Abstract
Purpose
Cardiac rehabilitation (CR) improves clinical outcomes in patients with cardiovascular disease (CDV). Patients with CVD often have multiple comorbidities, including obstructive sleep apnea (OSA), potentially affecting their ability to participate and achieve functional improvement during CR. We aimed to test the hypothesis that OSA reduces peak exercise capacity (EC) in patients undergoing CR and to explore if OSA treatment modifies this relationship.
Methods
Data from a retrospective cohort of CR patients was analyzed. OSA was defined as a respiratory event index > 5/h or physician diagnosis. Patients with OSA were considered “treated” if using continuous positive airway pressure regularly during the CR period. Change in METs was the primary study outcome.
Results
Among 312 CR patients, median age of 67 years, 103 (33%) had known OSA (30 treated, 73 untreated). Patients with OSA vs. those with no OSA were more likely to be obese and male; otherwise, groups were similar. Compared with the no OSA group, patients with OSA had lower pre-CR METs (3.3 [2.9–4.5] vs. 3.9 [3.1–5], P = .01) and lower post-CR METs (5.3 [4–7] vs. 6 [4.6–7.6], P = .04), but achieved a similar increase in METs post-CR (1.8 [0.6–2.6] vs. 2.0 [0.9–3], P = .22). Furthermore, compared to no OSA, pre-CR and post-CR METs tended to be similar in patients with treated OSA, but lower in untreated patients, with similar increases in METs across all groups, even when adjusting for covariates via multivariable regression.
Conclusion
OSA is prevalent in patients with CVD undergoing CR. CR substantially improves exercise capacity independent of OSA status, but screening for—and treatment of—OSA may improve the absolute exercise capacity achieved through CR.
Keywords: Obstructive sleep apnea, Cardiac rehabilitation, Exercise capacity
Introduction
Obstructive sleep apnea (OSA) is intricately linked with cardiovascular disease (CVD) outcomes [1–5]. Previous studies have shown that OSA impacts important cardiovascular endpoints such as blood pressure, left ventricular ejection fraction, vascular parameters, and risk of arrhythmias as well as increases the risk of cardiovascular events [6–9]. Although prospective observational cohort studies have shown an increased incidence of fatal and non-fatal cardiovascular events with untreated OSA [6], definitive data from randomized controlled trials showing the benefit of OSA therapy for hard cardiovascular outcomes are currently lacking [10]. On the other hand, treatment of OSA has been shown to improve daytime sleepiness and function which may be important determinants of how fully patients engage in medical interventions such as cardiac rehabilitation (CR) programs [11, 12]. Importantly, CR improves hard clinical outcomes such as hospital readmissions, quality of life, and mortality in patients with CVD and is thus a class I recommendation [13–16]. However, despite literature implicating OSA as a modulator of CVD risk and data linking CR to improved CVD outcomes, there remains a lack of rigorous studies regarding the impact of OSA on CR outcomes. We, and others, have observed that OSA is common in patients with CVD [17–20]. OSA has been associated with impaired exercise capacity (EC) in some, but not all, studies [21–24]. However, the mechanism underlying exercise impairment in OSA patients remains unclear, although endothelial dysfunction and deconditioning have been suggested. Thus, it remains unclear whether a diagnosis of OSA affects CR outcomes or whether treatment of OSA (e.g., continuous positive airway pressure [CPAP] which is the standard of care and most used treatment for OSA) influences CR outcomes. At least in theory, if OSA adversely affects CR outcomes, then treatment of OSA would be predicted to be beneficial from the standpoint of improved exercise capacity which could translate into improved outcomes following CR. This finding would also imply that patients planning to participate in CR would benefit from assessment of OSA status (sleep study or questionnaires [25]) prior to initiation of the CR program. Based on this framework, our objective was to test the hypothesis that OSA reduces exercise capacity in CVD patients undergoing CR and to explore if OSA treatment modifies this relationship.
Methods
We performed a retrospective chart review on a cohort of 312 consecutive adult patients with CVD who underwent CR at UCSD between 1/1/2018 and 3/15/2019 (IRB #190,538). Although some of our participants were involved in other as-yet unpublished analyses, none of the present findings has been previously published.
