Abstract
BACKGROUND AND PURPOSE:
Cigarette smoking is a known risk factor for cardiovascular disease, including ischemic stroke. The literature regarding the rate of persistent smoking after acute ischemic stroke and its effect on subsequent cardiovascular events is scarce. With this study we aimed to report the rate of persistent smoking after ischemic stroke and the association between smoking status and major cardiovascular outcomes.
METHODS:
This is a post-hoc analysis of the Secondary Prevention of Small Subcortical Strokes (SPS 3) trial. Patients were divided into four groups based on smoking status at trial enrollment: 1) never smokers, 2) former smokers, 3) smokers who quit at 3 months and 4) persistent smokers. The primary outcome is a major adverse cardiovascular events (MACE) composite of stroke (ischemic and hemorrhagic), myocardial infarction, and mortality. Outcomes were adjudicated after month 3 of enrollment until an outcome event or the end of study follow-up.
RESULTS
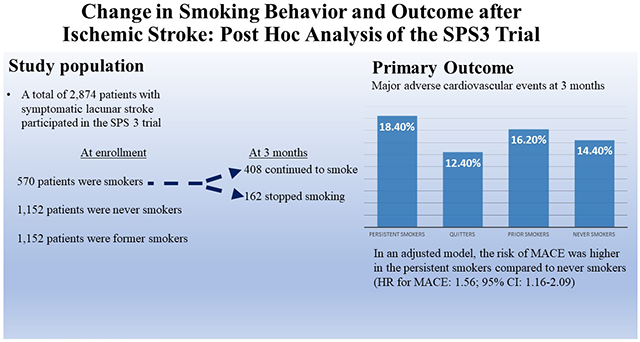
A total of 2,874 patients were included in the study. Of the total cohort, 570 patients (20%) were smokers at enrollment, of whom 408 (71.5%) patients continued to smoke and 162 (28.4%) quit smoking by 3 months. The MACE outcome occurred in 18.4%, 12.4%, 16.2% and 14.4%, respectively, in persistent smokers, smokers who quit, prior smokers, and never smokers. In a model adjusted for age, sex, race, ethnicity, education, employment status, history of hypertension, diabetes, hyperlipidemia, myocardial infarction, and intensive blood pressure randomization arm, the risk of MACE and death were higher in the persistent smokers compared to never smokers (HR for MACE: 1.56; 95% CI: 1.16-2.09; HR for death: 2.0; 95% CI, 2.18-3.12). The risk of stroke, and MI did not differ according to smoking status
CONCLUSION:
Compared to never smoking, persistent smoking after acute ischemic stroke was associated with an increased risk of cardiovascular events and death.
Clinical Trial Registration Information:
Keywords: stroke, smoking, death
Graphical Abstract
INTRODUCTION
Cigarette smoking is the leading contributor to death and disability in the US and globally,1 and is an established modifiable cardiovascular risk factor.1 Previous studies have consistently shown that smoking at the time of stroke presentation is associated with an increased risk of major cardiovascular events.2–7 A Meta-analysis of studies investigating the relationship between smoking and stroke reported an overall increased risk of stroke in current smokers compared to nonsmoker (OR, 1.61; P<0.001). 8 Notably, the risk of stroke in smokers is dependent on the duration and the intensity of smoking. The above mentioned meta-analysis found a 12% increased risk of stroke for each increment of 5 cigarettes per day. 8 Moreover, there is sound evidence from the cardiology literature suggesting an association between smoking behavior and risk of recurrent cardiovascular events. In a retrospective analysis of the Melbourne Interventional Group registry that included patients who suffered acute coronary syndrome, persistent smoking after acute coronary syndrome was associated with higher risk of major cardiovascular event compared to never smokers (Hazard Ratio 1.7, p<0.001). In contrast the results of major cardiovascular event in patients who quit smoking or who were former smokers was not significantly different than never smokers.9 The results were echoed in a retrospective analysis of the second manifestation or arterial disease (SMAR T) registry. 10 Despite the abundance of data regarding smoking and stroke risk, the data regarding smoking behavior after stroke including post-stroke smoking cessation and its association with future outcomes is scarce
The 2021 American Heart Association/American Stroke Association (AHA/ASA) secondary stroke prevention guidelines recommend that patients with stroke or transient ischemic attack (TIA) who continue to smoke should be advised to stop smoking (and, if unable, to reduce their daily smoking) to lower the risk of recurrent stroke (level of evidence B).11 The guidelines additionally noted the lack of studies that addressed the contribution of continued smoking to the risk of recurrent stroke and recommended development of a stroke registry to address this knowledge gap.11 A recently published systematic review and meta-analysis identified only two studies that assessed the effect of smoking cessation after ischemic stroke event on the risk of major cardiovascular events, one of which was an analysis of the Nanjing Stroke Registry Program in China, limiting generalizibility.12–14 In light of the paucity of evidence, we performed a post-hoc analysis of the Secondary Prevention of Small Subcortical Strokes (SPS 3)15 trial to assess the association of smoking status after a lacunar ischemic stroke on major adverse cardiovascular events (MACE) during follow-up.
