prevention. The literature provides examples of stroke LHS from single sites, regional systems, and national healthcare systems. Although several QI strategies were reported to be effective for advancing stroke LHSs, much less is known about patient-clinician and stakeholder engagement, governance and culture, or sophisticated application of data informatics to inform practice or improve the quality and value of care. The promise of LHSs can be realized and is still necessary in order to further advance stroke research and continue improvements in care and outcomes.
Supplementary Material
Acknowledgments
The authors thank Megan Reyneke from the Department of Medicine, School of Clinical Sciences at Monash Health for setting up the Qualtrics survey; Lachlan Dalli from the Department of Medicine, School of Clinical Sciences at Monash Health; Monash University, Australia for preparing the visual abstracts/infographics.
Sources of Funding
No specific funding for this article was obtained.
Disclosures
DAC acknowledges research fellowship support from the National Health and Medical Research Council (#1154273). RM was supported by various projects: IRENE COST Action #CA18118 (COST Association), IRIS-TEPUS #LTC20051 (INTER-EXCELLENCE INTER-COST Program of the Ministry of Education, Youth and Sports of the Czech Republic), STROCZECH within CZECRIN Large Research Infrastructure (#LM2018128; funded by the state budget of the Czech Republic), CARES CZ # NU21-09-00548 (Ministry of Health of the Czech Republic), and the INBIO Project (#CZ.02.1.01/0.0/0.0/16_026/0008451; European Regional Development Fund). BN has received honoraria from AstraZeneca and Bayer for Data Safety Monitoring Boards for the THALES and NAVIGATE-ESUS trials. MOO is supported by NIH grants: SIREN (U54HG007479), SIBS Genomics (R01NS107900), SIBS Gen Gen (R01NS107900-02S1), ARISES (R01NS115944-01), H3Africa CVD Supplement (3U24HG009780-03S5), CaNVAS (1R01NS114045-01), Sub-Saharan Africa Conference on Stroke (SSACS) 1R13NS115395-01A1 and Training Africans to Lead and Execute Neurological Trials & Studies (TALENTS) D43TW012030. AR reports grants from the Health Research Council of New Zealand and the Ministry of Health (NZ), travel grants from University of Texas (Austin) and Virginia Commonwealth University School of Medicine (United States) unrelated to this article, employment by Capital and Coast District Health Board. MFK acknowledges a Future Leader Fellowship (#105737) from the National Heart Foundation of Australia. MFK Member of Stroke Foundation Research and Advisory Committee and Australian Institute of Health and Welfare Cardiovascular Disease Expert Advisory Group. Associate Editor of the Health Information Management Journal.
Abbreviations
- Af
Africa
- Aus
Australia
- CZ
Czech Republic
- DNT
door-to-needle
- EMS
emergency medical services
- IVT
intravenous thrombolysis
- LHS
learning health system
- NZ
New Zealand
- PDSA
Plan-Do-Study-Act
- QI
quality improvement
- QIC
quality improvement collaboratives
- SW
Sweden
- TIA
transient ischemic attack
- VST
Victorian Stroke Telemedicine
- US
United States
References
- 1.Easterling D, Perry AC, Woodside R, Patel T, Gesell SB. Clarifying the concept of a learning health system for healthcare delivery organizations: Implications from a qualitative analysis of the scientific literature. Learn Health Syst. 2022;6:e10287. doi: 10.1002/lrh2.10287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley TJ, Vale L. What role for learning health systems in quality improvement within healthcare providers? Learn Health Syst. 2017;1:e10025. doi: 10.1002/lrh2.10025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enticott J, Johnson A, Teede H. Learning health systems using data to drive healthcare improvement and impact: a systematic review. BMC Health Serv Res. 2021;21:200. doi: 10.1186/s12913-021-06215-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menear M, Blanchette MA, Demers-Payette O, Roy D. A framework for value-creating learning health systems. Health Res Policy Syst. 2019;17:79. doi: 10.1186/s12961-019-0477-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maddox TM, Albert NM, Borden WB, Curtis LH, Ferguson TB, Kao DP, Marcus GM, Peterson ED, Redberg R, Rumsfeld JS, et al. The Learning Healthcare System and Cardiovascular Care: A Scientific Statement From the American Heart Association. Circulation. 2017;135:E826–E857. doi: 10.1161/cir.0000000000000480 [DOI] [PubMed] [Google Scholar]
