Abstract
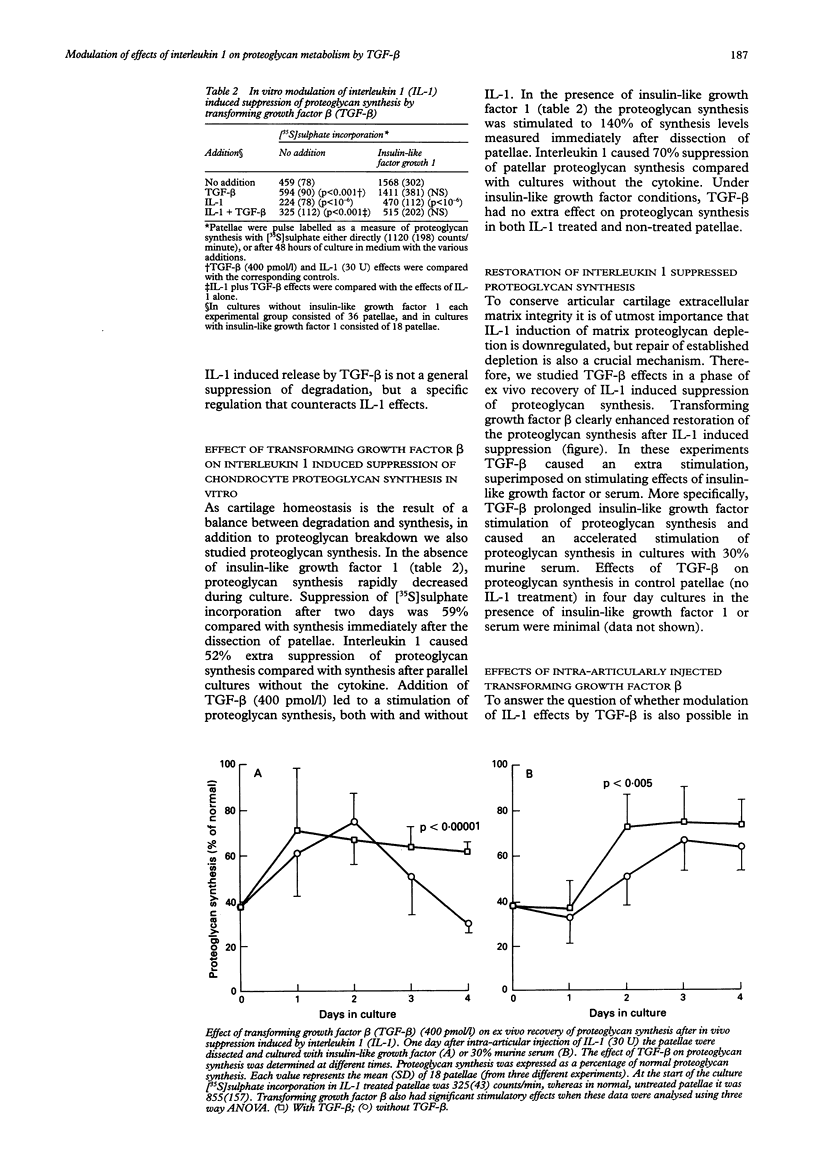
The modulation of interleukin 1 (IL-1) effects on proteoglycan metabolism in intact murine patellar cartilage by transforming growth factor beta (TGF-beta) was investigated in vitro and in vivo. In vitro TGF-beta (400 pmol/l) had no effect on basal proteoglycan degradation. Proteoglycan degradation induced by IL-1, however, was suppressed by TGF-beta in serum free medium alone and in medium supplemented with 0.5 micrograms/ml insulin-like growth factor 1. This suggests a specific regulatory role for TGF-beta under pathological conditions. In contrast with the suppression of breakdown, synthesis of proteoglycans was stimulated by TGF-beta for both basal and IL-1 suppressed proteoglycan synthesis in cultures without insulin-like growth factor. In the presence of insulin-like growth factor no extra effect of TGF-beta on proteoglycan synthesis was observed. With insulin-like growth factor, however, TGF-beta potentiated the ex vivo recovery of IL-1 induced suppression of proteoglycan synthesis. Analogous to the in vitro effects, TGF-beta injected intraarticularly suppressed IL-1 induced proteoglycan degradation. Furthermore, TGF-beta injected into the joint counteracted IL-1 induced suppression of proteoglycan synthesis. This indicates that in vivo also TGF-beta can ameliorate the deleterious effects of IL-1 on the cartilage matrix.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B., Manthey C. L., Hand A. R., Ohura K., Ellingsworth L., Wahl S. M. Rapid onset synovial inflammation and hyperplasia induced by transforming growth factor beta. J Exp Med. 1990 Jan 1;171(1):231–247. doi: 10.1084/jem.171.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews H. J., Edwards T. A., Cawston T. E., Hazleman B. L. Transforming growth factor-beta causes partial inhibition of interleukin 1-stimulated cartilage degradation in vitro. Biochem Biophys Res Commun. 1989 Jul 14;162(1):144–150. doi: 10.1016/0006-291x(89)91974-8. [DOI] [PubMed] [Google Scholar]
- Arner E. C., Pratta M. A. Independent effects of interleukin-1 on proteoglycan breakdown, proteoglycan synthesis, and prostaglandin E2 release from cartilage in organ culture. Arthritis Rheum. 1989 Mar;32(3):288–297. doi: 10.1002/anr.1780320310. [DOI] [PubMed] [Google Scholar]
- Bird T. A., Saklatvala J. Identification of a common class of high affinity receptors for both types of porcine interleukin-1 on connective tissue cells. Nature. 1986 Nov 20;324(6094):263–266. doi: 10.1038/324263a0. [DOI] [PubMed] [Google Scholar]
- Bryckaert M. C., Lindroth M., Lönn A., Tobelem G., Wasteson A. Transforming growth factor (TGF beta) decreases the proliferation of human bone marrow fibroblasts by inhibiting the platelet-derived growth factor (PDGF) binding. Exp Cell Res. 1988 Dec;179(2):311–321. doi: 10.1016/0014-4827(88)90270-4. [DOI] [PubMed] [Google Scholar]
- Campbell I. K., Last K., Novak U., Lund L. R., Hamilton J. A. Recombinant human interleukin-1 inhibits plasminogen activator inhibitor-1 (PAI-1) production by human articular cartilage and chondrocytes. Biochem Biophys Res Commun. 1991 Jan 15;174(1):251–257. doi: 10.1016/0006-291x(91)90513-7. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S., Harvey A. K. Transforming growth factor-beta is a potent inhibitor of IL-1 induced protease activity and cartilage proteoglycan degradation. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1352–1359. doi: 10.1016/s0006-291x(88)81024-6. [DOI] [PubMed] [Google Scholar]
