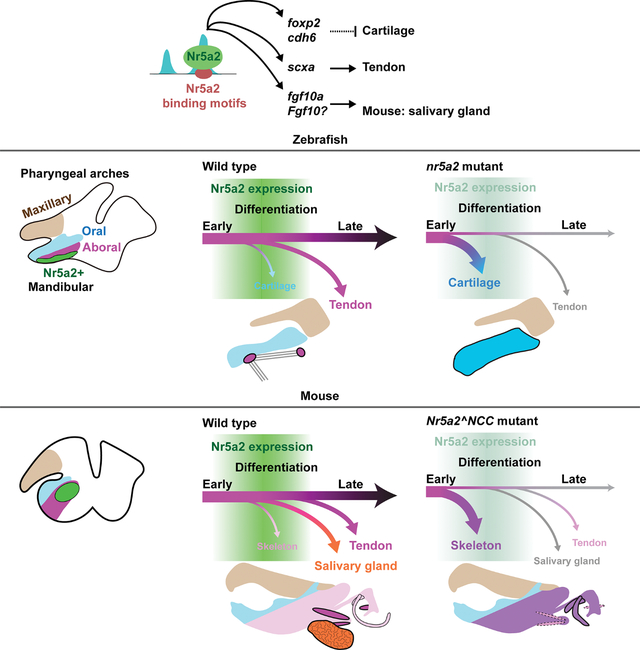
Summary
Organ development involves sustained production of diverse cell types with spatiotemporal precision. In the vertebrate jaw, neural-crest-derived progenitors produce not only skeletal tissues but also later-forming tendons and salivary glands. Here we identify the pluripotency factor Nr5a2 as essential for cell fate decisions in the jaw. In zebrafish and mice, we observe transient expression of Nr5a2 in a subset of mandibular post-migratory neural-crest-derived cells. In zebrafish nr5a2 mutants, nr5a2-expressing cells that would normally form tendons generate excess jaw cartilage. In mice, neural crest-specific Nr5a2 loss results in analogous skeletal and tendon defects in the jaw and middle ear, as well as salivary gland loss. Single-cell profiling identifies that Nr5a2, distinct from its roles in pluripotency, promotes jaw-specific chromatin accessibility and gene expression essential for tendon and gland fates. Thus, repurposing of Nr5a2 promotes connective tissue fates to generate the full repertoire of derivatives required for jaw and middle ear function.
eTOC Blurb
In this study Chen et al. identify a conserved function of the nuclear receptor Nr5a2 in patterning the zebrafish and mouse lower jaw and mouse middle ear. They find that Nr5a2 opens up regions of the genome essential for tendon and salivary gland development at the expense of skeleton formation.
Graphical Abstract:
Introduction
The gnathostome jaw is a remarkable evolutionary innovation that facilitated predation, feeding, and communication. In addition to the jaw skeleton (bone, cartilage, and teeth), multiple soft connective tissue components are required for its function. For example, tendons connect the jaw skeleton to musculature, ligaments stabilize the jaw joint, and salivary glands lubricate the oral cavity. Remarkably, the jaw skeleton, tendons, ligaments, and salivary gland mesenchyme all arise from cranial neural crest-derived cells (CNCCs) of the mandibular arch 1–5. CNCCs closer to the future oral cavity gives rise to Meckel’s cartilage, intramembranous regions of the dentary bone, and teeth, while CNCCs located in the more posterior (i.e. aboral) domain of the mandibular arch contribute to jaw tendons and salivary gland mesenchyme. In mammals, the more proximal domains of the mandibular arch also give rise to skeletal and connective tissue elements of the middle ear 6. The mechanisms that induce skeletal versus non-skeletal connective tissue fates from mandibular arch CNCCs remain unclear.
Transcription factors for skeletal and connective tissue fates in the jaw and middle ear, as well as the rest of the body, have been identified. Sox9 and Runx2 are essential for the specification of cartilage 7,8 and bone 9, respectively, and Scleraxis (Scx) is important for tendon and ligament formation 10,11. Sox9 is also regionally activated in distal mandibular epithelium, in part by a local mesenchymal source of Fgf10, to induce salivary gland development 12. However, we have an incomplete understanding of how the expression of lineage-specific transcription factors, such as Sox9 and Scx, is restricted to precise spatiotemporal mesenchymal domains. While the expression of these transcription factors is likely controlled by region-specific enhancers, the upstream factors controlling their region-specific expression have yet to be identified.
CNCCs contribute to a wide diversity of cell types 13. It remains debated, however, if their multilineage capacity is endowed during specification or acquired after their migration into the head (reviewed in 14). Whereas experiments in frog suggested that CNCCs might retain pluripotency from blastula stages 15, later studies in mouse support a reacquisition of multipotency at CNCC specification stages due to induction of pluripotency factors Oct4 and Sox2 16. Our recent single-cell genomic analysis of CNCC differentiation in zebrafish shows that the majority of enhancers required for CNCC lineage capacity gain accessibility after CNCCs migrate into the jaw-forming mandibular arch 17. This suggests that opening of lineage-specific enhancers, and therefore access to transcription factors, plays a major role in determining the range of potential fates for CNCCs in the developing face, yet few factors that regulate CNCC enhancer accessibility have been identified.
Through single-cell profiling of zebrafish CNCCs, we recently identified nr5a2 as a highly specific marker of the aboral domain of the mandibular arch of zebrafish 17. Nr5a2 is a member of the nuclear receptor transcription factor family and is considered orphan as a physiological ligand remains unknown. Nr5a2 functions to maintain Oct4 expression in mouse embryonic stem cells 18 and can replace Oct4 in reprogramming to induced pluripotent cells 19. Reflective of this key role in pluripotency, loss of Nr5a2 in mouse results in lethality at blastula stages, around embryonic (E) day 6.5 18,20. Nr5a2 also has roles in development of the liver 20, pancreas 21, and nervous system 22. Whereas zebrafish nr5a2 mutants have liver and pancreas defects, effects on CNCC differentiation and jaw development were not examined 23. Here we found that Nr5a2 plays a conserved role in the aboral mandibular domain of zebrafish and mouse embryos where it promotes tendon and salivary gland fates at the expense of skeletal fates. In particular, we found that Nr5a2 establishes accessibility of enhancers required for maintaining undifferentiated progenitors while priming them for later tendon and gland differentiation. These targets of Nr5a2 in CNCCs are distinct from those described in embryonic stem cells 19. Nr5a2 therefore promotes diverse connective tissue fates at the expense of skeletal fates in a restricted subset of postmigratory mandibular CNCCs through binding to genomic targets distinct from those important for pluripotency.
Results
Conserved expression of Nr5a2 within the zebrafish and mouse mandibular arches
In single-cell transcriptome and chromatin accessibility analysis of zebrafish CNCCs, we previously identified nr5a2 expression and the Nr5a2 DNA-binding motif as being highly enriched in the aboral domain of the mandibular arch (i.e. opposite to the oral epithelium) at 1.5 days post-fertilization (dpf) 17. In situ hybridization for nr5a2 mRNA identified highly specific expression in dlx2a+ mesenchyme of the mandibular arch at 1.5 dpf, posterior to the sox9a+ lower jaw Meckel’s cartilage at 2 and 3 dpf (Fig. 1A–C). Previously published in situ patterns indicate a lack of CNCC expression at stages earlier than 1.5 dpf 24, which agrees with bulk RNA sequencing studies of migratory CNCCs in zebrafish 25. We also generated a nr5a2:membrane-GFP knock-in reporter line (nr5a2mGFP) by inserting membrane-GFP in the 5’ untranslated region of the nr5a2 locus via homologous recombination. Consistent with endogenous expression, nr5a2mGFP labeled the aboral mandibular domain at 2 and 3 dpf, with mesenchymal nr5a2mGFP expression partially co-localizing with the tenocyte marker scxa:mCherry but not the cartilage maker col2a1a:mCherry (Fig. 1D; Fig. S1A; Fig. 2SA). We also observed weak nr5a2:mGFP expression in mesenchyme adjacent to cartilage in the neurocranium (Fig. S1B). Sequential live imaging of individuals identified that nr5a2mGFP expression precedes that of scxa:mCherry, with nr5a2mGFP being downregulated in scxa:mCherry+ tenocytes as they mature (Fig. S1C). Thus, nr5a2 expression is transiently upregulated in postmigratory mandibular CNCCs that are precursors for tendon but not cartilage cells.
Figure 1. Nr5a2 expression within the developing zebrafish and mouse face.
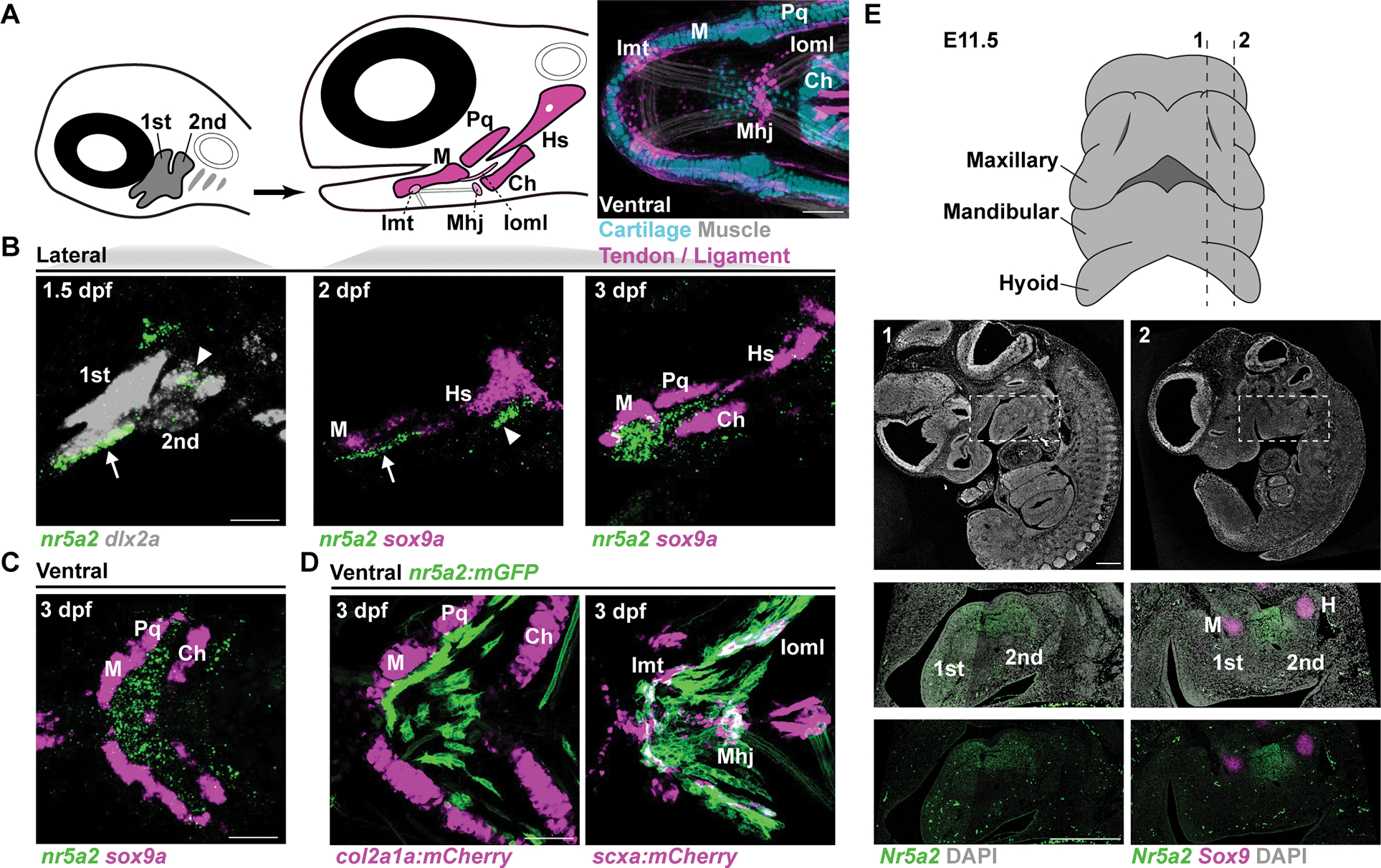
(A) Diagrams of zebrafish heads showing pharyngeal arch CNCCs (grey, numbered), facial cartilages (magenta), and tendons and ligaments (pink). Image at right is a ventral view of the lower jaw in transgenic animals at 6 dpf, with cartilage in blue (col2a1a:GFP), tendon and ligament in magenta (scxa:mCherry), and muscle in white (phalloidin). Ceratohyal cartilage (Ch), Hyosymplectic cartilage (Hs), Intermandibular tendon (Imt), Interopercular–mandibular ligament (Ioml), Mandibulohyoid junction tendon (Mhj), Meckel’s cartilage (M), Palatoquadrate cartilage (Pq).
(B,C) In situ hybridizations show expression of nr5a2 (green) in mandibular (arrow) and hyoid (arrowhead) arch CNCCs (dlx2a+) at 1.5 dpf, and posterior to developing sox9a+ M and Pq jaw cartilages at 2 and 3 dpf (lateral views in B and ventral view in C).
(D) Ventral views of the jaw at 3 dpf show nr5a2:membrane-GFP (mGFP) expression posterior to col2a1a:mCherry-NTR+ M and Pq chondrocytes and partially overlapping with the tenocyte and ligamentocyte marker scxa:mCherry at the Imt, Ioml, and Mhj.
(E) Diagram shows ventral view of jaw region in E11.5 mouse embryo with dashed lines indicating sagittal sections shown below (numbered). Overviews of sagittal sections with DAPI staining only (1, 2 - white). RNAscope in situ hybridizations of boxed regions show Nr5a2 expression at the posterior boundary of the mandibular (1st) arch and anterior boundary of the hyoid (2nd) arch, posterior to Sox9+ Meckel’s cartilage and anterior to Hyoid (H) cartilage. Similar staining was observed in multiple sections. Scale bars = 50 μm (A-D); 500 μm (E). See also Figure S1.
We next examined whether mandibular expression of Nr5a2 is conserved in mouse. Examination of mouse mandibular arch single-cell transcriptome data at E10.5 26 identified expression of Nr5a2 in a putative aboral domain complementary to an oral domain marked by Pitx1 expression (Fig. S1F), as in zebrafish 17. In situ hybridization at E11.5 and E12.5 identified mesenchymal expression of Nr5a2 at the posterior boundary of the mandibular arch and anterior boundary of the hyoid arch. As in zebrafish, mandibular Nr5a2 expression was observed just posterior to developing Sox9+ Meckel’s cartilage in mouse (Fig. 1E, Fig. S1D,E). In more lateral regions, we observed Nr5a2 expression between the first pharyngeal pouch and first pharyngeal cleft (Fig. S1D,E). At E14.5, Nr5a2 expression was observed in mesenchyme of the palate and flanking the basisphenoid-forming region of the skull base, in mesenchyme surrounding the malleus, incus, and external auditory meatus, and in salivary gland mesenchyme (Fig. S1G) 27, with single-cell transcriptome profiles of mouse submandibular glands 28 further confirming salivary gland mesenchyme expression that decreased from E12 to E16 (Fig. S1H). Nr5a2 is therefore expressed in the mandibular arches of both fish and mammals, though it appears to be more prominently restricted to the aboral domain in fish.