- 6.Platt JE, Raj M, Wienroth M. An Analysis of the Learning Health System in Its First Decade in Practice: Scoping Review. J Med Internet Res. 2020;22:e17026. doi: 10.2196/17026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLachlan S, Potts HWW, Dube K, Buchanan D, Lean S, Gallagher T, Johnson O, Daley B, Marsh W, Fenton N. The Heimdall Framework for Supporting Characterisation of Learning Health Systems. J Innov Health Inform. 2018;25:77–87. doi: 10.14236/jhi.v25i2.996 [DOI] [PubMed] [Google Scholar]
- 8.Zurynski Y, Smith CL, Vedovi A, Ellis LA, Knaggs G, Meulenbroeks I, Warwick M, Gul H, Pomare C, Braithwaite J. Mapping the Learning Health System: A Scoping Review of Current Evidence - A White Paper. In: Sydney, Australia: Australian Institute of Health Innovation; 2020:63. [Google Scholar]
- 9.Ellis LA, Sarkies M, Churruca K, Dammery G, Meulenbroeks I, Smith CL, Pomare C, Mahmoud Z, Zurynski Y, Braithwaite J. The Science of Learning Health Systems: Scoping Review of Empirical Research. JMIR Med Inform. 2022;10:e34907. doi: 10.2196/34907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enticott JC, Melder A, Johnson A, Jones A, Shaw T, Keech W, Buttery J, Teede H. A Learning Health System Framework to Operationalize Health Data to Improve Quality Care: An Australian Perspective. Front Med (Lausanne). 2021;8:730021. doi: 10.3389/fmed.2021.730021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foley T, Horwitz L, Zahran R. The Learning Healthcare Project: Realising the potential of leaning health systems. In: United Kingdom: Newcastle University; 2021:101. [Google Scholar]
- 12.Institute of Medicine. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 13.Kilkenny MF, Bravata DM. Quality Improvement. Stroke. 2021;52:1866–1870. doi: 10.1161/STROKEAHA.121.033451 [DOI] [PubMed] [Google Scholar]
- 14.Collantes MV, Zuniga YH, Granada CN, Uezono DR, De Castillo LC, Enriquez CG, Ignacio KD, Ignacio SD, Jamora RD. Current State of Stroke Care in the Philippines. Front Neurol. 2021;12:665086. doi: 10.3389/fneur.2021.665086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zusevics KL, Kaemmerer NN, Lang J, Link J, Bluma DD. A Unique Approach to Quality Improvement Within the Stroke System of Care Utilizing Developmental Evaluation. Health Promot Pract. 2021;22:224–235. doi: 10.1177/1524839919894305 [DOI] [PubMed] [Google Scholar]
- 16.Porat T, Marshall IJ, Sadler E, Vadillo MA, McKevitt C, Wolfe CDA, Curcin V. Collaborative design of a decision aid for stroke survivors with multimorbidity: a qualitative study in the UK engaging key stakeholders. BMJ Open. 2019;9:e030385. doi: 10.1136/bmjopen-2019-030385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrlich ME, Kolls BJ, Roettig M, Monk L, Shah S, Xian Y, Jollis JG, Granger CB, Graffagnino C. Implementation of Best Practices-Developing and Optimizing Regional Systems of Stroke Care: Design and Methodology. Am Heart J. 2020;222:105–111. doi: 10.1016/j.ahj.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 18.Rattray NA, Damush TM, Miech EJ, Homoya B, Myers LJ, Penney LS, Ferguson J, Giacherio B, Kumar M, Bravata DM. Empowering Implementation Teams with a Learning Health System Approach: Leveraging Data to Improve Quality of Care for Transient Ischemic Attack. J Gen Intern Med. 2020;35:823–831. doi: 10.1007/s11606-020-06160-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadler E, Porat T, Marshall I, Hoang U, Curcin V, Wolfe CDA, McKevitt C. Shaping innovations in long-term care for stroke survivors with multimorbidity through stakeholder engagement. PLoS One. 2017;12:16. doi: 10.1371/journal.pone.0177102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felipe