METHODS
Cohort
This is a post-hoc analysis of the SPS3 trial. In brief, the SPS3 was a randomized, multicenter trial that was conducted in 82 centers in North America, Latin America and Spain and recruited patients from March, 2003, and April, 2011. The trial was completed in April 2012. The trial enrolled patients if 1) they were 30 years of age or older, 2) had a MRI confirmed symptomatic lacunar stroke within the preceding 180 days, and 3) did not have major risk factors for cardioembolic stroke or surgically amenable ipsilateral carotid artery disease. Patients underwent simultaneous randomization to the antiplatelet intervention (aspirin and clopidogrel versus aspirin alone) and to one of the two systolic blood pressure target levels (<130 mm Hg vs. 130 to 149 mm Hg). The trial protocol and final results have been previously reported.15–17 IRB approval was not required for this retrospective study of deidentified data per University of Utah Institutional Review Board Guidelines. The data for this study was obtained from the publicly available NINDS archive. This study reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.18
Exposure and Outcomes
For the purposes of this study, we set the exposure period as the baseline study visit to month 3 (for the primary analysis), and captured smoking status during that time period. For sensitivity analysis, we changed the exposure period to 6 months. Patients who had a primary outcome or were lost to follow-up during the exposure period were excluded. Ethnicity was self-reported and participants who identified themselves as Latino or Spanish were classified as Hispanic. Patients were divided into four exposure groups based on the ordinal smoking status from baseline to month 3: 1) never smokers, 2) former smokers (patients who quit smoking prior to qualifying event), 3) smokers who quit during the exposure period and after qualifying event, 4) smokers who continued to smoke (persistent smokers). Clinic visits were monthly during the first 3 months after enrollment in the trial and every 3 months thereafter. Smoking status was assessed at baseline, 3months, and then every 3 months thereafter. Outcomes were adjudicated after month 3 until an outcome event or the end of follow-up. The primary outcome is a major adverse cardiovascular events (MACE), which is a composite of all-cause death, stroke (ischemic and hemorrhagic), and myocardial infarction. Ischemic stroke was defined clinically as a focal neurologic deficit of sudden onset and persisting for more than 24 hours. Intracranial hemorrhage included epidural, subdural, subarachnoid and intracerebral hemorrhage. All reported outcomes were confirmed by a central adjudication committee that was unaware of the treatment assignments.
For the purposes of the primary analysis, patients who experienced any of the outcomes were censored at the time of event. For patients who never experienced any of the outcomes were right censored at the final time point of contact or at study completion. As secondary outcomes, we explored the stroke, myocardial infarction, and death as individual outcomes, in which case the first event of any determined the time of censoring.