Requirement for nr5a2 in zebrafish lower jaw development
Given the restricted mandibular expression of nr5a2, we investigated its requirement in zebrafish jaw development. We first examined homozygous nr5a2oz3/oz3 mutant zebrafish, in which an early frameshift mutation results in premature truncation of the protein before the DNA-binding and ligand-binding domains 23 (Fig. S2A). Mutant fish displayed an open mouth, and skeletal staining showed an enlarged Meckel’s cartilage of the lower jaw, but no other craniofacial skeletal defects, at 6 and10 dpf (Fig. 2A; Fig. S2B). Repeated live imaging identified increased numbers of col2a1a:h2az2a-mCherry-2A-EGFP-CAAX+ Meckel’s chondrocytes in nr5a2 mutants versus wild-type sibling controls from 2.5 dpf, the earliest stage at which labeled chondrocytes appear, through 7 dpf (Fig. 2B,C). The numbers of proliferating Meckel’s chondrocytes, as assessed by anti-phospho-Histone-H3 staining, was unchanged at 2.5 and 3 dpf (Fig. S2C). Enlargement of Meckel’s cartilage in mutants is therefore likely due to increased specification and not increased proliferative expansion of chondrocytes.
Figure 2. Requirement of nr5a2 for zebrafish lower jaw development.
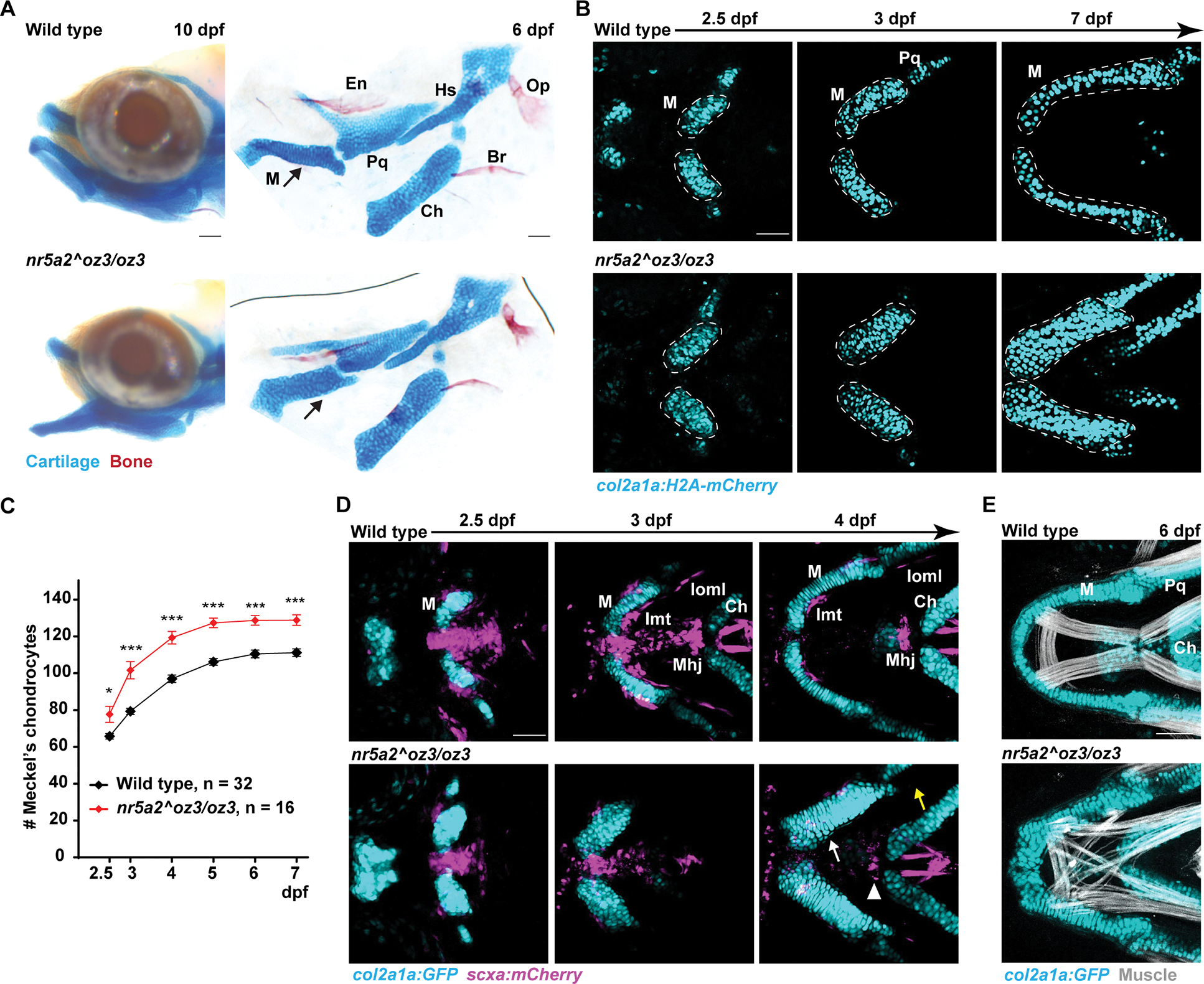
(A) Heads of 10 dpf zebrafish and flat-mount dissections of the first and second arch skeleton of 6 dpf zebrafish stained with Alcian Blue (cartilage) and Alizarin Red (bone). Arrows denote thickening of Meckel’s cartilage (M) in mutants. Branchiostegal ray bone (Br), Ceratohyal cartilage (Ch), Entopterygoid bone (En), Hyosymplectic cartilage (Hs), Opercle bone (Op), Palatoquadrate cartilage (Pq).
(B) Repeat imaging of individual animals shows progressive thickening of Meckel’s cartilage (outlined) in mutant versus control col2a1a:h2az2a-mCherry-2A-EGFP-CAAX+ fish. H2az2a-mCherry labels nuclei. EGFP-CAAX channel is not shown.
(C) Quantification of Meckel’s chondrocyte number. Difference of wild type and mutant chondrocyte numbers was compared by Wilcoxon rank-sum test at each stage. Error bars represent standard error of the mean. * = p < 0.05; *** = p < 0.0001.
(D) Repeated imaging of individuals shows thickening of the col2a1a:GFP+ Meckel’s cartilage and loss of the scxa:mCherry+ intermandibular tendon (Imt, white arrow), interopercular–mandibular ligament (Ioml, yellow arrow), and mandibulohyoid junction tendon (Mhj, arrowhead) in 4/4 mutants compared to 0/3 wild types. Note that the hyoid tendons (far right at 4 dpf) are unaffected in mutants, as is the midline scxa:mCherry expression at 2.5 and 3 dpf that does not contribute to tendons by 4 dpf in wild types.
(E) Phalloidin staining (white) shows that the jaw muscles connected to col2a1a:GFP+ Meckel’s cartilage are highly disorganized in 5/5 mutants compared to 0/8 wild types. Scale bars = 50 μm. See also Figure S2.
We next examined whether increased numbers of Meckel’s chondrocytes are accompanied by concomitant loss of other CNCC derivatives. Meckel’s cartilage is connected to lower jaw muscles by CNCC-derived scxa:mCherry+ tenocytes at 2.5 and 3 dpf, with these condensing at intermandibular tendons by 4 dpf (Fig. 1A, 2D). In nr5a2 mutants, we observed variable disorganization and loss of scxa:mCherry+ lower jaw tenocytes, which was accompanied by disorganization of the lower jaw muscles (Fig. 2D,E; Fig. S2D). The interopercular–mandibular ligament, which stabilizes the jaw joint, was also missing in 12/13 mutants at 6 dpf, yet hyoid arch-derived tendons were largely unaffected (Fig. 2D, Fig. S2E). The lower-jaw-specific expansion of cartilage, loss of tendon and ligament, and disorganization of muscle precisely correlates with the highly localized expression of nr5a2 in the aboral mandibular arch from which these structures derive.
Nr5a2 functions cell-autonomously in zebrafish CNCCs to specify tenocytes at the expense of chondrocytes
To investigate the cellular basis of jaw tenocyte loss in nr5a2 mutant zebrafish, we performed short-term lineage tracing with nr5a2mGFP. Homozygous nr5a2mGFP/mGFP fish displayed normal lower jaw cartilage and muscle and were adult viable; we did observe subtle jaw muscle defects in nr5a2mGFP/oz3 but not nr5a2+/oz3 fish, suggesting that the nr5a2mGFP allele is a weak hypomorph (Fig. S2B,D). To create a severe loss-of-function allele in cis to the mGFP insertion, we injected CRISPR-Cas9 reagents into nr5a2mGFP fish and obtained progeny with an in-frame deletion of three amino acids (nr5a2mGFP-DBD-del) that removes a critical zinc finger motif-forming cysteine residue in the DNA-binding domain (Fig. S2A). We observed similar enlargement of Meckel’s cartilage and muscle disorganization in nr5a2mGFP-DBD-del/mGFP-DBD-del and nr5a2oz3/oz3 fish, indicating that disruption of the DNA-binding domain generates a severe loss-of-function allele (Fig. S2B,D). The similarity of cartilage defects in the mGFP-DBD-del and early frameshift oz3 alleles also argues against genetic compensation, i.e. transcriptional adaptation in response to nonsense-mediated decay, in the oz3 allele 29.
In nr5a2mGFP-DBD-del/+ controls, mGFP+ cells contributed to scxa:mCherry+ jaw tenocytes but only rarely to Meckel’s chondrocytes at 3 dpf, which we confirmed by lack of co-expression of the cartilage marker col2a1a:mCherry-NTR+ at 2.5 dpf. In contrast in nr5a2mGFP-DBD-del/mGFP-DBD-del mutants, increased numbers of mGFP+ cells contributed to Meckel’s cartilage and co-expressed col2a1a:mCherry-NTR, with a concomitant decrease in contribution to scxa:mCherry+ jaw tenocytes (Fig. 3A,B). These observations indicate that nr5a2-expressing cells shift from a tenocyte to chondrocyte fate in the absence of Nr5a2.
Figure 3. Nr5a2 functions cell-autonomously in zebrafish CNCCs to promote tenocyte at the expense of chondrocyte fate.
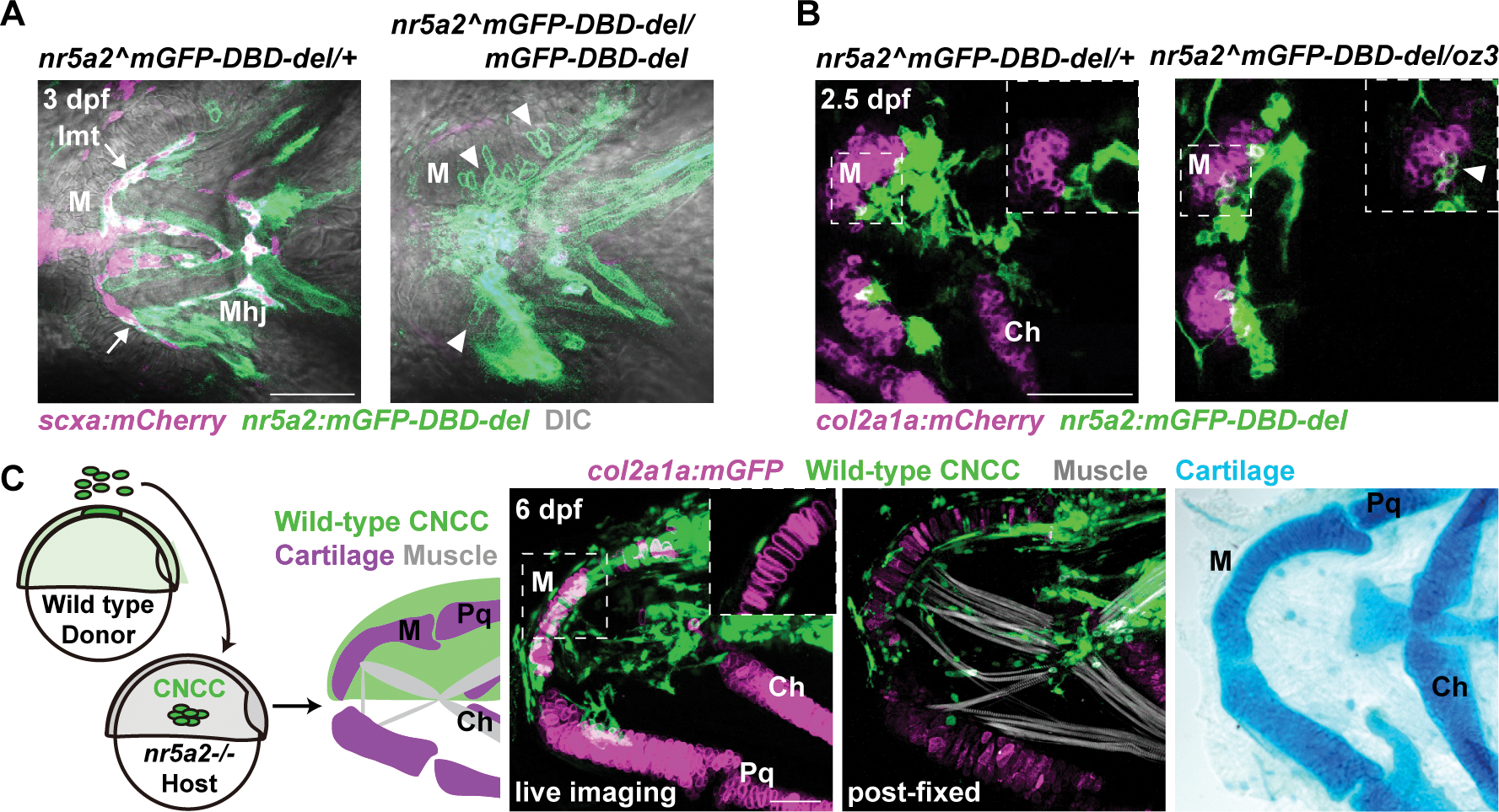
(A) In representative confocal sections at 3 dpf, nr5a2:mGFP-DBD-del+ cells contributed to 12+/−3.9 Meckel’s (M) chondrocytes (arrowheads) across 10 mutants (nr5a2mGFP-DBD-del/mGFP-DBD-del) versus 0.4+/−0.8 chondrocytes across 12 controls (nr5a2mGFP-DBD-del/+) (p = 4.76E-05, Wilcoxon rank-sum test). Reciprocally, nr5a2:mGFP-DBD-del+ cells contributed to 1.3+/− 0.7 scxa:mCherry+ intermandibular tendon (Imt) cells (arrows) in mutants versus 9.6+/−2.0 in controls (p = 7.2E-05, Wilcoxon rank-sum test). Mhj, mandibulohyoid junction.
(B) At 2.5 dpf, nr5a2:mGFP-DBD-del+ cells contributed to 6.1+/−3.5% col2a1a:mCherry-NTR+ Meckel’s chondrocytes (arrowhead, inset) across 6 mutants versus 1.2+/−1.7% chondrocytes across 10 controls (p = 0.0198, Wilcoxon rank-sum test). Lower magnification images are confocal projections and insets are digital sections. Ch, ceratohyal cartilage.
(C) Schematic shows shield-stage transplantation of BFP+ (green, actb2:LOXP-BFP-LOXP-DsRed) wild-type ectoderm cells into the CNCC precursor domain of nr5a2 mutant hosts. In 4/4 mutants, unilateral contribution of wild-type CNCCs rescued morphology of Meckel’s cartilage (magenta, col2a1a:h2az2a-mCherry-2A-EGFP-CAAX+ in fluorescent images, h2az2a-mCherry channel is not shown; Alcian Blue+ at right) and muscle organization (white, Phalloidin, in middle panel). Inset shows magnified digital section in which wild-type CNCCs non-cell-autonomously rescued Meckel’s cartilage. Note that BFP signal is decreased after the fixation step required for Phalloidin staining. Pq, palatoquadrate cartilage. Scale bars = 50 μm. See also Figure S3.
