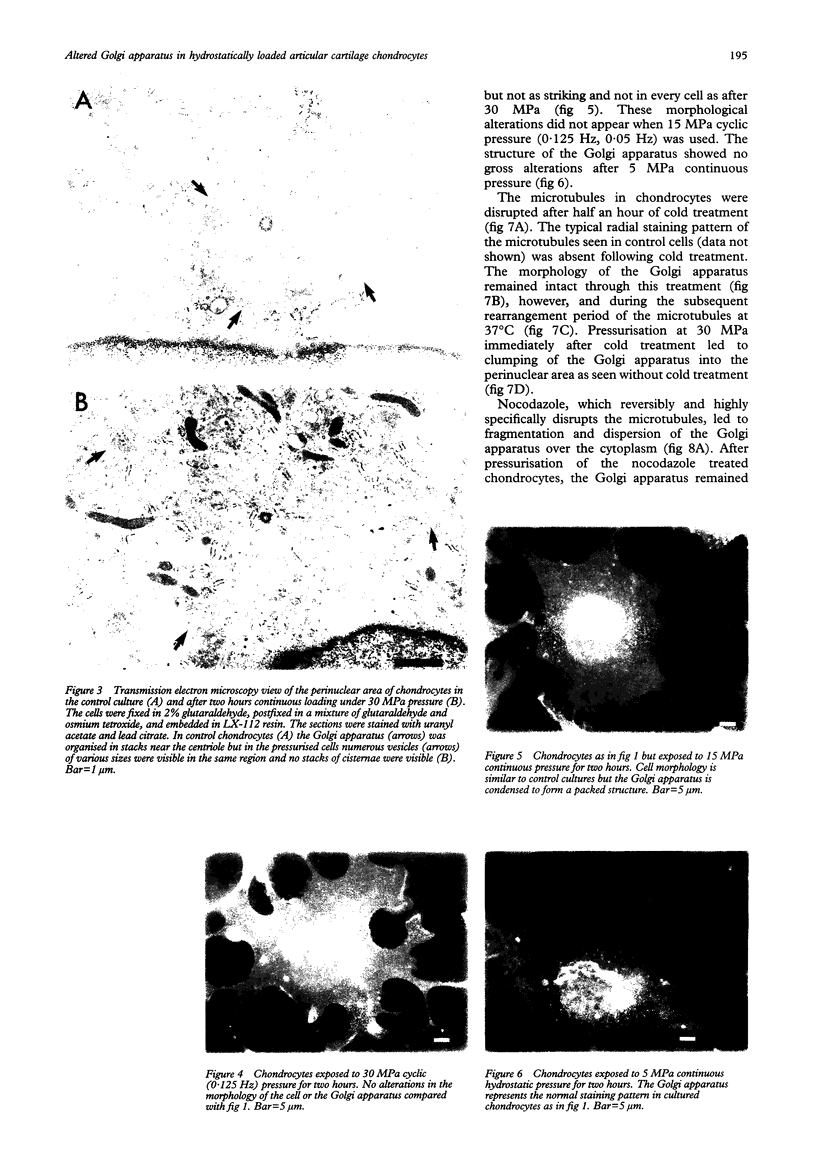
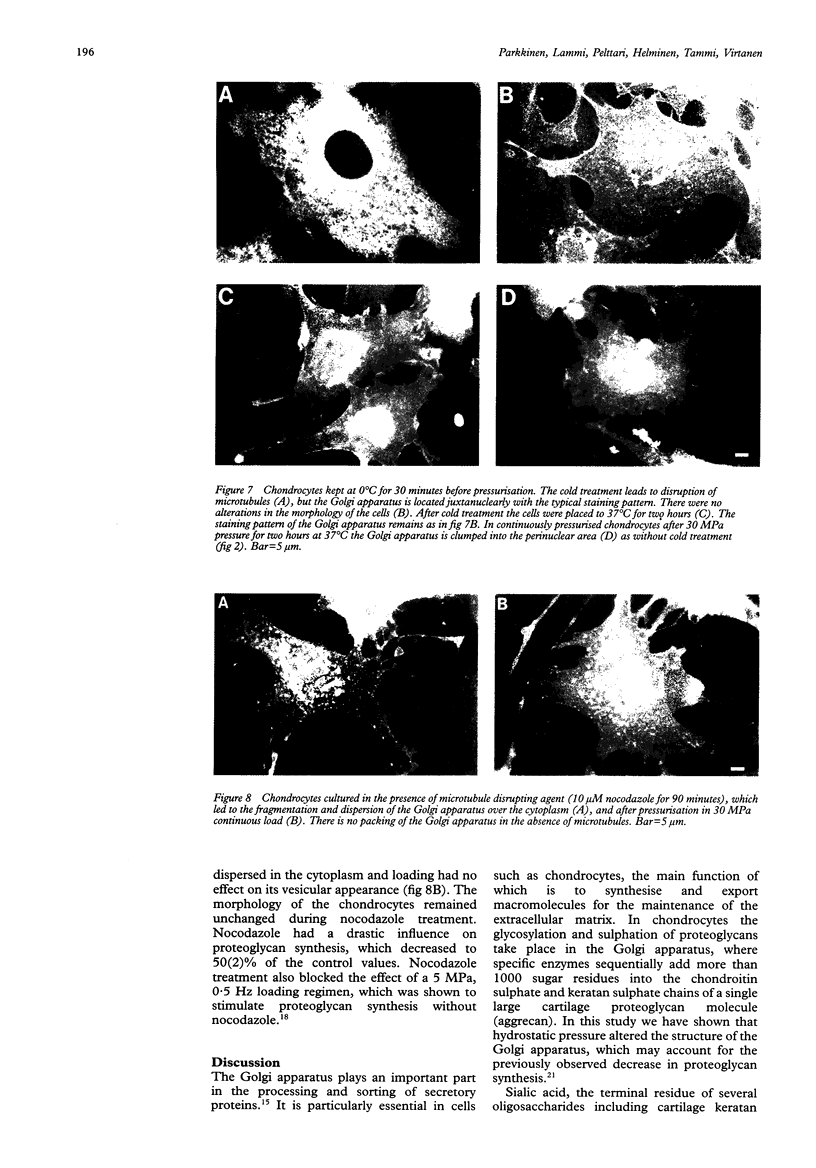
Abstract
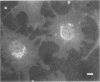
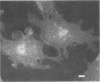
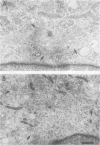
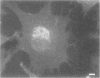
OBJECTIVES: Articular cartilage proteoglycan content is controlled by joint loading. This study aimed to elucidate the role of hydrostatic pressure in this regulation. METHODS: Primary cultures of chondrocytes from bovine articular cartilage, grown on coverslips, were subjected to 5, 15, or 30 MPa hydrostatic pressure, applied continuously or cyclically at 0.125 or 0.05 Hz. The Golgi apparatus was visualised either by a fluorochrome coupled wheat germ agglutinin or by transmission electron microscopy. Proteoglycan synthesis was studied by the incorporation of sulphur-35 labelled sulphate. RESULTS: After 30 MPa continuous hydrostatic pressure, the Golgi apparatus was observed in a compact form with a concomitant decrease in proteoglycan synthesis. The normal stacked appearance of the Golgi apparatus was no more visible in the electron microscopy preparation of the pressurised chondrocytes. This effect was reversible and was also noticed after 15 MPa continuous load, though to a minor extent. Cyclic pressures (5-30 MPa) caused no apparent change in the Golgi apparatus. The shape of some cells changed to a more retracted form after 30 MPa continuous pressure. Nocodazole, which causes disassembly of the microtubules, blocked the compacting influence of pressurisation on the Golgi apparatus, and reduced proteoglycan synthesis to about half of the control level. CONCLUSIONS: The packing of the Golgi apparatus is dependent on microtubules and may contribute to the inhibition of proteoglycan synthesis observed in articular cartilage subjected to high hydrostatic pressure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan V. J., Kreis T. E. A microtubule-binding protein associated with membranes of the Golgi apparatus. J Cell Biol. 1986 Dec;103(6 Pt 1):2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourns B., Franklin S., Cassimeris L., Salmon E. D. High hydrostatic pressure effects in vivo: changes in cell morphology, microtubule assembly, and actin organization. Cell Motil Cytoskeleton. 1988;10(3):380–390. doi: 10.1002/cm.970100305. [DOI] [PubMed] [Google Scholar]
- Dibb W., Morild E., Laerum O. D. Effects of high hydrostatic pressure on normal and neoplastic rat cells in culture. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;38(2):169–176. doi: 10.1007/BF02892812. [DOI] [PubMed] [Google Scholar]
