Abstract
Objectives
The World Health Organization priority zoonotic pathogen Middle East respiratory syndrome (MERS) coronavirus (CoV) has a high case fatality rate in humans and circulates in camels worldwide.
Methods
We performed a global analysis of human and camel MERS-CoV infections, epidemiology, genomic sequences, clades, lineages, and geographical origins for the period January 1, 2012 to August 3, 2022. MERS-CoV Surface gene sequences (4061 bp) were extracted from GenBank, and a phylogenetic maximum likelihood tree was constructed.
Results
As of August 2022, 2591 human MERS cases from 26 countries were reported to the World Health Organization (Saudi Arabia, 2184 cases, including 813 deaths [case fatality rate: 37.2%]) Although declining in numbers, MERS cases continue to be reported from the Middle East. A total of 728 MERS-CoV genomes were identified (the largest numbers were from Saudi Arabia [222: human = 146, camels = 76] and the United Arab Emirates [176: human = 21, camels = 155]). A total of 501 ‘S’-gene sequences were used for phylogenetic tree construction (camels [n = 264], humans [n = 226], bats [n = 8], other [n=3]). Three MERS-CoV clades were identified: clade B, which is the largest, followed by clade A and clade C. Of the 462 clade B lineages, lineage 5 was predominant (n = 177).
Conclusion
MERS-CoV remains a threat to global health security. MERS-CoV variants continue circulating in humans and camels. The recombination rates indicate co-infections with different MERS-CoV lineages. Proactive surveillance of MERS-CoV infections and variants of concern in camels and humans worldwide, and development of a MERS vaccine, are essential for epidemic preparedness.
Keywords: MERS-CoV, Genetic diversity, S-gene, Phylogenetic analysis, Zoonotic reservoir, MERS
Introduction
Three novel coronaviruses (CoVs) have emerged over the past two decades, causing lethal diseases in humans: SARS-CoV-1 [1,2], Middle East respiratory syndrome coronavirus (MERS-CoV) [3], [4], [5], [6], [7], and more recently, SARS-CoV-2 [8,9]. In 2002, SARS-CoV-1 jumped species from civet cats to humans and rapidly spread by human-to-human transmission across continents, causing 8098 cases and 774 deaths (9.6% case fatality rate [CFR]) [1,2]. It disappeared within 18 months, and no human cases of SARS-CoV-1 infection have been recorded since January 2004 [1].
SARS-CoV-2 was first reported in December 2019 from Wuhan, Hubei province in China [8,9] and subsequently spread worldwide, causing the unprecedented pandemic of COVID-19, with a CFR of 1.1% [9]. The emergence of genetic variants of concern (VOCs) in SARS-CoV-2 has generated serious dialogue regarding the evolution of new genotypes, transmissibility, and response to vaccines [9].
MERS-CoV was first detected in the clinical samples of a patient with pneumonia who died in a Jeddah hospital in Saudi Arabia in 2012 [3]. Since then, over 2500 cases of laboratory-confirmed MERS cases have been reported from 27 countries, with a high CFR of up to 35% [4], [5], [6]. MERS-CoV has been identified in dromedary camels in the Middle East, Africa, and South Asia [4,5]. Exposure to dromedaries is recognized as a risk factor for primary human MERS cases [4], [5], [6], [7].
The recombination in CoVs is of great evolutionary importance since it is associated with expansion in host range, transmissibility, global spread changes in pathogenicity and host response [10]. Although MERS-CoV is associated with a high mortality rate and it remains on the World Health Organization (WHO) blueprint list of priority pathogens, the scientific and political attention on it has been eclipsed by the unprecedented SARS-CoV-2 (COVID-19) pandemic. As with SARS-COV-2 [9], new MERS-CoV lineages and variants with more or less efficient transmission capabilities may emerge over time. The large, unexpected MERS-CoV outbreak in South Korea in 2015 highlighted the epidemic potential of MERS-CoV [10], [11], [12], [13].
MERS-CoV continues to circulate in camels and humans, remaining a threat to global health security [[4], [5], [6],[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]]. The information on the evolution of MERS-CoV lineages, clades and new variants in humans and animals remains scanty and major knowledge gaps remain. Thus, similar to the collective global efforts at genomic sequencing and surveillance of SARS-CoV-2 variants [9], increasing surveillance, genomic sequencing, and monitoring of MERS-CoV circulation in camels and humans are equally important. We performed a 10-year (2012-2022) analysis of MERS-CoV infections, epidemiology, sequences, lineages, clades, animal host, and geographical origins.
Methods
We searched the WHO, WHO Regional Office for the Eastern Mediterranean, United Nations Food and Agriculture Organization, United States Centers for Disease Control and Prevention, Public Health England (United Kingdom), World Organization for Animal Health, European Centre for Disease Prevention and Control, the Saudi Ministry of Health MERS portal, Program for Monitoring Emerging Diseases, and the National Center for Biotechnology Information (NCBI) database websites for English language publications related to MERS-CoV for the period of January 1, 2011 to August 3, 2022. We used the search terms “Middle East Respiratory Syndrome” OR “MERS-CoV” in combination with the terms “Coronavirus”, OR “Genomes” OR “Sequences”, OR “Epidemiology”, OR “Aetiology”, OR “Dromedaries”, OR “Camels”, OR “Bats”, OR “Transmission”, OR “Virology” OR “Diagnosis” OR “Nosocomial” OR “Hospital”.