- Chantry D., Turner M., Abney E., Feldmann M. Modulation of cytokine production by transforming growth factor-beta. J Immunol. 1989 Jun 15;142(12):4295–4300. [PubMed] [Google Scholar]
- Ding A., Nathan C. F., Graycar J., Derynck R., Stuehr D. J., Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990 Aug 1;145(3):940–944. [PubMed] [Google Scholar]
- Dingle J. T., Page Thomas D. P., King B., Bard D. R. In vivo studies of articular tissue damage mediated by catabolin/interleukin 1. Ann Rheum Dis. 1987 Jul;46(7):527–533. doi: 10.1136/ard.46.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois C. M., Ruscetti F. W., Palaszynski E. W., Falk L. A., Oppenheim J. J., Keller J. R. Transforming growth factor beta is a potent inhibitor of interleukin 1 (IL-1) receptor expression: proposed mechanism of inhibition of IL-1 action. J Exp Med. 1990 Sep 1;172(3):737–744. doi: 10.1084/jem.172.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elford P. R., Lamberts S. W. Contrasting modulation by transforming growth factor-beta-1 of insulin-like growth factor-I production in osteoblasts and chondrocytes. Endocrinology. 1990 Oct;127(4):1635–1639. doi: 10.1210/endo-127-4-1635. [DOI] [PubMed] [Google Scholar]
- Espevik T., Waage A., Faxvaag A., Shalaby M. R. Regulation of interleukin-2 and interleukin-6 production from T-cells: involvement of interleukin-1 beta and transforming growth factor-beta. Cell Immunol. 1990 Mar;126(1):47–56. doi: 10.1016/0008-8749(90)90299-7. [DOI] [PubMed] [Google Scholar]
- Fava R. A., Olsen N. J., Postlethwaite A. E., Broadley K. N., Davidson J. M., Nanney L. B., Lucas C., Townes A. S. Transforming growth factor beta 1 (TGF-beta 1) induced neutrophil recruitment to synovial tissues: implications for TGF-beta-driven synovial inflammation and hyperplasia. J Exp Med. 1991 May 1;173(5):1121–1132. doi: 10.1084/jem.173.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava R., Olsen N., Keski-Oja J., Moses H., Pincus T. Active and latent forms of transforming growth factor beta activity in synovial effusions. J Exp Med. 1989 Jan 1;169(1):291–296. doi: 10.1084/jem.169.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham H. D., Ray C. J., O'Sullivan F. X. Defective neutrophil function in the autoimmune mouse strain MRL/lpr. Potential role of transforming growth factor-beta. J Immunol. 1991 Jun 1;146(11):3911–3921. [PubMed] [Google Scholar]
- Harvey A. K., Hrubey P. S., Chandrasekhar S. Transforming growth factor-beta inhibition of interleukin-1 activity involves down-regulation of interleukin-1 receptors on chondrocytes. Exp Cell Res. 1991 Aug;195(2):376–385. doi: 10.1016/0014-4827(91)90387-a. [DOI] [PubMed] [Google Scholar]
- Hiraki Y., Inoue H., Hirai R., Kato Y., Suzuki F. Effect of transforming growth factor beta on cell proliferation and glycosaminoglycan synthesis by rabbit growth-plate chondrocytes in culture. Biochim Biophys Acta. 1988 Apr 2;969(1):91–99. doi: 10.1016/0167-4889(88)90092-4. [DOI] [PubMed] [Google Scholar]
