Summary
Neutrophils or polymorphonuclear neutrophils (PMNs) are an important component of innate host defense. These phagocytic leukocytes are recruited to infected tissues and kill invading microbes. There are several general characteristics of neutrophils that make them highly effective as antimicrobial cells. First, there is tremendous daily production and turnover of granulocytes in healthy adults—typically 1011 per day. The vast majority (~95%) of these cells are neutrophils. In addition, neutrophils are mobilized rapidly in response to chemotactic factors and are among the first leukocytes recruited to infected tissues. Most notably, neutrophils contain and/or produce an abundance of antimicrobial molecules. Many of these antimicrobial molecules are toxic to host cells and can destroy host tissues. Thus, neutrophil activation and turnover are highly regulated processes. To that end, aged neutrophils undergo apoptosis constitutively, a process that contains antimicrobial function and proinflammatory capacity. Importantly, apoptosis facilitates nonphlogistic turnover of neutrophils and removal by macrophages. This homeostatic process is altered by interaction with microbes and their products, as well as host proinflammatory molecules. Microbial pathogens can delay neutrophil apoptosis, accelerate apoptosis following phagocytosis, or cause neutrophil cytolysis. Here, we review these processes and provide perspective on recent studies that have potential to impact this paradigm.
Keywords: Bacteria, cytolysis, neutrophil turnover, phagocytosis, apoptosis, granulopoiesis
1 ∣. INTRODUCTION
1.1. ∣. Neutrophil development and lifespan
1.1.1 ∣. Development
Neutrophils are the most abundant white blood cell in mammals and play an essential role in host defense against the multitude of pathogens encountered throughout life.1 On the other hand, neutrophils have a very short lifespan in circulation, so they must be replaced in large numbers on a daily basis (~1011/day) in order to perform their host defense surveillance function. Neutrophils are produced in the bone marrow by a process known as granulopoiesis, and ~55-60% of the bone marrow is dedicated to the production of this one cell type.2 Granulopoesis involves a series of stages originating from common myeloid progenitor cells (CMPs) and is principally regulated by granulocyte colony stimulating factor (G-CSF).3-5 CMPs differentiate into granulocyte-macrophage progenitors (GMPs) and megakaryocyte/erythrocyte progenitors (MEPs), which in turn give rise to mature myeloid cells and platelets or red blood cells. Stimulation of GMPs with granulocyte macrophage colony-stimulating factor (GM-CSF) leads to the production of monoblasts and myeloblasts, which are unipotent stem cells. Myeloblasts differentiate into promyelocytes, which then are committed to form neutrophils. Promyelocytes are similar in shape to myeloblasts and have a round nucleus; however, they contain many cytoplasmic azurophil granules that contain myeloperoxidase. In addition, promyelocytes have microperoxisomes, which contain catalase and suggests that even at this early stage in development neutrophils express protective antioxidant enzymes.6 These cells are classified as blast cells and can still actively divide. Promyelocytes give rise to myelocytes, which are smaller than promyelocytes and have oval nuclei that are localized eccentrically to one side of the cell. Myelocytes reside in the bone marrow and are characterized by the appearance of secondary or specific granules containing lactoferrin. Myelocytes are also considered blast cells and continue to divide and contribute to the neutrophil mitotic pool. Myelocyte mitosis dilutes the azurophil granule contents in each daughter cell, and specific granules eventually become more numerous than the azurophil granules. Precursors at the next stage are known as metamyelocytes and are no longer able to undergo mitosis—they can only develop into mature neutrophils. Metamyelocytes have kidney bean-shaped nuclei and contain ~67% specific granules and 33% azurophil granules. These cells are much smaller than myelocytes and eventually develop into band cells, which are the penultimate immature neutrophil form. Band cells are smaller than metamyelocytes and contain eccentric curved nuclei that are not lobular and have the same distribution of azurophil and specific granules as metamyelocytes, as well as a third granule type known as gelatinase granules. Band cells can now enter the bloodstream, and approximately 2-6% of cells in circulation are bands cells, although their circulating numbers can increase significantly during severe infection or inflammatory events in a process known as bandemia. Interestingly, bandemia has been correlated with an increasing likelihood of bacteremia and in-hospital mortality.7,8 The final stage of neutrophil development is maturation into segmented cells or polymorphonuclear neutrophils (PMNs or neutrophils). PMNs are characterized by segmented nuclei connected by thin strands of chromatin. While PMN nuclei normally have 3-5 lobes, neutrophil hypersegmentation has been observed where 6 or more segments are present and is one of the earliest signs of megaloblastic anemia.9 On the other hand, PMN hypersegmentation can also be seen under other situations that are not as well understood.10,11 Throughout the course of granulopoiesis, additional changes in surface marker expression, cytoskeletal elements, and granule contents also occur. For example, N-formyl peptide receptors are not expressed on myeloblasts but gradually appear as these cells mature through later stages of development.12 Mature PMNs contain secretory vesicles in addition to the three granule types found in band cells. Secretory vesicles are regulated exocytic vesicles similar to granules but contain plasma proteins and membrane receptors, and it has been suggested that they are formed in mature PMNs by endocytosis.13 As expression of granule proteins changes during maturation from promyelocytes to band cells, the granules formed at each stage also seem to acquire different cargo, which may account for the different subsets of granules that are observed.14 Note that new stores of microbicidal components are not resynthesized in PMNs, and new granules do not form in mature cells, which is consistent with a terminally differentiated end cell that has a short lifespan. That said, circulating neutrophils retain some capacity for gene expression and protein synthesis, which is needed to maintain functional capacity for extended periods (hours) and facilitate apoptosis and turnover.15
Bone marrow contains a large reserve of PMNs, and their rate of release is a major determinant of the number of circulating neutrophils present. PMNs must migrate across the sinusoidal endothelium that separates the hematopoietic compartment from the circulation, and migration of PMNs, as well as egress of some band cells, appears to be regulated by the relative levels of activation or egress signals versus retention signals. For example, PMN CXCR2 activation promotes mobilization of PMNs into the circulation, whereas interaction of CXCL12 (a.k.a. stromal cell-derived factor 1, SDF-1) with PMN CXCR4 functions to retain band cells and PMNs in the bone marrow.16 Thus, the CXCR4 axis is considered to play a key role in PMN egress, and it has been proposed that CXCR4 upregulation on senescent PMNs leads to preferential homing of these cells back to the bone marrow where they are cleared from circulation by bone marrow stromal macrophages.17,18
The relatively short PMN lifespan explains the need for high steady-state production of these cells by the bone marrow. In healthy adults, neutrophil development time in the bone marrow prior to entering circulation (i.e., maturation from myeloblast to PMN) is estimated to be 5-8 days. Subsequently, PMNs circulate in the bloodstream for less than a day, with a circulating PMN half-life ranging from 6-19 hours, depending on how the analysis was performed.19 Circulating PMNs function as sentinels to detect sites of infection or tissue damage and are among the first-responder cells that are rapidly recruited to these sites, where they utilize an array of oxygen-dependent and oxygen-independent mechanisms to protect and defend the host.20,21 The circulating PMN surveillance function is aided by their ability to marginate and roll along the vessel walls 3, and it has been estimated that ~49% of the PMN circulating pool is freely flowing in the blood, whereas the remaining 51% is in the marginated pool, which can be readily mobilized into the bloodstream if necessary for recruitment to other sites in the body for host defense.22
1.1.2 ∣. Apoptosis and turnover
Since innate host responses are not specific to individual pathogens, host tissue can be damaged along with insulting microorganisms and, if chronic in nature, contribute to the pathogenesis associated with inflammatory diseases. Thus, PMNs are programmed for rapid cell death by constitutive or spontaneous apoptosis in an effort to prevent excessive inflammation and disease.23,24 This is the default fate of all neutrophils. Moreover, PMNs are programmed to aid in the resolution of inflammation and thus produce proresolving mediators relatively soon after they initiate the inflammatory response.25 Indeed, insufficient activation of proresolving mechanisms contributes to an excessive or prolonged inflammatory response to the initial insult.26-28 These observations support the concept that the innate immune system has developed to not only protect the host from infection or damaging insult, but also to confine this response and resolve it as soon as possible and thereby avoid collateral host tissue damage.26-28
The circulating pool of PMNs is freely able to move through tissues in their role as sentinels, and it has been estimated that PMNs migrating into tissues under homeostatic conditions may reside there for 2-3 days before they undergo apoptosis and are removed by the reticuloendothelial system.29 Although apoptosis and clearance of apoptotic cells was proposed by Kerr et al., the removal process for neutrophils was characterized by Newman et al., who demonstrated that aged or senescent neutrophils are ingested by inflammatory macrophages.30,31 Subsequent studies by Savill et al. reported that aged human neutrophils undergo apoptosis within 24 h in culture and are then phagocytosed by macrophages.23 These two landmark studies served as the springboard for many lines of investigation over the next three decades that have resulted in our current understanding this essential homeostatic process. Healthy cells have receptors that signal “don’t eat me”.32 By comparison, phagocytosis of apoptotic neutrophils involves ligation of receptors that facilitate the nonphlogistic recognition and uptake by macrophages and other mononuclear phagocytes.32 This process is known as efferocytosis (Fig. 1).32 Consistent with the non-inflammatory removal process, neutrophil proinflammatory and antimicrobial capacities decrease concomitant with apoptosis.24 For example, Whyte and colleagues found that human PMNs undergoing apoptosis had reduced responses to receptor-mediated stimuli, and decreased capacity for chemotaxis, degranulation, and phagocytosis.24 Kobayashi et al. verified this decreased function and extended the findings to include decreased proinflammatory capacity at the level of the neutrophil transcriptome.33,34 These processes work in concert to promote safe removal of ~1011 effete neutrophils on a daily basis. For more detail on specific mechanisms of apoptosis and efferocytosis, we refer the reader to reviews on these topics.32,35 It is noteworthy that the non-inflammatory removal of effete neutrophils by apoptosis contrasts sharply with the proinflammatory outcomes that are caused by neutrophil cytolysis.
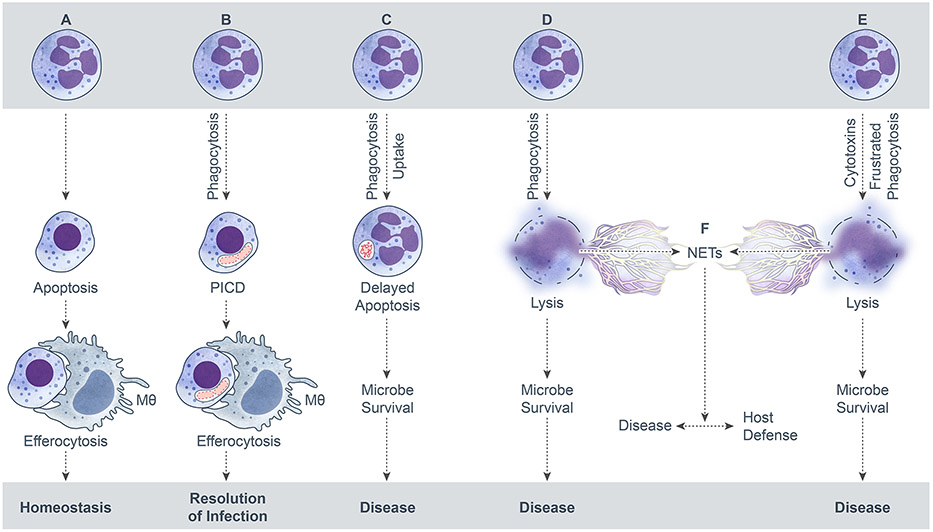
FIGURE 1.
Potential fates of neutrophils. (A) Apoptosis followed by efferocytosis. (B) PICD and efferocytosis. (C) Delayed neutrophil apoptosis and microbe survival. (D and E) Neutrophil cytolysis. (F). Formation of NETs by cytolysis. ©2017 Kobayashi, Malachowa, and DeLeo. Originally published in Front Cell Infect Microbiol. 2017; 7: 159. Published online 2017 May 1. doi:10.3389/fcimb.2017.00159.