We extracted the MERS-CoV genomic sequences deposited in the NCBI database across the world. The NCBI accession number, description of the genome, country and animal host origin, and date of collection and entry were noted. The sequences were stored as FASTA files in .txt format on normal Windows personal computer. The sequences of 501 of 728 ‘S’-gene sequences (4061 bp) were extracted. The ‘S’-gene has two subunits, S1 and S2. The S1 subunit consists of an N-terminal domain (NTD) and a C-terminal domain (CTD), which is a receptor-binding domain responsible for binding the host receptor dipeptidyl peptidase 4 [16]. In addition to loop, fusion peptide, transmembrane I, and cytoplasmic (CP) domain, the S2 subunit consists mainly of two conserved domains, heptad repeat 1 and heptad repeat 2 (HR2), which mediate the entry of MERS-CoV into the host cells.
Extracted ‘S’-gene sequences (Supplementary Table 1) were aligned with the Multiple Sequence Alignment Tool, using the MUSCLE program Molecular Evolutionary Genetics Analysis X (MEGAx, Ver.11.0, Pennsylvania State University, PA, USA). The best nucleotide substitution model was determined using general time reversible model general time reversible + G + I. The phylogenetic maximum likelihood tree was reconstructed using the phylogenetic estimation using maximum likelihood (PhyML Ver 3.0, Montpellier, France) for 1000 bootstrap pseudoreplicates with a discreet gamma distribution rate of five parameters. The rooted tree was annotated with the Tree of Life tool (iTOL Ver 6.6, European Molecular Biology Laboratory, Heidelberg, Germany).
Results
MERS cases
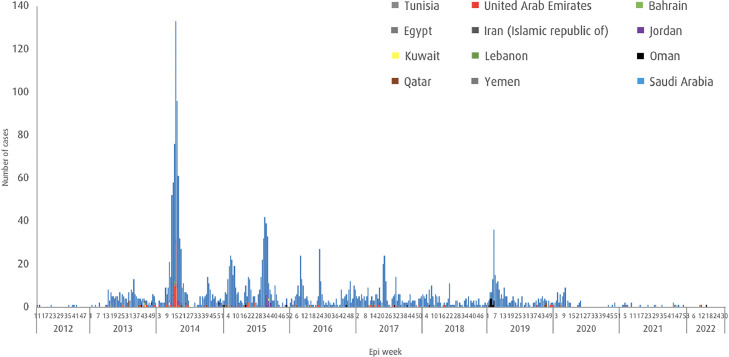
Since April 2012 up to August 3, 2022, a total of 2591 laboratory-confirmed human MERS cases were reported to the WHO. The majority of the cases were from Saudi Arabia, with 2184 cases and 813 related deaths (CFR: 37.2%) (Figure 1 ).
Figure 1.
Middle East respiratory syndrome cases reported to the World Health Organization.
Epi, epidemiology.
MERS-CoV ‘S’-gene sequences
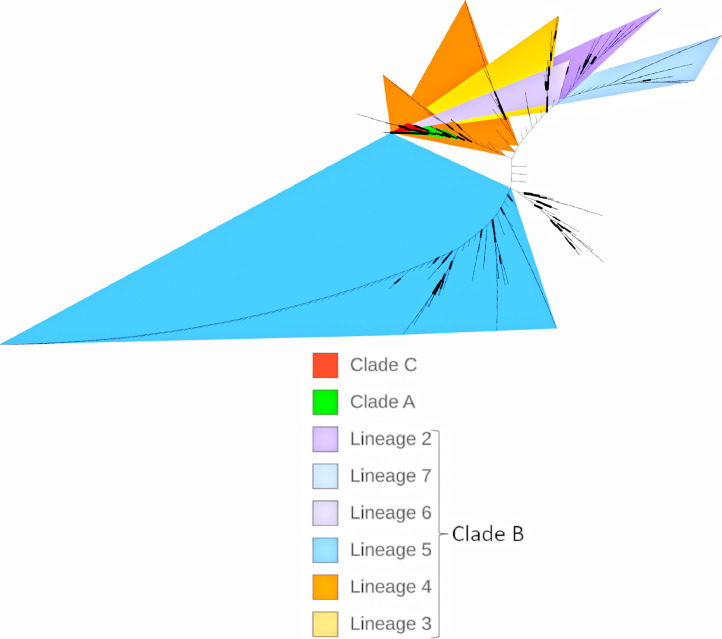
The sequences we extracted from GenBank were deposited from Saudi Arabia (222, human = 146, camels = 76), the United Arab Emirates (UAE) (176, human = 21, camels = 155), Jordan (n = 38), South Korea (n = 22), Nigeria (n = 9), Qatar (n = 5), Ethiopia (n = 4), Egypt (n = 3), Burkina Faso (n = 3), Oman (n = 2), and Morocco (n = 1). The sample origin was either from humans (n = 226) or dromedary camels (n = 264). They belonged to clades A (nine sequences), B (460 sequences), or C (five sequences), and 16 sequences were of an unclassified clade. Clade C entirely included the sequences from dromedary camels, whereas clade A included sequences from humans (n = 7) and camels (n = 2), clade B included sequences from humans (n=219) and camels (n = 241), and the unclassified clade included entirely dromedary sequences (Figure 2 , Figure 3 , Table 1 ).