Patients were eligible for CR due to at least one of the following: acute myocardial infarction within the last twelve months, stable angina, coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), heart valve repair or replacement, stable chronic systolic heart failure, heart transplant, and peripheral artery disease. Patients went through either standard CR (SCR) or the Ornish intensive CR (ICR) program [1, 26]. The details about both CR programs were presented previously [1]. Briefly, the ICR program (specifically, the Ornish program, Sharecare, Inc., Atlanta, GA, USA) included a structured class model twice a week (4 h) over nine weeks (18 sessions, 72 h in total) comprising supervised exercise training, a specialized plant-based diet, education on nutrition and a healthy lifestyle, stress management, and social support. Nutritional counseling about a specific plant-based diet along with written instructional materials was provided to patients. A whole food, low-fat, low in refined carbohydrates, nutritionally adequate, plant-based diet (consisting of fruits, vegetables, whole grains, legumes, and soy products) without caloric restriction was recommended. Stress management included stretching exercises, breathing techniques, meditation, progressive relaxation, and imagery. Social support was provided through regular group meetings led by a clinical psychologist. The SCR program involved exercise and educational sessions on nutrition and healthy lifestyle (1 h) 3 days per week over 12 weeks (36 sessions, 36 h in total). Healthy food choices were recommended, such as saturated fatty acids < 10% of total energy intake, 2–3 servings of fruit/day, 2–3 servings of vegetables/day, fish 1–2 times/week, < 5 g of salt/ day, 30–45 g of fiber/day (preferably from wholegrain products), and limited sugar-sweetened soft drinks and alcoholic beverages. The structured and supervised exercise included regular, moderate aerobic, and resistance/strength training. The exercise component was the same in both CR programs. Patients were individually prescribed exercise levels (typically walking) according to baseline exercise treadmill testing (ETT) results. The target training heart rate (HR) was 50–70% of the HR, at which symptoms and/or ECG changes occurred or 55–70% of predicted age-adjusted maximum HR based on patient conditioning level. Three of the 312 patients participated in CR twice, but we included only data from their first encounter in the analyses (i.e., 312 unique CR encounters). There were no other exclusion criteria.
Charts were manually reviewed, and data were collected on baseline demographics, comorbidities, and medications. In addition, characteristics related to CR were collected including the diagnosis that prompted a referral to CR, participation in SCR versus ICR, duration of CR (days), CR adherence (% of all attended CR sessions), and smoking status/alcohol use at the time of CR. For patients classified as having OSA, adherence/ treatment status was assessed by obtaining adherence data remotely through online cloud-based databases during the dates of the patient’s CR dates. If no data were found, physicians’ notes indicating treatment were used if available. The peak exercise capacity (EC) was quantified as metabolic equivalents (METS) during exercise treadmill testing (ETT) that was performed according to the standard Bruce protocol. Patients exercised as long as possible, depending on the conditioning level, to achieve at least 70–85% of the predicted age-adjusted maximum HR or until symptoms (such as dyspnea, fatigue, and chest pain), 1 mm ST depression in ECG, abnormal BP response, or ventricular ectopy occurred. The maximum METs value was calculated using a standard formula, and the person’s performance on a treadmill as the workload was increased.
OSA was defined as a respiratory event index (REI) > 5/h on in-laboratory polysomnography, home sleep study, or a physician diagnosis of OSA. OSA patients were considered “treated” if using continuous positive airway pressure (CPAP) > 4 h/night on > 70% of nights during the CR period, or if physician notes indicated treatment adherence and objective CPAP data were unavailable [27]. METs were assessed on treadmill exercise testing to peak exercise (70–85% of their age-adjusted maximum heart rate based on level of conditioning or until symptoms), which is consistent with standardized measurement methods in other cardiac rehabilitation studies [28]. Changes in METs were calculated by subtracting the METs documented on the first day of CR (“pre-CR”) from the METs documented on the last day of CR (“post-CR”).
Categorical data were summarized as percent (number) and compared using Fisher’s exact tests. Continuous data were summarized using the median (interquartile range [IQR]); for univariable analyses, we used Wilcoxon rank-sum tests for comparisons by OSA status and Kruskal–Wallis tests (followed by pairwise Wilcoxon rank-sum tests with p-values adjusted based on the method by Hochberg and Benjamini [29]) when making comparisons across OSA treatment groups. Given a large number of candidate covariates, we used multivariable regression with backward selection (based on Bayes’ information criterion) to adjust for major covariates. Pre/post-CR METs were log-transformed for multivariable analyses to increase normality. Candidate variables for all regression models included age, sex, body mass index (BMI), race, self-reported Hispanic status, underlying cardiac condition, smoking, alcohol use, asthma, and COPD. For post-CR METs and changes in METs, we further considered CR adherence, CR duration, and participation in the Ornish vs. standard program. All analyses were performed in R (3.6.1; R Foundation, Vienna, Austria), using P-values < 0.05 to judge statistical significance.
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request (Table 1).
Table 1.