To further test the sufficiency of nr5a2 in CNCCs for jaw patterning, we performed unilateral transplantation of wild-type BFP+ CNCC precursors into nr5a2 mutants (Fig. 3C). In contrast to contralateral mutant sides, 4/4 sides with mandibular contribution of wild-type BFP+ CNCCs displayed rescue of Meckel’s cartilage morphology based on col2a1a:h2az2a-mCherry-2A-EGFP-CAAX expression and Alcian Blue staining. We also observed rescue of lower jaw muscle organization in transplanted sides, with wild-type CNCCs forming cells with tenocyte morphology at the junction of muscle and cartilage. In two examples, wild-type CNCCs rescued Meckel’s cartilage morphology without contributing to chondrocytes, indicating a non-cell-autonomous role of Nr5a2 in patterning Meckel’s cartilage (Fig. 3C; Fig. S3A). Thus, Nr5a2 functions to suppress chondrocyte differentiation in cells adjacent to the developing Meckel’s cartilage.
Nr5a2 misexpression mildly suppresses chondrogenesis and disrupts tendon formation in the zebrafish lower jaw
To test whether Nr5a2 is sufficient to repress lower jaw chondrogenesis, we generated a UAS:nr5a2 transgenic line and crossed it to a CNCC mesenchyme-specific fli1a:Gal4VP16 driver 30, resulting in nr5a2 expression throughout CNCC mesenchyme. Compared to single-positive fli1:Gal4VP16 and UAS:nr5a2 controls, Meckel’s cartilage of fli1a:Gal4VP16; UAS:nr5a2 fish had an abnormal pointed morphology and a 12–16% reduction in chondrocyte number, as assessed by col2a1a:EGFP and nuclear DAPI staining (Fig. S3B). Misexpression of nr5a2 also resulted in ectopic scxa:mCherry+ tenocytes and disorganized lower jaw muscle fibers, with occasional fibers connecting to ectopic tenocytes; the number of scxa:mCherry+ tenocytes in the normal lower jaw attachment region, however, were unchanged (Fig. S3C). While tenocyte defects could reflect increased specification and/or disrupted migration and morphogenesis, our data show that Nr5a2 misexpression only mildly suppresses cartilage differentiation in the lower jaw.
Requirement for Nr5a2 in mouse NCCs for lower jaw and middle ear development
We next examined whether Nr5a2 has a similar requirement for development of mandibular arch-derived structures in mouse. As homozygous loss of Nr5a2 results in lethality at epiblast stages 20, we utilized the Wnt1-Cre driver and a Nr5a2-flox allele to delete both copies of Nr5a2 in NCCs (Nr5a2NCC). Nr5a2NCC animals died shortly after birth due to unidentified causes, with air bubbles apparent in their intestines (Fig. S4A). Whereas overall head length was similar between newborn controls and Nr5a2NCC animals, staining for cartilage and bone identified abnormalities in skeletal structures derived from the mandibular arch (Fig. 4A; Fig. S4F). While Meckel’s cartilage was largely unaffected at E14.5 (Fig. S4B), at birth the malleus cartilage, which derives from the proximal portion of Meckel’s, was enlarged. We also observed shortening and thickening of the angular process of the mandibular bone, as well as the tympanic ring and gonial bones of the middle ear (Fig. 4A; Fig. S4F). In the pterygoid plate of the cranial base, mutants displayed abnormal ossification at lateral edges and truncated medial bones (Fig. S4C) consistent with Nr5a2 expression in this region at E14.5 (Fig. S1G).
Figure 4. CNCC requirement for Nr5a2 in mouse mandibular arch development.
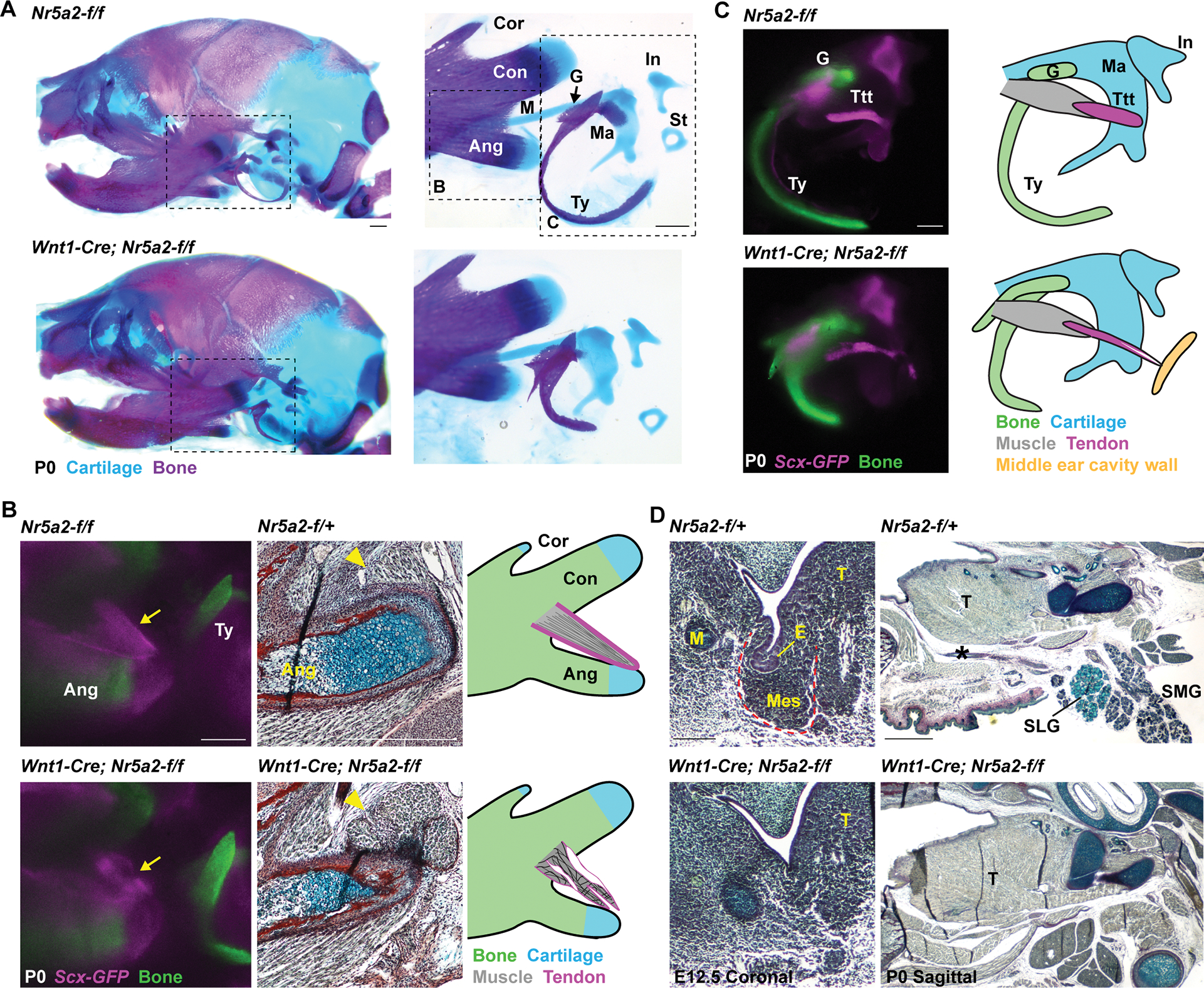
(A) Newborn (P0) skulls of control (Nr5a2-f/f) and Nr5a2NCC (Wnt1-Cre; Nr5a2-f/f) mice stained with Alcian Blue (cartilage) and Alizarin Red (bone). Dashed boxes designate magnified regions shown at right. Mutants display an enlarged malleus cartilage (Ma), and shorter and thicker lower jaw angular process (Ang), tympanic bone (Ty), and gonial bone (G). Consistent phenotypes were seen across 12 wild-type (Nr5a2-f/+, Nr5a2-f/f or Wnt1-Cre; Nr5a2-f/+) and 6 Nr5a2NCC heads. Condylar process (Con), coronoid process (Cor), incus (In), body of Meckel’s cartilage (M), stapes (St).
(B) Angular processes are labeled for tendon (Scx-GFP, magenta) and bone (Alizarin Red, green) in whole-mount imaging, and trichrome staining in sagittal sections. Diagrams depict the dysmorphic tendons (arrow) and detached muscle (yellow arrowhead) in 4/4 Nr5a2NCC mice.
(C) Dissected middle ears and accompanying diagrams show dysmorphology of the Scx-GFP+ (magenta) tensor tympanic tendon (Ttt, which connects the malleus (blue) to the tensor tympanic muscle (grey)) in Nr5a2NCC mice. Tendon defects were seen in 6/6 middle ears from Nr5a2NCC mice, with abnormal connections to the middle ear wall (yellow) in three ears. The abnormal gonial and tympanic bones are visualized by Alizarin Red staining (green).
(D) Trichrome staining of coronal sections shows absence of salivary gland mesenchyme (Mes, red outline) and epithelial invagination (E) at E12.5 in 2/2 Nr5a2NCC mice. Sagittal sections at birth (P0) show absence of the submandibular gland (SMG), sublingual gland (SLG), and salivary gland duct (asterisk) in 4/4 Nr5a2NCC newborn (P0) mice. The tongue (T) is unaffected. Scale bars = 500 μm. See also Figure S4.
We next used Scx-GFP to examine the effects of NCC-specific Nr5a2 deletion on tendon development. In Nr5a2NCC; Scx-GFP newborn mice, the tendons connecting to the angular process of the lower jaw were reduced and dysmorphic. Histology identified dysmorphology of the connective tissue at the muscle insertion site, and detachment of muscle fibers that was confirmed by MF20 staining (Fig. 4B; Fig. S4D–F). The tensor tympani tendon connecting to the malleus was similarly dysmorphic, in some cases with ectopic insertion into the wall of the middle ear cavity (Fig. 4C). In contrast, deletion of Nr5a2 from limb mesenchyme using Prrx1-Cre had no effect on the bones or Scx-GFP+ tendons of the forelimb and hindlimb (Fig. S4G,H), showing specificity of skeletal and tendon defects to the jaw and middle ear.
In addition to tendon defects, Nr5a2NCC animals had a complete absence of submandibular and sublingual salivary glands and their connecting ducts at birth (Fig. 4D; Fig. S4J), consistent with these arising from the Nr5a2-expressing mandibular domain. These defects are reflected by a complete loss of salivary gland mesenchyme and epithelial invagination at E12.5 (Fig. 4D). The parotid and lacrimal glands arising from the mid-oral and eye field, respectively, were unaffected (Fig. S4J). These findings identify a highly region-specific requirement for Nr5a2 in development of tendons and glands from the mandibular arch.
Single-cell multi-omics analysis of mandibular CNCC defects in nr5a2 mutants
We next sought to understand the mechanistic basis by which Nr5a2 regulates cell fate decisions in the jaw. Given its role in transcriptional regulation, we performed integrated single-nuclei analysis (10X Genomics Multiome) of mRNA expression (snRNAseq) and assay for transposase accessible chromatin (snATACseq) in nr5a2-expressing cells sorted from control (nr5a2mGFP-DBD-del/+) and mutant (nr5a2mGFP-DBD-del/oz3) fish heads at 2.5 dpf (Fig. 5A). After filtering for nuclei with high quality reads (see Methods), we recovered 4,304 control and 6,589 mutant cells with a median of 11,505 and 6,546 ATAC fragments and 1,411 and 821 genes per nucleus, respectively. UMAP clustering of integrated RNAseq and ATACseq data from control and mutant heads using Seurat (see Methods), combined with analysis of known cell type markers, identified major clusters of neurons and mesenchyme, consistent with expression of nr5a2mGFP in both these populations (Fig. S5A,B; Table S1). We also observed clusters of epithelial, vascular, and blood cells, likely representing contaminating cells. We then extracted and combined control and mutant mesenchyme clusters based on co-expression of fli1a and prrx1a, which resolved into 14 clusters upon sub-clustering (Fig. S5C; Table S1). For detailed analysis, we further extracted a cartilage cluster (acana+), a tendon cluster (tnmd+), and four mandibular arch mesenchyme clusters (based on co-expression of dlx2a, dlx5a, and hand2) (Fig 5B; Fig. S5C). The six remaining dlx2a-; dlx5a-mesenchyme clusters, which express lower levels of nr5a2 (Fig. S5C), may represent the neurocranium mesenchyme that we found also weakly expresses nr5a2mGFP (Fig. S1B).
Figure 5. Single-cell analysis of gene expression and chromatin changes in nr5a2 mutants.
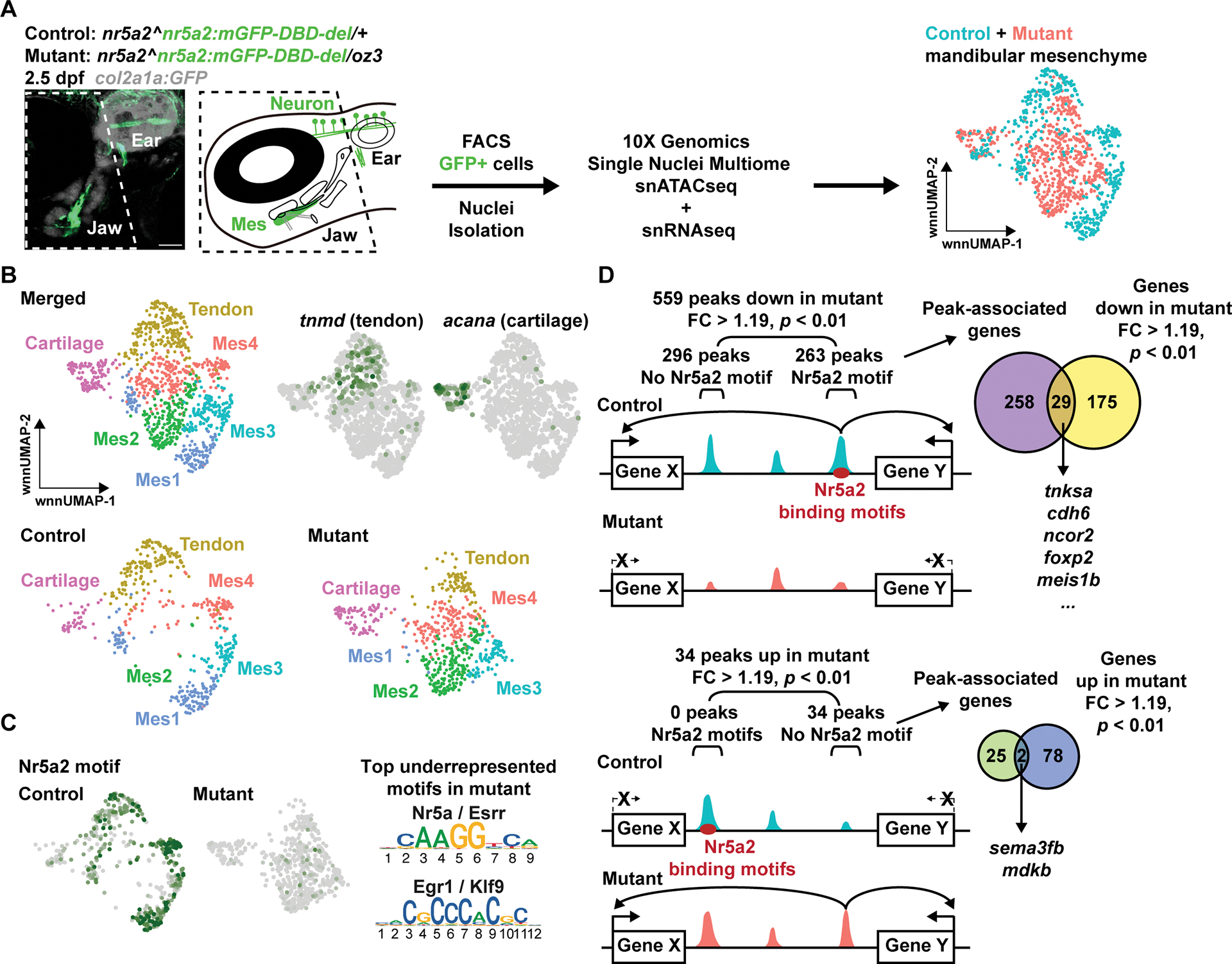
(A) Image of 2.5 dpf nr5a2:mGFP+ cells and scheme for jaw dissection (boxed region), FACS, and Multiome analysis. UMAPs show distribution of mandibular mesenchyme from controls and mutants. Scale bar = 50 μm.