In the absence of infection or injury, which results in the focused migration of PMNs to sites of infection or inflammation and an extended lifespan, PMNs exit the circulating/marginating pool and enter the liver, spleen, and bone marrow in approximately equal proportions where they undergo spontaneous apoptosis and are removed by macrophage efferocytosis (Fig. 1).36 Note that recognition and ingestion of apoptotic neutrophils by macrophages also induces macrophage G-CSF generation, which in turn stimulates granulopoiesis to replenish the pool of neutrophils.3,18 Thus, return of senescent PMNs to the bone marrow (and ultimate removal) is an essential process to maintain a robust pool of fresh PMNs and is facilitated by the upregulation of CXCR4 on these senescent cells that enhances homing to the bone marrow. Under homeostatic conditions, turnover rates from one body compartment to the next seem to be relatively stable. However, under acute inflammatory conditions or infections, PMNs are activated and rapidly mobilized from the blood compartment by chemoattractants, such as interleukin 8 (CXCL8), leukotriene B4 (LTB4), platelet-activating factor (PAF), and N-formyl peptide, which are generated at sites of infection/inflammation.1 This process may result in transient neutropenia that triggers an associated increase in release of PMNs and expansion of the circulating PMN pool or even bandemia in cases of severe infection to supplement the increased tissue demand until the inflammation/infection is controlled.7,8 The mechanisms controlling inflammation-associated circulating neutrophilia are not well understood; although acute mobilization of PMNs from the bone marrow seems to require the coordinated actions of G-CSF and CXC chemokines, with disruption of the CXCR4-SDF-1 retention mechanism and CXC chemokines stimulating neutrophil migration from the bone marrow.17,18,37 PMN priming is also associated with delayed apoptosis and enhanced lifespan, and there are a number of factors produced during the inflammatory response that can extend a PMN’s lifespan, including cytokines (e.g., G-CSF, GM-CSF), damage-associated molecular patterns (DAMPs), and pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS).38 Subsequent to the resolution of inflammation, which is facilitated by recruited PMNs themselves as well as the process of efferocytosis, activated neutrophils recruited to address the insult and clean up the associated tissue damage undergo apoptotic or necrotic cell death and are removed, whereas bone marrow PMN production returns to homeostasis.25,39,40
1.2 ∣. Neutrophil microbicidal mechanisms
Neutrophils utilize an extensive array of microbicidal mechanisms to destroy and remove infectious agents, and these mechanisms effectively utilize the numerous granule-packaged products developed during granulopoiesis. Neutrophil microbicidal mechanisms have been reviewed extensively (e.g., see refs.1,41,42) and are only briefly outlined here.
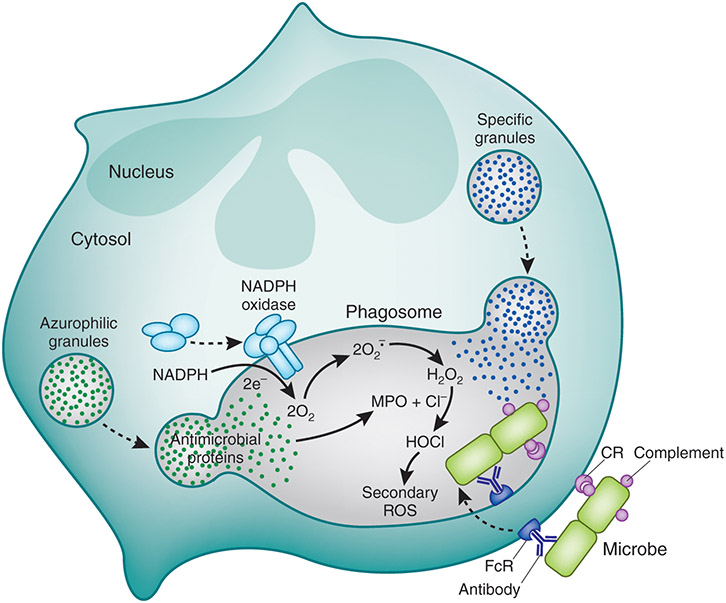
PMNs generate microbicidal reactive oxygen species (ROS) through the activation of a multi-protein enzyme complex known as the NADPH oxidase.43 The NADPH oxidase is expressed in neutrophils, as well as all other granulocytes, and plays an essential role in host defense against pathogenic microorganisms.43-45 It is generally accepted that the core NADPH oxidase enzyme complex is composed of five proteins (p22phox, p40phox, p67phox, and gp91phox) and a small GTPase (Rac1/2).43,46-49 Current nomenclature for the phagocyte NADPH oxidase proteins uses the suffix phox, which refers to phagocyte oxidase. The one exception is gp91phox, which is also known as NOX2. In resting cells, these proteins are segregated from each other in specific granule and secretory granule membranes, as well as cytosolic compartments, to protect the host from untimely oxidase activation and the production of toxic ROS.50-52 The neutrophil NADPH oxidase is activated in response to a wide range of stimuli, including bacteria, fungi, protozoan parasites, viruses, soluble proinflammatory molecules, and other foreign antigens. Depending on the stimulus, NADPH oxidase assembly and activation can occur at the plasma membrane (e.g., activation by soluble stimuli such as N-formyl peptides following priming) or in the phagosomal membrane (e.g., activation following phagocytosis of a pathogen or foreign antigen) (Fig. 2).53,54 Activation of the NADPH oxidase ultimately results in the formation of extracellular or intraphagosomal superoxide anion (O2•−), a process known as the respiratory burst. Although high concentrations of O2•− can accumulate in phagosomes where the pH is low and can be directly toxic to some pathogens by virtue of its ability to oxidize iron-sulfur clusters present in bacterial enzymes, it is generally thought that O2•− represents the precursor to more powerful ROS that play more prominent roles in pathogen killing.44 The initial production of O2•− is absolutely essential to human health, as demonstrated by chronic granulomatous disease (CGD), which is a rare immunodeficiency resulting from NADPH oxidase defects. Individuals with CGD are susceptible to severe, recurrent bacterial and fungal infections, as well as hyperinflammation.55-57 At physiologic pH, O2•− is rapidly converted to hydrogen peroxide (H2O2) through spontaneous or enzymatic dismutation by superoxide dismutase (SOD). SOD is abundant in PMNs and plays an essential role in protecting cells and tissues from excessive ROS, as do catalase and glutathione peroxidase. In addition to the production of ROS, PMN activation also results in the release of cytoplasmic granule contents into the phagosome, including high concentrations of MPO delivered from azurophil granules, which catalyzes the production of potent microbicidal ROS, such as hypochlorous acid (HOCl).44 Additional ROS and reactive nitrogen species (RNS) are also generated during the inflammatory response and are important in host defense, as well as in the pathogenesis of various inflammatory diseases.44,58
FIGURE 2.
Neutrophil activation during phagocytosis. ©2020 DeLeo and Nauseef. Originally published in Granulocytic Phagocytes. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th Edition, Eds. Bennett JE, Dolin R, and Blaser M. (2020) Elsevier Limited, Oxford, UK. pp. 83-98.
Although significantly diminished in capacity, CGD neutrophils are still able to kill a variety of microorganisms through the application of an array of oxygen-independent microbicidal antimicrobial peptides and enzymes that developed and were packaged into granules during granulopoiesis.4,55 In addition to their high levels of MPO, neutrophil azurophil granules contain four neutral proteinases (cathepsin G, elastase, NSP4, and proteinase 3), and to date all but NSP4 have demonstrated antimicrobial activity. Lysozyme is present in both the azurophil and specific granules and cleaves bacterial wall peptidoglycan and can participate with complement in bacterial permeabilization. Azurophil granules also contain several microbicidal cationic proteins, including bactericidal/permeability-increasing protein (BPI) and the α-defensins, which can permeabilize and kill a range of Gram-negative and Gram-positive bacteria, fungi, and enveloped viruses. Specific granules store unsaturated lactoferrin and lipocalin-2 to sequester iron and limit bacterial growth, transcobalamin II to sequesters cyanocobalamin from bacteria, lysozyme, collagenase, phospholipase A2, and hCAP-18, which is cleaved by elastase to generate bactericidal LL-37. Additionally, neutrophil specific granules contain a number of membrane proteins that are transported to the plasma or phagosomal membranes upon neutrophil activation. Altogether, it is clear that PMNs can bring to bear a powerful array of microbicidal mechanisms during an inflammatory response, yet these same mechanisms have the potential to cause significant host damage and must be contained or rapidly removed to maintain health of the host.42,52,59
2 ∣. MODULATION OF APOPTOSIS BY MICROORGANISMS
Neutrophil apoptosis is not an immutable process and can be delayed to promote enhanced clearance of microbial pathogens or induced/accelerated to facilitate turnover and resolution of infection and inflammation (Fig. 1). The ability of neutrophils to decipher and respond to environmental signals is paramount for their success as professional phagocytes. As PMNs are recruited to sites of infection, many of the signals they receive function as priming stimuli (reviewed in ref.60,61). Neutrophil priming agents—including many proinflammatory molecules— enhance microbicidal capacity and in general delay apoptosis. Although the delay of PMN apoptosis can be beneficial to the host by enabling increased bactericidal activity, the potential neutrophil accumulation comes with the risk of enhanced inflammatory capacity and damage to host tissue. Acute and chronic inflammatory disorders are often associated with excessive neutrophil accumulation. The infection site inflammatory milieu is complex, and neutrophils must decipher a multitude of signals from both host and bacteria-derived molecules that can be pro-survival or pro-death. In addition, bacterial pathogens can delay neutrophil apoptosis following uptake to facilitate intracellular survival or accelerate the process to evade PMN killing.
2.1 ∣. Inflammatory molecules that promote neutrophil survival
2.1.1 ∣. Cytokines, chemokines and proinflammatory molecules
Neutrophils receive a multitude of signals to indicate the presence of bacterial pathogens and to recruit them to sites of infection. Host immune cells in inflamed tissue produce proinflammatory mediators that generally operate as neutrophil priming agents and are known to delay PMN apoptosis. Begley et al. reported that neutrophil survival is enhanced by culture with GM-CSF and G-CSF.62 This observation was followed by studies showing that these proinflammatory cytokines, in addition to interleukin-1β (IL-1β), tumor necrosis factor (TNF), IL-6, interferon-γ (IFN-γ) and complement factor 5a (C5a), extend neutrophil survival by inhibiting programmed cell death / apoptosis.38,63 Indeed, it is now known that numerous proinflammatory molecules, including IL-8, IFN-α, GROα, PAF, fMLF, leptin, and LTB4 can delay neutrophil apoptosis.64-69 Interestingly, the effects of TNFα, an important PMN priming agent, are antiapoptotic at low concentrations (0.1 ng/mL) whereas high doses (10 ng/mL) result in induction of apoptosis.70 Neutrophil priming with TNF elicits multiple signaling pathways including p38, extracellular signal-regulated kinase (ERK) 1/2, phosphoinositide-3-kinase (PI3K) and nuclear factor- κB (NF-κB).71 High concentrations of TNFα trigger caspase-dependent acceleration of myeloid cell leukemia-1 (Mcl-1) turnover, whereas low concentrations stimulate expression of the antiapoptotic molecule Bfl-1.72
GM-CSF and other proinflammatory mediators delay neutrophil apoptosis largely by stabilizing Mcl-1, an anti-apoptotic member of the Bcl-2 family.73 Mcl-1 has a short half-life and is targeted rapidly by proteasomal degradation.74 Mcl-1 activity is sustained through activation of signaling pathways that involve PI3K/Akt and ERK.75 GM-CSF treatment results in enhanced expression of Mcl-1 in addition to the anti-apoptotic Bcl-2 family members Bcl-XL, and Bcl2A1.76 Of note, bronchiole alveolar lavage (BAL) neutrophils from patients with neutrophilic inflammatory lung disease demonstrate reduced intracellular levels of pro-apoptotic Bax and accompanying delayed neutrophil apoptosis compared to blood neutrophils from the same patients.77 Moreover, the BAL cells from patients released large amounts of G-CSF and GM-CSF compared to control individuals.
2.1.2 ∣. Host molecules and environmental factors at infection sites
Neutrophil trafficking from circulation to sites of infection involves signaling through a chemotactic gradient provided by proinflammatory cytokines and bacterial molecules. Interaction of neutrophil adhesins with cellular ligands can modulate apoptosis. For example, interaction of the neutrophil integrin α9β1 with endothelial cell molecule VCAM-1 delays both spontaneous and Fas-induced apoptosis.78 In addition, engagement of β2 integrins on the neutrophil surface delays spontaneous apoptosis and involves activation of survival pathways through Akt and MAPK-ERK.79,80 Neutrophil apoptosis is also delayed by ligation of soluble fibrinogen to CD11b by activation of NF-κB through ERK1/2-dependent signaling.81 Marginated neutrophils are exposed to a diversity of new stimuli in the inflammatory milieu at sites of infection, many of which delay apoptosis. Low oxygen tension is encountered in inflamed tissue and this hypoxic environment is well appreciated to delay neutrophil apoptosis and thereby extend survival.82,83 Neutrophil apoptosis is also delayed by mild extracellular acidosis.84 In addition, there are several reports that describe the anti-apoptotic effects of potentially neutrophil derived molecules such as heme, ATP, myeloperoxidase, and lactoferrin.85-88
2.2 ∣. Bacterial molecules that promote survival or extend the PMN lifespan
Neutrophils detect the presence of bacterial pathogens at least in part through ligation of surface receptors to microbial shed and secreted molecules. Several of these bacterial-derived components, such as LPS, lipoteichoic acid (LTA), peptidoglycan, CpG DNA and flagellin, have been reported to delay neutrophil apoptosis.89 The effects of LPS on neutrophil biology have been studied extensively, and endotoxin is classically described to be a potent neutrophil priming agent.90 LPS is recognized by a TLR4/MD2/CD14 complex, and LPS stimulation of neutrophils contributes to a delay in spontaneous apoptosis through a process involving NF-κB and PI3K activation.91-93 In addition, several bacterial toxins have been reported to delay neutrophil apoptosis, including S. epidermidis phenol soluble modulin (PSM), staphylococcal enterotoxins (A, B) and toxic shock syndrome toxin, E. coli O157:H7 verotoxin 2, E. coli Shiga toxin, and H. pylori water soluble surface protein.94-98
2.3 ∣. Influence of microorganisms on PMN fate
2.3.1 ∣. Bacteria that delay neutrophil apoptosis
The neutrophil raison d’être is to recognize and eliminate microbial pathogens by phagocytosis. The ability of inflammatory mediators and bacterial-derived products to enhance this process through extending neutrophil survival is critical to maximize host defense. On the other hand, the induction of neutrophil apoptosis following phagocytosis of microbes accounts for safe removal of effete PMNs through efferocytosis and is essential for resolution of infection and inflammation (Fig. 1). Although different types of microbial pathogens (e.g., bacteria, fungi, protozoa, and viruses) can influence PMN apoptosis directly or indirectly, for simplicity our discussion is focused primarily on bacterial pathogens.