Figure 2.
Distribution of different lineages and clades from sequences analyzed for the S-gene.
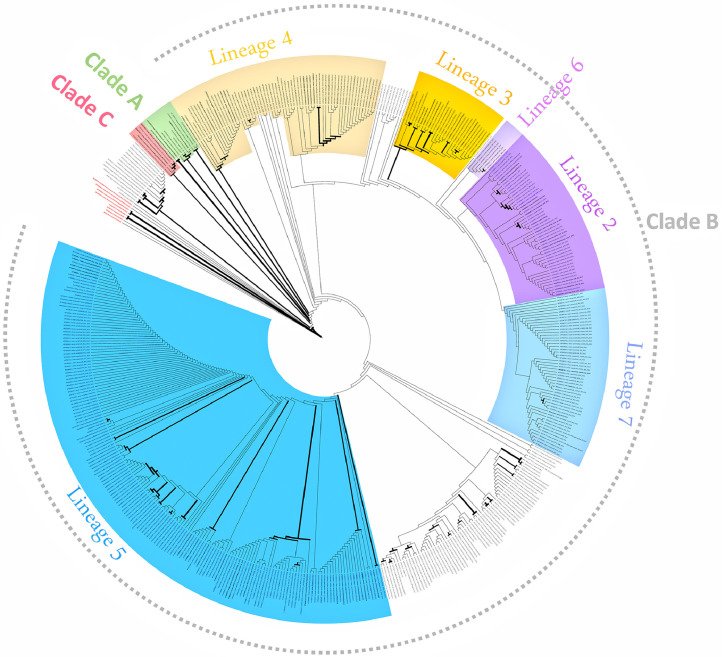
Figure 3.
Reconstructed maximum likelihood phylogenetic tree from Middle East respiratory syndrome CoV S-gene sequences using PhyML 3.3 with GTR+G+I nucleotide substitution model.
Table 1.
Middle East respiratory syndrome CoV variants by year of reporting, country, host, clade, and lineage.
| Function | Region | Mutation | Host | Clade | Lineage | Country | Year | Country | Region | Mutation | Host | Year |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | NTD | Val30Ala | Camels | C | - | Egypt | 2014 | Kenya | NTD | Val30Ala | Camels | 2019 |
| Ethiopia | 2017-2019 | Ap177Tyr | Camels | 2019 | ||||||||
| Val30Phe | Camels | B | 2,4,6,7 | UAE | 2014 | Ser32Pro | Camels | 2019 | ||||
| Ser32Pro | Camels | B | 2,4,6,7 | UAE | 2014-2015 | His215Tyr | Camels | 2019 | ||||
| Thr105Ile | Camels | B | 5 | Jordan | 2016 | |||||||
| Ser143Ala | Humans | B | 4 | Saudi Arabia | 2017 | |||||||
| CTD/RBD | Ser485Thr | Camels | B | 4.7 | UAE | 2015 | CTD/RBD | Ser416Phe | Camels | 2019 | ||
| Phe499Ser | Humans | B | 4 | Saudi Arabia | 2015-2017 | Leu476Phe | Camels | 2019 | ||||
| Ile568Thr | Camels | A | - | Saudi Arabia | 2017 | Pro673Arg | Camels | 2019 | ||||
| Humans | B | 4 | Saudi Arabia | 2017 | ||||||||
| Humans | B | 5 | South Korea | 2015 | ||||||||
| S2 | Loop | Pro758Ser | Camels | B | 5,6,7 | UAE | 2014-2015 | Loop | Ala1218Ser | Camels | 2019 | |
| Leu795Phe | Humans | B | 4 | Saudi Arabia | 2017-2018 | |||||||
| Gln883Arg | Humans | B | 4 | Saudi Arabia | 2014-2015 | |||||||
| UAE | ||||||||||||
| USA | ||||||||||||
| Jordan | ||||||||||||
| HR2 | Gly1276Ser | Camels | B | 3.4 | Saudi Arabia | 2014-2015 | HR1 | Gln1070Arg | Camels | 2019 | ||
| Cytoplasmic | Cys1387Phe | Camels | B | 5 | Saudi Arabia | 2014-2015 |
CTD, C-terminal domain; HR, heptad repeat; NTD, N-terminal domain; RBD, receptor-binding domain.
The clade distribution over the years showed that in 2012, only clade A (100%) was identified, then clade A (10%) declined in 2013, and the other clade B (80%) and an unclassified clade (10%) started to emerge. Clade B dominated in the years 2014 (98.7%), 2015 (97.4%), 2016 (86%), 2017 (95.3%), 2018 (93.3%), and 2019 (83.3%). Among 462 clade B lineages, lineage 5 (n = 177, 38.3%) was predominant, followed by lineage 4 (n = 65, 14.1%), lineage 7 (n = 53, 11.5%), lineage 2 (n = 51, 11.03%), lineage 3 (n = 28, 6.1%), and lineage 6 (n = 7, 1.5%), whereas 81 (17.5%) were unclassifiable. Lineage 1 was not identified (Figure 3).