RA, Plescia M, Peterman E, Tomlin H, Sells M, Easley C, Ahmed K, Presley-Cantrell L. A Public Health Framework to Improve Population Health Through Health Care and Community Clinical Linkages: The ASTHO/CDC Heart Disease and Stroke Prevention Learning Collaborative. Prev Chronic Dis. 2019;16:6. doi: 10.5888/pcd16.190065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holodinsky JK, Onaemo VN, Whelan R, Hunter G, Graham BR, Hamilton J, Schwartz L, Latta L, Peeling L, Kelly ME. Implementation of a provincial acute stroke pathway and its impact on access to advanced stroke care in Saskatchewan. BMJ Open Qual. 2021;10. doi: 10.1136/bmjoq-2020-001214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bravata DM, Myers LJ, Perkins AJ, Zhang Y, Miech EJ, Rattray NA, Penney LS, Levine D, Sico JJ, Cheng EM, et al. Assessment of the Protocol-Guided Rapid Evaluation of Veterans Experiencing New Transient Neurological Symptoms (PREVENT) Program for Improving Quality of Care for Transient Ischemic Attack: A Nonrandomized Cluster Trial. JAMA network open. 2020;3:e2015920. doi: 10.1001/jamanetworkopen.2020.15920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto K, Nohara Y, Wakata Y, Yamashita T, Kozuma Y, Sugeta R, Yamakawa M, Yamauchi F, Miyashita E, Takezaki T, et al. Impact of a learning health system on acute care and medical complications after intracerebral hemorrhage. Learn Health Syst. 2021;5:9. doi: 10.1002/lrh2.10223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Li Z, Wang Y, Zhao X, Liu L, Yang X, Wang C, Gu H, Zhang F, Wang C, et al. Chinese Stroke Center Alliance: a national effort to improve healthcare quality for acute stroke and transient ischaemic attack: rationale, design and preliminary findings. Stroke Vasc Neurol. 2018;3:256–262. doi: 10.1136/svn-2018-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganesh A, Lindsay P, Fang J, Kapral MK, Cote R, Joiner I, Hakim AM, Hill MD. Integrated systems of stroke care and reduction in 30-day mortality: A retrospective analysis. Neurology. 2016;86:898–904. doi: 10.1212/WNL.0000000000002443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang LX, Krumholz HM, Li X, Li J, Hu SS. Achieving best outcomes for patients with cardiovascular disease in China by enhancing the quality of medical care and establishing a learning health-care system. Lancet. 2015;386:1493–1505. doi: 10.1016/s0140-6736(15)00343-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapoor A, Verma A, Kim IJ, Kujbid N, Si K, Casaubon LK, Kapral MK, Fang J, Symons S, Swartz RH, et al. Multidisciplinary quality improvement initiative to optimize acute neurovascular imaging for transient ischemic attack or minor stroke. CJEM. 2021;23:820–827. doi: 10.1007/s43678-021-00180-1 [DOI] [PubMed] [Google Scholar]
- 28.Bravata DM, Myers LJ, Homoya B, Miech EJ, Rattray NA, Perkins AJ, Zhang Y, Ferguson J, Myers J, Cheatham AJ, et al. The protocol-guided rapid evaluation of veterans experiencing new transient neurological symptoms (PREVENT) quality improvement program: rationale and methods. BMC neurology. 2019;19:294. doi: 10.1186/s12883-019-1517-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penney LS, Homoya BJ, Damush TM, Rattray NA, Miech EJ, Myers LJ, Baird S, Cheatham A, Bravata DM. Seeding Structures for a Community of Practice Focused on Transient Ischemic Attack (TIA): Implementing Across Disciplines and Waves. J Gen Intern Med. 2021;36:313–321. doi: 10.1007/s11606-020-06135-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penney LS, Damush TM, Rattray NA, Miech EJ, Baird SA, Homoya BJ, Myers LJ, Bravata DM. Multi-tiered external facilitation: the role of feedback loops and tailored interventions in supporting change in a stepped-wedge implementation trial. Implement Sci Commun. 2021;2:82. doi: 10.1186/s43058-021-00180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damush TM, Miech EJ, Rattray NA, Homoya B, Penney LS, Cheatham A, Baird S, Myers J, Austin C, Myers LJ, et al. Implementation Evaluation of a Complex Intervention to Improve Timeliness of Care for Veterans with Transient Ischemic Attack. J Gen Intern Med. 2021;36:322–332. doi: 10.1007/s11606-020-06100-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labarthe DR, Biggers A, LaPier T, George MG, Paul Coverdell National Acute Stroke R. The Paul Coverdell National Acute Stroke Registry (PCNASR): a public health initiative. Am J Prev Med. 2006;31:S192–195. doi: 10.1016/j.amepre.2006.07.027 [DOI] [PubMed] [Google Scholar]