The relatively short neutrophil half-life is not conducive to long-term survival strategies employed by many intracellular pathogens. Notwithstanding, a limited number of bacterial pathogens have been shown conclusively to delay neutrophil apoptosis as a mechanism of replication and survival. Anaplasma phagocytophilum, the etiologic agent of human granulocytic anaplasmosis, was the first bacterial pathogen reported to delay PMN apoptosis.99 A. phagocytophilum is an obligate intracellular bacterium and is uniquely tropic for granulocytes, where it multiplies strictly within membrane-bound inclusions.100,101 In addition to A. phagocytophilum, several Chlamydia spp., including C. pneumoniae, C. trachomatis, and C. psittaci, have been reported to delay PMN apoptosis.102-104 Mechanisms underlying the delay in neutrophil apoptosis caused by A. phagocytophilum and C. pneumoniae are similar, and include increased phosphorylation of p38 mitogen-activated protein kinase (MAPK), activation of PI3K/Akt, and maintained expression of the antiapoptotic protein Mcl-1.105,106 Coxiella burnetii—the causative agent of the zoonotic disease Q fever in humans—also delays neutrophil apoptosis through stabilization of Mcl-1.107 Likewise, the facultative intracellular pathogen F. tularensis delays neutrophil apoptosis in a process specifically dependent on the activity of NF-κB, PI3Kα, and p38 MAPK.108,109 Several other important bacterial pathogens, including Neisseria gonorrhoeae, Neisseria meningitidis, Klebsiella pneumoniae, Treponema pallidum, and Mycobacterium tuberculosis, have also been reported delay neutrophil apoptosis.110-116
Some of the bacterial pathogens that prolong neutrophil survival either inhibit NADPH oxidase assembly and/or activation or have mechanisms to evade ROS-mediated killing. For example, intracellular A. phagocytophilum elicit negligible production of PMN ROS, which is consistent with reports that the bacterium interferes with assembly and activation of the NADPH oxidase in the inclusion membrane.117-120 C. burnetii disrupts assembly of the NADPH oxidase and produces an acid phosphatase that prevents translocation of p47phox to the phagosome membrane.121,122 F. tularensis disrupts the NADPH oxidase by multiple mechanisms including inhibition of oxidase assembly on the phagosome and diminished phosphorylation of p47phox and other PKC substrates.123,124 Thus, inhibition of the NADPH oxidase is a mechanism used by bacterial pathogens to subvert neutrophil microbicidal activity and thereby facilitate intracellular survival. These findings are compatible with multiple lines of evidence that suggest ROS are important in promoting phagocytosis-induced cell death (PICD).89
2.3.2 ∣. Phagocytosis-induced cell death (PICD)
Although neutrophils possess significant microbicidal capacity and kill most ingested microbes, at some point this capacity can be spent fully, and effete neutrophils must be removed safely. To that end, neutrophils can undergo PICD, a form of apoptosis dependent on production of NADPH oxidase-derived ROS. Watson et al. discovered that neutrophils undergo rapid apoptosis following phagocytosis of Escherichia coli.125 Induction of apoptosis in these studies was dependent on bacteria-to-neutrophil ratios of at least 10:1, was dose-dependent, and the level of neutrophil ROS produced correlated with apoptosis. Notably, apoptotic neutrophils underwent necrotic lysis after 24 h, findings consistent with the default outcome for these terminally differentiated cells in a closed system.125 Studies published later that year by Coxon et al. found that phagocytosis-induced apoptosis, later termed neutrophil PICD, requires CD11b/CD18 and NADPH oxidase-derived ROS.126 Subsequent work by that research group revealed PICD involves caspases 3 and 8, and the process could be modulated (enhanced or suppressed) by TNF or GM-CSF.127 Gamberale et al. then showed that precipitating immune complexes and antibody coated erythrocytes also triggered apoptosis in an ROS-dependent manner, albeit phagocytosis was not required.128
To gain a more comprehensive view of the molecular processes that occur following phagocytosis, we used a microarray-based approach to identify human PMN genes that accompany antibody- and complement-receptor mediated phagocytosis.15 Unexpectedly, the studies revealed that genes involved in apoptosis and cell fate were differentially expressed several hours after phagocytosis and were accompanied by neutrophil PICD. This led to the hypothesis that PICD is regulated in part at the level of gene expression and facilitates resolution of the inflammatory response.15 Based on these findings, we proposed that changes in the PMN transcriptome accompanying PICD comprise an apoptosis-differentiation program, a final stage of differentiation that promotes safe turnover of effete neutrophils.129 This idea was then extended to phagocytosis of bacterial pathogens.130 Indeed, phagocytosis of diverse bacterial species induced a common remodeling of the neutrophil transcriptome (the apoptosis-differentiation program) and ultimately PICD.130 PICD was induced by pathogens that were phagocytosed readily and killed by neutrophils. These findings provide strong support to the idea that the apoptosis-differentiation program and PICD contributes to the resolution of bacterial infection (Fig. 1). However, it is important to note that some bacterial pathogens alter or circumvent this process. For example, the epidemic USA300 strain (S. aureus) causes rapid neutrophil cytolysis after phagocytosis (see Section 3).
2.4 ∣. Inflammatory disease and immune disorders
Although delayed neutrophil apoptosis facilitates initiation of host defense against bacterial infection, failure to remove accumulating cells contributes to the pathology of inflammatory disease.131 For example, bacterial sepsis and sequelae, such as acute respiratory distress syndrome (ARDS), are associated with a profound delay in neutrophil apoptosis.132-134 Although the mechanisms responsible for the delay remain to be fully elucidated, pro-inflammatory cytokines such as GM-CSF are purportedly involved in sepsis.135,136 In addition, the pro-survival factor Mcl-1 and regulators, such as myeloid nuclear differentiation antigen (MNDA) and PD-L1, contribute to delay of PMN apoptosis during sepsis.137-139 Delayed neutrophil apoptosis is also described as a feature of other lung diseases, including chronic obstructive pulmonary disease (COPD), severe asthma, cystic fibrosis, and bronchiectasis.140-142 Neutrophil apoptosis is also delayed in blood and synovial fluid in patients with rheumatoid arthritis (RA).143,144 Both blood and synovial fluid from RA patients generally contain elevated levels of pro-inflammatory cytokines such as GM-CSF.145 In addition, RA blood neutrophils have higher levels of Mcl-1 and the hypoxic synovial environment promotes Mcl-1 expression thus facilitating survival.143,146 In conjunction with delayed neutrophil apoptosis during inflammatory disease, dysregulated neutrophil clearance contributes to disease. For example, efferocytosis is impaired in ARDS and contributes to lung pathology confounded by accumulation of neutrophils.147
3 ∣. NEUTROPHIL LYSIS (CYTOLYSIS)
3.1 ∣. Neutrophils and host tissue damage
Each of the aforementioned processes are coordinated to an extraordinary degree to regulate neutrophil homeostasis and turnover. This regulation is especially important during infection and other disease conditions. These processes provide multiple and often redundant mechanisms to prevent damage to host tissues that can be caused by cytotoxic molecules released during neutrophil activation or cytolysis.
3.1.1 ∣. Fundamental concepts for neutrophil-mediated host tissue damage
The path from quiescent to fully activated neutrophils normally occurs as a graded or stepwise process, whereby chemotactic molecules such as IL-8 or bacterial products including LPS prime neutrophils for enhanced function or activation by subsequent stimuli. These proinflammatory molecules cause mobilization of secretory vesicles and a subset of specific granules but do not typically activate neutrophils to release primary granules (which contain MPO and elastase) and/or produce NADPH oxidase-derived ROS. In addition, the vast majority of priming agents, including IL-8 or LPS, delay neutrophil apoptosis and turnover (see text above).38,66 Vesicle exocytosis and partial degranulation that occur during priming enrich the plasma membrane with receptors needed to interface with the host during the inflammatory response and facilitates recognition and killing of microbes (reviewed in ref.4). In addition to secretory vesicle exocytosis, priming causes partial assembly of NADPH oxidase, but the enzyme complex is neither fully assembled nor activated. This priming phenomenon is important in part because it contributes to the regulation of neutrophil activation, thereby preventing tissue damage at sites distal from infection. Despite the numerous host regulatory processes that exist to facilitate healthy neutrophil turnover, neutrophils contribute significantly to—or are the underlying cause of—host tissue damage and inflammatory disorders.148 In general, this occurs because the sheer number of neutrophils produced and/or recruited during some disease states overwhelms the capacity for turnover and removal, and interaction with some microbial pathogens causes neutrophil lysis directly. To best appreciate how microbes play an integral part in these inflammatory diseases, we need to first provide some background on how neutrophils cause host tissue damage.
Studies conducted decades ago by Golub and Spitznagel demonstrated that neutrophil granules alone can cause host tissue damage in vivo, and that there is a direct correlation between amount of granule protein present and tissue lesion size.149 Neutrophil elastase, collagenase, and gelatinase are among the most well-studied granule proteases. They are important for normal neutrophil functions, such as antimicrobial activity and neutrophil migration in tissues, but they can contribute significantly to host tissue damage. These proteases, which breakdown molecules in host connective tissues, are released from activated neutrophils by granule exocytosis (degranulation) or by cytolysis. However, it is important to note that granules are directed largely to fuse with phagosomes rather than the plasma membrane following phagocytosis of microbes. This directed phagosome-granule fusion helps prevent release of cytotoxins onto host tissues.
In addition to utilizing antimicrobial peptides and granule proteases for host defense, neutrophils generate significant levels of reactive active species (ROS).150 The phagocyte NADPH-dependent oxidase produces O2−, which in turn is converted to other ROS that contribute to antimicrobial activity, and assembly and activation of the NADPH oxidase occurs concomitant with granule exocytosis. Neutrophil ROS, such as HOCl or hydroxyl radical, are indiscriminating and react readily with host tissues vs. target microbes.151,152 Such molecules have the potential to cause host tissue damage. Therefore, activation of the NADPH complex is highly regulated and protein components of NADPH oxidase must fully assemble before O2− can be produced.43 During phagocytosis, the oxidase is directed to assemble in the phagosomal membrane and generate ROS into the phagocytic vacuole such that the vast majority of ROS are contained within the neutrophil phagosome and not released into host tissues.
In addition to physical containment of ROS within phagosomes, neutrophils employ biochemical detoxification as a redundant failsafe mechanism.148 For example, neutrophil lactoferrin and MPO have been shown to limit rather than promote formation of hydroxyl radicals, albeit the inhibition is context-dependent.153-155 Neutrophils also employ redox antioxidant systems that include catalase, SOD, peroxiredoxin, glutathione, and thioredoxin metabolism.129,156-161 There are also multiple host protease inhibitors, including α1-antitrypsin (SERPINA1), leukocyte elastase inhibitor (present in neutrophil cytoplasm and also known as SERPINB1), and α2-macroglobulin, which inhibit neutrophil elastase. The importance of α1-antitrypsin in protection against host tissue injury by neutrophil elastase is exemplified by genetic deficiency of α1-antitrypsin, in which case affected individuals often have severe inflammatory diseases, such as pulmonary emphysema and adult respiratory distress syndrome.162,163 Outside of genetic deficiency, elastase-mediated tissue injury can occur during inflammatory responses in which protease inhibiters are overwhelmed by neutrophil elastase and/or α1-antitrypsin is inactivated by PMN oxidants.148 Studies by Weiss and colleagues demonstrated that activated neutrophils produce ROS in quantities sufficient to inactivate protease inhibitors in an area surrounding the cell.164 Thus, a strict balance between active and inactivated elastase is an important component of the inflammatory response.
Notwithstanding the mechanisms that dampen the tissue destructive potential of neutrophils, these host leukocytes are the primary contributors to inflammatory diseases, including those caused by infection. It is important to reiterate that neutrophils are terminally differentiated leukocytes and undergo cytolysis if they are not removed from tissues by other phagocytic cells. In the absence of such removal, neutrophil cytolysis is inevitable, regardless of the cell death pathway (e.g., secondary necrosis after apoptosis, programmed necrosis/necroptosis, non-specific lysis, etc.). Therefore, if the influx of neutrophils exceeds the capacity for nonphlogistic removal by macrophages, they will lyse in tissues, causing host damage and triggering further inflammation. This problem is borne out in numerous inflammatory diseases, and is intriguing that this long-known inflammatory process is now conflated widely with formation of neutrophil extracellular traps (NETs) and the accompanying specialized form of cytolytic neutrophil death termed “NETosis”.