Mutations in MERS-CoV S1-NTD and CTD
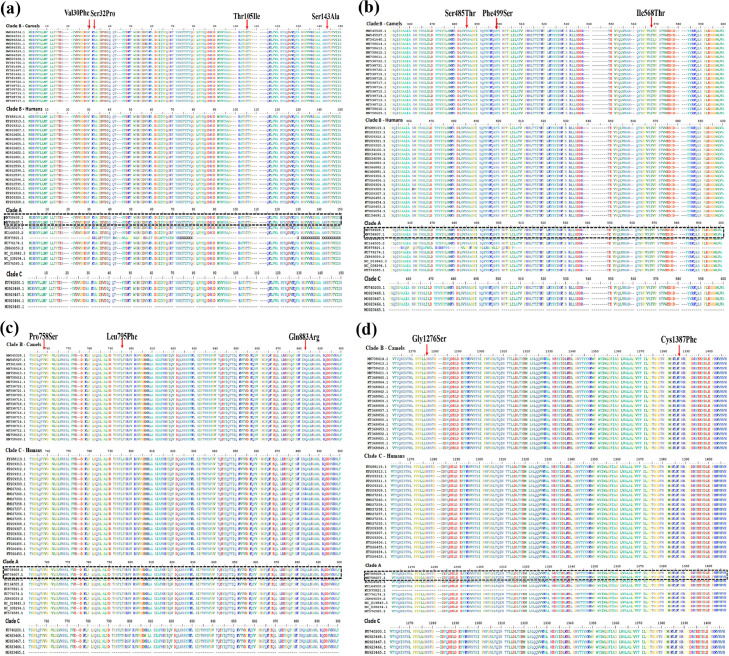
Three distinct mutations in the S1-NTD Val30Phe (n = 26, 29.2%), Ser32Pro (n = 44, 49.4%), Thr105Ile (n = 19, 21.3%) were observed only in the camel population. The mutation Ser143Ala (n = 12) is specific for humans (Table 1; Supplementary Table 2; Figure 4 a). Of those observed in camels, NTD Val30Phe and Ser32Pro are characteristic of clade B (lineages 2,4,6,7) and were documented only in UAE 2014-2015, whereas Thr105Ile is characteristic of clade B (lineage 5) and were only detected in Jordan in 2016. Another nonsynonymous mutation in the NTD Val30Ala (n = 5) is characteristic of clade C, which has been observed in camels from Egypt and Ethiopia. The Ser143Ala observed only in humans are distinct for clade B (Lineage 4) and were documented only in Saudi Arabia in 2017.
Figure 4.
Amino acids alignment view of (a) the S1-NTD region, (b) the S1-CTD region, (c) the S2-loop region and (d) the S2-HR2-CP region of the S-gene showing the amino acid substitutions.
CP, cytoplasmic; CTD, C-terminal domain; HR, heptad repeat; NTD, N-terminal domain.
Three distinct mutations in the S1-CTD Ser485Thr (n = 53) is predominantly found in camels, Phe499Ser (n = 22) is predominantly found in humans, and Ile568Thr (n = 17) is found in humans and camels (Table 1; Figure 4b). Ser485Thr observed in camels is characteristic of clade B (lineages 4, 7) and was documented only in UAE 2015. Phe499Ser is characteristic of clade B (lineages 4, 5) was observed only in Saudi Arabia (2015-2017). The Ile568Thr is characteristic of clade B in humans (lineages 4, 5) and clade A in camels (2017). The Ile568Thr was documented in humans in South Korea (2015) and later in Saudi Arabia in humans and camels (2017) (Table 1, Figure 4b).
The mutations Ser143Ala, Phe499Ser, Leu795Phe, and Gln883Arg were only identified in MERS-CoV from humans (mainly from Saudi Arabia). Ile568Thr was identified in both MERS-CoV from humans Korea (n = 16) and Saudi Arabia (n = 1) and in MERS-CoV from camels Saudi Arabia (n = 1). The rest of the mutations were detected only in MERS-CoV from dromedary camels.
A study from Kenya [17] showed a homogeneous pool of unique mutations, namely Ap177Tyr, Ser32Pro, His215Tyr, Ser416Phe, Leu476Phe, Pro673Arg, Gln1070Arg, and Ala1218Ser that were only identified in Kenya and one sequence from Ethiopia. They were lacking the other dromedary-specific mutations from the Arabian Peninsula, namely Val30Phe, Ser32Pro, Thr105Ile, Ser143Ala, Ser485Thr, Phe499Ser, Ile568Thr, Pro758Ser, Leu795Phe, Gln883Arg, Gly1276Ser, and Cys1387Phe nor the human mutations identified elsewhere.