- 33.California Acute Stroke Pilot Registry I. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64:654–659. doi: 10.1212/01.WNL.0000151850.39648.51 [DOI] [PubMed] [Google Scholar]
- 34.Stewart VT. Use of a prototype acute stroke registry to improve care: profile of receptive stroke programs. Am J Prev Med. 2006;31:S217–223. doi: 10.1016/j.amepre.2006.08.014 [DOI] [PubMed] [Google Scholar]
- 35.Stoeckle-Roberts S, Reeves MJ, Jacobs BS, Maddox K, Choate L, Wehner S, Mullard AJ. Closing gaps between evidence-based stroke care guidelines and practices with a collaborative quality improvement project. Jt Comm J Qual Patient Saf. 2006;32:517–527. doi: 10.1016/s1553-7250(06)32067-3 [DOI] [PubMed] [Google Scholar]
- 36.Ormseth CH, Sheth KN, Saver JL, Fonarow GC, Schwamm LH. The American Heart Association’s Get With the Guidelines (GWTG)-Stroke development and impact on stroke care. Stroke Vasc Neurol. 2017;2:94–105. doi: 10.1136/svn-2017-000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xian Y, Xu H, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Hernandez AF, Peterson ED, Schwamm LH, Fonarow GC. Achieving More Rapid Door-to-Needle Times and Improved Outcomes in Acute Ischemic Stroke in a Nationwide Quality Improvement Intervention. Stroke. 2022;53:1328–1338. doi: 10.1161/STROKEAHA.121.035853 [DOI] [PubMed] [Google Scholar]
- 38.Fonarow GC, Reeves MJ, Smith EE, Saver JL, Zhao X, Olson DW, Hernandez AF, Peterson ED, Schwamm LH, Committee GW-SS, et al. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in get with the guidelines-stroke. Circ Cardiovasc Qual Outcomes. 2010;3:291–302. doi: 10.1161/CIRCOUTCOMES.109.921858 [DOI] [PubMed] [Google Scholar]
- 39.Hu G, Zhou M, Liu J, Smith SC Jr., Ma C, Ge J, Huo Y, Fonarow GC, Hao Y, Liu J, et al. Smoking and Provision of Smoking Cessation Interventions among Inpatients with Acute Coronary Syndrome in China: Findings from the Improving Care for Cardiovascular Disease in China-Acute Coronary Syndrome Project. Glob Heart. 2020;15:72. doi: 10.5334/gh.784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis WR, Fonarow GC, Grau-Sepulveda MV, Smith EE, Bhatt DL, Hernandez AF, Olson D, Peterson ED, Schwamm LH. Improvement in use of anticoagulation therapy in patients with ischemic stroke: results from Get With The Guidelines-Stroke. Am Heart J. 2011;162:692–699 e692. doi: 10.1016/j.ahj.2011.07.019 [DOI] [PubMed] [Google Scholar]
- 41.Levi CR, Attia JA, D’Este C, Ryan AE, Henskens F, Kerr E, Parsons MW, Sanson-Fisher RW, Bladin CF, Lindley RI, et al. Cluster-Randomized Trial of Thrombolysis Implementation Support in Metropolitan and Regional Australian Stroke Centers: Lessons for Individual and Systems Behavior Change. J Am Heart Assoc. 2020;9:e012732. doi: 10.1161/JAHA.119.012732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machline-Carrion MJ, Santucci EV, Damiani LP, Bahit MC, Malaga G, Pontes-Neto OM, Martins SCO, Zetola VF, Normilio-Silva K, Rodrigues de Freitas G, et al. Effect of a Quality Improvement Intervention on Adherence to Therapies for Patients With Acute Ischemic Stroke and Transient Ischemic Attack: A Cluster Randomized Clinical Trial. JAMA Neurol. 2019;76:932–941. doi: 10.1001/jamaneurol.2019.1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong W, Lin L, Gong X, Chen Z, Chen Y, Yan S, Zhou Y, Zhang X, Hu H, Tong L, et al. Evaluation of a multicomponent intervention to shorten thrombolytic door-to-needle time in stroke patients in China (MISSION): A cluster-randomized controlled trial. PLoS Med. 2022;19:e1004034. doi: 10.1371/journal.pmed.1004034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Li Z, Zhao X, Wang C, Wang X, Wang D, Liang L, Liu L, Wang C, Li H, et al. Effect of a Multifaceted Quality Improvement Intervention on Hospital Personnel Adherence to Performance Measures in Patients With Acute Ischemic Stroke in China: A Randomized Clinical Trial. JAMA. 2018;320:245–254. doi: 10.1001/jama.2018.8802 [DOI] [PubMed] [Google Scholar]