3.1.2 ∣. Neutrophil extracellular traps (NETs), NETosis, and cytolysis
Brinkmann et al. reported that neutrophils stimulated with IL-8, LPS, or phorbol myristate acetate (PMA) produce extracellular traps.165 NETs in these studies were comprised of extracellular DNA, histones, and proteins from specific and azurophilic granules. In the original study, the authors demonstrated that NETs ensnare bacteria, including S. aureus, Shigella flexneri, and Salmonella typhimurium, and bacterial virulence molecules were degraded by the associated granule proteases.165 Brinkmann et al. also proposed the idea that NETs might moderate host tissue damage by containment of neutrophil cytotoxins.165 This initial report indicated NETs can form within 10 min of activation and in the absence of cell lysis (i.e., with viable neutrophils). However, subsequent work by the same research group showed that NETs form as the cell membrane ruptures.167 This cell death process was later termed “NETosis”, and at that time was reported as distinct from apoptosis and necrosis. As originally described by Fuchs et al., NETosis requires ROS.167 In contrast to the rapid formation of NETs (within 10 min) described in the first study by the same research group, S. aureus triggered formation of NETs only after an extended period of time in vitro (~120 min).167 Indeed, the authors found that within the first 60 min, neutrophils killed S. aureus following phagocytosis rather than forming NETs.167 Whether S. aureus-induced formation of NETs as reported by Fuchs et al. is distinct from lysis of human neutrophils that occurs following phagocytosis of S. aureus is discussed below. Although these two original reports by the same research group (Brinkmann et al. and Fuchs et al.) are seemingly discordant with regard formation of NETs from viable versus dead neutrophils, it is possible they report distinct phenomena. That is, NETs can form as an active process from viable neutrophils167 or they form as a result of neutrophil cytolysis, which can occur by multiple mechanisms. In keeping with the purpose of this article, which relates to the influence of microbes on neutrophil fate, our discussion here is limited to processes that involve neutrophil lysis. For a much more detailed view of live versus dead neutrophil NET formation, we refer the reader to recent articles by Yousefi et al. and Boltz et al.168,169
To gain an enhanced understanding of the stimuli that can trigger formation of NETs, Kenny et al. demonstrated that NETs form after neutrophil exposure to diverse stimuli.170 These stimuli activate distinct signaling pathways and each caused formation of NETs and concomitant cytolysis.170 There are also phenotypes associated with known cell death pathways that accompany or are the underlying basis of NET formation. For example, Desai et al. and Schreiber et al. reported programmed necrosis (necroptosis) leads to formation of NETs, whereas subsequent work by Amini et al. reported that NETs can form in the absence of RIPK3 and MLKL signaling (a hallmark of necroptosis).171-174 There is certainly no paucity of conditions and stimuli that have been linked to NET formation—and many of these reports are at variance with one another. For example, formation of NETs have been reported to be triggered by IL-8, LPS, PMA, calcium and potassium ionophores, bacteria, fungi, viruses, and protozoan parasites.170,175-177 ROS may or may not be required to trigger NETs, depending on the conditions or stimuli used. Interestingly, studies conducted many decades ago by Min-Fu Tsan demonstrated that neutrophil “autotoxicity” following activation with PMA requires ROS, a finding compatible with recent findings on PMA-induced cell death and NET formation.178 Studies that report the involvement of (or requirement for) MPO, elastase, and PAD4 for formation of NETs are also at variance.179-184 However, the unifying phenomenon for these studies is neutrophil cytolysis. Intriguingly, this is the default pathway for these terminally differentiated leukocytes. That is, in the absence of nonphlogistic removal, all neutrophils eventually undergo cytolysis. Indeed, the vast majority of studies report formation of NETs as occurring via neutrophil cytolysis.
Mechanisms of NET formation have been determined primarily by using in vitro assays with human neutrophils, and/or with mouse knock-out models. As with most in vitro approaches, it is difficult to reproduce conditions in humans, and this includes modeling inflammatory diseases in animal models. In addition, mouse disease models do not necessarily corroborate conditions in human disease. In the context of inflammatory diseases, this is in part due to differences between mouse and human neutrophils. Indeed, there are notable differences in NET formation in vitro between mouse and human neutrophils.185 Study of neutrophil processes, such as formation of NETs is feasible in vitro, but difficult or not possible in humans in vivo. Extracellular DNA, MPO, elastase, and citrullinated histones are commonly reported as markers of NETs in human tissues. However, the presence of these molecules in tissues does not indicate a specific process or the mechanism by which they were released. As stated above, MPO, elastase, and other granule constituents are exocytosed onto tissues by fully activated neutrophils. There can be multiple sources of extracellular DNA in inflamed tissues, including DNA released by lysed neutrophils. Thus, it is likely that a significant percentage of studies reporting NETs in inflammatory diseases are in fact reporting components of activated neutrophils and/or simply the remains of dead neutrophils. This notion is borne out in recent studies of severe COVID-19 cases.
Although neutrophils are known to contribute to severe respiratory diseases, the SARS-CoV-2 pandemic prompted much renewed interest in the specific role played by these host phagocytes. This is due largely to the observation that ICU patients with severe COVID-19 associated pneumonia have significantly increased neutrophil counts,186,187 which in turn led to the idea that neutrophilia observed in the patients with severe COVID-19 might contribute to formation of NETs.165,188 Indeed, subsequent studies demonstrated an association of COVID-19 severity and presence of NETs or at least components of NETs in the lungs.189,190 The confounding issue in this recent literature is the synonymous use of “NETosis” and formation of NETs. These are not synonymous terms, and we concur with Yousefi et al. in that such interchangeable use of the terms is inaccurate or misleading.169 Further, Boltz et al. and Galluzi et al. (and the Nomenclature Committee on Cell Death 2018) suggested the term “NETosis” should be avoided.168,191
3.2 ∣. Destruction of neutrophils by microbes
There are a relatively limited number of microbes known to kill neutrophils directly. However, if we consider formation of NETs by cytolysis, then by extension many varied microbial species influence killing of neutrophils. This topic is expansive, and a comprehensive discussion on each is beyond the scope of this review. Therefore, our discussion on this topic will focus solely on one model human pathogen that has well-known cytolytic capacity—the bacterium S. aureus.
3.2.1 ∣. S. aureus as a model human pathogen
S. aureus is among the most frequent causes of bloodstream, skin and soft tissue, and lower respiratory tract infections in much of the world.192,193 The microbe is carried asymptomatically in the nose by approximately 30% of healthy humans and is a notorious opportunistic pathogen. The high percentage human interaction is compounded by antibiotic resistance and most notably by methicillin-resistant S. aureus (MRSA). Healthcare-associated MRSA infections are typical of individuals with predisposing risk factors and treatment is often difficult. In contrast, community-associated MRSA (CA-MRSA) cause disease in otherwise healthy individuals.194 CA-MRSA emerged in the 1990s and then spread worldwide over the span of a decade. Although there has been a recent decrease in the number of hospital MRSA infections, the level of CA-MRSA infections, the vast majority of which are skin and soft tissue infections, has remained relatively constant.193 The molecular basis for the high virulence potential and success of CA-MRSA strains such as the USA300 epidemic strain is incompletely defined, although significant progress has been made. It has been well-documented that USA300 produces cytolytic toxins that can kill neutrophils and/or cause lysis of neutrophils after phagocytosis. We suggested previously that the enhanced ability of USA300 to circumvent killing by neutrophils, which includes neutrophil lysis, is linked to its success as a human pathogen.195
3.2.2 ∣. S. aureus cytotoxins and lysis of neutrophils
S. aureus is notorious for the ability to produce a large number of secreted virulence molecules. Many of these molecules have seemingly redundant functions, including pore-forming cytolytic toxins.196 Some of these cytolytic toxins such as α-hemolysin (α-toxin, Hla) and α-type phenol-soluble modulins (PSMα) kill a wide range of host cells, whereas others such as Panton-Valentine leukocidin (PVL) and leukocidin GH/AB (LukGH/LukAB) primarily target myeloid cells.196,197 Indeed, PVL and LukGH/AB are best known for their ability to cause neutrophil cytolysis. This host cell specificity is dictated by the expression of toxin surface receptors on the host cell.196 Differential expression of host cell receptors among animal species contributes to the observed differences in cytolytic capacity toward neutrophils from different animal species in vitro.
PVL has been associated historically with a subset of skin and soft tissue infections, such as furuncles, or with severe necrotizing pneumonia following influenza A virus infection.198-200 More recently, Shallcross et al. conducted a meta-analysis and found an association of PVL with skin and soft tissue infections (SSTIs) in general, but not with severe S. aureus infections (including pneumonia).201 The results of this study brought into question the idea that PVL is associated primarily with invasive S. aureus infections that have poor clinical outcomes.201 Consistent with these findings, Bae et al. analyzed data from a multinational phase three clinical trial and found presence of genes encoding PVL were not associated with clinical outcome in patients with complicated SSTIs.202 Rather, patients infected with PVL-positive S. aureus strains had significantly better clinical outcomes.202 Subsequent work by this group found that presence of PVL genes is not associated with clinical outcome in patients with hospital-acquired pneumonia caused by S. aureus.203
Although the relative contribution of PVL—if any—to S. aureus disease in humans remains incompletely determined, there is no question the toxin has the capacity to cause human neutrophil cytolysis in vitro.204 At sublytic concentrations (~1 nM), PVL primes human neutrophils for enhanced function, presumably because of the interaction with neutrophil C5aR1.205-207 At concentrations greater than 2 nM, purified PVL causes lysis of human neutrophils in vitro.206 Pilsczek et al. originally reported the ability of PVL (~10 nM) to cause formation of NETs and more recently Mazzoleni et al. described formation of NETs by “PVL NETosis”, a cytolytic process distinct from the original description of NETosis and not necessarily dependent on formation of membrane pores.208,209 This interesting finding merits further investigation. Given the capacity of PVL to cause lysis of neutrophils in vitro, one might predict this characteristic should translate to virulence in vivo. However, the translational component has been difficult to model from an animal infection standpoint, as S. aureus produces multiple virulence molecules, and PVL has tropism for human cells. Diep et al. used a rabbit model of severe USA300 respiratory tract infection to show that PVL-mediated lysis of neutrophils was the underlying cause of tissue destruction in the rabbit lung.210 This end-stage model of necrotizing pneumonia utilized a high bacterial inoculum (~1010 colony forming units) to demonstrate the potential of PVL to lyse neutrophils in vivo and in turn cause severe tissue damage.210 Interestingly, PVL-mediated neutrophil lysis data in vitro and tissue destruction in the rabbit severe pneumonia model do not corroborate the data with human clinical outcomes as described above. In the end, PVL is simply one of many S. aureus virulence molecules that have potential to contribute to human disease.
The contribution of LukGH/AB to human disease remains unknown, but patients with invasive S. aureus infections have elevated serum levels of anti-LukAB antibodies.211,212 The leukocidin elicits inflammation following administration in rabbit or monkey skin, but fails to contribute to virulence in a rabbit model of USA300 SSTI.213 Recently, LukAB was used as a component of a S. aureus toxoid vaccine that showed protection against primary and secondary infections.214 In vitro, LukGH/AB has cytolytic capacity toward human neutrophils that is similar to that of PVL.213 In addition, LukGH can cause formation of NETs by neutrophil cytolysis.215 Like PVL, LukGH/AB is a bi-component pore-forming toxin, but its target receptor is CD11b and thus distinct from that of PVL.216 Nonetheless, an attribute common to LukGH and PVL is formation of NETs by cytolysis. Importantly, Malachowa et al. demonstrated that non-specific pore formation caused by electropermeabilization of neutrophils led to formation of NETs that were identical to those caused by LukGH (i.e., comprised of DNA, MPO, and histones).215 This observation brings into question the now long-held idea that a unique or specific form of cell death is required for formation of NETs.