Statistical Analysis:
We report basic demographics and outcomes after stratification by the smoking status exposure defined in this study, and tested for intergroup difference with the chi-squared test for binary variables and ANOVA/Student’s t-test for interval variables. We fit Cox models to our primary and secondary outcomes. Model adjustments are as such, Model 1: unadjusted; Model 2: adjusted for age, sex, race, and ethnicity; Model 3: adjusted for age, sex, race, ethnicity, education, employment status, history of hypertension, diabetes, hyperlipidemia, myocardial infarction, and intensive blood pressure randomization arm. These covariates were chosen based on their possible confounding effects. Antiplatelet treatment arm is not available in the publicly available SPS3 data; therefore, it was not included in the adjusted analyses. We tested the proportional hazards assumption of our Cox models to ensure that the p value of individual covariates was >0.05 and the mean value was >0.05. We also plotted the Cox Snell residuals against the cumulative hazard and visually verified the goodness of fit. In addition, we tested for interactions between the covariates in the fully adjusted model (model 3) and the exposure of smoking status. We considered P<0.1 as significant interaction. We adjusted for multiplicity using Hommel’s multiple comparison procedure. 19 All analysis was performed in Stata 17.1 (StataCorp, College Station, TX).
RESULTS
A total of 2,874 patients were included (Figure 1), of which 36.9% were female, the mean age was 62.9±10.8 years, 57.2% were white, and 15.5% (445/2871) had the primary outcome of MACE during a mean of 41.6±24.7 months of follow-up. Baseline characteristics of the four groups are depicted in Table 1. In the full cohort, 570 patients (20%) were smokers at randomization, of whom 408 (71.5%) continued to smoke and 162 (28.4%) quit smoking at 3 months. Amongst the 408 patients who continued to smoke, the average daily number of cigarettes (n=402) was 11.7±10.1. Compared to the other groups, persistent smokers were younger and more likely to be male (Table1). Compared to patients who quit smoking, patients who persisted were less likely to be Hispanic (59.9% vs. 30.6%; p<0.01), more likely to be employed (35.8% vs. 44.9%, p<0.049), and more likely to have a college education (Table 1). In addition, duration of smoking was longer in patients who continued to smoke (35.6 years vs. 32.1 years; p=0.004). Over the course of 18 months of follow-up the percentage of baseline smokers who quit increased from 28.4% at 3 months to 34.0% at 18 months (Table 2). Of the participants classified as those who quit smoking by 3 months, 14.2% had resumed smoking by 6 months and 20.4% had resumed smoking by 12 months. The primary outcome occurred in 18.4%, 12.4%, 16.2% and 14.2%, respectively, in persistent smokers, smokers who quit, prior smokers, and never smokers (Table 1). In a fully adjusted model, the risk of the primary outcome was higher in the persistent smokers compared to never smokers (HR: 1.56; 95% CI: 1.16-2.09 (Table 3). We also found that the patients who quit smoking by 3 months did not have a significantly different risk of the primary outcome compared to never smokers (HR 1.25, 95% CI 0.77-2.02). In a sensitivity analysis, we changed the exposure period to 6 months and found that the results were consistent with the model for a 3 month exposure period (HR for persistent smokers = 1.49, 95% CI 1.08-2.06). In a sensitivity analysis, we compared patients who quit smoking and persistent smokers to former smokers. Persistent smokers had higher risk of primary outcome compared to former smokers (adjusted hazard ratio, 1.81; 95% CI 1.32-2.48). In contrast, there was no difference in the risk of primary outcome between patients who quit and former smokers (adjusted hazard ratio, 1.38; 95% CI 0.82-2.30)
Figure 1.