Mutations in the S2-loop and -heptad repeat 2
Five variants known so far in the S2 subunit (Figure 4c, d) are Pro758Ser (n = 50), Leu795Phe (n = 20), Gln883Arg (n = 14), Gly1276Ser (n = 23), and Cys1387Phe (n = 18), and all these variants belong to clade B. Pro758Ser, Leu795Phe, and Gln883Arg belong to the S2 loop, whereas Gly1276Ser and Cys1387Phe belong to the S2-HR2 and CP regions, respectively. Pro758Ser is only observed in camels (lineages 5, 6, and 7) and is representative of the UAE cluster (2014-2015), whereas the Leu795Phe observed in humans (lineage 4) is representative of the Saudi Arabia cluster (2017-2018). The Gln883Arg is observed in humans (lineage 4) and the sequence variants are representative of Saudi Arabia, the UAE, the United States, and Jordan (2014-2015). The S2-HR2 variant Gly1276Ser is observed in camels (lineages 3, 4) in Saudi Arabia (2014-2015). The S2-CP variant Cys1387Phe is distinctive in camels (lineage 5) in Saudi Arabia (2014-2015).
MERS-CoV Amino acids substitutions in camels in Kenya
The Val30Ala in the S1-NTD region is the only variant similar to the clade A camels of Egypt and Ethiopia. There are eight additional variants unique to the camels documented in Kenya in 2019: two variants in the S1-NTD (Asp177Tyr, His215Tyr), four from the S1 CTD region (Ser416Phe, Leu476Phe, Ala649Val, Pro673Arg), one from the S2 HR2 (Cln1070Arg), and r one from the loop region (Ala1218Se).
Discussion
The past 3 years of the COVID-19 pandemic have distracted global scientific and political attention from other infectious pathogens with epidemic potential. MERS-CoV remains on the WHO blueprint priority pathogens list for research and development [5,6], although no effective human or camel MERS vaccines have yet been developed. Whilst there have been a plethora of genomic studies on SARS-CoV-2 [8,9,25], there have been comparatively few reports of MERS-CoV genomic analyses from humans and camels ever since the first discovery of MERS-CoV in 2012 as a lethal human zoonosis [3,[5], [6], [7],[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24],[26], [27], [28]]. The emergence over time of several SARS-CoV-2 VOCs [9] with increased transmissibility have emphasized the importance of continued real-time surveillance and genomic analyses to detect the VOCs associated with epidemic potential. This will also allow effect of new dominant VOCs on effectiveness of COVID-19 vaccine effectiveness over time. We discuss findings and implications from our 10-year global analysis of human and camel MERS-CoV infections, epidemiology, genomic sequences, clades, lineages, and geographical origins for the period January 1, 2012 to August 3, 2022.
MERS-CoV epidemiology
MERS-CoV was first reported in Saudi Arabia in April 2012 [3,4]. As of February 1, 2023, a total of 2604 laboratory-confirmed cases of MERS have been reported worldwide in 27 countries, with 936 associated deaths (CFR of 35%) [5]. This represents 13 additional human MERS cases since August 3, 2022, the end of our study period. The majority of human MERS cases have been reported from the Middle East, particularly Saudi Arabia, reporting 2184 cases and 813 related deaths (CFR: 37.2%). In Europe, eight countries have reported confirmed MERS cases, all with direct or indirect connections to the Middle East [5,6,13,26]. The epidemic potential of MERS-CoV was illustrated by the largest outbreak outside the Arabian Peninsula, which occurred in South Korea in May 2015, arising from a returning traveler, resulting in 186 cases, including 38 deaths [11,12]. A total of 83% of transmission events were due to five superspreaders, and 44% of the 186 MERS cases were the patients who had been exposed to nosocomial transmission at 16 hospitals [11], [12], [13]. The South Korean government had quarantined 16,993 individuals for 14 days to control the outbreak.
Although there were numerous clinical reports on human MERS cases and human-to-human transmission before 2015 [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13],26], reports of new MERS cases to the WHO appear to be declining. Figure 1 shows the number of cases of laboratory-confirmed human MERS reported from April 2012 to August 2022. This may be due to the result of enhanced surveillance, heightened public, and camel owner awareness of MERS-CoV; strengthened capacities to detect cases early and contain hospital outbreaks; improved infection prevention and control measures (re-enforced during the COVID-19 pandemic); reducing human-to-human and camel-to-human transmission; more comprehensive investigations of cases at the time of detection; and effective communication about MERS to camel owners and public. Despite the reducing numbers of cases, it is important to note that human MERS cases continue to be reported sporadically, a decade after its first discovery as a new lethal human pathogen. Between January 1, 2022 and August 3, 2022, three MERS-CoV cases from Qatar, including one death, and one from Oman have been reported to the WHO [5,6]. All four cases were primary cases, having reported contact with camels. MERS-CoV also continues to circulate in camels in the Arabian Peninsula and Africa [20], [21], [22], [23], [24].
MERS-CoV in camels
Epidemiological and virological studies indicate that dromedary camels are the most likely source of human MERS-CoV infections [5,7,19,21,24]. The ancestral origin of MERS-CoV remains to be defined. In an analysis of different virus genomes, it is believed that MERS-CoV may have originated in bats and later transmitted to camels at some point over the centuries. However, the length of time MERS-CoV has been enzootic in dromedaries remains to be determined. MERS-CoV is sporadically transmitted from camels to humans. MERS-CoV has been identified in dromedary camels in several countries in the Middle East, Africa, and South Asia. The exact source and mechanism of primary MERS-CoV transmission to humans remain unknown. Dromedaries harbor several MERS-CoV lineages, which may serve as a reservoir [14] from which humans sporadically become infected through spillover zoonotic transmission [5,6,26]. The secondary and tertiary human-to-human transmission of MERS-CoV is well established, particularly during outbreaks within health care and household settings [5,[11], [12], [13]].