The S. aureus PSMα peptides (PSMs) are amphipathic peptides produced at relatively high levels in strains with high-virulence phenotypes, such as CA-MRSA strains.217 These peptide toxins are well characterized for their ability to cause neutrophil lysis by non-specific disruption of the plasma membrane.217 However, at sublytic concentrations they bind formyl-peptide receptor 2 (FPR2) on the surface of neutrophils. FPR2 binding in turn elicits changes in human neutrophil gene expression that are mediated by early growth response protein 1 (EGR1).218 PSMs prime neutrophils at low concentrations and promote neutrophil chemotaxis in vivo in mouse infection models.219 Studies in multiple animal infection models demonstrate that PSMs contribute significantly to CA-MRSA virulence.217,219,220 More recently, PSM production has been associated significantly with human skin and soft tissue infections.221 Inasmuch as PSMs cause neutrophil cytolysis, it is perhaps not unexpected that they cause rapid formation of NETs.222 Björnsdottir et al. reported that PSM-mediated NET formation did not require active cell signaling (e.g., NADPH oxidase-derived ROS, MPO, or elastase). Rather, the authors suggested NETs were the result of a “passive outcome of membrane disturbances” caused by PSMs.222
3.2.3 ∣. Lysis of neutrophils after phagocytosis of S. aureus
Despite the capacity of S. aureus to produce dozens of molecules that can potentially dampen the innate immune response, most S. aureus are ingested and killed by neutrophils. Indeed, neutrophils are known widely as the primary cellular defense against S. aureus infections. However, some S. aureus strains, such as the epidemic USA300 strain, have increased ability to circumvent killing by neutrophils and can cause leukocyte lysis after phagocytosis.195,223 This cytolytic phenomenon has the potential to influence virulence and strain success as a human pathogen.
Rogers and Tompsett demonstrated decades ago that coagulase-negative staphylococci (then S. albus; now S. epidermidis) and coagulase-positive staphylococci (S. aureus) are ingested rapidly by human PMNs in the presence of serum.224 They found that PMN phagocytosis and killing of S. epidermidis and S. aureus were comparable at early time points (~30 min).224 Subsequent work by Rogers and Melly reported similar ingestion within 60 min.225 Despite similar overall uptake by PMNs, there were significant differences in the fate of these microbes at later time points.224 Whereas S. epidermidis was killed and degraded by PMNs after phagocytosis, some of the ingested S. aureus survived within PMNs and caused leukocyte cytolysis within 3 or 4 h.224 Subsequent work in a rabbit bacteremia model by Rogers demonstrated that S. aureus is sequestered rapidly by circulating PMNs, which harbor viable organisms that contribute to persistence in the host.226,227 Studies conducted by Gresham et al. nearly 50 years later corroborated these findings with mouse neutrophils.228 Mouse PMNs containing viable S. aureus had the ability to establish infection in naive animals.228 Although PMN cytolysis was not evaluated, it is inferred as the means by which S. aureus escapes to cause infection. Therefore, this cytolytic process is potentially important for S. aureus pathogenesis and persistence in humans.
In studies published a number of years ago (c. 2005), we found that the epidemic USA300 CA-MRSA strain (named LAC in those studies) was ingested readily by human PMNs.195 However, ~30-50% of these organisms survived, and PMNs containing them underwent cytolysis within 4 or 5 h.195 These findings were compatible with the earlier studies by Rogers and colleagues. At the time, we hypothesized that the enhanced ability of USA300 to kill neutrophils after phagocytosis underlies the enhanced virulence phenotype this strain—i.e., the ability to cause infections in otherwise healthy individuals. We then characterized cellular and molecular features of neutrophils undergoing lysis after phagocytosis of USA300.223 At early time points after S. aureus uptake, the cellular phenotype is similar to that of neutrophils undergoing apoptosis, although lysis is neither delayed nor blocked by caspase inhibitors. The phenotype included membrane blebbing within 3 h and nuclear rounding and condensation by 4 h. However, the nucleus ultimately expands to fill the cytoplasm concomitant with rupture of the cell membrane.223 By microscopy analysis, the process bears striking resemblance to that of neutrophils forming NETs, as reported by Fuchs et al.166 We also determined that PMN lysis after phagocytosis is not dependent on a functioning NADPH oxidase, as cytolysis is virtually identical in neutrophils from patients with X-linked CGD.223 Transcriptome analyses were largely unrevealing to identify a specific mechanism, but neutrophil cytolysis was inhibited by actinomycin D or puromycin.223 The possible effect of these chemical inhibitors on S. aureus function was not examined at that time.
Further insights into the mechanism have been reported more recently by Greenlee-Wacker and colleagues. First, the authors discovered that neutrophils containing S. aureus are diverted from normal phagocytosis-induced apoptosis, and macrophage efferocytosis is decreased significantly.229 In addition, the authors demonstrated PMN lysis is in part inhibited by necrostatin-1, a RIPK-1 inhibitor often used to block necroptosis.229 Although these data provided support to the idea that neutrophils containing USA300 undergo programmed necrosis (necroptosis), a subsequent study by Greenlee-Wacker et al. indicated this form of neutrophil cytolysis requires RIPK-3 rather than RIPK-1.230 In any case, these findings are consistent with the ability of S. aureus to disrupt neutrophil homeostasis and promote disease.
3.2.3.2 ∣. Contribution of S. aureus molecules to PMN lysis after phagocytosis
Lysis of neutrophils after phagocytosis requires live S. aureus, and molecules produced by S. aureus contribute to the process.223 Multiple experimental approaches have demonstrated neutrophil lysis can be triggered by internalized S. aureus as opposed to cytolytic toxins secreted in the culture media. Production of cytolytic toxins within the phagosome is possible, and ligand binding regions of PVL and LukGH/AB receptors are accessible. However, these same receptors on the plasma membrane are not accessible from the cytoplasm (incorrect topology). Consistent with this notion, USA300 isogenic mutant strains lacking genes encoding PVL retain full capacity to cause lysis after phagocytosis.231 In contrast, S. aureus isogenic mutant strains lacking genes encoding LukGH/AB, PSMs or Hla have significantly reduced ability to cause neutrophil lysis after phagocytosis.232-236 At present, it is not clear how these cytolytic toxins contribute to cytolysis caused by ingested S. aureus. Recent studies by Rungelrath et al. demonstrated that USA300 is retained in intact phagosomes until the point of PMN lysis.236 Therefore, this cytolytic process does not requires escape of S. aureus from the phagosome. It is possible that these S. aureus toxins elicit cell death signals from within the phagosome, but more work is needed to resolve a specific contribution of S. aureus molecules to lysis after phagocytosis.
3.2.3.2 ∣. Why is neutrophil lysis after phagocytosis of S. aureus important?
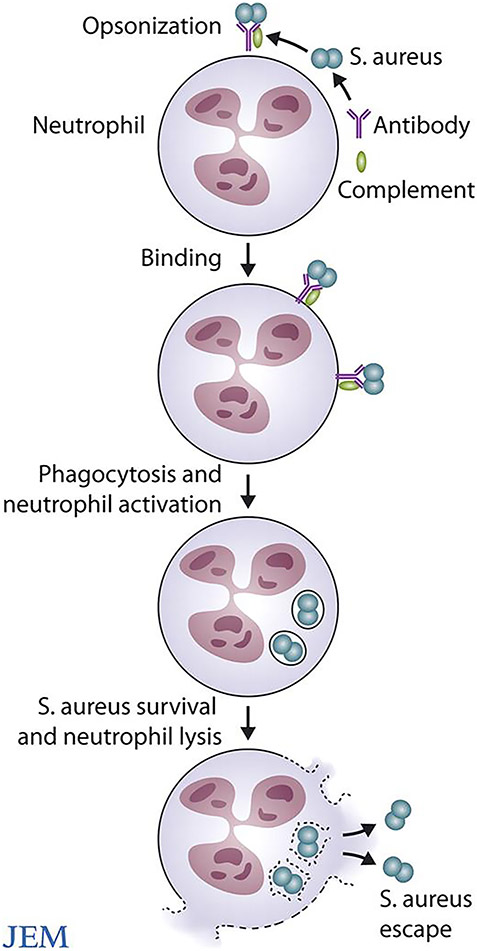
The landmark study by Rogers and Tompsett was one of the first reports of PMN lysis after phagocytosis of S. aureus. The implications of the Rogers and Tompsett study were seemingly underappreciated at the time they were published. Based on those studies and work by Jensen, Rogers stated, “attempts to control the problem of staphylococcal disease in man by evoking or reinforcing specific humoral immunity is theoretically unsound and offers little hope of success”.237,238 With regard to S. aureus opsonophagocytic vaccine approaches, the statement remains accurate. Human clinical trials that have been directed to promote S. aureus phagocytosis have failed or not met endpoint criteria. They have failed for two basic reasons: 1) phagocytosis of S. aureus is rapid and efficient in healthy individuals and 2) ingested S. aureus are not killed completely, and as a result some PMNs ultimately undergo cytolysis (Fig. 3). This is a major problem for a vaccine approach directed to enhance phagocytosis. In addition, lysis of neutrophils by ingested S. aureus likely contributes to (or is an underlying cause of) enhanced virulence phenotypes, persistent infections, and severe disease. Boosting the killing capacity of neutrophils toward ingested S. aureus would be ideal, but not practical or possible. Another solution is to moderate the severity of S. aureus infections by targeting virulence molecules. This vaccine approach has worked relatively well in animal infection models, and human clinical trials are in progress.
FIGURE 3.
Neutrophil cytolysis following phagocytosis of antibody and/or complement opsonized S. aureus. ©2008 DeLeo and Otto. Originally published in J Exp Med. 2008;205(2):271-274. doi:10.1084/jem.20080167.
4 ∣. CONCLUDING REMARKS
Neutrophil production and turnover are regulated to maintain immune system homeostasis and human health. Most bacteria are eliminated by neutrophils. However, pathogens such as S. aureus have the ability to alter normal neutrophil turnover, which in turn can have a negative influence on human health. Based on our current understanding of neutrophil biology and function, several questions and points merit further consideration.
Formation of NETs as a mechanism of host defense. Neutrophils cause host tissue damage and play a prominent role in severe inflammatory diseases. These processes are typically associated with neutrophil activation, ROS production, and exocytosis and/or cytolysis. The number of processes used by the host to regulate neutrophil activation and prevent cytolysis is extraordinary. Does a mechanism of host defense that requires neutrophil cytolysis fit with these key tenets of neutrophil biology?
Phagocytosis versus NETs in host defense. This is in essence comparison of a high concentration of microbicides contained in the phagocytic vacuole versus a relatively low concentration of extracellular molecules that by diffusion alone are far less effective as microbicides. The later can also damage host tissues. There can be no question that phagocytosis remains the primary means by which neutrophils eradicate bacterial pathogens. This topic has been discussed recently by DeLeo and Allen.239
Neutrophils are terminally differentiated phagocytes and removed daily in large numbers by efferocytosis. In the absence of efferocytosis and/or nonphlogistic removal, neutrophil will ultimately undergo cytolysis. This attribute might explain the ability of a wide range of stimuli and conditions to seemingly promote formation of NETs.
ACKNOWLEDGMENTS
FRD and SDK are supported by the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases (NIAID). MTQ is supported in part by National Institutes of Health IDeA Program Grant GM103474, USDA National Institute of Food and Agriculture, and the Montana State University Agricultural Experiment Station.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
Not applicable—no new data were generated.