(B) UMAPs show distribution of tendon, cartilage, and mandibular mesenchyme (Mes1–4) clusters from controls and mutants, and feature plots show expression of tendon (tnmd) and cartilage (acana) markers.
(C) Feature plots of the Nr5a2 binding motif (predicted by chromVAR) show its absence in mutant accessible chromatin. Top underrepresented motifs in mutants fall into two classes: Nr5a/Esrr and Egr1/Klf9.
(D) Schematic of regions with reduced or increased accessibility in mutants (> 1.19-fold change, p value < 0.01). Peaks were filtered based on predicted Nr5a2 motifs. We then intersected peaks with genes within 500 kb showing down or up regulation (> 1.19-fold change, p value < 0.01) to identify potential Nr5a2 target genes and their enhancers. See also Figure S5.
Differential analysis of snATACseq data by chromVAR identified that the top DNA-binding motif underrepresented in mutant versus control mandibular mesenchyme accessible regions was Nr5a2, followed by Egr1 (and versions of these) (Fig. 5C; Table S2). Feature plots of mandibular mesenchyme clusters confirmed that, while expression of nr5a2 persists in mutants, the Nr5a2 binding motif was nearly completely absent in mutant accessible chromatin regions (Fig. 5C; Fig. S5C). Whereas 559 regions displayed reduced accessibility in mutants, with 47% containing Nr5a2 motifs, only 34 regions had increased accessibility, with none containing Nr5a2 motifs (> 1.19 fold change, p < 0.01; Fig. 5D; Table S3). We also identified 204 genes that were downregulated in mutants, versus only 80 that were upregulated (> 1.19 fold change, p < 0.01; Fig. 5D; Table S1). Gene Ontogeny analysis of the 204 downregulated genes identified enrichment for biological processes that included cell adhesion, extracellular matrix organization, negative regulation of transcription, neural crest cell migration, cartilage development, and embryonic viscerocranium morphogenesis (Table S4). Although our data do not support upregulated genes being directly regulated by Nr5a2, we observed increased expression of igfbp5b and ogna in the mutant perichondrium (Fig. S6A). In contrast, expression of genes involved in regionalization of the mandibular arch mesenchyme along the dorsoventral (hand2) and oral-aboral (pitx1, gsc, msx1a) axes were unaffected in nr5a2 mutants at 1.5 dpf (Fig. S6B), suggesting that Nr5a2 functions largely downstream of initial regional patterning factors. These findings are consistent with a primary requirement for Nr5a2 to initiate or maintain open chromatin in mandibular mesenchyme to activate gene expression.
Identification of direct targets of Nr5a2 for diverse connective tissue fates
To identify potential Nr5a2 direct targets, we queried chromatin regions that lost chromatin accessibility in mutants, contained predicted Nr5a2 binding sites, and were located within 500 kb of a gene with decreased expression in mutants. The top five genes included tnksa (genomically linked to fgf10a), foxp2, and cdh6 (Fig. 5D; Table S5). We found fgf10a, foxp2, and cdh6 to be co-expressed with nr5a2 in the mesenchyme and perichondrium posterior to the developing lower jaw cartilage at 2.5 dpf, with foxp2 and fgf10a labelling largely distinct regions within a broader nr5a2+; cdh6+ domain (Fig. 6A–C; Fig. S6C). Perichondrium is a tissue surrounding the cartilage that is thought to house progenitors for tendon, cartilage, bone, and other connective tissues 31. In nr5a2 mutants, fgf10a, foxp2, and cdh6 expression was specifically reduced in the mesenchyme of the lower jaw, including the perichondrium associated with Meckel’s cartilage (Fig. 6A–C). In Fgf10−/− mice, all the salivary glands are lost 32, similar to the loss of the mandibular glands observed in Nr5a2NCC mice. In keeping with this result, Fgf10 expression was selectively lost in the mandibular domain of Nr5a2NCC mice at E12.5 (Fig. 6D).
Figure 6. Nr5a2 regulates jaw-specific enhancer accessibility and gene expression for perichondrium and tendon genes.
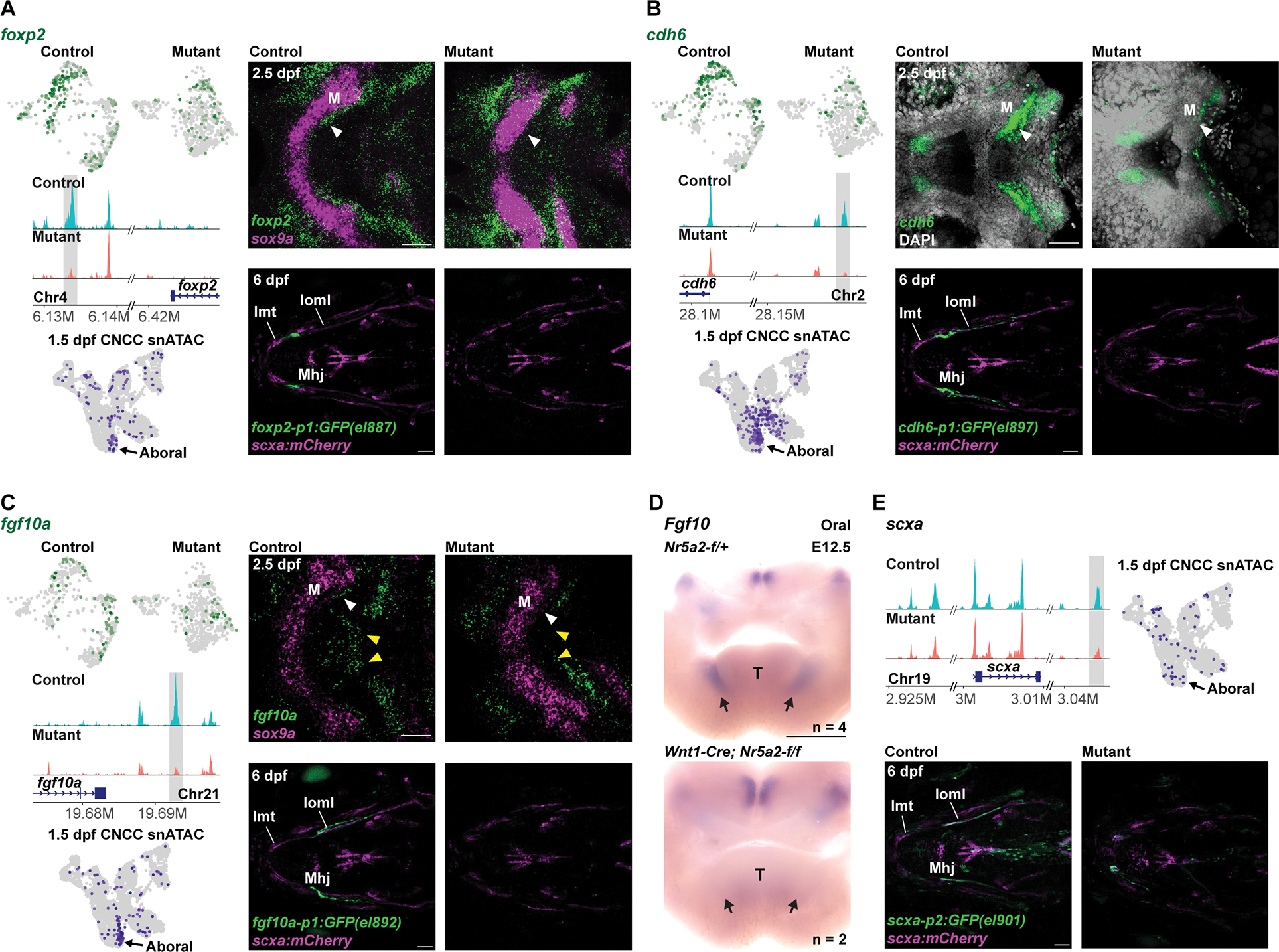
(A-C) For each gene, feature plots in top left show downregulation of transcripts in mutant (nr5a2:mGFP-DBD-del/oz3) versus control (nr5a2:mGFP-DBD-del/+) mandibular mesenchyme. Middle left shows genomic tracks of chromatin accessibility, with the Nr5a2 motif-harboring regions with decreased accessibility shown in grey. Bottom left shows feature plots of 1.5 dpf CNCC snATAC data 17 highlighting accessibility of the shaded regions in the aboral mandibular domain. Top right shows fluorescent in situ hybridizations of the mandibular and hyoid arches in ventral views, with sox9a expression labeling chondrocytes (A, C) or DAPI labeling all nuclei (B). White arrowheads denote loss of perichondrium expression in mutants, and yellow arrowheads loss of midline fgf10a expression. Bottom right shows transgenic lines in which GFP is driven by the highlighted genomic regions. For each line, GFP expression is observed posterior to Meckel’s (M) cartilage and partially overlapping with scxa:mCherry in 5/5 wild types and completely lost in 5/5 mutants. Intermandibular tendon (Imt), interopercular–mandibular ligament (Ioml), mandibulohyoid junction tendon (Mhj).
(D) Oral view of in situ hybridizations for Fgf10 in dissected mandibular arches from control (Nr5a2-f/+) and mutant (Wnt1-Cre; Nr5a2-f/f) mouse embryos at E12.5. Fgf10 expression is selectively lost in mandibular domains (arrows). T, tongue.
(E) Top left shows genome tracks of chromatin accessibility at the scxa locus, with the region containing Nr5a2 motifs and having decreased accessibility in mutants highlighted in grey. At top right, feature plot from 1.5 dpf CNCC snATAC data shows accessibility of this region in the aboral mandibular domain. At bottom, transgenic line in which GFP is driven by the highlighted genomic region shows expression in the scxa:mCherry+ Ioml ligament in 5/5 wild types and loss of Ioml expression in 5/5 nr5a2oz3/oz3 mutants. Scale bars = 50 μm (A-C, E); 500 μm (D). See also Figure S6 for additional examples of transgenic line expression and expanded genomic views of open chromatin.
We next focused on chromatin regions with reduced accessibility in mutants (> 1.19 fold change, p < 0.01) that contained predicted Nr5a2 binding sites. An unbiased analysis of the most highly significant regions identified regions near fgf10a (#3, p = 1.95E-15), cdh6 (#6, p = 7.19E-14), foxp2 (#10, p = 1.66E-12), and scxa (#43, p = 1.39E-08) (Table S3). Genome views confirmed reduced accessibility of these regions in mutants, and analysis of previously published snATACseq data of 1.5 dpf facial CNCCs 17 identified enriched accessibility of all four regions in the aboral mandibular domain (Fig. 6; Fig. S6F). Moreover, analysis of published chromatin accessibility data at NCC migration stages 33,34 identified that these regions gain accessibility only after migration into the arches (Fig. S6F). When combined with a minimal promoter in transgenic assays, all four regions drove highly specific expression of GFP in the mesenchyme posterior to Meckel’s cartilage, partially overlapping with scxa:mCherry expression in jaw tendons and ligaments (Fig. 6; Fig. S6D,E). In addition, GFP expression driven by all four regions was completely lost in mutants at 3 and 6 dpf, with the exception of a small amount of residual GFP expression driven by the scxa region (Fig. 6; Fig. S6D). These findings are consistent with Nr5a2 directly binding jaw-specific enhancers for fgf10a, cdh6, foxp2, and scxa to activate their expression.
Discussion
Here we uncovered a local and transient redeployment of the pluripotency factor Nr5a2 to preserve CNCC-derived progenitors for alternative non-skeletal fates in the vertebrate jaw and middle ear. Despite substantial differences in the types of structures arising from the aboral domain of the zebrafish and mouse mandibular arch, we found that Nr5a2 has a common role in repressing skeletogenesis and promoting tendon, gland, and potentially other mesenchymal derivatives in this domain. Transplantation rescue in zebrafish and conditional deletion experiments in mouse identified that Nr5a2 functions cell-autonomously in CNCCs for jaw and middle ear development.
In fish, nr5a2 was required for the accessibility of jaw-specific enhancers linked to uncommitted mesenchyme and tendon development. These enhancers were enriched for Nr5a2 binding sites, suggesting they are direct Nr5a2 targets. It also seems likely that Nr5a2 functions to open rather than simply maintain accessibility of these enhancers, as nr5a2 CNCC expression is not observed before 1.5 dpf in zebrafish 24 and the Nr5a2 target enhancers studied here do not gain accessibility until after NCC migration 33,34. These findings are consistent with the recently reported pioneer factor activity of Nr5a2 during zygotic genome activation in mice 35, although additional experiments will be needed to confirm this in CNCCs.
Our data support a highly localized role of Nr5a2 in the jaw to limit skeletal fate commitment while priming chromatin competency for connective tissue fates such as tendons and glands. The types of mandibular derivatives affected in zebrafish and mice lacking Nr5a2 reflect substantial evolutionary transitions, in particular the striking transformation of the fish jaw joint into the mammalian middle ear 36. Whereas in zebrafish nr5a2 mutants the entire Meckel’s cartilage was substantially enlarged, in Nr5a2NCC mice only the proximal portion of Meckel’s that forms the malleus cartilage of the middle ear was enlarged. These more localized phenotypes are reflected by the reduced expression domains and less prominent aboral restriction of Nr5a2 in mouse. In both species, however, the tendons and ligaments normally associated with the affected jaw and middle ear structures were missing or dysmorphic. Nr5a2 therefore functions similarly in fish and mice to repress chondrogenesis and promote tendon and ligament development, yet the requirement of Nr5a2 has shifted from the entire lower jaw of fish to a more localized region of the jaw joint and middle ear of mice.
In addition to enlargement of the malleus cartilage, Nr5a2NCC mice displayed shortening and thickening of several bones, including the angular process of the mandibular bone, and the tympanic and gonial bones. Whether this also reflects an expansion of the initial skeletogenic domain, which in turn depletes progenitors required for sustained growth, remains to be determined. Nr5a2NCC mice also had specific loss of the mandible-associated salivary glands (submandibular and sublingual), yet the lacrimal and parotid glands that form in different regions of the head were unaffected. Interestingly, despite fish lacking salivary glands, a conserved jaw-specific role of Nr5a2 in fgf10a/Fgf10 regulation could explain the selective loss of mandibular salivary glands in Nr5a2NCC mice. We also uncovered other predicted Nr5a2 target genes that we did not validate here, such as itga8 that is required in mandibular mesenchyme to promote continued morphogenesis of endodermal pouch epithelia 37. Nr5a2 may therefore promote additional connective tissue fates in the mandibular arch beyond tendon, ligament, and salivary gland mesenchyme.