- Finlay J. B., Repo R. U. Instrumentation and procedure for the controlled impact of articular cartilage. IEEE Trans Biomed Eng. 1978 Jan;25(1):34–39. doi: 10.1109/TBME.1978.326375. [DOI] [PubMed] [Google Scholar]
- Goldinger J. M., Kang B. S., Choo Y. E., Paganelli C. V., Hong S. K. Effect of hydrostatic pressure on ion transport and metabolism in human erythrocytes. J Appl Physiol Respir Environ Exerc Physiol. 1980 Aug;49(2):224–231. doi: 10.1152/jappl.1980.49.2.224. [DOI] [PubMed] [Google Scholar]
- Heinemann S. H., Conti F., Stühmer W., Neher E. Effects of hydrostatic pressure on membrane processes. Sodium channels, calcium channels, and exocytosis. J Gen Physiol. 1987 Dec;90(6):765–778. doi: 10.1085/jgp.90.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge W. A., Fijan R. S., Carlson K. L., Burgess R. G., Harris W. H., Mann R. W. Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci U S A. 1986 May;83(9):2879–2883. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurvelin J., Kiviranta I., Arokoski J., Tammi M., Helminen H. J. Indentation study of the biochemical properties of articular cartilage in the canine knee. Eng Med. 1987 Jan;16(1):15–22. doi: 10.1243/emed_jour_1987_016_006_02. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Sah R. L., Doong J. Y., Grodzinsky A. J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988 Oct;174(1):168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- Kiviranta I., Tammi M., Jurvelin J., Arokoski J., Sämänen A. M., Helminen H. J. Articular cartilage thickness and glycosaminoglycan distribution in the canine knee joint after strenuous running exercise. Clin Orthop Relat Res. 1992 Oct;(283):302–308. [PubMed] [Google Scholar]
- Kiviranta I., Tammi M., Jurvelin J., Sämänen A. M., Helminen H. J. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J Orthop Res. 1988;6(2):188–195. doi: 10.1002/jor.1100060205. [DOI] [PubMed] [Google Scholar]
- Lohmander S., Moskalewski S., Madsen K., Thyberg J., Friberg U. Influence of colchicine on the synthesis and secretion of proteoglycans and collagen by fetal guinea pig chondrocytes. Exp Cell Res. 1976 May;99(2):333–345. doi: 10.1016/0014-4827(76)90591-7. [DOI] [PubMed] [Google Scholar]
- Madsen K., Holmström S., Ostrowski K. Synthesis and secretion of proteoglycans by cultured chondrocytes. Effects of monensin, colchicine and beta-D-xyloside. Exp Cell Res. 1983 Oct 15;148(2):493–501. doi: 10.1016/0014-4827(83)90170-2. [DOI] [PubMed] [Google Scholar]