REFERENCES
- 1.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15(7):602–611. [DOI] [PubMed] [Google Scholar]
- 2.Bainton DF. Developmental Biology of Neutrophils and Eosinophils. In: Gallin JI, Goldstein IM, Snyderman R, eds. Inflammation: Basic Principles and Clinical Correlates. 2nd ed. New York: Raven Press; 1992:303–324. [Google Scholar]
- 3.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31(8):318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol Rev. 2016;273(1):11–28. [DOI] [PubMed] [Google Scholar]
- 5.Bainton DF, Ullyot JL, Farquhar MG. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971;134(4):907–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breton-Gorius J, Coquin Y, Guichard J. Cytochemical distinction between azurophils and catalase-containing granules in leukocytes. I. Studies in developing neutrophils and monocytes from patients with myeloperoxidase deficiency: comparison with peroxidase-deficient chicken heterophils. Lab Invest. 1978;38(1):21–31. [PubMed] [Google Scholar]
- 7.Hsueh L, Molino J, Mermel L. Elevated bands as a predictor of bloodstream infection and in-hospital mortality. Am J Emerg Med. 2021;41:205–208. [DOI] [PubMed] [Google Scholar]
- 8.Harada T, Harada Y, Morinaga K, Hirosawa T, Shimizu T. Bandemia as an Early Predictive Marker of Bacteremia: A Retrospective Cohort Study. Int J Environ Res Public Health. 2022;19(4):2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmel R. The laboratory diagnosis of megaloblastic anemias. West J Med. 1978;128(4):294–304. [PMC free article] [PubMed] [Google Scholar]
- 10.Sipahi T, Tavil B, Unver Y. Neutrophil hypersegmentation in children with iron deficiency anemia. Pediatr Hematol Oncol. 2002;19(4):235–238. [DOI] [PubMed] [Google Scholar]
- 11.Whitmore LC, Weems MN, Allen LH. Cutting Edge: Helicobacter pylori induces nuclear hypersegmentation and subtype differentiation of human neutrophils in vitro. J Immunol. 2017;198(5):1793–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan R, Griffin JD, Malech HL. Acquisition of formyl peptide receptors during normal human myeloid differentiation. Blood. 1987;70(4):1222–1224. [PubMed] [Google Scholar]
- 13.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5(14):1317–1327. [DOI] [PubMed] [Google Scholar]
- 14.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89(10):3503–3521. [PubMed] [Google Scholar]
- 15.Kobayashi SD, Voyich JM, Buhl CL, Stahl RM, DeLeo FR. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc Natl Acad Sci USA. 2002;99(10):6901–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–1910. [DOI] [PubMed] [Google Scholar]
- 17.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19(4):583–593. [DOI] [PubMed] [Google Scholar]
- 18.Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 2008;22(9):3111–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahoz-Beneytez J, Elemans M, Zhang Y, et al. Human neutrophil kinetics: modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood. 2016;127(26):3431–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malech HL, Deleo FR, Quinn MT. The role of neutrophils in the immune system: an overview. Methods Mol Biol. 2014;1124:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Othman A, Sekheri M, Filep JG. Roles of neutrophil granule proteins in orchestrating inflammation and immunity. FEBS J. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Athens JW, Haab OP, Raab SO, et al. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest. 1961;40:989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83(3):865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whyte MK, Meagher LC, MacDermot J, Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. J Immunol. 1993;150(11):5124–5134. [PubMed] [Google Scholar]
- 25.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira-Costa KM, Menezes GB, Paula Neto HA. Neutrophil accumulation within tissues: A damage x healing dichotomy. Biomed Pharmacother. 2022;145:112422. [DOI] [PubMed] [Google Scholar]
- 27.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. [DOI] [PubMed] [Google Scholar]
- 28.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saverymuttu SH, Peters AM, Keshavarzian A, Reavy HJ, Lavender JP. The kinetics of 111Indium distribution following injection of 111Indium labelled autologous granulocytes in man. Br J Haematol. 1985;61(4):675–685. [DOI] [PubMed] [Google Scholar]
- 30.Newman SL, Henson JE, Henson PM. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982;156(2):430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henson PM. Cell removal: efferocytosis. Annu Rev Cell Dev Biol. 2017;33:127–144. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi SD, Voyich JM, Braughton KR, DeLeo FR. Down-regulation of proinflammatory capacity during apoptosis in human polymorphonuclear leukocytes. J Immunol. 2003;170(6):3357–3368. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi SD, Voyich JM, Whitney AR, DeLeo FR. Spontaneous neutrophil apoptosis and regulation of cell survival by granulocyte macrophage-colony stimulating factor. J Leukoc Biol. 2005;78(6):1408–1418. [DOI] [PubMed] [Google Scholar]
- 35.McCracken JM, Allen LA. Regulation of human neutrophil apoptosis and lifespan in health and disease. J Cell Death. 2014;7:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cartwright GE, Athens JW, Wintrobe MM. The kinetics of granulopoiesis in normal man. Blood. 1964;24:780–803. [PubMed] [Google Scholar]
- 37.Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80(8):2012–2020. [PubMed] [Google Scholar]
- 39.Shim HB, Deniset JF, Kubes P. Neutrophils in homeostasis and tissue repair. Int Immunol. 2022;34(8):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filep JG, Ariel A. Neutrophil heterogeneity and fate in inflamed tissues: implications for the resolution of inflammation. Am J Physiol Cell Physiol. 2020;319(3):C510–C532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehman HK, Segal BH. The role of neutrophils in host defense and disease. J Allergy Clin Immunol. 2020;145(6):1535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liew PX, Kubes P. The neutrophil's role during health and disease. Physiol Rev. 2019;99(2):1223–1248. [DOI] [PubMed] [Google Scholar]
- 43.Nauseef WM. The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling. Curr Opin Immunol. 2019;60:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winterbourn CC, Kettle AJ, Hampton MB. Reactive oxygen species and neutrophil function. Annu Rev Biochem. 2016. [DOI] [PubMed] [Google Scholar]
- 45.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with non-phagocyte oxidases. J Leukoc Biol. 2004;76:760–781. [DOI] [PubMed] [Google Scholar]
- 47.Pick E. Cell-Free NADPH oxidase activation assays: a triumph of reductionism. Methods Mol Biol. 2020;2087:325–411. [DOI] [PubMed] [Google Scholar]
- 48.Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009;11(10):2607–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buvelot H, Jaquet V, Krause KH. Mammalian NADPH oxidases. Methods Mol Biol. 2019;1982:17–36. [DOI] [PubMed] [Google Scholar]
- 50.Leto TL, Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal. 2006;8(9-10):1549–1561. [DOI] [PubMed] [Google Scholar]
- 51.Chen K, Craige SE, Keaney JF Jr. Downstream targets and intracellular compartmentalization in Nox signaling. Antioxid Redox Signal. 2009;11(10):2467–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casas AI, Dao VT, Daiber A, et al. Reactive oxygen-related diseases: therapeutic targets and emerging clinical indications. Antioxid Redox Signal. 2015;23(14):1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valenta H, Erard M, Dupré-Crochet S, Nüβe O. The NADPH oxidase and the phagosome. Adv Exp Med Biol. 2020;1246:153–177. [DOI] [PubMed] [Google Scholar]
- 54.Dahlgren C, Karlsson A, Bylund J. Intracellular neutrophil oxidants: from laboratory curiosity to clinical reality. J Immunol. 2019;202(11):3127–3134. [DOI] [PubMed] [Google Scholar]
- 55.Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38(1):3–10. [DOI] [PubMed] [Google Scholar]
- 56.Segal BH, Veys P, Malech H, Cowan MJ. Chronic granulomatous disease: lessons from a rare disorder. Biol Blood Marrow Transplant. 2011;17(1 Suppl):S123–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rieber N, Hector A, Kuijpers T, Roos D, Hartl D. Current concepts of hyperinflammation in chronic granulomatous disease. Clin Dev Immunol. 2012;2012:252460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quinn MT, Ammons MC, Deleo FR. The expanding role of NADPH oxidases in health and disease: no longer just agents of death and destruction. Clin Sci (Lond). 2006;111(1):1–20. [DOI] [PubMed] [Google Scholar]
- 59.Häger M, Cowland JB, Borregaard N. Neutrophil granules in health and disease. J Intern Med. 2010;268(1):25–34. [DOI] [PubMed] [Google Scholar]
- 60.Walker BA, Ward PA. Priming and signal transduction in neutrophils. Biol Signals. 1992;1(5):237–249. [DOI] [PubMed] [Google Scholar]
- 61.Swain SD, Rohn TT, Quinn MT. Neutrophil priming in host defense: role of oxidants as priming agents. Antioxid Redox Signal. 2002;4(1):69–83. [DOI] [PubMed] [Google Scholar]
- 62.Begley CG, Lopez AF, Nicola NA, et al. Purified colony-stimulating factors enhance the survival of human neutrophils and eosinophils in vitro: a rapid and sensitive microassay for colony-stimulating factors. Blood. 1986;68(1):162–166. [PubMed] [Google Scholar]
- 63.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54(4):283–288. [PubMed] [Google Scholar]
- 64.Bruno A, Conus S, Schmid I, Simon HU. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol. 2005;174(12):8090–8096. [DOI] [PubMed] [Google Scholar]
- 65.Dunican AL, Leuenroth SJ, Ayala A, Simms HH. CXC chemokine suppression of polymorphonuclear leukocytes apoptosis and preservation of function is oxidative stress independent. Shock. 2000;13(3):244–250. [DOI] [PubMed] [Google Scholar]
- 66.Kettritz R, Gaido ML, Haller H, Luft FC, Jennette CJ, Falk RJ. Interleukin-8 delays spontaneous and tumor necrosis factor-alpha-mediated apoptosis of human neutrophils. Kidney Int. 1998;53(1):84–91. [DOI] [PubMed] [Google Scholar]
- 67.Murray J, Barbara JA, Dunkley SA, et al. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood. 1997;90(7):2772–2783. [PubMed] [Google Scholar]
- 68.Petrin D, Turcotte S, Gilbert AK, Rola-Pleszczynski M, Stankova J. The anti-apoptotic effect of leukotriene B4 in neutrophils: a role for phosphatidylinositol 3-kinase, extracellular signal-regulated kinase and Mcl-1. Cell Signal. 2006;18(4):479–487. [DOI] [PubMed] [Google Scholar]
- 69.Sakamoto E, Hato F, Kato T, et al. Type I and type II interferons delay human neutrophil apoptosis via activation of STAT3 and up-regulation of cellular inhibitor of apoptosis 2. J Leukoc Biol. 2005;78(1):301–309. [DOI] [PubMed] [Google Scholar]
- 70.van den Berg JM, Weyer S, Weening JJ, Roos D, Kuijpers TW. Divergent effects of tumor necrosis factor alpha on apoptosis of human neutrophils. J Leukoc Biol. 2001;69(3):467–473. [PubMed] [Google Scholar]
- 71.Vogt KL, Summers C, Chilvers ER, Condliffe AM. Priming and de-priming of neutrophil responses in vitro and in vivo. Eur J Clin Invest. 2018;48 Suppl 2:e12967. [DOI] [PubMed] [Google Scholar]
- 72.Cross A, Moots RJ, Edwards SW. The dual effects of TNFalpha on neutrophil apoptosis are mediated via differential effects on expression of Mcl-1 and Bfl-1. Blood. 2008;111(2):878–884. [DOI] [PubMed] [Google Scholar]
- 73.Chao JR, Wang JM, Lee SF, et al. Mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol Cell Biol. 1998;18(8):4883–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hornstein T, Lehmann S, Philipp D, et al. Staurosporine resistance in inflammatory neutrophils is associated with the inhibition of caspase- and proteasome-mediated Mcl-1 degradation. J Leukoc Biol. 2016;99(1):163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Derouet M, Thomas L, Cross A, Moots RJ, Edwards SW. Granulocyte macrophage colony-stimulating factor signaling and proteasome inhibition delay neutrophil apoptosis by increasing the stability of Mcl-1. J Biol Chem. 2004;279(26):26915–26921. [DOI] [PubMed] [Google Scholar]
- 76.Cowburn AS, Summers C, Dunmore BJ, et al. Granulocyte/macrophage colony-stimulating factor causes a paradoxical increase in the BH3-only pro-apoptotic protein Bim in human neutrophils. Am J Respir Cell Mol Biol. 2011;44(6):879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dibbert B, Weber M, Nikolaizik WH, et al. Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis: a general mechanism to accumulate effector cells in inflammation. Proc Natl Acad Sci USA. 1999;96(23):13330–13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ross EA, Douglas MR, Wong SH, et al. Interaction between integrin alpha9beta1 and vascular cell adhesion molecule-1 (VCAM-1) inhibits neutrophil apoptosis. Blood. 2006;107(3):1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pluskota E, Soloviev DA, Szpak D, Weber C, Plow EF. Neutrophil apoptosis: selective regulation by different ligands of integrin alphaMbeta2. J Immunol. 2008;181(5):3609–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitlock BB, Gardai S, Fadok V, Bratton D, Henson PM. Differential roles for alpha(M)beta(2) integrin clustering or activation in the control of apoptosis via regulation of akt and ERK survival mechanisms. J Cell Biol. 2000;151(6):1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubel C, Gomez S, Fernandez GC, Isturiz MA, Caamano J, Palermo MS. Fibrinogen-CD11b/CD18 interaction activates the NF-kappa B pathway and delays apoptosis in human neutrophils. Eur J Immunol. 2003;33(5):1429–1438. [DOI] [PubMed] [Google Scholar]
- 82.Leuenroth SJ, Grutkoski PS, Ayala A, Simms HH. The loss of Mcl-1 expression in human polymorphonuclear leukocytes promotes apoptosis. J Leukoc Biol. 2000;68(1):158–166. [PubMed] [Google Scholar]
- 83.Walmsley SR, Print C, Farahi N, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201(1):105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El Kebir D, de Oliveira Lima Dos Santos E, Mansouri S, Sekheri M, Filep JG. Mild acidosis delays neutrophil apoptosis via multiple signaling pathways and acts in concert with inflammatory mediators. J Leukoc Biol. 2017;102(6):1389–1400. [DOI] [PubMed] [Google Scholar]