General characteristics
| OSA (N = 103) | n | No OSA (N = 209) | n | P | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Median % | [IQR] (n) | Median % | [IQR] (n) | ||||
| General characteristics Age, years | 68 | [61 to 75] | 66 | [57 to 73] | .03 | ||
| Female sex | 17% | (17) | 34% | (71) | .001 | ||
| Body mass index, kg/m2 | 28.5 | [26 to 35] | 26.1 | [24 to 29] | < .001 | ||
| Ornish program, yes | 31% | (32) | 33% | (68) | .9 | ||
| Race | .5 | ||||||
| White | 78% | (80) | 71% | (148) | |||
| Black | 6% | (6) | 5% | (10) | |||
| Asian | 7% | (7) | 10% | (21) | |||
| Other | 10% | (10) | 14% | (30) | |||
| Hispanic | 10% | (3) | 10% | (21) | .4 | ||
| Reason for rehab | |||||||
| Stable angina pectoris | 18% | (19) | 8% | (16) | 0.007 | ||
| CABG | 14% | (14) | 14% | (30) | > .9 | ||
| PCI | 24% | (25) | 39% | (81) | .01 | ||
| Valvular surgery | 10% | (10) | 13% | (27) | .5 | ||
| Heart transplant/LVAD | 5% | (5) | 3% | (7) | .5 | ||
| Acute MI (past 12 months) | 14% | (14) | 14% | (30) | > .9 | ||
| Stable HFrEF | 25% | (26) | 16% | (34) | .07 | ||
| PAD | 3% | (3) | 1% | (3) | .4 | ||
| Days of CR | 84 | [58 to 119] | 91 | [58 to 126] | .6 | ||
| CR adherence, %sessions | 100 | [50 to 100] | 100 | [42 to 100] | .8 | ||
| Smoking status | .3 | ||||||
| Current | 2% | (2) | 1% | (3) | |||
| Former | 43% | (44) | 35% | (73) | |||
| Never/unclear | 55% | (57) | 64% | (133) | |||
| Alcohol status | .5 | ||||||
| Current | 47% | (48) | 41% | (86) | |||
| Former | 9% | (9) | 8% | (16) | |||
| Never/unclear | 45% | (46) | 51% | (107) | |||
| Asthma | 14% | (14) | 10% | (21) | .3 | ||
| COPD | 17% | (17) | 10% | (21) | .14 | ||
| Beta-blocker use | 75% | (77) | 70% | (147) | .5 | ||
| ACE-inhibitor use | 35% | (36) | 27% | (57) | .2 | ||
| MRA use | 25% | (26) | 21% | (44) | .5 | ||
| Sleep apnea severity REI, h−1 | 22 | [14 to 44] | 82 | 2.2 | [1 to 3.9] | 14 | < .001 |
| OAI, h−1 | 2 | [1 to 7] | 75 | 0.2 | [0 to 1.5] | 14 | < .001 |
| CAI, h−1 | 1 | [0 to 5] | 74 | 0 | [0 to 0.3] | 14 | < .001 |
| SpO2 nadir, % | 82 | [75 to 85] | 79 | 87 | [83 to 91] | 14 | < .001 |
| Time with SpO2 < 88%, %MT | 2 | [0 to 7] | 73 | 0 | [0 to 0] | 15 | < .001 |
| Mean CPAP usage, min | 328 | (223 to 414) | 22 | na | |||
| Residual REI on CPAP | 2.8 | [1.4 to 4.6] | 22 | na | |||
ACE, angiotensin-converting enzyme; REI, respiratory event index; CABG, coronary artery bypass graft; CAI/OAI, central/obstructive apnea index; HFrEF, heart failure with reduced ejection fraction; IQR, interquartile range; LVAD, left ventricular assist device; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; %MT, percent of monitoring time; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SD, standard deviation. Bolded entries are values meeting statistical significance
Results
Among 312 CR patients, 103 (33%; 95% confidence interval [CI]: 28 to 39) had known OSA (30 treated, 73 untreated). Of the 103 patients classified as OSA, the majority (82) had sleep studies available (either home sleep test or polysomnogram) and 21 had an OSA diagnosis based on physician notes. CR patients were mostly older (67 [59 to 73] years) men (72%). Patients with OSA were more likely to be male (83% vs. 66%, P = 0.001) and had a higher body mass index (BMI) (28.5 vs. 26.1 kg/m2, P < 0.001) than patients without OSA (no OSA); otherwise, groups were clinically similar (Table 2). Furthermore, in the subgroup of patients with OSA, those with treated OSA were more obese (BMI 32 kg/m2 vs. 27.6 kg/m2, P = 0.002) and had more severe OSA based on the available REI (43/h vs. 19/h, P = 0.002) than untreated OSA patients, but otherwise had similar baseline characteristics (Supplementary Table E1).
Table 2.