- Hopkins S. J., Humphreys M., Jayson M. I. Cytokines in synovial fluid. I. The presence of biologically active and immunoreactive IL-1. Clin Exp Immunol. 1988 Jun;72(3):422–427. [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. R., Steinberg J. J., Bednar M. S., Sledge C. B. Effect of purified human interleukin-1 on cartilage degradation. J Orthop Res. 1988;6(2):180–187. doi: 10.1002/jor.1100060204. [DOI] [PubMed] [Google Scholar]
- Inoue H., Kato Y., Iwamoto M., Hiraki Y., Sakuda M., Suzuki F. Stimulation of cartilage-matrix proteoglycan synthesis by morphologically transformed chondrocytes grown in the presence of fibroblast growth factor and transforming growth factor-beta. J Cell Physiol. 1989 Feb;138(2):329–337. doi: 10.1002/jcp.1041380216. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Taylor A. S., Delsing G. A., Roberts A. B., Sporn M. B., Fauci A. S. Further studies of the role of transforming growth factor-beta in human B cell function. J Immunol. 1989 Sep 15;143(6):1868–1874. [PubMed] [Google Scholar]
- Kim K. J., Abrams J., Alphonso M., Pearce M., Thorbecke G. J., Palladino M. A. Role of endogenously produced interleukin-6 as a second signal in murine thymocyte proliferation induced by multiple cytokines: regulatory effects of transforming growth factor-beta. Cell Immunol. 1990 Dec;131(2):261–271. doi: 10.1016/0008-8749(90)90253-n. [DOI] [PubMed] [Google Scholar]
- Lotz M., Kekow J., Carson D. A. Transforming growth factor-beta and cellular immune responses in synovial fluids. J Immunol. 1990 Jun 1;144(11):4189–4194. [PubMed] [Google Scholar]
- Luyten F. P., Hascall V. C., Nissley S. P., Morales T. I., Reddi A. H. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988 Dec;267(2):416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N., Mizel D., Wong H., Wahl L., Wahl S. TGF-beta regulates production of growth factors and TGF-beta by human peripheral blood monocytes. Growth Factors. 1990;4(1):27–35. doi: 10.3109/08977199009011007. [DOI] [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Campbell M. A., Bolis S., Milway V. E., Herington A. C. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. 1986 Dec 1;240(2):423–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Interleukin 1 derived from human endothelial cells enhances the binding and chemotactic step of T lymphocyte emigration. Clin Exp Immunol. 1988 Aug;73(2):250–254. [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Dinarello C. A., Ziff M. Interleukin-1 lymphocyte chemotactic activity in rheumatoid arthritis synovial fluid. Arthritis Rheum. 1986 Apr;29(4):461–470. doi: 10.1002/art.1780290402. [DOI] [PubMed] [Google Scholar]
- Miossec P., Naviliat M., Dupuy d'Angeac A., Sany J., Banchereau J. Low levels of interleukin-4 and high levels of transforming growth factor beta in rheumatoid synovitis. Arthritis Rheum. 1990 Aug;33(8):1180–1187. doi: 10.1002/art.1780330819. [DOI] [PubMed] [Google Scholar]
- Morales T. I., Roberts A. B. Transforming growth factor beta regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J Biol Chem. 1988 Sep 15;263(26):12828–12831. [PubMed] [Google Scholar]