- 85.Arruda MA, Barcellos-de-Souza P, Sampaio AL, Rossi AG, Graca-Souza AV, Barja-Fidalgo C. NADPH oxidase-derived ROS: key modulators of heme-induced mitochondrial stability in human neutrophils. Exp Cell Res. 2006;312(19):3939–3948. [DOI] [PubMed] [Google Scholar]
- 86.El Kebir D, Jozsef L, Pan W, Filep JG. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ Res. 2008;103(4):352–359. [DOI] [PubMed] [Google Scholar]
- 87.Francis N, Wong SH, Hampson P, et al. Lactoferrin inhibits neutrophil apoptosis via blockade of proximal apoptotic signaling events. Biochim Biophys Acta. 2011;1813(10):1822–1826. [DOI] [PubMed] [Google Scholar]
- 88.Vaughan KR, Stokes L, Prince LR, et al. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J Immunol. 2007;179(12):8544–8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DeLeo FR. Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis. 2004;9(4):399–413. [DOI] [PubMed] [Google Scholar]
- 90.Guthrie LA, McPhail LC, Henson PM, Johnston RB Jr. Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984;160(6):1656–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Francois S, El Benna J, Dang PM, Pedruzzi E, Gougerot-Pocidalo MA, Elbim C. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol. 2005;174(6):3633–3642. [DOI] [PubMed] [Google Scholar]
- 92.Sabroe I, Dower SK, Whyte MK. The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis. Clin Infect Dis. 2005;41 Suppl 7:S421–426. [DOI] [PubMed] [Google Scholar]
- 93.Sabroe I, Prince LR, Jones EC, et al. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170(10):5268–5275. [DOI] [PubMed] [Google Scholar]
- 94.Brigotti M, Carnicelli D, Ravanelli E, et al. Interactions between Shiga toxins and human polymorphonuclear leukocytes. J Leukoc Biol. 2008;84(4):1019–1027. [DOI] [PubMed] [Google Scholar]
- 95.Kim JS, Kim JM, Jung HC, Song IS, Kim CY. Inhibition of apoptosis in human neutrophils by Helicobacter pylori water-soluble surface proteins. Scand J Gastroenterol. 2001;36(6):589–600. [DOI] [PubMed] [Google Scholar]
- 96.Liles WC, Thomsen AR, O'Mahony DS, Klebanoff SJ. Stimulation of human neutrophils and monocytes by staphylococcal phenol-soluble modulin. J Leukoc Biol. 2001;70(1):96–102. [PubMed] [Google Scholar]
- 97.Liu J, Akahoshi T, Sasahana T, et al. Inhibition of neutrophil apoptosis by verotoxin 2 derived from Escherichia coli O157:H7. Infect Immun. 1999;67(11):6203–6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moulding DA, Walter C, Hart CA, Edwards SW. Effects of staphylococcal enterotoxins on human neutrophil functions and apoptosis. Infect Immun. 1999;67(5):2312–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoshiie K, Kim HY, Mott J, Rikihisa Y. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect Immun. 2000;68(3):1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carlyon JA, Fikrig E. Mechanisms of evasion of neutrophil killing by Anaplasma phagocytophilum. Curr Opin Hematol. 2006;13(1):28–33. [DOI] [PubMed] [Google Scholar]
- 101.Rikihisa Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin Microbiol Rev. 2011;24(3):469–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frazer LC, O'Connell CM, Andrews CW Jr., Zurenski MA, Darville T Enhanced neutrophil longevity and recruitment contribute to the severity of oviduct pathology during Chlamydia muridarum infection. Infect Immun. 2011;79(10):4029–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He Z, Xiao J, Wang J, et al. The Chlamydia psittaci inclusion membrane protein 0556 inhibits human neutrophils apoptosis through PI3K/AKT and NF-kappaB signaling pathways. Front Immunol. 2021;12:694573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Zandbergen G, Gieffers J, Kothe H, et al. Chlamydia pneumoniae multiply in neutrophil granulocytes and delay their spontaneous apoptosis. J Immunol. 2004;172(3):1768–1776. [DOI] [PubMed] [Google Scholar]
- 105.Sarkar A, Hellberg L, Bhattacharyya A, et al. Infection with Anaplasma phagocytophilum activates the phosphatidylinositol 3-Kinase/Akt and NF-kappaB survival pathways in neutrophil granulocytes. Infect Immun. 2012;80(4):1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sarkar A, Moller S, Bhattacharyya A, et al. Mechanisms of apoptosis inhibition in Chlamydia pneumoniae-infected neutrophils. Int J Med Microbiol. 2015;305(6):493–500. [DOI] [PubMed] [Google Scholar]
- 107.Cherla R, Zhang Y, Ledbetter L, Zhang G. Coxiella burnetii Inhibits neutrophil apoptosis by exploiting survival pathways and antiapoptotic protein Mcl-1. Infect Immun. 2018;86(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kinkead LC, Krysa SJ, Allen LH. Neutrophil survival signaling during Francisella tularensis infection. Front Cell Infect Microbiol. 2022;12:889290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwartz JT, Barker JH, Kaufman J, Fayram DC, McCracken JM, Allen LA. Francisella tularensis inhibits the intrinsic and extrinsic pathways to delay constitutive apoptosis and prolong human neutrophil lifespan. J Immunol. 2012;188(7):3351–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe. 2012;11(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cho C, Teghanemt A, Apicella MA, Nauseef WM. Modulation of phagocytosis-induced cell death of human neutrophils by Neisseria gonorrhoeae. J Leukoc Biol. 2020;108(5):1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee CH, Chuah SK, Tai WC, Chang CC, Chen FJ. Delay in human neutrophil constitutive apoptosis after infection with Klebsiella pneumoniae serotype K1. Front Cell Infect Microbiol. 2017;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Persson A, Blomgran-Julinder R, Eklund D, Lundstrom C, Stendahl O. Induction of apoptosis in human neutrophils by Mycobacterium tuberculosis is dependent on mature bacterial lipoproteins. Microb Pathog. 2009;47(3):143–150. [DOI] [PubMed] [Google Scholar]
- 114.Recher M, Malipiero U, Schaer DJ, et al. Inhibition of meningitis-associated neutrophil apoptosis by TNF-alpha depends on functional PI3-kinase in monocytes. J Leukoc Biol. 2013;93(2):259–266. [DOI] [PubMed] [Google Scholar]
- 115.Simons MP, Nauseef WM, Griffith TS, Apicella MA. Neisseria gonorrhoeae delays the onset of apoptosis in polymorphonuclear leukocytes. Cell Microbiol. 2006;8(11):1780–1790. [DOI] [PubMed] [Google Scholar]
- 116.Wang J, Lu S, Zheng K, et al. Treponema pallidum delays the apoptosis of human polymorphonuclear neutrophils through the intrinsic and extrinsic pathways. Mol Immunol. 2022;147:157–169. [DOI] [PubMed] [Google Scholar]
- 117.Borjesson DL, Kobayashi SD, Whitney AR, Voyich JM, Argue CM, Deleo FR. Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J Immunol. 2005;174(10):6364–6372. [DOI] [PubMed] [Google Scholar]
- 118.JW IJ, Mueller AC. Neutrophil NADPH oxidase is reduced at the Anaplasma phagocytophilum phagosome. Infect Immun. 2004;72(9):5392–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mott J, Rikihisa Y. Human granulocytic ehrlichiosis agent inhibits superoxide anion generation by human neutrophils. Infect Immun. 2000;68(12):6697–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang T, Malawista SE, Pal U, et al. Superoxide anion production during Anaplasma phagocytophila infection. J Infect Dis. 2002;186(2):274–280. [DOI] [PubMed] [Google Scholar]
- 121.Hill J, Samuel JE. Coxiella burnetii acid phosphatase inhibits the release of reactive oxygen intermediates in polymorphonuclear leukocytes. Infect Immun. 2011;79(1):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Siemsen DW, Kirpotina LN, Jutila MA, Quinn MT. Inhibition of the human neutrophil NADPH oxidase by Coxiella burnetii. Microbes Infect. 2009;11(6-7):671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McCaffrey RL, Allen LA. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J Leukoc Biol. 2006;80(6):1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McCaffrey RL, Schwartz JT, Lindemann SR, et al. Multiple mechanisms of NADPH oxidase inhibition by type A and type B Francisella tularensis. J Leukoc Biol. 2010;88(4):791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Watson RW, Redmond HP, Wang JH, Condron C, Bouchier-Hayes D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol. 1996;156(10):3986–3992. [PubMed] [Google Scholar]
- 126.Coxon A, Rieu P, Barkalow FJ, et al. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5(6):653–666. [DOI] [PubMed] [Google Scholar]
- 127.Zhang B, Hirahashi J, Cullere X, Mayadas TN. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J Biol Chem. 2003;278(31):28443–28454. [DOI] [PubMed] [Google Scholar]
- 128.Gamberale R, Giordano M, Trevani AS, Andonegui G, Geffner JR. Modulation of human neutrophil apoptosis by immune complexes. J Immunol. 1998;161(7):3666–3674. [PubMed] [Google Scholar]
- 129.Kobayashi SD, Voyich JM, Somerville GA, et al. An apoptosis-differentiation program in human polymorphonuclear leukocytes facilitates resolution of inflammation. J Leukoc Biol. 2003;73(2):315–322. [DOI] [PubMed] [Google Scholar]
- 130.Kobayashi SD, Braughton KR, Whitney AR, et al. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc Natl Acad Sci USA. 2003;100(19):10948–10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol. 2013;34(8):398–409. [DOI] [PubMed] [Google Scholar]
- 132.Ertel W, Keel M, Infanger M, Ungethum U, Steckholzer U, Trentz O. Circulating mediators in serum of injured patients with septic complications inhibit neutrophil apoptosis through up-regulation of protein-tyrosine phosphorylation. J Trauma. 1998;44(5):767–775; discussion 775-766. [DOI] [PubMed] [Google Scholar]
- 133.Taneja R, Parodo J, Jia SH, Kapus A, Rotstein OD, Marshall JC. Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential and reduced caspase-9 activity. Crit Care Med. 2004;32(7):1460–1469. [DOI] [PubMed] [Google Scholar]
- 134.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78(2):325–337. [DOI] [PubMed] [Google Scholar]
- 135.von Gunten S, Jakob SM, Geering B, Takala J, Simon HU. Different patterns of Siglec-9-mediated neutrophil death responses in septic shock. Shock. 2009;32(4):386–392. [DOI] [PubMed] [Google Scholar]
- 136.von Gunten S, Yousefi S, Seitz M, et al. Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood. 2005;106(4):1423–1431. [DOI] [PubMed] [Google Scholar]
- 137.Fotouhi-Ardakani N, Kebir DE, Pierre-Charles N, et al. Role for myeloid nuclear differentiation antigen in the regulation of neutrophil apoptosis during sepsis. Am J Respir Crit Care Med. 2010;182(3):341–350. [DOI] [PubMed] [Google Scholar]
- 138.Harter L, Mica L, Stocker R, Trentz O, Keel M. Mcl-1 correlates with reduced apoptosis in neutrophils from patients with sepsis. J Am Coll Surg. 2003;197(6):964–973. [DOI] [PubMed] [Google Scholar]
- 139.Wang JF, Wang YP, Xie J, et al. Upregulated PD-L1 delays human neutrophil apoptosis and promotes lung injury in an experimental mouse model of sepsis. Blood. 2021;138(9):806–810. [DOI] [PubMed] [Google Scholar]
- 140.Bedi P, Davidson DJ, McHugh BJ, Rossi AG, Hill AT. Blood neutrophils are reprogrammed in bronchiectasis. Am J Respir Crit Care Med. 2018;198(7):880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gray RD, Hardisty G, Regan KH, et al. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax. 2018;73(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Uddin M, Nong G, Ward J, et al. Prosurvival activity for airway neutrophils in severe asthma. Thorax. 2010;65(8):684–689. [DOI] [PubMed] [Google Scholar]
- 143.Wright HL, Chikura B, Bucknall RC, Moots RJ, Edwards SW. Changes in expression of membrane TNF, NF-{kappa}B activation and neutrophil apoptosis during active and resolved inflammation. Ann Rheum Dis. 2011;70(3):537–543. [DOI] [PubMed] [Google Scholar]
- 144.Wright HL, Lyon M, Chapman EA, Moots RJ, Edwards SW. Rheumatoid arthritis synovial fluid neutrophils drive inflammation through production of chemokines, reactive oxygen species, and neutrophil extracellular traps. Front Immunol. 2020;11:584116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wright HL, Bucknall RC, Moots RJ, Edwards SW. Analysis of SF and plasma cytokines provides insights into the mechanisms of inflammatory arthritis and may predict response to therapy. Rheumatology (Oxford). 2012;51(3):451–459. [DOI] [PubMed] [Google Scholar]
- 146.Cross A, Barnes T, Bucknall RC, Edwards SW, Moots RJ. Neutrophil apoptosis in rheumatoid arthritis is regulated by local oxygen tensions within joints. J Leukoc Biol. 2006;80(3):521–528. [DOI] [PubMed] [Google Scholar]
- 147.Mahida RY, Scott A, Parekh D, et al. Acute respiratory distress syndrome is associated with impaired alveolar macrophage efferocytosis. Eur Respir J. 2021;58(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320(6):365–376. [DOI] [PubMed] [Google Scholar]
- 149.Golub ES, Spitznagel JK. The role of lysosomes in hypersensitivity reactions: tissue damage by polymorphonuclear neutrophil lysosomes. J Immunol. 1965;95(6):1060–1066. [PubMed] [Google Scholar]
- 150.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281(52):39860–39869. [DOI] [PubMed] [Google Scholar]
- 151.Tauber AI, Babior BM. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977;60(2):374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Klebanoff SJ. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980;93(3):480–489. [DOI] [PubMed] [Google Scholar]
- 153.Britigan BE, Hassett DJ, Rosen GM, Hamill DR, Cohen MS. Neutrophil degranulation inhibits potential hydroxyl-radical formation. Relative impact of myeloperoxidase and lactoferrin release on hydroxyl-radical production by iron-supplemented neutrophils assessed by spin-trapping techniques. Biochem J. 1989;264(2):447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Winterbourn CC. Myeloperoxidase as an effective inhibitor of hydroxyl radical production. Implications for the oxidative reactions of neutrophils. J Clin Invest. 1986;78(2):545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Britigan BE, Rosen GM, Thompson BY, Chai Y, Cohen MS. Stimulated human neutrophils limit iron-catalyzed hydroxyl radical formation as detected by spin-trapping techniques. J Biol Chem. 1986;261(36):17026–17032. [PubMed] [Google Scholar]