- 45.Mikulik R, Bar M, Cernik D, Herzig R, Jura R, Jurak L, Neumann J, Sanak D, Ostry S, Sevcik P, et al. Stroke 20 20: Implementation goals for intravenous thrombolysis. Eur Stroke J. 2021;6:151–159. doi: 10.1177/23969873211007684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikulik R, Bar M, Belaskova S, Cernik D, Fiksa J, Herzig R, Jura R, Jurak L, Klecka L, Neumann J, et al. Ultrashort Door-to-Needle Time for Intravenous Thrombolysis Is Safer and Improves Outcome in the Czech Republic: Nationwide Study 2004 to 2019. J Am Heart Assoc. 2022;11:e023524. doi: 10.1161/JAHA.121.023524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang YJ, Li ZX, Gu HQ, Zhai Y, Zhou Q, Jiang Y, Zhao XQ, Wang YL, Yang X, Wang CJ, et al. China Stroke Statistics: an update on the 2019 report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol. 2022. doi: 10.1136/svn-2021-001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowther HJ, Harrison J, Hill JE, Gaskins NJ, Lazo KC, Clegg AJ, Connell LA, Garrett H, Gibson JME, Lightbody CE, et al. The effectiveness of quality improvement collaboratives in improving stroke care and the facilitators and barriers to their implementation: a systematic review. Implement Sci. 2021;16:16. doi: 10.1186/s13012-021-01162-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsieh FI, Jeng JS, Chern CM, Lee TH, Tang SC, Tsai LK, Liao HH, Chang H, LaBresh KA, Lin HJ, et al. Quality Improvement in Acute Ischemic Stroke Care in Taiwan: The Breakthrough Collaborative in Stroke. PLoS One. 2016;11:12. doi: 10.1371/journal.pone.0160426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popoola O, Ovbiagele B, Arulogun O, Akinyemi J, Akinyemi R, Uvere E, Akpa O, Salami A, Taiwo O, Olaniyan O, et al. African Rigorous Innovative Stroke Epidemiological Surveillance: Protocol for a Community-Based Mobile-Health Study. Neuroepidemiology. 2022;56:17–24. doi: 10.1159/000518885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bladin CF, Kim J, Bagot KL, Vu M, Moloczij N, Denisenko S, Price C, Pompeani N, Arthurson L, Hair C, et al. Improving acute stroke care in regional hospitals: clinical evaluation of the Victorian Stroke Telemedicine program. Med J Aust. 2020;212:371–377. doi: 10.5694/mja2.50570 [DOI] [PubMed] [Google Scholar]
- 52.Kim J, Tan E, Gao L, Moodie M, Dewey HM, Bagot KL, Pompeani N, Sheppard L, Bladin CF, Cadilhac DA. Cost-effectiveness of the Victorian Stroke Telemedicine program. Aust Health Rev. 2022;46:294–301. doi: 10.1071/AH21377 [DOI] [PubMed] [Google Scholar]
- 53.Cadilhac DA, Grimley R, Kilkenny MF, Andrew NE, Lannin NA, Hill K, Grabsch B, Levi CR, Thrift AG, Faux SG, et al. Multicenter, Prospective, Controlled, Before-and-After, Quality Improvement Study (Stroke123) of Acute Stroke Care. Stroke. 2019;50:1525–1530. doi: 10.1161/STROKEAHA.118.023075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ranta A, Thompson S, Harwood MLN, Cadilhac DA, Barber PA, Davis AJ, Gommans JH, Fink JN, McNaughton HK, Denison H, et al. Reducing Ethnic and Geographic Inequities to Optimise New Zealand Stroke Care (REGIONS Care): Protocol for a Nationwide Observational Study. JMIR Res Protoc. 2021;10:e25374. doi: 10.2196/25374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ranta A. Projected stroke volumes to provide a 10-year direction for New Zealand stroke services. N Z Med J. 2018;131:15–28. [PubMed] [Google Scholar]