- Mitchell D., Hardingham T. The effects of cycloheximide on the biosynthesis and secretion of proteoglycans by chondrocytes in culture. Biochem J. 1981 May 15;196(2):521–529. doi: 10.1042/bj1960521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalewski S., Thyberg J., Friberg U. Cold and metabolic inhibitor effects on cytoplasmic microtubules and the Golgi complex in cultured rat epiphyseal chondrocytes. Cell Tissue Res. 1980;210(3):403–415. doi: 10.1007/BF00220198. [DOI] [PubMed] [Google Scholar]
- Parkkinen J. J., Lammi M. J., Helminen H. J., Tammi M. Local stimulation of proteoglycan synthesis in articular cartilage explants by dynamic compression in vitro. J Orthop Res. 1992 Sep;10(5):610–620. doi: 10.1002/jor.1100100503. [DOI] [PubMed] [Google Scholar]
- Simon W. H. Scale effects in animal joints. I. Articular cartilage thickness and compressive stress. Arthritis Rheum. 1970 May-Jun;13(3):244–256. doi: 10.1002/art.1780130305. [DOI] [PubMed] [Google Scholar]
- Sämänen A. M., Tammi M., Jurvelin J., Kiviranta I., Helminen H. J. Proteoglycan alterations following immobilization and remobilization in the articular cartilage of young canine knee (stifle) joint. J Orthop Res. 1990 Nov;8(6):863–873. doi: 10.1002/jor.1100080612. [DOI] [PubMed] [Google Scholar]
- Sämänen A. M., Tammi M., Kiviranta I., Jurvelin J., Helminen H. J. Levels of chondroitin-6-sulfate and nonaggregating proteoglycans at articular cartilage contact sites in the knees of young dogs subjected to moderate running exercise. Arthritis Rheum. 1989 Oct;32(10):1282–1292. doi: 10.1002/anr.1780321014. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Moskalewski S. Microtubules and the organization of the Golgi complex. Exp Cell Res. 1985 Jul;159(1):1–16. doi: 10.1016/s0014-4827(85)80032-x. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Tartakoff A. M. The response of the Golgi complex to microtubule alterations: the roles of metabolic energy and membrane traffic in Golgi complex organization. J Cell Biol. 1989 Nov;109(5):2081–2088. doi: 10.1083/jcb.109.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco A., Hidalgo J., Müller M., Garcia-Herdugo G. Ultrastructural demonstration of lectin binding sites in the Golgi apparatus of rat epiphyseal chondrocytes. Histochemistry. 1988;89(2):177–184. doi: 10.1007/BF00489921. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Ekblom P., Laurila P. Subcellular compartmentalization of saccharide moieties in cultured normal and malignant cells. J Cell Biol. 1980 May;85(2):429–434. doi: 10.1083/jcb.85.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]