Differences in exercise performance based on OSA diagnosis
| Univariable analyses | Multivariable analyses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| OSA(N = 103) | No OSA(N = 209) | (OSA vs. no OSA) | (OSA vs. no OSA) | ||||||||
|
|
|
|
|
||||||||
| Median Mean | [IQR] (SD) | Median Mean | [IQR] (SD) | Δ Medians Δ Mean | P crude A | beta | SE | P adjusted | InterpretationB | ||
|
| |||||||||||
| Δ | (95% CI) | ||||||||||
| Pre-CR METs | 3.3 | [2.9 to 4.5] | 3.9 | [3.1 to 5] | − 0.6 | .01 | |||||
| Pre-CR METs | 3.8 | (1.32) | 4.2 | (1.52) | − 0.4 | .02 | |||||
| log(Pre-CR METs) | 1.27 | (0.33) | 1.37 | (0.33) | − 0.1 | .01 | − 0.12 | 0.04 | .003 | − 11.3% | (− 18 to − 3.9) C |
| Post-CR METs | 5.3 | [4 to 7] | 6 | [4.6 to 7.6] | − 0.7 | .04 | |||||
| Pre-CR METs | 5.7 | (2.37) | 6.3 | (2.46) | − 0.6 | .05 | |||||
| log(Post-CR METs) | 1.66 | (0.41) | 1.76 | (0.39) | − 0.1 | .04 | − 0.09 | 0.05 | .06 | − 8.7% | (− 17.1 to 0.4)D |
| Change in METs | 1.8 | [0.6 to 2.6] | 2 | [0.9 to 3] | − 0.2 | .22 | |||||
| Change in METs | 2.0 | (1.71) | 2.2 | (1.74) | − 0.2 | .38 | − 0.13 | 0.2 | .51 | − 0.13 | (− 0.52 to 0.26)E |
METs, metabolic equivalents; CR, cardiac rehabilitation; OSA, obstructive sleep apnea; IQR, interquartile range; CI, confidence interval; SD, standard deviation
Based on Wilcoxon rank-sum test or t-test
To promote normality, pre/post-CR METs were log-transformed for multivariable analyses; thus, the beta can be interpreted as a percentage difference based on the formula: [1-exp(beta)]*100; the change in METs did not require transformation; thus, the beta reflects the absolute difference between both groups after adjusting for covariates
Adjusted for sex
Adjusted for sex, body mass index, CR adherence (%sessions), Ornish program (yes/no)
Adjusted for baseline METs, CR adherence (%sessions) Bolded entries are values meeting statistical significance
Primary analyses: the effect of OSA diagnosis on exercise capacity
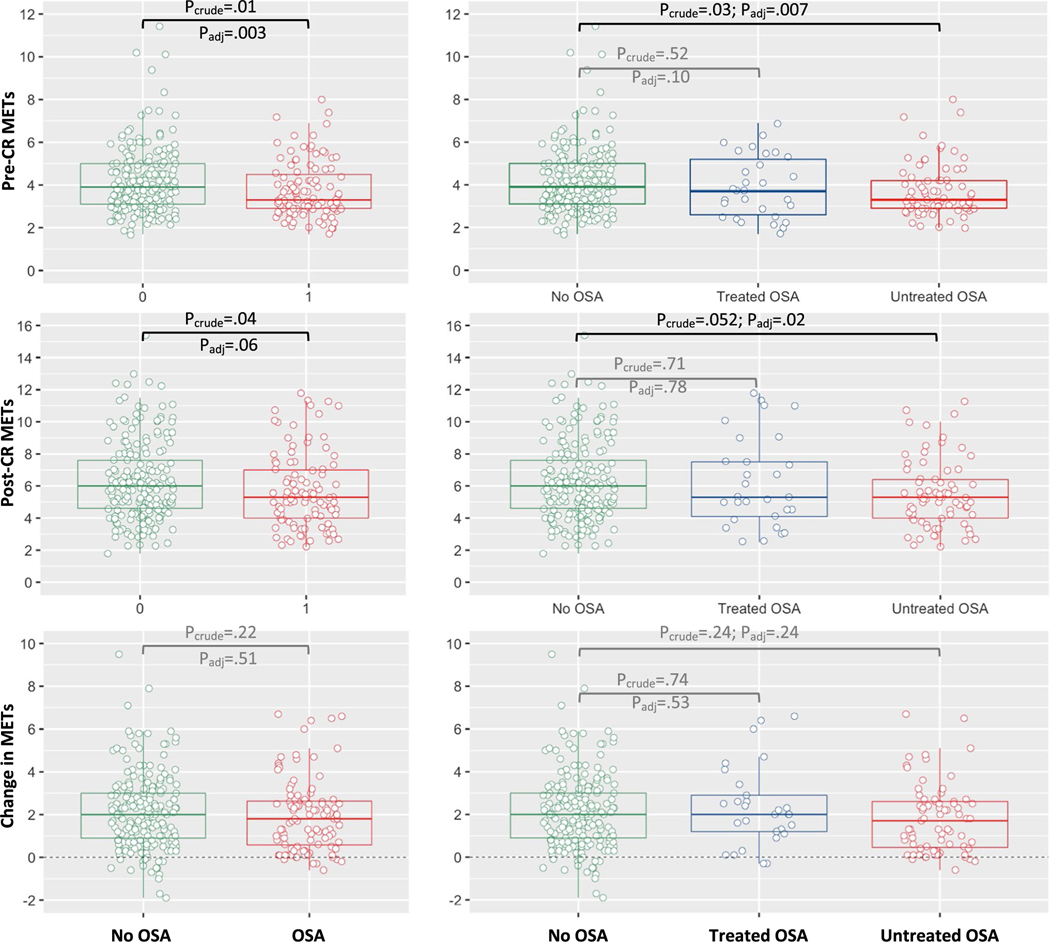
Compared with the no OSA group, OSA patients had lower median pre-CR (baseline) METs (3.3 vs. 3.9, P = 0.01) and lower post-CR METs (5.3 vs. 6, P = 0.04), but achieved a similar increase in METs during CR (1.8 vs. 2.0, Pbetween-group = 0.22; Pwithin-group < 0.001). In sensitivity analyses, results were similar when comparing (log-transformed) means using t-tests, suggesting the robustness of the results. Furthermore, results were also similar when adjusting for covariates: Compared to no OSA patients, OSA patients had 11.3% lower pre-CR METs (P = 0.003) and 8.7% lower post-CR METs (P = 0.06) which did not reach statistical significance, while the change in METs was similar in both groups (P = 0.51; for more details, see Table 2, Fig. 1).