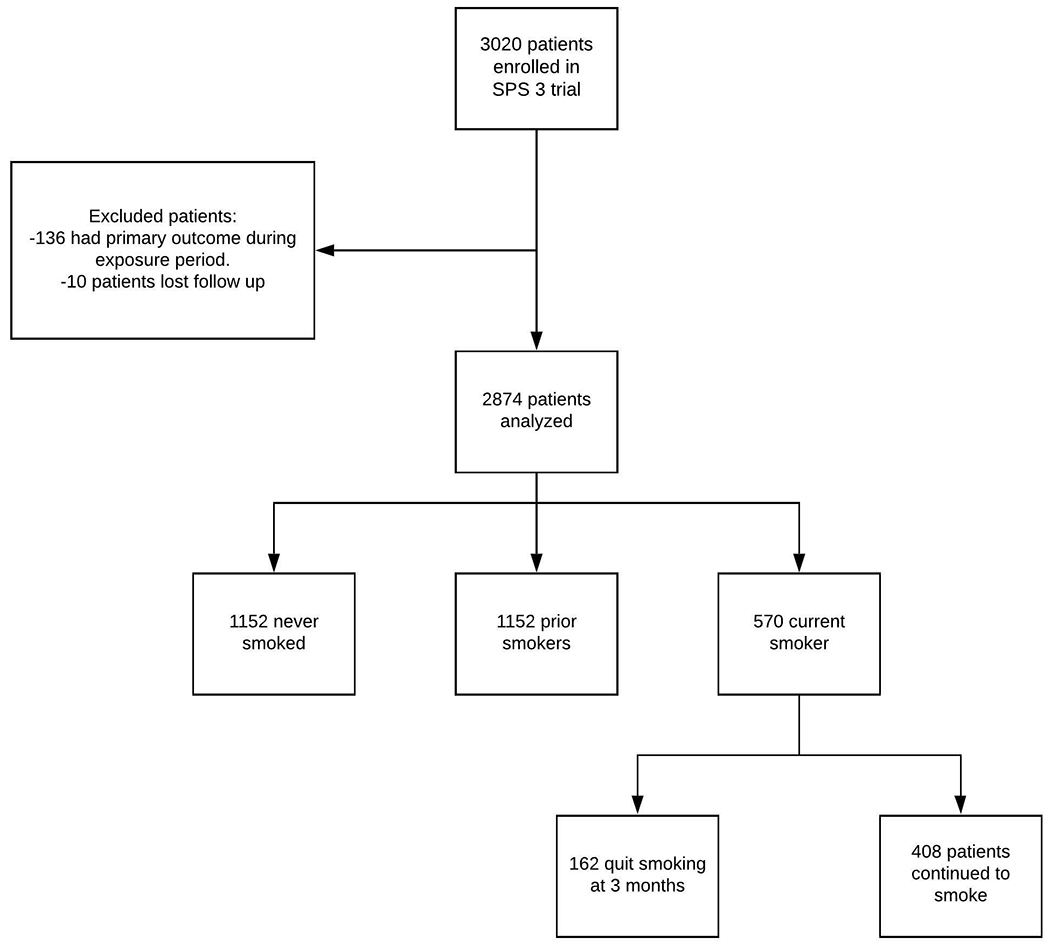
Flow chart
Table 1.
Baseline demographics and outcomes by smoking stratification.
| Variable* | Never smoker (n=1,152) |
Prior smoker (n=1,152) |
Smoker who quit by month 3 (n=162) |
Persistent smoker at month 3 (n=408) |
P value |
|---|---|---|---|---|---|
|
| |||||
| Age | 64.4±11.1 | 63.9±10.5 | 58.1±8.9 | 57.8±8.9 | <0.001 |
|
| |||||
| Female sex | 610 (53.0%) | 296 (25.7%) | 37 (22.8%) | 117 (28.7%) | <0.001 |
|
| |||||
| Race | <0.001 | ||||
| White | 596 (51.8%) | 683 (59.3%) | 104 (64.2%) | 262 (64.2%) | |
| Black | 150 (13.0%) | 165 (14.3%) | 35 (21.6%) | 106 (26.0%) | |
| Other | 406 (35.2%) | 304 (26.4%) | 23 (14.2%) | 40 (9.8%) | |
|
| |||||
| Hispanic ethnicity | 573 (49.7%) | 475 (41.2%) | 97 (59.9%) | 125 (30.6%) | <0.001 |
|
| |||||
| Education | <0.001 | ||||
| 0-4 years | 167 (14.5%) | 108 (9.4%) | 20 (12.3%) | 17 (4.2%) | |
| 5-8 years | 205 (17.8%) | 168 (14.6%) | 32 (19.8%) | 49 (12.0%) | |
| 9-12 years | 377 (32.7%) | 433 (37.6%) | 79 (48.8%) | 198 (48.5%) | |
| Any college | 403 (35.0%) | 443 (38.4%) | 31 (19.1%) | 144 (35.3%) | |
|
| |||||
| Employed | 649 (56.3%) | 637 (55.3%) | 58 (35.8%) | 183 (44.9%) | <0.001 |
|
| |||||
| Hypertension | 888 (77.1%) | 890 (77.3%) | 97 (60.0%) | 277 (67.9%) | <0.001 |
|
| |||||
| Diabetes | 419 (36.4%) | 390 (33.9%) | 41 (25.3%) | 104 (25.5%) | <0.001 |
|
| |||||
| Hyperlipidemia | 554 (48.1%) | 589 (51.1%) | 64 (39.5%) | 189 (46.3%) | 0.026 |
|
| |||||
| Prior myocardial infarction | 40 (3.5%) | 70 (6.1%) | 4 (2.5%) | 34 (8.3%) | <0.001 |
|
| |||||
| Years smoked | - | 24.0±14.8 | 32.1±14.9 | 35.6±12.1 | <0.001 |
|
| |||||
| Randomized to intensive blood pressure arm | 557 (48.4%) | 582 (50.5%) | 80 (49.4%) | 215 (52.7%) | 0.472 |
|
| |||||
| Systolic blood pressure during follow up (mean (SD)) | 134.7 (14.4) | 134.1 (14.1) | 133.4 (12.3) | 135.1 (14.9) | 0.403 |
|
| |||||
| Days between qualifying event and randomization | 75.5±48.3 | 78.3±46.7 | 70.0±45.5 | 75.5±46.3 | 0.147 |