MERS-CoV genomic diversity
Genome analysis is useful for understanding the recombinant events during outbreaks caused by one or more viral lineages. Some of these recombination events could lead to new viral lineages that have fitness advantages in terms of transmission patterns, replicative fitness, and infectivity. Epidemiological fitness is the ability of a virus to sustain itself in the environment, be it a serotype, a clade, a lineage, or a variant. We were able to extract 501 Surface gene (S-gene) sequences (around 4061 bp) from 728 deposited MERS-CoV sequences. The analyses of the MERS-CoV sequences by us and others have identified three major MERS-CoV clades: clades A, B, and C [14,15,[17], [18], [19], [20], [21], [22], [23], [24]]. The MERS-CoVs from the Arabian Peninsula are classified as either clade A or clade B, whereas all MERS-CoVs from African dromedary camels are classified as clade C viruses. In the Arabian Peninsula, there are six phylogenetic lineages of clade B strains circulating in both humans and dromedary camels. Lineage 5, also termed novel recombinant clade novel recombinant clade for the novel recombinant due to the presumed recombination between lineage 3 and 4, resulted in the formation of a circulating recombinant lineage clade during or before the year 2014 in dromedary camels. Since 2015, lineage 5 seems to have replaced all other MERS-CoV strains detected before 2015. Although MERS-CoV exhibits genomic diversity depending on the geographical region, all known MERS-CoVs are >99% identical at the nucleotide level [22]. Lineages are temporal relationships between ancestors and descendants, whereas clades are mono-, poly-, or para-phyletic groups of representatives of lineages that exist at a given time. Camels harbor several lineages, which likely serve as reservoirs and intermediate hosts for the transmission of the virus to humans [21], [22], [23], [24].
MERS-CoV co-infections and recombination events
With respect to MERS-CoV genomic architecture, the frequently observed break points occur at the junction between the ORF1b/S-gene. As observed in the SARS-CoV-2 genomes, in MERS-CoV, the substitutions in the S-gene were the major drivers of the fitness advantage [24]. Recombination events occur in the evolution and transmission of CoVs, including MERS-CoV, so positive selection sites in the spike protein of MERS-CoV in camels may have enabled spillover infection of humans [10,17,20]. For instance, MERS-CoV lineage 5 is reported to have evolved as a result of the recombination of lineages 3 and 4, with the genomic regions (ORF1ab and S-gene) originating from lineage 4 and the rest of the genome sequence representing lineage 3 [23]. The recombination rates in the MERS-CoV genome indicate a large number of MERS-CoV co-infections in some hosts. The S-gene appears to have had a strong positive selection when MERS-CoV was transmitted from its natural host (camels) to humans because six of the nine positive selection sites discovered in the spike protein are in its receptor-binding domain (Ser485Thr, Ser416Phe, Leu476Phe, Phe499Ser, Ile568Thr, Ser416Phe, Leu476Phe, Pro673Arg), that is in direct contact with host cells [25].
MERS-CoV Clades and lineages
Of three polyphyletic MERS-CoV clades, clades A and C are extinct and are now considered noncirculating strains [17,22,26]. Clade B has evolved further, with multiple viral lineages found in humans and dromedary camels. In 2016, clade B was described with five lineages [23] and in 2017, with seven lineages [22]. Lineage 1 was described in 2013 in Saudi Arabian and Qatari patients [27]. Lineage 2 represents the cluster of cases reported during the 2014 outbreak in the UAE [27], whereas lineage 3 has been found both in humans and camels throughout the Middle East, switching back and forth [27]. Similarly, lineage 4 represents a case cluster from the Hajj pilgrimage (the outbreak in Jeddah) in spring 2014 and was subsequently observed in Riyadh, Al-Kharj, and Madinah [19]. Interestingly, MERS-CoV lineage 5 evolved as a result of recombination of lineages 3 and 4 and was the source of major outbreaks in 2016 not only in Saudi Arabia but also to a greater extent in South Korea [23]. Lineage 5 was described to have higher replicative fitness and be more resistant to interferon pretreatment than others [28]. Lineage 6 is described as unique to the camel populations and has not yet been described in humans [22]. Lineage 7 has also been described mainly in dromedary camels; however, in 2015, a patient was reported from Germany who carried lineage 7 and had acquired the disease in the UAE [22].