Fig. 1.
Comparison of METs based on OSA diagnosis and treatment status. Compared to untreated OSA patients, patients with treated OSA had change in METs similar to patients without any OSA
Exploratory analyses: the effect of OSA treatment status on exercise capacity
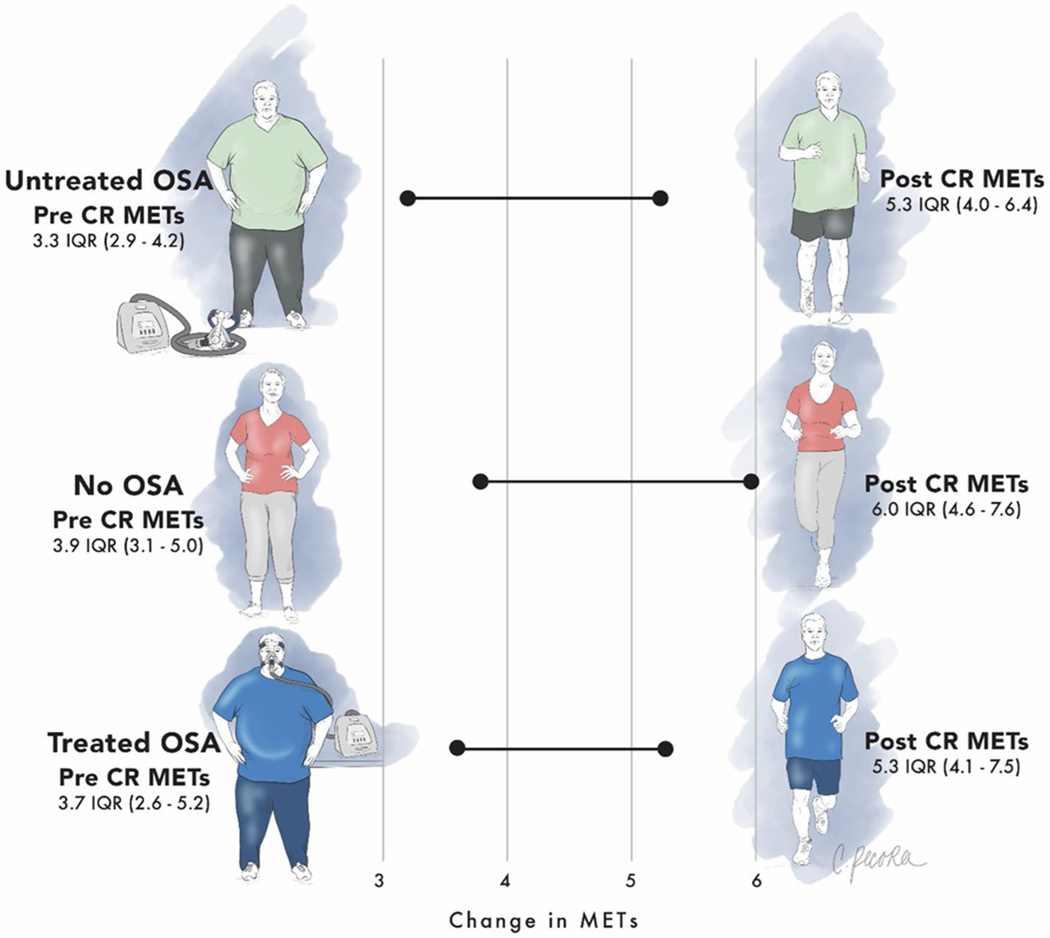
Compared with the no OSA group, untreated OSA patients had significantly lower median pre-CR (baseline) METs (3.3 vs. 3.9, P = 0.03) and lower post-CR METs (5.3 vs. 6, P = 0.052) which did not reach statistical significance, whereas treated OSA patients had similar pre-CR and post-CR METs as patients without OSA (P > 0.5). Both treated and untreated OSA patients achieved a clinically relevant increase in METs [30] during CR which was similar to patients without OSA (2.0 vs. 1.7 vs. 2, Pbetween-groups = 0.18; Pwithin-group < 0.001). Results were similar when adjusting for covariates: Compared with no OSA patients, untreated OSA patients had 11.7% lower pre-CR METs (P = 0.007) and 11.9% lower post-CR METs (P = 0.02), while changes in METs were similar across both groups (P > 0.2; for more details, see Table 3, Fig. 1). Key findings are illustrated in Fig. 2.
Table 3.