- 56.Adelman EE, Burke JF. Can Electronic Health Records Make Quality Measurement Fast and Easy? Circ Cardiovasc Qual Outcomes. 2017;10. doi: 10.1161/CIRCOUTCOMES.117.004180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bravata DM, Myers LJ, Cheng E, Reeves M, Baye F, Yu Z, Damush T, Miech EJ, Sico J, Phipps M, et al. Development and Validation of Electronic Quality Measures to Assess Care for Patients With Transient Ischemic Attack and Minor Ischemic Stroke. Circ Cardiovasc Qual Outcomes. 2017;10. doi: 10.1161/CIRCOUTCOMES.116.003157 [DOI] [PubMed] [Google Scholar]
- 58.Yu AYX, Liu ZA, Pou-Prom C, Lopes K, Kapral MK, Aviv RI, Mamdani M. Automating Stroke Data Extraction From Free-Text Radiology Reports Using Natural Language Processing: Instrument Validation Study. JMIR Med Inform. 2021;9:e24381. doi: 10.2196/24381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gremyr A, Andersson Gare B, Thor J, Elwyn G, Batalden P, Andersson AC. The role of co-production in Learning Health Systems. Int J Qual Health Care. 2021;33:ii26–ii32. doi: 10.1093/intqhc/mzab072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perito ER, Squires JE, Bray D, Bucuvalas J, Krise-Confair C, Eisenberg E, Gonzalez-Peralta RP, Gupta N, Hsu EK, Kosmach-Park B, et al. A Learning Health System for Pediatric Liver Transplant: The Starzl Network for Excellence in Pediatric Transplantation. J Pediatr Gastroenterol Nutr. 2021;72:417–424. doi: 10.1097/MPG.0000000000002974 [DOI] [PubMed] [Google Scholar]
- 61.Chuong KH, Mack DR, Stintzi A, O’Doherty KC. Human Microbiome and Learning Healthcare Systems: Integrating Research and Precision Medicine for Inflammatory Bowel Disease. OMICS. 2018;22:119–126. doi: 10.1089/omi.2016.0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fung-Kee-Fung M, Maziak DE, Pantarotto JR, Smylie J, Taylor L, Timlin T, Cacciotti T, Villeneuve PJ, Dennie C, Bornais C, et al. Regional process redesign of lung cancer care: a learning health system pilot project. Curr Oncol. 2018;25:59–66. doi: 10.3747/co.25.3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grob R, Gleason K, McLean P, McGraw S, Solomon M, Joffe S. Patients’ roles in governance of learning: Results from a qualitative study of 16 learning healthcare systems. Learn Health Syst. 2022;6:e10269. doi: 10.1002/lrh2.10269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Key KD, Lewis EY. Sustainable community engagement in a constantly changing health system. Learn Health Syst. 2018;2. doi: 10.1002/lrh2.10053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mullins CD, Wingate LT, Edwards HA, Tofade T, Wutoh A. Transitioning from learning healthcare systems to learning health care communities. J Comp Eff Res. 2018;7:603–614. doi: 10.2217/cer-2017-0105 [DOI] [PubMed] [Google Scholar]
- 66.Kilbourne AM, Jones PL, Atkins D. Accelerating implementation of research in Learning Health Systems: Lessons learned from VA Health Services Research and NCATS Clinical Science Translation Award programs. J Clin Transl Sci. 2020;4:195–200. doi: 10.1017/cts.2020.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scott P, Dunscombe R, Evans D, Mukherjee M, Wyatt J. Learning health systems need to bridge the ‘two cultures’ of clinical informatics and data science. J Innov Health Inform. 2018;25:126–131. doi: 10.14236/jhi.v25i2.1062 [DOI] [PubMed] [Google Scholar]
- 68.Bagot KL, Bladin CF, Vu M, Kim J, Hand PJ, Campbell B, Walker A, Donnan GA, Dewey HM, Cadilhac DA, et al. Exploring the benefits of a stroke telemedicine programme: An organisational and societal perspective. J Telemed Telecare. 2016;22:489–494. doi: 10.1177/1357633X16673695 [DOI] [PubMed] [Google Scholar]
- 69.Bagot KL, Cadilhac DA, Kim J, Vu M, Savage M, Bolitho L, Howlett G, Rabl J, Dewey HM, Hand PJ, et al. Transitioning from a single-site pilot project to a state-wide regional telehealth service: The experience from the Victorian Stroke Telemedicine programme. J Telemed Telecare. 2017;23:850–855. doi: 10.1177/1357633X17734004 [DOI] [PubMed] [Google Scholar]
- 70.Bagot KL, Cadilhac DA, Vu M, Moss K, Bladin CF, collaborators VST. Telemedicine in the acute health setting: A disruptive innovation for specialists (an example from stroke). J Telemed Telecare. 2015;21:443–448. doi: 10.1177/1357633X15610722 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.