- Nelson B. J., Ralph P., Green S. J., Nacy C. A. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-beta 1. J Immunol. 1991 Mar 15;146(6):1849–1857. [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redini F., Galera P., Mauviel A., Loyau G., Pujol J. P. Transforming growth factor beta stimulates collagen and glycosaminoglycan biosynthesis in cultured rabbit articular chondrocytes. FEBS Lett. 1988 Jul 4;234(1):172–176. doi: 10.1016/0014-5793(88)81327-9. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., McFall R. C. Platelet-derived growth factor and transforming growth factor-beta regulate plasminogen activator inhibitor-1 synthesis in vascular smooth muscle cells. J Biol Chem. 1991 May 25;266(15):9419–9427. [PubMed] [Google Scholar]
- Rooney M., Symons J. A., Duff G. W. Interleukin 1 beta in synovial fluid is related to local disease activity in rheumatoid arthritis. Rheumatol Int. 1990;10(5):217–219. doi: 10.1007/BF02274836. [DOI] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Rifkin D. B. The opposing effects of basic fibroblast growth factor and transforming growth factor beta on the regulation of plasminogen activator activity in capillary endothelial cells. J Cell Biol. 1987 Aug;105(2):957–963. doi: 10.1083/jcb.105.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers T. J., Wiltrout T. A., Bull C. A., Denn A. C., 3rd, Pilaro A. M., Lokesh B. Effect of cytokines on polymorphonuclear neutrophil infiltration in the mouse. Prostaglandin- and leukotriene-independent induction of infiltration by IL-1 and tumor necrosis factor. J Immunol. 1988 Sep 1;141(5):1670–1677. [PubMed] [Google Scholar]
- Schalkwijk J., Joosten L. A., van den Berg W. B., van Wyk J. J., van de Putte L. B. Insulin-like growth factor stimulation of chondrocyte proteoglycan synthesis by human synovial fluid. Arthritis Rheum. 1989 Jan;32(1):66–71. doi: 10.1002/anr.1780320111. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Rohloff N. A., Sam L. M., Justen J. M., Deibel M. R., Cornette J. C. Recombinant human interleukin-1 alpha and recombinant human interleukin-1 beta stimulate cartilage matrix degradation and inhibit glycosaminoglycan synthesis. Inflammation. 1989 Aug;13(4):367–382. doi: 10.1007/BF00914921. [DOI] [PubMed] [Google Scholar]
- Thompson K. L., Assoian R., Rosner M. R. Transforming growth factor-beta increases transcription of the genes encoding the epidermal growth factor receptor and fibronectin in normal rat kidney fibroblasts. J Biol Chem. 1988 Dec 25;263(36):19519–19524. [PubMed] [Google Scholar]
- Tsunawaki S., Sporn M., Ding A., Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988 Jul 21;334(6179):260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- Turner M., Chantry D., Katsikis P., Berger A., Brennan F. M., Feldmann M. Induction of the interleukin 1 receptor antagonist protein by transforming growth factor-beta. Eur J Immunol. 1991 Jul;21(7):1635–1639. doi: 10.1002/eji.1830210708. [DOI] [PubMed] [Google Scholar]