In-depth genomic analyses of MERS-CoV over time is necessary for understanding MERS-CoV evolution and spread in dromedaries and the adaptation, evolution, and spread during the spillover into humans with associated outbreaks. MERS-CoV has been shown to be closely related to the bat beta CoVs HKU4 and HKU5 [10]. Genomic studies of MERS-CoV from dromedary camels have been restricted in geographical coverage, and most reports are from the Arabian Peninsula or Africa [5,10,14,15,[17], [18], [19],21,22,29]. The phylogeographical analyses suggest that the current distribution of MERS-CoV clades in camels is not predominantly shaped by the transregional exchange. It appears that despite the steady import of camels from Africa infected with MERS-CoV clade C viruses, the African lineages do not appear to have established themselves in Saudi Arabian camels. An analysis of 238 MERS-CoV full genomes from dromedary camel MERS-CoV isolates from within Saudi Arabia and those from camels imported from Africa [15,20,21,26] showed that the Saudi Arabian MERS-CoV strains were phylogenetically distant from the African MERS-CoV clades. Thus, the Saudi Arabian camel MERS-CoV strains appear to maintain endemic status without the introduction of additional lineages. Introducing the MERS-CoV clade B strains to Africa through infected camels must be avoided because these strains might outcompete the African MERS-CoV clade C strains and pose a greater zoonotic and pandemic threat in Africa.
The MERS-CoV recombination rates indicate co-infections with different MERS-CoV lineages and adaptive potential in camels. Major knowledge gaps [30] exist on MERS-CoV epidemiology, prevalence, transmission dynamics, exact source, and mode(s) of transmission. More comprehensive controlled serological and genotypic cohort, cross-sectional, and follow-up studies of MERS-CoV in camels and exposed human populations may provide clues as to whether MERS-CoV subclinical or asymptomatic infections are ongoing locally or in other geographical regions. Future studies to understand the transmission dynamics, genomic evolution, and disease causation in humans will require global collaborative efforts to enable geographically wider and larger sampling studies. As with SARS-CoV [25], proactive, continuous surveillance and genomic analyses of MERS-CoV infections, including VOCs in camels and humans in geographically diverse regions, remain important to identify any changes in endemic and epidemic transmission potential.
Our study focused on the S region of the viral genome. Other genomic regions need to be investigated. ORF 4 is reported to have several deletion patterns in dromedaries from Africa. These are not found in dromedaries from the Arabian Peninsula. The role of these deletions on transmissibility needs to be further investigated to explain the lack of outbreaks in humans in Africa, despite the widespread presence of MERS-CoV in dromedary camels.
Conclusion
MERS-CoV remains a threat to global health security. Although the numbers of human MERS cases have declined over the past 5 years, new MERS-CoV variants continue to circulate in camels and humans. The recombination rates indicate co-infections with different MERS-CoV lineages and adaptive potential in camels. Proactive surveillance of MERS-CoV infections and VOCs in camels and humans worldwide, and development of a human MERS vaccine is essential for epidemic preparedness. MERS-CoV remains a WHO priority pathogen and further research and investments are required to understand the MERS-CoV evolution and circulation in dromedary camels and limit spillover infections to humans. Heightened vigilance and surveillance should be in place in lieu of the huge camel MERS-CoV reservoir.
Funding
This research work was funded by the Institutional Fund Projects under grant No. (IFPIP-021-140-2020). The authors gratefully acknowledge the technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Ethical approval
Because this was an analysis of existing data no ethical approval was required
Author contributions
AZ and EIA ideated and coordinated the study, and developed the first draft of the manuscript. TPV and IR performed the MERS-CoV genomic analysis. All authors contributed to developing the study protocol, interpreting data, developing figures/tables and writing the discussion.
Declaration of competing interests
The authors have no competing interests to declare.
Acknowledgments
Sir Zumla (AZ) is co-director and Prof Velavan (TPV) is co-investigator of the Pan African Network for Rapid Research, Response, and Preparedness for Infectious Diseases Epidemics Consortium (PANDORA-ID-NET), funded by the European and Developing Countries Clinical Trials Partnership the EU Horizon 2020 Framework Programme (EDCTP-RIA2016E-1609). They also acknowledge support from EDCTP-Central Africa and East African Clinical Research Networks (CANTAM-3, EACCR-3). TPV and IR acknowledge the support of PAN-ASEAN Coalition for Epidemic and Outbreak Preparedness (PACE-UP; DAAD, Project ID: 57592343). Sir Zumla is an NIHR Senior Investigator, a Mahathir Science Award, Sir Patrick Manson Medal and EU-EDCTP Pascoal Mocumbi Prize laureate.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.03.046.