Differences in exercise performance based on OSA treatment status
| Univariable analyses |
Multivariable analyses |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | [IQR] | Median | [IQR] | Δ Medians | P crude | P global | beta | SE | P adjusted | InterpretationA |
||
| Δ | (95% CI) | |||||||||||
| Pre-CR METs | ||||||||||||
| OSA treatment status | .03 | |||||||||||
| Untreated vs. no OSA | 3.3 | [2.9 to 4.2] | 3.9 | [3.1 to 5] | −0.6 | .03 | −0.13 | 0.05 | .007 | −11.7% | (− 19.3 to − 3.5) B | |
| Treated vs. no OSA | 3.7 | [2.6 to 5.2] | 3.9 | [3.1 to 5] | −0.2 | .52 | −0.11 | 0.07 | .10 | −10.1% | (−20.9 to 2.1)B | |
| Post-CR METs | ||||||||||||
| OSA treatment status | 0.065 | |||||||||||
| Untreated vs. no OSA | 5.3 | [4 to 6.4] | 6 | [4.6 to 7.6] | −0.7 | .052 | −0.13 | 0.05 | .02 | −11.9% | (− 20.5 to — 2.4) C | |
| Treated vs. no OSA | 5.3 | [4.1 to 7.5] | 6 | [4.6 to 7.6] | −0.7 | .71 | 0.02 | 0.08 | .78 | 2.3% | (−12.6 to −2.4)C | |
| Change in METs | ||||||||||||
| OSA treatment status | 0.18 | |||||||||||
| Untreated vs. no OSA | 1.7 | [0.5 to 2.6] | 2 | [0.9 to 3.0] | −0.3 | .24 | −0.27 | 0.23 | .24 | −0.27 | (−0.71 to 0.18)D | |
| Treated vs. no OSA | 2 | [1.2 to 2.9] | 2 | [0.9 to 3.0] | 0 | .74 | −0.20 | 0.32 | .53 | −0.20 | (−0.43 to 0.83)D | |
For abbreviations, see Table 2 footnote
To promote normality, pre/post-CR METs were log-transformed for multivariable analyses; thus, the beta can be interpreted as a percentage difference based on the formula: [l-exp(beta)]*100; the change in METs did not require transformation; thus, the beta reflects the absolute difference between both groups after adjusting for covariates
Adjusted for sex
Adjusted for sex, body mass index, CR adherence (%sessions), Ornish program (yes/no)
Adjusted for baseline METs, CR adherence (%sessions)
Bolded entries are values meeting statistical significance
Fig. 2.
Illustration of key results. Patients with treated OSA tended to have similar exercise capacity at baseline and follow-up as patients without any OSA
Discussion
Our results add to the literature with several novel findings. First, we found that clinical OSA was highly prevalent (33%) in the population of patients with CVD undergoing the comprehensive CR program, even with the limitation of using clinical diagnosis, as discussed below. In comparison, Spielmanns et al. screened for OSA (using an REI > 15/h) in their study of post-cardiac surgery patients entering CR and found a prevalence of 59% [31]. But to our knowledge, our study is the first to define this prevalence in a more generalizable cohort as it included all applicable referral types to CR (e.g., post-myocardial infarction, post-coronary revascularization, and heart failure). Second, the group with untreated OSA had substantially reduced exercise capacity at baseline and at the completion of CR as assessed by METs, whereas interestingly, patients with treated OSA tended to have a similar exercise capacity as those without OSA. Third, OSA diagnosis or treatment status did not impact on the post-CR change in METs implying that the CR intervention was still impactful. Taken all together, these findings suggest that identifying patients with OSA prior to initiation of CR with the intention of treating them prior to and during CR could be important to help patients realize optimal exercise capacity and CR outcomes.
Fourth, the above-mentioned results also support the notion that OSA itself does have effects on exercise capacity (EC), as suggested in previous studies. A few previous studies examining OSA and exercise training were typically small or focused on the relationship of exercise on sleep apnea and not vice versa. For example, Awad et al. showed that exercise duration was positively associated with protection from sleep-disordered breathing in a longitudinal epidemiologic study of 1521 adults [22]. Furthermore, in a study by Beitler et al. including 34 subjects, OSA was associated with impaired EC as defined by decreased p eakVO2 [23]. Similarly, Przyby-lowski et al. found in 111 OSA patients that a greater REI was associated with decreased peak VO2, elevated blood pressure response during exercise, and delayed recovery of blood pressure post-exercise [32]. A subsequent meta-analysis of studies that evaluated peak VO2 in OSA confirmed by a sleep study vs. no OSA controls found that peak VO2 was preserved in patients with moderate to severe OSA based on a patient-level meta-analysis while the study-level meta-analysis showed lower peak VO2 in severe OSA [33]. Of note, prior studies have shown some improvement in sleep apnea following cardiac rehabilitation, even in the absence of important weight loss, although the underlying mechanisms are unclear [34]. Thus, despite interesting findings, the literature in this area is relatively sparse, with some mixed results, and would likely benefit from larger-scale systematic studies.