Several of the direct target genes of Nr5a2, including foxp2 and cdh6, are co-expressed with nr5a2 in the lower jaw perichondrium, a tissue known to contain progenitors for diverse connective tissue fates 31. However, lineage tracing with nr5a2mGFP, as well as the finding that Nr5a2 functions non-cell-autonomously to pattern Meckel’s cartilage, show that Nr5a2 acts in tenocyte progenitors, rather than a bipotent cartilage-tendon progenitor, to repress chondrogenesis. Foxp2 has been shown to repress gene expression in the nervous system by closing chromatin 38, and members of the Foxp1/2/4 family can inhibit osteogenic differentiation in mouse 39. One possibility then is that Nr5a2 indirectly represses skeletogenesis through promoting jaw-specific expression of foxp2. Alternatively, lower jaw cartilage expansion in nr5a2 mutants could be an indirect consequence of disrupted tenocyte specification. Consistently, deletion of both Scleraxis genes in zebrafish results in a similar loss of tendons and expansion of Meckel’s cartilage as seen in nr5a2 mutants 10.
Our data argue against redeployment of Nr5a2 in the mandibular arch reflecting re-utilization of the Nr5a2-regulated pluripotency network. In embryonic stem cells, Nr5a2 regulates the expression of Oct4 and other pluripotency genes 18,20, in part by binding as a complex with the pluripotency factors Sox2 and Klf4 19. Although a recent study has shown that Oct4 and Sox2 have important roles in early neural crest formation 40, we had previously shown that zebrafish homologs of oct4, klf4, and most other pluripotency genes are not expressed in postmigratory CNCCs 17. Sox2 and Klf4 motifs are also not strongly enriched in Nr5a2-dependent chromatin regions in CNCCs. Instead, we found that Nr5a2 is repurposed to regulate a largely distinct set of genes in mandibular CNCCs important for perichondrium biology and formation of tendons, ligaments, and glands. Along with previous studies showing that pluripotency factors Sox2 and Oct4 bind targets in neural progenitors 41 and neural crest cells 40 distinct from those in embryonic stem cells, our findings highlight the context-dependent activity of transcription factors associated with pluripotency at later stages of development.
The role of nuclear receptor transcription factors in controlling cell fate decisions in postmigratory CNCCs may be a general theme. Nr2f (COUP-TF) family nuclear receptors have been shown to restrict progenitors from committing to cartilage fates in the upper jaw, thus facilitating later dermal bone formation 42. A key difference is that Nr2f factors are expressed during initial formation of CNCC mesenchyme and persist in specific jaw regions, whereas nr5a2 expression initiates only after migration of CNCCs to the mandibular arch. In the future, it will be interesting to examine what makes this class of transcription factor particularly well suited for regulating CNCC potential in diverse contexts.
Limitations of the study
Whereas we show that Nr5a2 is required for chromatin accessibility at jaw-specific enhancers, many of which contain predicted Nr5a2-binding sites, we did not directly test whether Nr5a2 binds and opens these enhancers during development. It will be important to use approaches such as Cleavage Under Targets and Release Using Nuclease (CUT&RUN) sequencing to profile which putative jaw-specific enhancers are directly bound by Nr5a2. Although Nr5a2 was required for jaw tendon patterning, misexpression of Nr5a2 in zebrafish did not result in a clear expansion of the tendon-forming domain. The expression domain of nr5a2 is also much broader than the tendon-forming domain in the fish jaw. One possibility is that Nr5a2 functions to prime jaw tendon enhancers through chromatin opening but does not activate them without the appropriate co-factors. For example, it would be interesting to test whether co-expression of Nr5a2 and the tendon-promoting factor Scx would be sufficient to synergistically induce ectopic tendons. In Nr5a2NCC mice, we observed expansion of cartilage and dysmorphology of bones in the middle ear. Given that Nr5a2 appears to function mainly as an activator and not a repressor of transcription in zebrafish, it remains unclear how Nr5a2 affects skeletal patterning. Lineage analyses of skeletal progenitors in Nr5a2-deficient fish and mice should help inform the cellular bases of skeletal defects, and genetic studies of Nr5a2 target genes such as foxp2 will be needed to address molecular mechanisms by which Nr5a2 patterns the jaw and middle ear skeleton.
STAR METHODS
Resource availability
Lead contact
Requests for material should be directed to J. Gage Crump (gcrump@usc.edu).
Materials availability
All transgenic lines and plasmids generated in this study will be available upon request.
Data and code availability
Single-cell RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. Microscopy data reported in this paper will be shared by the lead contact upon request.
No new code has been generated for this study.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rabbit-anti-phospho-histone H3 (Ser10) | Santa Cruz Biotechnology | sc-8656-R |
| chicken-anti-mCherry | Novus Biologicals | NBP2-25158 |
| chicken-anti-GFP | Abcam | ab13970 |
| mouse-anti-MF-20 | Developmental Studies Hybridoma Bank | MF 20 |
| rabbit-anti-Sox9 | Novus Biologicals | NBP1-85551 |
| Alexa Fluor 488 anti-chicken | ThermoFisher | A-11039 |
| Alexa Fluor 568 anti-chicken | ThermoFisher | A-11041 |
| Alexa Fluor 568 anti-rabbit | ThermoFisher | A11011 |
| Alexa Fluor 647 anti-rabbit | ThermoFisher | A-31573 |
| Alexa Fluor 647 anti-mouse | ThermoFisher | 21242 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Alexa Fluor 647 Phalloidin | ThermoFisher | A22287 |
| Critical Commercial Assays | ||
| RNAscope Multiplex Fluorescent Reagent Kit v2 Assay | Advanced Cell Diagnostics | Protocol 323100-USM and technical note MK 50-016 |
| Chromium Next GEM Single Cell Multiome ATAC + Gene Expression | 10X Genomics | Protocol CG000338 |
| Deposited Data | ||
| Raw sequencing fastq files and Cell Ranger ARC v2.0.0-processed Multiome data in this paper | GEO (Gene Expression Omnibus), this paper | GSE210251 |
| Published scRNAseq of submandibular salivary gland | GEO (Gene Expression Omnibus) (Hauser et al., 2020)28 | GSE150327 |
| Published scRNA-Seq of mouse mandible | FaceBase (Yuan et al., 2020)26 | Record ID 1-DTK2 |
| Published ATAC-Seq of zebrafish NCC | GEO (Gene Expression Omnibus) (Trinh et al., 2017)34 | GSE89670 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Nr5a2-flox: Nr5a2tm1Sakl | The Jackson Laboratory | JAX 024054 |
| Mouse: Wnt1-Cre: Wnt1-Cre1 | (Danielian et al., 1998)45 | N/A |
| Mouse: Prrx1-Cre: Tg(Prrx1-Cre)1Cjt | The Jackson Laboratory | JAX 005584 |
| Mouse: Scx-GFP: Tg(Scx-GFP)1Stzr | (Pryce et al., 2007)46 | N/A |
| Zebrafish: nr5a2oz3: nr5a2oz3 | (Nissim et al., 2016)23 | oz3 |
| Zebrafish: sox10:dsRed:Tg(sox10:DsRedExpress) | Our lab | el10 |
| Zebrafish: fli1a:GAL4:Tg(fli1:GAL4-VP16,myl7:EGFP) | Our lab | el360 |
| Zebrafish: sox10:mGFP:Tg(sox10:EGFP-CAAX) | Our lab | el375 |
| Zebrafish: col2a1a:GFP:TgBAC(col2a1a:EGFP) | Our lab | el483 |
| Zebrafish: col2a1a:mCherry:TgBAC(col2a1a:mCherry-NTR) | Our lab | el599 |
| Zebrafish: col2a1a:h2az2a-mCherry-2A-EGFP-CAAX:Tg(col2a1a:h2az2a-mCherry-2A-EGFP-CAAX) | Our lab | el690 |
| Zebrafish: scxa:mCherry:Tg(scxa:mCherry) | (Chen et al., 2020)43 | fb301 |
| Zebrafish: actb2:LOXP-BFP-LOXP-DsRed:Tg(actb2:LOXP-BFP-LOXP-DsRed) | (Kobayashi et al., 2014) 44 | sd27 |
| Zebrafish: nr5a2mGFP :Tg(nr5a2:GFP-CAAX) | This paper | el874 |
| Zebrafish: nr5a2mGFP-DBD-del:Tg(nr5a2:GFP-CAAX-DBD-del) | This paper | el875 |
| Zebrafish: UAS-nr5a2:Tg(UAS-nr5a2,cryaa:Cerulean) | This paper | el877 |
| Zebrafish: foxp2-p1:GFP:Tg(foxp2_p1-Mmu.E1b:GFP, cryaa:Cerulean) | This paper | el887,el888,el889 |
| Zebrafish: cdh6-p1:GFP:Tg(cdh6_p1-Mmu.E1b:GFP, cryaa:Cerulean) | This paper | el895,el896,el897 |
| Zebrafish: fgf10a-p1:GFP:Tg(fgf10a_p1-Mmu.E1b:GFP, cryaa:Cerulean) | This paper | el890,el891,el892 |
| Zebrafish: scxa-p2:GFP:Tg(scxa_p2-Mmu.E1b:GFP, cryaa:Cerulean) | This paper | el900,el901 |
| Oligonucleotides | ||
| RNAscope probe: cdh6 | Advanced Cell Diagnostics | 1225191-C4 |
| RNAscope probe: fgf10a | Advanced Cell Diagnostics | 1090101-C1, 1090101-C4 |
| RNAscope probe: foxp2 | Advanced Cell Diagnostics | 1181381-C1 |
| RNAscope probe: nr5a2 | Advanced Cell Diagnostics | 578901-C2 |
| RNAscope probe: ogna | Advanced Cell Diagnostics | 848241-C4 |
| RNAscope probe: sox9a | Advanced Cell Diagnostics | 543491-C3 |
| RNAscope probe: Nr5a2 | Advanced Cell Diagnostics | 543571 |
| RNAscope probe: Sox9 | Advanced Cell Diagnostics | 401051-C3 |
| guideRNA targeting sequences | Table S6 | N/A |
| In situ hybridization probes | Table S6 | N/A |
| Zebrafish transgenesis primers | Table S6 | N/A |
| Genotyping primers | Table S6 | N/A |
| Software and Algorithms | ||
| Cell Ranger ARC v2.0.0 | 10X Genomics | https://support.10xgenomics.com/single-cell-multiome-atac-gex/software/pipelines/latest/what-is-cell-ranger-arc |
| Seurat v4 | (Hao et al., 2021)55 | https://satijalab.org/seurat/ |
| Signac v1.6 | (Stuart et al., 2021)56 | https://satijalab.org/signac/ |
| chromVAR | (Schep et al., 2017)57 | https://greenleaflab.github.io/chromVAR/ |
| LinkPeaks | (Ma et al., 2020)58 | N/A |
| DAVID Bioinformatics Resources | (Huang et al., 2009)59; (Sherman et al., 2022)60 | https://david.ncifcrf.gov/ |
| Fiji | (Schindelin et al., 2012)61 | https://fiji.sc |
Experimental model and subject details
All experiments on zebrafish (Danio rerio) and mouse (Mus musculus) were approved by the Institutional Animal Care and Use Committee of the University of Southern California (IACUC protocols #20771 and #21151). Embryos and animals were humanely euthanized for experiments.
Zebrafish
Zebrafish were raised in vivarium under standard conditions at 28.5°C with health and water conditions monitored daily. Heterozygous mutant adult fish were raised in similar conditions and showed no visible defects, with homozygous mutant embryos produced by breeding for experimental analysis. Published mutant and transgenic lines include: nr5a2oz3 23; Tg(sox10:DsRedExpress)el10; Tg(fli1:GAL4-VP16,myl7:EGFP)el360; Tg(sox10:EGFP-CAAX)el375; TgBAC(col2a1a:EGFP)el483; TgBAC(col2a1a:mCherry-NTR)el559; Tg(col2a1a:h2az2a-mCherry-2A-EGFP-CAAX)el690; Tg(scxa:mCherry)fb301 43; and Tg(actb2:LOXP-BFP-LOXP-DsRed)sd27 (ubiquitous BFP reporter) 44. Transgenic lines generated in this study include Tg(nr5a2:GFP-CAAX)el874(mGFP); Tg(nr5a2:GFP-CAAX-DBD-del)el875 (mGFP-DBD-del); Tg(UAS:nr5a2,cryaa:Cerulean)el877; Tg(foxp2_p1-Mmu.E1b:GFP, cryaa:Cerulean)el887; el888; el889; Tg(cdh6_p1-Mmu.E1b:GFP, cryaa:Cerulean)el895; el896; el897; Tg(fgf10a_p1-Mmu.E1b:GFP, cryaa:Cerulean)el890; el891; el892; and Tg(scxa_p2-Mmu.E1b:GFP, cryaa:Cerulean)el900; el901. All transgenic lines generated in this study are on a Tubingen background. Developmental stages and numbers of embryos used are described for each experiment. As zebrafish develop gender differences after the stages studied here, the gender of embryos was undetermined. For oz3 allele genotyping, genomic DNA was amplified by PCR and digested by restriction enzyme BsrI. Wild type yields cut DNA products, while oz3 yields uncut product. For the nr5a2:mGFP-DBD-del allele, genotyping was based on a reduction in band size due to the 9 bp deletion.
Mice
Mice were raised in vivarium under standard conditions with health conditions monitored daily. All mice are in the C57BL/6 background. Alleles utilized in this study are Nr5a2-flox (Nr5a2tm1Sakl, JAX 024054, The Jackson Laboratory), Wnt1-Cre1 45, Prrx1-Cre (Tg(Prrx1-Cre)1Cjt, JAX 005584, The Jackson Laboratory), and Scx-GFP (Tg(Scx-GFP)1Stzr) 46. Developmental stages and numbers of embryos used are described for each experiment. Both male and female embryos were used. To conditionally delete both Nr5a2 alleles in mutants, Nr5a2-flox/flox was bred onto Wnt1-Cre or Prrx1-Cre drivers and their siblings were used as controls. Adult mice carrying a subset of transgenes and alleles were raised in similar conditions and showed no visible defects, with conditional mutant embryos produced by breeding for experimental analysis.
Method details
Zebrafish transgenesis
To generate nr5a2:mGFP, GFP-CAAX sequences were inserted just upstream of the translation start site (ATG) of nr5a2 by injecting a mix of Cas9 protein (final concentration of 800 ng/μl, Pnabio CP02), two guide RNAs (see Table S6 for sequences, final concentration of 200 ng/μl), and linearized dsDNA (final concentration of 20 ng/μl) of GFP-CAAX flanked by a left homology arm of 1020 bp and right homology arm of 1196 bp centered around the translation start site. One founder line was identified with mGFP expression recapitulating endogenous nr5a2 expression. To generate the nr5a2:mGFP-DBD-del allele, five guide RNAs targeting the DNA binding domain (DBD) of nr5a2 were injected with Cas9 protein into nr5a2:mGFP embryos. The deletion allele was identified by the smaller band size following PCR amplification, and sequencing identified an in-frame 9 bp deletion that removes a critical zinc finger motif-forming cysteine residue in the DNA-binding domain.