Appendix. Supplementary materials
References
- 1.World Health Organization . 2012. SARS (severe acute respiratory syndrome)https://www.who.int/ith/diseases/sars/en/ [accessed 02 September 2022] [Google Scholar]
- 2.Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 3.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.Memish ZA, Perlman S, Van Kerkhove MD, Zumla A. Middle East respiratory syndrome. Lancet. 2020;395:1063–1077. doi: 10.1016/S0140-6736(19)33221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . 2022. Middle East respiratory syndrome coronavirus (MERS-CoV)https://www.who.int/health-topics/middle-east-respiratory-syndrome-coronavirus-mers#tab=tab_1 [accessed 12 February 2023] [Google Scholar]
- 6.World Health Organization . 2023. Middle East Respiratory Syndrome- MERS situation 2023 update.https://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html [accessed 17 March 2023] [Google Scholar]
- 7.Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 8.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . 2022. Tracking SARS-CoV-2 variants 2021.https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ [accessed 04 September 2022] [PubMed] [Google Scholar]
- 10.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh MD, Park WB, Park SW, Choe PG, Bang JH, Song KH, et al. Middle East respiratory syndrome: what we learned from the 2015 outbreak in the Republic of Korea. Korean J Intern Med. 2018;33:233–246. doi: 10.3904/kjim.2018.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YJ, Cho YJ, Kim DW, Yang JS, Kim H, Park S, et al. Complete genome sequence of Middle East respiratory syndrome coronavirus KOR/KNIH/002_05_2015, isolated in South Korea. Genome Announc. 2015;3 doi: 10.1128/genomeA.00787-15. e00787–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui DS, Azhar EI, Kim YJ, Memish ZA, Oh MD, Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18:e217–e227. doi: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holloway P, Gibson M, van Doremalen N, Nash S, Holloway T, Letko M, et al. Risk factors for Middle East respiratory syndrome coronavirus infection among camel populations, Southern Jordan, 2014–2018. Emerg Infect Dis. 2021;27:2301–2311. doi: 10.3201/eid2709.203508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Z, Hui KPY, So RTY, Lv H, Perera RAPM, Chu DKW, et al. Phenotypic and genetic characterization of MERS coronaviruses from Africa to understand their zoonotic potential. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2103984118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park YJ, Walls AC, Wang Z, Sauer MM, Li W, Tortorici MA, et al. Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors. Nat Struct Mol Biol. 2019;26:1151–1157. doi: 10.1038/s41594-019-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngere I, Hunsperger EA, Tong S, Oyugi J, Jaoko W, Harcourt JL, et al. Outbreak of Middle East respiratory syndrome coronavirus in camels and probable spillover infection to humans in Kenya. Viruses. 2022;14:1743. doi: 10.3390/v14081743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifert SN, Schulz JE, Ricklefs S, Letko M, Yabba E, Hijazeen ZS, et al. Limited genetic diversity detected in Middle East respiratory syndrome-related coronavirus variants circulating in dromedary camels in Jordan. Viruses. 2021;13:592. doi: 10.3390/v13040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drosten C, Muth D, Corman VM, Hussain R, Al Masri M, HajOmar W, et al. An observational, laboratory-based study of outbreaks of middle East respiratory syndrome coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia, 2014. Clin Infect Dis. 2015;60:369–377. doi: 10.1093/cid/ciu812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu DKW, Hui KPY, Perera RAPM, Miguel E, Niemeyer D, Zhao J, et al. MERS coronaviruses from camels in Africa exhibit region-dependent genetic diversity. Proc Natl Acad Sci U S A. 2018;115:3144–3149. doi: 10.1073/pnas.1718769115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Kafrawy SA, Corman VM, Tolah AM, Al Masaudi SB, Hassan AM, Müller MA, et al. Enzootic patterns of Middle East respiratory syndrome coronavirus in imported African and local Arabian dromedary camels: a prospective genomic study. Lancet Planet Health. 2019;3:e521–e528. doi: 10.1016/S2542-5196(19)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusof MF, Queen K, Eltahir YM, Paden CR, Al Hammadi ZMAH, Tao Y, et al. Diversity of Middle East respiratory syndrome coronaviruses in 109 dromedary camels based on full-genome sequencing, Abu Dhabi, United Arab Emirates. Emerg Microbes Infect. 2017;6:e101. doi: 10.1038/emi.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabir JS, Lam TT, Ahmed MM, Li L, Shen Y, Abo-Aba SE, et al. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science. 2016;351:81–84. doi: 10.1126/science.aac8608. [DOI] [PubMed] [Google Scholar]
- 24.Ulrich L, Halwe NJ, Taddeo A, Ebert N, Schön J, Devisme C, et al. Enhanced fitness of SARS-CoV-2 variant of concern alpha but not Beta. Nature. 2022;602:307–313. doi: 10.1038/s41586-021-04342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Shen L, Gu X. Evolutionary dynamics of MERS-CoV: potential recombination, positive selection and transmission. Sci Rep. 2016;6:25049. doi: 10.1038/srep25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reusken CB, Messadi L, Feyisa A, Ularamu H, Godeke GJ, Danmarwa A, et al. Geographic distribution of MERS coronavirus among dromedary camels. Africa. Emerg Infect Dis. 2014;20:1370–1374. doi: 10.3201/eid2008.140590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotten M, Watson SJ, Zumla AI, Makhdoom HQ, Palser AL, Ong SH, et al. Spread, circulation, and evolution of the Middle East respiratory syndrome coronavirus. mBio. 2014;5 doi: 10.1128/mBio.01062-13. e01062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroeder S, Mache C, Kleine-Weber H, Corman VM, Muth D, Richter A, et al. Functional comparison of MERS-coronavirus lineages reveals increased replicative fitness of the recombinant lineage 5. Nat Commun. 2021;12:5324. doi: 10.1038/s41467-021-25519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azhar EI, Hashem AM, El-Kafrawy SA, Sohrab SS, Aburizaiza AS, Farraj SA, et al. Detection of the Middle East respiratory syndrome coronavirus genome in an air sample originating from a camel barn owned by an infected patient. mBio. 2014;5 doi: 10.1128/mBio.01450-14. e01450–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peiris M, Perlman S. Unresolved questions in the zoonotic transmission of MERS. Curr Opin Virol. 2022;52:258–264. doi: 10.1016/j.coviro.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.