Despite our study’s strengths including a substantial sample size of patients with CVD undergoing CR, we acknowledge a number of limitations. First, we relied on clinically diagnosed OSA, and thus, we likely missed subclinical disease, potentially biasing results toward the null. Moreover, we relied on physician diagnosis of OSA in a subset of our participants, recognizing that polysomnography would be the gold standard in this context. Nonetheless, our goal was to do a real-world study of clinical OSA, but we recognize that further work will be required to characterize the impact of subclinical OSA on the EC in patients with CVD more definitively. Second, we assessed EC by measuring METs using a treadmill stress test before and after CR which has been an-established clinical tool. However, we recognize that gold standard measurements would require more detailed exercise testing such as cardiopulmonary exercise testing [35]. Thus, again, we advocate for further research to understand the mechanisms underlying our new findings. Third, we conducted a retrospective analysis, and thus, we did not prospectively assign therapy or have the ability to randomize which patients might receive PAP therapy. Thus, we may suffer from the so-called healthy user effect, whereby CPAP may well ameliorate underlying OSA but may also be a marker of a patient who is motivated, affluent, and/or educated. Indeed, Platt et al. have shown that CPAP use is a marker of statin therapy, and thus, we advocate for randomized trials to draw firm conclusions in the future [36]. Fourth, the null finding for the change in METs could reflect a power issue. But reassuringly, based on post-hoc analyses, we had a power of > 0.98 to detect a medium effect size (Cohen’s d = 0.5) of 0.87 METs, and still a reasonable power of > 0.69 to detect even a small effect size (Cohen’s d = 0.3) of 0.52 METs. Despite these limitations, we believe that our findings are novel and will facilitate and stimulate further research in this area.
Our main conclusions from these findings are that sleep apnea is common in patients participating in cardiac rehabilitation, contributes to impaired exercise capacity, and patients might benefit from OSA screening prior to cardiac rehabilitation to optimize post-cardiac rehabilitation peak exercise capacity.
Supplementary Material
Funding
Dr. Sonners, Dr. Raphelson, Dr. Roberts, Dr. Sykes, and Dr. Swiatkiewicz have nothing to disclose. Dr. Schmickl is supported by NIH T32 grant HL134632, ATS ASPIRE Fellowship, and an unrestricted ATS foundation grant. Dr. Malhotra is funded by NIH. He reports medical education-related income from Livanova, Equillium, and Jazz. ResMed provided a philanthropic donation to UC San Diego. Dr. Taub has served as a consultant for Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi and is a shareholder in Epirium Bio and has received research grants from NIH (R01 DK118278-01 and R01 HL136407) American Heart Association (SDG #15SDG2233005) and Department of Homeland Security/FEMA (EMW-2016-FP-00788).
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11325-022-02704-0.
Declarations
Ethics approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution of UCSD and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Leung RS, Bradley TD (2001) Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 164(12):2147–2165 [DOI] [PubMed] [Google Scholar]
- 2.Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S, Initiative I (2017) Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation 136(19):1840–1850. 10.1161/CIRCULATIONAHA.117.029400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caples SM, Garcia-Touchard A, Somers VK (2007) “Sleep-disordered breathing and cardiovascular risk,” (in eng). Sleep 30(3):291–303 [DOI] [PubMed] [Google Scholar]
- 4.Caples SM, Kara T, Somers VK (2005) “Cardiopulmonary consequences of obstructive sleep apnea,” (in eng). Semin Respir Crit Care Med 26(1):25–32. 10.1055/s-2005-864208 [DOI] [PubMed] [Google Scholar]
- 5.Jordan AS, McSharry DG, Malhotra A (2014) Adult obstructive sleep apnoea. Lancet 383(9918):736–747. 10.1016/S0140-6736(13)60734-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin JM, Carrizo SJ, Vicente E, Agusti AG (2005) “Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study,” (in eng). Lancet 365(9464):1046–53. 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- 7.Montesi SB, Bajwa EK, Malhotra A (2012) “Biomarkers of sleep apnea,” (in eng). Chest 142(1):239–245. 10.1378/chest.11-2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montesi SB, Edwards BA, Malhotra A, Bakker JP (2012) The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 8(5):587–596. 10.5664/jcsm.2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Javaheri S, Somers VK (2011) “Cardiovascular diseases and sleep apnea,” (in eng). Handb Clin Neurol 98:327–345. 10.1016/B978-0-444-52006-7.00020-4 [DOI] [PubMed] [Google Scholar]
- 10.McEvoy RD et al. (2016) “CPAP for prevention of cardiovascular events in obstructive sleep apnea,” (in eng). N Engl J Med 375(10):919–31. 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 11.Jenkinson C (1999) Davies RJ, Mullins R, Stradling JR, “Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial,.” Lancet 353(9170):2100–2105 [DOI] [PubMed] [Google Scholar]