To generate zebrafish transgenic lines, donor plasmids (final concentration of 20 ng/μl) were co-injected with Tol2 transposase RNA (final concentration of 30 ng/μl) into one-cell-stage embryos. To make UAS:nr5a2 plasmid, the nr5a2 coding sequence was first amplified from cDNA made from Tubingen mRNA, and then combined with 10X UAS regulatory sequences and polyA entry vectors into a destination plasmid flanked by Tol2 transposase sequences (Tol2kit) 47 and containing the co-selection marker cryaa:Cerulean (“eye CFP”) using Gateway cloning (Thermofisher BP Clonase II 11789020, LR Clonase II plus 12538120). Three independent UAS:nr5a2 alleles were tested and gave similar phenotypes when combined with fli1a:Gal4VP16. To test putative enhancers of foxp2, cdh6, fgf10a, and scxa, accessible chromatin regions were cloned from Tubingen genomic DNA into a plasmid containing Mmu.E1b as a minimal promoter, GFP, and cryaa:Cerulean as a co-selection marker, flanked by Tol2 transposase sequences, using In-Fusion cloning (Takara Bio 638910). Two to three independent founder lines were identified for each construct, and consistent GFP expression patterns were observed in at least three embryos of each allele (see Fig. 6 and Fig. S6E).
mRNA in situ hybridization
Zebrafish embryos were fixed in 4% PFA/1X PBS at 4°C overnight and dehydrated through MeOH series (25%, 50%, 75% MeOH in PBSTw (0.25% Tween-20 in 1X PBS), 100% MeOH twice) 5 min each and stored in 100% MeOH at −80°C overnight or longer. In situ probes in this study include digoxigenin-labeled cdh6 48, gsc 49, igfbp5b 25, msx1a 50, nr5a2 17, pitx1 25, and Fgf10 27 (GenePaint Set ID MH359); dinitrophenol-labeled dlx2a 51, hand2 52, and sox9a 53; RNAscope probes of cdh6, fgf10a, foxp2, nr5a2, ogna, sox9a, Nr5a2, and Sox9 were synthesized by Advanced Cell Diagnostics.For whole-mount detection of cdh6, dlx2a, gsc, hand2, igfbp5b, msx1a, nr5a2, pitx1, and sox9a by fluorescent in situ, the full protocol is available online (https://wiki.zfin.org/display/prot/Triple+Fluorescent+In+Situ) with minor modifications: 1.5 dpf embryos were permeabilized with gentle shaking for 30 min and 2.5 dpf embryos for 1 hr with 1.25 μg/ml protease K. We used 1 ng/μl of each probe, 1:500 of anti-DIG-peroxidase (Roche 11207733910), 1:200 of anti-DNP-peroxidase (Akoya NEL747A001KT), 1:30 of Tyr-Cy3 (Akoya NEL704A001KT), and 1:30 of Tyr-Cy5 (Akoya NEL705A001KT). Counterstain of nuclei by DAPI staining was performed at 4°C overnight.
Whole-mount detection of cdh6, fgf10a, foxp2, nr5a2, ogna, and sox9a in zebrafish and detection of Nr5a2 and Sox9 on mouse sections were performed by RNAscope Multiplex Fluorescent Reagent Kit v2 Assay per manufacturer’s instructions (Advanced Cell Diagnostics, protocol 323100-USM, and technical note MK 50–016) with optimization. RNAscope on zebrafish embryos was performed in tubes (six 2.5 dpf embryos/ml). Target Retrieval was performed for 20 min at 100°C. We permeabilized 2.5 dpf embryos with Protease Plus for 15 min at 40°C. RNAscope on mouse embryos was performed on paraformaldehyde-fixed paraffin-embedded sections with sample preparation, paraffin embedding, and sectioning.
For Fgf10 in situs, mandibular arches of E12.5 mouse embryos were dissected and fixed in 4% PFA/1X PBS at 4°C overnight. Colorimetric whole-mount detection of Fgf10 was performed as described 54. In brief, embryos were dehydrated through MeOH series and stored at −20°C overnight, rehydrated through MeOH series to PBSTx (0.2% Triton-X in 1X PBS) before permeabilization by 20 μg/ml of protease K for 30 min with gentle shaking at RT (room temperature). Secondary fixation in 4% PFA/0.1% glutaraldehyde/1X PBS for 30 min was followed by two washes of PBSTx for 10 min at RT and prehyb incubation for 2 hr at 65°C. 1 ng/μl of probe was used for hybridization for 48 hr followed by 2X SSC and 0.2% SSC washes three each for 20 min at 65°C and two KTBT washes for 10 min each at RT. Blocking and anti-DIG-AP Fab incubation (1:2000, Roche 11093274910) were performed in 20% sheep serum/KTBT at 4°C overnight. We then washed five times with KTBT for 1 hr and twice with NTMT for 15 min before color development in 10 μl NBT/BCIP premix stock (Roche 11681451001) per ml of NTMT in the dark. When hybridization signal was optimal, we stopped the reaction with KTBT followed by PBS washes. Similar expression patterns were observed in at least three zebrafish embryos of wild type and mutant unless described in the text and panels. Numbers of mouse embryos examined are described in the figure legends.
Sectioning
For paraffin sections, we fixed E11.5 or E12.5 whole embryos or dissected newborn mouse heads in 4% PFA/1X PBS at 4°C overnight, followed by decalcification in 0.5 M EDTA (pH 8) (for newborn) and dehydration though EtOH series (1X PBS, two 50% EtOH, two 70% EtOH for 1 hr each, 95% EtOH overnight, 95% EtOH, three 100% EtOH for 1 hr each at RT in the second day, and then 100% EtOH overnight at 4°C). Before embedding with paraffin, samples were infused with Hemo-De (xylene substitute, Electron Microscopy Sciences 23412–01) and paraffin through a series of 50% EtOH/50% Hemo-De, four Hemo-De at RT. Sections were then placed in the vacuum incubator at 65°C and put through a series of 50% Hemo-De/50% paraffin and paraffin three times for 30 min each. After embedding in paraffin, paraffin blocks were left overnight at RT to harden. For mouse embryos, 5 μm thick sections were cut on a Thermo Scientific Shandon Finesse ME+ Microtome. For newborn mouse heads, 8 μm thick sections were cut with a Leica Microtome RM2245. For cryo-sectioning, E16.5 mouse heads were fixed in 4% PFA/1X PBS followed with 1X PBS wash at 4°C for 30 min each, and were then infused with sucrose (10% sucrose overnight and 30% sucrose until sinking at 4°C) and embedded in OCT (with a quick wash of OCT) (Sakura 4583). Cryo-blocks were frozen on dry ice and stored in −80°C. For cryo-sections, 10 μm thick sections were cut on a Leica Cryostat CM3050S.
Trichrome staining
Staining was performed via standard protocols. Coronal and sagittal paraffin sections were first de-paraffinized and rehydrated through an EtOH series (100%, 90%, 70%, and 50%) to water. The sections were stained with Alcian Blue (1% Alcian Blue 8GX (BDH) in 3% acetic acid, pH 2.5), Ehrlich’s hematoxylin (Solmedia), and differentiated in 2.5% phosphomolybdic acid (Acros Organic), with two rinses of water between each solution. Finally, the sections were stained with Sirius red (0.1% Direct Red 80 in saturated aqueous solution of picric acid, Sigma) followed by two 1% Acetic Acid washes prior to mounting and imaging.
Skeletal staining
For skeletal staining, zebrafish embryos were fixed in 2% PFA/1X PBS for 1 hr and washed with 100 mM Tris (pH 7.5)/10 mM MgCl2 for 10 min at RT. We then incubated embryos with Alcian stain (0.04% Alcian Blue (Anatech 862), 100 mM Tris (pH 7.5), 10 mM MgCl2, 80% EtOH) for cartilage staining overnight followed by de-staining in EtOH gradient (80%, 50%, 25% EtOH in 100 mM Tris (pH 7.5)/10 mM MgCl2) for 5 min each. Embryos were then incubated in 3% H2O2 /0.5% KOH with open lids under a light source until pigments bleached. To stain bone, we then washed in 25% glycerol/0.1% KOH for 10 min before adding Alizarin stain (0.01% Alizarin Red S (Sigma A5533), 25% glycerol, 100 mM Tris (pH 7.5)) for 30 min. Embryos were de-stained in 50% glycerol/0.1% KOH twice before whole-mount imaging. Zebrafish facial skeletons were dissected with fine insect pins and mounted on microscope slides for flat-mount imaging.
For whole-mount skeletal staining of E14.5 embryos and newborn mice, we skinned newborn animals and removed soft tissues before fixation in 95% EtOH for five days with occasional changes of fresh EtOH at RT. We then added Alcian stain (0.015% Alcian Blue (Alcian Blue 8GX (Sigma A3157), 75% EtOH, 20% Glacial acetic acid) for one day and de-stained in 95% EtOH for one day, replacing solution once. Soft tissues were cleared with 0.5% KOH for five days before addition of Alizarin stain (0.002% Alizarin Red S (Sigma A5533), 0.5% KOH) for four days. We then de-stained in 1% KOH for one day before transfer into a glycerol gradient (25%, 50%, 75% glycerol / 0.5% KOH and 100% glycerol) one day each for imaging and storage. E14.5 Meckel’s cartilages were dissected with fine insect pins and mounted on microscope slides for flat-mount imaging.
Freshly dissected, un-fixed Scx-GFP+ mouse heads were stained in Alizarin stain and washed in PBS for 30 min each. Reported phenotypes were observed in at least three zebrafish or mouse embryos unless described otherwise.
Immunostaining
For whole-mount immunostaining, zebrafish embryos were fixed in 4% PFA/1X PBS at 4°C overnight and washed by PBS and ddH2O each for 5 min. Permeabilization of embryos was performed in cold acetone at −20°C for 10 min, followed by washing in ddH2O for 5 min, rehydrating with PBS for 5 min, blocking for 3 hr at RT, a brief wash, and incubation with rabbit anti-phospho-histone H3 (Ser10) (1:200, Santa Cruz Biotechnology), chicken anti-mCherry (1:200, Novus Biologicals NBP2–25158), or chicken anti-GFP (1:200, Abcam ab13970) in 2% goat serum / PBDTx (1% BSA, 1% DMSO, 0.1% Triton-X in 1X PBS, pH 7.3) at 4°C overnight. We then washed three times for 20 min each with PBDTx before incubating with 1:300 secondary antibodies Alexa Fluor 647 anti-rabbit (ThermoFisher A-31573), Alexa Fluor 568 anti-chicken (ThermoFisher A-11041), or Alexa Fluor 488 anti-chicken (ThermoFisher A-11039), as well as DAPI, in 2% goat serum / PBDTx at 4°C overnight. Embryos were washed twice with PBSTx (0.1% Triton-X in 1X PBS) before imaging.
For staining of mouse cryo-sections, slides were rinsed with ddH2O, air dried at RT, and fixed in 4% PFA/1X PBS at 4°C for 10 min. Tissues were permeabilized in PBSTx for 10 min and washed twice with PBSTw (0.05% Tween-20 in 1X PBS). We then blocked for 1h at RT before incubating with mouse anti-MF-20 (1:300, Developmental Studies Hybridoma Bank MF 20), rabbit anti-Sox9 (1:800, Novus Biologicals NBP1–85551), or chicken anti-GFP (1:200, Abcam ab13970) at 4°C overnight. This was followed with incubation of 1:300 secondary antibodies Alexa Fluor 647 anti-mouse (ThermoFischer A-21242), Alexa Fluor 568 anti-rabbit (ThermoFischer A11011), or Alexa Fluor 488 anti-chicken (ThermoFisher A-11039) in 10% FBS/1% BSA/5% goat serum in PBSTw at RT for 1 hr. Slides were rinsed twice with ddH2O before mounting and imaging. Numbers of zebrafish and mouse embryos examined are described for each experiment in the figure panels or legends.
Muscle staining
To stain muscle, zebrafish embryos were fixed in 4% PFA/1X PBS for 1 hr at RT (or at 4°C overnight), and Scx-GFP+ mouse limbs were left un-fixed. Both samples were permeabilized with 0.5% Triton-X in PBS for at least 1 hr followed by phalloidin staining (1:100 in 1X PBS, Alexa Fluor 647 Phalloidin, ThermoFisher A22287) at 4°C overnight. Samples were rinsed several times with PBS before imaging. Phenotypes were observed in at least three zebrafish and mouse embryos unless described otherwise.
Transplantation
For CNCC transplants, BFP+ naïve ectoderm from shield-stage actb2:LOXP-BFP-LOXP-DsRed donors was transplanted into CNCC-forming regions of shield-stage col2a1a:h2az2a-mCherry-2A-EGFP-CAAX hosts. Transplantation was performed unilaterally with one side as control. Embryos were held in place with 2% methylcellulose in Ringer’s solution (116 mM NaCl, 2.6 mM KCl, 5 mM HEPES, pH 7.0) on glass slides flooded with Ringer’s solution. A fine glass needle was used to move small clumps of cells from donors to hosts. Animals were recovered in embryo medium with 50 U/ml Penicillin and 0.05 mg/ml Streptomycin, selected by contribution of BFP+ cells to pharyngeal arches on one side of the embryo at 1.5 dpf, and imaged at 6 dpf on a confocal microscope before fixation for phalloidin staining of muscle. The same embryos then underwent skeletal staining as described above.
Imaging
Images of live and fixed zebrafish, as well as sections after in situ hybridization, immunostaining, or phalloidin staining were captured on a Zeiss LSM800 confocal microscope using ZEN software. Skeletal staining, freshly dissected mouse tissues (salivary glands, stomach, and intestines), and mouse whole-mount in situs were imaged using a Leica S8APO stereo microscope. Images of flat-mount zebrafish skeletons were captured with a Leica DM2500 compound microscope. Fluorescence images of freshly dissected mouse skeletons after Alizarin Red staining were taken by a Leica MZ16F fluorescence microscope. Trichrome stains of mouse histological sections were imaged by a Nikon Eclipse 80i microscope.
Single nuclei isolation
Zebrafish embryos were screened for nr5a2:mGFP-DBD-del+ and scxa:mCherry+ with a fluorescence dissecting microscope and fin clips were collected for PCR genotyping of the mutant oz3 allele before 2 dpf. Control and mutant heads were decapitated between the eye and ear after anesthesia at 2.5 dpf. Separately for controls and mutants, 160 dissected heads (twenty heads pre tube) were washed twice with fresh and iced Ringer’s solution followed by dissociation at 28.5 °C for 40 min by mechanical and enzymatic digestion – nutating and pipetting every 5 min in pre-warmed protease solution (0.25% trypsin, 1 mM EDTA (pH 8.0), and 20 mg/mL Collagenase D (from stock of 400 mg/mL Collagenase D in HBSS) in PBS) until full dissociation. The reaction was stopped with 6X stop solution (6 mM CaCl2 and 30% fetal bovine serum (FBS) in PBS). Dissociated cells were collected by centrifugation for 5 min (2000 rpm at 4°C) and washed by suspension solution (1% FBS, 0.8mM CaCl2, 50 U/ml Penicillin, 0.05 mg/ml Streptomycin in Leibovitz Medium) twice and filtered by cell strainer before sorting. Live cells were fluorescence-activated cell sorted (FACS) to isolate 50,000 GFP+ cells that excluded the cytoplasmic stain Zombie Violet (control) or nuclear stain DAPI (mutant) into 0.04% BSA/ PBS at 4°C. Nuclei isolation was performed per manufacturer’s instructions (10X Genomics, protocol CG000169, low cell input protocol) with optimization for zebrafish mesenchyme. FACS cells were pelleted for 15 min (300 rcf at 4oC) and incubated with lysis buffer for 100 s on ice. Isolated nuclei were washed by Wash buffer and Nuclei buffer and checked for nuclear integrity with a fluorescence confocal microscope with DAPI staining before use in library construction.