|
| |||||
| Mean follow-up (months) | 41.7±24.4 | 42.7±24.4 | 38.8±23.5 | 39.4±26.5 | 0.052 |
|
| |||||
| Primary outcome | 164 (14.2%) | 186 (16.2%) | 20 (12.4%) | 75 (18.4%) | 0.133 |
|
| |||||
| Secondary outcomes | |||||
| Stroke | 92 (8.0%) | 97 (8.4%) | 13 (8.0%) | 37 (9.1%) | 0.922 |
| MI | 38 (3.3%) | 36 (3.1%) | 4 (2.5%) | 22 (5.3%) | 0.154 |
| Death | 73 (6.3%) | 88 (7.6%) | 7 (4.3%) | 32 (7.7%) | 0.286 |
Binary variables shown as n (%) and interval variables as mean±SD. Intergroup differences tested with the chi-squared test for binary variables and ANOVA test for interval variables.
no missing data.
Table 2.
Rates of smoking cessation in patients still smoking at study baseline.
| 3 months (n=570) |
6 months (n=543) |
12 months (n=495) |
18 months (n=433) |
|
|---|---|---|---|---|
| Quit smoking (%, n) |
28.4% (162) |
31.7% (172) |
32.7% (162) |
34.0% (147) |
| Persistent smoking (%, n) |
71.6% (408) |
68.3% (371) |
67.3% (333) |
66.0% (286) |
Table 3.
Hazard ratios for the primary outcome of major adverse cardiovascular events.
| Hazard ratio | 95% CI | Hommel P value | |
|---|---|---|---|
|
| |||
| Model 1 | |||
| Never smoker | Ref | - | - |
| Prior smoker | 1.11 | 0.90-1.36 | 0.944 |
| Smoker who quit | 0.94 | 0.59-1.50 | 0.797 |
| Persistent smoker | 1.35 | 1.03-1.78 | 0.031 |
|
| |||
| Model 2 | |||
| Never smoker | Ref | - | - |
| Prior smoker | 1.02 | 0.82-1.27 | 0.944 |
| Smoker who quit | 1.10 | 0.68-1.77 | 0.797 |
| Persistent smoker | 1.42 | 1.07-1.90 | 0.031 |
|
| |||
| Model 3 | |||
| Never smoker | Ref | - | - |
| Prior smoker | 0.99 | 0.80-1.24 | 0.944 |
| Smoker who quit | 1.25 | 0.77-2.02 | 0.797 |
| Persistent smoker | 1.56 | 1.16-2.09 | 0.009 |
Model 1: unadjusted; Model 2: adjusted for age, sex, race, and ethnicity; Model 3: adjusted for age, sex, race, ethnicity, education, employment status, history of hypertension, diabetes, hyperlipidemia, myocardial infarction, and intensive blood pressure randomization arm.