- 12.Weaver TE et al. (1997) An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 20(10):835–843 [PubMed] [Google Scholar]
- 13.Świątkiewicz I, Di Somma S, De Fazio L, Mazzilli V, Taub PR (2021) Effectiveness of intensive cardiac rehabilitation in highrisk patients with cardiovascular disease in real-world practice. Nutrients 13(11). 10.3390/nu13113883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan G et al. (2021) Cardiac rehabilitation: a bibliometric review from 2001 to 2020. Front Cardiovasc Med 8:672913. 10.3389/fcvm.2021.672913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor RS, Anderson L, Oldridge N, Thompson DR, Zwisler AD, Dalal H (2017) The efficacy of exercise-based cardiac rehabilitation: the changing face of usual care. J Am Coll Cardiol 69(9):1207–1208. 10.1016/j.jacc.2016.10.084 [DOI] [PubMed] [Google Scholar]
- 16.Anderson L et al. (2016) Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 67(1):1–12. 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 17.MacDonald M, Fang J, Pittman SD, White DP, Malhotra A (2008) “The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers,” (in eng). J Clin Sleep Med 4(1):38–42 [PMC free article] [PubMed] [Google Scholar]
- 18.Benjafield AV et al. (2019) Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 7(8):687–698. 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sin D, Fitzgerald F, Parker J (1999) Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 160:1101–1106 [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb DJ, Punjabi NM (2020) Diagnosis and management of obstructive sleep apnea: a review. JAMA 323(14):1389–1400. 10.1001/jama.2020.3514 [DOI] [PubMed] [Google Scholar]
- 21.Awad KM, Drescher AA, Malhotra A, and Quan SF (2012) “Effects of exercise and nutritional intake on sleep architecture in adolescents,” (in Eng). Sleep Breath. 10.1007/s11325-012-0658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awad KM, Malhotra A, Barnet JH, Quan SF, Peppard PE (2012) “Exercise is associated with a reduced incidence of sleep-disordered breathing,” (in eng). Am J Med 125(5):485–490. 10.1016/j.amjmed.2011.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beitler JR et al. (2014) “Obstructive sleep apnea is associated with impaired exercise capacity: a cross-sectional study,” J Clin Sleep Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jen R et al. (2021) Impact of obstructive sleep apnea on cardiopulmonary performance, endothelial dysfunction, and pulmonary hypertension during exercise. Respir Physiol Neurobiol 283:103557. 10.1016/j.resp.2020.103557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagappa M et al. (2015) “Validation of the STOP-Bang Questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis,” (in eng). PLoS ONE 10(12):e0143697. 10.1371/journal.pone.0143697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman AM, Taub PR, Lo HC, Ornish D (2019) Intensive cardiac rehabilitation: an underutilized resource. Curr Cardiol Rep. 21(4):19. 10.1007/s11886-019-1104-1 [DOI] [PubMed] [Google Scholar]
- 27.Cistulli PA et al. (2019) Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med 59:114–116. 10.1016/j.sleep.2019.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rengo JL, Khadanga S, Savage PD, Ades PA (2020) Response to exercise training during cardiac rehabilitation differs by sex. J Cardiopulm Rehabil Prev 40(5):319–324. 10.1097/HCR.0000000000000536 [DOI] [PubMed] [Google Scholar]
- 29.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I (2001) “Controlling the false discovery rate in behavior genetics research,” (in eng). Behav Brain Res 125(1–2):279–284. 10.1016/s0166-4328(01)00297-2 [DOI] [PubMed] [Google Scholar]
- 30.Martin BJ, Arena R, Haykowsky M et al. (2013) Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clin Proc 88(5):455–463. 10.1016/j.mayocp.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 31.Spielmanns M, Pantev S, Turk A, Barthelmes J, Schindler M, Hermann M (2021) “Does an undetected obstructive sleep apnea influence the natural course and success of cardiac rehabilitation after cardiac surgery?,” (in eng). Eur J Phys Rehabil Med 57(1):148–157. 10.23736/S1973-9087.20.06340-6 [DOI] [PubMed] [Google Scholar]
- 32.Przybyłowski T et al. (2007) “Exercise capacity in patients with obstructive sleep apnea syndrome,” (in eng). J Physiol Pharmacol 58(5):563–74 [PubMed] [Google Scholar]
- 33.Berger M et al. (2019) “Does obstructive sleep apnea affect exercise capacity and the hemodynamic response to exercise? An individual patient data and aggregate meta-analysis,” (in eng). Sleep Med Rev 45:42–53. 10.1016/j.smrv.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 34.Mendelson Monique et al. (2020) Long-term effects of cardiac rehabilitation on sleep apnea severity in patients with coronary artery disease. J Clin Sleep Med 16(1):65–71. 10.5664/jcsm.8124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolle JJ, Waxman AB, Van Horn TL, Pappagianopoulos PP, Systrom DM (2008) Exercise-induced pulmonary arterial hypertension. Circulation 118(21):2183–2189. 10.1161/CIRCULATIONAHA.108.787101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platt AB et al. (2010) “Adherence to sleep apnea therapy and use of lipid-lowering drugs: a study of the healthy-user effect,” (in eng). Chest 137(1):102–108. 10.1378/chest.09-0842 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.