- Tyler J. A. Insulin-like growth factor 1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J. 1989 Jun 1;260(2):543–548. doi: 10.1042/bj2600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., Wong H. L., Dougherty S. F., Ellingsworth L. R. Antagonistic and agonistic effects of transforming growth factor-beta and IL-1 in rheumatoid synovium. J Immunol. 1990 Oct 15;145(8):2514–2519. [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Bansal G., McCartney-Francis N., Ellingsworth L., Allen J. B. Bacterial cell wall-induced immunosuppression. Role of transforming growth factor beta. J Exp Med. 1988 Oct 1;168(4):1403–1417. doi: 10.1084/jem.168.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wong H. L., Dougherty S., McCartney-Francis N., Wahl L. M., Ellingsworth L., Schmidt J. A., Hall G., Roberts A. B. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988 May 1;140(9):3026–3032. [PubMed] [Google Scholar]
- Wahl S. M., McCartney-Francis N., Mergenhagen S. E. Inflammatory and immunomodulatory roles of TGF-beta. Immunol Today. 1989 Aug;10(8):258–261. doi: 10.1016/0167-5699(89)90136-9. [DOI] [PubMed] [Google Scholar]
- Wankowicz Z., Megyeri P., Issekutz A. Synergy between tumour necrosis factor alpha and interleukin-1 in the induction of polymorphonuclear leukocyte migration during inflammation. J Leukoc Biol. 1988 Apr;43(4):349–356. doi: 10.1002/jlb.43.4.349. [DOI] [PubMed] [Google Scholar]
- Wiseman D. M., Polverini P. J., Kamp D. W., Leibovich S. J. Transforming growth factor-beta (TGF beta) is chemotactic for human monocytes and induces their expression of angiogenic activity. Biochem Biophys Res Commun. 1988 Dec 15;157(2):793–800. doi: 10.1016/s0006-291x(88)80319-x. [DOI] [PubMed] [Google Scholar]
- de Vries B. J., van den Berg W. B., Vitters E., van de Putte L. B. Quantitation of glycosaminoglycan metabolism in anatomically intact articular cartilage of the mouse patella: in vitro and in vivo studies with 35S-sulfate, 3H-glucosamine, and 3H-acetate. Rheumatol Int. 1986;6(6):273–281. doi: 10.1007/BF00541319. [DOI] [PubMed] [Google Scholar]
- van Beuningen H. M., Arntz O. J., van den Berg W. B. In vivo effects of interleukin-1 on articular cartilage. Prolongation of proteoglycan metabolic disturbances in old mice. Arthritis Rheum. 1991 May;34(5):606–615. doi: 10.1002/art.1780340513. [DOI] [PubMed] [Google Scholar]
- van de Loo A. A., van den Berg W. B. Effects of murine recombinant interleukin 1 on synovial joints in mice: measurement of patellar cartilage metabolism and joint inflammation. Ann Rheum Dis. 1990 Apr;49(4):238–245. doi: 10.1136/ard.49.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg W. B., Kruijsen M. W., van de Putte L. B., van Beusekom H. J., van der Sluis-van der Pol M., Zwarts W. A. Antigen-induced and zymosan-induced arthritis in mice: studies on in vivo cartilage proteoglycan synthesis and chondrocyte death. Br J Exp Pathol. 1981 Jun;62(3):308–316. [PMC free article] [PubMed] [Google Scholar]
- van den Berg W. B., van de Loo F. A., Zwarts W. A., Otterness I. G. Effects of murine recombinant interleukin 1 on intact homologous articular cartilage: a quantitative and autoradiographic study. Ann Rheum Dis. 1988 Oct;47(10):855–863. doi: 10.1136/ard.47.10.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kraan P. M., Vitters E. L., van den Berg W. B. Inhibition of proteoglycan synthesis by transforming growth factor beta in anatomically intact articular cartilage of murine patellae. Ann Rheum Dis. 1992 May;51(5):643–647. doi: 10.1136/ard.51.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]