We tested for interactions between the covariates in the fully adjusted model and the exposure of smoking status. The only significant interaction (p<0.1) was between age and smoking, which had a p value of 0.082. After stratification by age categories (<60 vs. ≥60, n=1,150 vs. 1,724), the interaction term was highly significant (p<0.001). In the fully adjusted model, the hazard ratio for the primary outcome in younger patients (<60 years old) who persisted smoking, compared to never smokers, was 1.76 (95% CI 1.14-2.74), while in older patients (≥60 years old) it was 1.15 (95% CI 0.76-1.73). There was not a significant interaction with race (p= 0.56), ethnicity (p= 0.9), or education (p= 0.5).
For the secondary outcomes, there were 239 stroke events, 100 myocardial infarctions, and 200 death events. Although, it was not statistically significant, the independent rate of stroke, MI and death was numerically higher in persistent smokers compared to the other groups (Table 1). In the fully adjusted model, persistent smoking, compared to never smoking, had a significant association with death (HR 2.0, 95% CI 1.28-3.13), and the direction of effect remained consistent for the outcomes of stroke or myocardial infarction, but they lacked significance (Table 4).
Table 4.
Adjusted hazard ratios for the secondary outcomes.
| Outcome | Hazard ratio* | 95% CI | Hommel P value |
|---|---|---|---|
|
| |||
| Stroke | |||
| Never smoker | Ref | - | - |
| Prior smoker | 0.93 | 0.69-1.25 | 0.634 |
| Smoker who quit | 1.13 | 0.62-2.06 | 0.923 |
| Persistent smoker | 1.14 | 0.76-1.72 | 0.521 |
|
| |||
| Myocardial infarction | |||
| Never smoker | Ref | - | - |
| Prior smoker | 0.74 | 0.46-1.18 | 0.634 |
| Smoker who quit | 1.05 | 0.37-3.03 | 0.923 |
| Persistent smoker | 1.50 | 0.86-2.63 | 0.310 |
|
| |||
| Death | |||
| Never smoker | Ref | - | - |
| Prior smoker | 1.11 | 0.80-1.55 | 0.634 |
| Smoker who quit | 1.39 | 0.63-3.09 | 0.923 |
| Persistent smoker | 2.00 | 1.28-3.13 | 0.006 |
Adjusted for age, sex, race, ethnicity, education, employment status, history of hypertension, diabetes, hyperlipidemia, myocardial infarction, and intensive blood pressure randomization arm.
DISCUSSION
In this study, we demonstrated an association between persistent smoking after stroke and higher risk of composite outcome of death, stroke and myocardial infarction. In addition, persistent smokers had double the risk of death compared to never smokers. Interestingly, there was no significant difference in the risk of cardiovascular events or death between patients who were former smokers at baseline or those who quit smoking compared to never smokers which implies that smoking cessation after stroke may reduce the risk of death and cardiovascular events.
The data regarding the change of smoking behavior and its effect on outcome after ischemic stroke is scarce.15 A prior post-hoc analysis of the Insulin Resistance Intervention after Stroke (IRIS)14 trial compared cardiovascular outcomes of those who quit smoking at 6 months to those who continued.14 The study reported a higher rate of cardiovascular events and death in persistent smokers compared to those who quit smoking. In the present study, persistent smokers had higher risk of MACE compared to never smokers and former smokers. In both our study and the IRIS study, the higher risk of MACE in persistent smokers was mainly driven by higher risk of death which suggests that the harmful effect of persistent smoking after stroke is more related to the systemic complications of smoking rather than only the risk of recurrent stroke. The IRIS trial only included nondiabetic patients with a history of stroke. In contrast, our study included patients with lacunar stroke regardless of their diabetes status. Therefore, our results complement and support the previous study’s results.