Multi-omic library construction, sequencing, and alignment
Multi-omic libraries of snATACseq and snRNAseq from the same barcoded single nuclei were constructed per manufacturer’s instructions (10X Genomics, Chromium Next GEM Single Cell Multiome ATAC + Gene Expression, protocol CG000338). In brief, to capture accessible chromatin and transcripts from the same cells, accessible chromatin regions from isolated nuclei were first tagged by Tn5 transposase. Tagged chromatin and polyadenylated mRNA from the same nuclei were pulled down and barcoded with the same sequences within isolated GEMs to achieve single-nuclei separation. Chromatin fragments were further indexed with sample-specific i7 indexes and linked with Illumina P5 and P7 sequences. Reverse transcription was performed in GEMs to synthesize RNA:DNA hybrids from polyadenylated mRNA. cDNAs were further synthesized, fragmented, and indexed with sample-specific i7 and i5 indexes and linked with Illumina P5 and P7 sequences. Quality control of libraries was assessed with the 4200 TapeStation system and Qubit dsDNA HS assay kit. Libraries were sequenced on Illumina HiSeq (control) or NextSeq (mutant) platforms. For sequencing snATACseq libraries, both Read1 and Read2 were extended to 60 cycles, and for sequencing snRNAseq libraries, Read2 was extended to 102 cycles for longer coverage. Sequencing reads were aligned to customized genome (built with GRCz11.fa, GRCz11.104.gtf, and JASPAR2020.pfm, with addition of GFP-CAAX and mCherry gene information; the same genome build is used for analysis by Seurat/Signac) and peak calling was performed by Cell Ranger ARC v2.0.0 per manufacturer’s instructions to generate peak-by-cell and gene-by-cell count matrices.
Multi-omics data analysis
Multii-omics data were processed by Seurat v4 55 and Signac v1.6 56 packages in Rstudio following the standard workflow with optimization. To first identify the mesenchyme populations of individual libraries, Cell Ranger ARC-output count matrices in H5 format of individual libraries were used to create Seurat objects by “CreateSeuratObject” and “CreateChromatinAssay” functions. Quality control was performed by setting the thresholds of accessible region counts (nCount_ATAC) < 50000, transcript counts (nCount_RNA) < 7500, ratio of mononucleosome to nucleosome-free region (nucleosome_signal) < 2, and enrichment at TSS (TSS.enrichment) > 0.5. In order to compare accessible chromatin regions (peaks) between control and mutant, peaks-called were combined by “disjoin” function that splits peaks into overlapping and non-overlapping regions between the control and mutant libraries and filtered by new peak widths (peakwidths < 10000 & > 20). The disjoined-peak list was used to recalculate peak-by-cell count matrices by “CreateFragmentObject”, “FeatureMatrix”, and “CreateChromatinAssay” functions. For further analysis of ATAC data (peak-by-cell count matrices), 95% of most common peaks were selected by “FindTopFeatures (min.cutoff = 5)” function. Normalization and linear dimension reduction by LSI were performed by “RunTFIDF”, and “RunSVD” functions. RNA data (gene-by-cell count matrices) were normalized and scaled, and the top 3000 most common genes were selected by “SCTransform” function for dimension reduction by PCA (“RunPCA” function). To cluster cells with ATAC and RNA information in combination, “FindMultiModalNeighbors” (2:20 dimensions of LSI and 1:25 dimensions of PCA were used, with the first dimension of LSI excluded due to its high correlation with sequencing depth), “RunUMAP” (nn.name = “weighted.nn”), and “FindClusters” (resolution = 0.8, graph.name=”wsnn”, algorithm = 3) functions. Identities of clusters were identified by differentially enriched genes calculated by “FindAllMarkers” (test.use = “wilcox” (Wilcoxon Rank Sum test), logfc.threshold = 0.25 (log2 Fold Change), return.thresh = 0.01 (p value)) function. Clusters 0, 2, 5, 8, 20 of control library and clusters 2, 4,10,18 of mutant library were identified as mesenchyme populations for the high expression of fli1a, prrx1a, and other mesenchyme genes.
“Merge” function was used to combine count matrices of control and mutant mesenchyme clusters. Merged ATAC data were processed by “FindTopFeatures” (min.cutoff = 10) and LSI linear dimension reduction, and merged RNA data were processed and dimensionally reduced by PCA as described above. 2:30 dimensions of LSI and 1:30 dimensions of PCA were used to cluster cells with both ATAC and RNA information as described above. After filtering for artificial clusters with over- and under-accessible profiles, 14 sub-clusters of mesenchyme were identified and differentially enriched genes were calculated by “FindAllMarkers” (test.use = “wilcox”, logfc.threshold = 0.25, return.thresh = 0.01, min.pct = 0.25). To calculate motif enrichments of each cell by chromVAR 57, “AddMotifs” was first used to calculate motif enrichments of each accessible chromatin region (peak) followed by “RunChromVAR” to calculate motif activity in each mesenchyme cell. To identify accessibility of chromatin regions correlated with expression of genes whose TSS was located within 500 kb of the chromatin regions, “LinkPeaks” (method =”spearman”, min.cells = 5, pvalue_cutoff = 0.1, score_cutoff = 0.02) 58 was performed on merged mesenchyme.
For detailed analysis of mandibular arch clusters, we further extracted a cartilage cluster (acana+, cluster 11), a tendon cluster (tnmd+, cluster 2), and four mandibular arch mesenchyme (Mes1–4) clusters (dlx2a+; dlx5a+; hand2+; Hox-negative, clusters 8, 4, 7, and 1, respectively). UMAP visualization of mandibular arch clusters was generated by “FindMultiModalNeighbors” and “RunUMAP” using the same parameters as mesenchyme analysis above. To investigate the difference of mutant mandibular arch clusters compared to control, differential analysis was performed. Differential motif enrichments of mutant and control mandibular mesenchyme, excluding cartilage, were calculated by “FindMarkers” (test.use = “wilcox”, mean.fxn = rowMeans, fc.name = “avg_diff”, logfc.threshold = 0.25 (calculating average difference)) using “chromvar” matrix, differential accessible chromatin regions by “FindAllMarkers” (test.use = ‘LR’ (logistic regression), logfc.threshold = 0.25, return.thresh = 0.01, min.pct = 0.05, latent.vars = ‘nCount_ATAC’) on “ATAC” matrix, and differential gene expressions by “FindAllMarkers” (test.use = “wilcox”, logfc.threshold = 0.25, return.thresh = 0.01, min.pct = 0.25) on “SCT” matrix. Gene Ontology analysis (GO) of downregulated genes in mutants was performed using DAVID Bioinformatics Resources 59,60. To identify potential Nr5a2 direct targets, regions with less or more accessibility in mutants were divided into two groups: those that contain predicted Nr5a2 binding motifs and those that did not. Genes whose expression correlated with the accessibility of the Nr5a2 motif regions (predicted by “LinkPeaks” above) were intersected with genes with decreased or increased expression in mutants. To investigate the accessibility of enhancers in the published 1.5 dpf CNCC snATACseq data 17, the most overlapping chromatin regions (peaks) were identified and their activities were plotted for visualization.
Quantification and statistical analysis
Quantification of Meckel’s chondrocyte numbers across time was performed by counting nuclei labeled by the col2a1a:h2az2a-mCherry-2A-EGFP-CAAX transgene in serial confocal sections with Fiji software 61. Quantification of pHH3+ nuclei labeled by the col2a1a:h2az2a-mCherry-2A-EGFP-CAAX transgene in wild-type and mutant Meckel’s was counted in serial confocal sections. Wilcoxon rank-sum test was performed to compare the difference of wild-type and mutant chondrocyte numbers at each stage. Numbers of nr5a2:mGFP-DBD-del+ tenocytes and chondrocytes were counted by membrane-GFP+ cell outlines and chondrocyte numbers by col2a1a:mCherry-NTR labeling in serial confocal sections. Wilcoxon rank-sum test was performed to compare the difference of GFP+ tenocyte numbers and GFP+ chondrocyte proportion to total chondrocyte numbers in wild types and mutants. Quantification of Meckel’s chondrocyte and tenocyte numbers in fixed embryos of fli1a:Gal4VP16; UAS:nr5a2 and single-transgene animals were counted with nuclei labeled by DAPI within cells marked by chondrocyte or tenocyte transgenes across serial confocal sections. Measurements of skeleton and tendon length in newborn mice were measured by Fiji software. Differences across genotypes were calculated by a Tukey’s range test to account for multiple comparisons.
Supplementary Material
Table S1. Differentially expressed genes for control nr5a2:mGFP+ clusters, mutant nr5a2:mGFP+ clusters, combined control and mutant nr5a2:mGFP+ mesenchyme clusters, and nr5a2 mutant mandibular mesenchyme related to Figure 5A, 5D, S5
Table S2. Differential DNA-binding motifs in nr5a2 mutant mandibular mesenchyme, related to Figure 5C
Table S3. Differential chromatin accessibility in nr5a2 mutant mandibular mesenchyme, related to Figure 5D
Table S4. GO analysis of downregulated genes in nr5a2 mutant mandibular mesenchyme, related to Figure 5D
Table S5. Differentially accessible chromatin regions in nr5a2 mutant mandibular mesenchyme associated with differentially expressed genes and Nr5a2 motifs, related to Figure 5D, 6, S6D–F
Table S6. Oligonucleotide sequences, related to STAR Methods
Highlights.
Pluripotency gene Nr5a2 redeployed in neural crest-derived mesenchyme
Nr5a2 restricts cartilage specification in the fish jaw and mouse middle ear
Essential role for Nr5a2 in jaw tendon and salivary gland formation
Nr5a2 ensures jaw enhancer accessibility for non-skeletal multilineage potential
Acknowledgements
We thank Jenna Galloway for Tg(scxa:mCherry)fb301, the Broad CIRM Center Flow Cytometry Facility for FACS, the CHLA Center for Personalized Medicine Molecular Genomics Core for NGS sequencing, Megan Matsutani and Olivia Ruffins for fish care, and Samantha Brugmann for sharing the protocol of colorimetric whole-mount in situ for mouse embryos. Funding provided by NIDCR R21DE029656 and R35DE027550 to J.G.C. and HHMI Hannah H. Gray Fellows Program to D.T.F.
Footnotes
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper received support from a program designed to increase minority representation in their field of research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chai Y, Jiang X, Ito Y, Bringas P Jr., Han J, Rowitch DH, Soriano P, McMahon AP, and Sucov HM (2000). Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671–1679. 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 2.Chen JW, and Galloway JL (2014). The development of zebrafish tendon and ligament progenitors. Development 141, 2035–2045. 10.1242/dev.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crump JG, Swartz ME, Eberhart JK, and Kimmel CB (2006). Moz-dependent Hox expression controls segment-specific fate maps of skeletal precursors in the face. Development 133, 2661–2669. 10.1242/dev.02435. [DOI] [PubMed] [Google Scholar]
- 4.Platt JB (1893). Ectodermic origin of the cartilages of the head. Anat. Anz. 8, 506–509. [Google Scholar]
- 5.Schilling TF, and Kimmel CB (1994). Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 120, 483–494. [DOI] [PubMed] [Google Scholar]
- 6.Anthwal N, and Thompson H (2016). The development of the mammalian outer and middle ear. J Anat 228, 217–232. 10.1111/joa.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi W, Deng JM, Zhang Z, Behringer RR, and de Crombrugghe B (1999). Sox9 is required for cartilage formation. Nat Genet 22, 85–89. 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 8.Yan YL, Willoughby J, Liu D, Crump JG, Wilson C, Miller CT, Singer A, Kimmel C, Westerfield M, and Postlethwait JH (2005). A pair of Sox: distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development 132, 1069–1083. 10.1242/dev.01674. [DOI] [PubMed] [Google Scholar]
- 9.Ducy P, Zhang R, Geoffroy V, Ridall AL, and Karsenty G (1997). Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747–754. 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 10.Kague E, Hughes SM, Lawrence EA, Cross S, Martin-Silverstone E, Hammond CL, and Hinits Y (2019). Scleraxis genes are required for normal musculoskeletal development and for rib growth and mineralization in zebrafish. FASEB J 33, 9116–9130. 10.1096/fj.201802654RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, and Schweitzer R (2007). Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134, 2697–2708. 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 12.Chatzeli L, Gaete M, and Tucker AS (2017). Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development. Development 144, 2294–2305. 10.1242/dev.146019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bronner ME, and LeDouarin NM (2012). Development and evolution of the neural crest: an overview. Dev Biol 366, 2–9. 10.1016/j.ydbio.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabian P, and Crump JG (2022). Reassessing the embryonic origin and potential of craniofacial ectomesenchyme. Semin Cell Dev Biol. 10.1016/j.semcdb.2022.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buitrago-Delgado E, Nordin K, Rao A, Geary L, and LaBonne C (2015). Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science 348, 1332–1335. 10.1126/science.aaa3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zalc A, Sinha R, Gulati GS, Wesche DJ, Daszczuk P, Swigut T, Weissman IL, and Wysocka J (2021). Reactivation of the pluripotency program precedes formation of the cranial neural crest. Science 371. 10.1126/science.abb4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabian P, Tseng KC, Thiruppathy M, Arata C, Chen HJ, Smeeton J, Nelson N, and Crump JG (2022). Lifelong single-cell profiling of cranial neural crest diversification in zebrafish. Nat Commun 13, 13. 10.1038/s41467-021-27594-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu P, Goodwin B, Chung AC, Xu X, Wheeler DA, Price RR, Galardi C, Peng L, Latour AM, Koller BH, et al. (2005). Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol Cell Biol 25, 3492–3505. 10.1128/MCB.25.9.3492-3505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heng JC, Feng B, Han J, Jiang J, Kraus P, Ng JH, Orlov YL, Huss M, Yang L, Lufkin T, et al. (2010). The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell 6, 167–174. 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Pare JF, Malenfant D, Courtemanche C, Jacob-Wagner M, Roy S, Allard D, and Belanger L (2004). The fetoprotein transcription factor (FTF) gene is essential to embryogenesis and cholesterol homeostasis and is regulated by a DR4 element. J Biol Chem 279, 21206–21216. 10.1074/jbc.M401523200. [DOI] [PubMed] [Google Scholar]