- 156.de Souza LF, Pearson AG, Pace PE, et al. Peroxiredoxin expression and redox status in neutrophils and HL-60 cells. Free Radic Biol Med. 2019;135:227–234. [DOI] [PubMed] [Google Scholar]
- 157.Rister M, Baehner RL. The alteration of superoxide dismutase, catalase, glutathione peroxidase, and NAD(P)H cytochrome c reductase in guinea pig polymorphonuclear leukocytes and alveolar macrophages during hyperoxia. J Clin Invest. 1976;58(5):1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roos D, Weening RS, Wyss SR, Aebi HE. Protection of human neutrophils by endogenous catalase: studies with cells from catalase-deficient individuals. J Clin Invest. 1980;65(6):1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Voetman AA, Roos D. Endogenous catalase protects human blood phagocytes against oxidative damage by extracellularly generated hydrogen peroxide. Blood. 1980;56(5):846–852. [PubMed] [Google Scholar]
- 160.Roos D, Weening RS, Voetman AA, et al. Protection of phagocytic leukocytes by endogenous glutathione: studies in a family with glutathione reductase deficiency. Blood. 1979;53(5):851–866. [PubMed] [Google Scholar]
- 161.Iversen MB, Gottfredsen RH, Larsen UG, et al. Extracellular superoxide dismutase is present in secretory vesicles of human neutrophils and released upon stimulation. Free Radic Biol Med. 2016;97:478–488. [DOI] [PubMed] [Google Scholar]
- 162.Turino GM, Seniorrm, Garg BD, Keller S, Levi MM, Mandl I. Serum elastase inhibitor deficiency and alpha 1-antitrypsin deficiency in patients with obstructive emphysema. Science. 1969;165(3894):709–711. [DOI] [PubMed] [Google Scholar]
- 163.Talamo RC, Allen JD, Kahan MG, Auste KF. Hereditary alpha-1-antitrypsin deficiency. N Engl J Med. 1968;278(7):345–351. [DOI] [PubMed] [Google Scholar]
- 164.Weiss SJ, Regiani S. Neutrophils degrade subendothelial matrices in the presence of alpha-1-proteinase inhibitor. Cooperative use of lysosomal proteinases and oxygen metabolites. J Clin Invest. 1984;73(5):1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. [DOI] [PubMed] [Google Scholar]
- 166.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16(11):1438–1444. [DOI] [PubMed] [Google Scholar]
- 168.Boeltz S, Amini P, Anders HJ, et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019;26(3):395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yousefi S, Stojkov D, Germic N, et al. Untangling "NETosis" from NETs. Eur J Immunol. 2019;49(2):221–227. [DOI] [PubMed] [Google Scholar]
- 170.Kenny EF, Herzig A, Kruger R, et al. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schreiber A, Rousselle A, Becker JU, von Massenhausen A, Linkermann A, Kettritz R. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci USA. 2017;114(45):E9618–E9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Amini P, Stojkov D, Wang X, et al. NET formation can occur independently of RIPK3 and MLKL signaling. Eur J Immunol. 2016;46(1):178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Desai J, Foresto-Neto O, Honarpisheh M, et al. Particles of different sizes and shapes induce neutrophil necroptosis followed by the release of neutrophil extracellular trap-like chromatin. Sci Rep. 2017;7(1):15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Desai J, Kumar SV, Mulay SR, et al. PMA and crystal-induced neutrophil extracellular trap formation involves RIPK1-RIPK3-MLKL signaling. Eur J Immunol. 2016;46(1):223–229. [DOI] [PubMed] [Google Scholar]
- 175.Diaz-Godinez C, Carrero JC. The state of art of neutrophil extracellular traps in protozoan and helminthic infections. Biosci Rep. 2019;39(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8(4):668–676. [DOI] [PubMed] [Google Scholar]
- 177.Schonrich G, Raftery MJ. Neutrophil extracellular traps go viral. Front Immunol. 2016;7:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tsan MF. Phorbol myristate acetate induced neutrophil autotoxicity. J Cell Physiol. 1980;105(2):327–334. [DOI] [PubMed] [Google Scholar]
- 179.Metzler KD, Fuchs TA, Nauseef WM, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117(3):953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207(9):1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Martinod K, Witsch T, Farley K, Gallant M, Remold-O'Donnell E, Wagner DD. Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J Thromb Haemost. 2016;14(3):551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Neeli I, Radic M. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front Immunol. 2013;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kasperkiewicz P, Hempel A, Janiszewski T, et al. NETosis occurs independently of neutrophil serine proteases. J Biol Chem. 2020;295(51):17624–17631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun. 2009;1(3):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li S, Jiang L, Li X, et al. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020;5(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Radermecker C, Detrembleur N, Guiot J, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med. 2020;217(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Diekema DJ, Hsueh PR, Mendes RE, et al. The microbiology of bloodstream Infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 2019;63(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kourtis AP, Hatfield K, Baggs J, et al. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections - United States. MMWR Morb Mortal Wkly Rep. 2019;68(9):214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375(9725):1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Voyich JA, Braughton KR, Sturdevant DE, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175(6):3907–3919. [DOI] [PubMed] [Google Scholar]
- 196.Spaan AN, van Strijp JAG, Torres VJ. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol. 2017;15(7):435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dumont AL, Nygaard TK, Watkins RL, et al. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol. 2011;79(3):814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359(9308):753–759. [DOI] [PubMed] [Google Scholar]
- 199.Prevost G, Couppie P, Prevost P, et al. Epidemiological data on Staphylococcus aureus strains producing synergohymenotropic toxins. J Med Microbiol. 1995;42(4):237–245. [DOI] [PubMed] [Google Scholar]
- 200.Cribier B, Prevost G, Couppie P, Finck-Barbancon V, Grosshans E, Piemont Y. Staphylococcus aureus leukocidin: a new virulence factor in cutaneous infections? An epidemiological and experimental study. Dermatology. 1992;185(3):175–180. [DOI] [PubMed] [Google Scholar]
- 201.Shallcross LJ, Fragaszy E, Johnson AM, Hayward AC. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(1):43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bae IG, Tonthat GT, Stryjewski ME, et al. Presence of genes encoding the Panton-Valentine leukocidin exotoxin is not the primary determinant of outcome in patients with complicated skin and skin structure infections due to methicillin-resistant Staphylococcus aureus: results of a multinational trial. J Clin Microbiol. 2009;47(12):3952–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sharma-Kuinkel BK, Ahn SH, Rude TH, et al. Presence of genes encoding Panton-Valentine leukocidin is not the primary determinant of outcome in patients with hospital-acquired pneumonia due to Staphylococcus aureus. J Clin Microbiol. 2012;50(3):848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Woodin AM. Staphylococcal leukocidin. In: Montje TK, S. Ajl S, ed. Microbial Toxins. New York, NY, USA, and London, UK: Academic; 1970:327–355. [Google Scholar]
- 205.Zimmerli W, Reber AM, Dahinden CA. The role of formylpeptide receptors, C5a receptors, and cytosolic-free calcium in neutrophil priming. J Infect Dis. 1990;161(2):242–249. [DOI] [PubMed] [Google Scholar]
- 206.Graves SF, Kobayashi SD, Braughton KR, et al. Sublytic concentrations of Staphylococcus aureus Panton-Valentine leukocidin alter human PMN gene expression and enhance bactericidal capacity. J Leukoc Biol. 2012;92(2):361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Spaan AN, Henry T, van Rooijen WJM, et al. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe. 2013;13(5):584–594. [DOI] [PubMed] [Google Scholar]
- 208.Mazzoleni V, Zimmermann K, Smirnova A, Tarassov I, Prevost G. Staphylococcus aureus Panton-Valentine Leukocidin triggers an alternative NETosis process targeting mitochondria. FASEB J. 2021;35(2):e21167. [DOI] [PubMed] [Google Scholar]
- 209.Pilsczek FH, Salina D, Poon KK, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185(12):7413–7425. [DOI] [PubMed] [Google Scholar]
- 210.Diep BA, Chan L, Tattevin P, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci USA. 2010;107(12):5587–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sharma-Kuinkel BK, Tkaczyk C, Bonnell J, et al. Associations of pathogen-specific and host-specific characteristics with disease outcome in patients with Staphylococcus aureus bacteremic pneumonia. Clin Transl Immunology. 2019;8(7):e01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Thomsen IP, Dumont AL, James DB, et al. Children with invasive Staphylococcus aureus disease exhibit a potently neutralizing antibody response to the cytotoxin LukAB. Infect Immun. 2014;82(3):1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Malachowa N, Kobayashi SD, Braughton KR, et al. Staphylococcus aureus leukotoxin GH promotes inflammation. J Infect Dis. 2012;206(8):1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Karauzum H, Venkatasubramaniam A, Adhikari RP, et al. IBT-V02: A multicomponent toxoid vaccine protects against primary and secondary skin infections caused by Staphylococcus aureus. Front Immunol. 2021;12:624310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Malachowa N, Kobayashi SD, Freedman B, Dorward DW, DeLeo FR. Staphylococcus aureus leukotoxin GH promotes formation of neutrophil extracellular traps. J Immunol. 2013;191(12):6022–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.DuMont AL, Yoong P, Day CJ, et al. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci USA. 2013;110(26):10794–10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang R, Braughton KR, Kretschmer D, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13(12):1510–1514. [DOI] [PubMed] [Google Scholar]
- 218.Kretschmer D, Gleske AK, Rautenberg M, et al. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7(6):463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nguyen TH, Cheung GYC, Rigby KM, et al. Rapid pathogen-specific recruitment of immune effector cells in the skin by secreted toxins. Nat Microbiol. 2022;7(1):62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kobayashi SD, Malachowa N, Whitney AR, et al. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis. 2011;204(6):937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Berlon NR, Qi R, Sharma-Kuinkel BK, et al. Clinical MRSA isolates from skin and soft tissue infections show increased in vitro production of phenol soluble modulins. J Infect. 2015;71(4):447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bjornsdottir H, Dahlstrand Rudin A, Klose FP, et al. Phenol-soluble modulin alpha peptide toxins from aggressive Staphylococcus aureus induce rapid formation of neutrophil extracellular traps through a reactive oxygen species-independent pathway. Front Immunol. 2017;8:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kobayashi SD, Braughton KR, Palazzolo-Ballance AM, et al. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J Innate Immun. 2010;2(6):560–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rogers DE, Tompsett R. The survival of staphylococci within human leukocytes. J Exp Med. 1952;95(2):209–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Melly MA, Thomison JB, Rogers DE. Fate of staphylococci within human leukocytes. J Exp Med. 1960;112:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rogers DE. Studies on bacteriemia. I. Mechanisms relating to the persistence of bacteriemia in rabbits following the intravenous injection of staphylococci. J Exp Med. 1956;103(6):713–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rogers DE. The blood stream clearance of staphylococci in rabbits. Ann N Y Acad Sci. 1956;65(3):73–84. [DOI] [PubMed] [Google Scholar]
- 228.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164(7):3713–3722. [DOI] [PubMed] [Google Scholar]
- 229.Greenlee-Wacker MC, Rigby KM, Kobayashi SD, Porter AR, DeLeo FR, Nauseef WM. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J Immunol. 2014; 192(10):4709–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Greenlee-Wacker MC, Kremserová S, Nauseef WM. Lysis of human neutrophils by community-associated methicillin-resistant Staphylococcus aureus. Blood. 2017;129(24):3237–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Voyich JM, Otto M, Mathema B, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194(12):1761–1770. [DOI] [PubMed] [Google Scholar]
- 232.Pang YY, Schwartz J, Thoendel M, Ackermann LW, Horswill AR, Nauseef WM. agr-Dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J Innate Immun. 2010;2(6):546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Surewaard BG, de Haas CJ, Vervoort F, et al. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol. 2013;15(8):1427–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ventura CL, Malachowa N, Hammer CH, et al. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PloS One. 2010;5(7):e11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.DuMont AL, Yoong P, Surewaard BG, et al. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect Immun. 2013;81(5):1830–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rungelrath V, Porter AR, Malachowa N, et al. Further Insight into the mechanism of human PMN lysis following phagocytosis of Staphylococcus aureus. Microbiol Spectr. 2021;9(2):e0088821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jensen K. A normally occurring Staphylococcus antibody in human serum. APMIS. 2007;115(5):533–539; discussion 540-531. [DOI] [PubMed] [Google Scholar]
- 238.Rogers DE. Experimental observations on staphylococcal disease. Postepy Mikrobiologii. 1966;5:275–296. [Google Scholar]
- 239.DeLeo FR, Allen LH. Phagocytosis and neutrophil extracellular traps. Fac Rev. 2020;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable—no new data were generated.