Smoking status is influenced by individual factors such as age, race and socioeconomic status. According to CDC survey, smokers tend to be younger, male, less educated and have lower socioeconomic status than never smokers.20 In our study, the harmful effect of persistent smoking was not modified by race, educational status, sex or socioeconomic status. In contrast, age modified the effect of persistent smoking on outcome, and the harmful effect was more pronounced in younger patients
Cigarette smoking increases the risk of cardiovascular events through two main effects: an atherosclerotic and prothrombotic effect. Cigarette smoking impairs vasodilation, and induces inflammation which eventually leads to atherosclerosis.21 The prothrombotic effect of cigarette smoking is mediated by platelet dysfunction and alteration of antithrombotic and prothrombotic factors. The immediate reduction of cardiovascular events after smoking cessation is thought to be primarily related to alleviation of the prothrombotic effects of cigarette smoking. 21
Despite the rigorous risk factor management in the SPS3 trial, the rate of smoking cessation was low (28.4%) at 3 months and improved slightly at 18 months (34%). Moreover, 20% of patients who quit smoking resumed smoking within 12 months. Interestingly, persistent smokers in our study were more likely to be younger, have college education and to be employed. Moreover, as expected the duration of smoking was longer in patients who persisted than those who quit. It is conceivable that the rate of smoking cessation in real-world practice is even lower which highlights the need for more effective strategies to encourage smoking cessation among smokers presenting with ischemic stroke. Despite the efforts to identify an optimal smoking cessation pharmacotherapy agent, behavioral therapy remains the mainstem treatment of smoking cessation. A systematic review published in 2020 examined the available evidence regarding the efficacy and safety of smoking-cessation pharmacotherapies after stroke. The study identified 3 randomized controlled trials and one observational study that assessed the efficacy of pharmacotherapy compared to behavioral therapy. Overall there was a numerically higher smoking cessation with pharmacotherapy but no individual study reported statistically significant differences. Notably, the included studies suffered multiple limitations and their quality was judged as very low according to the GRADE assessment tool.22 Our study ,along with previous studies, highlights the importance of smoking cessation after stroke and the lack of an effective smoking cessation strategy. Future studies are needed to examine the effect of social, socioeconomic and structural factors on smoking behavior in “real-world” practice and to identify patients who are at risk for persistent smoking after stroke. Moreover, well-designed randomized controlled trials are urgently needed to identify the best smoking cessation strategy in patients who smoke and are hospitalized for cardiovascular events. The smoking cessation efforts should start during hospitalization and continue during follow up. It is possible that a combined pharmacotherapy and behavioral approach is needed to achieve the best results.
Limitations
The main limitation of this study is related to the post-hoc nature of our analysis.1) It is possible that the association between persistent smoking and cardiovascular outcomes noted in our study is also related to the fact that patients who continue to smoke are also less likely to be compliant with other secondary stroke interventions. Notably, the mean systolic blood pressure did not differ between groups. 2) Despite that the overall sample size being large, the sample size of patients who quit smoking is modest which could have contributed to the lack of association between smoking cessation and the risk of cardiovascular outcomes. 3) Furthermore, as noted in Table 2, smoking status continued to change after the exposure period, and we cannot rule out that persistent smokers group contained patients who quit smoking after 3 months. 4) Moreover, our study is limited to patients with lacunar stroke; therefore, our results may not apply to other stroke etiologies. Finally, it is unclear when former smokers quit smoking.
CONCLUSION
Persistent smoking compared to never smoking after lacunar stroke was associated with a significant increase in the risk of major cardiovascular events and death. The overall rate of smoking cessation after ischemic stroke was low, highlighting the need for more targeted and effective smoking cessation strategies.
Supplementary Material
Acknowledgment
We thank Alen Delic for his assistance with statistical analysis.
Disclosure
Dr. de Havenon reports grant support from NIH/NINDS, investigator initiated research support from AMAG and Regeneron, and equity in TitinKM and Certus.
Dr. Spiotta: Research grant to institution: Medtronic, Stryker, Penumbra ; Consulting: Cerenovus, Penumbra, RapidAI, Siemens, Stryker, Terumo. Dr. Sheth reports funding from Biogen, Novartis, Bard, Hyperfine, Astrocyte, Alva Health, NControl, and is DSMB Chair for Zoll.