- 21.Hale MA, Swift GH, Hoang CQ, Deering TG, Masui T, Lee YK, Xue J, and MacDonald RJ (2014). The nuclear hormone receptor family member NR5A2 controls aspects of multipotent progenitor cell formation and acinar differentiation during pancreatic organogenesis. Development 141, 3123–3133. 10.1242/dev.109405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stergiopoulos A, and Politis PK (2016). Nuclear receptor NR5A2 controls neural stem cell fate decisions during development. Nat Commun 7, 12230. 10.1038/ncomms12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nissim S, Weeks O, Talbot JC, Hedgepeth JW, Wucherpfennig J, Schatzman-Bone S, Swinburne I, Cortes M, Alexa K, Megason S, et al. (2016). Iterative use of nuclear receptor Nr5a2 regulates multiple stages of liver and pancreas development. Dev Biol 418, 108–123. 10.1016/j.ydbio.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertrand S, Thisse B, Tavares R, Sachs L, Chaumot A, Bardet PL, Escriva H, Duffraisse M, Marchand O, Safi R, et al. (2007). Unexpected novel relational links uncovered by extensive developmental profiling of nuclear receptor expression. PLoS Genet 3, e188. 10.1371/journal.pgen.0030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Askary A, Xu P, Barske L, Bay M, Bump P, Balczerski B, Bonaguidi MA, and Crump JG (2017). Genome-wide analysis of facial skeletal regionalization in zebrafish. Development 144, 2994–3005. 10.1242/dev.151712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan Y, Loh YE, Han X, Feng J, Ho TV, He J, Jing J, Groff K, Wu A, and Chai Y (2020). Spatiotemporal cellular movement and fate decisions during first pharyngeal arch morphogenesis. Sci Adv 6. 10.1126/sciadv.abb0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visel A, Thaller C, and Eichele G (2004). GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res 32, D552–556. 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauser BR, Aure MH, Kelly MC, Hoffman MP, Chibly AM, and Core G.a.C.B. (2020). Generation of a Single-Cell RNAseq Atlas of Murine Salivary Gland Development. iScience 23, 101838. 10.1016/j.isci.2020.101838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi A, Kontarakis Z, Gerri C, Nolte H, Holper S, Kruger M, and Stainier DY (2015). Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524, 230–233. 10.1038/nature14580. [DOI] [PubMed] [Google Scholar]
- 30.Xu P, Balczerski B, Ciozda A, Louie K, Oralova V, Huysseune A, and Crump JG (2018). Fox proteins are modular competency factors for facial cartilage and tooth specification. Development 145. 10.1242/dev.165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colnot C, Lu C, Hu D, and Helms JA (2004). Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol 269, 55–69. 10.1016/j.ydbio.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Entesarian M, Matsson H, Klar J, Bergendal B, Olson L, Arakaki R, Hayashi Y, Ohuchi H, Falahat B, Bolstad AI, et al. (2005). Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. Nat Genet 37, 125–127. 10.1038/ng1507. [DOI] [PubMed] [Google Scholar]
- 33.Lukoseviciute M, Gavriouchkina D, Williams RM, Hochgreb-Hagele T, Senanayake U, Chong-Morrison V, Thongjuea S, Repapi E, Mead A, and Sauka-Spengler T (2018). From Pioneer to Repressor: Bimodal foxd3 Activity Dynamically Remodels Neural Crest Regulatory Landscape In Vivo. Dev Cell 47, 608–628 e606. 10.1016/j.devcel.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trinh LA, Chong-Morrison V, Gavriouchkina D, Hochgreb-Hagele T, Senanayake U, Fraser SE, and Sauka-Spengler T (2017). Biotagging of Specific Cell Populations in Zebrafish Reveals Gene Regulatory Logic Encoded in the Nuclear Transcriptome. Cell Rep 19, 425–440. 10.1016/j.celrep.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gassler J, Kobayashi W, Gaspar I, Ruangroengkulrith S, Mohanan A, Gomez Hernandez L, Kravchenko P, Kummecke M, Lalic A, Rifel N, et al. (2022). Zygotic genome activation by the totipotency pioneer factor Nr5a2. Science 378, 1305–1315. 10.1126/science.abn7478. [DOI] [PubMed] [Google Scholar]
- 36.Gould SJ (1990). An earful of jaw. Natural History 3190, 12–23. [Google Scholar]
- 37.Talbot JC, Nichols JT, Yan YL, Leonard IF, BreMiller RA, Amacher SL, Postlethwait JH, and Kimmel CB (2016). Pharyngeal morphogenesis requires fras1-itga8-dependent epithelial-mesenchymal interaction. Dev Biol 416, 136–148. 10.1016/j.ydbio.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hickey SL, Berto S, and Konopka G (2019). Chromatin Decondensation by FOXP2 Promotes Human Neuron Maturation and Expression of Neurodevelopmental Disease Genes. Cell Rep 27, 1699–1711 e1699. 10.1016/j.celrep.2019.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao H, Zhou W, Yao Z, Wan Y, Cao J, Zhang L, Zhao J, Li H, Zhou R, Li B, et al. (2015). Foxp1/2/4 regulate endochondral ossification as a suppresser complex. Dev Biol 398, 242–254. 10.1016/j.ydbio.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hovland AS, Bhattacharya D, Azambuja AP, Pramio D, Copeland J, Rothstein M, and Simoes-Costa M (2022). Pluripotency factors are repurposed to shape the epigenomic landscape of neural crest cells. Dev Cell 57, 2257–2272 e2255. 10.1016/j.devcel.2022.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson KA, Nishi Y, Ma W, Vedenko A, Shokri L, Zhang X, McFarlane M, Baizabal JM, Junker JP, van Oudenaarden A, et al. (2012). Neural-specific Sox2 input and differential Gli-binding affinity provide context and positional information in Shh-directed neural patterning. Genes Dev 26, 2802–2816. 10.1101/gad.207142.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barske L, Rataud P, Behizad K, Del Rio L, Cox SG, and Crump JG (2018). Essential Role of Nr2f Nuclear Receptors in Patterning the Vertebrate Upper Jaw. Dev Cell 44, 337–347 e335. 10.1016/j.devcel.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen JW, Niu X, King MJ, Noedl MT, Tabin CJ, and Galloway JL (2020). The mevalonate pathway is a crucial regulator of tendon cell specification. Development 147. 10.1242/dev.185389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi I, Kobayashi-Sun J, Kim AD, Pouget C, Fujita N, Suda T, and Traver D (2014). Jam1a-Jam2a interactions regulate haematopoietic stem cell fate through Notch signalling. Nature 512, 319–323. 10.1038/nature13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danielian PS, Muccino D, Rowitch DH, Michael SK, and McMahon AP (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol 8, 1323–1326. 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 46.Pryce BA, Brent AE, Murchison ND, Tabin CJ, and Schweitzer R (2007). Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn 236, 1677–1682. 10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- 47.Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, and Chien CB (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236, 3088–3099. [DOI] [PubMed] [Google Scholar]
- 48.Liu Q, Liu B, Wilson AL, and Rostedt J (2006). cadherin-6 message expression in the nervous system of developing zebrafish. Dev Dyn 235, 272–278. 10.1002/dvdy.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schulte-Merker S, Hammerschmidt M, Beuchle D, Cho KW, De Robertis EM, and Nüsslein-Volhard C (1994). Expression of zebrafish goosecoid and no tail gene products in wild-type and mutant no tail embryos. Development 120, 843–852. 10.1242/dev.120.4.843. [DOI] [PubMed] [Google Scholar]
- 50.Akimenko MA, Johnson SL, Westerfield M, and Ekker M (1995). Differential induction of four msx homeobox genes during fin development and regeneration in zebrafish. Development 121, 347–357. 10.1242/dev.121.2.347. [DOI] [PubMed] [Google Scholar]
- 51.Akimenko MA, Ekker M, Wegner J, Lin W, and Westerfield M (1994). Combinatorial expression of three zebrafish genes related to distal-less: part of a homeobox gene code for the head. J Neurosci 14, 3475–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angelo S, Lohr J, Lee KH, Ticho BS, Breitbart RE, Hill S, Yost HJ, and Srivastava D (2000). Conservation of sequence and expression of Xenopus and zebrafish dHAND during cardiac, branchial arch and lateral mesoderm development. Mech Dev 95, 231–237. 10.1016/s0925-4773(00)00334-8. [DOI] [PubMed] [Google Scholar]
- 53.Yan YL, Miller CT, Nissen RM, Singer A, Liu D, Kirn A, Draper B, Willoughby J, Morcos PA, Amsterdam A, et al. (2002). A zebrafish sox9 gene required for cartilage morphogenesis. Development 129, 5065–5079. 10.1242/dev.129.21.5065. [DOI] [PubMed] [Google Scholar]
- 54.Elliott KH, Millington G, and Brugmann SA (2018). A novel role for cilia-dependent sonic hedgehog signaling during submandibular gland development. Dev Dyn 247, 818–831. 10.1002/dvdy.24627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, et al. (2021). Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e3529. 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stuart T, Srivastava A, Madad S, Lareau CA, and Satija R (2021). Single-cell chromatin state analysis with Signac. Nat Methods 18, 1333–1341. 10.1038/s41592-021-01282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schep AN, Wu B, Buenrostro JD, and Greenleaf WJ (2017). chromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat Methods 14, 975–978. 10.1038/nmeth.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma S, Zhang B, LaFave LM, Earl AS, Chiang Z, Hu Y, Ding J, Brack A, Kartha VK, Tay T, et al. (2020). Chromatin Potential Identified by Shared Single-Cell Profiling of RNA and Chromatin. Cell 183, 1103–1116.e1120. 10.1016/j.cell.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang d.W., Sherman BT, and Lempicki RA (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4, 44–57. 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 60.Sherman BT, Hao M, Qiu J, Jiao X, Baseler MW, Lane HC, Imamichi T, and Chang W (2022). DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 10.1093/nar/gkac194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Differentially expressed genes for control nr5a2:mGFP+ clusters, mutant nr5a2:mGFP+ clusters, combined control and mutant nr5a2:mGFP+ mesenchyme clusters, and nr5a2 mutant mandibular mesenchyme related to Figure 5A, 5D, S5
Table S2. Differential DNA-binding motifs in nr5a2 mutant mandibular mesenchyme, related to Figure 5C
Table S3. Differential chromatin accessibility in nr5a2 mutant mandibular mesenchyme, related to Figure 5D
Table S4. GO analysis of downregulated genes in nr5a2 mutant mandibular mesenchyme, related to Figure 5D
Table S5. Differentially accessible chromatin regions in nr5a2 mutant mandibular mesenchyme associated with differentially expressed genes and Nr5a2 motifs, related to Figure 5D, 6, S6D–F
Table S6. Oligonucleotide sequences, related to STAR Methods
Data Availability Statement
Single-cell RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. Microscopy data reported in this paper will be shared by the lead contact upon request.
No new code has been generated for this study.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rabbit-anti-phospho-histone H3 (Ser10) | Santa Cruz Biotechnology | sc-8656-R |
| chicken-anti-mCherry | Novus Biologicals | NBP2-25158 |
| chicken-anti-GFP | Abcam | ab13970 |
| mouse-anti-MF-20 | Developmental Studies Hybridoma Bank | MF 20 |
| rabbit-anti-Sox9 | Novus Biologicals | NBP1-85551 |
| Alexa Fluor 488 anti-chicken | ThermoFisher | A-11039 |
| Alexa Fluor 568 anti-chicken | ThermoFisher | A-11041 |
| Alexa Fluor 568 anti-rabbit | ThermoFisher | A11011 |
| Alexa Fluor 647 anti-rabbit | ThermoFisher | A-31573 |
| Alexa Fluor 647 anti-mouse | ThermoFisher | 21242 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Alexa Fluor 647 Phalloidin | ThermoFisher | A22287 |
| Critical Commercial Assays | ||
| RNAscope Multiplex Fluorescent Reagent Kit v2 Assay | Advanced Cell Diagnostics | Protocol 323100-USM and technical note MK 50-016 |
| Chromium Next GEM Single Cell Multiome ATAC + Gene Expression | 10X Genomics | Protocol CG000338 |
| Deposited Data | ||
| Raw sequencing fastq files and Cell Ranger ARC v2.0.0-processed Multiome data in this paper | GEO (Gene Expression Omnibus), this paper | GSE210251 |
| Published scRNAseq of submandibular salivary gland | GEO (Gene Expression Omnibus) (Hauser et al., 2020)28 | GSE150327 |
| Published scRNA-Seq of mouse mandible | FaceBase (Yuan et al., 2020)26 | Record ID 1-DTK2 |
| Published ATAC-Seq of zebrafish NCC | GEO (Gene Expression Omnibus) (Trinh et al., 2017)34 | GSE89670 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Nr5a2-flox: Nr5a2tm1Sakl | The Jackson Laboratory | JAX 024054 |
| Mouse: Wnt1-Cre: Wnt1-Cre1 | (Danielian et al., 1998)45 | N/A |
| Mouse: Prrx1-Cre: Tg(Prrx1-Cre)1Cjt | The Jackson Laboratory | JAX 005584 |
| Mouse: Scx-GFP: Tg(Scx-GFP)1Stzr | (Pryce et al., 2007)46 | N/A |
| Zebrafish: nr5a2oz3: nr5a2oz3 | (Nissim et al., 2016)23 | oz3 |
| Zebrafish: sox10:dsRed:Tg(sox10:DsRedExpress) | Our lab | el10 |
| Zebrafish: fli1a:GAL4:Tg(fli1:GAL4-VP16,myl7:EGFP) | Our lab | el360 |
| Zebrafish: sox10:mGFP:Tg(sox10:EGFP-CAAX) | Our lab | el375 |
| Zebrafish: col2a1a:GFP:TgBAC(col2a1a:EGFP) | Our lab | el483 |
| Zebrafish: col2a1a:mCherry:TgBAC(col2a1a:mCherry-NTR) | Our lab | el599 |
| Zebrafish: col2a1a:h2az2a-mCherry-2A-EGFP-CAAX:Tg(col2a1a:h2az2a-mCherry-2A-EGFP-CAAX) | Our lab | el690 |
| Zebrafish: scxa:mCherry:Tg(scxa:mCherry) | (Chen et al., 2020)43 | fb301 |
| Zebrafish: actb2:LOXP-BFP-LOXP-DsRed:Tg(actb2:LOXP-BFP-LOXP-DsRed) | (Kobayashi et al., 2014) 44 | sd27 |
| Zebrafish: nr5a2mGFP :Tg(nr5a2:GFP-CAAX) | This paper | el874 |
| Zebrafish: nr5a2mGFP-DBD-del:Tg(nr5a2:GFP-CAAX-DBD-del) | This paper | el875 |
| Zebrafish: UAS-nr5a2:Tg(UAS-nr5a2,cryaa:Cerulean) | This paper | el877 |
| Zebrafish: foxp2-p1:GFP:Tg(foxp2_p1-Mmu.E1b:GFP, cryaa:Cerulean) | This paper | el887,el888,el889 |
| Zebrafish: cdh6-p1:GFP:Tg(cdh6_p1-Mmu.E1b:GFP, cryaa:Cerulean) | This paper | el895,el896,el897 |
| Zebrafish: fgf10a-p1:GFP:Tg(fgf10a_p1-Mmu.E1b:GFP, cryaa:Cerulean) | This paper | el890,el891,el892 |
| Zebrafish: scxa-p2:GFP:Tg(scxa_p2-Mmu.E1b:GFP, cryaa:Cerulean) | This paper | el900,el901 |
| Oligonucleotides | ||
| RNAscope probe: cdh6 | Advanced Cell Diagnostics | 1225191-C4 |
| RNAscope probe: fgf10a | Advanced Cell Diagnostics | 1090101-C1, 1090101-C4 |
| RNAscope probe: foxp2 | Advanced Cell Diagnostics | 1181381-C1 |
| RNAscope probe: nr5a2 | Advanced Cell Diagnostics | 578901-C2 |
| RNAscope probe: ogna | Advanced Cell Diagnostics | 848241-C4 |
| RNAscope probe: sox9a | Advanced Cell Diagnostics | 543491-C3 |
| RNAscope probe: Nr5a2 | Advanced Cell Diagnostics | 543571 |
| RNAscope probe: Sox9 | Advanced Cell Diagnostics | 401051-C3 |
| guideRNA targeting sequences | Table S6 | N/A |
| In situ hybridization probes | Table S6 | N/A |
| Zebrafish transgenesis primers | Table S6 | N/A |
| Genotyping primers | Table S6 | N/A |
| Software and Algorithms | ||
| Cell Ranger ARC v2.0.0 | 10X Genomics | https://support.10xgenomics.com/single-cell-multiome-atac-gex/software/pipelines/latest/what-is-cell-ranger-arc |
| Seurat v4 | (Hao et al., 2021)55 | https://satijalab.org/seurat/ |
| Signac v1.6 | (Stuart et al., 2021)56 | https://satijalab.org/signac/ |
| chromVAR | (Schep et al., 2017)57 | https://greenleaflab.github.io/chromVAR/ |
| LinkPeaks | (Ma et al., 2020)58 | N/A |
| DAVID Bioinformatics Resources | (Huang et al., 2009)59; (Sherman et al., 2022)60 | https://david.ncifcrf.gov/ |
| Fiji | (Schindelin et al., 2012)61 | https://fiji.sc |