ABBREVIATION
- HR
Hazard ratio
- MACE
Major cardiovascular events
- SPS3
Small Subcortical Strokes
REFERENCES
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 2.Bonita R, Scragg R, Stewart A, Jackson R and Beaglehole R. Cigarette smoking and risk of premature stroke in men and women. Br Med J (Clin Res Ed). 1986;293:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Critchley JA and Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86–97. [DOI] [PubMed] [Google Scholar]
- 4.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–75. [DOI] [PubMed] [Google Scholar]
- 5.Rallidis LS, Sakadakis EA, Tympas K, Varounis C, Zolindaki M, Dagres N, Lekakis J. The impact of smoking on long-term outcome of patients with premature (≤ 35 years) ST-segment elevation acute myocardial infarction. American heart journal. 2015;169:356–362. [DOI] [PubMed] [Google Scholar]
- 6.Rea TD, Heckbert SR, Kaplan RC, Smith NL, Lemaitre RN, Psaty BM. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med. 2002;137:494–500. [DOI] [PubMed] [Google Scholar]
- 7.Wannamethee SG, Shaper AG, Whincup PH, Walker M. Smoking cessation and the risk of stroke in middle-aged men. JAMA. 1995;274:155–60. [PubMed] [Google Scholar]
- 8.Pan B, Jin X, Jun L, Qiu S, Zheng Q, Pan M. The relationship between smoking and stroke: A meta-analysis. Medicine (Baltimore). 2019;98:e14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yudi MB, Farouque O, Andrianopoulos N, Ajani AE, Kalten K, Brennan AL, Lefkovits J, Hiew C, Oqueli E, Reid CM, et al. The prognostic significance of smoking cessation after acute coronary syndromes: an observational, multicentre study from the Melbourne interventional group registry. BMJ Open. 2017;7:e016874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Berg MJ, van der Graaf Y, Deckers JW, de Kanter W, Algra A, Kappelle LJ, de Borst GJ, Cramer MM, Visseren FLJ, et al. Smoking cessation and risk of recurrent cardiovascular events and mortality after a first manifestation of arterial disease. Am Heart J. 2019;213:112–122. [DOI] [PubMed] [Google Scholar]
- 11.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Li S, Zheng K, Wang H, Xie Y, Xu P, Dai Z, Gu M, Xia Y, Zhao M, et al. Impact of Smoking Status on Stroke Recurrence. J Am Heart Assoc. 2019;8:e011696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein KA, Viscoli CM, Spence JD, Young LH, Inzucchi SE, Gorman M, Gerstenhaber B, Guarino PD, Dixit A, Furie KL, et al. Smoking cessation and outcome after ischemic stroke or TIA. Neurology. 2017;89:1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noubiap JJ, Fitzgerald JL, Gallagher C, Thomas G, Middeldorp ME, Sanders P. Rates, Predictors, and Impact of Smoking Cessation after Stroke or Transient Ischemic Attack: A Systematic Review and Meta-Analysis. J Stroke Cerebrovasc Dis. 2021;30:106012. [DOI] [PubMed] [Google Scholar]
- 15.SPS3 Investigators, Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. New England Journal of Medicine. 2012;367:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, Coffey C, McClure LA, Szychowski JM, Conwit R, et al. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. Int J Stroke. 2011;6:164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group SPSS, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 19.Wright SP. Adjusted p-values for simultaneous inference. Biometrics 1992: 1005–1013. [Google Scholar]
- 20.National Center for Health Statistics. Crude percentages of all types of heart disease for adults aged 18 and over US, 2015–2018. National Health Interview Survey. Generated interactively: Sun Jan 30 2022. and Source: National Center for Health Statistics NHIS, 2015-2018. [Google Scholar]
- 21.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–7. [DOI] [PubMed] [Google Scholar]
- 22.Parikh NS, Salehi Omran S, Kamel H, Elkind MSV, Willey JZ. Smoking-cessation pharmacotherapy for patients with stroke and TIA: Systematic review. J Clin Neurosci. 2020;78:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


